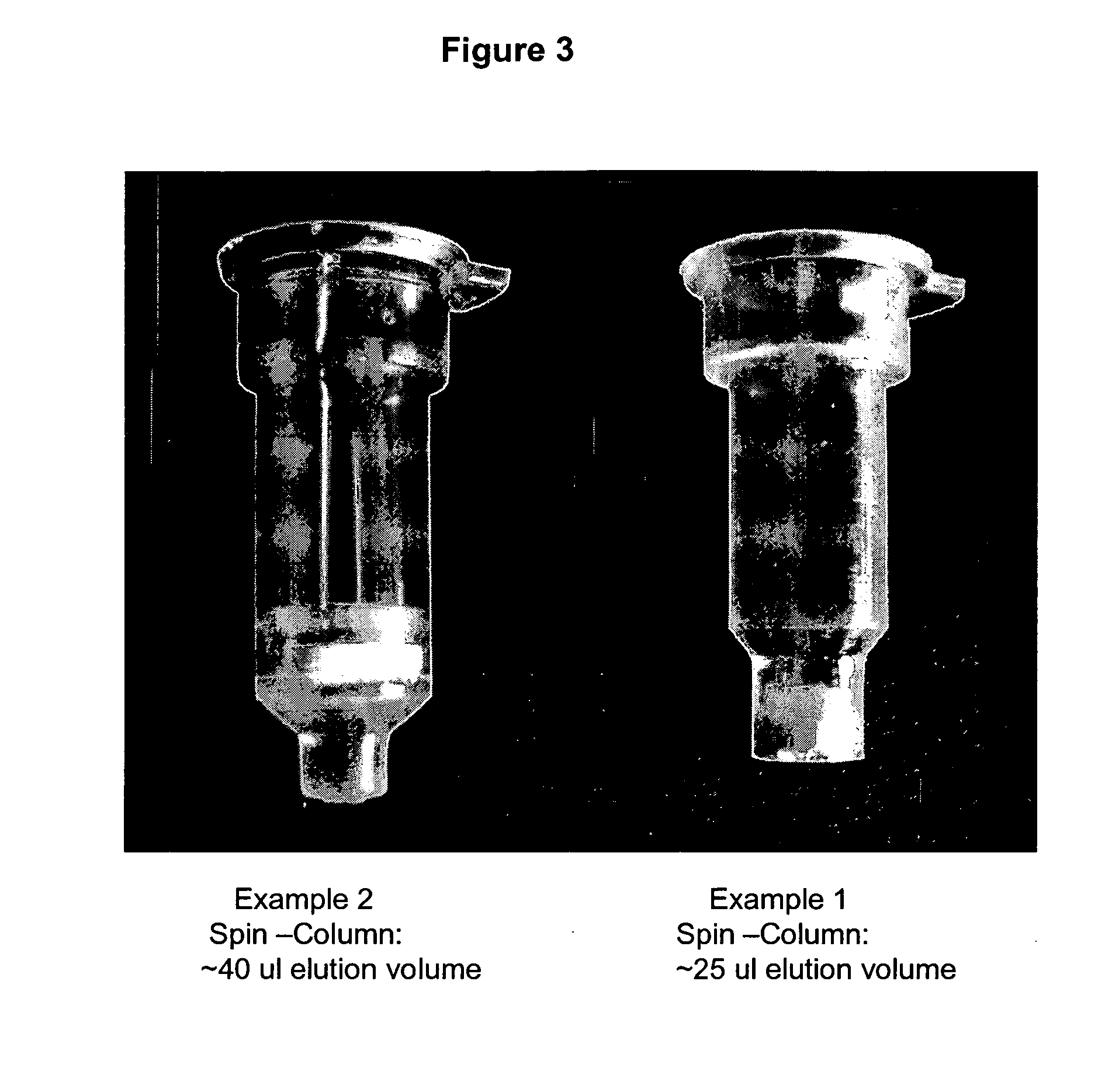
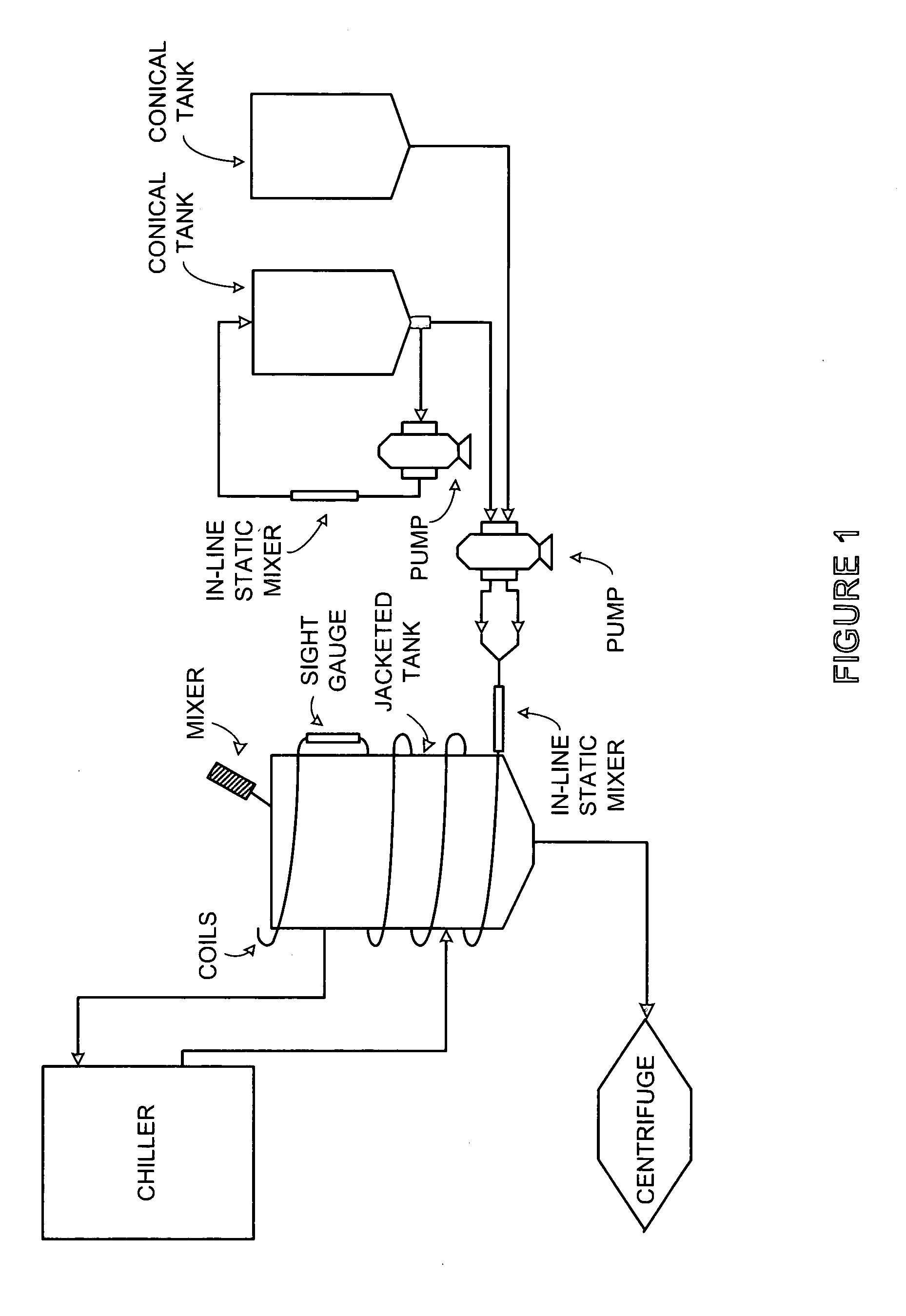
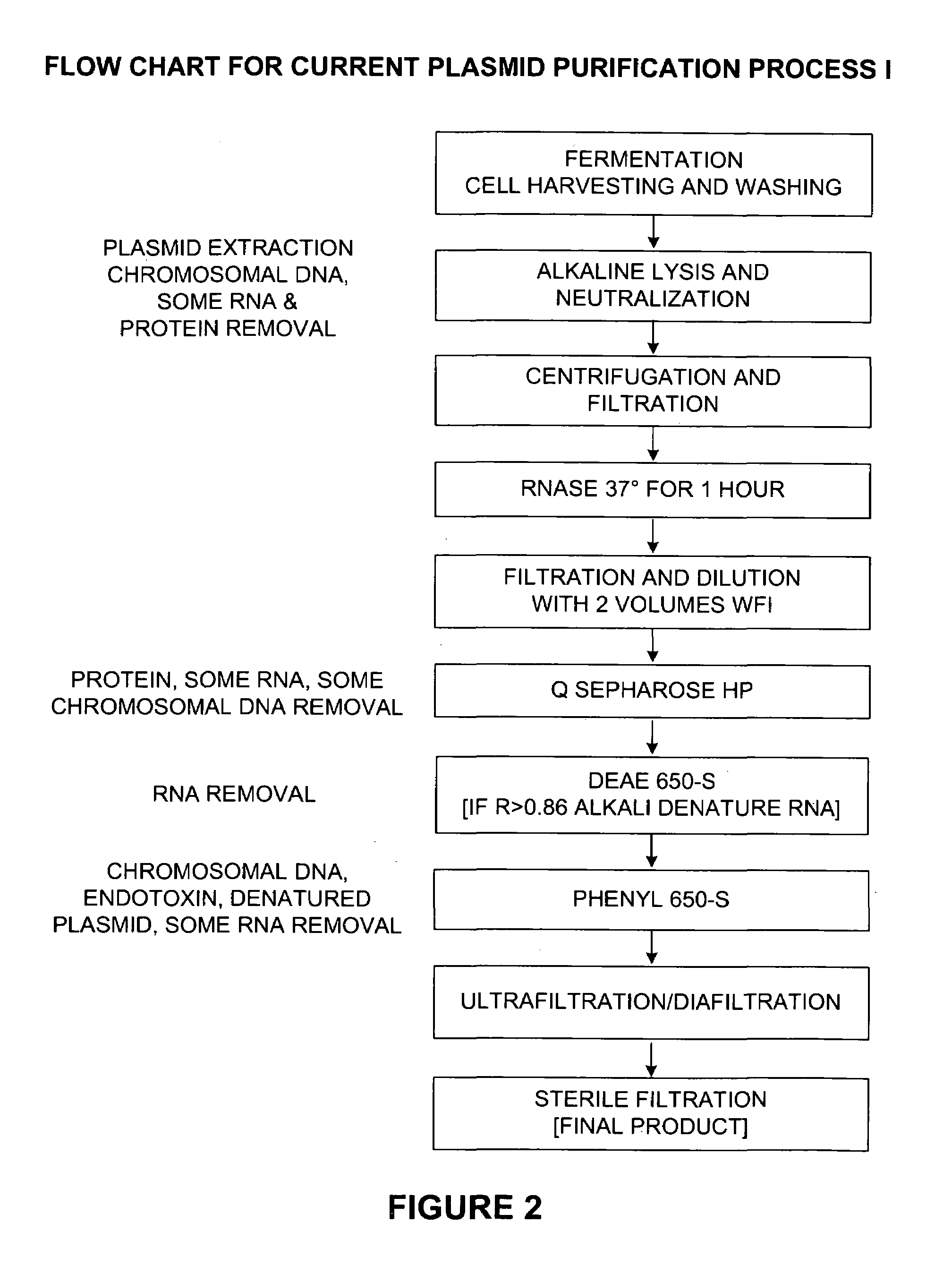
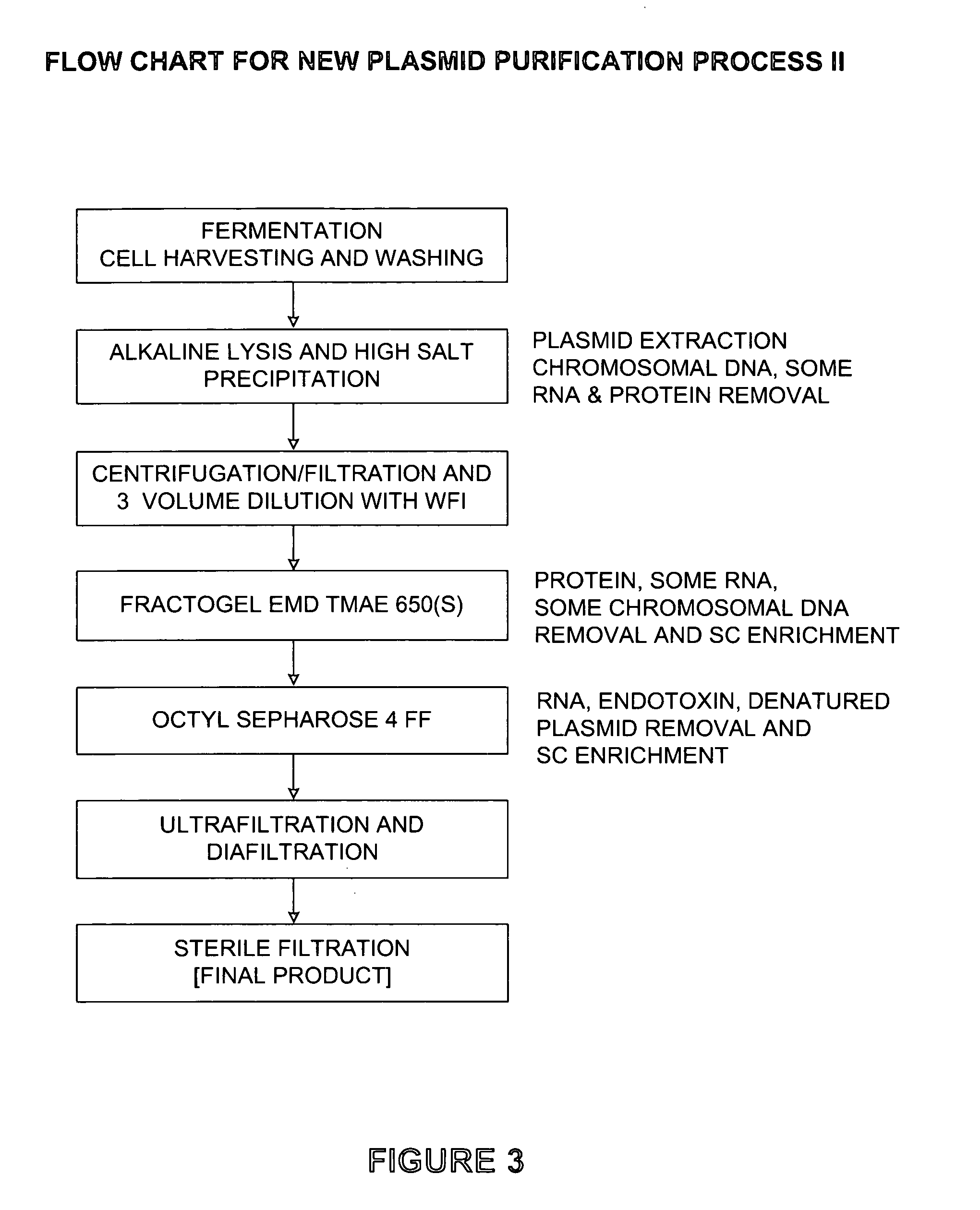
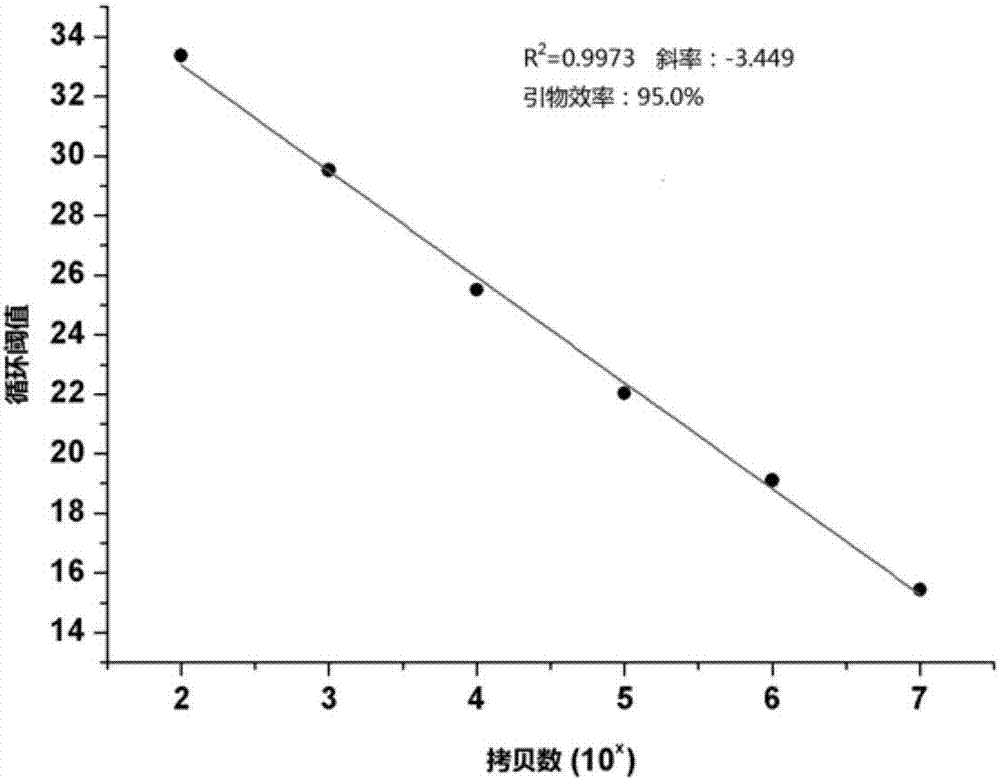
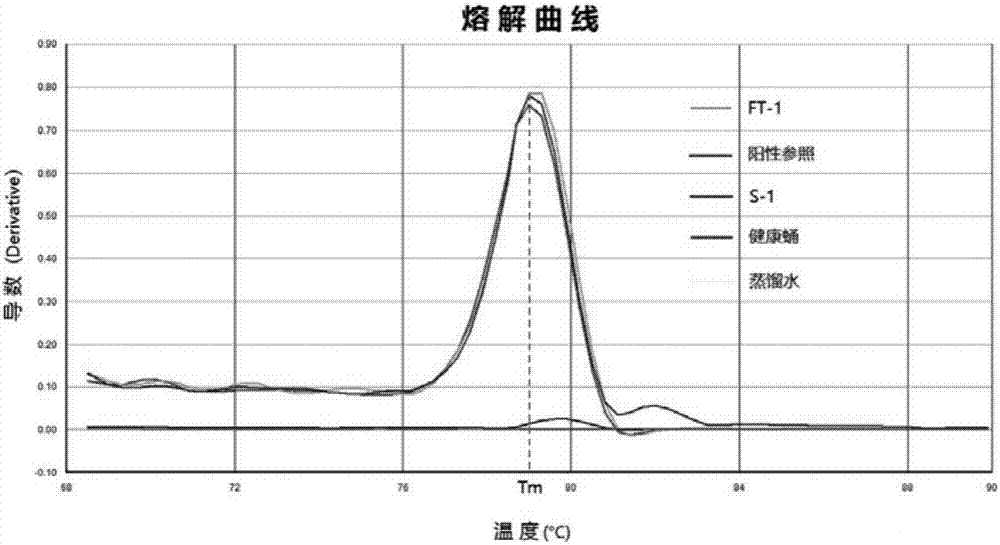
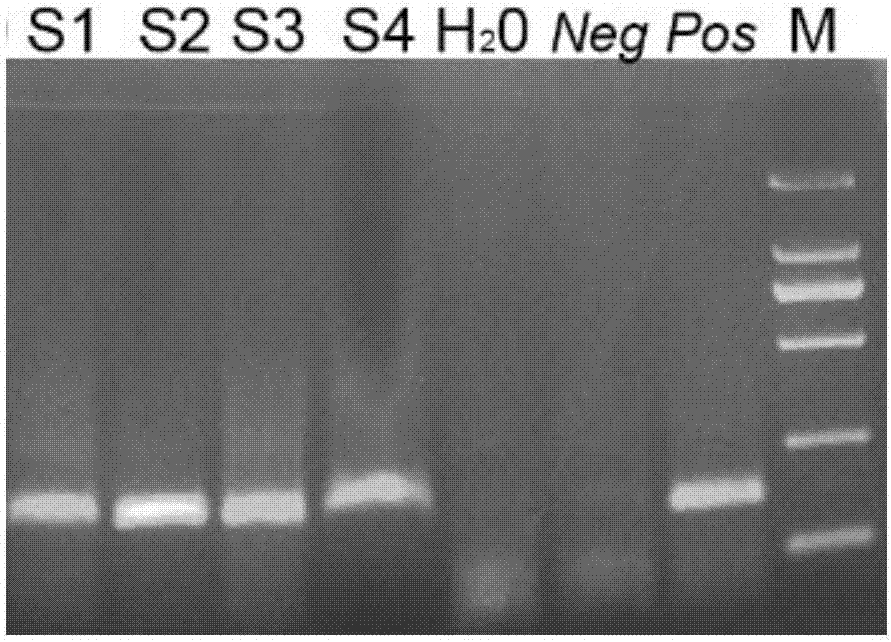
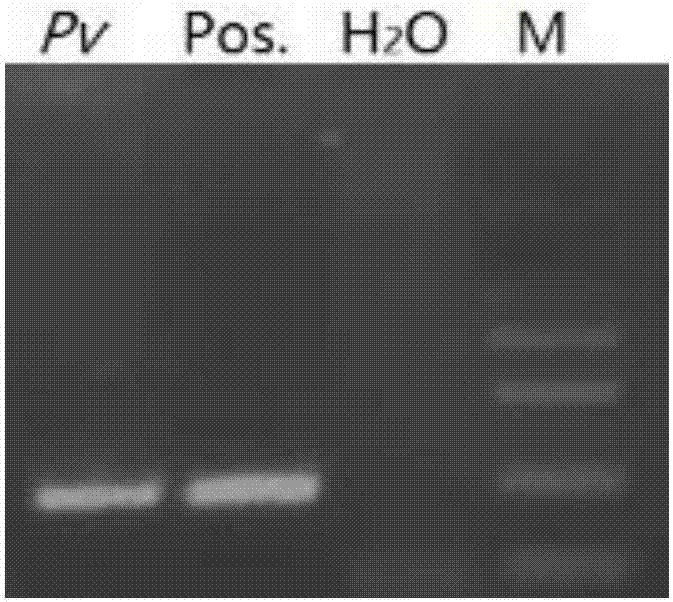
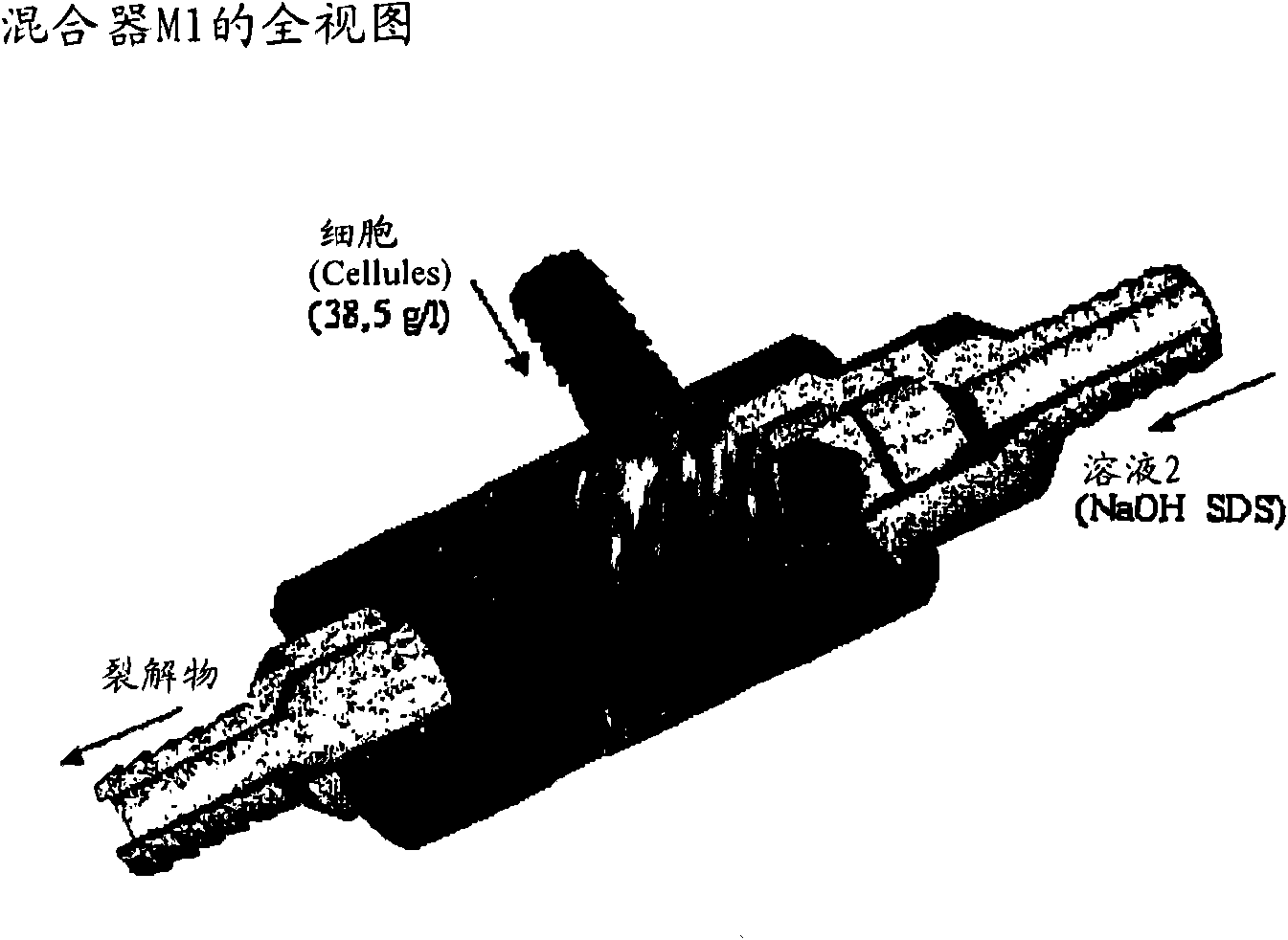
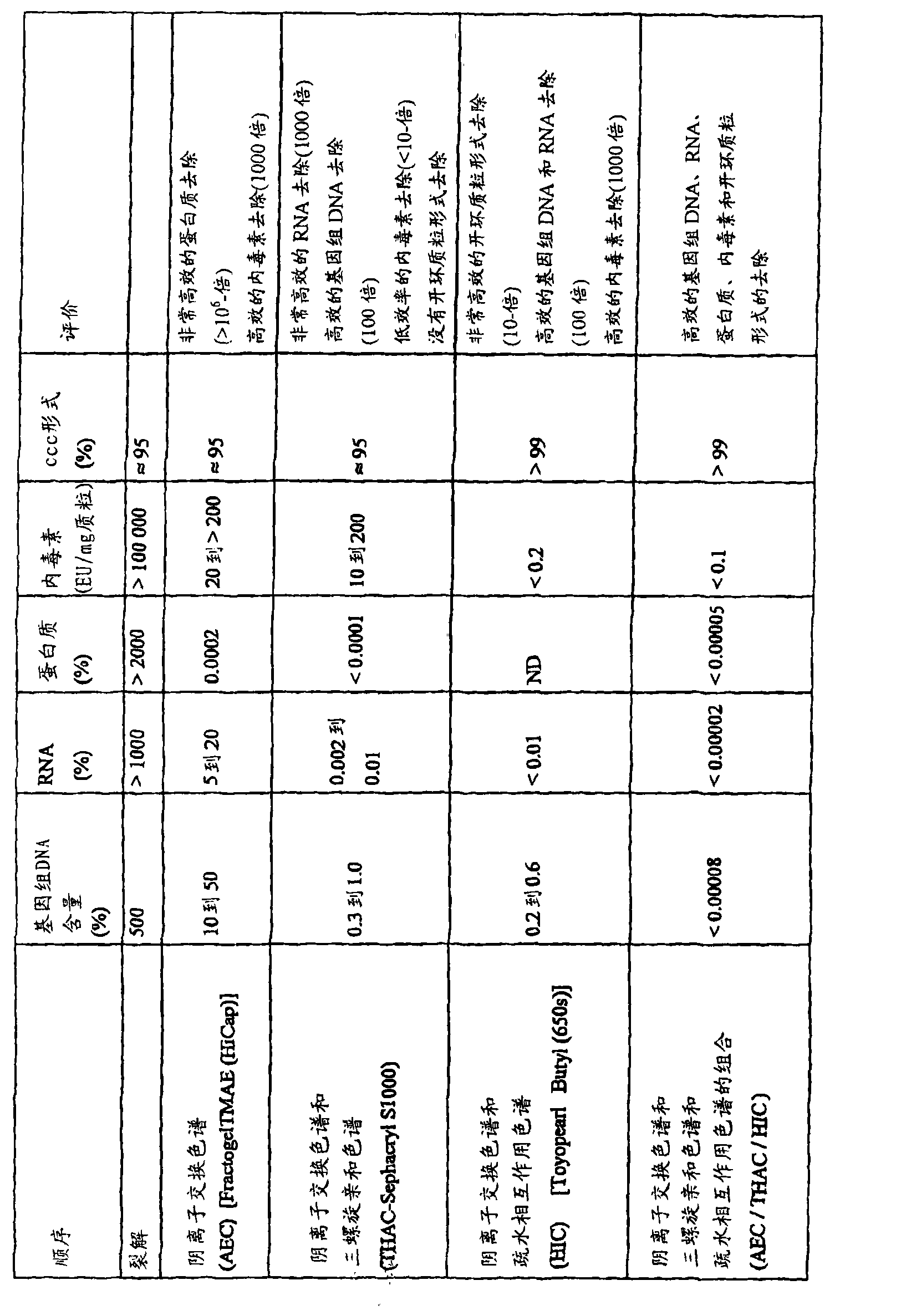
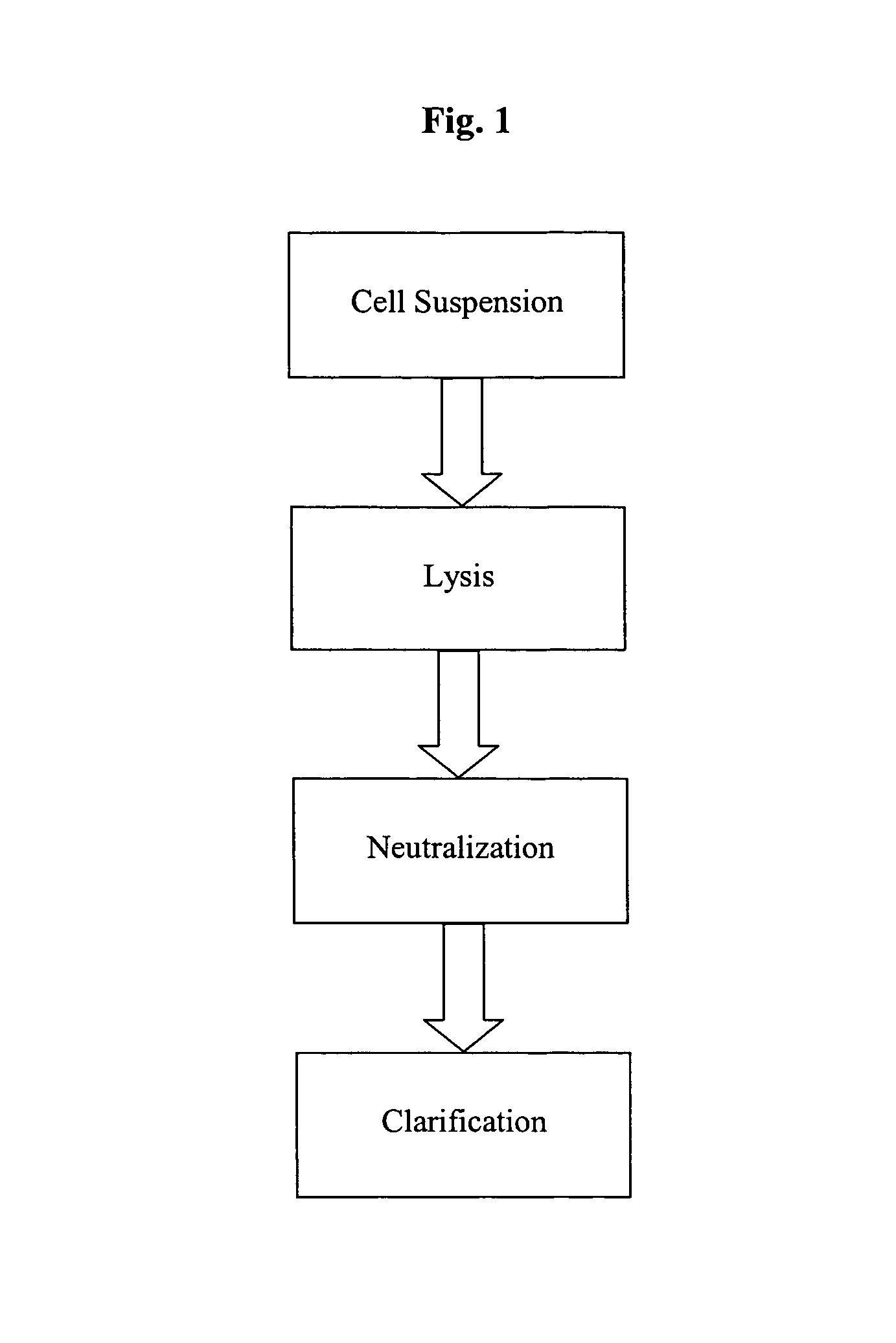
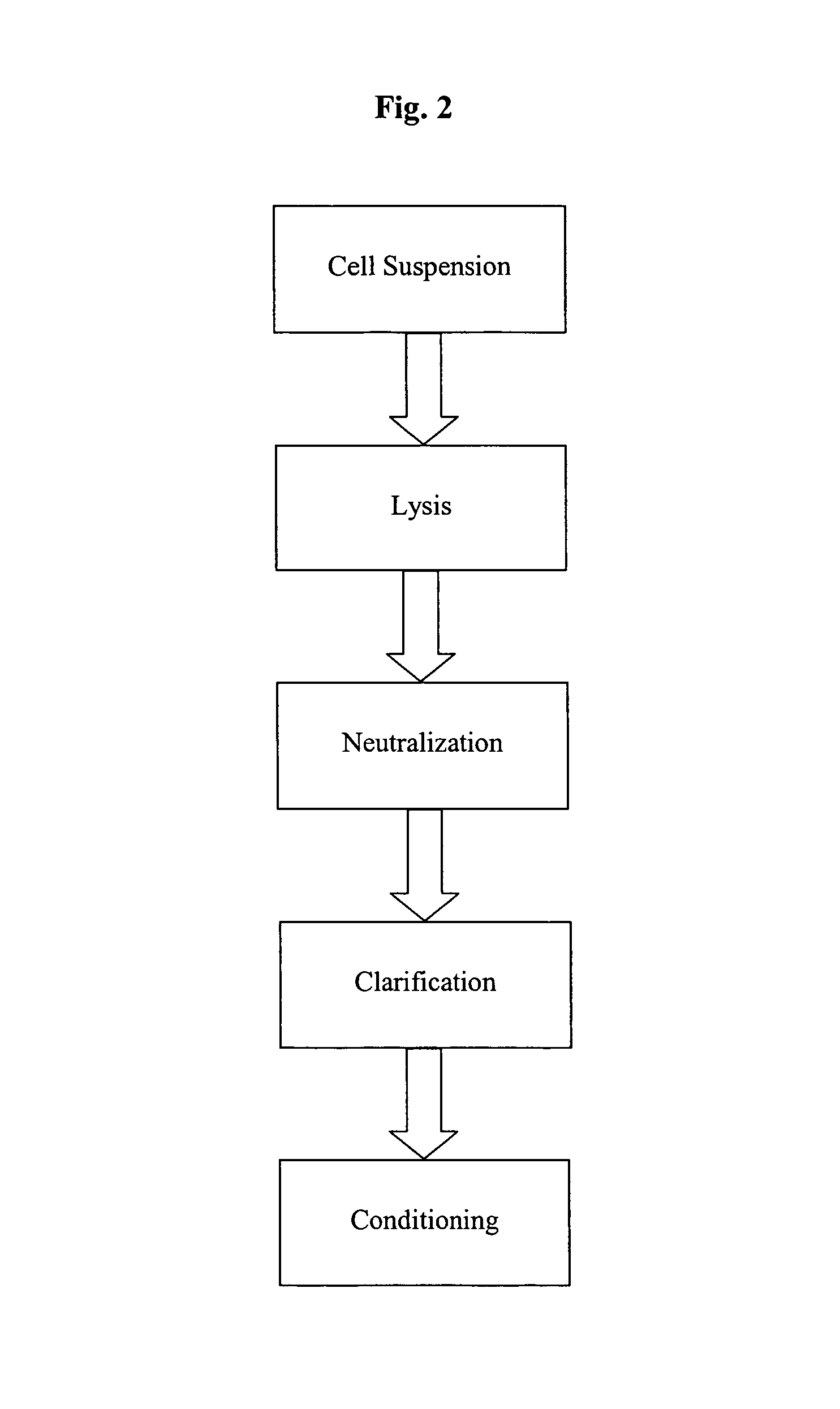
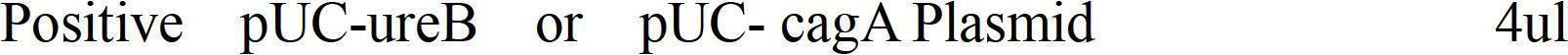Patents
Literature
71 results about "Alkaline lysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
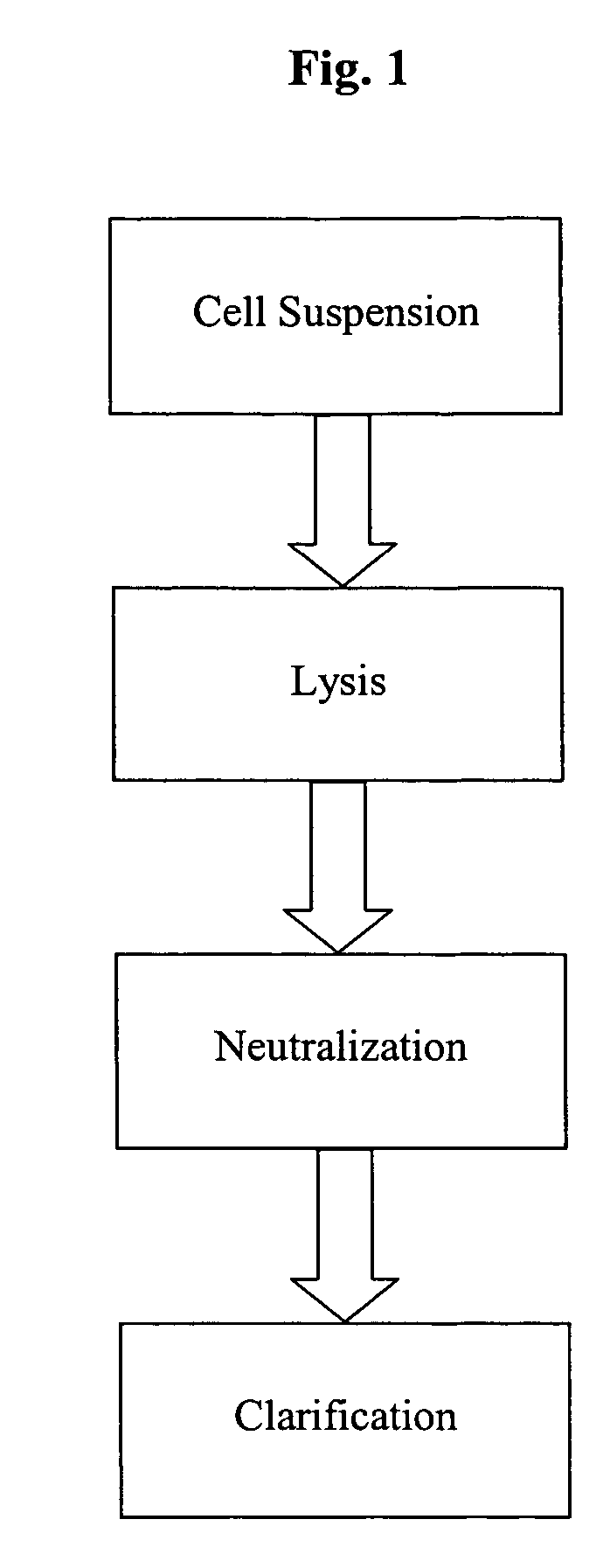
Alkaline lysis or alkaline extraction is a method used in molecular biology to isolate plasmid DNA from bacteria.
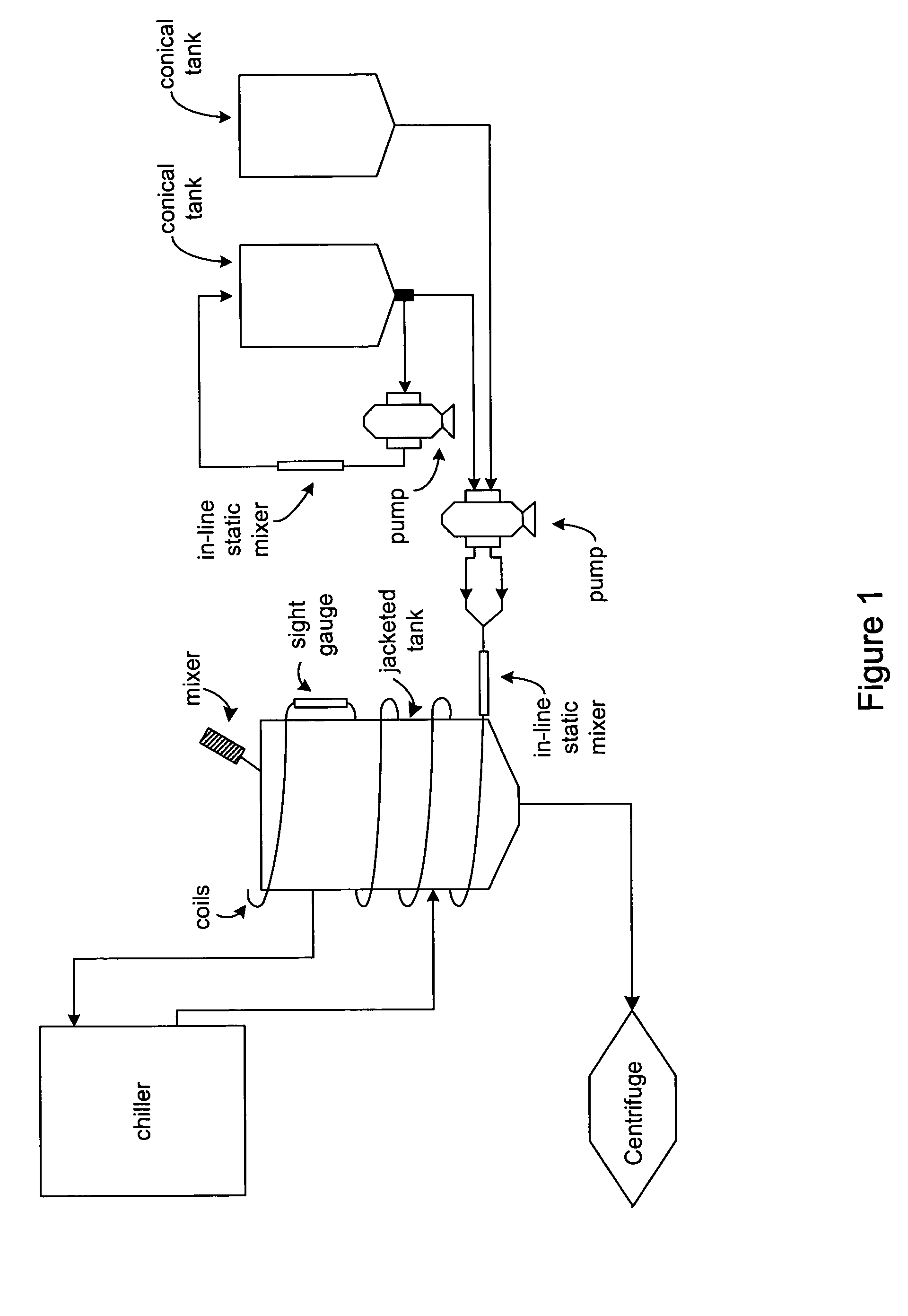
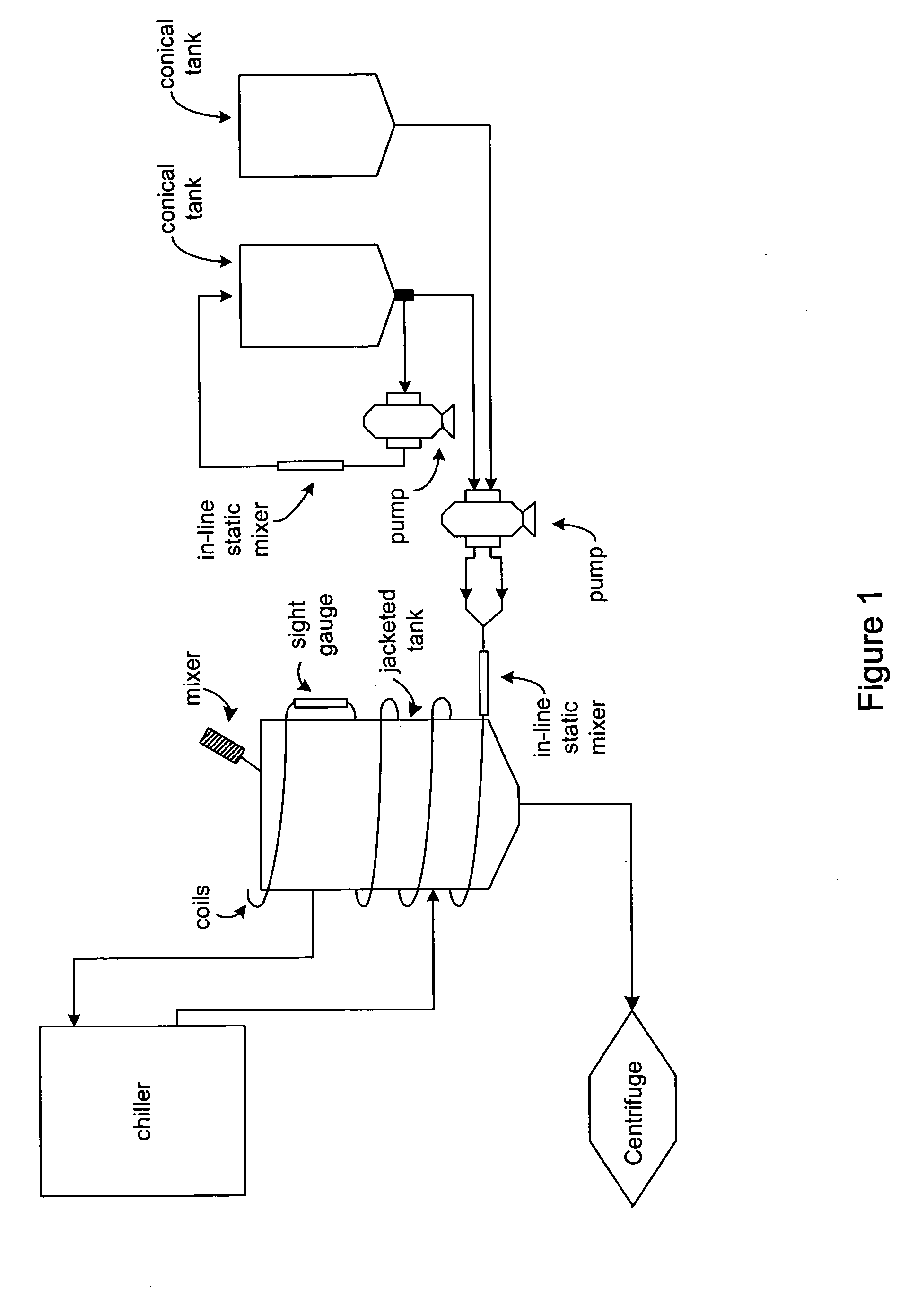
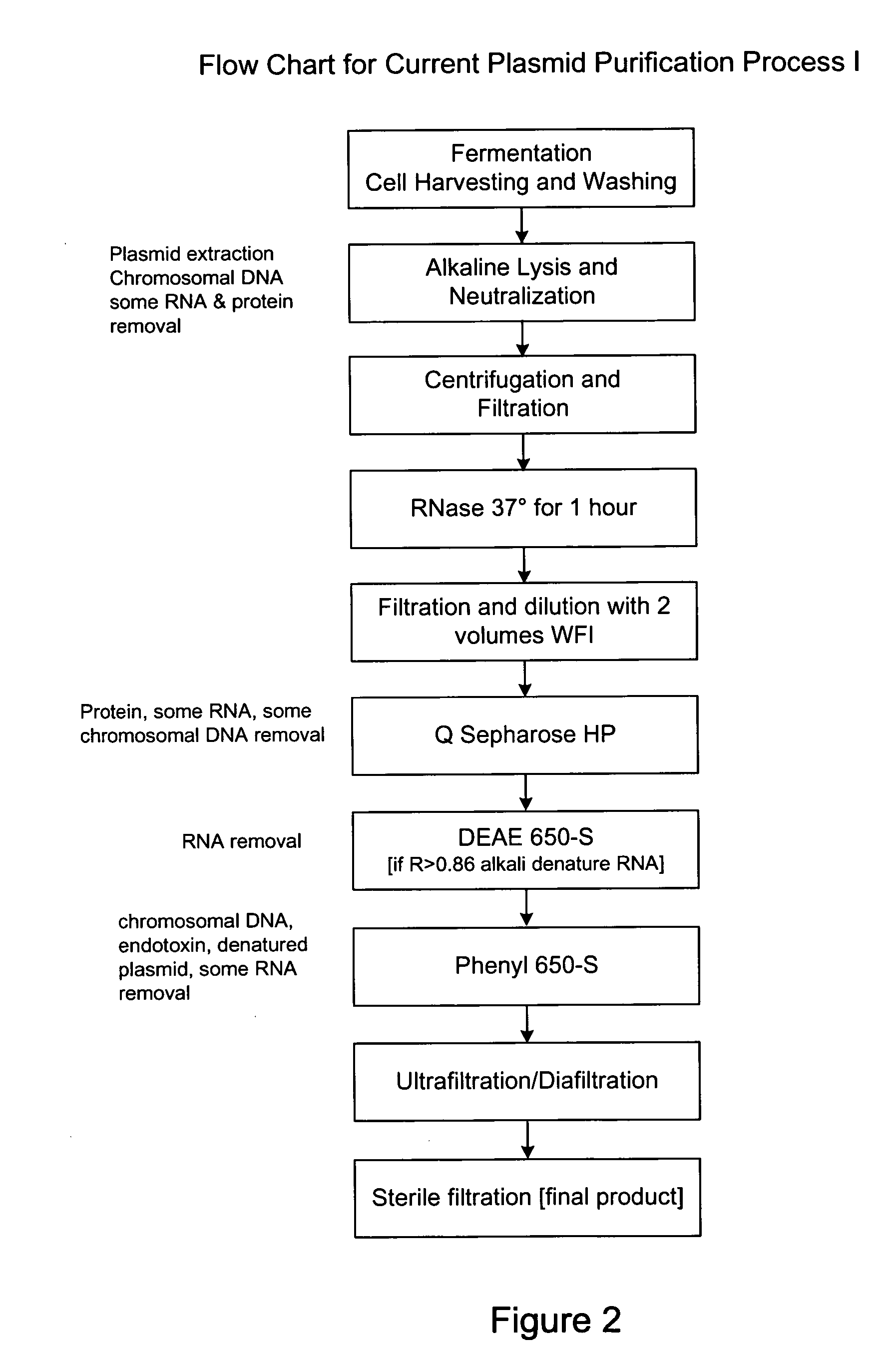
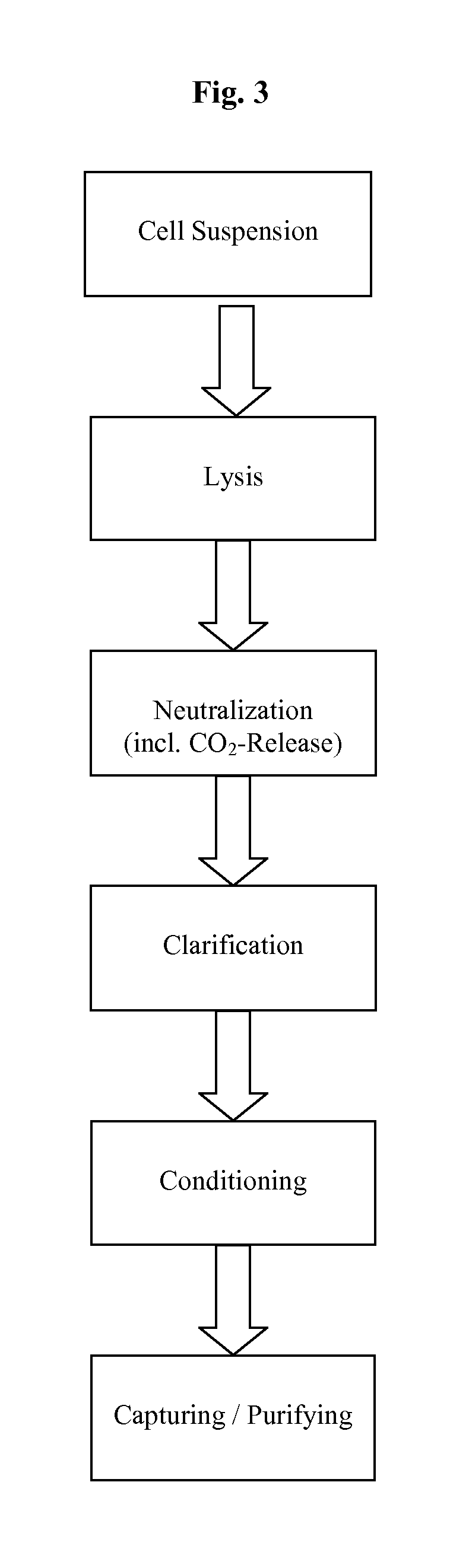
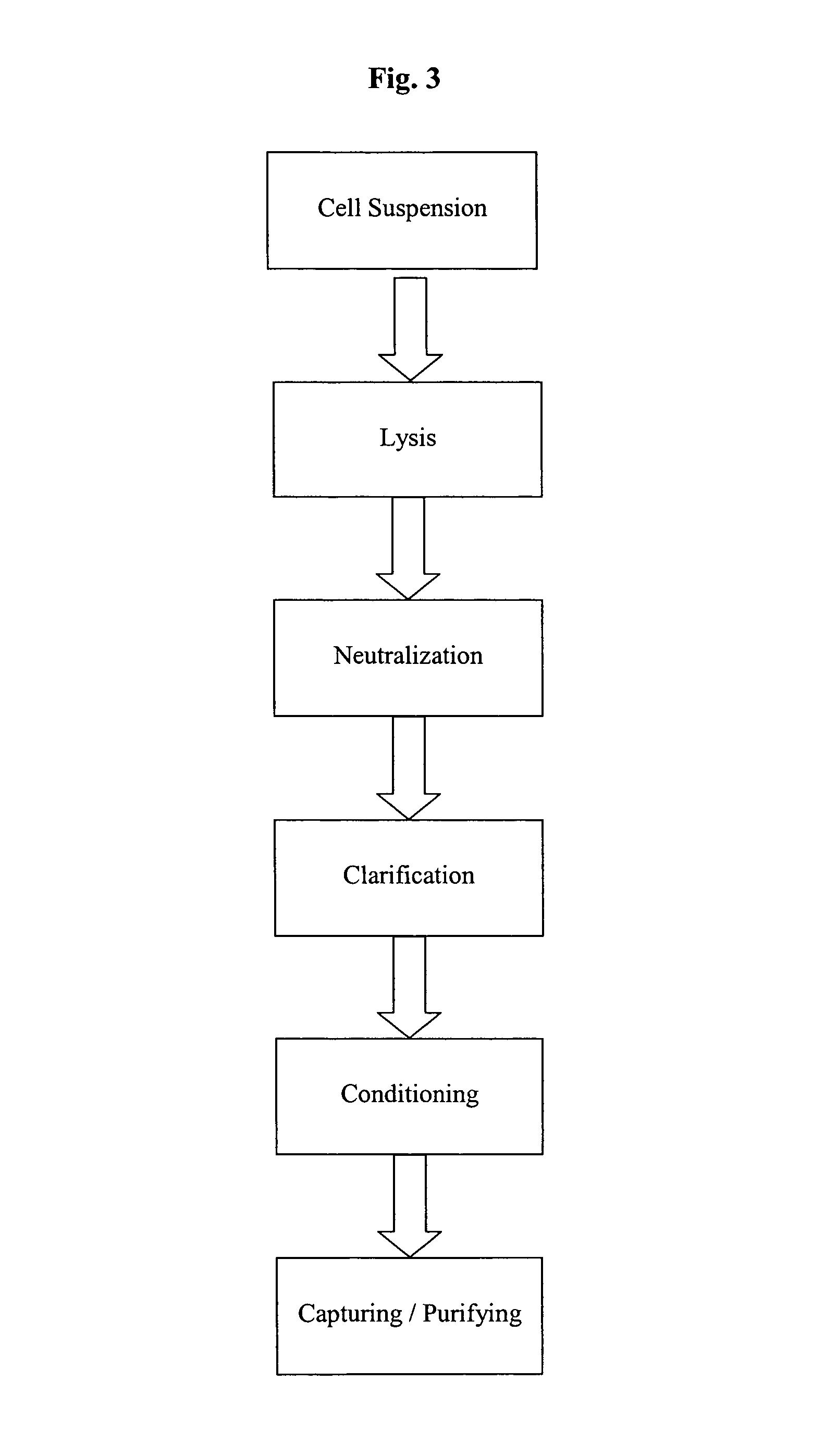
Process and equipment for plasmid purification
InactiveUS8236495B2Easy to operateConsistent levelCation exchanger materialsOrganic anion exchangersEscherichia coliLysis
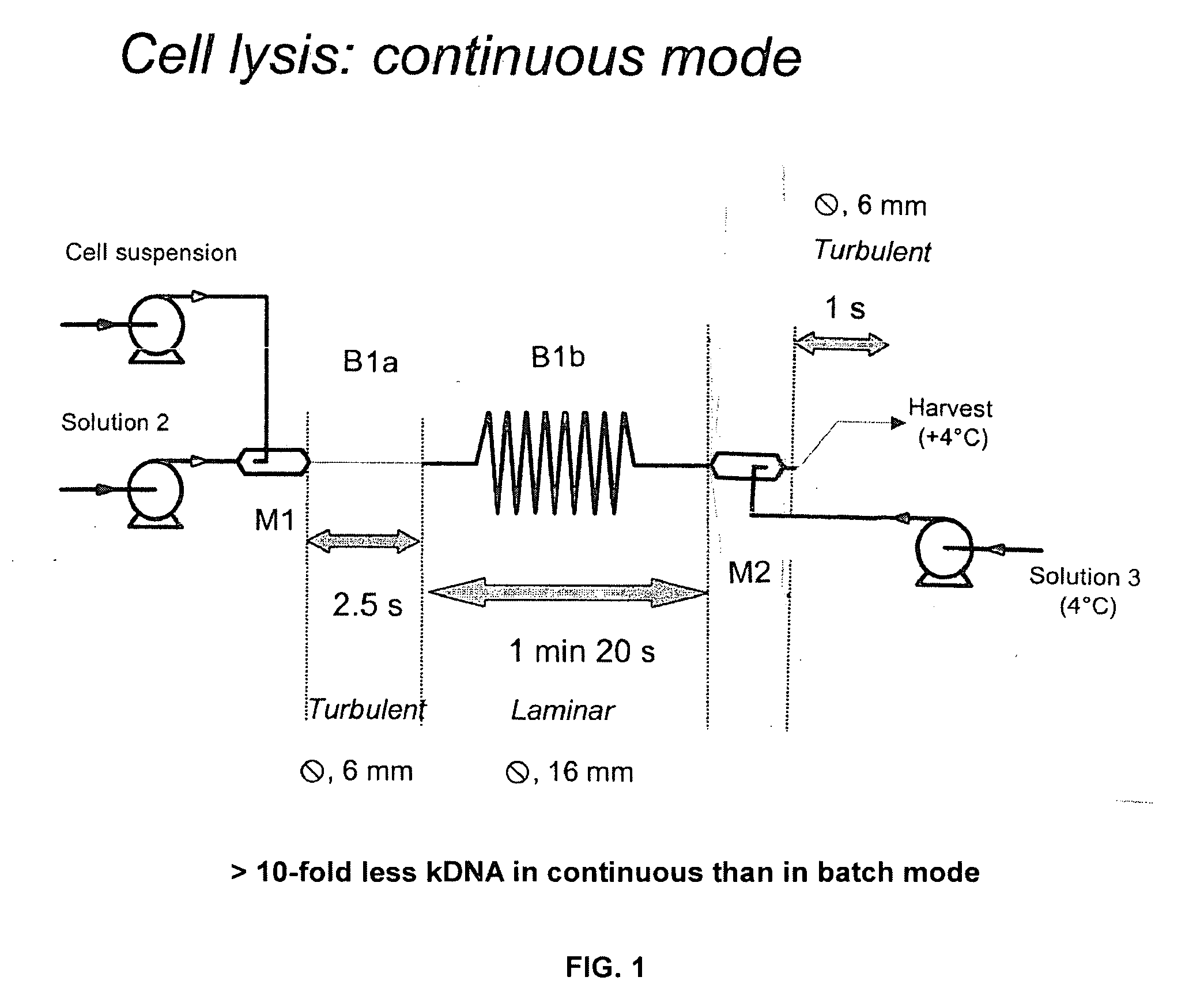
A scalable alkaline lysis process, including procedures and devices for the isolation of large quantities (grams and kilograms) of plasmid DNA from recombinant E. coli cells. Effective, controllable, and economical operation, and consistent low level of host chromosomal DNA in the final plasmid product. Involves a series of new unit operations and devices for cell resuspension, cell lysis, and neutralization.
Owner:URIGEN PHARMA INC
Process and equipment for plasmid purfication
InactiveUS20060106208A1Easy to operateConsistent levelCation exchanger materialsIon-exchanger regenerationLysisGram
A scalable alkaline lysis process, including procedures and devices for the isolation of large quantities (grams and kilograms) of plasmid DNA from recombinant E. coli cells. Effective, controllable, and economical operation, and consistent low level of host chromosomal DNA in the final plasmid product. Involves a series of new unit operations and devices for cell resuspension, cell lysis, and neutralization.
Owner:URIGEN PHARMA INC
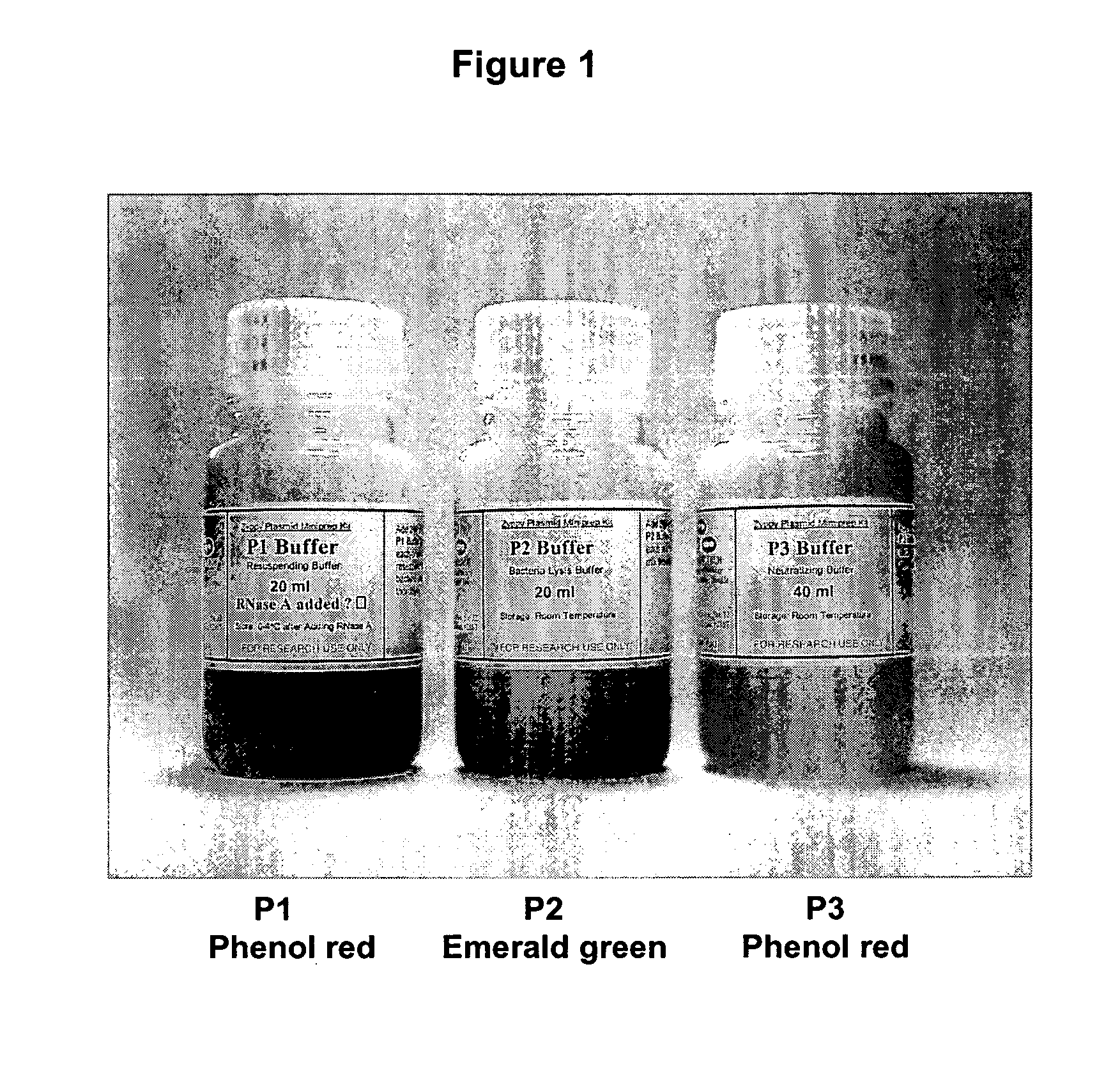
Isolation of nucleic acid using colored buffers
InactiveUS20070015169A1Optimization of bacterial lysisOptimization of neutralization stepSugar derivativesMicrobiological testing/measurementEscherichia coliMicroorganism
The present invention describes isolation of plasmid DNA or nucleic acid material from Escherichia coli (E. coli) or other species of microorganisms, or from other sources. The addition of indicator dyes to the alkaline lysis based purification buffers allows for increased ease of visual monitoring of the mixing and lysis steps of the procedure. The yield and quality of plasmid DNA is improved and the chance of error is reduced. The practice may be applied to other nucleic acid purification procedures.
Owner:ZYMO RES CORP
Process and equipment for plasmid purification
InactiveUS7026468B2Effective and controllable and economical operationConsistent low levelCation exchanger materialsIon-exchanger regenerationEscherichia coliLysis
A scalable alkaline lysis process, including procedures and devices for the isolation of large quantities (grams and kilograms) of plasmid DNA from recombinant E. coli cell. Effective, contgrollable, and economical operation, and consistent low level of host chromosomal DNA in the final plasmid product. Involves a series of new unit operations and devices for cell resuspension, cell lysis, and nuetralization.
Owner:URIGEN PHARMA INC
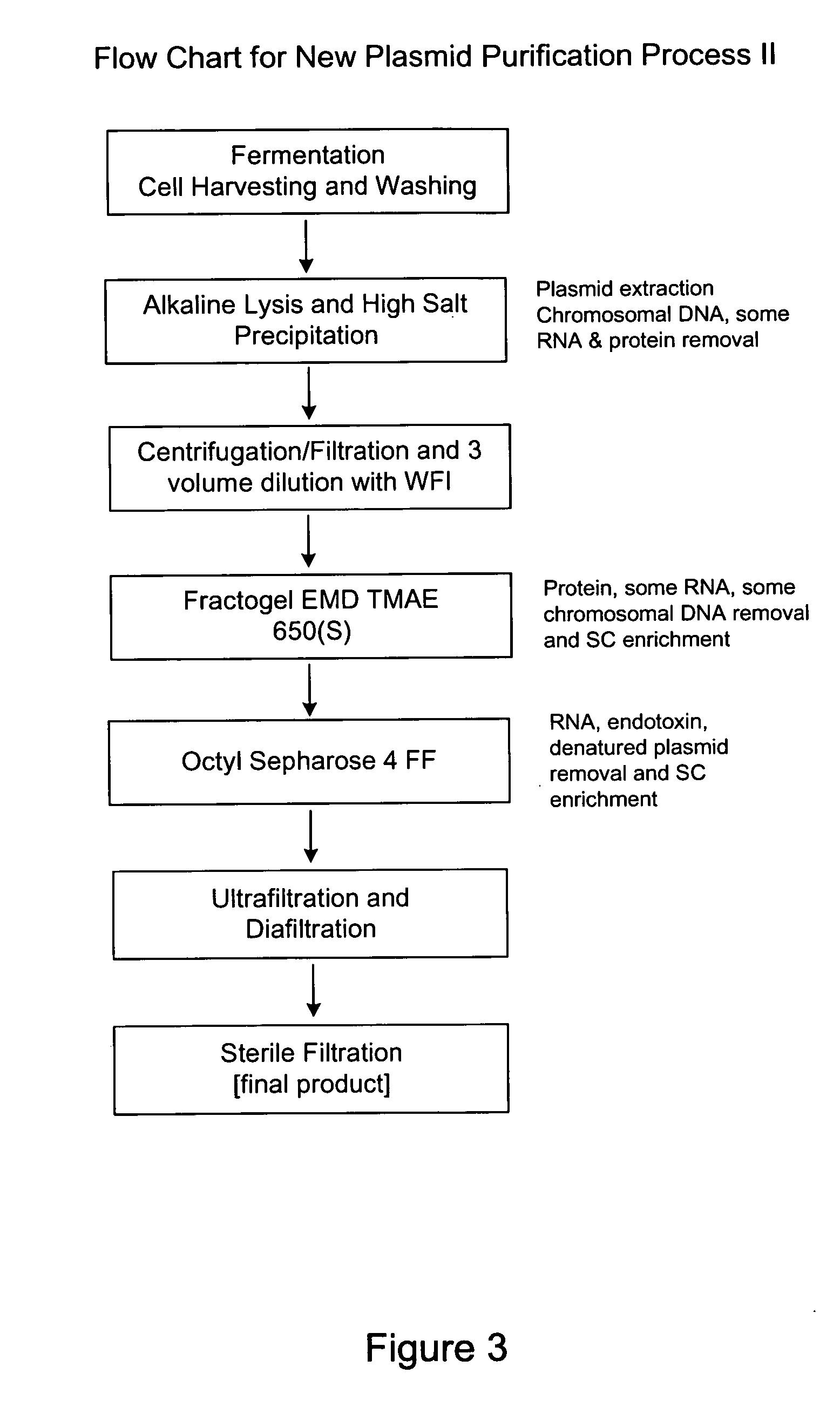
Methods and devices for producing biomolecules
ActiveUS20050026177A1Process is directionalMicrobiological testing/measurementNucleic acid reductionLysisCracking reaction
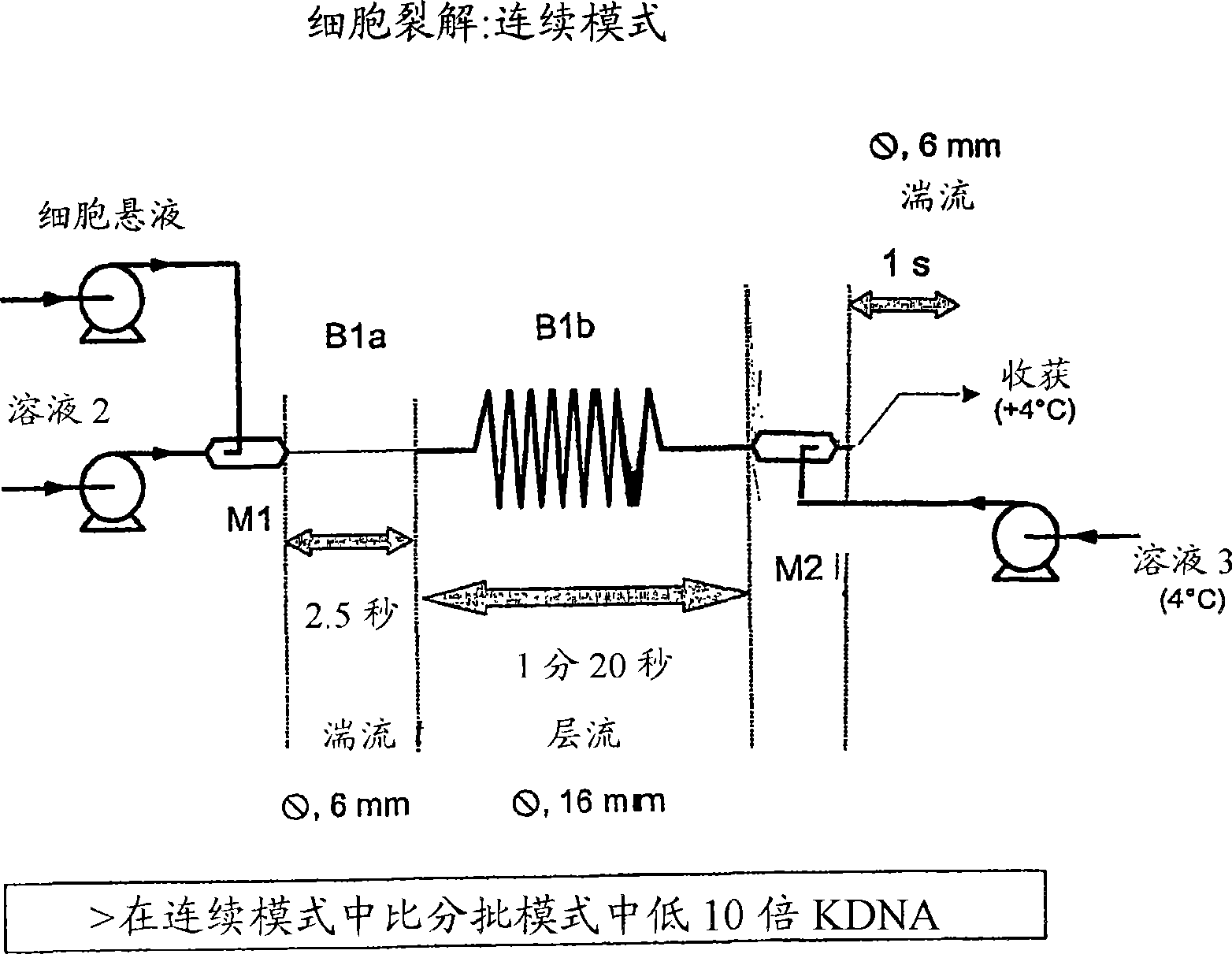
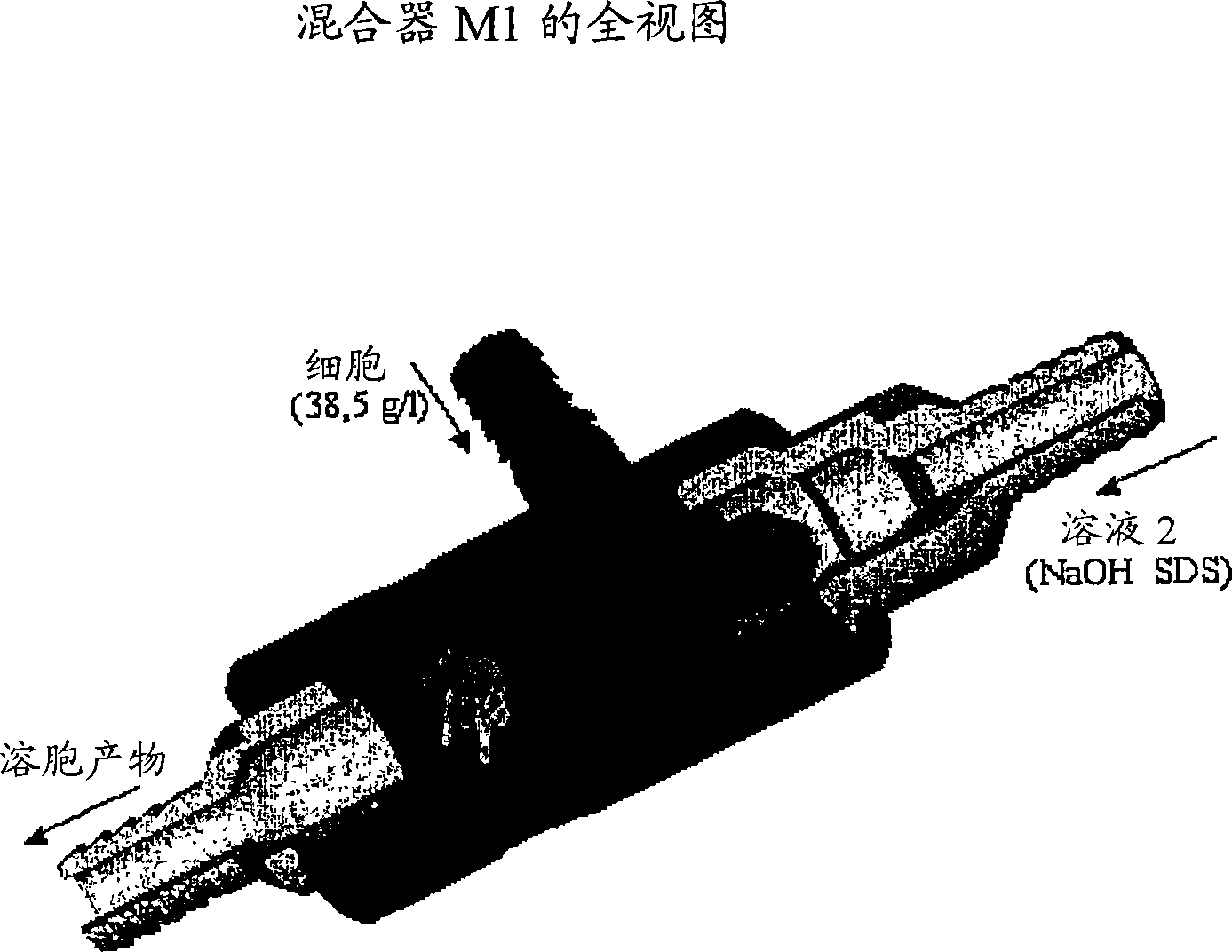
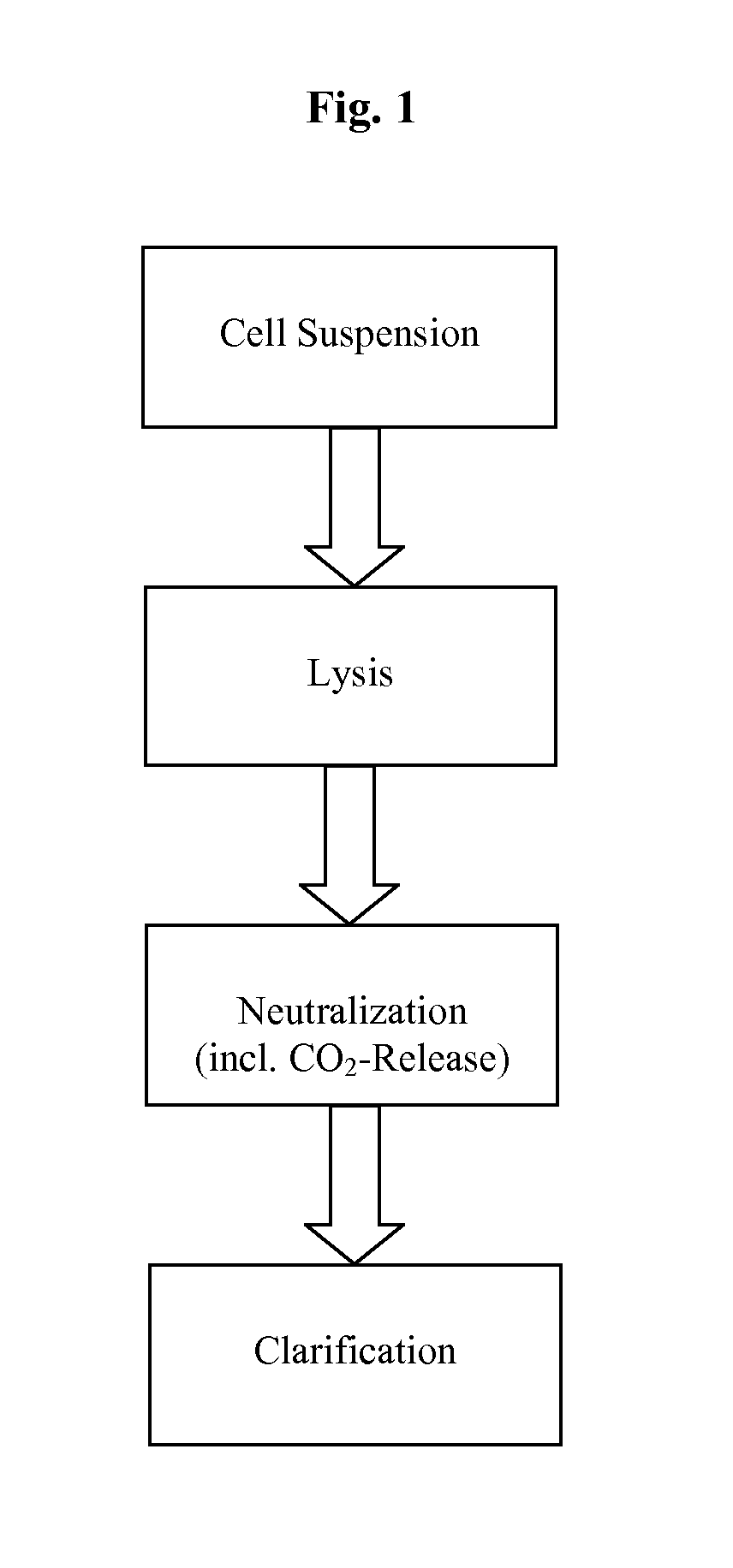
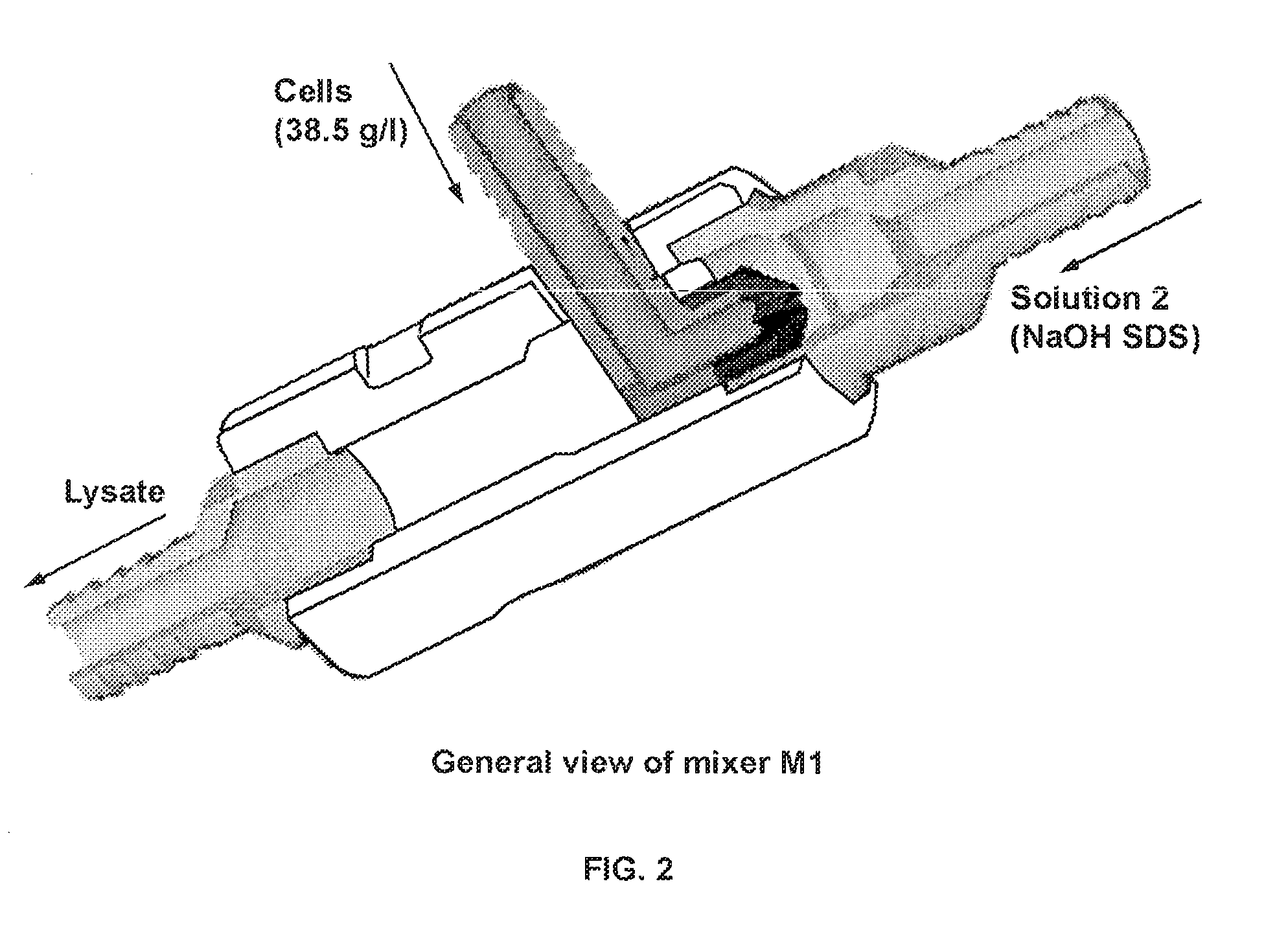
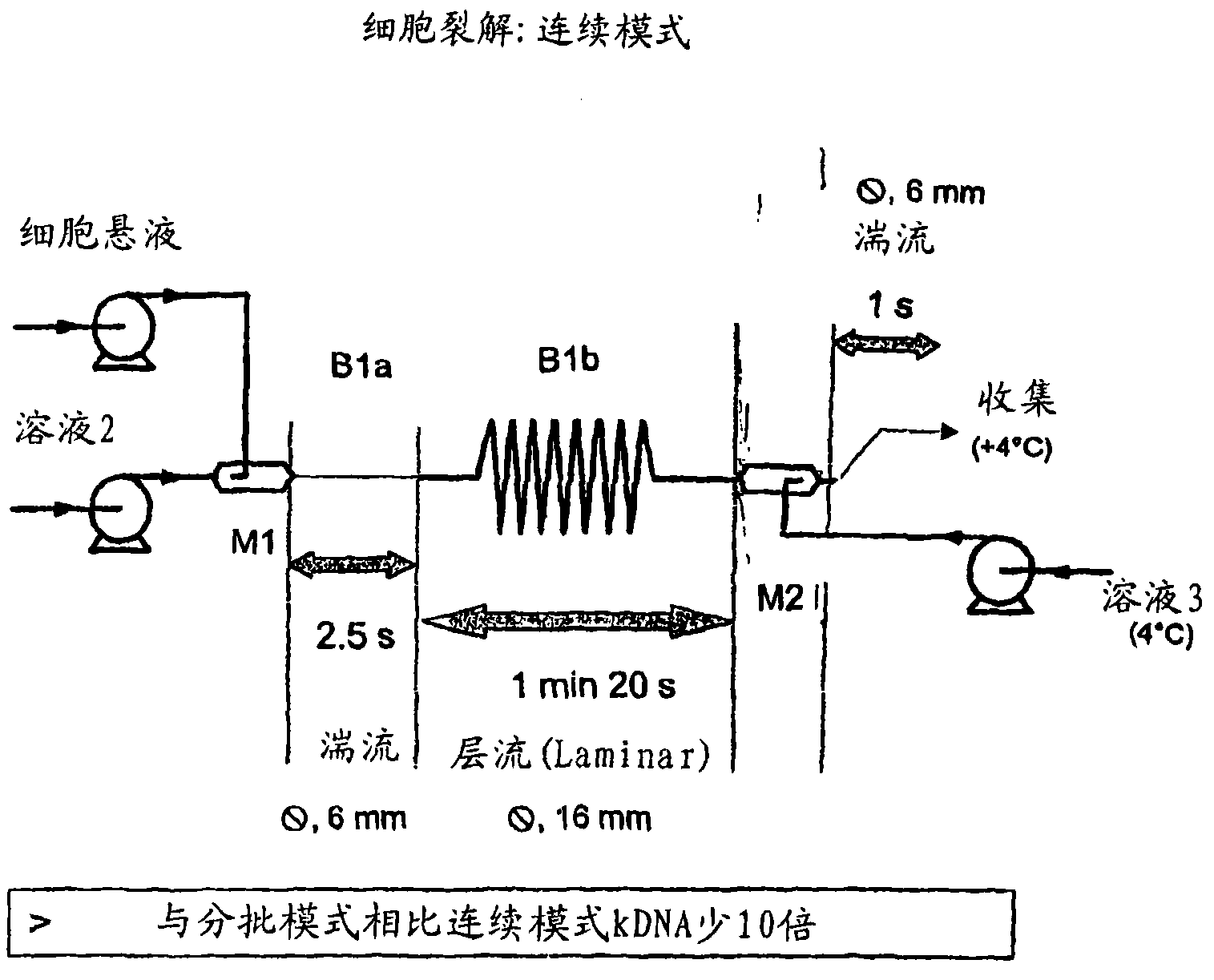
A scalable process and device for producing a biomolecule, in particular pharmaceutical grade plasmid DNA. The process includes the steps of alkaline lysis and a neutralization. For separating the lysate and the precipitate, the mixture is allowed to gently flow downward through a clarification reactor that is partially filled, in its lower part, with retention material like glass beads, whereby the precipitate is retained on top of and within the retention. In a preferred embodiment of the lysis step, cell suspension and alkaline lysis solution flow through a lysis reactor that is filled with particulate material like glass beads. The process can be run continuosly and fully automated.
Owner:BOEHRINGER INGELHEIM RCV GMBH & CO KG
Alkaline lysis system for preparing plasmid DNA and combined system
ActiveCN102212466AImprove stabilityImprove efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsShear stressUltrafiltration
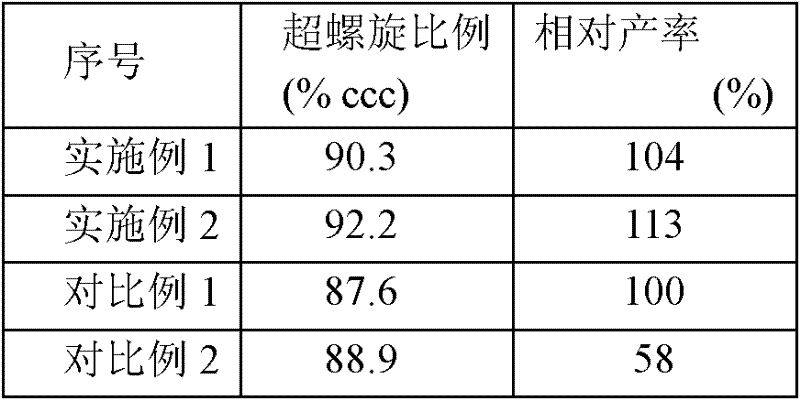
The invention provides an alkaline lysis system for preparing plasmid DNA and a combined system. The alkaline lysis system for preparing plasmid DNA contains a bacterial suspension storage tank, an alkaline lysate storage tank and a hollow ultrafiltration column, wherein the hollow ultrafiltration column contains a first cavity and a second cavity which are divided by an ultrafiltration membrane;the alkaline lysate storage tank is communicated with the first cavity; and the bacterial suspension storage tank is communicated with the second cavity. The alkaline lysis system provided by the invention adopts the hollow ultrafiltration column to mix bacterial suspension and alkaline lysate evenly and avoid the problems that the pH value is too high locally and the shear stress is too large owning to the conventional mixing method such as stirring; and the invention provides the alkaline lysis system for preparing plasmid DNA, and the system has good stability, high efficiency and high repeatability. Meanwhile, the plasmid DNA prepared by the alkaline lysis system has higher supercoiled DNA content which is up to more than 90%.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
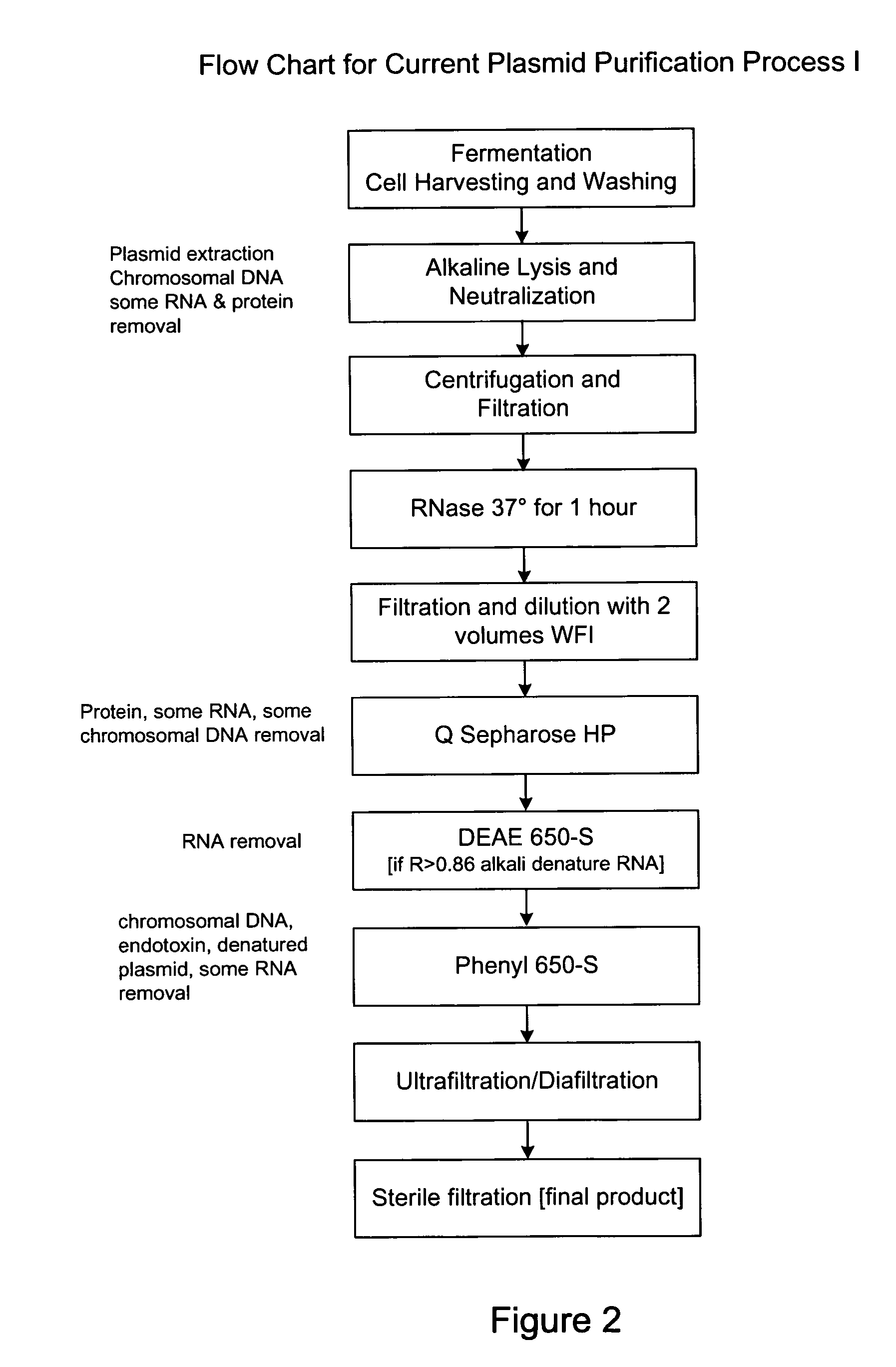
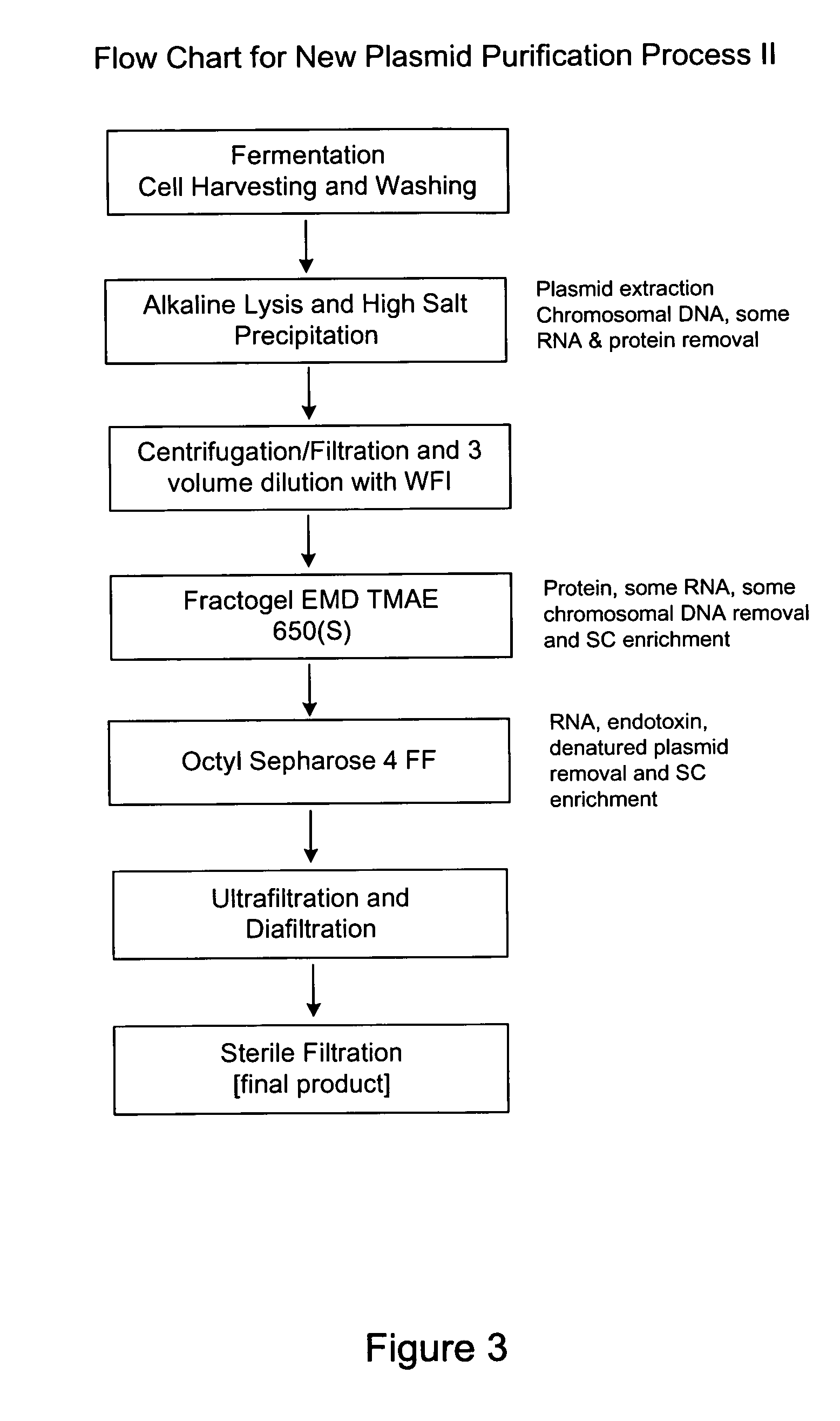
Method for purifying plasmid DNA
InactiveUS20070213289A1Yield maximizationProtozoaGenetic therapy composition manufactureLysisFiltration
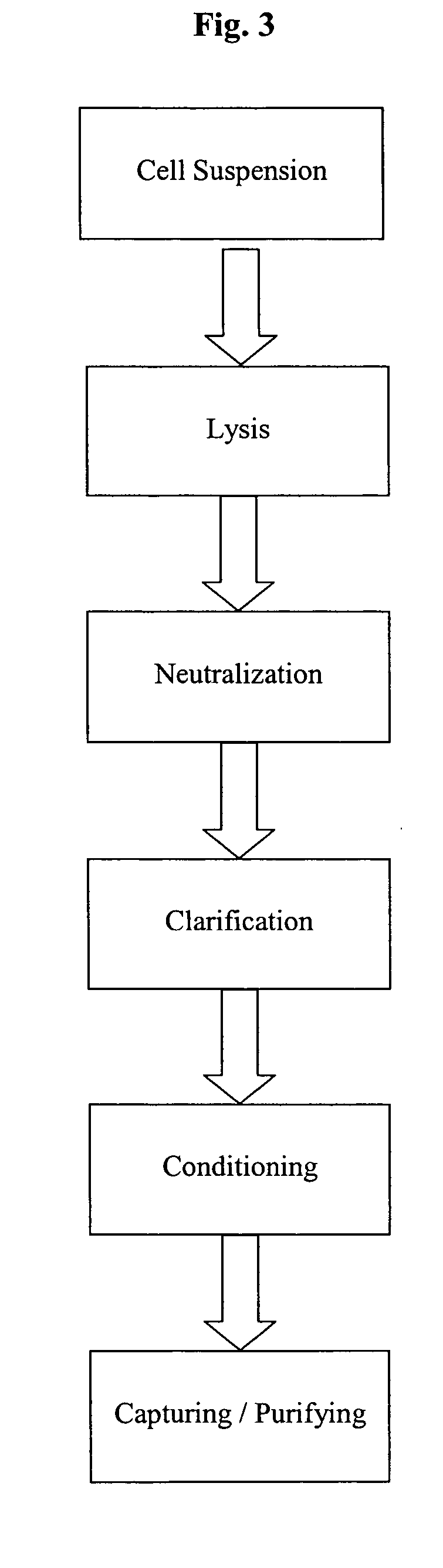
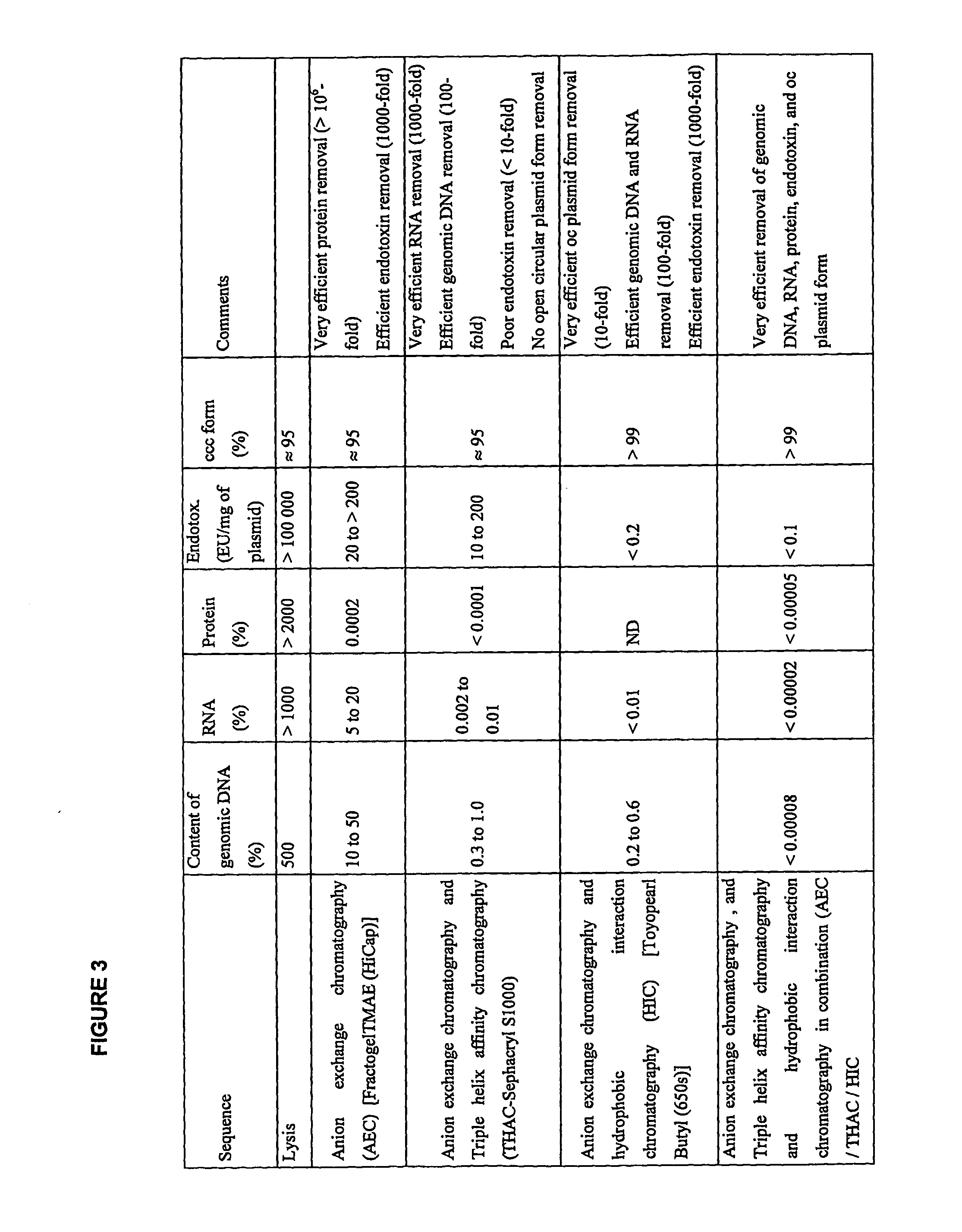
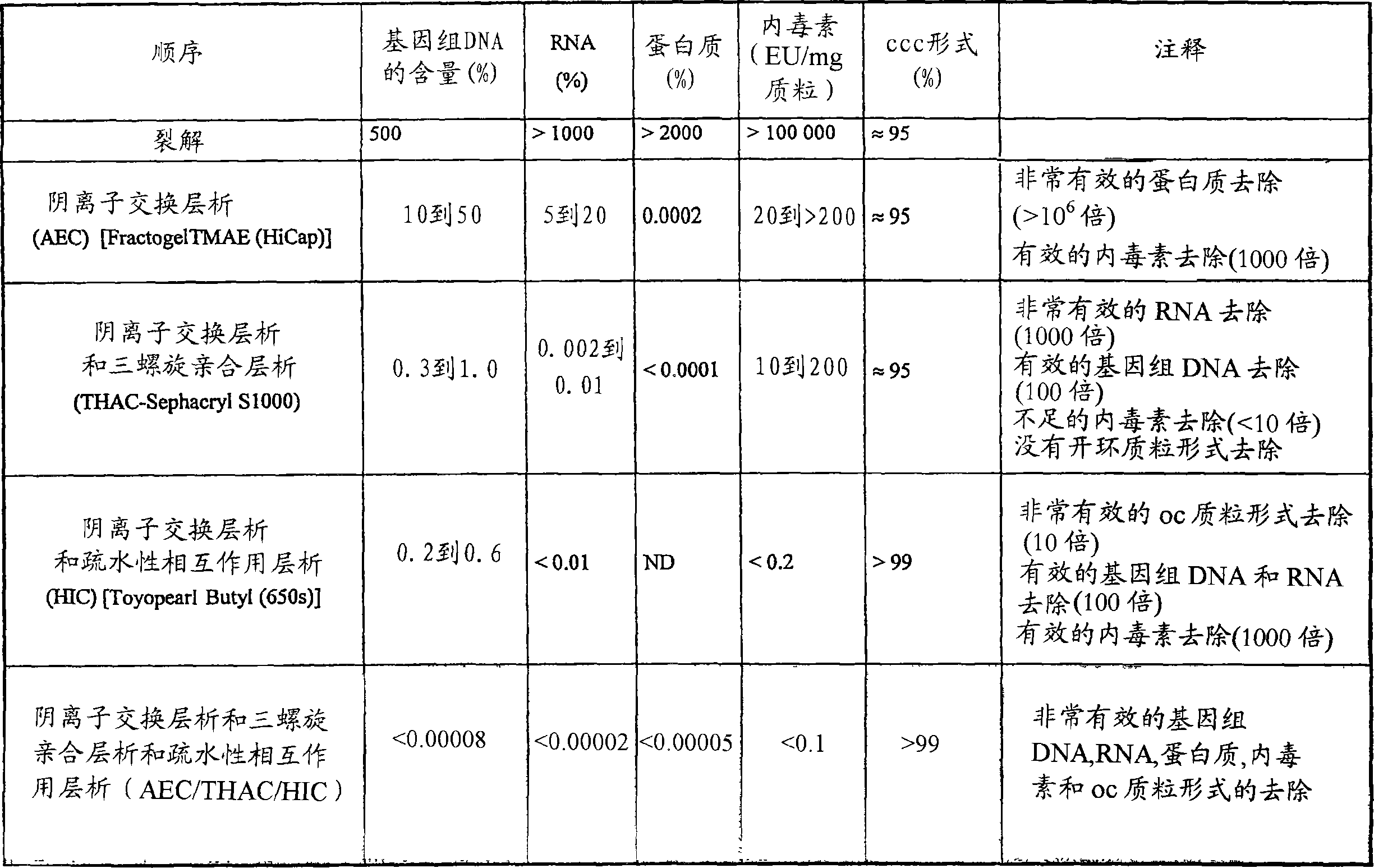
This invention provides a process for the continuous alkaline lysis of a bacterial suspension in order to harvest pDNA. It further provides for optional additional purification steps, including lysate filtration, anion exchange chromatography, triplex affinity chromatogragphy, and hydrophobic interaction chromatography. These optional purification steps can be combined with the continuous lysis in order to produce a highly purified pDNA product substantially free of gDNA, RNA, protein, endotoxin, and other contaminants.
Owner:AVENTIS PHARMA SA (US)
Kit for quickly extracting plant genome and applications thereof
InactiveCN101575597ASimple compositionConvenient amountSugar derivativesDNA preparationPlanting seedTarget gene
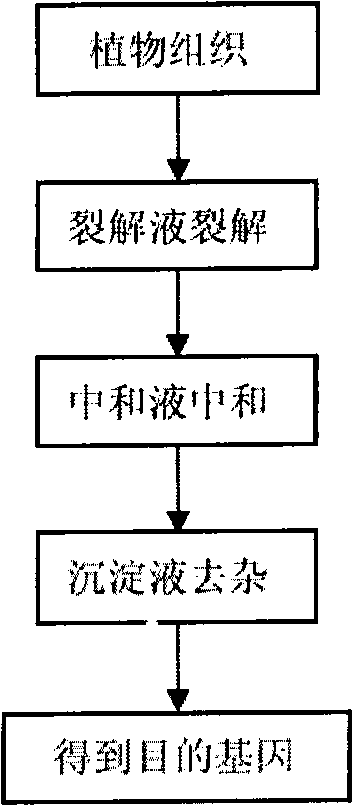
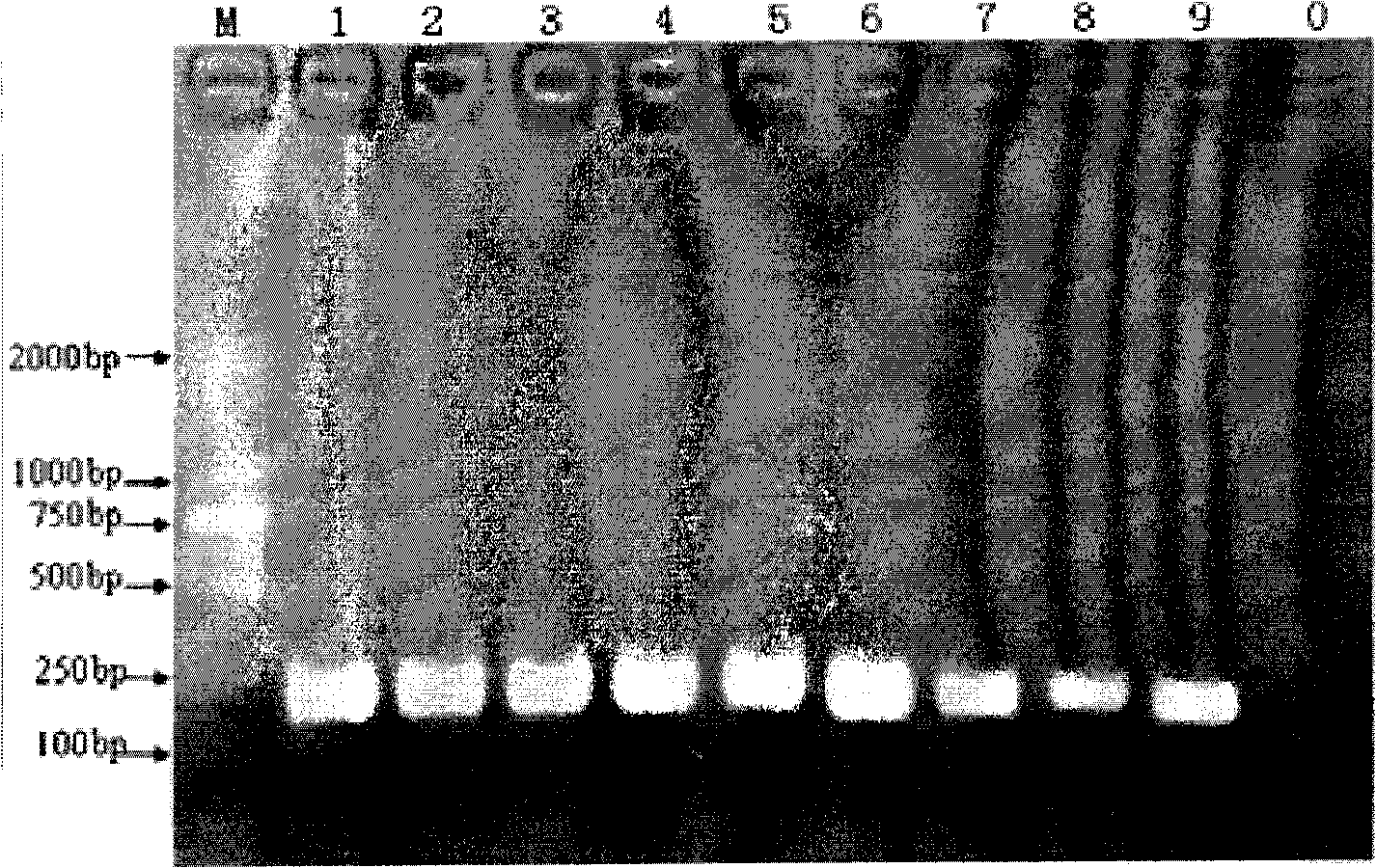
The invention discloses a kit which is suitable for PCR amplification and used for quickly extracting plant genomes, comprising a lysate, a neutralizer and a precipitation liquid. The kit improves and optimizes the traditional alkaline lysis method used for preparing genome and enlarges the applicable range of the alkaline lysis method used for preparing genome. The kit is widely applied to the extraction on the genome of various plants and can quickly extract the genome used for PCR template directly from plant seeds. The kit has obvious detection effect on the target gene of 600bp, has excellent amplification effects on endogenous gene and exogenous gene and can discover the samples with the components to be measured more than 1%. The extraction time for the whole genome is controlled within 21min, thus greatly shortening the extraction time of the genome.
Owner:CHINA AGRI UNIV
Isolation of nucleic acid using colored buffers
InactiveUS7754873B2Improve yield and qualityFast and reliable and efficientSugar derivativesMicrobiological testing/measurementVisual monitoringLysis
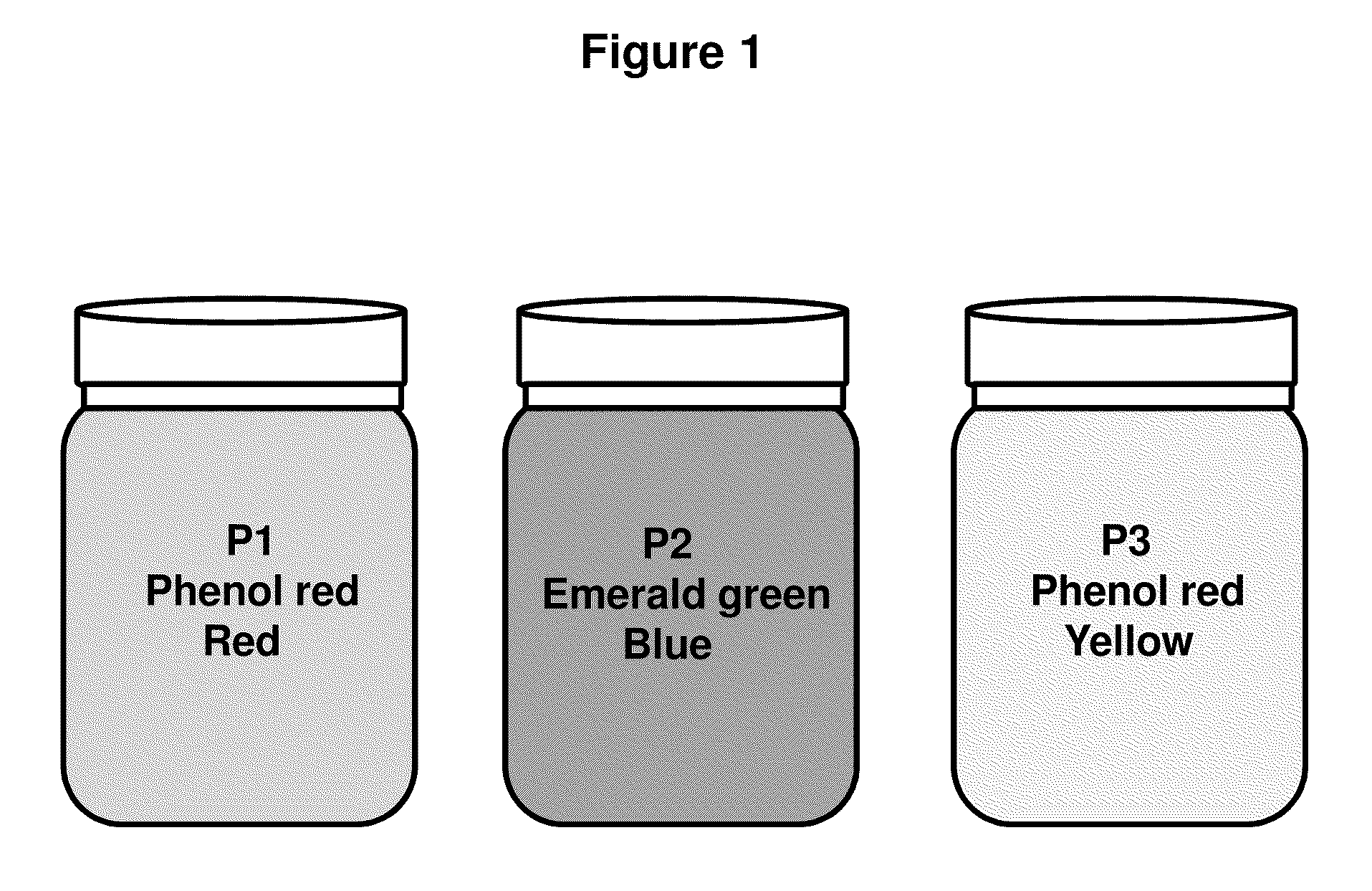
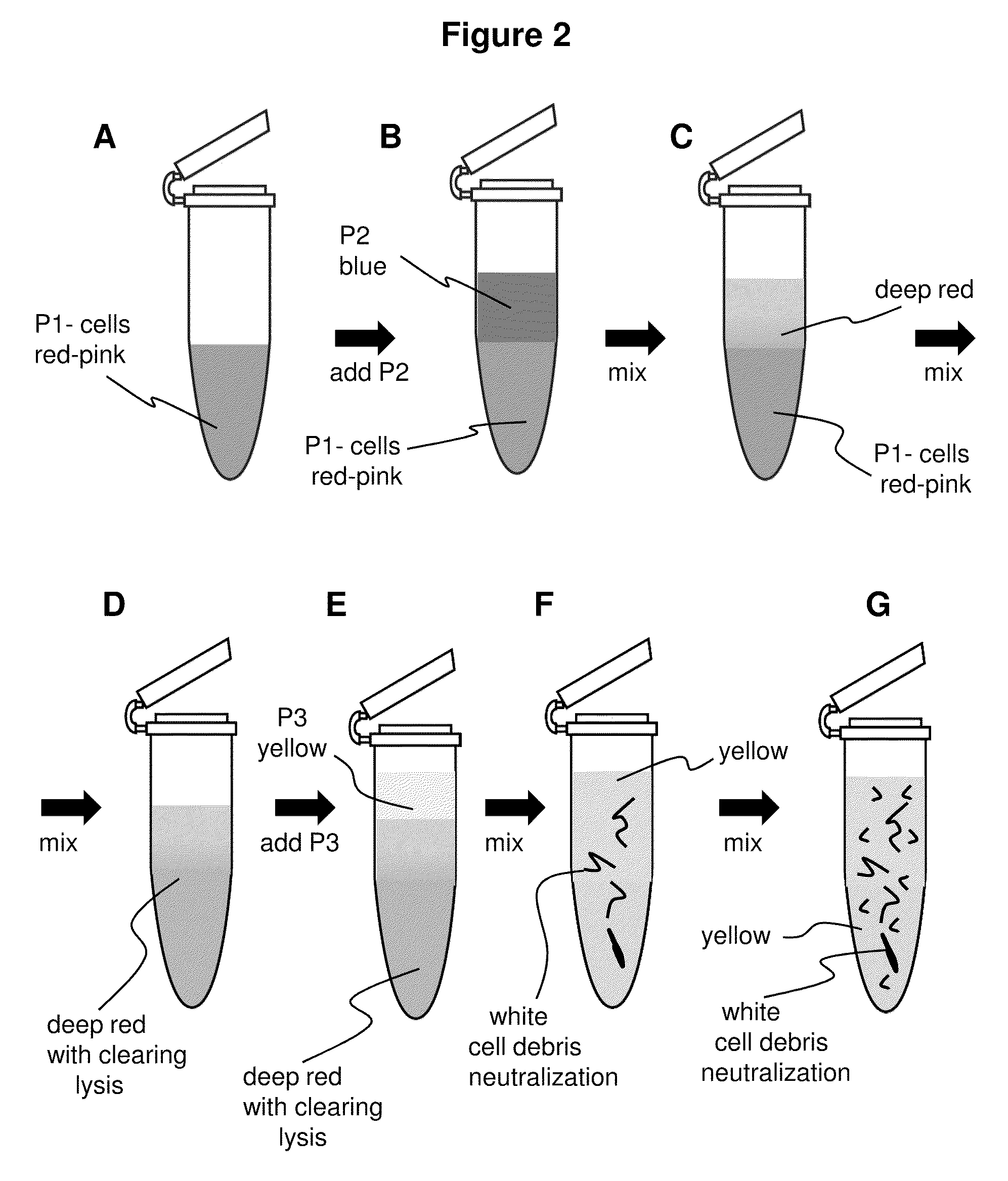
The present invention describes isolation of plasmid DNA from bacteria. The addition of dyes to the alkaline lysis based purification buffers (P1, P2, and P3) allows for improved visual monitoring of the steps of preparing a bacterial lysate filtrate coupled to filtration or spin-column chromatography. The method comprises the suspending of the bacterial cells with buffer P1 (suspension is red / pink); lysing the bacteria with buffer P2 (suspension goes from red / purple color to translucent / purple); precipitating cellular debris with buffer P3 (solution becomes yellow with debris suspension); centrifuging or filtering to product a lysate filtrate; binding the lysate filtrate to a DNA binding matrix; washing; and isolating the plasmid via chromatography. The yield and quality of plasmid DNA is improved due to more consistent lysis. Errors in buffer addition are reduced by visualizing the color as buffers are added and also of changes in color of the preparation at each step.
Owner:ZYMO RES CORP
Method of preparation of pharmaceutically grade plasmid DNA
InactiveCN1882682AImprove efficiencyBrief vigorous mixingGenetic therapy composition manufactureMicroorganism lysisLysisFiltration
This invention provides a process for the continuous alkaline lysis of a bacterial suspension in order to harvest pDNA. It further provides for optional additional purification steps, including lysate filtration, anion exchange chromatography, triplex affinity chromatography, and hydrophobic interaction chromatography. These optional purification steps can be combined with the continuous lysis in order to produce a highly purified pDNA product substantially free of gDNA, RNA, protein, endotoxin, and other contaminants.
Owner:AVENTIS PHARMA INC
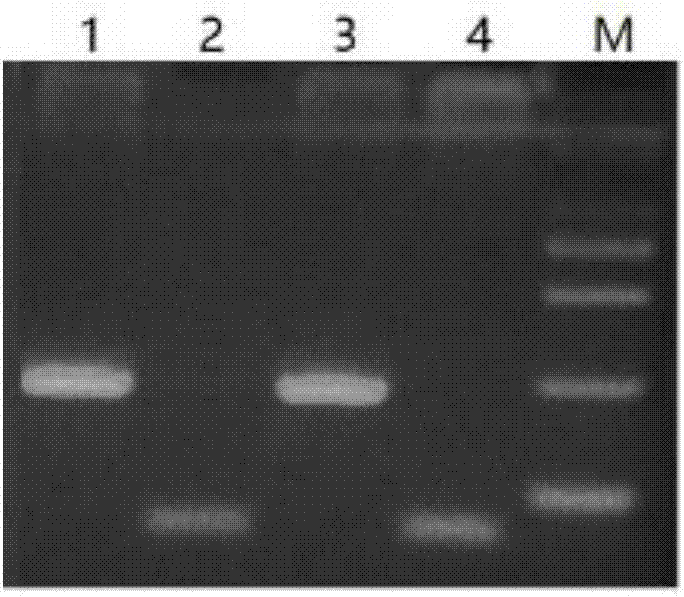
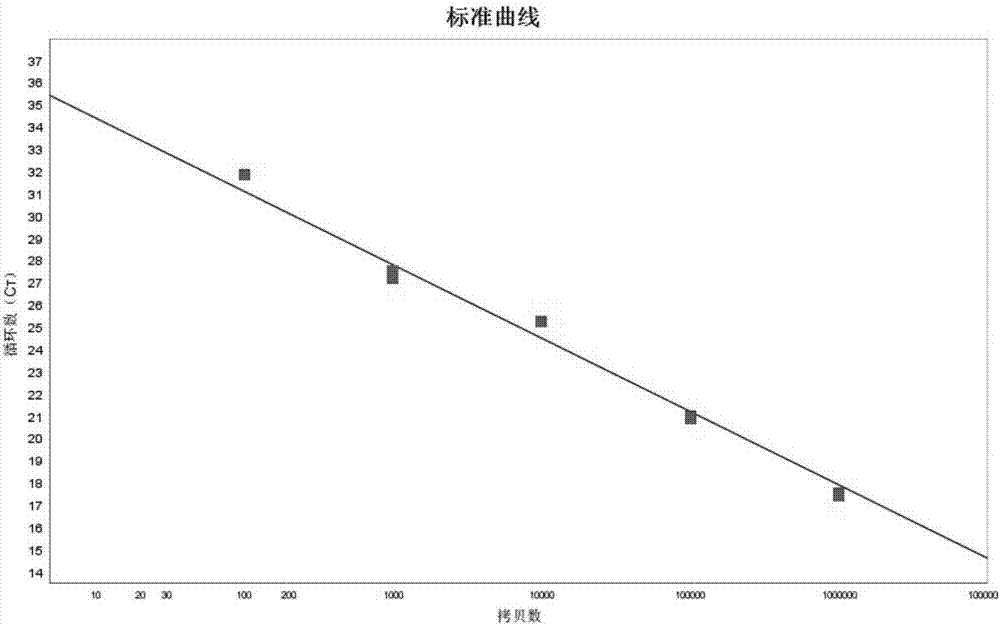
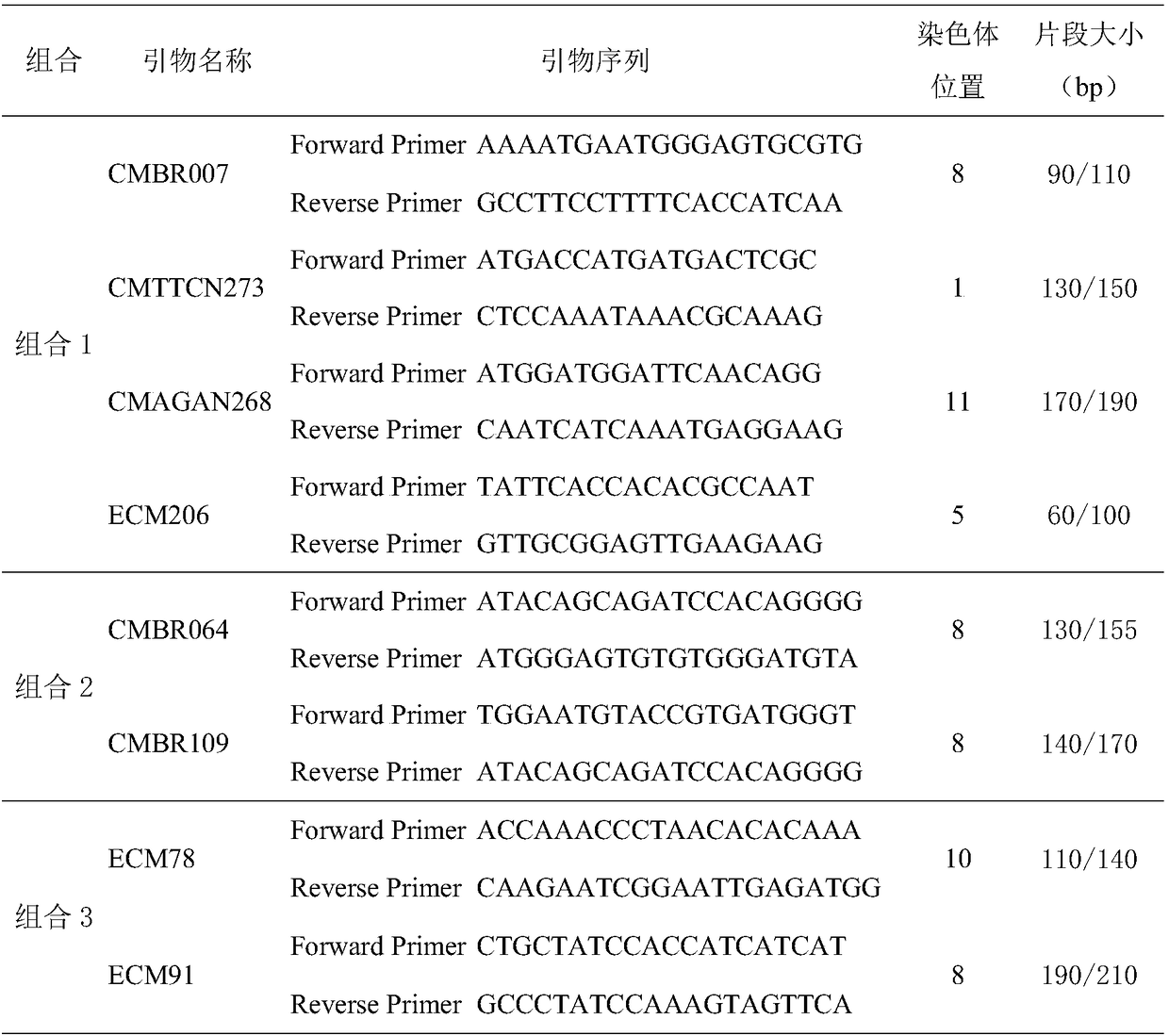
Simple sequence repeat-polymerase chain reaction (SSR-PCR)-based hybrid rape seed purity detection method
InactiveCN102321767AGood polymorphismStrong specificityMicrobiological testing/measurementDNA SolutionsBuffer solution
The invention discloses a simple sequence repeat-polymerase chain reaction (SSR-PCR)-based hybrid rape seed purity identification method, which comprises the following steps of: performing sprouting culture on hybrid rape sample seeds to be detected, performing alkaline lysis on the cultured seedlings, simultaneously performing ultrasonic disruption treatment, and adding an extracting buffer solution to obtain a genome DNA solution; performing PCR amplification on genome DNA by using an SSR primer sequence; performing voltage stabilizing electrophoretic separation on a PCR amplification product in agarose gel; performing imaging and tape reading on the PCR amplification product subjected to electrophoretic separation in a gel imaging system, comparing band characteristics of the sample seeds with those of parent seeds, counting seeds with the band characteristics of male parent and the band characteristics of female parent in the sample seeds, and obtaining the purity of the hybrid rape seeds to be detected according to a variety purity formula. The identification method has the advantages of quickness, simplicity, convenience, high throughput, low detection cost, high detection efficiency, stable and reliable detection results and the like.
Owner:湖南省作物研究所
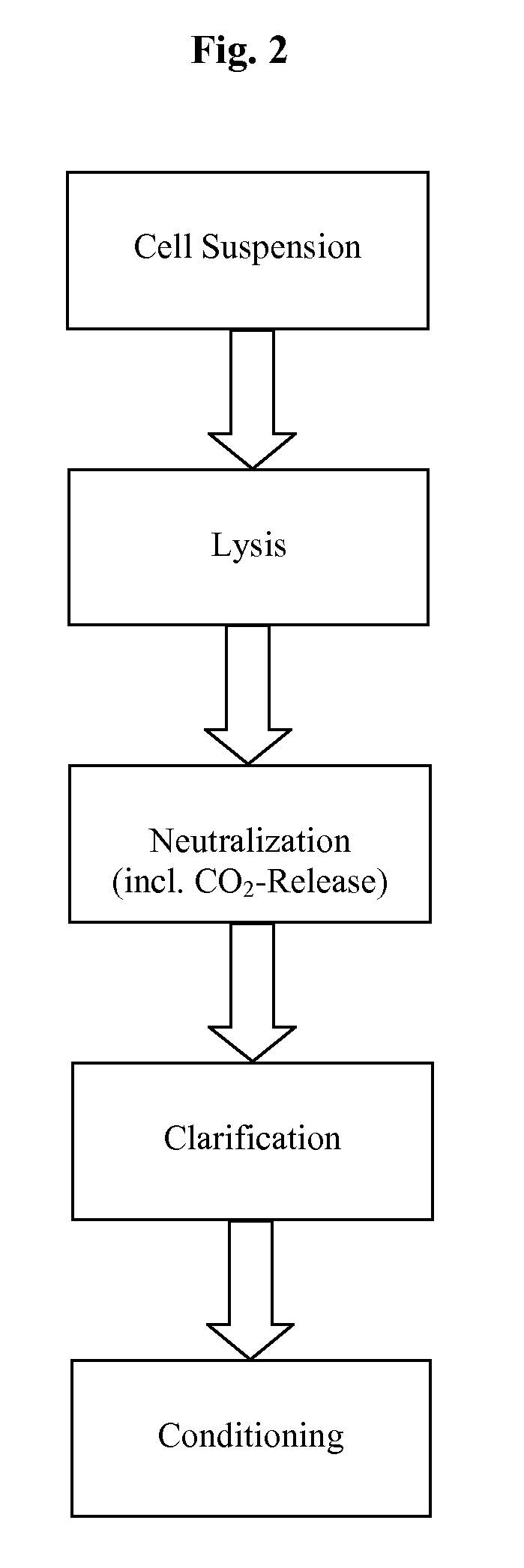
Methods and devices for producing biomolecules
InactiveUS20110124101A1Process is directionalSugar derivativesLoose filtering material filtersLysisPharmaceutical drug
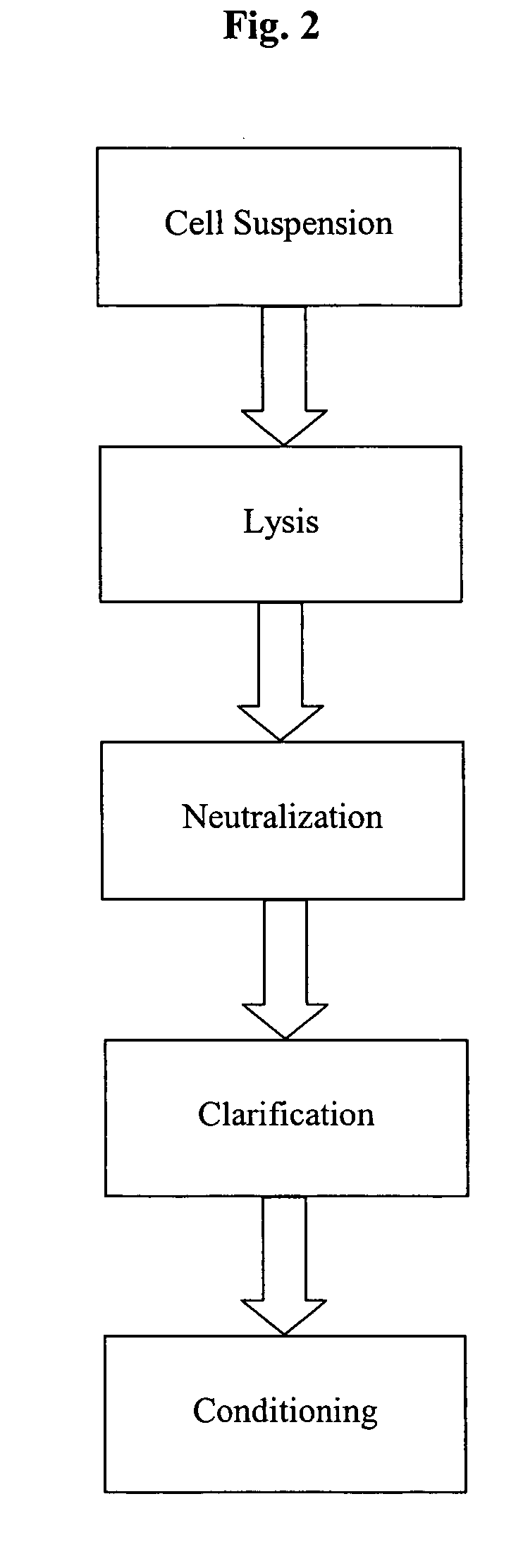
A scalable process and device for producing a bio molecule, in particular pharmaceutical grade plasmid DNA is described. The process includes the steps of alkaline lysis, neutralization and clarification and can be further extended. For separating the lysate and the precipitate an improved floatation method is disclosed. This method is based on attachment of CO2 bubbles on the precipitate floe. The CO2 is released from a carbonate salt during or after neutralization (acidification). The method of the invention is preferably carried out in an automated continuous mode applying devices for lysis and neutralization and a novel device for completely continuous clarification (separation of floes and clarified lysate).
Owner:BOEHRINGER INGELHEIM RCV GMBH & CO KG
Plasmid extraction kit and extraction method
The invention discloses a high-purity plasmid extraction kit. The high-purity plasmid extraction kit comprises an S I buffer solution, an S II buffer solution, an S III buffer solution, a PS cleaningsolution, an elution buffer solution, a TE buffer solution and a novel centrifugal column, wherein the S I buffer solution comprises Tris-HCl of 1 M, EDTA of 0.5 M and glucose of 1 M, and the pH valueis 8.0; the S II buffer solution comprises NaOH of 2 M and 10% SDS; the buffer solution S III comprises potassium acetate of 3 M, guanidine hydrochloride of 2 M and acetic acid of 2 M, and the pH value is 3.6-4.2; the PS cleaning solution is an isopropyl alcohol solution containing 5-10% of TritonX-114; and the elution buffer solution is 75% ethanol. The invention further provides a method for extracting a large number of plasmids by using the kit. According to the method, the novel centrifugal column is combined with a classic strong base-SDS bacterial cell cracking method, so that a plasmidDNA sample is centrifugally combined to a purification column, plasmid DNA can be fully eluted under a certain condition, rapid purification of plasmids is realized, phenol chloroform extraction is not needed in the whole process, high-purity plasmid DNA can be obtained, and the use of eukaryotic cell transfection can be met.
Owner:JIANGSU KEYGEN BIOTECH CORP LTD
Method of preparation for pharmaceutical grade plasmid DNA
InactiveUS20070111221A1Yield maximizationBioreactor/fermenter combinationsBiological substance pretreatmentsLysisFiltration
This invention provides a process for the continuous alkaline lysis of a bacterial suspension in order to harvest pDNA. It further provides for optional additional purification steps, including lysate filtration, anion exchange chromatography, triplex affinity chromatography, and hydrophobic interaction chromatography. These optional purification steps can be combined with the continuous lysis in order to produce a highly purified pDNA product substantially free of gDNA, RNA, protein, endotoxin, and other contaminants.
Owner:AVENTIS PHARMA SA (US)
Plasmid DNA isolation
InactiveUS20080206746A1Eliminate needBioreactor/fermenter combinationsBiological substance pretreatmentsA-DNABiochemistry
Owner:ZYMO RES CORP
Genetic engineering polypeptide vaccine adjuvant for aftosa, preparation method and application thereof
InactiveCN1994470AReduce dosageSufficient immunityGenetic material ingredientsAntiviralsEscherichia coliResistant genes
The invention relates to a method for preparing polypeptide vaccine agent used in foot-and-mouth disease gene project, wherein said agent is recombined particle that prepared by colonizing pig interferon-alpha gene via molecule biological technique into eucaryon expression carrier; the said agent is recombined particle that prepared by colonizing pig is one of interferon-alpha gene group; the eucaryon expression carrier is any DNA expression carrier with eucaryon starter, protocaryon copier, and protocaryon resistant gene; said inventive particles will transfer into bacillus coli to expand, via alkali crack method to obtain many particles, to be diluted by PBS buffer liquid into some density, to prepare inventive polypeptide vaccine agent. Animal test has proved that said agent is safe coupled with O-tyep vaccine of foot-and-mouth disease gene project, while the protection ration can reach 100% by one injection.
Owner:FUDAN UNIV
Extraction reagent and extraction method of hepatitis B virus DNA
The invention discloses an extraction method of hepatitis B virus deoxyribonucleic acid (DNA) and belongs to the field of nucleic acid extraction in molecular biology. An alkaline lysis technology is adopted according to particular characteristics of blood and viruses; impurities such as proteins, polysaccharides, lipids and the like are not required to be pre-removed; and the virus DNA is directly extracted through lysis buffer 1 and lysis buffer 2. The method has the characteristics of high efficiency, high speed, simplicity and no pollution, and the whole operation process only lasts for 50 minutes. The obtained hepatitis B virus DNA can be applied to molecular biology experiments such as the common polymerase chain reaction (PCR), fluorescence quantitative PCR and the like.
Owner:上海裕隆医学检验所股份有限公司
DNA molecular weight standard with even 200 bp gradient and rapid preparation method thereof
InactiveCN101575599AFast processHigh yieldFermentationDNA preparationEscherichia coliEnzyme digestion
The invention relates to a DNA molecular weight standard with an even 200 bp gradient and a rapid preparation method thereof. A super plasmid is constructed by a brand-new genetic engineering method; the DNA molecular weight standard with the even 200 bp gradient is prepared by methods of bacillus coli fermentation and restricted enzyme digestion and is a restricted enzyme digestion mixture comprising seven DNA bands with the lengths of 400 bp, 600 bp, 800 bp, 1000 bp, 14000 bp, 2000 bp and 3000 bp, and the densities (masses) of the seven DNA bands have a certain proportional relation of 2:3:4:5:7:10:15. The rapid preparation method of the DNA molecular weight standard with the even 200 bp gradient comprises the following steps: enabling the seven DNA bands to use a plasmid pYE 9600 as an enzyme digestion object, extracting a target plasmid pYE9600 by methods of bacillus coli fermentation and alkaline lysis, purifying the target plasmid pYE9600 and using a restricted enzyme EcoRI for enzyme digestion so as to release 200 bp serial sections contained in the target plasmid pYE9600.
Owner:UNIV OF JINAN
Nosema pernyi template DNA extraction method and application of nosema pernyi template DNA extraction method to molecular diagnosis
ActiveCN107400668ALow costLess distracting factorsMicrobiological testing/measurementMicroorganism based processesGermplasmDNA extraction
The invention discloses a rapid nosema pernyi template DNA extraction method and application thereof. The rapid nosema pernyi template DNA extraction method specifically comprises the step of releasing, enrichment, separation and purification of DNA through alkaline lysis and magnetic beads. According to the method, operation is simple and rapid, the cost is low, the flux is high, living pupa sampling can be achieved, the antijamming capability is high, the contamination risk is low, the quality of extracted DNA is high, automatic extraction can be achieved, and the method can be widely applied to molecular diagnosis; the detection flux and the operation convenience can be remarkably improved, the false positive rate of detection can be obviously decreased, and the detection sensitivity and accuracy and the detection efficiency are improved; and through an automatic series nucleic acid extraction instrument, a PCR and a preferred result-viewable PCR technique, a visual and automatic nosema pernyi analysis technique which can integrate batched DNA extraction on a porous plate and molecular diagnosis can be achieved. The rapid nosema pernyi template DNA extraction method and the application thereof have important significance for centralized quarantine of antheraea pernyi granulosis and conservation of genetic resources in the antheraea pernyi industry.
Owner:辽宁省农业科学院大连生物技术研究所
Method for quickly extracting plasmodium DNA in high through-put mode and application of method
ActiveCN107326024AHigh purityGuaranteed purityMicrobiological testing/measurementMicroorganism based processesPlasmodium DNAMagnetic bead
The invention discloses a method for quickly extracting plasmodium DNA in a high through-put mode and application of the method. Particularly, the template extracting method comprises the steps that release, enrichment, separation and purification of DNA are conducted through alkaline lysis and magnetic beads. The method is simple and fast in operation, low in cost, high in through-put, low in contamination risk, high in quality of the extracted DNA, and capable of achieving automation and being widely used in molecular detection, the detection through-put and the operation convenience can be remarkably improved, the false positive rate of detection is obviously reduced, and the detection sensitivity and accuracy and the detection efficiency are improved. The visible and automatic sample diagnosis technology that extraction and detection of a mass of DNA on a perforated plate are integrated can be achieved through series connection of an automatic nucleic acid extractor and a PCR instrument and the PCR technology which is visible in optimizing result. The problems that in an existing plasmodium detection technology, the preparation of the target DNA is complex, wastes time, is low in through-put and the like are solved, and the method has a wide application prospect.
Owner:辽宁省农业科学院大连生物技术研究所
High-flux identification method for purity of Xinjiang thick-peel muskmelon Huangpi 9818 hybrid variety based on SSR molecular marker
ActiveCN108531637ALow costImprove efficiencyMicrobiological testing/measurementHigh fluxElectrophoresis
The invention discloses a high-flux identification method for purity of Xinjiang thick-peel muskmelon Huangpi 9818 hybrid variety based on an SSR molecular marker. The method comprises the following steps: extracting DNA by adopting an alkaline lysis method, placing seeds in a 96-pore PCR plate, adding a solution buffer A, performing the hot bath for 10 minutes at 95 DEG C in a PCR instrument, then adding a buffer B, uniformly mixing, wherein the obtained solution can be used for PCR amplification. A polymorphism SSR primer is screened by utilizing parents, F1 is used for verification, 8 pairsof parental complementary type primers are screened. The sequencing and combination are performed according to the size of a PCR amplification product fragment, the PCR amplification can be separately performed, the PCR products are mixed, the electrophoresis is performed in a sheet of polyacrylamide gel, after the silver staining, a strip is read, and then the purity of the hybrid variety is identified. The identification result is basically consistent with the traditional purity identification result of the field hybrid variety. By adopting the method, the muskmelon Huangpin 9818 hybrid variety purity can be rapidly and accurately identified by virtue of high flux, the cost is low, and the application prospect is wide.
Owner:XINJIANG AGRI SCI ACAD CANTALOUPE RES CENT
Preparation process for gene vaccines containing UreB and CagA protein of helicobacter pylori
InactiveCN102688502AAddressing Low Immunity LevelsImprove immune efficiencyAntibacterial agentsBacterial antigen ingredientsAmpicillinGenetic engineering
The invention provides a preparation process for gene vaccines containing UreB and CagA protein of helicobacter pylori. The preparation process includes: establishing recombinant plasmid pIRES-UreB-CagA by recombinant plasmids pUC-UreB and pUC-CagA according to the genetic engineering technology, transforming, screening, amplifying and determining; chemically transforming attenuated Salmonella typhimurium LB500 to perform methylation; screening ampicillin resistant bacterial colony; amplifying selectively; extracting a small quantity of plasmids by the alkaline lysis method; obtaining definitive host bacteria SL7207 by further pulse cell transfection; picking resistant colone to amplify the same in LB culture solution containing 100ug / ml ampicillin to obtain protein; and finally obtaining helicobacter pylori vaccines by purifying the protein. The novel vaccines containing UreB and CagA protein of helicobacter pylori has immunization effects of both UreB and CagA protein, so that immunization efficacy can be improved to a high level.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
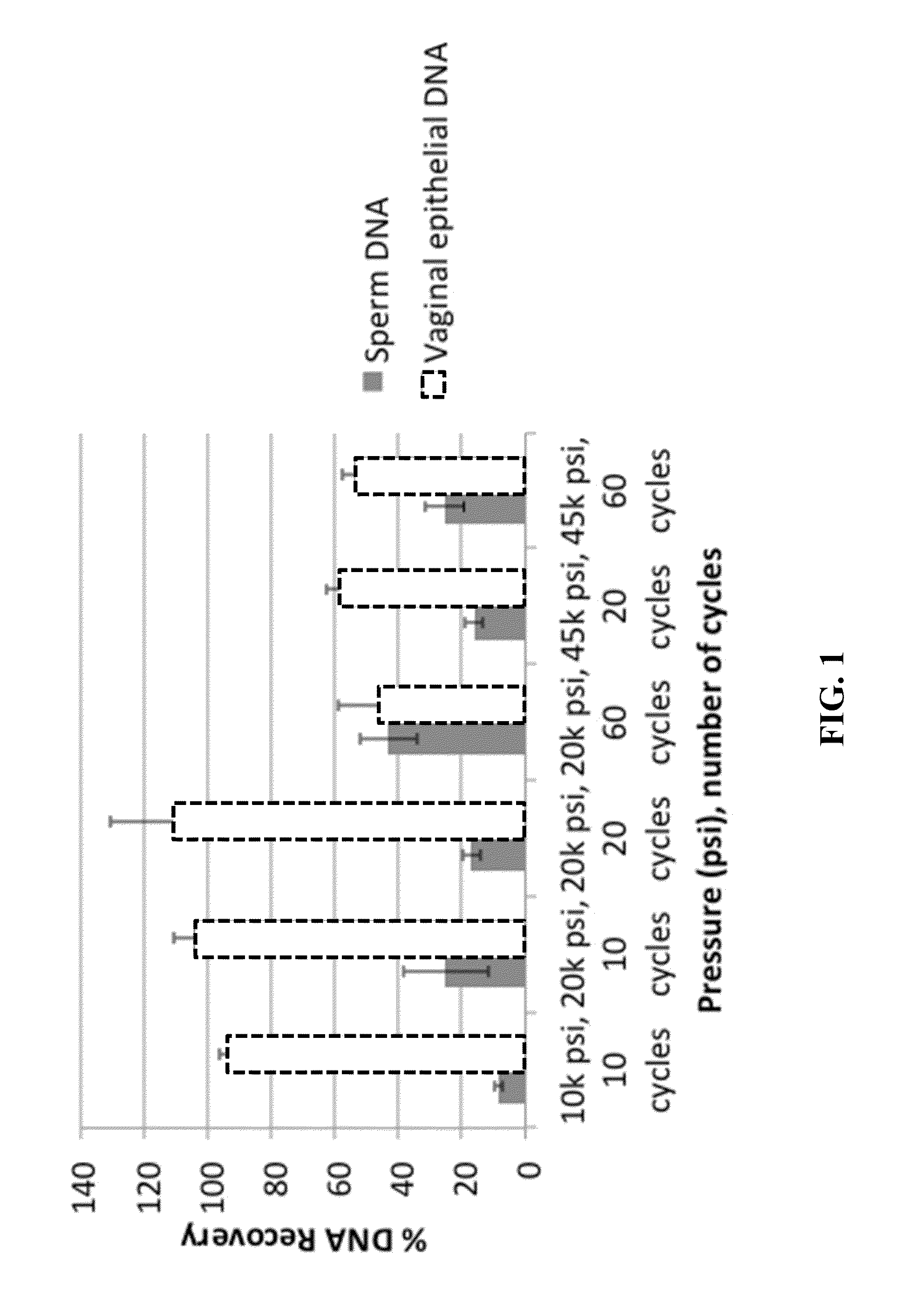
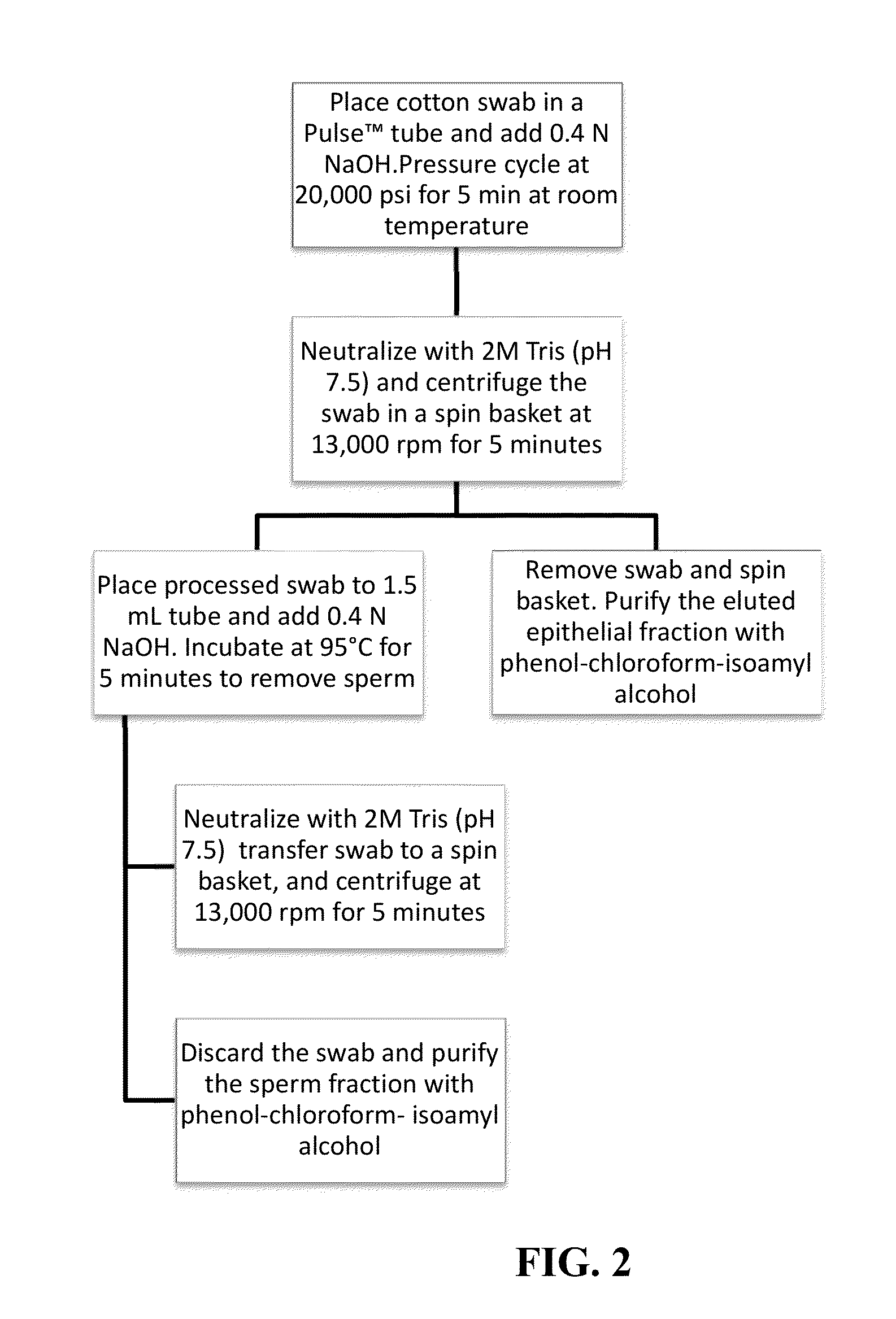
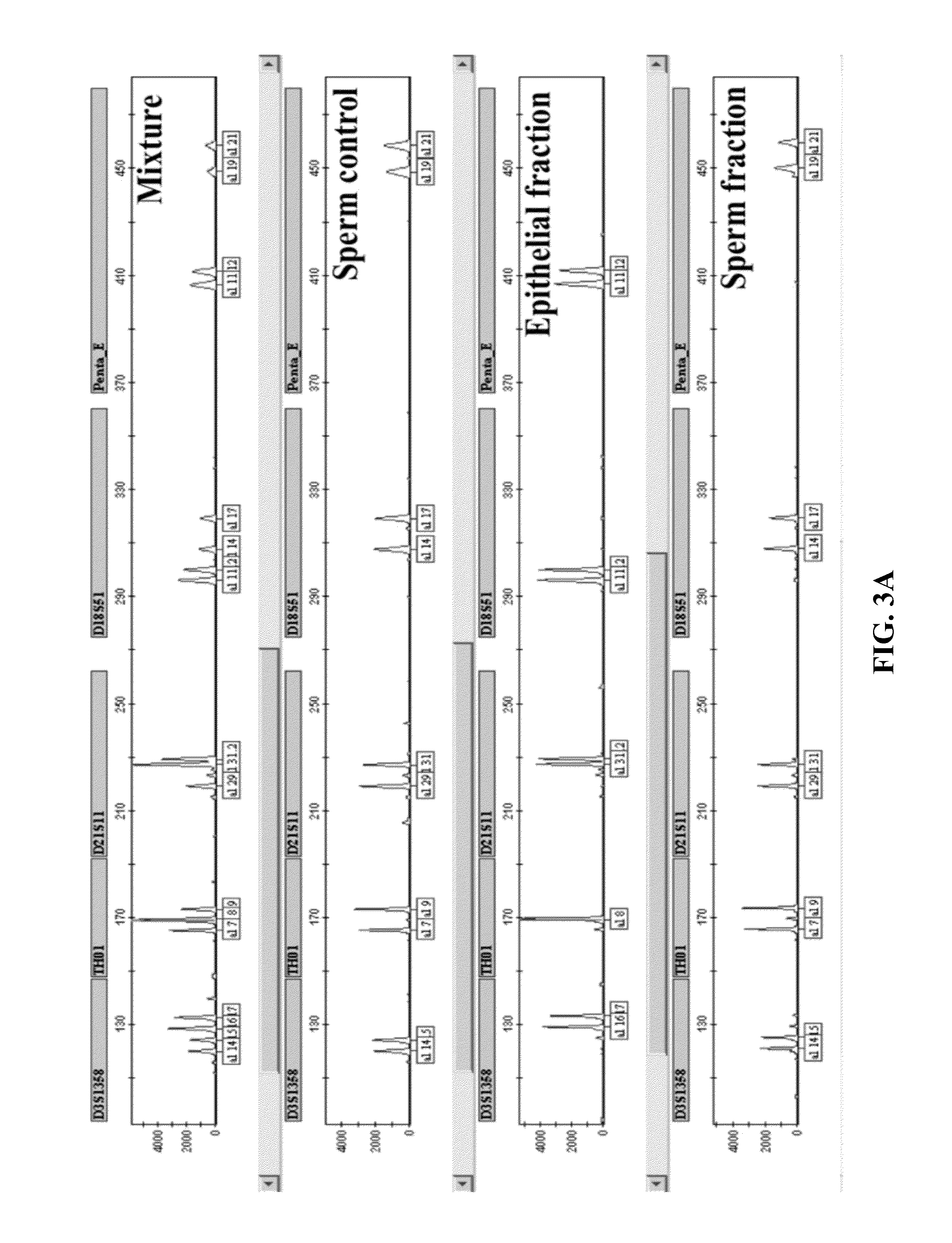
Materials and Methods for Achieving Differential Lysis of Mixtures with the Aid of Alkaline Lysis and Pressure Cycling Technology (PCT)
InactiveUS20150232917A1Improve efficiencyEfficient processingMicrobiological testing/measurementMicroorganism lysisLysisForensic dna
The subject invention provides a two-step protocol using pressure cycling technology (PCT) and alkaline lysis for differential extraction of mixtures. In a preferred embodiment the procedure is used in forensic DNA applications such as, for example, DNA testing in the case of rape.
Owner:PRESSURE BIOSCI +1
Method for purifying plasmid DNA
This invention provides a process for the continuous alkaline lysis of a bacterial suspension in order to harvest pDNA. It further provides for optional additional purification steps, including lysate filtration, anion exchange chromatography, triplex affinity chromatography, and hydrophobic interaction chromatography. These optional purification steps can be combined with the continuous lysis in order to produce a highly purified pDNA product substantially free of gDNA, RNA, protein, endotoxin, and other contaminants.
Owner:AVENTIS PHARMA INC
Methods and devices for producing biomolecules
ActiveUS8501402B2Process is directionalMicrobiological testing/measurementNucleic acid reductionLysisPartial filling
A scalable process and device for producing a biomolecule, in particular pharmaceutical grade plasmid DNA. The process includes the steps of alkaline lysis and a neutralization. For separating the lysate and the precipitate, the mixture is allowed to gently flow downward through a clarification reactor that is partially filled, in its lower part, with retention material like glass beads, whereby the precipitate is retained on top of and within the retention. In a preferred embodiment of the lysis step, cell suspension and alkaline lysis solution flow through a lysis reactor that is filled with particulate material like glass beads. The process can be run continuously and fully automated.
Owner:BOEHRINGER INGELHEIM RCV GMBH & CO KG
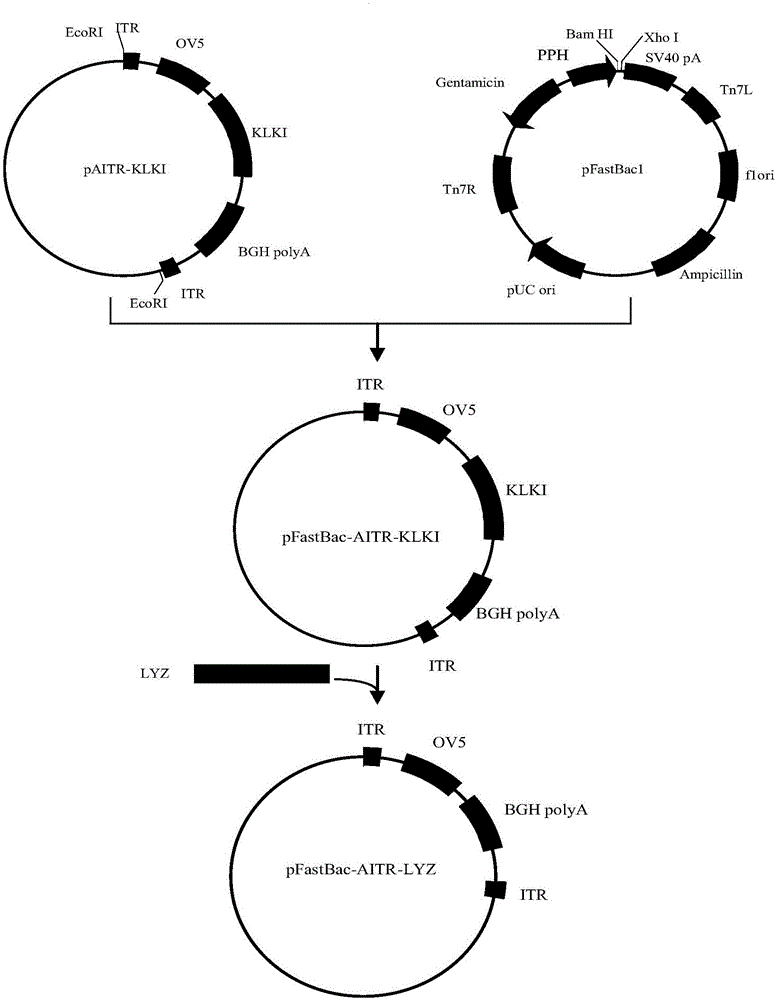
Method for preparing recombinant avian adeno-associated virus and use of recombinant avian adeno-associated virus
InactiveCN106591246AActiveInhibitionAntibacterial agentsAntimycoticsShuttle vectorEukaryotic plasmids
The invention discloses a method for preparing a recombinant avian adeno-associated virus. The method comprises 1, preparing three recombinant baculovirus transfer vectors by a DNA recombination technology, 2, respectively carrying out transposition on the three recombinant baculovirus transfer vectors to a DH10Bac competent cell, carrying out screening, and extracting three recombinant shuttle vector plasmid DNAs through an alkaline lysis method, 3, uniformly mixing the three recombinant shuttle vector plasmid DNAs and a transfection reagent PEI according to a certain proportion, transfecting a Sf9 insect cell with the mixture, and after transfection for three days, preparing the recombinant avian adeno-associated virus expressing a human lysozyme. The invention also provides the use of the recombinant avian adeno-associated virus expressing a human lysozyme. The titer of the recombinant avian adeno-associated virus is ten times that of a recombinant avian adeno-associated virus obtained by the conventional method. The human lysozyme expressed by the recombinant avian adeno-associated virus can produce antibacterial effects on the common pathogens.
Owner:JIANGSU AGRI ANIMAL HUSBANDRY VOCATIONAL COLLEGE
Reverse transcription method, reverse transcription kit and application thereof
PendingCN106867993AReduce the difficulty of operationShorten experiment timeDNA preparationLysisComplementary deoxyribonucleic acid
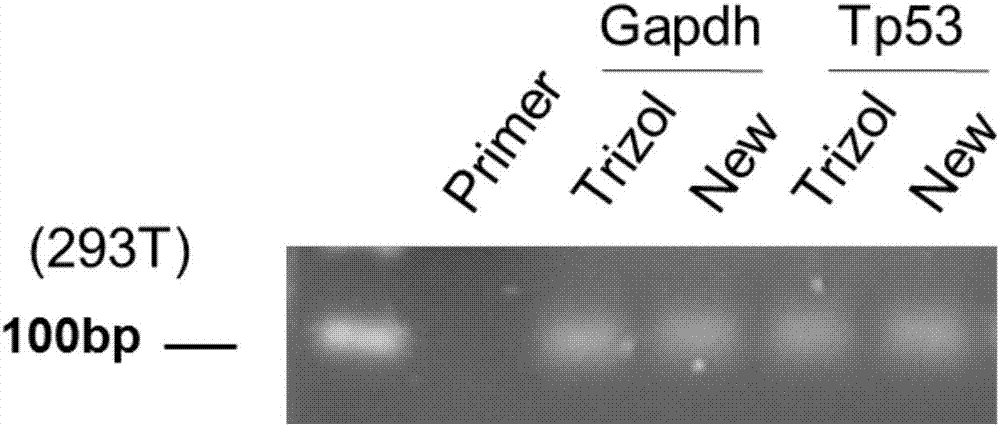
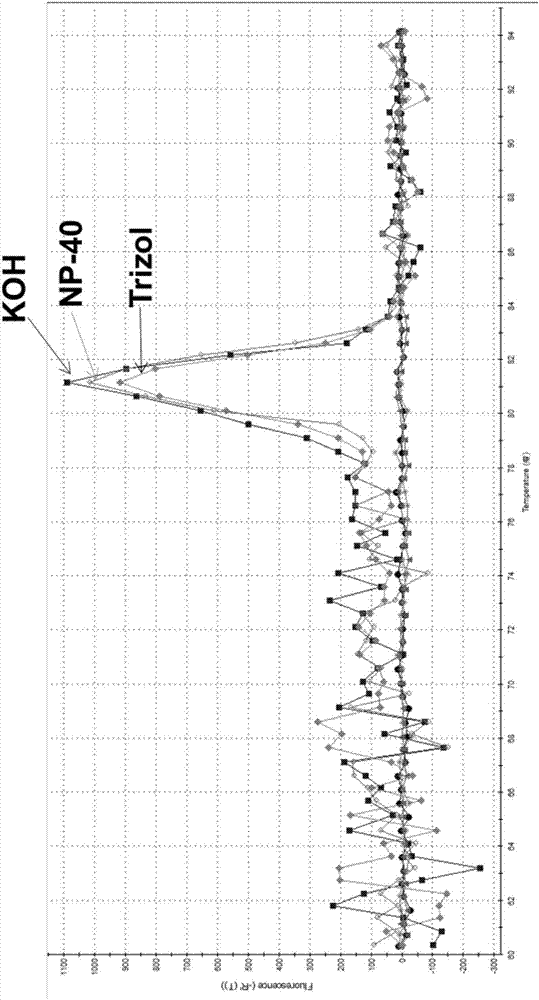
The invention relates to a reverse transcription method, a reverse transcription kit and application thereof. The reverse transcription kit contains NP-40 lysate or an alkaline lysis agent; the lysis agent is the NP-40 lysate; the formula of the NP-40 lysate comprises RNase-Free H2O, NP-40 and RNasin, wherein the mass concentration of the NP-40 is 0.2-5%, and the concentration of the RNasin is 1-10U; the formula of the alkaline lysis agent is prepared from RNase-Free H2O and KOH, wherein the concentration of the KOH is 0.00001-0.01M; the reverse transcription method comprises the steps of firstly, carrying out lysis on a sample by using the lysis agent; then, directly carrying reverse transcription on the sample, subjected to lysis, by using a reverse transcription reagent. The method is a brand-new method for acquiring complementary deoxyribonucleic acid (cDNA) from the sample; after the method is adopted, the operation process is simplified, the operation difficulty is reduced, the experimental time is greatly shortened, and the cost is lowered.
Owner:唐旌生物科技(上海)有限公司
Plasmid DNA alkali cracking equipment and use method thereof
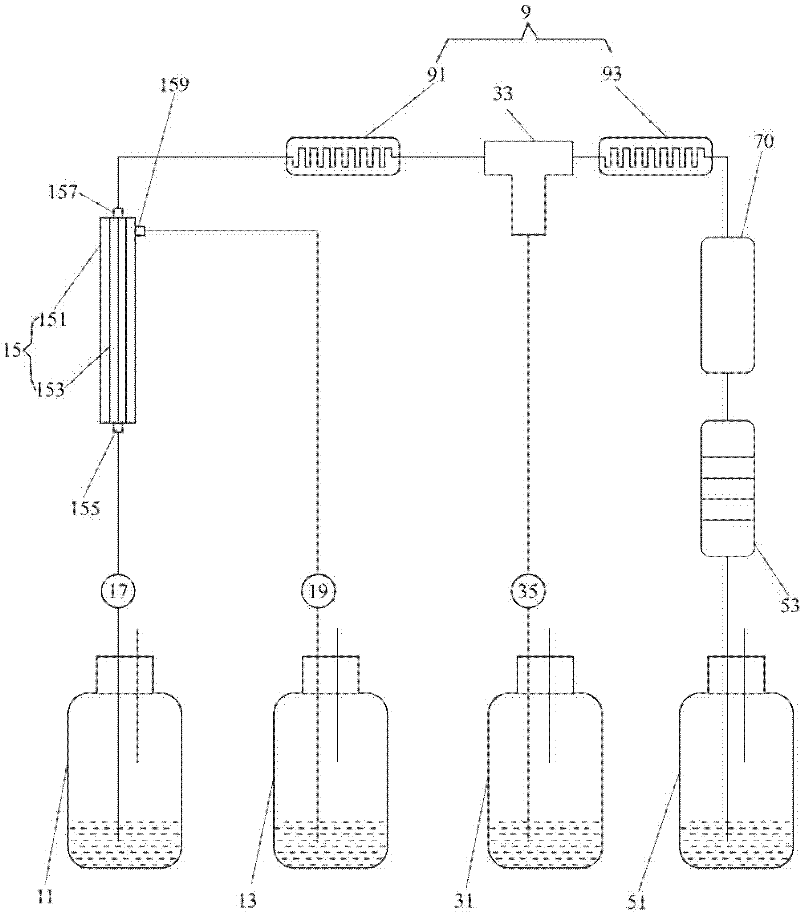
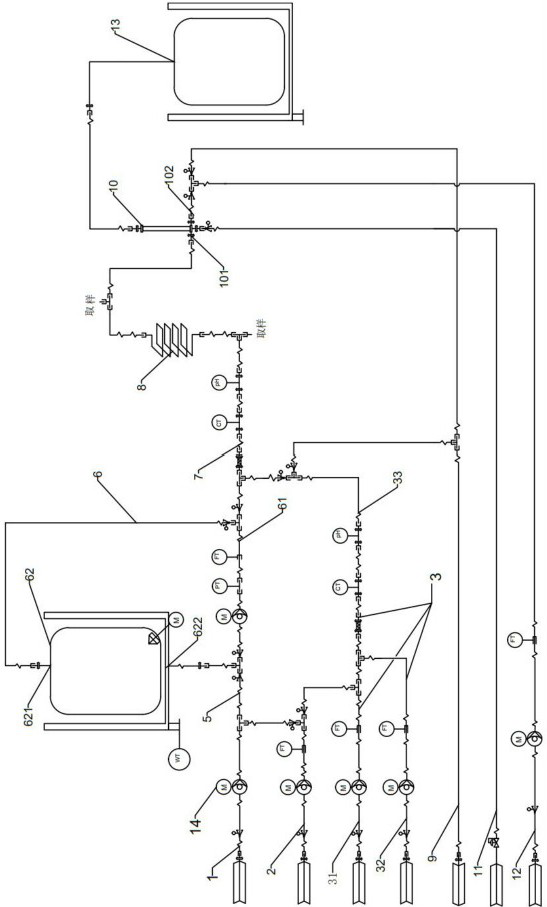
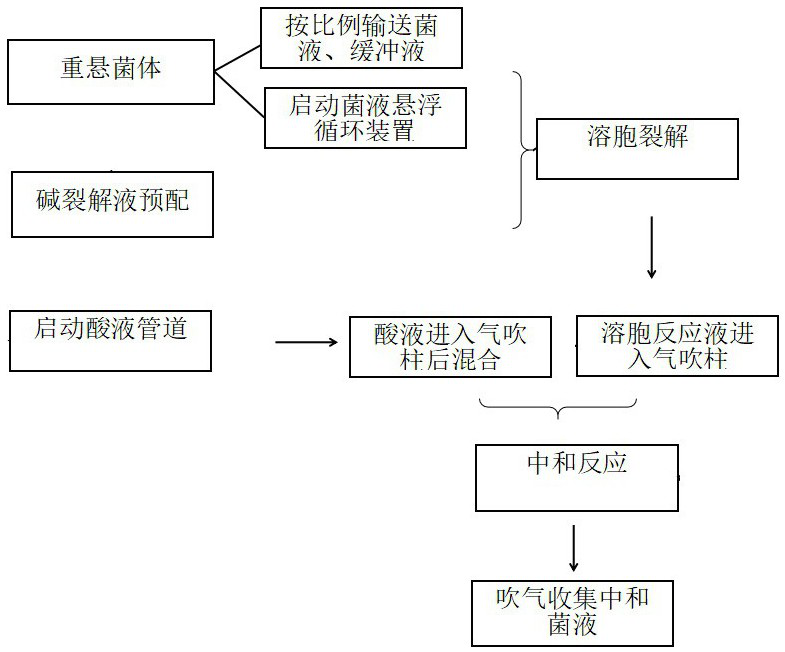
ActiveCN111733060AImprove resuspension effectAchieve serialization of resuspensionBioreactor/fermenter combinationsBiological substance pretreatmentsLysisMicrobiology
The invention discloses plasmid DNA alkali cracking equipment. The equipment comprises a bacterial liquid pipeline; a buffer solution pipeline; a bacteria liquid suspension circulating device comprising circulating pipelines which are connected end to end to form a loop, and connected with the bacteria liquid pipeline and the buffer solution pipeline, wherein a pump and a valve arranged on the circulating pipeline; an alkali lysate pre-preparation pipeline; a lysis reactor; a neutralization reactor; an acid liquor pipeline; and a collecting device. The invention also discloses a method for extracting plasmid DNA by using the method. The method comprises the following steps: resuspending thalli by using a bacterial liquid suspension circulating device, pre-preparing an alkali lysate, breaking cells, inputting an acid solution, neutralizing a bacterial liquid and the like. The bacterial liquid can repeatedly circulated in the bacterial liquid suspension circulating device, the flow is improved, and the bacterial liquid resuspension effect is improved; the flow is adjusted, and thus the bacterial liquid can reach the target flow, and is convenient to mix with the alkali lysate.
Owner:LISUI TECH SUZHOU
Pseudomonas Na+/H+ antiporter protein gene and its cloning process
InactiveCN1667122AImprove salt toleranceHighlight the function of pumping out of the cellMicrobiological testing/measurementFermentationAntiporterPseudomonas
This invention is fake unicell bacteria Na+ / H+ antiport albumen gene and its clone method, it relates to one kind of gene clone. One kind of fake unicell bacteria Na+ / H+ antiport albumen structure gene and one kind of quickly and economic clone method are provided, gene length is 1089bp, coding 362 amino acids. Procedures are that extra anti-salt fake unicell bacteria is inoculated and cultivated, whole DNA is extracted, primer is designed, PCR reaction and amplified product is gel imaged system sweep record, then the amplified about 1.1kb tripe is cut down. One gland piao lin deoxyribonucleotide (A) is added to end of PCR product, above clear liquid is abandoned, and 20muL weight distilled water is added to dissolve DNA. Carrier is connected with DNA to form single colony. White colony is selected and recombination particle is extracted by caustic cracking solution. Goal gene is identificated and anti-salt level of transformant is detected, clone gene of transformant is sequenced and weight plasmid of transformant is identificated, then colone gene is estimated.
Owner:XIAMEN UNIV
Method for extracting plasmids with endotoxin removed on large scale
ActiveCN107502606AImprove pollutionEliminate pollutionMicrobiological testing/measurementDNA preparationEndotoxin removalDigestion
The invention relates to a method for extracting plasmids with endotoxin removed on a large scale. The method comprises the steps of acquiring roughly-separated plasmid DNA by virtue of a classical alkaline lysis method, sequentially adding isopropanol, an NH4Ac solution, RNase A and NaCl, adding a mixed solution of PEG6000 and NaCl, dissolving and precipitating by virtue of a TE solution, repeatedly precipitating by virtue of absolute ethyl alcohol, washing and precipitating by virtue of 70% alcohol, simultaneously coordinating with centrifugation, drying, and finally dissolving and precipitating by virtue of the TE solution, so as to obtain target plasmids. According to the method, the pollution caused by endotoxin to plasmid DNA can be well removed, all reagents are standing chemical reagents in a laboratory, and the operation method is simple, easy to control and suitable for large-scale extraction of plasmids. The concentration of the plasmids can reach 5mg / mL, the endotoxin is lower than 5EU / mu g, and the plasmids can be applied to digestion, DNA sequencing, cell transfection, virus packaging and clinic animal immunization experiments.
Owner:上海埃秀马生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com