HIV composite multi-epitope DNA vaccine and application thereof
A DNA vaccine and multi-epitope technology, applied in the field of AIDS vaccine, can solve problems such as ignoring humoral immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
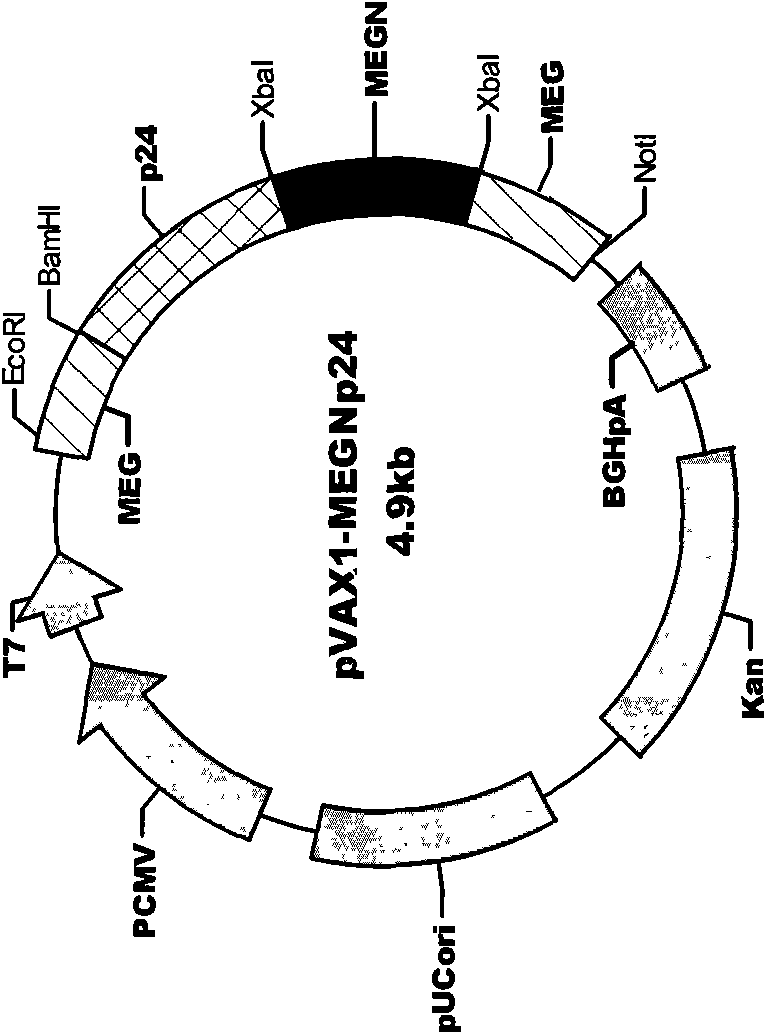
[0035] Example 1 Establishment of HIV Multi-epitope DNA Vaccine
[0036] On the basis of the HIV-1 MEGp24 epitope design completed earlier (Chinese patent application number: 03127002.6), the reverse vaccinology theory was further applied, and the authoritative American Los Alamos (Los Alamos) HIV molecular immunology database ( HIV Molecular Immunology Database), and referred to the HIV Epitope Database (HIV Epitope Database, http: / / lava.genetics.utah.edu / epitopeDBv3 / index.cfm) of Utah State University (The University of Utah), search HIV- 1 and HIV-2 CTL and CD8 T cell epitopes (http: / / hiv-web.lanl.gov / content / immunology / ctl_search). Based on the design concept of balancing the immune response and focusing on strengthening the induction of specific cellular immunity (CTL), the main structures and regulation of HIV-1 Env, Gag, Nef, Pol, Rt, Vpr, Tat and HIV-2 p24, p17 Protein-based 17 highly conserved CTL immunodominant epitopes. In terms of selection, the classic dominant ...
Embodiment 2
[0060] Example 2 Experimental Study on Mice Immunization of HIV Compound Multi-epitope DNA Vaccine
[0061] 1. Experimental grouping, mouse immunization and sampling
[0062] Twenty BALB / c female mice were randomly divided into 2 groups, 10 mice in each group, respectively pVAX1 empty plasmid control group and pVAX-MEGNp24 plasmid immunization group. Immunization was carried out three times with an interval of 14 days. Each time, 100 μg rDNA (dissolved in 100 μL sterile saline) was injected into the bilateral tibialis anterior muscle of the mice. Blood was collected 2 weeks after the second immunization, placed overnight at 4°C, centrifuged at 5000rpm for 10min, serum was collected, and stored at -20°C for testing. On the 10th day after the third immunization, the eyeballs were picked to take blood, and the cervical spine was dislocated to kill. The serum was coagulated and separated for ELISA to detect cytokines; at the same time, the spleen was aseptically collected to pre...
Embodiment 3
[0075] Example 3 Study on the immune effect of living cells of HIV compound multi-epitope DNA vaccine in vitro
[0076] 1. Sample collection:
[0077] The EDTA anticoagulated blood was sent to the laboratory for processing within 6 hours after collection, and the genetic background of the screened blood donors was HLA-0201.
[0078] 2. Human PBMC isolation:
[0079] (1) Transfer the anticoagulated blood to a 50 mL centrifuge tube and dilute it with 1640 medium (1:3).
[0080] (2) Add 15mL Ficoll Lymphocyte Layering Solution into the 50mL centrifuge tube.
[0081] (3) Slowly add the diluted blood to the surface of the layered liquid, taking care not to damage the liquid surface.
[0082] (4) Centrifuge at 700g for 30 minutes at 20°C.
[0083] (5) Aspirate the lymphocyte layer above the layered solution into a 50mL centrifuge tube, taking care not to absorb the erythrocyte layer.
[0084] (6) Add 40 mL of 1640 medium, and mix evenly by inverting. Centrifuge at 600g for 10 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com