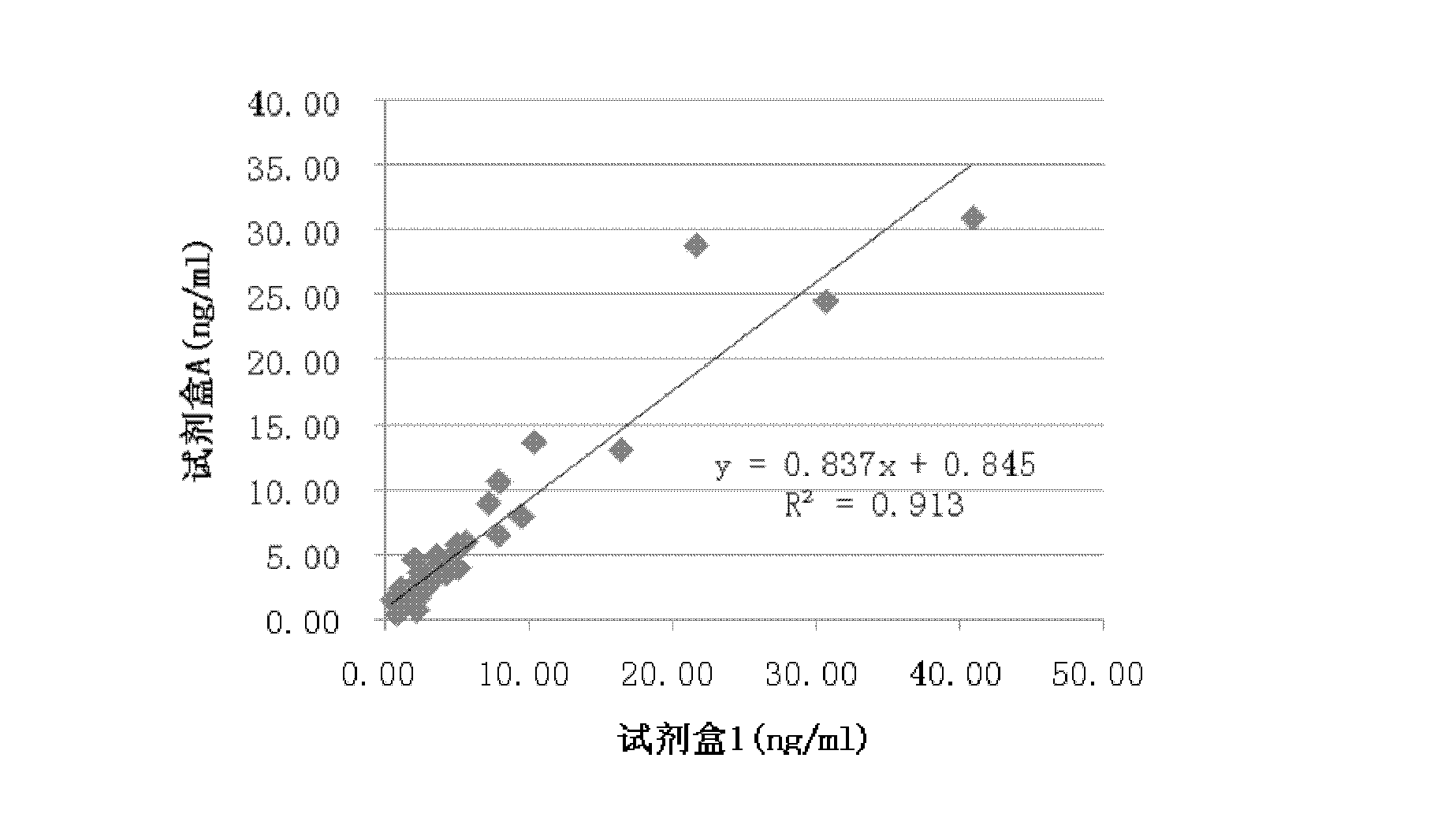
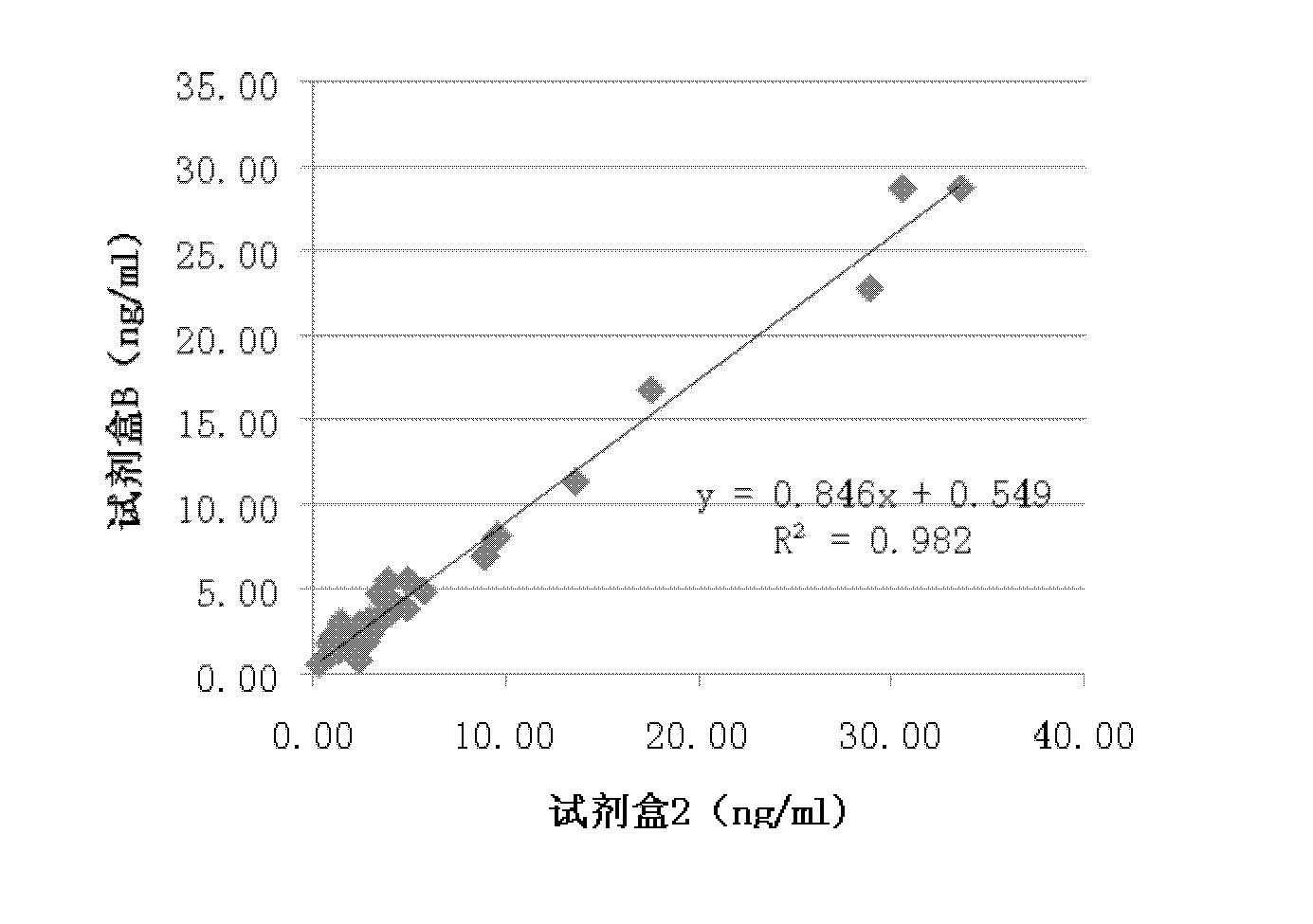
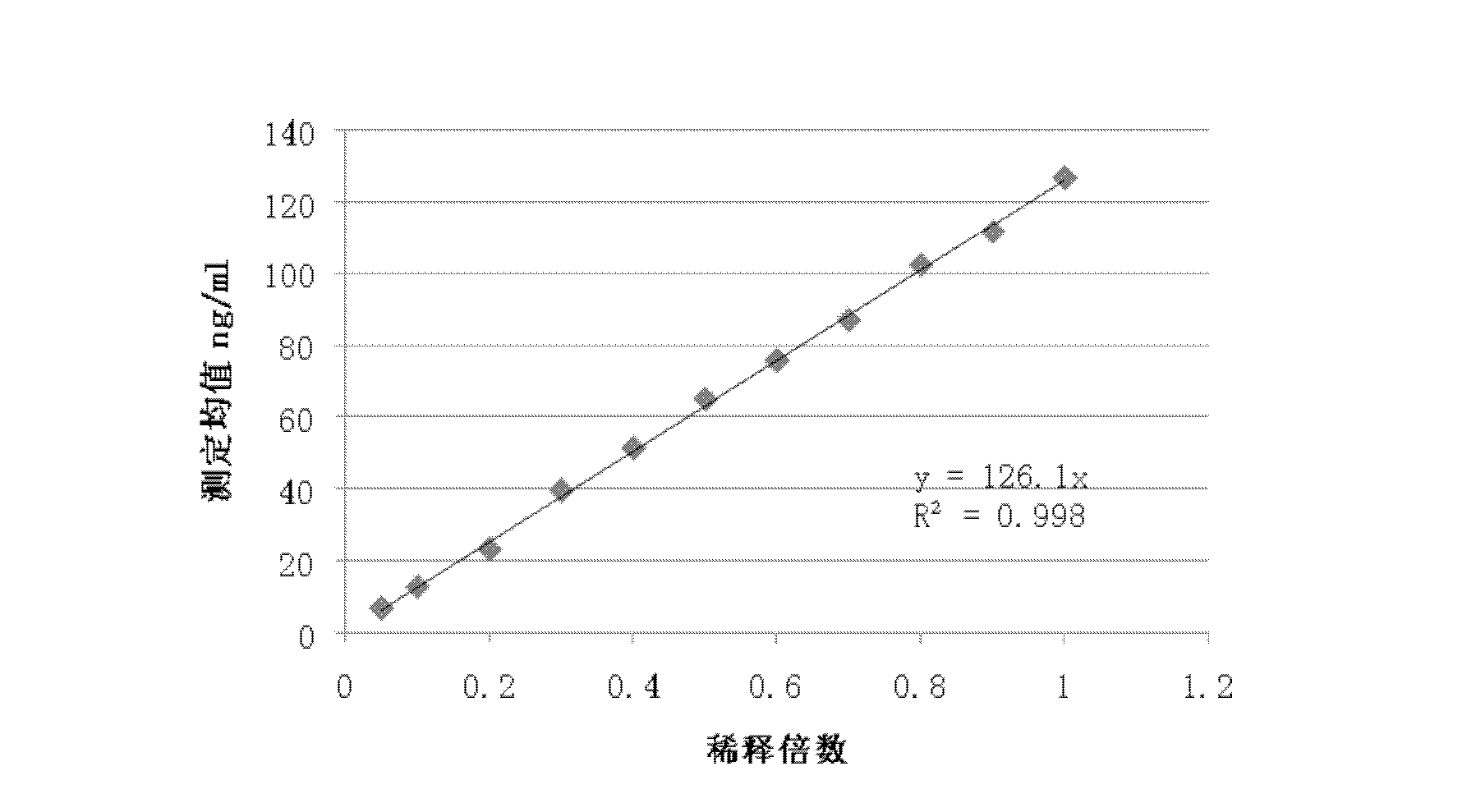
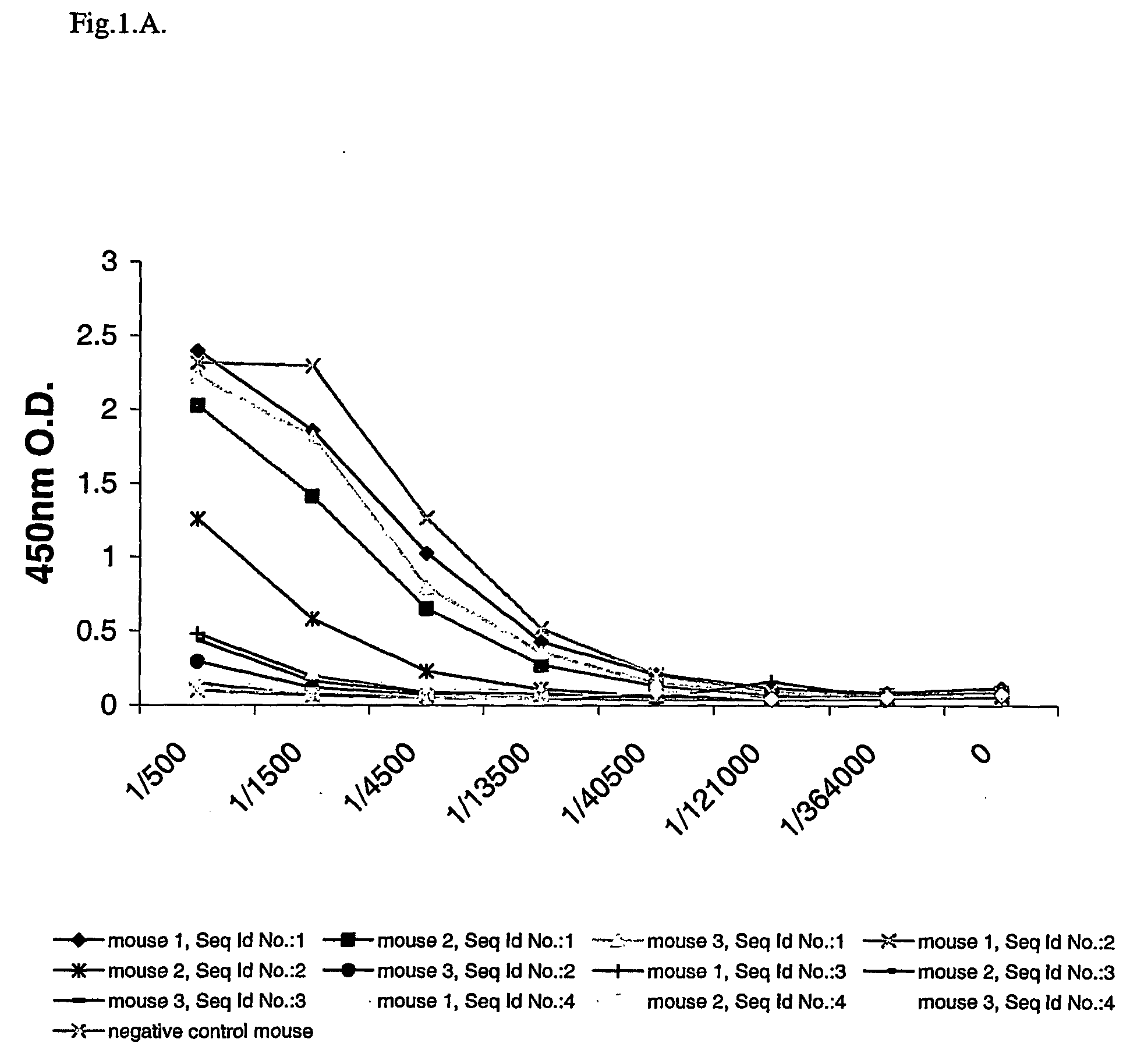
Patents
Literature
593 results about "Protein.monoclonal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
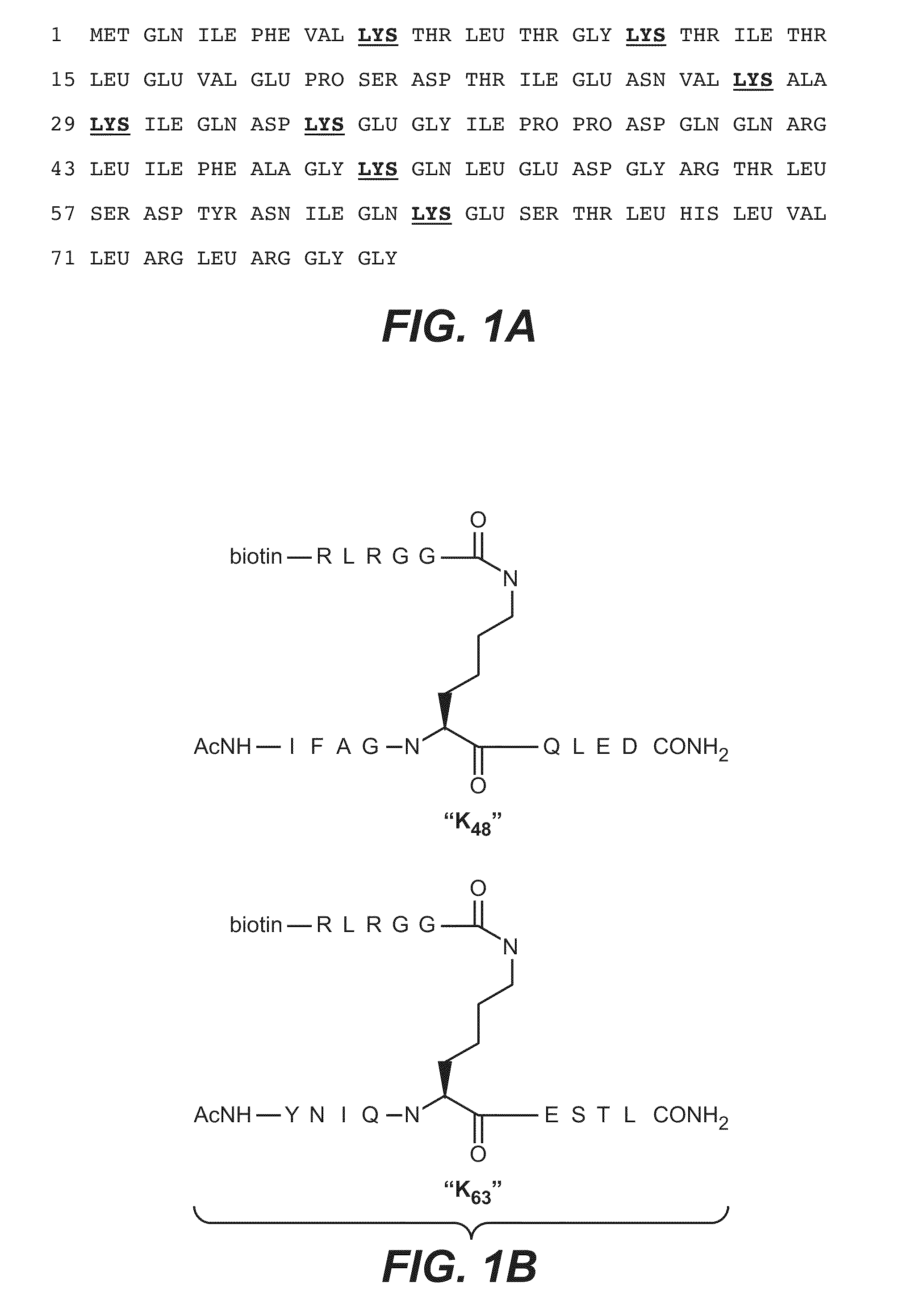
Monoclonal gammopathies are conditions in which abnormal proteins are found in the blood. These proteins grow from a small number of plasma cells in the bone marrow.
Kit for determining heart-type fatty acid binding protein in serum or urine by latex enhanced turbidimetric immunoassay
ActiveCN102628864AStrong specificityImprove accuracyColor/spectral properties measurementsBiological testingLatex particlePHA granule
The invention relates to a kit for determining heart-type fatty acid binding protein in serum or urine by latex enhanced turbidimetric immunoassay. Specifically, the kit for determining the heart-type fatty acid binding protein comprises a reagent R1, a reagent R2 and a calibrator, wherein the reagent R1 contains a reaction promoter, an antiseptic, a surfactant, a stabilizing agent, an electrolyte and a buffer; the reagent R2 contains latex particles with binding of anti-heart-type fatty acid binding protein monoclonal antibody and polyclonal antibody, an antiseptic, a surfactant, a stabilizing agent, an electrolyte and a buffer; and the calibrator contains an antiseptic, an electrolyte, a stabilizing agent, a heart-type fatty acid binding protein pure product and a buffer. By the complex coating method of latex particles with the monoclonal antibody and the polyclonal antibody, high sensitivity and wide linear range of the kit are guaranteed. Simultaneously, the kit also has advantages of high accuracy, good repeatability, strong singularity, easy operation and the like, and is applicable to an automatic biochemical analyzer which is commonly used in clinic.
Owner:BEIJING STRONG BIOTECH INC
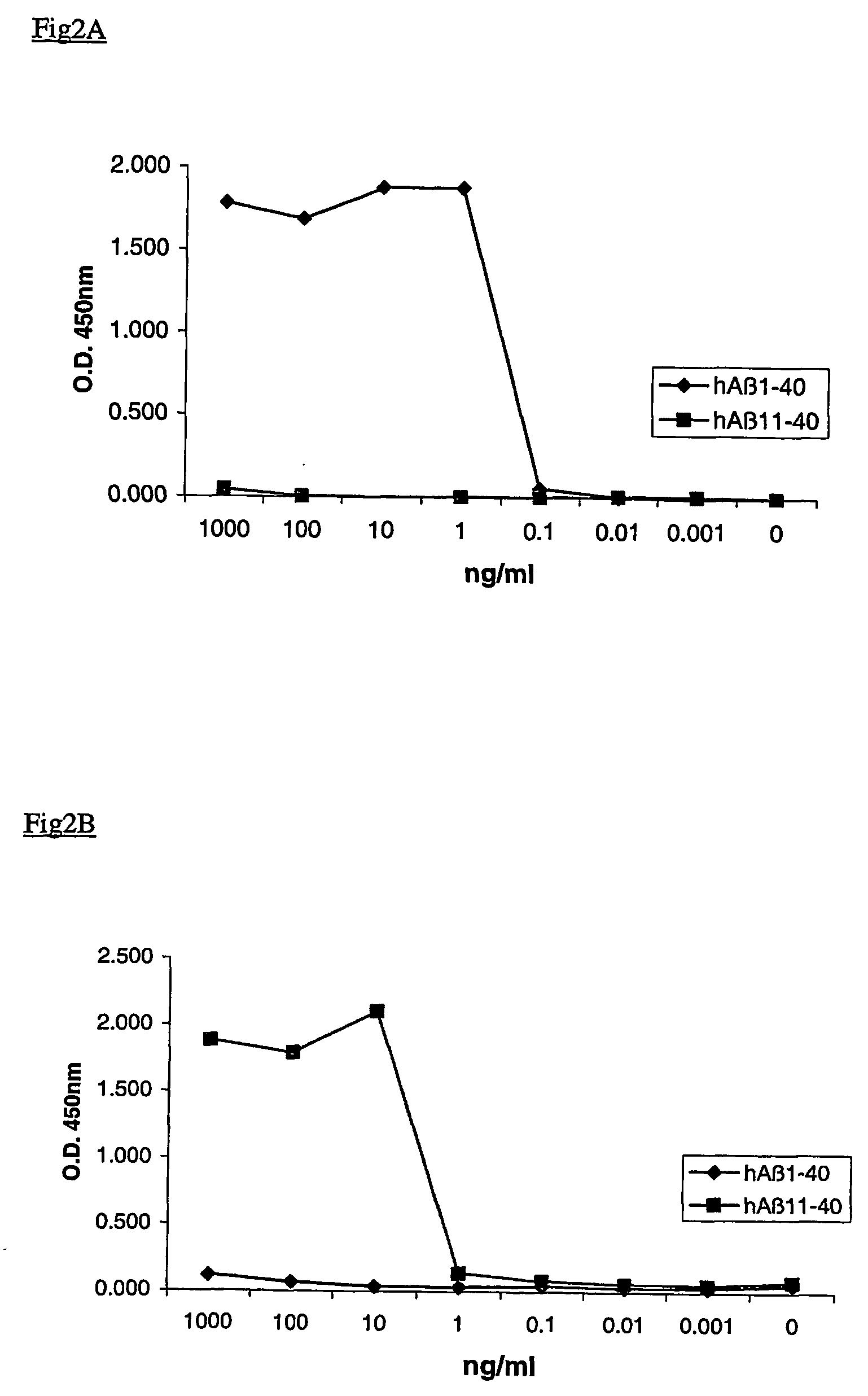
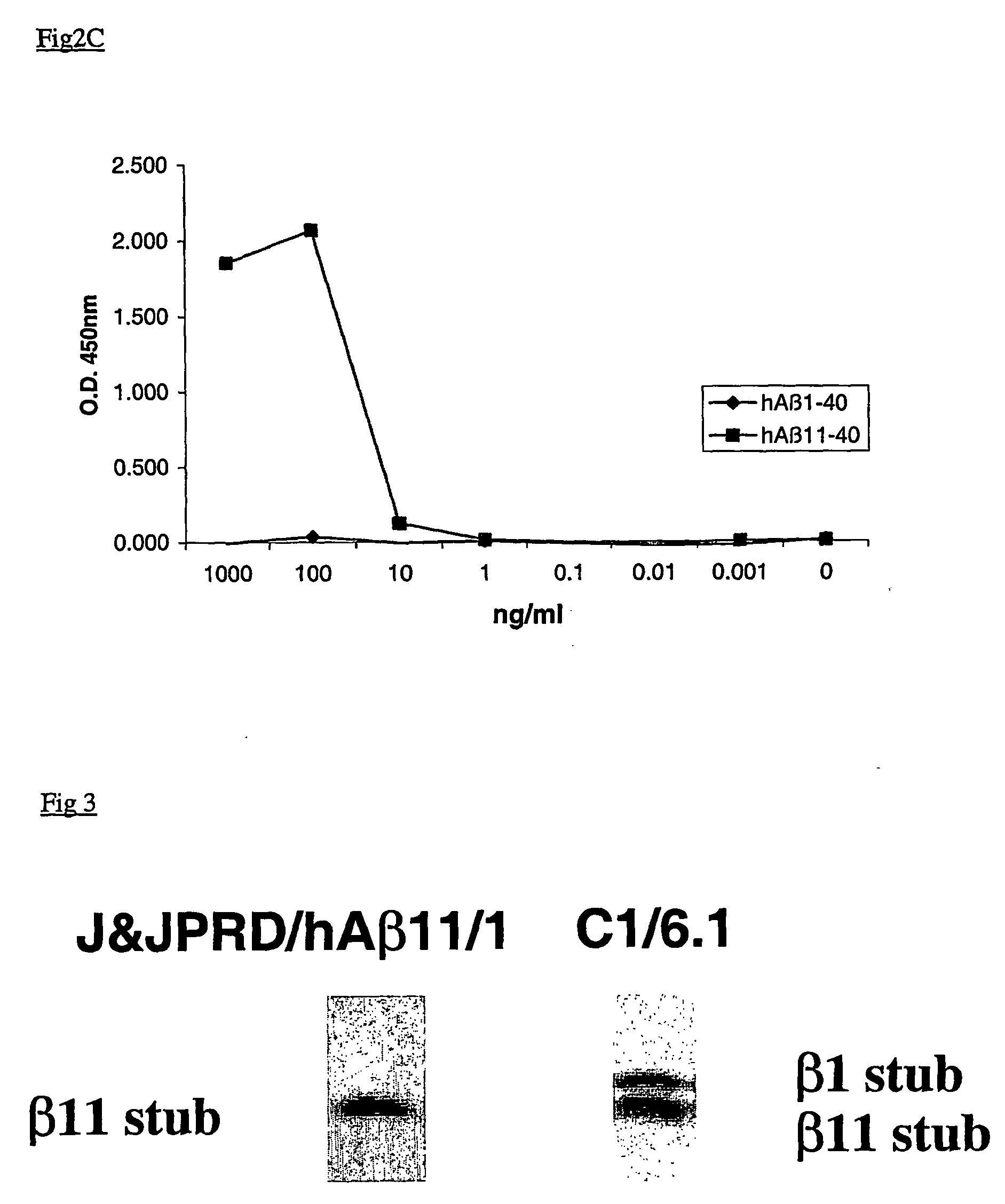
N-11 truncated amyloid-beta monoclonal antibodies, compositions, methods and uses
ActiveUS20060127954A1Inhibit the formation of amyloid plaquesAnimal cellsNervous disorderProtein.monoclonalAmyloid beta
This invention relates to antibodies, including specified portions or variants, specific for at least the human Amyloid-beta 11 N-terminal site, i.e. Aβ11-x peptides. It further provides methods of making and using said antibodies, including therapeutic formulations, administration and devices.
Owner:JANSSEN PHARMA NV
C reactive protein detection kit
ActiveCN103941017AImmunoglobulins against animals/humansBiological material analysisAntiendomysial antibodiesProtein.monoclonal
The invention relates to a C reactive protein detection kit, which contains a C reactive protein monoclonal antibody coated porous plate, an enzyme working solution, a sample diluent, an enzyme reaction substrate, a C reactive protein calibrator, and a C reactive protein quality control product. The kit provided by the invention can be used for quantitative detection of the CRP content in serum or plasma to realize early diagnosis of inflammation, and has the advantages of fast detection speed, high sensitivity, and good specificity.
Owner:BEIJING PERGRANDE BIOTECH DEV
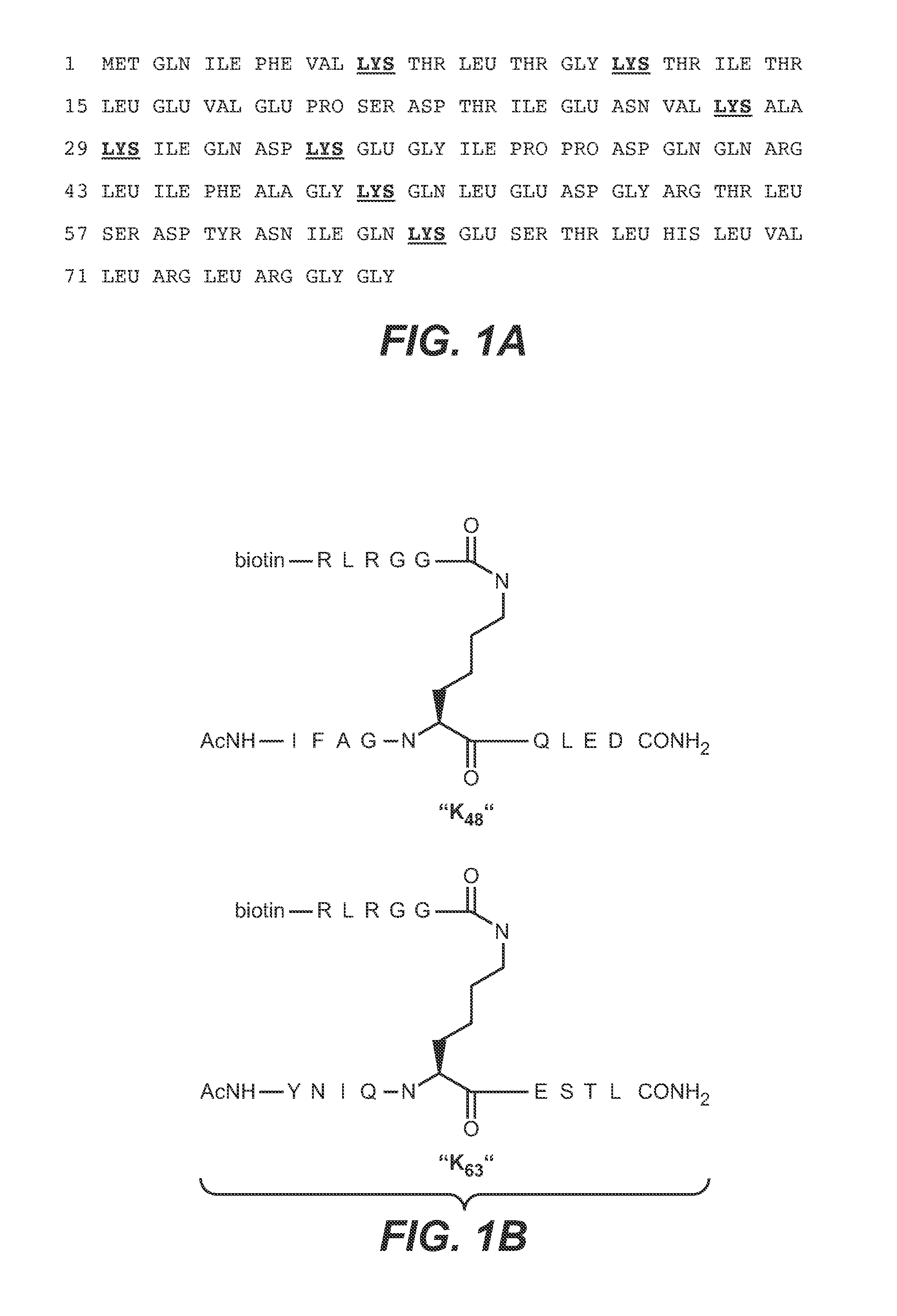
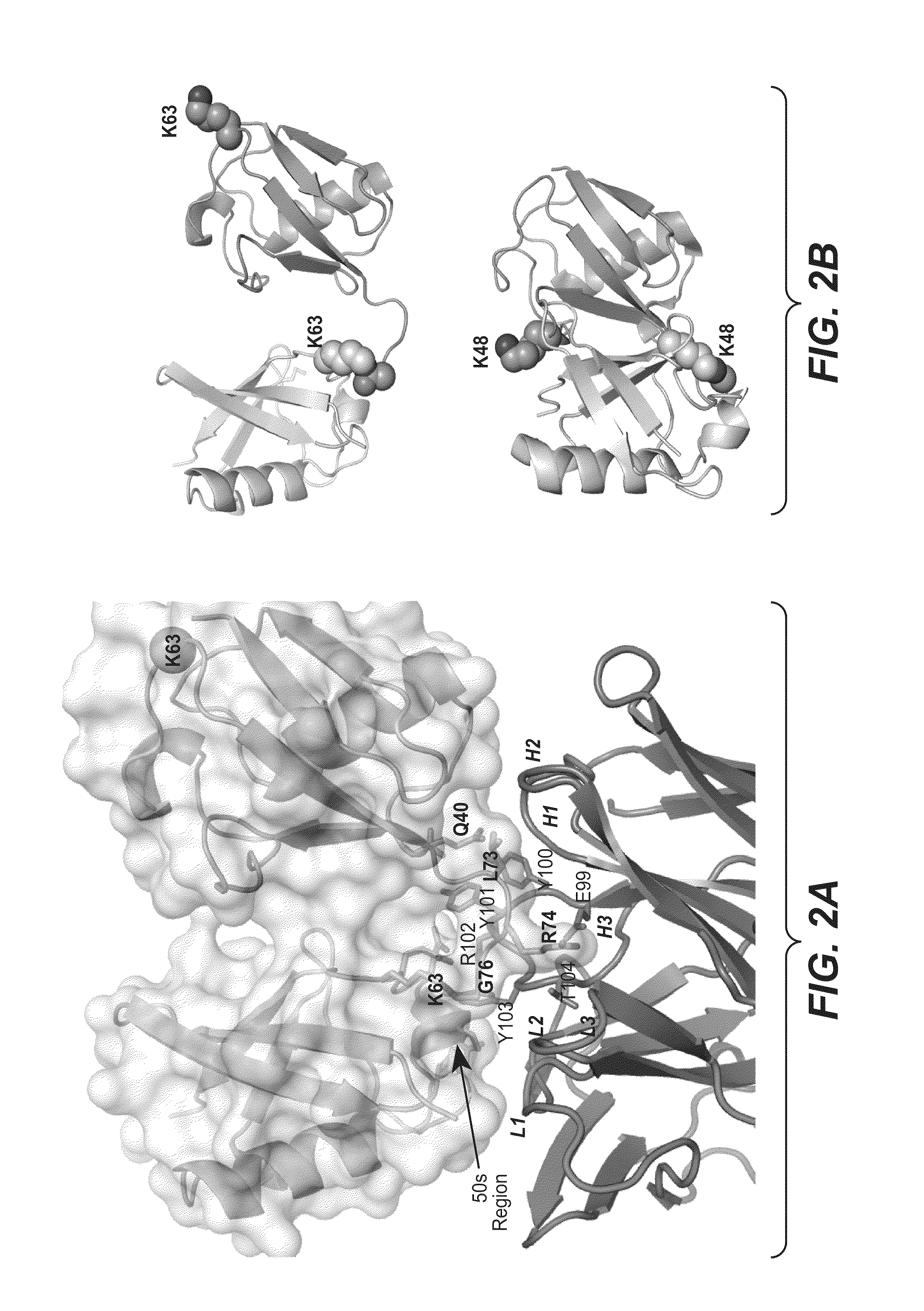
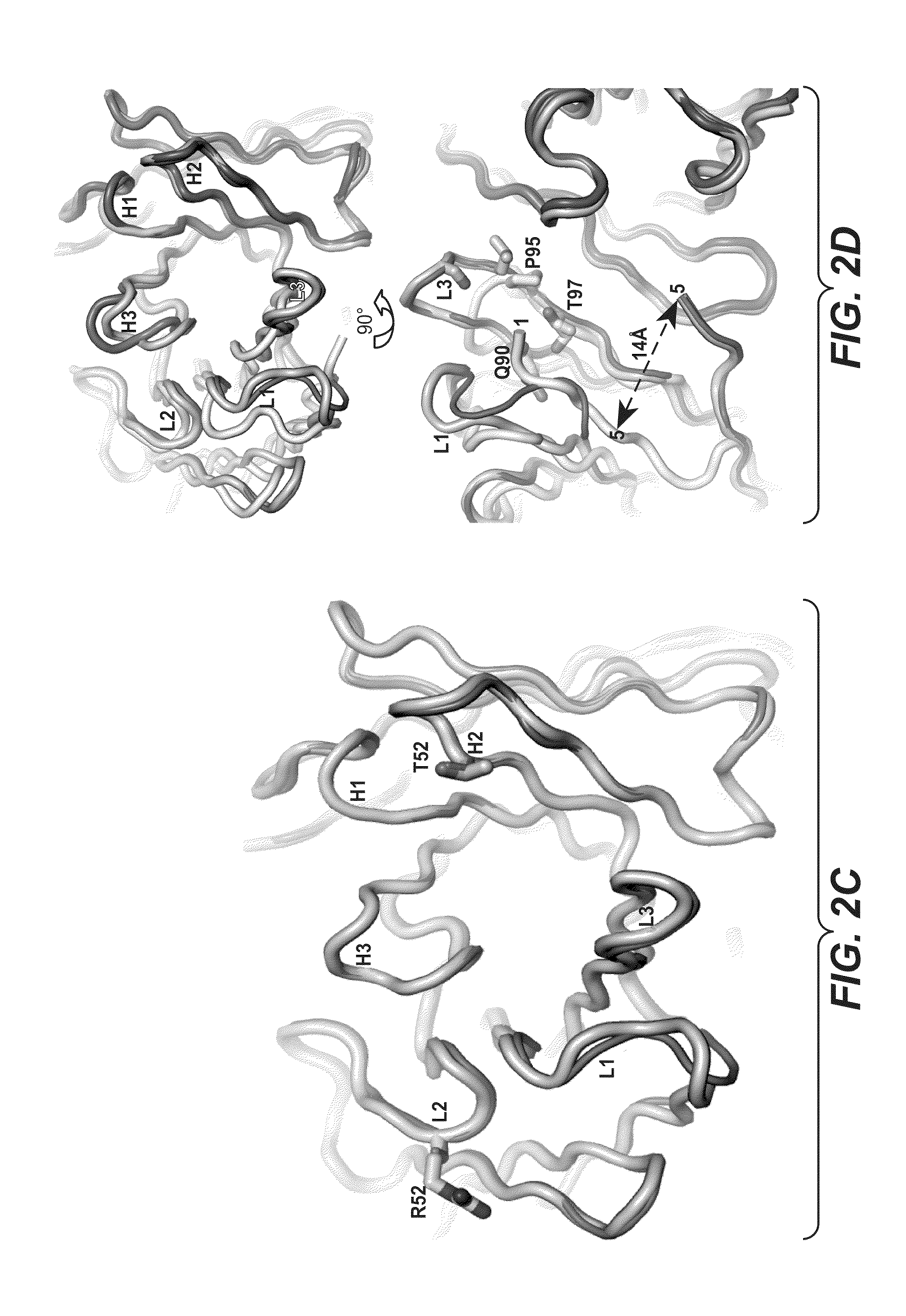
Methods and compositions for targeting polyubiquitin
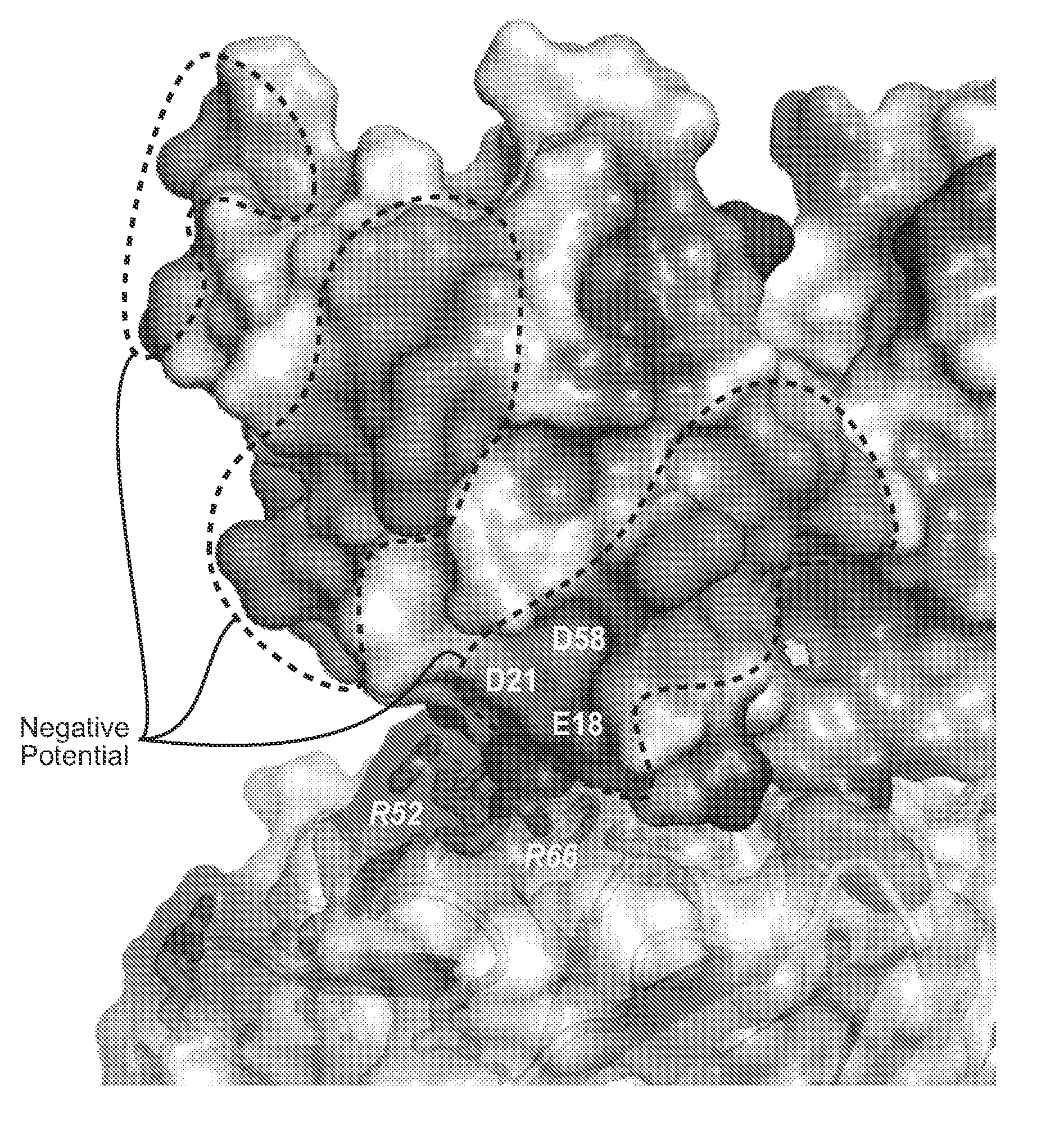
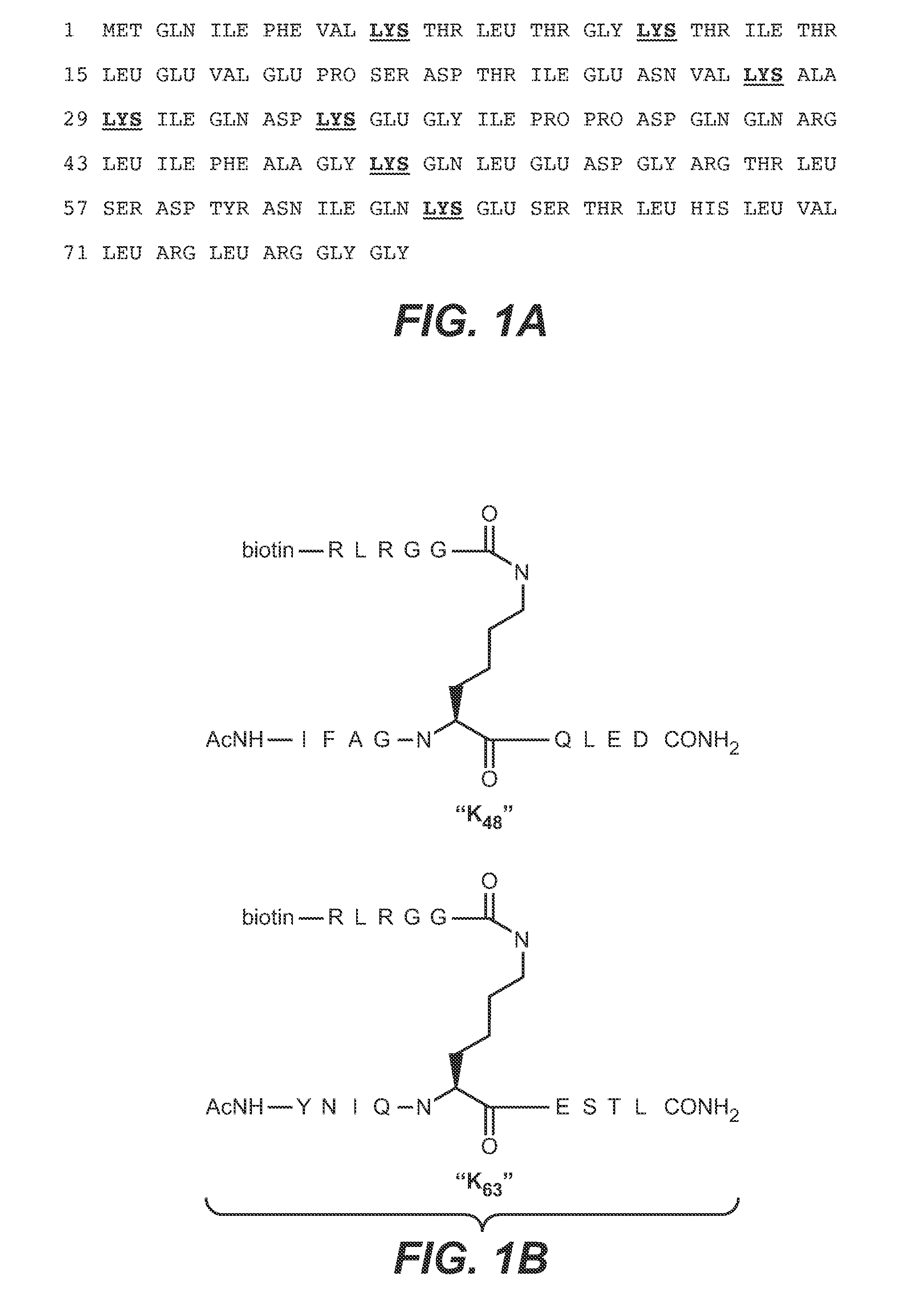
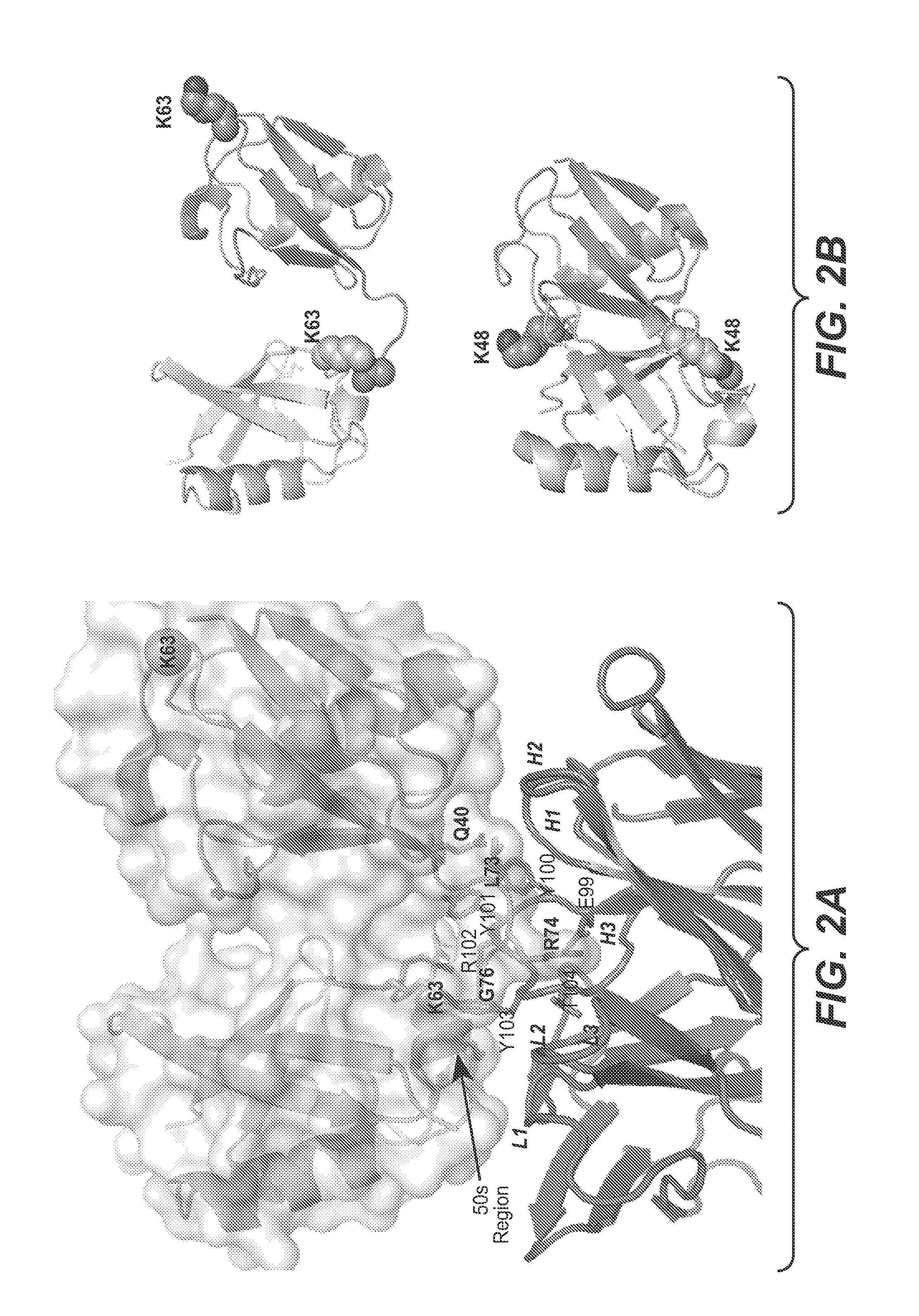
ActiveUS20090191209A1Improved electrostatic compatibilityNervous disorderAntipyreticProtein.monoclonalMonoclonal antibody
Anti-K63-linked polyubiquitin monoclonal antibodies, and methods for using the antibodies, are provided.
Owner:GENENTECH INC
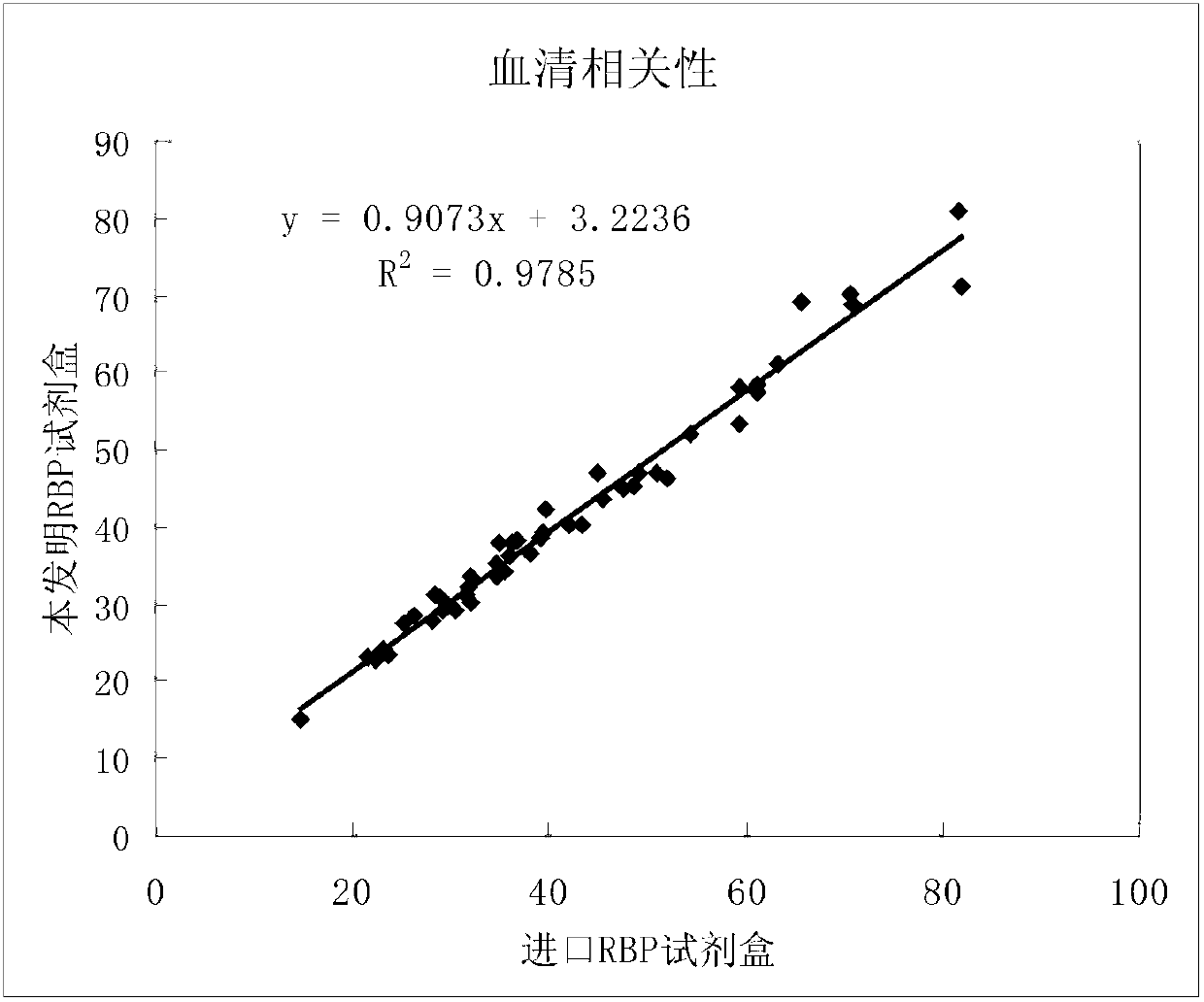
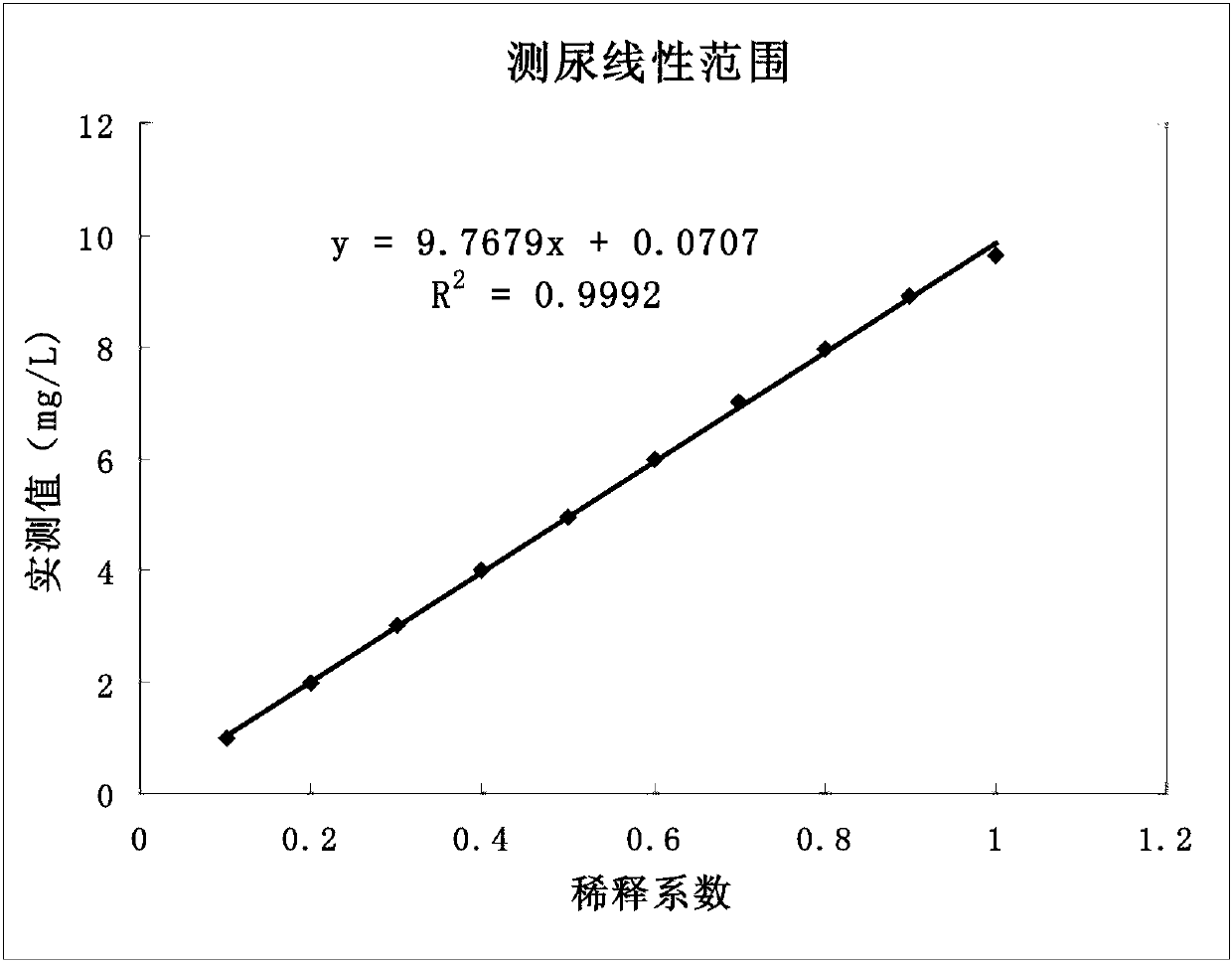
Kit for simultaneously detecting retinol-binding protein (RBP) in urine sample and serum sample
ActiveCN103134934AReduce dosageStrong specificityColor/spectral properties measurementsLatex particlePromotion effect
The invention provides a kit for simultaneously detecting RBP in a urine sample and a serum sample. The kit comprises a reagent R1 and a reagent R2, the reagent R1 is a polymer buffer solution having an agglomeration promotion effect, the reagent R2 is a buffer solution containing a latex-antibody crosslink, and the latex-antibody crosslink comprises anti-RBP polyclonal antibody marked large latex particles and anti-RBP monoclonal antibody marked small latex particles. The kit which adopts a composite monoclonal and polyclonal antibody sensitized latex enhanced immune detection method has the advantages of simultaneous satisfying of the requirements comprising good specificity, high sensitivity and wide linear range, small antibody application amount, and cost reduction, and can be simultaneously used for the clinic detection of the RBP in human blood and urine.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
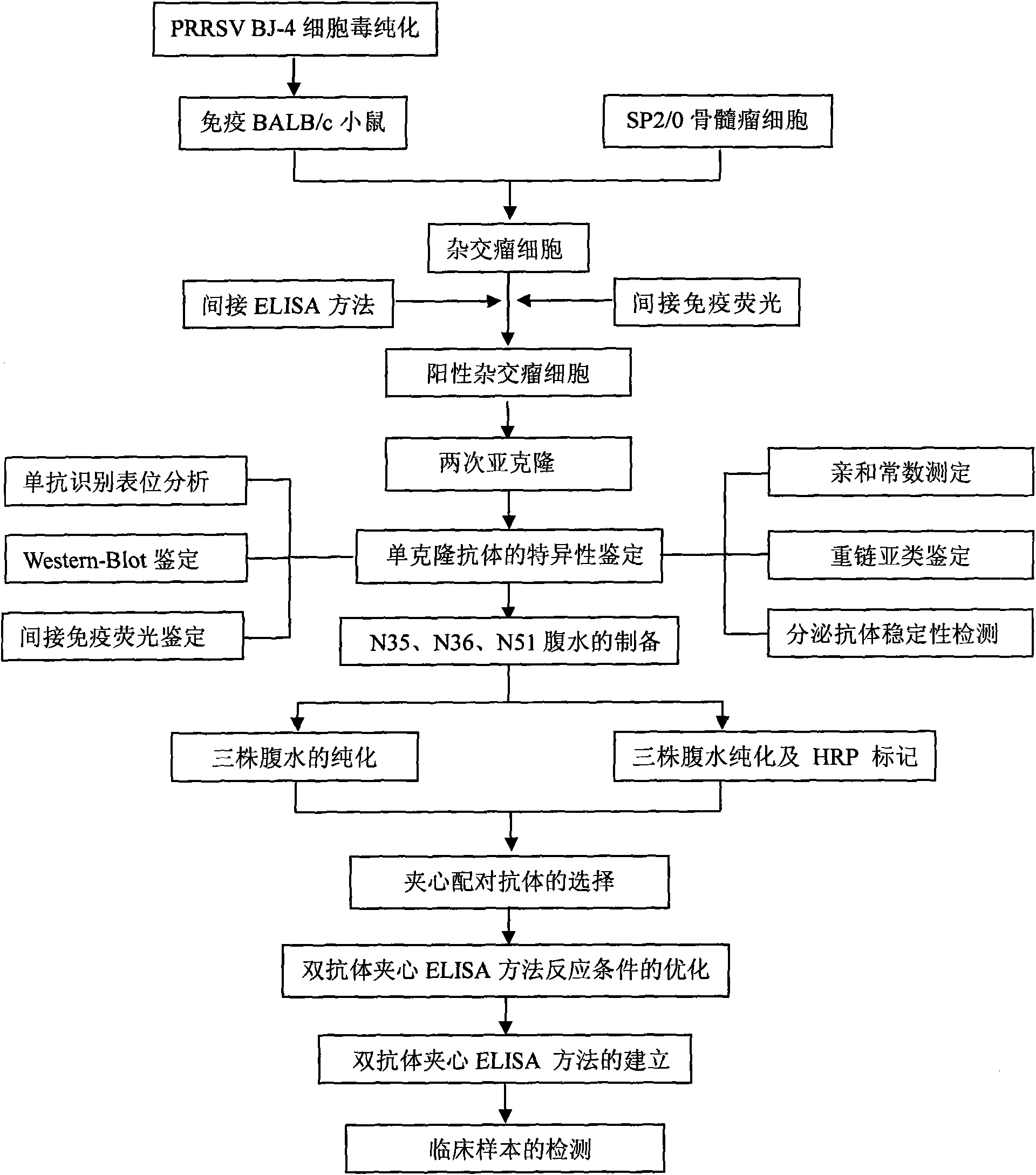
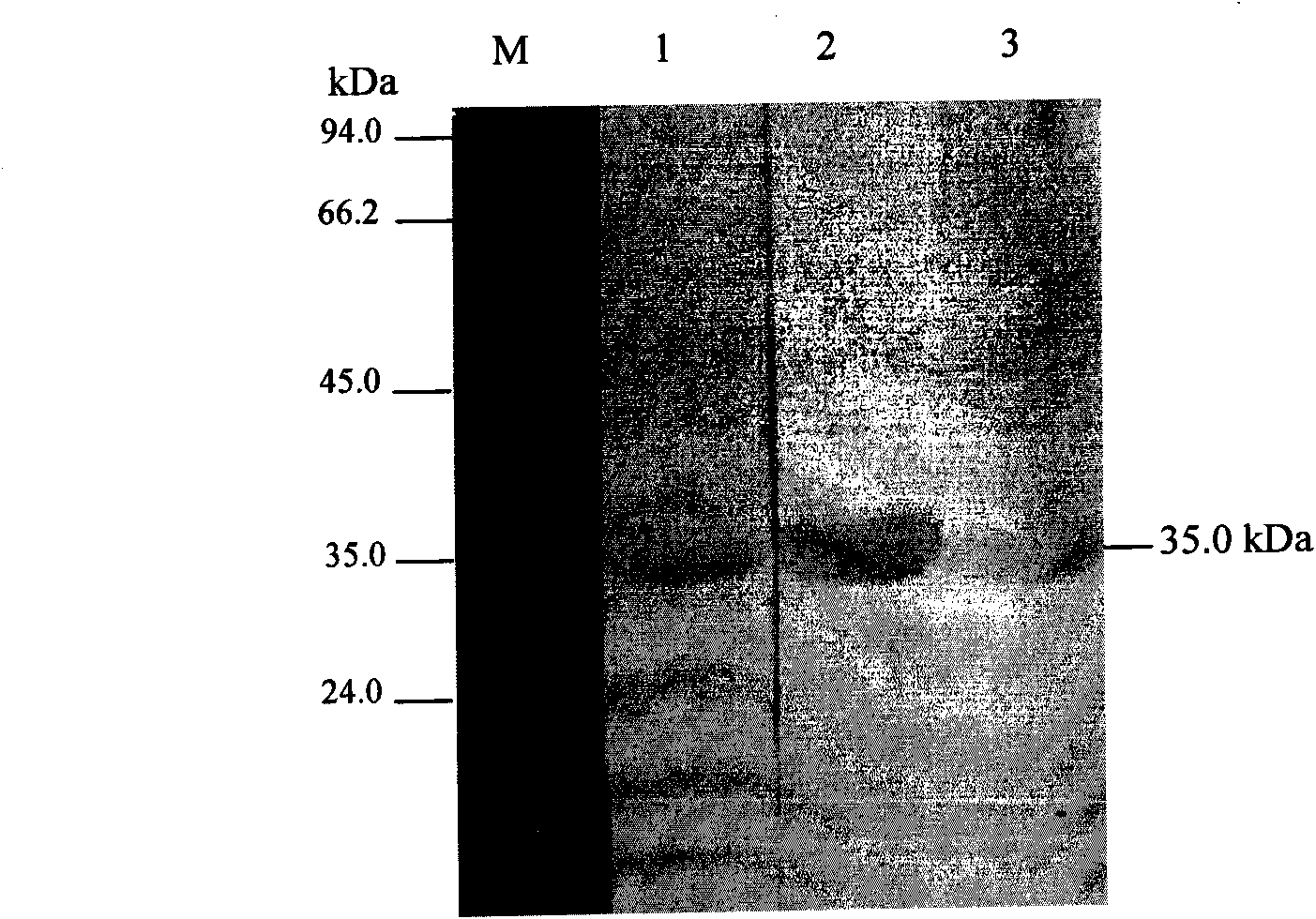
Porcine reproductive and respiratory syndrome virus (PRRSV) double-antibody sandwich ELISA kit
The invention provides a porcine reproductive and respiratory syndrome virus double-antibody sandwich ELISA kit. The kit comprises: an elisa plate coated with PRRSV N protein monoclonal antibody, an enzyme labeling PRRSV N protein monoclonal antibody, lysis solution and the like. A capture antibody and a detection antibody are respectively aimed at antigenic determinants with different N proteins.The kit provides a reliable means for quick detection of clinical PRRSV antigen. The kit can detects that blood serum only contains 0.2 TCID50 highly pathogenic PRRSV JXwn06 strain (non-highly pathogenic strain can be also be detected). Through detecting clinically collected 80 blood serum samples, compared with RT-PCR result, the specificity of the method is 88 percent, the sensitiveness is 90 percent and the coincidence rate of the specificity and the sensitiveness are 88.8 percent. The kit is convenient for operation, low in use cost, good repetitiveness and suitable for wide promotion andapplication.
Owner:CHINA AGRI UNIV
Anti-Golgi apparatus protein monoclonal antibody and use
InactiveCN101407544AHigh sensitivityQuantitatively accurateImmunoglobulins against animals/humansFermentationSerum igeSerum samples
The invention relates to an anti-Golgi protein antibody and an application thereof. The invention recombines human GP73 protein immunity animal to obtain an anti-GP73 polyclonal antibody and a monoclonal antibody which specifically aims at GP73 and builds a plurality of methods for detecting GP73 in clinical tissue sections and serum samples, such as immunohistochemical stain and double antibody sandwiched ELISA method and the like. Tests prove that the polyclonal and monoclonal antibodies can be used for preparing a plurality of GP73 detecting agents of different detecting methods.
Owner:曹伯良 +1
Anti-influenza A virus nucleoprotein monoclonal antibody, its preparation and application
ActiveCN102747040AHigh secretion yieldHigh affinityImmunoglobulins against virusesTissue cultureProtein.monoclonalCell strain
The invention discloses an anti-influenza A virus nucleoprotein monoclonal antibody with high affinity and high specificity, its preparation and application. The anti-influenza A virus nucleoprotein monoclonal antibody is secreted by a hybridoma cell strain H1N1-2F10, and the preservation number of the cell strain is CCTCC C201119. The invention also provides a kit prepared with monoclonal antibody for rapid detection of influenza A. The kit has the characteristics of simple operation, strong specificity and high sensitivity, etc.
Owner:GUANGDONG WESAIL BIOTECH CO LTD
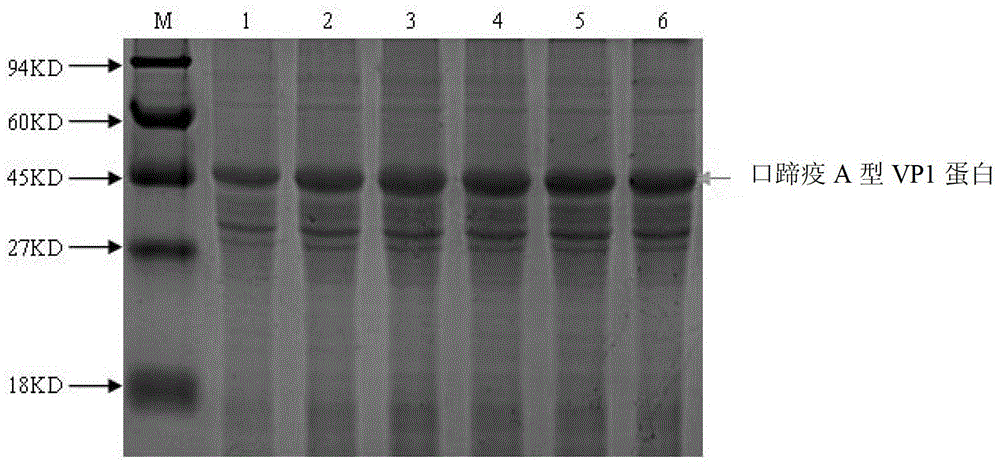
Competitive ELISA method based on foot-and-mouth disease A type VP1 protein and its monoclonal antibody
InactiveCN103554234AHigh purityImprove production efficiencySsRNA viruses positive-senseAntibody mimetics/scaffoldsAntigenDisease
The invention relates to a competitive ELISA method based on a foot-and-mouth disease A type VP1 protein and its monoclonal antibody, also relates to a preparation method of the foot-and-mouth disease A type VP1 protein, and a preparation method of the monoclonal antibody of the foot-and-mouth disease A type VP1 protein, and belongs to the technical field of animal immunological detection. In the invention, a primer pair C1 and C2 and a primer pair E1 and E2 are amplified to obtain a gene sequence of the foot-and-mouth disease A type VP1 protein, the foot-and-mouth disease A type VP1 protein is obtained by constructing an expression plasmid, introducing the expression plasmid into a prokaryotic expression host and carrying out inducible purification, the foot-and-mouth disease A type VP1 protein monoclonal antibody is obtained by treating the foot-and-mouth disease A type VP1 protein as an antigen through a hybridomas technology, and the competitive ELISA method used for detecting a foot-and-mouth disease A type antibody is established based on the foot-and-mouth disease A type VP1 protein and its monoclonal antibody. The detection method has a strong specificity and a good stability, and can be used for detecting a foot-and-mouth disease A type serum antibody. By comparing a result obtained through the detection method with a liquid phase blocking ELISA kit, the coincidence rate is 95.8%.
Owner:广西壮族自治区动物疫病预防控制中心
Methods and compositions for targeting polyubiquitin
ActiveUS7763245B2Expand accessRapid clearanceNervous disorderAntipyreticProtein.monoclonalMonoclonal antibody
Owner:GENENTECH INC
Methods and compositions for targeting polyubiquitin
ActiveUS8133488B2Improved electrostatic compatibilityNervous disorderAntipyreticProtein.monoclonalMonoclonal antibody
Anti-K63-linked polyubiquitin monoclonal antibodies, and methods for using the antibodies, are provided.
Owner:GENENTECH INC
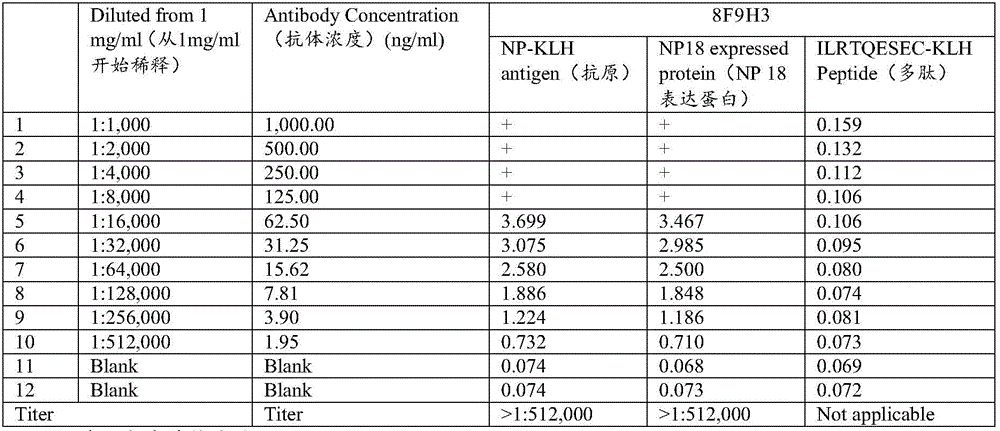
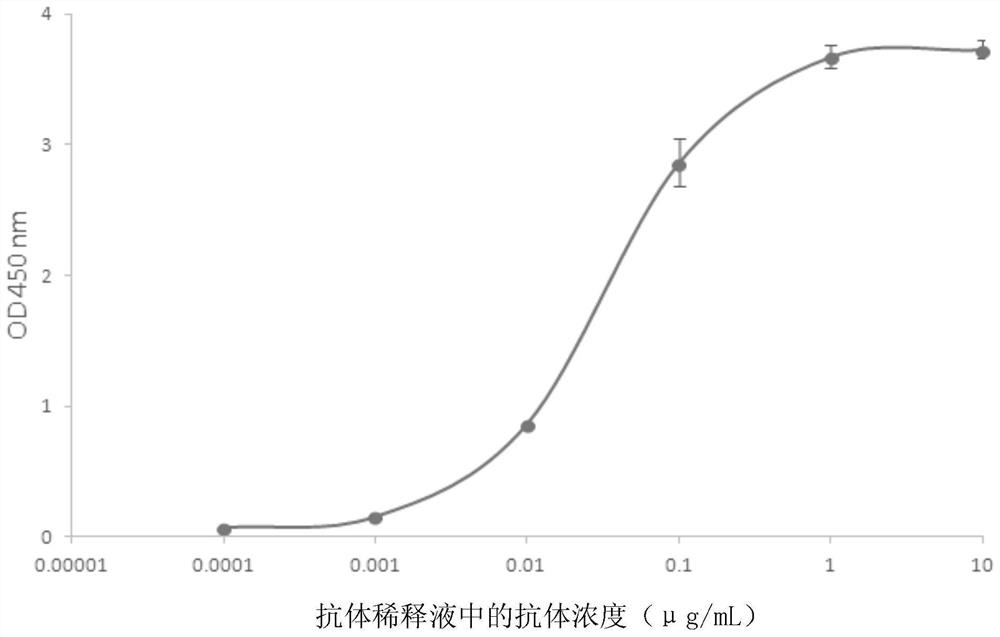
Monoclonal antibody for detecting novel coronavirus and application of preparation kit
ActiveCN111269313ARealize detectionStrong specificityBiological material analysisImmunoglobulins against virusesProtein.monoclonalTiter
The invention relates to a monoclonal antibody for detecting a novel coronavirus and an application of a preparation kit. An N protein is an important marker protein of coronavirus, and coronavirus detection can be realized by detecting the N protein. An anti-N protein monoclonal antibody obtained in the invention has characteristics of high specificity and mass production, and a titer of the obtained antibody is relatively high. The anti-N protein monoclonal antibody obtained in the invention can be specifically combined with the N protein, can be used for immunological detection of cells, has a wide market prospect and has important clinical significance.
Owner:BEIJING SUNGEN BIOMEDICAL TECH CO LTD
Blocking ELISA kit for detecting NDV (Newcastle disease virus) antibody
ActiveCN106596933ASimple and fast operationEasy to operateBiological material analysisElisa kitPositive control
The invention discloses a blocking ELISA kit for detecting an NDV (Newcastle disease virus) antibody. The blocking ELISA kit for detecting the NDV antibody comprises an ELISA plate coated with an NDV inactivated antigen, an NDV positive control serum, an NDV negative control serum and a horseradish peroxidase labeled NDV NP protein monoclonal antibody, wherein the horseradish peroxidase labeled NDV NP protein monoclonal antibody is secreted by a hybridoma cell strain with the preservation number being CCTCC NO: C2016180. The blocking ELISA kit for detecting the NDV antibody can detect serum samples which are infected with the suspected NDV and are from different species, can distinguish an MG7-deficient vaccine from an NDV serum after being infected with a wild virus, and has no cross reaction with a common avian viral pathogen positive serum, thereby being high in sensitivity and specificity, good in reproducibility and suitable for high-throughput detection of serum samples.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
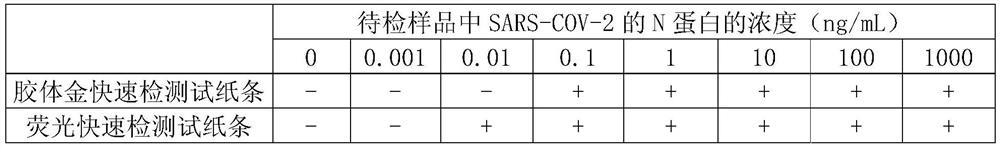
Hybridoma cell capable of secreting an anti-novel coronavirus N protein monoclonal antibody, onoclonal antibody and application
ActiveCN111733141AQuick monitoringIncreased sensitivityBiological material analysisImmunoglobulins against virusesProtein.monoclonalAntigen testing
The invention discloses a hybridoma cell capable of secreting an anti-novel coronavirus (SARS-COV-2) N protein monoclonal antibody, a monoclonal antibody and anapplication. The invention provides a hybridoma cell strain N-3G3, wherein the preservation number of strain is CCTCC NO: C202075. The invention also protects the monoclonal antibody secreted by the hybridoma cell strain N-3G3. The antibodywith high sensitivity and high specificity is the key to the development and implementation of an antigen detection technology. The specific antibody of the N protein of the SARS-CoV-2 is obtained onthe basis of a monoclonal antibody technology, and an SARS-COV-2 detection test strip is prepared from the N protein of the SARS-CoV-2. When the test strip provided by the invention is used for detecting the SARS-CoV-2, the operation is simple, the sensitivity is high, the specificity is strong, and the rapid monitoring and prevention of the SARS-COV-2 can be realized.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
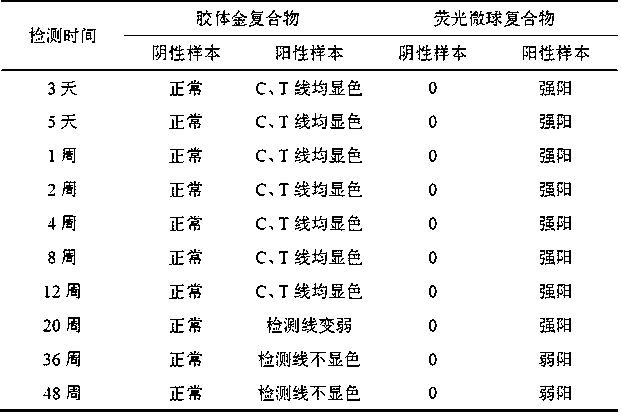
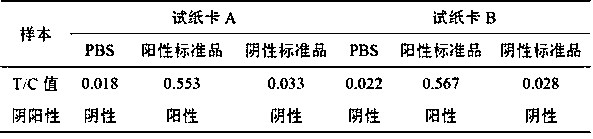
Fluorescence quantitative immunochromatography test paper strip for fast detecting zika virus NS1 protein and manufacturing method thereof
InactiveCN107102144ARapid Field DetectionSensitive Field DetectionBiological testingEpitopeProtein.monoclonal
The invention discloses a fluorescence quantitative immunochromatography test paper strip for fast detecting zika virus NS1 protein and a manufacturing method thereof. The test paper strip comprises a bottom plate, wherein a sample pad, a combination pad, a covering film and water suction paper are sequentially arranged on the bottom plate and are in sequential lap joint; a fluorescent microsphere marked zika NS1 protein monoclonal antibody and a fluorescent microsphere marked goat anti-chicken antibody are arranged on the combination pad; a zika NS1 protein monoclonal antibody with different epitope from the fluorescent microsphere marked zika NS1 protein monoclonal antibody on the combination pad covers a detection region of the covering film; a chicken IgY antibody covers a quality control region. The manufacturing method comprises the following steps of fluorescent microsphere cleaning and activation, fluorescent microsphere marked antibody preparation, fluorescent marked antibody combination pad preparation, covering film preparation and test paper strip assembly. The manufactured test paper strip can be used for fast, specially and precisely detecting the zika virus NS1 protein; the fast quantitative detection in field can be realized.
Owner:SHENZHEN ZIJIAN BIOTECH
Preparation method for PCV-II Cap protein monoclonal antibody, antibody and application
InactiveCN101768218AAvoid distortionThe ability to secrete antibodies is strong and stableImmunoglobulins against virusesFluorescence/phosphorescenceBALB/cIndirect elisa
The invention discloses a preparation method for a PCV-II Cap protein monoclonal antibody, an antibody and application. The invention adopts ultracentrifuged and purified PCV-II as an immunogen to immunize a BALB / c mouse by the conventional method, takes spleen cells of the immunized BALB / c mouse to fuse with SP2 / 0 cells, obtains two strains of hybridoma cells secreting the PCV2-Cap protein monoclonal antibodies by indirect ELISA screening, respectively names the two strains of hybridoma cells as 8-60 and 10-48, identifies biological characteristics of the two strains 8-60 and 10-48, and usesthe two strains 8-60 and 10-48 as the first antibodies to establish an indirect immunofluorescence diagnostic method. The result of the indirect immunofluorescence diagnostic method is basically consistent with that of the PCR diagnostic method, and the positive and negative coincidence rates are respectively 93.75 percent and 100 percent so as to provide reference for preventing and treating theporcine circovirus disease.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI +7
Vibrio parahaemolyticus flagellin monoclonal antibody and antigen capture ELISA (enzyme-linked immunosorbent assay) kit
InactiveCN102659942AImprove featuresImprove stabilityImmunoglobulins against bacteriaTissue cultureProtein.monoclonalVibrio parahemolyticus
The invention discloses a vibrio parahaemolyticus flagellin monoclonal antibody and an antigen capture ELISA (enzyme-linked immunosorbent assay) kit. The vibrio parahaemolyticus flagellin monoclonal antibody is produced by secreting of a hybridoma cell strain with the preservation number of CGMCC (China General Microbiological Culture Collection) No.6061. The vibrio parahaemolyticus flagellin monoclonal antibody can be used for detecting vibrio parahaemolyticus. The invention also discloses a vibrio parahaemolyticus flagellin capture ELISA (enzyme-linked immunosorbent assay) kit.
Owner:BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU INSPECTION & QUARANTINE TECH CENT
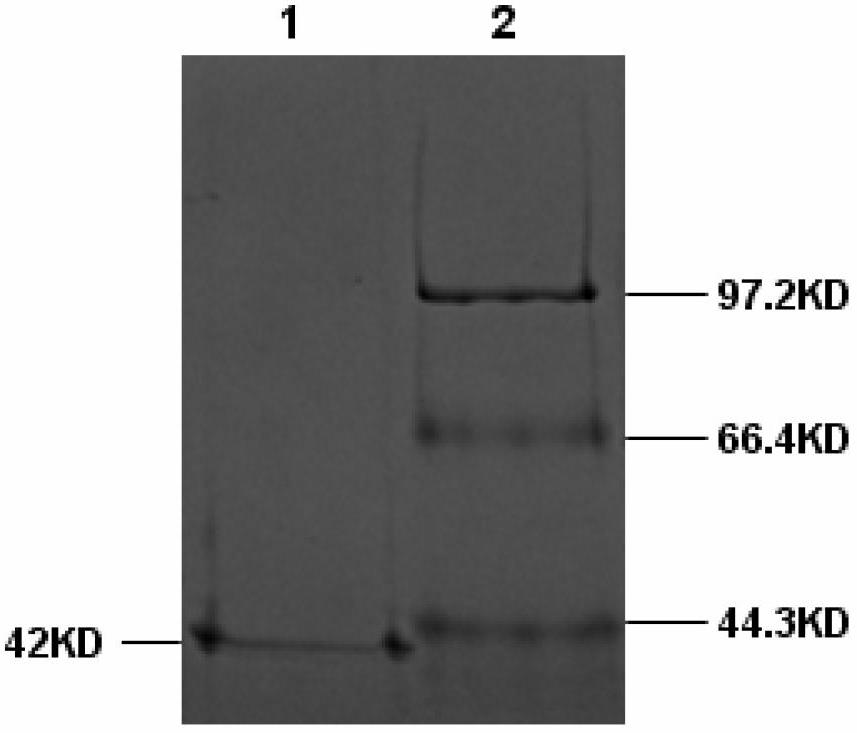
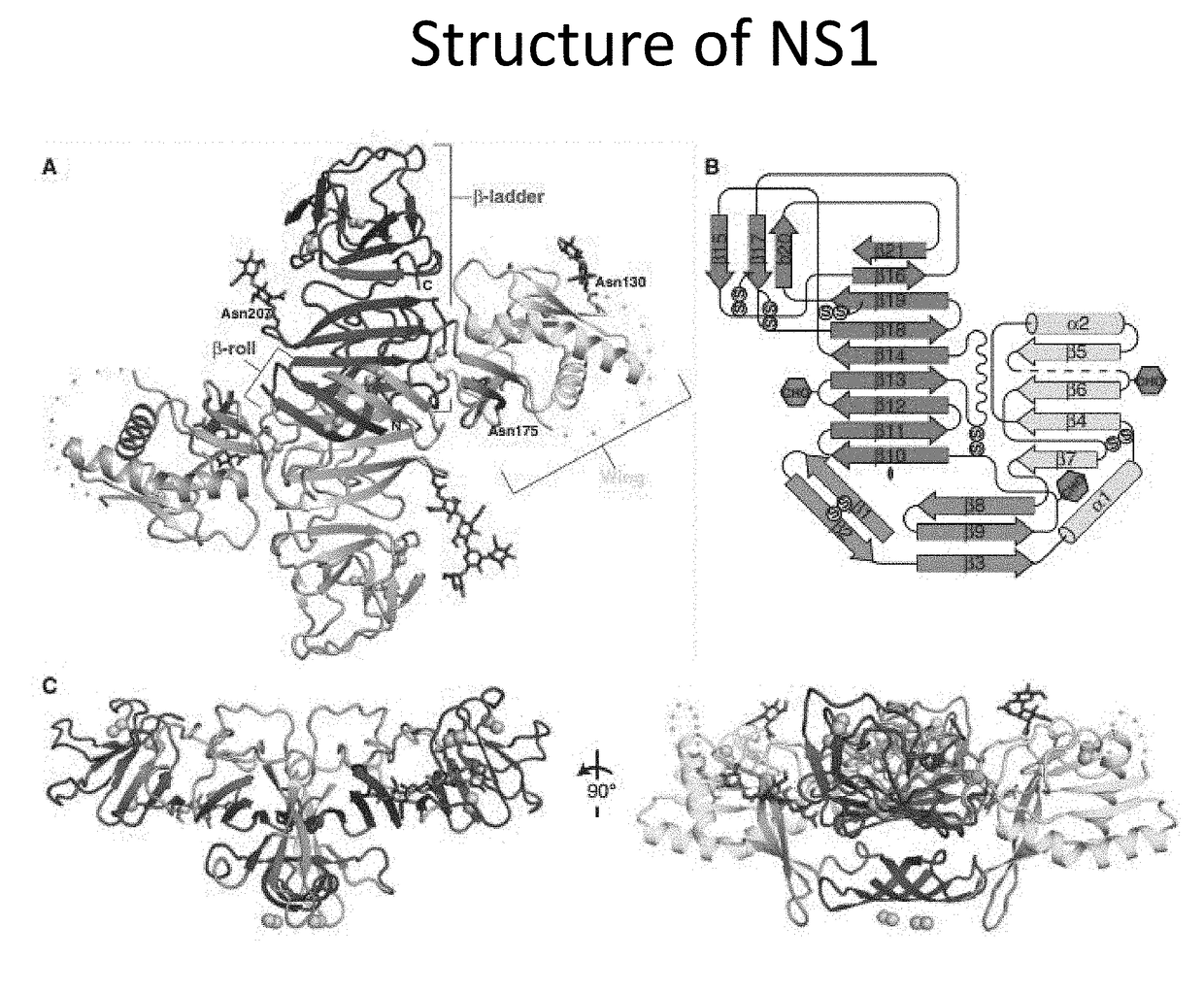
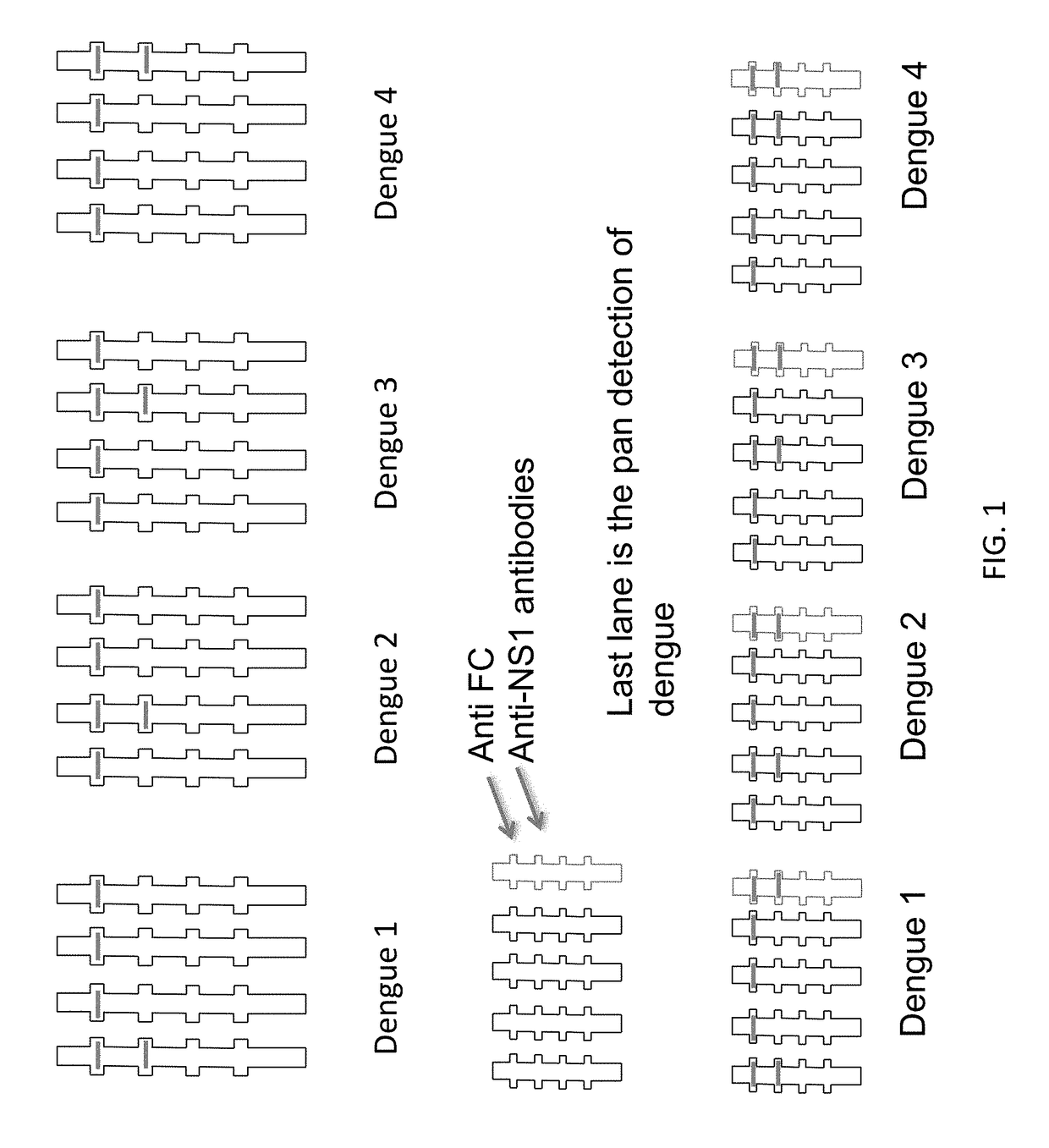
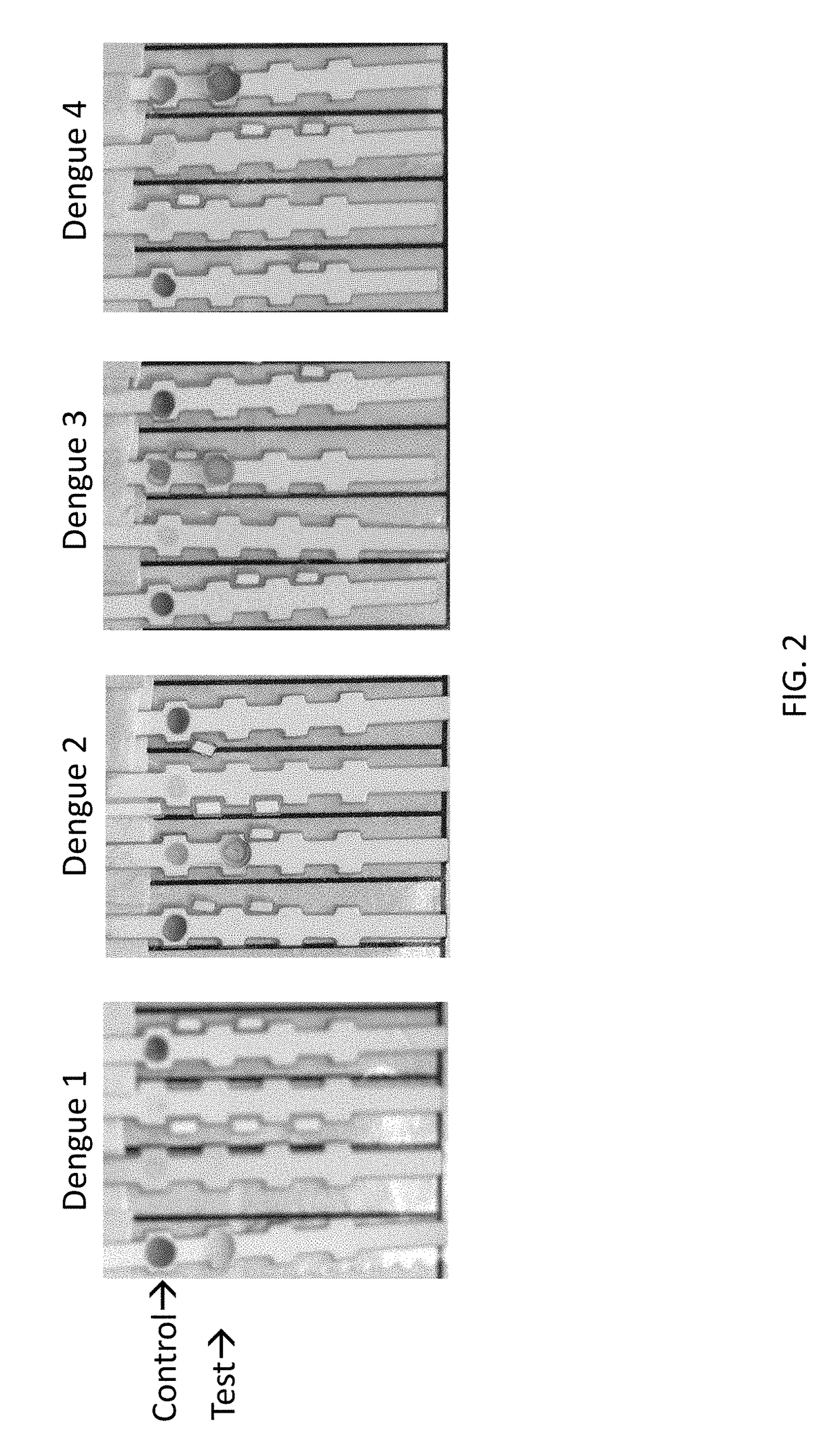
Anti-Dengue Virus NS1 Protein Monoclonal Antibodies
ActiveUS20170233460A1Improve developmentUseful in therapyImmunoglobulins against virusesAntibody ingredientsProtein.monoclonalSpecific detection
The present invention provides matched antibody pairs for the specific detection of one or more of the four dengue virus serotypes in a biological sample that may contain one or more of such dengue virus serotypes. Each matched antibody pair is capable of detecting not more than one serotype of dengue virus NS1 protein that may be present in the sample and will not cross react with other serotypes that may be present in the sample. Multiple matched pairs may be used to detect one or more dengue virus serotypes that may be present in a sample. Such matched pair antibodies, facilitate the development of confirmatory in vitro diagnostic tests such as sandwich immunoassays, that detect and distinguish the presence of one or more dengue virus serotypes in a biological sample, preferably a sample derived from human subject. The invention also provides kits comprising the matched antibody pairs of the invention and methods for using the kits for immunoassays for the specific detection of one or more serotypes of dengue virus in a patient population. The present invention also provides monoclonal antibodies specific for the NS1 protein of dengue virus and therapeutic compositions and methods for treating dengue virus infection.
Owner:THE FOOD & DRUG ADMINISTATION +1
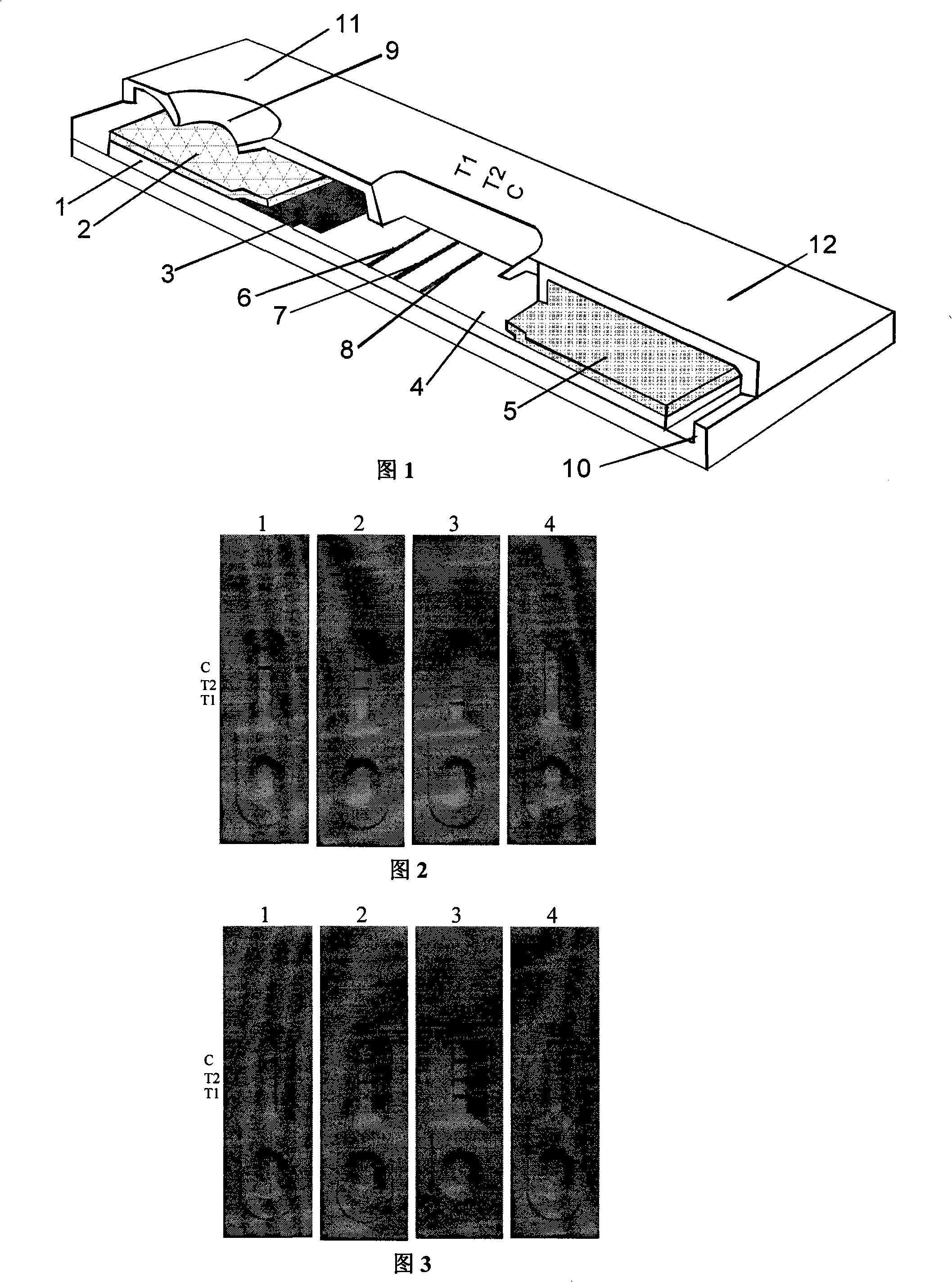
Porcine circovirus PCV1 and PCV2 identifying and detecting test paper card
The invention relates to a PCV1 and PCV2 differential detection paper card which can be used for rapid identification and detection of pig PCV2 infection. The reaction reagent carrier adsorption layer comprises a fiber layer, a gold-labeled fiber layer, a cellulose film and an absorbent-material layer; the gold-labeled fiber layer is a gold-labeled glass wool adsorbing colloidal gold-labeled anti-capsid protein monoclonal antibodies which can identify PCV1 and PCV2 common epitope, and the cellulose film is a nitrocellulose film which is orderly printed with a detection trace T1, a detection trace T2 and a control trace C; the detection trace T1 is a strip-shaped PVC1 detection trace printed and produced by monoclonal antibody solution which can identify PCV1-typed specific epitope; and T2is a strip-shaped PVC detection trace printed and produced by anti-capsid protein monoclonal antibody solution which can identify PCV1 and PCV2 common epitope, and the control trace C is a strip-shaped control trace which is printed and produced by anti-mouse IgG polyclonal antibody solution. The paper card has the advantages of strong specificity, high sensitivity, convenience and speediness andintuitive test result, and can be easily promoted for application in the production practice.
Owner:ZHEJIANG UNIV
Porcine circovirus type 2 antigen capture ELISA kit
The invention discloses a porcine circovirus type 2 antigen capture ELISA kit. The kit internally comprises an enzyme labeled monoclonal antibody which is secreted by a hybridoma cell strain with the preservation number of CGMCC NO.10205. The invention also discloses a capture ELISA method for rapidly detecting the porcine circovirus type 2 and established by utilizing the monoclonal antibody. A porcine circovirus type 2 polyclonal antibody and a porcine circovirus type 2 cap protein monoclonal antibody respectively act as a capture antibody and a detecting antibody. The method can be used for detecting multiple gene type PCV2 viruses; the detection sensibility is 400 TICD50 / ml; the porcine circovirus type 2 has no crossed reaction with other swine viruses, and the toxic value determination method coincidence rate is 88%. The result shows that the method has the advantages of operation simplicity, good specificity, high sensitiveness, short time consumption and the like, can be used for estimating the toxic value of viruses, and can conveniently and rapidly control the quality of PCV2 inactivated vaccine semi-finished products.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI +1
Test paper card for detecting swine transmissible gastroenteritis virus antibody and preparation and detection method of test paper card
InactiveCN107942061AImprove stabilityHigh sensitivityMaterial analysisCelluloseTransmissible gastroenteritis virus Antibody
The invention relates to a test paper card for detecting porcine transmissible gastroenteritis virus antibody, preparation and detection method thereof, and belongs to the field of immunological detection. The test paper card includes a jammed case and a test strip, and the test strip includes a bottom plate and sequentially lapped and pasted on the Absorbent pad, detection pad, binding pad and sample pad on the base plate; the detection pad is a nitrocellulose membrane with a quality control line C and a detection line T, the quality control line C is coated with goat anti-mouse monoclonal antibody, and the detection line T is Coated with inactivated porcine transmissible gastroenteritis virus; the binding pad is a glass cellulose membrane embedded with time-resolved fluorescent microsphere-labeled anti-E2 protein monoclonal antibody; the sample pad is dried glass after soaking in the sample treatment solution Cellulose film. The test paper card prepared by the invention has better stability and higher sensitivity, and can achieve the purpose of semi-quantitative detection through fluorescence signal analysis.
Owner:洛阳现代生物技术研究院有限公司
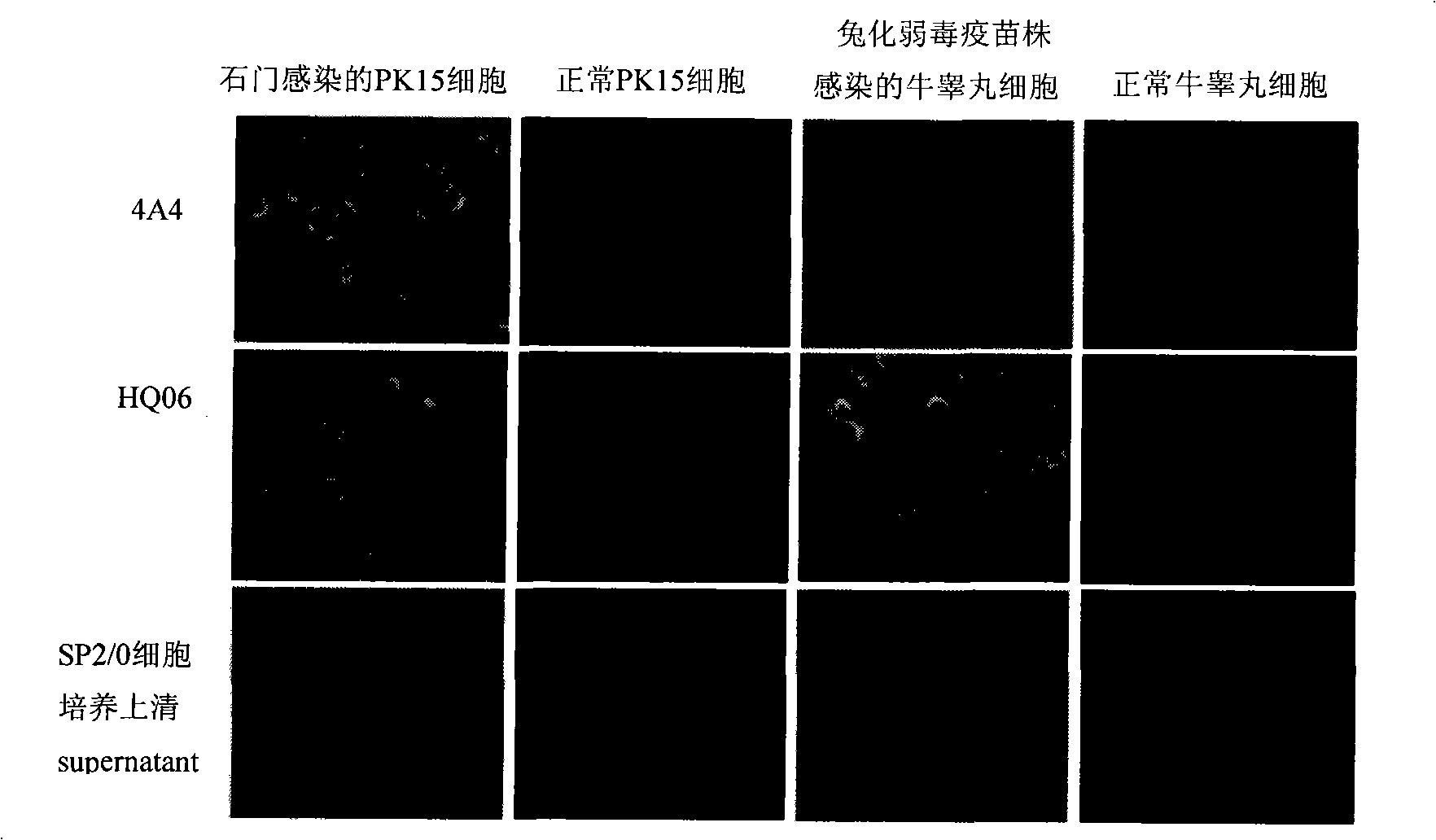
Monoclone antibody of swine fever virus resistant wild strain E2 protein, preparation method and application thereof
InactiveCN101294147ANeutralizing activityImmunoglobulins against virusesTissue cultureSwine Fever VirusCholera
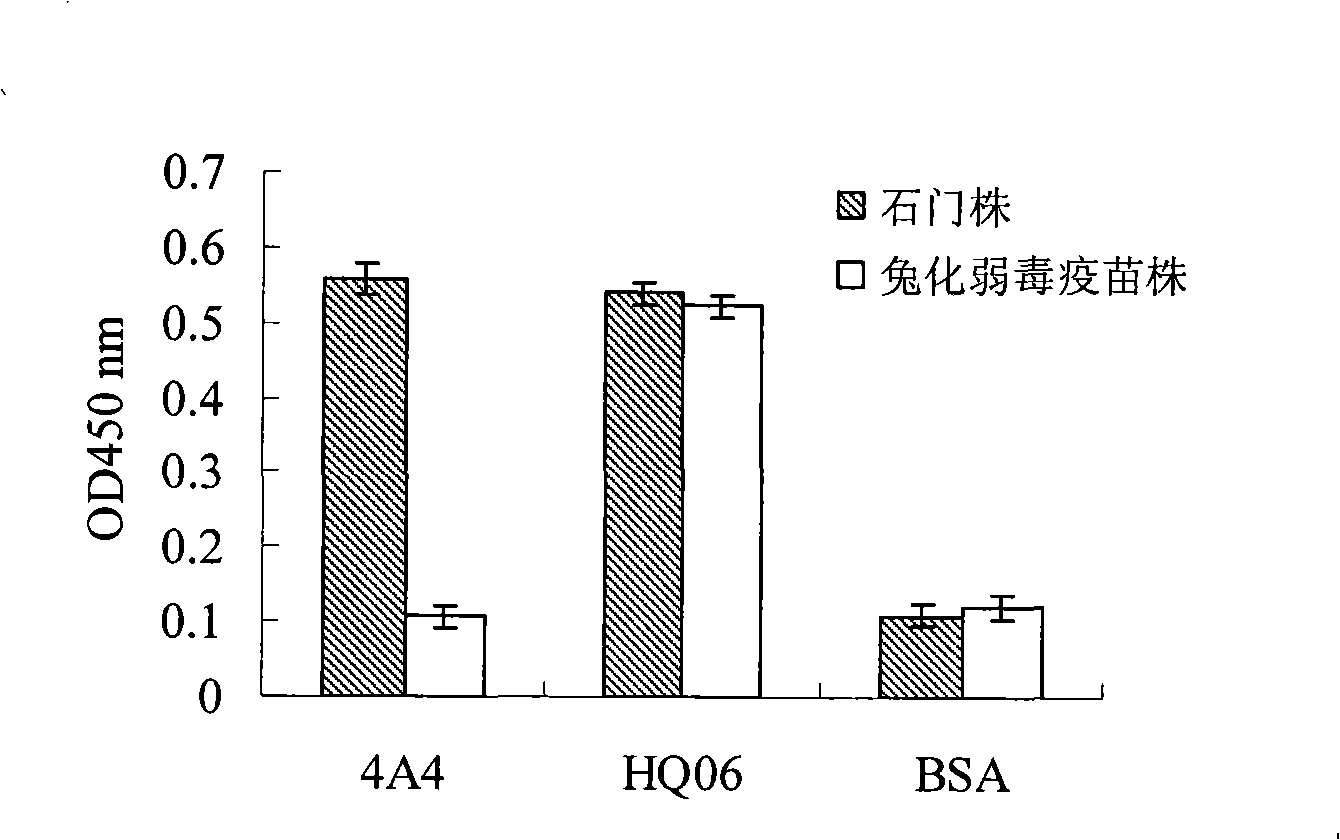
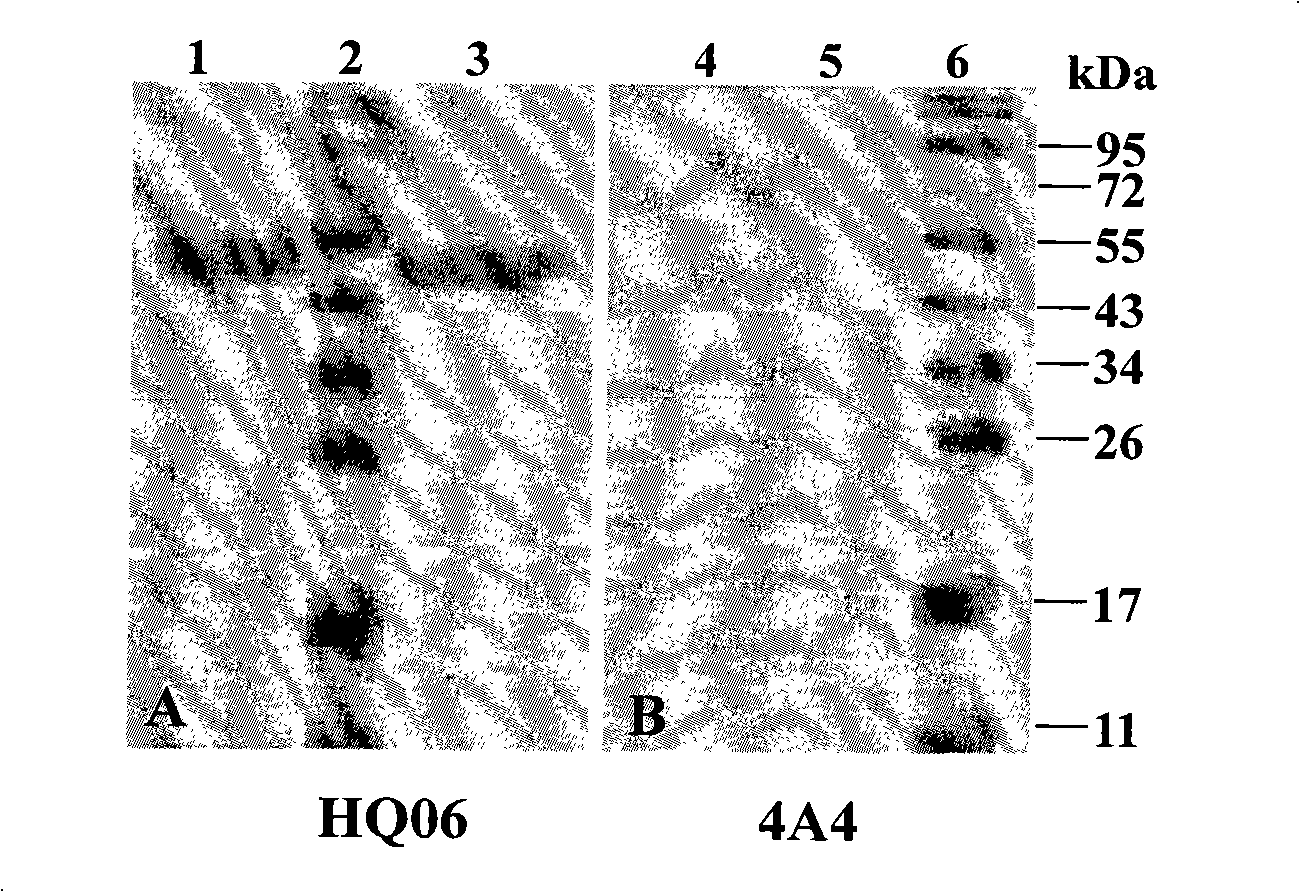
The invention discloses a monoclonal antibody against virulent strain E2 protein of classical swine fever virus and a hybridoma cell strain secreting the monoclonal antibody. The hybridoma cell strain is obtained by using hog cholera lapinized virus vaccine strain E2 protein expressed by Baculovirus as tolerogen, selecting Shimen strain E2 protein as immunogen, immunizing mouse by cyclophosphamide immunosuppression method, carrying out cell fusion, and sieving hybridoma cell strain capable of stably secreting monoclonal antibody against E2 protein. The monoclonal antibody can react with Shimen strain and can produce specific reaction with virulent strain of classical swine fever viruses of 1.1, 2.1, 2.2 and 2.3 gene sub-groups. The monoclonal antibody has neutralization activity and does not react with hog cholera lapinized virus vaccine strain, so that the monoclonal antibody can be used for differentiating virulent strain of classical swine fever virus and hog cholera lapinized virus vaccine strain, which establishes the foundation for establishing a method for differentiating wild virus infection of classical swine fever and vaccine immunity and for researching the molecular difference between CSFV virulent strain and mild strain.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Antihuman hemoglobin detection reagent strips and monoclone antibody contained therewith
InactiveCN1490407AMeet the inspection standardMeet the requirementsHybrid cell preparationImmunoglobulinsReagent stripProtein.monoclonal
A hybridoma cell strain B9 (CGMCC No.0970), its monoclonal antibody resistant to human haematoglobin, the reagent strip containing said monoclonal antibody for the colloidal gold immunochromatographic test, and the preparing process of said reagent strip are disclosed. Its advantages are high sensitivity and high specificity.
Owner:公安部第二研究所
Retinol detection kit and preparation method thereof
InactiveCN102841210AImprove stabilityImprove accuracyBiological testingVitamin A RetinolPolyethylene glycol
The invention relates to a detection kit for a retinol binding protein. The interior of a detection kit body comprises reagents R1 and reagents R2, wherein the reagents R1 comprise a buffer solution, polyethylene glycol, sodium azide, sodium ethylene diamine tetracetate and bovine serum albumin; and the reagents R2 comprise a buffer solution, latex grains combined with retinol binding protein monoclonal antibodies, tween-20, sodium azide, sodium ethylene diamine tetracetate and bovine serum albumin. A sample and the reagents are mixed at a certain volume ratio and generate a series of reactions, and then a reactant is placed under a semi / full-automatic biochemical analyzer, and the speed of absorbance change in the position of a main wavelength of 340nm is detected, so that the concentration magnitude of the retinol binding protein is measured and calculated. The detection kit has the advantages of accuracy, stability and convenience.
Owner:谢兵
Immune-fluorescence test strip component for rapidly detecting C-reactive protein quantitatively, detection card component produced by same and method for preparing same
ActiveCN102680702AHigh sensitivityImprove signal-to-noise ratioBiological testingFluorescence/phosphorescencePorphyrinIMMUNE FLUORESCENCE
The invention discloses an immune-fluorescence test strip component for rapidly detecting C-reactive protein quantitatively, a detection card component produced by the same and a method for preparing the same. The test strip component comprises a test strip and a platinum porphyrin marked specific antibody independently packed. The test strip comprises a bottom lining, a water absorption pad, coating analysis film and a sample pad. The coating analysis film is provided with a detection line and a quality control line, a specific antibody coated by the detection line is a C-reactive protein resistance monoclonal antibody, and a specific antibody coated by the quality control line is a rabbit intravenous gamma globulin (IgG) antibody; the detection card component comprises a test strip, a card box composed of a cover plate and a back plate and a latinum porphyrin marked specific antibody independently packed. The test strip component for detecting C-reactive protein in body fluid has the advantages of being simple in operation, rapid, sensitive, good in specificity and the like, and having good clinical application prospect.
Owner:GUANGZHOU HONGQI OPTICAL INSTR TECH
Anti-AKR1B10 protein monoclonal antibody and applications thereof
ActiveCN104650234AImmunoglobulins against enzymesMaterial analysisAntigenTime resolved fluorescence immunoassay
The invention relates to the field of medical biotechnology, and particularly relates to anti-human tumor specific antigen ketoreductase 1B10 (AKR1B10) protein monoclonal antibody, and a time resolved fluorescence immunoassay (TRFIA) kit used for screening, diagnosis, efficacy judgment, prognosis evaluation or recurrence monitoring of cancers.
Owner:湖南莱拓福生物科技有限公司
Preparation method of HRPII protein monoclonal antibody of plasmodium falciparum
ActiveCN101659975AGood repeatabilityAchieve serial expressionMicroorganism based processesFermentationChemical synthesisEscherichia coli
The invention relates to a preparation method of HRPII protein monoclonal antibody of plasmodium falciparum. The preparation method comprises the following steps of: adopting HRPII protein of plasmodium falciparum as target antigen and respectively analyzing and selecting two dominant antigen epitopes of A and B; respectively repeating the two dominant antigen epitopes of A and B, then continuously connecting four glycine and forming recombinant protein C; adopting most securest code of escherichia coli and converting the amino acid sequence of the recombinant protein C into corresponding nucleotide sequence; carrying out chemical synthesis to the former step to obtain the nucleotide sequence, and respectively adding enzyme cutting sites BamHI and EcoRI at the upstream and downstream thereof; inserting nucleotide fragment obtained by the former step into expression carrier PET-28a(+), constructing recombinant protein C expression carrier and inducing to express the recombinant proteinC in the escherichia coli BL21 (DE3); carrying out ultrasonic bacteria breaking and low-temperature centrifugation, then taking supernatant of the solution, affining a chromatographic column by nickel-agarose, eluting and obtaining purified recombinant protein C; after immunizing Balb / c mouse with the recombinant protein C for a plurality of times, taking and fusing spleen cells with sp2 / 0 myelomacells, and obtaining six hybridoma cell lines by multiple rounds of screening; and purifying monoclonal antibody, respectively marking horse radish peroxidase and prorating matching and combination of optimum monoclonal antibody by ELISA orthogonal experiment.
Owner:杭州新脉生物科技有限公司
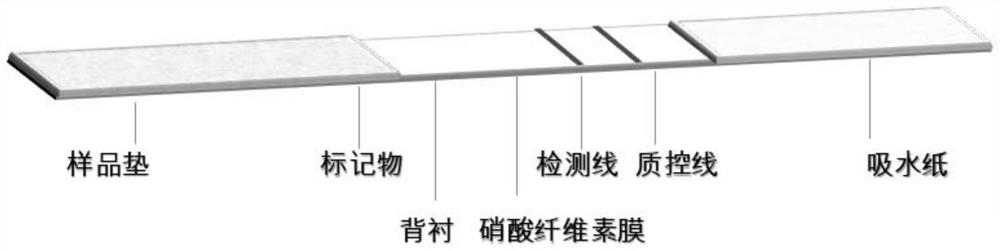
Immunochromatographic test paper for detecting novel coronavirus
ActiveCN111879933AAccurate identificationAvoid missing detectionMaterial analysisAgainst vector-borne diseasesBlood vesselAffitin
The invention relates to immunochromatographic test paper for detecting novel coronavirus SARS-CoV-2. The test paper comprises a substrate, and a sample pad, a combination pad, a nitrocellulose membrane and a water absorption pad which are arranged on the substrate and are sequentially and fixedly adhered to the substrate along the flowing direction of a to-be-detected liquid sample, the substrateis a PVC plate. The sample pad is coated with a biotin labeled anti-SARS-CoV-2N protein monoclonal antibody and biotin labeled angiotensin converting enzyme 2 (ACE2), the combination pad is coated with avidin crosslinked colloidal gold, the nitrocellulose membrane is provided with a detection line and a quality control line, the detection line is coated with an anti-SARS-CoV-2M protein monoclonalantibody, and the quality control line is coated with goat anti-mouse IgG. The immunochromatographic test paper provided by the invention achieves high-sensitivity, high-specificity, high-speed and convenient-to-operate detection, and can be applied to large-scale rapid screening of people in primary hospitals and communities.
Owner:广州德成生物科技有限公司
Method of examing staphylococcus aureus
InactiveUS20060051820A1High sensitivityQuickly and conveniently testedMaterial analysisProtein.monoclonalStaphylococcus aureus
This invention relates to a method for testing S. aureus in a specimen by an immunoassay using an antibody against protein A wherein at least one mouse IgG1 monoclonal antibody is used in the immunoassay, and a test kit for testing S. aureus in a specimen wherein the kit at least comprises a mouse IgG1 anti-protein A monoclonal antibody and a reagent for detecting the labeled protein A. Use the present invention enables detection of S. aureus in the sample in a short time and at a high sensitivity.
Owner:SEKISUI MEDICAL CO LTD
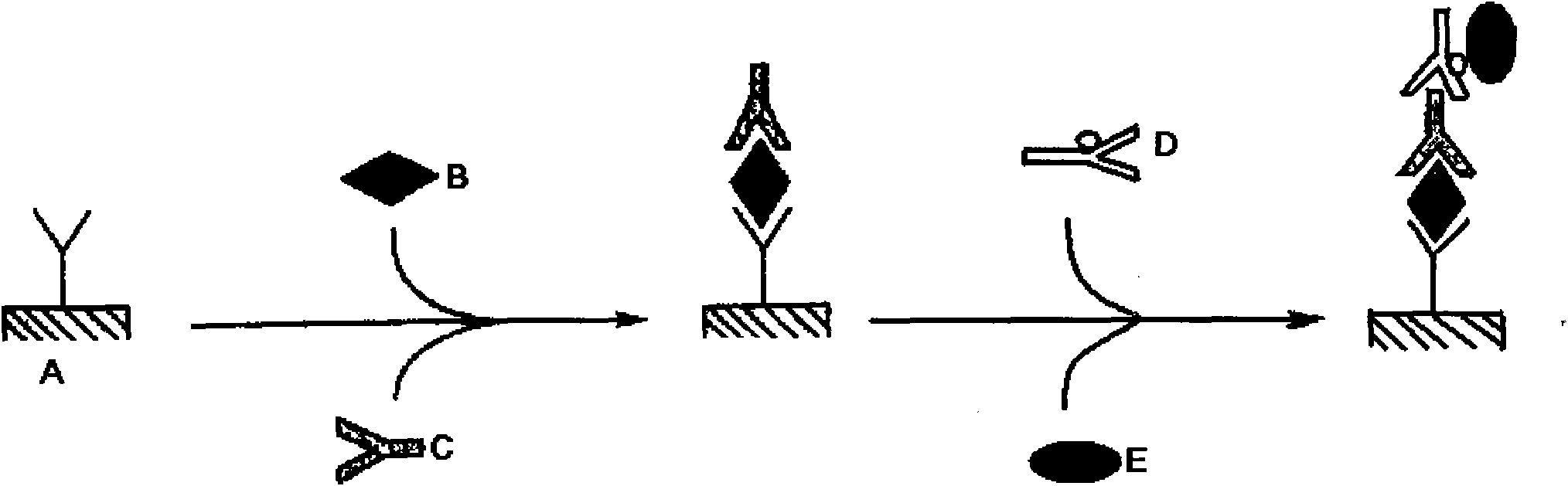
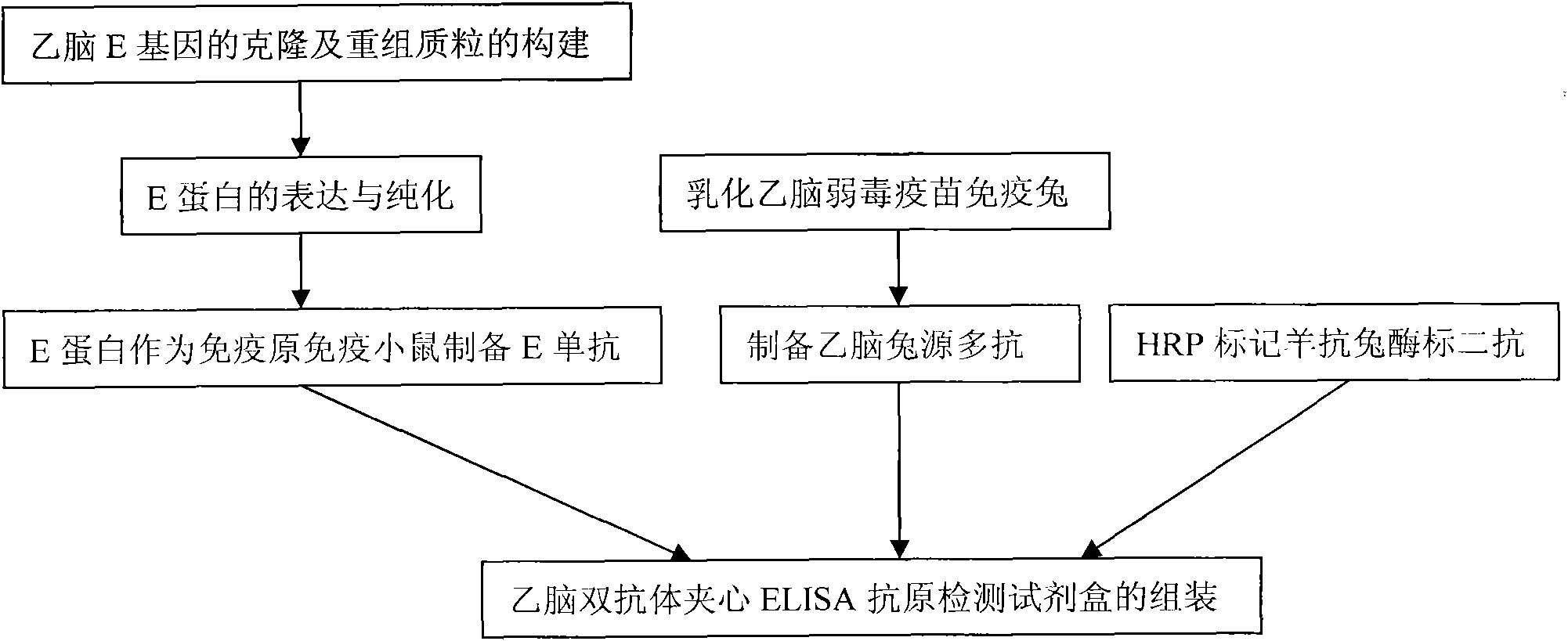
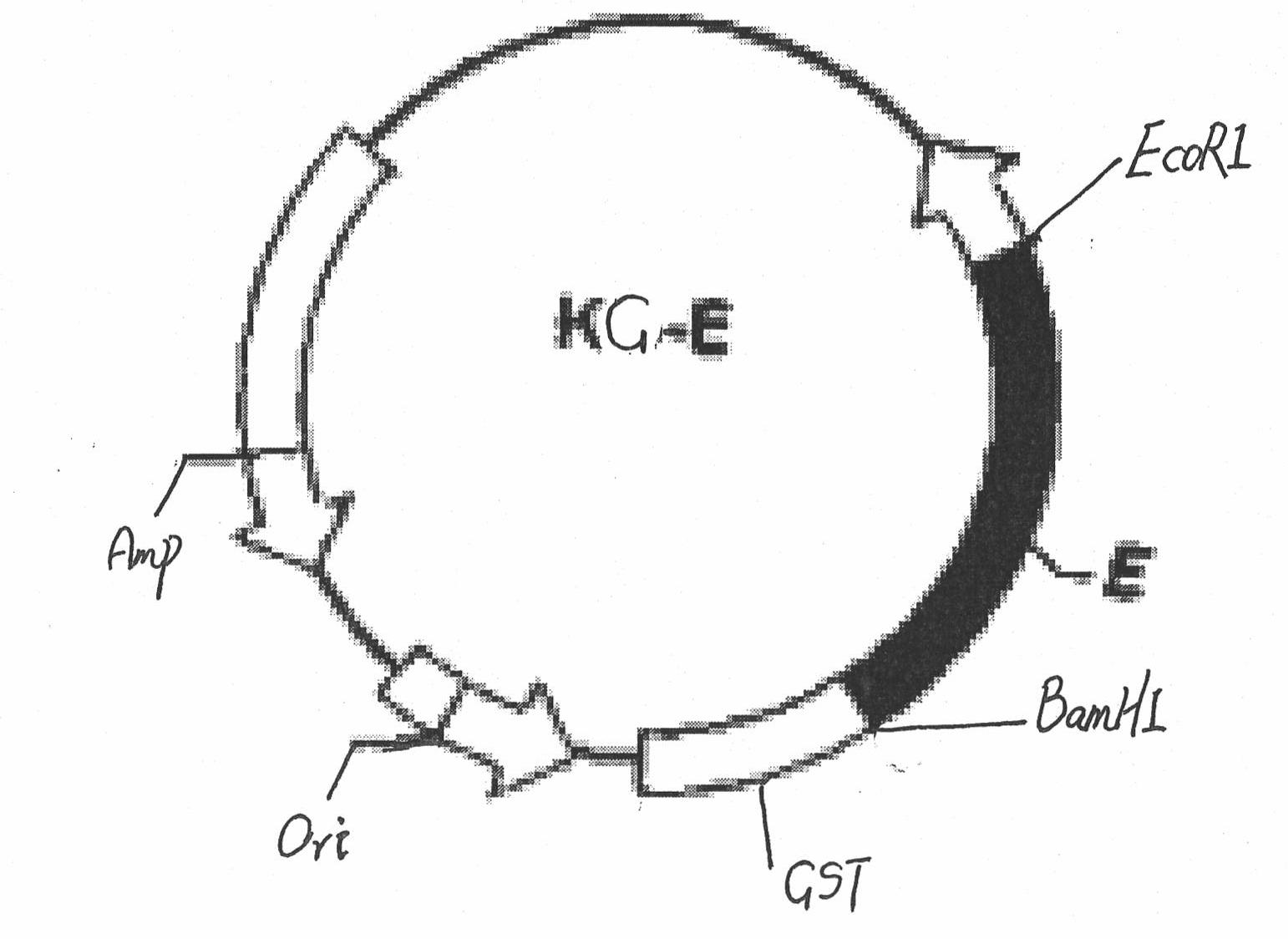
ELISA (Enzyme-Linked Immuno Sorbent Assay) kit for detecting Japanese encephalitis virus antigens in swine, human and mosquitoes and application
InactiveCN102464716AEfficient detectionShort period of bloodMicroorganism based processesImmunoglobulins against virusesAnimal virusProtein.monoclonal
The invention belongs to the technical field of animal virology and immunology, in particular to a double-antibody sandwich ELISA (Enzyme-Linked Immuno Sorbent Assay) kit for detecting Japanese encephalitis virus antigens in swine, human and mosquitoes. Core reagents of the kit comprise a Japanese encephalitis virus E protein monoclonal antibody serving as a primary antibody and a rabbit polyclonal antibody serving as a secondary antibody. The invention discloses a preparation and purification method of the Japanese encephalitis virus E protein monoclonal antibody and the rabbit polyclonal antibody. The invention also discloses a detection method of the double-antibody sandwich ELISA. A hybridoma cell strain secreting the monoclonal antibody is preserved in China Center for Type Culture Collection with CCTCC NO. C2010114.
Owner:HUAZHONG AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com