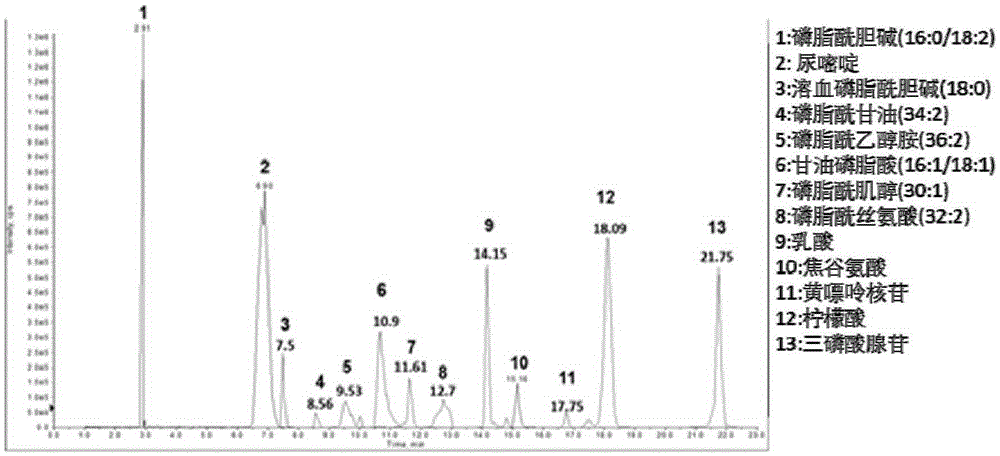
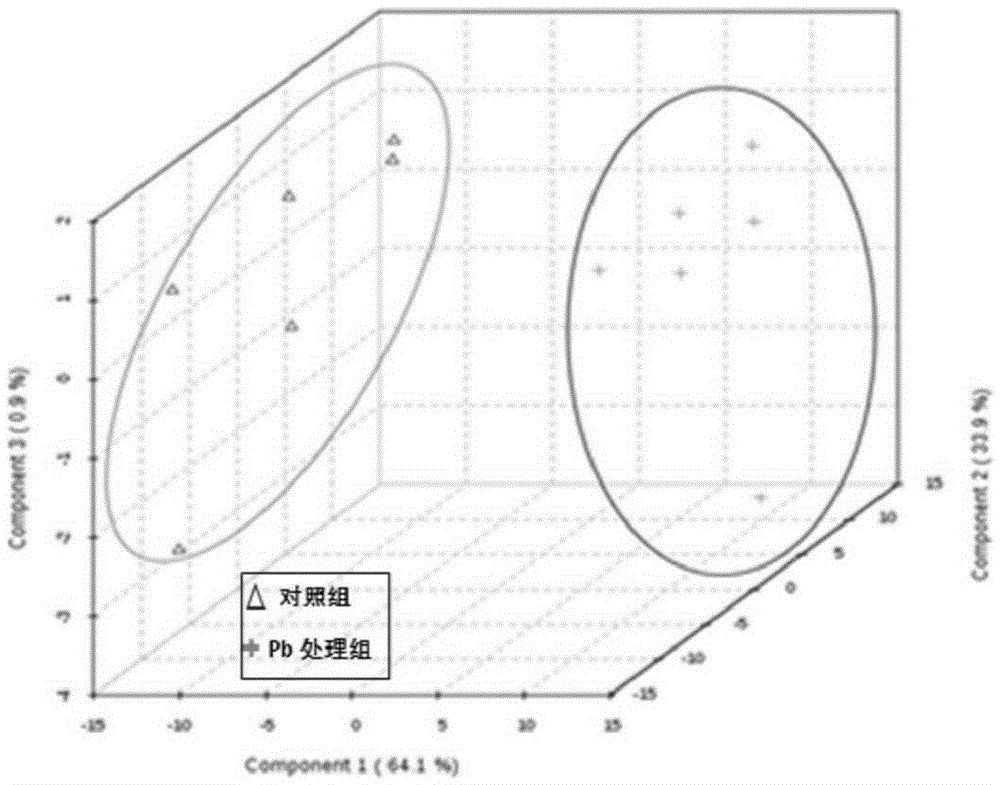
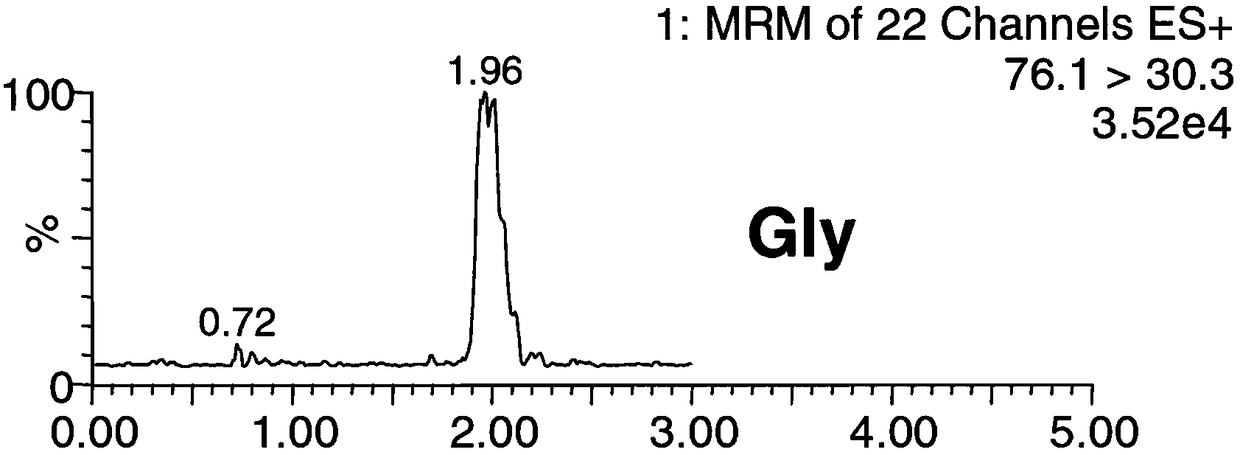
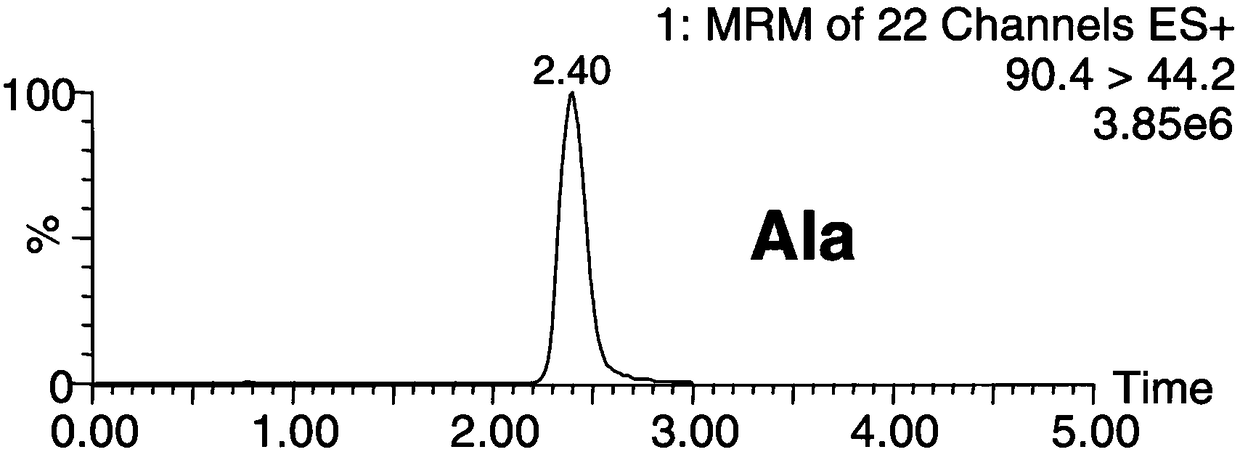
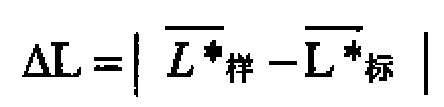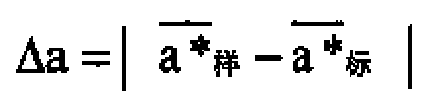Patents
Literature
1632 results about "Standard product" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition of Standardized Product: A standardized product is a product that regardless of what company furnishes the good, consumers regard the product furnished by all the companies as identical. Examples of standardized products include agricultural products (such as grain and milk), most mined minerals, and fish.
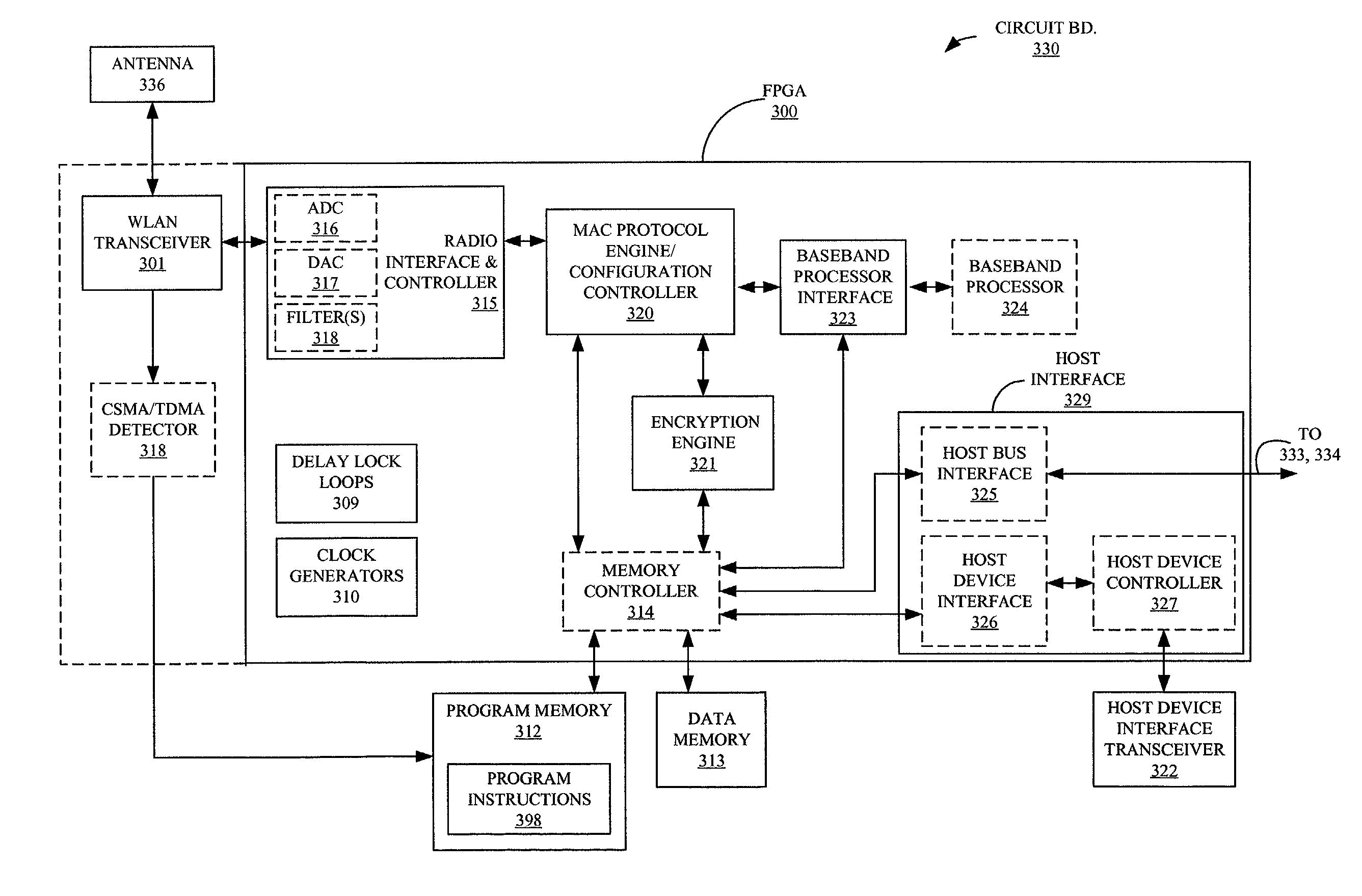
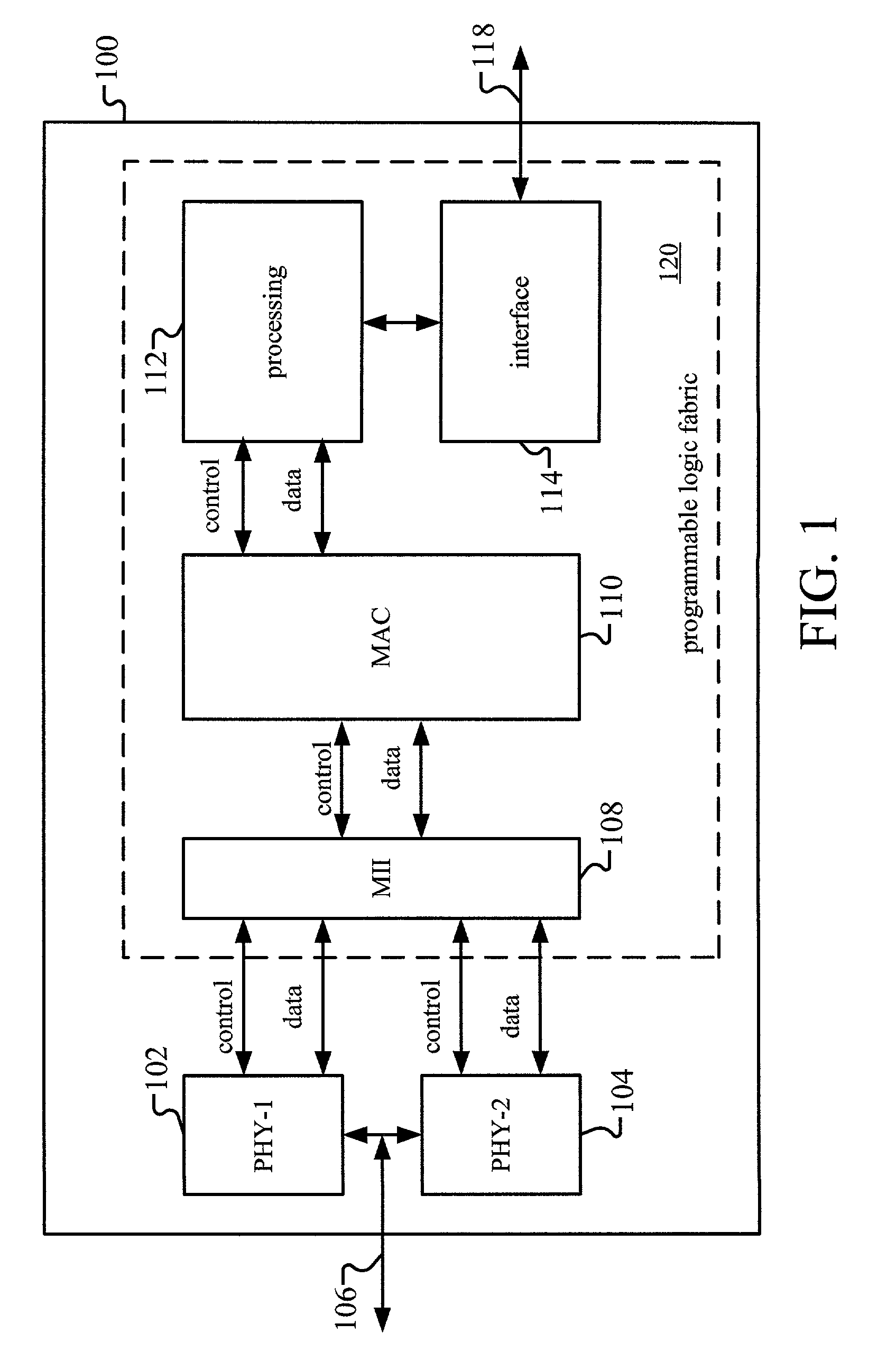
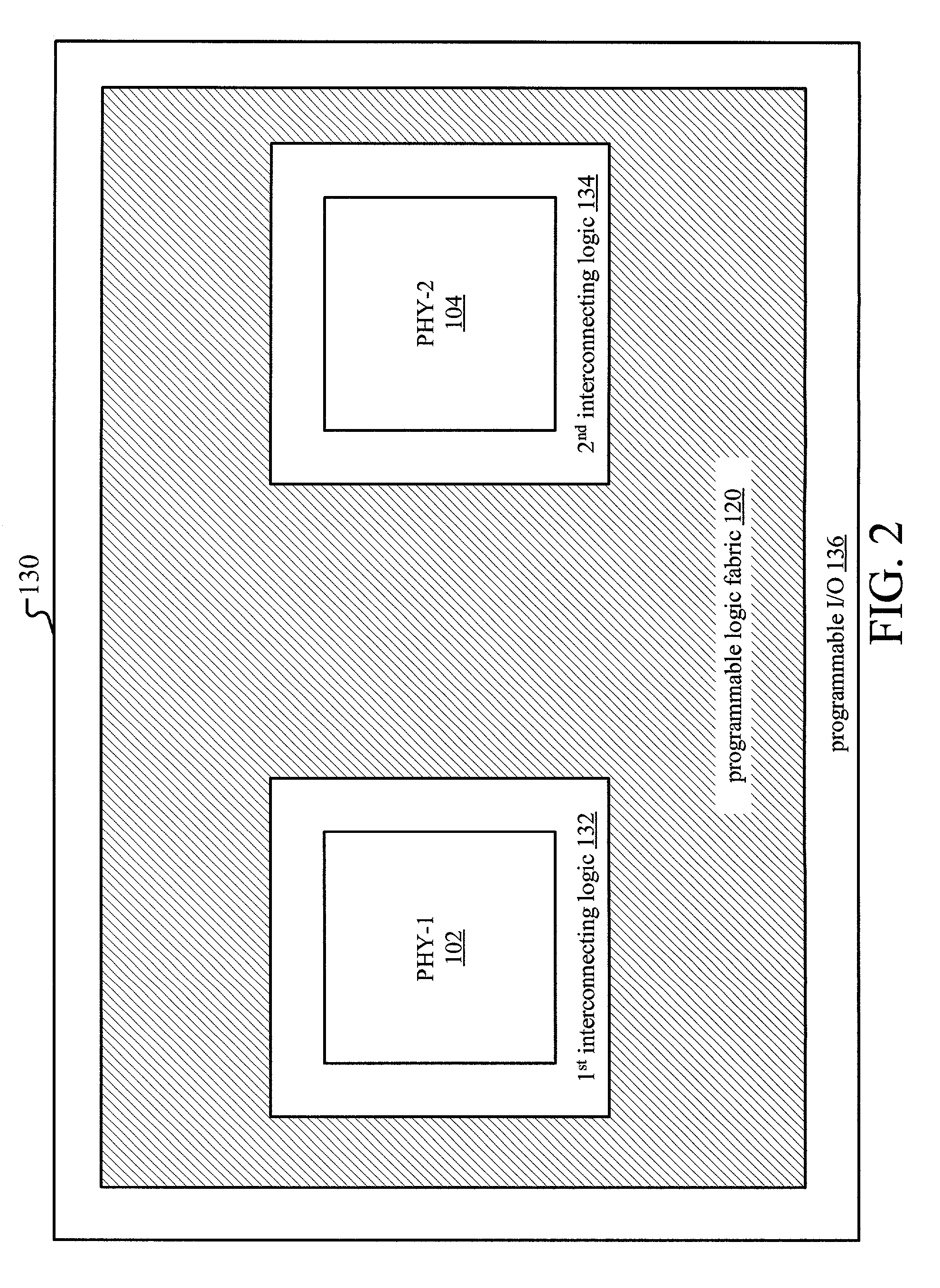
Programmable logic device for wireless local area network
ActiveUS20050084076A1Simple interfaceEasy accessInterconnection arrangementsData switching by path configurationProgram instructionProgrammable logic device
Method and apparatus for a wireless local area network programmable logic device is described. More particularly, a field programmable gate array (FPGA) is coupled to memory having programming instructions for configuring the FPGA with a medium access layer selected from more than one type of medium access layers. A physical layer is hardwired or embedded on the FPGA, or a separate integrated circuit for the physical layer is used. Additionally, the memory comprises programming instructions for a baseband controller, and may include programming instructions for a baseband processor, for configuring the FPGA in accordance therewith. In this manner, a single physical layer may be used with an FPGA to provide a multi-platform application specific standard product (ASSP). This is especially advantageous for providing multi-platform devices for use in countries or applications where one or more standards may be employed.
Owner:XILINX INC
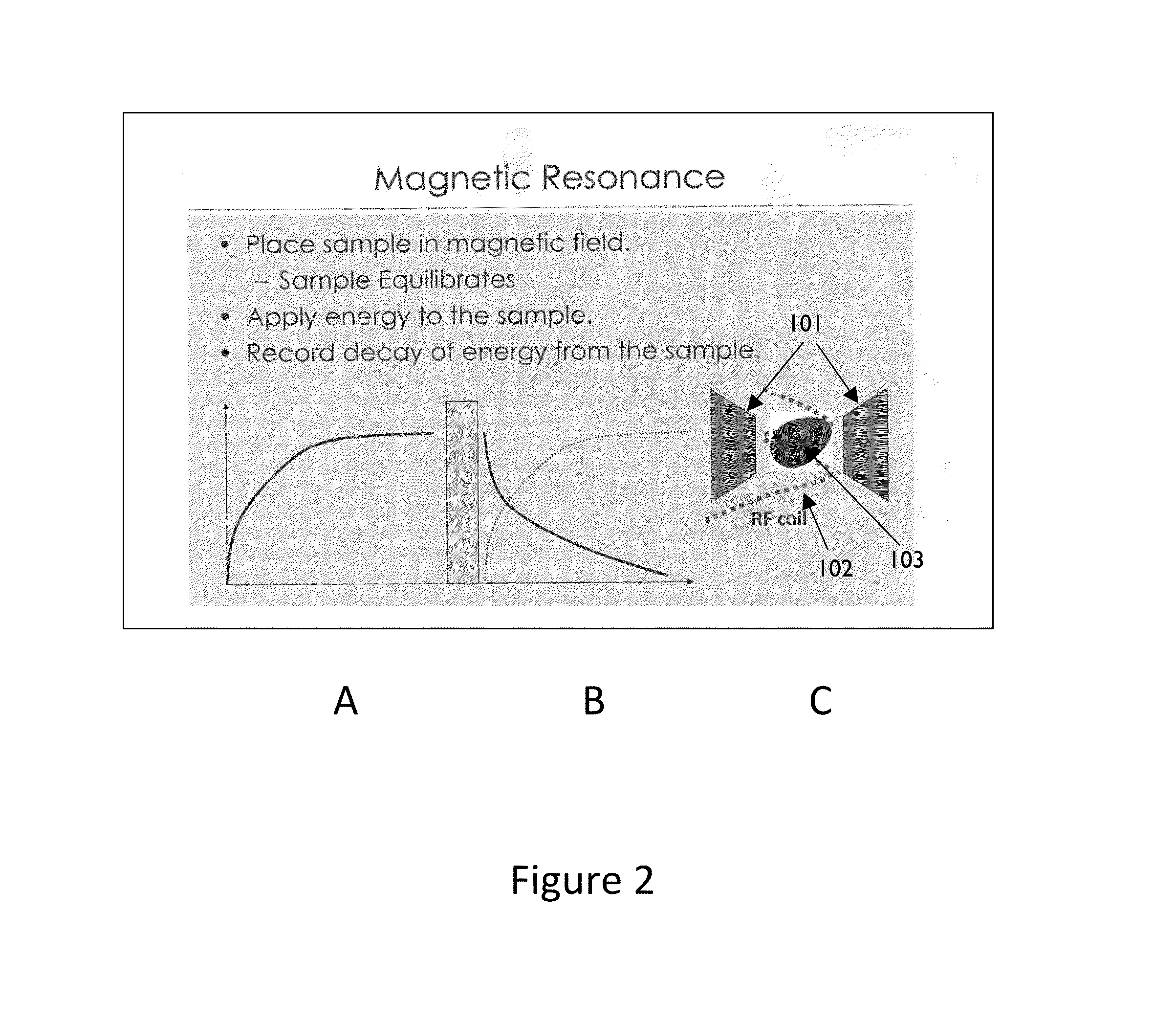
Non-invasive MRI system for analyzing quality of solid food products enveloped by flexible aluminum foil wrapper and methods thereof
InactiveUS20140050827A1Magnetic measurementsAnalysis using nuclear magnetic resonanceControl systemQuality control system
The present invention provides a quality control system characterized by:a. a line of substantially-solid food-products, said products at least partially enclosed in a flexible aluminum foil wrapper and randomly-orientated on said line;b. an MRI device adapted to image each of said products on said line; said MRI is in communication with a computer readable medium adapted to (i) store at least one computer-retrievable standardized parameter related with a standard product; (ii) analyze said MRI thereby providing an online parameter; (iii) compare said online parameter with said predefined standardized parameter; and thereby (iv), detect defects in said products.
Owner:ASPECT IMAGING
Raman spectroscopy for bioprocess operations
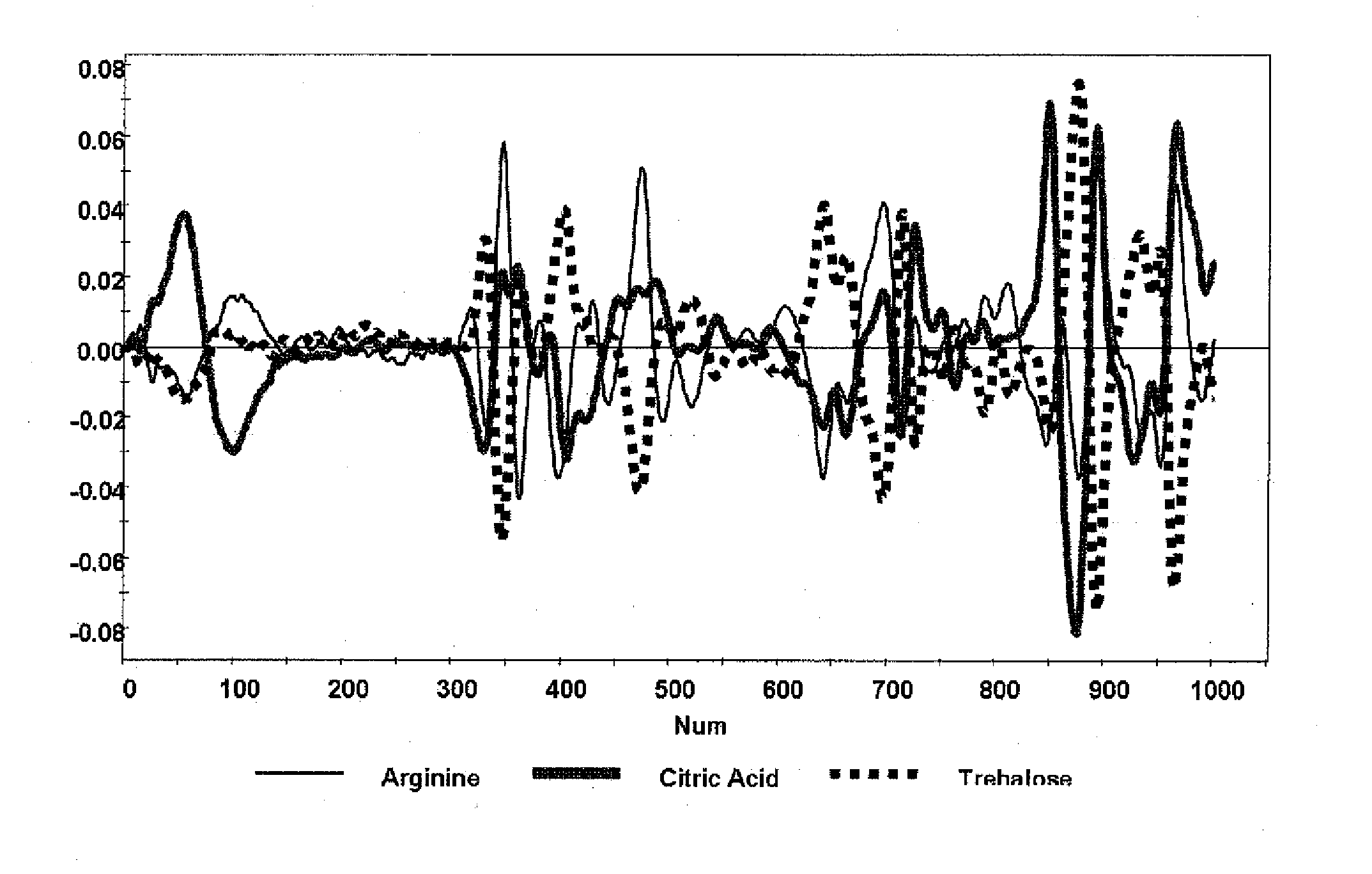
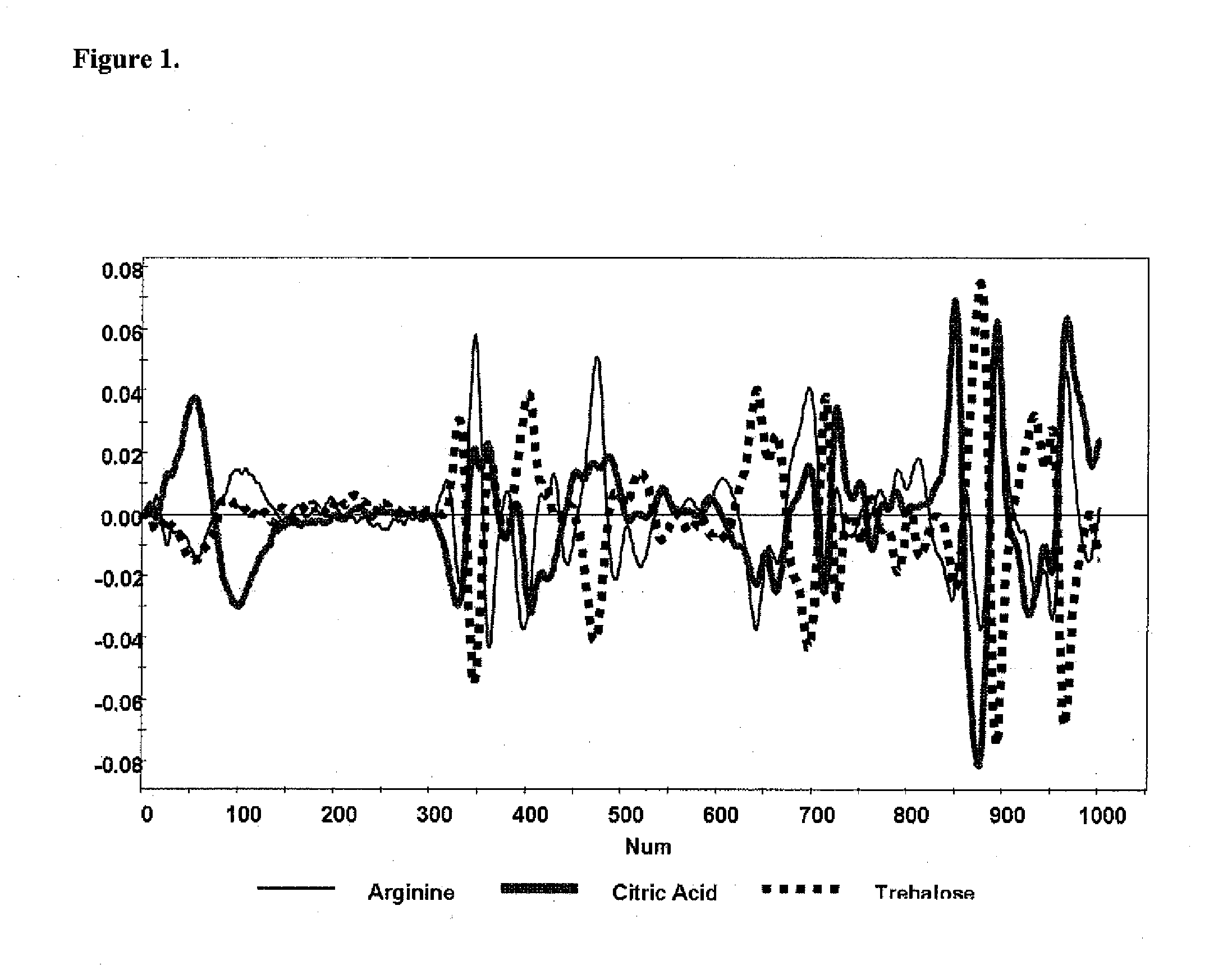
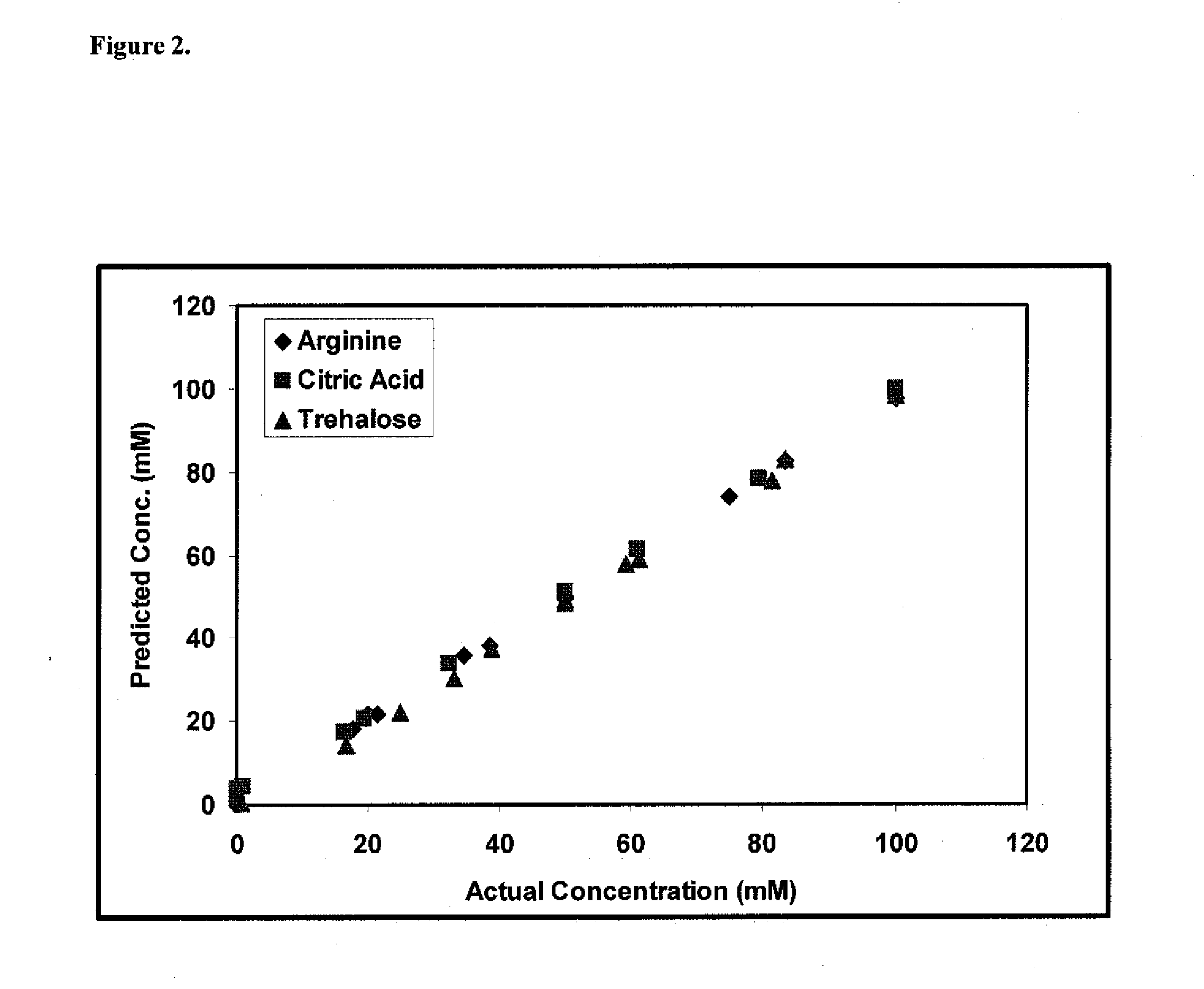
A method of characterizing a multi-component mixture for use in a bioprocess operation that includes providing a multi-component mixture standard with pre-determined amounts of known components; performing a Raman Spectroscopy analysis on the multi-component mixture standard; providing a multi-component test mixture from the bioprocess operation; performing a Raman Spectroscopy analysis on the multi-component test mixture; and comparing the analysis of the multi-component mixture standard and the multi-component test mixture to characterize the multi-component test mixture. In one embodiment, the multi-component mixture standard and the multi-component test mixture both comprise one or more of, at least two, at least three of, or each of, a polysaccharide (e.g. sucrose or mannitol), an amino acid (e.g., L-arginine, L-histidine or L-ornithine), a surfactant (e.g. polysorbate 80) and a pH buffer (e.g., a citrate formulation).
Owner:ABBVIE INC
Dot immuno gold directed infiltration detection kit and application thereof
InactiveCN102053153AReduce consumptionEasy to makeMicrobiological testing/measurementNanosensorsMicrosphereColloid
The invention relates to a dot immuno gold directed infiltration detection kit and application thereof. The kit comprises a dot immuno gold infiltration card, nano colloidal gold or a detection probe marked by latex microballoons, a negative standard product, a positive standard product and flushing liquor. The kit is technically characterized in that the dot immuno gold infiltration card is a dot immuno gold directed infiltration card which can be used for ensuring that a sample to be detected and the detection probe marked by the latex microballoons infiltrate sequentially along an area covered by a coated probe. The invention has the advantages of simpleness of manufacture, less reagent consumption, wide detection range and good maneuverability, and compared with a traditional dot immuno gold directed infiltration method, the detection sensitivity is obviously improved.
Owner:XIAN WEITONG BIOSCI
Improved prealbumin detection kit
The invention discloses an improved immune transmission turbidity reagent kit for detecting the prealbumin content in blood serum, comprising a reagent R1, a reagent R2 and a prealbumin standard. Through the specific reaction of an antigen-antibody, the reagent kit forms a small immune compound which is smaller than 19s and forms a large visual compound which is larger than 19s under the action of a flocculant to generate a certain turbidity, thereby being suitable for measuring the transmission turbidity. The reagent can effectively prevent false turbidity from generating, has high accuracy rate, favorable reproducibility, strong capacity of resisting disturbance and simple operation and is suitable for various fully automatic biochemical analysers. The titer containing in the reagent adopts stroma with whole serum, thereby the influence of stroma effect is avoided.
Owner:BEIJING STRONG BIOTECH INC
Product merchandising system
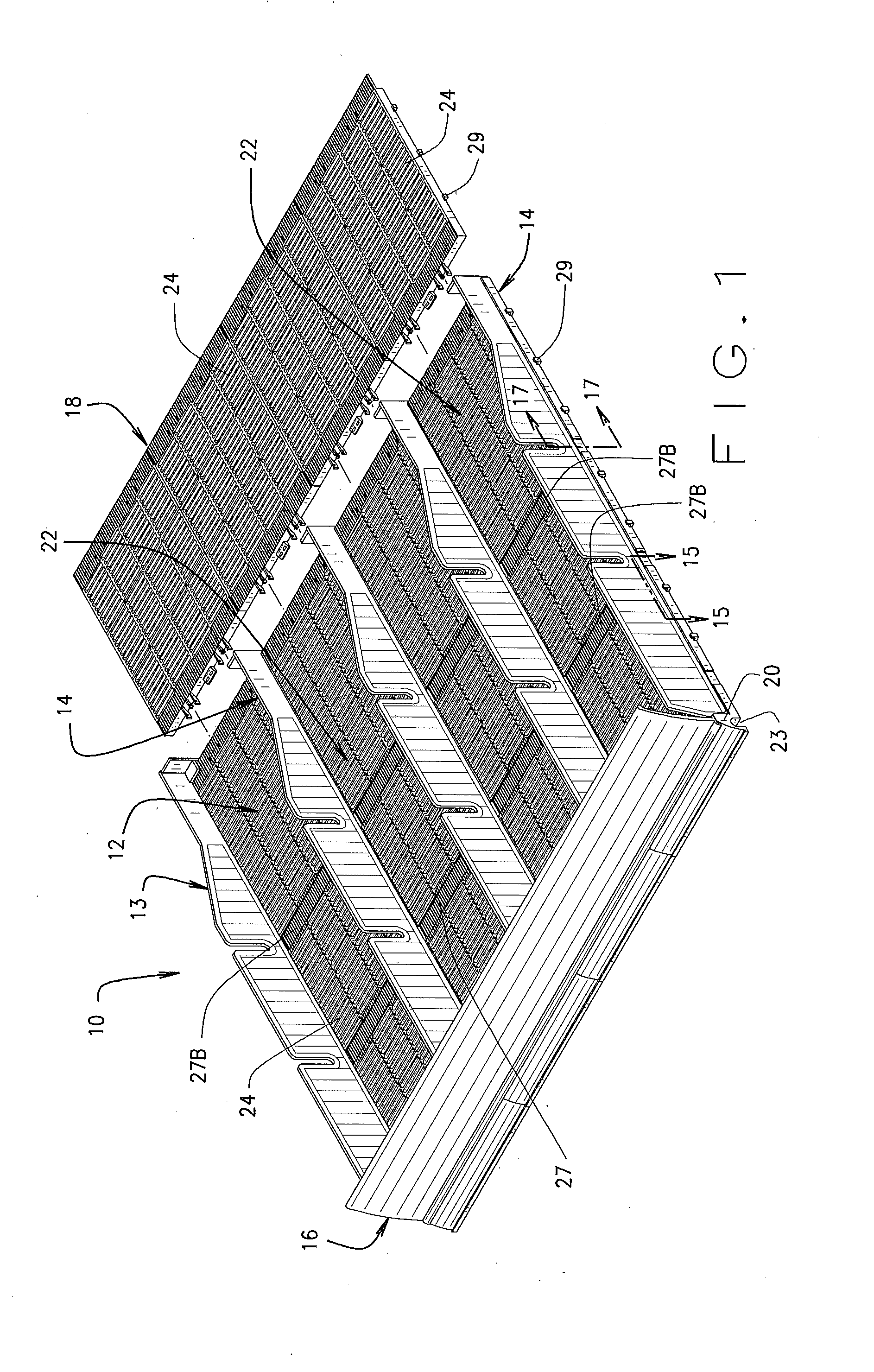
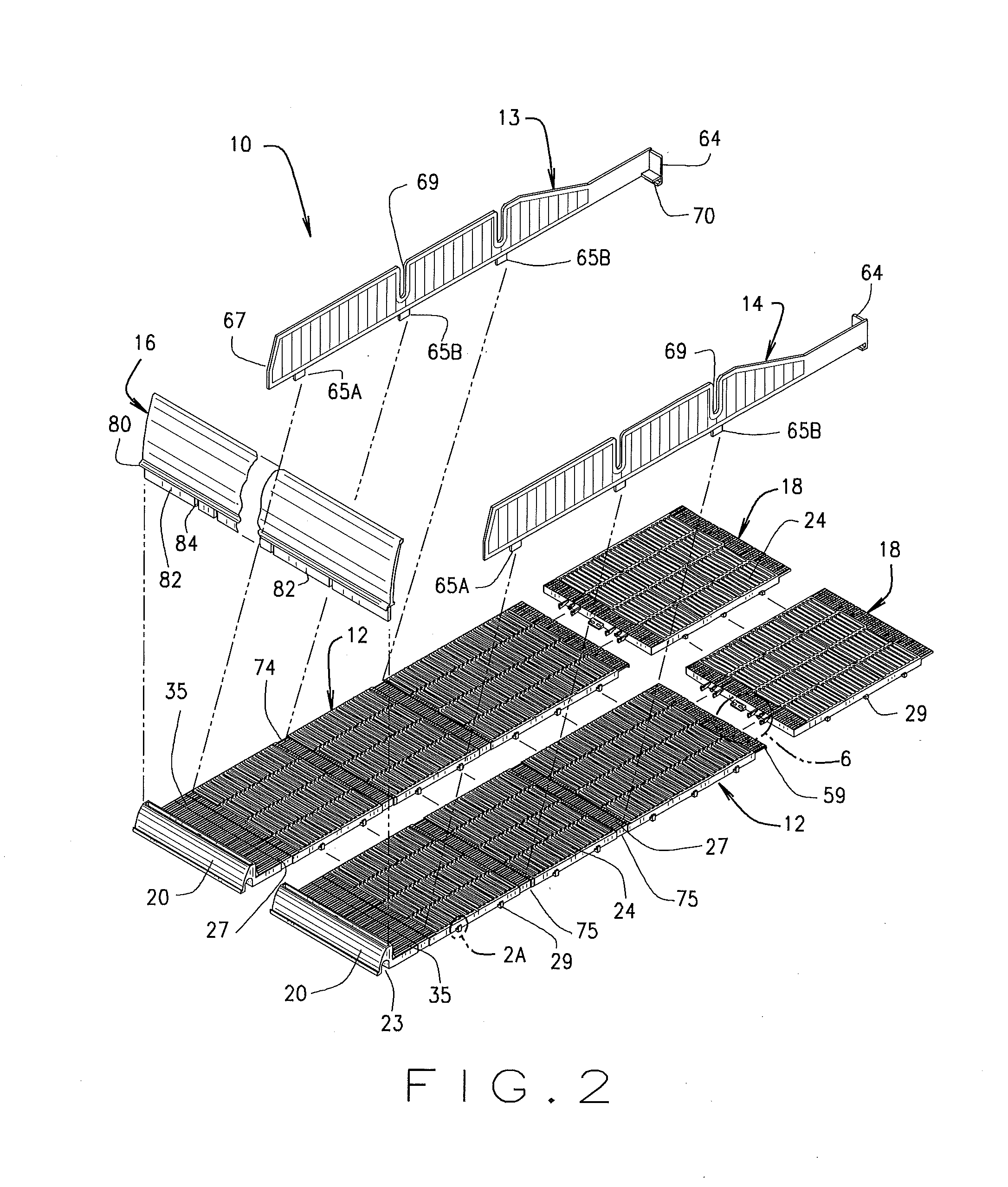
A variable shelf organizer system for displaying merchandise thereon including a roller glide floor member, a standard track glide floor member, a plurality of removably adjustable divider members and a front wall member. The divider members and front wall member are engageable with both floor members and when multiple divider members are engaged with either floor member, product channels are formed therebetween for holding and securing products of varying size and shape on either floor member. The roller glide floor member is best suited for heavier packaged products such as six-pack or twelve pack products and the track glide floor member is best suited for standard products. The width of the shelf system may be increased or decreased by joining or detaching similarly constructed floor members in a side-by-side relationship and the length of the shelf system may be increased by attaching one or more floor extension members to the floor members.
Owner:PRESENCE FROM INNOVATION
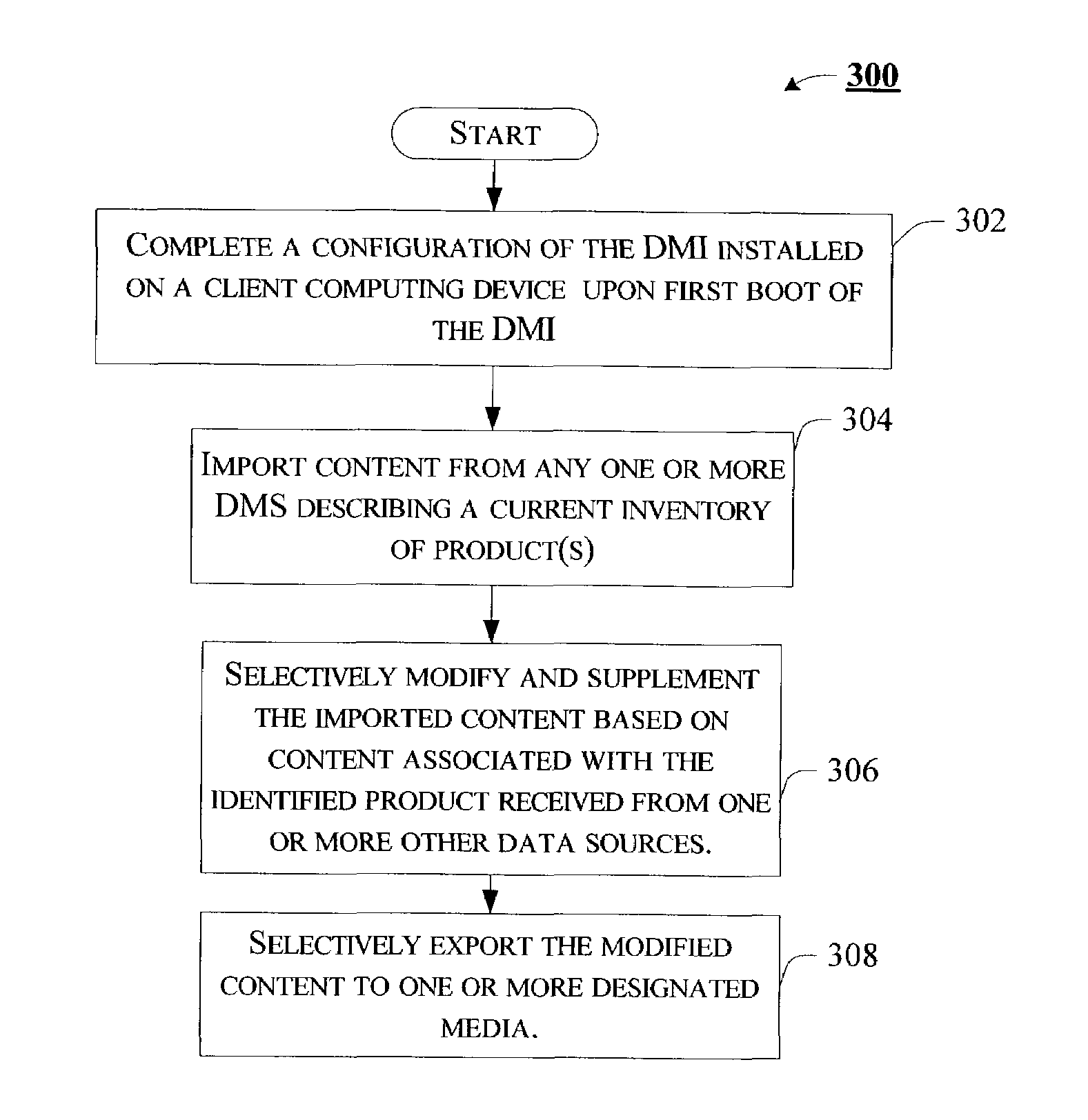
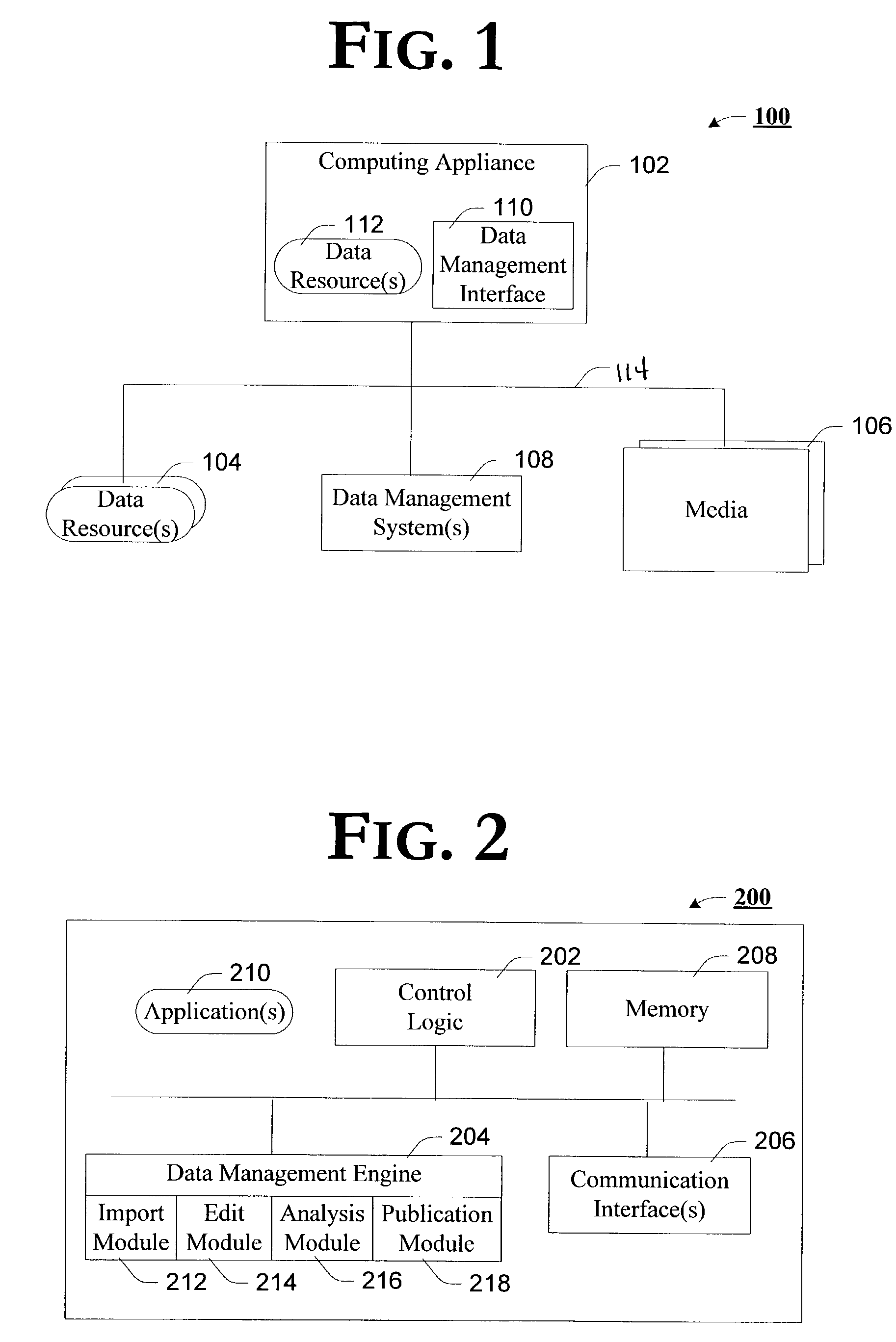
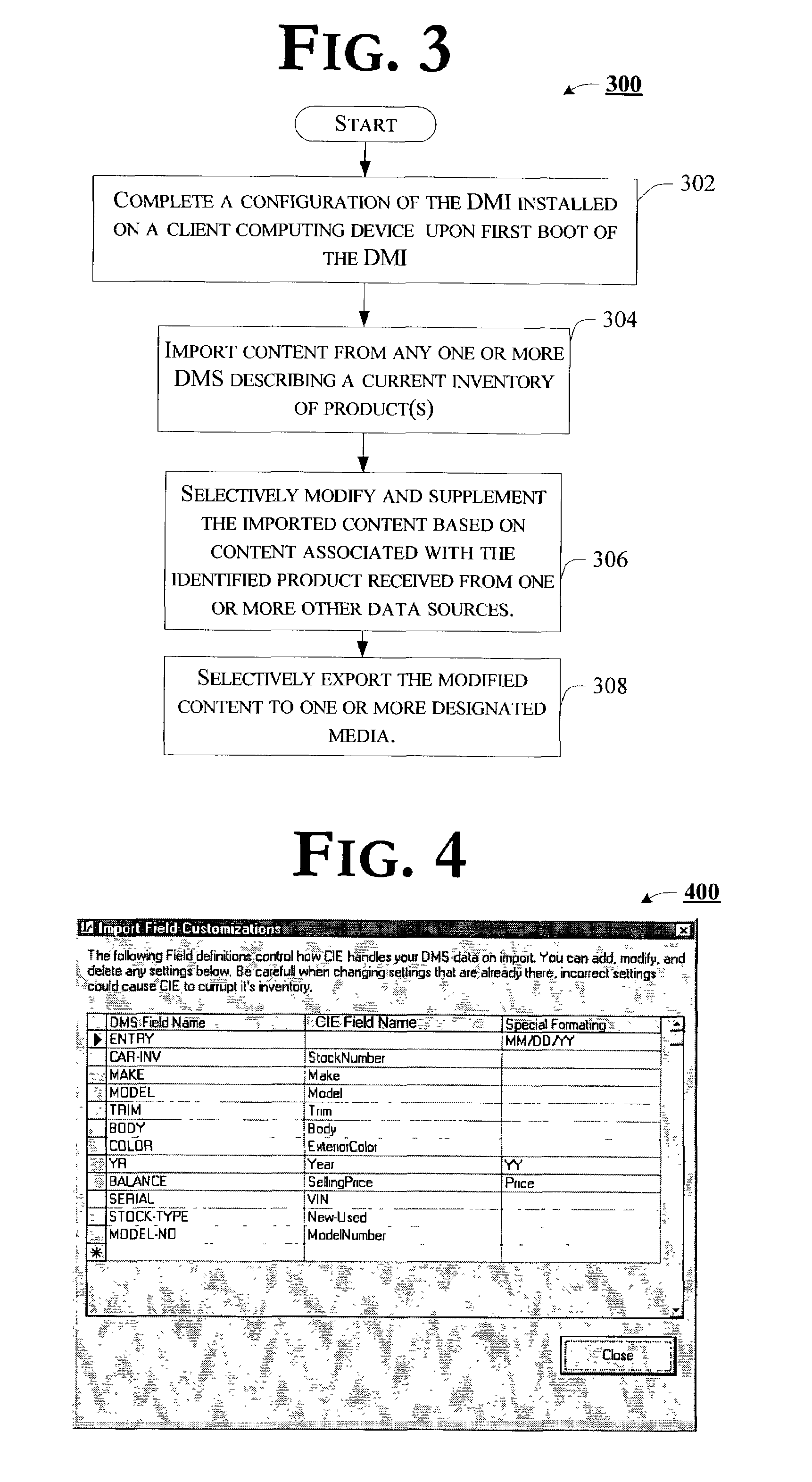
Data management interface capable of providing enhanced representation of imported electronic content
Embodiments of a data management interface (DMI) and associated methods are generally described. According to but one example embodiment, a method is introduced comprising importing content describing product inventory from one or more disparate data sources of such content, the content including one or more standard product descriptors that uniquely identify an individual instance of a product, selectively modifying the imported content based, at least in part, on content associated with the uniquely identified instance of the product obtained from an independent resource, and exporting the selectively modified content to any one or more of a number of media.
Owner:AUTODATA INC +1
Particle-enhanced turbidimetric immune assay kit for detecting adiponectin and preparation method thereof
ActiveCN101968492AAccurately reflectHigh sensitivityMaterial analysis by observing effect on chemical indicatorBiological testingPreservativeMonoclonal antibody
The invention relates to a particle-enhanced turbidimetric immune assay kit for detecting adiponectin and a preparation method thereof, in particular to a detection method of a turbidimetric immune assay. The particle-enhanced turbidimetric immune assay kit for detecting the adiponectin comprises a kit body, an application liquid bottle and a latex suspension bottle coating an antiviral adiponectin monoclonal antibody, wherein the application liquid bottle is filled with application liquid; the application liquid comprises a surfactant, a preservative, a macromolecular accelerator, sodium chloride and a buffering agent; the latex suspension bottle coating the antiviral adiponectin monoclonal antibody is filled with a latex suspension coating the antiviral adiponectin monoclonal antibody; and the latex suspension coating the antiviral adiponectin monoclonal antibody comprises latex coating the antiviral adiponectin monoclonal antibody, the surfactant, a stabilizer and the buffering agent. The preparation method comprises the following steps of: preparing a latex antibody; preparing the latex suspension coating the antiviral adiponectin monoclonal antibody; preparing the applicationliquid; and finally preparing adiponectin series standard products.
Owner:ANHUI IPROCOM BIOTECH CO LTD
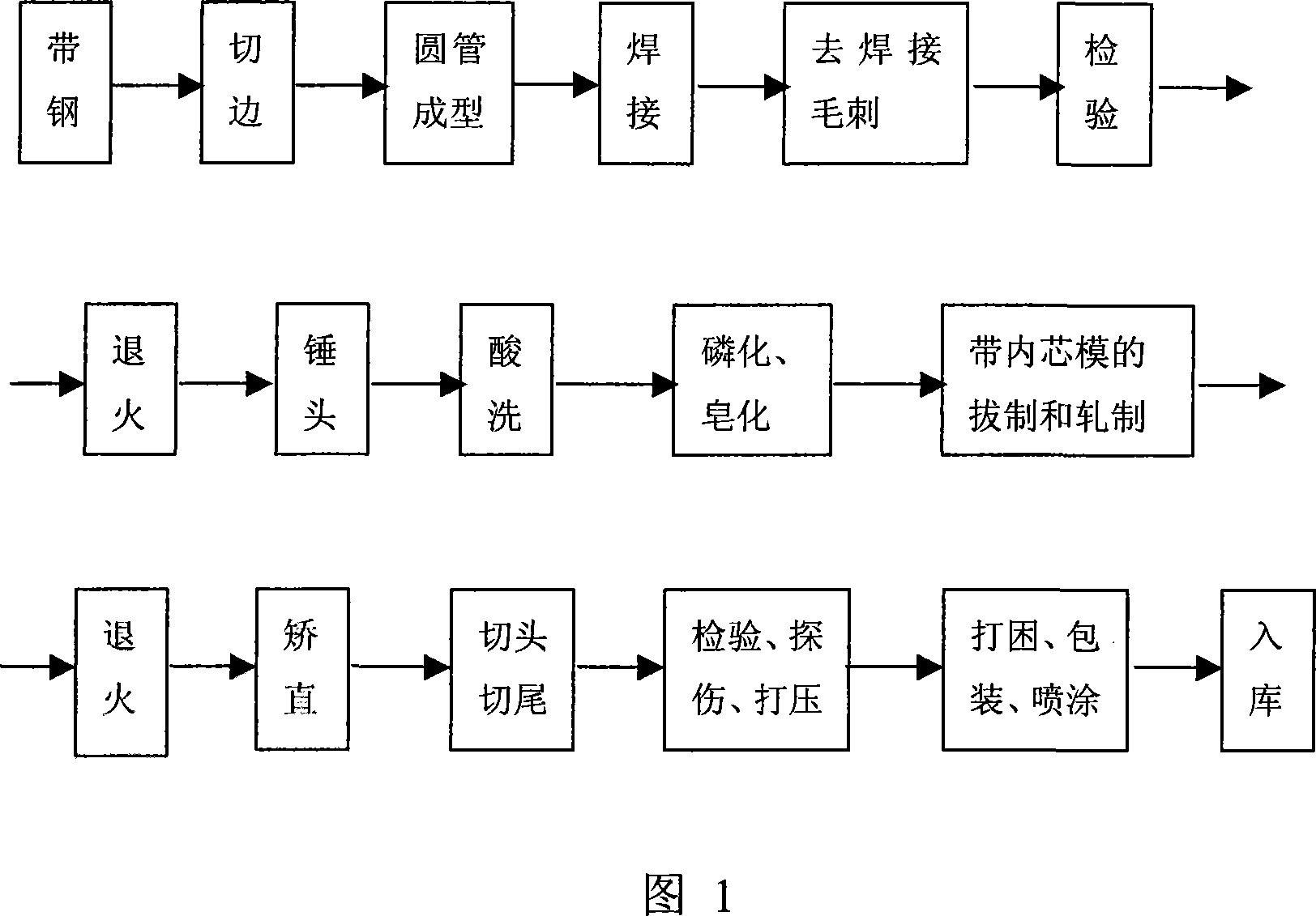
Cold drawing, cold rolling production method for high-accuracy weldless steel tube
InactiveCN101130197ASimple processLow costHigh frequency current welding apparatusChemical compositionStrip steel
The invention discloses a cold-drawing and cold-rolling manufacturing method of high-precision seamless steel pipe, which comprises the following steps: doing pipe formation procedure for the front part to cut edge of raw material steel, mould round tube, weld, remove the welded burr, detect the standard product to enter subsequent disposing procedure; doing back disposal procedure to heat, beat, wash through acid, phosphorized, saponify; rolling the mould with inner core; annealing; aligning; cutting head and tail; detecting flaw; bulging; testing; packing; storing. The invention saves energy and cost with simple operation and even wall thickness, which can replace common seamless steel pipe, low-middle pressure boiler pipe and steel pipe of the fluid completely.
Owner:李永立
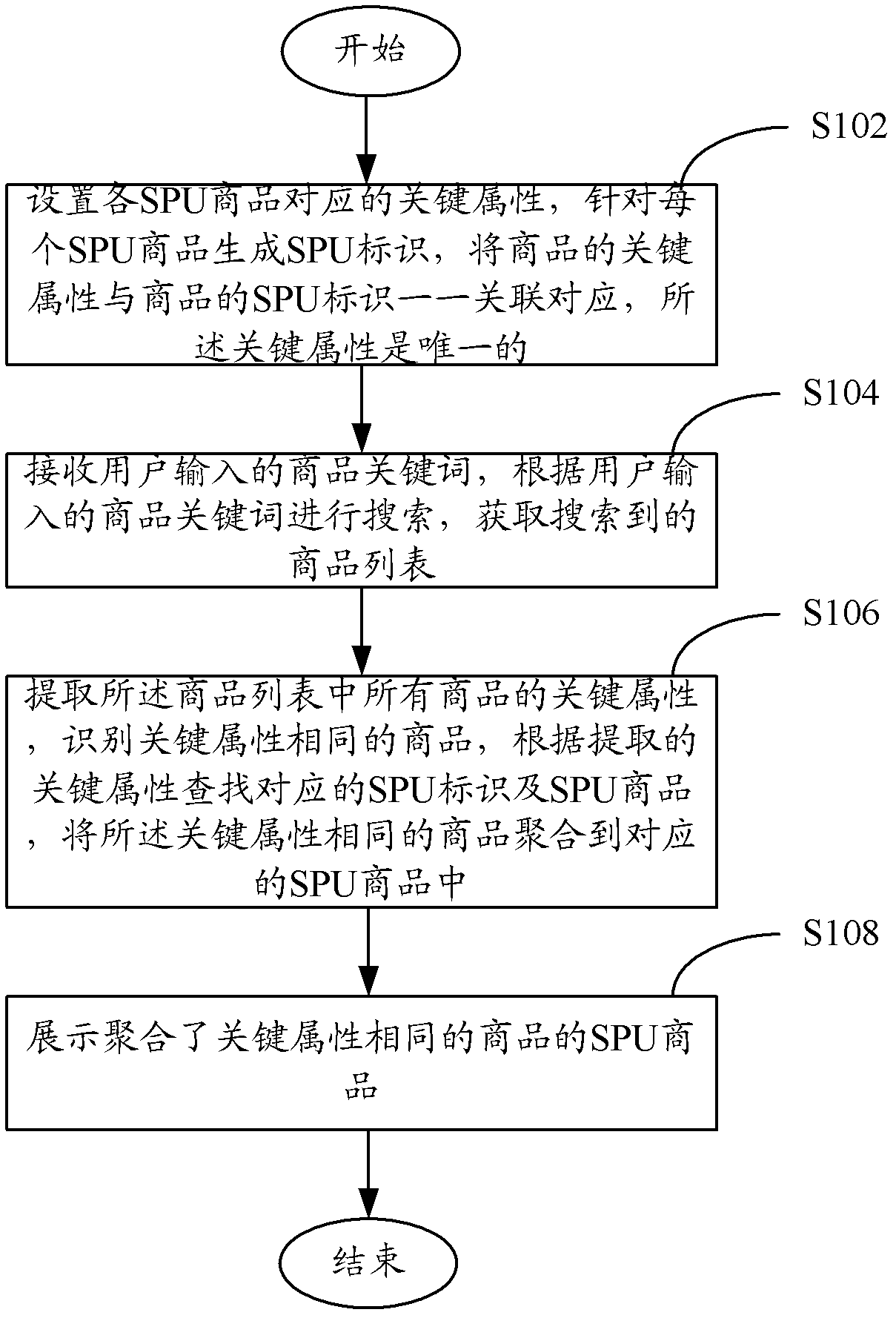
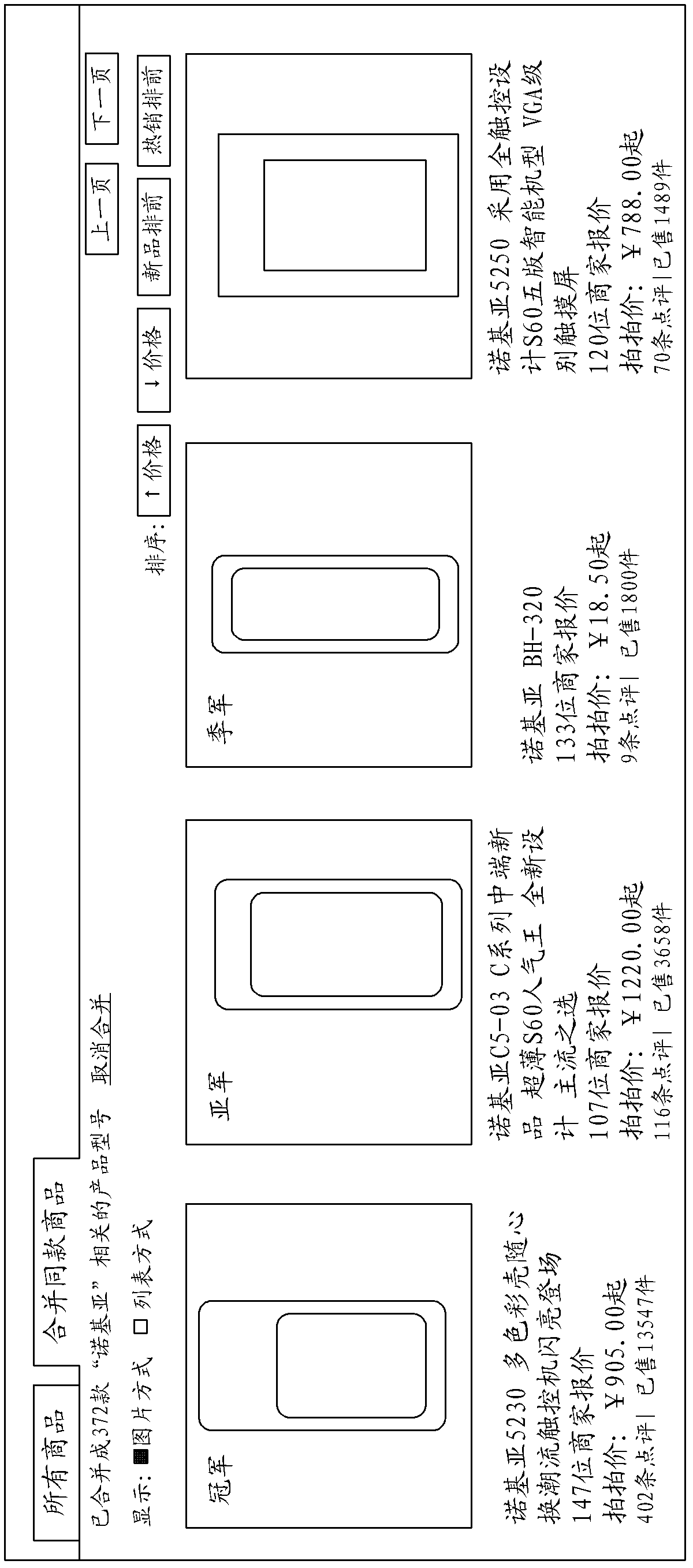
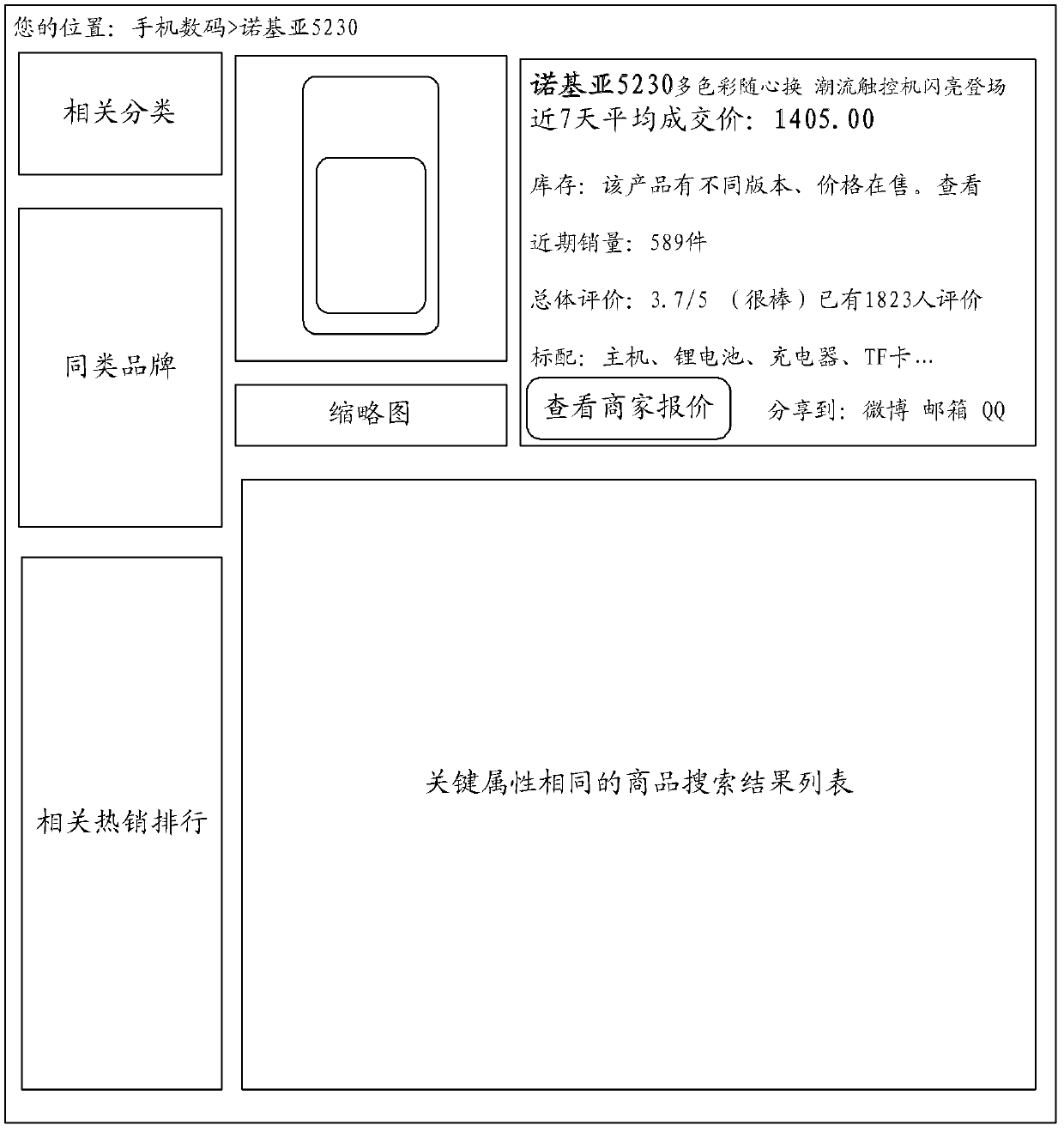
Method and system for showing commodity search result
The invention discloses a method for showing commodity search result. The method comprises the steps of: setting determinant attribute corresponding to each SPU (Standard Product Unit) commodity, generating a SPU identifier for each SPU commodity, building one-to-one association correspondence between the determinant attribute of the commodity and the SPU identifier of the commodity, wherein the determinant attribute is unique; receiving a commodity keyword input by a user, searching according to the commodity keyword input by the user to obtain the searched commodity list; extracting the determinant attributes of all the commodities in the commodity list, identifying the commodities with the same determinant attribute, finding corresponding SPU identifiers and SPU commodities according to the extracted determinant attributes, gathering the commodities with the same determinant attribute in the corresponding SPU commodity; and showing the SPU commodity in which the commodities with same determinant attribute are gathered. According to the method provided by the invention, the user can check and browse the commodity search result conveniently. Moreover, the invention further provides a system for showing the commodity search result.
Owner:BEIJING JINGDONG SHANGKE INFORMATION TECH CO LTD
Method for simultaneously measuring multiple residues of organic chloride and pyrethroid pesticides
InactiveCN101793879AMeet analysis requirementsEasy to operateComponent separationRetention timeSolid phase extraction
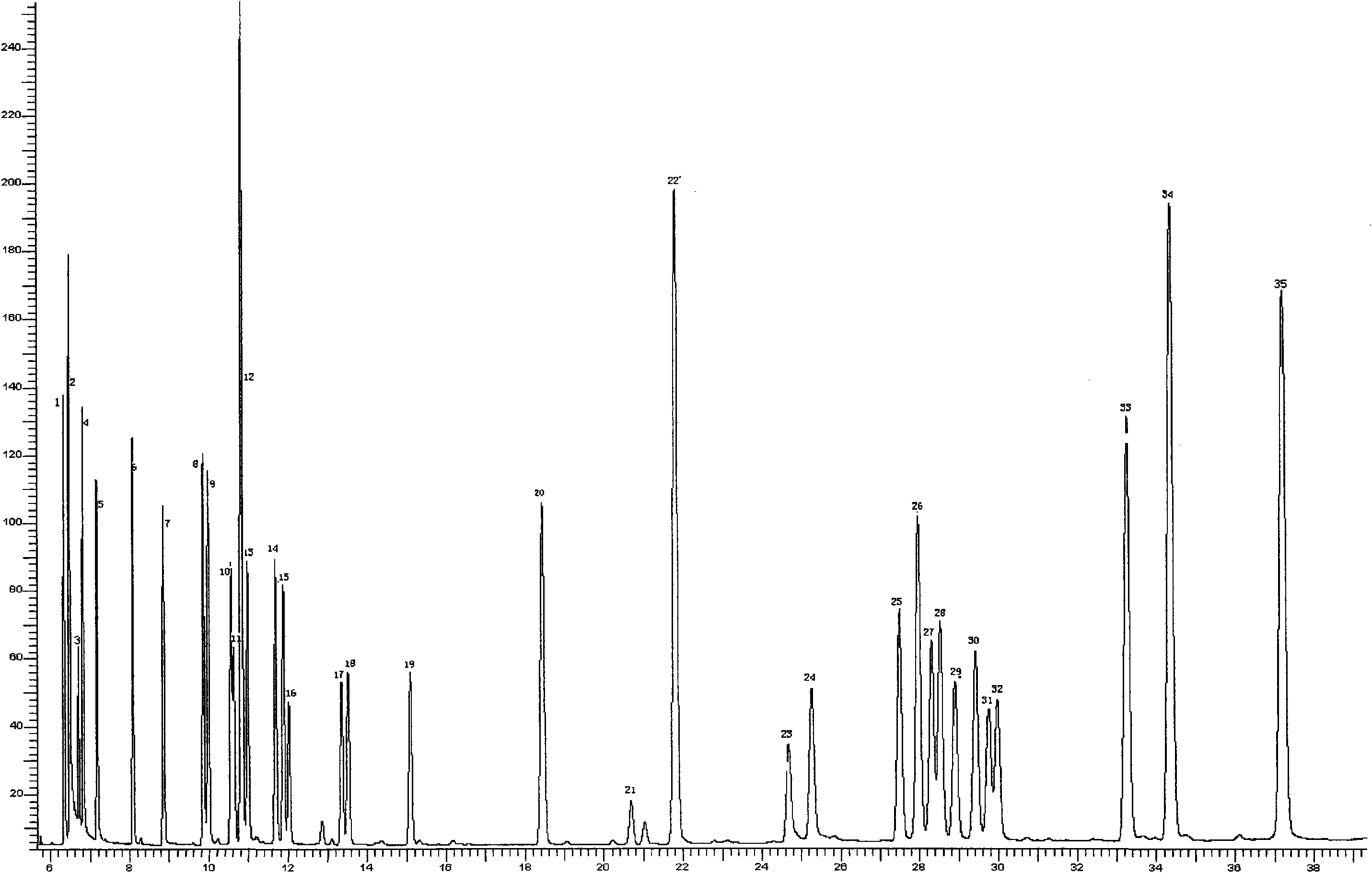
The invention discloses a method for simultaneously measuring multiple residues of organic chloride and pyrethroid pesticides. The method comprises the following working procedures: crushing a tobacco sample until grain diameters are less than 450 mu m; adding 10 to 20ml of extracting solution and internal standard solution into every program of tobacco sample; extracting the mixed solution by using ultrasonic wave; filtering the mixed solution by using a funnel filled with anhydrous sodium sulfate to obtain the extracting solution; adding the extracting solution into a Florisil solid extraction column for extracting and eluting; collecting an eluent; analyzing the eluent by using a gas chromatograph, wherein the analysis comprises the following conditions: adopting an Elite 5MS capillary column, high-purity N2 as a carrier gas, a constant flow mode in which the flow rate is 0.5 to 1.5 ml / min, a sample inlet temperature of between 280 and 320 DEG C, a detector temperature of between 330 and 370 DEG C, splitless sampling in which the sampling volume is between 1 and 2 mu L, and an initial temperature of 100 DEG C which rises from 100 to 180 DEG C at a speed of 8 DEG C / min, is kept for 4 minutes, rises from 180 to 270 DEG C at the speed of 5 DEG C / min and is kept for 15 minutes until all sample flows out completely; and determining the nature of the sample by using the retaining time of a standard sample and quantifying by using an internal standard method. In the method, ultrasonic wave is adopted for extracting and a solid extraction column is adopted for purifying, so that the method has the advantages of operating step simplification, high work efficiency, good purification effect, capability of simultaneously measuring multiple residues of 25 kinds of pesticides including 17 organic chloride pesticides of hexachlorocyclohexane, DDT and the like, seven pyrethroid pesticides such as fenpropathrin and the like, and one plant growth regulator.
Owner:YUNNAN ACAD OF TOBACCO AGRI SCI
Quantitative detection device and detection method of biological fluid samples
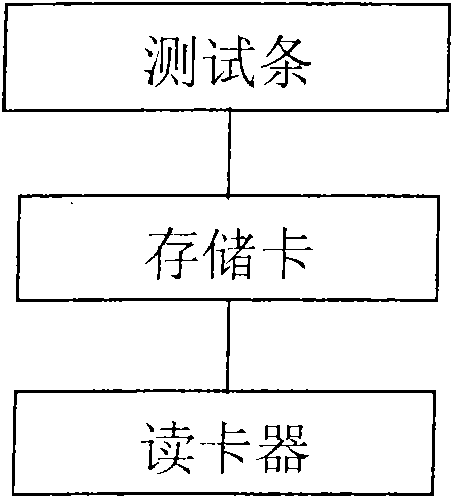
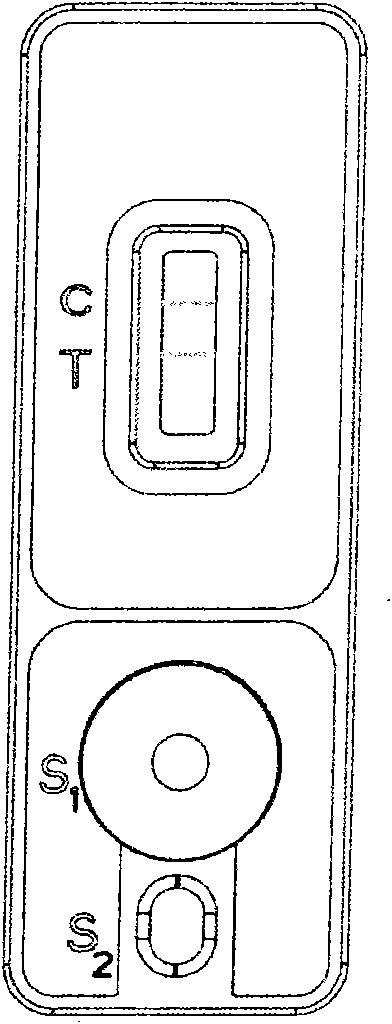
The invention discloses a quantitative detection device of biological fluid samples, which comprises a test strip arranged in a box body, a color intensity data memory and a color intensity data reader, wherein a substrate is arranged at the bottom layer of the test strip, and a porous test main film is arranged on the substrate; a second film is arranged at the upstream of the test main film, a sample application site and a tagged ligand site are arranged on the second film, and a biological fluid sample to be tested and compounds thereof and the tagged ligand can be promoted under the capillary action to flow; a third film is arranged at the downstream of the test main film, and the third film is used for adsorbing the biological fluid sample to be tested passing through the test main film at the downstream end of the test main film; and two sample injecting holes and a viewing window are arranged on the box cover of the box body. The detection device of the invention can be used for drawing a standard sample curve through the light reflection intensity of the standard sample with known concentration, thereby quickly, effectively and accurately carrying out quantitative analysis on the biological body fluid sample.
Owner:李金波
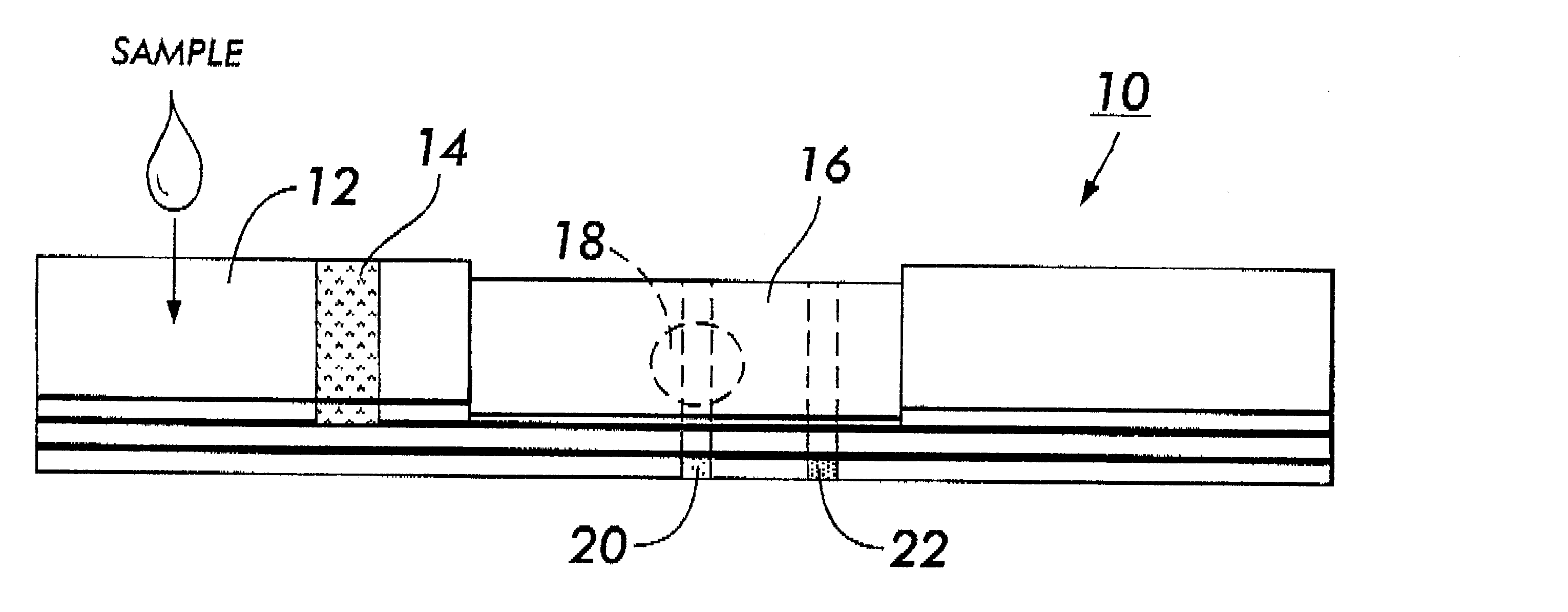
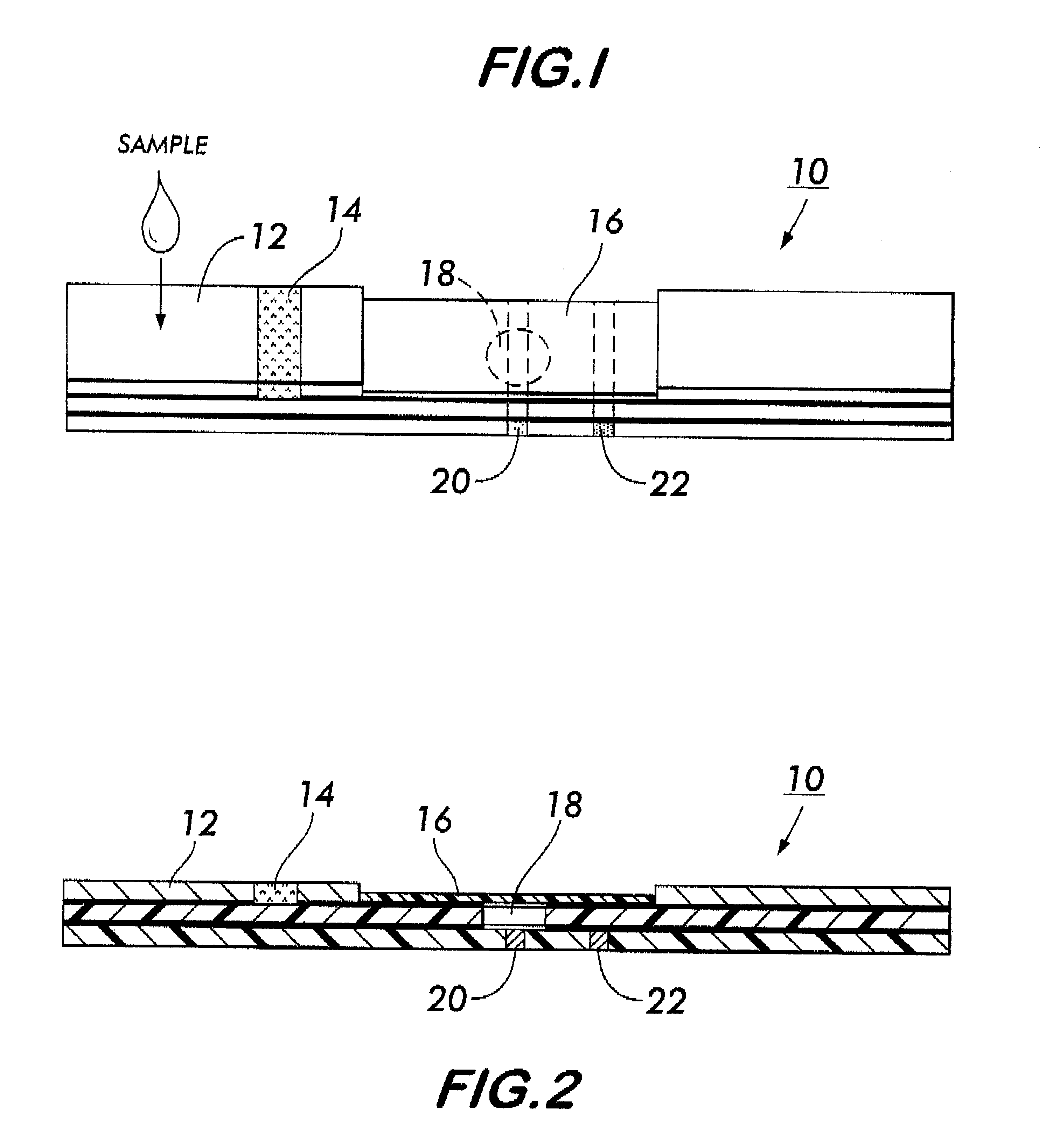
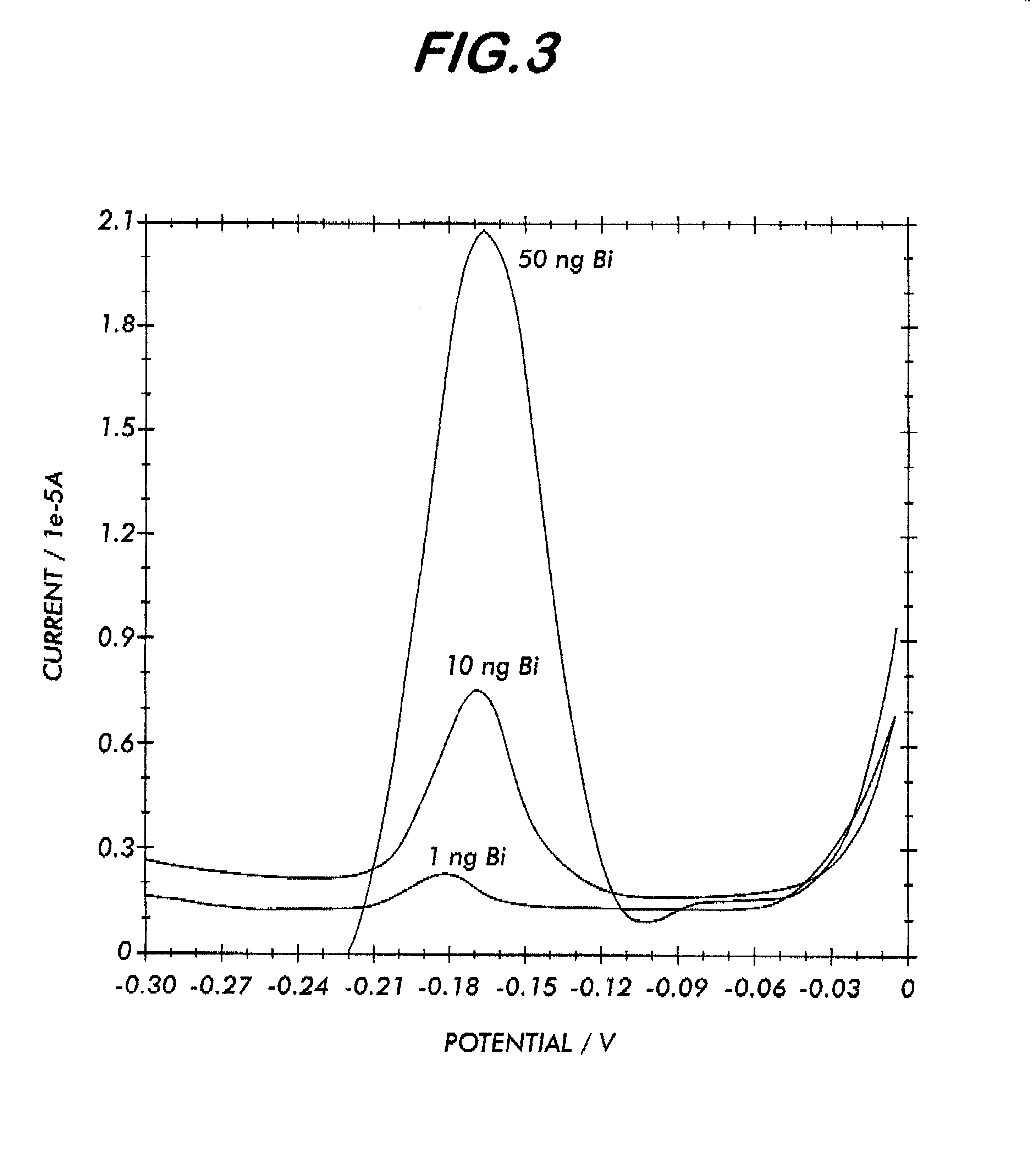
System for electrochemical quantitative analysis of analytes within a solid phase and affinity chromatographic test strip
InactiveUS6485983B1High sensitivityWide linear rangeVolume/mass flow measurementFluid pressure measurement by electric/magnetic elementsProstate cancerTime-Consuming
System, method, and test strip for solid phase, electrochemical, quantitative analysis of analytes contained in biological fluid samples. Preliminary to analysis, a test sample solution can be applied to a sample collection pad associated with the solid phase test environment of the test strip. The test sample solution and a test kit reagent are thereby initially contacted, under assay conditions, within this solid phase test environment, and caused to migrate along a fluid pathway therein. Irrespective of the assay format (competitive assay, sandwich assay, etc.), a test kit reagent (e.g. labeled substance) and the analyte of interest (e.g. proteins, hormones or enzymes, small molecules, polysaccharides, antibodies, nucleic acids, drugs, toxins, viruses or virus particles, portions of a cell wall and other compounds which have specific or characteristic markers that permit their identification), either interact with one another to form a complex, or, alternatively, compete with one another for interaction with another test kit reagent, resulting in the concentration of an indicator substance within a delimited area of the solid phase. Thereafter, the delimited area of the test strip is subjected to electrochemical analysis and the results determined by monitoring an electrochemical transition in the form of an indicator, or derivative of the indicator (e.g. indicator species), by potentiostatic or potentiometric quantitative analysis (e.g. anodic stripping voltammetry). This electrochemical transition of the indicator has a characteristic electrical fingerprint that can be measured and which, when compared to a standard, can be correlated with the concentration of the analyte in the sample. This method is suitable for the determination / monitoring of therapeutic range of drugs (anti-convulsants drugs), determination of critical and potentially dangerous levels of endogenous materials which are indicative of disease states (prostate cancer), and numerous other applications presently requiring elaborate and time-consuming clinical laboratory analysis.
Owner:INTEC SCI INC
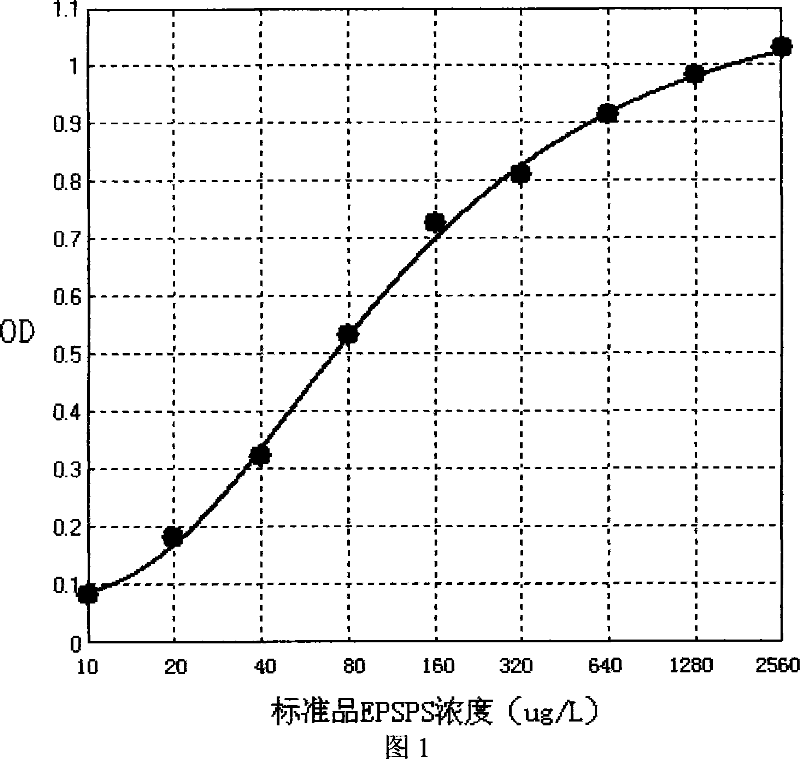
ELISA kit for detecting EPSPS gene in herbicide-tolerance soybeans and method of use thereof
This invention discloses one EPSPS gene enzyme immune agent case and its use method in the anti-herbicide bean, wherein, the case comprises anti-EPSPS multi-clone antigen, enzyme board with the antigen, enzyme antigens for two, gene switch bean standard product, non-transfer gene bean standard, intense wash liquid, develop liquid and terminal liquid. This invention method comprises the following steps: cloning CP4-EPSPS gene from the gene switch bean expressed in the bacillus coli; then through protein purification complex property to regroup EPSPS protein antibody immune animal to get special single clone antigen and multi-clone antigen; establishing double antigen clamp enzyme immune system test sample to determine its EPSPS protein content.
Owner:SOUTH CHINA AGRI UNIV
Method for rapidly, accurately and repeatedly determining foot-and-mouth disease vaccine antigen 146S
InactiveCN104634891AGood repeatabilityReduce mistakesComponent separationChromatographic separationPhosphate
The invention discloses a method for rapidly, accurately and repeatedly determining foot-and-mouth disease vaccine antigen 146S. A size exclusion high-efficiency liquid-phase chromatographic column in a molecular weight separation range of 2*10<4> to 1*10<7>Da is adopted to carry out the chromatographic separation on a detected sample on a high-efficiency liquid-phase chromatography. The operation pressure of the chromatography is 1.0MPa to 2.5MPa, the flow rate in the chromatographic column is 0.5 to 1.0 ml / min, a flow phase is phosphate buffer (pH 7.0 to 7.5) containing 0.1M sodium sulfate, and the column temperature is 15 to 25 DEG C. An ultraviolet and laser detector is used for detecting an optical signal of effluent at an outlet of the size exclusion high-efficiency liquid-phase chromatographic column, and a peak area of a sample can be analyzed by virtue of a computer software system of the high-efficiency liquid-phase chromatography. A standard curve of the absorption peak area and 146S concentration is established by virtue of a relation between the ultraviolet absorption peak and the concentration of different 146S standard products of different concentrations. Chromatograph is carried out on the detected sample through the size exclusion high-efficiency liquid-phase chromatographic column. The ultraviolet absorption peak area is measured, and the concentration of 146S in the detected sample can be acquired according to the standard curve.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Kit for performing retinol binding protein detection by using latex turbidimetry
InactiveCN102944679AHigh detection sensitivityGuaranteed SensitivityColor/spectral properties measurementsHydrogenTurbidimetry
The invention relates to the technical field of biotechnology, and particularly discloses a kit for detecting retinol binding protein content by using latex immunoturbidimetry. The kit comprises a reagent R1, a reagent R2 and a standard product, wherein the reagent R1 is buffer solution with pH (Potential of Hydrogen) value of 6-9; the reagent R2 is latex reagent coated by anti-retinol binding protein double antibodies; and the standard product is retinol binding protein solution with pH value of 5-8. According to the kit, the retinol binding protein content in a sample can be detected by using the latex immunoturbidimetry; the sensitivity is high and can reach 0.042 mg / L; and the kit has the advantages of high stability, easiness and quickness in operation, high specificity, low probability of interference, accurate quantification and broad application prospect.
Owner:BEIJING KANGMEI TIANHONG BIOTECH
Appearance color detection method for reconstituted tobacco by papermaking method
ActiveCN101482493AAvoid misjudgmentSolve the problem of inconsistent judgment resultsColor/spectral properties measurementsSpecial data processing applicationsPattern recognitionColor index
The invention discloses a paper-making method recreated tobacco leaf appearance color detection method, The sample color uniformity is presented by the absolute value of the difference between the single-piece sample measurement value and the mean value and the color difference of the sample to be tested is represented by the absolute value of the difference between the sample lightness value mean value, the mean value of the red and green value, the mean value of the yellow and blue value and the standard measurement value. If two or more color uniformity index values do not accord with the specification: Delta Ln<=2.0, Delta a n<=1.5, Delta bn <=1.5, thus the color uniformity is unqualified. If one or more color differential values do not accord with the specification: DeltaL<=2.5,Deltaa<=1.5,Deltab<=1.5, thus the color difference is unqualified. The said method can effectively prevent the misjudgement due to the human factor of the sense organ, thus the appearance color index is changed into quantified data index from qualitative description, therefore the accuracy and consistency of the judgement can be ensured.
Owner:CHINA TOBACCO YUNNAN REMFG TOBACCO CO LTD +1
Computer-implemented product valuation tool
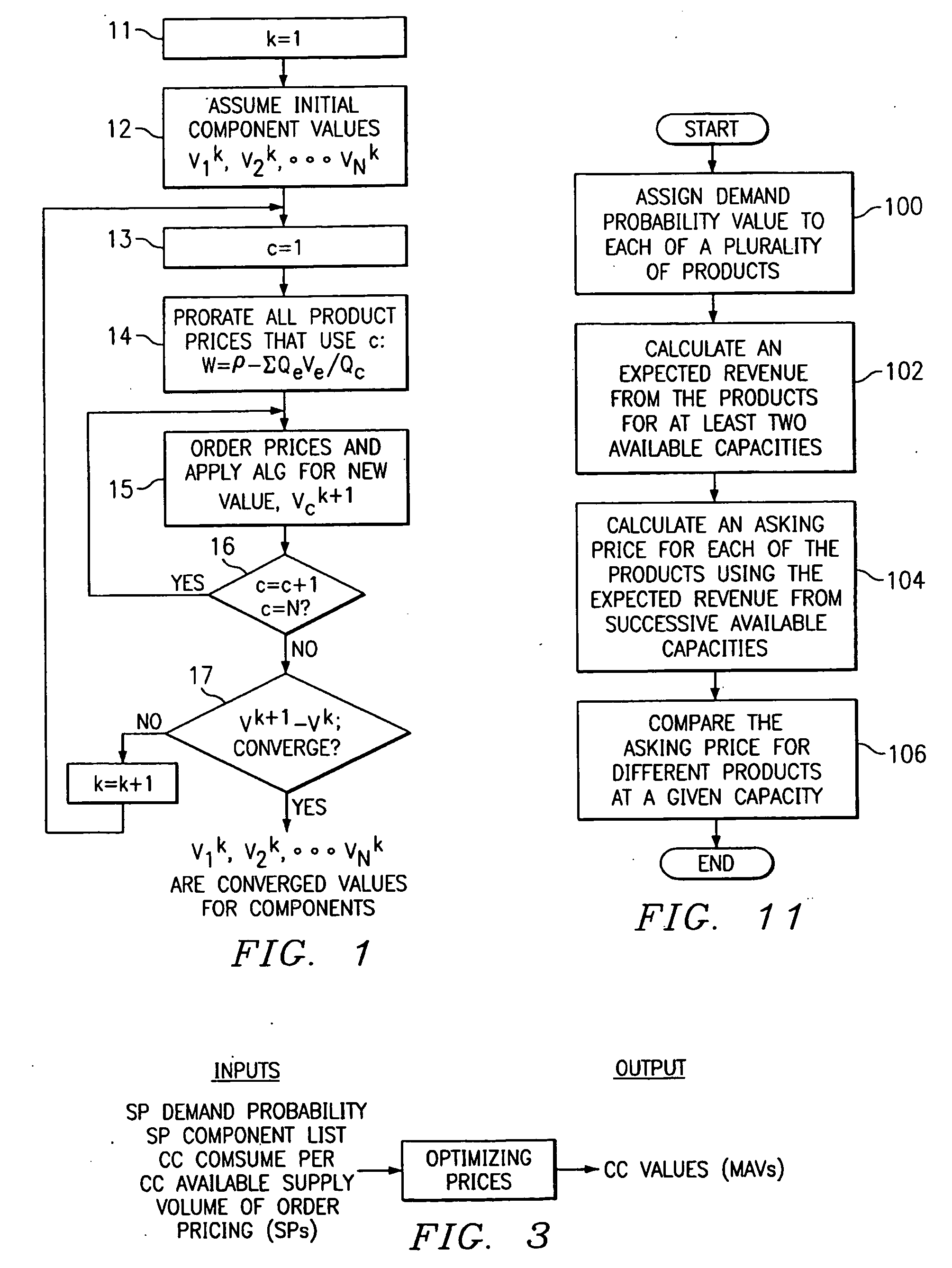
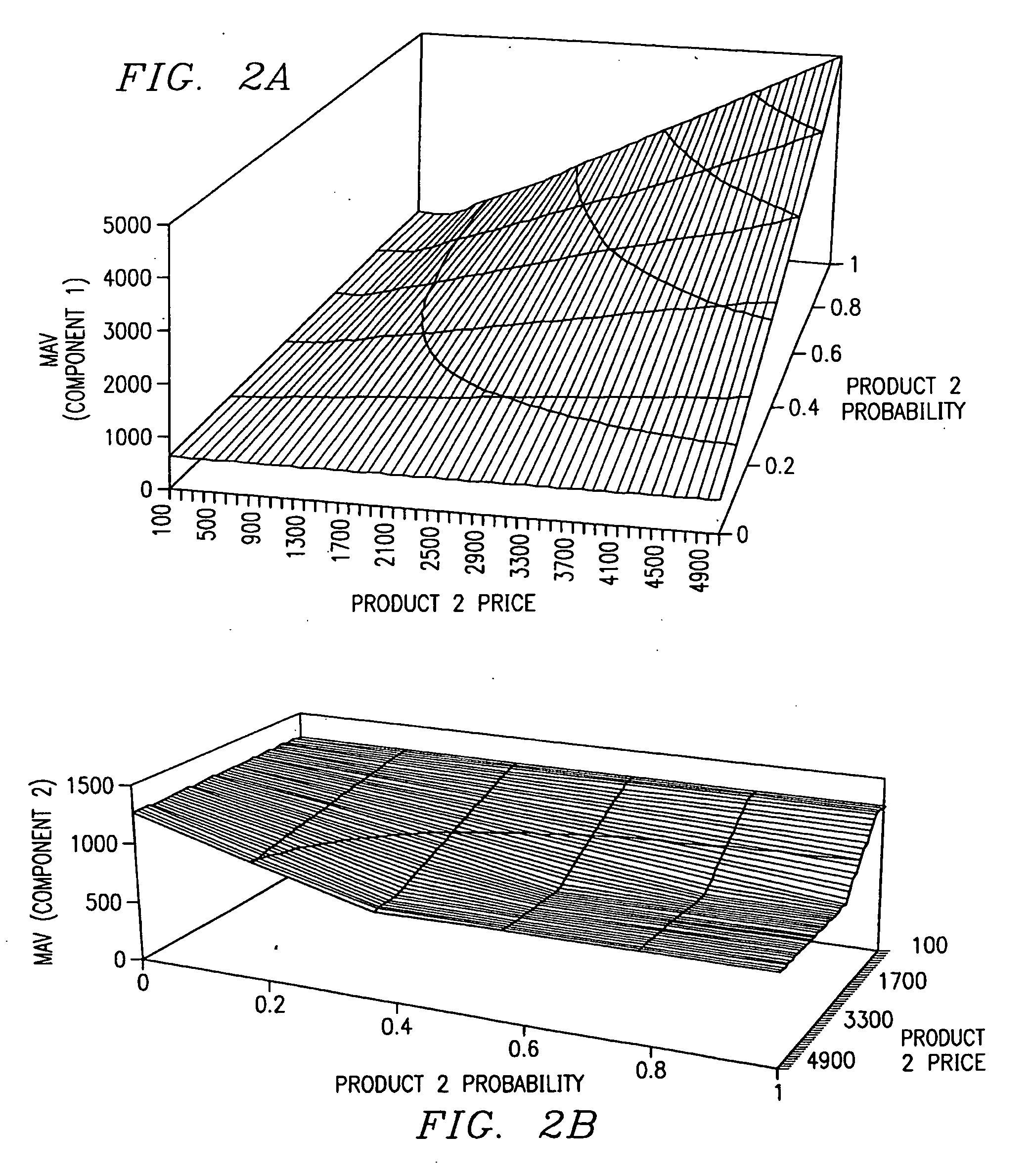
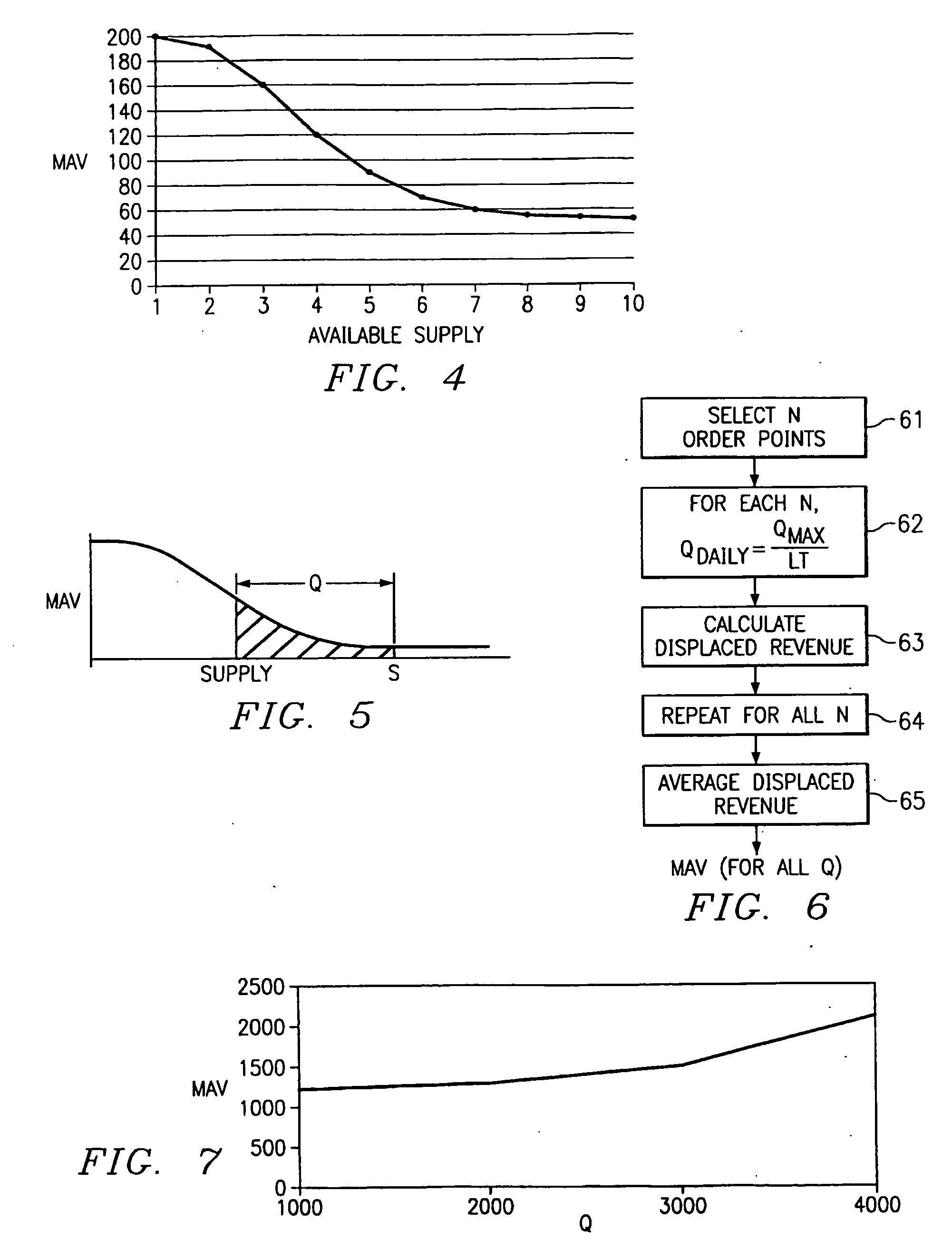
A method of valuing products based on demand probabilities. Products are designed by identifying product components, and combining the components in various combinations to provide standard and non-standard products. Components are valued using an algorithm that considers demand probability as well as known prices of standard products. The component values are added to determine product values and may be used to make pricing and order fulfillment decisions.
Owner:JDA SOFTWARE GROUP
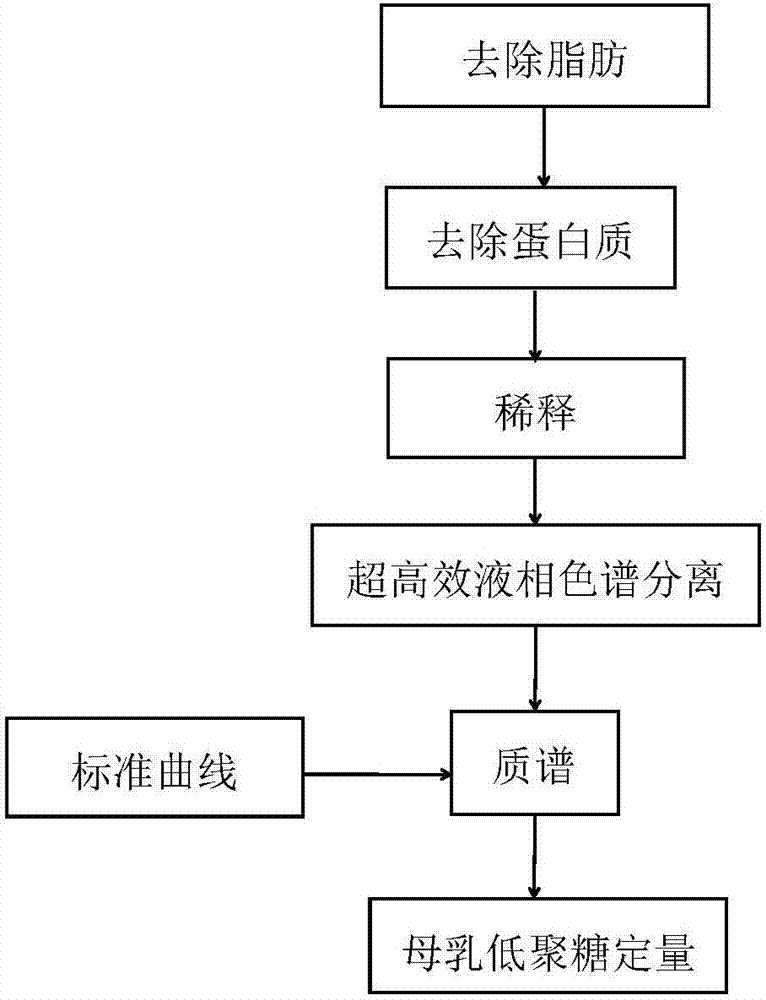
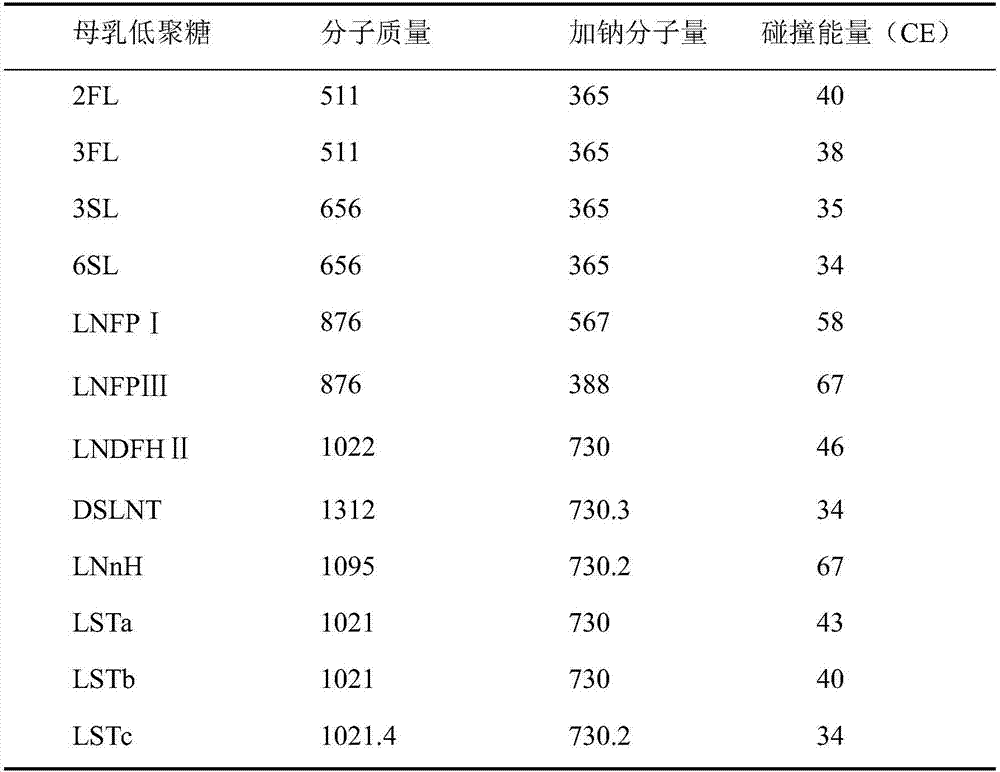
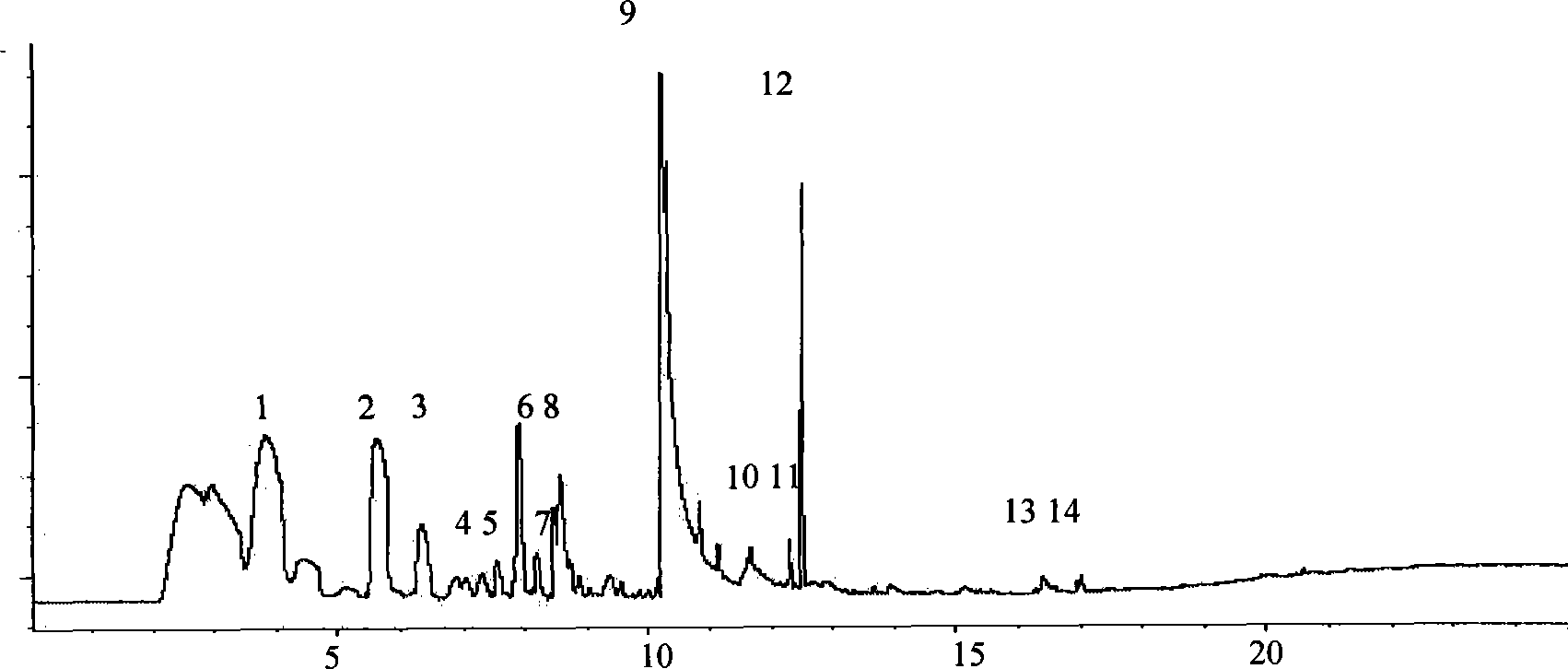
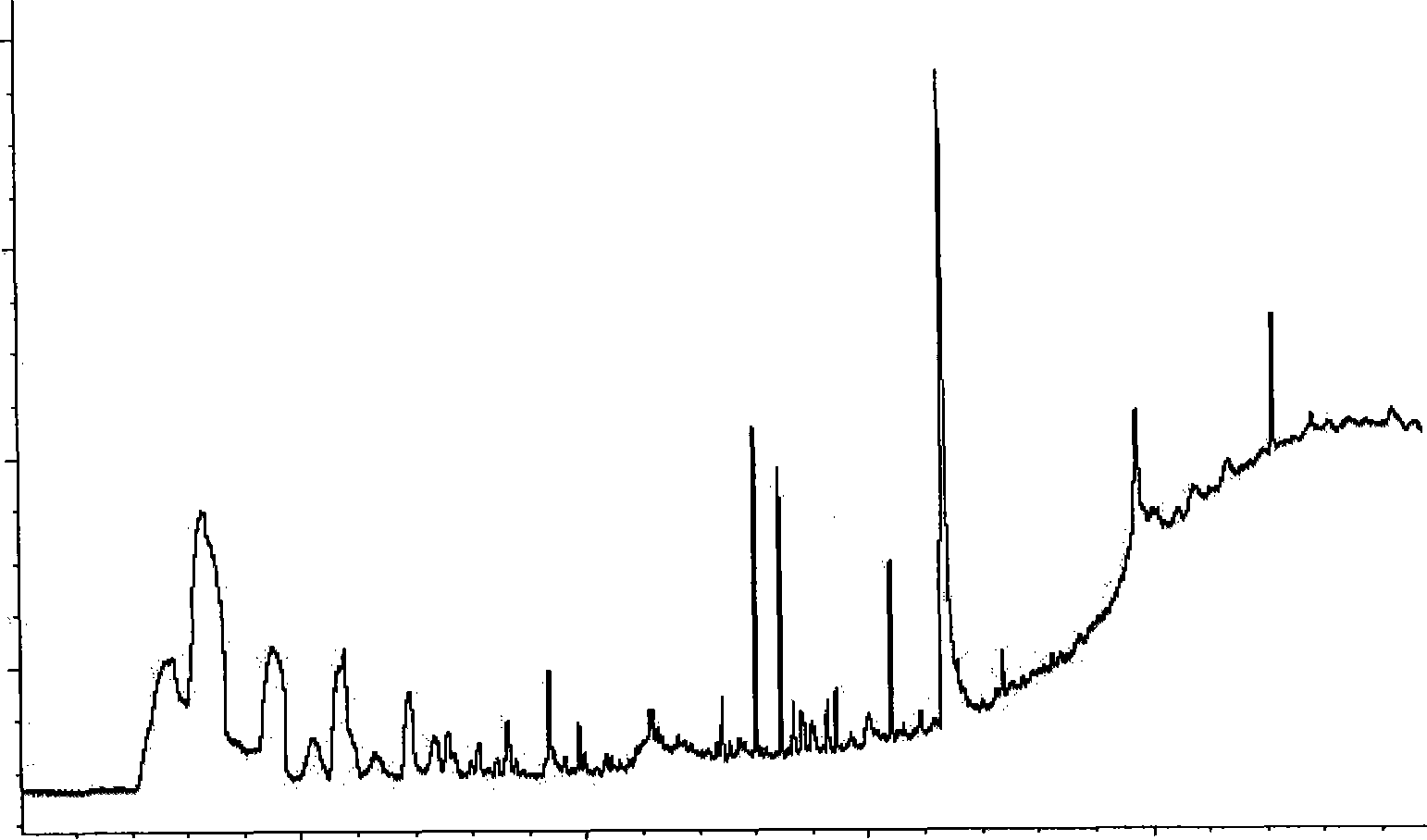
Quick qualitative and quantitative method for oligosaccharide in breast milk
ActiveCN107192771AAccurate detectionQuick checkComponent separationGradient elutionColumn temperature
The invention discloses a quick qualitative and quantitative method for oligosaccharide in breast milk. The quick qualitative and quantitative method mainly includes steps of 1, pretreating samples, to be more specific, removing fat and proteins from 150-250 micro-l of breast milk to obtain ultimate supernatant, adding ultra-pure water into the supernatant and diluting the supernatant to obtain the loaded samples; 2, establishing standard curves for standard substances by the aid of ultrahigh-performance liquid chromatography and mass spectrometry; 3, separating different components of the oligosaccharide in the breast milk in the loaded samples by the aid of ultrahigh-performance liquid mass spectrometry and carrying out quantitative analysis by the aid of mass spectrometry combined with the standard curves so as to obtain the content of the oligosaccharide in the breast milk. The ultrahigh-performance liquid chromatography is implemented by the aid of amino chromatographic columns with the sizes of 2.1*100 mm and 1.7 micrometers, 8-10 mmol / L of ammonium formate solution (A) and acetonitrile (B), and the ammonium formate solution (A) and the acetonitrile (B) are used as mobile phases; gradient elution programs are carried out by the aid of 95%-75% of B for 0-10 min or are carried out by the aid of 75% of B for 10-15 min or are carried out by the aid of 75%-65% of B for 15-20 min or are carried out by the aid of 65%-10% of B for 20-21 min or are carried out by the aid of 10% of B for 21-24 min or are carried out by the aid of 10%-95% of B for 24-25 min or are carried out by the aid of 95% of B for 25-35 min; the flow rates are 0.3 mL / min, and the column temperatures are 40-60 DEG C. The quick qualitative and quantitative method has the advantage that 12 types of oligosaccharide in the breast milk can be quickly detected by the aid of the quick qualitative and quantitative method and can be quantified by the aid of the quick qualitative and quantitative method.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
Gas-chromatography fingerprinting for rapidly identifying edible oil
InactiveCN101398412AHigh sensitivityGood precisionComponent separationTesting foodFood safetyGas liquid chromatographic
The invention provides a gas chromatographic fingerprint method for fast distinguishing edible oil, comprising the following steps of: A. establishing a fingerprinting for the volatile constituents of the edible oil: the edible oil with the same origin and brand is used as a proof sample and the gas chromatographic fingerprinting of the volatile constituents thereof is established by gas chromatographic analysis; and B. detecting samples: an edible oil sample to be detected is taken and treated with sampling with the proof sample under the same condition to obtain the fingerprinting of the detected sample, the fingerprinting of the two is taken as the qualitative base by the analysis of a direct observational method and fingerprinting software. By the observation of the methodology, the fingerprinting made by the volatile constituents of the edible oil proves that the precision, the stability and the reproducibility thereof all have better application prospect, thus being suitable for generalization in the technical field of food safety detection.
Owner:HEBEI UNIVERSITY
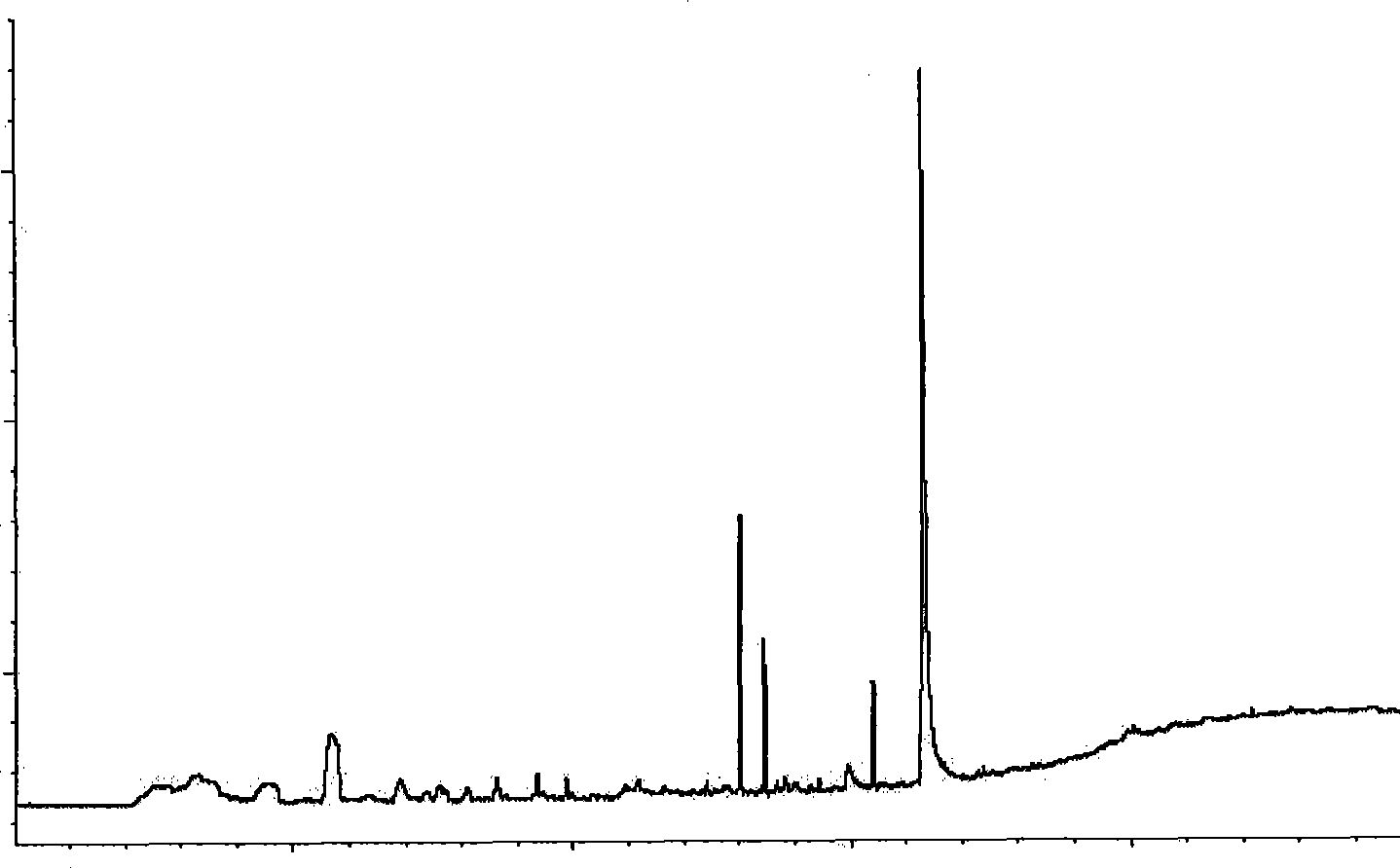
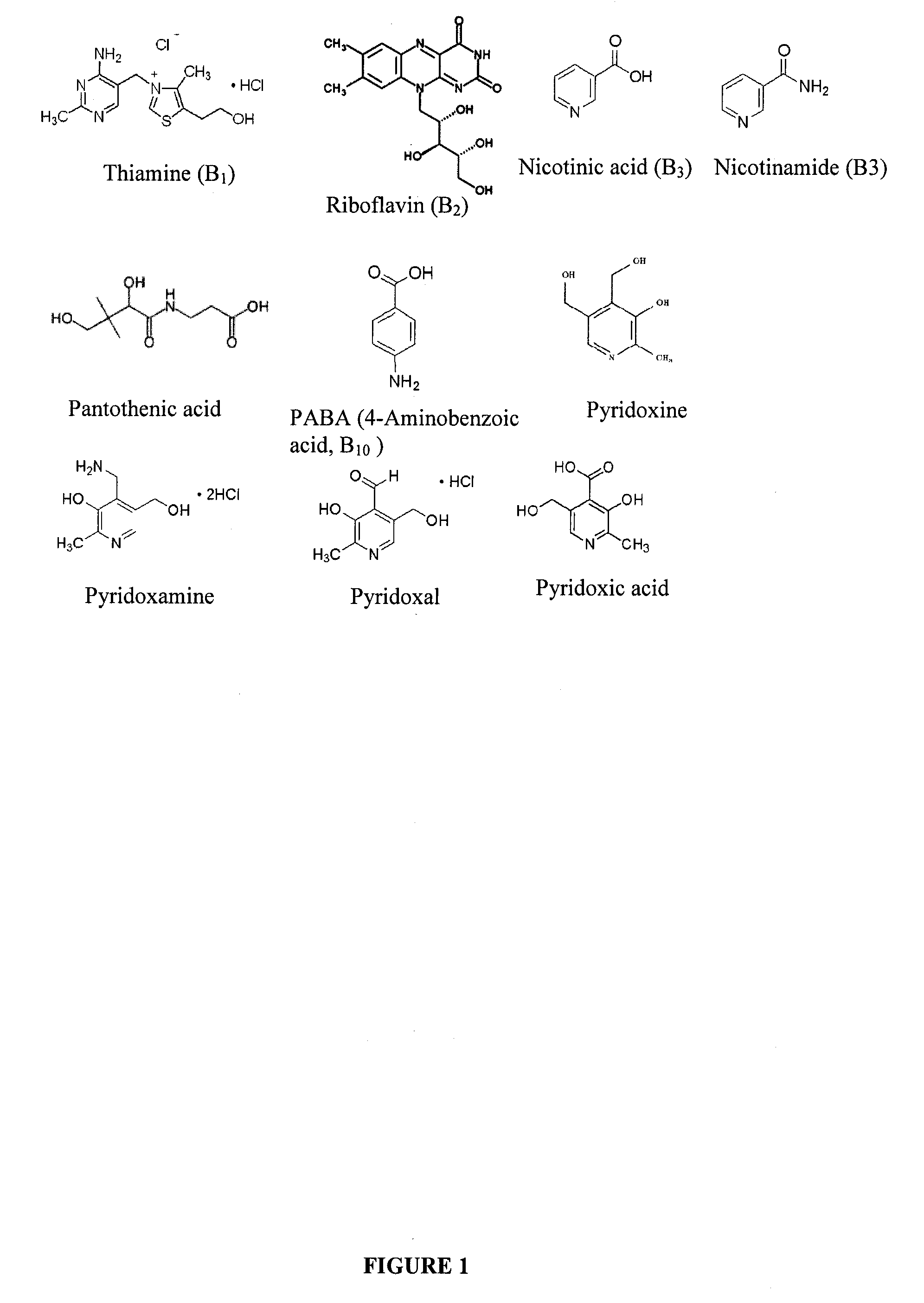
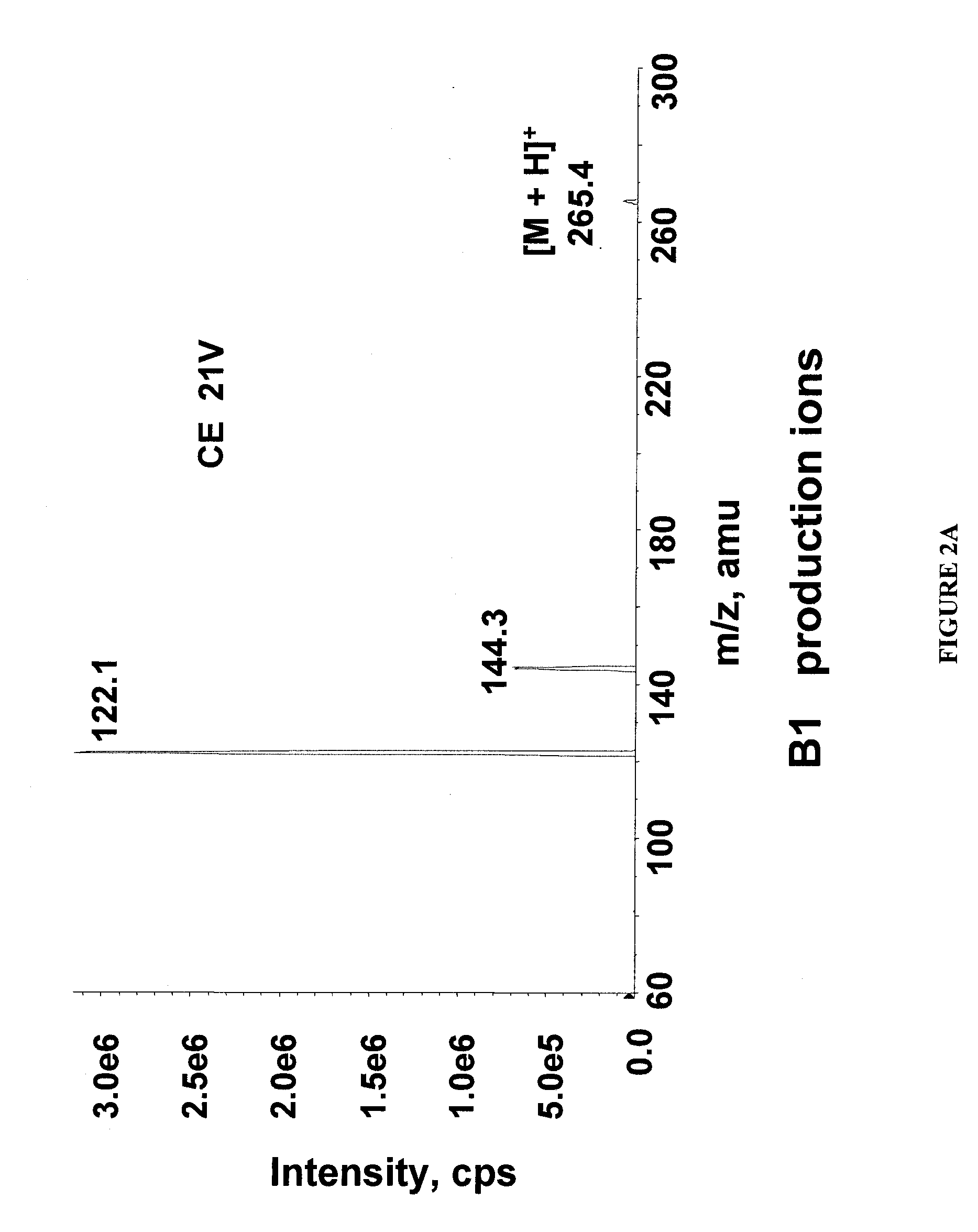
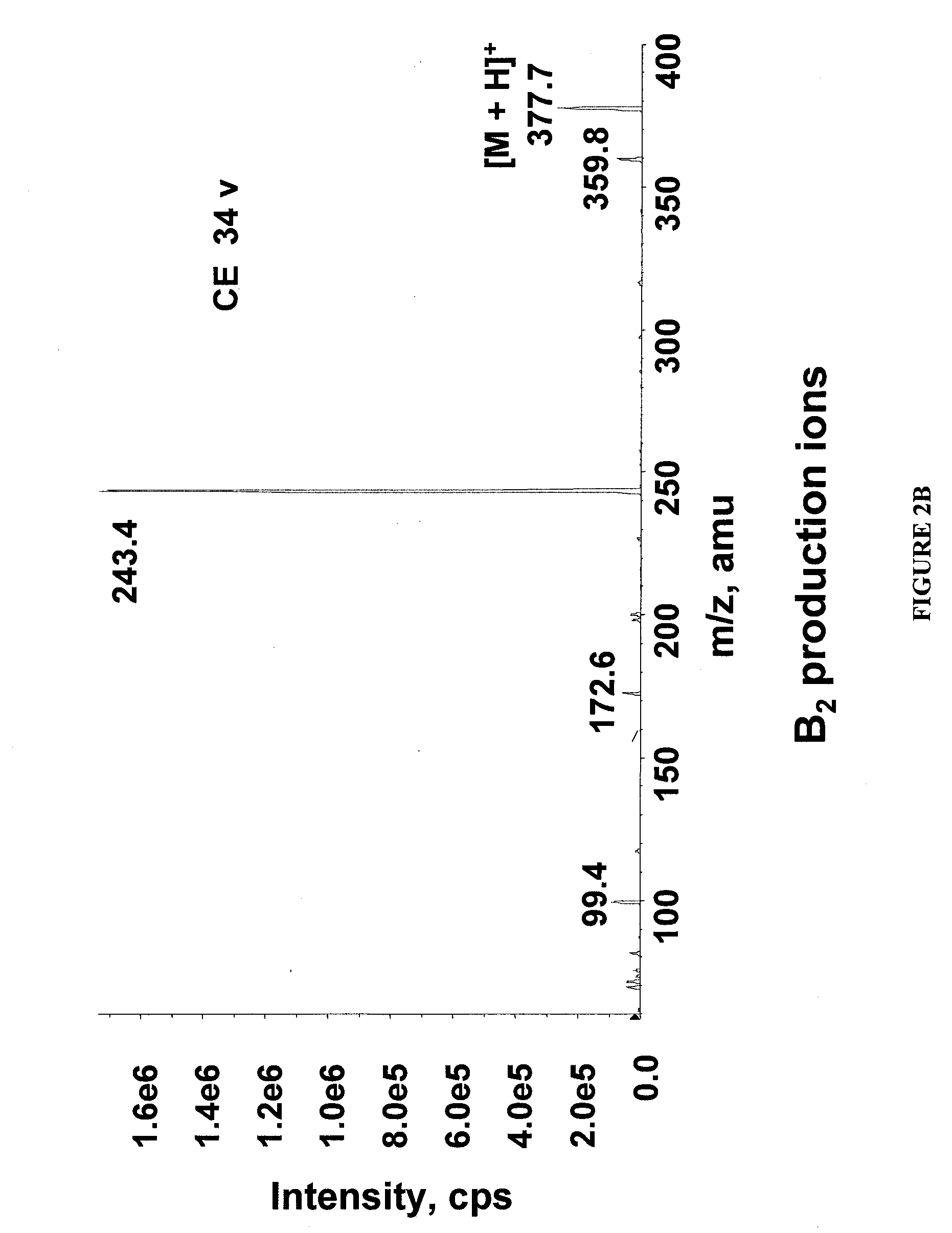
Methods for analysis of vitamins
InactiveUS20090203145A1Testing dairy productsBiological testingTest sampleMass Spectrometry-Mass Spectrometry
The invention relates to methods for determining the level of B vitamins in a test sample. The methods can include the steps of obtaining a water soluble vitamin fraction by extraction in acidic aqueous solution of a test sample, wherein the vitamin fraction comprises one or more B vitamins having at least a predetermined minimum concentration; adding a vitamin standard comprising one or more B vitamins of known quantity to a portion of the vitamin fraction of the test sample, wherein said vitamins in the vitamin standard comprise an isotope label and wherein the vitamin standard is added in an amount sufficient to determine the level of the corresponding vitamins in the test sample; and determining the level of one or more vitamins in the test sample corresponding to one or more of the vitamins in the vitamin standard using mass spectrometry.
Owner:COVANCE LAB
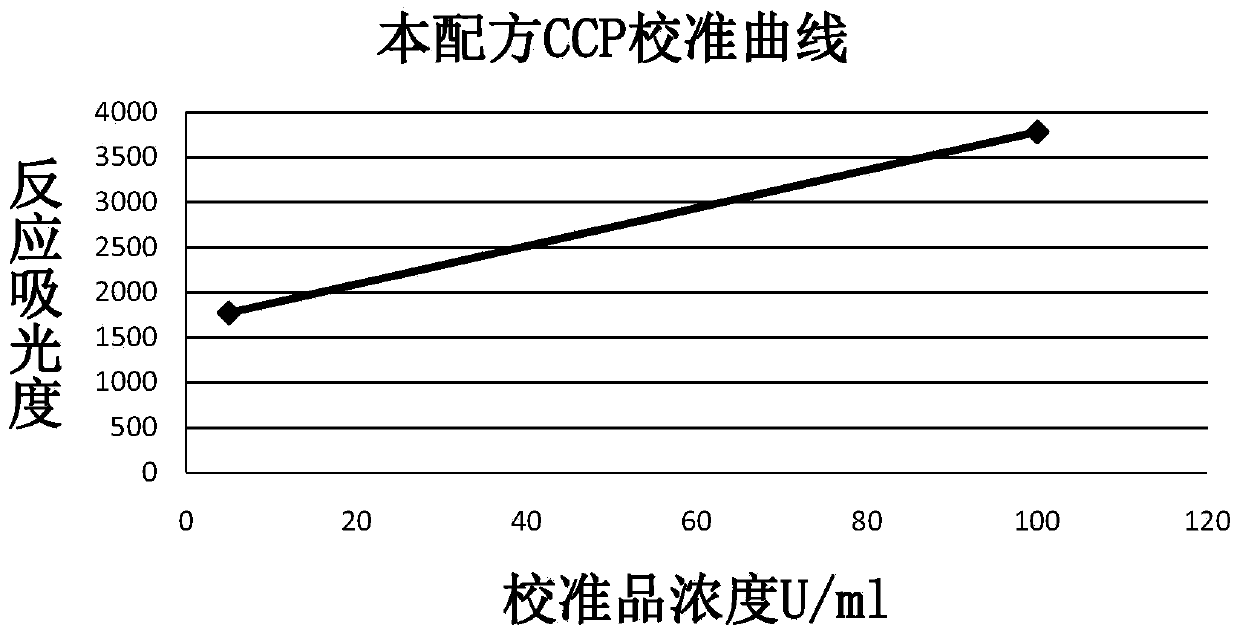
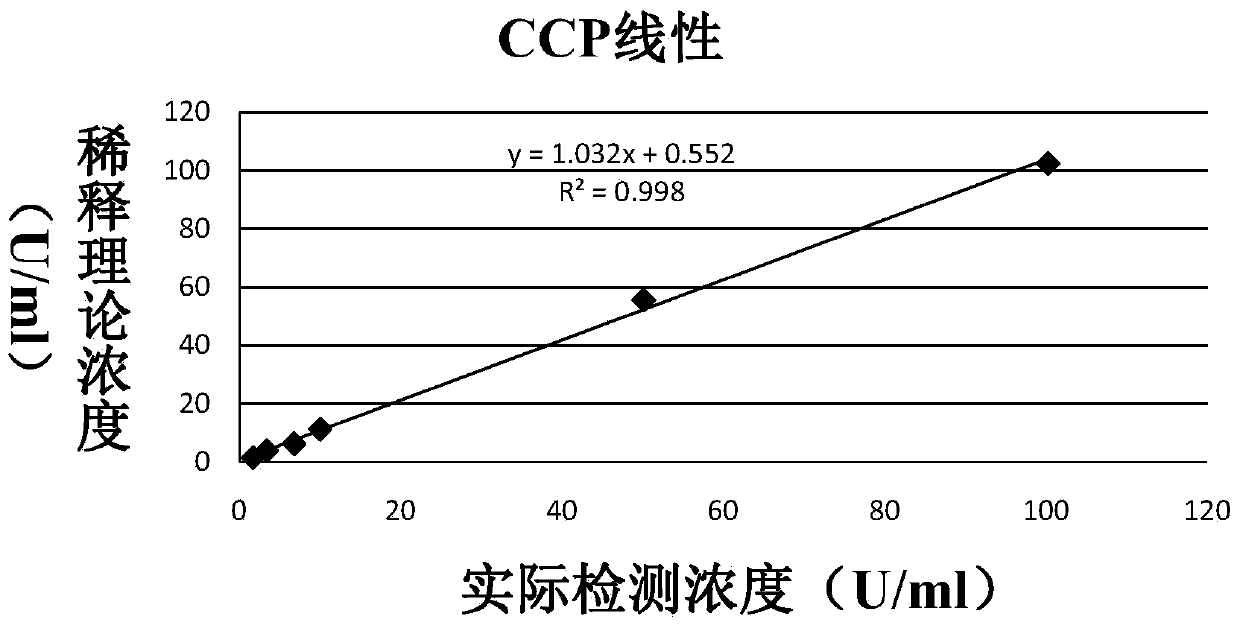
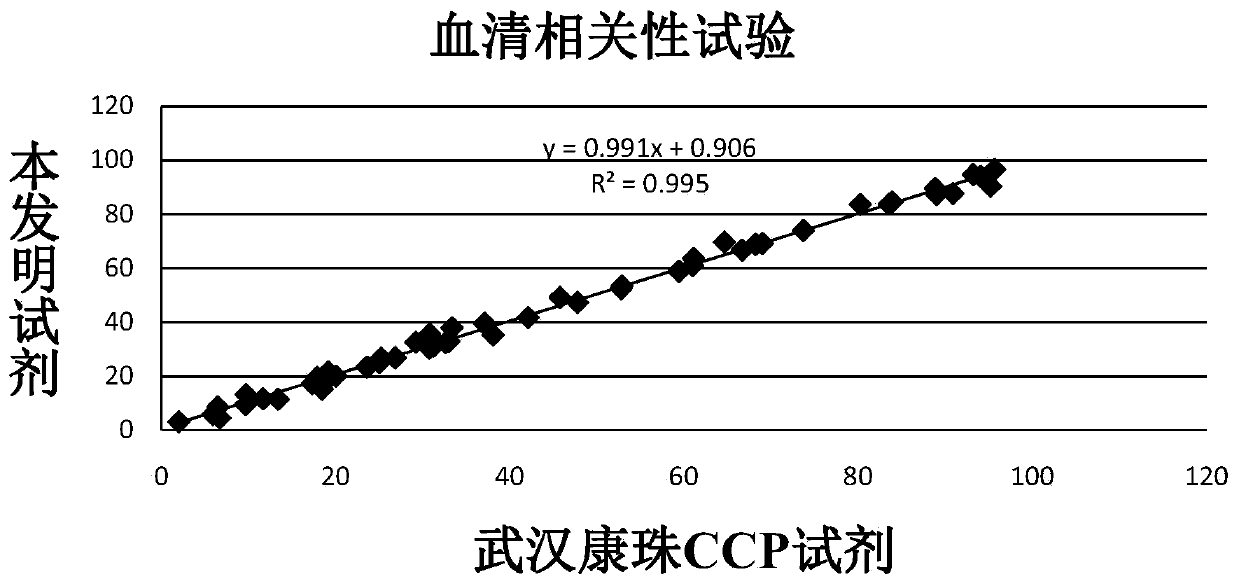
Anti-cyclic citrullinated peptide (CCP) antibody detection kit
ActiveCN104198725AHigh refractive indexHigh chemical inertnessBiological testingFluorescence/phosphorescenceAnti ccp antibodiesMicrosphere
The invention discloses an anti-cyclic citrullinated peptide (CCP) antibody detection kit. The kit comprises a reagent R1, a reagent R2, a standard substance and a standard diluent, wherein the reagent R1 is prepared by washing CCP antigen latex particles by 50mM Tris buffer solution containing 20-500mmol / L first stabilizer and 0.1-1% preservative and then dispersing; and CCP antigens and polystyrene latex microspheres are subjected to chemical crosslinking and then are closed, so that the CCP antigen latex particles are formed. For the kit, the latex-enhanced immunoturbidimetry is adopted for detecting the content of an anti-CCP antibody, the kit has the high sensitivity, the high stability and the high detection speed (the time from determination to result acquisition is only within 5-10min), can detect mass samples on a conventional biochemical analyzer and can greatly improve the detection efficiency.
Owner:上海睿康生物科技有限公司
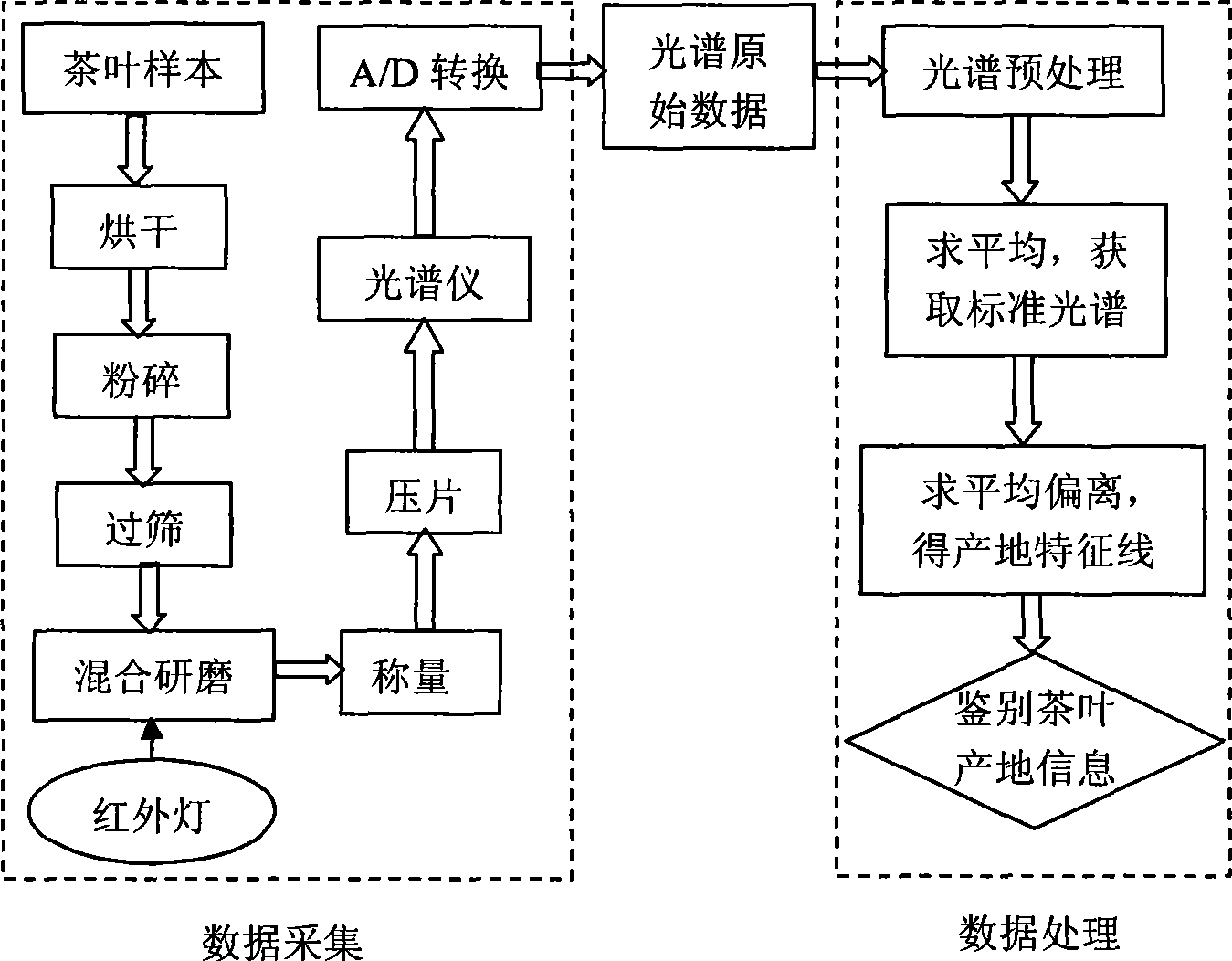
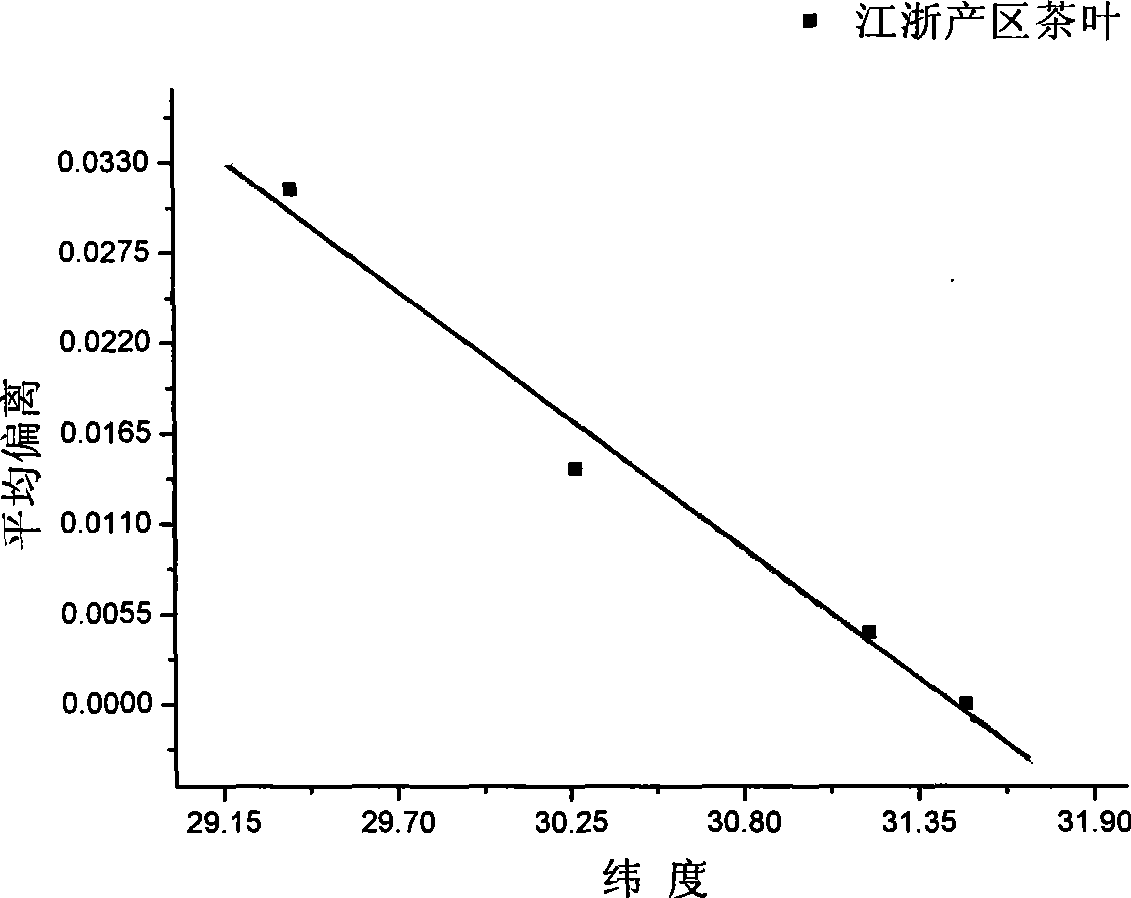
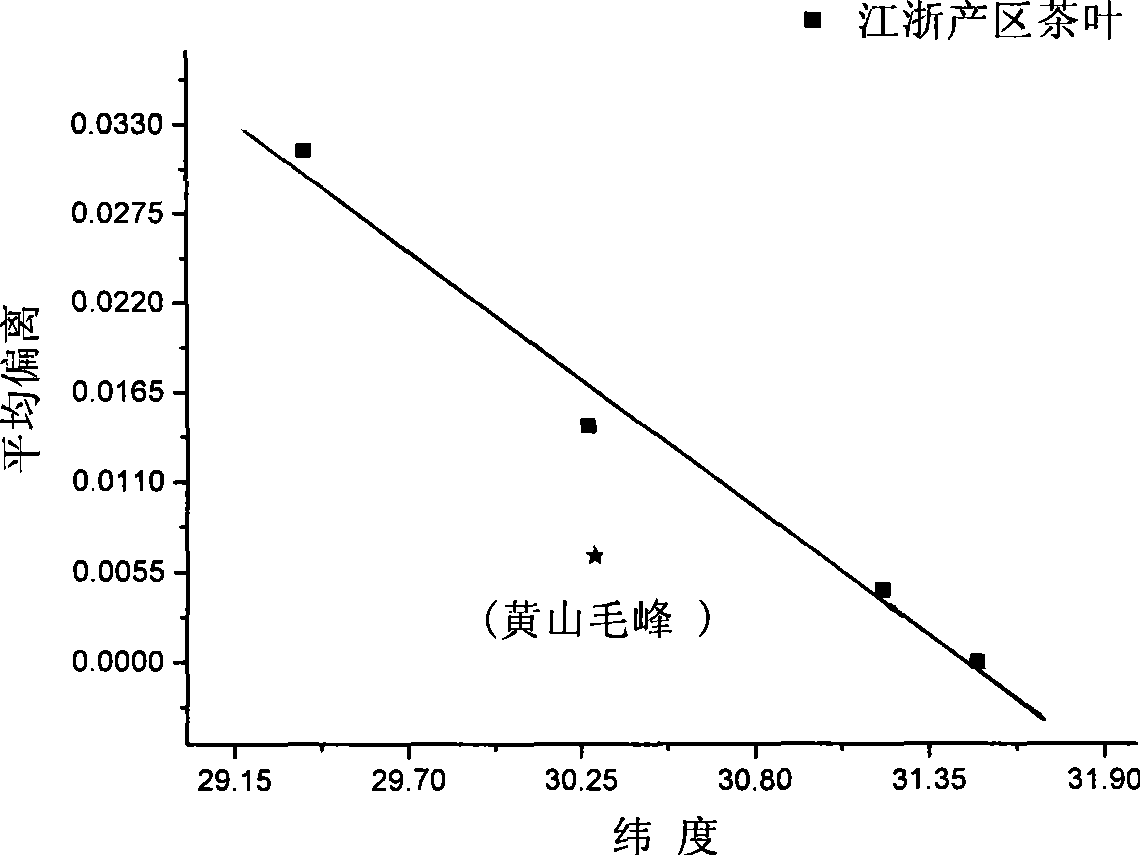
Method for identifying tea-leaf origin by infrared spectrum
InactiveCN101413883AObjective evaluation criteriaNo pollutionColor/spectral properties measurementsInfraredSimilarity theory
The invention provides a method for identifying production place of tea by using infrared spectrum technology, which comprises the following steps: production place typical lines of tea in place of origin are constructed; B. detecting sample: tea samples to be detected are taken and detected under the same condition with the standard sample respectively to obtain standard mid-infrared spectrums, average deviations between the tea samples and reference tea in production place are calculated according to the spectrums, and difference values between the average deviations and the production place typical lines are calculated, if the difference value is within standard deviation range, the tea sample belongs to the production place, otherwise, the tea sample does not belong to the production place. The method has the following advantages: 1. the analyzing result is not affected by subjective consciousness, thereby providing an impersonal evaluation criterion; 2. according to similarity theory, the average deviation can represent the difference between infrared spectrums of tea more accurately; 3. component separation, extraction and the like on tea are not needed before detecting infrared spectrum of the tea, which simplifies the operation, greatly improves the analyzing speed and reduces the detecting cost without environment pollution.
Owner:HEBEI UNIVERSITY
Method for measuring vitamins A, D and E in compound vitamin
InactiveCN102253130AEfficient separationQuality Implementation ControlComponent separationColor/spectral properties measurementsRetention timePeak area
The invention discloses a method for measuring vitamins A, D and E in a compound vitamin. The method comprises the following steps: (1) preparing solution of a sample to be measured; (2) preparing standard substance solution; (3) taking the solution of the sample to be measured, separating the solution prepared in the step (2) with a Waters reversed phase C18 chromatographic column and detecting the solution with a diode array detector to obtain a corresponding chromatogram and qualitatively deciding various vitamins in the solution of the sample to be measured according to the retention time of various vitamins in the standard substance solution; (4) respectively obtaining the mass concentrations of various vitamins in the solution of the sample to be measured via the proportional relationships of the peak areas of various vitamins in the chromatogram according to the concentrations of various vitamins in the standard substance solution; and (5) computing the contents of the vitamins in the solution of the sample to be measured through a formula according to the concentrations. The method has the following advantages: the method has the characteristic of simple, convenient, rapid and accurate measurement; and the analysis time is shortened and the organic reagent consumption is reduced while control of the raw materials of the health food is implemented.
Owner:TIANJIN TIANSHI BIOLOGICAL DEV +2
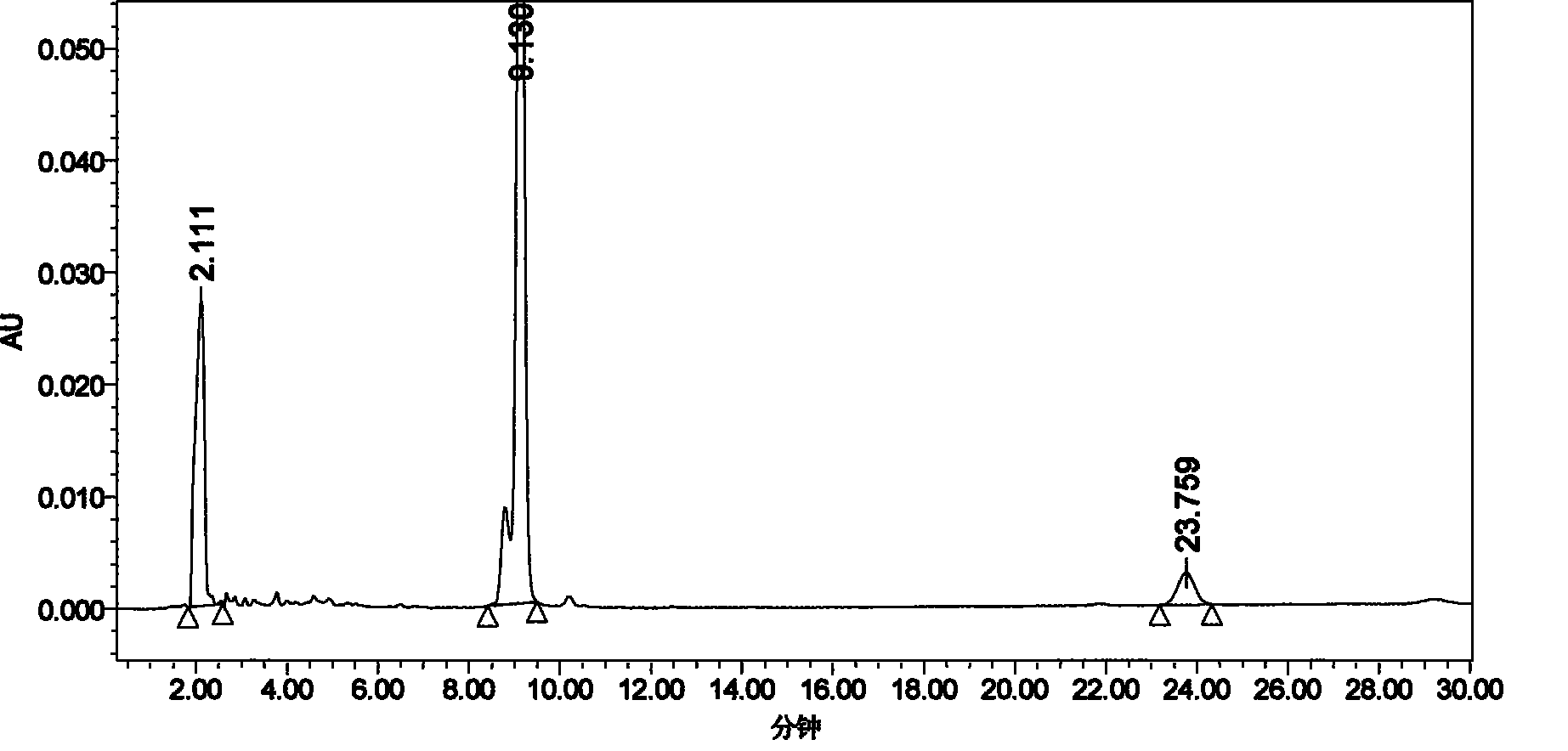
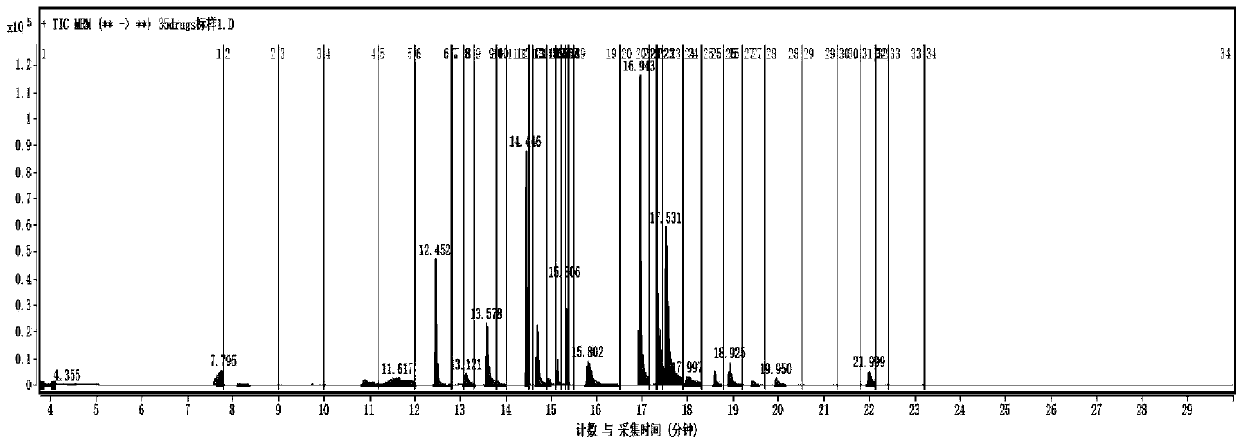
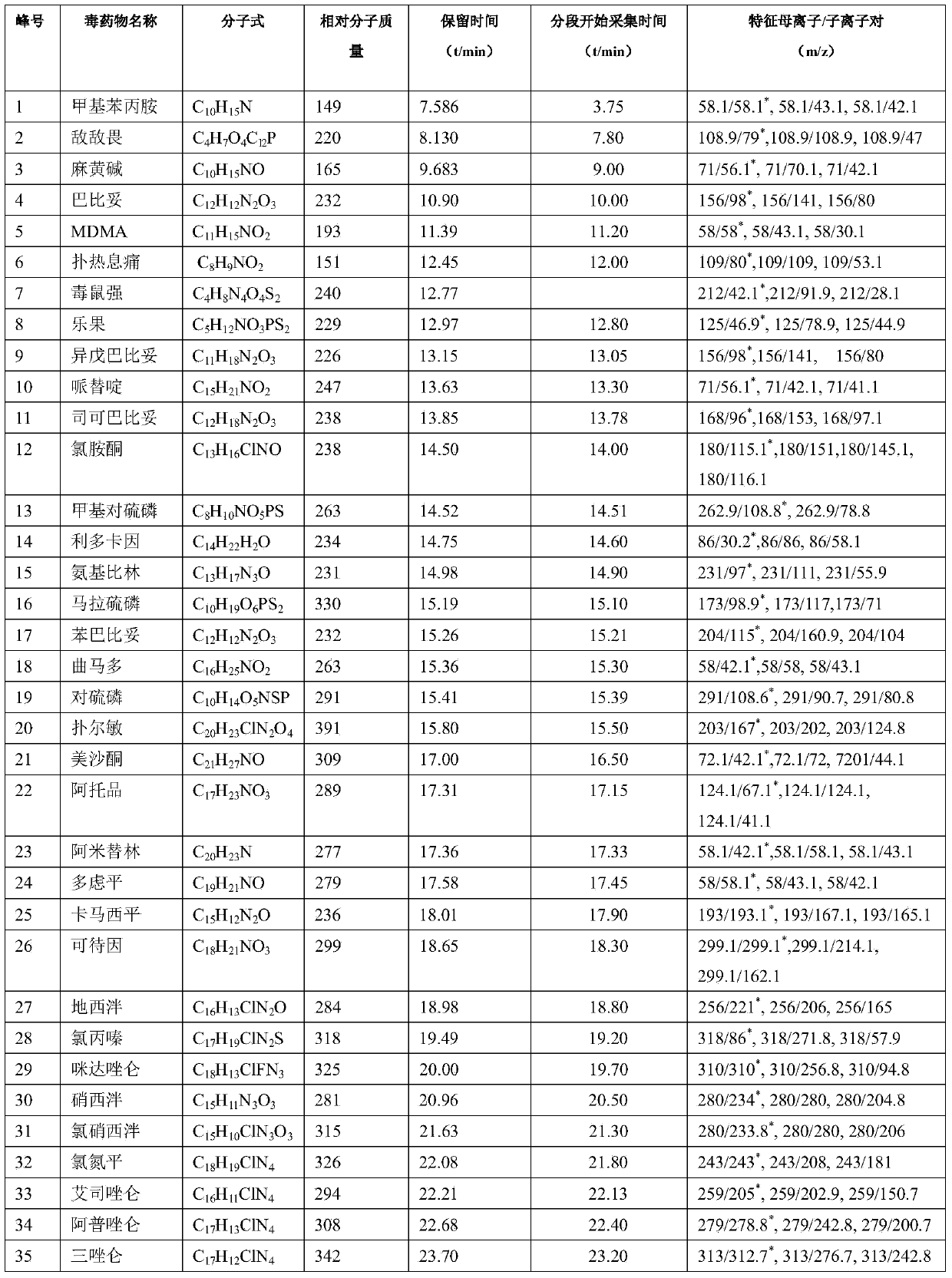
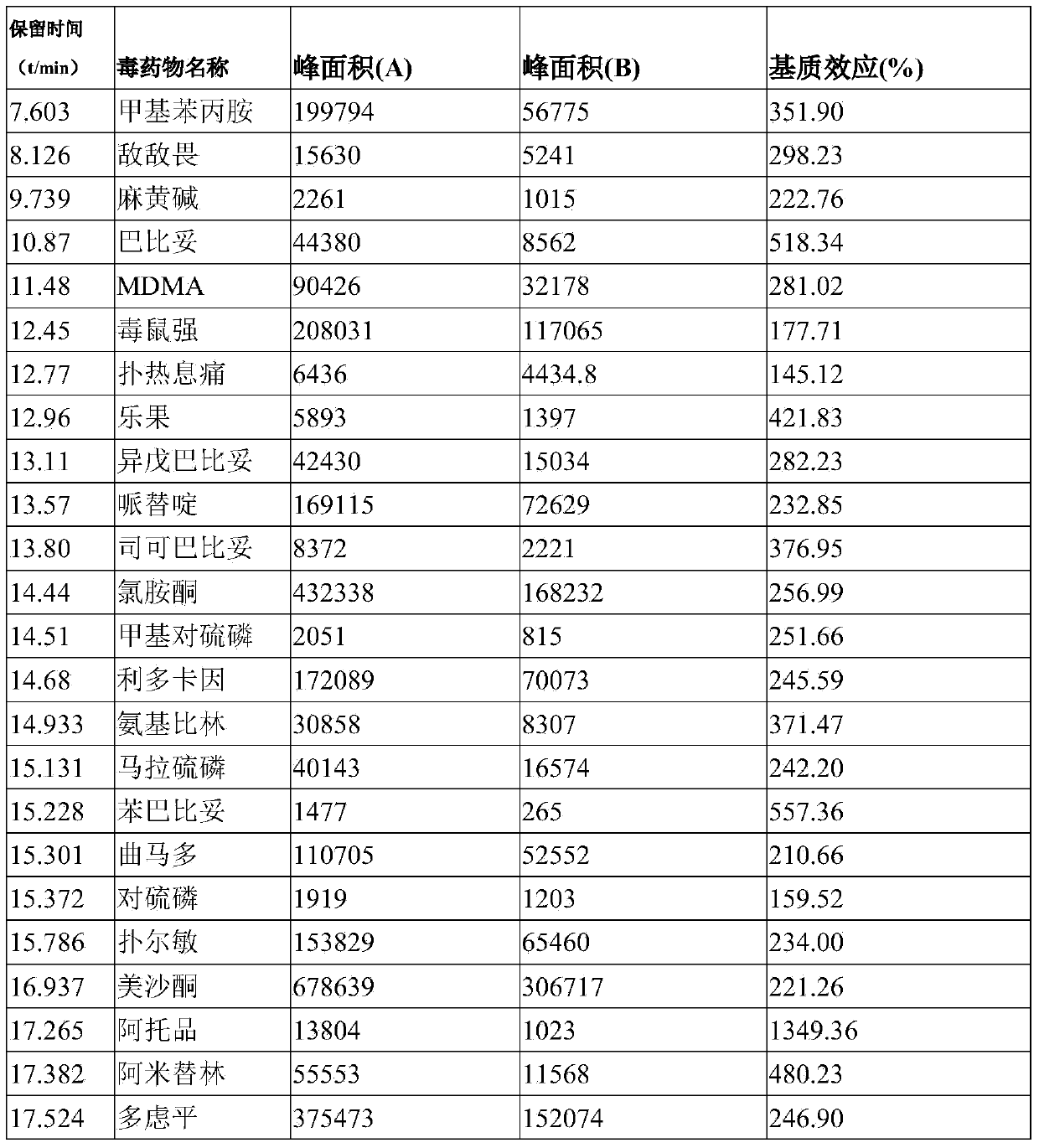
Series quadrupole-rod gas-chromatographic mass spectrometry detection method for 35 toxic medicaments in urine
InactiveCN103808846AAccurate Qualitative and Quantitative AnalysisEliminate distractionsComponent separationGas phaseRetention time
The invention discloses a series quadrupole-rod gas-chromatographic mass spectrometry detection method for 35 toxic medicaments in urine. The series quadrupole-rod gas-chromatographic mass spectrometry detection method comprises the steps of constructing a multiple-reaction detection method on a series quadrupole-rod gas chromatograph-mass spectrometer through standard substances of 35 toxicants (medicines), and determining 2-3 pairs of feature parent ions and daughter ions and the retention time of each toxicants (medicines); extracting a urine sample to be detected through diethyl ether, performing ultrasonic and centrifugal treatment, blow-drying and dissolving, and then qualitatively and quantitatively analyzing the toxicants (medicines) through a tandem mass spectrum MRM. By the adoption of a series quadrupole-rod gas-chromatographic-mass spectrometry / mass spectrometry method (GC-MS / MS), the method disclosed by the invention can quickly screen 35 conventional toxicants (medicines) in the urine of a human body; a detection result is accurate, sensitive and quick; the recycling rate of toxicants (medicines) is 80.82-118.56 percent; the detection limit is up to 0.0014-1.8 micrograms per microliter.
Owner:FUJIAN INT TRAVEL HEALTH CARE CENT
Anti-fake detection method, device and system
InactiveCN103646333AImprove accuracyImprove detection efficiencyCo-operative working arrangementsCommerceFiberRelevant information
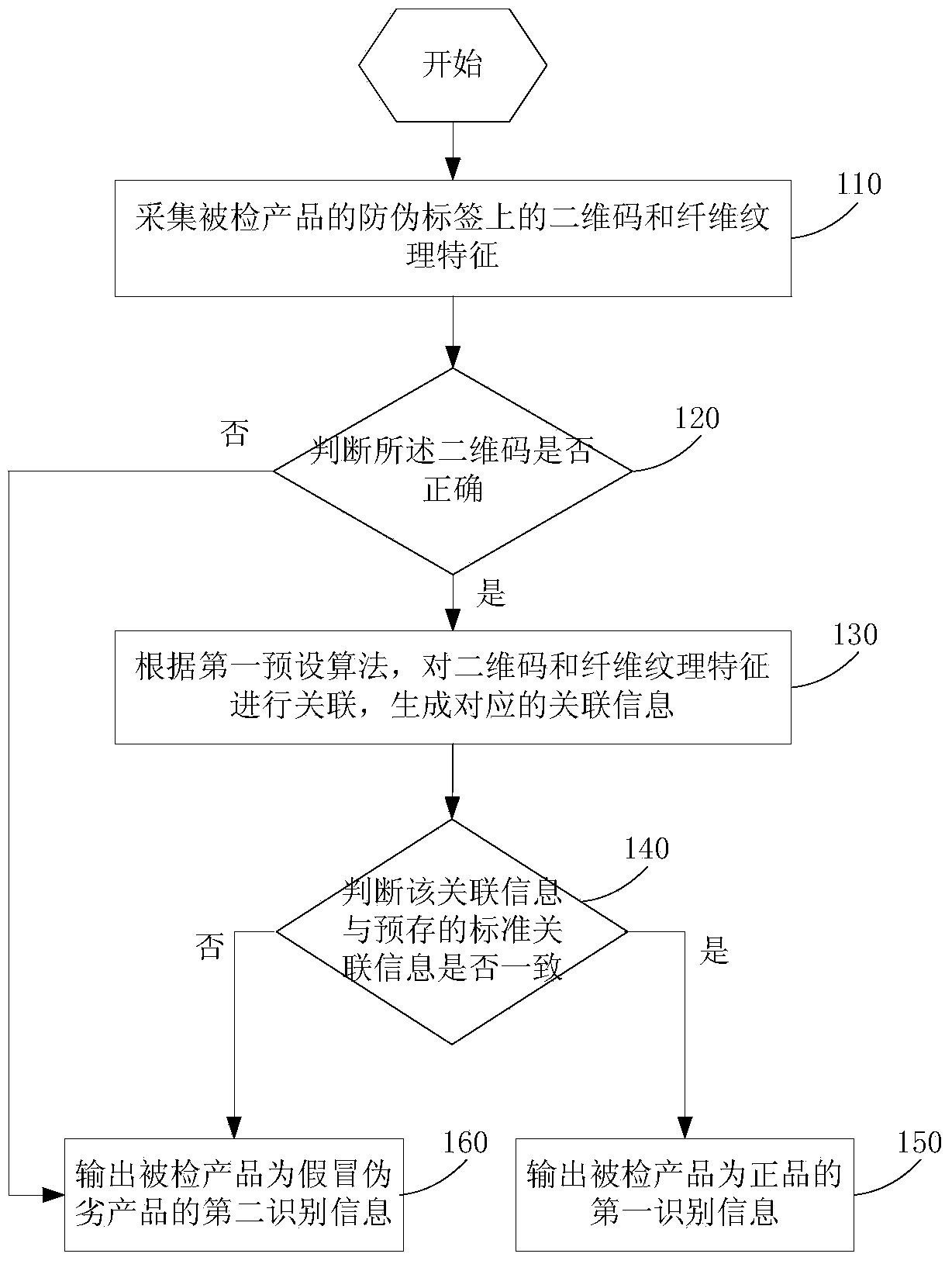
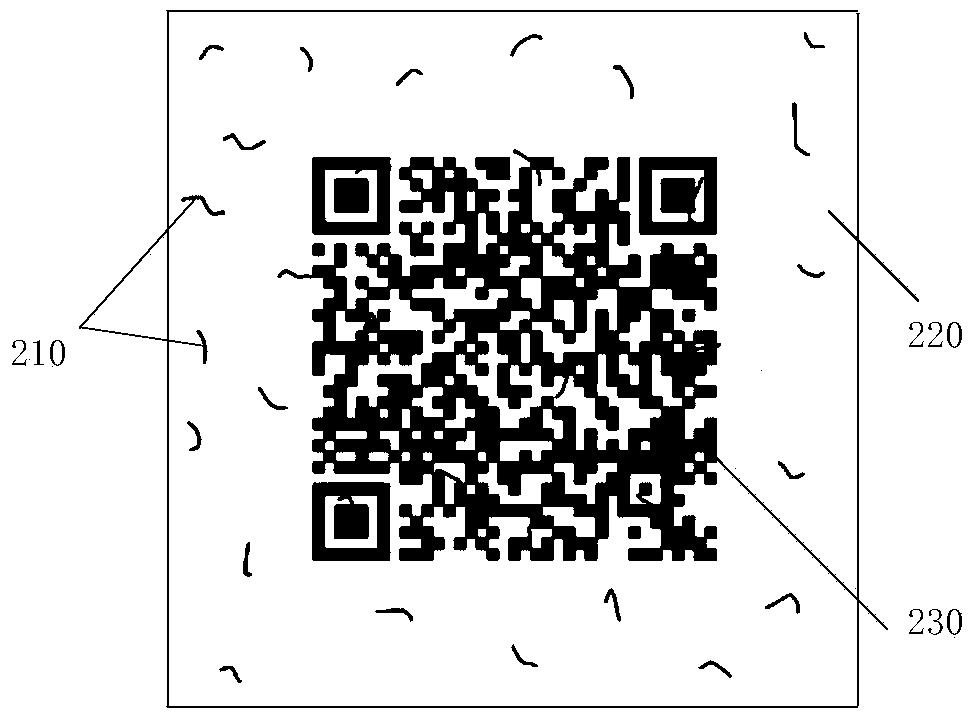
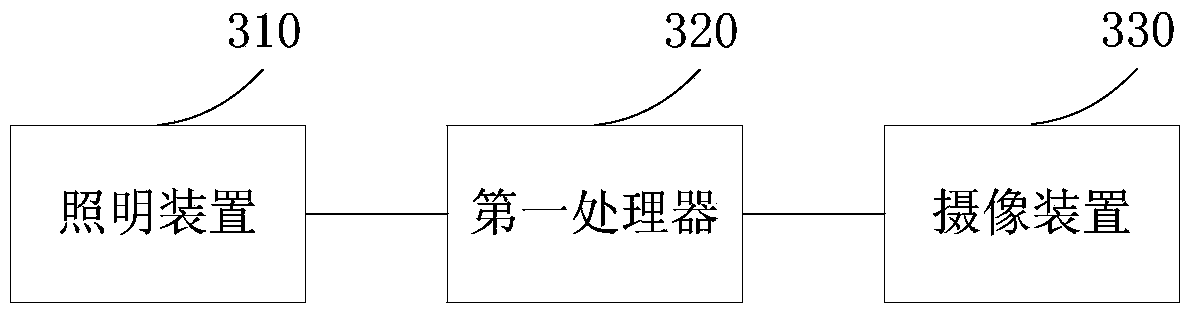
The invention provides an anti-fake detection method, device and system. Due to the fact that a two-dimensional code of an anti-fake label of a collected detected product contains relevant information of the detected product and is an identity identifier of the product, whether the product is a fake product can be identified by judging whether the two-dimensional code is correct. When the two-dimensional code is determined to be correct and correlation information between the two-dimensional code and fiber texture is consistent with standard correlation information, first identifying information that the detected product is a salable product is output; otherwise, second identifying information that the detected product is the fake product is output. Anti-fake detection accuracy is greatly improved, the anti-fake detection process is simplified, and detection working efficiency is improved. In addition, due to the fact that the two-dimensional code is printed on fragile label paper containing anti-fake fiber, the fact that the anti-fake label is transferred to the fake product is avoided, and the rights and the interests of consumers and standard product manufacturers are further guaranteed.
Owner:BEIJING VISION BRILLIANCE TECH CO LTD
Targeted metabo lomics analysis method for determining metabolites of living body
InactiveCN105301163AMeet the needs of analysisOvercoming repetitivenessComponent separationMaterial analysis by electric/magnetic meansQuadrupolePathway enrichment
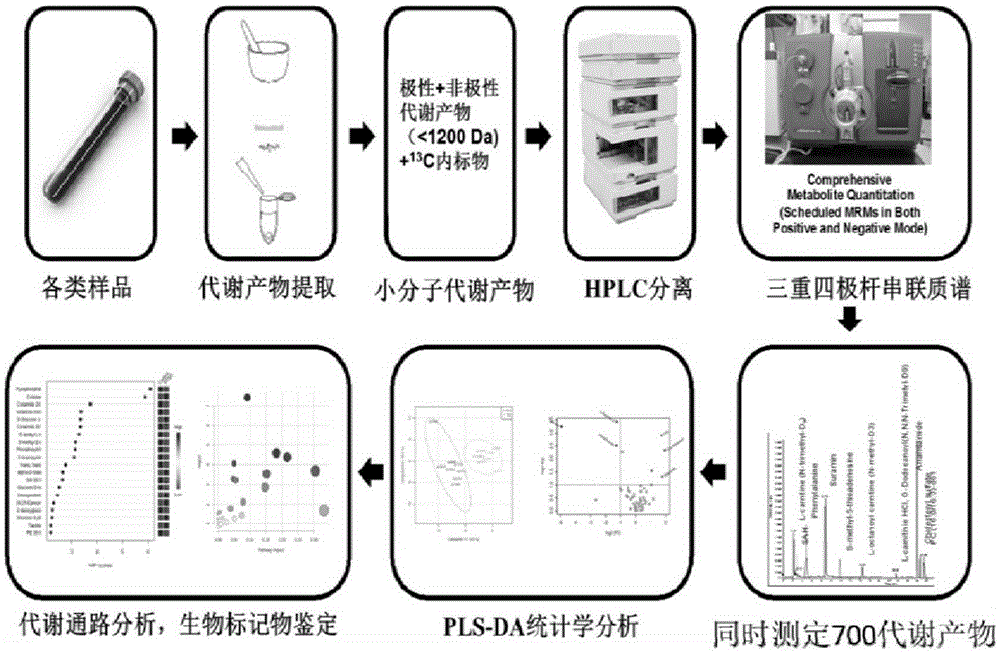
The invention provides a targeted metabo lomics analysis method for determining metabolites of a living body. The targeted metabo lomics analysis method can simultaneously determine 700 main metabolites of a living body based on series connection of ultra-high performance liquid chromatography and triple quadrupole and comprises the following steps: firstly, 700 main metabolites in a living body are screened; standard products of compounds are optimized to obtain optimal parent ions, daughter ions and mass spectrography conditions of the metabolites; the metabolites in samples are extracted by methanol, acetonitrile and water; liquid chromatograph conditions are optimized; a hydrophilic interaction is adopted to separate the metabolites; the separated metabolites enters a mass spectrum; the 700 main metabolites are determined at the same time by use of a real-time scheduled multi-reaction monitoring mode with quick positive and negative polarity switching function; the metabolite differences between all the samples are analyzed via partial least square analysis; changes of the metabolic pathway are found by pathway enrichment analysis. The targeted metabo lomics analysis method remarkably increases the number of detected compounds, and greatly shortens the analysis time.
Owner:TIANJIN SUNNYPEAK BIOTECH CO LTD
Method for simultaneously detecting forty types of amino acids in dried blood spots, blood and urine
InactiveCN108333268AAvoid distortionRealize real-time monitoringComponent separationThree levelChromatographic separation
The invention discloses a method for simultaneously detecting forty types of amino acids in dried blood spots, blood and urine. The method comprises the following steps of firstly, pretreating a biological sample by a simple liquid and liquid extracting method; performing chromatographic separation and mass spectrometric detection; using the relative retention time and qualitative ions as the qualitative basis for each type of amino acid, and using a standard product to manufacture a standard curve for quantifying. The method has the advantages that the accuracy and validity of the method shall be studied by three levels of quality control products, so as to avoid the distortion of detection results; the forty types of non-derived amino acids in one sample can be simultaneously detected bya HPLC-MS / MS (high performance liquid chromatography-mass spectrograph) technique for the first time; the operation is simple, convenient and rapid, the flux is high, and the cost is low; the level of amino acids in a human body can be effectively monitored in real time; the enough basis is provided for the clinical disease diagnosis; the method is suitable for being clinically popularized and applied.
Owner:JINAN YING SHENG BIOTECH
Simulated dose calibrator source standard for positron emission tomography radionuclides
ActiveUS20090194677A1Accurate activityCalibration apparatusPortable shielded containersEpoxyPositron emitters
A method for calibration and a calibrator source standard calibrated by the method are provided. The calibration method includes providing mock syringes, or other simulated dose container. A first of the mock syringes is filled with a short half life positron emitter. A second of the mock syringes is filled with a longer half life radionuclide set in a matrix material such as an epoxy. The activities of the two syringes can be determined, ideally in the same ion chamber, for example, against a radioactive source standard having a half life greater than the first and second radionuclides. This allows a conversion factor to be determined which can be used for a calibrator source standard formed as for the second mock syringe (i.e., with the same type of container containing the longer half life radionuclide set in a matrix material), when the calibrator source standard is used as a proxy for calibrating a calibrator for use in determining the activity of a dose container of the same configuration containing a dose of the short lived radionuclide.
Owner:RADQUAL
Geological calamity emergency monitoring, predicting and analyzing method
InactiveCN101477206ARich forecasting toolsPenetratingSeismologyRadio wave reradiation/reflectionRadarPredictability
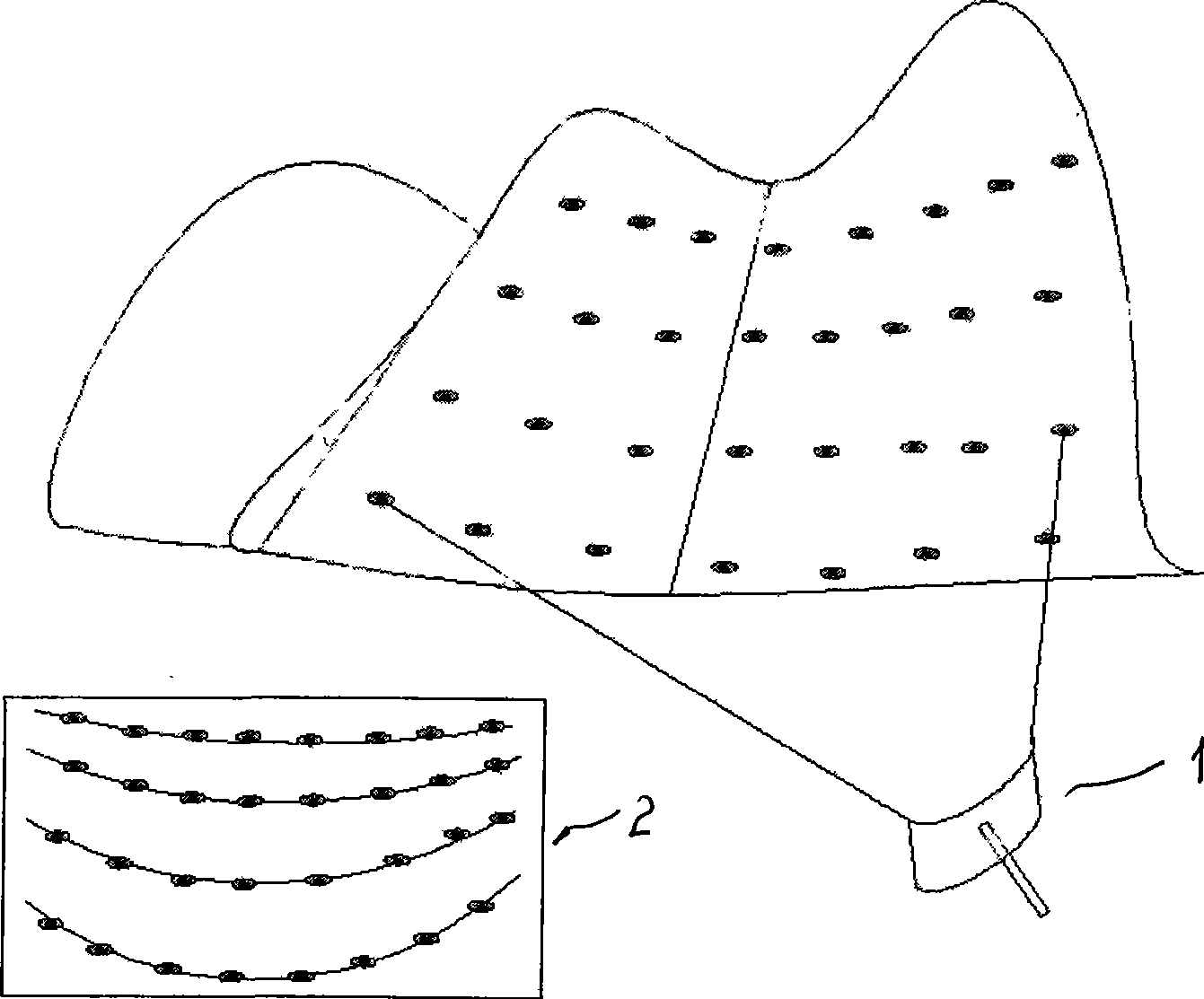
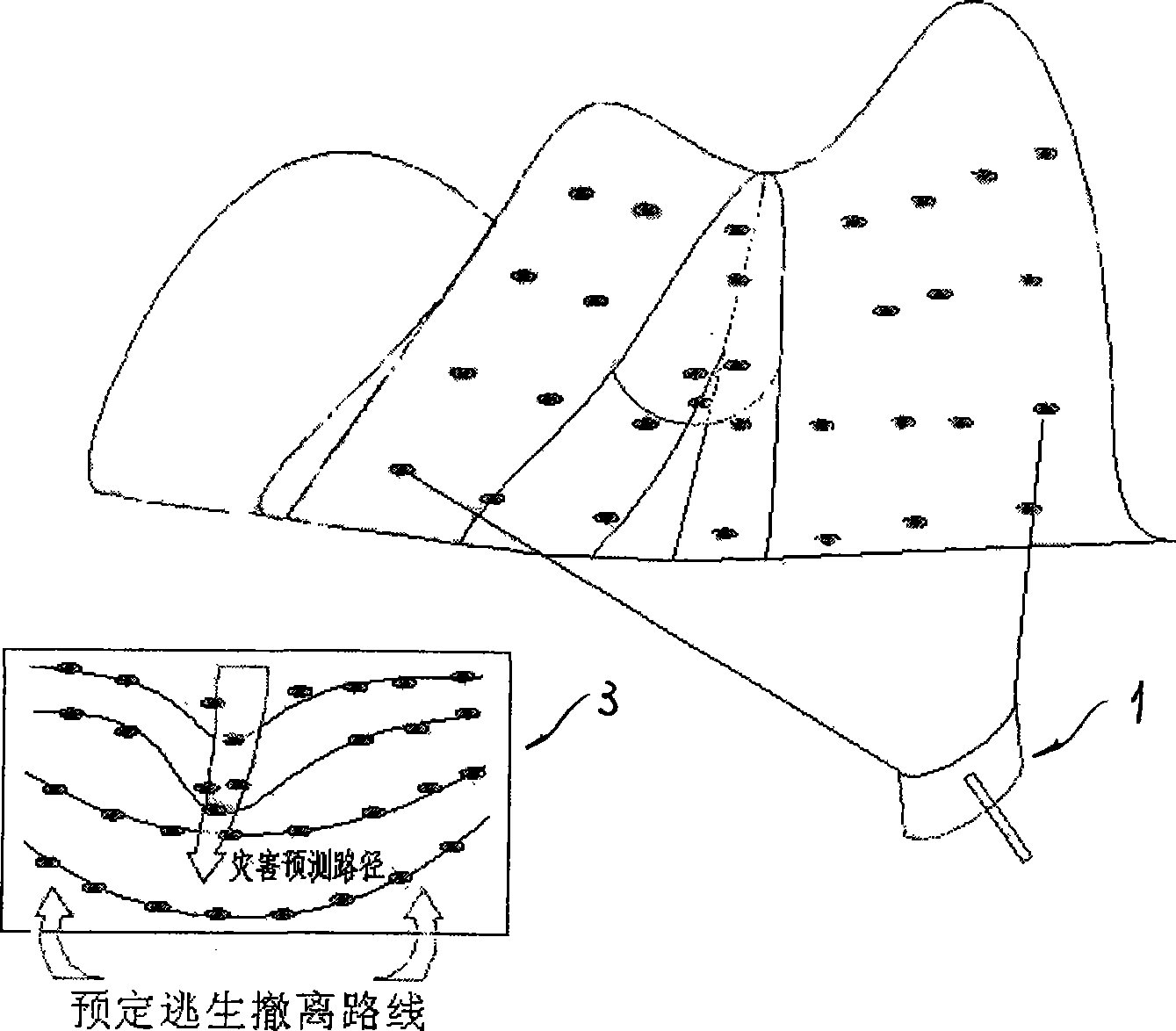
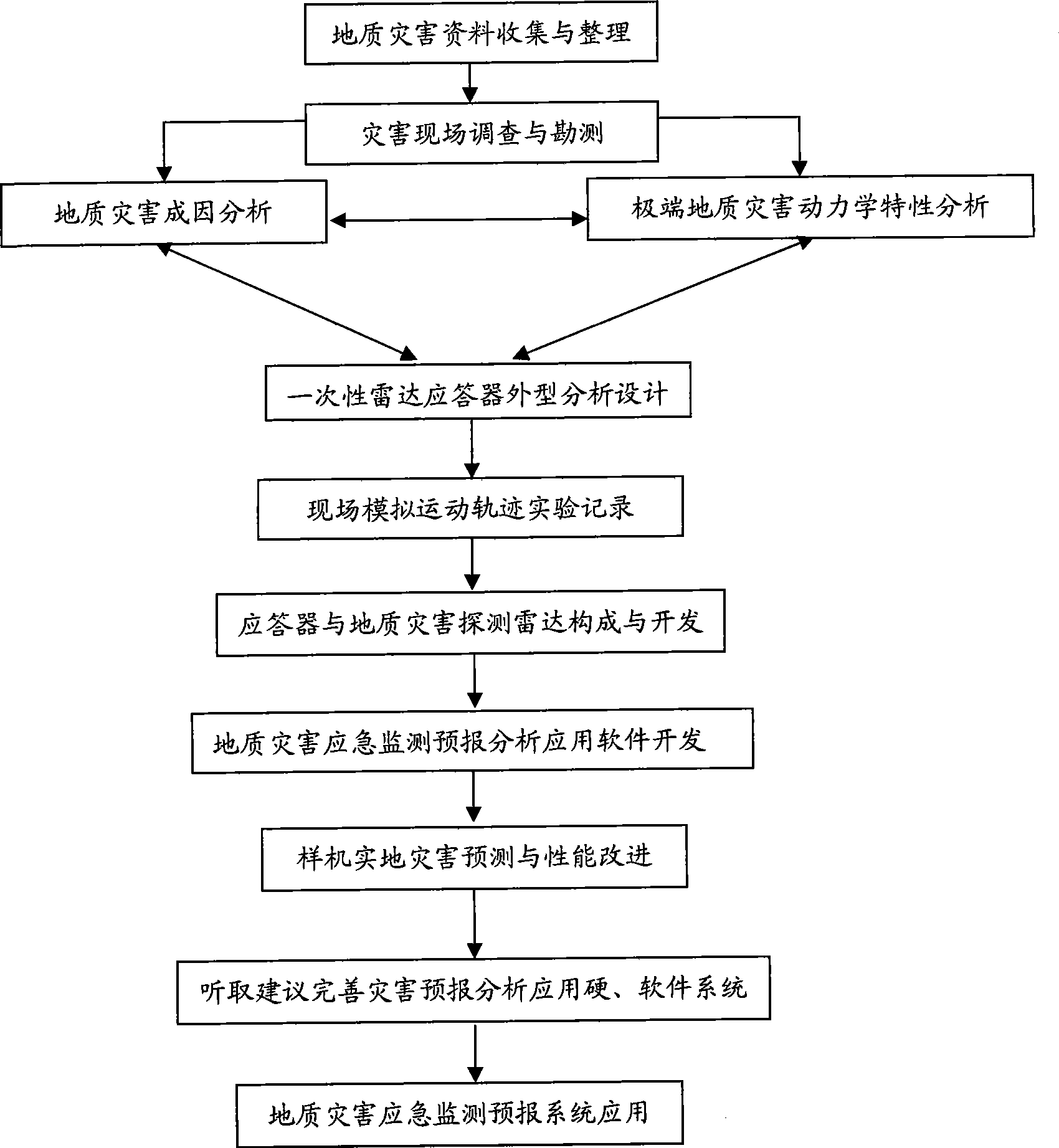
The invention discloses a geologic hazard emergency monitoring forecast system and a method for analyzing the forecast of the system. Disposable radar responders move together with landslide or debris flow, flood and the like on ground surface by irregularly launching the disposal radar responders in a geologic hazard region which is formed or is forecasted to be formed. Through technology of radar scanning, running tracks of the radar responders can be monitored and compared intuitively and quickly; the place and the time formed by the hazard can be further analyzed and determined combining with status of topography and landform; and basic causes and important information, such as a territorial scope which is about to be affected by the hazard, forecasted imminent time slice and safe escape route, and the like, can be acquired assorted with rainfall, earthquake and other information. The system and the method have a standard product structure and low cost, and have incomparable advantages on the forecast of geologic hazard which has clear cause, certain predictability, large scale and short-terming burst under quick response.
Owner:INST OF MOUNTAIN HAZARDS & ENVIRONMENT CHINESE ACADEMY OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com