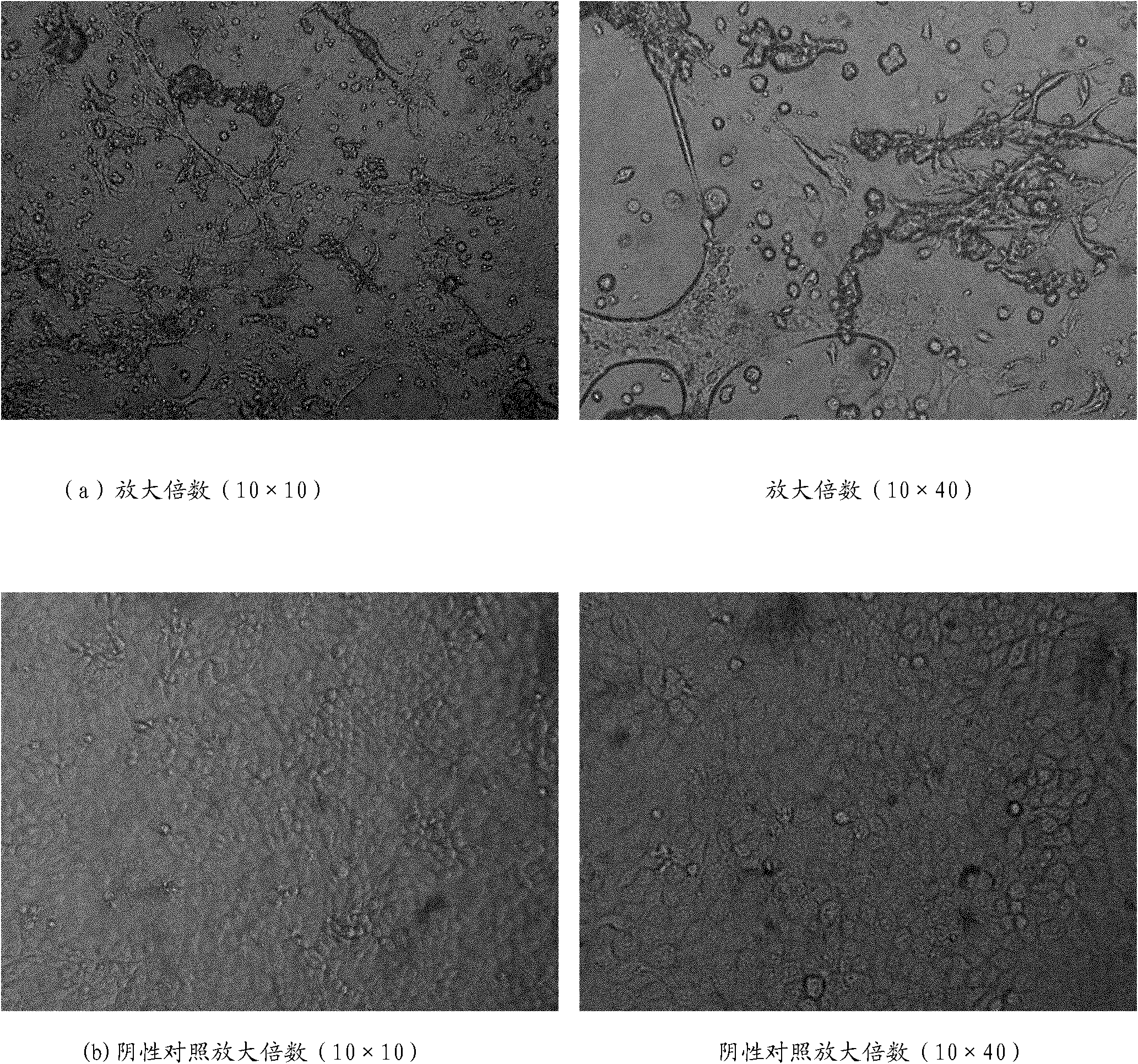
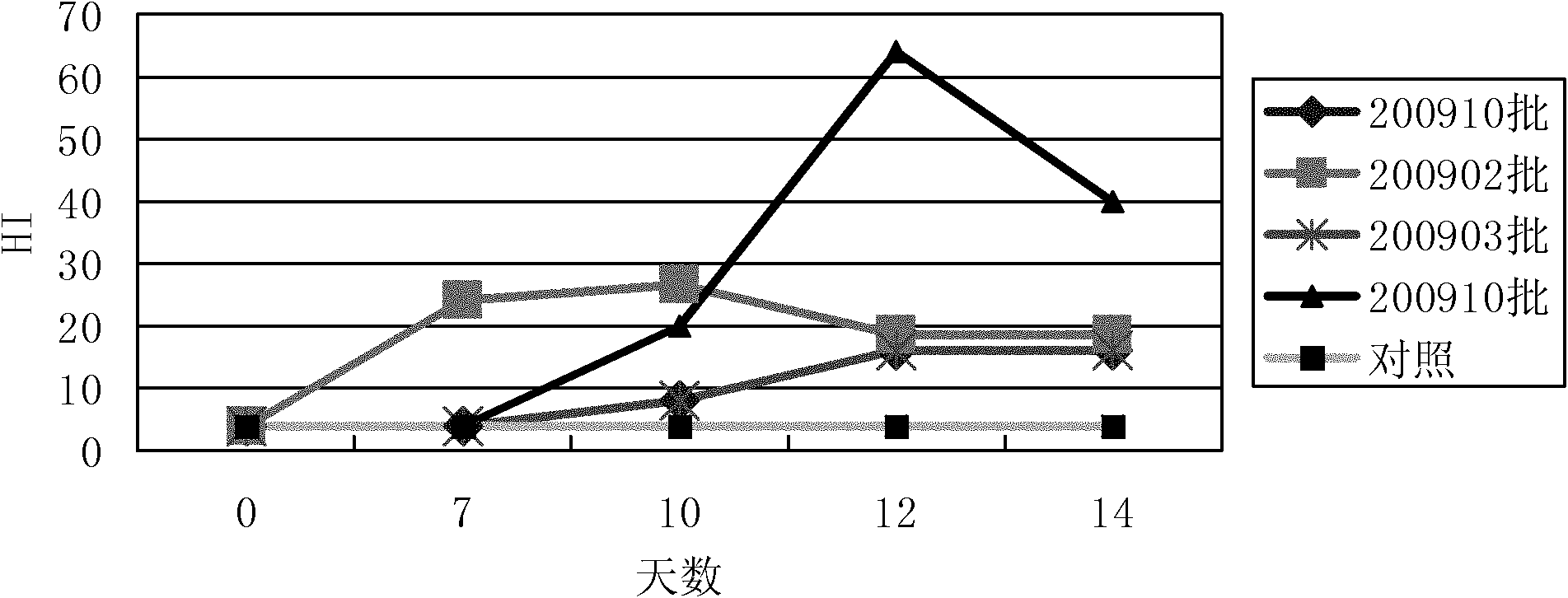
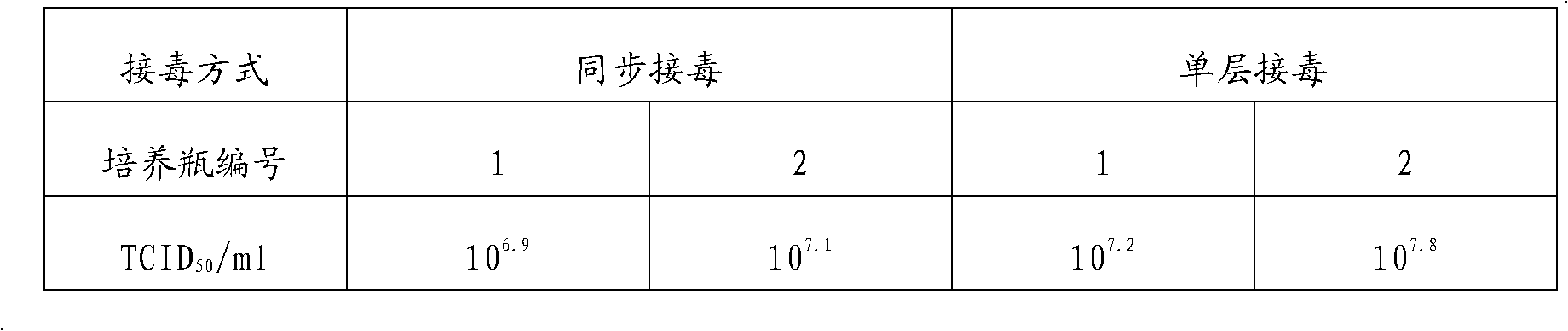
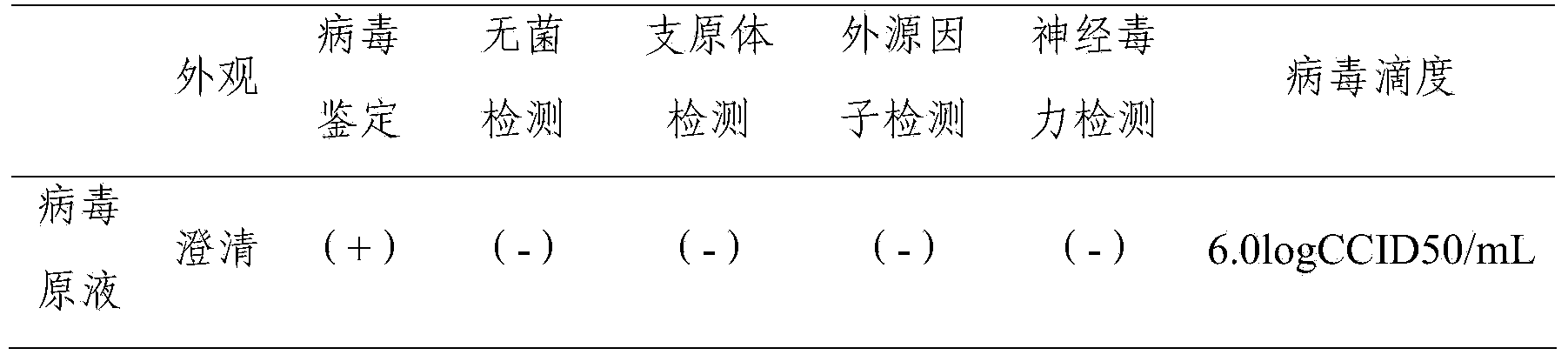
Patents
Literature
69 results about "Virus suspension" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
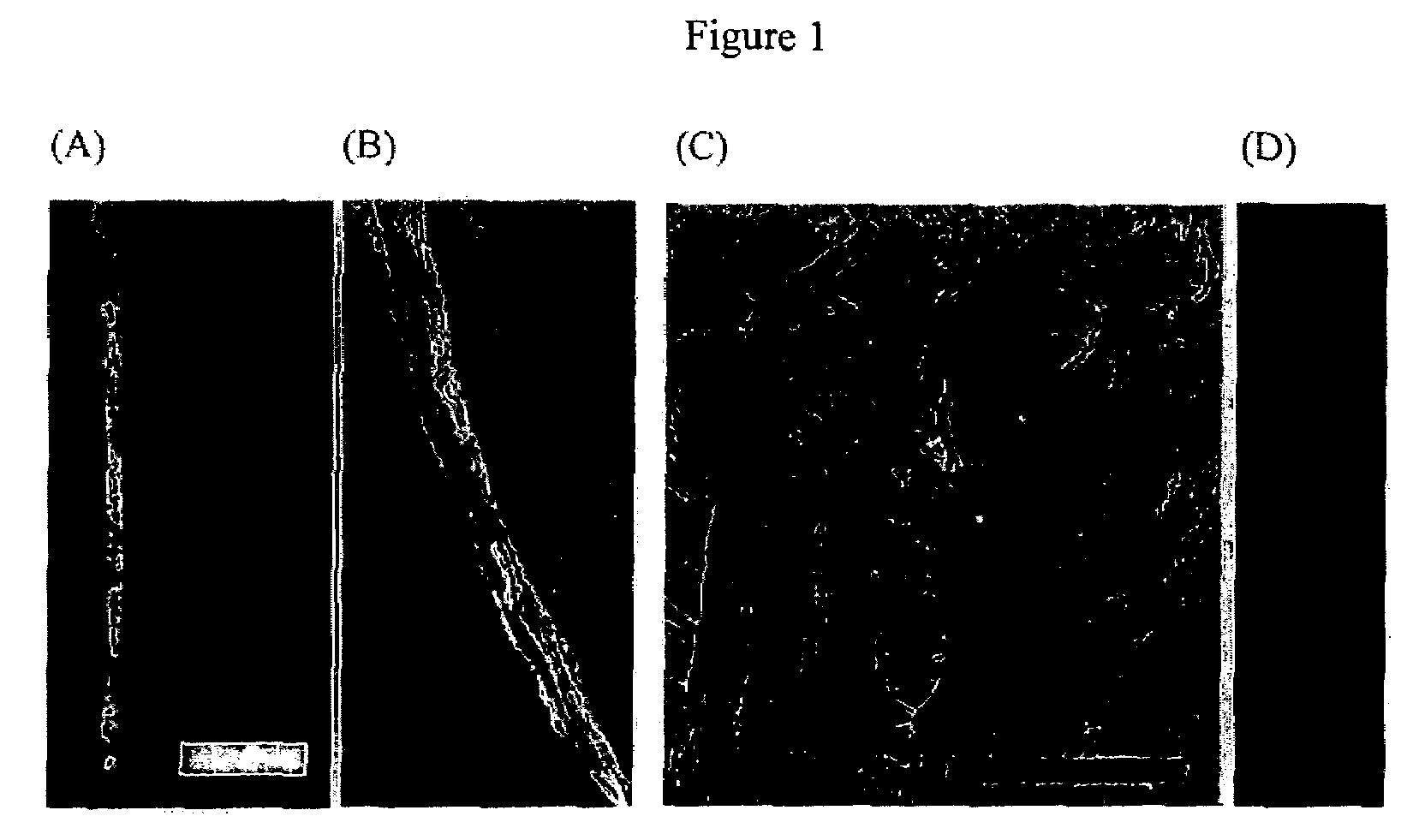
Viral fibers
InactiveUS20050180992A1Improve the level ofHigh surface energyMicroorganismsNanomedicineFiberLiquid crystalline
Long rod shaped M13 viruses were used to fabricate one dimensional (1D) micro- and nanosized diameter fibers by mimic the spinning process of the silk spider. Liquid crystalline virus suspensions were extruded through the micrometer diameter capillary tubes in cross-linking solution (glutaraldehyde). Resulting fibers were tens of micrometers in diameter depending on the inner diameter of the capillary tip. AFM image verified that molecular long axis of the virus fibers were parallel to the fiber long axis. Although aqueous M13 virus suspension could not be spun by electrospinning, M13 viruses suspended in 1,1,1,3,3,3-hexafluoro-2-propanol were spun into fibers. After blending with highly water soluble polymer, polyvinyl 2-pyrolidone (PVP), M13 viruses was spun into continuous uniform virus blended PVP (virus-PVP) fibers. Resulting virus-PVP electrospun fibers showed intact infecting ability to bacterial hosts after suspending in the buffer solution.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Univalent and bivalent inactivated vaccine for hand-foot-and-mouth disease and preparation method thereof
InactiveCN101695570AInactivation/attenuationMicroorganism based processesHand-foot-and-mouth diseaseCoxsackievirus a16
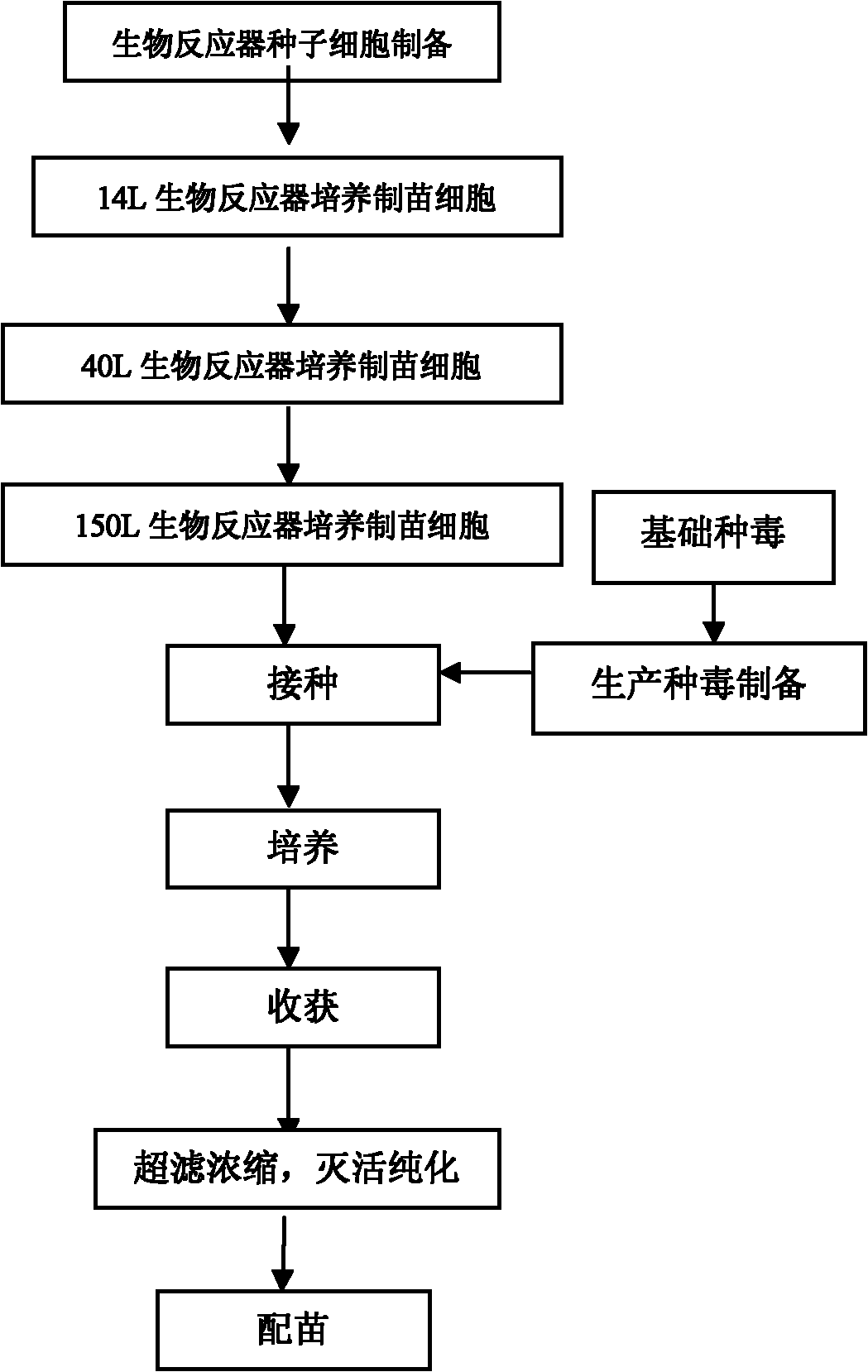
The invention discloses a univalent and bivalent inactivated vaccine for preventing Enterovirus 71 (EV71) and Coxsackie virus A16 (Cox.A16) of hand-foot-and-mouth disease, and a preparation method thereof. The preparation method comprises the following steps: screening vaccine strains for the hand-foot-and-mouth disease; establishing and verifying a vaccine strain third-stage seed lot library; culturing cells; inoculating and propagating virus; collecting virus suspension; inactivating the virus; ultra-filtering, concentrating and purifying the virus suspension to obtain vaccine stock solution; and finally preparing the univalent and bivalent inactivated vaccine. The univalent and bivalent inactivated vaccine has good application prospect for preventing the hand-foot-and-mouth disease.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Univalent and bivalent gene engineered subunit vaccine for hand-foot-and-mouth disease and preparation method thereof
InactiveCN101695569AInactivation/attenuationMicroorganism based processesHand-foot-and-mouth diseaseCoxsackievirus a16
The invention discloses a univalent and bivalent gene engineered subunit vaccine for preventing Enterovirus 71 (EV71) and Coxsackie virus A16 (Cox.A16) of hand-foot-and-mouth disease, and a preparation method thereof. The preparation method comprises the following steps: respectively obtaining recombinant baculovirus Bac-EV71-P1-3CD and Bac-Cox.A16-P1-3CD by gene engineering means, respectively efficiently coexpressing similar SeQ ID No.1 EV71 P1 and Se Q ID No.2 Cox.A16 P1 and 3CD proteins in insect cells, and respectively self-assembling into EV71 VLP and Cox.A16 VLP; establishing and verifying a vaccine strain third-stage seed lot library; culturing cells; inoculating and propagating virus; lysing the cells, ultra-filtering and purifying virus suspension; and further preparing the univalent and bivalent vaccine. The vaccine has good application prospect for preventing the hand-foot-and-mouth disease.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Viral fibers
Long rod shaped M13 viruses were used to fabricate one dimensional (1D) micro- and nanosized diameter fibers by mimic the spinning process of the silk spider. Liquid crystalline virus suspensions were extruded through the micrometer diameter capillary tubes in cross-linking solution (glutaraldehyde). Resulting fibers were tens of micrometers in diameter depending on the inner diameter of the capillary tip. AFM image verified that molecular long axis of the virus fibers were parallel to the fiber long axis. Although aqueous M13 virus suspension could not be spun by electrospinning, M13 viruses suspended in 1,1,1,3,3,3-hexafluoro-2-propanol were spun into fibers. After blending with highly water soluble polymer, polyvinyl 2-pyrolidone (PVP), M13 viruses was spun into continuous uniform virus blended PVP (virus-PVP) fibers. Resulting virus-PVP electrospun fibers showed intact infecting ability to bacterial hosts after suspending in the buffer solution.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST +1
Method for preparing live vaccines of hog cholera and product thereof
InactiveCN101879311ASmall batch-to-batch quality varianceStable production processInactivation/attenuationAntiviralsVaccine ProductionFreeze-drying
The invention discloses a method for preparing live vaccines of hog cholera and a product thereof. The preparation method comprises the following steps of: (1) culturing porcine passage cell lines; (2) inoculating the porcine passage cell lines with live vaccine production seed viruses of the hog cholera to obtain attenuated vaccine strains of the hog cholera; (3) performing virus multiplication on the attenuated vaccine strains of the hog cholera; (4) measuring the virus titer of multiplication virus suspension by adopting an immunofluorescence method; and (5) adding a freeze-drying protective agent and antibiotics into the virus suspension which is detected to be qualified for vaccine matching and freeze-drying. The preparation method has the advantages of producing the live vaccines of the hog cholera by using the cell lines so as to achieve small quality differences among batches and the characteristics of simple and stable process, easy operation, high yield, low cost, the feasibility and extendibility of industrial production and the like, and measuring the virus titer of the multiplication virus suspension by adopting the immunofluorescence method so as to achieve sensitive, fast, specific and accurate detection, high repeatability and reliable results. The live vaccines of the hog cholera prepared by the method can completely protect pigs from the attacks of violent hog cholera viruses.
Owner:武华
Broad spectrum bacilliform virus insecticide made of substitution host
The invention provides a broad-spectrum rod-shaped viral pesticide produced by substitute hosts, the components and the weight proportion of which are: 20 billion PIB / ml virus suspension 2.5 to 15 percent, 60nm silicon dioxide 0.1 to 0.5 percent, aromatic amino acid 0.1 to 0.15 percent, refined fluorescent whitening agent 0.5 to 1 percent, sodium lignin sulfonate 0.1 to 1 percent, chlorfluazuron 0.01 to 0.1 percent, glycerol 1.0 to 2.0 percent, and remaining no sterilized water; the virus suspension is received by the infection of nuclear polyhedrosis virus of cabbage armymoth and the substation for the host---cotton bollworm or beet armyworm. The pesticide not only maintains the biological pesticide properties of viral pesticides and has no harms to human beings and higher animals, but also is suitable for the the massive employments on vegetables, and has the wide insect disinfestation spectrum. Therefore, a broad-spectrum viral pesticide can prevent and control various harmful insects while with only a rod-shaped virus. The invention has the advantages of good insecticidal effect, fast insecticidal speed, and very good application prospect.
Owner:JIANGXI NEW DRAGON BIOTECH
Composition Useful as a Vaccine
The present invention relates to a composition comprising a viral antigen, a first protein and a second protein. Optionally, the composition also comprises three different disaccharaides, or, optionally, the composition comprises a primary sugar and at least one, preferably two secondary sugars. The present invention also relates to the use of a viral antigen, a first protein and a second protein for the manufacture of a composition, preferably a vaccine. The present invention furthermore relates to a method of treatment or prevention of virus associates diseases in humans. Moreover, the present invention relates to a method of adapting a virus to a suitable cell-line. The invention is also useful for the production of virus suspensions suitable for making stable, live / inactivated, monovalent and / or polyvalent, liquid / lyophilized rotavirus vaccine compositions for oral and / or nasal or any other suitable route of administration in human.
Owner:BHARAT BIOTECH INTERNATIONAL
Highly effective method for producing adenovirus
InactiveCN101235365AIncrease productionScale upGenetic material ingredientsViruses/bacteriophagesMicrobiologyBioreactor
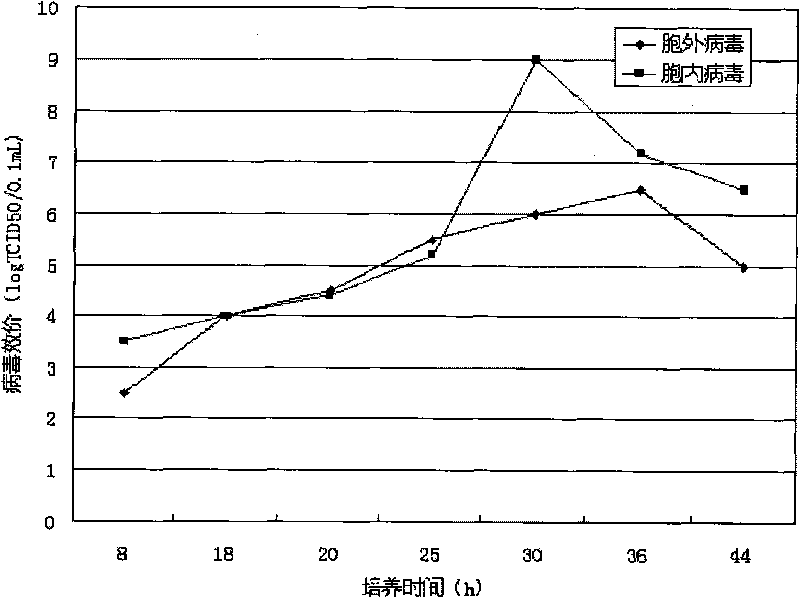
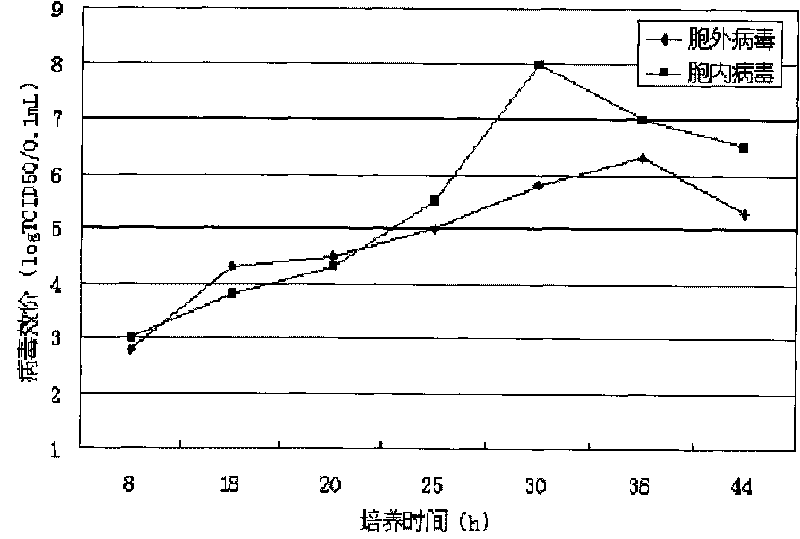
The invention relates to a method for producing adenovirus hominis, the method comprises the following steps: inoculating host cell, leading cells to grow in culture medium, replacing culture solution in a bioreactor, utilizing adenovirus hominis to infect the host cell, breeding virus, gathering virus suspension liquid and concentrating through monitoring virus concentration in the bioreactor, and the method also comprises utilizing chromatography to separate adenovirus hominis. The producing method can produce adenovirus hominis which is in accordance with the requirement of clinical medication in large scale. The method has the advantages of high yield, large scale and low production cost, which is suitable for industrial production.
Owner:TSINGHUA YUANXING BIO PHARM SCI & TECH
Vaccine produced by suspended microcarrier cell culture system and method for producing vaccine
ActiveCN101869702AReduce usageReduce exposureViral antigen ingredientsMicroorganism based processesAdjuvantFreeze-drying
The invention discloses a vaccine produced by a suspended microcarrier cell culture system and a method for producing the vaccine. The method comprises the following technical steps of: (1) inoculating cells for preparing the vaccine to a culture tank which contains a culture medium and a microcarrier; (2) uniformly mixing the cells and the microcarrier to make the cells attached to the microcarrier; (3) providing sufficient nutrient and gas for the cells at an appropriate temperature to make the cells continue growing on the microcarrier; (4) preparing virus suspension from viruses for preparing the vaccine, inoculating the virus suspension to the cells and continuing culturing, and harvesting virus liquid or the cells containing the viruses and replacing culture solution at intervals of1 to 3 days; and (5) after purifying the harvested virus liquid, inactivating the virus liquid as required, adding a proper adjuvant into the inactivated virus liquid, adding a proper freeze-drying protective agent into activated virus liquid, and quantitatively packaging after fully and uniformly mixing to obtain the vaccine. The method has the advantages of simple production process and capability of obviously improving the yield and quality of the vaccine.
Owner:香港维克贸易有限公司
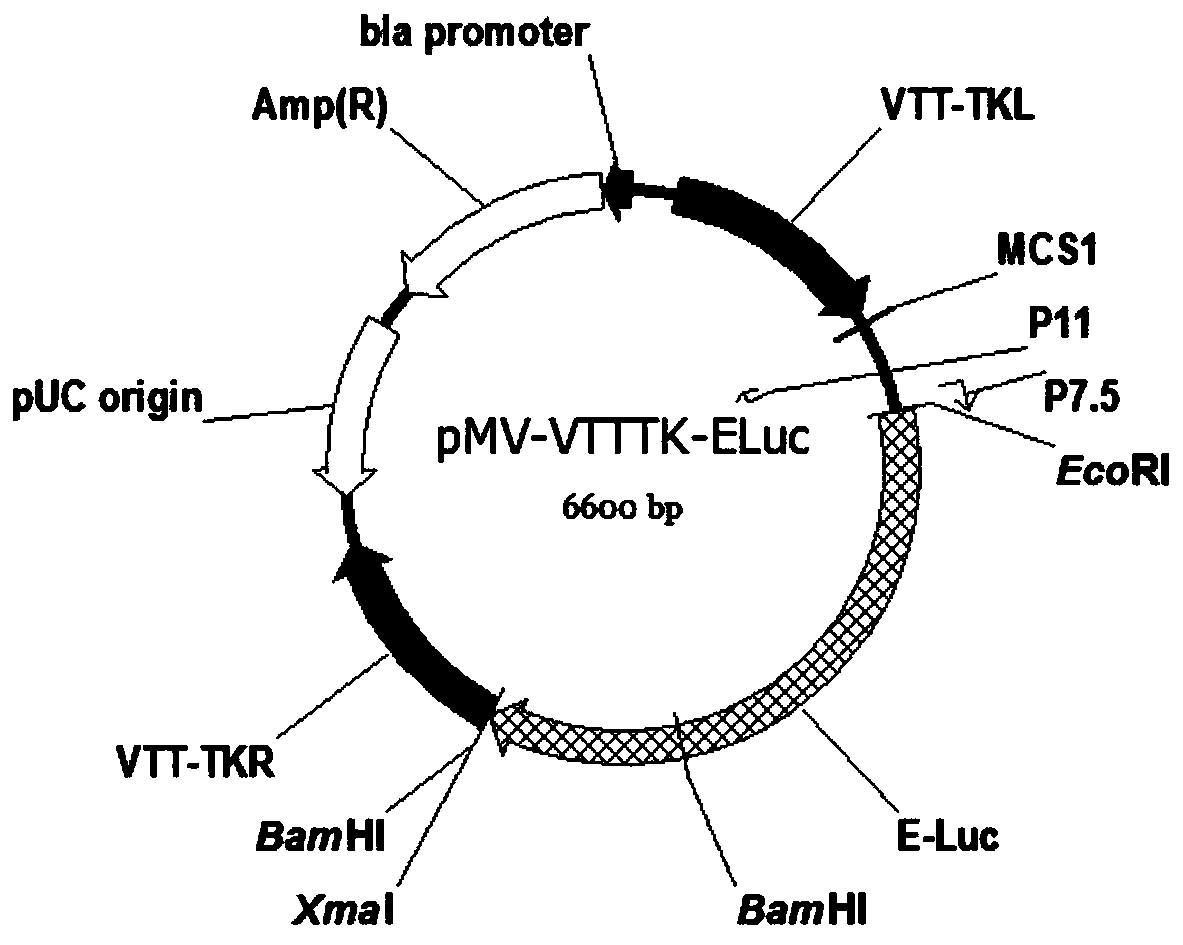
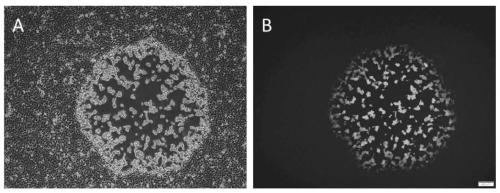
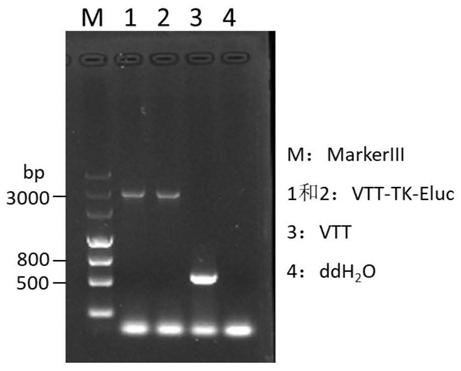
TK (Thymidine Kinase) gene removed recombinant VTT (Tian Tan strain) oncolytic VACA (Vaccinia Virus) and preparation and application thereof
PendingCN109810953ALow toxicityImprove tumor selectivityStable introduction of DNAViruses/bacteriophagesMelanomaWild type
The invention discloses a TK (Thymidine Kinase) gene removed recombinant VTT (Tian Tan strain) oncolytic VACA (Vaccinia Virus). A VTT oncolytic VACA is subjected to recombination transformation, a TKgene is removed, and an EGFP (Enhanced Green Fluorescent Protein) gene and a Luciferase gene are expressed. The invention further discloses a preparation method of the TK gene removed recombinant VTToncolytic VACA, which comprises the steps of: recombining a wild type VTT virus and a targeting plasmid aiming at the TK gene of the VTT virus in cells to obtain virus suspension; screening and purifying the virus suspension to obtain the attenuated VTT oncolytic VACA. According to the TK gene removed recombinant VTT oncolytic VACA, which is disclosed by the invention, the TK gene is used as an attenuation target, the EGFP gene and the Luciferase gene are expressed, virus virulence is reduced, and tumor selectivity of the virus is improved; the TK gene removed recombinant VTT oncolytic VACA has a function of monitoring distribution of virus particles in a body, provides a novel antitumor drug for the clinic, can be used for researching and developing a live vaccine and is applicable to various tumors such as the lung cancer, the liver cancer, the melanoma and the like.
Owner:西安彤盛生物科技有限公司
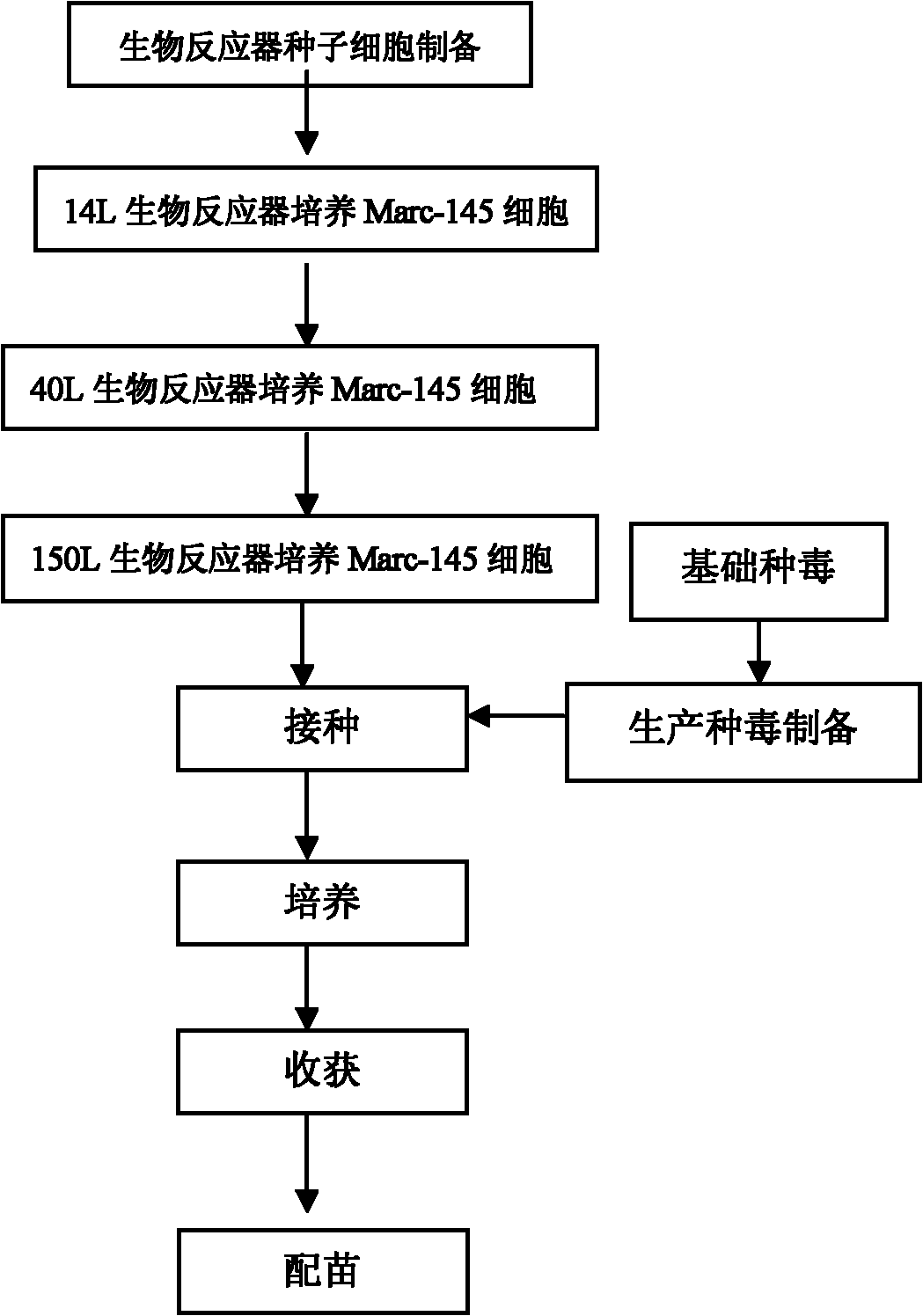
Method for industrially producing porcine Japanese encephalitis (JE) vaccines by utilizing bioreactor
InactiveCN102038943AHigh titerHigh degree of automation controlViral antigen ingredientsMicroorganism based processesAutomatic controlBottle
The invention provides a method for industrially producing porcine Japanese encephalitis (JE) vaccines by utilizing a bioreactor. The method comprises the following steps: (1) sterilizing a microcarrier and the bioreactor, then inoculating cells for vaccine preparation for culturing, inoculating JE viruses when dense monolayers are formed on the cells on the microcarrier, and continuing culturing to reproduce the viruses; (2) stopping culturing and harvesting virus suspension when cytopathy reaches more than 80%; (3) carrying out ultrafilter concentration as well as virus inactivation on the harvested virus suspension; and (4) purifying the inactivated viruses by adopting column chromatography to prepare inactivated vaccines. In the method, the technology of using the microcarrier in the bioreactor for culture is adopted to carry out high density culture of the cells to produce the porcine JE vaccines. Compared with the traditional spinner bottle production method, the method has the following advantages: the automatic control degree is high, so production can be monitored in real time; the labour is saved, thus reducing the cost; the land for production is few, thus being easy to enlarge the scale of production; and the produced viruses have high titer, so the batch-to-batch variation is small, the product quality is stable and the side reaction is low.
Owner:WUHAN CHOPPER BIOLOGY
Method for industrially producing porcine reproductive and respiratory syndrome (PRRS) vaccines by utilizing bioreactor
ActiveCN102038942AHigh titerHigh degree of automation controlViral antigen ingredientsMicroorganism based processesAdjuvantFreeze-drying
The invention relates to a method for industrially producing porcine reproductive and respiratory syndrome (PRRS) vaccines by utilizing a bioreactor. The method comprises the following steps: (1) sterilizing a microcarrier and the bioreactor, then adding cell growth liquid, inoculating cells for vaccine preparation for culturing, inoculating PRRS viruses when dense monolayers are formed on the cells on the microcarrier and continuing culturing to reproduce the viruses; (2) stopping culturing and harvesting virus suspension when cytopathy reaches more than 80%; and (3) adding a freeze-drying protective agent to the harvested virus suspension to prepare freeze-dried attenuated live vaccines or carrying out ultrafilter concentration as well as virus inactivation on the harvested virus suspension, purifying the inactivated viruses by adopting column chromatography, and adding adjuvants to prepare inactivated vaccines. Compared with the traditional spinner bottle production process, the method has the following advantages: the automation control degree is high, so production can be monitored in real time; the labour is saved, thus reducing the cost; the land for production is few, thus being easy to enlarge the scale of production; and the produced viruses have high titer, so the batch-to-batch variation is small, the product quality is stable and the side reaction is low.
Owner:WUHAN CHOPPER BIOLOGY
Animal rabies virus and vaccine and production method thereof
ActiveCN101979515AHigh infection efficiencyHigh titerInactivation/attenuationAntiviralsFreeze thawingAdjuvant
Owner:PULIKE BIOLOGICAL ENG INC
New Vaccine Formulations
ActiveUS20080187555A1Improve stabilityEnhance immune responseSsRNA viruses negative-senseAntibacterial agentsActive agentPorcine circovirus
Owner:MERIAL INC
Process for preparing subunit vaccine of reovirus antigen for grass carp
InactiveCN1616094AEasy to prepareEasy to operateViral antigen ingredientsAntiviralsInfected cellReovirus (antigen)
The preparation process of reovirus antigen subunit vaccine for grass carp includes the following steps: proliferation of GCRV873, which is the Hunan Shaoyang strain of reovirus, in grass carp kidney cell line; centrifugal purification and concentration of GCRV873 infected cell virus suspension; processing purified virus with surfactant to disintegrate complete virus structure to form viral nucleic acid and capsid protein subunit component; digesting with nuclease to degrade free virus genome dsRNA; dialysis to obtain reovirus protein subunit antigen preparation for grass carp containing no nuclease; and diluting with physiological saline to obtain the said vaccine. The vaccine of the present invention has the advantages of containing complete virus protein antigen component, containing no genetic virus matter RNA, simple preparation process, high product purity, etc.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Method of preparing foot-and-mouth disease vaccine by using BHK-21 adherent cells
ActiveCN103087995AIncrease productionIncrease the number ofMicroorganism based processesViruses/bacteriophagesPollutionBioreactor
The invention discloses a method of producing a foot-and-mouth disease vaccine by using a large-scale high-density BHK-21 cell adherent culture technology. The method comprises the following steps of: 1) thawing and primarily amplifying cells; 2) conducting large-scale and high-density cell culture in a medium-volume bioreactor, and after the culture is completed, diluting the high-density cell sap in a large cell culture pot; and 3) inoculating foot-and-mouth disease seed virus, harvesting all virus suspension under proper conditions, and preparing the foot-and-mouth disease vaccine through the following steps. The process method is free from the process of acclimating the adherent cells into suspension cells; the necessarily gradual amplification process in the existing technology of preparing the foot-and-mouth disease vaccine by conducting large-scale suspension culture of the BHK-21 cell can be avoided, the technology flow can be obviously simplified, the production cycle can be shortened, the escape of the foot-and-mouth disease virus can be furthest reduced, and the pollution possibility can be decreased.
Owner:杭州国牧生物科技有限公司
Preparation method and product of swine fever live vaccine
ActiveCN102294029ASmall batch-to-batch quality varianceStable production processInactivation/attenuationAntiviralsImmunofluorescenceVirus multiplication
The invention discloses a preparation method and product of a swine fever live vaccine. The preparation method comprises the following steps: (1) culturing a swine-derived continuous cell line; (2) inoculating the swine-derived continuous cell line into a seed virus for producing the swine fever live vaccine, thereby obtaining a swine fever weak-virus vaccine strain; (3) carrying out virus multiplication on the swine fever weak-virus vaccine strain; (4) determining the virus value of the multiplied virus suspension by an immunofluorescence method; and (5) adding a freeze-drying protective agent and antibiotics into the qualified virus suspension to carry out vaccine preparation and freeze-drying. Since the swine fever live vaccine is prepared from the cell line, the difference of quality among different batches is small, and the invention has the characteristics of simple and stable technique, high yield, low cost and the like, is easy to operate, and has feasibility and amplification of industrialized mass production. In addition, the immunofluorescence method, which has the advantages of high detection sensitivity, high speed, high specificity, high accuracy, high repetitiveness and reliable result, is used for determining the virus value of the multiplied virus suspension. The swine fever live vaccine disclosed by the invention can 100% protect the attack of swine fever strong virus.
Owner:华威特(江苏)生物制药有限公司
Newcastle disease (ND) vaccine, and its production method
InactiveCN102600465AGuaranteed to be pureEnsure safetyViral antigen ingredientsAntiviralsEmbryoControl quality
A production method of Newcastle disease (ND) vaccine includes (1) adding virus growth solution to chick-embryo-cultured inoculation subculture cell line of attenuated ND virus, and culturing to obtain cell-adapted vaccine seed virus; adding virus culture maintenance medium to inoculation subculture cell line of cell-adapted vaccine seed virus, and culturing to obtain proliferated virus suspension; (3) determining titer of the proliferated virus suspension, preparing vaccine from qualified suspension, sub-packaging, and lyophilizing. The production method has advantages of simple and stable production process, easy operation, high virus content, small batch difference, easily controlled quality, rapid and accurate titer determination, improved vaccine yield and quality, high vaccine safety, high immune efficacy, and complete immuno-protection effect against ND virus.
Owner:SINOVET BEIJING BIOTECH +1
Virus and vaccine of porcine reproductive and respiratory syndrome and preparation method of same
ActiveCN101979514AHigh viral titerHigh poison priceViral antigen ingredientsAntiviralsFreeze-dryingCells/microL
The invention discloses a method for preparing virus of porcine reproductive and respiratory syndrome on a large scale. In the method, the virus of the porcine reproductive and respiratory syndrome is prepared in a cell microcarrier suspension culture system by a bioreactor. The method comprises the following steps of: inoculating host cells for preparing the virus to a carrier tank containing culture solution and a microcarrier, and mixing the cells and the microcarrier uniformly to ensure that the cells are attached to the microcarrier; providing sufficient nutrients and appropriate gas environment for the cells under the appropriate culture environment to ensure that the cells are grown until the cells are in an amount which are 10 to 20 times of the inoculation concentration on the microcarrier; preparing virus suspension from the virus of the porcine reproductive and respiratory syndrome by using cell maintenance culture solution to ensure that the suspension is adsorbed to the cells; culturing the virus under the appropriate culture environment; culturing continuously for 2 to 3 days to obtain virus solution; and after the virus solution passes inspection, performing freeze thawing on the virus solution twice at the temperature of -20 DEG C, and inactivating and purifying to prepare an inactivated vaccine of the porcine reproductive and respiratory syndrome or adding a freeze-drying protective agent for freeze drying to prepare a live vaccine of the porcine reproductive and respiratory syndrome. The method has large production scale, high yield of single batch and low production cost.
Owner:PU LIKE BIO ENG
Method for preparing genotype F mumps attenuated live vaccine
InactiveCN103751772AGood humoral immunityImprove securitySsRNA viruses negative-senseViral antigen ingredientsFreeze thawingGenotype
The invention provides a method for preparing genotype F mumps attenuated live vaccine. The method comprises the following steps of inoculating mumps attenuated strain MHM-19 to human embryo lung diploid cell MHL-3 which is cultured to a monolayer; releasing virus through frozen-thawed cell in the peak of viral multiplication; centrifuging and removing cell debris; adding a protective agent to centrifuged virus suspension to obtain a semi-finished product of vaccine; and freezing and drying the semi-finished product to obtain the finished product of genotype F mumps attenuated live vaccine. After a rhesus monkey is injected with the prepared MHM-19 attenuated live vaccine for immunizing, good humoral immunity is induced to be produced, and the detection results obtained at different times after immunization show that the MHM-19 attenuated live vaccine has immunizing potency superior to that of reference mumps vaccine S79; in addition, the safety experiment shows that the rhesus monkey does not suffer from parotitis-related symptom after being injected with MHM-19 attenuated live vaccine, the pathological tissue nearly has no obvious difference with that of S79 strain by observing, so that the MHM-19 attenuated live vaccine has high safety.
Owner:BEIJING MINHAI BIOTECH
Ectropis oblique warren nuclear polyhedrosis virus insecticidal suspending agent and production method thereof
InactiveCN104430539AAvoid drug resistanceAvoid reduction in the number of natural enemiesBiocideAnimal repellantsFluorescencePolyvinyl alcohol
The invention mainly aims to provide an ectropis oblique warren nuclear polyhedrosis virus insecticidal suspending agent and a production method thereof. The insecticide consists of virus suspension, silicon dioxide, aromatic amino acids, a refined fluorescent whitening agent, sodium lignin sulfonate, polyvinyl alcohol, chlorfluazuron, glycerin and sterile water. The production method comprises the following steps: paired spawning on pupas by virtue of emerged adults, sterilizing the eggs, hatching larvae, feeding the larvae by using artificial feed, inoculating ectropis oblique warren virus on the feed surface to feed, grinding and filtering the larvae infected with the virus, centrifuging filtrate, collecting precipitates, and preparing the corresponding insecticide. By utilizing the insecticide, plant diseases and insect pests of ectropis oblique warren can be effectively controlled, the control cost is low, the environment friendliness is promoted, and the problems that the drug resistance of injurious insects is enhanced, the natural enemy number is reduced and pesticide residues in tea leaves are increased due to use of lots of chemical pesticides are solved.
Owner:INST OF MICROBIOLOGY JIANGXI ACADEMY OF SCI
Method for culture of Newcastle disease virus by suspension cells and application
PendingCN110129281AIncrease agglomeration rateSsRNA viruses negative-senseSerum freeNewcastle disease virus NDV
The invention relates to the field of virus suspension culture and provides a method for culture of Newcastle disease virus by suspension cells. The invention provides application of a DMEM culture medium to increasing of cell agglomeration rate in a process of virus culture with the suspension cells. The invention further provides a method for culturing gene VII-type Newcastle disease virus by the suspension cells. The method is characterized by including: culturing AGE1 cells until density reaches (8-12)*10<6> / ml, performing inoculation of the Newcastle disease virus, adding a serum-free DMEM culture medium accounting for 10%-20% of the volume of a cell culture system, daily adding pancreatic enzymes in final concentration of 8-16U / ml, culturing at a temperature of 33-37 DEG C, and collecting the virus. The AGE1 is used for culturing the gene VII-type Newcastle disease virus for the first time, and by adoption of the serum-free DMEM culture medium and other reasonable process parameters, high-titer Newcastle disease virus can be obtained.
Owner:成都史纪生物制药有限公司
Rabies virus SNK-CTN strain and application thereof
InactiveCN104357406AReduce contentReduce extractionMicroorganism based processesAntiviralsRabies virus strainDiploid cells
The invention relates to the field of microbes and biological pharmacy, and particularly relates to an adapted strain of a rabies virus strain (a CTN-1V strain) in a diploid cell MRC-5 strain. The preparation method of the rabies virus strain comprises the following steps: 1) taking MRC-5 cells in a cell bank for resuscitation, cultivating for 3 days for forming single layer cells, pouring away a cell culture fluid in an MRC-5 cell culture flask, adopting a 0.25% of trypsin digestive juice for digestion, dispersing into uniform cells, inoculating a rabies virus CTN-1V5 strain suspension according to the dosage of 0.01-1.0 MOI, cultivating at 37 DEG C for 2-3 days, replacing the suspension with a maintenance medium containing 2-4% of bovine serum, cultivating at 33-35 DEG C for 3-5 days to obtain a virus liquid, and cryopreserving at -70 DEG C to prepare a virus suspension; 2) adopting the method, and continuously subculturing for 30-32 generations on MRC-5 cells. The preservation code of the rabies vaccine virus strain (an SNK-CTN strain) is CGCC No8887.
Owner:SHINAIKE JIANGSU BIOLOGICAL PHARMA
Method for producing rabies virus antigens for animals at a large scale
The invention discloses a method for producing rabies virus antigens for animals at a large scale, which produces the rabies virus antigens at a large scale by utilizing a bioreactor by a cell microcarrier suspension culture system. The method comprises the following steps: inoculating cells for preparing the antigens into a carrier tank containing a culture solution and microcarriers to enable the cells to be attached to the microcarriers; in a proper culture environment, enabling the cells to grow on the microcarriers until the quantity of the cells is 5-40 times more than inoculum density; making rabies viruses into a virus suspension, and enabling the virus suspension to be adsorbed on the cells; culturing the viruses in the proper culture environment by using a cell maintenance culture solution; continuously culturing for 3-5 days and then harvesting a virus solution for the first time, wherein a semicontinuous process is adopted and the ratio of a changed solution is 50 percent; continue culturing for 9-11 days, and harvesting the changed solution once every 24 hours; mixing the harvested virus solution with the virus solution of the bioreactor; and carrying out freeze thawing at the temperature of -20 DEG C and inactivation purification to obtain the rabies virus antigens. The method has large production scale, high single-scale yield and relatively low production cost.
Owner:PU LIKE BIO ENG
Pseudorabies/porcine parvovirus infection combined inactivate vaccine and suspension culture preparation method
InactiveCN108421037AHigh antigen contentLarge batches of antigensViral antigen ingredientsInactivation/attenuationAdjuvantPorcine parvovirus antigen
The invention belongs to the technical field of veterinary biological products and particularly relates to a pseudorabies / porcine parvovirus infection combined inactivate vaccine and a suspension culture preparation method. The preparation method comprises the following steps: preparing a pseudorabies virus suspension culture antigen and a porcine parvovirus infection virus suspension culture antigen; proportionally mixing the inactivated pseudorabies virus and porcine parvovirus infection virus antigen solutions, and then adding an adjuvant to fully emulsify to obtain the pseudorabies / porcineparvovirus infection combined inactivate vaccine. The suspension culture processes of the pseudorabies virus antigen solution and the porcine parvovirus infection virus antigen solution are established, the virus antigen solutions prepared through the suspension culture process have the advantages of high antigen content, large batch of the antigen and stable batch, the preparation method greatlyreduces the use of manpower, the occupied area and space of a culture system is reduced, and the production cost of an enterprise is lowered; meanwhile, the pseudorabies / porcine parvovirus infectioncombined inactivate vaccine is used, two viruses are prevented through one syringe, the immunity times of an animal are reduced, the stress times of the animal are reduced, and the production cost ofthe vaccine and the culture cost of a farmer are greatly reduced.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Method for purifying slow virus
InactiveCN104371982AMeet production needsResidue reductionMicroorganism based processesRecovery/purificationPurification methodsFiltration
The invention relates to a method for purifying a slow virus. The method comprises the following steps: (S10) providing a cell culture containing the slow virus; (S20) carrying out centrifugal concentration on the cell culture, and filtering to obtain virus suspension; (S30) purifying the virus suspension by virtue of anion exchange chromatography; and (S40) purifying the virus suspension by virtue of gel filtration chromatography. A process for purifying the slow virus is easy to amplify, can be used for processing large amounts of virus liquid produced by cell factories or bioreactors on a large scale, can meet production requirements of slow virus liquid, is stable and is good in repeatability; furthermore, the purified slow virus liquid prepared by virtue of the method is not polluted by exogenous factors, is low in residues of cell host protein and host DNA, good in safety, and high in purity and titer and can completely meet requirements of clinic gene therapy.
Owner:武汉维诺赛生物技术有限公司
Buzurasuppressarianucleopolydro virus and beauveria bassiana insecticidal suspending agent, and preparation method thereof
PendingCN108617695AOvercome the shortcoming of short effective periodRich in componentsBiocideAnimal repellantsSuspending AgentsDrug resistance
The invention provides a buzurasuppressarianucleopolydro virus and beauveria bassiana insecticidal suspending agent, and a preparation method thereof. The effective components comprise a uzurasuppressarianucleopolydro virus suspension solution and beauveria bassiana powder at a weight ratio of 1:12-2:1. The buzurasuppressarianucleopolydro virus and beauveria bassiana insecticidal suspending agentpossess synergistic effect, the composition is improved, the suspensibility is better, the self life is longer, no adverse influence on the environment is caused, and the buzurasuppressarianucleopolydro virus and beauveria bassiana insecticidal suspending agent is a safe and nontoxic high efficiency biological pesticide capable of avoiding generation of drug resistance.
Owner:INST OF MICROBIOLOGY JIANGXI ACADEMY OF SCI +1
Preparation method of classical swine fever spleen-lymph-sourced compound living vaccine
InactiveCN104888213AEnhance humoral immunityBreak through interferenceOrganic active ingredientsAnthropod material medical ingredientsMucosal Immune ResponsesMaternal antibody
The invention relates to a preparation method of a classical swine fever spleen-lymph-sourced compound living vaccine and belongs to the technical field of veterinary biological products. The preparation method of the classical swine fever spleen-lymph-sourced compound living vaccine comprises the following process steps: selecting a domestic rabbit to be inoculated; inoculating; measuring the temperature and observing; harvesting; preparing virus suspension and inspecting; carrying out mixed adsorption on the harvested full-suspension virus bulk and chicken anti-hog cholera virus egg yolk antibody; and adding freezing and drying protecting agent containing an immunopotentiator into antigen antibody compound obtained through adsorption, carrying out split charging, freezing, and carrying out vacuum drying, so that the classical swine fever spleen-lymph-sourced compound living vaccine is obtained. By adopting the preparation method of the classical swine fever spleen-lymph-sourced compound living vaccine, an antibody acquiring route is simple, animal welfare can be improved, manpower and material resources are saved, production cost is greatly reduced, humoral immunity of the living vaccine can be greatly improved, and high neutralizing antibodies can be produced; and the classical swine fever spleen-lymph-sourced compound living vaccine can be applied to piglet immunization, maternal antibody interference can be avoided, no interference is produced to other vaccines, especially a mycoplasma hyopneumoniae vaccine and the like, after the classical swine fever spleen-lymph-sourced compound living vaccine is applied to piglets, and strong mucosal immune response can be induced, so that protection rate is improved.
Owner:浙江美保龙生物技术有限公司
Construction method of chronic hepatitis E virus mouse model
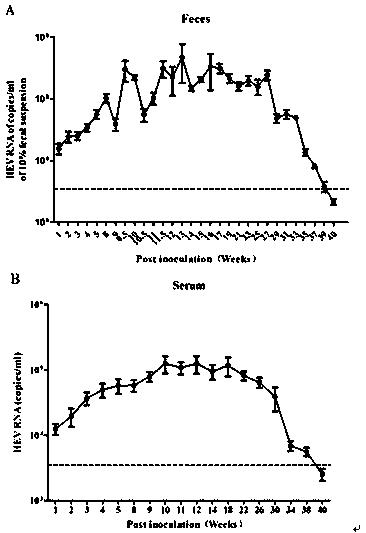
ActiveCN109769748ALow priceEasy to breedViral/bacteriophage medical ingredientsAnimal husbandryBALB/cFeces
The invention discloses a construction method of a chronic hepatitis E virus mouse model. In the method, HEV strains are derived from feces of chronically infected rhesus monkeys, and the rhesus monkeys continuously expel toxin for 93 weeks; after an HEV virus suspension is inoculated into BALB / c mice through caudal veins, HEV RNA can be continuously detected in feces and serum of the mice, and the HEV RNA has high virus copying property, and the continuous positive time can reach 36 weeks. The method for establishing the model is simple, can be used for researching the persistent infection mechanism of HEV, and can provide an important animal model for screening and evaluation of anti-HEV drugs.
Owner:KUNMING UNIV OF SCI & TECH
Removal method of residual disinfectant in virus inactivation test
InactiveCN110643742AShorten test timeImprove detection efficiencyMicrobiological testing/measurementVirus inactivationDisinfectant
The invention belongs to the technical field of disinfection, relates to a virus inactivation test, and particularly relates to a removal method of residual disinfectant in the virus inactivation test. The removal method comprises the steps of that (1) a preparation step is performed, specifically, a virus suspension is prepared; (2) a removal step is performed, specifically, first, disinfectant is sucked into a test tube, disinfectant removal treatment is performed after water bath, then ultracentrifugation is performed after 30-50 times dilution, then supernatant is removed, finally a cell maintenance solution is added for dilution and mixing again,, and the residual disinfectant is removed; and (3) a detection step is performed, specifically, virus suspension is sucked and added and mixed well, and a final sample is sucked for virus titer determination. According to the removal method of the residual disinfectant, the test time for disinfectant removal in the whole process can be shortened, the test efficiency of the virus inactivation test can be greatly improved, and the effects of disinfectants and neutralizers on cells and viruses in chemical neutralization methods are avoided.
Owner:GUANGDONG PROVINCIAL INST OF BIOLOGICAL PROD & MATERIA MEDICA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com