Preparation method of high-efficiency killer cell preparation adopting immunodetection point dual-block CTL (cytotoxic lymphocyte)
A technology of immune detection points and killing cells, which is applied in the direction of blood/immune system cells, cell culture active agents, biochemical equipment and methods, etc., can solve the problems of low clinical objective effective rate and low tumor killing efficiency, and achieve slowing down of T Effect of cell depletion, improvement of tumor killing effect, and improvement of clinical objective efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
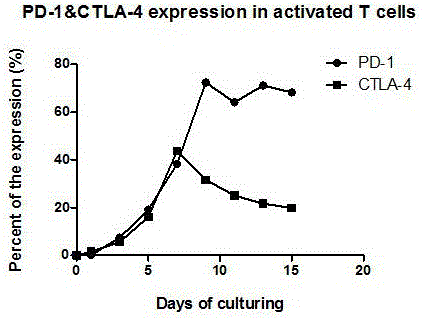
[0031] In this example 1, PD-1 and CTLA-4 antibodies were used to block the inhibitory signal of CTL preparations to prepare high-efficiency double-deleted CTL preparations. At the same time, the tumor cell surface PD-1 ligands PD-L1 and CTLA-4 were detected. The expression characteristics of ligands B7-1 and 2 (CD80, CD86), the specific steps are as follows:
[0032] 1) Acquisition of monocytes:
[0033] Intravenous extraction or blood component separator collects peripheral blood mononuclear cells of tumor patients (or collects cord blood hematopoietic stem cells to prepare allogeneic DC) 100ml, collects whole blood cells by centrifugation, transfers the lymphocyte separation solution, and centrifuges 2000rpm×15 with a horizontal rotor Minutes, aspirate the middle albuginea layer, and collect mononuclear cells.
[0034] 2) DC and T cell sorting:
[0035] The monocytes collected above were transferred to a culture flask containing serum-free medium and placed at 37°C, 5% CO 2 Incuba...
Embodiment 2
[0047] This example 2 is to use Dual-block CTL effector cell preparations to conduct laboratory killing experiments (in vitro) and animal experiments (in vivo) on target cells (breast cancer). At the same time, it is combined with DC-CIK and antigen-specific CTL The effector cells were subjected to a control study to verify the advantages of Dual-block CTL in killing efficiency.
[0048] 1. In vitro killing experiment research:
[0049] 1) Effector cells: Dual-block CTL (experimental group), T cells (control 1), DC-CIK (control 2), CTL (control 3).
[0050] 2) Target cell: breast cancer cell line (MDA-SB435S).
[0051] 3) Experimental grouping: Dual-block CTL (experimental group), T cell (control 1), DC-CIK (control 2), CTL (control 3), NS (negative control).
[0052] 4) In vitro killing and cytokine secretion experiments:
[0053] Using cytotoxicity analysis, inoculate MDA-SB435S target cells in 96-well plates, and add effector cells Dual-blockCTL, CTL, DC-CIK, T and NS, 3 wells per gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com