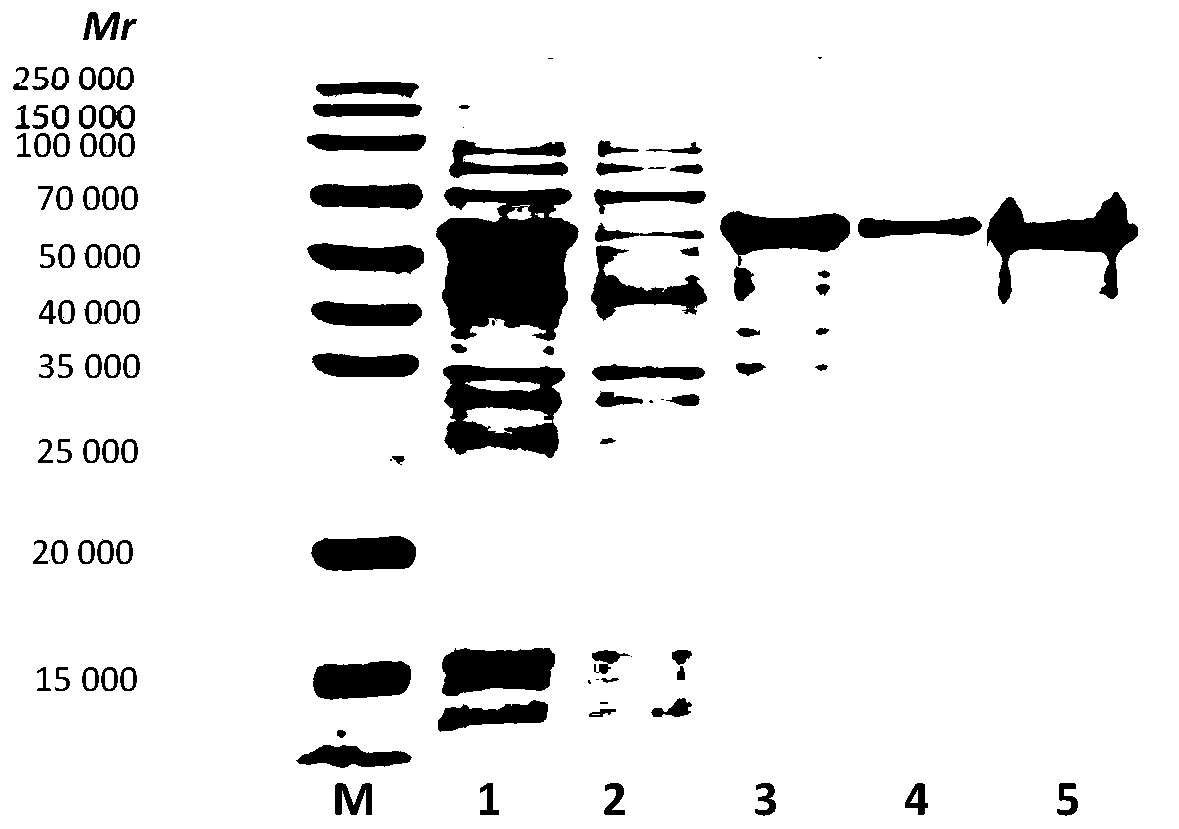
Patents
Literature
33results about How to "Guaranteed immune effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
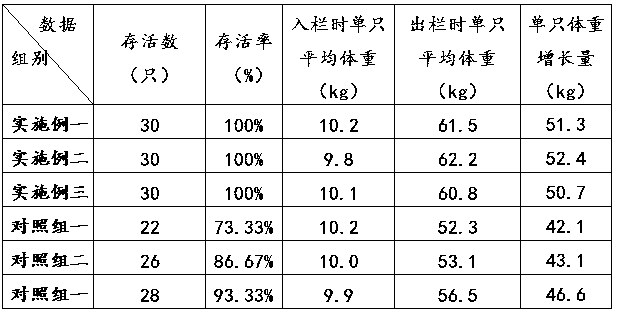
Follow-up infant formula
The invention discloses a follow-up infant formula which relates to a formula. The follow-up infant formula improves the immunity, promotes development of nerves and retina, enhances the resistance of body against pathogen, and promotes lipid metabolism and nutrition balance. Each ton of the follow-up infant formula disclosed by the invention consists of 638 kg of dry substances in raw milk serving as a raw material, 237 kg of desalted whey powder, 60 kg of soybean oil, 53 kg of fructooligosaccharide and 12 kg of composite nutrient; and the raw materials are mixed and subjected to sterilization concentrating and spray drying processes to obtain the follow-up infant formula. The follow-up infant formula disclosed by the invention has an active effect on the immune system, improves the immunity, promotes development of nerves and retina, enhances the resistance of body against pathogen, and promotes lipid metabolism and nutrition balance and the like. The follow-up infant formula disclosed by the invention is suitable for the infants of 6-12 months old.
Owner:张久民
Porcine epidemic diarrhea virus genetic engineering vaccine and preparation method thereof
ActiveCN109456412AEfficient assemblyImprove uniformityImmunoglobulins against virusesAntiviralsAntigenProtective antigen
The invention discloses a porcine epidemic diarrhea virus genetic engineering vaccine and a preparation method thereof, which belongs to the field of veterinary biological products. Core proteins of hepatitis B virus are used as a vector, a porcine epidemic diarrhea virus protective antigen is inserted between a 79 amino acid to 80 amino acid of the vector by using a molecular biology method to form the genetic engineering vaccine for preventing the porcine epidemic diarrhea virus. The preparation method of the vaccine is simple, a great amount of antigen proteins can be prepared, theconsumed time is short, the expression amount is high, the production cost is greatly reduced, and the mass production is facilitated. The porcine epidemic diarrhea sub-unit vaccine comprising HBcAg-PEDV-COE recombinant proteins prepared in the invention is good in immunity effect, small in immunity dose, and capable of effectively preventing the infection of the porcine epidemic diarrhea virus.
Owner:扬州优邦生物药品有限公司
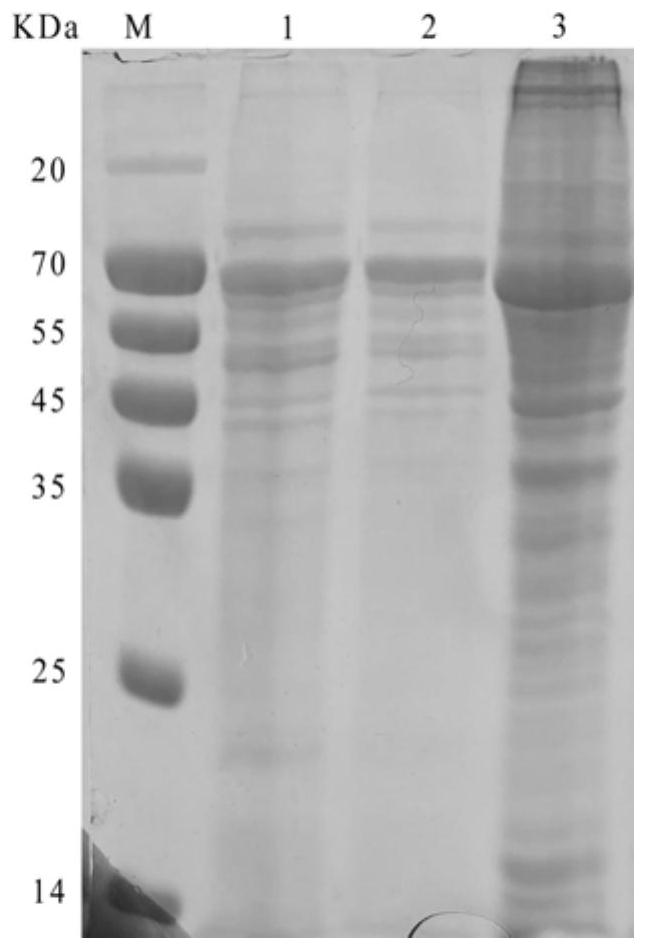
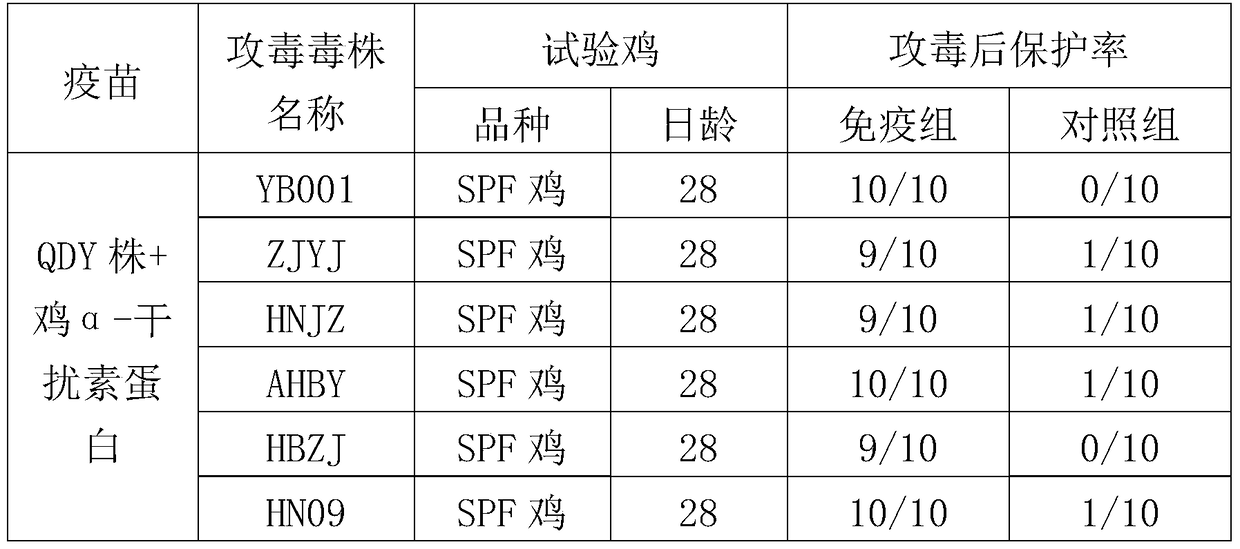
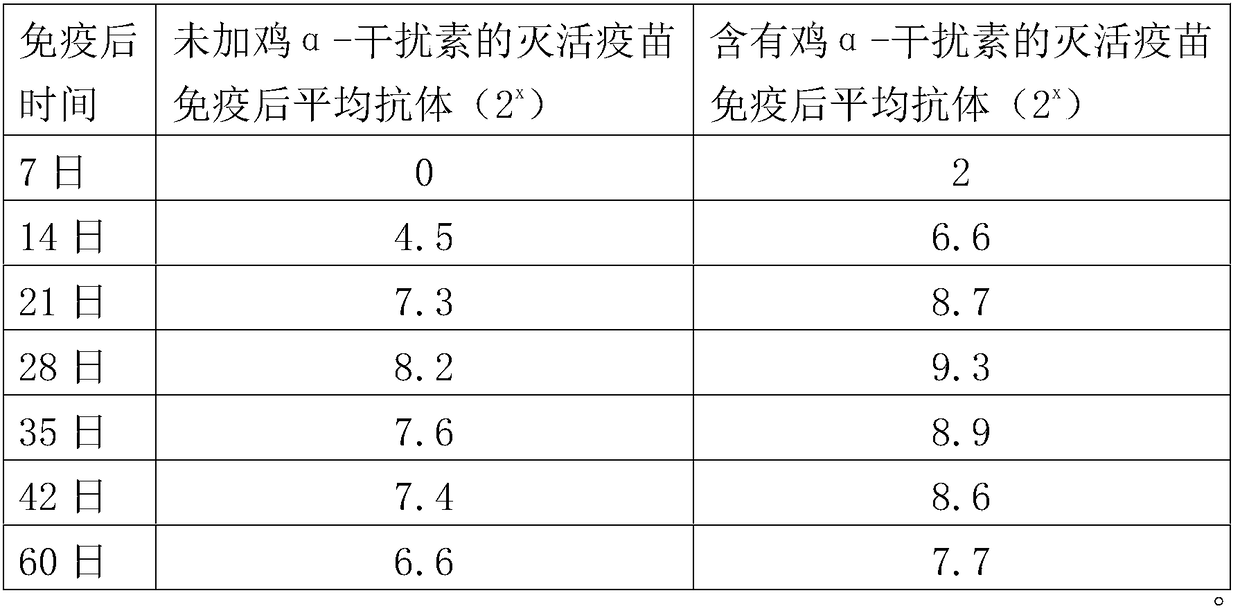
H9 subtype avian influenza virus inactivated vaccine including chicken a-interferon protein
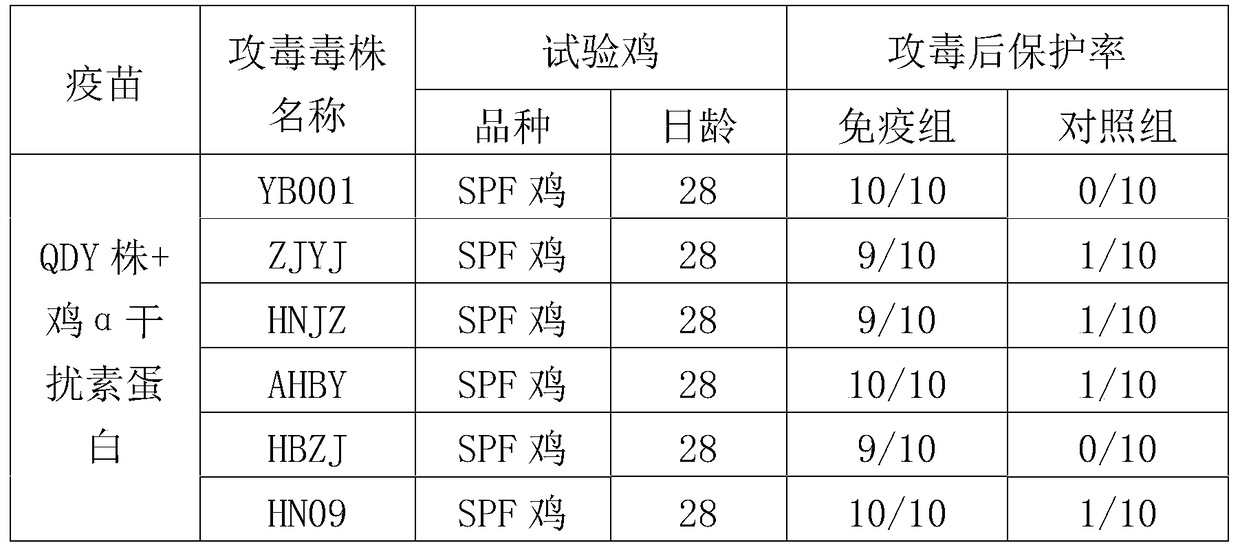
ActiveCN104940921AGuaranteed immune effectImprove securityPeptide/protein ingredientsAntiviralsDiseaseOil adjuvant
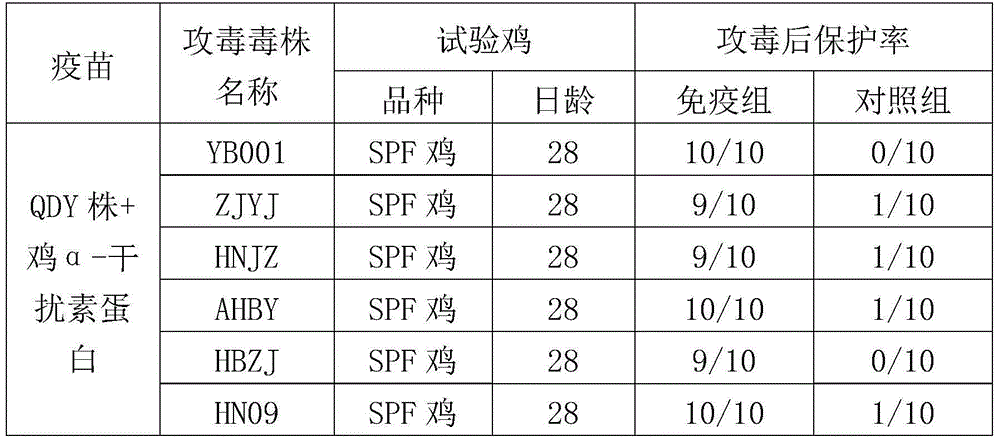
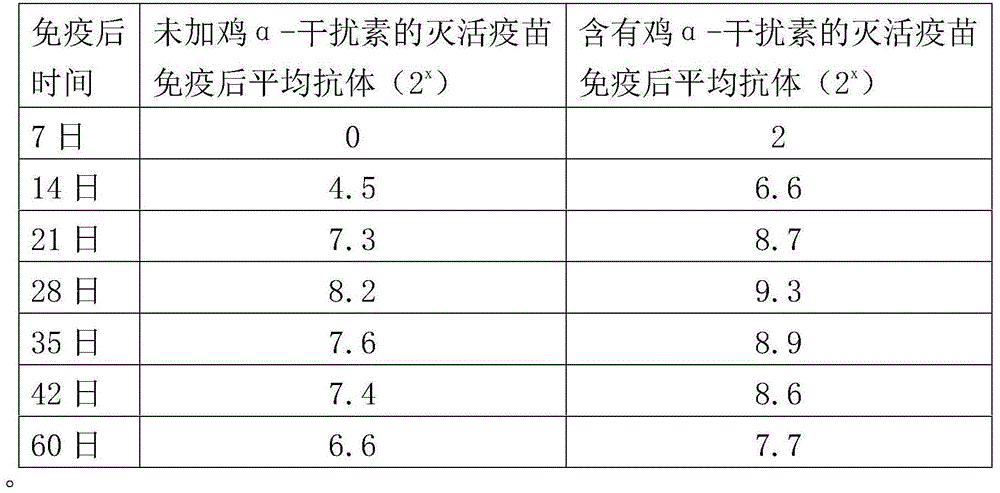
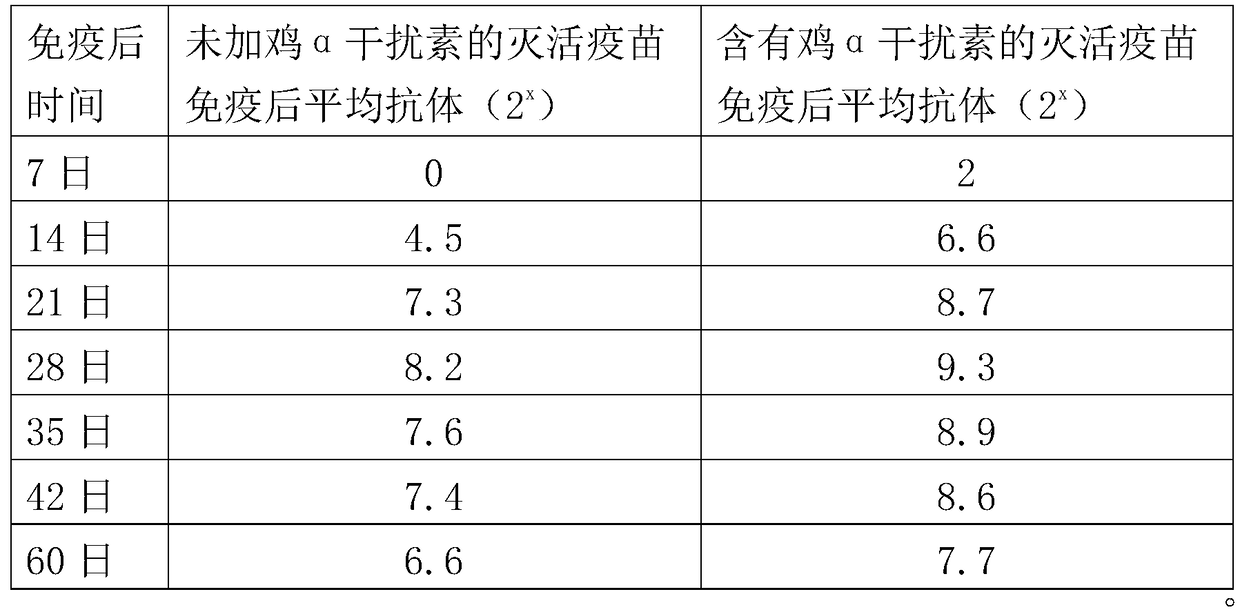
The invention provides an H9 subtype avian influenza virus inactivated vaccine including chicken a-interferon protein. The H9 subtype avian influenza virus inactivated vaccine comprises an antigen and a vaccine adjuvant, and the adopted antigen is an inactivated H9 subtype avian influenza virus QDY1 strain including the chicken a-interferon protein. A screened H9 subtype avian influenza virus has the advantages of being small in virulence and high in immunogenicity. The H9 subtype avian influenza virus (QDY strain) is inoculated into a chick embryo, then collects virus liquid, and is mixed with the extracted and purified chicken a-interferon protein after inactivation of formaldehyde solutions, and an oil adjuvant is added for emulsification to manufacture the vaccine. The prepared vaccine can stimulate an organism to generate an antibody fast, the level of the antibody is improved, the persistent period of the antibody is prolonged, and diseases caused by the H9 subtype avian influenza virus is prevented. The vaccine has the advantages of being efficient and good in safety.
Owner:YEBIO BIOENG OF QINGDAO
Non-reactive high-efficiency feed for piglet early weaning and production method of feed
InactiveCN104757310AImprove feed utilizationImprove coat colorAnimal feeding stuffEarly weaningLow protein
The invention discloses non-reactive high-efficiency feed for piglet early weaning. The feed consists of corns, broken rice, wheat middling, soybean meal, extruded soybeans, fermented soybean meal, fish meal, domey protein powder, low protein whey powder, chocolate feed powder, cane sugar, soybean oil, mountain flour, calcium hydrogen phosphate, salt, choline, nome selenium, zinc oxide, feed-bond, lysine, methionine, threonine, tryptophan, healthy acid, weaning powder, a sweetening agent, peony fragrance, feed complex enzyme, an antioxidant Yangliting, pig mineral substance premix and pig vitamin premix. According to the feed disclosed by the invention, the weaning stress, nutritional stress, temperature stress and environmental stress of the piglets can be effectively reduced, and the economic benefits are improved.
Owner:INST OF AGRI SCI ALONG YANGTZE RIVER IN JIANGSU
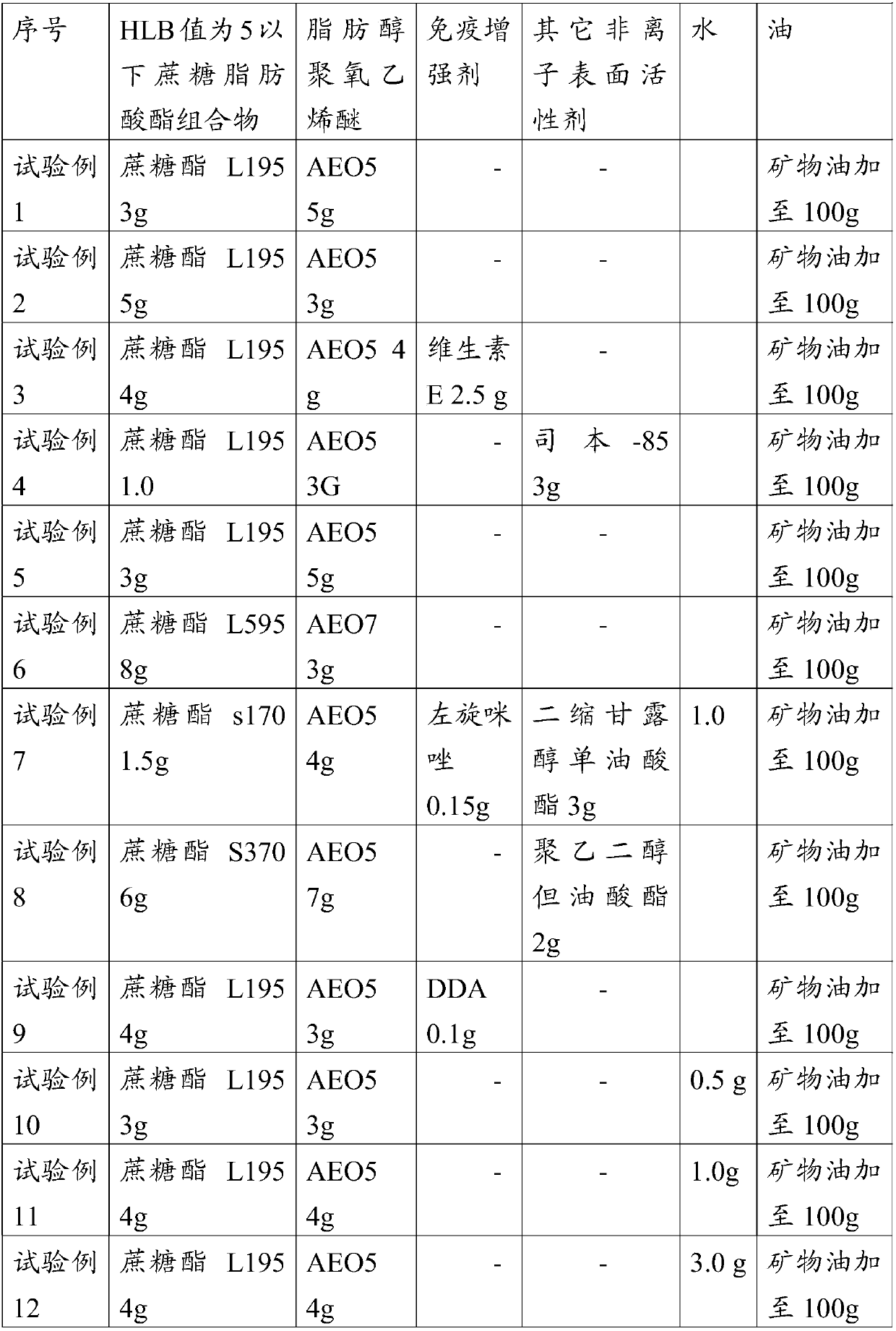
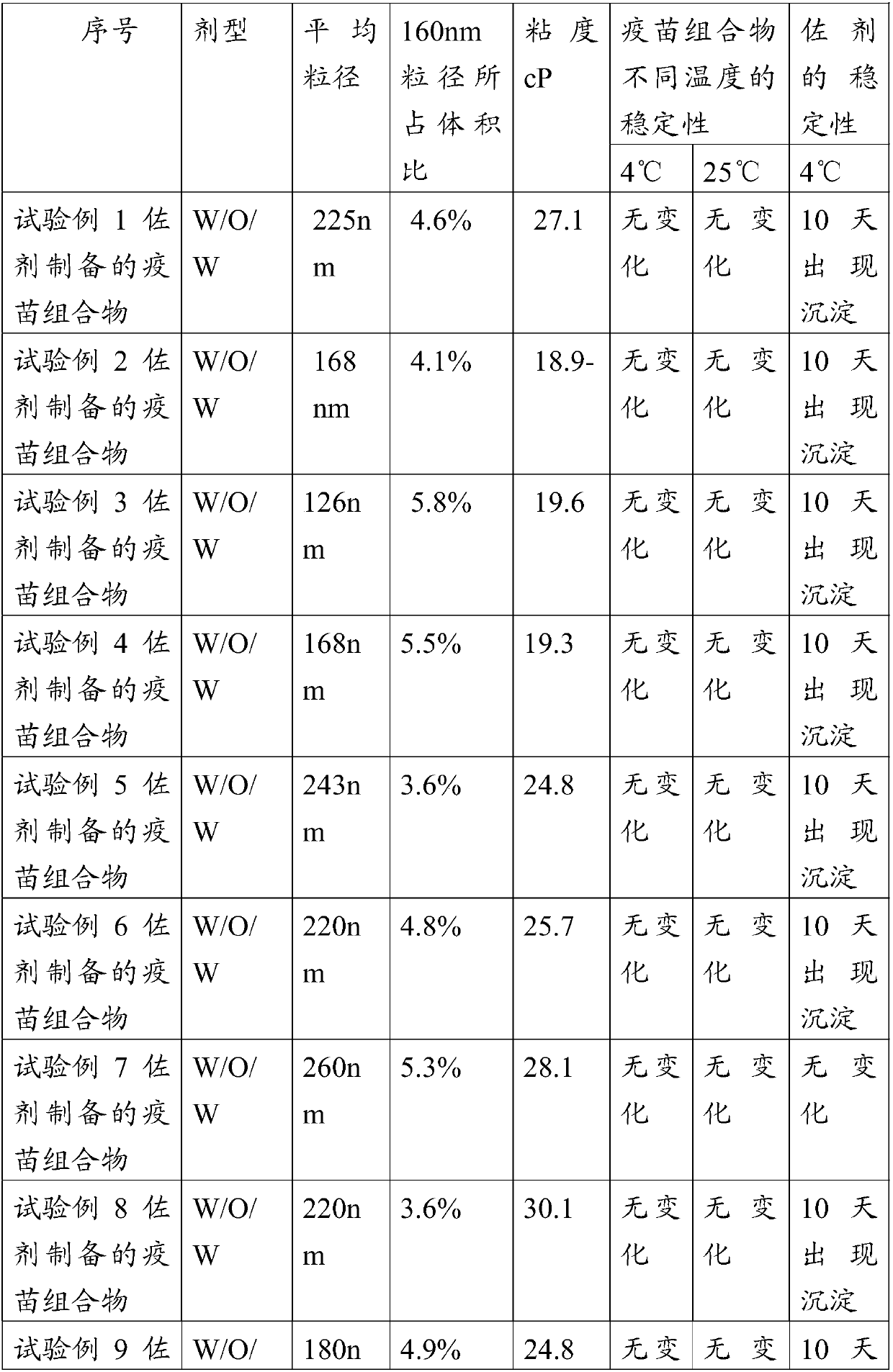
W/o/w adjuvant composition, vaccine composition prepared thereby, and preparation method of vaccine composition
ActiveCN107596364AReach the state of protection in timeProduce quicklySsRNA viruses positive-senseViral antigen ingredientsImmune effectsAdjuvant
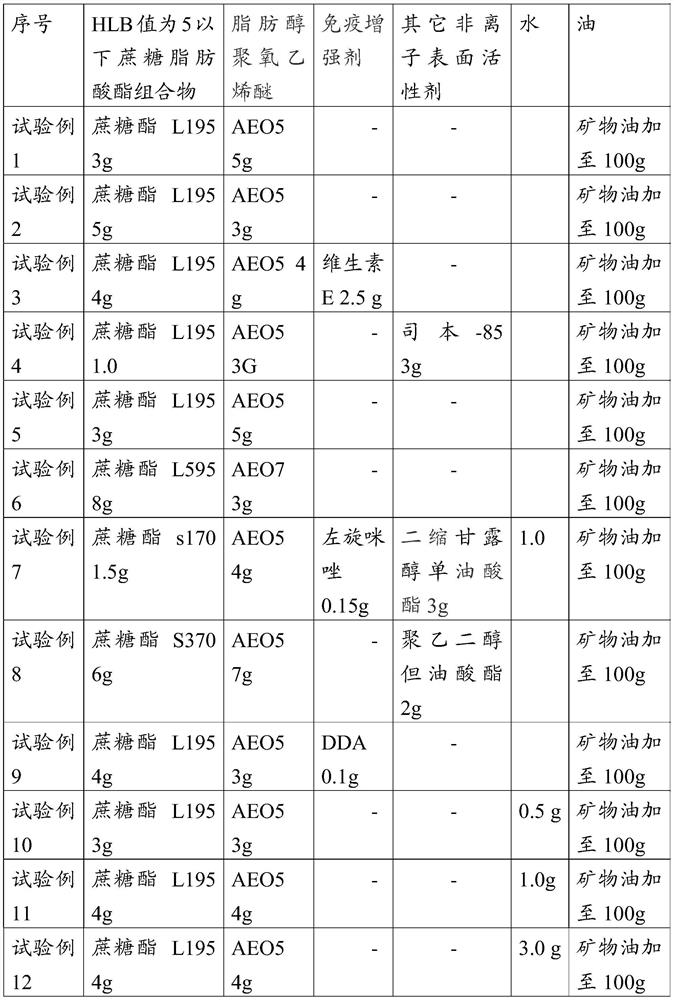
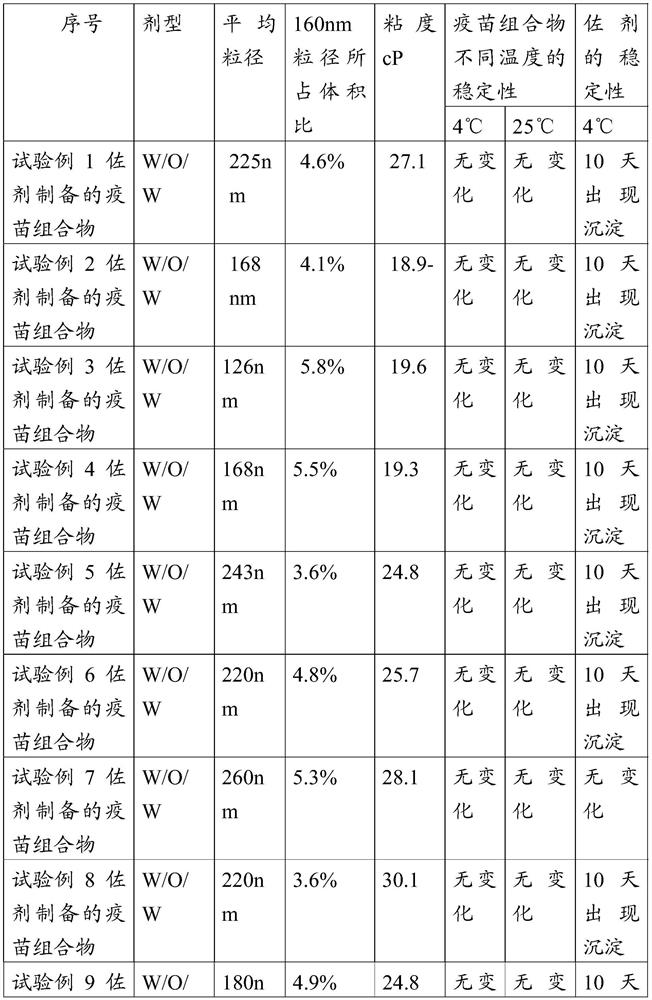
The invention relates to a w / o / w adjuvant composition. The w / o / w adjuvant composition comprises a sucrose fatty acid ester with HLB value of below 5, fatty alcohol-polyoxyethylene ether and oil. The vaccine composition prepared by the adjuvant composition is stable at different temperature and has no layering and demulsification phenomena, and the preparation condition of the vaccine is simple andmild. The prepared vaccine achieves the immune effect rapidly and does not have adverse effect during use.
Owner:LUOYANG SEIWEI BIOTECHNOLOGIES CO LTD
Preparation method of inactivated lyophilized vaccine for porcine circovirus type 2
InactiveCN104383527AHigh antigen contentImproving immunogenicityPowder deliveryViral antigen ingredientsMolecular sieveImmune effects
The invention provides a preparation method of an inactivated lyophilized vaccine for porcine circovirus type 2. The preparation method comprises the following steps: successively carrying out clarification procedure, ultrafiltration concentration and molecular sieve chromatography column purification on a virus culture solution, so that the impurity content of an antigen used for preparing the vaccine is effectively ensured to be reduced to the minimum, thus avoiding the phenomenon that side effects are generated after the product is used, and also being beneficial to ensuring the immune effect of the vaccine. In addition, according to the preparation method provided by the invention, as the inactivated antigen is directly prepared in a lyophilizing mode without adding an oil adjuvant, the problems of difficulty in injection and absorption do not exist. The PCV 2 inactivated lyophilized vaccine prepared by the preparation method is high in antigen content, immunogenicity and antigen purity, is good in safety, is low in side effect, is capable of rapidly generating antibody protection without an adjuvant and is easily injected and absorbed.
Owner:TIANJIN RINGPU BIO TECH
Process for preparing antibody for small molecular substance using nanoliposome immune adjuvant
InactiveCN101565726AGood biocompatibilityImprove stabilityImmunoglobulinsFermentationSolubilityRotary evaporator
The invention relates to a process for preparing antibody for small molecular substance using nanoliposome immune adjuvant, which belongs to the field of immunologic technique, including mixing soybean lecithin and cholesterol according to a weight ratio of 1.8-2.5 / 1, dissolving the mixture into a methylene dichloride solvent with W / V at 1 / 500-800, adding into a flask, vacuum evaporating at a temperature from 35 to 40 DEG C. on a rotary evaporator; charging an antigen solution with concentration ranging from 1.5 to 2.0mg / mL, and ratio to the embedding material ranging from 2.0 to 2.5 / 1, strongly swinging, wherein 20mM PBS with pH ranging from 6.8 to 7.2 is used as buffer; intravenous immune is carried out at the amount of 200-300 mu g per mice on each time; secondary booster immune; selecting mouse with high special antibody potency for cell fusion experiment, screening needed monoclonal antibody. The invention has advantage that: (1) the antibody is great in solubility with the embedding material, easy to be dissolved in the animal body and non-toxic; (2) liposome is rapidly captured by an immune cells when introduced into peripheral blood and distributed to lymph nodes and central immune organs, and antigen presenting is realized; (3) the antigen is embedded into the liposome, so that stability of antigen is increased, and immune effect is ensured.
Owner:NANCHANG UNIV
Application of swine fever and porcine pseudorabies live vaccine to preparation of medicament for treating or preventing swine fever and porcine pseudorabies
InactiveCN104288761AResolution timeGuaranteed immune effectAntiviralsRespiratory disorderInhalationSwine Fever Virus
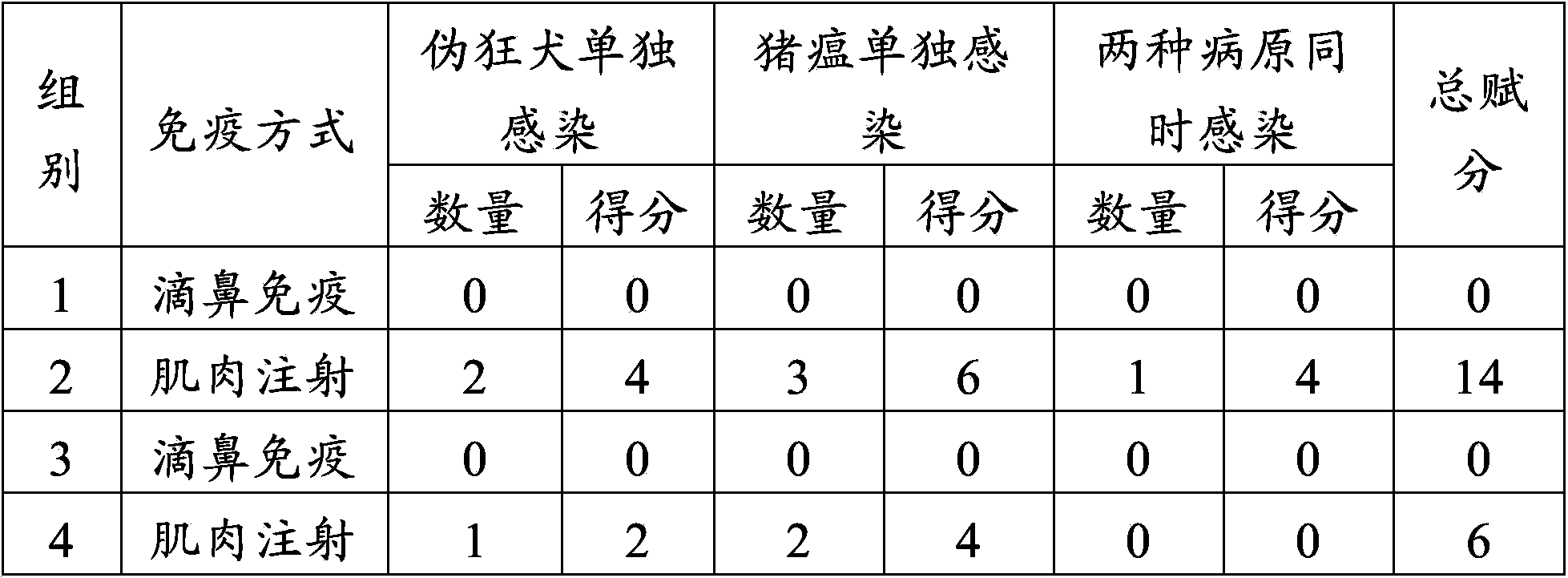
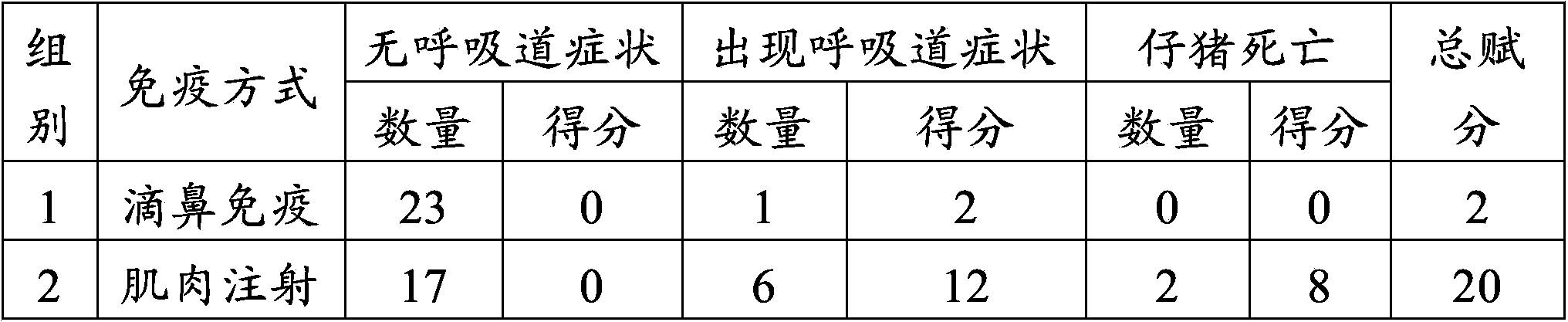
The invention provides application of swine fever and porcine pseudorabies live vaccines to preparation of medicaments for treating or preventing swine fever and porcine pseudorabies. The swine fever vaccine and porcine pseudorabies vaccine are used for immunization of piglets through nasal inhalation. The swine fever and porcine pseudorabies live vaccines can effectively prevent swine fever virus and porcine pseudorabies of piglets, at the same time significantly reduce the occurrence of porcine respiratory disease, and effectively prevent respiratory syndromes.
Owner:PU LIKE BIO ENG
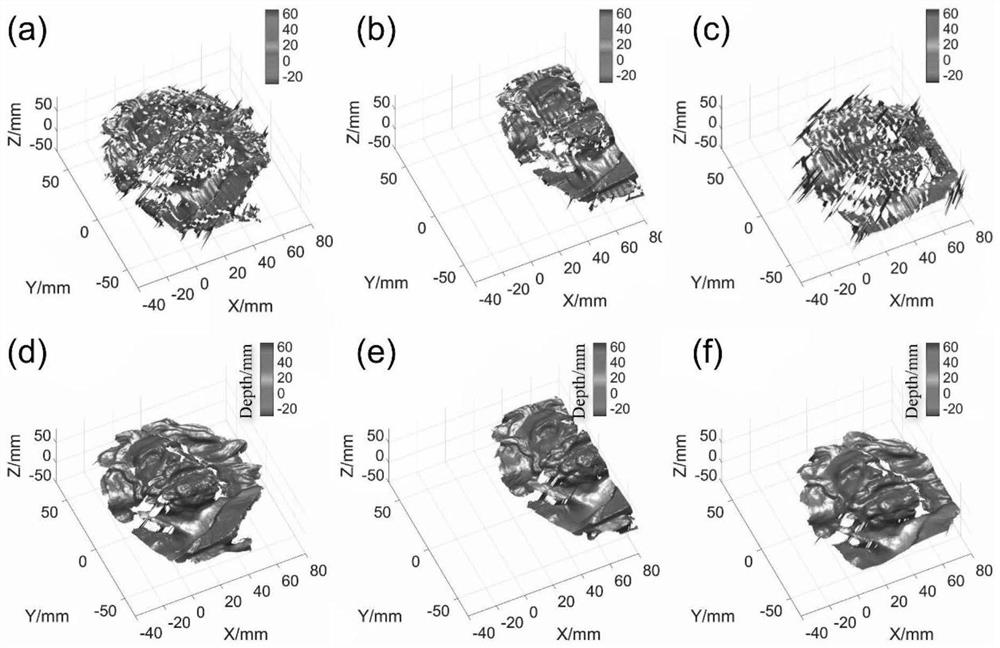
Single-frame fringe projection three-dimensional surface type measurement method
ActiveCN112833818AGuaranteed immune effectGuaranteed stabilityUsing optical meansFeature extractionComputer graphics (images)
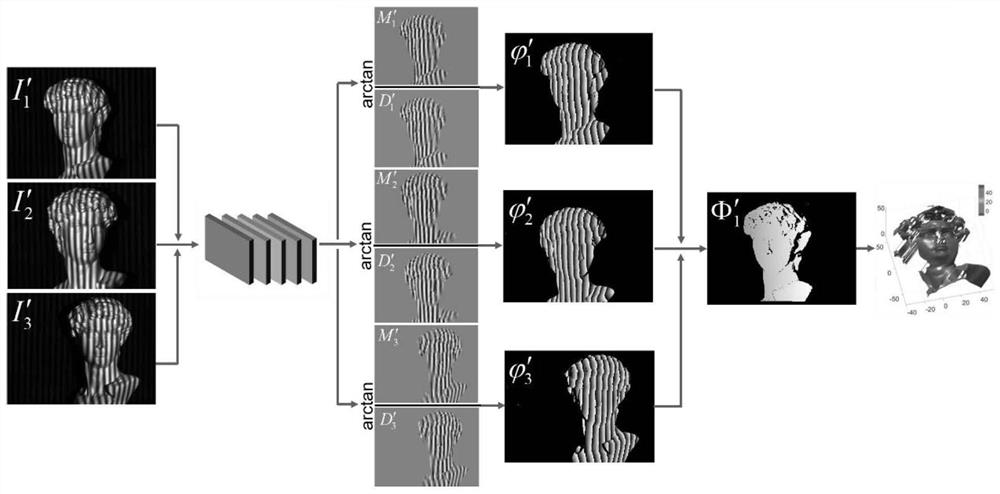
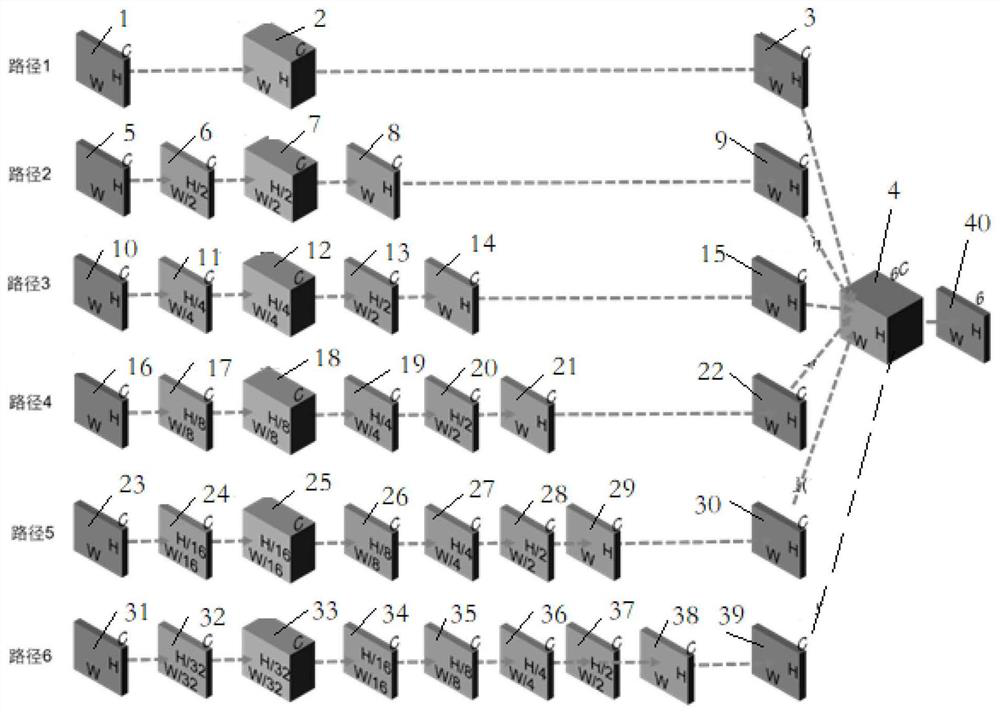
The invention provides a single-frame fringe projection three-dimensional surface type measurement method, wherein the method comprises the following steps: S1, constructing a deep convolutional neural network which has six paths, and defining the deep convolutional neural network as DL-net. According to the method, firstly, a deep learning method is utilized, high-quality phase information extraction of a single-frame fringe image at three camera visual angles is realized under the driving of a large amount of data, then, a calibration space epipolar relationship among multiple visual angles is utilized, and the similarity wrapping the phase information is used as measurement to realize robust phase unwrapping, and then the high precision absolute depth information of a measured object can be recovered; and the powerful feature extraction advantage of deep learning and the efficient phase unwrapping advantage of geometric constraint are combined, and high-quality phase extraction, robust phase unwrapping and high-precision absolute depth recovery are achieved on the premise of single-frame projection.
Owner:南京理工大学智能计算成像研究院有限公司
Northern Guizhou Ma goat breeding epidemic disease prevention and control technology
The invention belongs to the technical field of livestock breeding, and particularly relates to a Northern Guizhou Ma goat breeding epidemic disease prevention and control technology. The technology comprises the following steps that S1, a site is selected, and leymus chinensis is planted; S2, epidemic disease preventing medicine is prepared; S3, epidemic disease preventing forage grass is processed and prepared, medicine A and harvested and chopped forage grass are mixed to be uniform according to the proportion of 1:6, then ensiling, ammoniation and fermentation are conducted in sequence toobtain the epidemic disease preventing forage grass, medicine B is uniformly irrigated on outdoor farm grass through a water spraying device at night every 2-3 days, on the second day after irrigationis completed, and forage grass in the area not eaten in a short time is harvested and serves as epidemic disease preventing forage grass raw materials; S4, disinfection and source destroying are conducted; S5, desinsectization and medicated bath are conducted; S6, immunization is conducted. The technology is scientific, reasonable, high in prevention and control density and good in prevention andcontrol effect, fattening goats for slaughter do not have any infectious diseases or other diseases, the prevention and control density reaches 100%, and no dead angle exists.
Owner:仁怀市桑合牧业有限公司
Formula of traditional Chinese medicine for resisting diseases, enhancing immunity and improving production performance of meat poultry
InactiveCN108066485AGood control effectReduce moistureAntibacterial agentsDigestive systemDiseaseVaccination
The invention discloses a formula of a traditional Chinese medicine for resisting diseases, enhancing immunity and improving production performance of meat poultry. The traditional Chinese medicine isprepared from raw materials in parts by weight as follows: flos chrysanthemi, semen cassiae, fructus lycii, pearl, herba menthae, common macrocarpium fruit, poria cocos, cortex moutan radicis and rhizoma atractylodis. The traditional Chinese medicine has the following beneficial effects: the traditional Chinese medicine prevents and resists pestilences and has remarkable prevention and treatmenteffects on infectious proventriculitis, ventriculitis, colibacillosis, salmonellosis, enterotoxin syndrome, air sacculitis and other diseases; drug resistance is reduced, drug sensitivity is recovered, and bacteriosis can be controlled easily; immunosuppression is removed, the vaccination effect is guaranteed, and stress response to a vaccine is reduced; after the traditional Chinese medicine is used in the whole feeding period, the poultry meat contains little water, is rich in vitamins and amino acids and low in cholesterol content and has good flavor.
Owner:广安市广安区井河镇初级中学校
Immunization method of chicken embryos
InactiveCN102405853AStable temperatureReduce the chance of infectionAvicultureUnfertilized EggsAlcohol
The invention discloses an immunization method of chicken embryos, comprising the following immunization steps of (1) sealing, steaming and disinfecting the immunization space with potassium permanganate and formalin in each cubic meter; (2) disinfecting the chicken embryo air-chamber with alcohol of which the concentration is 75%; (3) candling the chicken embryos of which the age is 18-19 days to screen the dead eggs or the unfertilized eggs; (4) diluting the immunization vaccine with 200-220 milliliters of diluent in each 4000 dose; (5) immunizing the single strain with the immunization chicken embryo with the diluted vaccine, wherein the immunization part is the air-chamber part of the chicken embryo; and (6) sealing, which is to seal the immunization needle holes of the chicken embryo eggs which are immunized. In the invention, the immunization space and the immunization parts are firstly disinfected, so the infection possibility is reduced, and the temperature and the humidity of the immunization space are stabilized; and because of the immunization sealing, the immunization effect is ensured, the stress death of the chicken embryos is prevented, and the hatch loss is reduced.
Owner:林继恒
Novel coxsackie virus group A 6 recombinant subunit protein vaccine and preparation method thereof
ActiveCN111000991AImproving immunogenicityLow costSsRNA viruses positive-senseViral antigen ingredientsEscherichia coliCoxsackie Viruses
The invention provides a novel coxsackie virus group A 6 recombinant subunit protein vaccine and a preparation method thereof. The invention relates to the novel coxsackie virus group A 6 recombinantsubunit protein vaccine which is artificially designed. The vaccine comprises a recombinant protein formed by fusion design of a diphtheria toxin non-toxic mutant CRM197 protein with an immunologic adjuvant effect and a CV-A6 antigen structural protein. The recombinant protein is subjected to escherichia coli expression, chromatographic column separation, purification and compatibility, and finally the recombinant subunit protein vaccine with good immunogenicity is prepared. The vaccine can effectively activate an organism to generate a neutralizing antibody aiming at the coxsackie virus groupA 6, and can effectively prevent the infection of the coxsackie virus group A 6 on an organism. The vaccine disclosed by the invention realizes efficient expression of the target protein in an escherichia coli expression system by carrying out password liberation on an expression gene, so that the preparation efficiency is greatly improved, the production cost is reduced, and a foundation is laidfor popularization and immunization of large-scale crowds.
Owner:ZHEJIANG PUKANG BIOTECH
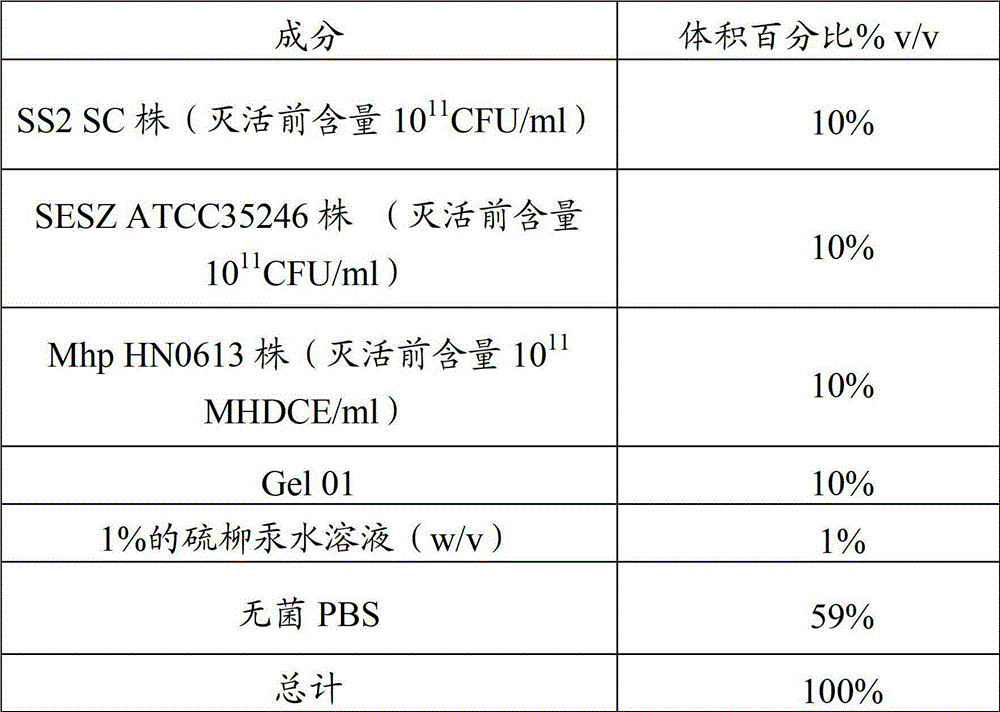
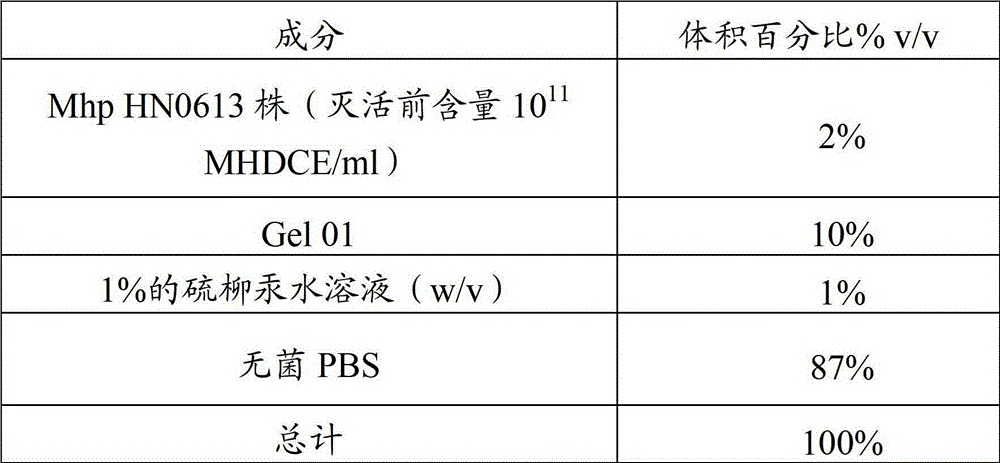
Vaccine composition containing swine mycoplasmal pneumonia antigen and swine streptococcosis antigen, and preparation method and application thereof
ActiveCN103861095APreserve immune efficiencyLow costAntibacterial agentsBacterial antigen ingredientsImmune effectsAdjuvant
The invention provides a vaccine composition containing a swine mycoplasmal pneumonia antigen and a swine streptococcosis antigen, and a preparation method and an application thereof. The vaccine composition includes an immunizing dose of the swine mycoplasmal pneumonia antigen, an immunizing dose of the swine streptococcosis antigen, and an adjuvant. The vaccine composition has a simple immunization program, can effectively control the swine mycoplasmal pneumonia antigen and the swine streptococcosis antigen, has an immune effect equivalent to the immune effect realized through respective injection of single vaccines, and also has the characteristics of small side reaction, long immune period, less time consumption, and less labor consumption; and the vaccine composition also has the advantages of simple production technology, low immune cost and strong practicality.
Owner:PU LIKE BIO ENG
Anti-Hib-RSV-meningococcus combination vaccine
InactiveCN108619505ALow costSimple production processAntibacterial agentsSsRNA viruses negative-senseBacteroidesLinker peptide
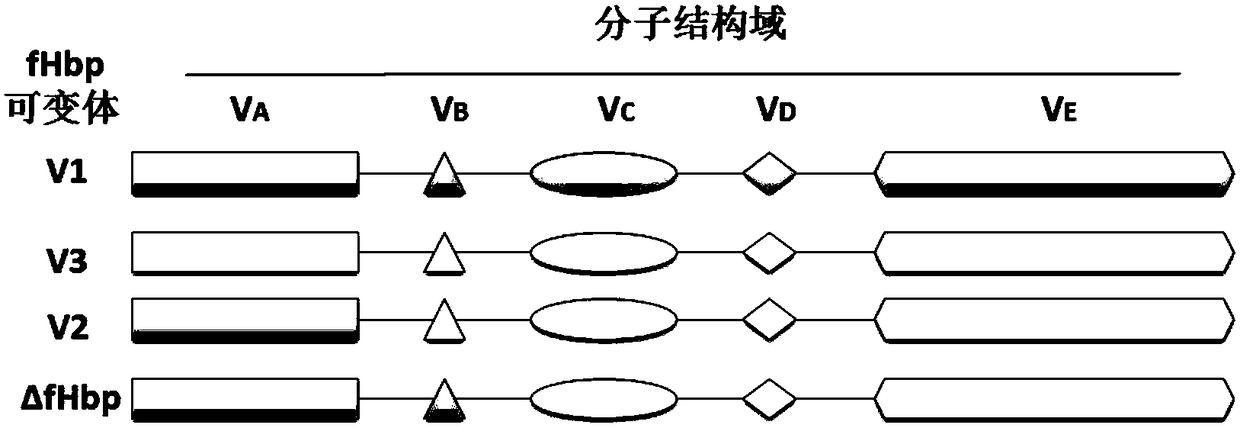
The invention discloses an anti-Hib-RSV-meningococcus combination vaccine comprising a Hib-meningococcus intermediate and an RSV immune intermediate, wherein the meningococcus immune intermediate comprises modified delta fHbp protein, a flexible linker peptide segment and NadA protein, the recombinant delta fHbp protein comprises VA and VB domains of fHbp variant V1 and VC, VD and VE domains of fHbp variant V3, and Hib capsular polysaccharides are conjugated to the delta fHbp protein and / or the NadA protein after being activated; the antigen of the RSV immune intermediate is an RSV membrane surface fusion protein F and / or attachment protein G which can cause body immune response, the nucleic acid sequence expressing the protein antigen is recombined on a nucleic acid vector, the nucleic acid sequence of the recombinant protein takes attenuated intracellular bacteria as a bacteria vector; the immune intermediates of the components are separately prepared and then mixed for use.
Owner:BRAVOVAX
Canine distemper virus strain, bivalent vaccine based on canine distemper virus and canine parvovirus and application
PendingCN114317459ALow costIncrease productivityMicroorganism based processesAntiviralsLaboratory cultureAntigen
The invention provides a canine distemper virus strain, a bivalent vaccine based on a canine distemper virus and a canine parvovirus and application of the bivalent vaccine, and belongs to the technical field of vaccines. The biosafety problem of bivalent vaccine virus seed enhancement and the immune protection effect problem of young dogs are solved. The invention provides a canine distemper virus which is named as a canine distemper virus CDV-SN strain and has a preservation number of CGMCC (China General Microbiological Culture Collection Center) NO.23205. The invention also provides a bivalent vaccine based on the canine distemper virus and the canine parvovirus disease, and the bivalent vaccine comprises a stock solution of the canine distemper virus CDV-SN and a stock solution of recombinant baculovirus CPV-2b-VP2, and the mixing ratio of the stock solution to the stock solution of the recombinant baculovirus CPV-2b-VP2 is 1: 1. Compared with the existing product canine distemper virus and parvovirus disease bivalent live vaccine, the product has the advantages of good safety, high antigen concentration and high purity, and can be used as a preferred vaccine for basic immunity of puppies to reduce the influence of maternal antibodies on the immune effect.
Owner:CHANGCHUN SR BIOLOGICAL TECH
Recombinant herpesvirus turkey live vector vaccine capable of simultaneously expressing classical strain and variant infectious bursal disease virus VP2 protein
ActiveCN114107227AGuaranteed immune effectImprove securityViral antigen ingredientsVirus peptidesVector vaccineVariant strain
The invention discloses a recombinant herpesvirus turkey live vector vaccine capable of simultaneously expressing classical strain and variant strain infectious bursal disease virus VP2 protein, and belongs to the field of veterinary biological products. According to the invention, a VP2 protein and LTB fusion expression cassette containing an infectious bursal disease virus classical strain is knocked into a US2 virus replication non-essential region of a turkey herpesvirus, and a VP2 protein and LTB fusion expression cassette of a variant strain is inserted into a US10 virus replication non-essential region. The finally obtained recombinant virus simultaneously and efficiently expresses LTB-VP2 fusion antigen protein of the IBDV classic strain and the variant strain in CEF cells. The vaccine prepared by the invention can improve the antibody level after immunization, improve the uniformity of the antibody after immunization and ensure the immunization effect of the vaccine, has the advantages of high efficiency, good safety and lifelong immunization after one-time inoculation, does not cause clinical reaction and pathological damage to chicks, and has the vaccine protection rate of 100%.
Owner:扬州优邦生物药品有限公司
Artificial avian paramyxovirus
InactiveCN109929815APrevent proliferationGuaranteed immune effectViruses/bacteriophagesHemagglutininImmune effects
The invention belongs to the field of biological medicine and discloses an artificial avian paramyxovirus. The genome of the artificial virus contains a backbone sequence (which is the genomic sequence of a certain avian paramyxovirus) and a partial sequence of an influenza virus hemagglutinin gene (which does not encode a polypeptide anchored to the viral envelope). Unlike previously similar artificial avian paramyxoviruses, the artificial avian paramyxovirus does not alter their capsular structure. Therefore, if the artificial virus is used as an influenza vaccine (being a live vaccine) strain for humans and animals, it can be ensured that the artificial virus normally proliferates in an inoculated animal in terms of the structure of the viral envelope, thereby ensuring its immune effect. In addition to inserting partial sequences of influenza virus genes into the genome of the artificial virus, it is also possible to insert a protein coding sequence of another class of viruses, so that an animal inoculated with the artificial virus also produces a specific immune response against another type of viruses.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
A strain of h9 subtype avian influenza virus
ActiveCN104946600BGuaranteed immune effectImprove securityMicroorganism based processesAntiviralsOil adjuvantImmune effects
The invention aims at providing an H9 subtype avian influenza virus strain. Vaccine is prepared by the virus strain to solve the problem that existing H9 subtype avian influenza virus vaccine is poor in immune effect caused by a novel virus. The H9 subtype avian influenza virus QDY strain (Avian Influenza Virus) is preserved in the typical culture preservation center in China in Wuhan University on April 29, 2015, and the preservation number is CCTCC NO: V201517. After the H9 subtype avian influenza virus (QDY strain) is inoculated to a chick embryo, a virus solution is collected, after ultrafiltration concentration and formaldehyde solution inactivation are performed, oil adjuvant is added, and then mixing and emulsifying are performed to prepare the vaccine. According to the prepared vaccine, the antibody level after immunization can be improved, the antibody uniformity after immunization can be improved, and the immune effect of the vaccine can be guaranteed; the vaccine has the advantages of being efficient and good in safety.
Owner:YEBIO BIOENG OF QINGDAO
Syringe needle connection exchanging device for animal
ActiveCN101361681BKeep healthyEnsure safetyInfusion syringesIntravenous devicesVaccinationDrug effect
The invention discloses an apparatus for receiving and replacing syringe needles for animals, which comprises a main shaft, a nut, a pin, containing grooves, a frame for receiving and replacing needles, marks, a handle, a base, syringe needles, an upper cover, a nick of the upper cover, a spring, a concave and convex contact face at the upper end of the frame for receiving and replacing needles, and a annular groove. Pairs of containing grooves that are considered as main units for containing as well as receiving and replacing needles are arranged on the frame for receiving and replacing needles, and each pair of the containing grooves can contain sterilized needles and used needles respectively. The application of the apparatus for receiving and replacing syringe needles for animals can meet the requirements of 'one animal, one syringe needle' on animal epidemic prevention of China, which avoids the spread of epidemic caused by replacing needles through hand clamps in the traditionalinjection method. The apparatus for receiving and replacing syringe needles for animals not only can render a quick replacement of vaccination and medical needles, improve the working efficiency of hand-operated needle replacement; but also can avert any possibility of spread of animal epidemic caused by the repeated use of needles in the process of disease prevention and treatment; and at the same time meet the requirements of using the vaccination and liquid medicine in a limited time to reduce the losses caused by the failure of immunization and decline of drug effect.
Owner:黄海波 +1
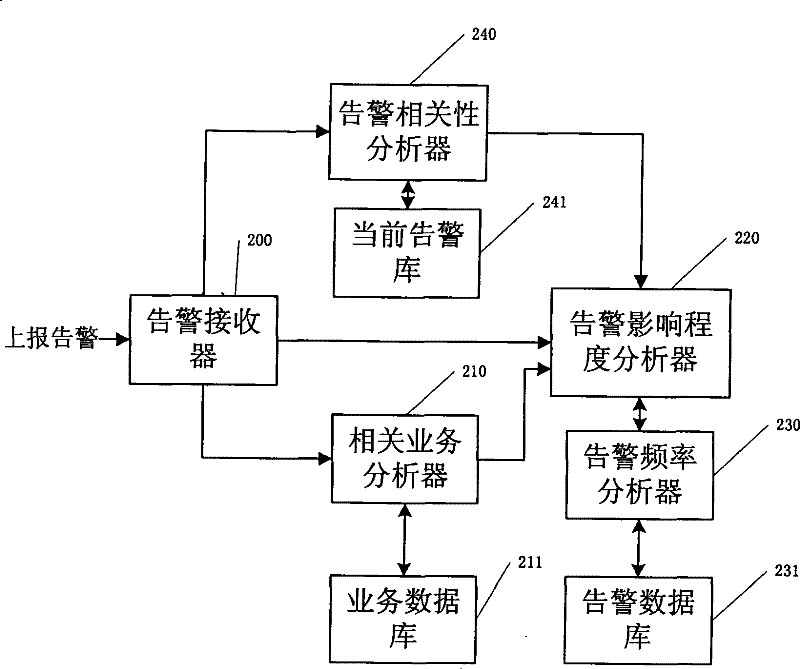
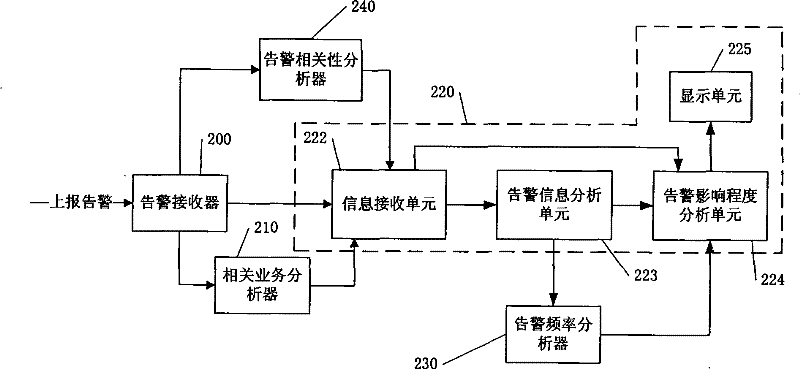
Dynamic analysis system and method for network alarm
ActiveCN101277218BGuaranteed recovery effectGuaranteed immune effectData switching networksRelevant informationLinked list
The invention discloses a dynamic analyse system of network alarm which is used for dynamic analysing influence degree of alarm to service network. The dynamic analyse system includes: the corresponding service analyzer is used for generating corresponding service link list; the alarm frequency analyzer is used for receiving alarm information came from the alarm influence degree analyzer and timesection used for calculating alarm frequency, and calculates alarm frequency generated in present alarm port; the alarm influence degree analyser is used for colligating present alarm information andthe alarm frequency to calculate influence degree of present alarm. Comparing with prior art, the system and method provided by the invention can make manager finding key problem of threatening and influencing network developing smoothly. On the one hand, manager can achieve maximum results with little effort and reduce labour cost, on the other hand, recovery capability and immunity capability of network can be solved early.
Owner:ZTE CORP
A Porcine Parvovirus Subunit Vaccine
ActiveCN105879024BGuaranteed immune effectImprove securityViral antigen ingredientsAntiviralsPichia pastorisOil adjuvant
The invention aims at providing porcine parvovirus subunit vaccine. By means of the porcine parvovirus subunit vaccine, the problem that existing vaccine is not good in immune effect is solved. Antigen used in the vaccine is porcine parvovirus VP2 protein expressed by recombined pichia pastoris X33-VP2, the strain was preserved in China Center for Type Culture Collection on 9, Marh, 2016, and the preservation number is CCTCC M 2016098. According to the porcine parvovirus subunit vaccine, after a culture medium is inoculated, the recombined pichia pastoris X33-VP2 is subjected to inducible expression, after extraction, purification and inactivation via a formaldehyde solution are conducted, oil adjuvant is added, and emulsifier is mixed, so that the vaccine is obtained. By means of the subunit vaccine, uniformity of immunized antibody can be improved, the immune effect of the vaccine is guaranteed, and the vaccine has the advantages of being high in efficiency and good in safety.
Owner:YEBIO BIOENG OF QINGDAO
A h9 subtype avian influenza virus inactivated vaccine containing chicken α-interferon protein
ActiveCN104940921BGuaranteed immune effectImprove securityPeptide/protein ingredientsAntiviralsDiseaseOil adjuvant
Owner:YEBIO BIOENG OF QINGDAO
A kind of vaccine composition and its preparation method and application
ActiveCN104288762BLow costReduce the number of vaccinationsAntibacterial agentsAntiviralsAntigenDisease
Owner:PU LIKE BIO ENG
A dual vaccine against avian metapneumovirus and h9 subtype avian influenza virus
ActiveCN105833263BGuaranteed immune effectImprove securitySsRNA viruses negative-senseViral antigen ingredientsF proteinTGE VACCINE
The invention provides a bivalent vaccine of an acian metapneumovirus and an H9subtype avian influenza virus. The bivalent vaccine comprises an antigen and an vaccine adjuvant, wherein the antigen is an H9 subtype avian influenza virus strain and acian metapneumovirus F protein; thepreservation numberof the H9 subtype avian influenza virus strain is CCTCC NO.V201517; and the amino acid sequence of the acian metapneumovirus F protein is SEQ ID NO:1. The H9 subtype avian influenza virus strain QDY and the acian metapneumovirus F protein antigen solution are mixed at the ratio, and then an oil adjuvant is added to the mixture to be mixed and emulsified into the vaccine. According to the prepared vaccine, the immunized antibody level can be improved, the uniformity of the immunized antibody is improved, the immune effect of the vaccine is ensured, and the vaccine has the advantages of high efficiency and good safety.
Owner:YEBIO BIOENG OF QINGDAO
Anti-porcine circovirus and porcine infectious pleuropneumonia vaccine composition and preparation
ActiveCN103656634BImprove immunityGuaranteed immune effectAntibacterial agentsAntiviralsAntigenImmune effects
The invention relates to a bivalent vaccine composition resisting type-2 porcine circovirus and porcine infectious pleuropneumonia infection and a preparation method. The bivalent vaccine composition comprises a type-2 porcine circovirus antigen and an actinobacillus pleuropneumoniae antigen; the immune procedure is simple; the type-2 porcine circovirus related diseases and porcine infectious pleuropneumonia can be effectively prevented at the same time. Compared with a single vaccine, the bivalent vaccine composition does not generate an immune interference phenomenon while the immune effect is not reduced; moreover, the actinobacillus pleuropneumoniae can enhance the immune effect of the type-2 porcine circovirus antigen. The preparation process of the bivalent vaccine composition is simple, the immune cost is low, and the practicability is high.
Owner:PULIKE BIOLOGICAL ENG INC
Vaccine composition containing swine mycoplasma pneumonia antigen and porcine streptococcal disease antigen and its preparation method and application
ActiveCN103861095BLow costReduce the number of vaccinationsAntibacterial agentsBacterial antigen ingredientsAntigenImmune effects
The invention provides a vaccine composition containing a swine mycoplasmal pneumonia antigen and a swine streptococcosis antigen, and a preparation method and an application thereof. The vaccine composition includes an immunizing dose of the swine mycoplasmal pneumonia antigen, an immunizing dose of the swine streptococcosis antigen, and an adjuvant. The vaccine composition has a simple immunization program, can effectively control the swine mycoplasmal pneumonia antigen and the swine streptococcosis antigen, has an immune effect equivalent to the immune effect realized through respective injection of single vaccines, and also has the characteristics of small side reaction, long immune period, less time consumption, and less labor consumption; and the vaccine composition also has the advantages of simple production technology, low immune cost and strong practicality.
Owner:PU LIKE BIO ENG
A kind of subunit vaccine for preventing type 4 avian adenovirus and its preparation method and application
ActiveCN108558989BGuaranteed immune effectImprove securityViral antigen ingredientsVirus peptidesAntigenAdjuvant
The invention discloses a subunit vaccine for preventing type-IV aviadenovirus as well as a preparation method and application thereof, which belongs to the field of veterinary biological products. The preparation method comprises the following steps: cloning and amplifying a C-end and an N-end structural domain genes of FadV 4C Hexon genes; establishing a recombinant baculovirus rBac-HEXON-P10-C-PH-N capable of co-expressing two antigen proteins by using an insect cell-baculovirus bidirectional expression system, wherein the recombinant virus efficiently expresses the FAdV 4C Hexon-C and Hexon-N antigen proteins in the insect cell HF; and extracting, purifying, BEI inactivation, and emulsifying by adding adjuvant, thus obtaining the vaccine. The preparation method is simple, a great amount of FAdV 4C Hexon-C and Hexon-N proteins can be simultaneously prepared, the consumed time is short, the expression amount is high, the production cost is greatly reduced, and the mass production canbe realized. The obtained recombinant subunit vaccine is good in immunity effect, small in immunity dose and capable of preventing the infection of the aviadenovirus.
Owner:扬州优邦生物药品有限公司
A w/o/w adjuvant composition, prepared vaccine composition and preparation method thereof
ActiveCN107596364BGuaranteed immune efficiencyNo effect on activitySsRNA viruses positive-senseViral antigen ingredientsAdjuvantSucrose
The invention relates to a w / o / w adjuvant composition, which comprises a sucrose fatty acid ester composition with an HLB value of 5 or less, fatty alcohol polyoxyethylene ether and oil. The vaccine composition used for the preparation of the adjuvant composition of the present invention is stable at different temperatures without delamination and demulsification, and the preparation conditions of the vaccine are simple and mild. The prepared vaccine quickly achieves the immune effect, and the vaccine has no adverse reaction when used.
Owner:LUOYANG SEIWEI BIOTECHNOLOGIES CO LTD
A Type b Avian Metapneumovirus Subunit Vaccine
ActiveCN105770884BGuaranteed immune effectImprove securitySsRNA viruses negative-senseViral antigen ingredientsPichia pastorisF protein
The invention provides a type B acian metapneumovirus subunit vaccine.The type B acian metapneumovirus subunit vaccine is prepared from an antigen and a vaccine adjuvant, wherein inactivated type B acian metapneumovirus F protein serves as the antigen, and the amino acid sequence of the type B acian metapneumovirus F protein is SEQ ID NO:1.According to the type B acian metapneumovirus subunit vaccine, genetic engineering pichia pastoris X33-F for expressing the type B acian metapneumovirus F protein is inoculated into a culture medium, the type B acian metapneumovirus F protein is efficiently expressed under the action of methyl alcohol, the type B acian metapneumovirus F protein is extracted and purified and then added into a formaldehyde solution to be inactivated, then an oil adjuvant is added for mixing emulsification, and the vaccine is prepared.The prepared vaccine can increase the level of an immunized antibody and improve the uniformity of the immunized antibody, and the immune effect of the vaccine can be ensured; the vaccine has the advantages of being efficient and good in safety.
Owner:YEBIO BIOENG OF QINGDAO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com