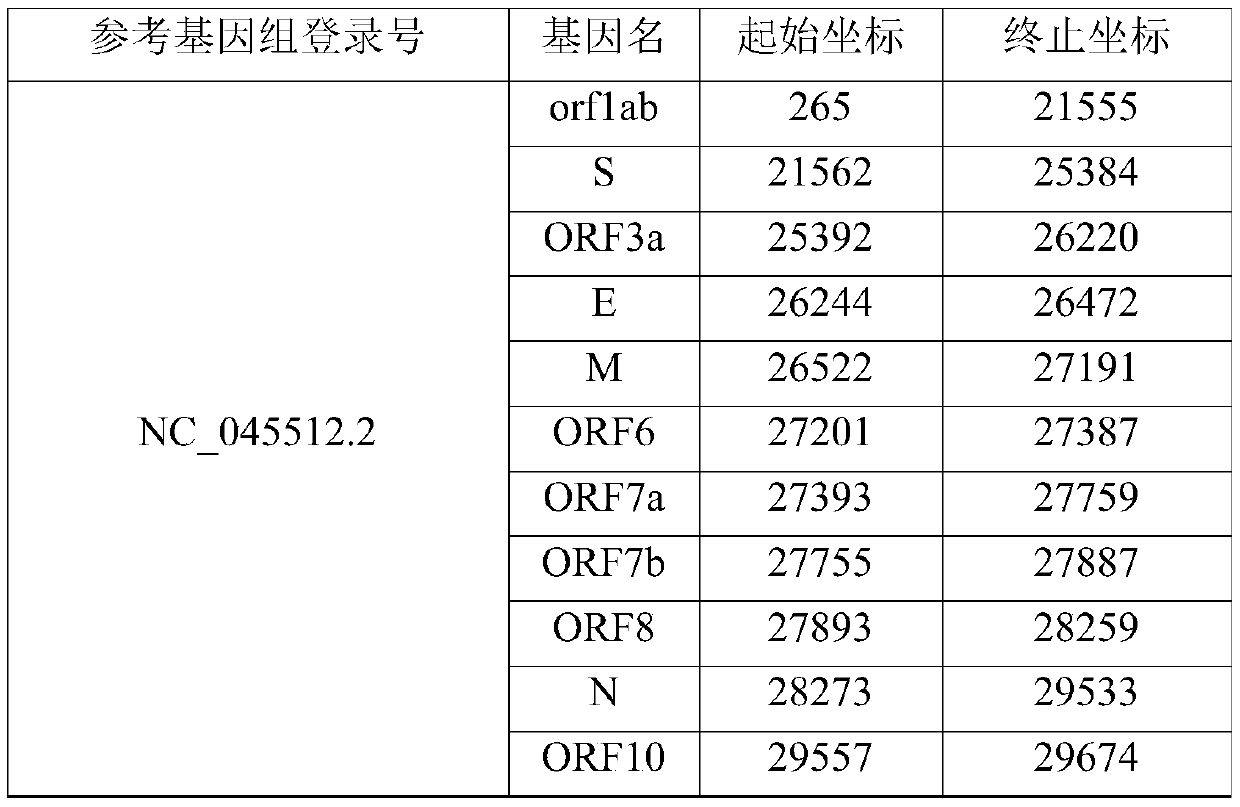
Patents
Literature
155 results about "Viral culture" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Viral culture is a laboratory test in which samples are placed with a cell type that the virus being tested for is able to infect. If the cells show changes, known as cytopathic effects, then the culture is positive.
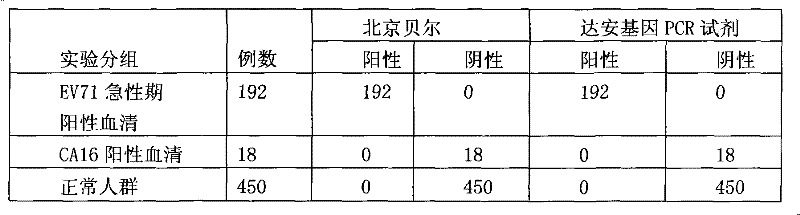
Diagnostic reagent kit (enzyme-linked immunosorbent assay (ELISA)) for enterovirus (EV) 71-type antibody (immune globulin M (IgM))
InactiveCN102243232AEasy to operateAvoid distractionsColor/spectral properties measurementsEnterovirusAbzyme
The invention relates to the field of biomedicine, in particular to an enzyme-linked immunization diagnostic reagent kit for detecting an enterovirus (EV) 71-type antibody (immune globulin M (IgM)), and a preparation method and application of the diagnostic reagent kit. The probability of hand-foot-and-mouth disease and severe infection (viral encephalitis, viral cerebrospinal meningitis and pulmonary edema) caused by EV71 type is relatively higher, and case fatality rate is relatively higher and can be 10 to 25 percent. The enzyme-linked immunization diagnostic reagent kit of the EV71-IgM antibody can be used for diagnosing the infection of the EV71 type. According to related documents about the detection of the EV71-IgM, EV71 virus cultures serving as indirect enzyme-linked immuno sorbent assay (ELISA) of envelope antigens has defects in such aspects as specificity, sensitivity and stability, and due to high cultivation cost and low efficiency, a large amount of virus cannot be supplied to the market. In order to overcome the defects, the invention provides the reagent kit which is used for detecting the EV71-IgM in human blood serum, required by clinical examination, simple and convenient to operate and applicable to all medical disease control departments, and the preparation method and the application of the reagent kit. The invention has the technical scheme that: firstly, the human blood serum is added into a micro-pore plate, wherein the IgM antibody is obtained by an anti-mu chain which is pre-enveloped on the micro-pore plate, and other uncombined components are washed and removed; secondly, an enzyme labeling object is added, the EV71-IgM in the obtained IgM can be combined with the specificity of an EV71 recombinant antigen which is labeled by horse radish peroxidase (HRP), and after washing, the HRP can react with substrates which are added subsequently; and finally, the aim of detecting the EV71-IgM antibody is fulfilled.
Owner:BEIJING BEIER BIOENG
Permissive cells and uses thereof
ActiveUS20100158947A1Compound screeningSsRNA viruses positive-senseVaccine ProductionFamily Arteriviridae
Owner:UNIV GENT ENGLISH TRANSLATION BEING GHENT UNIV
ST cell adapting to full-suspension culture, and application thereof, and vaccine virus culturing method
ActiveCN105112352ASolve the requirements of cultivating large-scale industrializationHigh degree of automationMicroorganism based processesVertebrate cellsVaccine virusEngineering
The invention relates to an ST cell adapting to full-suspension culture, and an application in vaccine virus culturing, and belongs to biotechnical field. The ST cell adapting to full-suspension culture is named as a swine testicle cell suspension adaptation strain ST-2014S, and is preserved in China Center for Type Culture Collection on May 13, 2015 with the preservation number of CCTCC C201567. The invention also discloses a method for culturing a vaccine virus through using the full-suspension adaptation ST cell. Compared with vaccine viruses in the prior art, the full-suspension adaptation ST cell cultured vaccine virus has the advantages of high automation degree, no need of vector intervention, realization of suspension culture in a serum-free or low-serum medium, meeting of large-scale industrial requirements of virus culture, development of a large-scale production method meeting GMP production technology requirements, and very good industrial application prospect.
Owner:郑州爱科生物科技有限公司
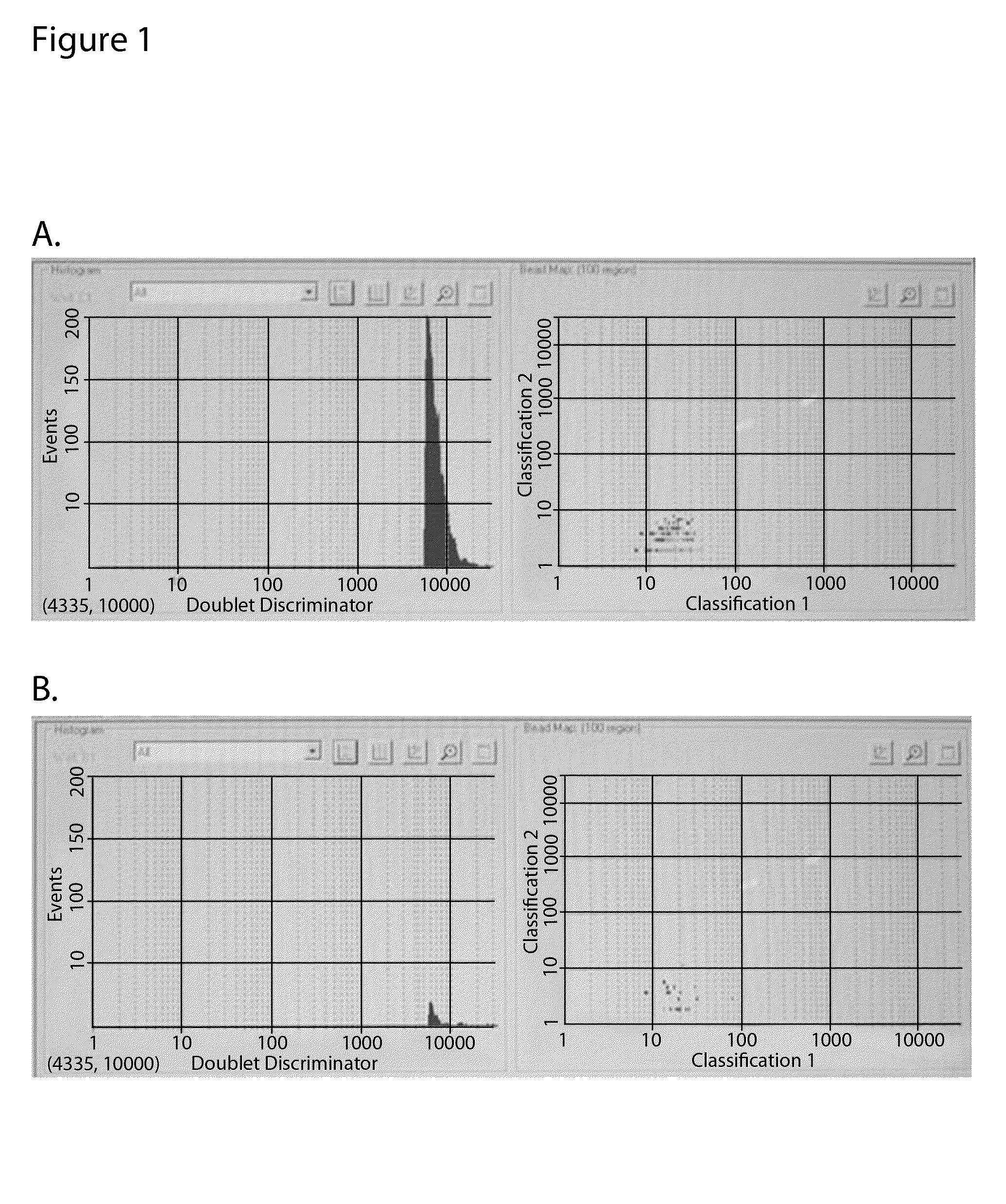
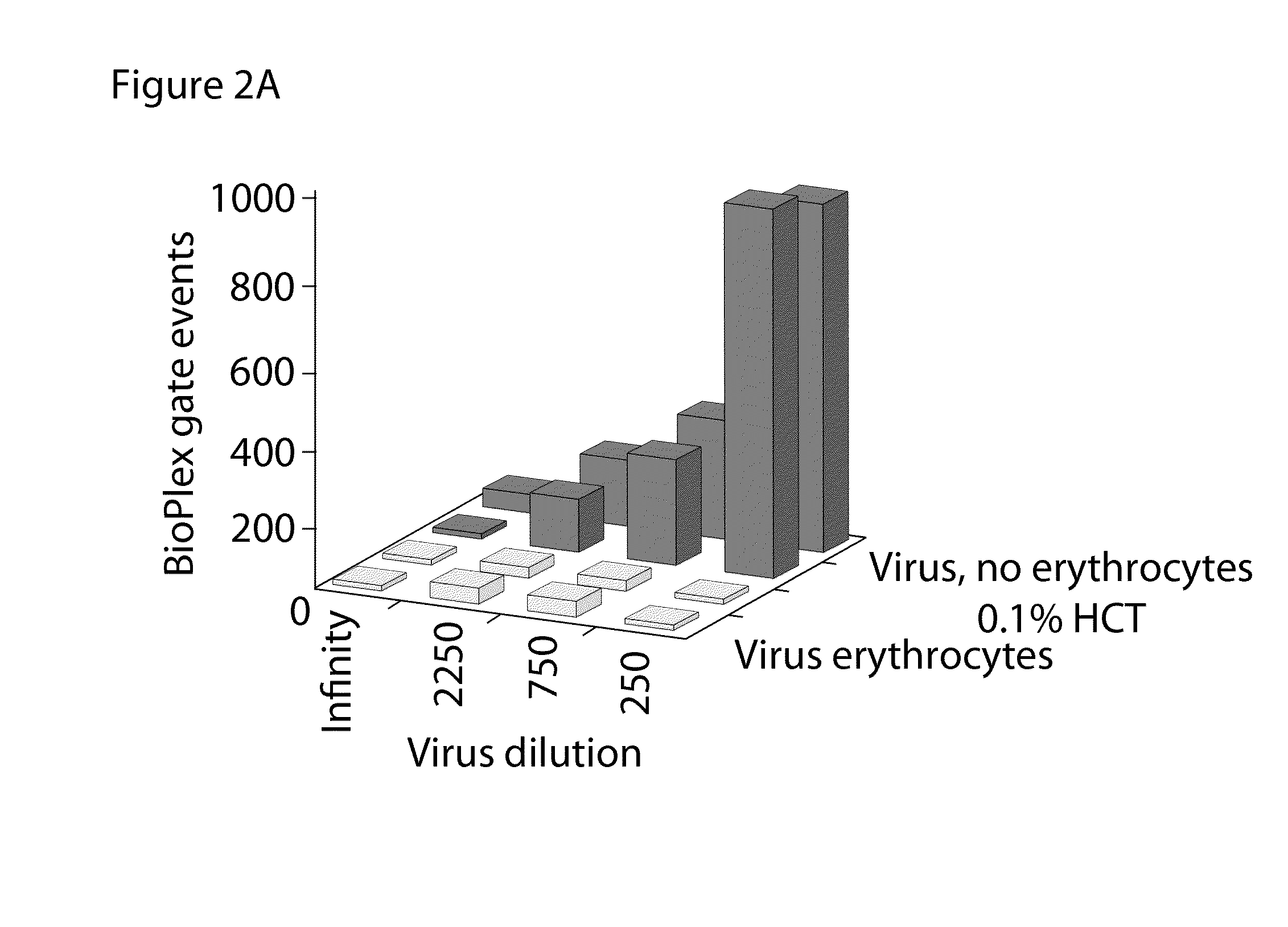
Bead Array Reader Based-Hemagglutination and Hemagglutination Inhibition Assay
InactiveUS20090325148A1High sensitivityMicrobiological testing/measurementBiological material analysisViral VaccineAnti viral immunity
Hemagglutination assays and hemagglutination inhibition assays were introduced in medical and virology practice more than 60 years ago. Since then, these assays have become important tools for measuring concentrations and strengths of viral cultures, the efficacy of the anti-viral immunization, and for studying the neutralizing capacity of virus-specific antibodies. The present invention comprises an improved hemagglutination inhibition assay (HAI), with at least about a 10-fold increase in sensitivity versus the traditional the HAI, to provide more accurate measurements of components in, for example, fluids from the in vitro MIMIC® system when assessing the effects of anti-viral vaccines (e.g., for seasonal influenza).
Owner:SANOFI PASTEUR VAX DESIGN
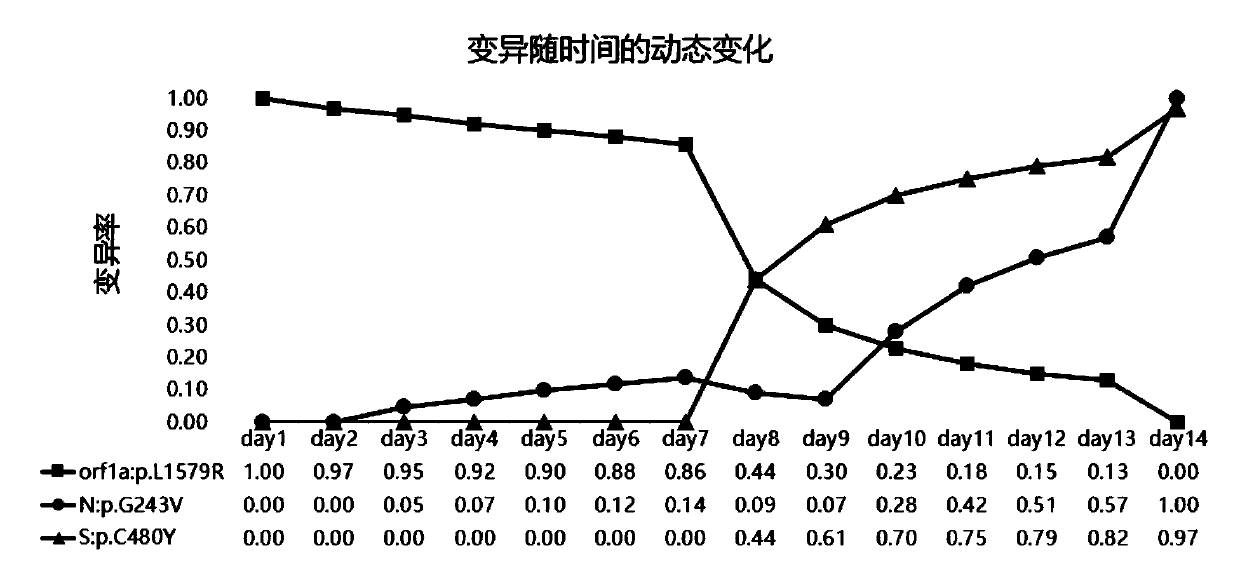
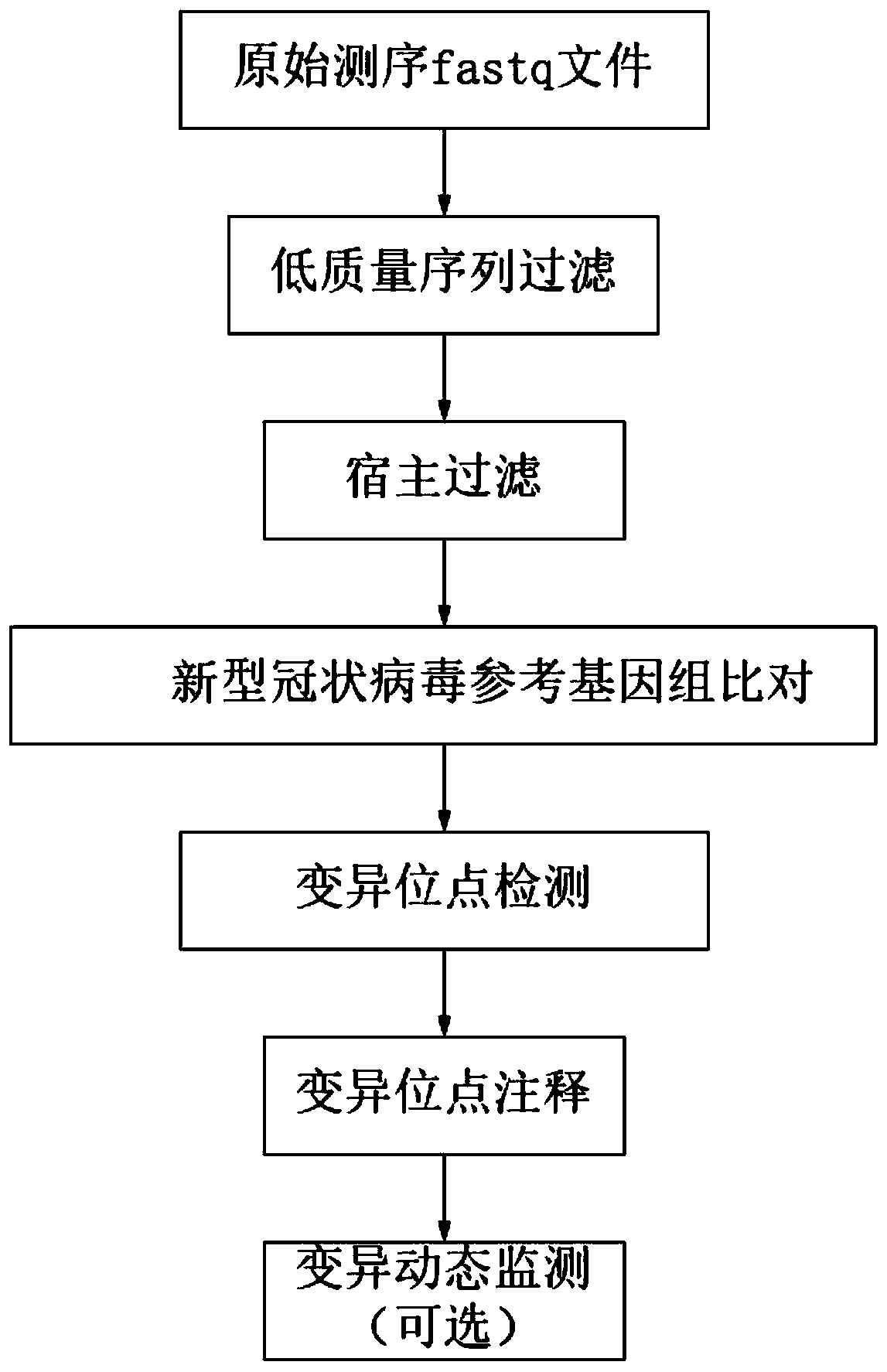
Novel coronavirus variation analysis method and application
ActiveCN111445955AAccurate NotesImprove accuracySequence analysisInstrumentsGenomic sequencingMedicine
The invention relates to a novel coronavirus variation analysis method and application, and belongs to the technical field of gene sequencing analysis. The method comprises the steps of data acquisition, data filtering, data comparison, variation detection, coordinate analysis, coordinate correction and variation annotation. According to the method, variation detection can be carried out on pure virus culture sequencing data, variation detection can also be carried out on metagenome sequencing data, and the application range is wider; and meanwhile, ribosome transcoding can be accurately annotated, and joint mutation can be accurately annotated, so that the mutation detection accuracy is improved; and in addition, the virus variation can be dynamically monitored.
Owner:广州微远医疗器械有限公司 +3
Pig Getah virus strain, vaccine composition, and preparation method and application thereof
ActiveCN106636012AImproving immunogenicityReduce yieldSsRNA viruses positive-senseViral antigen ingredientsMicroorganismAdjuvant
The invention discloses a newly separated pig Getah virus HNJZ-S1 strain. The microbial preservation number of the pig Getah virus HNJZ-S1 strain is CGMCC NO 12550; the pig Getah virus is separated from a naturally infected pig group for the first time in China, and has good immunogenicity. The invention also discloses a vaccine composition which is prepared from the pig Getah virus HNJZ-S1 strain, and a preparation method thereof. The vaccine composition contains inactivated pig Getah virus strain HNJZ-S1 strain and a pharmaceutically acceptable adjuvant; the preparation method for the vaccine composition comprises the steps of virus culturing, virus liquid inactivating, aqueous phase preparing, oil phase preparing emulsifying and the like. The vaccine composition has good immunogenicity, and can produce high immunity after immunization; the attacking protecting rate is high; after inoculation, the abortion rate and the stillbirth rate of sows are obviously reduced; the incidence of piglets is remarkably reduced; the popularizing and spreading of the pig Getah virus can be effectively prevented; the vaccine composition has a broad application prospect.
Owner:HENAN AGRICULTURAL UNIVERSITY
Large-scale full-suspension culture method of porcine circovirus type 2
ActiveCN107312746AReduce dosageImprove immunityViral antigen ingredientsMicroorganism based processesEngineeringPorcine kidney
The invention discloses a large-scale full-suspension production method of a porcine circovirus type 2. The inventors of the invention domesticate a porcine kidney cell adaptable to large-scale serum-free full-suspension culture, which is named as sPK15-YP, is collected in the China General Microbiological Culture Collection Center and has the culture collection number of CGMCC NO.13846. The sPK15-YP cell adaptable to full-suspension culture is used for achieving serum-free large-scale culture of the porcine circovirus type 2 (PCV2), so that the conventional spinner bottle culture technology is replaced, the human resource is reduced, the product quality is improved, and the bottleneck that the virus content is low during PCV2 full-virus culture is solved; by a full-suspension sPK15-YP cell technology, a high-potency PCV2 semifinished product is prepared; by a hollow fiber method, a PCV2 virus culture solution is concentrated and purified to obtain a more pure PCV2 virus concentrated antigen. By the large-scale full-suspension production method, a solid foundation is laid for studying a PCV2 multivalent vaccine, the dosage of the vaccine is reduced, the stress of a swine herd is reduced and the immune level of the swine herd is enhanced.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Method for cultivating ginger-free viruses and cultivating virus-free seedlings by means of propagating and rooting synchronously
InactiveCN102144565AInhibition of replicationEfficient removalPlant tissue cultureHorticulture methodsShoot apexBud
Owner:CHINA PHARM UNIV
Production method of canine parvovirus inactivated vaccine by utilizing bioreactor
InactiveCN102861329AAvoid process problemsAvoid timeAntiviralsAntibody medical ingredientsAntigenAdjuvant
The invention discloses a production method of a canine parvovirus inactivated vaccine by utilizing a bioreactor. The production method comprises the following steps: (1) culturing a vaccine producing cell by applying a microcarrier and chip carrier system of the bioreactor; (2) vaccinating a canine parvovirus and performing virus multiplication culture; (3) harvesting virus culture fluid; and (4) inactivating the virus fluid with BEI (binary ethyleneimine), and adding adjuvant to prepare the vaccine. The canine parvovirus inactivated vaccine has the advantages of high density of cultured cell, high virus titer, uniform and stable quality, controllable process and high production efficiency, and the defects of large difference among product batches, low antigen content and low production efficiency in a conventional spinner bottle or chick embryo production process can be overcome.
Owner:WUHAN CHOPPER BIOLOGY
Method for preparing pseudorabies virus
ActiveCN101979518ANo pollution in the processIncrease productionRecovery/purificationGene defectHigh density
The invention relates to a method for producing a gene defect pseudorabies virus by culturing a passage cell in a tidal biological reactor. The method comprises the following steps of: (1) subcultring a recovery cell; (2) performing high-density cell culturing by using a specific biological reactor and a carrier system, attaching the cell on a vector, circulating a culture medium, filling oxygen and adjusting pH value and glucose; (3) performing virus infection by using the biological reactor, replacing the culture medium by using a virus culture medium, and inoculating the virus into the virus culture medium so as to ensure that the virus is fully adsorbed on the cell; (4) culturing and amplifying the virus under a specific condition; and (5) harvesting viruses so as to realize mass production.
Owner:PU LIKE BIO ENG
Production of vaccines
InactiveUS20060063261A1Inhibit bindingSsRNA viruses negative-senseSsRNA viruses positive-senseSerum freeMammal
Means and methods for producing mammalian viruses, the method comprising infecting a culture of immortalized human cells with a virus, incubating the culture infected with virus to propagate the virus under conditions that permit growth of the virus, and to form a virus-containing medium, and removing the virus-containing medium. The viruses can be harvested and be used for the production of vaccines. Advantages include that human cells of the present invention can be cultured under defined serum-free conditions and the cells show improved capability for propagating virus. Methods are provided for producing, in cultured human cells, influenza virus and vaccines derived thereof. This method eliminates the necessity of using whole chicken embryos for the production of Influenza vaccines. The method also provides for the continuous or batch-wise removal of culture media. As such, the present invention allows the large-scale continuous production of viruses to a high titer.
Owner:JANSSEN VACCINES & PREVENTION BV
Method for preparing pseudorabies vaccines
InactiveCN101979519ANo pollution in the processIncrease productionAntiviralsRecovery/purificationHigh densityVector system
The invention relates to a method for producing pseudorabies vaccines by culturing passage cells by using a tidal bioreactor, which comprises the following steps of: (1) subculturing recovered cells; (2) performing high-density culture on the cells by using the specific bioreactor and a vector system to attach the cells onto vectors, and performing circulation, oxygenation and pH value and glucose regulation on a culture medium; (3) performing virus infection by using the bioreactor, replacing the culture medium with a virus culture medium, inoculating viruses and completely absorbing the viruses onto the cells; (4) culturing and amplifying the viruses under specific condition; (5) harvesting the viruses; and (6) adding a lyophilization protecting agent, and performing uniform mixing, quantitative subpackaging and lyophilization to obtain the pseudorabies vaccines.
Owner:PU LIKE BIO ENG
SARS-CoV-2 inactivated vaccine and preparation method of vaccine
ActiveCN111569058AReduce Biosecurity RisksLow impurity contentSsRNA viruses positive-senseViral antigen ingredientsVirus inactivationTGE VACCINE
The invention relates to a SARS-CoV-2 inactivated vaccine and a preparation method of the vaccine. The preparation method comprises the following steps: step (1) recovery and large-scale culture of Vero cells; step (2) inoculation of working seeds, batch of virus seeds, virus culture and one-time harvest virus liquid, namely, the virus harvest liquid; step (3) obtaining an inactivated virus concentrated solution after a first virus inactivation, concentration and a second virus inactivation; step (4) obtaining a vaccine stock solution after purifying the inactivated virus concentrated solution; and step (5) preparation of semi-finished product and sub-package after the stock solution is verified to be qualified.
Owner:中国生物技术股份有限公司 +1
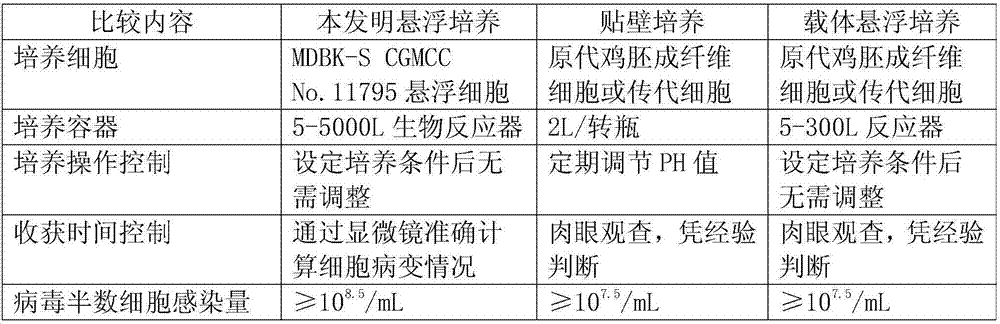
Application of bovine kidney cells capable of being subjected to suspension culture to virus culture and vaccine production
ActiveCN107201333AEliminate repetitive domestication workThe production scale-up process is simpleSsRNA viruses negative-senseSsRNA viruses positive-senseLiquid ChangeVaccine Production
The invention discloses application of bovine kidney cells capable of being subjected to suspension culture to virus culture and vaccine production. The application has the advantages that a large quantity of manual operation for cell digestion, liquid change, frequent seed separation and the like can be omitted by bovine kidney cell (MDBK) suspension culture methods, the suspension culture methods are simple, convenient and speedy, processes can be automated by the aid of reactor culture, the cell yield of the unit volume can be increased, and the technical difficult problem of difference between adherent culture cell batches can be solved.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Preparation method of mink canine distemper virus live vaccine and vaccine prepared by same
InactiveCN105816869AIncrease culture densityIncrease productionSsRNA viruses negative-senseViral antigen ingredientsFreeze thawingSide effect
The invention relates to the field of veterinary biological products and particularly relates to a preparation method of a mink canine distemper virus live vaccine. The method comprises the following steps: inoculating a bioreactor with sensitive cells for vaccine preparation, and culturing by using a micro-carrier; after the sensitive cells are cultured by over 50% and grow into a dense single layer, inoculating the bioreactor with canine distemper virus for enrichment culture; harvesting the virus culture liquid and micro-carrier; performing freeze-thawing and removing the micro-carrier and cell debris to obtain virus liquid; and blending the virus liquid to obtain the vaccine. By improving the reaction conditions of each step and optimizing the production flow, the method provided by the invention realizes the technical effects of short production cycle, high virus titer, stable product quality, increased production efficiency and low side effect.
Owner:INST OF SPECIAL ANIMAL & PLANT SCI OF CAAS +1
MDCK cell line, method for duplicating viruses and application of method
InactiveCN108359632AReduce the risk of contamination from external sourcesIncrease the culture speedSsRNA viruses negative-senseArtificial cell constructsVaccine ProductionMicrobiology
The invention relates to the field of cell culture, in particular to an MDCK cell line, a method for duplicating viruses and application of the method. The cell line is preserved in China Center for Type Culture Collection, the preservation number is CCTCC NO: C201857, and the preservation time is February 10, 2018. The MDCK cell line provided by the invention is not only suitable for the full-suspension culture, but also can adopt animal-source-free serum culture medium to culture, so that the pollution risk of an external source in the virus culture or vaccine production can be reduced.
Owner:吉林冠界生物技术有限公司
Production of vaccines
InactiveUS20060051747A1Inhibit bindingSsRNA viruses negative-senseSsRNA viruses positive-senseSerum freeHuman cell
Means and methods for producing mammalian viruses, the method comprising infecting a culture of immortalized human cells with a virus, incubating the culture infected with virus to propagate the virus under conditions that permit growth of the virus, and to form a virus-containing medium, and removing the virus-containing medium. The viruses can be harvested and be used for the production of vaccines. Advantages include that human cells of the present invention can be cultured under defined serum-free conditions and the cells show improved capability for propagating virus. Methods are provided for producing, in cultured human cells, influenza virus and vaccines derived thereof. This method eliminates the necessity of using whole chicken embryos for the production of Influenza vaccines. The method also provides for the continuous or batch-wise removal of culture media. As such, the present invention allows the large-scale continuous production of viruses to a high titer.
Owner:JANSSEN VACCINES & PREVENTION BV
Method for amplifying porcine circovirus type 2 by applying bioreactor and flaky vector
InactiveCN103614344AReduce labor intensityMechanizationMicroorganism based processesViruses/bacteriophagesPorcine circovirusBottle
The invention relates to a method for amplifying porcine circovirus type 2 by applying a bioreactor and a flaky vector, and belongs to the technical field of biological products. The method comprises the following steps: (1) seed cell preparation; (2) bioreactor preparation; (3) PK-15 cell suspension culture; (4) PK-15 cell suspension virus culture; (5) cell suspension virus culture and continuous harvesting; and (6) virus liquid culture period and harvest yield. The method disclosed by the invention exceeds multiple technical bottlenecks of a spinner bottle production process and provides a process which obtains high-titer porcine circovirus type 2 (PCV2) virus liquid with high efficiency, sustainability and large-scale production by carrying out suspension culture on a PK15 cell by utilizing the flaky vector.
Owner:扬州优邦生物药品有限公司
Method for suspension culture of Newcastle disease virus (NDV) by using full-suspension passage cell line
InactiveCN108220227AAvoid the risk of foreign virusesEliminate hidden dangersSsRNA viruses negative-senseBioreactor/fermenter combinationsNewcastle disease virus NDVTiter
The invention relates to a method for suspension culture of Newcastle disease virus (NDV) by using a full-suspension passage cell line. The method comprises the following steps: inoculating the full-suspension EB66 cell line having a growth density of 8*10<6> cells / mL to 16*10<6> cells / mL with the NDV by using a two-stage culture method, continuously culturing after the virus is inoculated, and sampling every 12 hours so as to determine the EID50 of the virus; harvesting the virus when the titer of the virus is maximum, and storing the harvested virus so as to complete the culture of the NDV.The method adopts the duck embryonic stem cell EB66 passage cell line to culture the chicken NDV, thus effectively improving the titer and purity of the obtained virus, further enabling produced vaccines to have higher quality, and lowering the production cost.
Owner:ZHAOQING INST OF BIOTECHNOLOGY CO LTD +2
Newcastle disease (ND) vaccine, and its production method
InactiveCN102600465AGuaranteed to be pureEnsure safetyViral antigen ingredientsAntiviralsEmbryoControl quality
A production method of Newcastle disease (ND) vaccine includes (1) adding virus growth solution to chick-embryo-cultured inoculation subculture cell line of attenuated ND virus, and culturing to obtain cell-adapted vaccine seed virus; adding virus culture maintenance medium to inoculation subculture cell line of cell-adapted vaccine seed virus, and culturing to obtain proliferated virus suspension; (3) determining titer of the proliferated virus suspension, preparing vaccine from qualified suspension, sub-packaging, and lyophilizing. The production method has advantages of simple and stable production process, easy operation, high virus content, small batch difference, easily controlled quality, rapid and accurate titer determination, improved vaccine yield and quality, high vaccine safety, high immune efficacy, and complete immuno-protection effect against ND virus.
Owner:SINOVET BEIJING BIOTECH +1
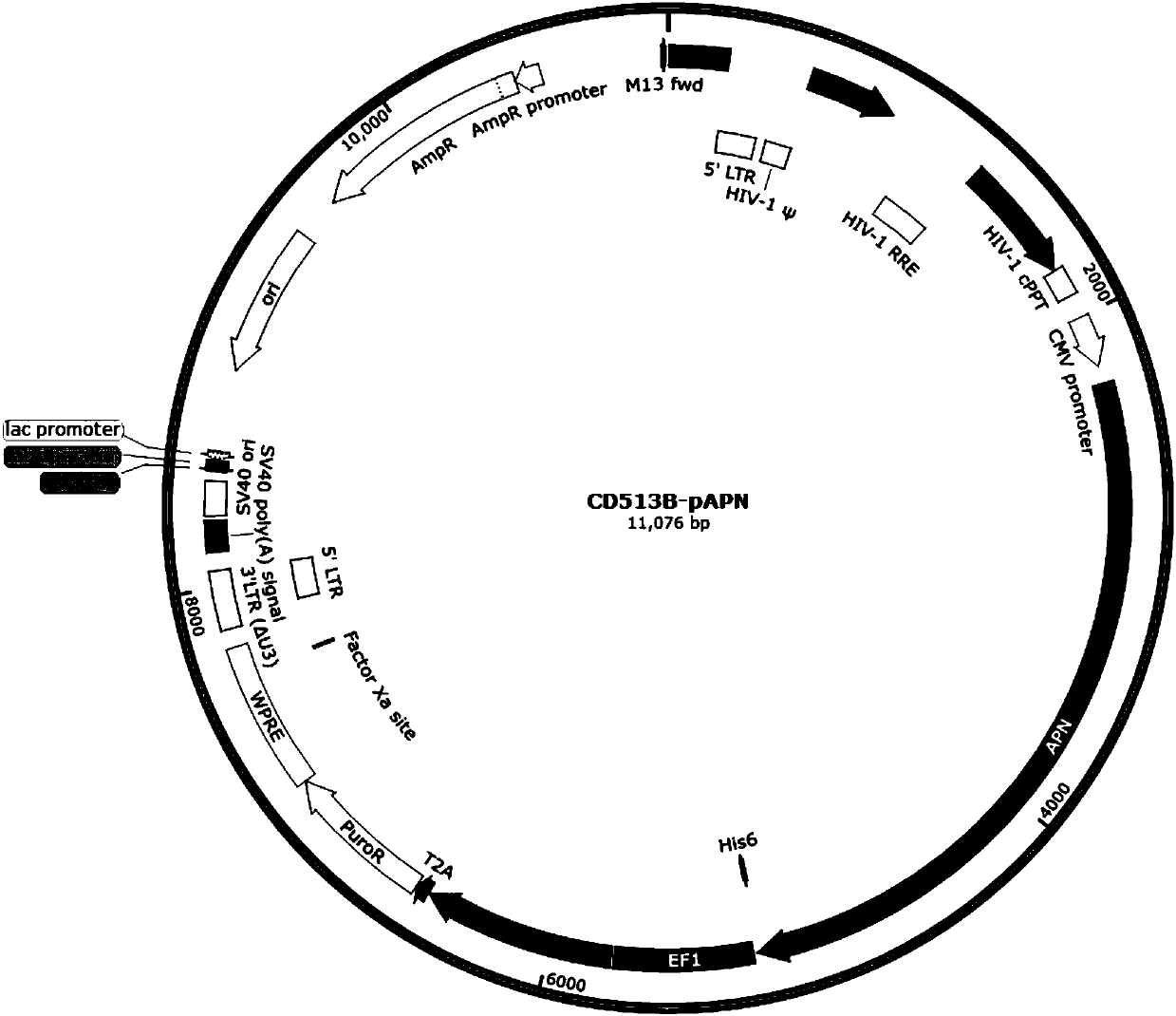
Vero-pAPN (Porcine Aminopeptidase N) cell line and preparation method thereof
InactiveCN107794244ALow efficiencyStable expressionFermentationVector-based foreign material introductionAminopeptidase NTransfer mode
The invention relates to a Vero-pAPN (Porcine Aminopeptidase N) cell line. An adopted construction method is a slow virus mediating method. Compared with a Vero cell line which is constructed by parental Vero cells and an instant transfer mode and is used for expressing pAPN, cells of the cell line disclosed by the invention can stably express APN protein at a higher level and improve the PEDV (porcine epidemic diarrhea virus) breeding titer, and become host cells more suitable for multiplication of PEDV. The cells can be applied to subsequent virus culture, separation and purification, and provide host raw materials for preparation of vaccines.
Owner:杨凌凯瑞生物科技有限公司
Vero E6 cell strain adapting to full-suspension culture and application thereof
ActiveCN107267443AHigh degree of automationSolve the requirements of cultivating large-scale industrializationArtificial cell constructsViruses/bacteriophagesSerum freeVaccine virus
The invention discloses a vero E6 cell strain adapting to full-suspension culture, the vero E6 cell strain is named as vero E6-s, and is preserved in China Center for Type Culture Collection, the preservation number is CCTCC2017101, the vero E6 cell strain is classified and named as Vero cell suspension adaptive strain VeroE6-S, and the preservation date is June 29, 2017. Compared with the prior art, the vero E6 cell strain adapting to the full-suspension culture has high degree of automation for culture of vaccine viruses, can be suspended and cultured in a serum-free or low-serum medium without carrier intervention, and solves large-scale industrialization requirements of virus cultivation, a large-scale production method meeting GMP production technology requirements can be developed, and the large-scale production method has a good prospect of industrialization.
Owner:郑州爱科生物科技有限公司
Method for producing human diploid cell encephalitis B inactivated vaccine
InactiveCN102406927AImprove quality and safetyImprove quality stabilityViral antigen ingredientsAntiviralsUltrafiltrationDiploid cells
The invention relates to the technical field of biology, in particular to a method for producing a human diploid cell encephalitis B inactivated vaccine. The method sequentially comprises the following steps of: resuscitating and subculturing a human diploid cell strain; culturing the human diploid cell strain by using a multi-stage bioreactor; inoculating an encephalitis B virus strain, and performing virus multiplication culture; harvesting a virus culture solution; and inactivating the virus culture solution, performing ultrafiltration, purifying, adding a glycoprotein protective agent, and preparing the vaccine. Human diploid cells are used as a cell matrix, so that the vaccine is safe and is free from exogenous factor pollution; the human diploid cells are cultured by the bioreactor through stage-by-stage linear amplification, so that the large-scale production of the vaccine with low cost, high quality, safety and stability is easy to implement; and the titer of the virus harvested solution is subjected to sampling inspection every day, so that the high expression of the harvested virus culture is ensured, and production quality is ensured.
Owner:CHENGDU KANGHUA BIOLOGICAL PROD
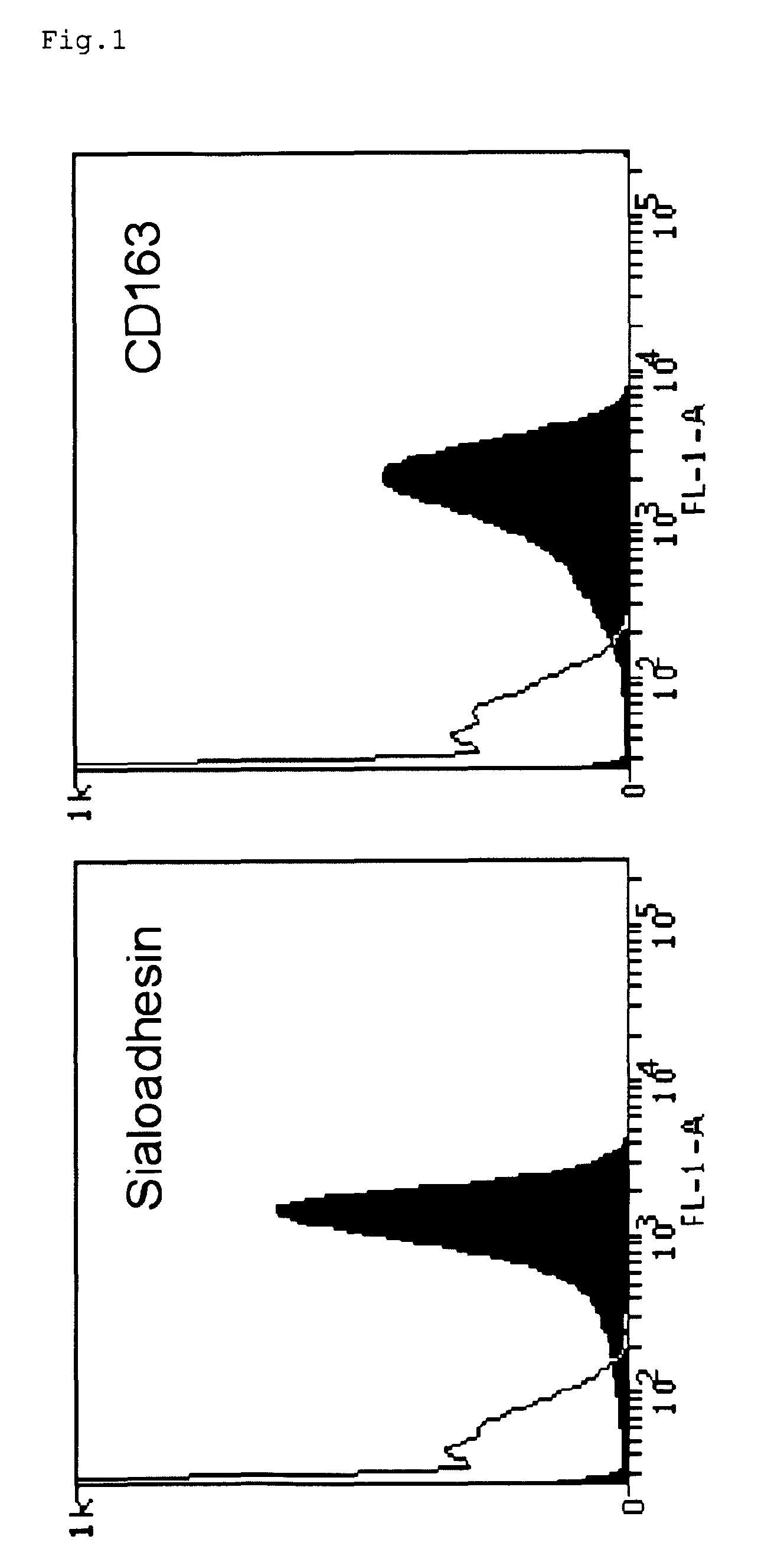
Permissive cells and uses thereof
ActiveUS8420373B2Enhanced virusPermissivity of partially susceptible cells can be increasedCompound screeningSsRNA viruses positive-senseVaccine ProductionFamily Arteriviridae
The invention relates generally to the field of virology. More particularly, the present invention relates to methods for determining the permissiveness of a cell for a virus that is a member of the family Arteriviridae or Coronaviridae or Asfarviridae, in particular for Porcine Reproductive and Respiratory Syndrome Virus (PRRSV). The invention further provides methods and compositions related to the generation of host cells permissive for a virus that is a member of the family Arteriviridae or Coronaviridae or Asfarviridae, in particular for Porcine Reproductive and Respiratory Syndrome Virus (PRRSV). Methods of using said cells thus identified or thus generated, in preparing a culture of a virus that is a member of the family Arteriviridae or Coronaviridae or Asfarviridae, as well as the use of said virus for the purpose of vaccine production or diagnosis, are also provide by the present invention.
Owner:UNIV GENT ENGLISH TRANSLATION BEING GHENT UNIV
Fast and sensitive method of detecting virus infection titer of attenuated live hepatitis A vaccine
ActiveCN1772920ACalculation of infectivity titersReduce usageMicrobiological testing/measurementAgainst vector-borne diseasesHepatitis A vaccinePcr method
The present invention provides one kind of fast and sensitive method for detecting virus infection titer of attenuated live hepatitis A vaccine. The method determines the existence of the virus via detecting the negative brand RNA intermediate of marker appearing during duplicating hepatitis A virus, and is superior to available inverse transcription PCR method and cell culturing method. The method has culture period shortened to 8 days, short detection period, no need of replacing maintaining liquid, less contamination possibility, less cell post-treating steps, high work efficiency and capacity of providing virus heredity information via sequencing the PCR product.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Infectious bursal disease virus Vero cell-adapted strain and application thereof
InactiveCN103923885AStable proliferationImprove securityViral antigen ingredientsMicroorganism based processesOil emulsionEmbryo
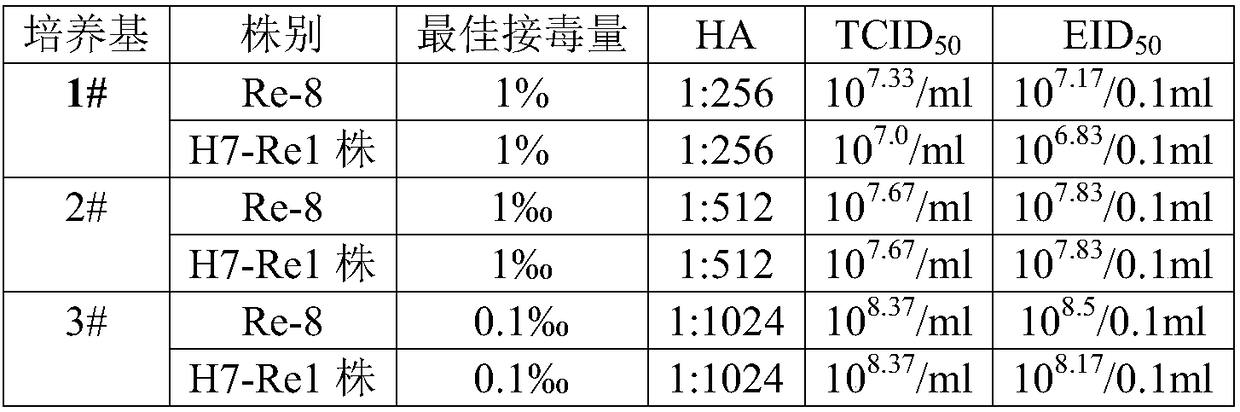
The invention provides an infectious bursal disease virus Vero cell-adapted strain and belongs to the field of bioengineering. The related infectious bursal disease virus Vero cell-adapted strain is named Ck / Jiangsu / NJ-23 / 2008 and has a collection No. of CGMCCNO.8852. The infectious bursal disease virus Vero cell-adapted strain which can efficiently multiply on serum-free cultured Vero cell is finally obtained through wild strain separation, chick embryo passage, Vero cell passage adaption; the infectious bursal disease virus Vero cell-adapted strain is subjected to continuous passage culture on the serum-free cultured Vero cell and TCID50 can be kept to be higher than 108.5 / mL. Virus culture solution is inactivated and prepared into oil emulsion; after the prepared oil emulsion is used to immunize chicken, detection proves that the prepared oil emulsion has good immunogenicity. The infectious bursal disease virus (IBDV) strain and the production process thereof are simple, safe and efficient and suitable for industrial culture.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Method for preparing swine fever live vaccines
InactiveCN103157105AIncreased sensitivityUniform stateAntiviralsRecovery/purificationPublic healthTreatment fever
The invention relates to a method for preparing swine fever live vaccines, and belongs to the field of biotechnology. The method for preparing the swine fever live vaccines includes the following steps: (1) cell inoculation, (2) culturing of cells used for vaccine preparation, (3) virus inoculation, (4) virus culture and harvest, and (5) vaccine preparation. Cells used for vaccine preparation are screened, adaptability between the cells and virus is strengthened, a riptide perfusion type bioreactor culture system is used for improving multiplication titer and harvest yield of the virus, and a whole production process does not involve other biosafety and public health problems and is suitable for large scale production.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD
Method for preparing duck tembusu virus inactivated vaccine and duck tembusu virus inactivated vaccine
ActiveCN108721615AImprove adaptabilityHigh virus contentSsRNA viruses positive-senseViral antigen ingredientsDiseaseSide reaction
The invention discloses a method for preparing a duck tembusu virus inactivated vaccine and the duck tembusu virus inactivated vaccine. A cell line used for virus inoculation is EB66 cell line (a duckembryonic stem cell-derived cell strain), and a bioreactor is adopted for virus culture in a serum-free full suspension manner; and the method comprises the following steps: 1) breeding of virus species; 2) establishment of virus seed batches; 3) preparation of cell venom; 4) inactivation of viruses; and 5) emulsification and other steps to complete the preparation of the vaccine. The method provided by the invention has the characteristics that the prepared cell venom is high in virus content, the production process is stable, intelligent control is achieved, large-scale serum-free suspension culture is realized, the operation is easy, and the cost is low; and the prepared duck tembusu virus inactivated vaccine has the advantages of safety, low side reactions, high immune efficacy, smallbatch-to-batch difference, small number of tests, low cost and the like, and is an ideal vaccine for preventing the occurrence and the prevalence of a duck tembusu virus disease in the waterfowl industry.
Owner:BEIJING ACADEMY OF AGRICULTURE & FORESTRY SCIENCES +1
Method of utilizing stirred bioreactor to produce infectious Bursal disease virus
InactiveCN105368794AExpand the scope of operationWell mixedMicroorganism based processesViruses/bacteriophagesCell seedingViral culture
The invention provides a method of utilizing a stirred bioreactor to produce an infectious Bursal disease virus. Bioreactor microcarrier cell culture technology is used to replace conventional roller bottle culture, so that the problems of low production efficiency, unstable product quality and low virus titer can be solved. On this basis, biological characteristics of the infectious Bursal disease virus and DF1 cells are combined, and proper conditions are matched from the perspectives of microcarrier adding amount, cell inoculation density, virus inoculation amount, cell density during virus inoculation, virus collection time and reactor operation parameters, so that virus culture efficiency is improved remarkably, and unit culture titer is improved by 10-100 times. In addition, compared with roller bottle culture, the method utilizing the bioreactor has the advantages that culture scale is large, and parameter control is comprehensive, so that systematic risk of being polluted is lowered, quality stability is improved, and the method has a wide application prospect.
Owner:TIANJIN RINGPU BIO TECH
Method for preparing rabies vaccine
ActiveCN103285390AIncrease in sizeImprove the growing environmentAntiviralsTissue/virus culture apparatusVaccinationOrganism
The invention discloses a method for preparing a rabies vaccine, and belongs to the technical field of organisms. The method comprises the following steps of: a, cell inoculation; b, cultivation of cells for seeding; c, virus vaccination, d, virus cultivating and harvesting; and e, vaccine preparation. By adopting the method, the virus is rejuvenated; the reproductive capacity of the virus is enhanced; the multiplication titer and the harvesting yield of the virus are improved by adopting a torrent perfusion-type bioreactor culture system; the entire production process is not involved with other problems of biosafety and public health; and the method is suitable for mass production.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com