Respiratory syncytial virus sub-units vaccine, preparation and application
A subunit vaccine, syncytial virus technology, applied in the field of respiratory syncytial virus vaccine and its preparation, vaccine preparation of avirulent E. To enhance the degree of disease and other issues, to achieve the effect of strengthening local mucosal immune function and systemic immune function, eliminating pathological reactions, and improving expression levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Truncated RSV Membrane Protein G and Mutant G CTL Acquisition of genes:
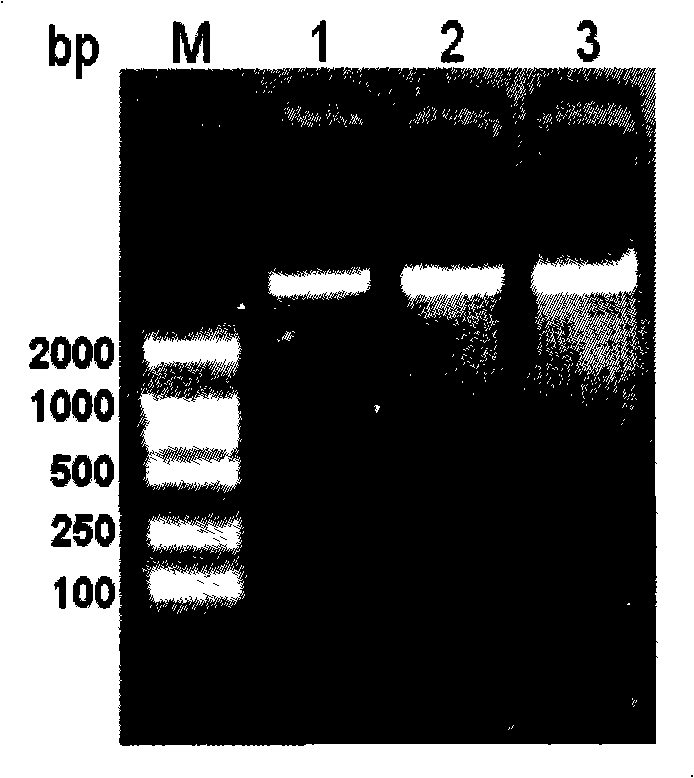
[0047] Entrusted a DNA synthesis company to synthesize the entire gene sequence of RSV G protein nt288~690, a total of 303 bp, and primers gc1: 5′-CTTTCTTTTTCCAGGTAAGTTGGATTGTTGCTGCAGATGC-3′; gc2: 5′-GAAAGTATTTTATAAAAGAATACCAAACAAAAAACCTG-3′; gp10: 5'-GCGGATCCTTAGTGGTGGTGGTGGTGGTGAGGCTTGGTGGTAGGTACTTC-3'. Among them, gp1 and gp10 are used to amplify the G protein gene, and introduce Nde I and BamH I restriction sites and 6×HIS tags, and gc1 and gc2 are used to mutate the CX3C motif in the G gene and replace it with a CTL epitope. Primers (gp1, gp10) were used for PCR amplification, and the PCR product (about 320bp) was the truncated G gene. G was obtained by overlap extension PCR method CTL Gene, that is, use the G gene synthesized by the whole gene as a template, and use primers gp1 / gc1 and gc2 / gp10 to amplify, respectively, to obtain fragments g11 and g210. amplified to obtain the...
Embodiment 2
[0048] Embodiment 2: recombinant expression vector G / pET22b and GCTL Construction of / pET22b:
[0049] G gene and G CTL After gene purification, digest with Nde I and BamH I at 37°C for 2 hours, recover the purified fragment, and connect with the purified expression vector pET22b double digestion product with T4 DNA ligase at 16°C overnight, and transform the ligated product by heat shock method CaCl 2 Prepared E.coli DH5α, coated with Amp + -LB plate, cultured at 37°C for 16 hours, picked several single colonies to inoculate Amp + -LB culture medium, cultured with shaking at 37°C for 12 hours, extracted the plasmid by alkaline lysis, and identified the G gene and G CTL Gene, and the sequencing result of a professional DNA sequencing company is correct. Construct the correct recombinant plasmid and transform E.coli BL21(DE3) again using the above method to induce the expression of truncated G protein and G CTL protein.
Embodiment 3
[0050] Example 3: G protein and G CTL Induced expression of protein:
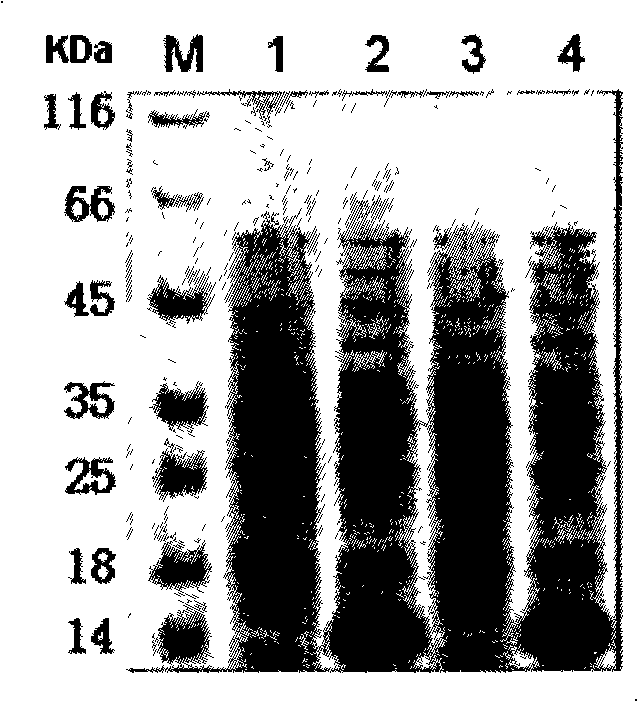
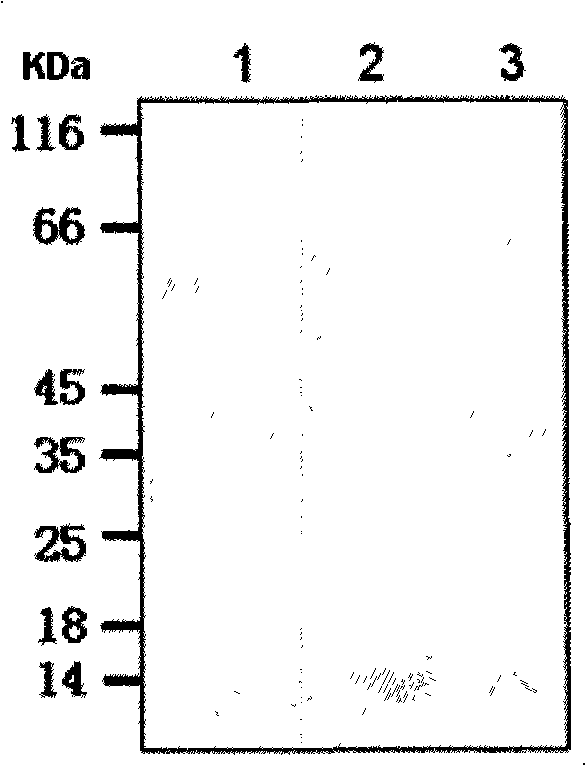
[0051] Single colonies of recombinant engineered bacteria G / pET22b / BL21 and GCTL / pET22b / BL21 were picked and inoculated into Amp + -In LB culture medium, cultivate overnight at 37°C with shaking, and inoculate fresh Amp at 1% the next day + -In LB culture medium, shake culture at 37°C until OD600 is about 1.0, then add 0.5mmol IPTG to induce expression at 37°C for 4 hours. After induction, an appropriate amount of samples were taken for identification by SDS-PAGE and Western blot. The results showed that: G protein and G CTL The apparent molecular weights of the proteins are 14.2KDa and 14.7KDa respectively, which are basically consistent with the theoretically calculated values, and the relative contents of the total protein in the bacteria are 18.4% and 19.4% respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com