Porcine circovirus type 2 subunit vaccine and preparation method thereof
A porcine circovirus and subunit vaccine technology, applied in the biological field, can solve the problems of difficult inactivated vaccines and attenuated vaccines, low value-added titers, etc., and achieve the effects of safe and reliable expression products, convenient preparation, and high biological activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation method of porcine circovirus type 2 subunit vaccine
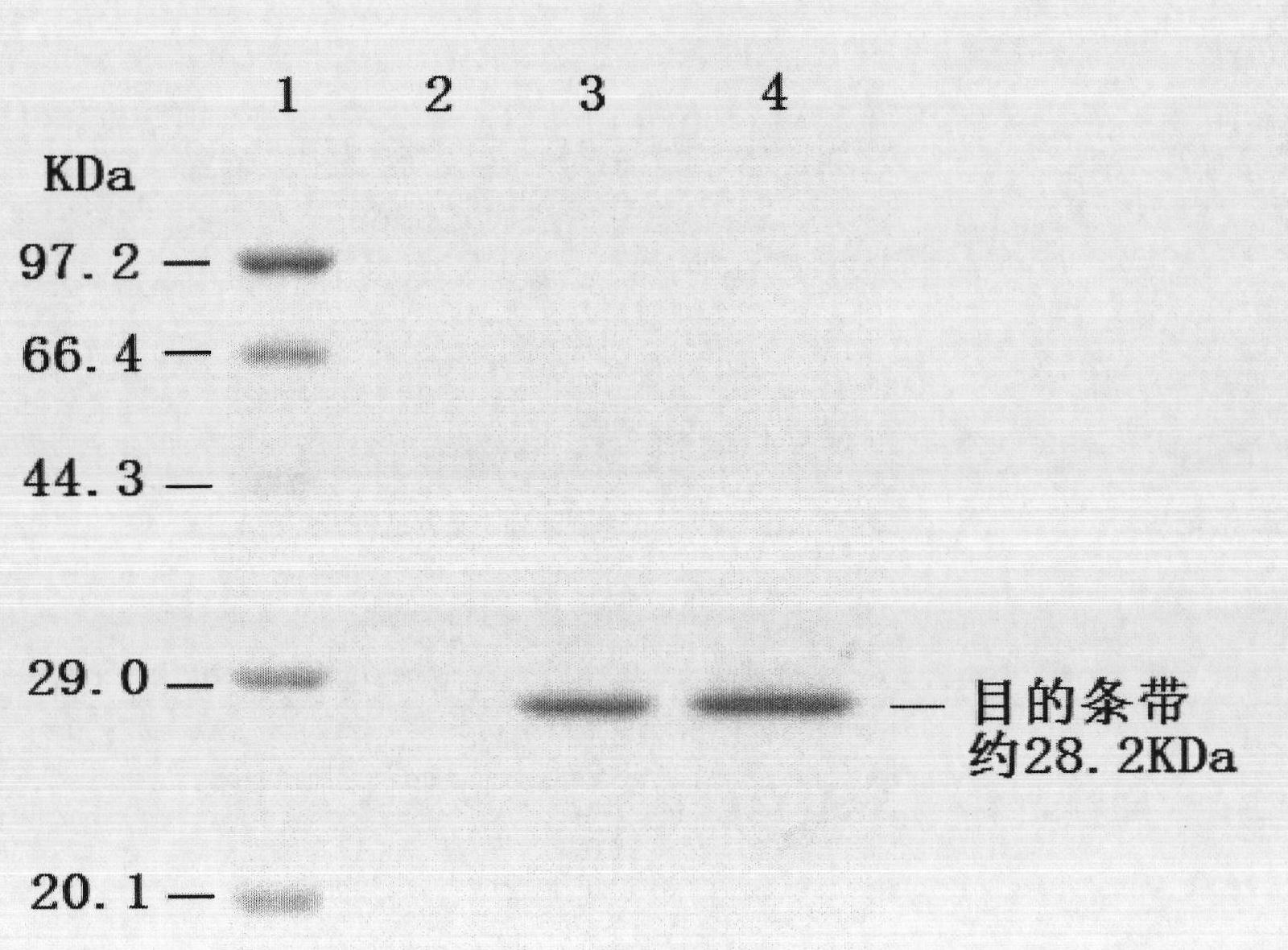
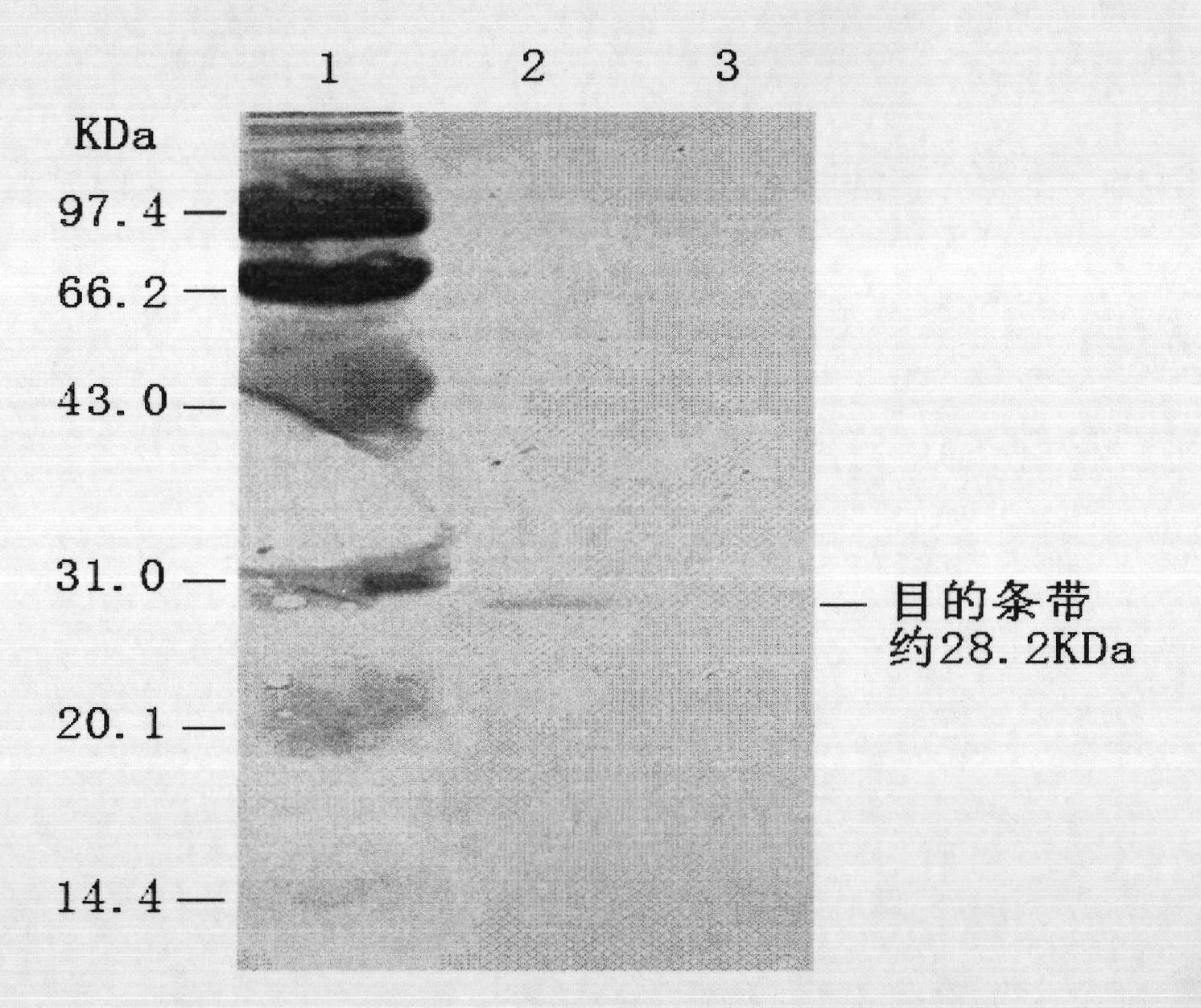
[0040] 1. Clone the porcine circovirus type 2 ORF2 gene and introduce the melittin signal peptide gene sequence, and transfer it into the pMD-18T vector to obtain a recombinant vector containing the porcine circovirus type 2 ORF2 gene:
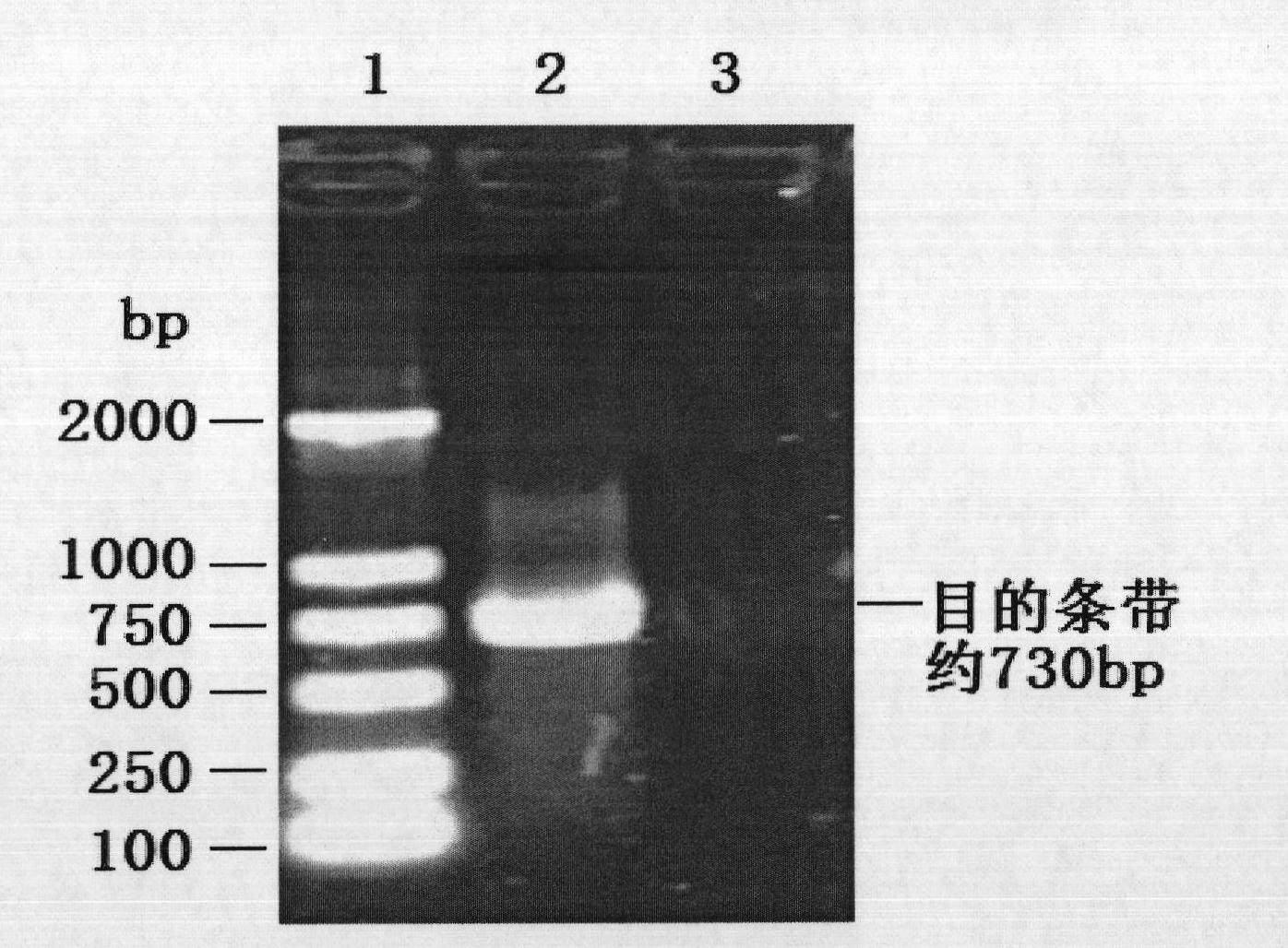
[0041]First design primer Spe-ATG (5'-cgactagtatgacgtatccaaggaggcgt-3') and primer Hind-TAA (5'-gcaagctttatcattaagggttaagttggg-3'), use the genomic DNA of the PCV2-SH strain whose preservation number is CGMCC No.2389 as a template, and amplify The PCV2 ORF2 gene was amplified, and the amplified ORF2 gene was cloned into the pMD-18T vector to obtain the recombinant vector pT-ORF2. The size of the PCR product is about 730bp, see the attached results figure 1 . Among them, the first hole is DNA Marker2000; the second hole is PCV2 ORF2 amplification product; the third hole is PCR negative control. The PCR product was sequenced. According to the results of electro...
Embodiment 2
[0065] Efficacy detection of embodiment 2 PCV2 subunit vaccine:
[0066] Pigs from a pig farm in Luoyang were tested for major pathogens and related antibodies, and 10-14 day-old pigs negative for major pathogens such as porcine circovirus, swine fever virus, porcine blue ear disease virus, and porcine parvovirus were selected.
[0067] Qualified piglets were randomly divided into 9 groups, 10 in each group, and 7 groups were given subunit vaccines containing different amounts of recombinant ORF2 protein (comprising 0.2 μg / head, 1 μg / head, 2 μg / head, respectively) of recombinant ORF2 protein. head portion, 4 μg / head portion, 8 μg / head portion, 16 μg / head portion and 40 μg / head portion), one group was used as a challenge control, and the other group was used as a negative control. Among them, group 1 was given a subunit vaccine containing 0.2 μg / head of recombinant ORF2 protein; group 2 was given a subunit vaccine containing 1 μg / head of recombinant ORF2 protein; group 3 was gi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com