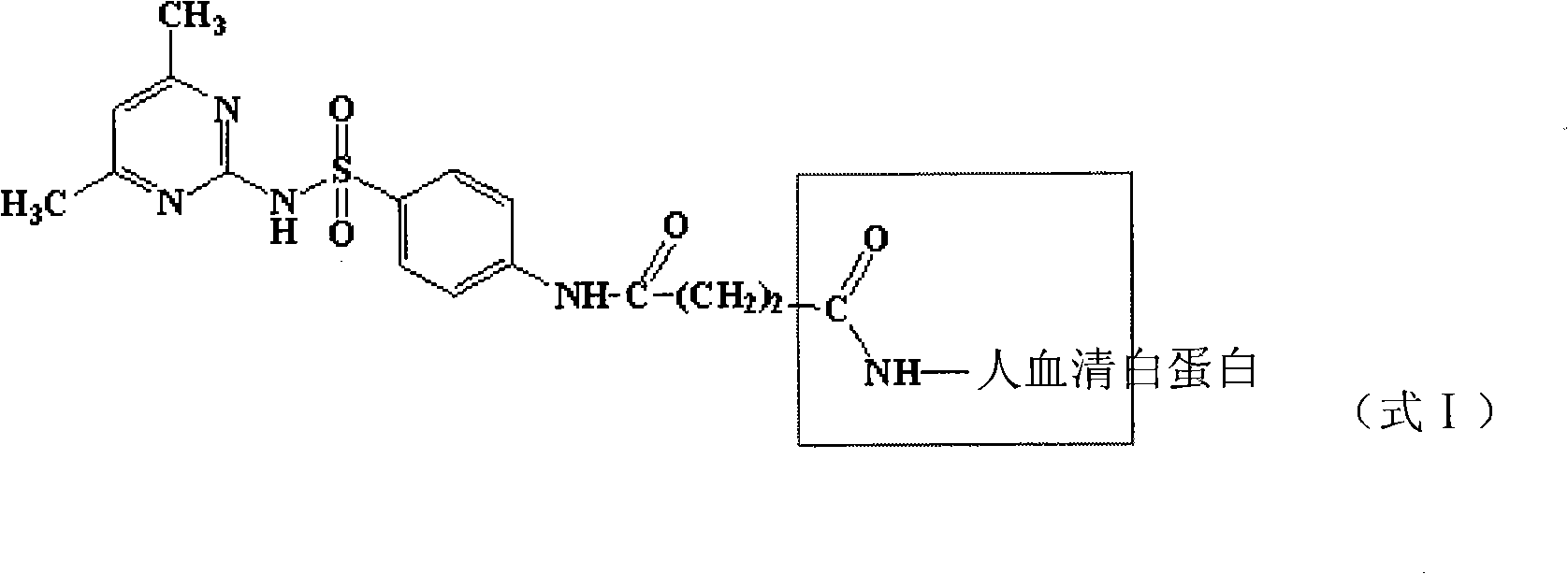
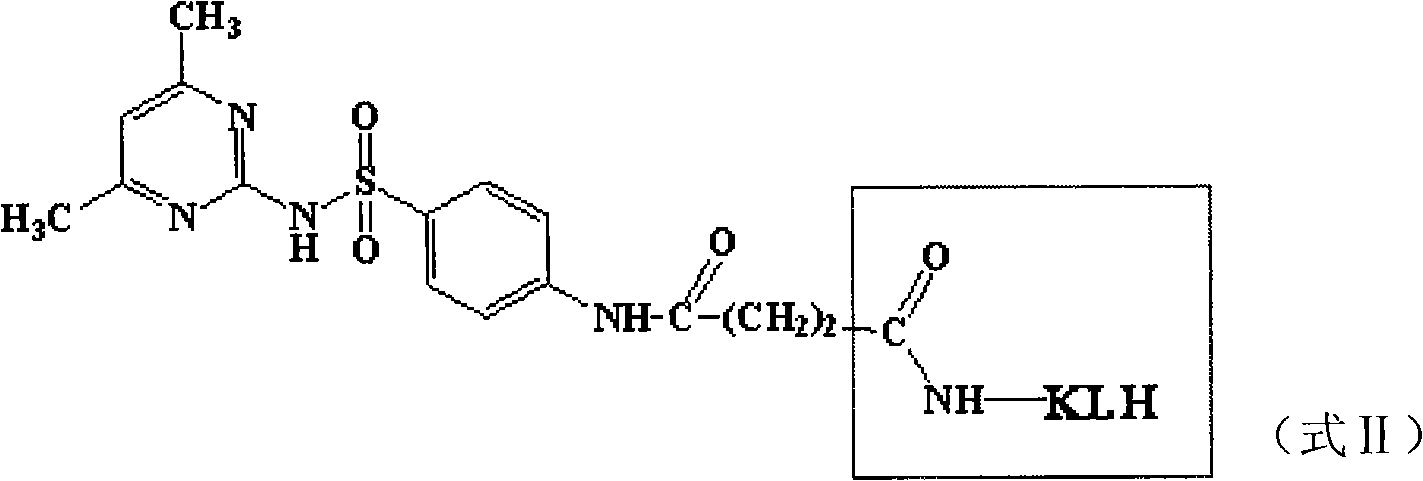
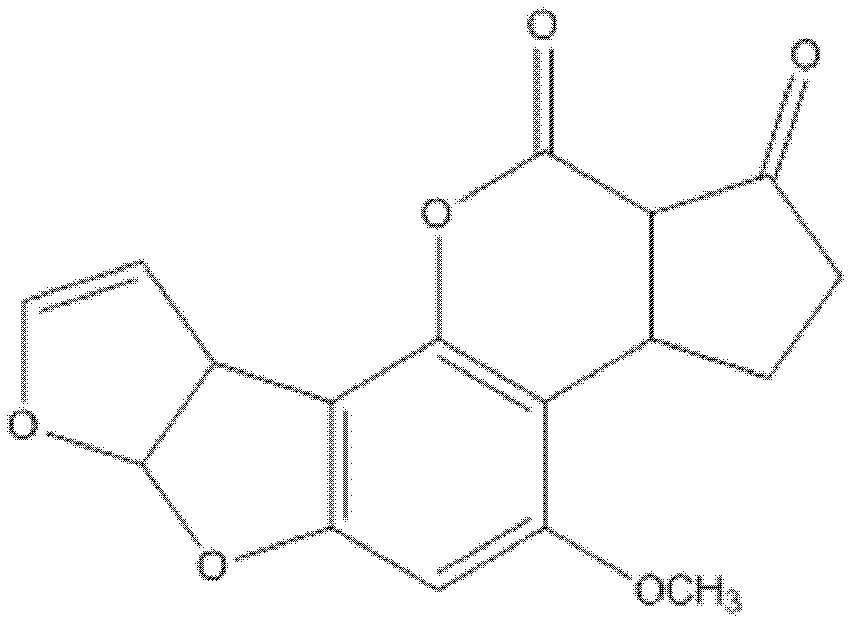
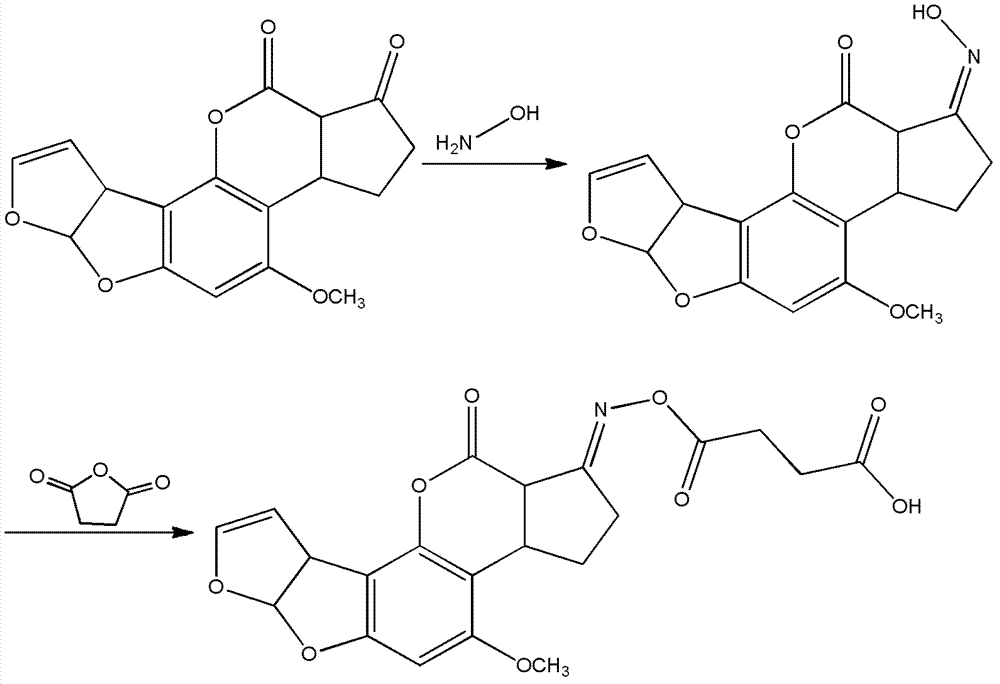
Patents
Literature
1314 results about "Elisa kit" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
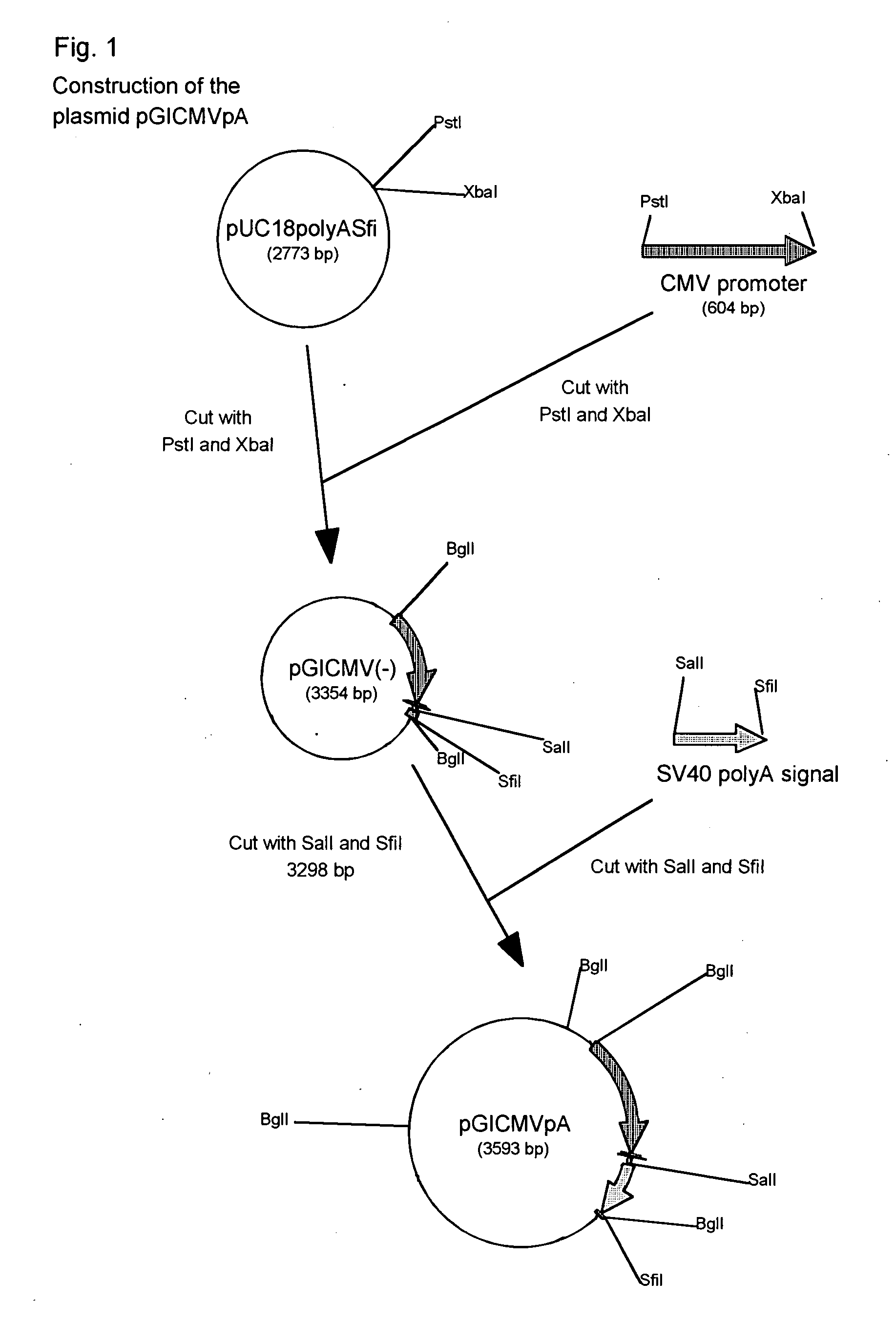
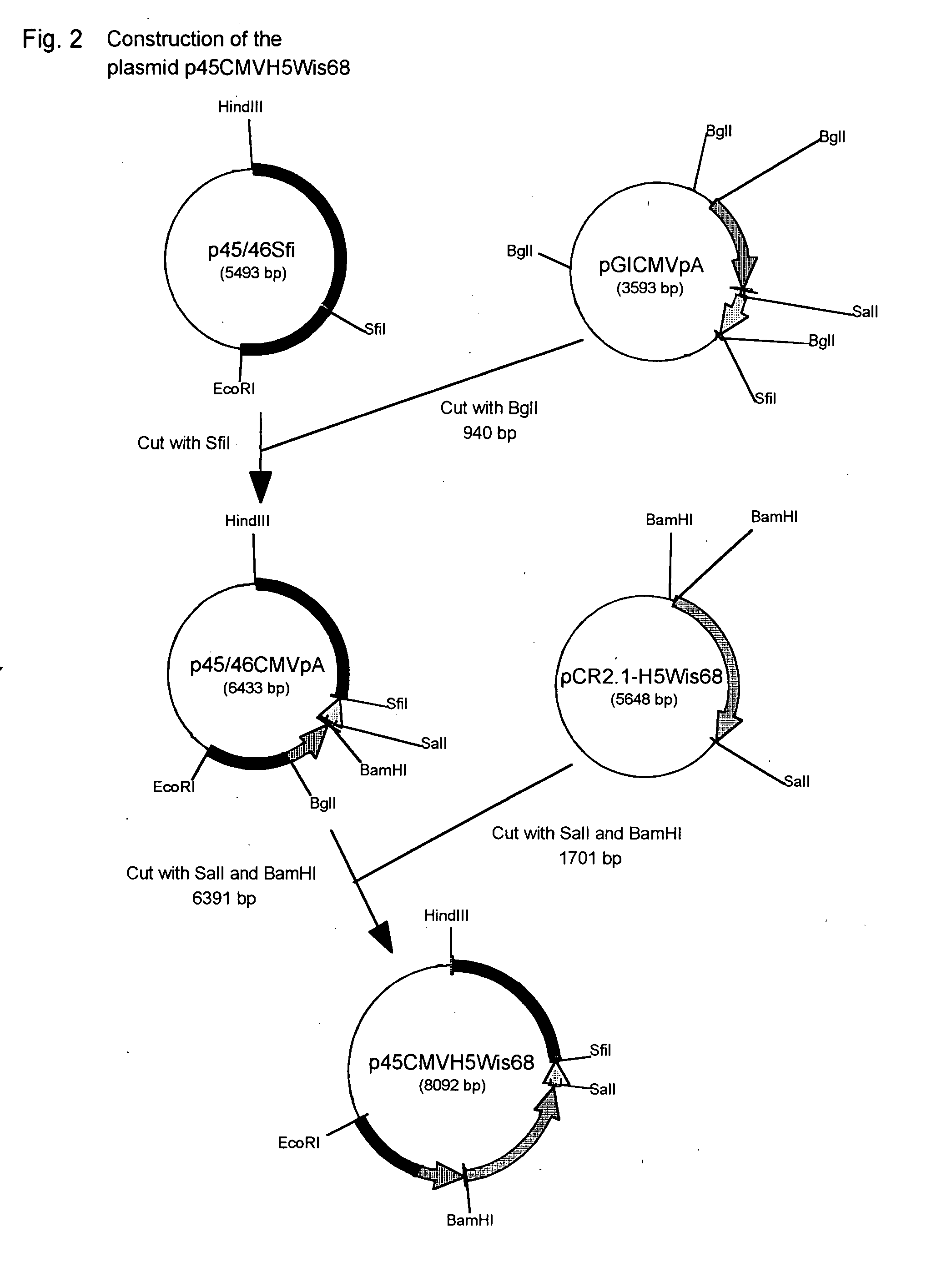
Method of selectively assaying adiponectin multimers
ActiveUS20070042424A1Accurate assessmentPeptide/protein ingredientsMicrobiological testing/measurementElisa kitFiltration
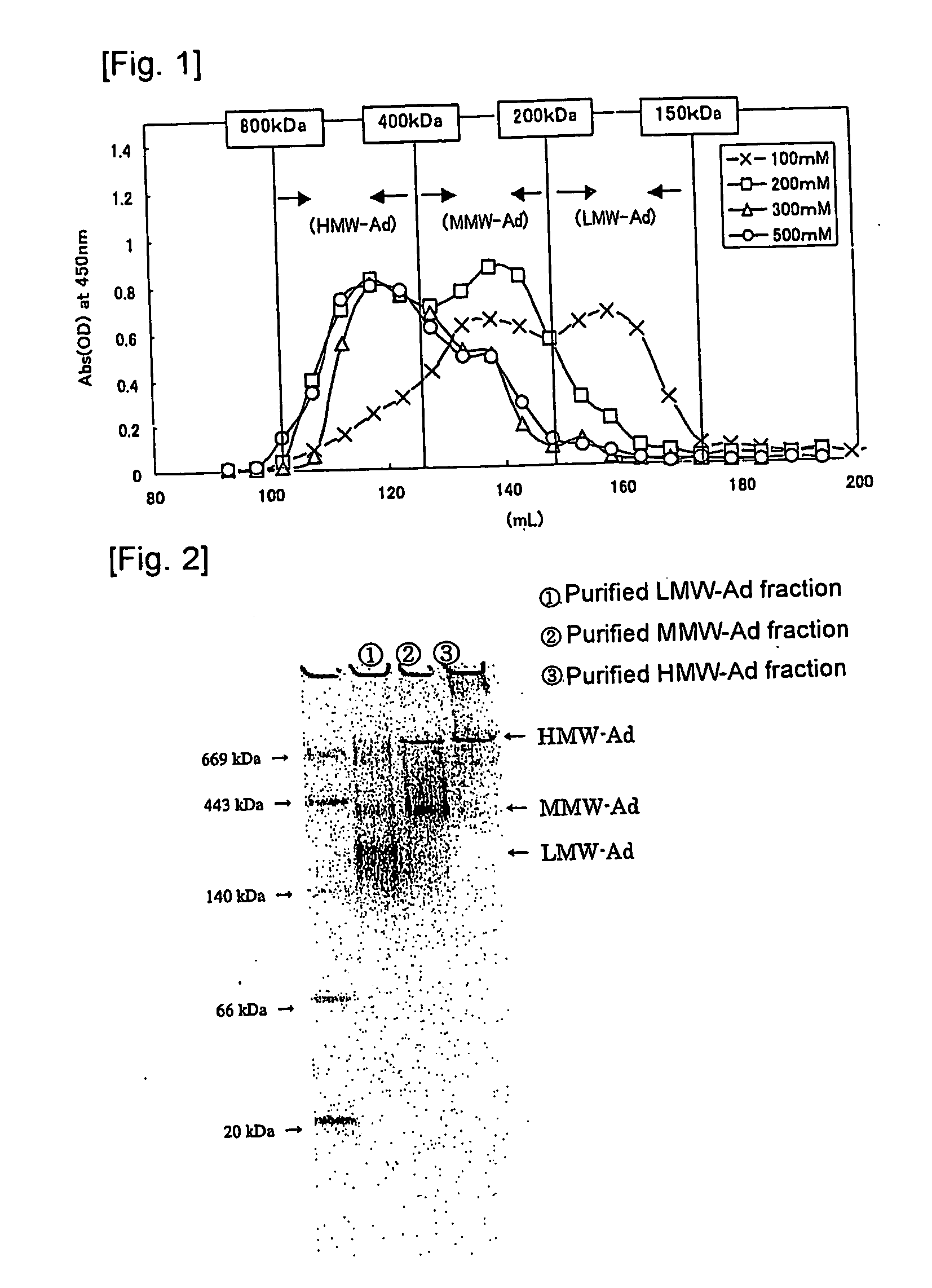
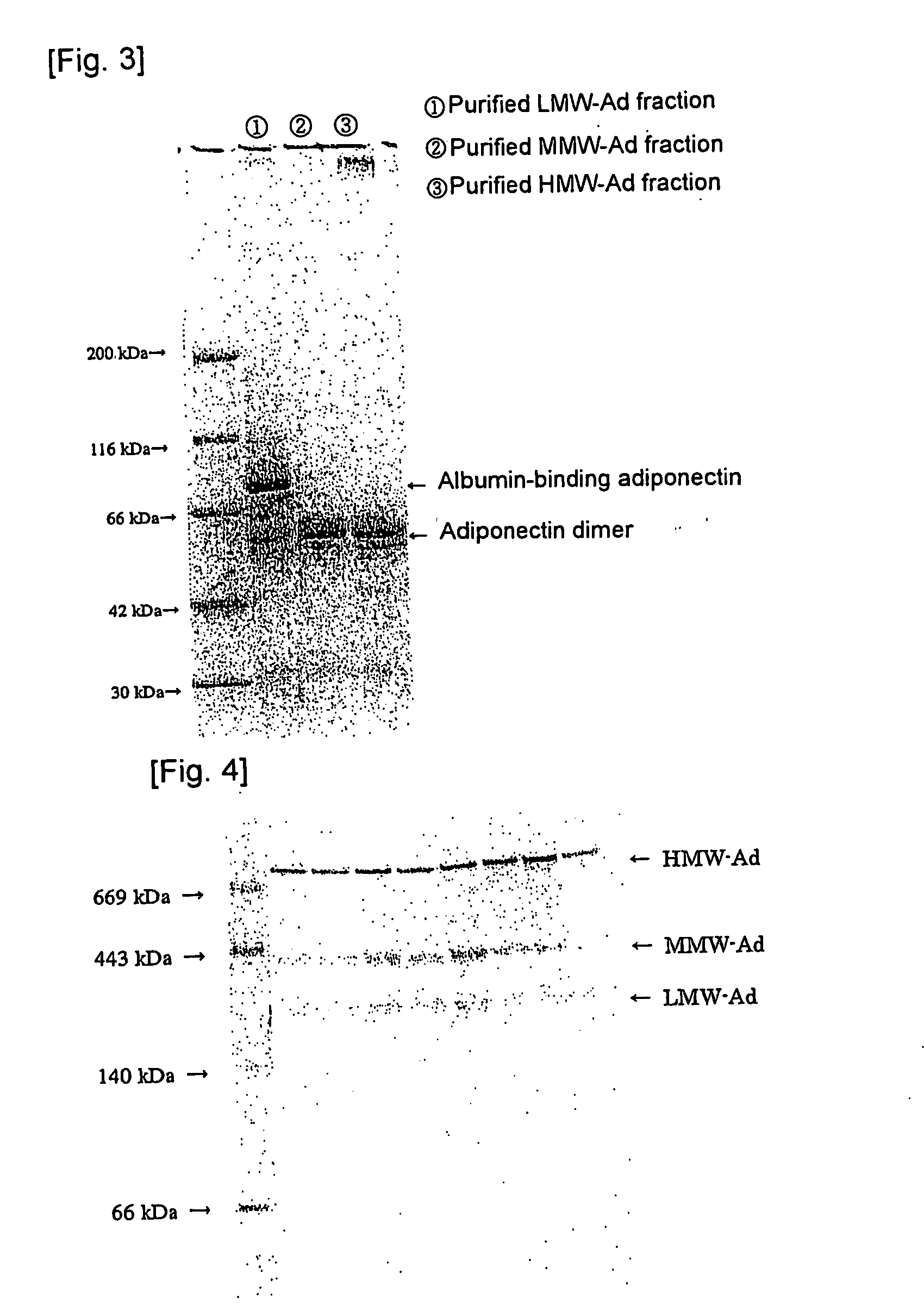
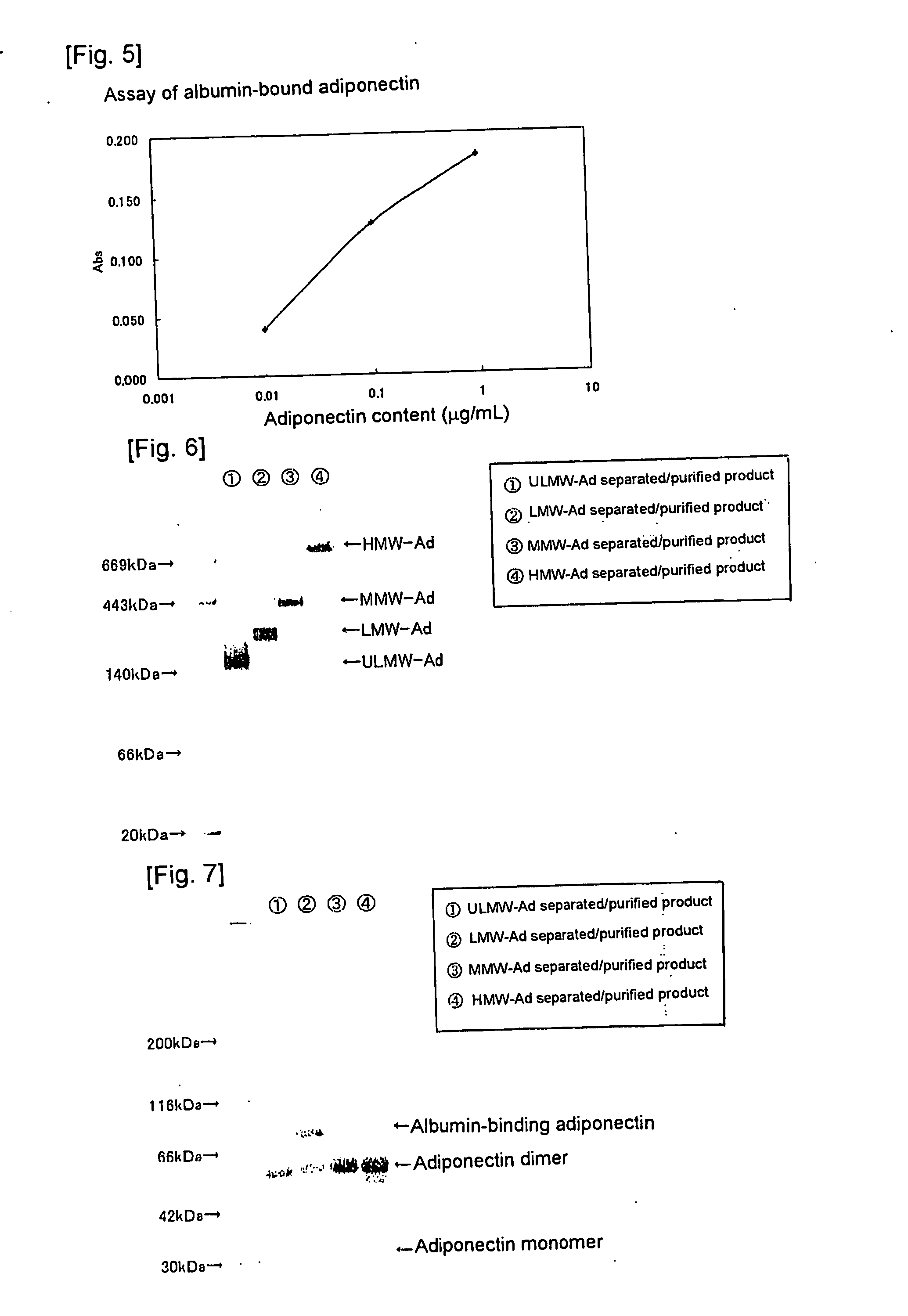
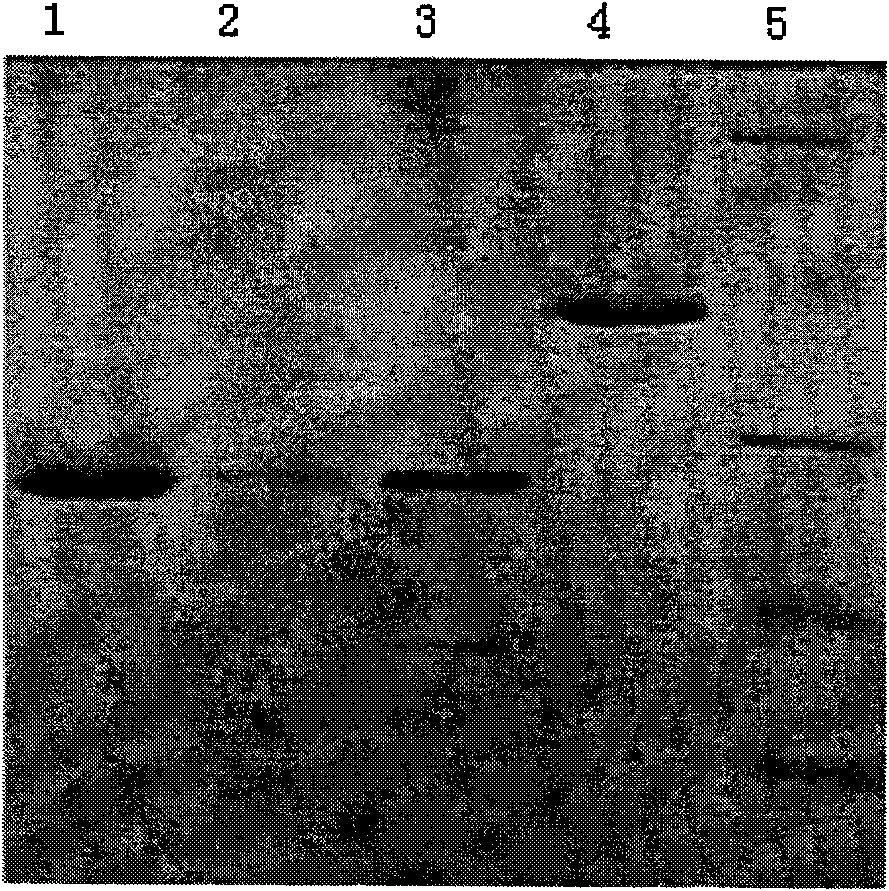
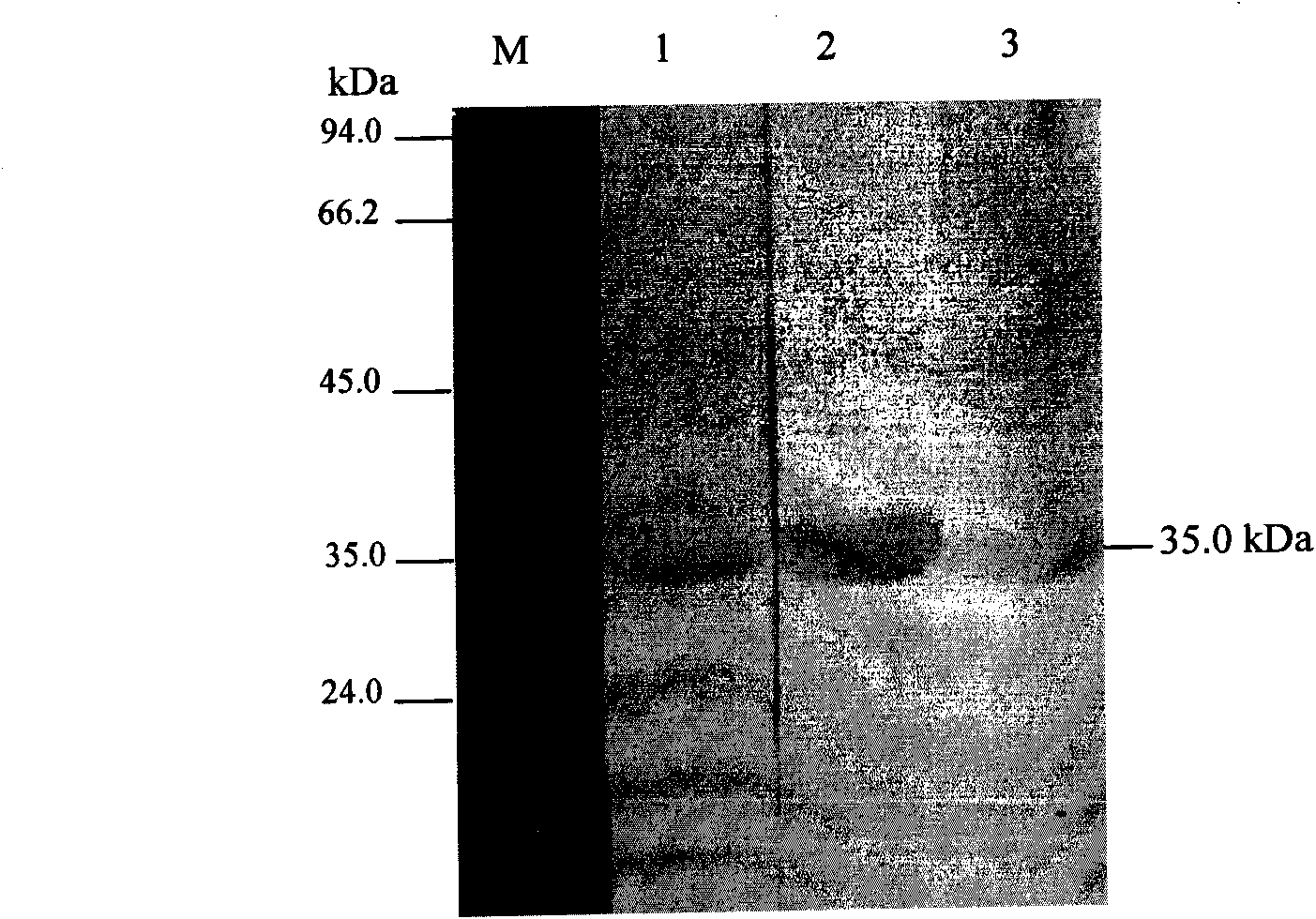
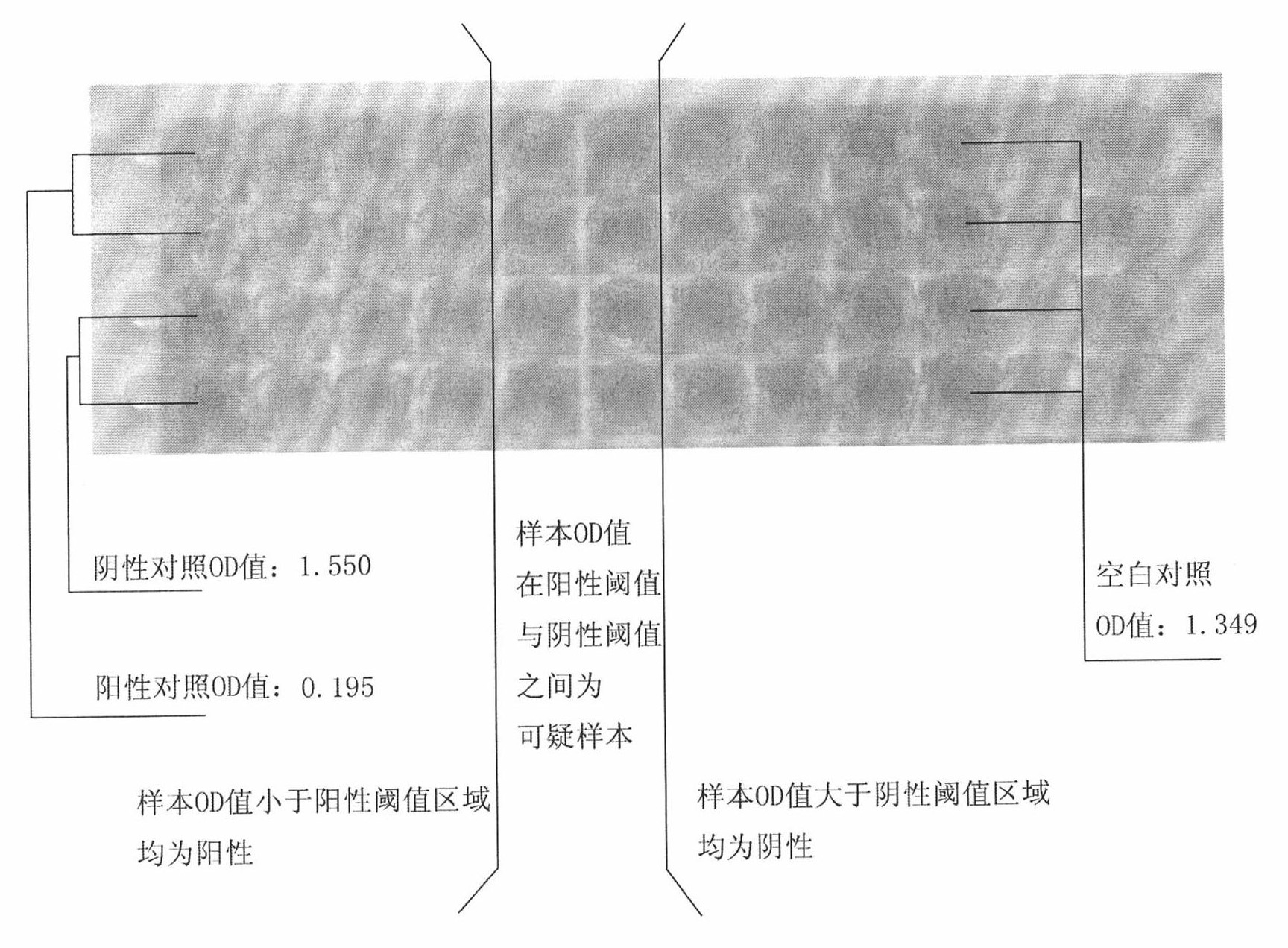
We have discovered a “human adiponectin ELISA kit”, which can specifically measure adiponectin in human blood serum (or blood plasma) or in extracted fluid from lipocytes or culture supernatant fluid. After fractioning human serum by use of gel filtration column, each fraction was measured by the present kit, and as a result, adiponectin immunity activity was detected as a plurality of peaks at above 670 kD of molecular weight. From the results, it is presumed that adiponectin is present in the blood as a polymer or forms a complex with polymer proteins.
Owner:SEKISUI MEDICAL CO LTD +1
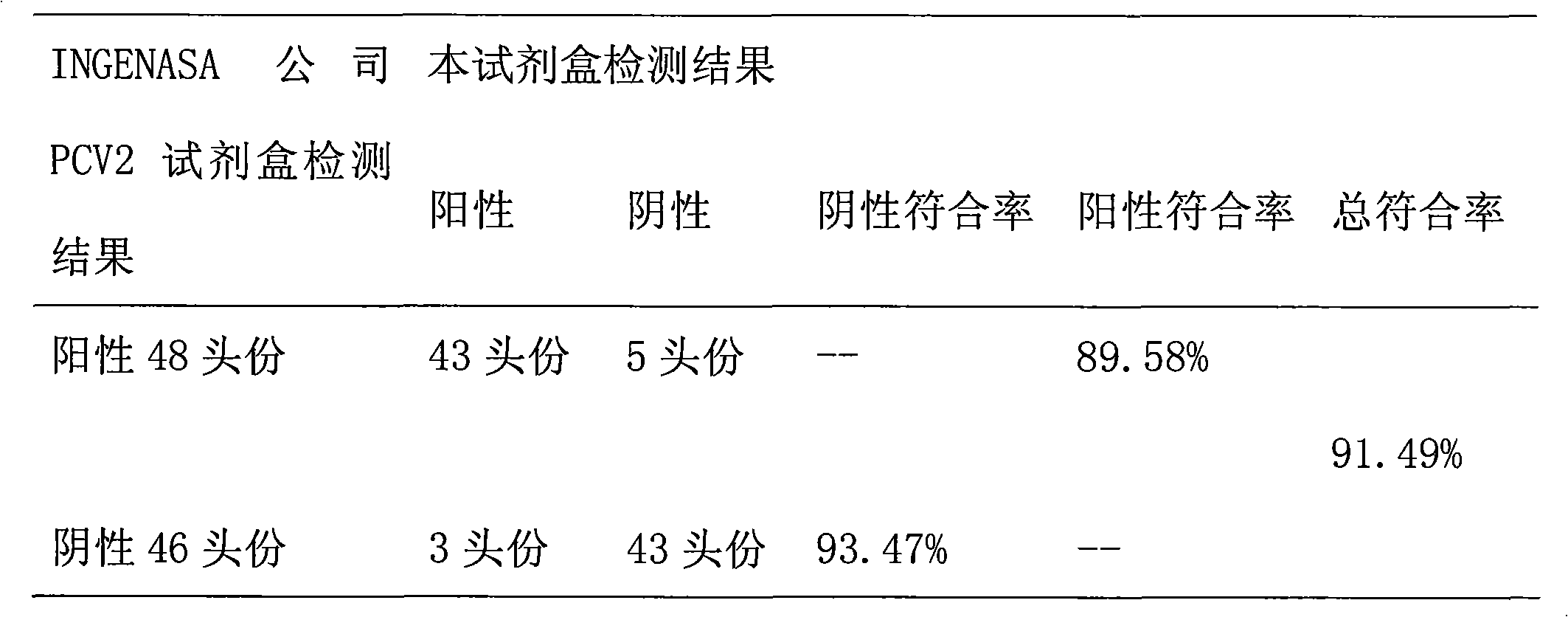
ELISA kit for detecting porcine circovirus antibody II
ActiveCN101629956ASimilar sensitivityStrong specificityMicroorganism based processesBiological testingSerum igeElisa kit
The invention relates to an ELISA kit for detecting porcine circovirus antibodies II, wherein, the kit is developed according to the double antigen sandwich ELISA principle. The ELISA kit comprises a prepacked ELISA antigen plate bar, a cleaning solution, 100 times concentrated enzyme labeled antigen, an enzyme labeled dilution, a zymolyte coloration, a stopping solution, standard PCV2 negative sera, and standard PCV2 positive sera. The kit replaces enzyme labeled anti-antibodies with the enzyme labeled antigen. The enzyme labeled antigen can not combine with antibodies absorbed with ELSA plate without specificity. Meanwhile, the serum specimen to be detected does not need to be pre-diluted, and the serum specimen can be directly mixed with the enzyme labeled antigen with working concentration to be directly used for determination. The ELISA kit has the advantages of simple operation and shorter detecting time.
Owner:北京金诺百泰生物技术有限公司
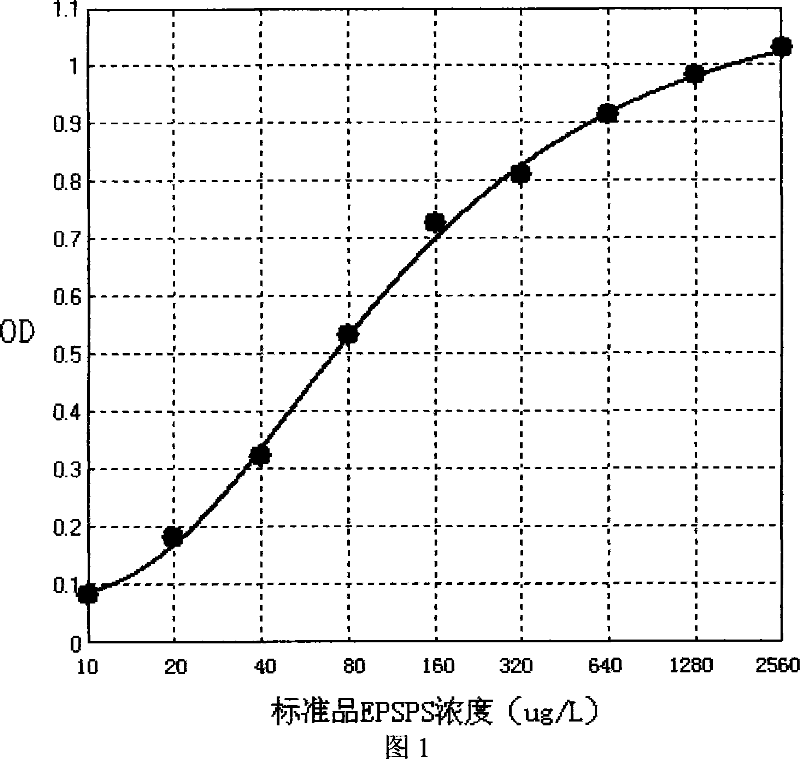
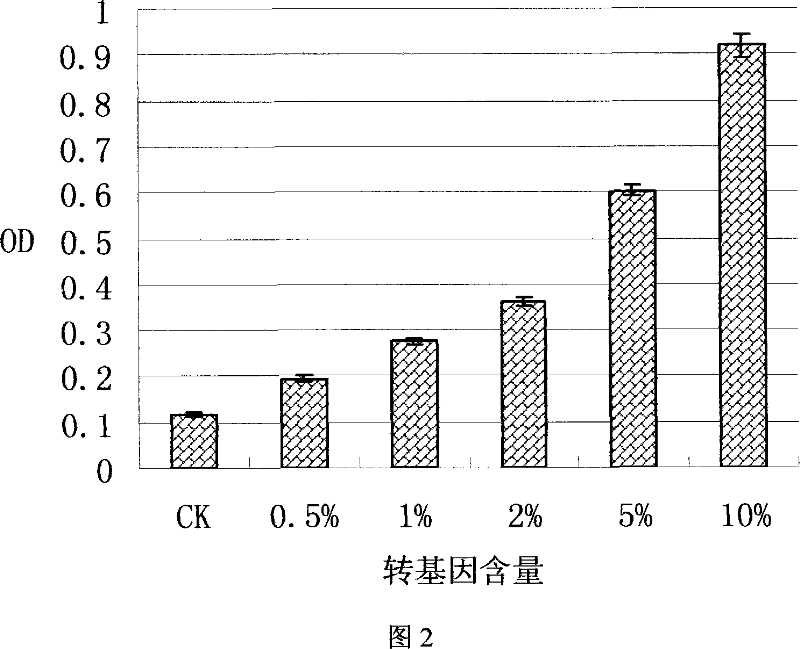
ELISA kit for detecting EPSPS gene in herbicide-tolerance soybeans and method of use thereof
This invention discloses one EPSPS gene enzyme immune agent case and its use method in the anti-herbicide bean, wherein, the case comprises anti-EPSPS multi-clone antigen, enzyme board with the antigen, enzyme antigens for two, gene switch bean standard product, non-transfer gene bean standard, intense wash liquid, develop liquid and terminal liquid. This invention method comprises the following steps: cloning CP4-EPSPS gene from the gene switch bean expressed in the bacillus coli; then through protein purification complex property to regroup EPSPS protein antibody immune animal to get special single clone antigen and multi-clone antigen; establishing double antigen clamp enzyme immune system test sample to determine its EPSPS protein content.
Owner:SOUTH CHINA AGRI UNIV
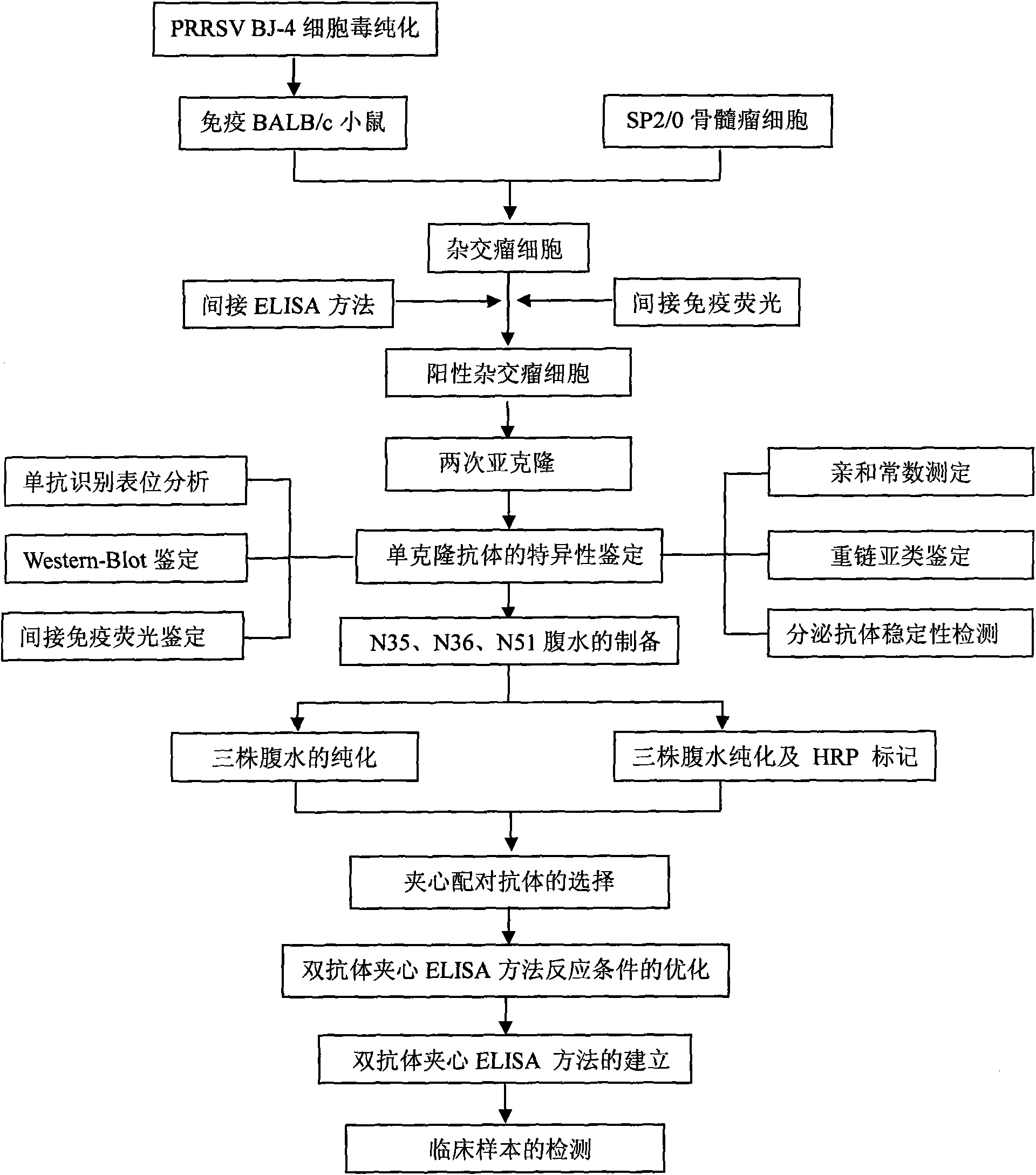
Porcine reproductive and respiratory syndrome virus (PRRSV) double-antibody sandwich ELISA kit
The invention provides a porcine reproductive and respiratory syndrome virus double-antibody sandwich ELISA kit. The kit comprises: an elisa plate coated with PRRSV N protein monoclonal antibody, an enzyme labeling PRRSV N protein monoclonal antibody, lysis solution and the like. A capture antibody and a detection antibody are respectively aimed at antigenic determinants with different N proteins.The kit provides a reliable means for quick detection of clinical PRRSV antigen. The kit can detects that blood serum only contains 0.2 TCID50 highly pathogenic PRRSV JXwn06 strain (non-highly pathogenic strain can be also be detected). Through detecting clinically collected 80 blood serum samples, compared with RT-PCR result, the specificity of the method is 88 percent, the sensitiveness is 90 percent and the coincidence rate of the specificity and the sensitiveness are 88.8 percent. The kit is convenient for operation, low in use cost, good repetitiveness and suitable for wide promotion andapplication.
Owner:CHINA AGRI UNIV
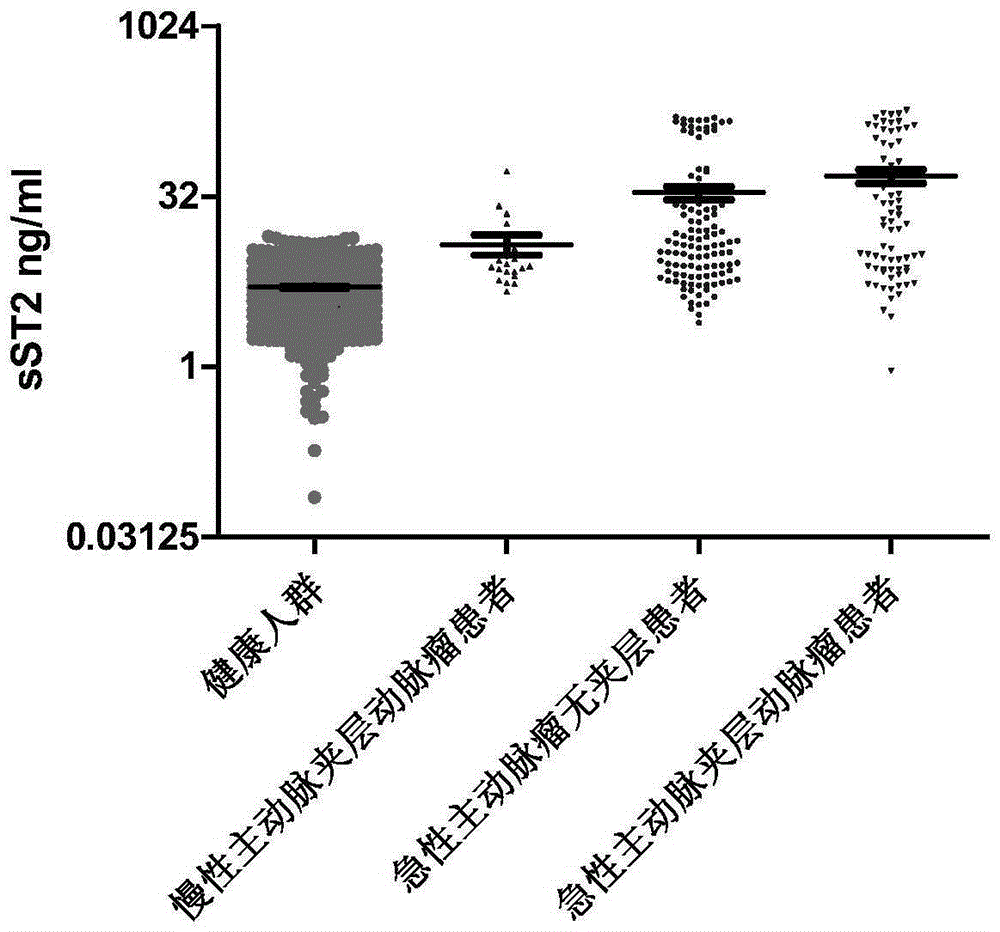
Kit and method for detecting sST2 (soluble ST2) in blood of abdominal aortic aneurysm and/or aortic dissection patient
ActiveCN105259353AQuantitativeEasy to detectImmunoglobulins against cell receptors/antigens/surface-determinantsDisease diagnosisAortic dissectionDisease
The invention discloses a kit and a method for detecting sST2 (soluble ST2) in blood of an abdominal aortic aneurysm and / or aortic dissection patient. According to the kit and the method, a pair of antibodies for identifying different epitopes of sST2 is assembled to obtain a double-antibody sandwich ELISA and immune colloidal gold test strip, so as to realize quantitive and qualitative detection of sST2. Tests prove that the ELISA kit and the immune colloidal gold test strip have relatively high specificity and sensitivity in an abdominal aortic aneurysm marker-sST2 protein of human, are simple to operate, can be utilized for detecting abdominal aortic aneurysm, aortic dissection and other diseases in scientific research and clinical application, and have the functions of auxiliary diagnosis, guide treatment and prognosis judgment.
Owner:华新安平(北京)生物医药有限公司
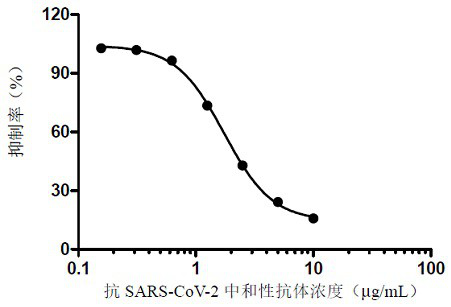
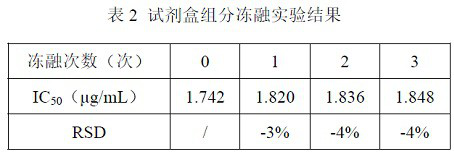
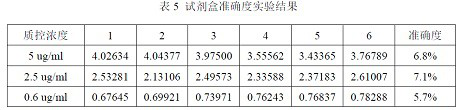
ELISA kit for detecting titer of novel coronavirus neutralizing antibody
ActiveCN111781354AThe pre-processing process is simpleShort timeImmunoassaysElisa kitHorseradish peroxidase
Owner:ACROBIOSYSTEMS INC
Indirect competitive ELISA kit for detecting malachite green in aquatic product
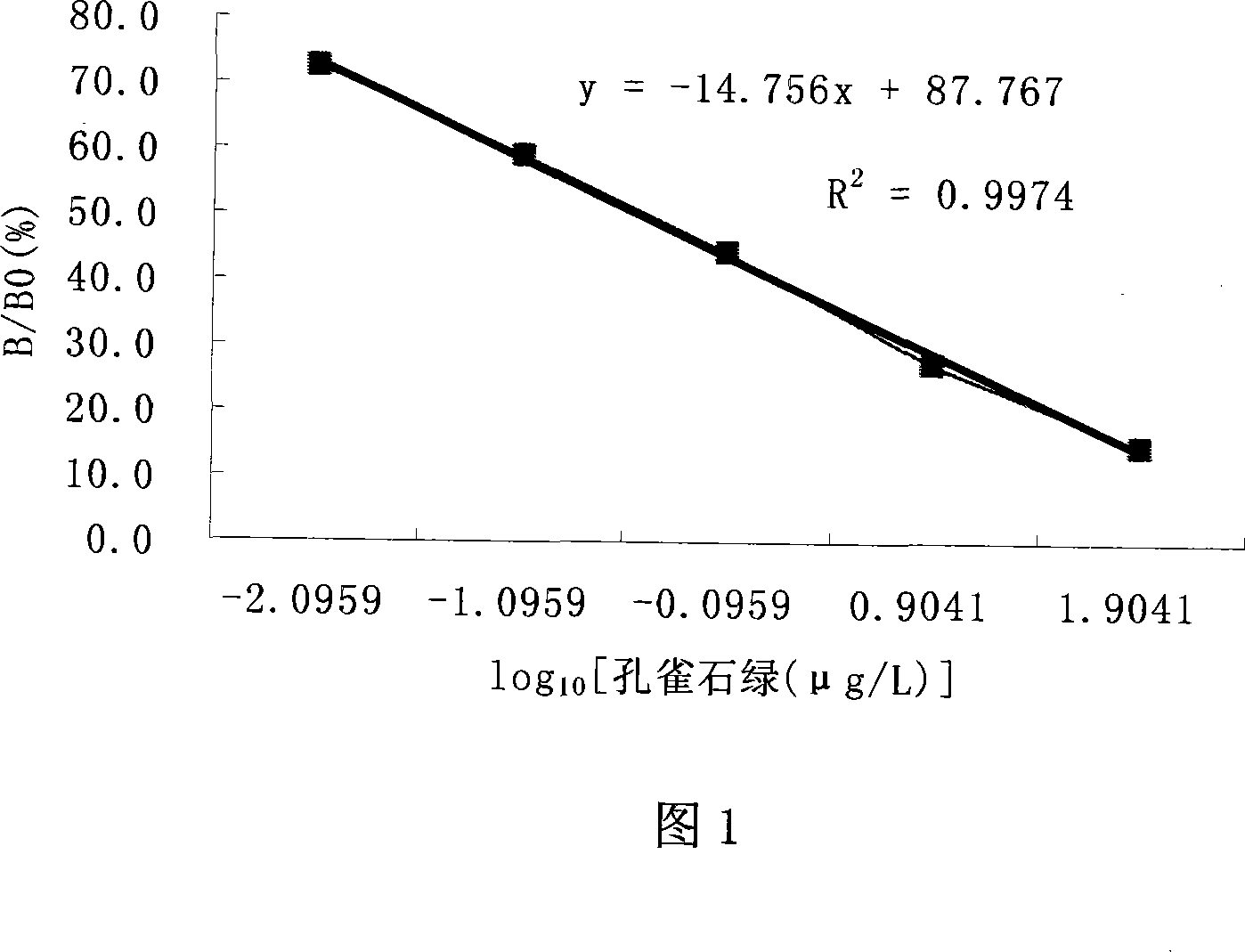
InactiveCN1766623AEasy to operateStrong specificityTesting medicinal preparationsMalachite greenElisa kit
The invention relates to a colorless green malachite green indirect compete ELISA test agent box of an aquatic product. The test plate of the agent box is a split 96 holes enzyme mark plate which coats colorless green malachite green and albumin coupling material; the anti-colorless green malachite green antibody is a monoclonal antibody which is prepared by the colorless green malachite green and the albumin coupling material immune mouse; the tested sample uses ethyl hexoate and cyclohexane to extract after uniform and adjusts PH value for detecting. The quoting standard of the sample detecting is that it is a regression curve which uses the logarithm value of the sample density as abscissa and uses the degradation rate as ordinate. We could read the density of the corresponding sample from the degradation rate of each sample. The sensibility of the test method is 0.0023ª–g / ml; the test range is 0.0016-1ª–g / ml.
Owner:SHANGHAI ENTRY EXIT INSPECTION & QUARANTINE BUREAU OF P R C +1
Malachite green vestigial ELISA detection kit and usage method thereof
The invention discloses an enzyme immunoassay of testing the bice green residues in animal derived food, which comprises an enzyme label plate covering bice green antigen, enzyme label bice green antibody working solution, bice green standard solution, substrate solution, substrate buffer solution, reaction termination solution, concentration washing liquid and sample dilute solution. The invention further discloses a method for using the immunoassay to test bice green residues, which comprises sample pretreatment, testing via the immunoassay, processing and analyzing result. The inventive immunoassay of bice green test uses direct competition enzyme-linked immunoassay adsorption analysis technique, with high sensitivity, high stability, simplified operation, reduced reaction time, reduced error caused by complex operation, reduced cost, wide application for testing samples and high practicality.
Owner:SOUTH CHINA AGRI UNIV
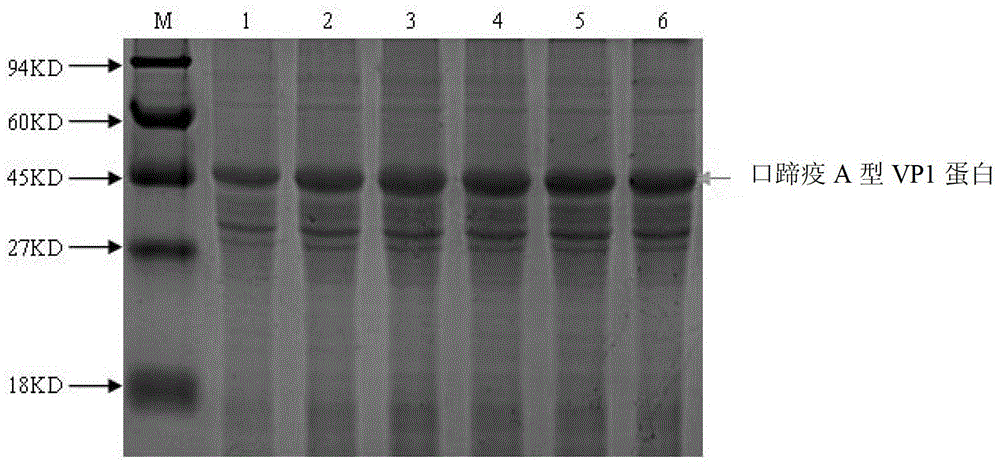
Competitive ELISA method based on foot-and-mouth disease A type VP1 protein and its monoclonal antibody
InactiveCN103554234AHigh purityImprove production efficiencySsRNA viruses positive-senseAntibody mimetics/scaffoldsAntigenDisease
The invention relates to a competitive ELISA method based on a foot-and-mouth disease A type VP1 protein and its monoclonal antibody, also relates to a preparation method of the foot-and-mouth disease A type VP1 protein, and a preparation method of the monoclonal antibody of the foot-and-mouth disease A type VP1 protein, and belongs to the technical field of animal immunological detection. In the invention, a primer pair C1 and C2 and a primer pair E1 and E2 are amplified to obtain a gene sequence of the foot-and-mouth disease A type VP1 protein, the foot-and-mouth disease A type VP1 protein is obtained by constructing an expression plasmid, introducing the expression plasmid into a prokaryotic expression host and carrying out inducible purification, the foot-and-mouth disease A type VP1 protein monoclonal antibody is obtained by treating the foot-and-mouth disease A type VP1 protein as an antigen through a hybridomas technology, and the competitive ELISA method used for detecting a foot-and-mouth disease A type antibody is established based on the foot-and-mouth disease A type VP1 protein and its monoclonal antibody. The detection method has a strong specificity and a good stability, and can be used for detecting a foot-and-mouth disease A type serum antibody. By comparing a result obtained through the detection method with a liquid phase blocking ELISA kit, the coincidence rate is 95.8%.
Owner:广西壮族自治区动物疫病预防控制中心
Competitive ELISA kit for peste-des-petits-ruminants antibody detection and preparation method thereof
ActiveCN102967710ANo cross reactionStrong specificityImmunoglobulins against virusesBiological testingSerum igeElisa kit
Belonging to the field of biotechnologies, the invention discloses a competitive ELISA kit for detection of a peste-des-petits-ruminants virus antibody. The kit comprises a detection system composed of a coating antigen reaction solution and a monoclonal antibody reaction solution. The kit adopts prokaryotically expressed peste-des-petits-ruminants Nigeria 75 / 1 strain N protein as the coating antigen and employs a monoclonal antibody against N protein as the competitive antibody. The antibody against a peste-des-petits-ruminants virus in sheep serum is detected according to a competitive ELISA principle. The kit provided in the invention can rapidly and specifically detect the peste-des-petits-ruminants virus antibody in serum, and simultaneously has the advantages of large-scale production of monoclonal antibodies, good reaction specificity, high sensitivity, simple operation, low cost, stable, reliable and easily observable reaction results, thus being very suitable for import and export quarantine of sheep, food hygiene and screening of large batches of samples in livestock breeding farms, and being easy for large-scale popularization and application.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
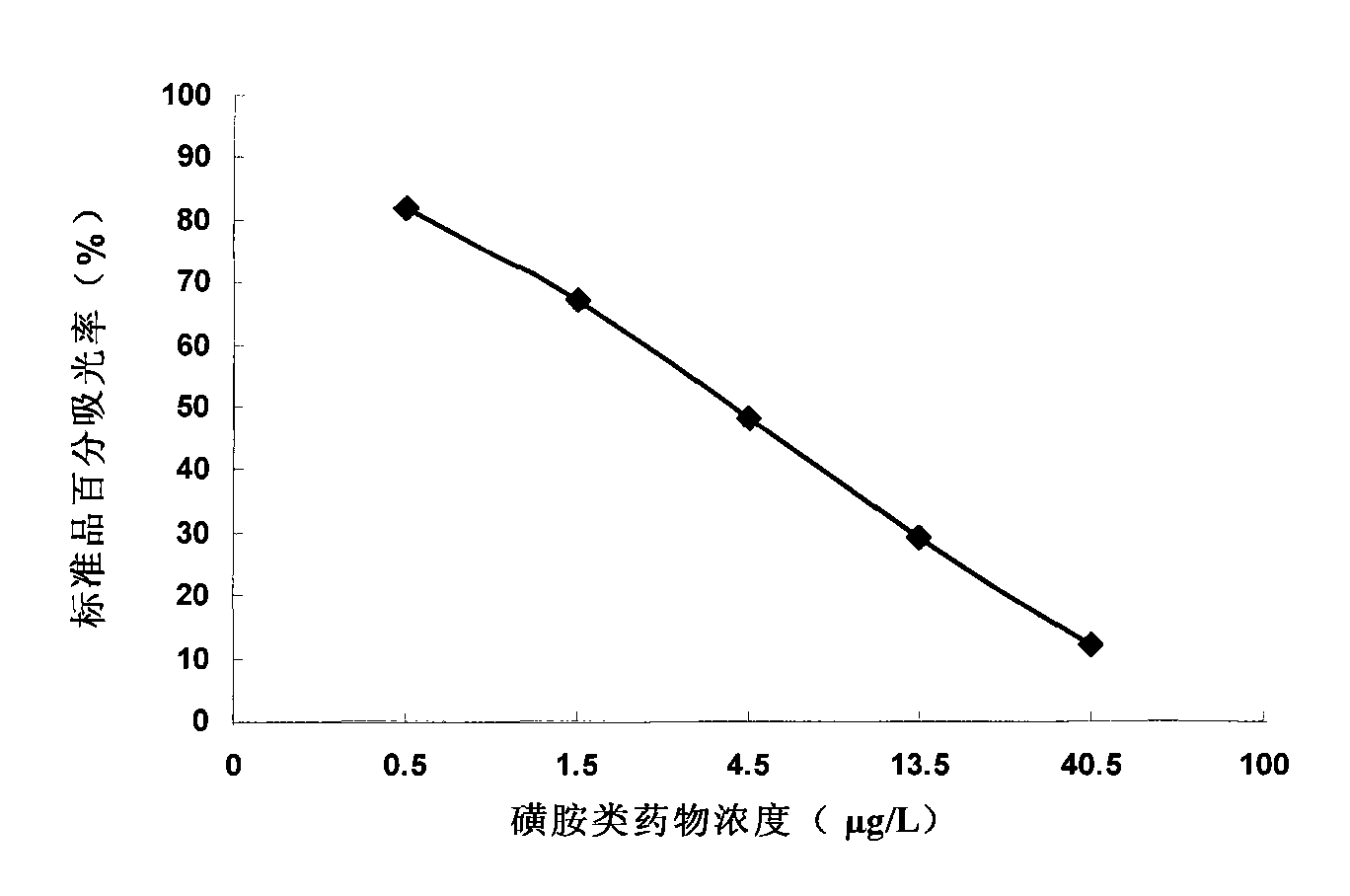
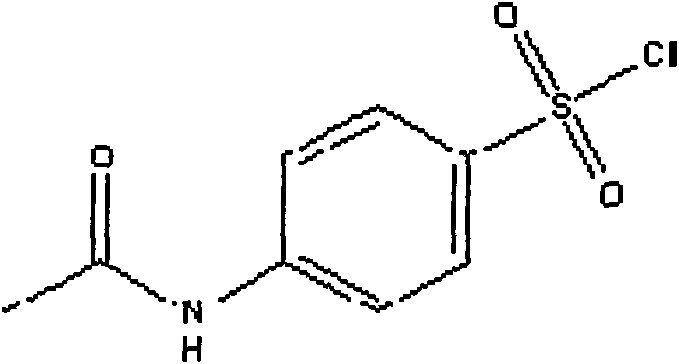
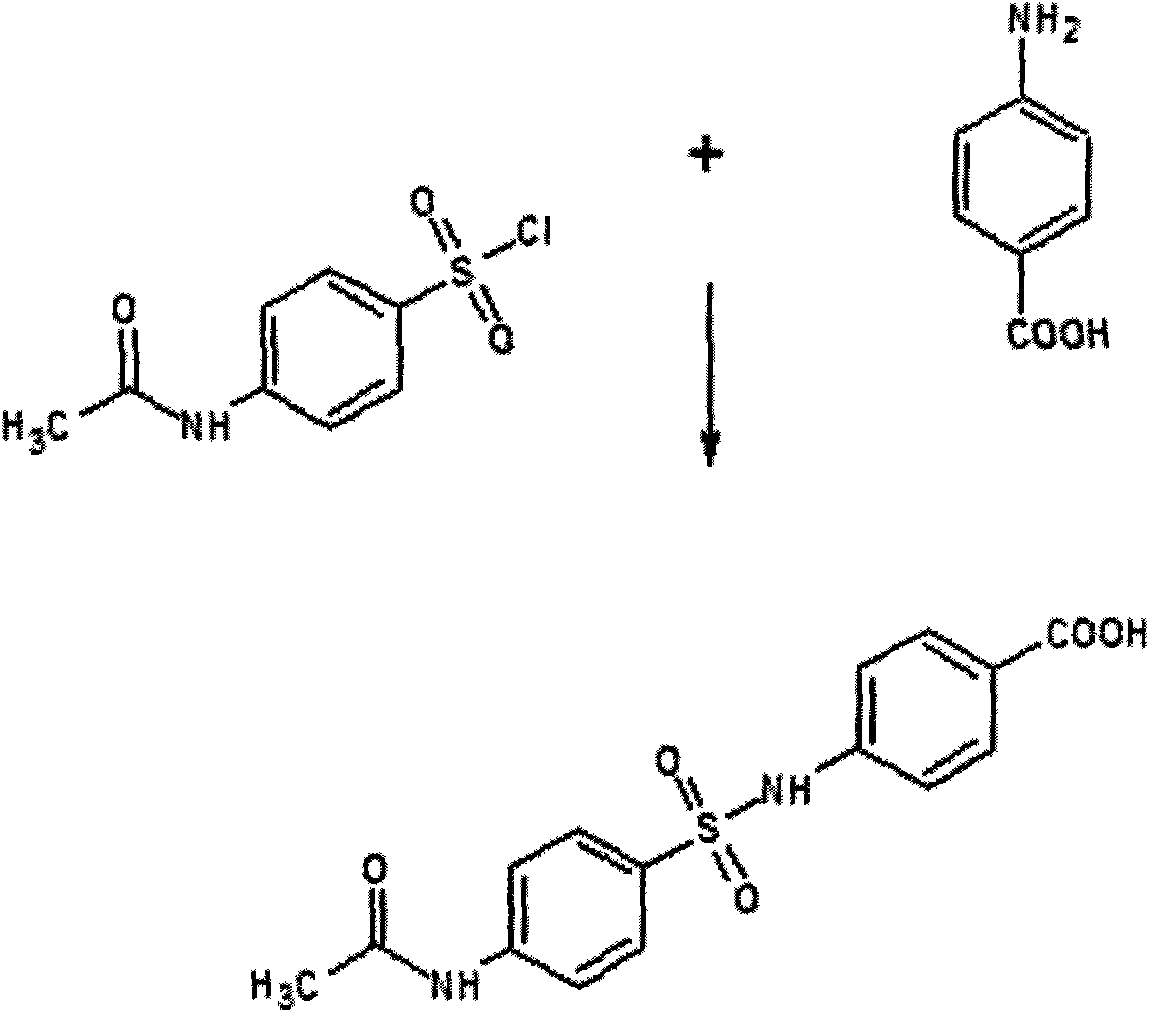
Method for detecting sulfanilamide medicine and special enzyme-linked immunoassay reagent kit thereof
The invention discloses a method for detecting a sulfanilamide medicine and a special enzyme-linked immunoassay reagent kit thereof. The enzyme-linked immunoassay reagent kit comprises a sulfanilamide medicine monoclonal antibody and the sulfanilamide medicine. The sulfanilamide medicine monoclonal antibody is secreted by the hybridoma cell line SAs of a sulfanilamide monoclonal antibody, whose preservation number is CGMCC No. 3393. In the enzyme-linked immunoassay reagent kit of the invention, an indirect competition ELISA (Enzyme-Linked Immuno Sorbent Assembly) method is mainly used for qualitatively or quantitatively detecting the content of the sulfanilamide medicine in products (especially milk, pork, chicken, eggs, honey, fish, shrimp and the like) eaten by animals or people. The reagent kit and the detection method of the invention have low requirements on the pretreatment of samples and simple pretreatment process of the samples and can detect mass samples quickly at the same time. By using the sulfanilamide medicine monoclonal antibody with high specificity, the detection method is convenient and simple, and the invention has the characteristics of high specificity, high sensibility, high precision, high accuracy and the like.
Owner:北京维德维康生物技术有限公司
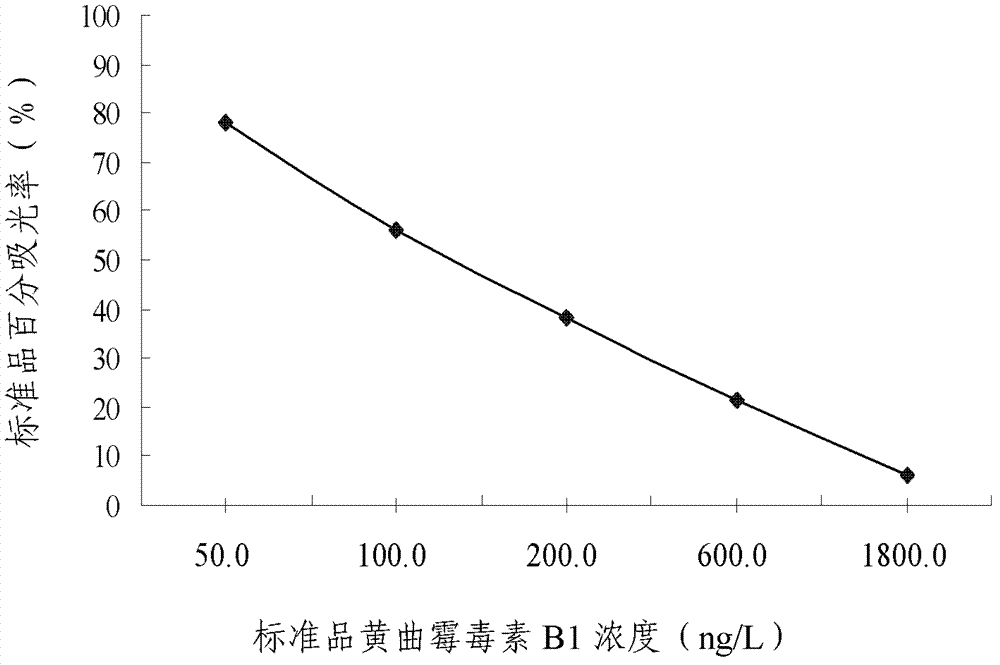
Enzyme-linked immunosorbent assay kit for detecting aflatoxin B1-containing medicine and application for same
The invention provides an enzyme-linked immunosorbent assay kit for detecting an aflatoxin B1-containing medicine and an application for the same. The enzyme-linked immunosorbent assay (ELISA) kit comprises an ELISA plate coated with a coating antigen, an enzyme label, aflatoxin B1 specific antibody working solution (contained in the case that the coating antigen on the ELISA plate and the enzyme label are enzyme-labelled antibodies or enzyme-labelled antigens), aflatoxin B1 standard substance solution, substrate developing solution, stopping solution, concentrated washing solution and concentrated compound solution. The method for detecting aflatoxin B1 by virtue of the kit provided by the invention comprises the following steps of: performing sample pre-treatment at first, and then detecting by virtue of the kit, and finally analysing the detected result. The enzyme-linked immunosorbent assay kit provided by the invention can be used for detecting the residual amount of aflatoxin B1 in samples such as oil, peanuts and grains, as well as is simple and convenient to operate, low in expense, high in sensitivity, capable of being monitored in the field, and suitable for screening lots of samples.
Owner:BEIJING KWINBON BIOTECH
ELISA kit for distinctively detecting antibodies of classical swine fever (CSF) vaccine immunity and wild virus infection and preparation method thereof
InactiveCN101900731AConvenient prevention and controlImprove purification effectMaterial analysisElisa kitStructural protein
The invention relates to an ELISA kit for distinctively detecting antibodies of classical swine fever (CSF) vaccine immunity and wild virus infection and a preparation method thereof. An indirect ELISA kit or a blockage ELISA kit is formed by expressing and purifying classical swine fever virus (CSFV) non-structural protein NS3, coating a solid-phase carrier and assembling with other matched reagents. The kit has the characteristic of distinctively detecting different antibodies generated by the CSF vaccine immunity and the wild virus infection. The kit can distinctively diagnose the CSF vaccine immunity and the wild virus infection.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Method for detecting pig plague virus specific antibody and its ELISA reagent kit
InactiveCN101144818AGuaranteed specificityGuaranteed Differential DiagnosisMaterial analysis by observing effect on chemical indicatorElisa kitSwine Fever Virus
The invention discloses a method for detecting the specific antibody of classical swine fever virus and a special ELISA kit thereof. The kit includes a classical swine fever virus antigen and an enzyme-labeled classical swine fever virus single-epitope specific antibody; the said swine fever virus antigen is a polypeptide containing one or more than one amino acid residue sequence described in sequence 1. The detection reagent of the classical swine fever virus specific antibody of the present invention can carry out effective detection to the classical swine fever virus specific antibody by solid-phase antigen competition ELISA (blocking method); The B cell epitope ensures the differential diagnosis, and the high degree of conservation of the epitope among various strains ensures the specificity of detection.
Owner:TSINGHUA UNIV +2
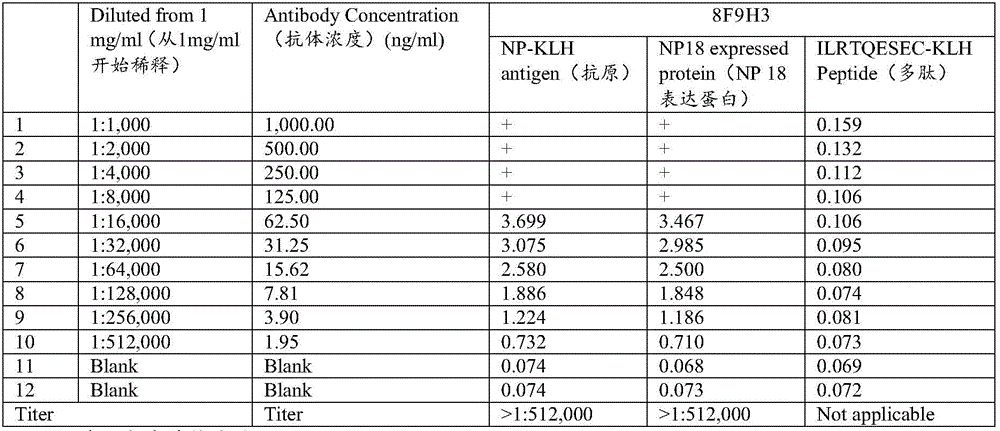
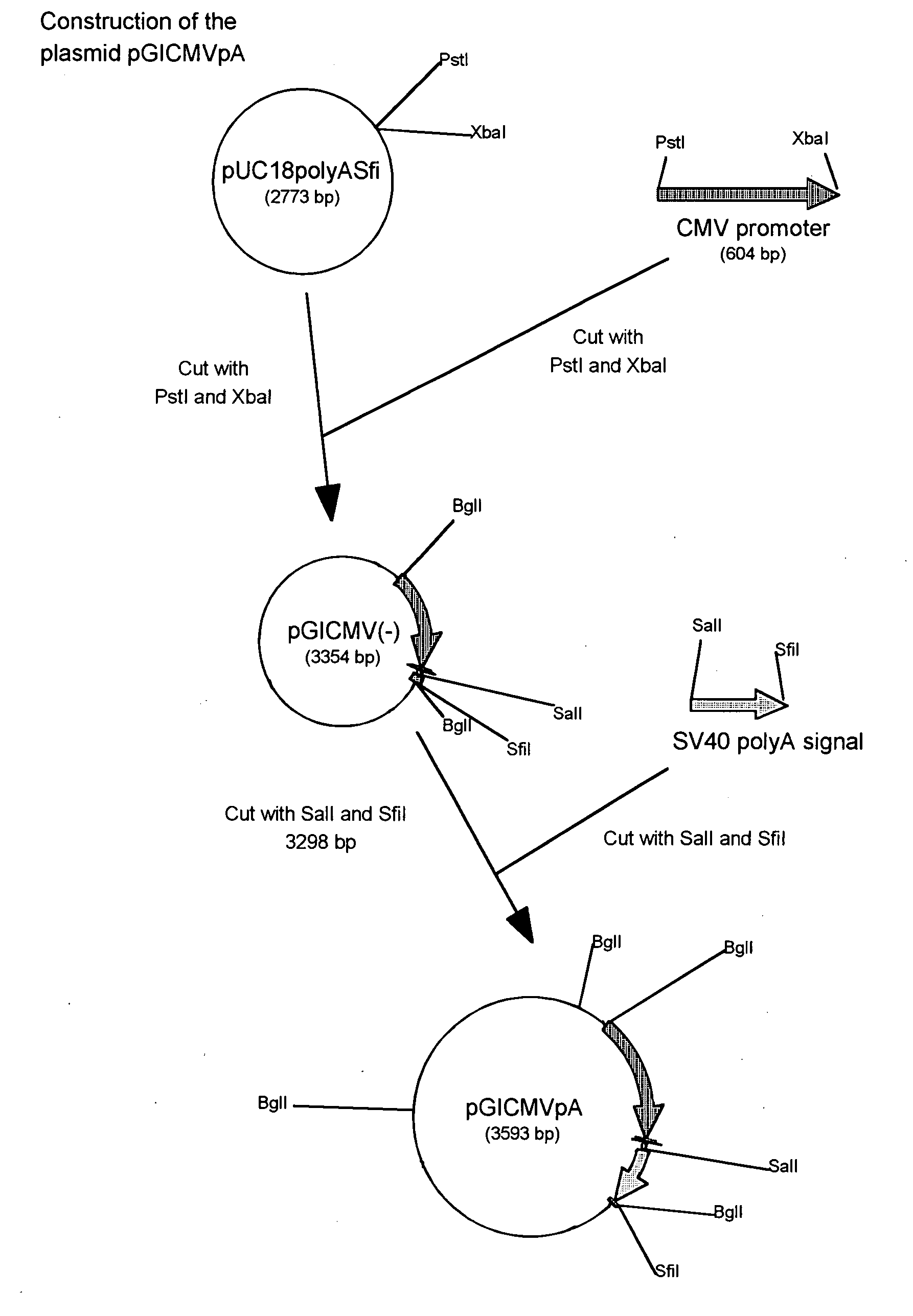
Human adiponectin ELISA kit
The invention relates to human adiponectin monoclonal antibody engineering in the field of medical immunology and relates to preparation of a human adiponectin ELISA kit by employing a hybridoma cell strain XA187 No.1 and a hybridoma cell strain XA187 No.19, and application of the kit to detection of human adiponectin content.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Pig mycoplasma pneumoniae recombination antigen ELISA detection reagent kit
The invention discloses a pig mycoplasma pneumonia recombination antigen ELISA detection Kit. The Kit is provided with an antibody detection plate, enzyme conjugate treatment fluid, a positive control, a negative control, sample diluent, 10x condensed cleaning solution, developing solution A, developing solution B and termination solution. The detection plate of the Kit is a detachable 96-pore enzyme label plate enveloped by the mutational pig mycoplasma pneumonia membrane protein P46 gene protein antigen, the enzyme conjugate treatment fluid is a rabbit anti-pig antibody labeled by horse radish peroxidase, the positive control serum is taken from a pig which is detected positive through indirect hemagglutination and the ELISA Kit of IDEXX and has obvious pig mycoplasma pneumonia lesions in the lungs after anatomy, and the negative control serum is taken from a pig which is detected negative through indirect hemagglutination and the ELISA Kit of IDEXX and has no pig mycoplasma pneumonia lesions in the lungs after anatomy. The pig mycoplasma pneumonia recombination antigen ELISA detection Kit has the advantages of strong specificity, high sensitivity, simple operation, easy large-scale generation and application, and broad market prospect.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Blocking ELISA kit for detecting NDV (Newcastle disease virus) antibody
ActiveCN106596933ASimple and fast operationEasy to operateBiological material analysisElisa kitPositive control
The invention discloses a blocking ELISA kit for detecting an NDV (Newcastle disease virus) antibody. The blocking ELISA kit for detecting the NDV antibody comprises an ELISA plate coated with an NDV inactivated antigen, an NDV positive control serum, an NDV negative control serum and a horseradish peroxidase labeled NDV NP protein monoclonal antibody, wherein the horseradish peroxidase labeled NDV NP protein monoclonal antibody is secreted by a hybridoma cell strain with the preservation number being CCTCC NO: C2016180. The blocking ELISA kit for detecting the NDV antibody can detect serum samples which are infected with the suspected NDV and are from different species, can distinguish an MG7-deficient vaccine from an NDV serum after being infected with a wild virus, and has no cross reaction with a common avian viral pathogen positive serum, thereby being high in sensitivity and specificity, good in reproducibility and suitable for high-throughput detection of serum samples.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Biologically safe Africa swine fever antigen multifactorial serum for ELISA diagnosis
The invention relates to a biologically safe Africa swine fever antigen multifactorial serum for ELISA (enzyme-linked immuno sorbent assay) diagnosis. A technical scheme adopted in the invention includes: adopting gene-expressed structural protein P72, K205R, P54, and A104R, conducting chemical purification, carrying out coating with a Freund's incomplete adjuvant, performing intramuscular immunization on laboratory swine in three batches, collecting swine blood after one month, separating serum, implementing serological testing, and conducting subpackaging and preservation. The serum is subpackaged into ELISA kits to undergo test according to conventional ELISA test methods.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
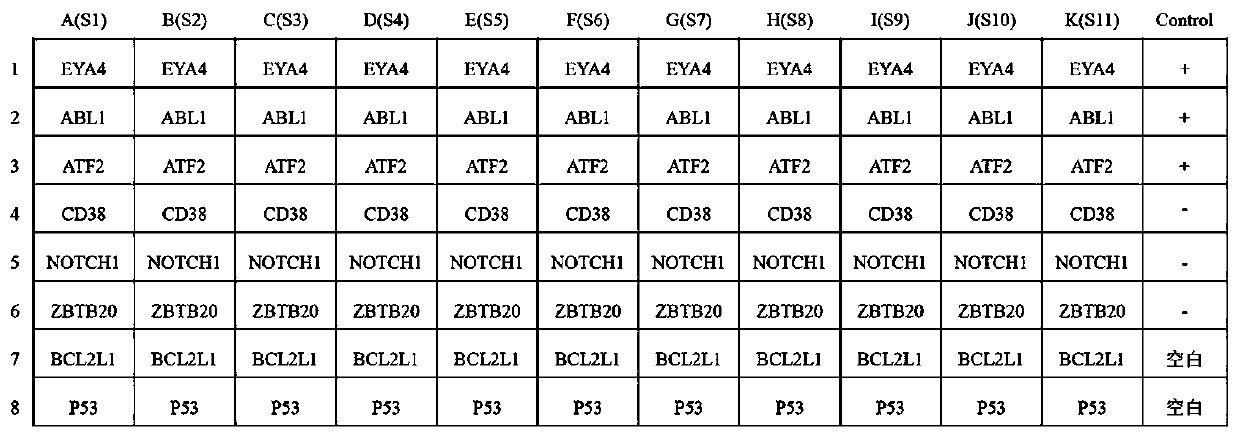
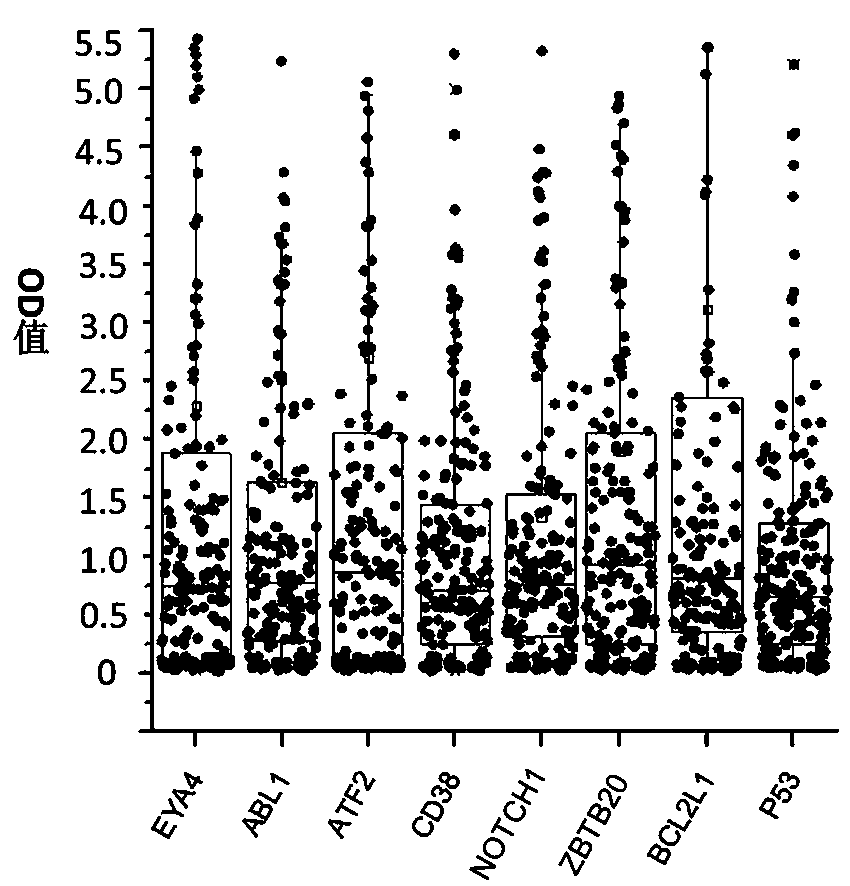
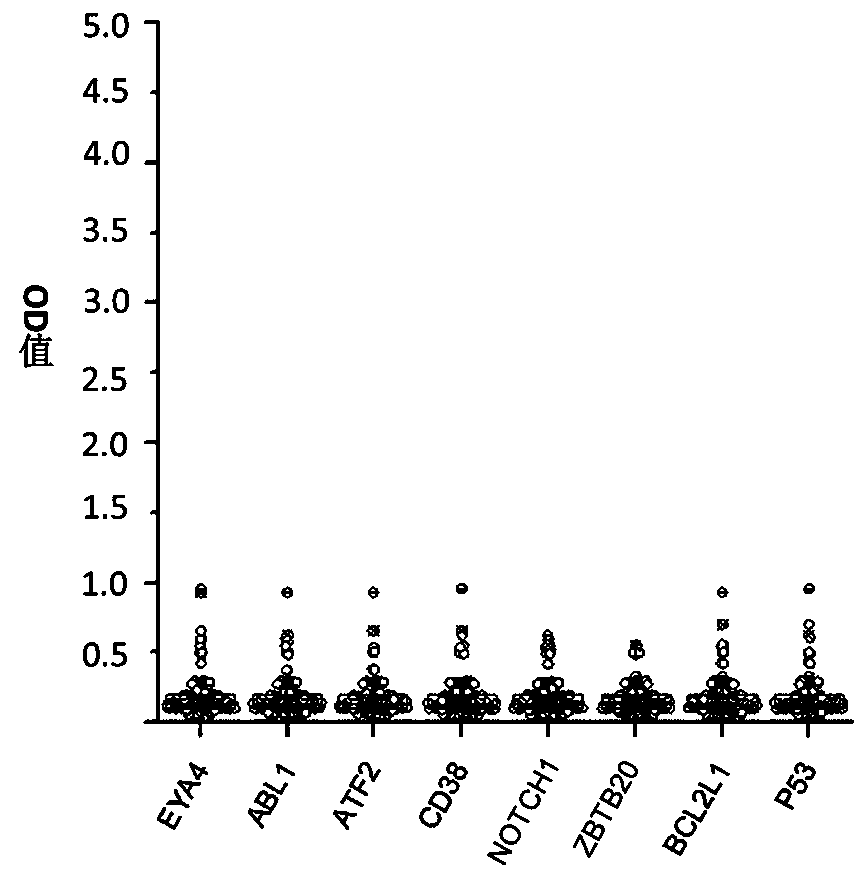
Autoantibody joint detection ELISA kit for screening early esophageal cancer
ActiveCN110187108AEfficient detectionHigh detection sensitivityMaterial analysisHigh risk populationsElisa kit
The invention belongs to the technical field of tumor medicine, and particularly discloses an autoantibody joint detection ELISA kit for screening early esophageal cancer. The kit comprises a solid-phase carrier and tumor-associated antigens coated on the solid-phase carrier, wherein the tumor-associated antigens are EYA4, ABL1, ATF2, CD38, NOTCH1, ZBTB20, BCL2L1 and P53. Furthermore, the kit alsocomprises a sample diluent, a second antibody, a second antibody diluent, positive control serum, negative control serum, a color developing solution, a stop solution and a washing solution. The ELISA kit provided by the invention can effectively detect esophageal cancer, especially early esophageal cancer, has the detection sensitivity of 94% and the specificity of 79%, can be used for large-scale screening of asymptomatic people in high-incidence areas of esophageal cancer, and is beneficial to screening and early discovery of asymptomatic high-risk population.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV +1
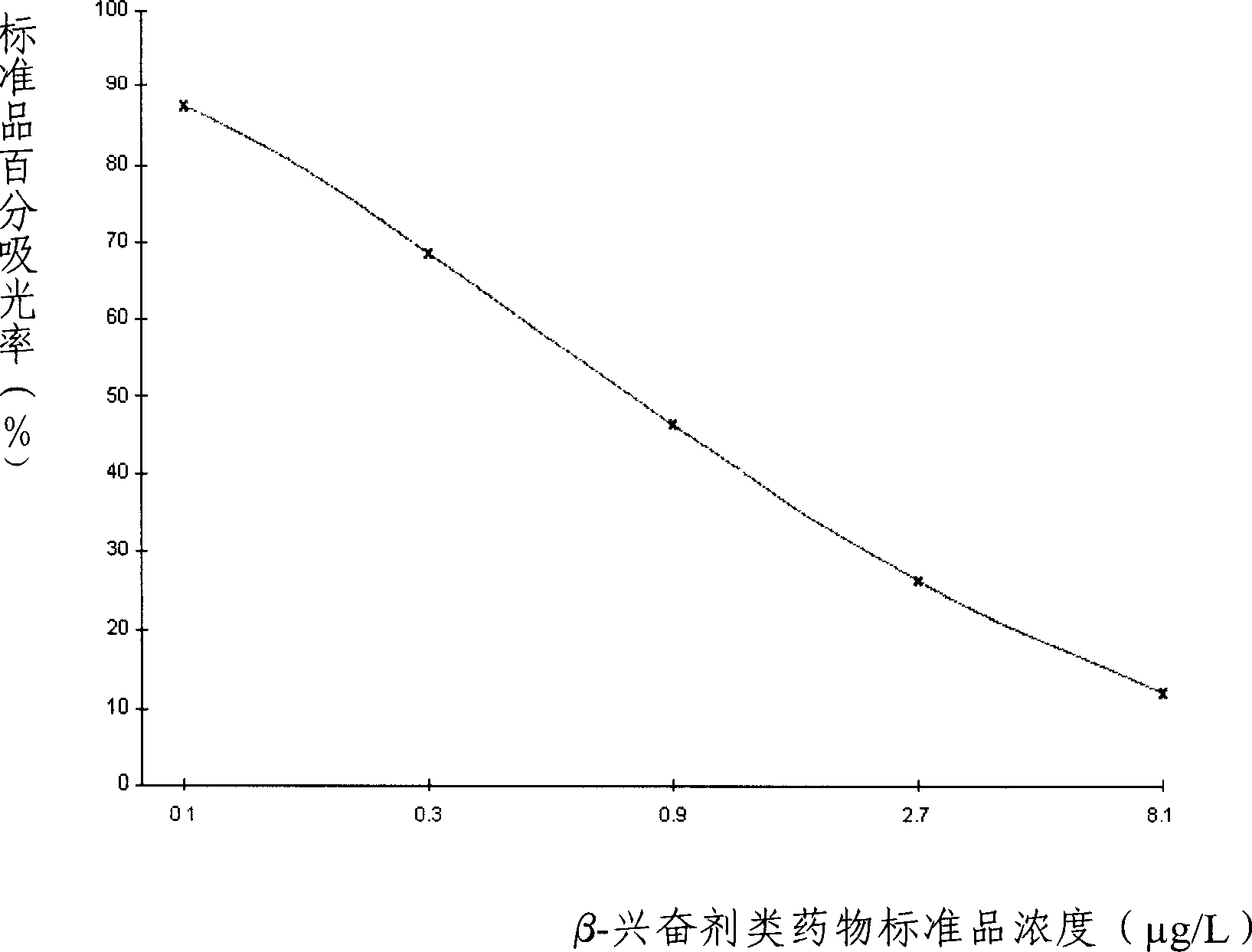
ELISA kit for detecting beta-stimulants and detection method thereof
ActiveCN1766617ALow pre-processing requirementsThe pre-processing process is simpleBiological testingElisa kitStimulant
The invention relates to an enzyme immune agent box for detecting ª‰-excitant drugs, which comprises: enzyme mark plate which coats ª‰-excitant drugs, enzyme mark antibody, clenobuterol standard solution, base material color developing solution, ª‰-excitant drugs antibody working solution, compression cleaning liquid, ending solution and compression twin solution. The invention also discloses a method for applying the detecting method, which comprises: first doing sample front process, then using the agent box to detect, at last analyzing the detected result.
Owner:BEIJING WANGER BIOTECH
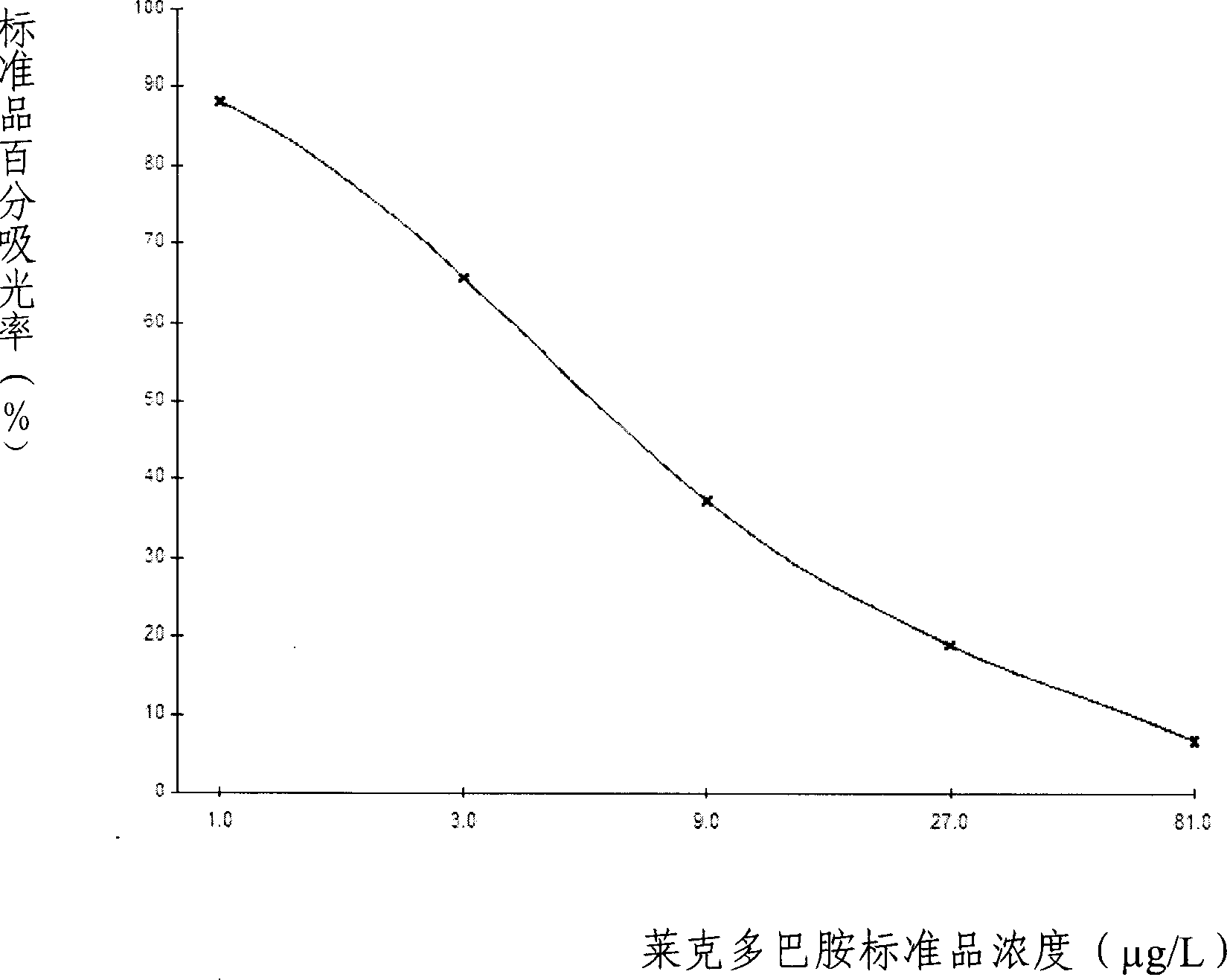
ELISA kit for detecting ractopamine in animal derived food
The invention discloses an enzyme immune agent box for detecting lake drugs of animal foodstuff; it also provides a method for using the agent to detect the lake drugs residue. The agent box comprises: enzyme mark plate which coats lake drugs antigen or antibody, lake drugs mouse monoclonal antibody or polyclonal antibody working solution, enzyme mark antibody or enzyme mark lake drugs antigen, lake drugs standard solution, base material color developing solution, compression cleaning liquid, ending solution and compression twin solution. The invention also discloses a method for applying the detecting method, which comprises: first doing sample front process, then using the agent box to detect, at last analyzing the detected result.
Owner:BEIJING WANGER BIOTECH
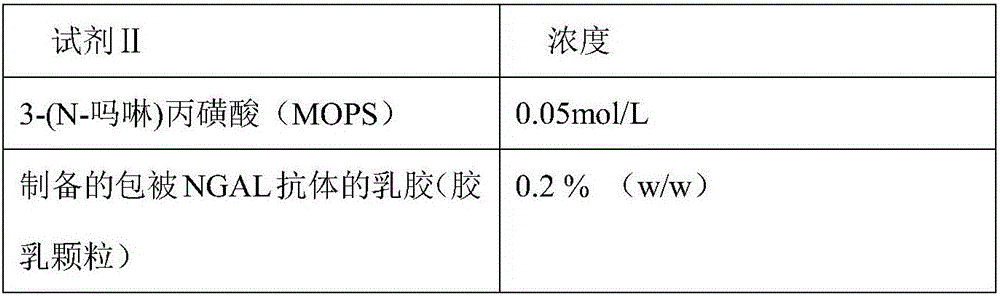
Neutrophil gelatinase-associated lipocalin (NGAL) protein level ELISA kit
ActiveCN106814193ASpeed up dissociationColor/spectral properties measurementsBiological testingElisa kitBinding site
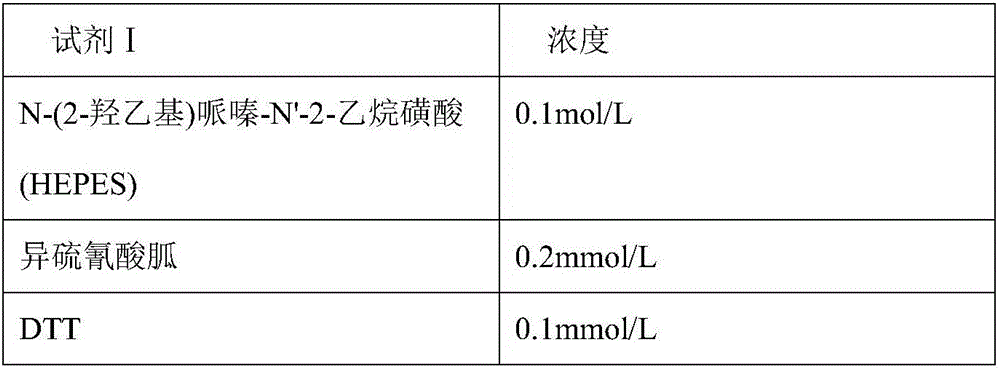
Disclosed is a neutrophil gelatinase-associated lipocalin (NGAL) protein level ELISA kit which is composed of a reagent I and a reagent II. The reagent I comprises a slow-release agent and a denaturant; the reagent II comprises latex particles coated with NGAL antibodies. Aggregated protein is denatured to some extent after being added with the denaturant, physical and (or) chemical binding site is exposed, chemical binding action of the chemical binding site is fractured through sulfydryl dissociation agent , while physical binding action of the physical binding site is dispersed by surface active agent, so that the aggregated protein is disaggregated. Therefore, it is quite important to choose the appropriate types and concentrations of denaturant, sulfydryl dissociation agent, and surface active agent; the aggregate is disaggregated without interference on following immunological detection. According to the method, the the appropriate types and concentrations of denaturant, sulfydryl dissociation agent and surface active agent are determined and chosen specifically, which solves the technical problem above.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
ELISA test box for detecting zearalenone and preparing and detecting method thereof
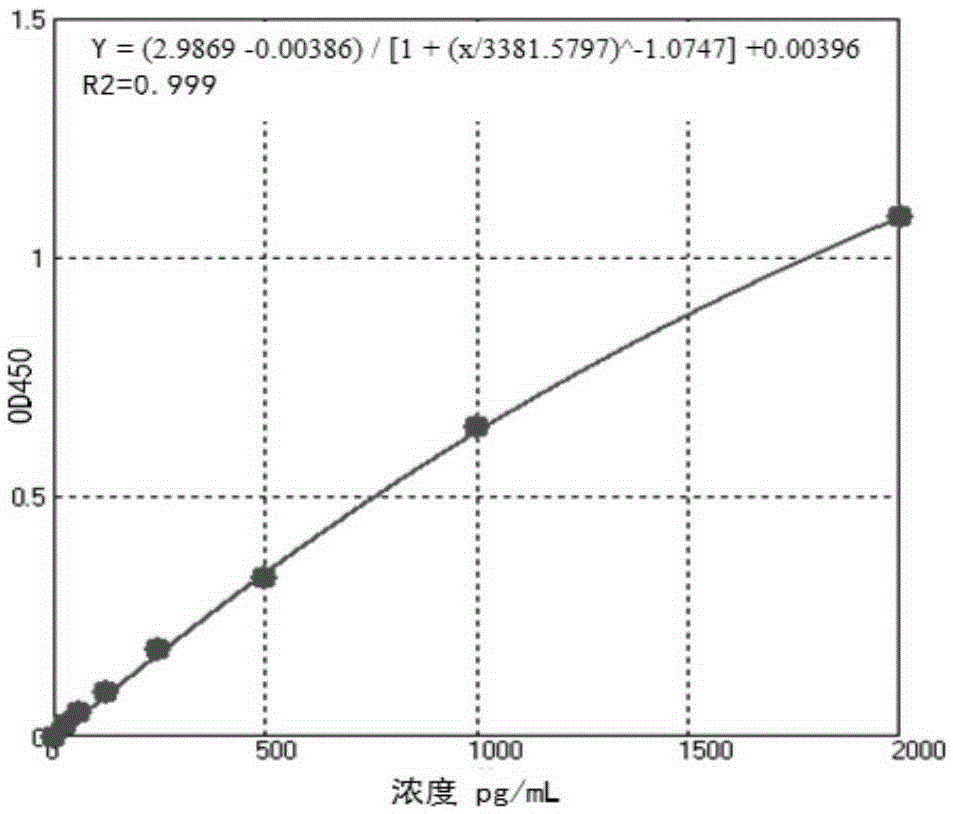
The invention relates to an ELISA kit for detecting zearalenone, the detection is rapid, sensitive, accurate, quantitative, simple in operation, low in requirements on sample purity and strong in specificity, thereby being particularly applicable to the detection of large quantities of samples; and the invention also provides a preparation of the kit and a detection method. The kit comprises washing liquid, color developing liquid A, color developing liquid B and stop solution, and the kit is characterized in that: the kit also comprises a coated plate, a zearalenone standard product, a zearalenone monoclonal antibody freeze-dried product and an enzyme-labeled goat anti-mouse antibody free-dried product; when in detection, the coated plate is taken, 50mu1-100mu1 of the ZEN standard product or a well processed sample is added into the respective micropores, 50mul-100mul of the anti-ZEN antibody is added, the incubation is carried out at 35 DEG C-45 DEG C for about 0.5 hour-1 hour, the washing liquid is used for washing for 3 times-5 times, 50mu1-100mu1 of the horseradish peroxidase (HRP)-goat anti-mouse antibody is added, the incubation is carried out at about 35 DEG C-45 DEG C for about 0.5 hour-1 hour, the washing liquid is used for washing for 3 times-5 times, 50mu1-100mu1 of the color developing liquid A and 50mu1-100mu1 of the color developing liquid B are added, the mixture is placed still in the dark for 10 minutes-20 minutes, then the stop solution is added, the absorbance value is measured at 450nm, and the ZEN content in the sample is calculated from a standard curve.
Owner:BEOSON JIANGSU FOOD SAFETY TECH CO LTD
Cyclic chimeric citrullinated peptide antigen and application thereof
InactiveCN104262489AImprove stabilityIncrease exposureBiological testingHybrid peptidesPeptide antigenDisulfide bonding
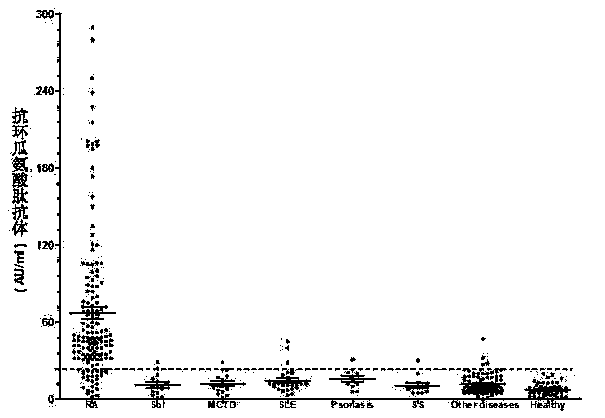
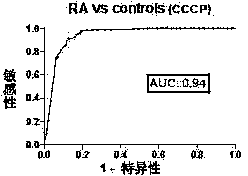
The invention discloses a cyclic chimeric citrullinated peptide antigen and an application thereof. The preparation of the cyclic chimeric citrullinated peptide antigen comprises the following steps: firstly connecting and jogging three small-molecular antigen peptides, namely a citrullinated peptide1, a citrullinated peptide 2 and a citrullinated peptide 3 derived from a silk polymerizing protein / an intermediate filament protein, and then synthetizing a cyclic polypeptide with a similar protein beta-corner structure by forming a disulfide bond through two cysteines inserted into the end N and the end C of a chimeric peptide. The cyclic chimeric citrullinated peptide antigen coats a solid-phase vector to prepare an indirect enzyme linked immunosorbent assay kit used for detecting the hypotype of multiple anti-citrullinated protein antibodies contained in RA (Rheumatoid Arthritis) serum. The cyclic chimeric citrullinated peptide antigen and the ELISA kit thereof which are disclosed by the invention have the advantages of simple preparation and experimental operation process, good result repeatability, qualification or quantification and wide clinical application and scientific research value and are outstandingly enhanced in detection sensibility and diagnosis value on RA compared with an international similar kit.
Owner:陈仁奋
Enzyme-linked immunosorbent inspect kit for inspecting sulfa drugs and method thereof
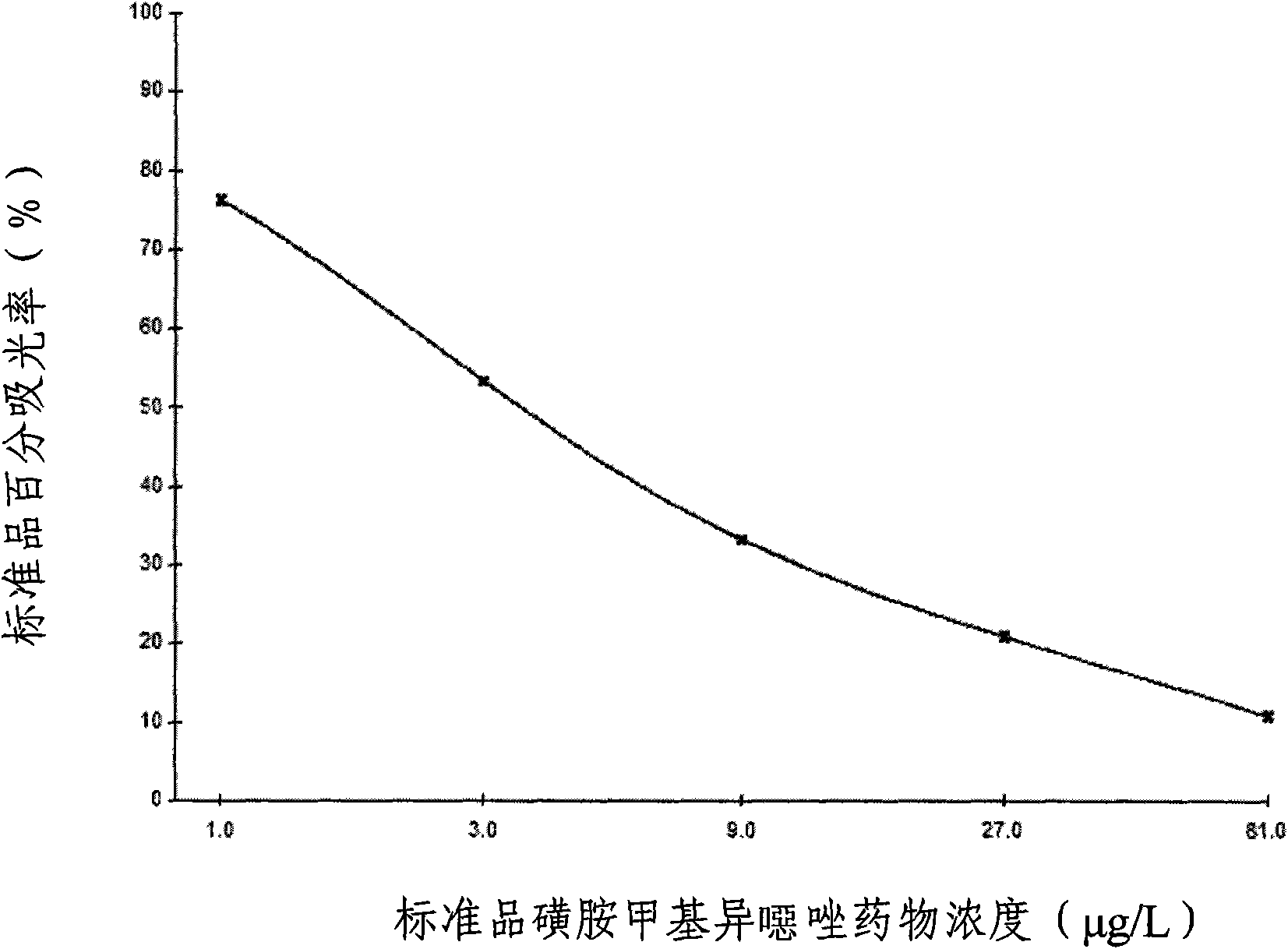
The invention provides an enzyme-linked immunosorbent kit for inspecting sulfa drugs, comprising an ELISA plate which is coated with coating antigen, an enzyme label, sulfa drug specific antibody working liquid (being contained when the antigen is coated on the ELISA plate and the enzyme label is enzyme labeling antibody or antibody is coated on the ELISA plate and the enzyme label is enzyme labeling antigen), sulfamethoxy-isoxazole standard product solution, substrate color development solution, stop solution, concentrated washing liquid and concentrated complex solution. The invention further discloses a method which applies the enzyme-linked immunosorbent kit for inspecting the sulfa drugs, and the method comprises the steps of firstly carrying out the pre-treatment on a sample, then using the kit for inspecting and finally analyzing the inspection result. The provided enzyme-linked immunosorbent kit can be used for inspecting the residual amount of the sulfa drugs in animal tissues(chicken, pork, fish and shrimp), honey, eggs, milk, feeds and other samples, the operation is simple, the cost is low, the sensitivity is high and the enzyme-linked immunosorbent kit can be monitore d on-site and is applicable in screening mass samples.
Owner:BEIJING WANGER BIOTECH
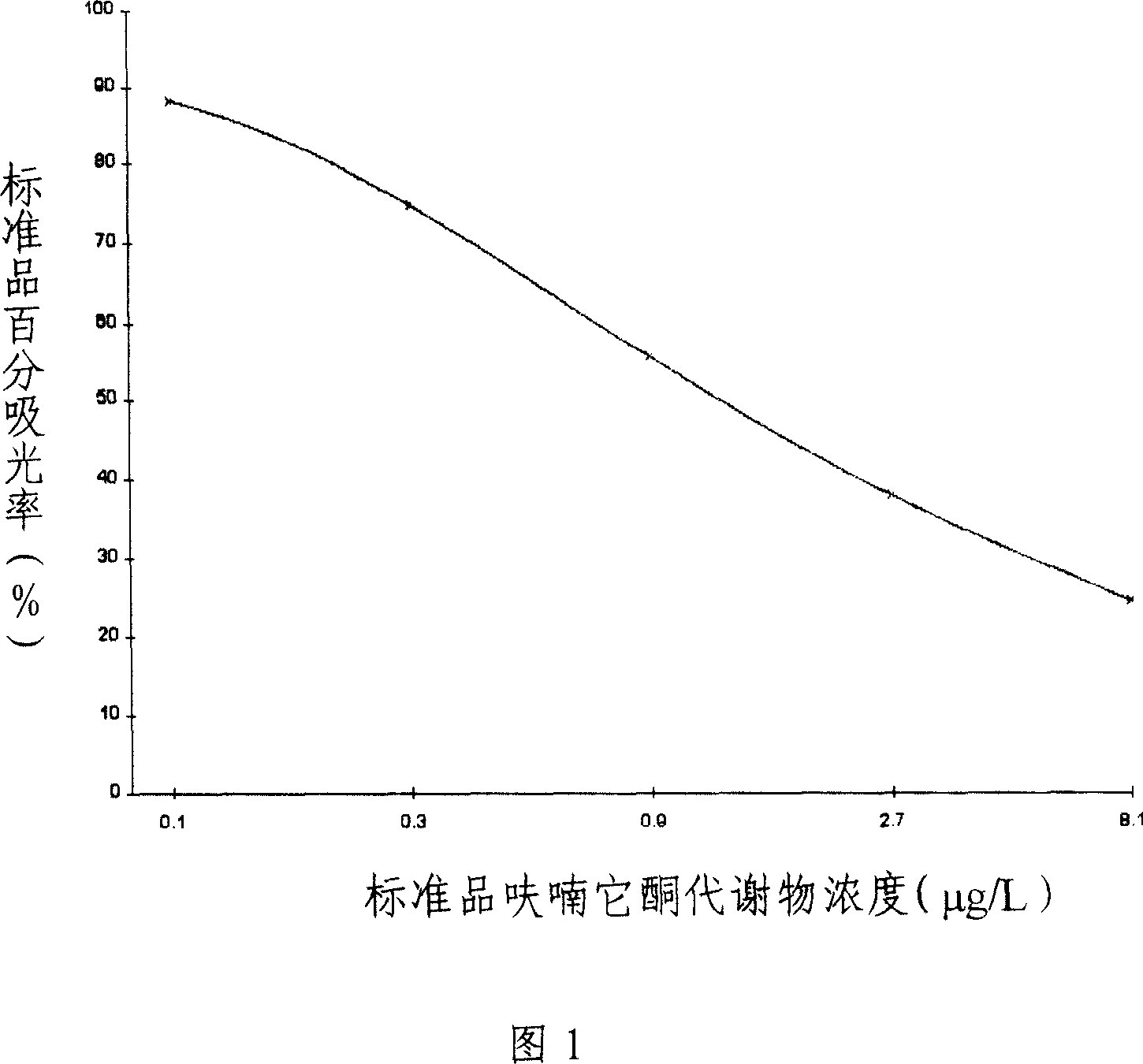
Enzyme linked immunosorbent reagent casing for detecting furantoin metabolite and uses thereof
InactiveCN101013130AConducive to preservationImprove stabilityMaterial analysisSite monitoringShrimp
The invention provides a detecting furanketone metabolite ELISA kit, which contains: an ELISA plate covered by the original coated, enzyme marker, furanketone metabolite specific antibody or furanketone metabolite derivative antibody, furanketone metabolite standard solution or furanketone metabolite derivative standard solution, the substrate color solution, the termination solution, the condensed washing solution, the condensed complex solution. The invention also provides a method to apply the above ELISA kit detecting furanketone metabolite, which includes steps: firstly, sample pre-treatment, and then using the kit for testing, and finally analyzing testing results. The invention is to provide the furanketone metabolite residues in the ELISA kit for detection of animal derived foods, such as chicken, pork, fish, shrimp, milk, honey, egg, and other samples, and the detection method is simple, low cost, high sensitivity, and it can monitor on the scene and suitable for screening large number of samples.
Owner:BEIJING WANGER KANGTAI BIOTECH +1
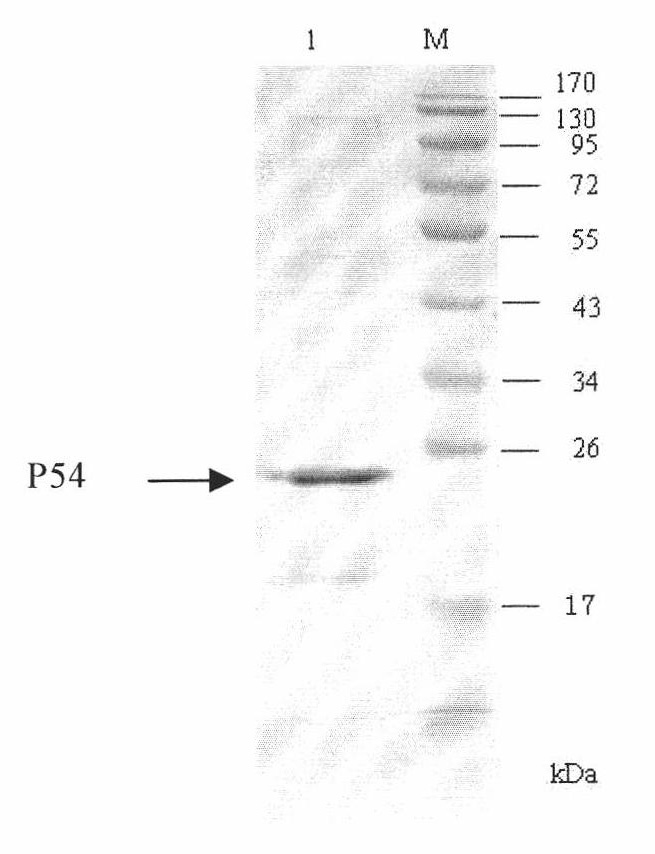
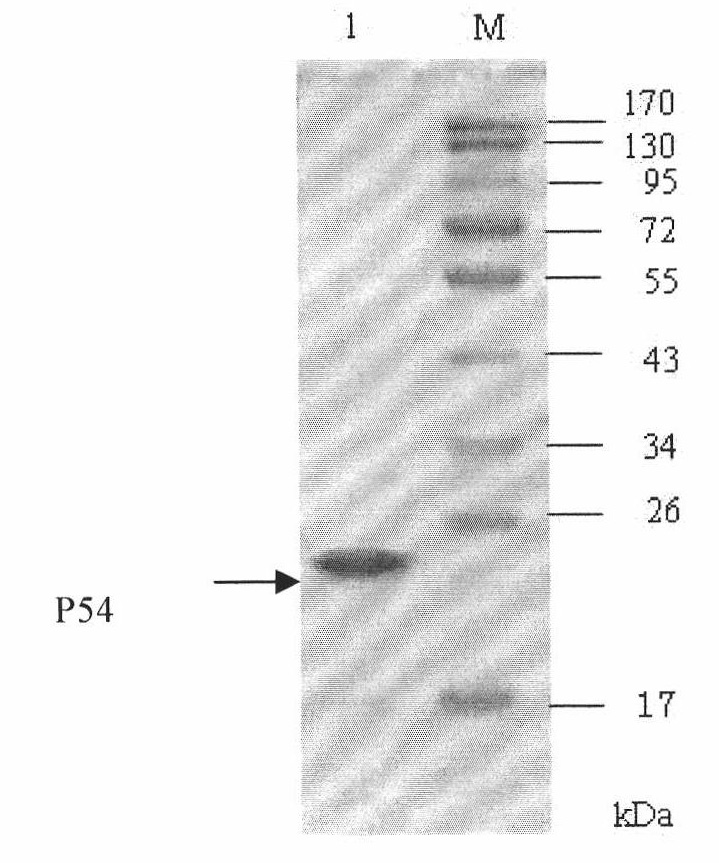
Competitive ELISA (Enzyme-Linked Immuno Sorbent Assay) kit for detecting antibody of African swine fever virus and application thereof
The invention discloses a competitive ELISA (Enzyme-Linked Immuno Sorbent Assay)kit for detecting an antibody of an African swine fever virus and application thereof, belonging to the technical field of organisms. The kit is used for detecting an antibody of the African swine fever virus in pig serum by adopting prokaryotically expressed recombinant P54 protein as an envelope antigen according toa competitive ELISA principle. The envelope antigen in a 96 pore plate in the kit is prokaryotically expressed recombinant P54 protein and has favorable antigenicity. The enzyme-linked immuno kit provided by the invention comprises the P54 protein enveloped 96 pore plate, positive control, negative control, a horseradish peroxidase marked monoclonal antibody, a concentrated cleaning solution, serum diluent, a TMB substrate and a stopping solution. The kit can be used for screening samples in bulk, main reagents in the kit are provided in a working solution way, and the use is convenient.
Owner:ANIMAL & PLANT & FOOD INSPECTION CENT OF TIANJIN ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Human oophoroma tumor marker HE4 enzymoimmunoassay kit
InactiveCN101525614ASave foreign exchangeImprove stabilityMicroorganism based processesFermentationElisa kitPeroxidase
The invention discloses a human oophoroma tumor marker HE4 enzymoimmunoassay kit, a preparation method and an application thereof. A PCR overlapping method is adopted to fully manually synthesize an HE 4 gene by a synthesized HE4 gene prime; the gene is constructed into a Rho GEX-4T1 expression vector for protein expression; the expressed HE4 fusion protein is restricted by enzyme and purified to obtain an HE4 purified product which immunes animals to obtain a polyclonal antibody and a monoclonal antibody; a rabbit antihuman HE4 polyclonal antibody is used to wrap and close an ELISA plate; the rabbit polyclonal antibody is used as a capture antibody, rat antihuman HE4 monoclonal antibody labeled by horseradish peroxidase is used as a detection antibody so as to be assembled into a double-antibody sandwiched ELISA kit for detecting HE4. The kit has the advantages of high detection sensitivity and high specificity, is suitable for the early diagnosis of oophoroma and provides oophoroma patients for curative effect observation and prognosis judgment with an easy, convenient, rapid, economical and reliable detection method.
Owner:大连美亿德生物科技有限公司
Turkey herpesvirus vectored recombinant containing avian influenza genes
InactiveUS20080241188A1Easy to distinguishEasy to detectSsRNA viruses negative-senseVectorsVaccinationElisa kit
The present invention provides a recombinant turkey herpesvirus modified by the presence of the cDNA encoding the hemagglutinin protein of avian influenza virus under a promoter. A poultry vaccine comprising the recombinant turkey herpesvirus described in the present invention can induce serological responses that may be easily detected by the hemagglutination inhibition assay but not by commercially available diagnostic ELISA kits; thus enabling easy differentiation between vaccination and field infection.
Owner:ZEON CORP +1
Indirect ELISA kit for detecting African swine fever virus antibody and application thereof
InactiveCN102236017AImmunoglobulinsMaterial analysisAfrican swine fever virus AntibodyPositive control
The invention discloses an indirect ELISA kit for detecting an African swine fever virus antibody and an application thereof, and belongs to the technical field of biology. The kit adopts prokaryotic expression recombinant P30 protein as a coating antigen, and detects the antibody of African swine fever virus in porcine serum based on the indirect ELISA principle. The coating antigen in a 96-well plate of the kit is prokaryotic expression recombinant P30 protein which has good antigenicity. The enzyme-linked immunoassay kit provided by the invention comprises a 96-well plate coated with P30 protein, a positive control, a negative control, a horseradish peroxidase-labeled rabbit anti-porcine IgG polyclonal antibody, a concentrated washing liquid, a serum diluent, a TMB substrate, and a terminating liquid. The kit of the invention is applicable to the screening of large quantities of samples, and main reagents in the kit are provided in a form of operating fluid which is convenient for use.
Owner:陈文刚
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com