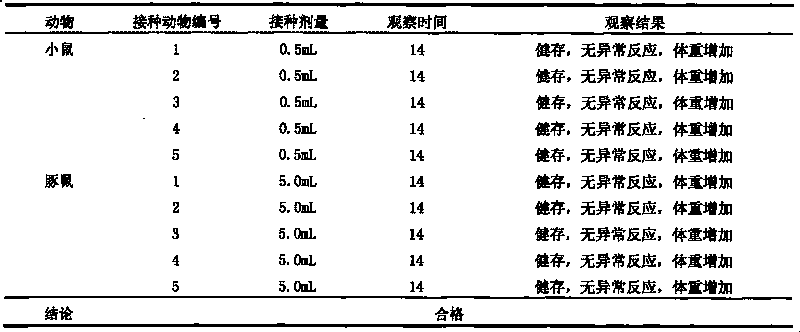
Patents
Literature
463 results about "Recombinant baculovirus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Therapy of cancer by insect cells containing recombinant baculovirus encoding genes
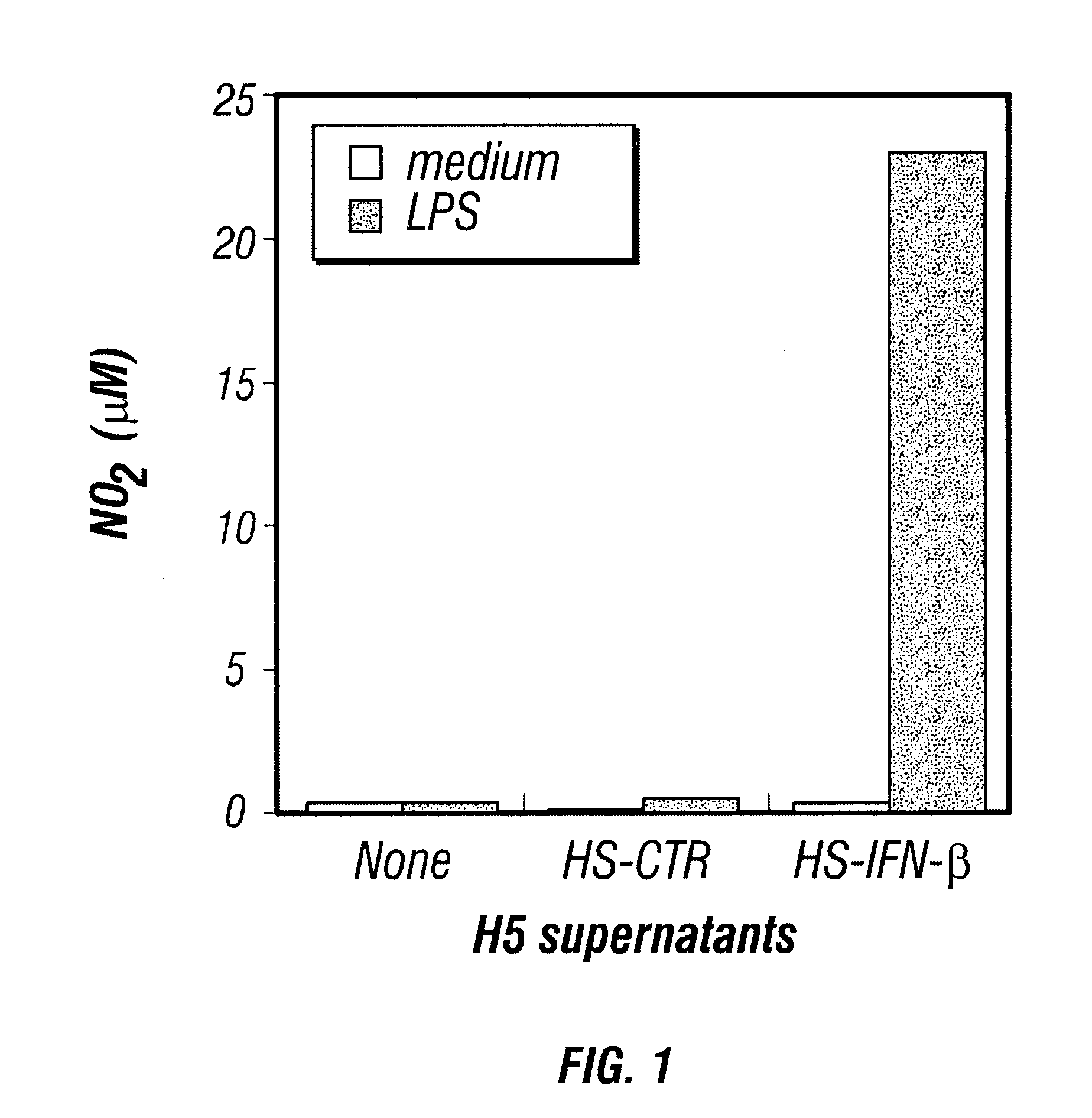
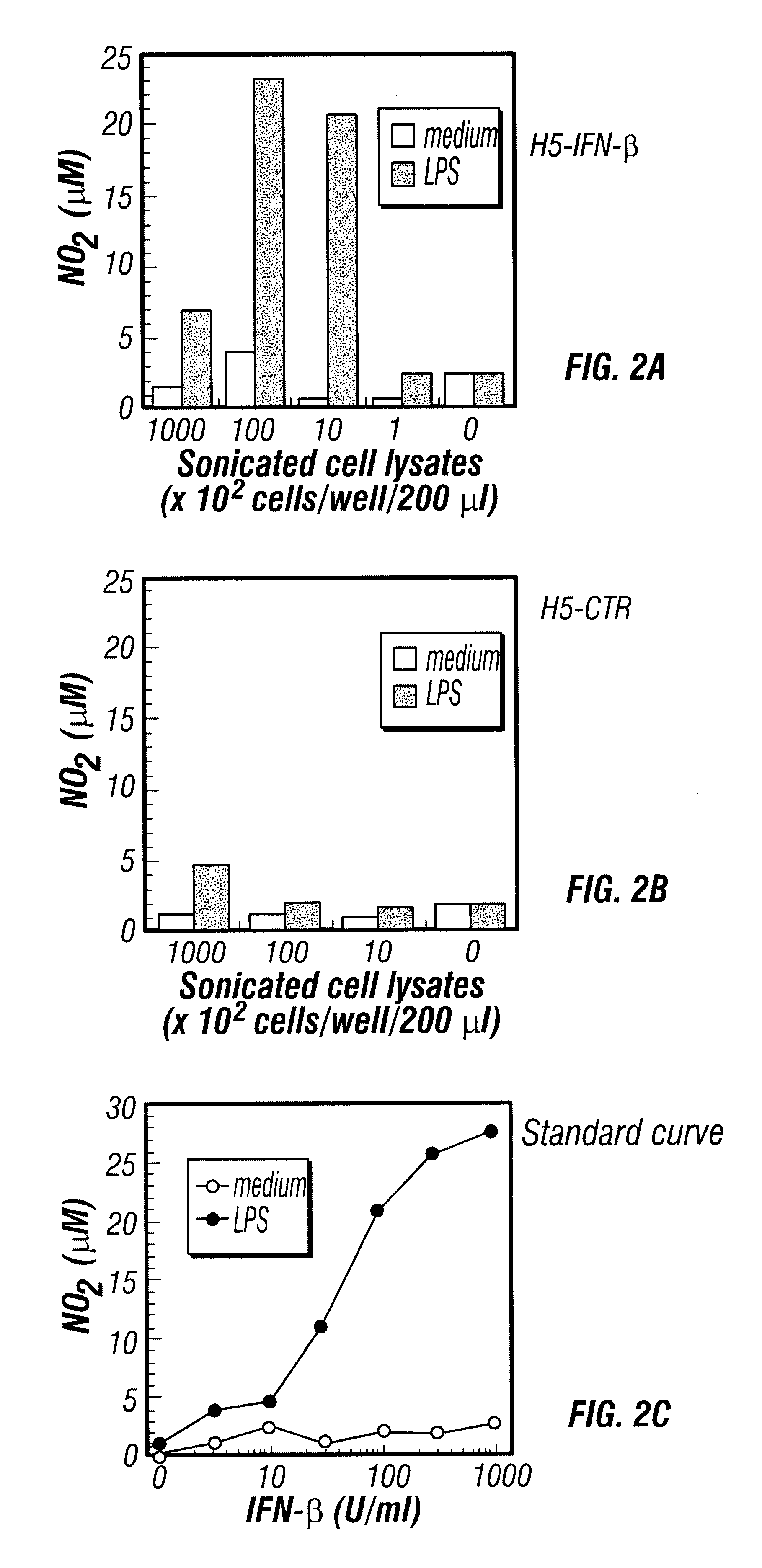
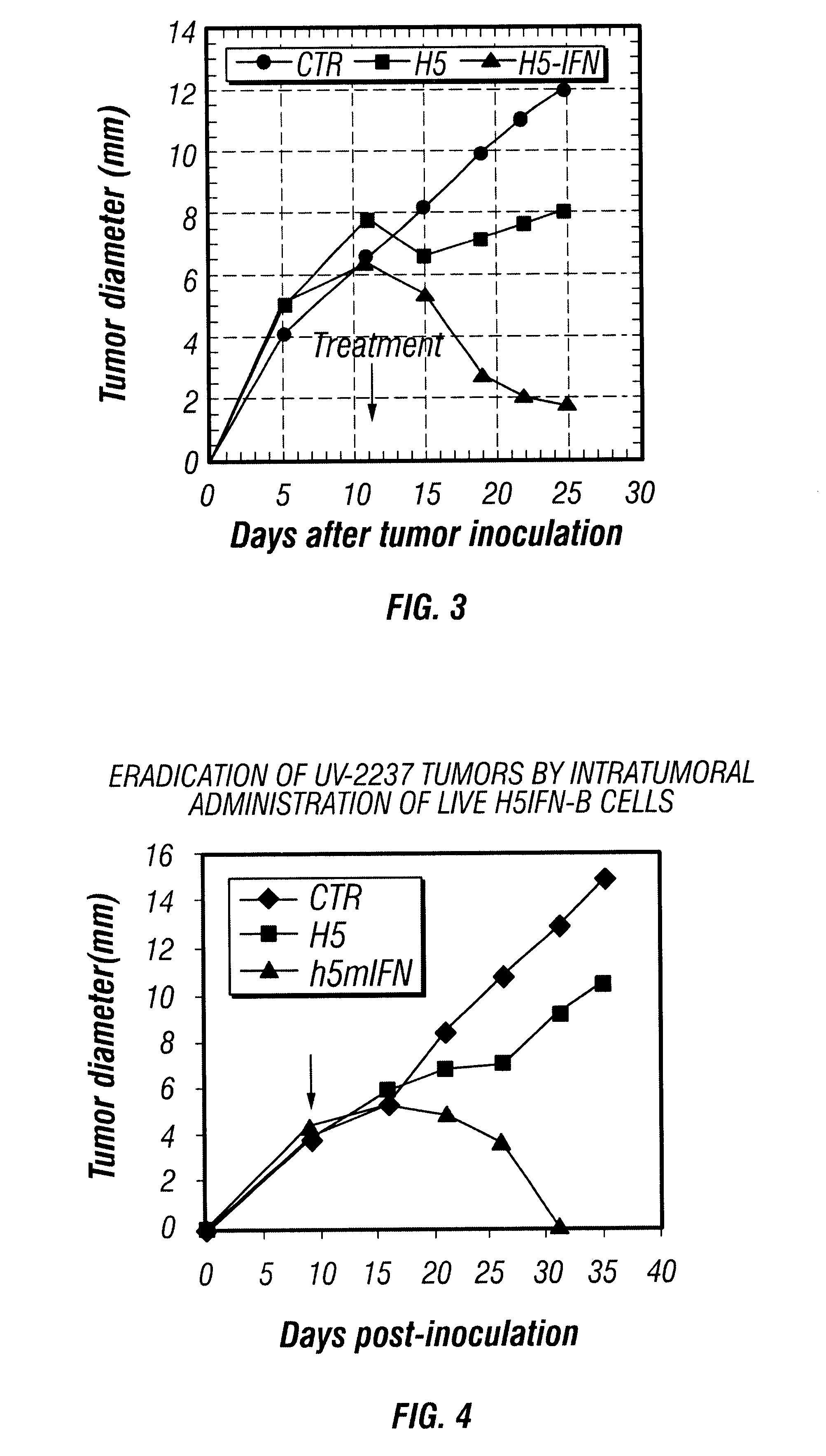
Provided are compositions and methods of use for insect cells comprising baculovirus encoding non-surface expressed proteins and peptides. The claimed invention particularly relates to compositions comprising insect cells containing baculovirus that express cytokines. Such compositions may be administered by, for example, direct intratumoral injection into tumors in mammals, resulting in tumor reduction or recission. Another aspect of the claimed invention concerns methods of promoting resistance to the reoccurence of tumors in mammals who have undergone such tumor recission. In a specific aspect of the claimed invention, the mammals are human subjects presenting with various forms of cancer.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
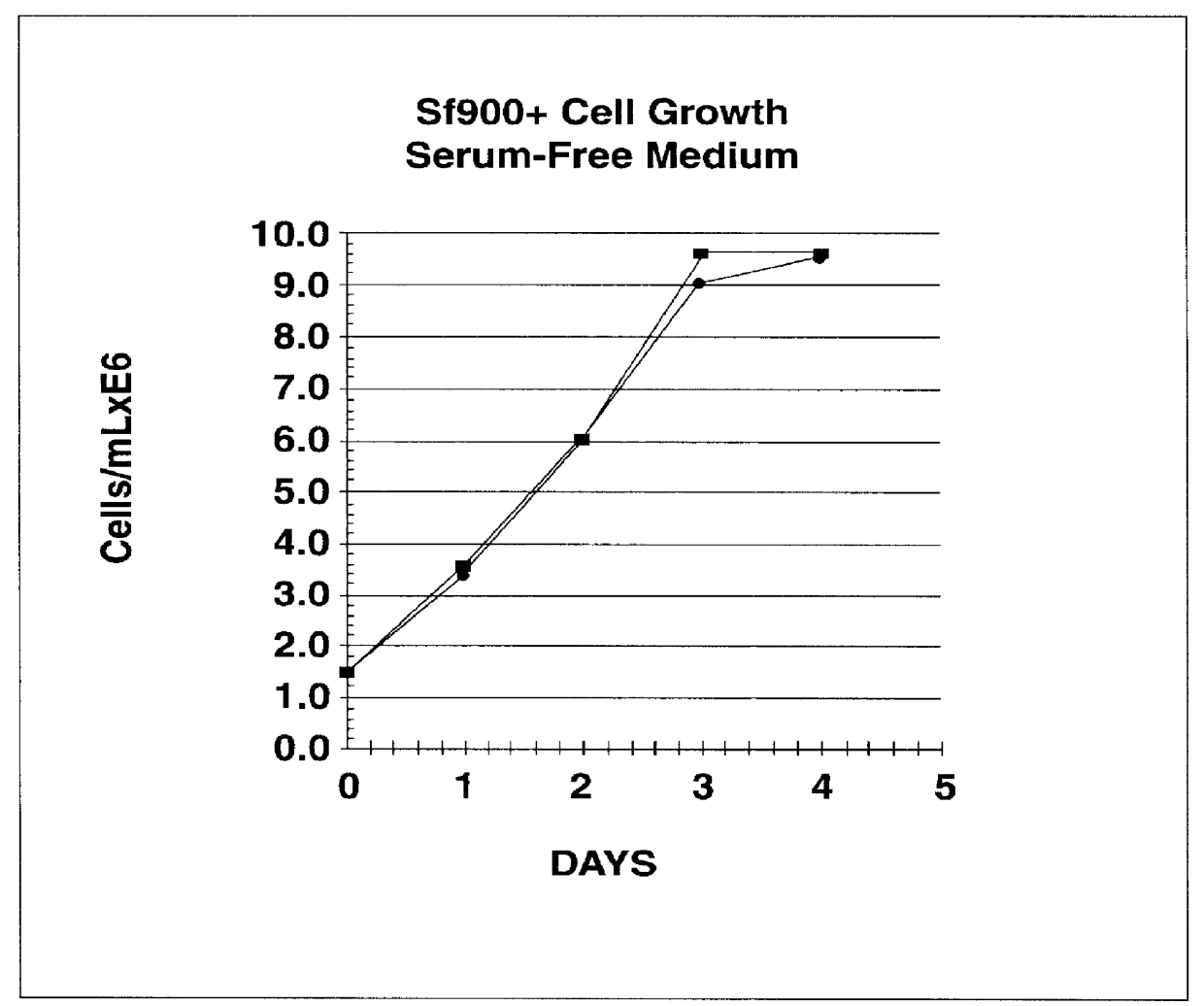
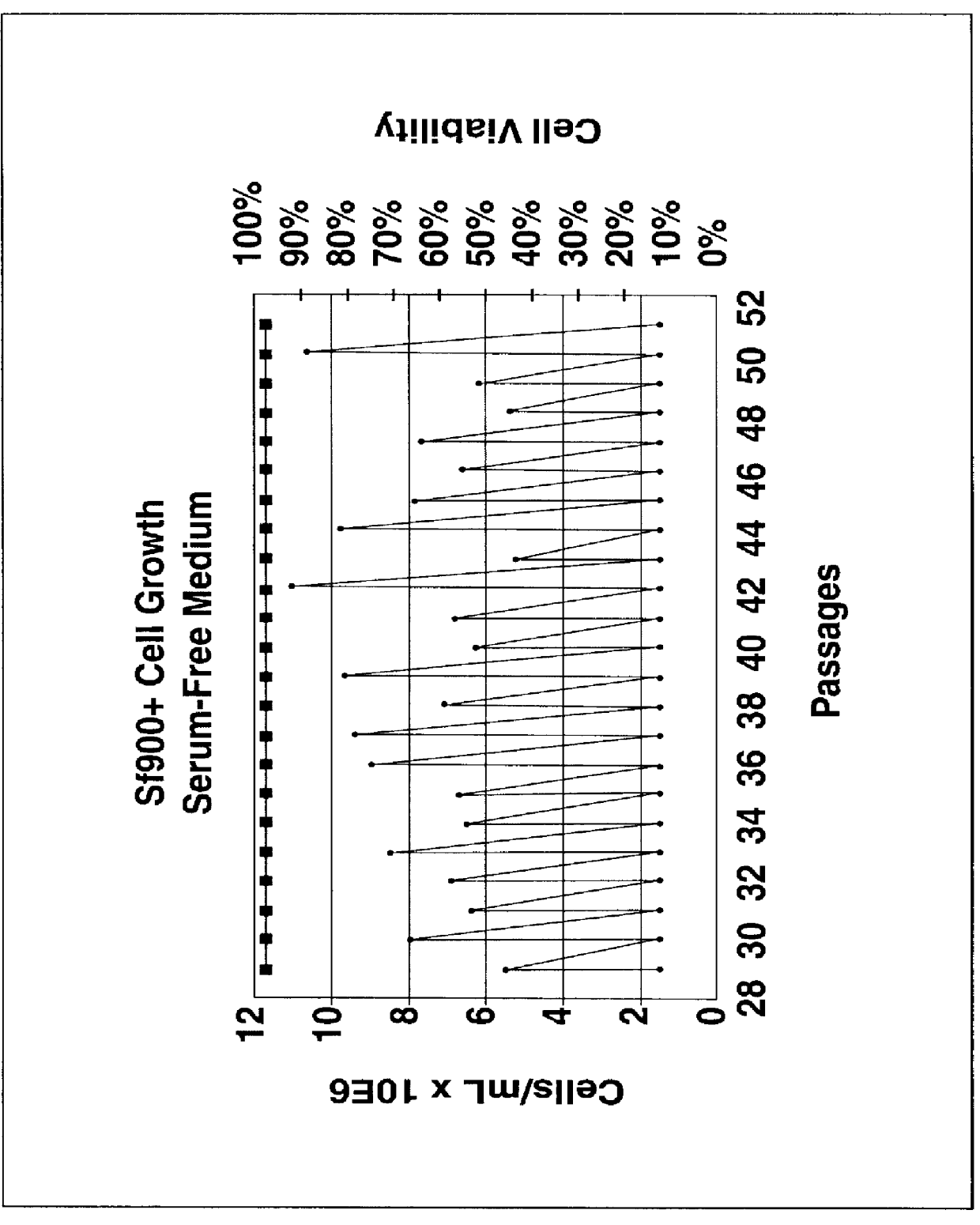
Spodoptera frugiperda single cell suspension cell line in serum-free media, methods of producing and using
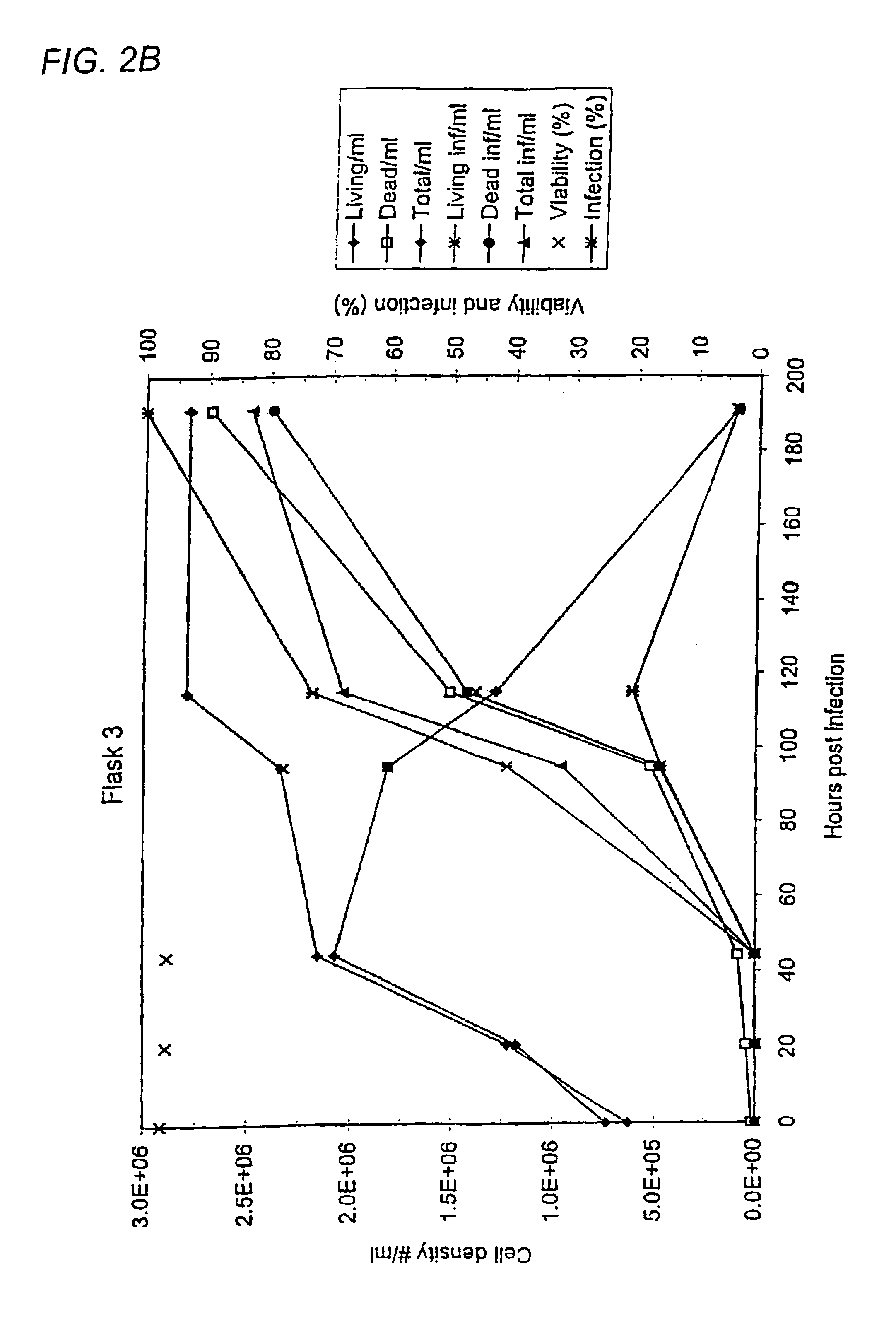
InactiveUS6103526AAvoid infectionHigh densityConnective tissue peptidesInvertebrate cellsSerum free mediaAdjuvant
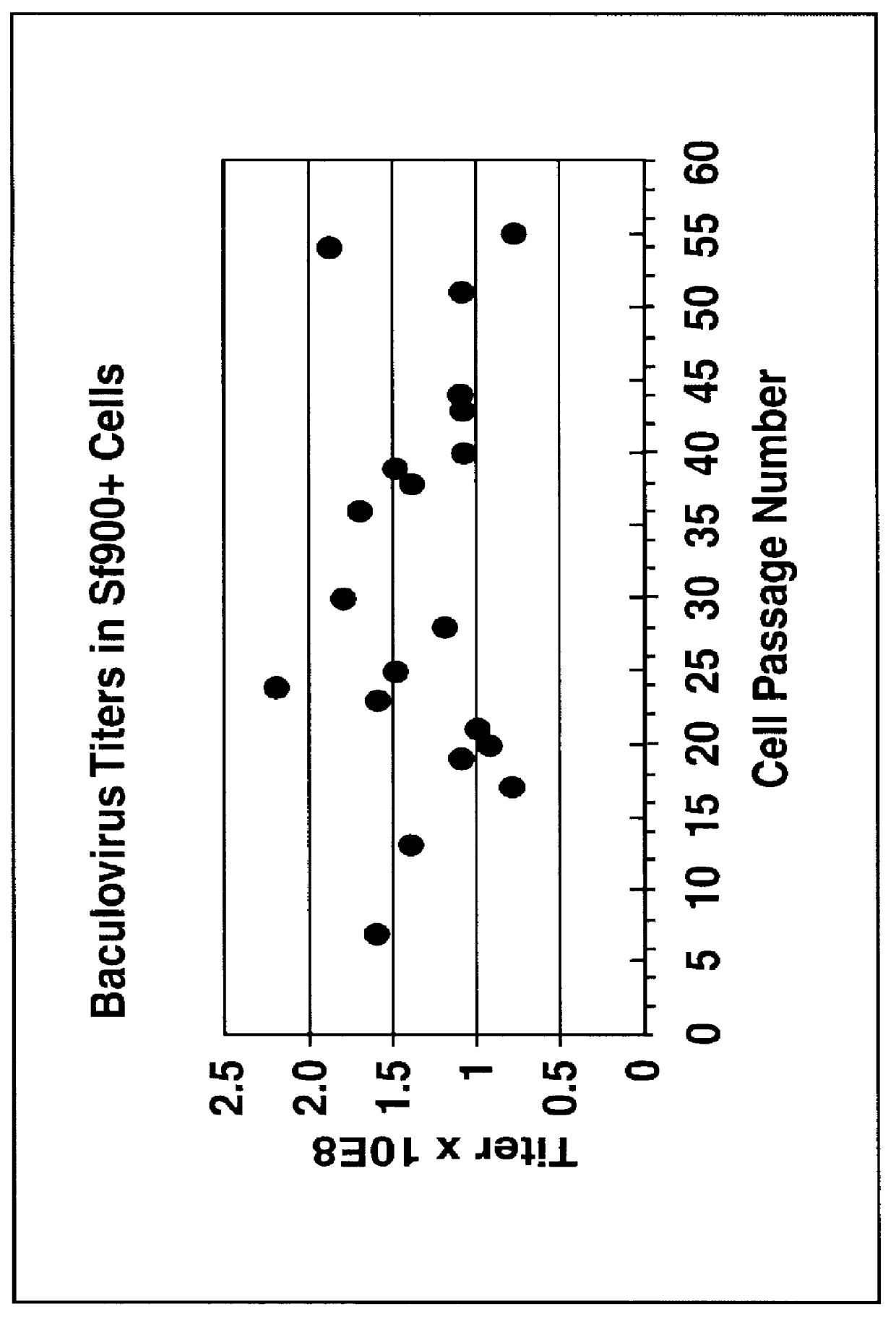
Disclosed and claimed is a new insect cell line, Sf900+, ATCC CRL-12579. The insect cell line was established from Lepidoptera, Noctuidae, Spodoptera frugiperda Sf-9 (ATCC CRL-1711) through multiple rounds of limiting dilution and selection in a serum-free insect medium supplemented with added human insulin. The insect cell line is useful in BEVS or as an adjuvant and has many characteristics and advantages. Also disclosed and claimed are recombinant proteins from recombinant baculovirus expression in insect cells such as Sf900+ cells, for instance, HA, NA, EPO, CD4, CEA, and thrombospondin.
Owner:PROTEIN SCI
Insect cells or fractions as adjuvant for antigens
Disclosed and claimed is an adjuvant for immunogenic, immunological, antigenic or vaccine compositions. The adjuvant is composed of insect cells or fractions thereof. Disclosed and claimed are also methods for preparing and using the adjuvant and compositions containing the adjuvant. Advantageously, a recombinant baculovirus containing DNA encoding and expressing an epitope of interest or antigen can be infected into insect cells such as insect cells derived from a Lepidopteran species such as S. frugiperda for expression, and the infected insect cells or a fraction thereof can be used with the expressed epitope of interest or antigen as an inventive antigen or in an inventive immunological, antigen or vaccine composition.
Owner:MERIAL LTD
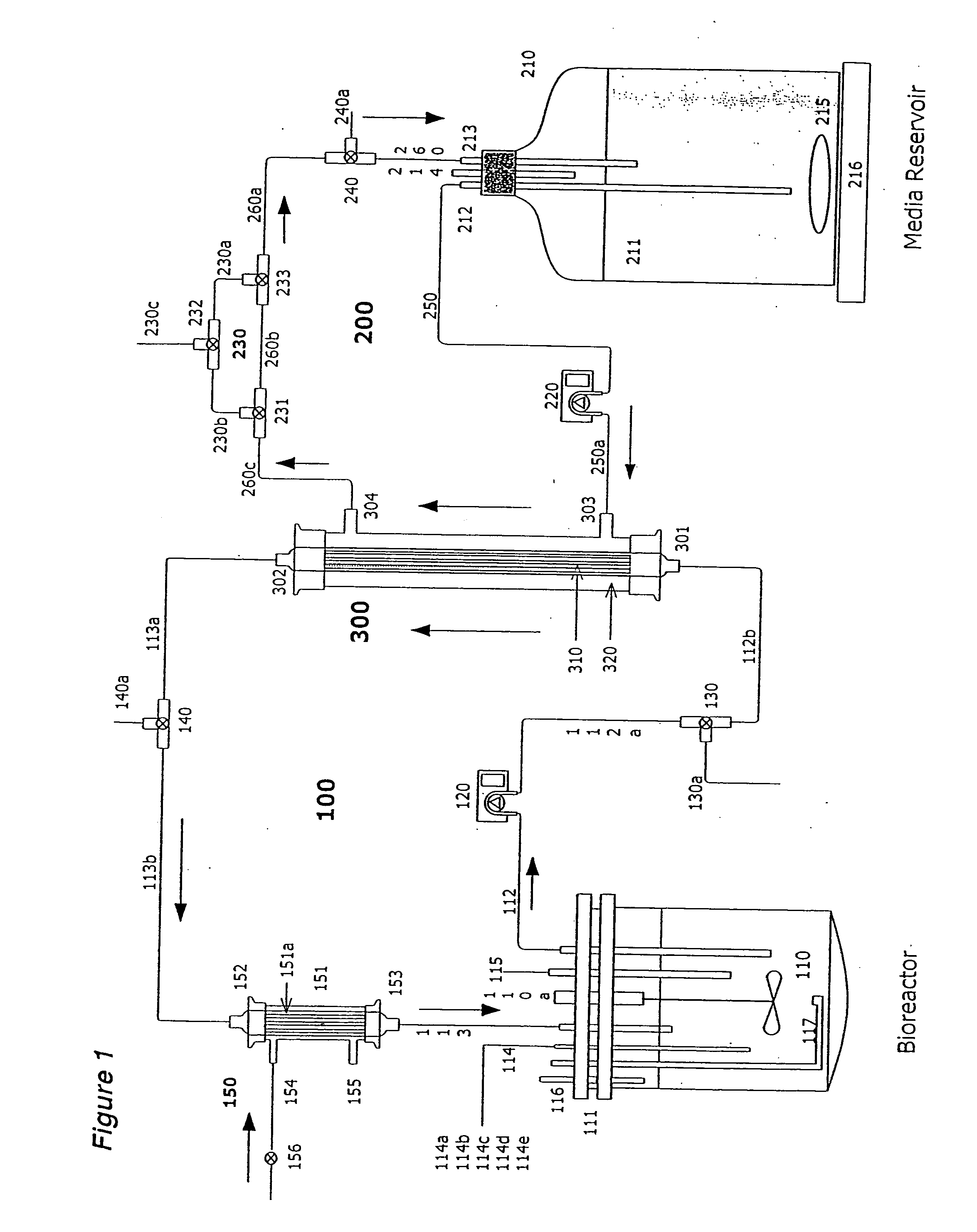
Apparatus and methods for producing and using high-density cells and products therefrom
InactiveUS20060019385A1High densityQuick exchangeAnimal cellsBioreactor/fermenter combinationsHigh cellHigh density
Disclosed and claimed is apparatus and methods for the growth of cells to high density, products therefrom and uses thereof. Also disclosed and claimed is the use of this method for the growth to high-density insect cells, such as the Spodoptera frugiperda Sf900+ cell line (ATCC: CRL 12579). Further disclosed is the infection of Sf900+ cells at high cell density with wild type and recombinant baculoviruses to produce baculovirus and DNA or gene or expression products.
Owner:PROTEIN SCI
Porcine circovirus II-type recombinant baculovirus as well as preparation method and application thereof
ActiveCN103122352AImprove expression levelHigh expressionGenetic material ingredientsAntiviralsEscherichia coliSpecific immunity
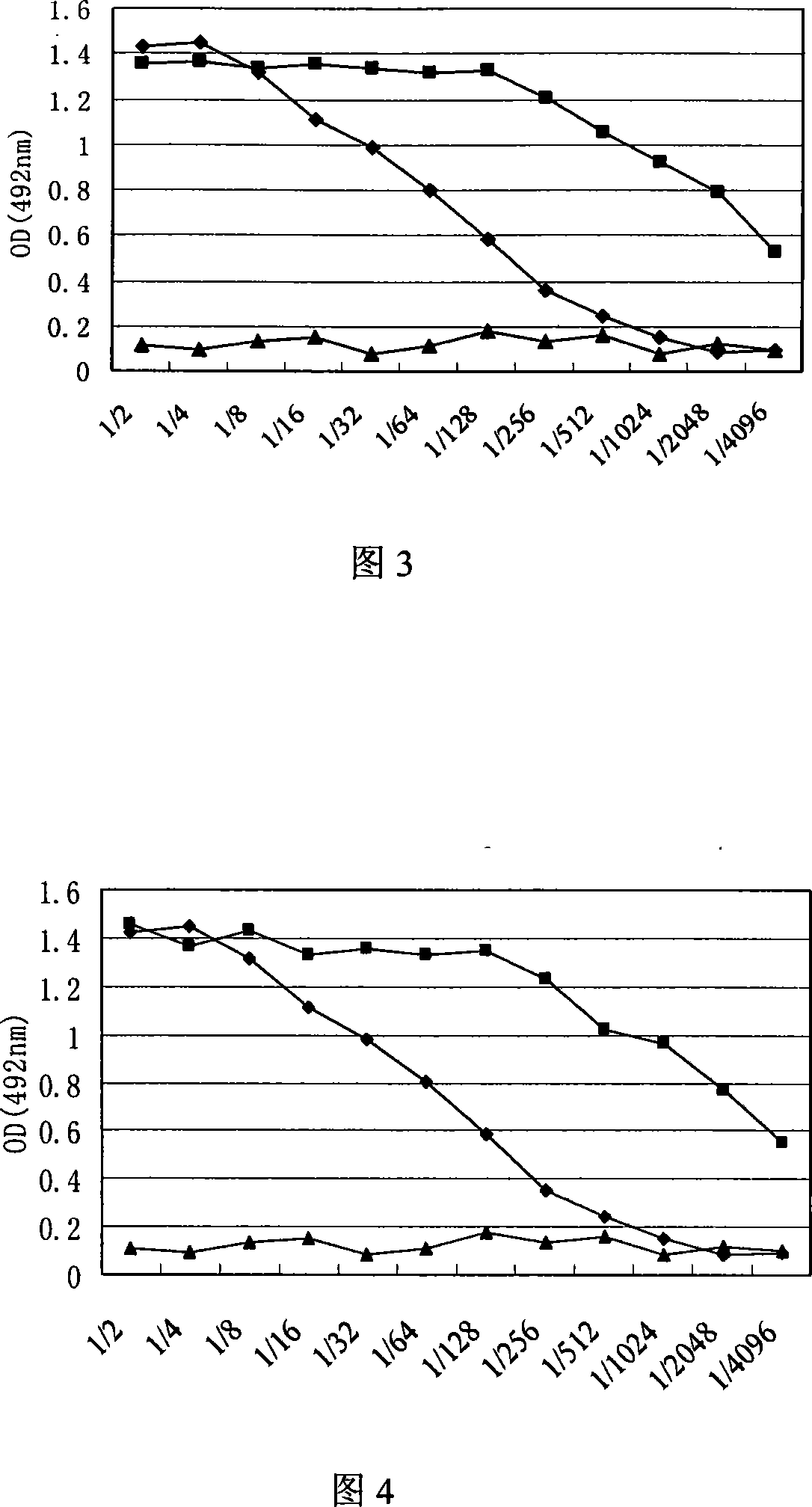
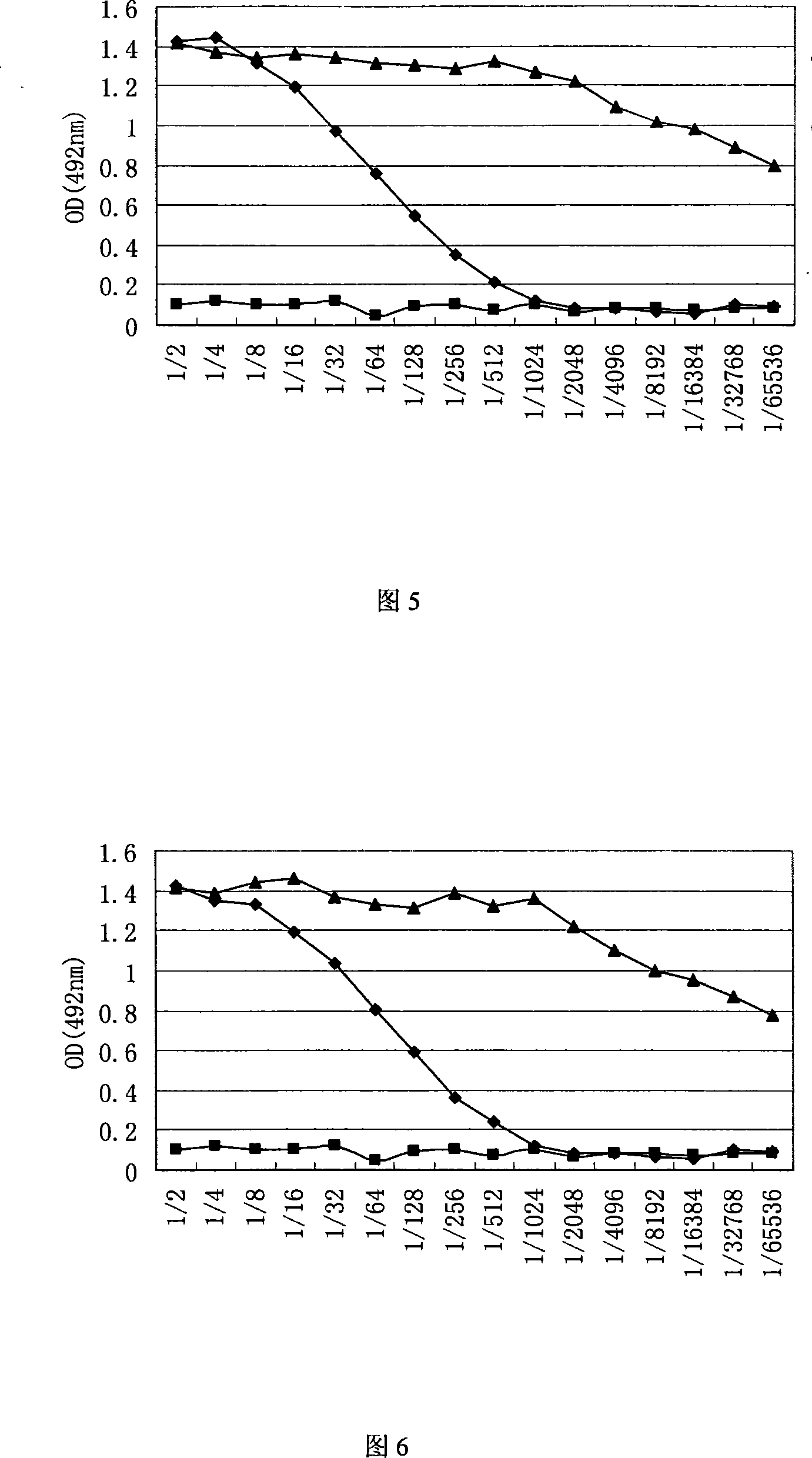
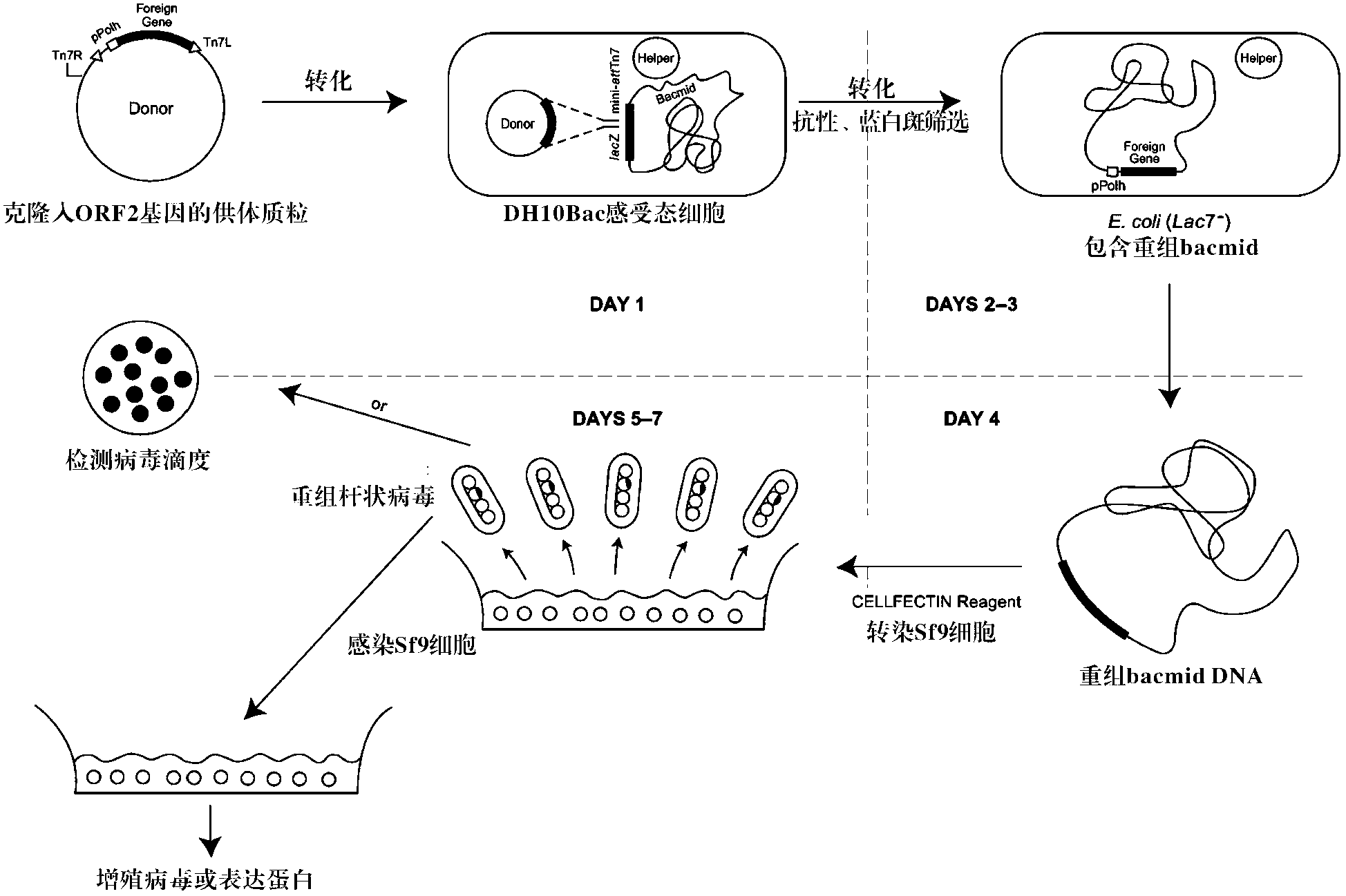
The invention discloses porcine circovirus II-type recombinant baculovirus as well as a preparation method and application thereof. ORF2 gene is artificially synthesized by referring to a PCV2b isolated strain ORF2 gene sequence; the synthesized ORF2 gene is connected to pFBDPHmHNM1P10eGFP plasmid by adopting the plasmid as a framework vector, so that a baculovirus transfer vector pFBDPHm 30RF2 is obtained. The baculovirus transfer vector pFBDPHm30RF2 is mixed with DH10Bac escherichia coli competent cells, and the positive bacterial colony is selected to obtain a recombinant rod granule rBac-PVR30RF2; the rod granule is transferred with a sf9 cell to obtain the recombinant baculovirus QP-Ac-30RF2. The recombinant baculovirus can be used for efficiently expressing the PCV20RF2 protein and forming virus-like particles. The VLP which is expressed and packaged by the recombinant baculovirus disclosed by the invention is used for preparing inactivated vaccine, and the organism is induced to generate specific immunity response after a 28-day-aged piglet is immunized, and the pig body can be completely protected from virulent attacks of the porcine circovirus.
Owner:HUAZHONG AGRI UNIV
Snow mountain virus genome sequence, virus-like particles and methods of use
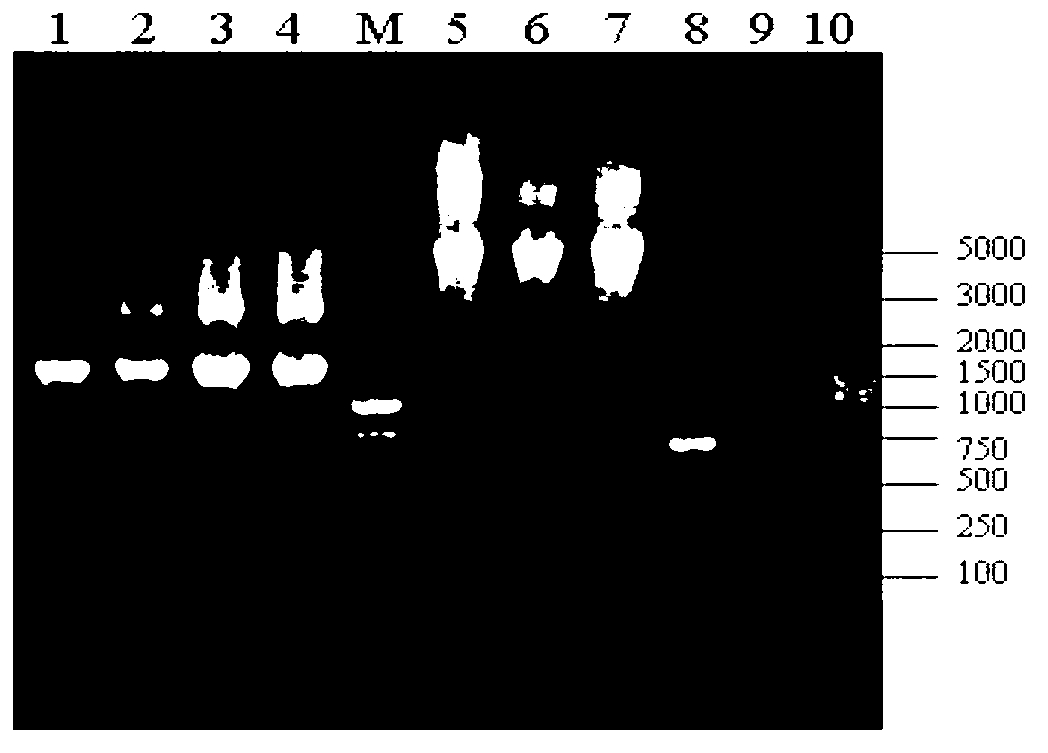
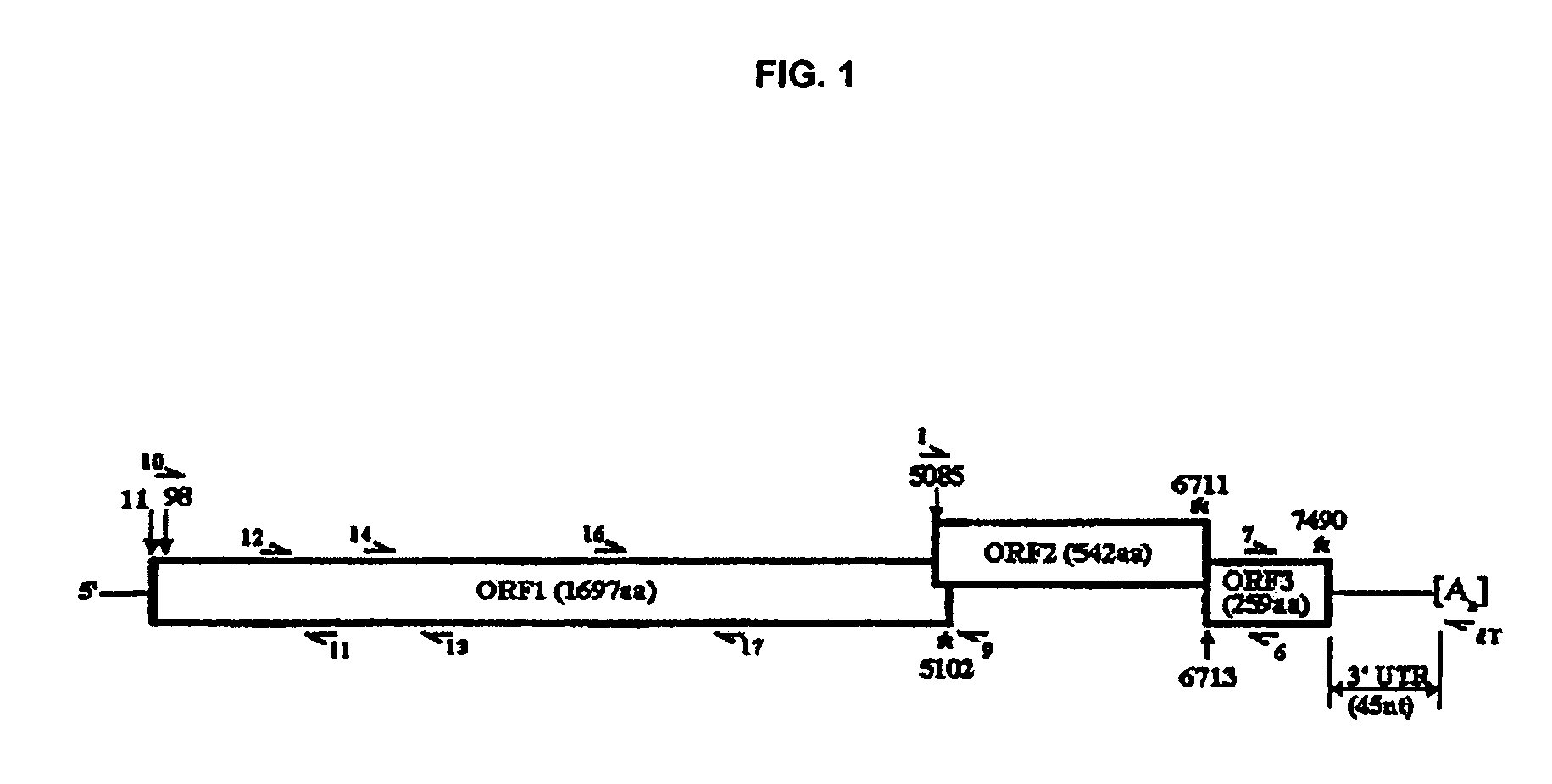
Snow Mountain Virus (SMV) belongs to the Norovirus genus of the Caliciviridae family. SMV is a genogroup II (GII) reference strain of human enteric caliciviruses associated with epidemic gastroenteritis. The positive sense RNA genome sequence of SMV was determined to be 7,537 nucleotides in length excluding the 3′ polyadenylated tract. The genome is organized into three open reading frames. Pairwise sequence alignments showed SMV ORF1 is highly conserved with other GII noroviruses, and most closely related to GII strains Melksham and Hawaii viruses. Comparative sequence analyses showed the SMV is a recombinant norovirus. VP1 / NP2 proteins assembled into virus-like particles (VLPs) when expressed in insect cells by a recombinant baculovirus. Characterization of one clone that expressed VP1 but failed to assemble into VLPs, identified histidine residue 91 as important for particle assembly.
Owner:MONTANA STATE UNIVERSITY
Porcine circovirus type 2 subunit vaccine and preparation method thereof
InactiveCN101884787AImprove biological activityHighly species-specificViral antigen ingredientsVirus peptidesOpen reading frameAntigenicity
The invention mainly aims to provide a porcine circovirus type 2 (PCV2) subunit vaccine and a preparation method thereof. Particularly, a baculovirus vector expression system is utilized to express a large amount of recombinant open reading frame type 2 (ORF2) protein in insect cells, so that the subunit vaccine with good immunity effect is developed. A bac-to-bac baculovirus expression system is adopted to perform whole gene amplification on the porcine circovirus type 2 ORF2 gene, and a melittin signal peptide nucleotide sequence is introduced into a terminal 5', so that the recombinant baculovirus of an open reading frame containing the melittin signal peptide nucleotide sequence and a PVC2ORF2 gene sequence is established, wherein the infected insect cell expresses the recombinant ORF2 protein with efficient and high PCV2 antigenicity; and thus the subunit vaccine containing the PCV2 recombinant ORF2 protein is prepared. The inoculation experiments of piglets show that the subunit vaccine has a good immunity protection effect.
Owner:PU LIKE BIO ENG
Porcine epidemic diarrhea recombinant baculovirus gene engineering subunit vaccine, preparation method and application thereof
InactiveCN103585625AImprove abilitiesTargetedMicroorganism based processesAntiviralsGenetic engineeringTGE VACCINE
The invention belongs to the technical field of biological vaccine preparation, and particularly relates to a porcine epidemic diarrhea (PED) recombinant baculovirus gene engineering subunit vaccine, a preparation method and an application thereof. According to the present invention, S1 gene and M gene of the current new PEDV epidemic strain are selected as reference sequences, a baculovirus expression system is adopted to express S1 protein or partial S1 protein and M protein, and the obtained recombinant protein is prepared into a subunit vaccine for effectively controlling PED occurrence; with the PED recombinant baculovirus gene engineering subunit vaccine produced by using the method, the defect of the current PEDV traditional vaccine is solved; and the PED recombinant baculovirus gene engineering subunit vaccine can be used for prevention and treatment of PEDV infections and related diseases caused by PEDV, and can further be used for preparation of coating antigen of PEDV detection antibody ELISA kits.
Owner:SOUTH CHINA AGRI UNIV
Recombinant baculovirus strain of porcine circovirus type 2 Cap protein expression, construction method and application thereof
InactiveCN101358182AImprove immune activityViral antigen ingredientsAntiviralsMicroorganism preservationImmunocompetence
The present invention discloses a recombinant baculovirus strain rBac / PCV2Cap (microorganism preservation number: CGMCC NO.2083) efficiently expressing Porcine circovirus type 2 Cap protein and applications thereof. The recombinant baculovirus strain rBac / PCV2Cap constructed by the present invention can efficiently express recombinant PCV2-Cap protein in insect cells, and the expressed recombinant Cap protein, which has good immunocompetence and antigenicity, can serve as a subunit vaccine used to prevent the related plague caused by Porcine circovirus type 2 infection as well as a detecting and diagnostic antigen for Porcine circovirus type 2 serum antibody.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Method for assembling foot and mouth disease virus hollow capsid in insect with acidproof improvement
The present invention discloses a method for assembling foot-and-mouth disease virus empty capsids in insect cells via the alteration of acid-resistance. The method for assembling foot-and-mouth disease virus empty capsids in insect cells includes the following steps: (1) the altered P12A gene and the non-structural protein gene 3C of foot-and-mouth disease virus are introduced into bacteria via baculovirus vectors for recombination to produce recombinant rhabdovirus A; (2) the DNA of the recombinant rhabdovirus A is used to transfect the insect cells, so that the foot-and-mouth disease virus empty capsids are obtained. The method assembles the integral foot-and-mouth disease virus empty capsids in the insect cells for the first time, lays a foundation for the research and the development of gene-engineered subunit vaccines and novel diagnostic reagents.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Porcine O-type foot-and-mouth disease virus recombinant baculovirus as well as preparation method and application thereof
InactiveCN103122353AImprove expression levelHigh expressionGenetic material ingredientsAntiviralsEscherichia coliShuttle vector
The invention discloses porcine O-type foot-and-mouth disease virus recombinant baculovirus as well as a preparation method and application thereof. Sequences of VP0, VP1 and VP3 genes are artificially synthesized by referring to an FMDV (Foot And Mouth Disease Virus) O-type epidemic strain gene sequence; the VP0, VP1 and VP3 genes are connected to pFBDPHmHNM1P10eGFP plasmid by adopting the plasmid as a framework vector, so that a baculovirus transfer vector pFBDPHmVP013 is obtained. The baculovirus transfer vector pFBDPHmVP013 is mixed with DH10Bac escherichia coli competent cells, and the positive bacterial colony is selected to obtain a recombinant shuttle vector Bacmid; the shuttle vetcor Bacmid is transferred with a sf9 cell, and the recombinant baculovirus QP-Ac-FVLP is obtained by collecting the cell supernatant. The recombinant baculovirus can be used for efficiently expressing FMDVVP0, Vp1 and Vp3 proteins and forming virus-like particles. And the virus-like particles are used for preparing subunit vaccine, so that the organism is induced to generate specific immunity response after the mouse is immunized.
Owner:HUAZHONG AGRI UNIV
Univalent and bivalent gene engineered subunit vaccine for hand-foot-and-mouth disease and preparation method thereof
InactiveCN101695569AInactivation/attenuationMicroorganism based processesHand-foot-and-mouth diseaseCoxsackievirus a16
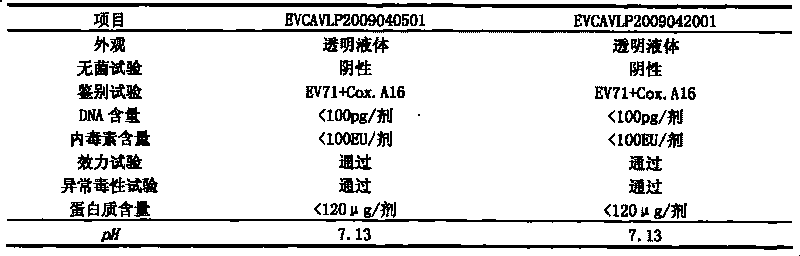
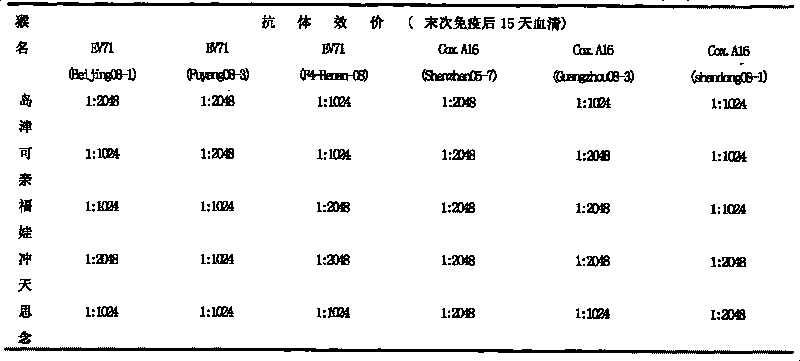
The invention discloses a univalent and bivalent gene engineered subunit vaccine for preventing Enterovirus 71 (EV71) and Coxsackie virus A16 (Cox.A16) of hand-foot-and-mouth disease, and a preparation method thereof. The preparation method comprises the following steps: respectively obtaining recombinant baculovirus Bac-EV71-P1-3CD and Bac-Cox.A16-P1-3CD by gene engineering means, respectively efficiently coexpressing similar SeQ ID No.1 EV71 P1 and Se Q ID No.2 Cox.A16 P1 and 3CD proteins in insect cells, and respectively self-assembling into EV71 VLP and Cox.A16 VLP; establishing and verifying a vaccine strain third-stage seed lot library; culturing cells; inoculating and propagating virus; lysing the cells, ultra-filtering and purifying virus suspension; and further preparing the univalent and bivalent vaccine. The vaccine has good application prospect for preventing the hand-foot-and-mouth disease.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Method for efficiently preparing porcine circovirus type 2 empty capsid particles
The invention provides a method for efficiently preparing porcine circovirus 2 type empty capsid, which comprises the following steps: cloning different viral strain lines of antigen genes cap required by forming circovirus empty capsid and artificially reconstructed cap mutant into a domestic silkworm baculovirus carrying vector to obtain a homologous recombinant vector, recombining or transpositioning the homologous recombinant vector and parent virus DNA (deoxyribonucleic acid) in an insect cell or bacterium to obtain recombinant baculovirus, and infecting the insect with the recombinant baculovirus containing the antigen gene; and culturing the infected insect host to express the corresponding circovirus 2 type empty capsid, determining the information required by efficiently assembling the virus empty capsid by comparison and experimentation, and obtaining a recombinant viral strain line capable of efficiently expressing and assembling virus empty capsid, thereby carrying out mass production on the circovirus 2 type empty capsid. The porcine circovirus 2 type empty capsid produced by the method can be used for preparing vaccines for preventing and treating porcine circovirus disease after being subjected to primary purification.
Owner:CHINA INST OF VETERINARY DRUG CONTROL +1
African swine fever P30 protein recombinant baculovirus expression vector and preparation method thereof
The invention provides African swine fever P30 protein recombinant baculovirus expression vector and a preparation method thereof. The method comprises: amplifying in plasmid PCR-4TOPO-P30 of ASFV (African swine fever virus) P30 full-length gene to obtain P39 gene, linking the amplified P30 gene to a baculovirus vector pFastBac 1 to construct recombinant baculovirus vector pFastBac1-ASFV-P30, converting into competent Escherichia coli cells DH10Bac to obtain recombinant shuttle bacmid rBacmid-ASFV-P30, transfecting to insect cells Sf9 after verification is correct to obtain recombinant baculovirus, and passage amplifying the recombinant baculovirus, linking baculovirus high in titer and containing ASFV P30 gene to High Five insect cells for eukaryotic expression of ASFV P30. The African swine fever P30 protein recombinant baculovirus expression vector is constructed by using the method, a recombinant baculovirus expression system is used to express African swine fever P30 protein in insect cells, and basis is laid for African swine fever ELISA (enzyme-linked-immunosorbent serologic assay) detections.
Owner:QINGDAO AGRI UNIV
Recombinant baculovirus and application thereof
ActiveCN106544325AIncrease production capacitySuitable for large-scale scale-up productionMicroorganism based processesNucleic acid vectorBiotechnologyViral vector
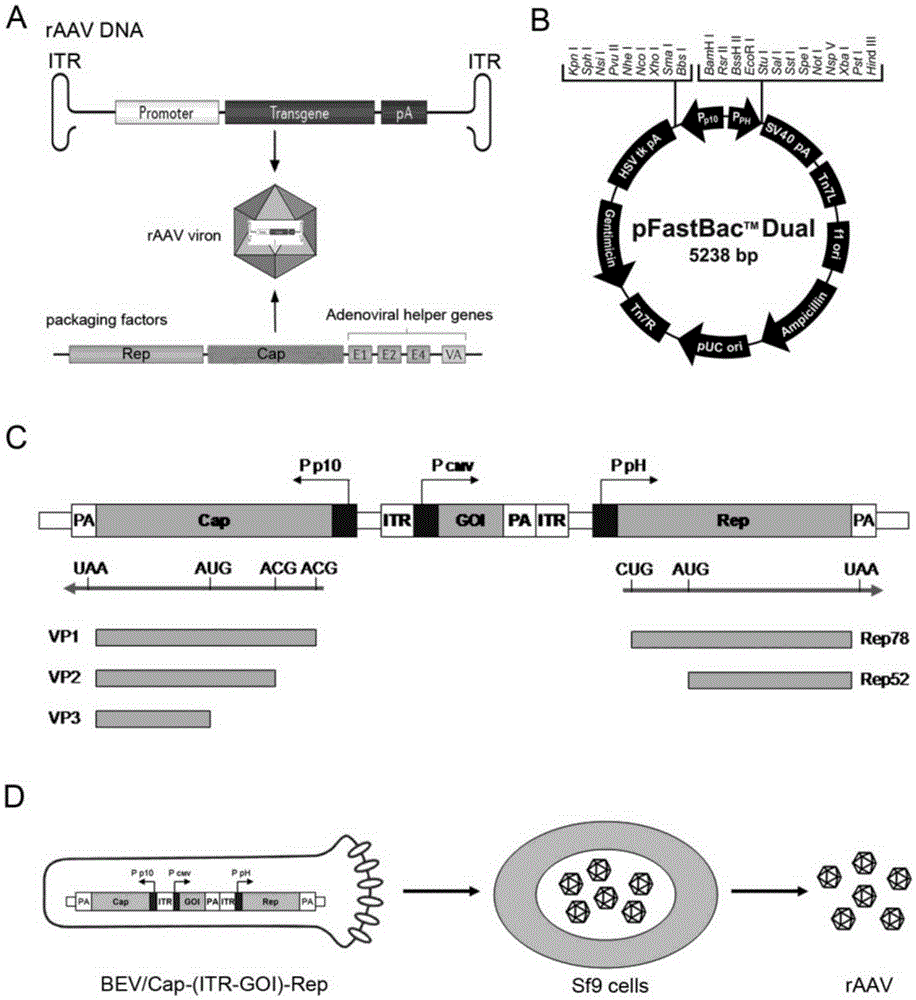
The invention discloses recombinant baculovirus and application thereof. The recombinant baculovirus integrates Rep genes and Cap genes of AAVs (Adeno Associated Viruses) and ITR core expression elements. The recombinant baculovirus is applied to preparation of an rAAV (Recombinant Adeno Associated Virus) carrier for gene treatment; the flexibility is high; the universality is high; the virus quality is high; the yield is high; the recombinant baculovirus is applicable to large-scale amplification production; and the difficult problem of rAAV large-scale preparation can be effectively solved.
Owner:布林凯斯(深圳)生物技术有限公司
2 type subunit vaccine for porcine circovirus as well as preparation method and application thereof
InactiveCN102517331AQuick responseHigh activityViral antigen ingredientsVirus peptidesImmune effectsVirus-like particle
The invention relates to a 2 type subunit vaccine for a porcine circovirus as well as a preparation method and application thereof. A recombinant bacilliform virus contains double promoters (a polyhedrin promoter and a P10 promoter), a coding gene of a Cap protein with double copying can be expressed, and the expression efficiency of the protein is obviously enhanced; moreover, the Cap protein expressed by an inserted foreign gene does not contain an excess sequence, virus-like particles (VLPs) can be effectively formed, and the immunogenicity of an expressed protein is enhanced; furthermore, a produced antigen has high content; and according to the 2 type subunit vaccine for the porcine circovirus, which is disclosed by the invention, the productivity ratio and the quality of a viral protein of the 2 type subunit vaccine for the porcine circovirus are obviously enhanced, and a prepared vaccine composition has the advantages of stable and persistent immune effect, high safety and the like.
Owner:WUHAN CHOPPER BIOLOGY
Method for preparing rabies virus antigen
ActiveCN101307317AReduce consumptionImprove securityAntiviralsDepsipeptidesAntigenBaculovirus expression
The invention provides a method for making rabies virus antigen. The method comprises the following steps that: the antigen gene of rabies virus or the combined expression combination of the antigen gene is respectively cloned in a baculovirus carrier so as to obtain a transfer expression carrier; the transfer expression carrier and baculovirus undergo cotransfection so as to carry out homologous recombination or transposition, thereby obtaining recombined baculovirus; the recombined baculovirus is used to infect insect host and cell; the infected insect host is cultured to express corresponding rabies antigen; and the expressed antigen is ingathered and purified so as to obtain rabies virus antigen. The method adopts a baculovirus expression system to make safe and efficient rabies virus antigen in a domestic silkworm bioreactor; moreover, due to having extremely high safety, the made antigen can be directly used to make injection vaccine and oral vaccine used for animal immunization. The method can substantially reduce the production cost of rabies virus antigen, and has the advantages of safety, high efficiency, less energy consumption and low cost, etc.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI +1
Method for expression of PCV 2 Cap protein by pFast Bac Dual baculovirus
InactiveCN102839195AEfficient expressionHighly efficient expression systemMicroorganism based processesFermentationEscherichia coliShuttle vector
The invention discloses a method for expression of a PCV 2 (Porcine circovirus type2) Cap protein by a pFast Bac Dual baculovirus. The method comprises the following steps of: amplifying a gene fragment of an encoded PCV 2 Cap protein with a His tag; connecting the gene fragment to a pFast Bac Dual plasmid so as to obtain a recombinant transfer plasmid pFast Bac-p10-ORF2-pH-ORF2; transforming Escherichia coli DH10Bac with the recombinant transfer plasmid, and carrying out blue-white selection to obtain a recombinant shuttle vector Bac-p10-ORF2-pH-ORF2; transfecting an insect cell with the recombinant shuttle vector so as to obtain a recombinant baculovirus Ac.Dual-Cap; and poisoning the insect cell with the recombinant baculovirus, performing cultivation, then collecting the insect cell, and purifying an expression product so as to obtain the recombinant Cap protein. The method disclosed in the invention solves the problem of low expression level of the Cap protein in eukaryotic cells. The recombinant Cap protein in the invention is designed with a His tag, thus being beneficial to the follow-up purification. And the recombinant Cap protein has biological activity superior to that of a Cap protein expressed by a prokaryotic expression system, thus being applicable to establishment of epidemiological diagnosis methods and reseach as well as development of PCV2 subunit vaccines.
Owner:SOUTH CHINA AGRI UNIV
SARS vaccine and its preparation method
InactiveCN101007168AImprove securityTo achieve the purpose of surface displayAntiviralsRespiratory disorderSurface displayCompetent cell
The invention discloses a SARS vaccine and preparing method, which comprises the following steps: 1) constructing external baculoviral surface display carrier of S protein of SARS coronary virus; 2) transmitting the carrier into susceptive cell with baculoviral genome plasmid Bacmid; obtaining recombinant baculoviral genome plasmid; 3) using the plasmid to infect insect cell; purifying the recombinant plasmid; obtaining the product.
Owner:PEKING UNIV
Apparatus and methods for producing and using high-density cells and products therefrom
InactiveUS7598075B2Quick exchangeImprove performanceBioreactor/fermenter combinationsBiological substance pretreatmentsHigh cellHigh density
Disclosed and claimed is apparatus and methods for the growth of cells to high density, products therefrom and uses thereof. Also disclosed and claimed is the use of this method for the growth to high-density insect cells, such as the Spodoptera frugiperda Sf900+ cell line (ATCC: CRL 12579). Further disclosed is the infection of Sf900+ cells at high cell density with wild type and recombinant baculoviruses to produce baculovirus and DNA or gene or expression products.
Owner:PROTEIN SCI
Preparation method and application of classical swine fever virus recombinant subunit vaccine
InactiveCN104826100ANo risk of contaminationImprove securityAntiviralsAntibody medical ingredientsProtein targetVaccine Production
The invention discloses a preparation method and application of a classical swine fever virus recombinant subunit vaccine with the amino acid sequence shown as SEQ ID No.1. The preparation method of the classical swine fever virus recombinant subunit vaccine typically includes the following steps: classical swine fever E2 truncated protein (TE2) coding gene is cloned into baculovirus vector pFastBacTM1, and is then transfected into Sf9 insect cells to obtain recombinant baculovirus capable of expressing protein TE2. The high five insect cells in logarithmic growth phase are infected by the recombinant baculovirus, so that a large amount of the protein TE2 can be expressed in a cell culture supernatant. Finally, the cell culture supernatant is recovered and purified to obtain a large amount of the recombinant protein TE2 with the purity more than 90%. According to the method, the target protein can be harvested from the cell culture supernatant, the time of protein purification is reduced, consumption of a large amount of time can be avoided, and the vaccine production process can be simplified. Under the premise of simplification of the vaccine production process, the recombinant protein TE2 has the advantages of strong immunogenicity and high safety, and the animal experiments prove that the recombinant protein can effectively stimulate the body to produce a highly effective humoral immune response.
Owner:NOVO BIOTECH CORP
Method for producing porcine parvovirus antigen and its product
ActiveCN102382845AImprove immune activityReduce manufacturing costGenetic material ingredientsAntiviralsAntigenBaculovirus expression
The invention discloses a method for producing a porcine parvovirus antigen and its product. The method comprises the following steps: porcine parvovirus capsid protein VP2 gene or optimized VP2 gene is cloned in a baculovirus carrier so as to obtain a transfer expression carrier; the constructed transfer expression carrier and baculovirus DNA are carried out cotransfection to obtain recombined baculovirus; the recombined baculovirus is used to infect insect host and cell; the infected insect host is cultured to express corresponding porcine parvovirus capsid protein; and the expressed antigen is ingathered and purified so as to obtain the porcine parvovirus antigen. The method adopts a baculovirus expression system to make safe and efficient porcine parvovirus antigen capsid particles in a domestic silkworm bioreactor; the prepared purified antigen by the method has high safety, and can be directly produced to vaccines for animal immunity. The method for producing porcine parvovirus antigen has the advantages of high expression efficiency, high immunization activity of the expressed antigen, low production cost, large scale production realization and the like.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Method for preparing foot-and-mouth disease antigen
ActiveCN101121938APromote safe productionReduce consumptionSsRNA viruses positive-senseVirus peptidesAntigenTransfer vector
The invention provides a method for expressing foot-and-mouth disease antigens in insects using recombinant baculoviruses, which includes: cloning different gene combinations of foot-and-mouth disease into baculovirus delivery vectors to construct transfer vectors; using the constructed transfer vectors to transfer Infect the baculovirus and perform DNA recombination to obtain the recombinant baculovirus; infect the insect host with the recombinant baculovirus; culture the infected insect host to express the foot-and-mouth disease antigen; collect and purify the expressed foot-and-mouth disease antigen. The method of the present invention uses a baculovirus expression system to safely and efficiently produce foot-and-mouth disease antigens in a silkworm bioreactor. The prepared antigens are extremely safe and can directly produce vaccines to immunize animals. The method of preparing foot-and-mouth disease antigen of the present invention does not require investment in building a factory, has no three wastes, consumes very little energy such as electricity and water resources, and its production cost is also significantly lower than the traditional method of preparing foot-and-mouth disease antigen. It is safe, efficient, has low energy consumption and low cost. Low and many advantages.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI +1
Recombinant baculovirus expressing manually modified and synthesized influenza A H1N1 virus HA-NA-M1 gene
InactiveCN101624580AImprove expression levelImprove screening efficiencyGenetic material ingredientsVirus peptidesInfluenza A (H1N1) virusSapovirus
The invention relates to the field of virology, in particular to a recombinant baculovirus which is manually modified and synthesized and contains a main immunogenic gene HA-NA-M1 of an influenza A H1N1 virus. The strain QP-Ac-HNM1 belongs to the baculovirus (Baculovirus) and is preserved in the China Center for Type Culture Collection (CCTCC) with the preserving number of CCTCC-V200912. The recombinant virus is capable of synchronously expressing the HA and NA of the influenza A H1N1 virus and M1 proteins to form virus particles which can be used for developing vaccines so as to prevent human beings and swine from being infected with the influenza A H1N1 virus.
Owner:HUAZHONG AGRI UNIV
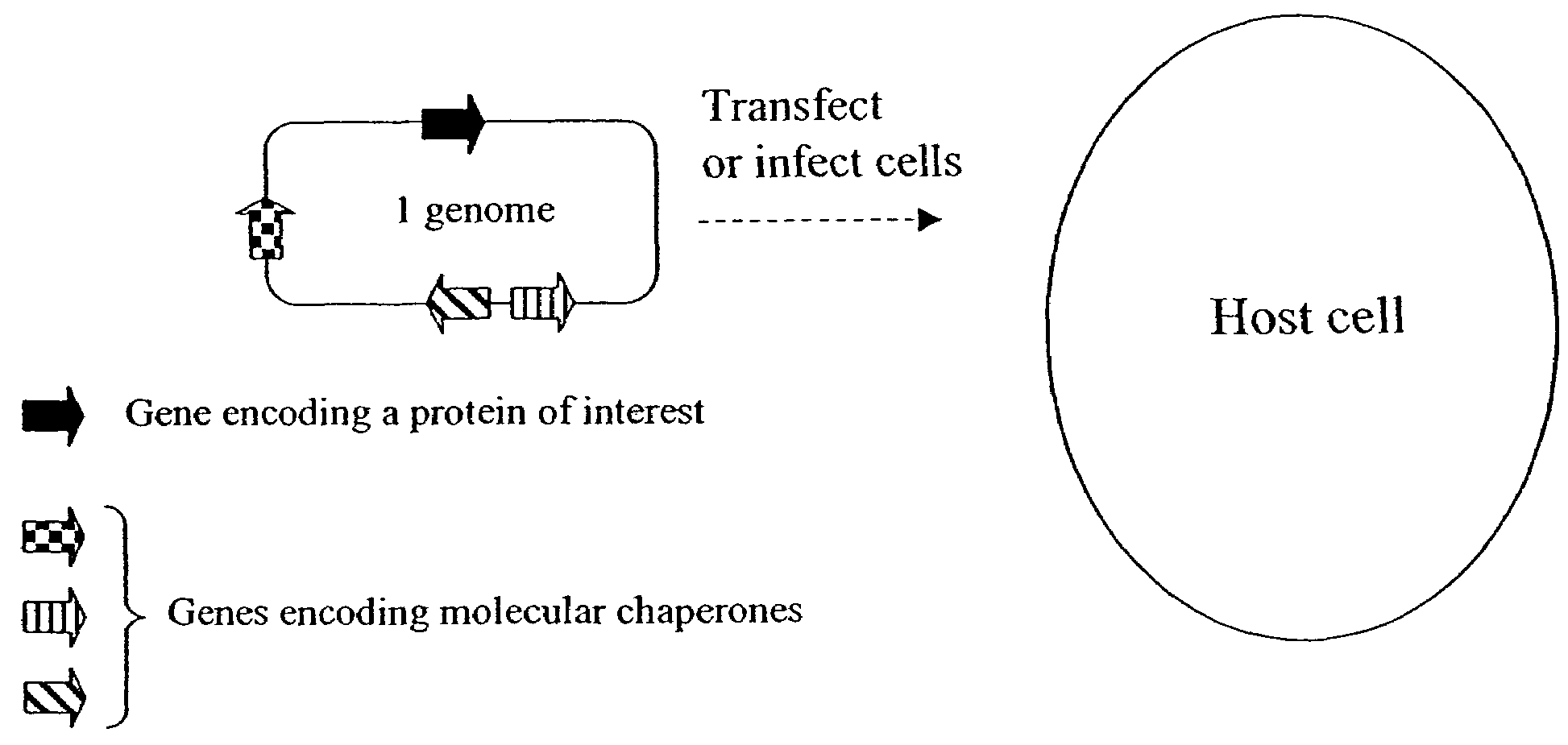
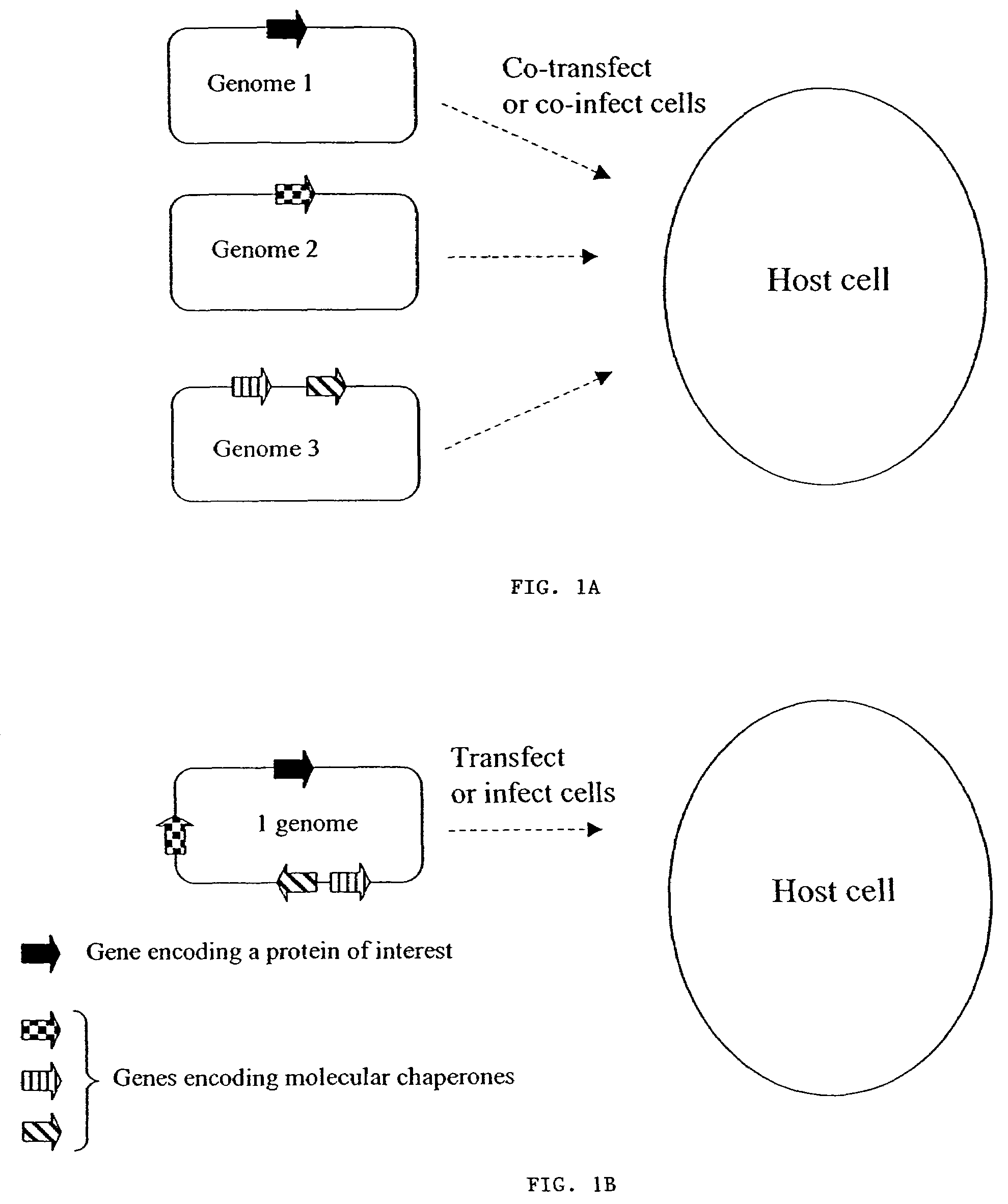
Chaperone expression genomes
InactiveUS7226781B1InterestingEnhance interestPeptide/protein ingredientsAntibody mimetics/scaffoldsForeign proteinChaperonin
A recombinant genome comprising polynucleotides encoding at least two additional molecular chaperones and a protein of interest, recombinant baculovirus vectors providing molecular chaperones and a method for producing a foreign protein using said genomes and vectors.
Owner:BELYAEV ALEXANDER S
Recombinant virus for expressing swine fever virus E2 gene, and preparation method and application thereof
ActiveCN104178505AImprove expression levelHigh expressionViral antigen ingredientsAntiviralsPig farmsSwine Fever Virus
Owner:HUAZHONG AGRI UNIV
E2 subunit vaccine comprising recombinant pestivirus E2 protein
InactiveUS6919085B2Increased and improved yieldProduce improveSsRNA viruses positive-senseViral antigen ingredientsCell culture mediaProtein C
The invention relates to a method of increasing protein expression in baculo vector virus expression systems. The invention provides a method to produce a recombinant protein in insect cell culture which comprises selecting a recombinant baculovirus expressing said protein, growing insect cells in growth medium in a culture vessel and infecting the cells with an inoculum of at least one baculovirus at a cell density of 1×105 to 5×106 cells / ml with an m.o.i of <0.01. The invention also provides a method to produce recombinant pestivirus E2 or Em9 protein or fragments thereof in insect cell culture characterized by a final concentration of the protein fragments in the growth medium at harvest of at least 100 μg / ml. The invention also provides a method of producing recombinant FSH, α-units and / or β-units, and complexes and fragments thereof, at a concentration in the growth medium at harvest of at least 15 μ / ml.
Owner:STICHTING INST VOOR DIERHOUDERIJ & DIERGEZONDHEID +1
Recombinant baculovirus expressing novel duck reovirus outer capsid proteins and construction method thereof
InactiveCN103642758AIncrease productionKeep aliveViral antigen ingredientsViral/bacteriophage medical ingredientsOuter capsidVirus
The invention relates to a recombinant baculovirus expressing novel duck reovirus NDRV outer capsid proteins. The recombinant baculovirus is vAcNDRV-[sigma]B / [sigma]C, and co-expresses the NDRV outer capsid protein [sigma]B and the NDRV outer capsid protein [sigma]C. The invention also relates to a construction method of the recombinant baculovirus. The construction method includes performing RT-PCR amplification to respectively obtain specific gene fragments of the [sigma]B and the [sigma]C; obtaining a co-expression carrier pFbNDRV-[sigma]B / [sigma]C through gene cloning and screening; transforming a DH10Bac cell with the screened recombinant co-expression carrier to obtain a transformed AcNDRV-[sigma]B / [sigma]C Bacmid; and transfecting the transformed AcNDRV-[sigma]B / [sigma]C Bacmid to an sf9 insect cell to obtain a recombinant virus vAcNDRV-[sigma]B / [sigma]C in the sf9 insect cell.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Porcine circovirus type 2 subunit vaccine, and preparation method and application thereof
ActiveCN102925486AImprove expression efficiencyImproving immunogenicityViral antigen ingredientsVirus peptidesImmune effectsVirus-like particle
The invention relates to a porcine circovirus type 2 subunit vaccine, and a preparation method and an application thereof. The recombinant baculovirus contains double promoters (a polyhedrin protein promoter and a P10 promoter), and can express double copies of Cap protein coding genes, such that protein expression efficiency is substantially improved. Also, Cap protein expressed by an inserted exogenous gene does not contain excess sequences, such that virus-like particles (VLPs) can be effectively formed, expressed protein immunogenicity is improved, and the content of produced antigen is high. According to the porcine circovirus type 2 subunit vaccine provided by the invention, protein yield and quality of porcine circovirus type 2 subunit vaccine are substantially improved, and prepared vaccine compositions have the advantages of stable and long-lasting immune effect, high safety, and the like.
Owner:WUHAN CHOPPER BIOLOGY
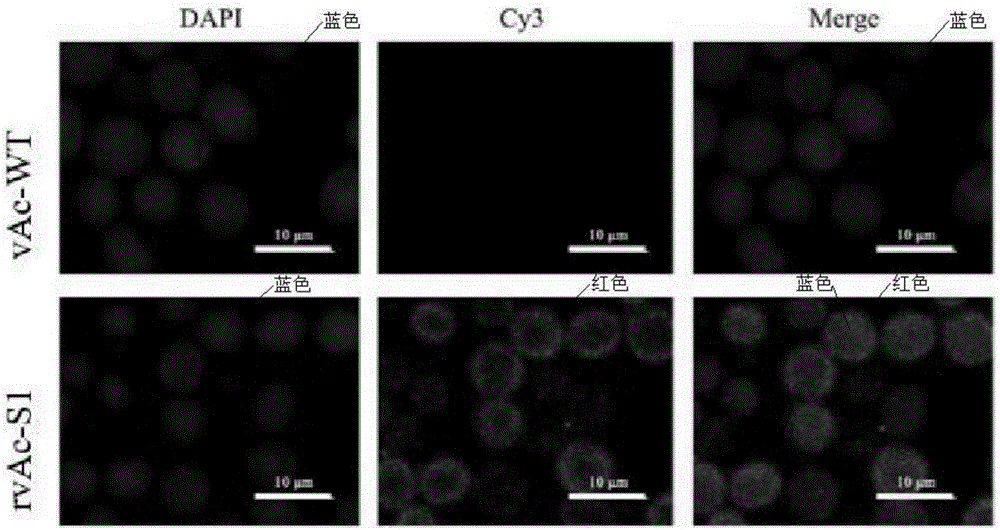
Recombinant baculovirus with surface displaying porcine epidemic diarrhea virus S protein
InactiveCN106085969AImprove Surface DisplayHigh expressionSsRNA viruses positive-senseViral antigen ingredientsSurface displayViral Vaccine
The invention provides a recombinant baculovirus with the surface displaying a porcine epidemic diarrhea virus S protein, and a preparation method thereof. The virus adopts PEDV spike protein S1 gene as antigen gene, the recombinant virus is constructed by using an insect baculovirus vector expression system, and an S1 protein is successfully expressed and displayed on the surface of the virus. The recombinant virus is used to immunize and inoculate mice as a PEDV pseudo-virus vaccine, and serum neutralization test and lymphocyte propagation experiment analysis shows that the recombinant virus can arouse an effective immune protection effect.
Owner:杭州洪晟生物技术股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com