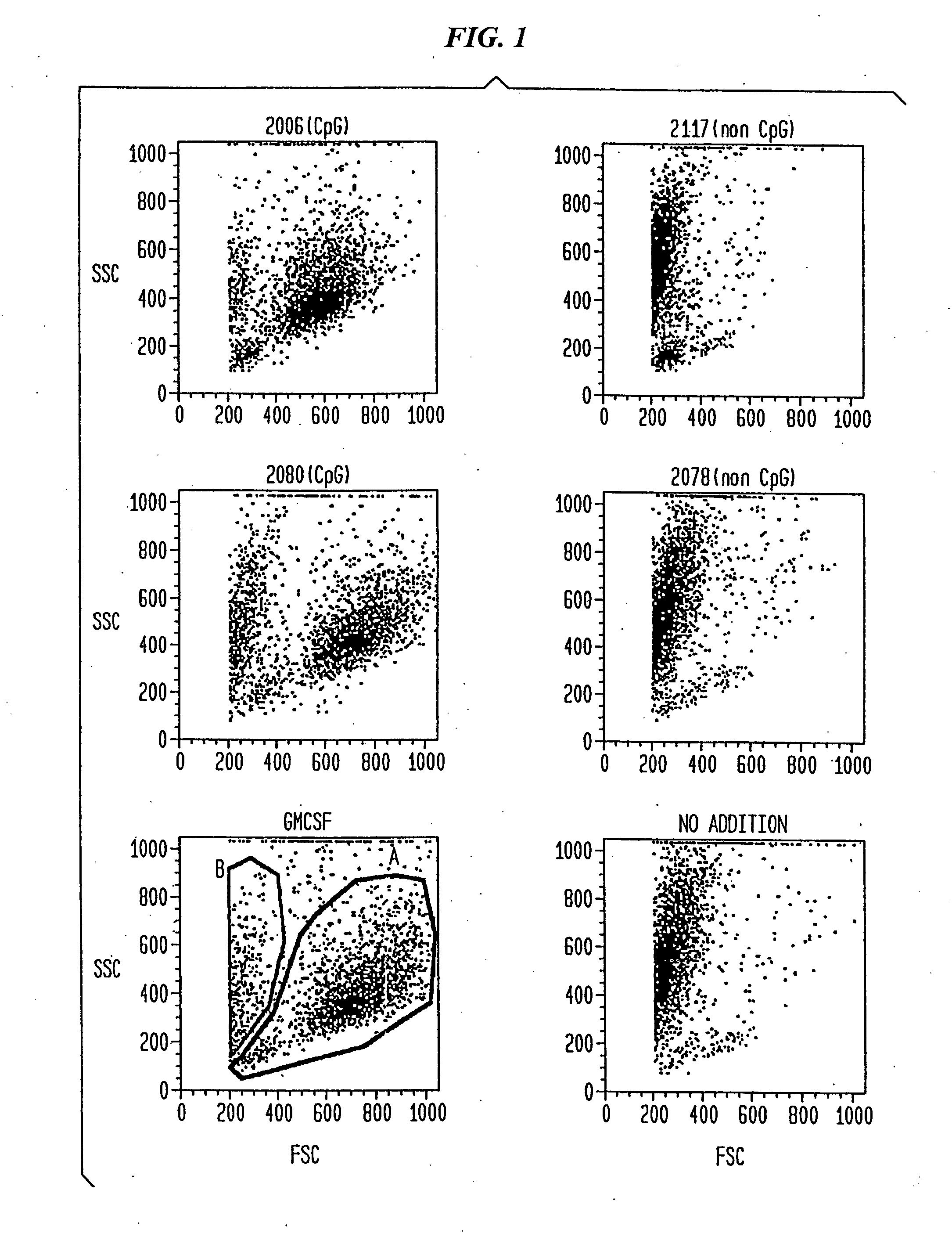
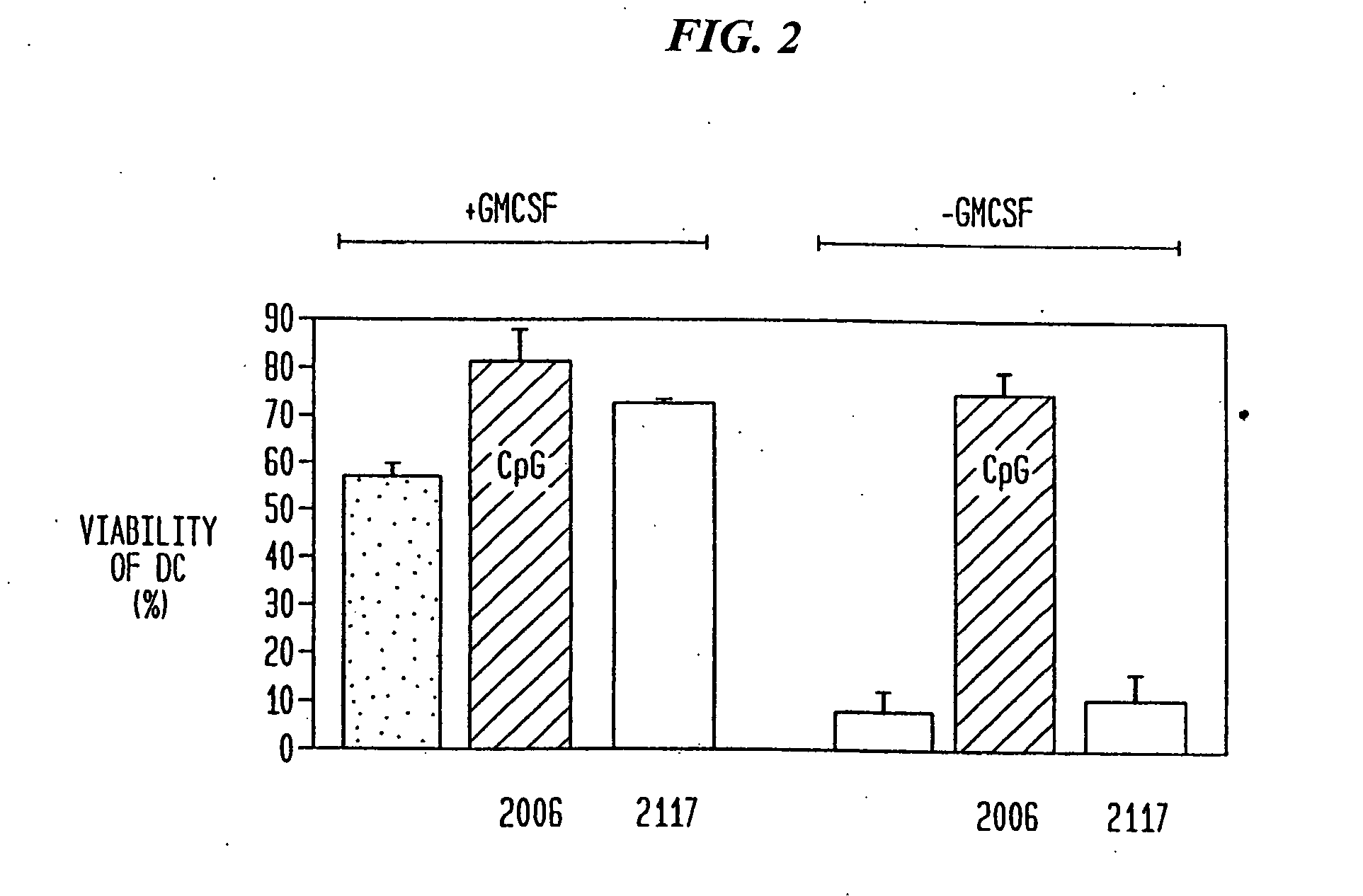
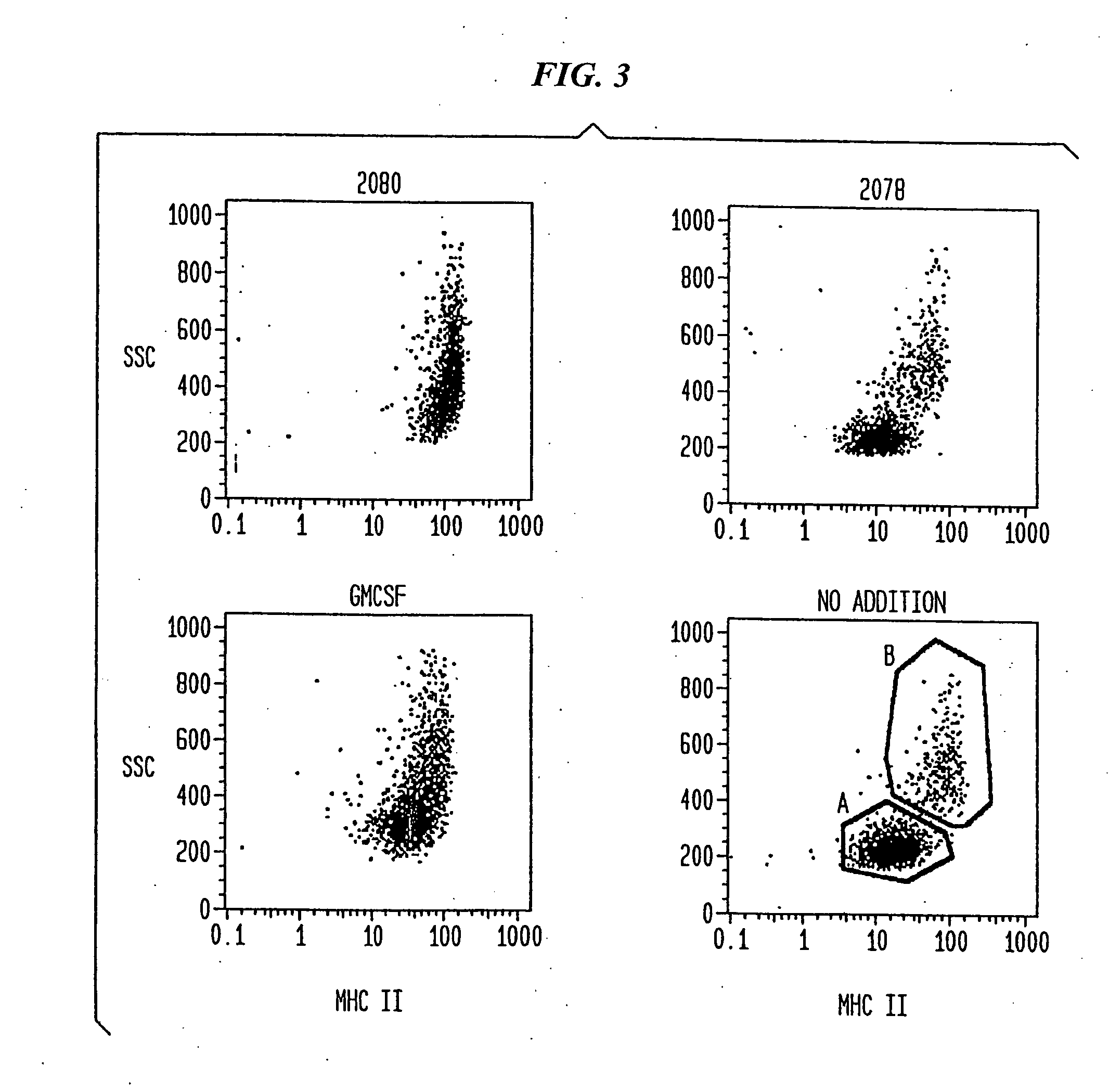
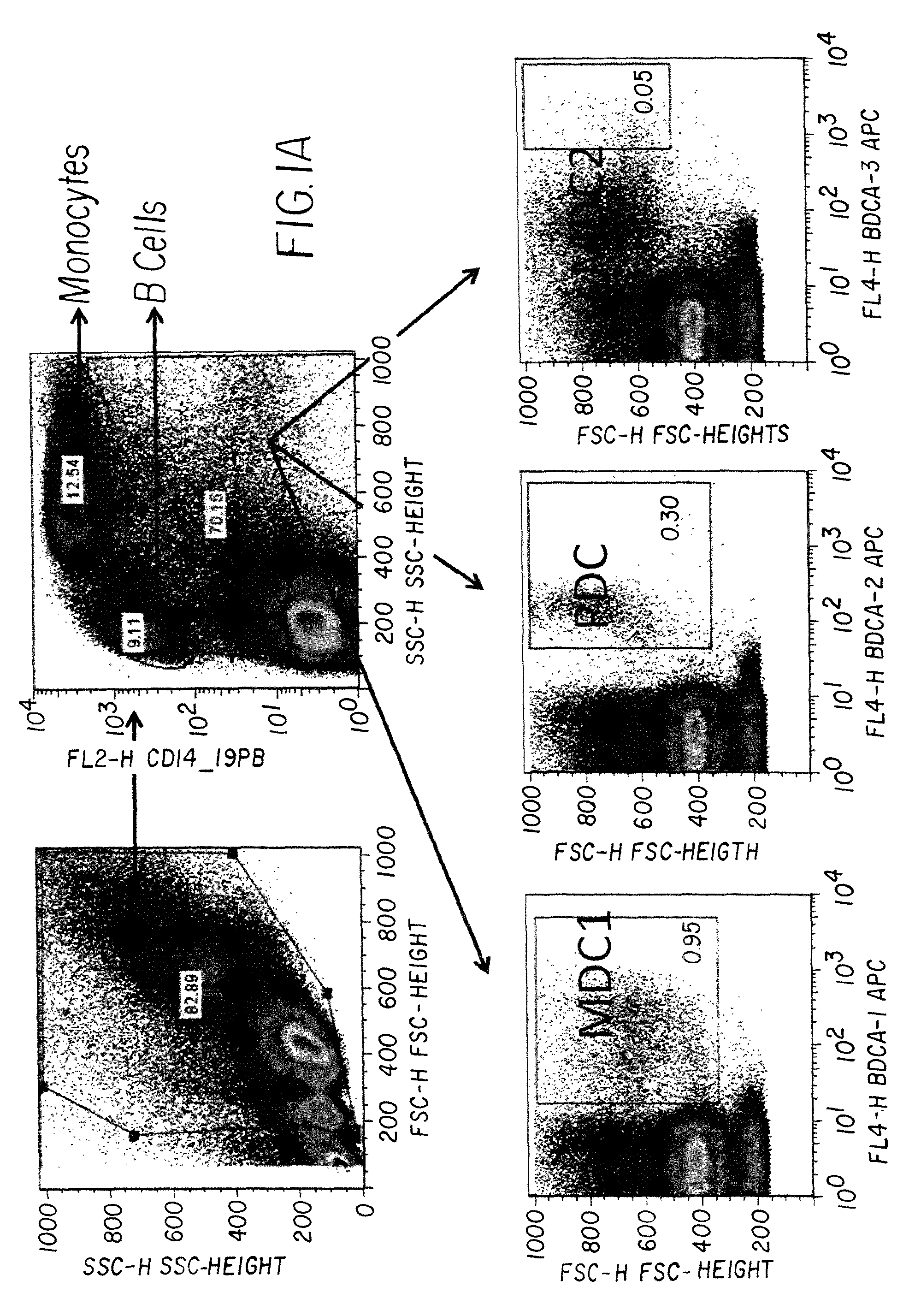
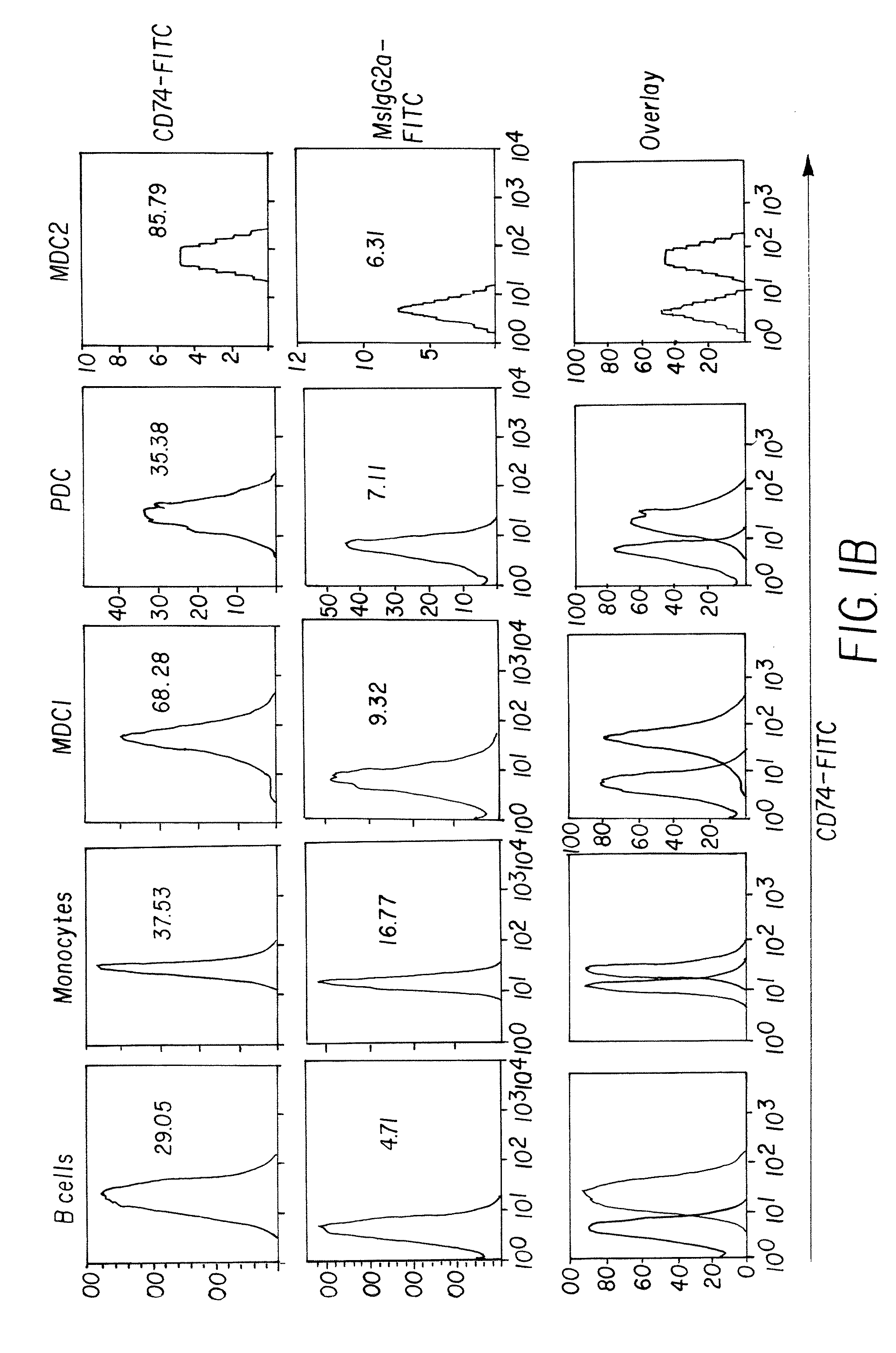
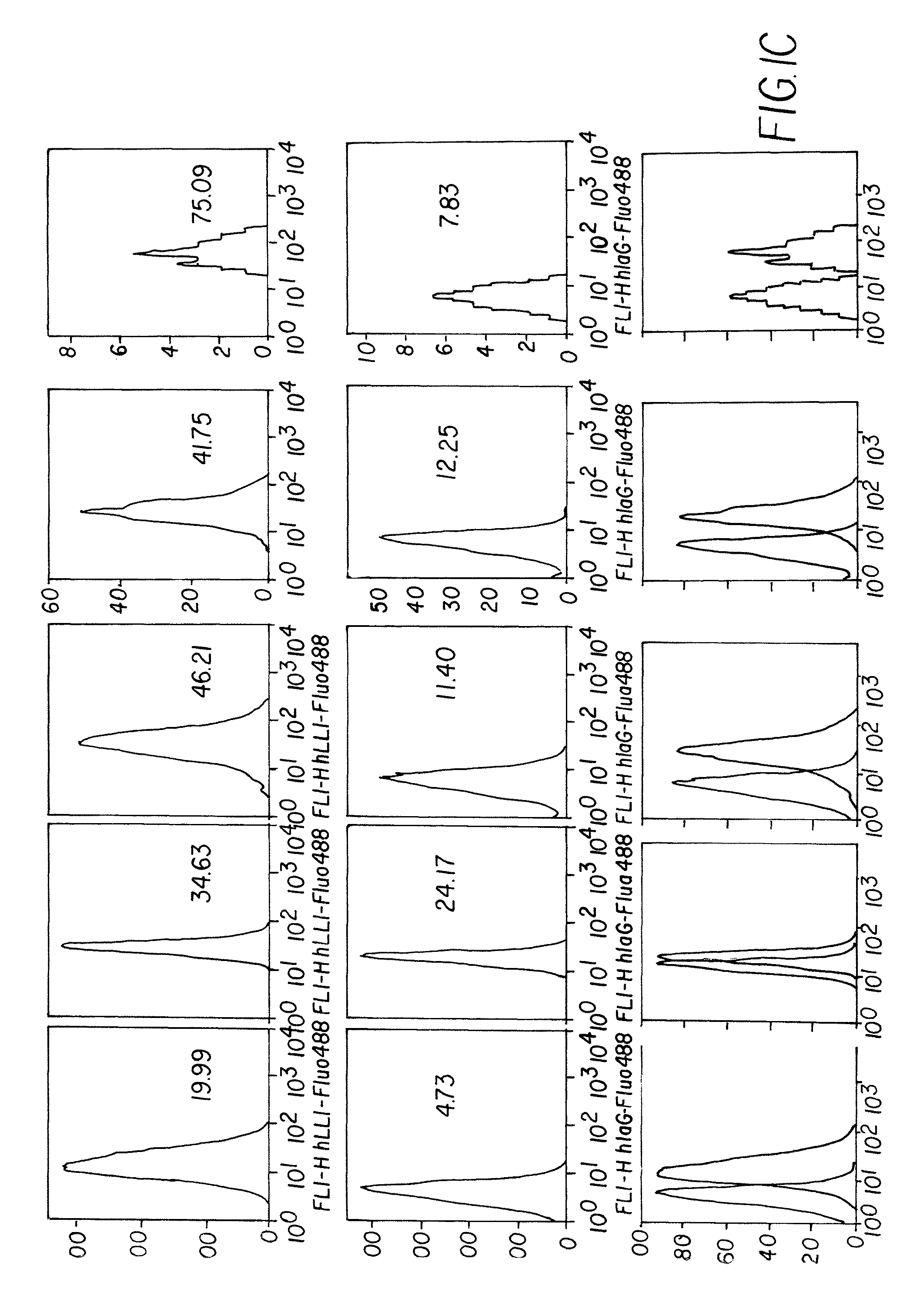
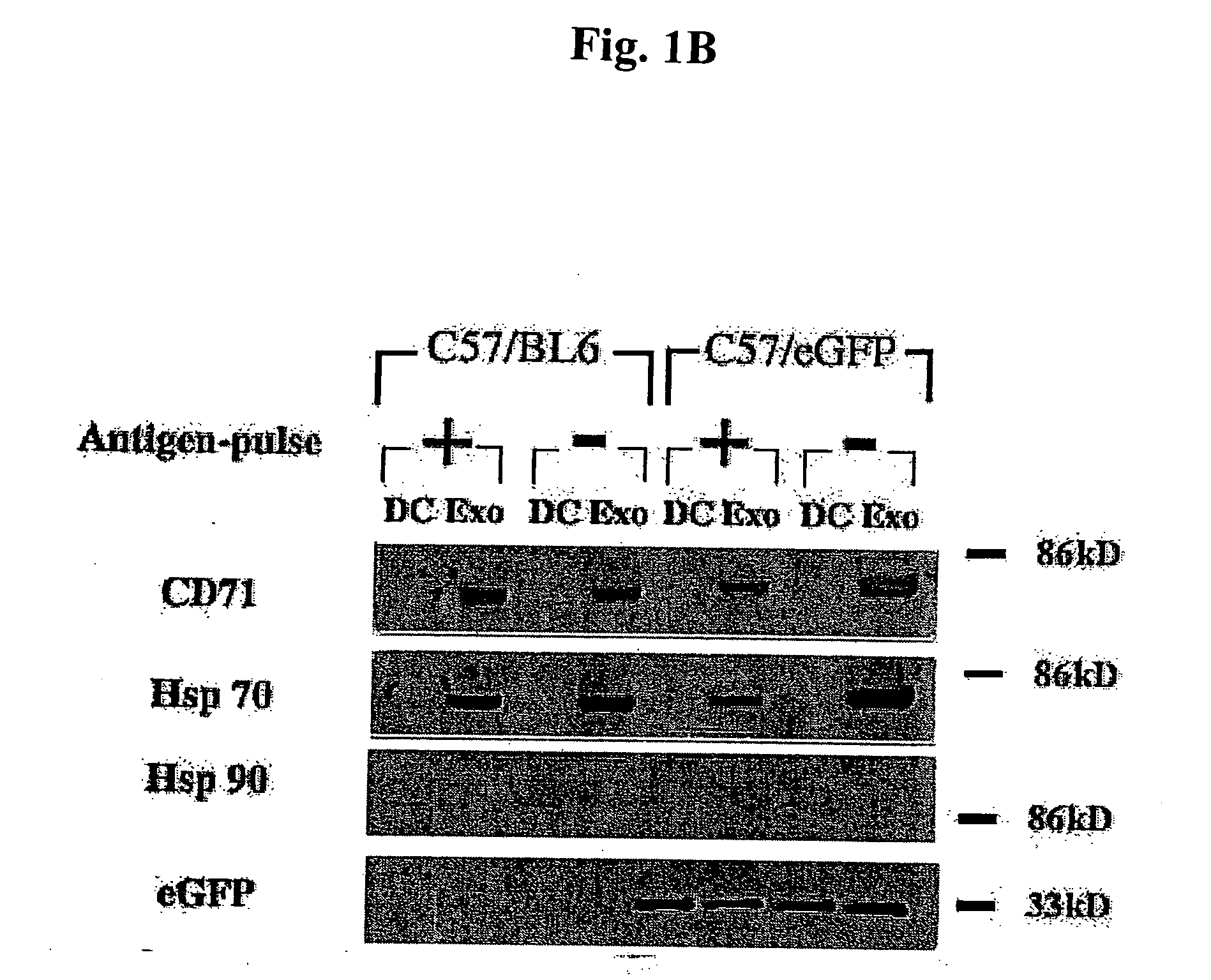
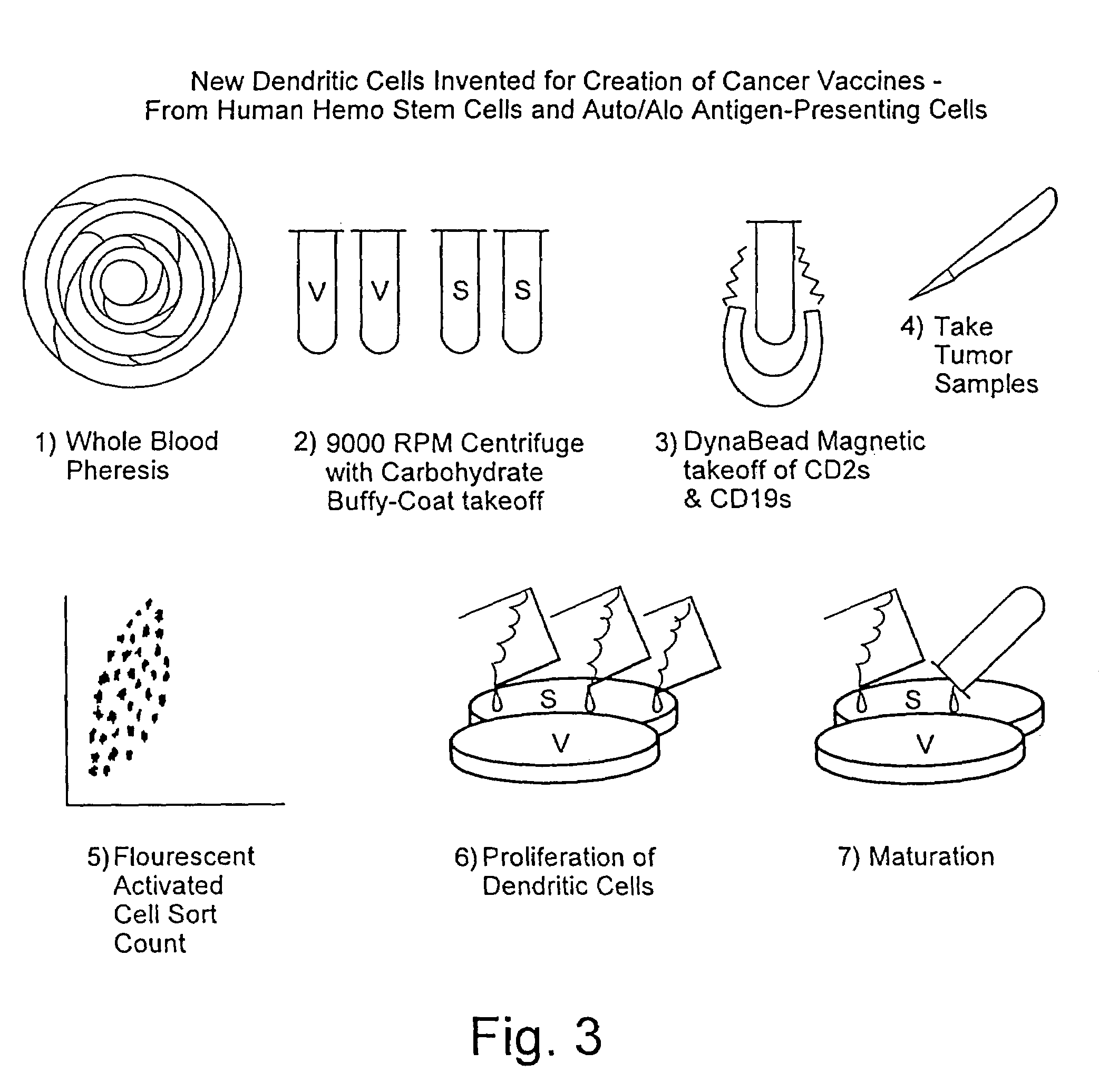
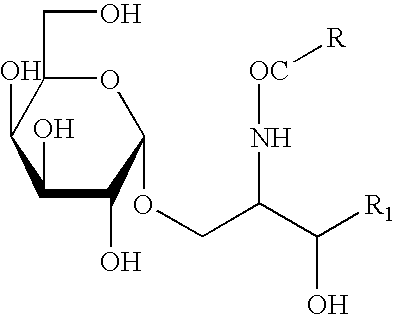
Patents
Literature
1435 results about "Dendritic cell" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
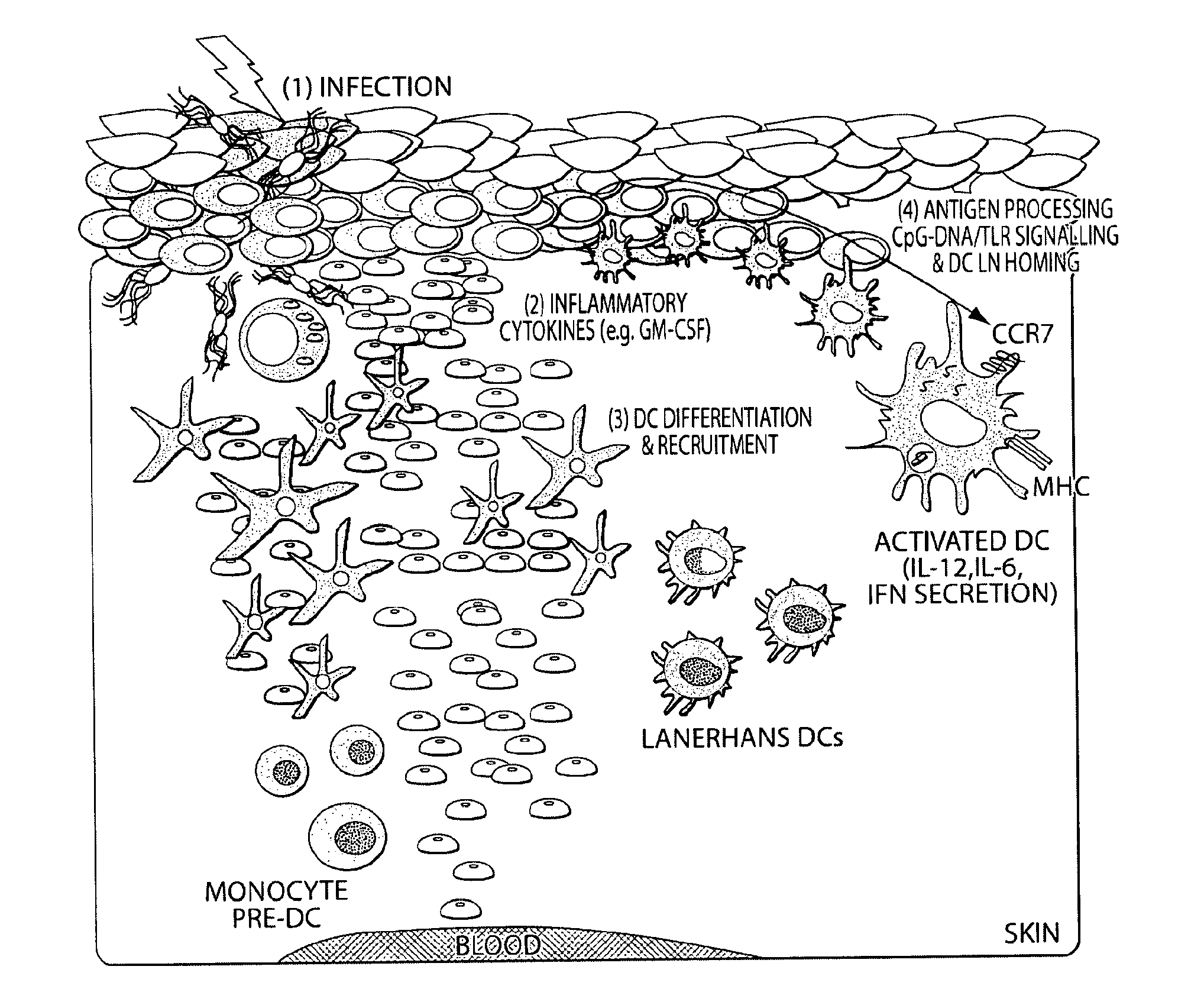
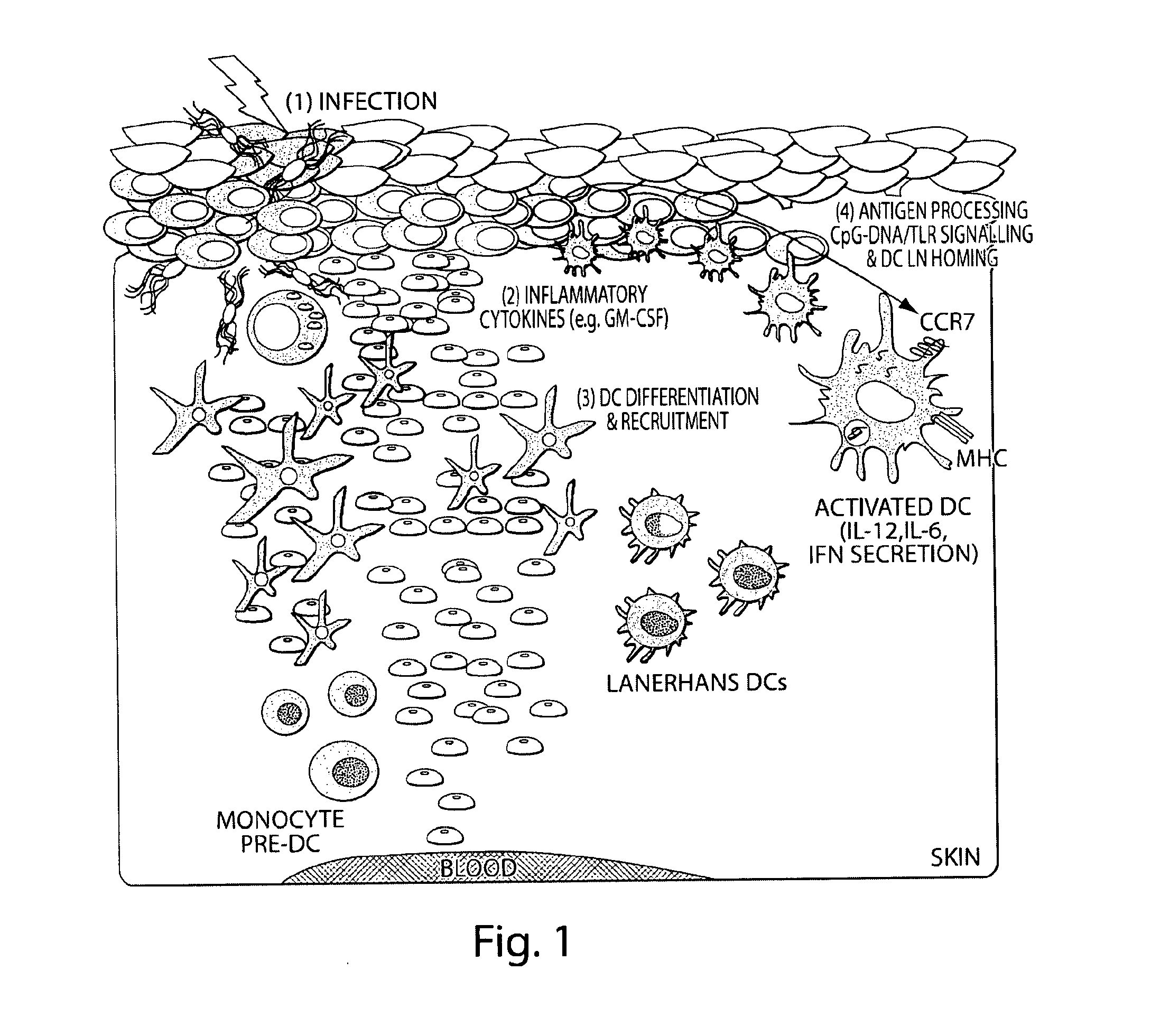
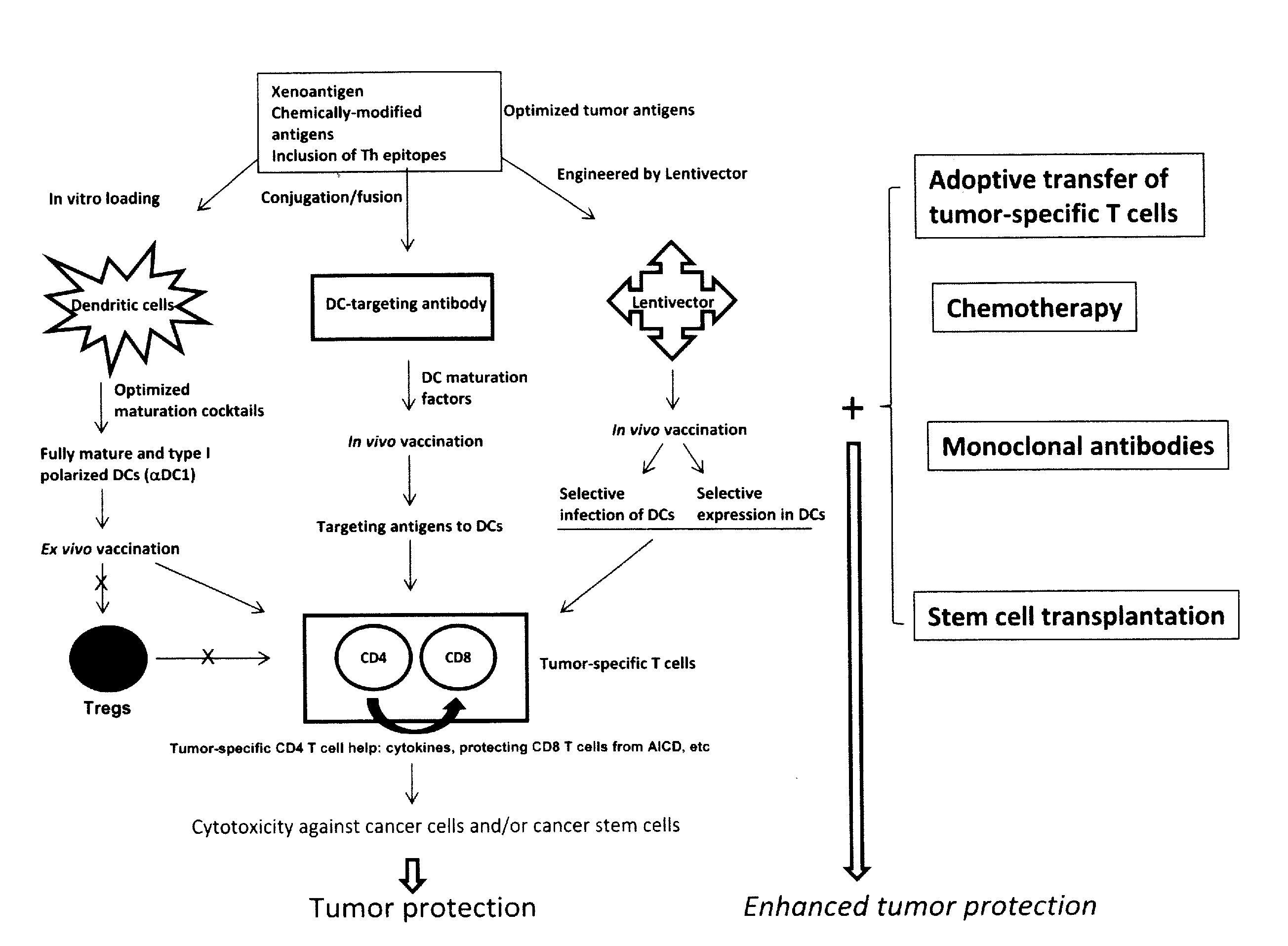
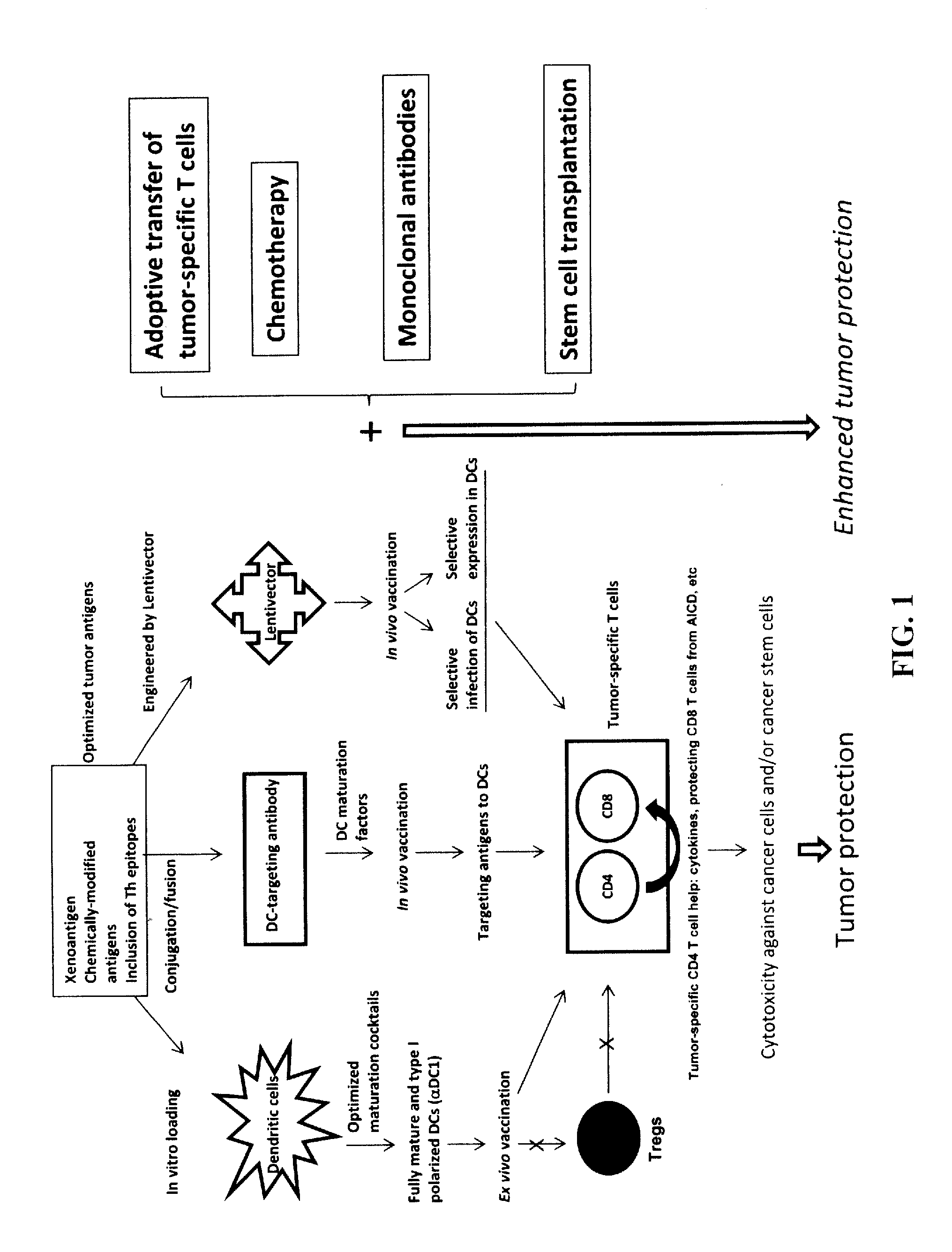
Dendritic cells (DCs) are antigen-presenting cells (also known as accessory cells) of the mammalian immune system. Their main function is to process antigen material and present it on the cell surface to the T cells of the immune system. They act as messengers between the innate and the adaptive immune systems.
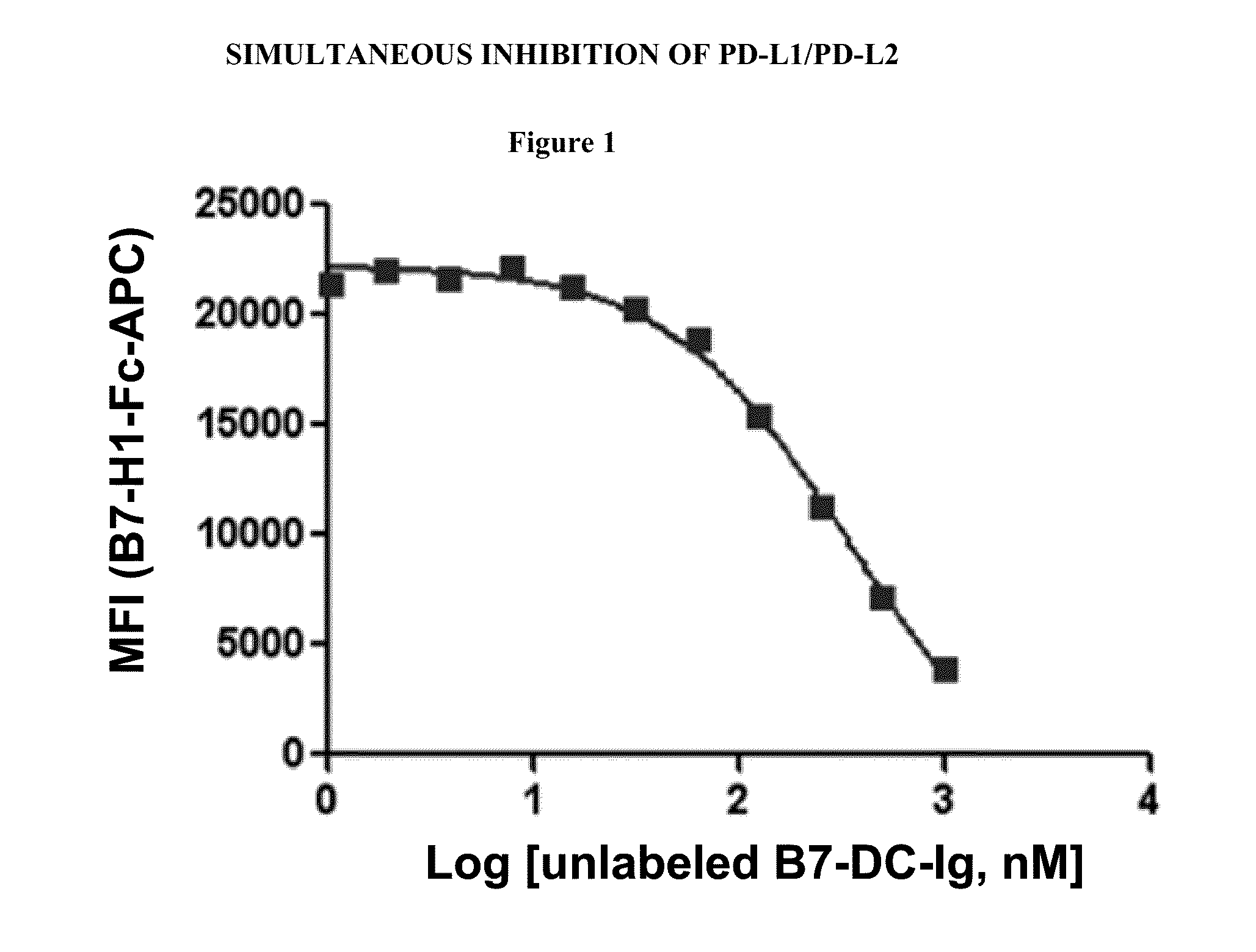
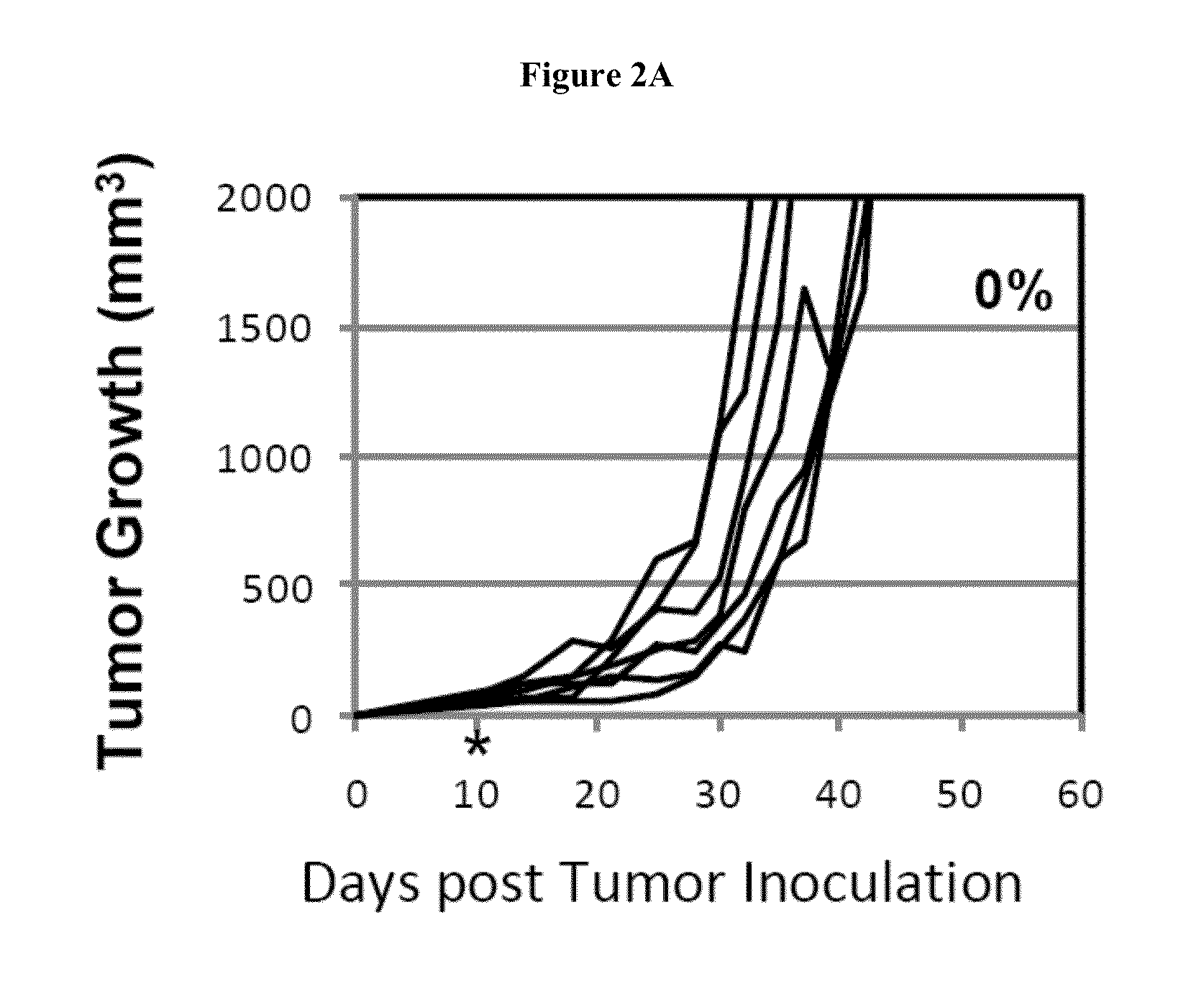
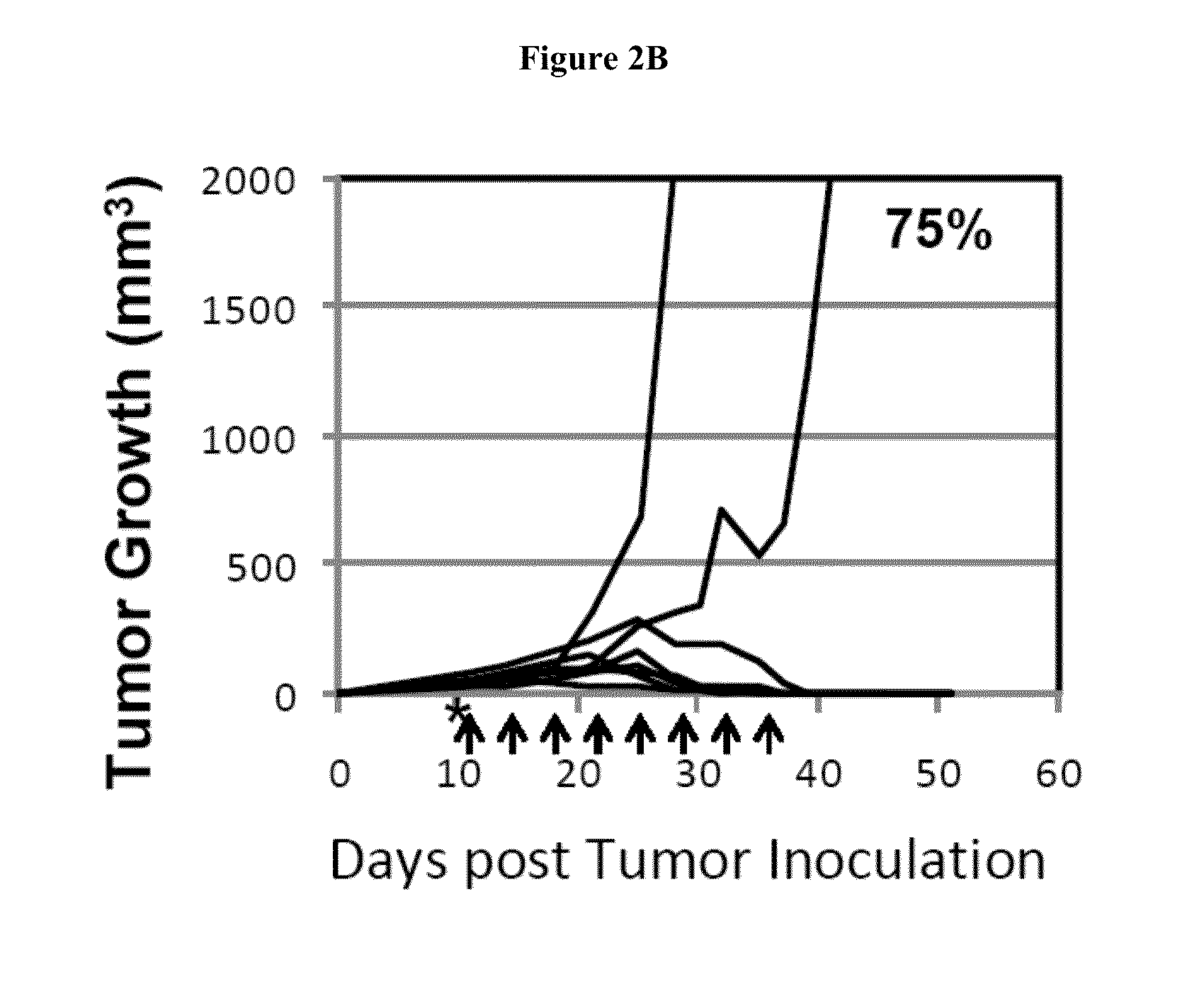
Simultaneous inhibition of pd-l1/pd-l2
InactiveUS20130017199A1Increase frequencyIncrease percentageAntibacterial agentsAntimycoticsDiseaseDendritic cell
Methods and compositions for treating an infection or disease that results from (1) failure to elicit rapid T cell mediated responses, (2) induction of T cell exhaustion, T cell anergy or both, or (3) failure to activate monocytes, macrophages, dendritic cells and / or other APCs, for example, as required to kill intracellular pathogens. The method and compositions solve the problem of undesired T cell inhibition by simultaneously inhibiting the PD-1 ligands, PD-L1 and PD-L2. The immune response can be modulated by providing antagonists which bind with different affinity, by varying the dosage of agent which is administered, by intermittent dosing over a regime, and combinations thereof, that provides for dissociation of agent from the molecule to which it is bound prior to being administered again. In some cases it may be particularly desirable to stimulate the immune system, then remove the stimulation.
Owner:AMPLIMMUNE
Compositions and methods for priming monocytic dendritic cells and t cells for th-1response
InactiveUS20050059151A1Mammal material medical ingredientsBlood/immune system cellsDendritic cellMonocyte
The present invention provides compositions and methods for inducing maturation of immature dendritic cells (DC) and for priming those cells for inducing a type 1 immune response. The present invention also provides dendritic cell populations useful for activating and for preparing T cells polarized towards production of type 1 cytokines and / or a type 1 response. Similarly, activated, polarized T cell populations, and methods of making the same are provided.
Owner:NORTHWEST BIOTHERAPEUTICS INC
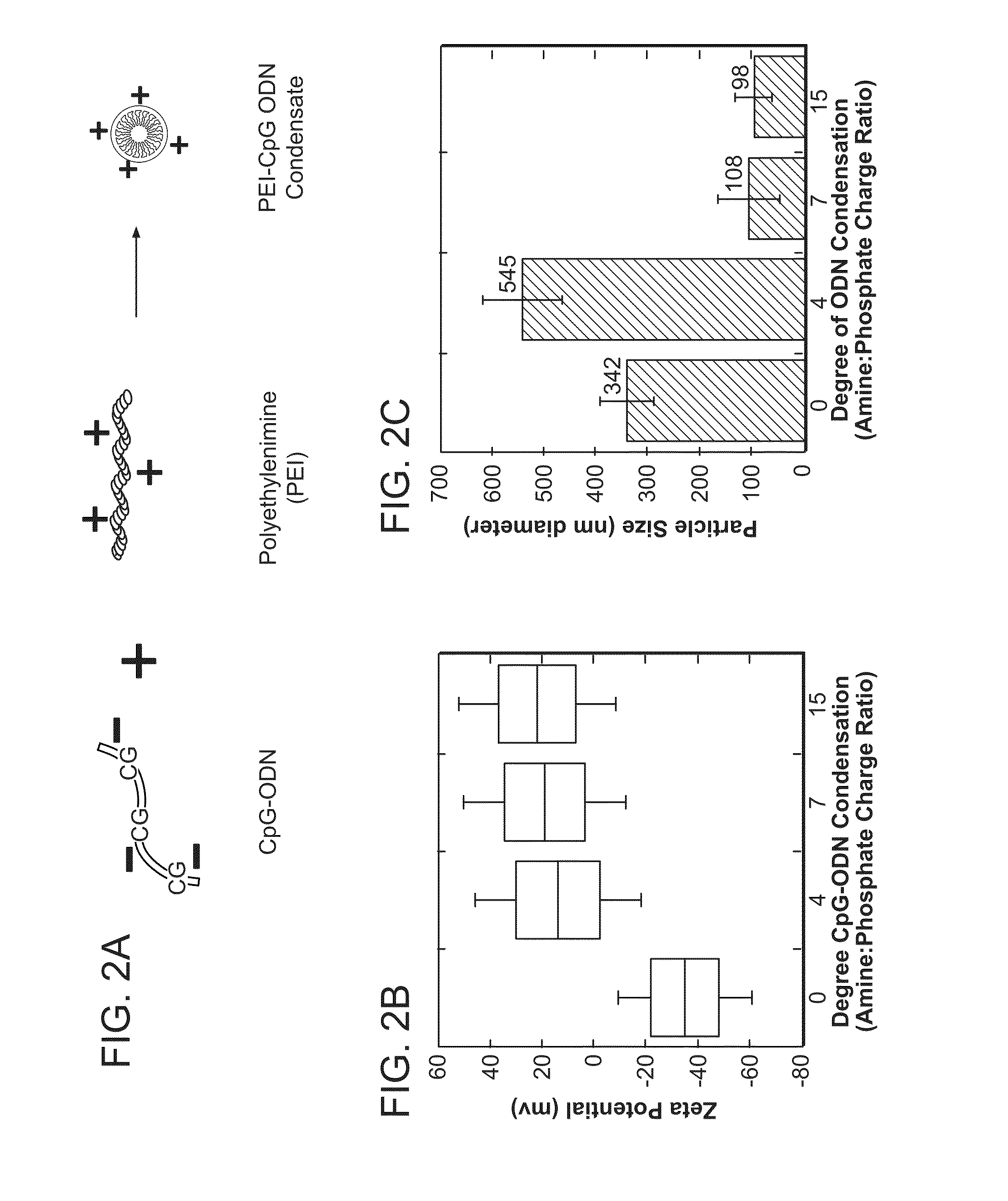
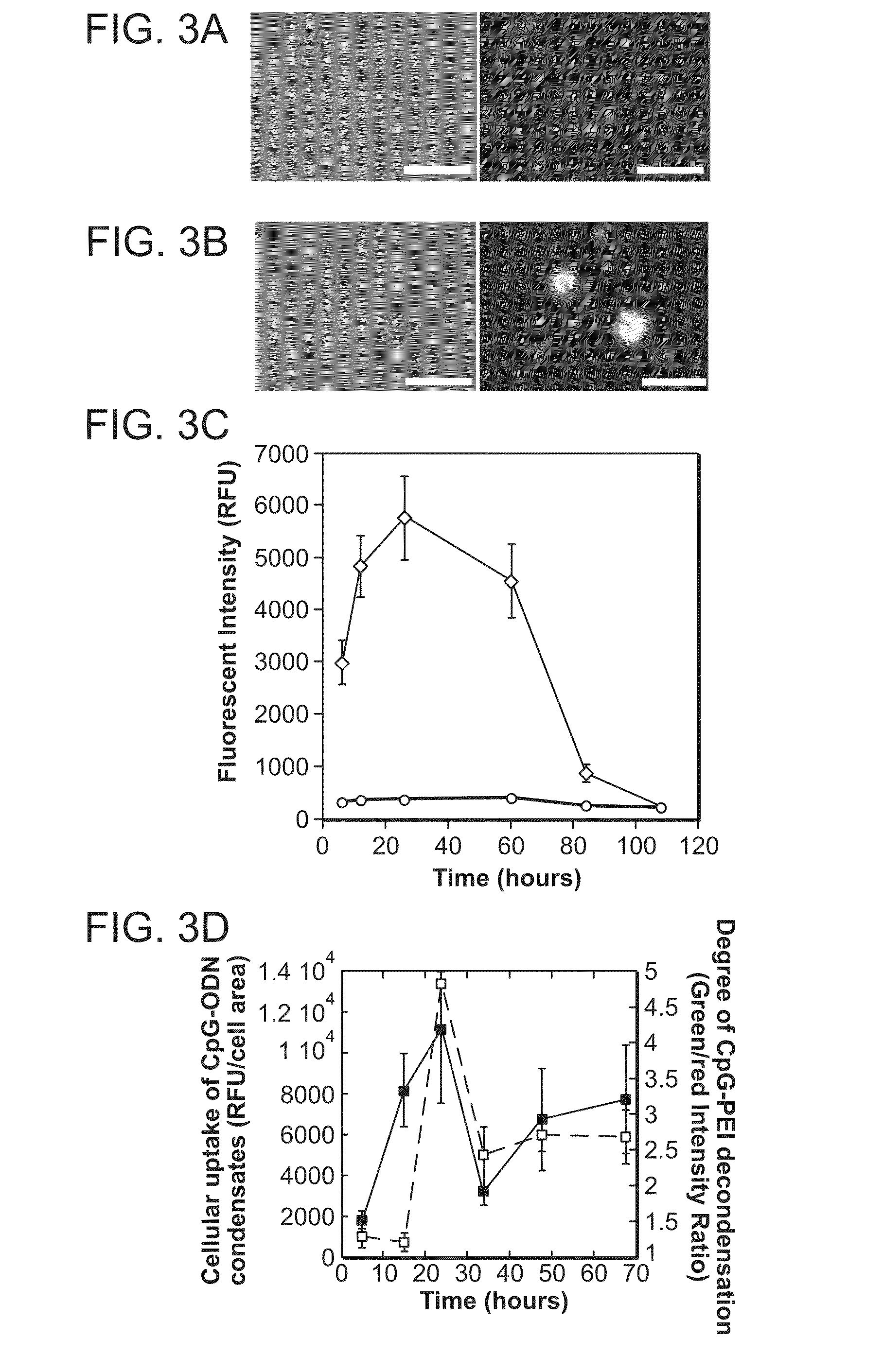
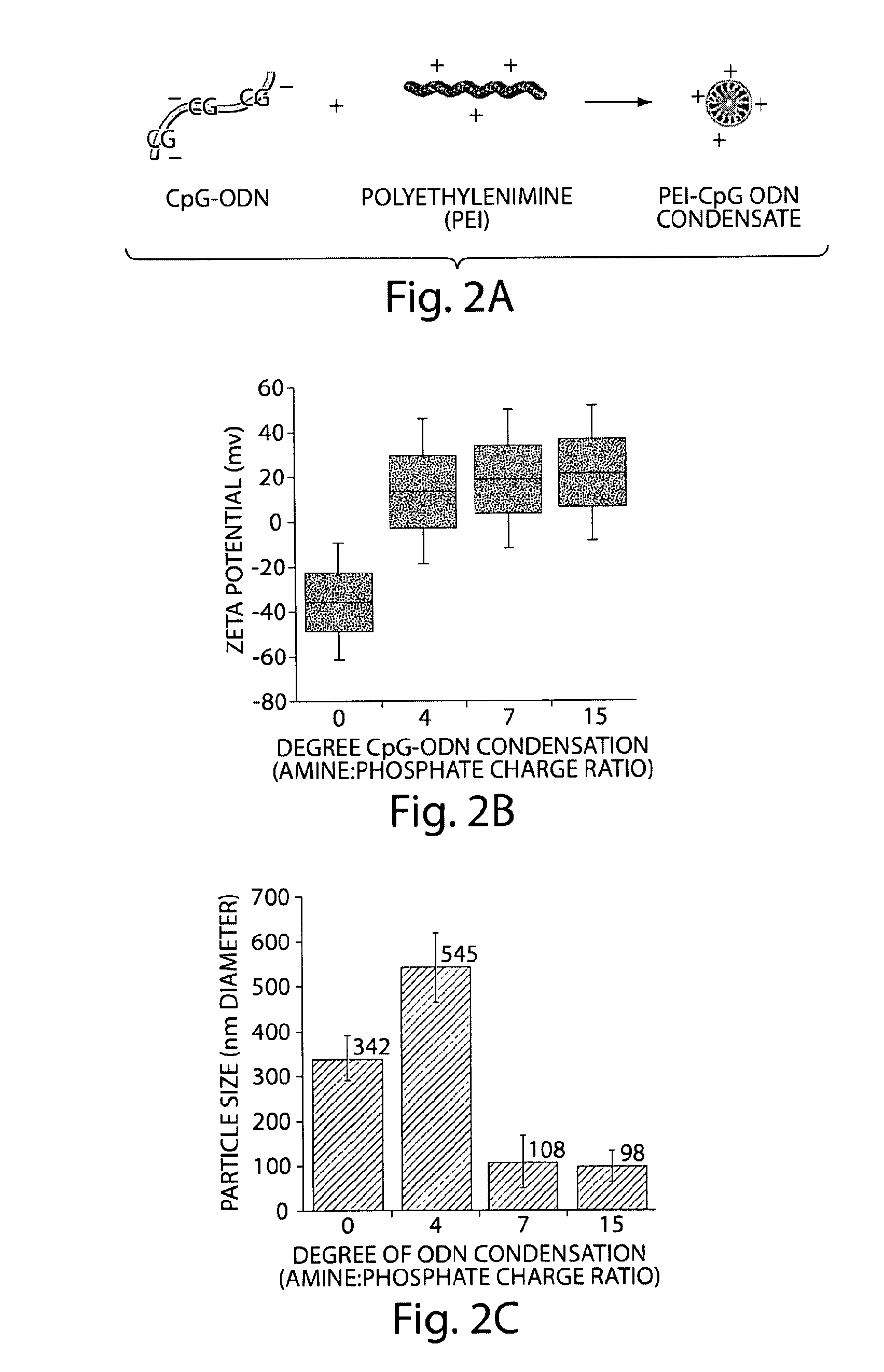
Immunostimulatory nucleic acid molecules for activating dendritic cells
InactiveUS20070065467A1Th responseEffective adjuvantOrganic active ingredientsSugar derivativesDendritic cellPyrimidine Nucleotides
The present invention relates generally to methods and products for activating dendritic cells. In particular, the invention relates to oligonucleotides which have a specific sequence including at least one unmethylated CpG dinucleotide which are useful for activating dendritic cells. The methods are useful for in vitro, ex-vivo, and in vivo methods such as cancer immunotherapeutics, treatment of infectious disease and treatment of allergic disease.
Owner:UNIV OF IOWA RES FOUND
Dock-and-lock (DNL) vaccines for cancer therapy
Owner:IBC PHARMACEUTICALS INC
Immunosuppressive exosomes
InactiveUS20060116321A1Suppress undesirable immune responseStimulate immune responseNervous disorderPeptide/protein ingredientsCytokine SuppressionDendritic cell
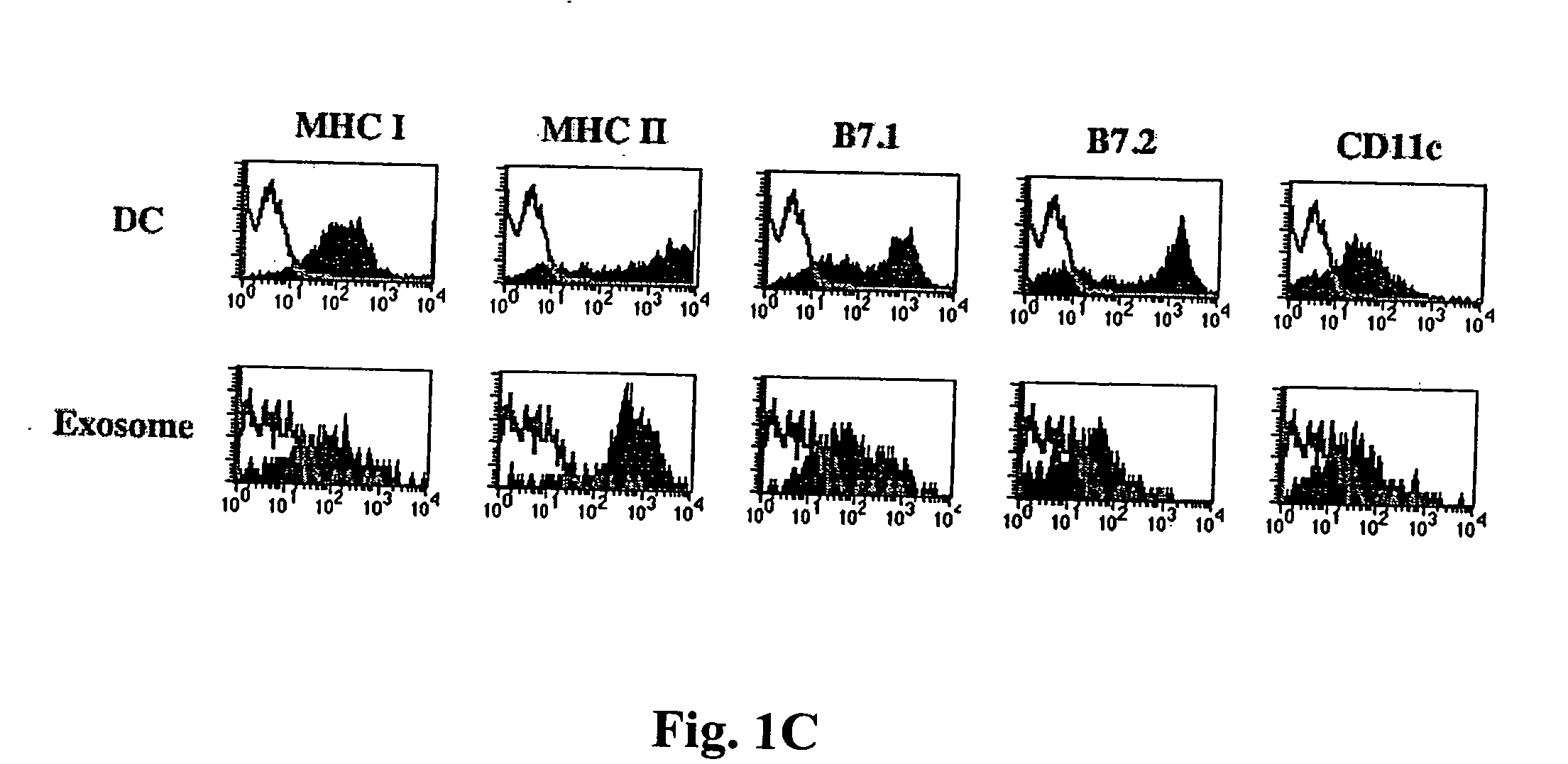
The present invention relates to methods and compositions for use in mediating an immunosuppressive reaction. The compositions of the invention comprise exosomes having immunosuppressive activity. Such exosomes may be derived from a variety of different cell types, including antigen presenting cells such as dendritic cells and macrophages. Prior to isolation of exosomes, the cells may be genetically engineered to express molecules capable of enhancing the immunosuppressive activity of said exosomes and / or may be exposed to one or more agents, such as cytokines or cytokine inhibitors, which are also capable of enhancing the immunosuppressive activity of exosomes. The present invention also relates to the use of such exosomes for the treatment of diseases and disorders associated with undesirable activation of the immune system. The present invention also includes exosomes isolated directly from serum that have been shown to be immunosuppressive.
Owner:PITTSBURGH UNIV OF THE +1
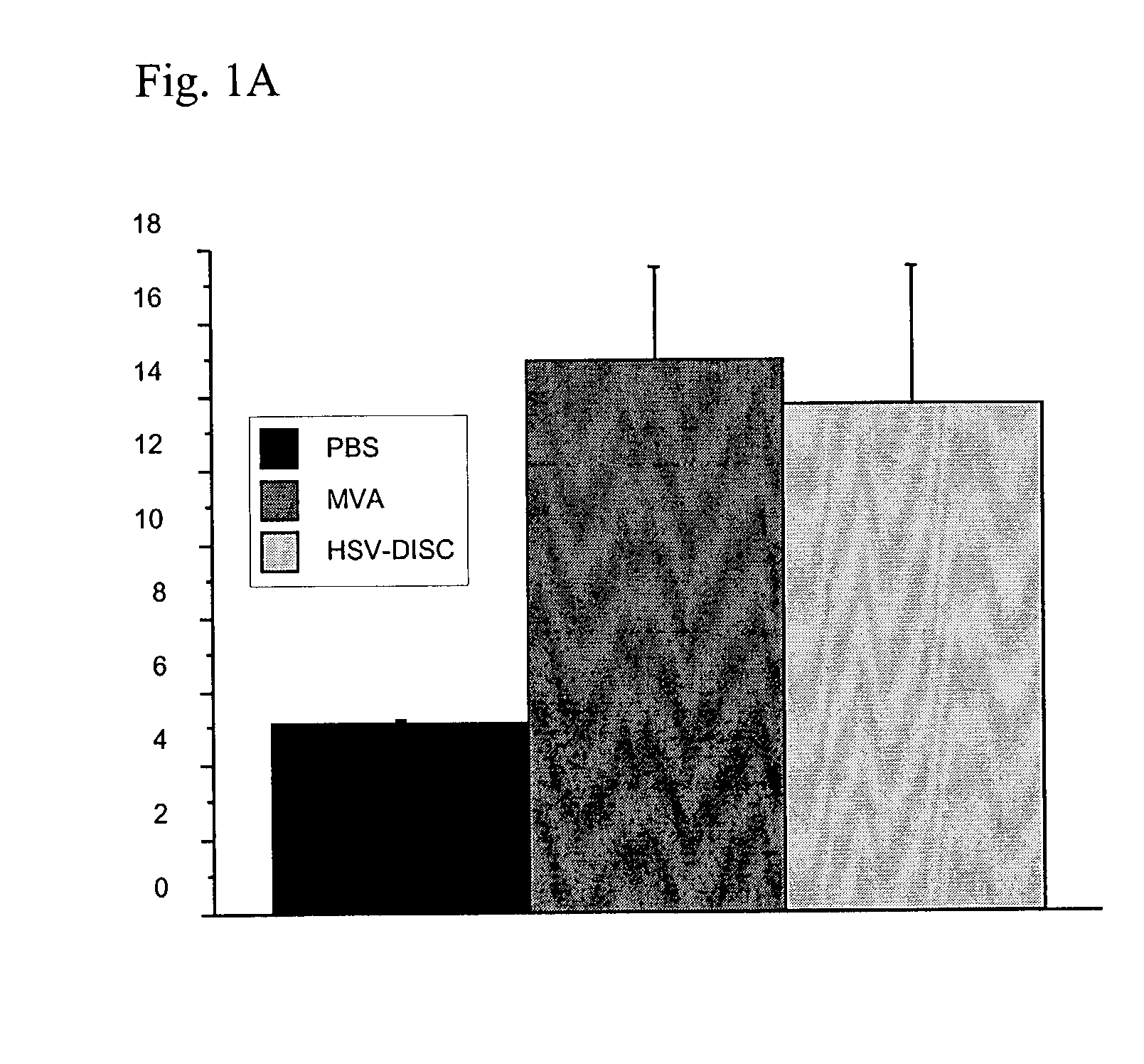
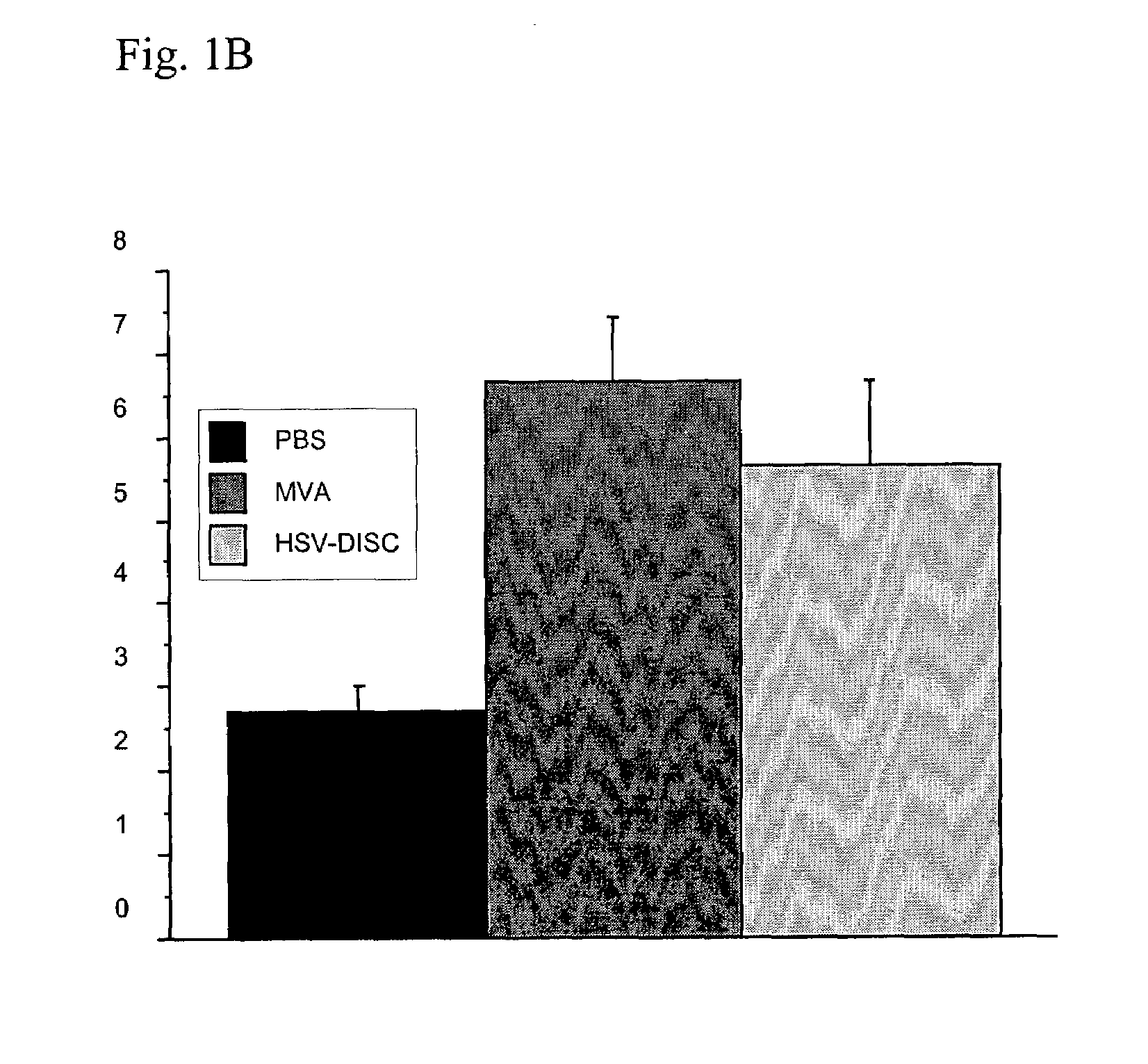
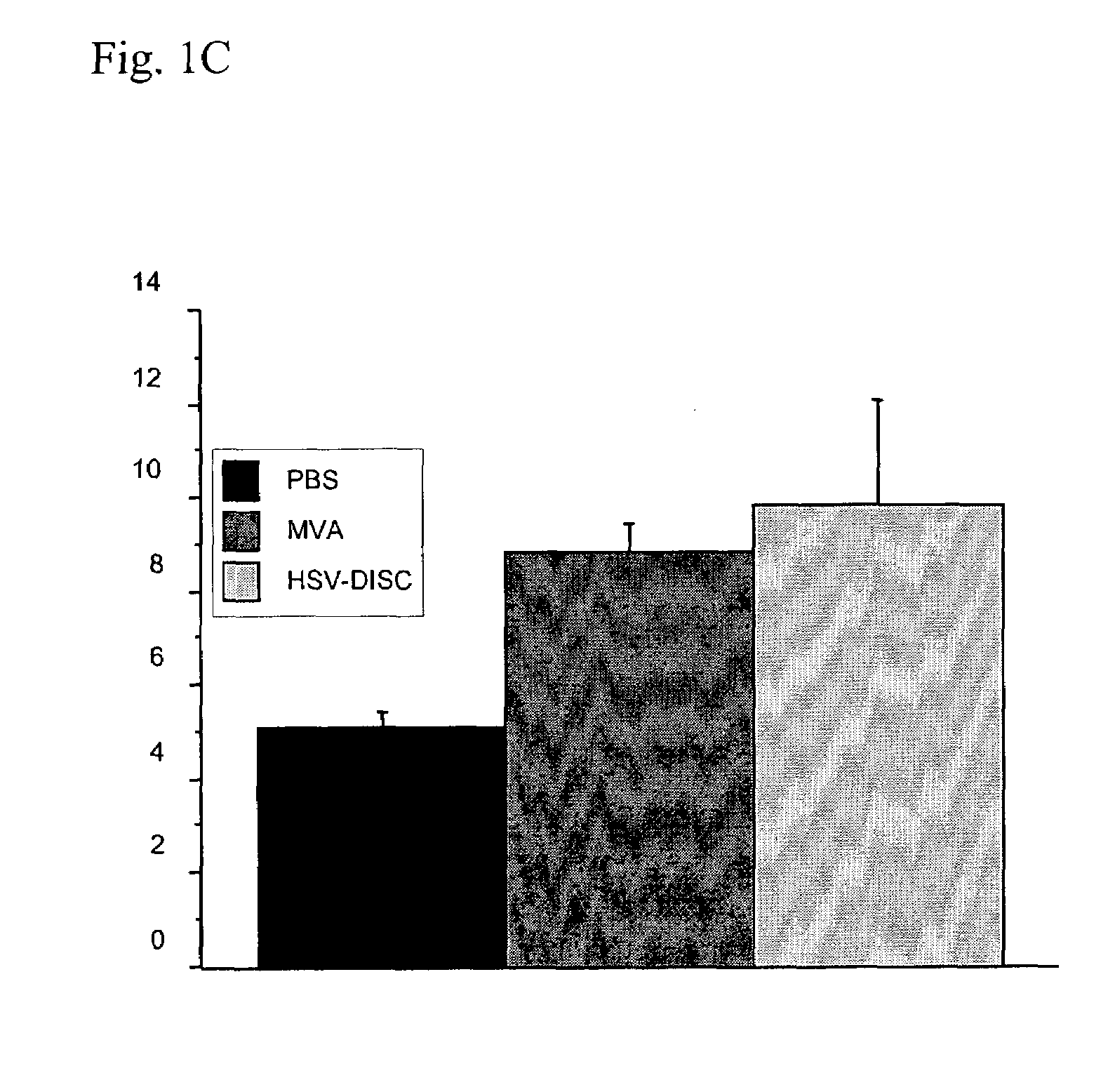
Modified vaccinia virus ankara for the vaccination of neonates
Owner:BAVARIAN NORDIC AS
Compounds for targeted immunotherapy
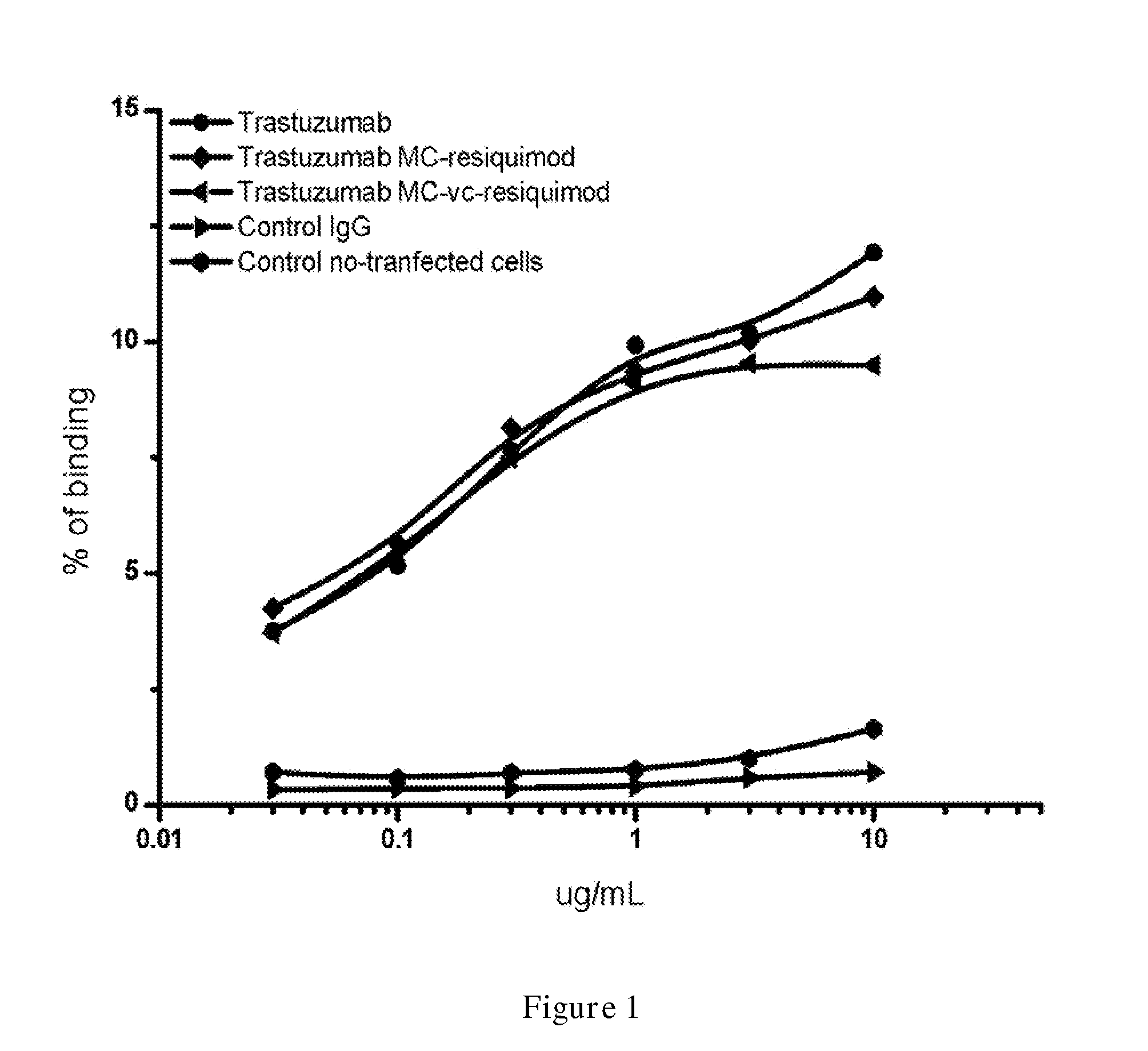
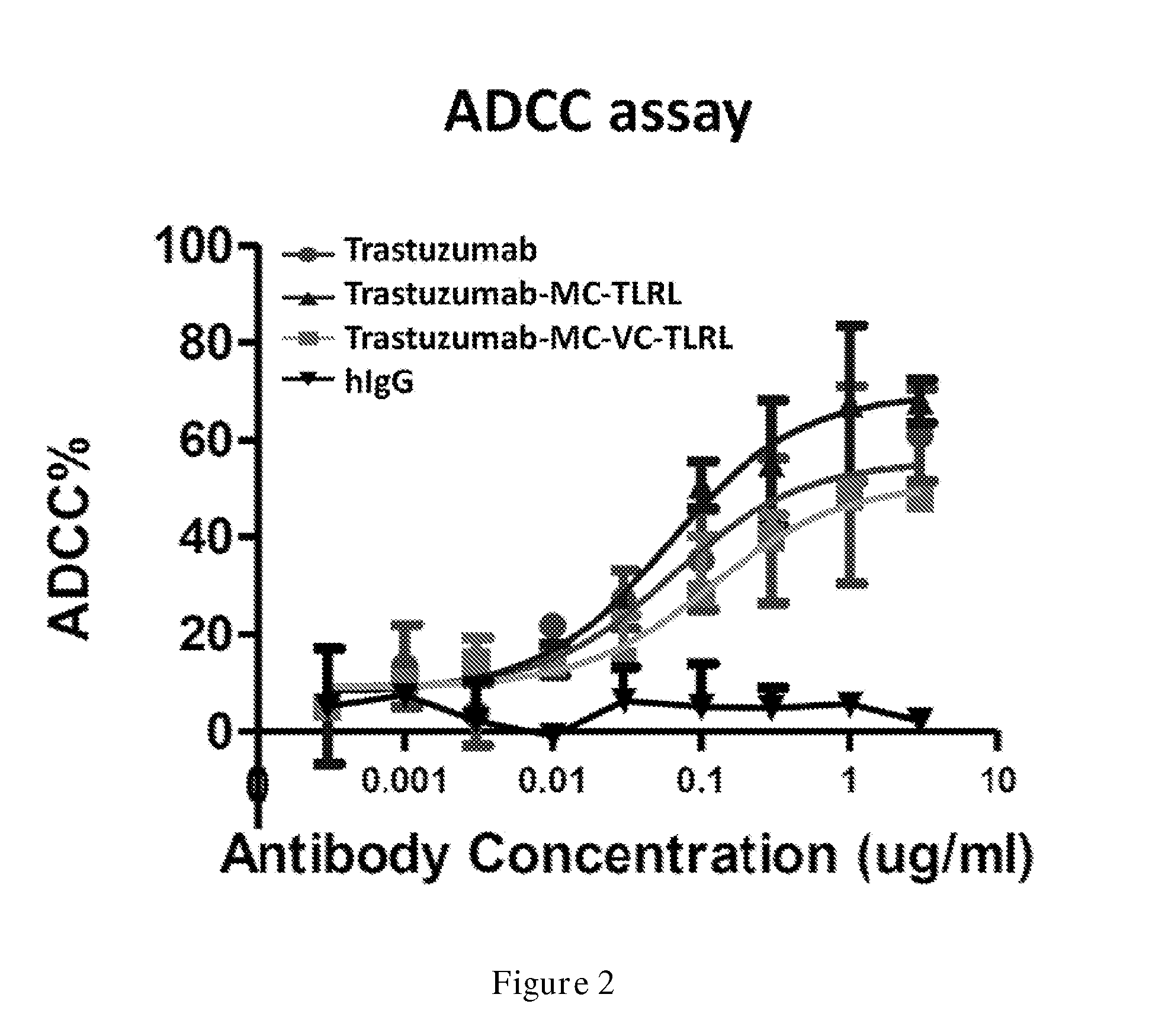
Compounds for targeted immunotherapy, compositions comprising the compounds and use of the compounds in the treatment of diseases such as cancer are disclosed. The compounds having the structure of formula TM-Ln-AM, wherein TM is a targeting moiety, AM is an activating moiety that is capable of activating a human dendritic cell, NK cell, or tumor cell, or a combination thereof, Ln is a linker, and n is an integer selected from 0 and 1.
Owner:BIRDIE BIOPHARM INC
Controlled Delivery of TLR Agonists in Structural Polymeric Devices
ActiveUS20130202707A1Increase successStimulate immune responsePowder deliveryOrganic active ingredientsTlr agonistsDendritic cell
The present invention comprises compositions, methods, and devices for creating an stimulating an antigen-specific dendritic cell immune response. Devices and methods provide prophylactic and therapeutic immunity to subjects against cancer and infectious agents.
Owner:DANA FARBER CANCER INST INC +1
Recombinant vector expressing multiple costimulatory molecules and uses thereof
InactiveUS7211432B2Enhance immune responseFacilitated DiffusionBiocideGenetic material ingredientsDendritic cellBiological activation
The present invention is a recombinant vector encoding and expressing at least three or more costimulatory molecules. The recombinant vector may additionally contain a gene encoding one or more target antigens or immunological epitope thereof. The synergistic effect of these costimulatory molecules on the enhanced activation of T cells is demonstrated. The degree of T-cell activation using recombinant vectors containing genes encoding three costimulatory molecules was far greater than the sum of recombinant vector constructs containing one costimulatory molecule and greater than the use of two costimulatory molecules. Results employing the triple costimulatory vectors were most dramatic under conditions of either low levels of first signal or low stimulator to T-cell ratios. This phenomenon was observed with both isolated CD4+ and CD8+ T cells. The recombinant vectors of the present invention are useful as immunogenes and vaccines against cancer and pathogenic micro-organisms, and in providing host cells, including dendritic cells and splenocytes with enhanced antigen-presenting functions.
Owner:UNITED STATES OF AMERICA
Induced activation in dendritic cell
ActiveUS7404950B2Enhance and regulate immune responseVirusesPeptide/protein ingredientsDiseaseDendritic cell
Owner:BAYLOR COLLEGE OF MEDICINE
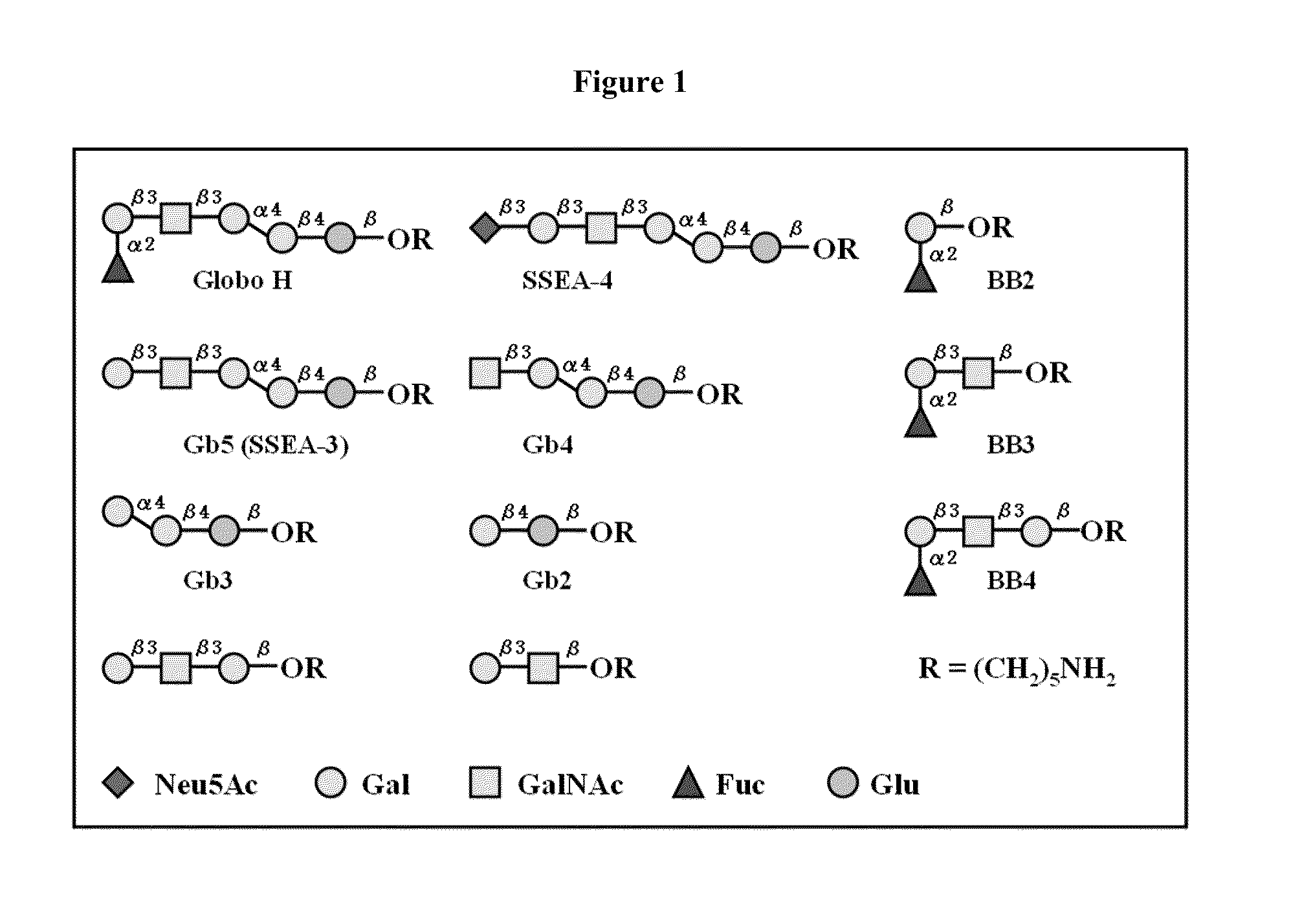
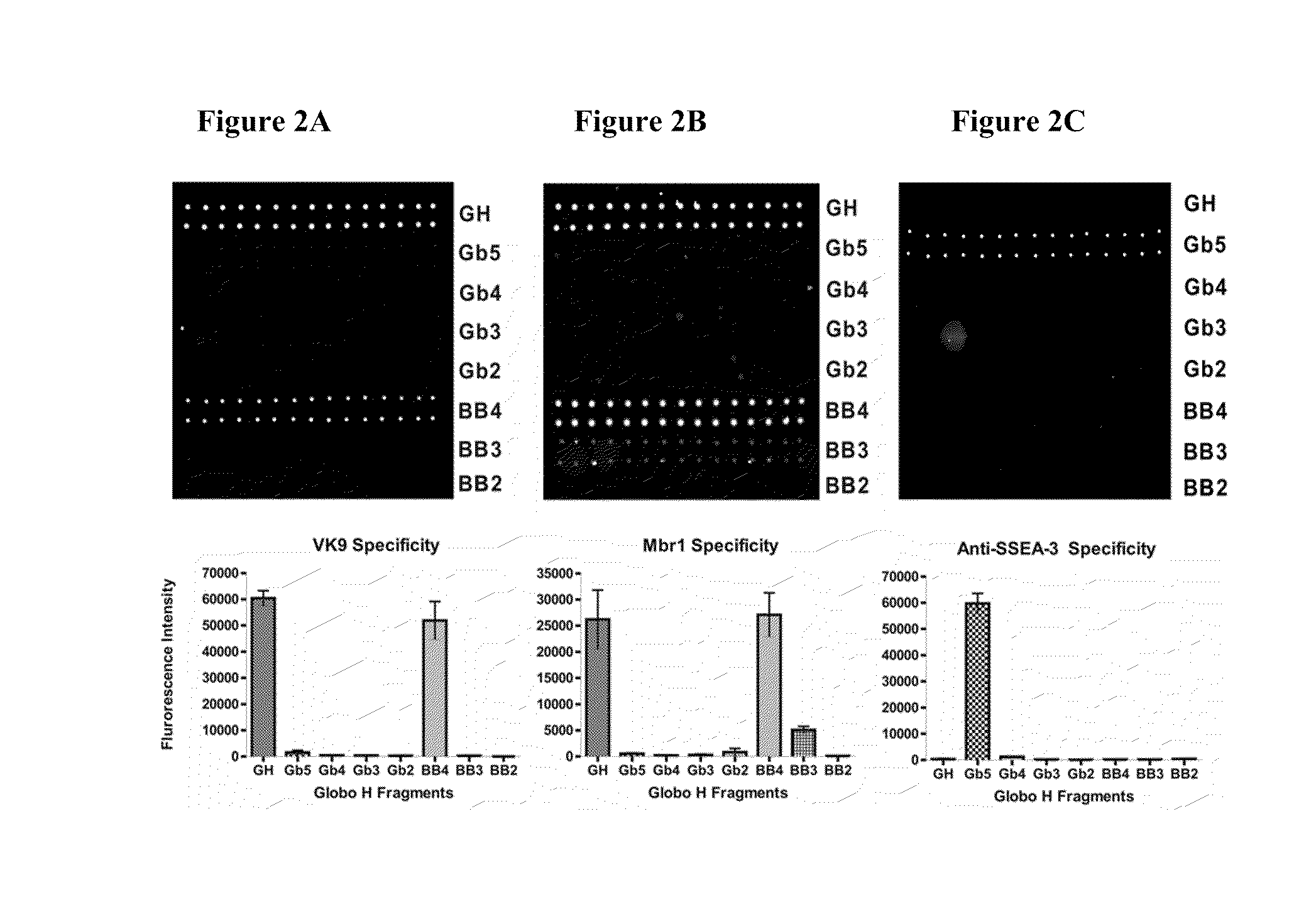
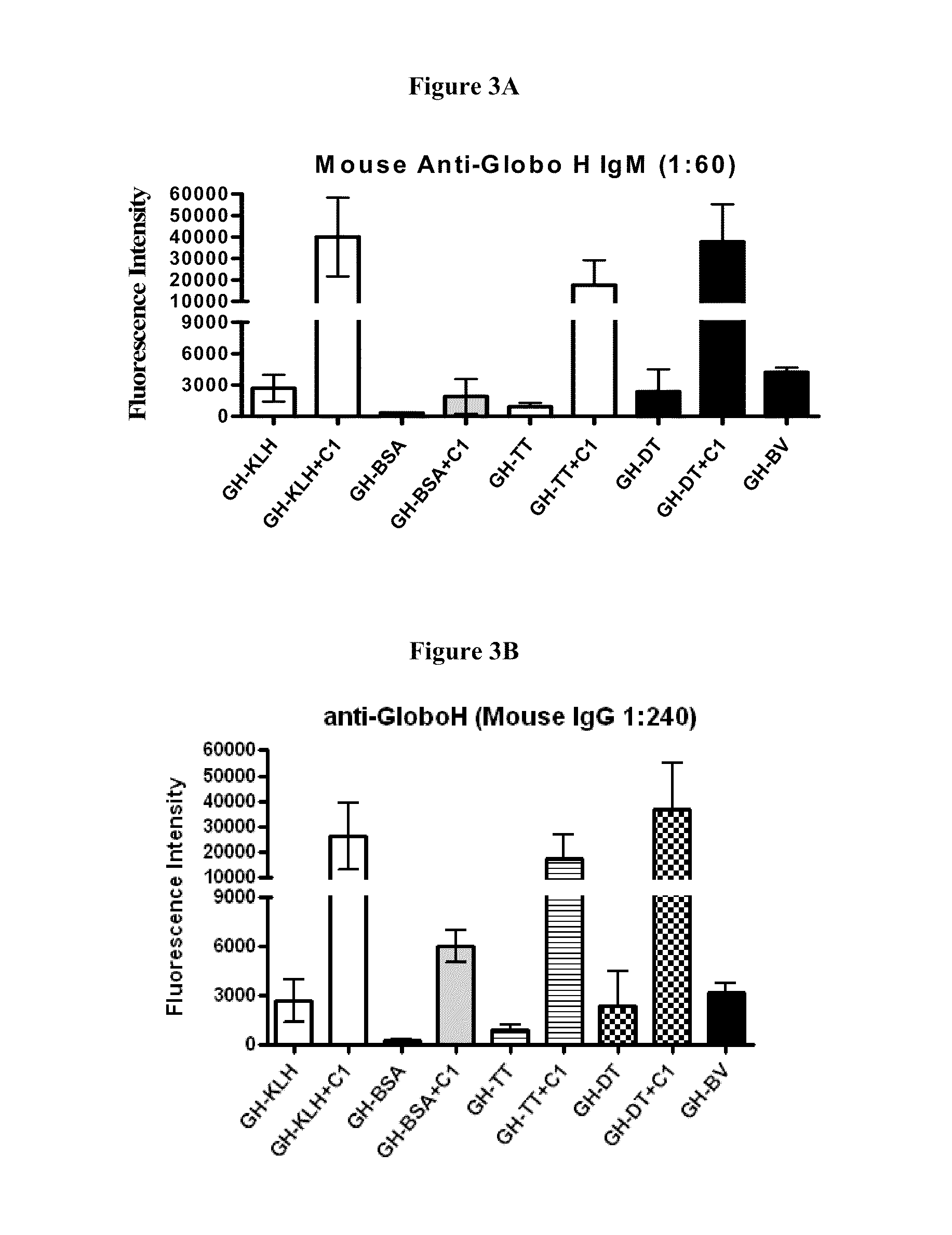
Globo h and related Anti-cancer vaccines with novel glycolipid adjuvants
ActiveUS20100136042A1Shrink tumorInhibit tumor growthOrganic active ingredientsSugar derivativesDendritic cellAdjuvant
Owner:ACAD SINIC
Continuous Cell Programming Devices
ActiveUS20120100182A1Easy to controlClinical effectiveness of has been limitedOrganic active ingredientsPeptide/protein ingredientsDendritic cellProtective immunity
The present invention comprises compositions, methods and devices for creating an infection-mimicking environment within a polymer scaffold to stimulate antigen-specific dendritic cell activation. Devices of the present invention are used to provide protective immunity to subjects against infection and cancer.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE +1
Method for stimulating an immune response
InactiveUS6977073B1Stimulating directed immune responseStimulate immune responseBiocideArtificial cell constructsAbnormal tissue growthHematopoietic cell
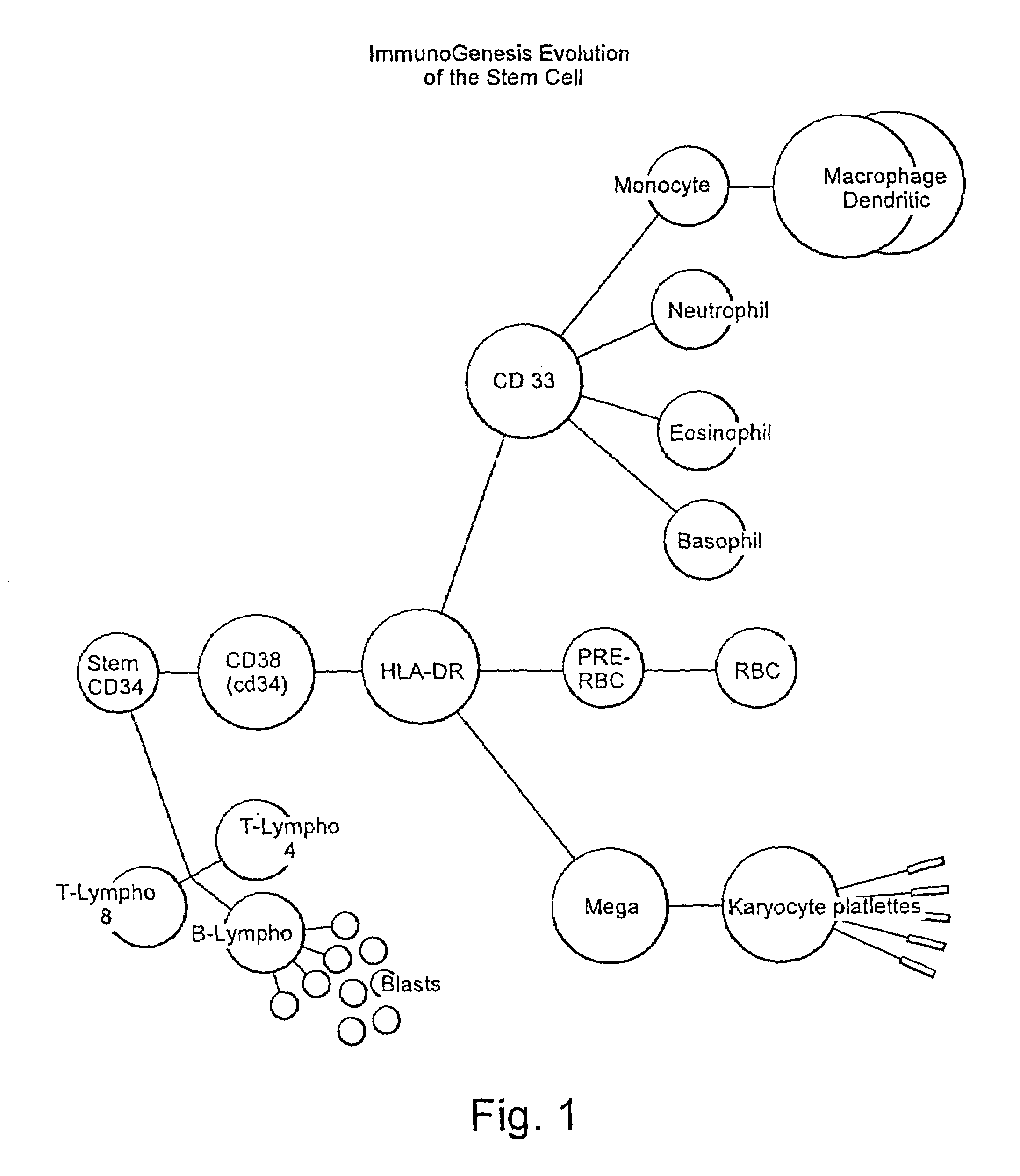
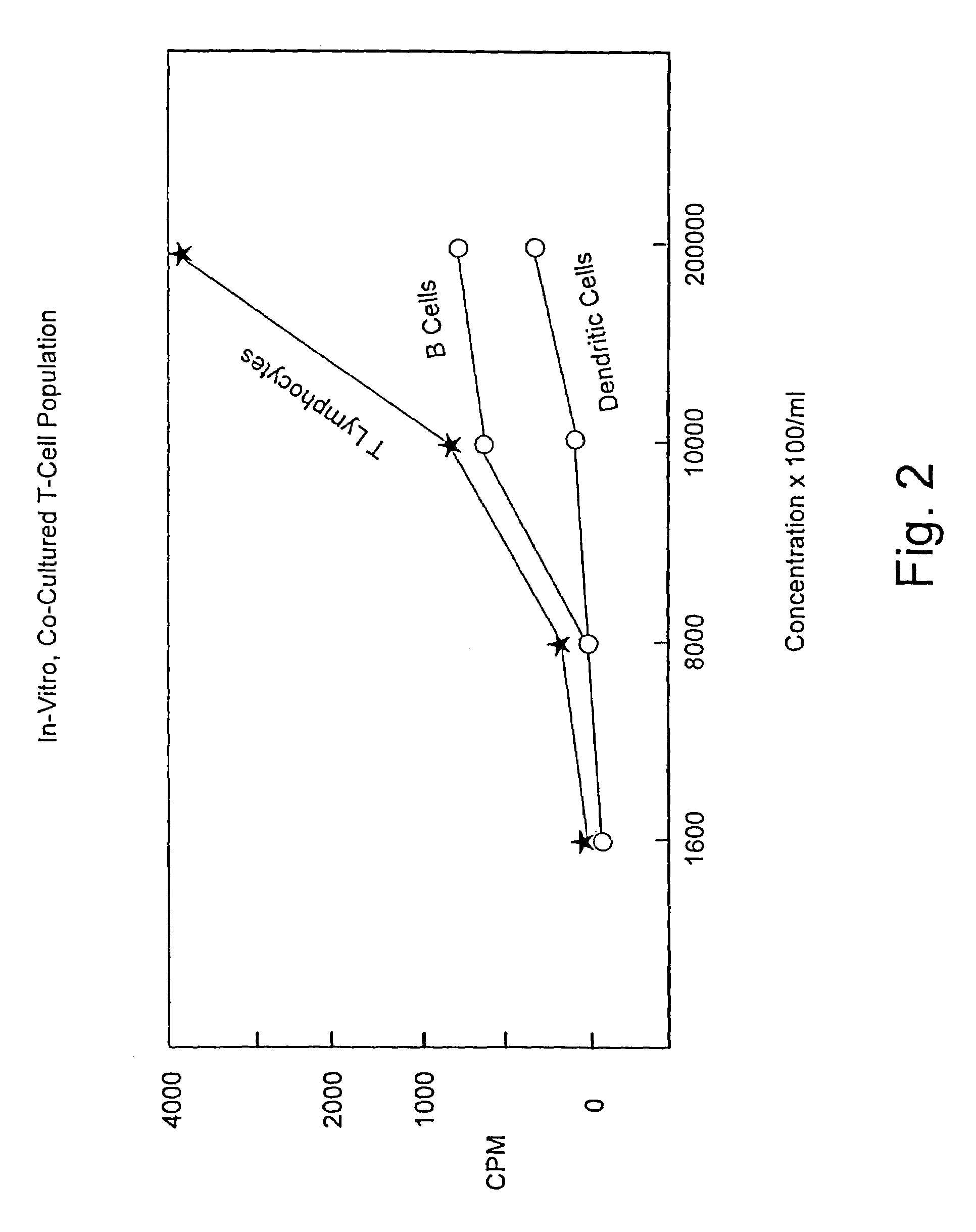
A method is described whereby dendritic cells derived from the CD34+ and CD 34−hematopoietic cell lineages are directed to become programmable antigen presenting cells. The programmed cells may be pulsed with tumor cell RNA or tumor cell RNA expression products. The protocol provides for directing the maturation of dendritic cells to become antigen presenting cells. The protocol further provides for isolating tumor cell RNA from biopsy material that has been prepared in paraffin block storage. The directed dendritic cell is provided with a plurality of tumor markers by using tumor RNA in toto, the poly A+RNA fraction or the expression product of such RNA. Once activated the dendritic cells are incubated with T4 and T8 lymphocytes to stimulate and sensitize the T lymphocytes which upon introduction either into a donor host or a nondonor recipient will provide immune response protection.
Owner:CEZAYIRLI CEM
Recombinant vector expressing multiple costimulatory molecules and uses thereof
InactiveUS6969609B1Enhance immune responseFacilitated DiffusionBiocideGenetic material ingredientsDendritic cellBiological activation
The present invention is a recombinant vector encoding and expressing at least three or more costimulatory molecules. The recombinant vector may additionally contain a gene encoding one or more target antigens or immunological epitope thereof. The synergistic effect of them costimulatory molecules on the enhanced activation of T cells is demonstrated. The degree of T-cell activation using recombinant vectors containing genes encoding three costimulatory molecules was far greater than the sum of recombinant vector constructs containing one costimulatory molecule and greater that the use of two costimulatory molecules. Results employing the triple costimulatory vectors were most dramatic under conditions of either low levels of first signal or low stimulator to T-cell ratios. This phenomenon was observed with both isolated CD4+and CD8+T cells. The recombinant vectors of the present invention are useful as immunogenes and vaccines against cancer and pathogenic micro-organisms, and in providing host cells, including dendritic cells and splenocytes with enhanced and antigen-presenting functions.
Owner:THE GOVERMENT OF THE UNITED STATES OF AMERICA REPRESENTED BY THE SEC DEPT OF HEALTH & HUMAN SERVICES (SEE PF37)
Novel Strategies for Improved Cancer Vaccines
The present invention concerns methods and compositions for forming anti-cancer vaccine complexes. In preferred embodiments, the anti-cancer vaccine complex comprises an antibody moiety that binds to dendritic cells, such as an anti-CD74 antibody or antigen-binding fragment thereof, attached to an AD (anchoring domain) moiety and a xenoantigen, such as CD20, attached to a DDD (dimerization and docking domain) moiety, wherein two copies of the DDD moiety form a dimer that binds to the AD moiety, resulting in the formation of the vaccine complex. The anti-cancer vaccine complex is capable of inducing an immune response against xenoantigen expressing cancer cells, such as CD138negCD20+ MM stem cells, and inducing apoptosis of and inhibiting the growth of or eliminating the cancer cells.
Owner:IBC PHARMACEUTICALS INC
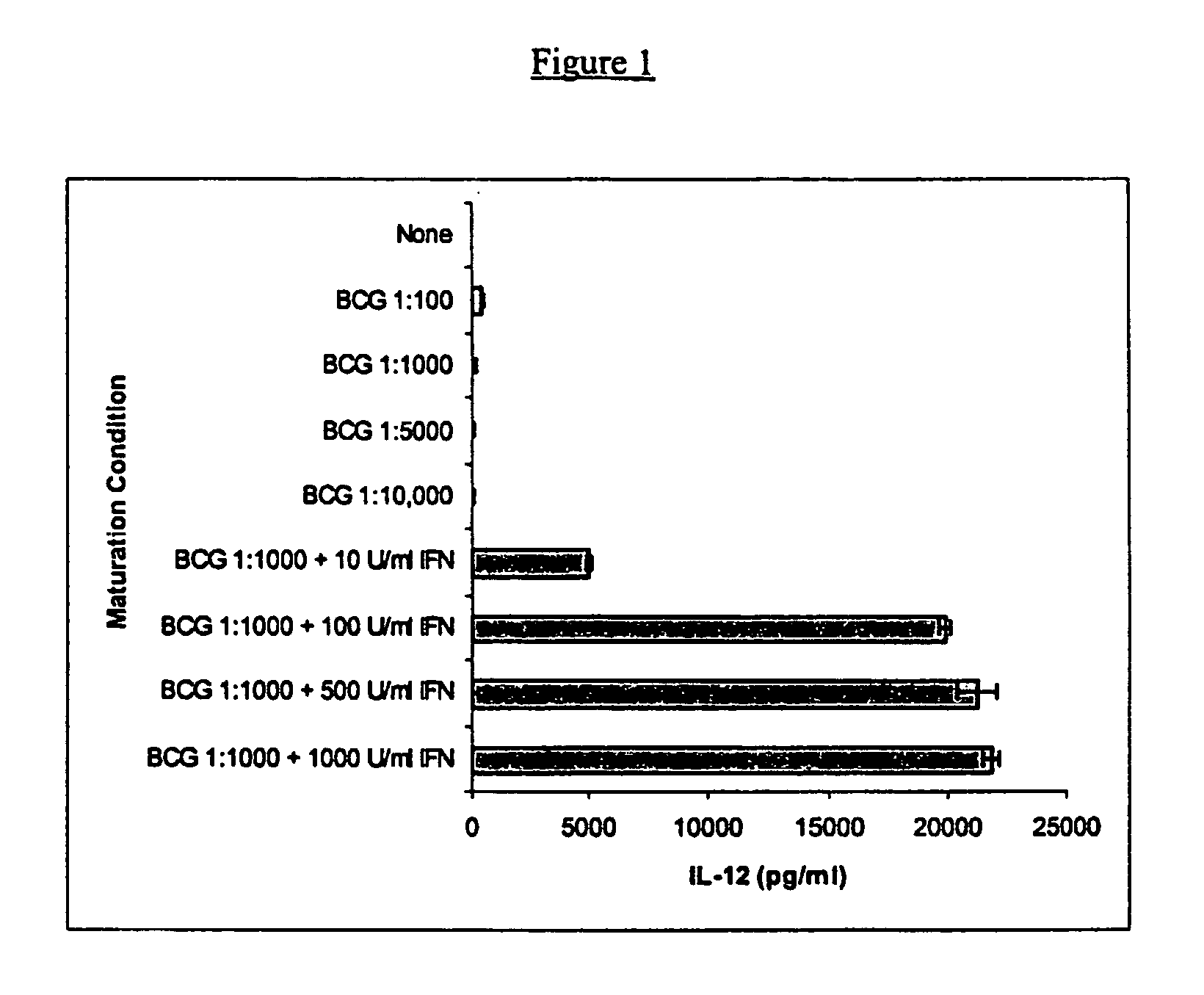
Mature dendritic cell compositions and methods for culturing same
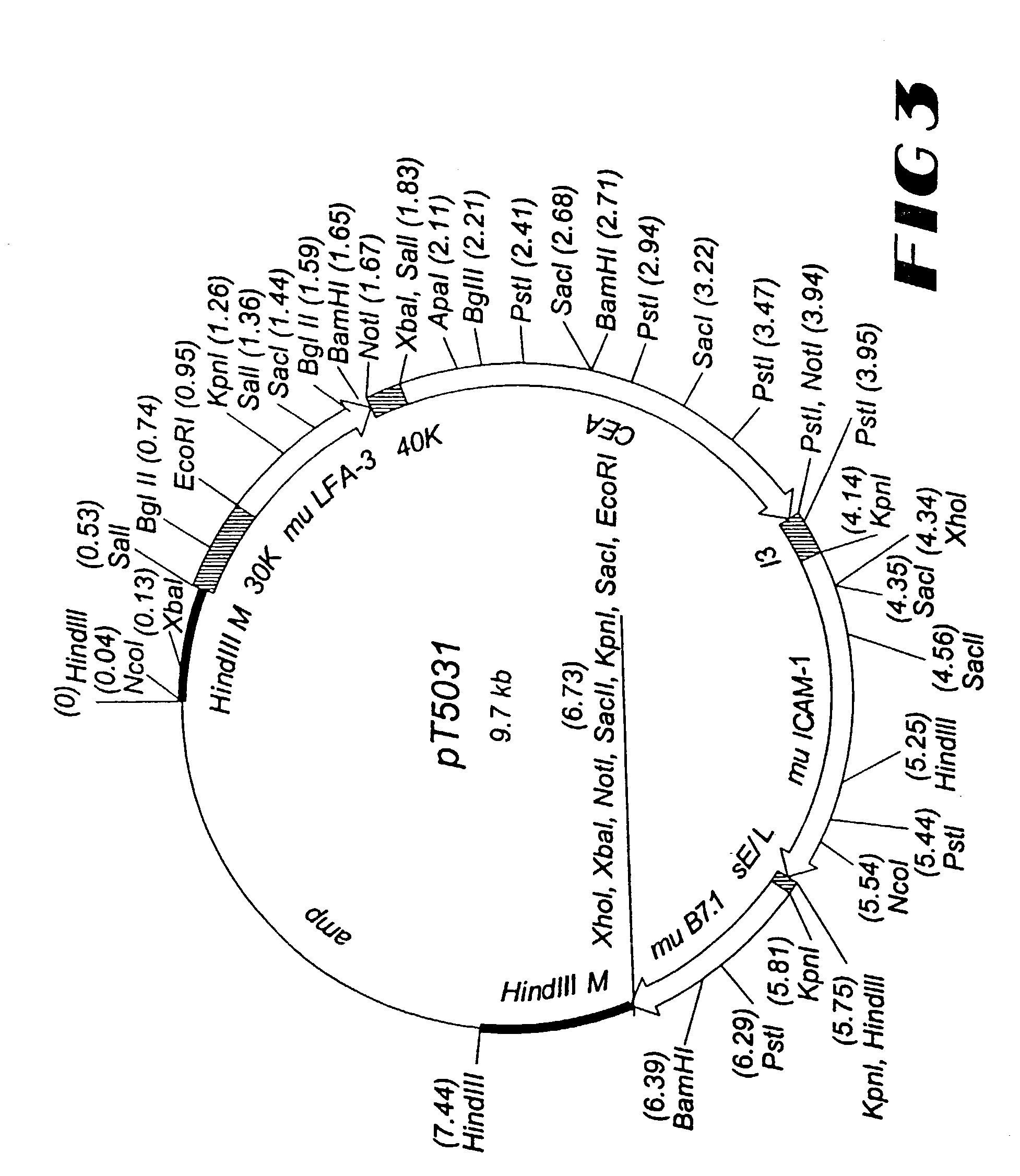
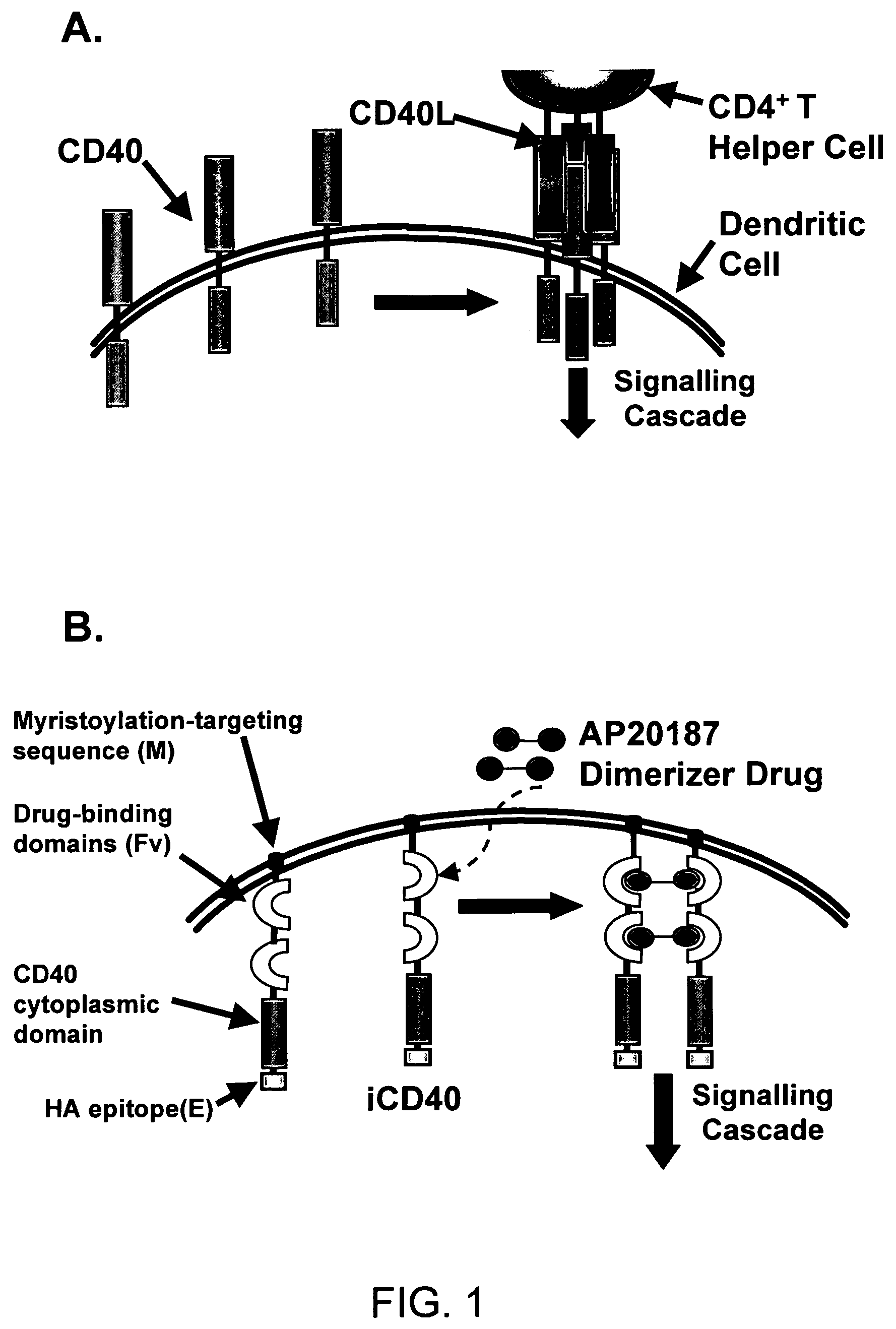
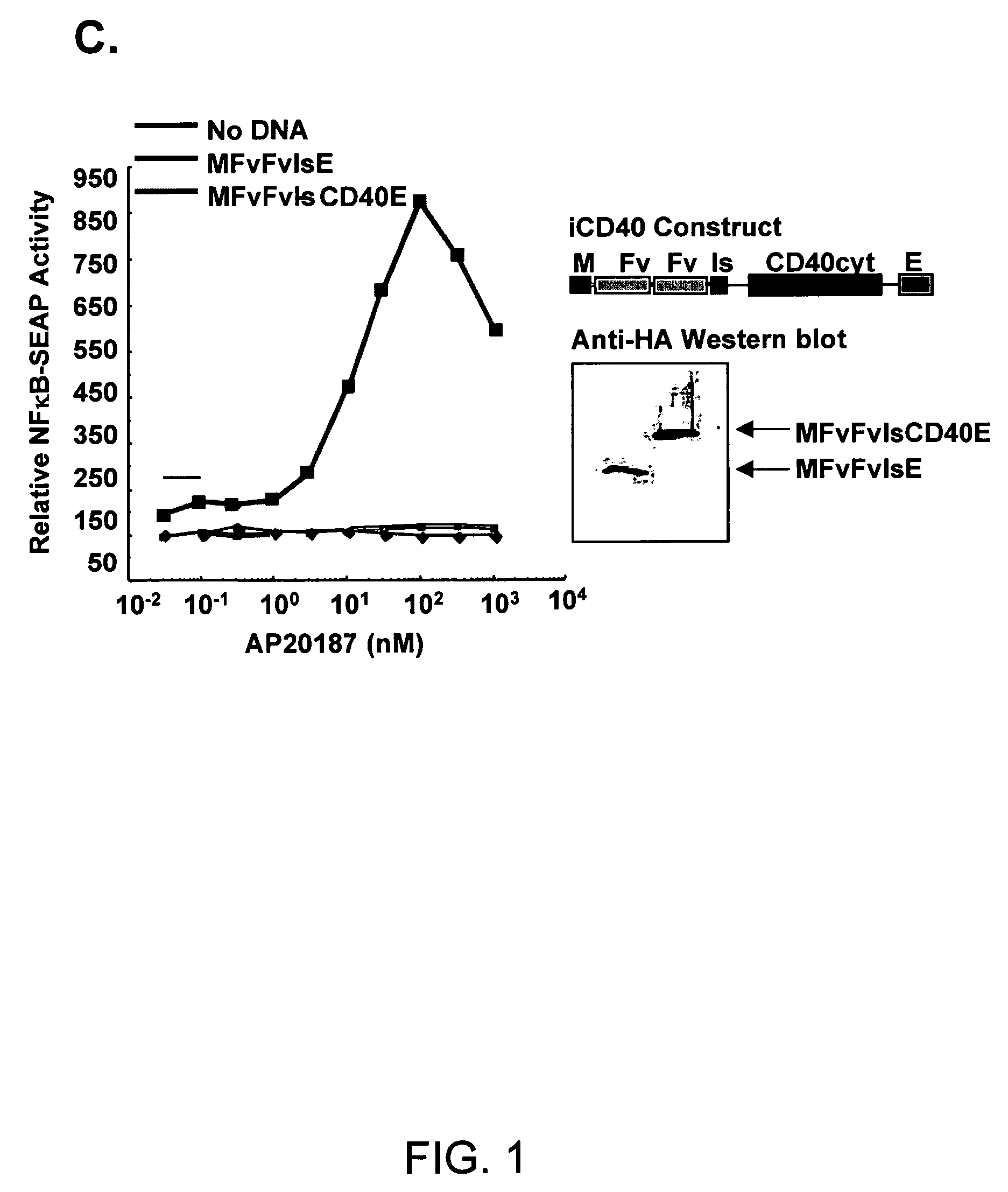
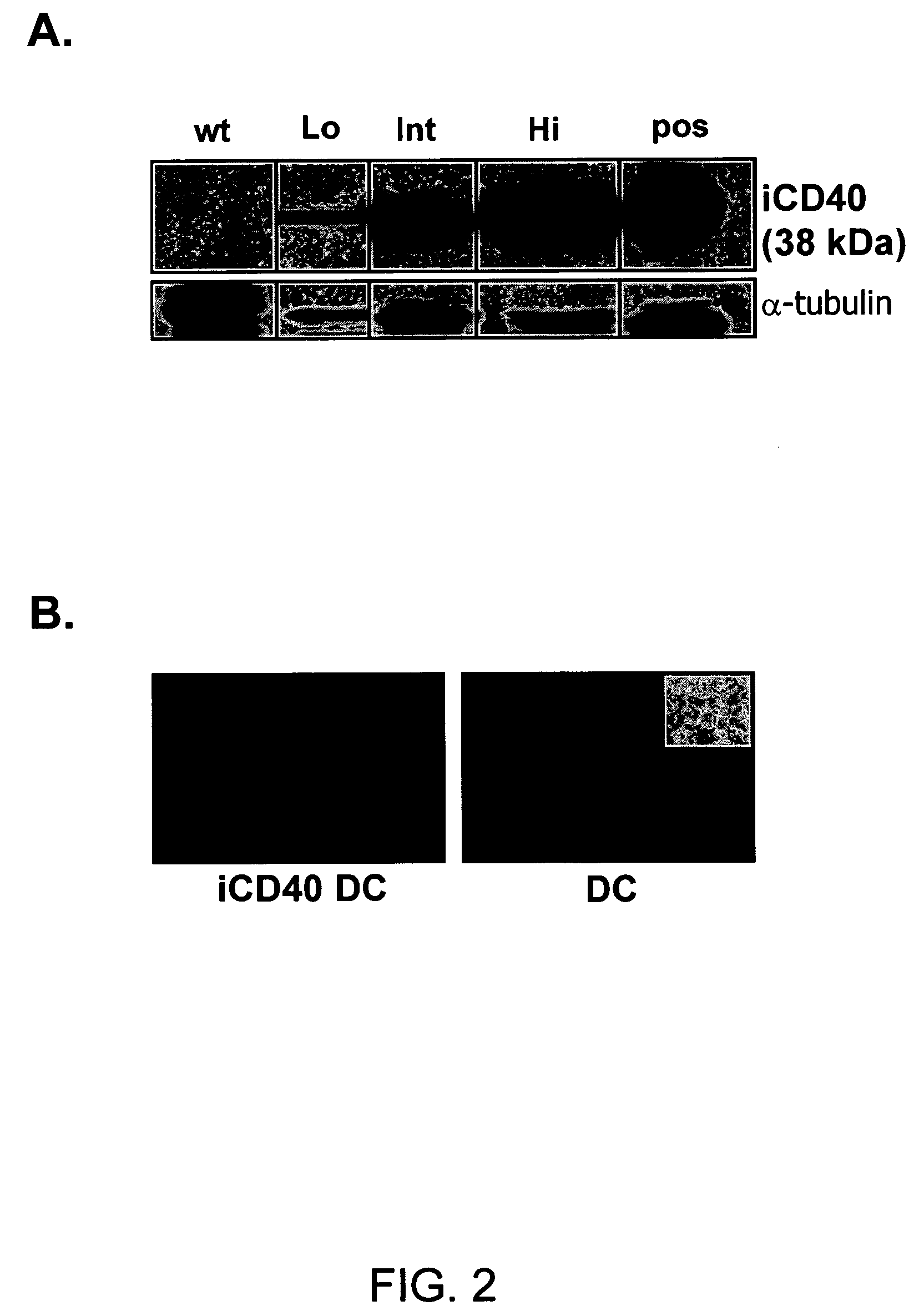
This invention provides methods to prepare and use immunostimulatory cells for enhancing an immune response. The invention provides a method for preparing mature dendritic cells (DCs), comprising the sequential steps of: (a) signaling isolated immature dendritic cells (iDCs) with a first signal comprising an interferon gamma receptor (IFN-γR) agonist and / or a tumor necrosis factor alpha receptor (TNF-αR) agonist to produce signaled dendritic cells; and (b) signaling said signaled dendritic cells with a second transient signal comprising an effective amount of a CD40 agonist to produce CCR7+ mature dendritic cells. Also provided by this invention are enriched populations of dendritic cells prepared by the methods of the invention. Such dendritic cells have enhanced immunostimulatory properties and increased IL-12 secretion and / or decreased IL-10 secretion. CD40 signaling can be initiated by one or more of polypeptide translated from an exogenous polynucleotide encoding CD40L (e.g., mRNA or DNA), an agonistic antibody to CD40 receptor or by CD40 ligand polypeptide. The enriched populations can be further modified by the administration of an immunogen to the DC. The DC will take up and process the immunogen on its cell surface.
Owner:COIMMUNE INC +1
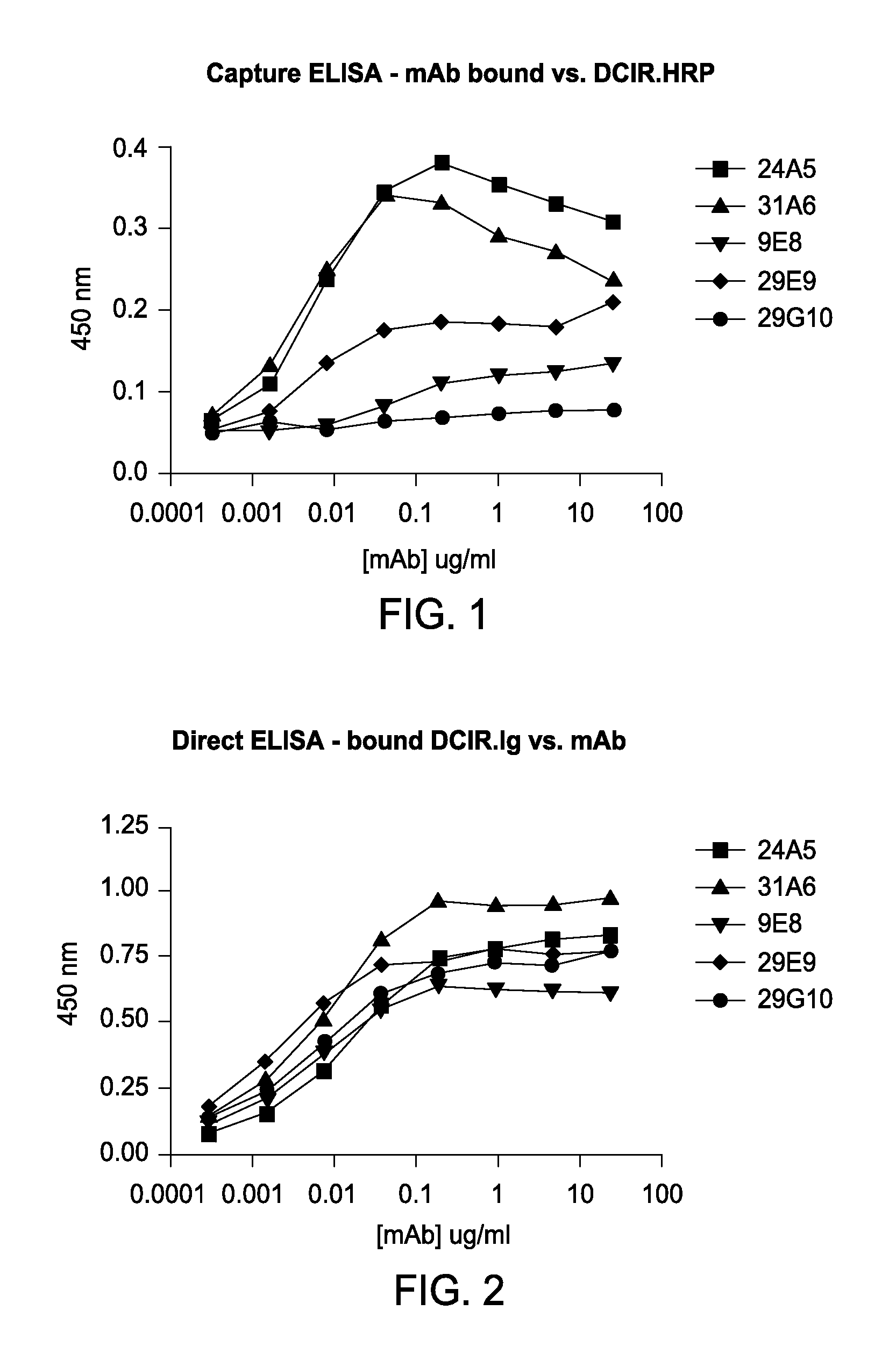
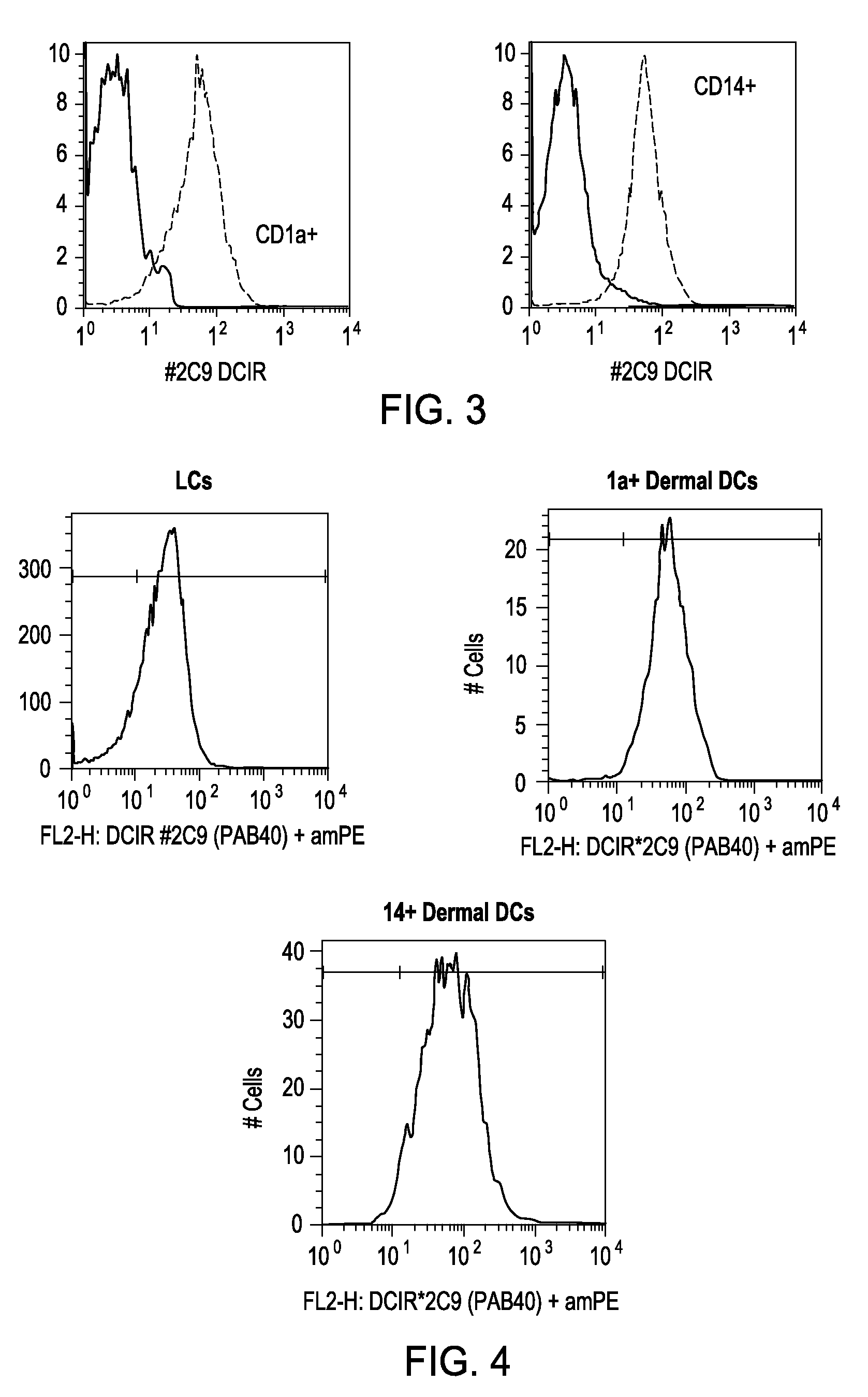
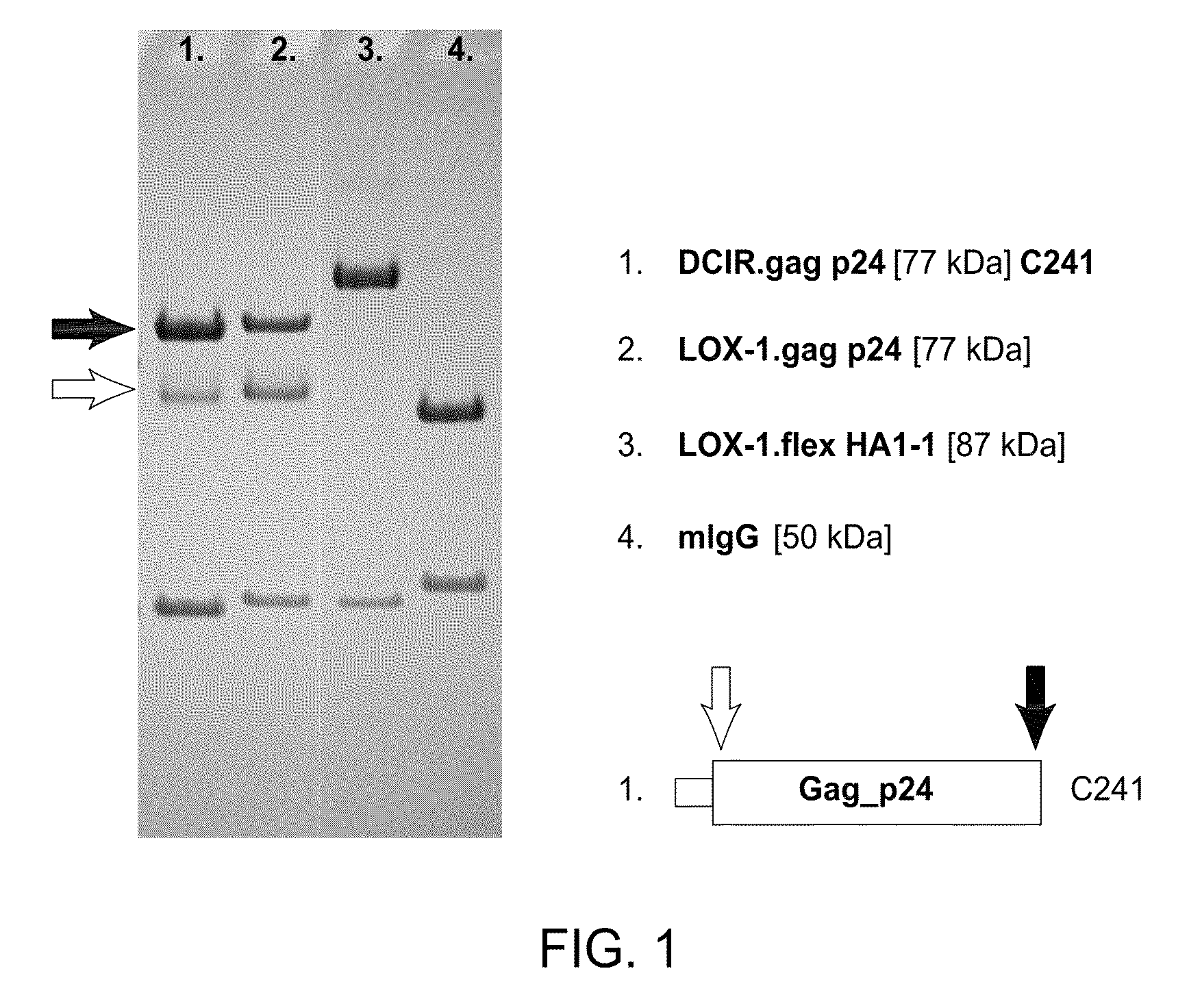
Vaccines Based on Targeting Antigen to DCIR Expressed on Antigen-Presenting Cells
ActiveUS20080241170A1Improve efficiencyAntibacterial agentsSsRNA viruses negative-senseDendritic cellAntibody antigen
The present invention includes compositions and methods for increasing the effectiveness of antigen presentation using a DCIR-specific antibody or fragment thereof to which an antigen is attached that forms an antibody-antigen complex, wherein the antigen is processed and presented by a dendritic cell that has been contacted with the antibody-antigen complex.
Owner:BAYLOR RES INST
Methods and compositions for immunotherapy and detection of inflammatory and immune-dysregulatory disease, infectious disease, pathologic angiogenesis and cancer
InactiveUS20060140936A1Antibacterial agentsOrganic active ingredientsDendritic cellAutoimmune condition
Methods and compositions for immunotherapy of inflammatory and immune-dysregulatory diseases, using multispecific antagonists that target at least two different markers are disclosed. The different targets include (i) proinflammatory effectors of the innate immune system, (ii) coagulation factors, and (iii) targets specifically associated with an inflammatory or immune-dysregulatory disorder, with a pathologic angiogenesis or cancer, or with an infectious disease, wherein the targets included in group (iii) are neither a proinflammatory effector of the immune system nor a coagulation factor. When the multispecific antagonist reacts specifically with a target associated with an inflammatory or immune-dysregulatory disorder, with a pathologic angiogenesis or cancer, or with an infectious disease, it also binds specifically with at least one proinflammatory effector of the immune system or at least one coagulation factor. Thus, the multispecific antagonist contains at least one binding specificity related to the diseased cell or condition being treated and at least one specificity to a component of the immune system, such as a receptor or antigen of B cells, T cells, neutrophils, monocytes and macrophages, and dendritic cells, a modulator of coagulation, or a proinflammatory cytokine. The multispecific antagonists are used in the treatment of various diseases that are generated or exacerbated by, or otherwise involve, proinflammatory effectors of the innate immune system or coagulation factors. Such diseases more particularly include acute and chronic inflammatory disorders, autoimmune diseases, giant cell arteritis, septicemia and septic shock, coagulopathies (including diffuse intravascular coagulation), neuropathies, graft versus host disease, infectious diseases, acute respiratory distress syndrome, granulomatous diseases, transplant rejection, asthma, cachexia, myocardial ischemia, and atherosclerosis. Other diseases also responsive to these therapies include cancers and conditions with pathological angiogenesis.
Owner:IMMUNOMEDICS INC
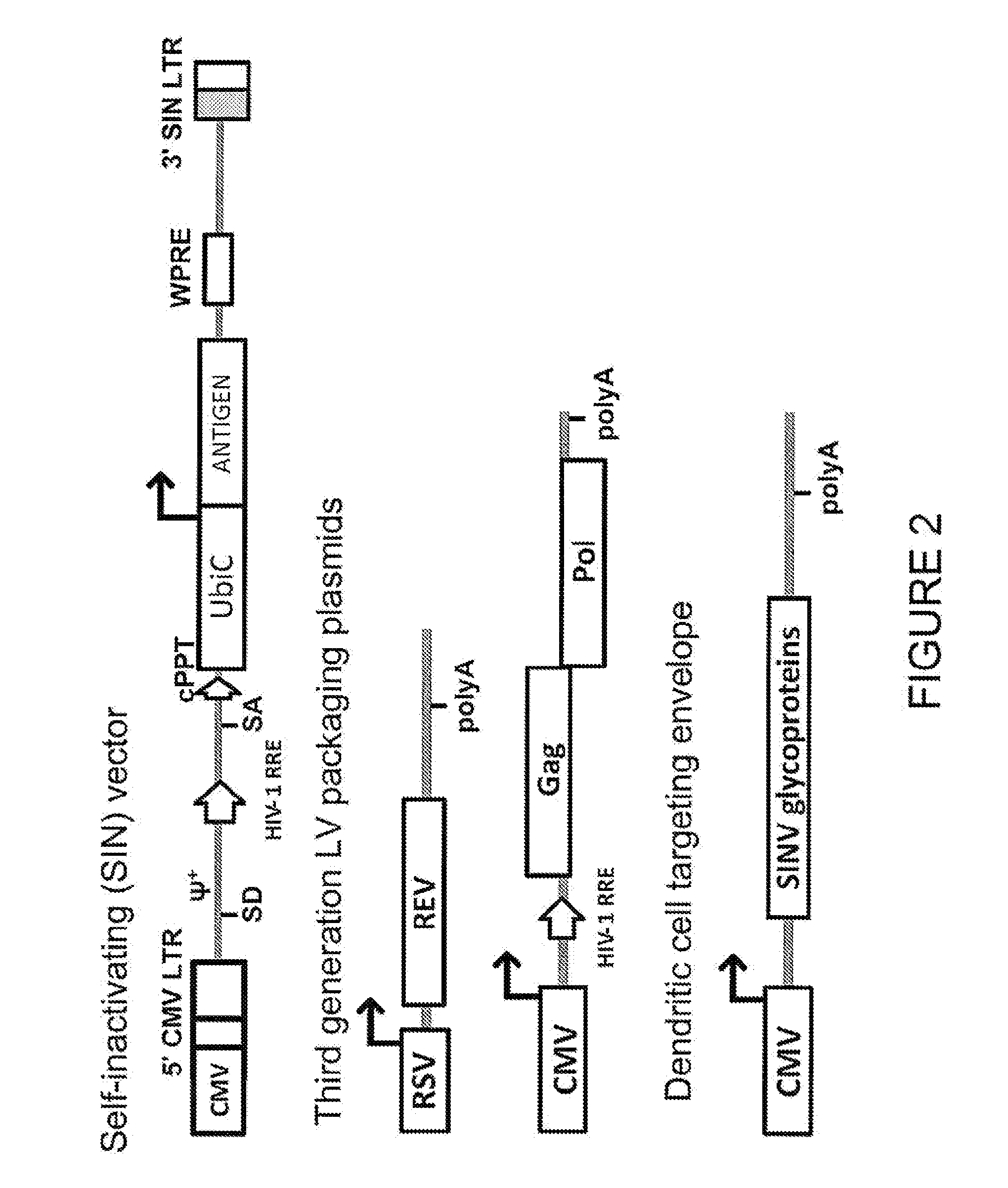
Lentiviral vectors pseudotyped with a sindbis virus envelope glycoprotein
InactiveUS20110064763A1Promote infectionSsRNA viruses positive-senseAntiviralsDendritic cellSindbis virus
Lentiviral vector particles comprising a Sindbis virus E2 glycoprotein variant and a lentiviral vector genome comprising a sequence of interest are provided. A lentiviral vector particle comprising: (a) an envelope comprising a Sindbis virus E2 glycoprotein variant; and (b) a lentiviral vector genome comprising a sequence of interest; wherein the E2 glycoprotein variant facilitates infection of dendritic cells by the lentiviral vector particle, and wherein the E2 glycoprotein variant has reduced binding to heparan sulfate compared to a reference sequence (HR strain).
Owner:IMMUNE DESIGN CORP
HIV vaccine based on targeting maximized gag and nef to dendritic cells
ActiveUS20100135994A1Improve efficiencyIncrease flexibilityBiocidePeptide/protein ingredientsCyclin D1Dendritic cell
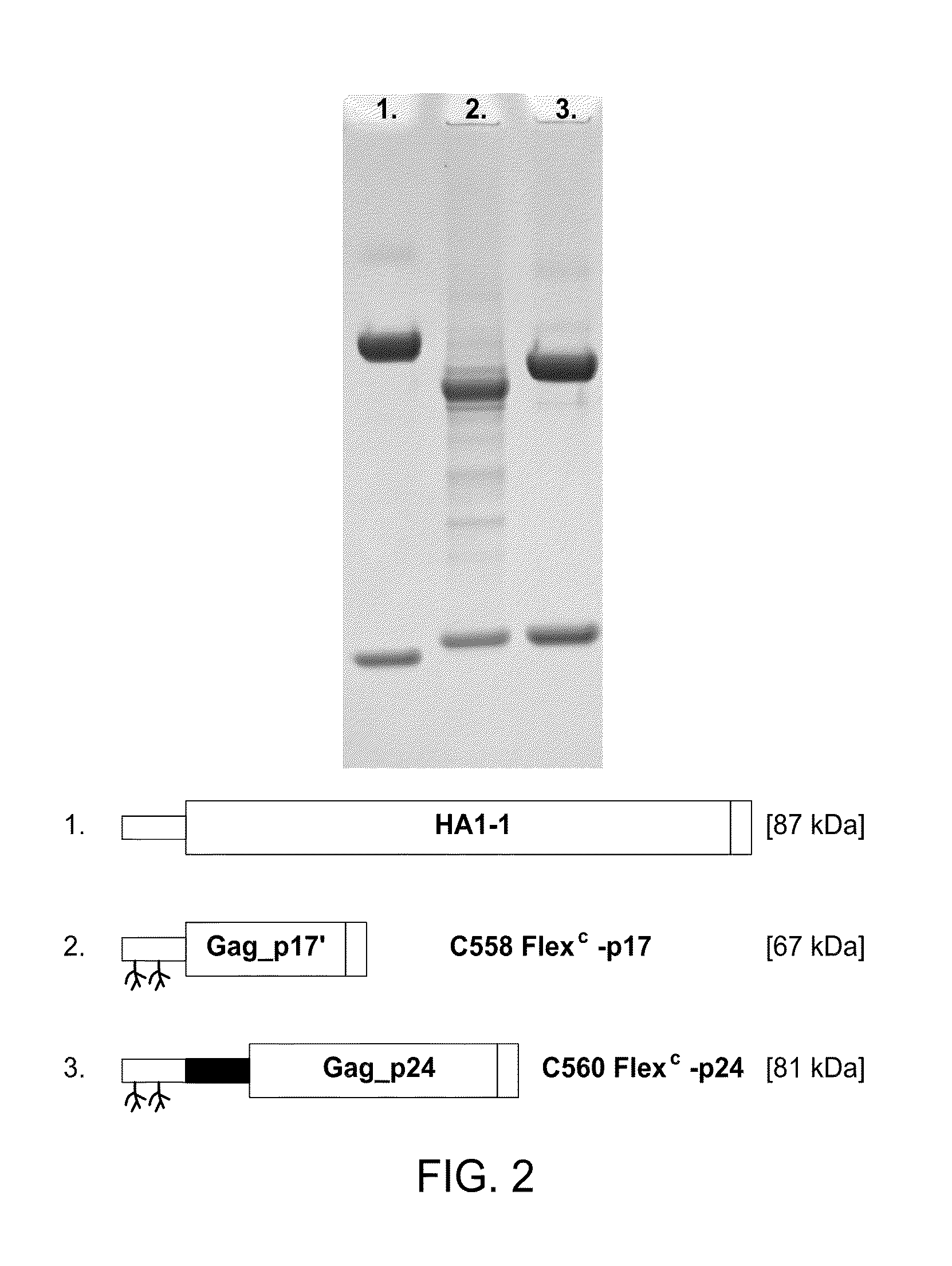
The present invention includes compositions and methods for making and using a vaccine that includes a DC-specific antibody or fragment thereof to which an engineered Gag antigen is attached to form an antibody-antigen complex, wherein the Gag antigen is less susceptible to proteolytic degradation by eliminating one or more proteolytic sites or a DC-specific antibody or fragment thereof to which an engineered Nef antigen is attached to form an antibody-antigen complex, wherein the Nef antigen comprises one or more codon usage optimization that increase antibody-antigen complex secretion, or both, wherein the vaccine is able to elicit an HIV-specific T cell immune response to Gag p17, Gag p24, Nef and / or Cyclin D1.
Owner:BAYLOR RES INST
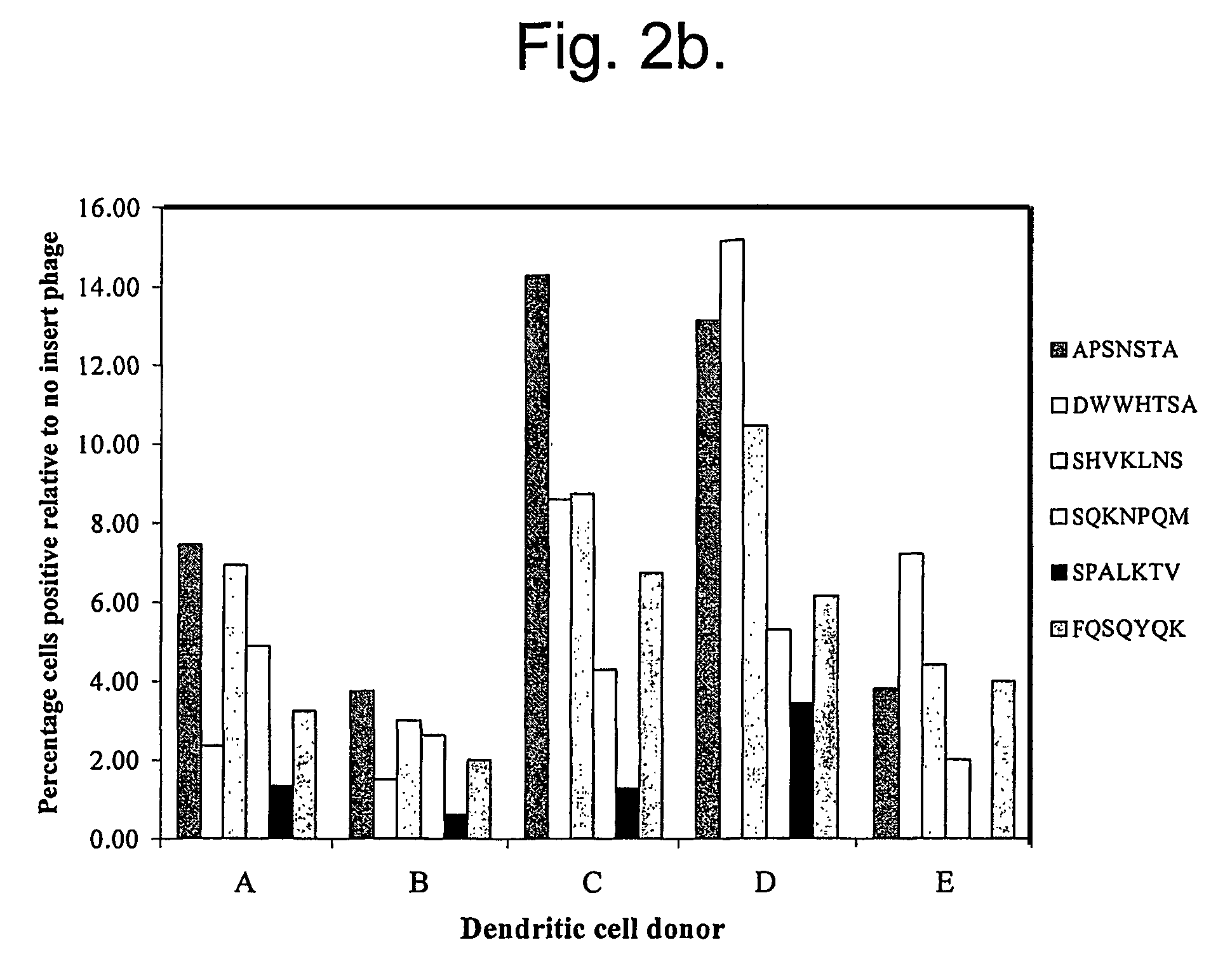
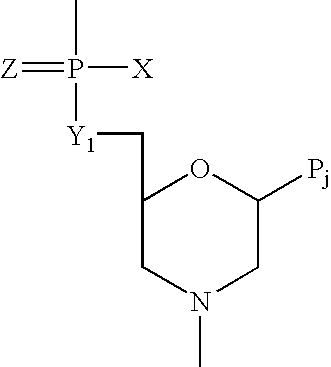
Peptide ligands
A peptide consisting of or comprising an amino acid sequence selected froma) PX1X2X3T [SEQ.ID.NO.:1];b) PSX4S [SEQ.ID.NO.:2];c) QX5X6X7Q [SEQ.ID.NO.:3];d) SX8S [SEQ.ID.NO.:4],in which X1, X2 and X3, which may be the same or different, each represents an amino acid residue;X4 represents an amino acid residue; andX5 and X7, which may be the same or different, each represents an amino acid residue, X6 represents an amino acid residue having an amide side chain; andX8 represent an amino acid having an aliphatic side chain, which peptide binds to dendritic cells and also to other types of cells. The peptide may be used a target non-viral and viral vectors to such cells.
Owner:RYBOQUIN COMPANY
Combination therapy for the prevention or treatment of cancer, inflammatory disorders or infectious diseases in a subject
InactiveUS20030035790A1Good treatment effectReduce dosageVirusesPeptide/protein ingredientsDendritic cellT cell
The present invention relates to compositions comprising compounds which augment activated immune cells, such as T-cells, dendritic cells and natural killer ("NK") cells, and methods for the treatment or prevention of diseases and disorders, including cancer, inflammatory disorders, and infectious diseases, in a subject comprising the administration of said compositions to said subject. In particular, the present invention relates to methods for the treatment or prevention of diseases and disorders, including cancer, inflammatory disorders, and infectious diseases, in a subject comprising administrating to said subject one or more compounds that activate one or more cytokine receptors and one or more compounds that activate one or more co-stimulatory molecules expressed by activated immune cells. The present invention also relates to compositions and kits comprising a compound that activates one or more cytokine receptors and a compound that activates one or more co-stimulatory molecules expressed by activated immune cells.
Owner:MT SINAI SCHOOL OF MEDICINE
Transcutaneous immunization without heterologous adjuvant
InactiveUS20060002949A1Increase skin hydrationIncrease local concentrationSsRNA viruses negative-senseAntibacterial agentsDendritic cellSpecific immunity
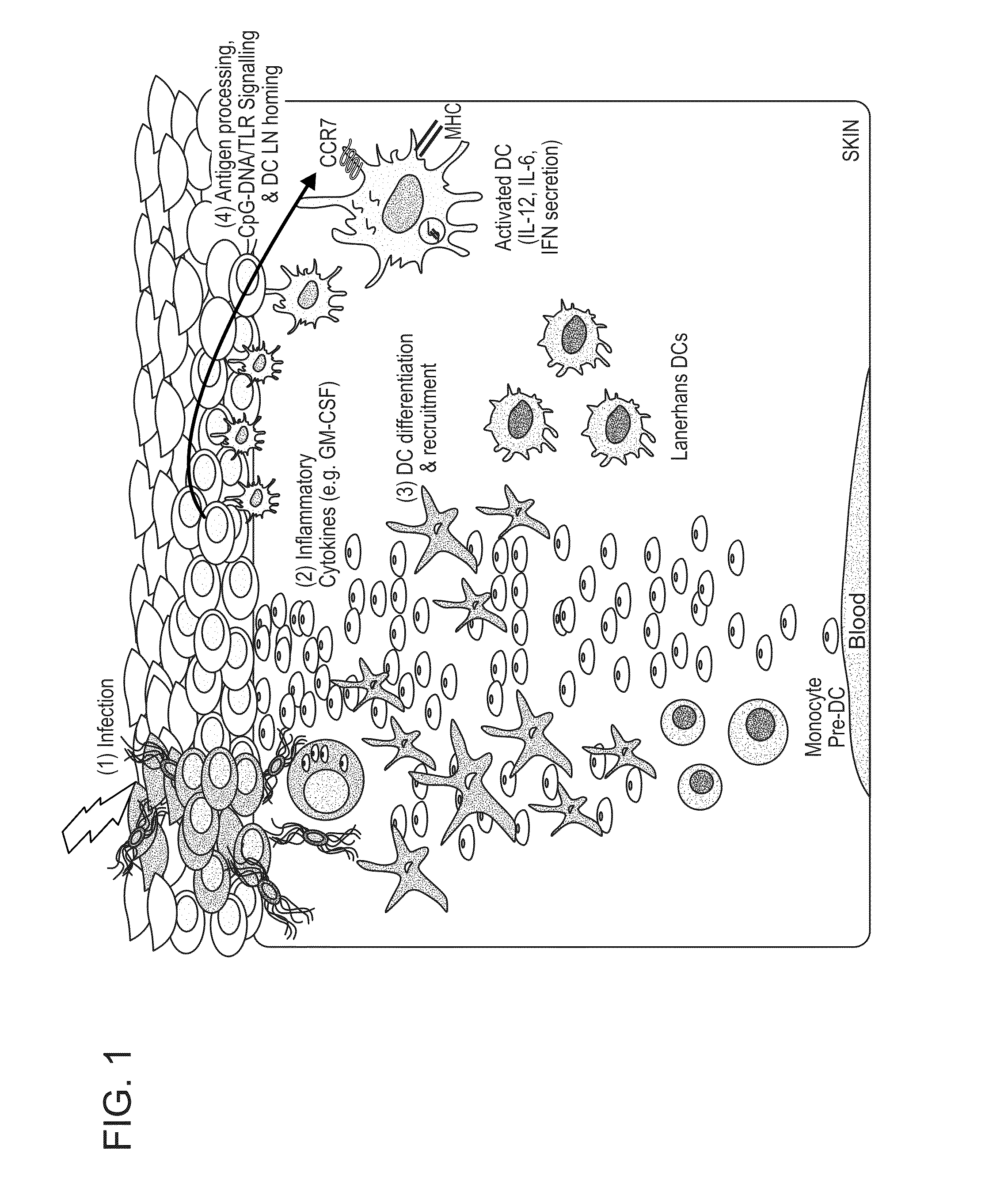
Transcutaneous immunization can deliver antigen to the immune system through the stratum corneum without physical or chemical penetration to the dermis layer of the skin. This delivery system induces an antigen-specific immune response without the use of a heterologous adjuvant. Although perforation of intact skin is not required, superficial penetration or micropenetration of the skin can act as an enhancer; similarly, hydration may enhance the immune response. This system can induce antigen-specific immune effectors after epicutaneous application of a formulation containing one or more antigens. The formulation may initiate processes such as antigen uptake, processing, and presentation; Langerhans cell activation, migration from the skin to other immune organs, and differentiation to mature dendritic cells; contacting antigen with lymphocytes bearing cognate antigen receptors on the cell surface and their stimulation; and combinations thereof. Systemic and / or regional immunity may be induced. Immune responses that provide prophylactic and / or therapeutic treatments are preferred. Antigenic activities in the formulation may be found in the same molecule, two or more different molecules dissociated from each other, or multiple molecules in a complex formed by covalent or non-covalent bonds. For antigens which are proteinaceous, they may be provided in the formulation as a polynucleotide for transcutaneous genetic immunization. Besides simple application of a dry or liquid formulation to the skin, patches and other medical devices may be used to deliver antigen for immunization.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE OFFICE OF THE COMMAND JUDGE ADVOCATE
Antisense oligomers and methods for inducing immune tolerance and immunosuppression
ActiveUS20060276425A1Reduce capacityIncrease productionBiocideOrganic active ingredientsExtracellularDendritic cell
A method and composition for inducing human dendritic cells to a condition of reduced capacity for antigen-specific activation of T cells, and, in mature dendritic cells, increased production of extracellular IL-10 is disclosed. A population of dendritic cells is exposed to a substantially uncharged antisense compound, including partially positively charged, containing 12-40 subunits and a base sequence effective to hybridize to an expression-sensitive region of a preprocessed or processed human CD86 transcript identified, in its processed form, by SEQ ID NO:33, to form a duplex structure between said compound and transcript having a Tm of at least 45° C. Formation of the duplex blocks expression of full-length CD86 in said cells, which in turn leads to reduced capacity for antigen-specific activation of T cells, and, in mature dendritic cells, increased production of extracellular IL-10.
Owner:SAREPTA THERAPEUTICS INC
Pd-1 antagonists and methods for treating infectious disease
InactiveUS20110159023A1Rapid induction of protectionRobust effector responseAntibacterial agentsOrganic active ingredientsDiseaseDendritic cell
Methods and compositions for treating an infection or disease that results from (1) failure to elicit rapid T cell mediated responses, (2) induction of T cell exhaustion, T cell anergy or both, or (3) failure to activate monocytes, macrophages, dendritic cells and / or other APCs, for example, as required to kill intracellular pathogens. The method and compositions solve the problem of undesired T cell inhibition by binding to and blocking PD-1 to prevent or reduce inhibitory signal transduction, or by binding to ligands of PD-1 such as PD-L1, thereby preventing (in whole or in part) the ligand from binding to PD-1 to deliver an inhibitory signal. The immune response can be modulated by providing antagonists which bind with different affinity (i.e., more or less as required), by varying the dosage of agent which is administered, by intermittent dosing over a regime, and combinations thereof, that provides for dissociation of agent from the molecule to which it is bound prior to being administered again (similar to what occurs with antigen elicitation using priming and boosting). In some cases it may be particularly desirable to stimulate the immune system, then remove the stimulation.
Owner:AMPLIMMUNE
Methods for using dendritic cells to activate gamma/delta-T cell receptor-positive T cells
InactiveUS6821778B1Control progressIncrease the number ofArtificial cell constructsBlood/immune system cellsAdoptive cellular immunotherapyDendritic cell
This invention relates to methods of using human dendritic cells to present antigens for the induction of antigen-specific T cell-mediated immune responses. In particular, it relates to the isolation of dendritic cells from human blood, exposing the cells to antigens, co-culturing the antigen-pulsed dendritic cells with gammadelta-T cell receptor-positive-T cells (gammadelta-TCR<+> T cells) obtained from unprimed or weakly primed individuals for the stimulation of antigen-specific T cell proliferative and cytotoxic activities. The dendritic cell antigen presentation system described herein has a wide range of applications, including but not limited to, activation and expansion of large numbers of antigen-specific major histocompatibility complex-unrestricted T cells for use in adoptive cellular immunotherapy against infectious diseases and cancer.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Adjuvanting Material
InactiveUS20080233143A1SsRNA viruses negative-senseOrganic active ingredientsLipid formationAdjuvant
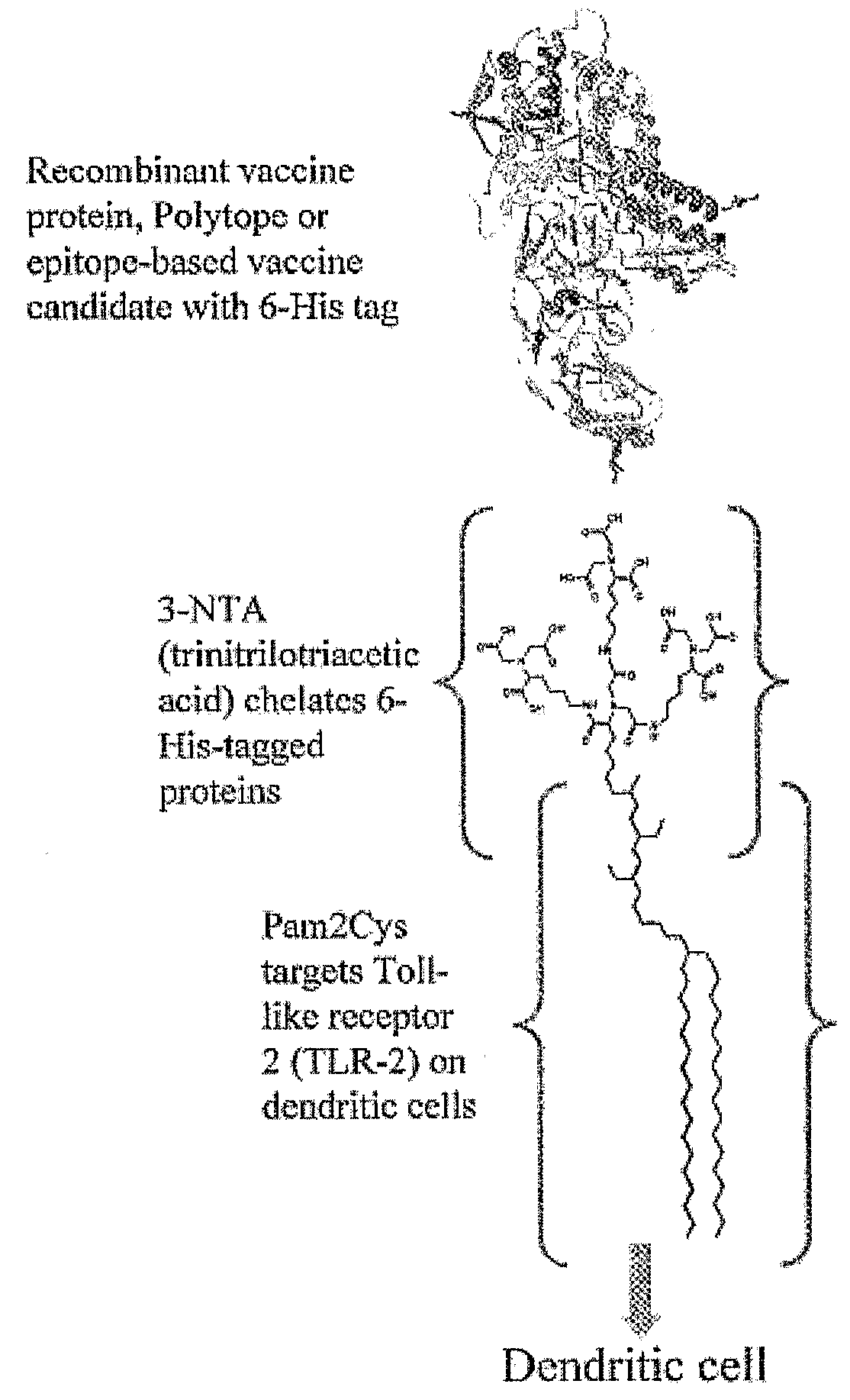
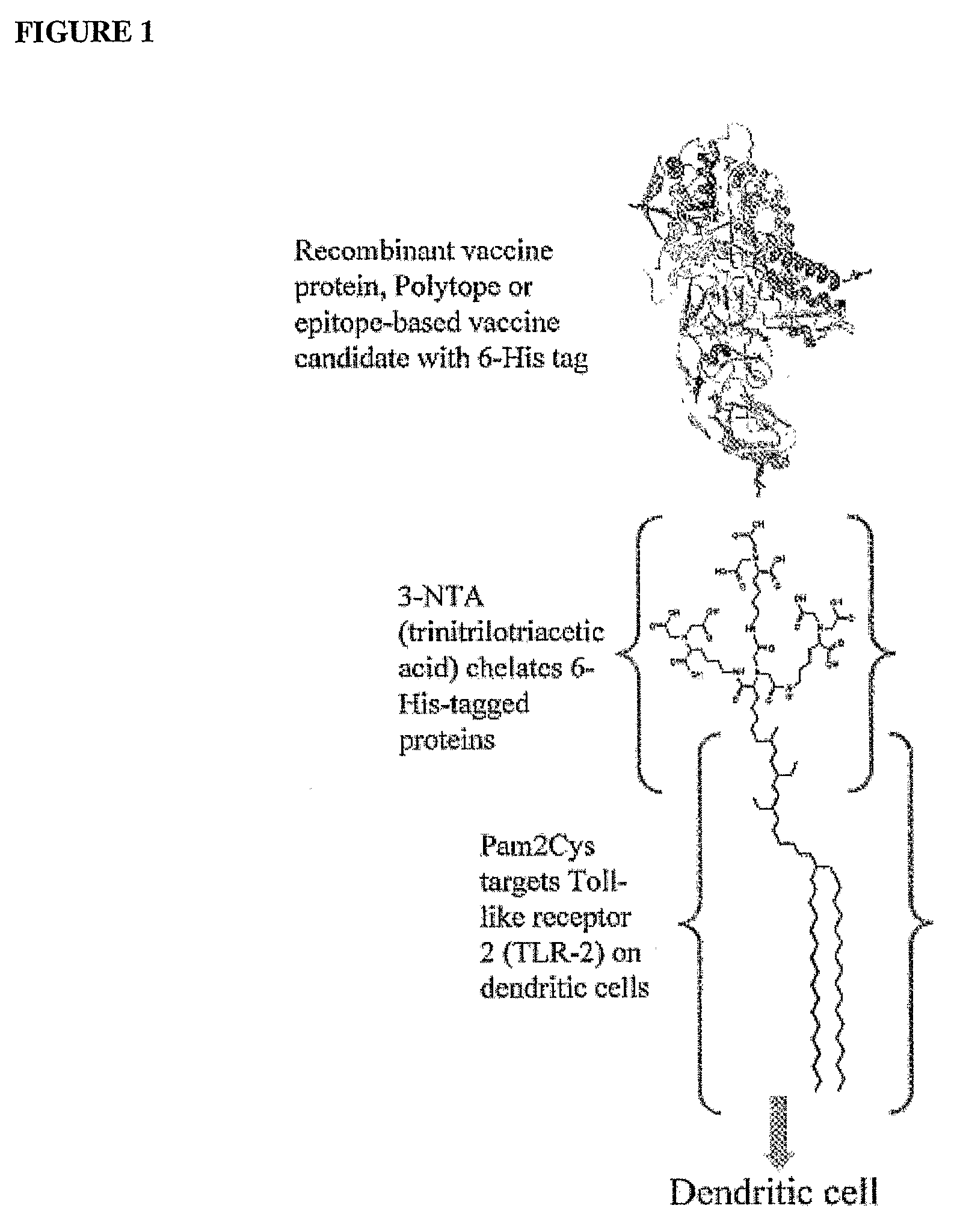
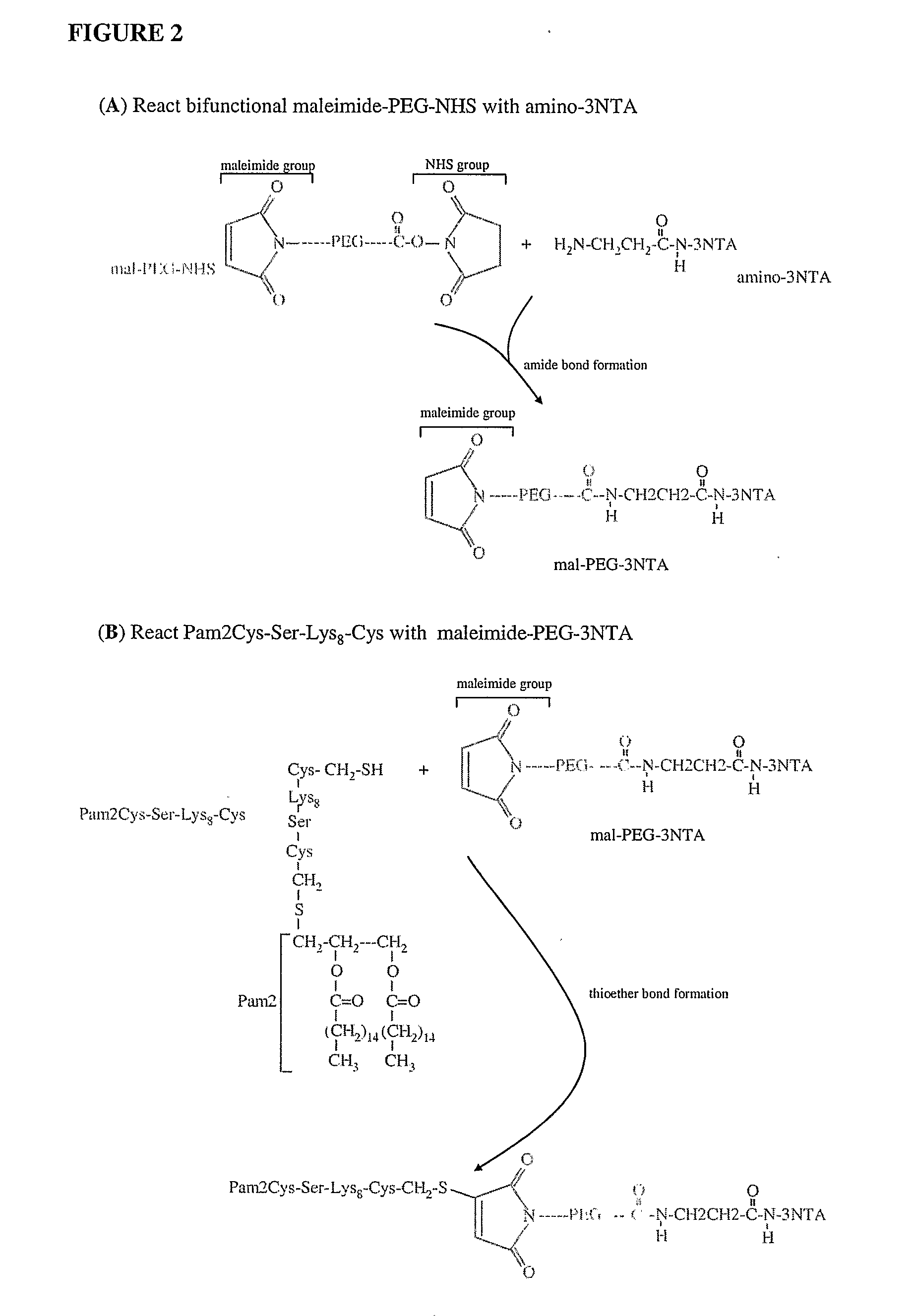
The present invention provides an adjuvanting material, the adjuvanting material comprising a lipid dendritic cell targeting moiety to which is covalently linked a metal chelating group. Further, the present invention provides an immunogenic composition comprising (a) a lipid dendritic cell targeting moiety to which is covalently linked a metal chelating group; (b) an antigen comprising a metal affinity tag; and optionally (c) metal ions, whereby the antigen is linked to the lipid dendritic cell targeting moiety via the interaction between the metal affinity tag and the metal chelating group.
Owner:LIPOTEK PTY LTD
Molecular vaccines employing nucleic acid encoding anti-apoptotic proteins
InactiveUS20070026076A1Increase the number ofEasy to demonstratePowder deliveryVirusesDendritic cellVaccine Potency
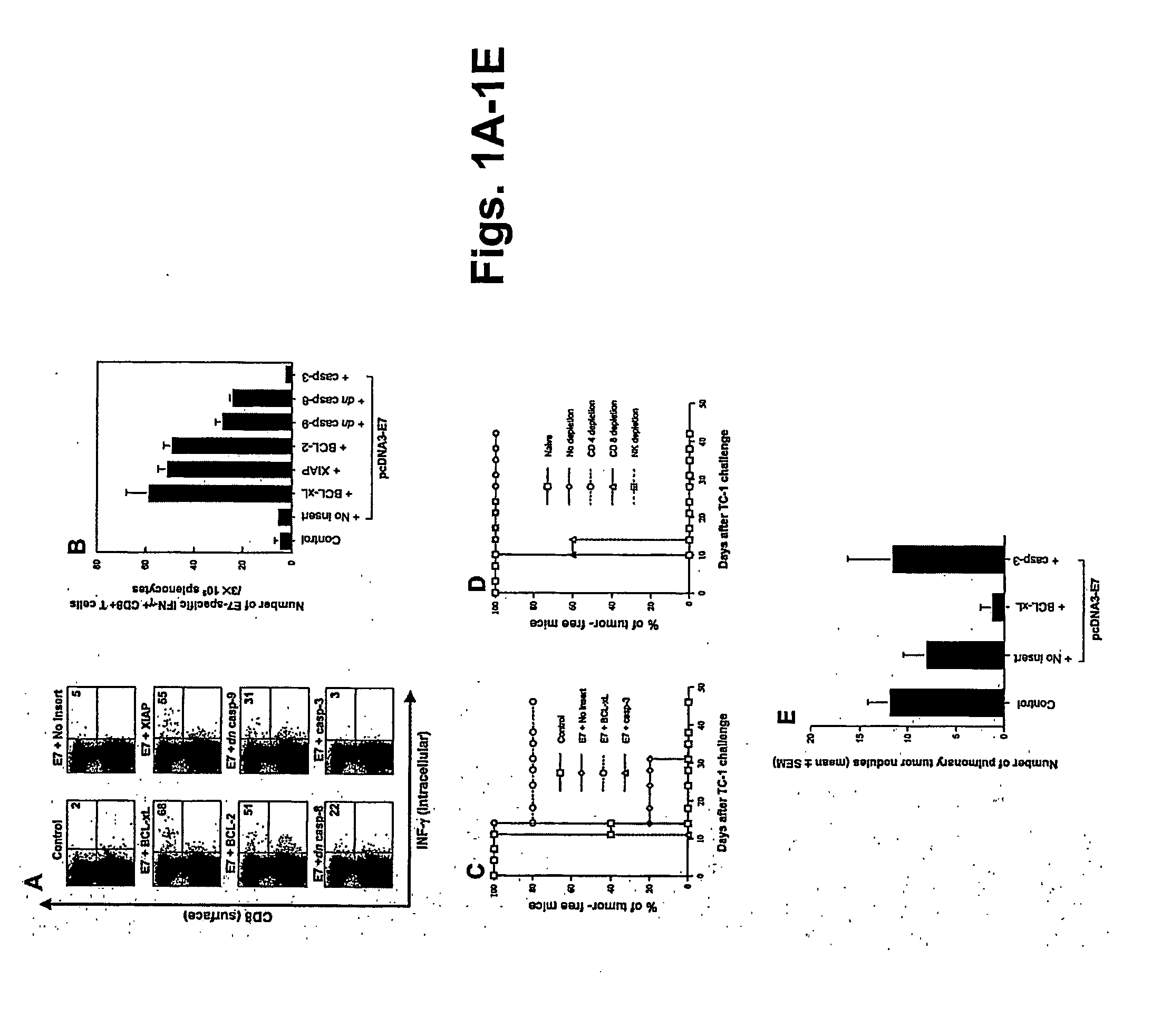
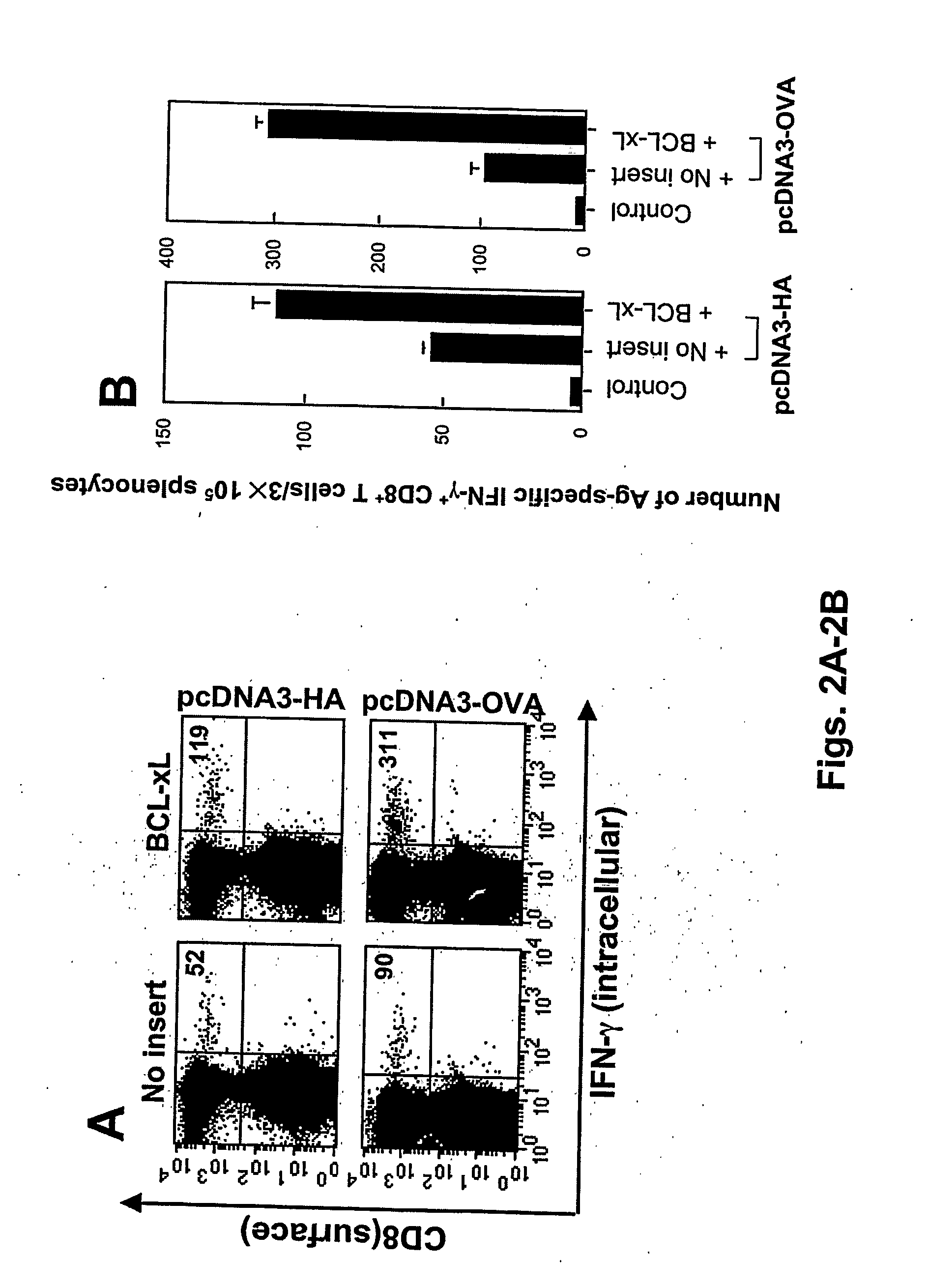
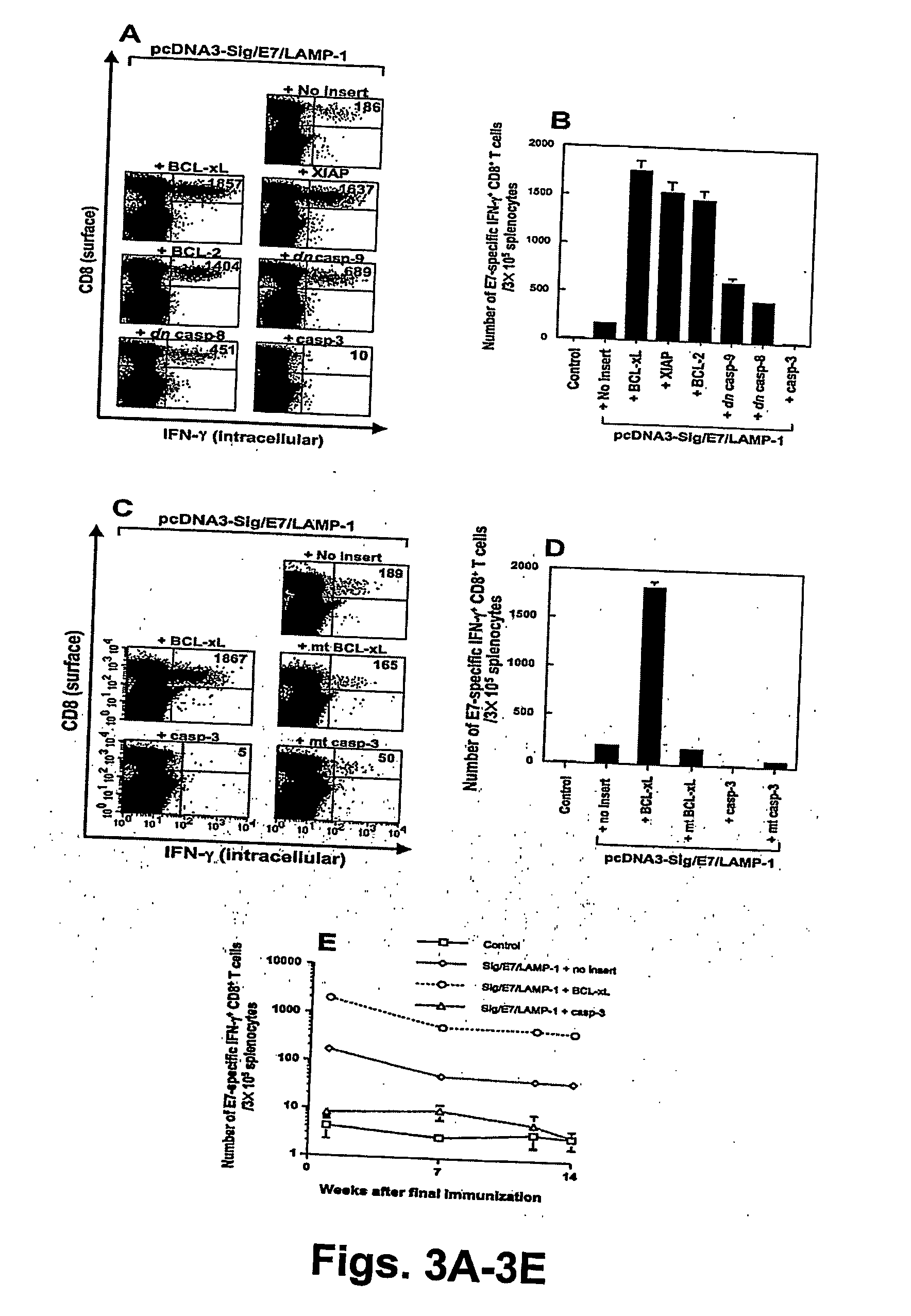
T cell immune responses are enhanced by presentation of antigen to CD8+ T cells using a chimeric nucleic acid immunogen or vaccine that links DNA encoding an antigen with DNA encoding a polypeptide that targets or translocates the antigenic polypeptide to which it is fused (immunogenicity-potentiating polypeptides or “IPP”). By inhibiting apoptosis in the vicinity of a T cell responses to such a nucleic acid immunogen, even more potent immune responses are attained. The present strategy prolongs the survival of DNA-transduced cells, including dendritic cells (DCs), thereby enhancing the priming of antigen-specific T cells and increase potency. Co-delivery of DNA encoding an inhibitor of apoptosis, including (a) BCL-xL, (b) BCL-2, (c) XIAP, (d) dominant negative caspase-9, or (e) dominant negative caspase-8, or (f) serine protease inhibitor 6 (SPI-6) which inhibits granzyme B, with DNA encoding an antigen, prolongs the survival of transduced DCs and results in significant enhancement of antigenspecific T cell immune responses that provide potent antitumor effects. Thus, co-administration of a DNA vaccine encoding antigen linked to an IPP along with one or more DNA constructs encoding an anti-apoptotic protein provides a novel way to enhance vaccine potency.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
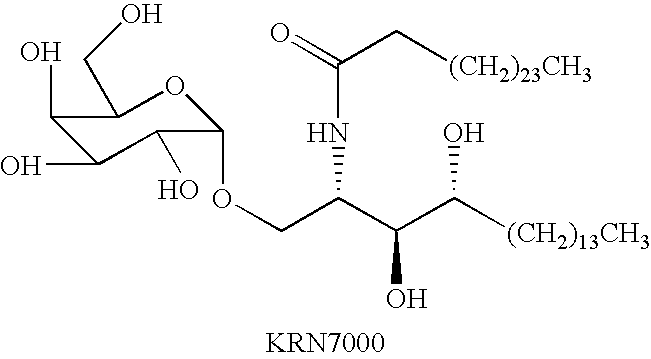
Ceramide derivatives as modulators of immunity and autoimmunity
α-Galactosylceramides and glycosylceramides (“ceramide-like glycolipids”) that modulate NK T cells. The ceramide-like glycolipids vary in the cytokines induced in NK T cells and vary in the antigen-presenting cells that are capable of efficiently presenting the compounds to NK T cells. Pharmaceutical compositions of the ceramide-like glycolipids are provided, as are pharmaceutical compositions of the ceramide-like glycolipids combined with dendritic cells. Methods utilizing the ceramide-like glycolipids in vaccines, to activate NK T cells, to stimulate the immune system, and to treat mammals are also provided. The invention also provides methods of evaluating a compound for its ability to activate an NK T cell in the presence of a cell expressing a CD1d protein.
Owner:ALBERT EINSTEIN COLLEGE OF MEDICINE OF YESHIVA UNIV
Method for treatment of blood tumor using anti-TIM-3 antibody
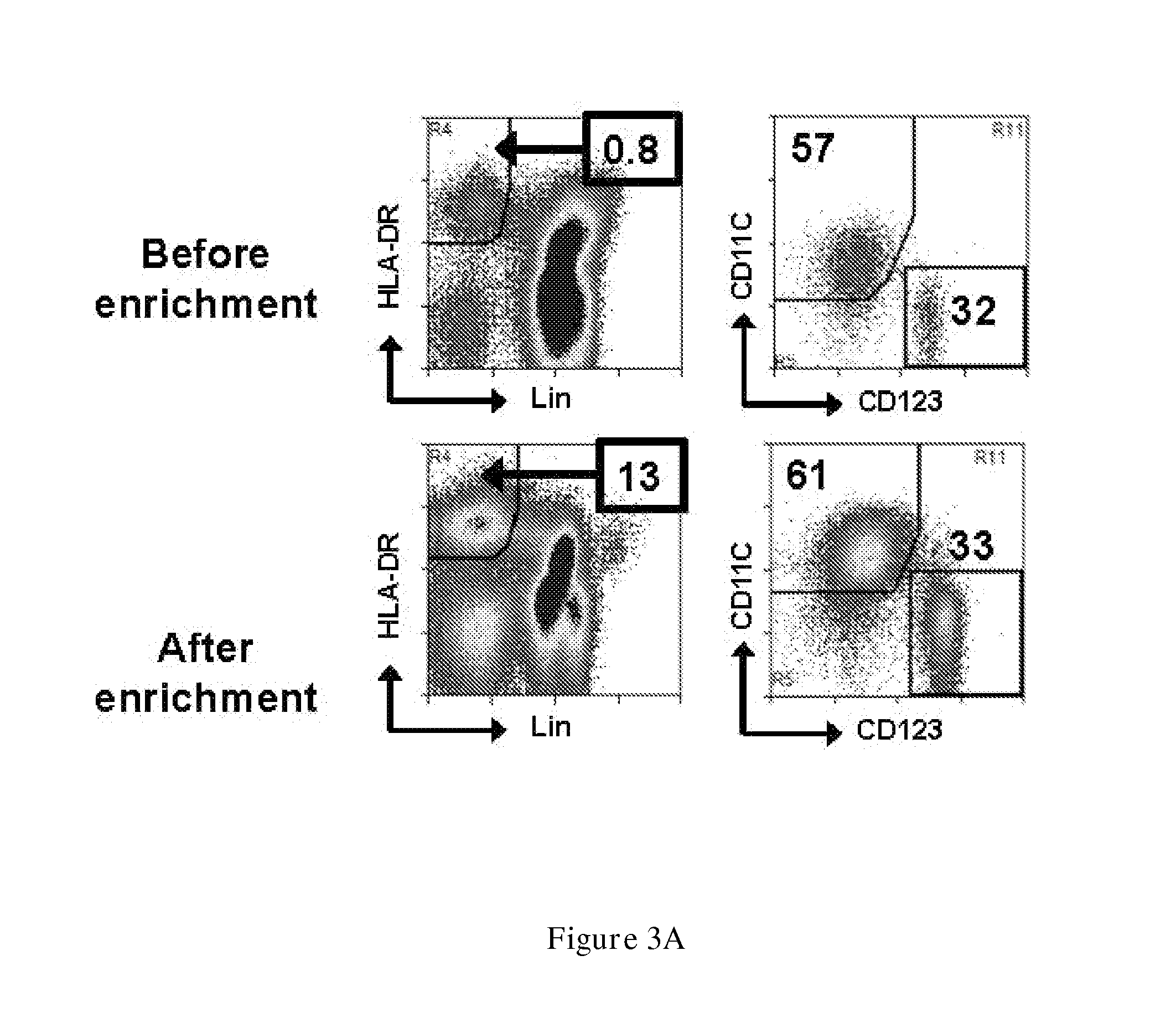
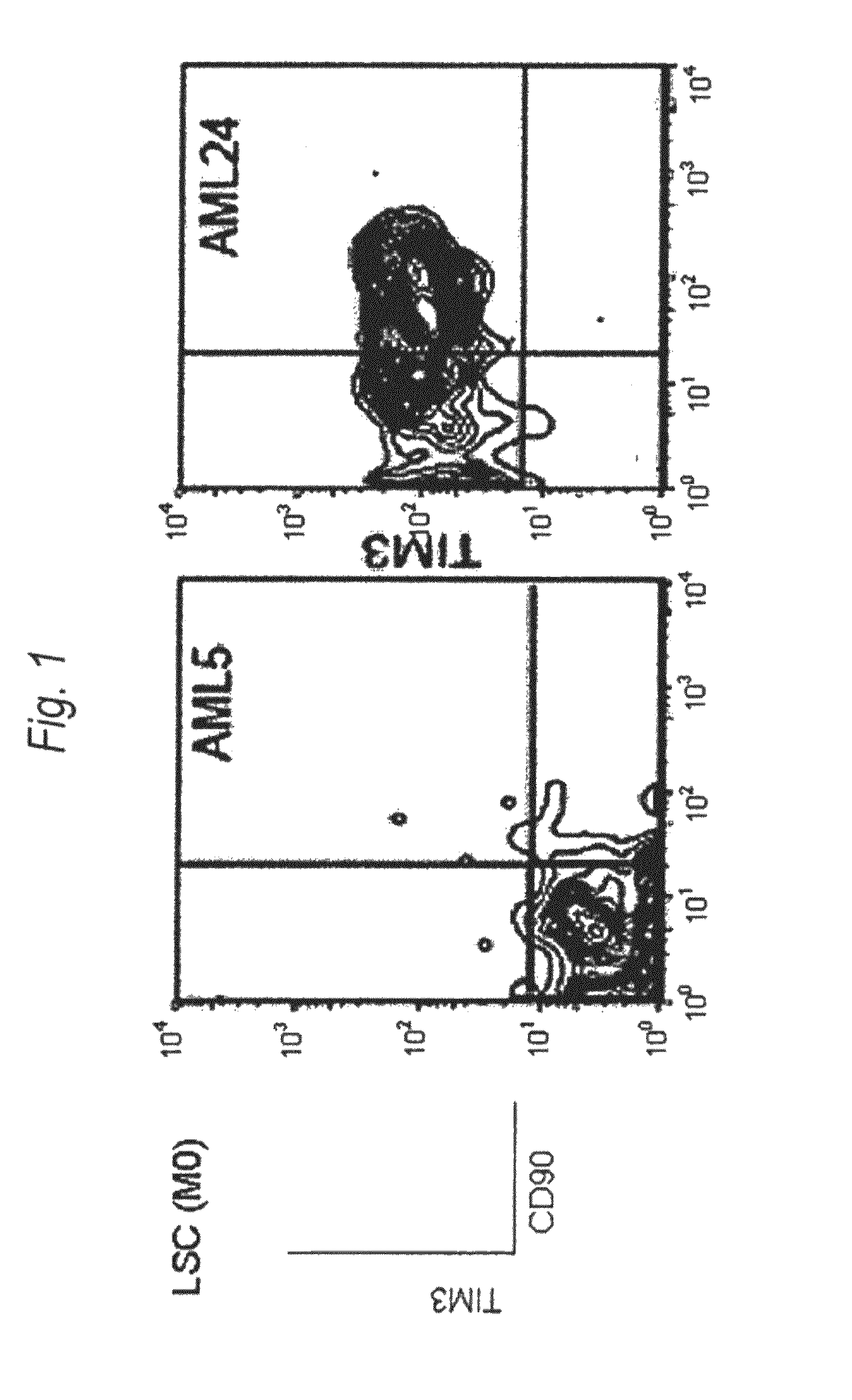
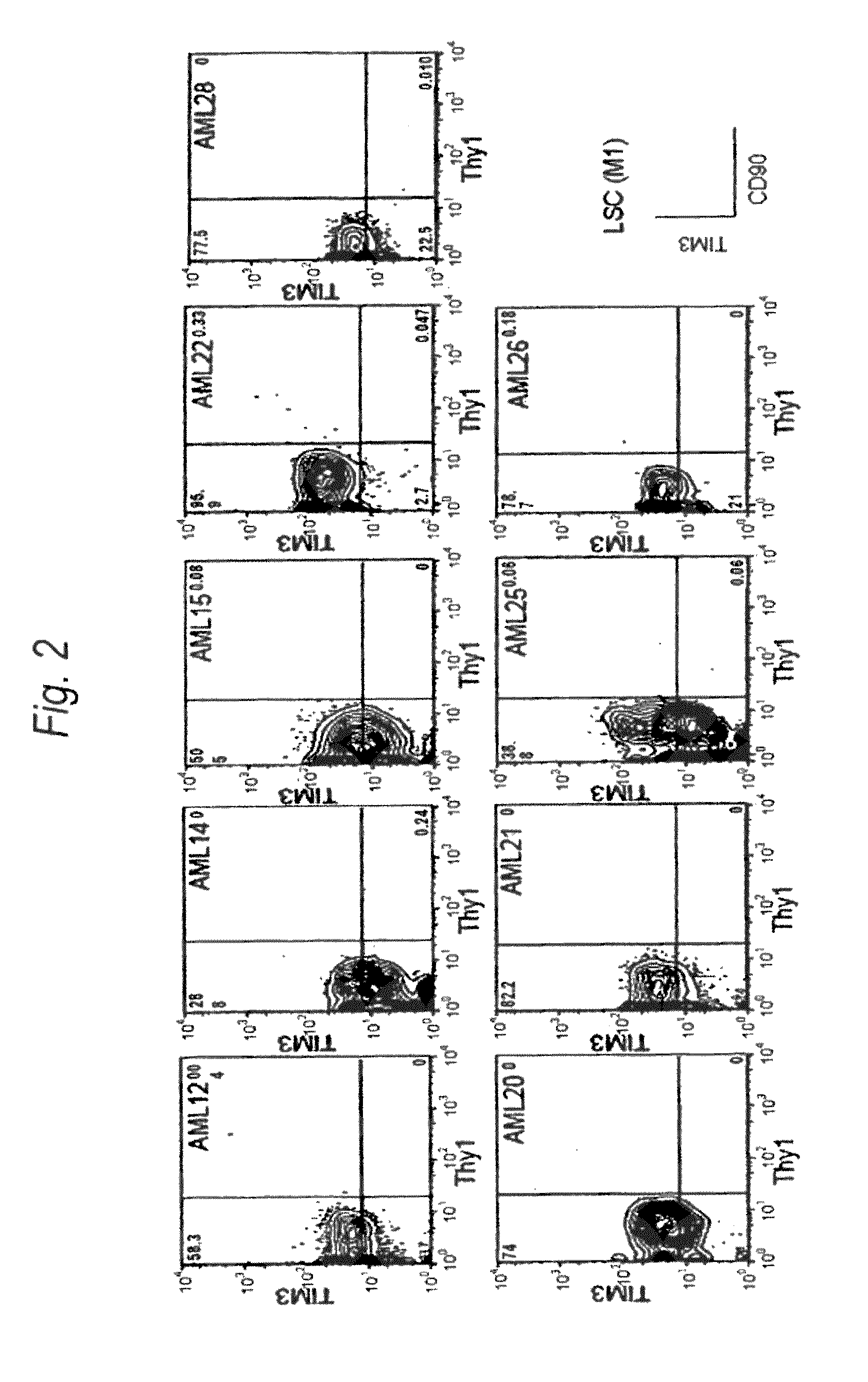
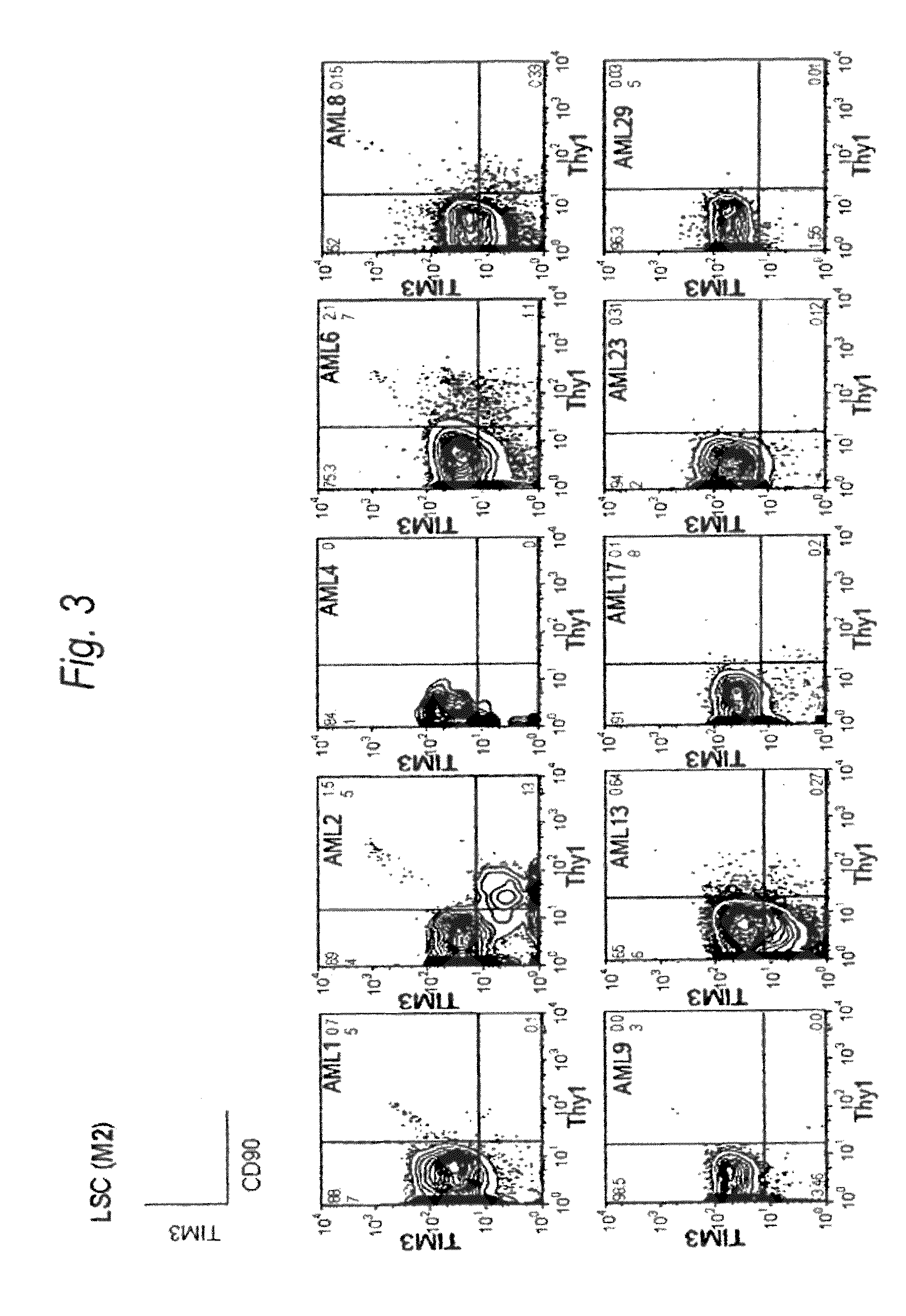
Disclosed is a therapeutic method comprising administering a TIM-3 antibody to a subject who is suspected to be suffering from blood tumor and in whom TIM-3 has been expressed in a Lin(−)CD34(+)CD38(−) cell fraction of bone marrow or peripheral blood or a subject who has been received any treatment for blood tumor. Also disclosed is a composition for preventing or treating blood tumor, which comprises a TIM-3 antibody as an active ingredient. Conceived diseases include those diseases which can be treated through the binding or targeting of the TIM-3 antibody to blood tumor cells (AML cells, CML cells, MDS cells, ALL cells, CLL cells, multiple myeloma cells, etc.), helper T cell (e.g., Th1 cells, Th17 cells), and antigen-presenting cells (e.g., dendritic cells, monocytes, macrophages, and cells resembling to the aforementioned cells (hepatic stellate cells, osteoclasts, microglial cells, intraepidermal macrophages, dust cells (alveolar macrophages), etc)), all of which are capable of expressing TIM-3. The diseases for which the therapeutic use is to be examined include blood diseases in which the expression of TIM-3 is observed in bone marrow or peripheral blood, particularly blood tumor.
Owner:KYOWA HAKKO KIRIN CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com