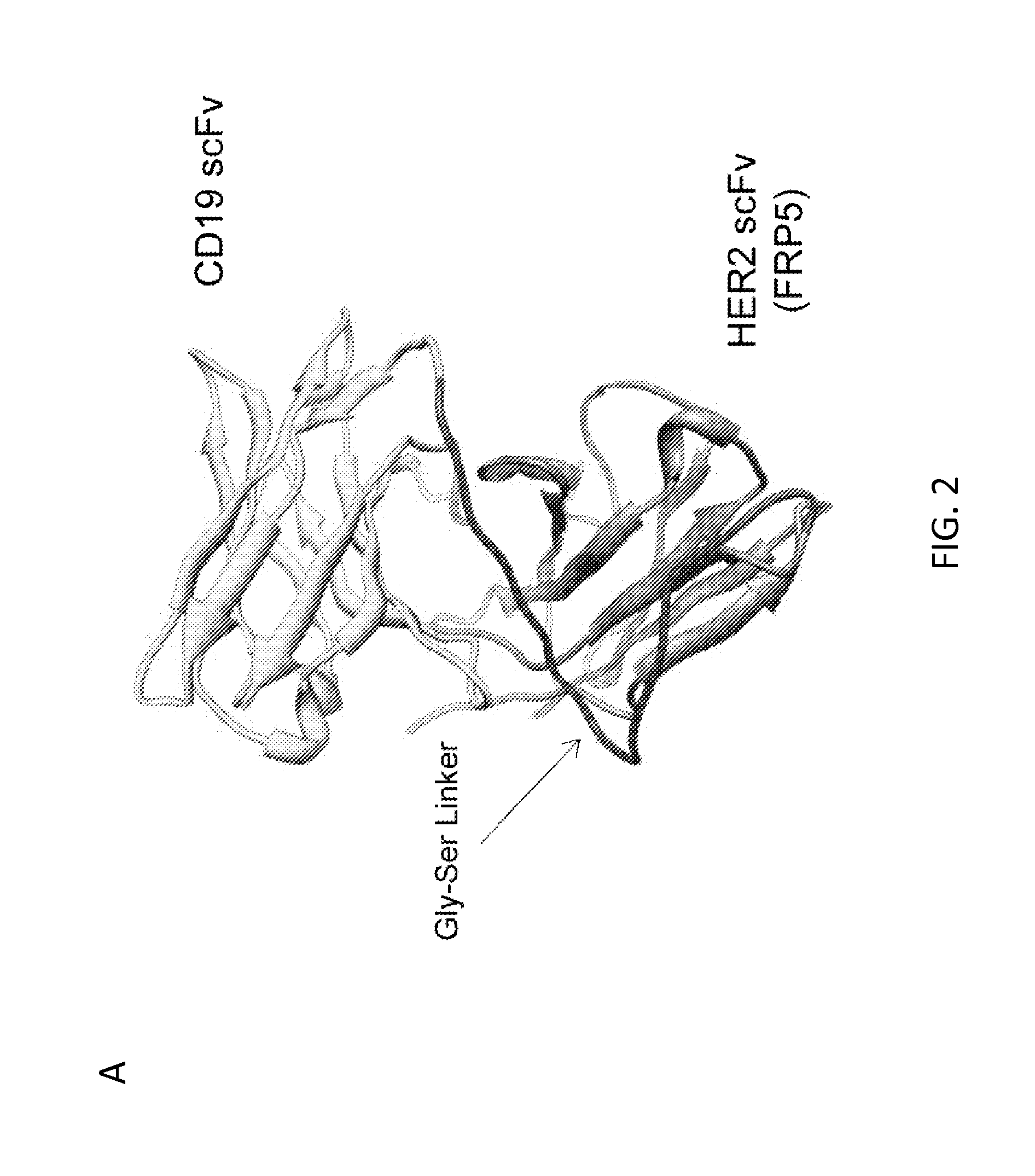
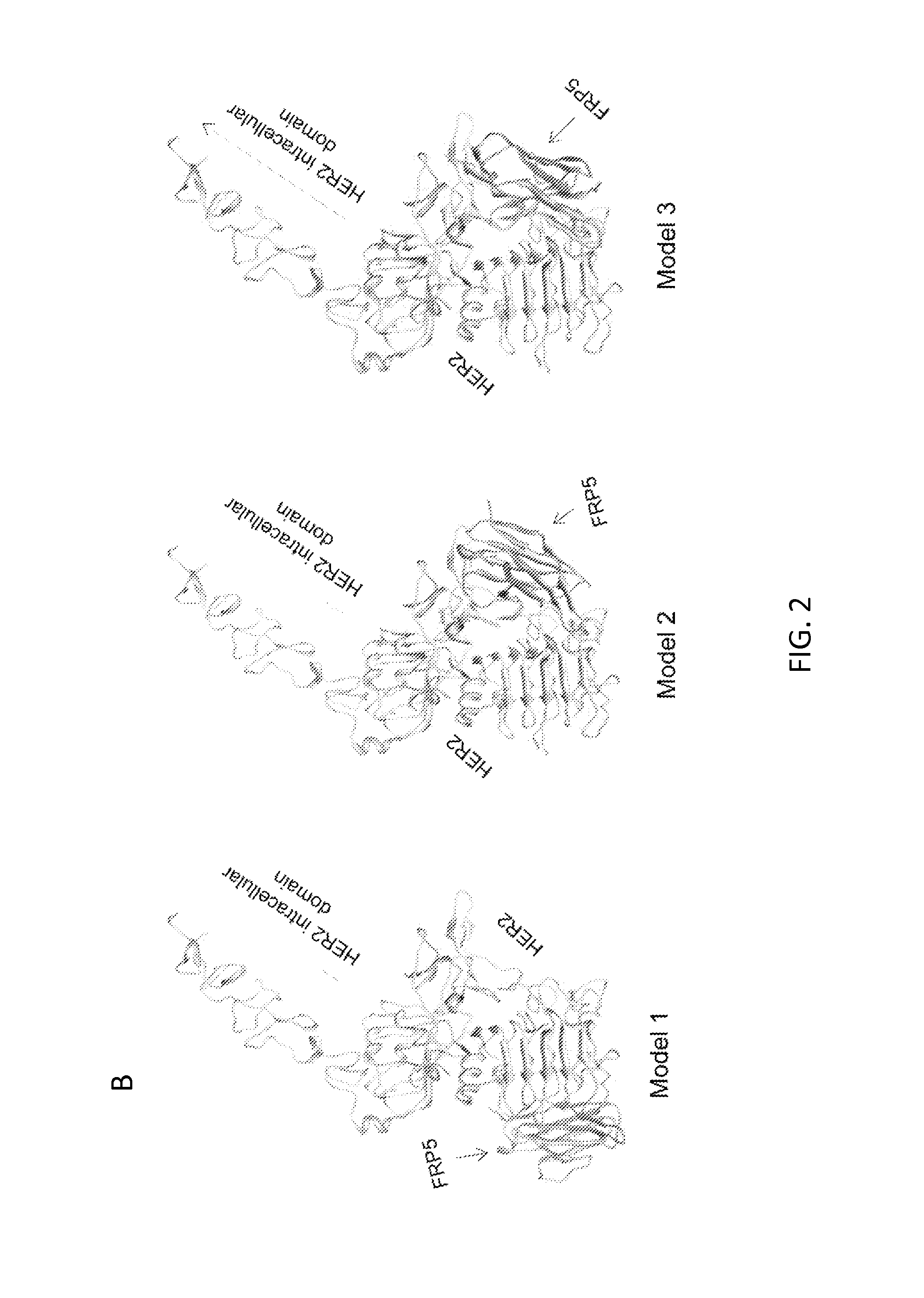
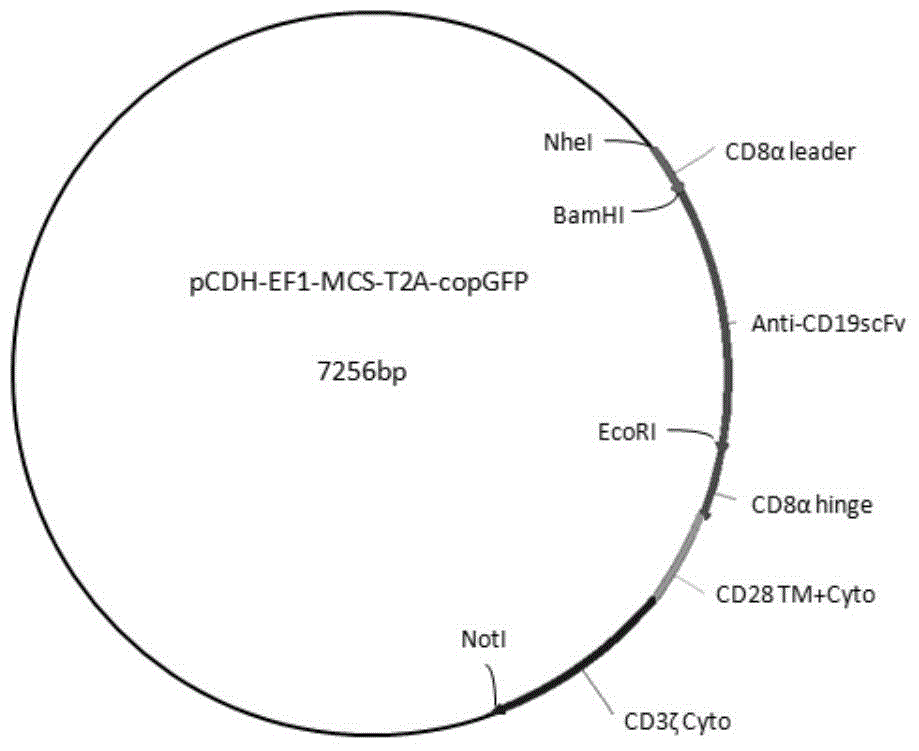
Patents
Literature
1388 results about "T lymphocyte" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Artificial antigen presenting cells and methods of use thereof
InactiveUS20020131960A1Palliating their conditionReduce riskBiocideCompound screeningEpitopeAccessory molecule
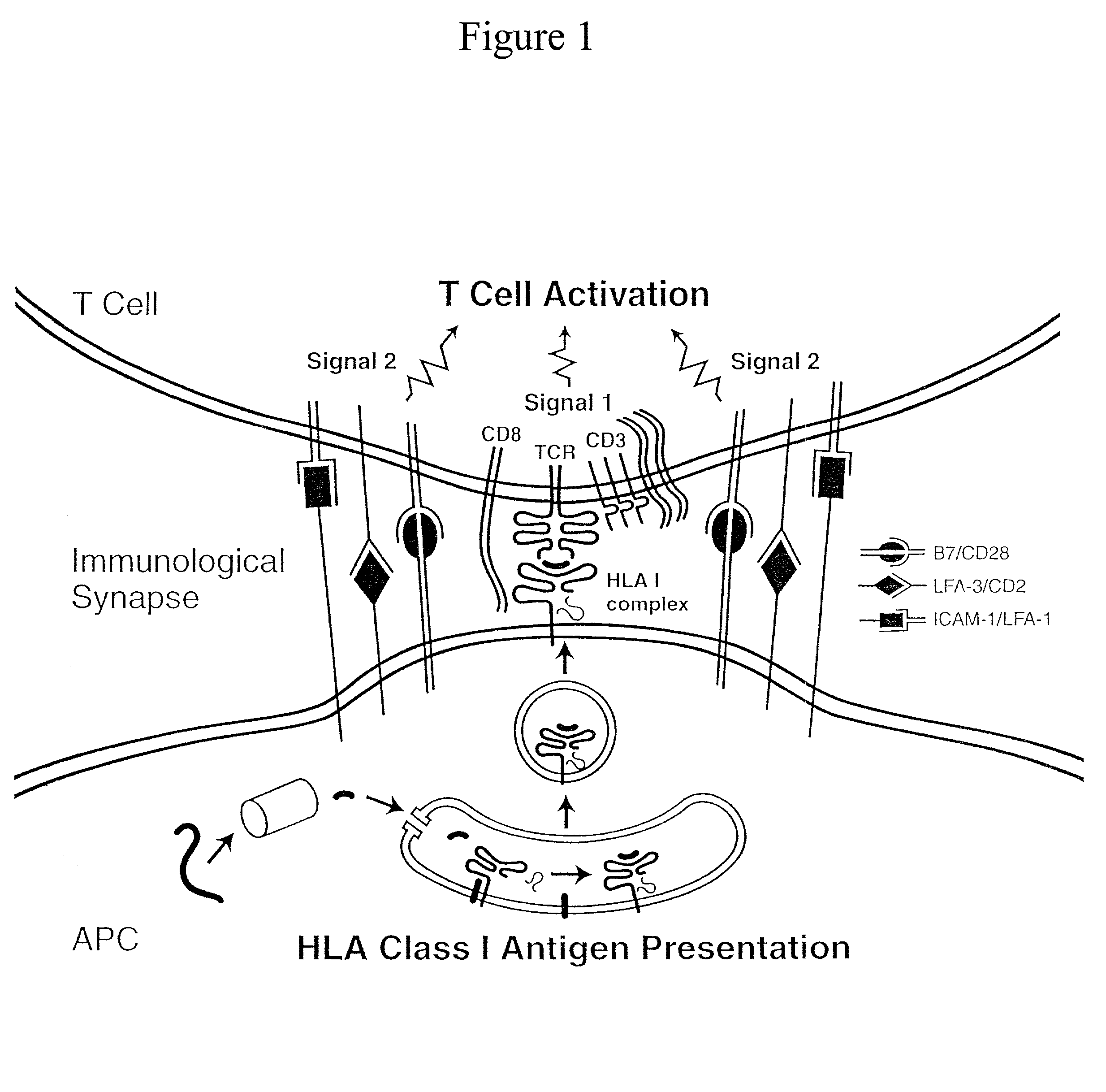
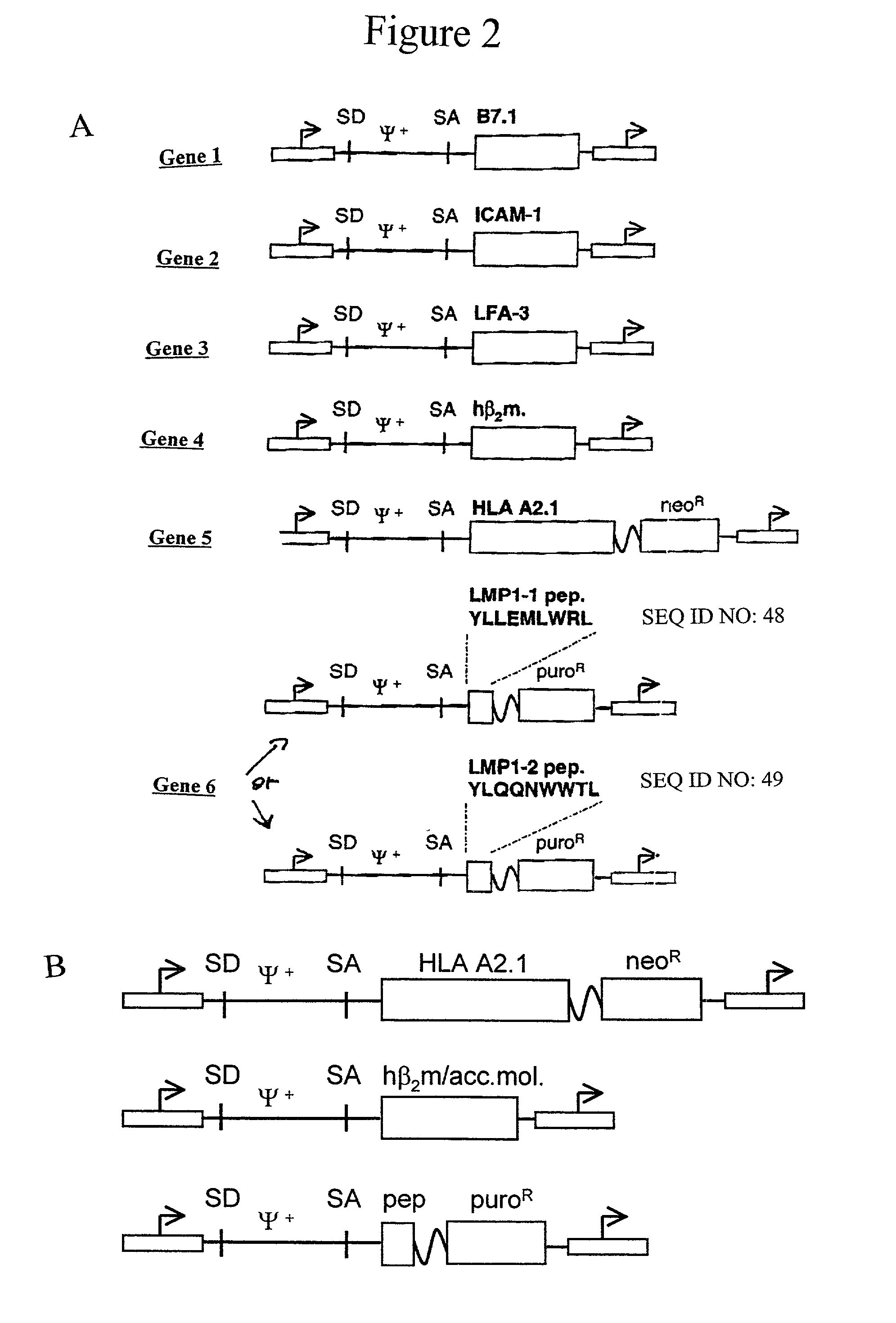
The invention provides an artificial antigen presenting cell (AAPC) comprising a eukaryotic cell expressing an antigen presenting complex comprising a human leukocyte antigen (HLA) molecule of a single type, at least one exogenous accessory molecule and at least one exogenous T cell-specific epitope. Methods of use for activation of T lymphocytes are also provided.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Constitutive expression of costimulatory ligands on adoptively transferred T lymphocytes
ActiveUS8252592B2Increase self-toleranceIncrease and reduces immune responseFused cellsFermentationRegulatory T cellLymphocyte
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Chimeric antigen receptor for bispecific activation and targeting of t lymphocytes
InactiveUS20130280220A1Increase T cell activationEffectively offsetting tumor escapeBiocideGenetic material ingredientsLymphocyteT lymphocyte
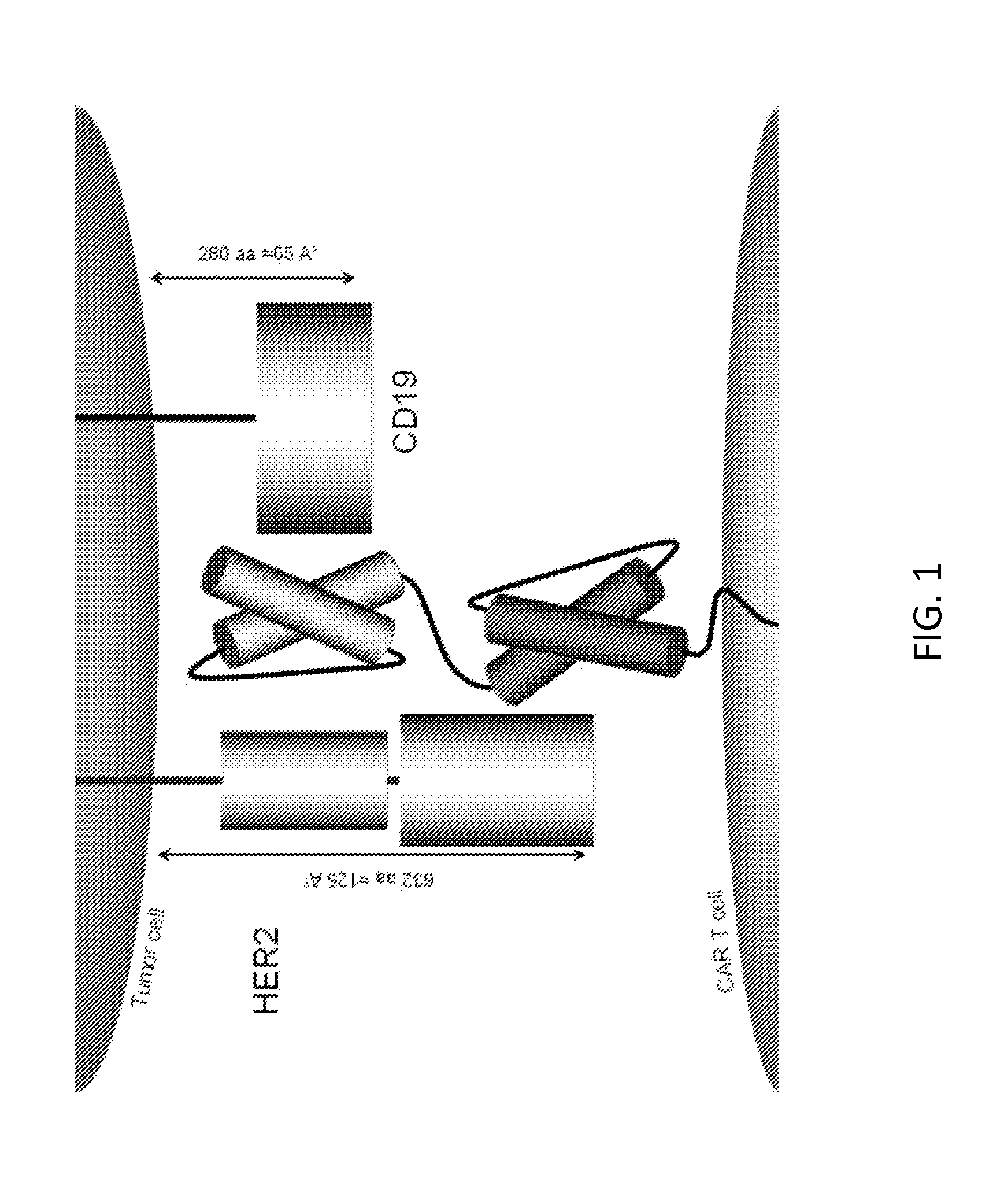
Embodiments of the invention include methods and compositions related to improved cells encoding a chimeric antigen receptor that is specific for two or more antigens. In certain aspects the receptor encompasses two or more non-identical antigen recognition domains. The antigens are tumor antigens, in particular embodiments.
Owner:BAYLOR COLLEGE OF MEDICINE
Cytotoxic t-lymphocyte-inducing immunogens for prevention, treatment, and diagnosis of cancer
The present invention relates to compositions and methods for the prevention, treatment, and diagnosis of cancer, especially carcinomas, such as breast carcinoma. The invention discloses peptides, polypeptides, and polynucleotides that can be used to stimulate a CTL response against breast or cancer.
Owner:IMMUNOTOPE
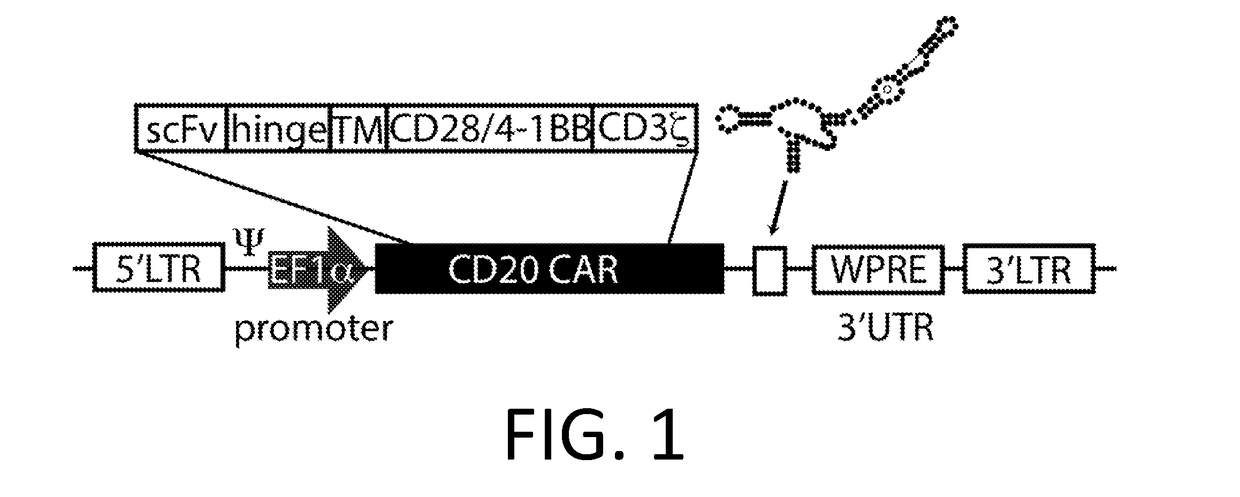
Chimeric antigen receptor hCD19scFv-CD8a-CD-28-CD3zata and application thereof
ActiveCN104788573AConfirmed specific killing effectMammal material medical ingredientsHybrid peptidesAntigen receptorHeavy chain
The invention discloses a chimeric antigen receptor hCD19scFv-CD8a-CD-28-CD3zata and application thereof. The chimeric antigen receptor is serially connected by an anti-human CD19 monoclonal antibody H119a light chain and heavy chain variable region (hCD19scFv), human CD8a hinge region, a human CD28 transmembrane region, an intracellular region and a human CD3zata intracellular region. The chimeric antigen receptor is used for modifying T lymphocyte, and the modified T lymphocyte (CAR-T cell) can be used for treating the tumor with positive surface CD19.
Owner:JUVENTAS CELL THERAPY LTD
Small cell lung cancer associated antigens and uses therefor
InactiveUS7314721B2Tumor rejection antigen precursorsPeptide/protein ingredientsSerum igeADAMTS Proteins
Cancer associated antigens have been identified by autologous antibody screening of libraries of nucleic acids expressed in small cell lung cancer cells using antisera from cancer patients. The invention relates to nucleic acids and encoded polypeptides which are cancer associated antigens expressed in patients afflicted with small cell lung cancer. The invention provides, among other things, isolated nucleic acid molecules, expression vectors containing those molecules and host cells transfected with those molecules. The invention also provides isolated proteins and peptides, antibodies to those proteins and peptides and cytotoxic T lymphocytes which recognize the proteins and peptides. Fragments of the foregoing including functional fragments and variants also are provided. Kits containing the foregoing molecules additionally are provided. The molecules provided by the invention can be used in the diagnosis, monitoring, research, or treatment of conditions characterized by the expression of one or more cancer associated antigens.
Owner:NEW YORK HOSPITAL CORNELL MEDICAL CENT +2
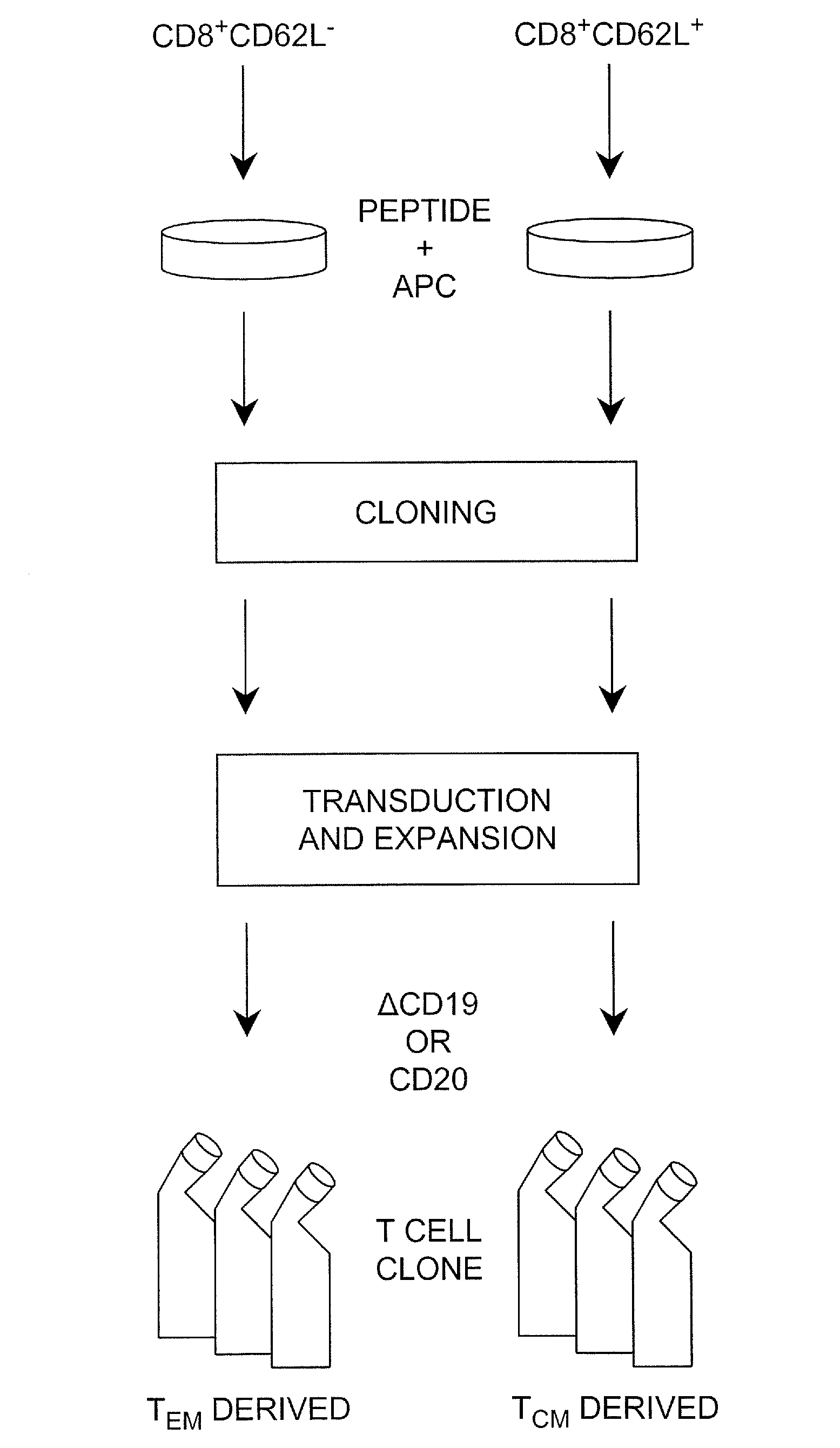
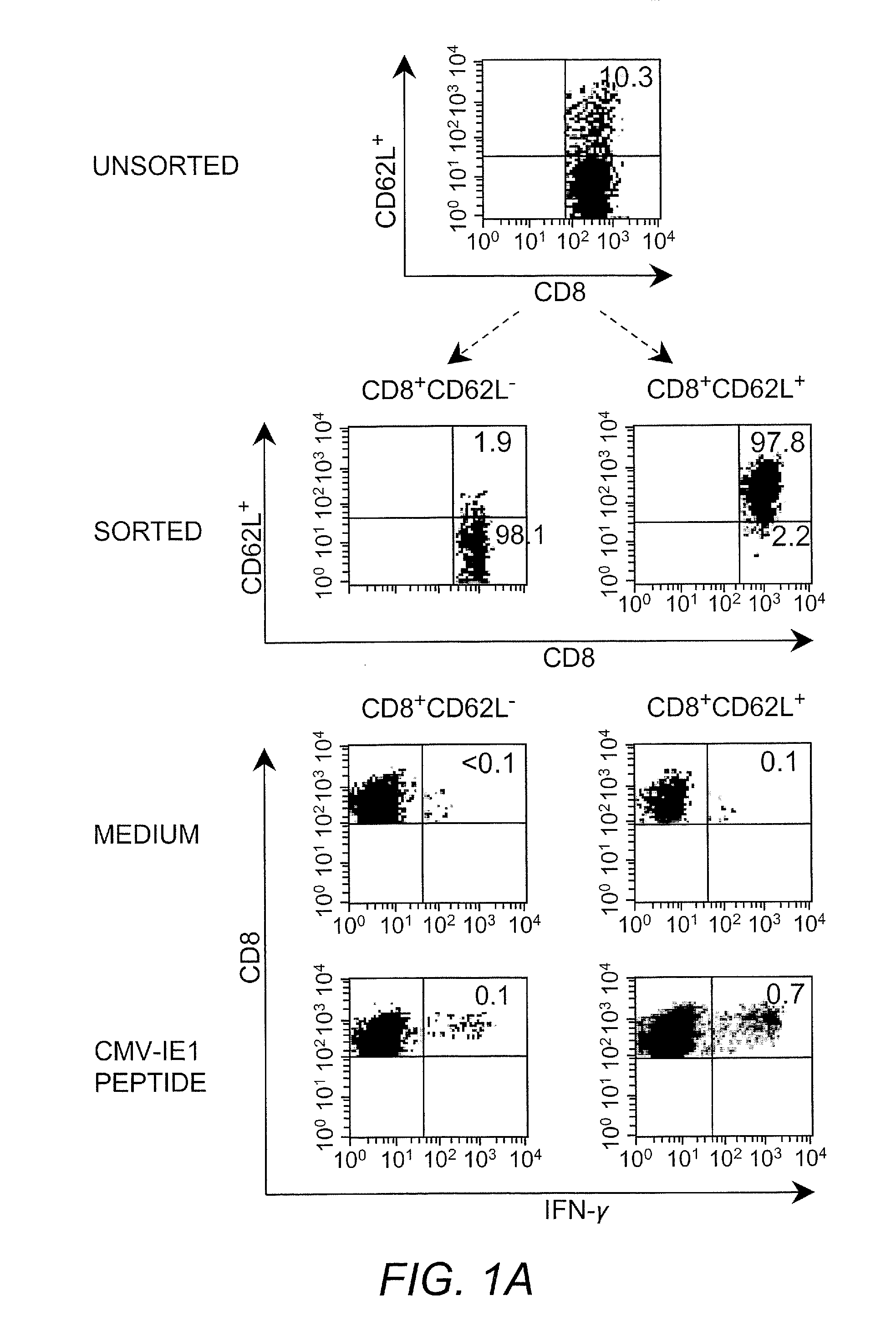
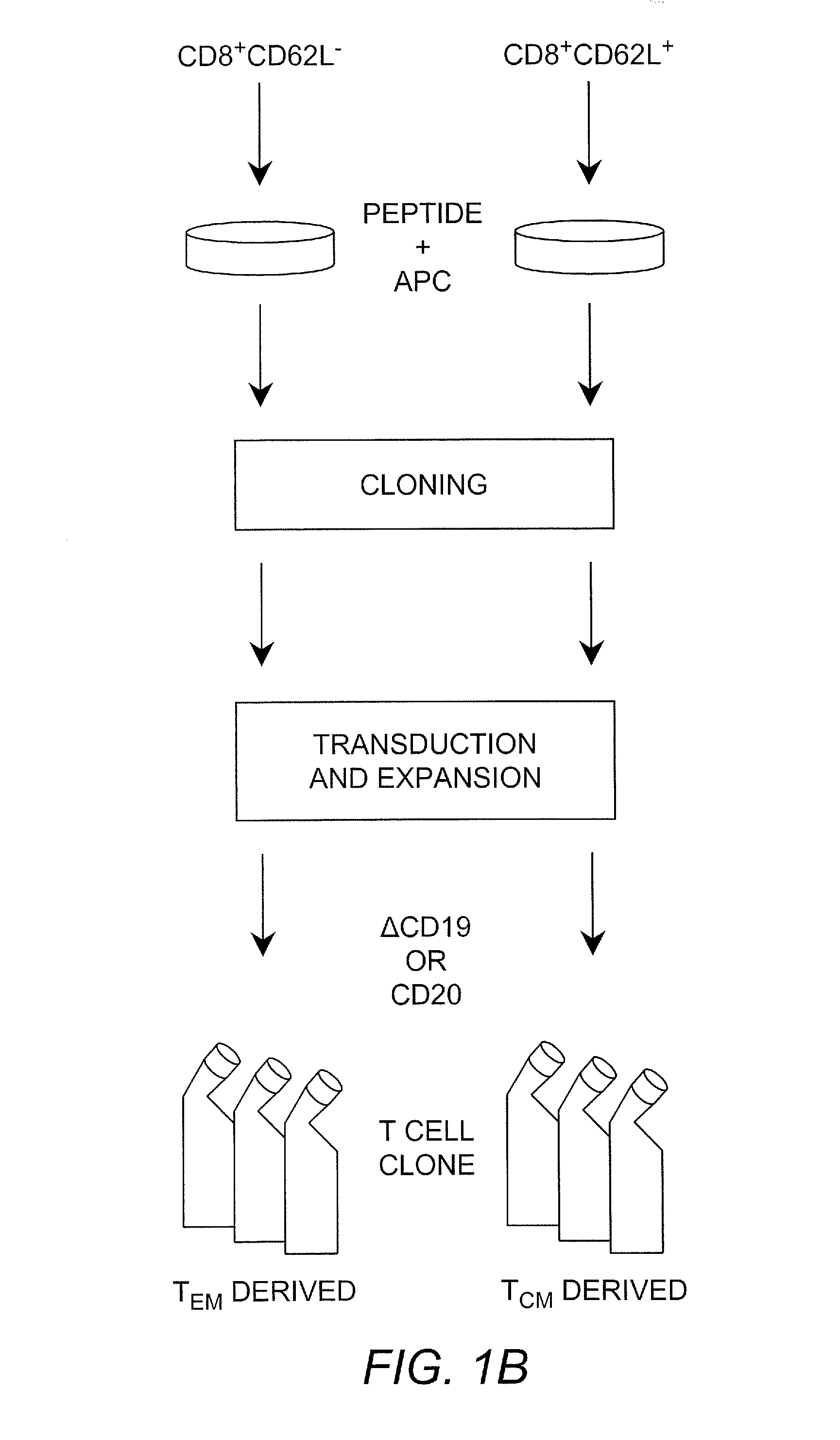
Adoptive transfer of cd8 + t cell clones derived from central memory cells
InactiveUS20080131415A1Increased proliferationBiocideArtificial cell constructsWhite blood cellPharmaceutical formulation
The present invention provides a method of carrying out adoptive immunotherapy in a primate subject in need thereof by administering the subject a cytotoxic T lymphocytes (CTL) preparation in a treatment-effective amount. The method comprises administering as the CTL preparation a preparation consisting essentially of an in vitro expanded primate CTL population, the CTL population enriched prior to expansion for central memory T lymphocytes, and depleted prior to expansion of effector memory T lymphocytes. In some embodiments, the method may further comprise concurrently administering Interleukin-15 to the subject in an amount effective to increase the proliferation of the central memory T cells in the subject. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:CITY OF HOPE +1
Method for assaying for natural killer, cytotoxic T-lymphocyte and neutrophil-mediated killing of target cells using real-time microelectronic cell sensing technology
ActiveUS7468255B2Microbiological testing/measurementBiological material analysisEffector cellNeutrophil granulocyte
The present invention includes a method of measuring cytolytic activity including providing a device capable of monitoring cell-substrate impedance operably connected to an impedance analyzer, adding target cells to at least one well of the device, adding effector cells to the at least one well, monitoring impedance of the at least one well and optionally determining a cell index from the impedance, wherein monitoring impedance includes measuring impedance during at least one time point before and at least one time point after adding effector cells, and determining viability of said target cells after adding effector cells by comparing the impedance or optionally the cell index at the at least one time point after adding effector cells to the impedance or optionally the cell index at the at least one time point before adding effector cells.
Owner:AGILENT TECH INC
A cell therapy method for the treatment of tumors
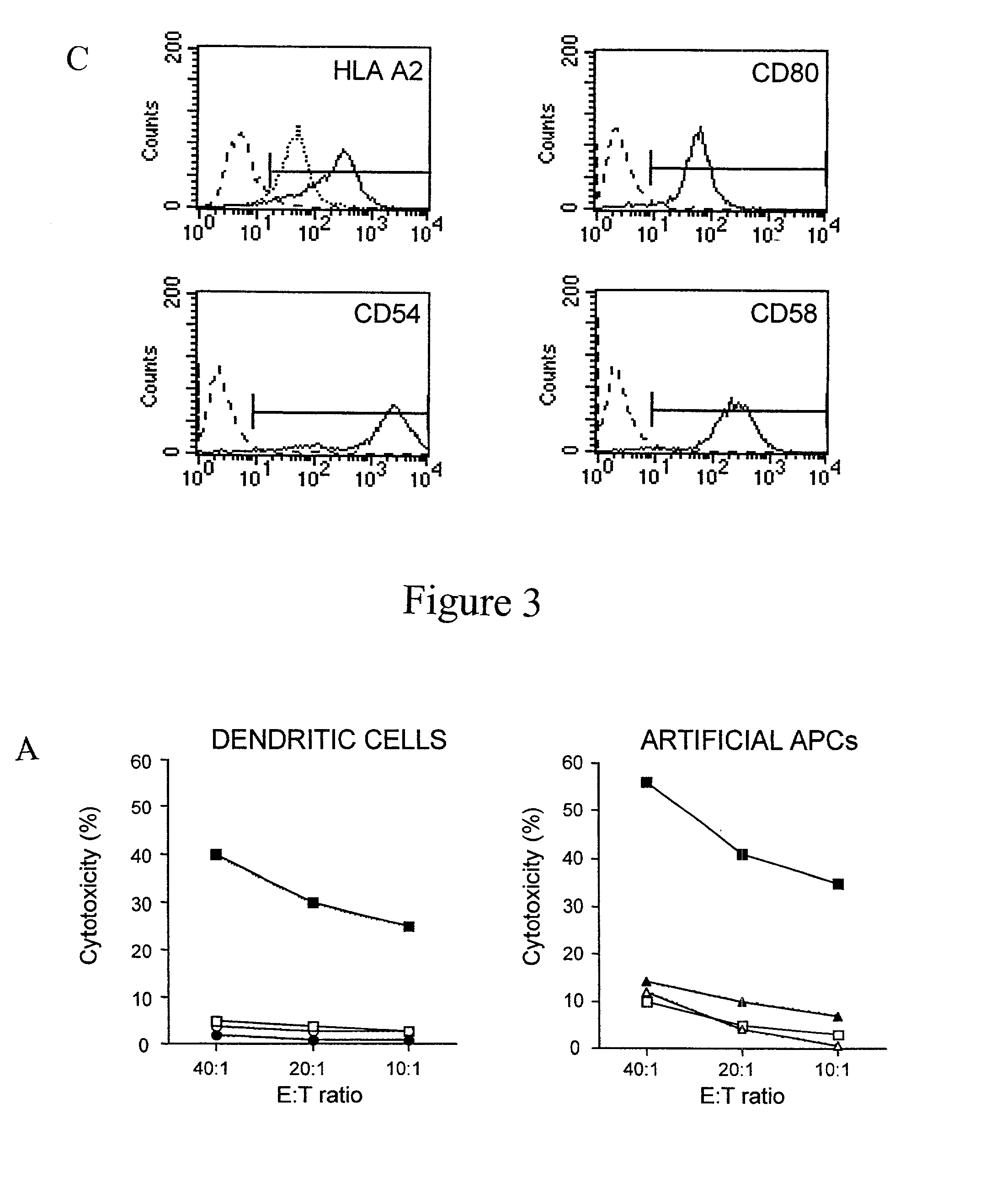
T cell responses are often diminished in humans with a compromised immune system. We have developed a method to isolate, stimulate and expand naïve cytotoxic T lymphocyte precursors (CTLp) to antigen-specific effectors, capable of lysing tumor cells in vivo. This ex vivo protocol produces fully functional effectors. Artificial antigen presenting cells (AAPCs; Drosophila melanogaster) transfected with human HLA class I and defined accessory molecules, are used to stimulate CD8+ T cells from both normal donors and cancer patients. The class I molecules expressed to a high density on the surface of the Drosophila cells are empty, allowing for efficient loading of multiple peptides that results in the generation of polyclonal responses recognizing tumor cells endogenously expressing the specific peptides. The responses generated are robust, antigen-specific and reproducible if the peptide epitope is a defined immunogen. This artificial antigen expression system can be adapted to treat most cancers in a significant majority of the population.
Owner:JANSSEN PHARMA INC
Chimeric immunoreceptor useful in treating human cancers
InactiveUS20090257994A1Negligible toxicityPotent and selectiveBiocidePeptide/protein ingredientsIntracellular signallingMalignancy
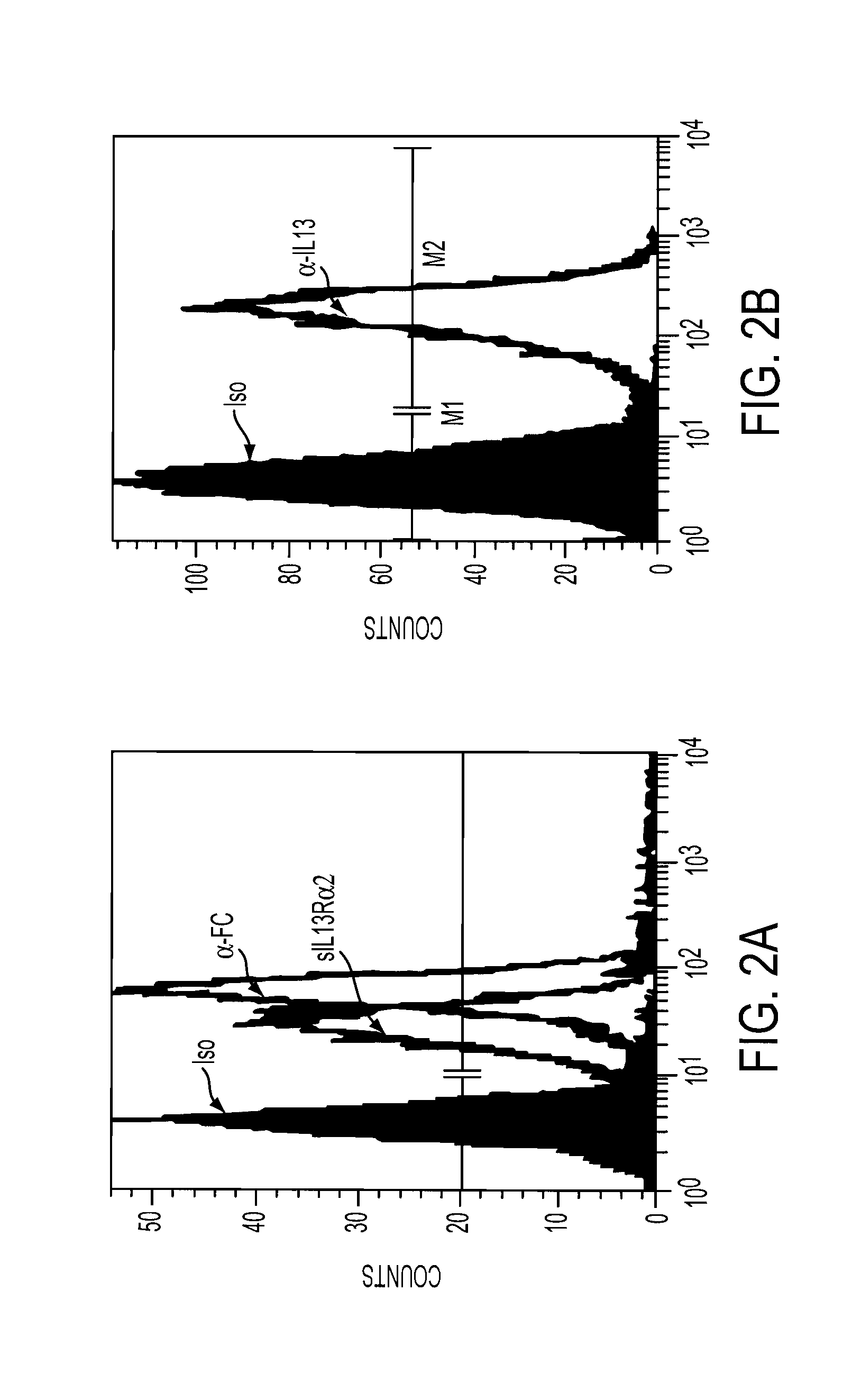
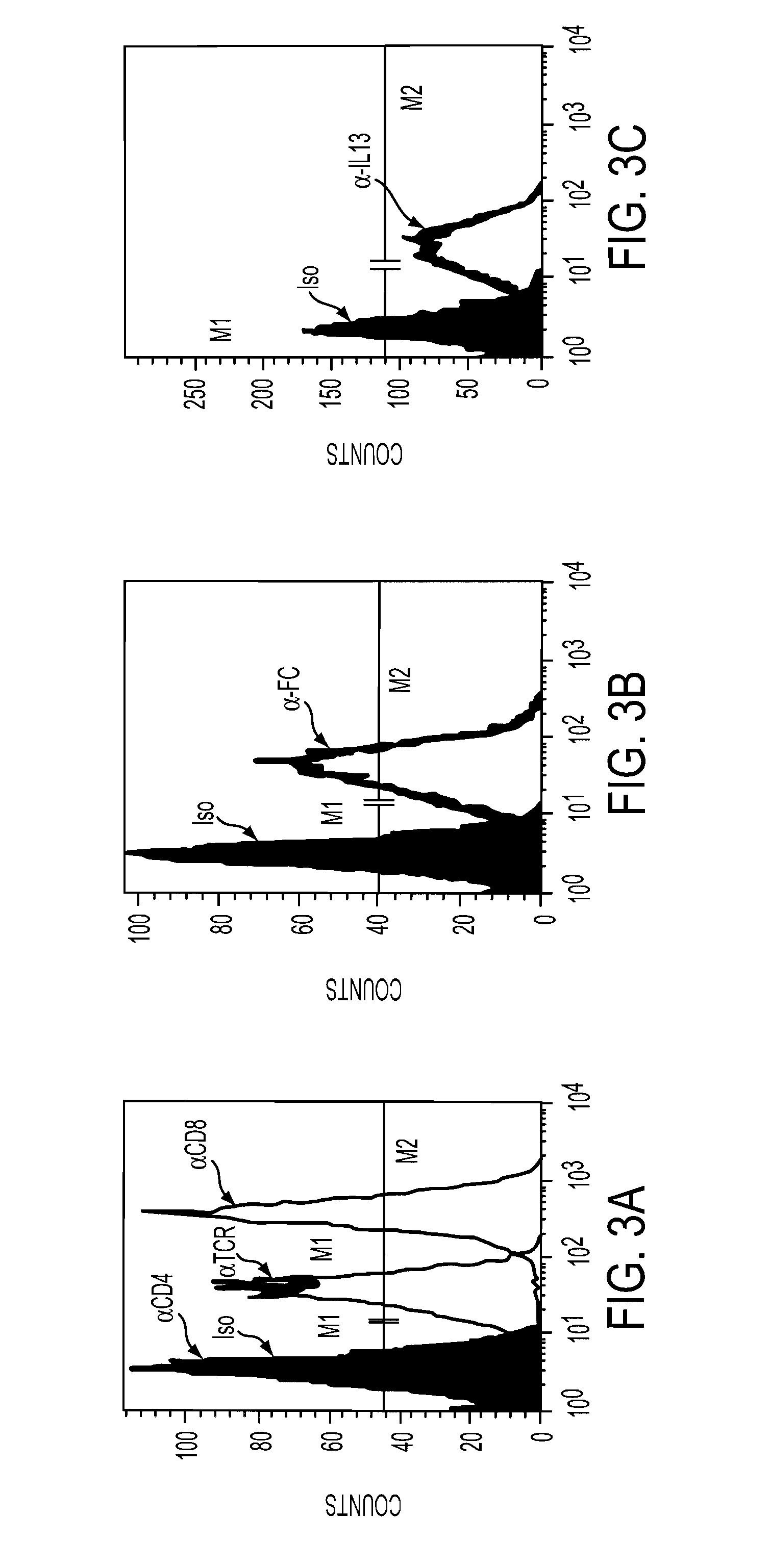
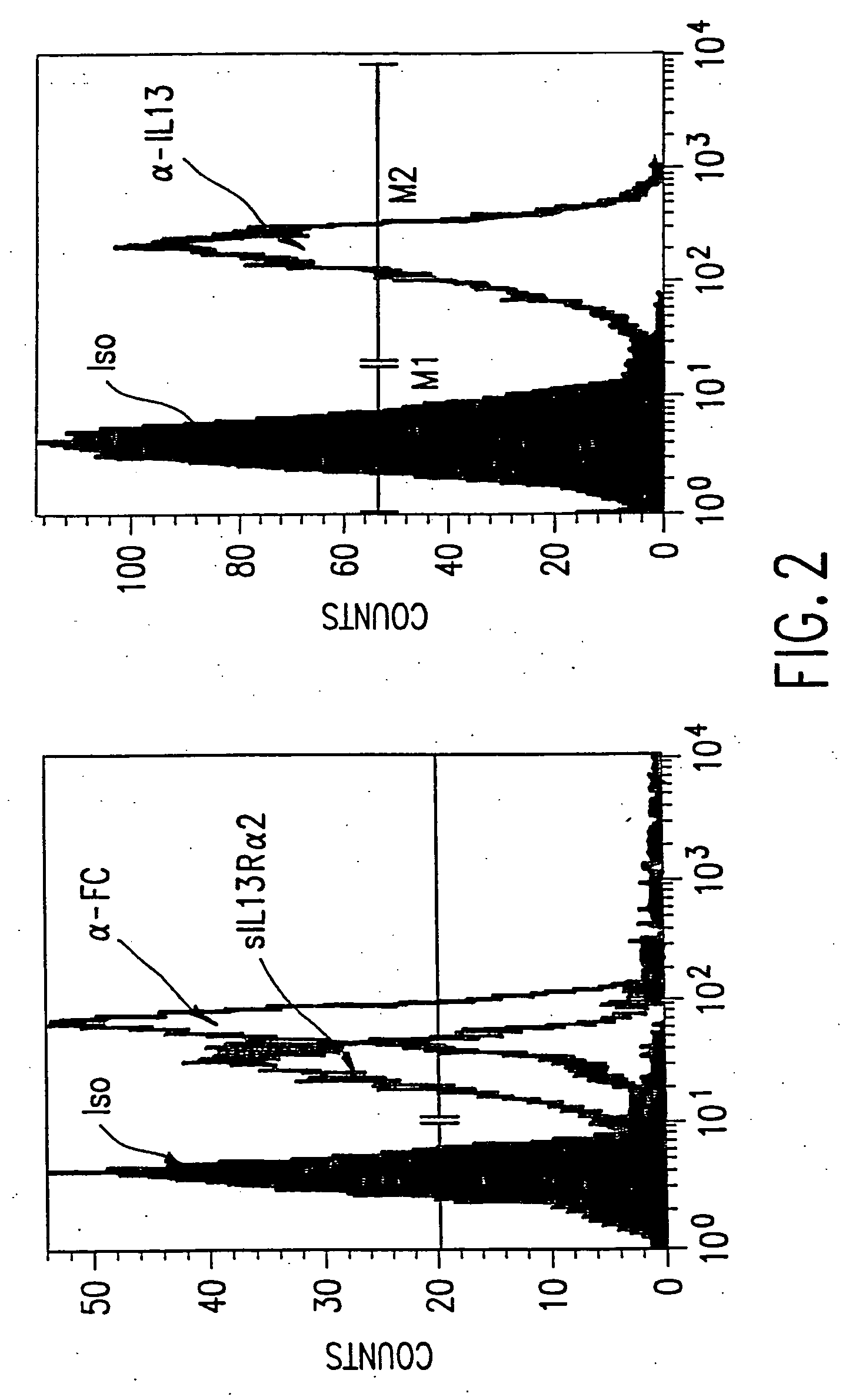
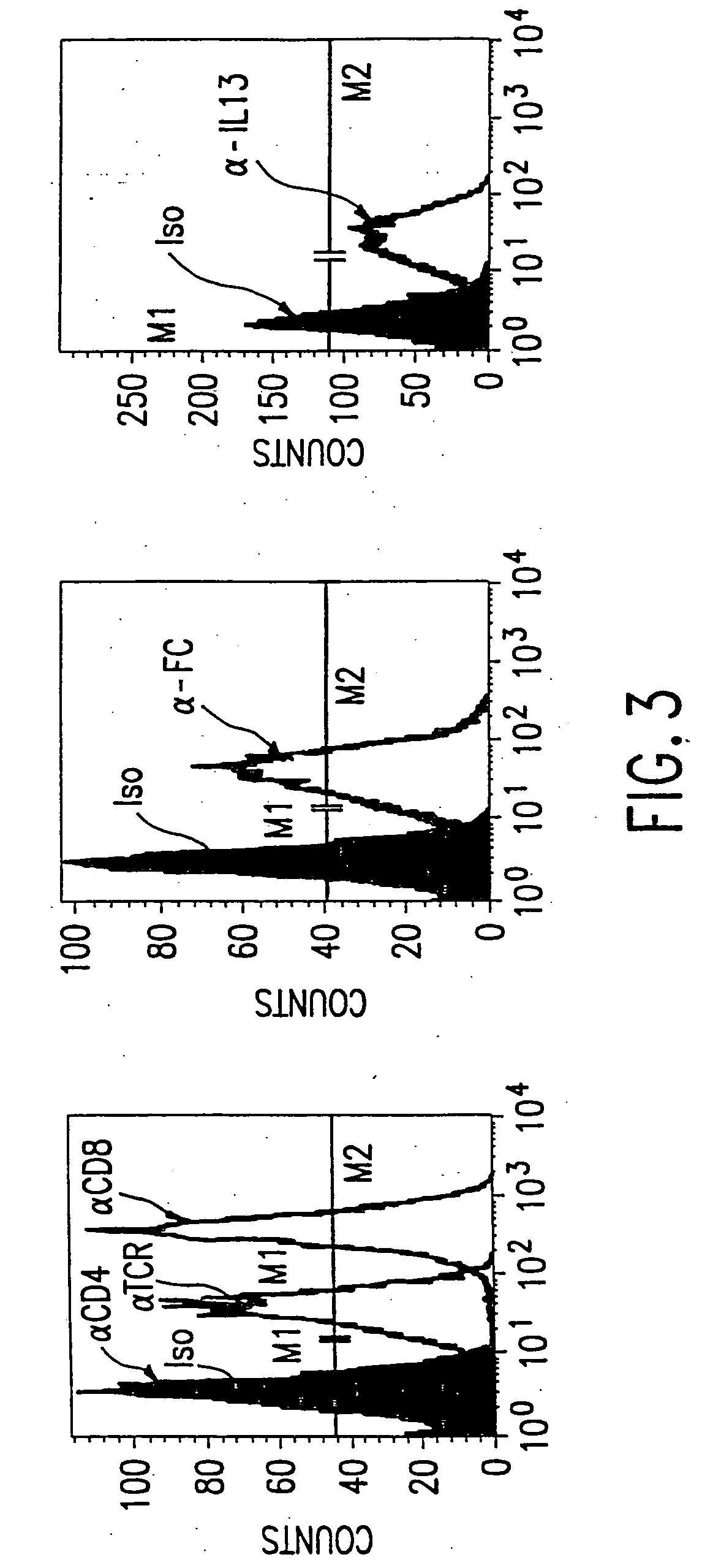
The present invention relates to chimeric transmembrane immunoreceptors, named “zetakines,” comprised of an extracellular domain comprising a soluble receptor ligand linked to a support region capable of tethering the extracellular domain to a cell surface, a transmembrane region and an intracellular signalling domain. Zetakines, when expressed on the surface of T lymphocytes, direct T cell activity to those specific cells expressing a receptor for which the soluble receptor ligand is specific. Zetakine chimeric immunoreceptors represent a novel extension of antibody-based immunoreceptors for redirecting the antigen specificity of T cells, with application to treatment of a variety of cancers, particularly via the autocrin / paracrine cytokine systems utilized by human malignancy. In a preferred embodiment is a glioma-specific immunoreceptor comprising the extracellular targetting domain of the IL-13Rα2-specific IL-13 mutant IL-13(E13Y) linked to the Fc region of IgG, the transmembrane domain of human CD4, and the human CD3 zeta chain.
Owner:CITY OF HOPE
Method for stimulating an immune response
InactiveUS6977073B1Stimulating directed immune responseStimulate immune responseBiocideArtificial cell constructsAbnormal tissue growthHematopoietic cell
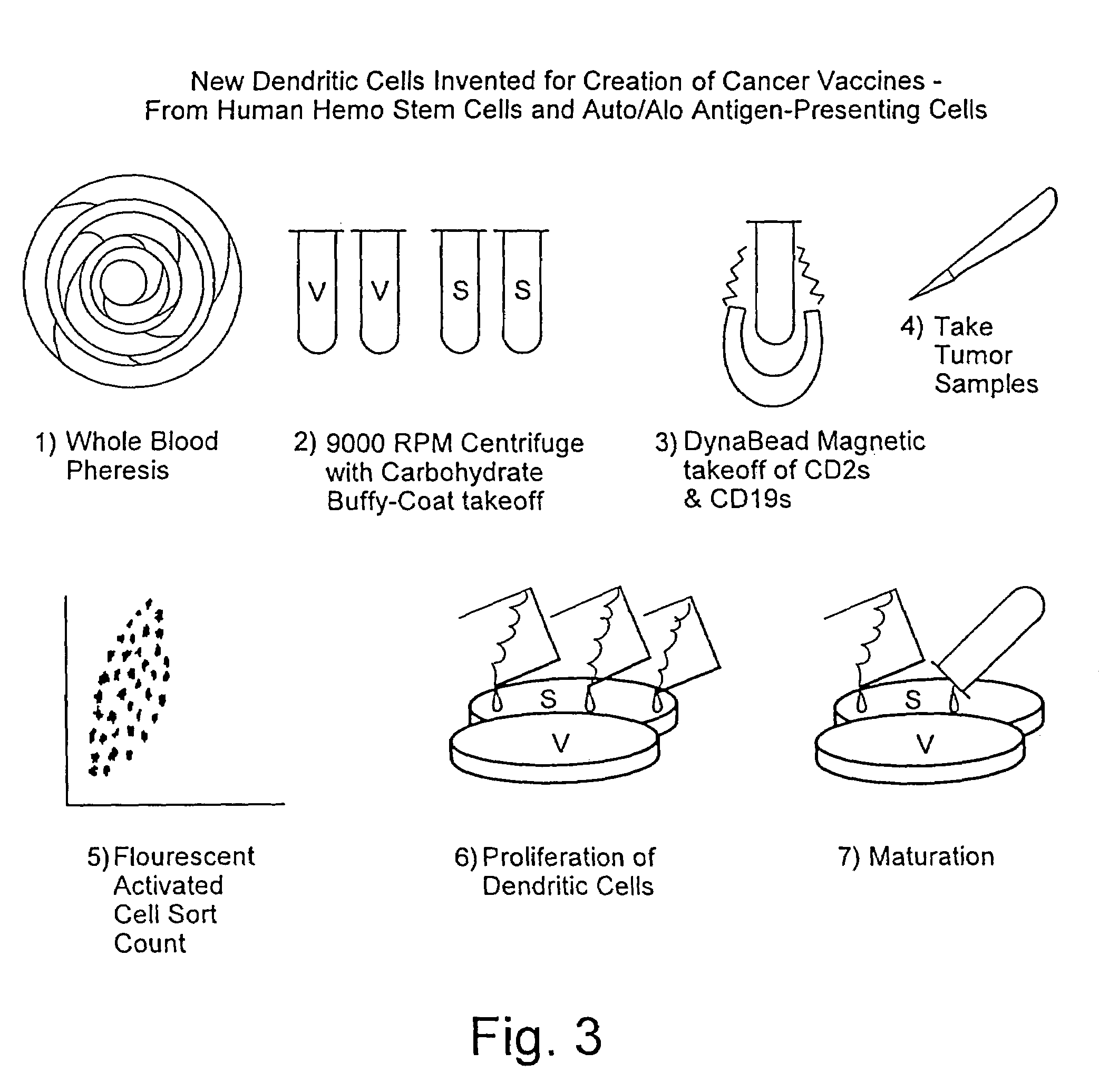
A method is described whereby dendritic cells derived from the CD34+ and CD 34−hematopoietic cell lineages are directed to become programmable antigen presenting cells. The programmed cells may be pulsed with tumor cell RNA or tumor cell RNA expression products. The protocol provides for directing the maturation of dendritic cells to become antigen presenting cells. The protocol further provides for isolating tumor cell RNA from biopsy material that has been prepared in paraffin block storage. The directed dendritic cell is provided with a plurality of tumor markers by using tumor RNA in toto, the poly A+RNA fraction or the expression product of such RNA. Once activated the dendritic cells are incubated with T4 and T8 lymphocytes to stimulate and sensitize the T lymphocytes which upon introduction either into a donor host or a nondonor recipient will provide immune response protection.
Owner:CEZAYIRLI CEM
Compositions and methods using complexes of heat shock protein 90 and antigenic molecules for the treatment and prevention of neoplastic diseases
The present invention relates to methods and compositions for eliciting an immune response and the prevention and treatment of primary and metastatic neoplastic diseases and infectious diseases. The methods of the invention comprise administering a composition comprising an effective amount of a complex, in which the complex consists essentially of a heat shock protein (hsp) noncovalently bound to an antigenic molecule. "Antigenic molecule" as used herein refers to the peptides with which the hsps are endogenously associated in vivo as well as exogenous antigens / immunogens (i.e., with which the hsps are not complexed in vivo) or antigenic / immunogenic fragments and derivatives thereof. In a preferred embodiment, the complex is autologous to the individual. The effective amounts of the complex are in the range of 10-600 micrograms for complexes comprising hsp7o, 50-1000 micrograms for hsp9o, and 10-600 micrograms for gp96. The invention also provides a method for measuring tumor rejection in viva in an individual, preferably a human, comprising measuring the generation by the individual of MHC Class I-restricted CD8+ cytotoxic T lymphocytes specific to the tumor. Methods of purifying hsp7o-peptide complexes are also provided.
Owner:FORDHAM UNIVERSITY
Compositions for inducing immune responses
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Compositions for inducing immune responses
ActiveUS20050107322A1Avoid inductionBacterial antigen ingredientsViral antigen ingredientsAdjuvantLactide
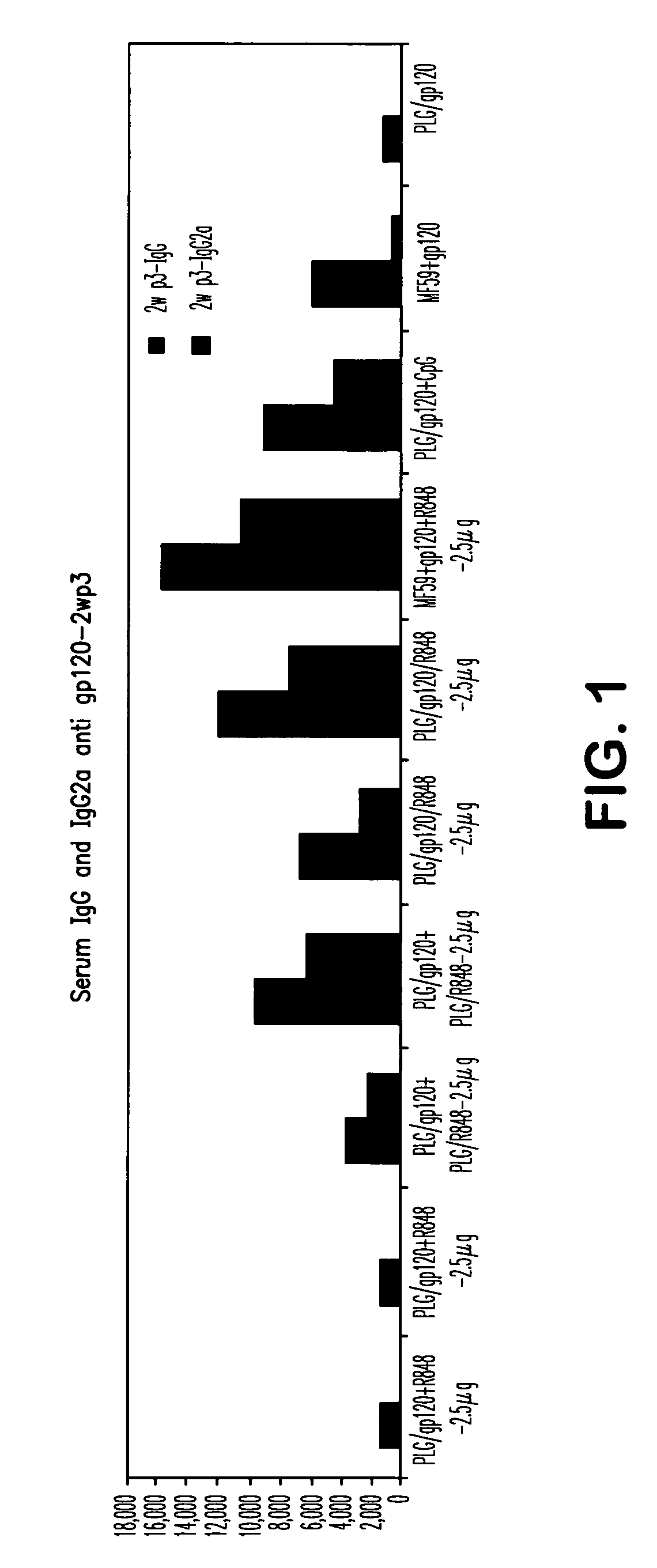
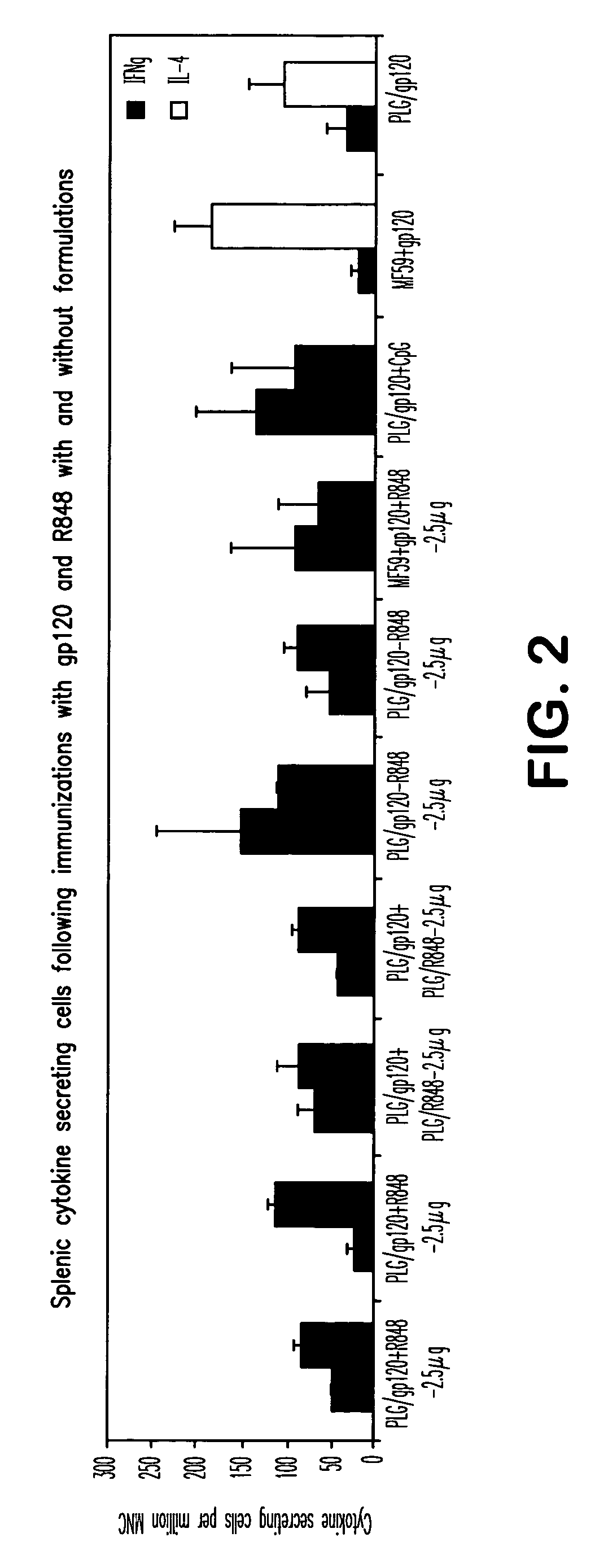
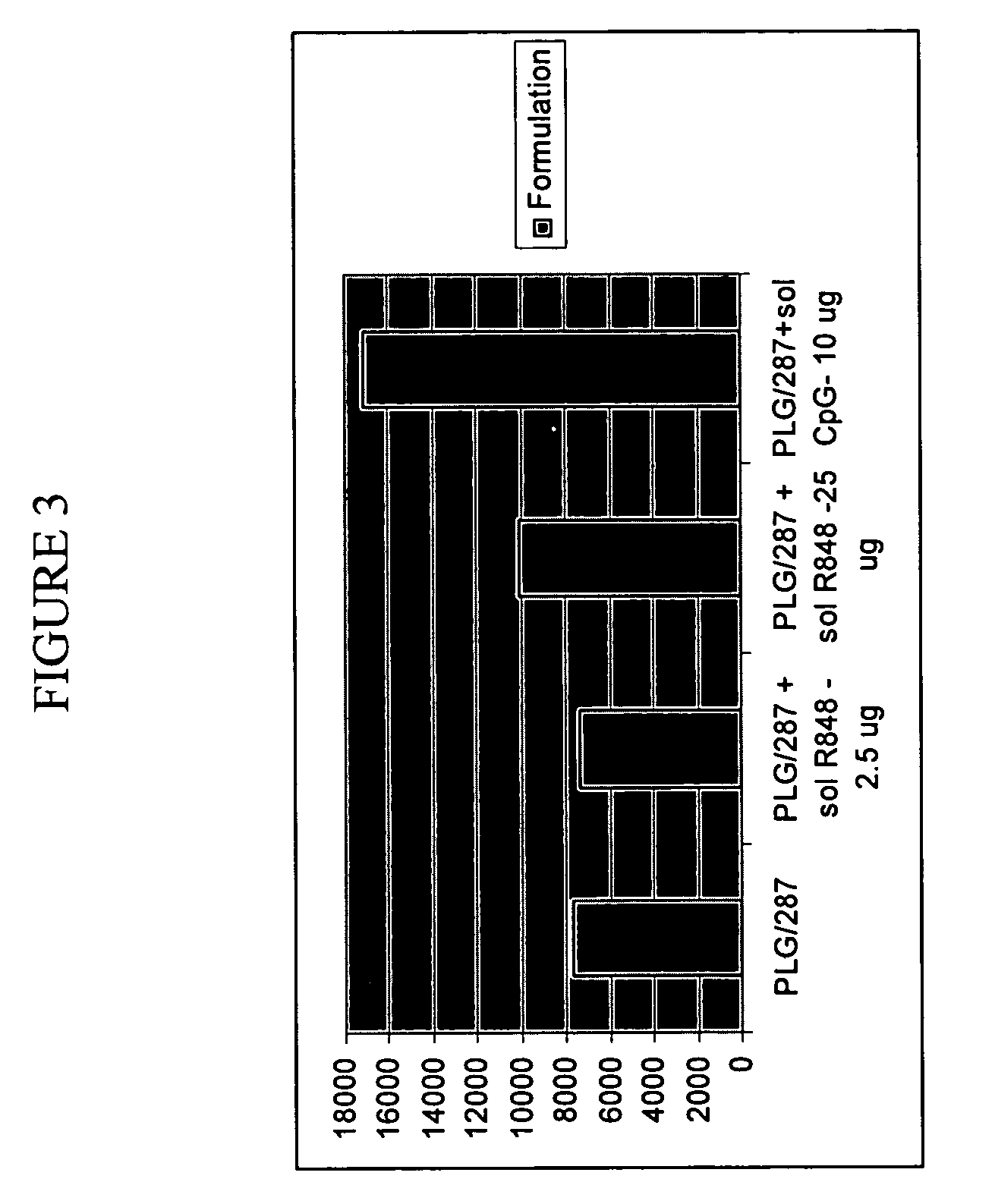
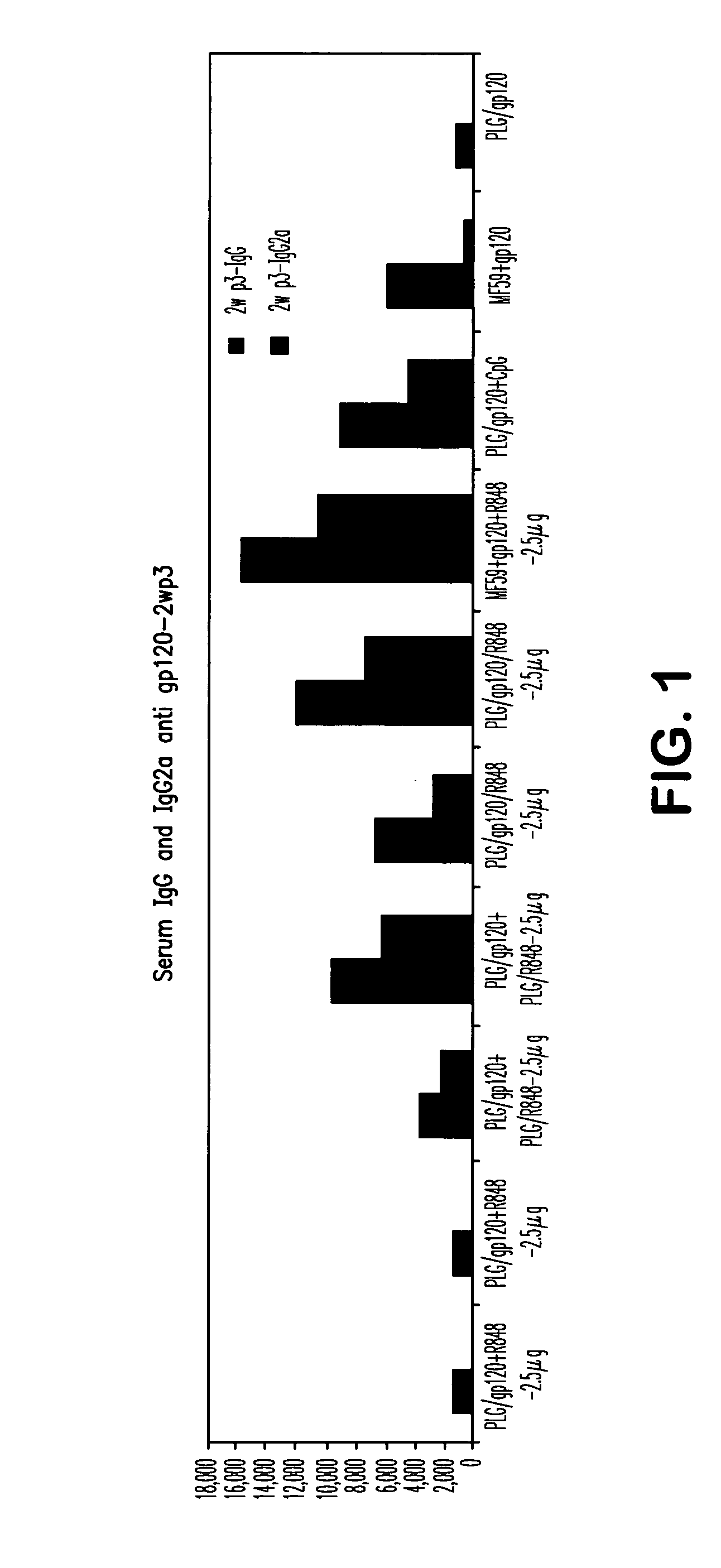
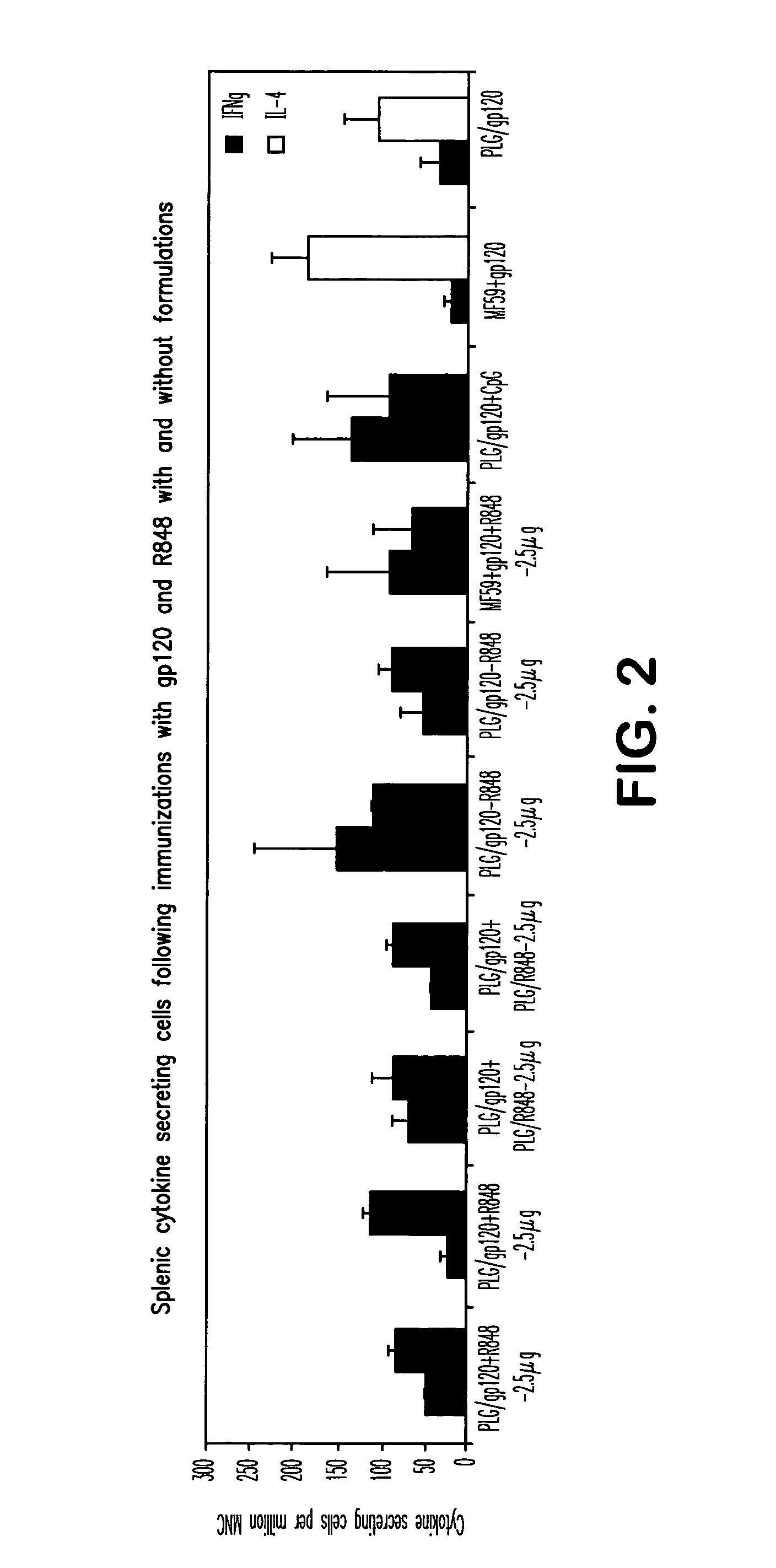
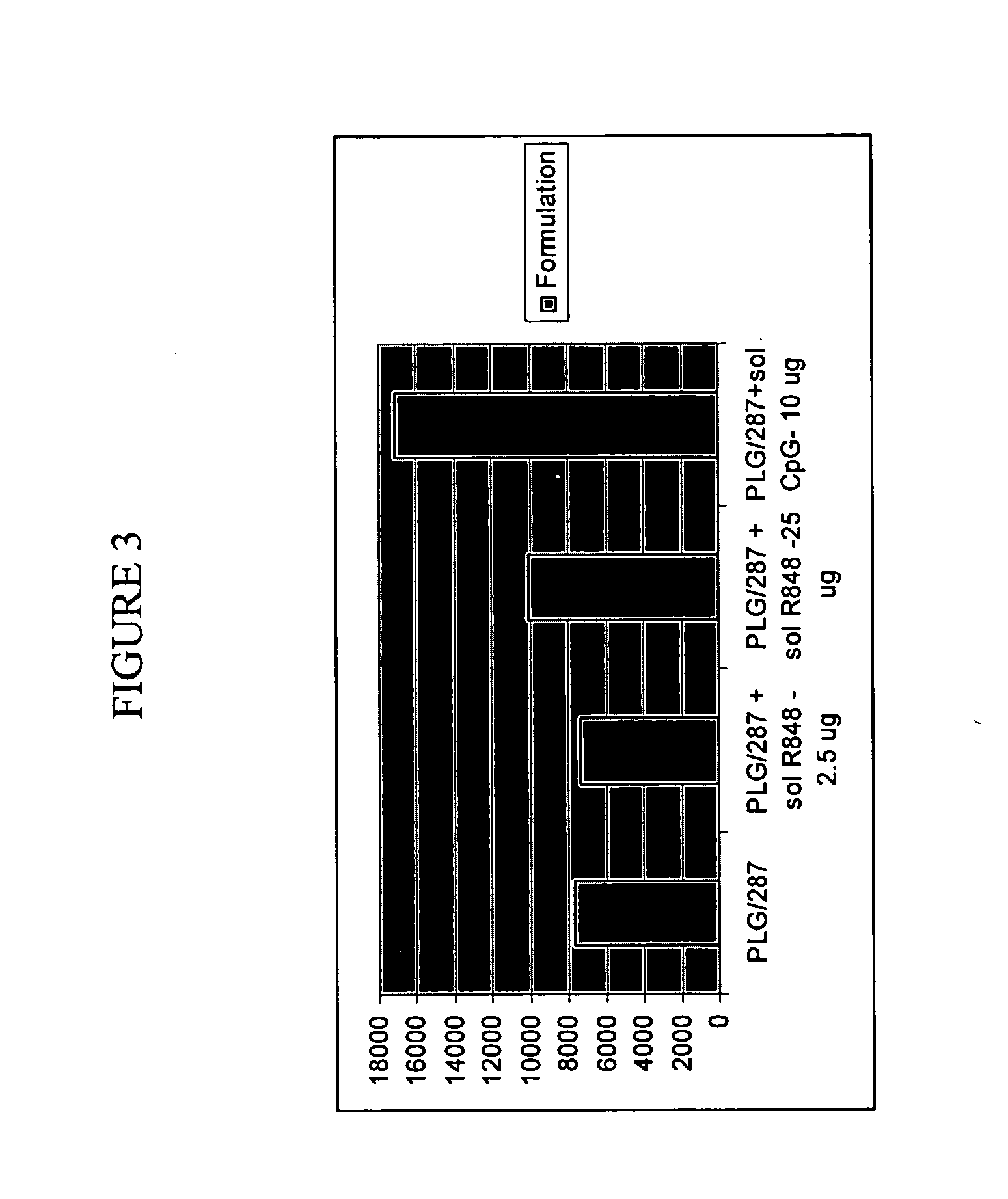
The invention provides, inter alia, immunogenic compositions comprising a first antigen, at least two adjuvants, wherein a first adjuvant comprises a polymer derived from poly(lactides) and / or poly(lactide-co-glycolides), and wherein a second adjuvant comprises an imidazoquinoline, wherein said first antigen is encapsulated within, adsorbed or conjugated to, co-lyophilized or mixed with said first adjuvant, and a pharmaceutically acceptable excipient, wherein said composition elicits a cellular immune response when administered to a vertebrate subject. The invention also provides methods of producing immunogenic compositions, methods for producing a cytotoxic-T lymphocyte (CTL) response in a vertebrate subject, and methods of immunization.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Peptide sequences specific for the hepatic stages of P. falciparum bearing epitopes capable of stimulating the T lymphocytes
The present invention relates to an in vitro diagnostic method for malaria in an individual comprising placing a tissue or a biological fluid taken from an individual in contact with a molecule or polypeptide composition, wherein said molecule or polypeptide composition comprises one or more peptide sequences bearing all or part of one or more T epitopes of the proteins resulting from the infectious activity of P. falciparum, under conditions allowing an in vitro immunological reaction to occur between said composition and the antibodies that may be present in the tissue or biological fluid, and in vitro detection of the antigen-antibody complexes formed. The invention further relates to a polypeptide comprising at least one T epitope from a liver-stage specific protein produced by P. falciparum and a vaccine composition directed against malaria comprising a molecule having one or more peptide sequences bearing all or part of one or more T epitopes resulting from the infectious activity of P. falciparum in the hepatic cells.
Owner:INST PASTEUR
Method for enhancing the antibody-dependent cellular cytotoxicity (ADCC) and uses of T cells expressing CD16 receptors
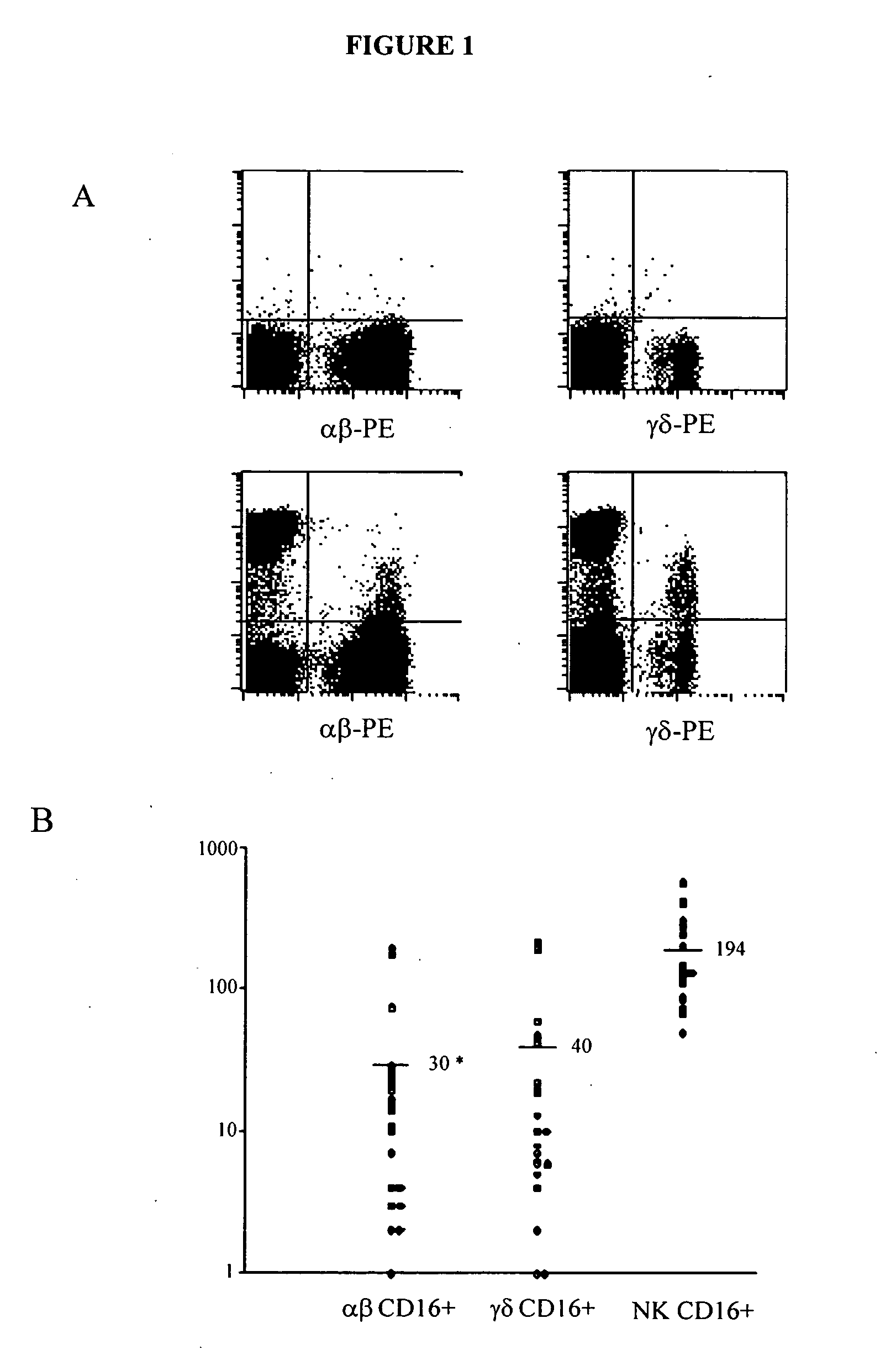
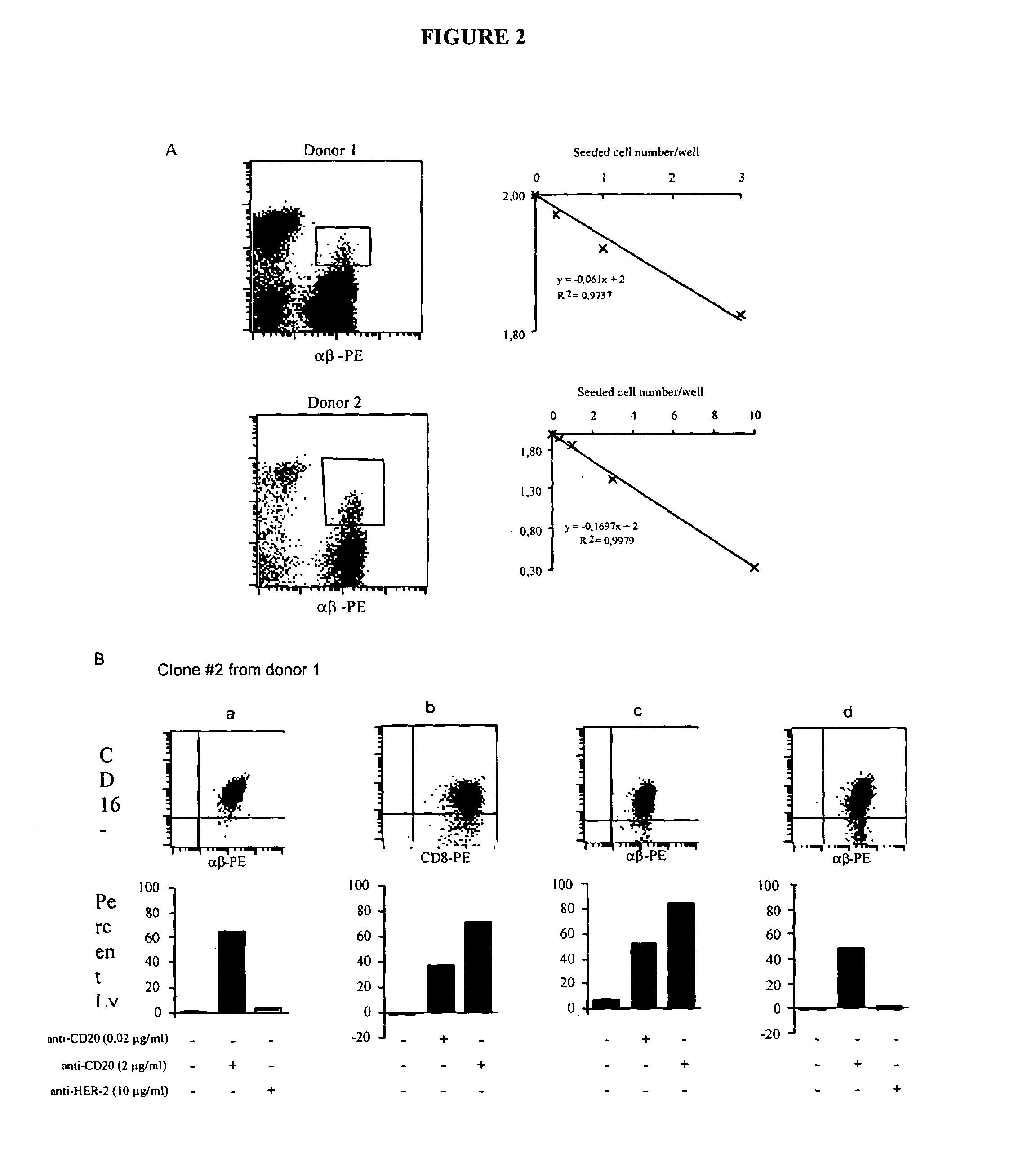
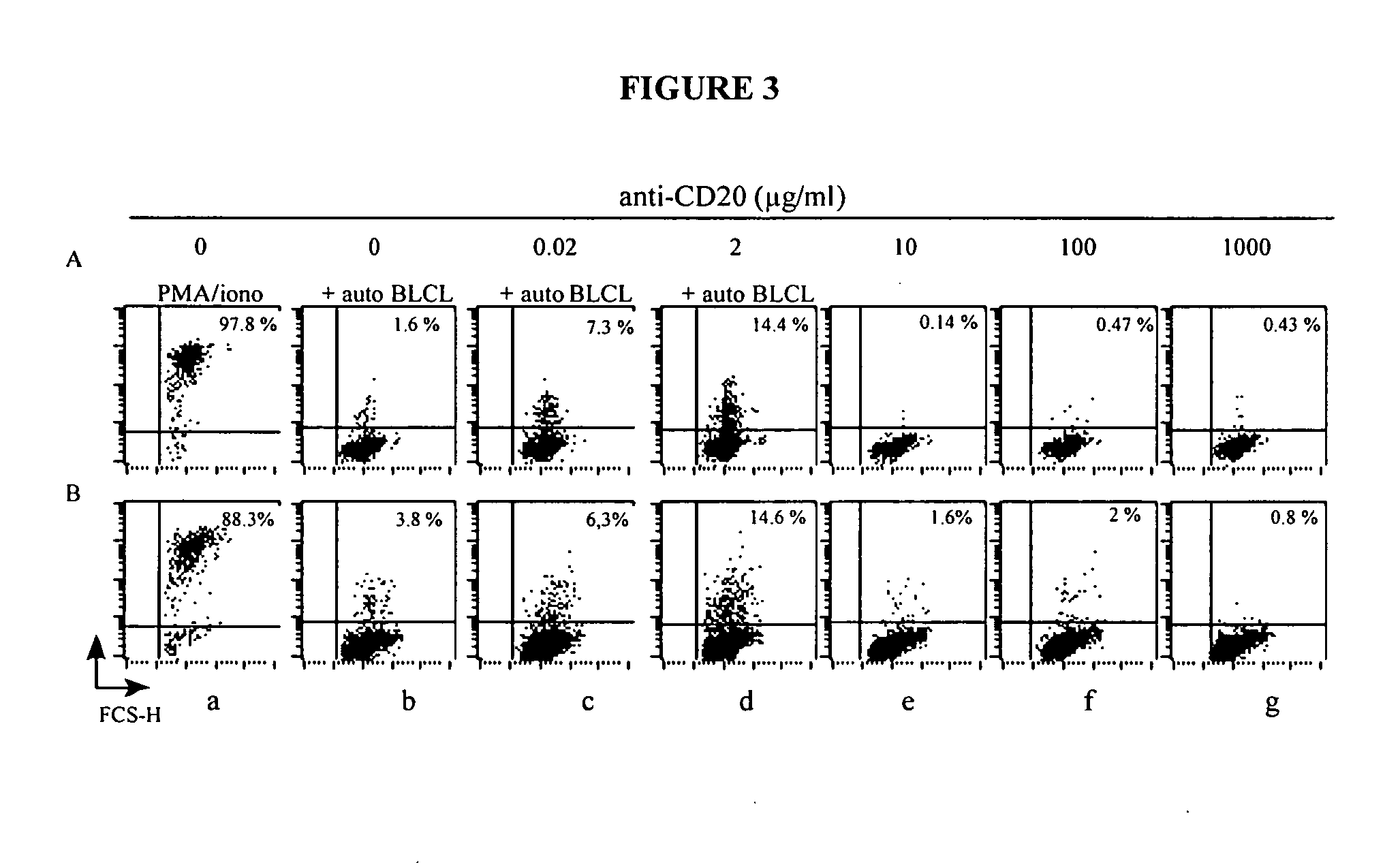
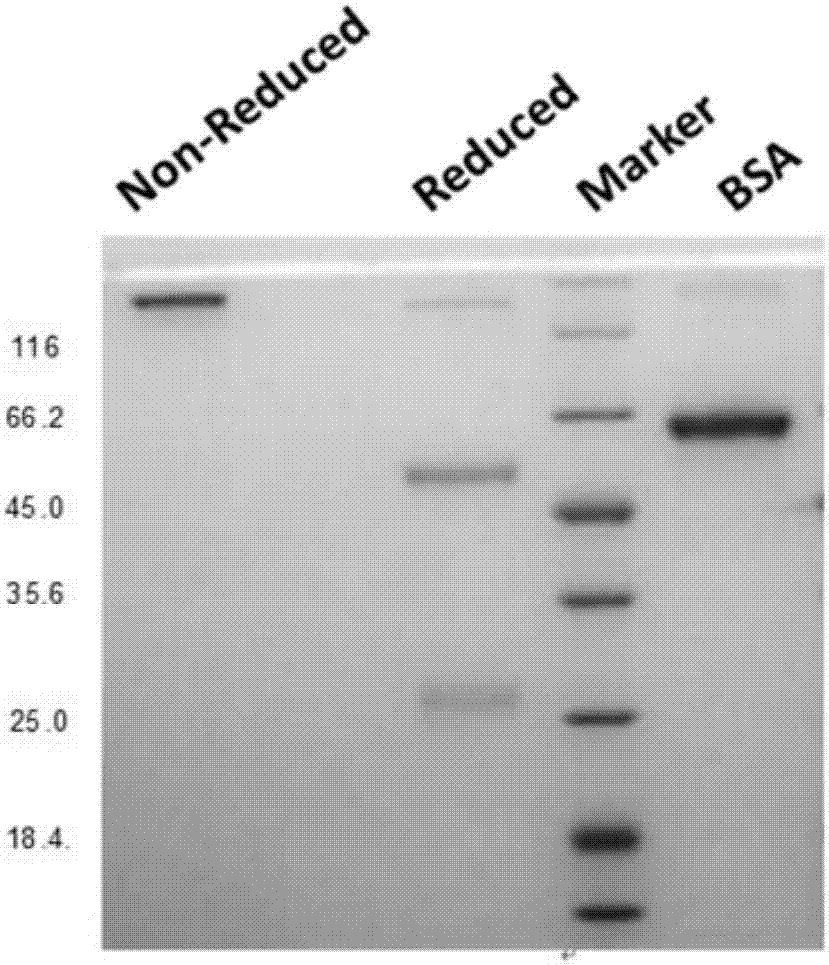
A method is provided for enhancing ADCC in an individual in need thereof, comprising the administration of T lymphocytes expressing a CD16-like receptor in said individual. Said method for enhancing ADCC may be used to treating cancers, autoimmune diseases or infections.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
Anti-CTLA4-anti-PD-1 bifunctional antibody, pharmaceutical composition and use thereof
ActiveCN106967172ABinding blockRelief of immunosuppressionHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsMolecular ImmunologyT lymphocyte
Belonging to the field of tumor treatment and molecular immunology, the invention relates to an anti-CTLA4-anti-PD-1 bifunctional antibody, a pharmaceutical composition and use thereof. Specifically, the anti-CTLA4-anti-PD-1 bifunctional antibody comprises: a PD-1 (programmed death-1) targeted first protein functional region, and a CTLA4 (cytotoxic T lymphocyte sociated antigen 4) targeted second protein functional region. The bifunctional antibody provided by the invention can well bind to CTLA4 and PD-1 specifically, specifically remove the immunosuppression of CTLA4 and PD-1 on the body, and activate T lymphocytes, thus having good application prospect.
Owner:AKESO PHARMA INC
Tumor precision T cell containing efficient killing starting mechanism and application of tumor precision T cell
ActiveCN105331586AActivate proliferationActivate growthImmunoglobulins against cell receptors/antigens/surface-determinantsMammal material medical ingredientsAntigen receptorsT lymphocyte
The invention belongs to the fields of immunology and cell biology, and relates to a tumor precision T cell containing an efficient killing starting mechanism and an application of the tumor precision T cell, in particular to a CAR (chimeric antigen receptor) having moderate-affinity binding characteristic with a broad-spectrum expression membrane antigen on the tumor cell surface as well as a new-generation tumor precision T cell, namely, Baize T. T cell activation is started rapidly under the action of the CAR having the moderate-affinity binding characteristic, an activation signal of the CAR is superposed with a TCR (T cell receptor) signal with tumor antigen natural recognition capacity in a CTL (tumor-specific cytotoxic T lymphocyte), the CTL is activated to proliferate and grow in a tumor microenvironment, and a tumor cell is killed preciously by the tumor-antigen-specific TCR. The tumor precision T cell has broad anti-tumor application prospect.
Owner:SHANGHAI CELL THERAPY RES INST +2
Chimeric antigen receptor and its use
InactiveCN103145849ASpeed up entryGood treatment effectGenetic material ingredientsAntiviralsLatent Membrane Protein-1Single-Chain Antibodies
The invention belongs to the biotechnical field of tumors, and discloses a preparation method of a chimeric antigen receptor and an application of the chimeric antigen receptor. The chimeric antigen receptor is formed through the structural series connection of a single chain antibody of a human anti-EB virus latent membrane protein 1, CH2CH3 of a human antibody IgG1, and intracellular signals of immune co-stimulating signal molecules CD28, CD134 and CD3zeta. The chimeric antigen receptor is used for modifying T lymphocytes, and the modified lymphocytes can be used for treating EB virus related tumors and preparing EB virus related tumor resisting medicines.
Owner:SINOBIOWAY CELL THERAPY CO LTD
Anti-PD1 monoclonal antibody, pharmaceutical composition and uses thereof
ActiveCN106977602ABinding blockRelief of immunosuppressionImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsMolecular ImmunologyAntigen Binding Fragment
The present invention belongs to the field of tumor treatment and molecular immunology, and relates to an anti-PD1 monoclonal antibody, a pharmaceutical composition and uses thereof. In particularly, the present invention relates to a monoclonal antibody or an antigen-binding fragment thereof, wherein the heavy chain variable region of the monoclonal antibody comprises CDR having an amino acid sequence represented by SEQ ID NO:9-11, and / or the light chain variable region of the monoclonal antibody comprises CDR having an amino acid sequence represented by SEQ ID NO:12-14. According to the present invention, the monoclonal antibody can well and specifically bind to PD1, can specifically release the immunosuppression of PD1 on body, and can activate T lymphocytes.
Owner:CTTQ AKESO (SHANGHAI) BIOMED TECH CO LTD
Thieno 2,4-substituted pyrimidine compound, and pharmaceutical composition and application thereof
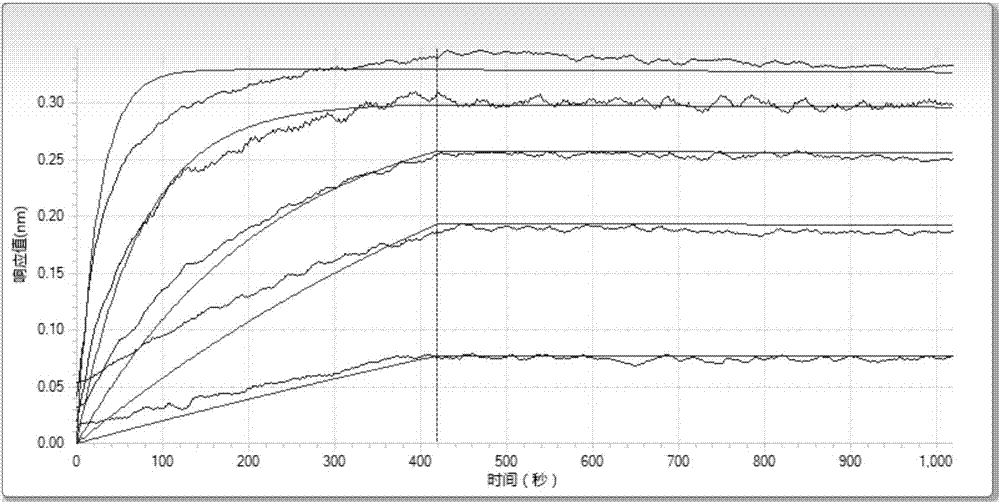
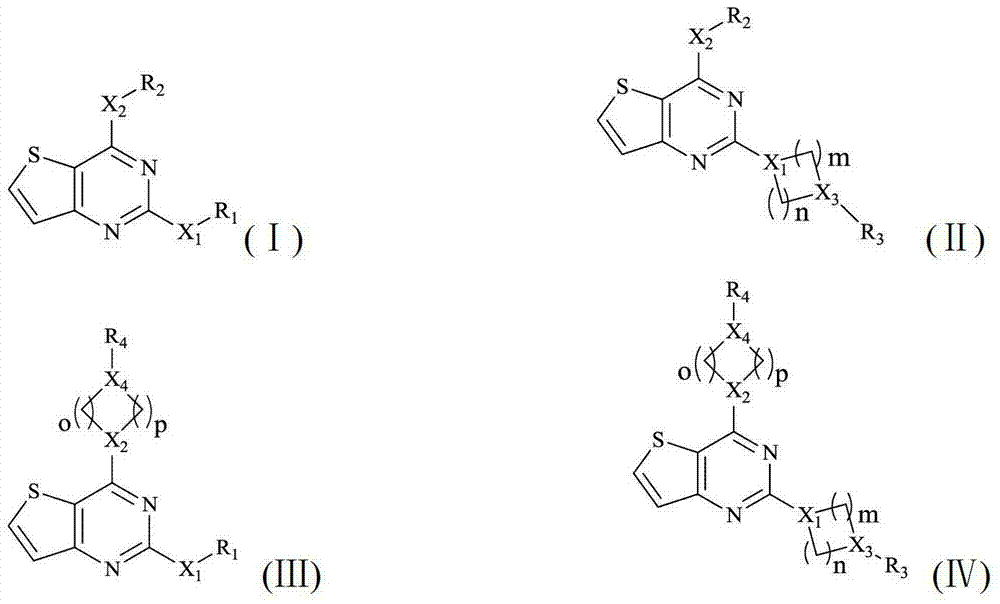
InactiveCN103242341AInhibit abnormal expressionPrevent proliferationOrganic active ingredientsNervous disorderDiseaseCancer cell
The invention discloses a thieno 2,4-substituted pyrimidine compound of which the general formulas are (I), (II), (III) and (IV), or pharmaceutically acceptable salt or stereisomer or a prodrug molecule, and a pharmaceutical composition and application thereof. The thieno 2,4-substituted pyrimidine compound can effectively restrain abnormal expression of Aurora kinase, has specific inhibited effect on Aurora-A and Aurora-B, can be applied to a novel field of molecular targeting treatment, and has strong inhibitory activity on an excessive hyperplasia disease, especially cervical tumor cells, human macrophage line leukemia cell, and human t-lymphocyte line cancer cell.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
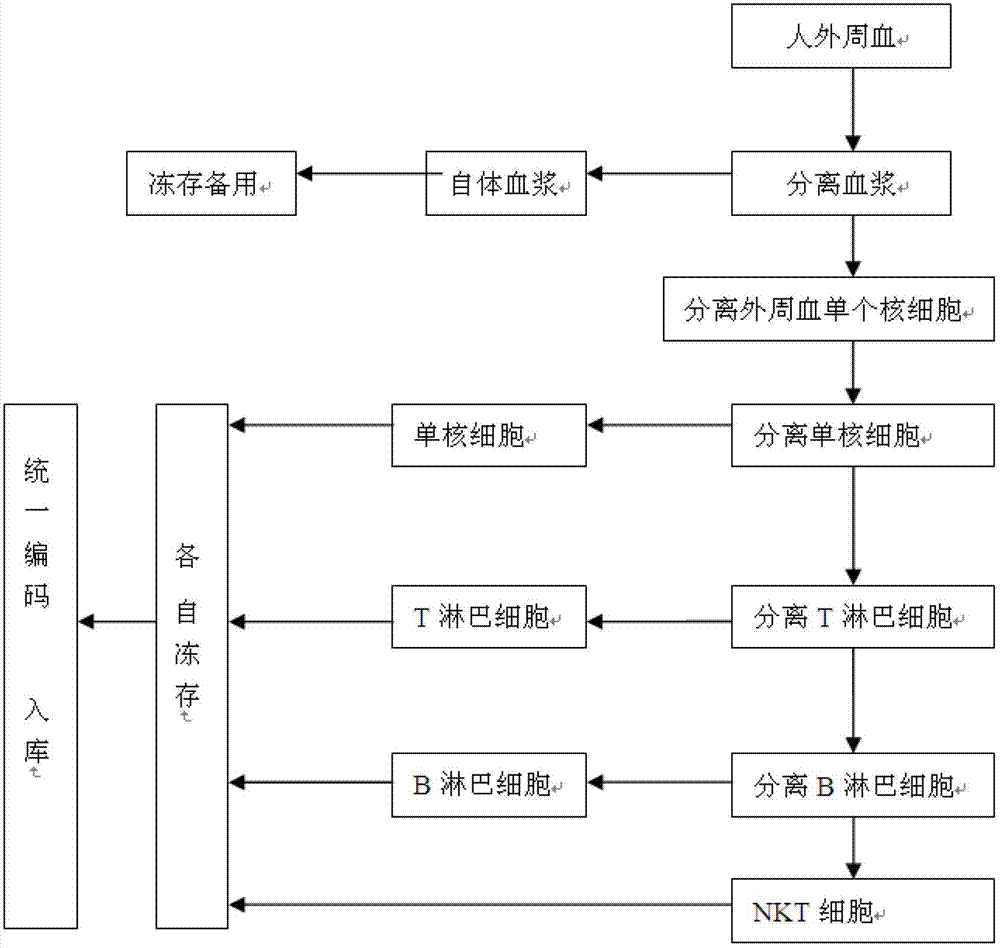
Method for constructing human peripheral blood immune cell bank
ActiveCN102758259AHigh activityHigh purityMicroorganism librariesBlood/immune system cellsPeripheral blood mononuclear cellNatural Killer Cell Inhibitory Receptors
The invention discloses a method for constructing a human peripheral blood immune cell bank. The method comprises the following steps of: collecting human peripheral blood, separating autologous plasma, separating a peripheral blood mononuclear cell, separating a mononuclear cell by the peripheral blood mononuclear cell and freezing, separating a T lymphocyte by the peripheral blood mononuclear cell and freezing, separating a B lymphocyte by the peripheral blood mononuclear cell and freezing, separating an NK cell by the peripheral blood mononuclear cell and freezing, and encoding and puttingin storage. According to the invention, immune cells of health or young people are separated and are respectively independently frozen, and relative numbers are stored and put into storage, so that the stored human immune cells have the characteristics of high activity, high purity and convenience for use, and fetal calf serum can be replaced by the human autologous plasma, so that introduction of a foreign protein can be avoided. Meanwhile, the method, provided by the invention, has the advantages of low cost, low requirements on laboratory conditions, and wide application.
Owner:济南赛尔生物科技股份有限公司
PDL-1 antibody, pharmaceutical composition thereof and application of PDL-1 antibody
ActiveCN107151269ABinding blockRelief of immunosuppressionAntibody mimetics/scaffoldsMicroorganism based processesMolecular ImmunologyHeavy chain
The invention belongs to the field of oncotherapy and molecular immunology, and relates to a PDL-1 antibody, a pharmaceutical composition thereof and application of the PDL-1 antibody, in particular to a monoclonal antibody of PDL-1 or an antigen binding fragment thereof. A variable region of the heavy chain of the monoclonal antibody comprises CDR of which the amino acid sequence is SEQ ID NO: 15-17; and a variable region of the light chain of the monoclonal antibody comprises CDR of which the amino acid sequence is SEQ ID NO: 18-20. The monoclonal antibody can be well combined to PDL-1 specifically, inhibition of PDL-1 to body immunity is relieved specifically, and T lymphocyte is activated.
Owner:SICHUAN KELUN BIOTECH BIOPHARMACEUTICAL CO LTD
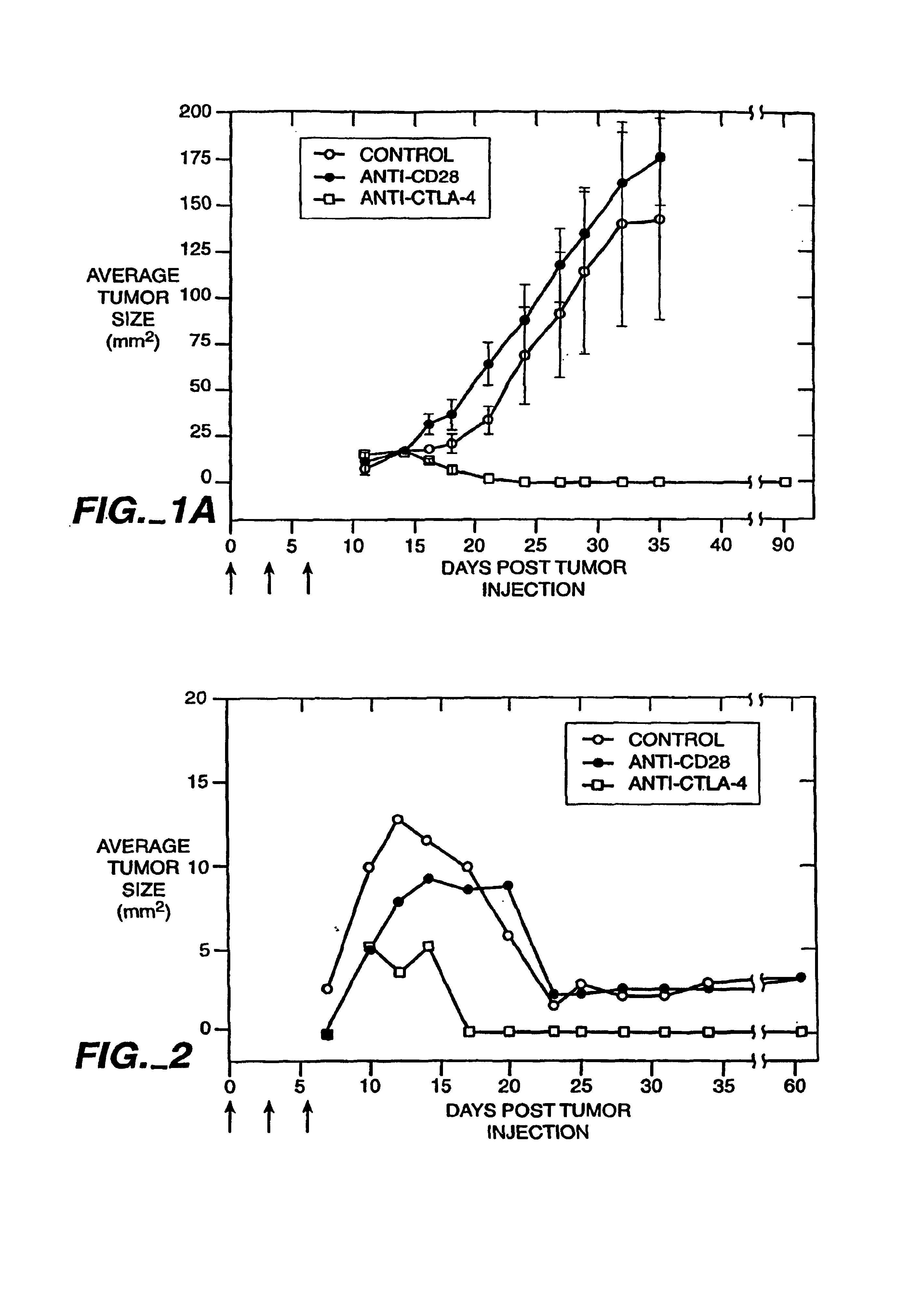
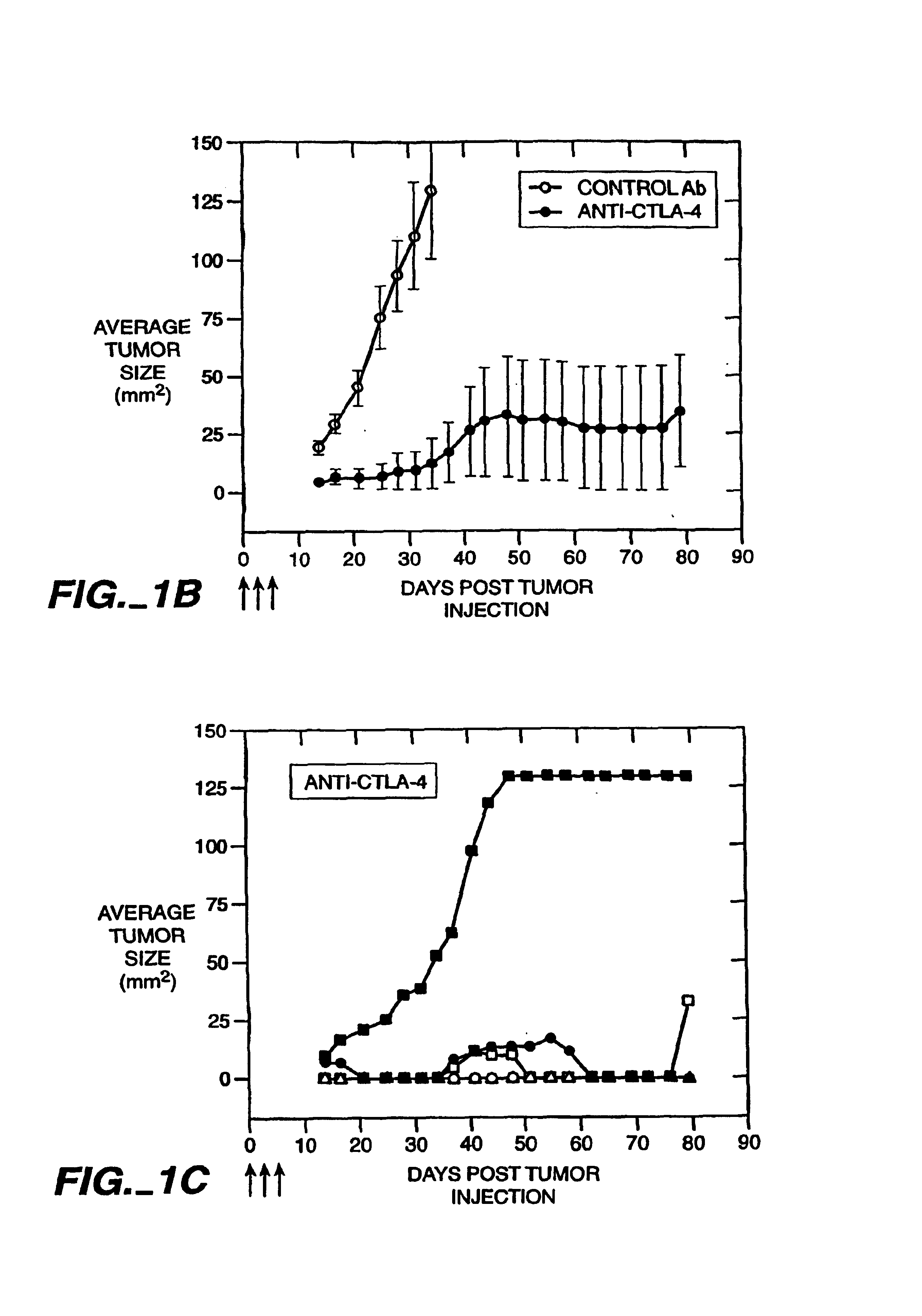
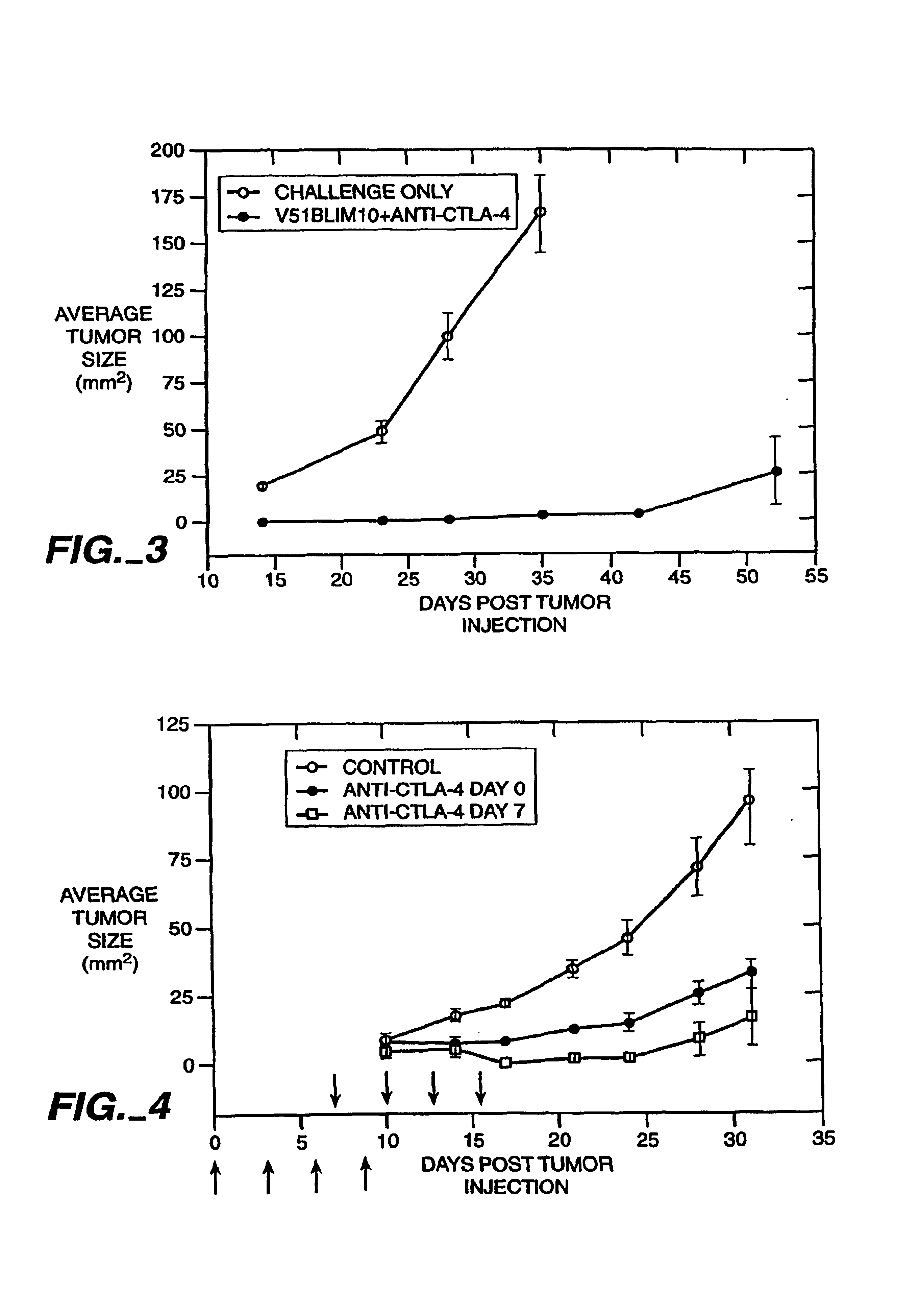
Blockade of T lymphocyte down-regulation associated with CTLA-4 signaling
InactiveUS7229628B1Lack of responsivenessPrevent proliferationOrganic active ingredientsPeptide/protein ingredientsAdjuvantActivation cells
Owner:RGT UNIV OF CALIFORNIA
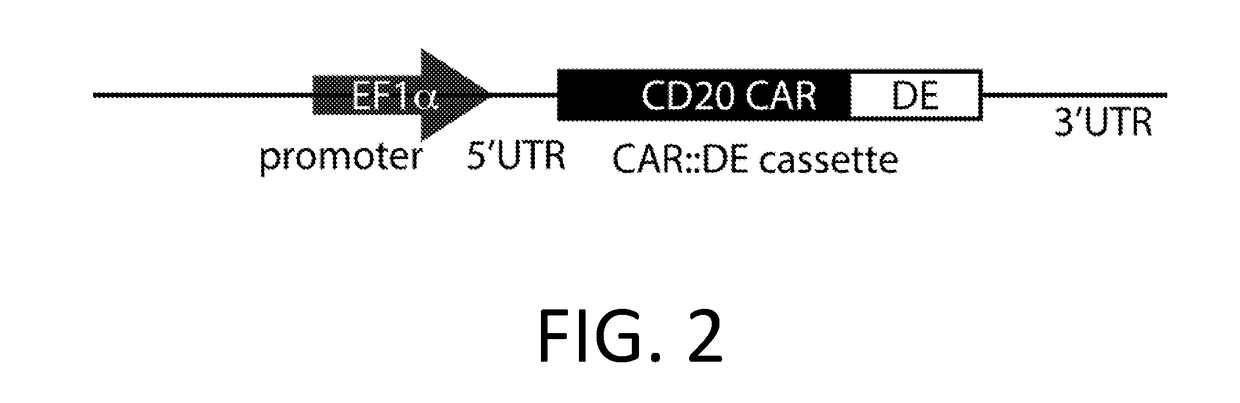
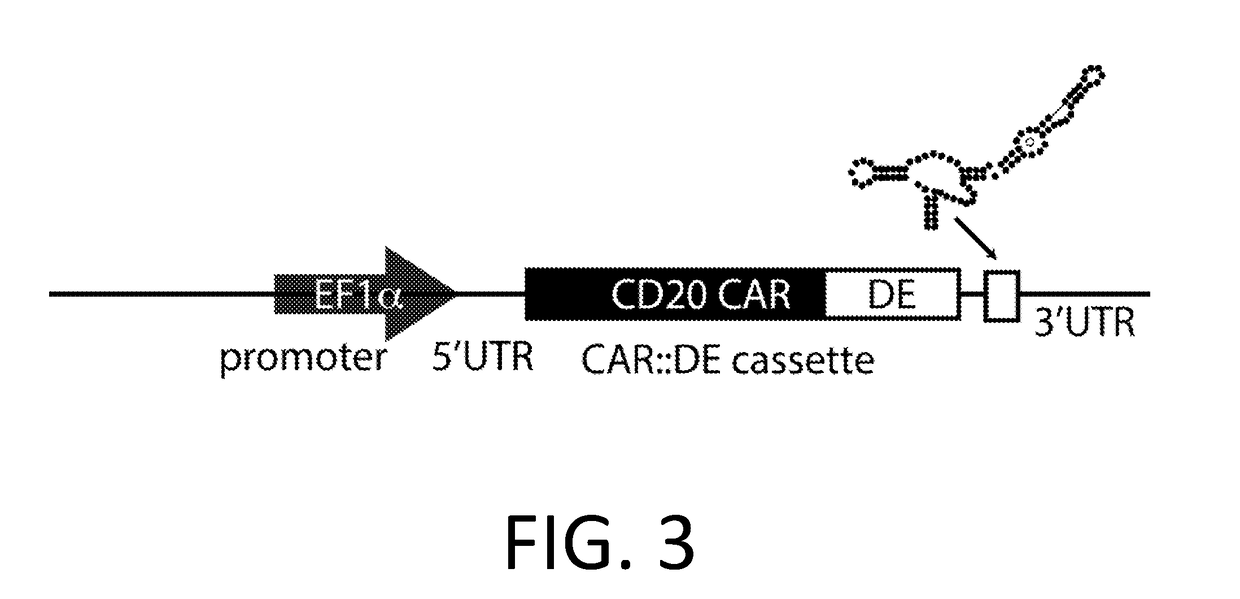
Smart CAR Devices and DE CAR Polypeptides for Treating Disease and Methods for Enhancing Immune Responses
ActiveUS20170157176A1Increase killIncrease the number of grainsPolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsDiseaseApoptosis
In an aspect, the present invention relates generally to the field of treating disease with CAR devices, Smart CAR devices, DE CAR devices, and / or Smart-DE CAR devices. The present invention also relates generally to the genetic modification of cytotoxic T-lymphocytes to reduce target cell killing by apoptosis and / or increase production of lytic proteins at desired times. In an aspect, the invention relates to the use of these genetically modified T-lymphocytes and / or natural killer cells with CAR devices, Smart CAR devices, DE CAR devices, and / or Smart-DE CAR devices to enhance the immune response against a disease.
Owner:CHIMERA BIOENG INC
Product and process for T lymphocyte immunosuppression
InactiveUS6264950B1Peptide/protein ingredientsAntibody mimetics/scaffoldsMajor histocompatibilityActivation cells
The present invention relates to a product and process for suppressing an immune response using a T lymphocyte veto molecule capable of blocking cell surface molecules responsible for T cell activation. Disclosed is a CD4 or CD2 molecule, associated with an immunoglobulin molecule capable of binding to a major histocompatibility antigen. Also disclosed is a method to produce a T lymphocyte veto molecule, a therapeutic composition comprising a T lymphocyte veto molecule and methods to use T lymphocyte veto molecules in therapeutic processes requiring suppression of an immune response.
Owner:NAT JEWISH MEDICAL & RES CENT
Superior molecular vaccine based on self-replicating RNA, suicidal DNA or naked DNA vector, that links antigen with polypeptide that promotes antigen presentation
InactiveUS7557200B2Efficiently presentedImprove effectivenessSsRNA viruses positive-senseAntibody mimetics/scaffoldsDiseaseMHC class I
Improved molecular vaccines comprise nucleic acid vectors that encode a fusion polypeptide that includes polypeptide or peptide physically linked to an antigen. The linked polypeptide is one that (a) promotes processing of the expressed fusion polypeptide via the MHC class I pathway and / or (b) promotes development or activity of antigen presenting cells, primarily dendritic cells. These vaccines employ one of several types of nucleic acid vectors, each with its own relative advantages: naked DNA plasmids, self-replicating RNA replicons and suicidal DNA-based on viral RNA replicons. Administration of such a vaccine results in enhance immune responses, primarily those mediated by CD8+ cytotoxic T lymphocytes, directed against the immunizing antigen part of the fusion polypeptide. Such vaccines are useful against tumor antigens, viral antigens and antigens of other pathogenic microorganisms and can be used in the prevention or treatment of diseases that include cancer and infections.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Chimeric immunoreceptor useful in treating human cancers
InactiveUS20060067920A1Negligible toxicityPotent and selectiveBiocideAntibody mimetics/scaffoldsIntracellular signallingAutocrine paracrine
The present invention relates to chimeric transmembrane immunoreceptors, named “zetakines,” comprised of an extracellular domain comprising a soluble receptor ligand linked to a support region capable of tethering the extracellular domain to a cell surface, a transmembrane region and an intracellular signalling domain. Zetakines, when expressed on the surface of T lymphocytes, direct T cell activity to those specific cells expressing a receptor for which the soluble receptor ligand is specific. Zetakine chimeric immunoreceptors represent a novel extension of antibody-based immunoreceptors for redirecting the antigen specificity of T cells, with application to treatment of a variety of cancers, particularly via the autocrin / paracrine cytokine systems utilized by human maligancy. In a preferred embodiment is a glioma-specific immunoreceptor comprising the extracellular targetting domain of the IL-13Rα2-specific IL-13 mutant IL-13(E13Y) linked to the Fc region of IgG, the transmembrane domain of human CD4, and the human CD3 zeta chain.
Owner:CITY OF HOPE
Methods for improving immunotherapy by enhancing survival of antigen-specific cytotoxic T lymphocytes
InactiveUS20070003531A1Improve survival rateBiocideOrganic active ingredientsT lymphocyteImmunotherapy
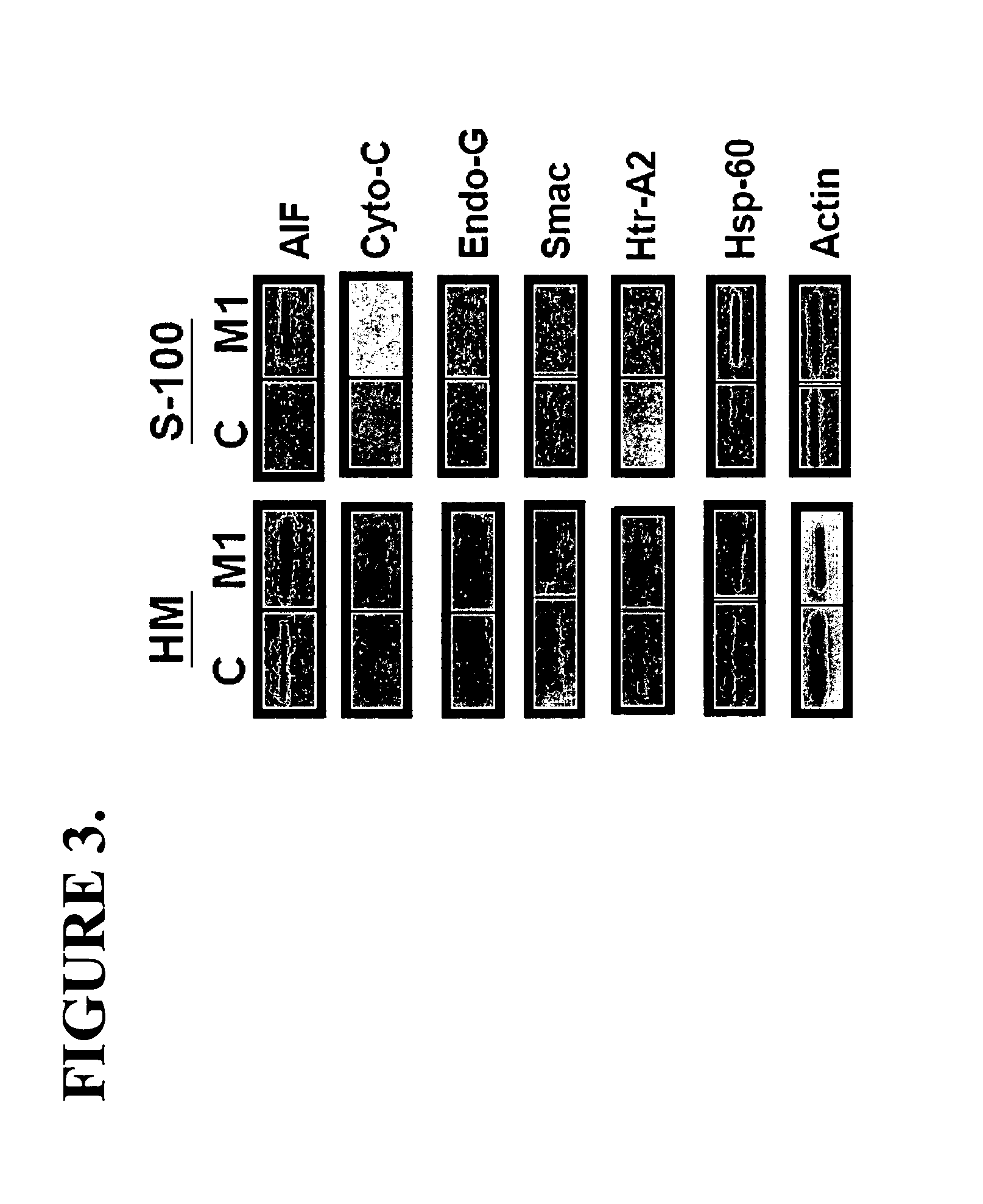
The invention relates generally to methods for enhancing immunity by improving the survival of activated T cells comprising the administration to a cell or a subject in need thereof an effective amount of an inhbitor of JNK and / or AIF. In other aspects the invention relates to the administration of an effective amount of an enzymatic nucleic acid, a pyrazoloanthrone or derivative, or combinations thereof to reduce activation induced cell death (AICD), programmed cell death (PCD), or both of antigen specific T cells.
Owner:UNIV OF CONNECTICUT
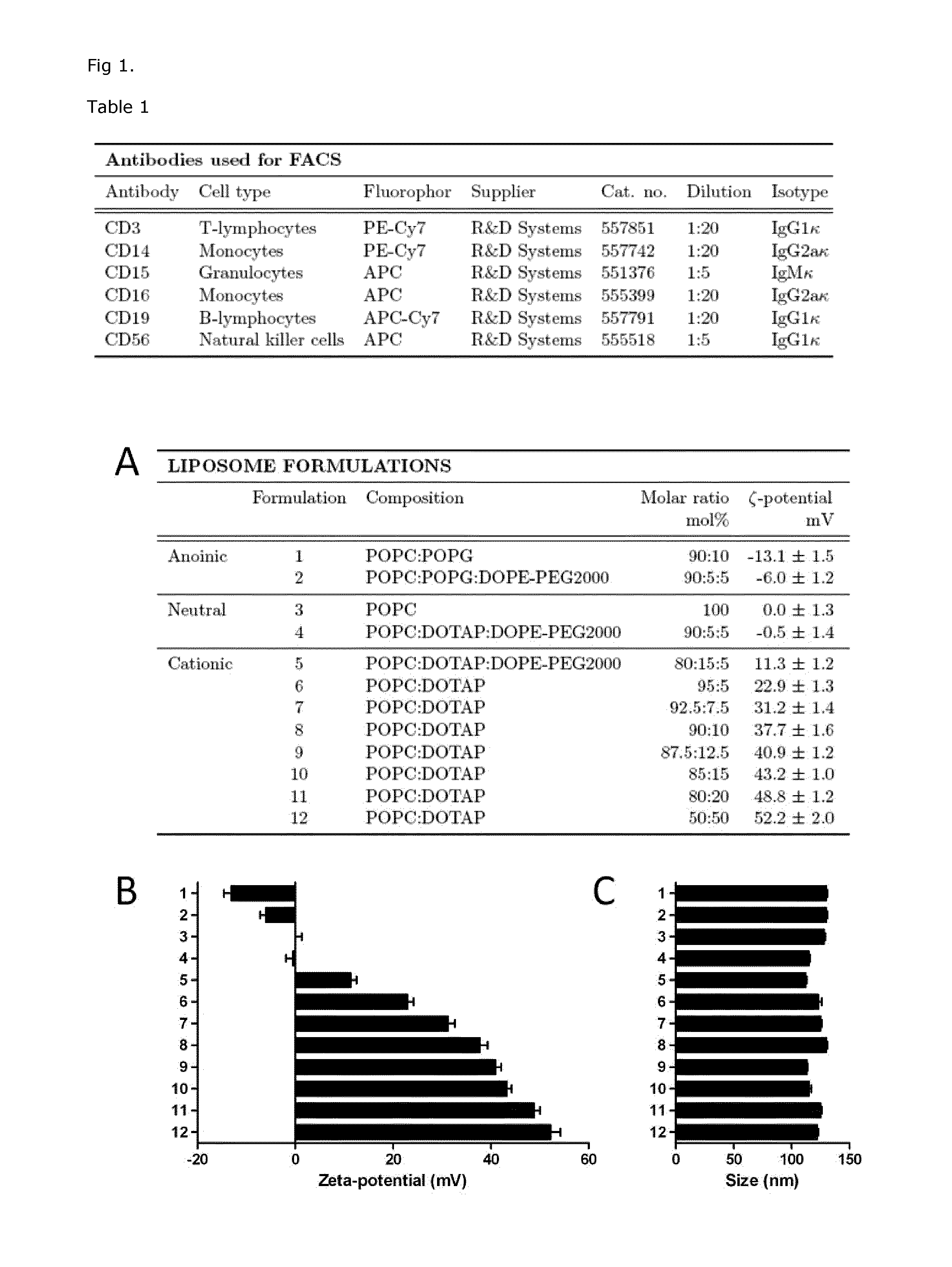
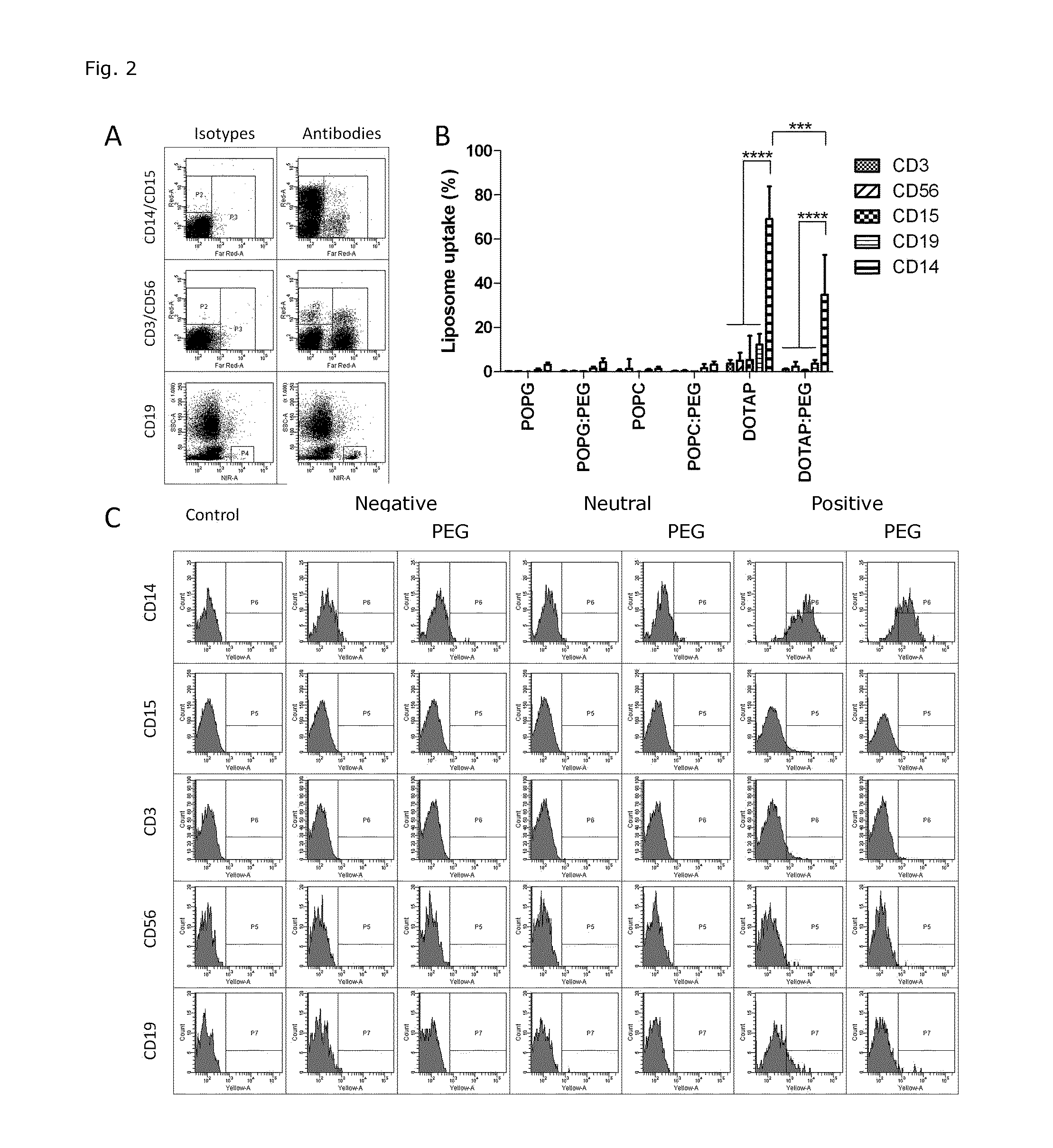
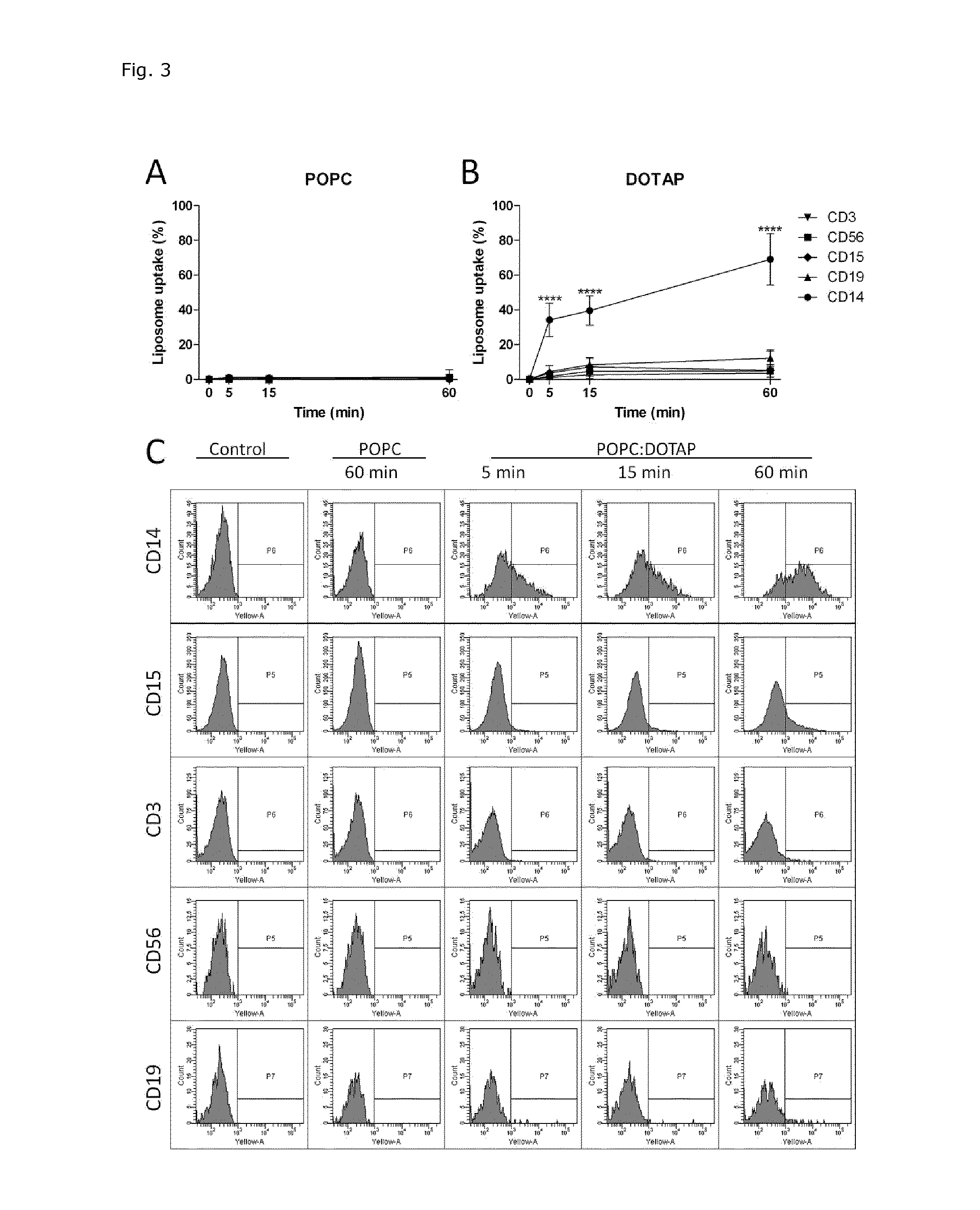
Cationic Liposomal Drug Delivery System for Specific Targeting of Human CD14+ Monocytes in Whole Blood
This invention concerns a liposome comprising lipids and at least one active ingredient, wherein at least one of the lipids is a cationic lipid; said liposome exhibiting a net positive charge at physiological conditions at which said liposome preferentially adheres to monocytes in freshly drawn blood when compared to adherence to granulocytes, T-lymphocytes, B-lymphocytes and / or NK cells in freshly drawn blood, to a lipid-based pharmaceutical composition comprising said liposomes and their use in monocytic associated prophylaxis, treatment or amelioration of a condition such as cancer, an infectious disease, an inflammatory disease, an autoimmune disease or allergy.
Owner:BIONEER +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com