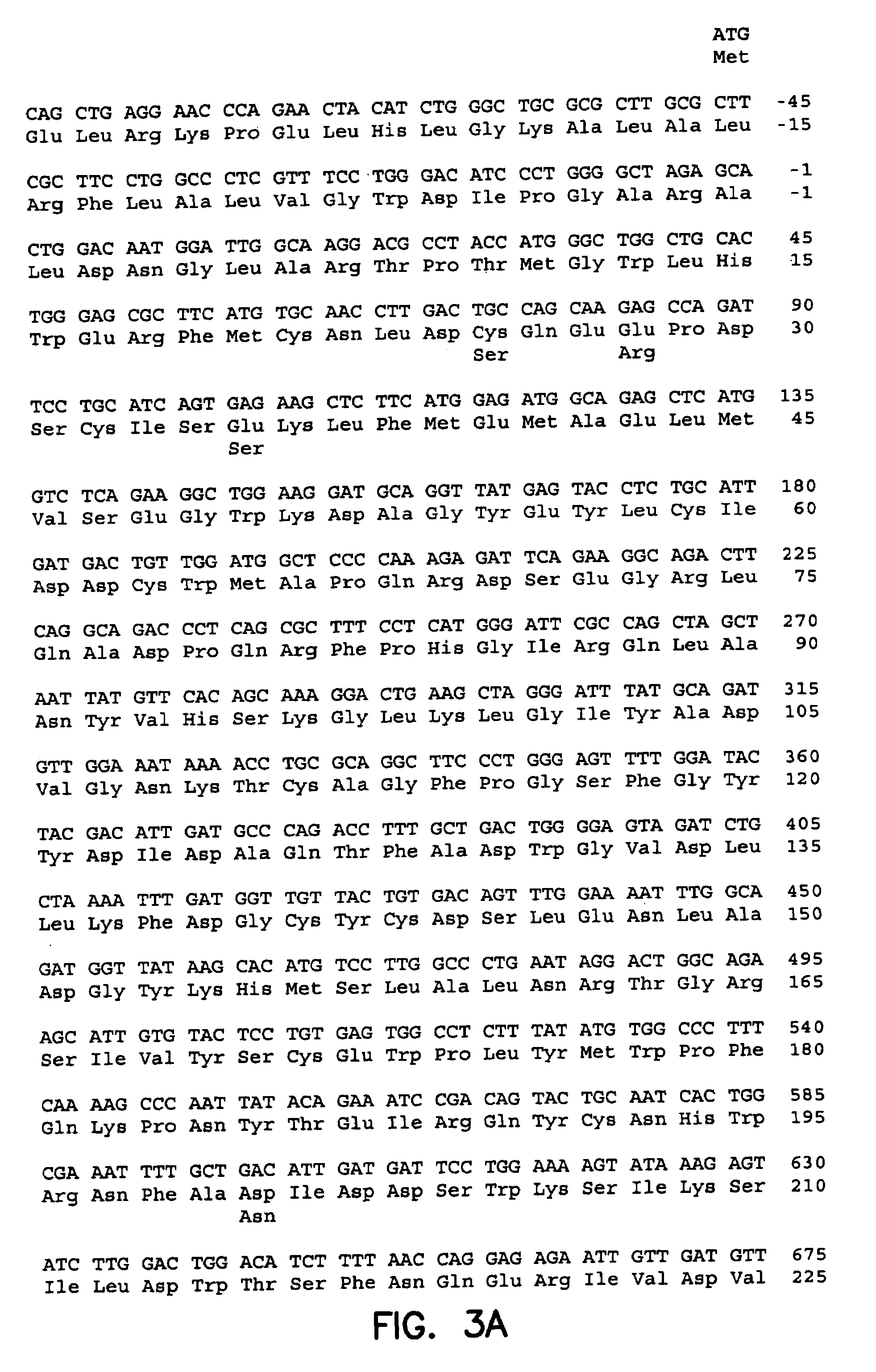
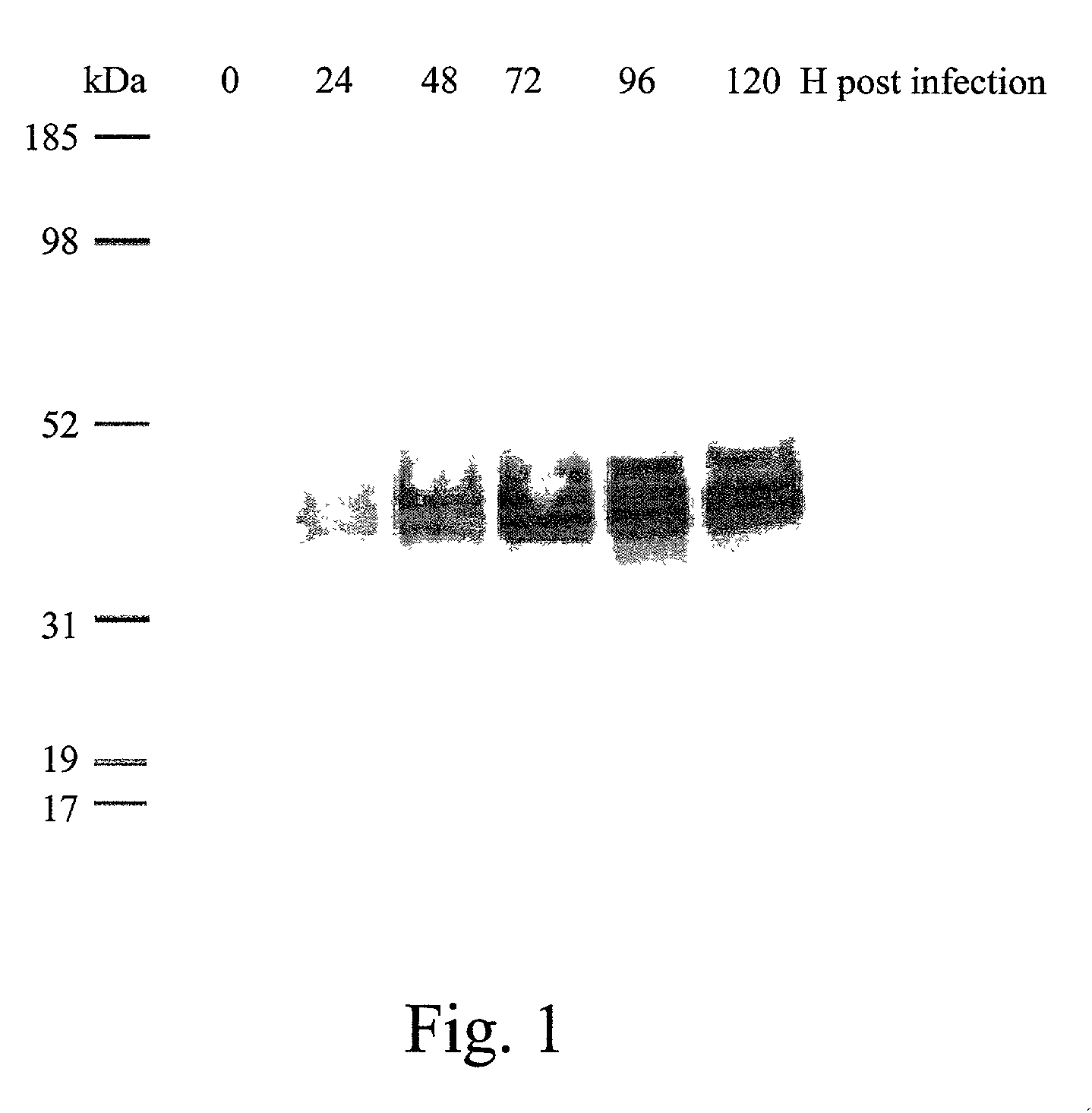
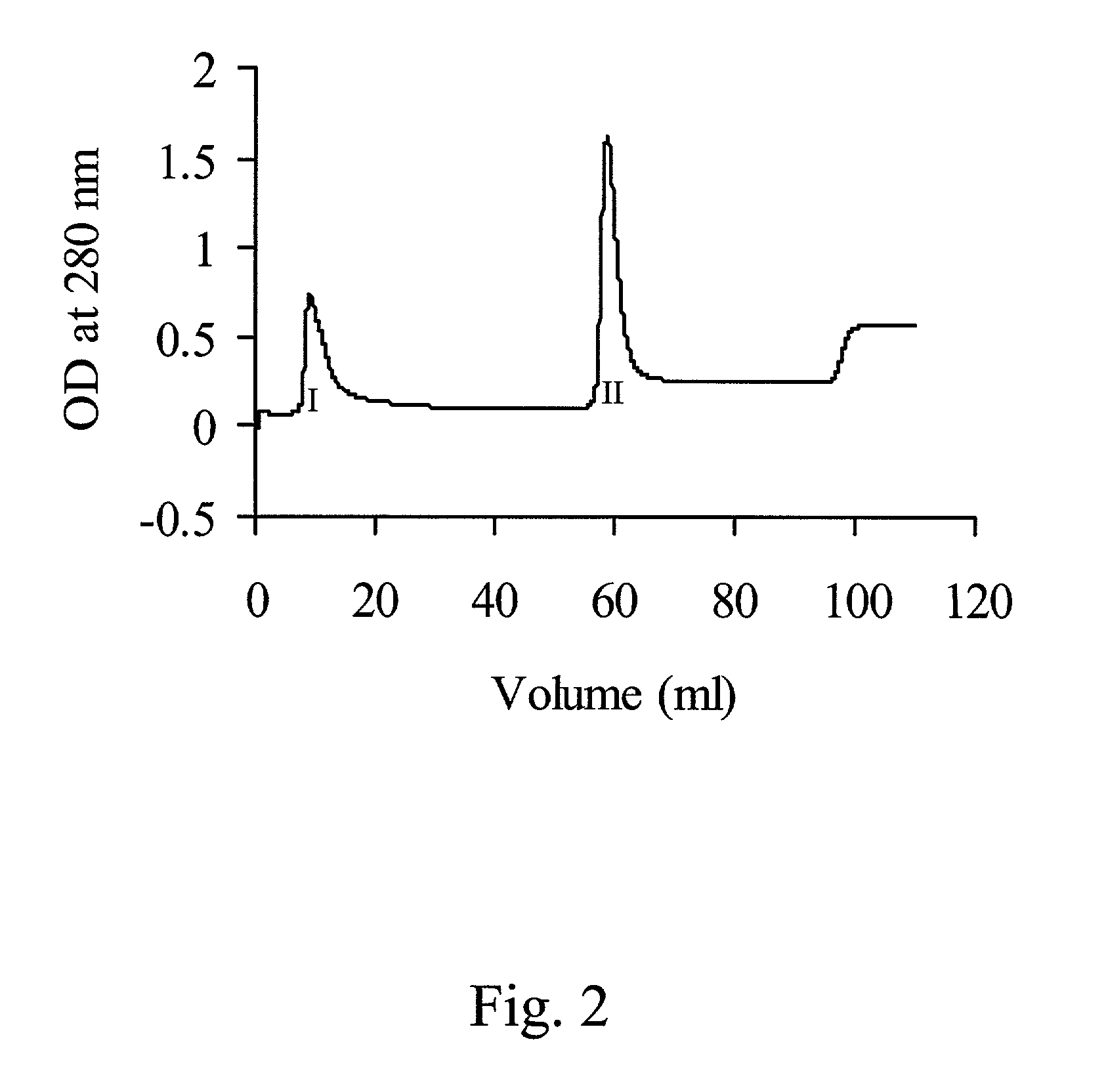
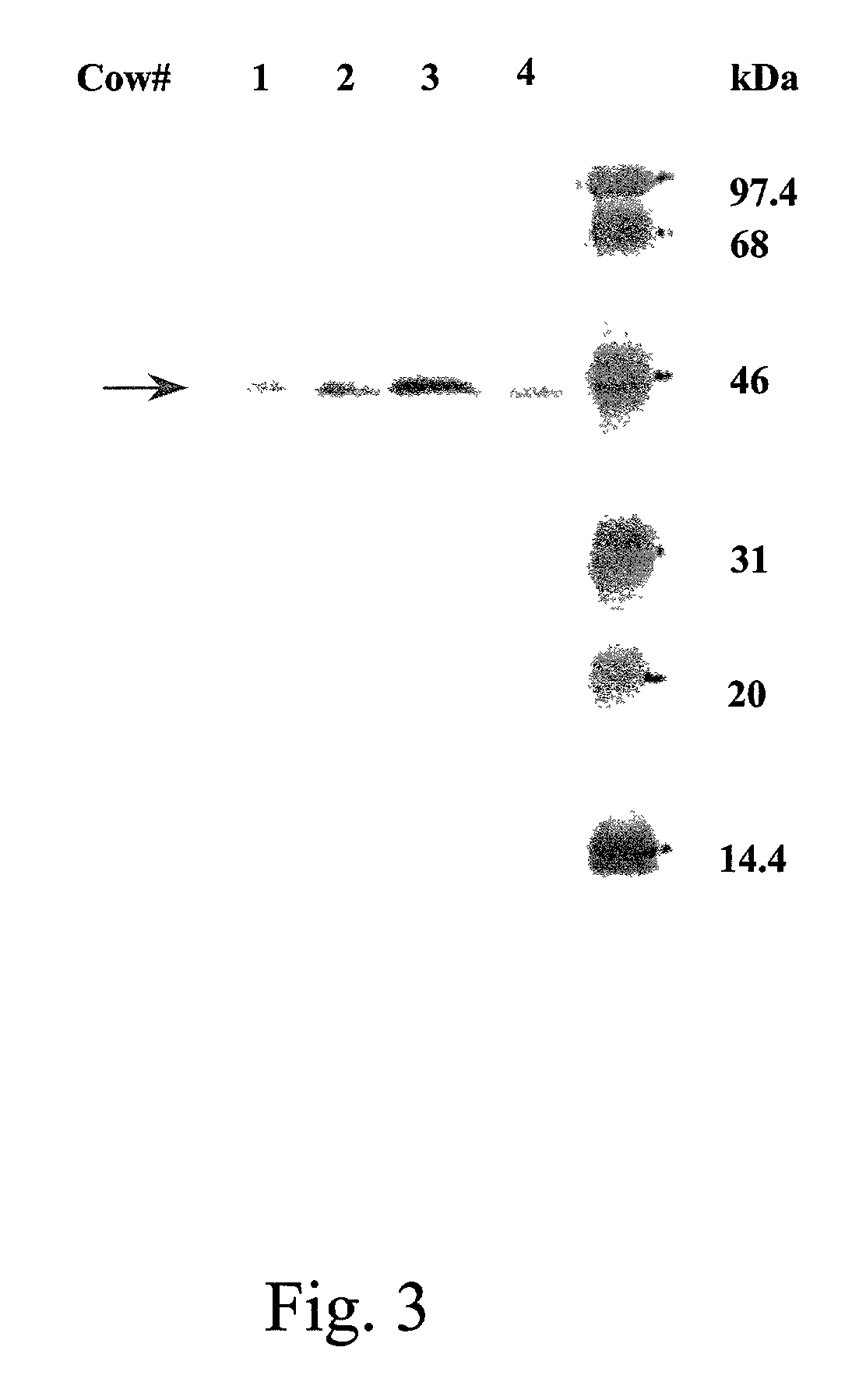
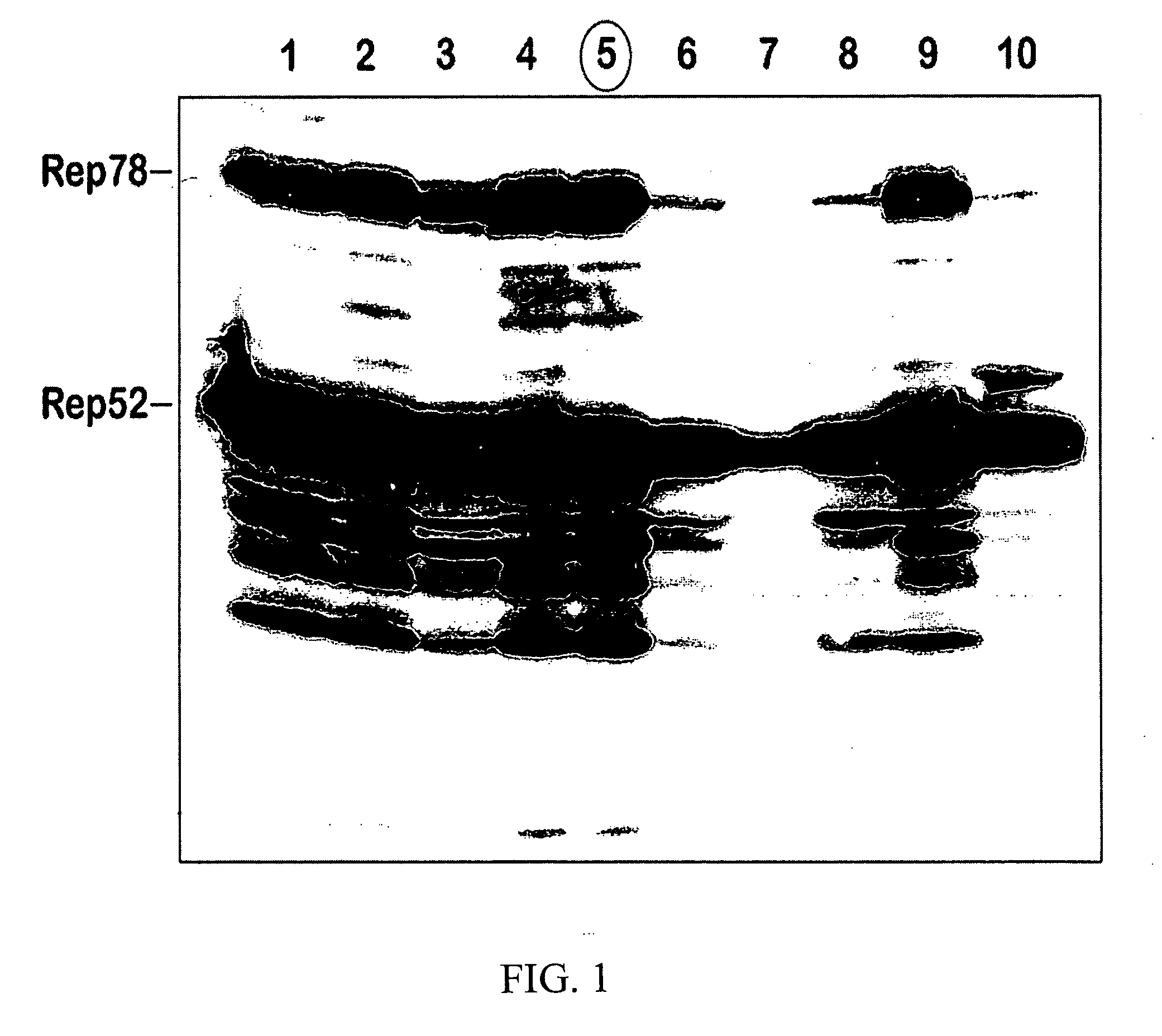
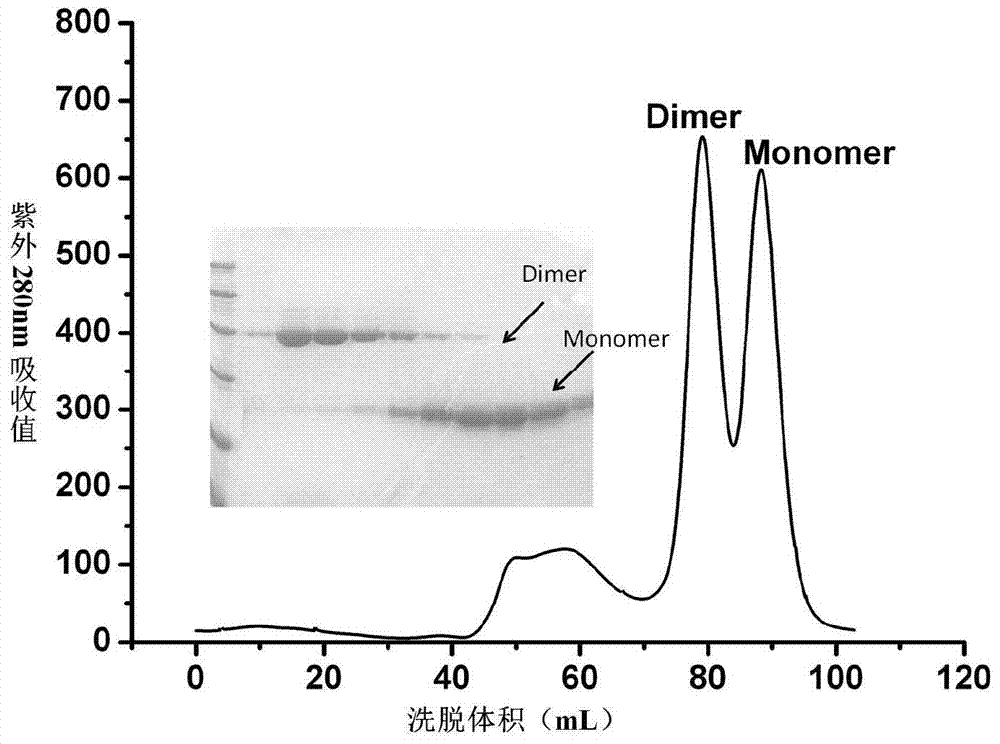
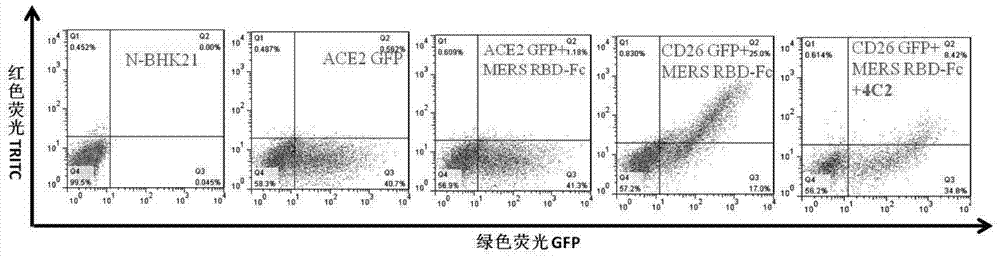
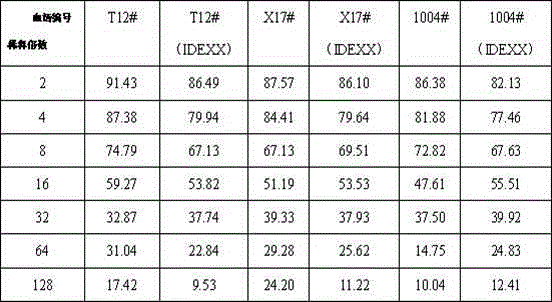
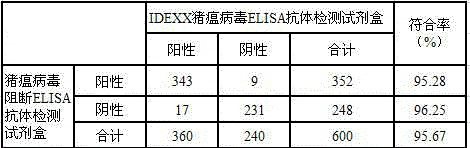
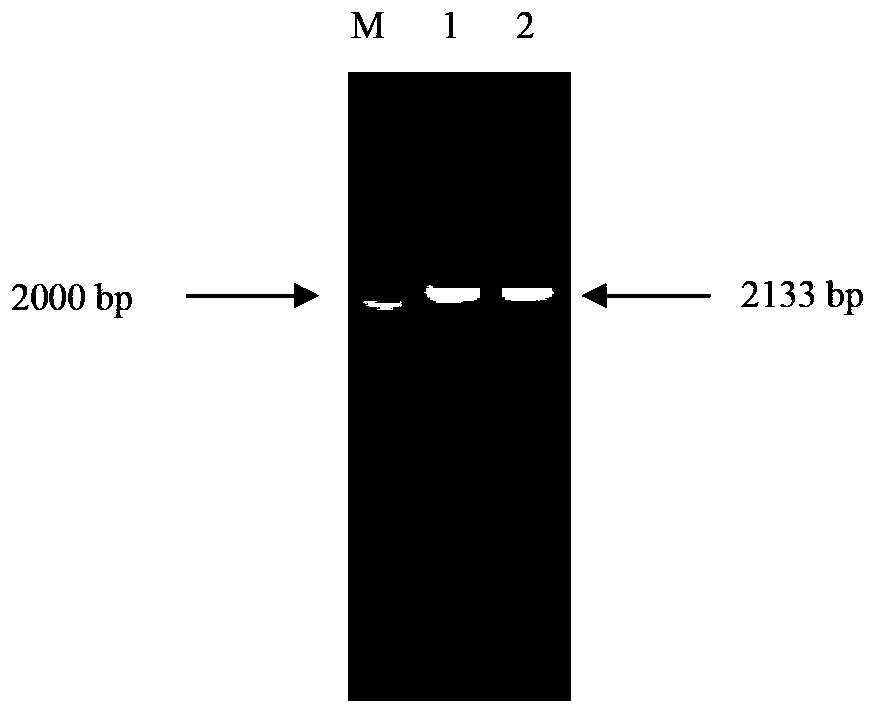
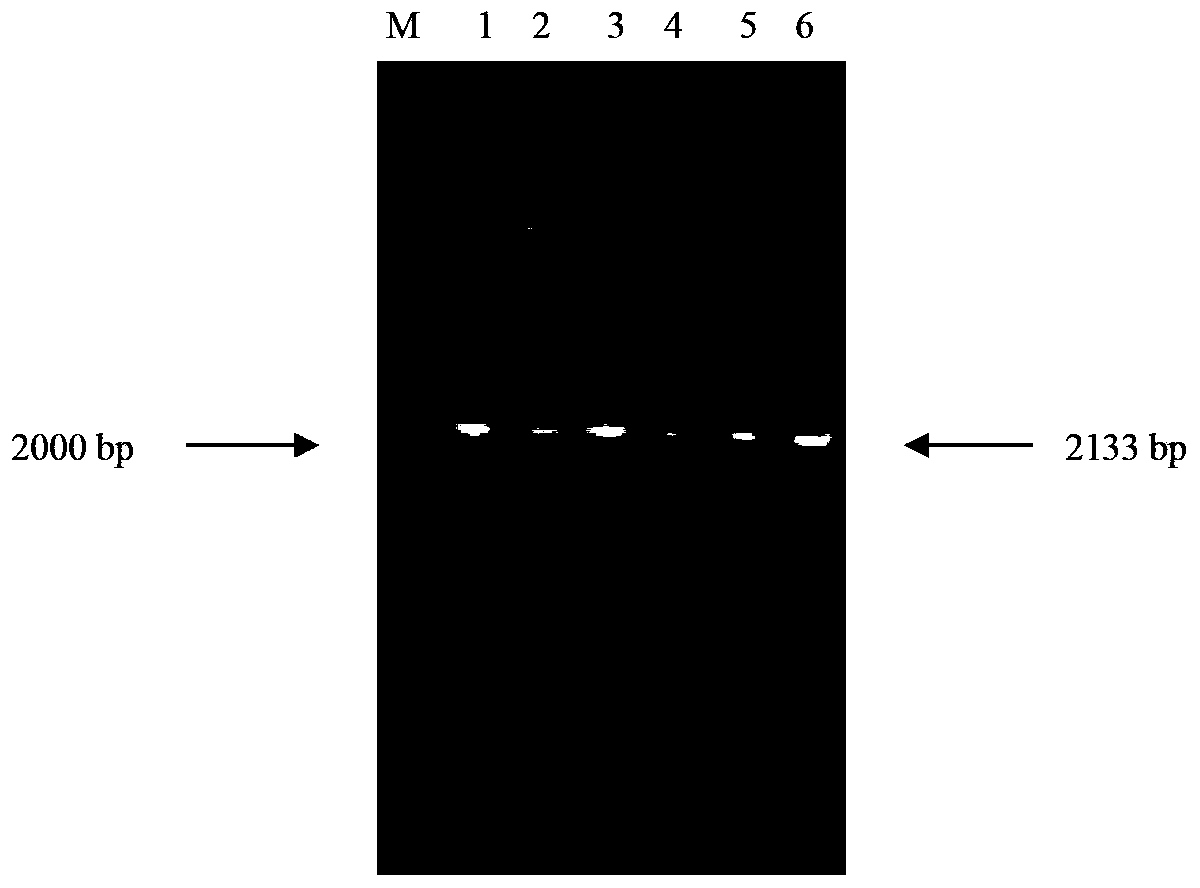
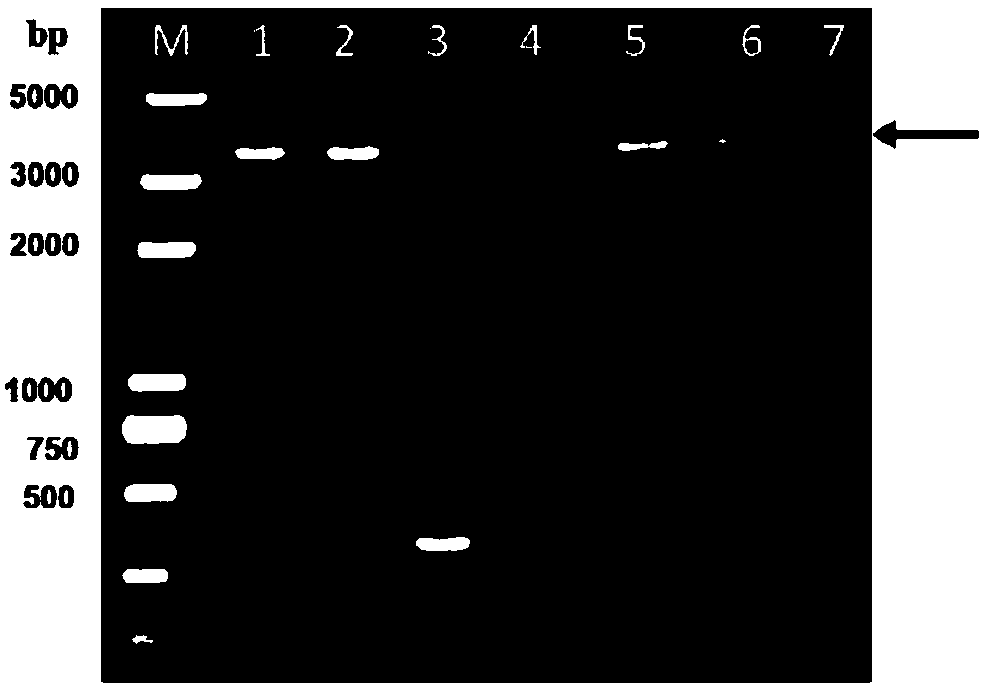
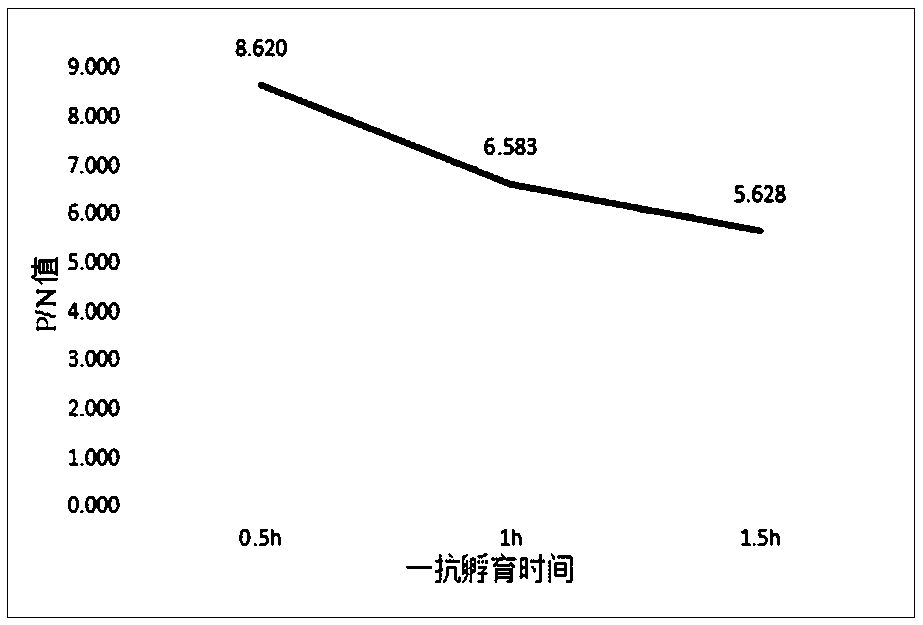
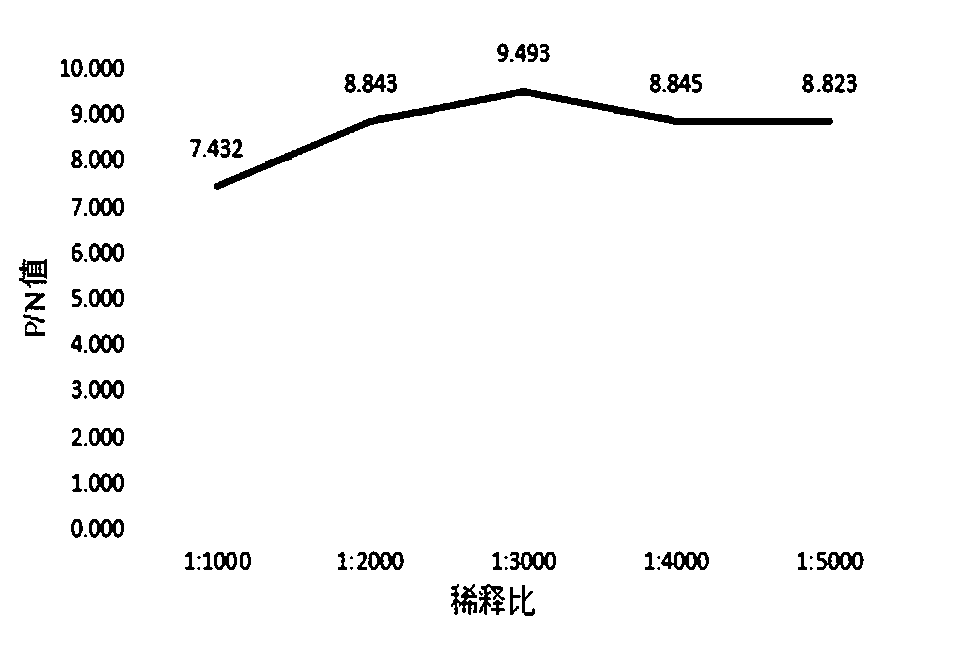
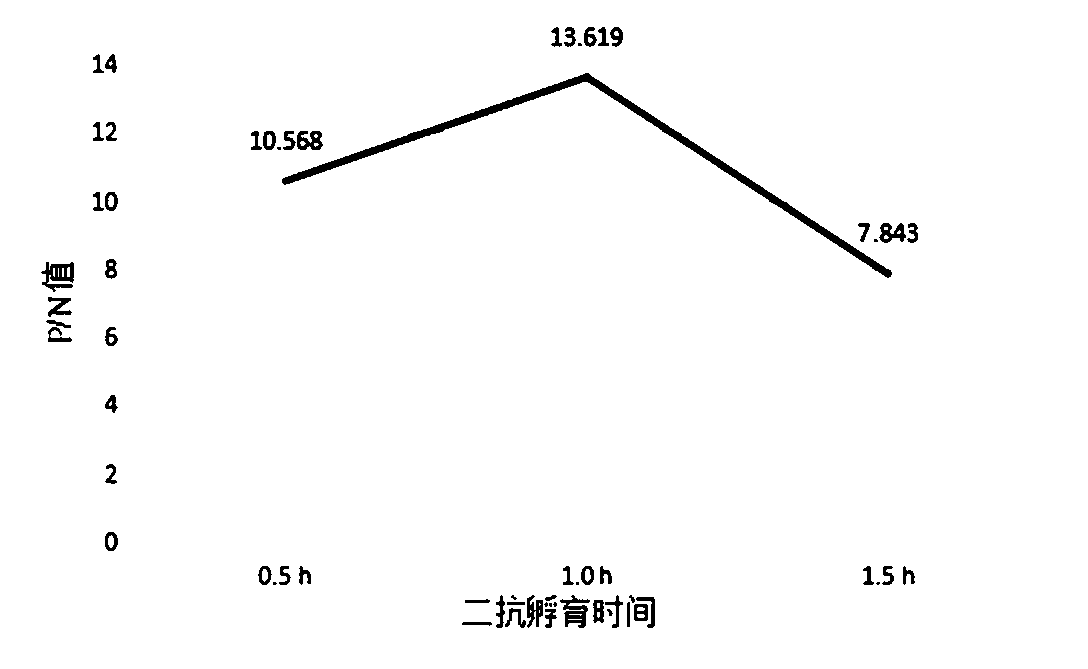
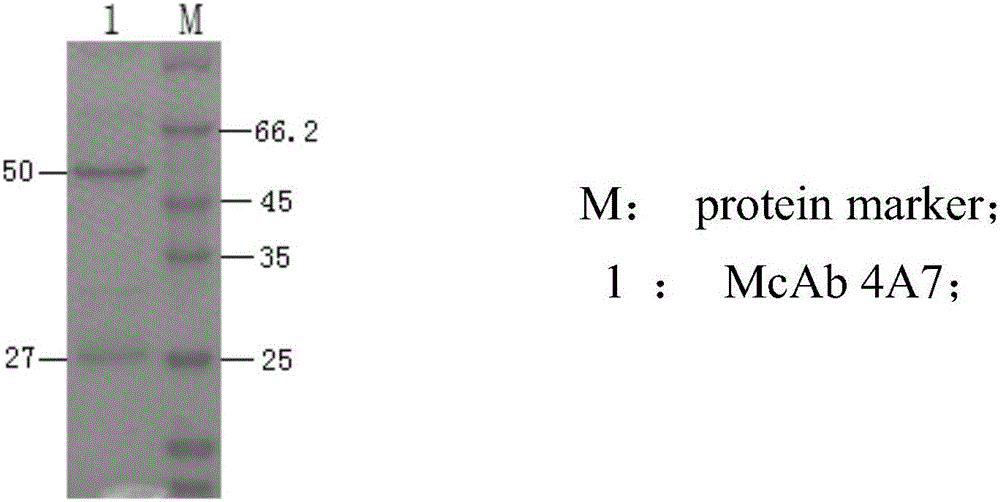Patents
Literature
251 results about "Baculovirus expression" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
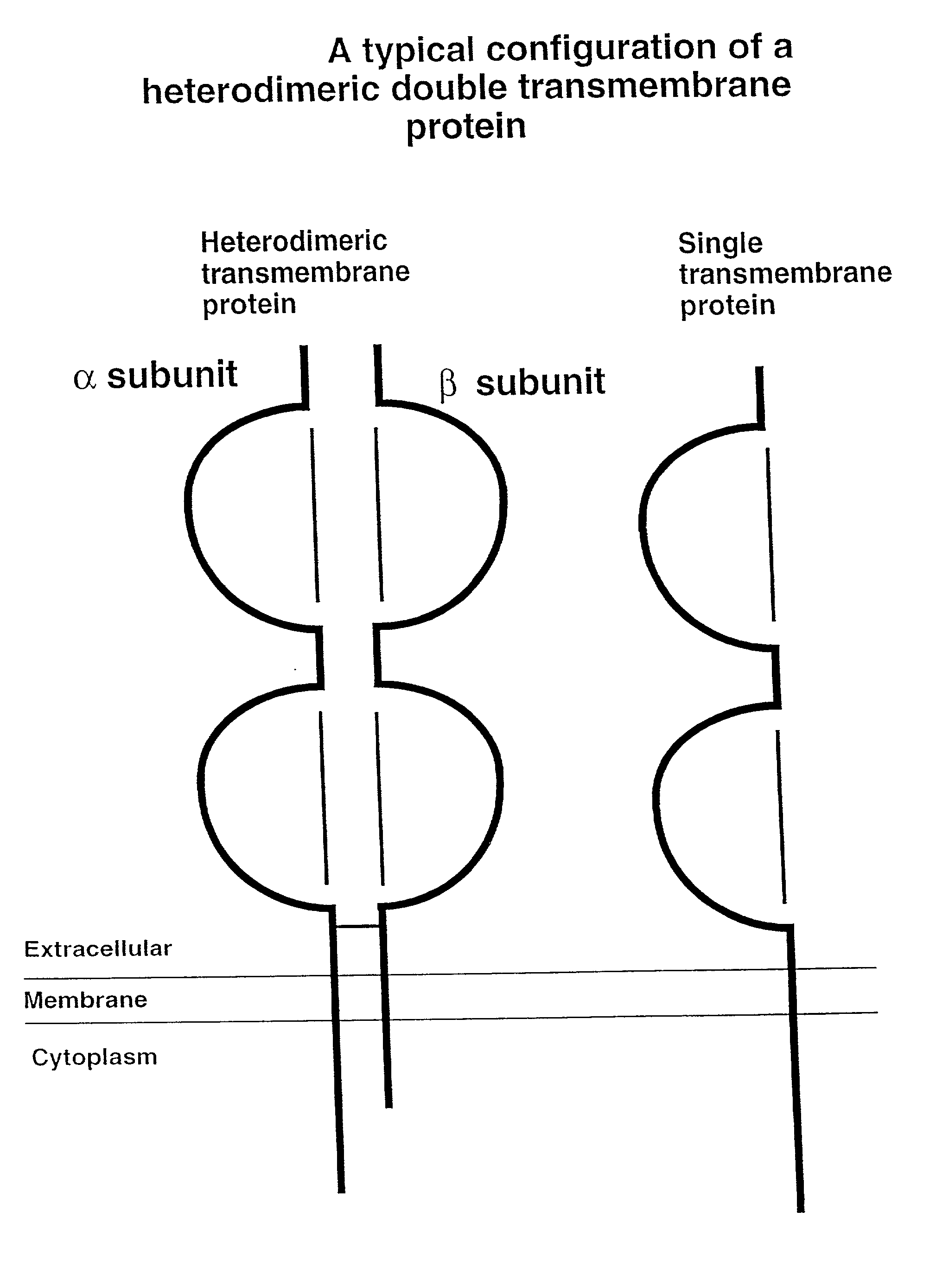
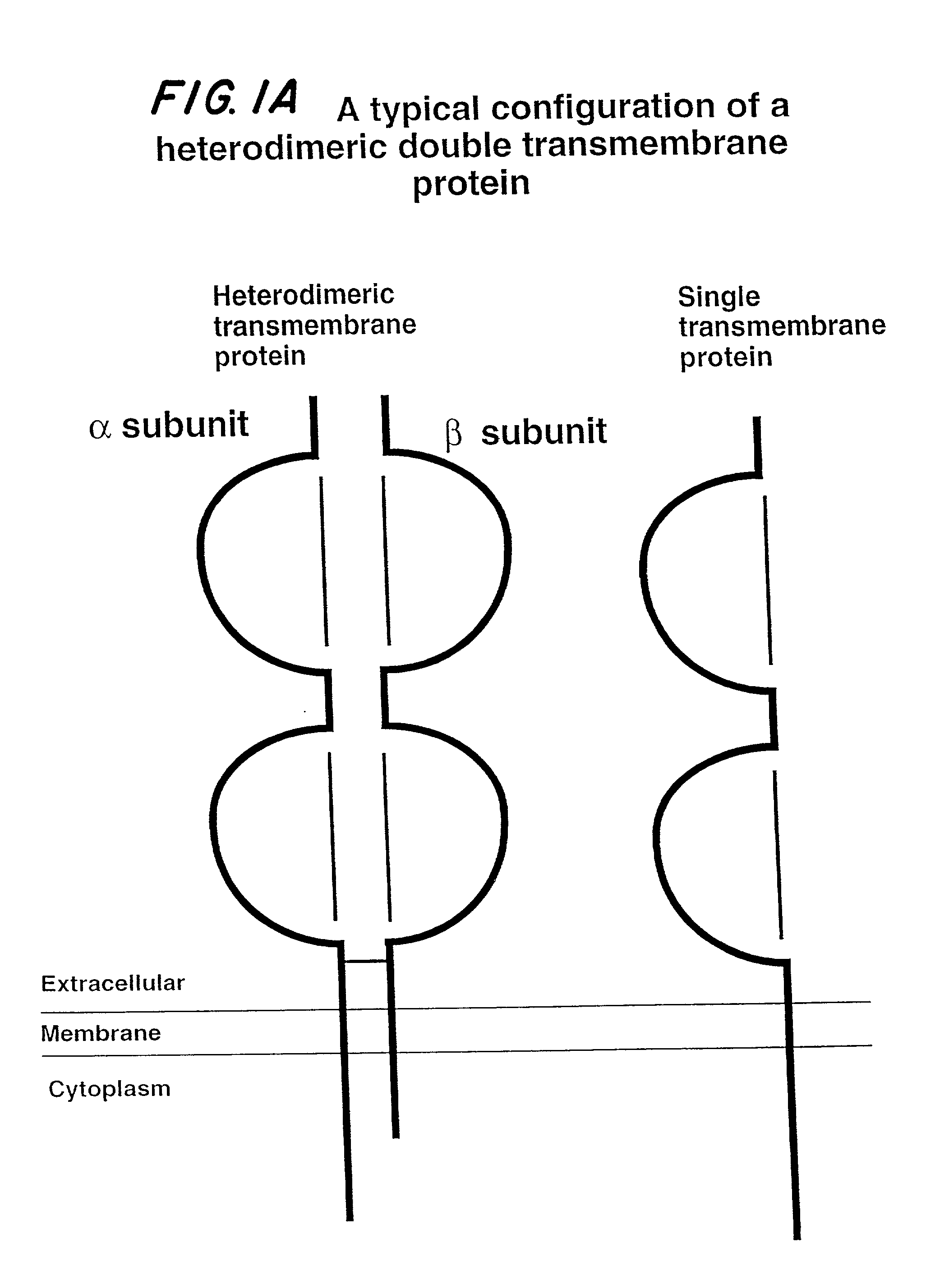
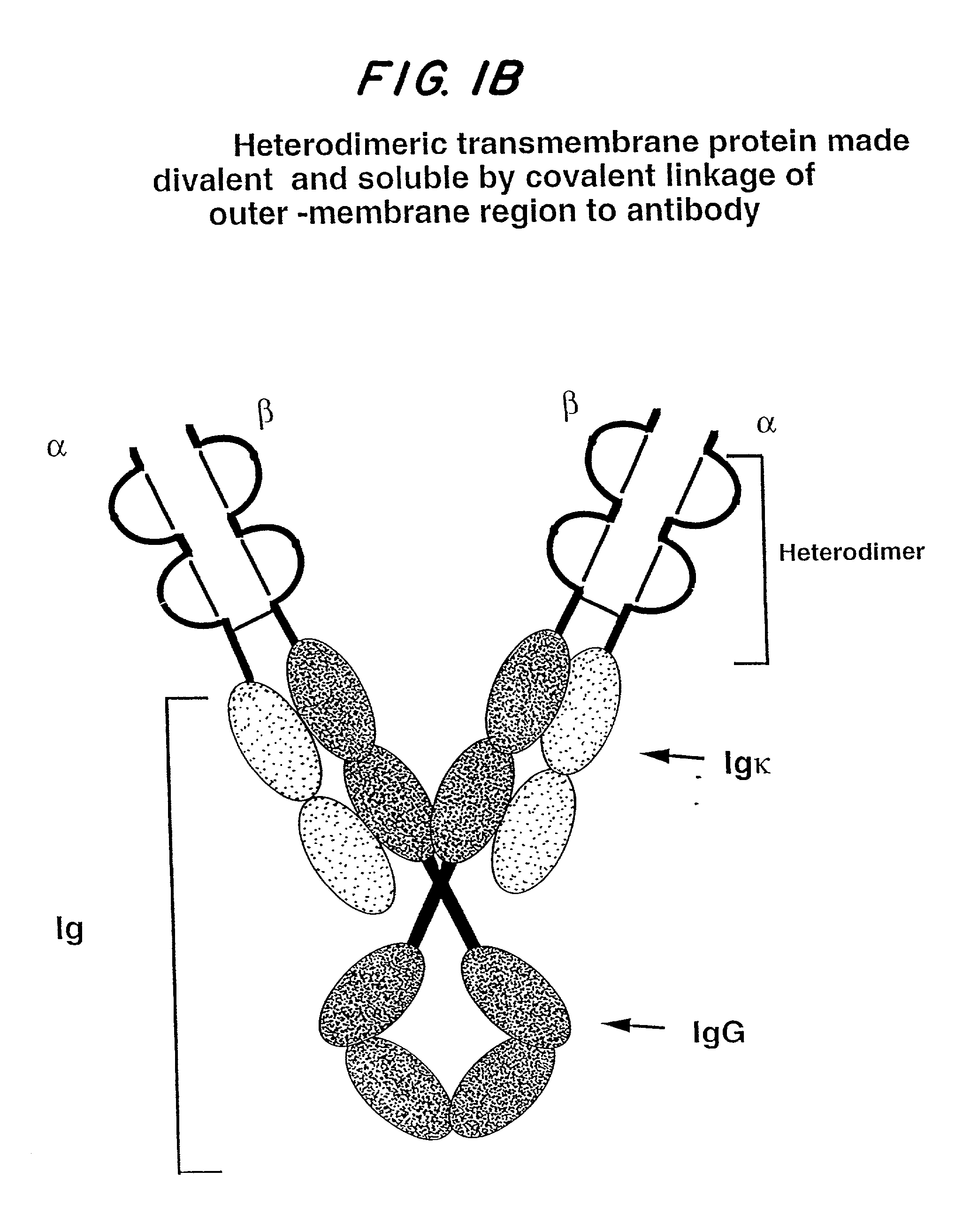
Soluble divalent and multivalent heterodimeric analogs of proteins
InactiveUS20020127231A1Well representedHigh affinityVirusesPeptide/protein ingredientsADAMTS ProteinsSpecific immunity
Specificity in immune responses is in part controlled by the selective interaction of T cell receptors with their cognate ligands, peptide / MHC molecules. The discriminating nature of this interaction makes these molecules, in soluble form, good candidates for selectively regulating immune responses. Attempts to exploit soluble analogs of these proteins has been hampered by the intrinsic low avidity of these molecules for their ligands. To increase the avidity of soluble analogs for their cognates to biologically relevant levels, divalent peptide / MHC complexes or T cell receptors (superdimers) were constructed. Using a recombinant DNA strategy, DNA encoding either the MHC class II / peptide or TCR heterodimers was ligated to DNA coding for murine Ig heavy and light chains. These constructs were subsequently expressed in a baculovirus expression system. Enzyme-linked immunosorbant assays (ELISA) specific for the Ig and polymorphic determinants of either the TCR or MHC fraction of the molecule indicated that infected insect cells secreted approximately 1 .mu.g / ml of soluble, conformnationally intact chimeric superdimers. SDS PAGE gel analysis of purified protein showed that expected molecular weight species. The results of flow cytometry demonstrated that the TCR and class II chimeras bound specifically with high avidity to cells bearing their cognate receptors. These superdimers will be useful for studying TCR / MHC interactions, lymphocyte tracking, identifying new antigens, and have possible uses as specific regulators of immune responses.
Owner:SCHNECK JONATHAN +1
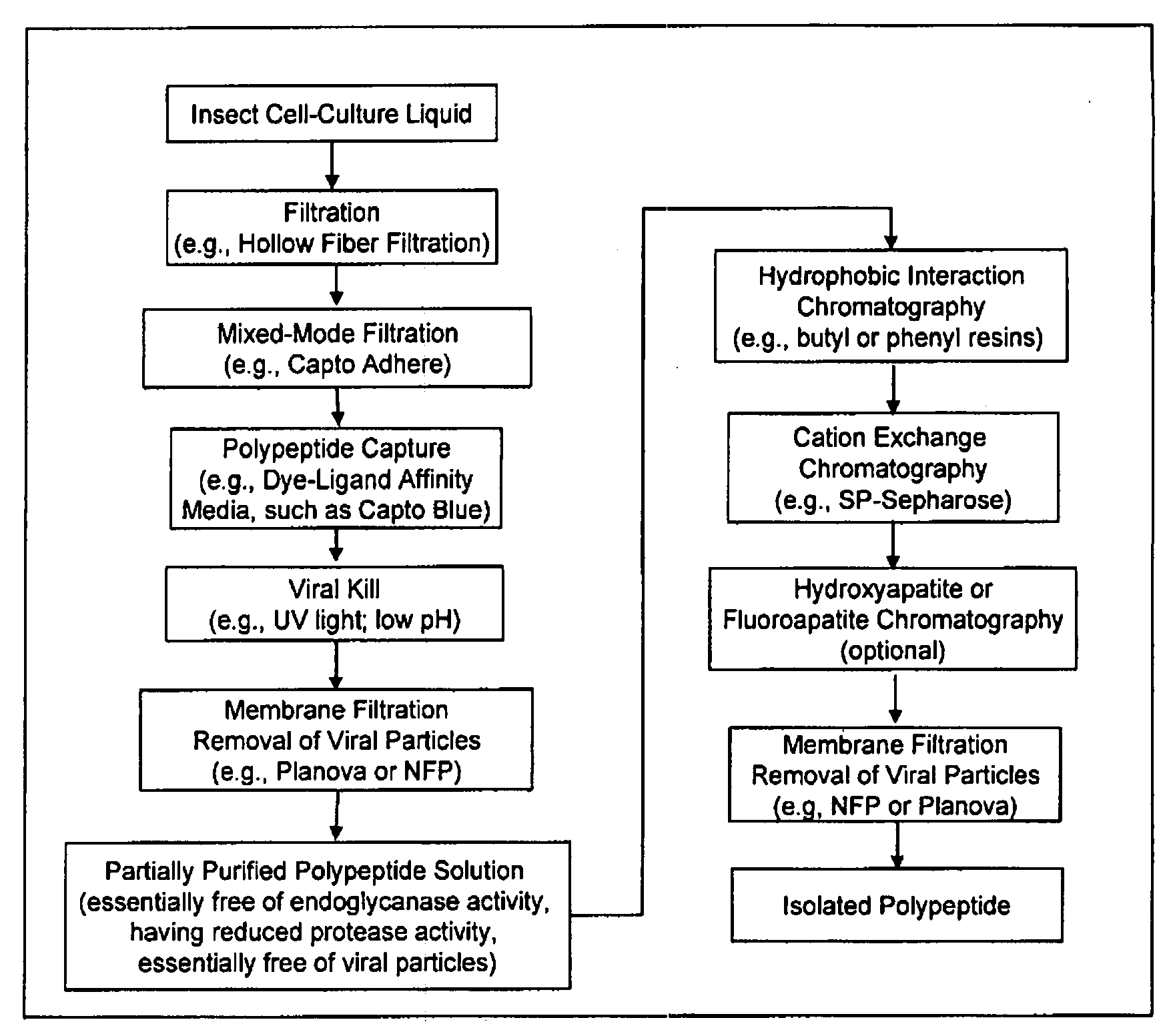
Manufacturing process for the production of polypeptides expressed in insect cell-lines
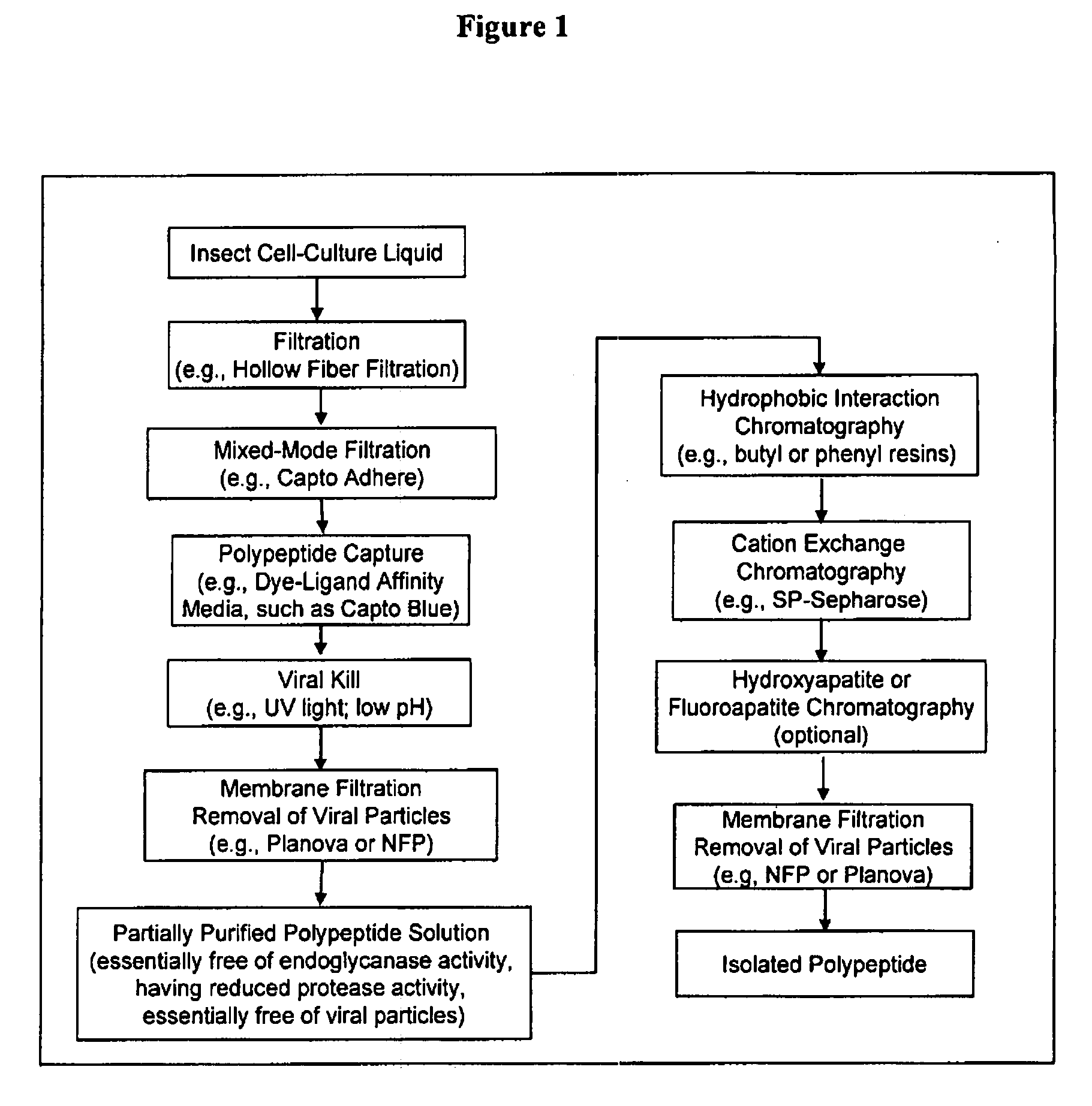
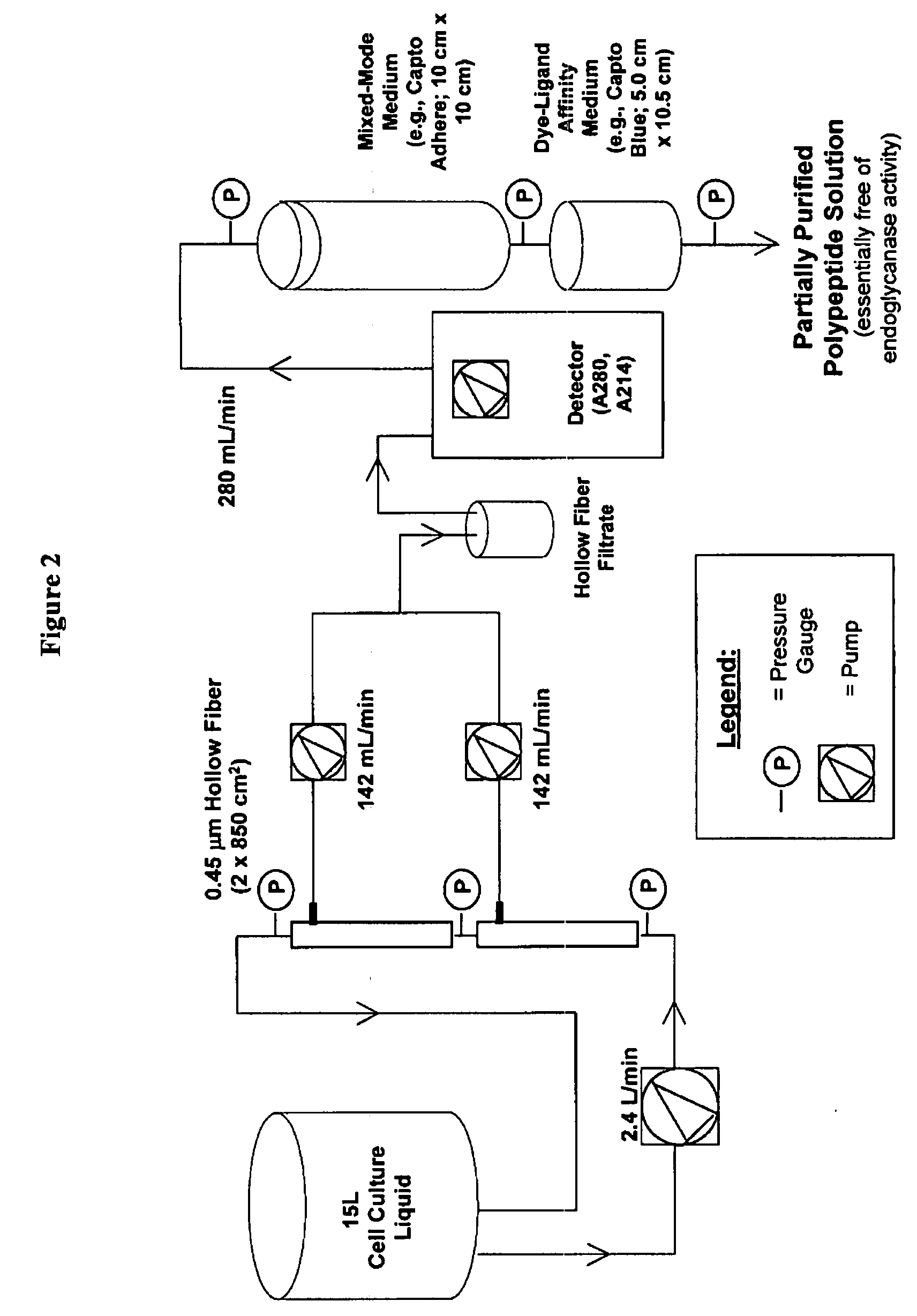
InactiveUS20080207487A1Promote recoveryReduce manufacturing costPeptide/protein ingredientsDepsipeptidesFiberCulture fluid
The present invention provides a manufacturing method for polypeptides that are produced in insect cells using a baculoviral expression system. In one example, the insect cell culture is supplemented with a lipid mixture immediately prior to infection (e.g., one hour prior to infection). The polypeptides are isolated from the insect cell culture using a method that employs anion exchange or mixed-mode chromatography early in the purification process. This process step is useful to remove insect-cell derived endoglycanases and proteases and thus reduces the loss of desired polypeptide due to enzymatic degradation. In another example, mixed-mode chromatography is combined with dye-ligand affinity chromatography in a continuous-flow manner to allow for rapid processing of the insect-cell culture liquid and capture of the polypeptide. In yet another example, a polypeptide is isolated from an insect cell culture liquid using a process that combines hollow fiber filtration, mixed-mode chromatography and dye-ligand affinity in a single unit operation producing a polypeptide solution that is essentially free of endoglycanase and proteolytic activities. In a further example, the isolated polypeptides are glycopeptides having an insect specific glycosylation pattern, which are optionally conjugated to a modifying group, such as a polymer (e.g., PEG) using a glycosyltransferase and a modified nucleotide sugar.
Owner:NOVO NORDISK AS
Recombinant alpha-galactosidase A therapy for Fabry disease
InactiveUS7011831B2Peptide/protein ingredientsGenetic material ingredientsBaculovirus expressionInsect cell culture
Fabry disease results from an X-linked deficiency in the enzyme α-galactosidase A. The present invention is directed to recombinant human α-galactosidase A and provides baculovirus expression vectors and recombinant virus that provide stable expression of extracellular and intracellular levels of this enzyme in an insect cell culture. The recombinant-derived enzyme can be used in enzyme replacement therapy to treat Fabry patients. Composition useful in therapeutic administration of α-galactosidase A are also provided.
Owner:SHELBYZYME
Porcine circovirus type 2 subunit vaccine and preparation method thereof
InactiveCN101884787AImprove biological activityHighly species-specificViral antigen ingredientsVirus peptidesOpen reading frameAntigenicity
The invention mainly aims to provide a porcine circovirus type 2 (PCV2) subunit vaccine and a preparation method thereof. Particularly, a baculovirus vector expression system is utilized to express a large amount of recombinant open reading frame type 2 (ORF2) protein in insect cells, so that the subunit vaccine with good immunity effect is developed. A bac-to-bac baculovirus expression system is adopted to perform whole gene amplification on the porcine circovirus type 2 ORF2 gene, and a melittin signal peptide nucleotide sequence is introduced into a terminal 5', so that the recombinant baculovirus of an open reading frame containing the melittin signal peptide nucleotide sequence and a PVC2ORF2 gene sequence is established, wherein the infected insect cell expresses the recombinant ORF2 protein with efficient and high PCV2 antigenicity; and thus the subunit vaccine containing the PCV2 recombinant ORF2 protein is prepared. The inoculation experiments of piglets show that the subunit vaccine has a good immunity protection effect.
Owner:PU LIKE BIO ENG
Use of recombinant bovine CD14 in the treatment and prevention of coliform mastitis in dairy cows
InactiveUS6984503B1High affinityEnhanced ability to enhance activationBacteriaSugar derivativesBaculovirus expressionInduced infections
Studies in mice and humans indicate that membrane CD14 (mCD14) on the cell surface of monocytes, macrophages, and PMN mediates the activation of these cells by LPS. The soluble CD14 (sCD14) present in the circulation also binds to LPS and blocks LPS binding to mCD14. To determine the role of a recombinant bovine soluble CD14 polypeptide in cellular activation by LPS, a recombinant bovine soluble CD14 polypeptide, rbosCD14, was cloned and expressed in a baculovirus expression system. Results indicated that rbosCD14 inhibited the LPS-induced increase in CD18 expression and TNFα mRNA in vitro and reduced mortality in mice injected with LPS. Further, rbosCD14 sensitized mammary epithelial cells to low concentrations of LPS resulting in recruitment of white blood cells and prevention of LPS-induced infection.
Owner:UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF AGRI THE
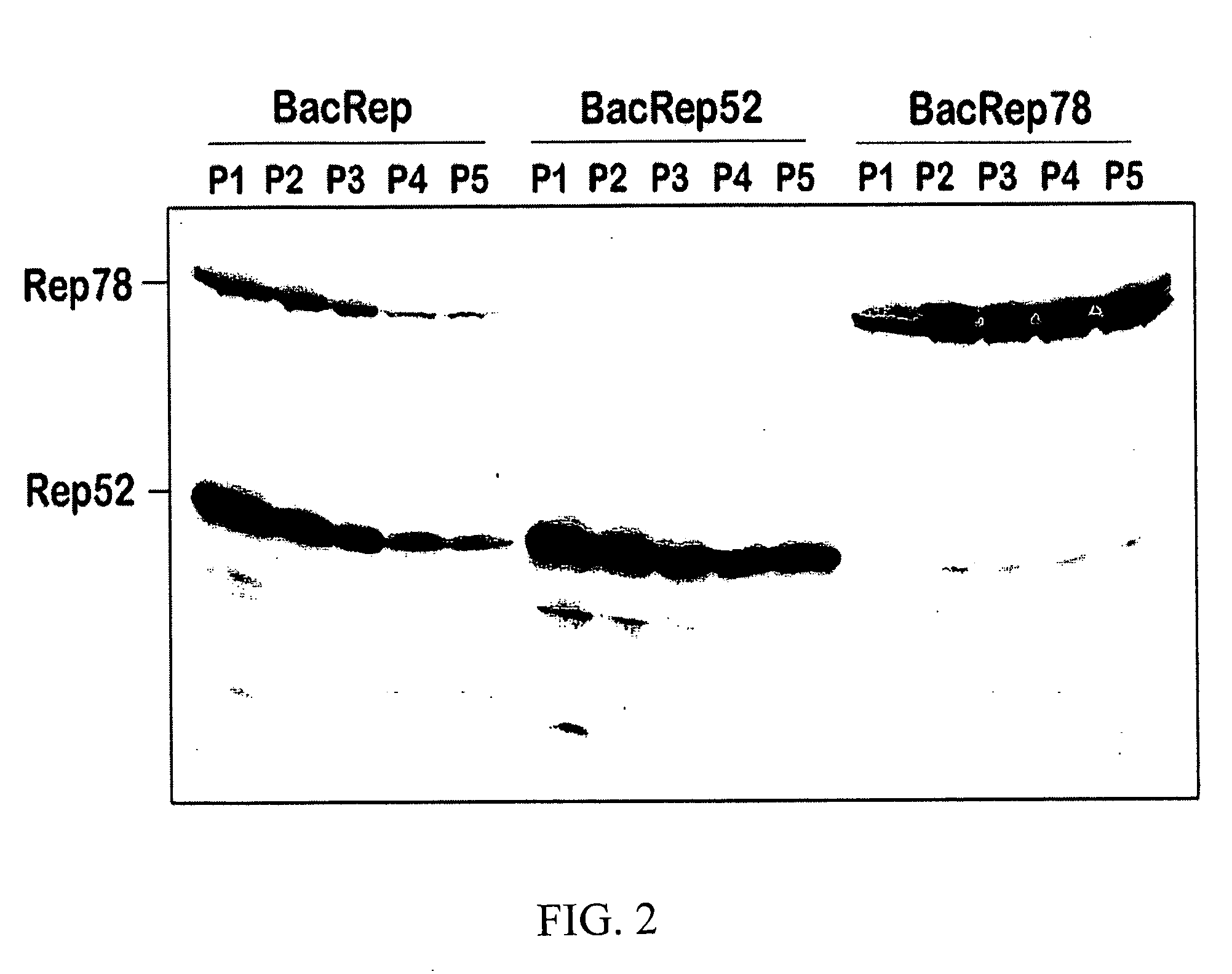
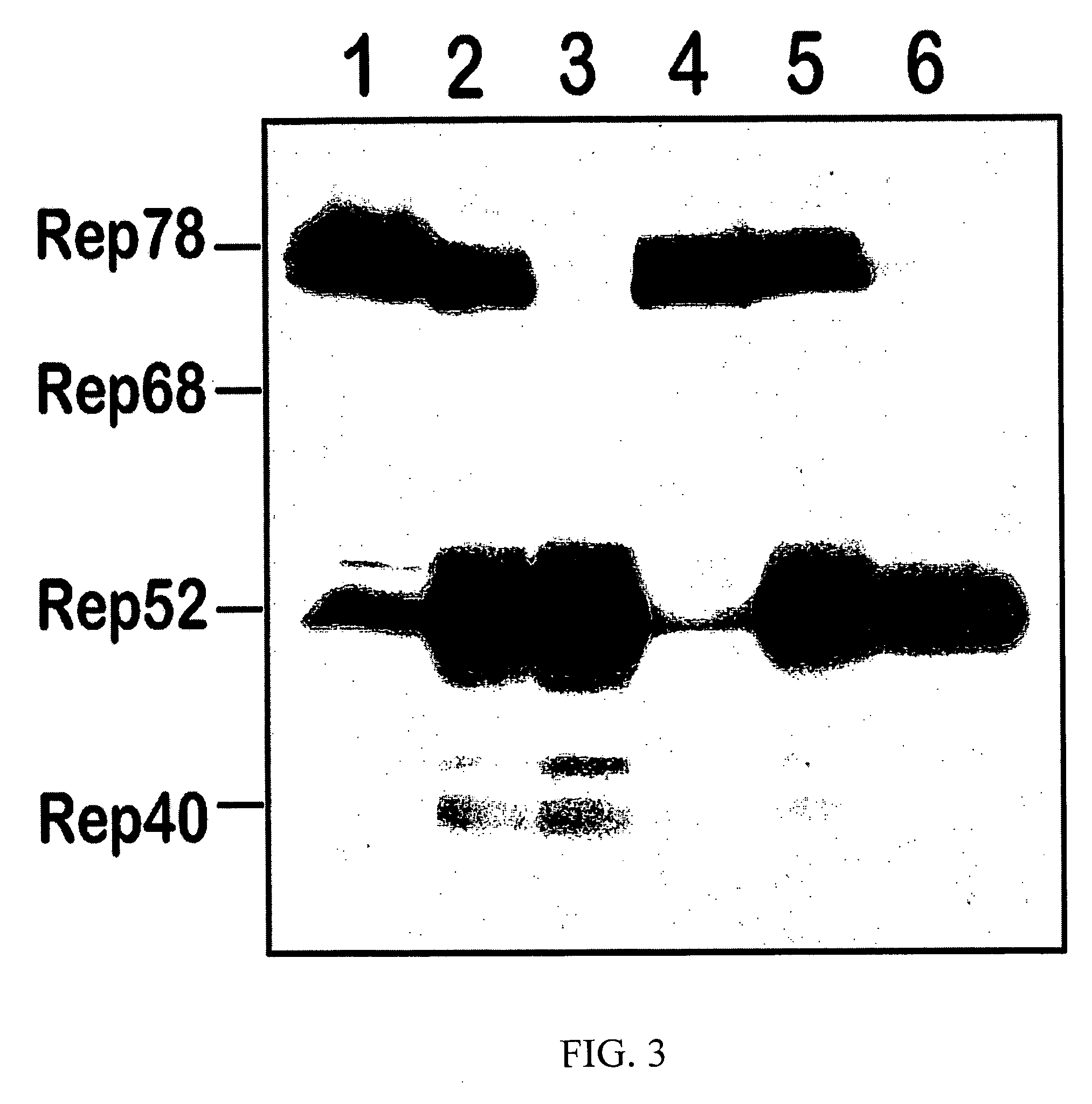
Modified baculovirus expression system for production of pseudotyped rAAV vector
InactiveUS20060166363A1Reduce lossesEfficient and high productionAnimal cellsSugar derivativesBaculovirus expressionHelper virus
The invention provides modifications to a baculovirus-based recombinant adeno associated virus (AAV) system including enhancement of the helper virus stability and construction of novel baculovirus vectors for rAAV pseudotyping. The modified system extends the flexibility of rAAV vector production and promotes the utility of AAV as, a clinically applicable gene therapy vector.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Porcine epidemic diarrhea recombinant baculovirus gene engineering subunit vaccine, preparation method and application thereof
InactiveCN103585625AImprove abilitiesTargetedMicroorganism based processesAntiviralsGenetic engineeringTGE VACCINE
The invention belongs to the technical field of biological vaccine preparation, and particularly relates to a porcine epidemic diarrhea (PED) recombinant baculovirus gene engineering subunit vaccine, a preparation method and an application thereof. According to the present invention, S1 gene and M gene of the current new PEDV epidemic strain are selected as reference sequences, a baculovirus expression system is adopted to express S1 protein or partial S1 protein and M protein, and the obtained recombinant protein is prepared into a subunit vaccine for effectively controlling PED occurrence; with the PED recombinant baculovirus gene engineering subunit vaccine produced by using the method, the defect of the current PEDV traditional vaccine is solved; and the PED recombinant baculovirus gene engineering subunit vaccine can be used for prevention and treatment of PEDV infections and related diseases caused by PEDV, and can further be used for preparation of coating antigen of PEDV detection antibody ELISA kits.
Owner:SOUTH CHINA AGRI UNIV
Middle east and respiratory syndrome coronavirus antibody and preparation method thereof
ActiveCN103864924APrevent intrusionHigh affinityImmunoglobulins against virusesAntibody ingredientsMiddle East respiratory syndromeBaculovirus expression vector system
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
African swine fever P30 protein recombinant baculovirus expression vector and preparation method thereof
The invention provides African swine fever P30 protein recombinant baculovirus expression vector and a preparation method thereof. The method comprises: amplifying in plasmid PCR-4TOPO-P30 of ASFV (African swine fever virus) P30 full-length gene to obtain P39 gene, linking the amplified P30 gene to a baculovirus vector pFastBac 1 to construct recombinant baculovirus vector pFastBac1-ASFV-P30, converting into competent Escherichia coli cells DH10Bac to obtain recombinant shuttle bacmid rBacmid-ASFV-P30, transfecting to insect cells Sf9 after verification is correct to obtain recombinant baculovirus, and passage amplifying the recombinant baculovirus, linking baculovirus high in titer and containing ASFV P30 gene to High Five insect cells for eukaryotic expression of ASFV P30. The African swine fever P30 protein recombinant baculovirus expression vector is constructed by using the method, a recombinant baculovirus expression system is used to express African swine fever P30 protein in insect cells, and basis is laid for African swine fever ELISA (enzyme-linked-immunosorbent serologic assay) detections.
Owner:QINGDAO AGRI UNIV
Humanized monoclonal antibody and application thereof
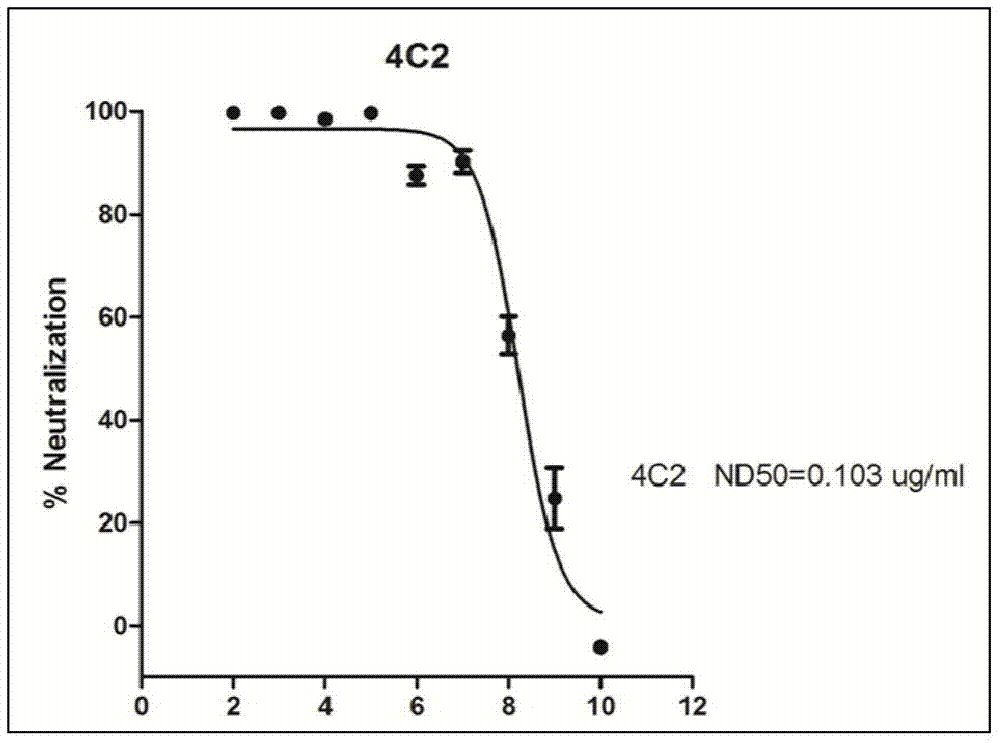
ActiveCN107586335AStrong neutralizing activityImmunoglobulins against virusesAntiviralsBaculovirus expressionHumanized antibody
The present invention discloses a humanized monoclonal antibody and an application thereof, belonging to the technical field of medicine. In the invention, the humanized transformation is carried outon a rat monoclonal antibody 2A10G6, the rat monoclonal antibody 2A10G6 is expressed by baculovirus, and the humanized antibody h2A10G6 is obtained. The h2A10G6 antibody of the present invention has high affinity and neutralization activity against yellow fever virus, dengue fever and West Nile virus, and can be applied to clinical treatment and prevention of yellow fever virus, dengue virus and West Nile virus.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI +1
Method for preparing rabies virus antigen
ActiveCN101307317AReduce consumptionImprove securityAntiviralsDepsipeptidesAntigenBaculovirus expression
The invention provides a method for making rabies virus antigen. The method comprises the following steps that: the antigen gene of rabies virus or the combined expression combination of the antigen gene is respectively cloned in a baculovirus carrier so as to obtain a transfer expression carrier; the transfer expression carrier and baculovirus undergo cotransfection so as to carry out homologous recombination or transposition, thereby obtaining recombined baculovirus; the recombined baculovirus is used to infect insect host and cell; the infected insect host is cultured to express corresponding rabies antigen; and the expressed antigen is ingathered and purified so as to obtain rabies virus antigen. The method adopts a baculovirus expression system to make safe and efficient rabies virus antigen in a domestic silkworm bioreactor; moreover, due to having extremely high safety, the made antigen can be directly used to make injection vaccine and oral vaccine used for animal immunization. The method can substantially reduce the production cost of rabies virus antigen, and has the advantages of safety, high efficiency, less energy consumption and low cost, etc.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI +1
Method for producing porcine parvovirus antigen and its product
ActiveCN102382845AImprove immune activityReduce manufacturing costGenetic material ingredientsAntiviralsAntigenBaculovirus expression
The invention discloses a method for producing a porcine parvovirus antigen and its product. The method comprises the following steps: porcine parvovirus capsid protein VP2 gene or optimized VP2 gene is cloned in a baculovirus carrier so as to obtain a transfer expression carrier; the constructed transfer expression carrier and baculovirus DNA are carried out cotransfection to obtain recombined baculovirus; the recombined baculovirus is used to infect insect host and cell; the infected insect host is cultured to express corresponding porcine parvovirus capsid protein; and the expressed antigen is ingathered and purified so as to obtain the porcine parvovirus antigen. The method adopts a baculovirus expression system to make safe and efficient porcine parvovirus antigen capsid particles in a domestic silkworm bioreactor; the prepared purified antigen by the method has high safety, and can be directly produced to vaccines for animal immunity. The method for producing porcine parvovirus antigen has the advantages of high expression efficiency, high immunization activity of the expressed antigen, low production cost, large scale production realization and the like.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Method for preparing foot-and-mouth disease antigen
ActiveCN101121938APromote safe productionReduce consumptionSsRNA viruses positive-senseVirus peptidesAntigenTransfer vector
The invention provides a method for expressing foot-and-mouth disease antigens in insects using recombinant baculoviruses, which includes: cloning different gene combinations of foot-and-mouth disease into baculovirus delivery vectors to construct transfer vectors; using the constructed transfer vectors to transfer Infect the baculovirus and perform DNA recombination to obtain the recombinant baculovirus; infect the insect host with the recombinant baculovirus; culture the infected insect host to express the foot-and-mouth disease antigen; collect and purify the expressed foot-and-mouth disease antigen. The method of the present invention uses a baculovirus expression system to safely and efficiently produce foot-and-mouth disease antigens in a silkworm bioreactor. The prepared antigens are extremely safe and can directly produce vaccines to immunize animals. The method of preparing foot-and-mouth disease antigen of the present invention does not require investment in building a factory, has no three wastes, consumes very little energy such as electricity and water resources, and its production cost is also significantly lower than the traditional method of preparing foot-and-mouth disease antigen. It is safe, efficient, has low energy consumption and low cost. Low and many advantages.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI +1
Genetic engineering subunit vaccine for porcine circovirus as well as preparation method and application of genetic engineering subunit vaccine
PendingCN108619503AReduce virus contentHigh antigen purityViral antigen ingredientsAntiviralsSolubilityEscherichia coli
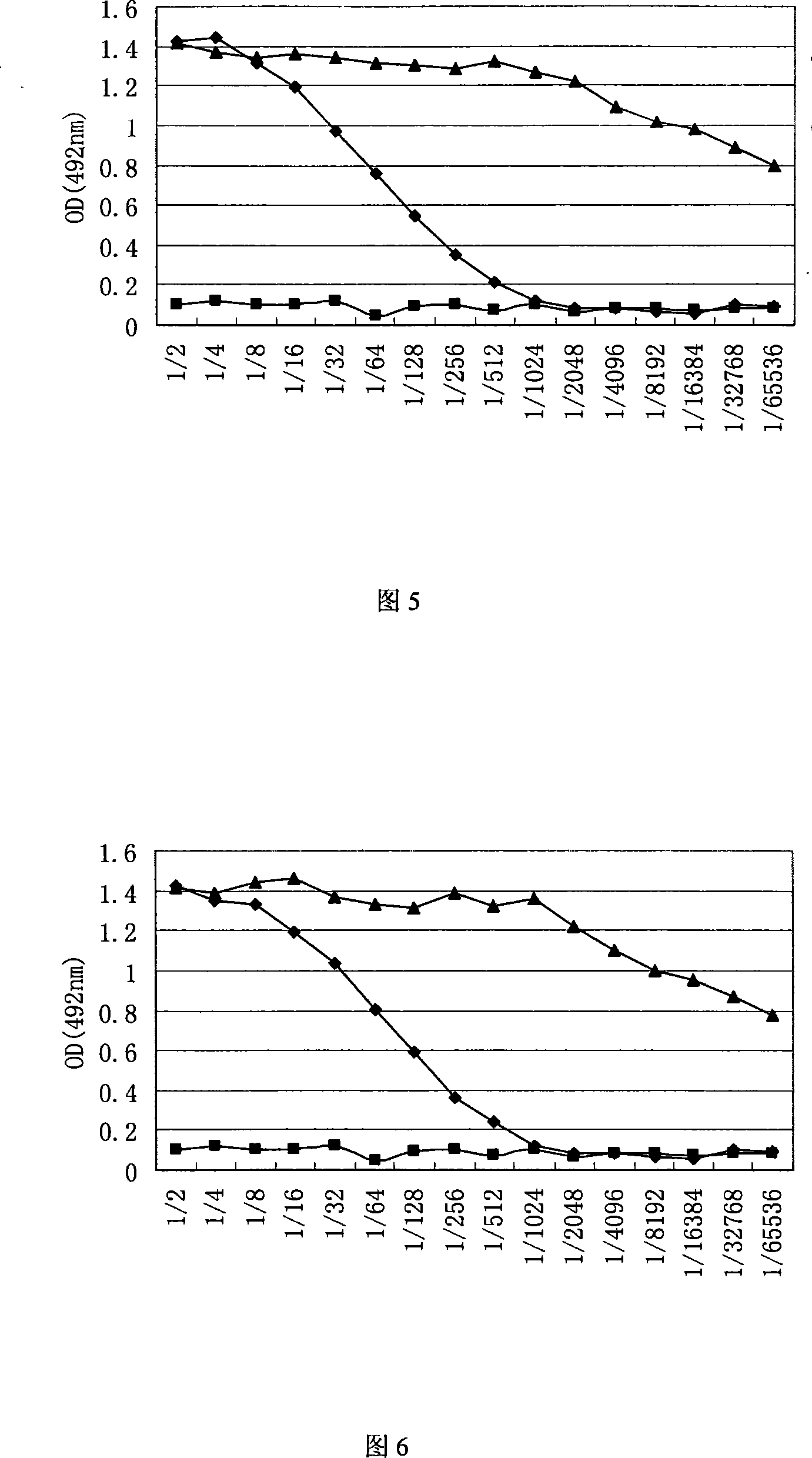
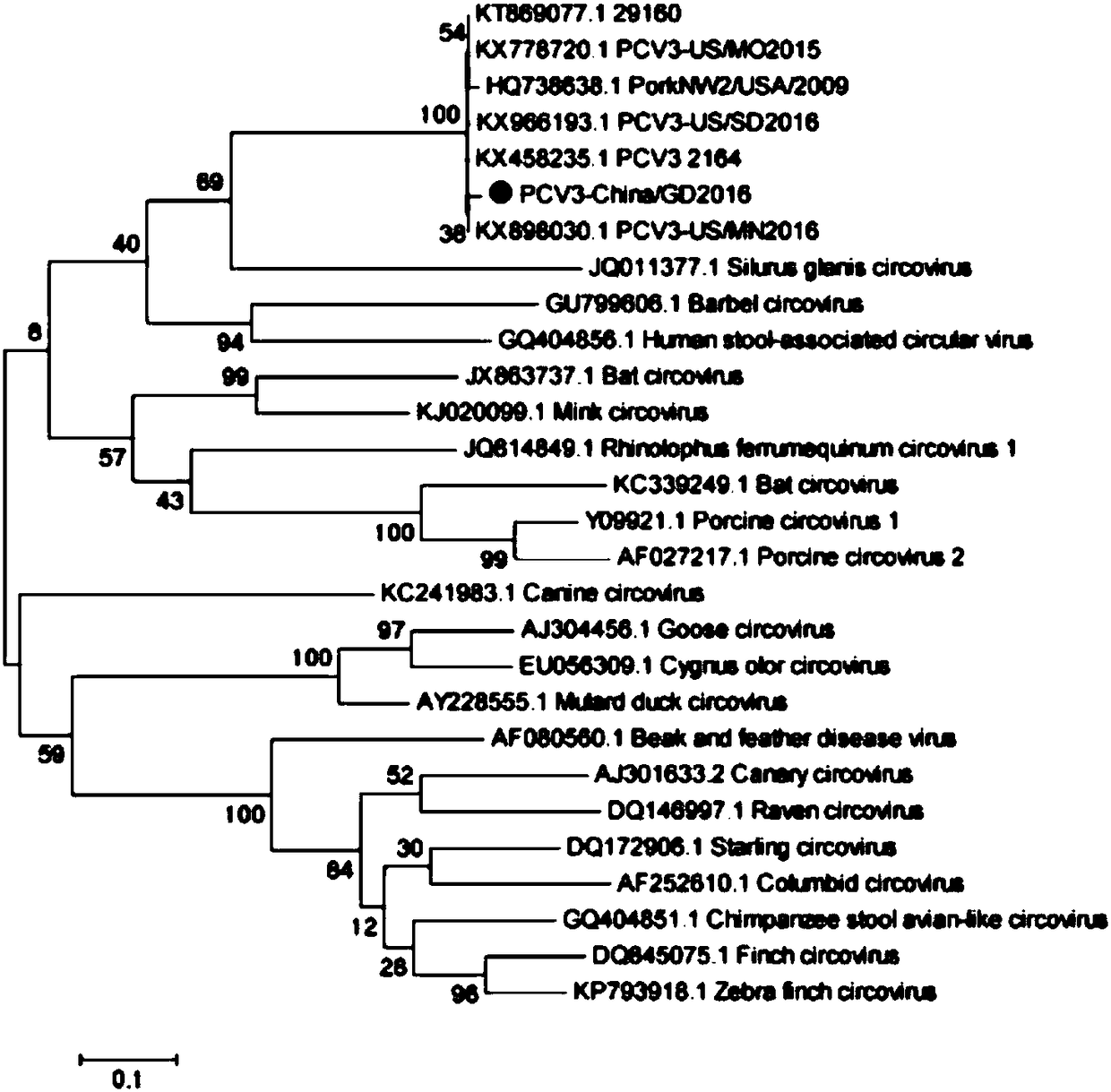
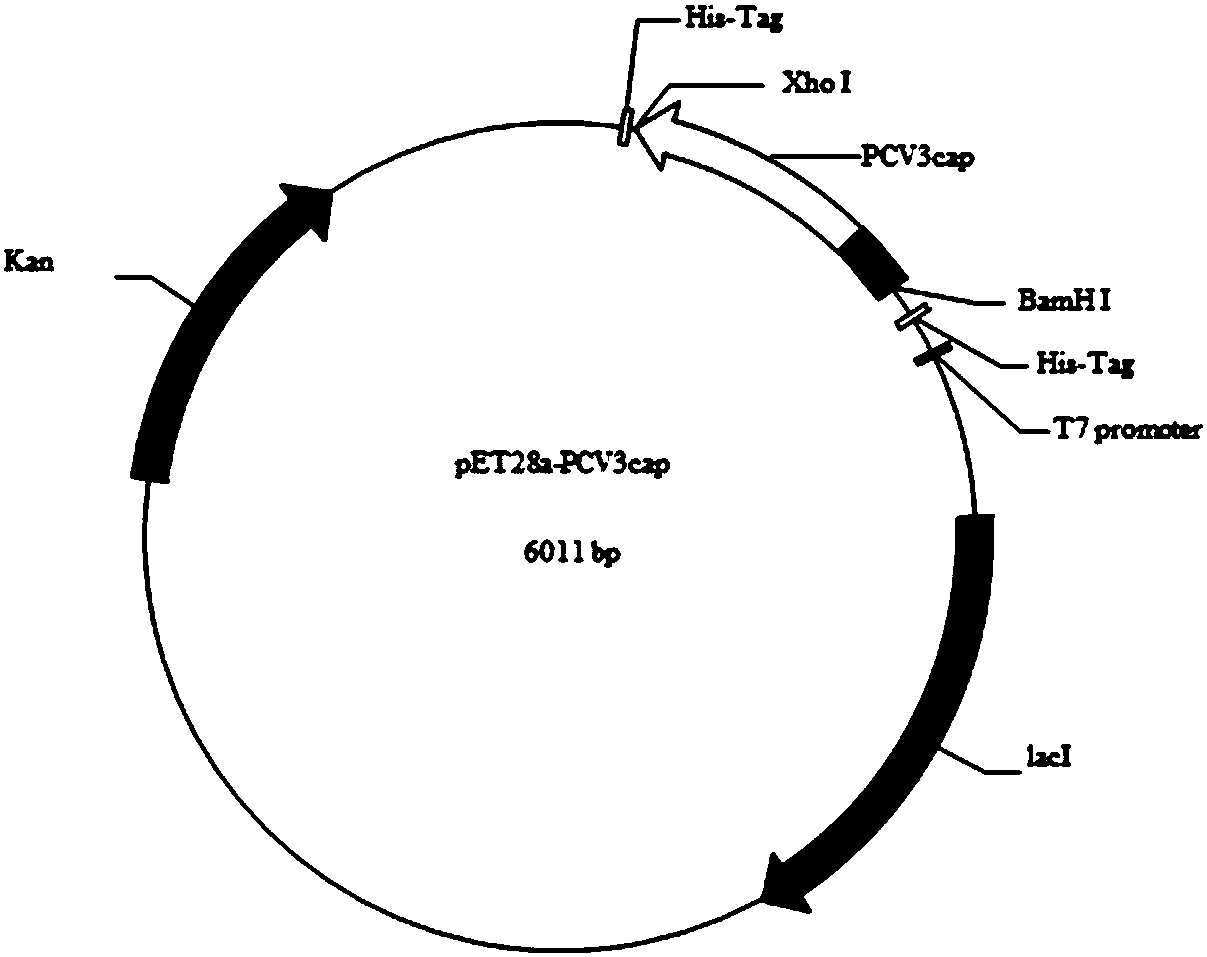
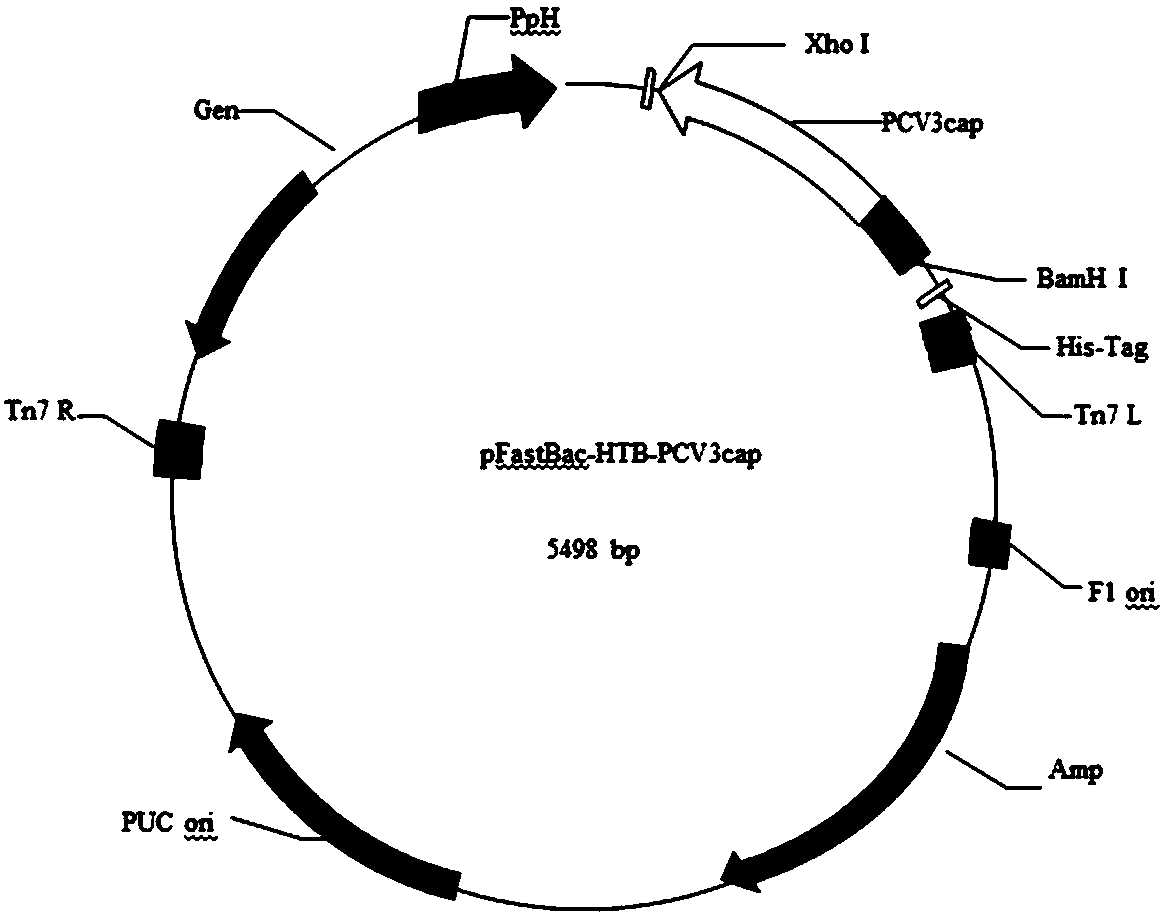
The invention discloses a genetic engineering subunit vaccine for a porcine circovirus as well as a preparation method and application of the genetic engineering subunit vaccine. By cloning the nucleocapsid protein of the novel porcine circovirus 3 (PCV3), a PCV-Cap protein with higher purity is successfully expressed by using an escherichia coli or baculovirus expression system. The subunit vaccine for the PCV3 is successfully developed for the first time by using the PCV-Cap protein; the prepared vaccine is high in antigen purity, good in safety and strong in immunogenicity, and has no pathogenicity to pigs and other animals; the antigen has good solubility in a neutral PH buffer solution; furthermore, the preparation method is simple and low in cost, thus being suitable for large-scaleindustrial production; an effective and powerful means is provided for the prevention and control of novel PCV, and the genetic engineering subunit vaccine has a wide application prospect in the fieldof the prevention and control of the PCV3.
Owner:SOUTH CHINA AGRI UNIV
Recombination expression and application of Chinese prawn antibacterial peptide gene
InactiveCN1459506AMicrobiological testing/measurementPeptide preparation methodsBiotechnologyEscherichia coli
The recombination, expression and application of Chinese prawn's antibacterial peptide gene are disclosed. Its recombination expression method includes configuring recombinant carrier (pET series, pGEX-4T series, pPIC series, pAO815, and baculovirus expression carrier), transferring the carrier to colibacillus and yeast and transfecting insect cells, screening engineered bacterial strain or cell, and inducing expression. The expressed product can be used to prepare feed additive, antistaling agent, cosmetics and medical products.
Owner:SHANDONG UNIV +1
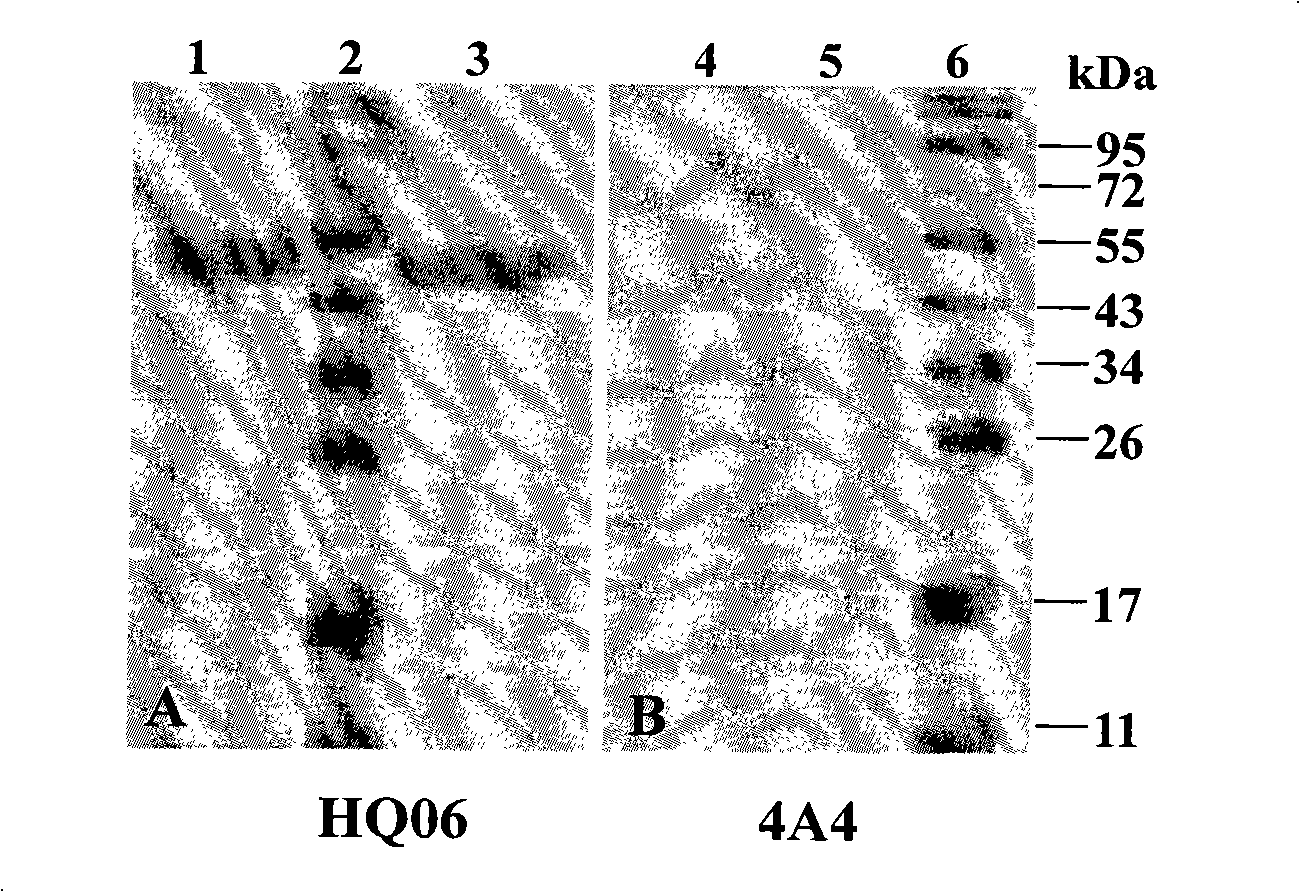
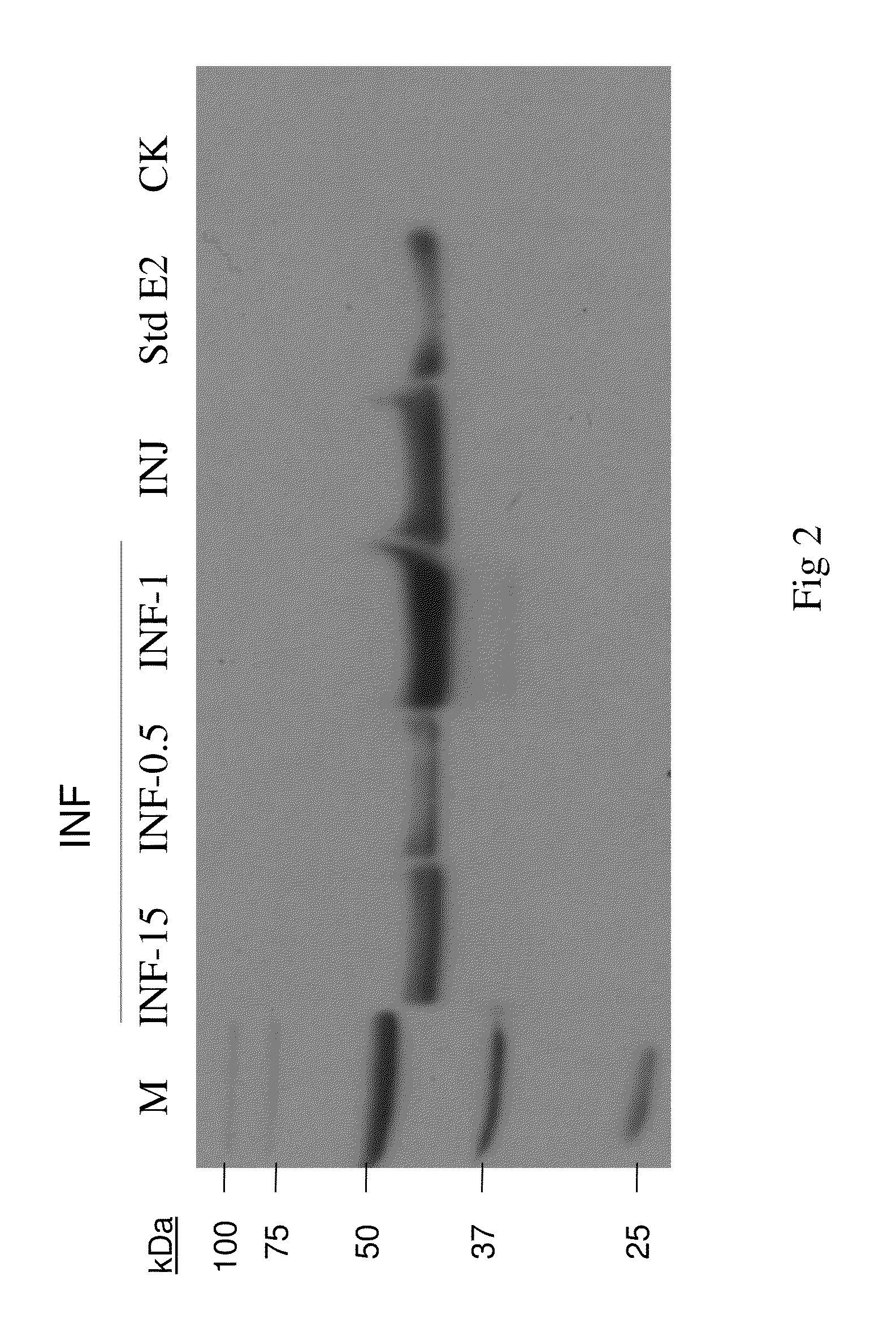
Monoclone antibody of swine fever virus resistant wild strain E2 protein, preparation method and application thereof
InactiveCN101294147ANeutralizing activityImmunoglobulins against virusesTissue cultureSwine Fever VirusCholera
The invention discloses a monoclonal antibody against virulent strain E2 protein of classical swine fever virus and a hybridoma cell strain secreting the monoclonal antibody. The hybridoma cell strain is obtained by using hog cholera lapinized virus vaccine strain E2 protein expressed by Baculovirus as tolerogen, selecting Shimen strain E2 protein as immunogen, immunizing mouse by cyclophosphamide immunosuppression method, carrying out cell fusion, and sieving hybridoma cell strain capable of stably secreting monoclonal antibody against E2 protein. The monoclonal antibody can react with Shimen strain and can produce specific reaction with virulent strain of classical swine fever viruses of 1.1, 2.1, 2.2 and 2.3 gene sub-groups. The monoclonal antibody has neutralization activity and does not react with hog cholera lapinized virus vaccine strain, so that the monoclonal antibody can be used for differentiating virulent strain of classical swine fever virus and hog cholera lapinized virus vaccine strain, which establishes the foundation for establishing a method for differentiating wild virus infection of classical swine fever and vaccine immunity and for researching the molecular difference between CSFV virulent strain and mild strain.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
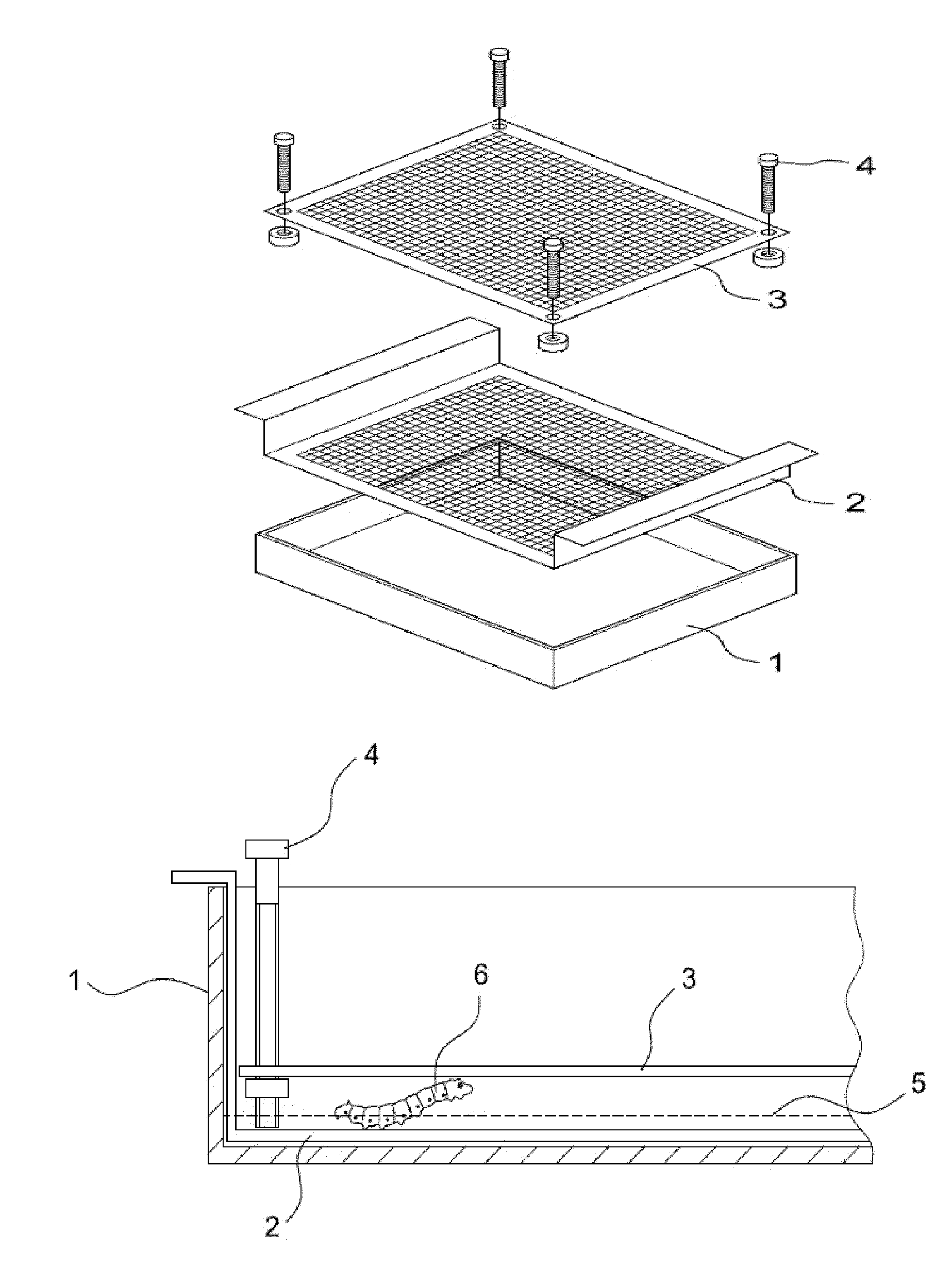
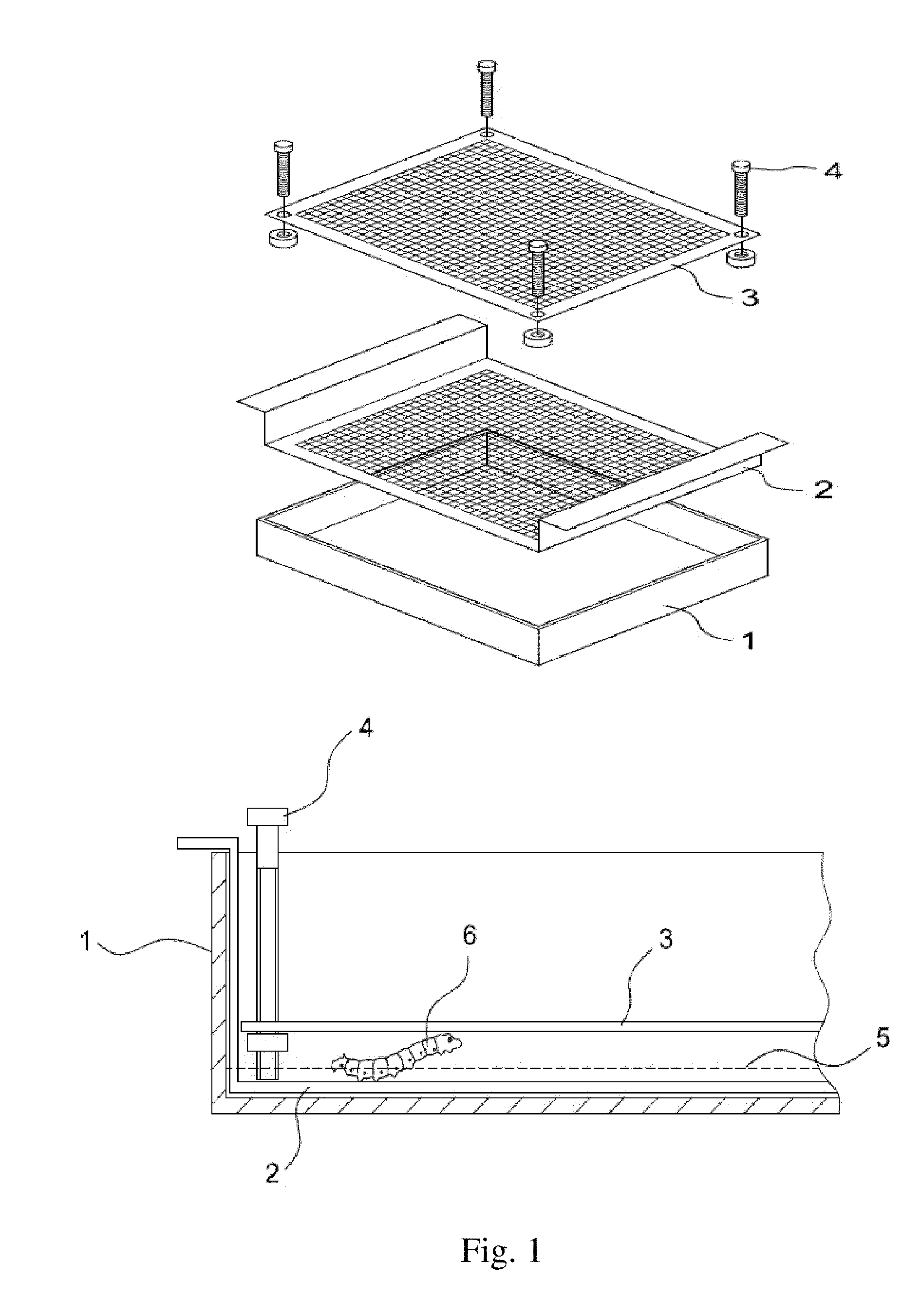
Insect Infection Method for Production of Proteins
The present invention provides an insect infection method for use in the production of a protein with a baculovirus expression vector in the insect, the method comprising the steps of:(g) providing a plurality of insect larvae or pupae;(h) providing a solution comprising a wild type baculovirus or a baculovirus expression vector having a desired gene encoding a protein;(i) stressing the insect larvae or pupae;(j) soaking the insect larvae or pupae in the solution for an appropriate time so that they are infected with the wild type baculovirus or the baculovirus expression vector; and(k) incubating the infected larvae or pupae for production of the protein; and(l) harvesting the protein.The method can treat a great quantity of larvae simultaneously, achieve batch infection of larvae or pupae, save manpower and effectively infect larvae or pupae at a high infection rate.
Owner:MIAOLI DISTRICT AGRI RES & EXTENSION STATION COUNCIL AGRI EXECUTIVE YUAN
Hog cholera virus inhibition ELISA antibody detection kit and application thereof
ActiveCN104483490AAchieve mass productionIncreased sensitivitySsRNA viruses positive-senseVirus peptidesPositive controlHorse radish peroxidase
The invention discloses a hog cholera virus inhibition ELISA antibody detection kit and application thereof. The kit comprises hog cholera virus E2 protein which is expressed by virtue of a baculovirus expression system and a cell suspension culture process; the hog cholera virus E2 protein is purified to serve as a coated plate antigen and an immunogen to prepare a monoclonal antibody of the hog cholera virus E2 protein, and horse radish peroxidase (a hybridoma cell strain 4A7 CCTCC NO.C2014229) is marked. The kit further comprises an antigen coated plate, a positive control, a negative control, sample diluent, a scrubbing solution, a developing solution A, a developing solution B and a stop solution. According to the application of the hog cholera virus inhibition ELISA antibody detection kit in the detection of the hog cholera virus antibody, the kit is used for detecting other positive serums except hog cholera viruses, the cross reaction is avoided, the specificity is strong, the sensitivity is high, the detection time is short, and the repeatability is good; compared with an import kit, the detection coincidence rate reaches above 95%.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Anti-chicken infectious bursal disease recombinant protein subunit vaccine
ActiveCN103360498AEnhance immune responseImprove protectionViral antigen ingredientsAntiviralsFlagellinVaccine Immunogenicity
The invention provides an anti-chicken infectious bursal disease (IBD) recombinant protein subunit vaccine. The vaccine is a fusion protein having high immunogenicity of Salmonella typhimurium flagellin and an infectious bursal disease virus (VP2). The above flagellin + VP2 fusion protein is obtained through the expression of a recombinant baculovirus containing a flagellin + VP2 gene by utilizing a Bac-to-Bac baculovirus expression system. The recombinant baculovirus obtained through the system has a short period, and the expressed flagellin + VP2 fusion protein has high immune protection force.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES
Production of papillomavirus capsid protein and virus-like particles
ActiveUS8062642B1High expressionPeptide/protein ingredientsViral antigen ingredientsBaculovirus expressionVirus-like particle
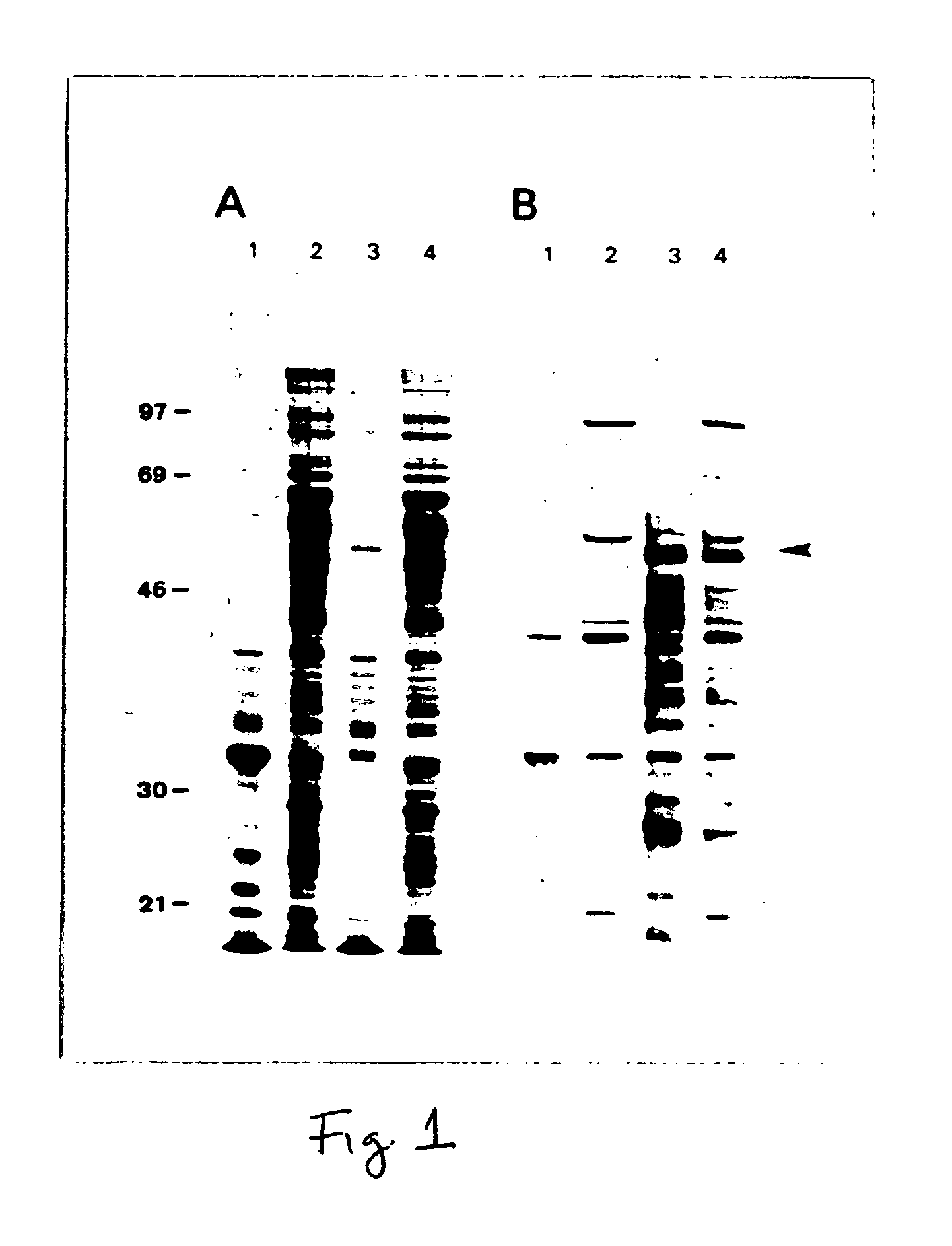
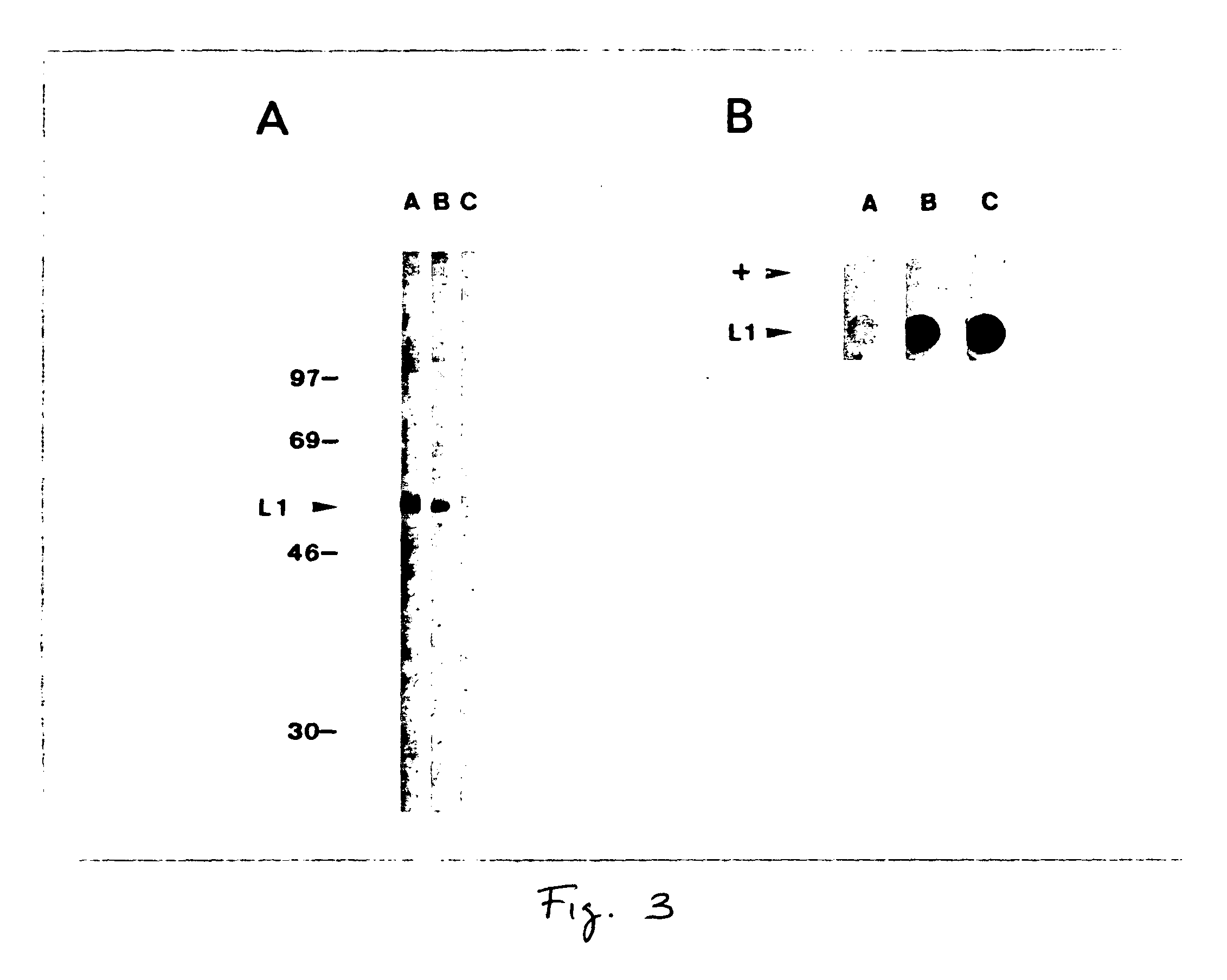
The present invention is directed to a method of expressing the papillomavirus capsid protein coding sequence in a cell using an expression system under conditions facilitating expression of the protein in the cell.In another aspect of the invention, it has been discovered that virus-like particle(s) (VLPs), fragment(s), capsomer(s) or portion(s) thereof are formed from the papillomavirus capsid protein. It was further discovered that the virus-like particle(s) comprises antigenic characteristics similar to those of native infectious papillomavirus particles.In an embodiment of the invention, there is provided a method of expressing the L1 major capsid protein of human papillomavirus type-11 (HPV-11) in Sf-9 insect cells using the baculovirus expression system, and the production of HPV-11 virus-like particles.
Owner:UNIVERSITY OF ROCHESTER
Antineoplastic invasion transfer function of snake venom metalloprotease inhibitors BJ46a and uses thereof
InactiveCN101429525AInhibitory activityObvious anti-tumor invasion and metastasis effectPeptide/protein ingredientsGenetic material ingredientsAbnormal tissue growthIn vivo
The invention discloses a snake venom metal protease inhibitor BJ46a capable of resisting attack and transference of tumor and application thereof, and belongs to the field of medical organism. In the invention, according to the BJ46a gene sequence (AF294836) in GenBank, the BJ46a full gene is designed and synthesized. An expression vector cloned to baculovirus can generate the recombined BJ46a protein capable of inhibiting the activity of the substrate metal protease in Sf9 insect cells, and in vivo and in vitro experiments show that the BJ46a protein has the functions of resisting the attack and transference of melanoma cells B16. The inhibitor adopts the genetic transfection technique, and can establish B16 / pcDNA3.1HisC-BJ46a cell strains with stable transfection, and the in vivo and in vitro experiments prove that the BJ46a can inhibit the attack and transference of B16 cells at the gene level. The inhibitor is applied to preventing and treating the attack and transference of tumor, and has great application prospect.
Owner:FUJIAN MEDICAL UNIV
GPV (gosling plague virus) subunit vaccine as well as preparation method and application thereof
InactiveCN107551267AGuaranteed immune effectImprove securityAntiviralsAntibody medical ingredientsBaculovirus expressionAntigen
The invention provides a preparation method of a GPV (gosling plague virus) subunit vaccine. The preparation method comprises steps as follows: a GPV VP3 protein complete gene is cloned; a recombinantbaculovirus of the antigen protein is constructed by utilizing an insect cell-baculovirus expression system (BAC TO BAC) and the recombinant virus efficiently expresses the GPV VP3 antigen protein ininsect cells Sf9; after extraction, purification and BEI inactivation, an adjuvant is added for emulsification, and the vaccine is prepared. The preparation method is simple, a large amount of the GPV VP3 protein can be prepared, short time is consumed, expression quantity is high, production cost is greatly reduced, and large-scale production is facilitated. The GPV subunit vaccine containing the VP3 protein prepared with the method is good in immunization effect, low in immunizing dose and high in safety and can effectively prevent GPV infection.
Owner:扬州优邦生物药品有限公司
ELISA kit of IBDV antibodies, test method and effective antibody titer determination method
The invention discloses an ELISA kit of IBDV antibodies, a test method and an effective antibody titer determination method. The ELISA kit comprises (1) an enzyme-labeled board coated with IBDV antigens and (2) goat anti-chicken Ig Y diluted according to the ratio of 1:3,000 and marked with HRP, wherein the enzyme-labeled board coated with the IBDV antigens is made by diluting the concentration ofthe IBDV antigens to 10ng / pore and then coating corresponding pores in a reaction board with diluent, the IBDV antigens are rVP2 proteins prepared through a baculovirus expression system, and a detection result is judged according to an S / P value obtained through an ELISA detection method. When the detection result is positive according to the S / P value obtained through the ELISA detection method, it is indicated that effective antibodies are in a high level and can neutralize IBDVs, and therefore it is needless to perform vaccine immunization again; and when the detection result is negativeaccording to the S / P value, it is indicated that the antibodies are in a low level without protective force and are prone to being infected by viruses, and therefore it is needed to perform vaccine immunization again. As a result, the established ELISA kit has a high antibody positivity detection rate and can guide vaccine immunization on production to reduce economic losses.
Owner:XINXIANG UNIV
Antigenic epitope simulative peptide of classical swine fever virus (CSFV) E2 protein, and preparation method and application thereof
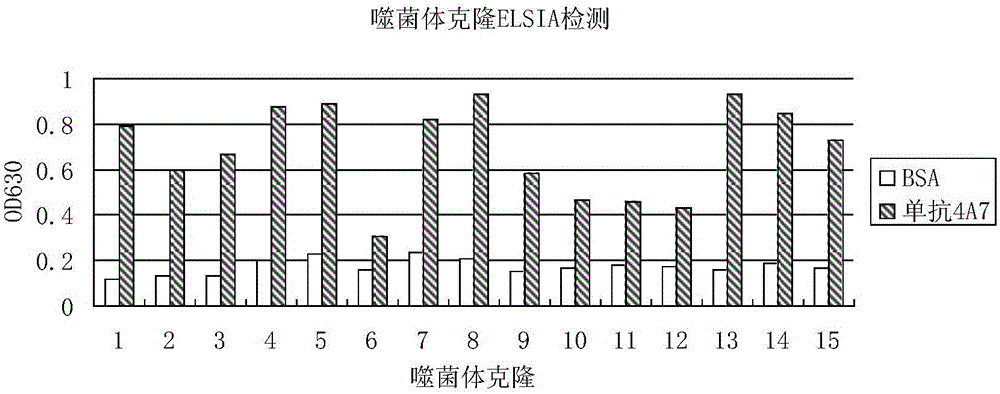
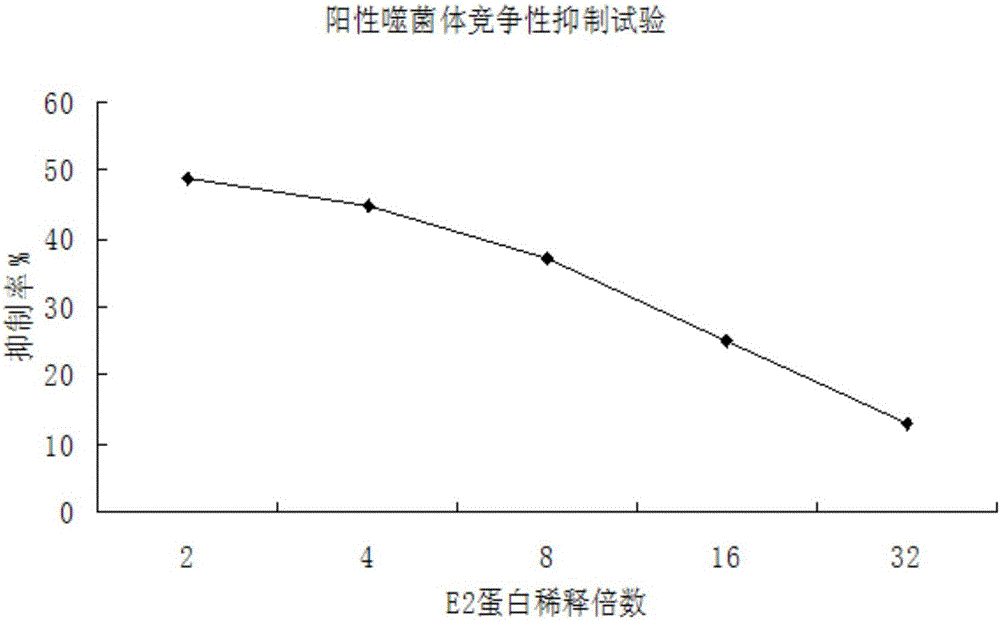
InactiveCN106188250ACreate pollutionEnsure processing safetySsRNA viruses positive-senseVirus peptidesChemical synthesisBALB/c
The invention discloses an antigenic epitope simulative peptide of classical swine fever virus (CSFV) E2 protein, and a preparation method and an application thereof. The preparation method includes the steps of: A) immunizing a BALB / C mouse with the CSFV E2 protein that is expressed by baculovirus to prepare monoclonal antibodies aiming to the E2 protein, and screening and identifying the monoclonal antibodies to obtain a monoclonal antibody 4A7 that has a blocking effect; B) purifying the monoclonal antibody 4A7 and coating an ELISA plate with the monoclonal antibody, and performing screening with a phage 12 peptide library, wherein the screening stress is increased round by round, thereby finally obtaining positive clone having high affinity; and C) performing DNA sequencing and sequence comparison analysis, which prove that the positive clone product is not completely consistent with the sequence of the original CSFV E2 protein; and D) performing chemical synthesis to the twelve amino acid sequences that are screened before according to a peptide synthetic technology. The invention also provides an application of the simulative peptide of the CSFV E2 protein in preparation of an ELISA antibody medicine detection kit for treating or preventing swine fever. The synthesized short peptide is used for detection of the swine fever antibody as a coating antigen. The simulative peptide has low cost and high purity in large-scale production.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Chimeric antigens for eliciting an immune response
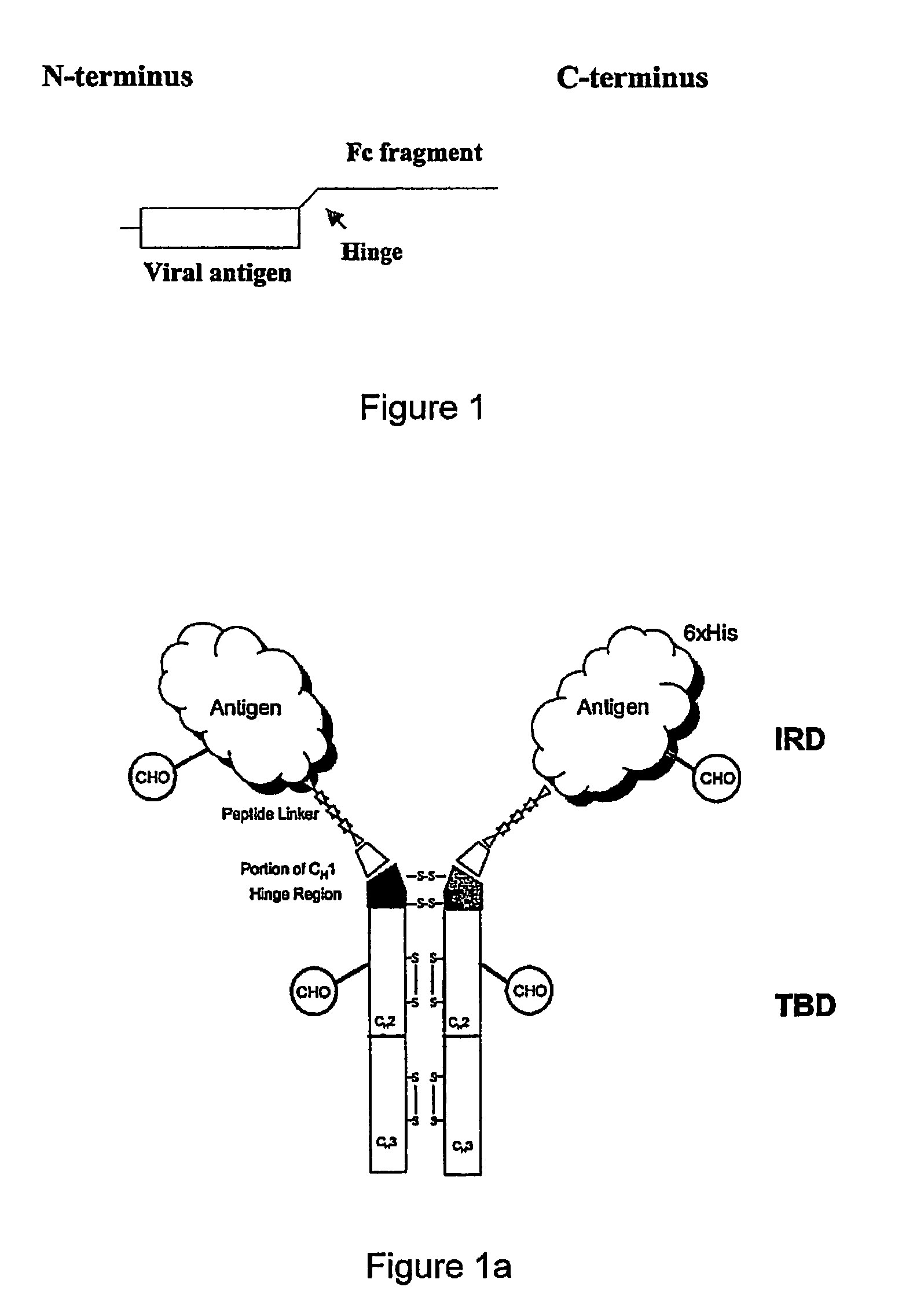
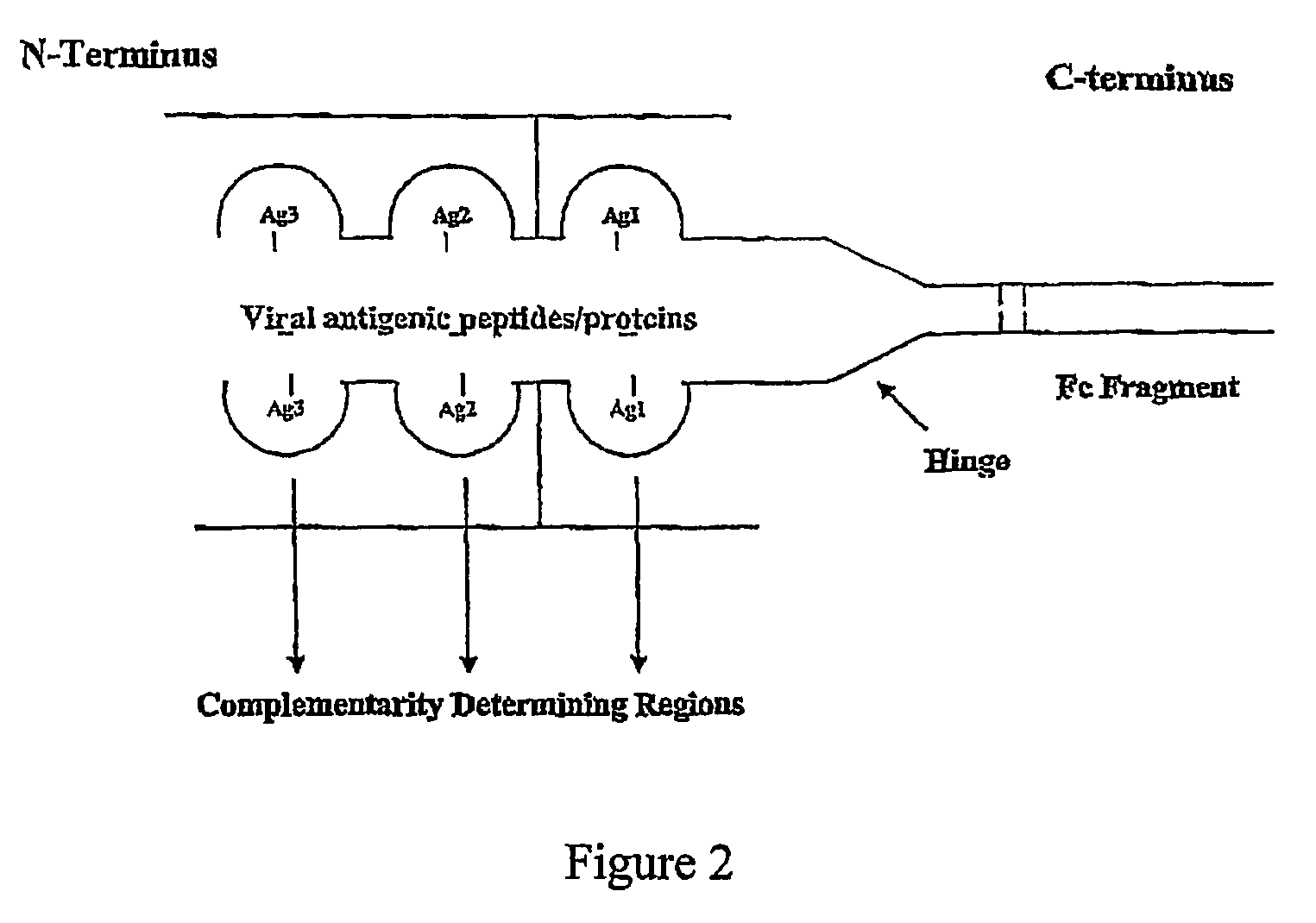
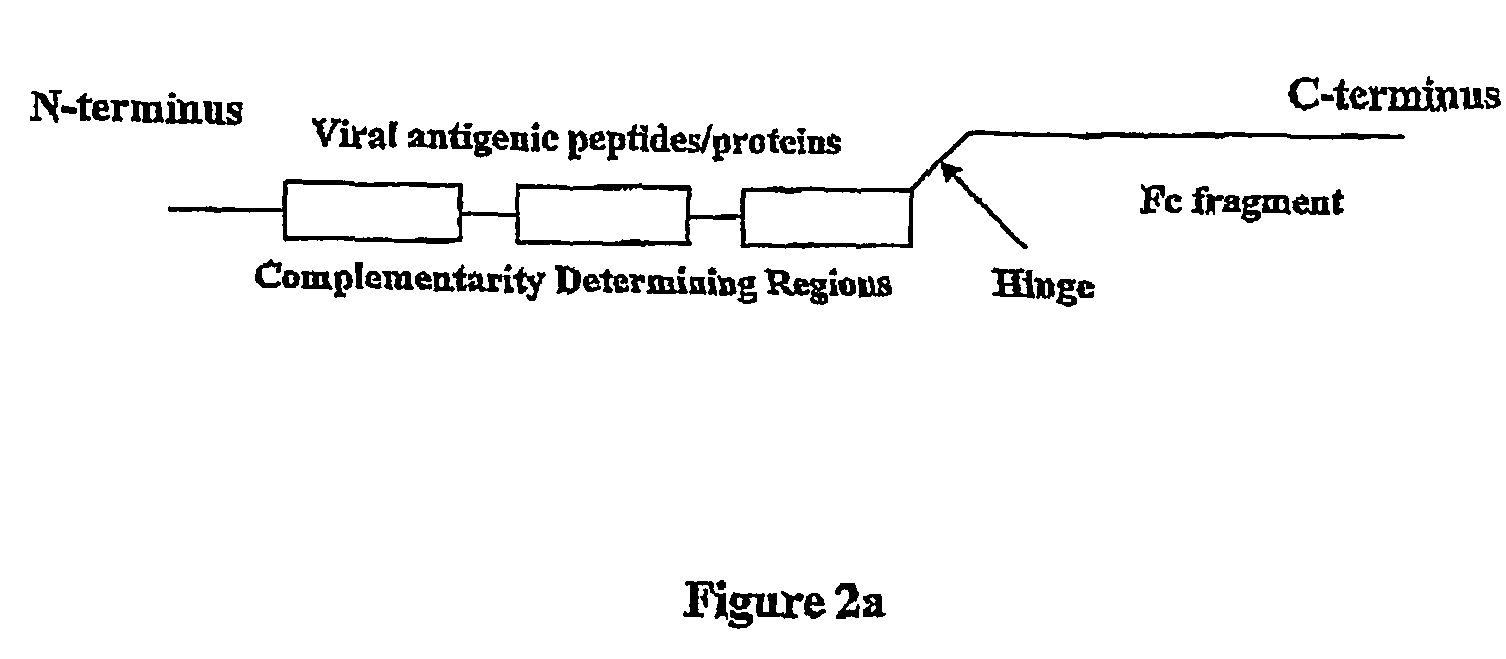
ActiveUS8029803B2Effective presentationImprove efficiencyBiocideSsRNA viruses positive-senseMHC class IHepatitis B immunization
Disclosed herein are the nucleotide sequences, deduced amino acid sequences as well as methods and compositions necessary to elicit immune responses against chronic Hepatitis B infections in animals and humans. Immune response is enhanced by fusing relevant viral antigens with xenotypic immunoglobulin heavy chain region through a peptide linker and producing the fusion proteins in Baculovirus expression system to incorporate high mannose glycosylation. By virtue of the antibody component, the fusion proteins bind to Fc receptors on the surface of antigen presenting cells, are taken up, processed and derived peptides are presented on MHC Class I, which elicit a CTL (Th1) response. In a similar fashion, due to cross priming and presentation on MHC Class II, will elicit a humoral (Th2) response. In addition, disclosed are the methods of cloning, expression and production of the fusion proteins.
Owner:KAIMI BIOMEDICINE (CHENGDU) CO LTD
Novel antibody ELISA kit for porcine epidemic diarrhea
InactiveCN106950369AImprove stabilityImprove featuresBiological material analysisAntigenPositive control
The present invention discloses a novel antibody ELISA kit for porcine epidemic diarrhea, wherein the novel antibody ELISA kit comprises an enzyme label plate coated with porcine epidemic diarrhea antigen, a negative control serum, a positive control serum, a 20X concentrated lotion, an enzyme labeled antigen, a substrate buffer liquid A, a substrate liquid B, and a terminating liquid. According to the present invention, the PEDV-S protein obtained by the baculovirus expression system is used as the coating antigen, and is labeled with horseradish peroxidase through a sodium iodate method to obtain the enzyme labeled antigen; and the novel antibody ELISA kit developed based on the enzyme labeled antigen can detect the IgG in the pig serum and can further detect the IgA in the colostrum, is firstly created in domestic and foreign, has advantages of good specificity, good sensitivity, short detection time, long storage time and good stability, can simultaneously detect a large number of samples, and provides important practical significance for the solving of the detection of the porcine epidemic diarrhea virus antibody in the large-batch pig serum samples.
Owner:SOUTH CHINA AGRI UNIV
Dual-host recombination rhabdovirus expression vector and construction method and application thereof
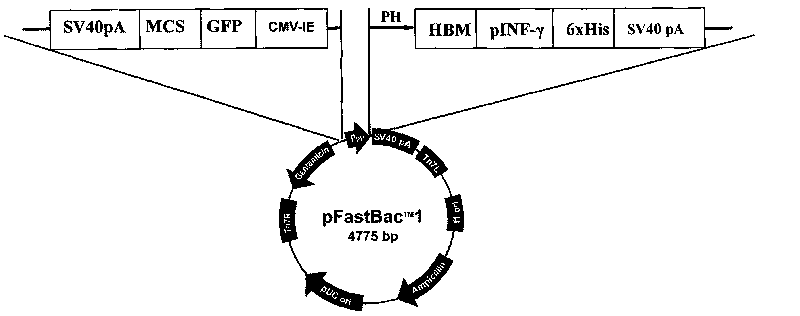
InactiveCN101724651ASave resourcesLow costGenetic material ingredientsGenetic engineeringAgricultural scienceShuttle vector
The invention discloses a dual-host recombination rhabdovirus expression vector and a construction method and application thereof, belonging to the technical field of gene engineering. The dual-host recombination rhabdovirus expression vector is provided with a recognizable Polyhedrin promoter of an insect cell and a recognizable CMV-IE promoter of an animal cell. Dual-host recombination rhabdovirus can be used for preparing a functional protein on the insect cell or can express a target foreign protein on the animal cell and can be used for gene therapy and nonreplication vector vaccine. The construction method of the dual-host recombination rhabdovirus expression vector comprises the following steps of: taking a transfer vector pFas in a Bac-to-Bac expression system as a base; introducing a CMV-IE promoter expression kit on the upstream of the Polyhedrin promoter; constructing the transfer vector; transforming a DH10Bac competent cell; recombining a dual-host Bacmid shuttle vector; and then transfecting insect cells in a logarithmic phase to obtain the dual-host recombination rhabdovirus expression vector.
Owner:HENAN AGRICULTURAL UNIVERSITY
Recombinant limulus three-factor reagent and method for detecting endotoxin with same
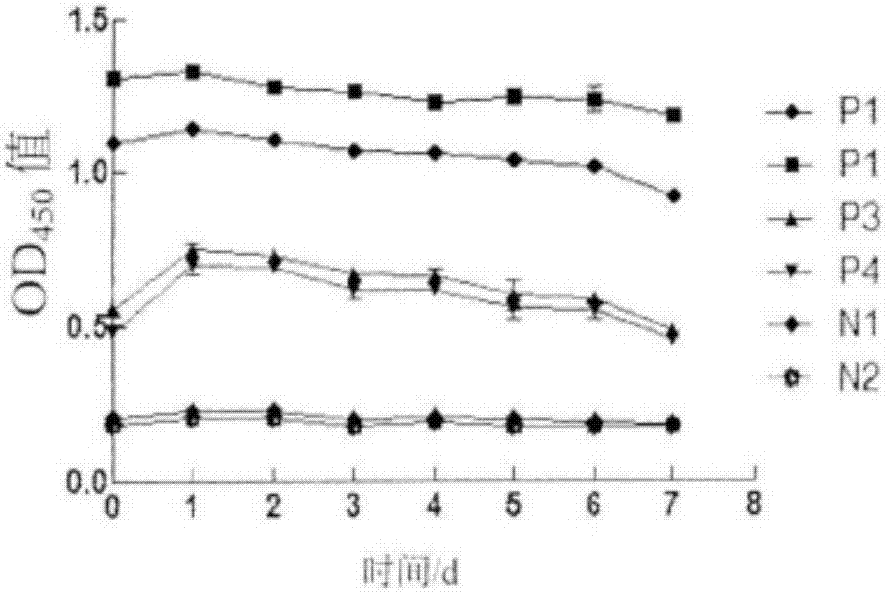
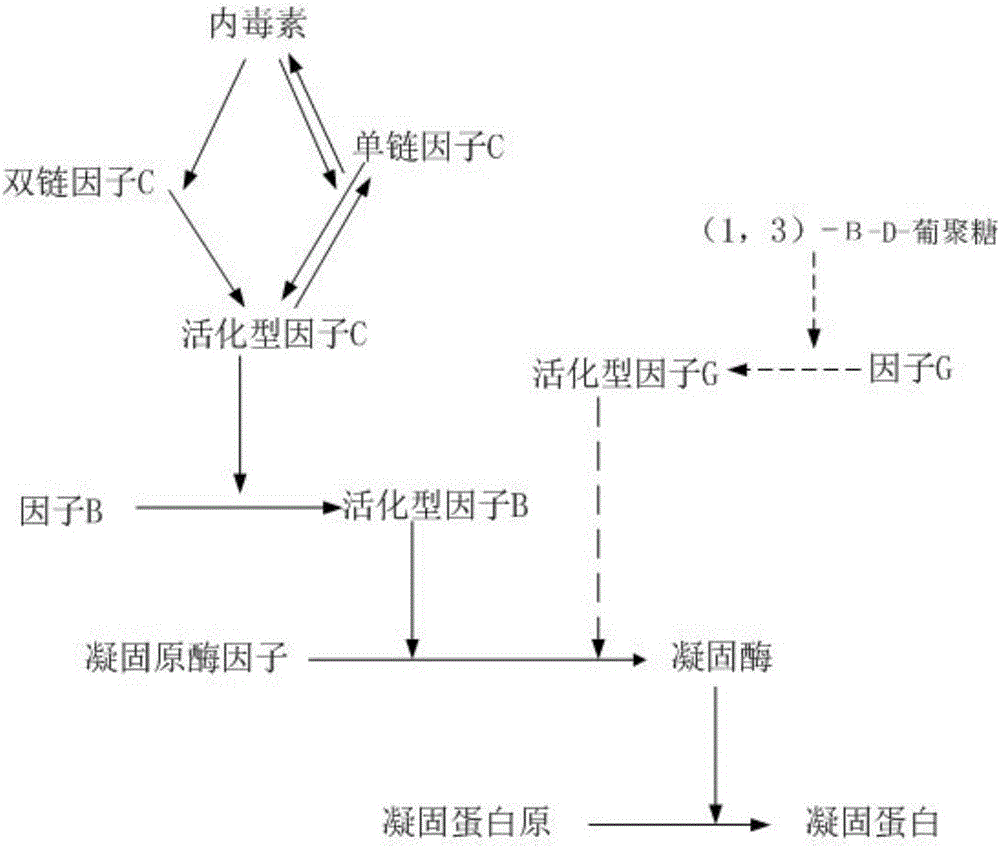
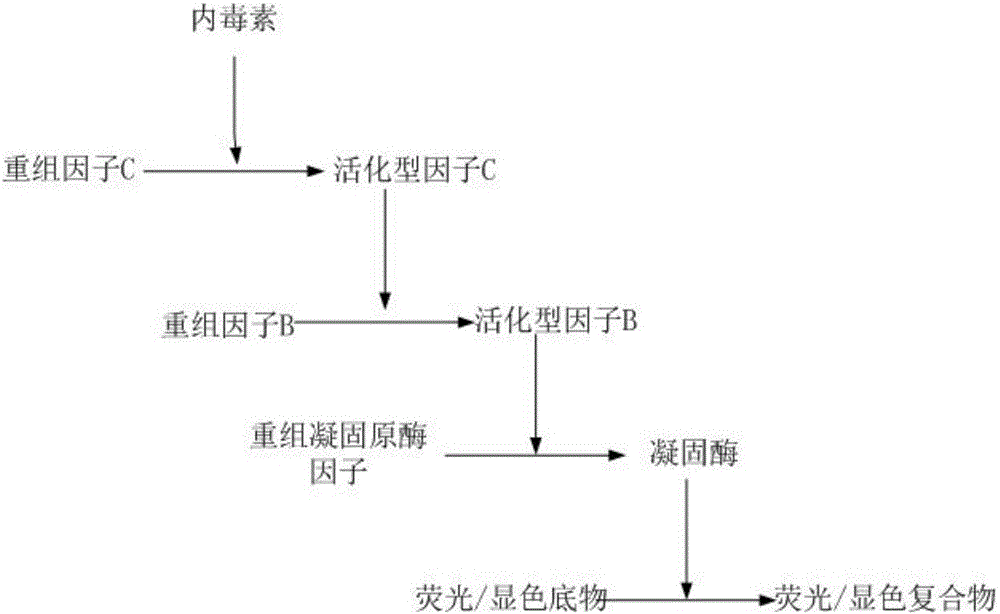
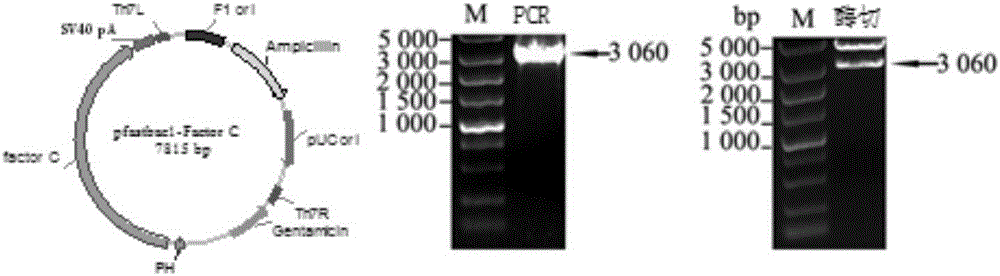
ActiveCN105866080AEfficient and sensitive detectionSolve the problem of a sharp decrease in the number ofFluorescence/phosphorescenceBacteroidesLimulus factor C
The invention discloses a recombinant limulus three-factor reagent which comprises the following components: endotoxin-free water, a heat-resource-free Tris buffer liquid, Tween-20, a fluorescent substrate, a recombinant limulus factor C, a recombinant limulus factor B and a recombinant limulus proclotting enzyme factor. The invention provides the recombinant limulus three-factor reagent and a method for detecting endotoxin with the same, three recombinant limulus factors (the proclotting enzyme factor, the recombinant limulus factor B and the recombinant limulus factor C) can be expressed by using an insect baculovirus expression system, the reagent can be used for detecting bacterial endotoxin, and by adopting the method, bypass interference of a factor G can be eliminated, so that the possibility of false positive caused by glucan is effectively avoided; moreover, the defects that the number of limulus is limited and different batches of limulus reagents are not stable are avoided, and thus the reagent can be used as a novel endotoxin detection reagent.
Owner:XIAMEN BIOENDO TECH CO LTD
Establishment of protein secreted expression vector and application of same
The invention provides a baculovirus expression vector capable of realizing secreted expression of protein and a method for establishing the same, as well as a method for producing protein by using the vector.
Owner:INST OF PATHOGEN BIOLOGY CHINESE ACADEMY OF MEDICAL SCI
Novel water soluble composite immune adjuvant and porcine circovirus disease vaccine
ActiveCN107375922AGood immune protectionStrong immune adjuvant effectViral antigen ingredientsAntiviralsDiseaseBaculovirus expression
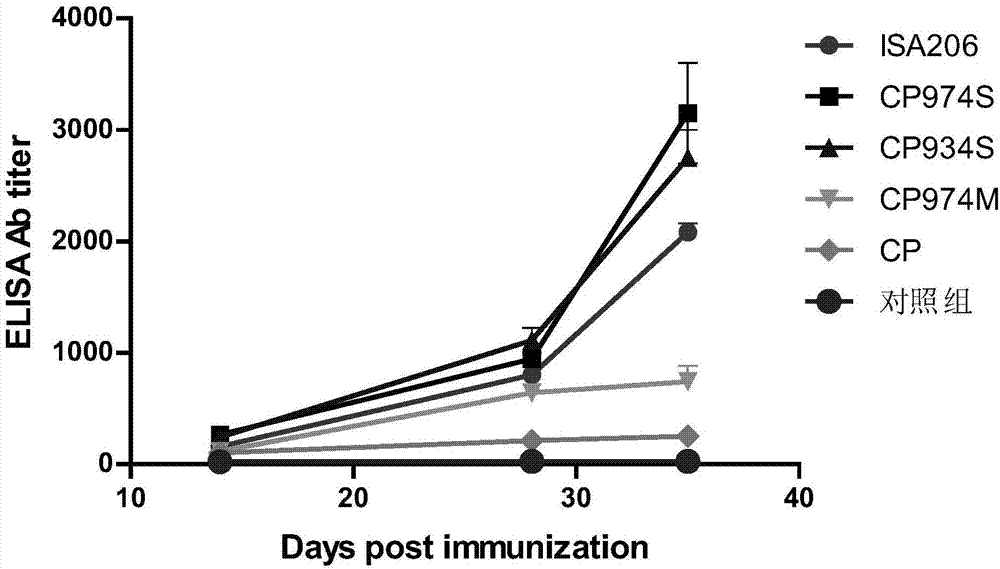
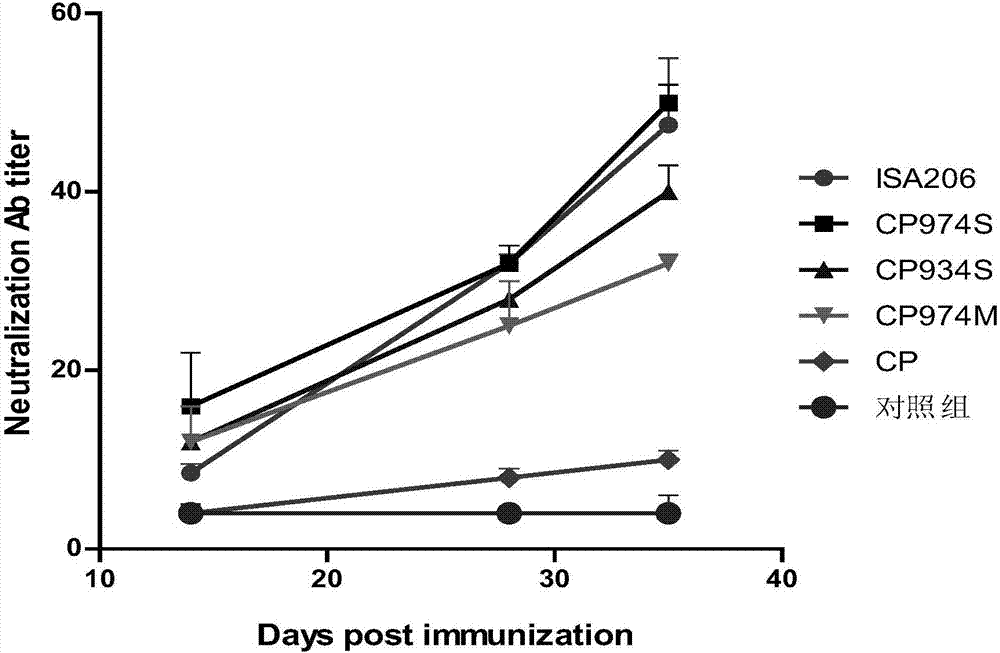
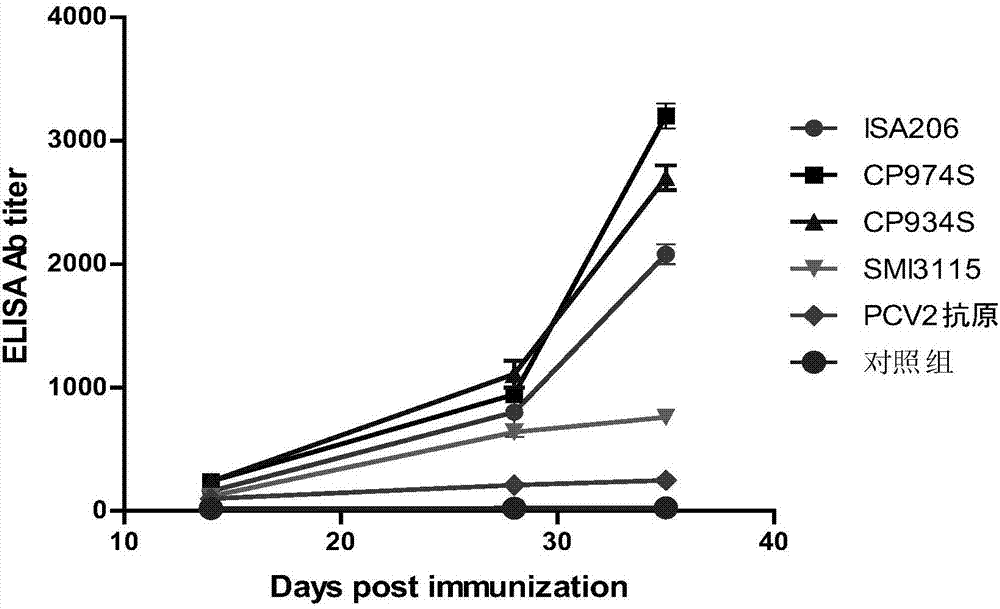
The invention discloses a novel water soluble composite immune adjuvant and a porcine circovirus disease vaccine. Four aqueous adjuvants (CP974S, CP934S, CP974M, and CP) are screened and designed to prepare a PCV2 inactivated vaccine. The results of mouse immunity tests and pig immunity tests show that CP974S and CP934S have a strong immune adjuvant effect on the PCV2 inactivated vaccine, and the pig immunity protective effect of CP974S aqueous adjuvant is better than the PCV2 imported ISA206 adjuvant inactivated vaccine. A baculovirus expressed PCV2Cap recombinant protein is taken as the basis to prepare different adjuvant vaccines. The results of mouse immunity tests and pig immunity tests show that two aqueous adjuvants (CP974S and CP934S) also have a strong immunity adjuvant effect on the PCV2Cap recombinant protein and the pig immunity effect of CP974S adjuvant vaccine is better than imported PCV2Cap subunit vaccine. The CP974S aqueous adjuvant fills the gap of water soluble adjuvant for pigs in China. The developed PCV2 vaccine is safe and effect and has an important application prospect.
Owner:NANJING AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com