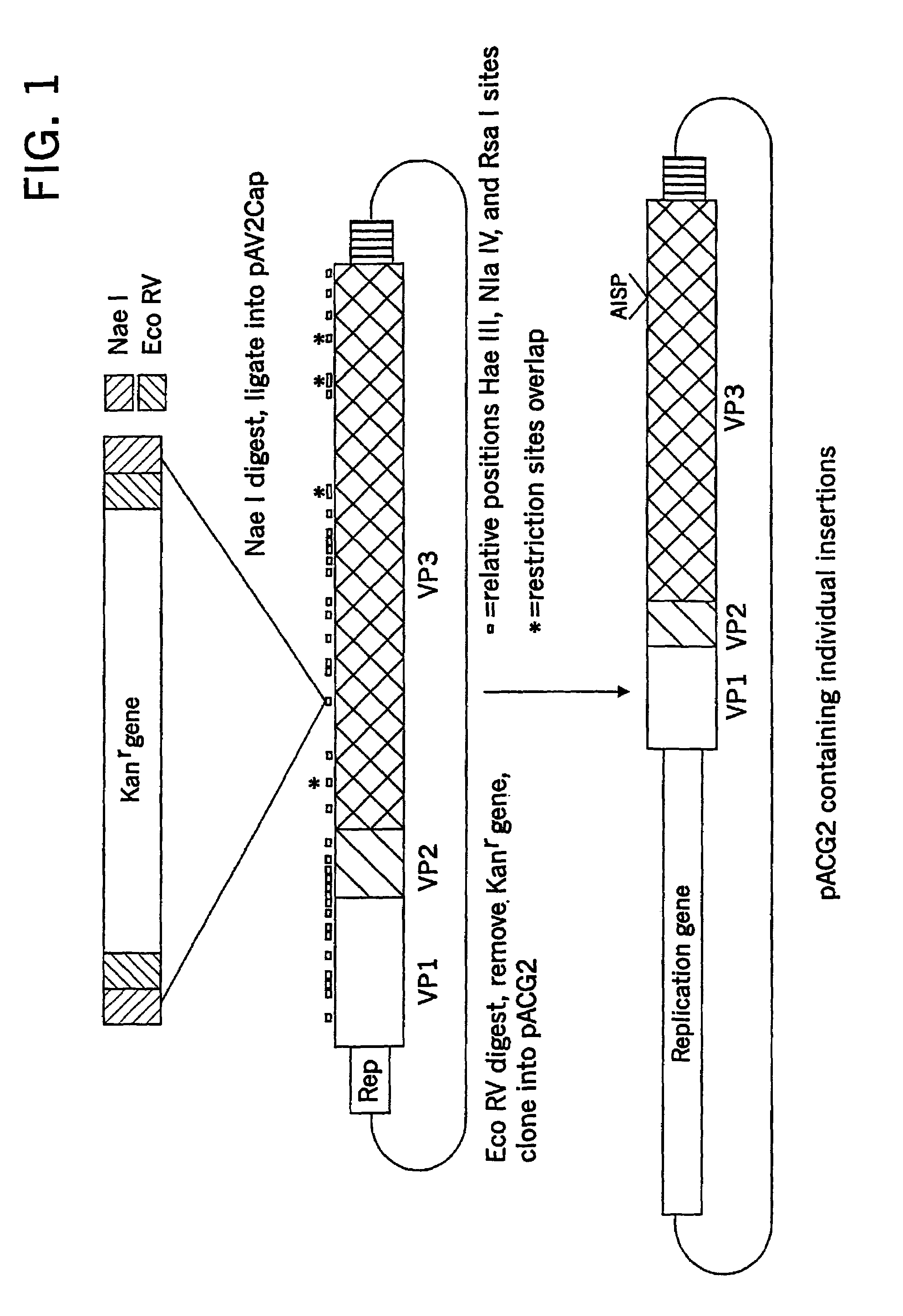
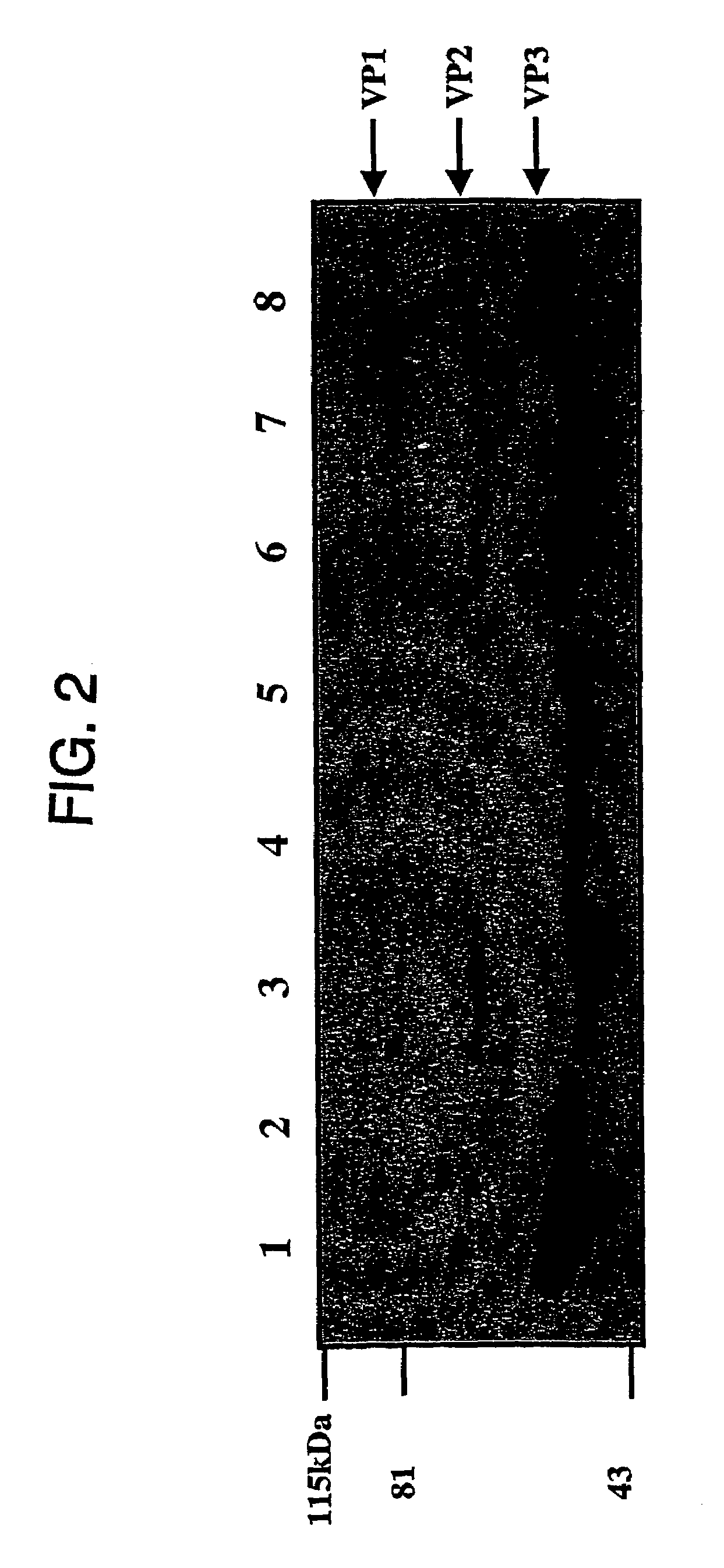
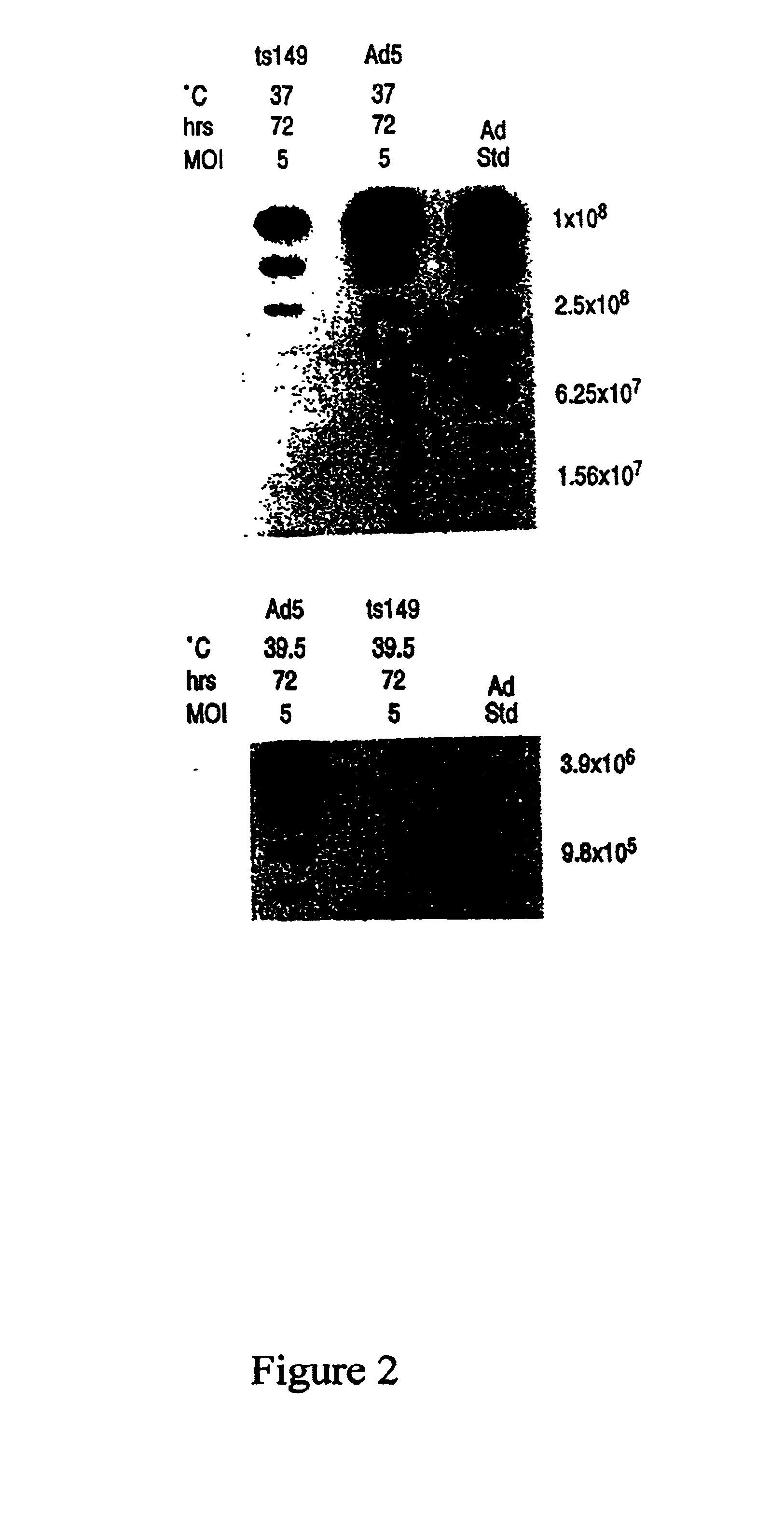
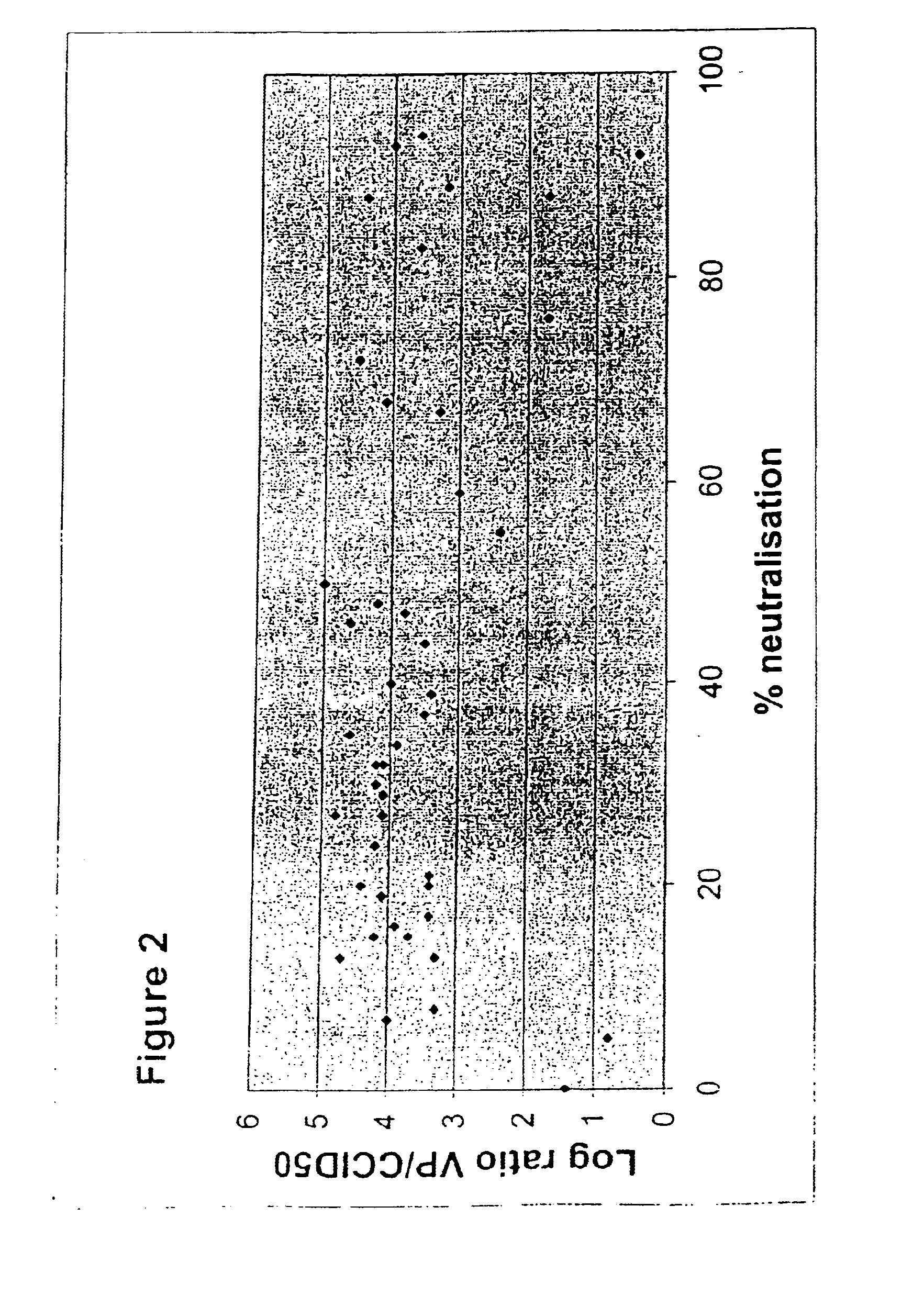
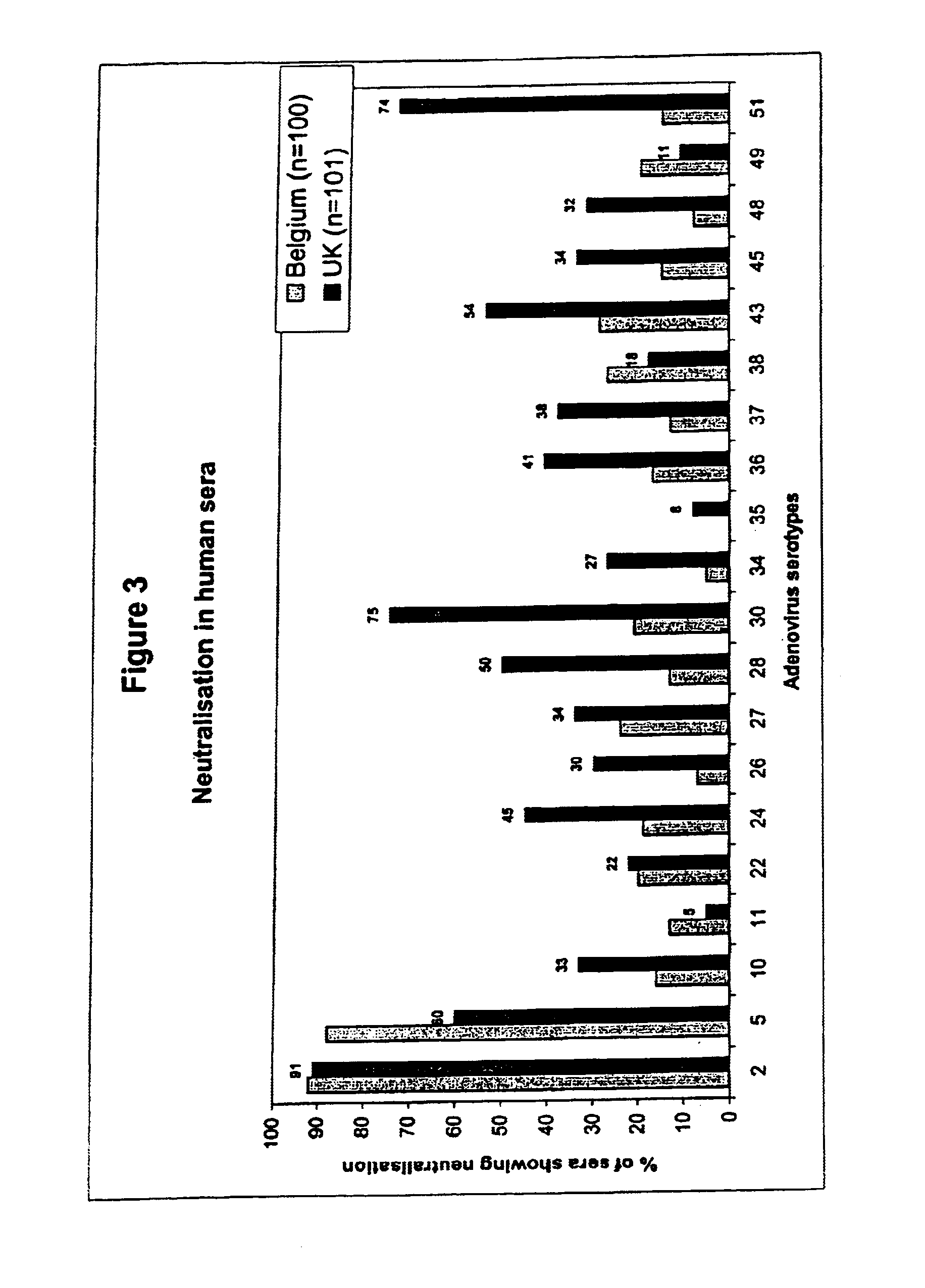
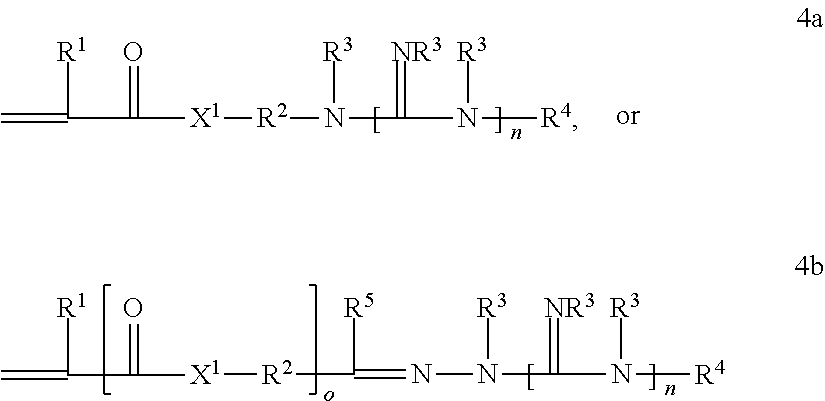
Patents
Literature
1398results about "Recovery/purification" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Virus vectors and methods of making and administering the same
The present invention provides genetically-engineered parvovirus capsids and viruses designed to introduce a heterologous gene into a target cell. The parvoviruses of the invention provide a repertoire of vectors with altered antigenic properties, packaging capabilities, and / or cellular tropisms as compared with current AAV vectors.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Methods for generating high titer helper-free preparations of released recombinant AAV vectors
InactiveUS6989264B2Genetic therapy composition manufactureGroup 5/15 element organic compoundsGene deliveryHeterologous
This invention provides methods and compositions for producing high titer, substantially purified preparations of recombinant adeno-associated virus (AAV) that can be used as vectors for gene delivery. At the onset of vector production, AAV producer cells of this invention typically comprise one or more AAV packaging genes, an AAV vector comprising a heterologous (i.e. non-AAV) transgene of interest, and a helper virus such as an adenovirus. The AAV vector preparations produced are generally replication incompetent but are capable of mediating delivery of a transgene of interest (such as a therapeutic gene) to any of a wide variety of tissues and cells. The AAV vector preparations produced according to this invention are also substantially free of helper virus as well as helper viral and cellular proteins and other contaminants. The invention described herein provides methods of producing rAAV particles by culturing producer cells under conditions, such as temperature and pH, that promote release of virus. Also provided is a quantitative, high-throughput assay useful in the assessment of viral infectivity and replication, as well as in the screening of agent that affect viral infectivity and / or replication.
Owner:TARGETED GENETICS CORPORTION
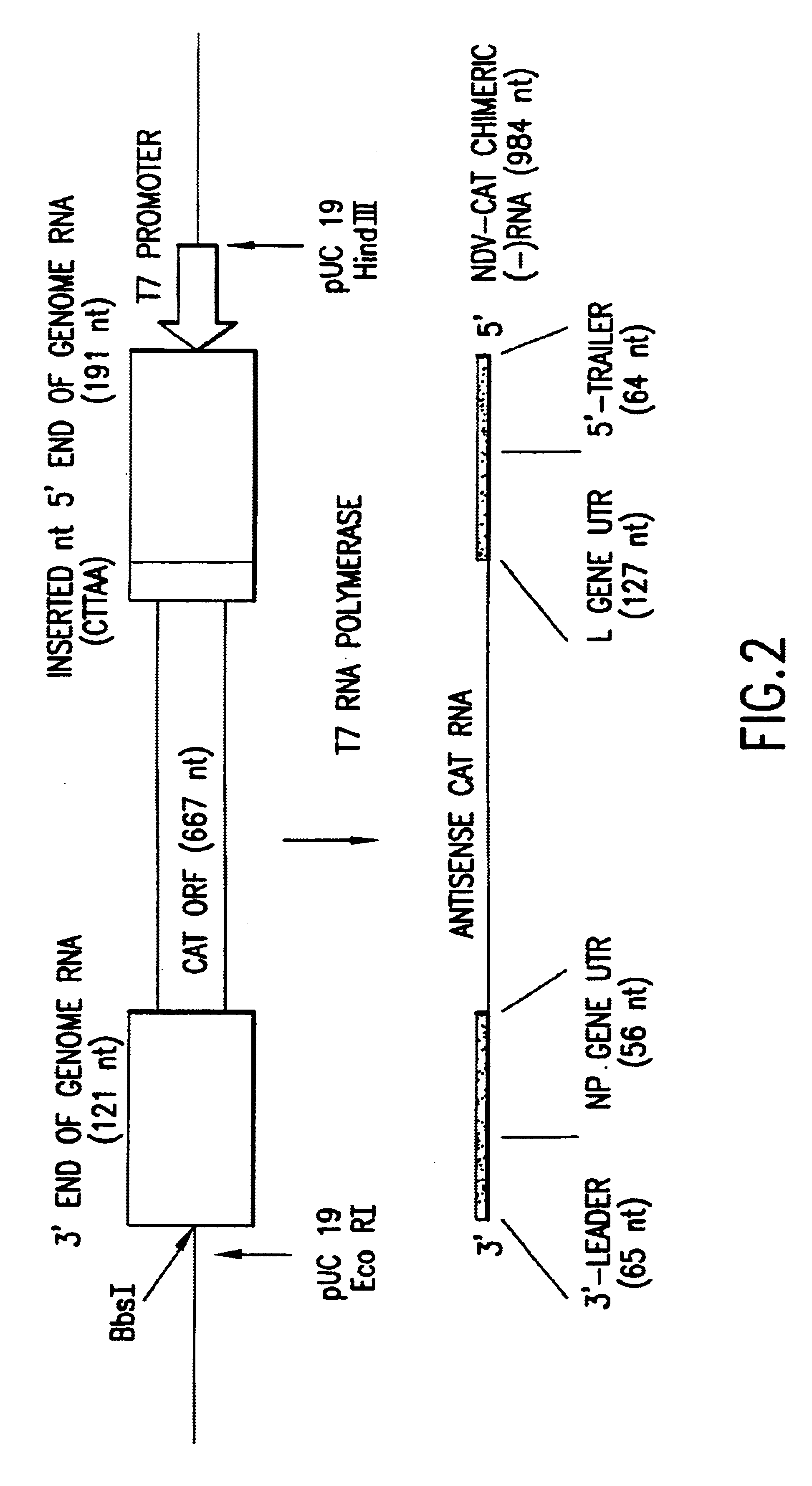
Helper-free rescue of recombinant negative strand RNA virus
Owner:MT SINAI SCHOOL OF MEDICINE
Helper-free rescue of recombinant negative strand RNA viruses
InactiveUS6544785B1SsRNA viruses negative-senseGenetic material ingredientsNegative strandNucleic acid sequence
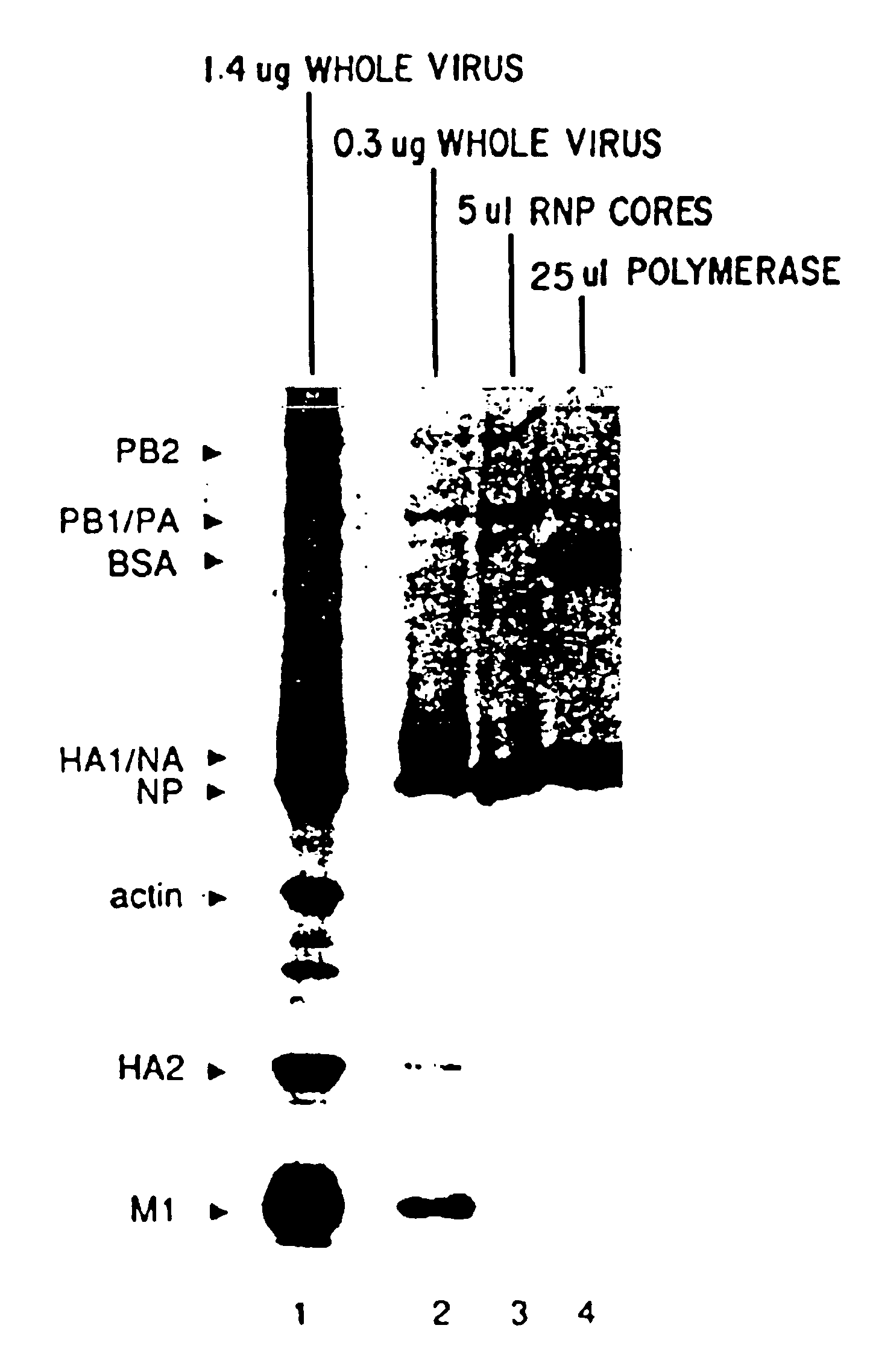
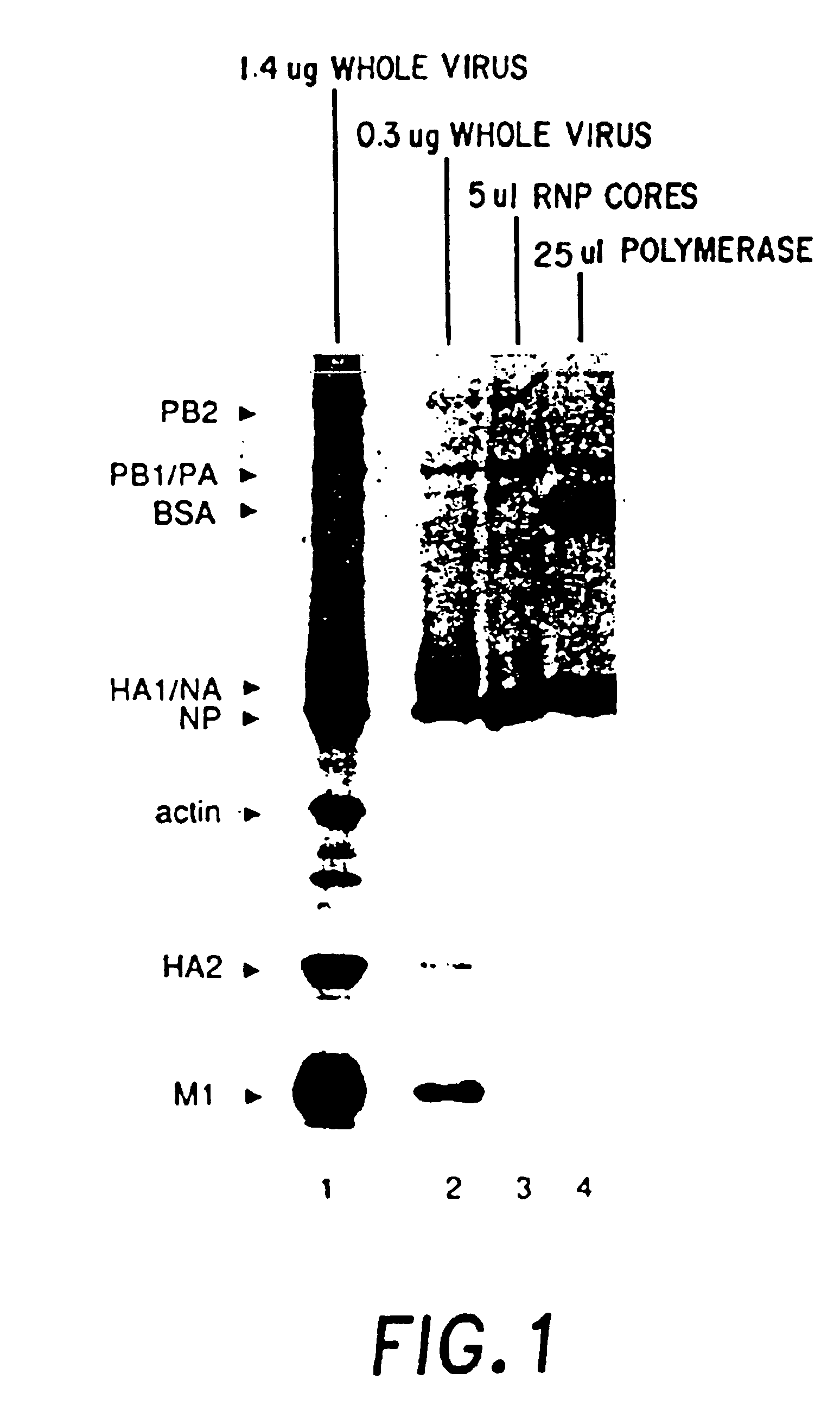
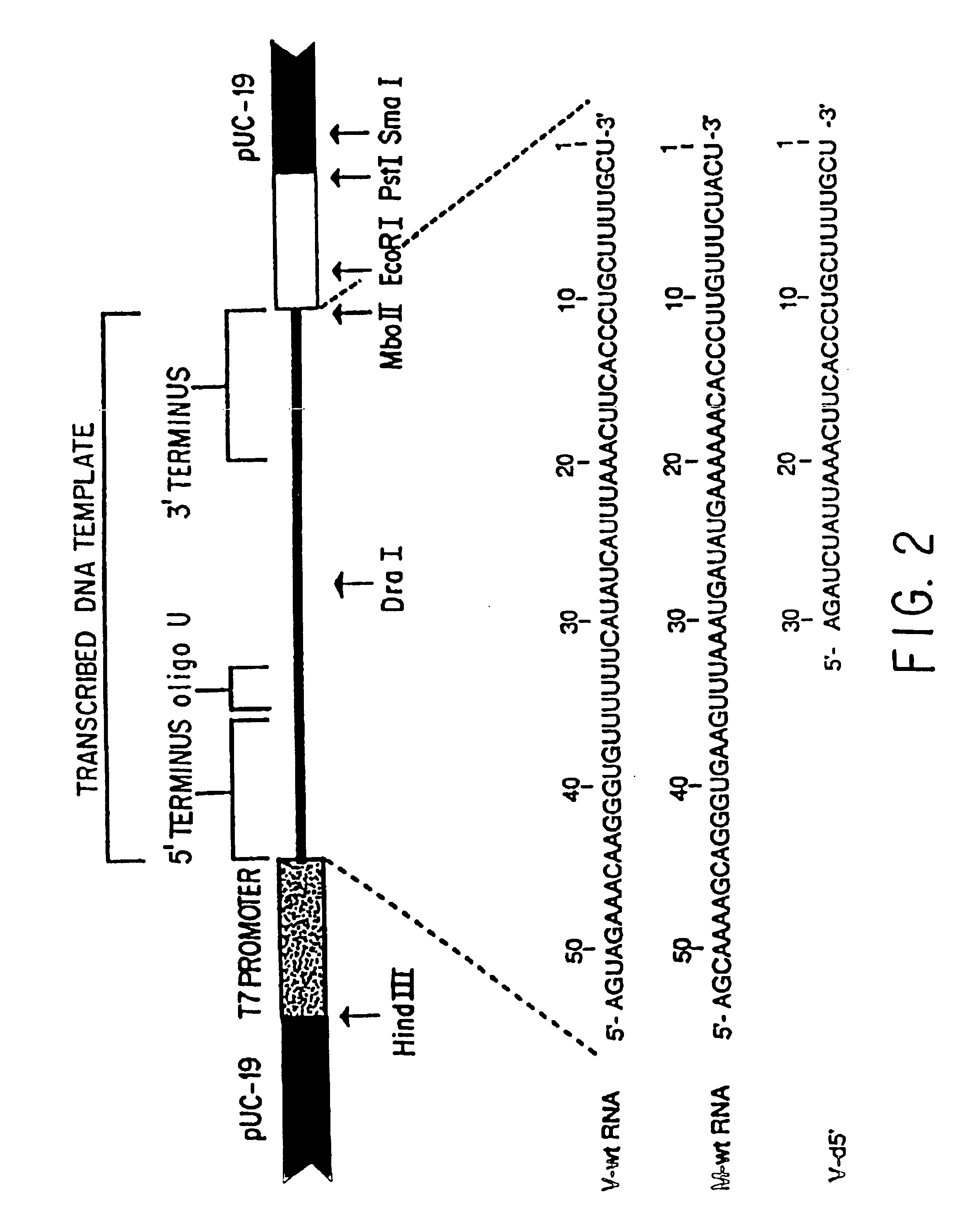
The present invention relates methods of generating infectious negative-strand virus in host cells by an entirely vector-based system without the aid of a helper virus. In particular, the present invention relates methods of generating infectious recombinant negative-strand RNA viruses intracellularly in the absence of helper virus from expression vectors comprising cDNAs encoding the viral proteins necessary to form ribonucleoprotein complexes (RNPs) and expression vectors comprising cDNA for genomic viral RNA(s) (vRNAs) or the corresponding cRNA(s). The present invention also relates to methods of generating infectious recombinant negative-strand RNA viruses which have mutations in viral genes and / or which express, package and / or present peptides or polypeptides encoded by heterologous nucleic acid sequences. The present invention further relates the use of the recombinant negative-strand RNA viruses or chimeric negative-strand RNA viruses of the invention in vaccine formulations and pharmaceutical compositions.
Owner:MT SINAI SCHOOL OF MEDICINE
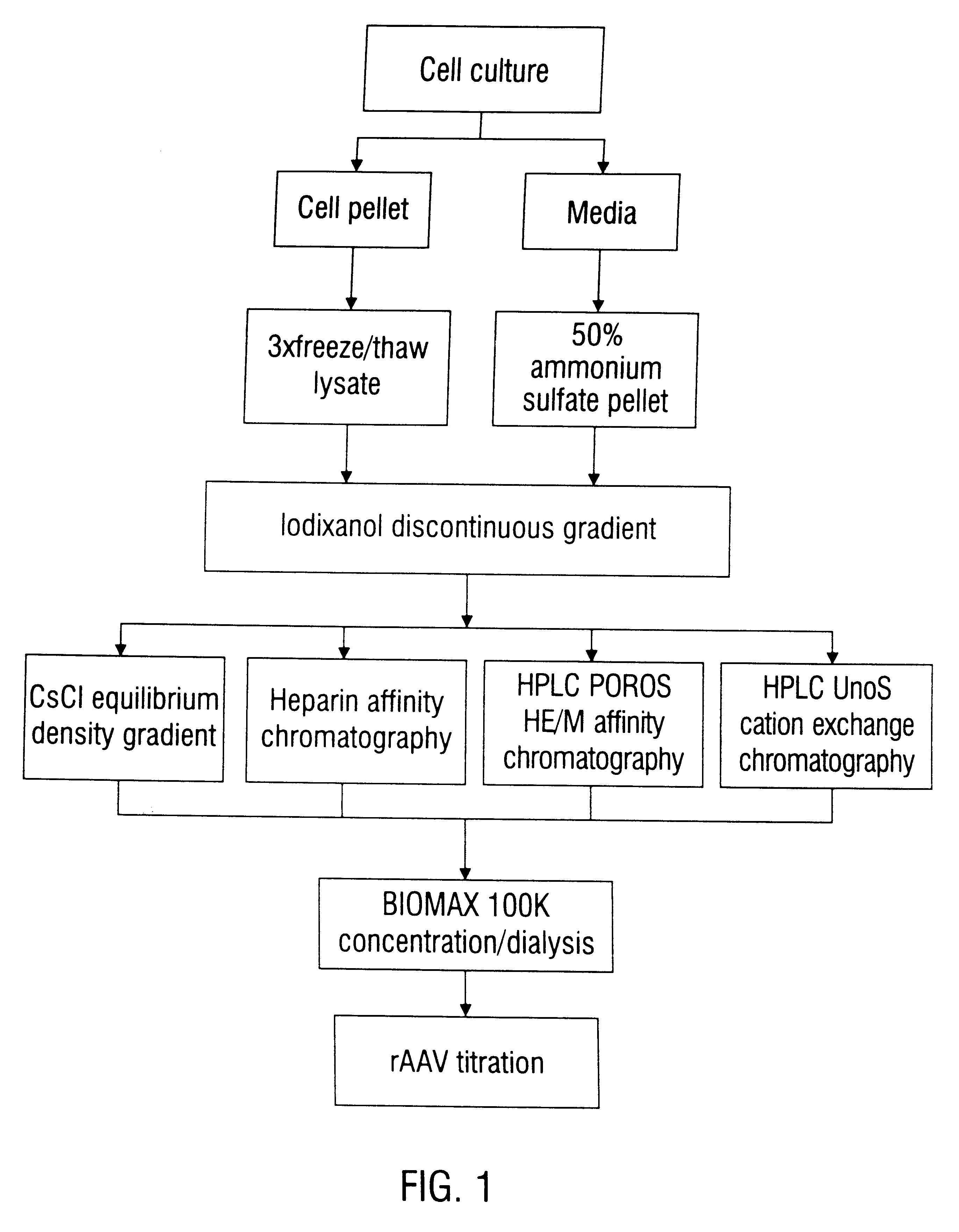
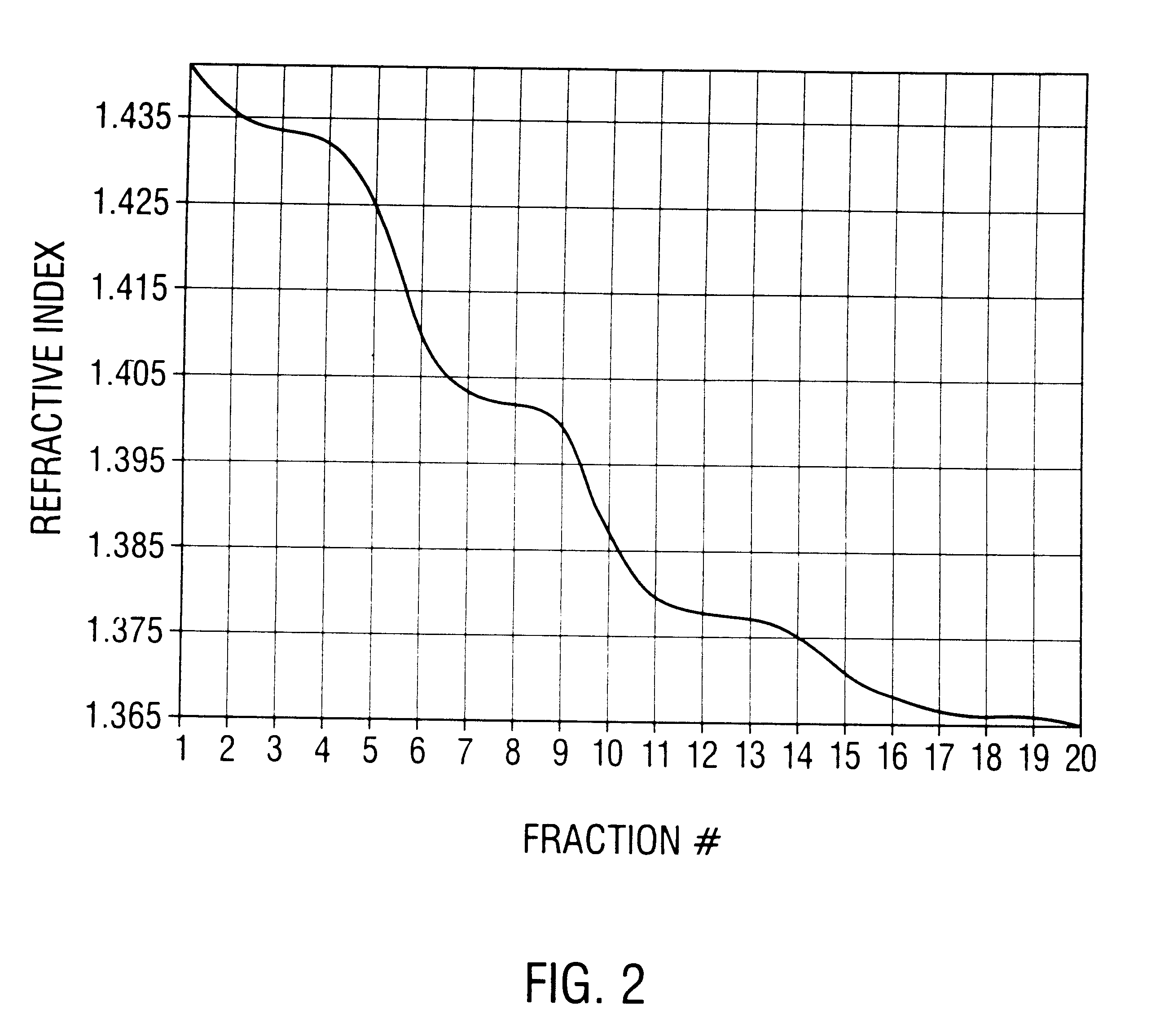
Method of preparing recombinant adeno-associated virus compositions
InactiveUS6660514B1Reduce concentrationImprove yield and recoveryMicrobiological testing/measurementEnzymologyBiochemistryAdeno-associated virus
Disclosed are methods for the isolation and purification of high-titer recombinant adeno-associated virus (rAAV) compositions. Also disclosed are methods for reducing or eliminating the concentration of helper adenovirus in rAAV samples. Methods are disclosed that provide highly-purified rAAV stocks having titers up to about 10<13 >particles / ml at particle-to-infectivity ratios of less than 100 in processes that are accomplished about 24 hours or less.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Compositions and methods for helper-free production of recombinant adeno-associated viruses
InactiveUS6953690B1Efficient productionIncrease the number ofBiocideGenetic therapy composition manufactureMammalWild type
A method for producing recombinant adeno-associated virus in the absence of contaminating helper virus or wild-type virus involves culturing a mammalian host cell containing a transgene flanked by adeno-associated virus (AAV) inverse terminal repeats and under the control of regulatory sequences directing expression thereof, an AAV rep sequence and an AAV cap sequence under the control of regulatory sequences directing expression thereof, and the minimum adenovirus DNA required to express an E1a gene product, an E1b gene product and an E2a gene product, and isolating therefrom a recombinant AAV which expresses the transgene in the absence of contaminating helper virus or wildtype AAV. This method obviates a subsequent purification step to purify rAAV from contaminating virus. Also provided are various embodiments of the host cell.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Method for the preparation of purified HCV RNA by exosome separation
InactiveUS7198923B1Simple preparation processPromote progressSsRNA viruses positive-senseMicrobiological testing/measurementExosomeBlood plasma
Owner:GRIFOLS WORLDWIDE OPERATIONS +1
Methods of generating chimeric adenoviruses and uses for such chimeric adenoviruses
A method for providing an adenovirus from a serotype which does not grow efficiently in a desired cell line with the ability to grow in that cell line is described. The method involves replacing the left and right termini of the adenovirus with the corresponding termini from an adenovirus which grow efficiently in the desired cell line. At a minimum, the left terminus spans the 5′ inverted terminal repeat, the left terminus spans the E4 region and the 3′ inverted terminal repeat. The resulting chimeric adenovirus contains the internal regions spanning the genes encoding the penton, hexon and fiber from the serotype which does not grow efficiently in the desired cell. Also provided are vectors constructed from novel simian adenovirus sequences and proteins, host cells containing same, and uses thereof.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Method for the production and purification of adenoviral vectors
InactiveUS20080050770A1Peptide/protein ingredientsGenetic material ingredientsSerum igeUltracentrifuge
Owner:JANSSEN VACCINES & PREVENTION BV
Mutant adeno-associated virus virions and methods of use thereof
ActiveUS20050053922A1Reduce the binding forceAltered infectivityAntibacterial agentsVirusesReassortant VirusesNeutralizing antibody
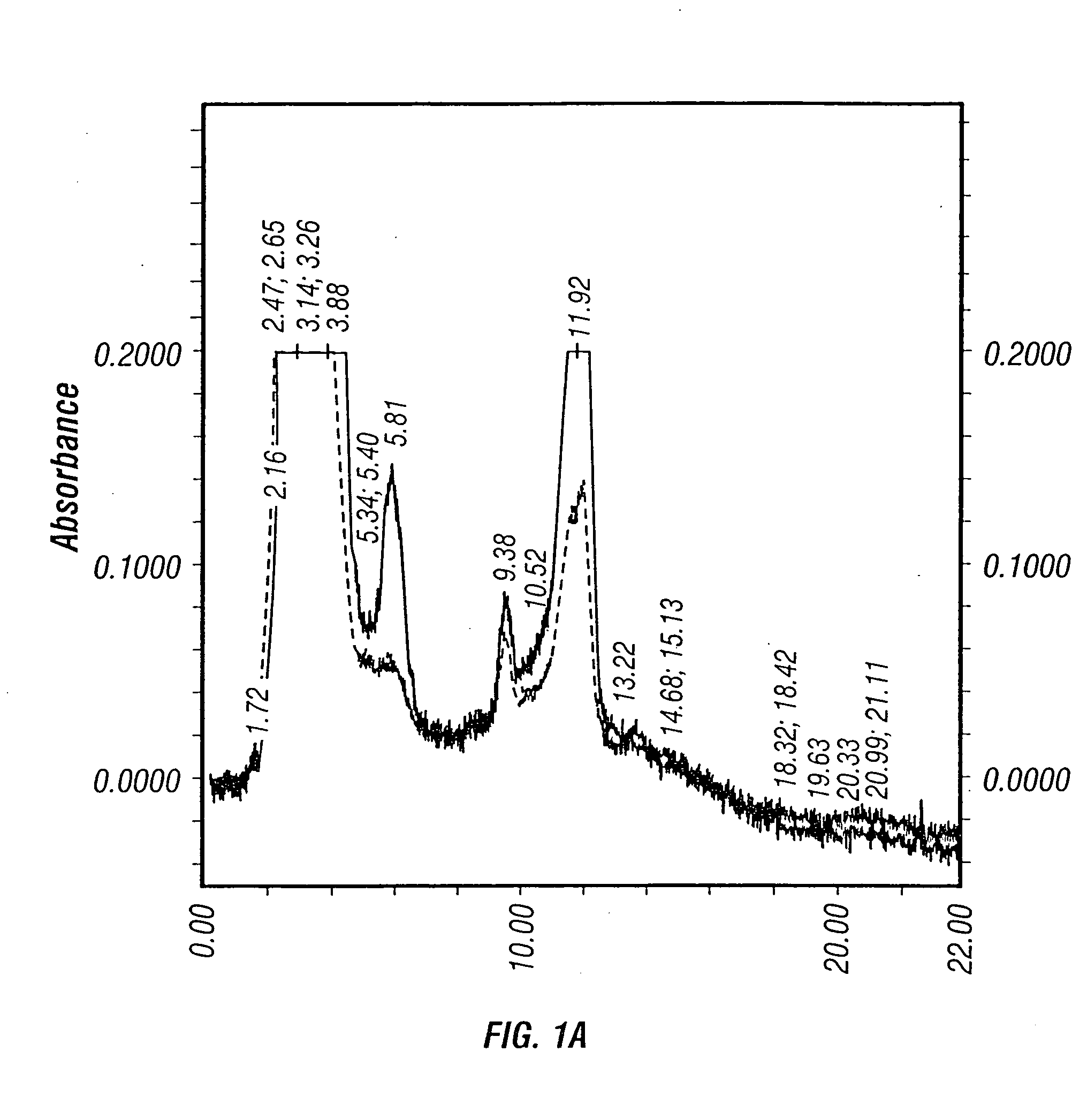
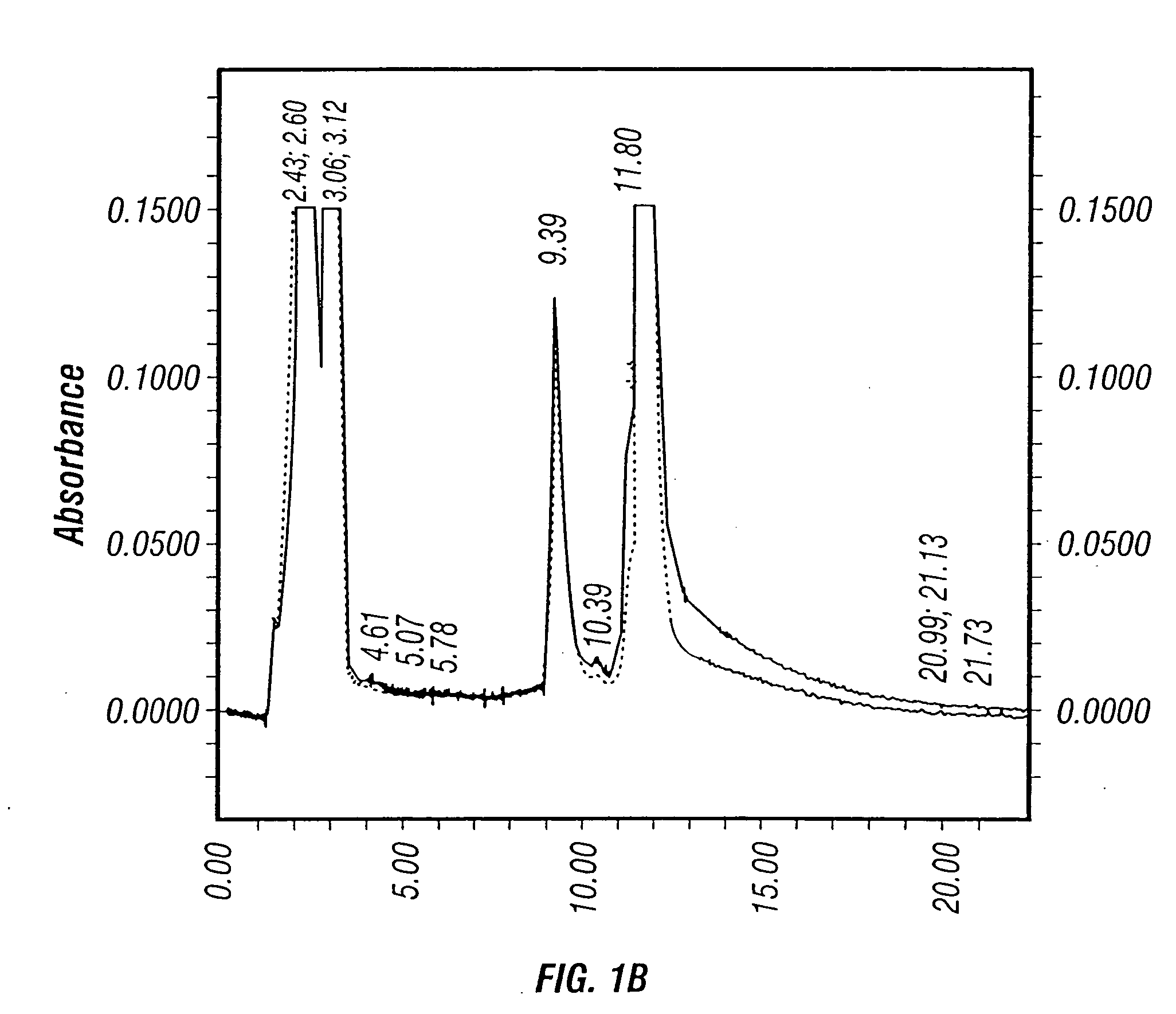
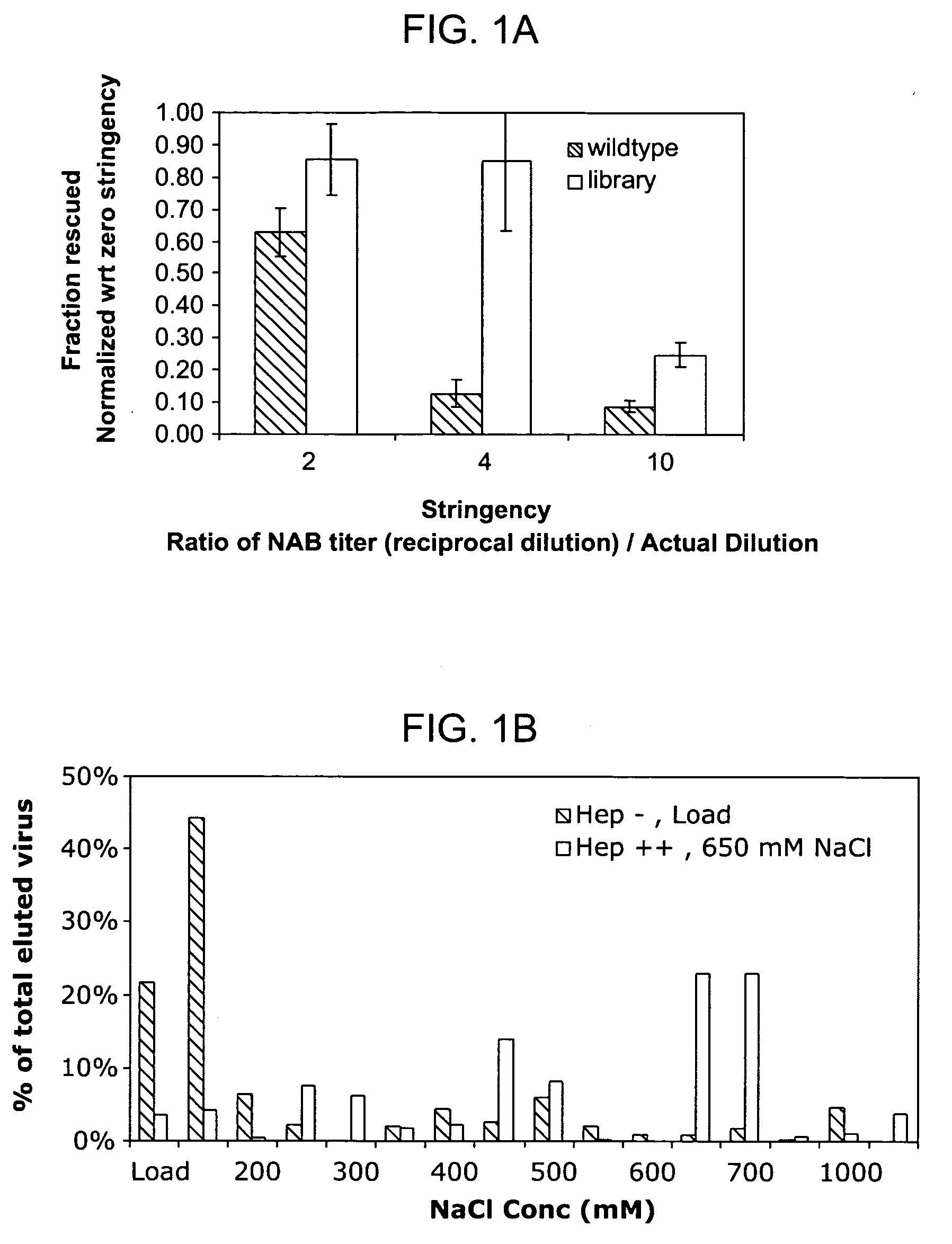
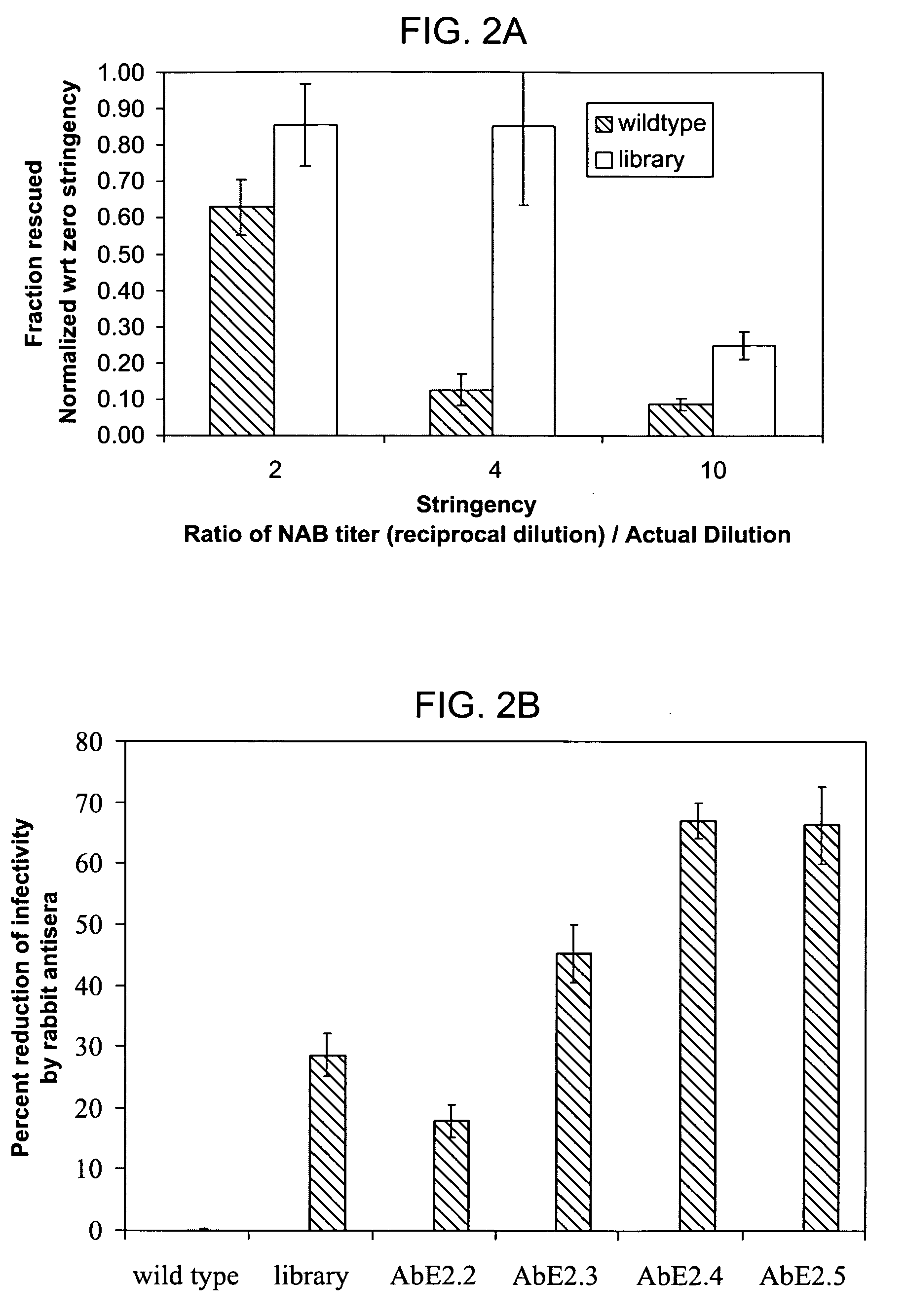
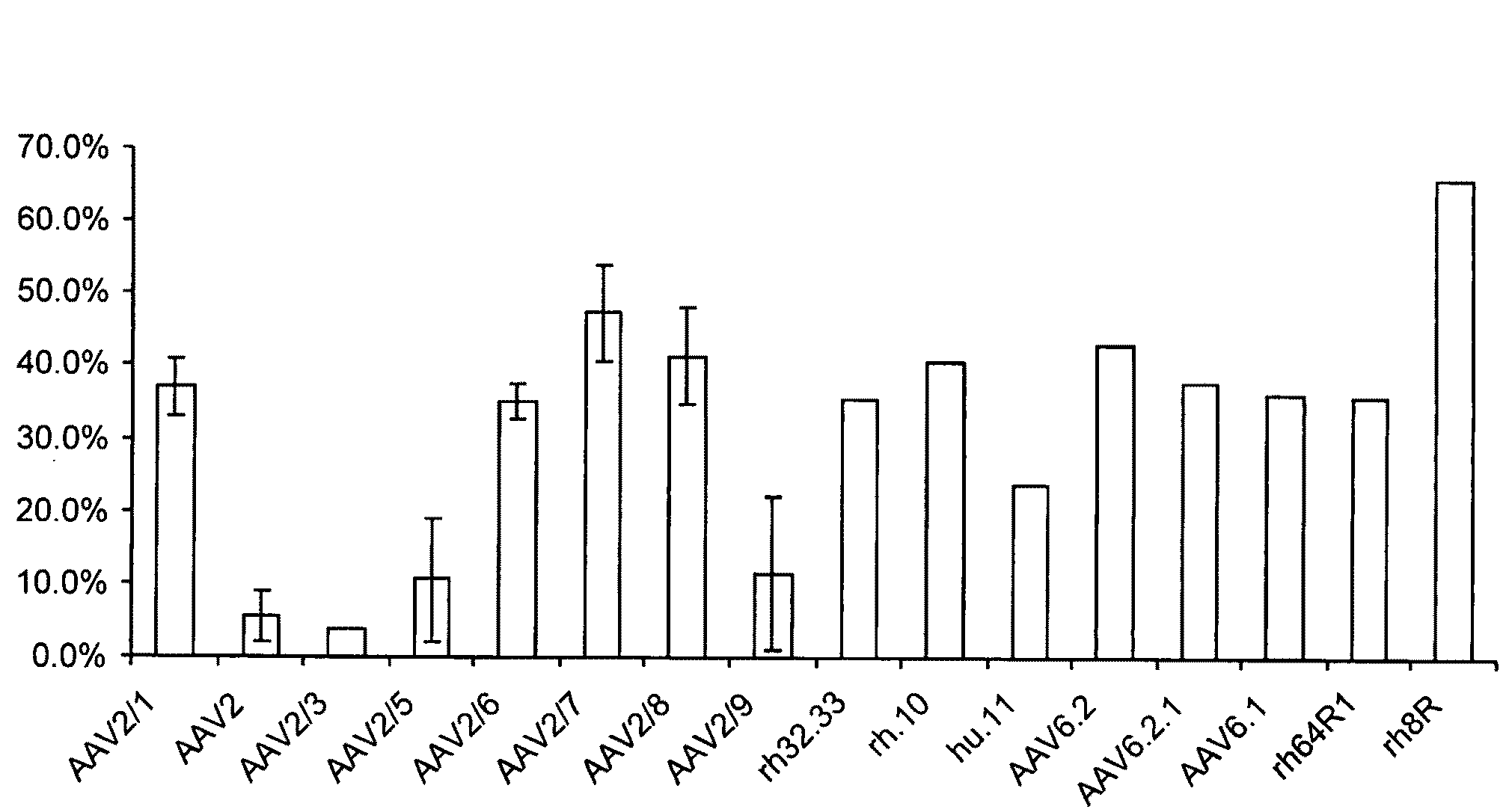
The present invention provides mutant adeno-associated virus (AAV) that exhibit altered capsid properties, e.g., reduced binding to neutralizing antibodies in serum and / or altered heparin binding and / or altered infectivity of particular cell types. The present invention further provides libraries of mutant AAV comprising one or more mutations in a capsid gene. The present invention further provides methods of generating the mutant AAV and mutant AAV libraries, and compositions comprising the mutant AAV. The present invention further provides recombinant AAV (rAAV) virions that comprise a mutant capsid protein. The present invention further provides nucleic acids comprising nucleotide sequences that encode mutant capsid proteins, and host cells comprising the nucleic acids. The present invention further provides methods of delivering a gene product to an individual, the methods generally involving administering an effective amount of a subject rAAV virion to an individual in need thereof.
Owner:INTEGRATIVE GENE THERAPEUTICS +1
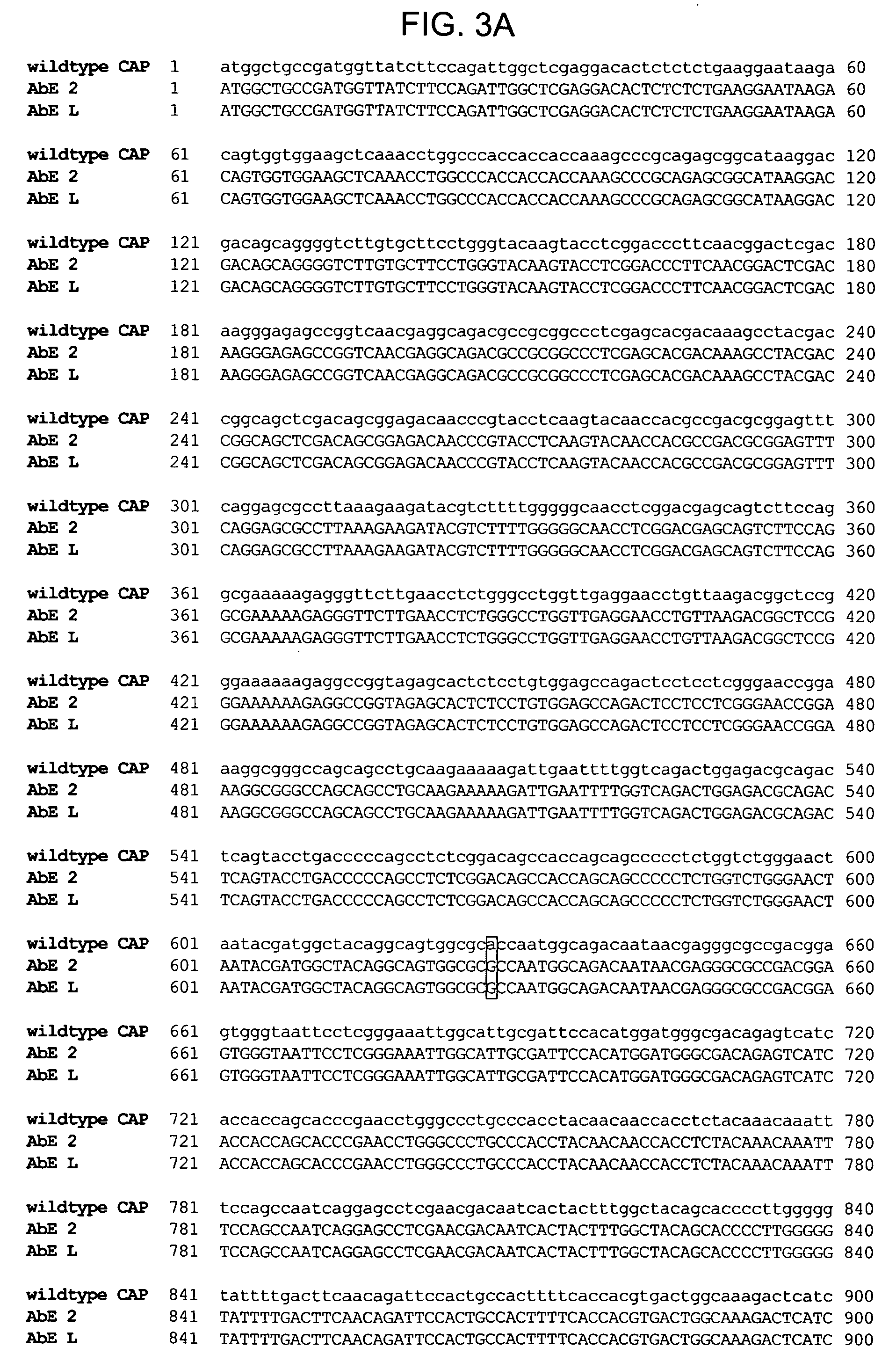
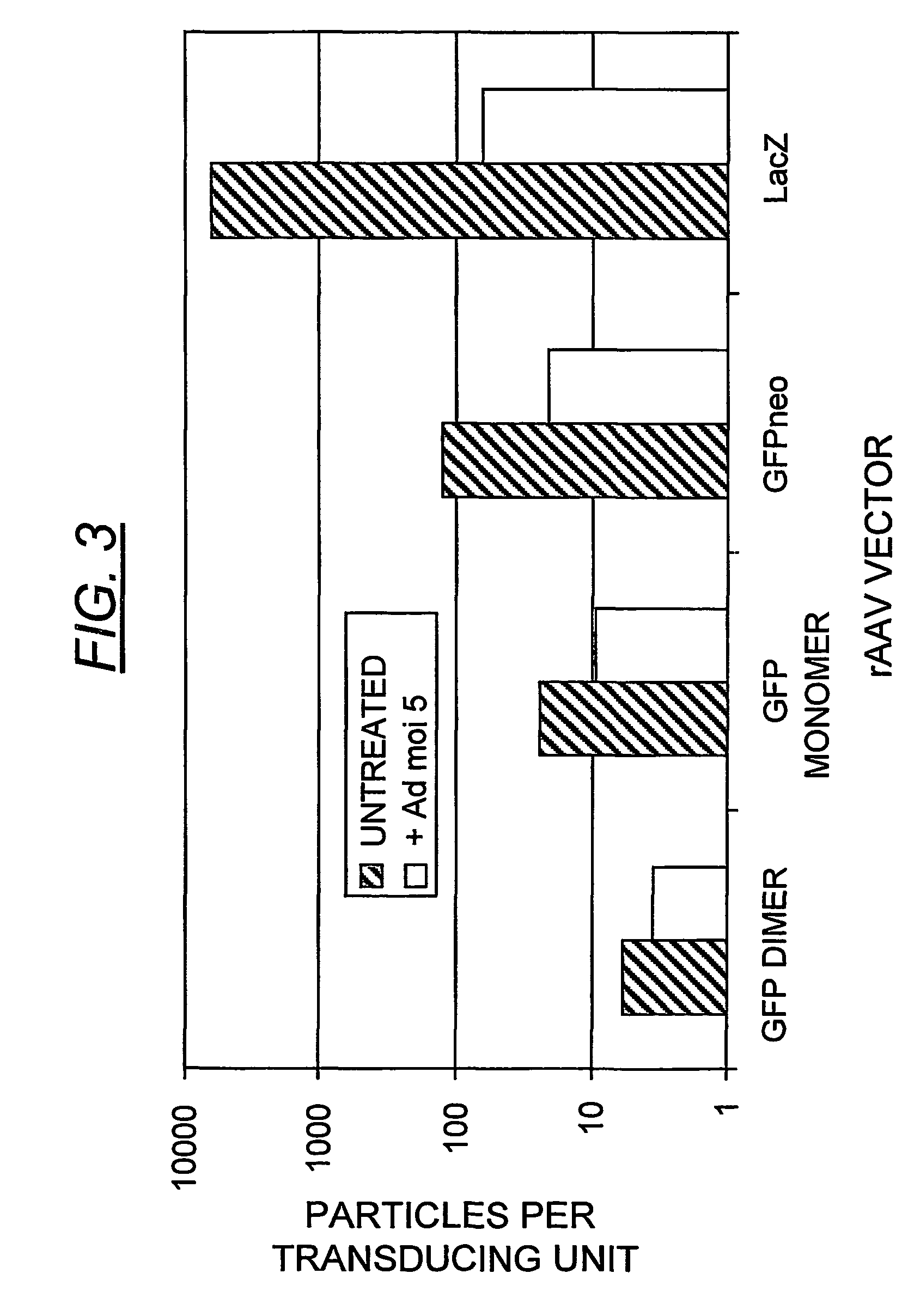
Duplexed parvovirus vectors
InactiveUS7465583B2High transduction efficiencyRapid onsetBiocidePeptide/protein ingredientsGeneticsViral vector
The present invention provides duplexed parvovirus vector genomes that are capable under appropriate conditions of forming a double-stranded molecule by intrastrand base-pairing. Also provided are duplexed parvovirus particles comprising the vector genome. Further disclosed are templates and methods for producing the duplexed vector genomes and duplexed parvovirus particles of the invention. Methods of administering these reagents to a cell or subject are also described. Preferably, the parvovirus capsid is an AAV capsid. It is further preferred that the vector genome comprises AAV terminal repeat sequences.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
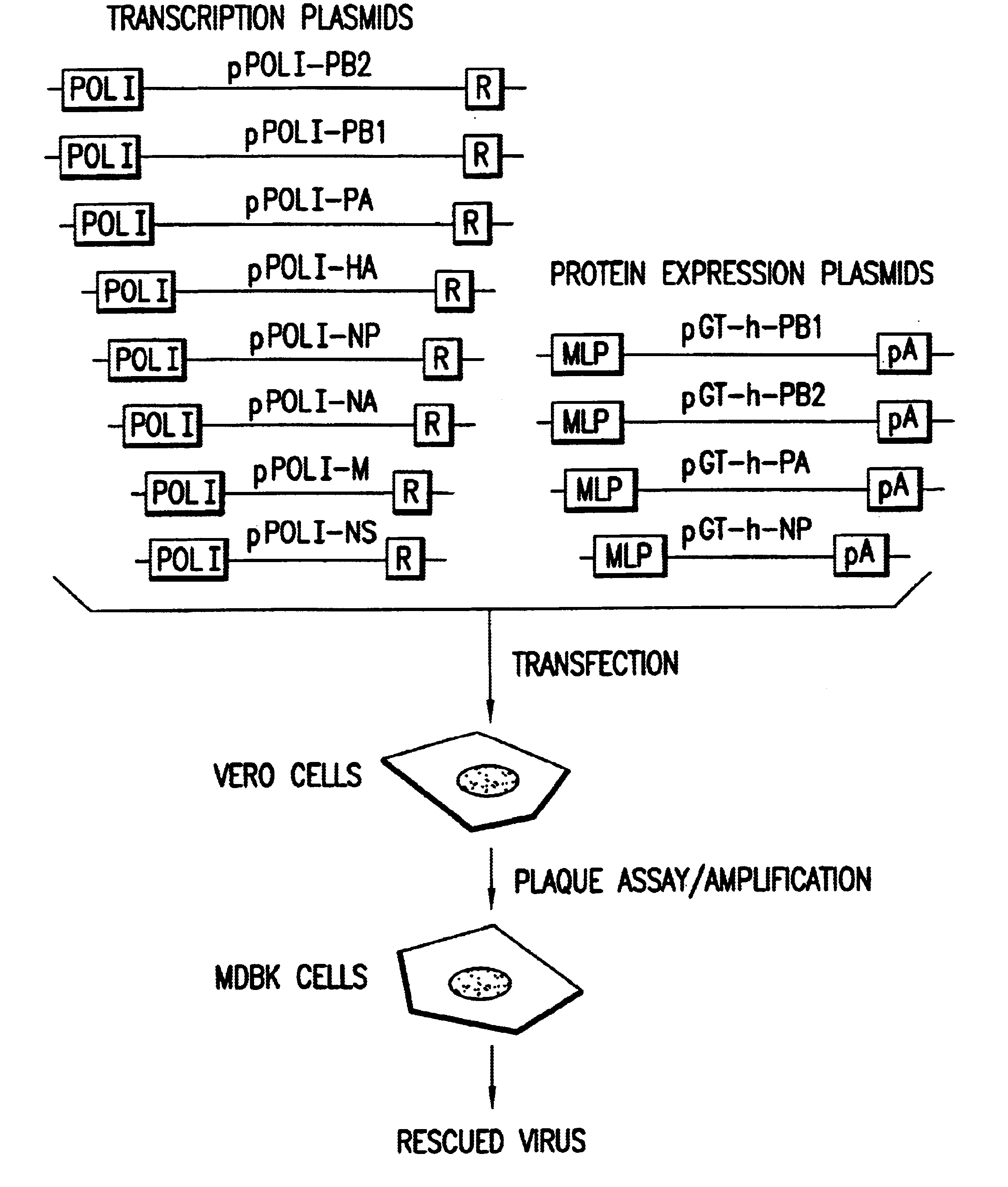
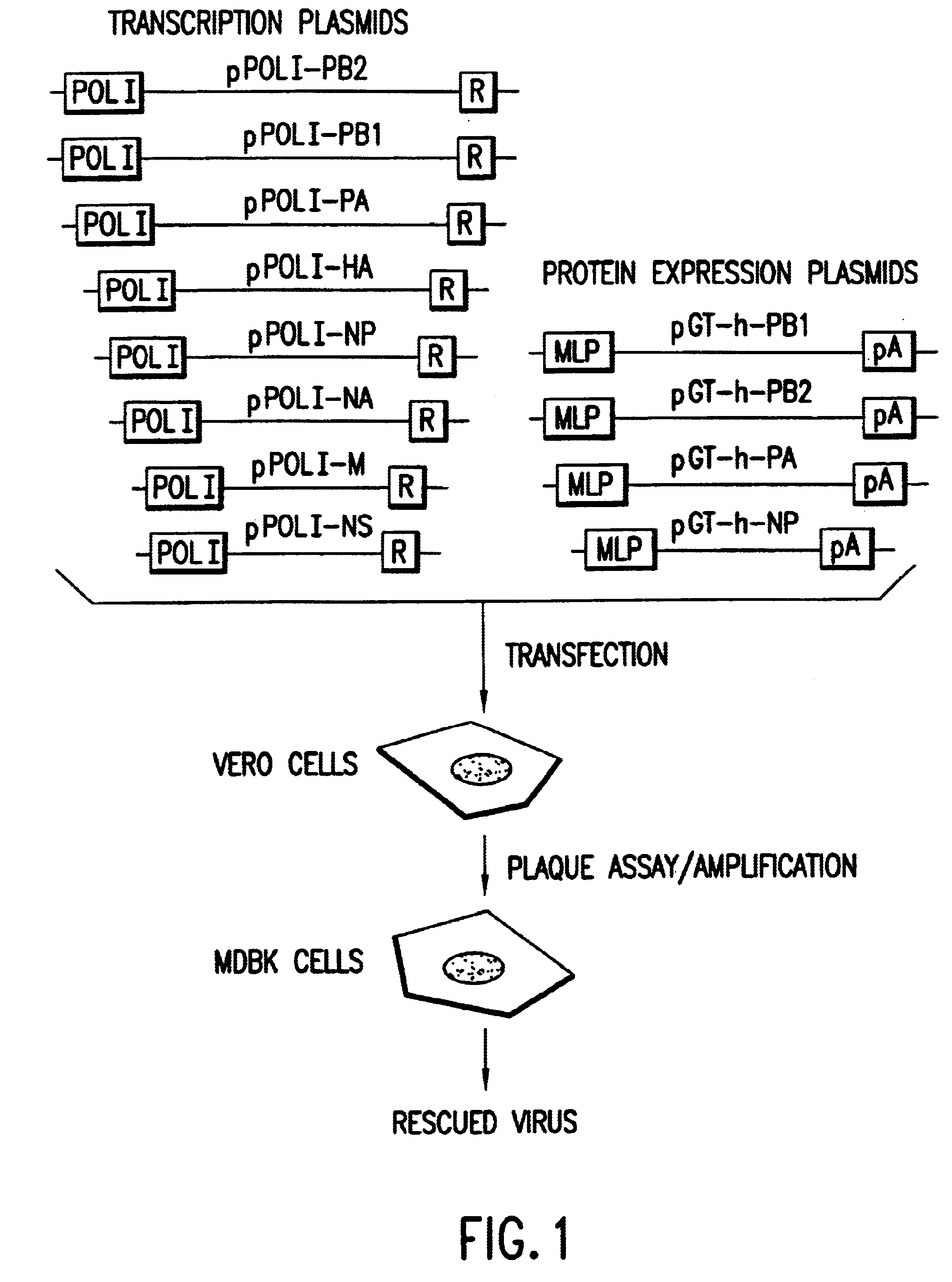
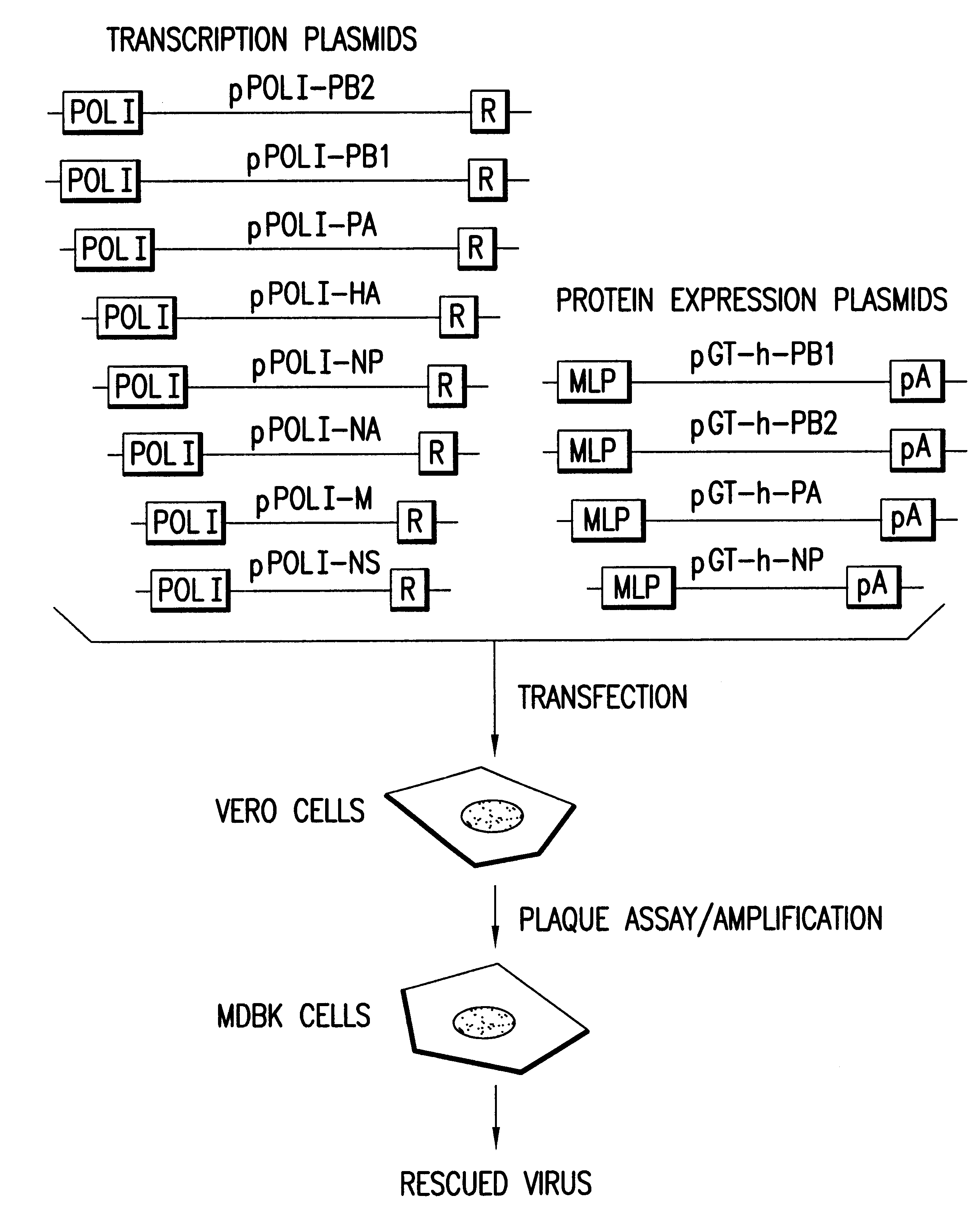
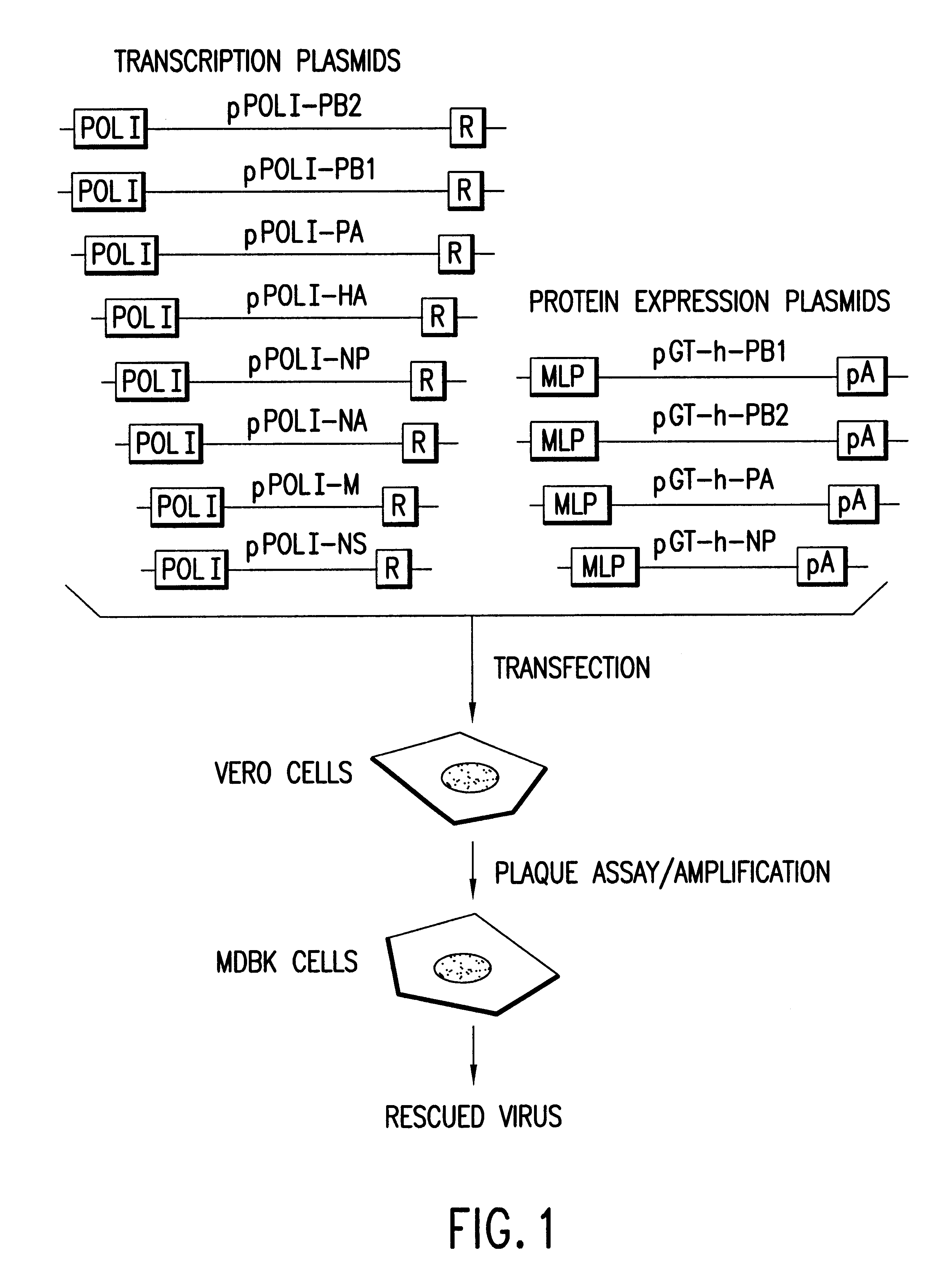
Multi plasmid system for the production of influenza virus
InactiveUS20050266026A1Easy to copyEnhanced ability to replicateSsRNA viruses negative-senseVectorsEmbryonated chicken eggCold adapted
Vectors and methods for the production of influenza viruses suitable as recombinant influenza vaccines in cell culture are provided. Bi-directional expression vectors for use in a multi-plasmid influenza virus expression system are provided. Additionally, the invention provides methods of producing influenza viruses with enhanced ability to replicate in embryonated chicken eggs and / or cells (e.g., Vero and / or MDCK) and further provides influenza viruses with enhanced replication characteristics. A method of producing a cold adapted (ca) influenza virus that replicates efficiently at, e.g., 25° C. (and immunogenic compositions comprising the same) is also provided.
Owner:MEDIMMUNE LLC
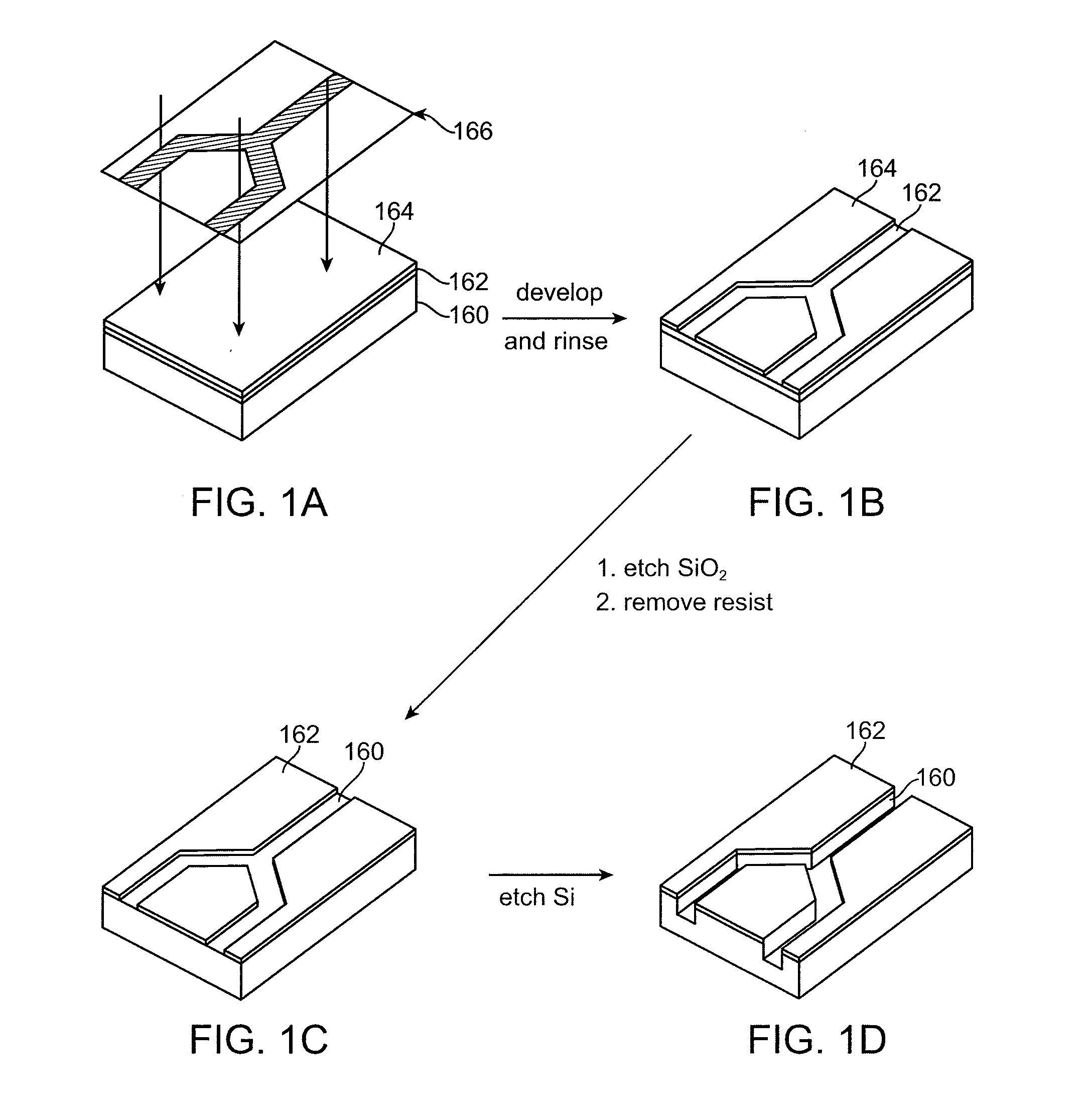
Microfabricated Crossflow Devices and Methods
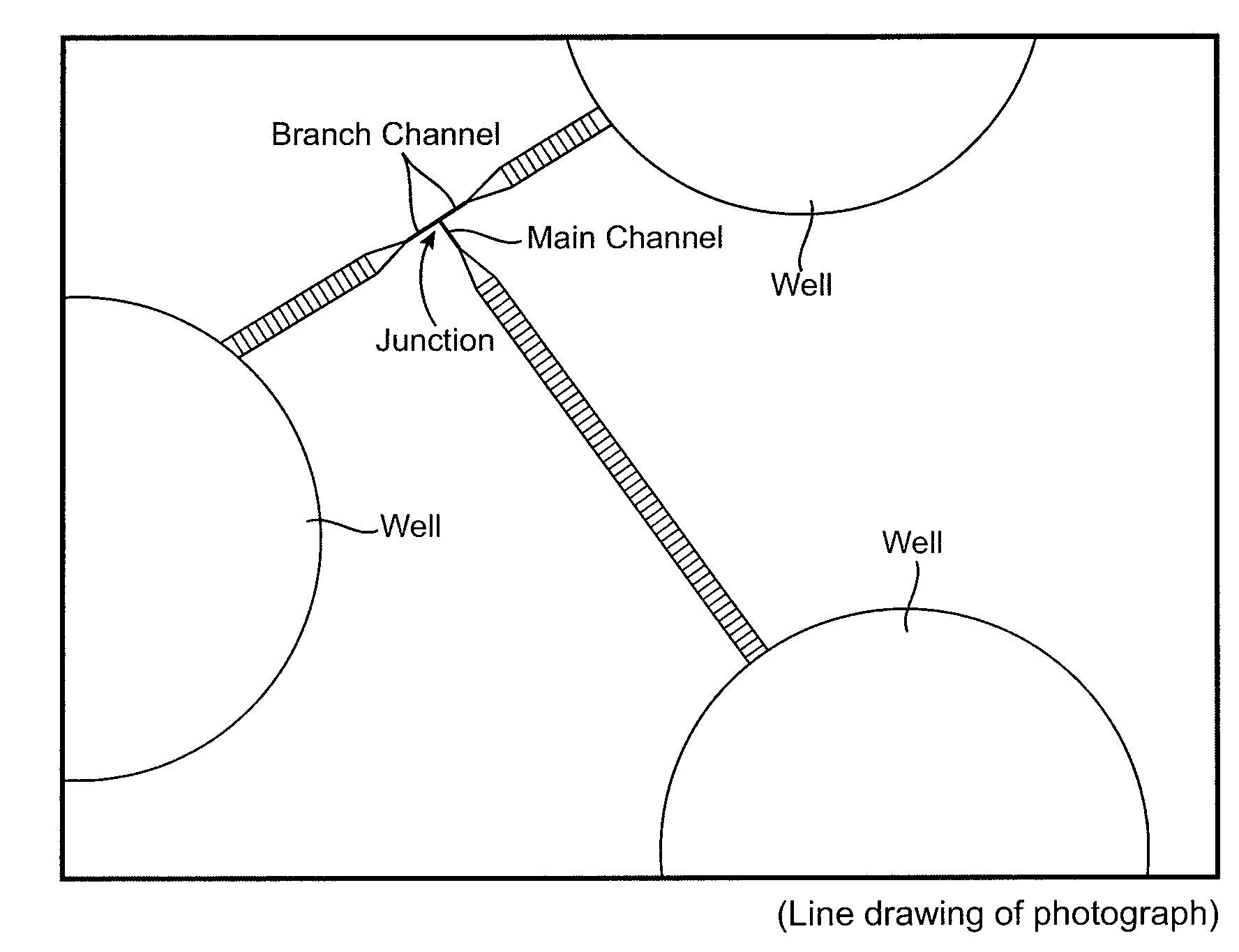
ActiveUS20090035838A1High sensitivityIncrease the number ofBioreactor/fermenter combinationsFixed microstructural devicesMain channelBiological materials
A microfluidic device for analyzing and / or sorting biological materials (e.g., molecules such as polynucleotides and polypeptides, including proteins and enzymes; viruses and cells) and methods for its use are provided. The device and methods of the invention are useful for sorting particles, e.g. virions. The invention is also useful for high throughput screening, e.g. combinatorial screening. The microfluidic device comprises a main channel and an inlet region in communication with the main channel at a droplet extrusion region. Droplets of solution containing the biological material are deposited into the main channel through the droplet extrusion region. A fluid different from and incompatible with the solution containing the biological material flows through the main channel so that the droplets containing the biological material do not diffuse or mix. Biological material within the droplets can be analyzed and / or sorted by detecting a predetermined characteristic of the biological sample in each droplet and sorting the droplet accordingly.
Owner:CALIFORNIA INST OF TECH
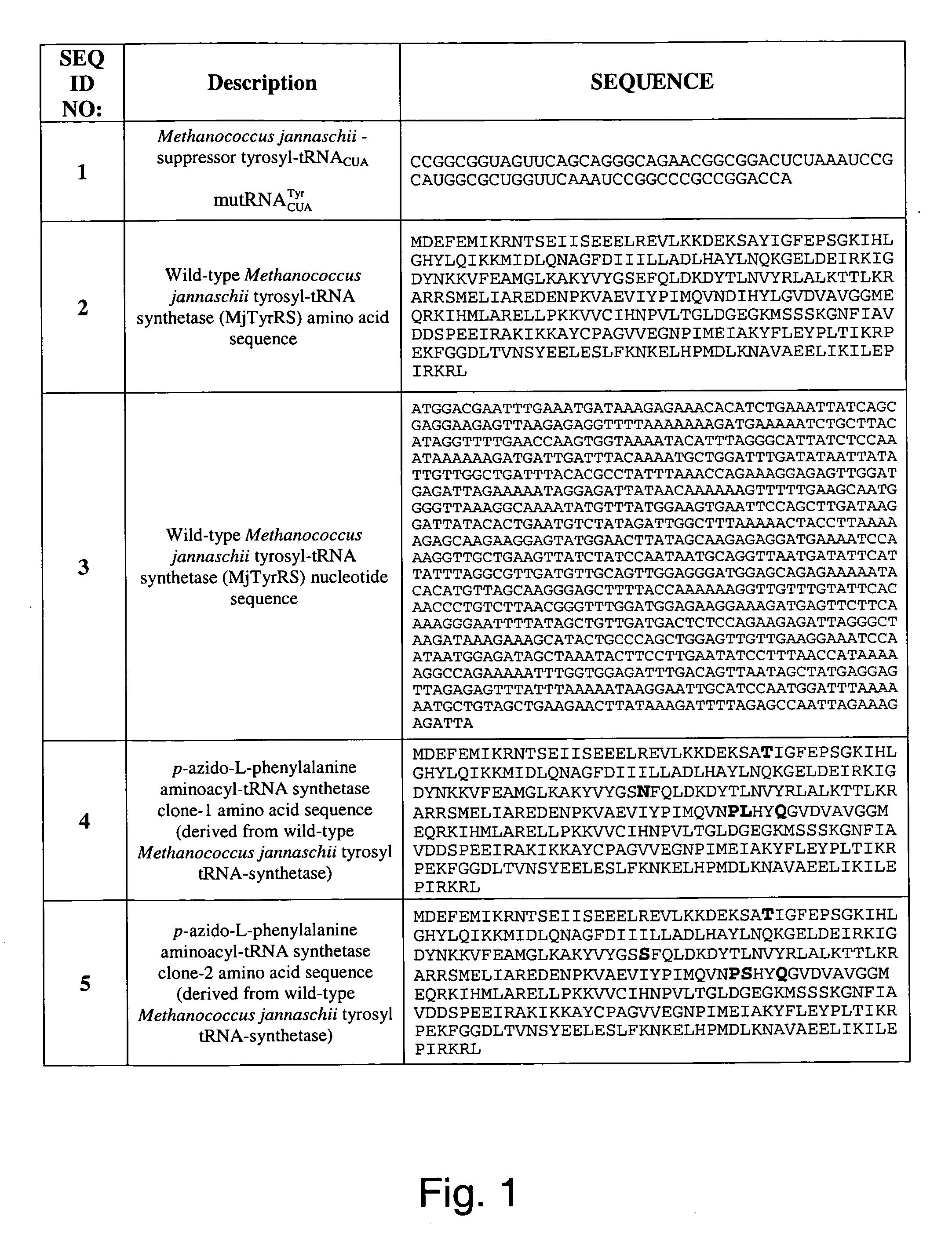
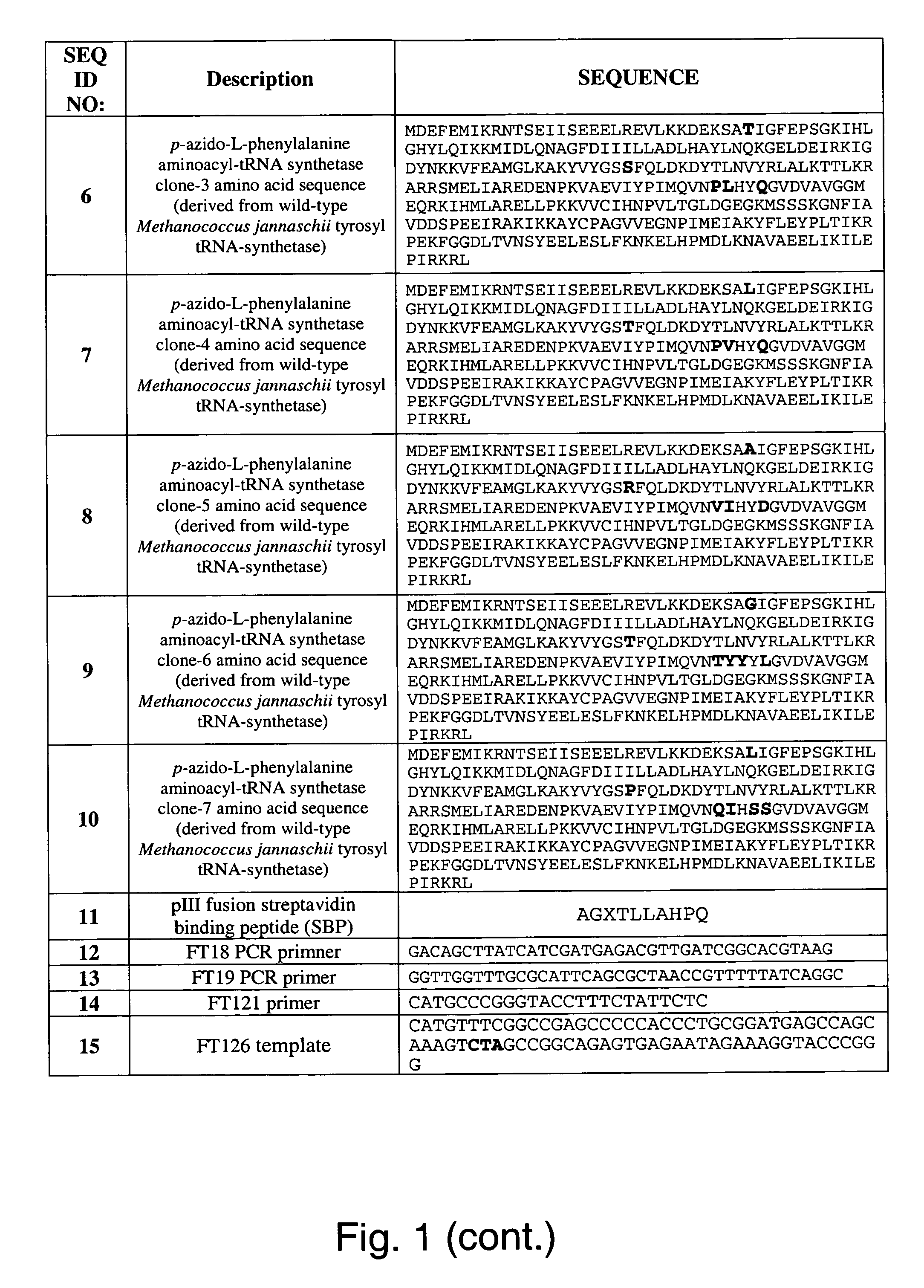
Selective posttranslational modification of phage-displayed polypeptides
ActiveUS20070178448A1Easy to detectEasy to quantifyAntibody mimetics/scaffoldsVirus peptidesArylCycloaddition
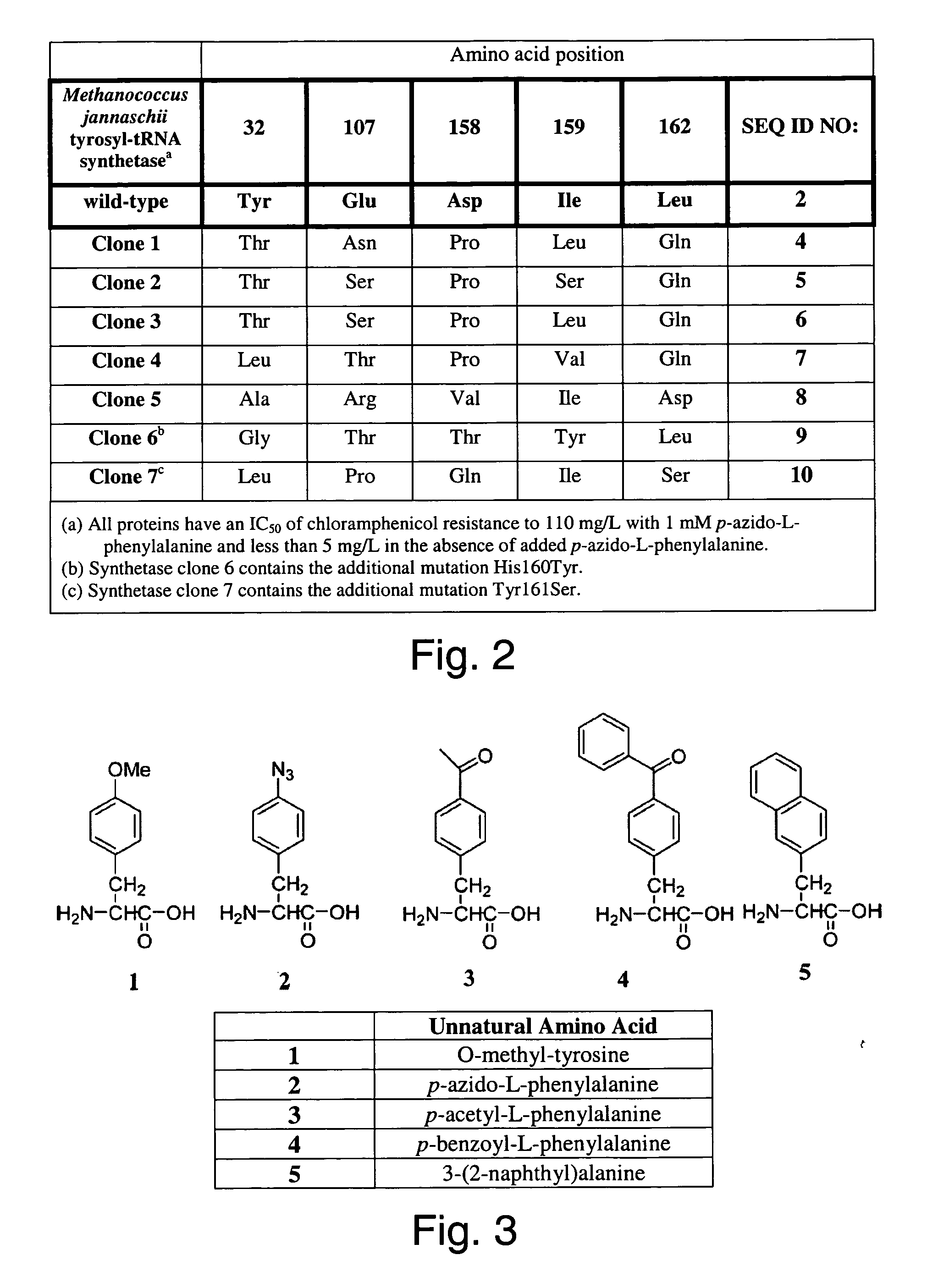
The invention relates to posttranslational modification of phage-displayed polypeptides. These displayed polypeptides comprise at least one unnatural amino acid, e.g., an aryl-azide amino acid such as p-azido-L-phenylalanine, or an alkynyl-amino acid such as para-propargyloxyphenylalanine, which are incorporated into the phage-displayed fusion polypeptide at a selected position by using an in vivo orthogonal translation system comprising a suitable orthogonal aminoacyl-tRNA synthetase and a suitable orthogonal tRNA species. These unnatural amino acids advantageously provide targets for posttranslational modifications such as azide-alkyne [3+2] cycloaddition reactions and Staudinger modifications.
Owner:THE SCRIPPS RES INST
Multi plasmid system for the production of influenza virus
InactiveUS20050158342A1Easy to copyEnhanced ability to replicateSsRNA viruses negative-senseVectorsEmbryonated chicken eggEukaryotic plasmids
Vectors and methods for the production of influenza viruses suitable as recombinant influenza vaccines in cell culture are provided. Bi-directional expression vectors for use in a multi-plasmid influenza virus expression system are provided. Additionally, the invention provides methods of producing influenza viruses with enhanced ability to replicate in embryonated chicken eggs and / or cells (e.g., Vero and / or MDCK) and further provides influenza viruses with enhanced replication characteristics. In addition, the present invention includes an improved method of rescue, wherein animal cells (e.g., SF Vero cells) are electroporated with plasmids and vectors of the invention.
Owner:MEDIMMUNE LLC
Scalable Production Method for AAV
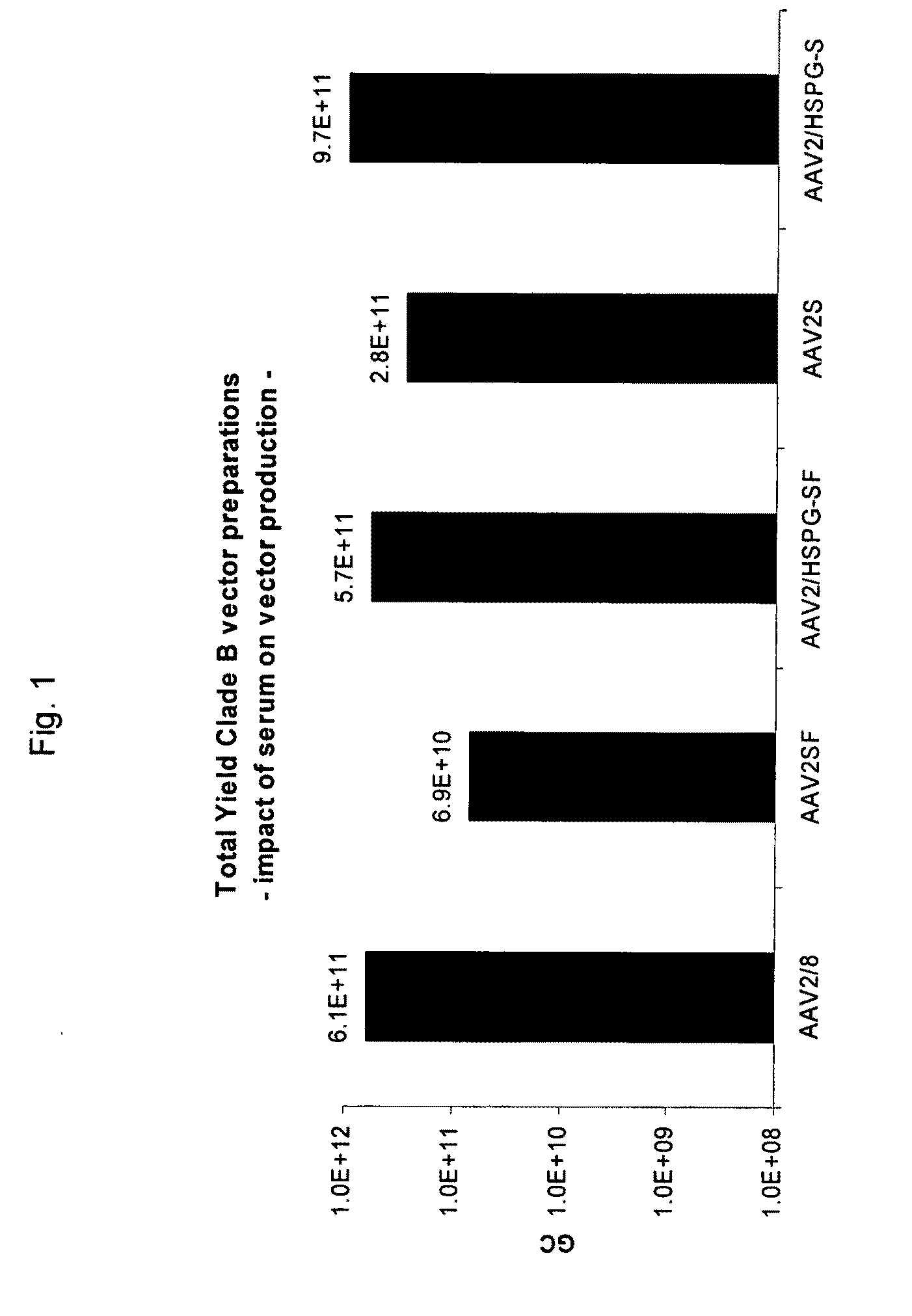
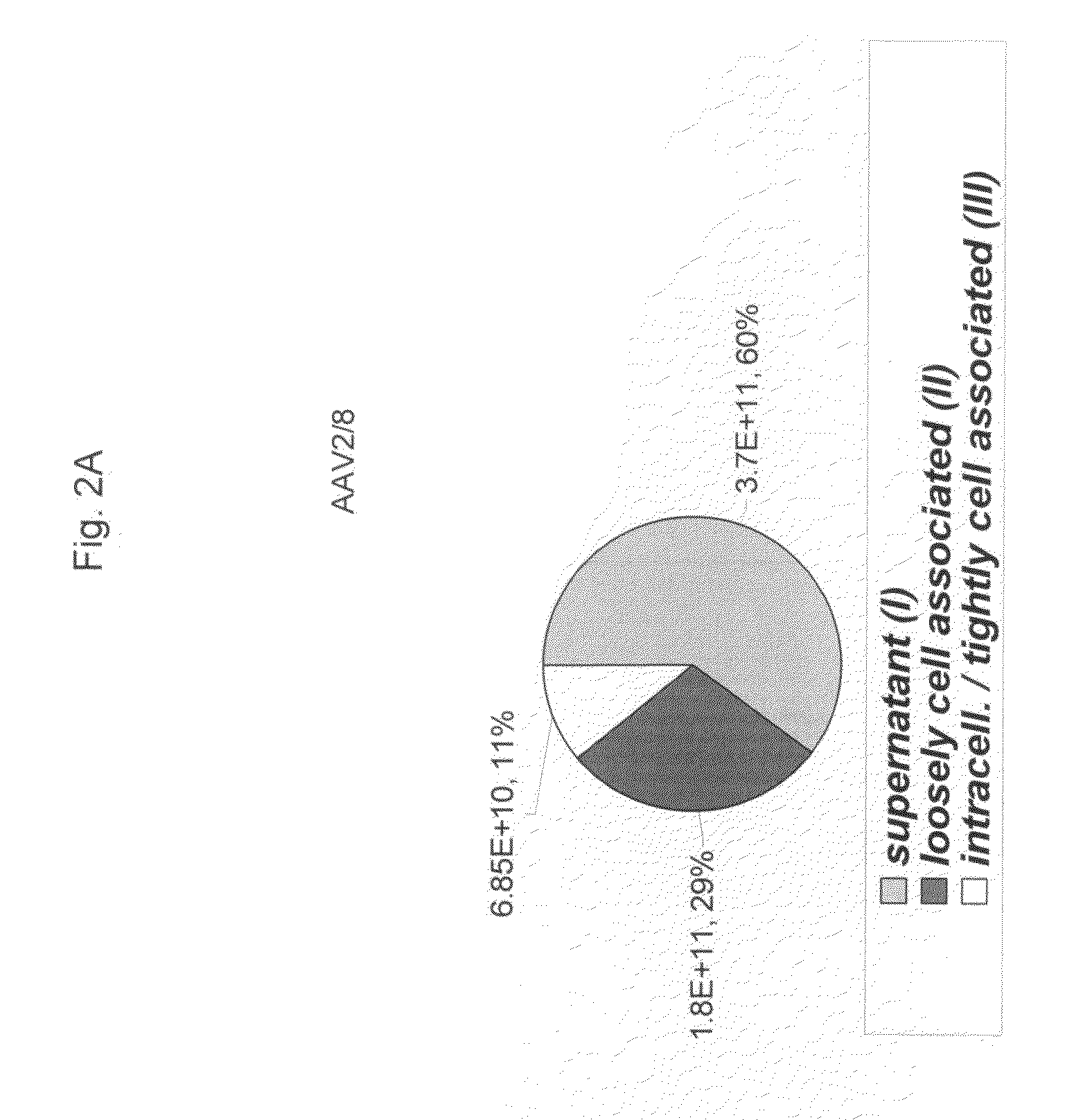
ActiveUS20090275107A1Reduce and eliminate bindingHigh yieldGenetic therapy composition manufactureVirus peptidesLysisBinding site
A method for producing AAV, without requiring cell lysis, is described. The method involves harvesting AAV from the supernatant. For AAV having capsids with a heparin binding site, the method involves modifying the AAV capsids and / or the culture conditions to ablate the binding between the AAV heparin binding site and the cells, thereby allowing the AAV to pass into the supernatant, i.e., media. Thus, the method of the invention provides supernatant containing high yields of AAV which have a higher degree of purity from cell membranes and intracellular materials, as compared to AAV produced using methods using a cell lysis step.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Recombinant negative strand RNA virus expression systems and vaccines
Owner:MEDIMMUNE VACCINES
Delivery of proteins using adeno-associated virus (AAV) vectors
ActiveUS20120232133A1Reduce the risk of infectionAvoid virus infectionOrganic active ingredientsSugar derivativesAdeno-associated virusVirology
Disclosed herein are compositions, systems and methods for delivery of proteins of interest using adeno-associated virus (AAV) vectors.
Owner:CALIFORNIA INST OF TECH
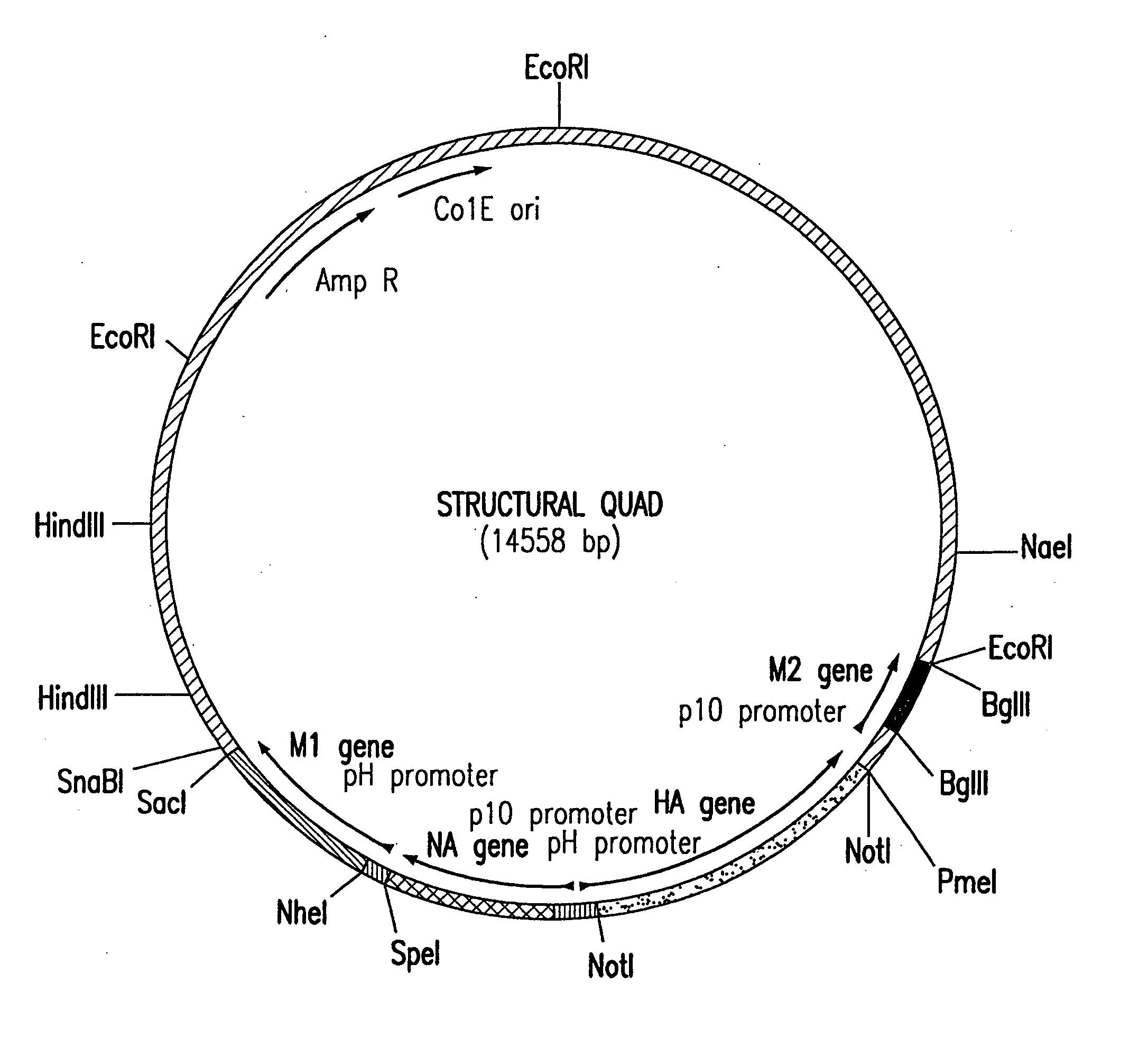
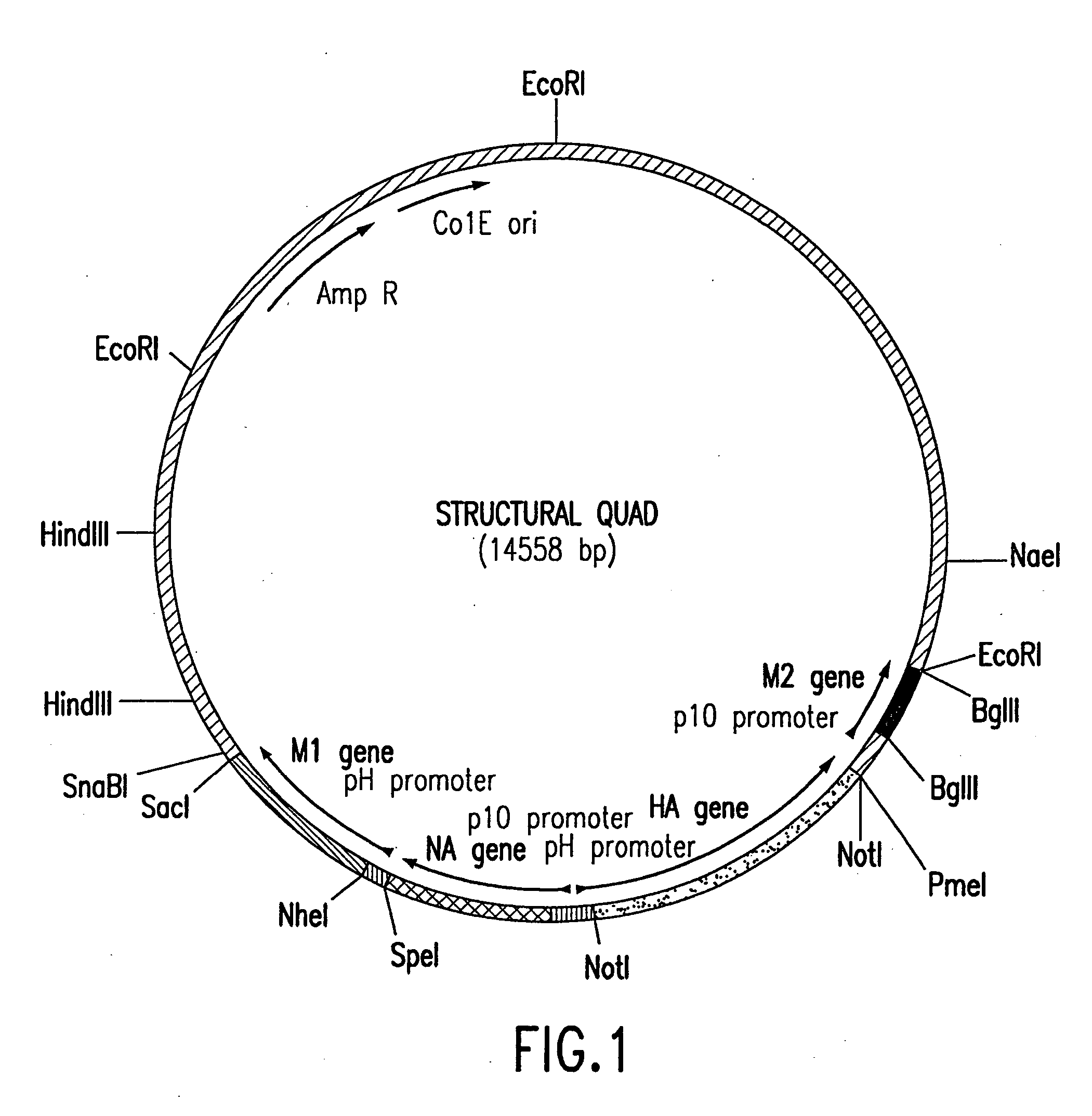
Assembly of wild-type and chimeric influenza virus-like particles (VLPs)
InactiveUS20050186621A1Minimal numberSsRNA viruses negative-senseFungiHeterologousVirus-like particle
Influenza virus-like particles (VLPs) comprising the structural proteins HA, NA, M1 and M2 are described. VLPs are also generated containing M1 alone, as are VLPs with M1 and any one or two of HA, NA and M2. VLPs with HA from one influenza subtype and NA from a different influenza subtype are also described, as are VLPs in which a portion or all of HA or NA is replaced by a heterologous moiety not produced by influenza virus, so as to comprise chimeric VLPs.
Owner:WYETH HOLDINGS LLC
Porcine circovirus 2 type inactivated vaccine
InactiveCN101240264ASimple processEasy to operateViral antigen ingredientsMicroorganism based processesAdjuvantVaccine Production
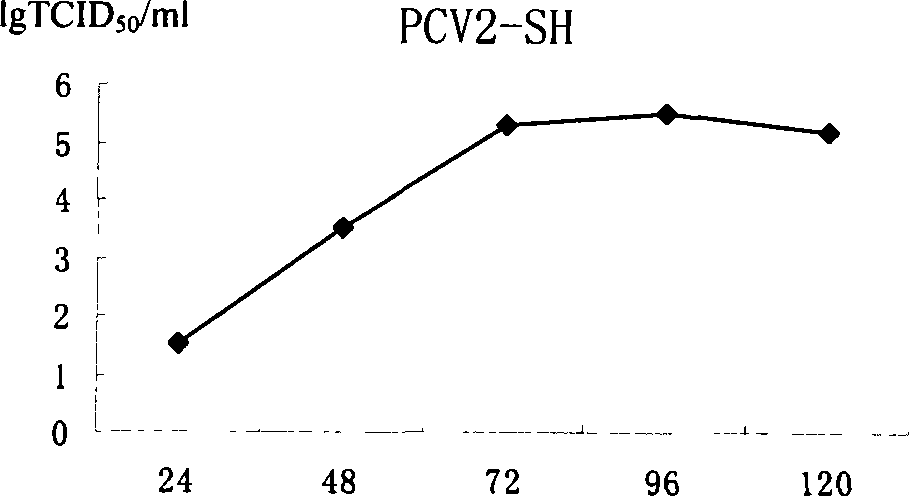
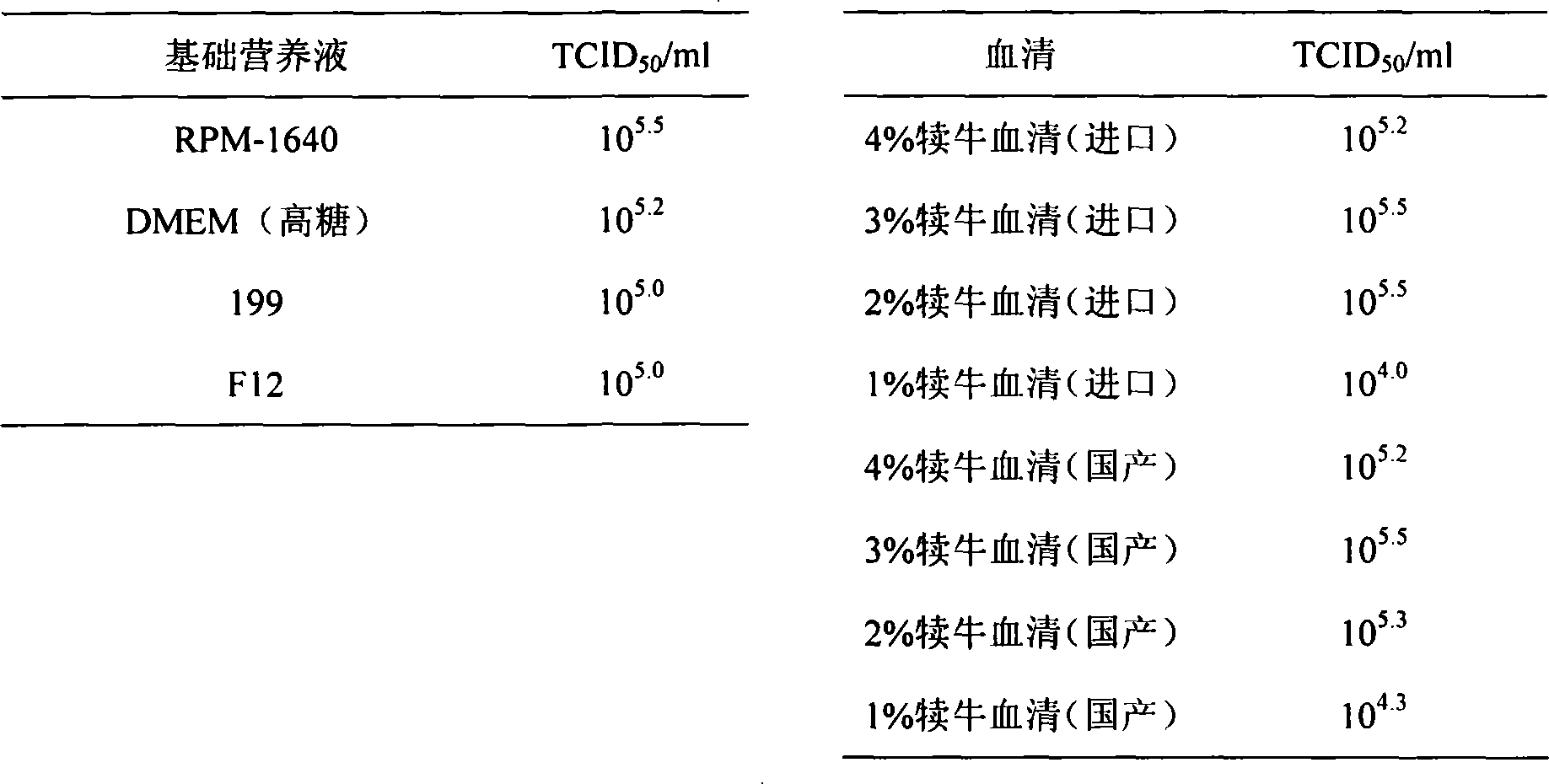
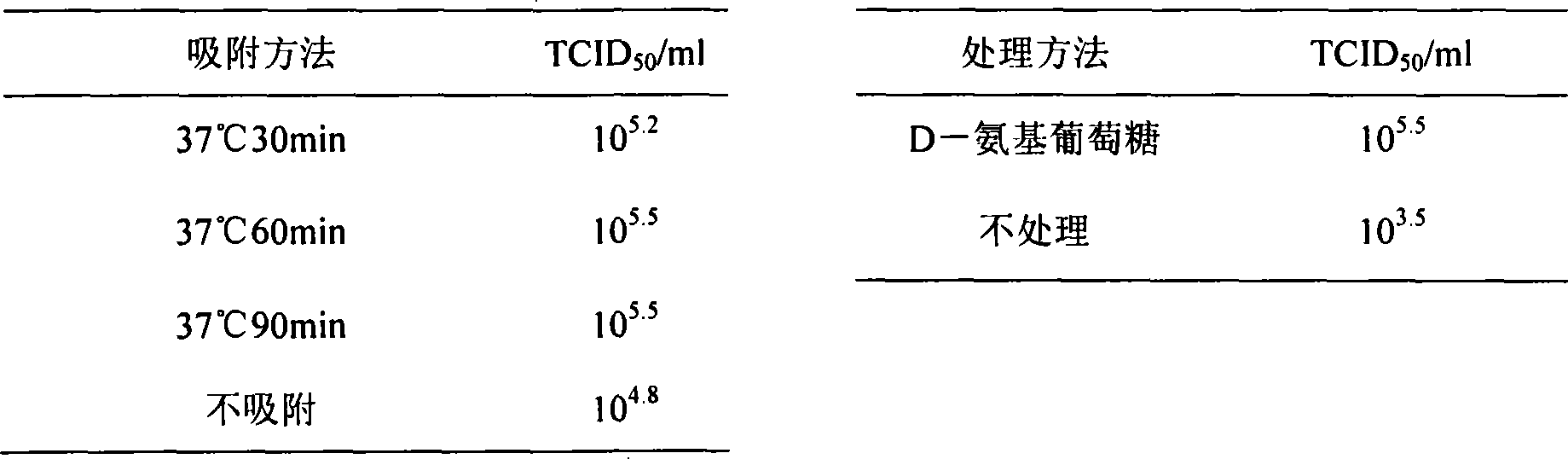
The pig circular ring virus 2 type (PVC2) inactivated vaccine (SH individual plant) of the invention belongs to biotechnology field. The pig circular ring virus 2 type poisonous individual plant SH belongs to circular ring virus section circular ring virus genus which has been preserved in Wuhan institute of virology, Chinese academy of sciences. The shanghai separated individual plant SH of purified PCV2 virus is obtained by gathering raw material from hogpen which happened bad weaning piglet multisystem exhaustion failure syndrome in Shanghai in 2002 year, separating, appraising and purifying virus. The PCV2-SH plant is proliferated in mass in PK-15 cell, inactivated through methyl aldehyde and emulsified with liquid paraffine adjuvant to prepare conventional liquid paraffin(e) adjuvant immunomodulators for vaccines. The laboratory has trial-manufactured five lots vaccines successfully which are good safety and also can induce pig bring immune protection effect, made out a draft rules for vaccines production and testing. The inactivated vaccine proved by every aspects experiment has met state biological products standard completely.
Owner:NANJING AGRICULTURAL UNIVERSITY
Treatment of AMD using aav sflt-1
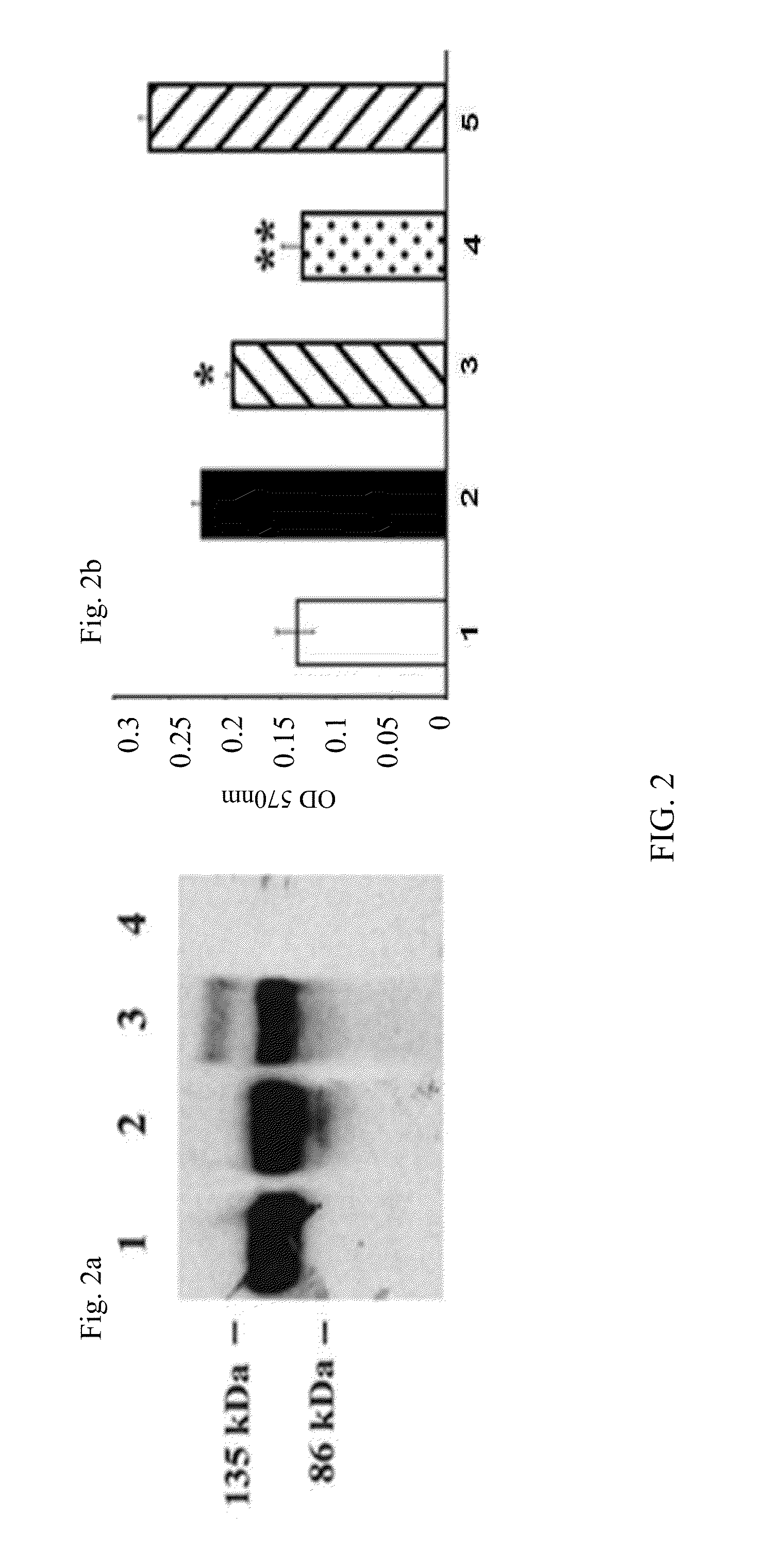
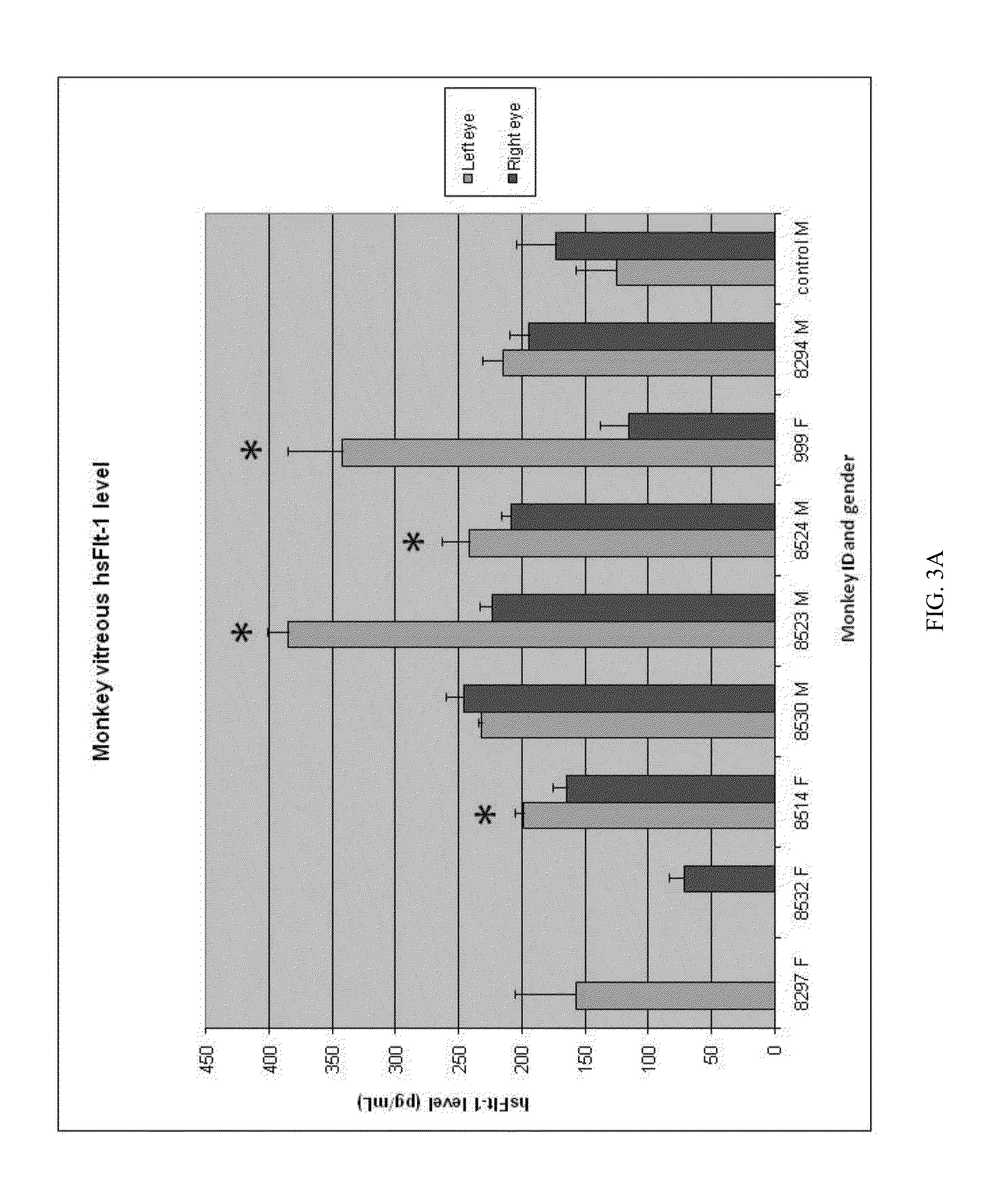
InactiveUS20130323302A1Compounds screening/testingOrganic active ingredientsOcular neovascularizationMedicine
The present disclosure provides compositions and methods for the prevention or treatment of ocular neovascularization, such as AMD, in a human subject, by administering subretinally a pharmaceutical composition comprising a pharmaceutically effective amount of a vector comprising a nucleic acid encoding soluble Fms-related tyrosine kinase-1 (sFlt-1) protein to the human subject.
Owner:AVALANCHE AUSTRALIA
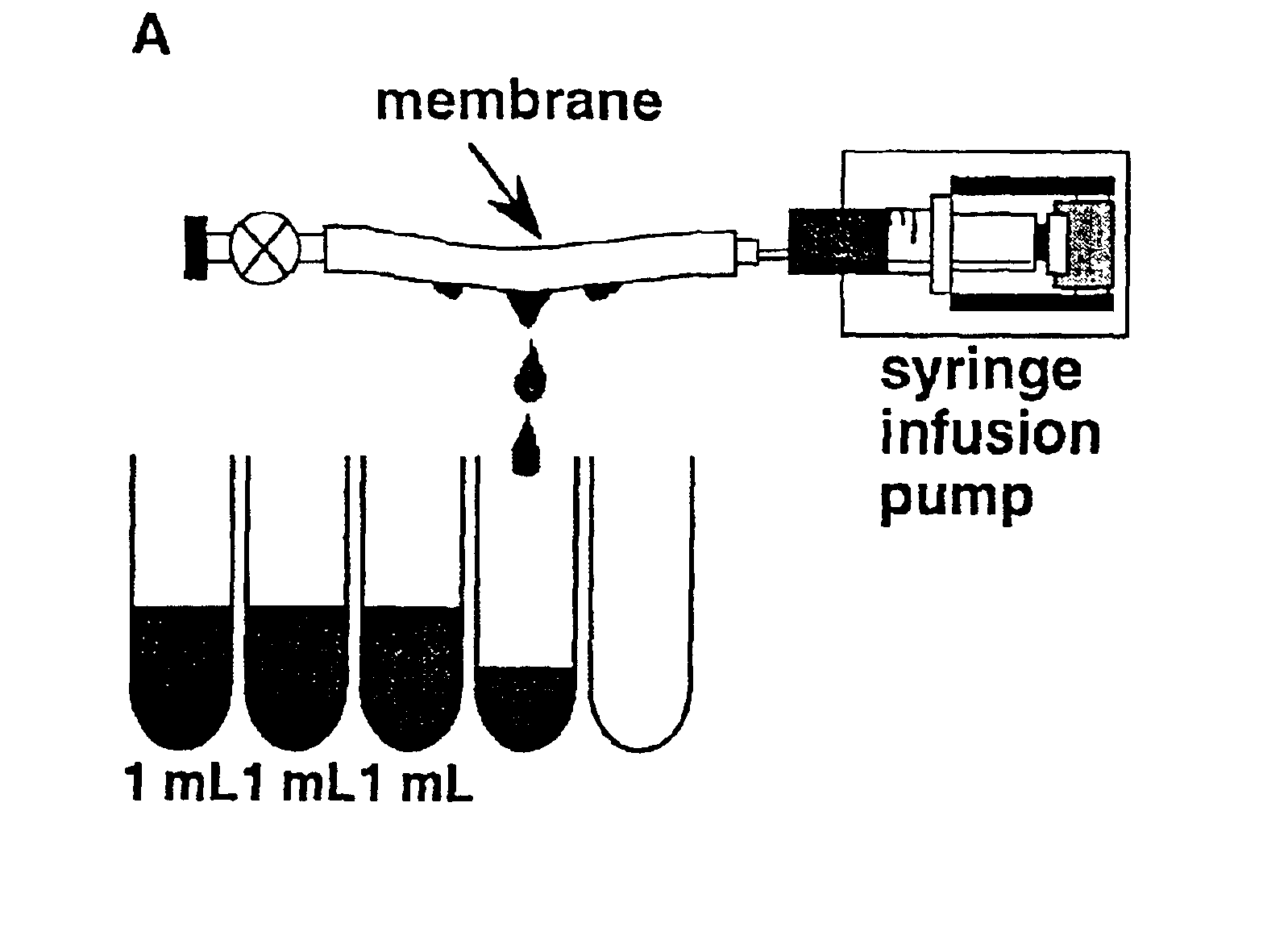
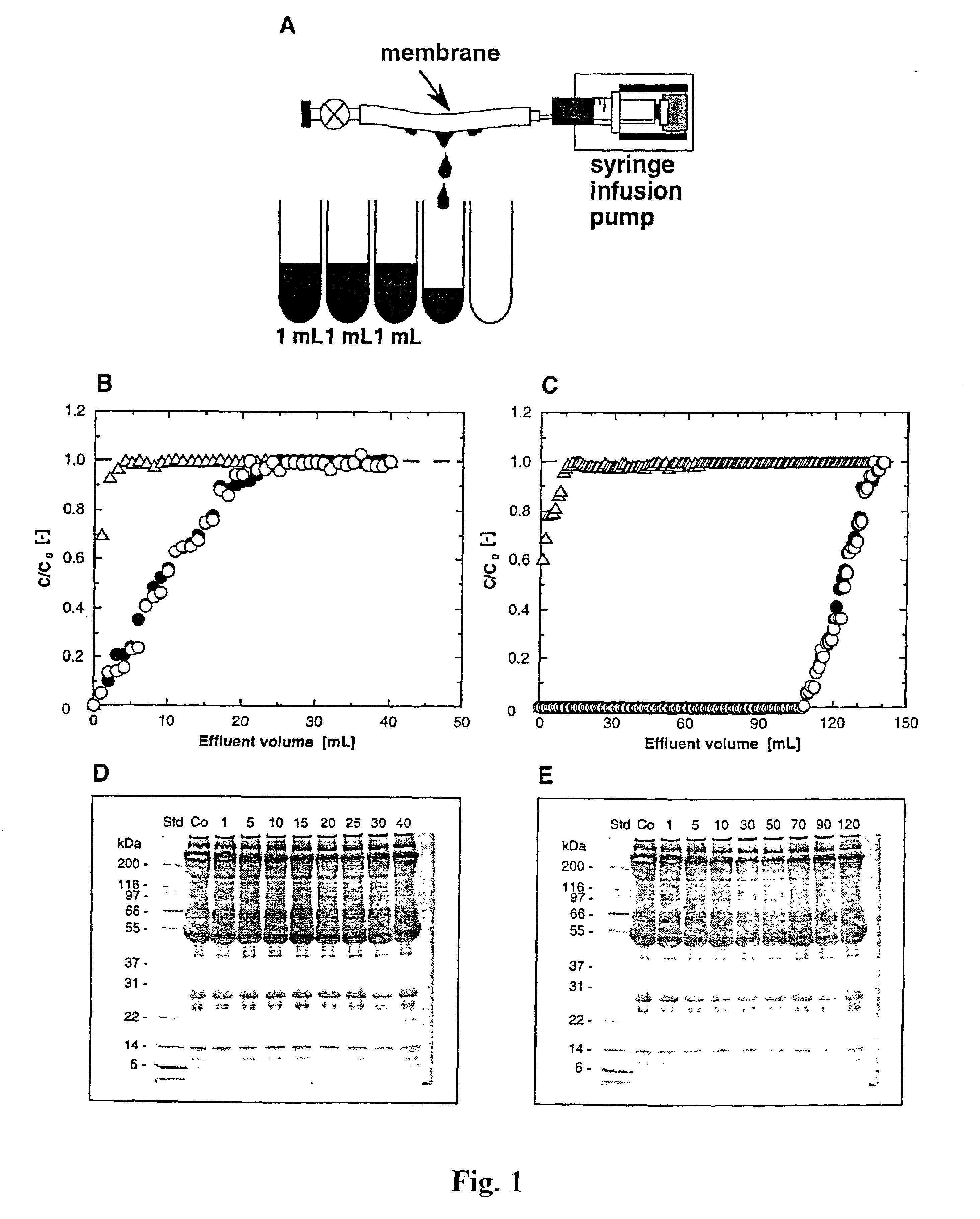
Methods for removal, purification, and concentration of viruses, and methods of therapy based thereupon
InactiveUS6861001B2Effect clotting timeEasy to zoom inBioreactor/fermenter combinationsBiological substance pretreatmentsSide chainBlood plasma
The invention is based on the discovery that certain membranes, which include side chains or molecular “brushes” having, for example, tertiary amino functional groups, can be used as highly effective filters to capture viruses / virus particles from liquids without removal of proteins. New methods based on this discovery include removing viruses from liquids such as blood or plasma, removing viruses from pharmaceuticals, concentrating and / or purifying viruses, e.g., for use in gene therapy, and producing recombinant viruses in new bioreactors. The invention also includes new methods of therapy or adjunct therapy for viral infections, in which a patient's blood or plasma is filtered through the membranes to remove viruses to reduce the viral load. The invention also includes new bioreactors and viral filters containing the membranes.
Owner:THE GENERAL HOSPITAL CORP
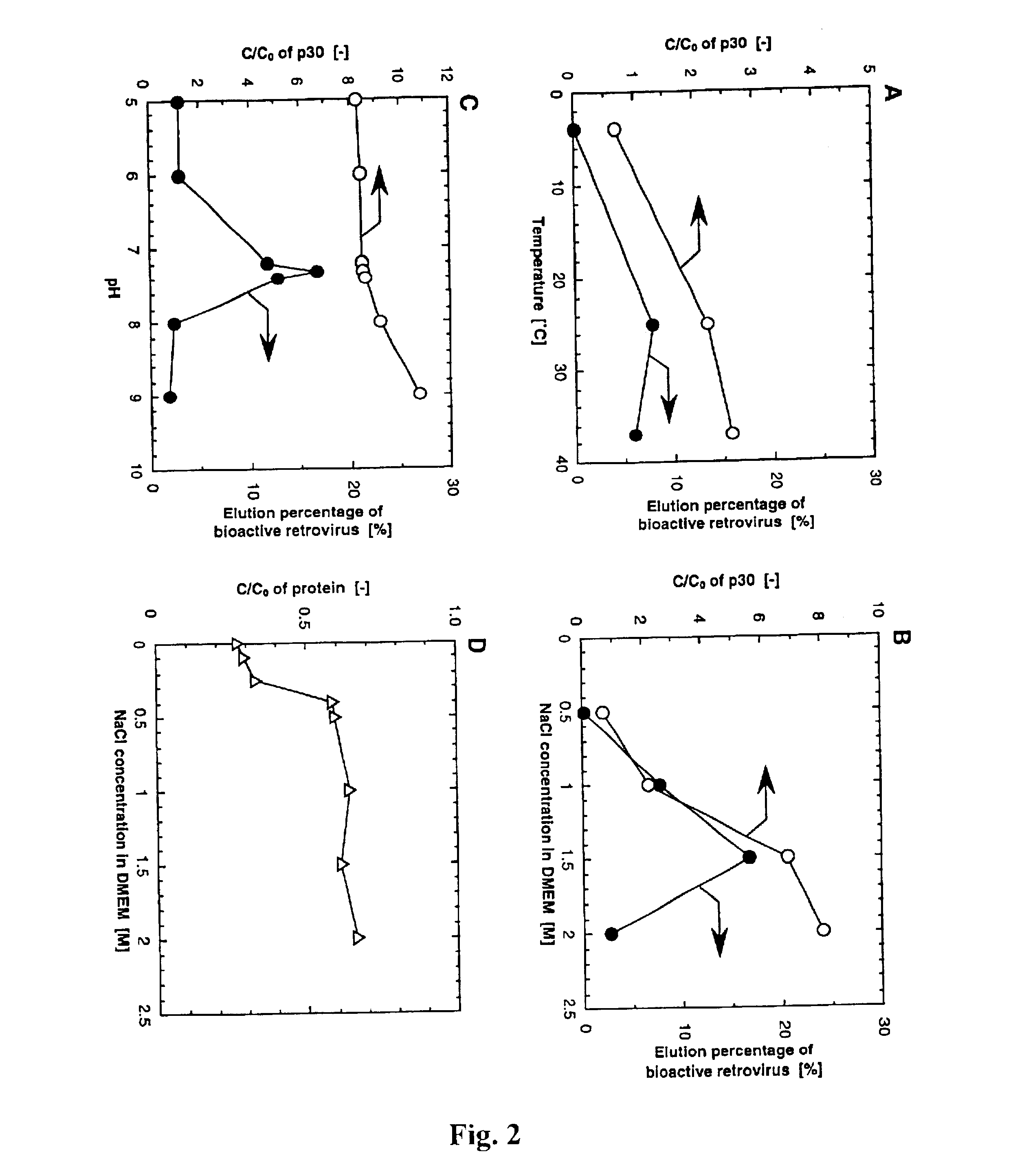
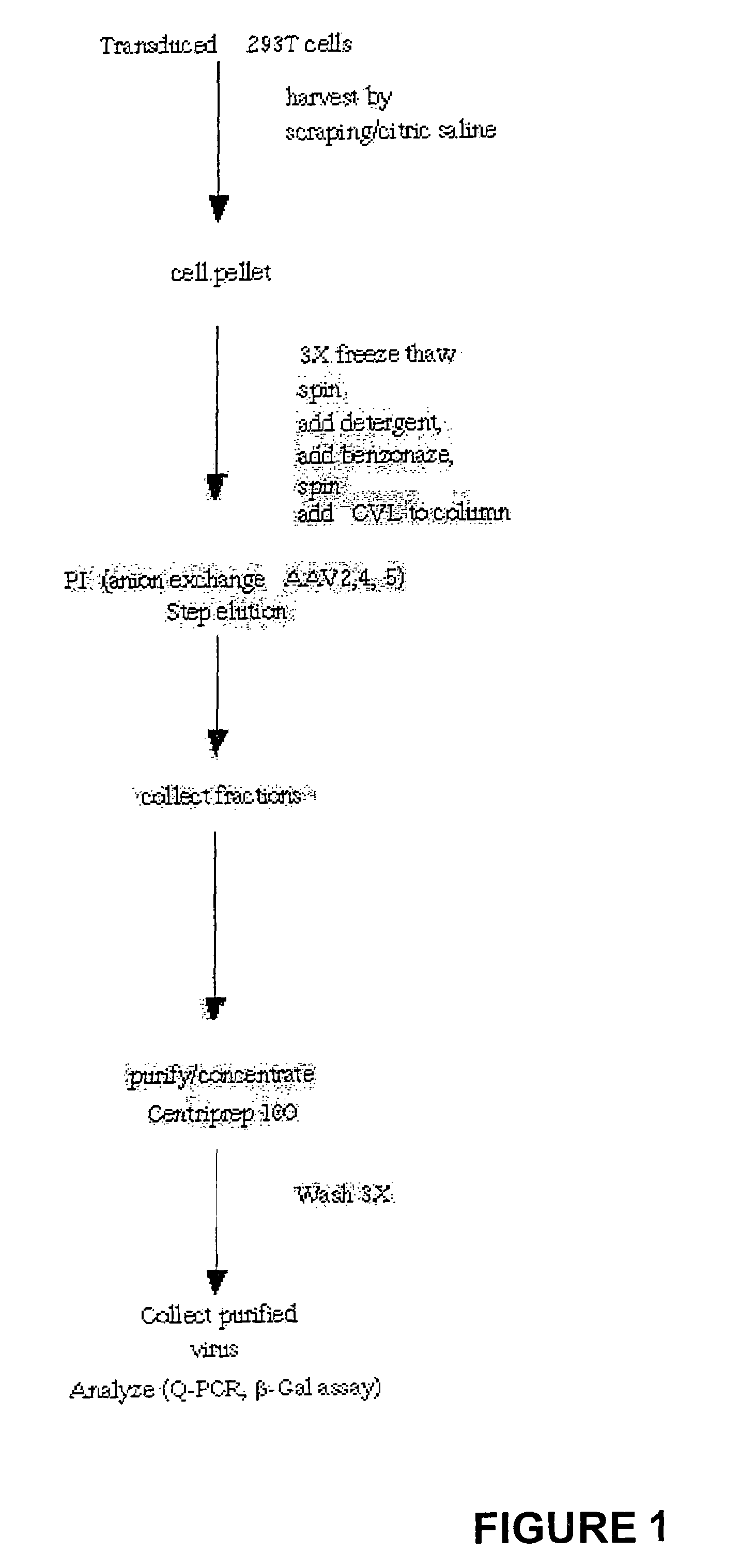
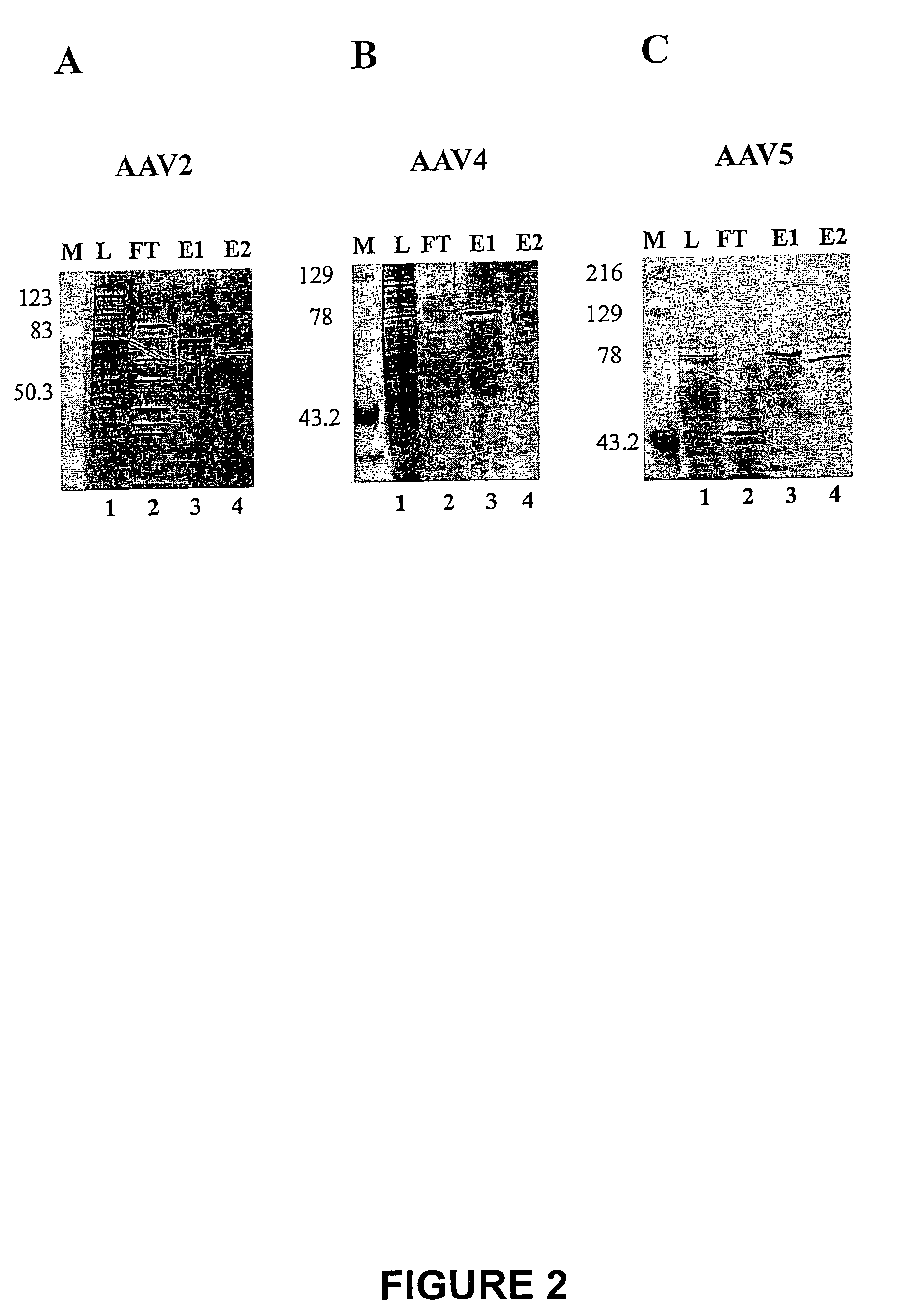
Scalable purification of AAV2, AAV4 or AAV5 using ion-exchange chromatography
The present invention provides methods of purifying adeno-associated virus (AAV) particles. These AAV particles include AAV2, AAV4 and AAV5 particles. The present invention also provides AAV particles purified by the methods of the present invention.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE
Method for the production and purification of adenoviral vectors
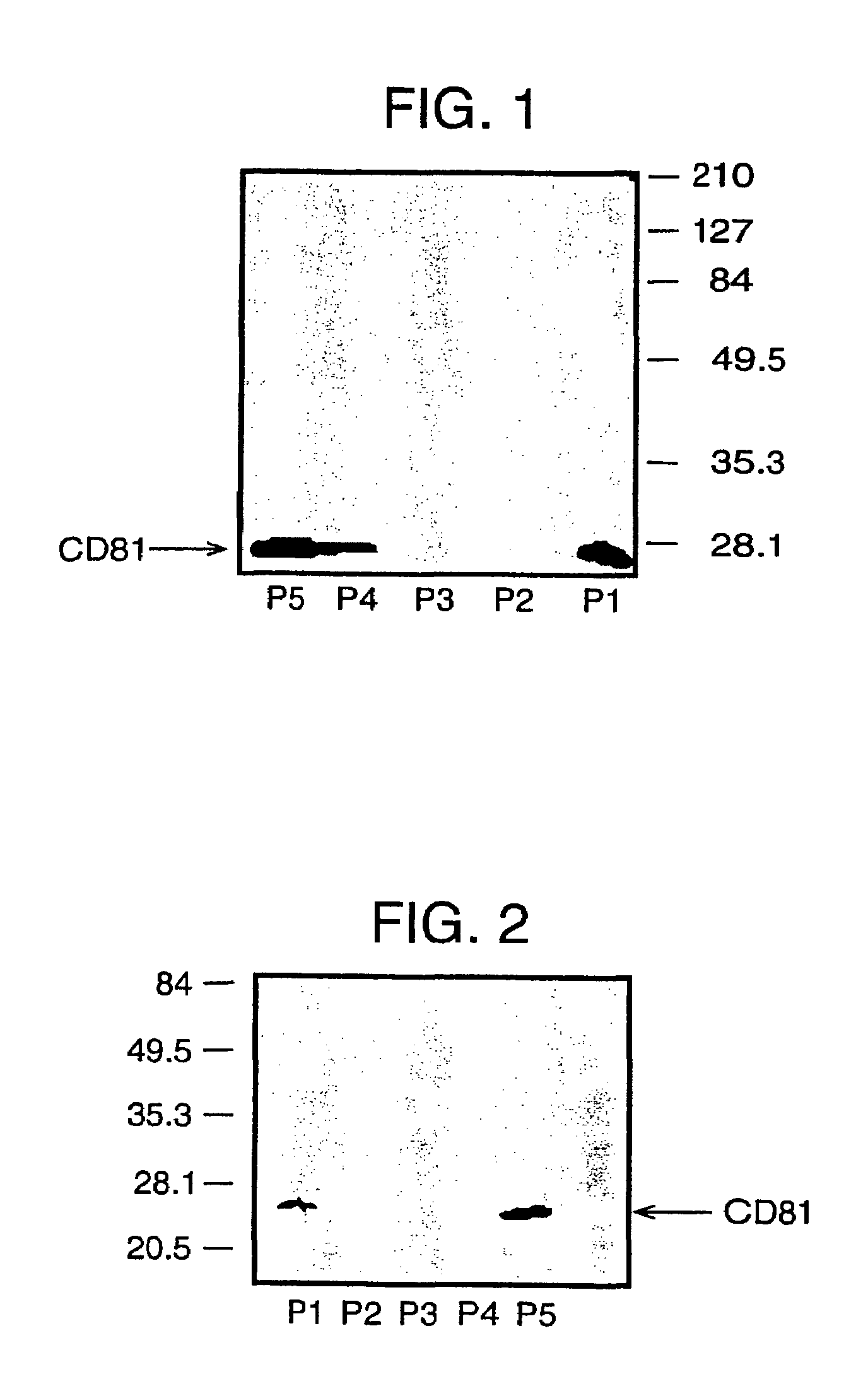
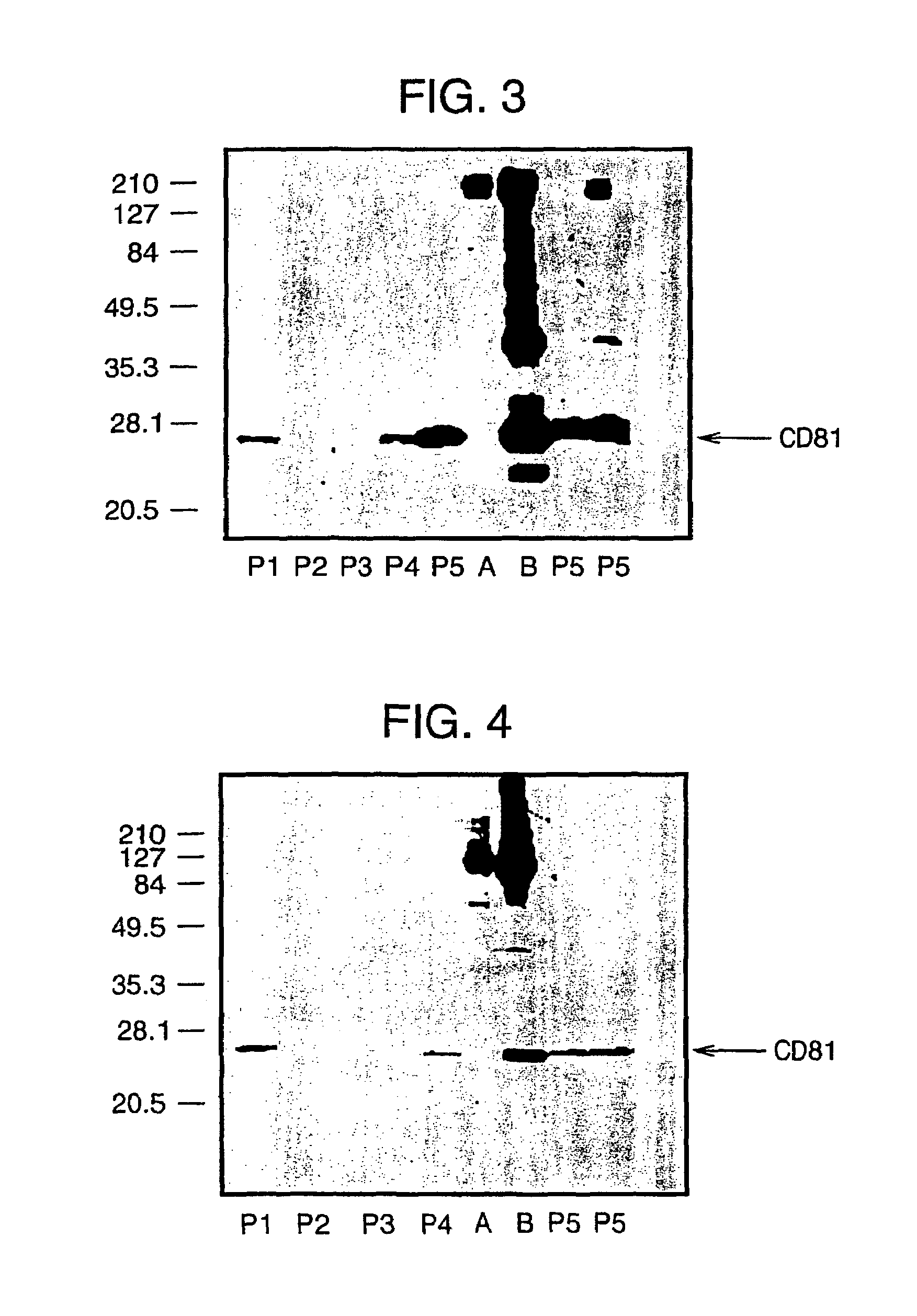
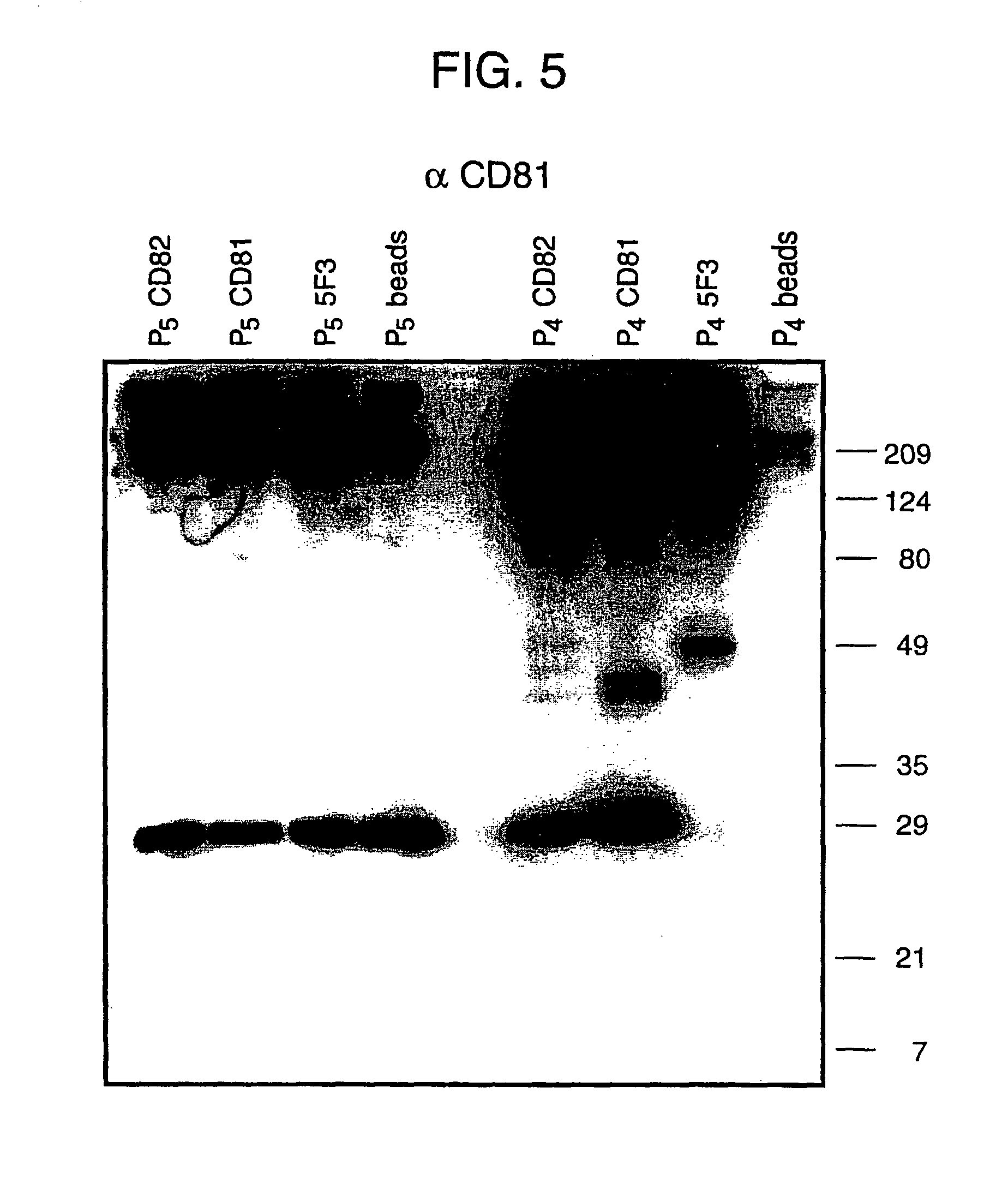
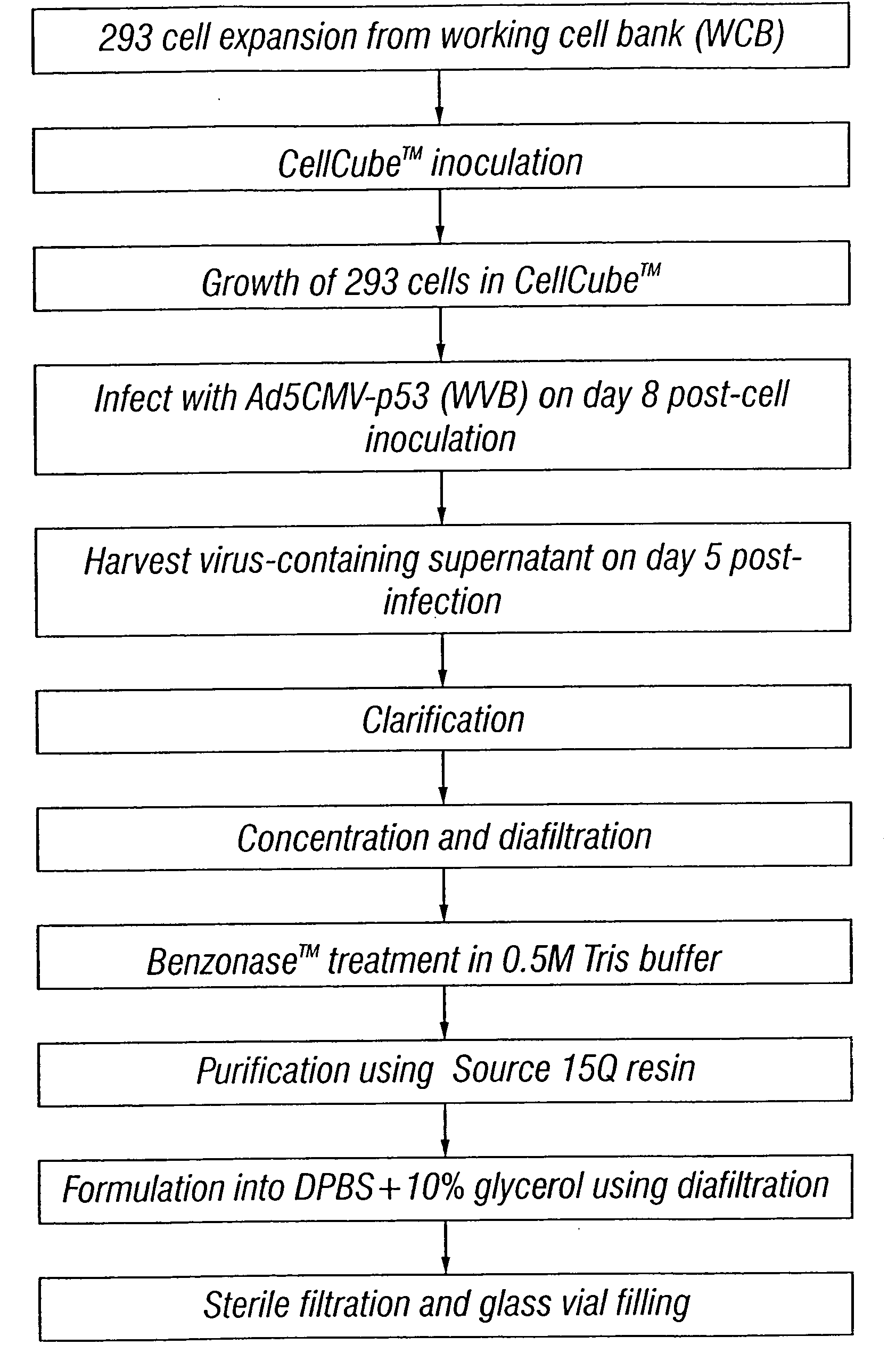
The present invention addresses the need to improve the yields of viral vectors when grown in cell culture systems. In particular, it has been demonstrated that for adenovirus, the use of low-medium perfusion rates in an attached cell culture system provides for improved yields. In other embodiments, the inventors have shown that there is improved Ad-p53 production cells grown in serum-free conditions, and in particular in serum-free suspension culture. Also important to the increase of yields is the use of detergent lysis. Combination of these aspects of the invention permits purification of virus by a single chromatography step that results in purified virus of the same quality as preparations from double CsCl banding using an ultracentrifuge.
Owner:JANSSEN VACCINES & PREVENTION BV
Isolated porcine respiratory and reproductive virus, vaccines and methods of protecting a pig against a disease caused by a porcine respiratory and reproductive virus
InactiveUS6110467AEfficient responseAntibacterial agentsSsRNA viruses positive-senseInfectious agentRespiratory disease
The present invention provides a vaccine which protects pigs from a virus and / or an infectious agent causing a porcine respiratory and reproductive disease, a method of protecting a pig from a disease caused by a virus and / or an infectious agent which causes a respiratory and reproductive disease, a method of producing a vaccine against a virus and / or an infectious agent causing a porcine reproductive and respiratory disease, and a biologically pure sample of a virus and / or infectious agent associated with a porcine respiratory and reproductive disease, particularly the Iowa strain of porcine reproductive and respiratory syndrome virus (PRRSV), and an isolated polynucleotide which is at least 90% homologous with a polynucleotide obtained from the genome of a virus and / or infectious agent which causes a porcine respiratory and reproductive disease.
Owner:ZOETIS WHC 2
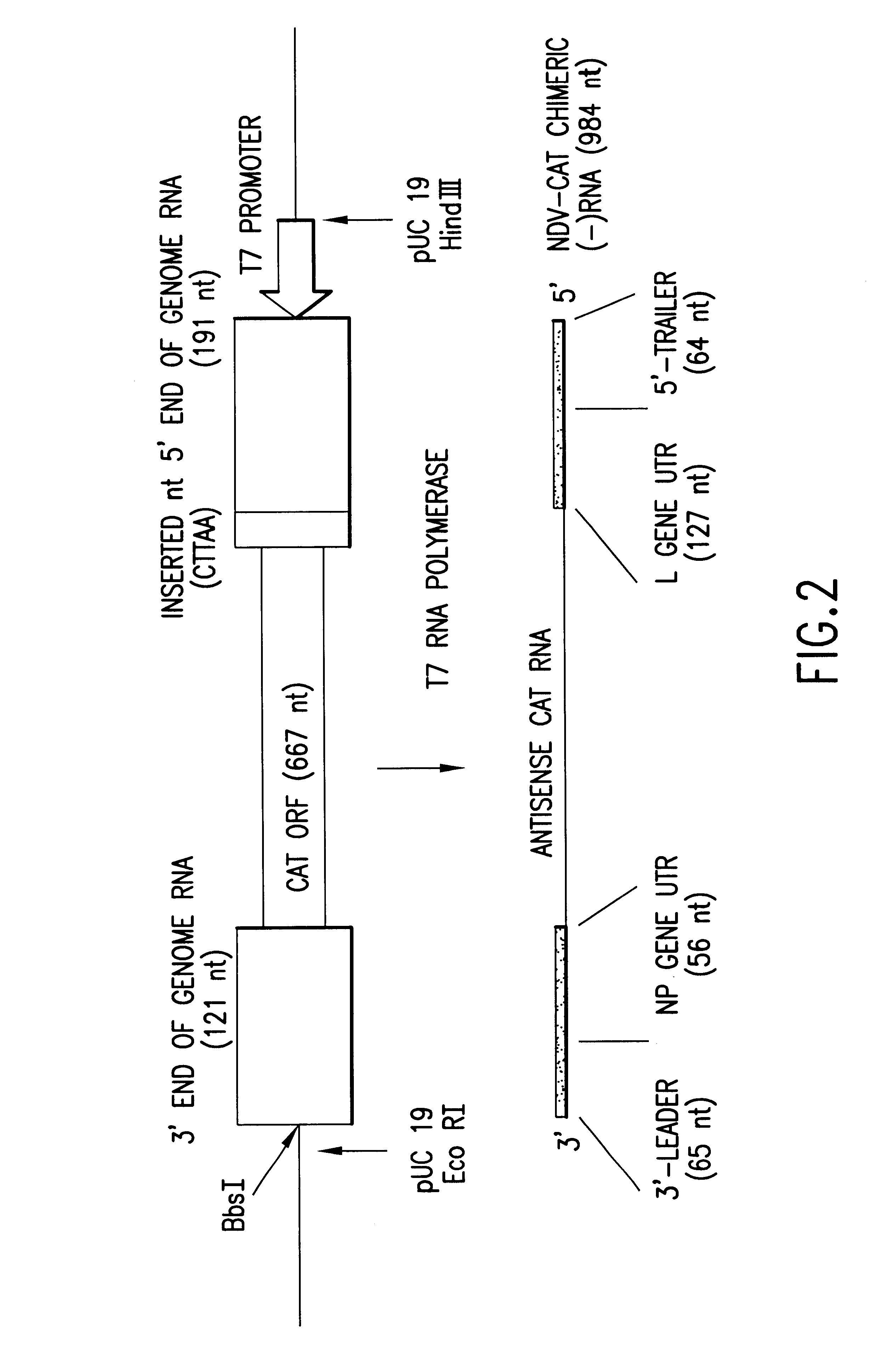
Recombinant vesiculoviruses and their uses
The invention provides recombinant replicable vesiculoviruses. The invention provides a method which, for the first time, successfully allows the production and recovery of replicable vesiculoviruses, as well as recombinant replicable vesiculoviruses, from cloned DNA, by a method comprising expression of the full-length positive-strand vesiculovirus antigenomic RNA in host cells. The recombinant vesiculoviruses do not cause serious pathology in humans, can be obtained in high titers, and have use as vaccines. The recombinant vesiculoviruses can also be inactivated for use as killed vaccines.
Owner:YALE UNIV
Chimeric Newcastle disease viruses and uses thereof
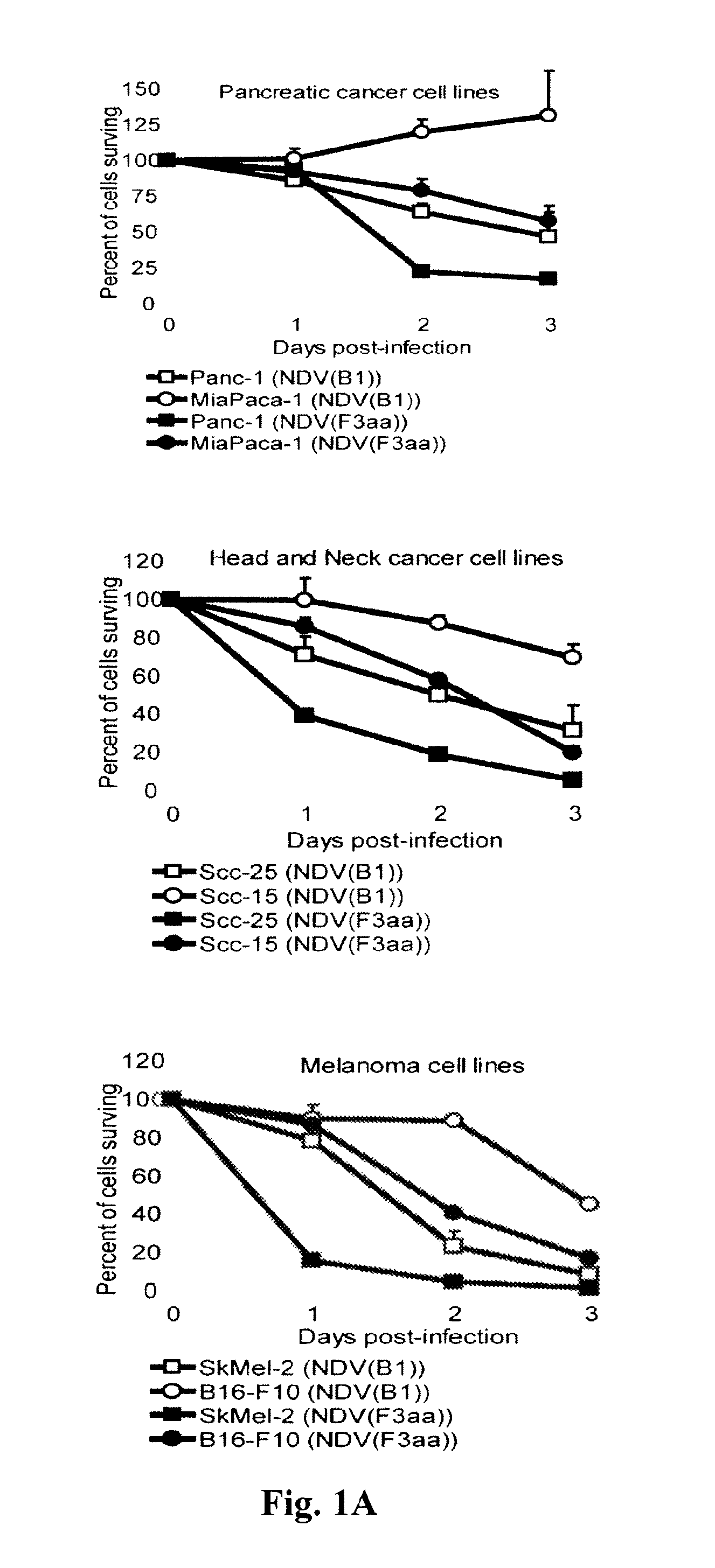
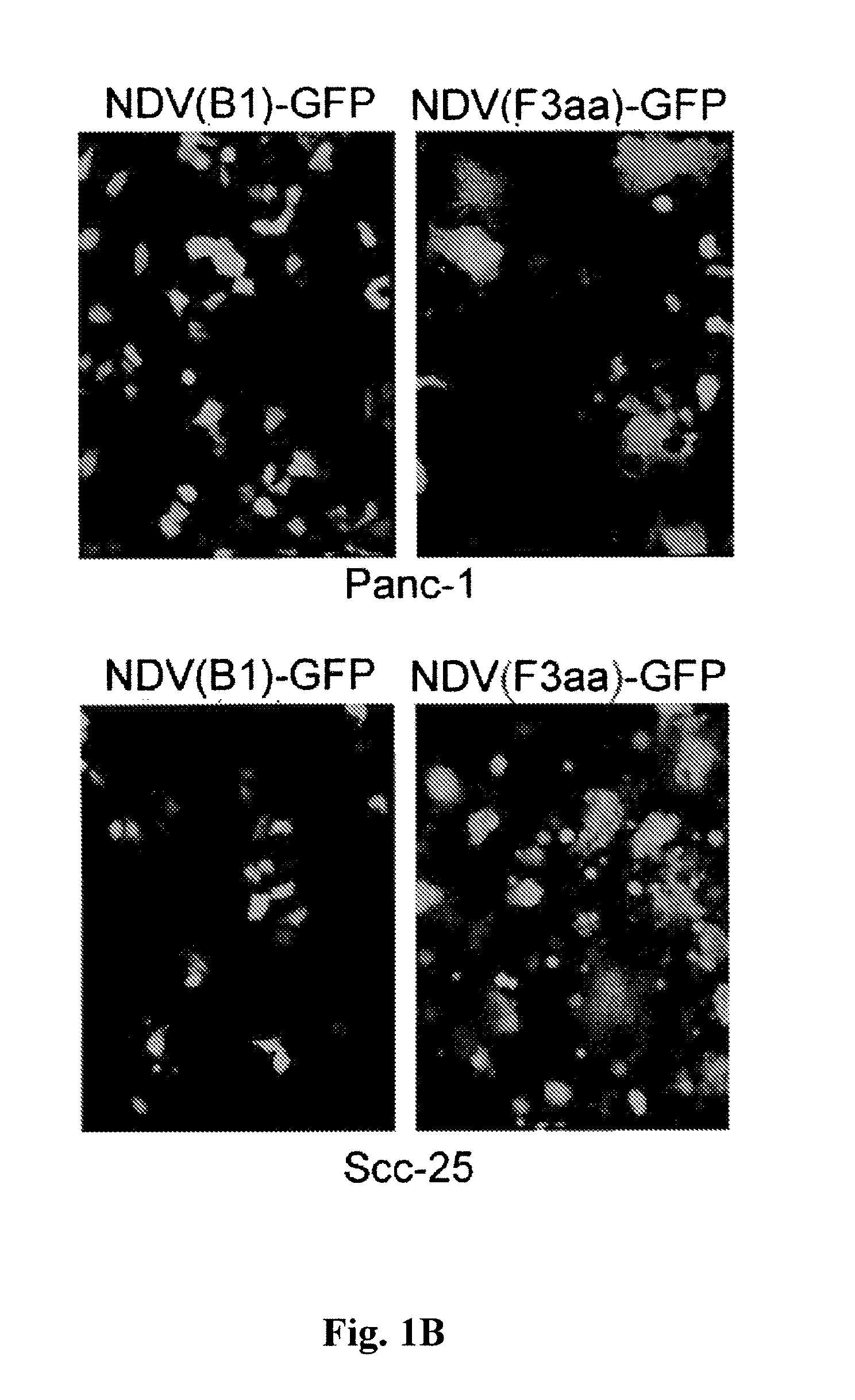
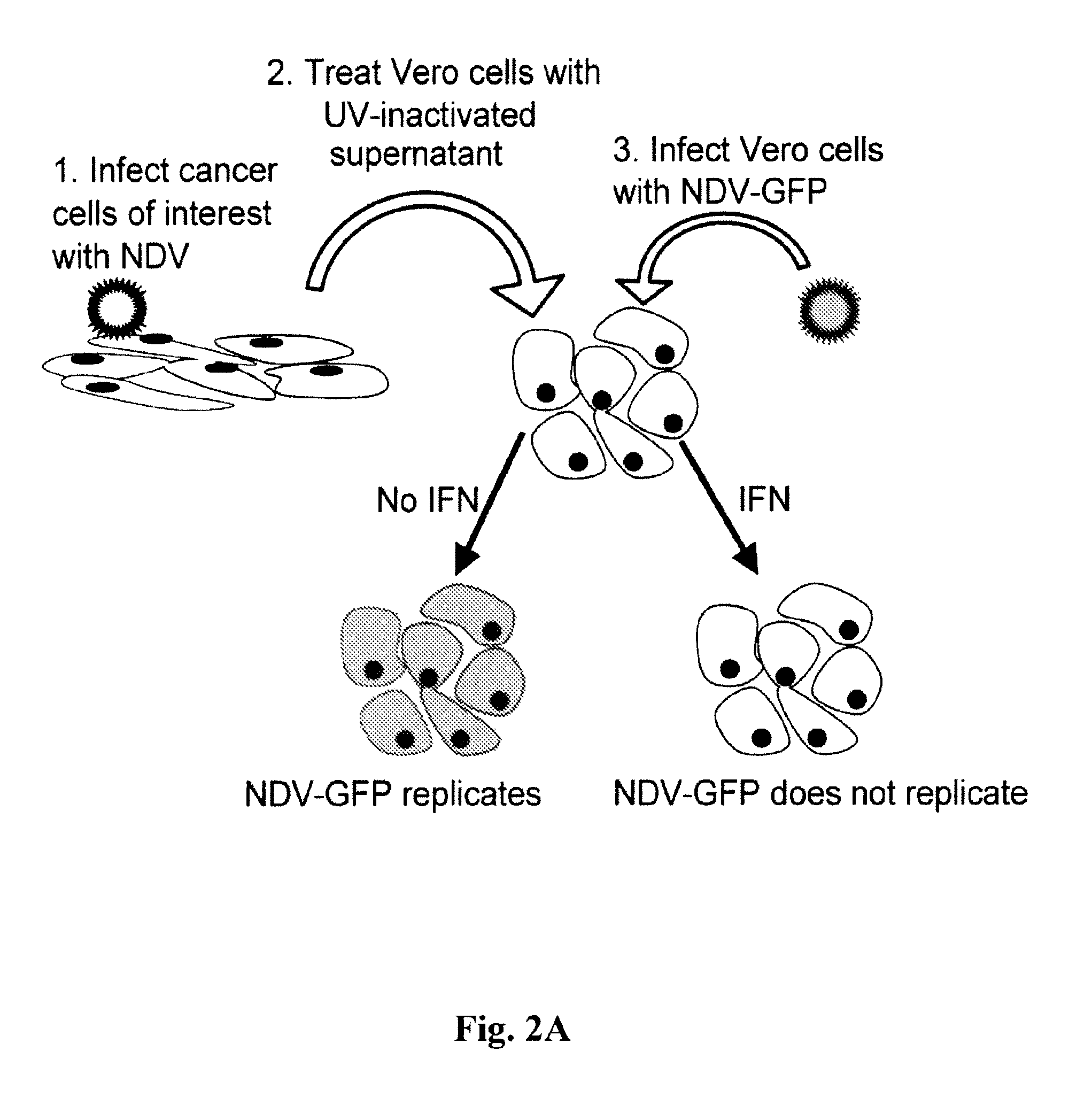
ActiveUS8591881B2Prevents progression and worseningReduce severitySsRNA viruses negative-senseBiocideNewcastle disease virus NDVAntagonist
Described herein are chimeric Newcastle disease viruses engineered to express a heterologous interferon antagonist and compositions comprising such viruses. The chimeric Newcastle disease viruses and compositions are useful in the treatment of cancer.
Owner:MT SINAI SCHOOL OF MEDICINE +1
Complementing cell lines
InactiveUS6974695B2Low efficiencyEfficient disseminationBiocideGenetic material ingredientsHeterologousVaccination
A packaging cell line capable of complementing recombinant adenoviruses based on serotypes from subgroup B, preferably adenovirus type 35. The cell line is preferably derived from primary, diploid human cells (e.g., primary human retinoblasts, primary human embryonic kidney cells and primary human amniocytes) which are transformed by adenovirus E1 sequences either operatively linked on one DNA molecule or located on two separate DNA molecules, the sequences being operatively linked to regulatory sequences enabling transcription and translation of encoded proteins. Also disclosed is a cell line derived from PER.C6 (ECACC deposit number 96022940), which cell expresses functional Ad35 E1B sequences. The Ad35-E1B sequences are driven by the E1B promoter or a heterologous promoter and terminated by a heterologous poly-adenylation signal. The new cell lines are useful for producing recombinant adenoviruses designed for gene therapy and vaccination. The cell line can also be used for producing human recombinant therapeutic proteins such as human growth factors and human antibodies. In addition, the cell lines are useful for producing human viruses other than adenovirus such as influenza virus, herpes simplex virus, rotavirus, measles virus.
Owner:JANSSEN VACCINES & PREVENTION BV
Ligand functional substrates
A substrate comprising a crosslinked polymer primer layer, and grafted thereto a ligand-functionalized polymer is provided. The grafted polymer has the requisite affinity for binding neutral or negatively charged biomaterials, such as cells, cell debris, bacteria, spores, viruses, nucleic acids, and proteins, at pH's near or below the pI's of the biomaterials.
Owner:3M INNOVATIVE PROPERTIES CO
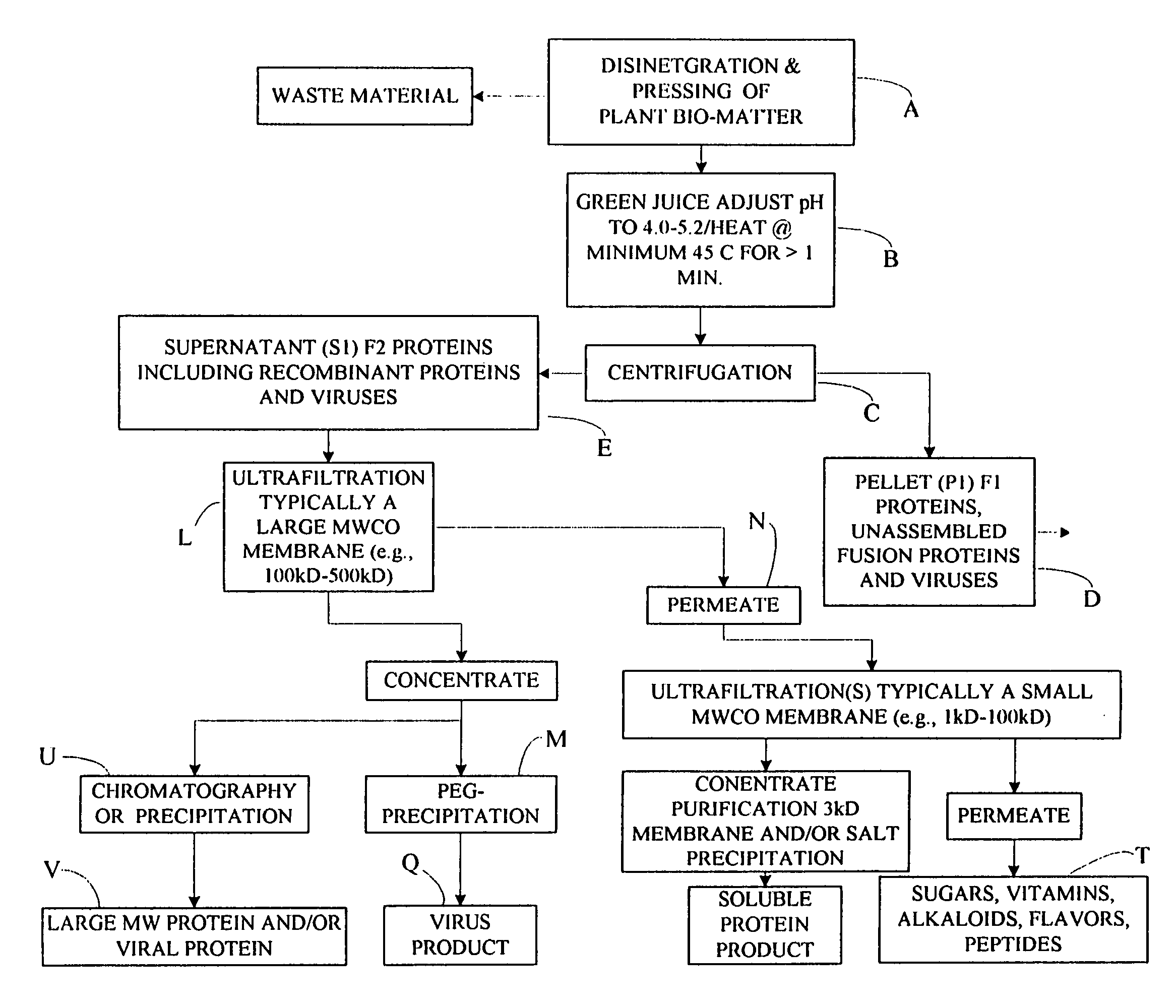
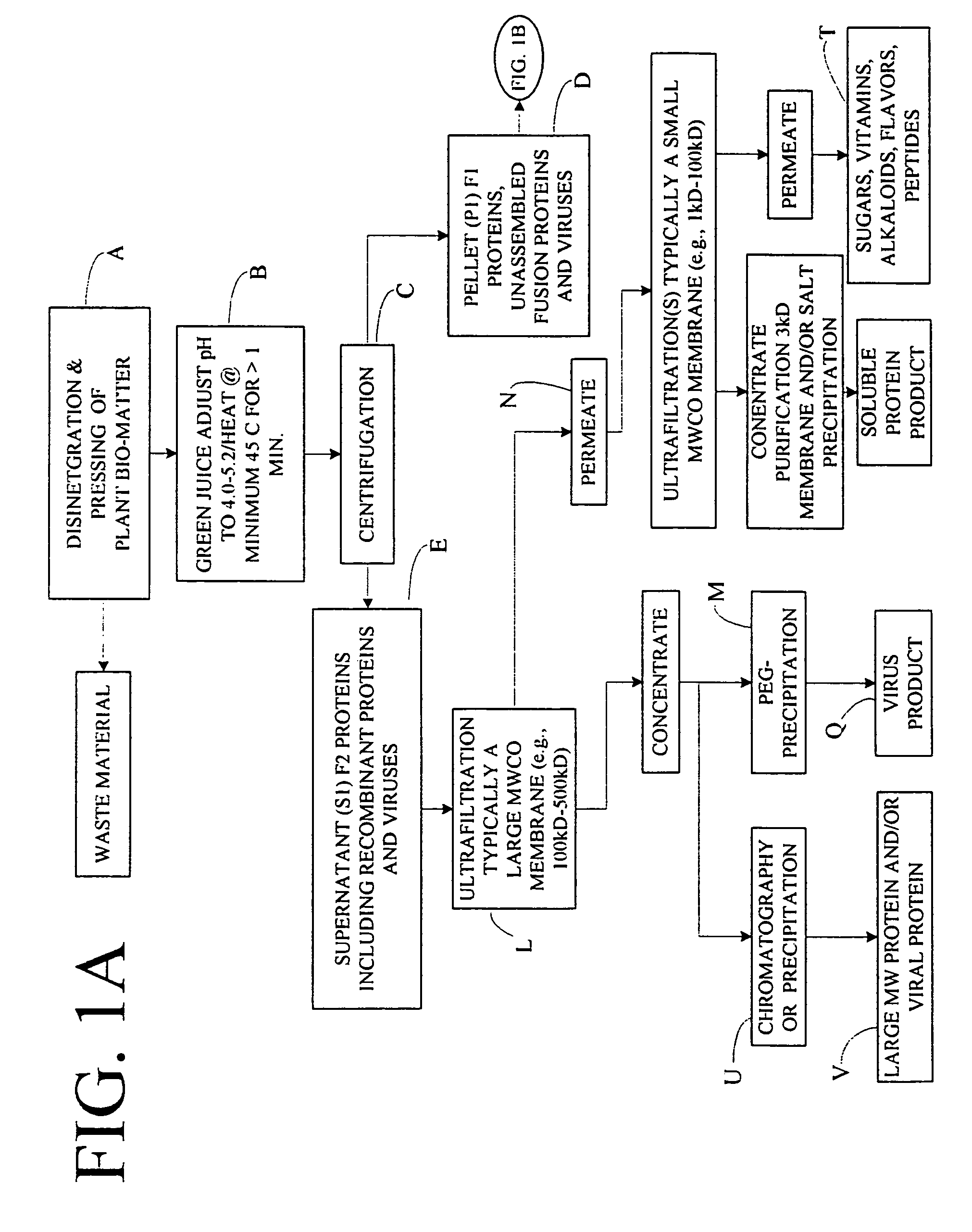
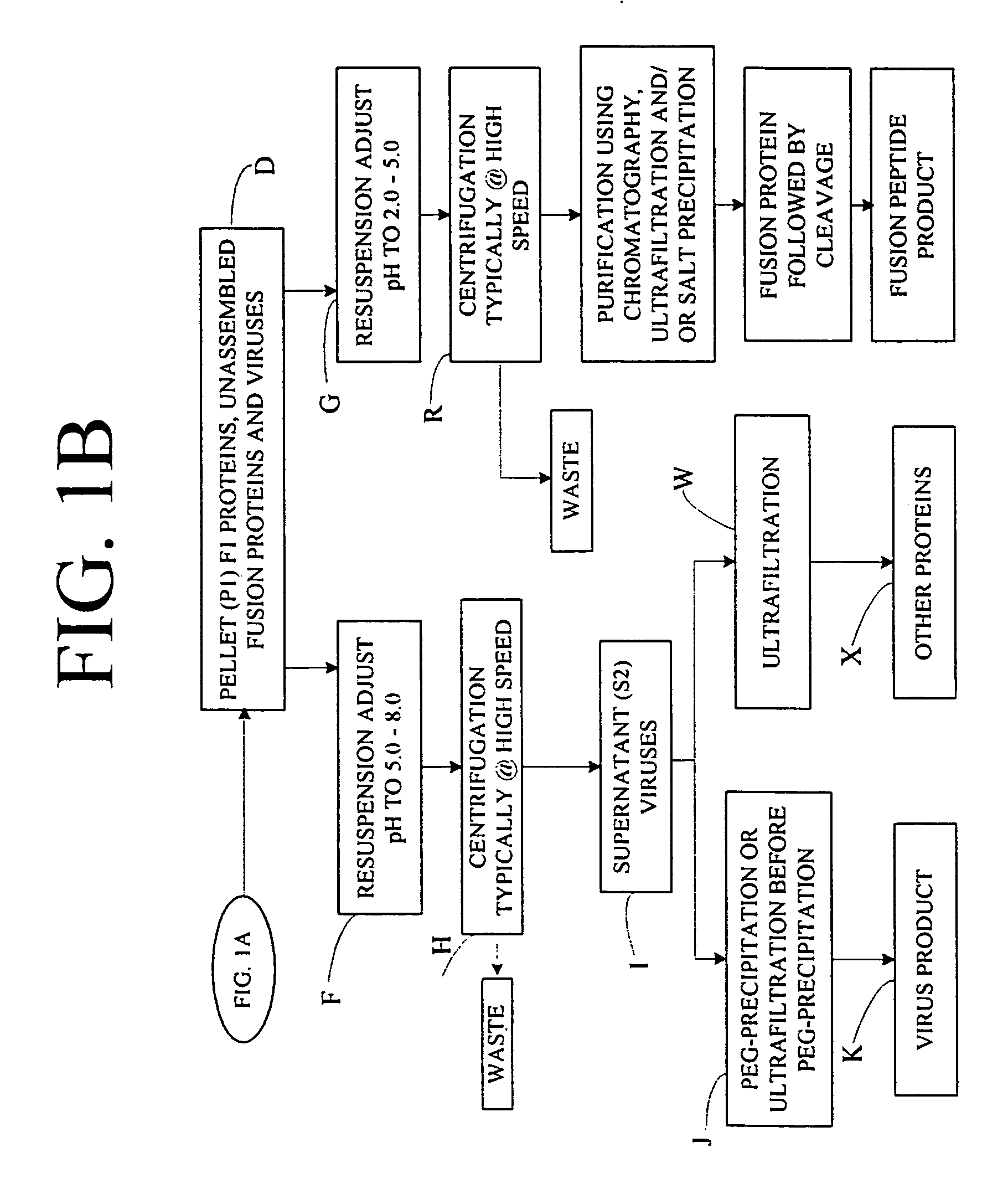
Flexible processing apparatus for isolating and purifying viruses, soluble proteins and peptides from plant sources
InactiveUS7048211B2Efficient and flexibleEasy to processCosmetic preparationsBioreactor/fermenter combinationsCentrifugationUltrafiltration
A flexible automated apparatus for isolating and purifying viruses, proteins and peptides of interest from a plant material is disclosed, the apparatus being applicable for large scale purification and isolation of such substances from plant material. The flexible automated apparatus provides an efficient apparatus for isolating viruses, proteins and peptides of interest with little waste material. The automated apparatus for isolating viruses, proteins and peptides of interest includes a grinding apparatus for homogenizing a plant to produce a green juice, a means for adjusting the pH of and heating the green juice, a means for separating the target species, either virus or protein / peptide, from other components of the green juice by one or more cycles of centrifugation, resuspension, and ultrafiltration, and finally purifying virus particles by such procedure as PEG-precipitation or purifying proteins and peptides by such procedures as chromatography and / or salt precipitation.
Owner:KENTUCKY BIOPROCESSING
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com