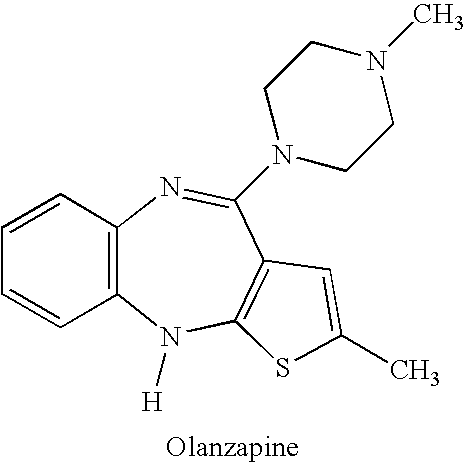
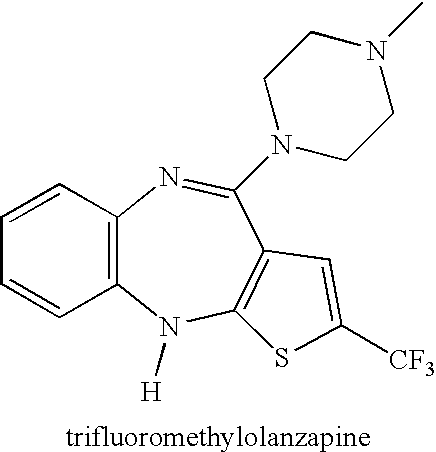
Patents
Literature
414 results about "Posttranslational modification" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
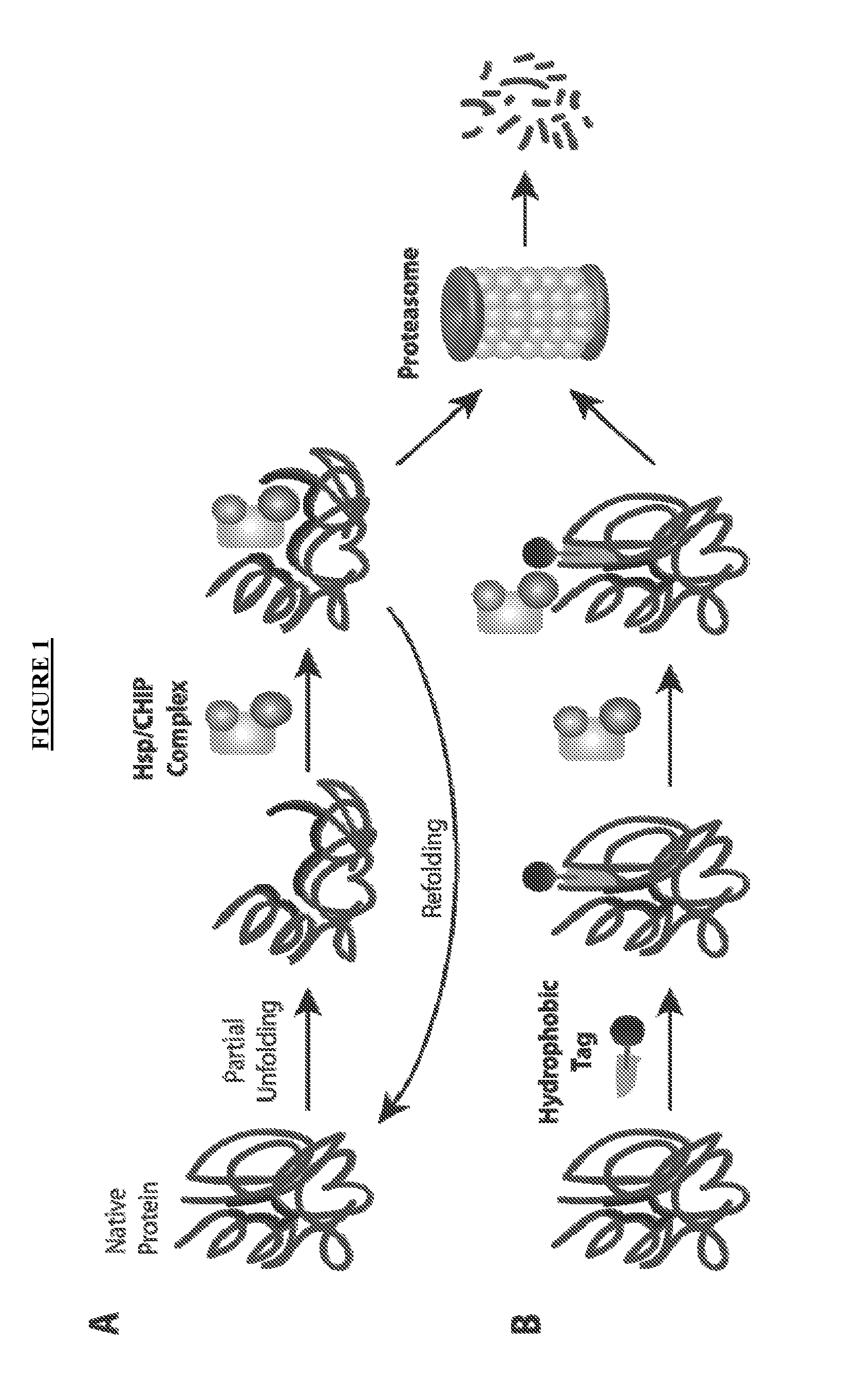
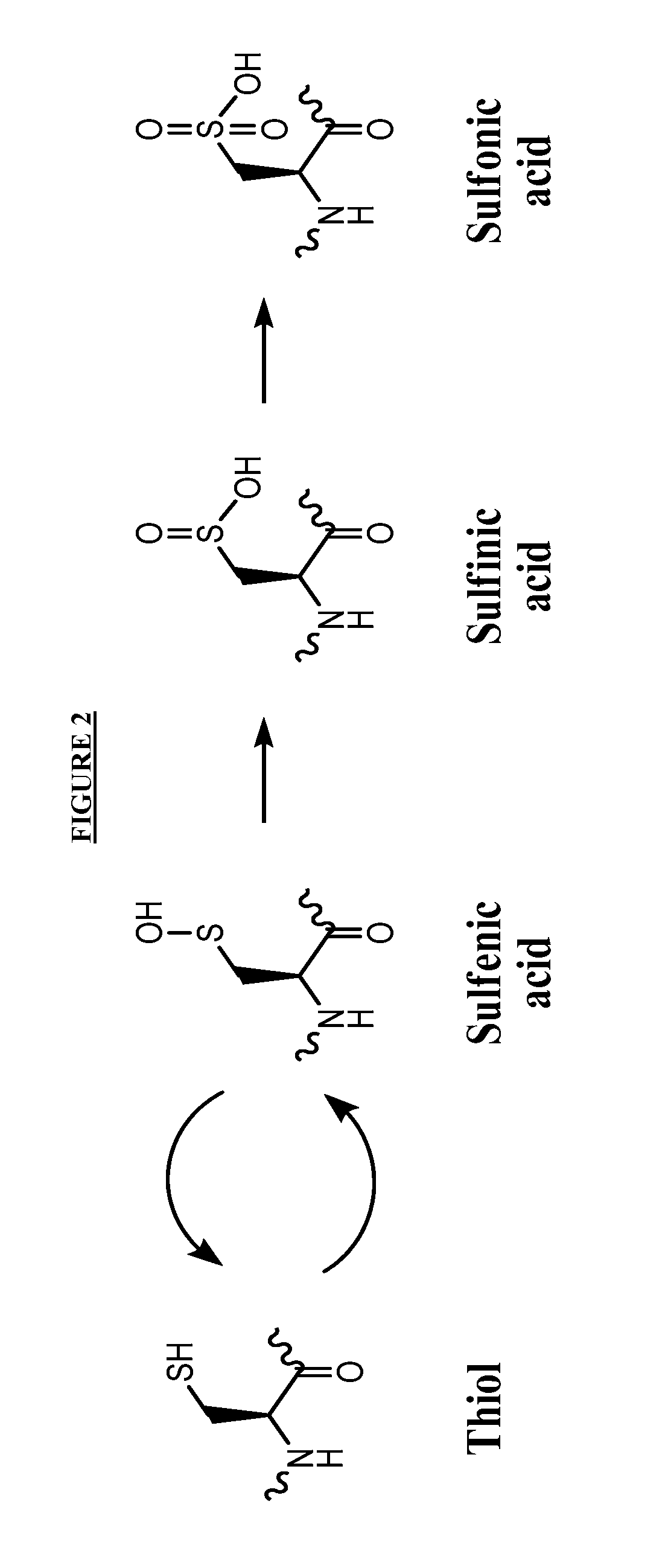
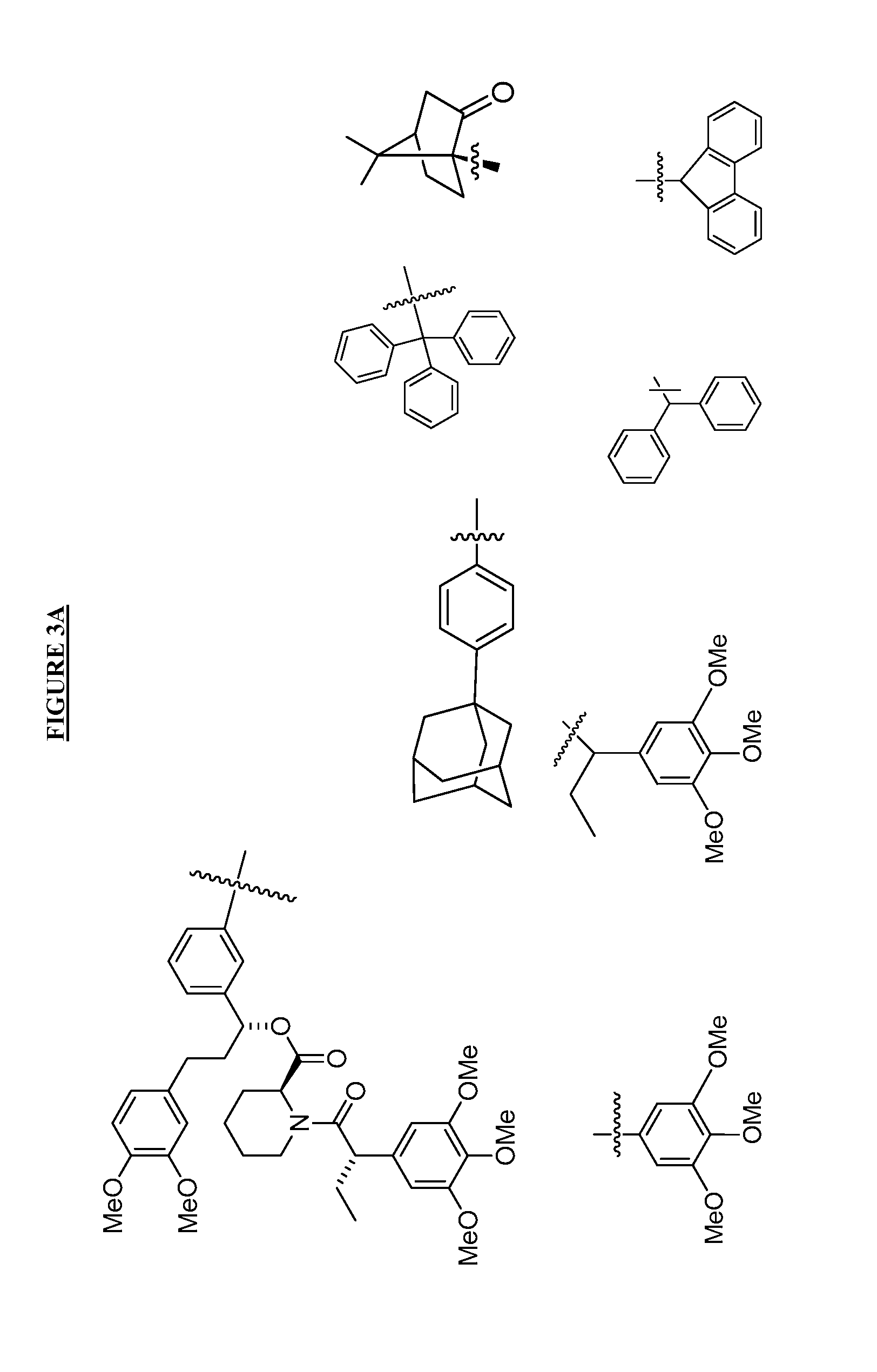
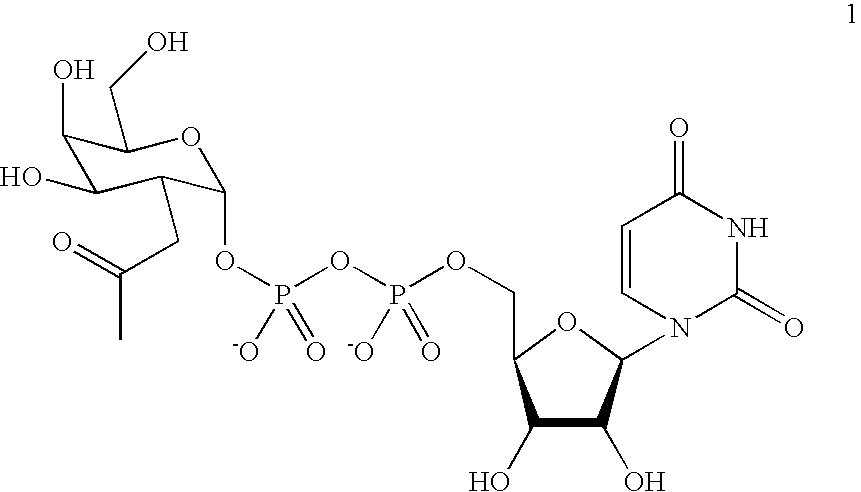
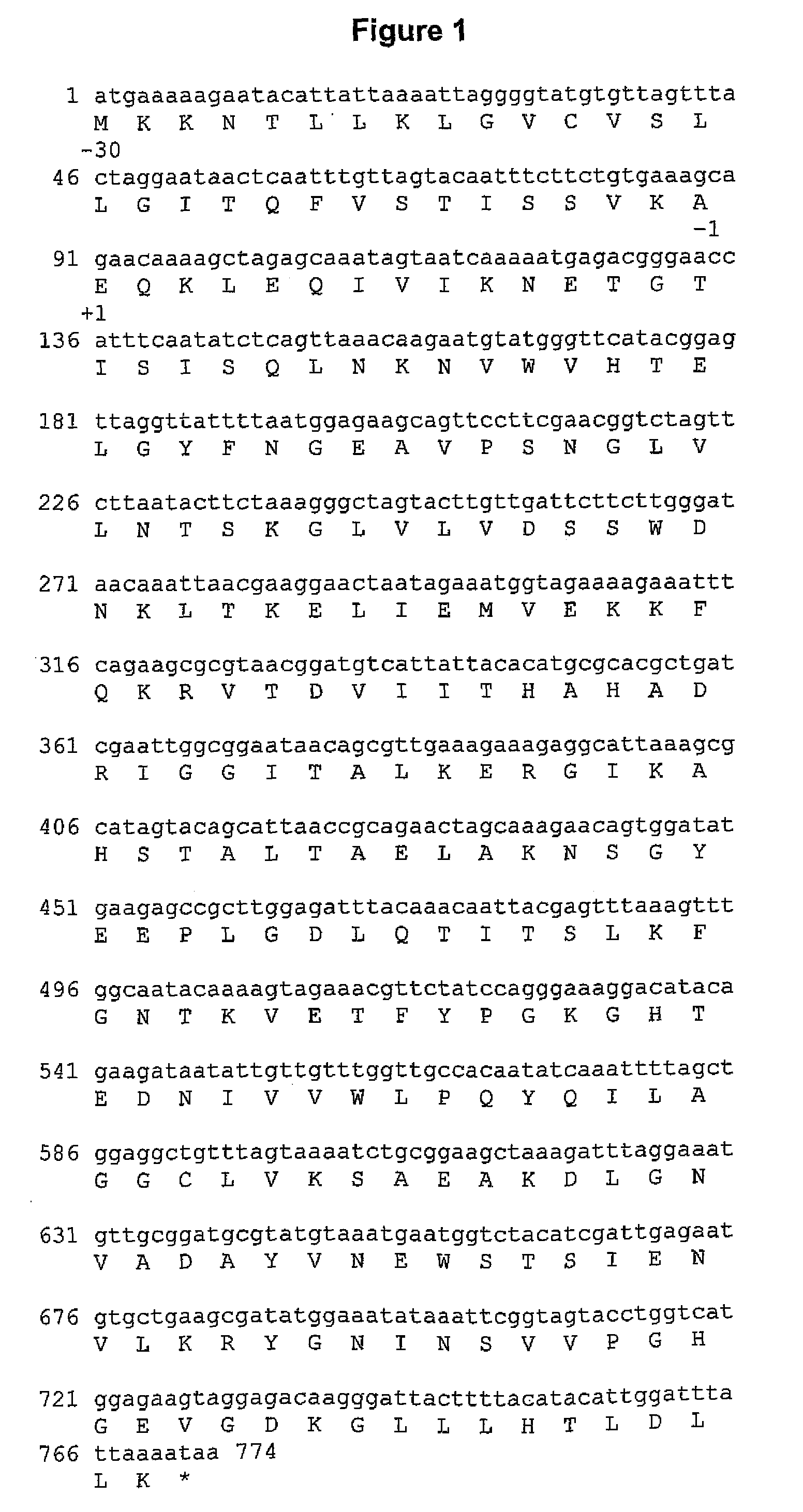
Post-translational modification (PTM) refers to the covalent and generally enzymatic modification of proteins following protein biosynthesis. Proteins are synthesized by ribosomes translating mRNA into polypeptide chains, which may then undergo PTM to form the mature protein product. PTMs are important components in cell signaling, as for example when prohormones are converted to hormones.
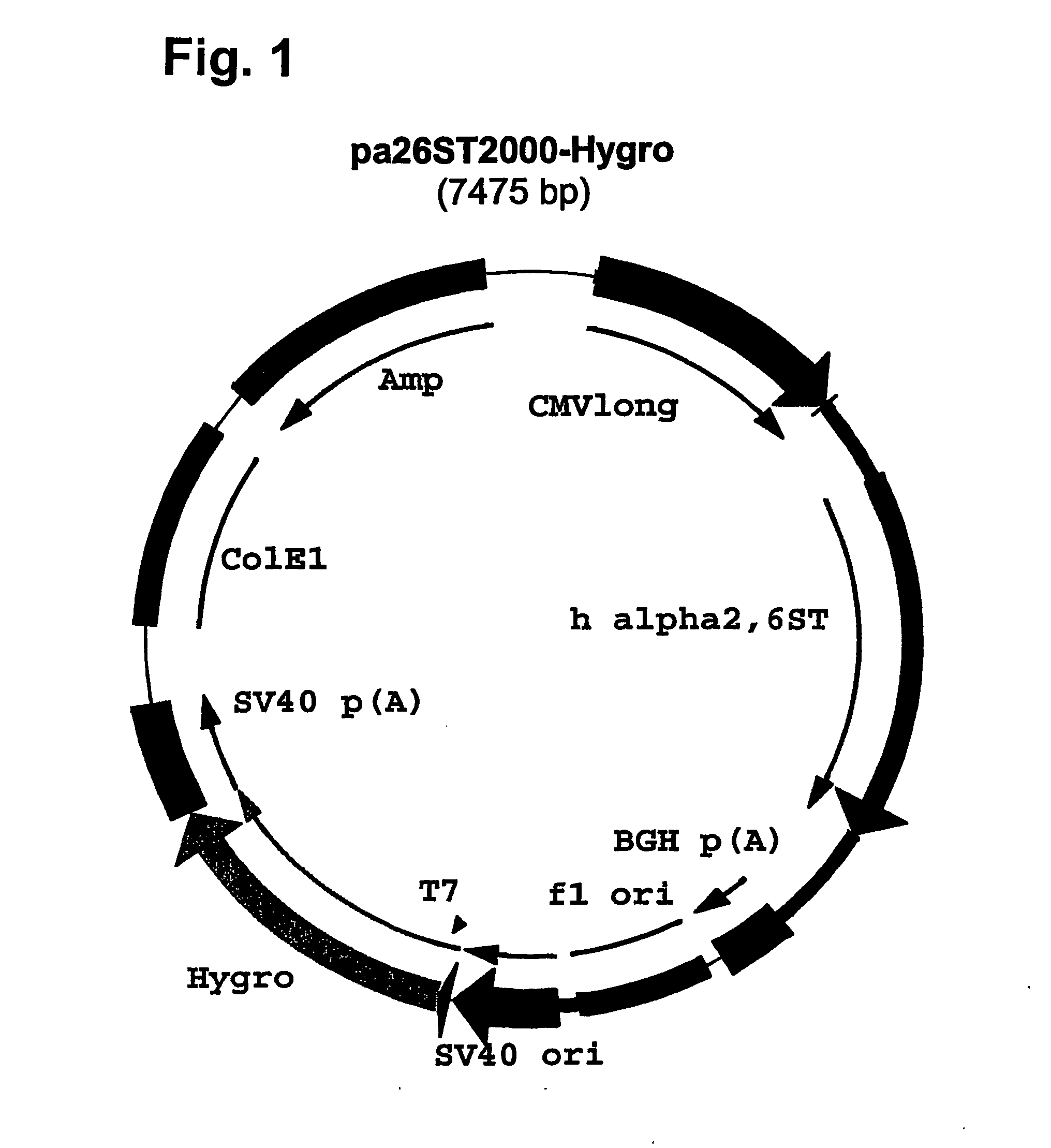
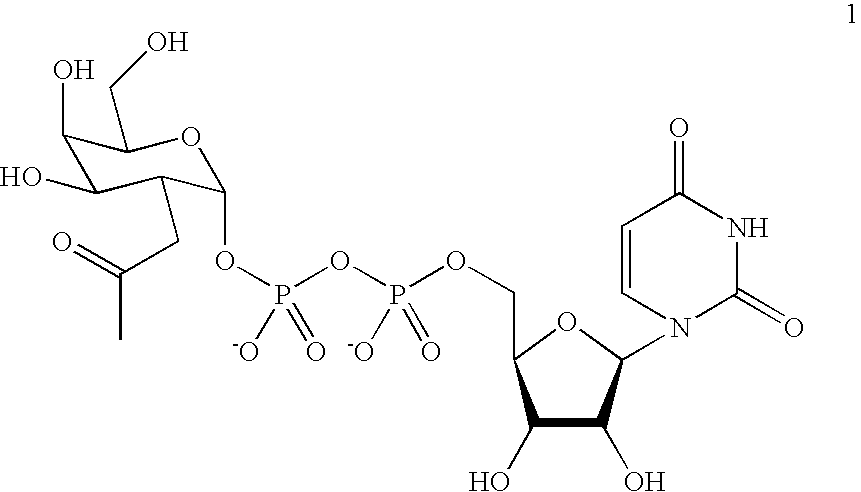
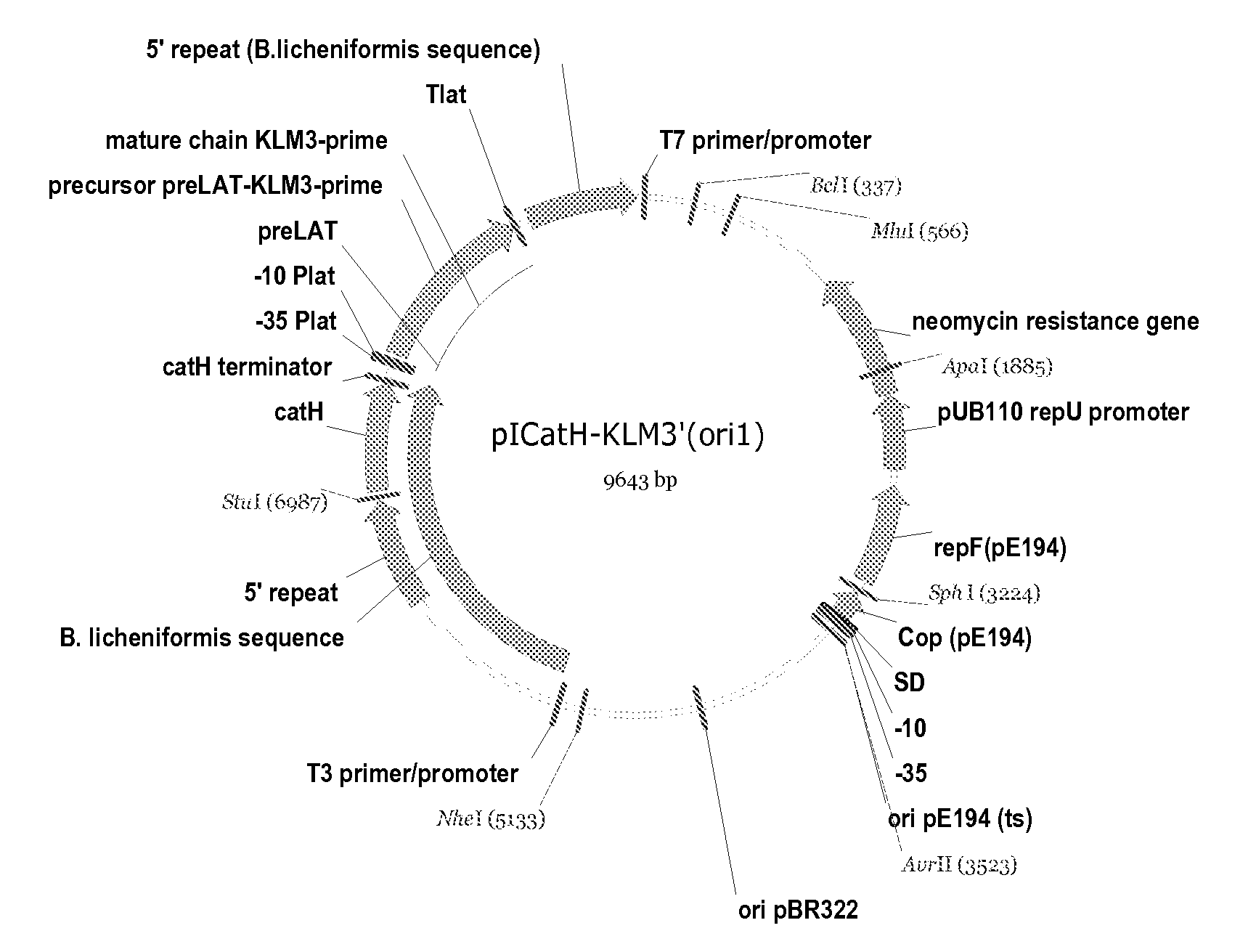
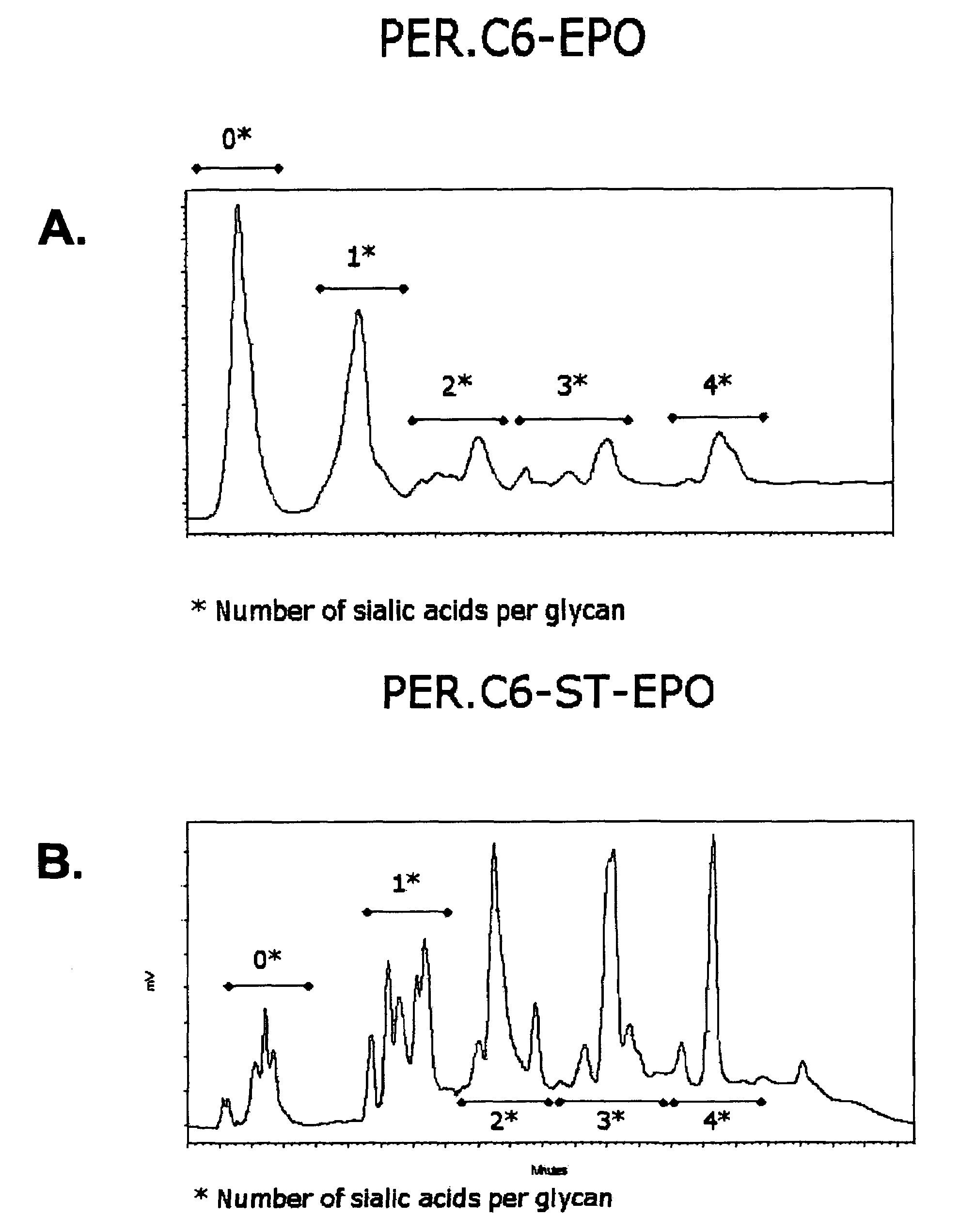
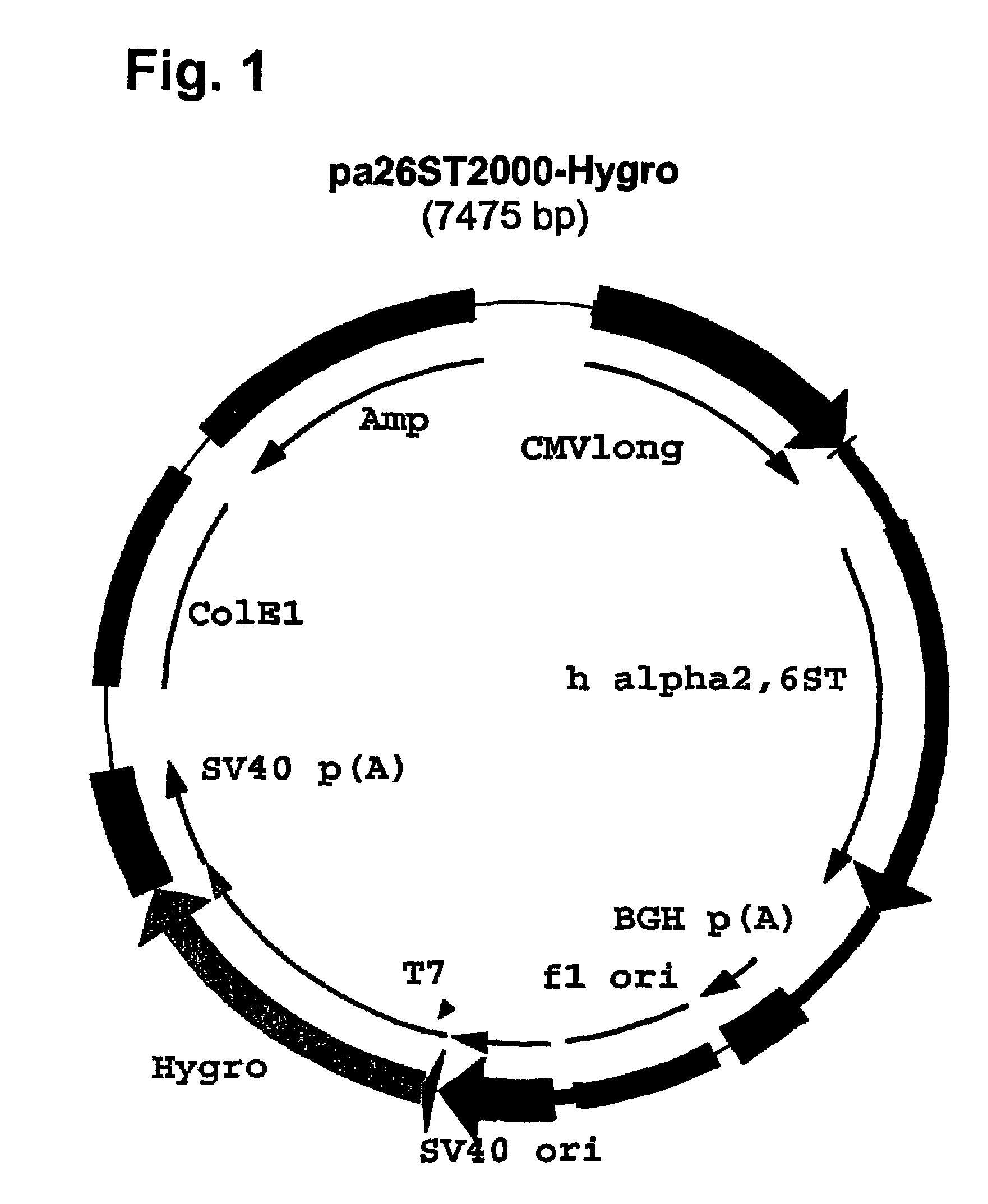
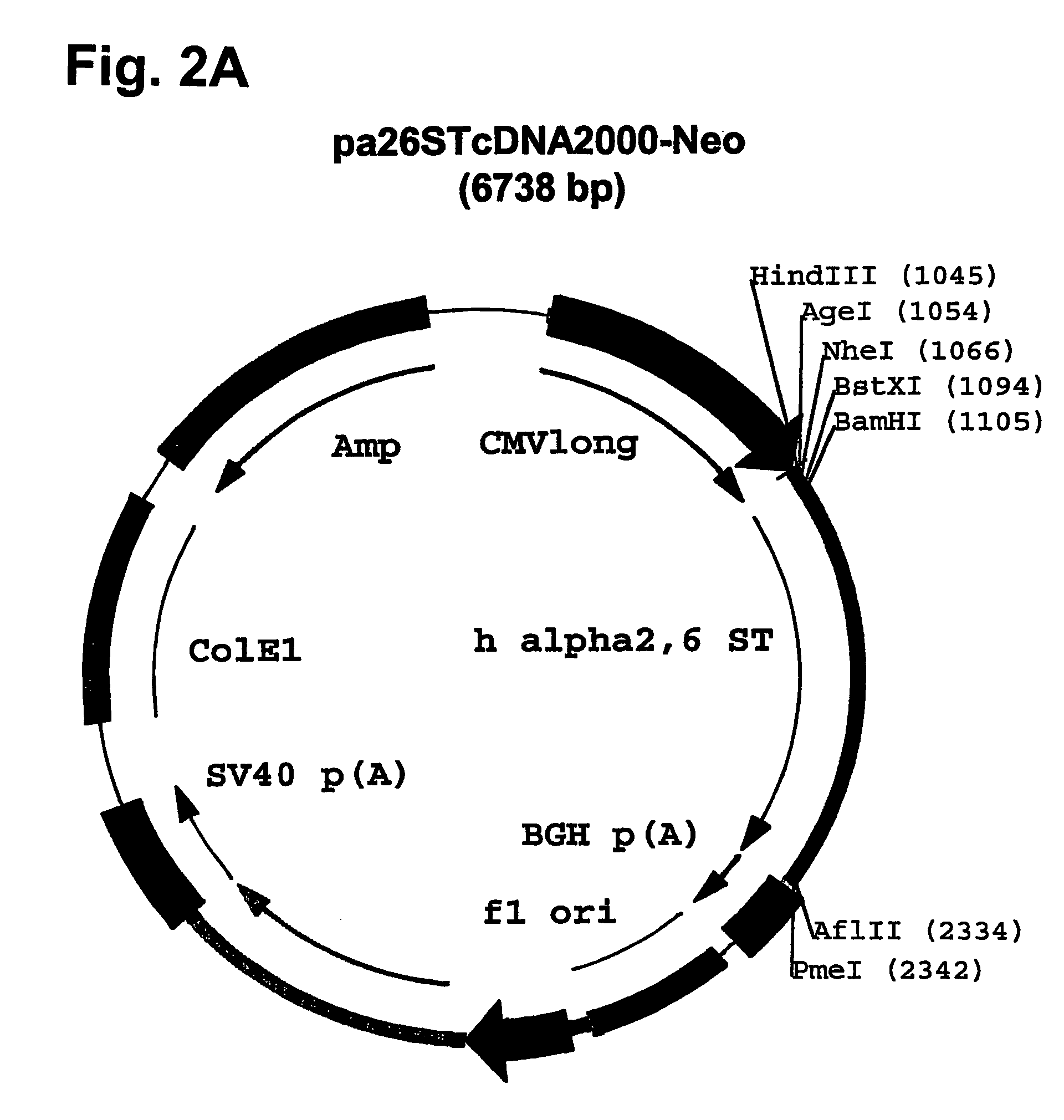
Compositions of erythropoietin isoforms comprising Lewis-X structures and high sialic acid content
InactiveUS20050181359A1Presence can be undesiredImprove system reliabilityOrganic active ingredientsBiocideHeterologousE1A Protein
Disclosed are immortalized human embryonic retina cells, having a nucleic acid sequence encoding an adenoviral E1A protein integrated into the genome of the cells, and further comprising a nucleic acid sequence encoding an enzyme involved in post-translational modification of proteins, such as a sialyltransferase, wherein said nucleic acid sequence encoding the enzyme involved in post-translational modification of proteins is under control of a heterologous promoter. Methods for producing recombinant proteins from such cells and obtaining such recombinant proteins having increased sialylation are provided as are novel compositions of isoforms of erythropoietin .
Owner:JANSSEN VACCINES & PREVENTION BV
Advanced drug development and manufacturing
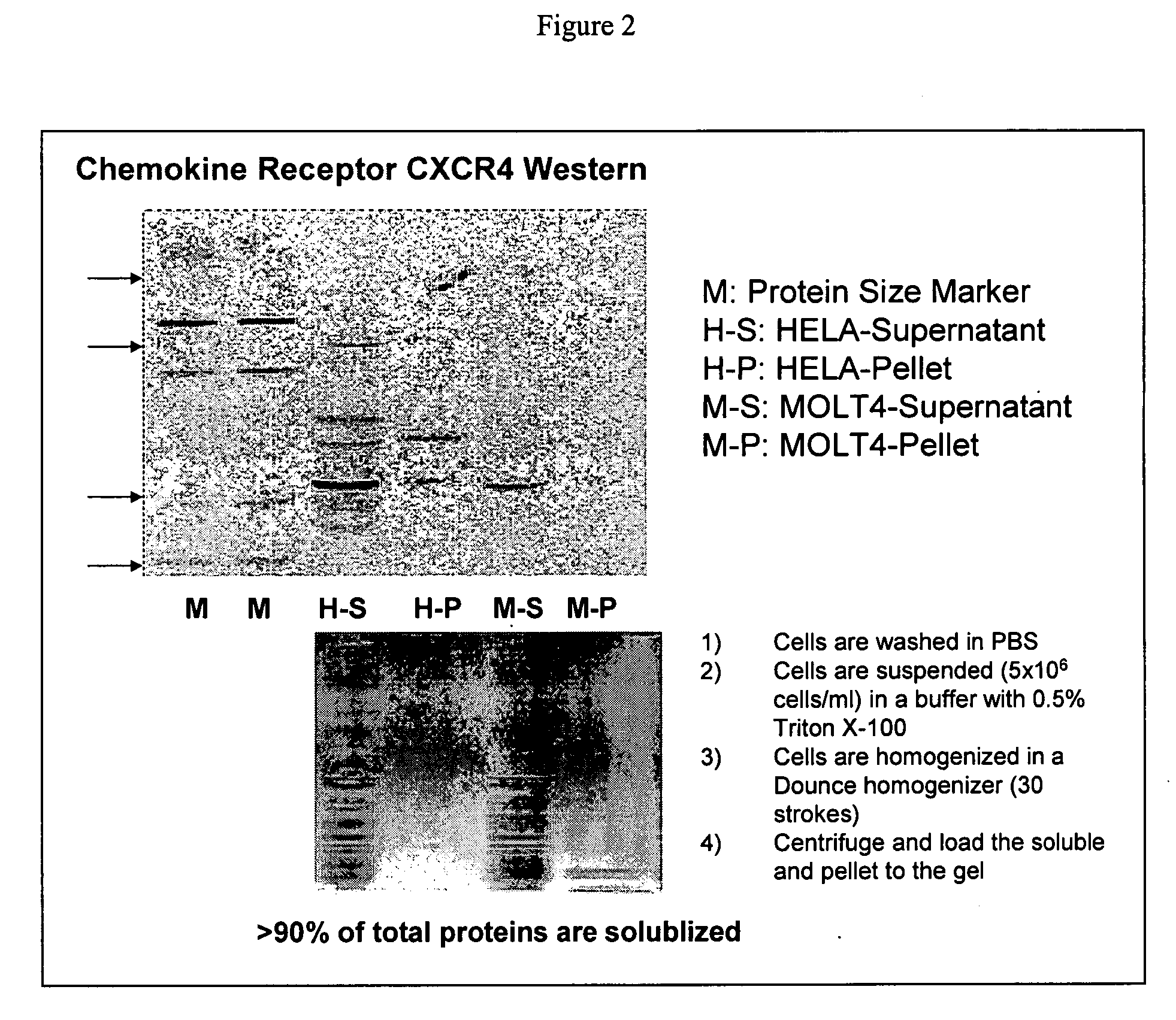
X-ray fluorescence (XRF) spectrometry has been used for detecting binding events and measuring binding selectivities between chemicals and receptors. XRF may also be used for estimating the therapeutic index of a chemical, for estimating the binding selectivity of a chemical versus chemical analogs, for measuring post-translational modifications of proteins, and for drug manufacturing.
Owner:RGT UNIV OF CALIFORNIA
Recombinant protein production in a human cell
InactiveUS6855544B1Easy to handleLarge-scale (continuous) productionSsRNA viruses negative-senseSugar derivativesHamsterHuman cell
Methods and compositions for the production of recombinant proteins in a human cell line. The methods and positions are particularly useful for generating stable expression of human recombinant proteins of interest that are modified post-translationally, for example, by glycosylation. Such proteins may have advantageous properties in comparison with their counterparts produced in non-human systems such as Chinese Hamster Ovary cells.
Owner:JANSSEN VACCINES & PREVENTION BV
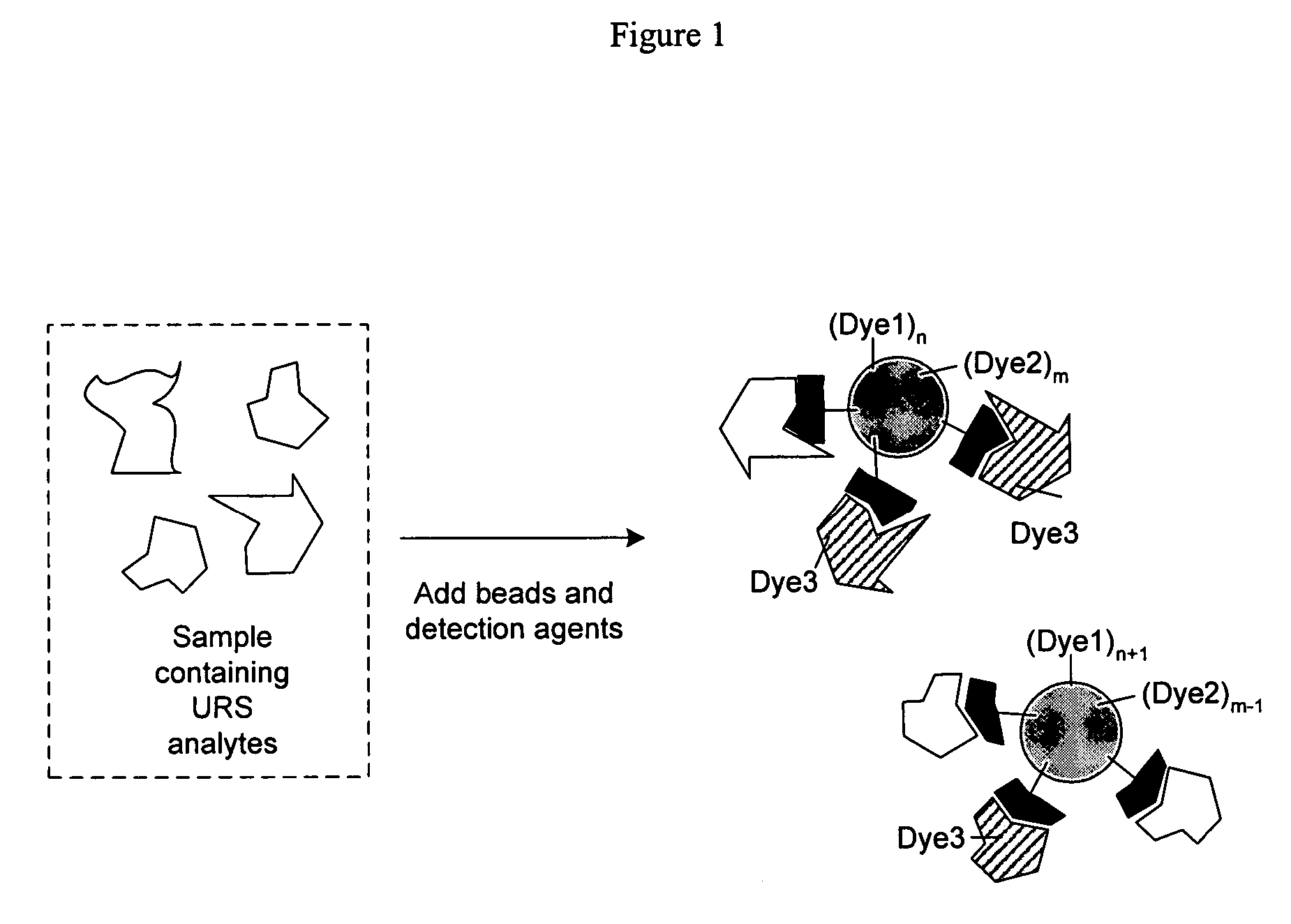
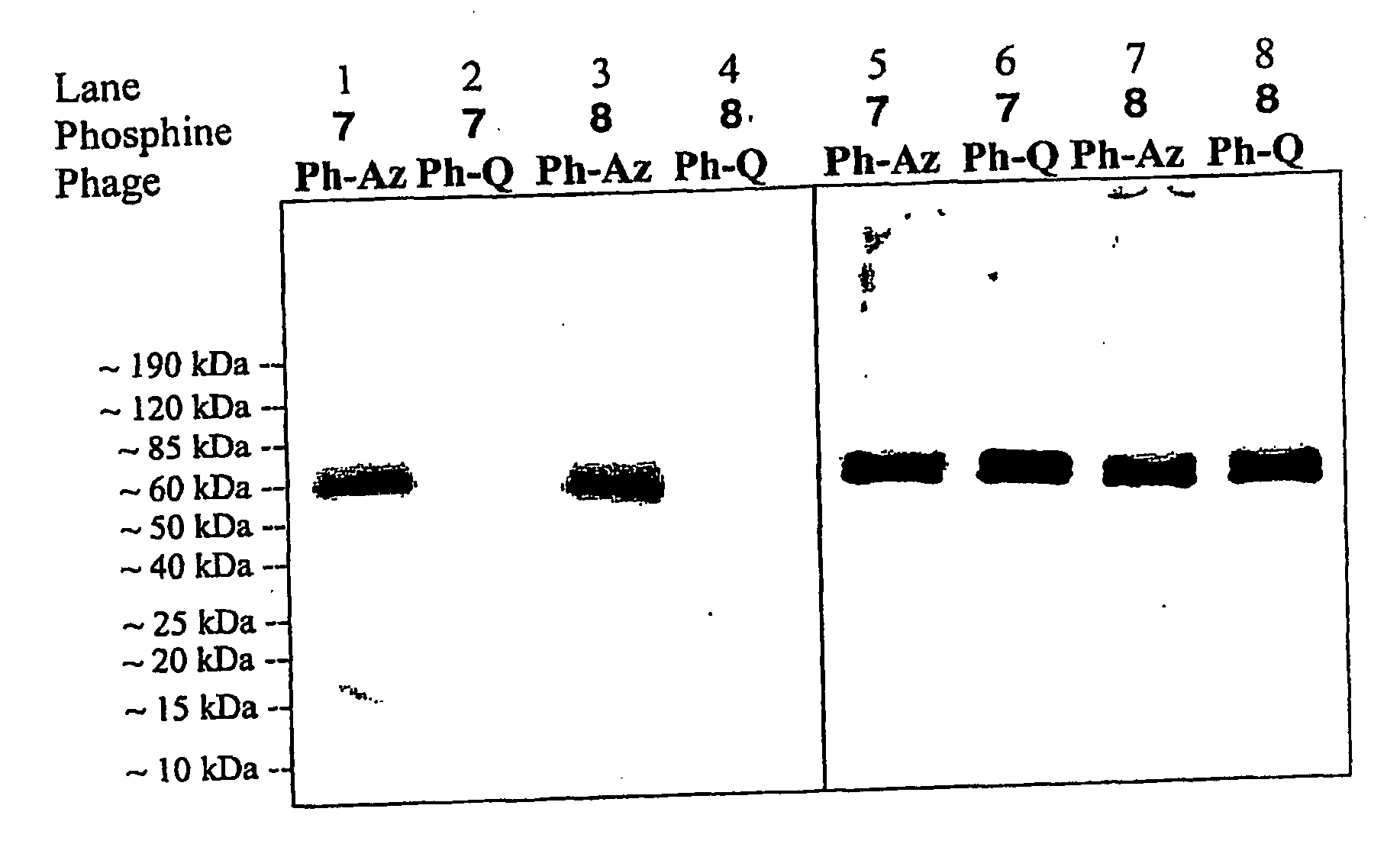
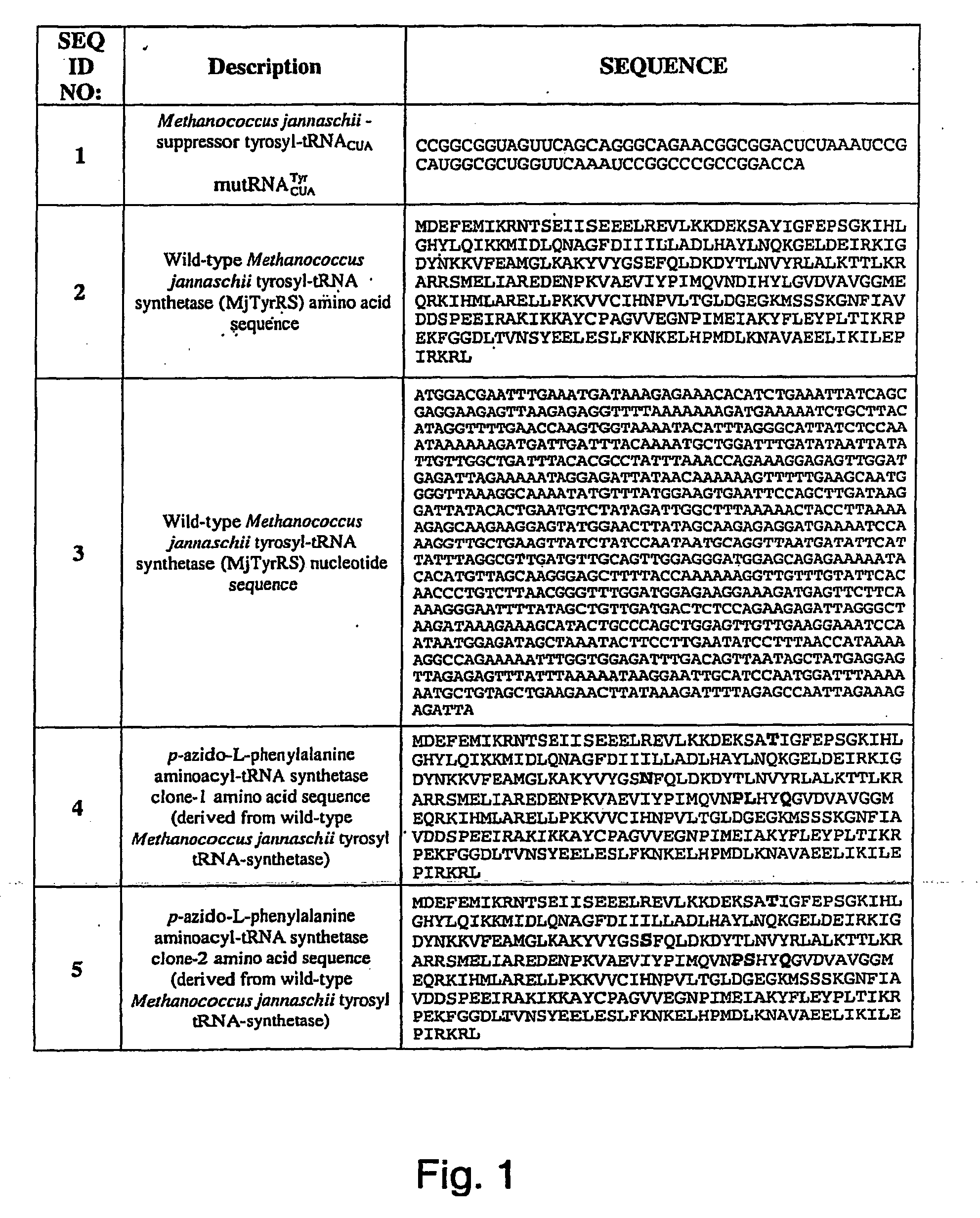
Selective posttranslational modification of phage-displayed polypeptides
ActiveUS20070178448A1Easy to detectEasy to quantifyAntibody mimetics/scaffoldsVirus peptidesArylCycloaddition
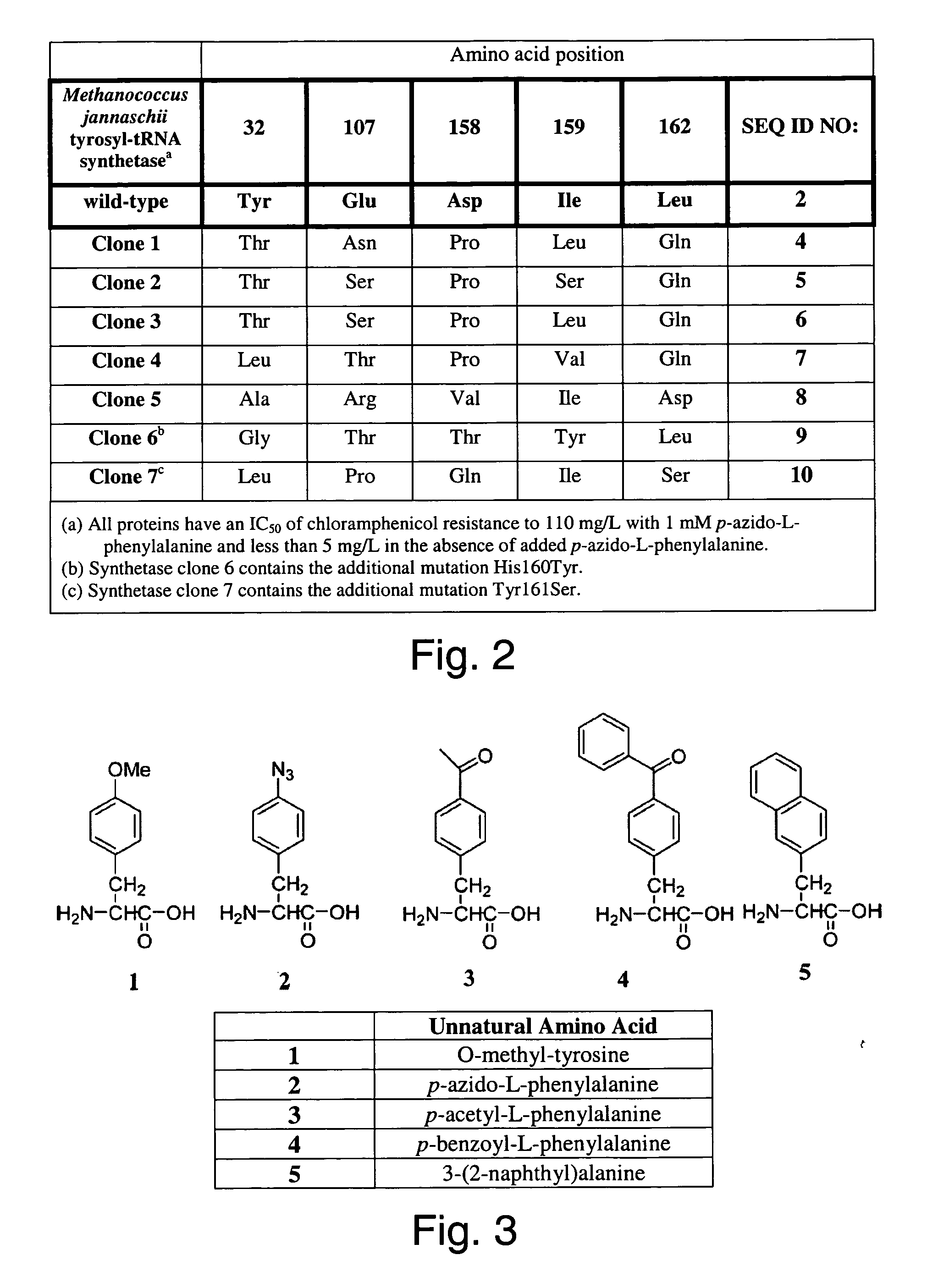
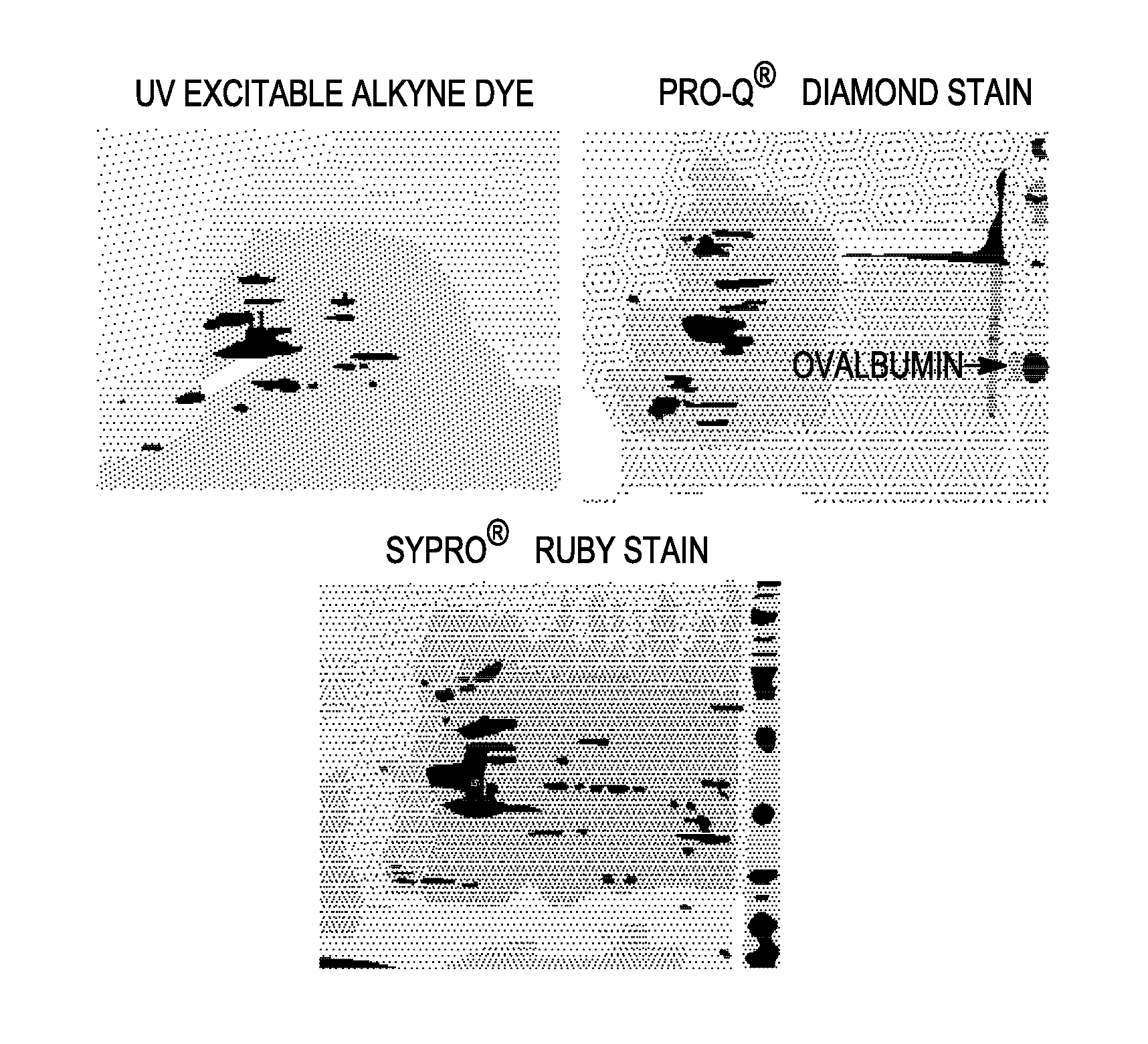
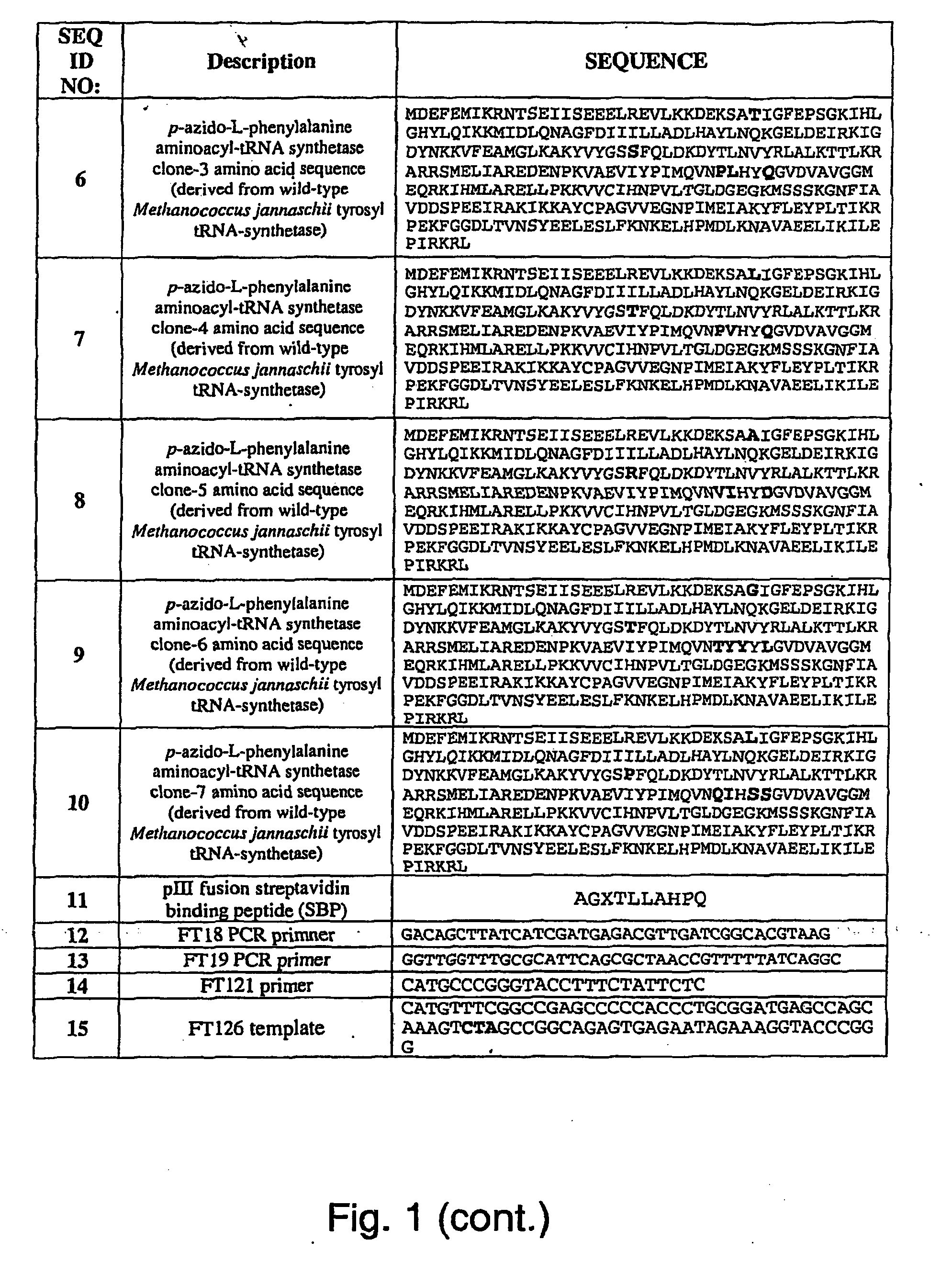
The invention relates to posttranslational modification of phage-displayed polypeptides. These displayed polypeptides comprise at least one unnatural amino acid, e.g., an aryl-azide amino acid such as p-azido-L-phenylalanine, or an alkynyl-amino acid such as para-propargyloxyphenylalanine, which are incorporated into the phage-displayed fusion polypeptide at a selected position by using an in vivo orthogonal translation system comprising a suitable orthogonal aminoacyl-tRNA synthetase and a suitable orthogonal tRNA species. These unnatural amino acids advantageously provide targets for posttranslational modifications such as azide-alkyne [3+2] cycloaddition reactions and Staudinger modifications.
Owner:THE SCRIPPS RES INST
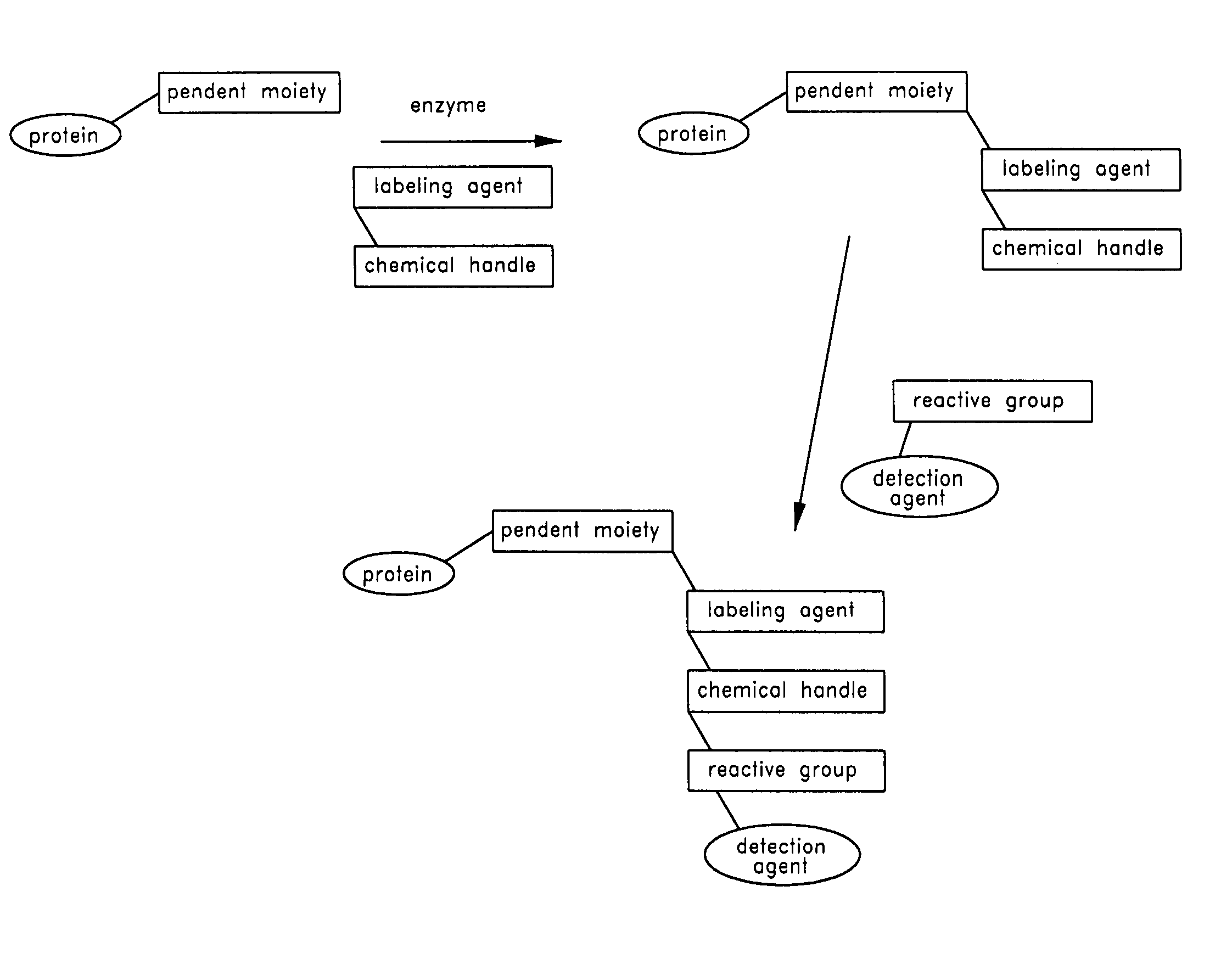
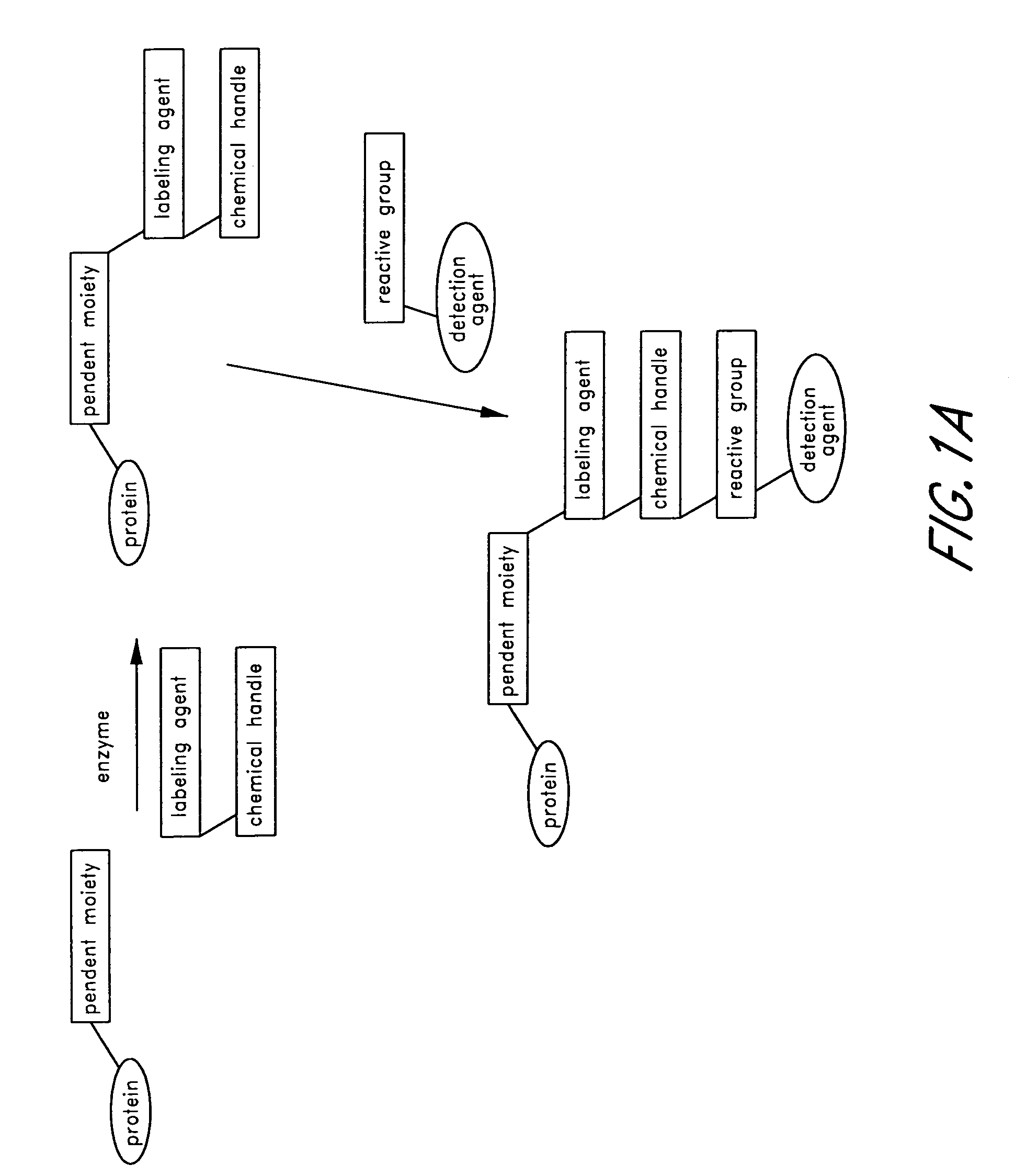
Labeling and detection of post translationally modified proteins
Provided in certain embodiments are new methods for forming azido modified biomolecule conjugates of reporter molecules, carrier molecules or solid support. In other embodiments are provided methods for enzymatically labeling a biomolecules with an azide group.
Owner:LIFE TECH CORP
Proteome epitope tags and methods of use thereof in protein modification analysis
InactiveUS20060014212A1High clinical application valueReliable detectionLibrary screeningNanoinformaticsEpitopePost translational
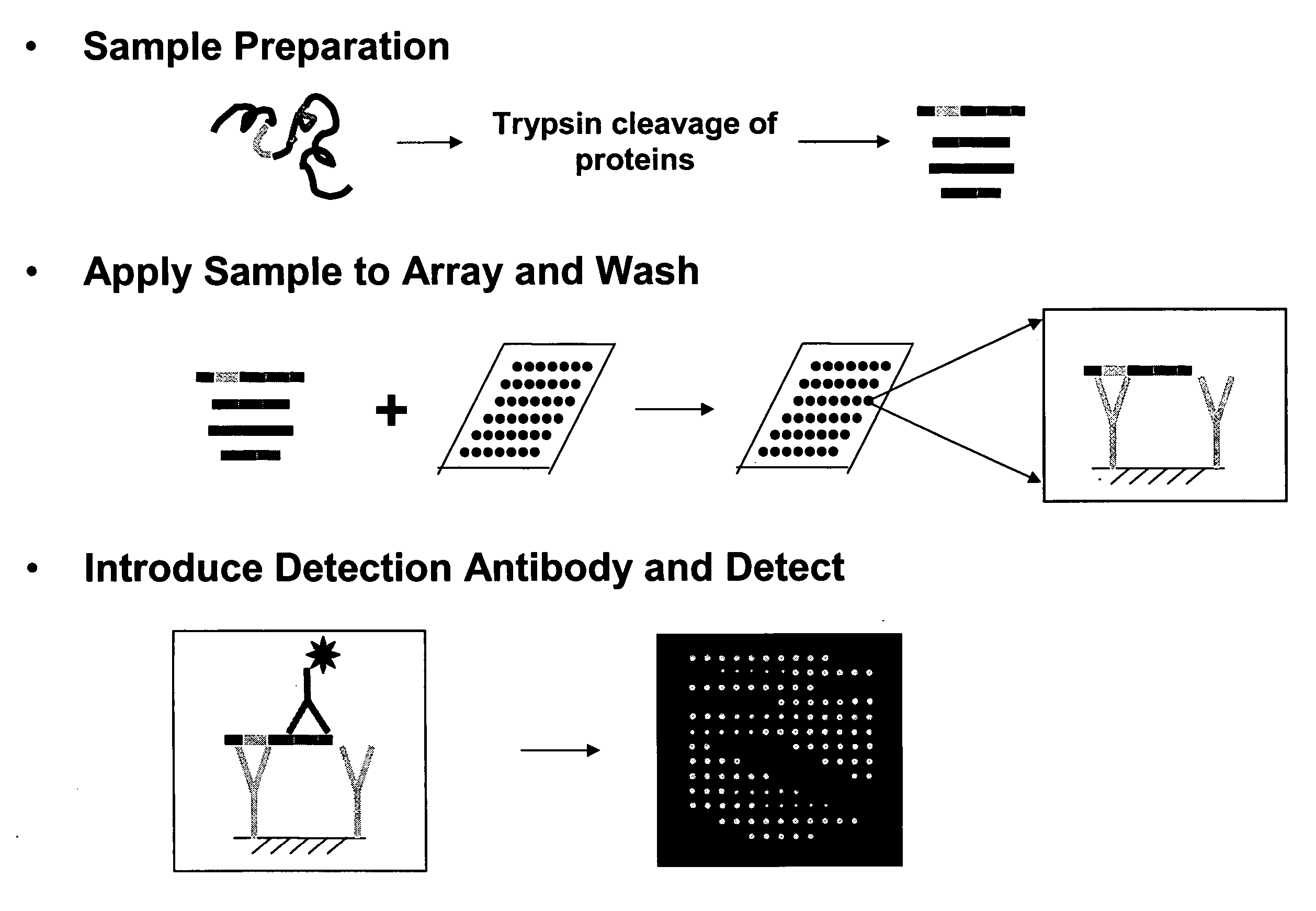
Disclosed are reagents and methods for reliably detecting the presence and measuring the amount of proteins, including proteins with various post-translational modifications (phosphorylation, glycosylation, methylation, acetylation, etc.) in a sample by the use of one or more capture agents that recognize and interact with recognition sequences uniquely characteristic of a protein or a set of proteins (Proteome Epitope Tags, or PETs) in the sample. Arrays comprising these capture agents or PETs are also provided.
Owner:EPITOME BIOSYST
Selective Posttranslational Modification of Phage-Displayed Polypeptides
ActiveUS20090137424A1High selectivityPreserves viral infectivityAntibody mimetics/scaffoldsMicrobiological testing/measurementArylCycloaddition
The invention relates to posttranslational modification of phage-displayed polypeptides. These displayed polypeptides comprise at least one unnatural amino acid, e.g., an aryl-azide amino acid such asp-azido-L-phenylalanine, or an alkynyl-amino acid such as para-propargyloxyphenylalanine, which are incorporated into the phage-displayed fusion polypeptide at a selected position by using an in vivo orthogonal translation system comprising a suitable orthogonal aminoacyl-tRNA synthetase and a suitable orthogonal tRNA species. These unnatural amino acids advantageously provide targets for posttranslational modifications such as azide-alkyne [3+2]cycloaddition reactions and Staudinger modifications.
Owner:THE SCRIPPS RES INST
Automated identification of peptides
InactiveUS20020102610A1Quick identificationCharacter and pattern recognitionBiological testingPresent methodPost translational
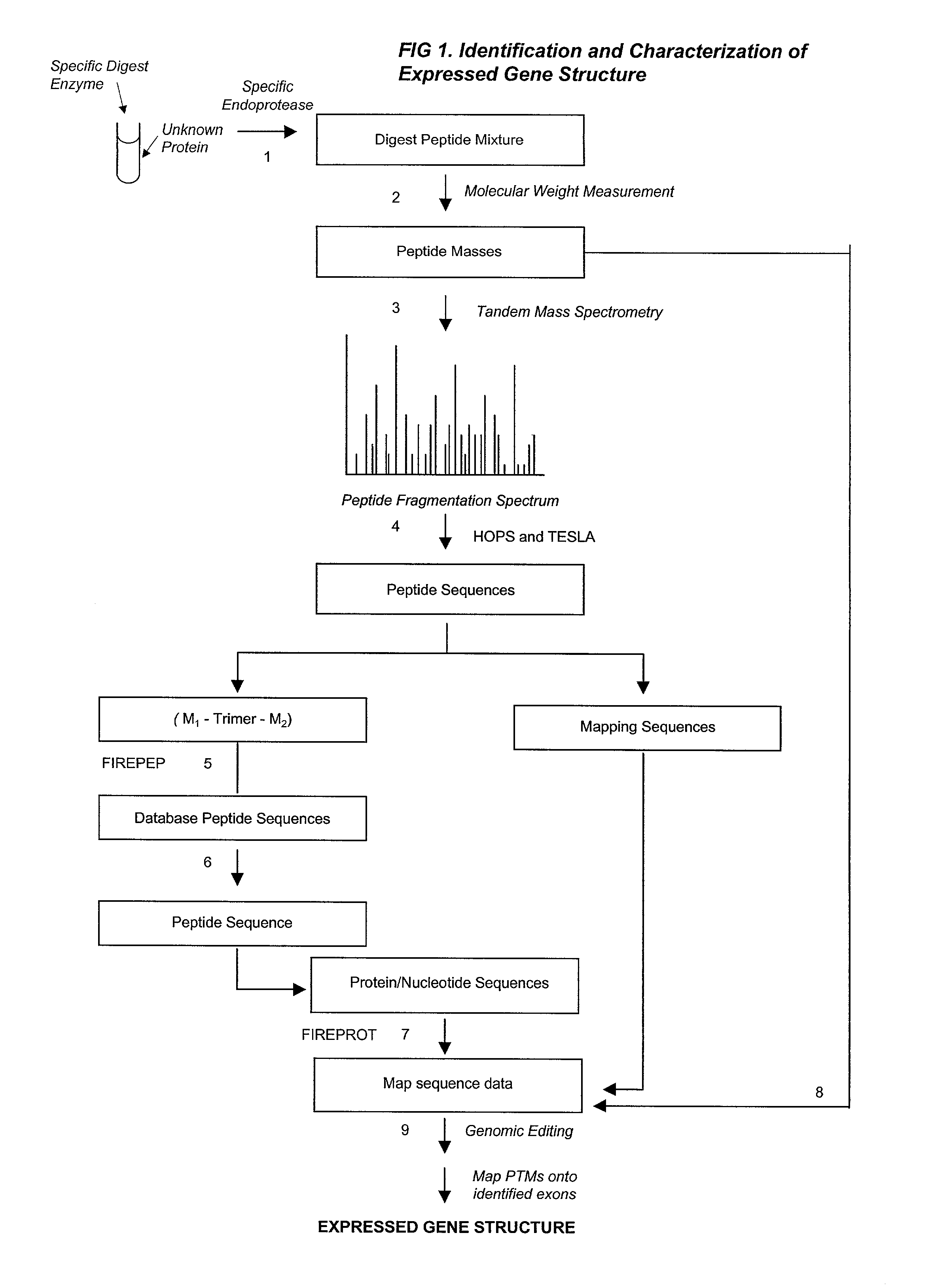
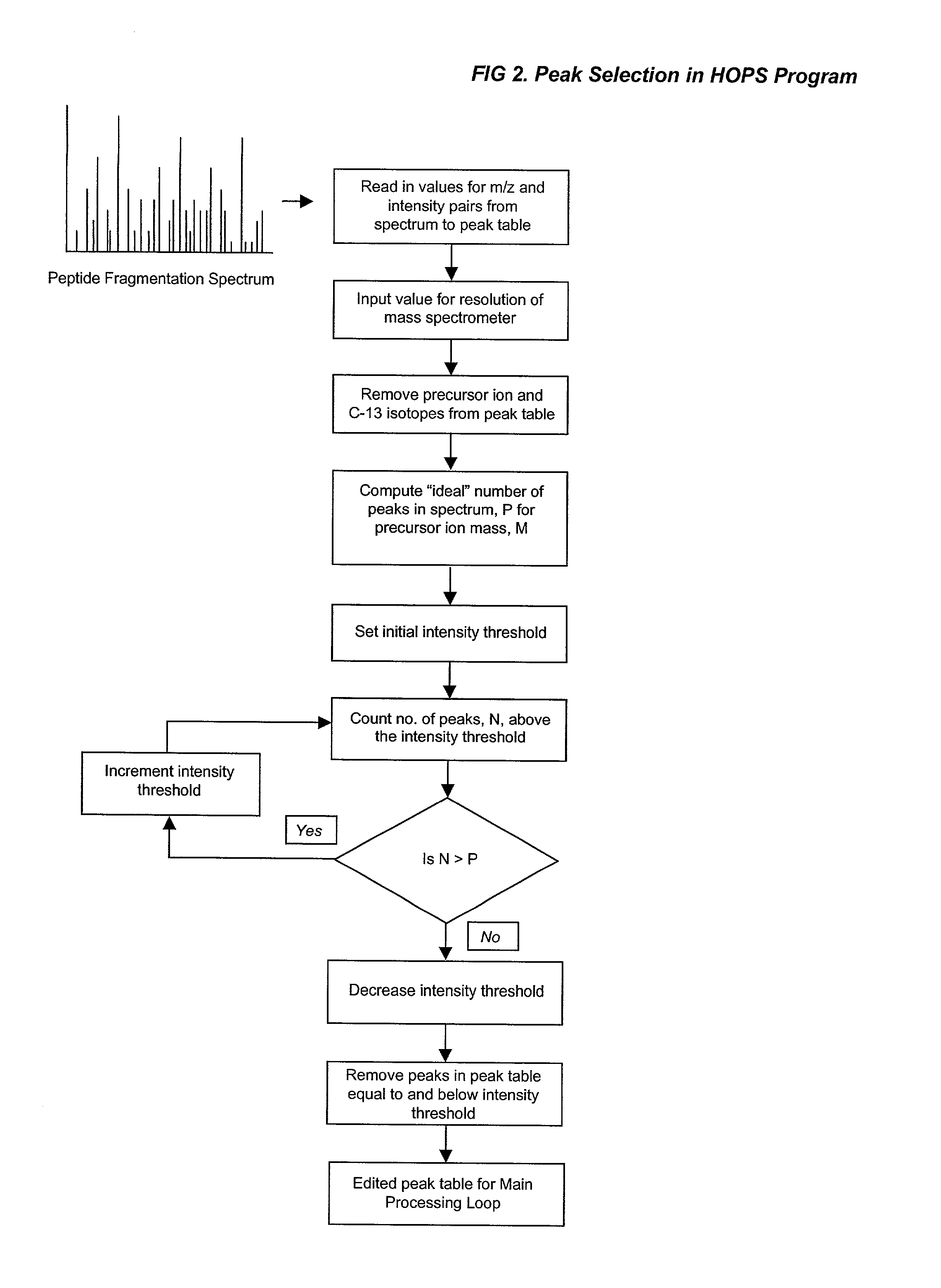
A fully automated, user-independent method is described for computer-mediated interpretation of data derived by mass spectrometry of an experimental peptide to identify and characterize a corresponding peptide sequence in a peptide database. The method identifies the corresponding sequence if it is present in the database, without the need for a skilled observer to choose from amongst a list of possible matches. By using an automated back-read process, the present method can uniquely identify a corresponding peptide sequence in a database based on a single matching peptide sequence. The method also permits mapping of mass spectral data to sequences in peptide or nucleotide databases for unambiguous identification of exons; determining a correct reading frame; identifying artefacts and errors in sequences; identifying mutations and polymorphisms; identifying post-translational modifications; and identifying exon-intron boundaries.
Owner:OXFORD GLYCOSCI UK
Method and compositions for the detection of protein glycosylation
ActiveUS20050130235A1Sugar derivativesMicrobiological testing/measurementPost translationalProtein insertion
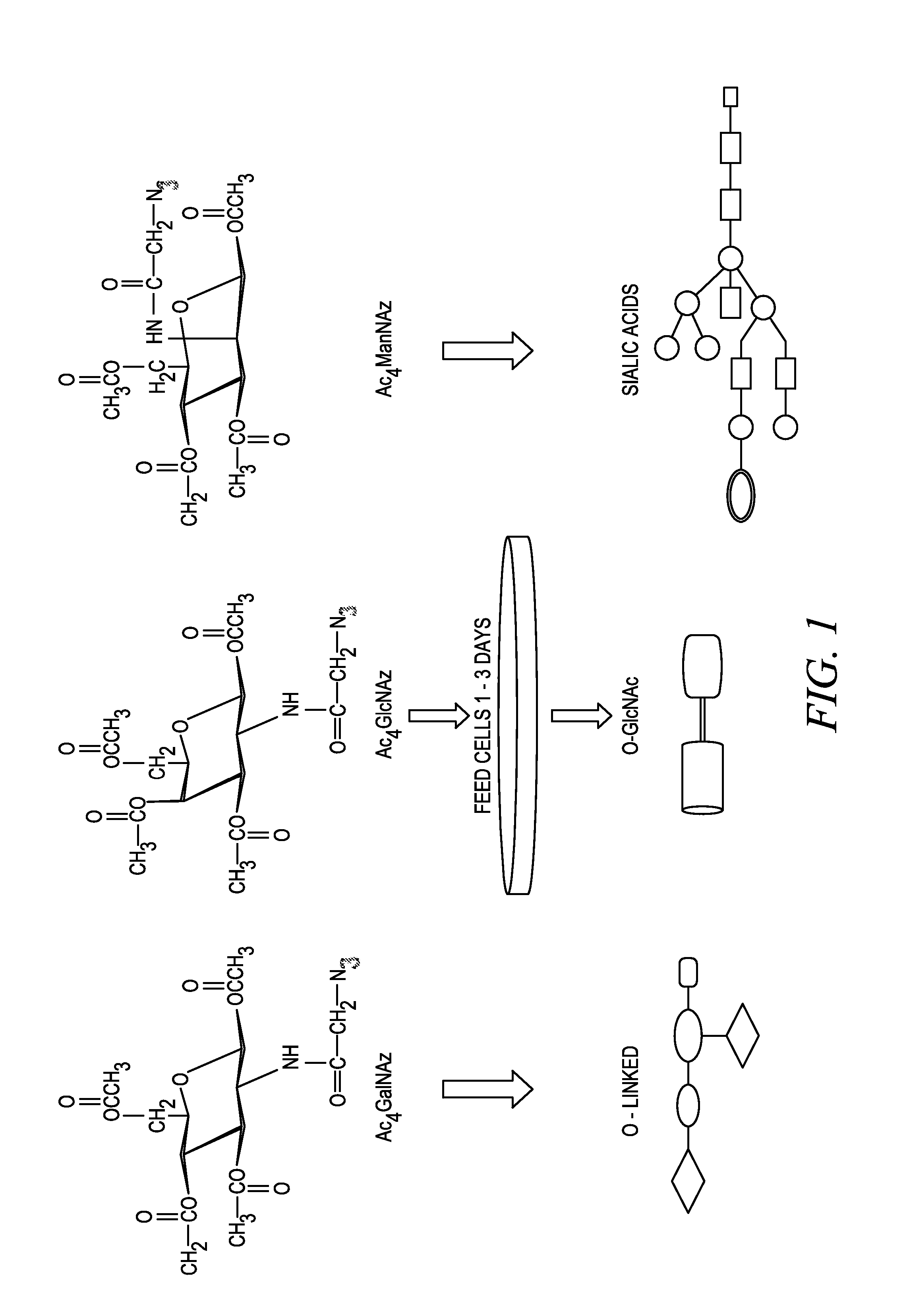
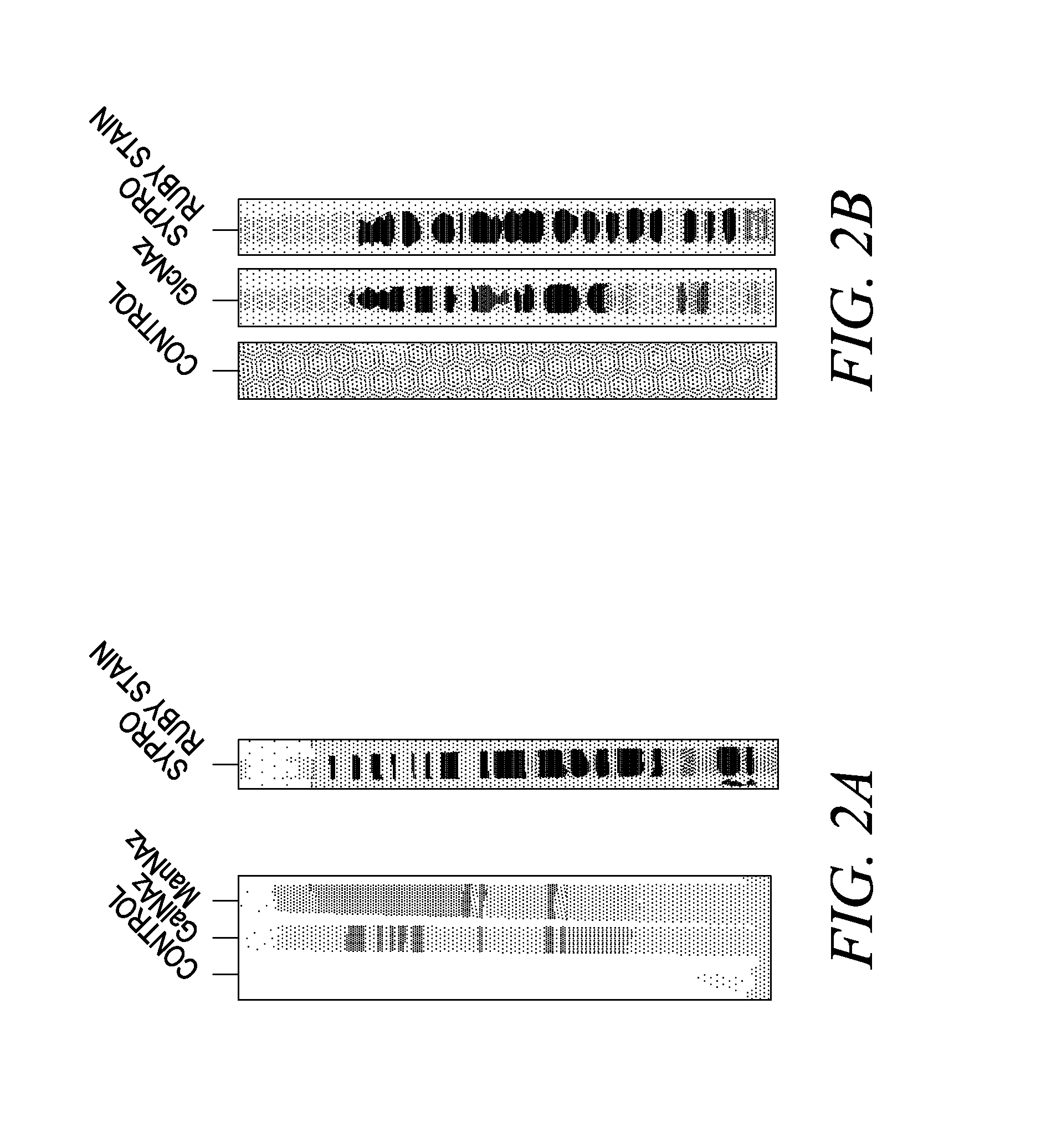
The invention provides methods and compositions for the rapid and sensitive detection of post-translationally modified proteins, and particularly of those with post-translational glycosylations. The methods can be used to detect O-GlcNAc posttranslational modifications on proteins on which such modifications were undetectable using other techniques. In one embodiment, the method exploits the ability of an engineered mutant of β-1,4-galactosyltransferase to selectively transfer an unnatural ketone functionality onto O-GlcNAc glycosylated proteins. Once transferred, the ketone moiety serves as a versatile handle for the attachment of biotin, thereby enabling detection of the modified protein. The approach permits the rapid visualization of proteins that are at the limits of detection using traditional methods. Further, the preferred embodiments can be used for detection of certain disease states, such as cancer, Alzheimer's disease, neurodegeneration, cardiovascular disease, and diabetes.
Owner:CALIFORNIA INST OF TECH
Post-translational modifications and Clostridial neurotoxins
InactiveUS7223577B2Increase and decrease persistenceIncrease or decrease stabilityPeptide/protein ingredientsDepsipeptidesDiseasePost translational
The present invention discloses modified neurotoxins with altered biological persistence. In one embodiment, the modified neurotoxins are derived from Clostridial botulinum toxins. Such modified neurotoxins may be employed in treating various conditions, including but not limited to muscular disorders, hyperhidrosis, and pain.
Owner:ALLERGAN INC
Linker peptides and polypeptides comprising same
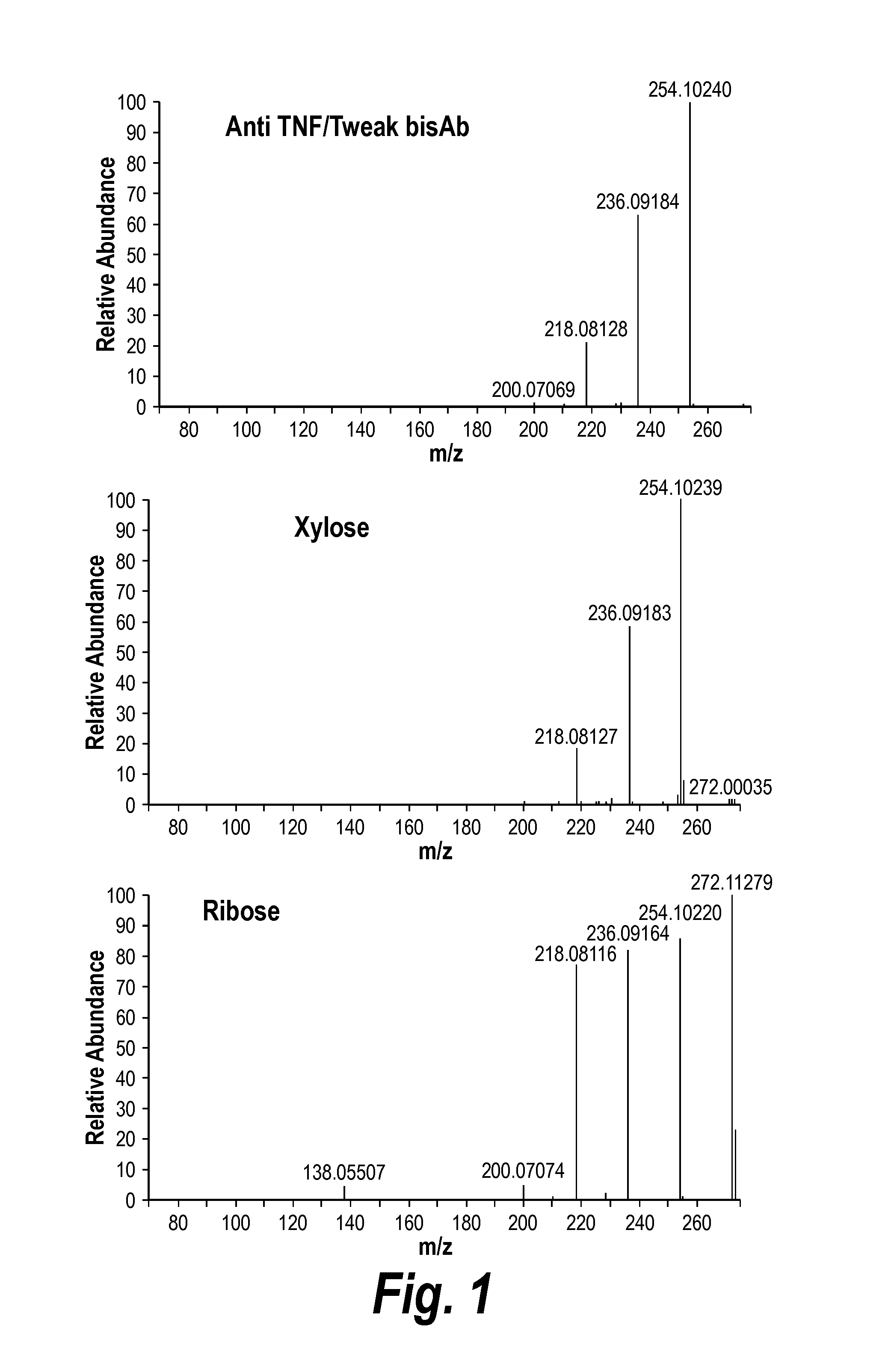
ActiveUS20140079701A1Reduce aggregationImproves pH stabilityBacteriaAntibody mimetics/scaffoldsXylosyltransferaseLinker peptide
The invention is based, at least in part, on the finding that linker peptides which lack the amino acid sequence GSG reduce or eliminate the addition of posttranslational modifications to the polypeptides which comprise them. More specifically, the novel linker peptides disclosed herein reduce the ability of enzymes to link carbohydrate adducts to polypeptides comprising these linker peptides, e.g., reduce the ability of xylosyltransferase to link xylose to polypeptides. These novel linker peptides, molecules comprising same, and methods of their use are described.
Owner:BIOGEN MA INC
Methods and compositions for analyzing proteins
InactiveUS7255999B2Electrolysis componentsMaterial analysis by observing effect on chemical indicatorBinding siteElectrophoresis
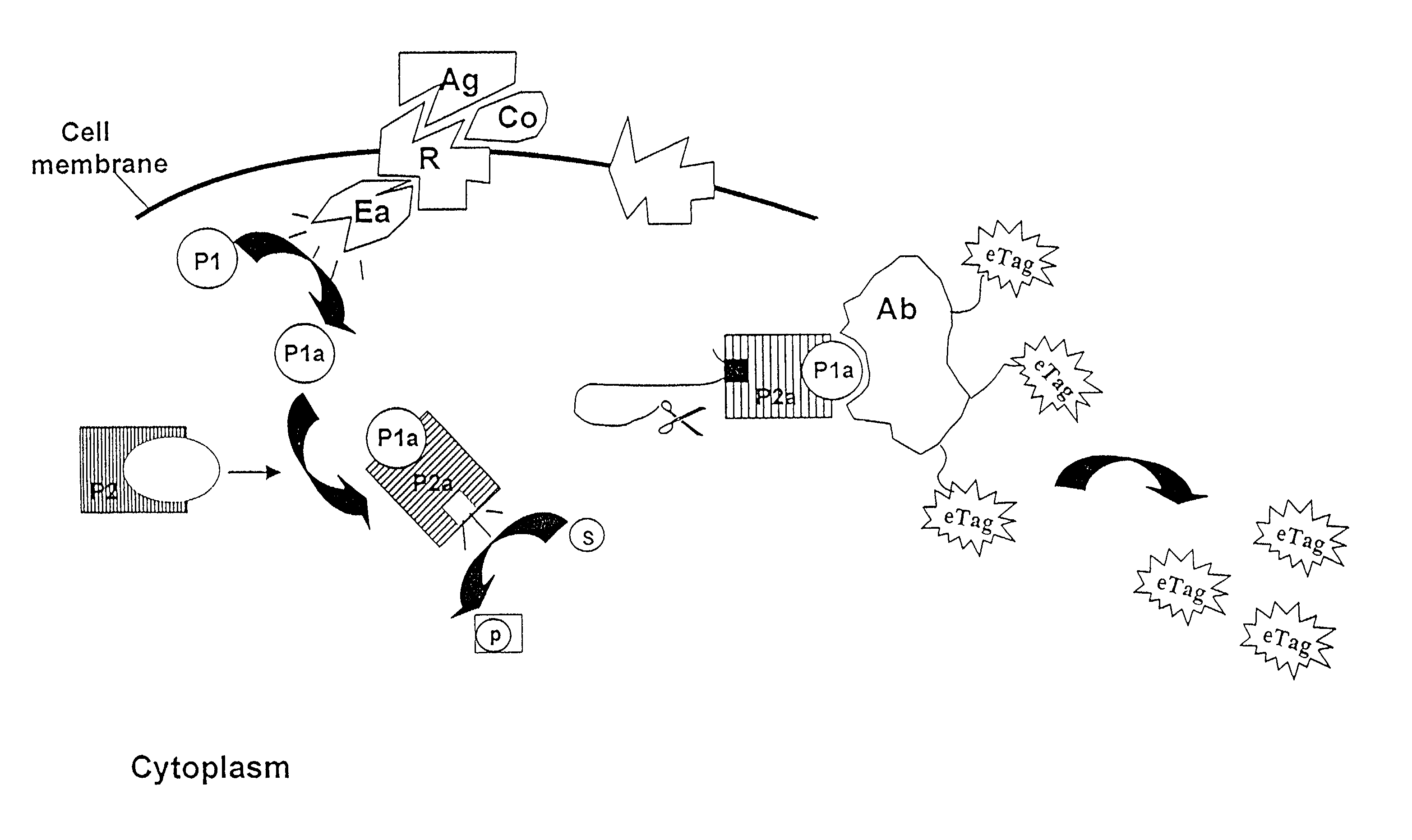
Methods, compositions and kits are disclosed for determining one or more target polypeptides in a sample where the target polypeptides have undergone a post-translational modification. A mixture comprising the sample and a first reagent comprising a cleavage-inducing moiety and a first binding agent for a binding site on a target polypeptide is subjected to conditions under which binding of respective binding moieties occurs. The binding site is the result of post-translational modification activity involving the target polypeptide. The method may be employed to determine the target polypeptide itself. In another embodiment the presence and / or amount of the target polypeptide is related to the presence and / or amount and / or activity of an agent such as an enzyme involved in the post-translational modification of the target polypeptide. The interaction between the first binding agent and the binding site brings the cleavage-inducing moiety into close proximity to a cleavable moiety, which is associated with the polypeptide and is susceptible to cleavage only when in proximity to the cleavage-inducing moiety. In this way, an electrophoretic tag for each of the polypeptides may be released. Released electrophoretic tags are separated and the presence and / or amount of the target polypeptides are determined based on the corresponding electrophoretic tags.
Owner:MONOGRAM BIOSCIENCES
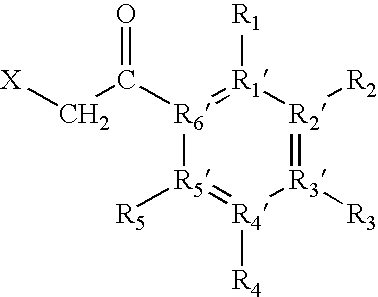
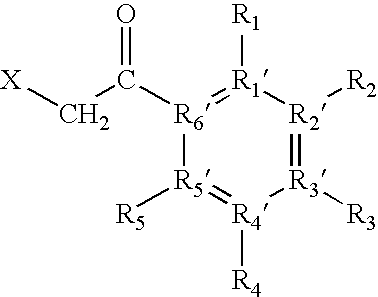
Modified beta-lactamase and method for its preparation
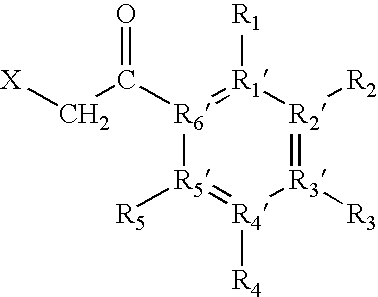
The invention relates to targeted post translational modifi-cation of metallo-beta-lactamase by truncation and inser-tion of a dipeptide at the amino terminal end to reduce amino terminal heterogeneity in a recombinant DNA pro-duction system. A protein K-T-E-ΔBL is expressed, and modified by host proteases to E-ΔBL. Appropriate nucleotide molecules, vectors and hosts are also de-scribed. E-ΔBL is useful in a pharmaceutical composition for treating antibiotic induced adverse effects in the intes-tine of patients treated with beta-lactam antibiotics.
Owner:SYNTHETIC BIOLOGICS INC
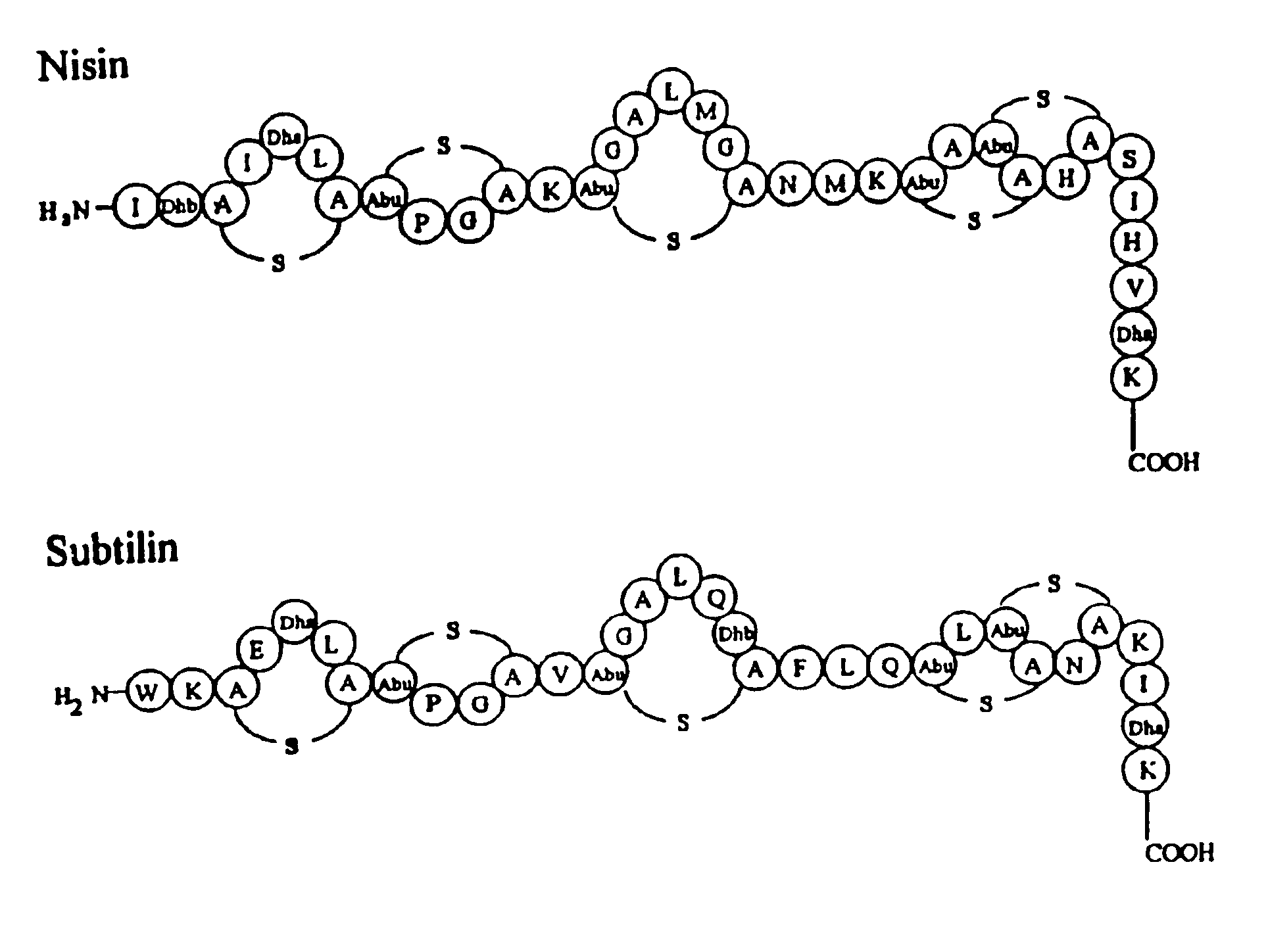
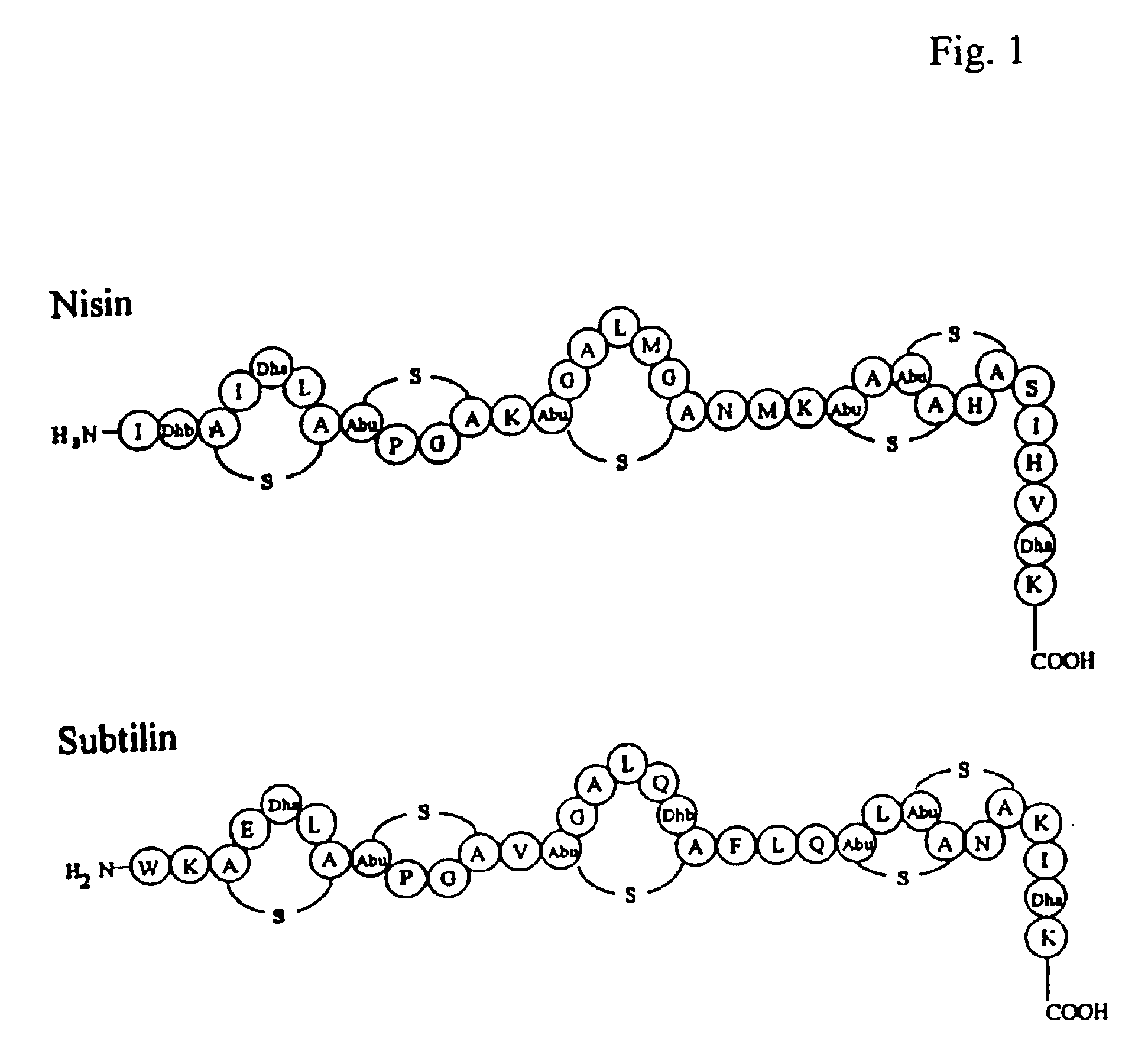
Export and modification of (poly)peptides in the lantibiotic way
InactiveUS6861236B2Avoid insufficient puritySubstantial measurePolypeptide with localisation/targeting motifSaccharide peptide ingredientsDehydroalanineSulfur
The invention includes a method for harvesting a polypeptide produced by a host cell, wherein the polypeptide has not undergone intracellular posttranslational modification, such as dehydration of a serine or a threonine, and / or thioether bridge formation. The invention also includes a method for producing thioether-containing peptides and dehydroalanine / dehydrobutyrine-containing.peptides, wherein thioether rings may be formed extracellularly.
Owner:APPLIED NANOSYST
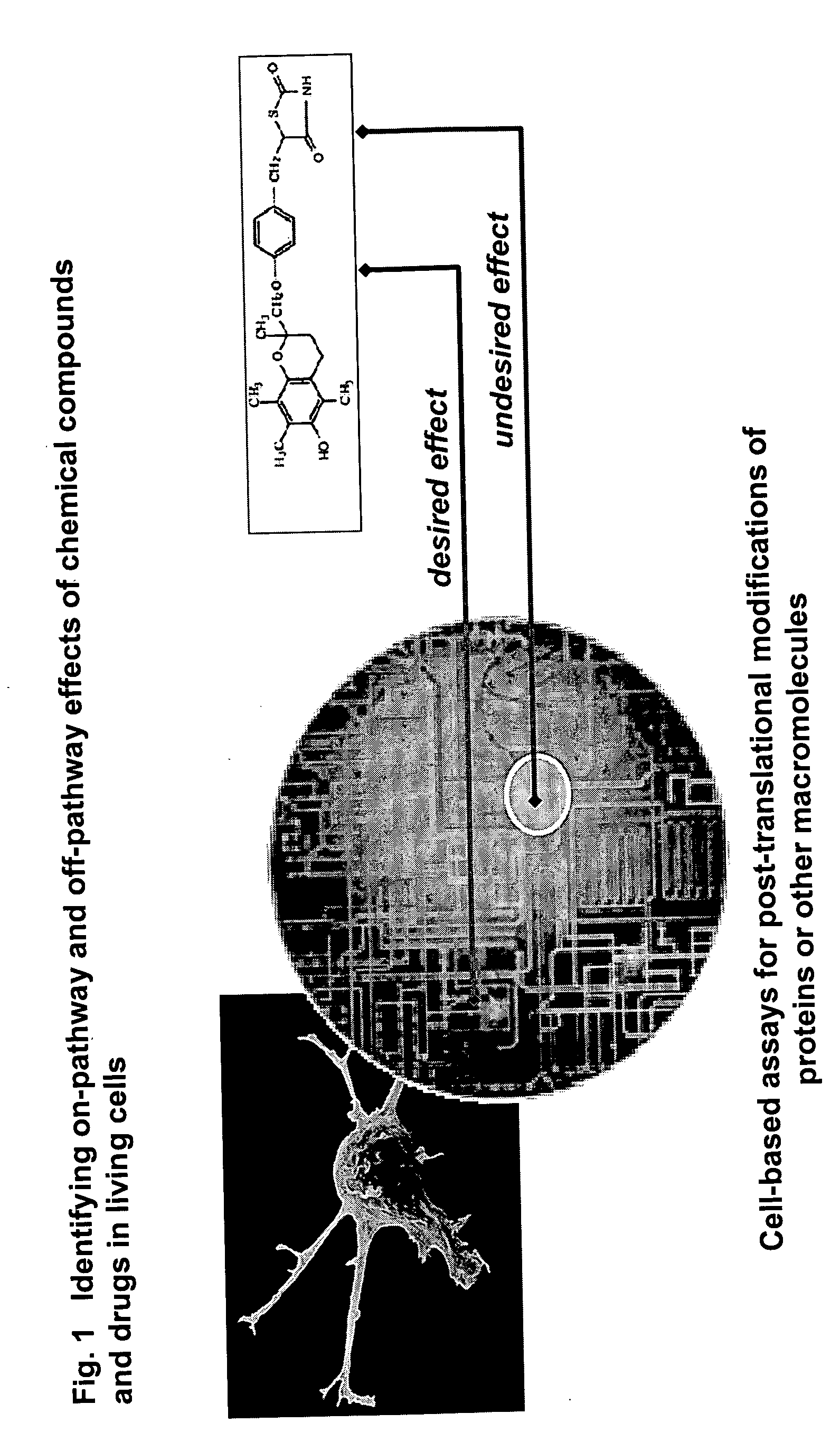
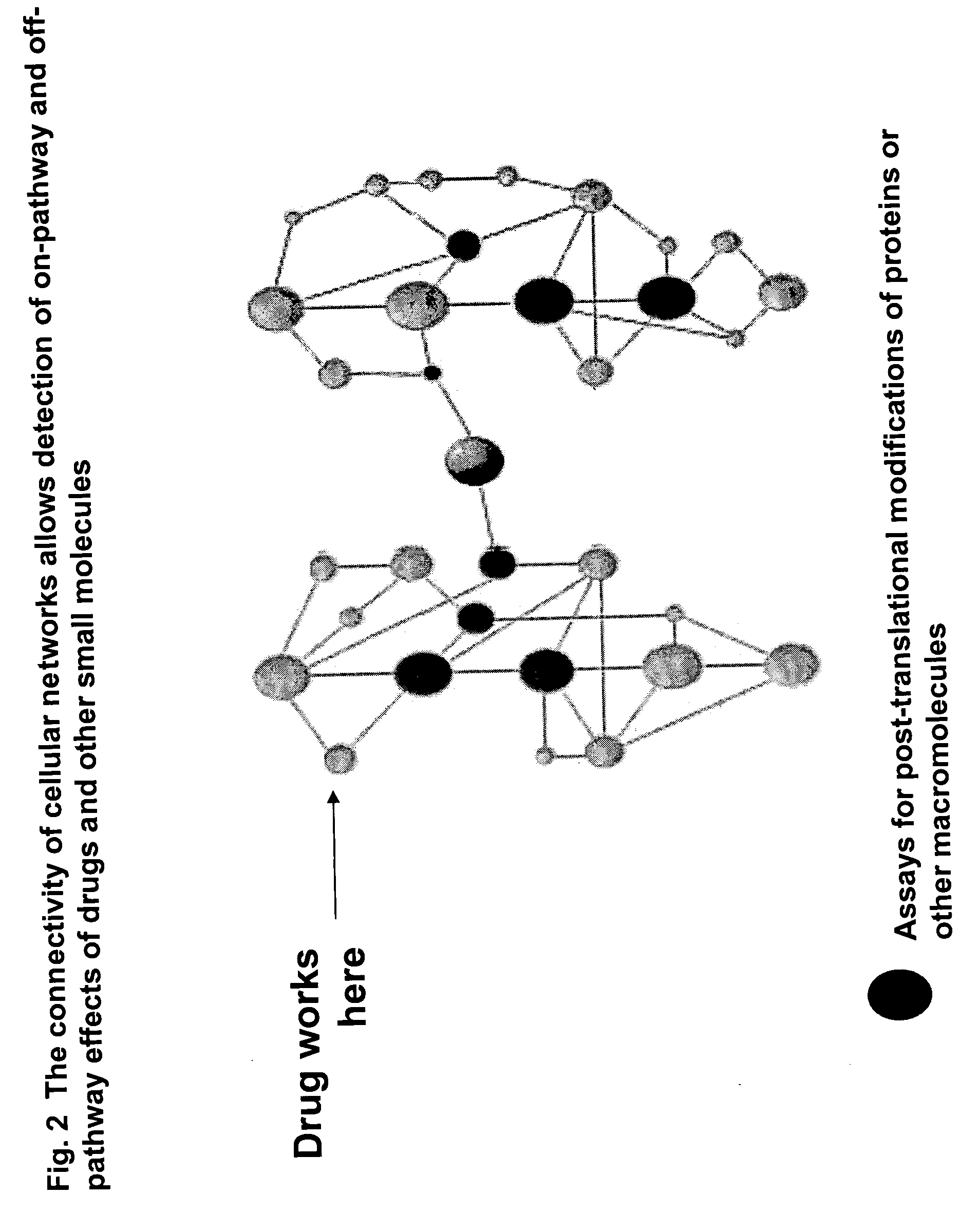
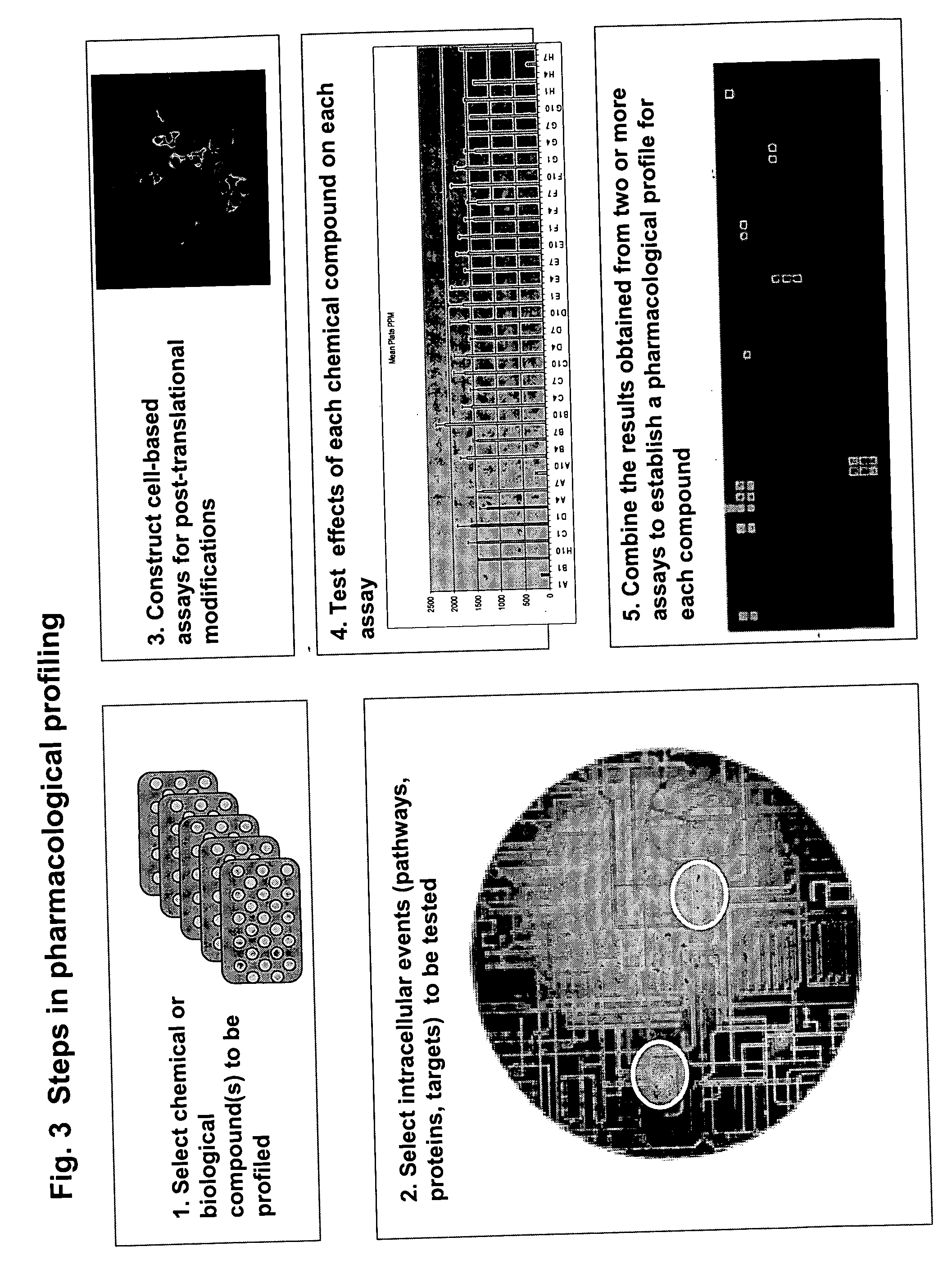
Pharmacological profiling of drugs with cell-based assays
InactiveUS20060040338A1Enable optimizationBioreactor/fermenter combinationsCompound screeningPost translationalAssay
The instant invention provides a method for establishing safety profiles for chemical compounds, as well as pharmacological profiling said method comprising (A) testing the effects of said chemical compounds on the amount and / or post-translational modifications of two or more macromolecules in intact cells; (B) constructing a pharmacological profile based on the results of said tests; and (C) comparing said profile to the profile(s) of drugs with established safety characteristics. Additionally, the invention is also directed to a composition comprising an assay panel, said panel comprising at least one high-content assay for the amount and / or post-translational modification of a protein and at least one high-content assay for the amount and / or subcellular location of a protein-protein interaction.
Owner:ODYSSEY THERA INC
Method for analysing amino acids, peptides and proteins
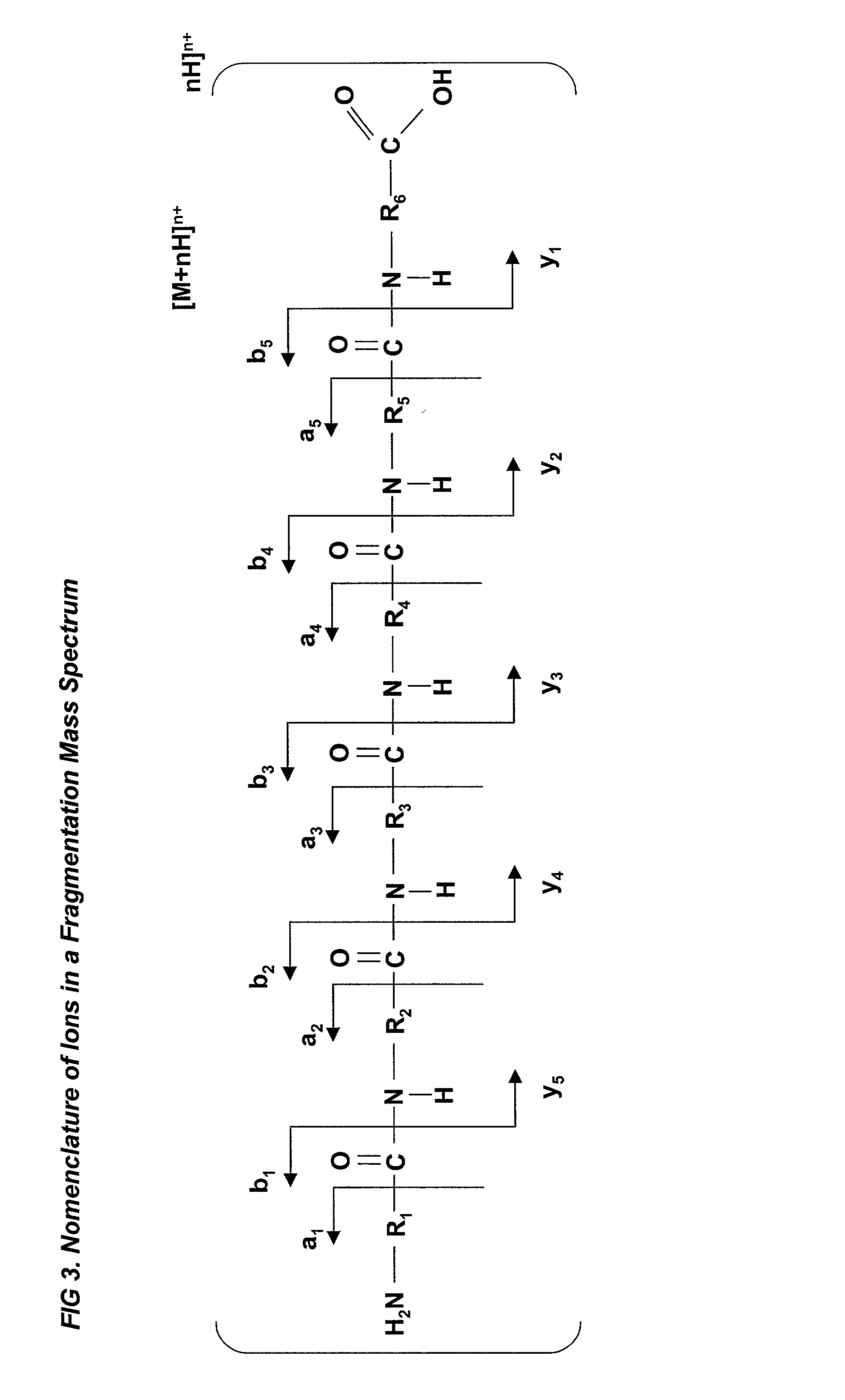
The invention provides methods, reagents and kits for amino acid, peptide and protein identification, differential quantitation and for the analysis of post translational modification and cross-linking status, comprising: derivatizing a mixture of amino acids peptides or proteins, to form at least one amino acid peptide or protein containing a fixed-charge ion, other than at the C-terminal or N-terminal end thereof; introducing the mixture of amino acids peptides or proteins containing the fixed charge derivatized amino acid peptide or protein to a mass spectrometer; passing the mixture of amino acids peptides or proteins containing the fixed charge derivatized amino acid peptide or protein through a first mass resolving spectrometer to select precursor protein or peptide ions having a first desired mass-to-charge ratio; subjecting the precursor ions of the first mass to charge ratio to dissociation to form a product ion having a second mass-to-charge ratio that is characteristic of a fragmentation occurring at a site adjacent to the fixed charge; and detecting the product ions having the second mass-to-charge ratio. The product ion having the second mass-to-charge ratio may be either a product ion formed by neutral loss of the fixed charge from the precursor ion, or a product ion formed by charged loss of the fixed charge from the precursor ion.
Owner:LUDWIG INST FOR CANCER RES
Variant polypeptides and methods of making
InactiveUS20080070287A1High hydrolytic activityIncreased activity transferase activityBacteriaHydrolasesBacillus licheniformisLipid formation
The present invention relates to a method for the production of a variant lipid acyltransferase comprising the steps of: (i) selecting a parent enzyme which is a lipid acyltranserase enzyme; (ii) modifying one or more amino acids to produce a variant lipid acyltransferase; (iii) testing the activity of the variant lipid acyltransferase on a galactolipid and / or phospholipid and / or triglyceride substrate; (iv) selecting a variant enzyme with enhanced activity towards galactolipids compared with the parent enzyme; (v) providing a Bacillus licheniformis cell; (vi) transforming the Bacillus licheniformis cell with a heterologous nucleotide sequence encoding said variant lipid acyltransferase; and (iii) expressing said variant lipid acyltransferase in the cell under the control of a promoter sequence. The variant lipid acyltransferase can undergo post-translations modification, truncation and / or clipping, i.e., to remove a signal peptide. In addition, the present invention further relates to the use of Bacillus licheniformis to express a lipid acyltransferase, a Bacillus licheniformis host cell comprising a heterologous lipid acyltransferase and a vector comprising a nucleotide sequence encoding a lipid acyltransferase operably linked to a promoter sequence homologous to B. licheniformis.
Owner:DUPONT NUTRITION BIOSCIENCES APS
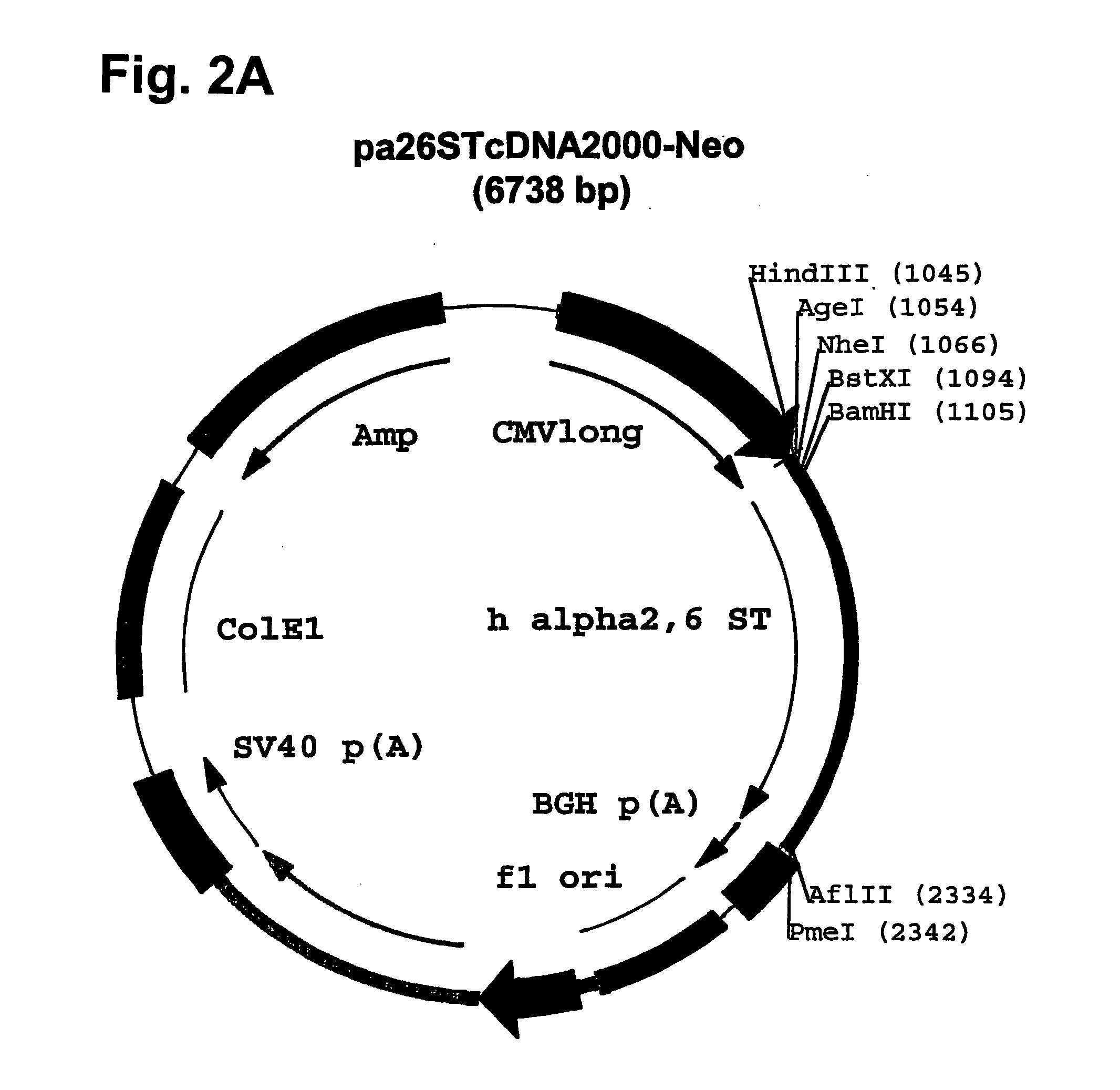
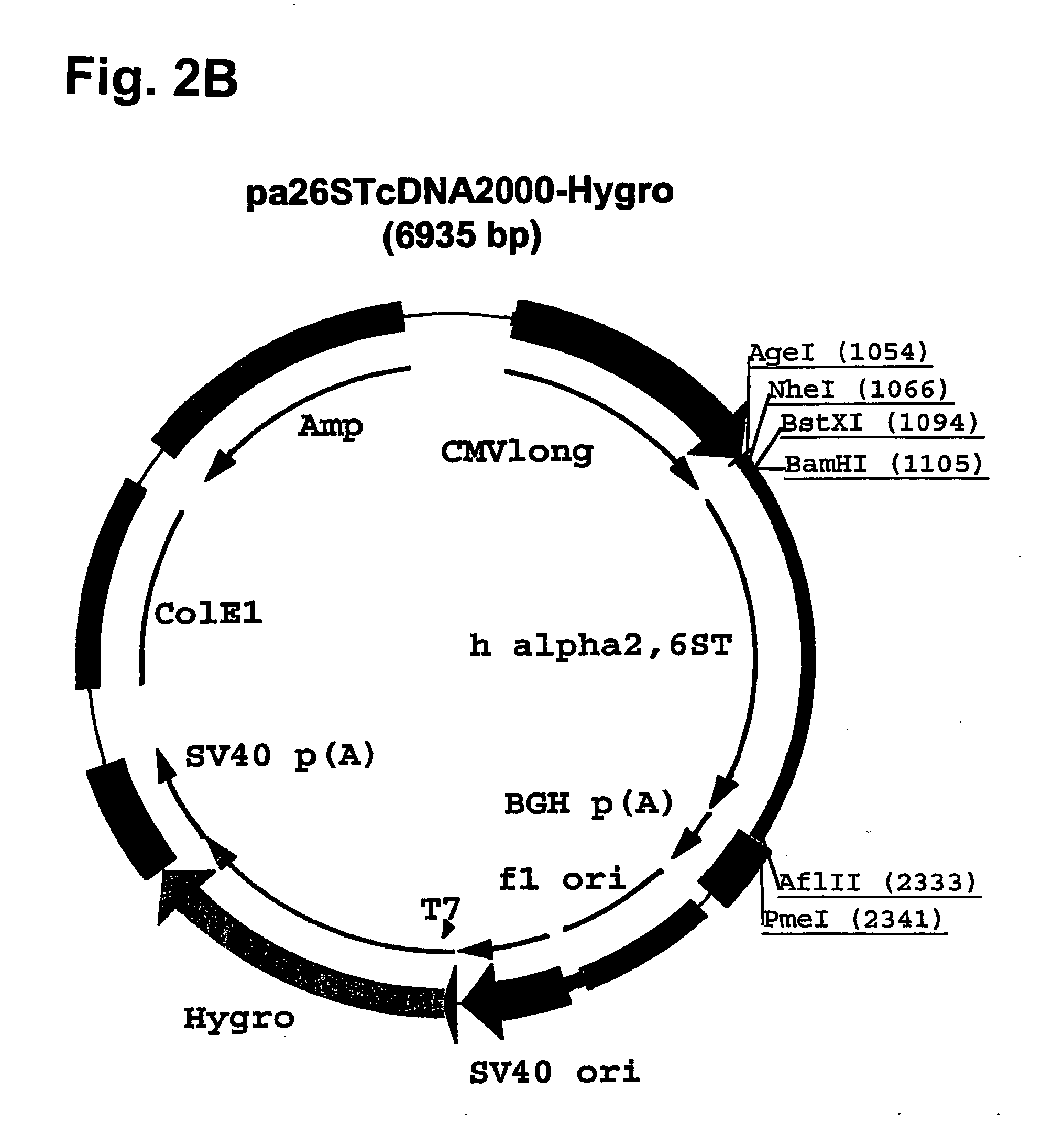
Compositions of erythropoietin isoforms comprising Lewis-X structures and high sialic acid content
InactiveUS7297680B2Improve system reliabilitySimplifies isolationOrganic active ingredientsVirusesHeterologousE1A Protein
Disclosed are immortalized human embryonic retina cells, having a nucleic acid sequence encoding an adenoviral E1A protein integrated into the genome of the cells, and further comprising a nucleic acid sequence encoding an enzyme involved in post-translational modification of proteins, such as a sialyltransferase, wherein said nucleic acid sequence encoding the enzyme involved in post-translational modification of proteins is under control of a heterologous promoter. Methods for producing recombinant proteins from such cells and obtaining such recombinant proteins having increased sialylation are provided as are novel compositions of isoforms of erythropoietin.
Owner:JANSSEN VACCINES & PREVENTION BV
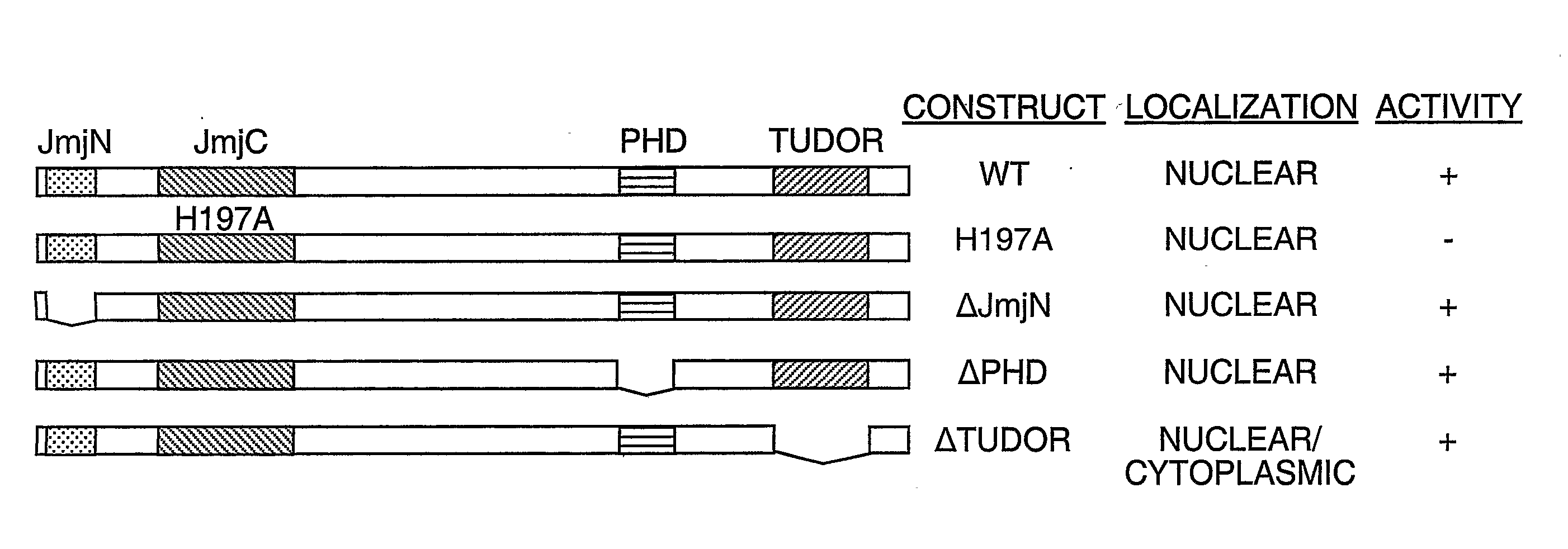
Protein demethylases comprising a jmjc domain
Post-translational modification, including protein methylation, plays an important role in regulating protein function. The present invention provides a novel assay for evaluating demethylase activity and the discovery of a family of protein demethylases comprising a novel demethylase motif.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL
Compositions and Methods for Detection, Prognosis and Treatment of Breast Cancer
InactiveUS20090118175A1Improved prognosisOrganic active ingredientsSugar derivativesDiseasePost translational
The present invention relates to methods of detection, prognosis and treatment of breast cancer using a plurality genes or gene products present in normal and neoplastic cells, tissues and bodily fluids. Gene products relate to compositions comprising the nucleic acids, polypeptides, antibodies, post translational modifications (PTMs), variants, derivatives, agonists and antagonists of the invention and methods for the use of these compositions. Additional uses include identifying, monitoring, staging, imaging and treating cancer and non-cancerous disease states in breast as well as determining the effectiveness of therapies alone or in combination for an individual. Therapies include gene therapy, therapeutic molecules including but not limited to antibodies, small molecules and antisense molecules.
Owner:MACINA ROBERTO A
Modified beta-lactamase and method for its preparation
The invention relates to targeted post translational modification of metallo-beta-lactamase by truncation and insertion of a dipeptide at the amino terminal end to reduce amino terminal heterogeneity in a recombinant DNA production system. A protein K-T-E-ΔBL is expressed, and modified by host proteases to E-ΔBL. Appropriate nucleotide molecules, vectors and hosts are also described. E-ΔBL is useful in a pharmaceutical composition for treating antibiotic induced adverse effects in the intestine of patients treated with beta-lactam antibiotics.
Owner:SYNTHETIC BIOLOGICS INC
Expression of triple-helical collagen-like products in e.coli
InactiveUS20120116053A1Facilitates protein foldingConnective tissue peptidesCell receptors/surface-antigens/surface-determinantsRepetitive SequencesCollagenan
Recombinant bacterial triple-helical collagen-like proteins comprising two or more repetitive sequences of Gly-Xaa-Yaa yielding high-stability polymeric constructs without the need for post-translational modifications and which may incorporate one or more functional domains of biological or structural importance. The polymers are capable of high-yield production for a variety of applications
Owner:RUTGERS THE STATE UNIV
Modulation of XBP-1 activity for treatment of metabolic disorders
InactiveUS20060063187A1High activityHigh expressionCompound screeningPeptide librariesSignal transductionMetabolic disorder
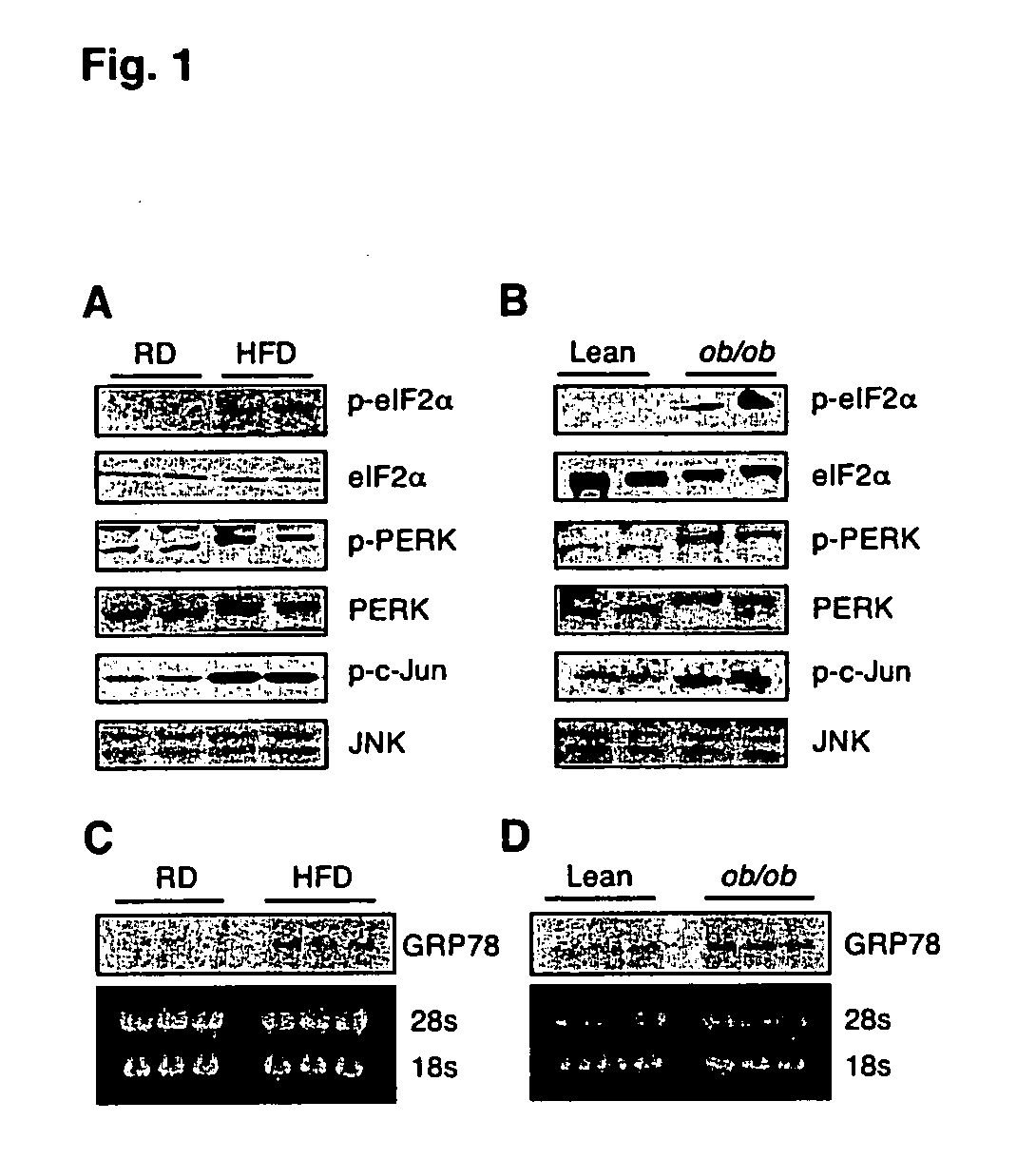
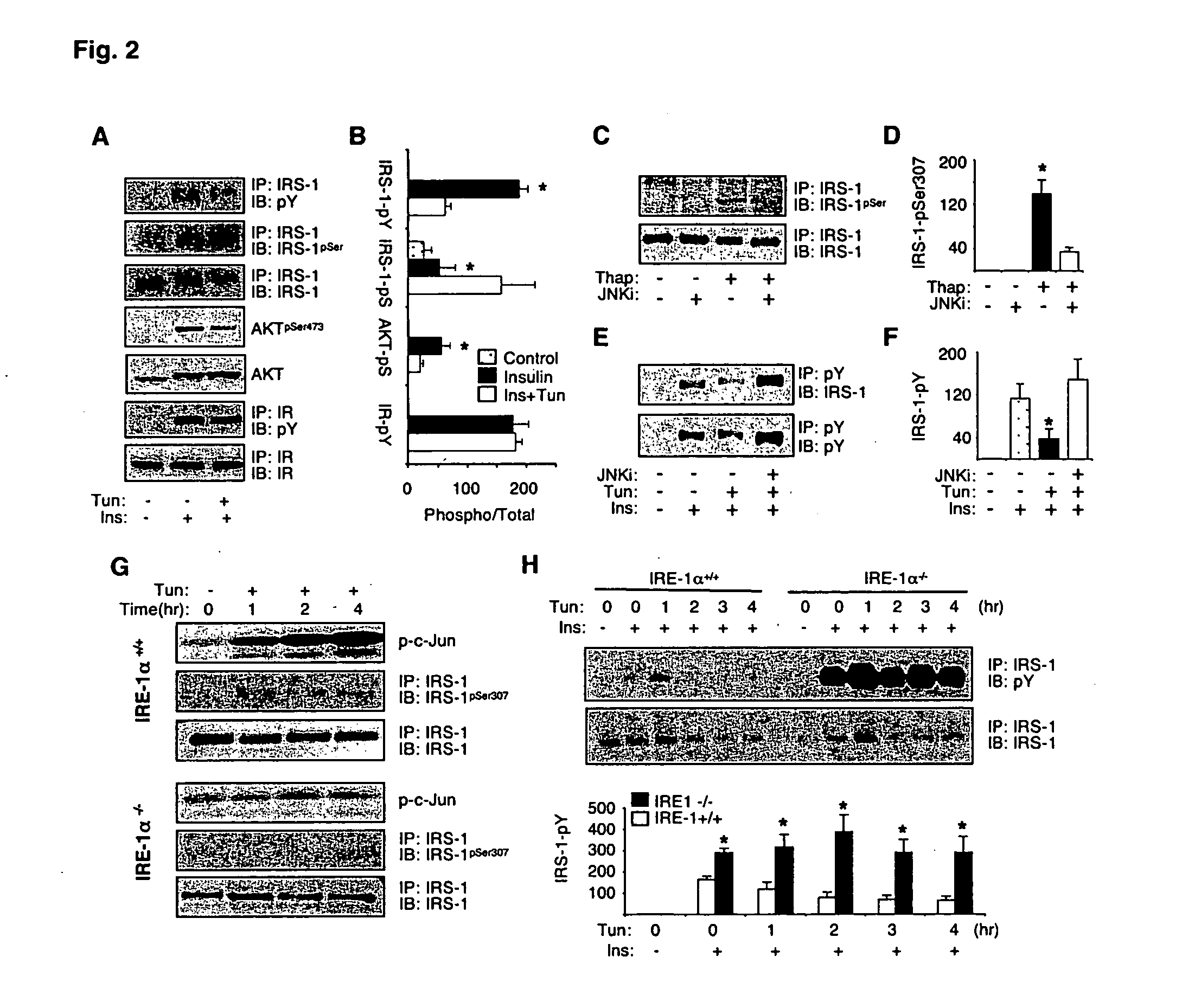
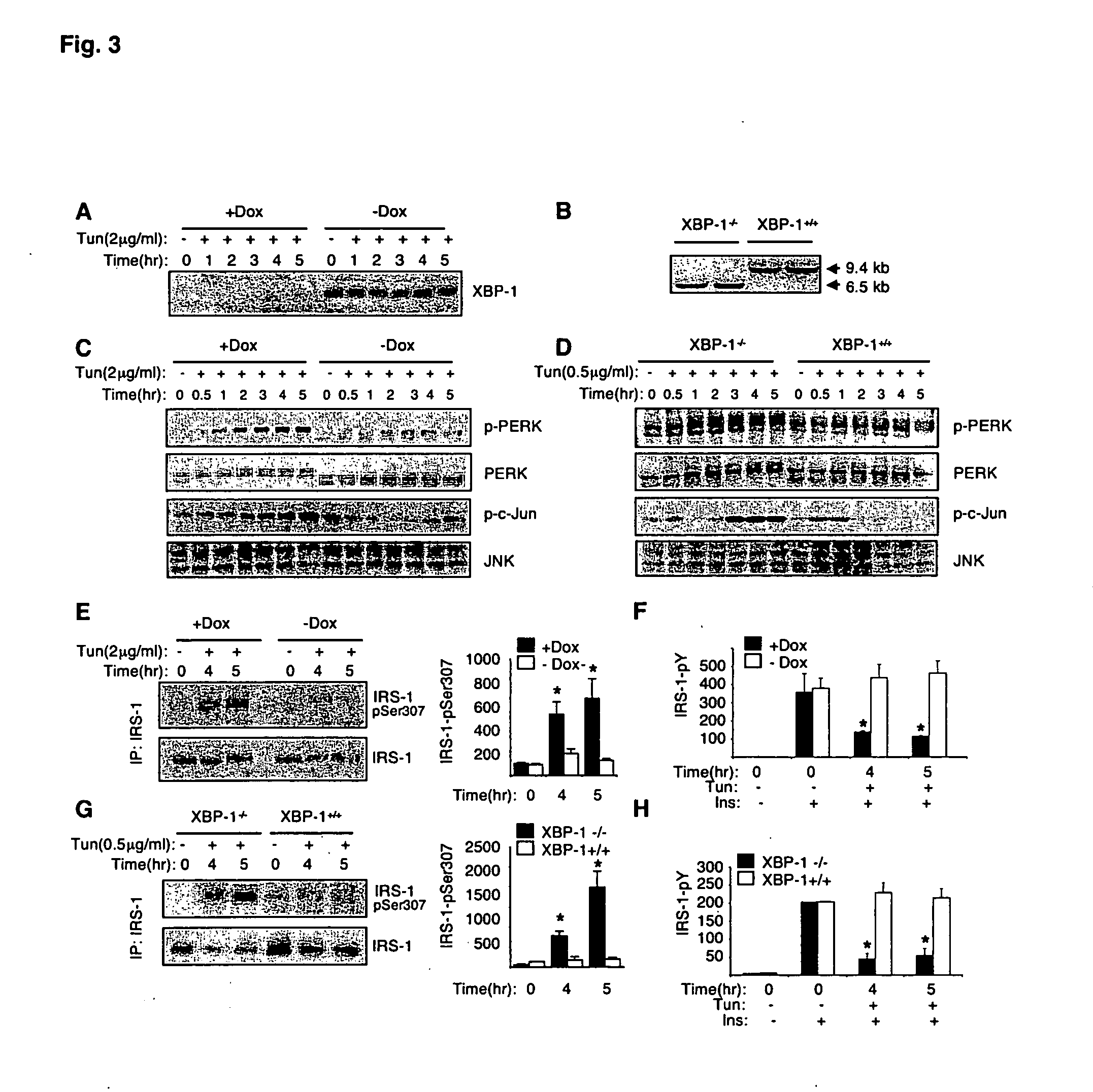
The invention provides methods and compositions for modulating the expression, processing, post-translational modification, stability and / or activity of XBP-1 protein, or a protein in a signal transduction pathway involving XBP-1 to treat metabolic disorders, e.g., type II diabetes. The present invention also pertains to methods for identifying compounds that modulate the expression, processing, post-translational modification, and / or activity of XBP-1 protein or a molecule in a signal transduction pathway involving XBP-1.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
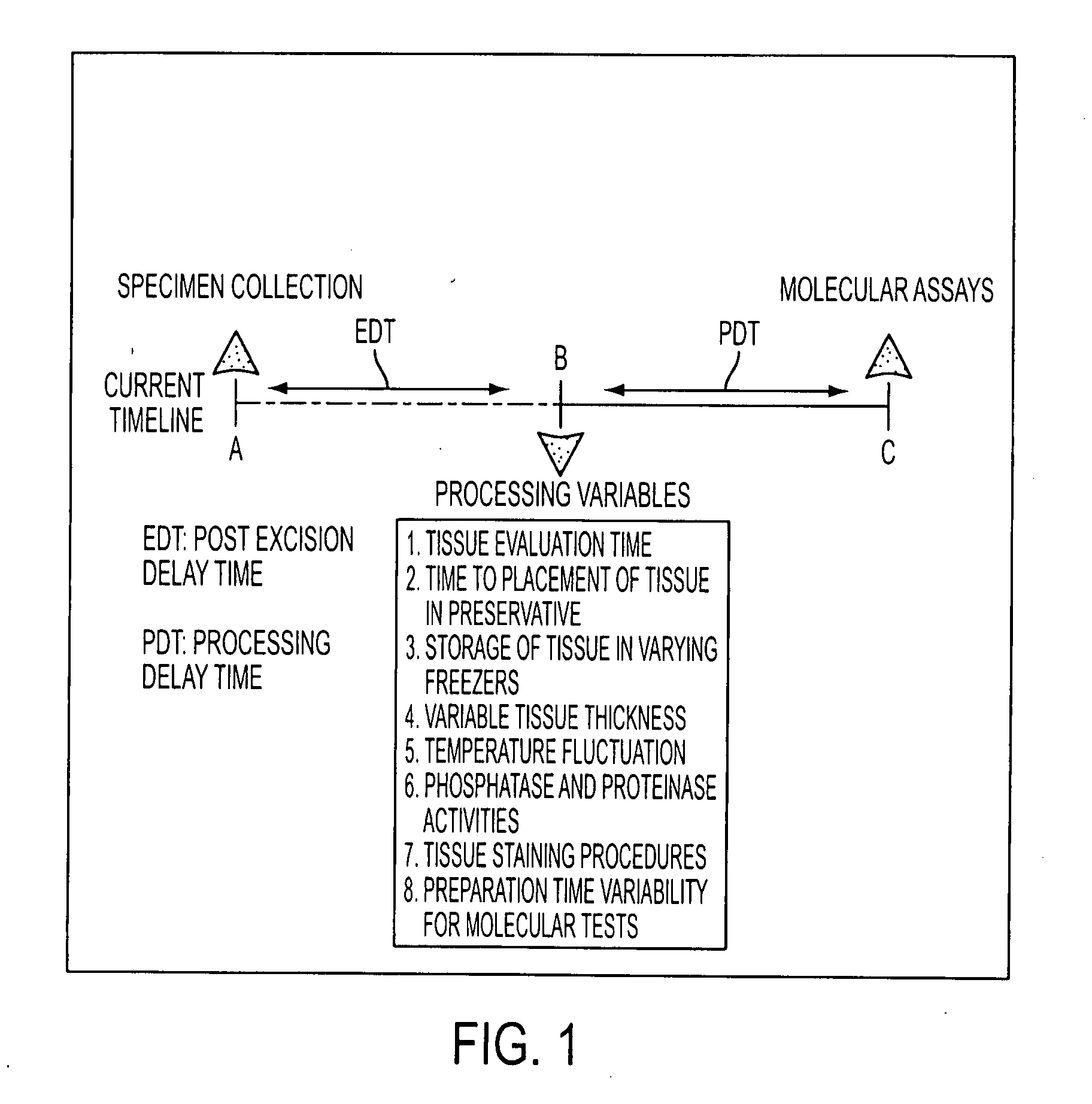
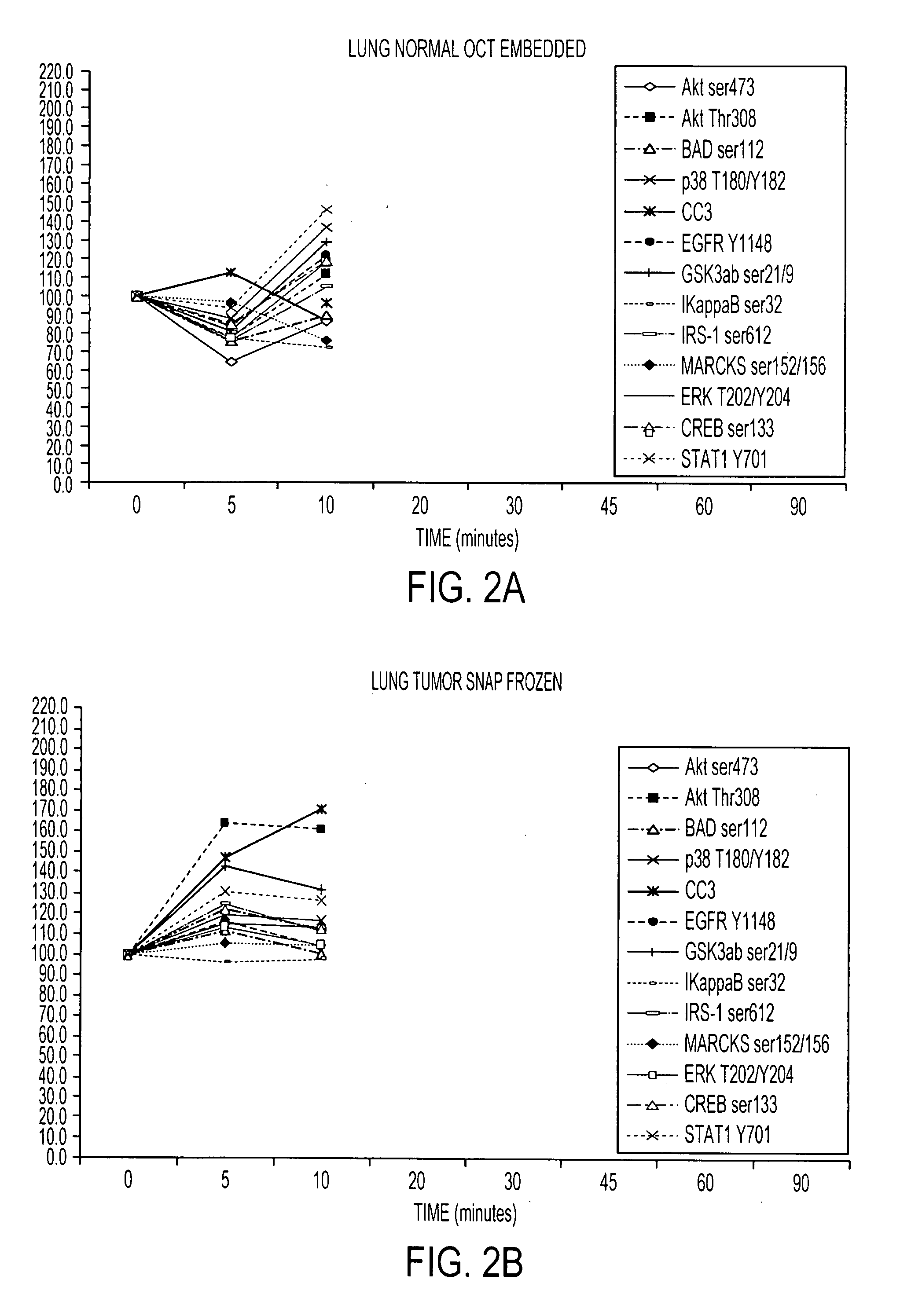
Tissue preservation and fixation method
ActiveUS20100068690A1Bioreactor/fermenter combinationsMaterial nanotechnologyPopulation sampleMolecular analysis
This invention relates, e.g., to a composition that, at room temperature, when contacted with a sample comprising phosphoproteins, can fix and stabilize cellular phosphoproteins, preserve cellular morphology, and allow the sample to be frozen to generate a cryostat frozen section suitable for molecular analysis. The composition comprises (1) a fixative that is effective to fix the phosphoproteins, and that has a sufficient water content to be soluble for a stabilizer and / or a permeability enhancing agent); (2) a stabilizer, comprising (a) a kinase inhibitor and (b) a phosphatase inhibitor and, optionally, (c) a protease (e.g., proteinase) inhibitor; and (3) a permeability enhancing agent (e.g. PEG). Methods are described for preserving phosphoproteins, using such a composition. Also described are endogenous surrogate markers for monitoring protein degradation, including the loss of posttranslational modifications (such as phosphorylation), e.g. the following removal of a cell or tissue from a subject; and exogenous molecular sentinels (e.g. phosphoproteins attached to magnetic nanoparticles) that allow one to evaluate the processing history of a cellular or tissue population sample.
Owner:GEORGE MASON UNIVERSITY
Method and compositions for the detection of protein glycosylation
The invention provides methods and compositions for the rapid and sensitive detection of post-translationally modified proteins, and particularly of those with post-translational glycosylations. The methods can be used to detect O-GlcNAc post-translational modifications on proteins on which such modifications were undetectable using other techniques. In one embodiment, the method exploits the ability of an engineered mutant of β-1,4-galactosyltransferase to selectively transfer an unnatural ketone functionality onto O-GlcNAc glycosylated proteins. Once transferred, the ketone moiety serves as a versatile handle for the attachment of biotin, thereby enabling detection of the modified protein. The approach permits the rapid visualization of proteins that are at the limits of detection using traditional methods. Further, the preferred embodiments can be used for detection of certain disease states, such as cancer, Alzheimer's disease, neurodegeneration, cardiovascular disease, and diabetes.
Owner:CALIFORNIA INST OF TECH
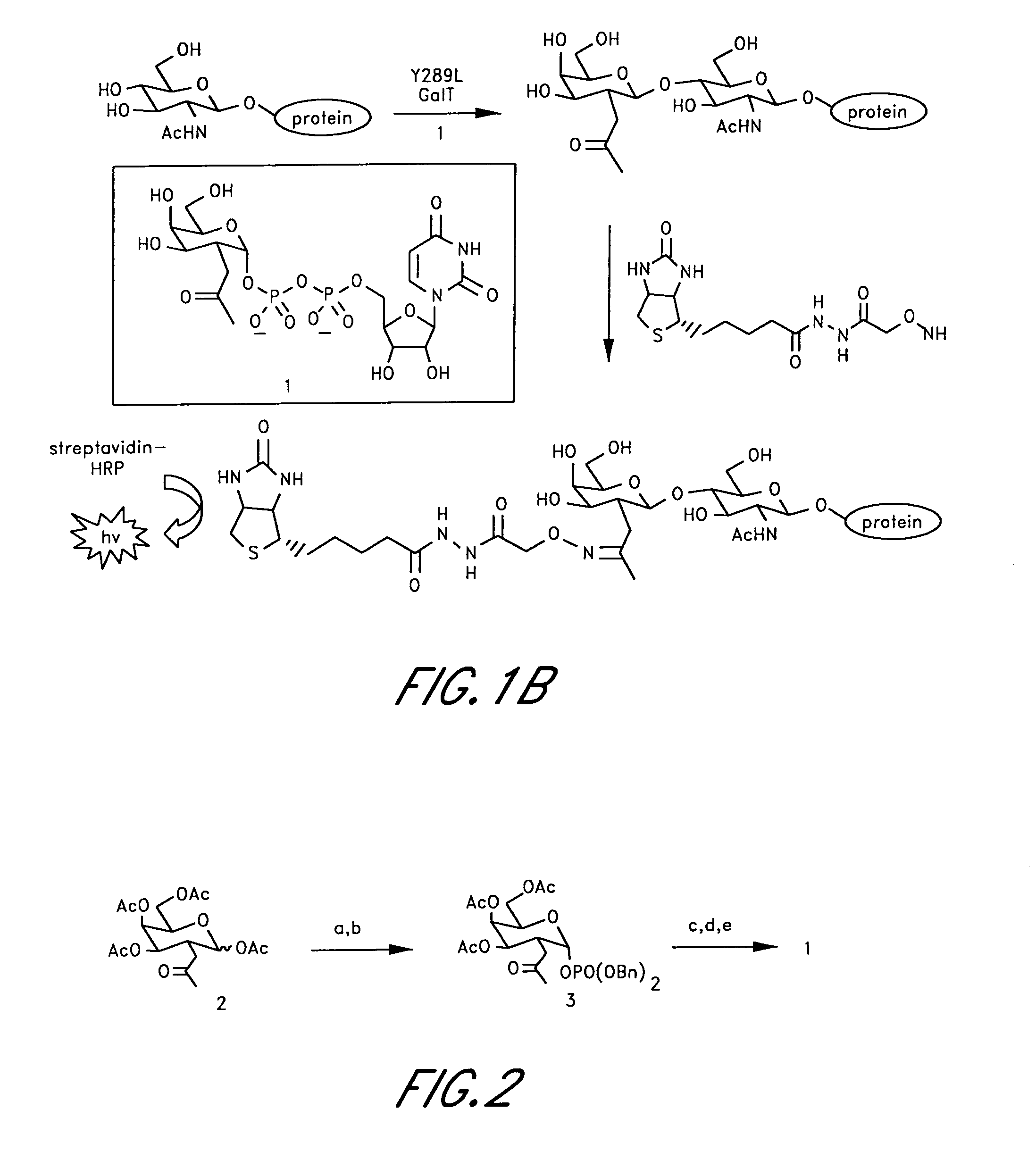
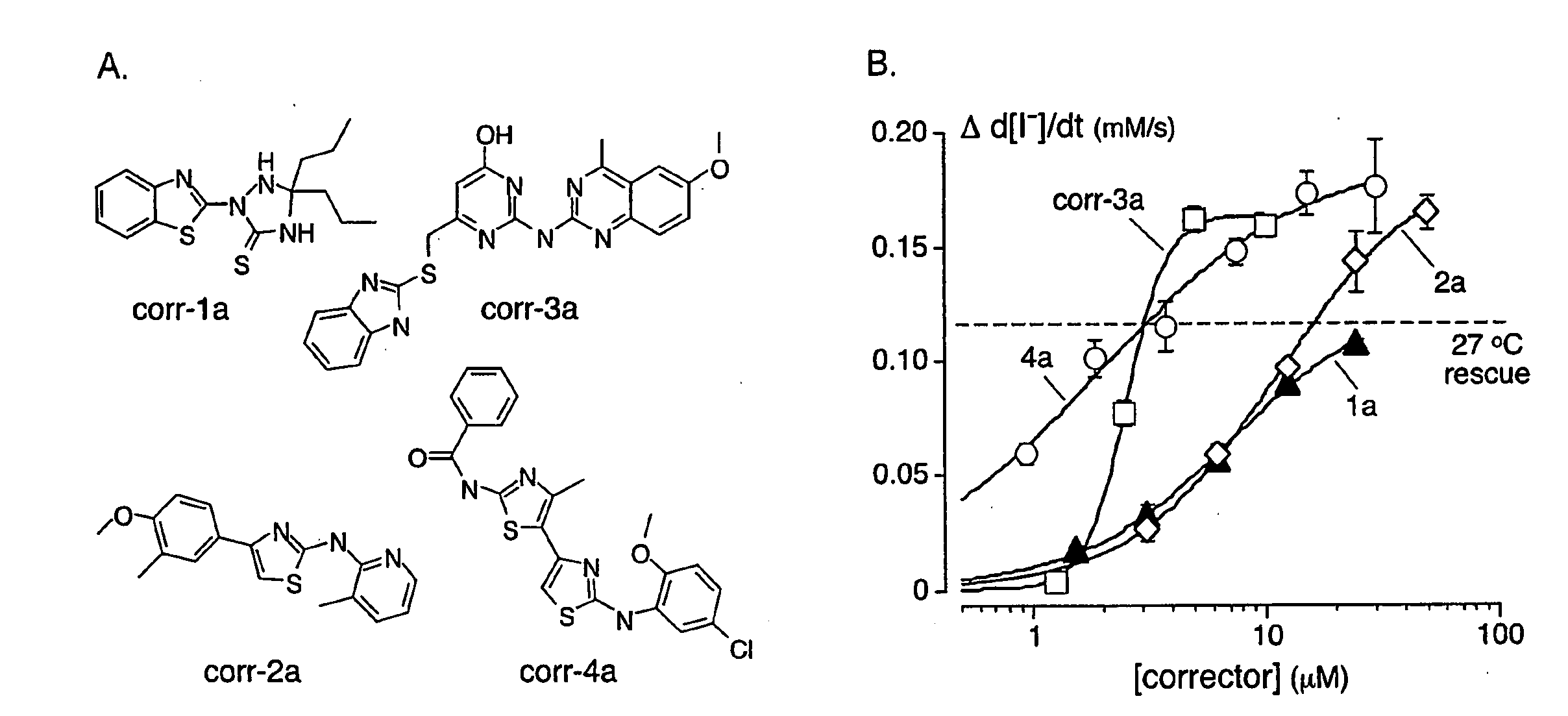
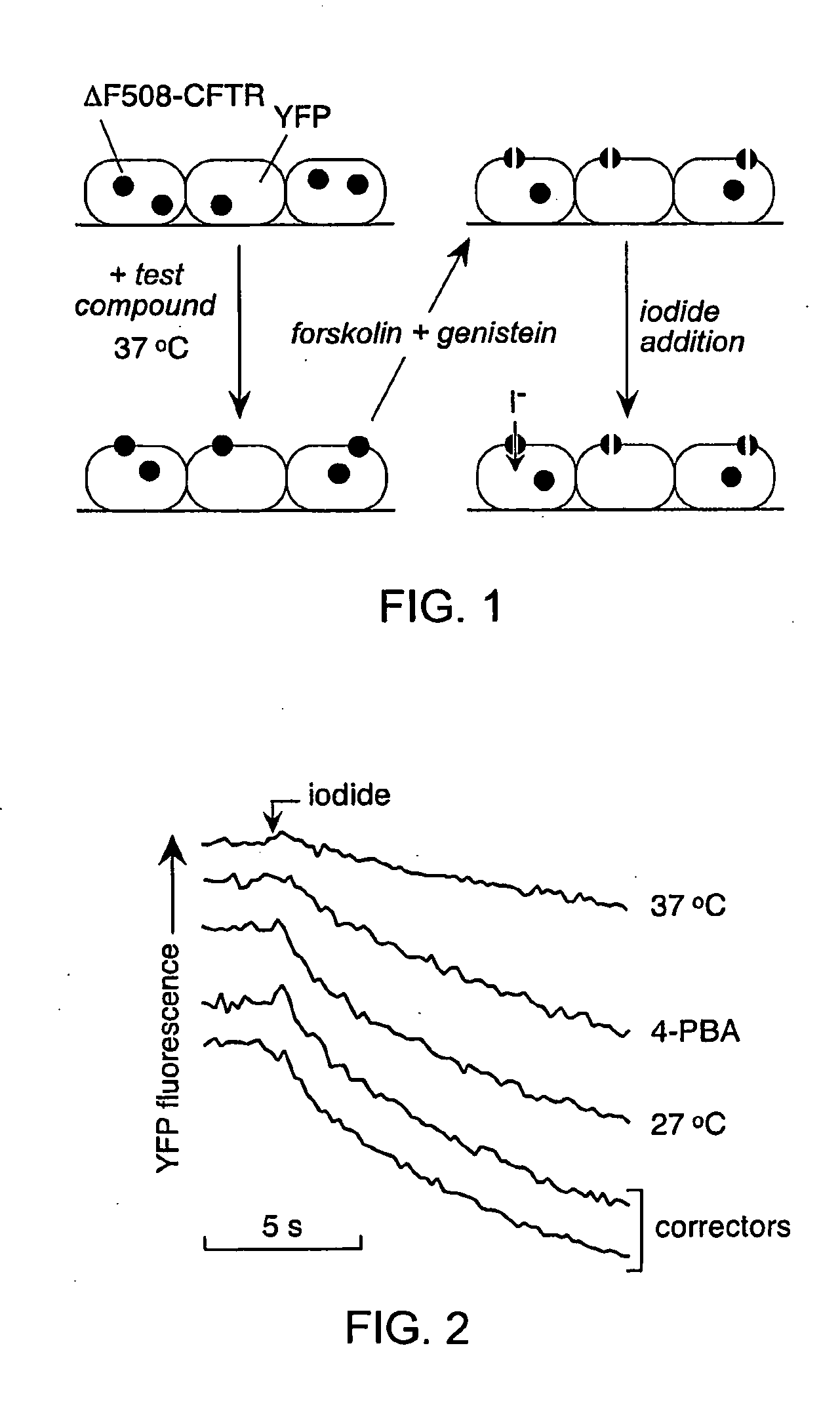
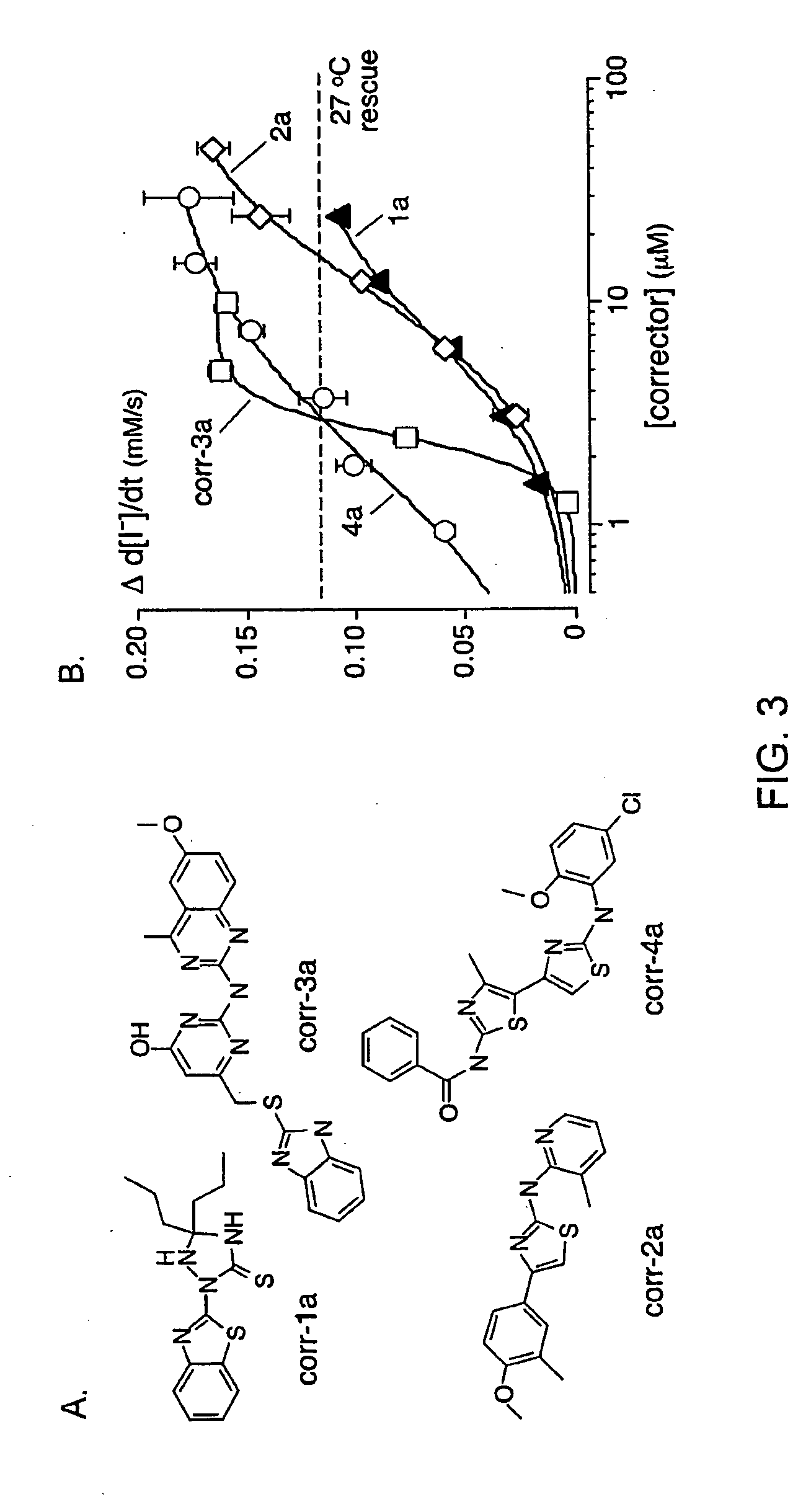
Compounds Having Activity in Correcting Mutant-Cftr Processing and Uses Thereof
ActiveUS20080318984A1Good cell permeabilityImprove ion permeabilityBiocideOrganic chemistryMutantCystic fibrosis lungs
The invention provides compositions, pharmaceutical preparations and methods for correcting cellular processing (e.g., folding, trafficking, or post-translational modification) of a mutant-cystic fibrosis transmembrane conductance regulator protein (e.g., ΔF508 CFTR) that are useful for the treatment of cystic fibrosis (CF). The compositions and pharmaceutical preparations of the invention may comprise one or more aminobenzothiazole-containing compounds, aminoarylthiazole-containing compounds, quinazolinylaminopyrimidinone-containing compounds, bisaminomethylbithiazole-containing compounds, or phenylaminoquino-line-containing compounds of the invention, or an analog or derivative thereof.
Owner:RGT UNIV OF CALIFORNIA
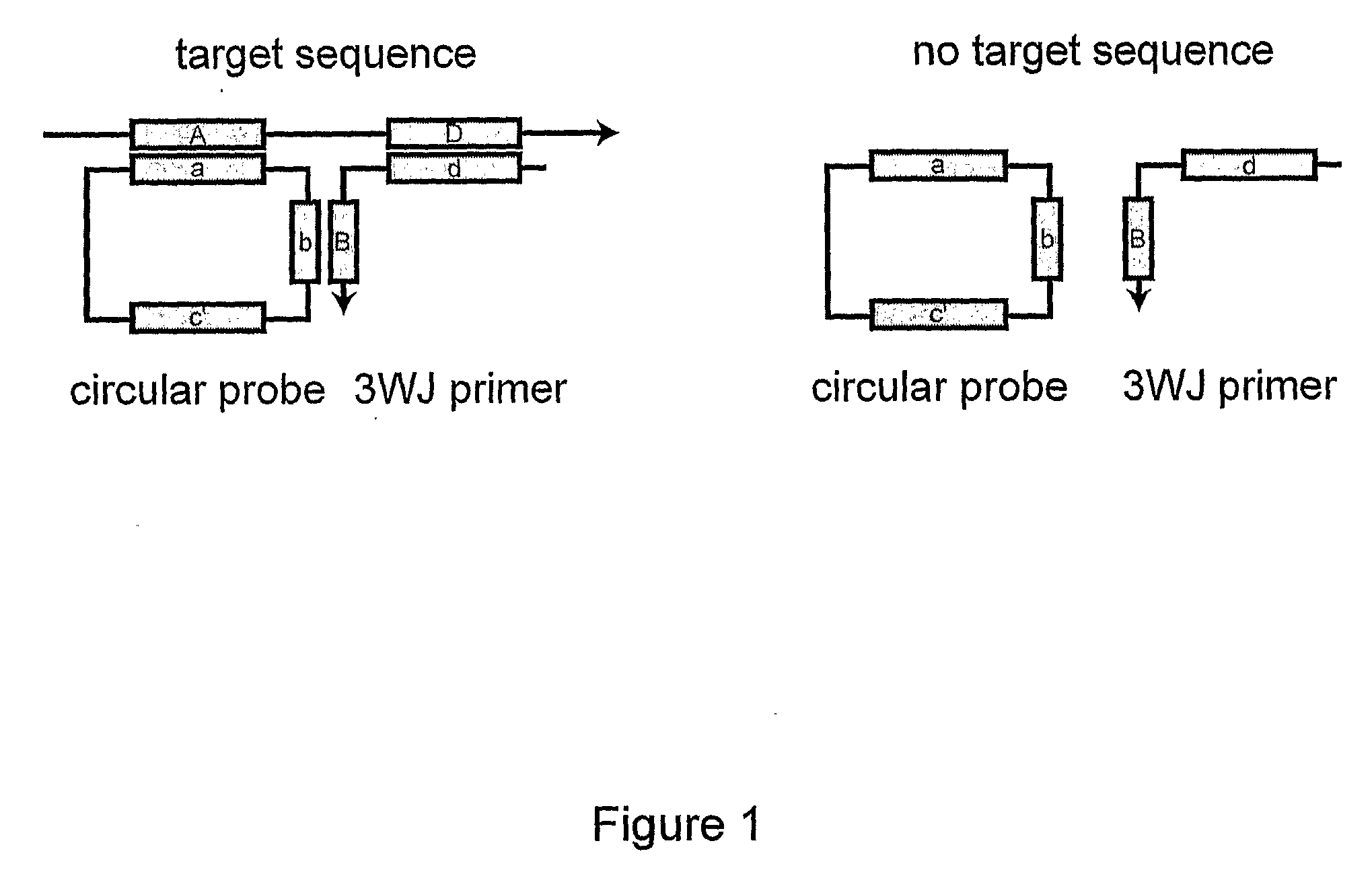
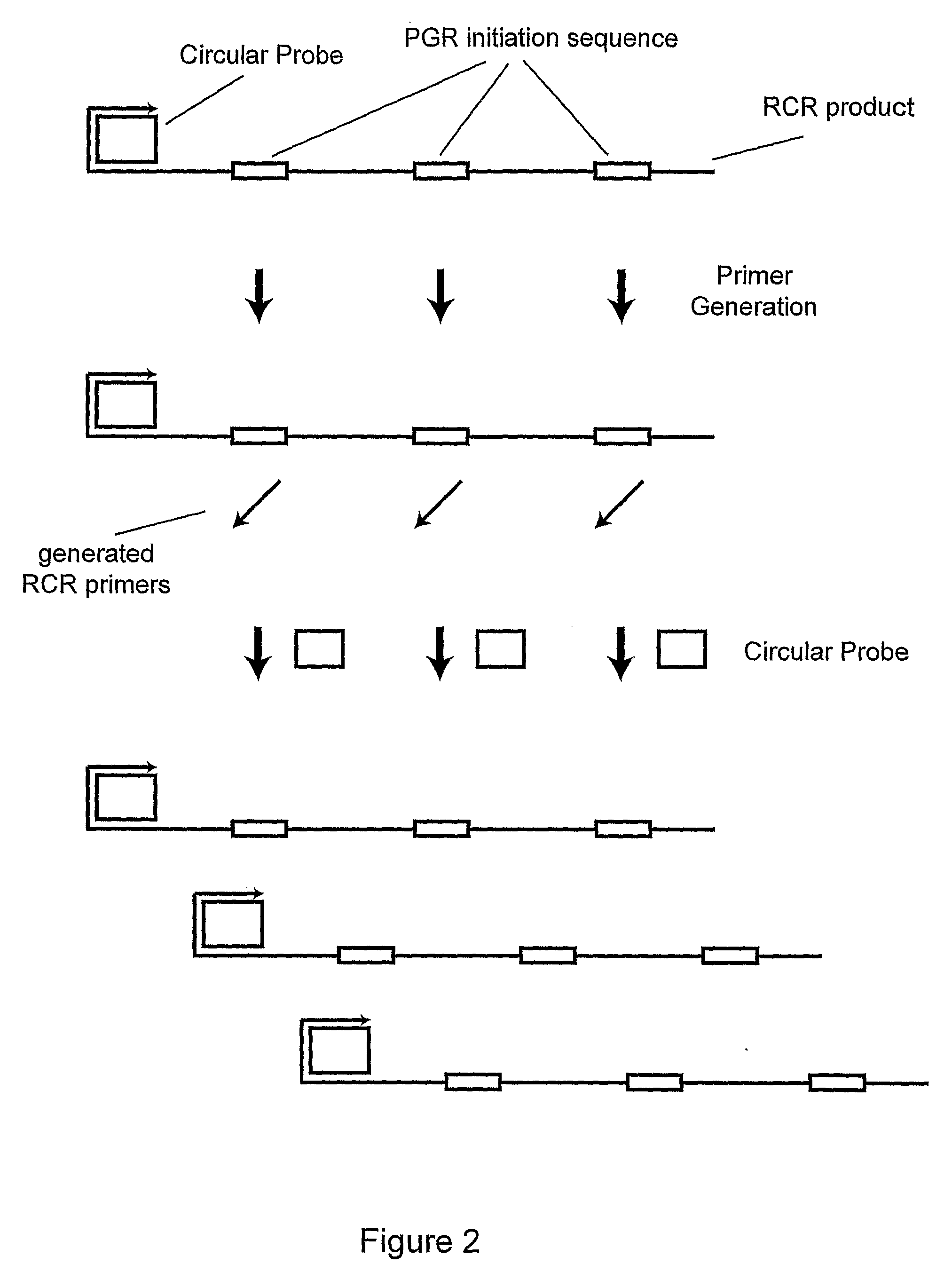
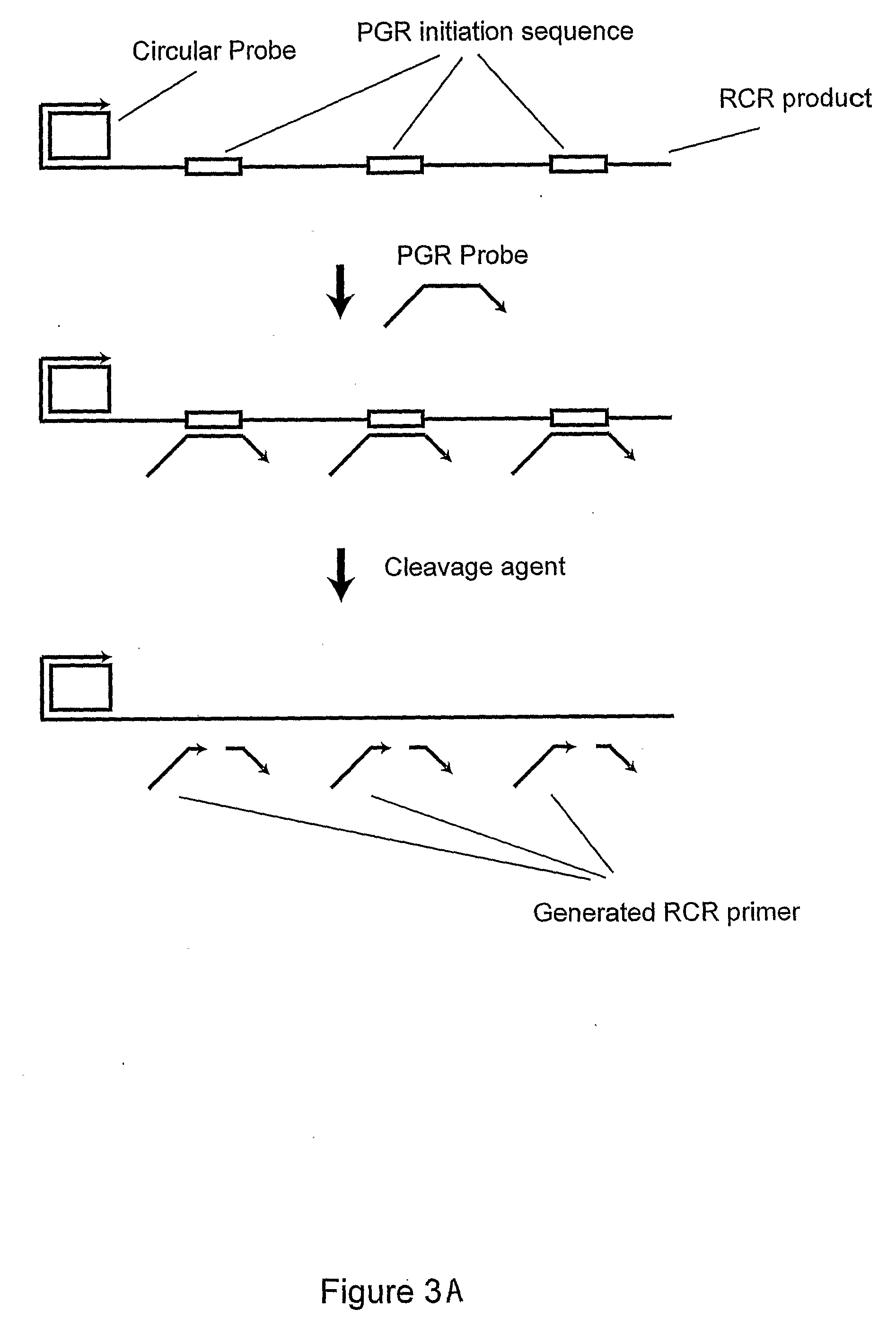
Primer generation rolling circle amplification
InactiveUS20090233277A1Sugar derivativesMicrobiological testing/measurementDNA methylationNucleotide
A method of amplifying a nucleic acid is provided which comprises: generating a first nucleic acid primer from a first nucleic acid sequence; combining the first nucleic acid primer with a first polymerase and a first circular nucleic acid probe, wherein the first circular nucleic acid probe contains at least one antisense sequence to a second nucleic acid sequence and at least one antisense sequence to the first nucleic acid primer; producing at least one repeat of a sequence copy of the first circular nucleic acid probe by rolling circle amplification using the first polymerase, wherein the sequence copy contains at least the second nucleic acid sequence; generating a second nucleic acid primer from the second nucleic acid sequence; combining the second nucleic acid primer with a second polymerase and a second circular nucleic acid probe, where the second circular nucleic acid probe contains at least one antisense sequence to the second nucleic acid primer; and producing at least one repeat of a sequence copy of the second circular nucleic acid probe by rolling circle amplification using the second polymerase. The method may be employed to detect molecules of interest such as nucleic acid sequences, DNA methylation, single nucleotide polymorphisms (SNP), proteins and posttranslational modifications. Furthermore, a ribbon probe is provided that comprises a circular nucleic acid probe and a nucleic acid lock probe, wherein: the nucleic acid lock probe contains at least a cleavable linker, and the circular nucleic acid probe and the lock probe are unable to dissociate without cleaving the cleavable linker.
Owner:HITACHI CHEM CO LTD +1
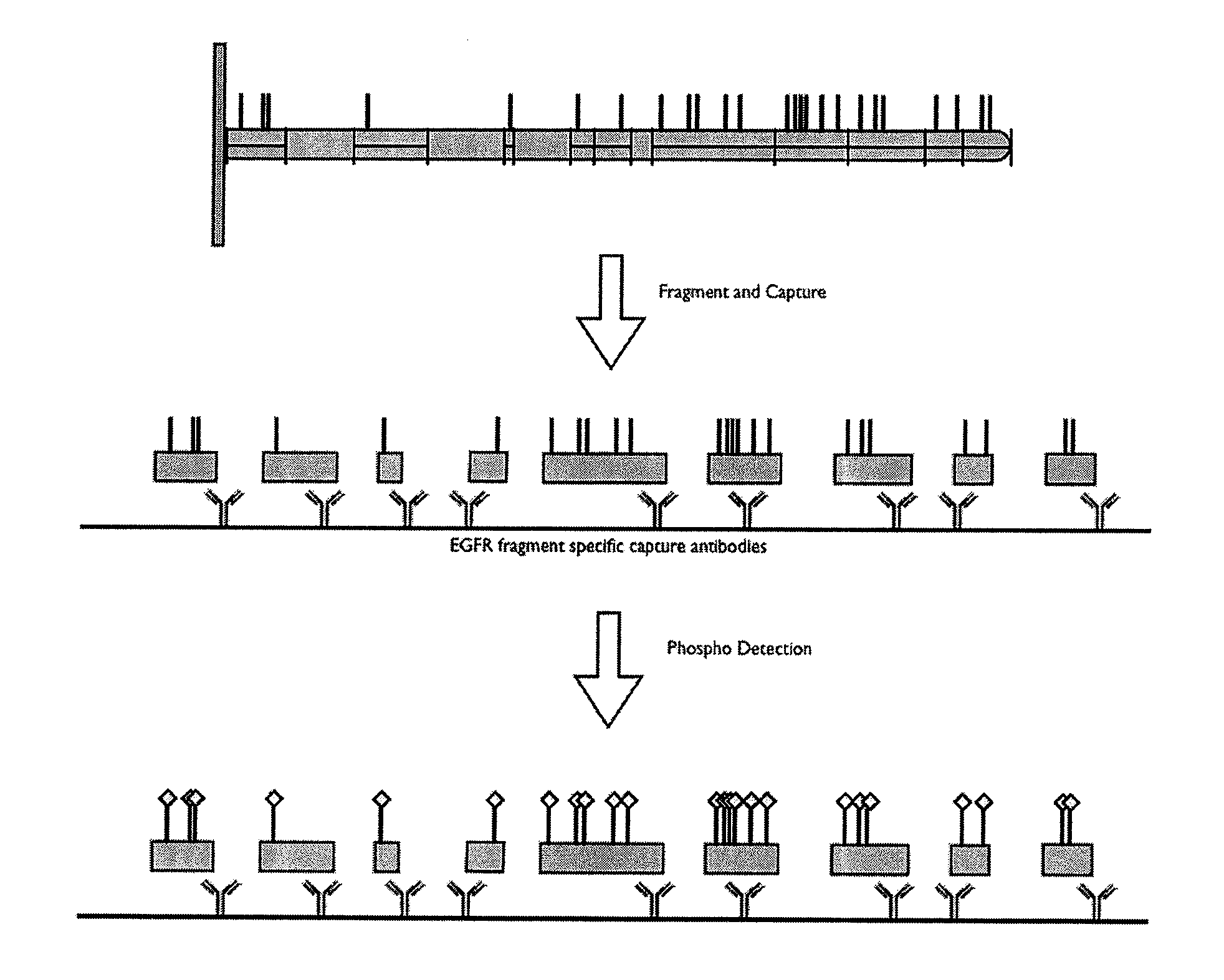
Post translational modification pattern analysis
InactiveUS20100331200A1Increase flexibilityReliably retrievedLibrary screeningBiological testingPost translationalProtein target
This invention relates to methods and apparatus for detecting the pattern of post translational modification in a protein or in a plurality of proteins in a sample. One or more target proteins are subjected to predetermined proteolysis to yield plural peptide fragments comprising potential post translational modification sites. The fragments and the state of such sites are analyzed to yield a post translational pattern for the protein or proteins.
Owner:MILLIPORE CORP
Preparation method of recombinant porcine circovirus type 2 Cap antigen
InactiveCN102127533AEasy to operateSimple and fast operationViral antigen ingredientsMicroorganism based processesEscherichia coliTGE VACCINE
The invention discloses a preparation method of a recombinant porcine circovirus type 2 Cap antigen, which comprises the following steps: using a yeast expression system to amplify the PCV2-ORF2 gene; then, constructing an expression engineering bacterium: inserting an amplification sequence into a yeast expression vector to form a recombinant expression vector, linearizing, and transforming into a pichia pastoris host cell so as to finish the construction of the expression engineering bacterium; and finally, carrying out secretory expression on the recombinant microzyme to obtain the recombinant porcine circovirus type 2 Cap antigen protein. The yeast expression system can carry out processing, folding and posttranslational modification on the expressed protein, so that the expressed protein has biological activity. Compared with Escherichia coli, the yeast expression system has more advantages; and compared with a mammal cell expression system, the yeast expression system is more convenient to operate. Compared with Saccharomyces cerevisiae, the pichia pastoris can not be easily subjected to hyperglycosylation, thereby facilitating the separation and purification steps. By using the yeast expression system, the invention has the advantages of simple operation process, high expression quantity and low production cost, can implement large-scale production, and is beneficial to the popularization and application of the porcine circovirus subunit vaccine.
Owner:SOUTH CHINA AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com