Patents
Literature
1260 results about "Serum samples" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Non-invasive detection of fetal genetic traits
InactiveUS20050164241A1Facilitates non-invasive detectionComponent separationOther chemical processesPregnancyNon invasive
Blood plasma of pregnant women contains fetal and (generally>90%) maternal circulatory extracellular DNA. Most of said fetal DNA contains ≦500 base pairs, said maternal DNA having a greater size. Separation of circulatory extracellular DNA of <500 base pairs results in separation of fetal from maternal DNA. A fraction of a blood plasma or serum sample of a pregnant woman containing, due to size separation (e.g. by chromatography, density gradient centrifugation or nanotechnological methods), extracellular DNA substantially comprising ≦500 base pairs is useful for non-invasive detection of fetal genetic traits (including the fetal RhD gene in pregnancies at risk for HDN; fetal Y chromosome-specific sequences in pregnancies at risk for X chromosome-linked disorders; chromosomal aberrations; hereditary Mendelian genetic disorders and corresponding genetic markers; and traits decisive for paternity determination) by e.g. PCR, ligand chain reaction or probe hybridization techniques, or nucleic acid arrays.
Owner:SEQUENOM INC
Process for discriminating between biological states based on hidden patterns from biological data
The invention describes a process for determining a biological state through the discovery and analysis of hidden or non-obvious, discriminatory biological data patterns. The biological data can be from health data, clinical data, or from a biological sample, (e.g., a biological sample from a human, e.g., serum, blood, saliva, plasma, nipple aspirants, synovial fluids, cerebrospinal fluids, sweat, urine, fecal matter, tears, bronchial lavage, swabbings, needle aspirantas, semen, vaginal fluids, pre-ejaculate.), etc. which is analyzed to determine the biological state of the donor. The biological state can be a pathologic diagnosis, toxicity state, efficacy of a drug, prognosis of a disease, etc. Specifically, the invention concerns processes that discover hidden discriminatory biological data patterns (e.g., patterns of protein expression in a serum sample that classify the biological state of an organ) that describe biological states.
Owner:ASPIRA WOMENS HEALTH INC +1
Non-invasive detection of fetal genetic traits
ActiveUS20080071076A1Facilitates non-invasive detectionSugar derivativesOther chemical processesPregnancyNon invasive
Blood plasma of pregnant women contains fetal and (generally>90%) maternal circulatory extracellular DNA. Most of said fetal DNA contains .Itoreq.500 base pairs, said maternal DNA having a greater size. Separation of circulatory extracellular DNA of .Itoreq.500 base pairs results in separation of fetal from maternal DNA. A fraction of a blood plasma or serum sample of a pregnant woman containing, due to size separation (e.g. by chromatography, density gradient centrifugation or nanotechnological methods), extracellular DNA substantially comprising .Itoreq.500 base pairs is useful for non-invasive detection of fetal genetic traits (including the fetal RhD gene in pregnancies at risk for HDN; fetal Y chromosome-specific sequences in pregnancies at risk for X chromosome-linked disorders; chromosomal aberrations; hereditary Mendelian genetic disorders and corresponding genetic markers; and traits decisive for paternity determination) by e.g. PCR, ligand chain reaction or probe hybridization techniques, or nucleic acid arrays.
Owner:SEQUENOM INC
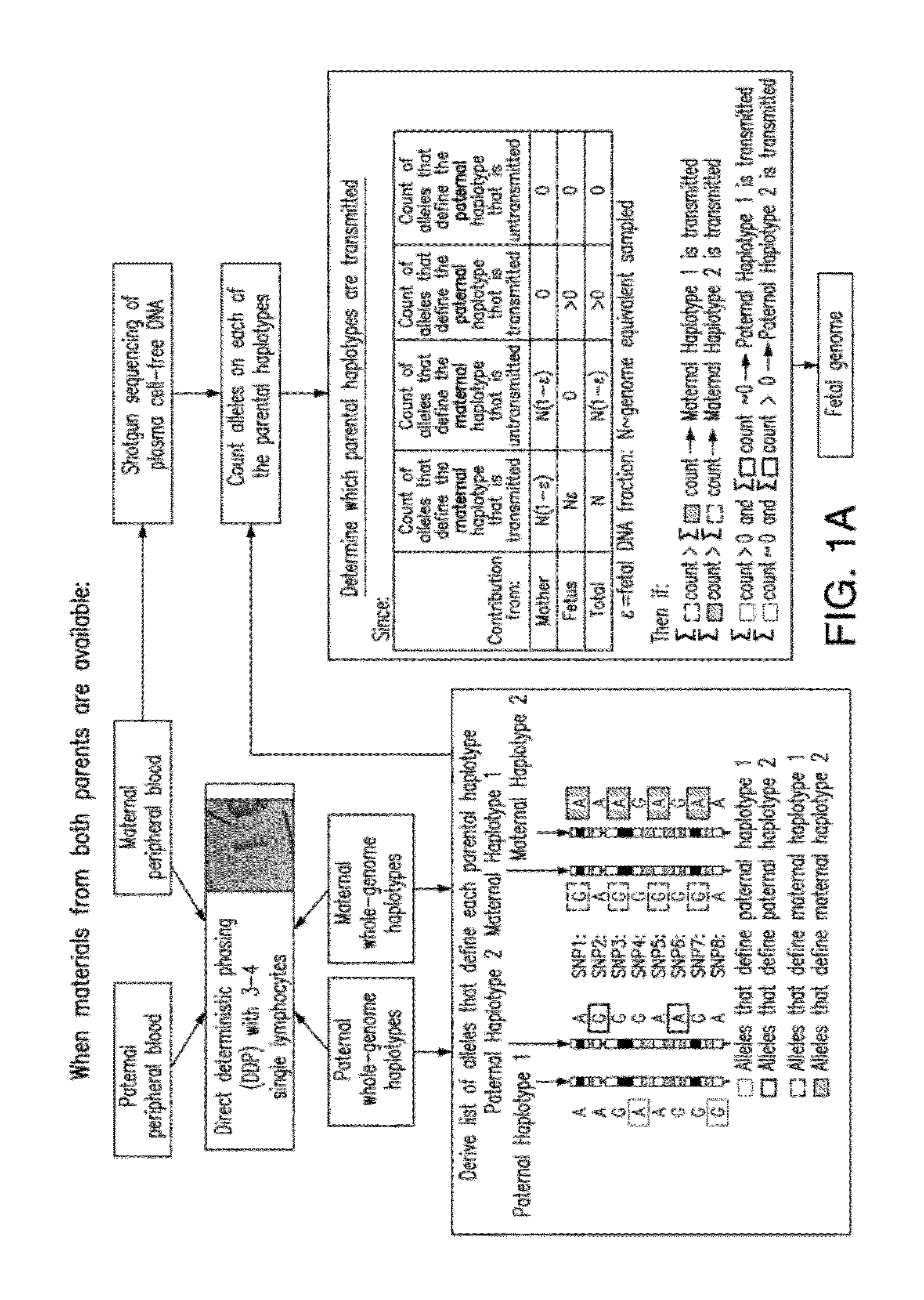
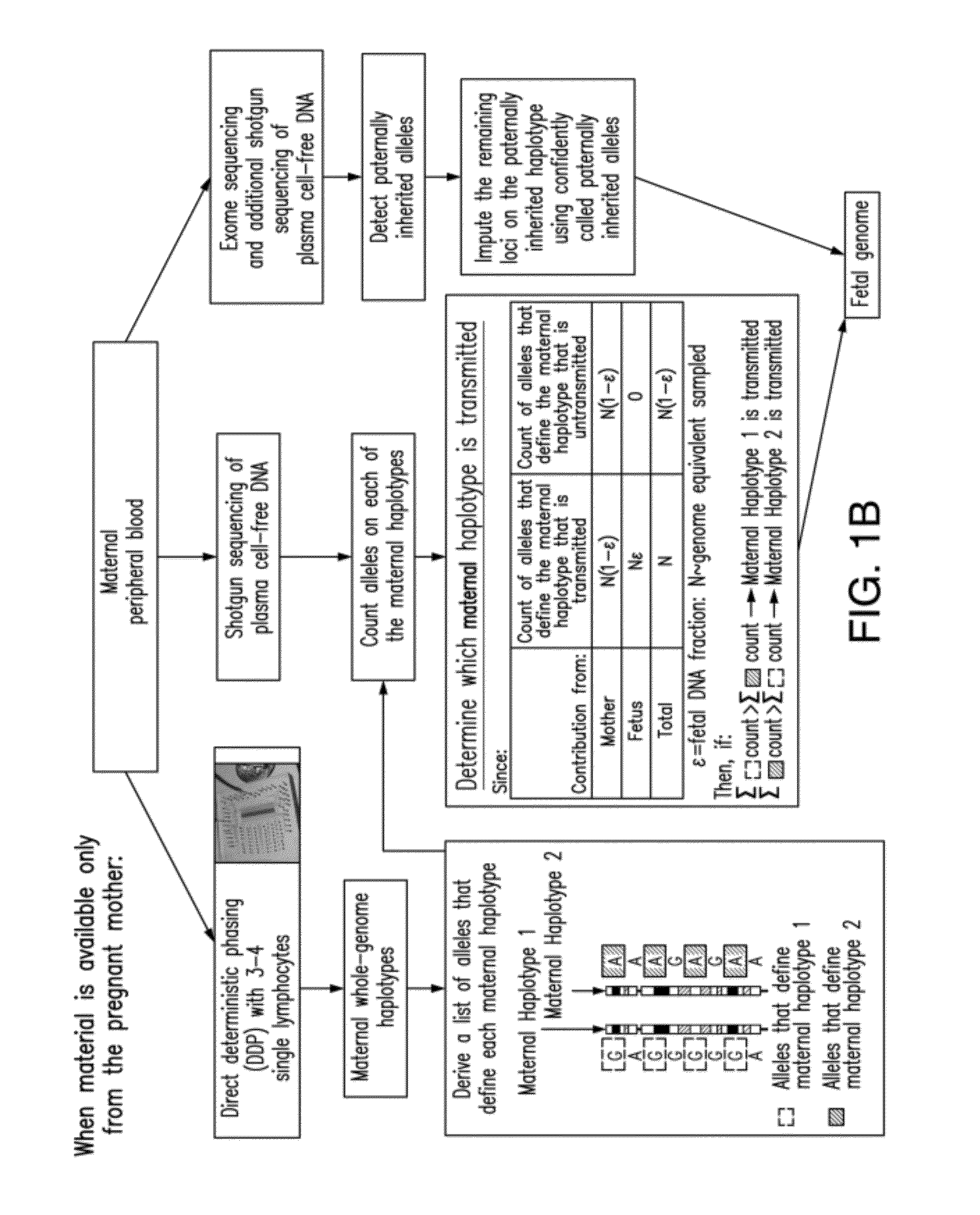
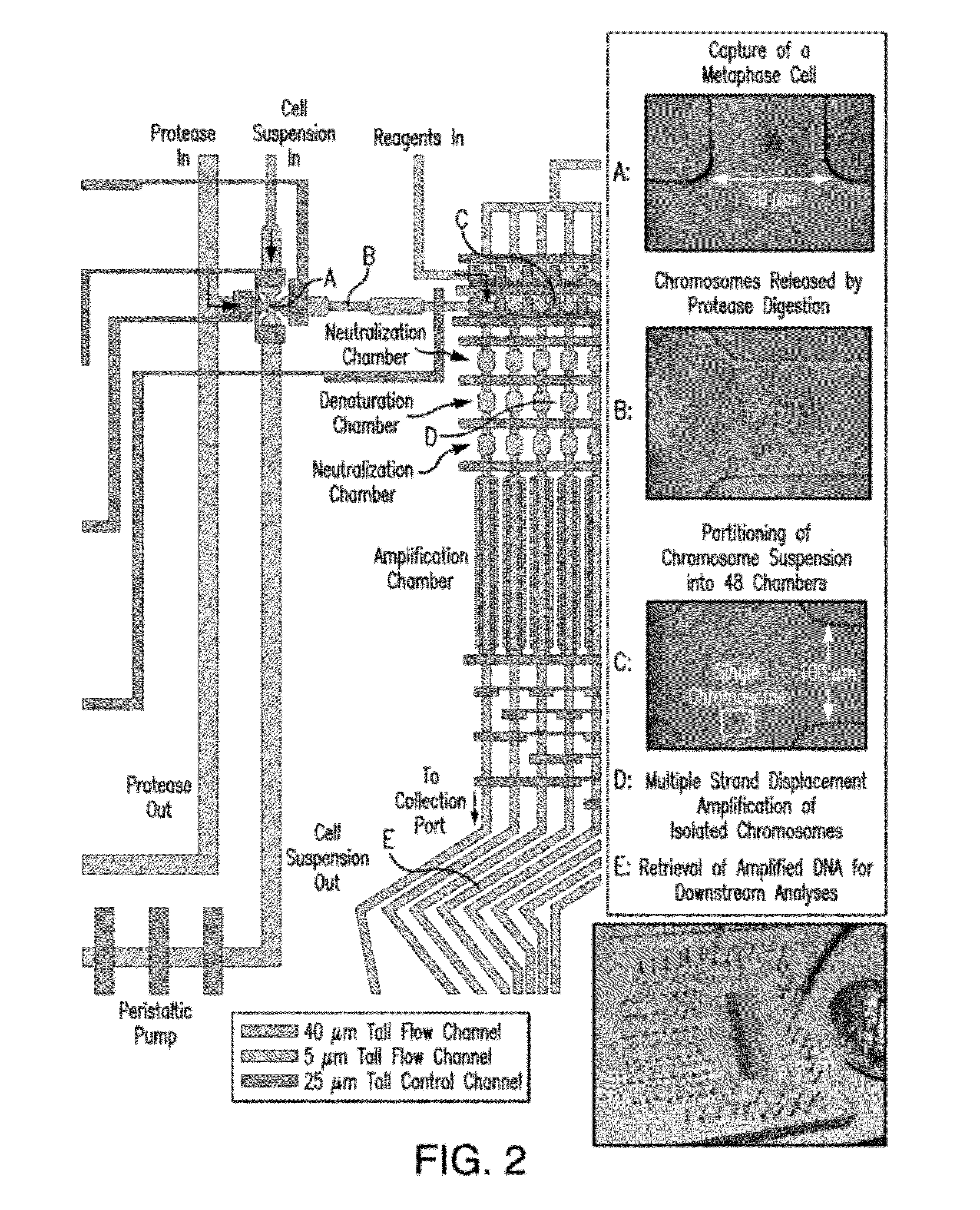
Non-invasive determination of fetal inheritance of parental haplotypes at the genome-wide scale
ActiveUS20120196754A1Reduce riskNon-invasively determiningBioreactor/fermenter combinationsBiological substance pretreatmentsSequence analysisSerum samples
The present invention provides a method, device and a computer program for haplotyping single cells, such that a sample taken from a pregnant female, without directly sampling the fetus, provides the ability to non-invasively determine the fetal genome. The method can be performed by determining the parental and inherited haplotypes, or can be performed merely on the basis of the mother's genetic information, obtained preferably in a blood or serum sample. The novel device allows for sequence analysis of single chromosomes from a single cell, preferably by partitioning single chromosomes from a metaphase cell into long, thin channels where a sequence analysis can be performed.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
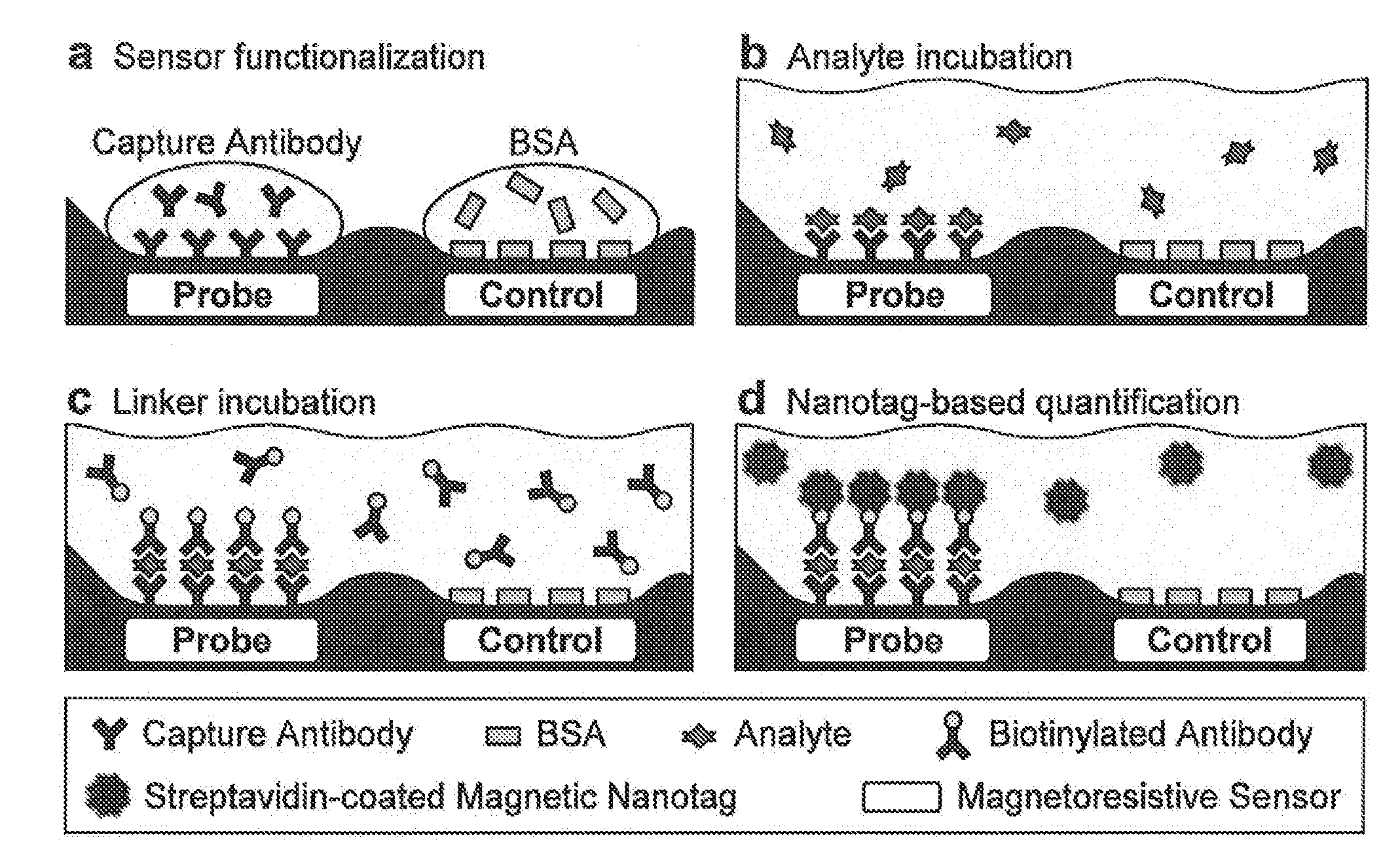
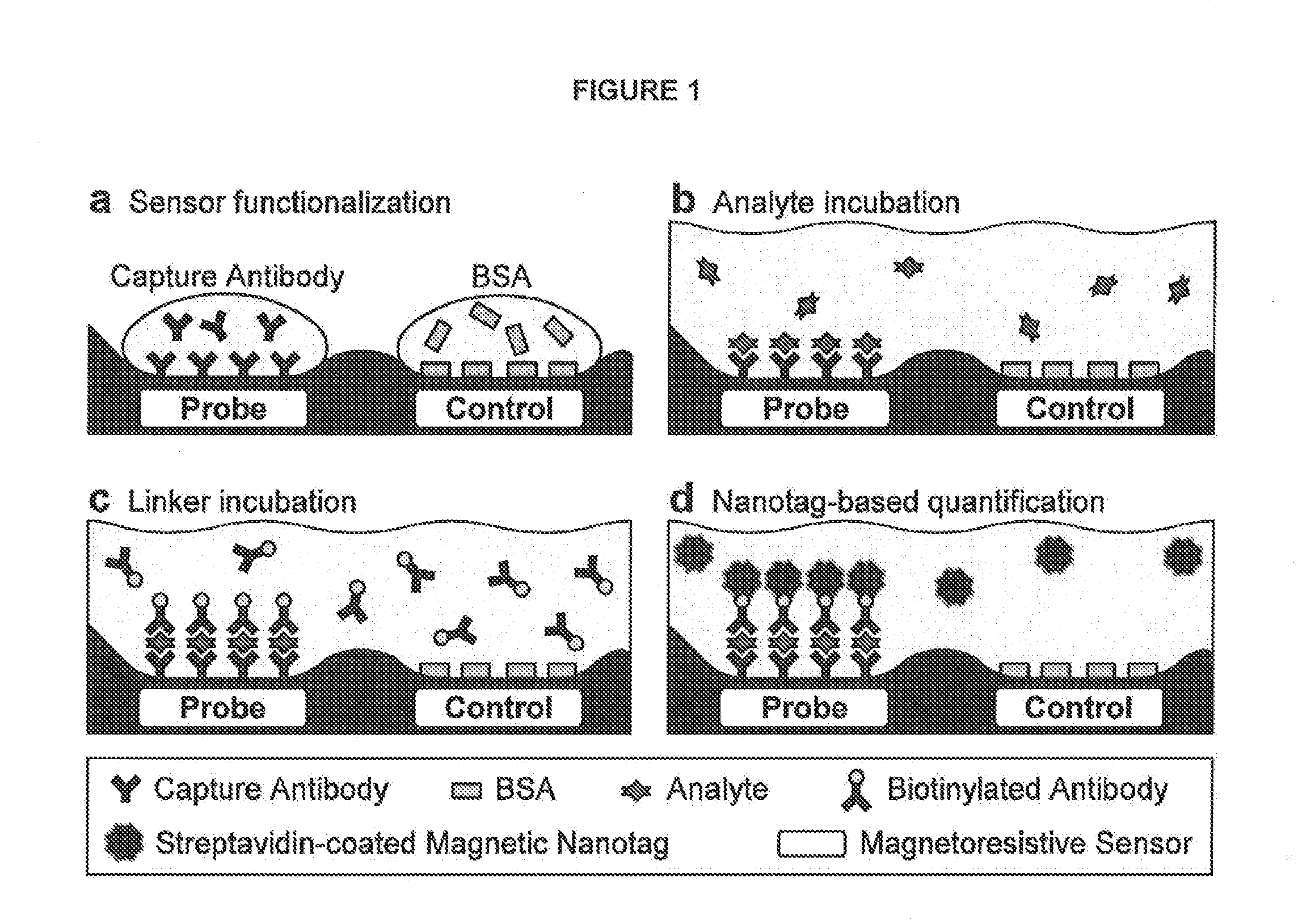
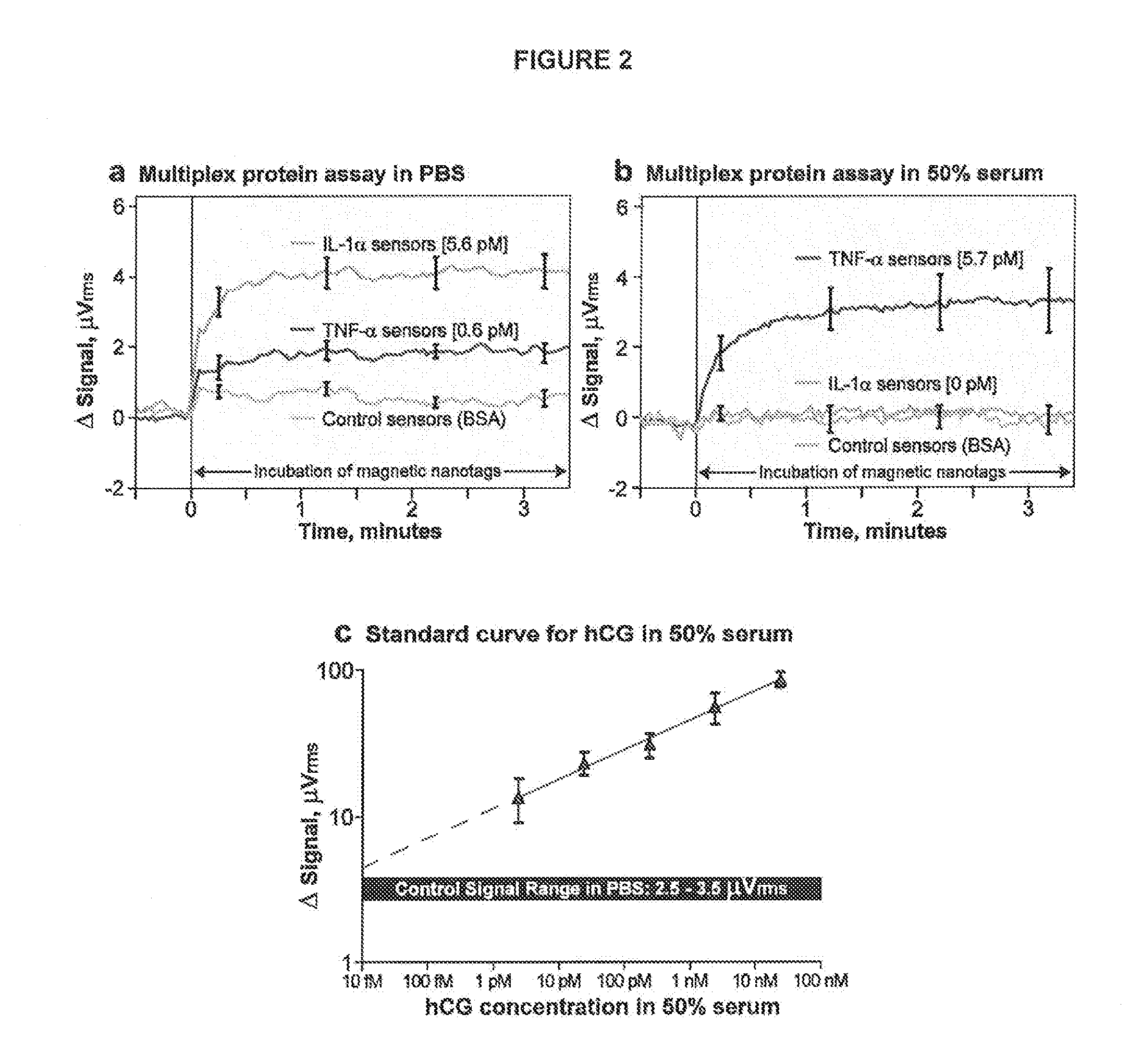
Analyte detection with magnetic sensors
ActiveUS20090104707A1Material analysis by electric/magnetic meansBiological testingDisease markersAnalyte
Methods for analyte detection with magnetic sensors are provided. Aspects of the methods include producing a magnetic sensor device having a magnetically labeled analyte from a sample, such as a serum sample, bound to a surface of a magnetic sensor thereof; and obtaining a signal, e.g., a real-time signal, from the magnetic sensor to determine whether the analyte is present in the sample. Also provided are devices, systems and kits that find use in practicing the methods of the invention. The methods, devices, systems and kits of the invention find use in a variety of different applications, including detection of biomarkers, such as disease markers.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV +1
Reagents and methods for diagnosing, imaging and treating atherosclerotic disease
The invention provides a novel human Mab Fab, cloned by phage display, and its use in diagnostic and therapeutic methods. In particular the invention provides a method for analyzing the OxLDL components of atherosclerotic plaques in vivo and a means to determine their relative pathology. As the method is based on a human Fab rather than a mouse Mab, the progress or regression of the disease may be monitored over time. The antibody may also be used for the analysis of surgical or serum samples ex vivo for the presence of OxLDL. The antibody may also be used to target therapeutic agents to the site of atherosclerotic plaques or may have use as a therapeutic agent itself.
Owner:RGT UNIV OF CALIFORNIA
Methods and compositions for the detection of ovarian disease
InactiveUS20090081685A1Easy diagnosisIncrease chanceMicrobiological testing/measurementBiological material analysisDiseaseComplement system
Methods and compositions for identifying ovarian cancer in a patient sample are provided. The methods of the invention comprise detecting overexpression of at least one biomarker in a body sample, wherein the biomarker is selectively overexpressed in ovarian cancer. In preferred embodiments, the body sample is a serum sample. The biomarkers of the invention include any genes or proteins that are selectively overexpressed in ovarian cancer, including, for example, acute phase reactants, lipoproteins, proteins involved in the regulation of the complement system, regulators of apoptosis, proteins that bind hemoglobin, heme, or iron, cytostructural proteins, enzymes that detoxify metabolic byproducts, growth factors, and hormone transporters. In some aspects of the invention, overexpression of a biomarker of interest is detected at the protein level using biomarker-specific antibodies or at the nucleic acid level using nucleic acid hybridization techniques. Kits for practicing the methods of the invention are further provided.
Owner:TRIPATH IMAGING INC
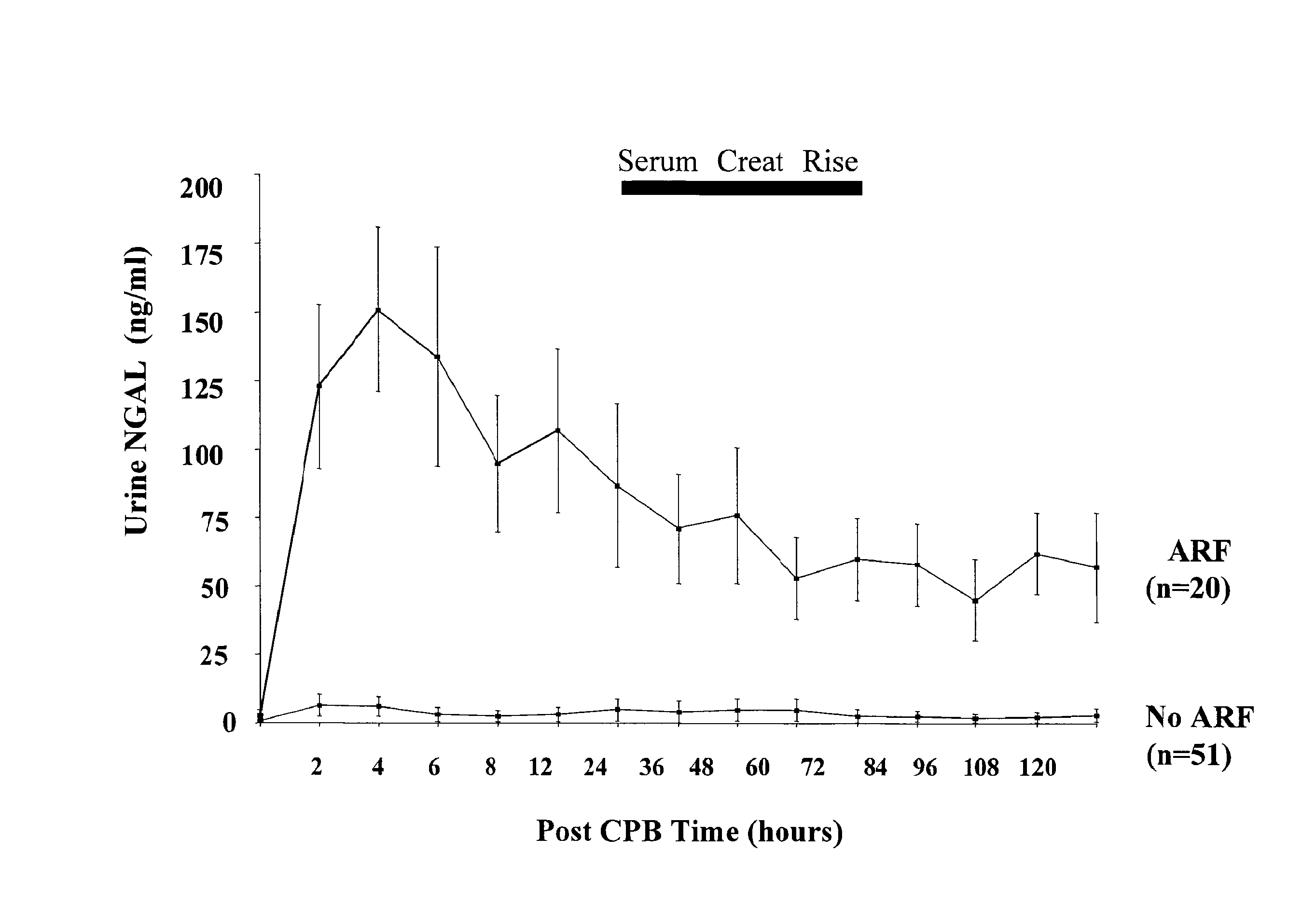
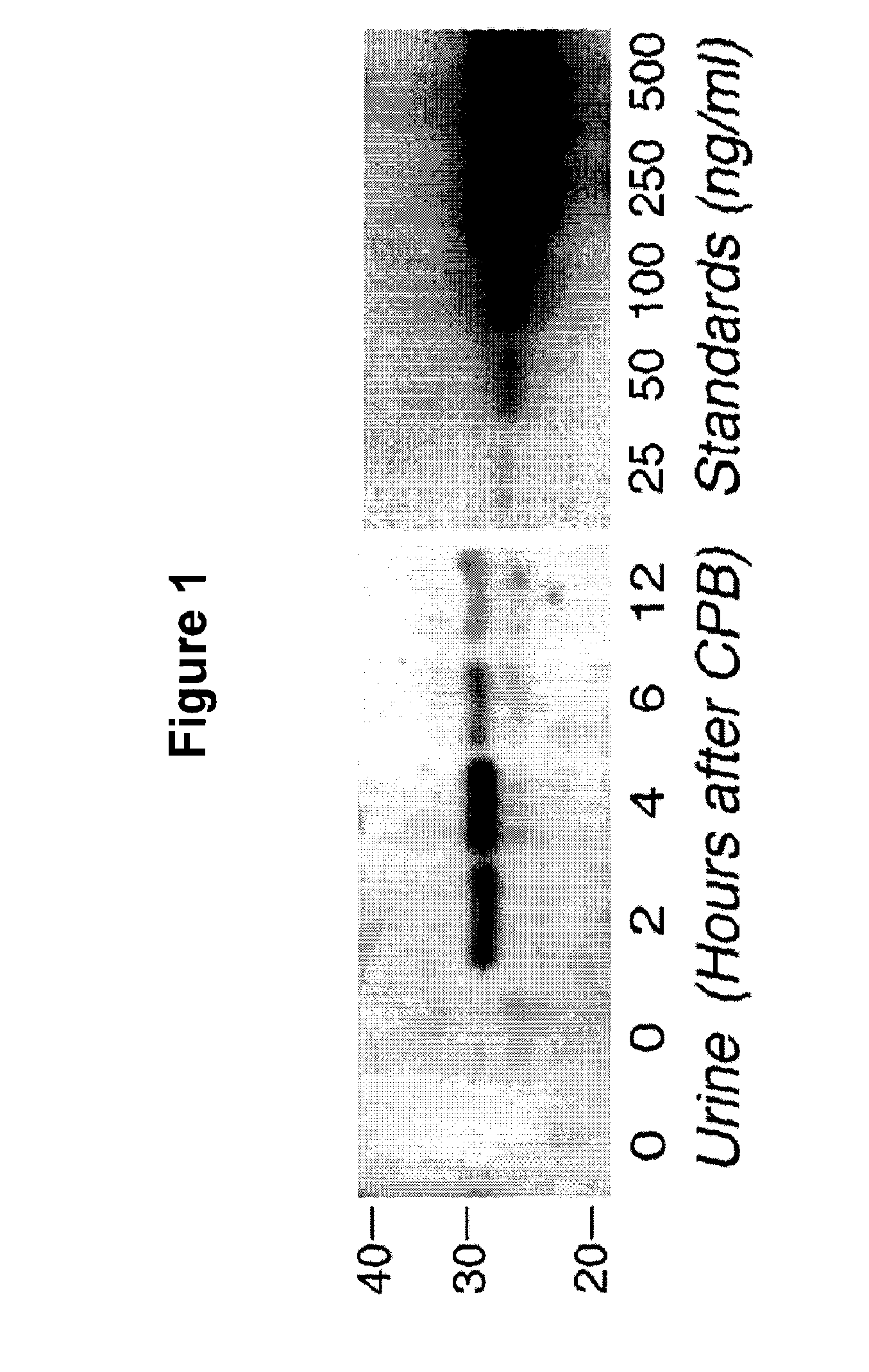
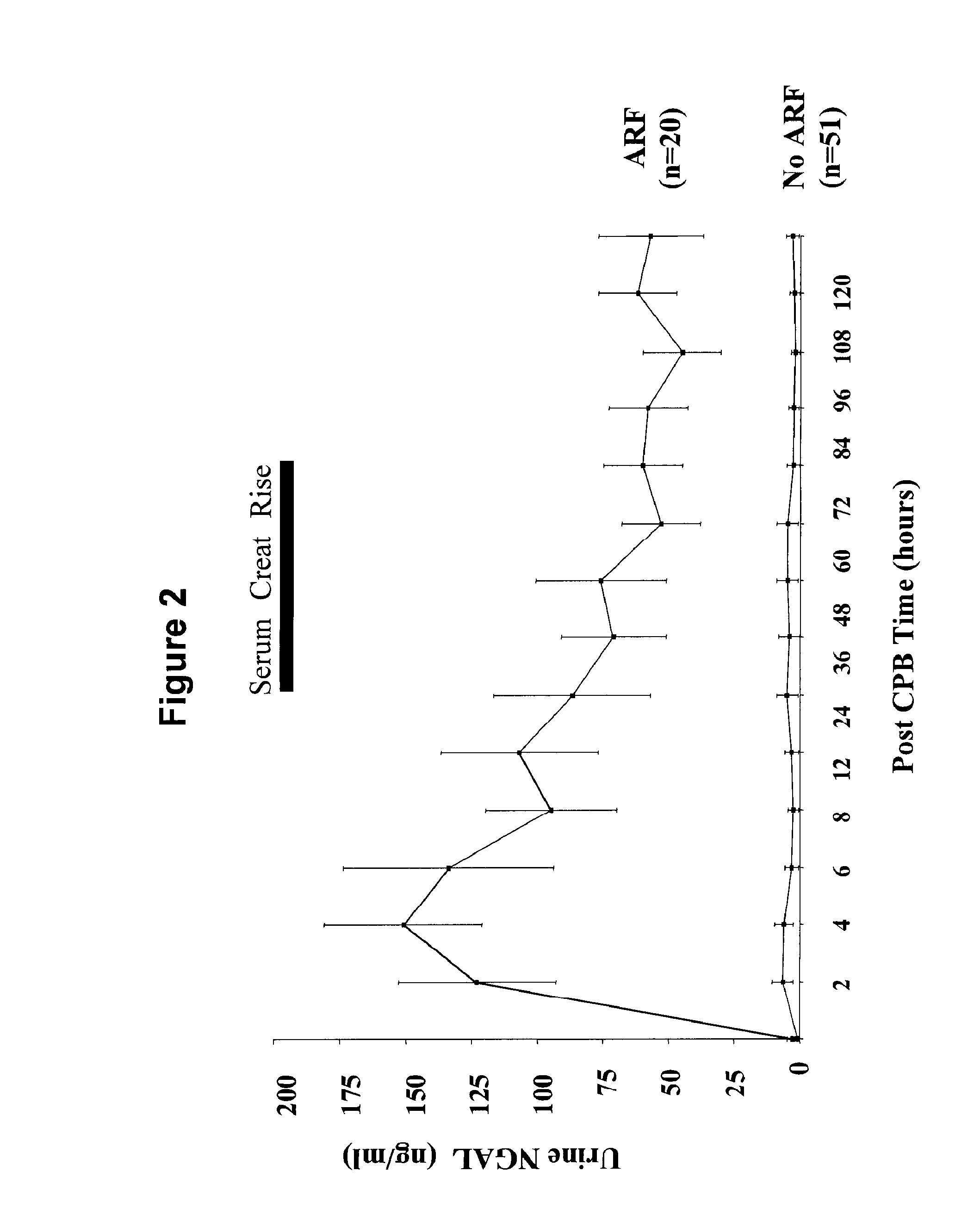
Method for the early detection of renal injury
A method and kit for detecting the immediate or early onset of renal disease and injury, including renal tubular cell injury, utilizing NGAL as an immediate or early on-set biomarker in a sample of blood serum. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the blood serum following renal tubule cell injury. NGAL protein expression is detected predominantly in proximal tubule cells, in a punctuate cytoplasmic distribution reminiscent of a secreted protein. The appearance NGAL in the serum is related to the dose and duration of renal ischemia and nephrotoxemia, and is diagnostic of renal tubule cell injury and renal failure. NGAL detection is also a useful marker for monitoring the nephrotoxic side effects of drugs or other therapeutic agents.
Owner:DEVARAJAN PRASAD +1
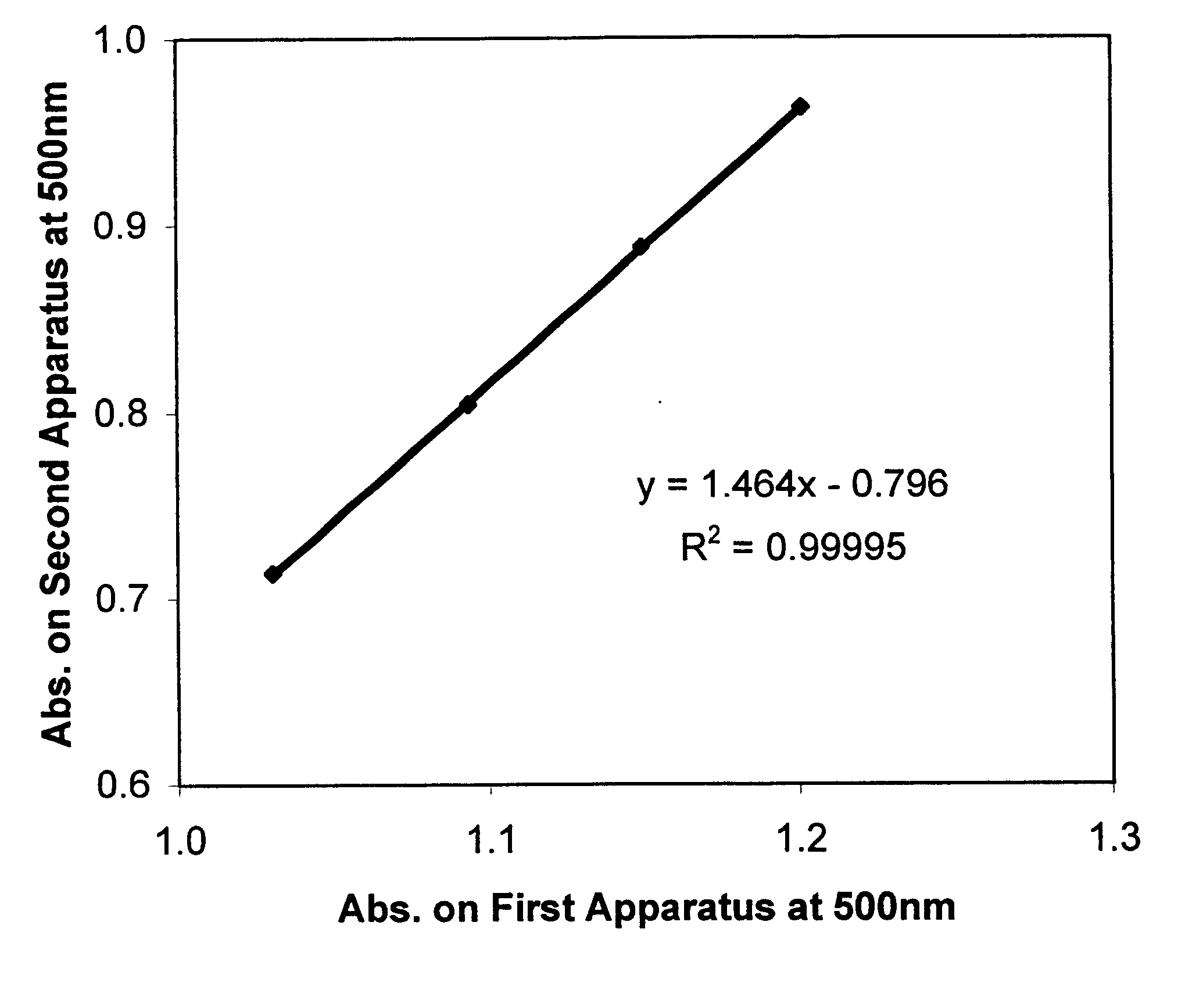
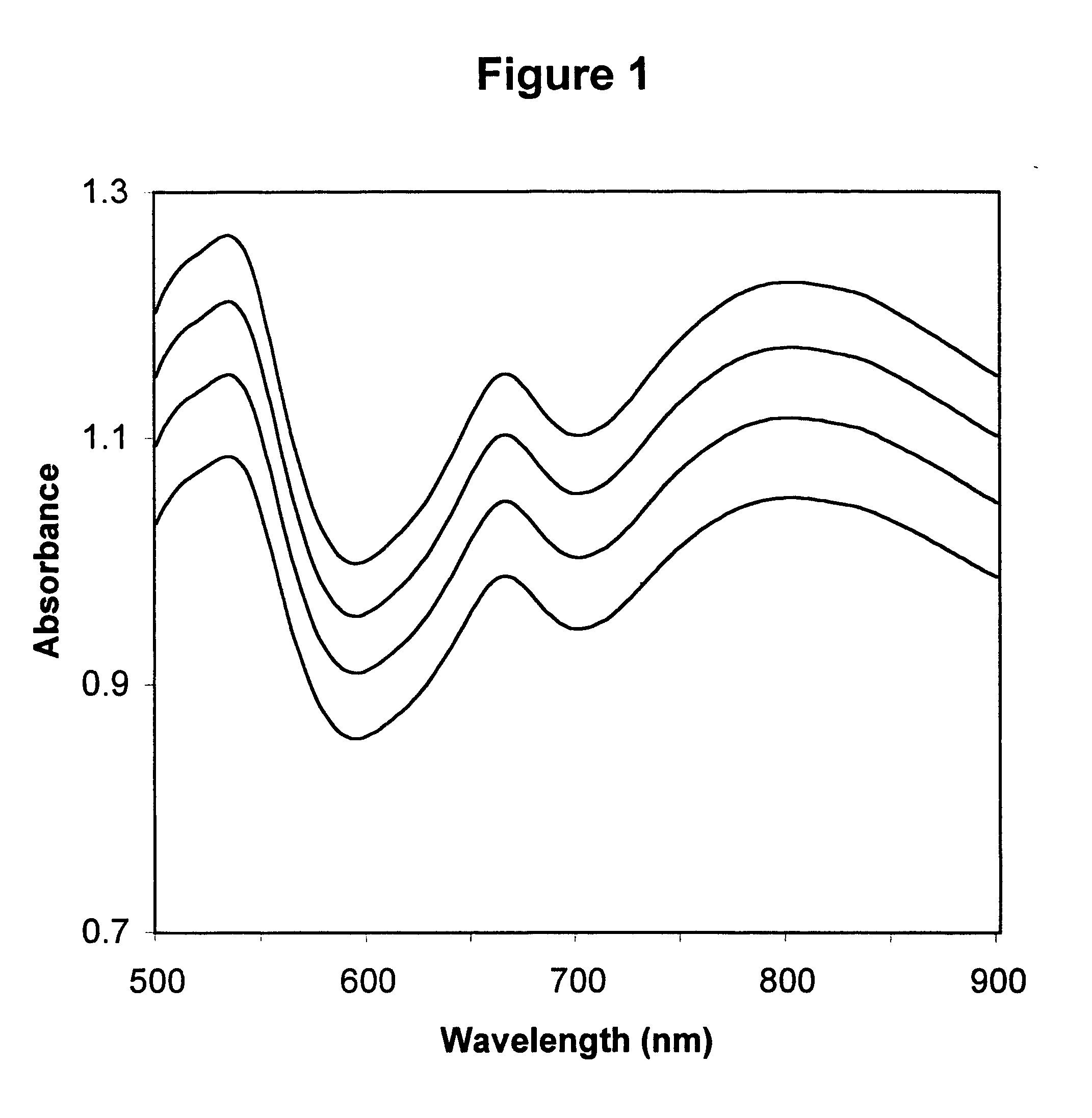
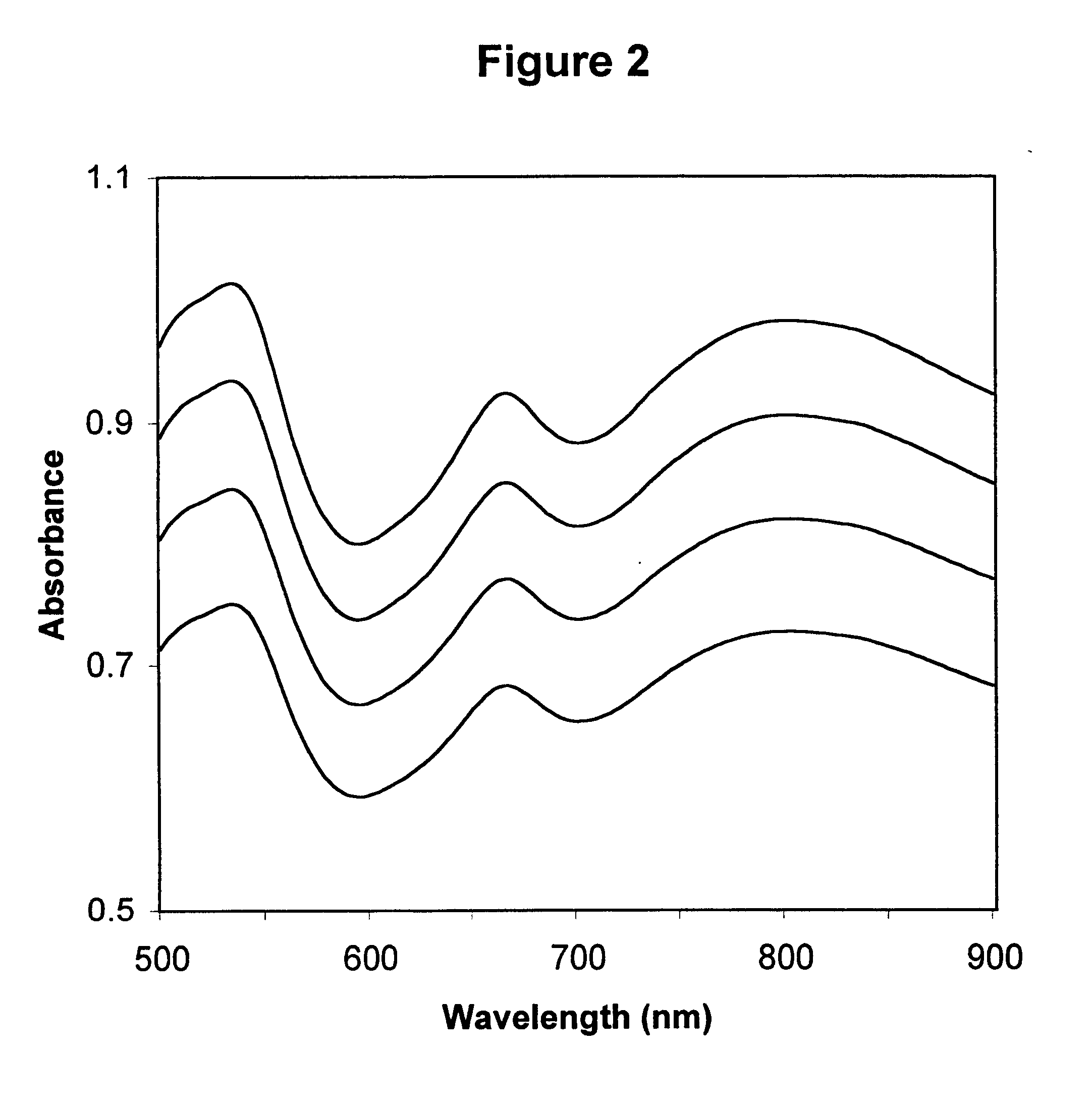
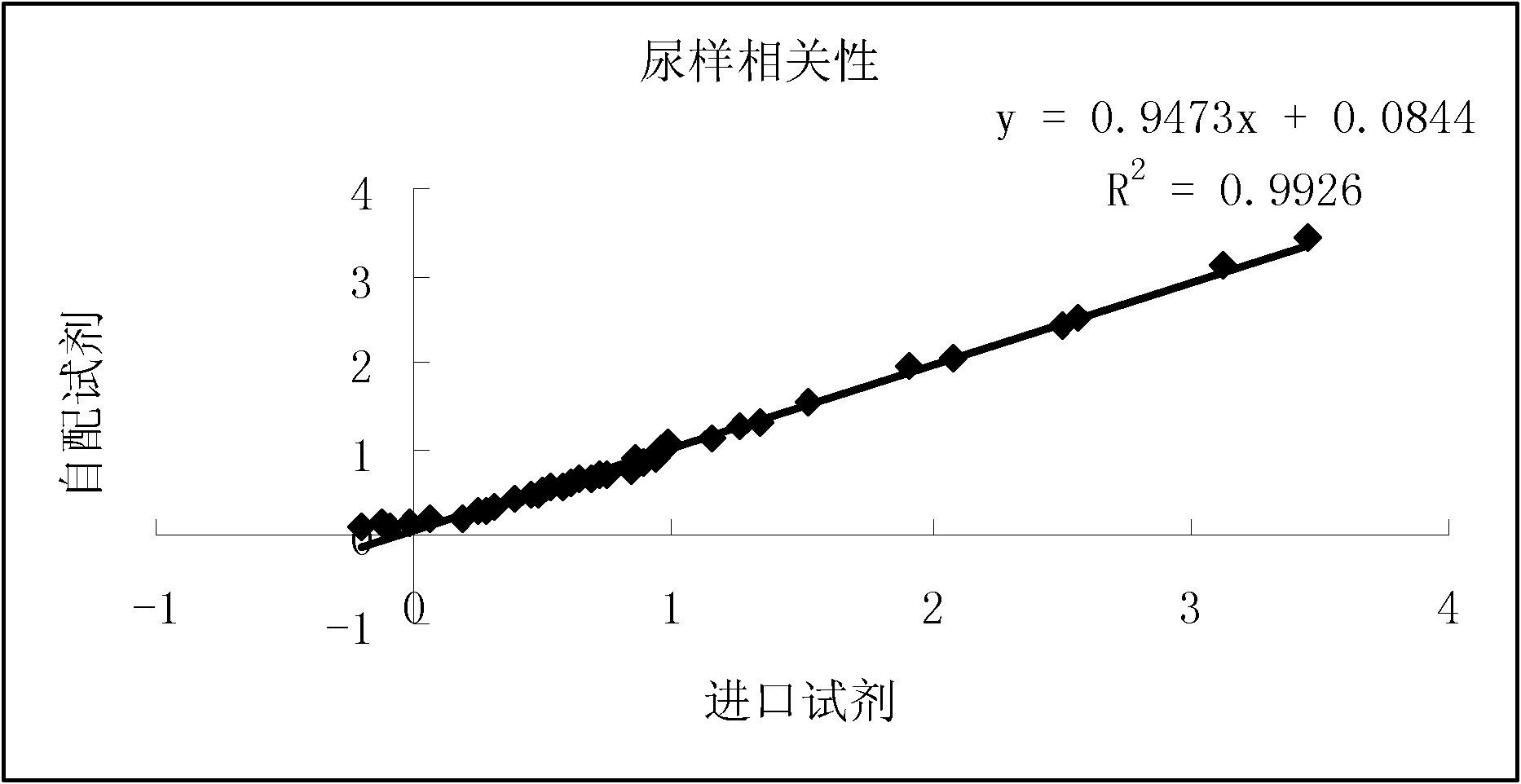
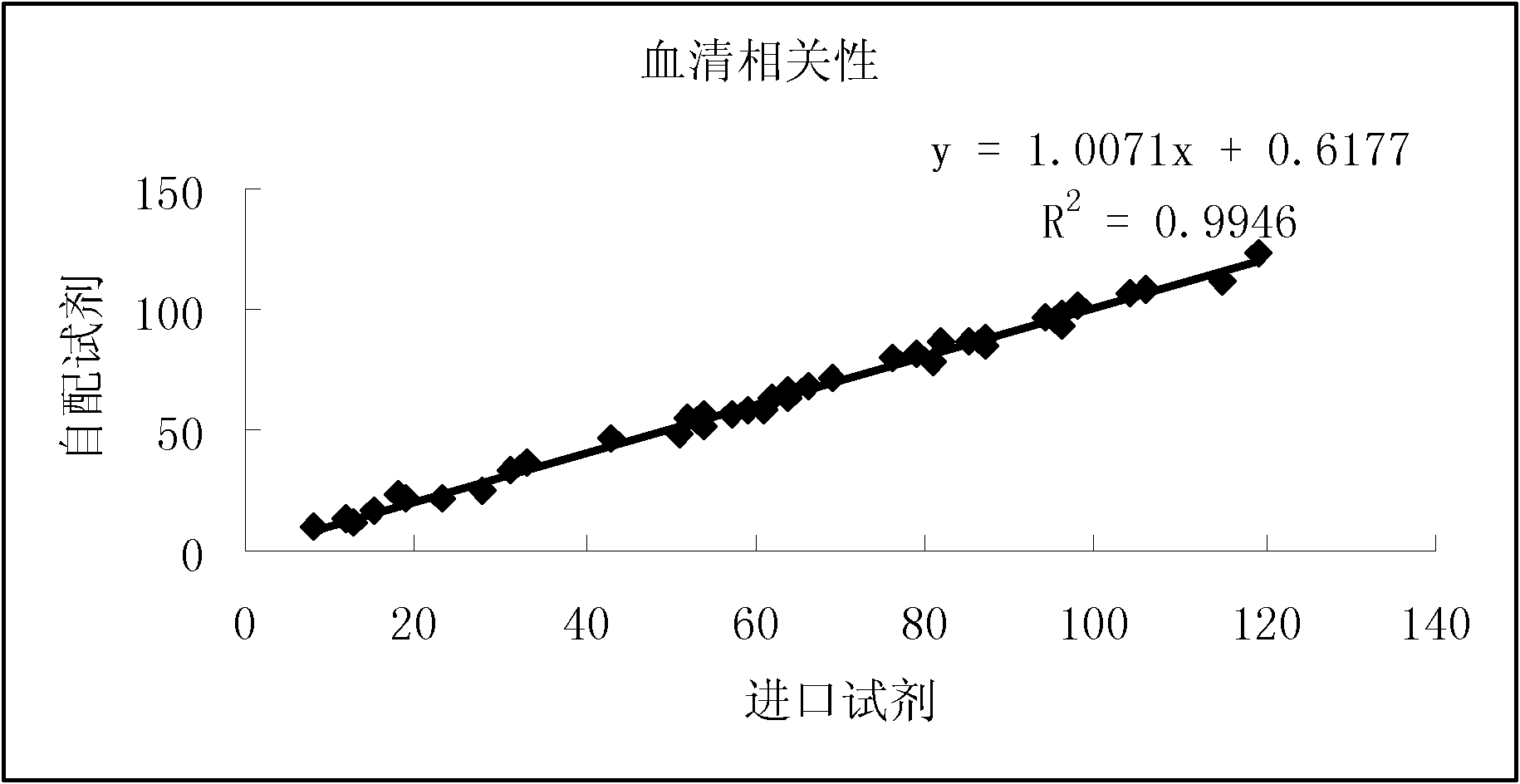
Method for calibrating spectrophotometric apparatus with synthetic fluids to measure plasma and serum analytes
InactiveUS6470279B1Testing/calibration apparatusScattering properties measurementsSample MeasureAnalyte
Described is a method for calibrating a spectrophotometric apparatus which is used to measure analytes in plasma and / or serum samples based on the calibration of a First Apparatus, and for recalibrating such apparatus, including recalibration of the First Apparatus all using synthetic calibrators. These apparatus use absorption of radiation to measure analytes in serum or plasma samples. The method described includes using synthetic calibrators which are submitted to the First Apparatus for measurement and compared with measurements of similar calibrators in a Second Apparatus and using the comparison to derive concentrations of analytes in samples measured on the Second Apparatus. As an alternative to making all apparatus identical, in terms of wavelength calibration, the absorbances of all apparatus should be mapped onto a standard set of wavelengths.
Owner:TYCO HEALTHCARE GRP LP
Methods and devices for quantitative detection of prostate specific membrane antigen and other prostatic markers
The invention provides for the detection and quantification of PSMA, PSMA', and other prostatic markers in serum samples as well as in other types of samples for use in differentiating prostate cancer, benign prostatic hyperplasia, and negative diagnoses. The diagnostic detection of nucleic acids, such as mRNAs, which encode prostatic markers in cell lysates and other sample sources is also provided. In addition to the multiplexed detection / quantification of these protein- and nucleic acid-based markers, the invention also includes biochips, kits and integrated systems.
Owner:EASTERN VIRGINIA MEDICAL SCHOOL
Method for detecting multiple lipid-soluble vitamins in blood sample simultaneously
ActiveCN105158394AEasy to handleImprove throughputComponent separationChromatographic separationThree level
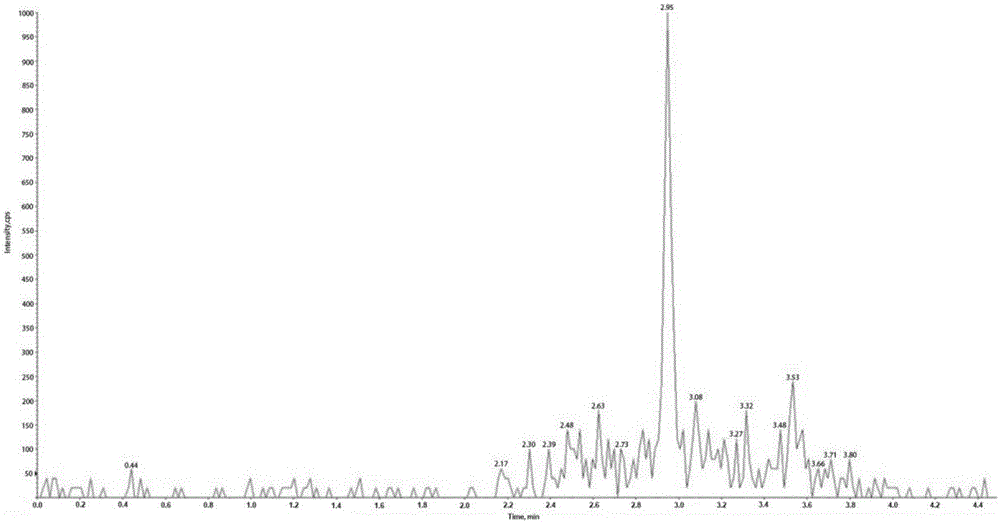
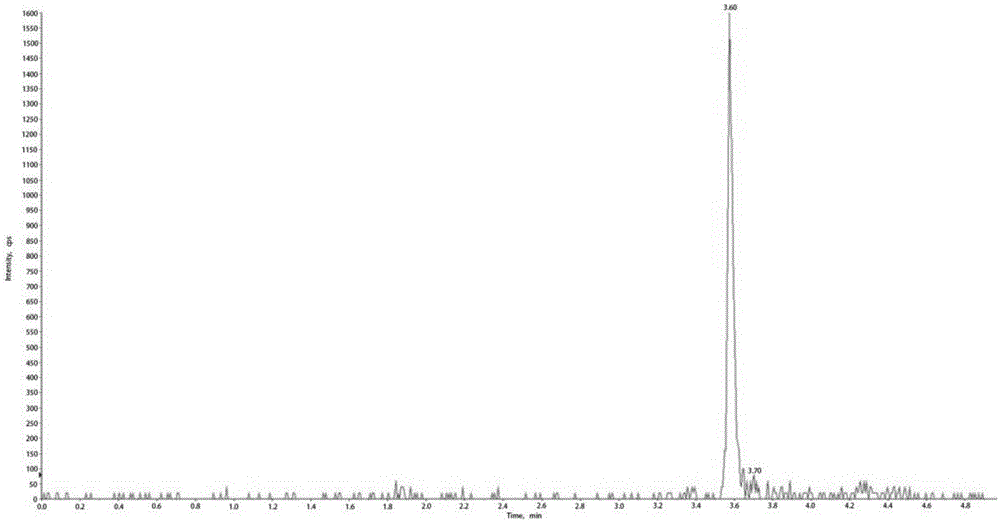
The invention discloses a method for detecting multiple lipid-soluble vitamins in a blood sample simultaneously. The method comprises the steps of preprocessing a biological sample by a simple liquid-liquid extraction method, then carrying out chromatographic separation and mass spectrometric detection, respectively selecting a pair of qualitative ions and a pair of quantitative ions according to each vitamin, wherein the relative retention time and qualitative ion pairs of the various vitamins are taken as qualitative basis, and a standard curve for quantitation is formed by standard substances. Meanwhile, according to the method, three levels of quality control substances are used for inspecting the accuracy and effectiveness of the method, and the distortion of the detection result is avoided. According to the method, the purpose that six lipid-soluble vitamins in one serum sample can be simultaneously detected can be realized for the first time by applying an LC-MS technology, the influence caused by interferents can be alleviated, the operation is simple, convenient and rapid, the analysis time is only 4.8min, the throughput is high, the cost is low, the levels of lipid-soluble vitamins in a human body are effectively monitored, and the method has guiding significance on the reasonable and safe supplementation of vitamins, and is liable to clinical promotion and popularization.
Owner:JINAN YING SHENG BIOTECH
Peptides and substances, methods and devices using same for diagnosing and treating neurodegenerative disorders
A method of identifying an existence, non-existence, type or state of a neurodegenerative disorder in an individual. The method is effected by (a) immunoreacting with a serum sample derived from the individual at least one peptide representing at least one epitope derived from an endogenous protein to which at least one antibody is produced in vivo at onset or during progression of the neurodegenerative disorder, the at least one peptide being selected such that the at least one antibody being capable of immunobinding with the at least one peptide; and (b) detecting a presence, absence or degree of the immunobinding to thereby identify the existence, non-existence, type or state of the neurodegenerative disorder.
Owner:RAMOT UNIV AUTHORITY FOR APPLIED RES & INDAL DEVMENT
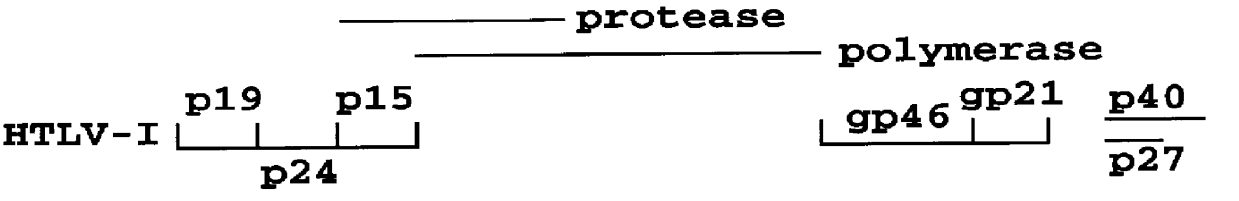
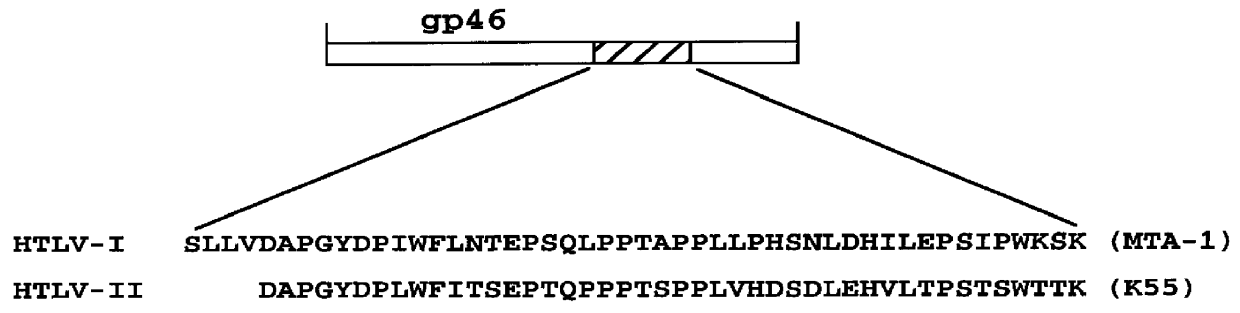
HTLV-I/HTLV-II assay and method
InactiveUS6110662AMicrobiological testing/measurementBiological material analysisPeptide antigenSerum samples
Method and assay kit for positively identifying HTLV-I and HTLV-II infection from human serum samples. The kit includes peptide antigens from the C-terminal regions of HLTV-I p19 and HTLV-II p21 gag proteins, and peptide antigens from the HLTV-I and HTLV-II env proteins immobilized on a solid support. After reaction of the serum sample with the solid support, an antibody-detection reagent in the kit is added to the support, to detect binding of human serum antibodies to each of the peptide antigens separately. The test allows positive identification of HTLV-I or HTLV-II when antibody binding to each HTLV-I or HTLV-II gag and env peptide antigen, respectively, is observed. Also disclosed is a kit for screening human sera for evidence of HTLV-I or HTLV-II infection.
Owner:GENELABS TECH INC +1
Double antibody latex enhanced retinol binding protein detection kit
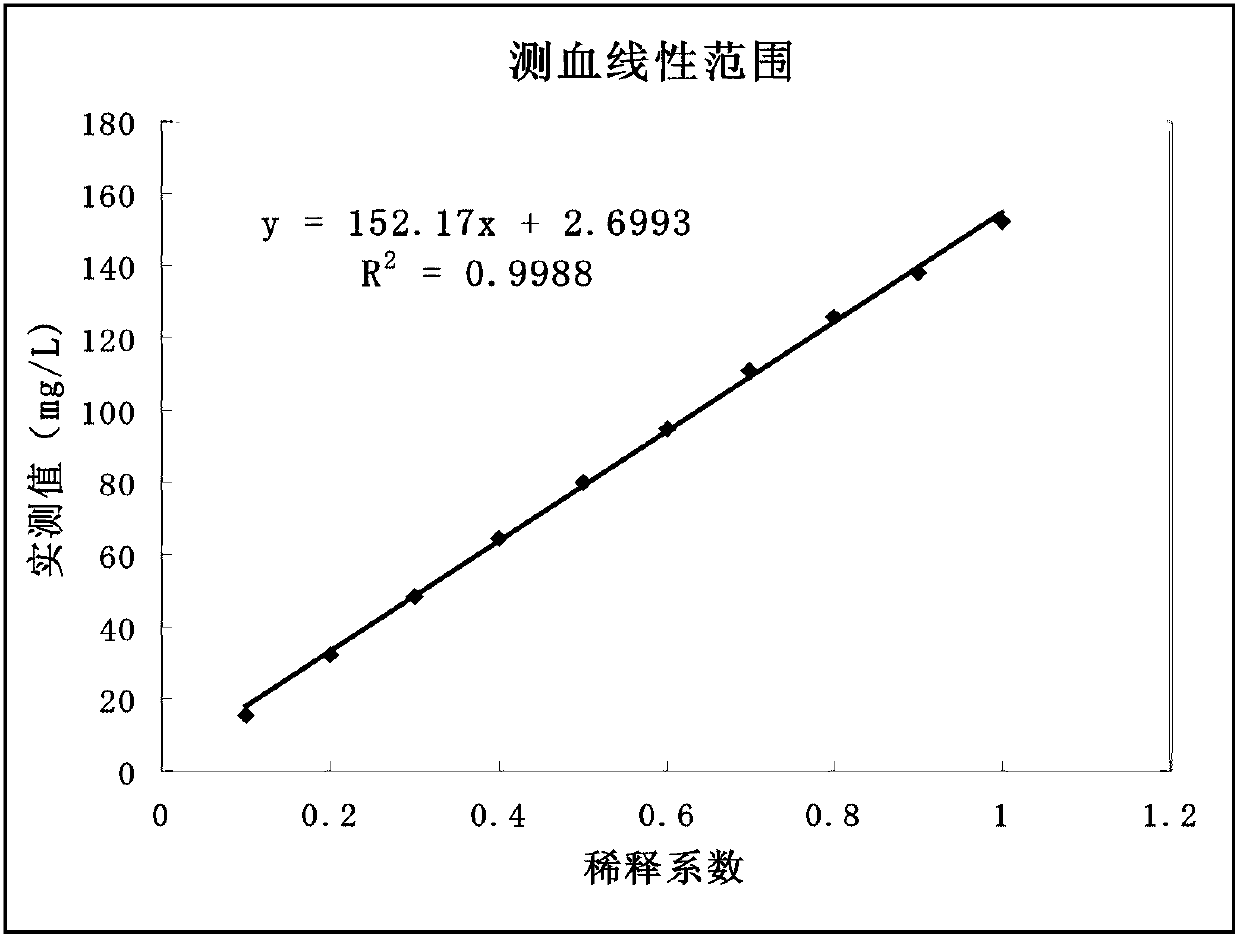
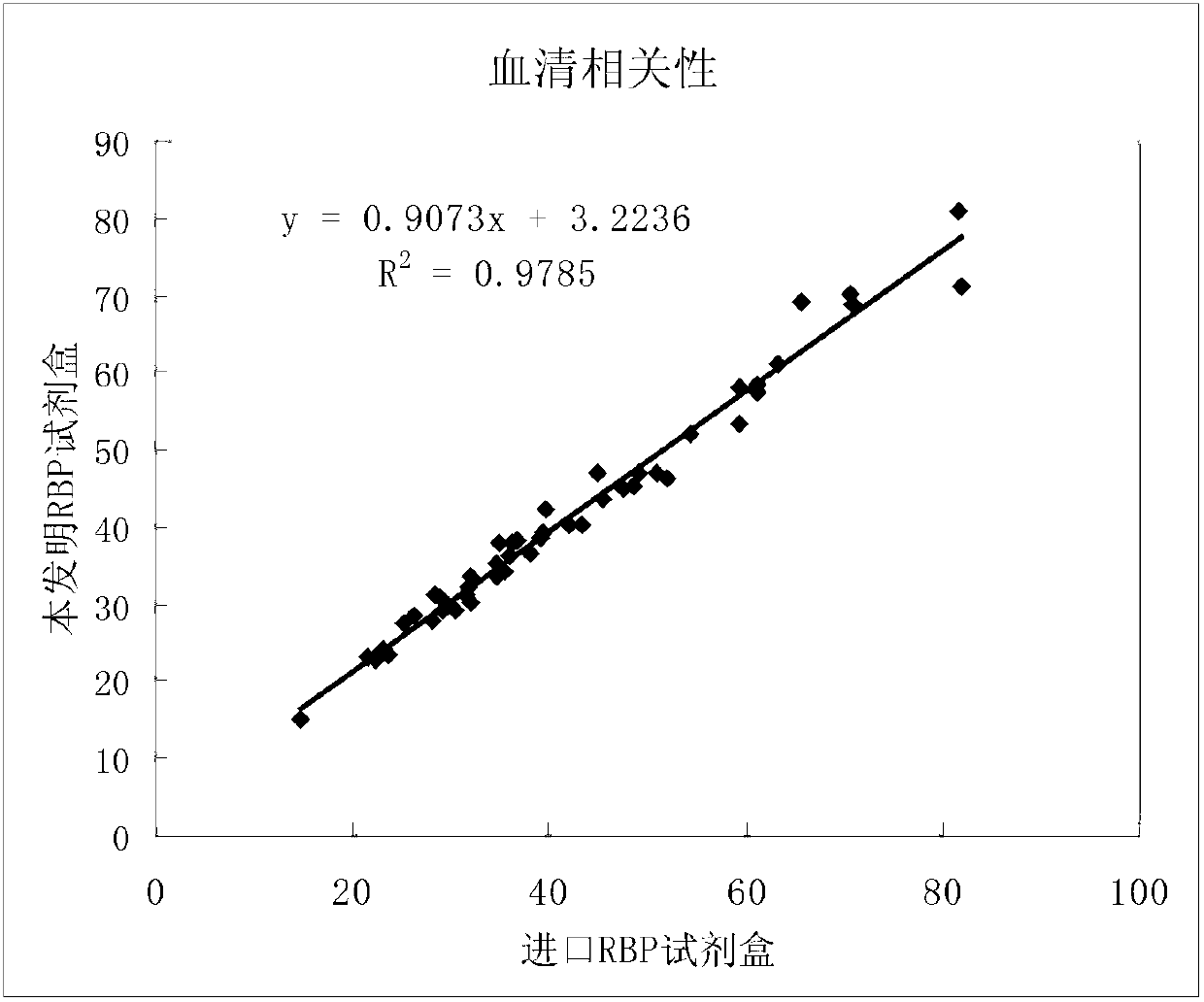
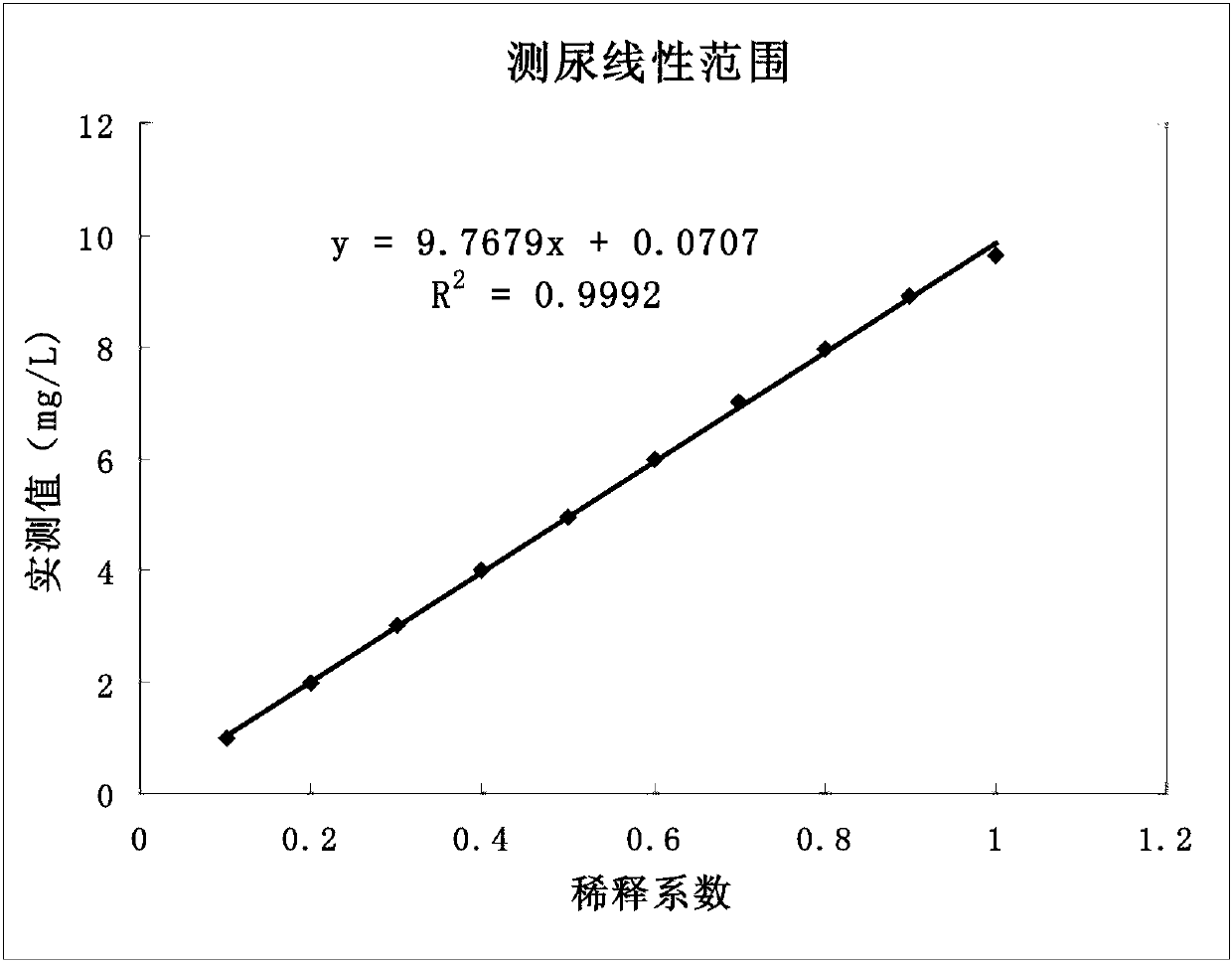
The invention relates to a double antibody latex enhanced retinol binding protein detection kit. More specifically, the invention discloses a double antibody coating latex enhanced immunoturbidimetry kit for detecting retinol binding protein. The kit contains a reagent 1, a reagent 2 and a calibrator. Paring monoclonal antibody A and B are respectively coated on latex particles. Coated antibody is bonded with RBP to be detected, and a plurality of bonders are aggregated together to form detectable turbidity change. The detection kit provided by the invention has high sensitivity, can be used to detect urine samples and serum samples and can be used as nutritive index to detect RBP in serum. Simultaneously, through the detection of RBP in urine, the kit is sensitive to the damage degree of renal proximal tubule.
Owner:BEIJING STRONG BIOTECH INC
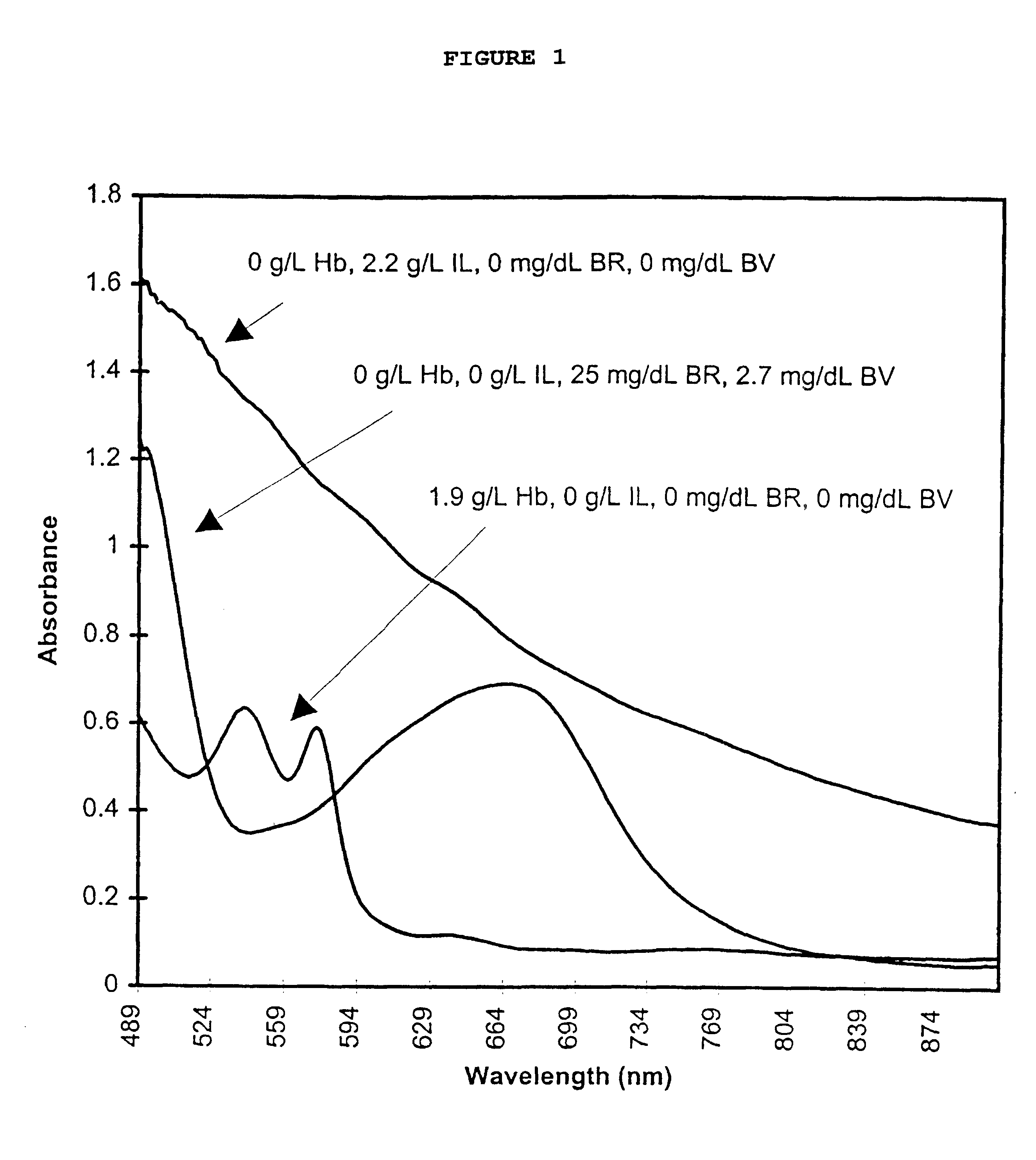
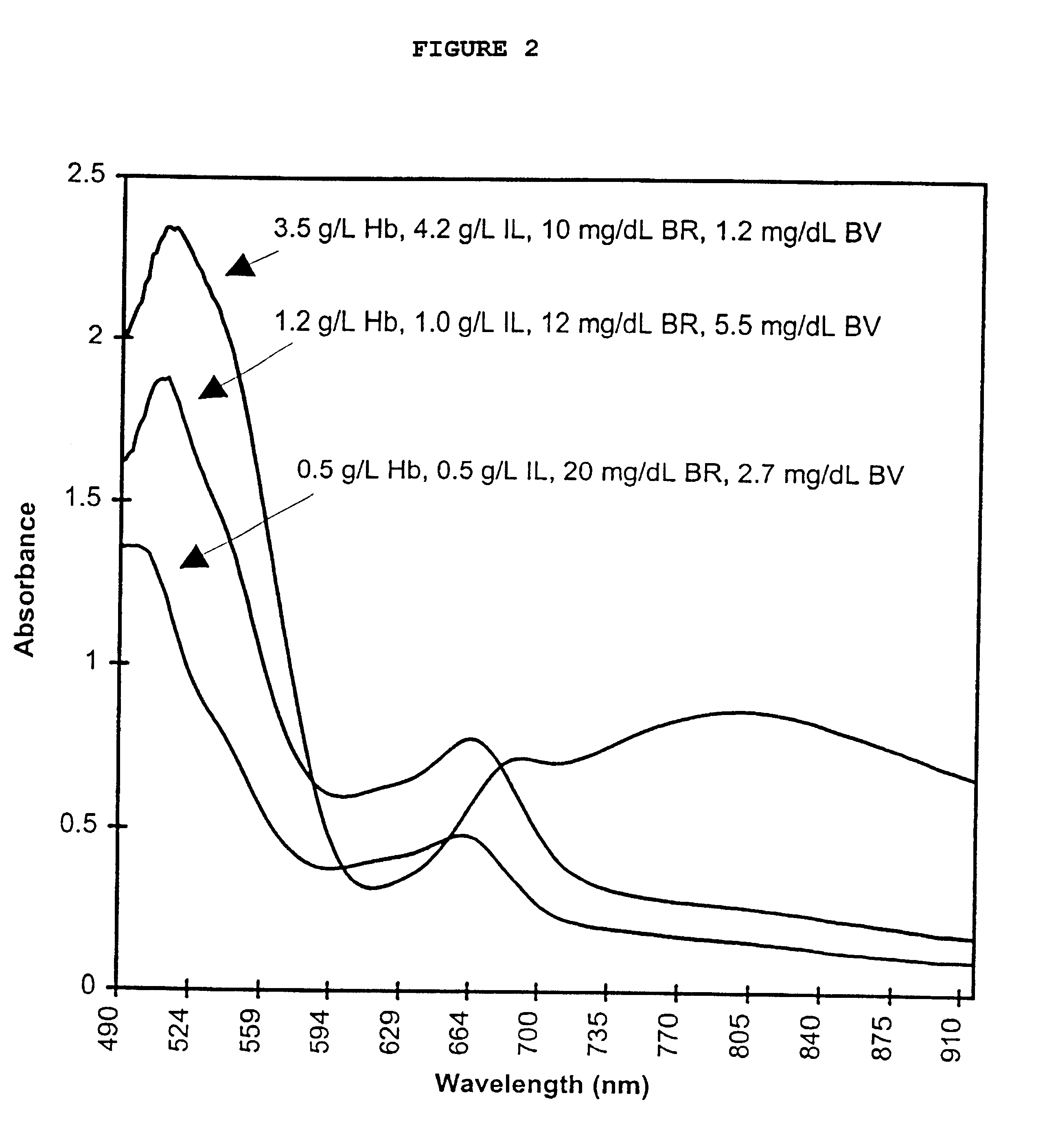
Calibrator material for instruments which measure interferents in serum and plasma specimens
InactiveUS6372503B1Increase absorbanceLeft outBiological testingSpecial data processing applicationsBILIRUBINAEMIAHemolysis
A quality control material is disclosed which is used to monitor the calibration or used for recalibration of instruments used to screen for interferents in serum or plasma specimens. In particular, the quality control material disclosed is used to monitor instrument calibrations or used for recalibration for instruments which assess the amount of hemolysis, turbidity, bilirubinemia and biliverdinemia, either separately, or any two, or any three, or all four simultaneously, in plasma or serum samples. The quality control material does not contain any blood products such as plasma lipids, bile pigments, or hemoglobin, is stable at room temperature, and is ready for use with up to four constituents.
Owner:TYCO HEALTHCARE GRP LP
Micro-fluidic chip for glycosylated hemoglobin immunodetection
The invention provides a micro-fluidic chip for glycosylated hemoglobin immunodetection in serum, comprising a sample introduction pool, a micro-fluid channel, a reaction tank, a detection tank, a waste liquor tank and a pump valve interface, wherein the sample introduction pool, the micro-fluid channel, the water liquor tank and the pump valve interface are serially connected by the micro-liquid channel; antigens or antibodies necessary for protein detection are fixed in the reaction and detection tanks in advance, a serum sample solution to be detected successively flows in the reaction tank by virtue of the sample introduction pool and the micro-fluid channel by an externally connected pump valve system, completes antigen or antibody specific reaction in the reaction tank and agglomerates, and the reaction product is subjected to absorbancy analysis to obtain the immune agglutination reaction and detection of the sample to be detected. The chip is convenient for sample introduction, less in sample consumption, high in reaction efficiency and short in detection time.
Owner:ZHEJIANG PUSHKANG BIOTECHNOLOGY CO LTD
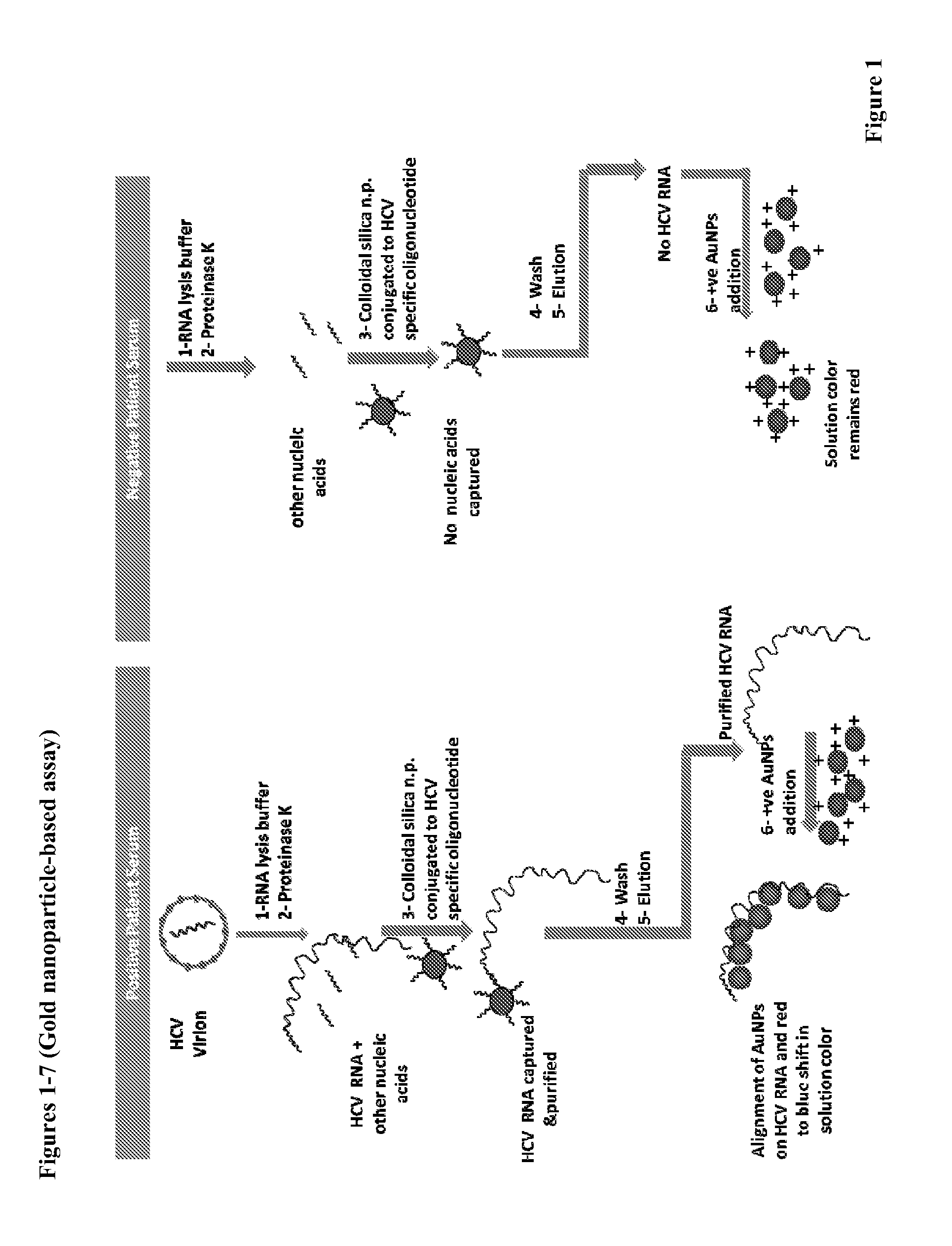
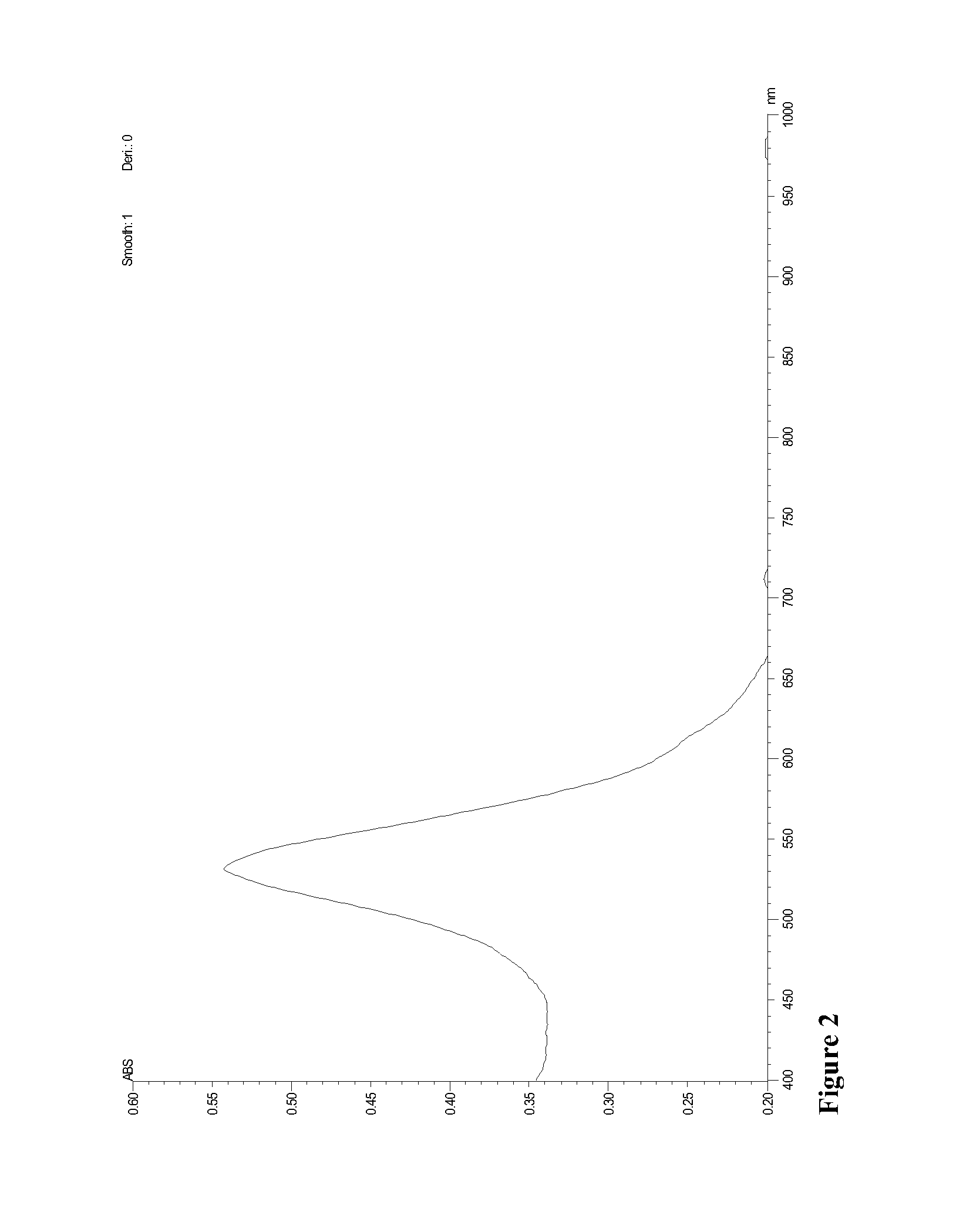
Direct detection of disease biomarkers in clinical specimens using cationic nanoparticle-based assays & versatile and green methods for synthesis of anisotropic silver nanostructures
InactiveUS20150017258A1Enhances thermalImprove electricityBiocideInorganic active ingredientsSilica nanoparticlesPurification methods
A gold nanoparticle-based assay for the detection of a target molecule, such as Hepatitis C Virus (HCV) RNA in serum samples, that uses positively charged gold nanoparticles (AuNPs) in solution based format. The assay has been tested on 74 serum clinical samples suspected of containing HCV RNA, with 48 and 38 positive and negative samples respectively. The developed assay has a specificity and sensitivity of 96.5% and 92.6% respectively. The results obtained were confirmed by Real-Time PCR, and a concordance of 100% for the negative samples and 89% for the positive samples has been obtained between the Real-Time PCR and the developed AuNPs based assay. Also, a purification method for the HCV RNA has been developed using HCV RNA specific probe conjugated to homemade silica nanoparticles. These silica nanoparticles have been synthesized by modified Stober method. This purification method enhanced the specificity of the developed AuNPs assay. The method can detect a target molecule, such as HCV RNA in serum, by employing modified silica nanoparticles to capture the target from a biological sample followed by detection of the captured target molecule using positively charged AuNPs. The assay is simple, cheap, sensitive and specific. Another aspect of the invention is anisotropic silver nanoparticles and methods of their use.
Owner:AMERICAN UNIV OF CAIRO AUC
Method for detection of colorectal cancer in human samples
InactiveUS20070117164A1Reduce signalingReduce intensityBiological material analysisSerum samplesMass Spectrometry-Mass Spectrometry
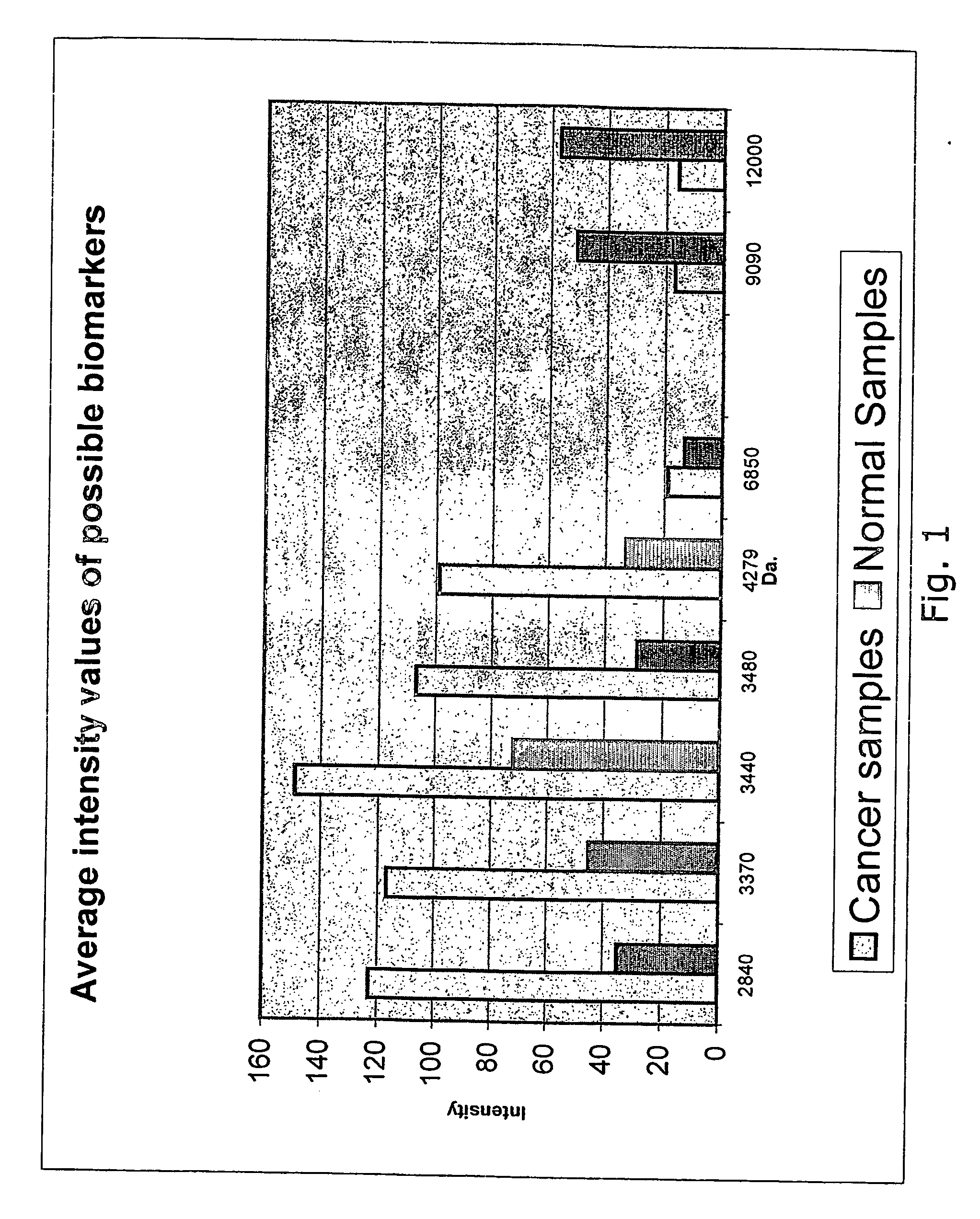
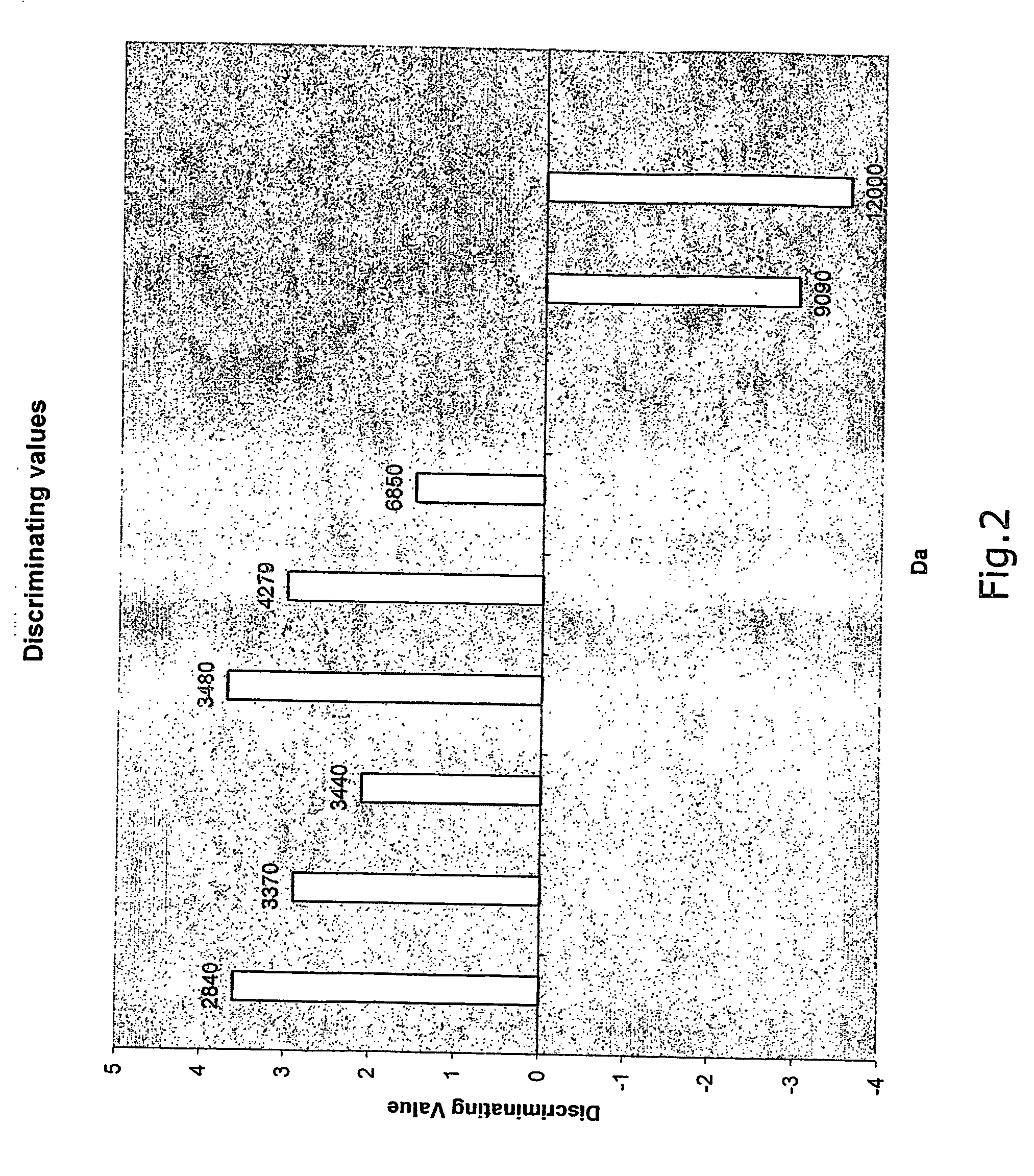
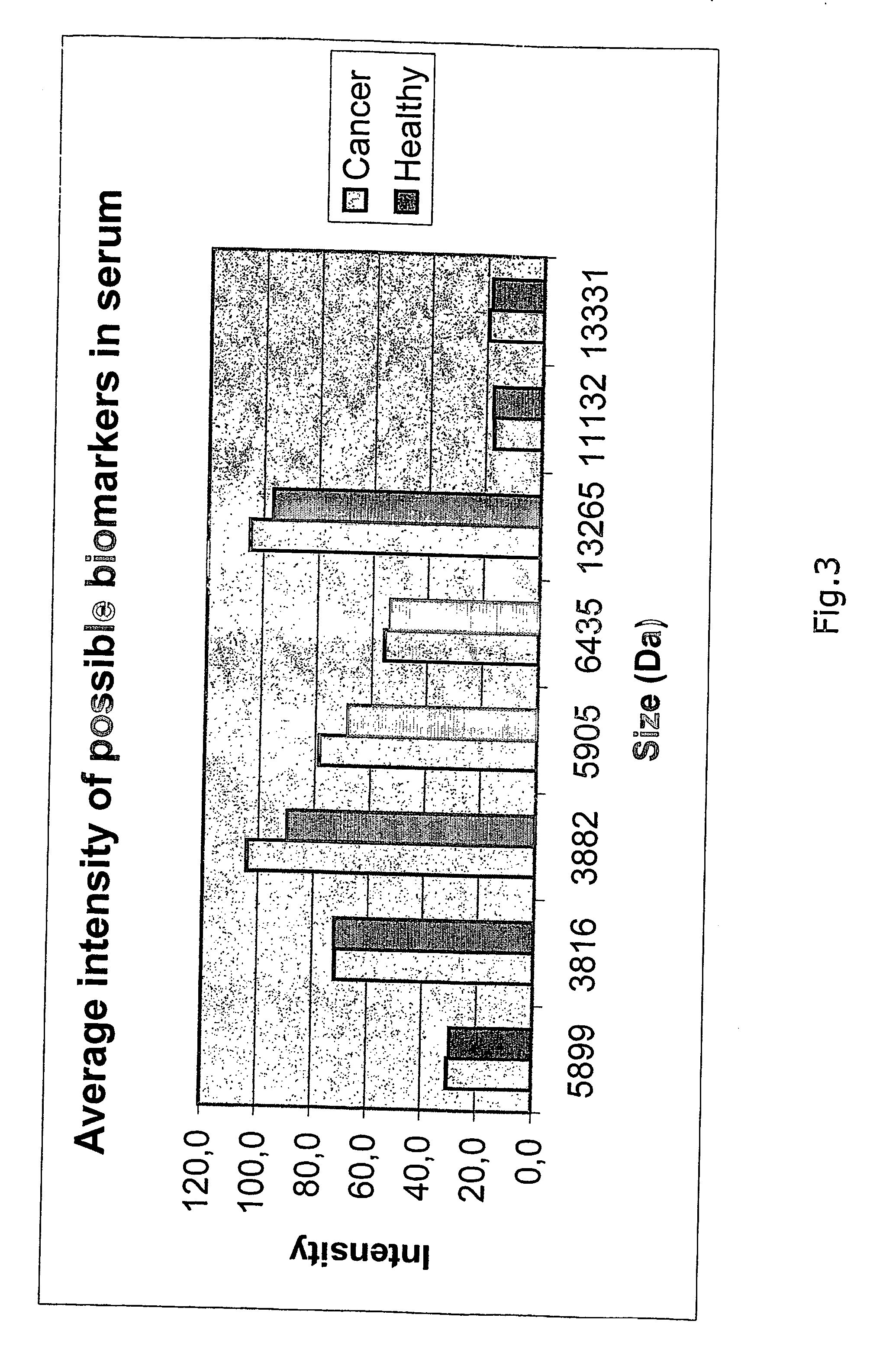
The present invention relates to a method of diagnosing colorectal cancer in human samples using several novel protein markers. The markers have been identified by assaying a number of tissue and serum samples from healthy individuals and persons diagnosed with colorectal cancer by means of protein chip technology using mass spectrometry. Differential expression pattern of these markers are indicative of a person having colorectal cancer patient. The diagnosis is based on comparing at least one intensity value, obtained using the method, to a reference value.
Owner:RASKOV HANS HENRIK +1
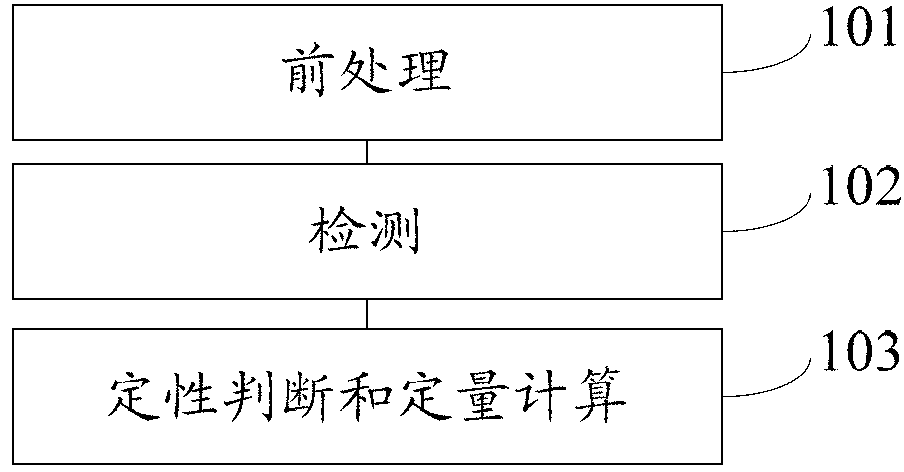
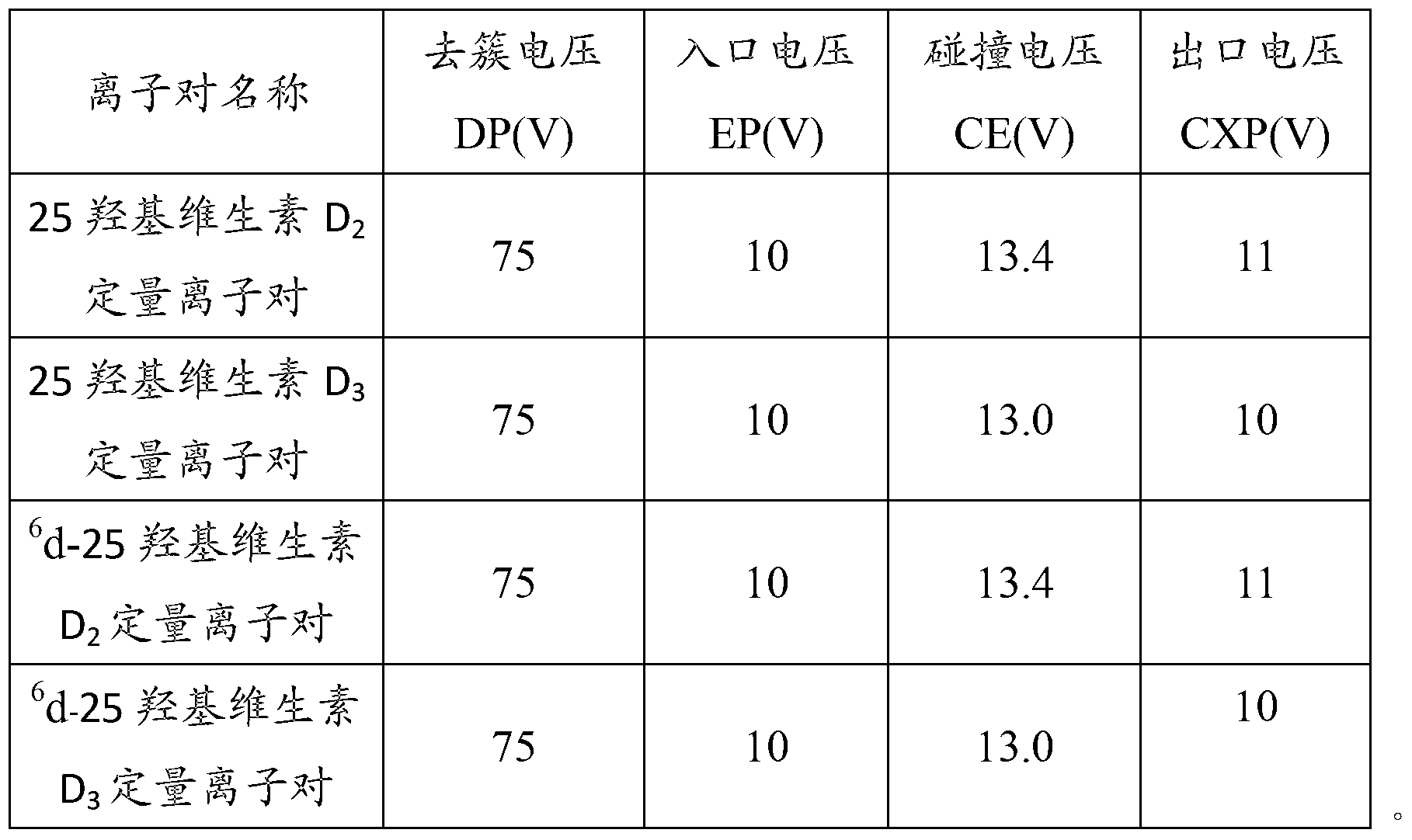
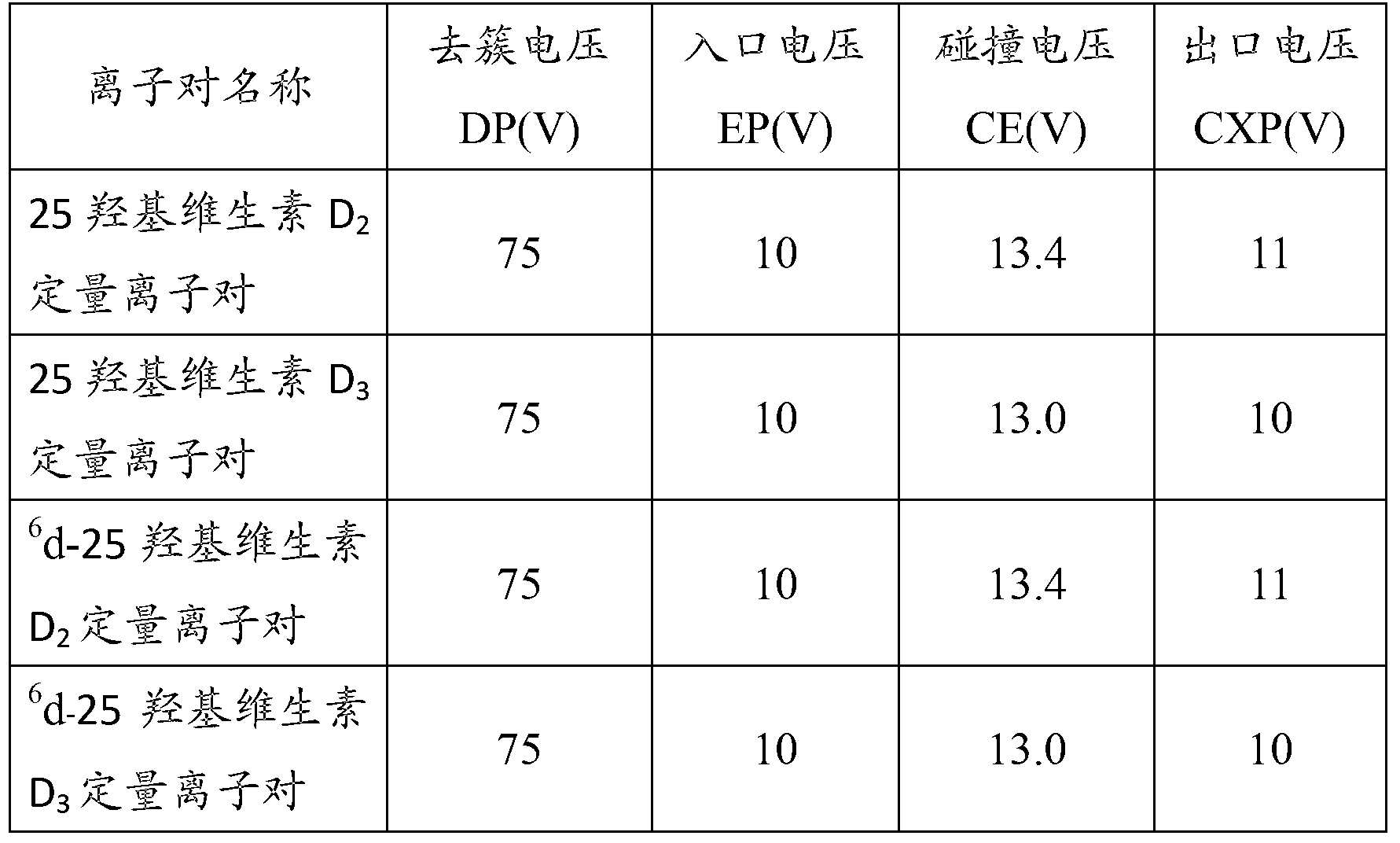
Method for detecting 25(hydroxyl)vitamin D by using high-pass liquid chromatography-tandem mass spectrometry
ActiveCN103308621AThe pre-processing process is simpleStrong specificityComponent separationRetention timeMass analyzer
The embodiment of the invention provides a method for detecting 25(hydroxyl)vitamin D by using a high-pass liquid chromatography-tandem mass spectrometry. The method comprises the following steps of: adding an acetonitrile solution containing an internal standard substance of the 25(hydroxyl)vitamin D into a human serum sample to carry out protein precipitation; sufficiently and uniformly mixing the solution, and then, adding an n-hexane extracting solvent; sufficiently and uniformly mixing the solution, then centrifuging the solution, movably taking a supernatant and drying the supernatant, and adding a complex solution to obtain a sample to be detected; detecting the sample to be detected by using a high-pass liquid chromatography-tandem quadrupole mass spectrometer; and quantifying according to the relative retention time of 25(hydroxyl)vitamin D2 and / or 25(hydroxyl)vitamin D3 and the detected abundance ratio of quantitative ion pairs by using an internal standard curve method. According to the embodiment of the invention, the method has the advantages of simplicity in pretreatment, strong specificity and matrix interference resistance, short detection time, high pass, high detection precision and low cost.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
LC/MS (liquid chromatography-mass spectrometer) metabonomics analysis method based on serum of GDM (gestational diabetes mellitus) patient
InactiveCN103592389AComprehensively reflect the variation statusNo noise disturbanceComponent separationMetaboliteOriginal data
The invention discloses an LC / MS (liquid chromatography-mass spectrometer) metabonomics analysis method based on serum of a GDM (gestational diabetes mellitus) patient. The method comprises the steps as follows: serum samples of a normal pregnant woman and a pregnant woman with GDM are collected, processed and separated by a chromatographic column, and mass spectrometric detection analysis is adopted; original data of an instrument is extracted and aligned after LC / MS detection analysis, and data free of noise disturbance and capable of being used for statistical analysis is obtained; and a multi-dimensional statistical model is established and visually displays metabolic profiling difference between the two groups, so that differential metabolites of the two groups are obtained, and the substances are subjected to preliminary evaluation. With the adoption of the method, the variation condition of the metabolites of the GDM patient can be presented comprehensively and synthetically, and the method can be used for providing powerful technical support for early diagnosis and prognosis of GDM.
Owner:HUZHOU CENT HOSPITAL
Microfluidic microbead array chip and application thereof in virus analysis
ActiveCN101709261AEasy to makeFast preparationBioreactor/fermenter combinationsBiological substance pretreatmentsMicrosphereMicrostructure
The invention discloses a microfluidic microbead array chip and application thereof in virus analysis, and belongs to the field of biological application of a miniaturized total analysis system. The microfluidic microbead array chip consists of a microbead fixed microstructure array, a microbead transport channel, a reagent transport channel and a microstructure array coverage channel. When high-sensitive virus analysis is performed, virus molecules in a serum sample is prepared into a biotin doped target RNA sequence through PCR and in vitro transcription process, and flows to a testing areaof the chip to react with a functional microbead array; function modified microspheres can specifically identify and capture target sequences; a quantum dot labeled reagent are introduced; surfaces of micro-particles are combined with quantum dots because of specifically capturing; and detection and quantitation are performed by a fluorescence imaging method. The microfluidic microbead array chipprovided by the invention has the advantages of simple process, high detecting sensitivity and quick detection, and provides an effective studying and detecting means for high-sensitive virus analysis.
Owner:香港城市大学深圳研究院
Preparation and application of Au@Pd core-shell material constructed lung cancer tumor marker immunosensor
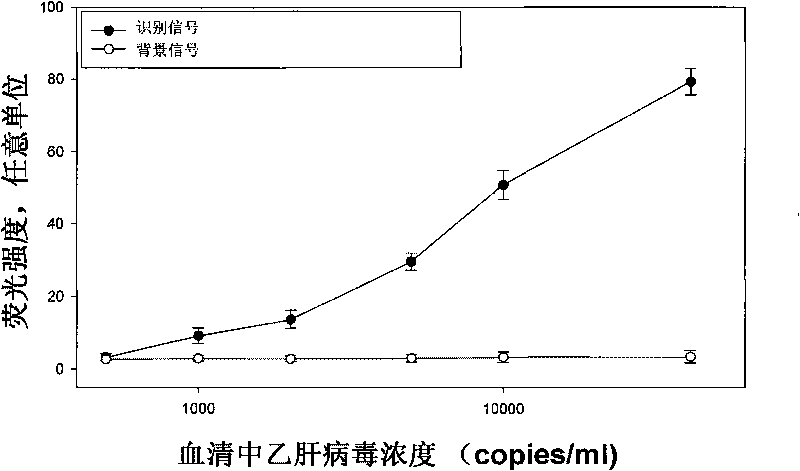
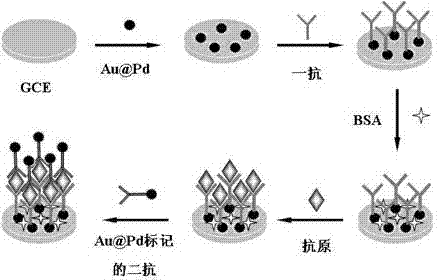
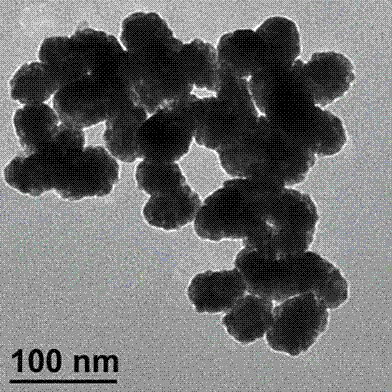
InactiveCN102818893ALarge specific surface areaImprove conductivityMaterial analysis by electric/magnetic meansAntigenSerum samples
Belonging to the technical field of novel functional materials, biosensing and clinical examination, the invention relates to preparation and application of an Au@Pd core-shell material constructed lung cancer tumor marker immunosensor. The method of the invention makes use of the characteristics of a large specific surface area, good biocompatibility, high catalytic efficiency and the like of anAu@Pd core-shell nano-material, and substantially improves the sensitivity of the immunosensor. The method adopts the Au@Pd core-shell nano-material to immobilize a first antigen and mark a second antigen, and by means of layer-by-layer self-assembly, a lung cancer tumor marker electrochemical immunosensor can be constructed. The Au@Pd core-shell material constructed lung cancer tumor marker immunosensor provided in the invention has the advantages of high sensitivity, good specificity, as well as easy operation, and can realize sensitive, rapid, and accurate detection of a variety of lung cancer tumor markers in a serum sample, thus having important significance for early diagnosis of lung cancer.
Owner:UNIV OF JINAN
Kit for simultaneously detecting retinol-binding protein (RBP) in urine sample and serum sample
ActiveCN103134934AReduce dosageStrong specificityColor/spectral properties measurementsLatex particlePromotion effect
The invention provides a kit for simultaneously detecting RBP in a urine sample and a serum sample. The kit comprises a reagent R1 and a reagent R2, the reagent R1 is a polymer buffer solution having an agglomeration promotion effect, the reagent R2 is a buffer solution containing a latex-antibody crosslink, and the latex-antibody crosslink comprises anti-RBP polyclonal antibody marked large latex particles and anti-RBP monoclonal antibody marked small latex particles. The kit which adopts a composite monoclonal and polyclonal antibody sensitized latex enhanced immune detection method has the advantages of simultaneous satisfying of the requirements comprising good specificity, high sensitivity and wide linear range, small antibody application amount, and cost reduction, and can be simultaneously used for the clinic detection of the RBP in human blood and urine.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Human body important parasite antigen chip and method for making same
The invention relates to the technical field of biology, in particular to an antigen chip used for detecting human parasites antigens in serum and a preparation method thereof. The invention spots antigen array of parasites which are seriously harmful to human body, Schistosoma, Lung fluke, Clonorchis sinensis, Cysticercosis, Sparganum mansoni, Trichina, Angiostrongylus cantonensis, Toxoplasma gondii, and the like, on a solid phase membrane carrier, and specific gamma immunoglobulin (IgG) of human parasites in serum is detected by a specially made vertical flow chip detect device, and the detecting marker is colloid gold second antibody conjugate or colloid gold protein A conjugate of fresh color. IgG antibodies in serum specially bind with the antigens on the membrane in percolation process layer upon layer, and human IgG which specially binds with the antigens is detected by the colloid gold marker. Detecting of a plurality of parasites antigen indexes in serum sample can be finished within several minutes. The device and the method have the advantages of high throughput, simple operation, convenient use and being suitable for parasitic diseases clinical test and serum-epidemiological investigation.
Owner:ZHEJIANG ACAD OF MEDICAL SCI
Porcine reproductive and respiratory syndrome virus (PRRSV) double-antibody sandwich ELISA kit
The invention provides a porcine reproductive and respiratory syndrome virus double-antibody sandwich ELISA kit. The kit comprises: an elisa plate coated with PRRSV N protein monoclonal antibody, an enzyme labeling PRRSV N protein monoclonal antibody, lysis solution and the like. A capture antibody and a detection antibody are respectively aimed at antigenic determinants with different N proteins.The kit provides a reliable means for quick detection of clinical PRRSV antigen. The kit can detects that blood serum only contains 0.2 TCID50 highly pathogenic PRRSV JXwn06 strain (non-highly pathogenic strain can be also be detected). Through detecting clinically collected 80 blood serum samples, compared with RT-PCR result, the specificity of the method is 88 percent, the sensitiveness is 90 percent and the coincidence rate of the specificity and the sensitiveness are 88.8 percent. The kit is convenient for operation, low in use cost, good repetitiveness and suitable for wide promotion andapplication.
Owner:CHINA AGRI UNIV
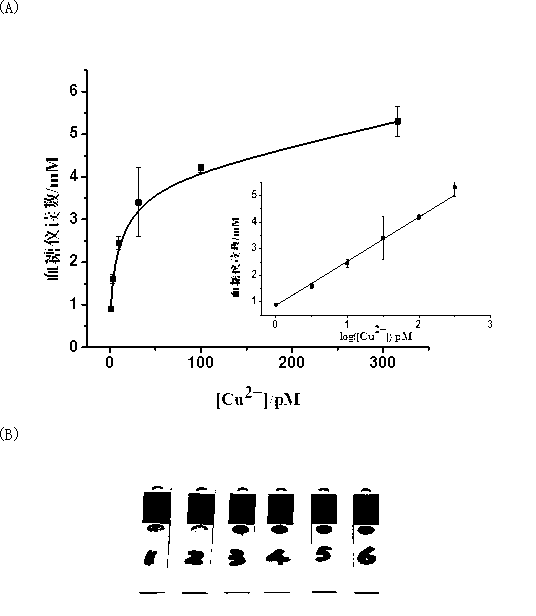
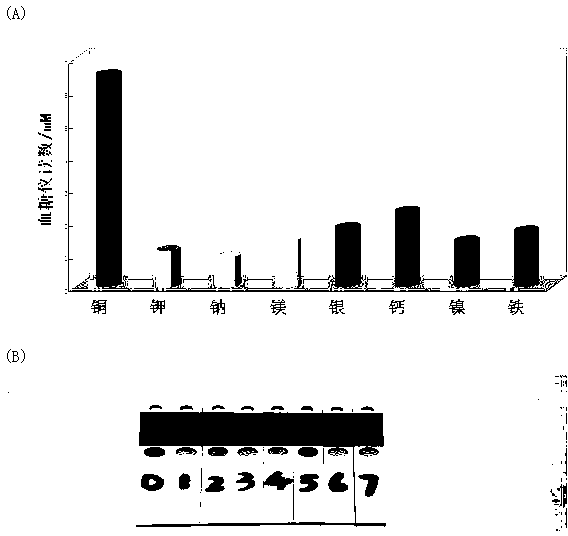
Portable copper ion concentration detection method based on click chemistry
ActiveCN103163130AGood linear responseEasy to operateMaterial analysis by observing effect on chemical indicatorDNA/RNA fragmentationMagnetic beadSodium ascorbate
The invention provides a portable copper ion concentration detection method based on click chemistry and belongs to the field of analytical chemistry. Under the condition that sodium ascorbate reduces cupric into cuprous as a catalyst, a 1,3-dipole cycloaddition reaction is carried out between NDA modified with an azide group and DNA modified with an alkynyl group, so that DNA modified with cane sugar invertase is fixed on magnetic beads; The magnetic beads are separated from a solution, the cleaned magnetic beads are dissolved into a cane sugar solution to carry out enzymatic hydrolysis, and then a reaction solution after enzymatic hydrolysis is measured by adopting a blood glucose meter, so that copper ion concentration can be detected. The portable copper ion concentration detection method provided by the invention takes the blood glucose meter as a platform and can portably and quickly detect the copper ion concentration on site; and the portable copper ion concentration detection method can be applied to copper ion detection in a human body serum sample, and the portable copper ion concentration detection method has the advantages of easy operation, low cost, high sensitivity and good specificity.
Owner:FUZHOU UNIV
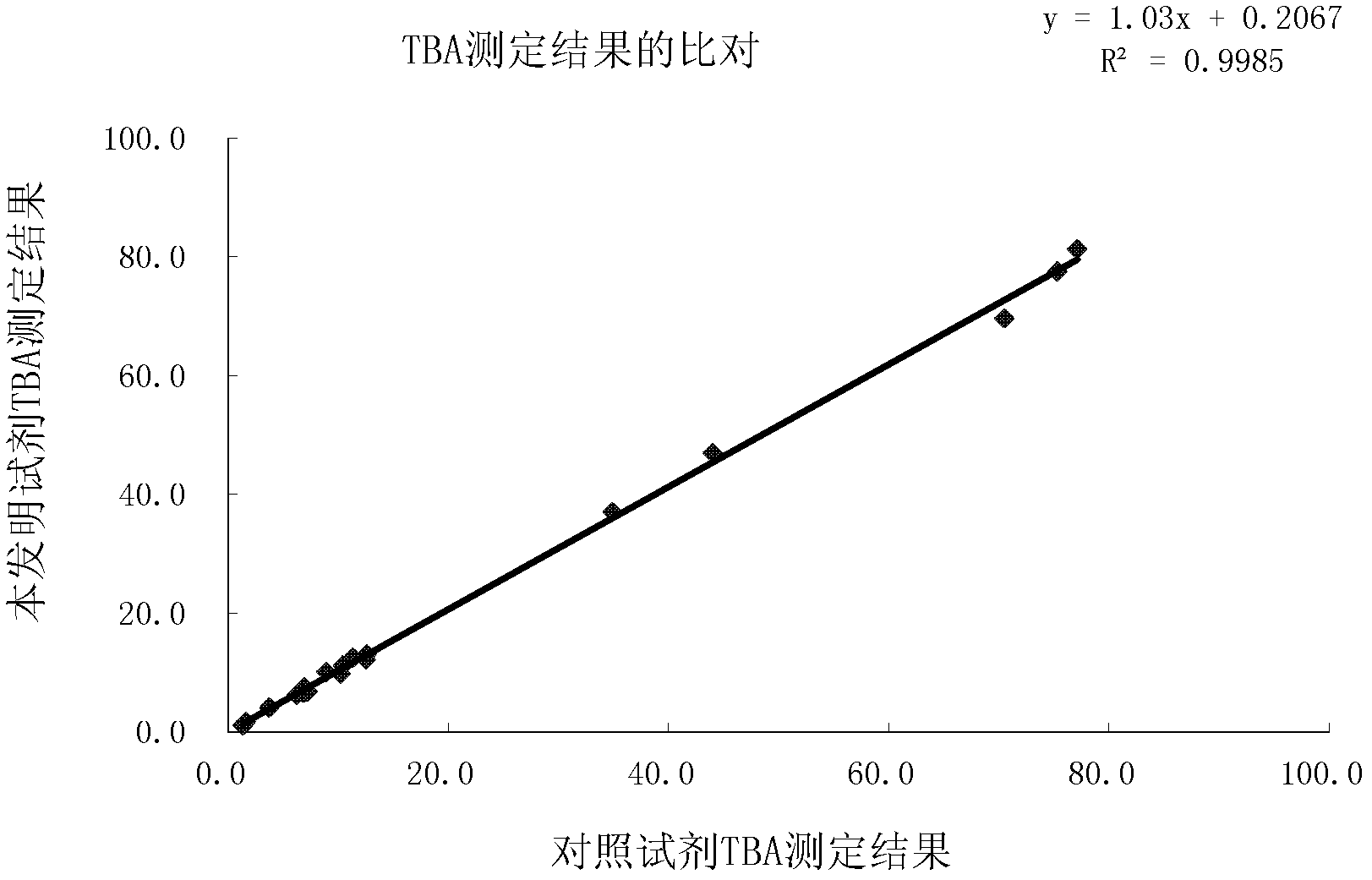
Total bile acid quantitative determination method and determination reagent kit
InactiveCN102539791AColor/spectral properties measurementsBiological testingSerum samplesQuantitative determination
The invention provides a reagent for quantitatively determining content of total bile acid in a human serum sample through an enzymological method. The reagent is suitable for an automatic biochemical analyzer to automatically and quantitatively determine the total bile acid. The reagent consists of a solution reagent 1 and a reagent 2 which are separately placed, wherein the reagent 1 contains oxidized thio-nicotinamide-adenine dinucleotide (Thio-NAD), buffer and stabilizer; and the reagent 2 contains reduced coenzyme I (NADH), hydroxysteroiddehydrogenase (HSD), stabilizer and buffer. The invention additionally provides a reagent kit with the reagent 1 and the reagent 2, and a method for determining the content of total bile acid in the serum. The reagent, the reagent kit and the method provided by the invention have the advantages of high sensitivity, low cost, simplicity, convenience and rapidness in operation and easiness in popularization.
Owner:宁波天康生物科技有限公司
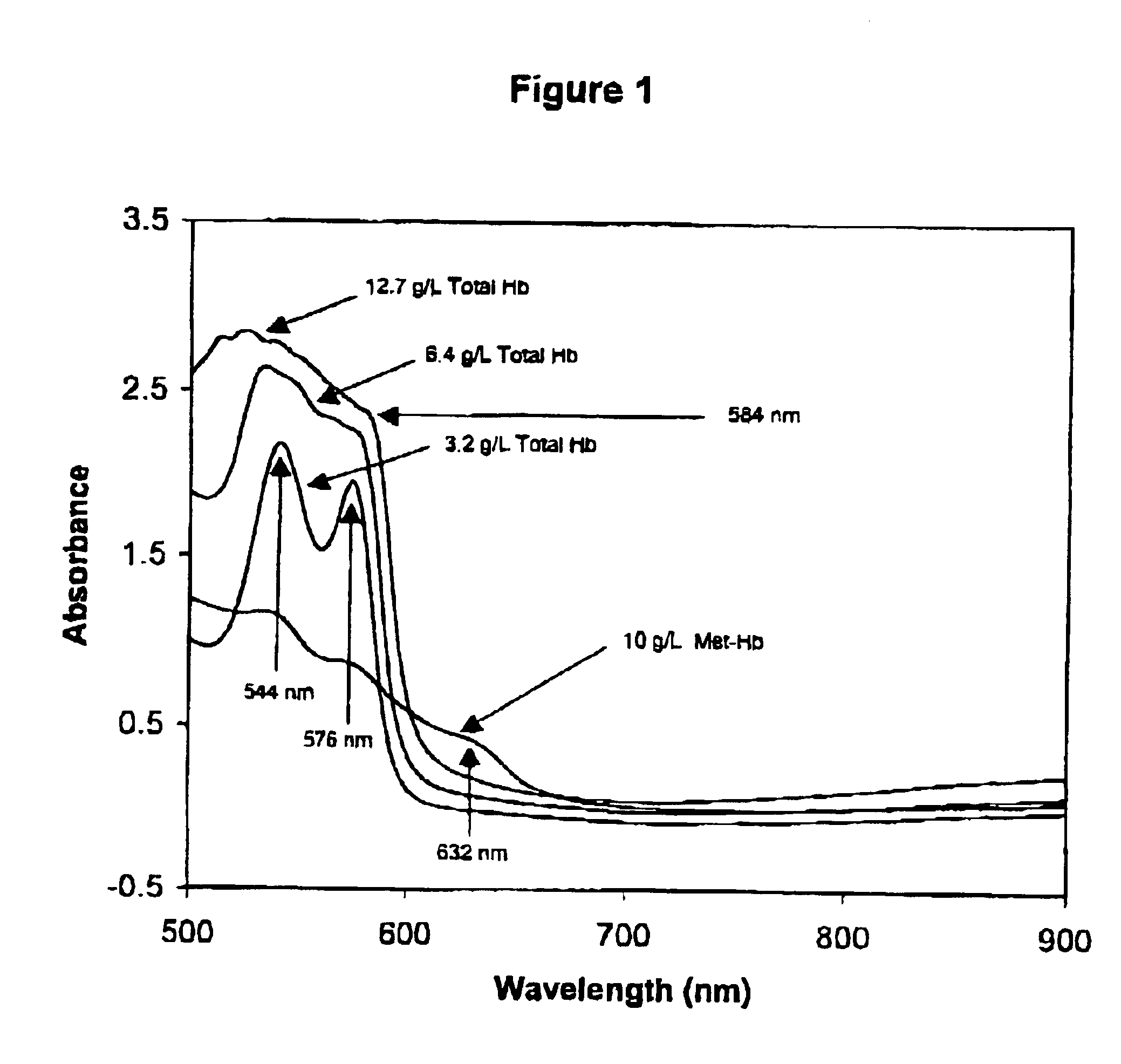
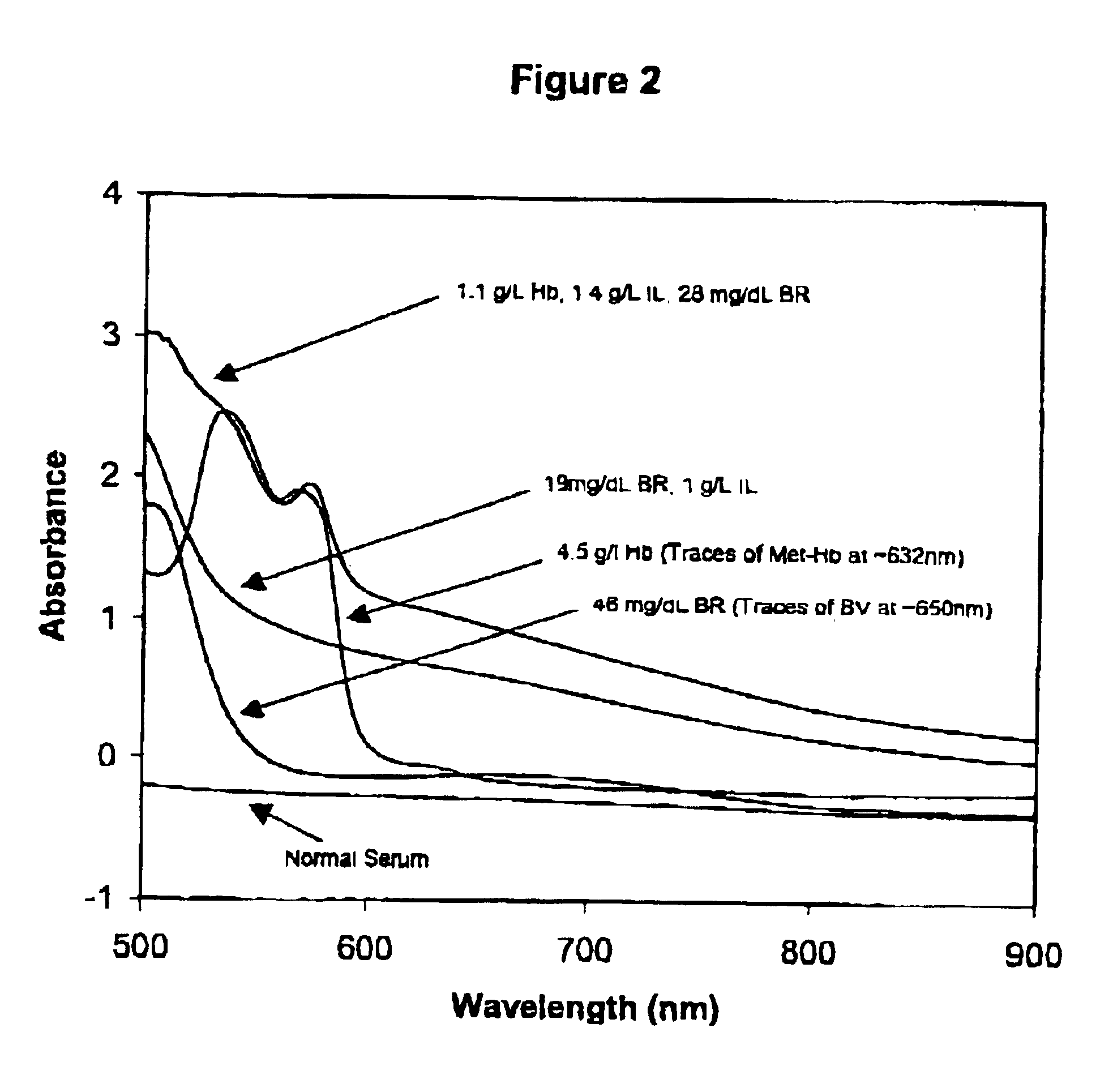
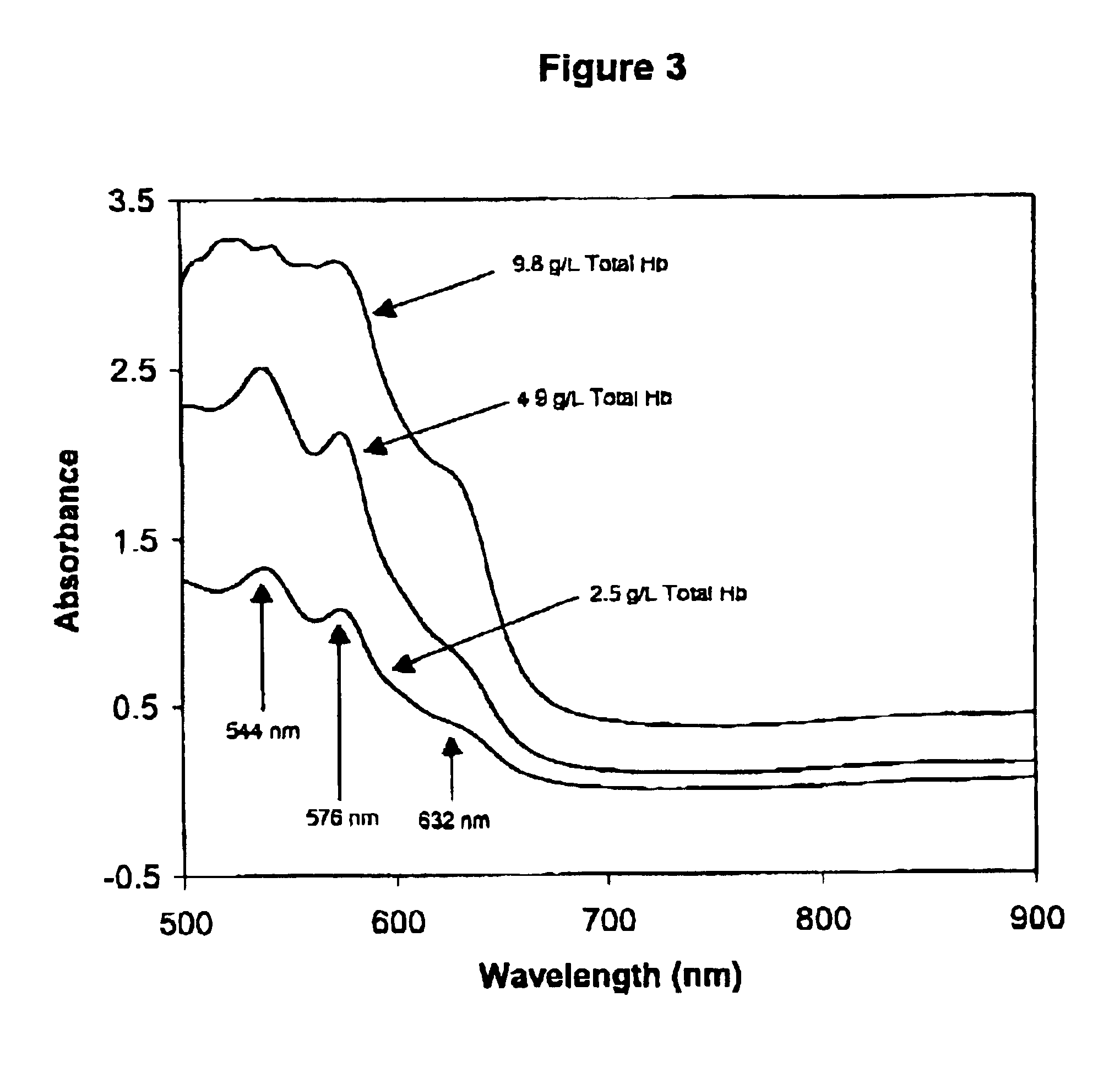
Method for monitoring degradation of Hb-based blood substitutes
InactiveUS6949384B2Analysis by material excitationAnalysis by subjecting material to chemical reactionPlasma samplesSerum samples
The present invention provides a method for monitoring degradation of Hb-based blood substitutes, in a sample. This method involves determining a concentration of met-Hb within the sample, by applying a calibration algorithm for met-Hb to an absorbance obtained from the sample at one or more than one wavelengths, and using the concentration of met-Hb, as a measurement of degradation of the Hb-based blood substitutes. Using this assay, a concentration of met-Hb that is equal to or greater than 3% may be used as an indicator of degradation of Hb. Alternatively, by obtaining samples over a period of time, the concentration of met-Hb and the concentration of Hb-based blood substitute may be determined in each of these samples, and an increase in the concentration of met-Hb over the period of time is an indicator of degradation of Hb. The sample may be a whole blood sample, a serum sample, or a plasma sample obtained from a patient transfused with one or more than one Hb-based blood substitutes, a stock Hb-based blood substitute sample, or a body part of a patient.
Owner:COVIDIEN LP
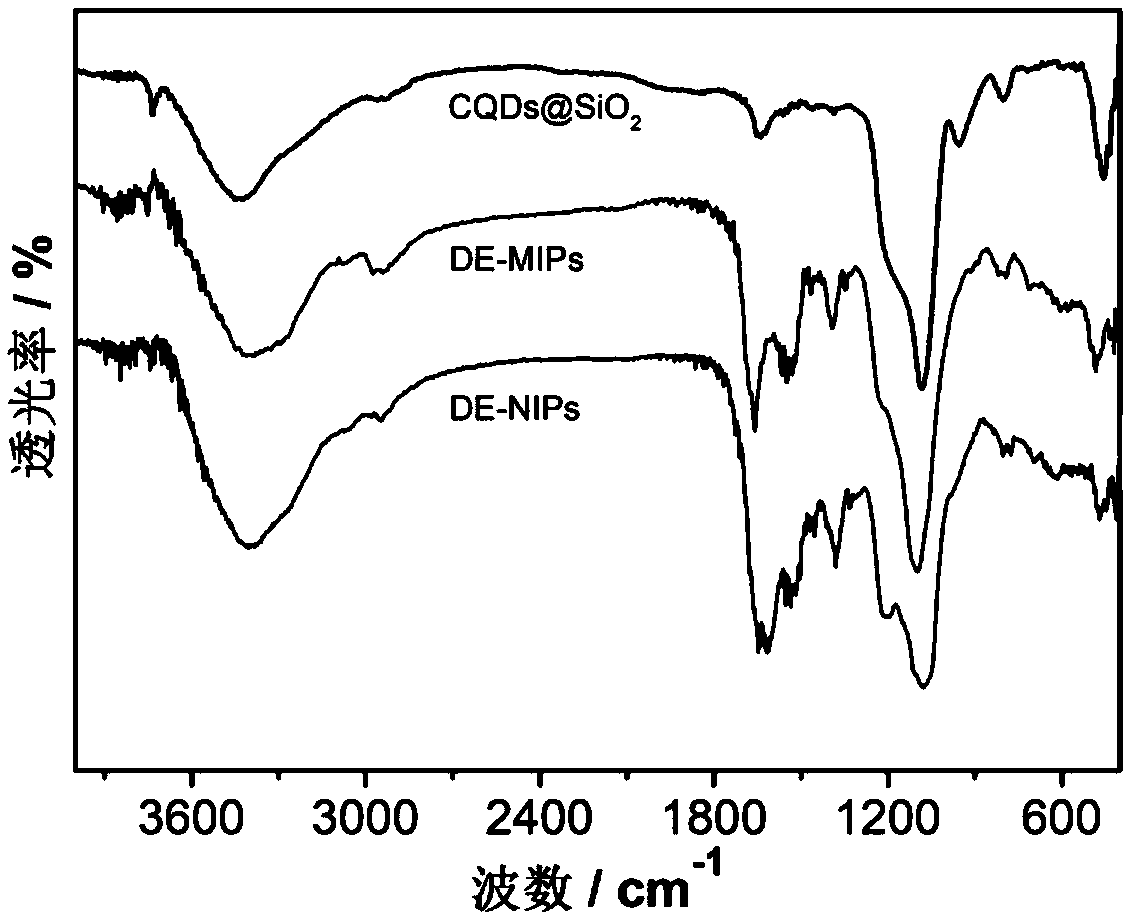
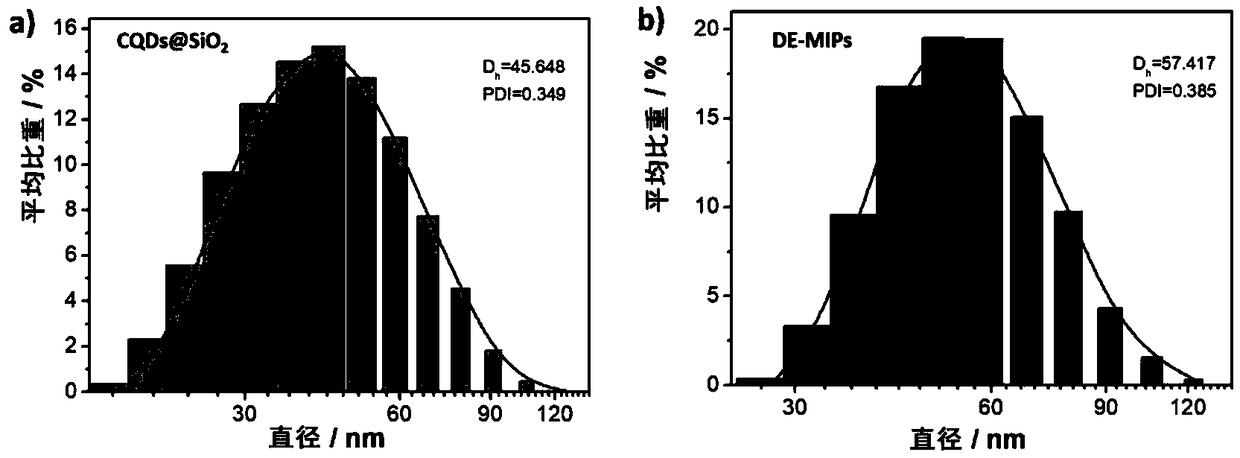
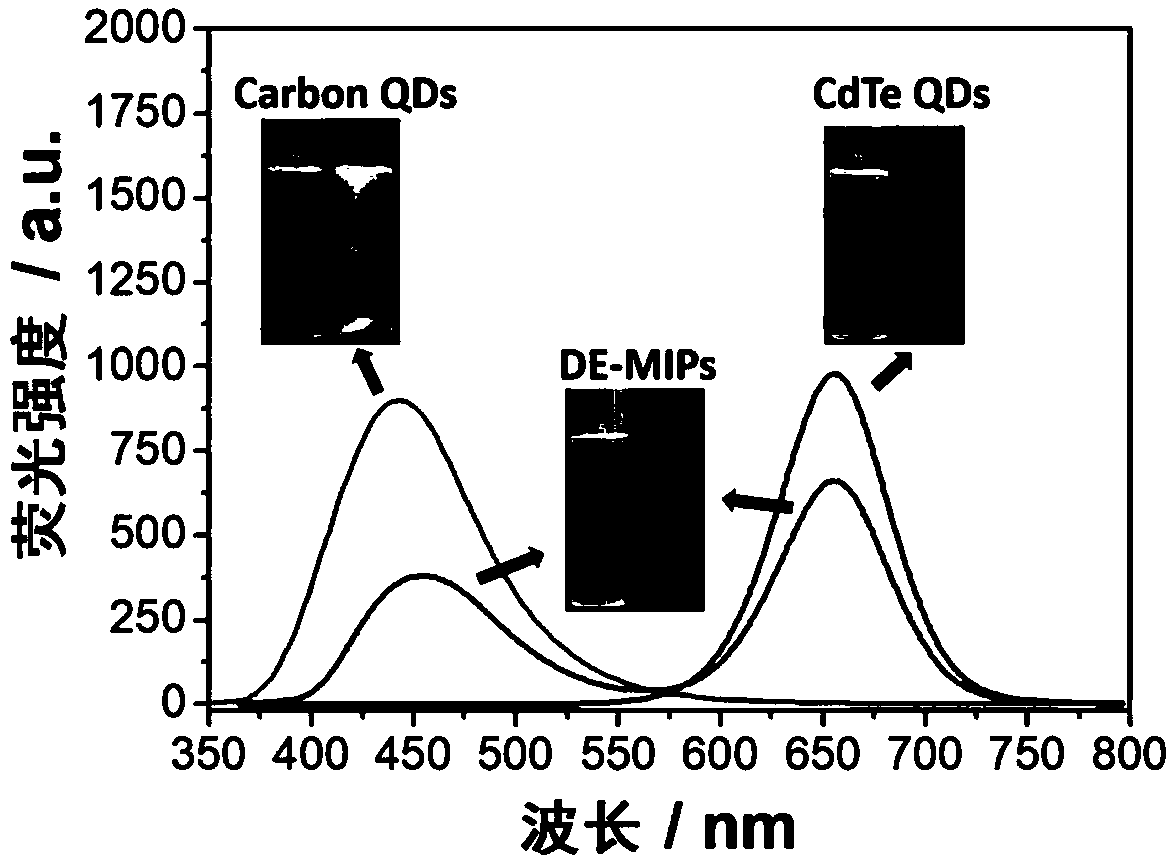
Dual-emission fluorescent molecularly imprinted polymer nanoparticles and preparation method and application thereof
ActiveCN109370565AHigh selectivityAvoid interferenceMaterial nanotechnologyFluorescence/phosphorescenceCadmium CationSilicon dioxide
The invention relates to the technical field of preparation of bio-functional materials, in particular to dual-emission fluorescent molecularly imprinted polymer nanoparticles and a preparation methodand application thereof. carbon quantum dots are coated with silica nanospheres to form a core of a ratio fluorescence probe; red cadmium telluride quantum dots are used as response signals, acrylamide and 4-vinylbenzeneboronic acid are used as bifunctional monomers, N,N-methylene bisacrylamide is used as a crosslinker, dopamine is used as templating molecules, dual-emission fluorescent molecularly imprinted polymer nanoparticles having specific recognition sites for dopamine are synthesized in an alcohol phase under initiating of azodiisobutyronitrile, and fluorescent detection test paper with visualizing effect is prepared then by means of impregnating so as to provide visual detection for DA (dopamine); the fluorescent detection test paper is applied to the detection of DA in a human serum sample, and the results prove that the fluorescent detection test paper prepared herein is suitable for half-quantitative detection of DA in actual complex samples.
Owner:JIANGSU UNIV
Anti-Golgi apparatus protein monoclonal antibody and use
InactiveCN101407544AHigh sensitivityQuantitatively accurateImmunoglobulins against animals/humansFermentationSerum igeSerum samples
The invention relates to an anti-Golgi protein antibody and an application thereof. The invention recombines human GP73 protein immunity animal to obtain an anti-GP73 polyclonal antibody and a monoclonal antibody which specifically aims at GP73 and builds a plurality of methods for detecting GP73 in clinical tissue sections and serum samples, such as immunohistochemical stain and double antibody sandwiched ELISA method and the like. Tests prove that the polyclonal and monoclonal antibodies can be used for preparing a plurality of GP73 detecting agents of different detecting methods.
Owner:曹伯良 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com