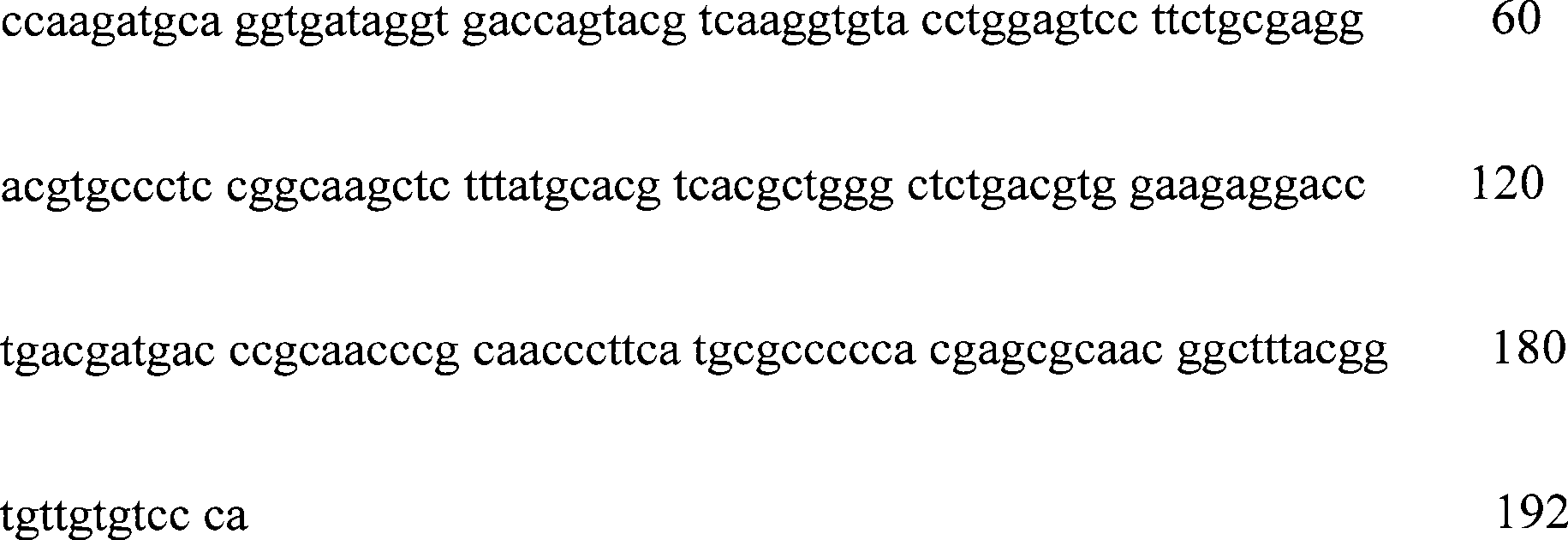Patents
Literature
154 results about "Eb virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Epstein-Barr virus (EBV) is a member of the herpesvirus family that can infect humans. EBV infections are very common — you’ve probably already contracted the virus without even knowing it. The condition that you may associate EBV infection with is infectious mononucleosis, or mono.
Anti EB virus resulted tumour polypeptide, and its use and preparing method
ActiveCN1641024ADelay drug resistanceLow chance of developing drug resistancePeptide/protein ingredientsAntibody mimetics/scaffoldsEscherichia coliNucleotide
The present invention provides one kind of gene, recombinant plasmid and polypeptide of EB virus resisting tumor polypeptide. The coding inducing polypeptide gene and coliform group gene are connected via operation to obtain polynucleotide sequence expressing recombinant antitumor polypeptide. The nucleotide sequence of the coding inducing polypeptide is inserted into coliform group gene by means of double strand oligomeric nucleotide point mutation technology to form the said recombinant plasmid. The obtained recombinant plasmid is transfected into engineering colibacillus TG1 to obtain engineering bacillus cell capable of generating recombinant antitumor polypeptide; and through mass proliferation, centrifugal precipitation and other steps, recombinant antitumor polypeptide is obtained. The antitumor polypeptide has specific targeting property, high efficiency of killing solid tumor, no attack to health cell, low toxicity and less negative reaction.
Owner:畿晋庆堂国际生物技术有限公司
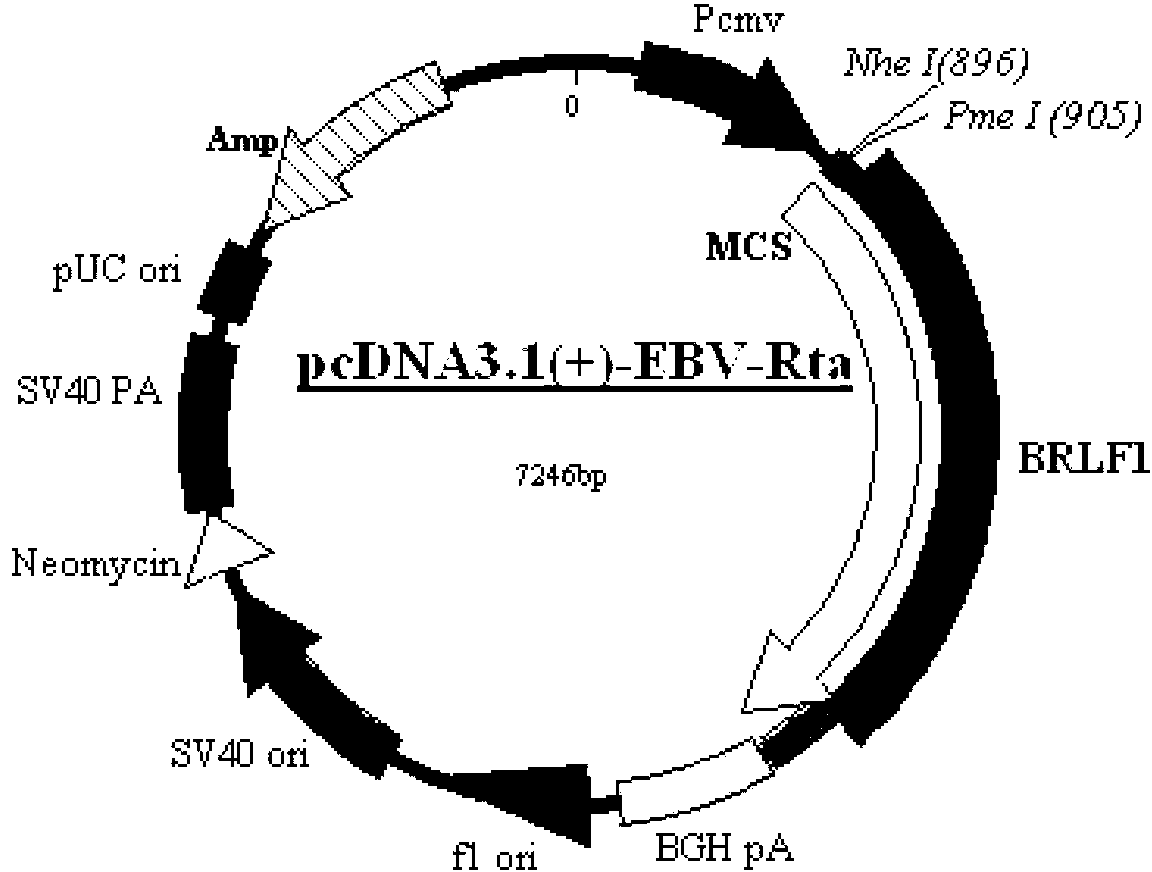
ELISA reagent kit for screening, diagnosis and treatment effect forecast of nasopharyngeal carcinoma
The present invention discloses two antigen protein fragments of protein product Rta of nasopharyngeal specific gene BRLF1, encoding gene, recombinant carrier, fusion protein (GST and GST-R185-R150), nasopharyngeal cancer diagnosis agent containing the compounds and the ELISA agent box, as well as the application of the compounds in detection of EB virus in vitro. The ELISA agent box of the present invention can be used for nasopharyngeal cancer screening, early diagnosis and forecasts of treatment results. The present invention has the advantages of simplicity, sensitivity and specificity, and is suitable for early diagnosis and forecasts of nasopharyngeal carcinoma, and the large-scale screening of high-risk groups. The present invention is easy to be widely promoted and used; the use is safe and clean; the present invention has broad market prospects.
Owner:同昕生物技术(北京)有限公司
Reagent box for enzyme linked immunosorbent assay of EB virus protease and its preparation
A method for preparing EB viral protein enzyme-linked immunosorbent diagnostic kit includes selectng EBNAl (BKRF1) prote, Zta (BZLF1) protein and VCA-p18 protin in EB viral protein for diagnosing nasopharyngeal carcinoma of serodiagnosis as target antigen for detecting antibody level in blood serum, using glutathione-transferase gene fusion system to carry on clone, presentation and purification of EB viral protein for creating diagnostic kit.
Owner:SINOCLONE LTD
Recombination plasmid related with EB virus specificity TCR gene and method for constructing anti-EBV specific CTL
ActiveCN101182531AHigh feasibilitySafe and efficient transfection methodFermentationVector-based foreign material introductionAntigenDisease
The invention discloses a recombinant plasmid relevant to the EB virus characteristic TCR gene and a method for reverting normal T cell to obtain the anti-EBV characteristic CTL. The recombinant plasmid consists of TCRV Alpha 15 gene, pIRES vector and TCR V Beta 1gene; the TCRV Alpha 15-p-IRES-TCR V V Alpha 1gene recombinant plasmid relevant to the clone of EB virus characteristic cell toxin T cell of the invention is delivered to the peripheral blood T cell to obtain a large number of high-efficiency cell toxin T cells of the antigen relevant to the characteristic EV virus; in this way, the cell of EBV<+> can be killed on purpose and the safety property is high; therefore, the invention has comparatively good prospects for curing diseases relevant to the EB virus.
Owner:JINAN UNIVERSITY
Compounds having virus resistance and composition thereof
ActiveCN103211829ATherapeuticPreventiveOrganic active ingredientsAntiviralsHuman papillomavirusViral infectious disease
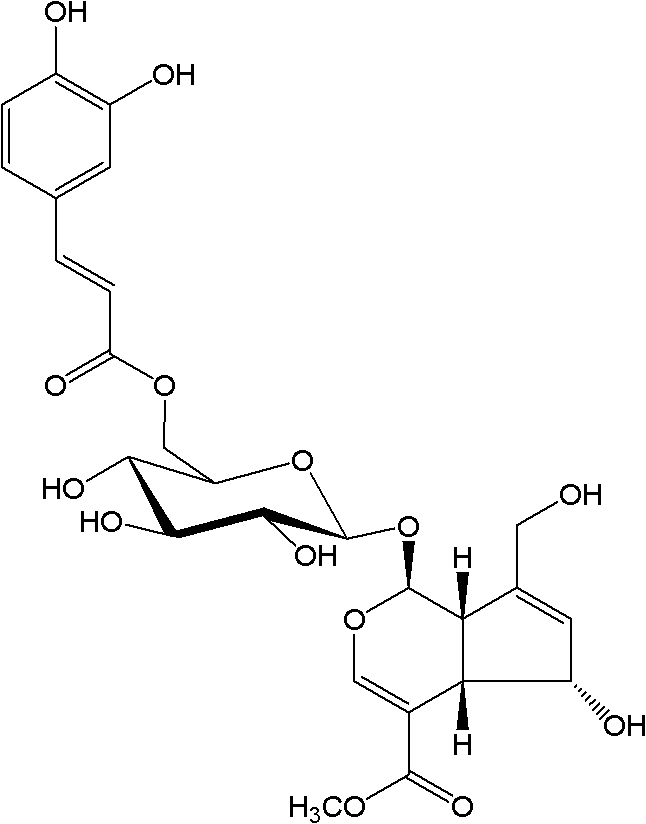
The invention discloses compounds having virus resistance and a composition thereof. The composition comprises one or more of the 13 compounds comprising 6'-O-caffeoyl deacetyl asperulosidic acid methyl ester. The compounds having virus resistance and the composition thereof can be extracted from plants and can also be prepared by synthesis. The composition comprising the compounds having virus resistance can prevent and treat viral infection-caused diseases such as influenza, myocarditis, conjunctivitis, viral pneumonia, encephalitis, leukemia, hepatitis B, hepatitis C, AIDS, genitourinary system infection, EB virus infection, human papillomavirus infection and cytomegalovirus infection. The composition can be prepared into an oral preparation, an injection, a spray, a subcutaneous injection and an anus suppository.
Owner:樊向德 +1
Fusion protein comprising Fc domain of IgG and extracellular domain of EB virus envelope glycoprotein
ActiveCN109824779AImproving immunogenicityHigh infection blocking efficiencyAntiviralsAntibody ingredientsDiseaseImmunogenicity
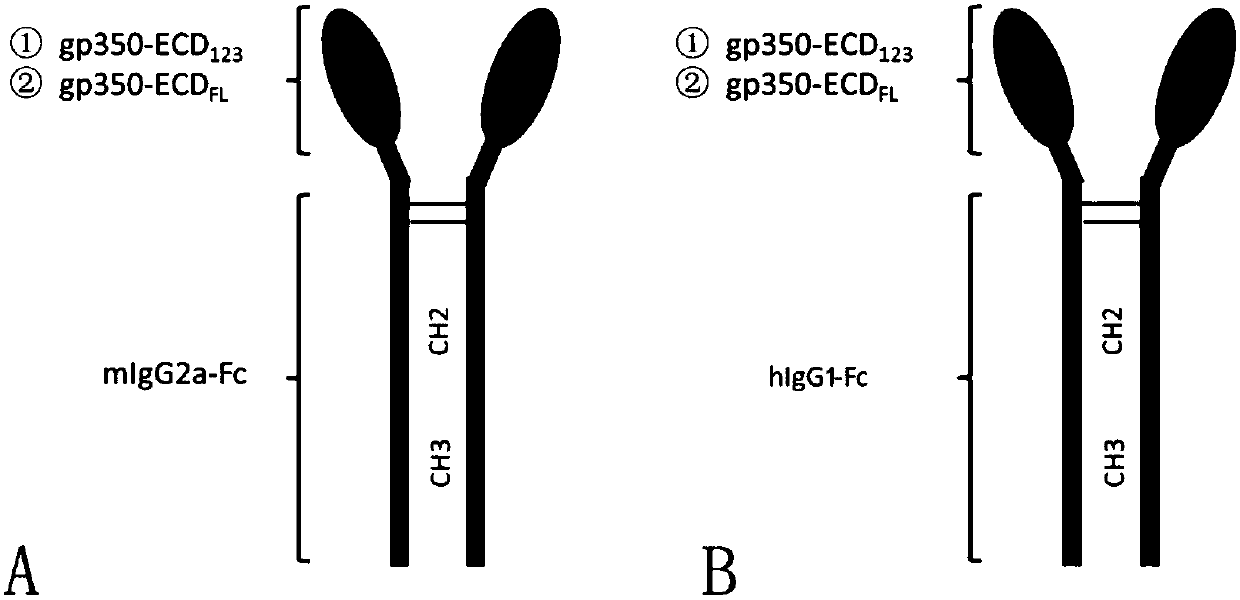
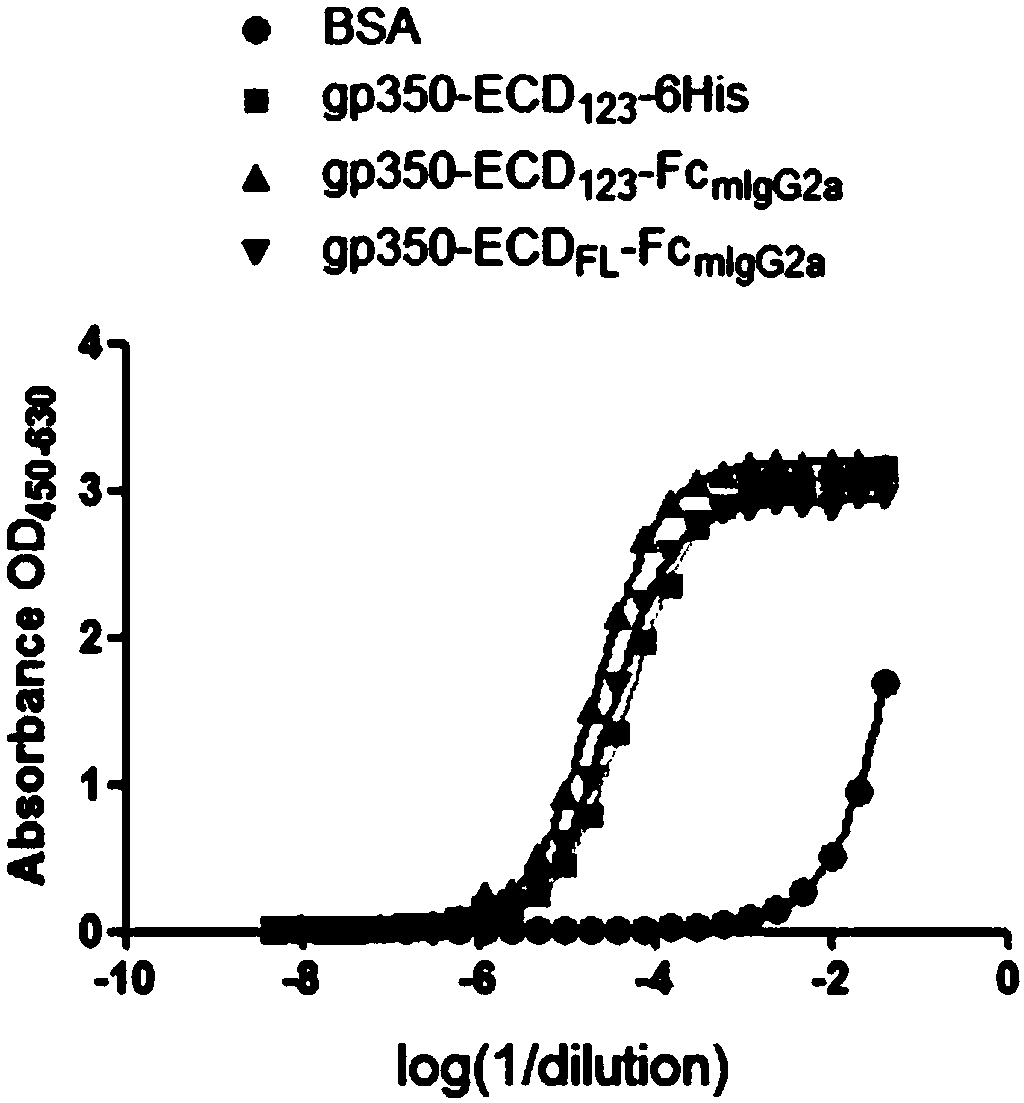
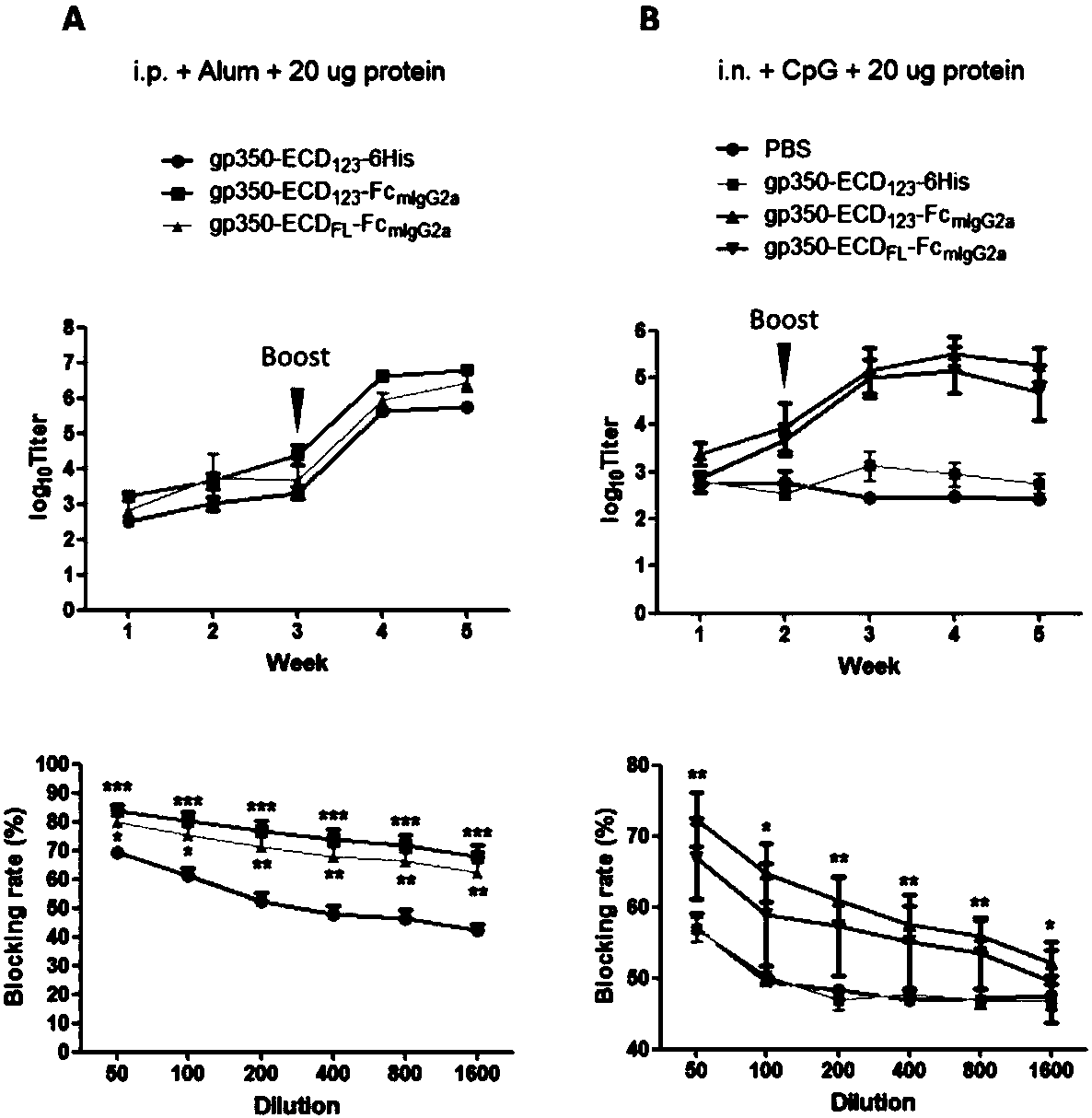
The invention discloses a fusion protein comprising an Fc domain of IgG and an extracellular domain of an EB virus envelope glycoprotein. The fusion protein is represented by the following formula: P-E-F, wherein P represents a secretion signal peptide, E represents an amino acid sequence of the extracellular domain of the EB virus envelope glycoprotein gp350, and F represents the amino acid sequence of the Fc domain of the IgG. It is found for the first time that after the fusion of the Fc domain of the immunoglobulin IgG with the envelope glycoprotein gp350 from the surface of the EB virus,the immunogenicity in vivo is significantly improved. After immunization with the fusion protein, the total serum titer an immunized animal, the serum specific neutralizing antibody titer and the serum in vitro viral infection blocking efficiency are significantly higher than that of a non-fused control protein. The fusion protein using the Fc domain of the immunoglobulin IgG and the EB virus membrane glycoprotein is adopted as an evidence for the efficacy of an EB virus vaccine and has important practical and theoretical significance and application prospects for prevention and treatment of EB virus-related diseases.
Owner:SUN YAT SEN UNIV +1
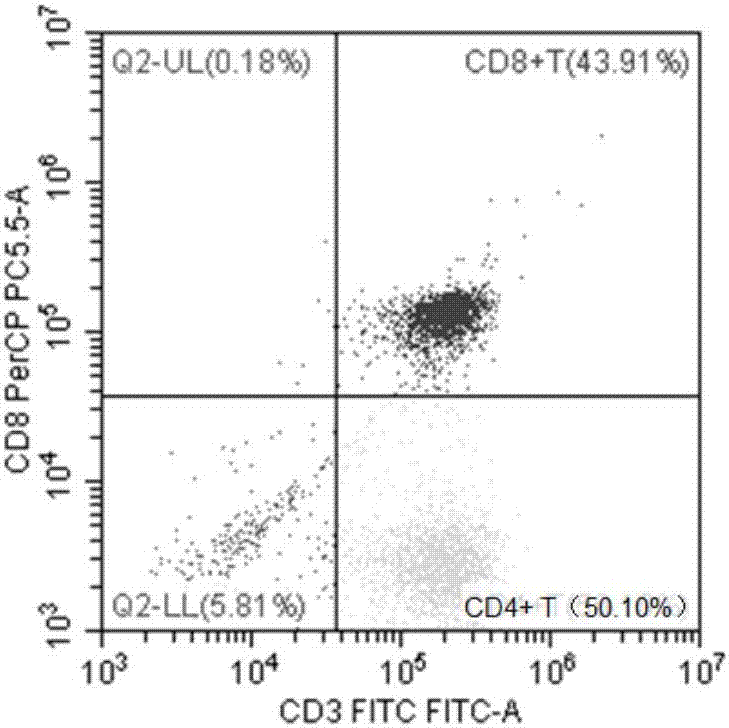
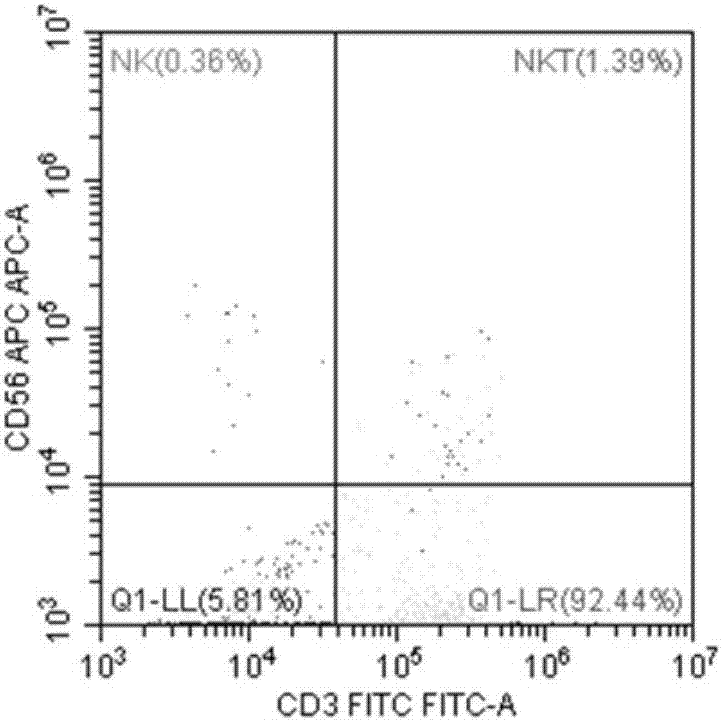
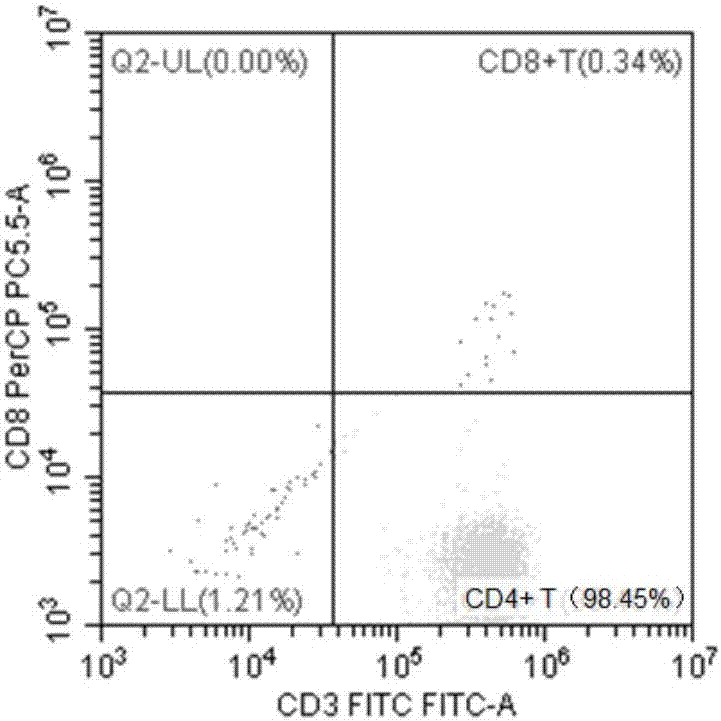
Identification method of EB virus infected lymphocyte subpopulation and application thereof
ActiveCN106947835APrecise positioningEffective treatmentMicrobiological testing/measurementMicroorganism based processesEbv infectionMonocyte
The invention provides an identification method of an EB virus infected lymphocyte subpopulation and an application thereof and relates to the technical field of medical detection. The identification method of the EB virus infected lymphocyte subpopulation provided by the invention can accurately position the cell type of EBV infection by sorting monocytes of peripheral blood of an EBV infected patient in combination with a flow cytometry and a real-time fluorescent quantitative PCR technology, and is helpful for a clinician to formulate a therapeutic regimen for the cell type with EBV infection so as to efficiently and accurately treat diseases caused by EBV infection. In addition, by applying the identification method provided by the invention, target cells infected with EBV can be determined, preparation of related therapeutic drugs can be guided, and the targeted drugs are prepared with a clear target.
Owner:BEIJING FRIENDSHIP HOSPITAL CAPITAL MEDICAL UNIV
EB virus VCA-IgA antibody detection reagent and preparation method thereof
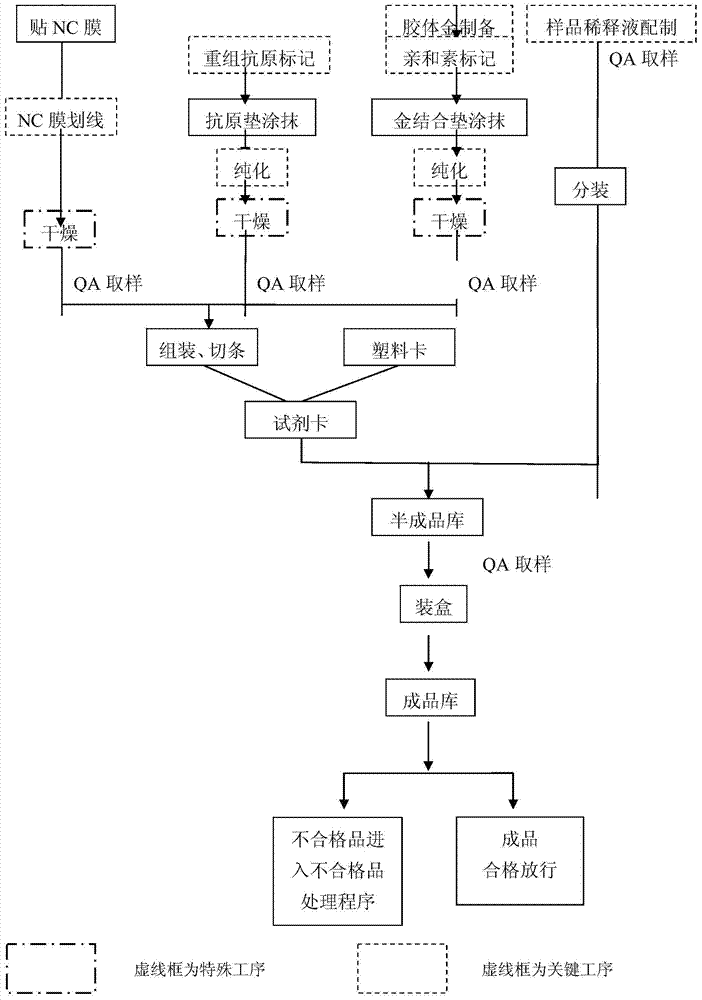
The invention provides an EB virus VCA-IgA antibody detection reagent which at least comprises a reactive film, an antigen pad and a gold-labeled pad, wherein a detection line and a quality control line are marked on the reactive film; the detection line contains a mouse anti-human IgA monoclonal antibody; the quality control line contains biotin; the antigen pad contains a biotin-labeled recombinant EB virus VCA antigen; and the gold-labeled pad contains an avidin complex labeled by colloidal gold. The detection kit disclosed by the invention has the characteristics of rapidness, simplicity, accuracy and high accuracy.
Owner:中山生物工程有限公司
Method for detecting infectious disease pathogens and kit
ActiveCN101545014AThe detection process is fastImprove efficiencyMicrobiological testing/measurementMicroorganism based processesCytomegalic inclusion diseaseBiology
The invention discloses a method for detecting infectious disease pathogens possibly existing in a biological sample. The infectious disease pathogens comprise EB virus, herpes simplex virus, cytomegalic inclusion disease virus and adenovirus. The method comprises the following steps: expanding nucleic acid fragments of the biological sample, and detecting the nucleic acid fragments by using a probe. The invention also provides primers used for expanding and the probe used for detection. The invention also provides a kit comprising the primers. The method has the advantages of high sensitivity, strong specificity, simple operation and wide sample range, can simultaneously detect various infectious disease pathogens, and is suitable for early diagnosis of respiratory infectious diseases.
Owner:海康生物科技(北京)有限公司
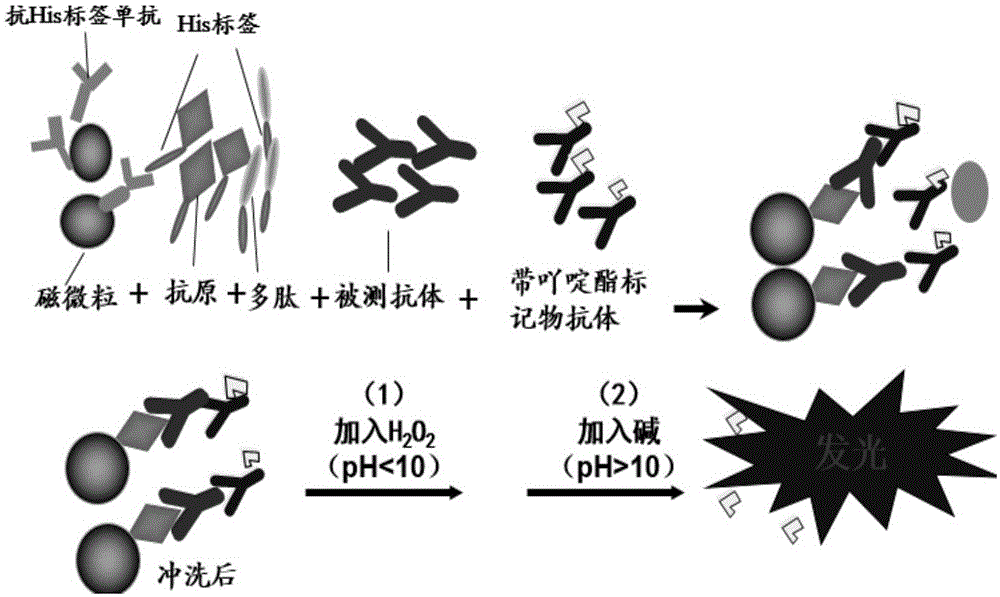
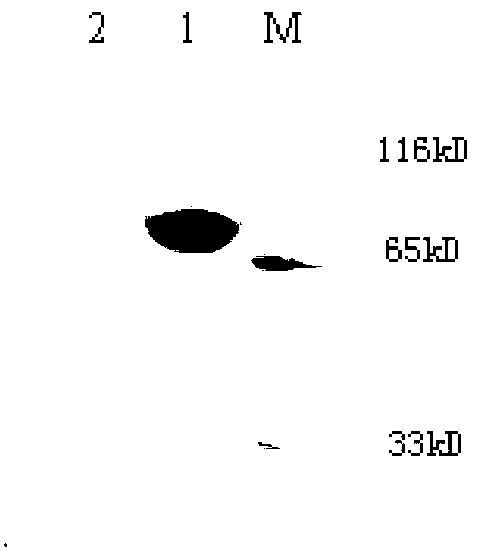
Chemiluminescence kit for detecting EB virus Rta/IgG antibody and use thereof
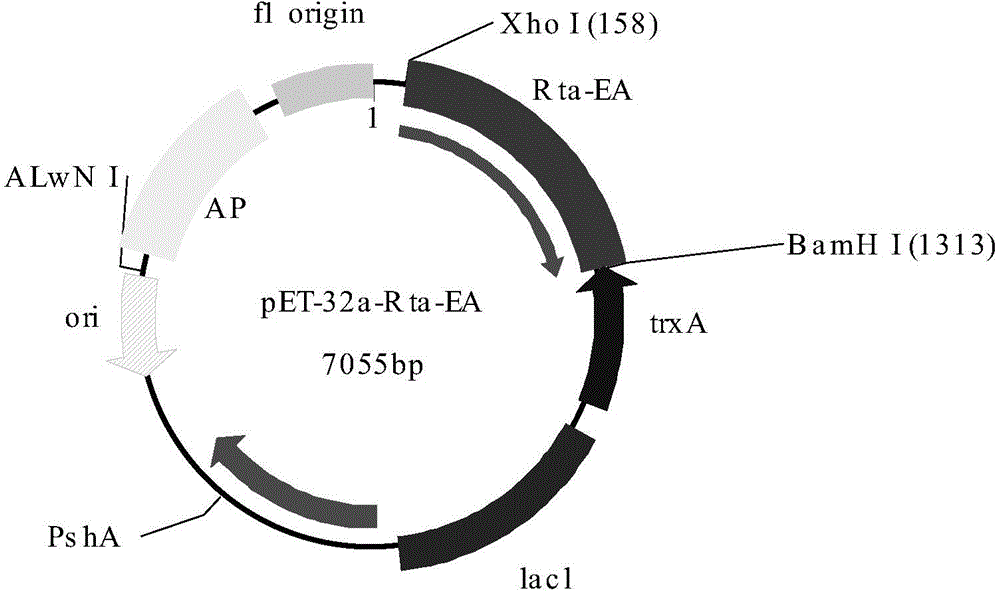
The invention provides a chemiluminescence kit for detecting EB virus Rta / IgG antibody and a use thereof and belongs to the fields of biotechnology, medical immunology and external serology diagnosis. The chemiluminescence kit comprises magnetic particles coated with an anti-His label monoclonal antibody, a Rta protein with a His label, a specific synthetic polypeptide with a His label and an acridinium ester-labeled anti-human IgG antibody. The specific synthetic polypeptide has an amino acid sequence shown in the formula of SEQ ID NO: 1. The invention also provides a method for detecting an EB virus Rta / IgG antibody by the chemiluminescence kit. The chemiluminescence kit and the detection method can realize fast, accurate, efficient, sensitive and specific detection of an EB virus Rta protein antibody in a sample to be detected, realize multiple sample treatment, shorten detection time and improve detection efficiency.
Owner:同昕生物技术(北京)有限公司
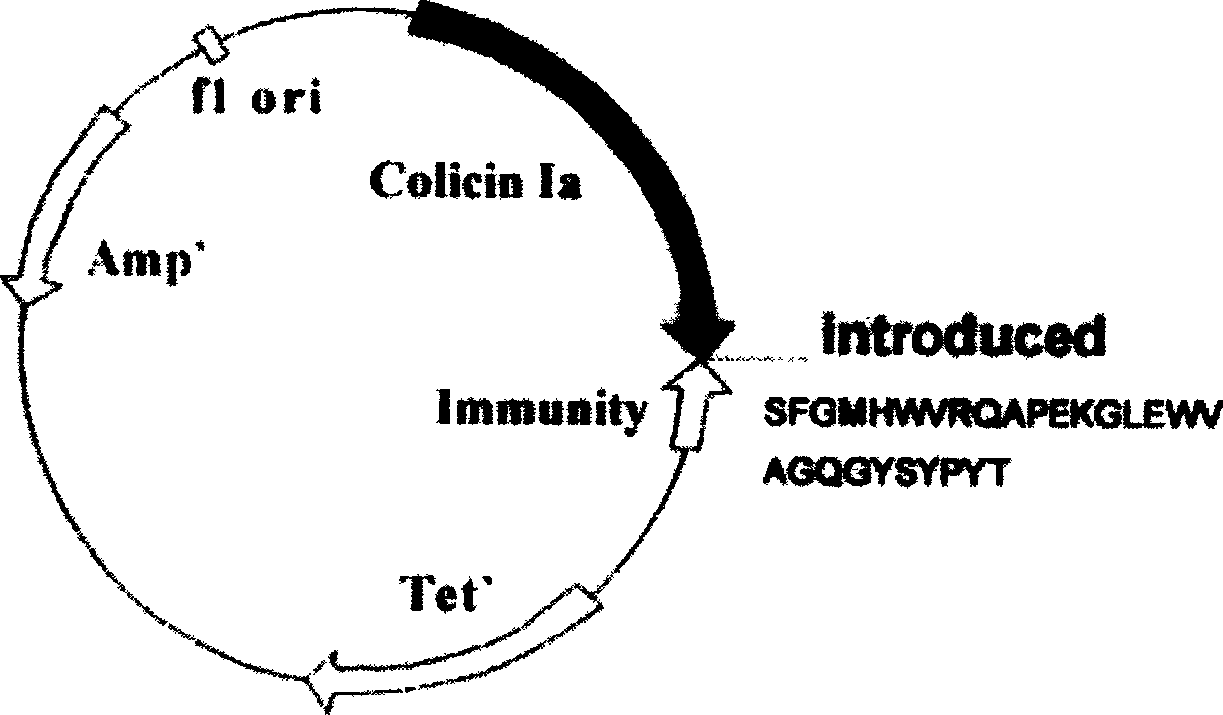
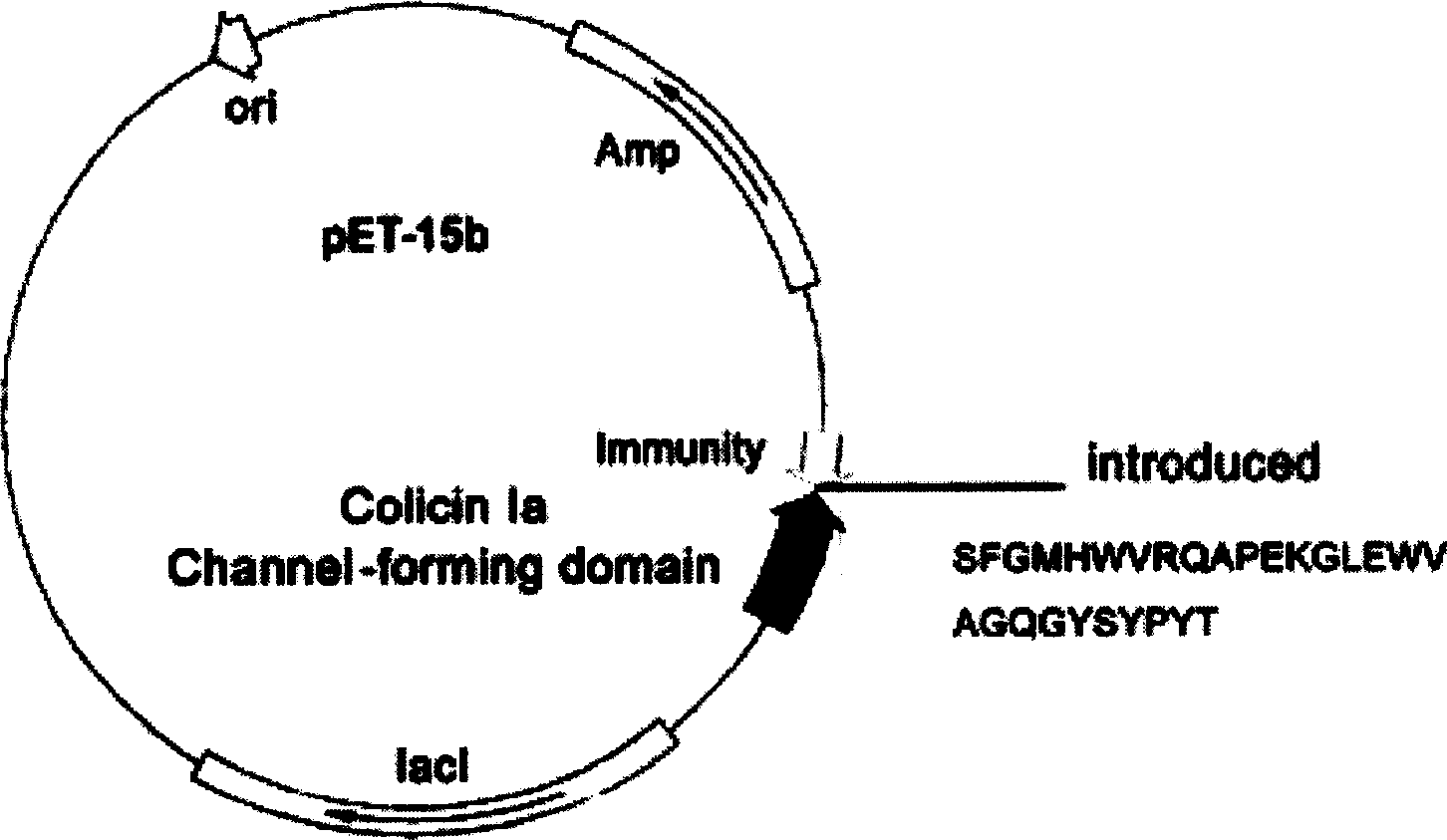
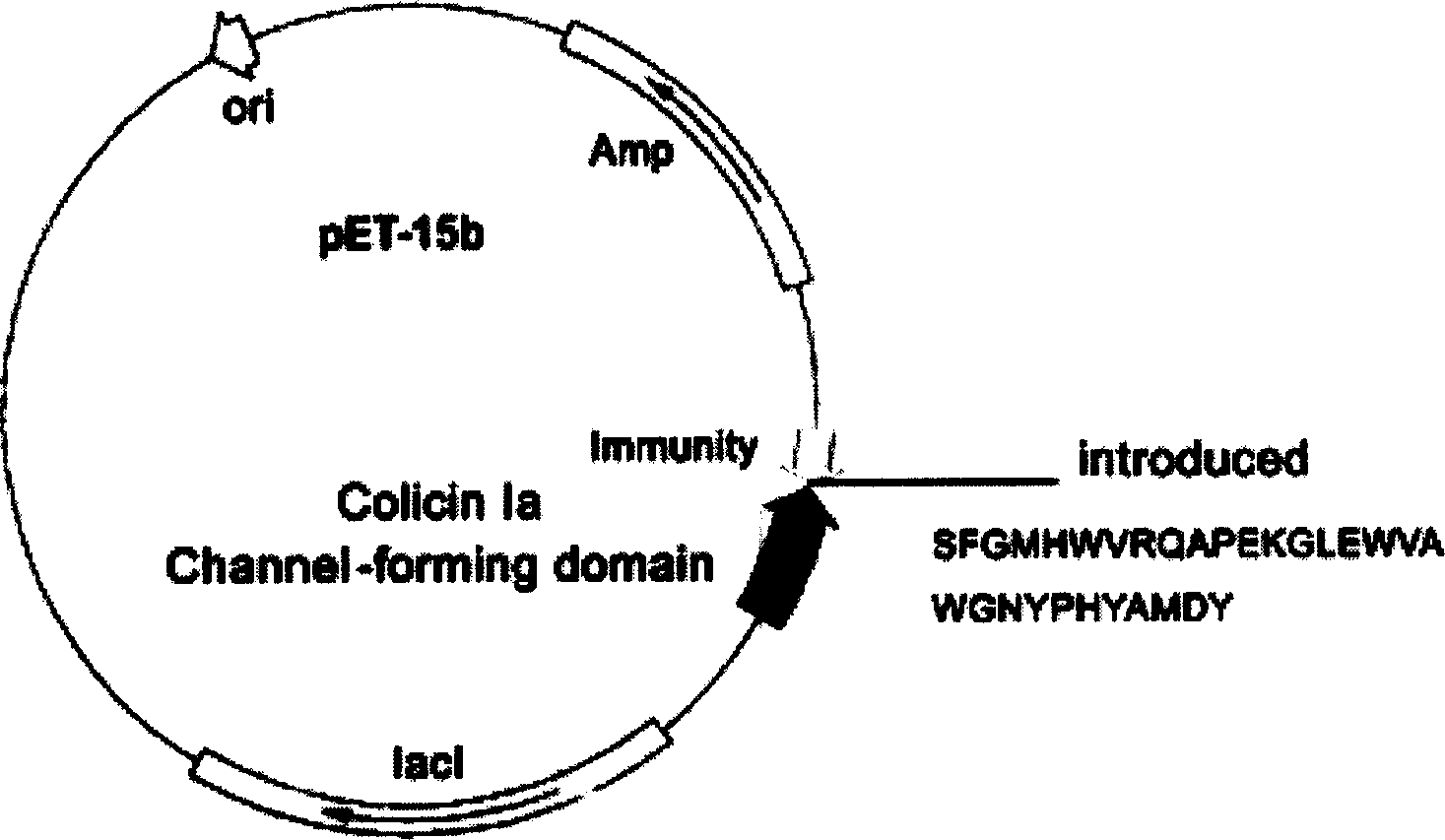
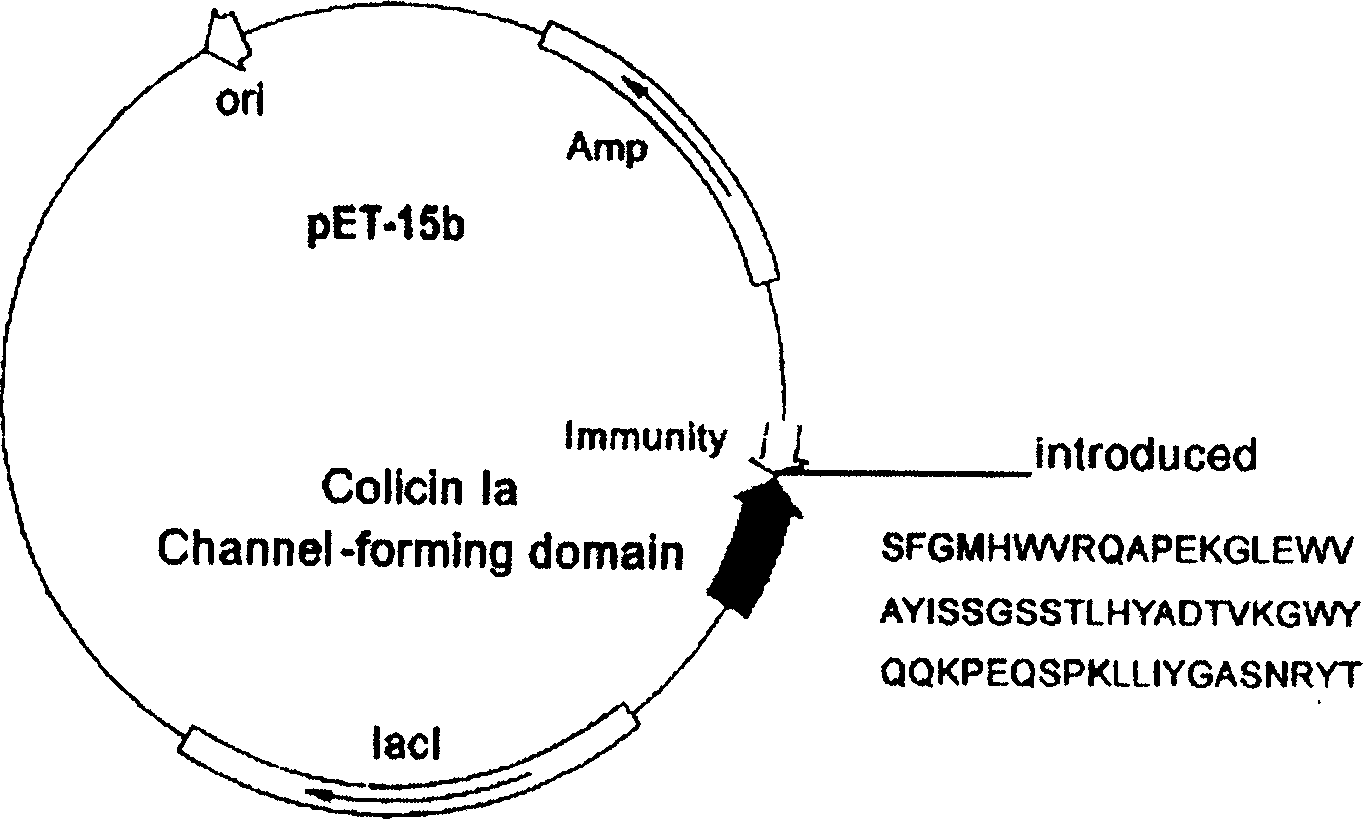
Novel polypeptide for resisting tumors caused by EB (Epstein-Barr) viruses, and application and preparation method thereof
InactiveCN102101888ALow immunogenicityImprove securityAntibody mimetics/scaffoldsVirus peptidesAntiendomysial antibodiesAntibody mimetic
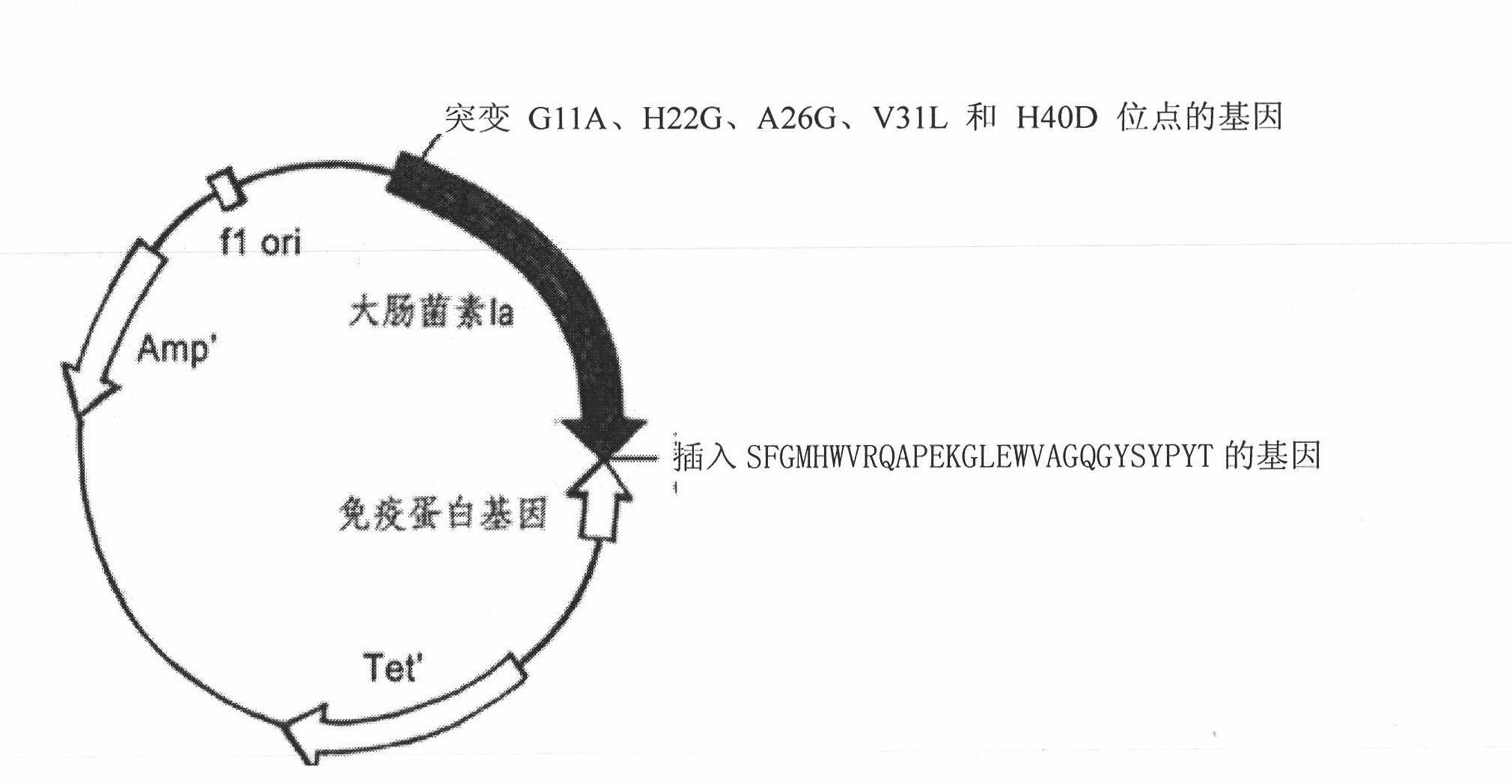
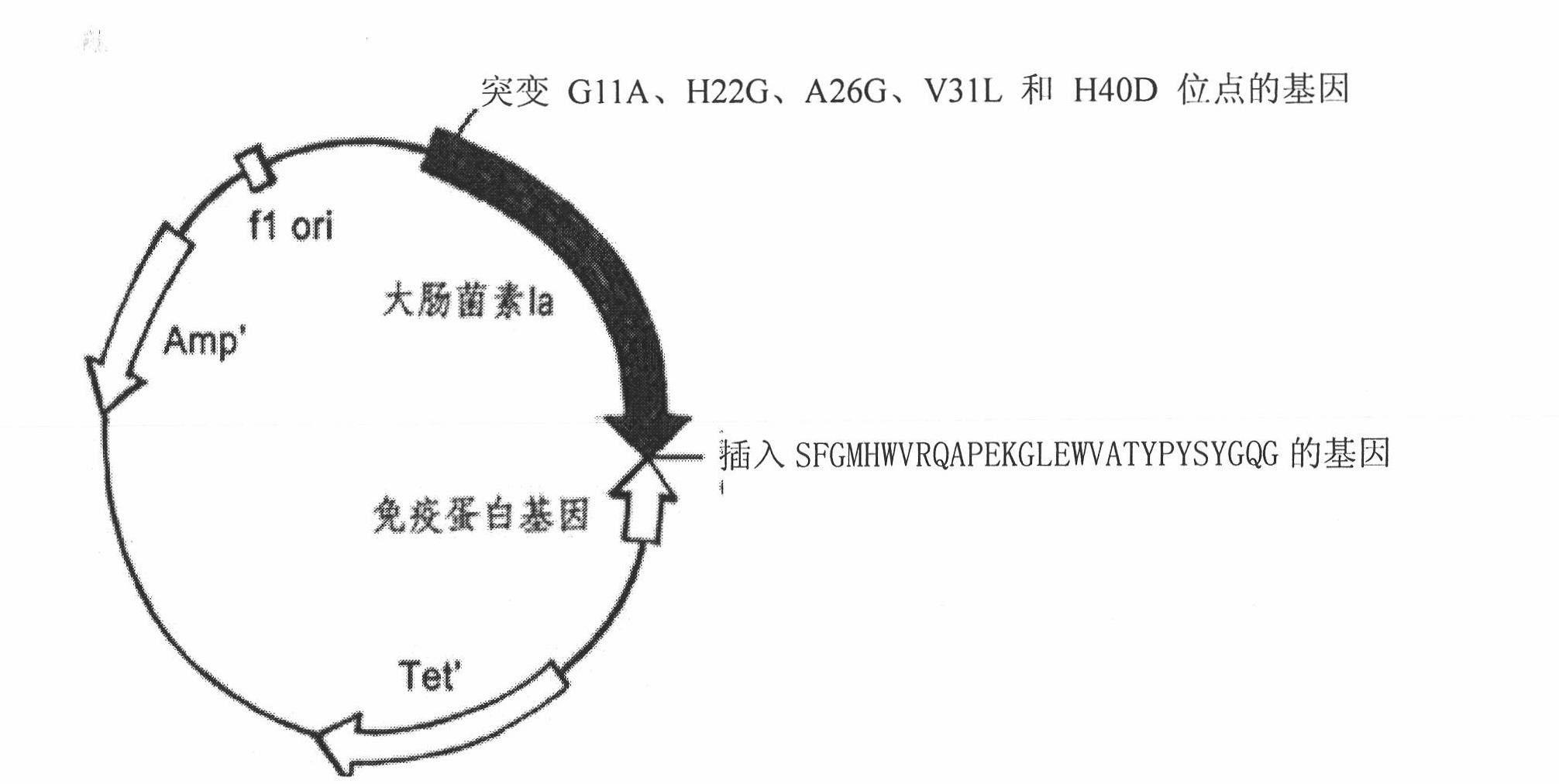
The invention relates to a novel polypeptide for resisting tumors caused by EB (Epstein-Barr) viruses, and application and a preparation method thereof, belonging to the fields of antineoplastic medicaments. The novel polypeptide is composed of a colicine allosteric polypeptide capable of forming ion channels, and an antibody polypeptide for resisting EB viruses or a mimic antibody polypeptide ofan anti-EB virus antibody, wherein the colicine allosteric polypeptide capable of forming ion channels is formed by mutating amino acid residues G11A, H22G, A26G, V31L and H40D with wild type colicine E1, Ia, Ib, A, B and N or a peptide chain of aqueous pore canal domain thereof; and the amino acid sequence of the anti-EB virus antibody is identical to that of a monoclonal antibody secreted by ATCC HB-168 hybridoma. The invention provides an improved medicament, which has the advantages of strong lethality, high safety, high specificity and low sensitization possibility, for treating tumors caused by EB viruses, and a preparation method thereof.
Owner:AMERICAN PHEROMYCIN BIOTECHNOLOGY CO
Artificial antigen and kit for joint detection of Rta protein antibody of epstein-barr (EB) virus and early antigen ethyl acrylate (EA) antibody of EB virus
ActiveCN104086657ASimple stepsImprove understandingMicroorganism based processesBiological testingTrue positive rateNasopharyngeal carcinoma
The invention relates to the technical fields of biotechnology, medical immunology and in vitro serological diagnosis, and particularly relates to an artificial antigen and a kit for joint detection of an Rta protein antibody of an epstein-barr (EB) virus and an early antigen ethyl acrylate (EA) antibody of the EB virus. The Rta protein antibody of the EB virus and the early antigen EA antibody existing in a to-be-detected sample can be accurately, efficiently, sensitively and specifically detected by the artificial antigen provided by the invention. By adopting the kit prepared from the artificial antigen, compared with the traditional detection method, the sensitivity and the specificity of nasopharynx cancer diagnosis are improved, the detection time is shortened, a patient can be timely treated, and the pain is relieved.
Owner:同昕生物技术(北京)有限公司
EB virus gene quick diagnosis kit based on loop-mediated equal-temperature amplification technology
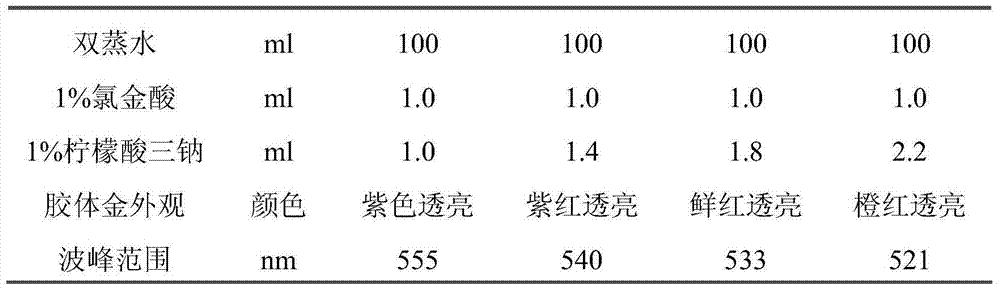
The invention relates to a kit checking EB virus with mediated isothermal amplification technology, and belongs to biological checking agent field. The kit comprises a set of primer, DNA polyase, reacting liquid, cracking liquid (1) and (2), coloured solution and positive contrast liquid; said a set of promer comprises two pair of primers, and the squence is: outer primer1: GCATGTTTGAGTCCCGCTG, outer primer 2: CCATTGCTGTGGAAGTGGC;inner primer 1: CACCCAACAAGGCAGGACGCTTTTGCTGAACATTAGCATCCCGG; inner primer2: GTGCCTGGGCCTGCAATTTACTTTTGCGATCTGGTGGGCATTC, or outer primer 1: TCAACAGATAATCCACCCGC, outer primer 2: TAGTAAATTGCAGGCCCAGG; inner primer 1: TCAAACATGCCAGAGGCGTTGGTTTTTGGCGTGGAAGTTAATGTCC; inner primer 2: GCGACCACCAGCTCTGTCATTTTTGAGCCTTGCACCCAACAAG. The kit needs no special agent and device, can fast detect EB virus in the sample, and is characterized by high speciality, high sensitivity, simple determination method and high correcting rate.
Owner:THE SECOND PEOPLES HOSPITAL OF SHENZHEN
Kit for genotyping VZV, production method of kit and application of kit
InactiveCN105132584ANo cross reactionStrong specificityMicrobiological testing/measurementMicroorganism based processesHuman DNA sequencingChemical structure
The invention provides a kit for genotyping VZV (Varicella-Zoster Viruses). The kit is characterized by comprising a nucleotide sequence shown as the Table 3 in the specification, and specific primers and specific probes corresponding to clade1-5 type VZV of chemical structures. The kit has the function of detecting various kinds of VZV DNA (Deoxyribonucleic Acid); the detection sensitivity is 10<2> copies / reaction; no cross reaction with human genome, herpes simplex viruses type I / type II, cytomegaloviruses and EB viruses exists; and the kit is applicable to the virus gene diagnosis of clinical VZV infected persons, and can also be used for the epidemiology survey of different Clade types of VZV.
Owner:CHENGDU MILITARY GENERAL HOSPITAL OF PLA
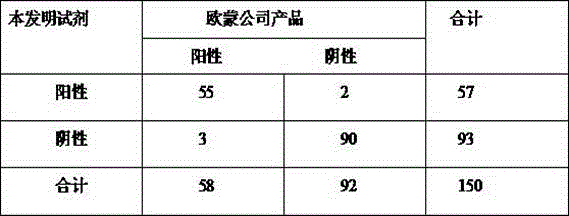
Application of latent membrane protein 2A of recombined EB virus for testing nasopharyngeal carcinoma
InactiveCN1687779AHigh clinical diagnostic valueColor/spectral properties measurementsBiological testingSerum igeAntigen
This invention relates to how to diagnose NPC. It shows the use of LMP2A-the membrane protein infected by recombined EBV in NPC diagnoses. It uses LMP2A as antigen, and detect serum sample by ELISA method. If the result is greater than the critical value, it is light demanding. This invention can be used to the early diagnoses of NPC, it is quick and convenient.
Owner:NANJING MEDICAL UNIV
CBA reagent kit for detecting EB virus gp78 antibody and method for making same
The invention discloses a CBA kit used for detecting anti-EB virus gp78 antibody, which includes coupling superbead, the superbead is made by coupling blank superbead and NeutrAvidin in a coupling way, the NeutrAvidin binds with the bioepiderm labeled EB virus gp78 antigen which is coupled with Biotin. The gp78 antibody CBA kit is suitable for crown screening, evaluation of the high incidence of nasopharyngeal carcinoma, diagnose and prognosis effect appraisal, observation, furthermore, the kit can be applied directly in nasopharyngeal carcinoma early diagnosis, judge prognosis of patients, etc. with the characteristics of rapid, objectivity and accurate.
Owner:SUN YAT SEN UNIV CANCER CENT
Hypersensitivity Epstein-Barr (EB) virus fluorescence quantitative polymerase chain reaction (PCR) kit for locked nucleotide acid (LNA) and detection method and application thereof
InactiveCN101899528AShort detection timeEasy to operateMicrobiological testing/measurementMicroorganism based processesForward primerPositive control
The invention provides a hypersensitivity Epstein-Barr (EB) virus fluorescence quantitative polymerase chain reaction (PCR) kit for locked nucleotide acid (LNA). The kit comprises LNA-TaqMan reaction liquid, a positive control sample, a forward primer, a reverse primer, and an LNA-TanMan fluorescent probe, wherein the forward primer is 5'-AATTTTTTCTGCTAAGCCCAACA-3'; the reverse primer is 5'-ACGGGTGGGTGTGTGTAGTGT-3'; and the LNA-TanMan fluorescent probe is 5'-FAM-CCACCACACCCAGGC-MGB3'. The invention also provides a hypersensitivity EB virus fluorescence quantitative PCR detection method for the LNA and the application of the kit in the detection of EB viruses, the diagnosis and treatment of nasopharyngeal darcinoma, prognosis judgment and the monitoring of recurrence and transfer after the treatment of the nasopharyngeal darcinoma. The kit of the invention has the advantages of rapidness, high sensitivity, simple operation process, large detection sample size and safety.
Owner:广州达健生物科技有限公司
Recombinant protein vaccine, recombinant expression vector containing genes for coding recombinant protein vaccine and application of recombinant protein vaccine
ActiveCN104707135AInhibition of activationActivation blockBacteriaMicroorganism based processesDiseaseTreatment effect
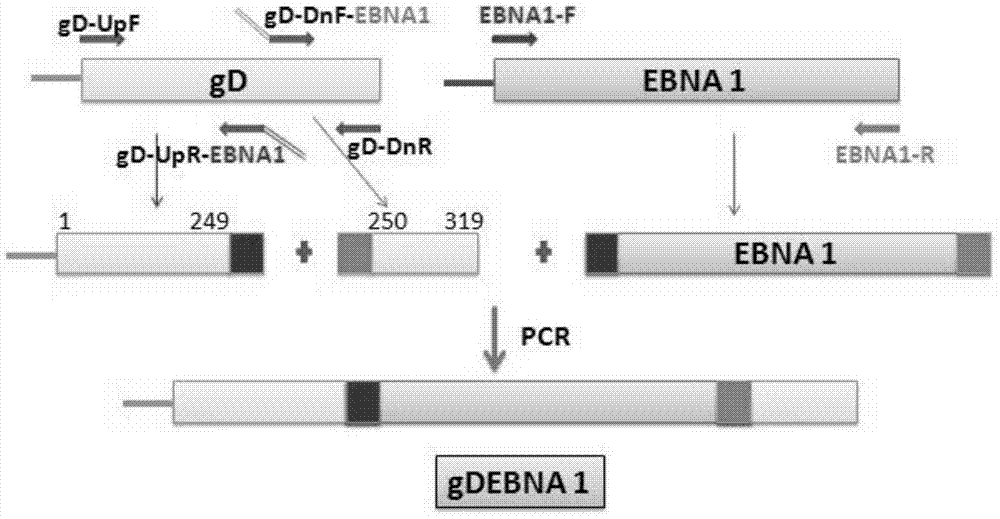
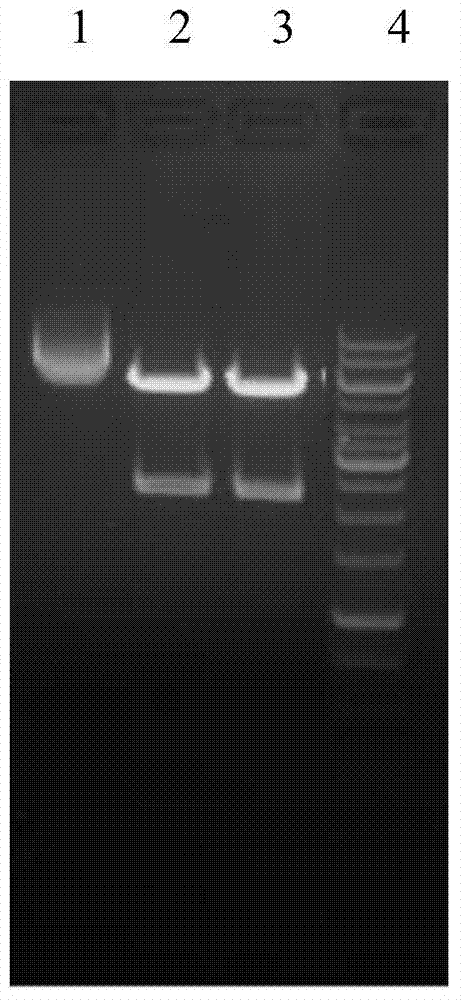
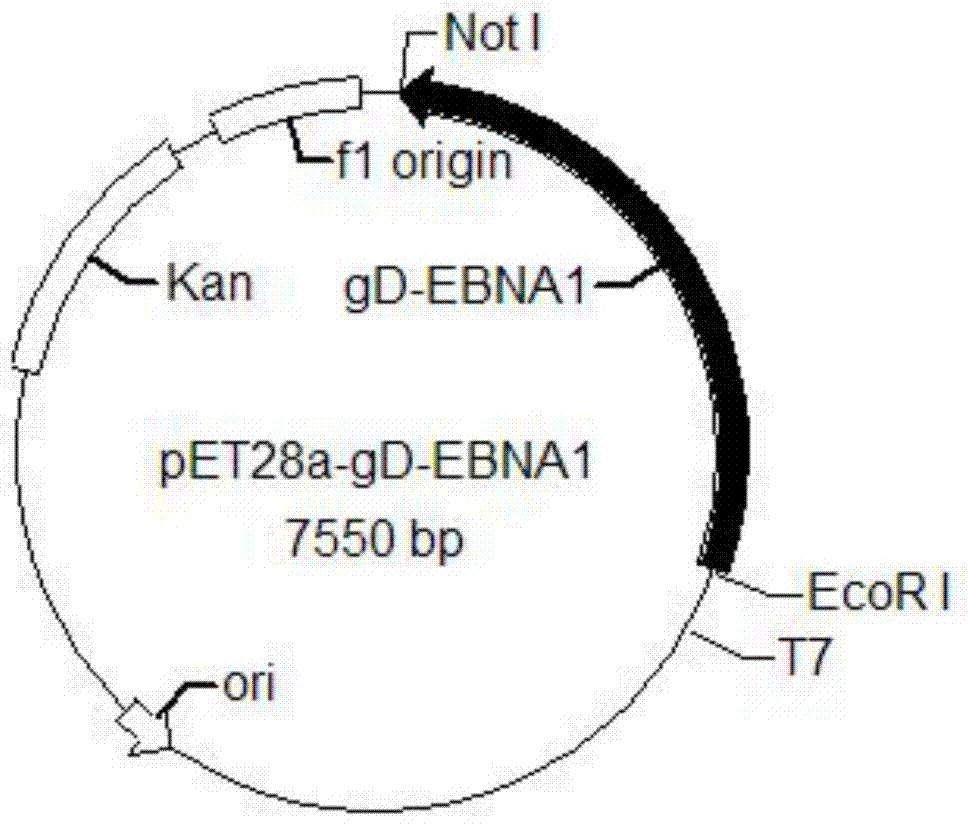
The invention provides a recombinant protein vaccine. The recombinant protein vaccine contains an epitope of EB virus antigen 1 (EBNA1) and an epitope of human herpes virus glycoprotein; fusion proteins can effectively activate a specific cell toxic T lymphocyte (CTL) reaction, directly restrain the expression EBNA1, and give play to treatment effects on EB virus-associated tumors; an immunosuppression path can be directly blocked through herpes virus glycoprotein, and the activity of regulatory T cells is improved. In this way, by means of the recombinant protein vaccine, EB virus infections and diseases related to the EB virus infections and tumors related to the EB virus infections can be prevented or treated in a multi-way manner more comprehensively. The invention further provides a recombinant expression vector containing genes for coding the recombinant protein vaccine and application of the recombinant protein vaccine.
Owner:SHENZHEN INST OF ADVANCED TECH
Preparation method of EB (Epstein-Barr) virus antigen and quick detection kit for detecting EB virus antibody prepared from antigen
InactiveCN106188248AHigh antigen yieldImproving immunogenicityVirus peptidesDsDNA virusesEscherichia coliInclusion bodies
The invention relates to an EB virus gene engineering artificial expression antigen and a method for preparing the antigen. The method comprises the following steps: artificially synthesizing a fused EB virus capsid proteantigen gene sequence, establishing a prokaryotic expression vector, expressing the EB virus capsid proteantigen in Escherichia coli, and renaturating the inclusion body by a dialyssis process, a gradient dilution process and gelchromatography to obtain the recombinant EB virus capsid proteantigen with the three-dimensional structure and immunocompetence. The invention also relates to a quick detection method for detecting an EB virus antibody. The method comprises the following step: using the EB virus capsid proteantigen. The invention also relates to a quick detection kit for EB virus antibody detection. The kit comprises the EB virus capsid proteantigen which can be directly used for whole blood detection. The kit comprises a rheumatism factor treatment pad which can be used for removing rheumatism factors in a sample and directly detecting IgM in the sample. The invention provides an EB virus antigen which has high specificity. The invention also provides a method for preparing the antigen, a method for quickly detecting the EB virus antibody and a kit for quickly detecting the EB virus antibody.
Owner:LANZHOU YAHUA BIOTECH
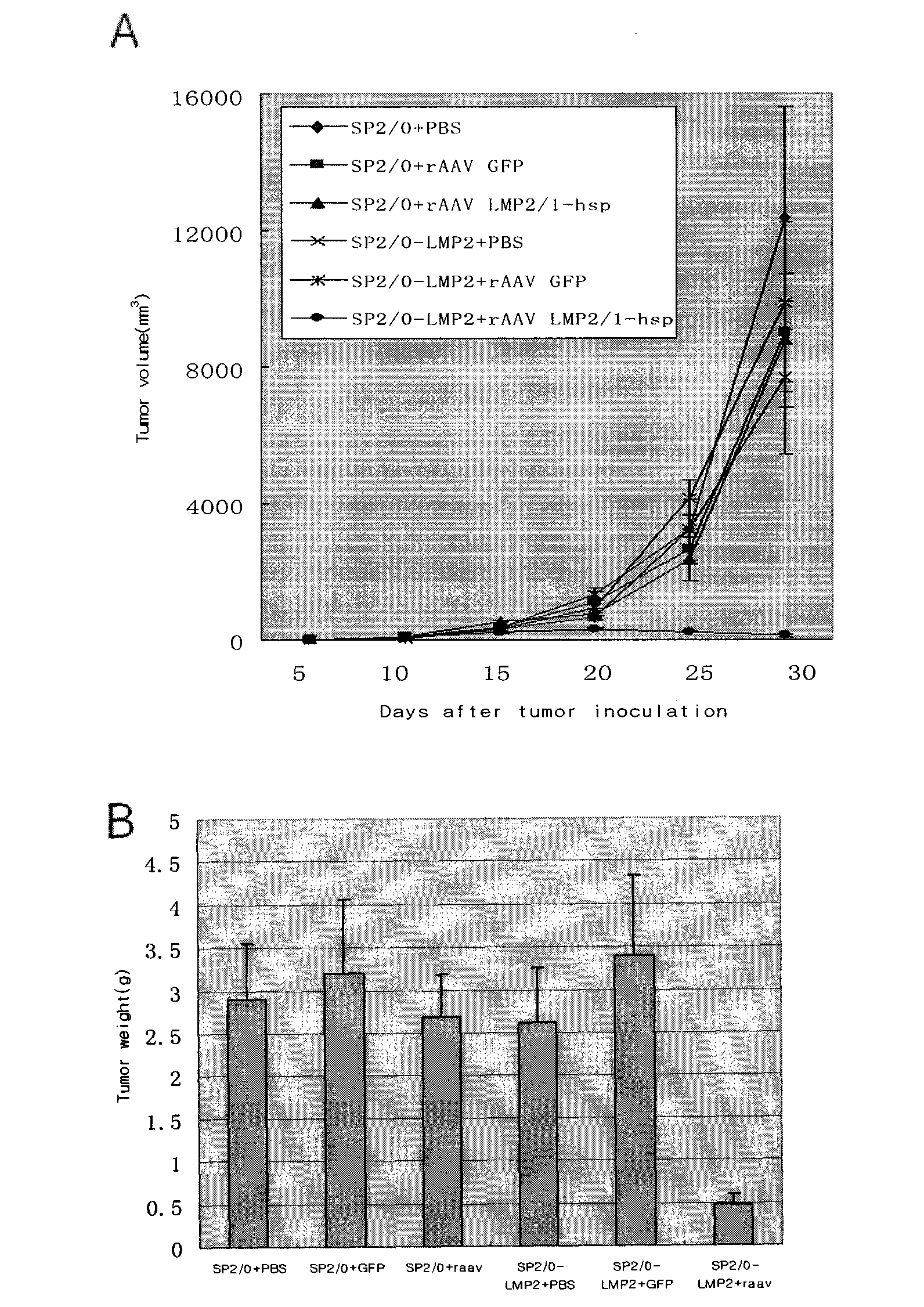
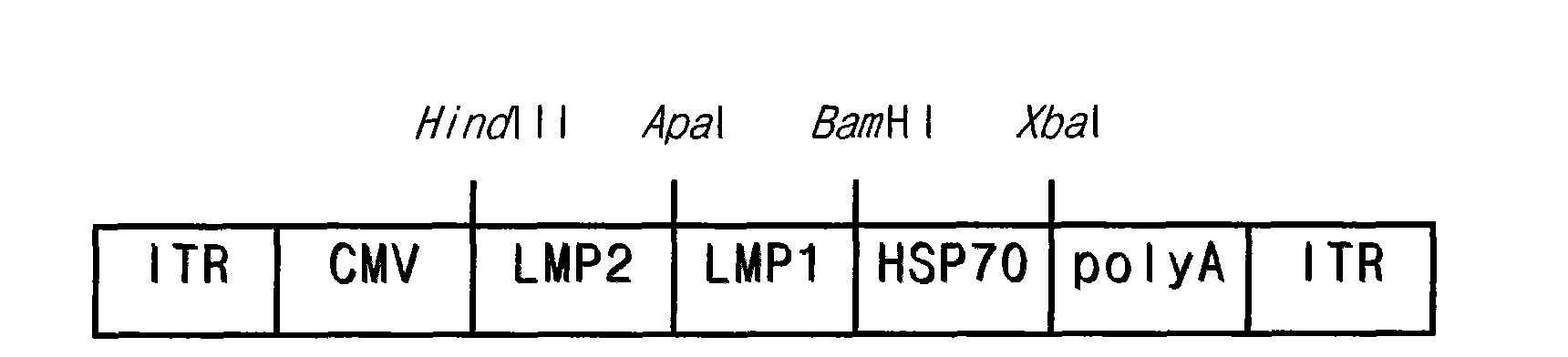
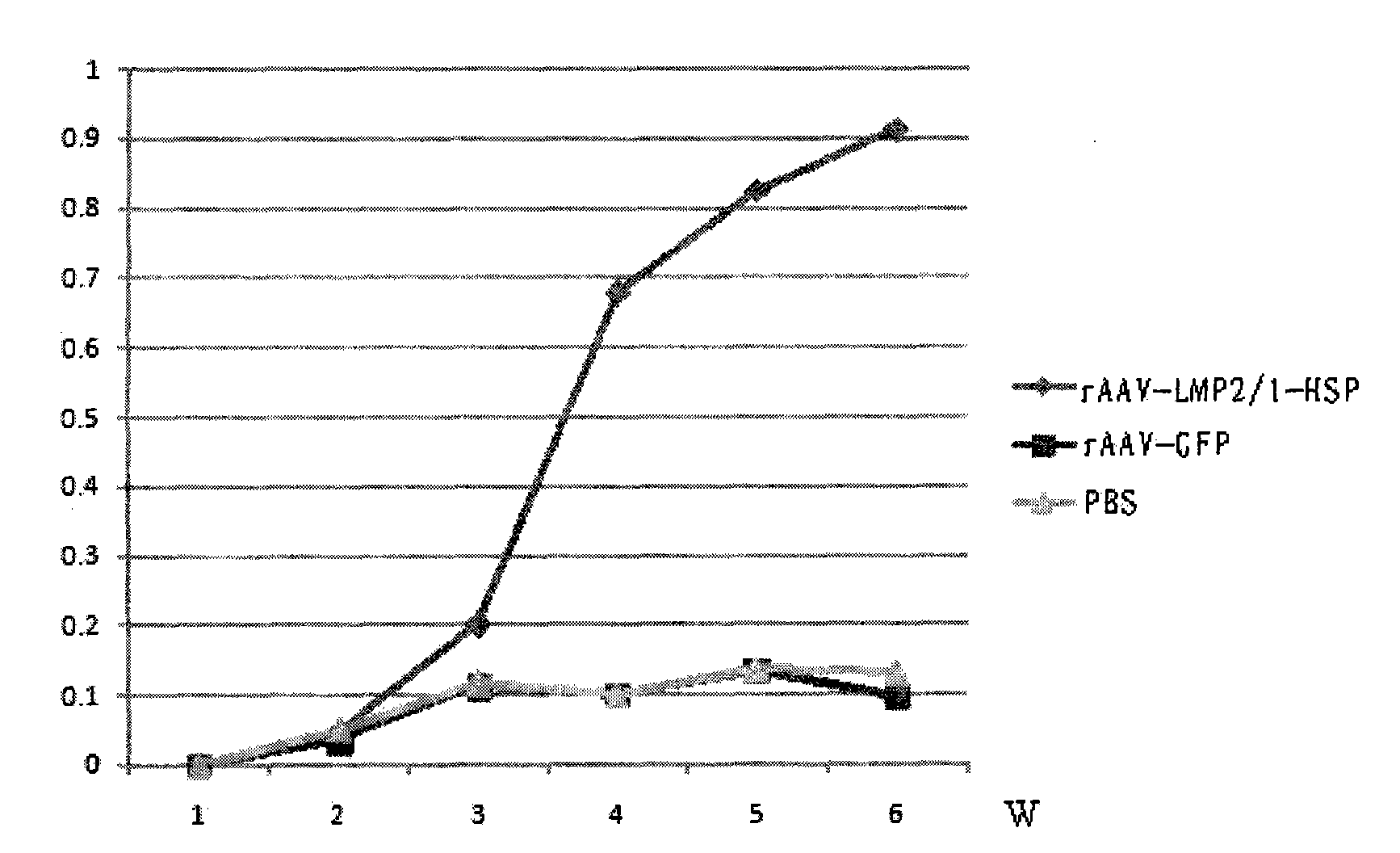
Preparation method of recombinant adeno-associated viruses containing EB virus latent membrane protein 1 and 2 genes and application thereof
ActiveCN101775375AEfficient transductionLow toxicityGenetic material ingredientsMicroorganism based processesLatent Membrane Protein-1Eukaryotic plasmids
The invention provides a preparation method of recombinant adeno-associated viruses containing EB virus latent membrane protein 1 and 2 genes and application thereof. The recombinant adeno-associated viruses of the invention carry DNA sequences of encoding EB virus latent membrane protein 1 and 2 as shown in CCTCC NO: V200907, wherein the virus titer of the recombinant adeno-associated viruses is not smaller than 1*10<11> / ml viral particles. The recombinant adeno-associated viruses can induce the EB virus specific cell cytotoxic lymphocyte reaction in vivo. The preparation method of the invention comprises the following steps: 1) cloning DNA sequences for encoding the proteins LMP1, LMP2 and HSP70 to proper adeno-associated virus carrier plasmids to obtain recombinant adeno-associated virus carrier plasmids containing the DNA encoding sequences of LMP1, LMP2 and HSP70; and 2) using the recombinant adeno-associated virus carrier plasmids in the first step and auxiliary plasmids for cotransfecting proper incasing cells to obtain required recombinant adeno-associated viruses.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH
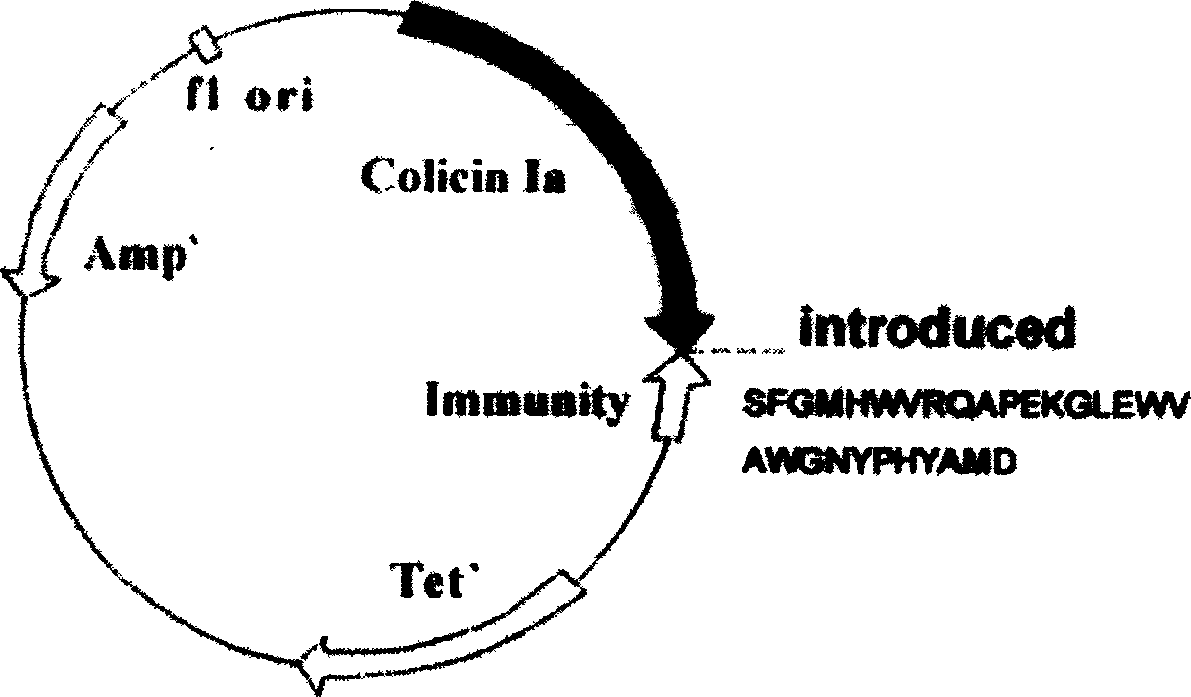
Miniaturized polypeptide of anti EB Virus tumour, application and preparation method
InactiveCN1661012ADelay drug resistanceLow chance of developing drug resistanceAntibody mimetics/scaffoldsImmunoglobulins against virusesEukaryotic plasmidsWilms' tumor
A miniature polypeptide resisting to EB virus tumor, its gene and its recombinant plasmid are disclosed. Its preparing process includes linking the gene for coding and leading peptide with the plasmid carrying the colicin ion channel structure domain gene, transferring engineering bacteria for amplifying, and separating and purifying by His-tag column. It can be used to kill tumor cells.
Owner:PROTEIN DESIGN LAB LTD
Chinese hamster ovary (CHO) cell line capable of expressing Rta albumen of Elzatein-Barn (EB) virus stably and efficiently, creation method and application thereof and cell base built by CHO cell line
ActiveCN103131674AConducive to screeningEasy diagnosisMicroorganism librariesVector-based foreign material introductionDihydrofolate reductaseCell strain
The invention relates to a Chinese hamster ovary (CHO) cell line capable of showing Rta albumen of Elzatein-Barn (EB) virus stably and efficiently, a creation method and application thereof and a cell base built by the CHO cell line, and belongs to the technical field of biology. Preservation number of the recombination cell line CHO / RTA is CGMCC6955, the CHO cell base expressing the Rta albumen of the EB virus can be used for practical production and formed by the recombination cell line CHO / RTA. The invention further provides a method for creating the CHO cell line. Dihydrofolate reductase defect type Chinese hamster ovary cells (CHO / dhfr-) serve as host cells, and the cell line capable of expressing the Rta albumen of the EB virus stably and efficiently is obtained after screening and amplification of the host cells.
Owner:同昕生物技术(北京)有限公司
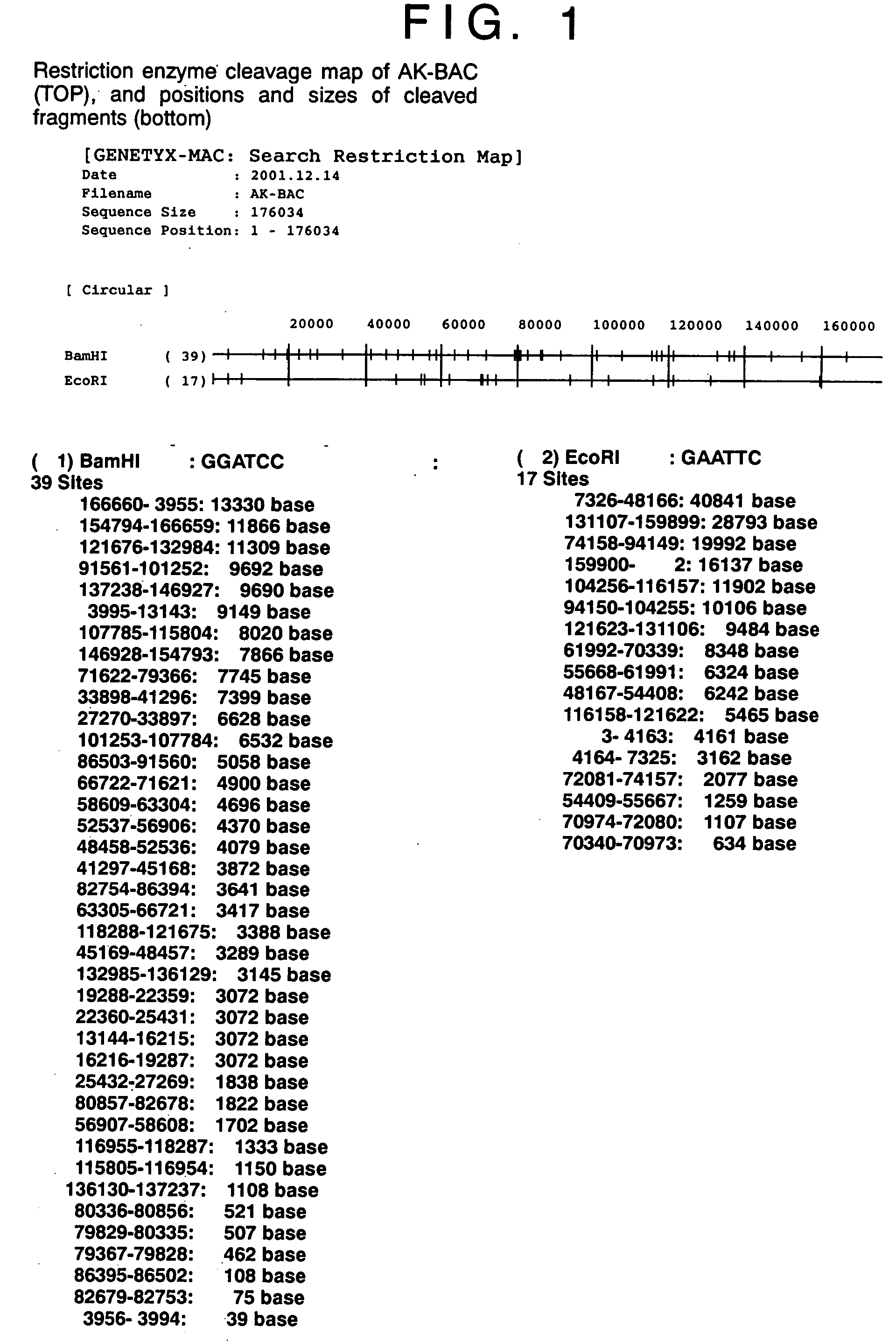
Double-stranded cyclic dna capable of proliferating as a bacterial e coli chromosome
InactiveUS20050106733A1Easy to modifyEasy to produceBacteriaInactivation/attenuationEscherichia coliGenomic DNA
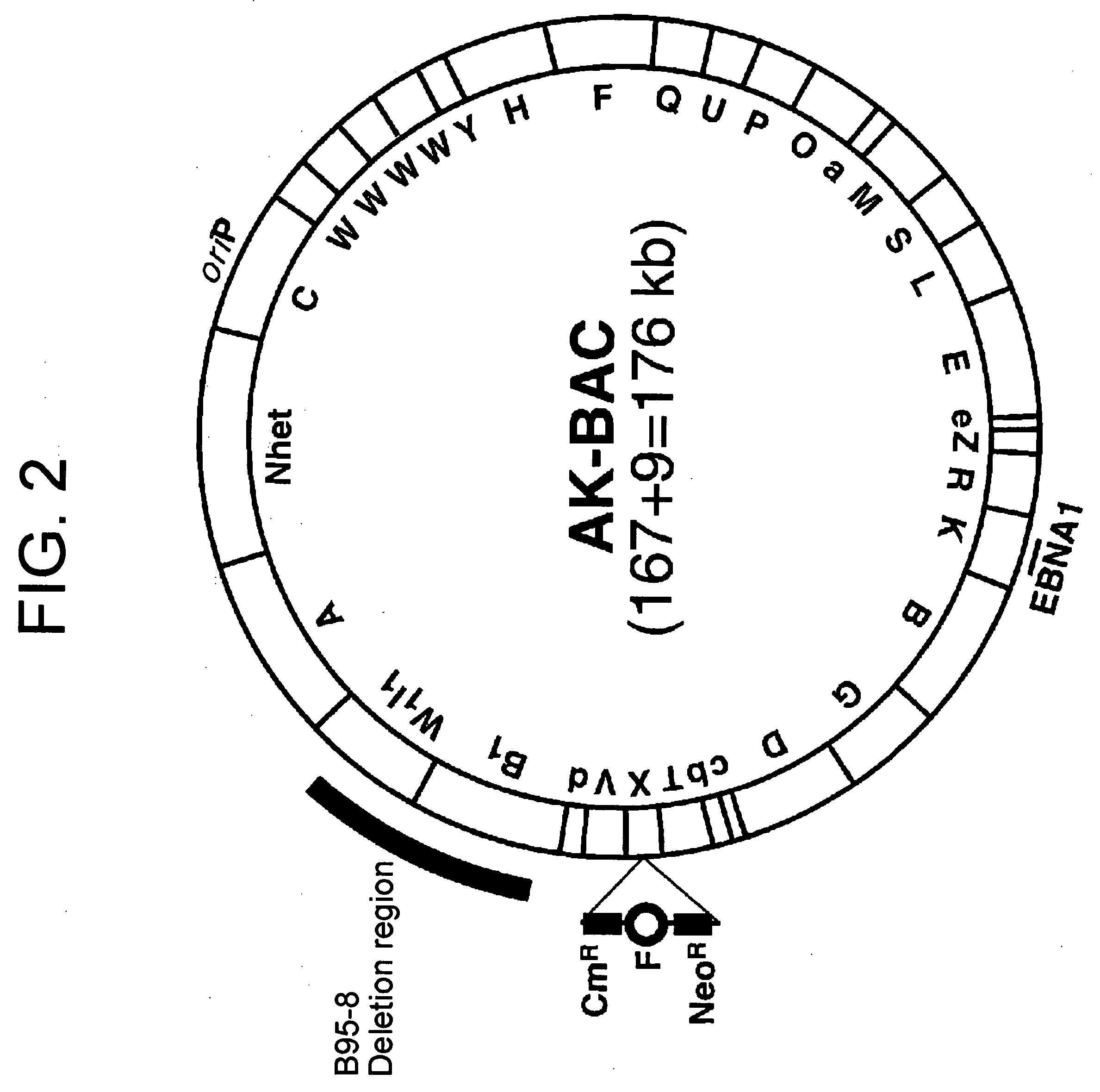
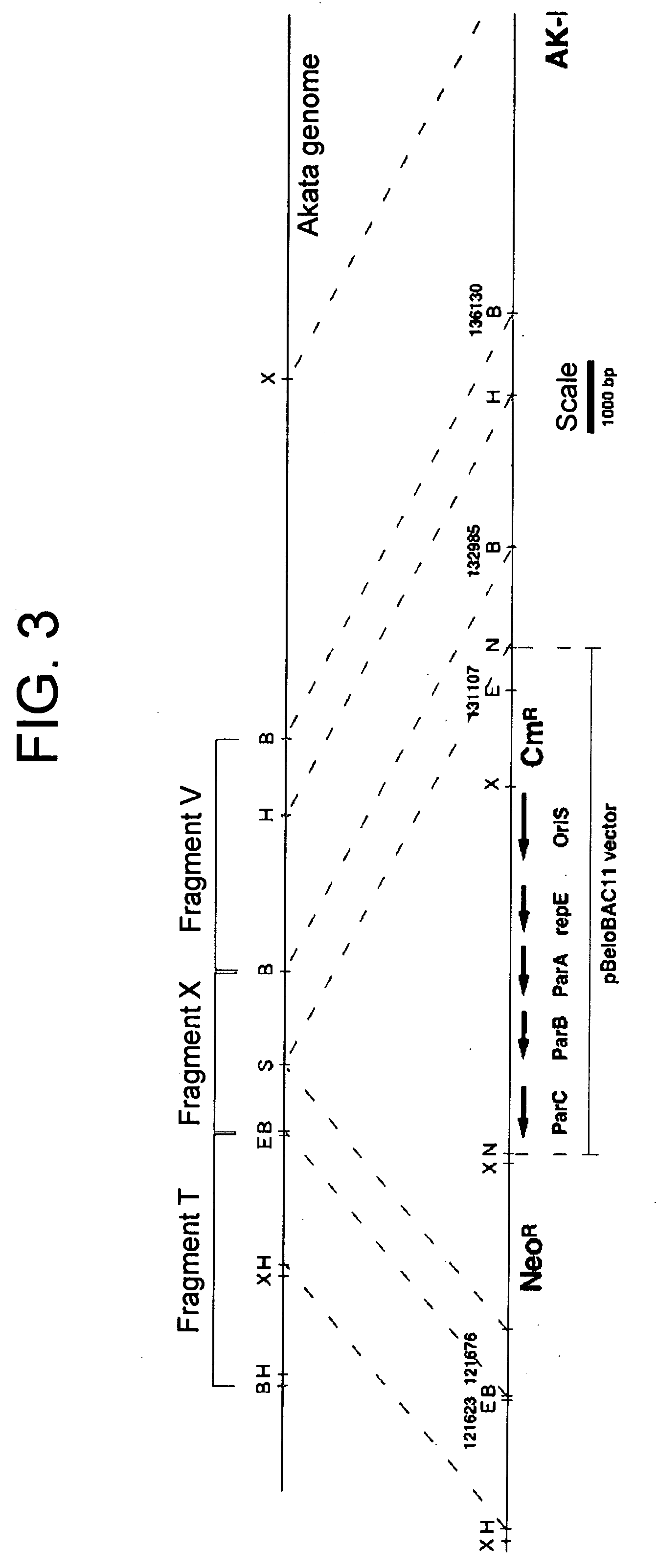
(PROBLEM) To establish a system enabling modification and proliferation of EB virus circular DNA in Escherichia coli and large-scale production of recombinant EB virus virions in the cells. (Means for Resolution) The present invention enables modification and proliferation of a circular EB virus genome derived from Akata cells in Escherichia coli by inserting a DNA sequence of a bacterial artificial chromosome (BAC) into a circular EB virus DNA in Akata cells via homologous recombination. The recombinant EB virus can be produced in a large quantity by introducing the resulting genomic DNA into Akata cells.
Owner:EVEC
Kit for detecting an EB viral capsid antigen IgA antibody
InactiveCN109633170AAvoid featuresAvoid sensitivityChemiluminescene/bioluminescenceBiological testingMicroparticleBlood plasma
The invention discloses a kit for detecting an EB viral capsid antigen (VCA) IgA antibody. The kit comprises a magnetic particle suspension taking a VCA gene recombinant antigen fragment as a coatingantigen, an anti-human IgA monoclonal antibody enzyme conjugate marked by horse radish peroxidase, a sample diluent and a chemiluminescent substrate solution. Magnetic particles are adopted as a solid-phase carrier of the antibody, and a chemiluminescence immunoassay technology is utilized, so that single-person random, rapid and automatic detection can be realized on a full-automatic chemiluminescence analyzer. Due to the fact that an indirect method is adopted in the technical principle, a plurality of dominant antigen fragments most valuable to previous infection and recent infection are selected from more than 30 EB virus target antigen proteins, and the problem that specificity and sensitivity are affected due to incomplete sites of conventional kits on the market is solved. The kit is suitable for the qualitative detection of the EB VCA IgA antibody in a human serum or plasma sample, and assists the clinical diagnosis of nasopharyngeal carcinoma.
Owner:AUTOBIO DIAGNOSTICS CO LTD
Monoclonal antibody IgM type RhD blood type shaped reagent
InactiveCN101178409ASave human serumAvoid infectious agentsBiological testingSodium phosphatesPotassium
The invention provides an RhD blood grouping reagent with a monoclonal antibody IgM type, which consists of anti-D monoclonal cell culture concentrated solution and dilution solution, the anti-D monoclonal cell culture concentrated solution is prepared by using the following method, firstly, the invention includes an establishing process of a hybrid cell system, the establishing process of the hybrid cell system includes a process of making use of lymph cells transformed by EB virus to hybridize with the mouse bone marrow cells, the hybrid monoclonal cell system is established by the process, secondly, the invention includes a culture process of the hybrid monoclonal cell system, the culture solution of the monoclonal cells is collected to carry out the concentration by the culture of the hybrid monoclonal cell system, so as to obtain the anti-D monoclonal cell culture concentrated solution; the dilution solution consists of sodium chloride, sodium phosphate with 12 water molecules, potassium dihydrogen phosphate, stabilizer, sodium azide, Tween 20 and distilled water; as the monoclonal antibody is adopted, the invention has strong specific antibody response and high accuracy rate of the detection.
Owner:上海血液生物医药有限责任公司
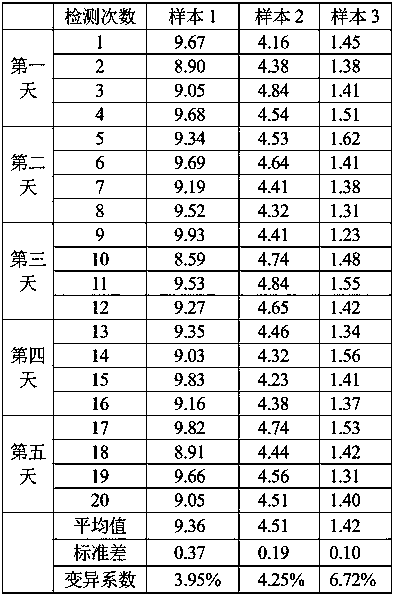
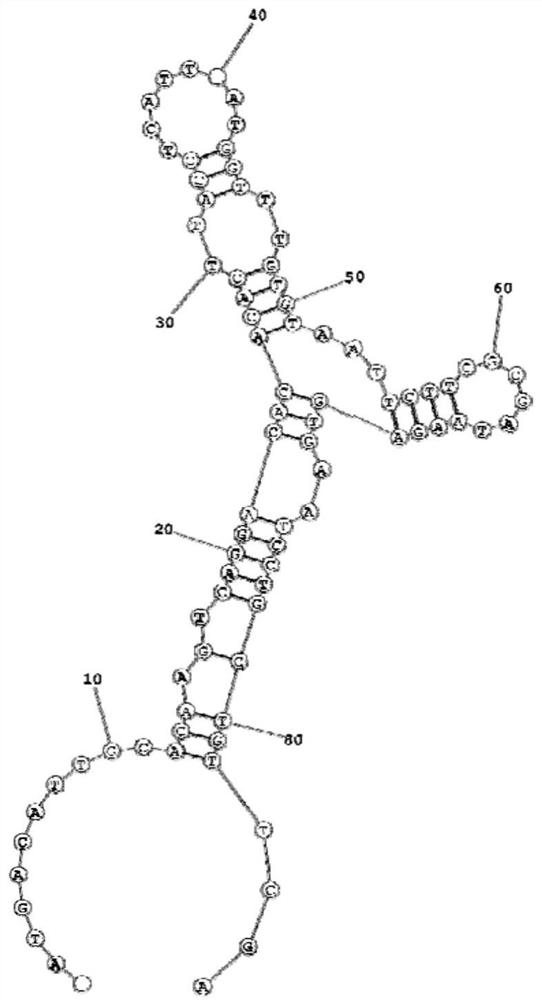
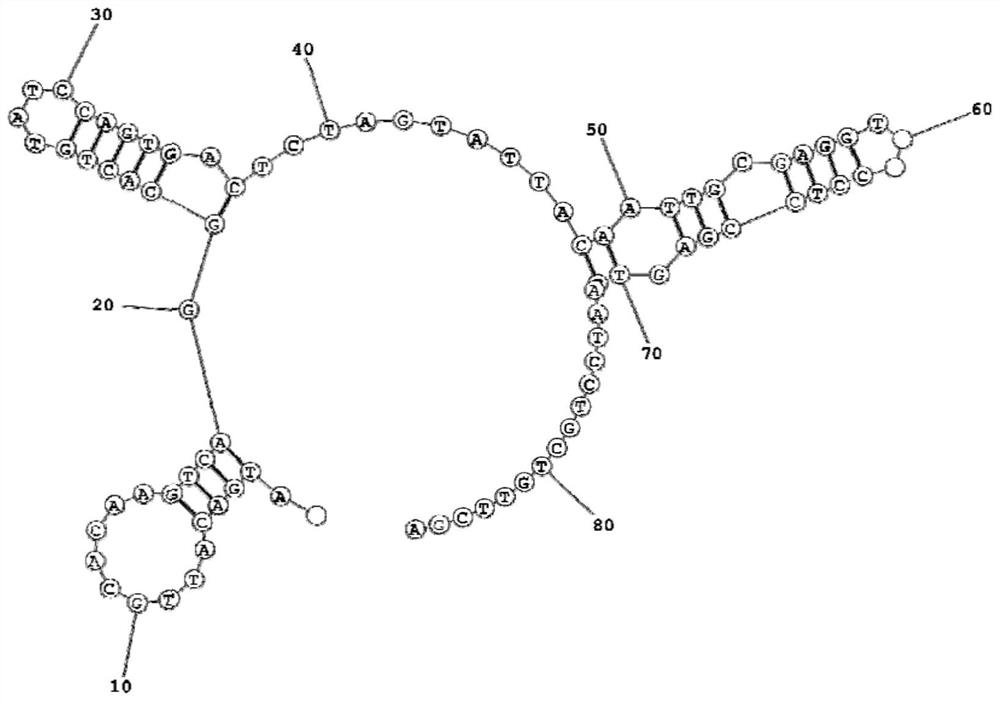
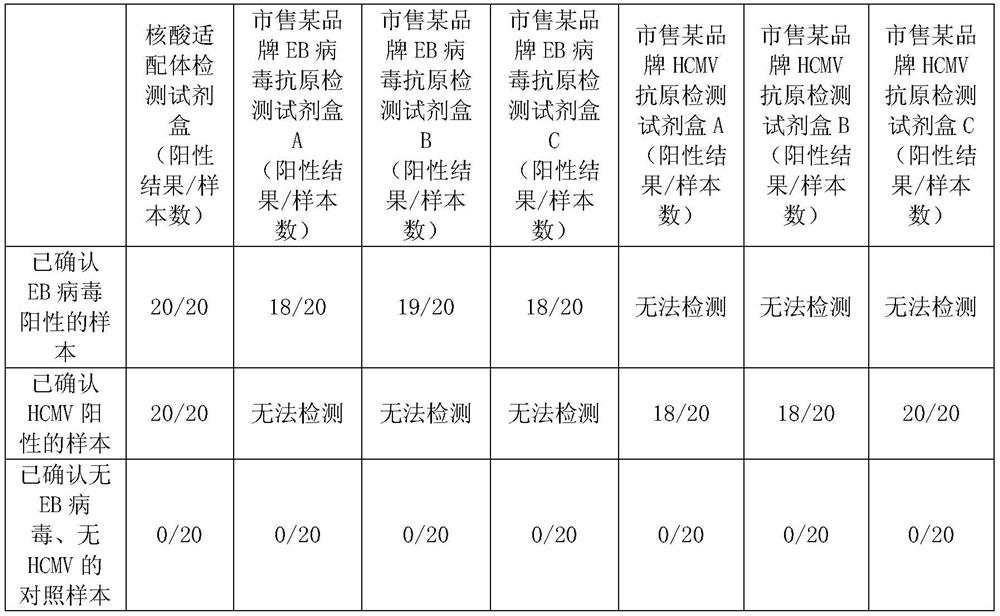
Kit for detecting EB virus/HCMV and application thereof
The invention provides a nucleic acid aptamer detection kit for EB virus and HCMV, and also provides a preparation method and application of the kit. The kit has the characteristics of simplicity in operation, quick response, high accuracy and high sensitivity during detection, and can be combined with detection devices such as a microplate reader, a flow cytometry and a fluorescence microscope torealize accurate detection of the two viruses. The kit is relatively high in sensitivity, good in specificity, wide in measurement range and simple to operate, can be used for simultaneously detecting two viruses, and has a good market prospect.
Owner:WUHAN UNIV
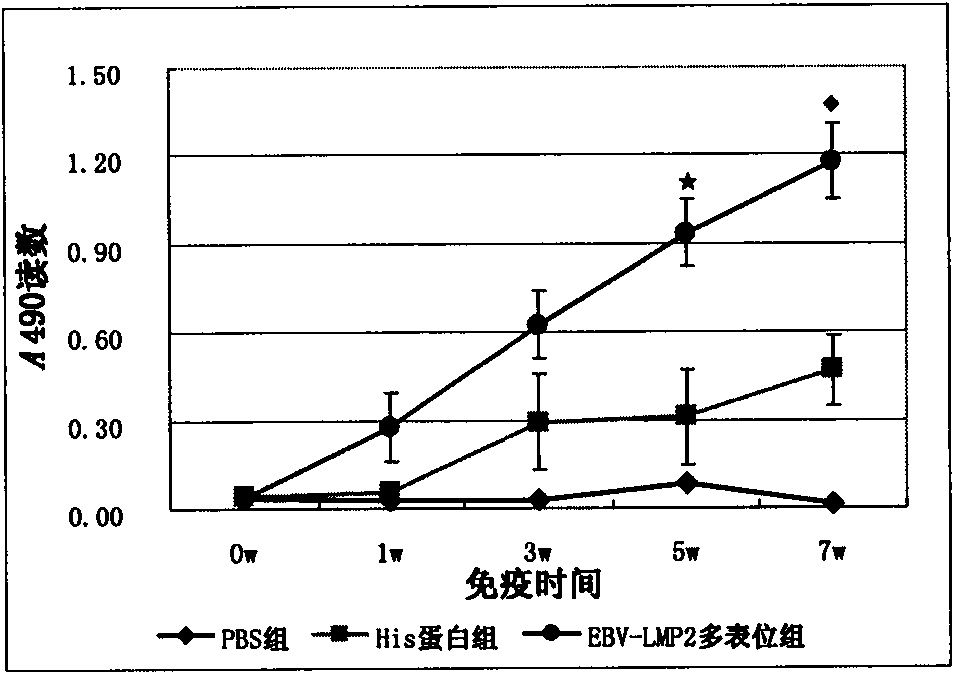
Multi-epitope recombinant protein of epstein-barr (EB) virus latent membrane protein 2 and application thereof
The invention relates to preparation and application of multi-epitope recombinant protein of epstein-barr (EB) virus latent membrane protein 2. The invention discloses the multi-epitope recombinant protein rich in a plurality of CTL epitopes, Th epitopes and B cell epitopes obtained by screening based on the full-length EB virus latent membrane protein 2. The invention also discloses the coding nucleic acid of the protein, and comprises a nucleic acid recombinant vector and a host cell. The invention also discloses the application of the protein in the aspects of preventing, treating and diagnosing EB virus infection and related disease thereof. The protein of the invention has very strong immunogenicity and antigenicity and good application prospect.
Owner:WENZHOU MEDICAL UNIV
Application method of EB virus encoded microRNA BART10
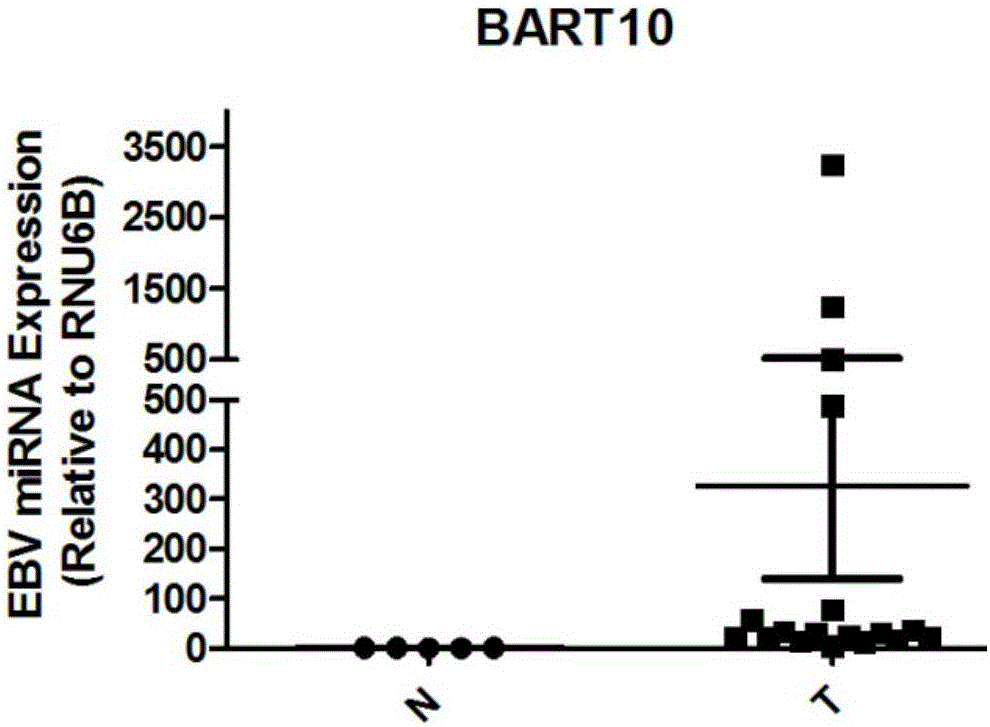
InactiveCN105154586AGood value for moneyMicrobiological testing/measurementDNA/RNA fragmentationLymphatic SpreadNasopharyngeal carcinoma
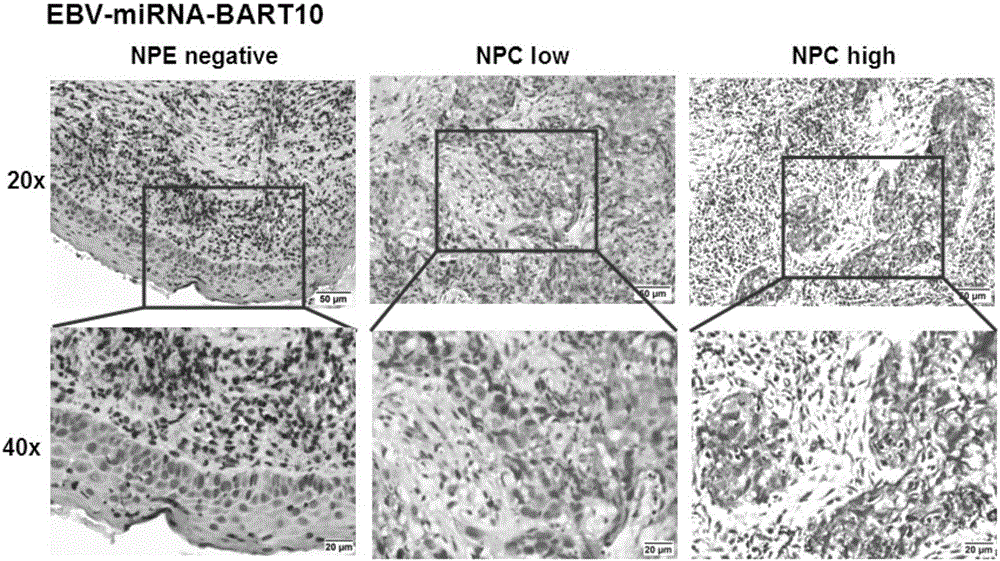
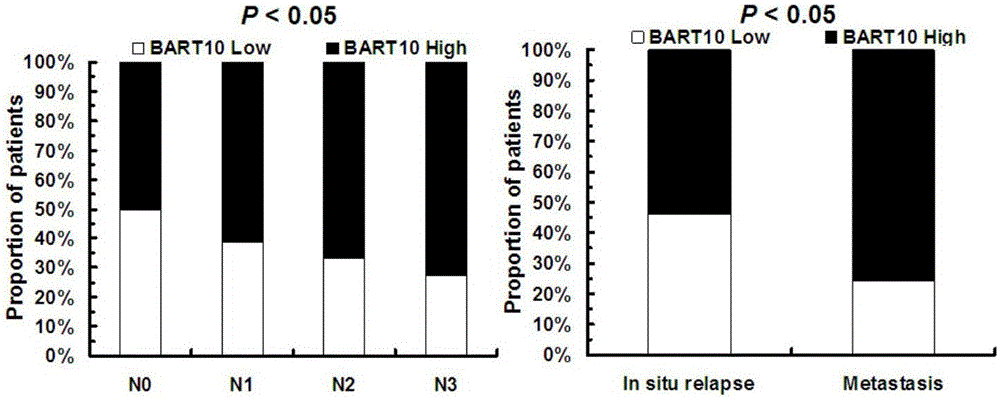
The invention discloses an application of EB virus encoded microRNA BART10 (EBV-miR-BART10) in preparing a prediction preparation for recurrence and metastasis of nasopharyngeal carcinoma. The research proves that the expression level of EBV-miR-BART10 in the nasopharyngeal carcinoma tissue is in positive correlation with the lymphatic metastasis and the distant metastasis of the nasopharyngeal carcinoma patient; if the expression of the EBV-miR-BART10 in the nasopharyngeal carcinoma tissue is up-regulated, the nasopharyngeal carcinoma patient with higher EBV-miR-BART10 expression have larger possibilities of recurrence and metastasis than the nasopharyngeal carcinoma patient with lower EBV-miR-BART10 expression, and the prognosis is worse, therefore, the application of the expression of the EBV-miR-BART10 in the predication of recurrence and metastasis of the nasopharyngeal carcinoma patient can provide powerful biomolecular basis for the prognosis of the nasopharyngeal carcinoma patient, and thus the application method has profound clinical significances and important popularization and application prospects.
Owner:CENT SOUTH UNIV
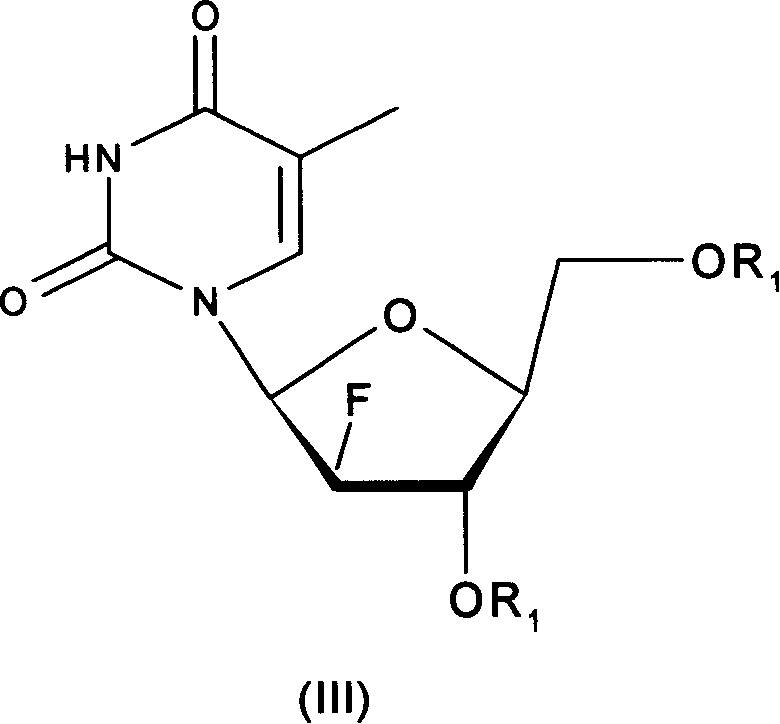
L-nucleoside prodrug
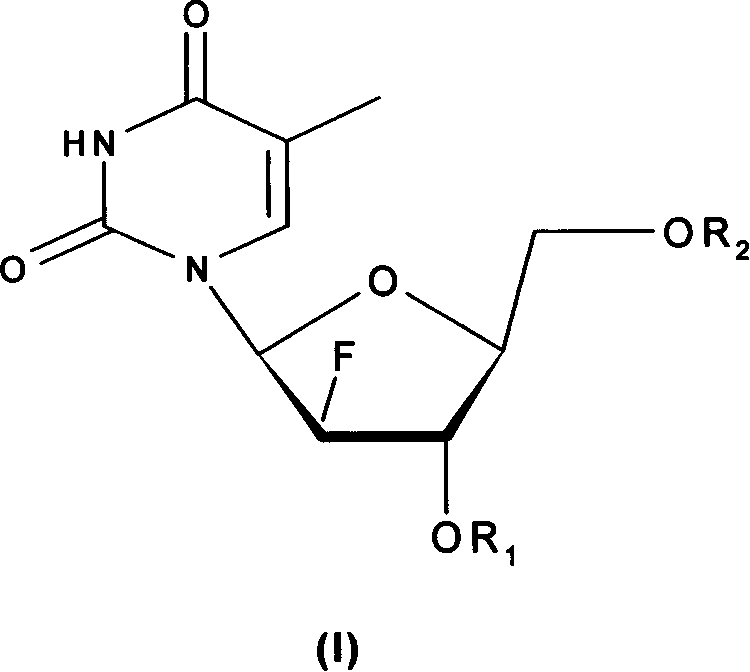
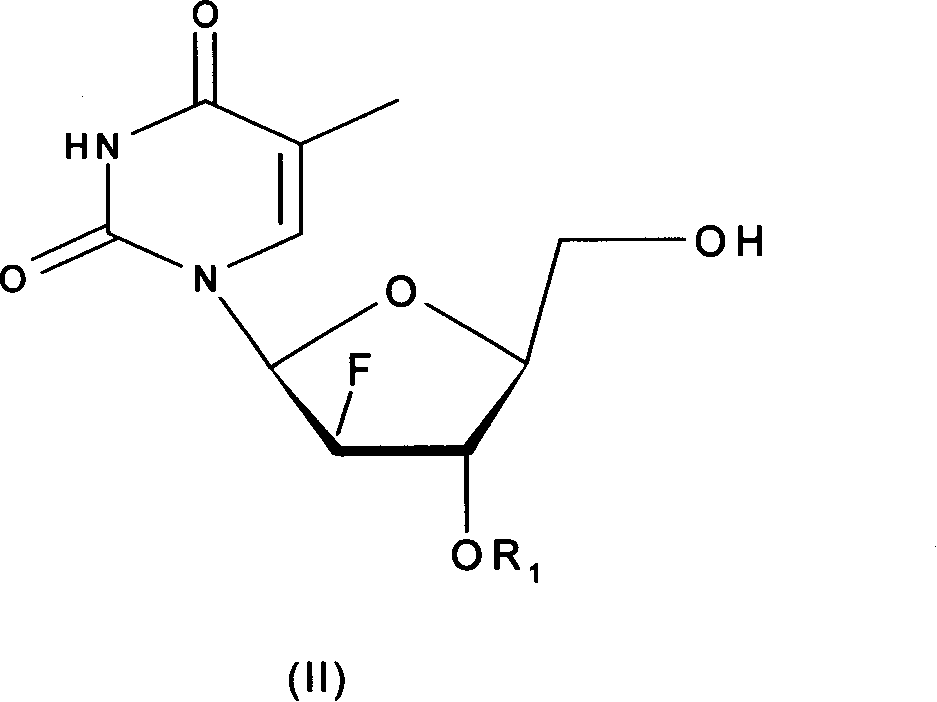
The invention relates to the compounds and their salts of type (I), among them R1 and R2 can be the same or different, R1 is the amino acid residue, alkoxyl formyl, organic acid acyl, phosphoryl and alkyl. R2 is H, amino acid residues, alkyloxyethyl formyl, organic carboxylic acid acyl, phosphoryl, and alkyl. These compounds have the function of anti - hepatitis B virus (HBV), anti-EB virus (EBV) and anti - hepatitis D virus (HDV) role. The invention also covers the preparation methods and compounds in the preparation of antiviral drugs.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Chimeric particle containing dominant epitope peptide of EB virus membrane surface glycoprotein gp350 and coding gene and application of chimeric particle
ActiveCN110615848AImproving immunogenicityHigh total titerAntibody mimetics/scaffoldsViral antigen ingredientsDiseaseImmunogenicity
The invention discloses a chimeric particle containing dominant epitope peptide of an EB virus membrane surface glycoprotein gp350 and a coding gene and application of the chimeric particle. Accordingto the chimeric particle and the coding gene and application thereof, it is found for the first time that the immunogenicity of polypeptide epitopes can be significantly improved by combining a plurality of dominant epitopes in a certain sequence and then conducting granulation display, immunized mice show that the total anti-titer of induced serum, the titer of neutralizing antibodies in the serum, and the efficiency of the serum in blocking viral infection at the cellular level are significantly improved. The invention provides a candidate vaccine form which can be used for developing and preventing EB virus infection, and important practical and theoretical significance and application prospects for prevention of EB virus-related diseases are achieved.
Owner:SUN YAT SEN UNIV CANCER CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com