Preparation method of inactivated lyophilized vaccine for porcine circovirus type 2
A porcine circovirus, inactivation technology, applied in freeze-drying delivery, antiviral agents, viral antigen components, etc., can solve the problems of poor immune effect, high side reaction rate, difficult to absorb, etc., and achieve easy injection and high antigen content. , the effect of easy absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 (vaccine preparation method)
[0031] 1 Instruments and reagents
[0032] 1.1 The instrument used in the embodiment is as follows:
[0033] Milipore’s 0.5-0.2um double-layer filter element; Cobetter sleeve; Milipore’s cross-cut UF concentration membrane package, 100KD; GE’s XK50 / 100 column; GE’s AKTA Primer plus chromatography system; Bio-Rad Protein detector; Pudong small freeze dryer.
[0034] 1.2 The reagents used in the embodiment are as follows:
[0035] (1) 100L porcine circovirus type 2 virus liquid, 10 7.2 TCID 50 / ml, produced by our company;
[0036] (2) 100L of 1M NaOH; 100L of 0.1M PBS; 100L of high-purity water;
[0037] (3) Trehalose, sucrose, lactose, skim milk, glycine.
[0038] 2 operation steps
[0039] 2.1 Clarification of porcine circovirus type 2 venom
[0040] Under sterile conditions, the harvested venom is filtered through 8 layers of sterile gauze, and the primary filtered venom is clarified through a 0.5um-2um double-layer...
Embodiment 2
[0079] Embodiment 2 (vaccine safety test prepared in embodiment 1)
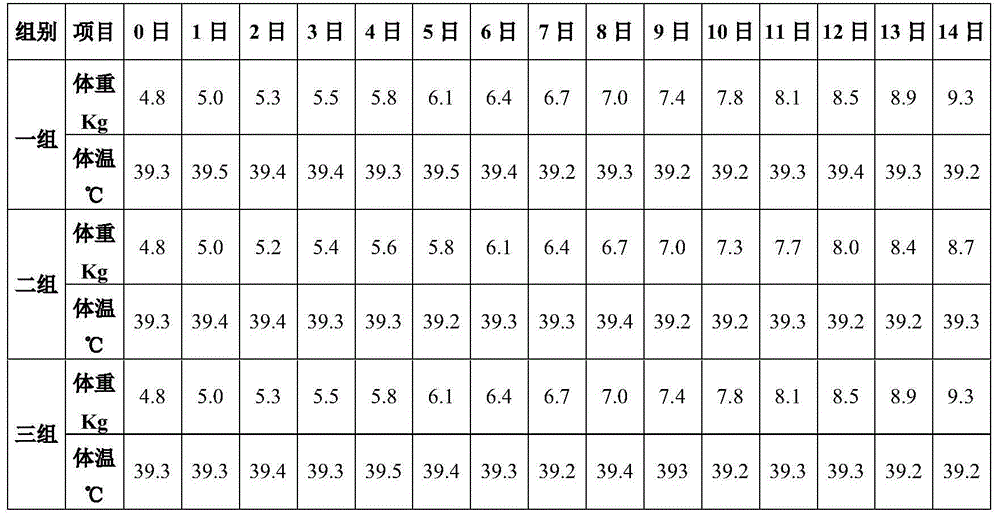
[0080] The PCV2 inactivated freeze-dried vaccine 1 prepared by the above process, the PCV2 white oil adjuvant inactivated vaccine 2 prepared by the conventional process, and sterile 0.01M PBS solution were used as experimental reagents; 20 days old PCV2 ELISA antibody negative, PCV2 antigen negative healthy easy 15 affected piglets were used as experimental animals. The specific operation steps are as follows:
[0081] 1 animal group
[0082] 15 piglets were randomly divided into 3 groups, 5 piglets in each group.
[0083] 2 vaccine immunization
[0084] Inject the two vaccines and PBS into the experimental piglets intramuscularly in the neck, as follows:
[0085] In the first group, 4ml PCV2 inactivated freeze-dried vaccine 1 was injected intramuscularly into the neck of each piglet, a total of 5 piglets;
[0086] The second group, the PCV2 white oil adjuvant inactivated vaccine 2 that every head piglet...
Embodiment 3
[0095] Embodiment 3 (the vaccine potency test that embodiment 1 prepares)
[0096] 1 Mice challenge test
[0097] PCV2 inactivated freeze-dried vaccine 1, PCV2 white oil adjuvanted inactivated vaccine 2 prepared by conventional techniques, and sterile 0.01M PBS solution were used as experimental reagents; 30 6-week-old healthy Balb / C mice were used as experimental animals. The specific experimental steps are as follows:
[0098] 1.1 Animal grouping
[0099] Thirty Balb / C mice were randomly divided into 3 groups, 10 in each group.
[0100] 1.2 Vaccine Immunization
[0101] Two kinds of vaccines and PBS were intraperitoneally injected into experimental mice, as follows:
[0102] In the first group, each mouse was intraperitoneally injected with 0.2ml PCV2 inactivated freeze-dried vaccine 1, a total of 10 mice;
[0103] The second group, each mouse was intraperitoneally injected with 0.2ml PCV2 white oil adjuvant inactivated vaccine 2 prepared by conventional technology, tot...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com