Enzyme linked immunosorbent reagent casing for detecting furantoin metabolite and uses thereof
A furanotazone and metabolite technology, applied in the field of enzyme-linked immunosorbent assay kits, can solve the problems of complex instruments and equipment, unsuitable for on-site monitoring, screening of a large number of samples, and tedious processes, so as to overcome technical problems and reduce the lower limit of sample detection. , the effect of improving the detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1 Preparation of kit components
[0067] 1. Antigen Synthesis
[0068] a. Synthesis of furaltadone metabolite derivative hapten
[0069] The furaltadone metabolite and m-carboxybenzaldehyde are reacted in water for derivatization to obtain the furaltadone metabolite derivative hapten.
[0070] Preparation process of furaltadone metabolite derivative hapten:
[0071] Dissolve 2000mg of furaltadone metabolite in 20ml of pure water, dissolve 1500mg of m-carboxybenzaldehyde in 30ml of water, add it to the furaltadone metabolite solution and react at room temperature for 48 hours to obtain a light yellow precipitate which is the furaltadone metabolite Derivative haptens. Repeatedly washing with 50ml of water for 5-6 times, drying to obtain the furaltadone metabolite derivative hapten.
[0072] b. Preparation of immunogenic furaltadone metabolite derivative conjugated antigen
[0073] The immunogen is obtained by coupling the furaltadone metabolite derivative ...
Embodiment 2
[0096] Example 2 Construction of an enzyme-linked immunosorbent assay kit for detecting furaltadone metabolites
[0097] An enzyme-linked immunosorbent assay kit for detecting furaltadone metabolites was constructed to include the following components:
[0098] (1) Enzyme plate coated with anti-furaltadone metabolite antigen;
[0099] (2) Goat anti-mouse anti-antibody labeled with horseradish peroxidase;
[0100] (3) Monoclonal antibody to furaltadone metabolite;
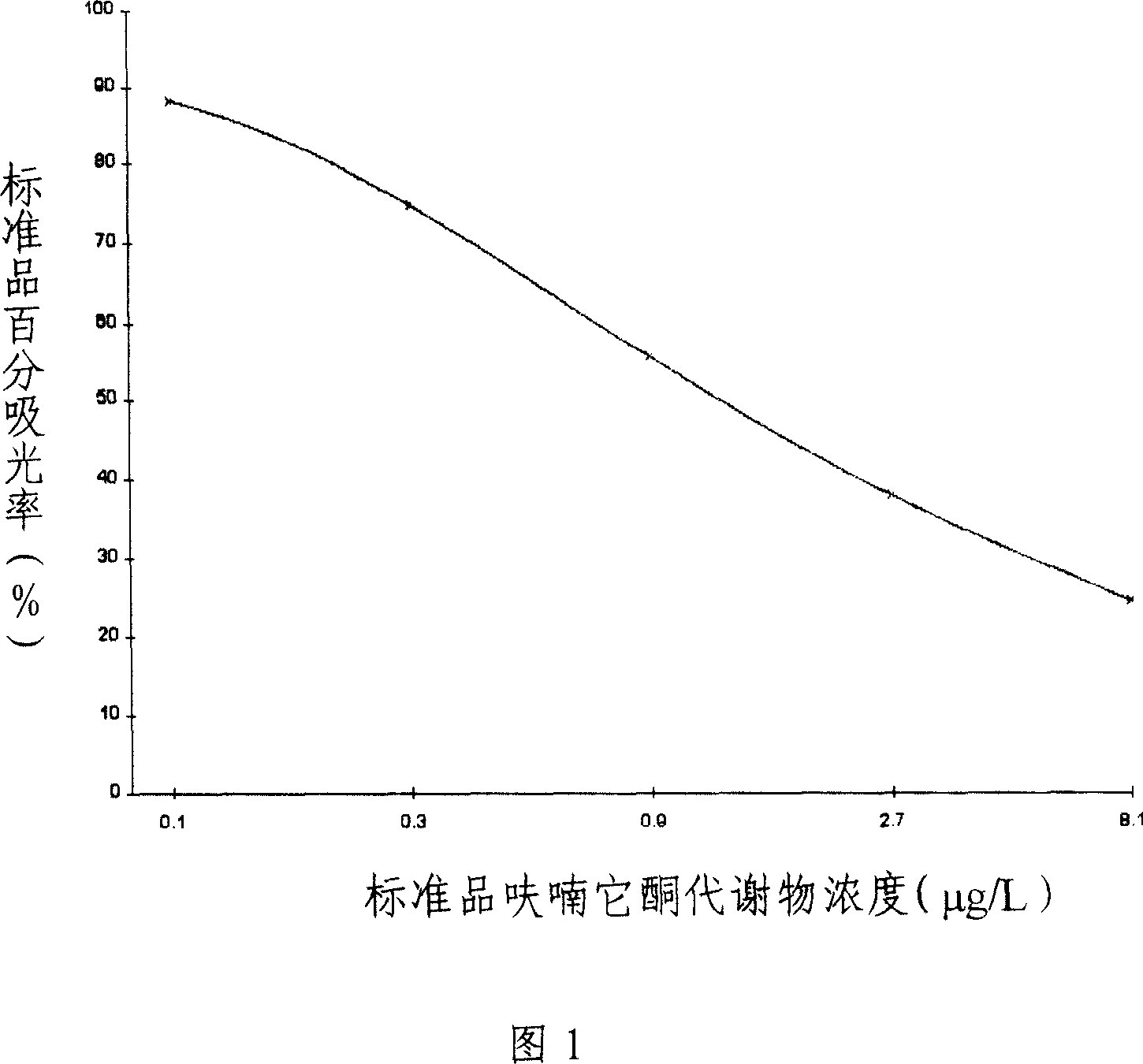
[0101] (4) 6 bottles of furaltadone standard solution, the concentrations were 0 μg / L, 0.1 μg / L, 0.3 μg / L, 0.9 μg / L, 2.7 μg / L, 8.1 μg / L;
[0102] (5) The substrate chromogenic solution is composed of A liquid and B liquid, the substrate chromogenic liquid A liquid is carbamide peroxide, and the substrate chromogenic liquid B liquid is tetramethylbenzidine;
[0103] (6) The stop solution is 2mol / L hydrochloric acid;
[0104] (7) The concentrated washing solution is 0.01M, pH7.6, containing 0.8% Tween 20 and 1‰ so...
Embodiment 3
[0106] Example 3 Detection of furaltadone metabolite residues in samples
[0107] 1. Sample pretreatment
[0108] Animal tissues (chicken, pork, fish and shrimp)
[0109] Take 1±0.05g tissue sample homogenate, add 4ml distilled water, 0.5ml 1M HCl and 100μl 10mM 2-nitrobenzaldehyde, shake well; incubate overnight at 37°C (about 16h); add 5ml 0.1M K 2 HPO 4 , 0.4ml 1M NaOH and 5ml ethyl acetate, shake vigorously for 30s; centrifuge at room temperature (20~25℃ / 68~77) over 3000g for 10min; take out 2.5ml ethyl acetate and blow it with nitrogen at 50℃ Dry or evaporate to dryness with a rotary evaporator; dissolve the dry matter with 1ml of n-hexane, and mix well with 1ml of the diluted complex solution; centrifuge at room temperature (20~25℃ / 68~77) over 3000g for 10min; use 50μl of the lower layer liquid for analysis.
[0110] 2. Detection with kit
[0111] Add 50 μl of a series of standard solutions or sample solutions to the microwells of the microtiter plate coated with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com