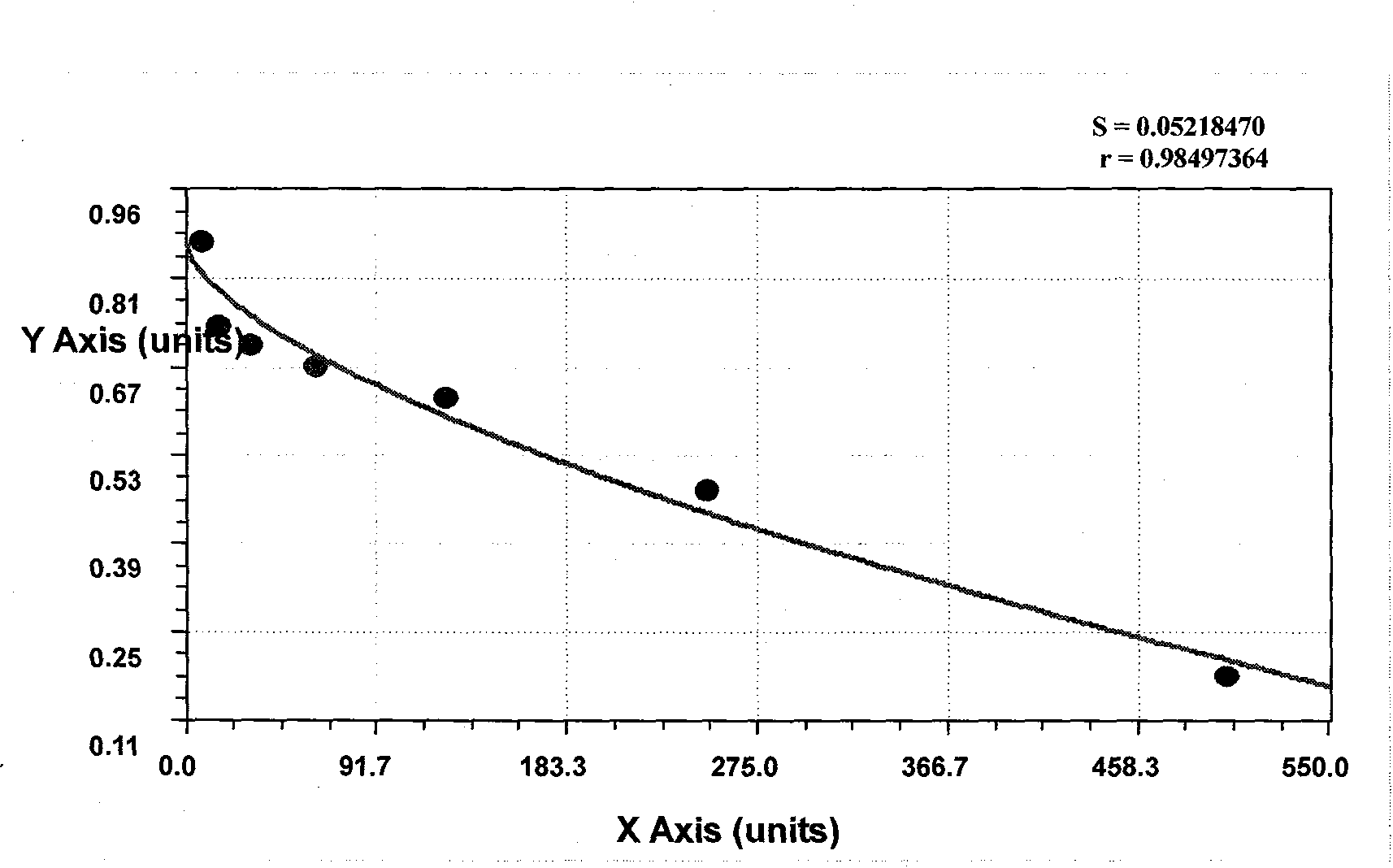
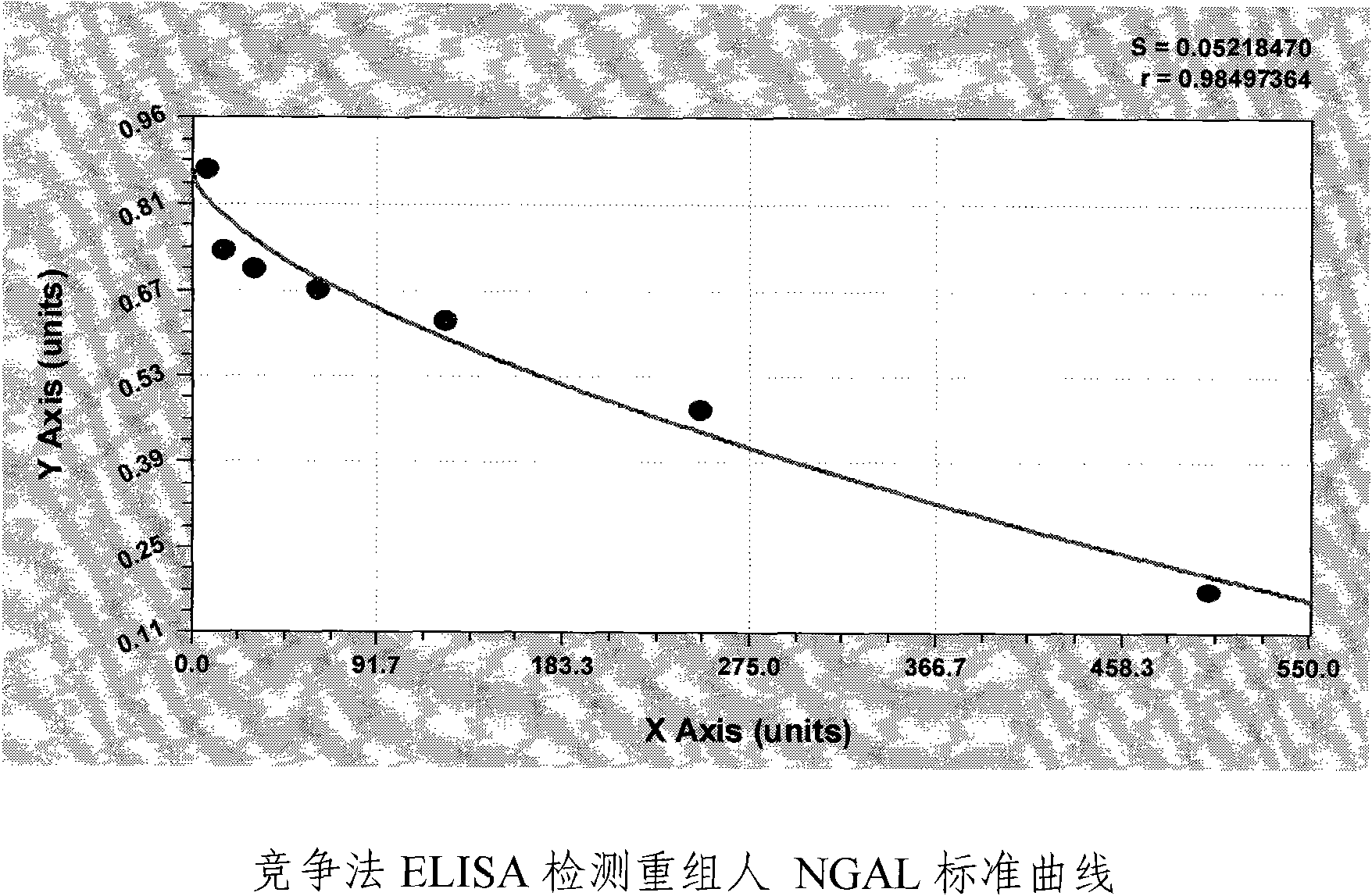
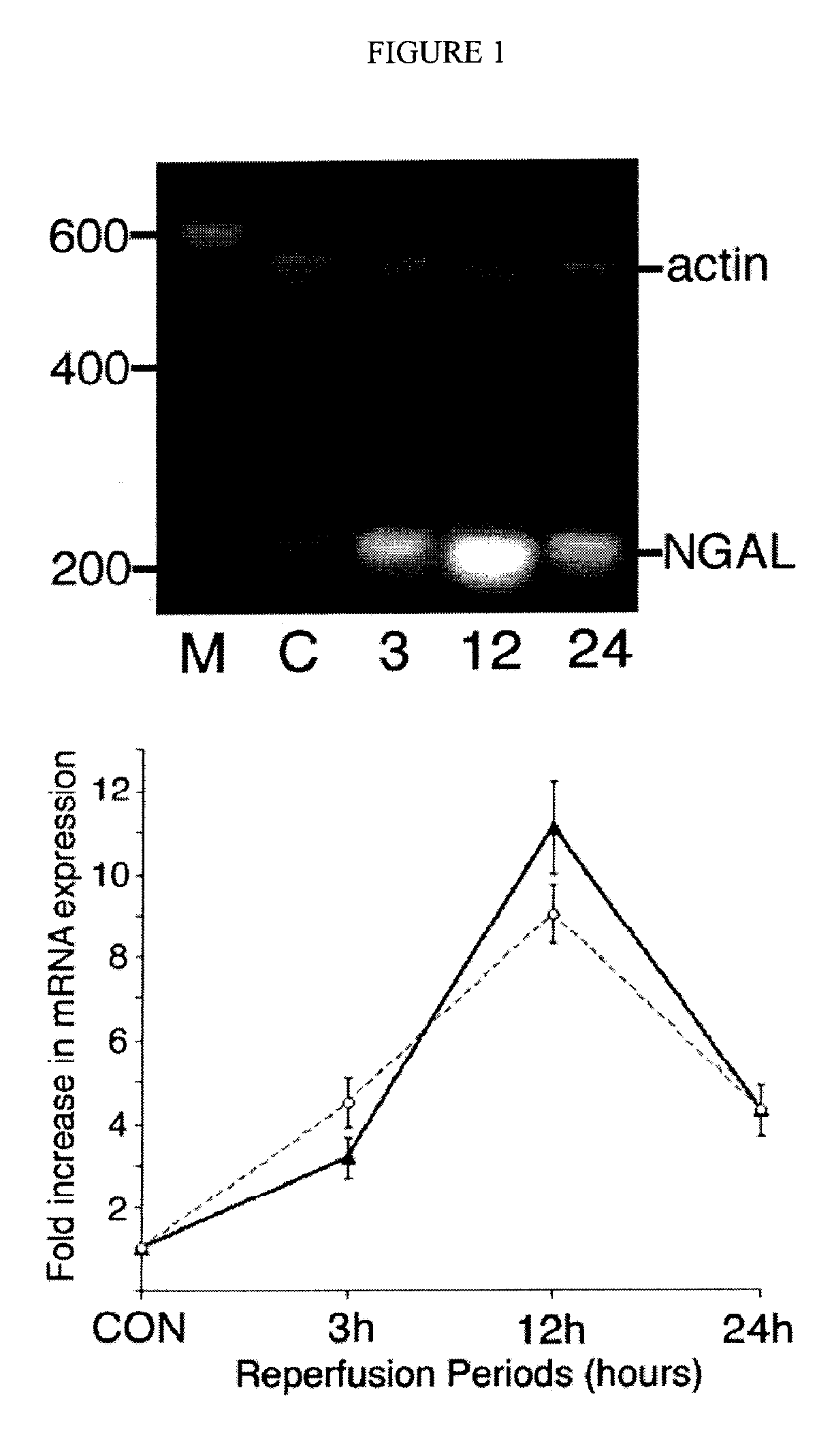
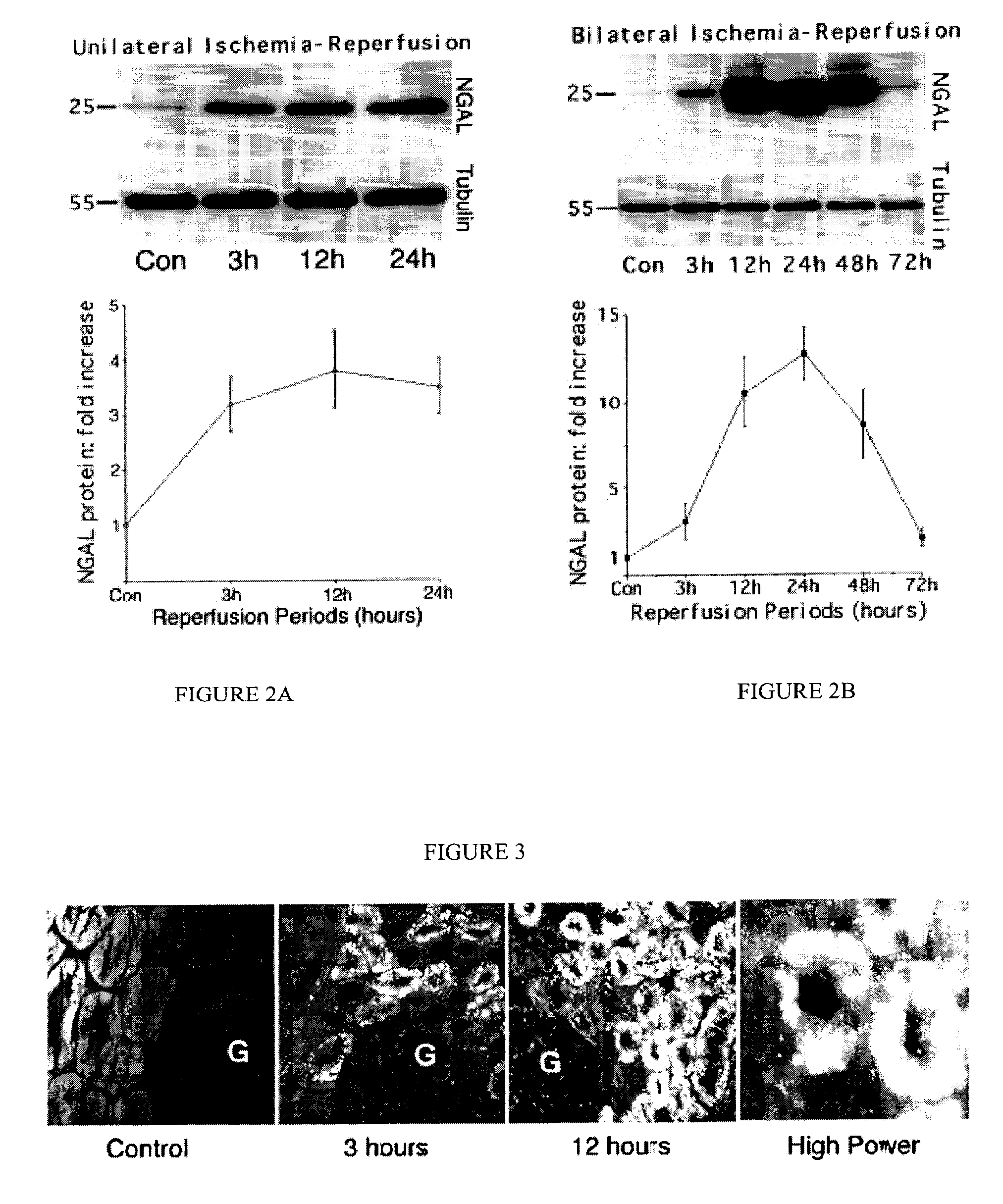
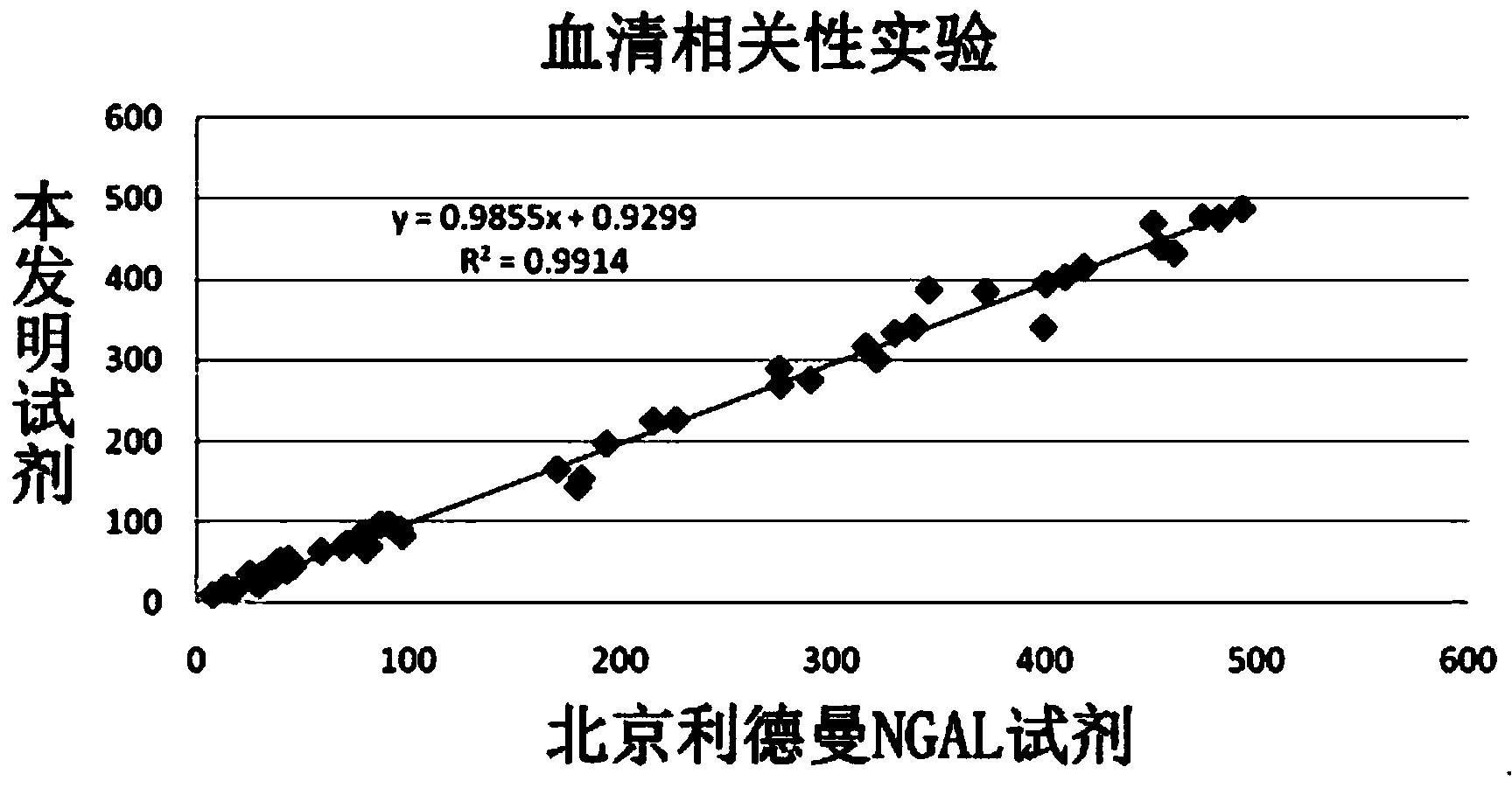
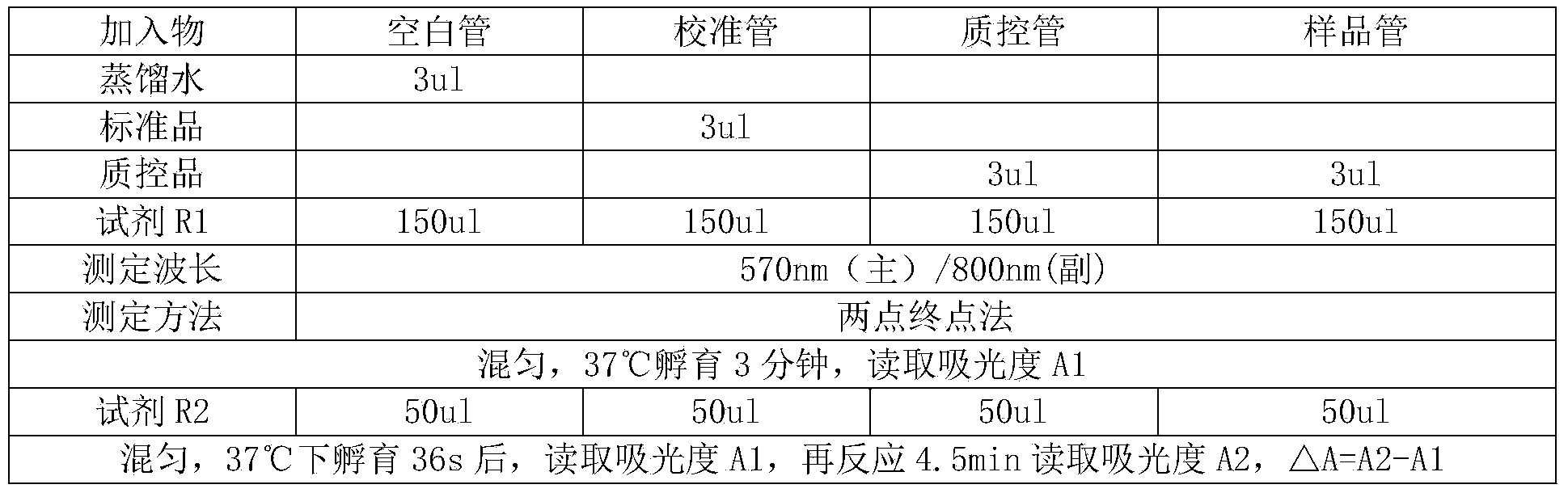
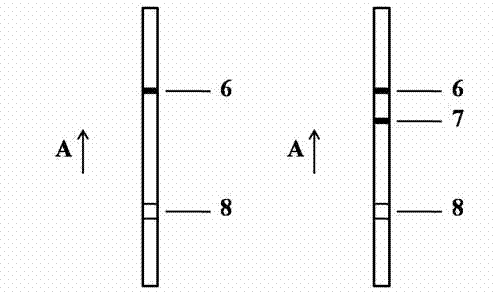
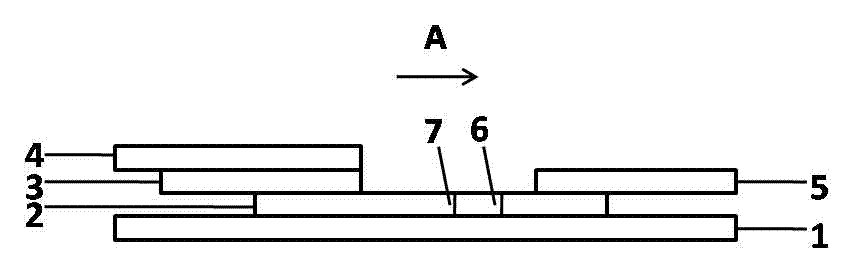
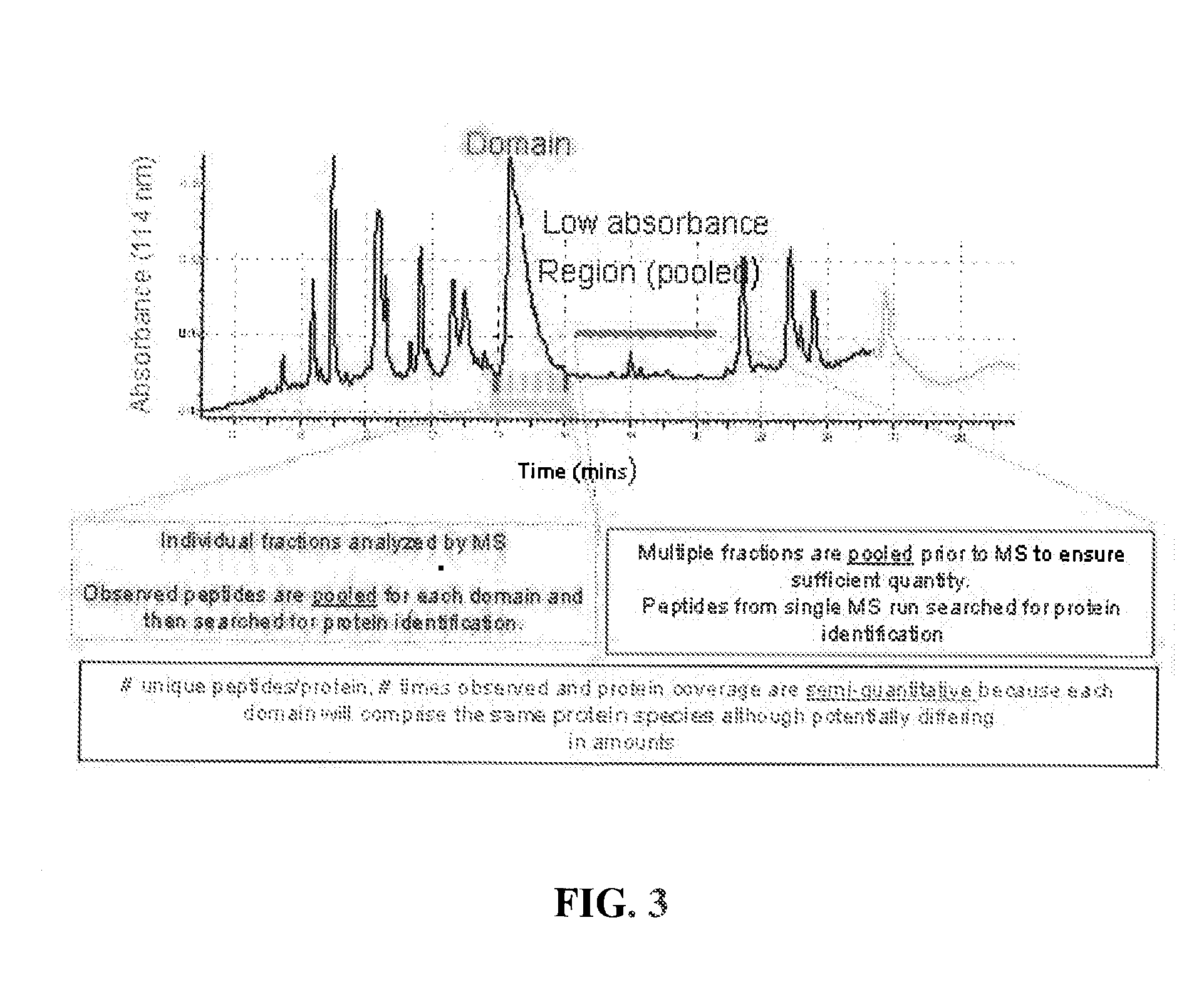
Patents
Literature
124 results about "NGAL Protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lipocalin-2 (LCN2), also known as oncogene 24p3 or neutrophil gelatinase-associated lipocalin (NGAL), is a protein that in humans is encoded by the LCN2 gene. NGAL is involved in innate immunity by sequestrating iron that in turn limits bacterial growth.
Method for the early detection of renal injury
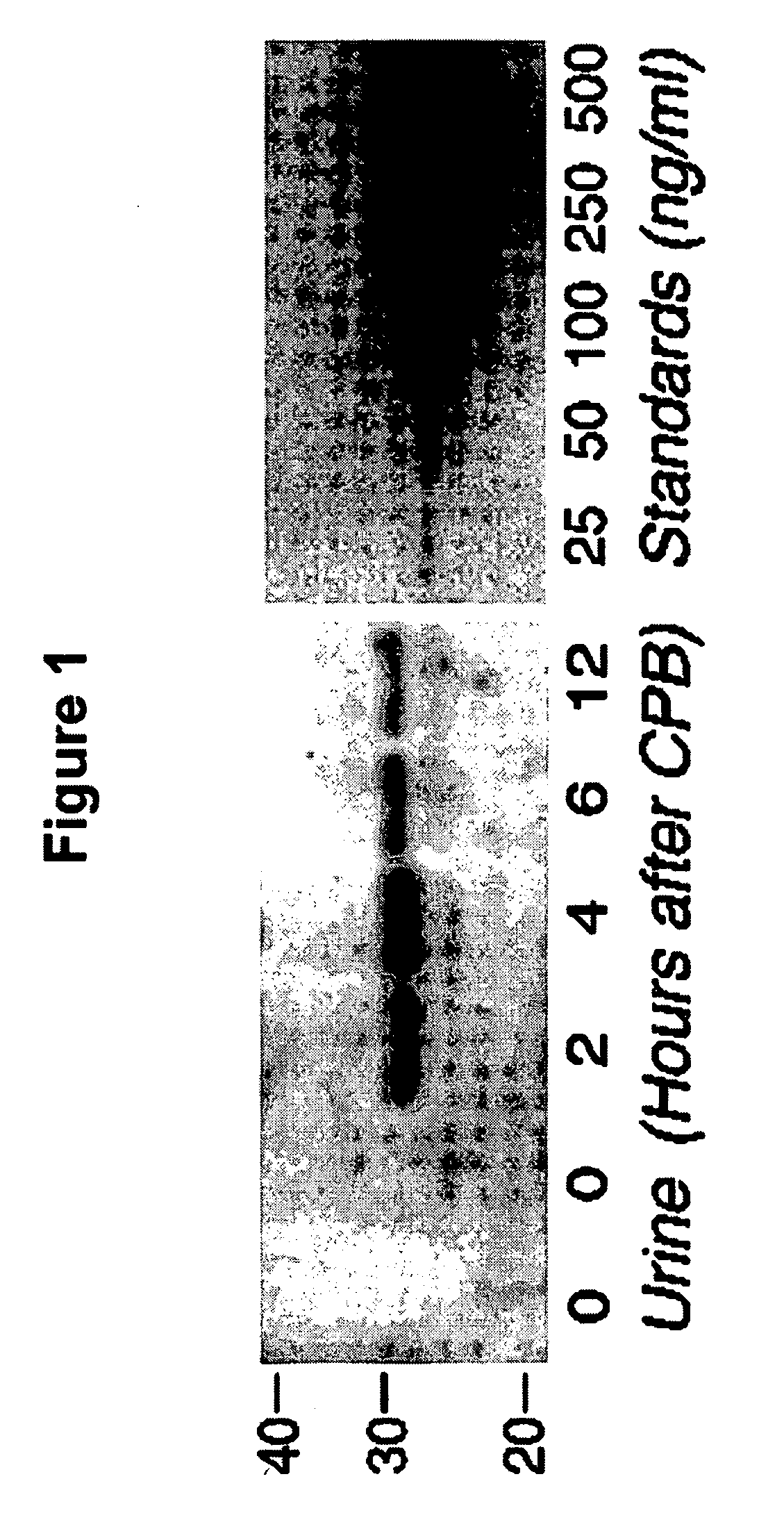
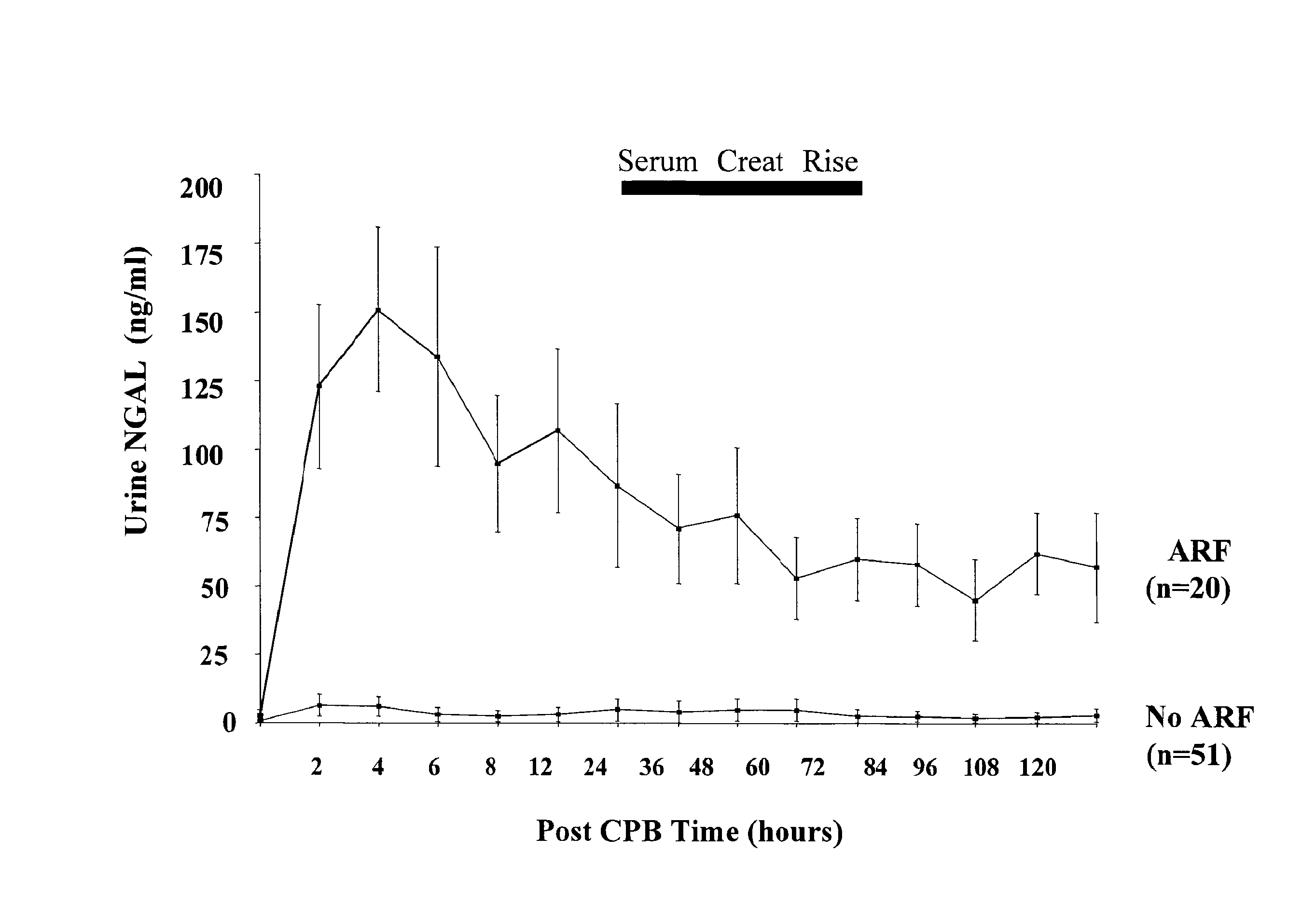
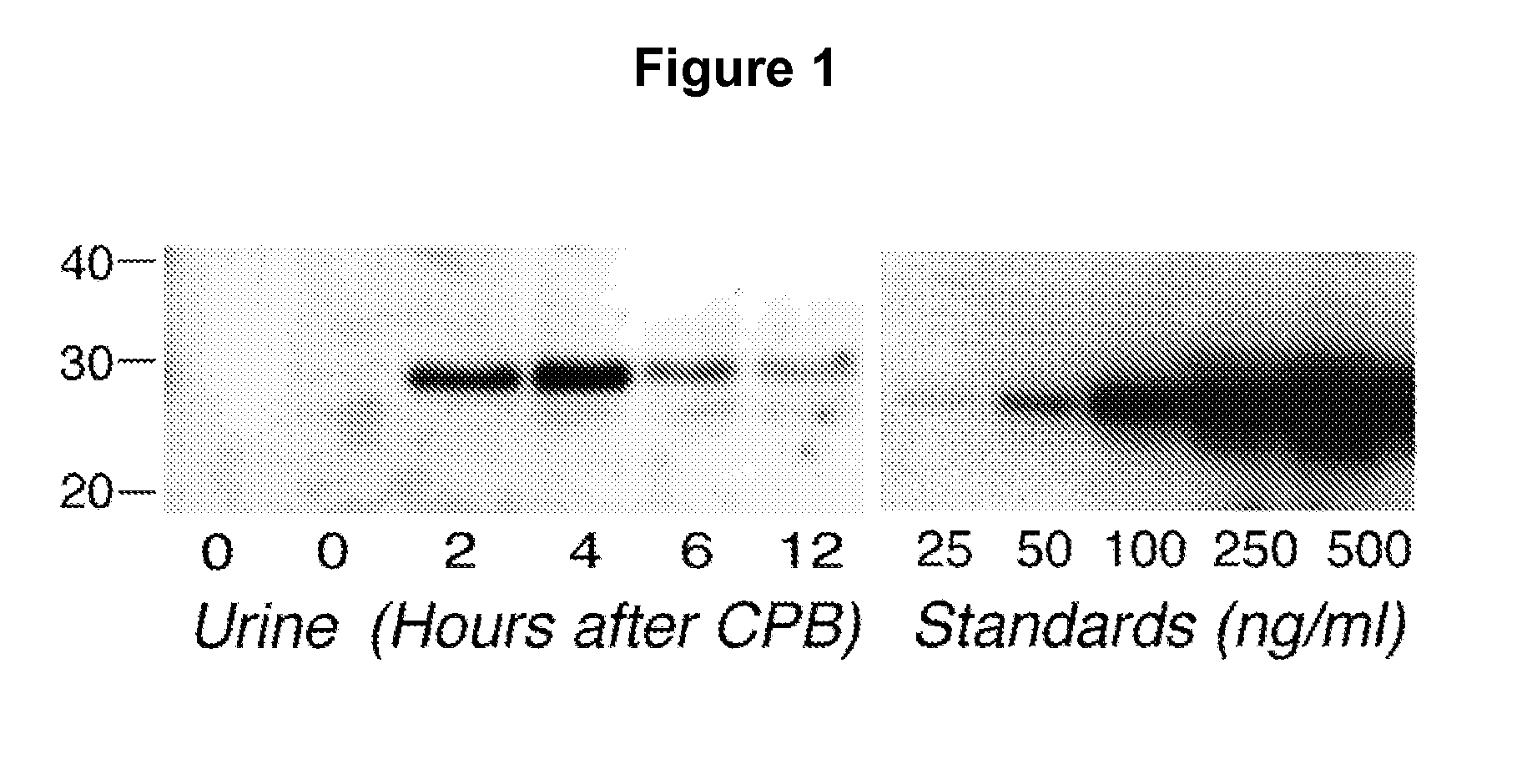
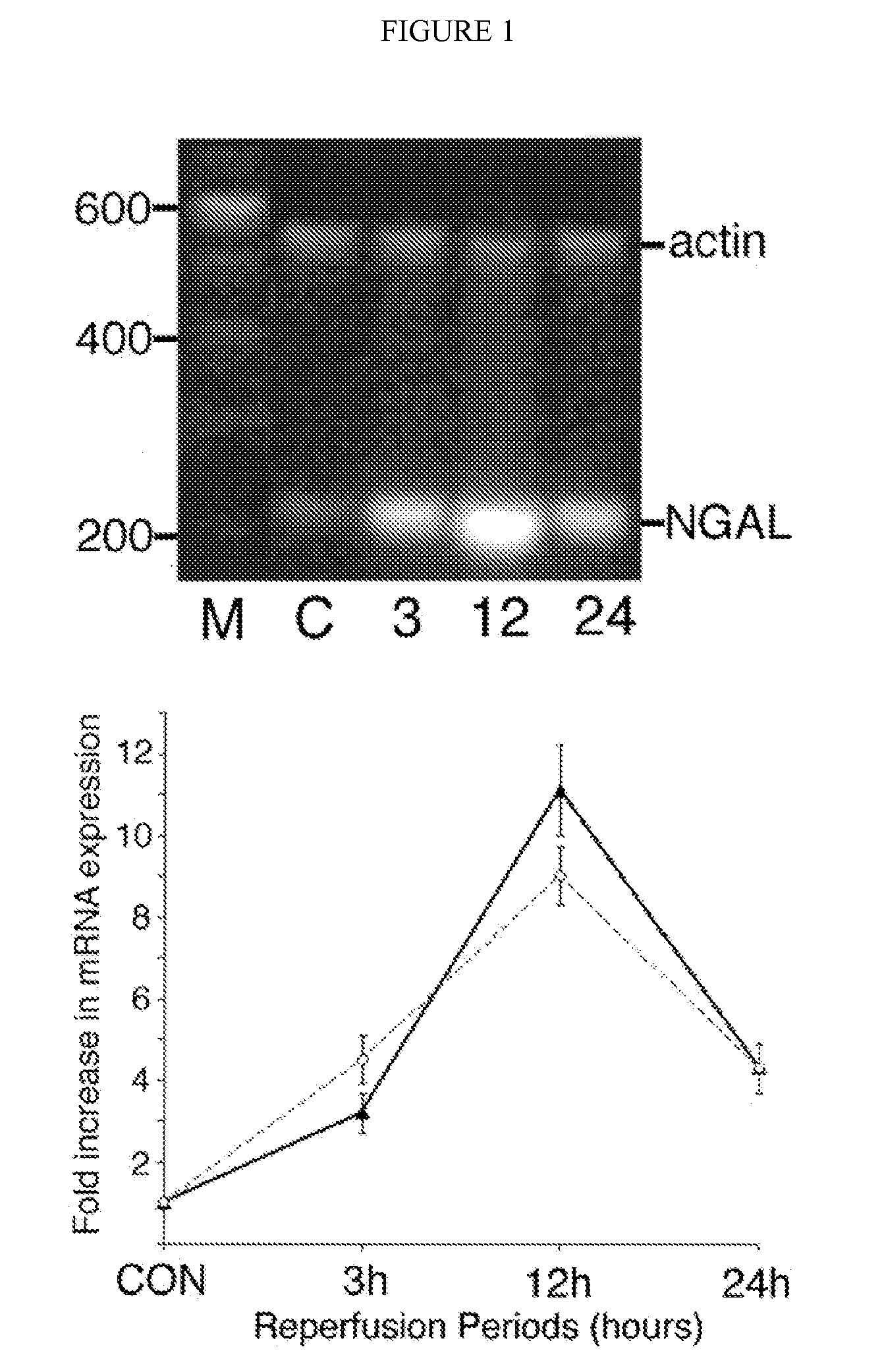
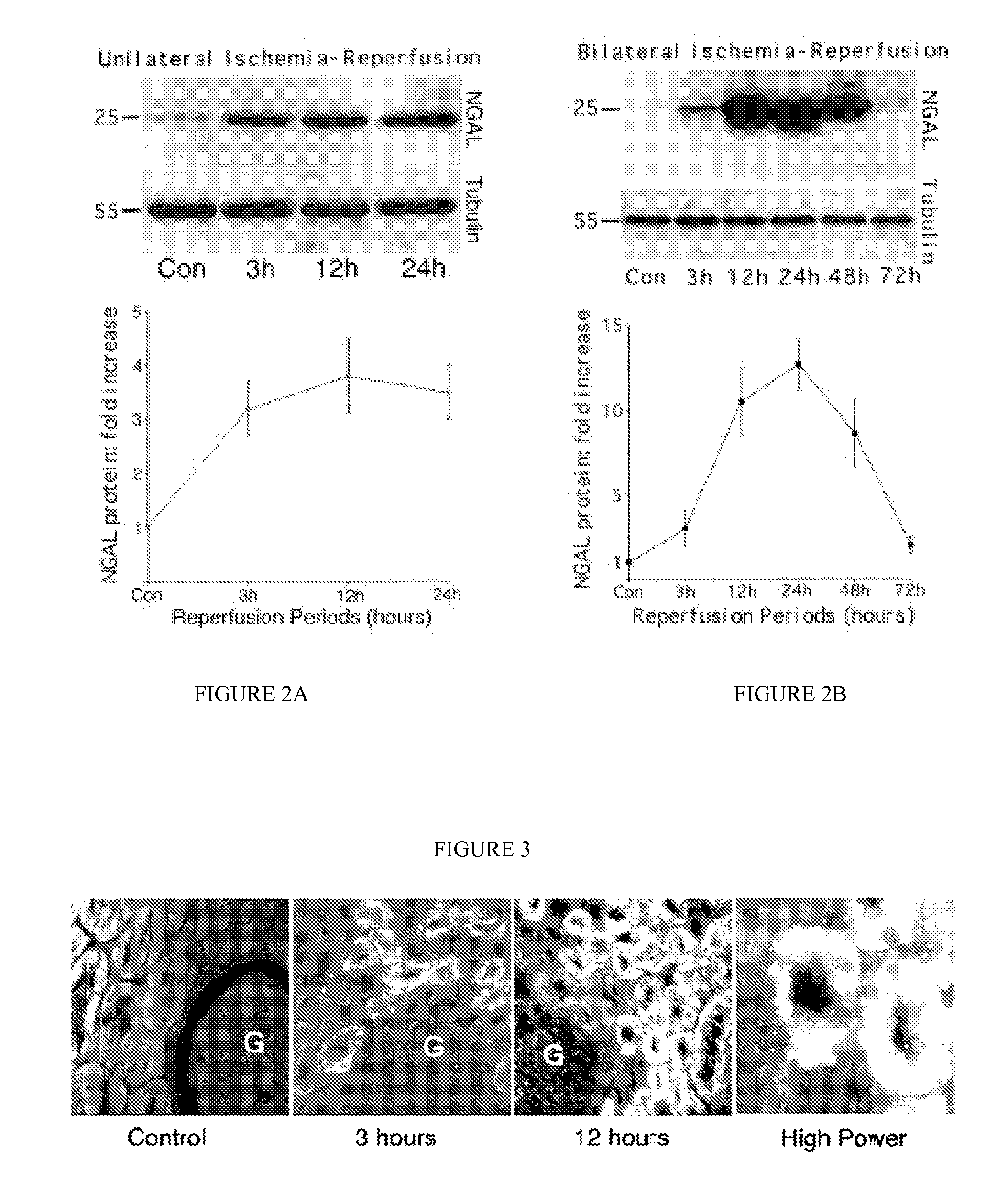
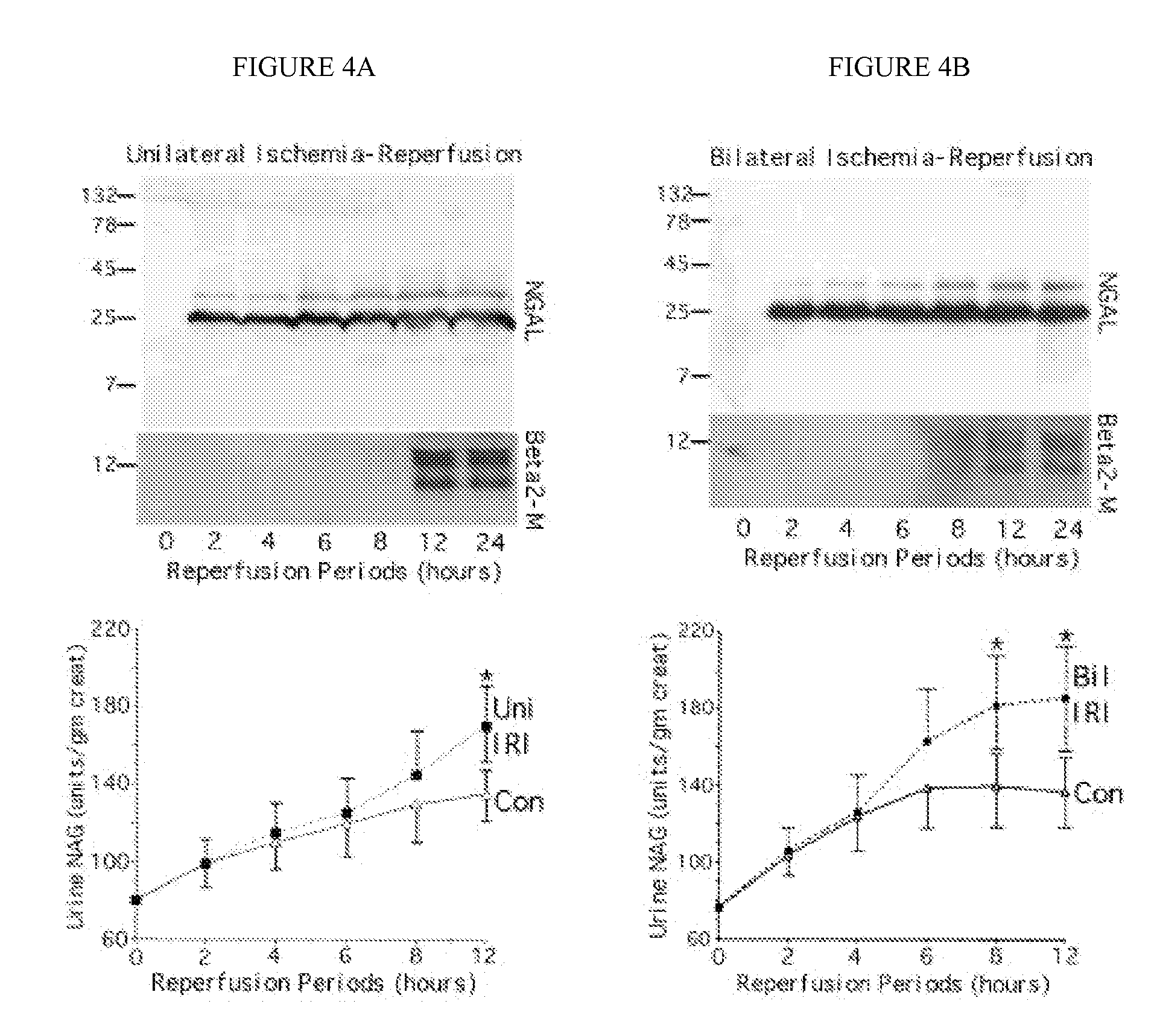
A method and kit for detecting the immediate or early onset of renal disease and injury, including renal tubular cell injury, utilizing NGAL as an immediate or early on-set biomarker in a sample of blood serum. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the blood serum following renal tubule cell injury. NGAL protein expression is detected predominantly in proximal tubule cells, in a punctuate cytoplasmic distribution reminiscent of a secreted protein. The appearance NGAL in the serum is related to the dose and duration of renal ischemia and nephrotoxemia, and is diagnostic of renal tubule cell injury and renal failure. NGAL detection is also a useful marker for monitoring the nephrotoxic side effects of drugs or other therapeutic agents.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
Methods And Compositions For Monitoring And Risk Prediction In Cardiorenal Syndrome
ActiveUS20080254485A1Reduce riskIncreased riskMicrobiological testing/measurementDisease diagnosisMedicineMortality rate
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects. In particular, the invention relates to methods and compositions selected to monitor cardiorenal syndrome using assays that detect NGAL, preferably together with assays that detect natriuretic peptides such as BNP. Such methods and compositions can provide early indications of a deterioration in cardiorenal syndrome status, including prognosis regarding mortality and worsening renal function.
Owner:ALERE SAN DIEGO INC
Diagnosis and monitoring of chronic renal disease using ngal
InactiveUS20080090304A1Difficult to levelImprove the level ofDisease diagnosisBiological testingRegimenProper treatment
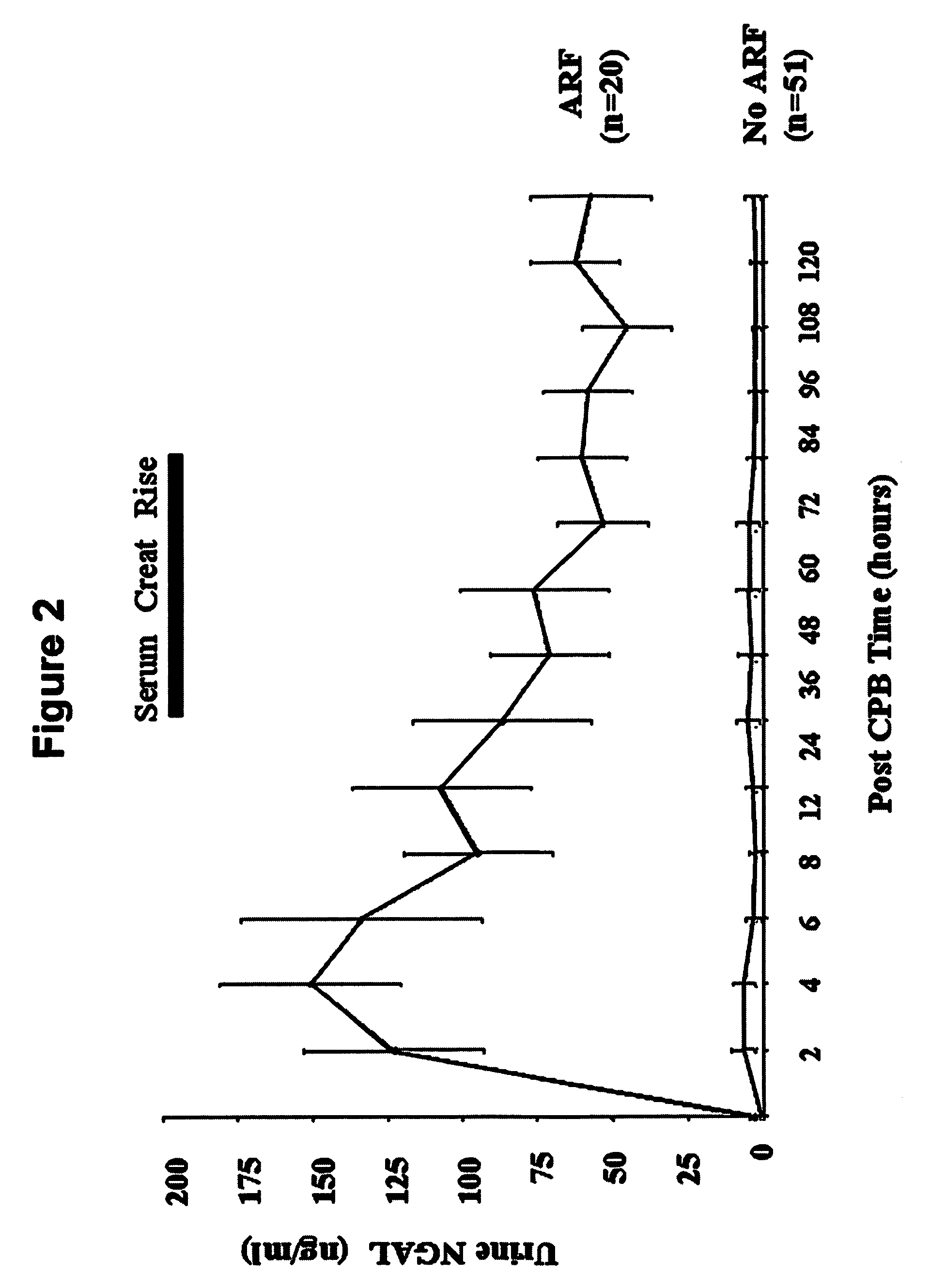
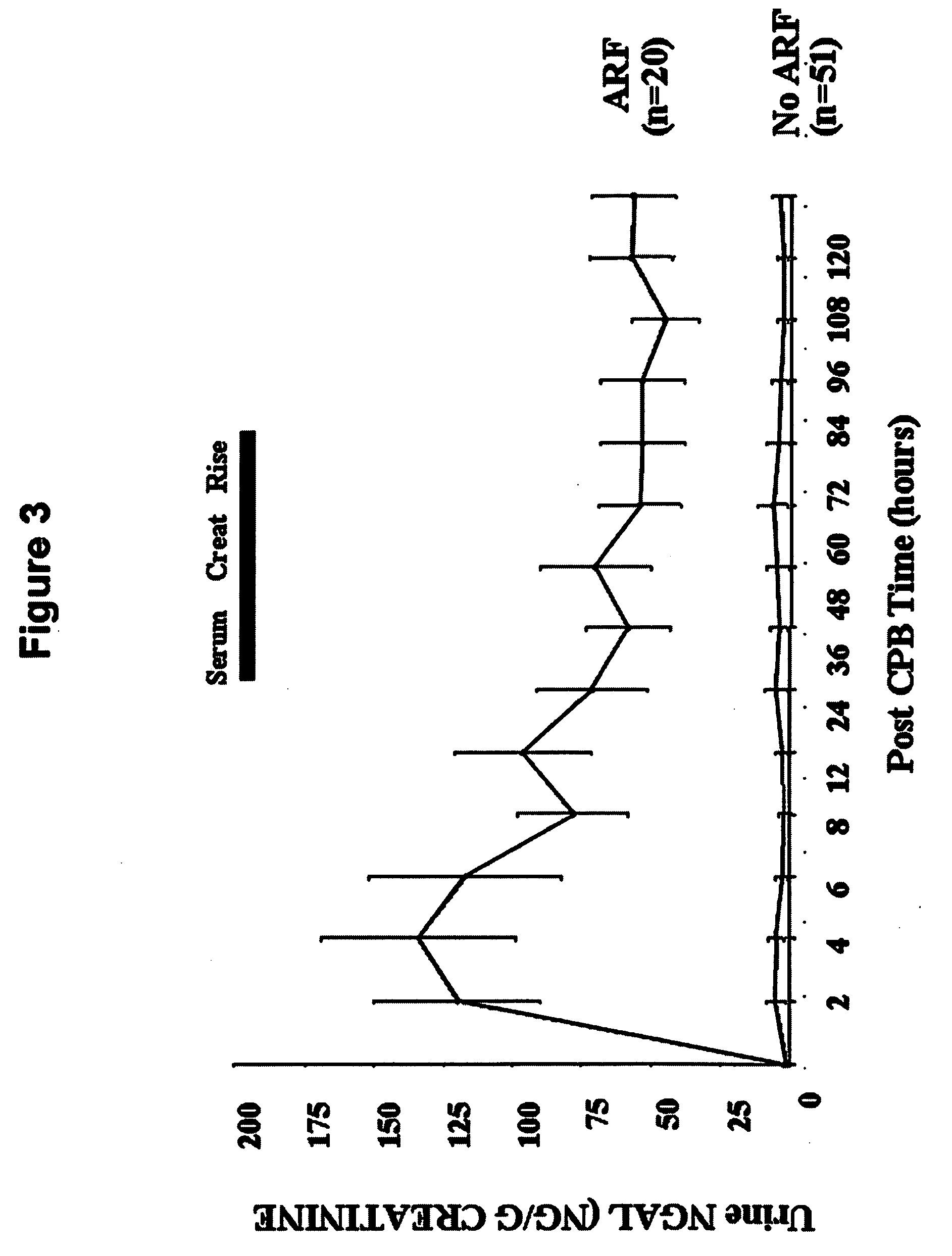
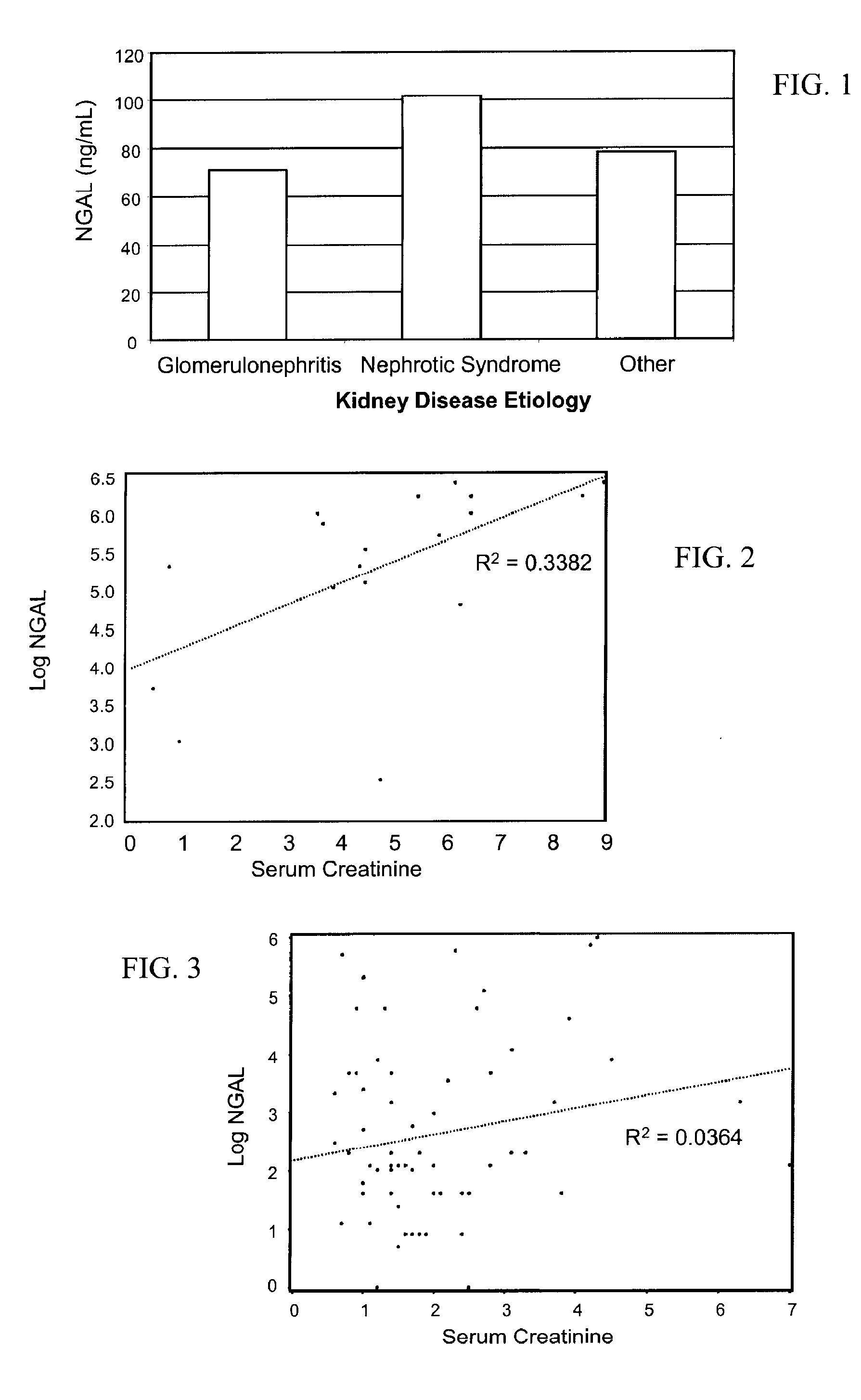
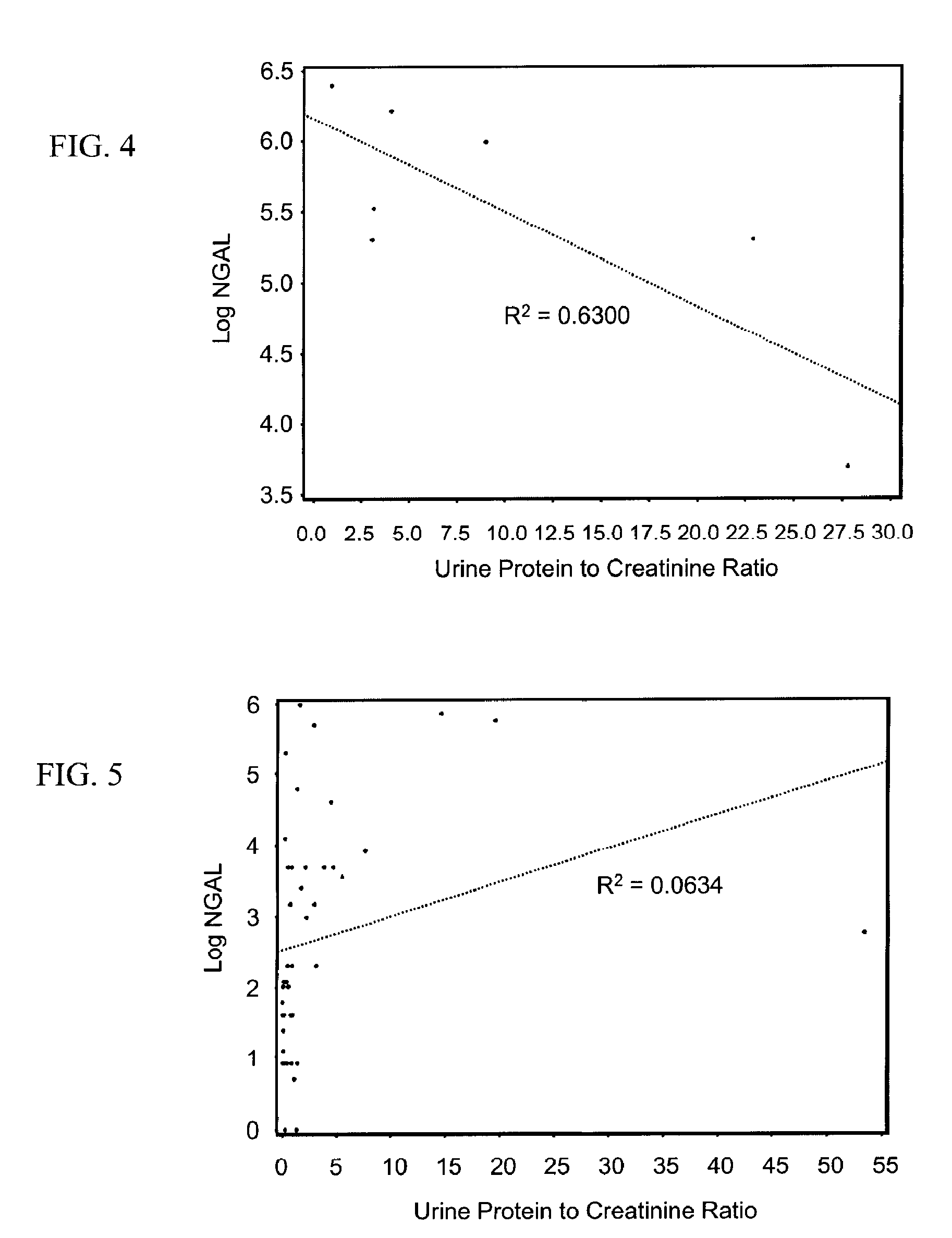
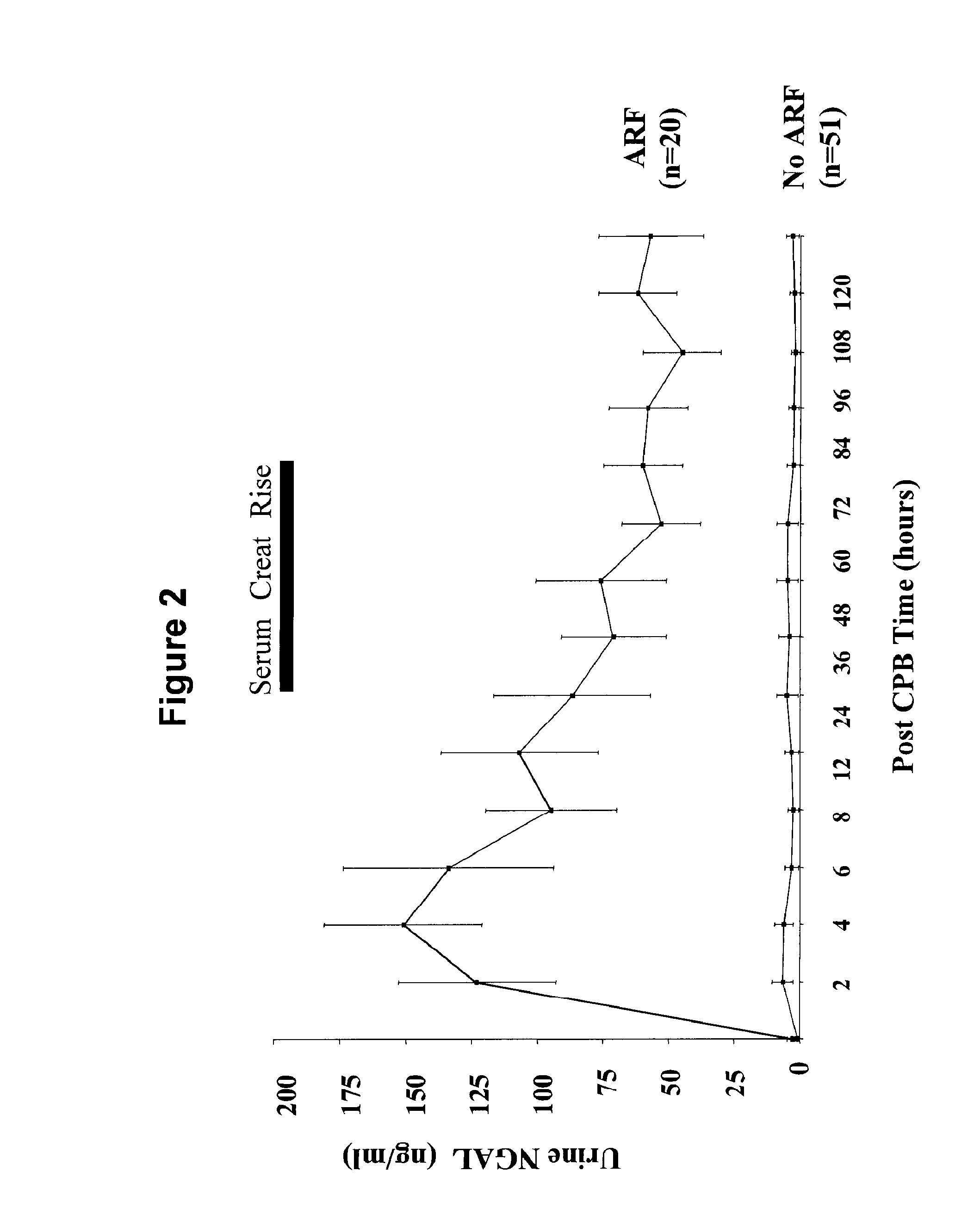
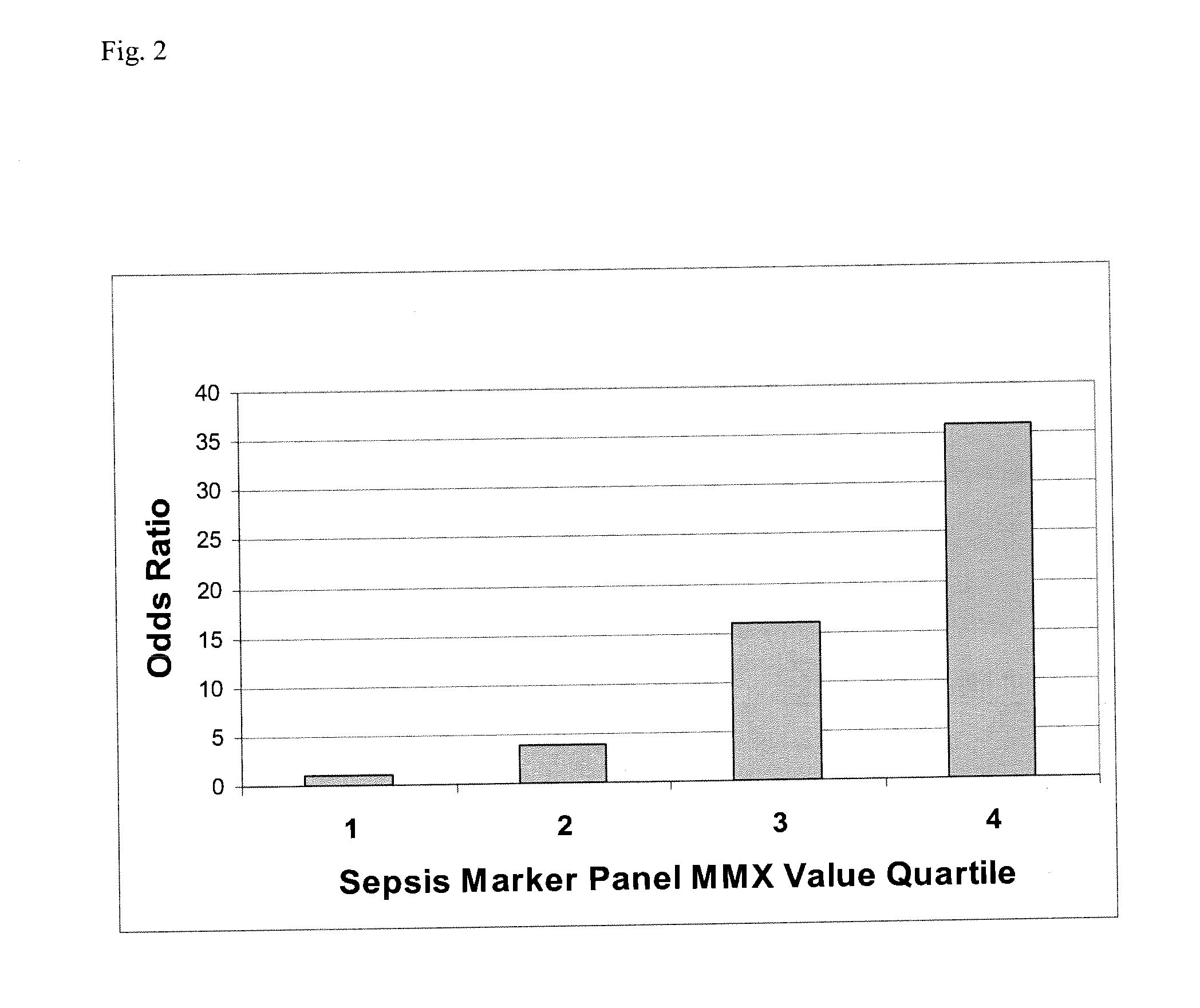
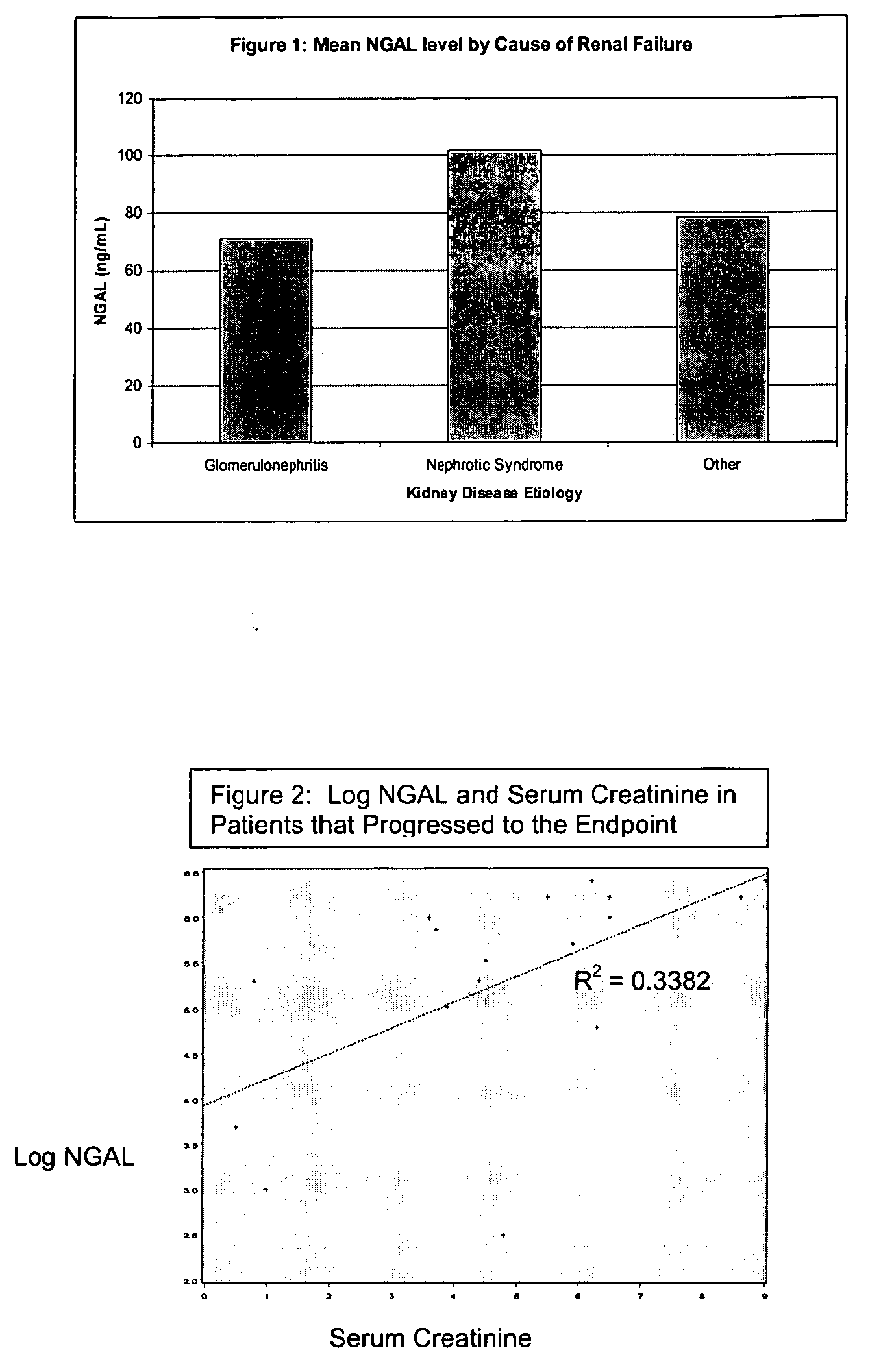
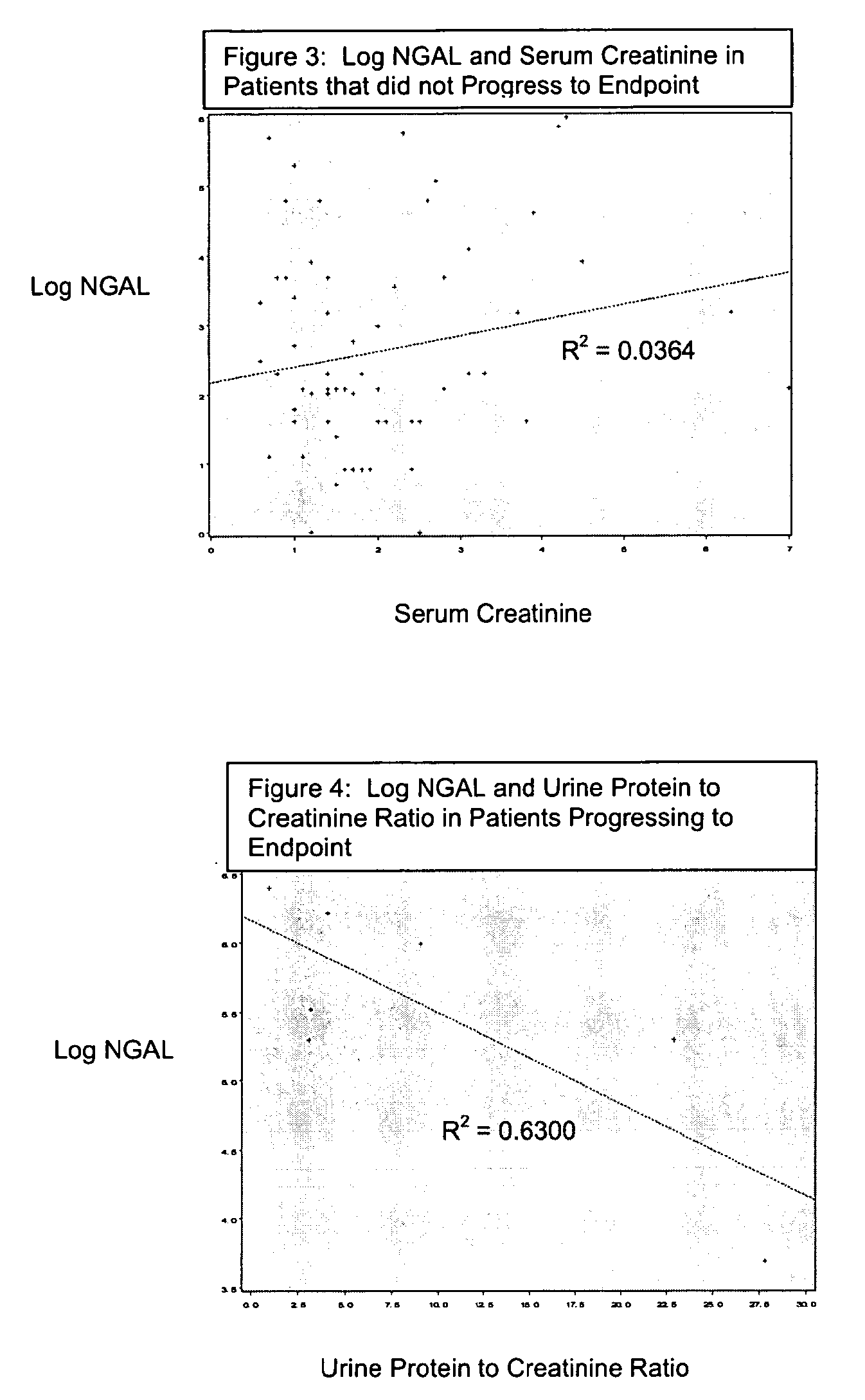
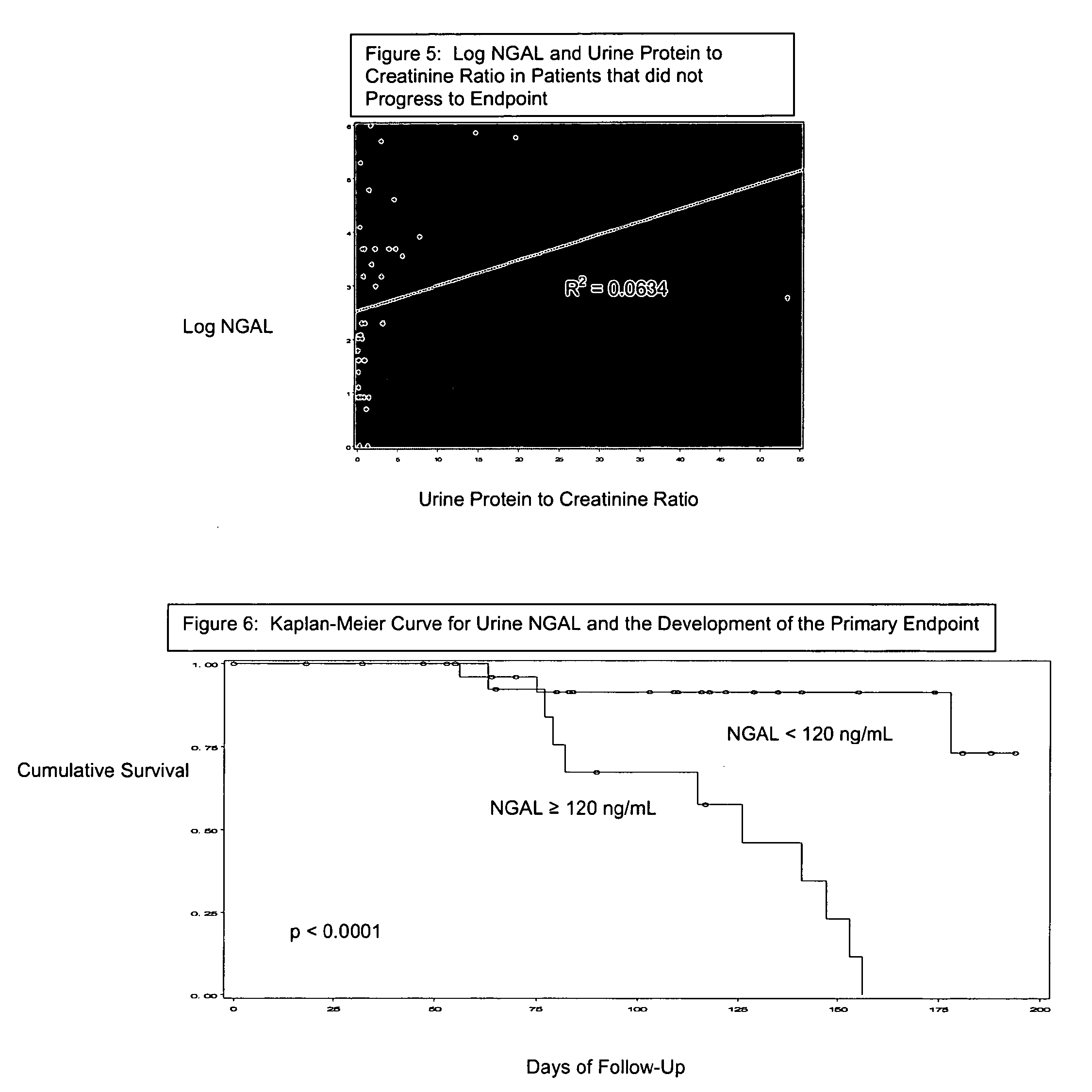
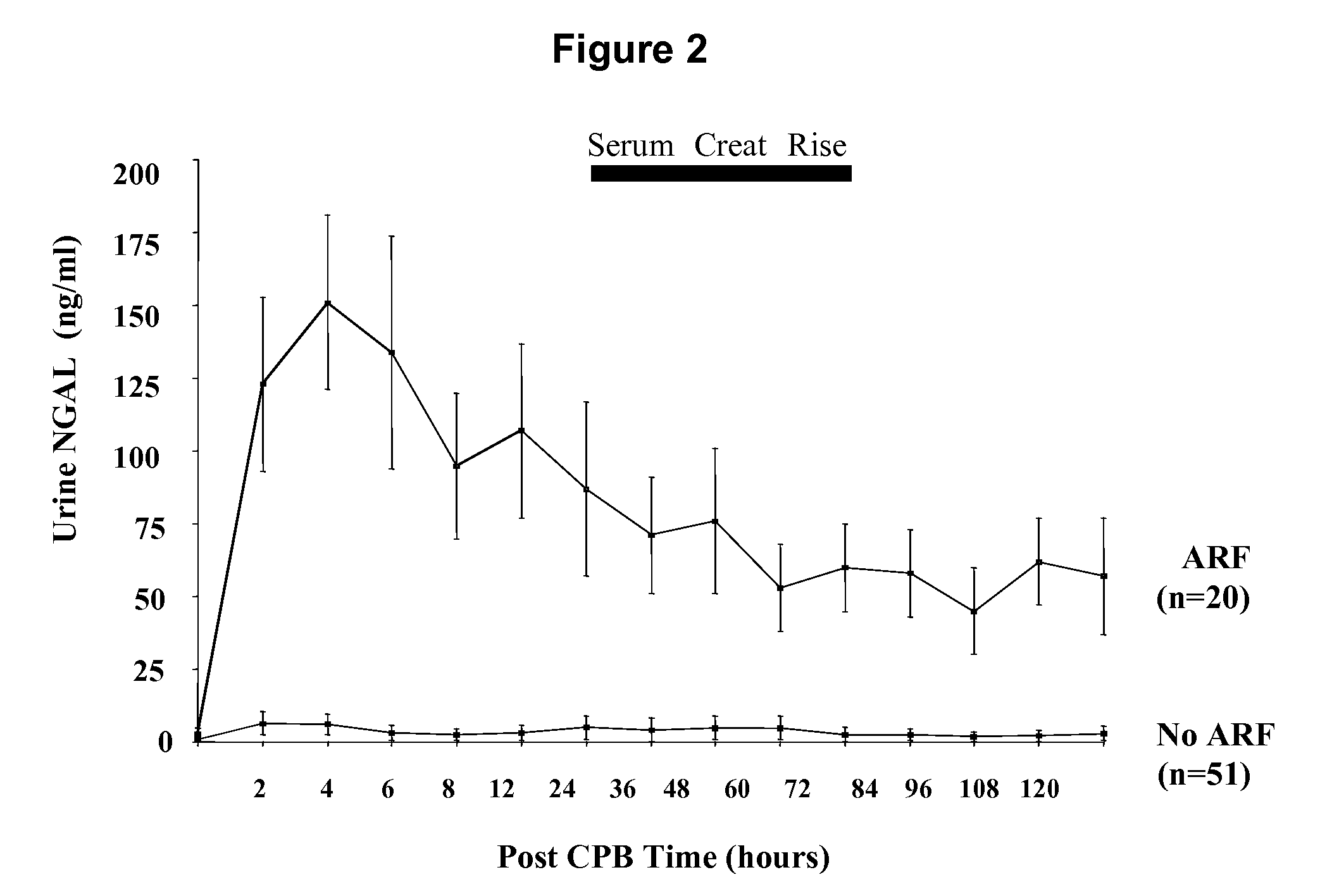
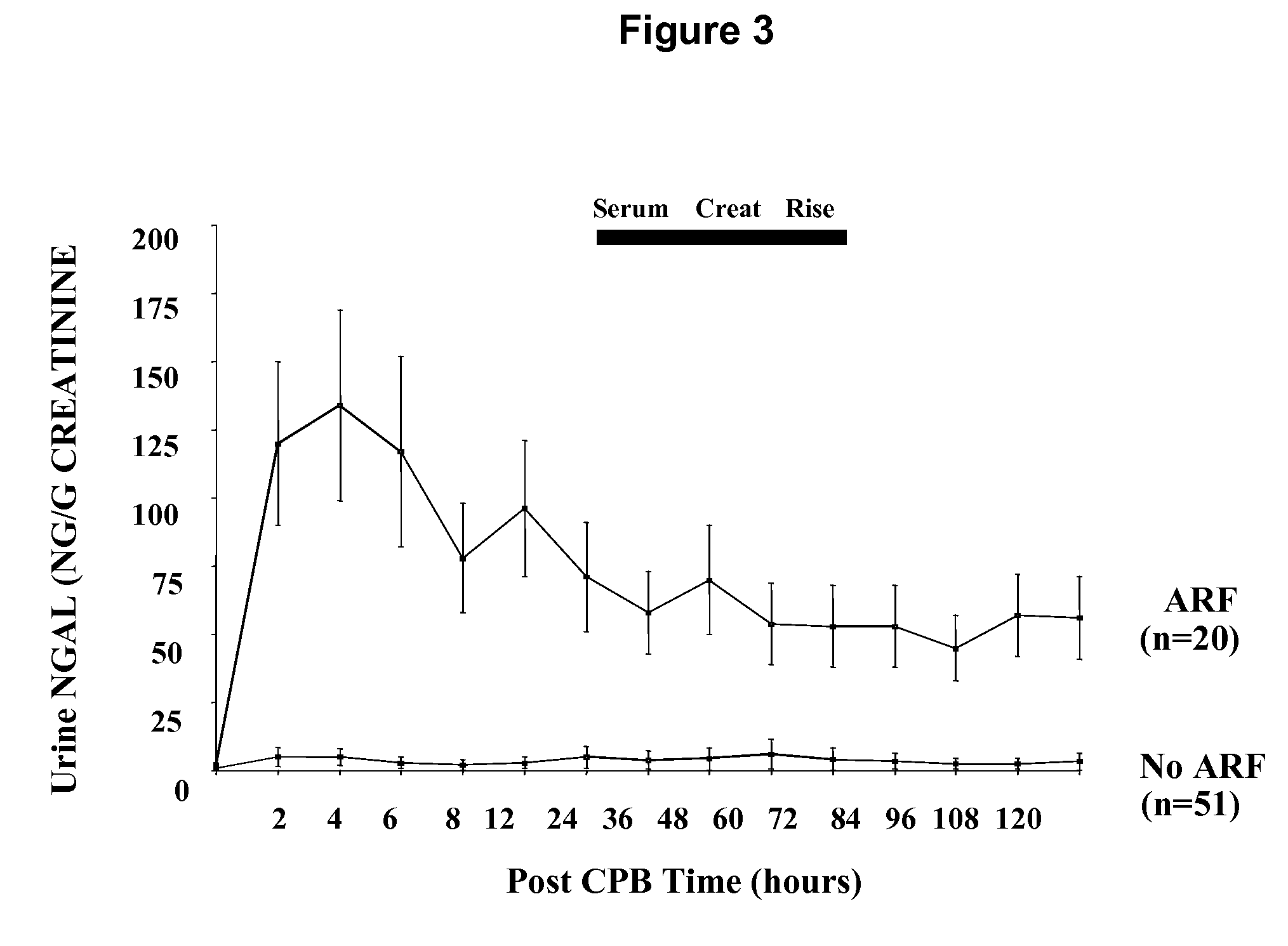
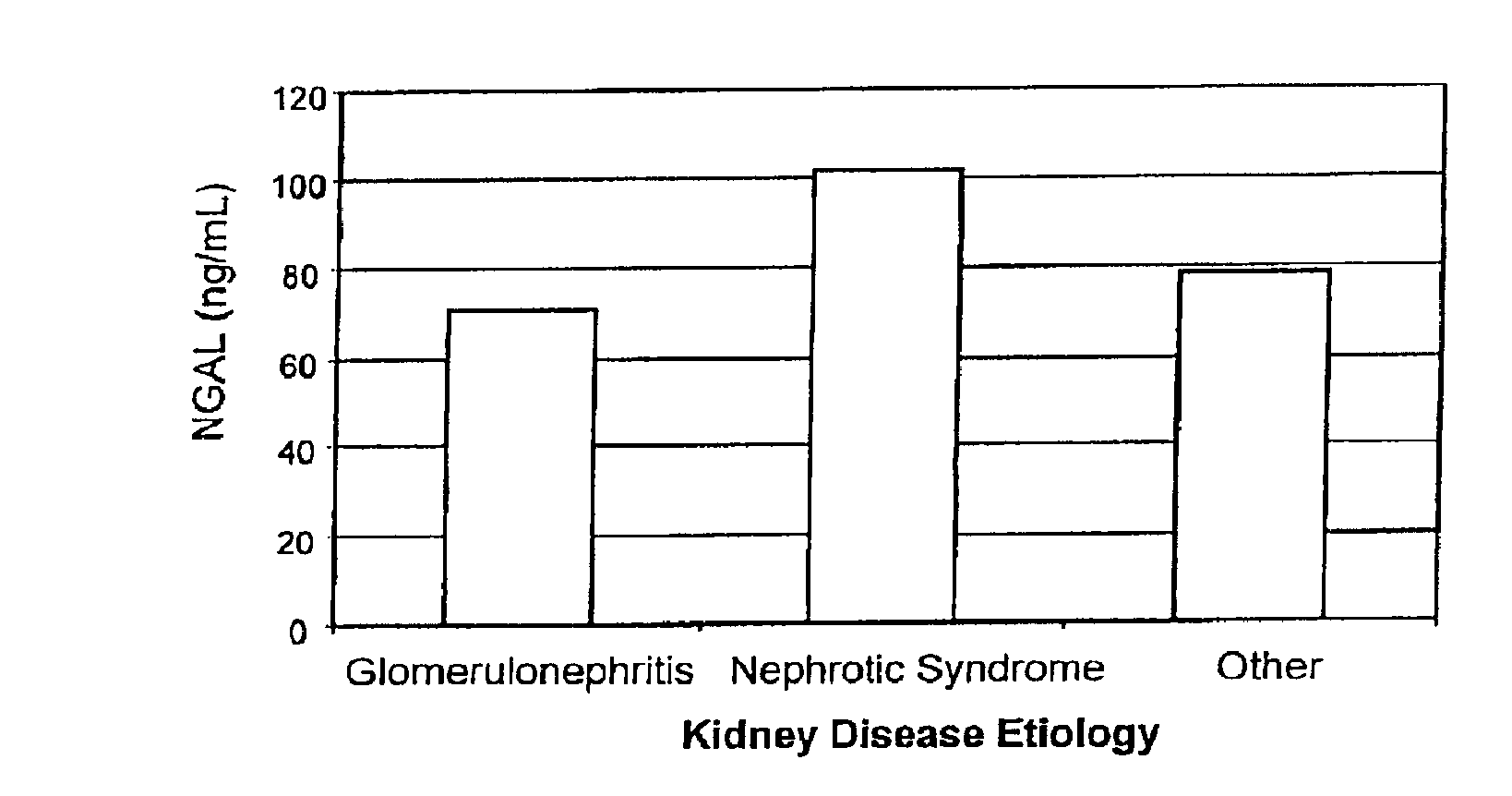
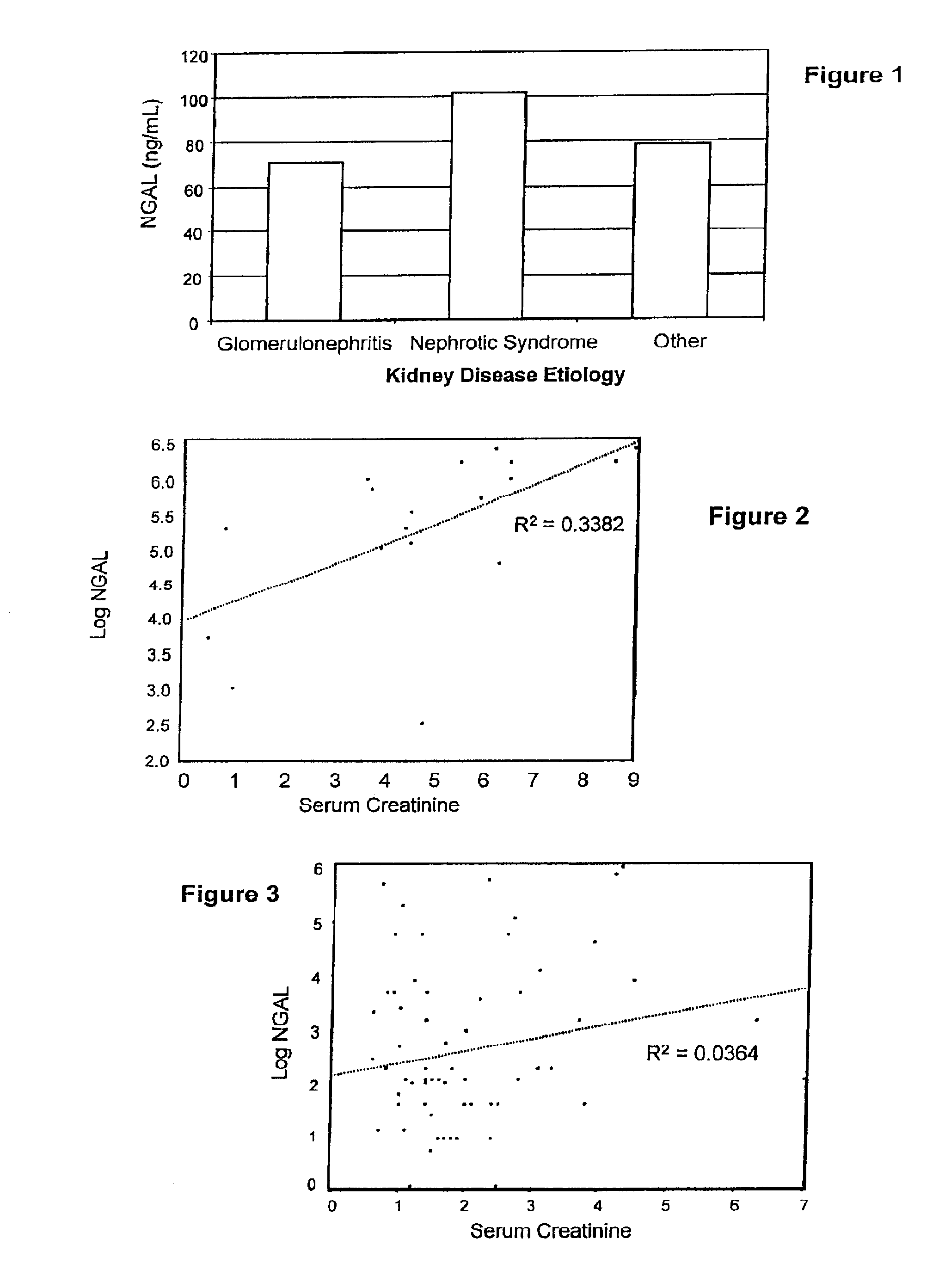
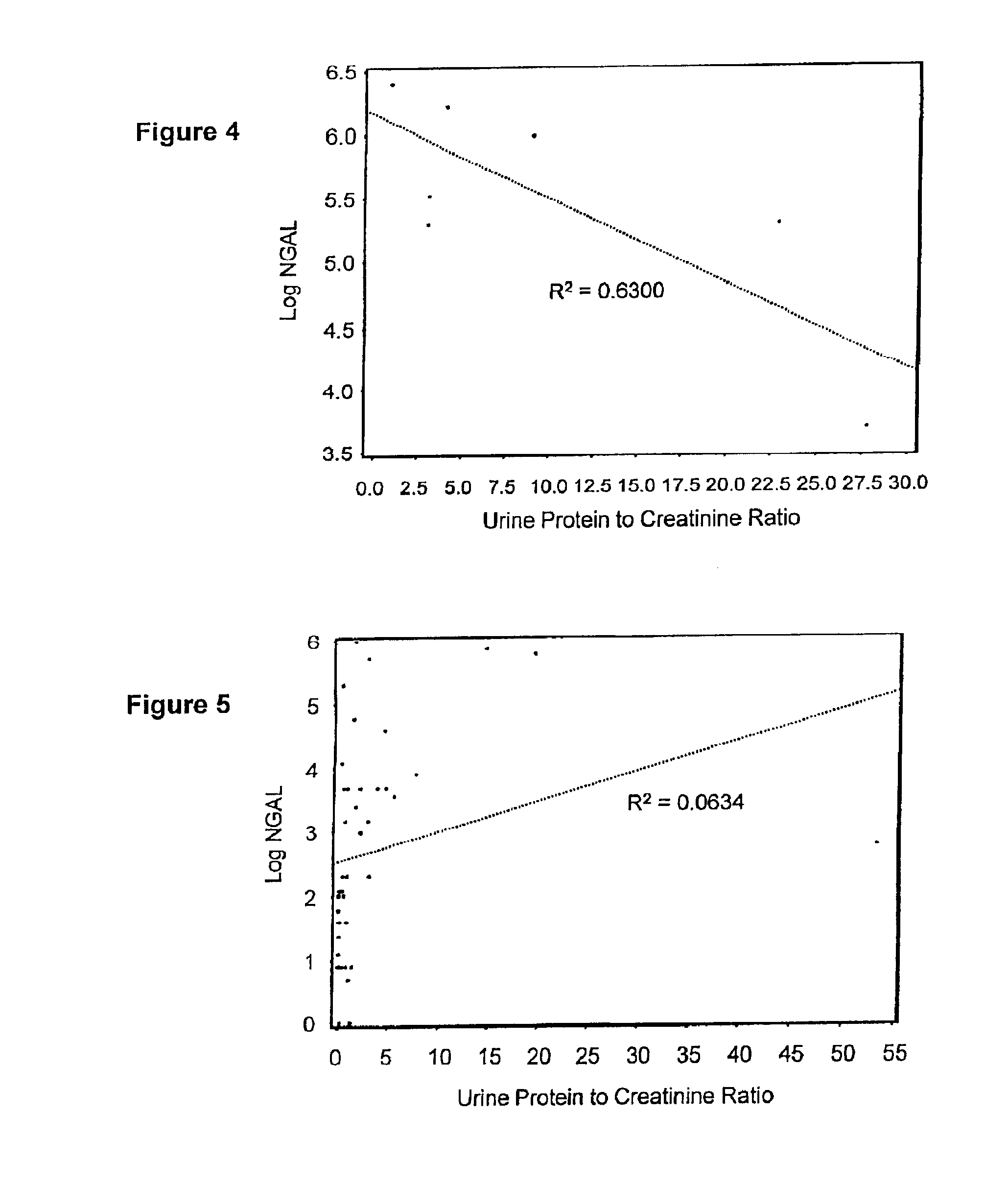
A method of assessing the ongoing kidney status of a mammal afflicted with or at risk of developing chronic renal injury or disease, including chronic renal failure (CRF) by detecting the quantity of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in urine, serum or plasma samples at discrete time periods, as well as over time. Incremental increases in NGAL levels in CRF patients over a prolonged period of time are diagnostic of worsening kidney disease. This increase in NGAL precedes and correlates with other indicators of worsening chronic renal disease or CRF, such as increased serum creatinine, increased urine protein secretion, and lower glomerular filtration rate (GFR). Proper detection of worsening (or improving, if treatment has been instituted) renal status over time, confirmed by pre- and post-treatment NGAL levels in the patient, can aid the clinical practitioner in designing and / or maintaining a proper treatment regimen to slow or stop the progression of CRF.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Method for the early detection of renal injury
A method and kit for detecting the immediate or early onset of renal disease and injury, including renal tubular cell injury, utilizing NGAL as an immediate or early on-set biomarker in a sample of blood serum. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the blood serum following renal tubule cell injury. NGAL protein expression is detected predominantly in proximal tubule cells, in a punctuate cytoplasmic distribution reminiscent of a secreted protein. The appearance NGAL in the serum is related to the dose and duration of renal ischemia and nephrotoxemia, and is diagnostic of renal tubule cell injury and renal failure. NGAL detection is also a useful marker for monitoring the nephrotoxic side effects of drugs or other therapeutic agents.
Owner:DEVARAJAN PRASAD +1
Diagnosis and monitoring of chronic renal disease using ngal
InactiveUS20080014644A1Monitor effectivenessEarly detectionDisease diagnosisBiological testingRegimenProper treatment
A method of assessing the ongoing kidney status of a mammal afflicted with or at risk of developing chronic renal injury or disease, including chronic renal failure (CRF) by detecting the quantity of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in urine, serum or plasma samples at discrete time periods, as well as over time. Incremental increases in NGAL levels in CRF patients over a prolonged period of time are diagnostic of worsening kidney disease. This increase in NGAL precedes and correlates with other indicators of worsening chronic renal disease or CRF, such as increased serum creatinine, increased urine protein secretion, and lower glomerular filtration rate (GFR). Proper detection of worsening (or improving, if treatment has been instituted) renal status over time, confirmed by pre- and post-treatment NGAL levels in the patient, can aid the clinical practitioner in designing and / or maintaining a proper treatment regimen to slow or stop the progression of CRF.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Innovative discovery of therapeutic, diagnostic, and antibody compositions related to protein fragments of phenylalanyl-beta-trna synthetases
ActiveUS20130202575A1Altering abilityImprove purification effectPeptide/protein ingredientsAntipyreticNucleotideNGAL Protein
Owner:ATYR PHARM INC +1
A method and kit for detecting the early onset of renal tubular cell injury
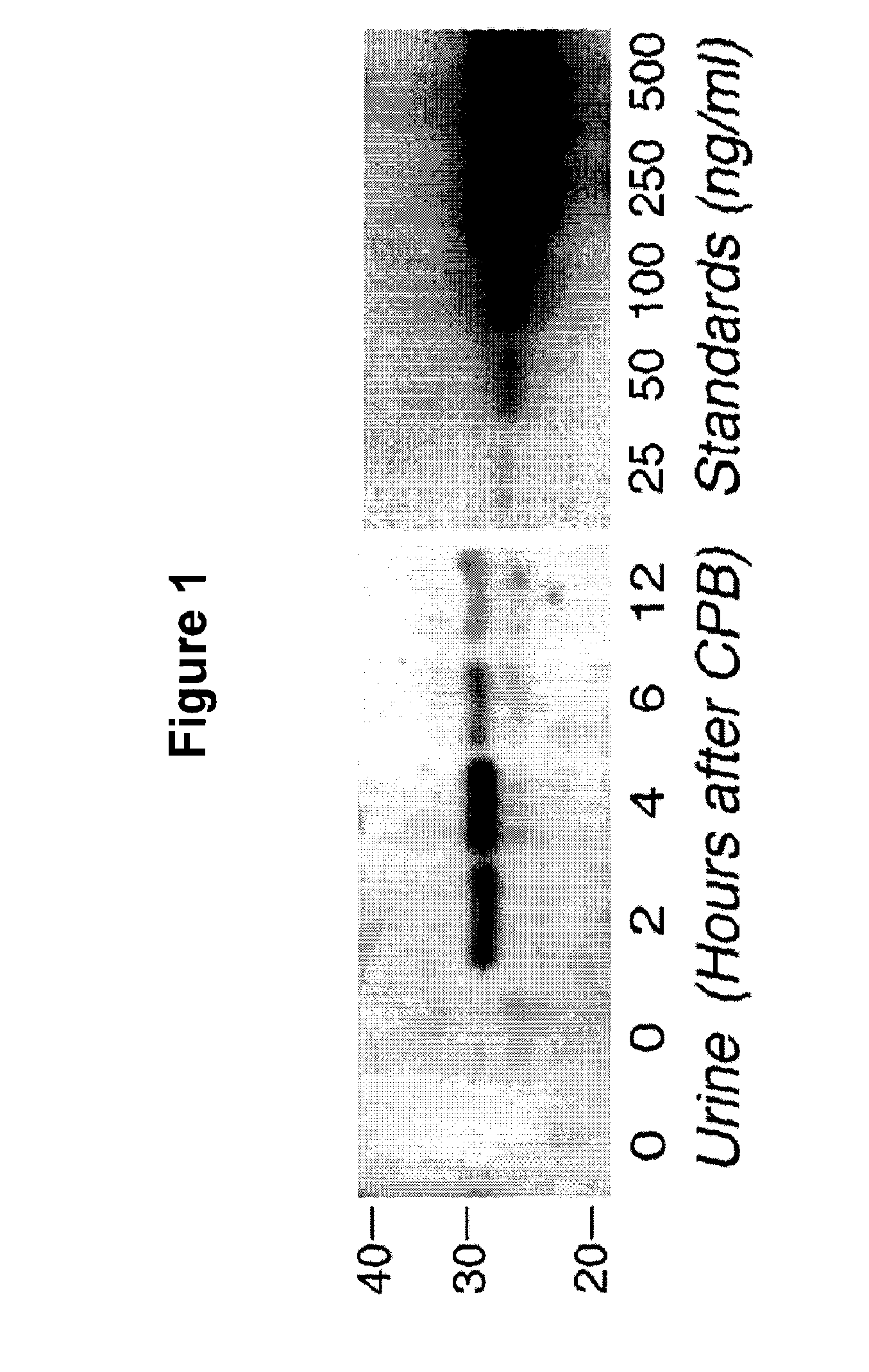
An early-onset method and kit for detecting renal tubular cell injury, using NGAL as an early urinary biomarker. NGAL is a small secreted polypeptide that is protease resistant and thus readily detected in urine following tubular cell injury. NGAL protein expression was mainly detected in the proximal tubule cells, which resembled a secreted protein with a punctate distribution in the cytoplasm. Apparent NGAL in urine correlates with the amount and duration of renal ischemia and nephrotoxicemia and is a diagnostic feature of tubular cell injury and renal failure. NGAL detection is also a useful marker for monitoring nephrotoxic side effects of drugs or other therapeutic agents.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
Methods and compositions for diagnosis and/or prognosis in systemic inflammatory response syndromes
Owner:BIOSITE INC
Detection of NGAL in chronic renal disease
Methods of assessing the ongoing kidney status in a subject afflicted with chronic renal failure (CRF) by detecting the quantity of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in fluid samples over time is disclosed. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the urine and serum as a result of chronic renal tubule cell injury. Incremental increases in NGAL levels in CRF patients over a prolonged period of time are diagnostic of worsening kidney disease. This increase in NGAL precedes and correlates with other indicators of worsening CRF, such as increased serum creatinine, increased urine protein secretion, and lower glomerular filtration rate (GFR). Proper detection of worsening (or improving, if treatment has been instituted) renal status over time, confirmed by pre- and post-treatment NGAL levels in the patient, can aid the clinical practitioner in designing and / or maintaining a proper treatment regimen to slow or stop the progression of CRF.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Kit for detecting NGAL content and preparation method thereof
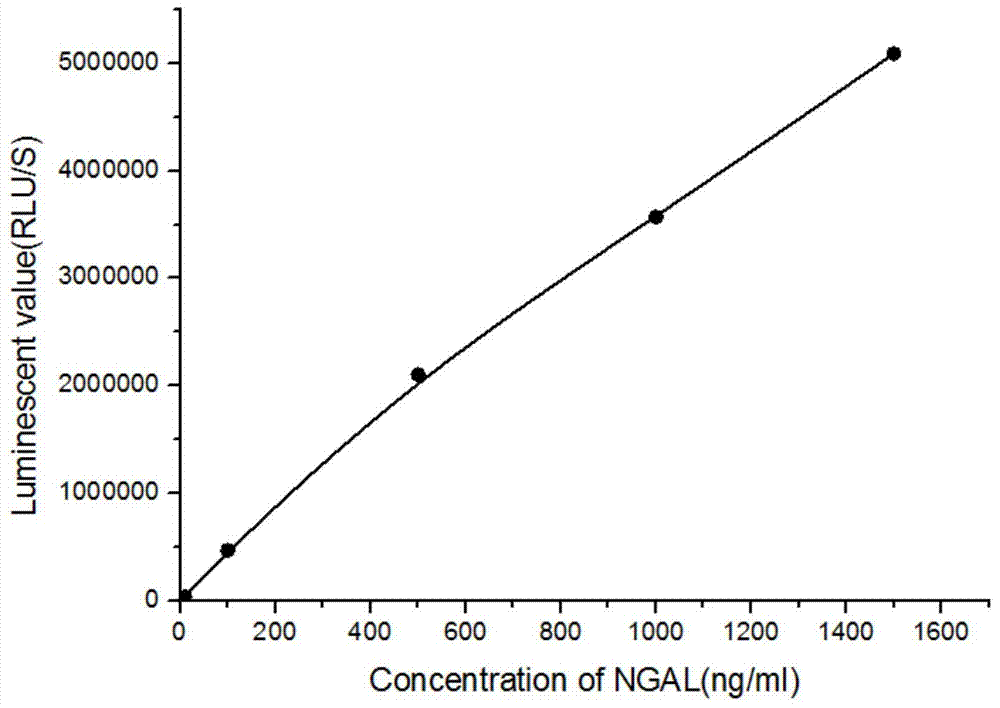
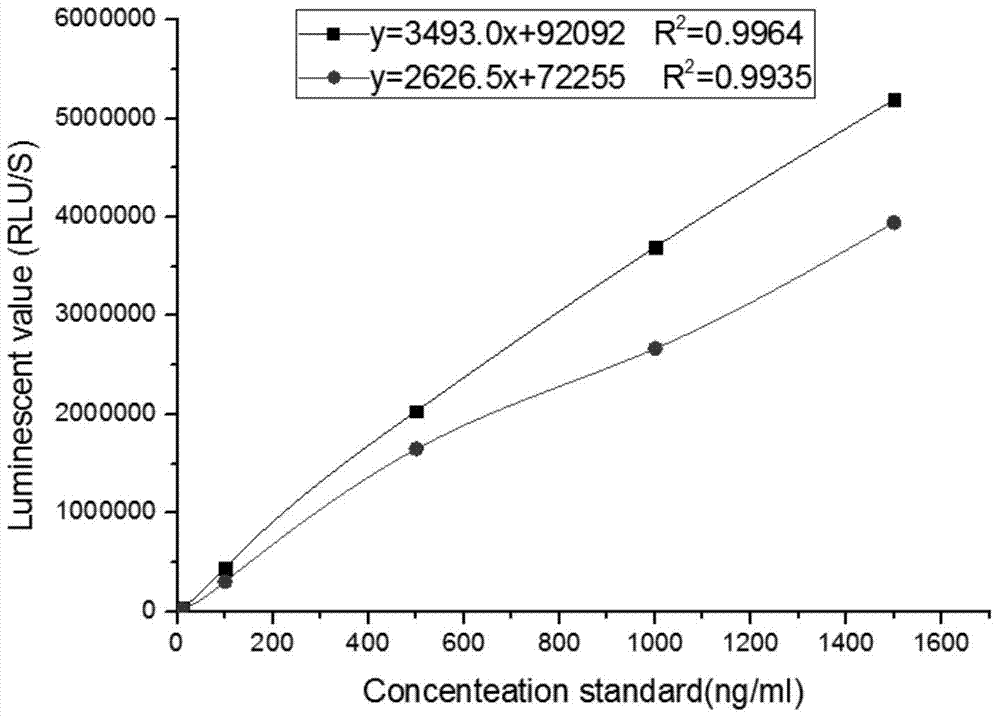
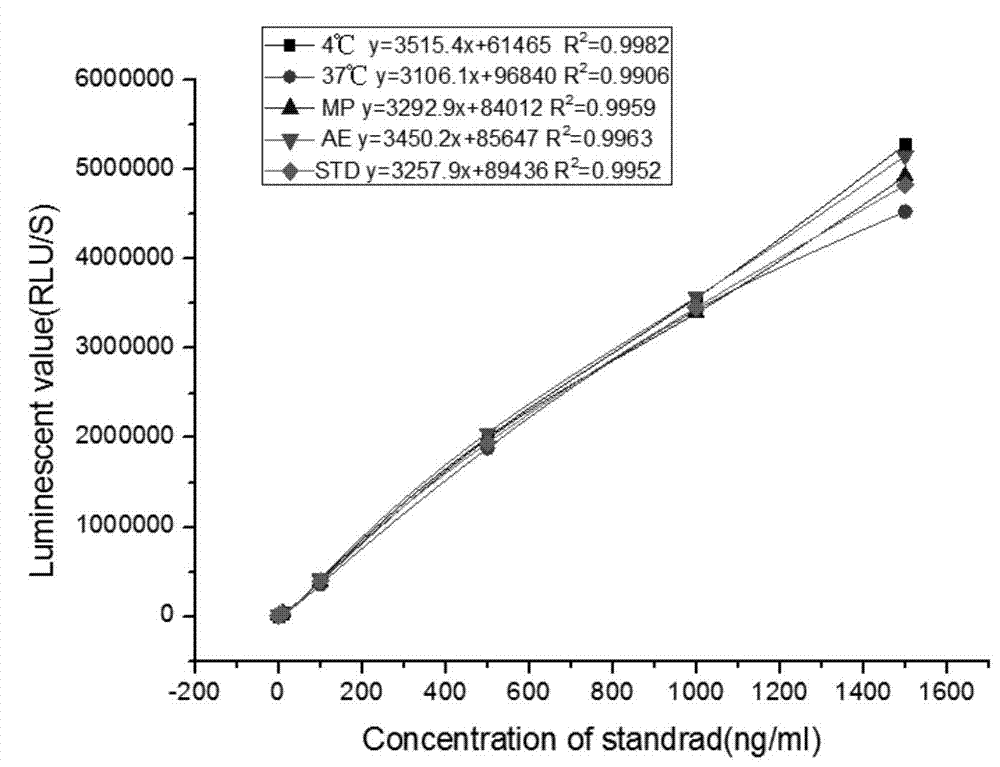
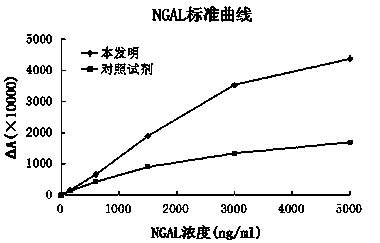
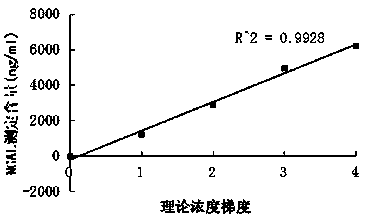
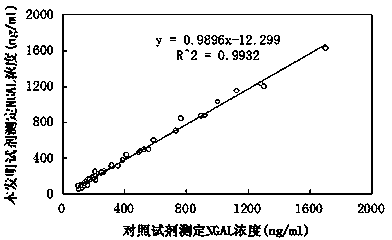
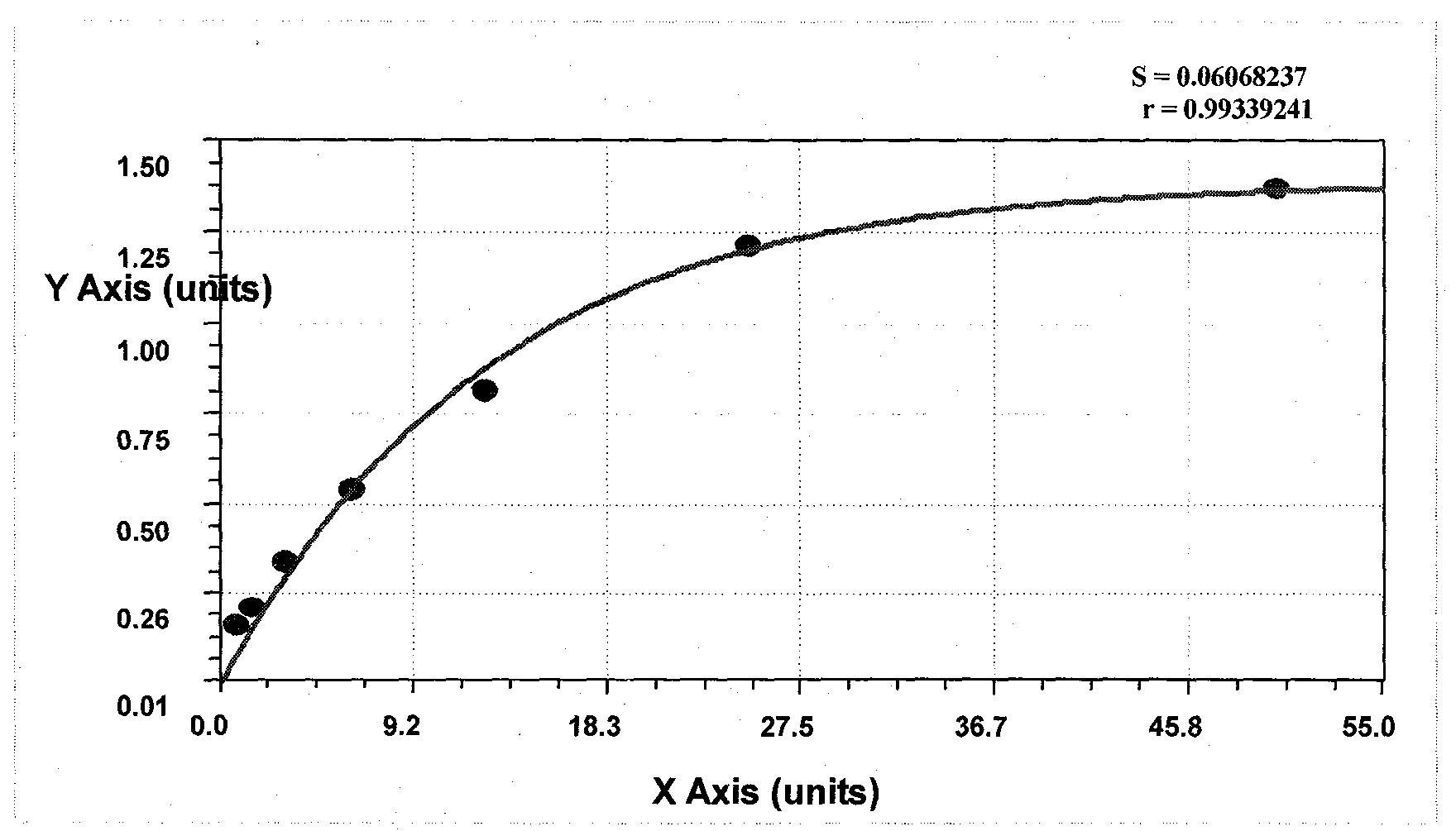
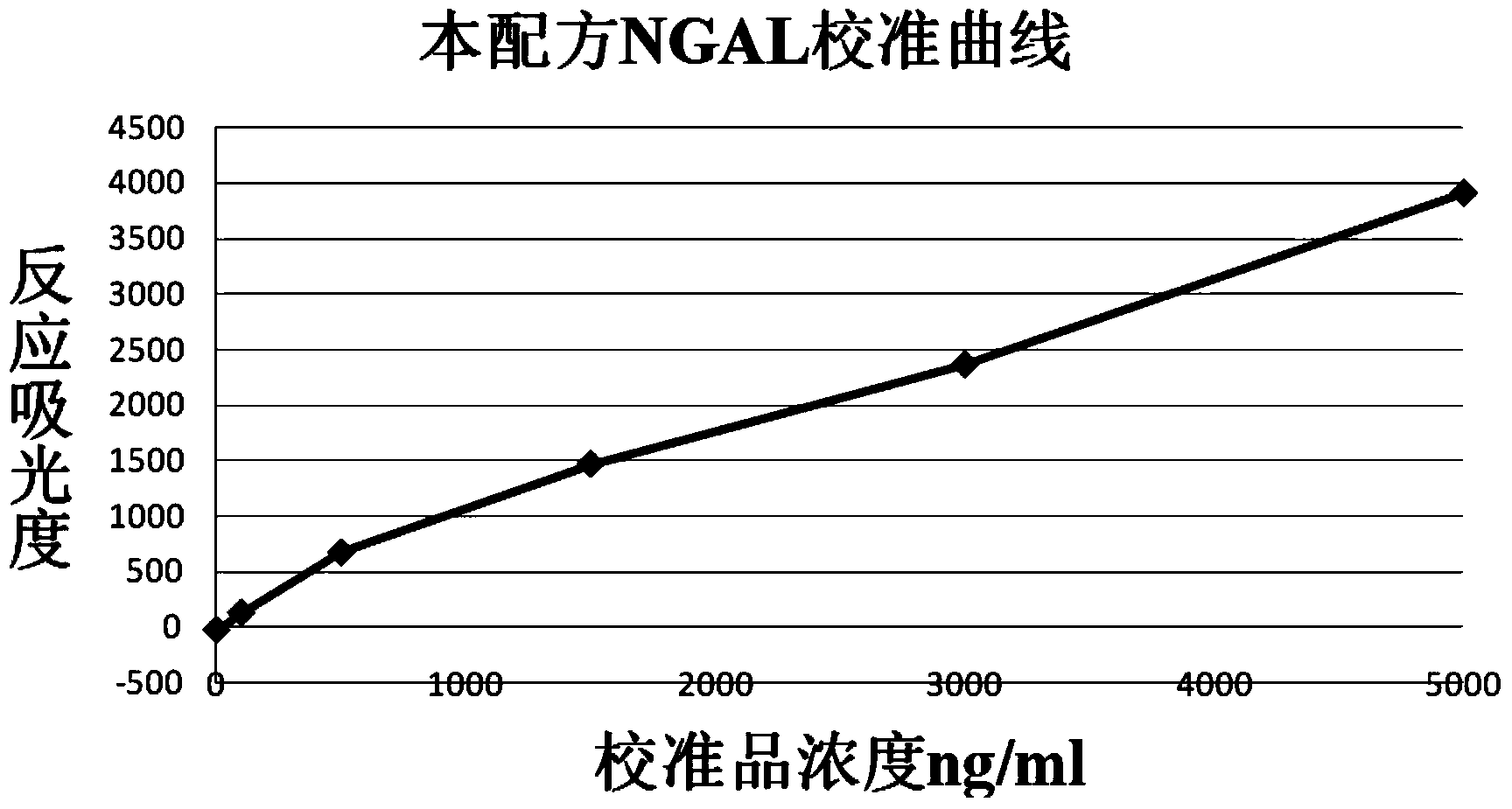
The invention relates to a kit for detecting neutrophil gelatinase-associated lipocalin content based on chemiluminescence immunoassay. According to the invention, by employing a double-antibody sandwich immunization analysis method, a chemiluminescence magnetic microspheres immunization technology is used, anti-NGAL antibody-coated magnetic microspheres for specifically combining with NGAL antigen of a standard substance / sample in a reaction cup, then are reacted to another strain anti-NGAL antibody labelled with acridine salt to form an immunization compound, through an acid-base chemical reaction of a pre-Trigger and a Trigger, relative light unit (RLU / s) of the chemiluminescence reaction can be measured; the NGAL antigen content in the sample is in direct proportion to the relative light unit (RLU / s) measured by an optical system, determination of NGAL content in an urine specimen can be determined through standard curve fitting; and the method has the obvious advantages of high sensitivity, strong specificity, good stability, simple operation and low cost.
Owner:GUANGZHOU DARUI BIOTECH
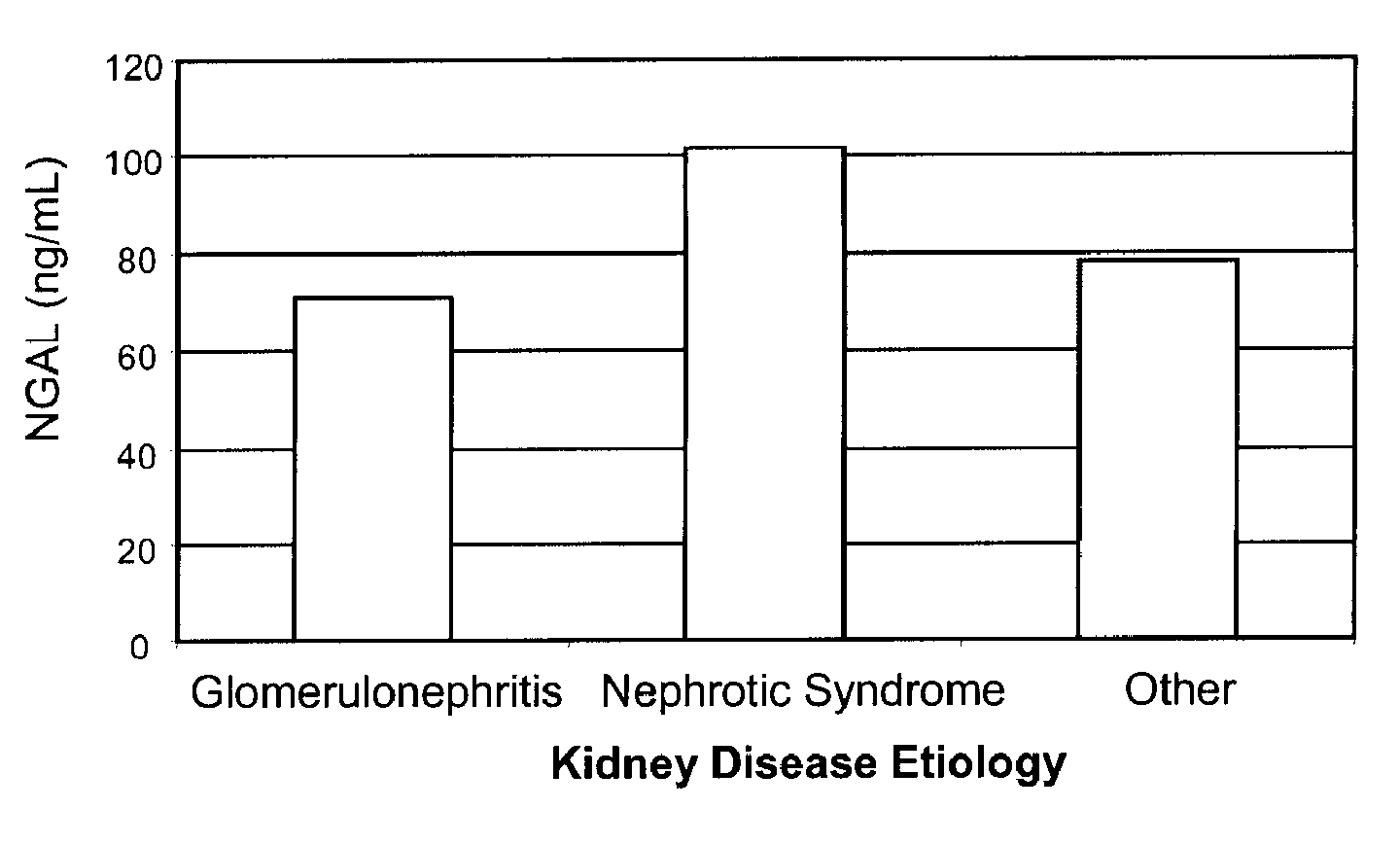
Determination of Neutrophil Gelatinase-Associated Lipocalin (NGAL) as a Diagnostic Marker for Renal Disorders
Methods for diagnosing renal disorders by measuring human neutrophil gelatinase-associated lipocalin (NGAL) are provided.
Owner:ANTIBODYSHOP
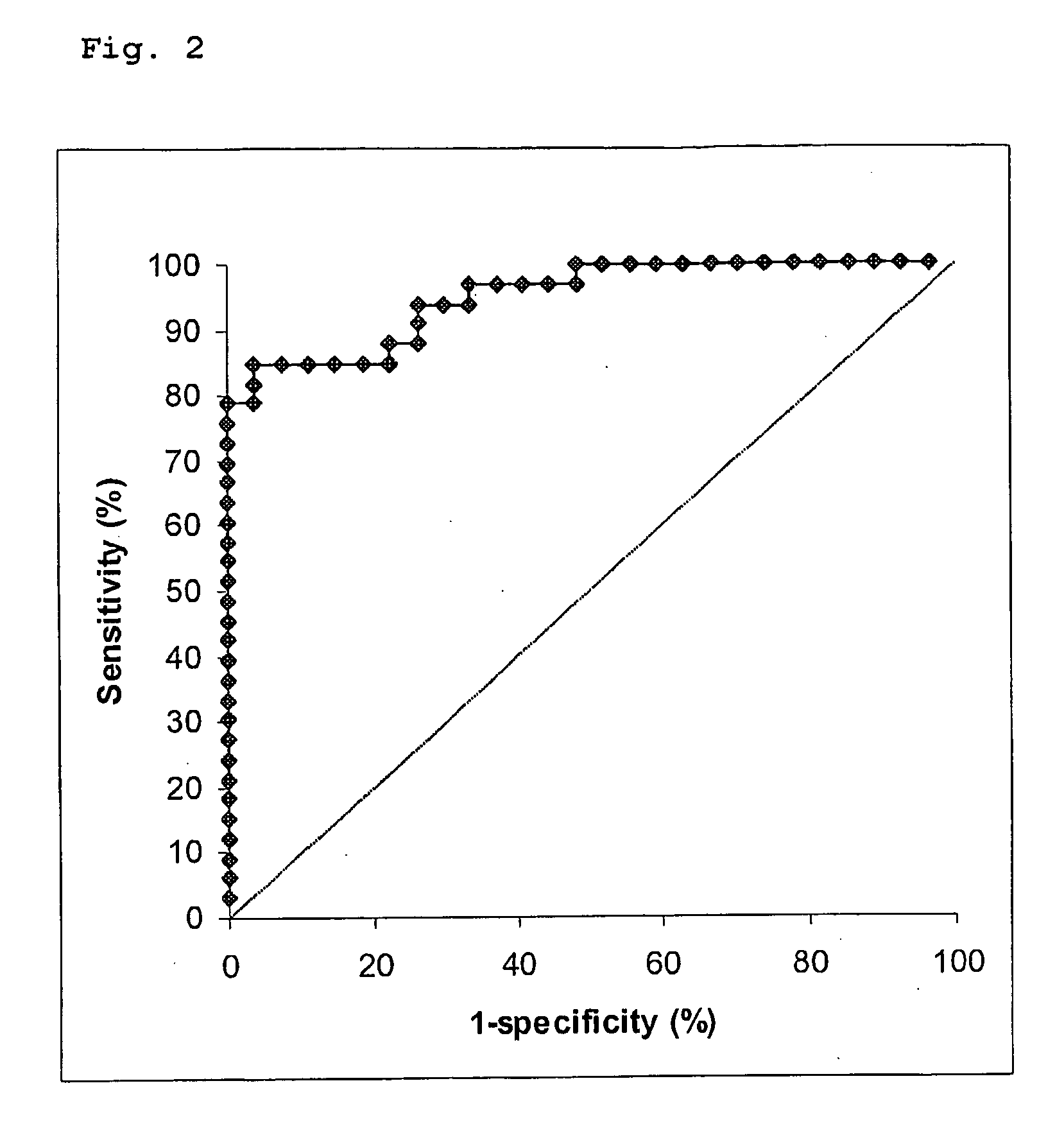
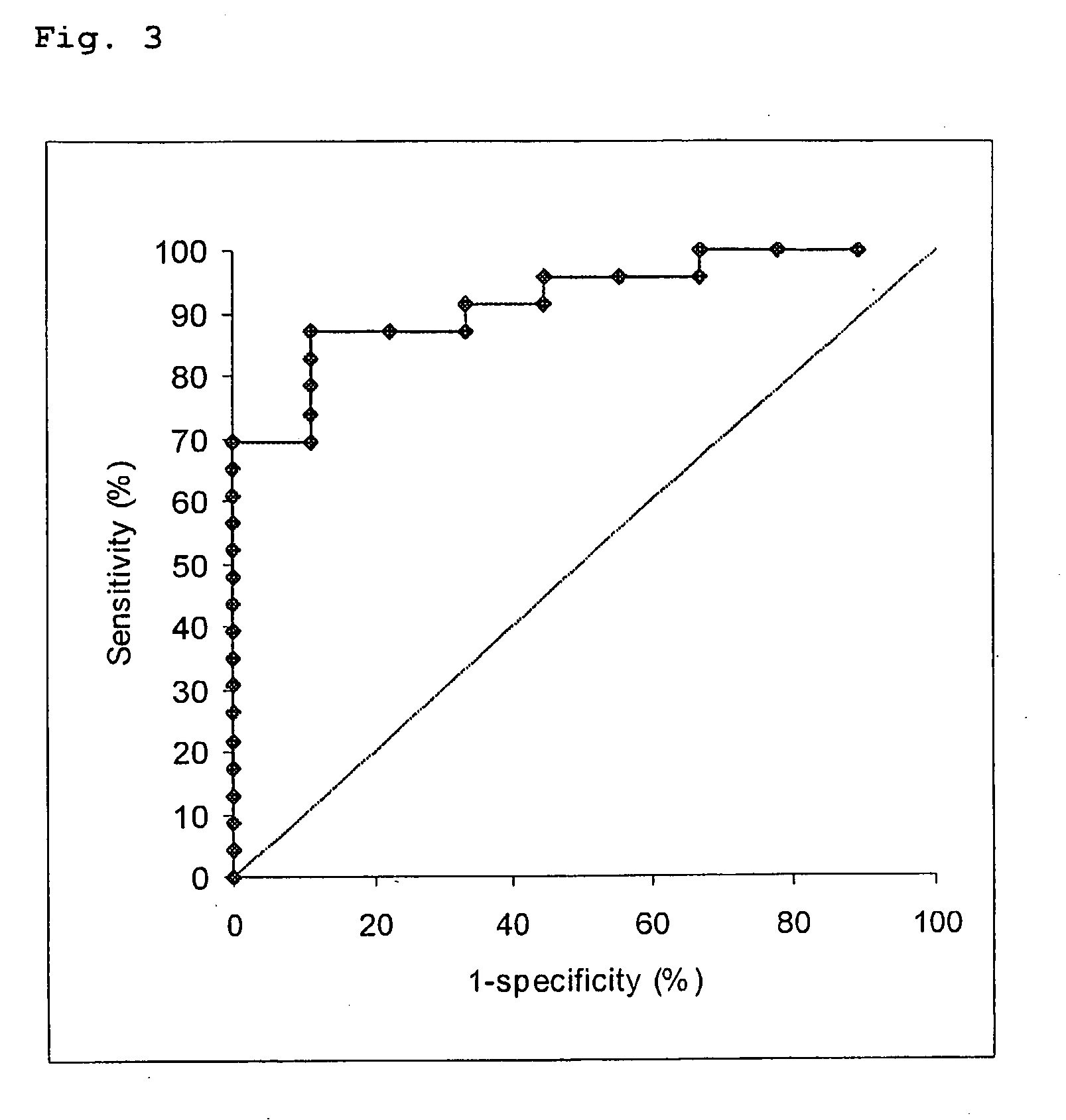
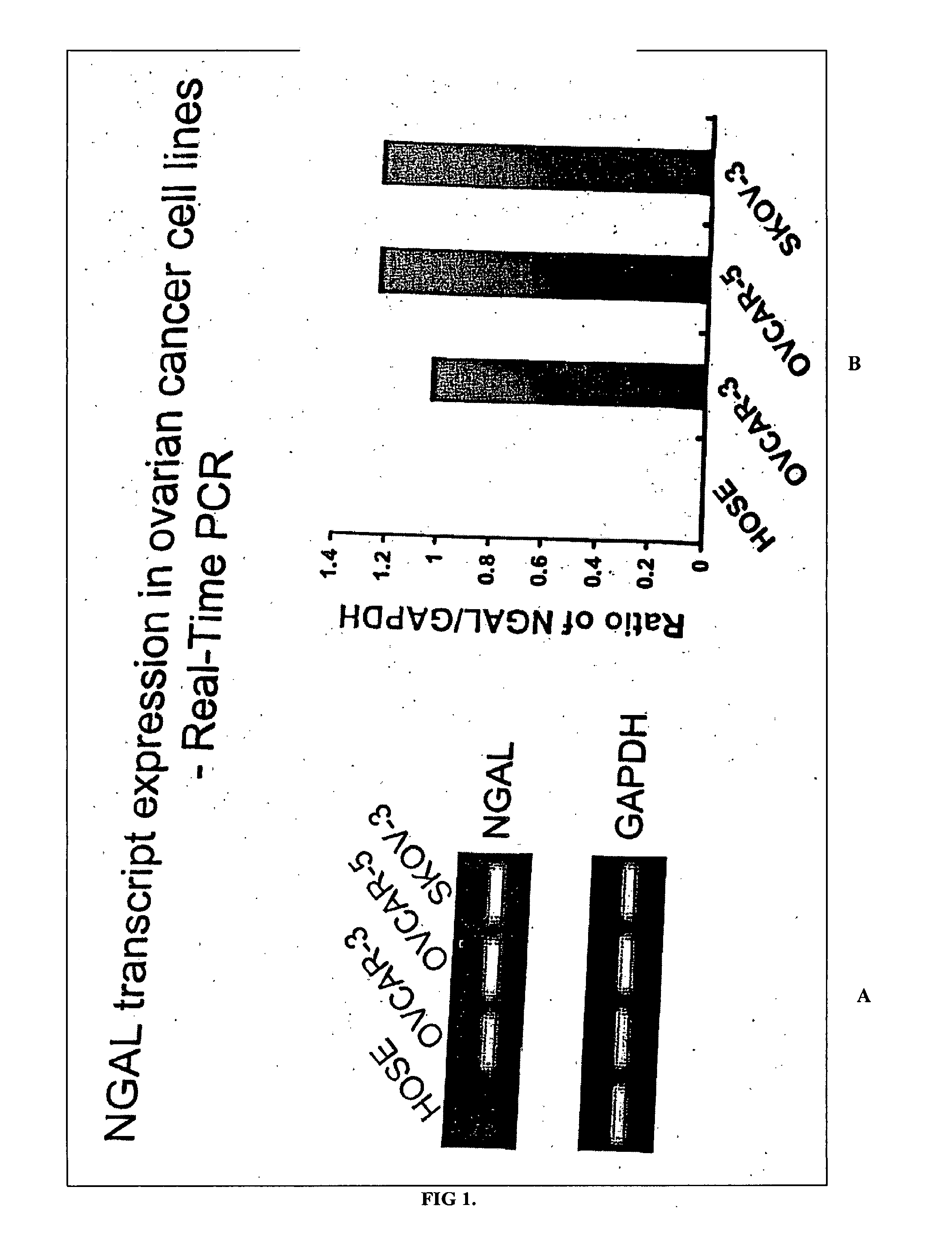
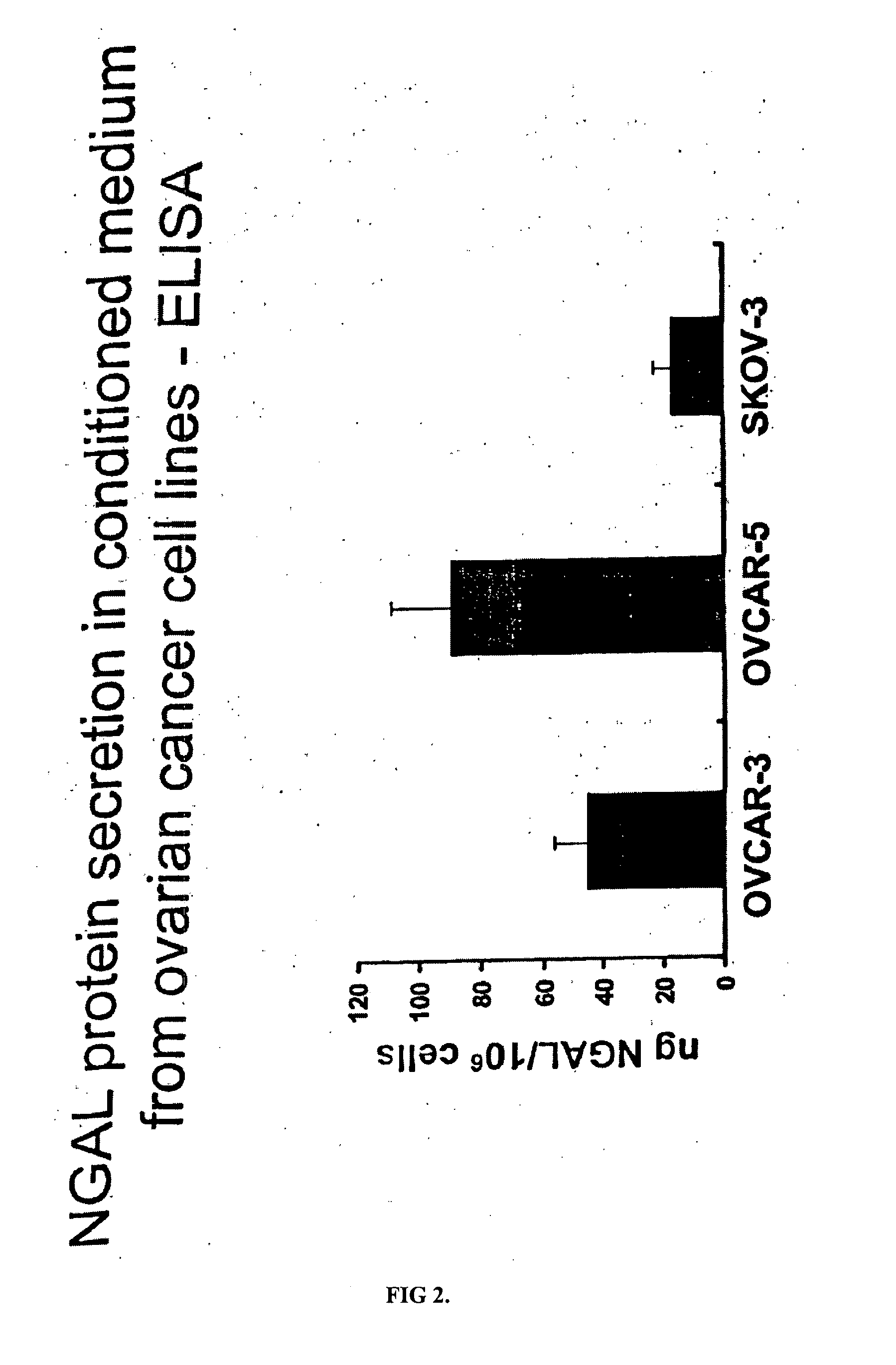
Free NGAL as a biomarker for cancer
Uncomplexed neutrophil gelatinase associated lipocalin (NGAL) is present at increased levels in individuals with atypical ductal hyperplasia (ADH), a major risk factor for future breast cancer development; in individuals that have ovarian cancer; and in individuals that have breast cancer, both invasive and noninvasive. Accordingly, the present invention is directed to measuring uncomplexed NGAL levels in urine as a primary screen to determine if an individual is either at risk of developing, or has developed cancer, e.g., cancer of epithelial origin including breast and ovarian cancer.
Owner:CHILDRENS MEDICAL CENT CORP
Method and kit for detecting the early onset of renal tubular cell injury
InactiveUS20070254370A1Organic active ingredientsMicrobiological testing/measurementSide effectNGAL Protein
A method and kit for detecting the early onset of renal tubular cell injury, utilizing NGAL as an early urinary biomarker. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the urine following renal tubule cell injury. NGAL protein expression is detected predominantly in proximal tubule cells, in a punctate cytoplasmic distribution reminiscent of a secreted protein. The appearance NGAL in the urine is related to the dose and duration of renal ischemia and nephrotoxemia, and is diagnostic of renal tubule cell injury and renal failure. NGAL detection is also a useful marker for monitoring the nephrotoxic side effects of drugs or other therapeutic agents.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Kit for detecting content of neutrophil gelatinase-associated lipocalin (NGAL)
ActiveCN104198732AComposition is simpleGood test sensitivityBiological testingLatex particlePolyethylene glycol
The invention discloses a kit for detecting the content of neutrophil gelatinase-associated lipocalin (NGAL). The kit comprises a reagent R1, a reagent R2 and an NGAL reagent reference standard substance, wherein the reagent R1 comprises the following components: 10mM-100mM of Tris, 50mM-200mM of NaCl, 0.05%-1% of BSA (Bovine Serum Albumin), 0.01%-0.1% of Tween-20, 0.5%-3% of PEG (Polyethylene Glycol) and 0.1% of NaN3; the reagent R2 comprises the following components: 10mM-100mM of Tris, 50mM-200mM of NaCl, 0.05%-1% of BSA, 0.01%-0.1% of Tween-20, 0.1% of NaN3, 1%-10% of saccharose and 0.1%-1% of NGAL antibody sensitization latex particles; the NGAL reagent reference standard substance comprises the following components: 10mM-100mM of Tris, 50mM-200mM of NaCl, 0.05%-1% of BSA, 0.01%-0.1% of Tween-20, 0.5mM-5mM of EDTA (ethylenediamine tetraacetic acid) and 0.1% of NaN3. The kit is simple in reagent composition, high in test sensitivity, wide in linear range, high in stability, low in test cost, high in accuracy and convenient to popularize.
Owner:NINGBO RUI BIO TECH
Genome editing of cognition related genes in animals
The present invention provides genetically modified animals and cells comprising edited chromosomal sequences encoding proteins that are associated with cognitive disorders. In particular, the animals or cells are generated using a zinc finger nuclease-mediated editing process. Also provided are methods of assessing the effects of agents in genetically modified animals and cells comprising edited chromosomal sequences associated with cognitive disorders.
Owner:SIGMA ALDRICH CO LLC
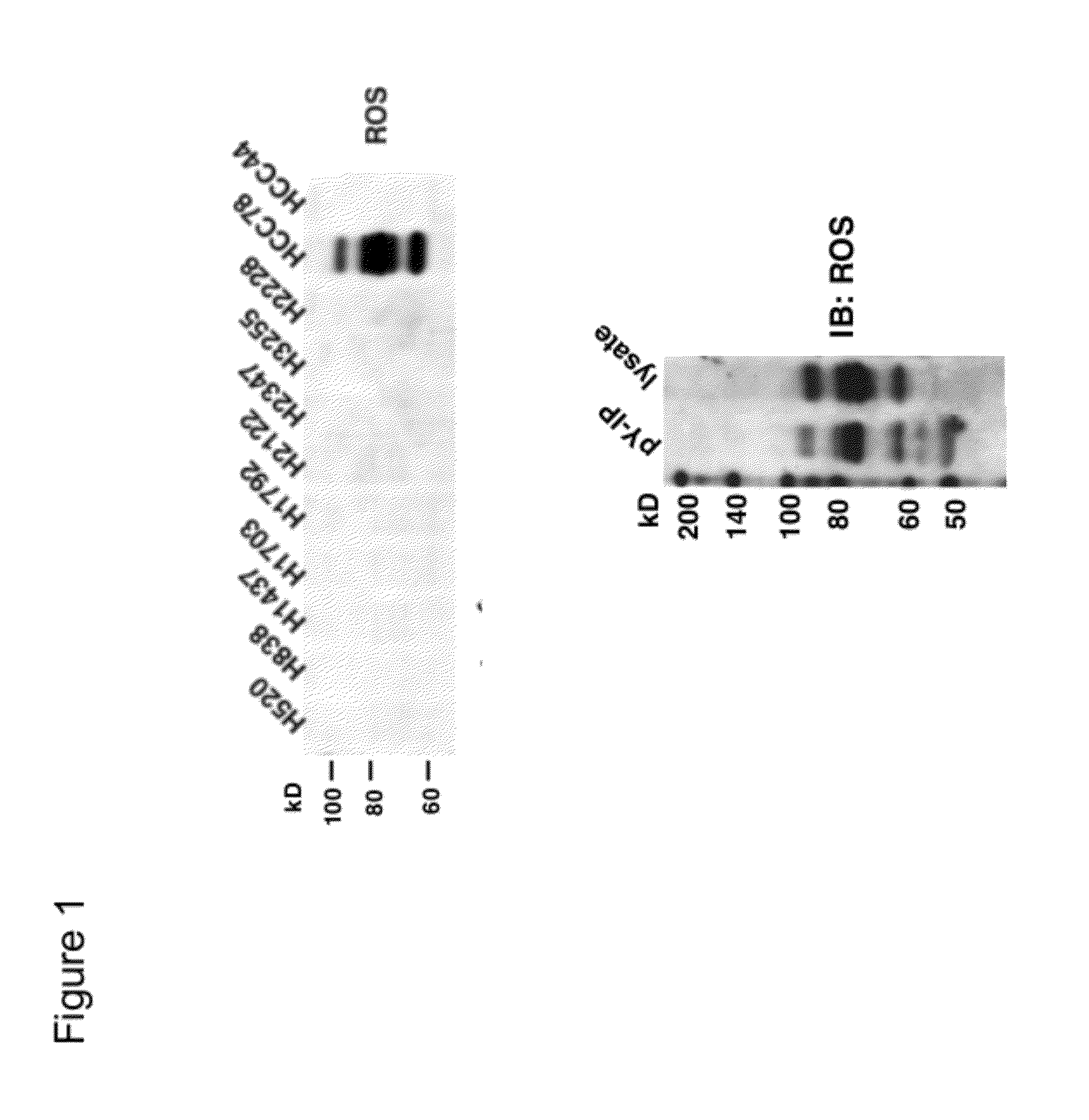
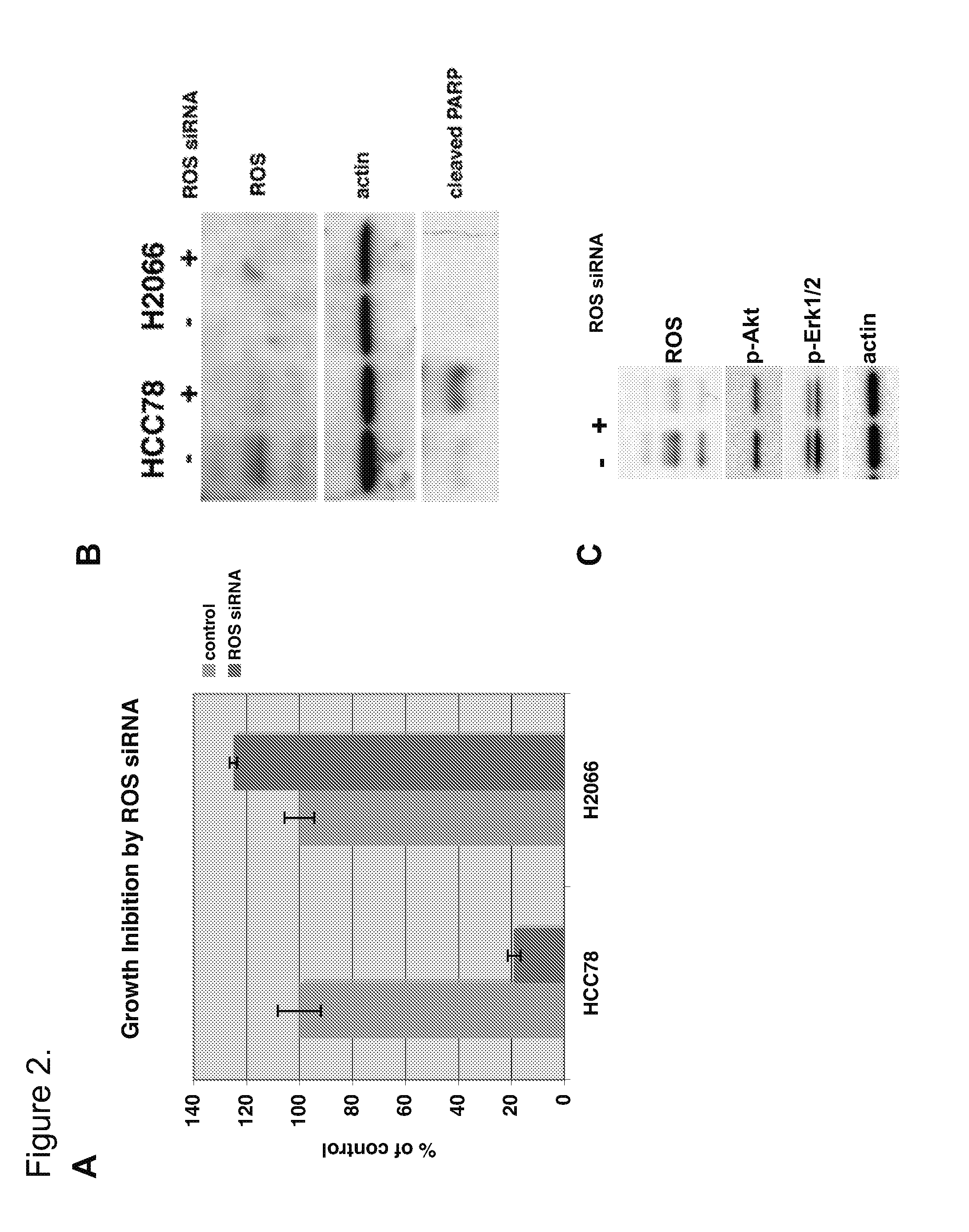
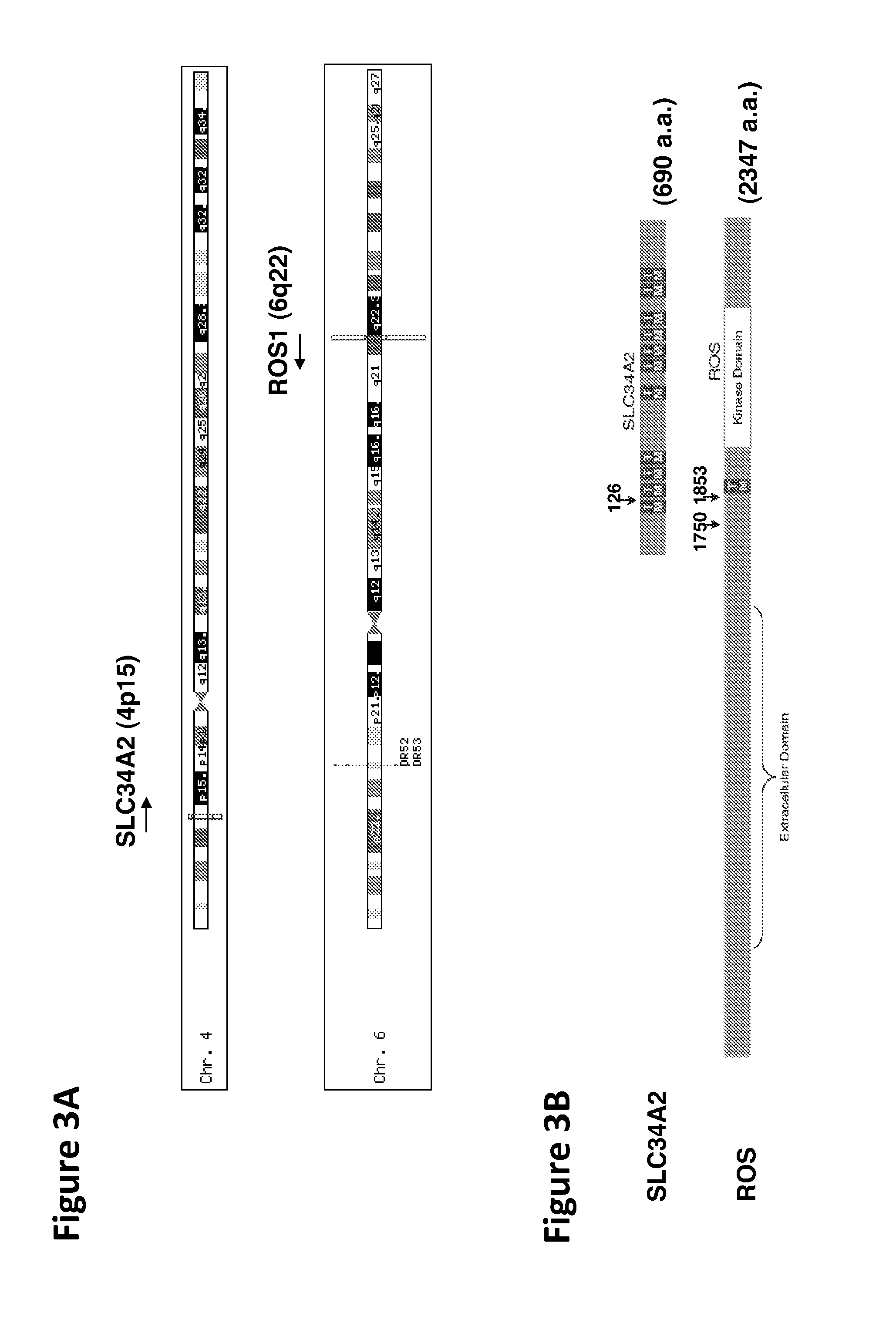
ROS Kinase in Lung Cancer
InactiveUS20120208824A1Inhibit expressionBiocideMicrobiological testing/measurementKinase activityNucleotide
The invention provides the identification of the presence of polypeptides with ROS kinase activity in mammalian lung cancer. In some embodiments, the polypeptide with ROS kinase activity is the result of a fusion between a ROS-encoding polynucleotide and a polynucleotide encoding a second (non-ROS) polypeptide. Three different fusion partners of ROS are described, namely proteins encoded by the FIG gene, the SLC34A2 gene, and the CD74 gene. The invention enables new methods for determining the presence of a polypeptide with ROS kinase activity in a biological sample, methods for screening for compounds that inhibit the proteins, and methods for inhibiting the progression of a cancer (e.g., an lung cancer).
Owner:CELL SIGNALING TECHNOLOGY
Method for the early detection of renal injury
InactiveUS20090142774A1Bioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseSide effect
A method and kit for detecting the immediate or early onset of renal disease and injury, including renal tubular cell injury, utilizing NGAL as an immediate or early on-set biomarker in a sample of blood serum. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the blood serum following renal tubule cell injury. NGAL protein expression is detected predominantly in proximal tubule cells, in a punctuate cytoplasmic distribution reminiscent of a secreted protein. The appearance NGAL in the serum is related to the dose and duration of renal ischemia and nephrotoxemia, and is diagnostic of renal tubule cell injury and renal failure. NGAL detection is also a useful marker for monitoring the nephrotoxic side effects of drugs or other therapeutic agents.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Method for detecting neutrophil gelatinase-associated lipocalin (NGAL) in sample
The invention relates to a method for detecting neutrophil gelatinase-associated lipocalin (NGAL) in a sample. The method comprises the following steps of: reacting an antibody of the NGAL with human NGAL of a specimen to be detected to form a compound which causes turbidity change; characterizing the change by using light projection intensity or light scattering intensity; and finding out the content of the human NGAL in the specimen to be detected from the concentration of the NGAL standard product and a standard curve of corresponding light scattering intensity or light transmission intensity. The method has the advantages of high sensitivity, high recovery rate and good repeatability, and a detection measure having the advantages of simplicity, convenience, good repeatability, strong adaptability and high speed is determined for measuring the NGAL in the samples of urine or blood plasma, and the like.
Owner:WUHAN LIFE ORIGIN BIOTECH LTD
Method and kit for detecting the early onset of renal tubular cell injury
InactiveUS20090181407A1Organic active ingredientsMicrobiological testing/measurementSide effectNGAL Protein
A method and kit for detecting the early onset of renal tubular cell injury, utilizing NGAL as an early urinary biomarker. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the urine following renal tubule cell injury. NGAL protein expression is detected predominantly in proximal tubule cells, in a punctate cytoplasmic distribution reminiscent of a secreted protein. The appearance NGAL in the urine is related to the dose and duration of renal ischemia and nephrotoxemia, and is diagnostic of renal tubule cell injury and renal failure. NGAL detection is also a useful marker for monitoring the nephrotoxic side effects of drugs or other therapeutic agents.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Methods, Devices and Kits for Detecting or Monitoring Acute Kidney Injury
ActiveUS20110287455A1High sensitivityEasy to detectPeptide librariesChemiluminescene/bioluminescenceEpitopeGelatinase
Methods for detecting acute kidney injury in an individual comprise (a) contacting a body fluid sample from the individual with an assay device including neutrophil gelatinase-associated lipocalin (NGAL) antibody and a detectable label, to allow complexing of NGAL protein in the sample with NGAL antibody, and determining an amount of complex formed between NGAL protein from the sample and NGAL antibody in the assay device using the detectable label, wherein NGAL antibody in the device has binding capacity with more than two NGAL protein epitopes, and wherein the amount of the formed complex represents a level of acute kidney injury. Methods for determining an origin of NGAL protein in a sample from an individual include the step of determining relative amounts of monomeric, dimeric and heterodimeric forms of NGAL protein in the sample and allow improved diagnosis and therefore better targeted treatment.
Owner:FUTURE MEDICAL DIAGNOSTICS CO LTD
Method and kit for detecting the early onset of renal tubular cell injury
InactiveUS20090123941A1Bioreactor/fermenter combinationsOrganic active ingredientsSide effectNGAL Protein
A method and kit for detecting the early onset of renal tubular cell injury, utilizing NGAL as an early urinary biomarker. NGAL is a small secreted polypeptide that is protease resistant and consequently readily detected in the urine following renal tubule cell injury. NGAL protein expression is detected predominantly in proximal tubule cells, in a punctate cytoplasmic distribution reminiscent of a secreted protein. The appearance NGAL in the urine is related to the dose and duration of renal ischemia and nephrotoxemia, and is diagnostic of renal tubule cell injury and renal failure. NGAL detection is also a useful marker for monitoring the nephrotoxic side effects of drugs or other therapeutic agents.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Method for diagnosing, evaluating or testing cancer and foreseeing cancer severity
The invention relates to a method for diagnosing, evaluating or testing a cancer and foreseeing the severity of the cancer. The testing method comprises the following steps: an antibody of anti-human tropic neutrophil gelatinase-associated lipocalin (NGAL) is reacted with the human NGAL of a specimen to be tested to form a compound which causes turbidity change; light protection intensity or light scattering intensity is used for presenting the change; the human NGAL content of the specimen to be tested is tested through a standard curve of the concentration of human NGAL standard to relevant light protection intensity or light scattering intensity; and finally the cancer severity is measured based on the tested NGAL content. The method has the advantages of high flexibility, high recovery and good repetitiveness, determines a testing method with great convenience, good repetitiveness and strong suitability for the NGAL in urine or blood plasma of a cancer patient, and provides possibility for the clinical directive function of human NGAL and further research of the functions of the human NGAL.
Owner:CUSABIO TECH LLC
Latex enhanced immunoturbidimetry NGAL detection kit
InactiveCN104215769AGuaranteed uniformityGuaranteed stabilityBiological testingLatex particleNGAL Protein
The invention provides a latex enhanced immunoturbidimetry NGAL detection kit. The latex enhanced immunoturbidimetry NGAL detection kit comprises a reagent R1, a reagent R2 and a standard substance; the reagent R1 comprises a buffer solution, a surfactant, a chelating agent, an accelerator and an antiseptic; the reagent R2 comprises the buffer solution, NGAL antibody-coated latex particles, and an unrelated protein; and the standard substance is a solution containing 6 NGAL recombinant protein concentration gradients. Compared with kits in the prior art, the neutral granulocyte gelatinase related lipid transporter content detection kit used on a fully automatic biochemical analyzer has the advantages of rapid determination of the content of the NGAL in urine or blood plasma, guarantee of the sensitivity and the linearity, and improved sensitivity, accuracy and linearity of the determination result.
Owner:上海睿康生物科技有限公司
Methods and compositions for monitoring and risk prediction in cardiorenal syndrome
ActiveUS7842472B2Reduce riskIncreased riskMicrobiological testing/measurementDisease diagnosisMedicineMortality rate
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects. In particular, the invention relates to methods and compositions selected to monitor cardiorenal syndrome using assays that detect NGAL, preferably together with assays that detect natriuretic peptides such as BNP. Such methods and compositions can provide early indications of a deterioration in cardiorenal syndrome status, including prognosis regarding mortality and worsening renal function.
Owner:ALERE SAN DIEGO INC
Colloidal gold test strip for detecting NGAL (Neutrophil Gelatinase Associated Lipocalin) and preparation method thereof
InactiveCN102759623AQuick forecastThe detection method is simpleBiological testingNGAL ProteinBioinformatics
The invention discloses a colloidal gold test strip for detecting neutrophil gelatinase associated lipocalin (NGAL) and a preparation method thereof. The colloidal gold test strip comprises a plastic bottom liner, a nitrocellulose membrane, a gold mark pad and a water sucking pad; a quality control line and a detection line which are mutually parallel are arranged on the nitrocellulose membrane; and the quality control line contains a rabbit anti-human IgG antibody, the detection line contains a NGAL (Neutrophil Gelatinase Associated Lipocalin) monoclonal antibody, and the gold mark pad contains another NGAL monoclonal antibody marked by colloidal gold. The colloidal gold test strip has the advantages of being capable of carrying out early prediction on acute renal failure, having strong specificity, being accurate in results, simple in operation and suitable for rapid diagnosis at bedsides.
Owner:GETEIN BIOTECH
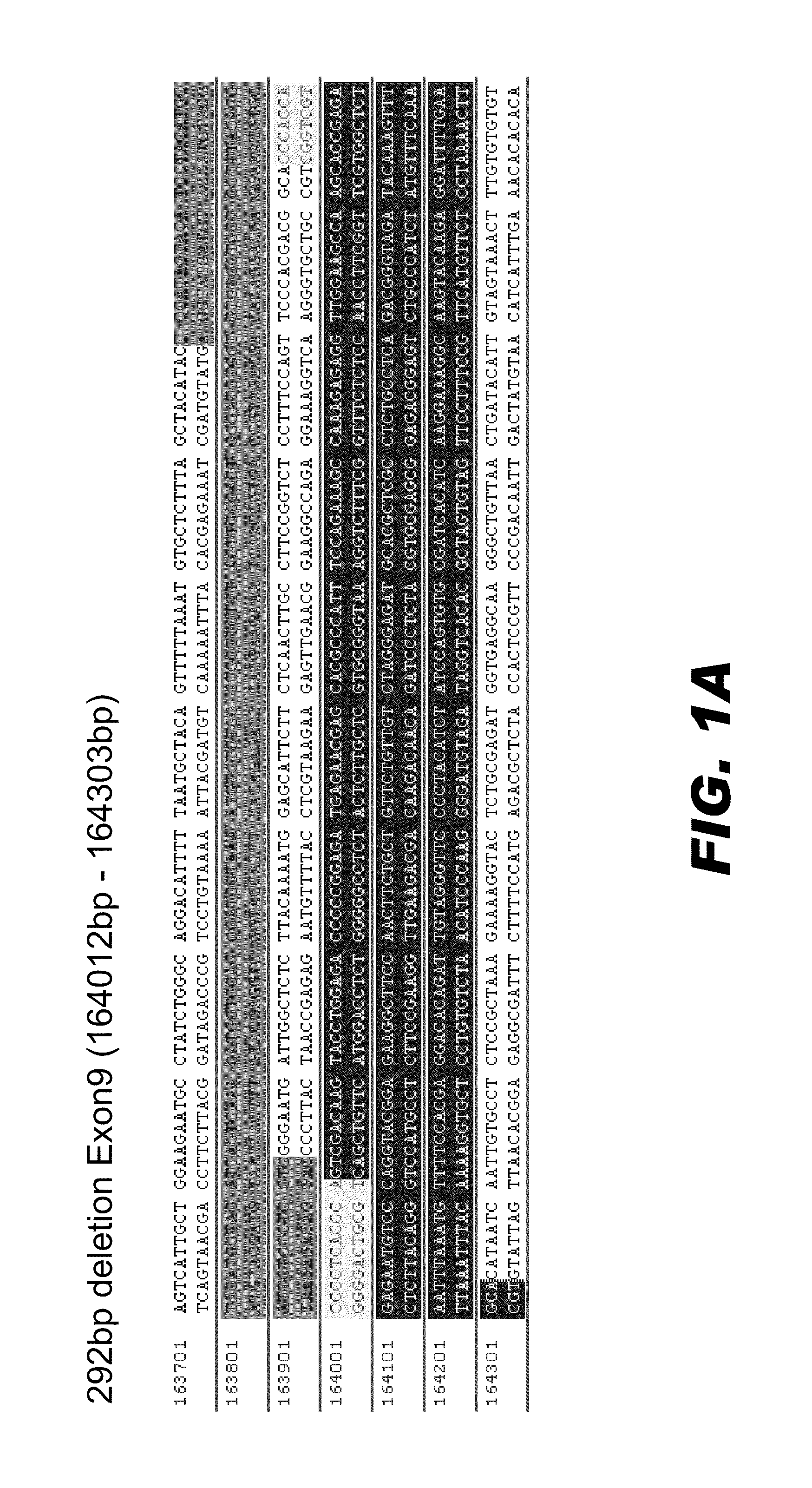
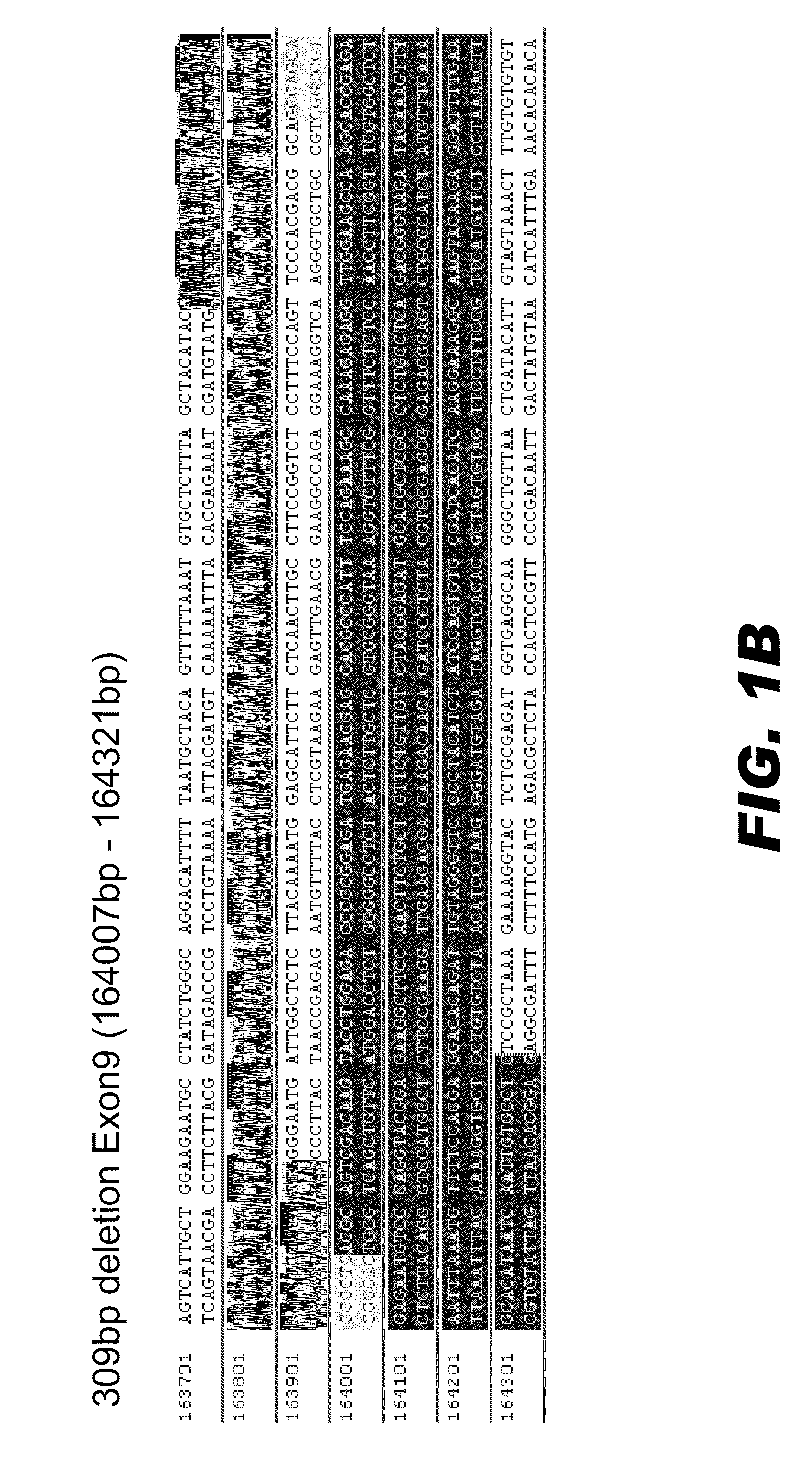
Transcriptional control element of human lung carcinoma cell NGAL gene promoter region
InactiveCN1974768AFermentationVector-based foreign material introductionRegulation of gene expressionTranscriptional Regulatory Elements
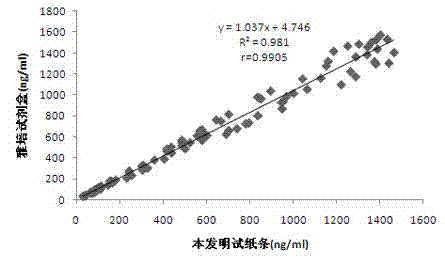
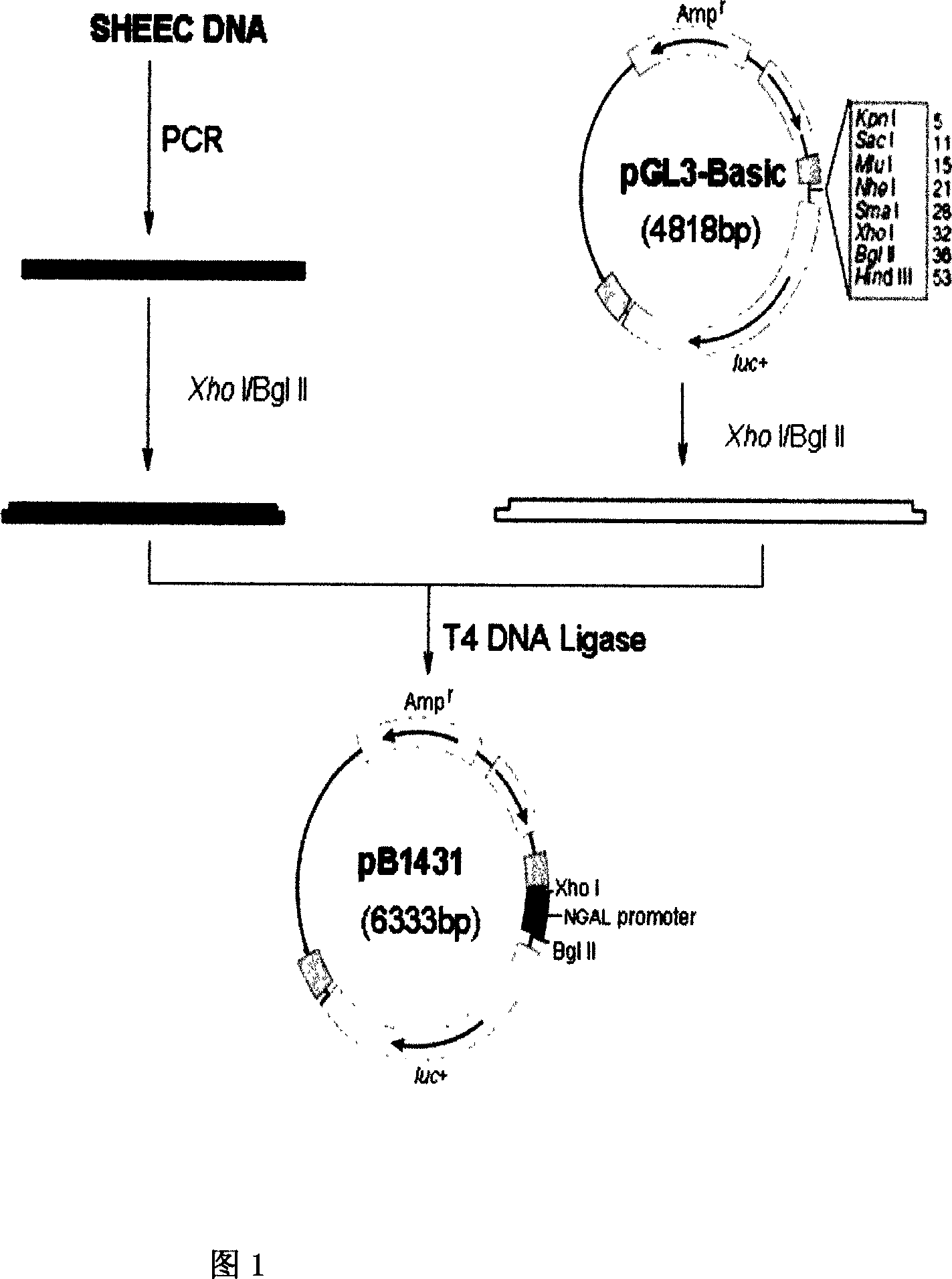
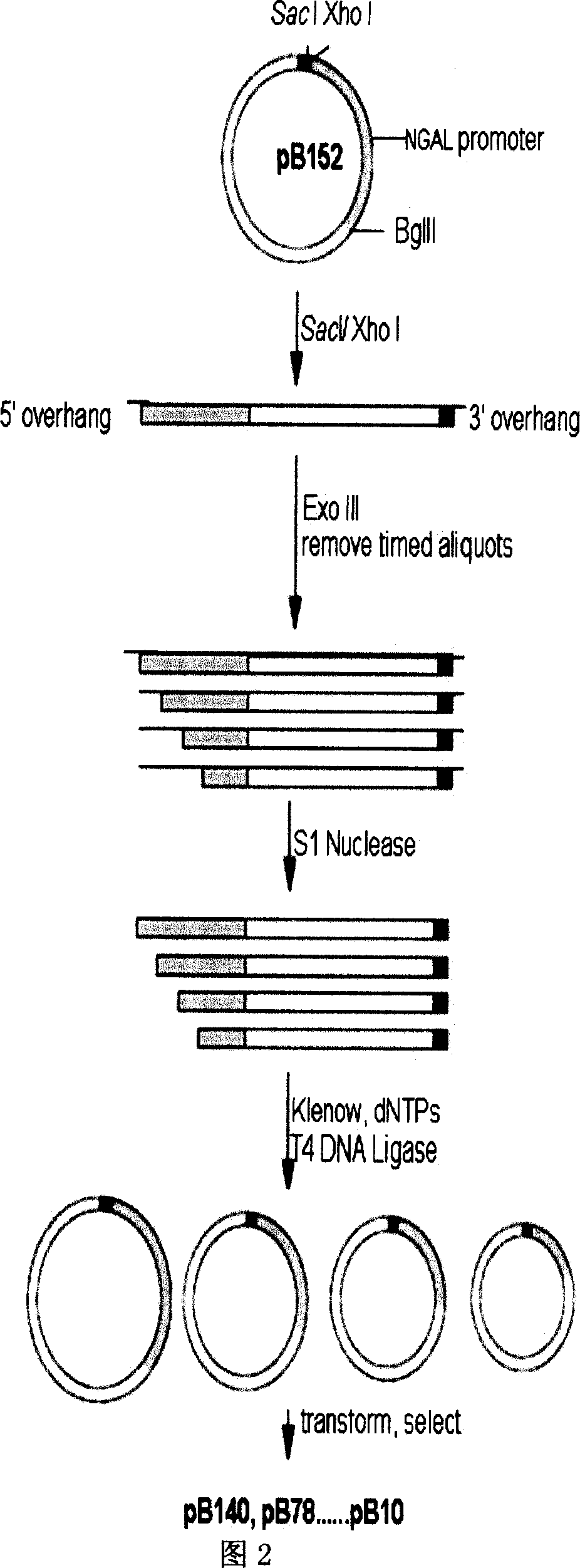
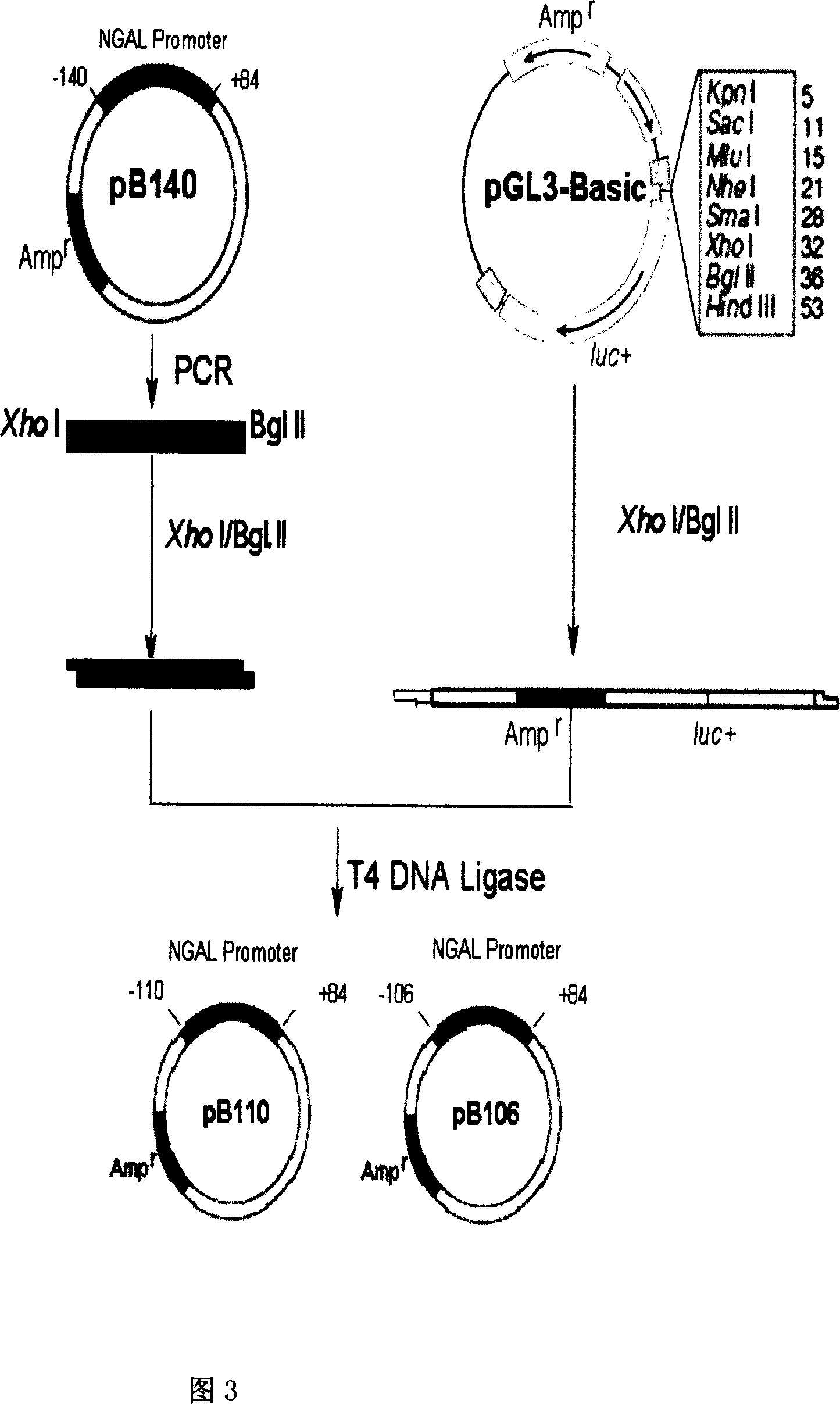
The present invention relates to gene expression controlling technology, and discloses one kind of transcriptional control element of human lung carcinoma cell NGAL gene promoter region. The present invention identifies human lung carcinoma cell NGAL gene promoter region for the first time, inserts the promoter sequence with gradually deleting 5' end sequence and partial modified controlling element to the upstream of the report gene to constitute serial eukaryotic expression plasmids, transfects mammal cell for instantaneous expression, and determines the key control element of human NGAL gene promoter in lung carcinoma cell transcription activity. The present invention is significant in the research of NGAL in the tumor cell expression control mechanism and NGAL targeting clinical treatment.
Owner:SHANTOU UNIV MEDICAL COLLEGE
Diagnosis and monitoring of chronic renal disease using ngal
InactiveUS20100234765A1Difficult to levelImprove the level ofDisease diagnosisDiagnostic recording/measuringRegimenProper treatment
A method of assessing the ongoing kidney status of a mammal afflicted with or at risk of developing chronic renal injury or disease, including chronic renal failure (CRF) by detecting the quantity of Neutrophil Gelatinase-Associated Lipocalin (NGAL) in urine, serum or plasma samples at discrete time periods, as well as over time. Incremental increases in NGAL levels in CRF patients over a prolonged period of time are diagnostic of worsening kidney disease. This increase in NGAL precedes and correlates with other indicators of worsening chronic renal disease or CRF, such as increased serum creatinine, increased urine protein secretion, and lower glomerular filtration rate (GFR). Proper detection of worsening (or improving, if treatment has been instituted) renal status over time, confirmed by pre- and post-treatment NGAL levels in the patient, can aid the clinical practitioner in designing and / or maintaining a proper treatment regimen to slow or stop the progression of CRF.
Owner:BARASCH JONATHAN MATTHEW +3
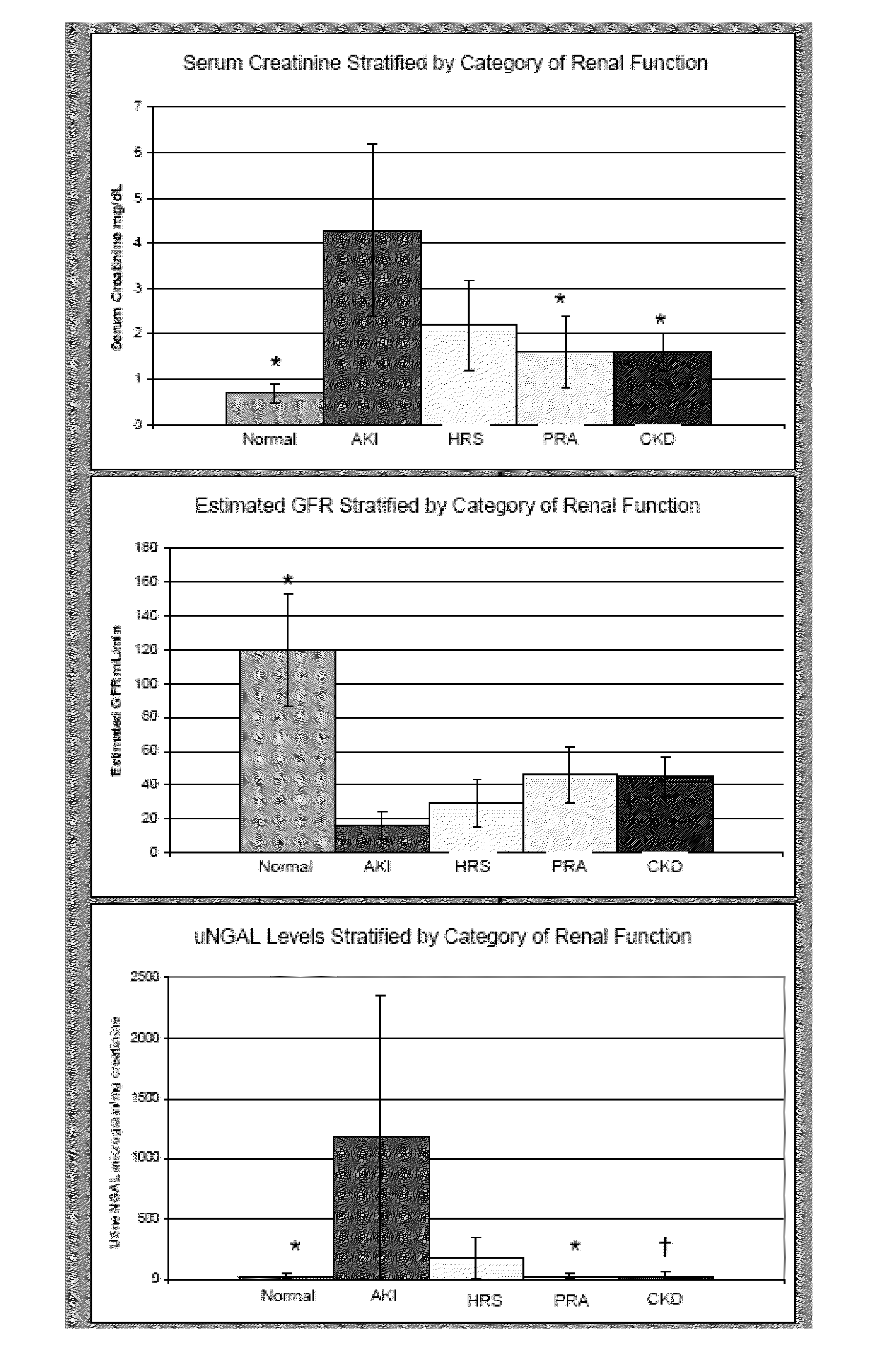
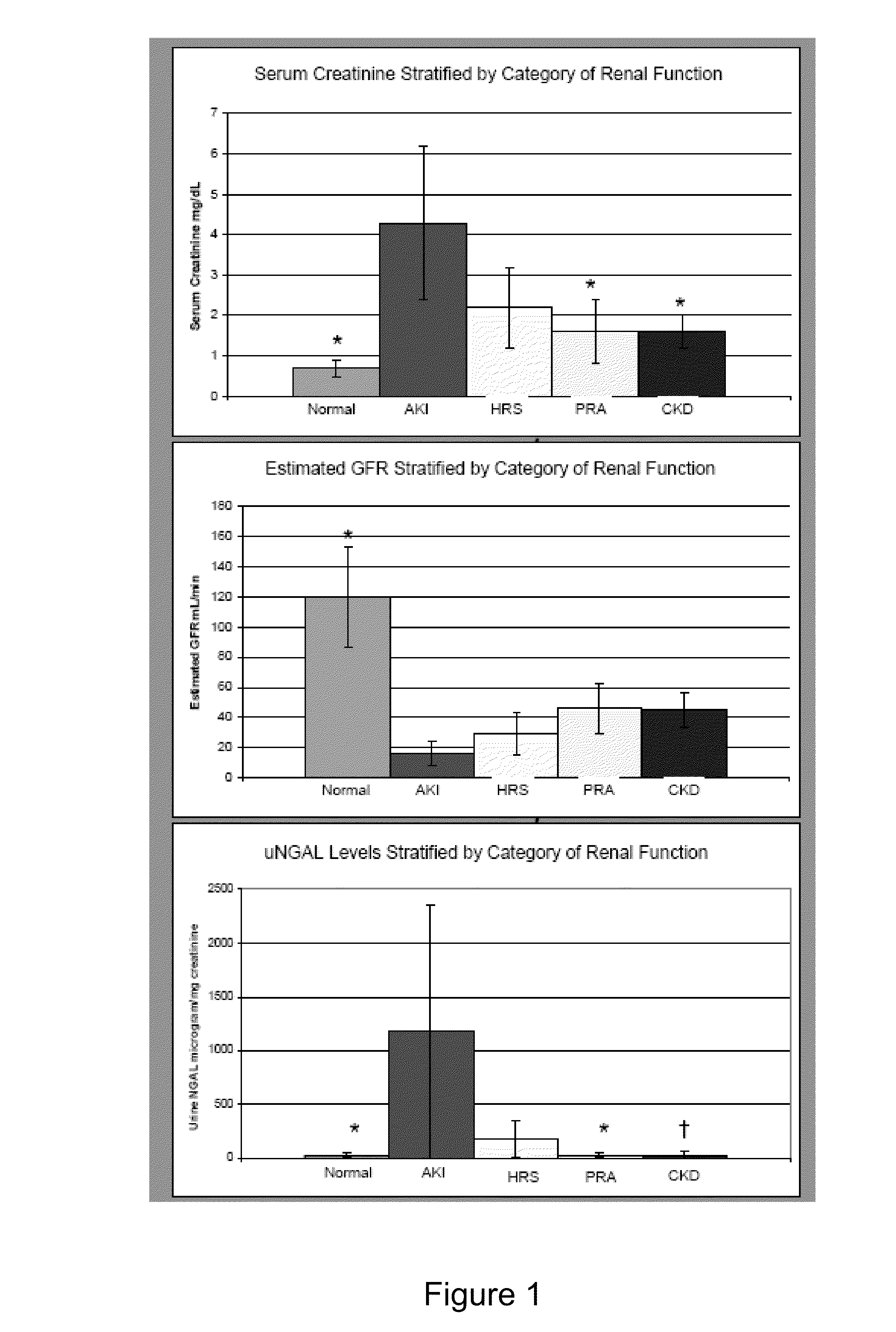
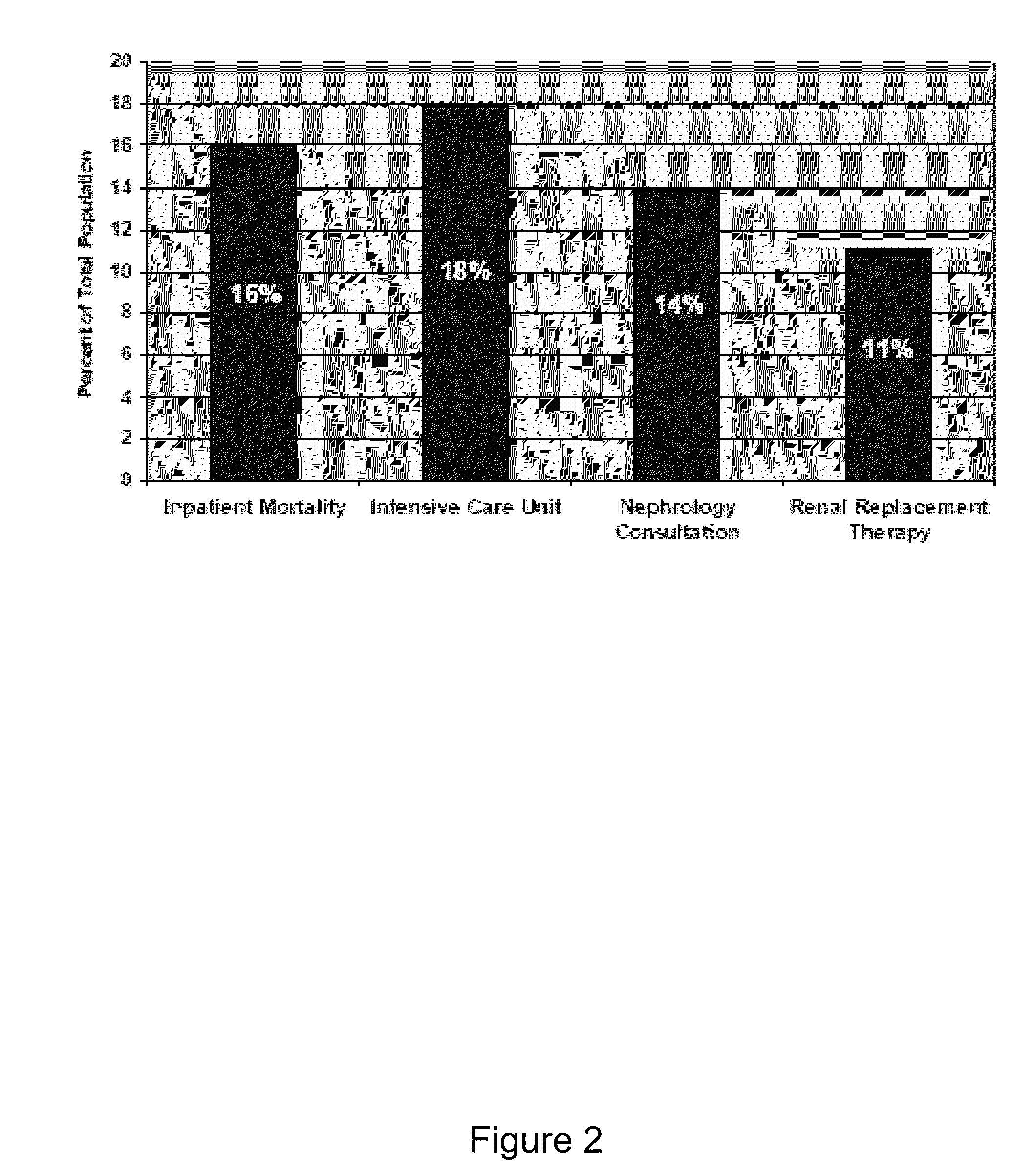
Use of urinary ngal to distinguish kidney disease and predict mortality in subjects with cirrhosis
InactiveUS20100233740A1Increase opportunitiesLow chanceDisease diagnosisBiological testingMortality rateNGAL Protein
In one embodiment, the present invention is directed to methods for diagnosis of acute kidney injury (AKI) and hepatorenal syndrome (HRS) in cirrhosis patients, and to methods for distinguishing between AKI and / or HRS and / or other kidney diseases in cirrhosis subjects. In another embodiment, the present invention is directed to prognostic methods for predicting disease-specific mortality in cirrhosis patients. In some aspects, the diagnostic and prognostic methods of the invention are based on determining whether a bodily fluid sample, such as a urine sample, contains an amount of NGAL protein that exceeds or is less than a certain threshold level, or that falls within a certain range.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Biomarkers for myocardial ischemia
ActiveUS20120009174A1Inhibition of polymerizationPromote degradationOrganic active ingredientsParticle separator tubesExtracellular matrix protein 1Cardiac muscle
This invention relates, e.g., to a method for determining if a subject has myocardial ischemia, comprising (a) providing a blood sample obtained from a subject suspected of having myocardial ischemia; (b) determining in the sample the amount of one or more of the following proteins: (i) Lumican and / or (ii) Extracellular matrix protein 1 and / or (iii) Carboxypeptidase N; and (c) comparing the amount(s) of the protein(s) to a baseline value that is indicative of the amount of the protein in a subject that does not have myocardial ischemia, wherein a statistically significantly increased amount of the protein(s) compared to the baseline value is indicative of myocardial ischemia. Other proteins indicative of myocardial ischemia are also described, as are methods for treating a subject based on a diagnostic procedure of the invention, and kits for carrying out a method of the invention.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
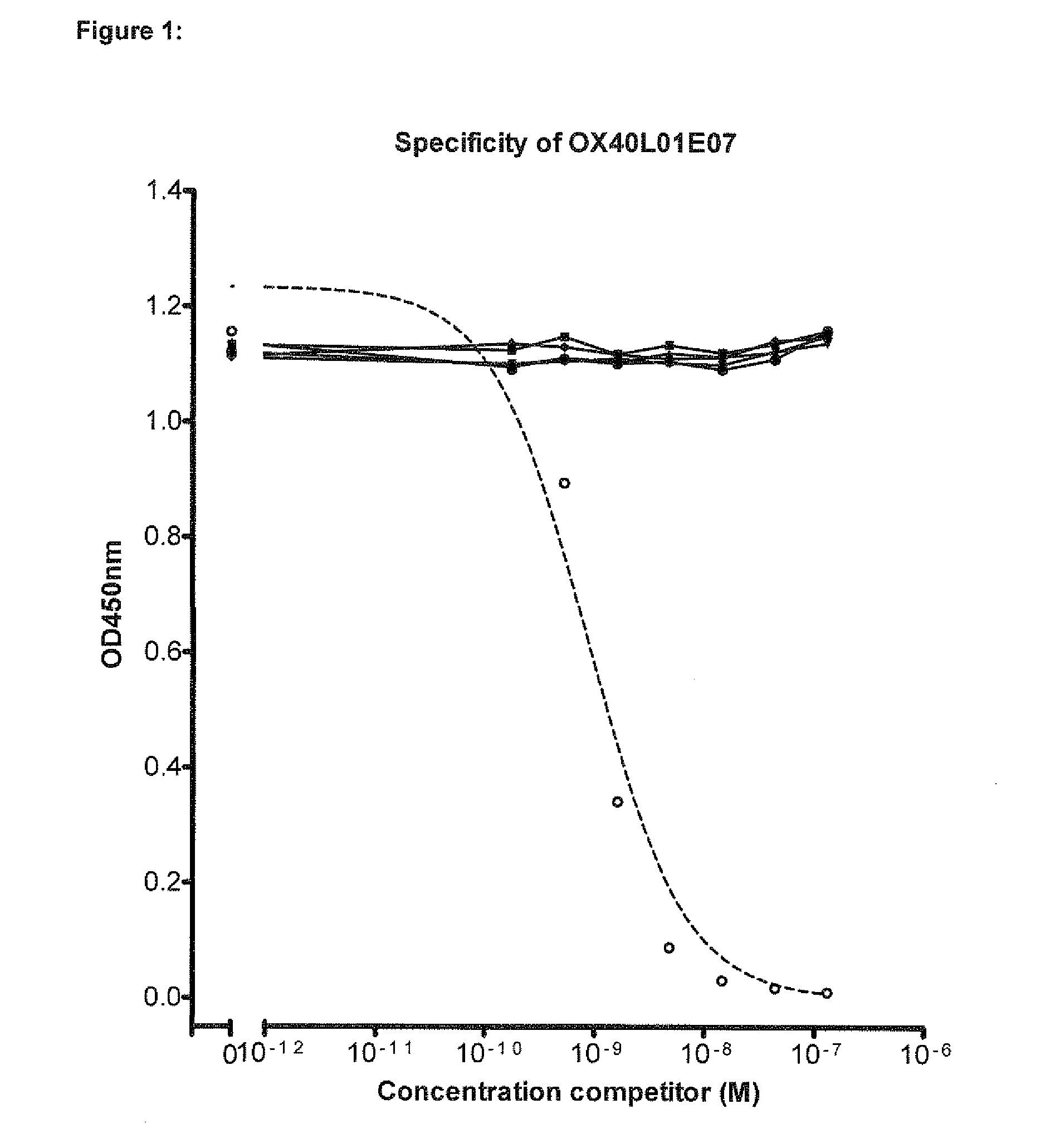
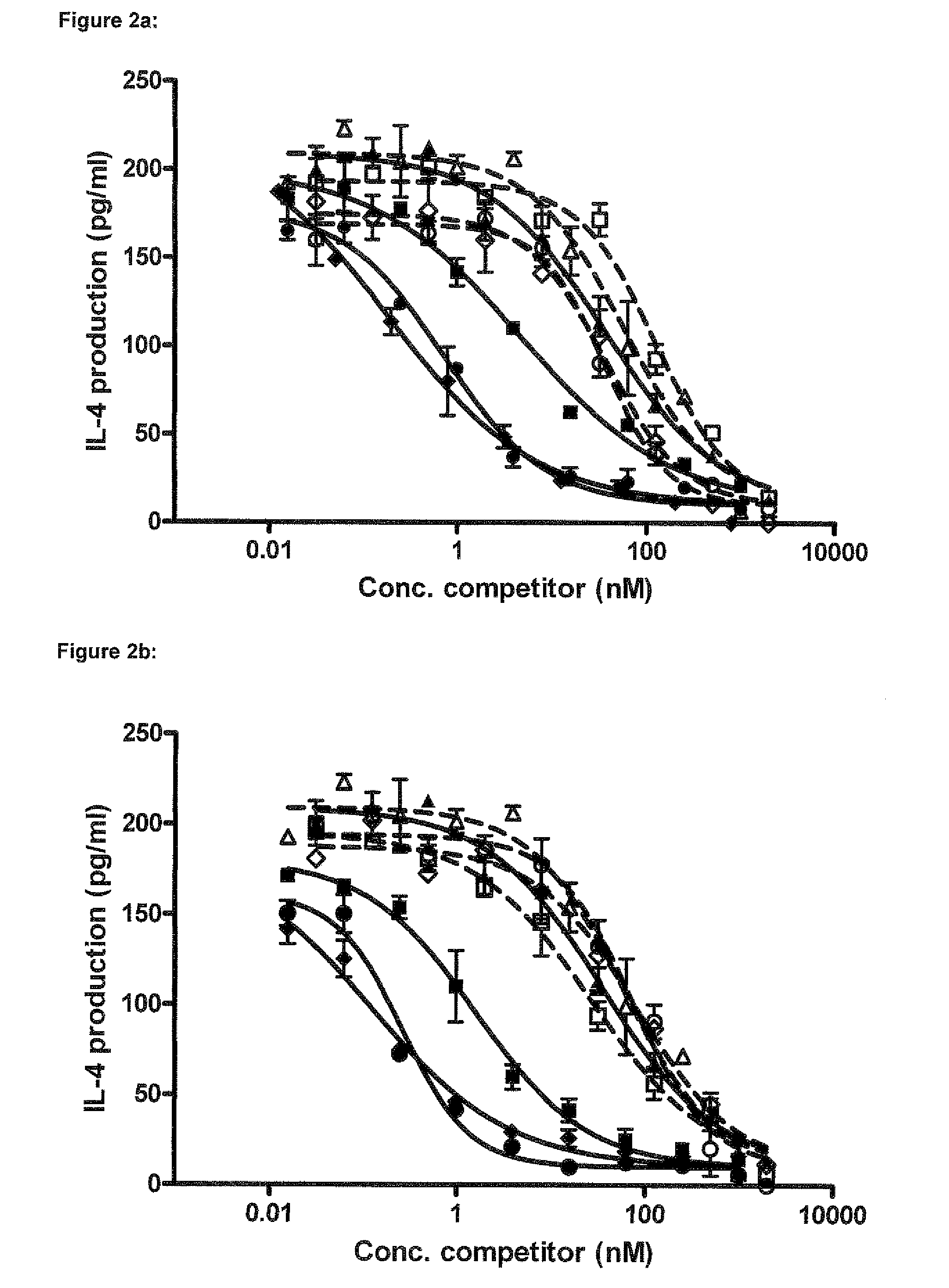
Single variable domain antibodies against ox40l, constructs and therapeutic use
The present invention relates to immunoglobulin single variable domain sequences that are directed against (as de-fined herein) OX40L, as well as to compounds or constructs, and in particular proteins and polypeptides, that comprise or essentially consist of one or more such immunoglobulin single variable domain sequences. In particular these immunoglobulin single variable domain sequences can block binding of OX40L to OX40. The immunoglobulin single variable domains, compounds and constructs can be used for prophylactic, therapeutic or diagnostic purposes, such as for the treatment of inflammatory disease and / or disorder such as e.g. asthma, allergic asthma, chronic colitis, Crohn's disease, inflammatory bowel disease, and / or arthrosclerosis.
Owner:ABLYNX NV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com