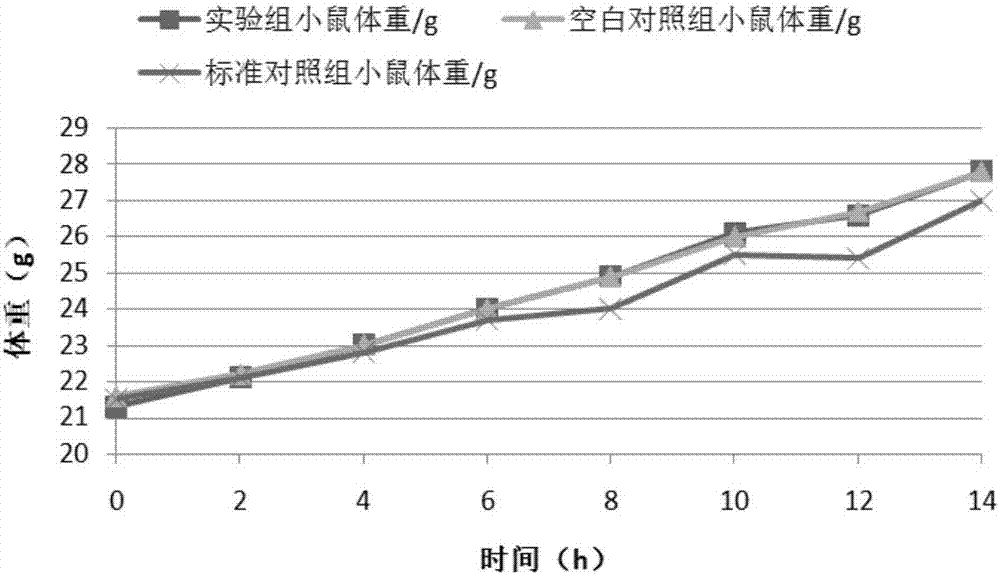
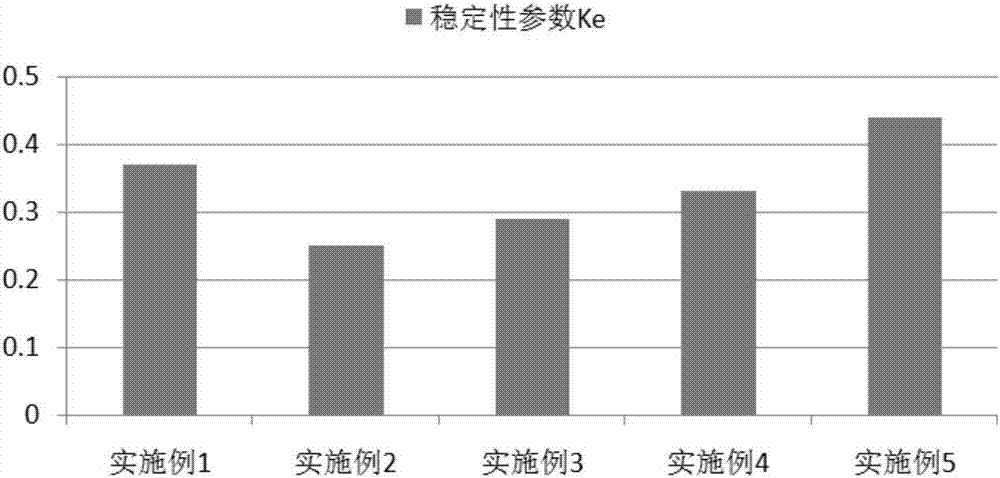
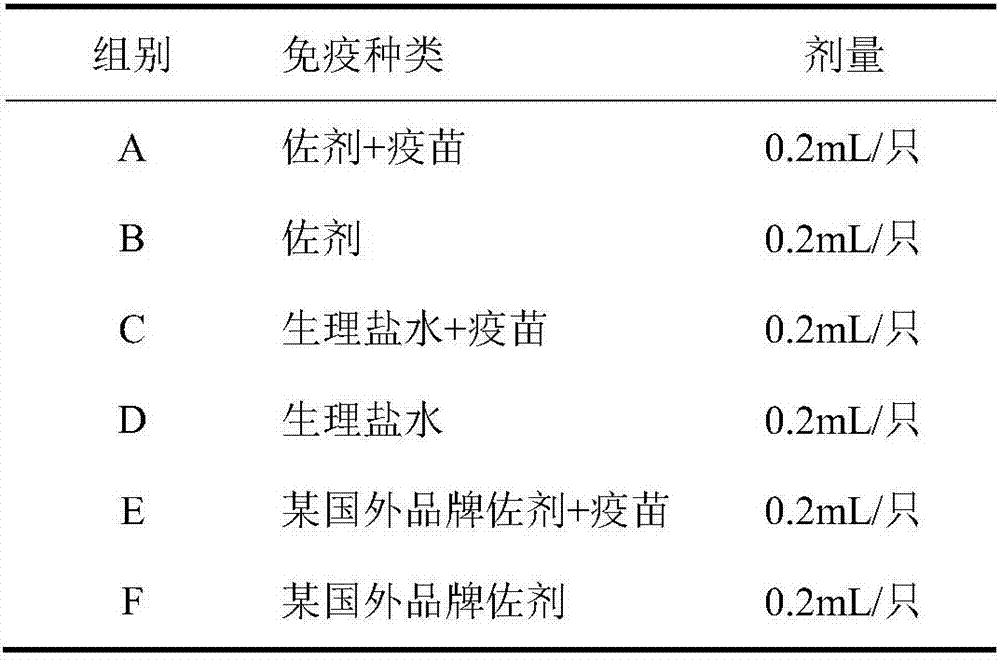
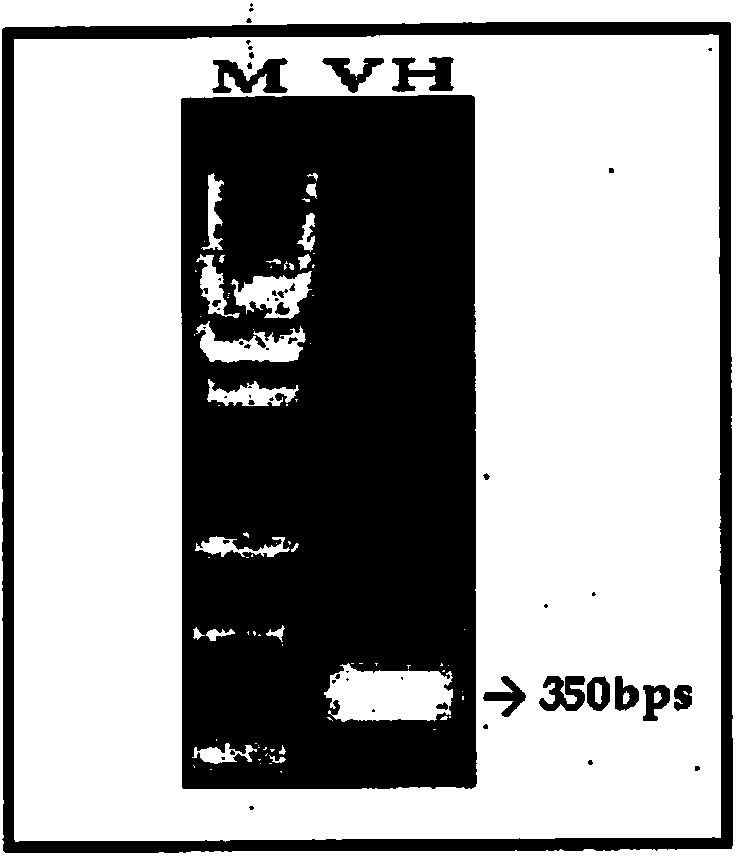
Patents
Literature
79 results about "Vaccine Potency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
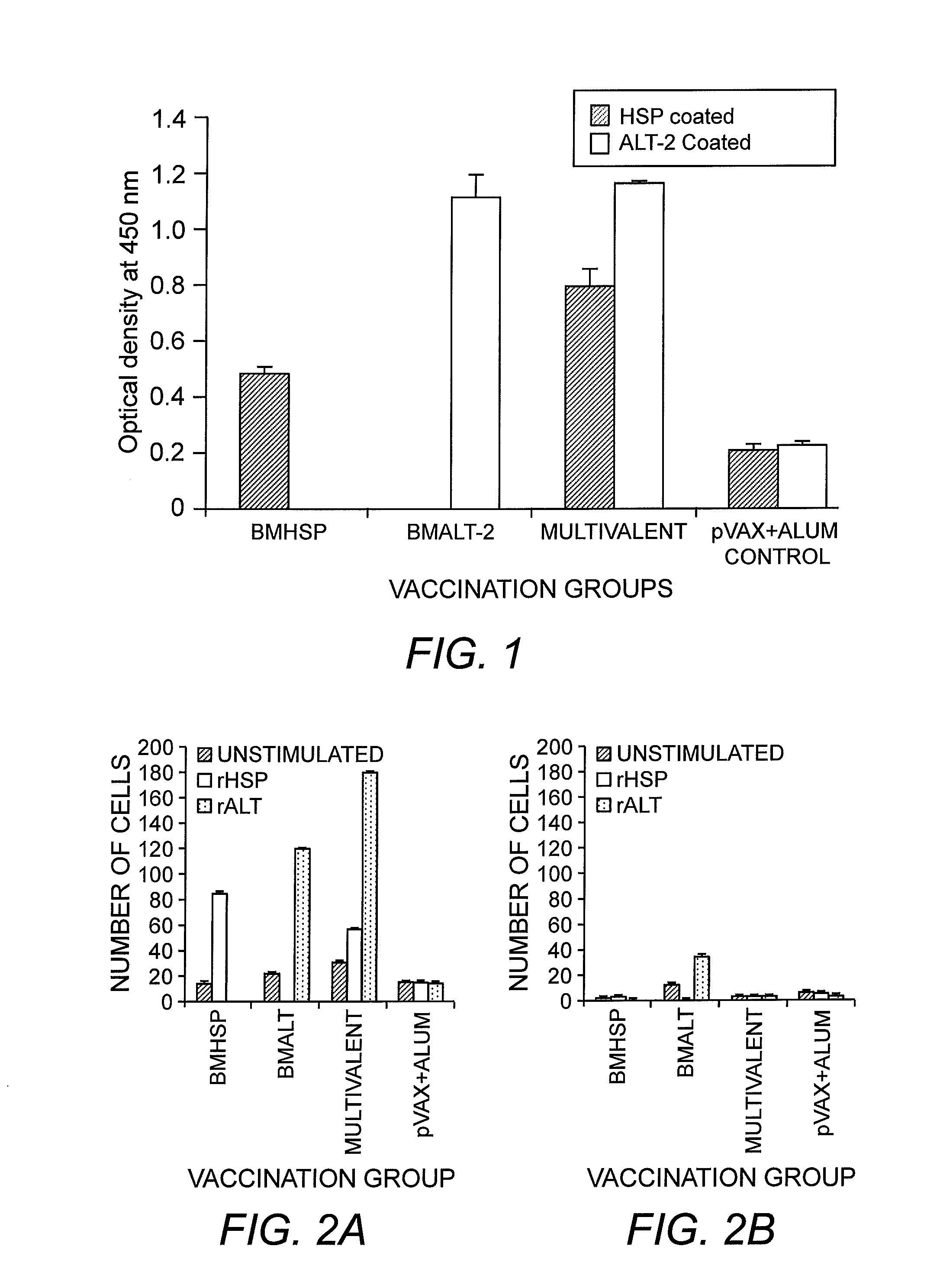
A quantitative measure of the specific ability of the vaccine product to achieve an intended biological effect defined in a suitable biological assay based on the attribute of the product that is linked to the relevant biological properties.
Molecular vaccines employing nucleic acid encoding anti-apoptotic proteins
InactiveUS20070026076A1Increase the number ofEasy to demonstratePowder deliveryVirusesDendritic cellVaccine Potency
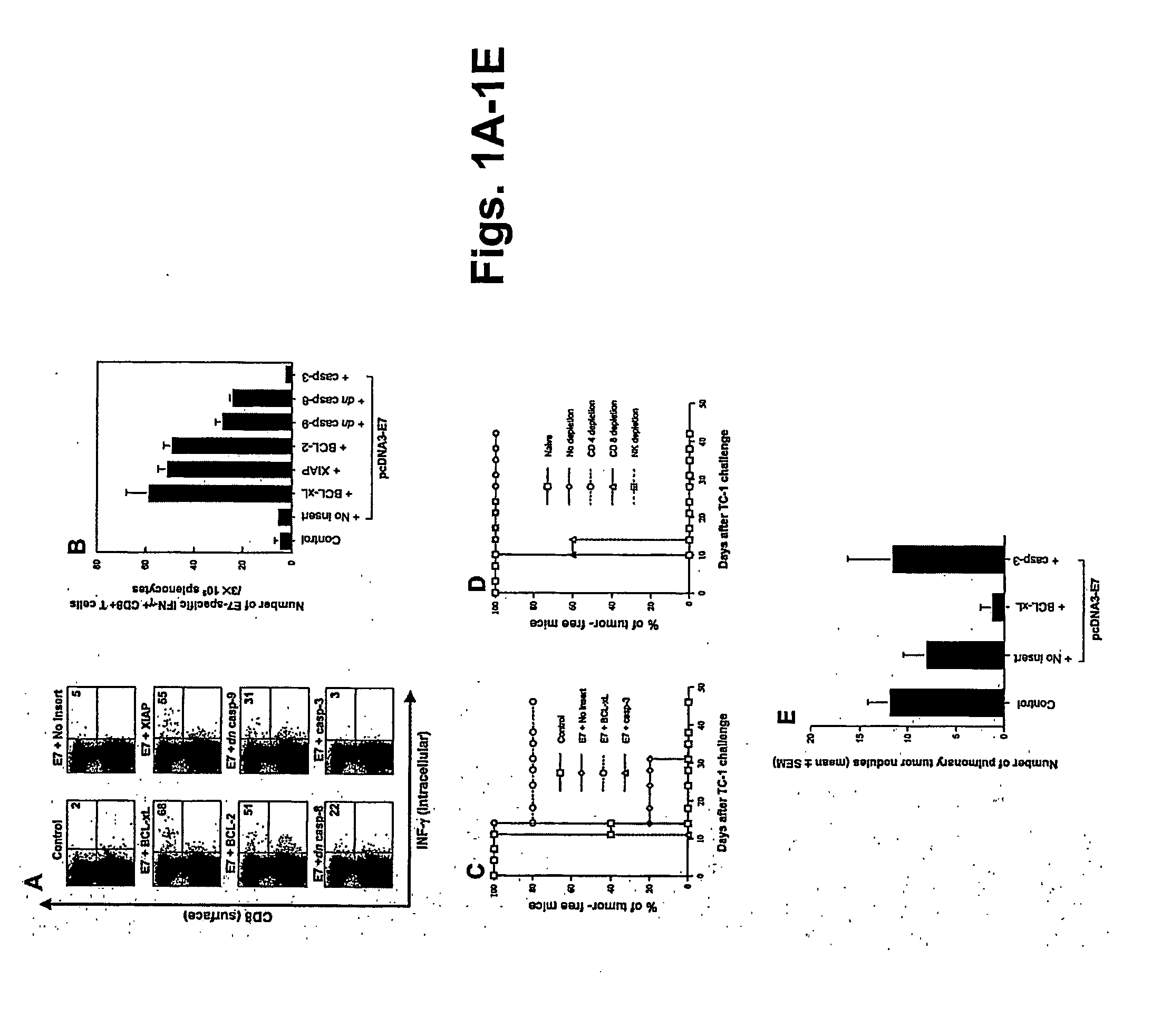
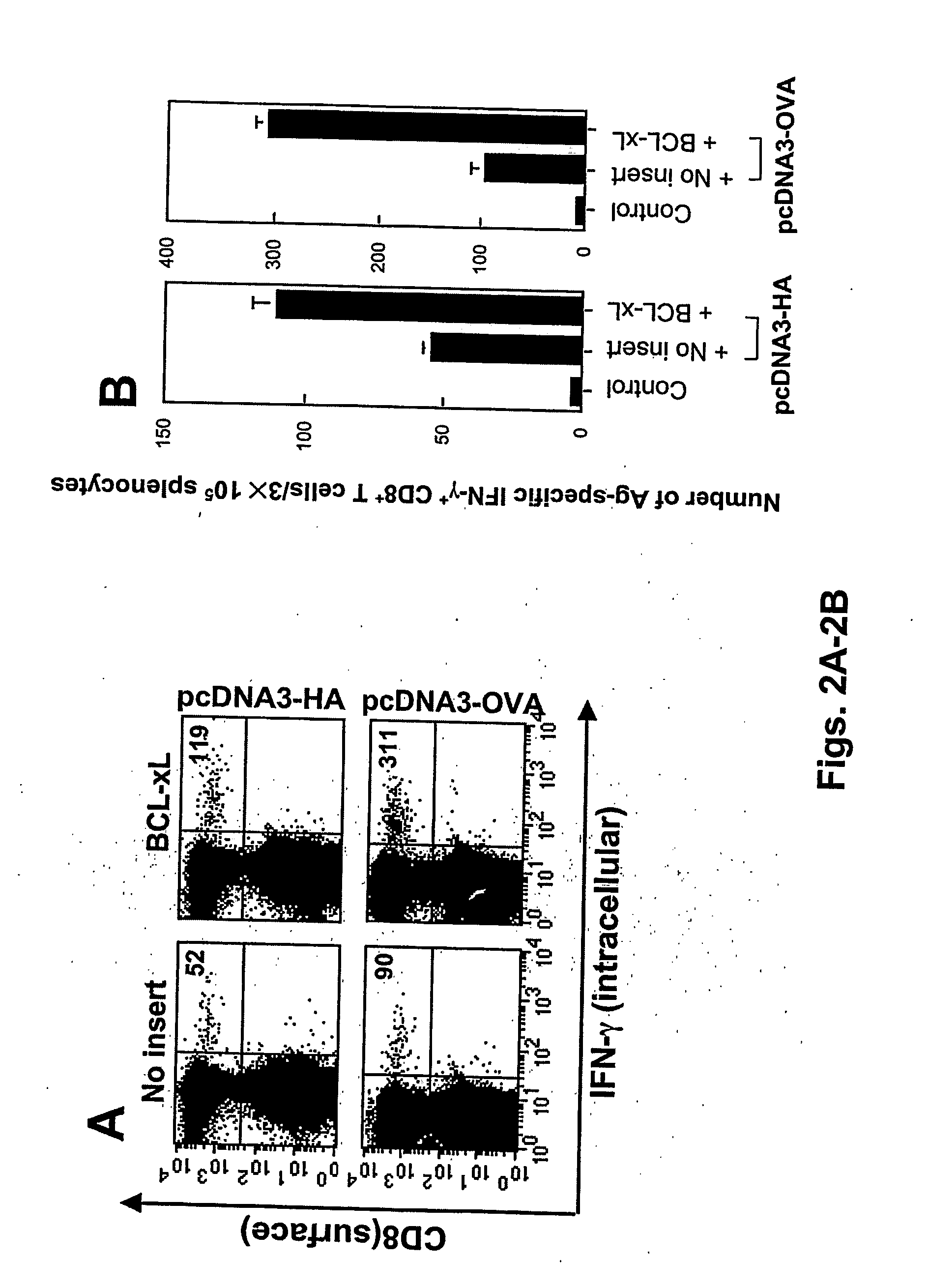
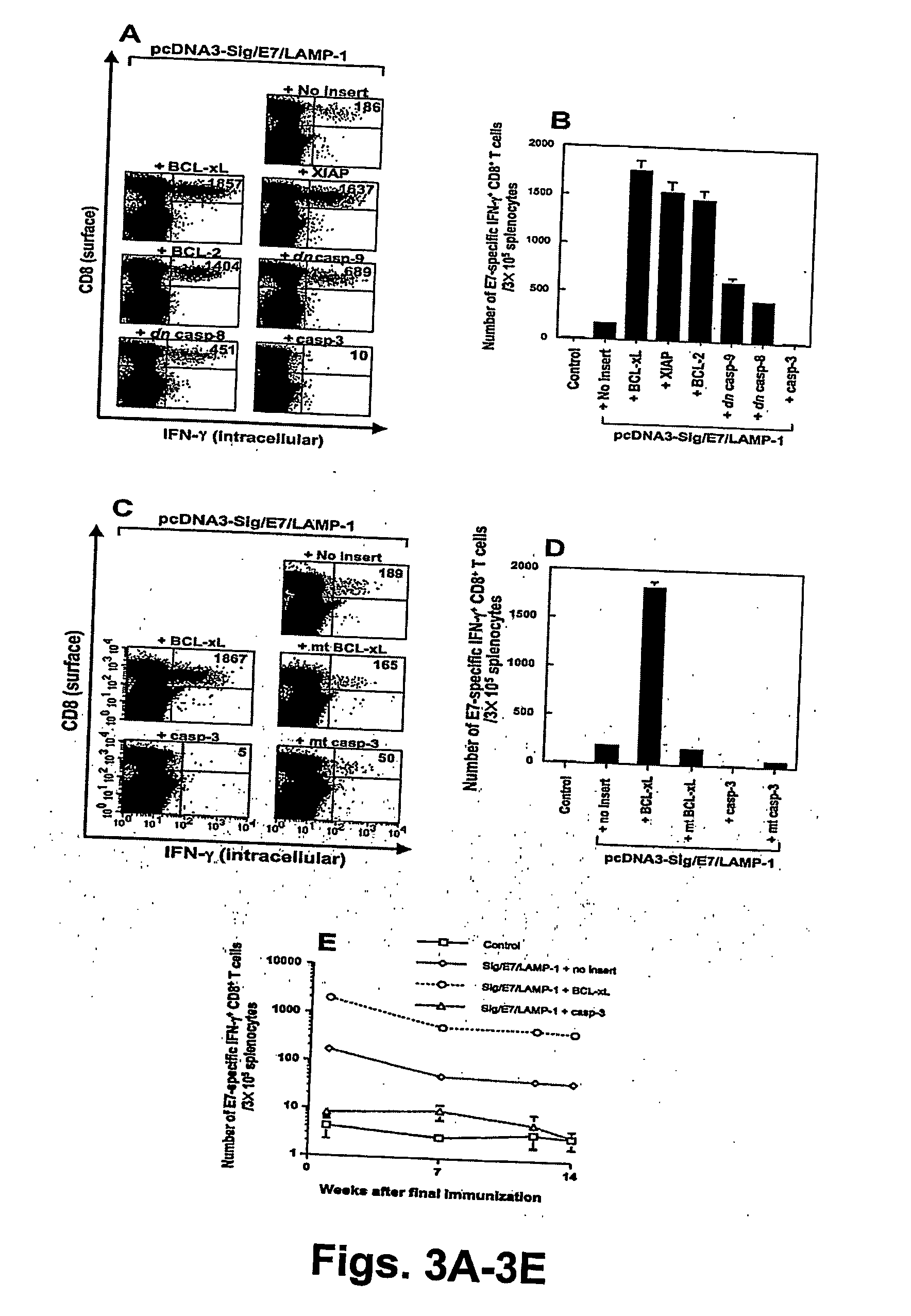
T cell immune responses are enhanced by presentation of antigen to CD8+ T cells using a chimeric nucleic acid immunogen or vaccine that links DNA encoding an antigen with DNA encoding a polypeptide that targets or translocates the antigenic polypeptide to which it is fused (immunogenicity-potentiating polypeptides or “IPP”). By inhibiting apoptosis in the vicinity of a T cell responses to such a nucleic acid immunogen, even more potent immune responses are attained. The present strategy prolongs the survival of DNA-transduced cells, including dendritic cells (DCs), thereby enhancing the priming of antigen-specific T cells and increase potency. Co-delivery of DNA encoding an inhibitor of apoptosis, including (a) BCL-xL, (b) BCL-2, (c) XIAP, (d) dominant negative caspase-9, or (e) dominant negative caspase-8, or (f) serine protease inhibitor 6 (SPI-6) which inhibits granzyme B, with DNA encoding an antigen, prolongs the survival of transduced DCs and results in significant enhancement of antigenspecific T cell immune responses that provide potent antitumor effects. Thus, co-administration of a DNA vaccine encoding antigen linked to an IPP along with one or more DNA constructs encoding an anti-apoptotic protein provides a novel way to enhance vaccine potency.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
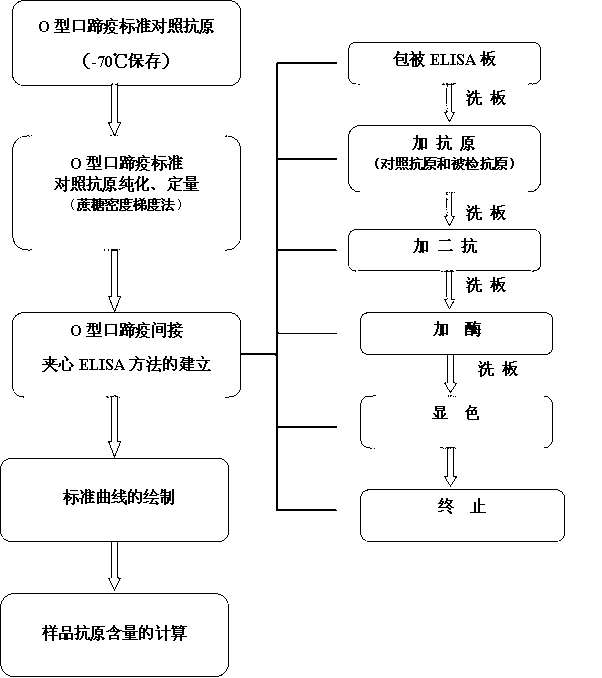
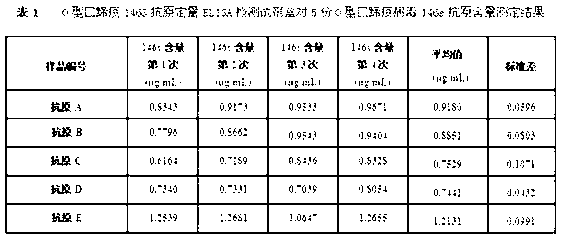
O type foot-and-mouth disease 146S antigen quantitative ELISA detection kit and method for using same
ActiveCN103076451ASolve efficiency problemsSolving the power test substitution problemMaterial analysisDiseaseVaccine Potency
The invention discloses an O type foot-and-mouth disease 146S antigen quantitative ELISA (enzyme-linked immuno sorbent assay) detection kit and a method for using the same. The kit comprises an ELISA plate, an O type foot-and-mouth disease standard reference antigen, a demulsifier, an O type foot-and-mouth disease rabbit antiserum, an O type foot-and-mouth disease guinea pig antiserum, a rabbit anti-guinea pig-horse radish peroxidase conjugate, a guinea pig antiserum dilute solution, a 25-fold PBST (phosphate buffer solution tween) concentrated solution, a carbonate buffer solution capsule, a citric acid-phosphate buffer solution tablet, an OPD (o-phenylenediamine) tablet, a stop solution, a plate sealing membrane, a moving liquid tank and a 96-mesh U-shaped dilution plate. The kit is an organic combination of a sucrose density gradient centrifugation method and an indirect sandwich ELISA method, integrates the advantages of the sucrose density gradient centrifugation method and the indirect sandwich ELISA method, is simple to operate and good in stability, is suitable for batch detection, can be used for distinguishing serum types, and is an ideal substitution method for antigen quantitative and vaccine efficacy detection.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
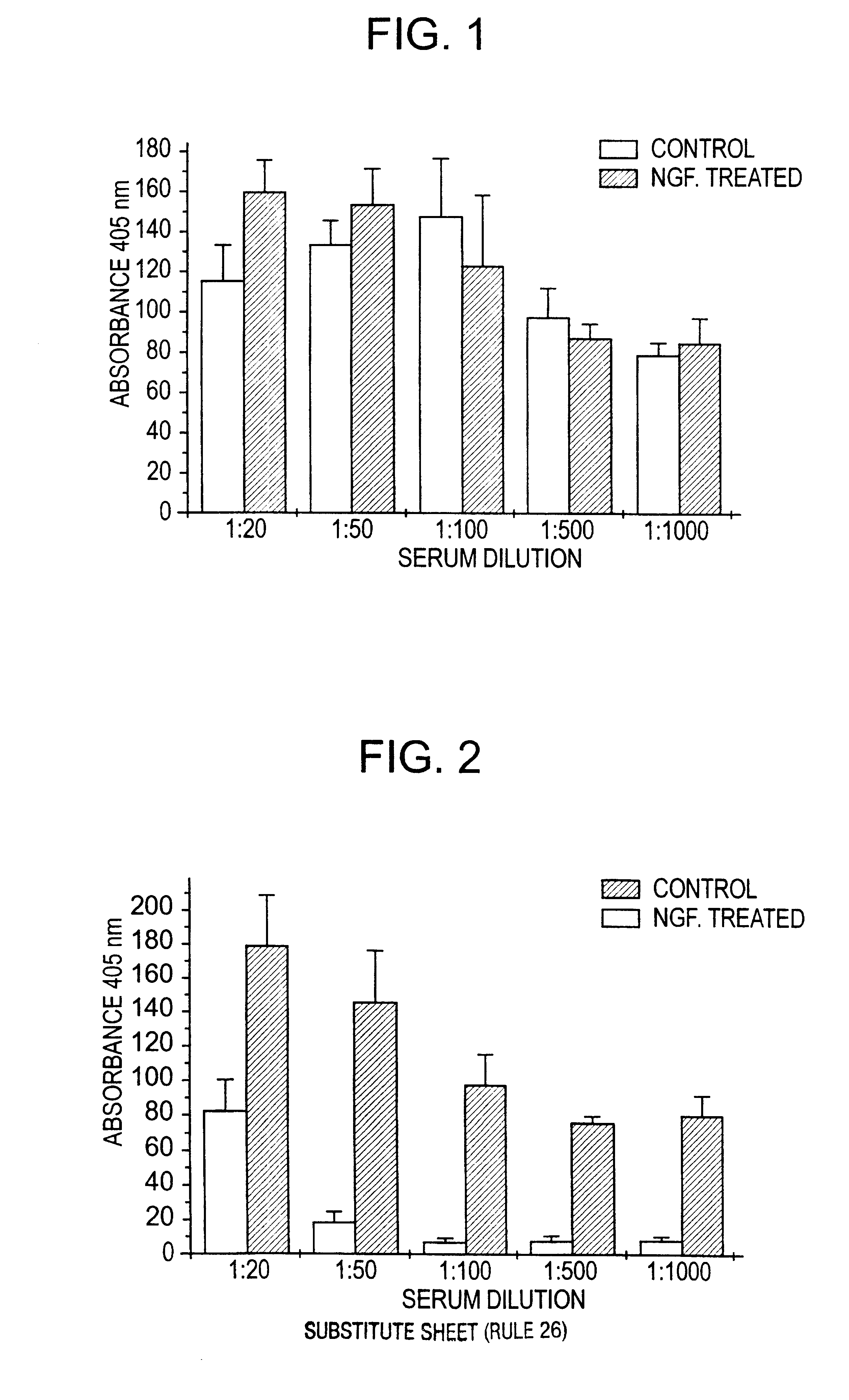
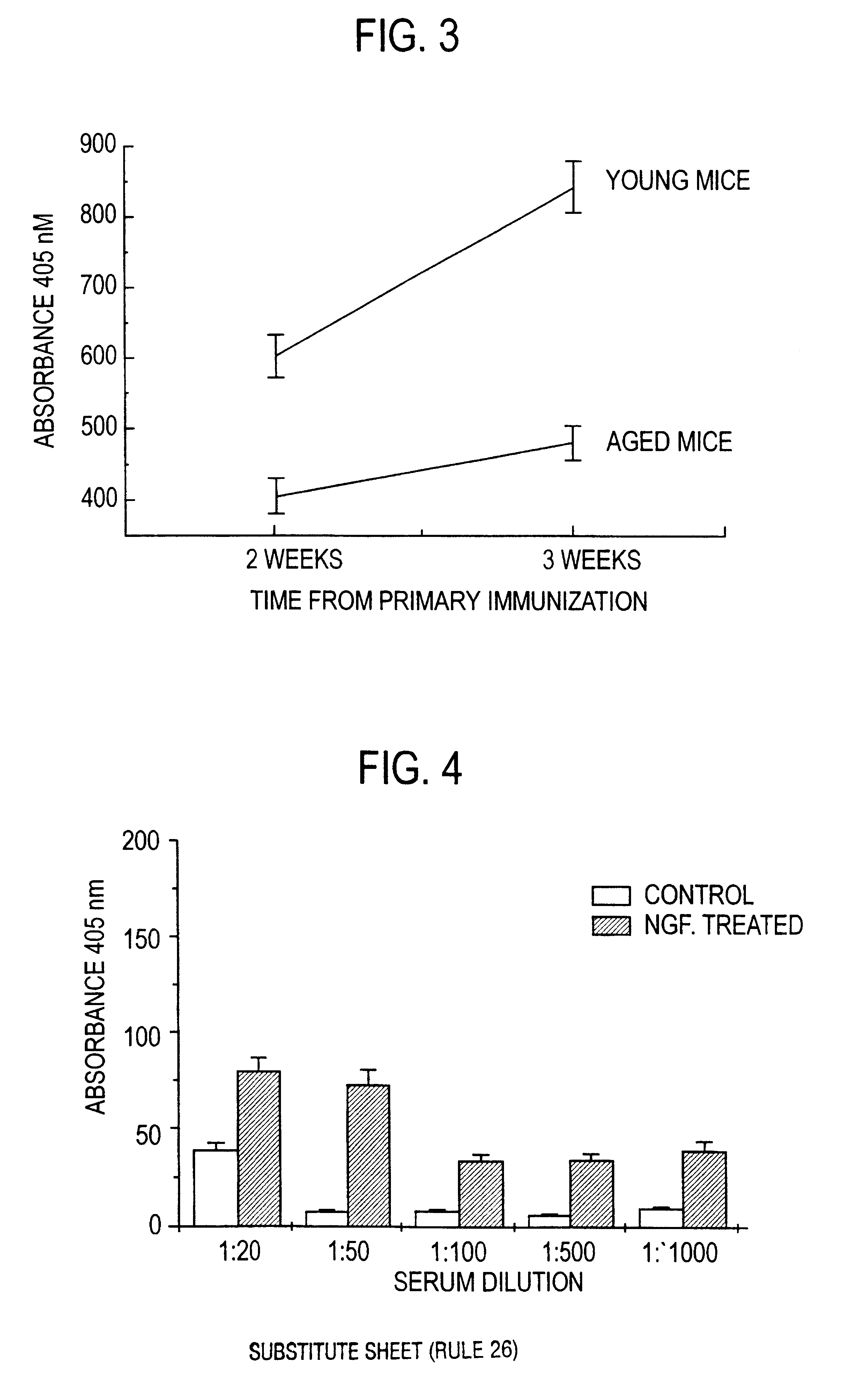
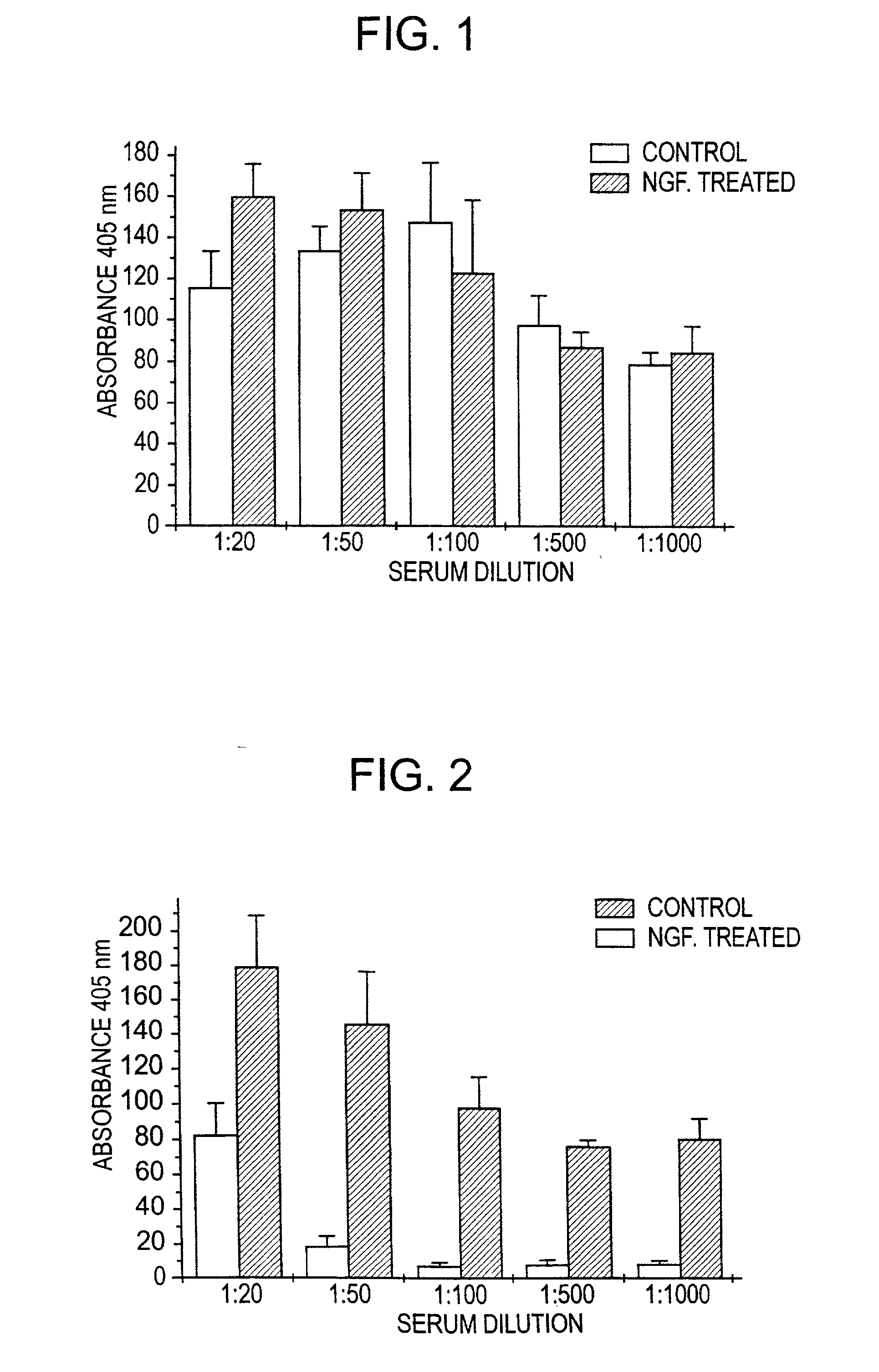
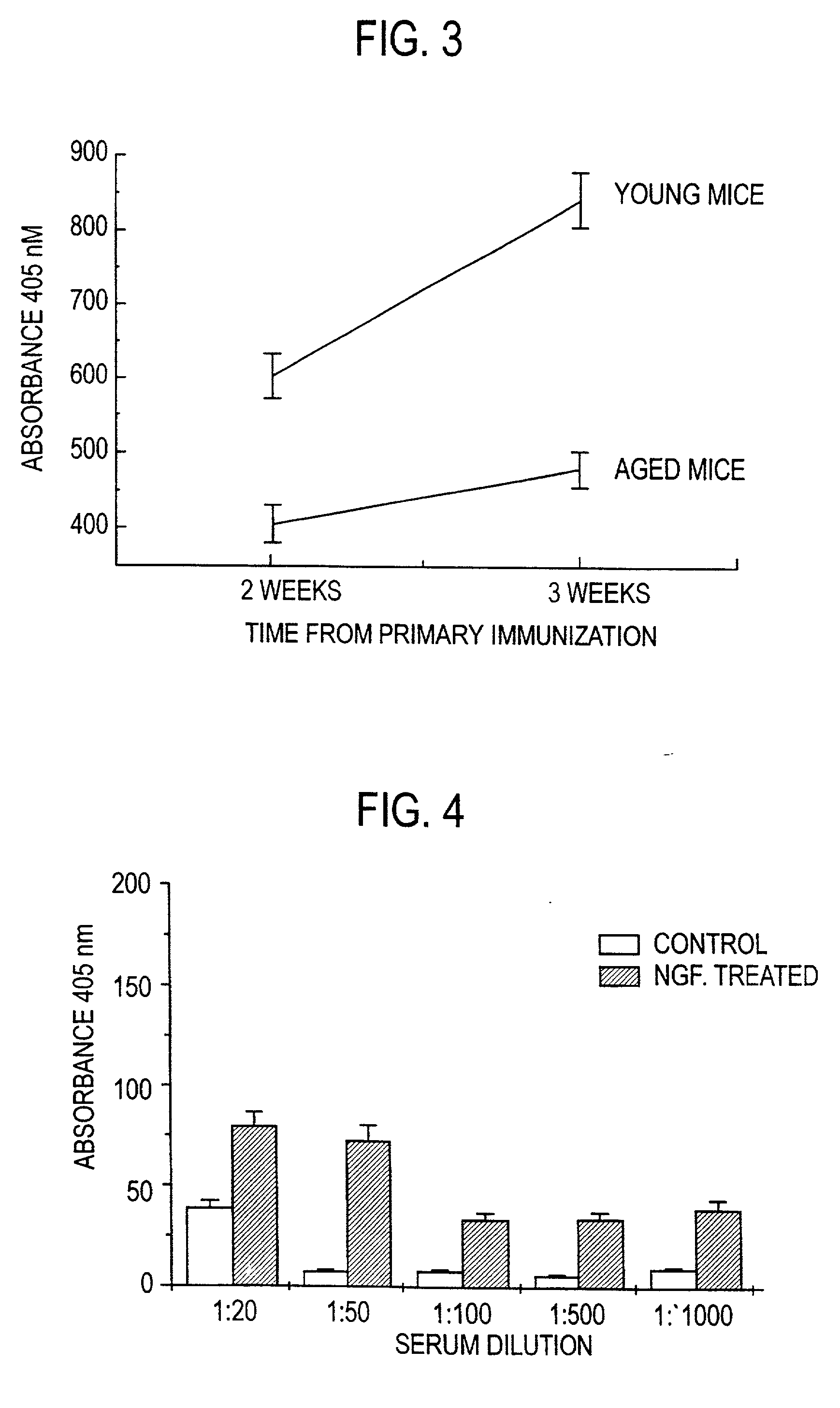
Nerve growth factor as a vaccine adjuvant
A vaccination method utilizes a pharmaceutical combination for enhancing vaccine effectiveness. The method utilizes an immune response-triggering vaccine capable of stimulating production in an immunodeficient animal antibodies to a disease-causing agent foreign to the animal. As an adjuvant, a vaccine effectiveness-enhancing amount of Nerve Growth Factor (NGF) is administered, which enhances production and affinity of the antibodies in the animal, in response to the vaccine.
Owner:PROTECHTION UNLIMITED
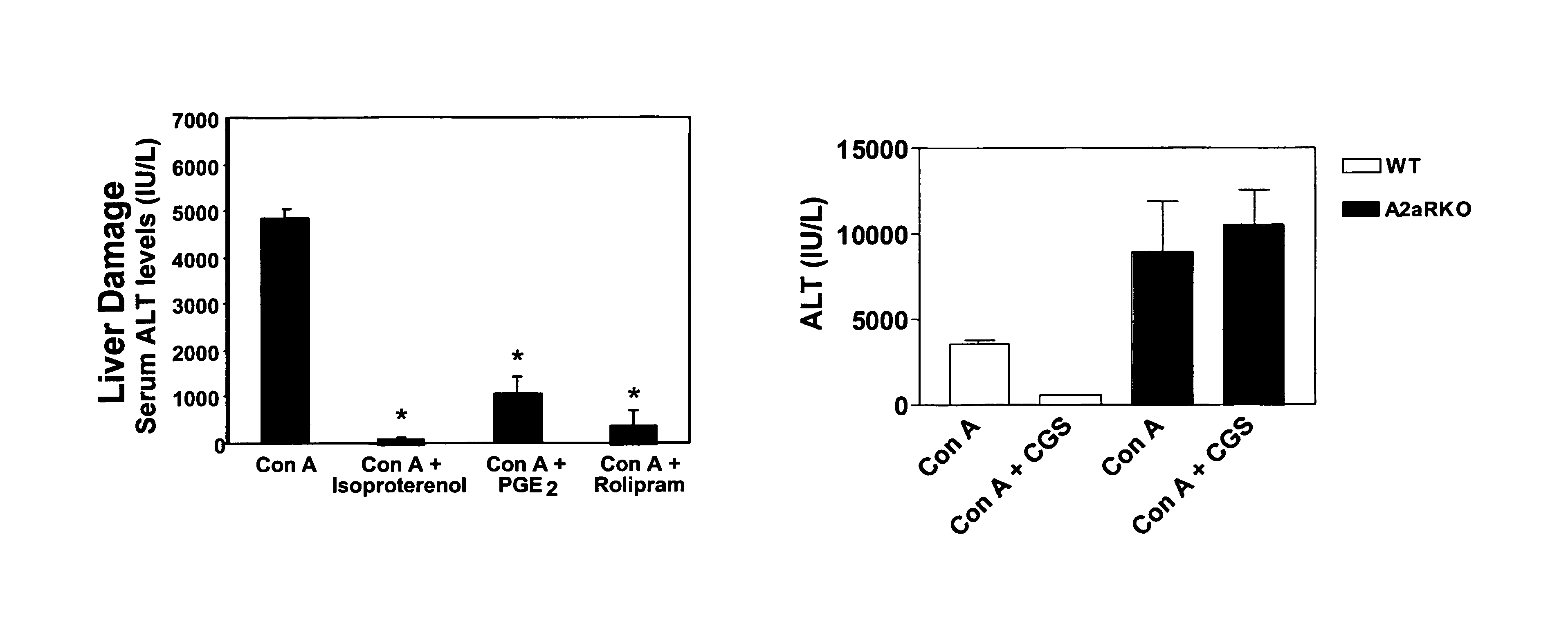
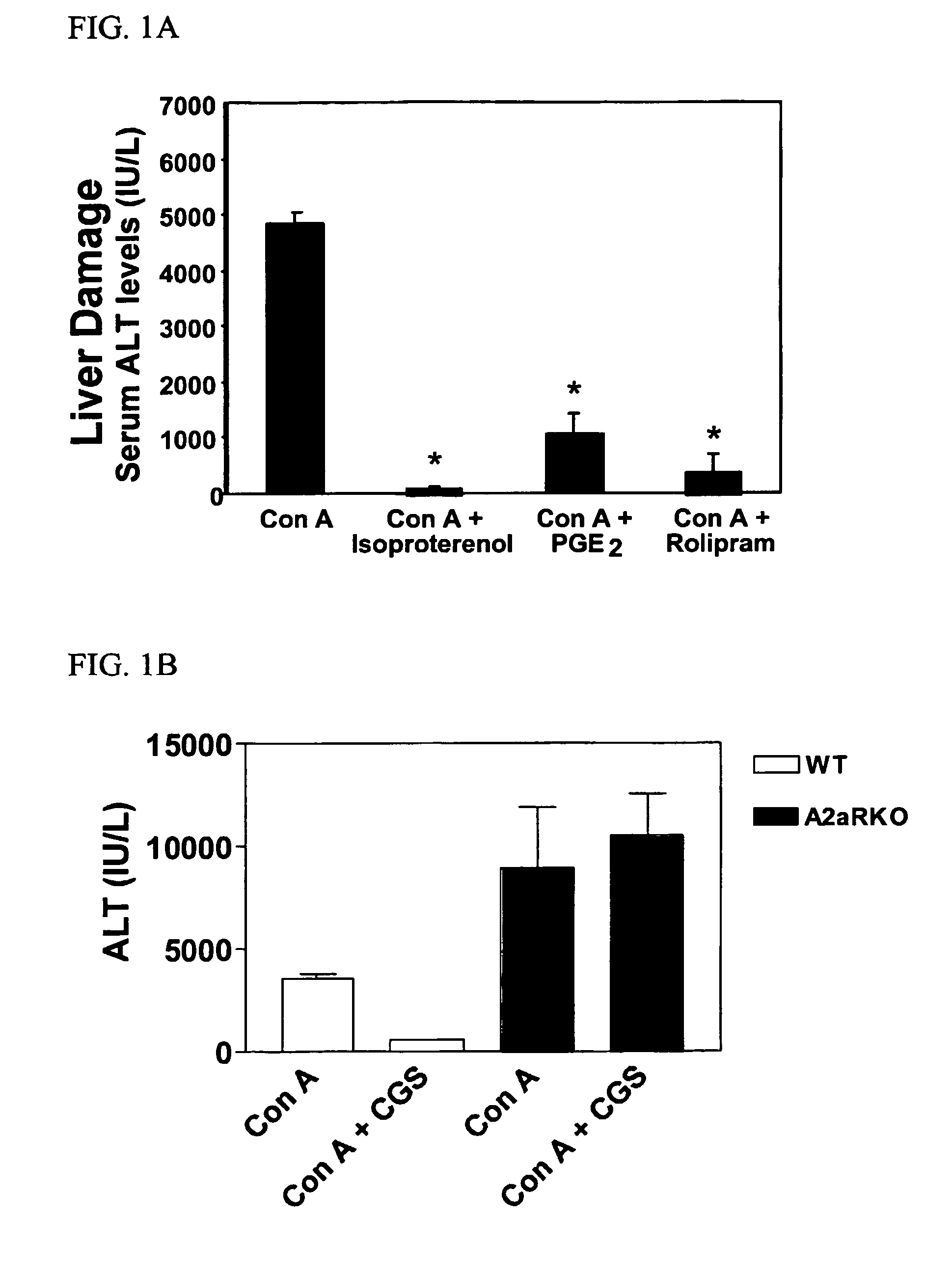
Methods for using extracellular adenosine inhibitors and adenosine receptor inhibitors to enhance immune response and inflammation
ActiveUS8080554B2Suppress immune responseEnhance immune responseAntibacterial agentsBiocideVaccine PotencyTumor destruction
A method is provided herein to increase an immune response to an antigen. The method includes administering an agent that inhibits extracellular adenosine or inhibits adenosine receptors. Also disclosed are methods to increase the efficacy of a vaccine and to increase an immune response to a tumor antigen or immune cell-mediated tumor destruction.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US SEC THE DEPT OF
Ii-key enhanced vaccine potency
InactiveUS20080095798A1Improve effectivenessEasy to manageAntibacterial agentsSsRNA viruses negative-senseHemagglutininVaccine Potency
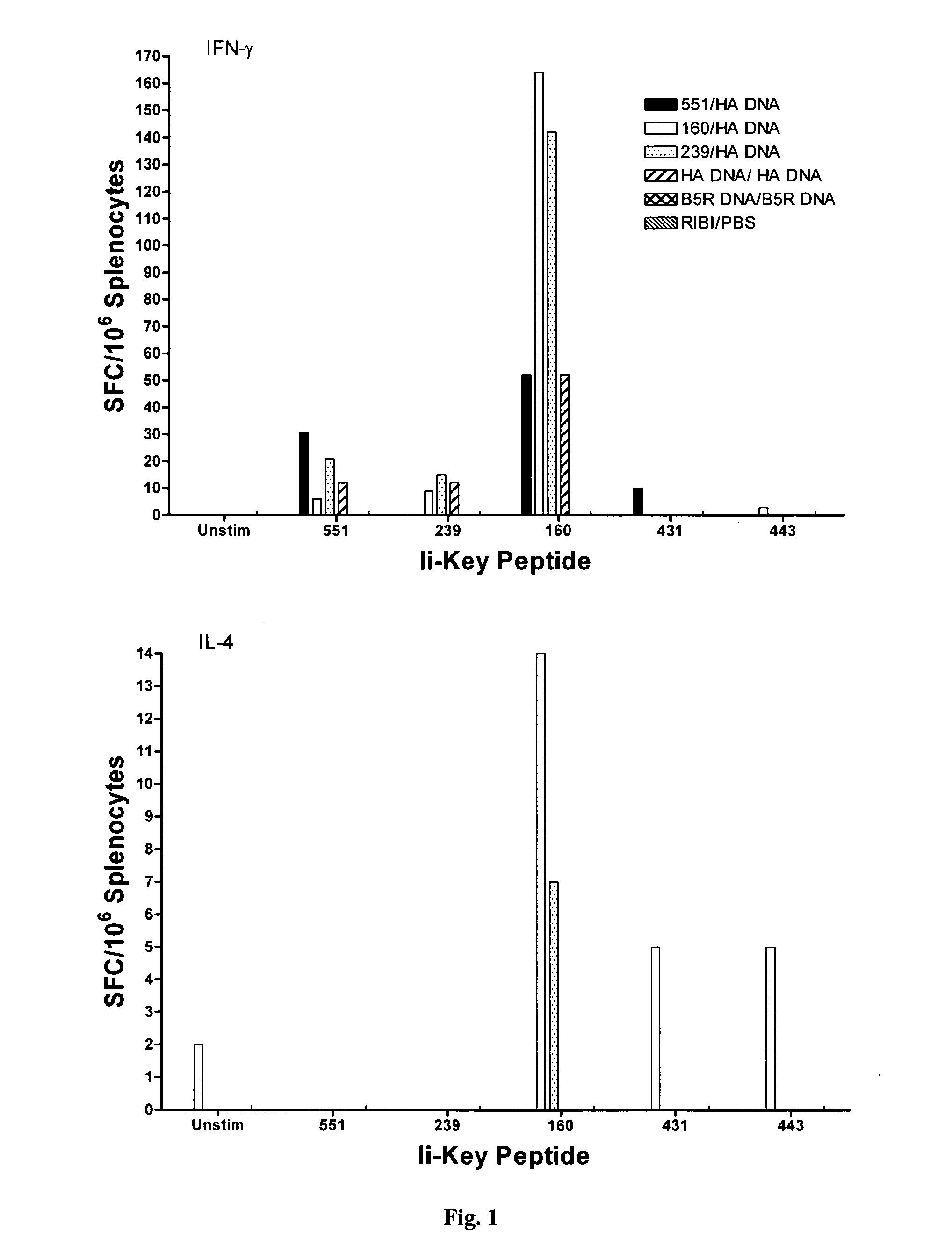
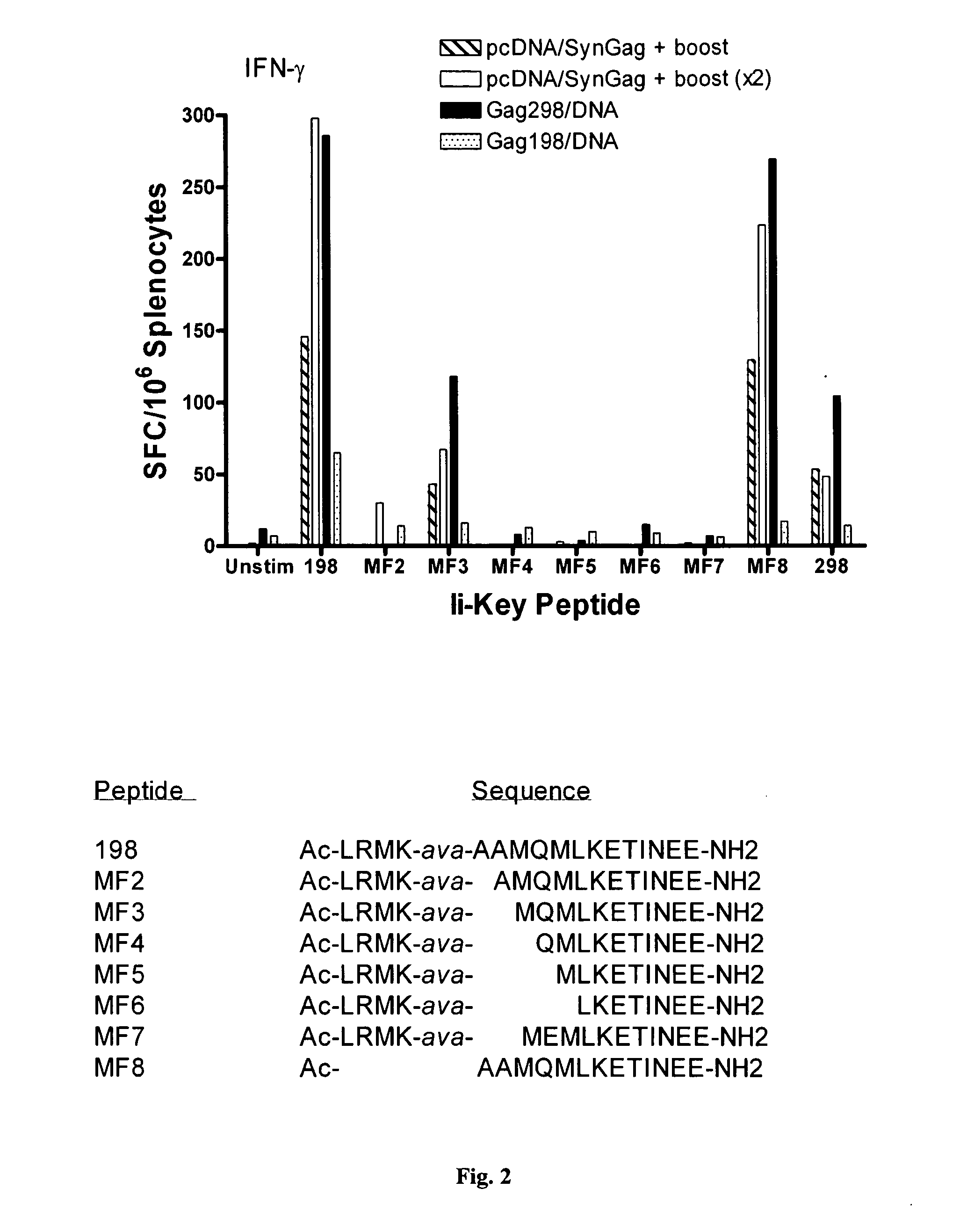
Disclosed is a method for increasing vaccine potency whereby a subject's immune system is first primed with an Ii-Key hybrid peptide construct before the subject subsequently receives a vaccine for a pathogen of interest. The vaccine may be comprised of a protein or portion thereof that is encoded by the genome of the pathogen. The vaccine may also be a DNA vaccine comprised of DNA encoding a protein of the pathogen. The Ii-Key hybrid peptide construct includes the LRMK residues of Ii-Key protein and an MHC Class II epitope of the protein or portion thereof which is used in the vaccine. The Ii-Key construct may be administered in the form of a nucleic acid construct encoding the Ii-Key hybrid peptide. Priming with Ii-Key peptides enhances the immunogenicity of rHA protein and HA and HIV DNA vaccines. Methods are described relating to the use of Ii-Key hybrid constructs in vaccine protocols wherein the pathogen is HIV or Influenza A, including H5N1. Methods and compositions are described wherein the MHC Class II epitope of the Ii-Key hybrid is hemagglutinin encoded by Influenza A or the Gag protein encoded by HIV.
Owner:ANTIGEN EXPRESS
Stabilization of alum-adjuvanted immunologically active agents
InactiveUS20060067943A1Improve the level ofEnhancement is mitigated or avoidedBiocideOrganic active ingredientsAdjuvantAlum adjuvant
A composition and method for formulating and delivering an adjuvanted immunological active agent, especially a vaccine, wherein adjuvant coagulation and concomitant loss of vaccine efficacy enhancement is mitigated or avoided. The adjuvanted, immunologically-active agent can be subjected to freezing, drying, freeze-drying, or lyophilization, and when reconstituted, retains a high level of potency. The present invention further provides for a composition and method for formulating and delivering a stable, adjuvanted, immunologically-active agent capable of being deposited on a transdermal delivery device or microprojection or array thereof.
Owner:ALZA CORP
Adjuvant Composition and Methods for Its Use
InactiveUS20070218086A1Enhance and augment immune responseReasonable yieldBacterial antigen ingredientsVirus peptidesVaccine PotencyLymphocyte
The present invention is directed to a vaccine adjuvant which improves the vaccine potency. More specifically, the present invention is directed to the use of a γδT lymphocyte activator as vaccine adjuvant to promote and enhance antigen specific immunological responses, as well as a vaccine composition comprising a γδT lymphocyte activator.
Owner:INNATE PHARMA SA
Compositions and methods for potentiating immune response, enhancing immunotherapy, and increasing vaccine potency
ActiveUS20140377250A1Improving immunogenicityEnhancing the potency of vaccines and cancer immunotherapies in a subjectOrganic active ingredientsBacterial antigen ingredientsAdjuvantVaccine Potency
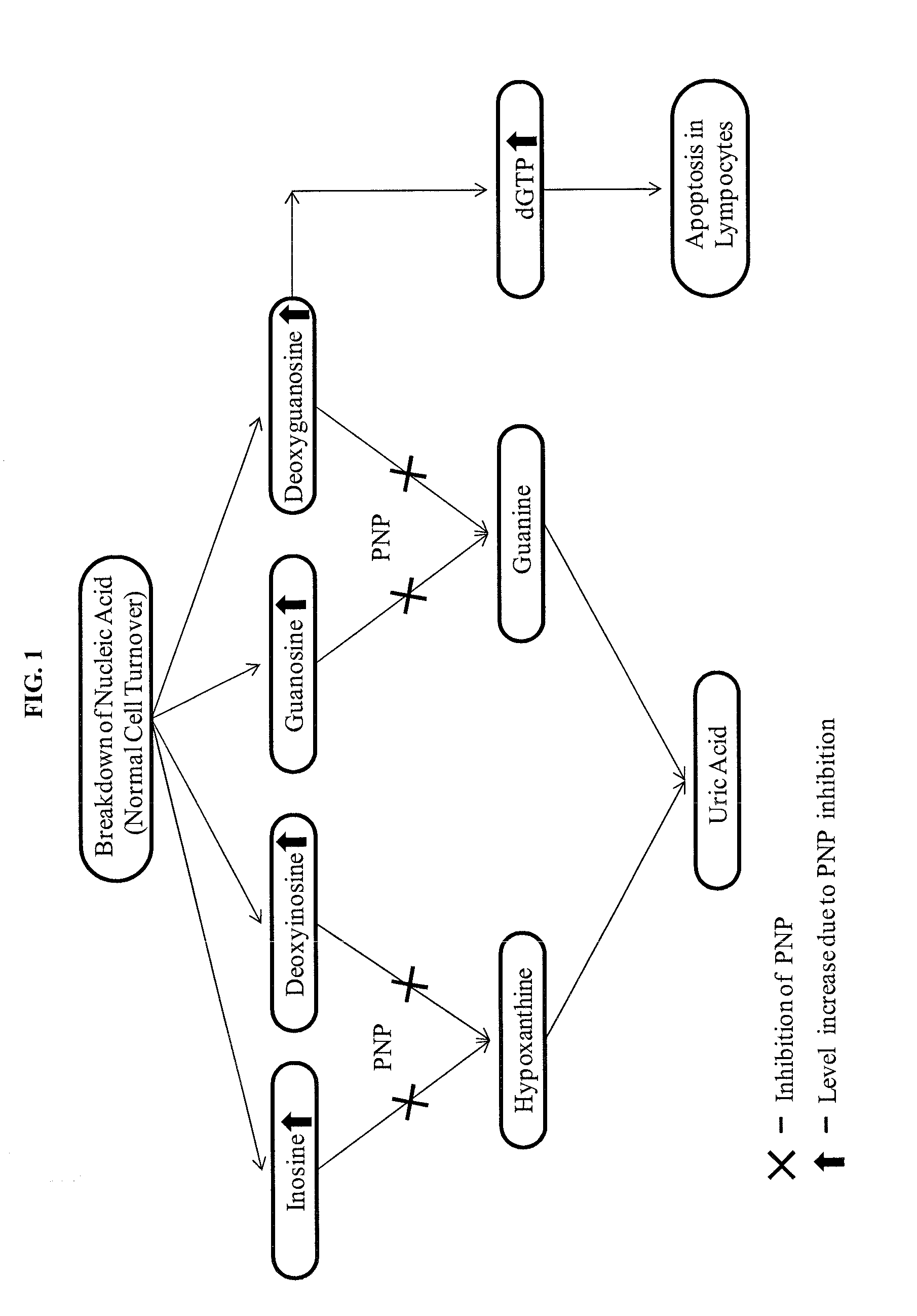
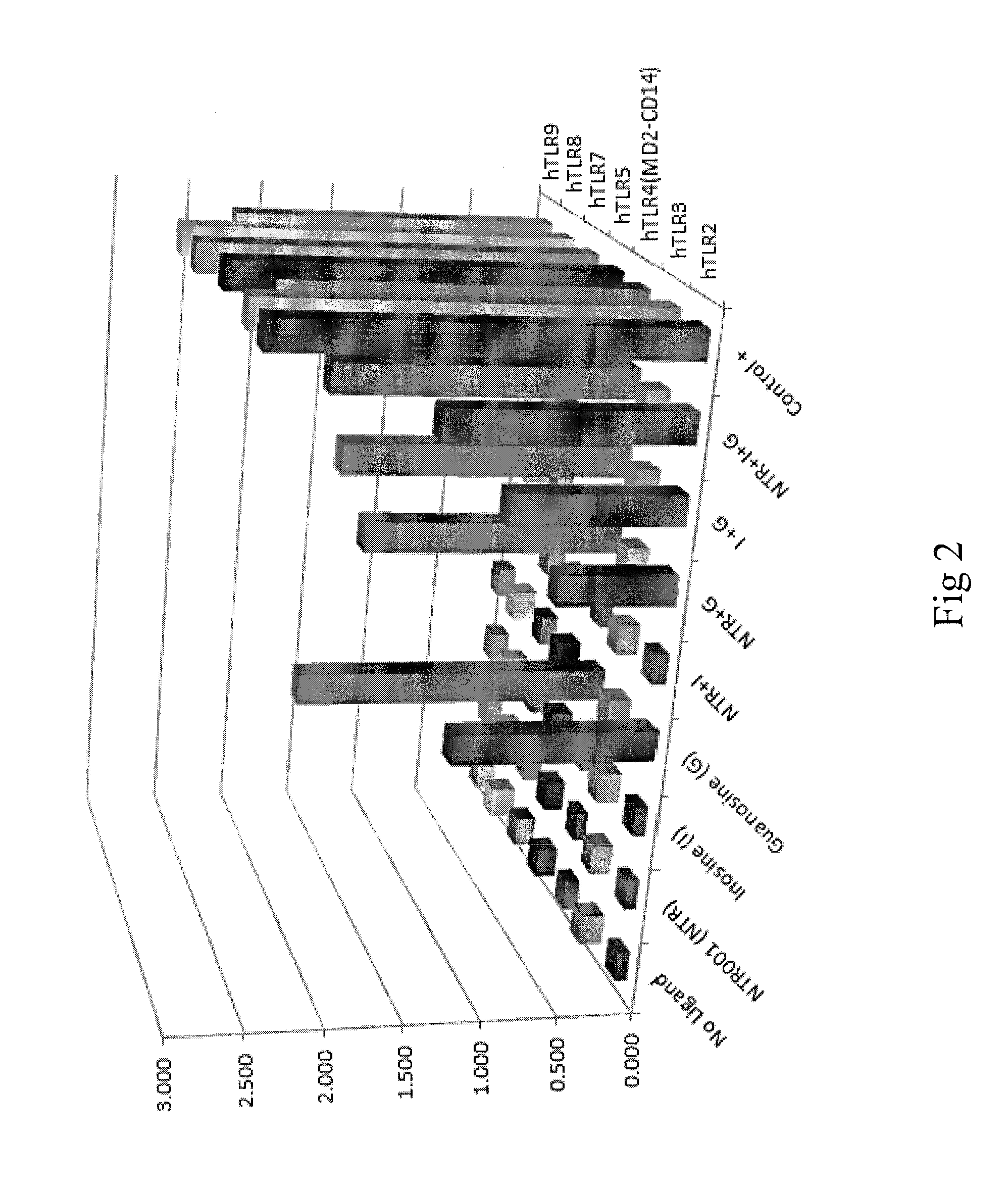
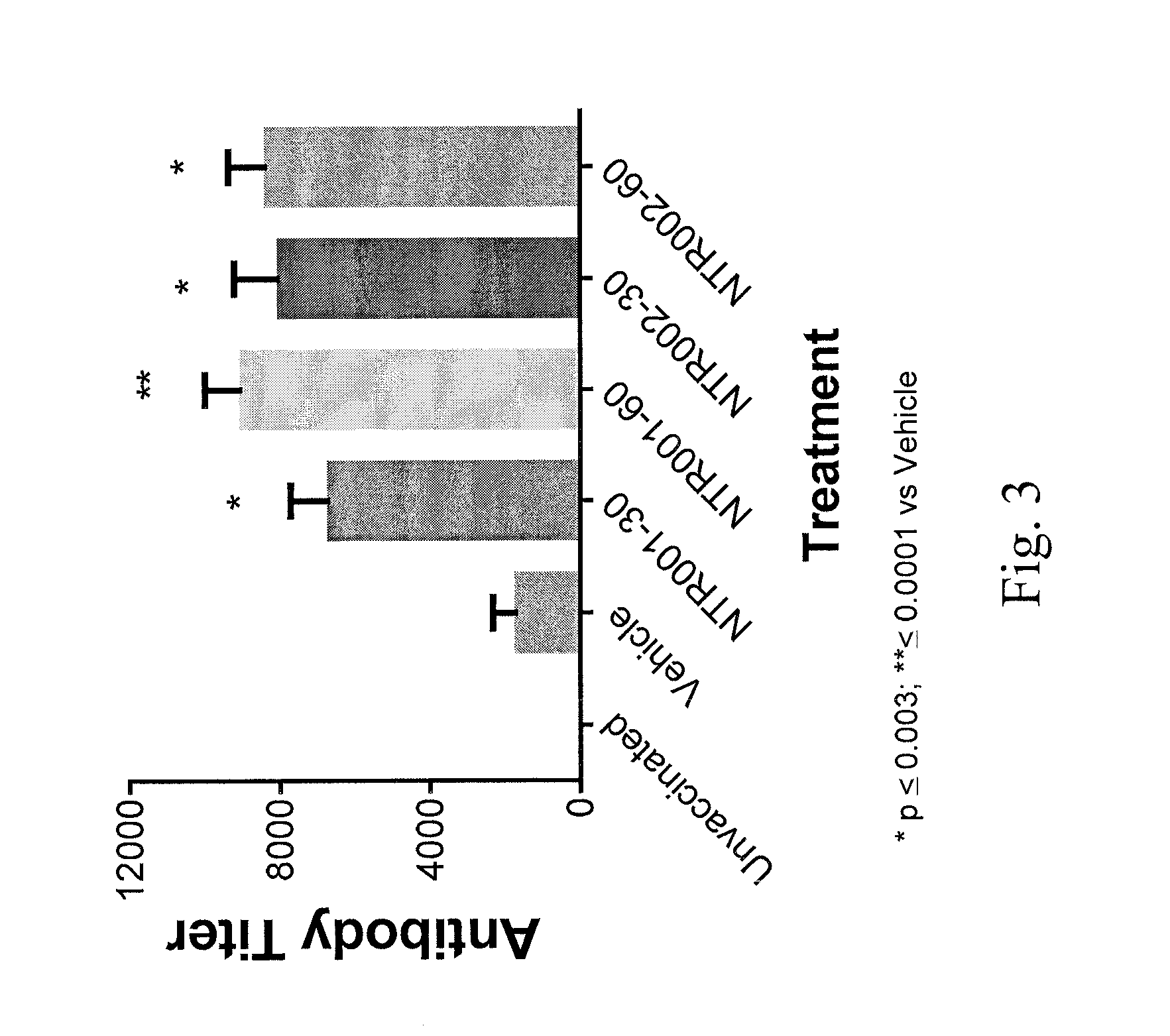
Compositions including at least one PNP inhibitor or at least one PNP inhibitor in combination with one or more agents identified as endogenous adjuvants useful for enhancing the potency of vaccine and cancer immunotherapies being administered for the prevention or treatment of infectious diseases or cancer. The compositions may be formulated as pharmaceutical dosage forms and components may be assembled as kits. Methods for increasing the levels of endogenous adjuvants to enhance the immunogenicity of an antigen as well as to augment the potency of vaccine and cancer immunotherapies are also disclosed.
Owner:NITOR THERAPEUTICS
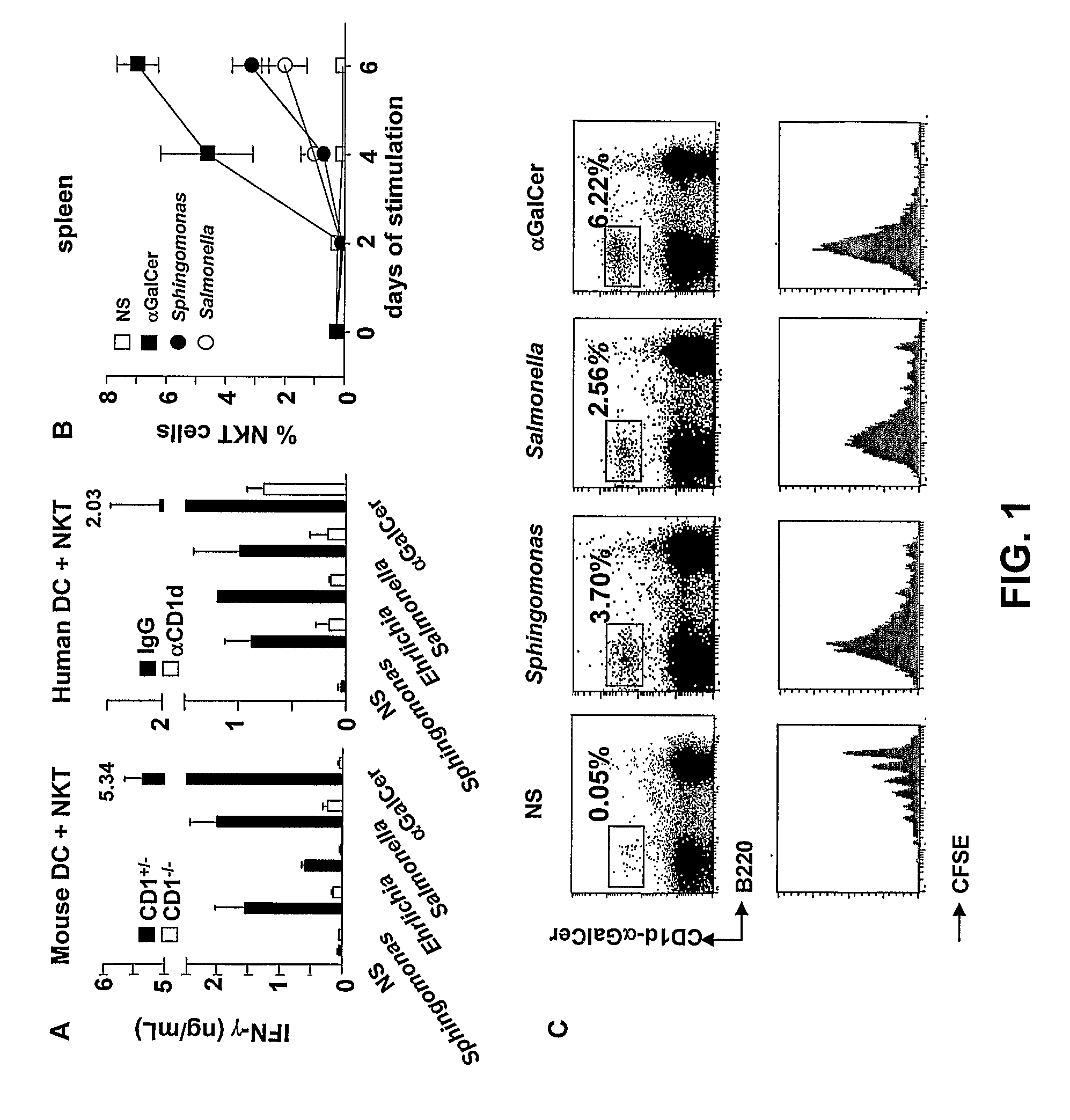
Bacterial Glycolipid Activation of Cd1d-Restricted Nkt Cells
InactiveUS20080279894A1Improving vaccine efficacyPromoting tumor rejectionAntibacterial agentsOrganic active ingredientsBacilliWilms' tumor
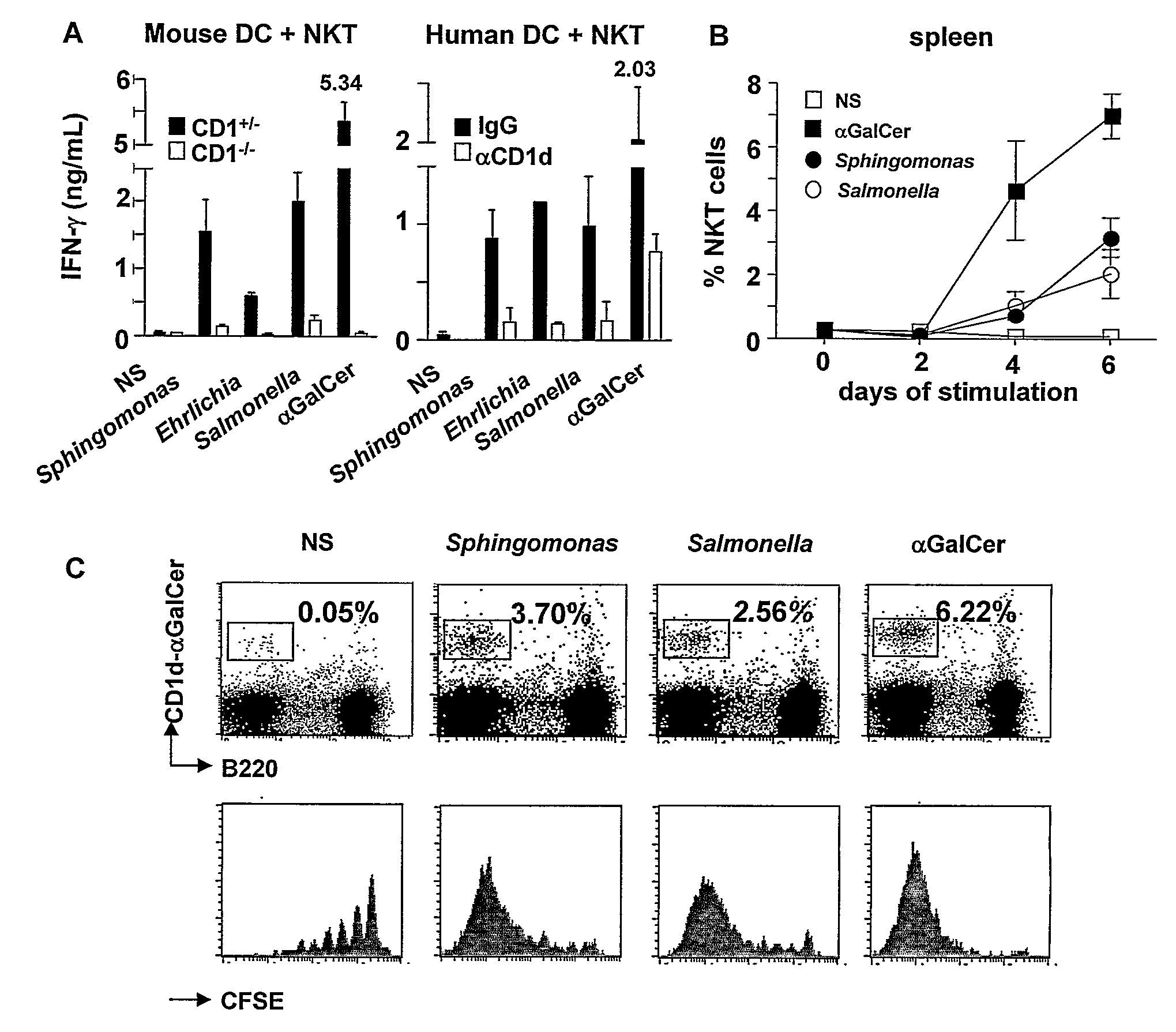
Disclosed are methods for activating an NKT cell, methods of stimulating an immune response in a subject, methods of improving vaccine efficacy, and methods of treating an infection. Also disclosed are methods of promoting tumor rejection, treating cancer, modulating autoimmunity and inhibiting allergen-induced hypersensitivity in subjects. The methods include contacting an NKT cell with a bacterial glycolipid complexed with a CD1 molecule to activate the NKT cell. The bacterial glycolipid may be derived from a member of the Class Alphaproteobacteria.
Owner:THE SCRIPPS RES INST +2
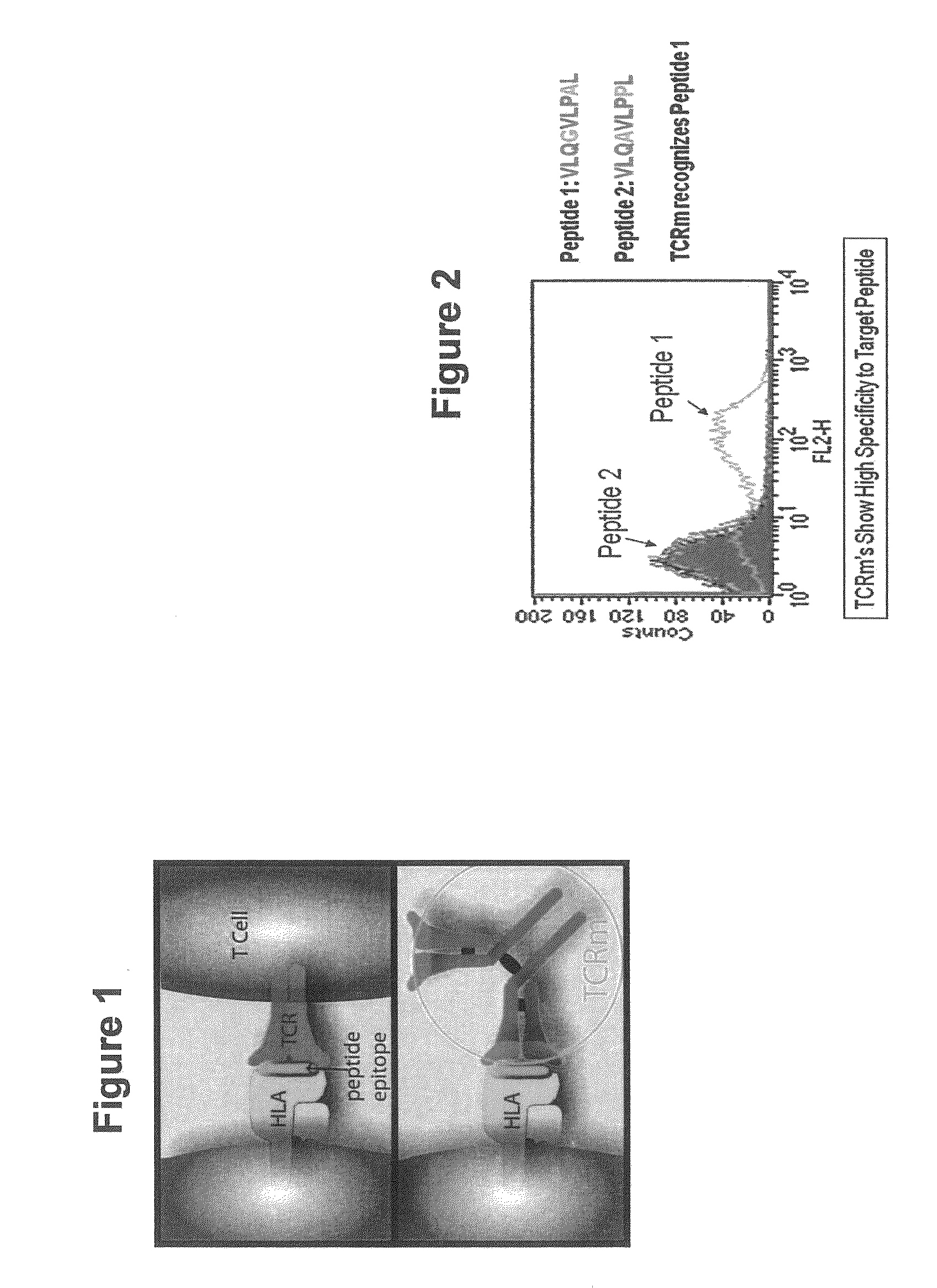
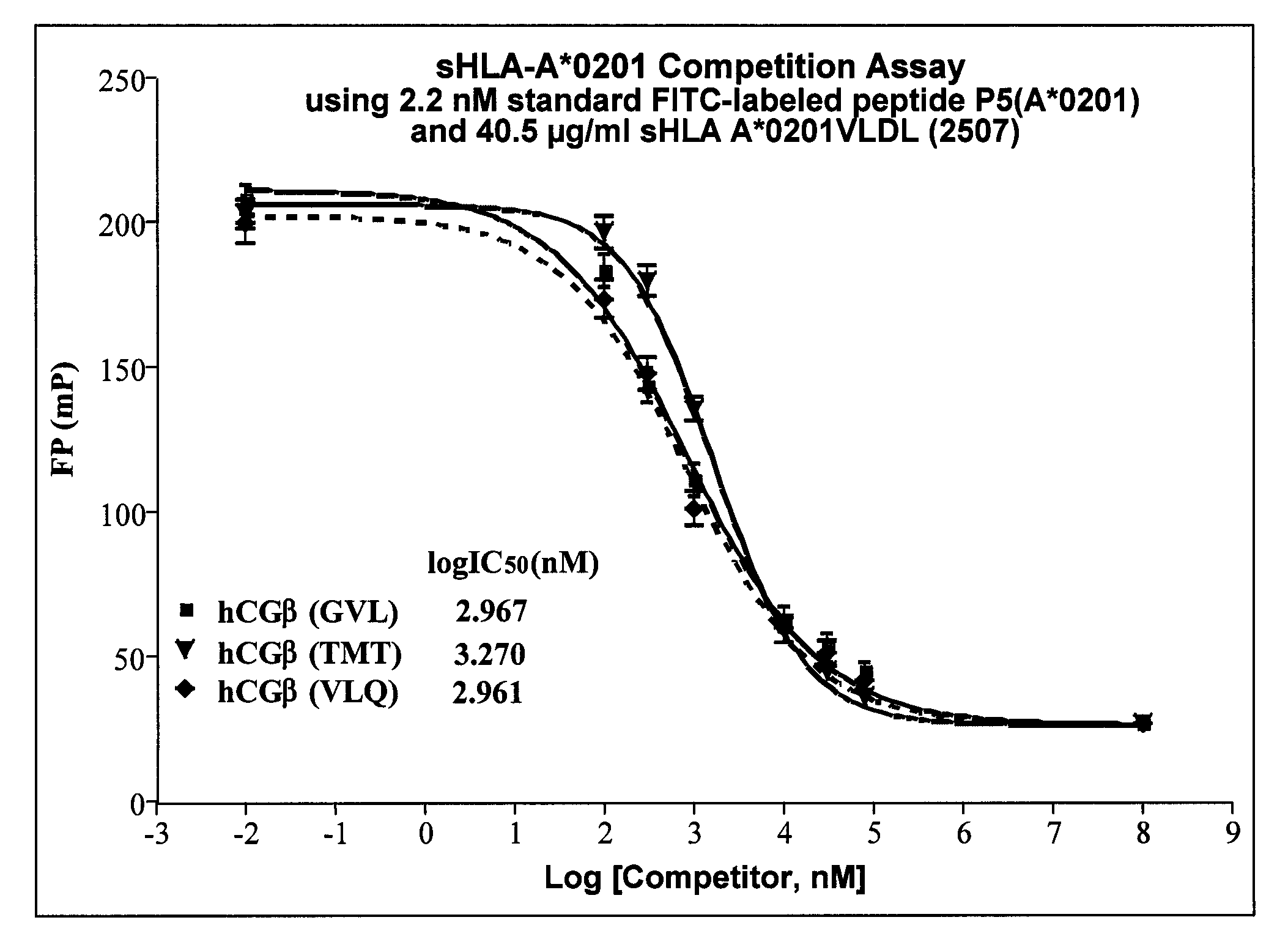
Methods of assaying vaccine potency
InactiveUS20090233318A1Immunoglobulin superfamilyCancer antigen ingredientsConjugate vaccineVaccine Potency
The present invention is related to methods of assaying potency of a vaccine composition, wherein the potency is a pre-defined minimum level of potential biological activity for the vaccine composition. The method includes providing a vaccine composition and delivering same to an antigen presenting cell, wherein the vaccine composition is processed into peptides and the peptides are presented by MHC complexes on the cell surface. An agent, such as a T cell receptor mimic, that is reactive against a specific peptide / MHC complex is provided and reacted with the vaccine-treated antigen presenting cell, whereby the agent binds to the cell surface of the vaccine-treated antigen presenting cell if the specific peptide / MHC complex recognized by the agent is present on the cell surface. A density of the specific peptide / MHC complex on the surface of the vaccine-treated antigen presenting cell is measured by agent binding. The potency of the vaccine is then determined based upon the measured density of specific peptide / MHC complex present on the surface of the vaccine-treated antigen presenting cell.
Owner:RECEPTOR LOGIC
Lipid and Nitrous Oxide Combination as Adjuvant for the Enhancement of the Efficacy of Vaccines
InactiveUS20090010964A1Improve actionStimulate immune responseAntibacterial agentsBacterial antigen ingredientsAntigenAdjuvant
The invention provides for a method of enhancing immunological responses to an antigen in a vaccine formulation, and for a vaccine formulation that provides for an enhanced immunological response to an antigen. In the method and formulation the antigen is administered with an adjuvant which adjuvant comprises a solution of nitrous oxide gas in a pharmaceutically acceptable carrier solvent for the gas and which adjuvant includes at least one fatty acid or ester or other suitable derivative thereof selected from the group consisting of oleic acid, linoleic acid, alpha-linolenic acid, gamma-linolenic acid, arachidonic acid, eicosapentaenoic acid [C20: 5ω3], decosahexaenoic acid [C22: 6ω3], ricinoleic acid and derivatives thereof selected from the group consisting of the C1 to C6 alkyl esters thereof, the glycerol-polyethylene glycol esters thereof and the reaction product of hydrogenated natural oils composed largely of ricinoleic acid based oils, such as castor oil with ethylene oxide.
Owner:EXHAUSTO +1
Methods of assaying vaccine potency
InactiveUS20090075304A1Immunoglobulin superfamilyCancer antigen ingredientsConjugate vaccineVaccine Potency
The present invention is related to methods of assaying potency of a vaccine composition, wherein the potency is a pre-defined minimum level of potential biological activity for the vaccine composition. The method includes providing a vaccine composition and delivering same to an antigen presenting cell, wherein the vaccine composition is processed into peptides and the peptides are presented by MHC complexes on the cell surface. An agent, such as a T cell receptor mimic, that is reactive against a specific peptide / MHC complex is provided and reacted with the vaccine-treated antigen presenting cell, whereby the agent binds to the cell surface of the vaccine-treated antigen presenting cell if the specific peptide / MHC complex recognized by the agent is present on the cell surface. A density of the specific peptide / MHC complex on the surface of the vaccine-treated antigen presenting cell is measured by agent binding. The potency of the vaccine is then determined based upon the measured density of specific peptide / MHC complex present on the surface of the vaccine-treated antigen presenting cell.
Owner:RECEPTOR LOGIC
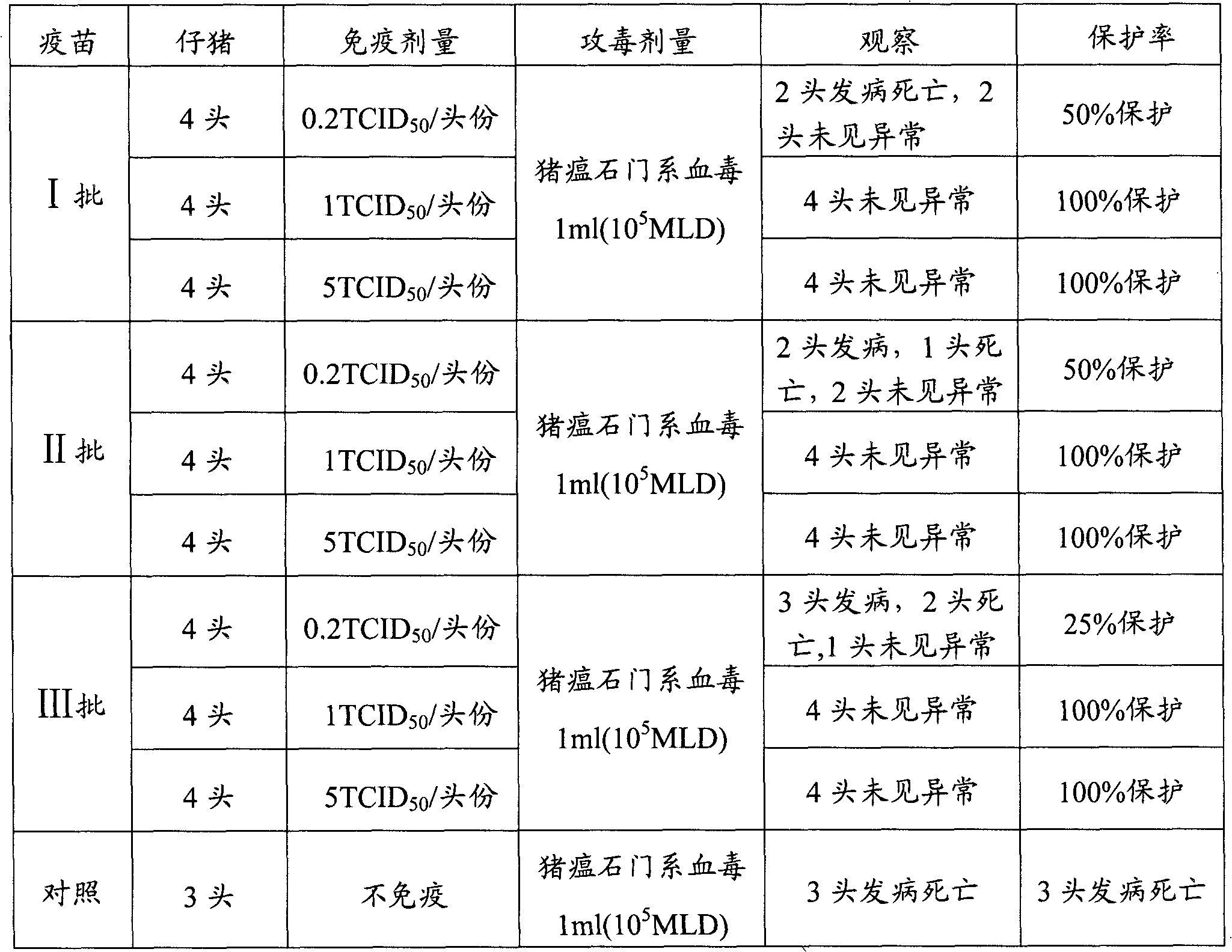
Porcine circovirus-II vaccine potency test method
InactiveCN101871941AShort detection timeShorten the timeBiological material analysisWhite mousePresent method
The invention belongs to the technical field of veterinary medicaments and new biological medicaments and relates to a porcine circovirus-II vaccine potency test method. The method comprises: immunizing a white mouse with a porcine circovirus-II inactivated vaccine; and determining the PCV2 specific antibody content of the serum of the white mouse to evaluate the potency of the vaccine. When the vaccine potency test method is used, and the problems of time-consuming and labor-consuming test pig screening, large inter-batch test result difference and the like of the conventional porcine circovirus-II inactivated vaccine potency test are solved. The test method of the invention has the advantages of short test period, simple operation, low cost, accurate result and high repeatability. The method has a high application value and a promising market prospect.
Owner:PU LIKE BIO ENG
Method for testing efficacy of swine fever live vaccine
ActiveCN101846683AShort detection timeEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceVaccine PotencyEfficacy
The invention belongs to the technical field of new biological veterinary medicaments, and in particular relates to a method for testing the efficacy of a swine fever live vaccine. The efficacy of the live vaccine is evaluated by detecting the virus content of the live vaccine through indirect immunofluorescence (IFA). The method for testing the efficacy of the swine fever live vaccine has the advantages of short test time, simple operation, low cost, accurate obtained result, high repeatability, excellent application value and wide market prospects.
Owner:PU LIKE BIO ENG
Pro-Apoptotic Bacterial Vaccines To Enhance Cellular Immune Responses
InactiveUS20120276144A1Diminishing intracellular survivalReduced activityAntibacterial agentsBacterial antigen ingredientsVaccine PotencyVaccine efficacy
Whole-cell vaccines and methods for their use in producing protective immune responses in vertebrate hosts subsequently exposed to pathogenic bacteria. The present invention involves a method of enhancing antigen presentation by intracellular bacteria in a manner that improves vaccine efficacy. After identifying an enzyme that has an anti-apoptotic effect upon host cells infected by an intracellular microbe, the activity of the enzyme is reduced, thereby modifying the microbe so that it increases immunogenicity. Also, the present invention provides a method of incrementally modifying enzyme activity to produce incrementally attenuated mutants of the microbe from which an effective vaccine candidate can be selected.
Owner:VANDERBILT UNIV
Oil-in-water vaccine adjuvant and preparation method thereof
InactiveCN107050449AEnhance immune stimulationImprove the effectiveness of anti-virus protectionSsRNA viruses positive-senseViral antigen ingredientsWater basedVaccine Potency
The invention provides an oil-in-water vaccine adjuvant and a preparation method thereof. According to the technical scheme, category and composition of surfactant are optimized; the stable oil-in-water adjuvant is prepared by virtue of a one-step process, so that autologous cellular and humoral immunity stimulation capacities of a vaccine are enhanced; and especially, the vaccine adjuvant also simultaneously comprises other water-based adjuvant ingredients which are effective on enhancing efficacy of the vaccine, so that attacking protection efficacy of the inactivated vaccine is further improved, and technical problems of an existing oil-in-water vaccine which is low in immunological activity and short in immunity persistence are solved. The preparation method of the nano vaccine adjuvant provided by the invention is simple in process and low in cost, and the obtained vaccine adjuvant is stable in dosage form. The prepared oil-in-water nano emulsion (the vaccine adjuvant) is long in stable period, easy for preservation, good in immunization effect, long in protection term, easy for injection and quite low in side reactions.
Owner:天津三江永利生物科技有限公司
Recombinant human bivalent diabody against rabies virus and uses thereof
InactiveCN103596975ASsRNA viruses negative-senseImmunoglobulins against virusesVaccine PotencyViral glycoprotein
The present invention provides recombinant human bivalent diabody against rabies virus capable of recognizing rabies virus glycoprotein and neutralizing rabies viruses and a method for production thereof. The present invention further provides polynucleotide encoding the recombinant bivalent diabody. The bivalent diabody disclosed in the present invention is also useful for quantitation of the rabies virus glycoprotein for evaluating the vaccine quality and predicting the vaccine potency.
Owner:INDIAN IMMUNOLOGICALS LIMITED
Nerve growth factor as a vaccine adjuvant
A vaccination method utilizes a pharmaceutical combination for enhancing vaccine effectiveness. The method utilizes an immune response-triggering vaccine capable of stimulating production in an immunodefficient animal of antibodies to a disease-causing agent foreign to the animal. As an adjuvant, a vaccine effectiveness-enhancing amount of Nerve Growth Factor (NGF) is administered, which enhances production and affinity of the antibodies in the animal, in response to the vaccine.
Owner:TORCIA MARIA
Vaccine and methods for detecting and preventing filariasis
The present invention is a multivalent vaccine for immunizing an animal against filariasis. In some embodiments, the antigens of the multivalent vaccine are protein-based, DNA-based, or a combination thereof. This invention also provides a method and kit for detecting a filarial nematode and determining vaccine efficacy.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Methods and compositions for enhancing immune response and for the production of in vitro Mabs
InactiveUS7790167B2Eliminates immune responseEnhance and alter and suppress immune responsePowder deliveryBiocideDendritic cellVaccine Potency
The methods and compositions of the present invention are directed to enhancing an immune response and increasing vaccine efficacy through the simultaneous or sequential targeting of specific immune system components. More particularly, specific immune components, such as macrophages, dendritic cells, B cells and T cells, are individually activated by component-specific immunostimulating agents. One such component-specific immunostimulating agent is an antigen-specific, species-specific monoclonal antibody. The invention is also directed to a method for the in vitro production of the antigen-specific, species-specific monoclonal antibodies which relies upon the in vitro conversion of blood-borne immune cells, such as macrophages and lymphocytes. Vaccine efficacy is enhanced by the administration of compositions containing component-specific immunostimulating agents and other elements, such as antigens or carrier particles, such as colloidal methods, such as gold.
Owner:CYTIMMUNE SCI
Method for increasing tumor cell immunogenicity using heat shock protein
InactiveUS20080075705A1Maximize delivery of cellReduce cancer cell growthBiocideEnergy modified materialsHeat shockVaccine Potency
A method for induction of endogenous heat shock protein in tumor cells using a simple heat shock treatment which provides a simple and inexpensive method for augmenting antitumor vaccine potency. The method comprises administering to a mammal tumor cells which have been subject to a heat shock condition sufficient to cause induction of endogenous heat shock protein therein. Also disclosed is a composition for enhancing tumor cell immunogenicity comprising a therapeutically effective amount of attenuated tumor cells which have been subject to a heat shock condition.
Owner:TRAN ANNIE CHEN
Recombinant epsilon toxin and alpha toxin fusion protein vaccine of non-toxic clostridium perfringens and production method of fusion protein vaccine
ActiveCN109745554AHigh expressionNo virulenceAntibacterial agentsBacterial antigen ingredientsDiseaseEscherichia coli
The invention relates to a recombinant epsilon toxin and alpha toxin fusion protein vaccine of non-toxic clostridium perfringens and a production method of the vaccine. According to an ETX mutant of non-toxic clostridium perfringens and C end fusion protein rETXm3CPAC of CPA, the non-toxic ETX mutant ETXm3 is in serial connection with a C end (CPAC) of CPA, soluble expression is achieved in escherichia coli BL21(DE3), the spatial conformation of natural toxin protein can be reserved to the highest extent, and accordingly the immunogenicity of escherichia coli can be maintained; the influence of a complex technology of inclusion body denaturation and renaturation on immunogenicity of the antigen protein is also avoided, the time of preparing the vaccine is shortened, and the production costis reduced. The C end of rETXm3CPAC contains six histidine (6*His) labels, and convenience is provided for protein purification; the obtained toxin fusion protein is completely free of toxicity in amouse body and has good safety, immunogenicity and immune protection performance in a rabbit model. The vaccine also has the advantages that the preparation technology is good, the efficacy of the vaccine is excellent, and A-type and D-type clostridium perfringens diseases are prevented at the same time.
Owner:CHINA INST OF VETERINARY DRUG CONTROL
Vaccines for the treatment of cancer and compositions for enhancing vaccine efficacy
InactiveUS20150343040A1Improve therapeutic efficacyInhibit progressAntibacterial agentsPeptide/protein ingredientsCancer preventionCancer cell
The present invention relates to the treatment and prevention of cancer. The present invention relates to vaccines comprising solubilized components of cancer cells or cancer-associated cells. Moreover, the present invention also relates to methods of producing vaccines from biological samples comprising cancer cells or cancer-associated cells and using said vaccines for the treatment or prevention of cancer in subjects. The present invention also relates to methods of producing vaccines, in particular, autologous vaccines. The present invention also relates to therapeutic uses of mesenchymal stem cells and to methods of treatment and or prevention that comprise administering mesenchymal stem cells to a subject. The present invention also relates to methods of enhancing the efficacy of vaccines and methods for the treatment and prevention of cancer, and to compositions and kits suitable for use in the methods.
Owner:CELL IDEAS +1
Reagents and methods for detecting influenza virus proteins
ActiveUS8669046B2Simplify the linkImprove solubilitySsRNA viruses negative-sensePeptide/protein ingredientsInfluenza virus nucleoproteinVaccine Potency
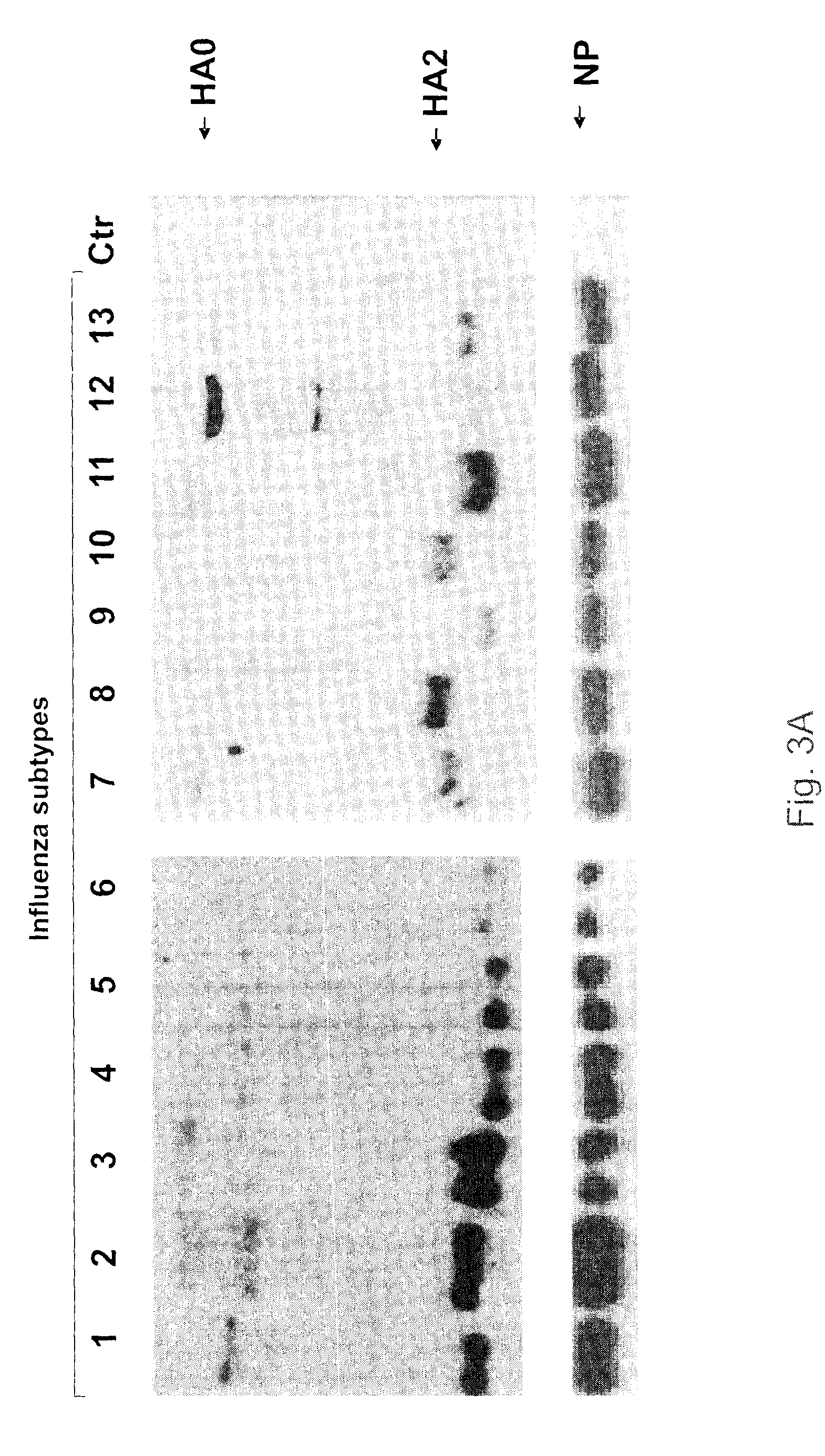
Antibodies that specifically bind to a peptide having an amino acid sequence as found at the N-terminus of the HA2 fusion peptide of the influenza A virus may be raised by inoculating a mammal with a conjugate of the peptide. In one embodiment, the conjugate comprises the peptide linked to a spacer (e.g. 6-aminocaproic acid) and a carrier protein (e.g. KLH). The antibodies may be used as a universal reagent for detecting HA proteins of influenza viruses. The antibodies are useful as versatile reagents for laboratory research and vaccine potency determination, especially in the event of pandemic influenza outbreaks.
Owner:HER MAJESTY THE QUEEN & RIGHT OF CANADA REPRESENTED BY THE MIN OF HEALTH
Helicobacter pylori mutant strain capable of stimulating immune response and construction method and application thereof
PendingCN111849850AHas adjuvant activityGood service for antigen presentationAntibacterial agentsBacterial antigen ingredientsVaccine PotencyVaccine efficacy
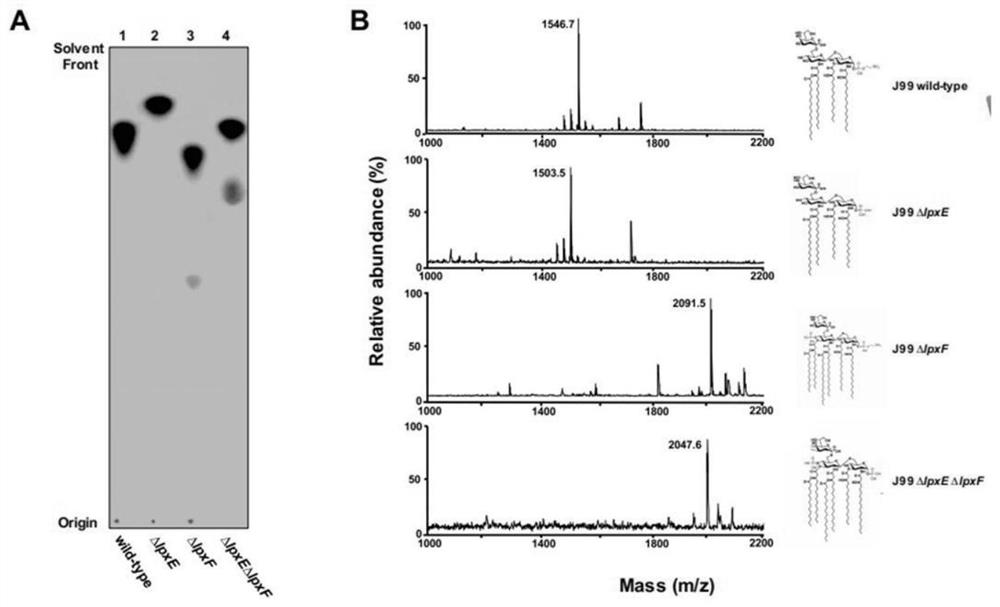
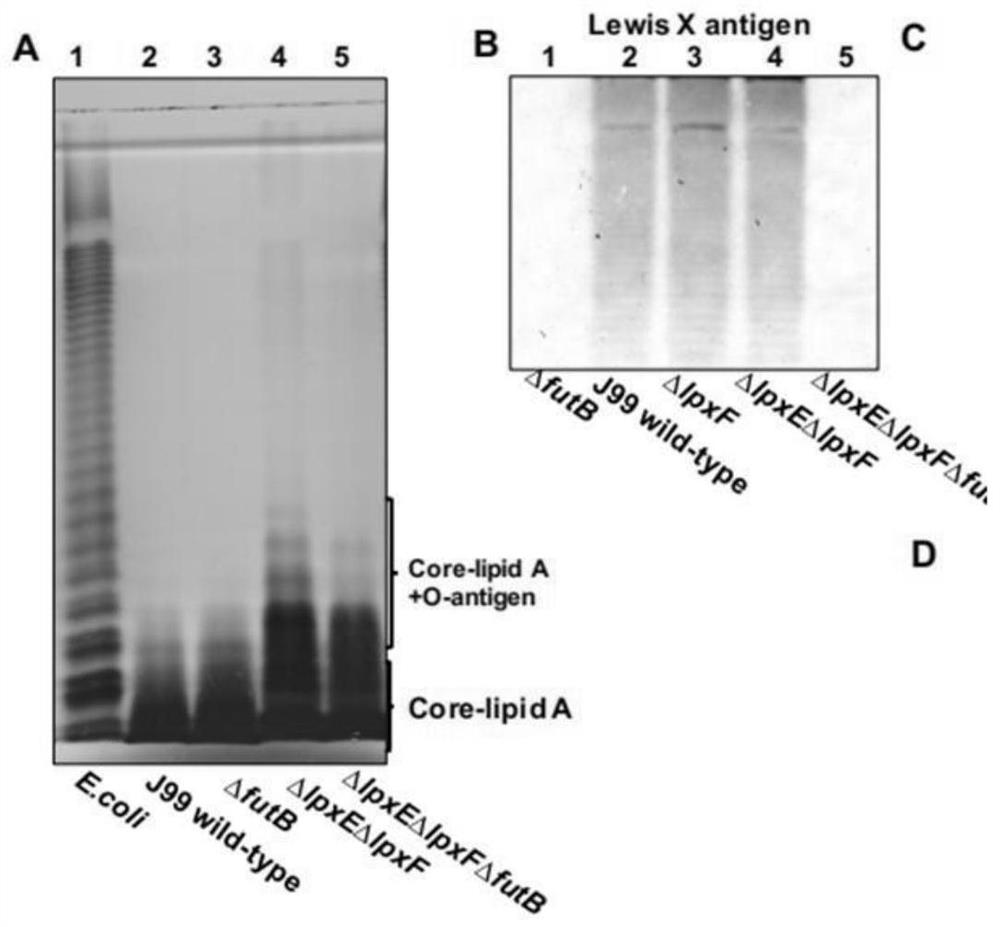
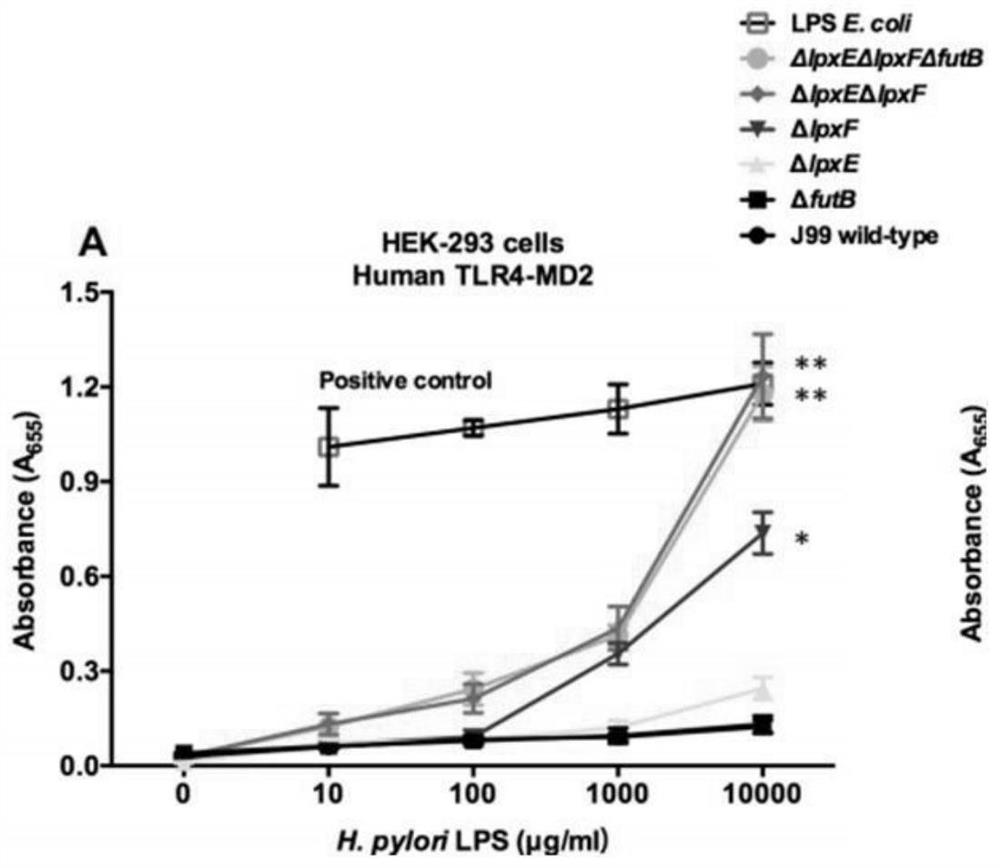
The invention provides a helicobacter pylori mutant strain capable of stimulating immune response and a construction method and application of the helicobacter pylori mutant strain, and belongs to thetechnical field of bioengineering. The mutant strain provided by the invention does not contain a gene futB for encoding glycosyltransferase in an O antigen synthesis process, meanwhile, a lipid A structure is modified, related synthesis genes lpxE and lpxF are knocked out, and the preservation number of the strain is CCTCC NO: M 2020028. The helicobacter pylori outer membrane vesicle vaccine canefficiently stimulate a host to generate immune response and secrete the outer membrane vesicle, so that the helicobacter pylori outer membrane vesicle has the characteristic of high vaccine efficacy. Meanwhile, the method can effectively enable helicobacter pylori lipid A to be recognized by TLR4 again, enables lipid A to have adjuvant activity, and can better serve antigen presentation. The method can be used for constructing novel helicobacter pylori antigen presenting plasmids, antigen protein is presented to periplasmids of bacteria or exposed to the surface of an outer membrane of the bacteria through different strategies, and therefore the efficiency of immunoreaction generated by host recognition of a target antigen is improved.
Owner:NANCHANG UNIV
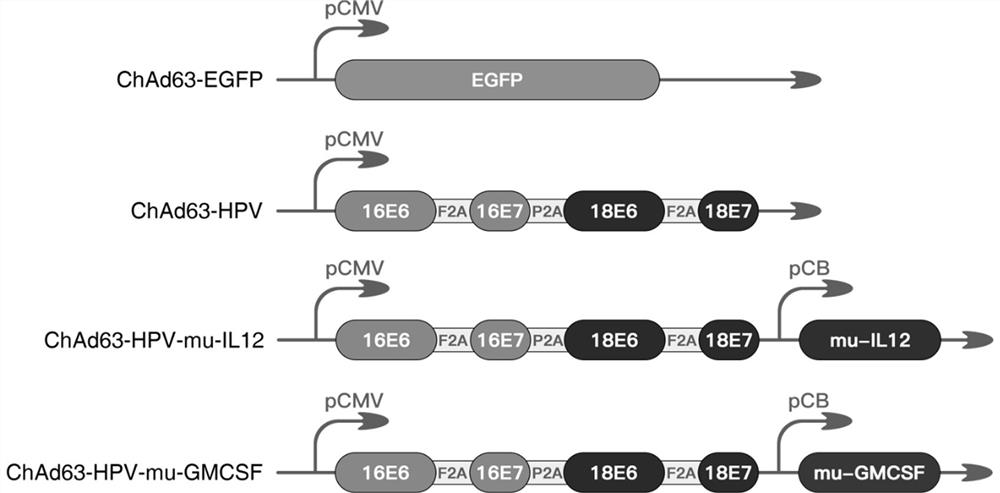
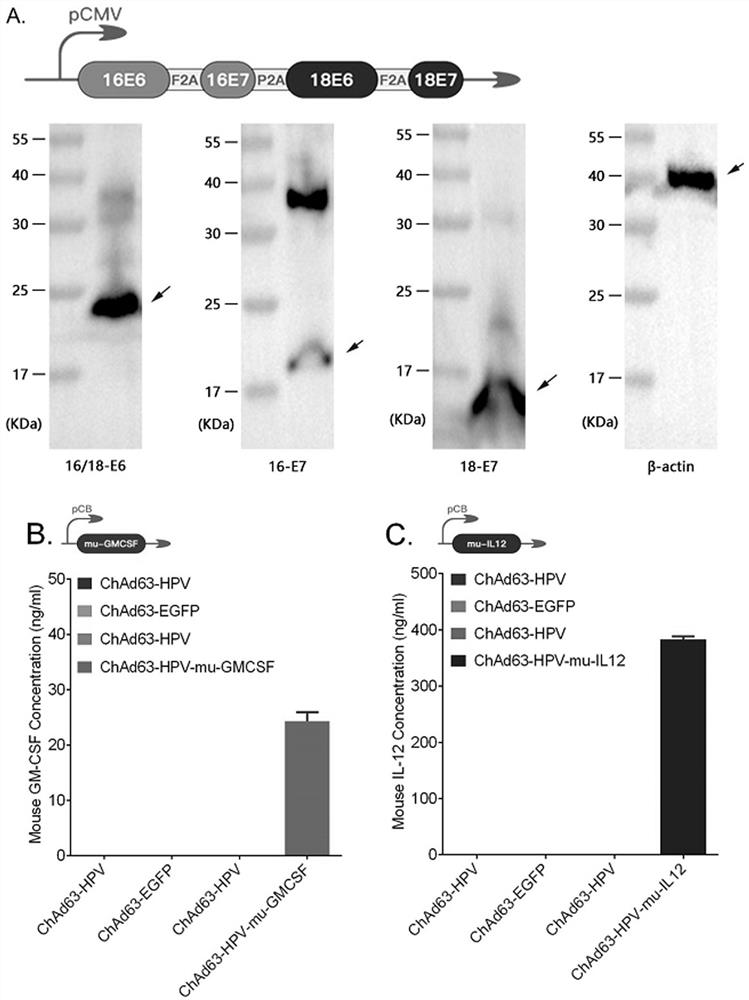
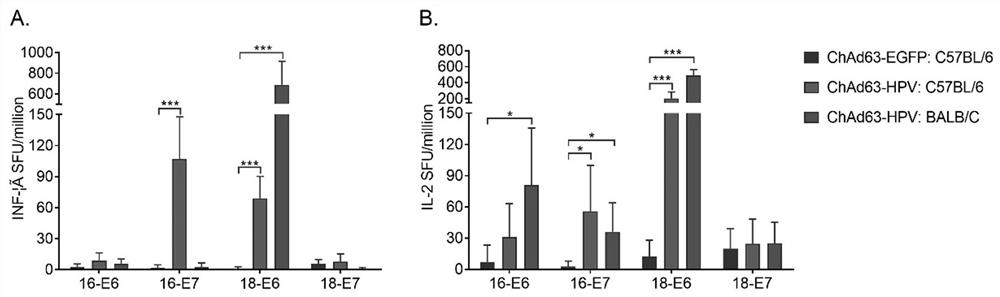
Therapeutic HPV vaccine based on chimpanzee adenovirus vector as well as preparation method and application of therapeutic HPV vaccine
InactiveCN112138150AEasy to makeImprove securityViral antigen ingredientsVirus peptidesVaccine PotencyAdjuvant
The invention discloses a therapeutic HPV vaccine based on a chimpanzee adenovirus vector as well as a preparation method and application of the therapeutic HPV vaccine. The therapeutic HPV vaccine isan immunogenic HPV recombinant adenovirus vaccine obtained by packaging and processing HPV virus vaccine vector plasmids, and the HPV virus vaccine vector is a replication-defective chimpanzee adenovirus vector. The HPV therapeutic vaccine is constructed by using the replication-defective chimpanzee adenovirus vector, the influence of the pre-stored immunity of a human adenovirus vector in a human body on the vaccine efficacy is overcome, and a vaccine product which is simple to prepare, low in cost, good in safety and free of adjuvants is expected to be obtained. The therapeutic HPV vaccinehas good immunogenicity and anti-tumor efficacy, can induce high-level antigen-specific T cell reaction and anti-tumor efficacy in a mouse body through single muscle immunization, and is expected to show good humoral and cellular immunotherapy effect can be embodied in human clinical tests.
Owner:IMMUNE PATH BIOTECHNOLOGY SUZHOU CO LTD
Method of screening
A method of detecting the presence of a functionally inhibitory immunointeractive molecule in a biological sample. Preferably, the immunointeractive molecule is directed to a pathogen derived antigen and, more particularly, a parasite derived antigen and, even more particularly, a Plasmodium drived antigen. The method of the present invention facilitates detection of the presence of functionally inhibitory immunointeractive molecules, both in vitro and in vivo, and is useful for qualitatively and / or quantitatively assessing the immune status of individuals who have been previously infected with a parasite, predicting the immune status of individuals vaccinated with an antigen based vaccine, determining the relative contribution of a specific immunoreactivity of antibody to the total inhibitory antibody elicited by combination vaccines which include two or more antigens, assessing vaccines to determine the efficacy of different forms of an antigen, determining vaccine potency, assessing the protective potential of certain immunoreactivities of antibodies and determining the importance of parasite inhibitory antibodies.
Owner:WALTER & ELIZA HALL INST OF MEDICAL RES
Use of cyclodextrins in diets, water or vaccine adjuvants to boost the immune system of fish
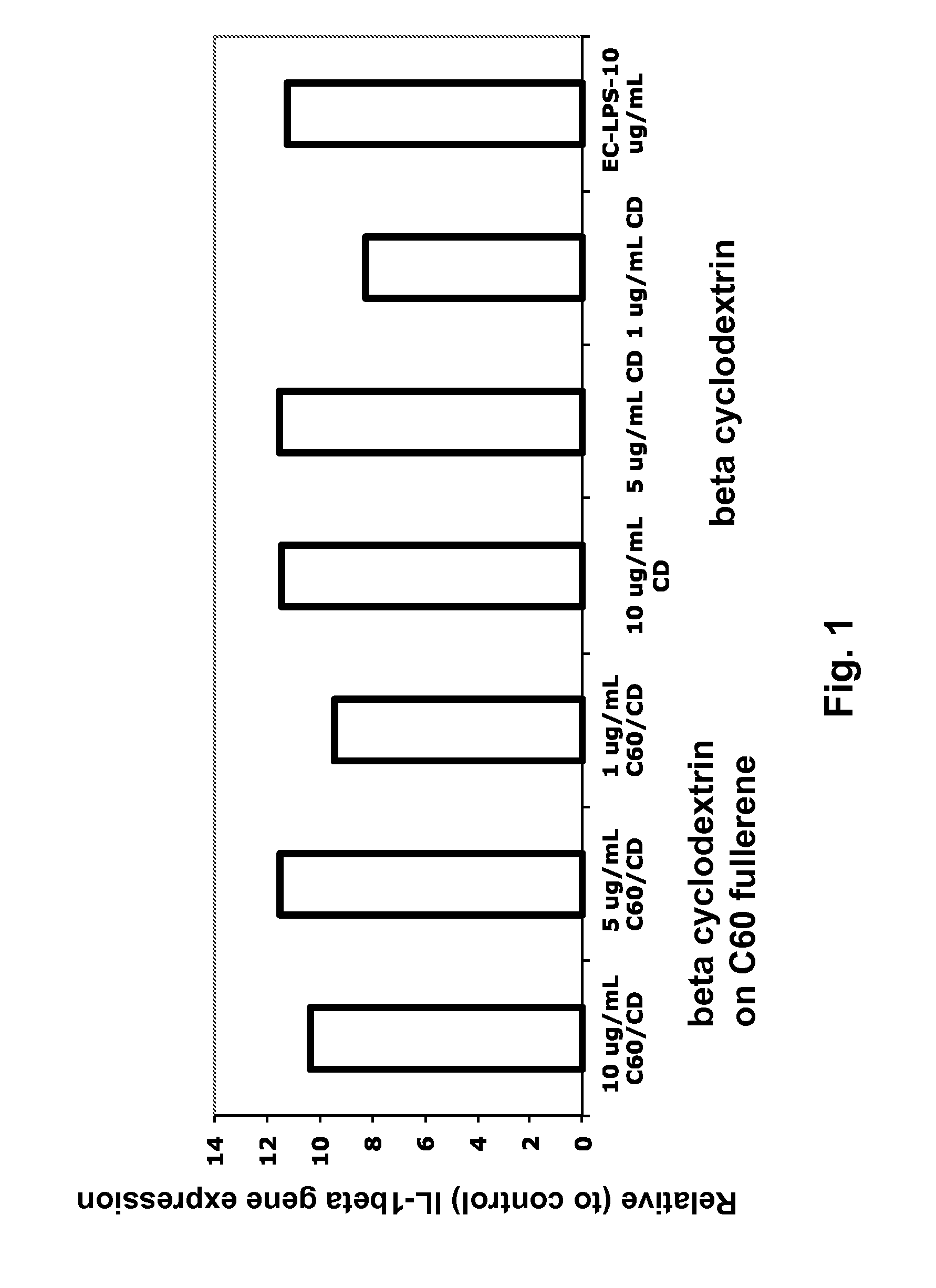
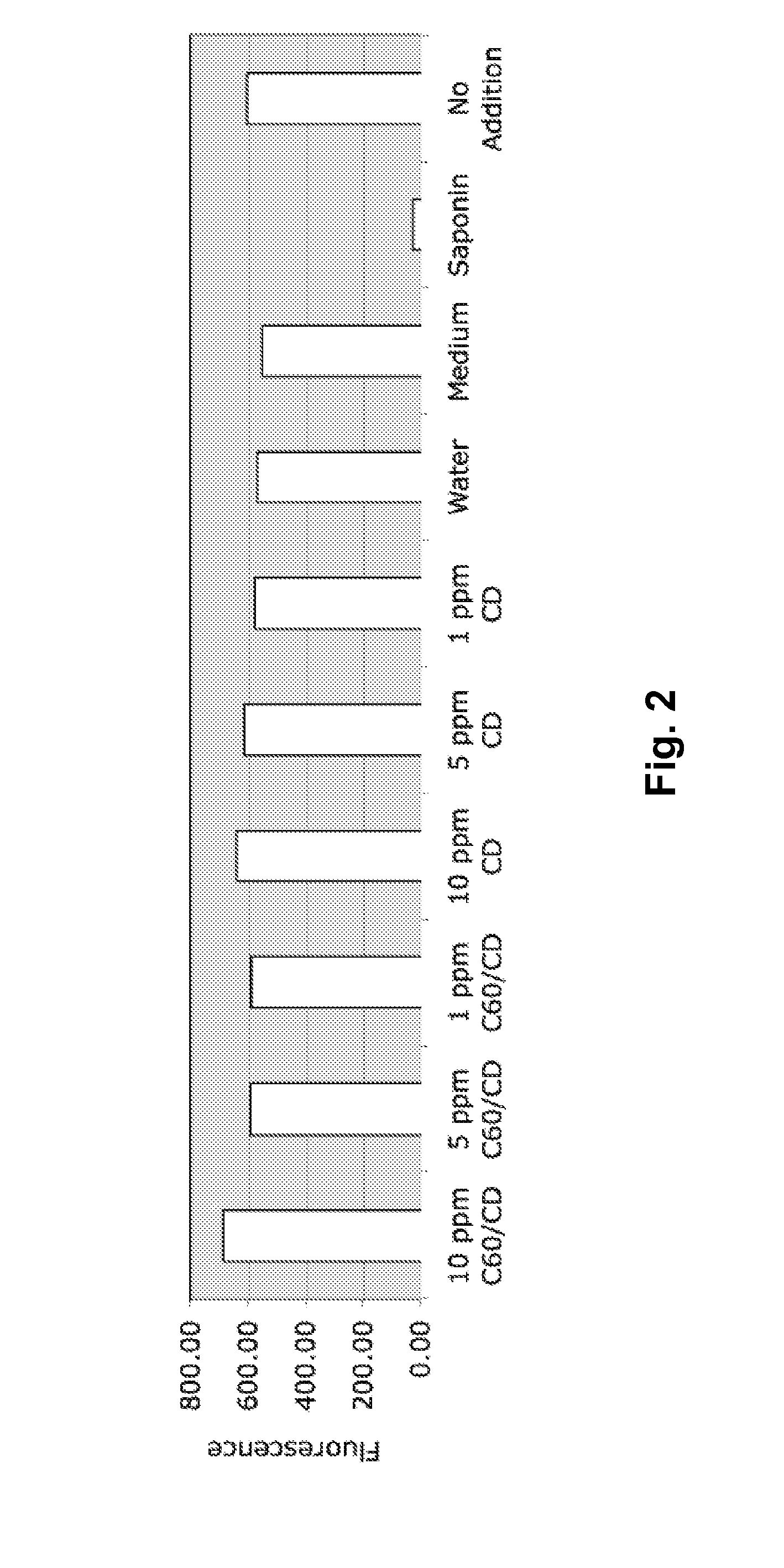
InactiveUS20140086961A1Good curative effectOrganic active ingredientsAnimal feeding stuffCyclodextrinFishery
The present disclosure describes compositions containing cyclodextrin and methods of using cyclodextrins to stimulate or enhance the immune system and response in fish. Methods of enhancing the efficacy of a fish vaccine by administering cyclodextrin to the fish are also described.
Owner:AUTONOMOUS UNIVERSITY OF BARCELONA +1
Pro-apoptotic bacterial vaccines to enhance cellular immune responses
InactiveUS8021671B2Improves vaccine efficacyDiminishing intracellular survivalAntibacterial agentsBiocideBacteroidesApoptosis
Whole-cell vaccines and methods for their use in producing protective immune responses in vertebrate hosts subsequently exposed to pathogenic bacteria. The present invention involves a method of enhancing antigen presentation by intracellular bacteria in a manner that improves vaccine efficacy. After identifying an enzyme that has an anti-apoptotic effect upon host cells infected by an intracellular microbe, the activity of the enzyme is reduced, thereby modifying the microbe so that it increases immunogenicity. Also, the present invention provides a method of incrementally modifying enzyme activity to produce incrementally attenuated mutants of the microbe from which an effective vaccine candidate can be selected.
Owner:VANDERBILT UNIV +1
Nucleic acid vaccine composition comprising a lipid formulation, and method of increasing the potency of nucleic acid vaccines
PendingUS20200046830A1Enhance immune responseImprove the level ofSsRNA viruses negative-senseOrganic active ingredientsVaccine PotencyTGE VACCINE
A nucleic acid vaccine composition comprising one or more of a plasmid-based nucleic acid vaccine and immunotherapy, as well as a lipid formulation, is provided. In addition, the present invention provides a method of enhancing the potency of plasmid-based DNA vaccines and immunotherapies, by formulating a vaccine and / or immunotherapy in a lipid formulation, which is stable when refrigerated or stored frozen, is then delivered to a vaccinee by either needle / syringe, jet injection, or microneedles. The lipid formulation of the present invention comprises one or more lipid excipients selected from 1,2-Distearoyl-sn-glycero-3-phosphocholine, Cholest-5-en-3β-ol, 1,2-Dimyristoyl-rac-glycero-3-methylpolyoxyethlene, and or more symmetric ionizable cationic lipids. The present invention increases vaccine potency dramatically. It was unexpectedly discovered that the level of immunogen, or immune response molecules, produced in vivo is increased (versus administering merely the vaccine or immunotherapy) and, in the case of a vaccine immunogen, the immune response is enhanced.
Owner:THE SEC OF THE ARMY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com