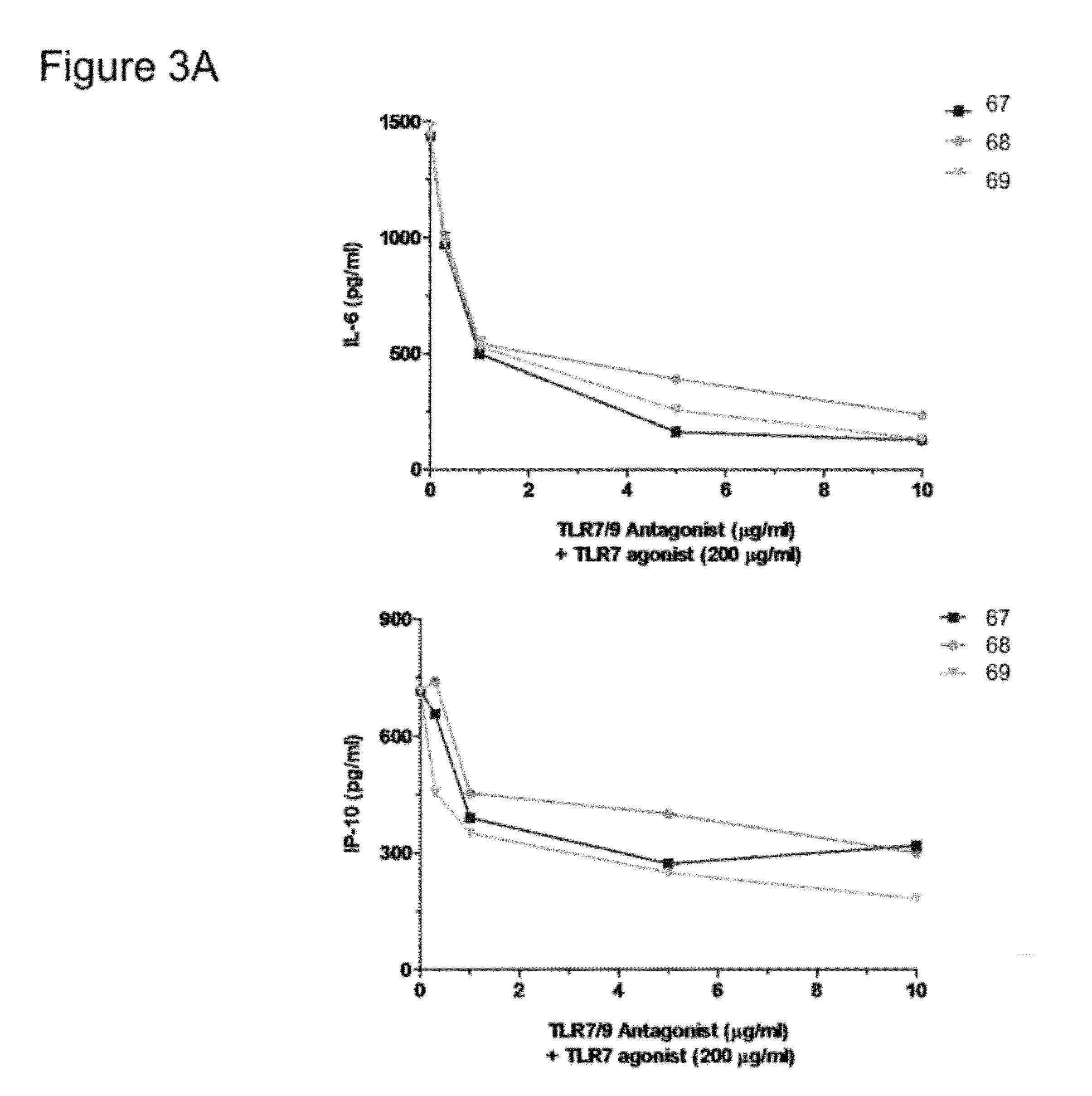
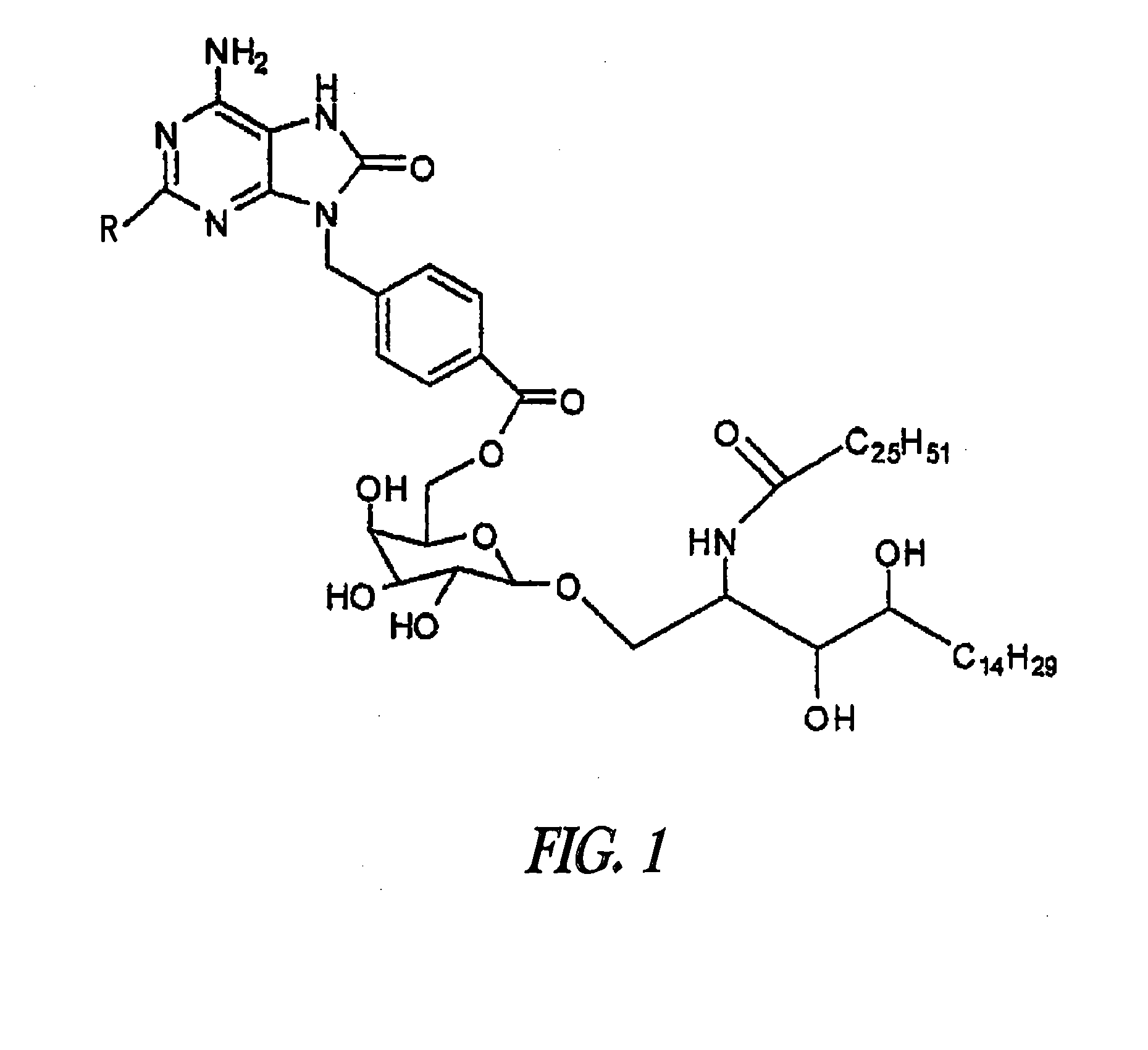
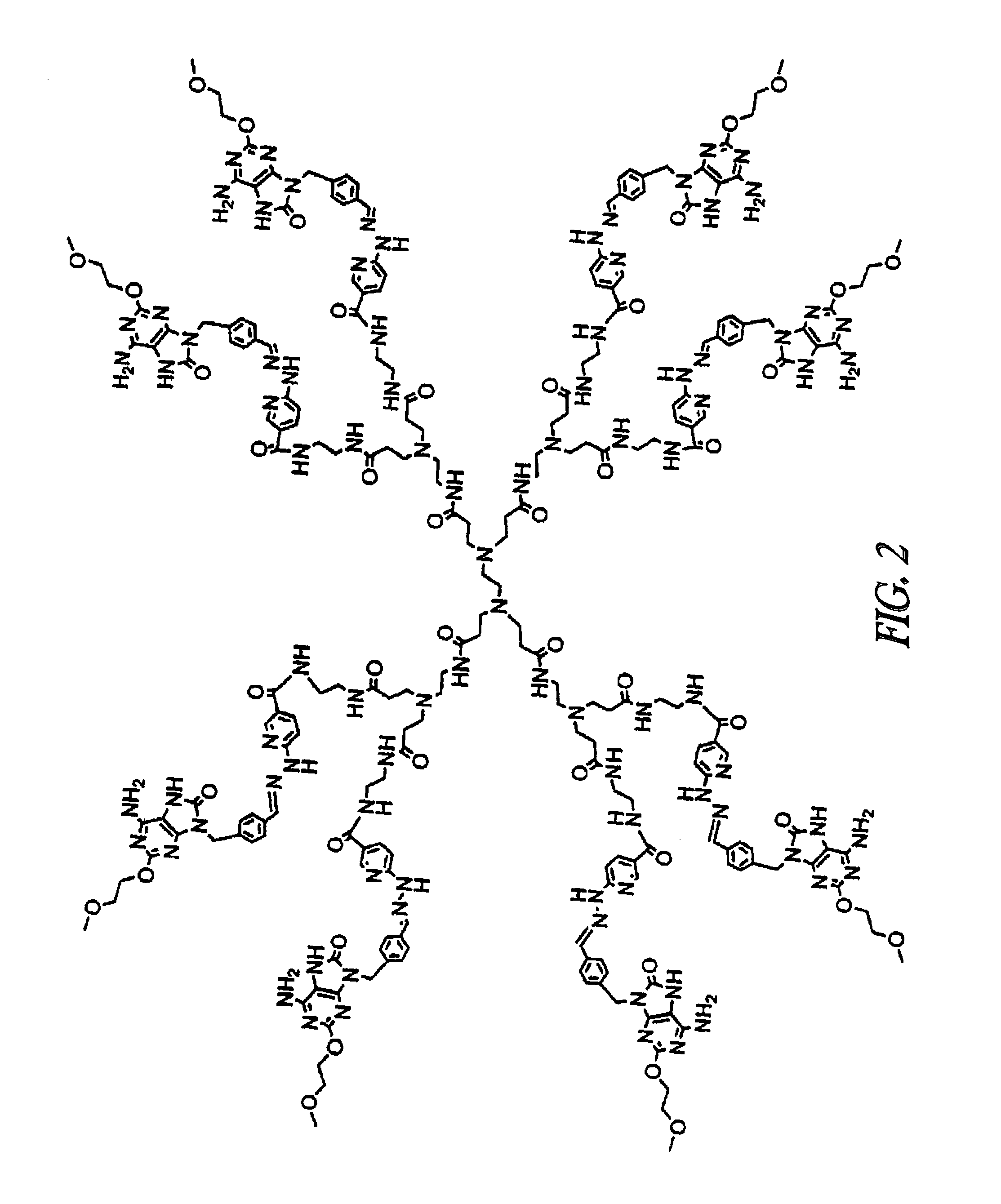
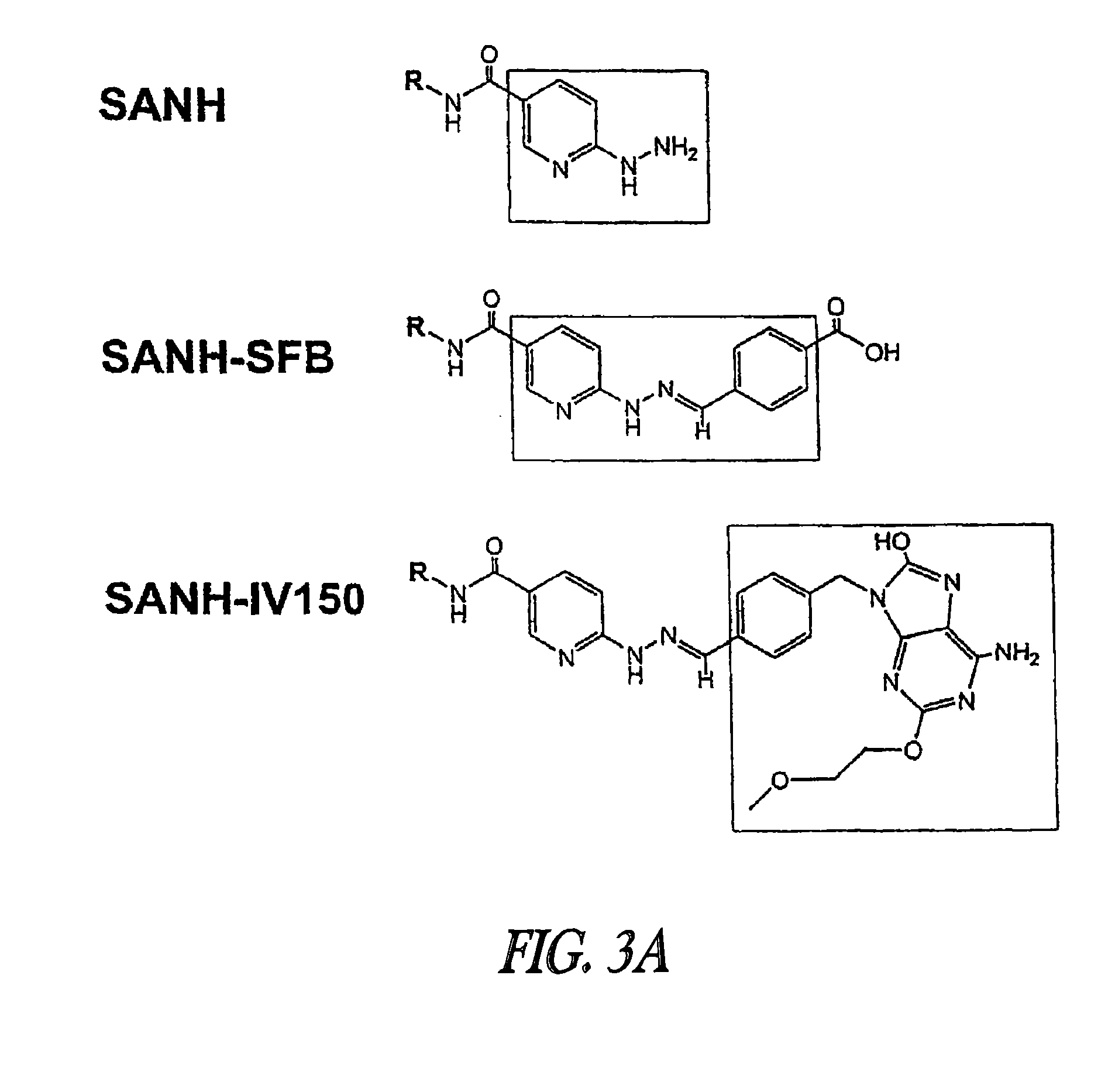
Patents
Literature
82 results about "Tlr agonists" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
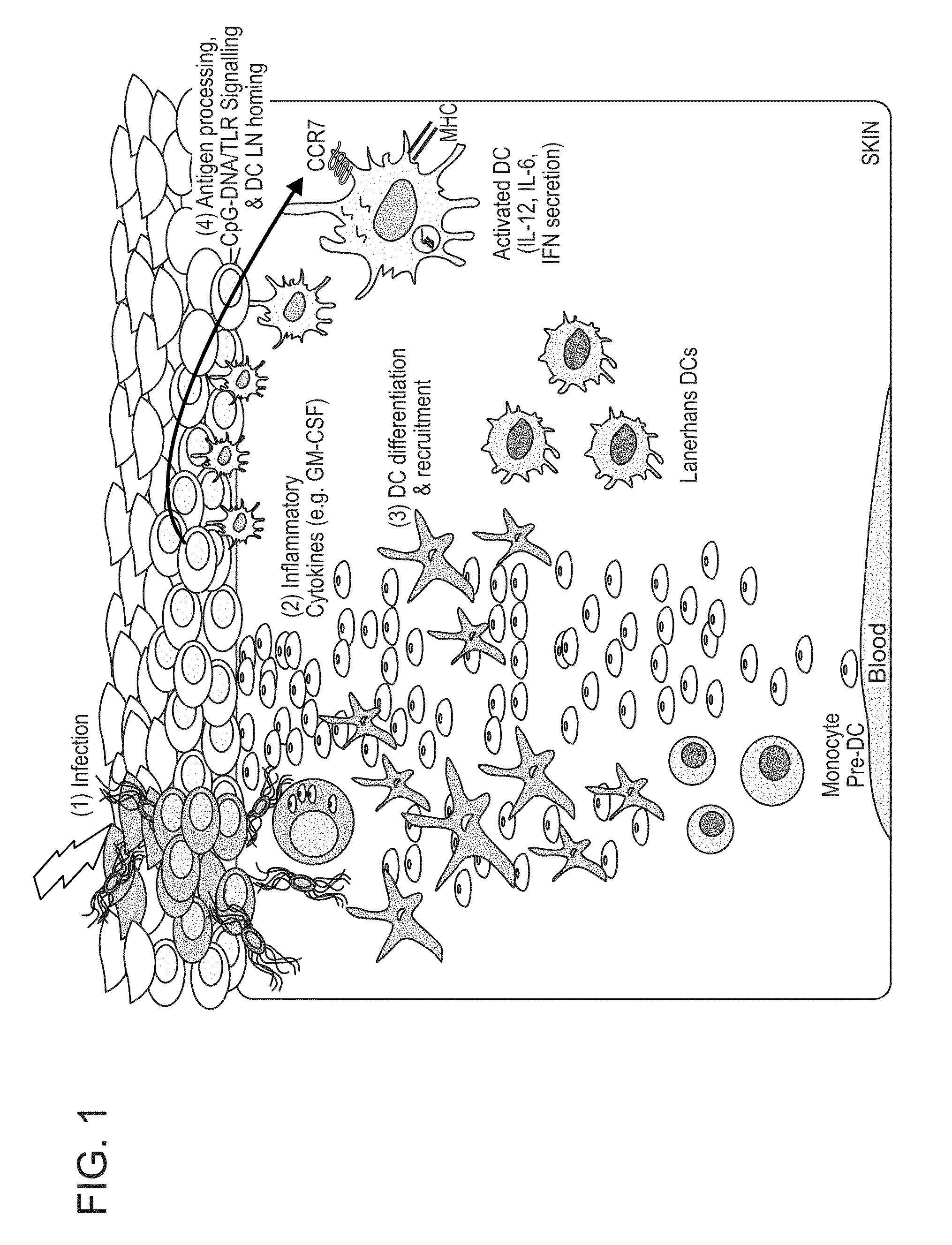
Therapeutic Use of a TLR Agonist and Combination Therapy
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Vaccine delivery compositions and methods of use
InactiveUS20080160089A1Easy to produceImprove efficiencySsRNA viruses negative-sensePowder deliveryPolyesterMHC class I
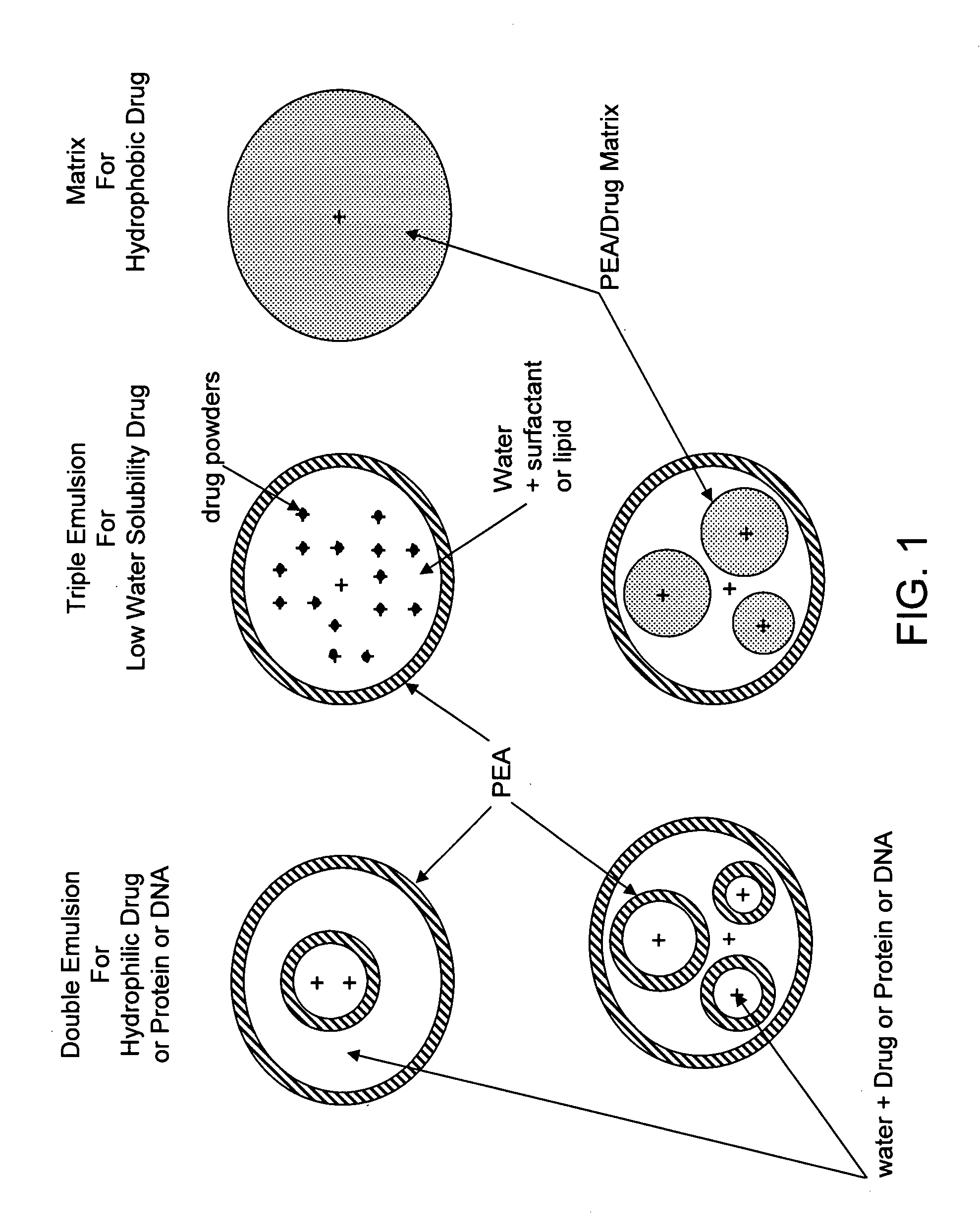
The present invention provides synthetic vaccines against a variety of pathogenic organisms and tumor cells in humans and other mammals based on biodegradable polymers containing polyester amide (PEA), polyester urethane (PEUR), and polyester urea (PEU) and immunostimulatory adjuvants. The vaccines can be formulated as a liquid dispersion of polymer particles or molecules in which are dispersed an immunostimulatory adjuvant, such as a TLR agonist, and whole protein or peptidic antigens containing MHC class I or class II epitopes derived from organism or tumor cell proteins. Methods of inducing an immune response via intracellular mechanisms to the pathogenic organism or tumor cells specific for the antigen in the invention compositions are also included.
Owner:MEDIVAS LLC
Adjuvant combinations comprising a microbial tlr agonist, a cd40 or 4-1bb agonist, and optionally an antigen and the use thereof for inducing a synergistic enhancement in cellular immunity
InactiveUS20080241139A1Enhanced T cell responseImprove responseAntibacterial agentsAntimycoticsDiseaseYeast
Adjuvant combinations comprising at least one microbial TLR agonist such as a whole virus, bacterium or yeast or portion thereof such a membrane, spheroplast, cytoplast, or ghost, a CD40 or 4-1BB agonist and optionally an antigen wherein all 3 moieties may be separate or comprise the same recombinant microorganism or virus are disclosed. The use of these immune adjuvants for treatment of various chronic diseases such as cancers and HIV infection is also provided.
Owner:UNIV OF COLORADO THE REGENTS OF
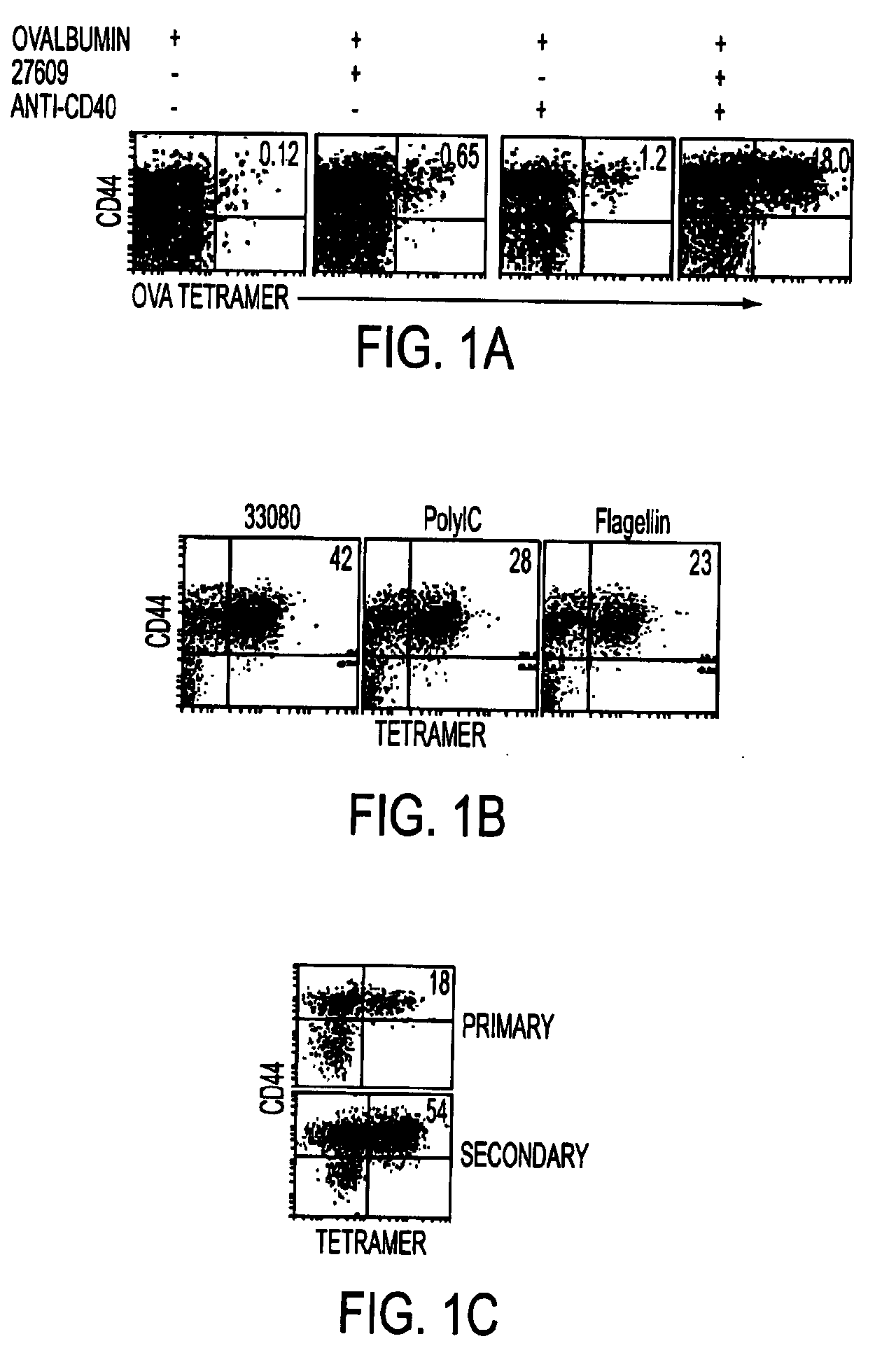
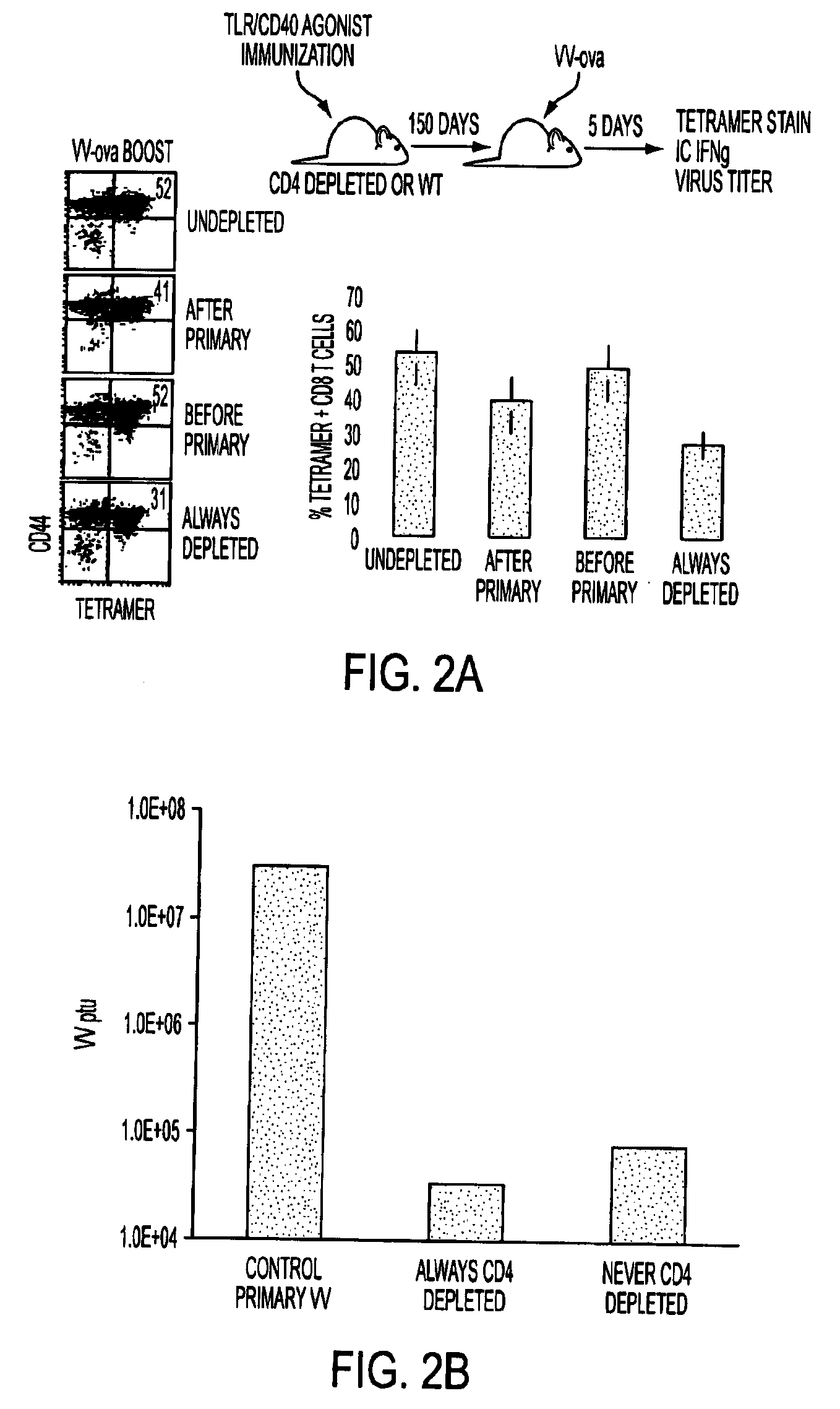
Tlr agonist (flagellin)/cd40 agonist/antigen protein and DNA conjugates and use thereof for inducing synergistic enhancement in immunity
InactiveUS20090004194A1Improve immunityEnhance cellular immune responseAntibacterial agentsFungiDiseaseTlr agonists
Owner:UNIV OF COLORADO THE REGENTS OF
Controlled Delivery of TLR Agonists in Structural Polymeric Devices
ActiveUS20130202707A1Increase successStimulate immune responsePowder deliveryOrganic active ingredientsTlr agonistsDendritic cell
The present invention comprises compositions, methods, and devices for creating an stimulating an antigen-specific dendritic cell immune response. Devices and methods provide prophylactic and therapeutic immunity to subjects against cancer and infectious agents.
Owner:DANA FARBER CANCER INST INC +1
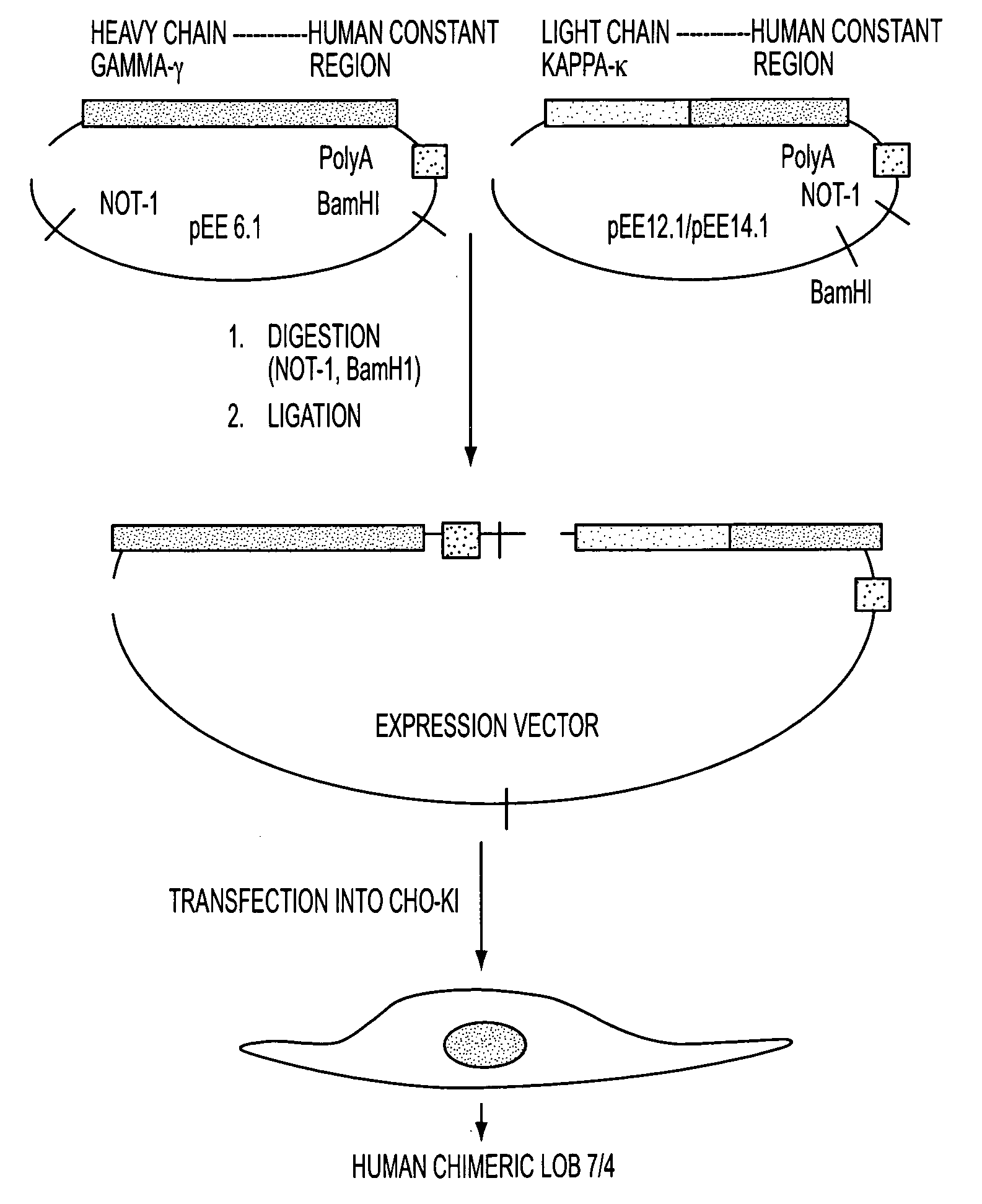
Human therapies using chimeric agonistic Anti-human cd40 antibody
Methods of human therapy using a chimeric anti-CD40 antibody, LOB 7 / 4 or humanized variants thereof, are provided. This CD40 antibody elicits agonistic effects on immunity when used as a monotherapy especially when used in the treatment of human lymphomas and leukemias and other solid tumors In addition, this agonistic CD40 antibody when administered in combination with certain molecules such as TLR agonists or interferons, e.g., alpha and beta interferon, elicits a synergistic effect on immunity.
Owner:UNIV OF SOUTHAMPTON
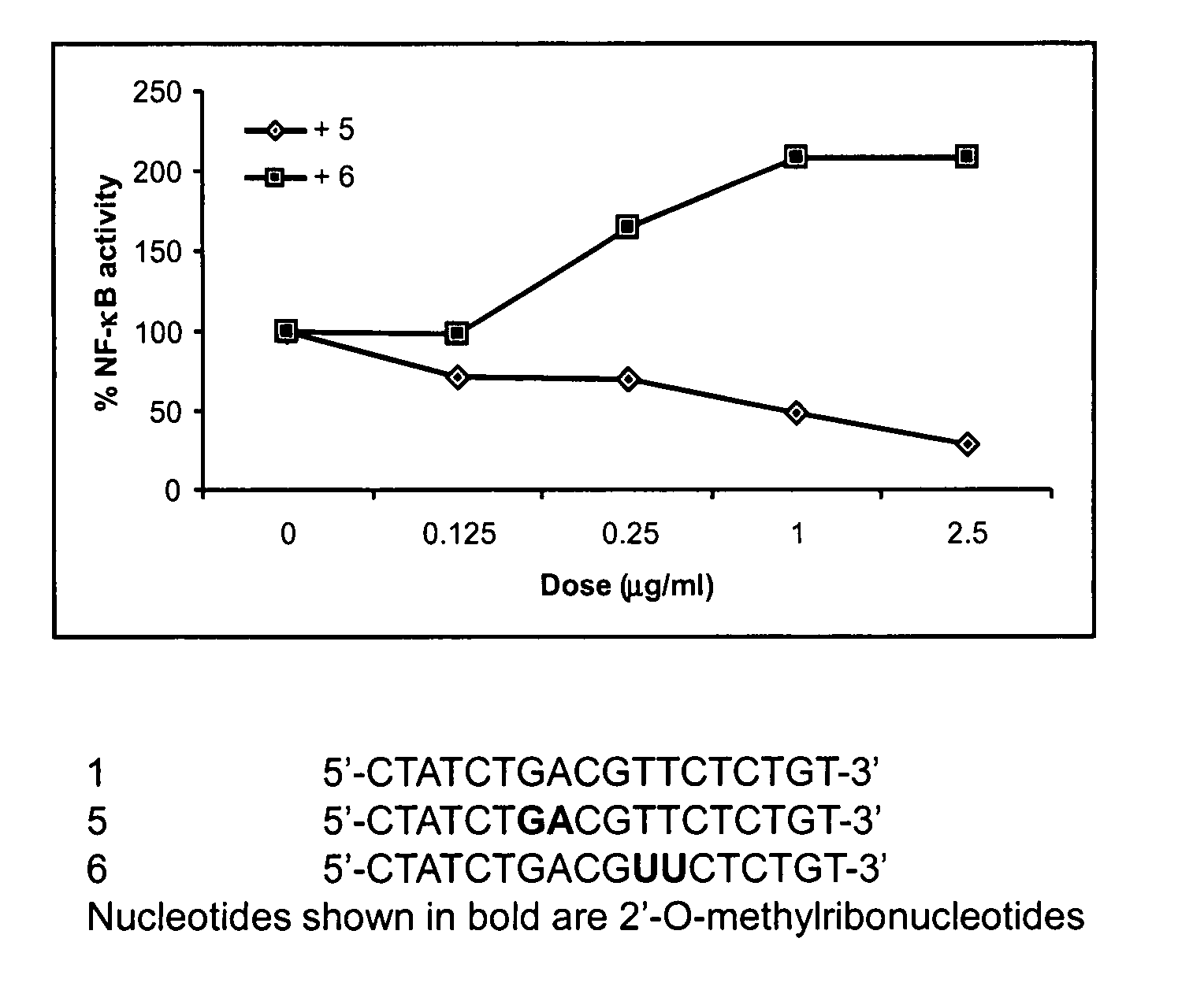
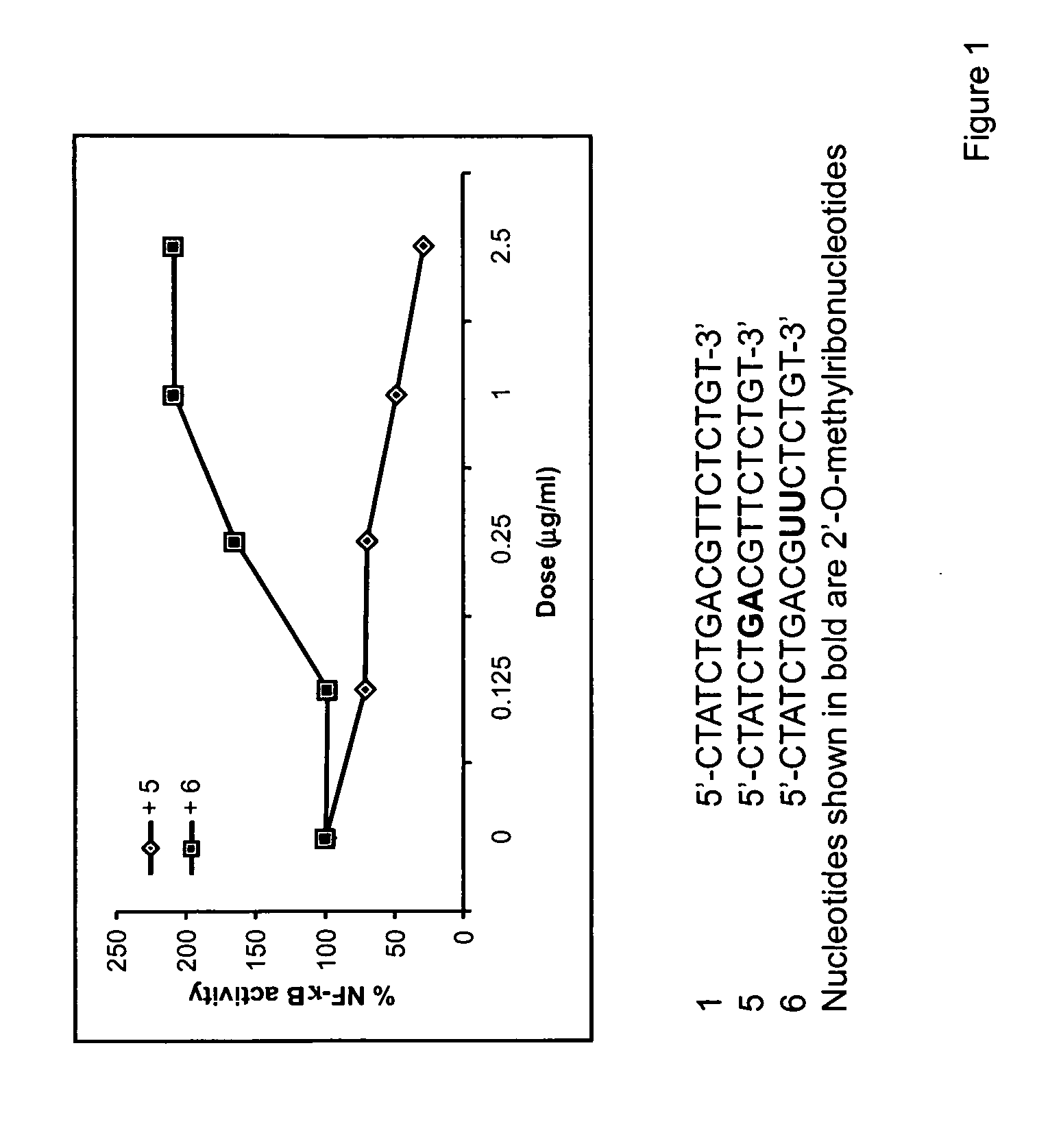
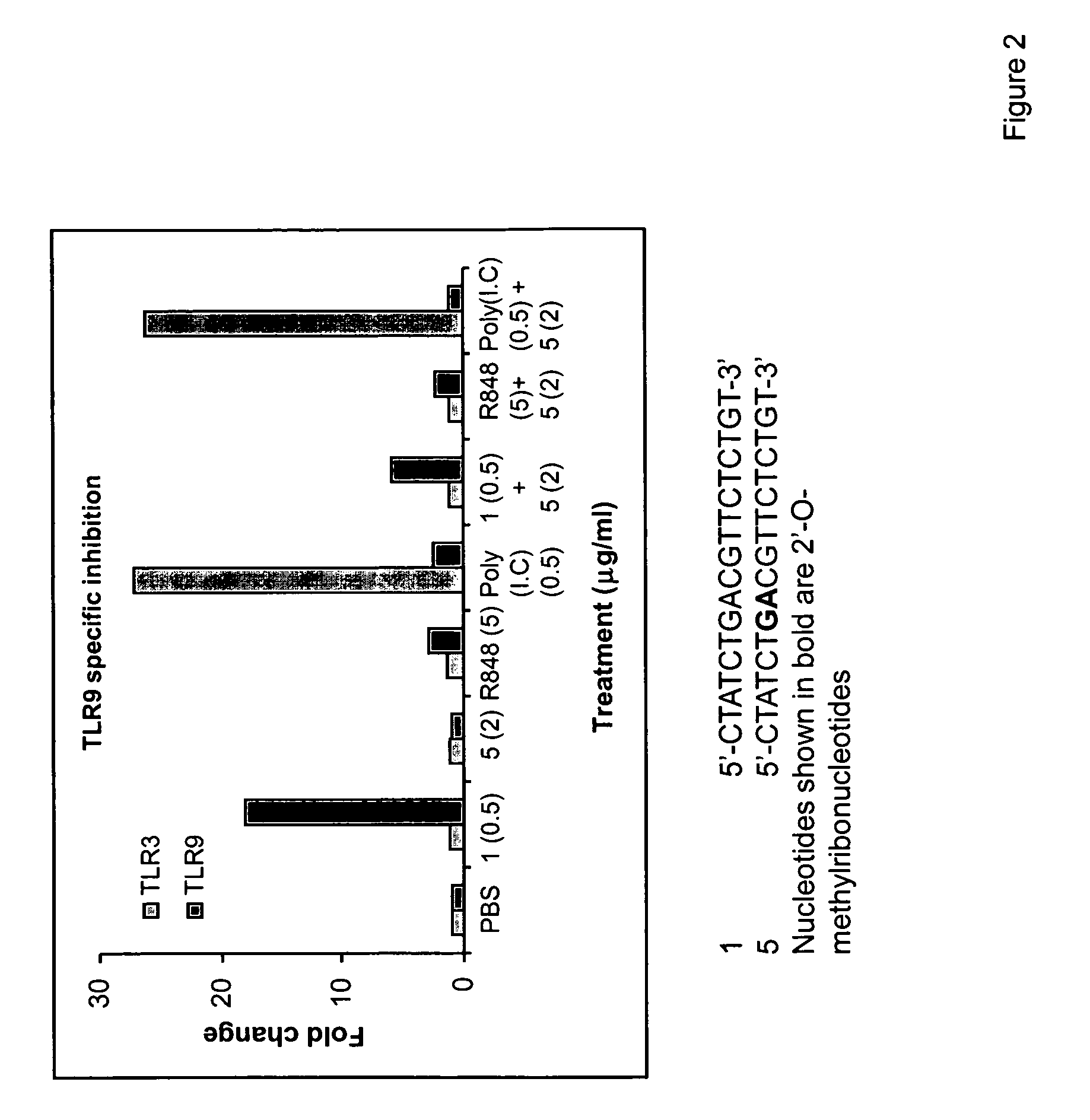
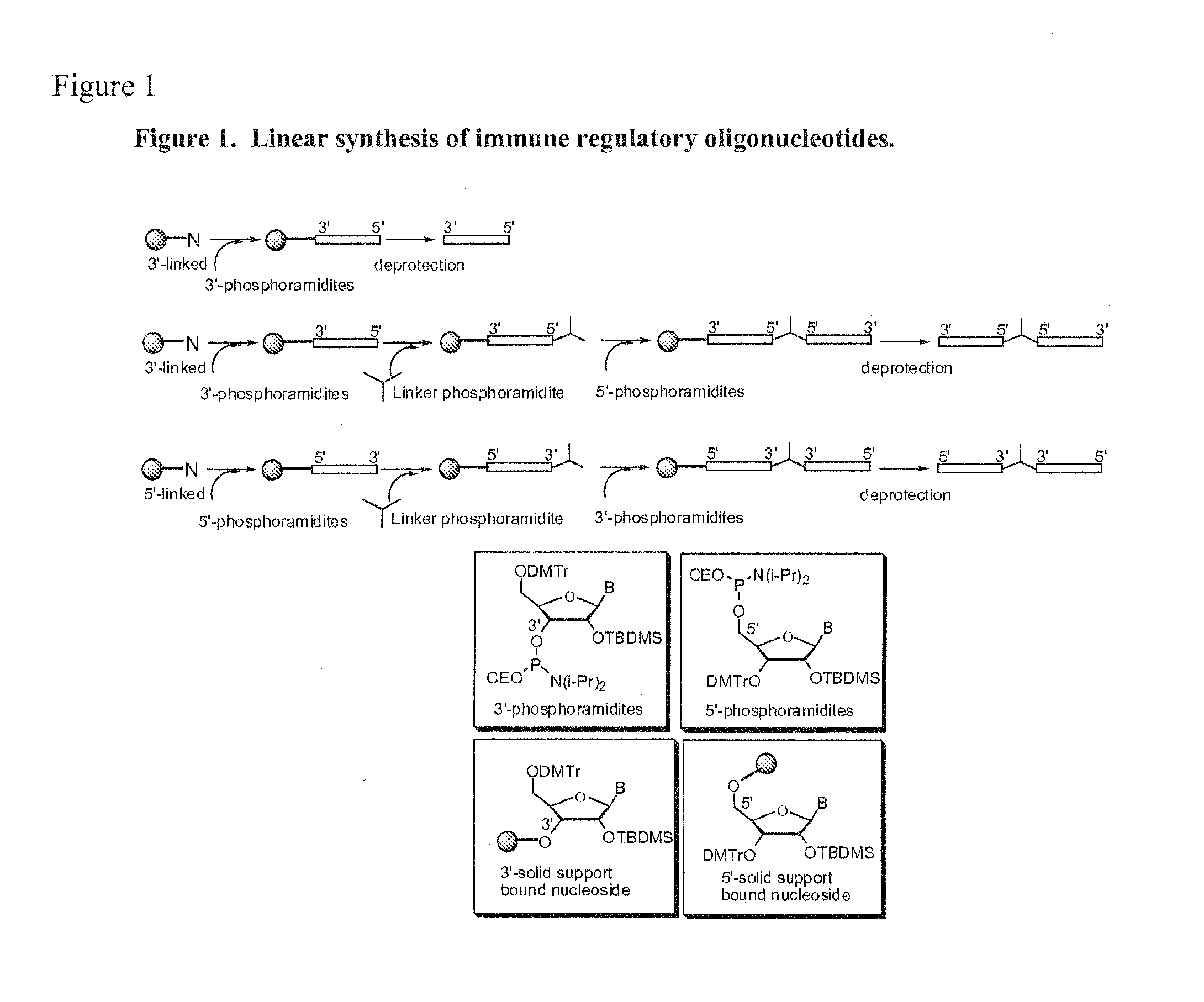
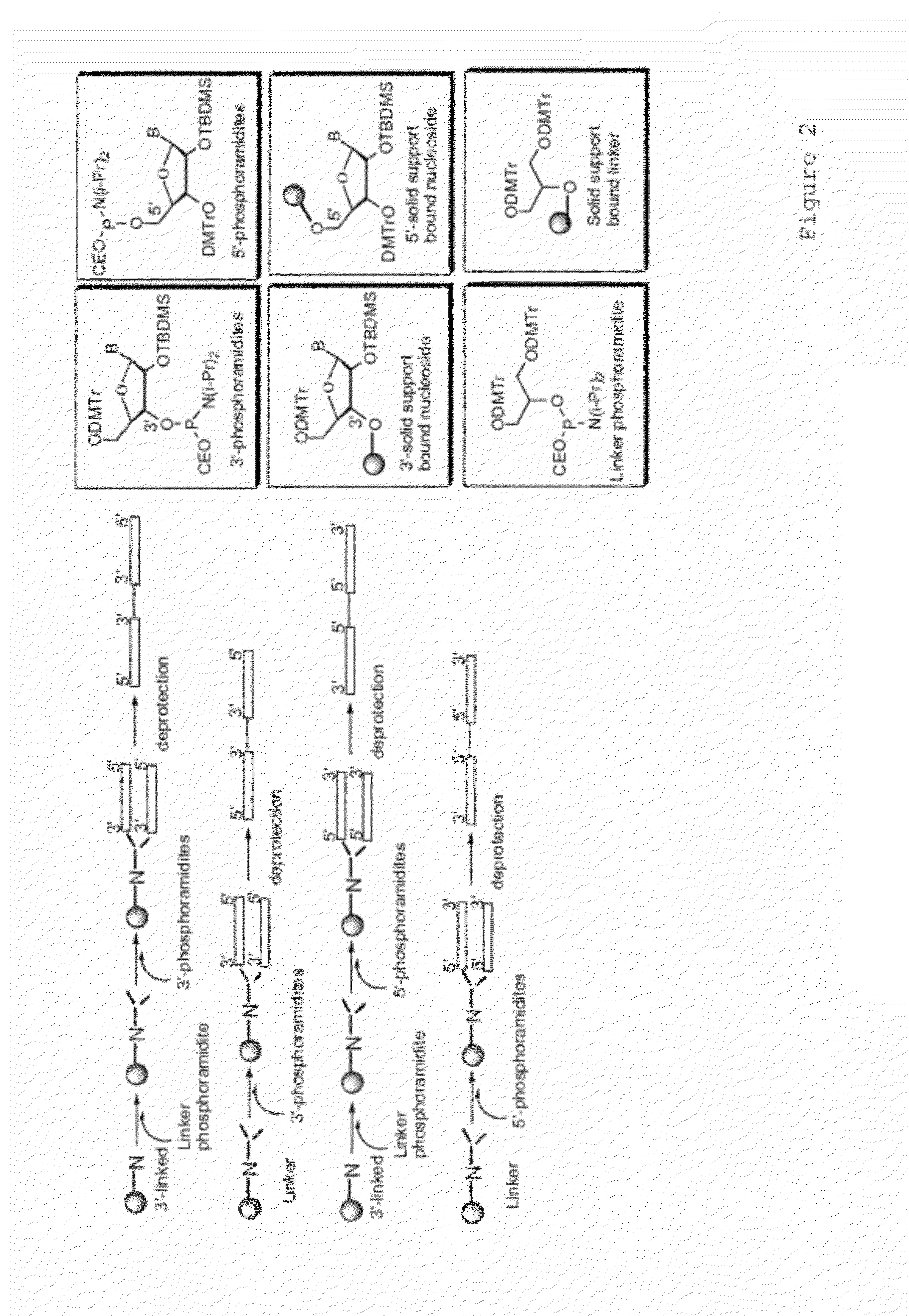
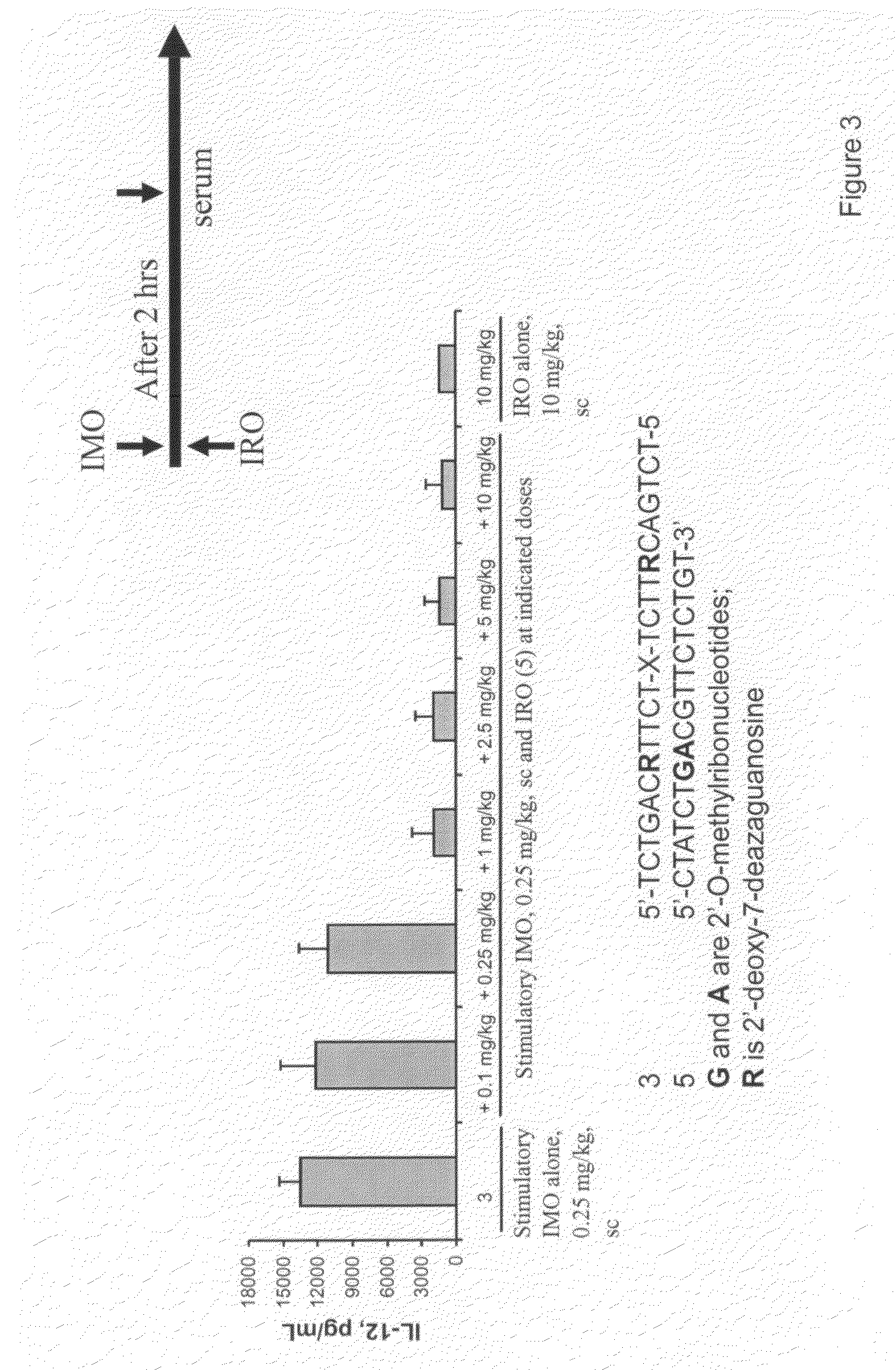
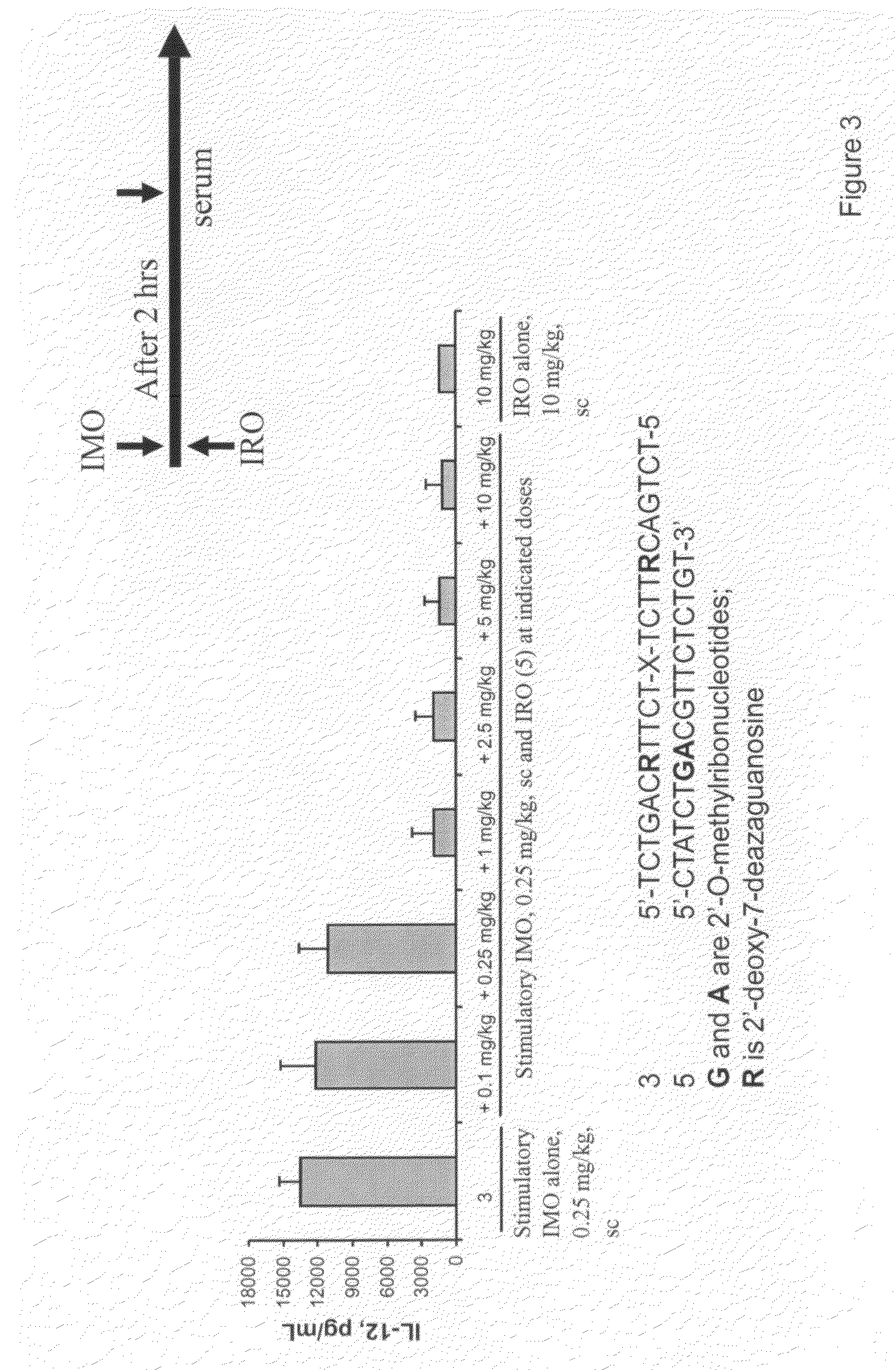
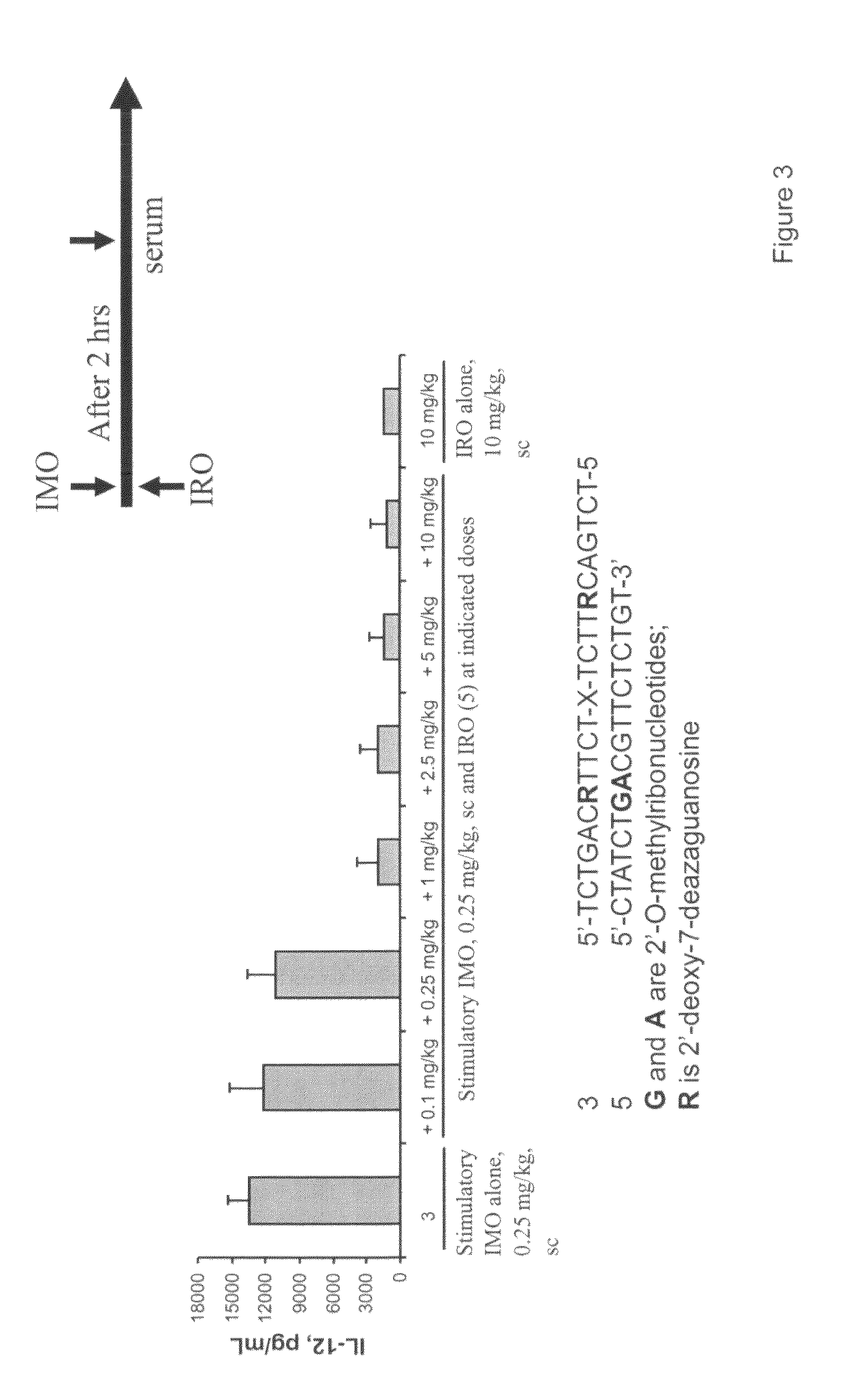
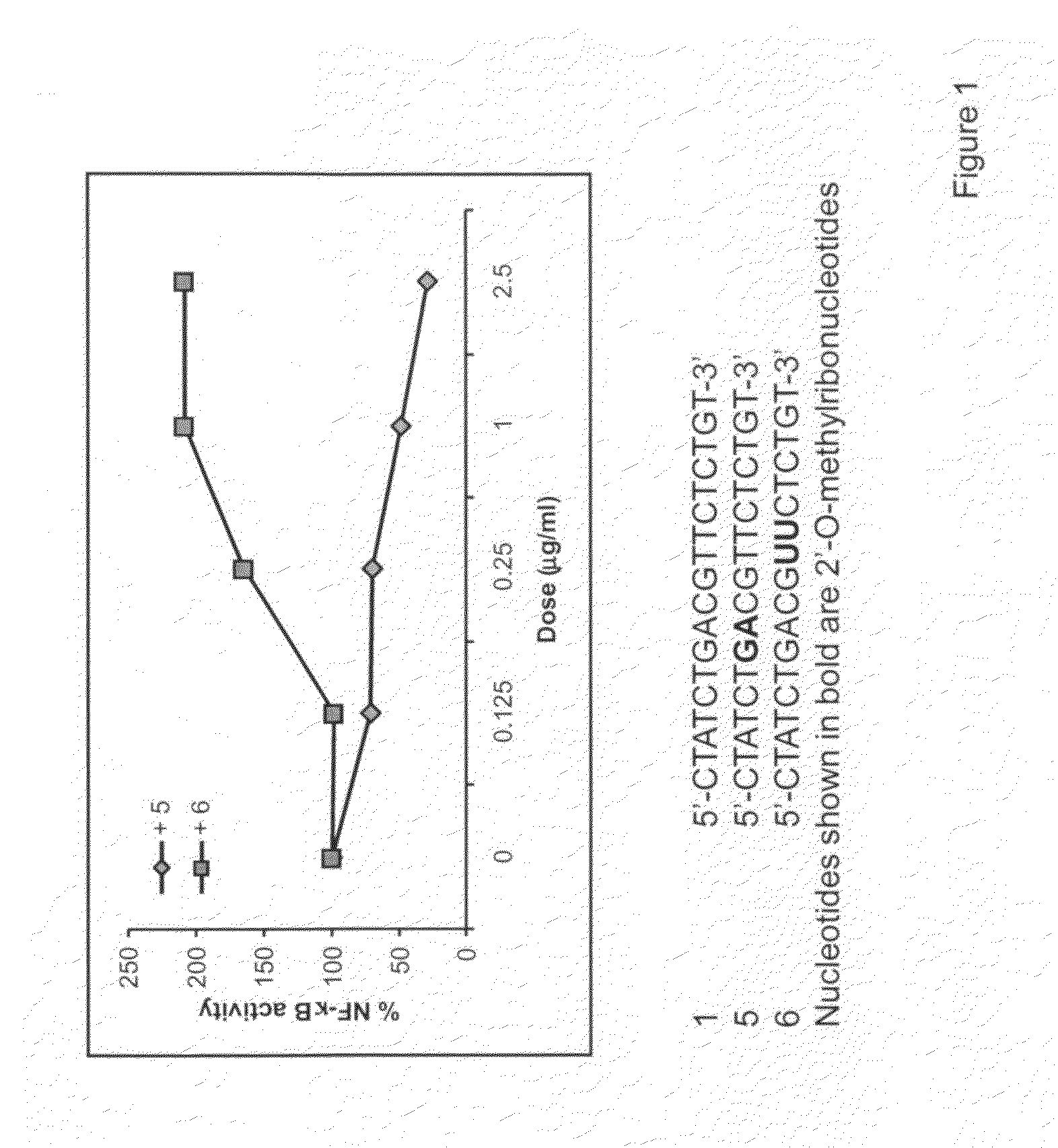
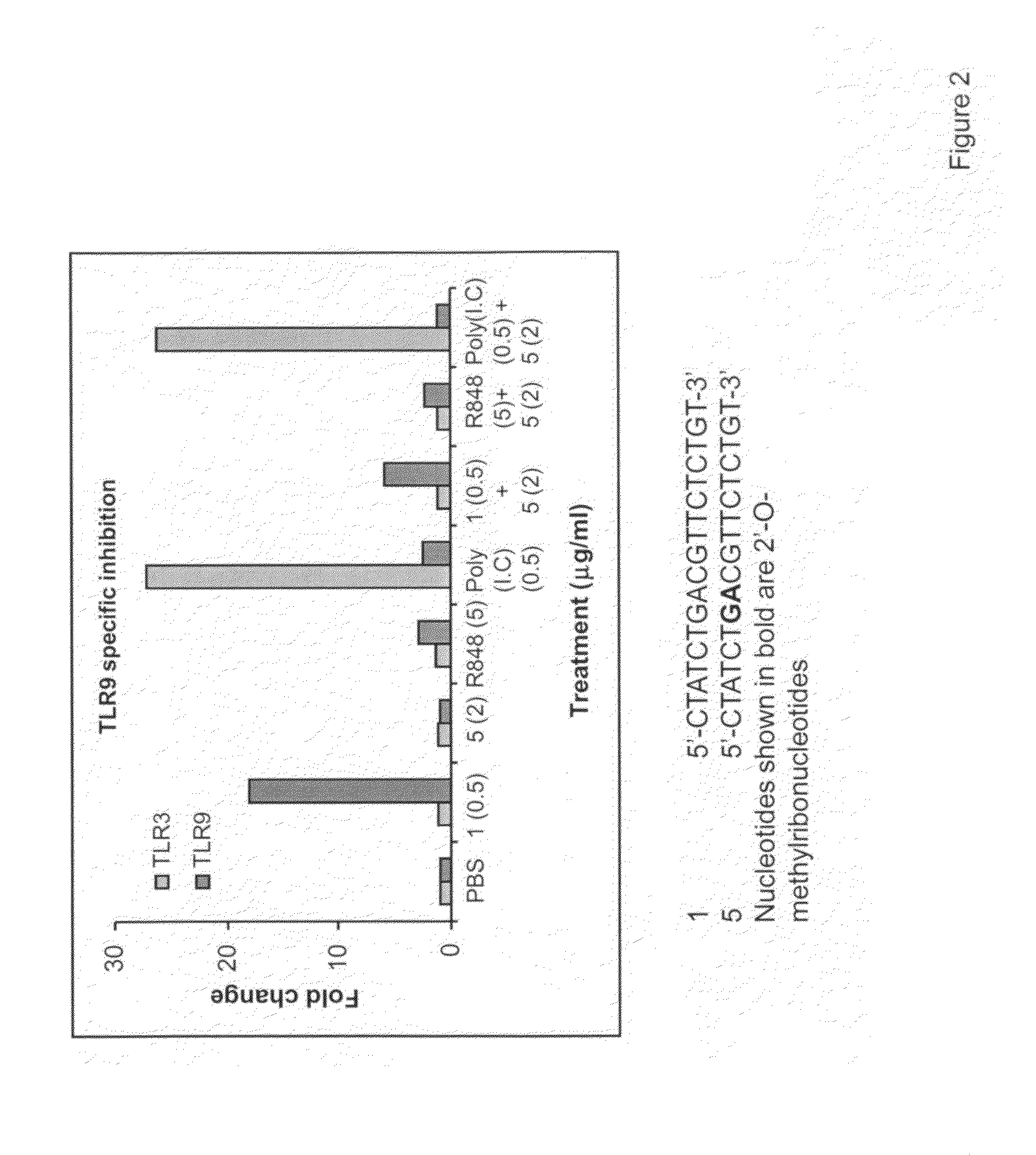
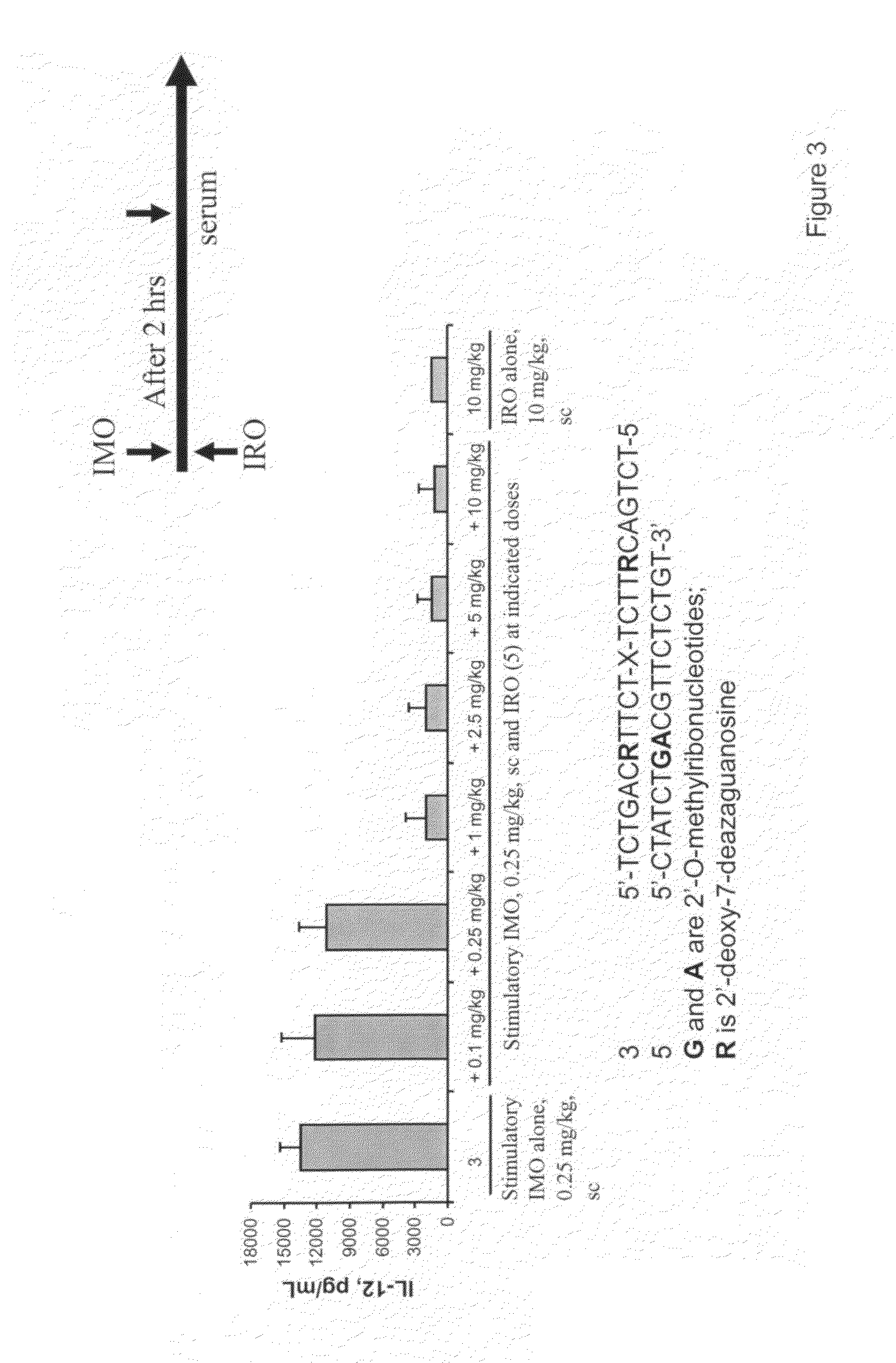
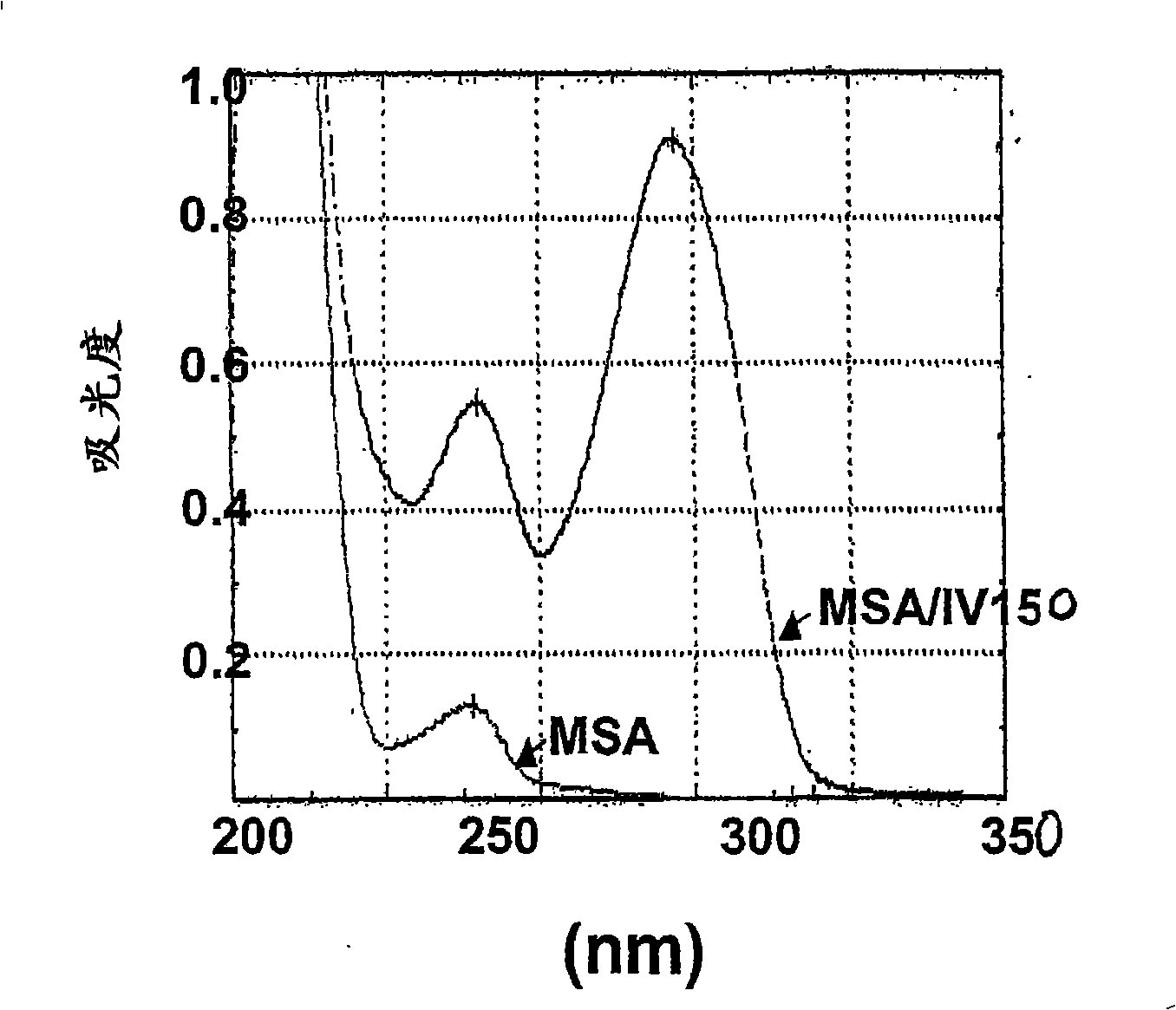
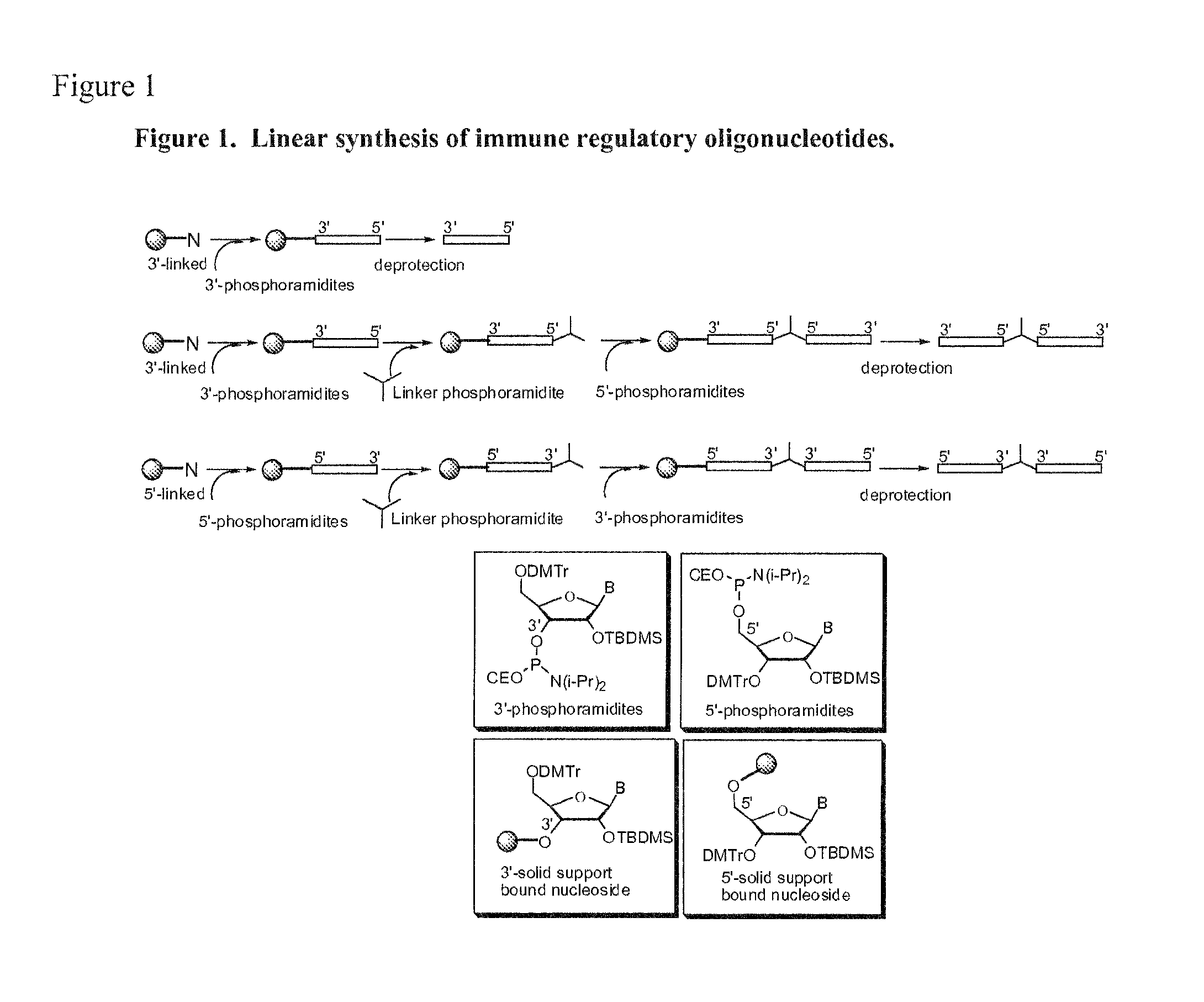
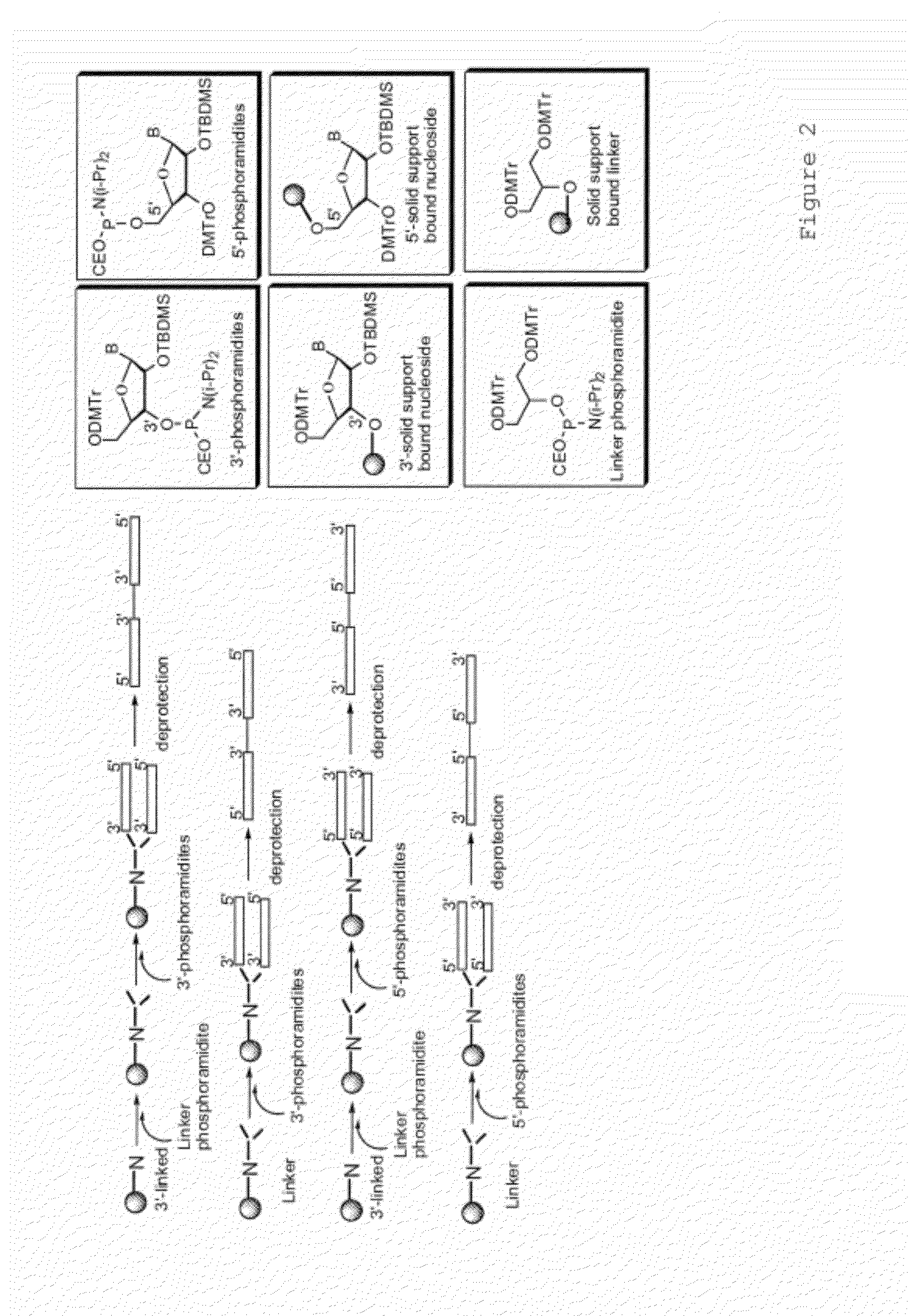
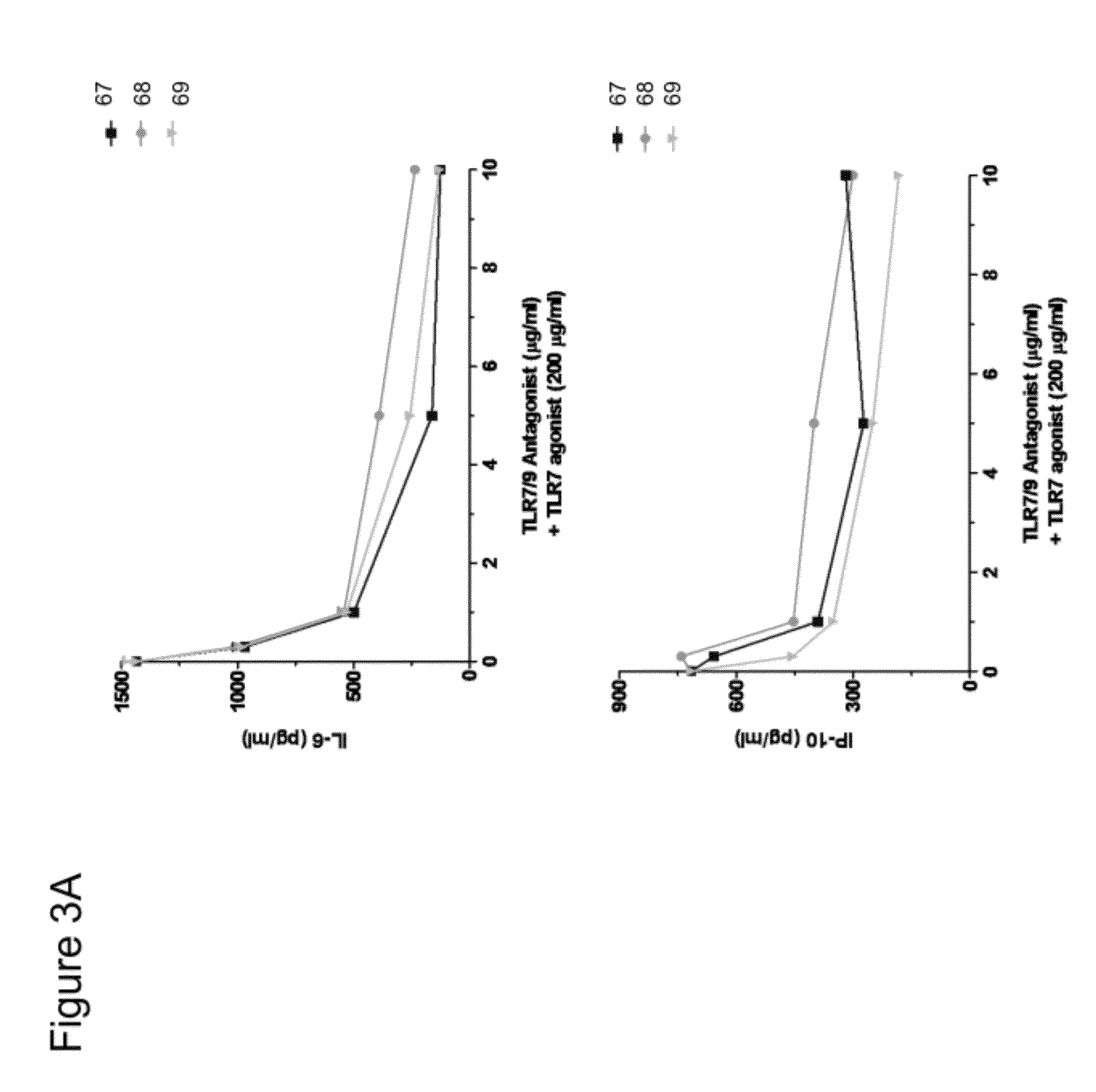
Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response
ActiveUS20090060898A1Avoid immune responseInhibitory activityOrganic active ingredientsAntipyreticTlr agonistsAutoimmune disease
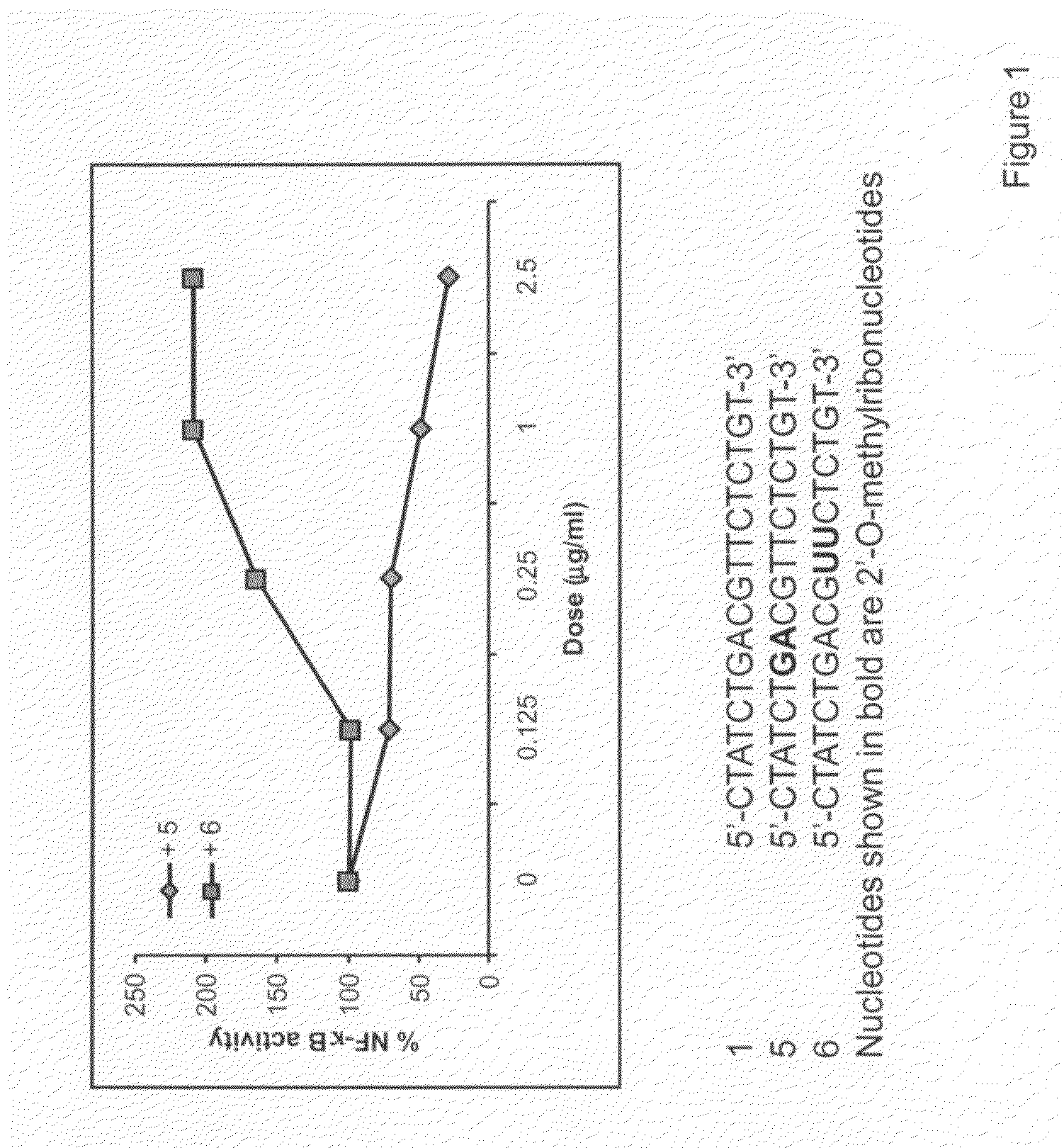
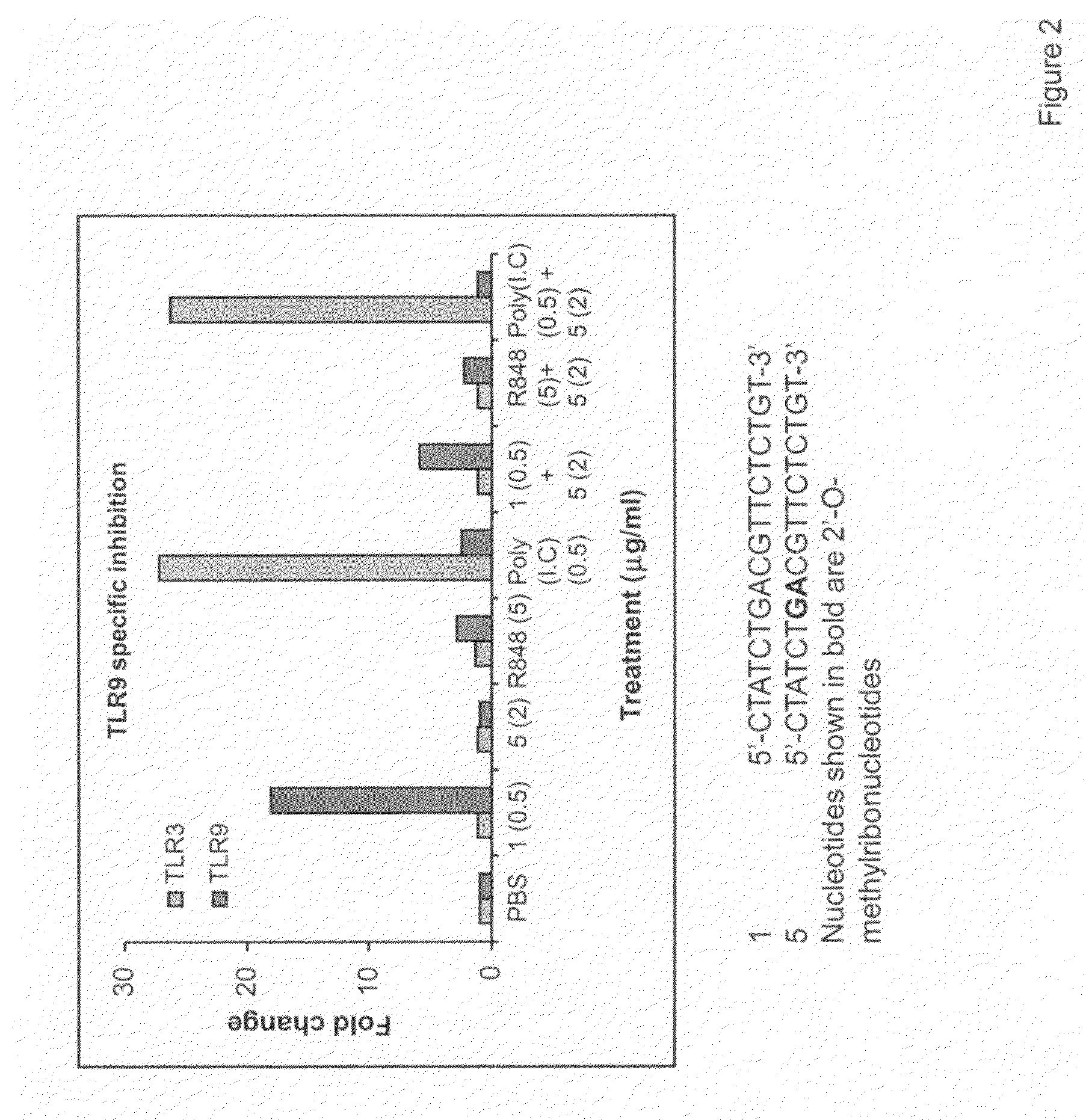
The invention provides novel immune regulatory oligonucleotides (IRO) as antagonist of TLRs and methods of use thereof. These IROs have unique sequences that inhibit or suppress TLR-mediated signaling in response to a TLR ligand or TLR agonist. The methods may have use in the prevention and treatment of cancer, an autoimmune disorder, airway inflammation, inflammatory disorders, infectious disease, skin disorders, allergy, asthma or a disease caused by a pathogen.
Owner:IDERA PHARMA INC
Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response
ActiveUS20120128699A1Antagonize inhibit suppress preventAntagonize, inhibit suppress or prevent the cytokine and chemokine responseNervous disorderAntipyreticTlr agonistsAutoimmune disease
The invention provides novel immune regulatory oligonucleotides (IRO) as antagonist of TLRs and methods of use thereof. These TROs have unique sequences that inhibit or suppress TLR-mediated signaling in response to a TLR ligand or TLR agonist. The methods may have use in the prevention and treatment of cancer, an autoimmune disorder, airway inflammation, inflammatory disorders, infectious disease, skin disorders, allergy, asthma or a disease caused by a pathogen.
Owner:IDERA PHARMA INC
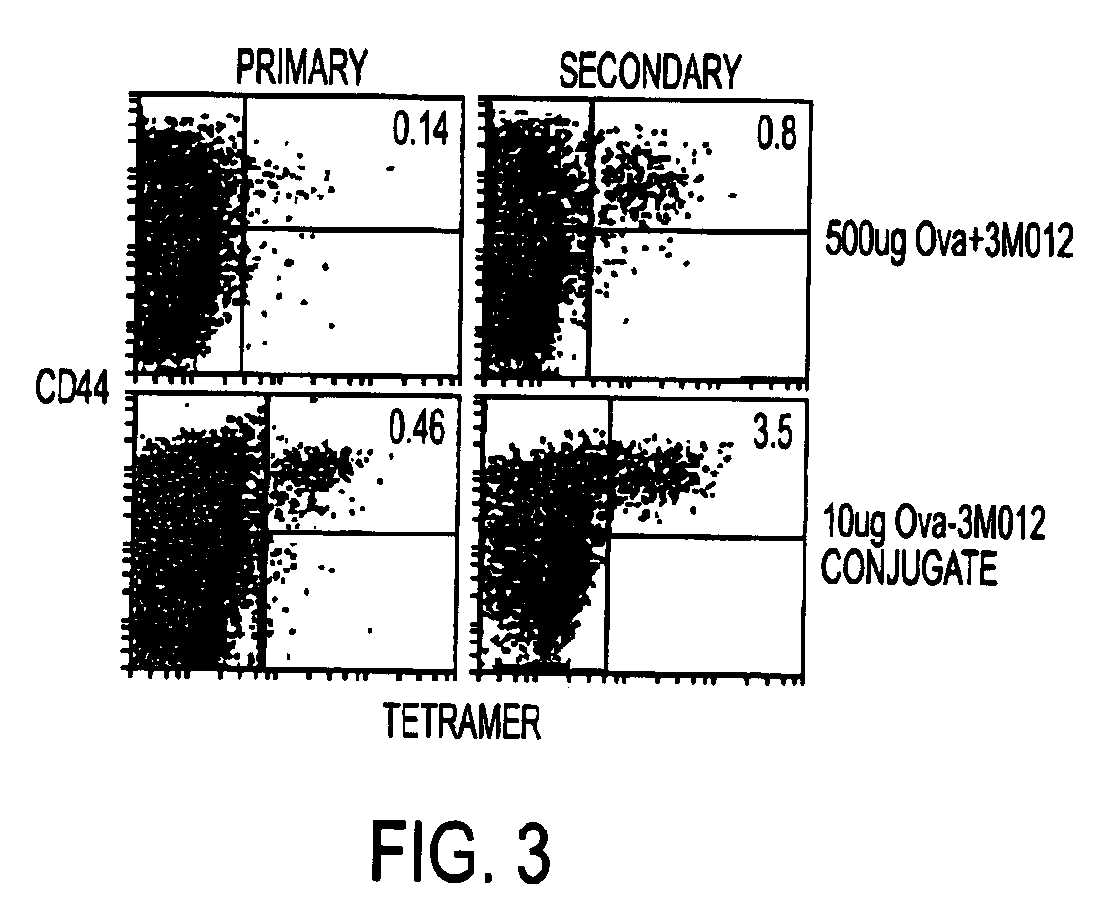
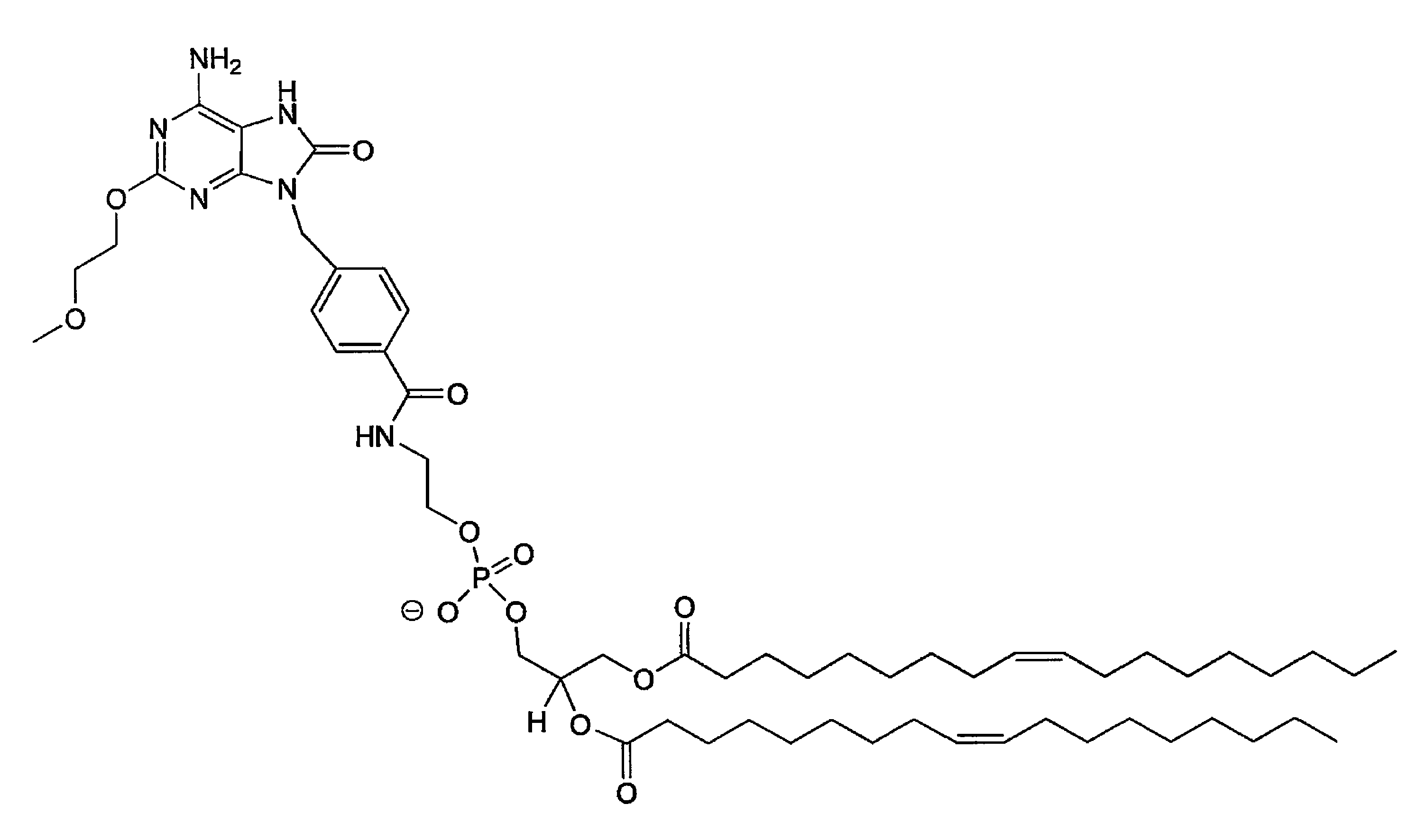
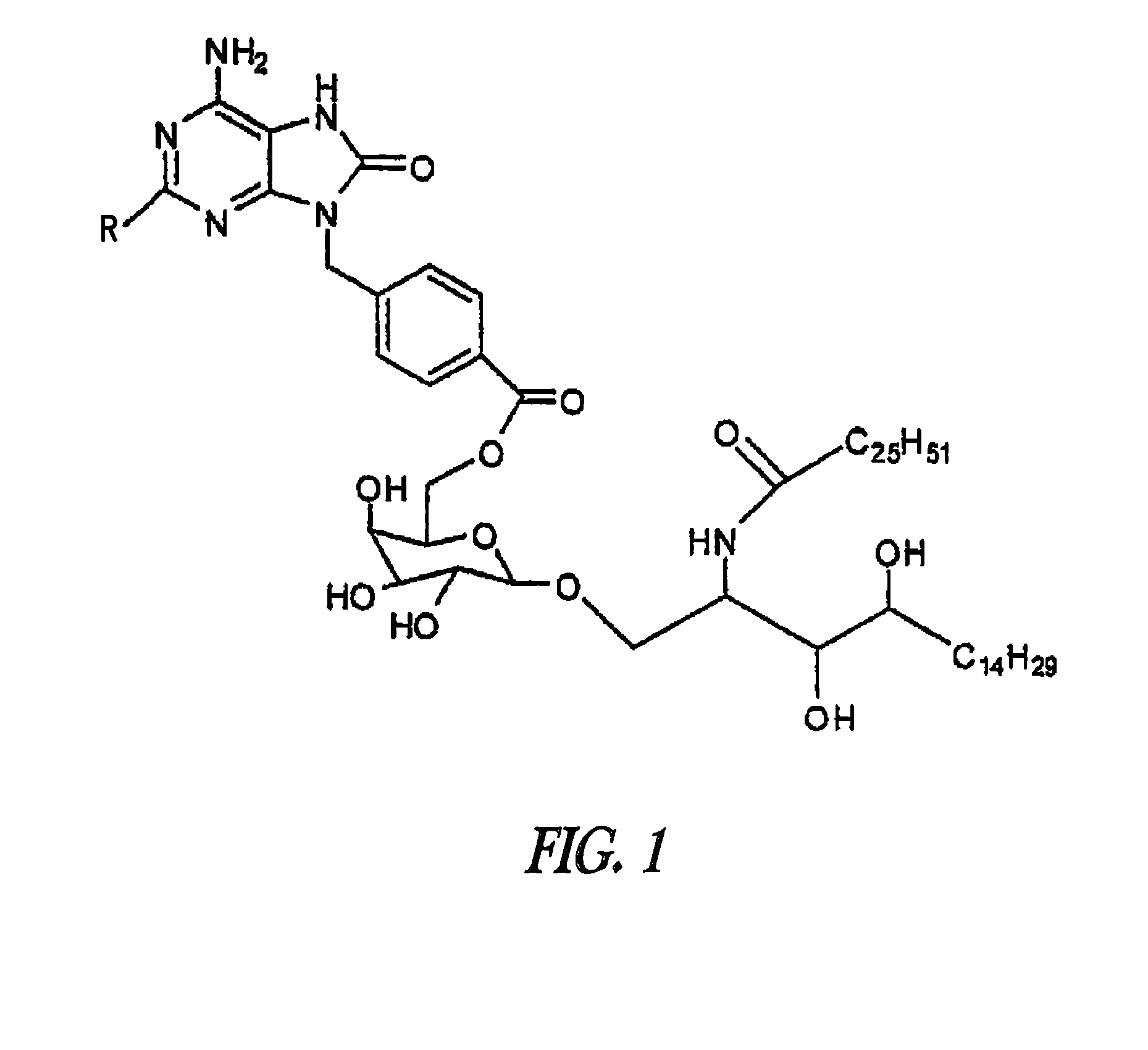
Conjugates of synthetic tlr agonists and uses therefor
ActiveUS20120148660A1Stimulate immune responseUndesirable systemic side effectAntibacterial agentsOrganic active ingredientsTlr agonistsChemistry
The invention provides TLR agonists and conjugates thereof useful in vaccines and to prevent, inhibit or treat a variety of disorders including pathogen infection and asthma.
Owner:RGT UNIV OF CALIFORNIA
Tlr agonist (flagellin)/cd40 agonist/antigen protein and DNA conjugates and use thereof for inducing synergistic enhancement in immunity
Owner:KEDL ROSS
Immunotherapy of cancer through combination of local and systemic immune stimulation
Provided herein are compositions and methods for immunotherapy of cancers, said methods combining systemic vaccination with a recombinant expression vector encoding a viral antigen and / or a cancer specific tumor antigen(s) to induce activated CD8 T-cells, with a local (e.g., intratumoral) immune stimulation using standard or modified application of a TLR agonist, to induce local inflammatory and innate immune responses which recruit T-cells to the tumor.
Owner:IMMUNE DESIGN CORP
Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response
ActiveUS20090087388A1Avoid immune responseInhibitory activityOrganic active ingredientsCosmetic preparationsTlr agonistsAutoimmune disease
The invention provides novel immune regulatory oligonucleotides (IRO) as antagonist of TLRs and methods of use thereof. These IROs have unique sequences that inhibit or suppress TLR-mediated signaling in response to a TLR ligand or TLR agonist. The methods may have use in the prevention and treatment of cancer, an autoimmune disorder, airway inflammation, inflammatory disorders, infectious disease, skin disorders, allergy, asthma or a disease caused by a pathogen.
Owner:IDERA PHARMA INC
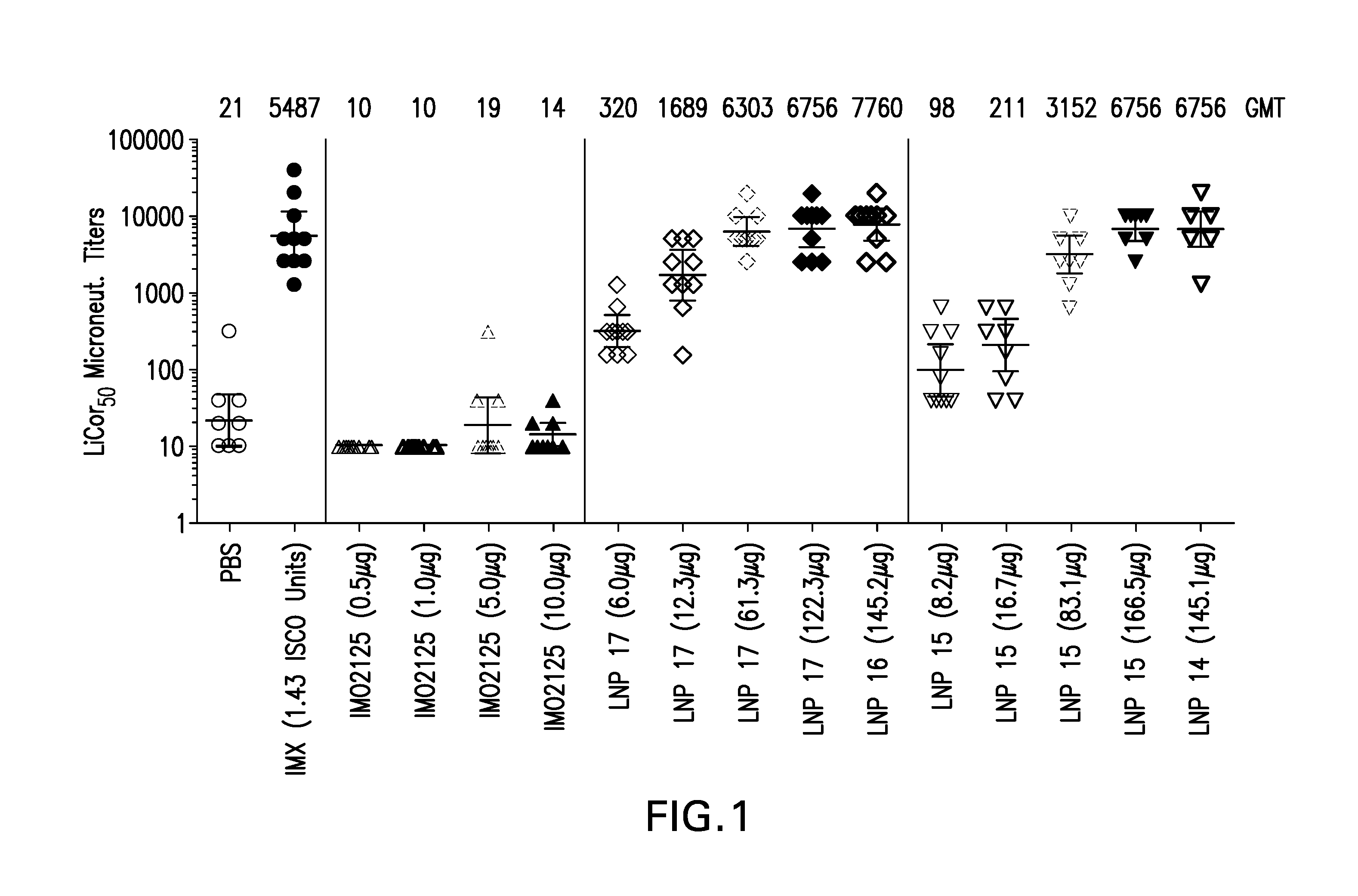
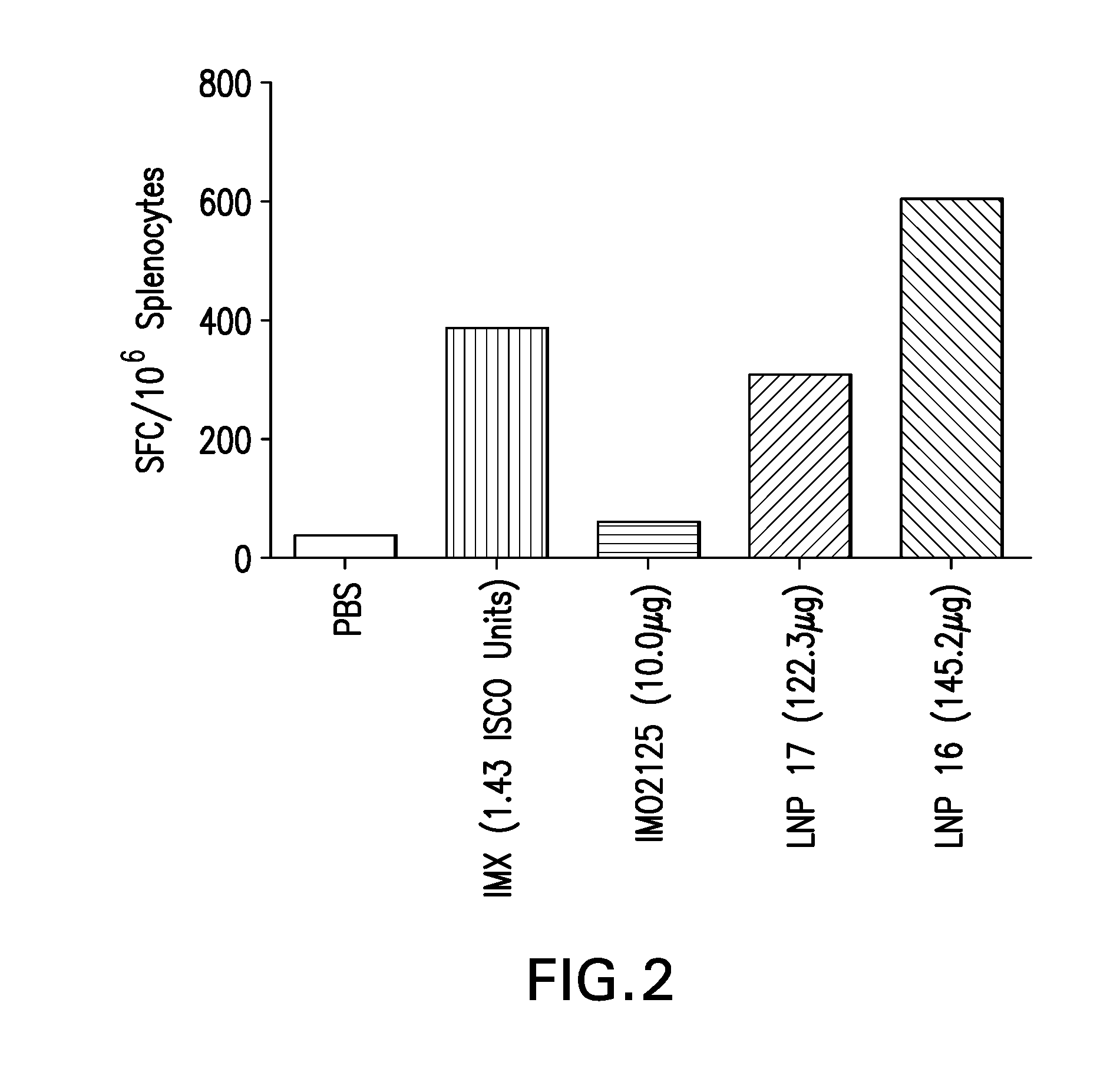
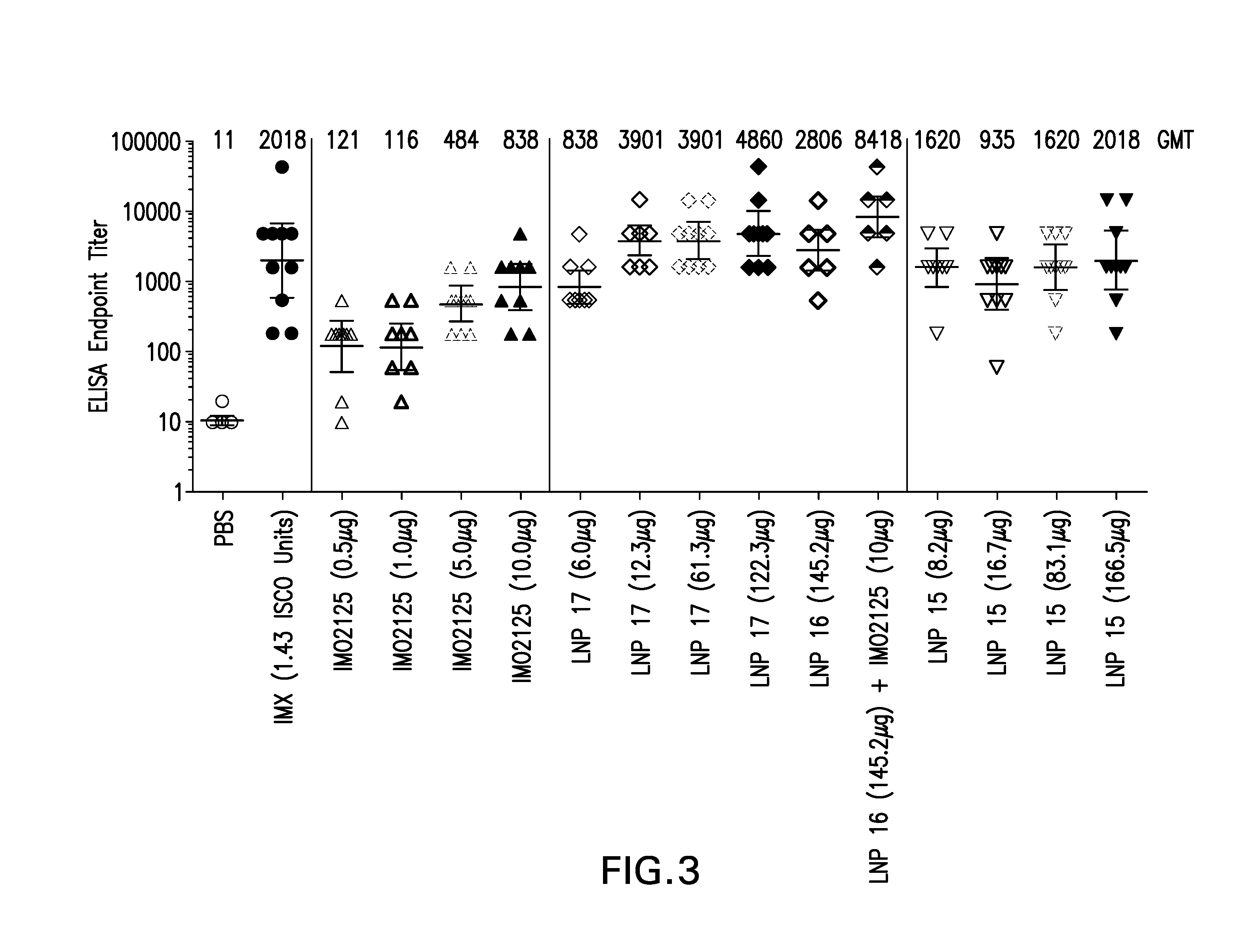
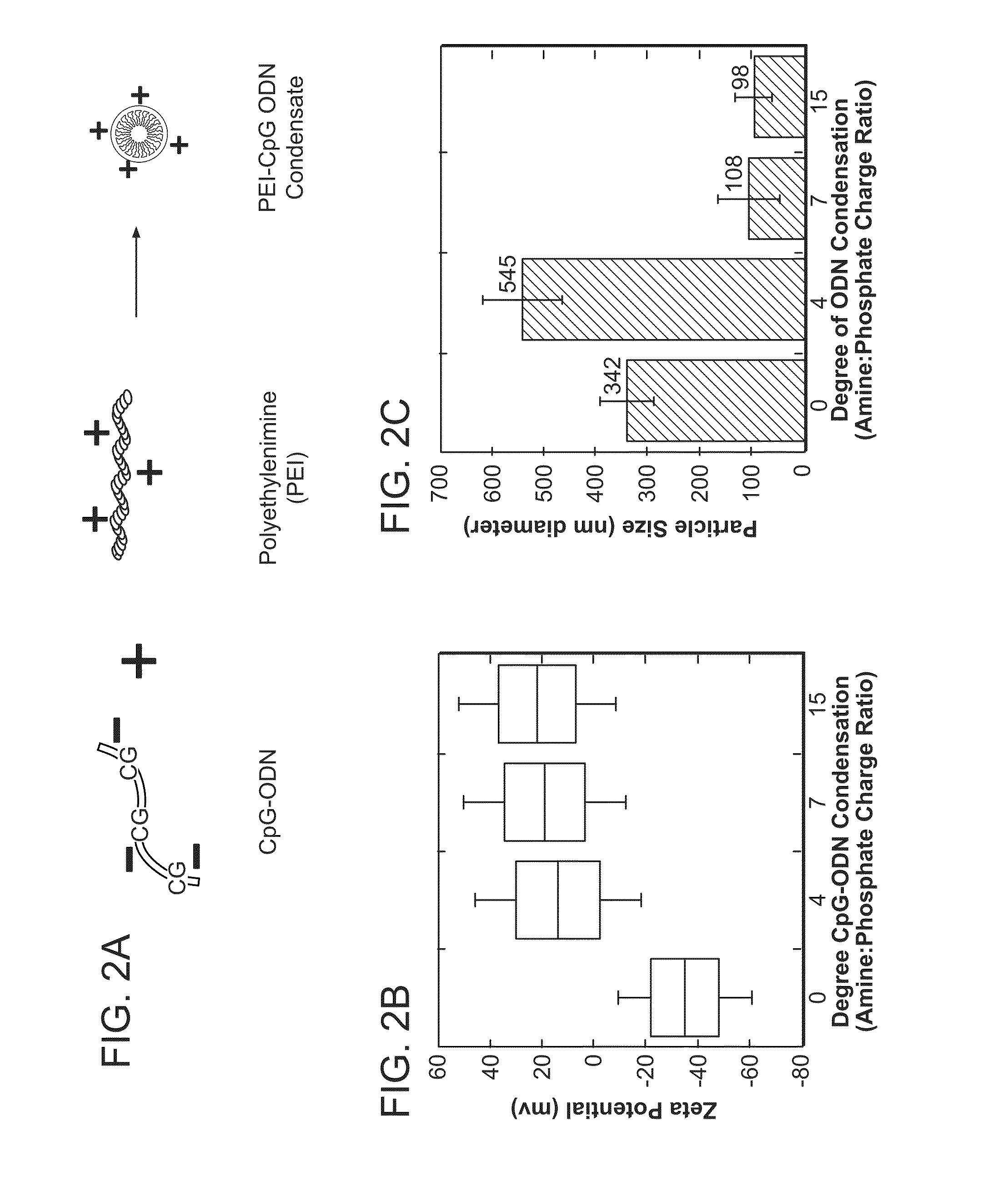
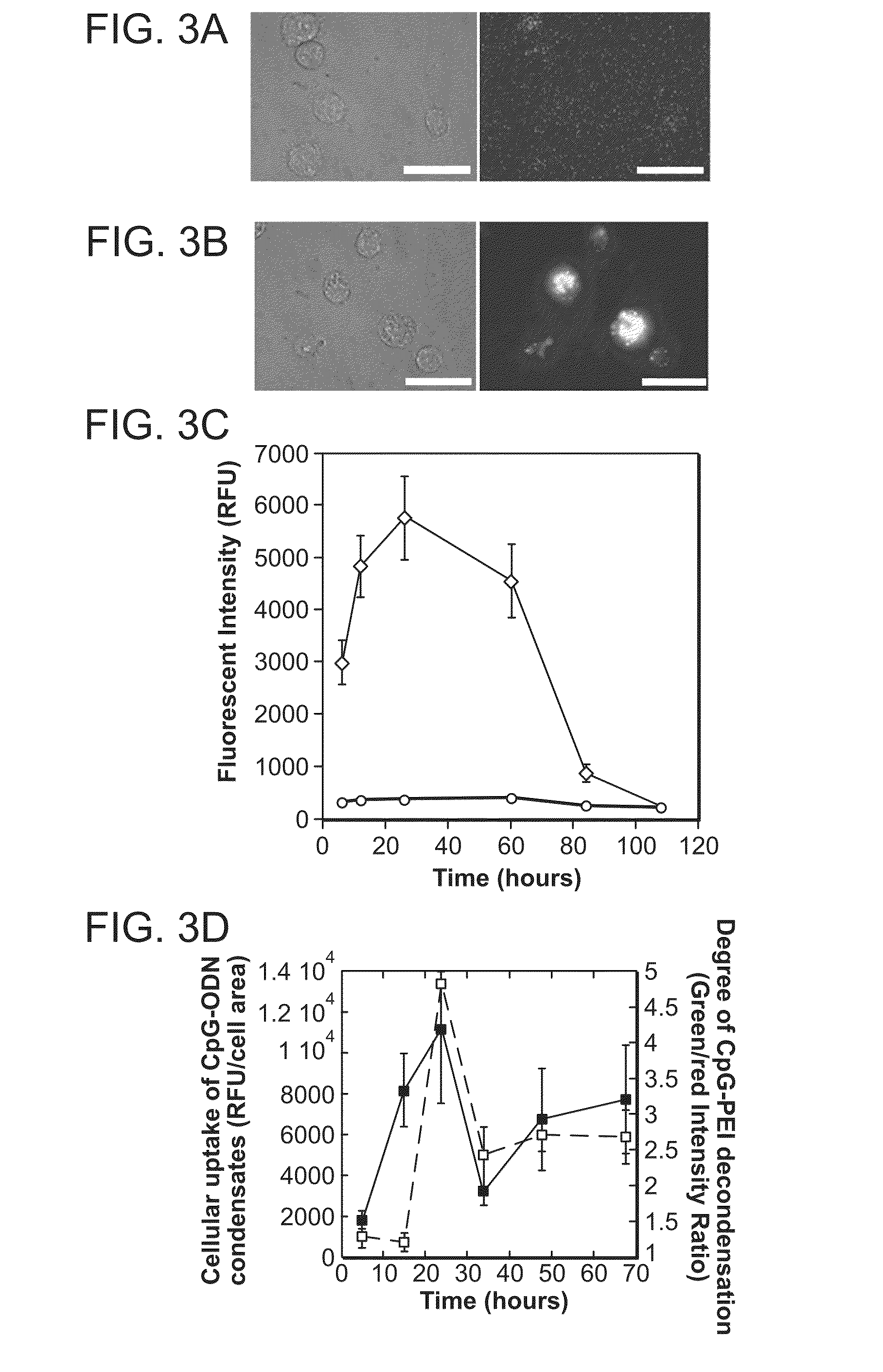
Lipid nanoparticle vaccine adjuvants and antigen delivery systems
The instant invention provides for novel lipid nanoparticle (LNP) formulations, containing cationic lipids, for use as vaccine adjuvants and / or as antigen delivery systems. It is an object of the instant invention to provide LNP formulations that demonstrate enhancements in humoral and cellular immunogenicity of vaccine antigens, particularly subunit vaccine antigens, when utilized alone or in combination with immunostimulatory agents (e.g. small molecule or oligonucleotide TLR agonists). The instant invention further identifies physical and chemical properties of the LNP formulations that can be manipulated to enhance antigen efficiency and adjuvant tolerability in vivo.
Owner:MERCK SHARP & DOHME LLC
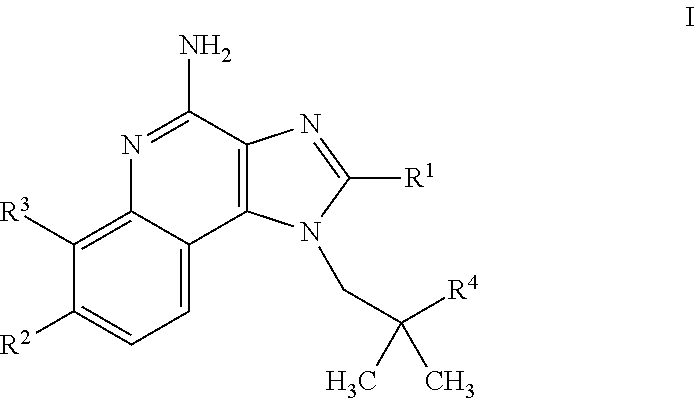
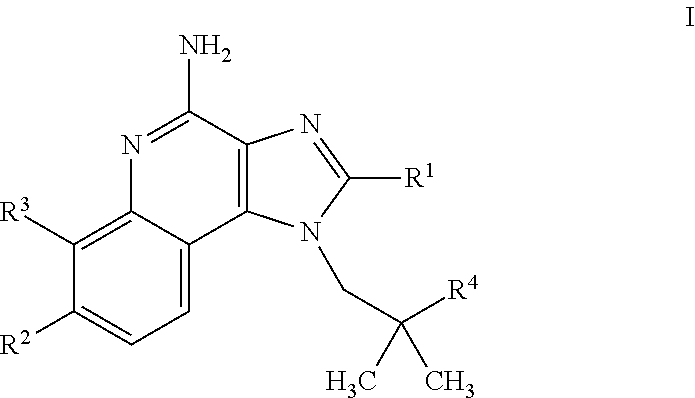
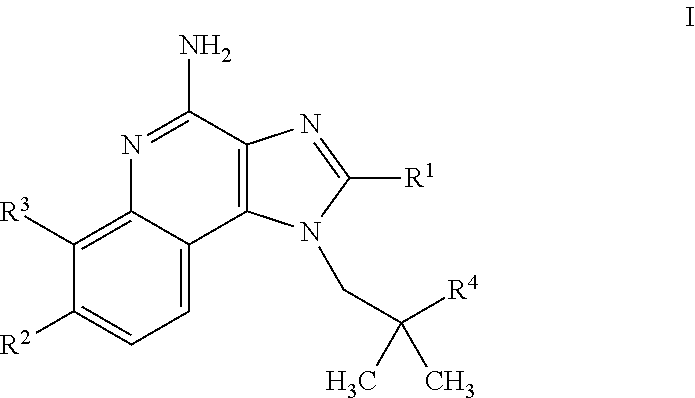
4-amino-imidazoquinoline compounds
This invention relates to novel 4-amino-imidazoquinoline compounds of the formulawherein R1 to R4 are as defined in the description and in the claims, as well as pharmaceutically acceptable salts thereof. These compounds are TLR agonists and may therefore be useful as medicaments for the treatment of diseases such as cancer or infectious diseases.
Owner:F HOFFMANN LA ROCHE & CO AG
Control of cellular redox levels
Disclosed herein are compositions and methods for regulating redox status and / or reducing oxidative stress in a subject, the methods and compositions comprising TLR agonists comprising bacterial lysates and / or lysate fractions. Also disclosed are compositions and methods comprising bacterial lysates and / or lysate fractions formulated or administered in combination with one or more other therapeutic or pharmaceutical agents.
Owner:伊丽莎白麦克纳
Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response
The invention provides novel immune regulatory oligonucleotides (IRO) as antagonist of TLRs and methods of use thereof. These IROs have unique sequences that inhibit or suppress TLR-mediated signaling in response to a TLR ligand or TLR agonist. The methods may have use in the prevention and treatment of cancer, an autoimmune disorder, airway inflammation, inflammatory disorders, infectious disease, skin disorders, allergy, asthma or a disease caused by a pathogen.
Owner:IDERA PHARMA INC
Controlled delivery of TLR agonists in structural polymeric devices
ActiveUS9370558B2Easy to controlClinical effectiveness of has been limitedPowder deliveryOrganic active ingredientsDendritic cellTlr agonists
The present invention comprises compositions, methods, and devices for creating an stimulating an antigen-specific dendritic cell immune response. Devices and methods provide prophylactic and therapeutic immunity to subjects against cancer and infectious agents.
Owner:DANA FARBER CANCER INST INC +1
Conjugates of synthetic TLR agonists and uses therefor
ActiveUS8357374B2Undesirable systemic side effectStimulate immune responseBiocideAntipyreticTlr agonistsChemistry
The invention provides TLR agonists and conjugates thereof useful in vaccines and to prevent, inhibit or treat a variety of disorders including pathogen infection and asthma.
Owner:RGT UNIV OF CALIFORNIA
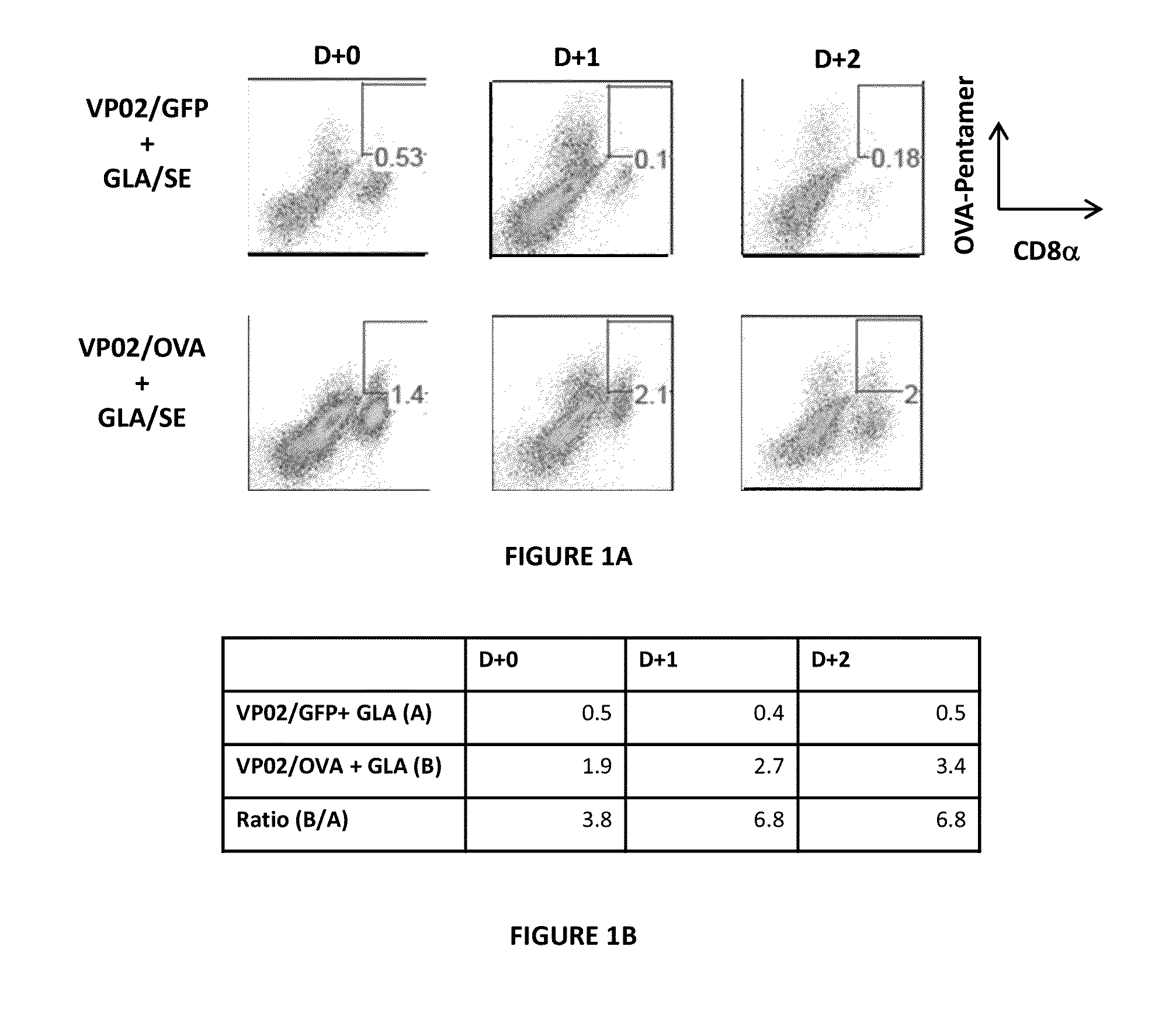
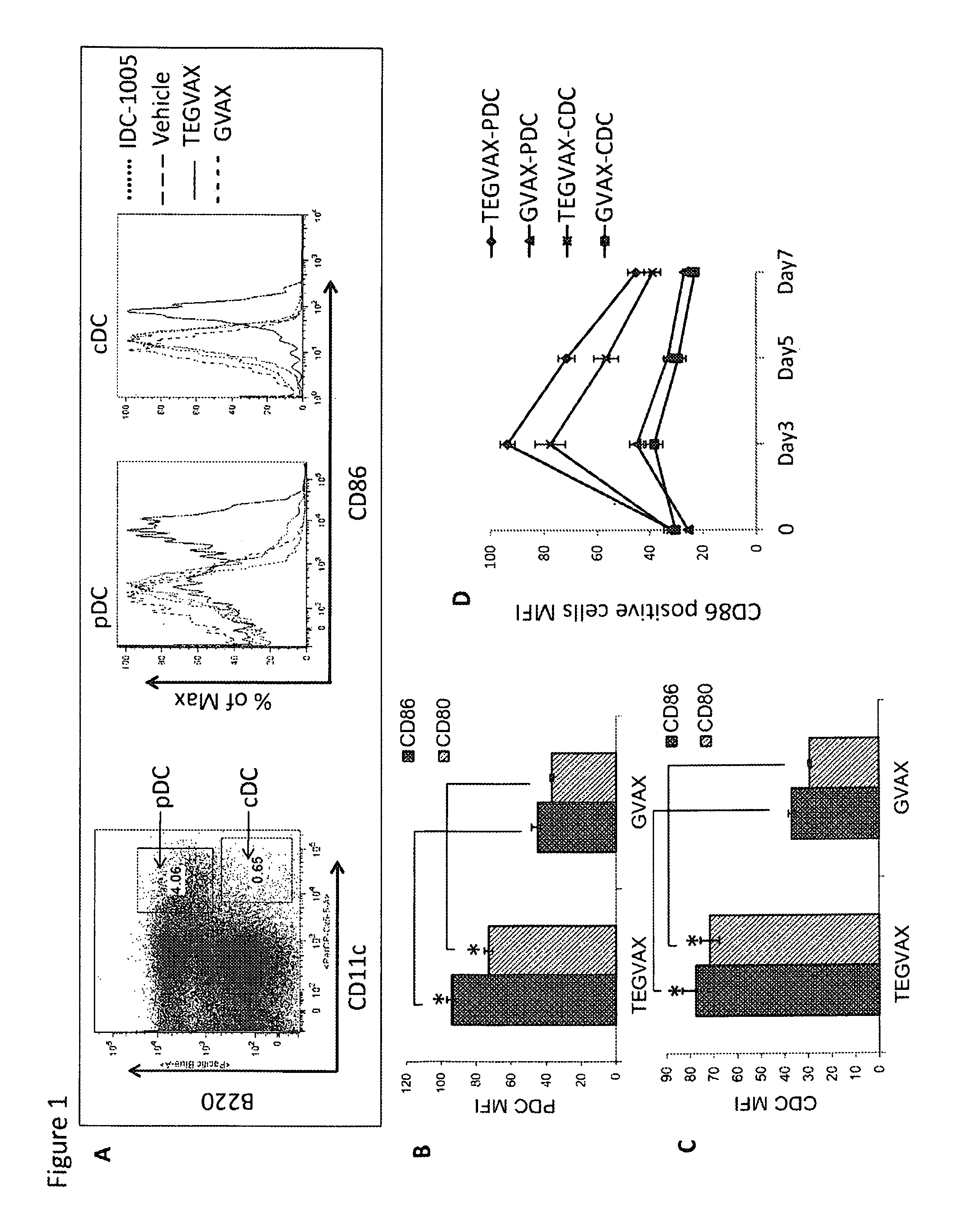
Cancer immunotherapy
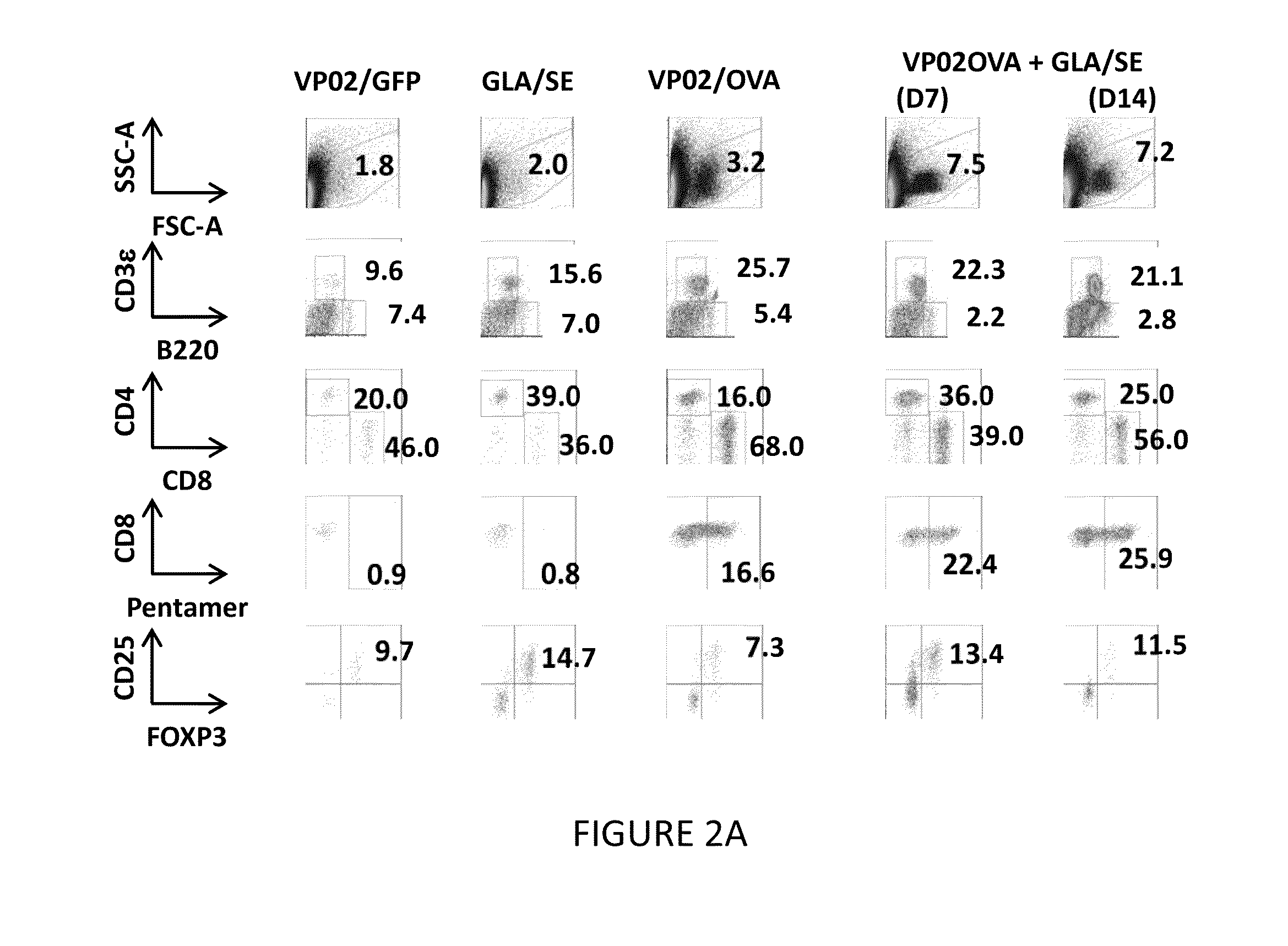
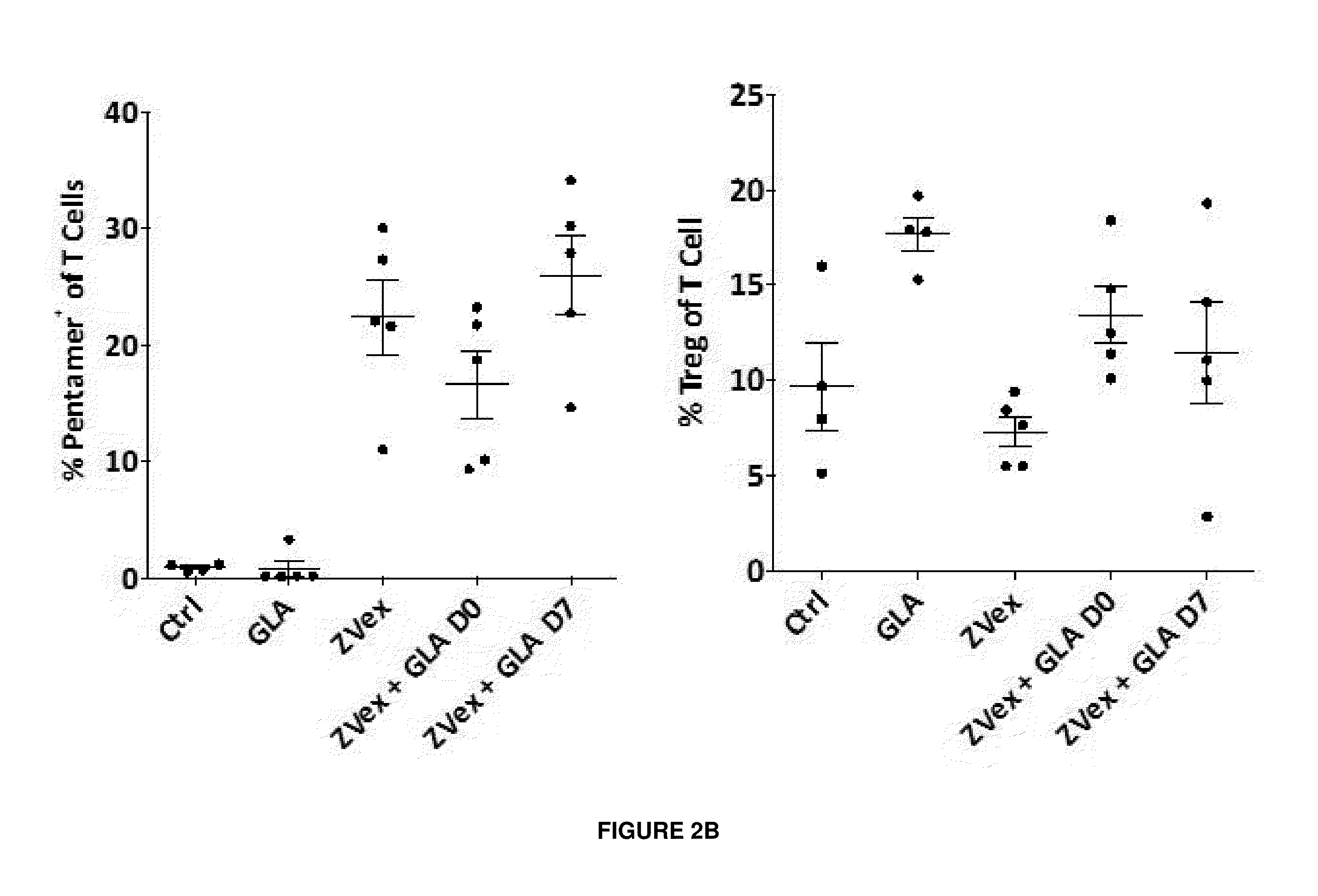
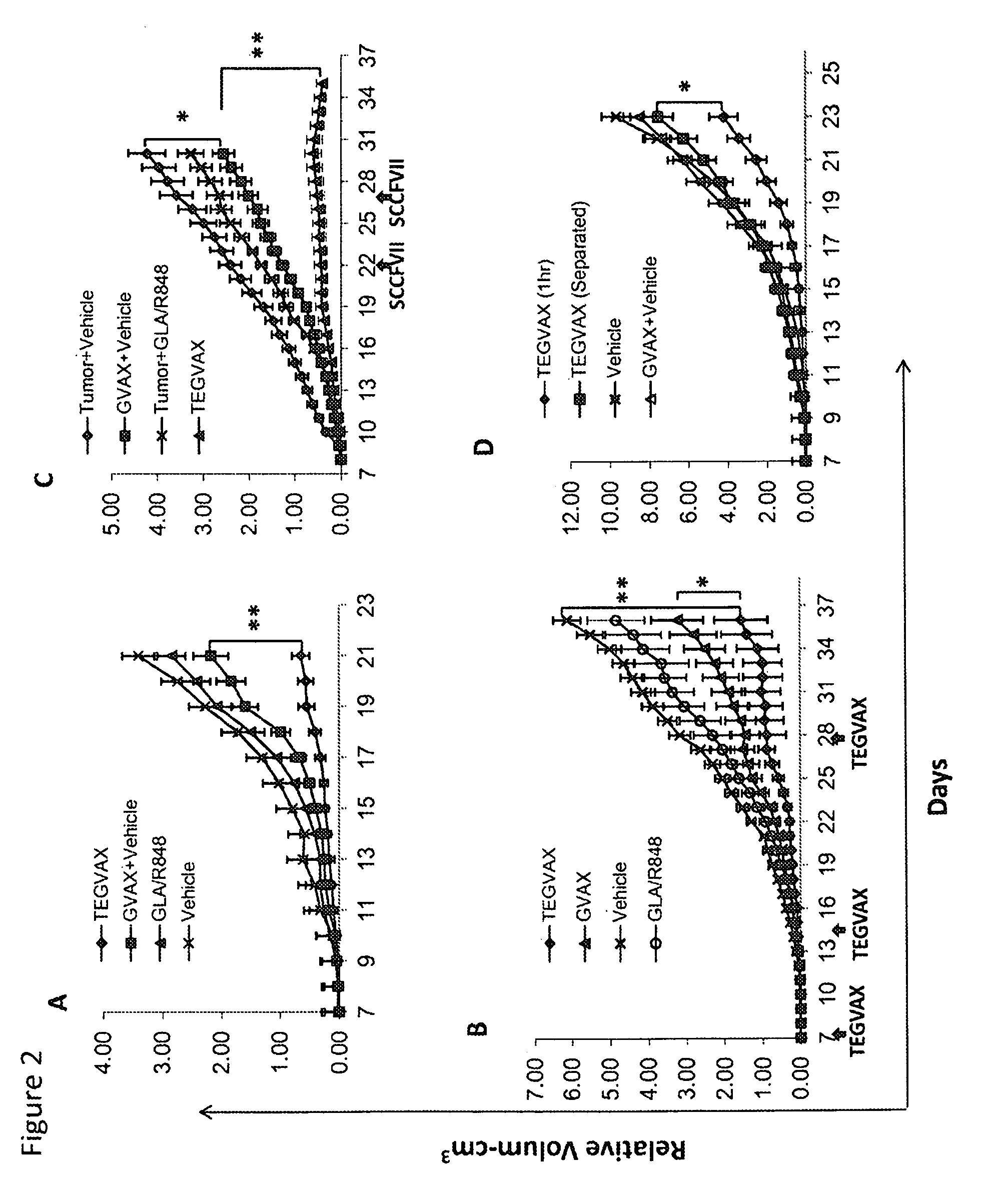
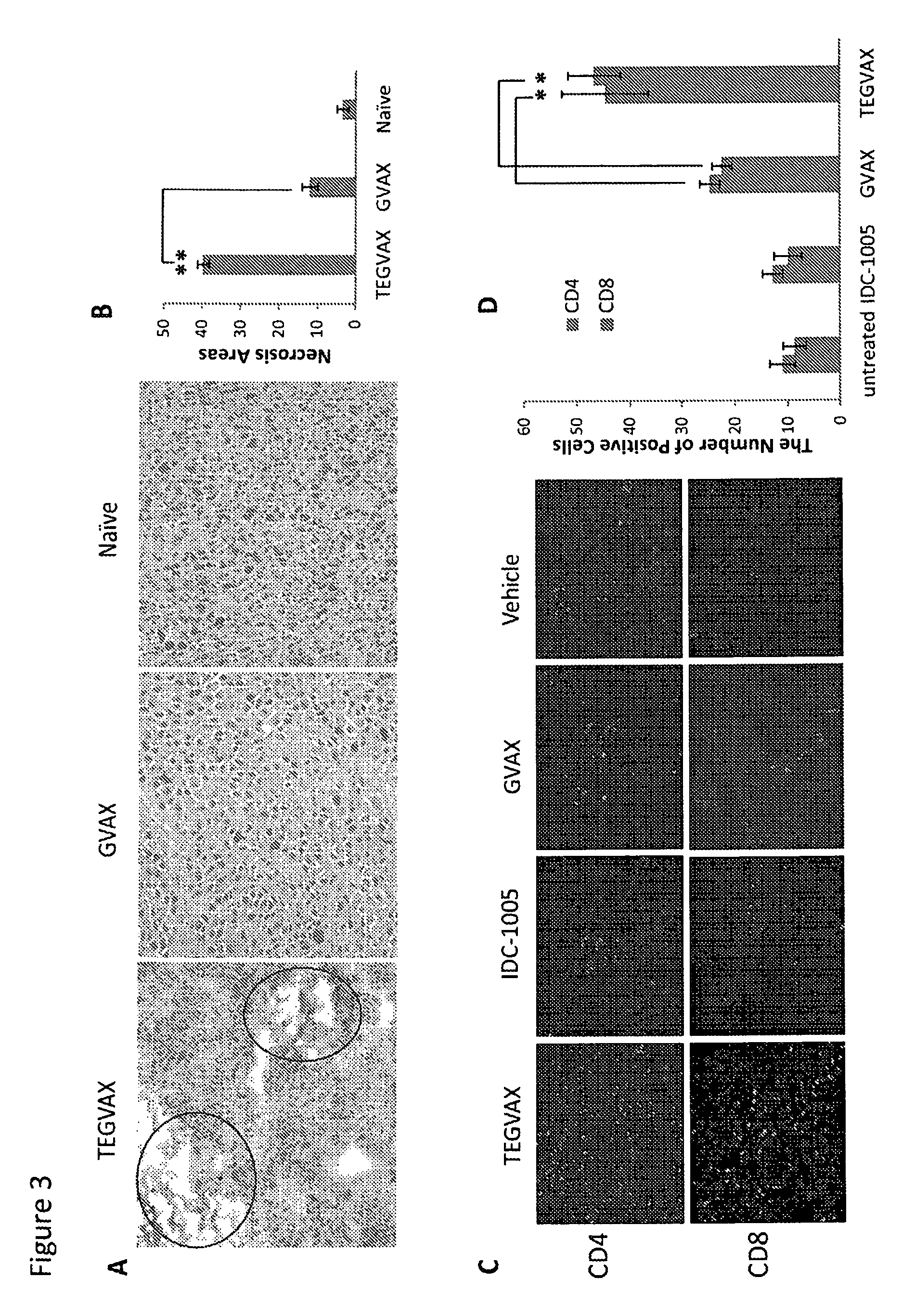
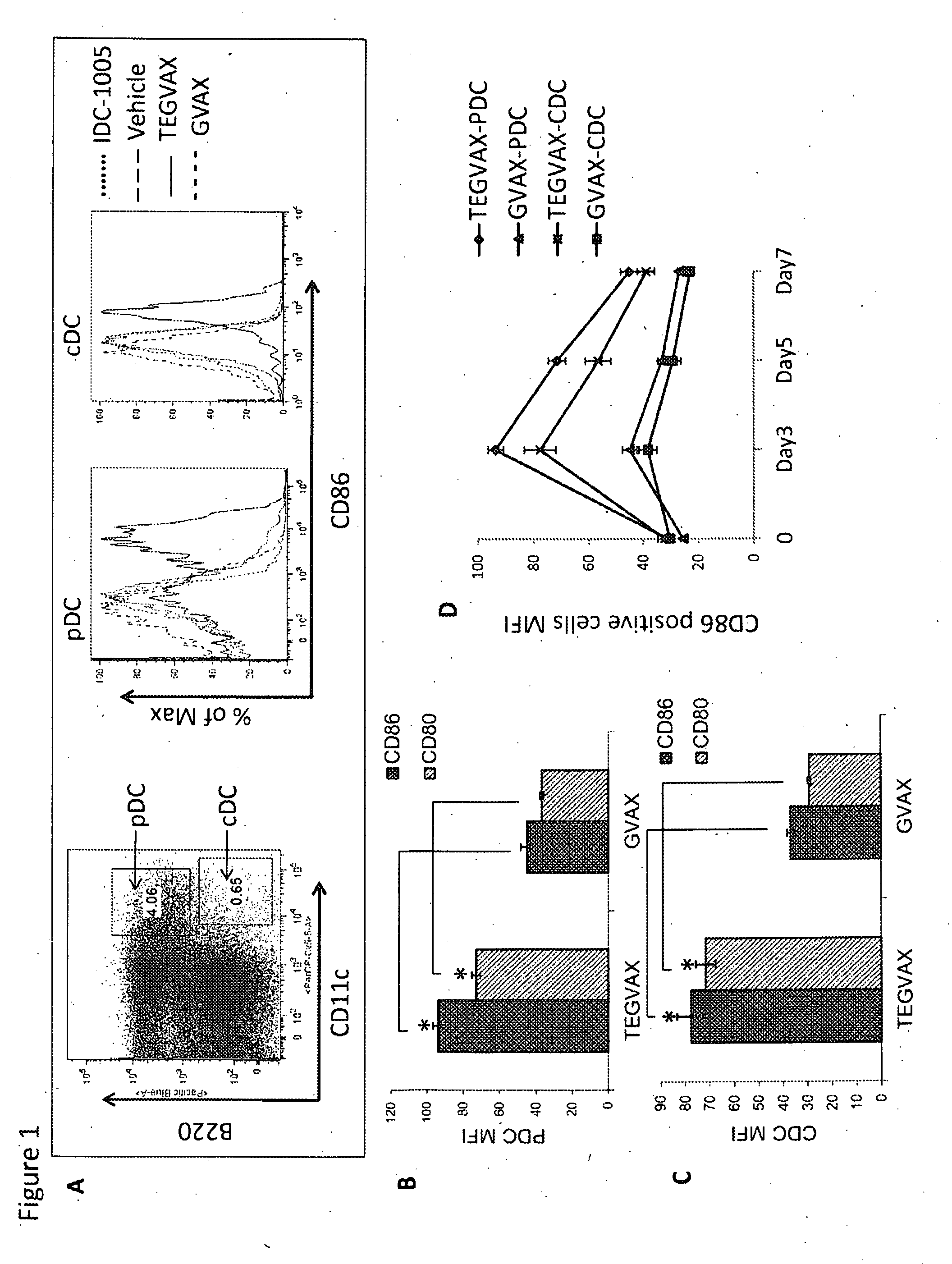
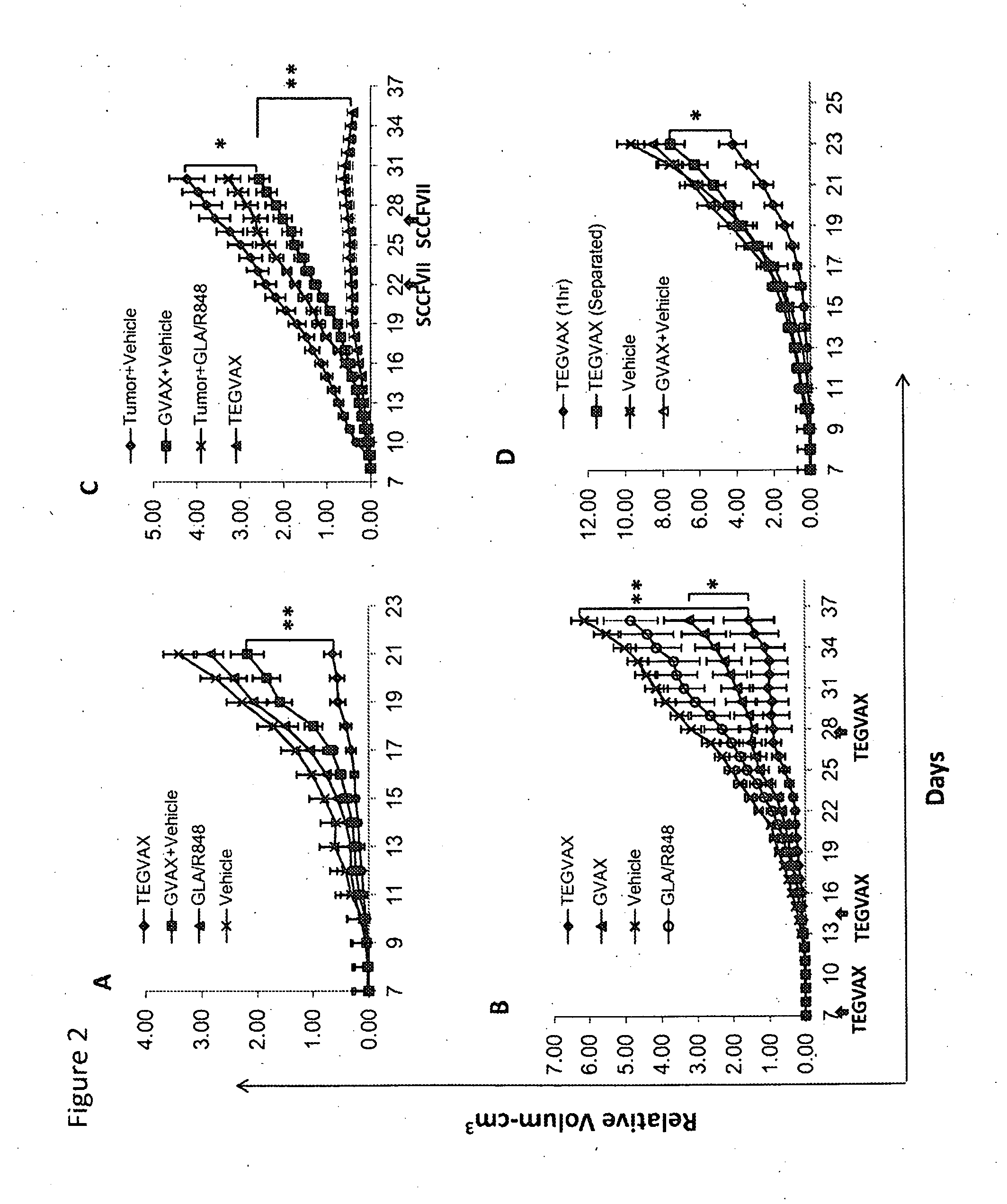
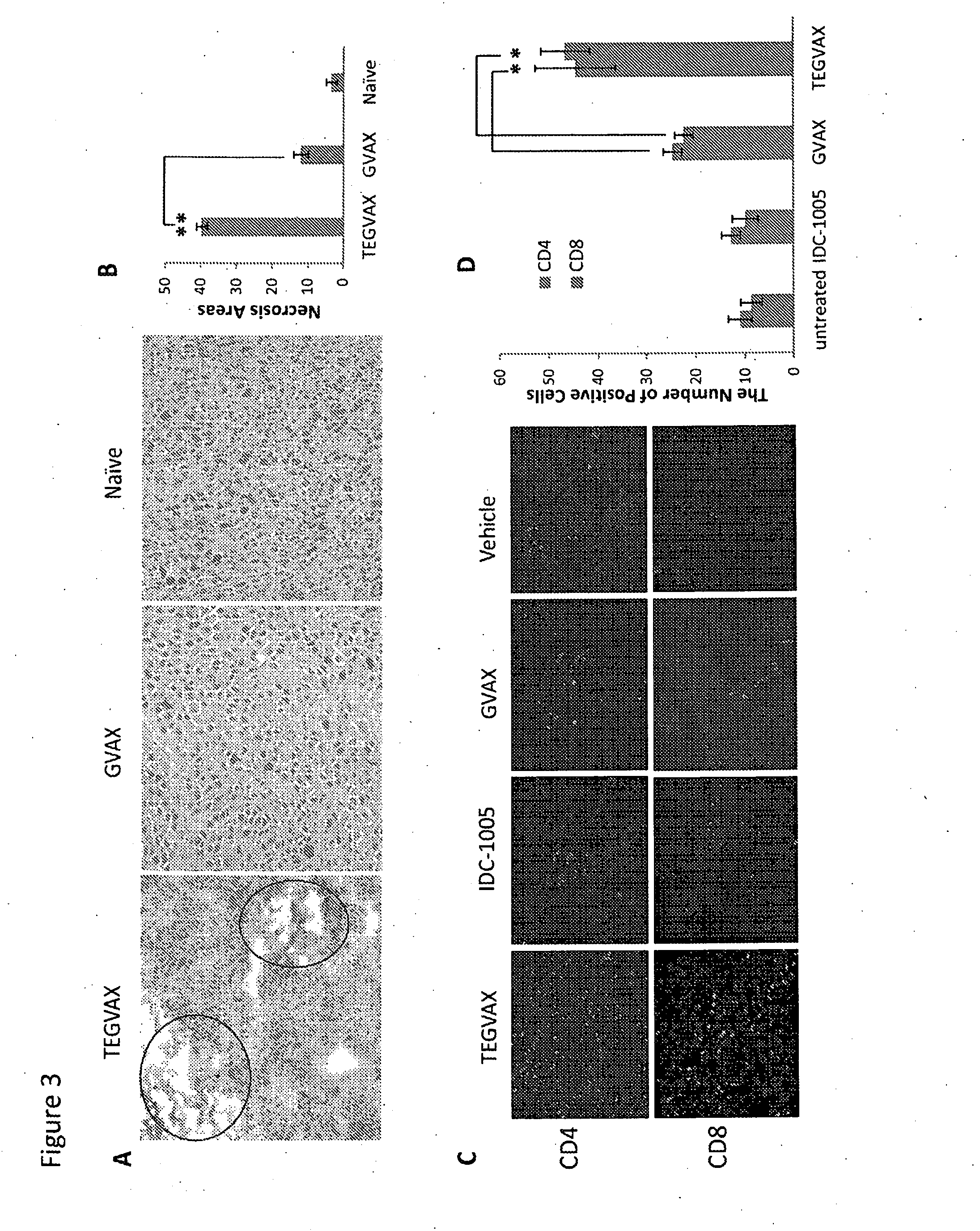
We formulated multiple TLR agonists into GVAX (lethally irradiated tumor cell vaccines engineered to secrete GM-CSF). Specifically, GLA and R848, TLR4 and TLR7 / 8 agonists found to be safe in patients, were formulated with GVAX (TEGVAX—for TLR agonists enhanced GVAX), and this formulation was effective in producing anti-tumor responses in 3 different preclinical models, including palpable B16. These anti-tumor responses were correlated with increased CD4 and CD8 T-cells that can secrete IFNγ circulating in the tumor microenvironment as well as significantly higher level of p15E specific CTL mediated cell killing in mice treated with TEGVAX in comparison to controls. When combined with anti-PD-1 antibody, TEGVAX was able to induce regression of established B16 tumors.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method for in-vitro amplification of CD8+T cell and cell subset of CD8+T cell
ActiveCN106834228AImprove efficiencyImprove repair functionCell dissociation methodsBlood/immune system cellsTlr agonistsMagnetic bead
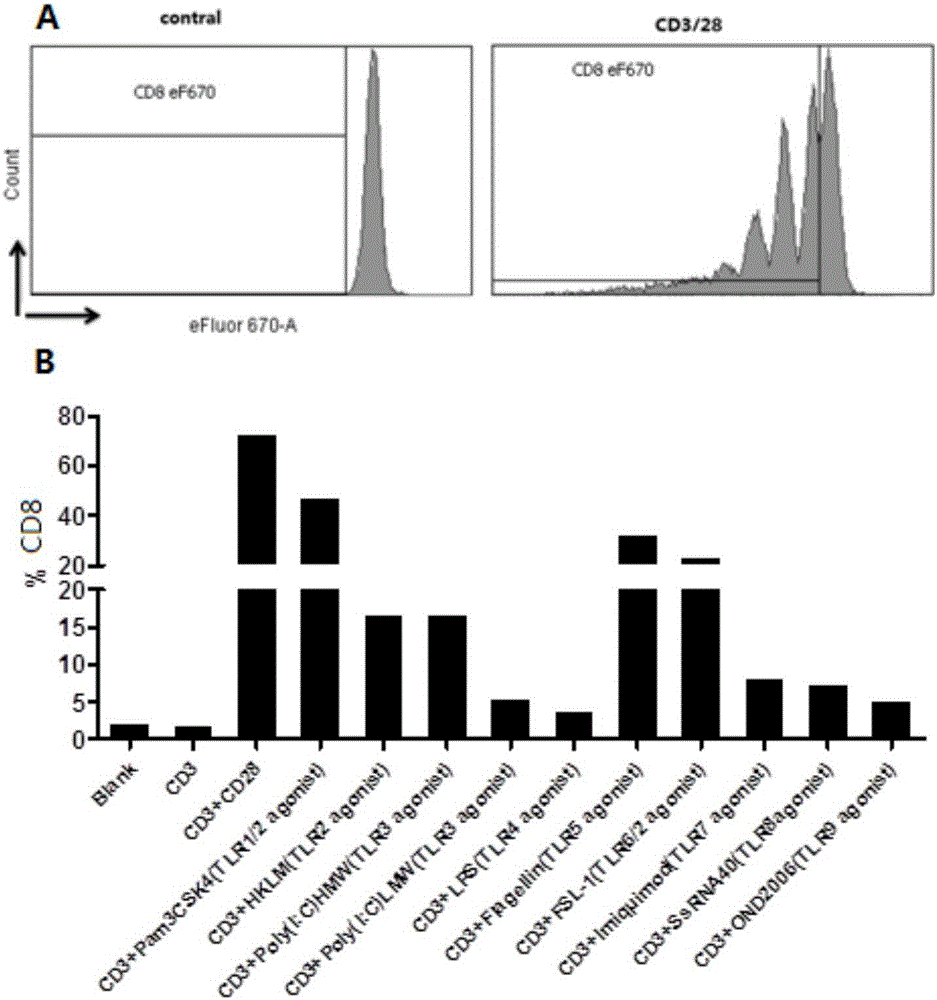
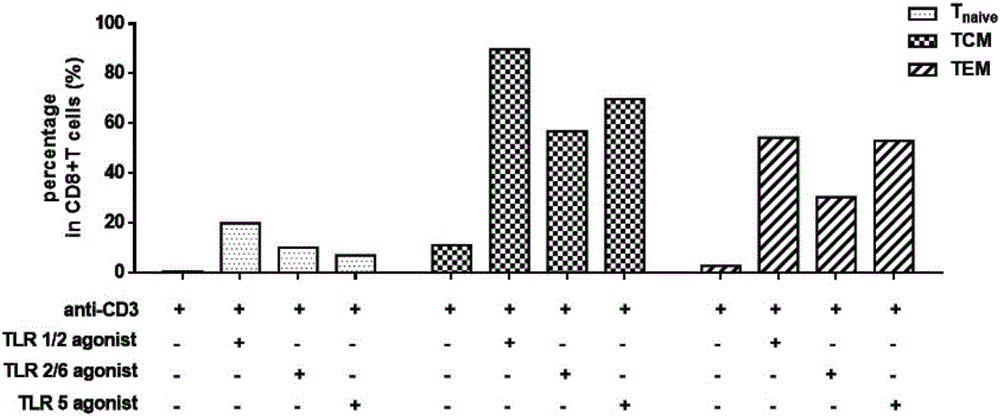
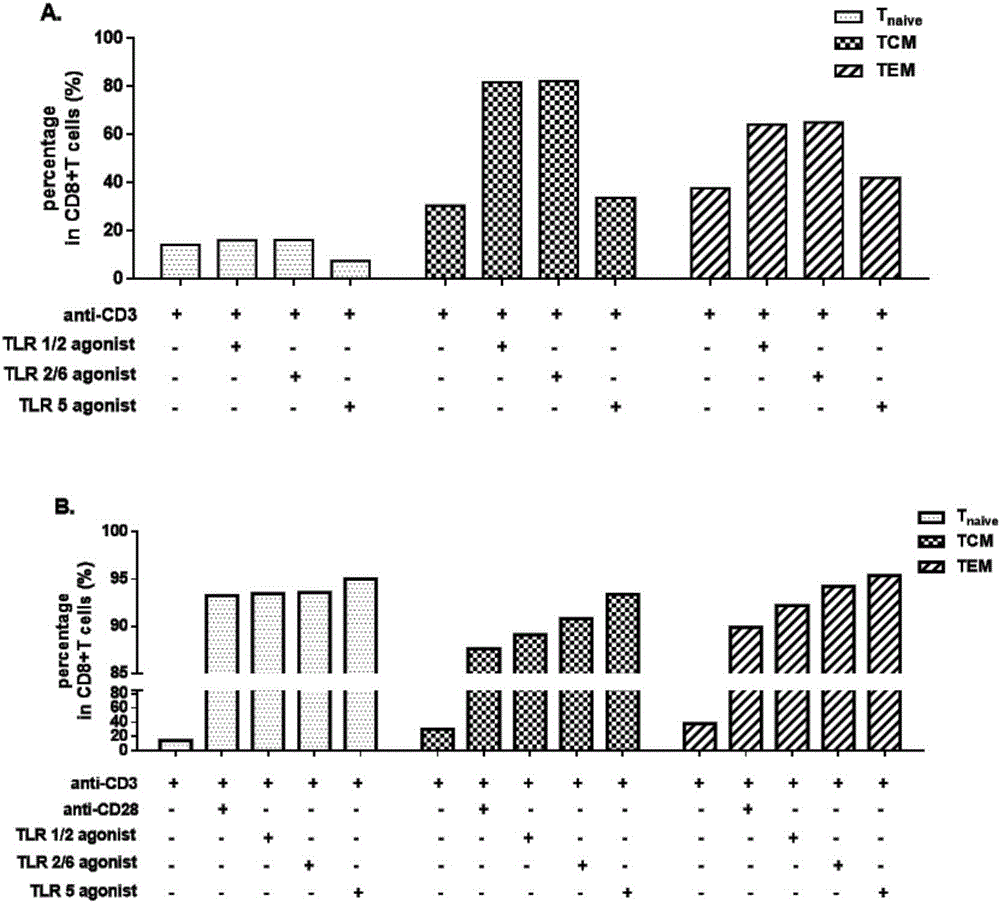
The invention establishes a method for in-vitro amplification of a CD8+T cell and a cell subset of the CD8+T cell. By adding a TLR1 / 2 agonist, a TLR2 / 6 agonist and a TLR5 agonist into an in-vitro culture system for conventionally culturing and amplifying the CD8+T cell or conjunctively utilizing the agonists, the amplification efficiency of the CD8+T cell can be remarkably improved; besides, by utilizing a TLC agonist, the functional CD8+T cell subsets which are difficult to amplify under a conventional culture condition are amplified, such as PD-1+CD8+T cells and TEM CD8+T cells; and the CD8+T cells and the functional cell subsets can be rapidly and largely propagated under the common and continuous stimulation of a TLRs agonist, recombinant cell factors IL-2, IL-7 and IL-15, an anti-human CD3 antibody and anti-human CD28 antibody coated magnetic beads.
Owner:SHANGHAI INNOVATIONAL CHANGAN BIOLOGICAL TECH CO LTD
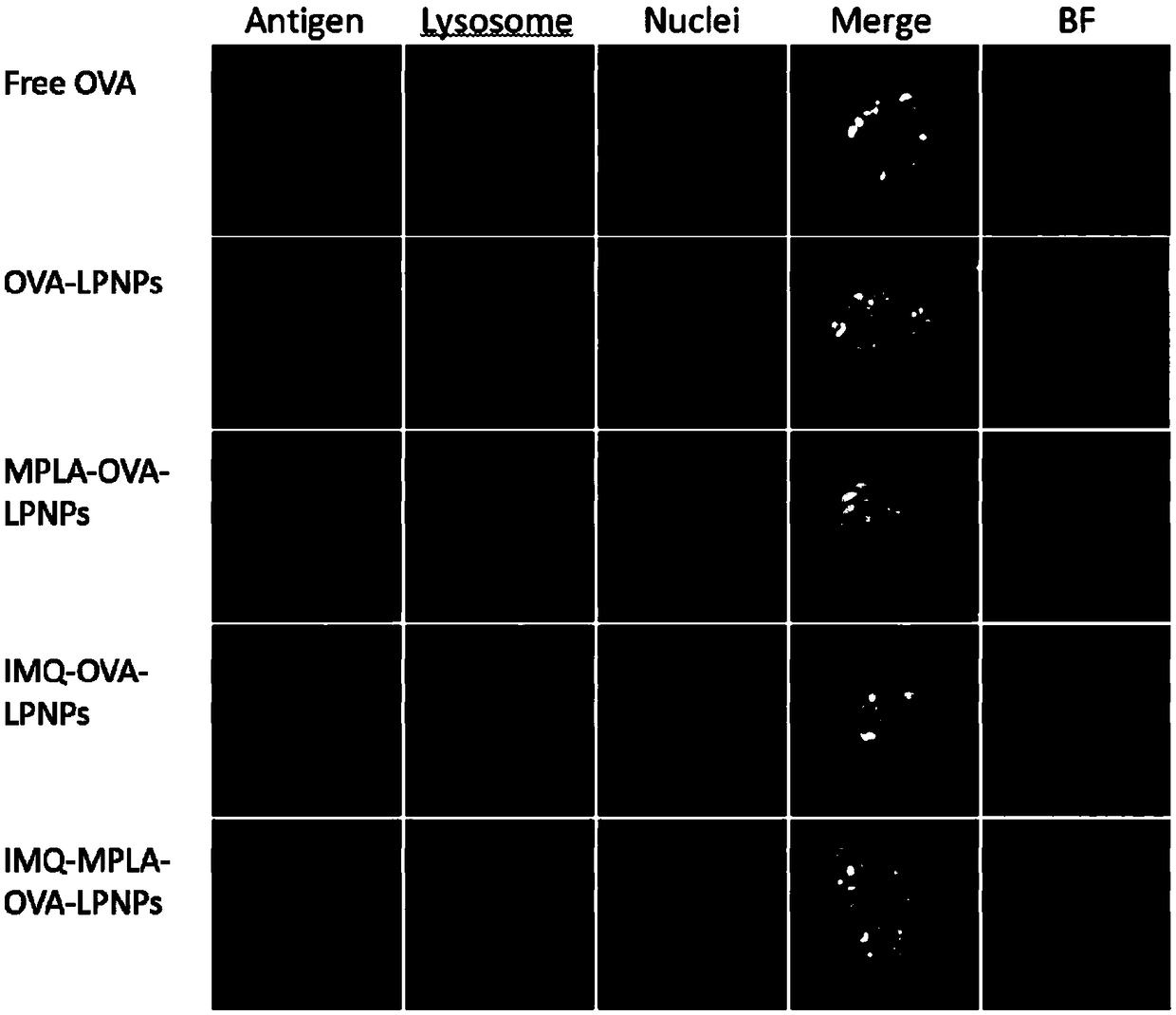
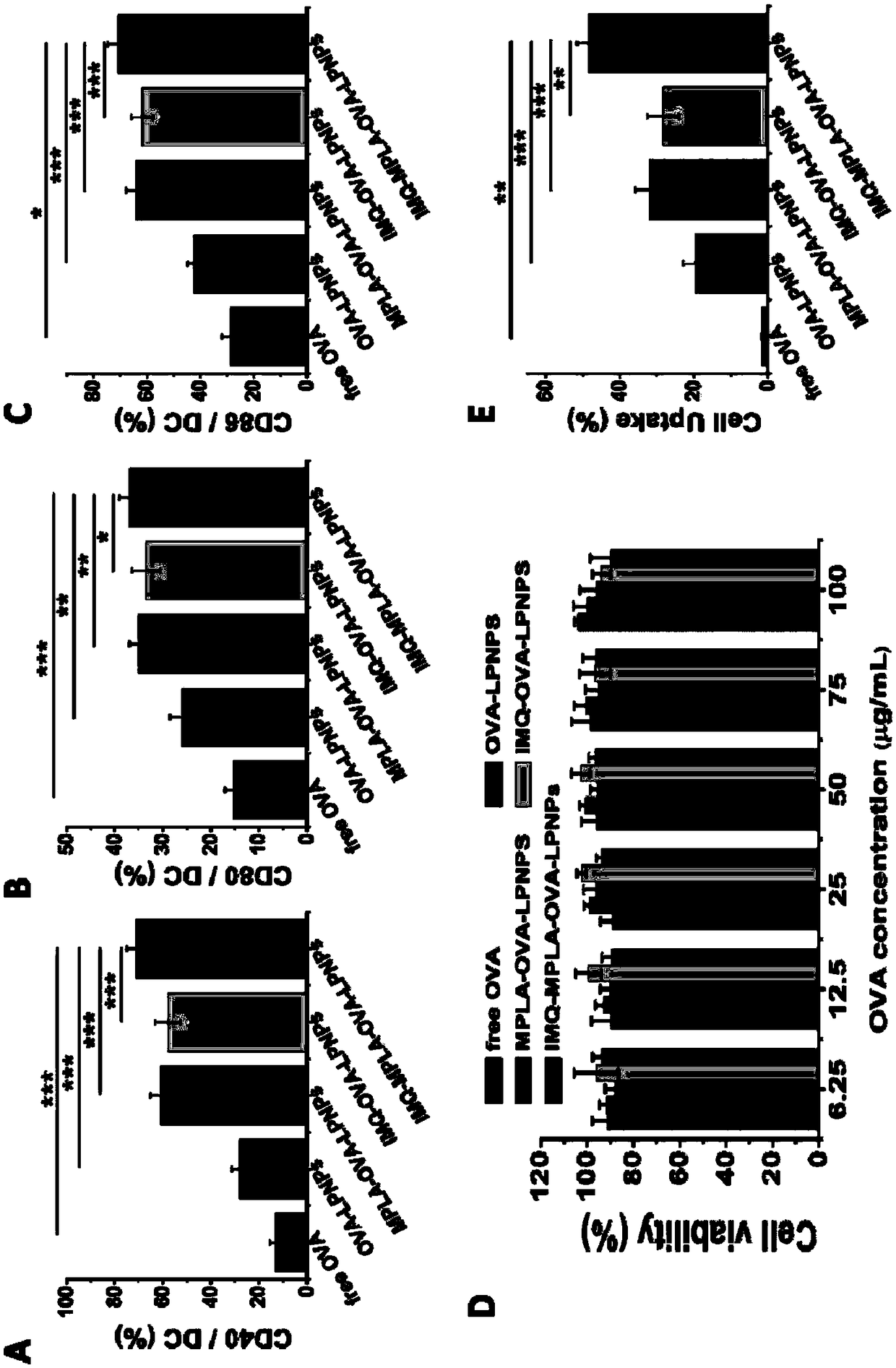
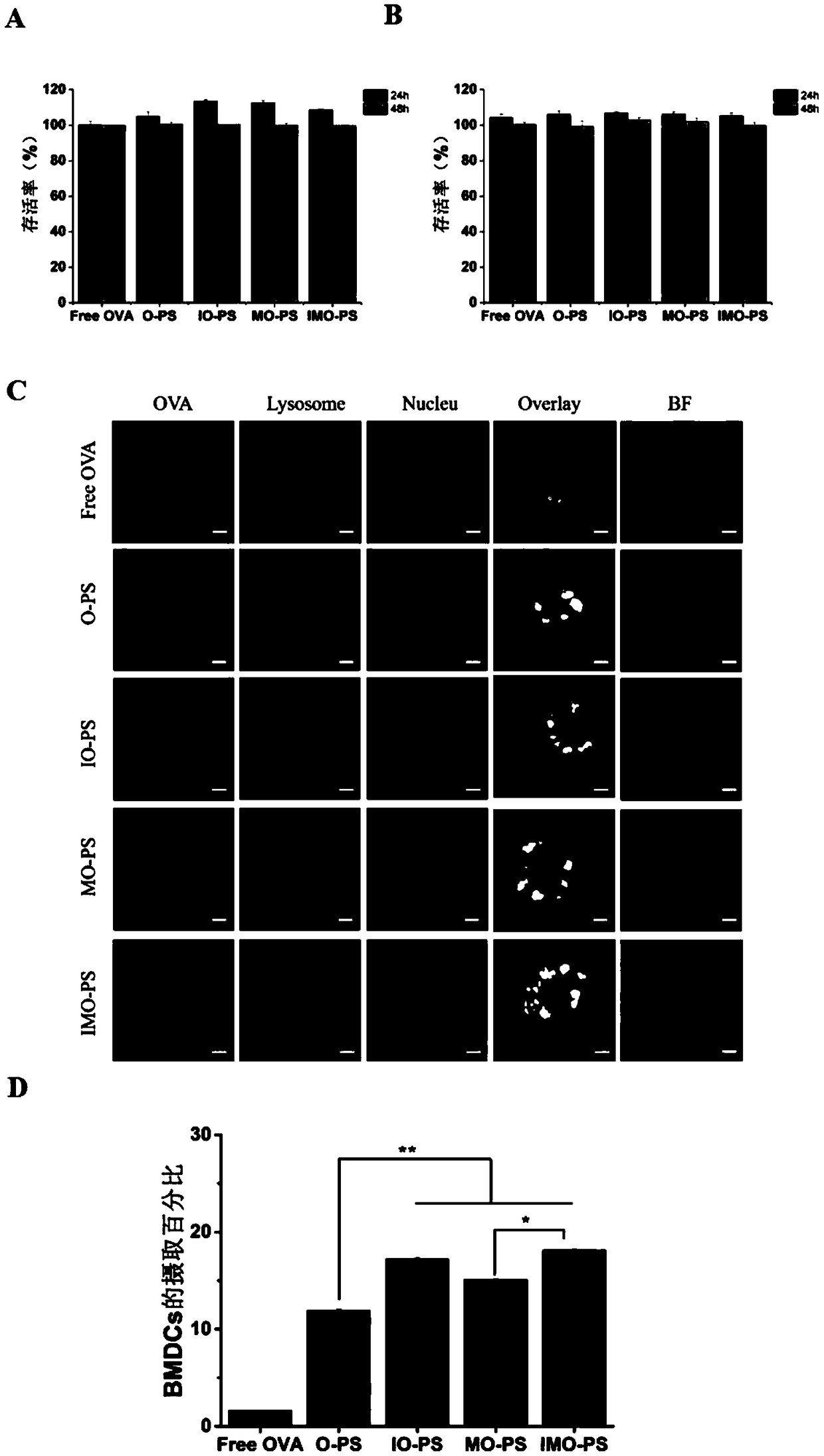
Antigen and TLR agonist targeting co-loaded cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant, and preparation method and application thereof
ActiveCN108992666AStrong activationRealize the loadCancer antigen ingredientsPharmaceutical non-active ingredientsDendritic cellPhospholipid
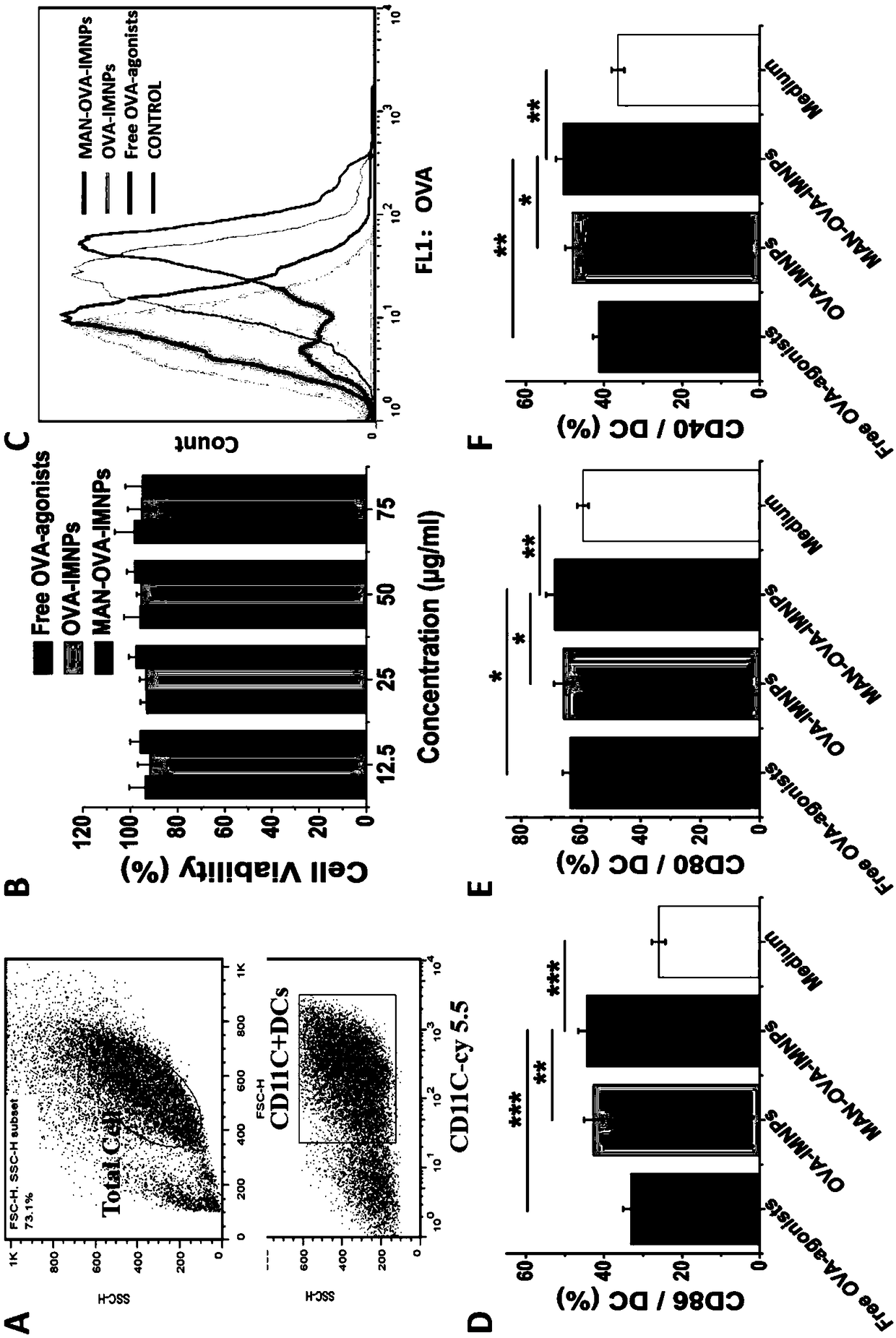
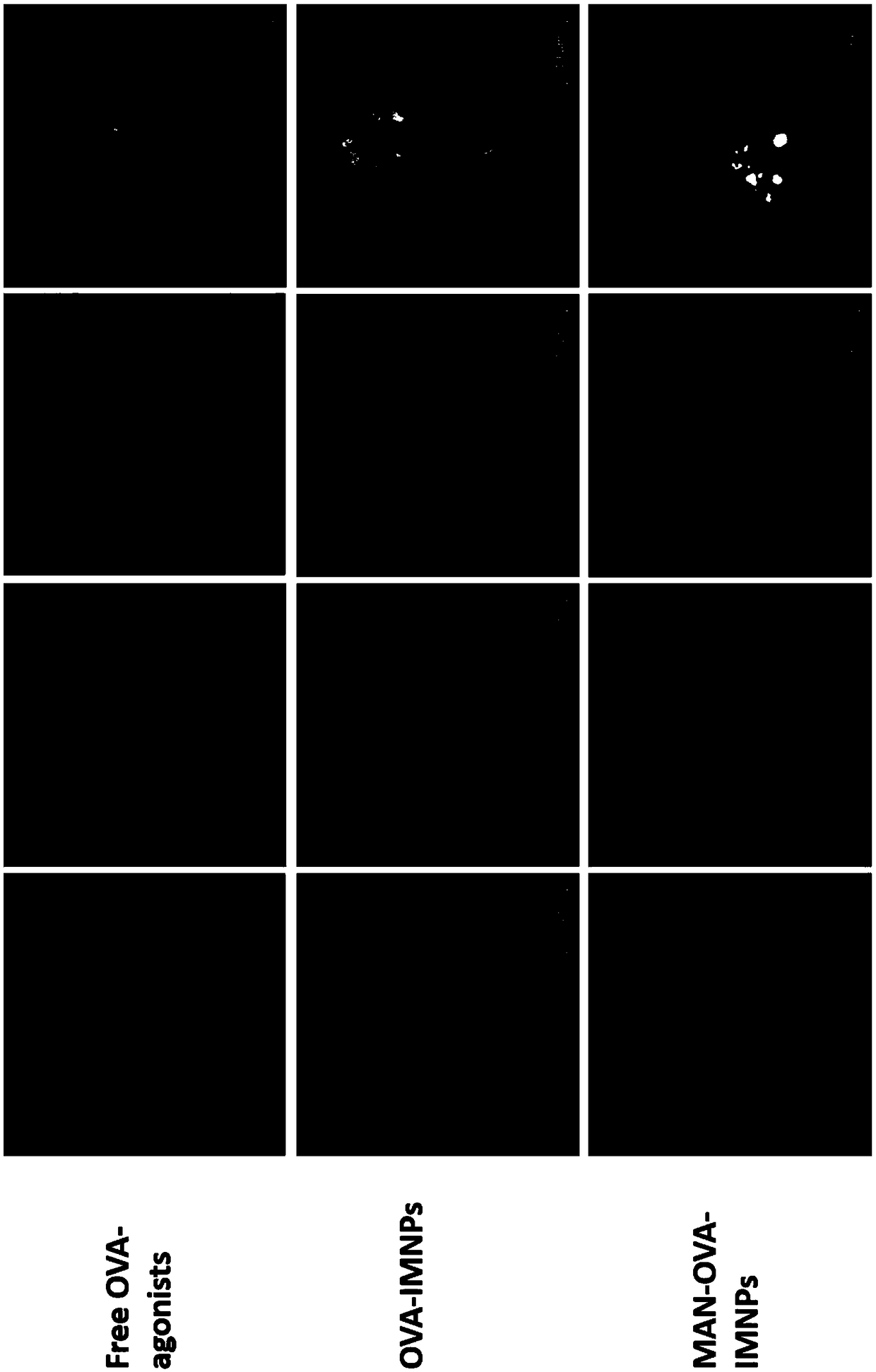
The invention relates to an antigen and TLR agonist targeting co-loaded cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant, and a preparation method and an application thereof. A hydrophobic inner core is encapsulated with a TLR7 agonist, a phospholipid layer is encapsulated with a TLR4 agonist, an antigen is adsorbed by cationic phospholipids in the phospholipid layer, and ligation of a mannose ligand makes the vaccine adjuvant like a specific targeting antigen to have the dendritic cell presenting ability, so the DC cell intake and the maturation promoting effect are enhanced; and the antigen is protected by hybridized nanoparticles, the antigen uptake by DC cells is improved, immune response after antigen stimulation is significantly enhanced by the TLR agonist, and thecross-presentation of the antigen is significantly improved, so vaccine adjuvant has a strong potent T cell killing effect, can induce cytokine secretion, has a long-acting memory T cell response, andhas an excellent prevention effect on tumors.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response
The invention provides novel immune regulatory oligonucleotides (IRO) as antagonist of TLRs and methods of use thereof. These IROs have unique sequences that inhibit or suppress TLR-mediated signaling in response to a TLR ligand or TLR agonist. The methods may have use in the prevention and treatment of cancer, an autoimmune disorder, airway inflammation, inflammatory disorders, infectious disease, skin disorders, allergy, asthma or a disease caused by a pathogen.
Owner:IDERA PHARMA INC
Adjuvant combinations comprising a microbial tlr agonist, a cd40 or 4-1bb agonist, and optionally an antigen and the use thereof for inducing a synergistic enhancement in cellular immunity
Adjuvant combinations comprising at least one microbial TLR agonist such as a whole virus, bacterium or yeast or portion thereof such a membrane, spheroplast, cytoplast, or ghost, a CD40 or 4-1BB agonist and optionally an antigen wherein all 3 moieties may be separate or comprise the same recombinant microorganism or virus are disclosed. The use of these immune adjuvants for treatment of various chronic diseases such as cancers and HIV infection is also provided.
Owner:DELUCIA DAVE
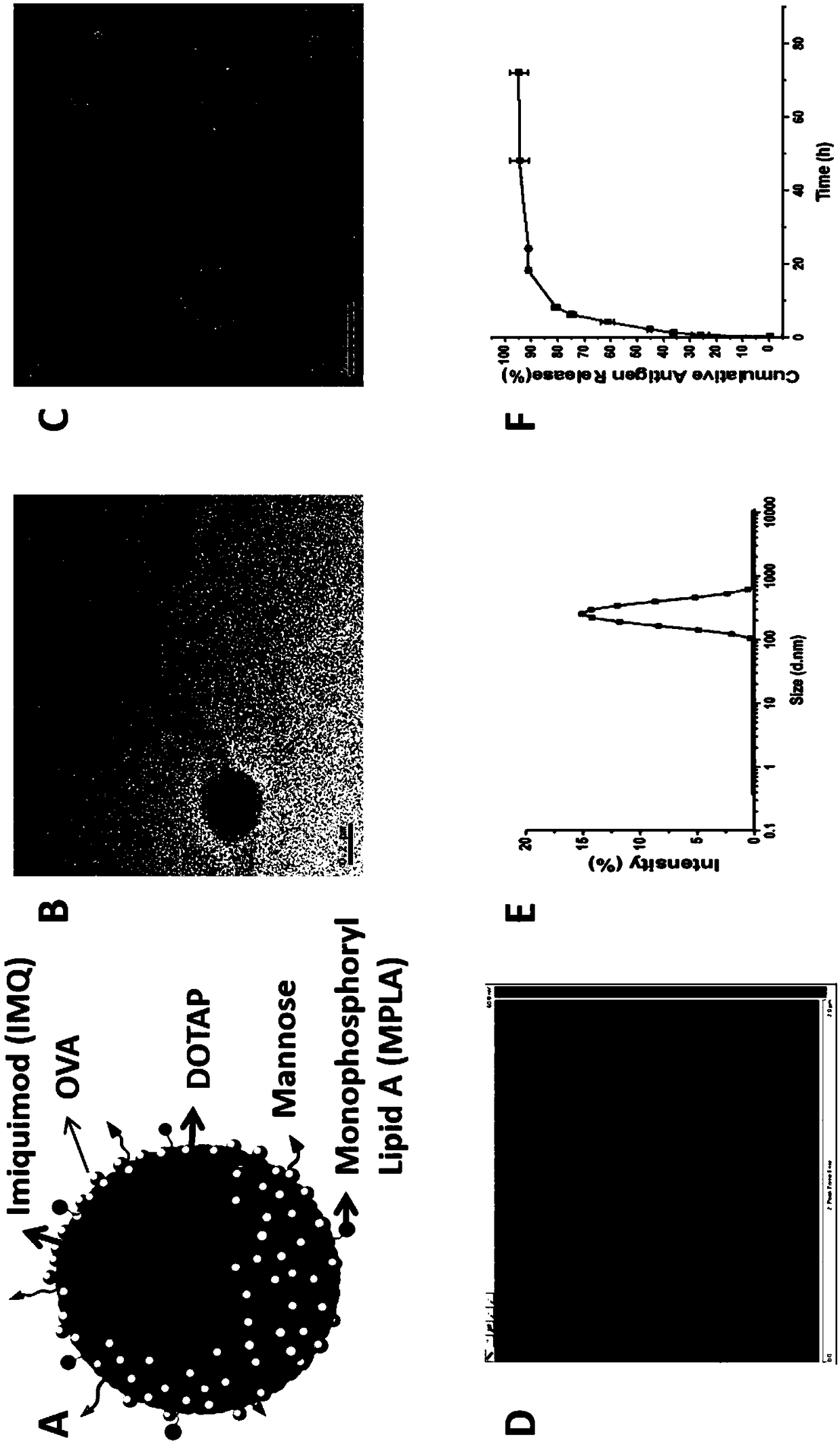
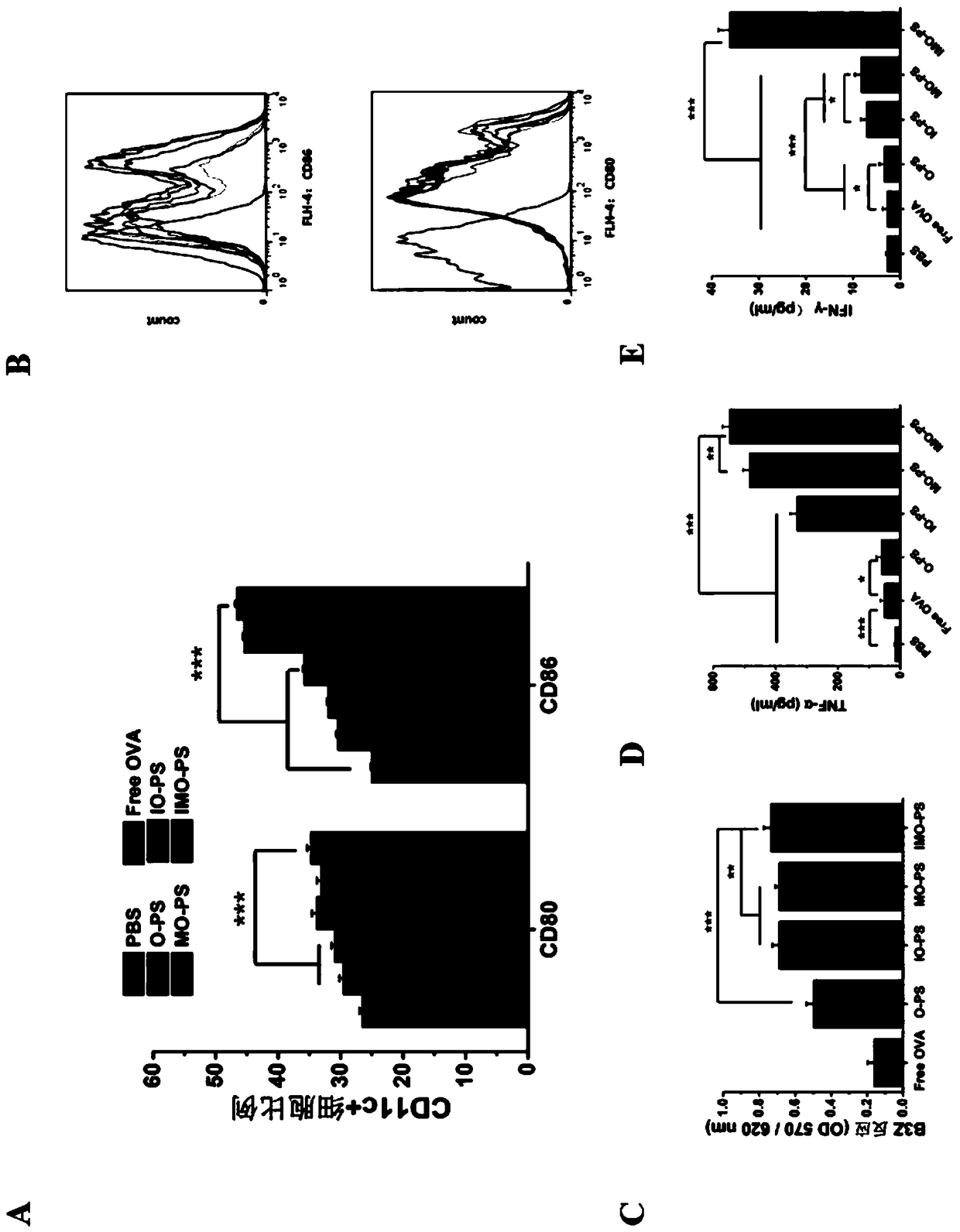
Cationic phospholipid-polymer hybridized nanoparticle vaccine adjuvant of common-carrier antigen, MPLA (Monophosphoryl Lipid A) and IMQ (Imiquimod) as well as preparation method and application thereof
ActiveCN108743939AGood biocompatibilityPromote degradationPharmaceutical non-active ingredientsAntibody medical ingredientsDendritic cellPhospholipid
The invention relates to a cationic phospholipid-polymer hybridized nanoparticle vaccine adjuvant of a common-carrier antigen, MPLA (Monophosphoryl Lipid A) and IMQ (Imiquimod) as well as a preparation method and application thereof. The vaccine adjuvant is characterized in that the IMQ as a TLR7 agonist is loaded on a hydrophobic core; the MPLA as a TLR4 agonist is loaded in a phopholipid layer;cationic phospholipid DOTAP (1,2-dioleoy-3-trimethylammonium-propane) in the phopholipid layer is used for adsorbing an antigen; the antigen is protected through hybridized nanoparticles, and the ingestion of the antigen by dendritic cells is improved; immune response after antigen stimulation is improved remarkably through the TLR agonist, and cross-presentation of the antigen is improved remarkably. The hybridized nanoparticles as the vaccine adjuvant can load the antigen and different types of TLR agonists simultaneously, can deliver the antigen through a plurality of immune paths, and promotes the DC activation and maturation. The cross-presentation level is raised, a strong and powerful T-cell killing effect is achieved, cell factor secretion is induced, a long-term memory T-cell reaction is generated, and higher prevention capability for tumors is achieved.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response
The invention provides novel immune regulatory oligonucleotides (IRO) as antagonist of TLRs and methods of use thereof. These IROs have unique sequences that inhibit or suppress TLR-mediated signaling in response to a TLR ligand or TLR agonist. The methods may have use in the prevention and treatment of cancer, an autoimmune disorder, airway inflammation, inflammatory disorders, infectious disease, skin disorders, allergy, asthma or a disease caused by a pathogen.
Owner:IDERA PHARMA INC
DCs vaccine based on phospholipid hybrid polymersome jointly encapsulating antigen and dual immunoagonists and preparation method and application thereof
ActiveCN108938568AMaximize Targeting EffectAchieving ImmunotherapyCancer antigen ingredientsPharmaceutical non-active ingredientsT lymphocyteBiological activation
The invention relates to a DCs vaccine based on phospholipid hybrid polymersome jointly encapsulating an antigen and dual immunoagonists, a preparation method and application thereof. The phospholipidhybrid polymersome which can jointly load a model antigen OVA and two types of TLR agonists (TLR7 / 8 and TLR4) is used for stimulation in vitro of the DCs so as to realize the effective phagocytosis of DCs cells. The rapid and long-term immunostimulatory effect on the DCs is achieved by the internal and external co-loading of the OVA antigen. The synergistic effect of the two types of TLR agonistssignificantly enhances the immune response after antigen stimulation; the phospholipid hybrid polymersome which jointly encapsulates the antigen and the dual immunoagonists can effectively promote the activation and maturation of the DCs, increases the level of cross-presentation, promotes the migration of the DC vaccine to secondary lymphoid organs, and produces a strong specific cytotoxic T lymphocytes (CTLs) killing effect, thereby effectively killing tumor cells and realizing the immunotherapy of the DCs vaccine on tumors.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response
The invention provides novel immune regulatory oligonucleotides (IRO) as antagonist of TLRs and methods of use thereof. These IROs have unique sequences that inhibit or suppress TLR-mediated signaling in response to a TLR ligand or TLR agonist. The methods may have use in the prevention and treatment of cancer, an autoimmune disorder, airway inflammation, inflammatory disorders, infectious disease, skin disorders, allergy, asthma or a disease caused by a pathogen.
Owner:IDERA PHARMA INC
Tlr agonists
The present invention provides TLR agonist conjugates (compounds) and compositions, as well as methods of using them. The compounds of the invention are broad-spectrum, long-lasting, and non-toxic combination of synthetic immunostimulatory agents, which are useful for activating the immune system of a mammal, preferably a human and can help direct the pharmacophore to the receptor within the endosomes of target cells and enhance the signal transduction induced by the pharmacophore.
Owner:RGT UNIV OF CALIFORNIA
Immune regulatory oligonucleotide (IRO) compounds to modulate toll-like receptor based immune response
ActiveUS8486908B2Antagonize, inhibit suppress or prevent the cytokine and chemokine responseOrganic active ingredientsPeptide/protein ingredientsTlr agonistsAllergy
The invention provides novel immune regulatory oligonucleotides (IRO) as antagonist of TLRs and methods of use thereof. These IROs have unique sequences that inhibit or suppress TLR-mediated signaling in response to a TLR ligand or TLR agonist. The methods may have use in the prevention and treatment of cancer, an autoimmune disorder, airway inflammation, inflammatory disorders, infectious disease, skin disorders, allergy, asthma or a disease caused by a pathogen.
Owner:IDERA PHARMA INC
Cancer immunotherapy
InactiveUS20140341978A1Skin cancer vaccineMammal material medical ingredientsAbnormal tissue growthTumor response
We formulated multiple TLR agonists into GVAX (lethally irradiated tumor cell vaccines engineered to secrete GM-CSF). Specifically, GLA and R848, TLR4 and TLR7 / 8 agonists found to be safe in patients, were formulated with GVAX (TEGVAX—for TLR agonists enhanced GVAX), and this formulation was effective in producing anti-tumor responses in 3 different preclinical models, including palpable B16. These anti-tumor responses were correlated with increased CD4 and CD8 T-cells that can secrete IFNγ circulating in the tumor microenvironment as well as significantly higher level of p15E specific CTL mediated cell killing in mice treated with TEGVAX in comparison to controls. When combined with anti-PD-1 antibody, TEGVAX was able to induce regression of established B16 tumors.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com