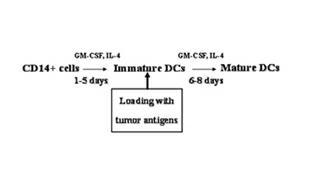
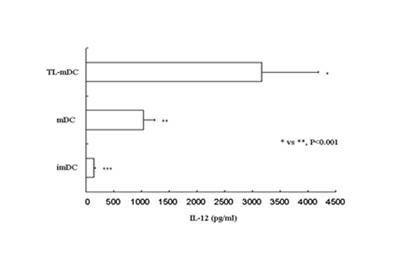
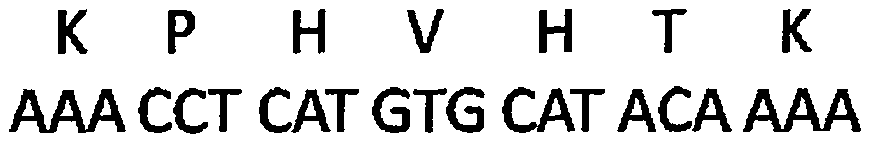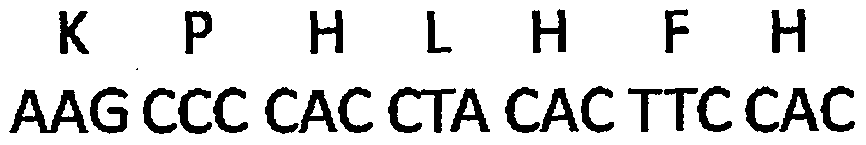Patents
Literature
100 results about "Dc vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of dendritic cell (DC) vaccine loaded with autologous tumor associated holoantigen
ActiveCN102091327ASignificant technological progressConvenient for clinical operationBlood/immune system cellsAntibody medical ingredientsCytotoxicityT lymphocyte
The invention belongs to preparation of biological cell formulations, and in particular relates to a preparation method of dendritic cell (DC) vaccine loaded with autologous tumor associated holoantigen. The preparation method comprises the following steps: preparing the autologous tumor associated holoantigen, collecting and separately culturing DCs, impacting the DCs by the autologous tumor associated holoantigen, maturing the DCs and preparing autologous tumor antigen specific DC vaccine. The invention solves the problems in the prior art that the immunogenicity of tumor antigen is not strong enough, the antigen target spots are incomplete, the tumor antigens of most tumor patients are difficult to acquire, and the like. The DC vaccine provided by the invention has the advantages of effectively inducing tumor antigen specific cytotoxic T lymphocyte (CTL) in vitro and in vivo, efficiently generating specific cytotoxicity on tumors, having high overall effective rate and no obvious toxic side effects in clinical application, and the like.
Owner:玥特农生物科技河北有限责任公司
Preparation of specific tumor killing cell
ActiveCN102526716ABreak immune tolerancePowerful killing functionMammal material medical ingredientsBlood/immune system cellsCell immunityT lymphocyte
The invention relates to an antitumor cell immunotherapy technology, in particular to preparation of a specific tumor killing cell. The preparation method disclosed by the invention comprises the following steps of: 1, sampling a single prokaryotic cell from peripheral blood; 2, separating a DC (Dendritic Cell) from a T cell; 3, maturing the DC and preparing a DC vaccine; 4, preparing a CIK (Cytokine Induced Killer); 5, preparing a CTL (cytotoxic T lymphocyte); and 6, preparing a specific DC-CIK-CTL cell preparation.
Owner:玥特农生物科技河北有限责任公司
Preparation method for dendritic cell of umbilical cord blood source and dendritic cell vaccine
ActiveCN102676455ABlood/immune system cellsAntibody medical ingredientsHematopoietic cellCell culture media
The invention discloses a preparation method for the dendritic cell (DC) of an umbilical cord blood source and a dendritic cell (DC) vaccine, which relates to a preparation method for the dendritic cell. According to the method, various cell factors are adopted to induce DC obtained by umbilical cord blood separation, and then the DC is stimulated by a tumor specific antigen so as to improve the specific antigen presentation capability of the DC; and a stem cell growth factor and Flt3-L are added into a cell culture medium so as to effectively accelerate a hematopoietic cell in the umbilical cord blood to induce and proliferate to an immune cell. The DC vaccine prepared with the method has the specific antigen presentation capability, can be combined with a CIK (cytokine induced killer) cell to mutually treat the malignant tumor when being used as a tumor immunotherapy product, and is used as an important adjuvant therapy after operations and chemoradiotherapy. Recurrence and metastasis after the operations can be effectively prevented, and toxic and side effects caused by the chemoradiotherapy on patients are lowered so as to improve the treatment effect.
Owner:北京和泽普瑞生物科技有限公司 +1
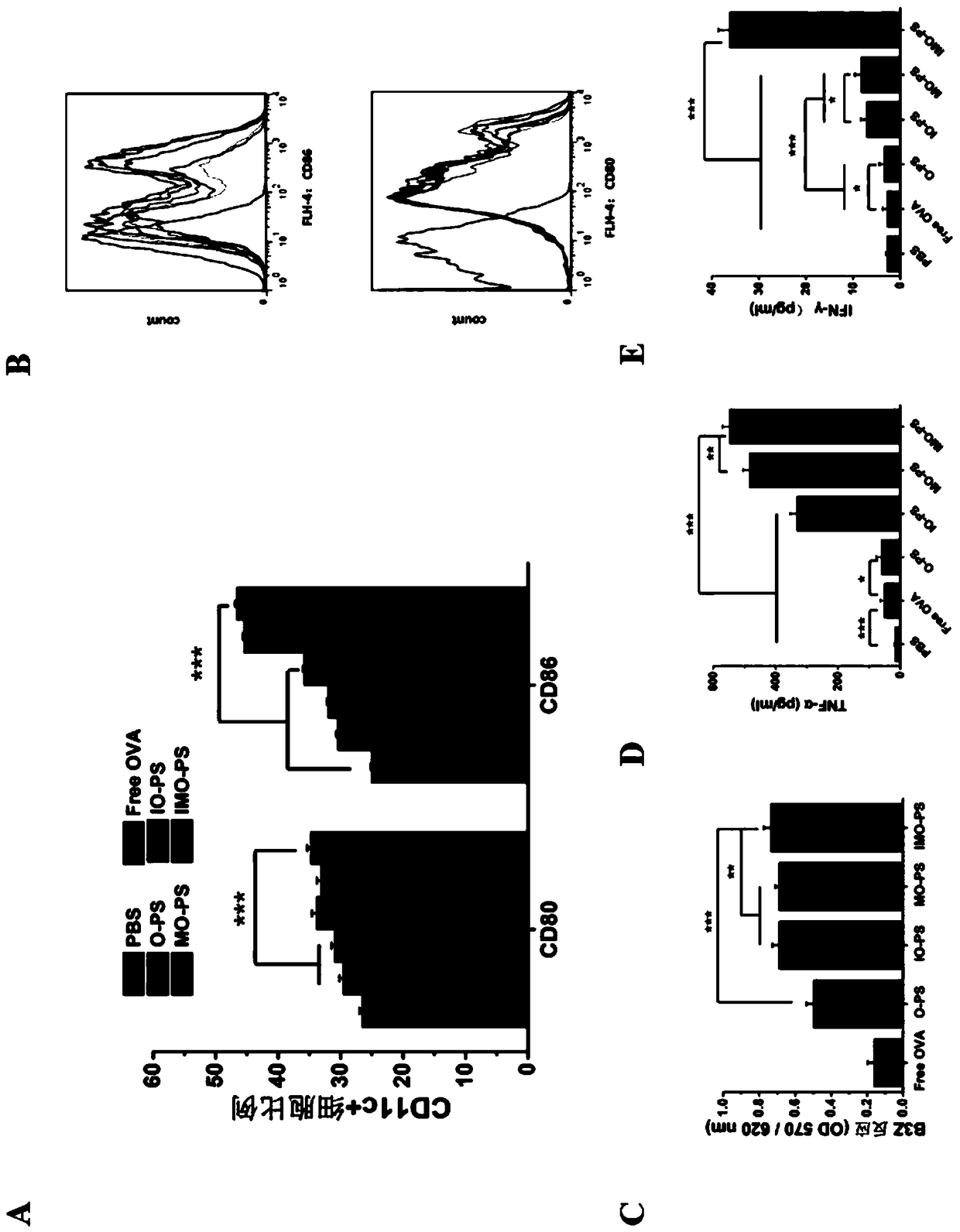
DCs vaccine based on phospholipid hybrid polymersome jointly encapsulating antigen and dual immunoagonists and preparation method and application thereof
ActiveCN108938568AMaximize Targeting EffectAchieving ImmunotherapyCancer antigen ingredientsPharmaceutical non-active ingredientsT lymphocyteBiological activation
The invention relates to a DCs vaccine based on phospholipid hybrid polymersome jointly encapsulating an antigen and dual immunoagonists, a preparation method and application thereof. The phospholipidhybrid polymersome which can jointly load a model antigen OVA and two types of TLR agonists (TLR7 / 8 and TLR4) is used for stimulation in vitro of the DCs so as to realize the effective phagocytosis of DCs cells. The rapid and long-term immunostimulatory effect on the DCs is achieved by the internal and external co-loading of the OVA antigen. The synergistic effect of the two types of TLR agonistssignificantly enhances the immune response after antigen stimulation; the phospholipid hybrid polymersome which jointly encapsulates the antigen and the dual immunoagonists can effectively promote the activation and maturation of the DCs, increases the level of cross-presentation, promotes the migration of the DC vaccine to secondary lymphoid organs, and produces a strong specific cytotoxic T lymphocytes (CTLs) killing effect, thereby effectively killing tumor cells and realizing the immunotherapy of the DCs vaccine on tumors.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
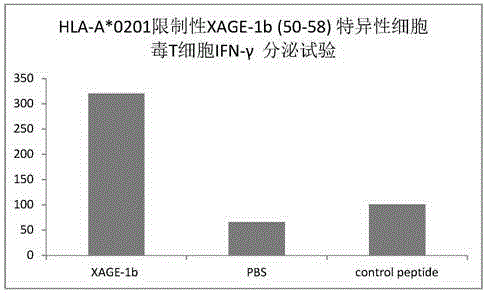
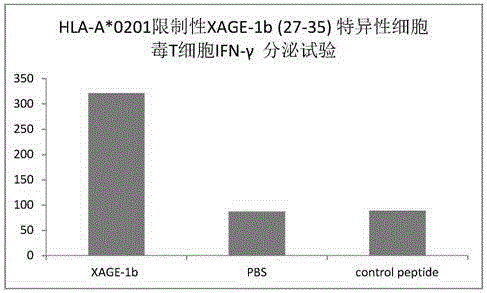
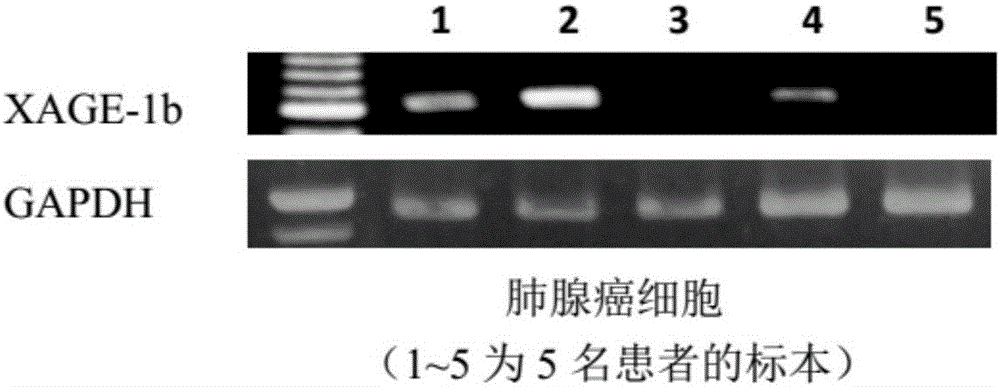
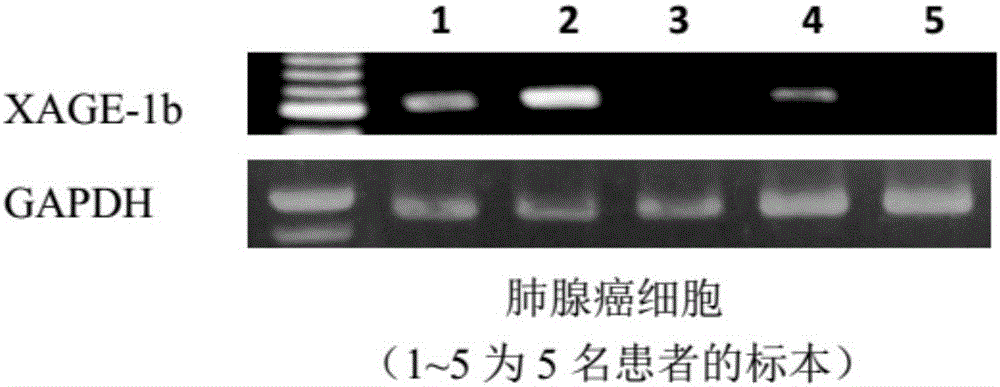
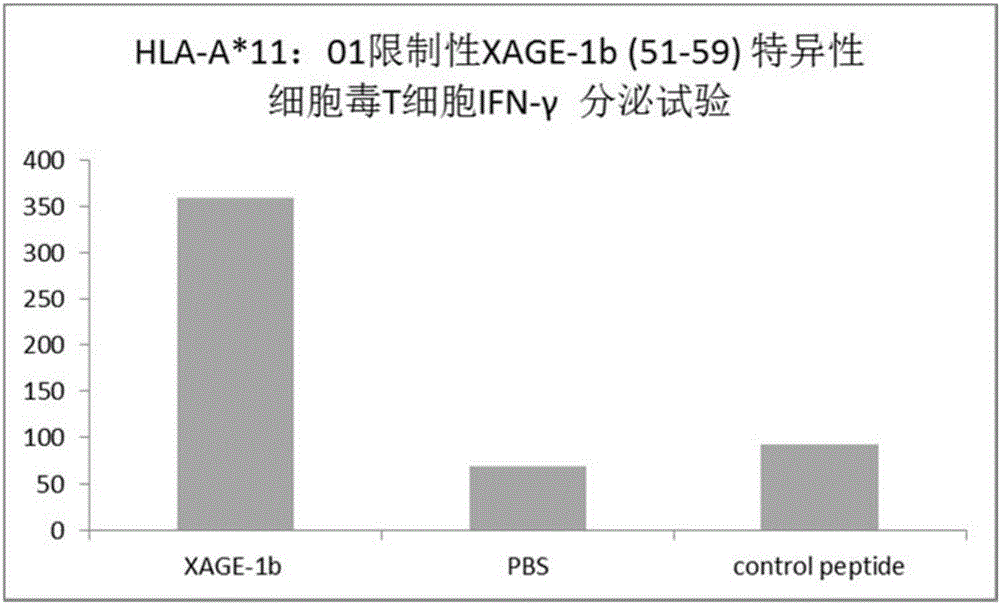
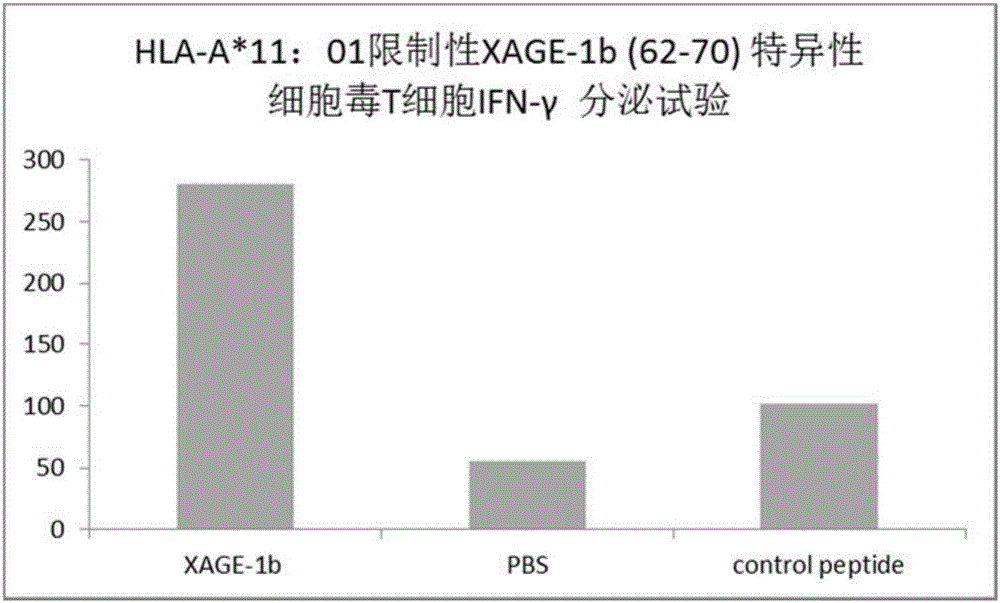
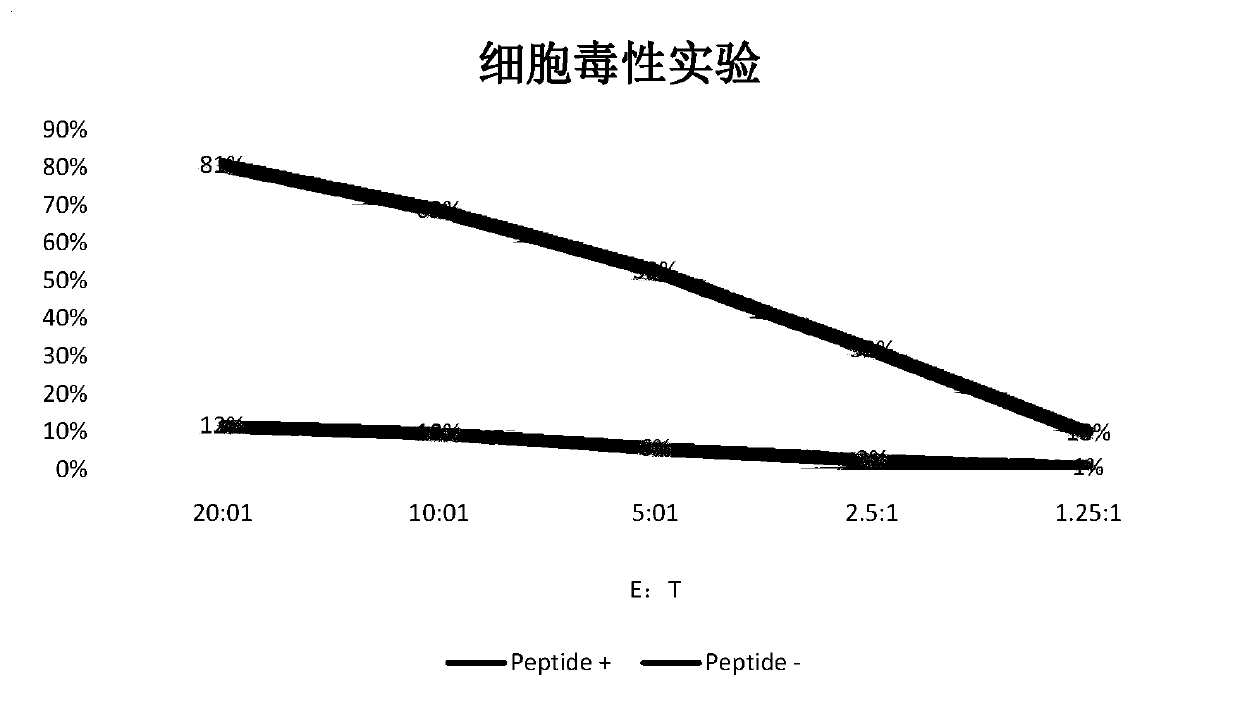
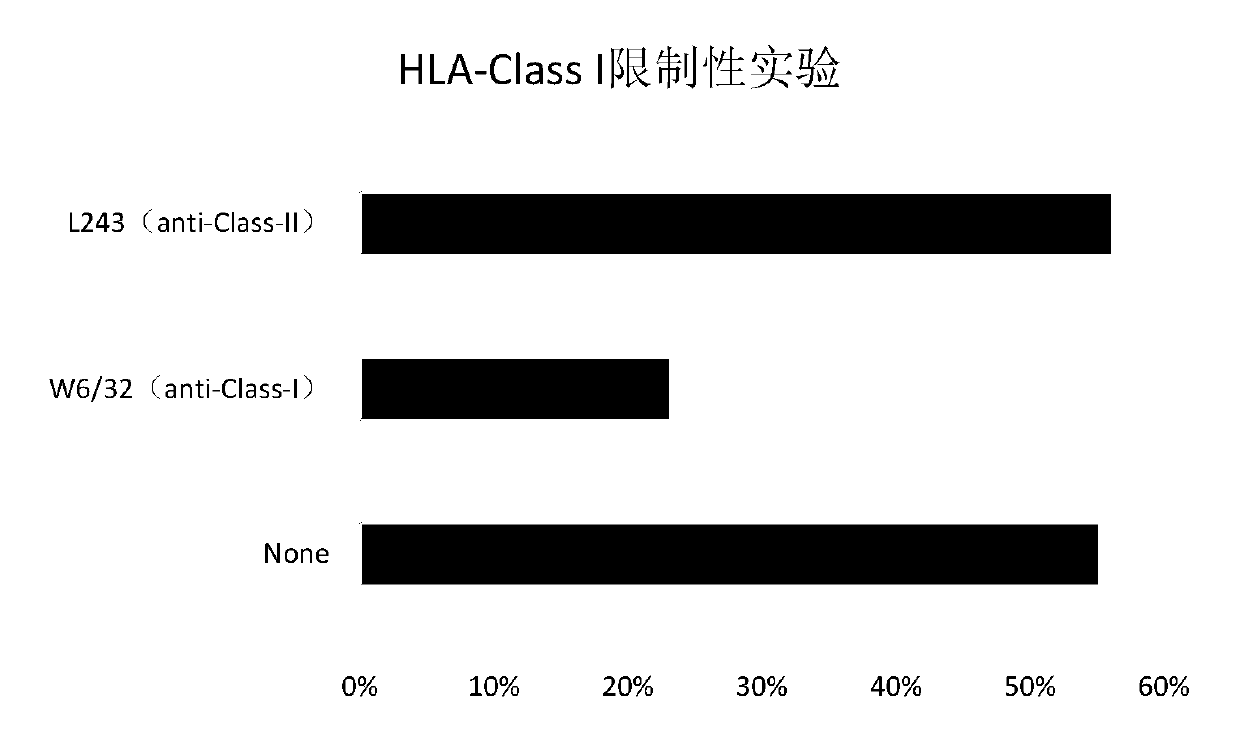
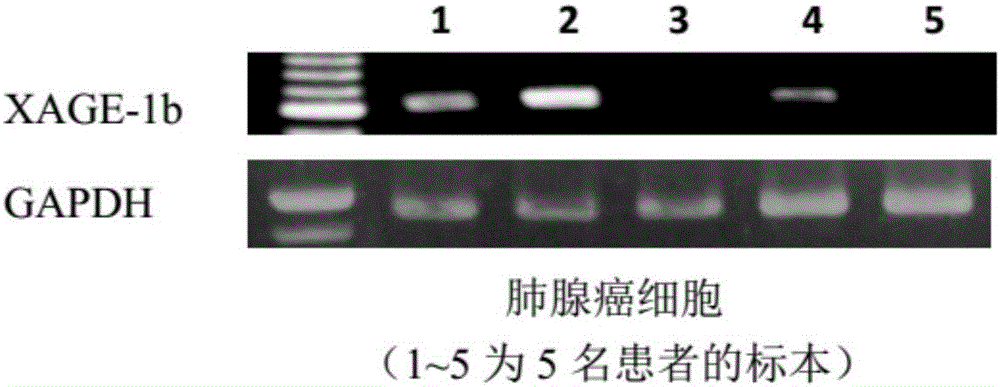
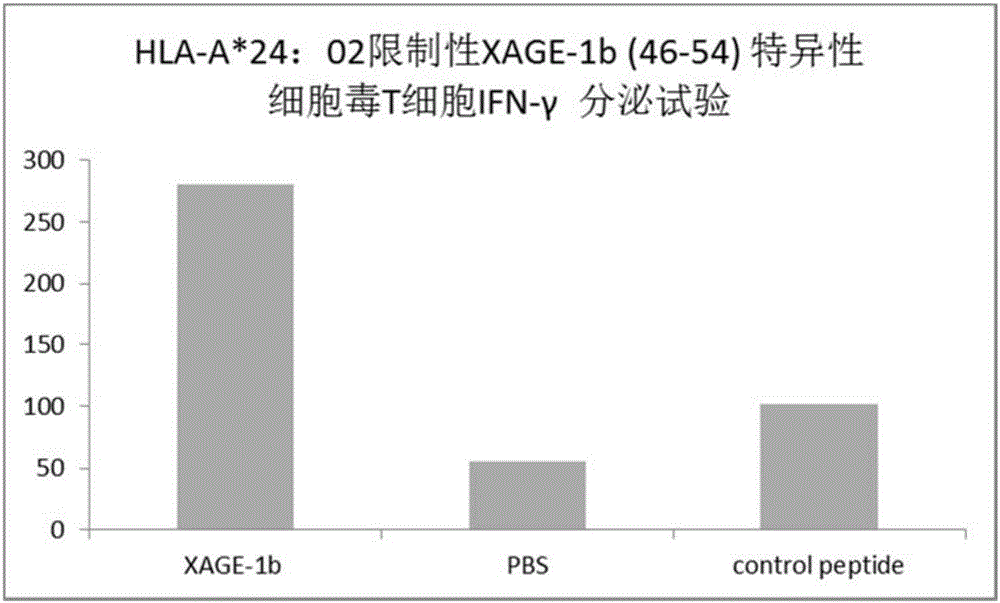
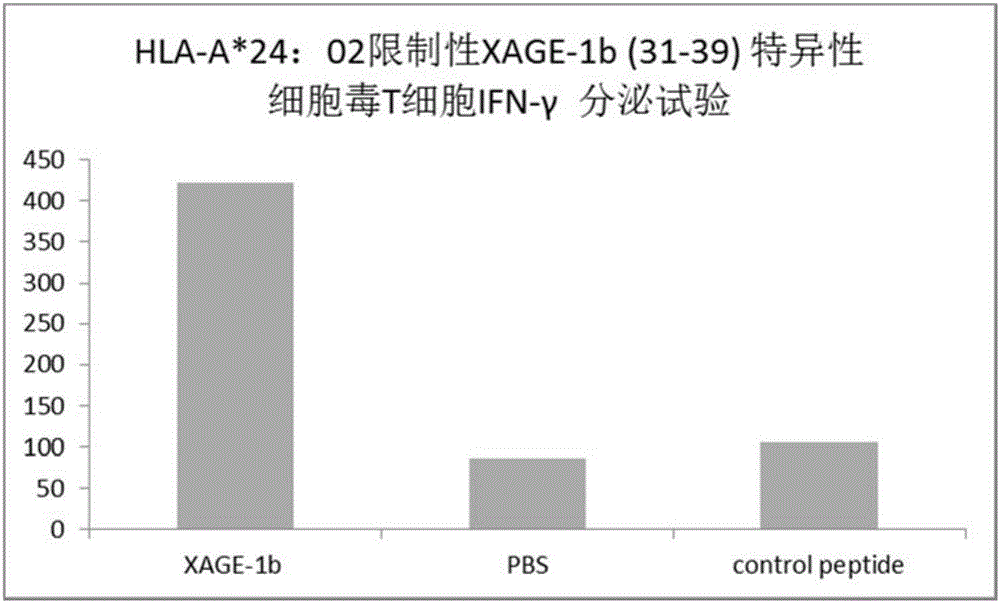
Tumor-associated antigen XAGE-1b short-peptide and application thereof
ActiveCN106279392AGood specific killing effectPromote clinical translationTumor rejection antigen precursorsTumor specific antigensCD8Dc vaccine
The invention discloses a tumor-associated antigen XAGE-1b short-peptide and application thereof. The sequences of the short-peptide is one of the SEQ ID NO:2-SEQ ID NO:15. The CTL induced by the XAGE-1b antigen peptide does not produce an immune response to testicular cells, and only kills tumor cells. The obtained CTL clone has a good specific killing effect on tumor cells. The selected XAGE-1b antigen peptide has comparable affinity to HLA on DC cells and can effectively stimulate and induce specific CTLs, indicating that the selected XAGE-1b antigen peptide has good potential of polypeptide vaccine and DC vaccine. By cloning XAGE-1b antigen peptide specific TCR gene, constructing viral vector, transducing peripheral circulation CD8+T cells, TCR gene modified T cells (TCR-T) clone is obtained, and also has specific killing effect on tumor cells which is similar to parental CTL, suggesting that the XAGE-1b short-peptide has good clinical application and transformation prospect.
Owner:安军 +1
Method for preparing dendritic cell vaccine
InactiveCN103948917AThe ingredients are clear and singleHigh purityBlood/immune system cellsAntibody medical ingredientsCord blood stem cellCD8
The invention belongs to the cellular immunity field, and concretely relates to a preparation method of dendritic cell vaccine. The method comprises the following steps: separating cord blood to obtain cord blood monocyte; screening DC cell from the cord blood monocyte, culturing and performing amplification, then performing single epitope multiple antigen peptide (SEA-MVP) for loading, and then incubating overnight to obtain the DC vaccine. The method has the beneficial effect that the single epitope multiple antigen peptide (SEA-MVP) is used for loading DC cells, the single epitope multiple antigen peptide (SEA-MVP) is obtained by in vitro synthesis, the composition is clear and single, the purity is high; alkaline amino acid is taken as a frame for connecting the single epitope, so that the orientation of the connected epitope peptide has consistency and the epitope peptide is easily recognized and presented by DC cells, the sensitivity and singularity are strong, the loading efficiency is high, and immune tolerance is not generated; compared with the current method, the antineoplastic cell capability of the dendritic cell vaccine for activating the CD4+ and CD8+ cells is increased by more than ten thousand times.
Owner:江苏和泽生物科技有限公司
Multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine for HCV (hepatitis C viruses)
The invention discloses a multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine for HCV (hepatitis C viruses), relating to the technical field of virology and immunology. The multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine is characterized in that two CTL epitopes (namely, a NS4B (1793-1801) SMMAFSAAL and a P7 (774-782) AAWYIKGRL) are used for constructing recombinant adenoviruses, then the recombinant adenoviruses are used for infecting human dendritic cells so as to prepare a multi-epitope DC vaccine. Detection results indicate that the multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine for HCV (hepatitis C viruses) disclosed by the invention has an immunogenicity.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Method for preparing tumor-specific DC vaccine by applying CD34+ cells of umbilical cord blood
ActiveCN103405759ABreak immune toleranceBlood/immune system cellsAntibody medical ingredientsAntigenDc vaccine
The invention discloses a method for preparing a tumor-specific DC vaccine by applying CD34+ cells in umbilical cord blood. The method comprises (1) a step of preparing autologous tumor-related holoantigen; (2) a step of obtaining the umbilical cord blood; (3) a step of obtaining mononuclear cells derived from the umbilical cord blood; (4) a step of purifying CD34+ cells in the mononuclear cells derived from the umbilical cord blood; (5) performing induction culture for a precursor DC; (6) a step of performing amplification and culture of an immature DC; and (7) a step of preparing the DC vaccine.
Owner:玥特农生物科技河北有限责任公司
Tumor-associated antigen XAGE-1b short peptide and application thereof
ActiveCN106243213AGood specific killing effectPromote clinical translationTumor rejection antigen precursorsBiological material analysisT lymphocyteCD8
The invention discloses a tumor-associated antigen XAGE-1b short peptide and an application thereof. The sequence of the short peptide is selected from SEQ ID NO: 2-SEQ ID NO: 13. Cytotoxic T lymphocytes (CTLs) induced by the XAGE-1b antigen peptides do not generate immune response to testicular cells, and only kill tumor cell. The induced CTL clones have good tumor cell specific killing effect. Meanwhile, in the process of establishing the CTLs, the screened XAGE-1b antigen peptides are found to have appropriate affinity with HLA on DC cells and can effectively stimulate and induce the generation of specific CTLs, and it is proved that the XAGE-1b antigen peptides have good potential of a polypeptide vaccine and a DC vaccine. XAGE-1 b antigen peptide specific TCR genes are cloned, a virus carrier is constructed, the genes are transduced to CD8+T cells from peripheral circulation to obtain TCR gene modified T cell (TCR-T) clones, and also the specific killing effect on tumor cells similar to parental CTLs is obtained, and thus the tumor-associated antigen XAGE-1b short peptide has good clinical conversion and practical application prospect.
Owner:珠海美烨生物科技有限公司
Preparation method of high-efficiency killer cell preparation adopting immunodetection point dual-block CTL (cytotoxic lymphocyte)
ActiveCN106177931AReduce exhaustEnhance tumor killing effectCancer antigen ingredientsBlood/immune system cellsTumor specificInterleukin 4
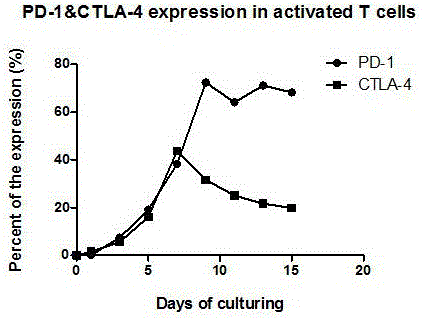
The invention discloses a preparation method of a high-efficiency killer cell preparation adopting an immunodetection point dual-block CTL (cytotoxic lymphocyte). The preparation method comprises steps as follows: step 1), collecting mononuclear cells in peripheral blood; step 2), performing DC (dendritic cell) separation and T cell separation on the separated mononuclear cells with an adherence method; step 3), adding adhering DCs to a serum-free culture medium containing GM-CSF (granulocyte-macrophage colony-stimulating factor) and IL-4 (interleukin-4) for preparation of a DC vaccine; step 4), adding suspending T cells to CD3 and IL-2 for T cell culture and amplification; step 5), adding antigen-supported DCs to the amplified T cells for preparation of the CTL, and adding PD-1 (Programmed death 1) and CTLA-4 (cytotoxic t lymphocyte associated antigen-4) antibodies for preparation of the cell preparation with the dual-block CTL effect. According to the novel technology and the novel method provided by the invention, an immune brake effect is effectively regulated and T cell depletion is retarded by blocking inhibitory molecules on the tumor specific CTL surfaces, so that the tumor cytotoxicity of CTL effector cells is substantially improved.
Owner:浓孚雨医药河北有限公司
Cell lysis solution, kit and application of cell lysis solution to preparation of tumor whole cell antigen loaded DC tumor vaccine
InactiveCN106620681ADoes not destroy higher order conformationsMaintain antigenicityCancer antigen ingredientsAntineoplastic agentsTreatment effectCellular antigens
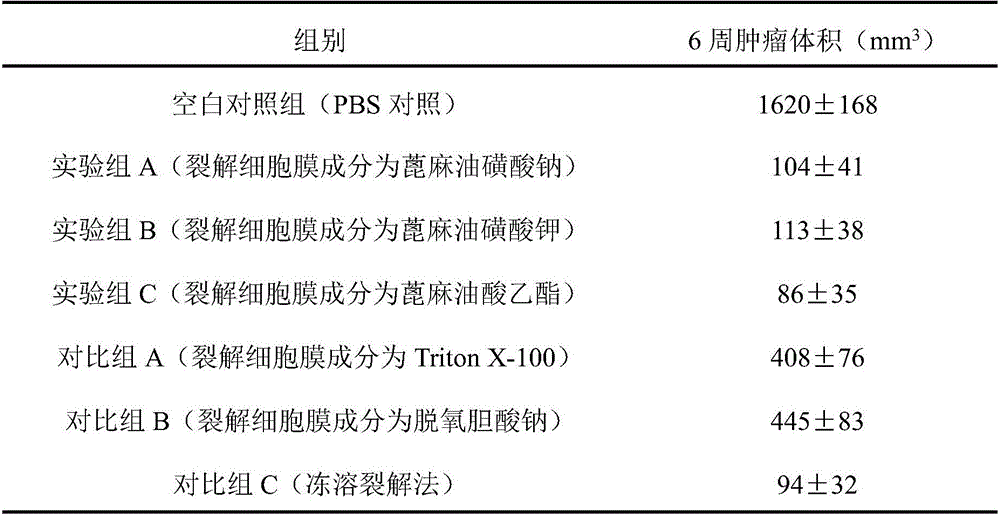
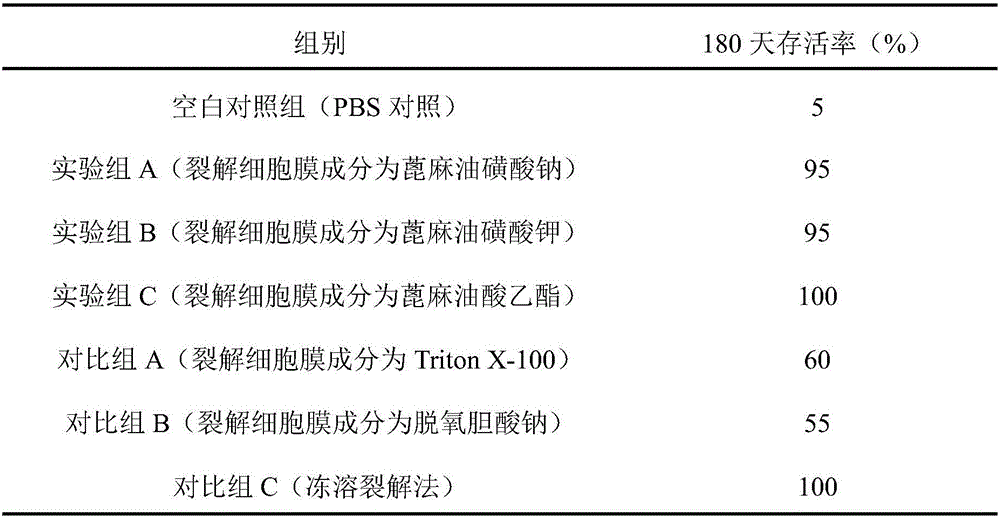
The invention discloses a cell lysis solution, a kit and application of the cell lysis solution to the preparation of a tumor whole cell antigen loaded DC tumor vaccine. The cell lysis solution comprises a buffer system component, an isotonic system component, a lysis cell membrane component, a protein stabilizer and a protease inhibitor, wherein the lysis cell membrane component is selected from a compound castor oil sulfonate or ricinoleate. The cell lysis solution has the advantages that the compound castor oil sulfonate or ricinoleate can mildly induce tumor cell membrane lysis without destroying the high-level conformation of cell membrane protein and cytoplasm protein, the cell lysis solution prepared by the compound castor oil sulfonate or ricinoleate can keep the antigenicity of lysate to the maximum extent, and the lysate sensitized DC vaccine obtained by co-culturing DC with the tumor cell lysate split by the cell lysis solution has an excellent antitumor effect; compared with a freezing-dissolving lysis method commonly used in the prior art, the cell lysis solution is evidently better than a traditional lysis solution and has a tumor preventing and treating effect close to the freezing-dissolving lysis.
Owner:南京佰泰克生物技术有限公司
DC vaccine for treating chronic hepatitis B
InactiveCN1981866AImprove efficiencyImprove immunityPharmaceutical delivery mechanismAntiviralsHepatitis B virus core AntigenChronic hepatitis
A therapeutic DC vaccine for preventing and treating chronic hepatitis B is a hepatitis B specific DC vaccine carried by both HBsAg and HBcAg. The dendritic cells coming from mononuclear cells are carried by both recombinant HBV surface antigen and recombinant HBV core antigen.
Owner:解放军三〇二医院生物治疗研究中心
DC vaccine for novel corona viruses, and preparation method and application of DC vaccine
InactiveCN111729079AShorten the timeIncrease lethalitySsRNA viruses positive-senseAntibody mimetics/scaffoldsAntigenIn vitro test
The invention provides a DC vaccine for novel corona viruses. The DC vaccine comprises COVID-19 S proteins, chemotactic factors CCL19. The DC vaccine prepared from self peripheral blood is high in safety and does not have exclusive reactions. Compared with other vaccines, the DC vaccine is longer in duration time, can faster recognize antigens, can start up killing and damaging functions of T cells, and can produce antibodies at the same time. During in vitro tests, when the DC vaccine is united with CIK, killing and damaging to target cells can be started within 4h. The 2019nCOV-S-CCL19 DC vaccine prepared by the invention is united with the CIK cells to achieve good killing and damaging experiment effects; in vitro tests, when the 2019nCOV-S-CCL19 DC vaccine is united with the CIK cellsfor use, the 2019nCOV-S-CCL19 DC vaccine, the CIK cells and target cells are mixed for 24h, then detection is performed, when an effector target ratio is 10:1, the cytotoxicity to the target cells isas high as 93.2%, and when the effector target ratio is 5:1, the cytotoxicity to the target cells is as high as 68.6%.
Owner:SHANDONG XINRUI BIOTECH CO LTD
Method for production of dendritic cell
InactiveCN101755045APowerful immune induction functionLess DCBlood/immune system cellsCell culture active agentsDc vaccinePrecursor cell
The invention provides a method for producing a dendritic cell (DC), which involves the step of culturing a DC precursor cell in the presence of two or more cytokines; a DC produced by the method; and use of the DC. The method can produce a large quantity of a DC precursor cell which is highly capable of being differentiated into a DC. The method can also produce a large quantity of a DC from a small quantity of a DC precursor cell. Therefore, it becomes possible to readily increase the number of DCs to be administered in the anti-tumor immunotherapy or the treatment of an infectious disease utilizing a DC, resulting in the enhancement of the efficacy of a DC vaccine.
Owner:DNAVEC CORP
Application method of tumor cell-derived exosome antigen in DC vaccine
PendingCN110604813AEfficient removalImprove the activation efficiency of source DCCancer antigen ingredientsAntineoplastic agentsAntigenMedicine
The invention discloses an application method of a tumor cell-derived exosome antigen in a DC vaccine. The invention discloses a preparation method of a tumor vaccine, which comprises the following step: sensitizing DC cells by using a tumor cell exosome to obtain the tumor vaccine. The tumor cell supernatant exosome provided by the invention has good stability, low toxicity and good cell compatibility, and is convenient to operate and apply and popularize in clinical tumor immunotherapy, and the method is a simple, feasible and practical application technology and method. The problems that inexisting DC vaccine construction, antigen sources are lacked, compatibility is poor, instability is caused, and an external DC antigen carrier has biotoxicity and is difficult to operate are solved.
Owner:SHENZHEN PEOPLES HOSPITAL
Genetically modified dendritic cell vaccine
ActiveCN109957548AEfficient DCGenetically modified cellsCancer antigen ingredientsHigh concentrationBULK ACTIVE INGREDIENT
The invention relates to the fields of biotechnology and medicine, and provides a modified dendritic cell (DC); a dendritic cell is infected with MG-7Ag antigen mimic epitope tandem sequences loaded with a lentivirus vector, and a target antigen sequence is integrated into a dendritic cell genome. The invention provides a rapid culture scheme of the dendritic cell derived from a peripheral blood monocyte in vitro, and also provides a vaccine with an active ingredient of the modified dendritic cell. The vaccine is used for tumor prevention and active immunotherapy. The MG-7Ag antigen sequence-modified DC vaccine can obtain high-purity CTL cells and efficient target cell killing ability after DC-CTL co-culture, and the co-culture supernatant contains high-concentration IFN[gamma] secretion.After tumor attack, the tumorigenic volume of mice in a DC-CTL group is significantly smaller than that of mice in a control group, and the DC vaccine has great potential value in immunotherapy of MG-7Ag positive tumor.
Owner:上海尚泰生物技术有限公司
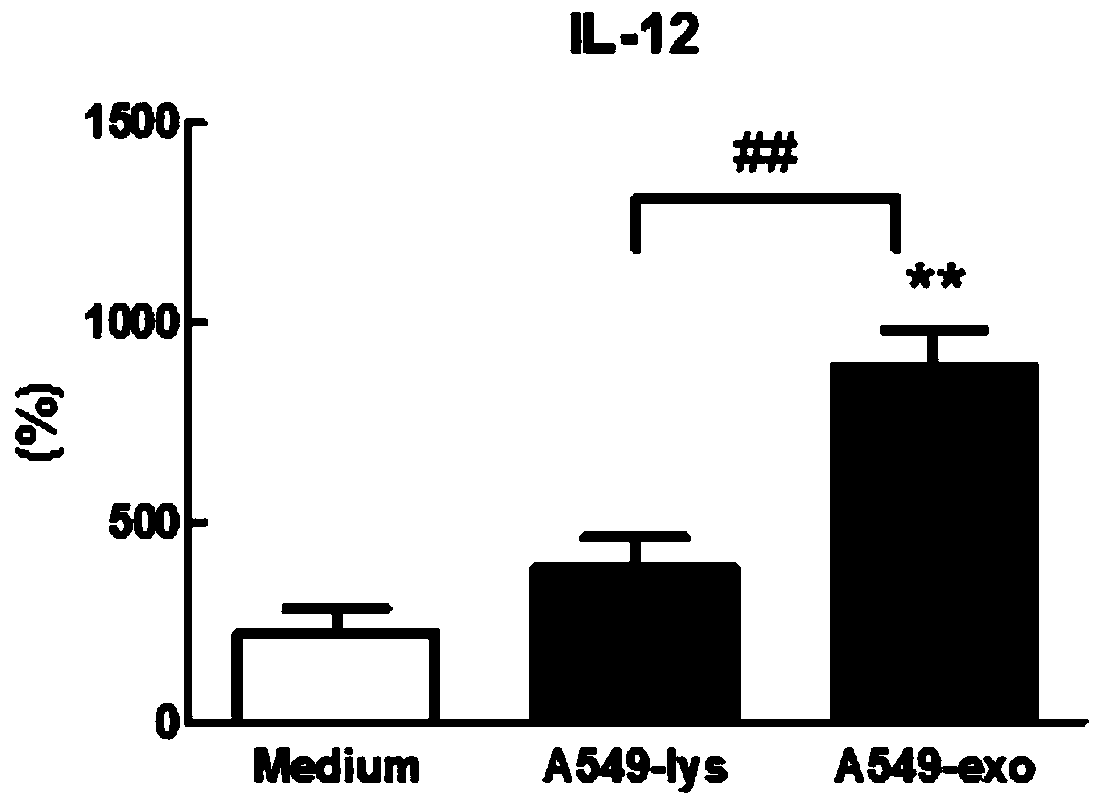
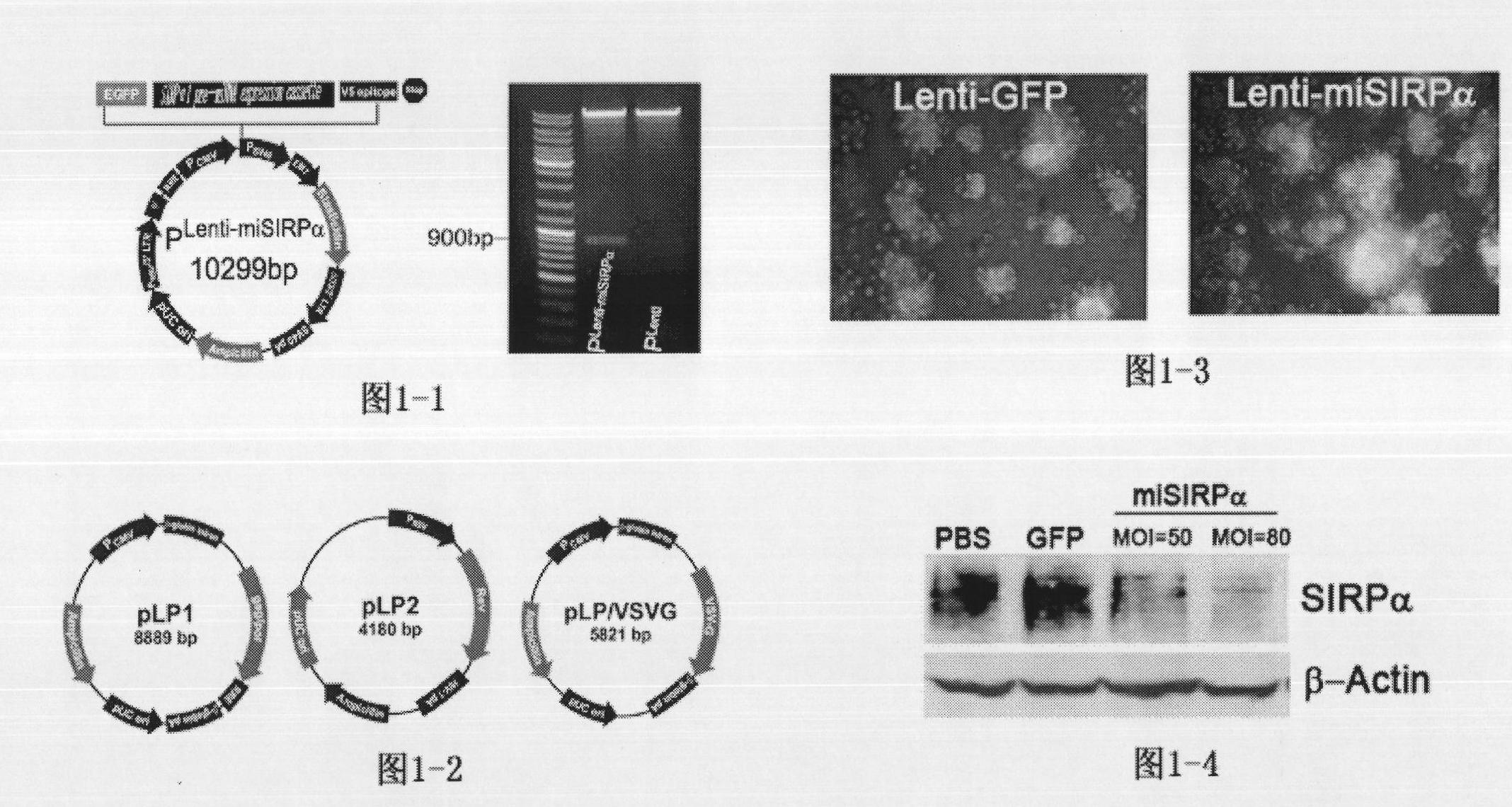
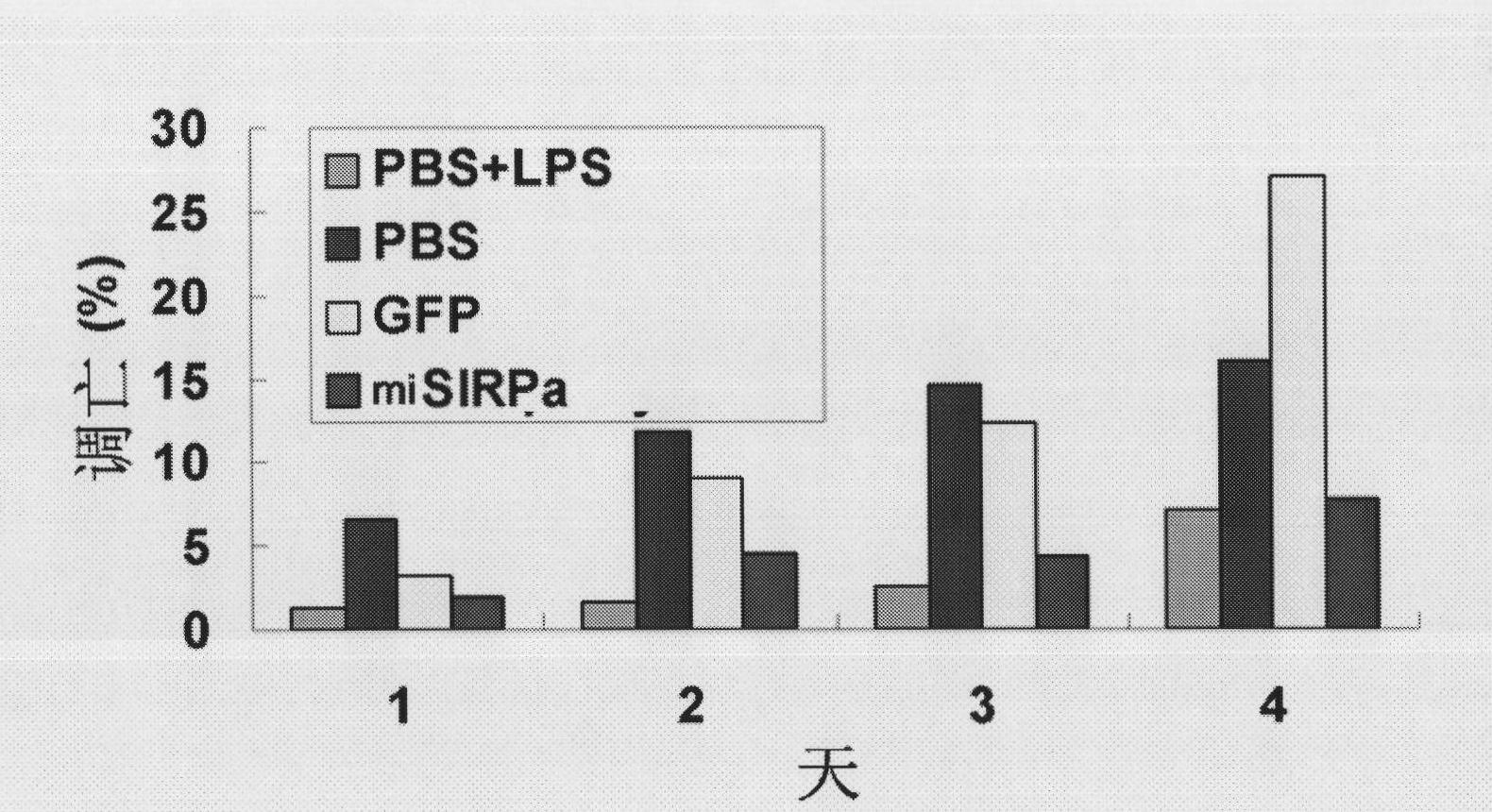
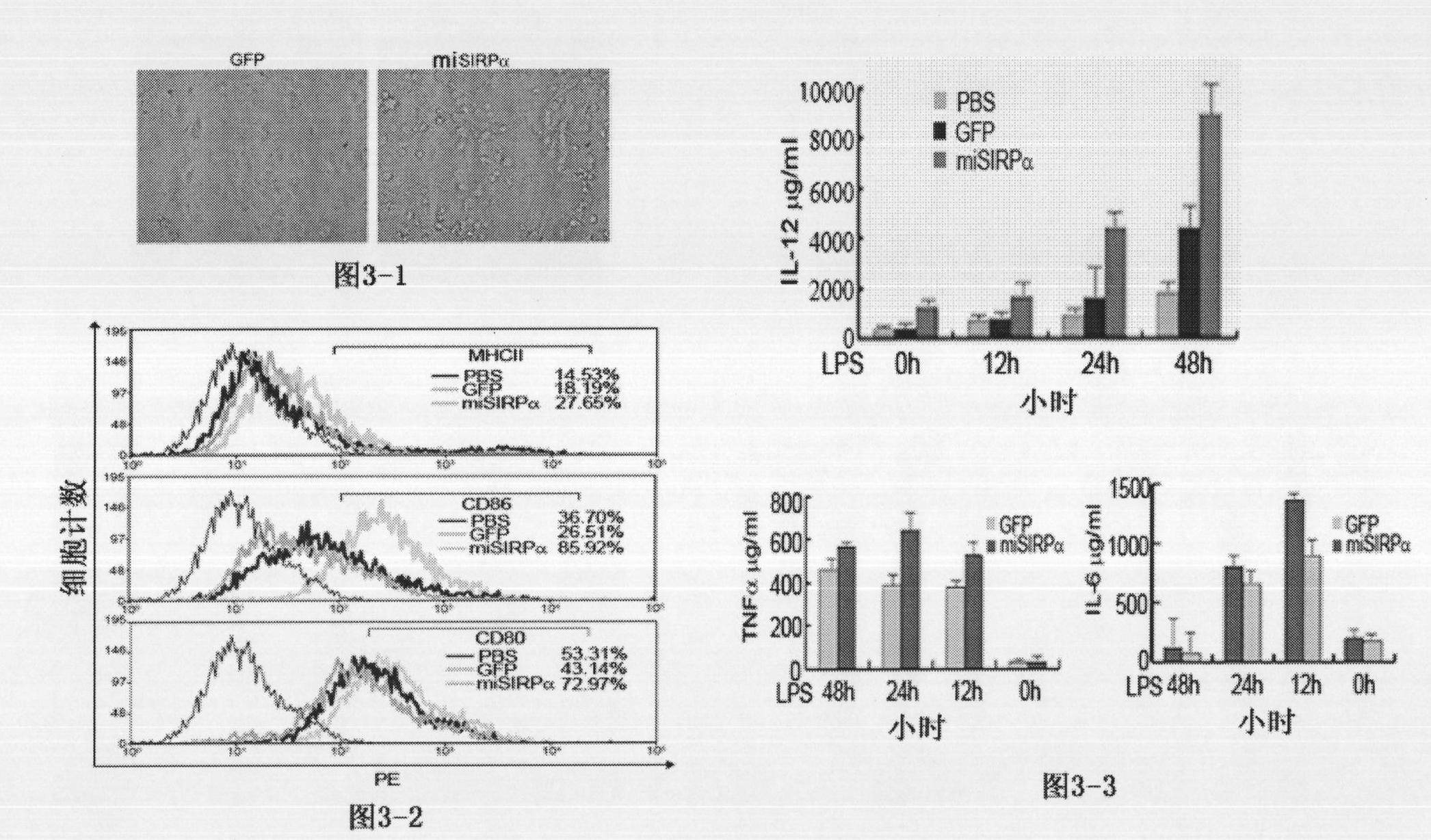
Application of signal adjusting protein alpha in preparation of DC vaccine for preventing and treating tumors
InactiveCN101780283AHigh activityFunction increaseGenetic material ingredientsFermentationAbnormal tissue growthSignal-regulatory protein alpha
The invention belongs to the technical field of medical bio-engineering, and particularly relates to new application of the signal adjusting protein alpha(SIRP alpha). The signal adjusting protein alpha(SIRP alpha) belongs to a member of the immunoglobulin super family(IGSF). In-vitro biological experiments and in-vivo animal experiments prove that in the application of the signal adjusting protein alpha(SIRP alpha) in the preparation of the DC vaccine for preventing and treating tumors of the invention, the SIRP alpha signal path negatively regulates the activity and antigen presentation functions of the DC. The invention further constructs a tumor DC vaccine which can interfere with the SIRP alpha expression by the mediation of a lentiviral vector, and the tumor DC vaccine can obviously improve the DC activation level and the antigen presentation function of the DC and be used for preventing and treating mouse tumors and is a novel tumor vaccine. The invention provides a new idea for clinical treatment application of the SIRP alpha.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Immunocyte medicine containing B cell vaccine loaded with new antigen
PendingCN110272874AGood cancer preventionTumor rejection antigen precursorsTumor specific antigensAbnormal tissue growthTherapeutic effect
The invention provides an immunocyte medicine containing a B cell vaccine (neoB) loaded with a tumor new antigen, and a medicine combination for united use of neoB and tumor specificity T cells. The neoB vaccine can have higher ex vivo expansion properties than a DC vaccine, has higher effect of continuous activating of in vivo T cells under the condition of repeated infusion, and can become a preventive or therapeutic vaccine or an immunocyte medicine to be applied to tumor resisting treatment suitable for any cancer kind. According to the medicine combination for united use of neoB and tumor specificity T cells, disclosed by the invention, the neoB can be used for further stimulating the tumor specificity T cells in bodies, so that the number of the tumor specificity T cells in bodies can be amplified, and the treatment effect is increased.
Owner:CHINEO MEDICAL TECH CO LTD
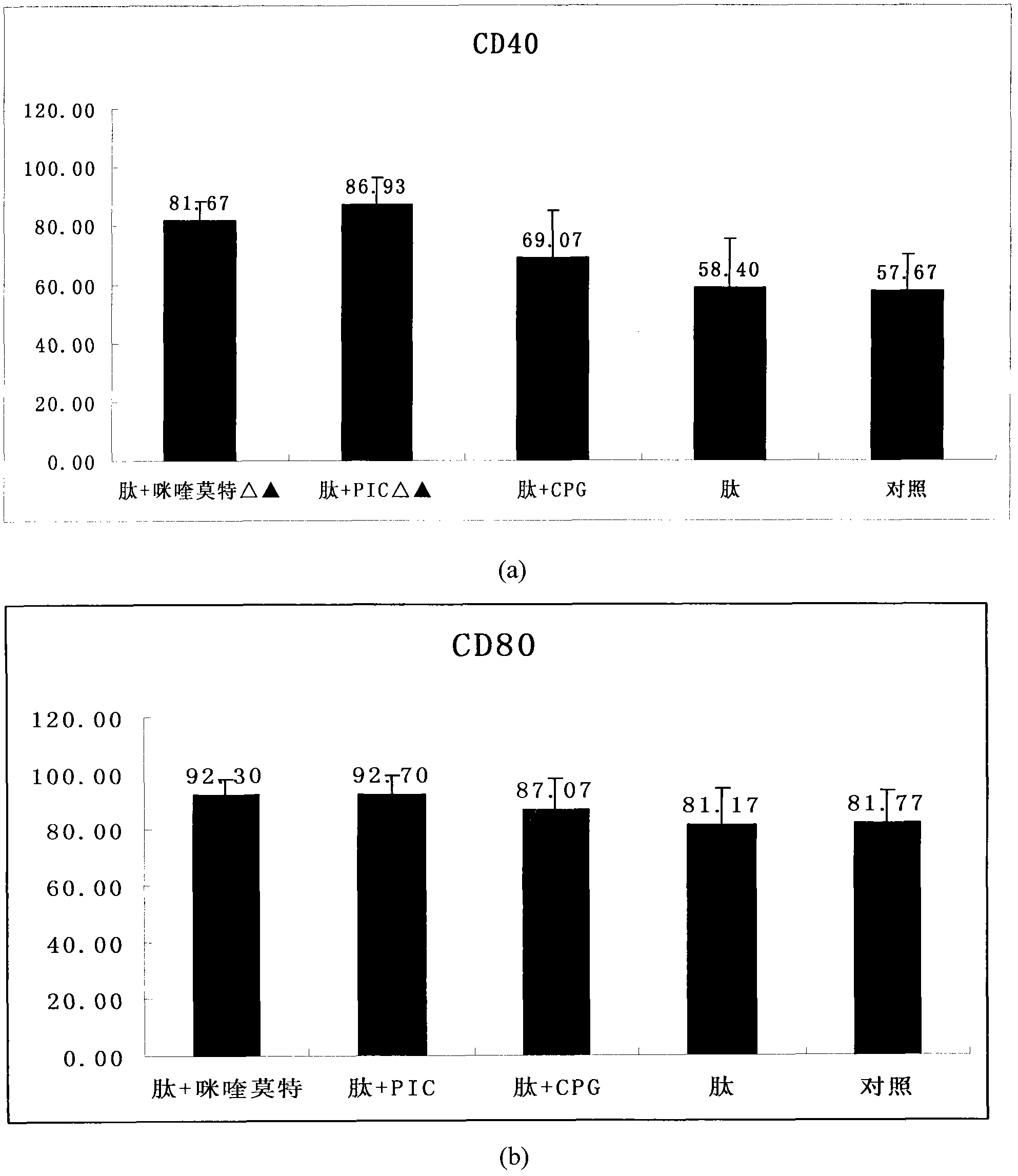
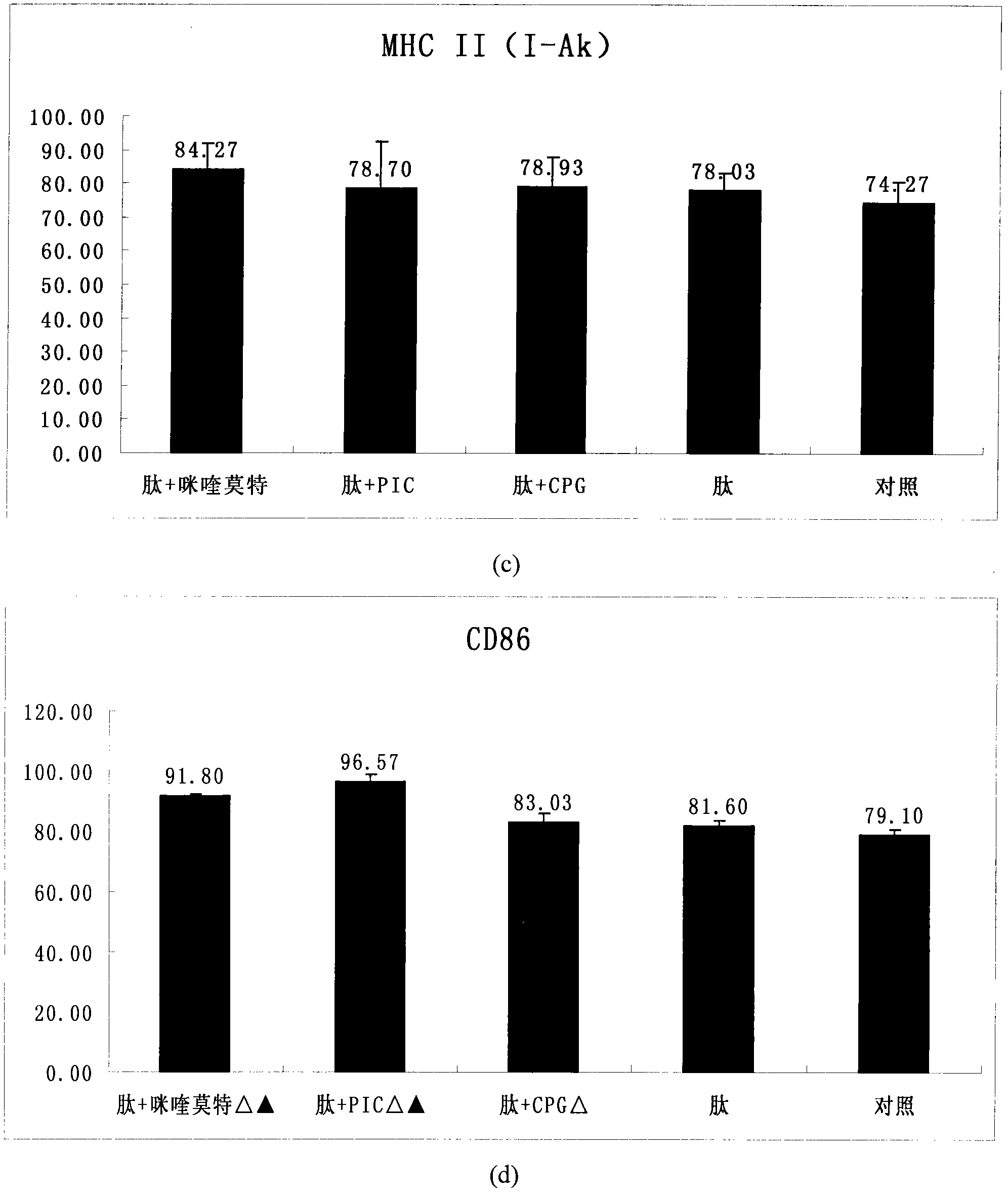
HPV (human papillomavirus) peptide/DC (dendritic cell) mixed vaccine and preparation thereof
InactiveCN102008721APromote maturityImprove the level ofViral antigen ingredientsAntiviralsAdjuvantHuman papillomavirus
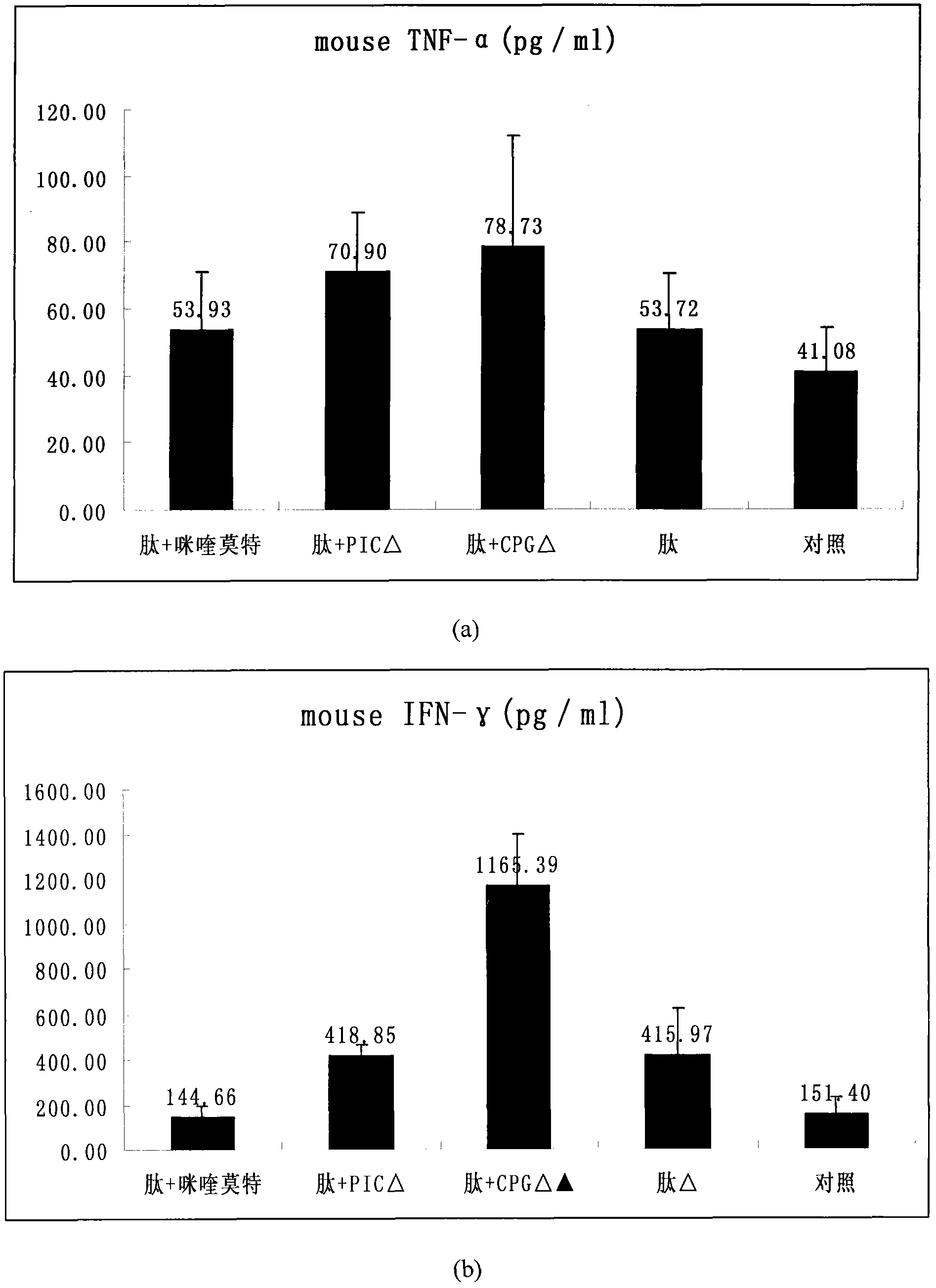
The invention provides an HPV (human papillomavirus) peptide / DC (dendritic cell) mixed vaccine prepared in the presence of a TLR (toll-like receptor) agonist. The mixed vaccine comprises one part of HPV11E77-15, one part of mouse bone marrow-derived dendritic cells which are cultured in vitro and one part of TLR9 agonist as an adjuvant, wherein the dendritic cells are extracted from mouse bone marrow and further cultured in vitro, the co-incubation with one section of HPV11E7CTL (cytotoxic T lymphocyte) epitope peptide with strongest immunogenicity is firstly carried out, the co-incubation with the TLR ligand CpG and the like is further respectively carried out for promoting the maturation of the DC, the degree of maturation can be confirmed by detecting the change of a marker on the surface of the DC after incubation, and the DC vaccine which is simulated to mature can be used for immunizing mice. The TLR ligand and the HPV11E7 peptide are utilized jointly to promote the degree of maturation of the DC, the level of TNF-alpha (tumor necrosis factor-alpha) and IFN-gamma (immunoreactive fibronectin-gamma) of factors of secretory cells of T cells can be particularly improved after combination of the CpG, and the HPV peptide / DC mixed vaccine can be used for preventing and treating genital warts and cervical cancer.
Owner:ZHEJIANG UNIV
Tumor-associated antigen XAGE-1b nonapeptide and application thereof
InactiveCN109970846AGood specific killing effectPromote clinical translationTumor rejection antigen precursorsBiological material analysisT lymphocyteCD8
The invention discloses tumor-associated antigen XAGE-1b nonapeptide and application thereof. The sequence of XAGE-1b oligopeptide is RQKKIRIQL. CTLs (cytotoxic T lymphocytes) induced by the XAGE-1b antigen peptide do not produce immune responses to testicular cells, but only kill tumor cells. Induced CTL clones have a good tumor cell specificity lethal effect. In the process of establishing the CTLs, the XAGE-1b antigen peptide screened out by the inventor has proper affinity with HLAs (human leukocyte antigens) on DC cells and can effectively stimulate and induce the specific CTLs, thereby having a good potential of serving as a polypeptide vaccine and a DC vaccine. Virus carriers are created by cloning specific TCR (T cell receptor) genes of the XAGE-1b antigen peptide, and peripheral circulation CD8+T cells are transduced to obtain clones of TCR gene modified T cells (TCR-T); the clones also have the tumor cell specificity lethal effect same as the parental CTLs, thereby having a good prospect in clinical transformation and practical application.
Owner:广州美萨生物科技有限公司
Fused gene, fused protein, recombinant vector and general DC vaccine of coronavirus and preparation methods for fused gene, fused protein, recombinant vector and general DC vaccine of coronavirus
ActiveCN112592928AEffective stimulationSsRNA viruses positive-senseViral antigen ingredientsVirus ProteinViral Vaccine
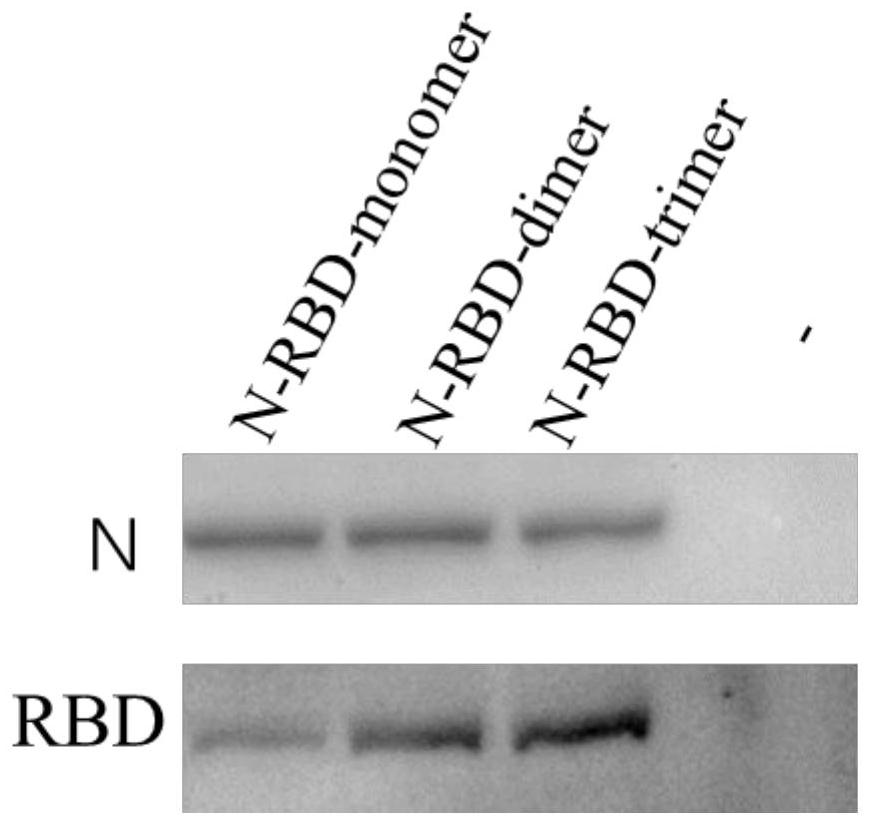
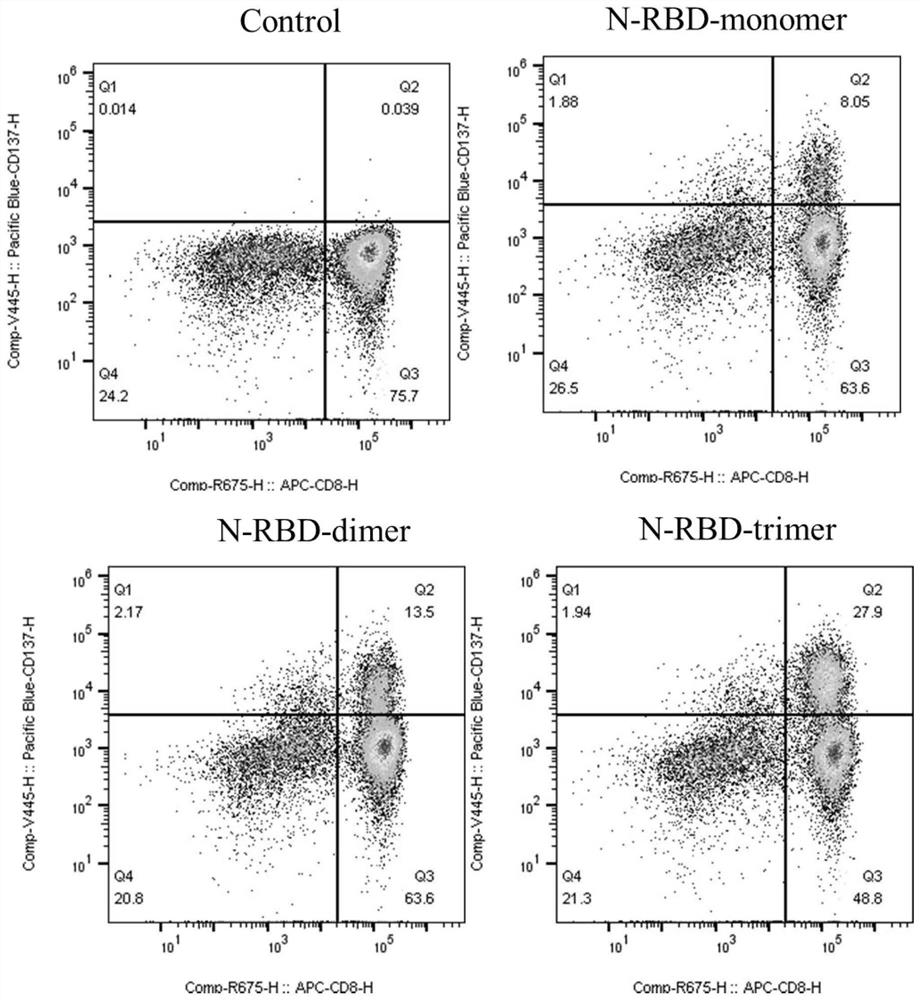
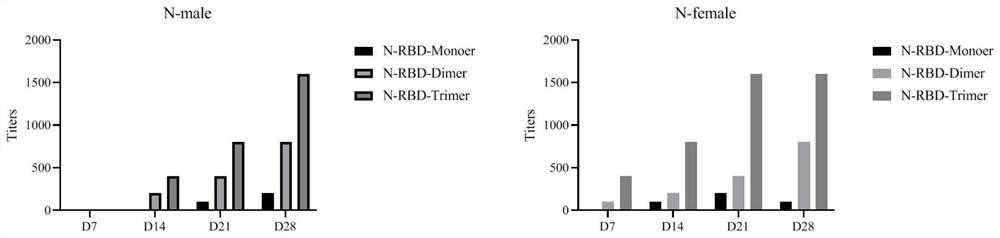
The invention provides a fused gene, fused protein, recombinant vector and general DC vaccine of coronavirus and application of the fused gene, fused protein, recombinant vector and general DC vaccineof the coronavirus and belongs to the technical field of preparation of viral vaccines. The fused gene comprises a gene for encoding a protein N of a COVID-19 virus and a gene for encoding a RBD monomer, dimer or trimer protein of the COVID-19 virus. A general DC cell vaccine of the coronavirus is obtained through expressing the fused gene in an antigen presentation cell line. The general DC vaccine can be used for effectively activating T cells. According to records of embodiments, the general DC vaccine provided by the invention can be used for effectively activating specific antibodies ofmacaca fascicularis to the proteins N and RBD of the COVID-19 virus.
Owner:BEIJING DCTY BIOTECH CO LTD +1
Antigen sensitized dendritic cell preparing method
InactiveCN105132373AThe method is safe and effectiveEasy to promote practiceBlood/immune system cellsAdditive ingredientHuman albumin
The invention relates to an antigen sensitized dendritic cell (DC) preparing method. On one hand, a culture medium for preparing antigen sensitized DCs is provided and is composed of a basal culture medium and other ingredients, the basal culture medium and the other ingredients can be conveniently mixed into the culture medium for culturing the DCs before using, the other ingredients comprise pharmaceutical grade human albumin, recombinant human insulin and the like, and the culture medium has the advantages of being definite in ingredient, free of ingredients of animal origin and the like; on the other hand, the invention provides a method for preparing the antigen sensitized DCs through the culture medium, and the method includes the steps that a single karyocyte is cultured and induced through the culture medium to obtain immaturate DC cells; strong immunogenicity tumor antigens are prepared through high hydrostatic pressure, astragalus polysaccharide is united to serve as an immunologic adjuvant, antigen sensitization is conducted on the immaturate DCs, and the antigen sensitized DCs are obtained. The preparing method has the advantages of being safe, efficient, easy to implement and the like. The DC vaccines for specific therapy can be provided for clinic through the method.
Owner:MINZU UNIVERSITY OF CHINA
Method for production of dendritic cell
InactiveUS20100184214A1Improve abilitiesUseful for immunotherapyArtificial cell constructsBlood/immune system cellsEpidermal Dendritic CellsDc vaccine
The present invention provides methods for producing DCs, which comprise the step of culturing DC precursor cells in the presence of multiple cytokines, dendritic cells produced thereby, and uses thereof. The methods of the present invention enable production of large quantities of DC precursors with a high ability to differentiate into DCs. The present invention enables one to obtain large quantities of DCs from a small number of DC precursor cells, and therefore makes it easier to increase the number of DCs for administration in DC-based anti-tumor immunotherapy, treatment of infection, and such. Thus, an enhancement is expected for the effect of DC vaccines.
Owner:DNAVEC CORP
Efficient tumor antigen loaded DC vaccine and method for induced proliferation of tumor antigen specific CTL by same
ActiveCN108379569AImproving immunogenicityExpand sourceCancer antigen ingredientsBlood/immune system cellsSide effectPD-L1
The invention provides an efficient tumor antigen loaded DC vaccine and a method for induced proliferation of tumor antigen specific CTL by the same. The DC vaccine is prepared by steps: (1) tumor antigen preparation; (2) acquisition, separation and efficient culture and proliferation of DC cells; (3) adoption of a tumor antigen and an activator for sensitization and activation of the DC cells; (4) DC cell surface PD-L1 molecule closing. The invention further provides a sequential blood drawing method for in-vitro induced proliferation of CTL by the DC vaccine and closing of surface PD-1 molecules of CTL subjected to in-vitro induced proliferation. Problems of low immunogenicity of existing tumor antigens, incompleteness of antigen targets, difficulty in acquisition of tumor antigens of patients and insufficiency in DC and CTL in-vitro proliferation are solved. The DC vaccine is effective in in-vitro and in-vivo induction of tumor antigen specific CTL and capable of efficiently generating specific killing effects on tumors without evident toxic and side effects, and a promising clinical application prospect is achieved.
Owner:北京百益宁医学科技有限责任公司
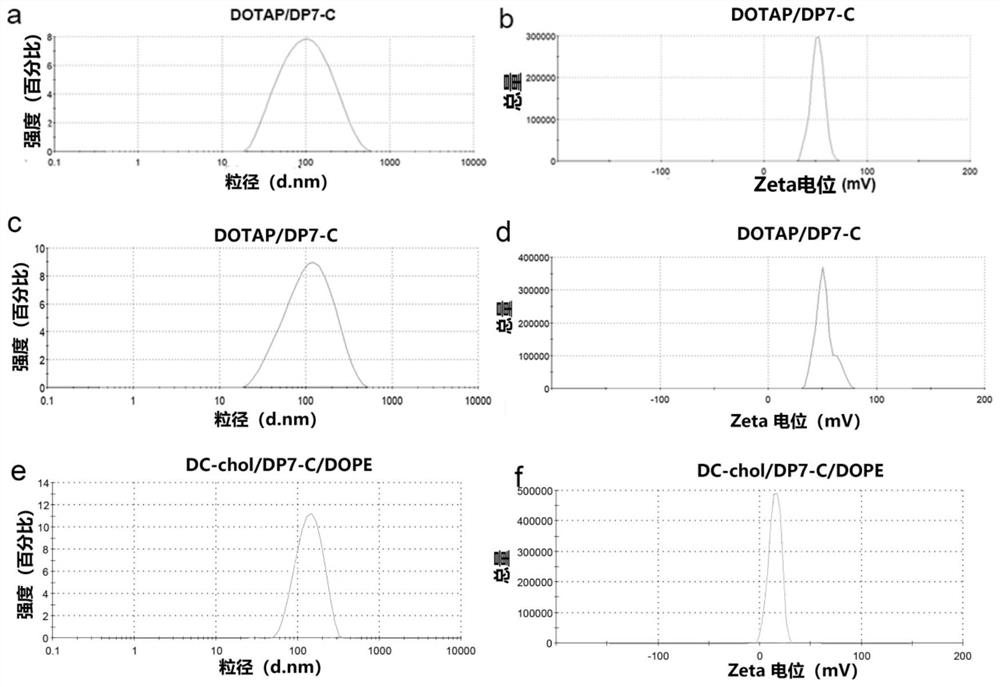
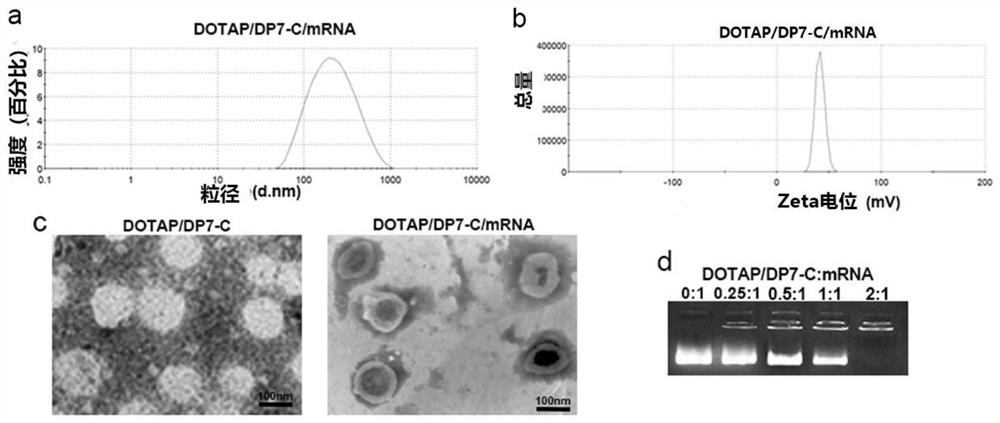
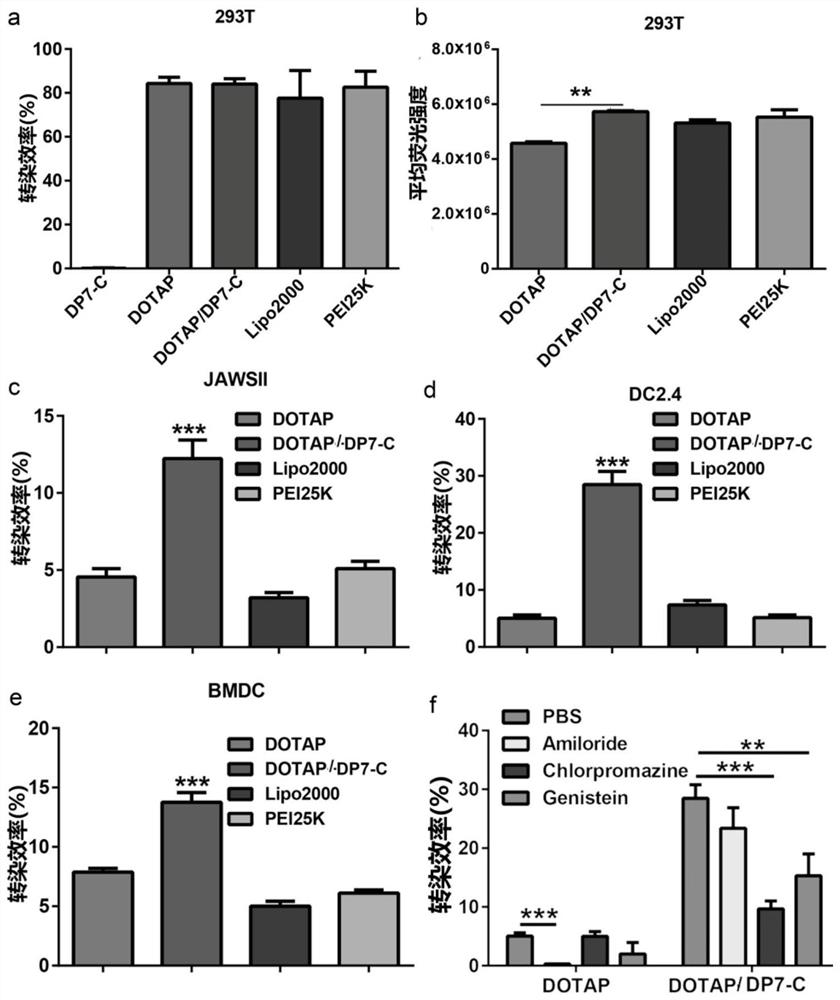
Polypeptide-modified liposome, mRNA delivery system and dendritic cell vaccine
PendingCN113855634AImprove the efficiency of mRNA deliveryImprove immunityCancer antigen ingredientsWhole-cell/virus/DNA/RNA ingredientsAntigenLipofectamine
The invention belongs to the field of biological medicines and particularly relates to a liposome modified by a hydrophobic-modified polypeptide, an mRNA delivery system and a dendritic cell vaccine. The technical problem to be solved by the invention is to improve the immune effect of mRNA-based vaccines and mRNA-sensitized DCs vaccines. The technical scheme for solving the technical problem is to provide the liposome modified by a hydrophobic-modified cationic polypeptide. The polypeptide-modified liposome disclosed by the invention can be used for efficiently delivering nucleic acid into cells; and especially, mRNA of a coding antigen can be efficiently delivered into DCs, the efficiency of delivering the mRNA to the DCs by the cationic liposome is obviously increased, the immune effect of the mRNA vaccines and the mRNA-sensitized DC vaccines is enhanced, and good clinical application prospects are achieved.
Owner:SICHUAN UNIV
Composition of DC vaccine and NKG2A antagonist and application of composition in anti-breast cancer or liver cancer
PendingCN110575537AInhibit and eliminate proliferative growthInhibition and Elimination of DiffusionLiver cancer vaccineCancer antigen ingredientsSide effectTreatment effect
The invention provides a composition of a DC vaccine and an NKG2A antagonist and application of the composition in anti-breast cancer or liver cancer. The DC vaccine in the composition is different from the early traditional tumor cell full-antigen-loaded DC vaccine, has specific targeting antigens, can more accurately treat tumors and reduce the killing side effects on normal cells; and secondly,the composition further contains a novel immune checkpoint inhibitor NKG2A antagonist which enables tumor cells with CD94-NKG2A / HLA-Ereceptor-ligand on the surface to be recognized and killed by NK cells and T cells, and an antigen presentation function of dendritic cells is enhanced, so that some cells which are not subjected to presentation and antigen by the DC vaccine are captured by an immune system again, the treatment effect on breast and liver cancer is enhanced, effect is faster than the conventional DC vaccine, the time of duration is longer, and the immunotherapy process is promoted.
Owner:刘慧宁
Chronic hepatitis B treatment DC vaccine
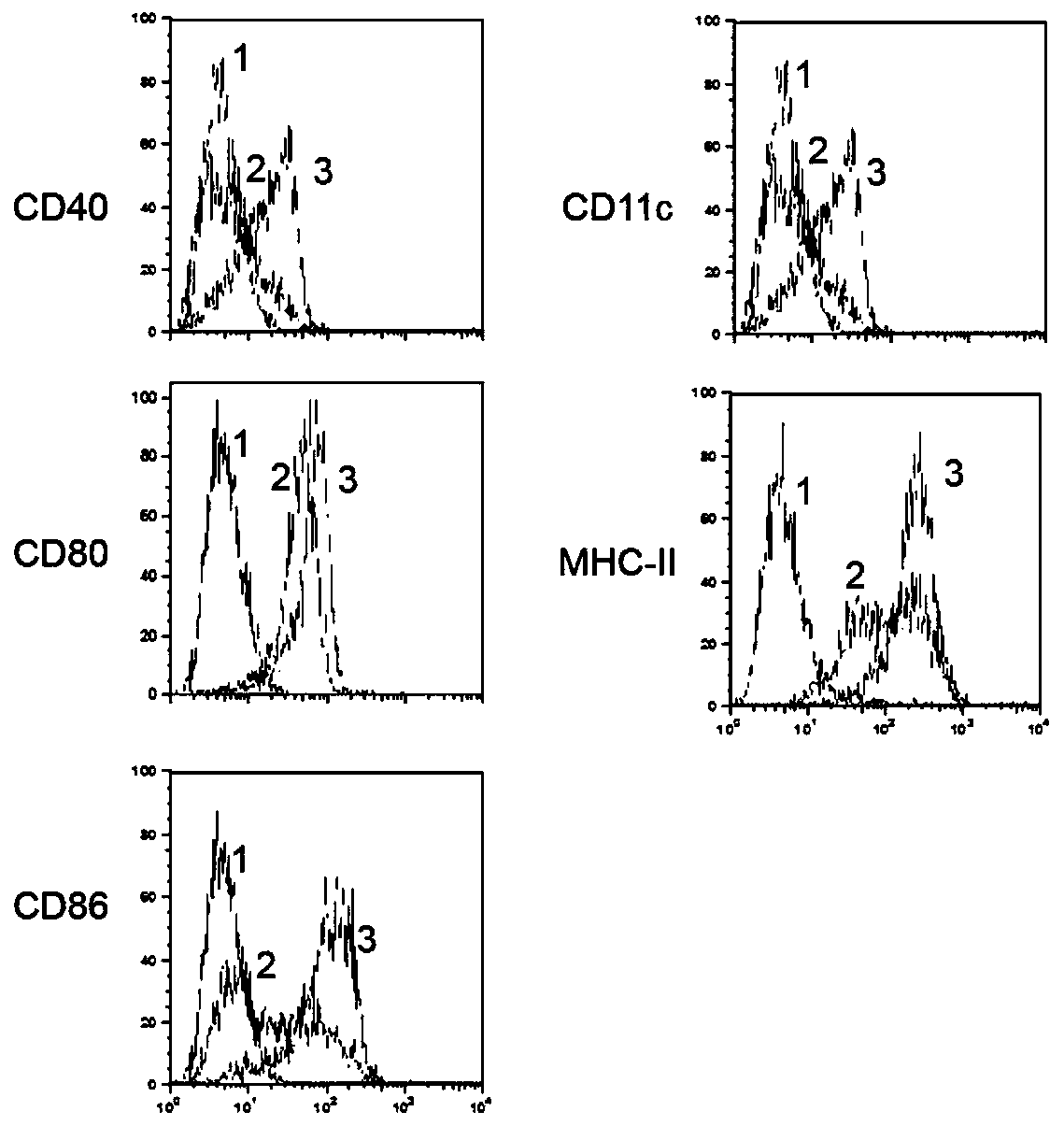
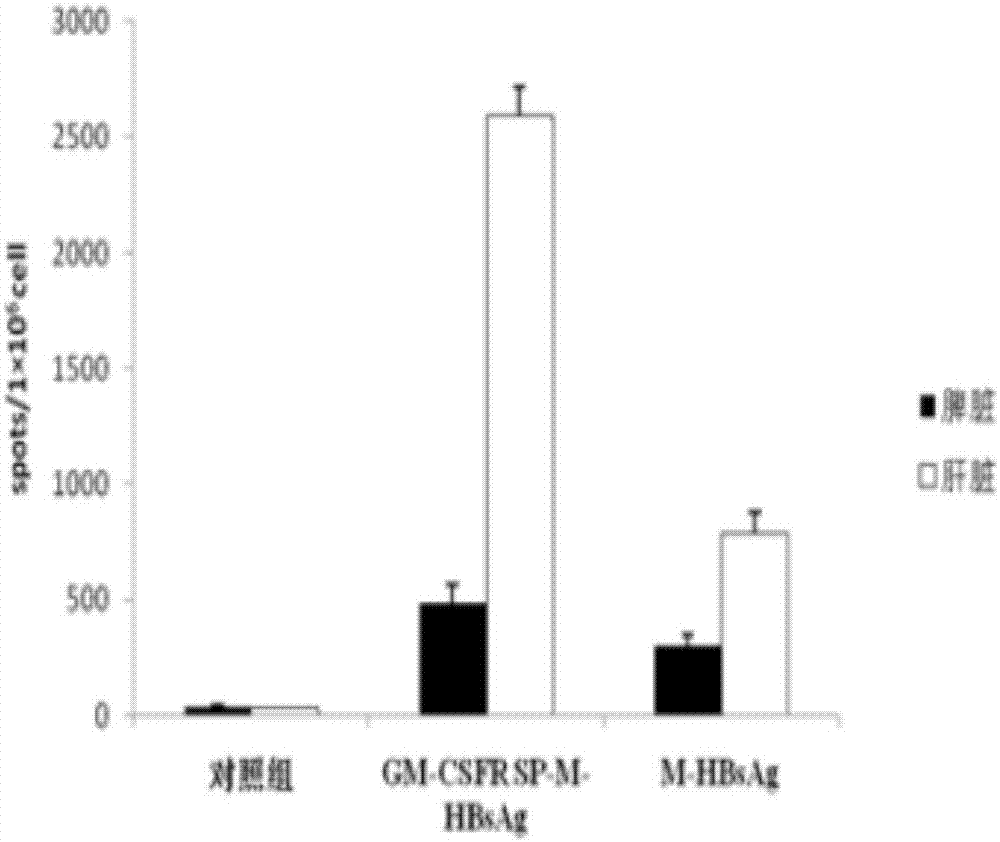
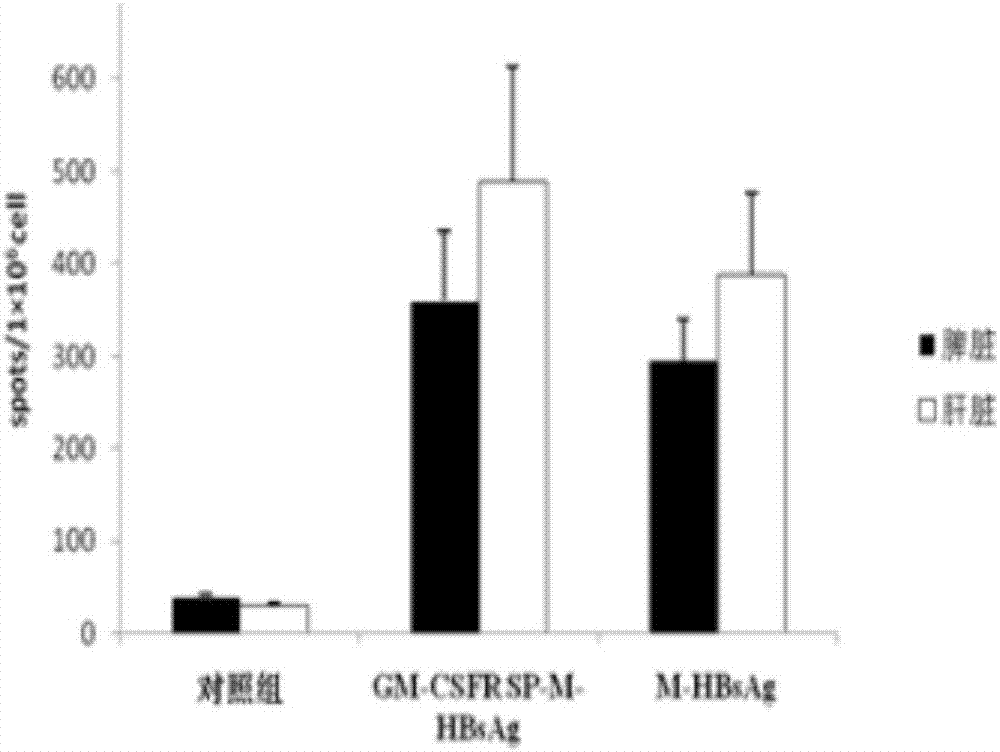
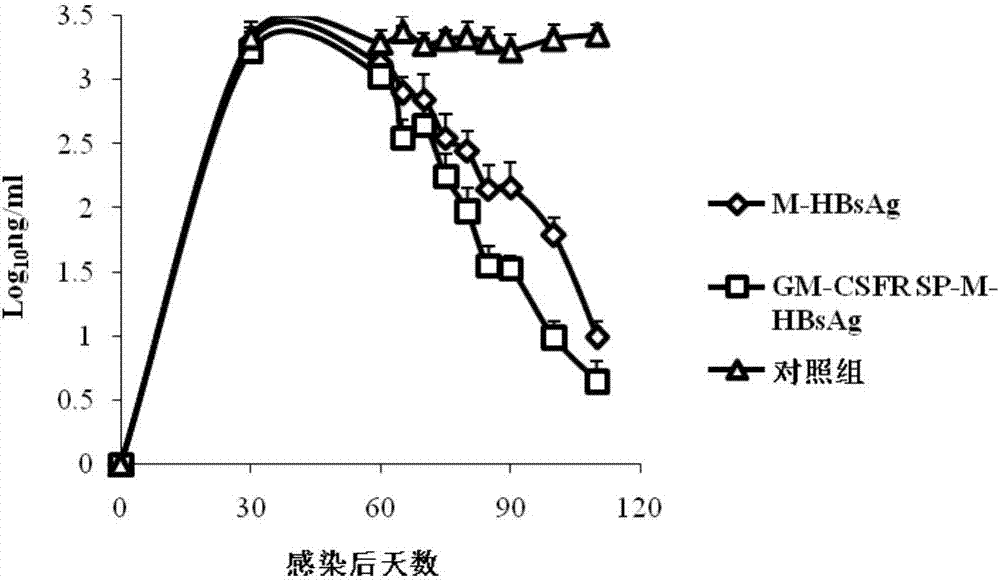
ActiveCN107335054AFree from infectionImprove expression efficiencyPolypeptide with localisation/targeting motifViral antigen ingredientsHBsAgChronic hepatitis
The invention discloses a chronic hepatitis B treatment DC vaccine, which comprises genetically modified dendritic cells, wherein the genetically modified dendritic cells contain a fusion gene fragment for the granulocyte-macrophage cluster factor receptor signal peptide guided encoding of the preS2 region and the S region of M-HBsAg. According to the present invention, when the body is immunized with the chronic hepatitis B treatment DC vaccine, the specific antibodies against the pres2 antigen can be produced, can well neutralize HBV viruses so as to protect the host from the HBV infection, can induce the serological transformation from HBsAg to HBsAb, can reduce the hepatitis B virus infection in the liver, and can enhance the immunization effect.
Owner:SHANDONG XINRUI BIOTECH CO LTD
Tumor associated antigen XAGE-1b short peptide and application thereof
ActiveCN106279391AGood specific killing effectPromote clinical translationTumor rejection antigen precursorsBiological material analysisCD8Dc vaccine
The invention discloses a tumor associated antigen XAGE-1b short peptide and application thereof. The sequence of the short peptide is one of SEQ ID NO:2-SEQ ID NO:15. CTL established by induction of XAGE-1b antigen peptide cannot generate an immunologic response to testis cells and only kills tumor cells. CTL clone obtained by induction has a favorable specific tumor cell killing effect; meanwhile, during a CTL establishment process, the inventor discovers that the screened XAGE-1b antigen peptide has appropriate appetency with HLA on DC cells and is capable of effectively stimulating and inducing production of specific CTLs, which indicates that the XAGE-1b antigen peptide has favorable polypeptide vaccine and DC vaccine potentials. By virtue of cloning the specific TCR genes of the XAGE-1b antigen peptide, a virus carrier is established, peripheral circulation CD8+T cells are transduced, TCR gene modified T cell (TCR-T) clone is obtained and also has a similar specific tumor cell killing effect with the parent-generation CTL, which indicates that the TCR gene modified T cell clone has favorable clinical conversion and practical application prospects.
Owner:广州美萨生物科技有限公司
Tumor composite antigen, dendritic cell multivalent vaccine and application of dendritic cell multivalent vaccine
PendingCN114246942AFast evolutionLower immune resistanceAnimal cellsStomach cancer vaccineIn vitro stimulationCancer cell
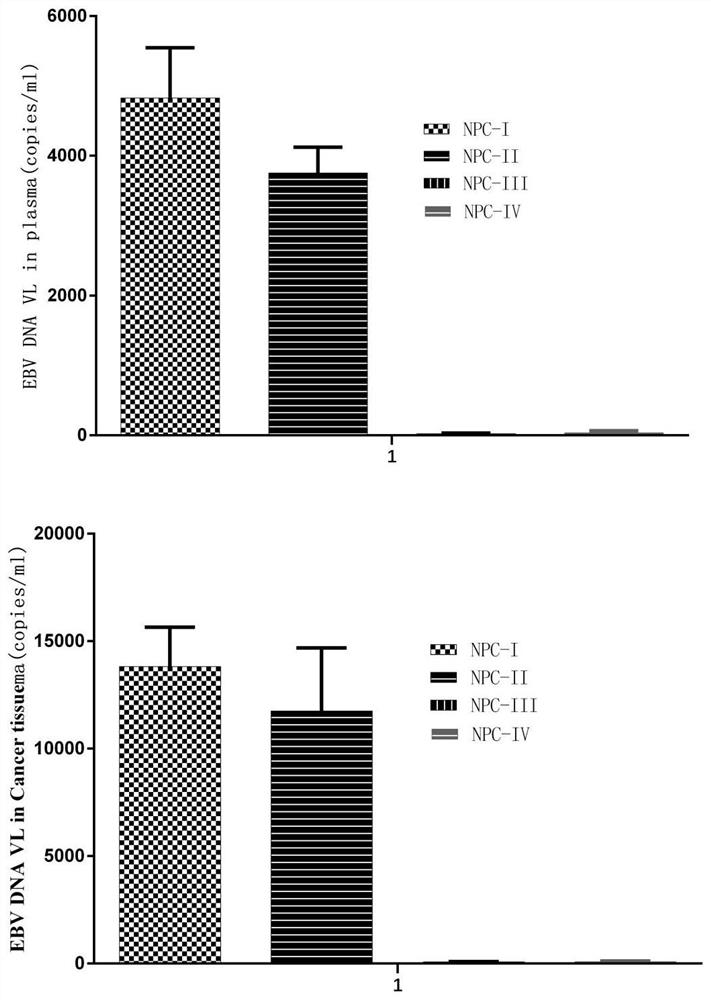
The invention discloses a tumor composite antigen, a dendritic cell multivalent vaccine and application of the tumor composite antigen and the dendritic cell multivalent vaccine. Dendritic cells of a patient are stimulated in vitro, multiple tumor cell lysates with super immunogenicity aiming at different EBV related tumors are loaded, mature dendritic cells are formed under induction of multiple cell factors and specific agonists, a complete DC vaccine with corresponding cancer antigens is formed, the DC vaccine is transfused back to a human body to activate an immune system, and the EBV related tumors are subjected to tumor immunogenicity. Natural immunity (such as induction of NK cells) is stimulated, lymphocytes are stimulated to generate acquired immune response, cytotoxic T cells are generated to kill cancer cells, and the cancer cells are accurately killed together; compared with radiotherapy and chemotherapy, the composition is particularly safe and almost has no side effect; and the dendritic cell vaccine has a preparation cycle of about 1 week, and is short in time and low in cost.
Owner:刘慧宁 +1
Mannan-modified hTRT (human Telomerase Reverse Transcriptase) gene-carrying and adeno-associated virus (AAV)-inducing targeting dendritic cell (DC) vaccine and preparation method thereof
InactiveCN102552892APowerful killing effectImprove anti-tumor effectBlood/immune system cellsPharmaceutical non-active ingredientsAbnormal tissue growthReverse transcriptase
The invention belongs to the technical field of medical biological engineering, and relates to a novel dendritic cell (DC) vaccine preparation technology. In the technology, an hTRT (human Telomerase Reverse Transcriptase) gene is inserted into an adeno-associated virus (AAV), and mannan surface modification is performed, so that an infected DC cell can be targeted in vitro. Along with stable expression of the hTRT gene in the DC, the DC can be continuously stimulated by a large quantity of hTRT antigen peptides. An hTRT antigen is a tumor-related antigen, so that a lymphocyte stimulated by the DC vaccine almost acts strong killing effects on all tumor cells. The vaccine prepared with a method disclosed by the invention has the characteristics of strong inducting function, high safety, low price, easiness for large-scale production, wide clinical application and the like. The problems of short half-life period, low transduction efficiency and poor activation-immune effect cell ability existing in the conventional DC transfected with an in-vitro antigen peptide are solved.
Owner:郑骏年
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com