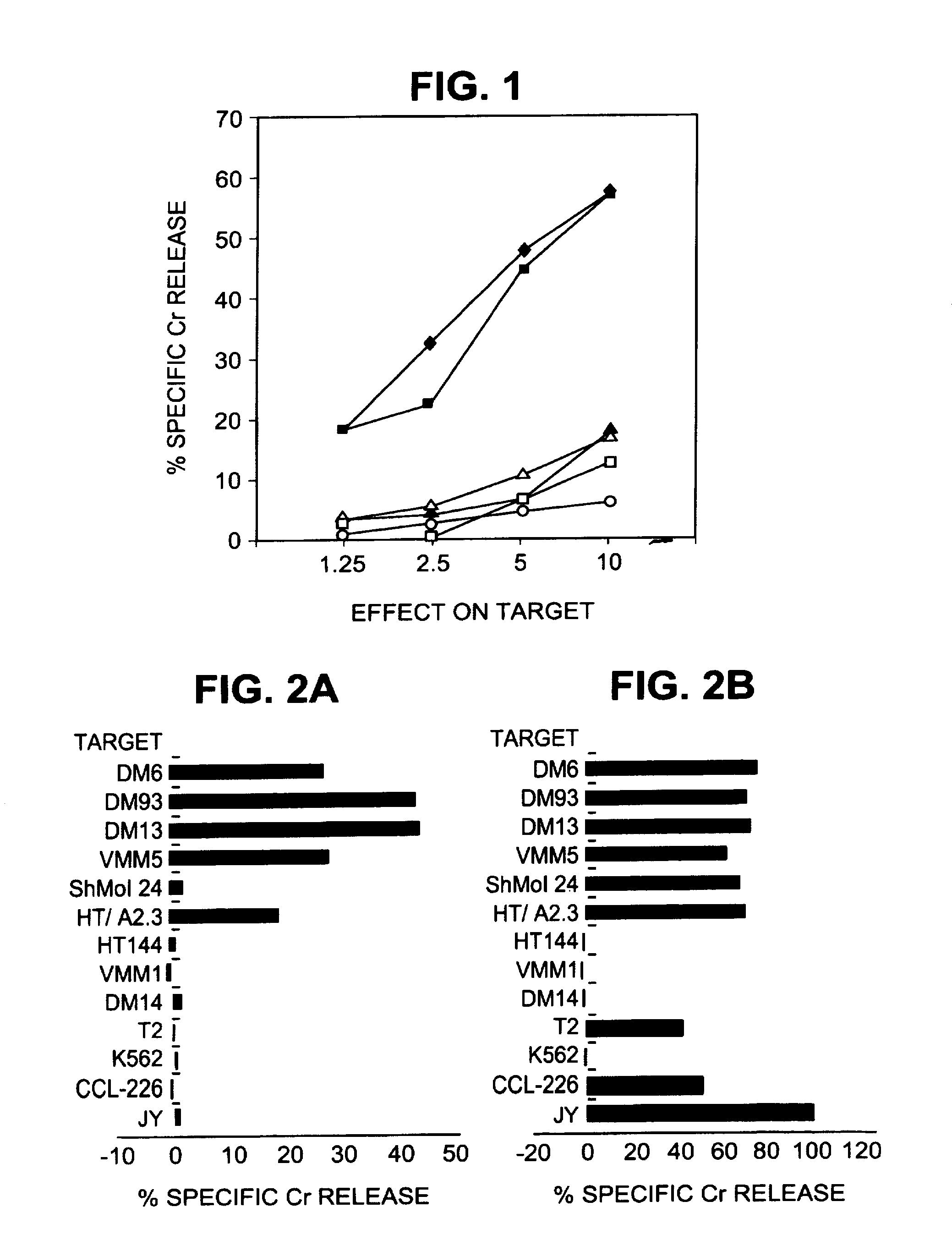
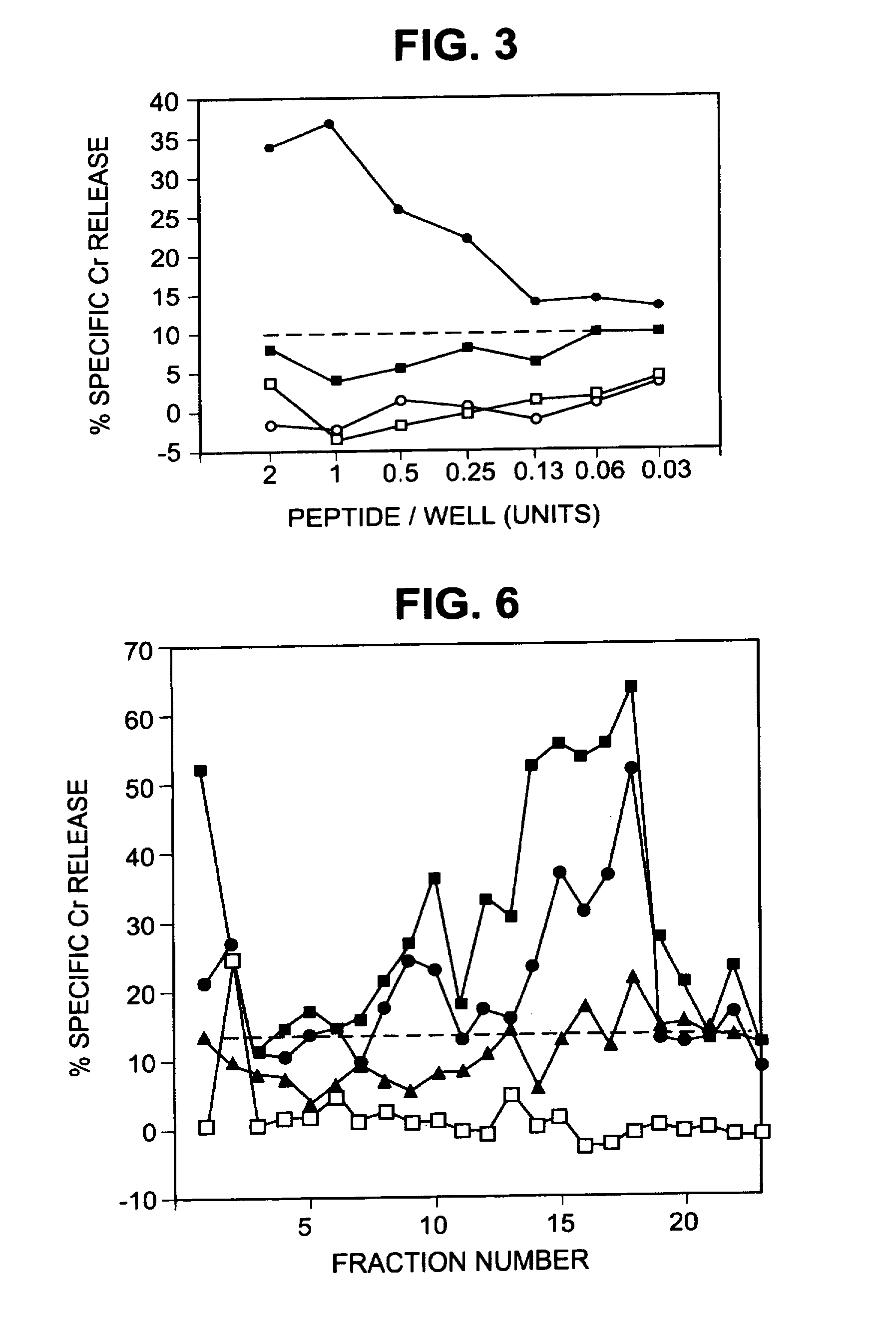
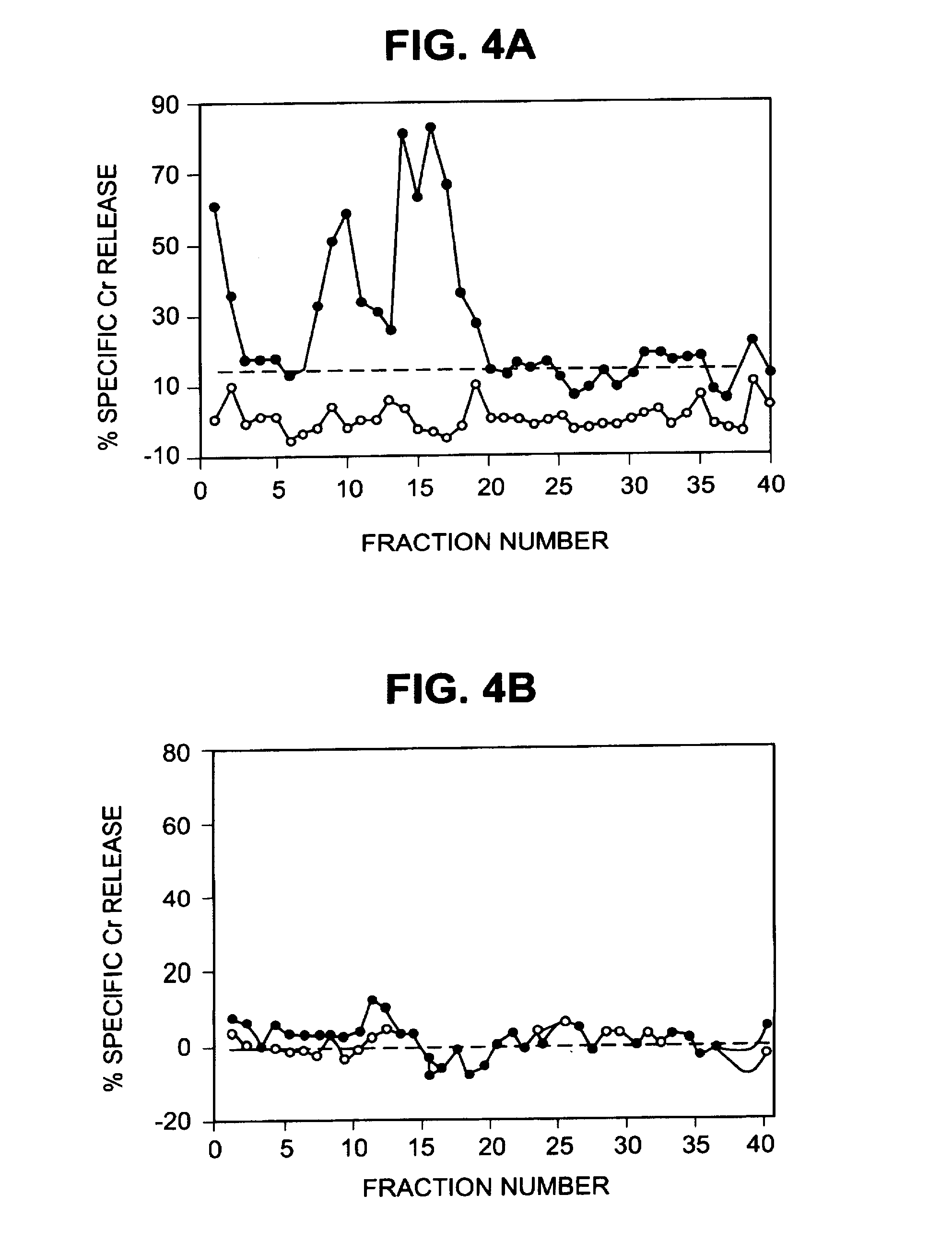
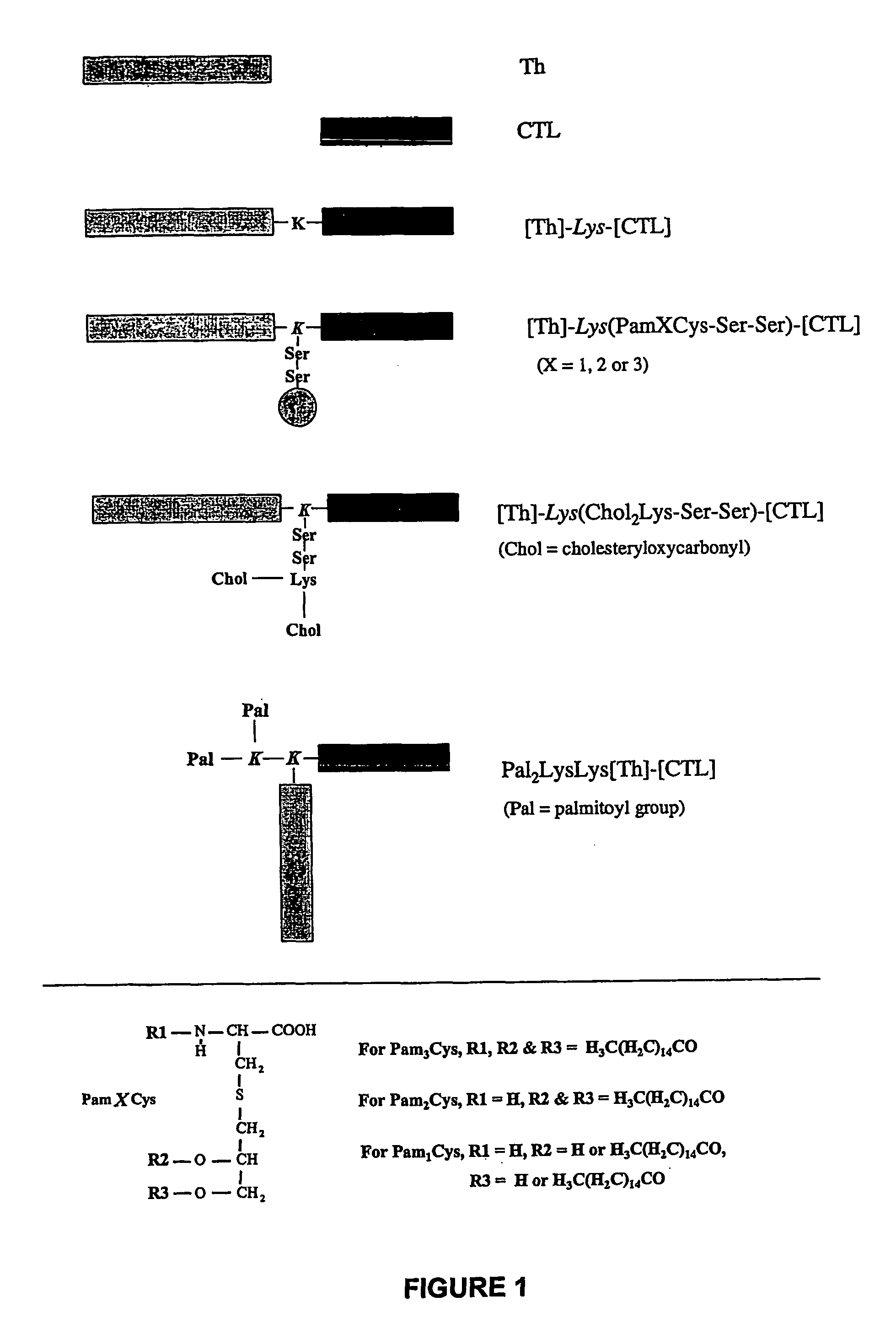
Patents
Literature
207 results about "Ctl epitope" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
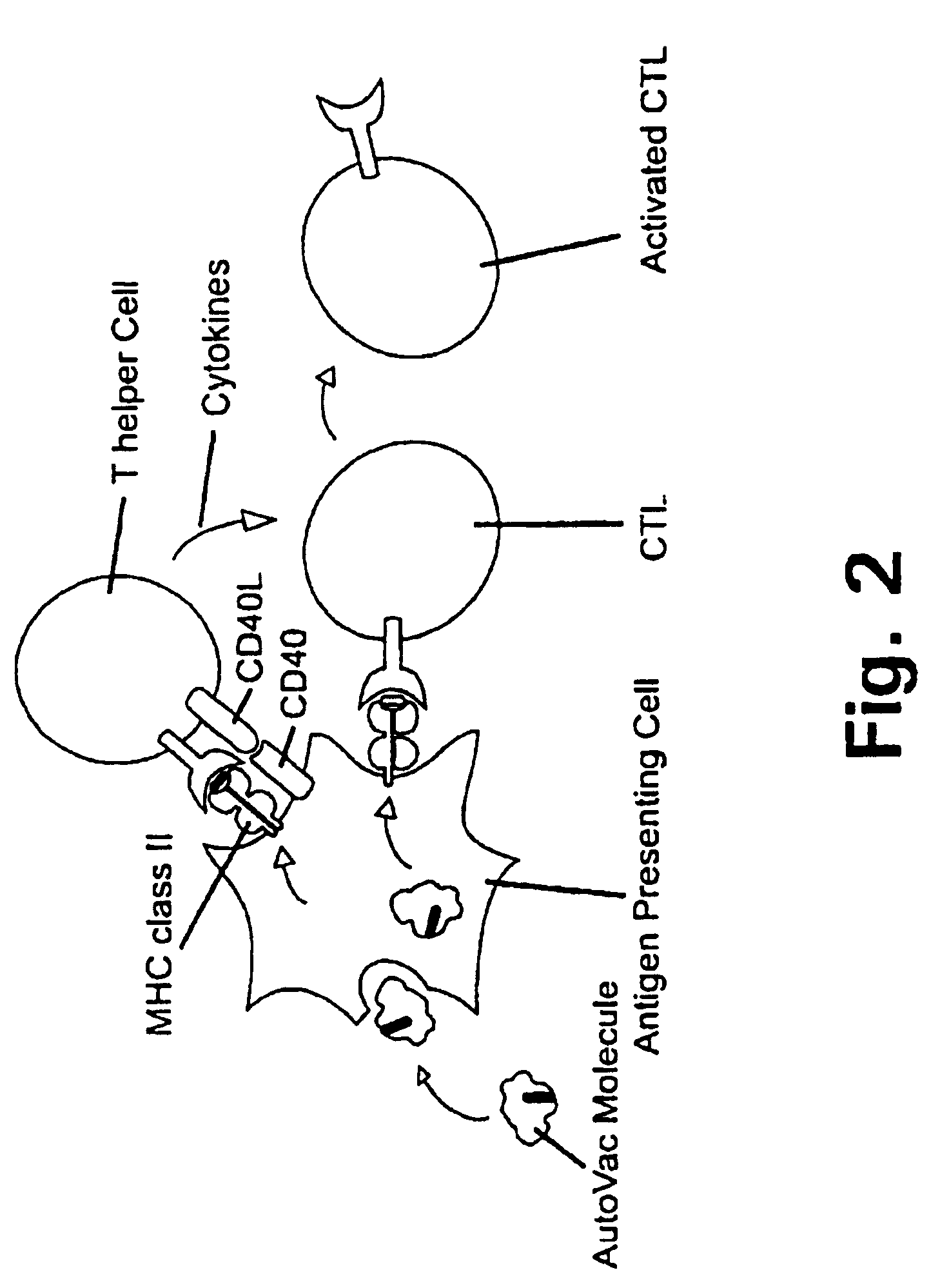
Novel methods for therapeutic vaccination
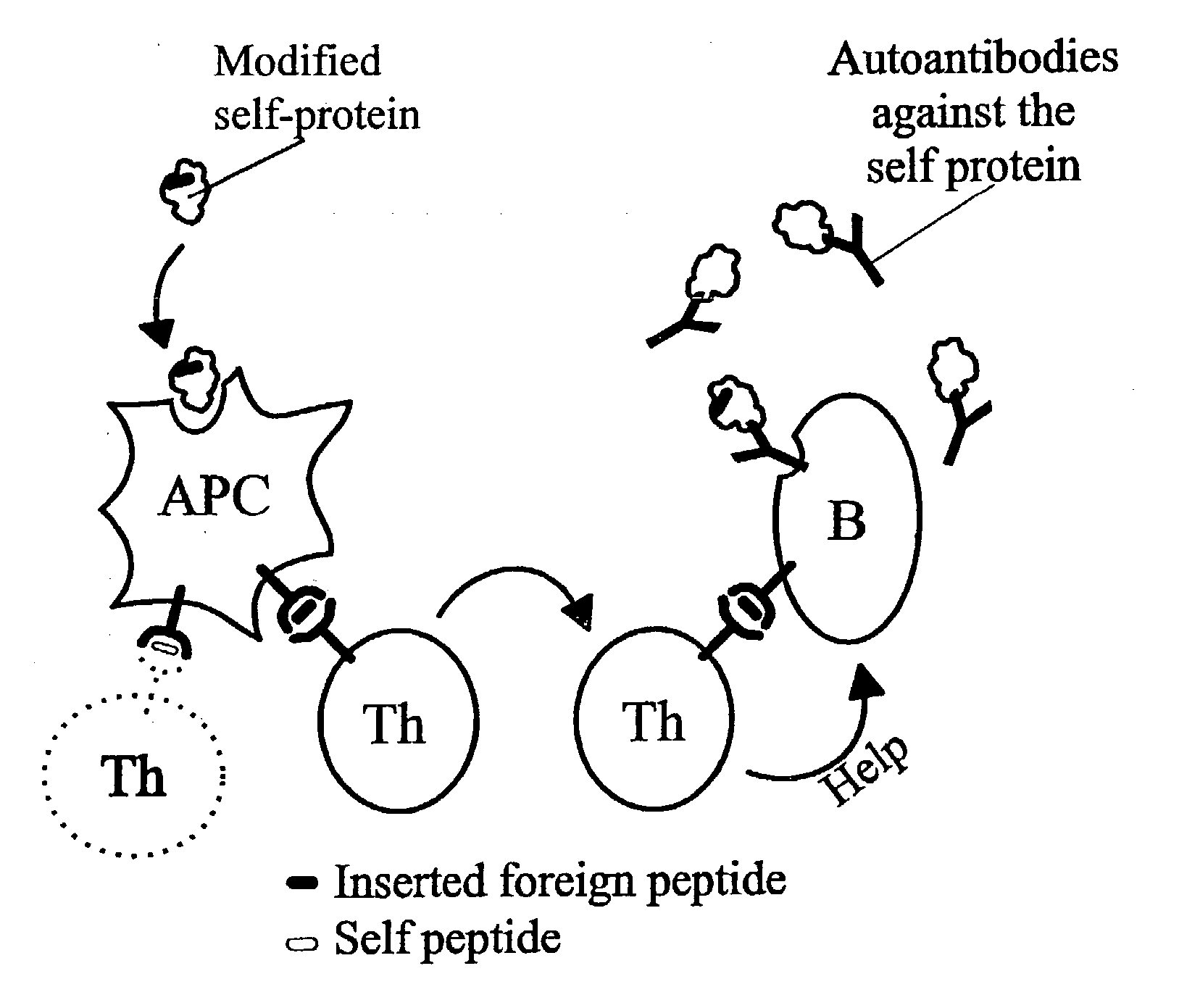
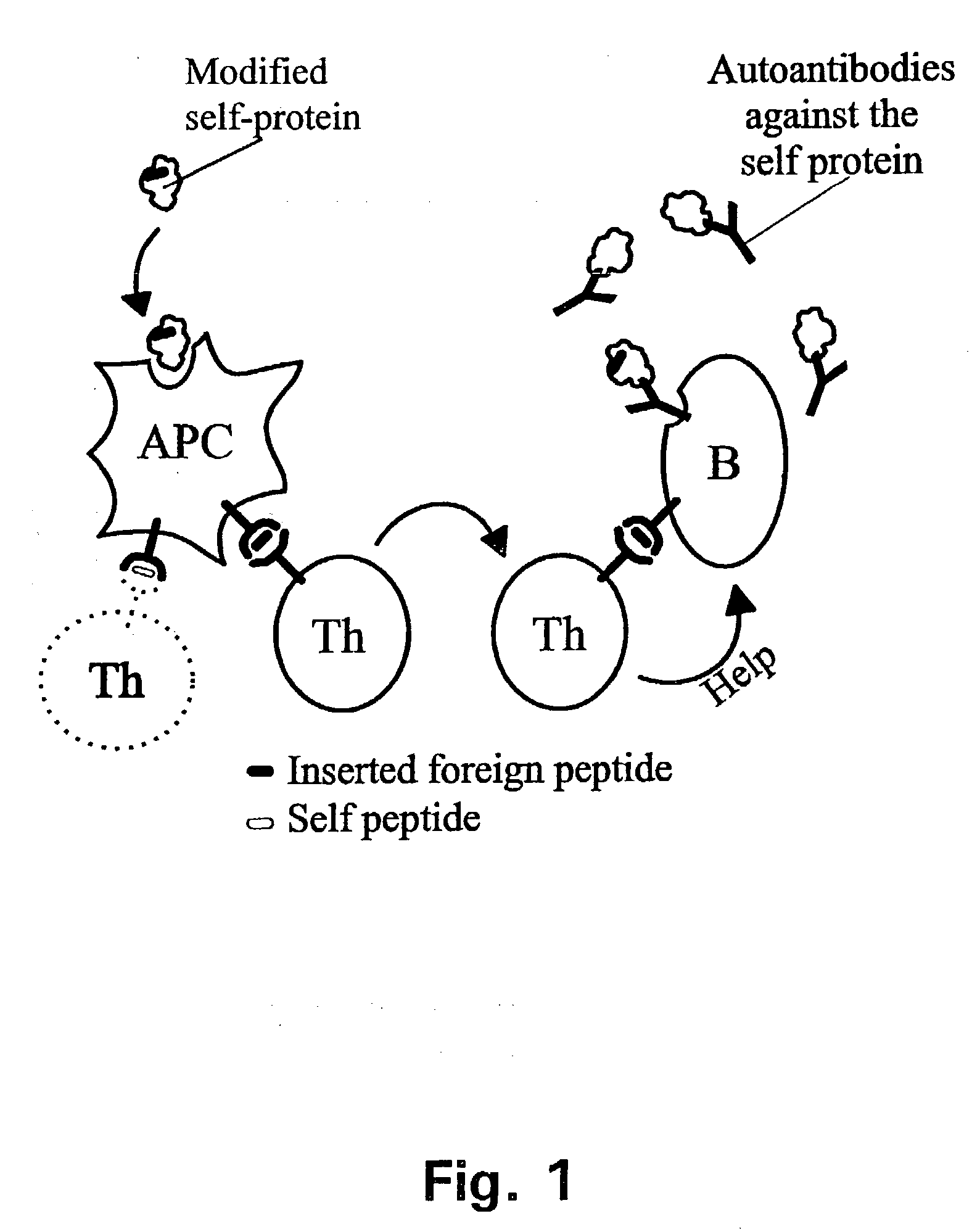
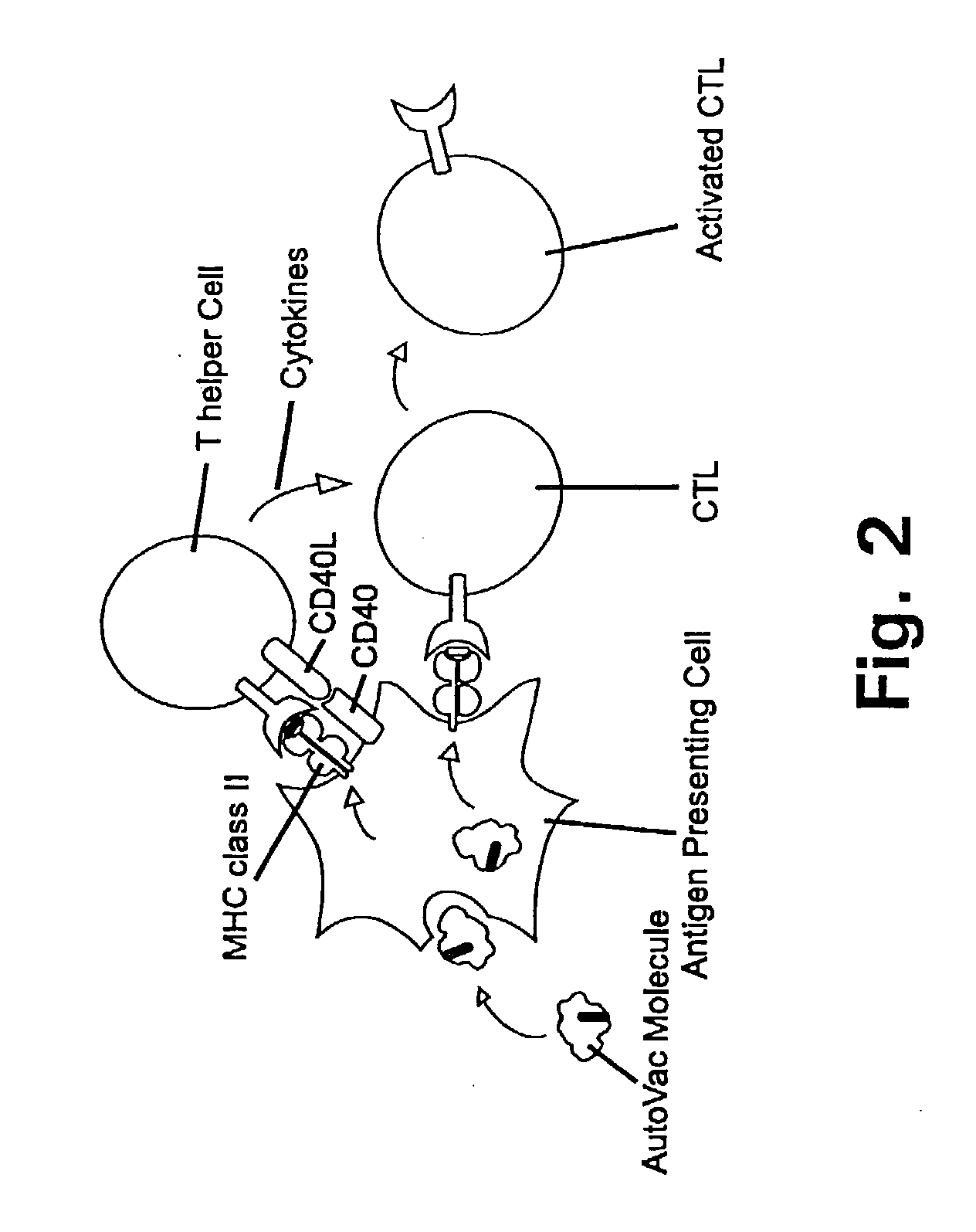
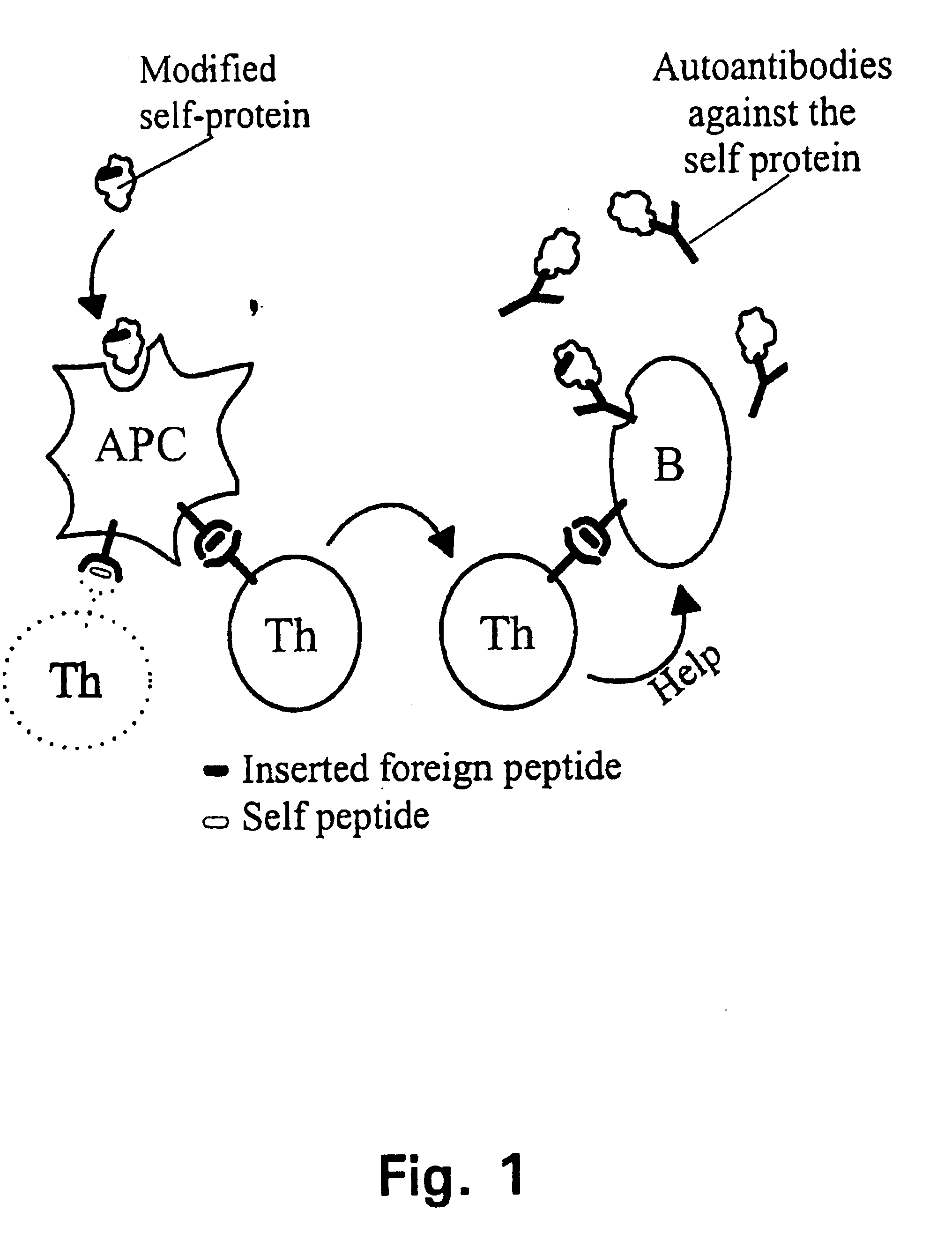
A method is disclosed for inducing cell-mediated immunity against cellular antigens. More specifically, the invention provides for a method for inducing cytotoxic T-lymphocyte immunity against weak antigens, notably self-proteins. The method entails that antigen presenting cells are induced to present at least one CTL epitope of the weak antigen and at the same time presenting at least one foreign T-helper lymphocyte epitope. In a preferred embodiment, the antigen is a cancer specific antigen, e.g. PSM, Her2, or FGF8b. The method can be exercised by using traditional polypeptide vaccination, but also by using live attenuated vaccines or nucleic acid vaccination. The invention furthermore provides immunogenic analogues of PSM, Her2 and FGF8b, as well as nucleic acid molecules encoding these analogues. Also vectors and transformed cells are disclosed. The invention also provides for a method for identification of immunogenic analogues of weak or non-immunogenic antigens.
Owner:BAVARIAN NORDIC AS
Immunodeficiency recombinant poxvirus
InactiveUS6596279B1SsRNA viruses negative-senseSsRNA viruses positive-senseFeline immunodeficiency virusCtl epitope
Attenuated recombinant viruses containing DNA encoding an immunodeficiency virus and / or CTL antigen, as well as methods and compositions employing the viruses, expression products therefrom, and antibodies generated from the viruses or expression products, are disclosed and claimed. The recombinant viruses can be NYVAC or ALVAC recombinant viruses. The DNA can code for at least one of: HIV1gag(+pro)(IIIB), gp120(MN)(+transmembrane), nef(BRU)CTL, pol(IIIB)CTL, ELDKWA or LDKW epitopes, preferably HIV1gag(+pro)(IIIB), gp120(MN) (+transmembrane), two (2) nef(BRU)CTL and three (3) pol(IIIB)CTL etpitopes; or two ELDKWA in gp120 V3 or another region or in gp160. The two (2) nef(BRU)CTL and three (3) pol(IIIB)CTL epitopes are preferably CTL1, CTL2, pol1, pol2 and pol3. The recombinant viruses and gene products therefrom and antibodies generated by the viruses and gene products have several preventive, therapeutic and diagnostic uses. DNA from the recombinant viruses are useful as probes or, for generating PCR primers or for immunization. Also disclosed and claimed are HIV immunogens and modified gp160 and gp120.
Owner:VIROGENETICS
Novel methods for therapeutic vaccination
A method is disclosed for inducing cell-mediated immunity against cellular antigens. More specifically, the invention provides for a method for inducing cytotoxic T-lymphocyte immunity against weak antigens, notably self-proteins. The method entails that antigen presenting cells are induced to present at least one CTL epitope of the weak antigen and at the same time presenting at least one foreign T-helper lymphocyte epitope. In a preferred embodiment, the antigen is a cancer specific antigen, e.g. PSM, Her2, or FGF8b. The method can be exercised by using traditional polypeptide vaccination, but also by using live attenuated vaccines or nucleic acid vaccination. The invention furthermore provides immunogenic analogues of PSM, Her2 and FGF8b, as well as nucleic acid molecules encoding these analogues. Also vectors and transformed cells are disclosed. The invention also provides for a method for identification of immunogenic analogues of weak or non-immunogenic antigens.
Owner:BAVARIAN NORDIC AS
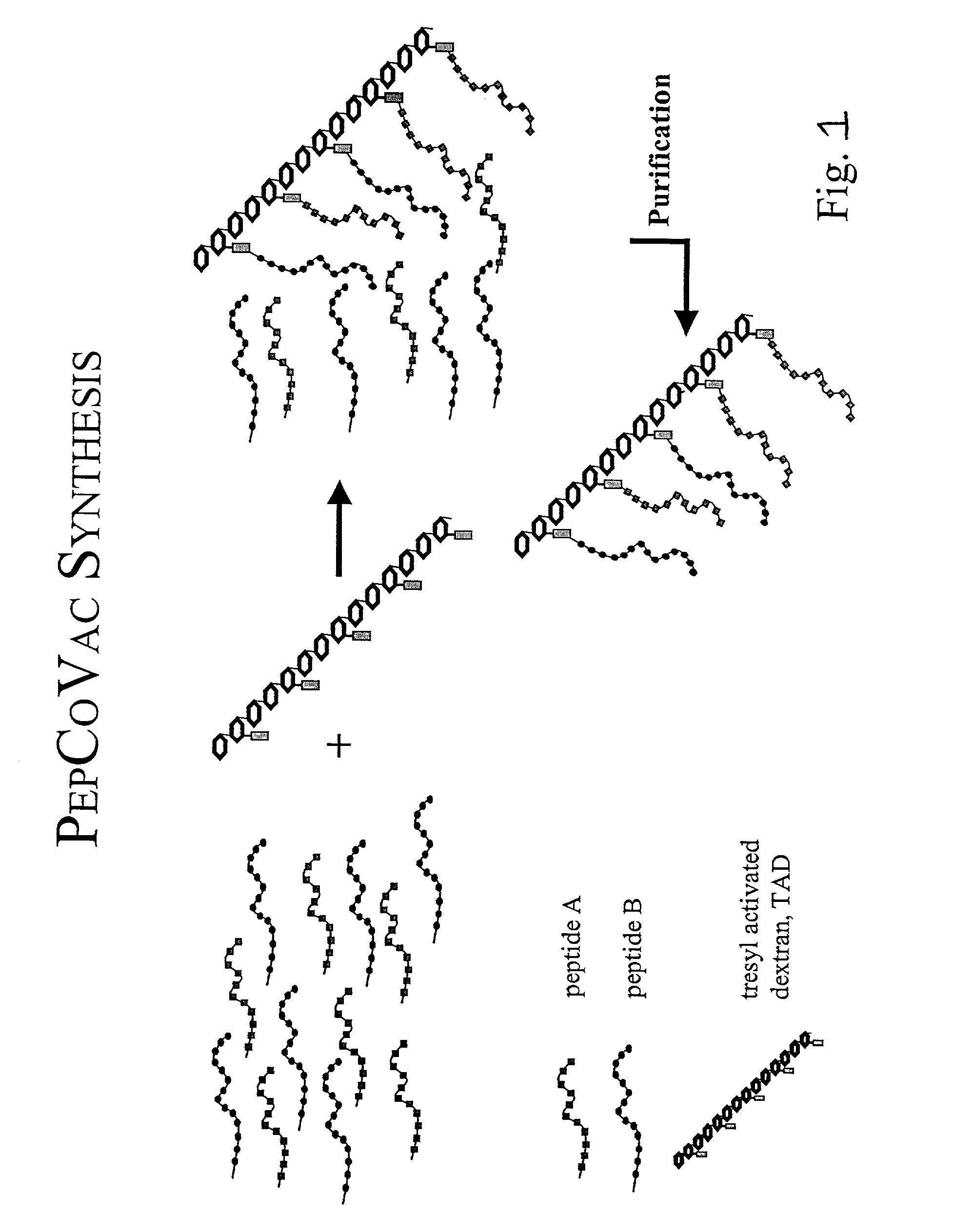
Synthetic vaccine agents
InactiveUS7097837B2Promote absorptionImprove responsePeptide/protein ingredientsAntibody mimetics/scaffoldsCtl epitopeHelper epitope
The present invention provides for novel immungens that are comprised of an activated polyhydroxypolymer backbone to which is attached 2 separate antigenic determinants. The 1st antigenic determinant includes a B-cell or CTL epitope and the 2nd antigenic determinant includes a T-helper epitope. In preferred embodiments, the antigenic determinants are derived from different molecules and species. Exemplary immunogens of the invention are constituted of a linear tresyl-activated dextran backbone to which is coupled B-cell or CTL epitopes of an antigen and to which is also coupled universal T-helper epitopes. Also disclosed are immunogenic compositions comprising the immunogens, methods of immunization and a method for identification of suitable immunogens of the invention.
Owner:BEALE STREET 143 INVEST APS
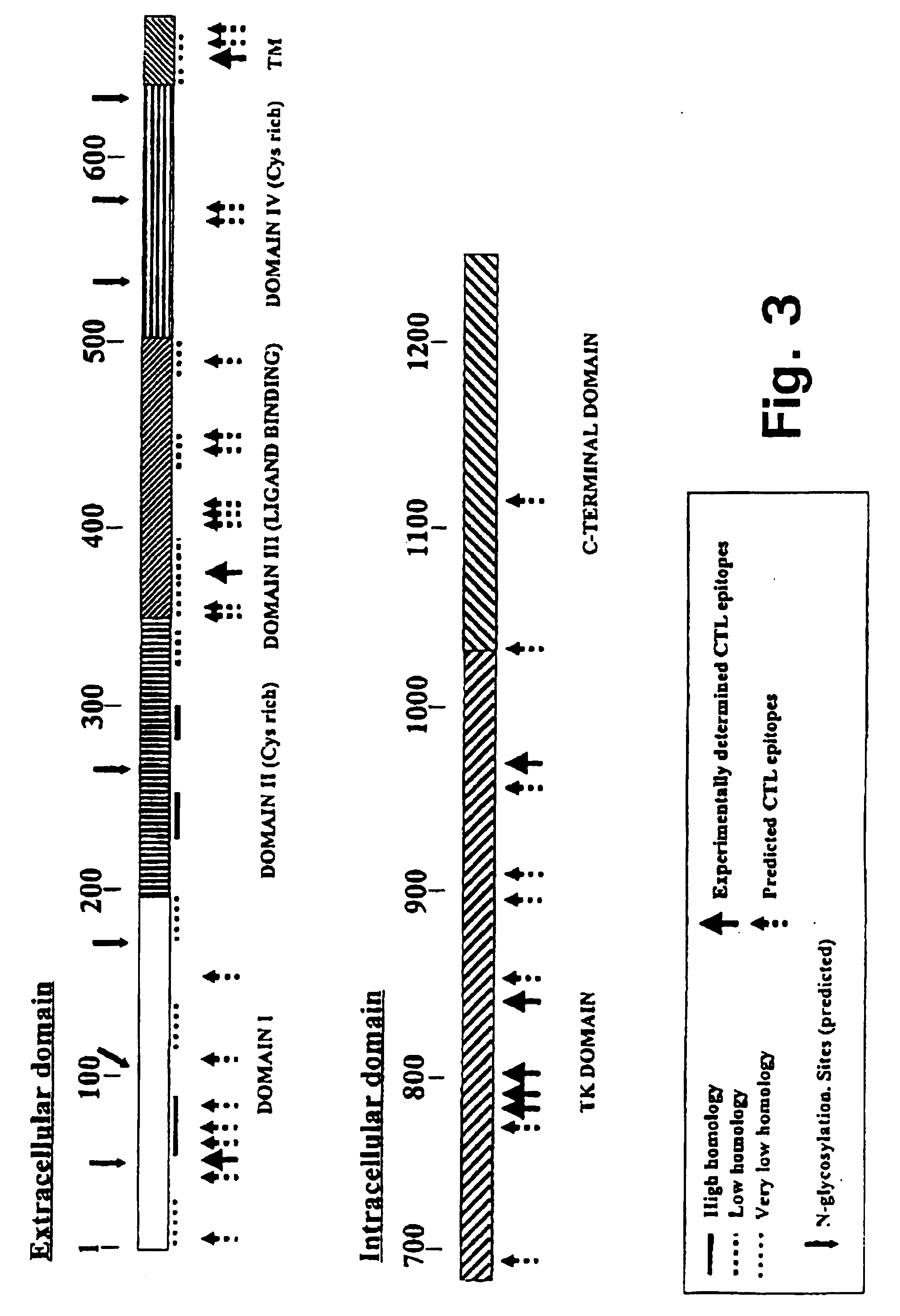
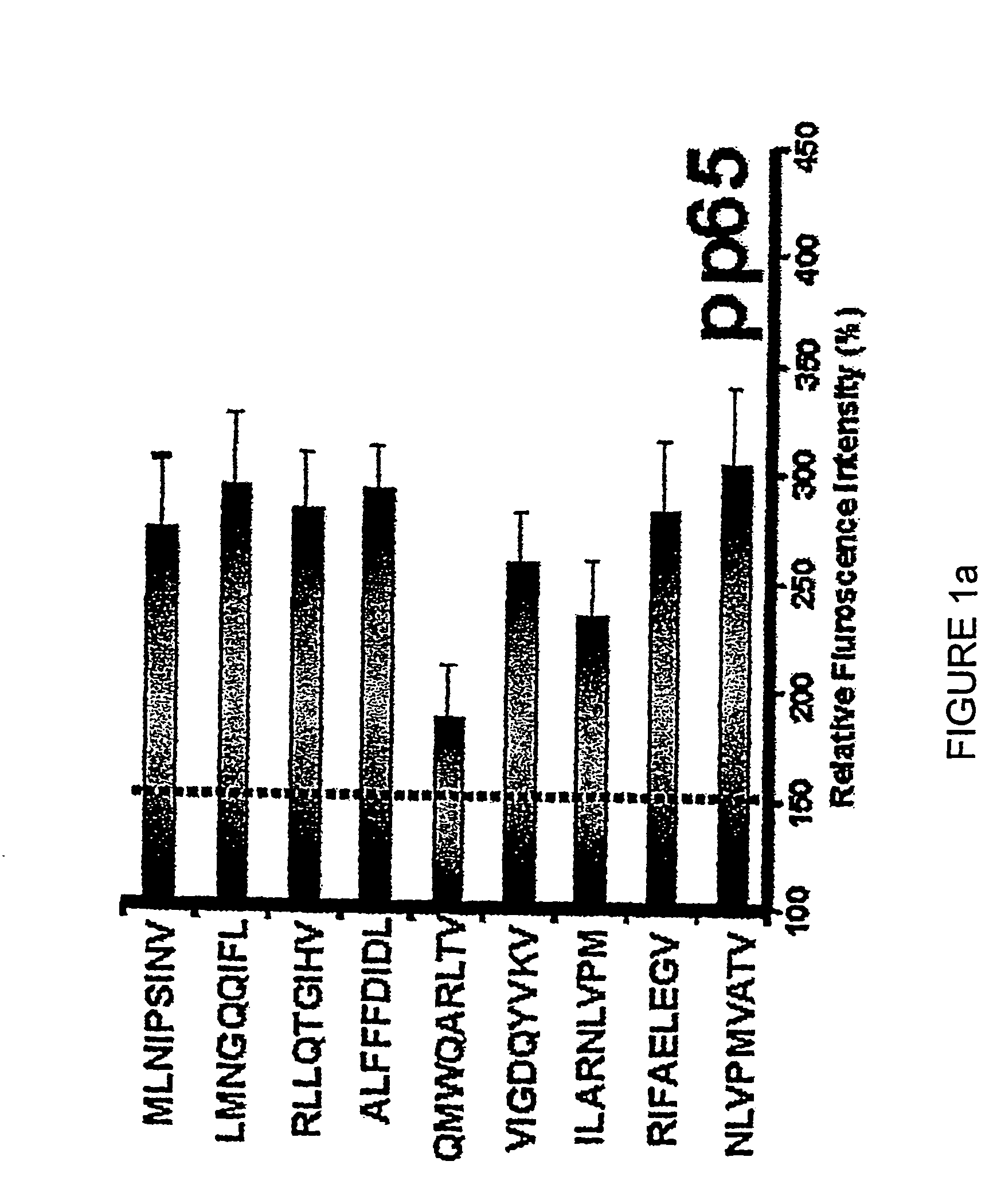
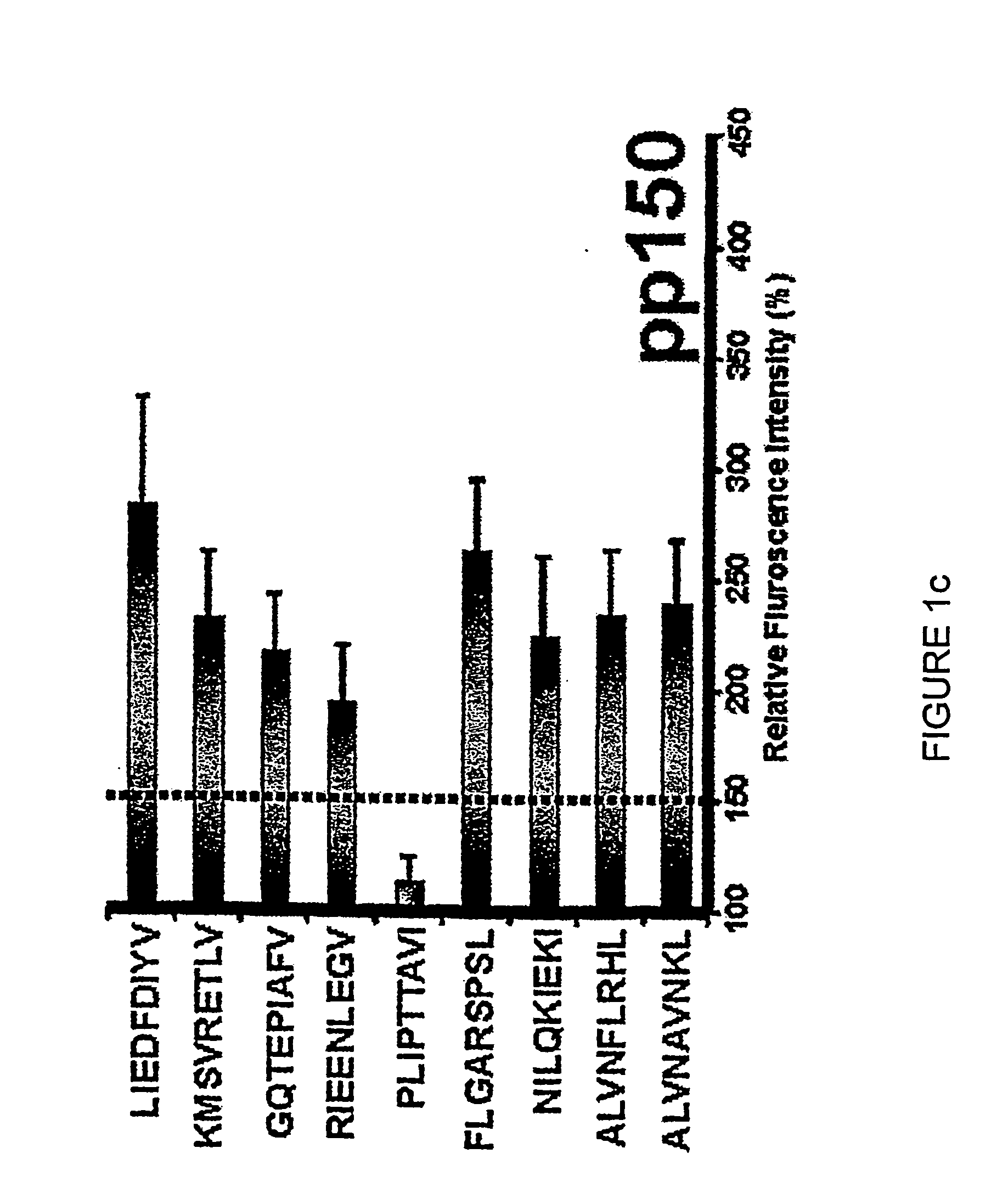
Novel human cytomegalovirus (hcmv) cytotoxic t cell epitopes, polyepitopes compositions comprising same and diagnostic and prophylactic and therapeutic uses therefor
InactiveUS20050019344A1Minimizing formulation difficultyReduce in quantityViral antigen ingredientsMicrobiological testing/measurementCtl epitopeVaccination
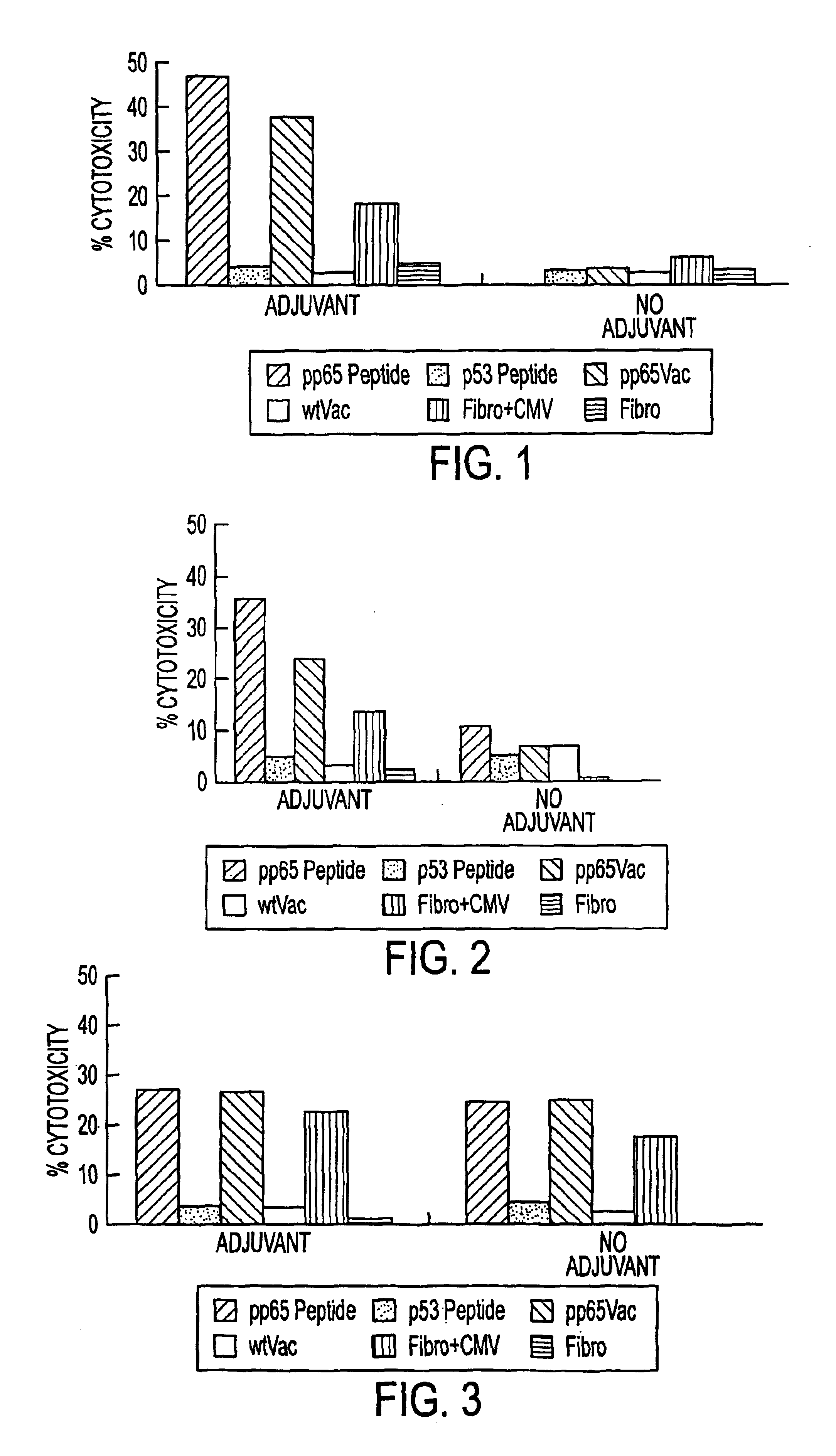
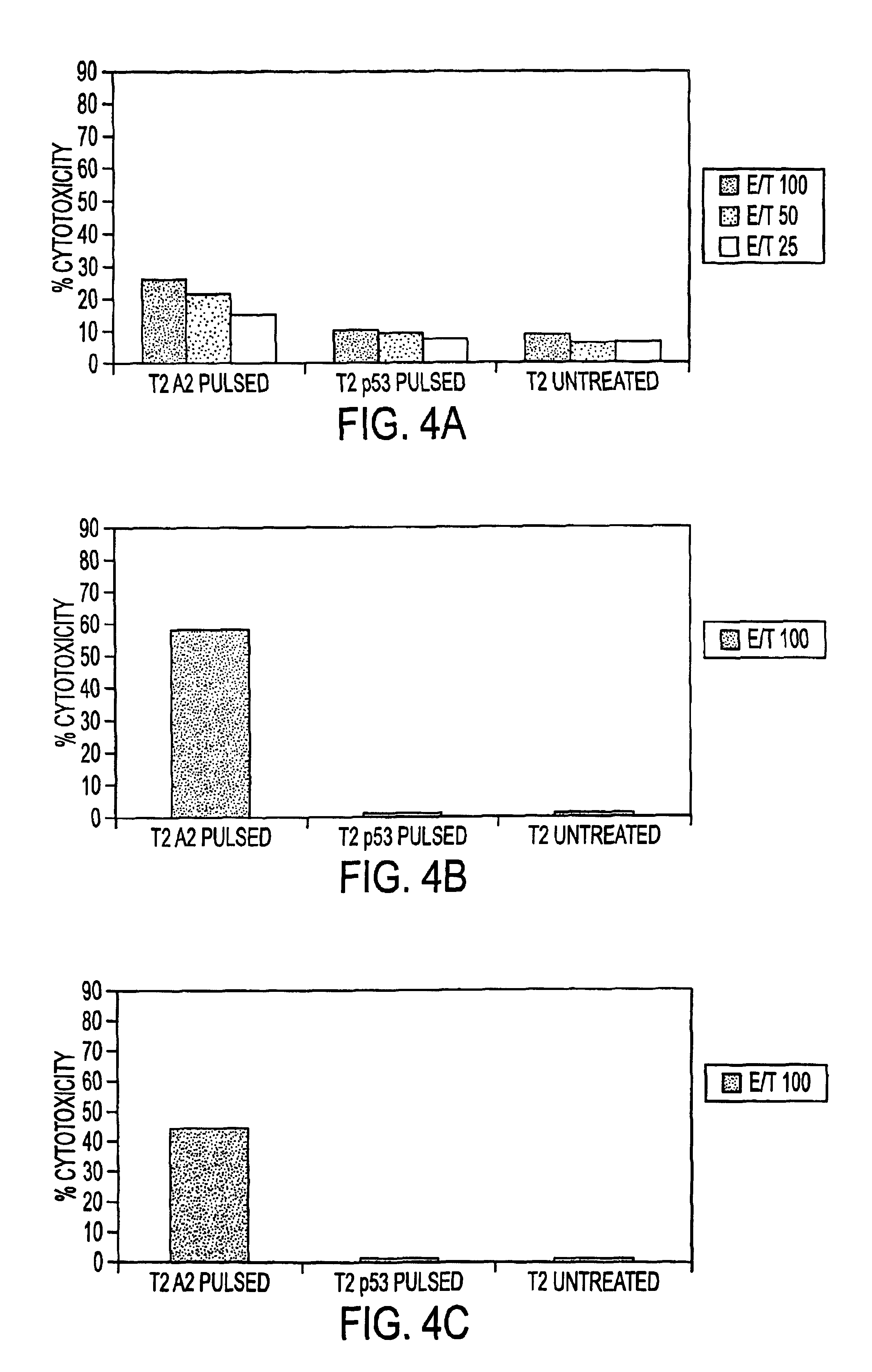
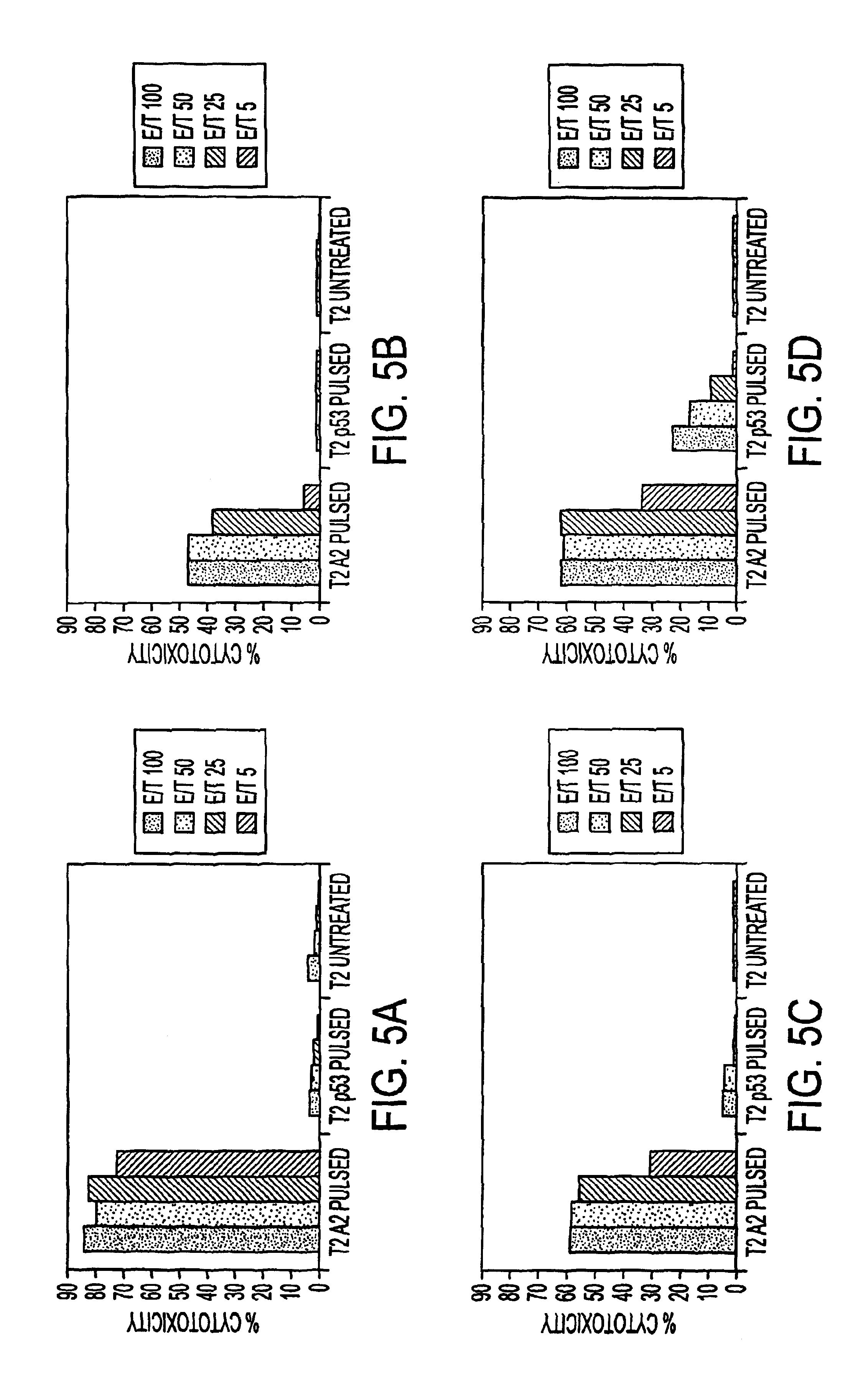
The present invention provides CTL epitope peptides and polyepitope peptides from 14 distinct antigens of human cytomegalovirus (HCMV) that are restricted through HLA the most commonly prevalent class I alleles in different ethnic populations of the world. These epitopes provide an important platform for CTL epitope-based vaccines against HCMV. The present invention further provides vaccine composiitons comprising the subject epitope and polyepitope peptides and methods for vaccination of humans and for the adoptive transfer of HCMV-specific T cells to human subjects. The present invention further provides reagents and methods for determining the HCMV status or level of HCMV-specific immunity of a subject.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Mixed lipopeptide micelles for inducing an immune response
The invention concerns mixed micelles or micro-aggregates for inducing an immune response containing at least a first lipopeptide comprising a CTL epitope and at least a first lipid motif; and a second lipopeptide comprising at least an auxiliary T epitope and at least a lipid motif, whereof the type can be different from the first lipopeptide motif. Said micelles can be used as medicines and vaccines.
Owner:INSTITUT PASTEUR DE LILLE +2
Immuno-reactive peptide CTL epitopes of human cytomegalovirus
The invention provides a plurality of peptides (and immunologically functional variants thereof) which are immunogenic epitopes recognized by CD8<+> class I MHC restricted cytotoxic T-lymphocytes of patients harboring latent human cytomegalovirus (HCMV) infection. The peptides are capable of activating CTLs and CTLps in the absence of active viral replication, and thus are useful for eliciting a cellular immune response against HCMV by normal and immunodeficient subjects. Peptide and lipopeptide vaccines, with and without adjuvants, also are disclosed. Cellular vaccines comprising the peptides form a further embodiment of this invention.
Owner:CITY OF HOPE
Synthetic vaccine agents
InactiveUS20040191264A1Promote absorptionImprove responseBacterial antigen ingredientsSnake antigen ingredientsCtl epitopeHelper epitope
The present invention provides for novel immungens that are comprised of an activated polyhydroxypolymer backbone to which is attached 2 separate antigenic determinants. The 1st antigenic determinant includes a B-cell or CTL epitope and the 2nd antigenic determinant includes a T-helper epitope. In preferred embodiments, the antigenic determinants are derived from different molecules and species. Exemplary immunogens of the invention are constituted of a linear tresyl-activated dextran backbone to which is coupled B-cell or CTL epitopes of an antigen and to which is also coupled universal T-helper epitopes. Also disclosed are immunogenic compositions comprising the immunogens, methods of immunisation and a method for identification of suitable immunogens of the invention.
Owner:PHARMEXA
Mutated ras peptides for generation of CD8+Cytotoxic T lymphocytes
InactiveUS7709002B1Growth inhibitionAvoid it happening againBacterial antigen ingredientsPeptide/protein ingredientsCtl epitopeMutated Ras Peptide
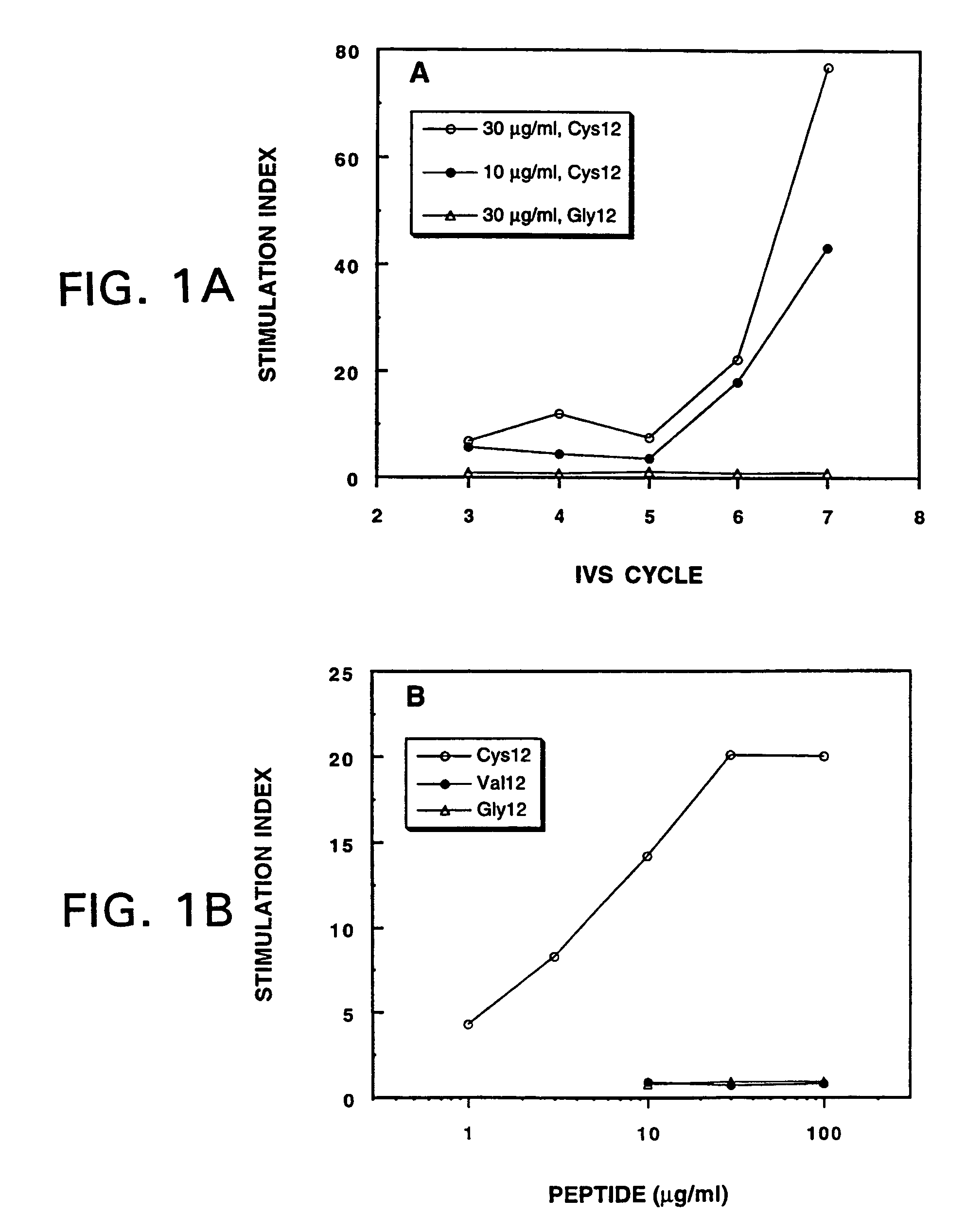
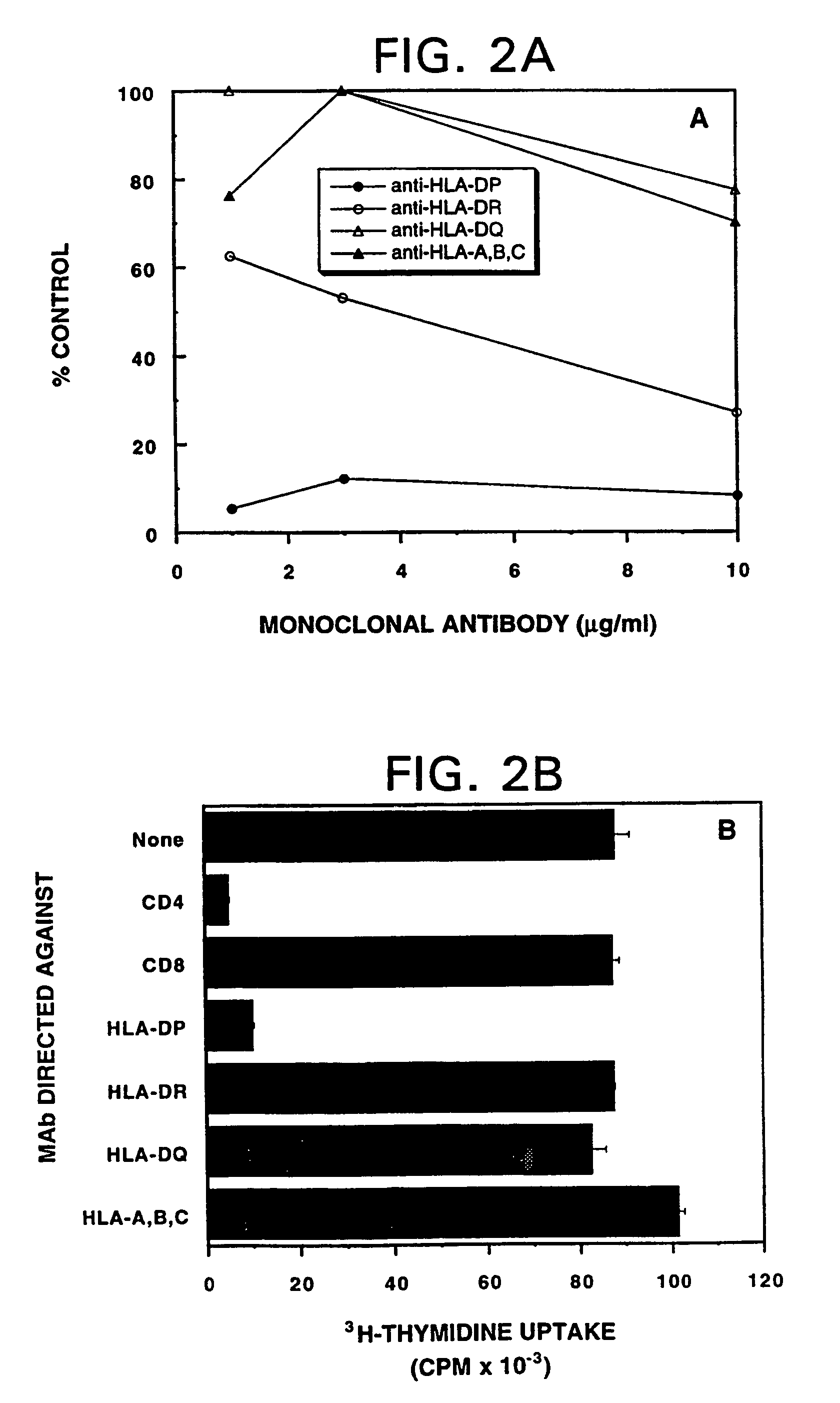
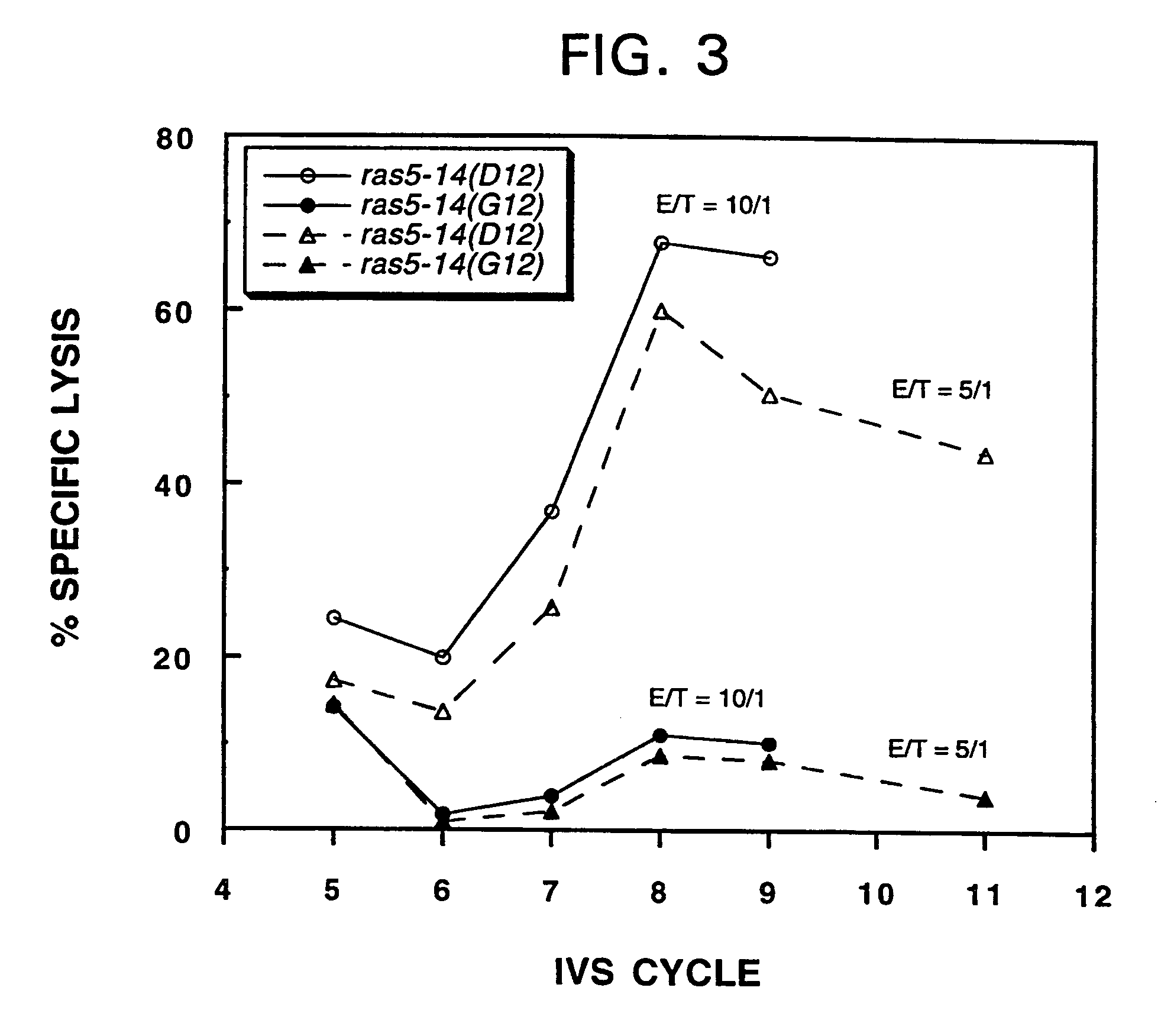
Mutant ras oncogene peptides may induce specific anti-ras cellular immune responses in vaccinated patients. Moreover, a human CD8+ CTL epitope(s) reflecting a specific point mutation in the K-ras oncogene at codon 12 was identified. The mutant ras peptide has implications for both active and passive immunotherapies in selected carcinoma patients. A nested 10-mer peptide was identified [i.e., ras5-14(Asp12)], which was shown to bind to HLA-A2 and display specific functional capacity for expansion of the in-vivo-primed CD8+ CTL precursors.
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
T cell receptors specific for immunodominant ctl epitopes of hcv
InactiveUS20130011375A1Improve isolationPromote productionFungiVirusesCtl epitopeImmunologic function
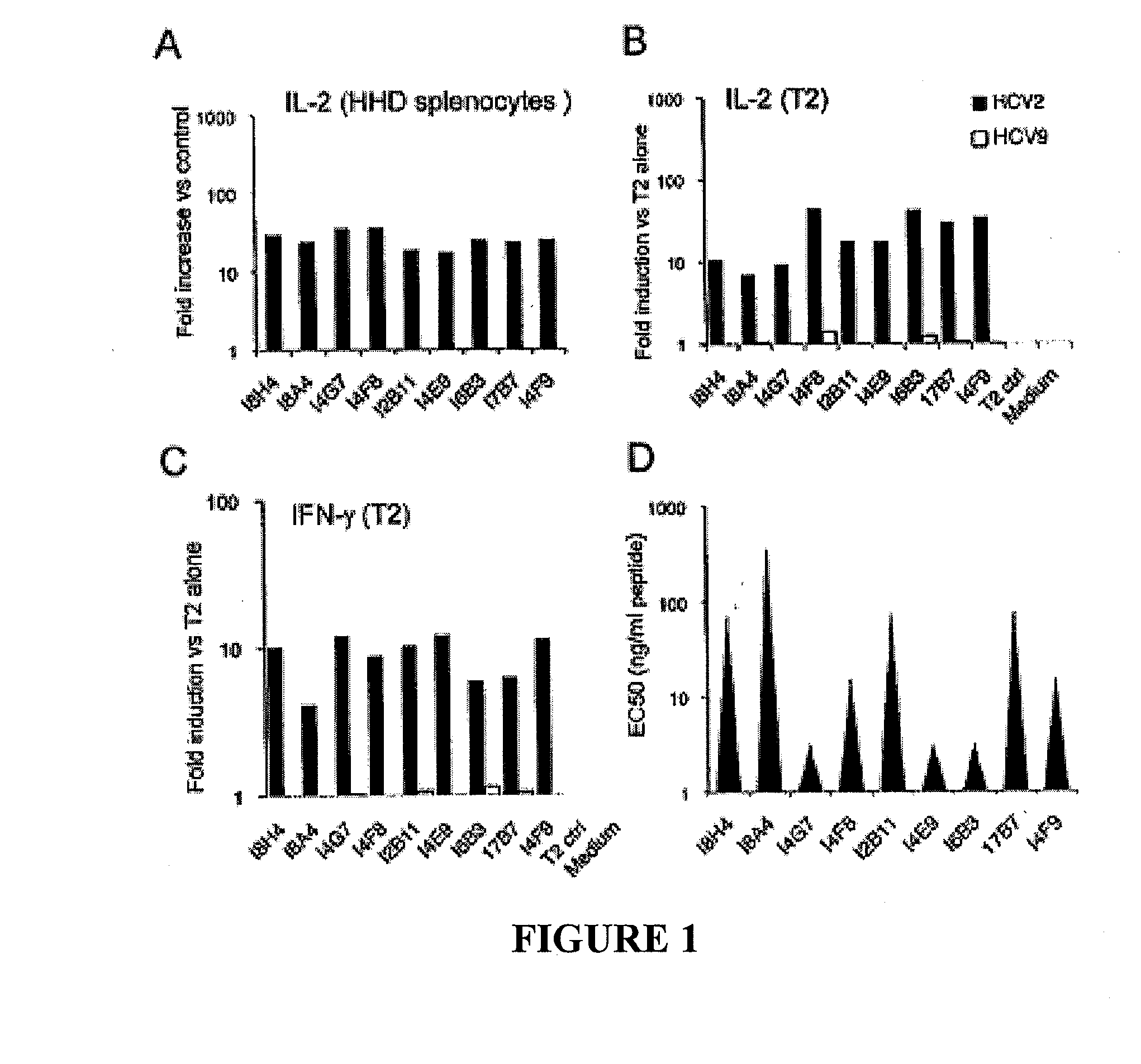
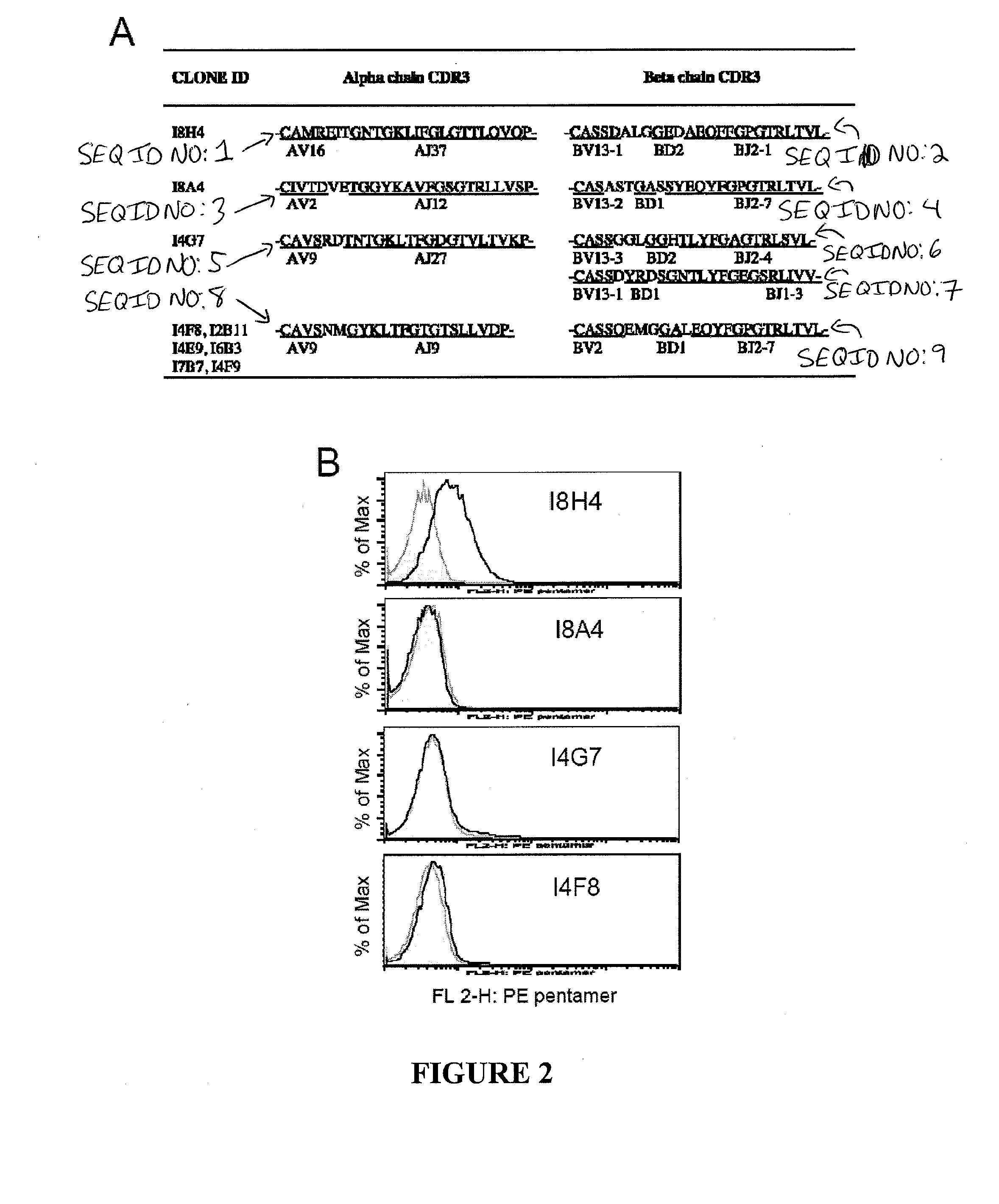
The present invention relates generally to the field of immunology. More particularly, aspects of the invention concern the discovery of several T cell receptors (TCRs) that are specific for an immunodominant CTL epitope of hepatitis C virus (HCV). Embodiments include TCRs, DNAs encoding TCRs, methods of making TCRs, and methods of using TCRs to treat, prevent or inhibit hepatitis C virus (HCV) proliferation.
Owner:TRIPEP
Human cytomegalovirus (HCMV) cytotoxic T cell epitopes, polyepitopes compositions comprising same and diagnostic and prophylactic and therapeutic uses therefor
InactiveUS7524503B2Minimize difficultyReduce in quantityPeptide/protein ingredientsVirus peptidesCtl epitopeVaccination
The present invention provides CTL epitope peptides and polyepitope peptides from 14 distinct antigens of human cytomegalovirus (HCMV) that are restricted through HLA the most commonly prevalent class I alleles in different ethnic populations of the world. These epitopes provide an important platform for CTL epitope-based vaccines against HCMV. The present invention further provides vaccine compositions comprising the subject epitope and polyepitope peptides and methods for vaccination of humans and for the adoptive transfer of HCMV-specific T cells to human subjects. The present invention further provides reagents and methods for determining the HCMV status or level of HCMV-specific immunity of a subject.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Hepatitis C virus-derived peptides capable of inducing cytotoxic T lymphocyte responses
InactiveUS7220420B2Strengthen and boostSubstantial homologySsRNA viruses positive-sensePeptide/protein ingredientsCtl epitopeChronic hepatitis
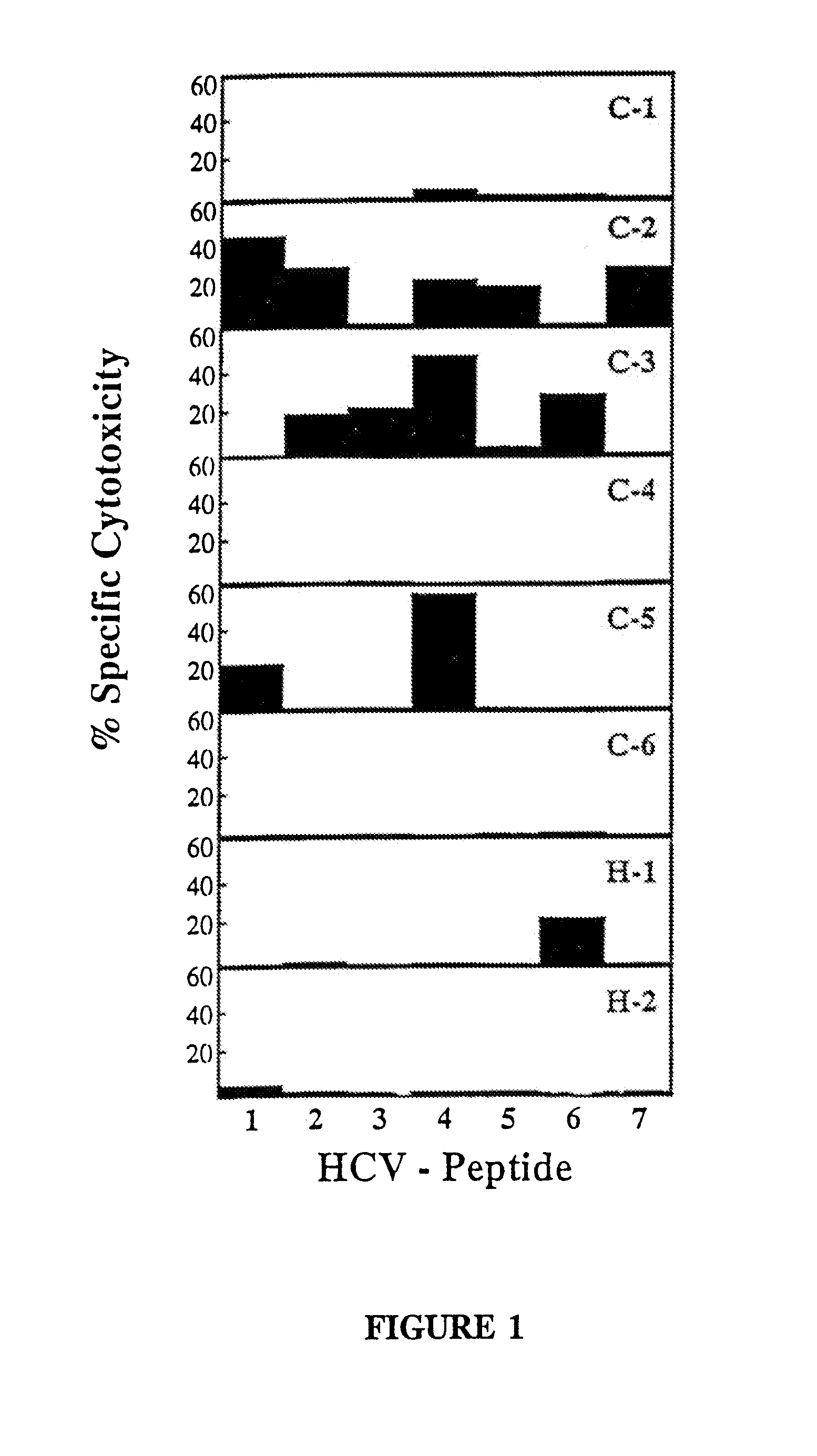
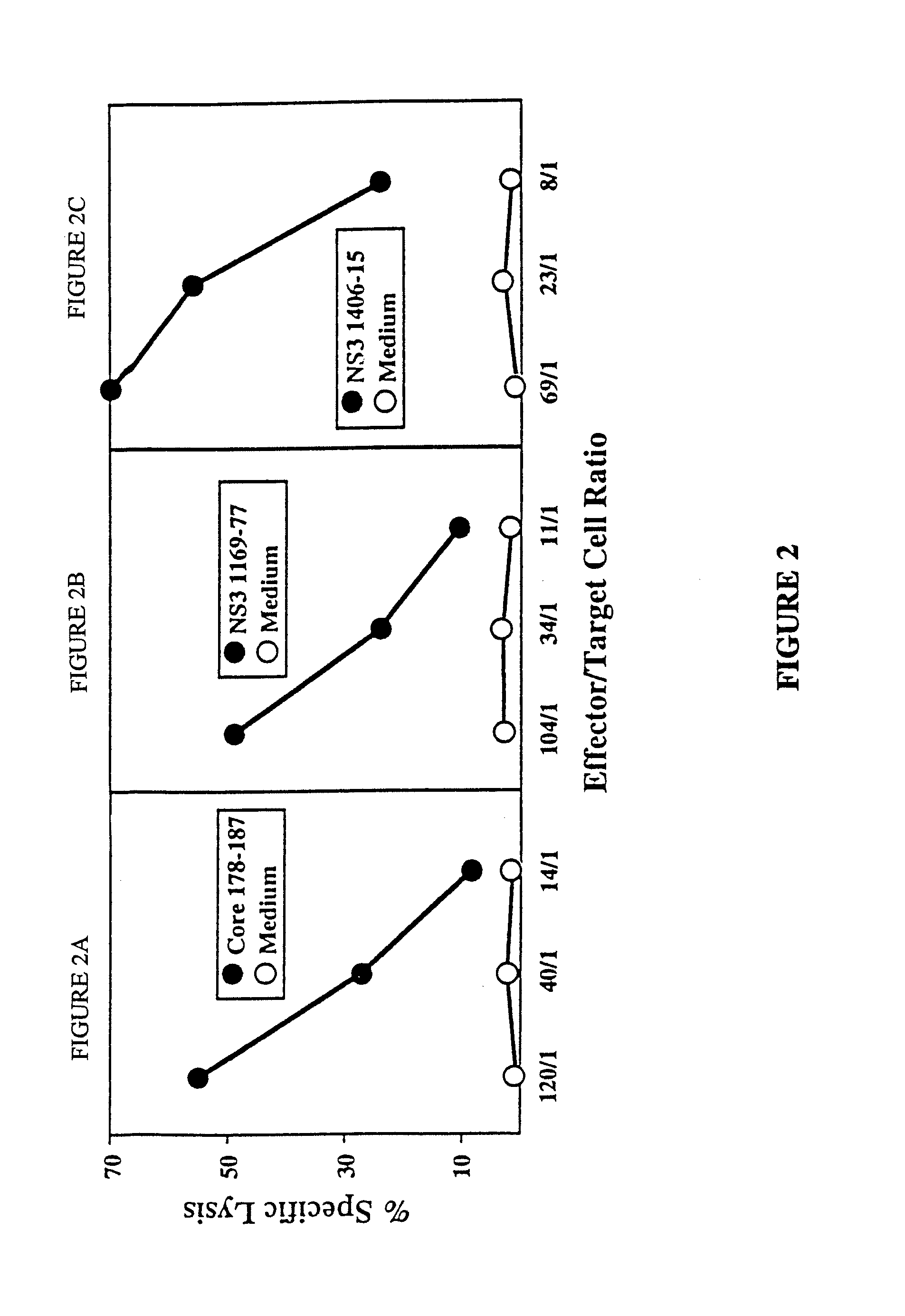
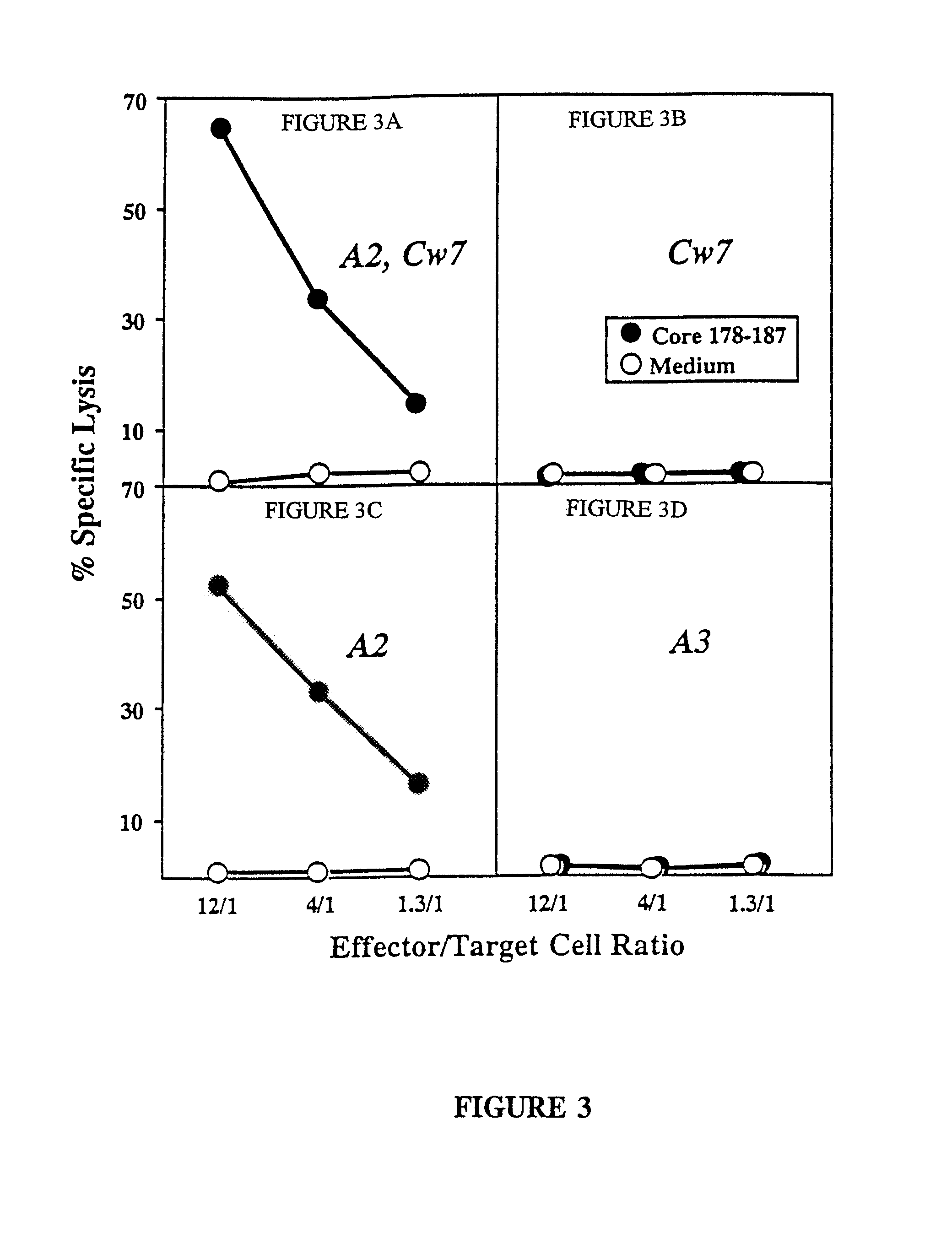
The present invention is directed to a molecule comprising a polypeptide having substantial homology with a CTL epitope selected from the group consisting of ADLMGYIPLV (Core131-140; SEQ ID NO:1), LLALLSCLTV (Core178-187; SEQ ID NO:2), QLRRHIDLLV (SEQ ID NO:55), LLCPAGHAV (NS31169-1177; SEQ ID NO:26), KLVALGINAV (NS31406-1415; SEQ ID NO:28), SLMAFTAAV (NS41789-1797; SEQ ID NO:34), LLFNILGGWV (NS41807-1816; SEQ ID NO:35), and ILDSFDPLV (NS52252-2260; SEQ ID NO:42). Such molecules are used for the treatment and prevention of acute or chronic HCV hepatitis; suitable pharmaceutical compositions and methods using such compositions are disclosed.
Owner:THE SCRIPPS RES INST
Toxicity T cell position vaccine of the cell for treating Hepatitis B and the preparing method
The invention relates to bio-pharmaceutical engineer technology domain. At present, the therapeutic drugs for HBV infection mainly depend on IFN-Alpha and nucleotide analog, which can not kill virus thoroughly with worse remote therapeutic effect. The invention provides a cytotoxic T cell epitope vaccine for hepatitis B, comprising a fused polypeptide formed by connecting 21 CTL epitopes selected from CTL epitopes data of HBV antigen and two universal epitopes of auxiliary T lymphocyte. Saccharomyces Serevisiae is selected to express the fused antigen and mixes with immunologic adjvant. The said vaccine can activate and enhance the cellular immune response of patient with chronic HBV infection. The vaccine can also promote the immune elimination of HBV, and can be used for treatment of chronic hepatitis B.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
NY-ESO-1 tumour antigen mimic epitope and use thereof
InactiveCN101381402AImproving immunogenicityStrong specificityPeptidesAntibody medical ingredientsCtl epitopePredictive methods
The invention discloses epitope for an NY-ESO-1 tumor antigen. The amino acid sequence of the mimic epitope is Ser-Leu-Leu- Met-Phe-Ile-Thr-Trp-Cys, namely SLLMFITWC; the mimic epitope can raise a CTL immunity response, can undergo a cross reaction with a natural epitope, has the characteristics of strong immunogenicity, immune tolerance breaking, strong specificity, safety, low cost, and easy synthesis and storage, and can be used to prepare tumor-therapeutic polypeptide vaccine. The invention also discloses a method for predicting the mimic epitope, which comprises steps of the establishment of a structural model of a TCR-pMHC complex, the analysis of TCR binding sites of the epitope, and the analysis of amino acid replacement of the TCR binding sites of the epitope. The method is also applicable to the computer-aided modification of CTL epitopes of other antigens, and can provide a useful tool for the design and research of therapeutic polypeptide vaccine.
Owner:ARMY MEDICAL UNIV
CTL epitopes from EBV
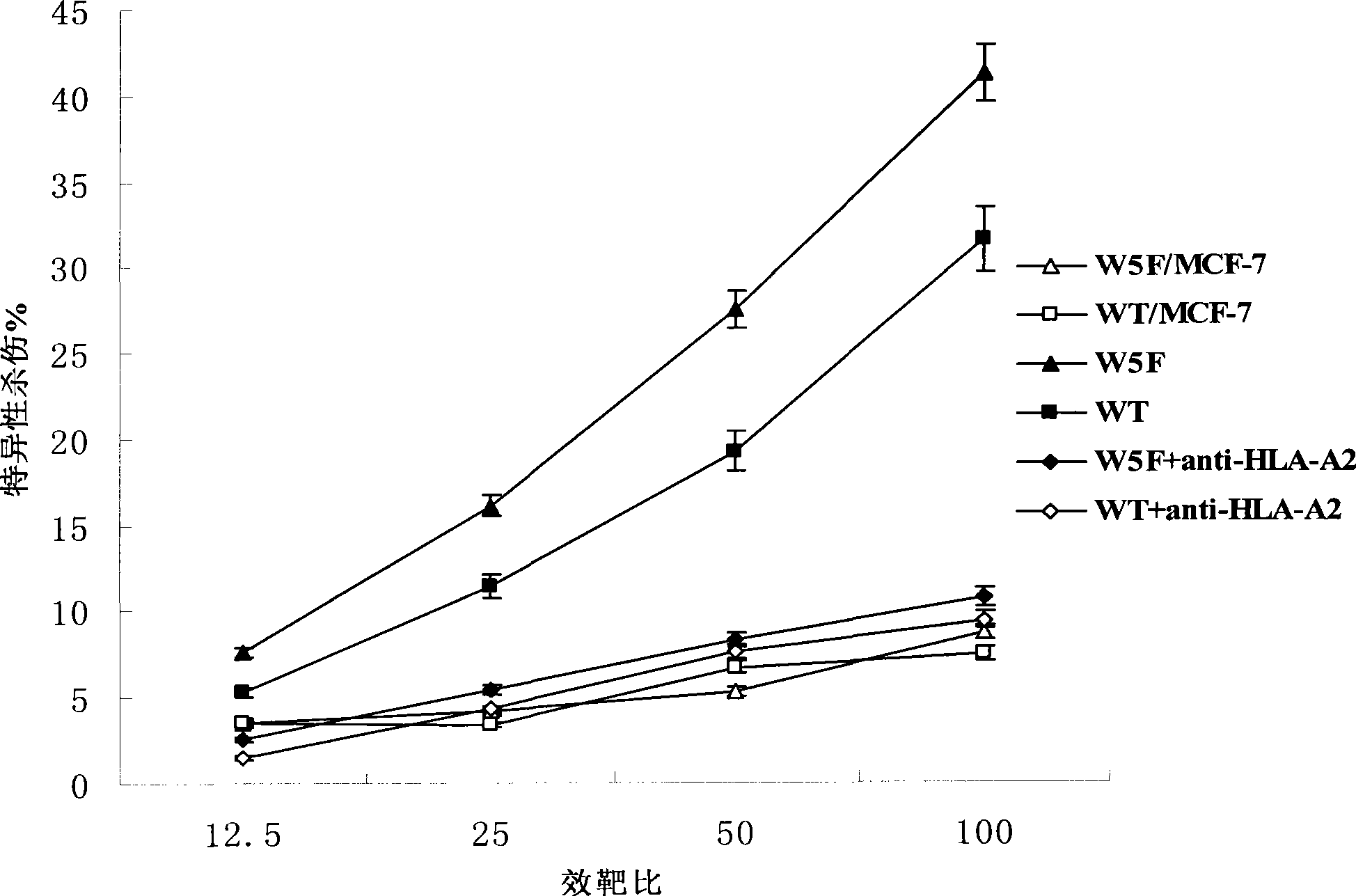
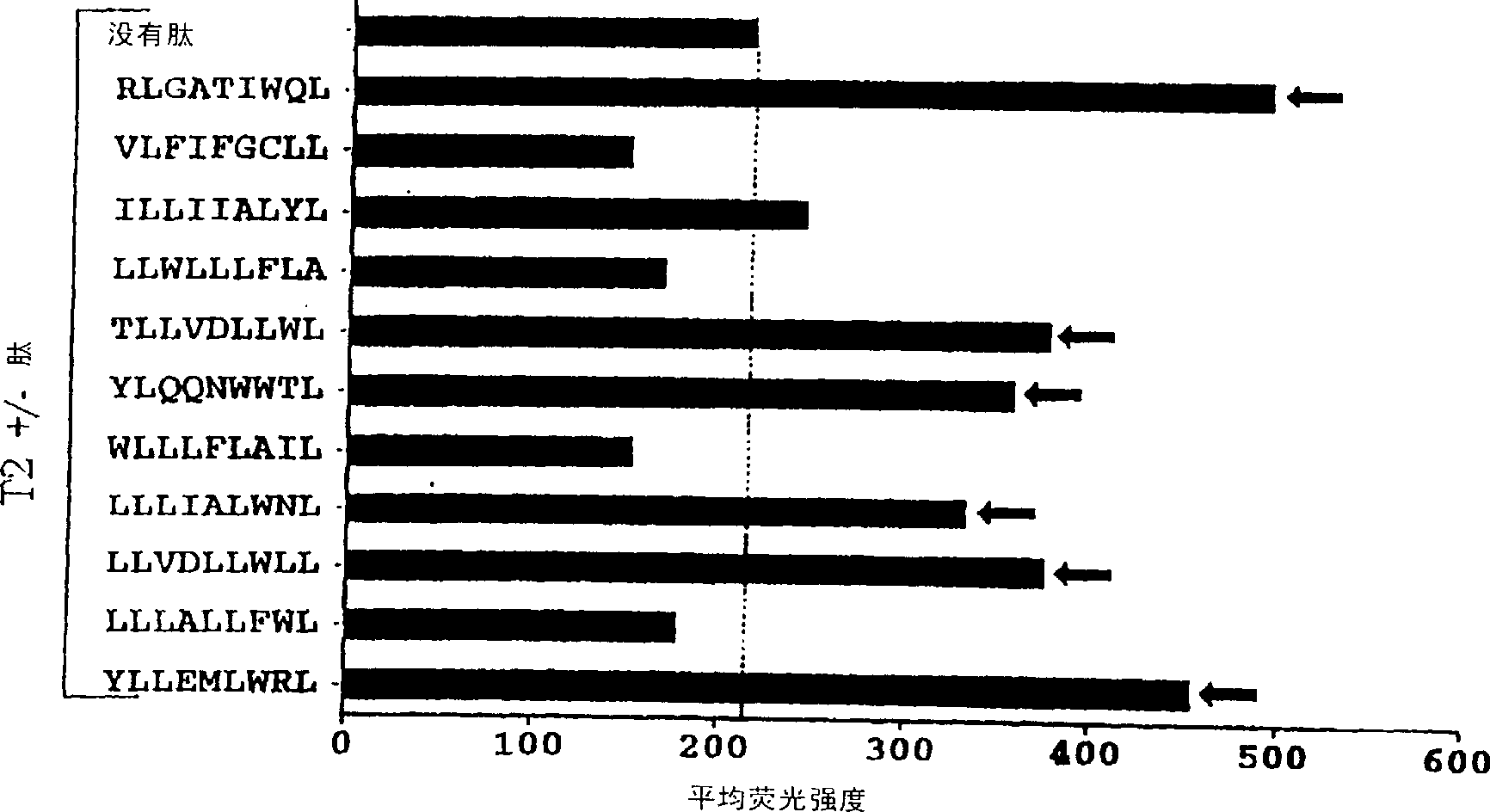
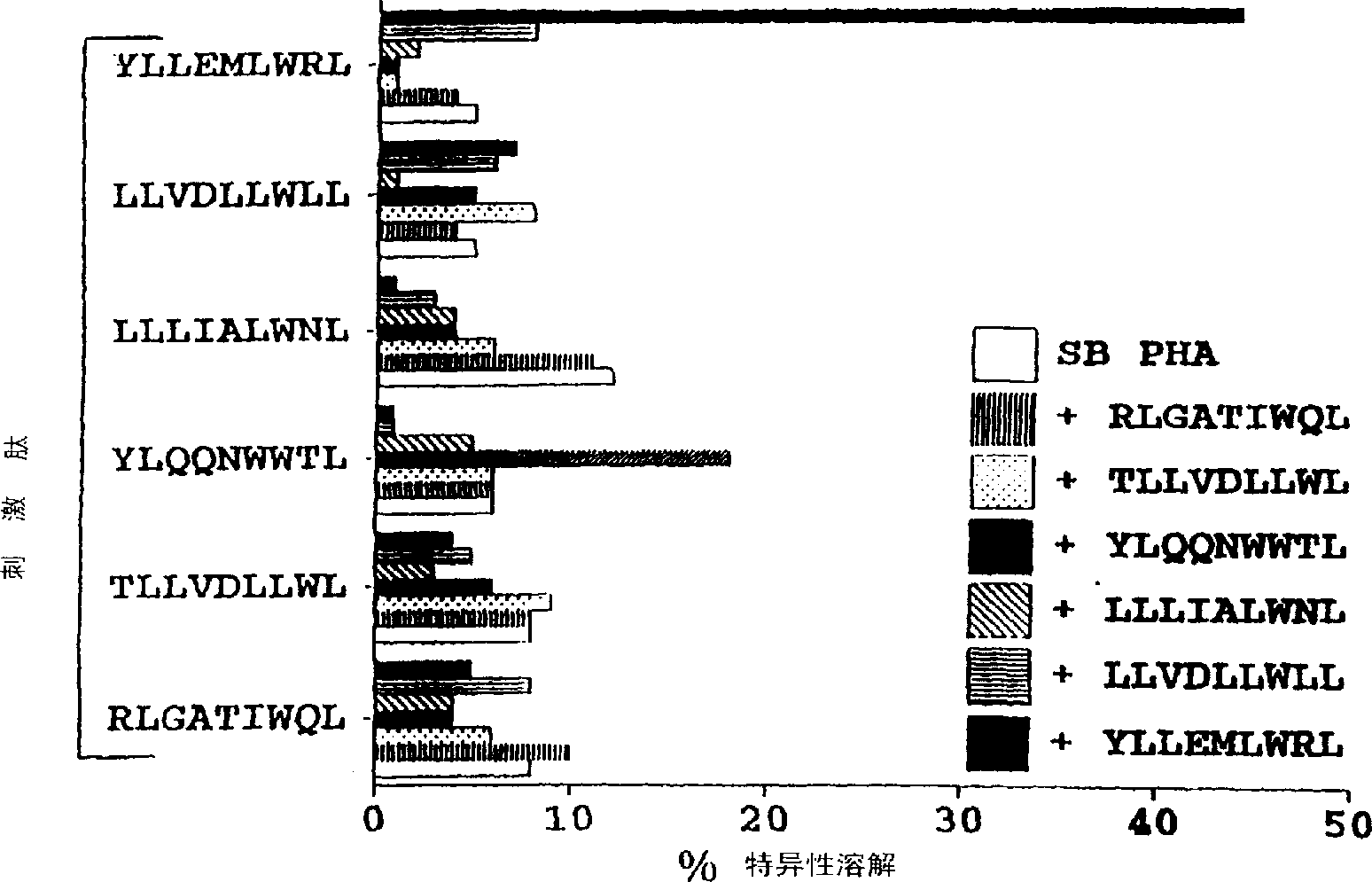
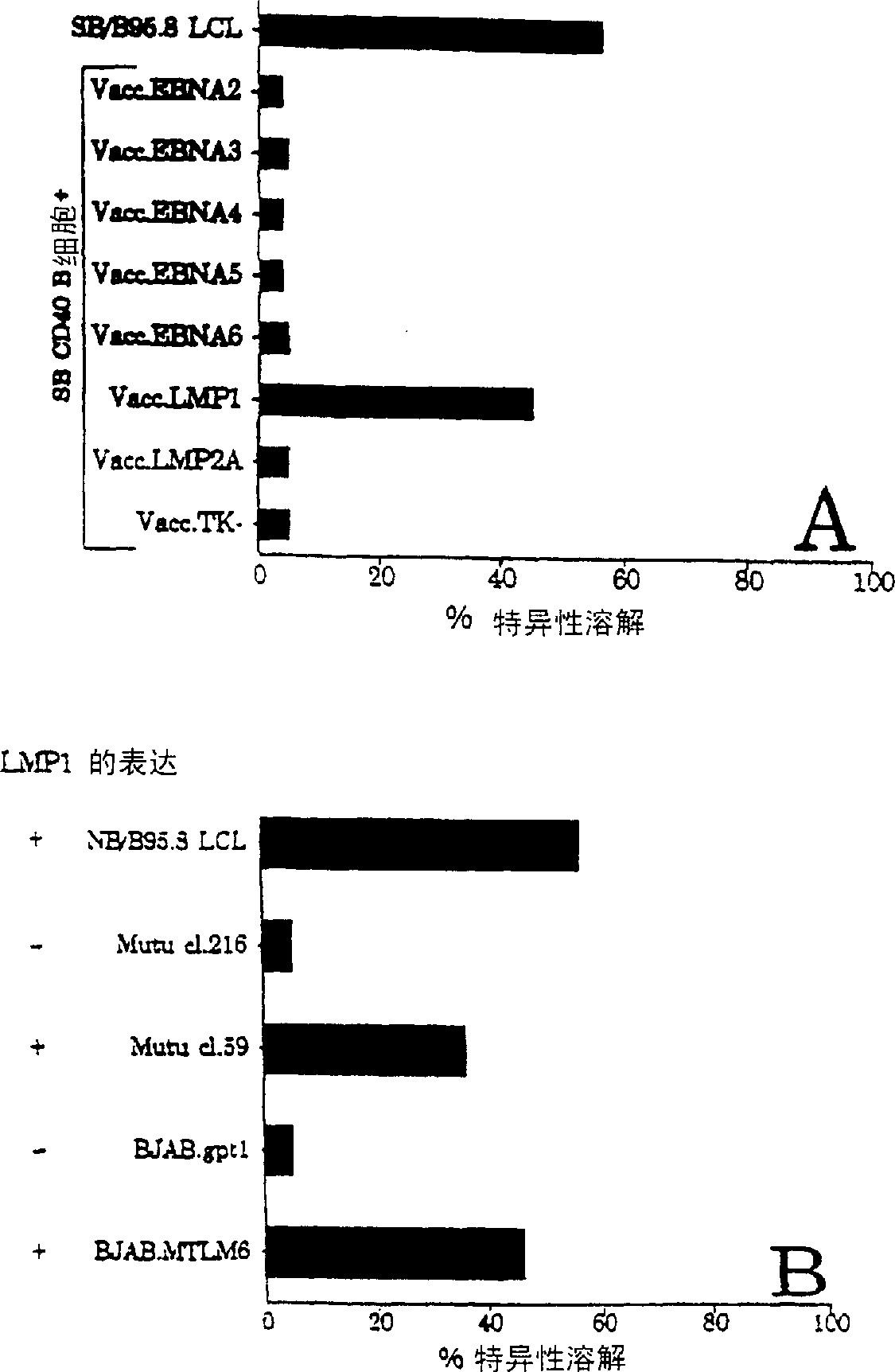
The present invention provides cytotoxic Epstein-Barr virus (EBV) T-cell epitopes derived from EBV structural antigens. Preferred epitopes include YLLEMLWRL (SEQ ID NO:1), YFLEILWGL (SEQ ID NO:32), YLLEILWRL (SEQ ID NO:33), YLQQNWWTL (SEQ ID NO:6), LLLALLFWL (SEQ ID NO:2), LLVDLLWLL (SEQ ID NO:3), LLLIALWNL (SEQ ID NO:4), WLLLFLAIL (SEQ ID NO:5), TLLVDLLWL (SEQ ID NO:7), LLWLLLFLA (SEQ ID NO:8), ILLIIALYL (SEQ ID NO:9), VLFIFGCLL (SEQ ID NO:10), RLGATIWQL (SEQ ID NO:11), ILYFIAFAL (SEQ ID NO:15), SLVIVTTFV (SEQ ID NO:17), LMIIPLINV (SEQ ID NO:20), TLFIGSHVV (SEQ ID NO:24), LIPETVPYI (SEQ ID NO:26), VLQWASLAV (SEQ ID NO:27) and QLTPHTKAV (SEQ ID NO:29). The present invention also provides methods of treating or preventing EBV infection in subjects which involve administration of EBV cytotoxic T-cell epitopes.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Peptides recognized by melanoma-specific A1-, A2- and A3-restricted cytoxic lymphocytes, and uses therefor
Melanoma-specific, A1- and A3-restricted CTL epitopes have been identified in tyrosinase and pMel-17, respectively, and may be used in the design of vaccines.
Owner:RHEARG +1
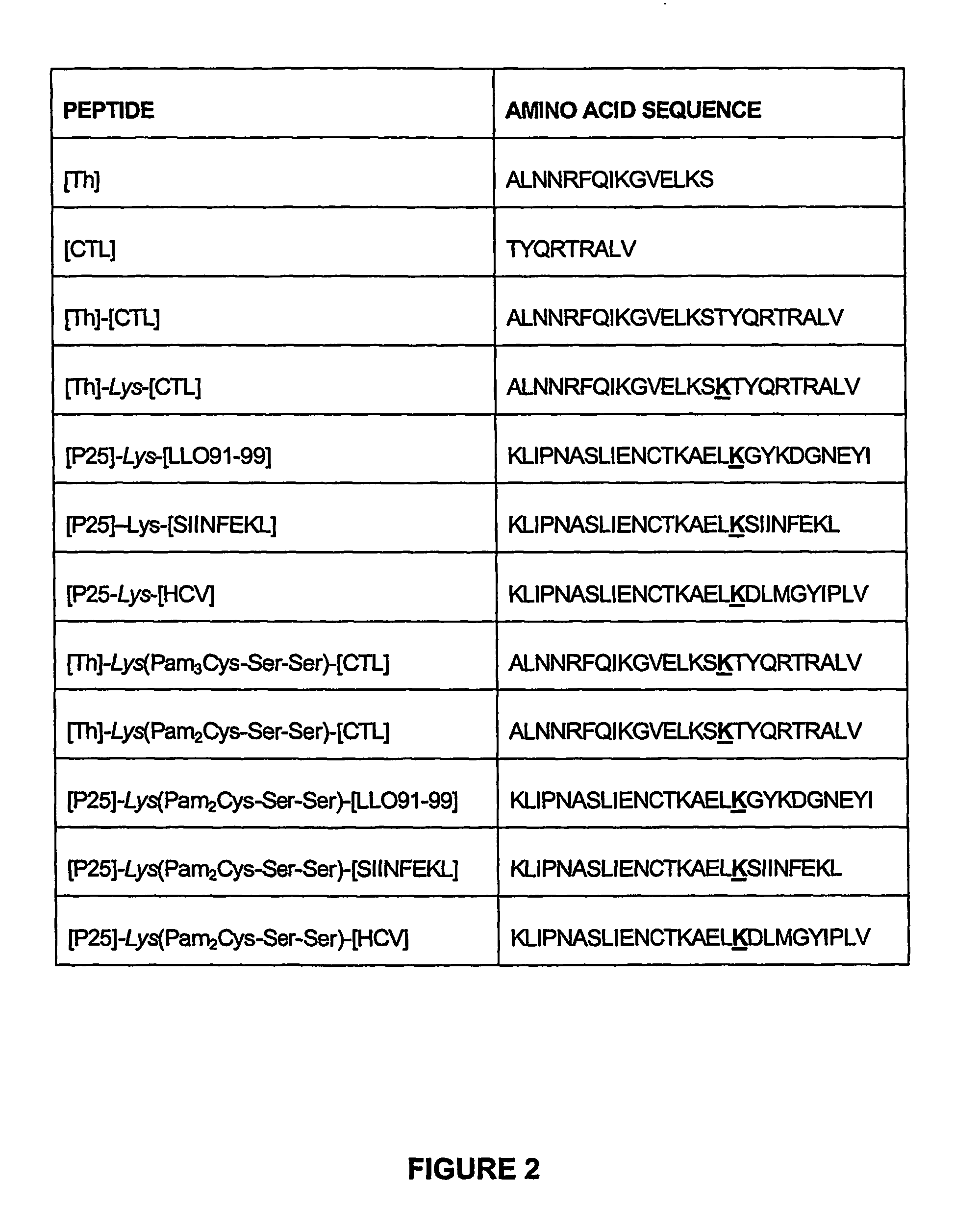
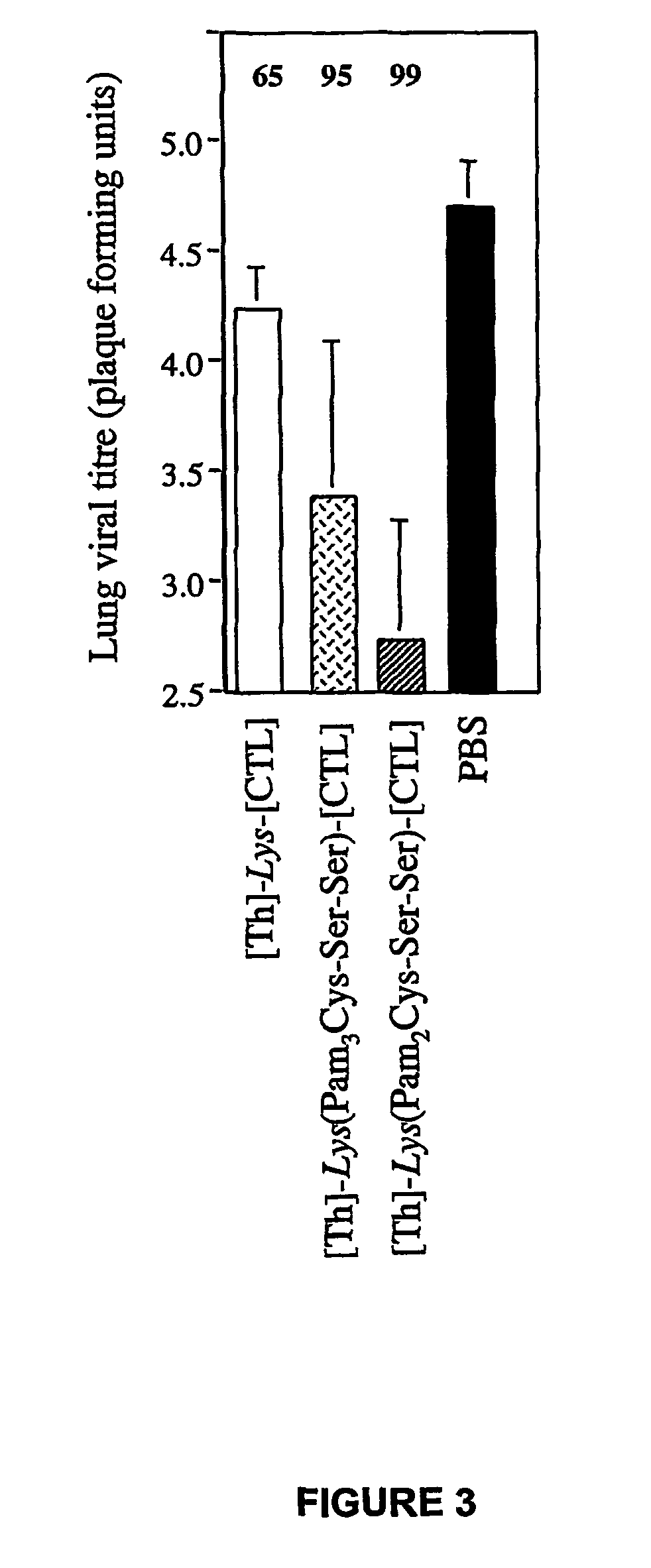
Immunogenic lipopeptides comprising T-helper and cytotoxic T lymphocyte (CTL) epitopes
InactiveUS7833532B2Easy to synthesizeIncrease profitSsRNA viruses negative-senseBiocideSynthetic ImmunogensCtl epitope
The present invention provides synthetic immunogenic lipopeptide molecules comprising co-linear T-helper and CTL epitopes, and methods for their production and use in the generation of primary and secondary immune responses, and for the vaccination of animal subjects against particular CTL epitopes. More particularly, the present invention provides highly soluble lipopeptides wherein the lipid moiety is attached to the terminal side-chain group of an internal lysine or lysine analog, preferably to the terminal side-chain group of an internal diamino acid residue. Preferably the internal lysine or lysine analog is positioned between the T-helper epitope and the CTL epitope.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
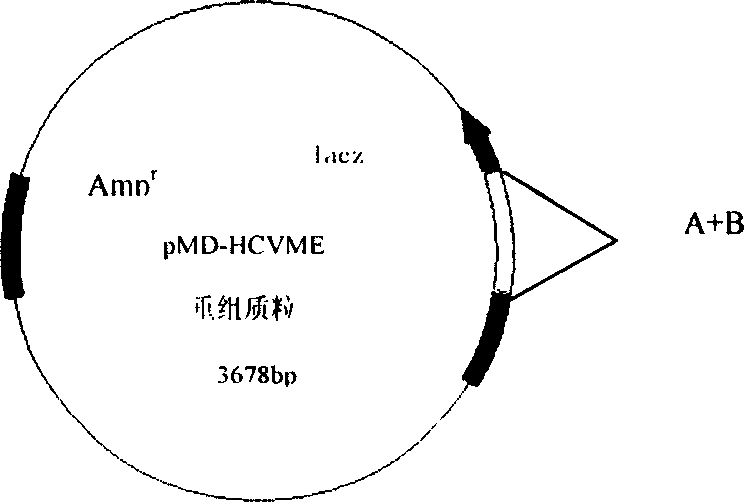
Gene cloning of polyepitope antigen of hepatitis C virus and its coding sequence
InactiveCN1609213AAvoid the disadvantage of being prone to mutationValid submissionGenetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCtl epitopeGene clone
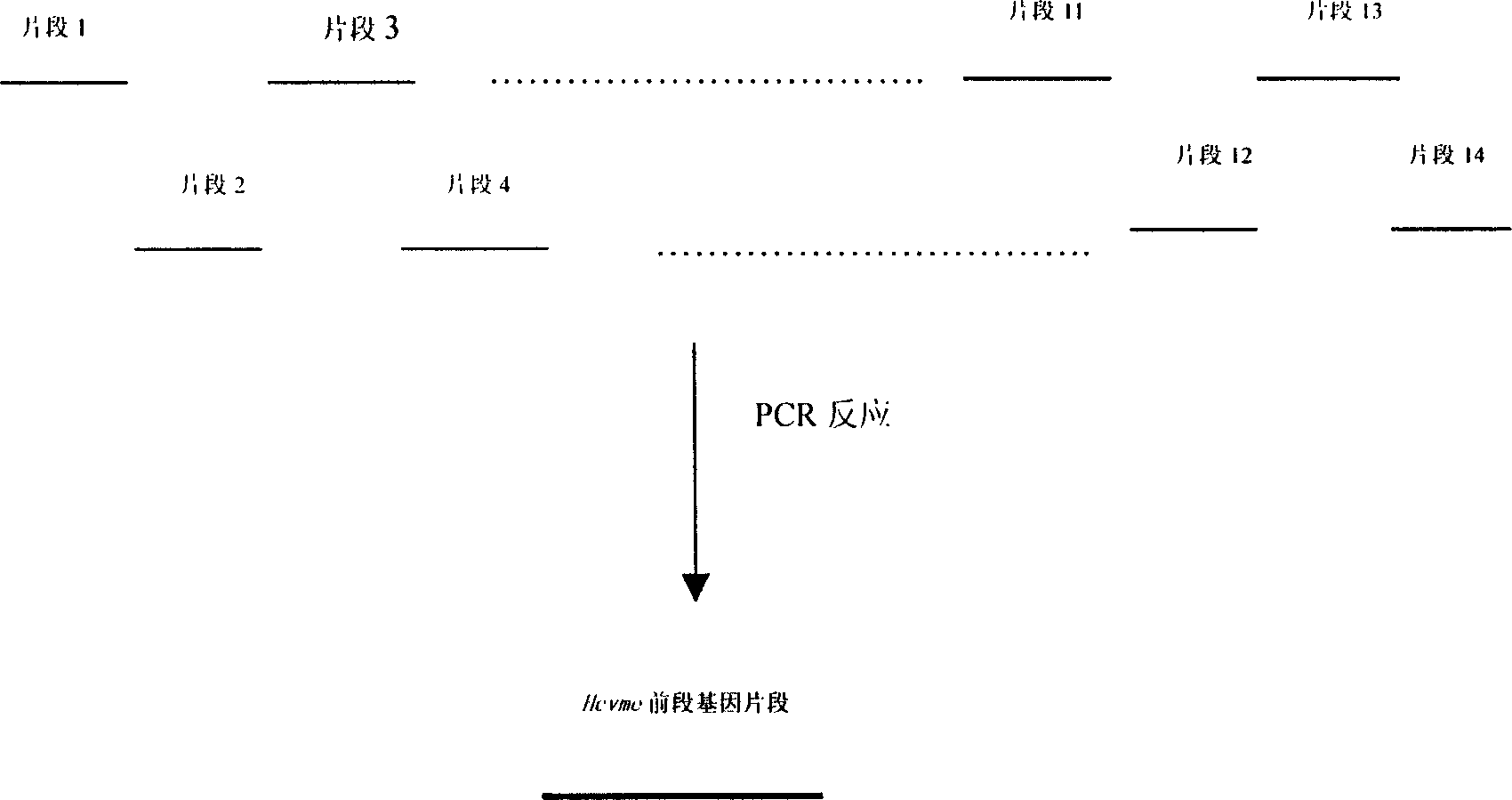
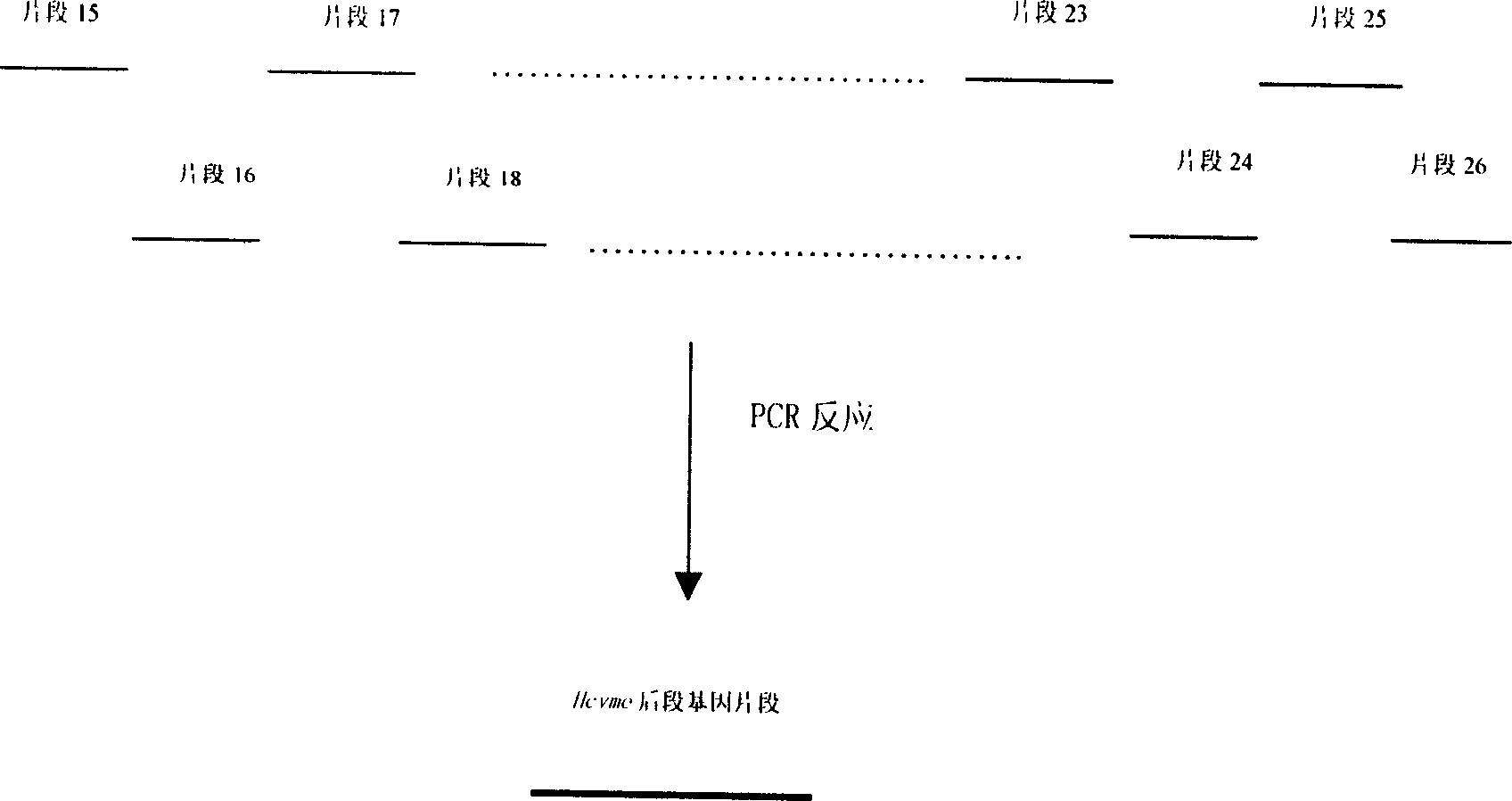
The present invention relates to biomedicine engineering technology, and is the gene cloning of polyepitope antigen of hepatitis C virus hcvme and the coded polyepitope antigen HCVME. The hcvme includes 9 epitope genes of HVR1 simulating B cell and 4 epitope genes of T cell in E2 region of HCV genome, and the 4 epitope genes of T cell includes 2 of conservative CTL epitope in region C, 1 of conservative CTL epitope in region NS3 and 1 of conservative Th epitope in region NS3. Serial tests show that hcvme has high cross reaction with karyogamy expressed product GST-HCVME of gst gene and positive hepatitis C antibody serum, and the GST-HCVME immunized male rabbit serum has high distinction frequency on natural HVR1 synthetic peptide. Therefore, hcvme and its expressing product HCVME may be used in preparing nucleic acid vaccine and polypeptide vaccine for hepatitis C and the test reagent for HCV antigen and antibody.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Synthetic conjugate of CpG single-stranded DNA and T-help/CTL fusion peptide
ActiveUS20050158336A1Improve efficiencySimple compositionAntibody mimetics/scaffoldsGenetic material ingredientsAntigenCtl epitope
Highly effective vaccine compositions are constructed according to the methods of this invention. The methods are amenable to use with any peptidic antigen sequence and involve covalent attachment of an immunostimulatory nucleotide sequence to an antigenic peptide sequence. Preferred antigenic peptides are fusion peptides made up of one or more CTL epitope peptides in sequence fused to a T helper peptide.
Owner:CITY OF HOPE
Respiratory syncytial virus sub-units vaccine, preparation and application
InactiveCN101264323ALimit developmentEnhance cellular immune responseDepsipeptidesAntiinfectivesEscherichia coliCtl epitope
The invention relates to a respiratory syncytial virus vaccine, and the preparation method and application, in particular to an application of escherichia coli to express and recombinant respiratory syncytial virus G protein and mutation G protein, and preparation vaccine with nontoxic typed escherichia coli heat labile enterotoxin, for human or animal preventive inoculation, and for respiratory syncytial virus resistance, belonging to the field of biotechnology. The respiratory syncytial virus vaccine is characterized in that: the respiratory syncytial virus subunits vaccine comprises lopped respiratory syncytial virus RSV protein G, and further comprises nontoxic typed escherichia coli heat labile enterotoxin LT adjuvant. The vaccines are lopped respiratory syncytial virus RSV protein G containing amino acid between aa130 and 230 of the original G protein, and substitutes the amino acid CAWIC (CX3C module ordered) between aa182 and 186 with the amino acid YLEKESIYY (CTL epitope) on the RSV M protein, forming the GCIL protein. The respiratory syncytial virus vaccine has the advantages of remarkable practical significance for preventing human respiratory syncytial virus infection.
Owner:KUNMING UNIV OF SCI & TECH
Tuberculosis medicament resistance related tuberculosis-resisting cytotoxic T lymphocyte (CTL) epitope peptide derived from refflux protein and application thereof
The invention discloses a tuberculosis medicament resistance related tuberculosis-resisting cytotoxic T lymphocyte (CTL) epitope peptide derived from refflux protein, namely, nonapeptide. The amino acid sequence of the nonapeptide is as follows: P5: YLGGTTGPV, or P6: YIVGFCLLV, or P7: TLTWLFAFV, or P8: GLVAGLSAV, or P9: ALGMLIAGL, or P10: MLIAGLPCL, or P11: LLCAIFAEV, or P12: RLWPTVGCL. Accordingto the invention, the HLA-A*0201 restrictive CTL epitope of a tuberculosis medicament resistance related protein antigen is predicted and analyzed by applying SYFPEITHI, BIMAS and NetCTL1.2 databasesand using an immunoinformatics mean according to a primary structure of the antigen so that the epitope peptide is obtained by virtue of selection, and the identified nonapeptide is not reported in documents. The epitope peptide is identified through an in-vitro enzyme linked immunospot (ELISPOT) experiment; and according to the result, a theoretical basis is provided for developing tuberculosis vaccine based on the medicament resistance related protein antigen and more information is provided for designing a tuberculosis polyepitope peptide vaccine based on mixed T cell epitope.
Owner:ZHENGZHOU UNIV
Multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine for HCV (hepatitis C viruses)
The invention discloses a multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine for HCV (hepatitis C viruses), relating to the technical field of virology and immunology. The multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine is characterized in that two CTL epitopes (namely, a NS4B (1793-1801) SMMAFSAAL and a P7 (774-782) AAWYIKGRL) are used for constructing recombinant adenoviruses, then the recombinant adenoviruses are used for infecting human dendritic cells so as to prepare a multi-epitope DC vaccine. Detection results indicate that the multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine for HCV (hepatitis C viruses) disclosed by the invention has an immunogenicity.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
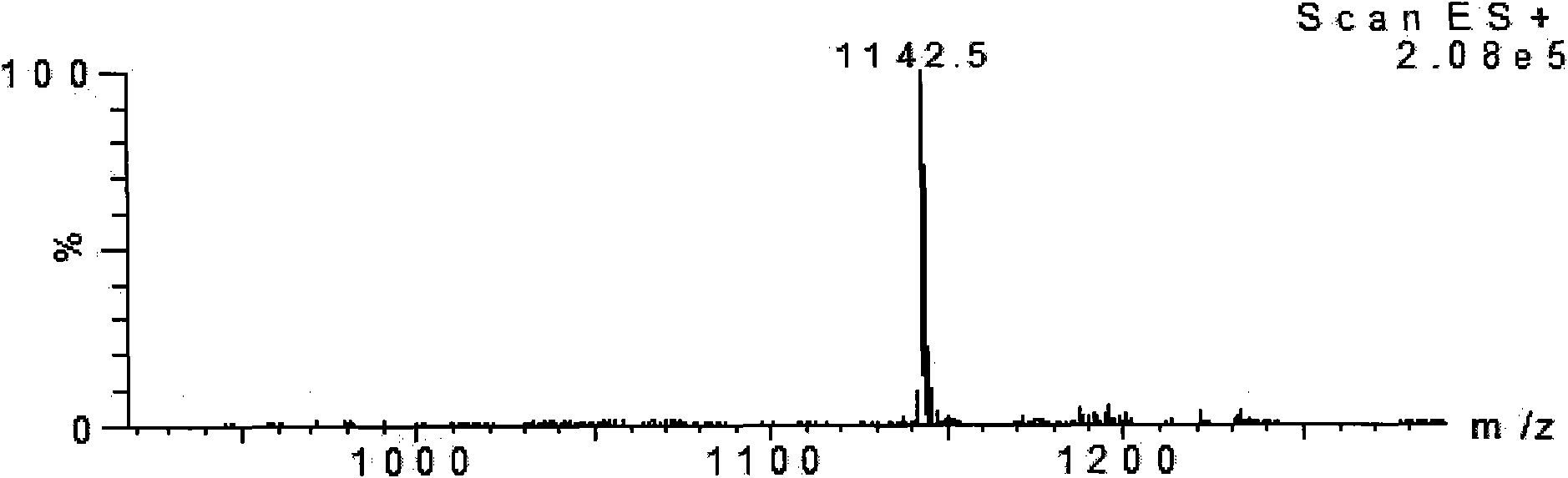
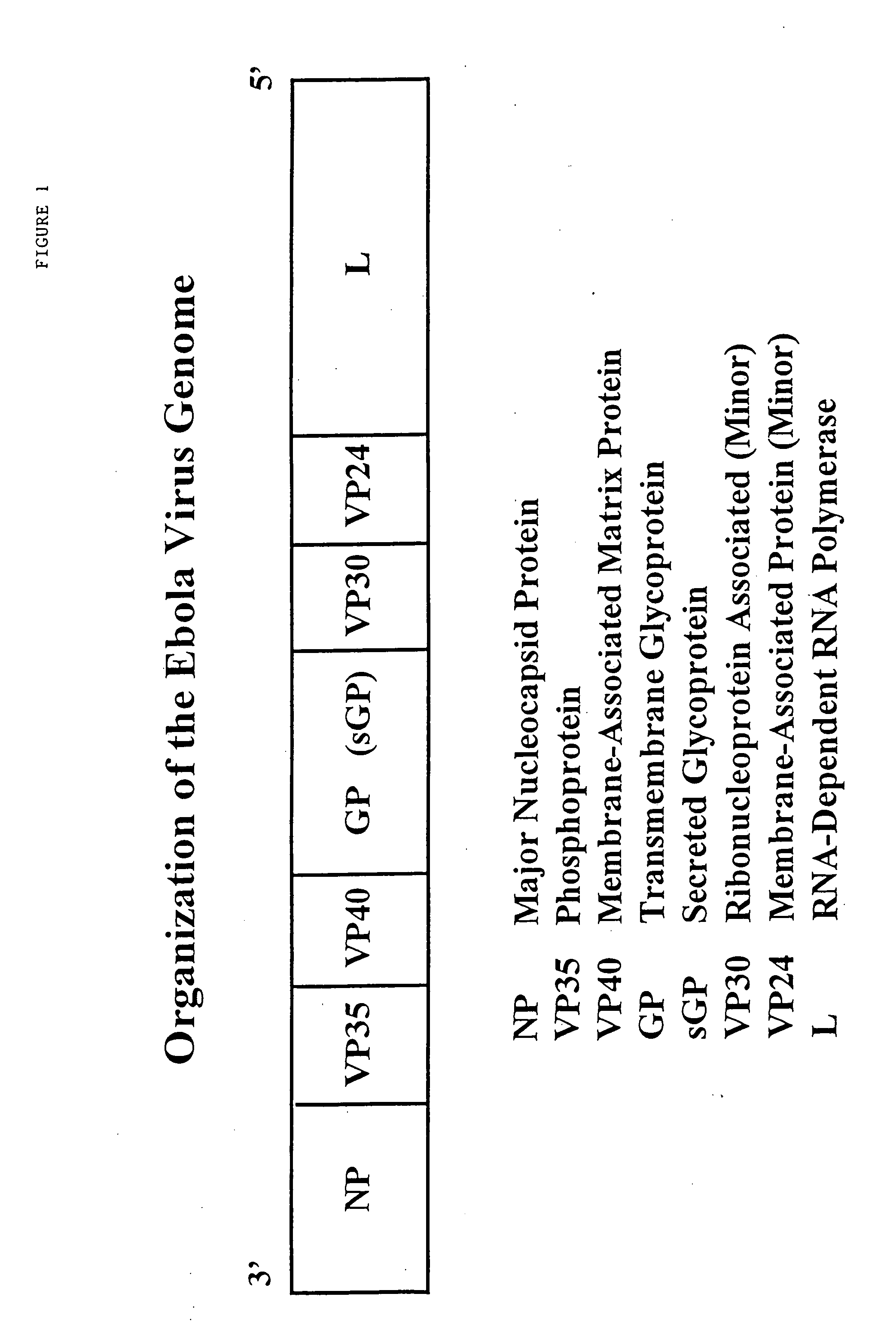
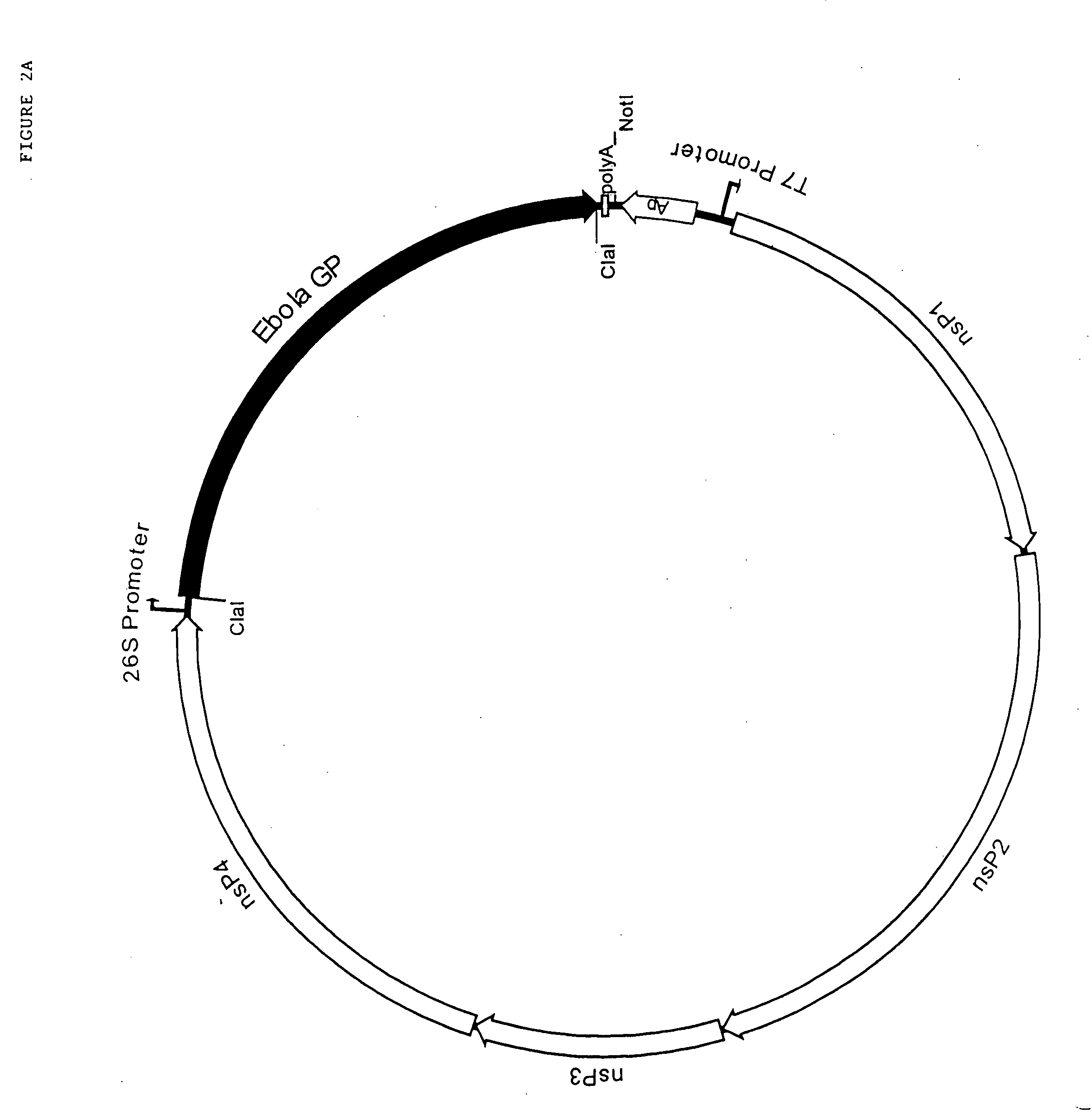
Ebola peptides and immunogenic compositions containing same
InactiveUS7267823B2Effective amountSsRNA viruses negative-senseSsRNA viruses positive-senseCtl epitopeImmunogenicity
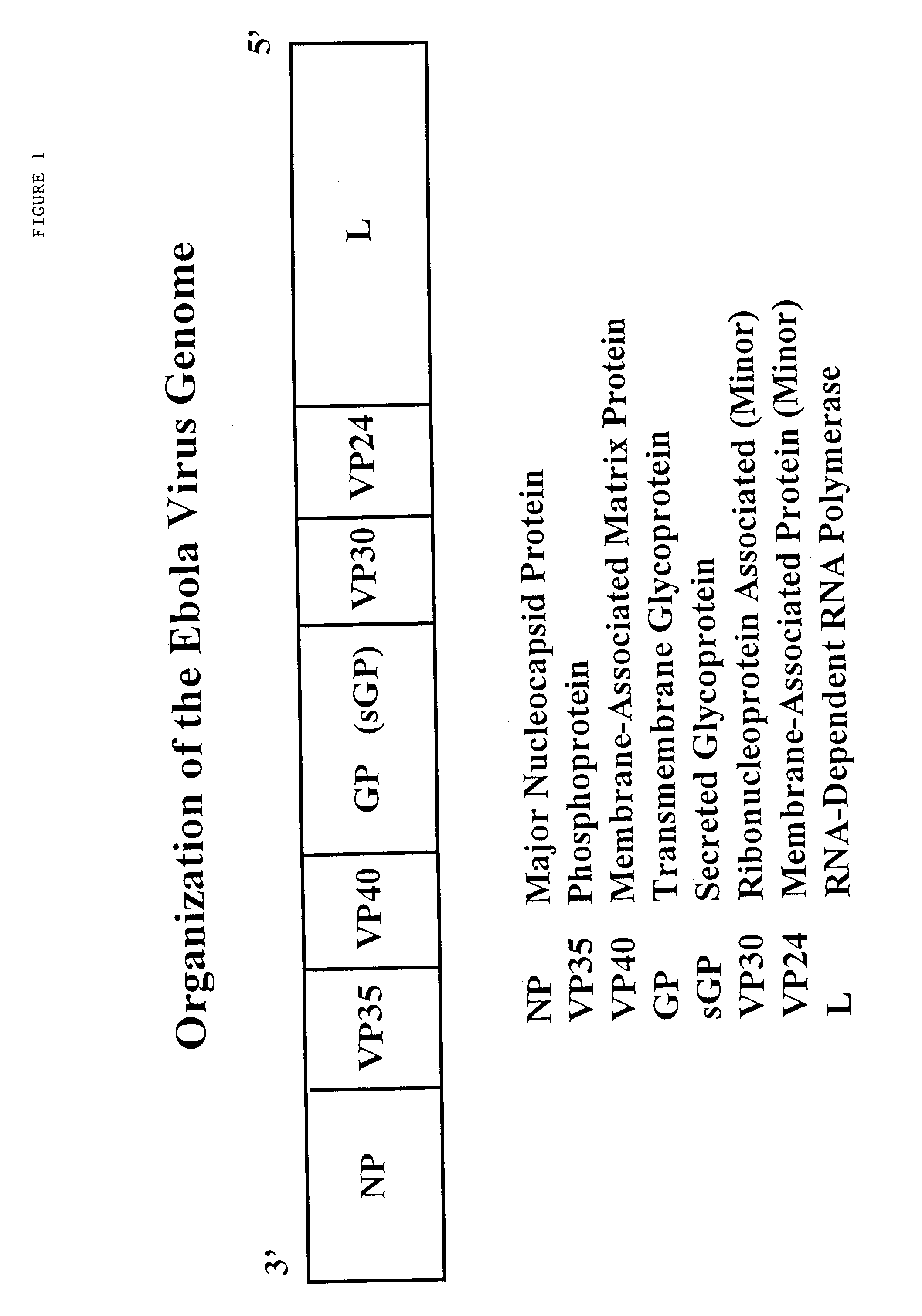
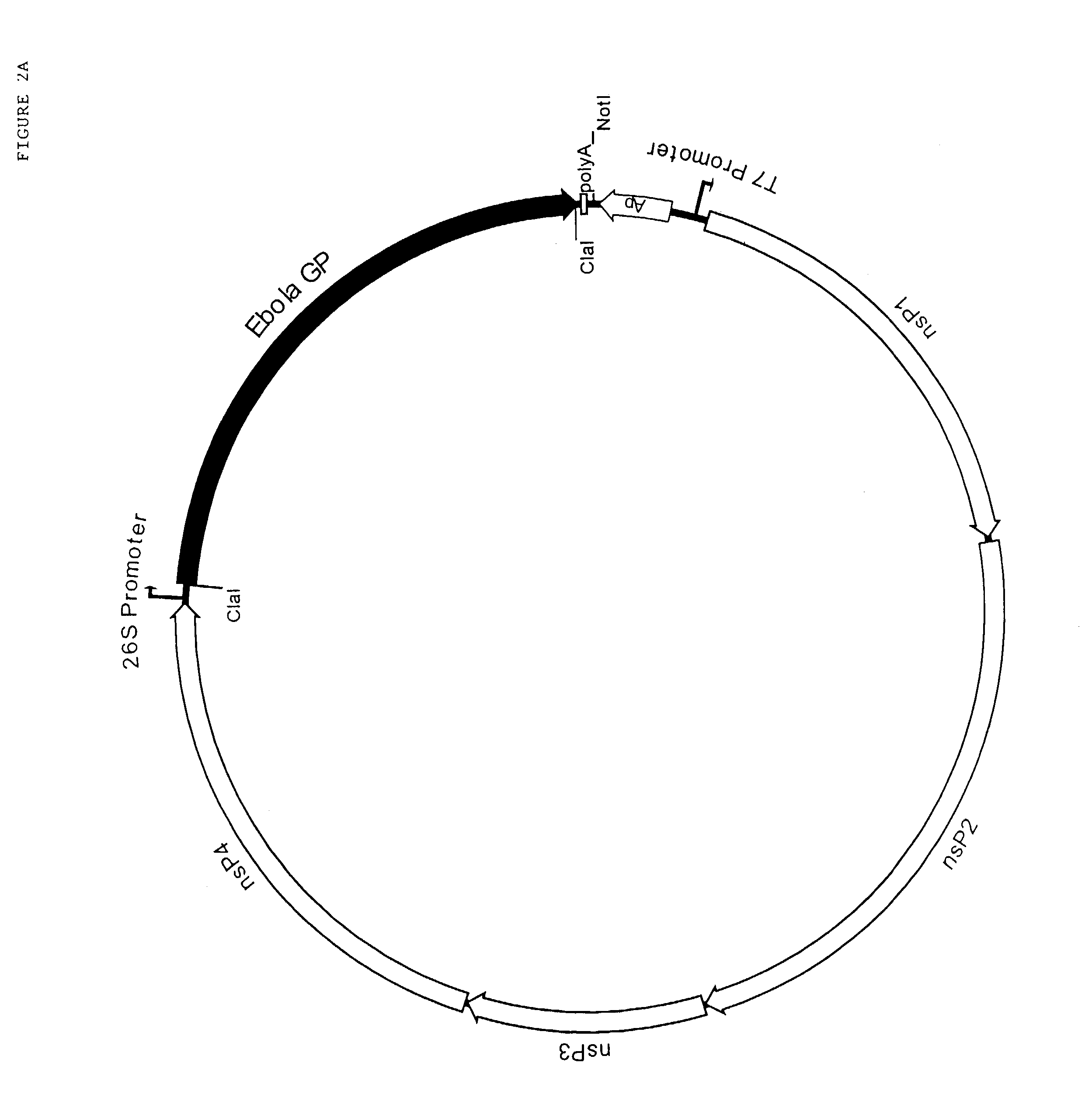
Using CTL epitopes to the Ebola GP, NP, VP24, VP30, VP35 and VP40 virion proteins, a method and composition for use in inducing an immune response which is protective against infection with Ebola virus is described.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Cysteine-depleted peptides recognized by A3-restricted cytotoxic lymphocytes, and uses therefor
Cysteine-depleted CTL epitopes can elicit a stronger or more specific CTL response than the native, cysteine-containing CTL epitope of a disease associated antigen.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND +2
MAGE (Melanoma Antigen Gene)-4 anti-tumor CTL (Cytotoxic T Lymphocyte) epitope peptide and application thereof
ActiveCN101870725APeptide preparation methodsAntibody medical ingredientsCtl epitopeAbnormal tissue growth
The invention relates to an MAGE (Melanoma Antigen Gene)-4 anti-tumor CTL (Cytotoxic T Lymphocyte) epitope peptide. The epitope peptide is a nonapeptide, and the sequence of the nonapeptide is a-b-Leu-Glu-His-Val-Val-Arg-c, wherein a is Lys or Tyr, b is Val or Leu, and c is Val or Leu; the sequence of an epitope peptide P286 is Lys-Val-Leu-Glu-His-Val-Val-Arg-Val; the sequence of an anti-tumor CTL epitope peptide P286-1Y2L is Tyr-Leu-Leu-Glu-His-Val-Val-Arg-Val; and the sequence of an anti-tumor CTL epitope peptide P286-1Y2L9L is Tyr-Leu-Leu-Glu-His-Val-Val-Arg-Leu. The specific CTL induced in the body of a transgenic mouse by the anti-tumor CTL epitope peptide has certain kill rate to tumor cells EC-9706; and the epitope peptide can be used for preparing therapeutic tumor polypeptide vaccines.
Owner:SHANGHAI LONGYAO BIOTECH CO LTD
Immunogenic compositions and vaccines for ebola
InactiveUS20100034843A1Efficient injectionSsRNA viruses negative-senseSsRNA viruses positive-senseCtl epitopeImmunogenicity
Using CTL epitopes to the Ebola GP, NP, VP24, VP30, VP35 and VP40 virion proteins, a method and composition for use in inducing an immune response which is protective against infection with Ebola virus is described.
Owner:ARMY GOVERNMENT OF THE UNITED STATES AS REPRESENTED BY THE SEC OF THE
HIV composite multi-epitope DNA vaccine and application thereof
InactiveCN101579528AExtensive cross-reactivityShow feasibilityGenetic material ingredientsAntiviralsCtl epitopeVaccination
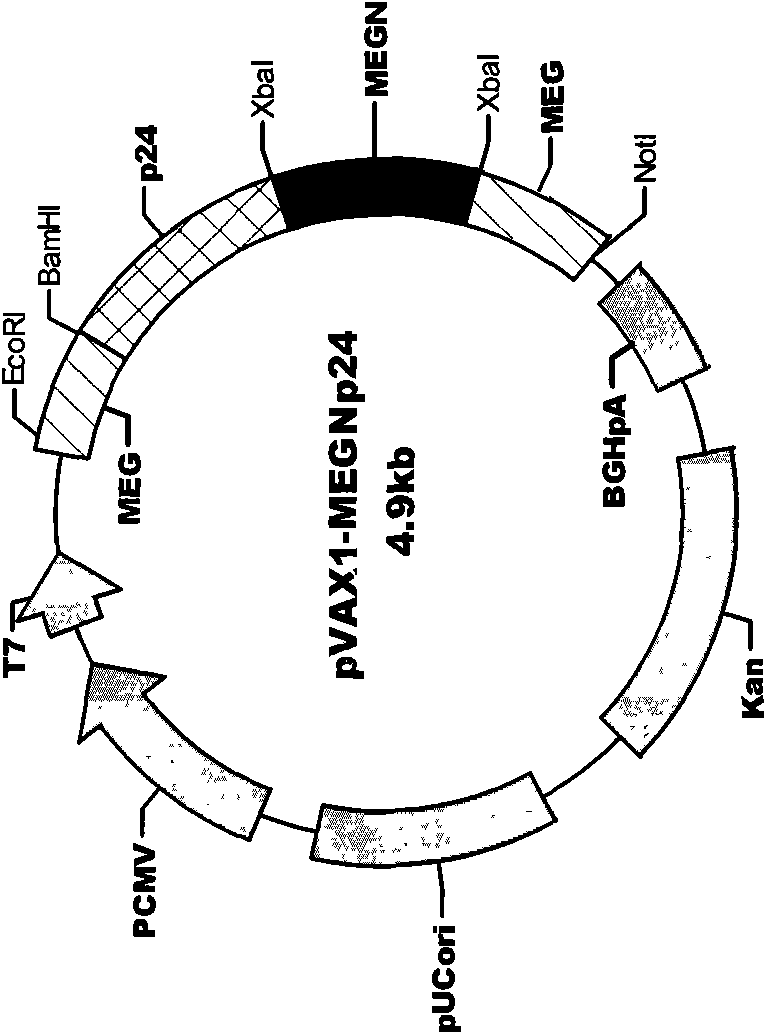
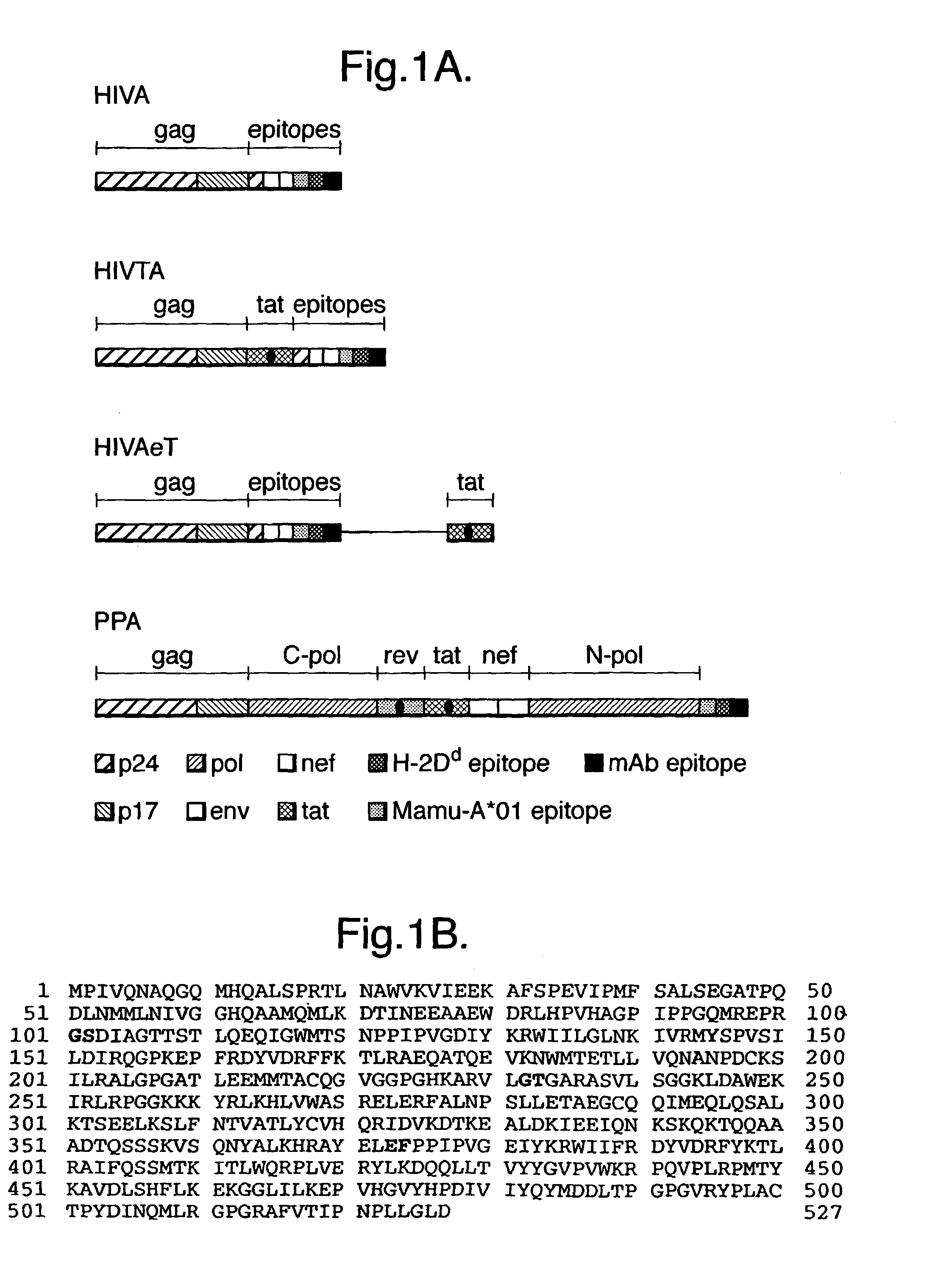
The invention discloses an HIV composite multi-epitope DNA vaccine which takes HIV-1 capsid protein p24 as a vector backbone molecule and ER signal peptide as a homing sequence, and contains twenty-six highly conservative immunodominant epitopes in an HIV genome; wherein, the HIV composite multi-epitope DNA vaccine comprises twenty-nine epitopes which are three neutralizing antibody epitopes, twenty-three CTL epitopes, one HIV-1 isolate common antibody epitope, one MHC nonrestrictive T helper lymphocyte epitope and one spasmotoxin B cell epitope. The vaccine can be used for vaccination of healthy people and immunization therapy of people who are infected by AIDS virus, thus having double effects of prevention and treatment.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Chimeric human immunodeficiency virus (HIV) immunogens comprising GAG P24-P17 fused to multiple cytotoxic T lymphocyte (CTL) epitopes
InactiveUS7993651B2Improve presentation efficiencyPrevent myristylationBacterial antigen ingredientsBacteriaCtl epitopeHIV Proteins
Disclosed is an immunogen in sterile form suitable for administration to a human subject, the immunogen comprising: at least a portion of the gag protein of HIV, said gag protein being from an HIV clade or having a consensus sequence for one or more HIV clades, and comprising at least parts of p17 and p24; and a synthetic polypeptide comprising a plurality of amino acid sequences, each sequence comprising a human CTL epitope of an HIV protein, and wherein a plurality of HIV proteins are represented in the synthetic polypeptide, said CTL epitopes being selected to stimulate an immune response to one or more HIV clades of interest.
Owner:MEDICAL RESEARCH COUNCIL +2
HTNV-NP (Hantaan virus nucleoprotein)-specific CTL (cytotoxic T lymphocyte) epitope peptides and application thereof
The invention discloses HTNV-NP (Hantaan virus nucleoprotein)-specific CTL (cytotoxic T lymphocyte) epitope peptides and application thereof. The CTL epitope peptides have amino acid sequences shown in SEQ ID NO:1-12. Especially, HLA-I (human leucocyte antigen-I) molecule restricted epitope polypeptide in the HTNV-NP-specific CTL epitope peptides can induce CD8+T lymphocyte to generate strong cellular immune response and secrete high-level IFN-gamma. The HTNV-NP-specific CTL epitope peptides can be used for preparing CTL epitope peptide vaccines or for inducing the generation of CTL epitope peptide-specific CTL, or for preparing CTL epitope peptide-sensitized antigen presenting cells and have bright development and application prospects in the field of specific immunization therapy of HFRS (hemorrhagic fever with renal syndrome).
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Novel human cytomegalovirus (HCMV) cytotoxic T cell epitopes, polyepitopes, compositions comprising same and diagnostic and prophylactic and therapeutic uses therefor
InactiveUS20100183647A1Minimize difficultyReduce in quantityBiocidePeptide/protein ingredientsCtl epitopeVaccination
The present invention provides CTL epitope peptides and polyepitope peptides from 14 distinct antigens of human cytomegalovirus (HCMV) that are restricted through HLA the must commonly prevalent class I alleles in different ethnic populations of the world. These epitopes provide an important platform for CTL epitope-based vaccines against HCMV. The present invention further provides vaccine compositions comprising the subject epitope and polyepitope peptides and methods for vaccination of humans and for the adoptive transfer of HCMV-specific T cells to human subjects. The present invention further provides reagents and methods for determining the HCMV status or level of HCMV-specific immunity of a subject.
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com