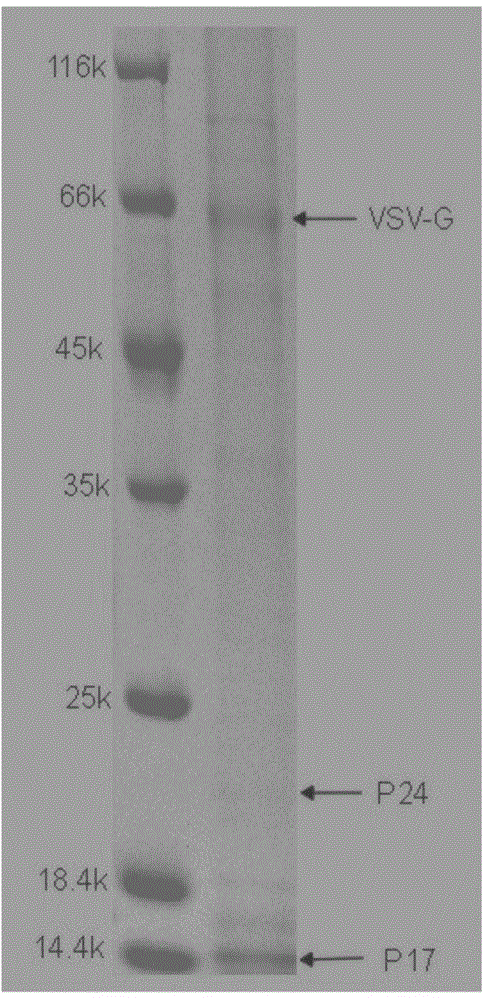
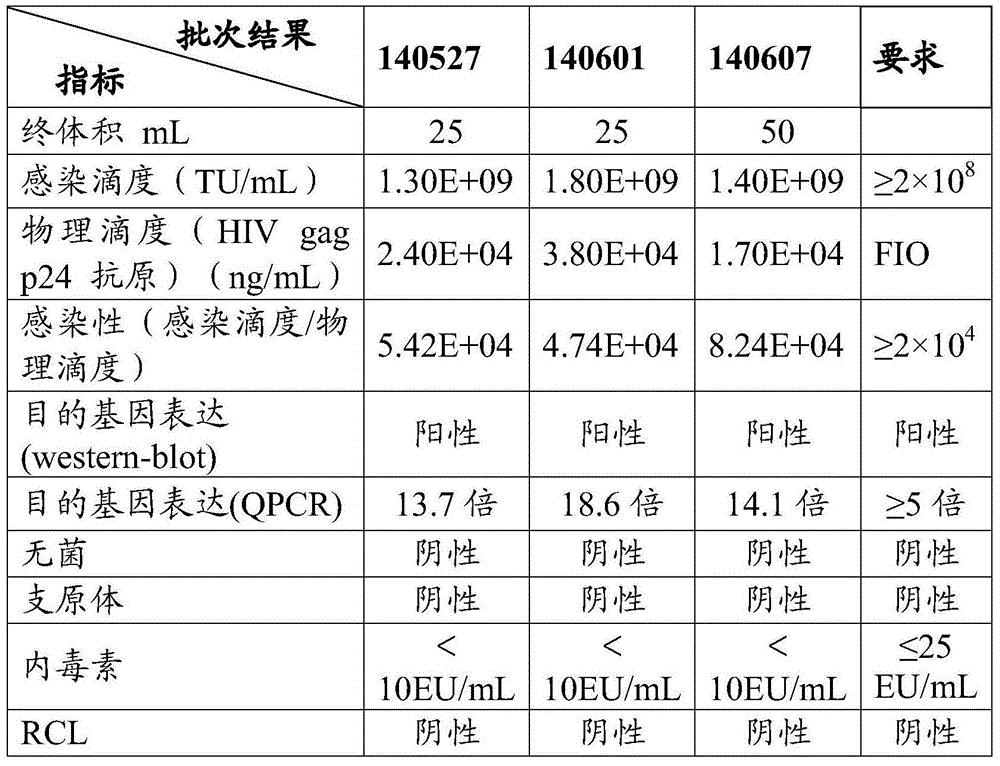
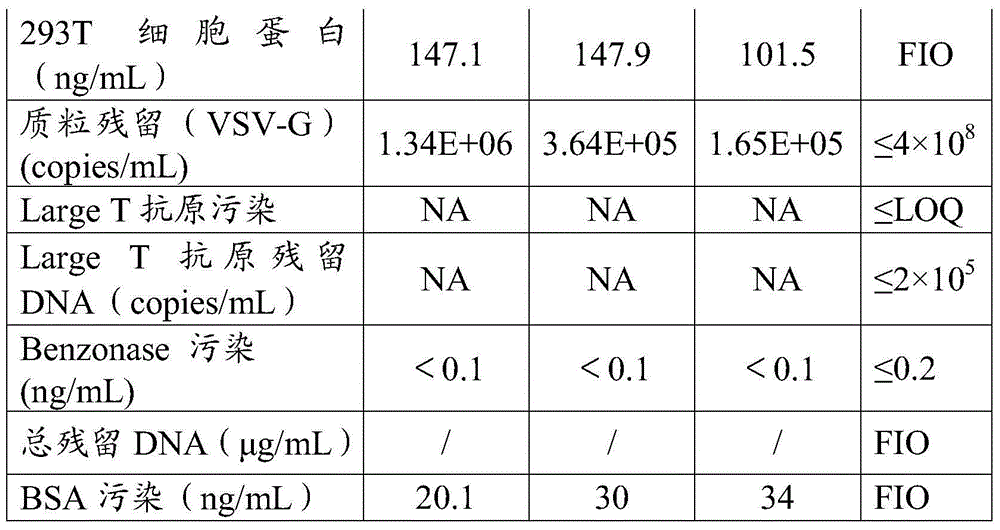
Method for purifying slow virus
A purification method, lentivirus technology, applied in the biological field, to achieve the effect of low host DNA residue, high purity and titer, and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the preparation of virus liquid
[0040] (1) Large-scale culture of packaging cells.
[0041] First, the frozen 293T cells were taken out from the liquid nitrogen tank, and immediately placed in a 37°C water bath for recovery. After it is completely melted, add 5mL DMEM complete medium (DMEM+10%FBS+P / S) and mix well, centrifuge at 1000rpm for 5min, discard the supernatant, mix well with 5mL DMEM complete medium for the precipitate, and transfer to T- 25cm 2 In a cell culture flask, cultivate overnight at 37°C in a 5% CO2 incubator; then, when the above cells grow to 80% confluence, discard the medium, add 5mL PBS, shake slightly, discard the PBS, and add 1mL 0.25 %EDTA-trypsin, put it at room temperature for 2-3min, when the cells are completely detached from the bottle wall, add 10mL DMEM complete medium, mix well, centrifuge at 1000rpm for 5min, discard the supernatant, and culture the cell pellet completely with 5mL DMEM Mix well, transfer to T-75cm ...
Embodiment 2
[0048] Embodiment 2: the purification of virus liquid:
[0049] (5) Nuclease treatment of virus liquid.
[0050] After the virus liquid in Example 1 was concentrated and filtered in step (4), 250 U / mL Benzonase was added and reacted in a water bath at 37°C for 6 hours.
[0051] (6) The virus liquid is purified by anion exchange chromatography and concentrated by centrifugation.
[0052] Specifically, the steps are as follows: ① first wash the 500mL HiTrap Q-XL strong negative spin column with 1L ddH2O, and set the flow rate to 50mL / min. ②Equilibrate a 500mL HiTrap Q-XL strong negative spin column with 1L loading buffer Buffer A (50mM Tris-HCl, pH 8.0), and set the flow rate to 50mL / min. ③ Load the virus supernatant after 0.45um filtration, and set the flow rate to 50mL / min. ④ Rinse 500mL HiTrap Q-XL strong negative spin column with 1.5L loading buffer Buffer A (50mM Tris-HCl, pH8.0) until the baseline is stable to remove non-specifically bound proteins, and set the flow rat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com