Multi-epitope recombinant protein of epstein-barr (EB) virus latent membrane protein 2 and application thereof
A latent membrane protein, Epstein-Barr virus technology, applied in the fields of application, viral peptides, antiviral agents, etc., can solve the problems of difficult to reach a large sensitive population, weak immunogenicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0094] The present invention also provides a kit (kit) for detecting diseases related to Epstein-Barr virus infection. The kit contains: a solid phase carrier coated with the multi-epitope Recombinant protein. The preparation method of the kit (kit) comprises: (1) coating the multi-epitope recombinant protein on a solid-phase carrier (such as an ELISA reaction plate) to obtain the multi-epitope recombinant protein coated and (2) placing the solid phase carrier coated with the multi-epitope recombinant protein obtained in (1) into a kit, so as to obtain a kit for detecting diseases related to Epstein-Barr virus infection. The kit may also include reagents (such as enzyme-linked immunosorbent reagents) for detecting antigen-antibody reactions, or reagents for gene amplification (such as PCR reagents) in appropriate containers, and / or also Instructions for use (book) are included.
[0095] Detection purpose
[0096] The multi-epitope recombinant protein of the invention can be...
Embodiment 1
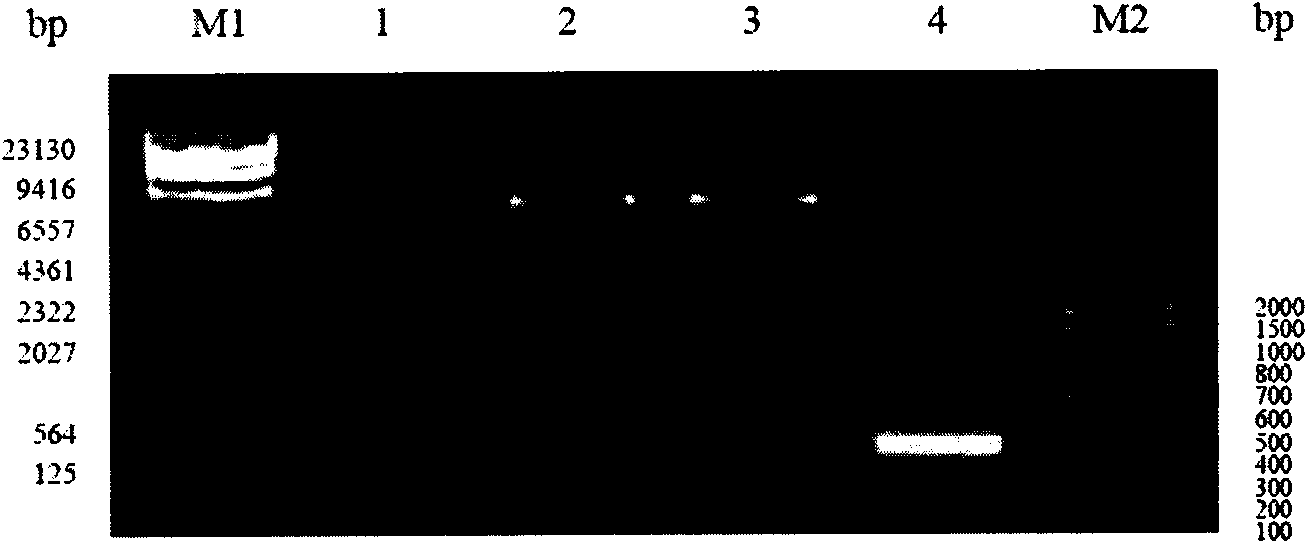
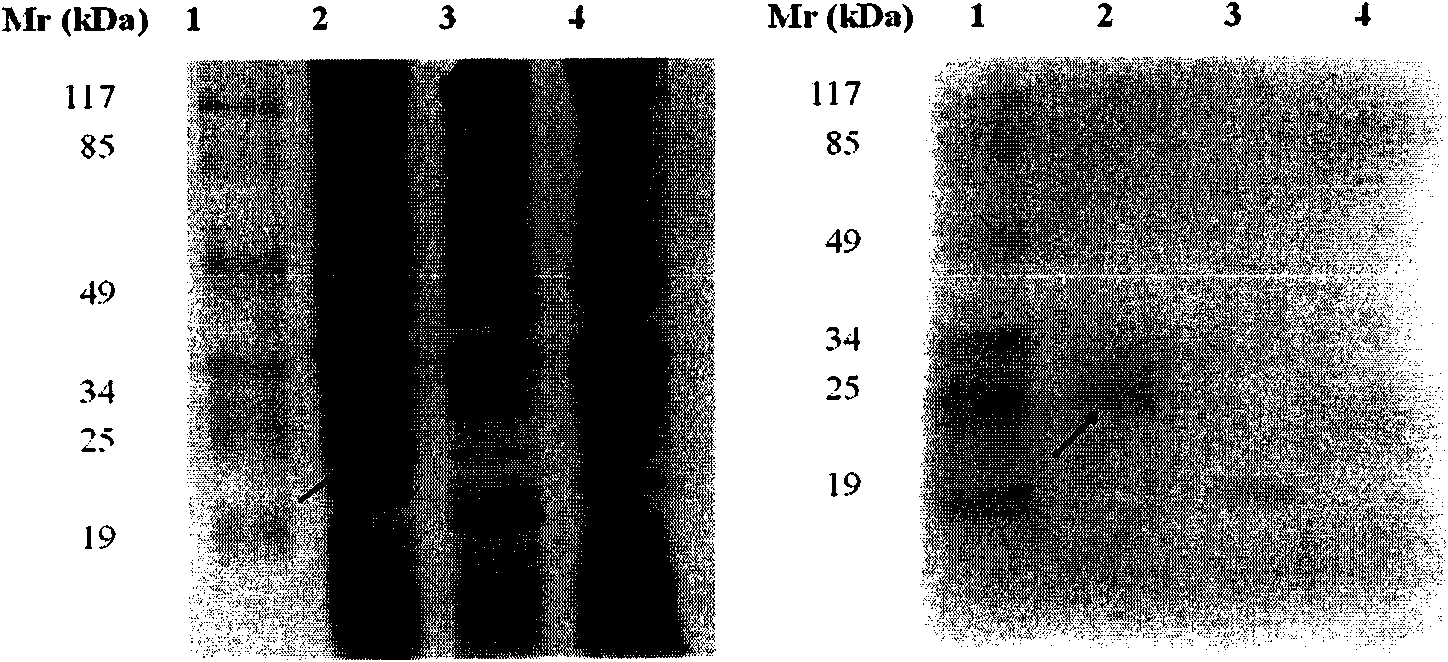
[0106] Example 1, Preparation and identification of EBV-LMP2 multi-epitope recombinant protein
[0107] 1. Design of EBV-LMP2 multi-epitope recombinant protein gene
[0108] Apply the network resource database (Genbank, Swiss-Prot) EBV latent membrane protein 2 (LMP2) gene and amino acid sequence; according to the most common HLA genes in the Chinese Han population are HLA-A*02, HLA-A*24, HLA-B* 58 and HLA-DRB 1*15, HLA-DRB 1*03 genes (Zeng Xuehui, Xiao Lulu, Li Jiang et al.; Study on the correlation between HLA-A, B, DRB1 allelic polymorphisms and nasopharyngeal carcinoma in southern China[J ]. Journal of Cellular and Molecular Immunology, 2007 (9): 819-821; Song Yonghong, Ma Chunhong, Lu Hongjuan, etc.; Study on HLA gene polymorphisms of the Han population in northern China [J]. Shandong University Journal (Medical Edition), 2007 (6 ): 546-553.), using the online software (SYFPEITHI, EXPASY) and the biological software DNASTAR to predict the above-mentioned HLA gene-restric...
Embodiment 2
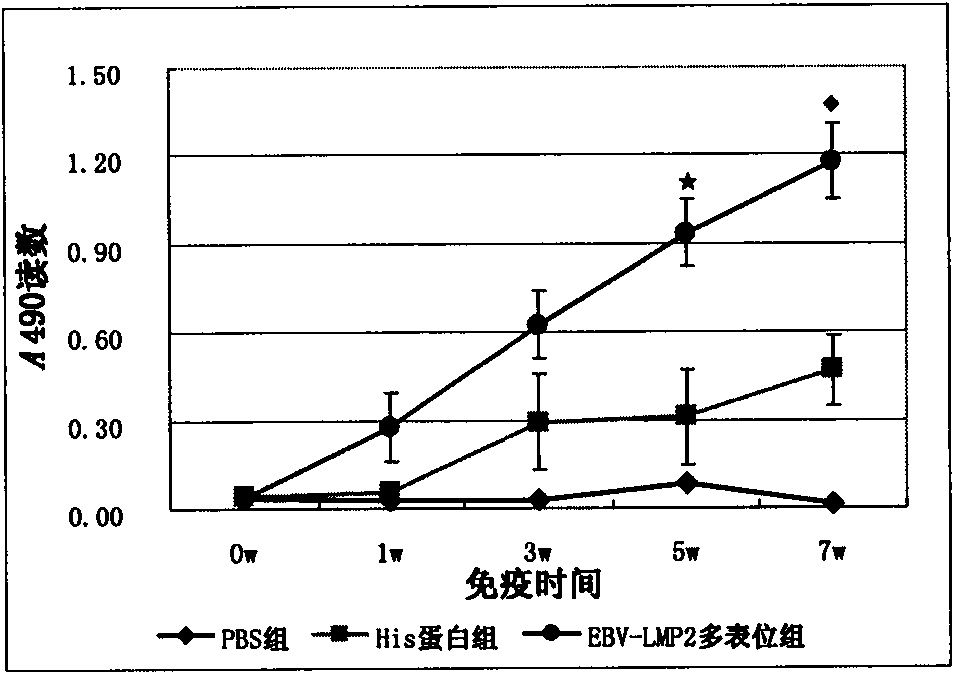
[0129] Example 2, Immunogenicity Research of EBV-LMP2 Multi-epitope Recombinant Protein
[0130] Female BALB / c mice aged 6-8 weeks were randomly divided into 3 groups, 9 mice in each group: the first group was the EBV-LMP2 multi-epitope protein immunization group, and the second group was the pET32a(+) empty vector control group; Group 3 was the PBS blank control group. Multi-epitope protein and Freund's adjuvant (FCA) 1:1 (W / W) were fully emulsified evenly, and at 0, 2, and 4 weeks respectively, 50 μg of multi-epitope protein or empty carrier protein was added to the back of each mouse. Spot immunize mice. The effects of humoral immunity and cellular immunity were tested on the immunized mice, that is, the titer and maintenance time of specific serum IgG and vaginal secretion sIgA, and the specificity of CTL was detected by lactate dehydrogenase (LDH) release method for cellular immunity lethal activity.
[0131] At 0, 1, 3, 5, and 7 weeks, the blood of each group of mice ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com