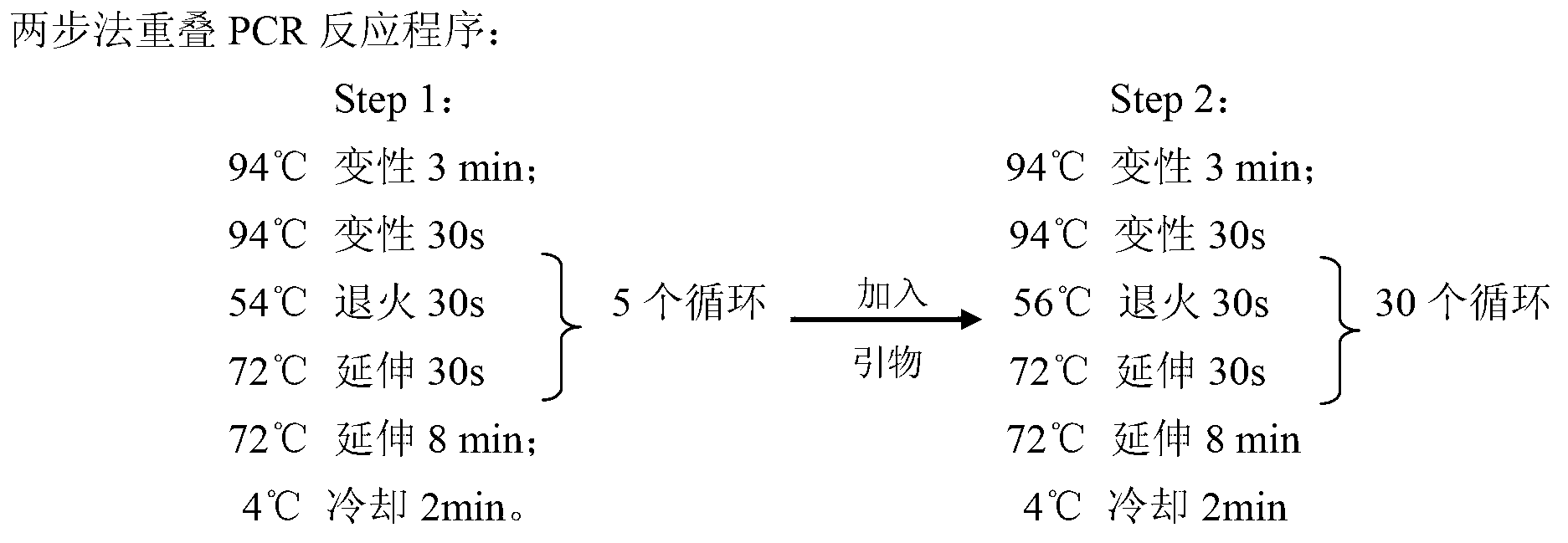
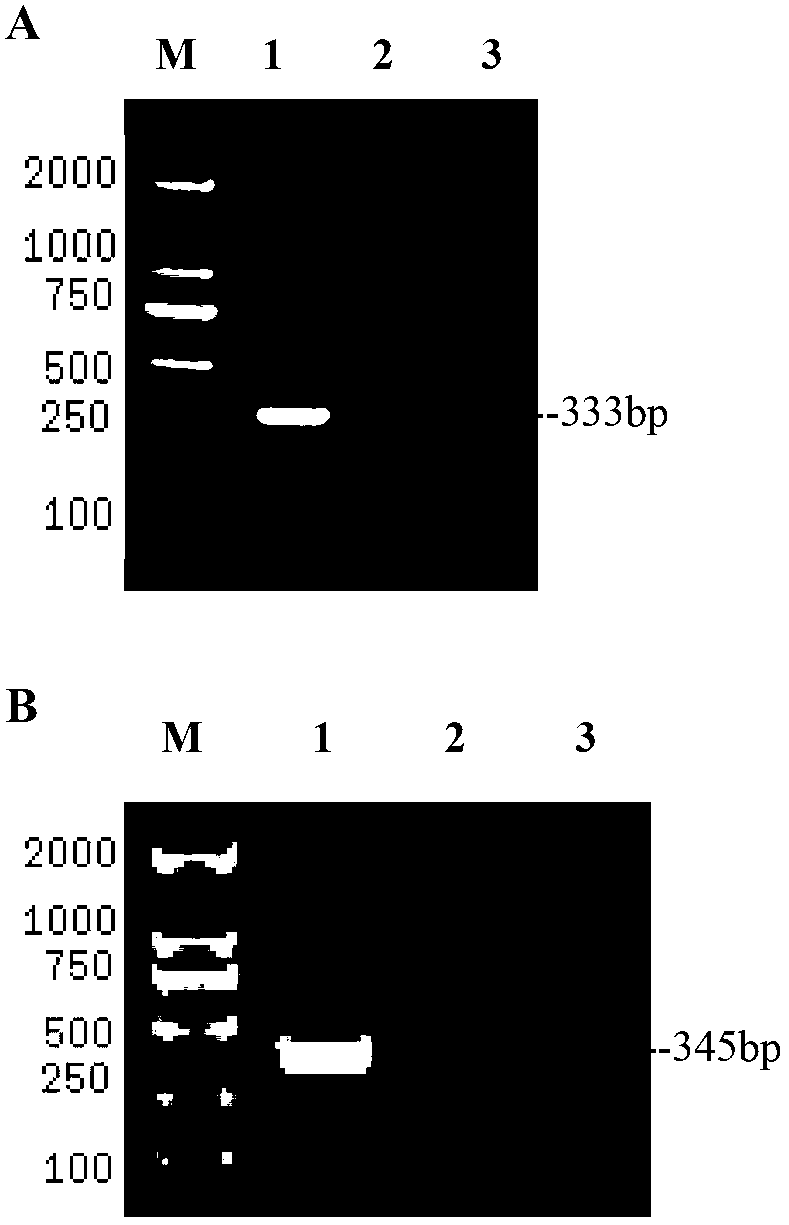
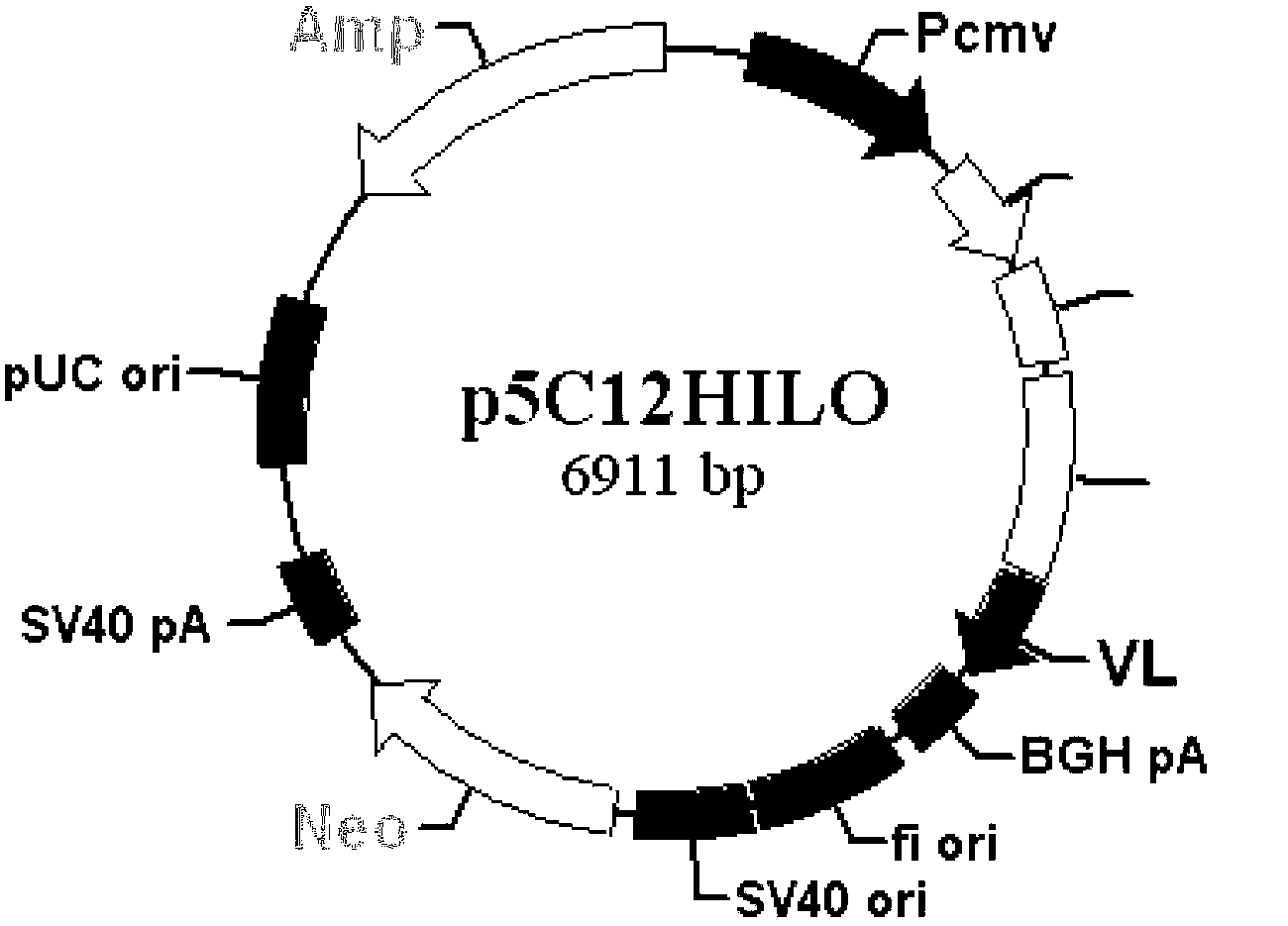
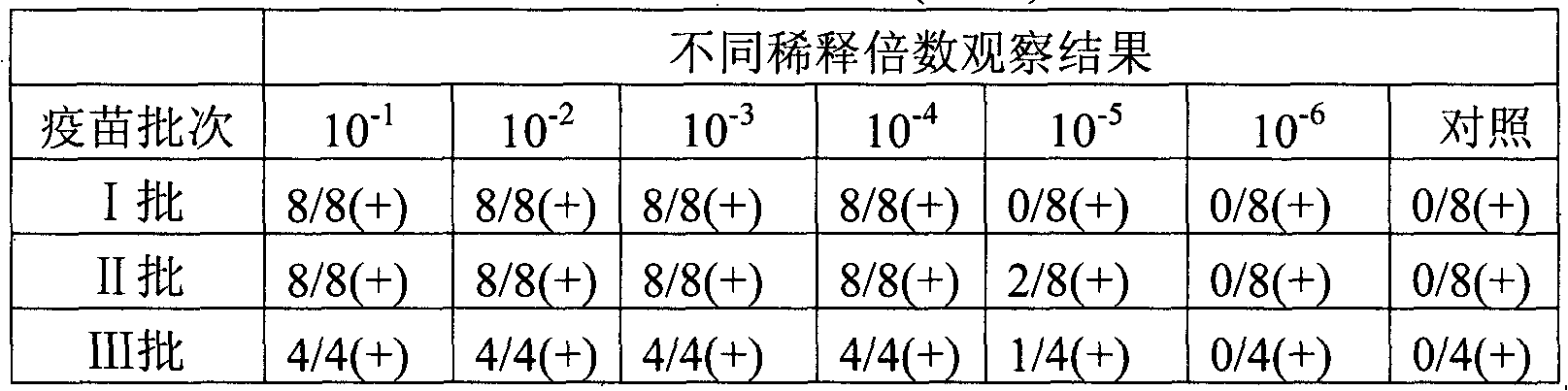
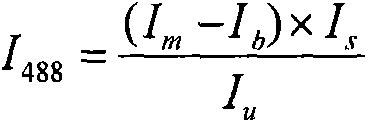Patents
Literature
166 results about "Indirect immunofluorescence" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Indirect immunofluorescence: Indirect fluorescence is a double antibody technique. the unlabelled antibodies which have bound to the antigens are visualized by a fluorescent antiglobulin reagent directed at the unlabelled antibodies.
Fusion protein construct and method for inducing HIV-specific serum IgG and secretory IgA antibodies in-vivo
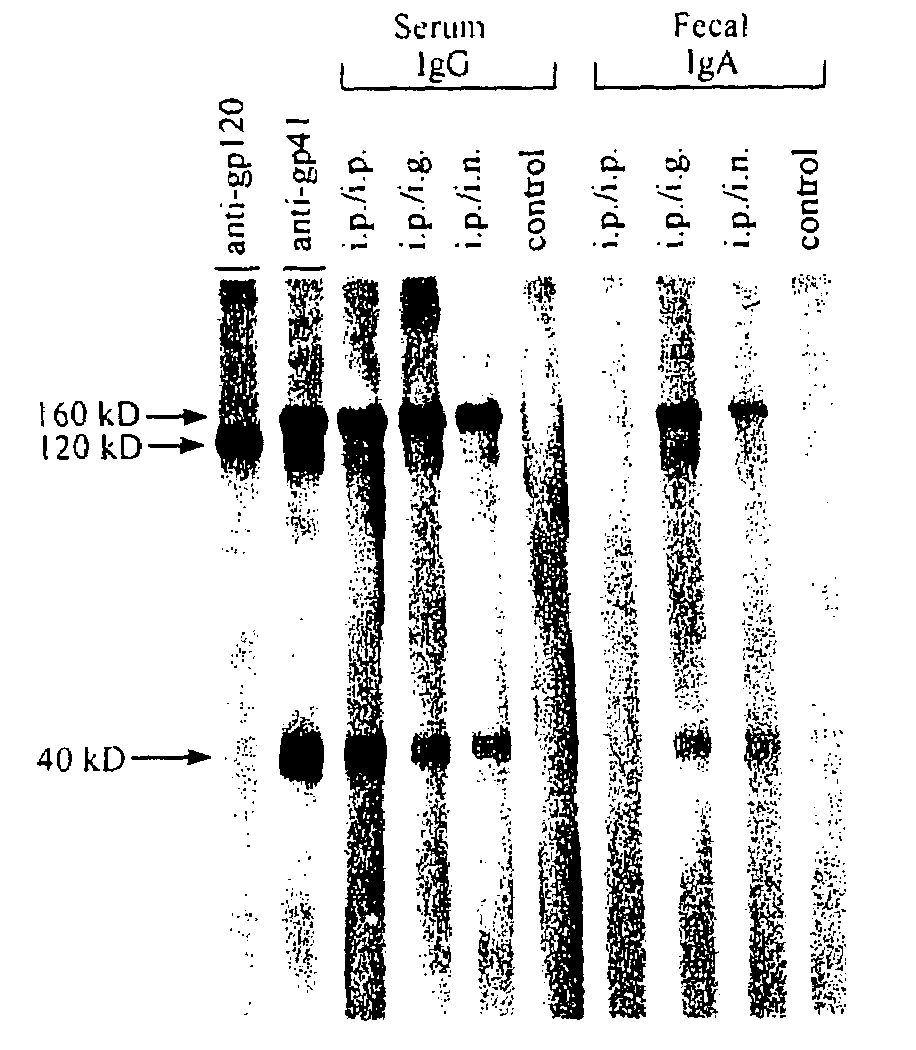
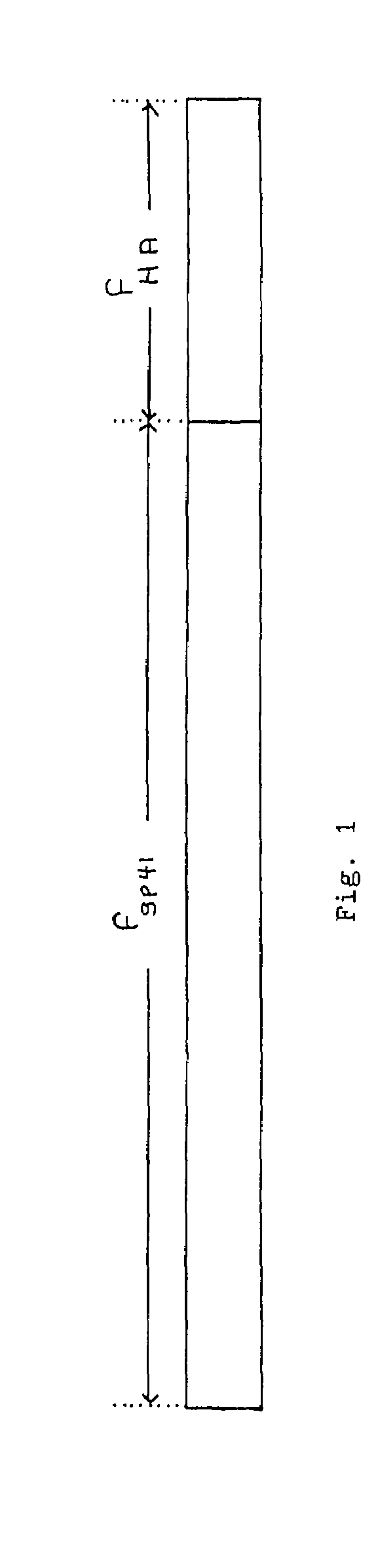
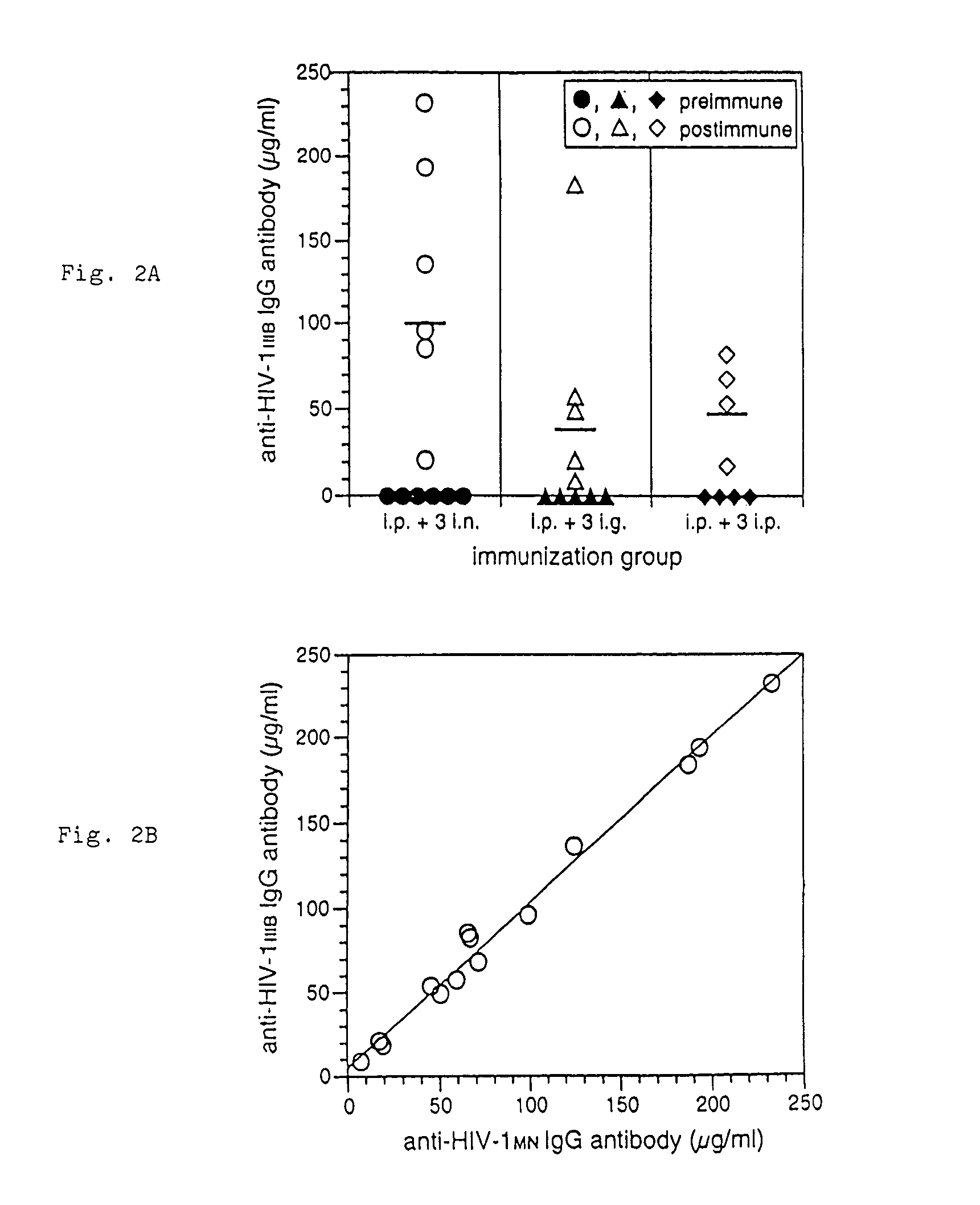
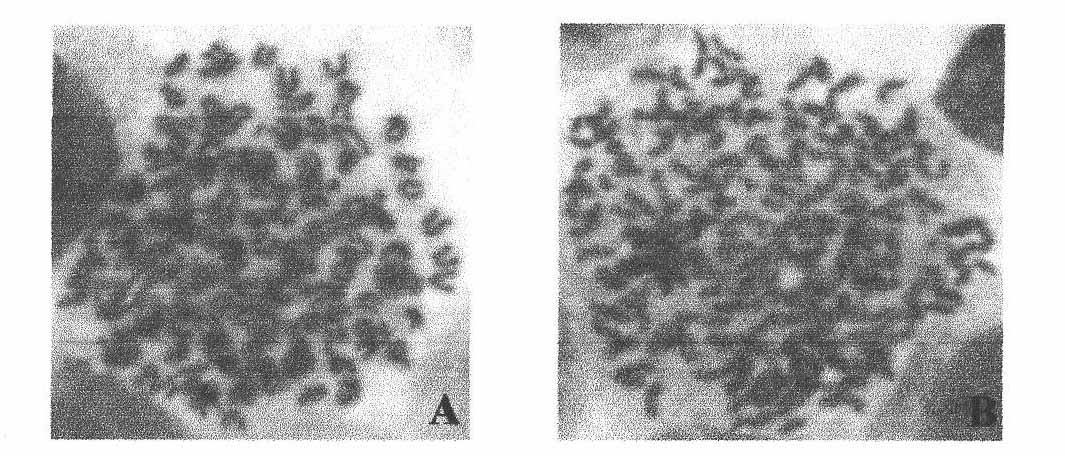
The present invention provides a fusion protein construct (gp41HA) consisting of the ectodomain of the HIV-1IIIB envelope glycoprotein gp41 fused to a fragment of the influenza virus HA2 hemagglutinin protein. Immunization in-vivo via an intraperitoneal prime followed by intranasal or intragastric boosts with gp41HA induces high concentrations of serum IgG antibodies and fecal IgA antibodies that reacted with gp41 in HIV-1IIIB viral lysate and are cross-reactive with gp41 in HIV-1MN lysate. Followup analyses by indirect immunofluorescence showed that both serum IgG and fecal IgA recognized human peripheral blood mononuclear cells infected with either syncytium-inducing (SI) or non-syncytium-inducing (NSI) North American HIV-1 field isolates, but not uninfected cells.
Owner:CHILDRENS MEDICAL CENT CORP
Preparation method for PCV-II Cap protein monoclonal antibody, antibody and application
InactiveCN101768218AAvoid distortionThe ability to secrete antibodies is strong and stableImmunoglobulins against virusesFluorescence/phosphorescenceBALB/cIndirect elisa
The invention discloses a preparation method for a PCV-II Cap protein monoclonal antibody, an antibody and application. The invention adopts ultracentrifuged and purified PCV-II as an immunogen to immunize a BALB / c mouse by the conventional method, takes spleen cells of the immunized BALB / c mouse to fuse with SP2 / 0 cells, obtains two strains of hybridoma cells secreting the PCV2-Cap protein monoclonal antibodies by indirect ELISA screening, respectively names the two strains of hybridoma cells as 8-60 and 10-48, identifies biological characteristics of the two strains 8-60 and 10-48, and usesthe two strains 8-60 and 10-48 as the first antibodies to establish an indirect immunofluorescence diagnostic method. The result of the indirect immunofluorescence diagnostic method is basically consistent with that of the PCR diagnostic method, and the positive and negative coincidence rates are respectively 93.75 percent and 100 percent so as to provide reference for preventing and treating theporcine circovirus disease.
Owner:INST OF ANIMAL HUSBANDRY & VETERINARY FUJIAN ACADEMY OF AGRI SCI +7
Anti-flavivirus envelope E protein monoclonal antibody and application thereof
ActiveCN101891806AGood broad-spectrum anti-flavivirus effectInfection fromFungiBacteriaCell strainProtein.monoclonal
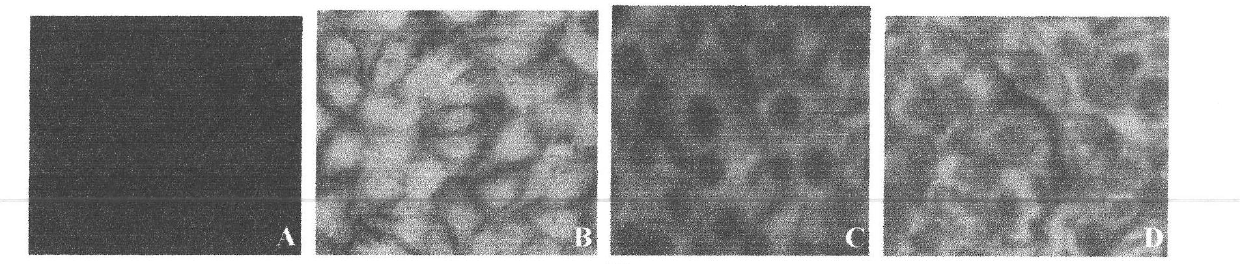
The invention discloses an anti-flavivirus envelope E protein monoclonal antibody and application thereof. The anti-flavivirus envelope E protein monoclonal antibody of the invention is secreted by a mouse source hybrid tumour cell strain D2-2A10G6 with the preservation number of CGMCC No. 3292. The anti-flavivirus monoclonal antibody of the invention can be specifically combined with the function epitope of flavivirus envelope E protein. The amino acid sequence of the functional epitope of envelope E protein is SEQ ID NO: 17. The monoclonal antibody of the invention is subject to screening by indirect immunofluorescent method, and indirect enzyme-linked immunization is used for evaluating the specificity and affinity when being combined with antigen. By adopting the monoclonal antibody or immunoconjugate of the invention, flavivirus infection cell can be blocked and suckling mouse can be protected from virus attack, thus achieving the effect of inhibiting virus infection. The monoclonal antibody or immunoconjugate of the invention also can be used for flavivirus envelope E protein detection.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
HCMVPP65 antigenemia indirect immunofluorescence method detection reagent kit
InactiveCN101261272AImprove featuresIncreased sensitivityFluorescence/phosphorescenceBALB/cFluorescence
The invention discloses a HCMVPP65 antigenemia indirect immune fluorescence method detection kit, which uses cytomegalovirus-AD169 virus strain pp65 protein as the immunogen to immune a Balb / c mouse. The spleen cells of the immunized mouse and the myeloma cells of the mouse which belongs to the same type with the immune mouse are conventionally integrated, by indirect ELISA screening and finite dilution cloning, the hybridoma cell lines of the mouse cytomegalovirus pp65 protein cloning antibody are obtained, and the characteristics of the hybridoma cell lines are identified by ELISA, immune fluorescence experiment and other methods; two monoclonal antibodies that stably secrete the pp65 protein are established successfully and named respectively as 1A6 and 4A8. A monoclonal antibody which differs from the former report and aims at the pp65 protein of the cytomegalovirus (CMV) is prepared and a method used for preparing erythrocyte fast pyrolysis is established; compared with other detection kits which belongs to the same kind, the detection kit of the invention is faster, simpler and more convenient and has higher specificity and sensitivity.
Owner:天津市秀鹏生物技术开发有限公司
Monoclonal antibody of immunoglobulin of anti lymphocyst vitos of Pacific fluke, and preparation method
ActiveCN101003572AInnovative designAchieve purificationImmunoglobulins against animals/humansBiological testingBALB/cCell engineering
This invention discloses monoclonal antibody against anti-LCDV immunoglobulin of Paralichthys olivaceus, which is excreted by hybridoma JF-lgM-H (CCTCC-C200631). The method comprises: immunizing Paralichthys olivaceus with LCDV inactivated by formalin to prepare antiserum, purifying Paralichthys olivaceus immunoglobulin, immunizing Balb / c mice as antigen, preparing hybridoma cells by cell engineering method, and screening the monoclonal antibody by immunoassay. Indirect ELISA and indirect immunofluorescent antibody assay show that this monoclonal antibody is located on the heavy chain (7-80 kDa) of the anti-LCDV immunoglobulin. The monoclonal antibody can be used for preparing reagents for detecting LCDV infection in early stage, and evaluating the immune effects of LCDV vaccine inactivated by formalin.
Owner:OCEAN UNIV OF CHINA
Recombinant protein subunit vaccine for resisting porcine circovirus serotype 2
ActiveCN103739717AEnhance immune responseGood protectionViral antigen ingredientsAntiviralsOrf2 geneFlagellin
The invention provides a recombinant protein subunit vaccine for resisting a porcine circovirus serotype 2. The recombinant protein subunit vaccine is a fused protein of a salmonella typhosa flagellin with relatively high immunogenicity and a porcine circovirus serotype 2 Cap protein. Manually coded Flagellin-ORF2 (Open Reading Frame) and Flagellin-delta ORF2 genes are fused and cloned in a pFastBac expression vector, and recombined and cloned together with ORF2 and deltaORF2 (pFastBac vector) for transfecting Sf9 cell, four fused proteins are expressed by using a baculovirus system and identified by using immunofluorescence assay (IFA) and Western-blot. A period of recombining baculovirus by the system is short, and the expressed flagellin+Cap fused protein is high in immune protection force.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Antinuclear antibody quantitative detection kit and use method thereof
The present invention provides a kit for quantitative detection of homogeneous or speckled antinuclear antibody, and a use method thereof. The kit comprises: (1) a Hep-2 cell antigen sheet; (2) a fluorescence labeled secondary antibody; (3) fluorescence intensity calibration microspheres; (4) a buffer; (5) a sheet sealing agent; and (6) an experimental operation and quantitative fluorescence intensity analysis instruction. According to the present invention, the (1) Hep-2 cell antigen sheet provided by the kit, the (2) fluorescence labeled secondary antibody provided by the kit, and a serum sample to be detected are subjected to an indirect immunofluorescence reaction, the (3) fluorescence intensity calibration microspheres are added to the (1) Hep-2 cell antigen sheet to be detected, observing and microscopic image shooting are performed through a fluorescence microscope, a microscopic image analysis software is used to carry out fluorescence intensity analysis, and the fluorescence intensity value is converted into the corresponding serum antinuclear antibody titer value according to the (6) experimental operation and quantitative fluorescence intensity analysis instruction provided by the kit, such that the quantitative detection of the homogeneous or speckled antinuclear antibody in the serum sample is achieved.
Owner:NAT INST OF METROLOGY CHINA
Staphylococcal enterotoxin gene engineering reshaped antibody and its preparation method and use
InactiveCN103224560AOvercome the defects in the detection meansImmunoglobulins against bacteriaBiological testingAntiendomysial antibodiesGenetic engineering
The invention discloses a staphylococcal enterotoxin gene engineering reshaped antibody and its preparation method and use. The staphylococcal enterotoxin gene engineering reshaped antibody has an amino acid sequence shown in the formula of SEQ ID No.1 in the sequence table. Through construction of light and heavy chain eukaryotic co-expression vectors of the staphylococcal enterotoxin monoclonal antibody, a high-efficiency expression and stable-secretion mammalian cell line and the gene engineering reshaped antibody having high singularity and strong affinity are obtained. The staphylococcal enterotoxin gene engineering reshaped antibody can be used in staphylococcal enterotoxin detection, cell indirect immunofluorescence detection and flow cytometry detection.
Owner:TIANJIN UNIV
Staphylococcal enterotoxin micromolecule antibody and its preparation method and use
InactiveCN103224561AImmunoglobulins against bacteriaVector-based foreign material introductionDisulfide bondingNatural antibody
The invention discloses a staphylococcal enterotoxin micromolecule antibody and its preparation method and use. The staphylococcal enterotoxin micromolecule antibody has an amino acid sequence shown in the formula of SEQ ID No.1 in the sequence table. The preparation method comprises the following steps of designing and constructing eukaryotic mono-promoter co-expression vectors of staphylococcal enterotoxin monoclonal antibody light chain and heavy chain variable region genes, introducing cysteine residues to C- ends of the light chain and the heavy chain to form interchain disulfide bonds by an intracellular peptide fragment self-assembling principle, and simulating antigen binding domain spatial conformation of natural antibodies to obtain high-efficiency expression stable-secretion mammal cell line and the high-singularity strong-affinity micromolecule antibody. The staphylococcal enterotoxin micromolecule antibody can be used for staphylococcal enterotoxin detection, cell indirect immunofluorescence detection and flow cytometry detection.
Owner:TIANJIN UNIV
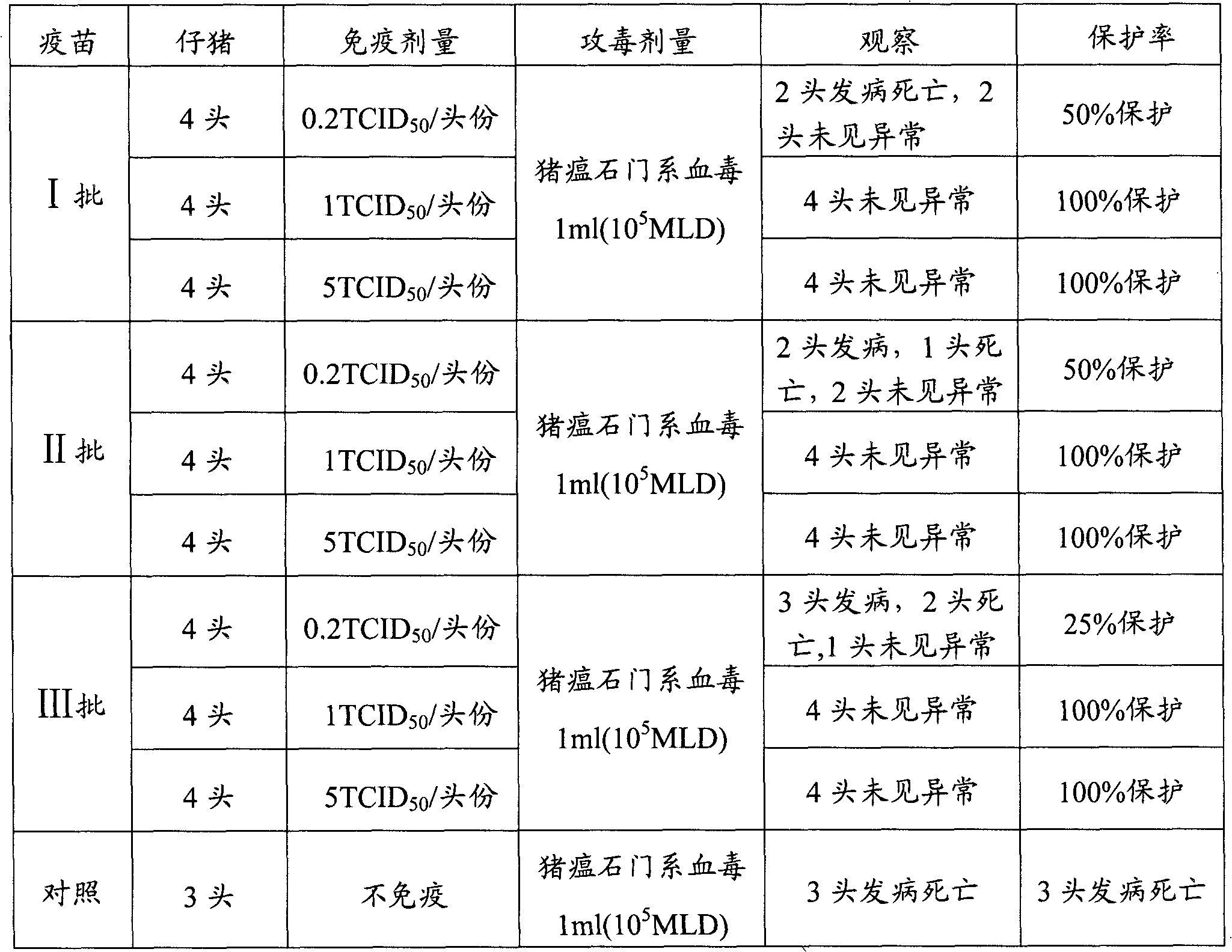
Method for testing efficacy of swine fever live vaccine
ActiveCN101846683AShort detection timeEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceVaccine PotencyEfficacy
The invention belongs to the technical field of new biological veterinary medicaments, and in particular relates to a method for testing the efficacy of a swine fever live vaccine. The efficacy of the live vaccine is evaluated by detecting the virus content of the live vaccine through indirect immunofluorescence (IFA). The method for testing the efficacy of the swine fever live vaccine has the advantages of short test time, simple operation, low cost, accurate obtained result, high repeatability, excellent application value and wide market prospects.
Owner:PU LIKE BIO ENG
Method for preparing allophycocyanin-marked fluorescent antinuclear antibody
InactiveCN101980019AExcellent fluorescent dyeNo toxicityPeptide preparation methodsAlgae/lichens peptidesAnion-exchange chromatographyHigh pressure
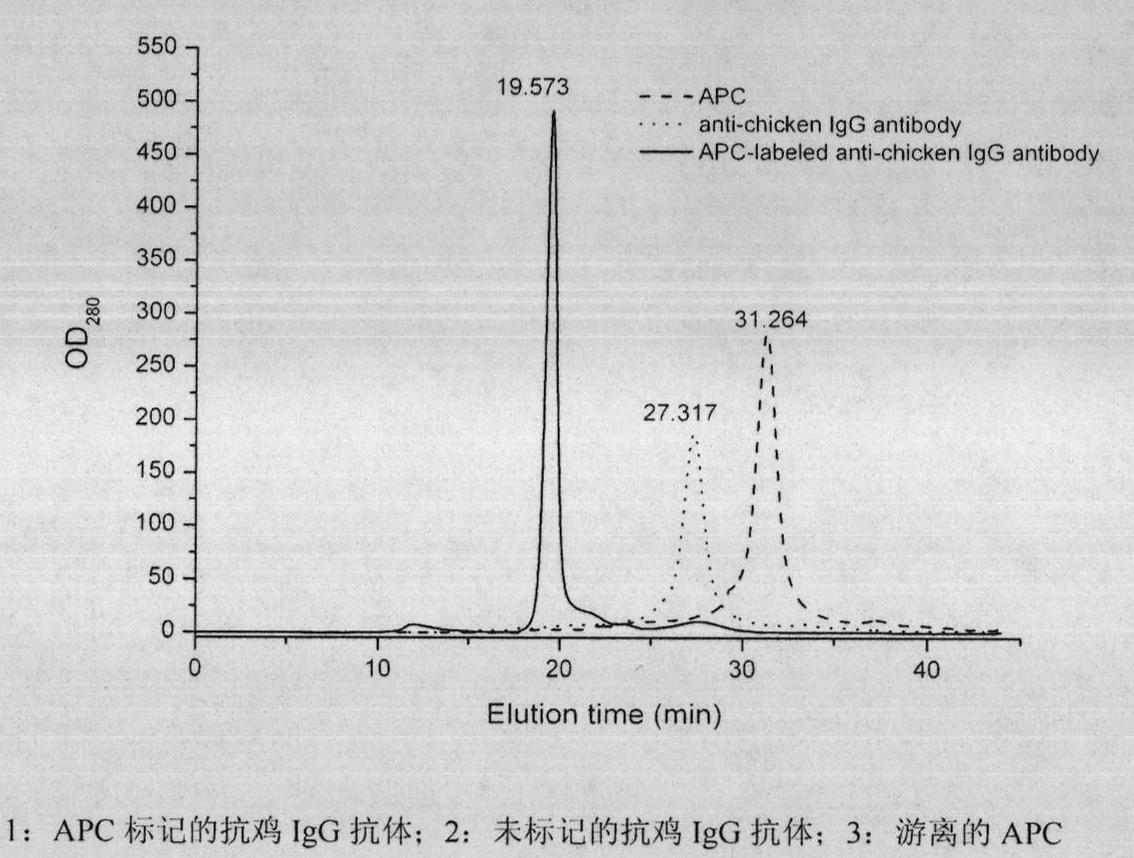
The invention relates to a method for preparing an allophycocyanin(APC)-marked fluorescent antinuclear antibody, which comprises: separating and purifying spirulina APC by anion exchange chromatography; crosslinking APC and antinuclear antibody derivatives in a liquid phase in a proper molar ratio; and preparing the APC-marked fluorescent antinuclear antibody by purification by high-pressure liquid chromatogram. The fluorescent antinuclear antibody prepared by the method has high crosslinking efficiency, high purity, bright red fluorescence, high stability and high sensibility, and can be used as a common fluorescent probe for indirect immunofluorescent detection of infectious diseases in animals such as chickens and pigs.
Owner:QILU UNIV OF TECH
Preparation method of PCV2 (Porcine Circovirus2)-D
The invention discloses a preparation method of PCV2 (Porcine Circovirus2)-D. The microorganism preservation number of PCV2 (Porcine Circovirus2) is CGMCC (China General Microbiological Culture Collection Center) No.7245. According to the preparation method, the preference of genetic codon of the PCV2 is modified, and the preferred codon is modified into non-preference codon, so that the expressions of the components of the virus are reduced, further, the copy rate of the virus in host cells is lowered, the virus is weakened, and the PCV2-D is obtained by genetic modification. Furthermore, the proliferation speed of the PCV2-D is compared with that of wild type strains at a cellular level. The detection of PCR (Polymerase Chain Reaction) and IFA (Indirect Immunofluorescence Assay) prove that the PCV2-D can be infected and copied in cells, and has certain infection.
Owner:SHANGHAI ACAD OF AGRI SCI
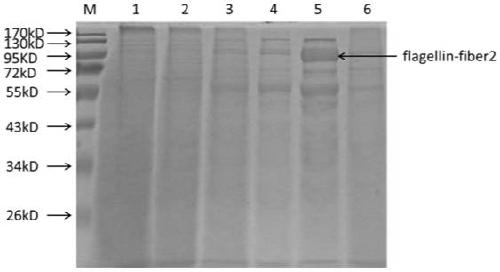
Flaggelin-fiber2 fusion protein, and preparation method and application thereof
ActiveCN109867727AEnhance immune responseStrong immune adjuvant effectAntiviralsAntibody medical ingredientsWestern blotFlagellin
The invention provides a flaggelline-fiber2 fusion protein as well as a preparation method and application thereof. The fusion protein is a fusion protein of a fowl adenovirus type 4 fiber2 protein with relatively high immunoprotection and a salmonella typhimurium flagellin. The preparation method comprises the following steps: cloning an artificially coded flaggin-fiber2 gene into a pFastBac-HA expression vector through fusion; carrying out gene transposition to form recombinant Bacmid, transfecting the recombinant Bacmid into Sf9 insect cells, expressing the fusion protein by utilizing a baculovirus system, and conducting identifying by virtue of indirect immunofluorescence IFA and Western blot. The period of obtaining the recombinant baculovirus through the system is short, and when SPFchicken are immunized by the flaggin-fibe2 fusion protein, results show that the flaggin-fibe2 fusion protein expressed by the baculovirus system has high immunoprotection capability.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Method for detecting virus content of swine fever live vaccine through indirect immunofluorescence
InactiveCN103698518AThe test result is accurateShort detection timeFluorescence/phosphorescenceFluorescenceVaccine virus
The invention belongs to the technical field of biological quarantine and particularly relates to a method for detecting the virus content of a swine fever live vaccine through indirect immunofluorescence. The method for detecting the virus content of the swine fever live vaccine through indirect immunofluorescence comprises the following steps: (1) preparing subculture cells; (2) measuring the virus content of the swine fever live vaccine in the subculture cells; (3) fluorescently dyeing virus-inoculated cells. According to the method, the viruses of the live vaccine in the subculture cells are detected through indirect immunofluorescence so as to achieve the purpose of rapidly, accurately, simply and effectively detecting the virus content of the swine fever live vaccine.
Owner:SHANDONG BINZHOU BOLAIWEI BIOTECH
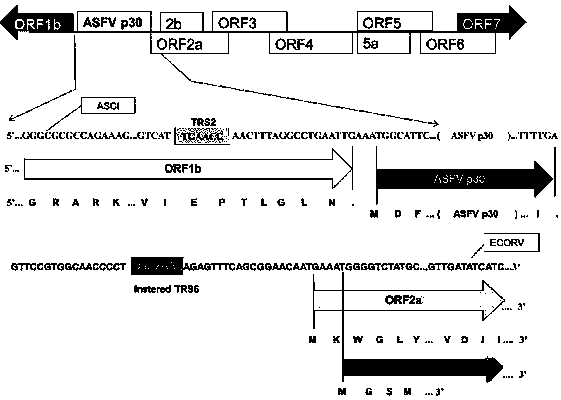
Construction method and application of recombinant porcine reproductive and respiratory syndrome virus capable of expressing African swine fever virus p12 or p17 proteins
InactiveCN110904153AMaintain genetic stabilitySsRNA viruses positive-senseViral antigen ingredientsClassical swine fever virus CSFVForeign protein
The invention provides a construction method of porcine reproductive and respiratory syndrome virus (PRRSV) recombinant plasmid for expressing ASFV (African swine fever virus) p12 protein or p17 protein. The invention also provides a genetic engineering vaccine constructed according to the recombinant plasmid and a construction method. Indirect immunofluorescence is carried out on rPRRSV-p12 and rPRRSV-p17 infection holes, and it is found that specific fluorescence for resisting PRRSV N protein and specific fluorescence for resisting ASFV p12 protein or p17 protein appear in the visual field.It shows that the recombinant PRRSV expressing African swine fever virus p12 protein or p17 protein can ensure that the p12 or p17 protein of exogenous protein ASFV can be well, efficiently and stablyexpressed. Researches show that p12 and p17 are important antigen proteins and can be used for ASF vaccine development.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Recombinant expression protein of 1301 ORF136 genes of herpesvirus cyprinid type 3, antibody and application of antibody
InactiveCN107056898AHigh purityMaintain biological activitySerum immunoglobulinsVirus peptidesNucleotideWestern blot
The invention discloses a recombinant expression protein of 1301 ORF136 genes of herpesvirus cyprinid type 3, a polyclonal antibody and an application of the polyclonal antibody. A full-length amino acid sequence of the recombinant expression protein of 1301 ORF136 genes of the herpesvirus cyprinid type 3 is as shown in SEQ ID NO:2, and a full-length nucleotide sequence encoding the protein is as shown in SEQ ID NO:1. A pET32a-ORF136 recombinant prokaryotic expression vector is constructed by selecting one part of gene sequence of ORF136; the recombinant expression protein is obtained through IPTG-induced expression; the obtained polyclonal antibody is subjected to western blot analysis by adopting a purified CyHV-3 virus and a KS cell infected by the CyHV-3; and the detection application of the antibody is further verified by adopting an indirect immunofluorescence assay. An important material is provided for construction of an ORF136 protein function research and CyHV-3 serological diagnosis method through preparation of the polyclonal antibody of the recombinant expression protein of the ORF136 genes.
Owner:PEARL RIVER FISHERY RES INST CHINESE ACAD OF FISHERY SCI
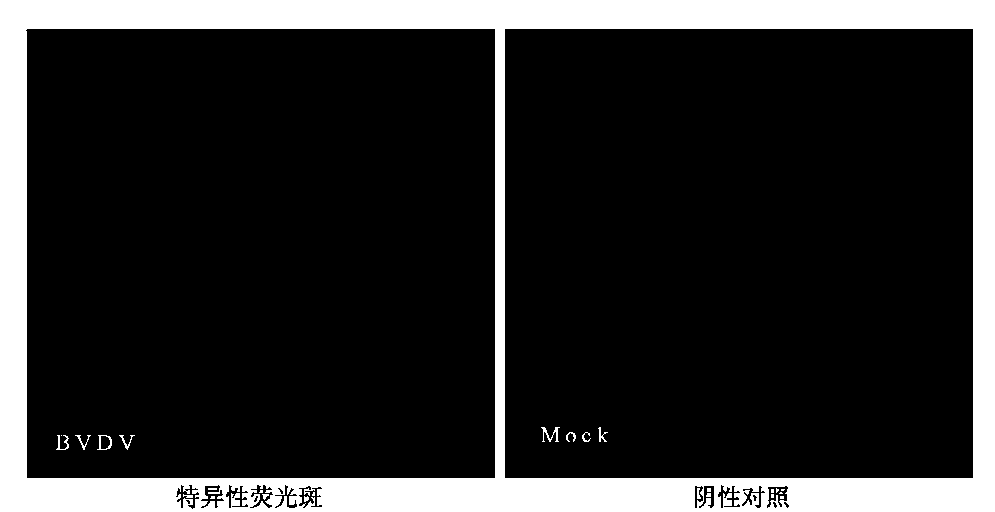
Method for detecting bovine viral diarrhea virus by virtue of indirect immunofluorescence
Owner:成都史纪生物制药有限公司
Method for determining baculovirus titer
InactiveCN103364560AGood repeatabilityThe test result is accurateMaterial analysisCytopathic effectFluorescence microscope
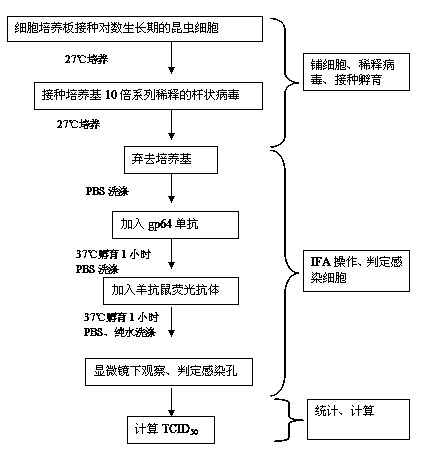
The invention discloses a method for determining baculovirus titer. The method comprises the following steps: paving a cell culture plate with insect cells in the logarithmic phase at the cell density of 2*104-4*104 / 0.1mL / hole, placing the cell culture plate into an incubator for culture and sticking the insect cells to hole bottom; inoculating baculovirus to be detected into the insect cell plate and culturing in the incubator; removing a medium in the cell plate, fixing, washing the cell plate, adding gp64 McAb for incubation, washing the cell plate, adding goat-anti-mouse fluorescent antibody for incubation, washing the cell plate, and observing under a fluorescence microscope; and calculating TCID50 of the virus. The method for determining baculovirus titer is combined with indirect immunofluorescence, is a TCID50 determination method for directly judging whether each hole cell is infected and has good repeatability. Obtained detection results are more accurate. It is not necessary to combine cytopathic effect for determination, and there is no influence from aspects of subjective and experience factors.
Owner:WUHAN CHOPPER BIOLOGY
African swine fever virus antibody ELISA detection kit and preparation method thereof
ActiveCN111929433AImprove the detection rateHigh sensitivityMaterial analysisClassical swine fever virus CSFVAfrican swine fever virus Antibody
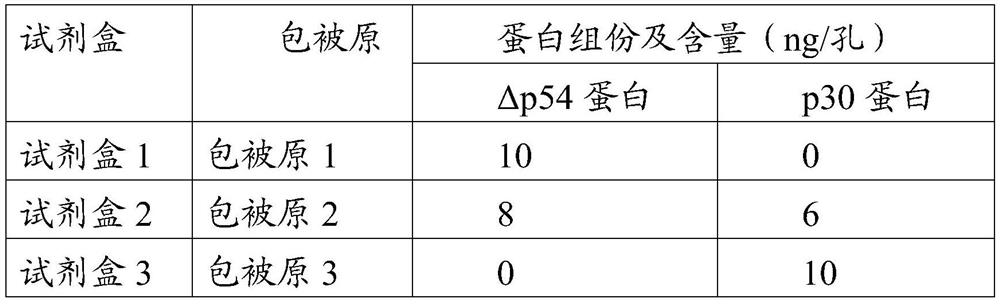
The invention provides an African swine fever virus antibody ELISA (Enzyme Linked Immuno Sorbent Assay) detection kit. A support medium of the kit is coated with an African swine fever virus antigen,and the African swine fever virus antigen is African swine fever virus p30 and / or protein delta p54 protein. The African swine fever virus p30 protein is as shown in SEQ ID No. 1, and the African swine fever virus delta p54 protein is as shown in SEQ ID No. 2. The African swine fever virus antibody ELISA detection kit disclosed by the invention is good in specificity, sensitivity and repeatability, the sensitivity of the kit coated with the two antigens is equivalent to that of indirect immunofluorescence detection, antibody positive can be detected earlier at a low antibody level in the earlystage, a basis is provided for clinical swine herd infection conditions, and the kit plays an important role in preventing and controlling African swine fever virus infection.
Owner:LUOYANG PULIKE WANTAI BIOTECH
Antibody indirect immunofluorescence test method for distinguishing immune animal infected with influenza A virus
InactiveCN101833002AGuaranteed to fold correctlyGood antigenicityVirus peptidesViruses/bacteriophagesSerum igeImmunofluorescence
The invention relates to an antibody indirect immunofluorescence test method for distinguishing whether an immune animal is infected with influenza A virus or not. The method comprises the following steps of: expressing NA protein of influenza A virus as antigen with insect baculovirus; establishing the antibody indirect immunofluorescence test method; and detecting the serum antibody of the NA protein of the influenza A virus. The expressed protein can be directly used as the antigen without purification. The method established by the invention is a detection method matched with the marking vaccine for the influenza A virus, which is used for distinguishing whether the animal vaccinated with the influenza A virus marking vaccine, such as birds, pigs and the like, are infected with corresponding subtype influenza virus or not; as a screening method, the method can be used for detecting the clinical serum sample and determining whether the animal vaccinated with the influenza A virus marking vaccine, such as the birds, pigs and the like, are infected with the corresponding subtype influenza virus or not; and therefore, corresponding preventative measures can be taken as soon as possible so as to eliminate and kill the corresponding epidemic clinical influenza A virus.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY +1
Immunofluorescence reagent for detecting E-type enterovirus and detection kit thereof
InactiveCN105548550ARapid diagnosisShort detection timeBiological material analysisImmunofluorescenceFluorescence
The invention discloses an immunofluorescence reagent for detecting an E-type enterovirus and a detection kit thereof. A direct immunofluorescence detection method set up by applying the immunofluorescence reagent for detecting the E-type enterovirus can be used for clinically fast diagnosing E-type enterovirus infection and used for positioning and authenticating an E-type enterovirus antigen in tissue. The time for direct immunofluorescence detection is short, and a result can be reported within 40 min; specificity is high, and the immunofluorescence reagent only reacts with the E-type enterovirus; the immunofluorescence reagent is high in repeatability and accuracy, the coincidence rate between results of immunofluorescence reagent and results of an indirect immunofluorescence method is 90% or more, and the immunofluorescence reagent can be used for fast diagnosing E-type enterovirus infection.
Owner:JILIN UNIV
Chicken C-type acian metapneumovirus strain(aMPV-JCX) and application thereof
The invention provides a chicken C-type acian metapneumovirus strain(aMPV-JCX) and application thereof. The preservation number of the aMPV / C-JCX strain is CGMCC No.10900. The virus is a cell culture adaptive strain, a preparation method of the cell culture adaptive strain comprises the steps that tissue grinding fluid of a sick chicken infected by aMPV / C-JCX which is firstly obtained through separation domestically is collected to be inoculated into single-layer Vero cells which grow being adherent to the wall, a cell culture serves as an inoculum for the next round of passage, sequentially continuous passage is conducted, and the cell culture adaptive strain is obtained. In the adaptive process, the early-generation culture cycle is 7-9 days, the culture cycle is shortened to 3-5 days when the 60th generation is cultured, and an obvious cytopathic effect can be observed. It is verified through a transmission electron microscope, indirect immunofluorescence and a western-blotting method that the aMPV / C-JCX can be effectively cultured. The titer 1 g TCID50 of the virus is equal to 10<-4.2> / 0.1 ml. It is verified through SPF chickens that the virus virulence is weak, good immunogenicity and protectiveness are achieved, and the cell-adapted virus is an ideal candidate strain for researching and producing viral vaccine.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
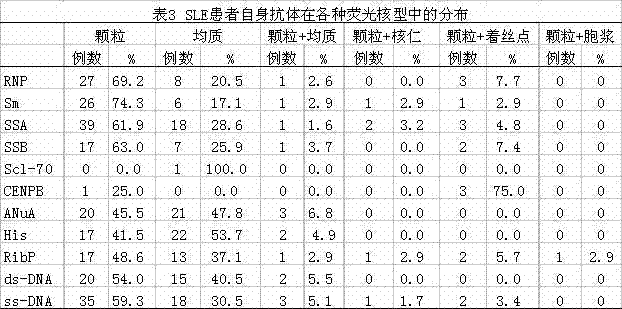
Method for detecting autoantibodies of systemic lupus erythematosus patient
InactiveCN102818890AAids in clinical diagnosisAids in healingMaterial analysisImmunofluorescenceAssay
The invention discloses a method for detecting autoantibodies of a systemic lupus erythematosus patient. The method includes steps of: detecting antinuclear antibodies and double-strand deoxyribonucleic acid (DNA) of the systemic lupus erythematosus patient respectively in an indirect immunofluorescence method, measuring antinuclear antibodies spectrums in a euroimmun western blotting method, and finally detecting single-strand DNA of the systemic lupus erythematosus patient through an enzyme-linked immunosorbent assay. The method for detecting autoantibodies of the systemic lupus erythematosus patient simplifies the existing detection method, improves detection efficiency and accurate diagnosis rate, and is beneficial to clinical diagnosis and treatment of systemic lupus erythematosus.
Owner:SUZHOU HEALTH COLLEGE
Construction method and application of recombinant porcine reproductive and respiratory syndrome virus expressing African swine fever virus p30 protein
PendingCN110628817AMaintain genetic stabilitySsRNA viruses positive-senseViral antigen ingredientsGenetic engineeringIndirect immunofluorescence
The invention provides a construction method of porcine reproductive and respiratory syndrome virus (PRRSV) recombinant plasmid expressing African swine fever virus (ASFV) p30 protein, and a genetically engineering vaccine constructed based on the recombinant plasmid and a construction method thereof. Indirect immunofluorescence is carried out on rPRRSV-p30 infected wells, it is found that the specific fluorescence of anti-PRRSV N protein and anti-ASFV p30 protein appears in the field of vision. Protein samples of rPRRSV-p30 infected wells are collected and analyzed by Western blot, results show that p30 bands of the expected size are found. It is suggested that the recombinant PRRSV expressing African swine fever virus p30 protein can ensure good, efficient and stable expression of foreign protein ASFV p30 protein. However, some studies show that p30 is an important antigen protein, and can be used in the development of ASF vaccine.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Autoimmune encephalitis antibody transient transfection and stable transfection detection method and application thereof
PendingCN113755443ABiological material analysisMicroorganism based processesImmunofluorescenceTransient transfection
The invention provides an autoimmune encephalitis antibody transient transfection and stable transfection detection method and application thereof. Specifically, the invention provides a recombinant cell for expressing mutant NMDAR, the recombinant cell expresses fusion protein of exogenous mutant NMDAR and fluorescent protein, and the transfection survival rate is high. The recombinant cell provided by the invention can be used for detecting antibodies related to autoimmune encephalitis, including NMDAR, AMPAR1, AMPAR2, LGI1, Caspr2 and GABABR. The invention also provides a method for detecting anti-autoimmune encephalitis by using the transfected cell line through an indirect immunofluorescence method based on the recombinant transfected cell line provided by the invention. The method disclosed by the invention has high sensitivity and specificity, and can be used for accurately detecting the types of pathogenic antibodies so as to help a patient to carry out targeted immunotherapy and recover health.
Owner:陈向军
Monoclonal antibody with rock bream iridovirus ORF049L recombinant proteins and preparation method thereof
InactiveCN103288954AOvercoming the shortcomings of detectionStrong specificityImmunoglobulins against virusesProtein.monoclonalProkaryotic expression
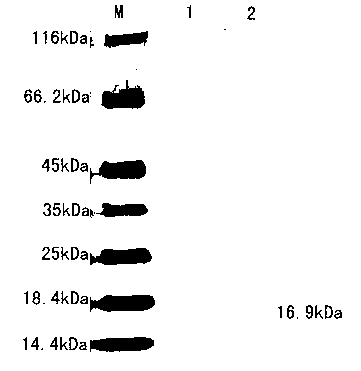
The invention discloses a monoclonal antibody with rock bream iridovirus ORF049L recombinant proteins. The monoclonal antibody is produced by secreting rock bream iridovirus ORF049L recombinant protein hybridoma of which the preservation number is CGMCC (China General Microbiological Culture Collection Center) No.7304. A preparation method of the monoclonal antibody comprises the following steps of: obtaining the rock bream iridovirus ORF049L recombinant proteins with the molecular weight of 16.9 kDa through gene cloning and prokaryotic expression; taking the obtained recombinant proteins as antigens to immune mice; performing cell fusion on mice spleens and myeloma cells, and screening and secreting the hybridoma of the rock bream iridovirus ORF049L recombinant protein monoclonal antibody by an immunoblotting method, a dot immunobinding assay and an indirect immunofluorescence; and cloning positive hybridoma by a finite dilution method.
Owner:DALIAN OCEAN UNIV +1
Method for detecting efficacy of classical swine fever live vaccines
ActiveCN103336116AShort detection timeEasy to operateMaterial analysisImmunofluorescenceRepeatability
The invention belongs to the technical field of novel biological medicines of veterinary medicines, and in particular relates to a method for detecting the efficacy of classical swine fever live vaccines. According to the method, the efficacy of live vaccines can be evaluated by detecting the virus content of live vaccines by an indirect immunofluorescence (IFA) method. The method is short in detection time, simple to operate, low in cost, accurate in result and good in repeatability, and has a good application value and wide market prospect.
Owner:PU LIKE BIO ENG
Swine fever and porcine pseudorabies bivalent vaccine as well as preparation method and application thereof
InactiveCN103505724ASolve the problem of low early potencyImprove securityAntiviralsAntibody medical ingredientsDiseaseRabies
The invention provides a swine fever and porcine pseudorabies bivalent vaccine. The swine fever and porcine pseudorabies bivalent vaccine contains at least one swine fever virus antigen and at least one porcine pseudorabies virus antigen, wherein the two antigens coordinate well, are excellent in immune effect and can promote each other. The swine fever and porcine pseudorabies bivalent vaccine is simple in preparation method, is convenient and efficient in immunization and has the advantages that immunization cost is reduced, an immunization procedure is simplified and economy and reliability are realized compared with a vaccine which can be used for preventing and treating more than two diseases only when immunization is carried out in steps and at least two injections are taken and an immune method of the vaccine in the prior art. The immune effect of the swine fever and porcine pseudorabies bivalent vaccine is better than that of a single vaccine and better in safety and avoids adverse effects caused by multiple immunizations. Besides, the invention also provides a simple testing method for determining swine fever effect in the bivalent vaccine by adopting an indirect immunofluorescence method, so that quality of bivalent live vaccines in each batch is guaranteed, and economic benefit is obviously increased.
Owner:PU LIKE BIO ENG
G type enterovirus direct immunofluorescent reagent and kit
ActiveCN105974119AStrong specificityHigh sensitivityDisease diagnosisBiological testingImmunofluorescenceFluorescence
The invention discloses a G type enterovirus detecting immunofluorescent reagent and a G type enterovirus immunofluorescent detecting kit. The immunofluorescent reagent is a VP1 monoclonal antibody capable of being labeled with a fluorescent dye, the VP1 monoclonal antibody is obtained by utilizing VP1 protein of the G type enterovirus and adopting a hybridoma monoclonal antibody preparation technology, and the amino acid sequence of the VP1 protein is shown as the sequence table SEQ ID NO.2. The immunofluorescent reagent and the detecting kit can be used for rapid diagnosis of G type enterovirus infection in clinic, and can also be used for localization and identification of a G type enterovirus pathogen in tissue; the direct immunofluorescent detecting time is short, and report results can be generated in 40 min; the specificity is strong, and reaction can only be conducted with the G type enterovirus; the repeatability is good, the accuracy is high, the coincidence rate to an indirect immunofluorescence method can reach 90% or above, and the immunofluorescent reagent and the detecting kit can be used for rapid diagnosis of the G type enterovirus infection.
Owner:JILIN UNIV
Monoclonal antibody of outer membrane protein of chlamydia abortus and application thereof
ActiveCN102220285AStrong specificityGood fluorescence propertiesImmunoglobulins against bacteriaTissue cultureFluorescenceIMMUNE FLUORESCENCE
The invention relates to monoclonal antibody of N-terminal protein of chlamydia abortus POMP18D as well as a preparation method and application thereof. The monoclonal antibody is secreted by a hybridoma cell strain 1F10D4 with the collection number of CGMCC No.4658. The invention also provides application of the monoclonal antibody in direct fluorescence immune or indirect fluorescence immune detection of chlamydia abortus. The monoclonal antibody of N-terminal protein of chlamydia abortus POMP18D has strong specificity and fluorescent characteristic, and is suitable for being used as a fluorescence antibody to establish a direct fluorescence detection method or indirect immune fluorescence detection method.
Owner:CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com