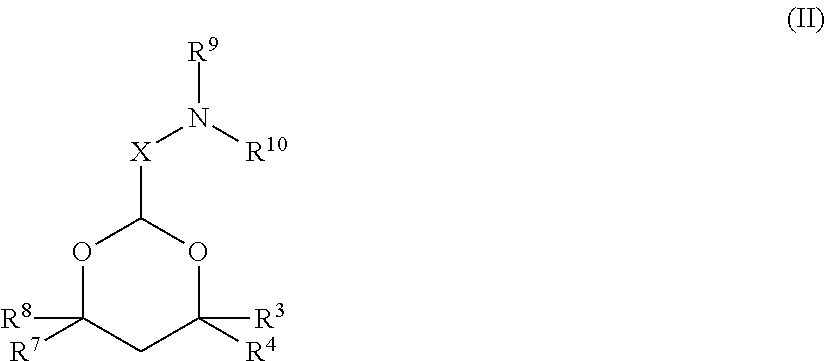Patents
Literature
369 results about "Therapeutic index" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
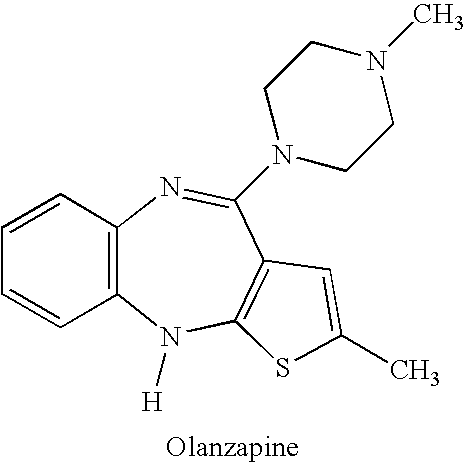
The therapeutic index (TI; also referred to as therapeutic ratio) is a quantitative measurement of the relative safety of a drug. It is a comparison of the amount of a therapeutic agent that causes the therapeutic effect to the amount that causes toxicity.The related terms therapeutic window or safety window refer to a range of doses which optimize between efficacy and toxicity, achieving the greatest therapeutic benefit without resulting in unacceptable side-effects or toxicity.
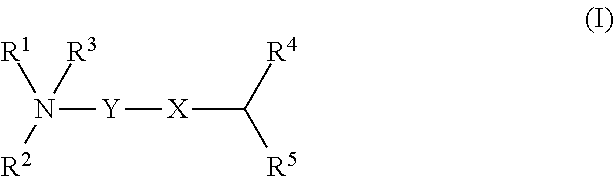
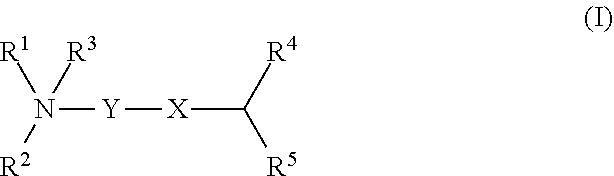
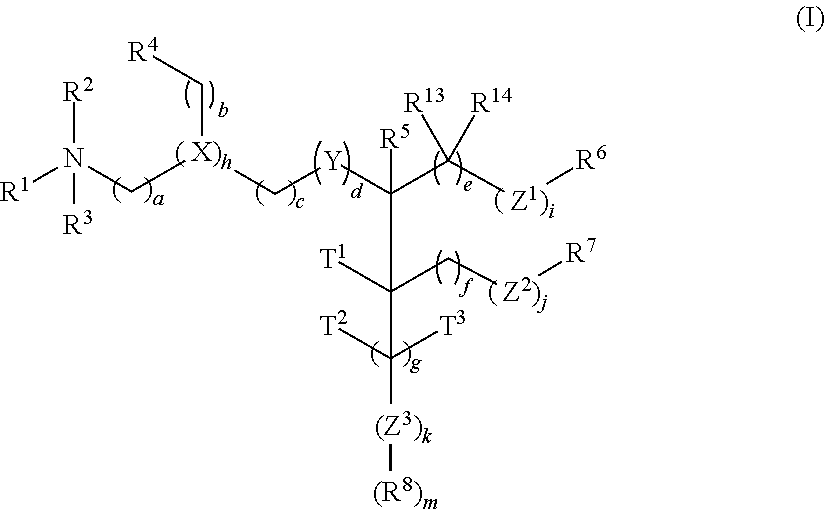
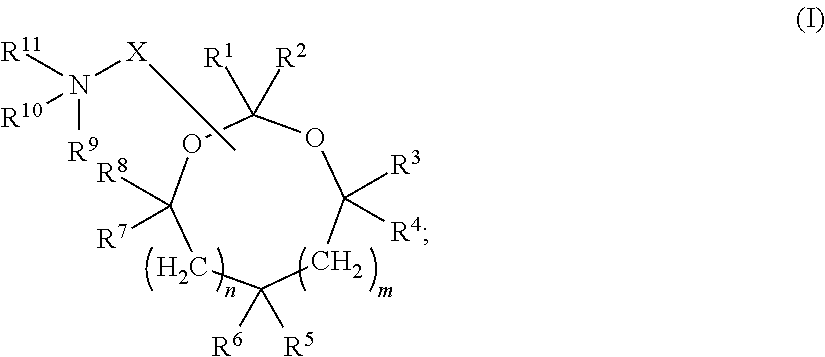
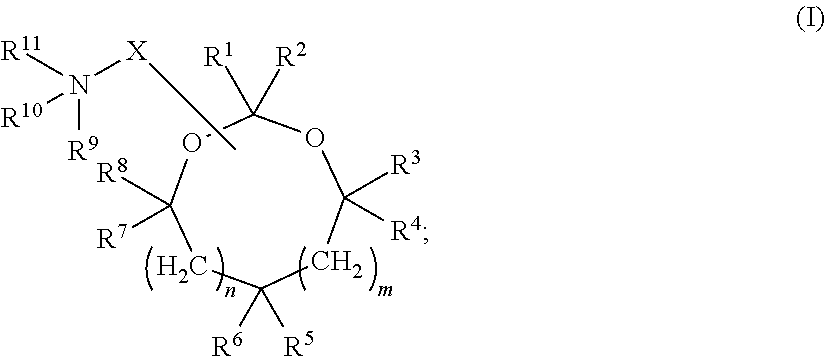
Cationic lipids and methods for the delivery of therapeutic agents
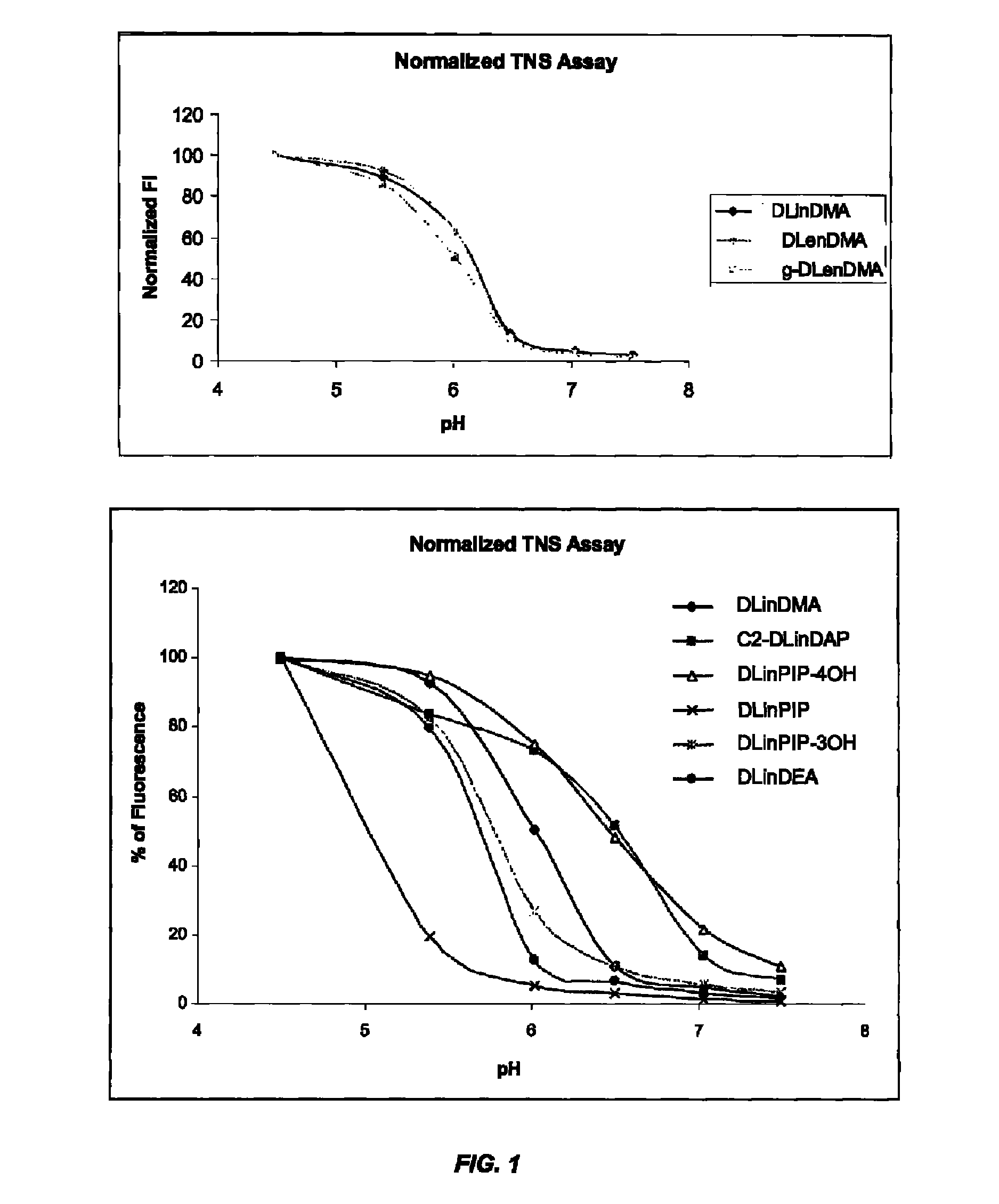
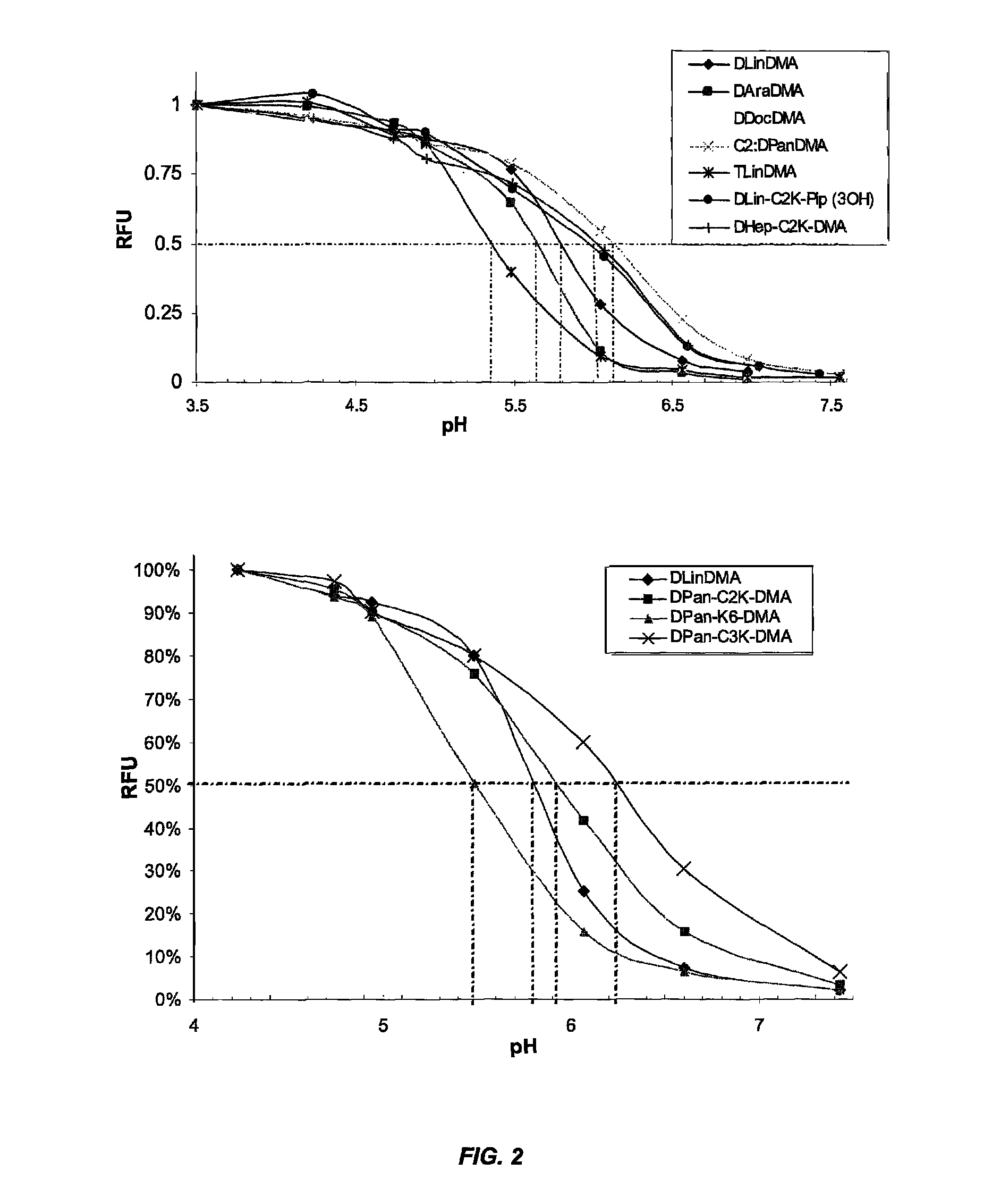
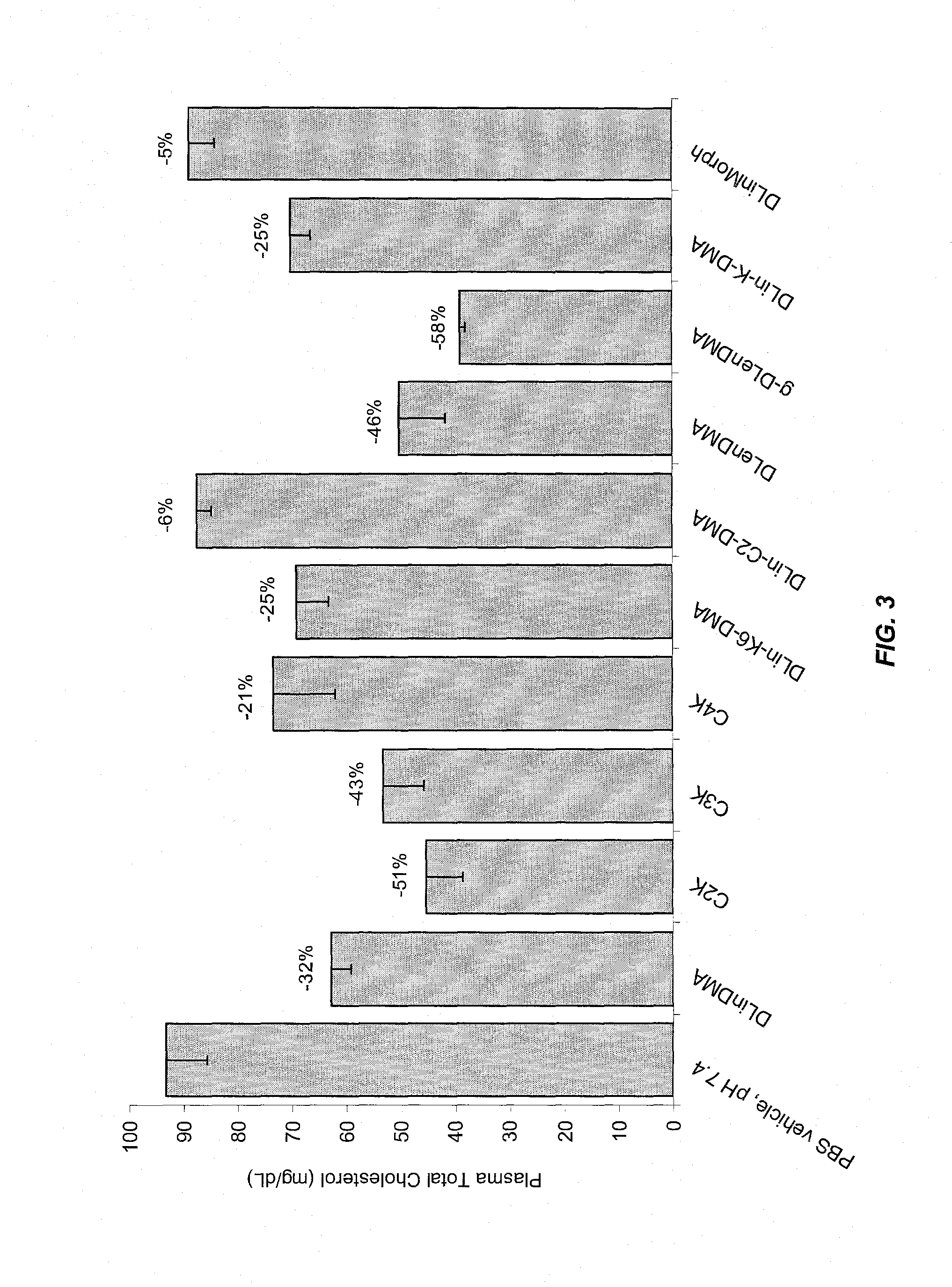
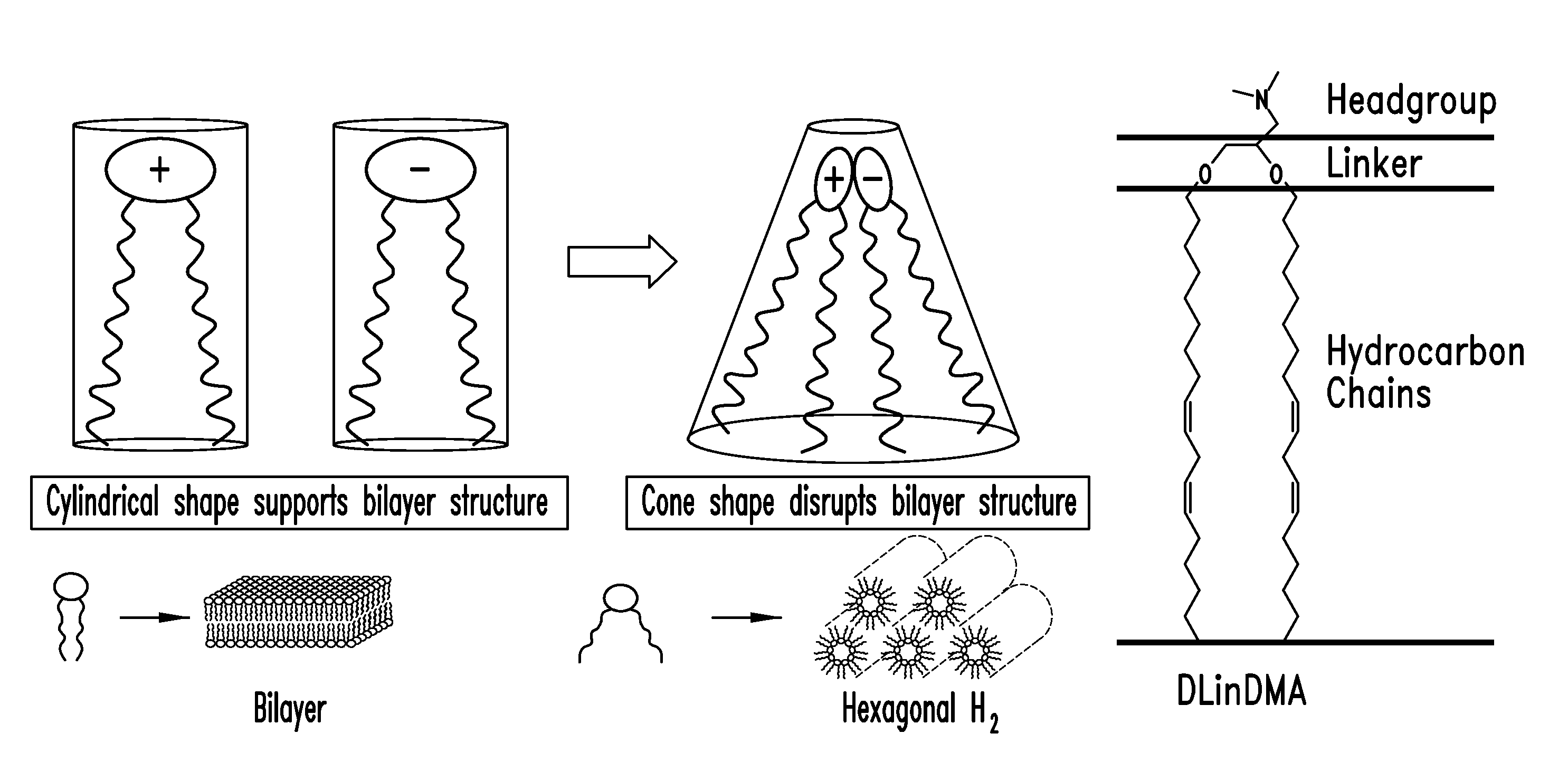
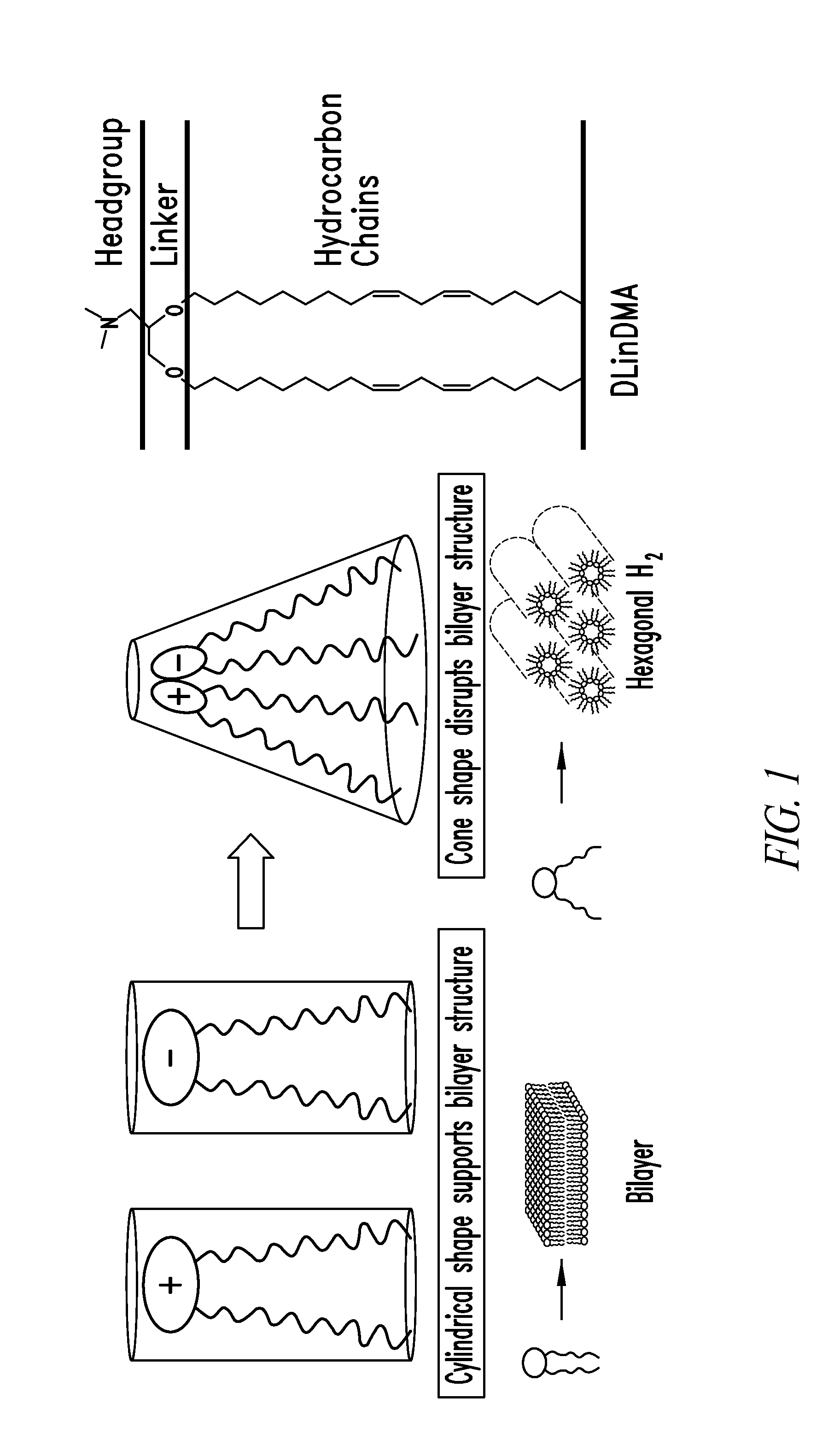
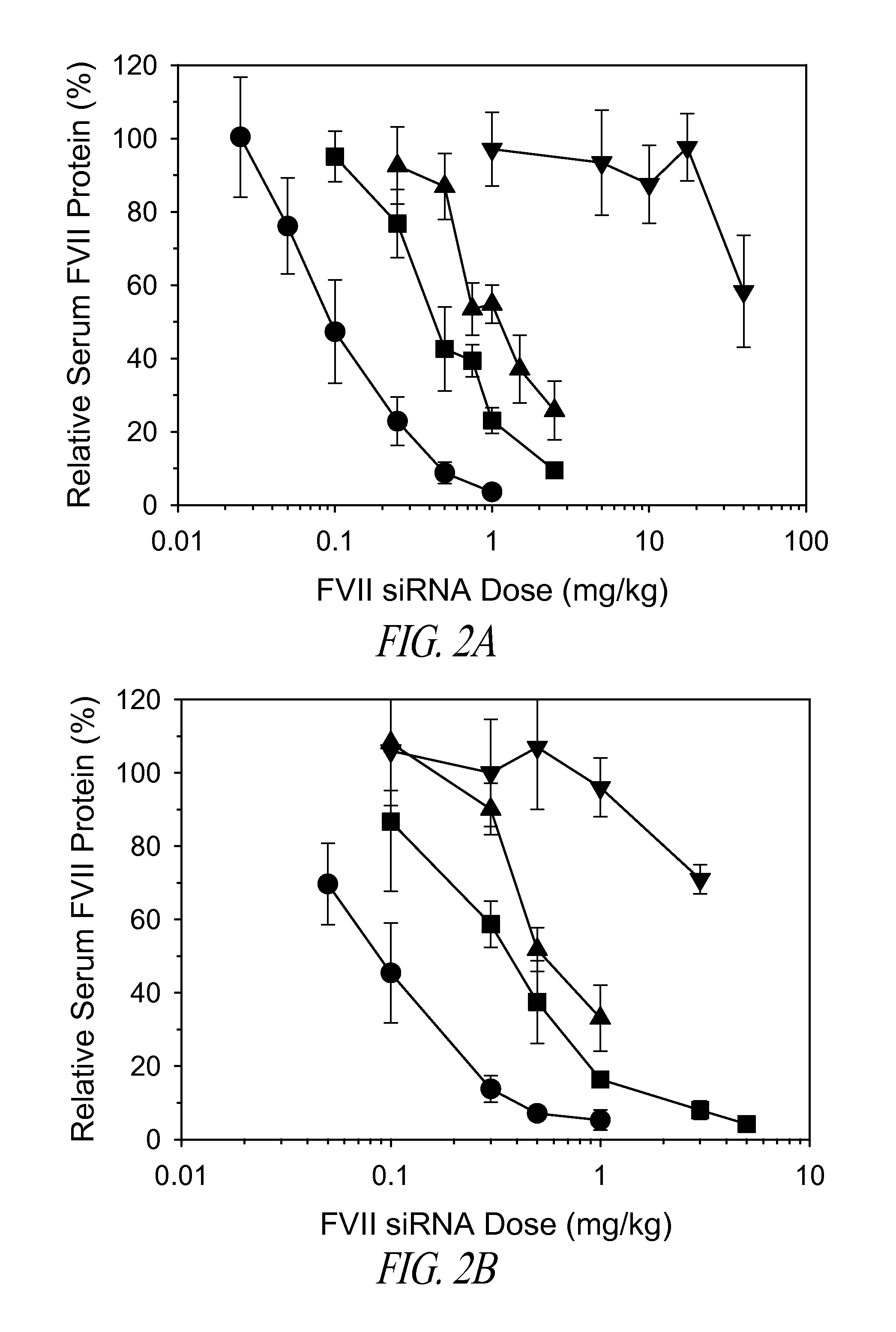
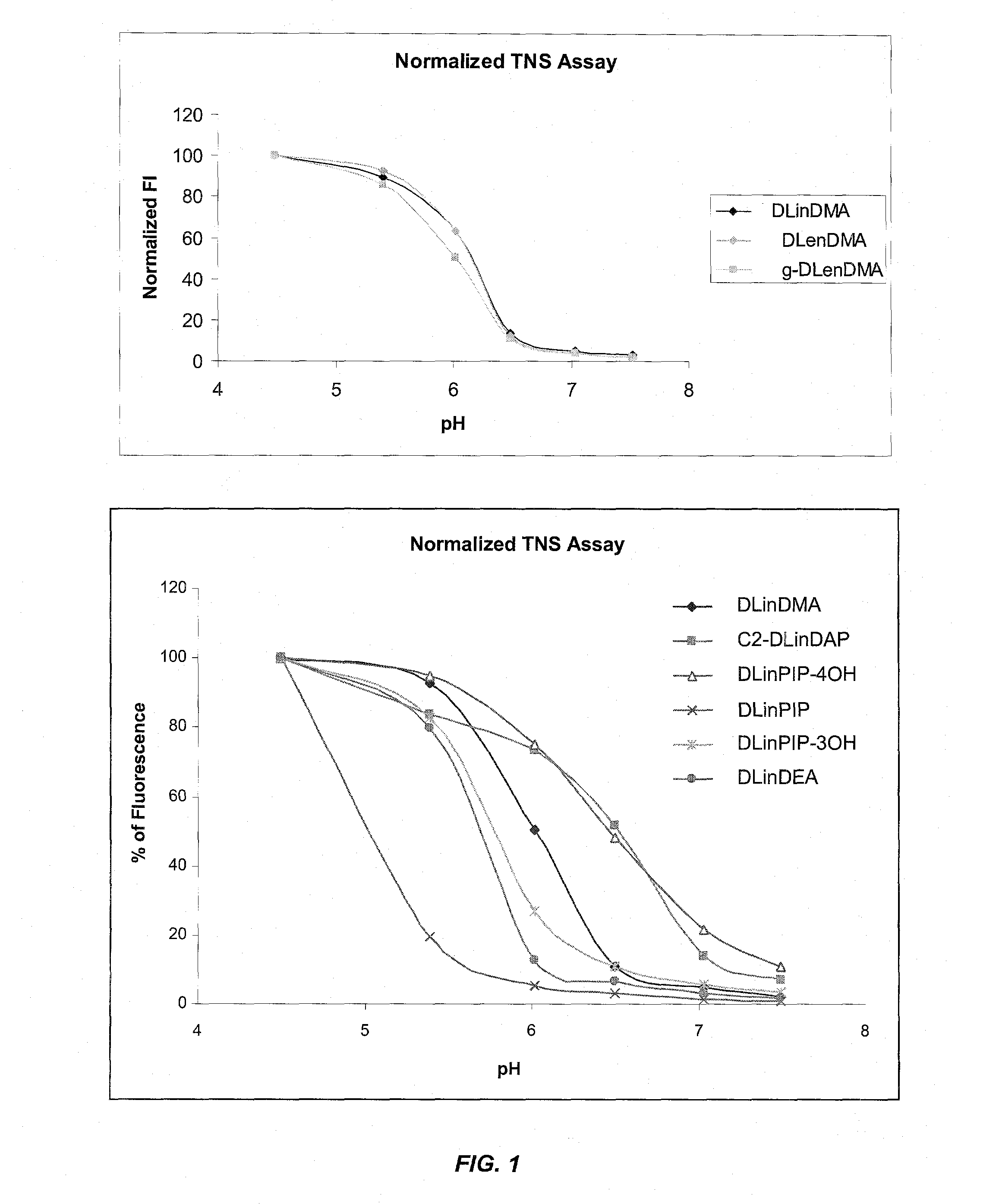
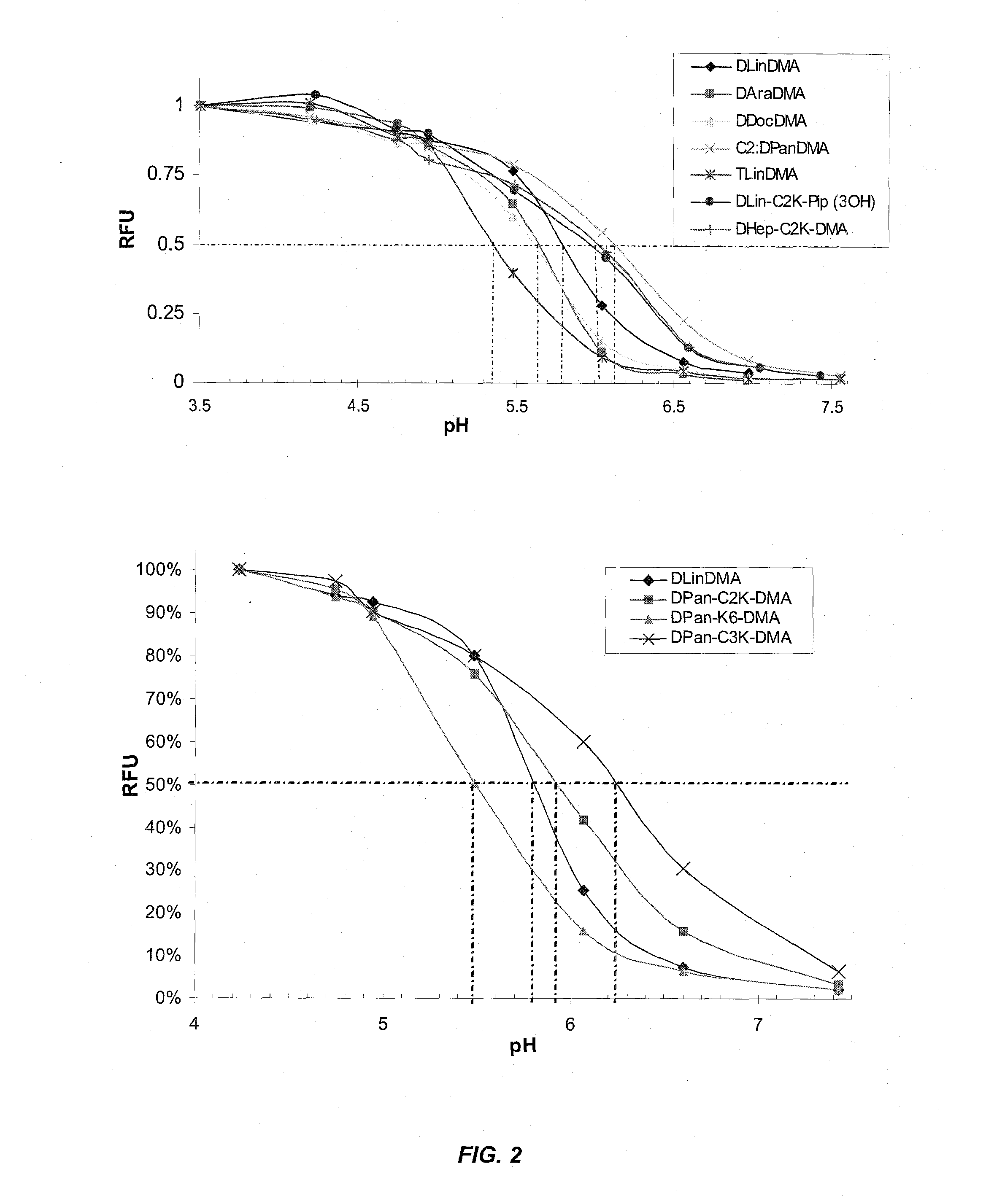
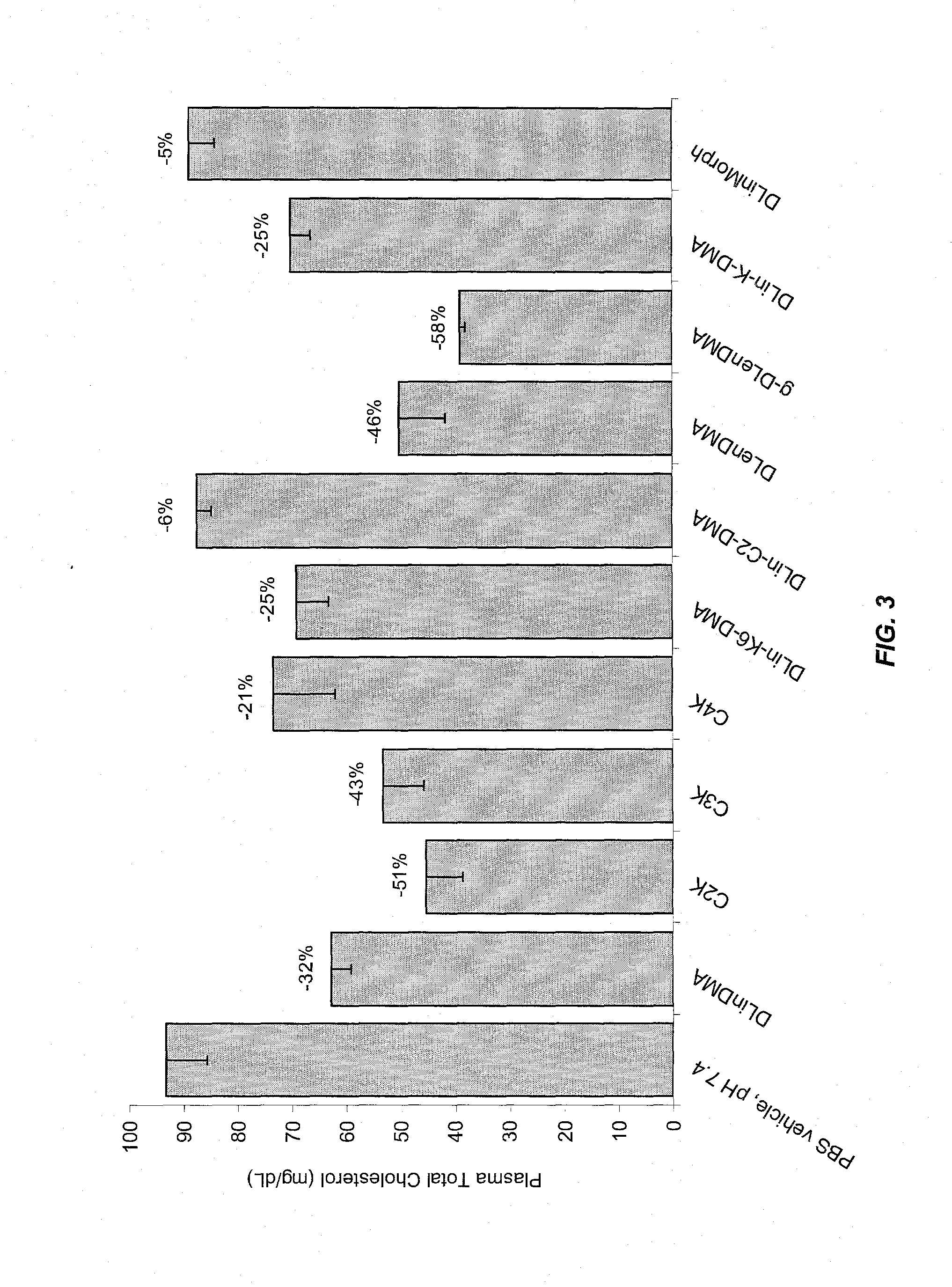
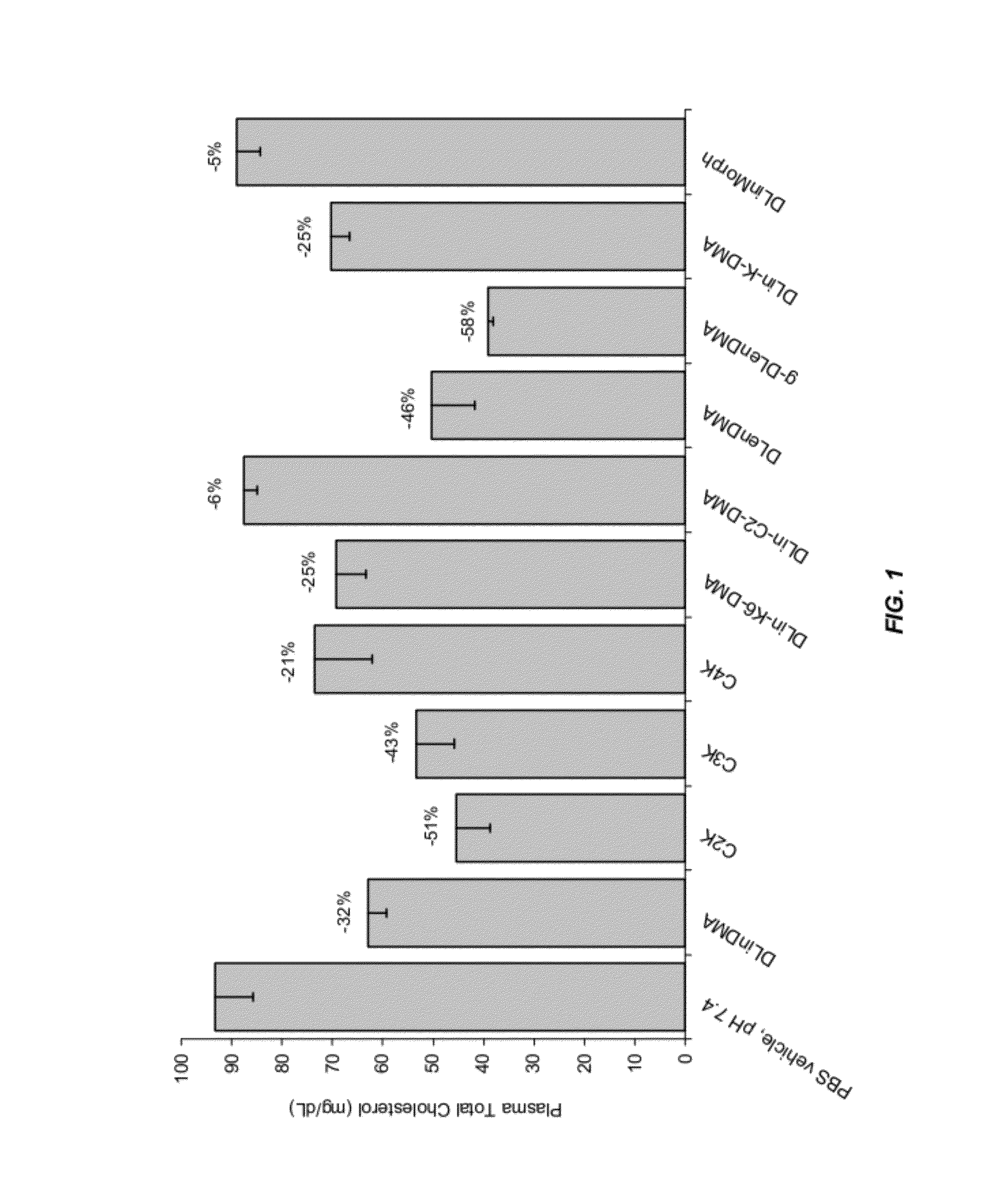
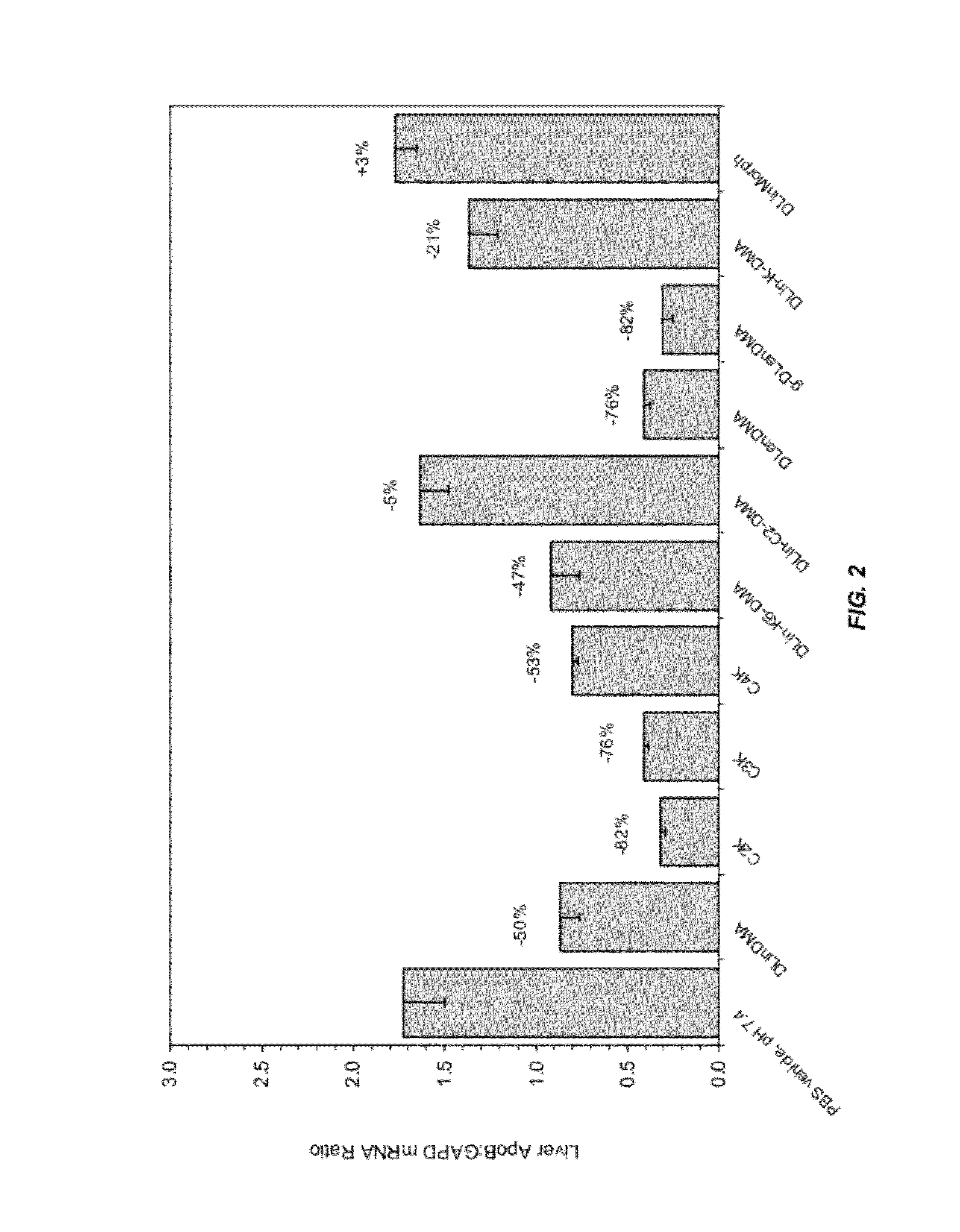
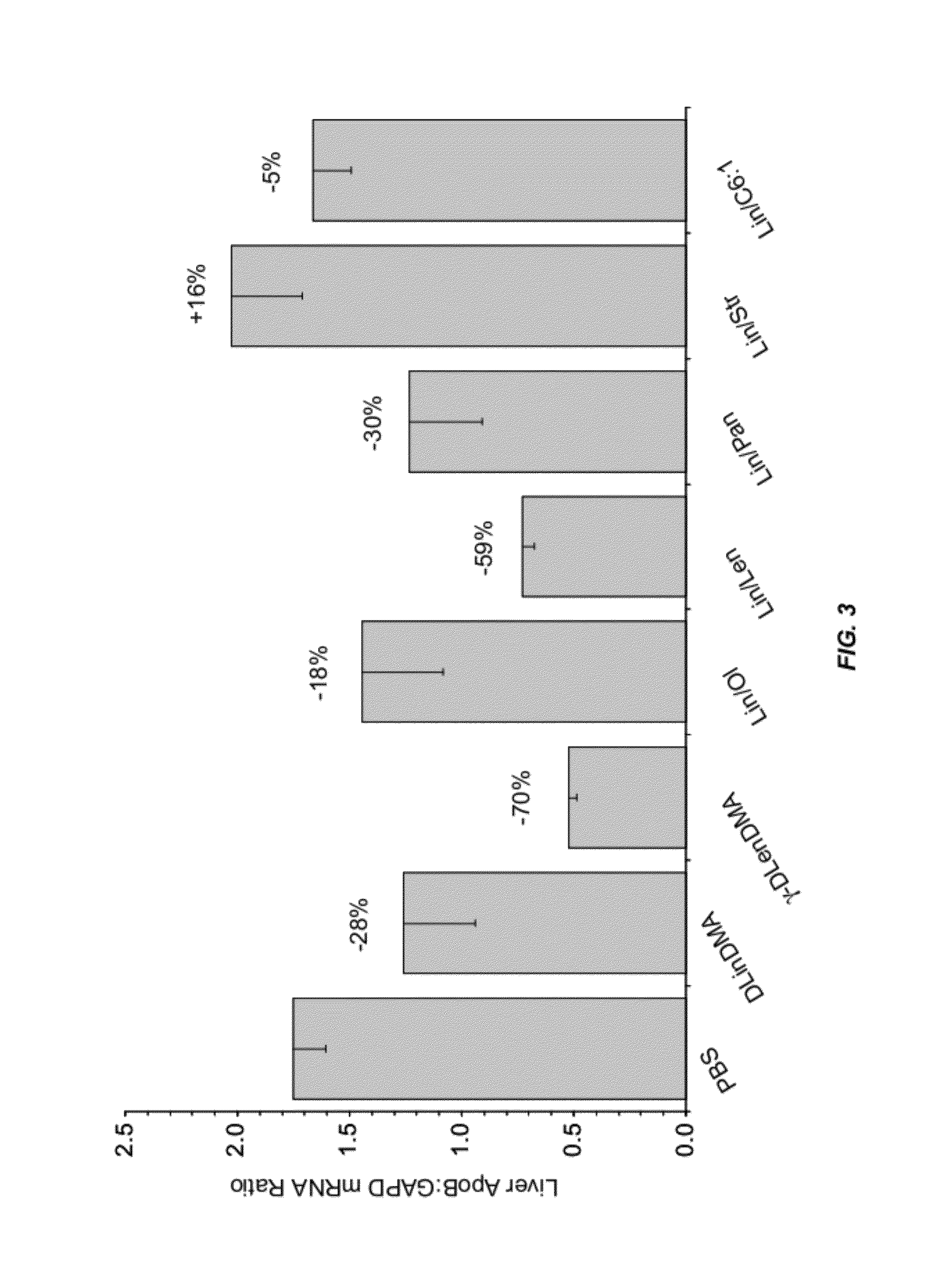
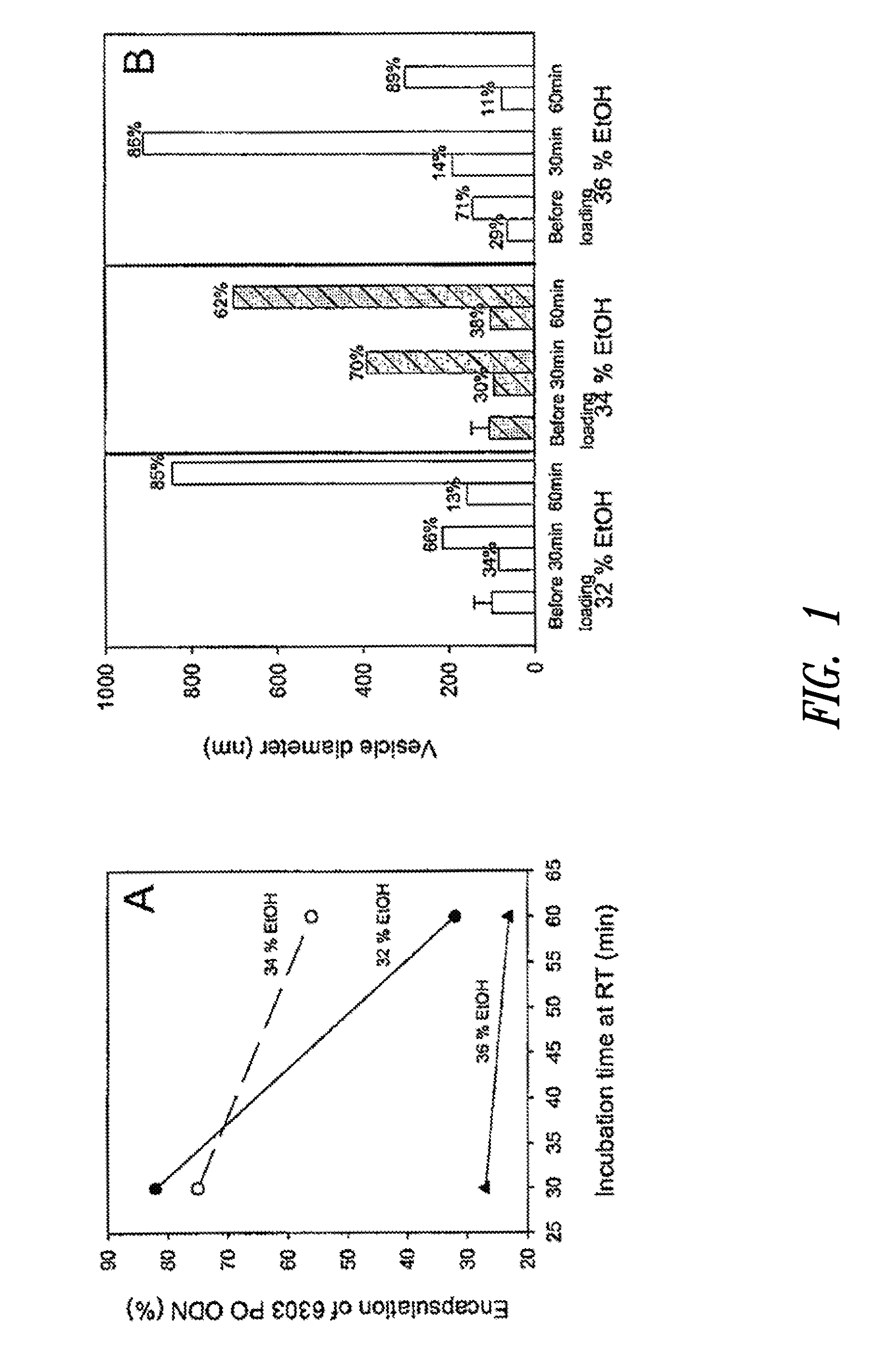
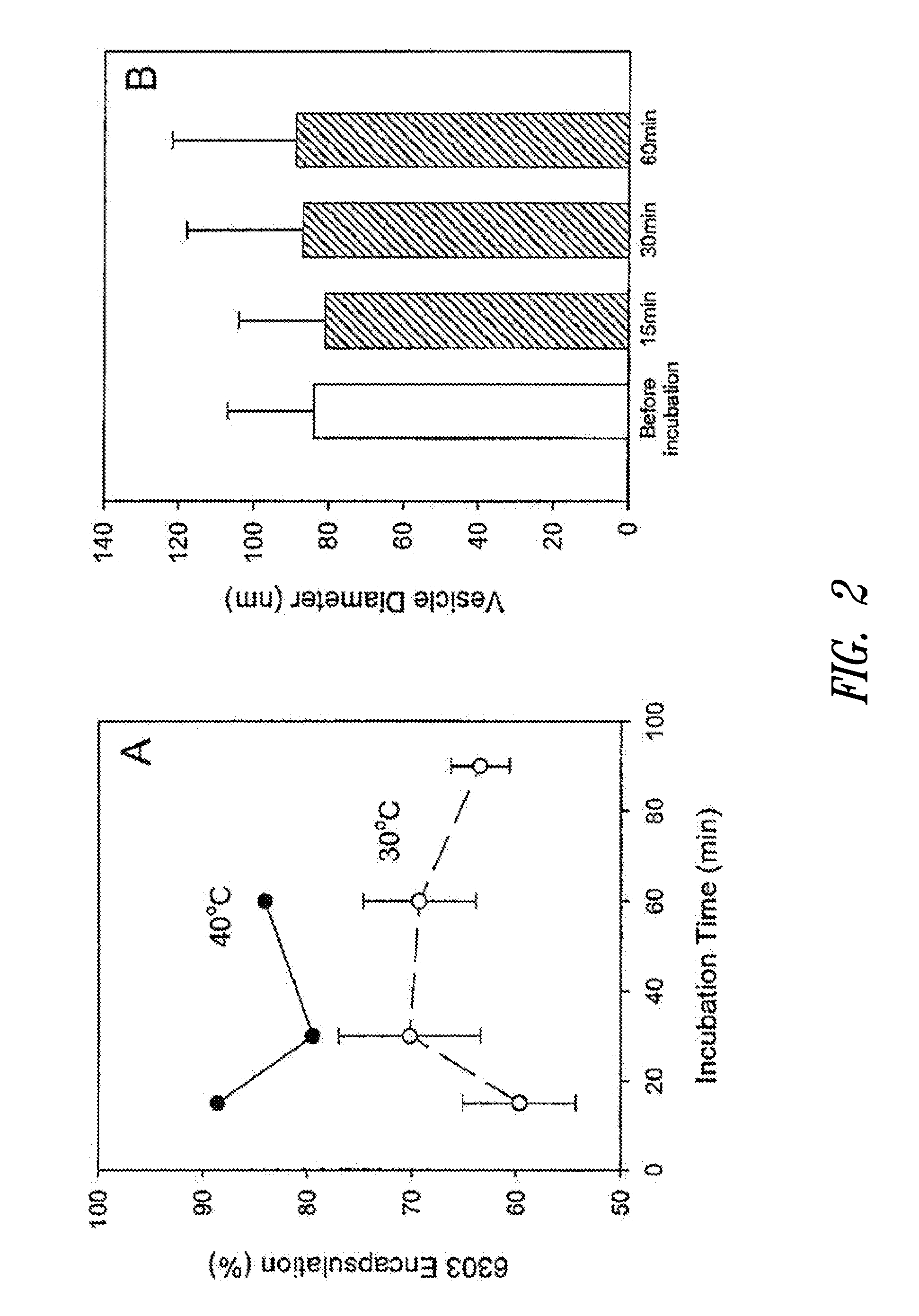
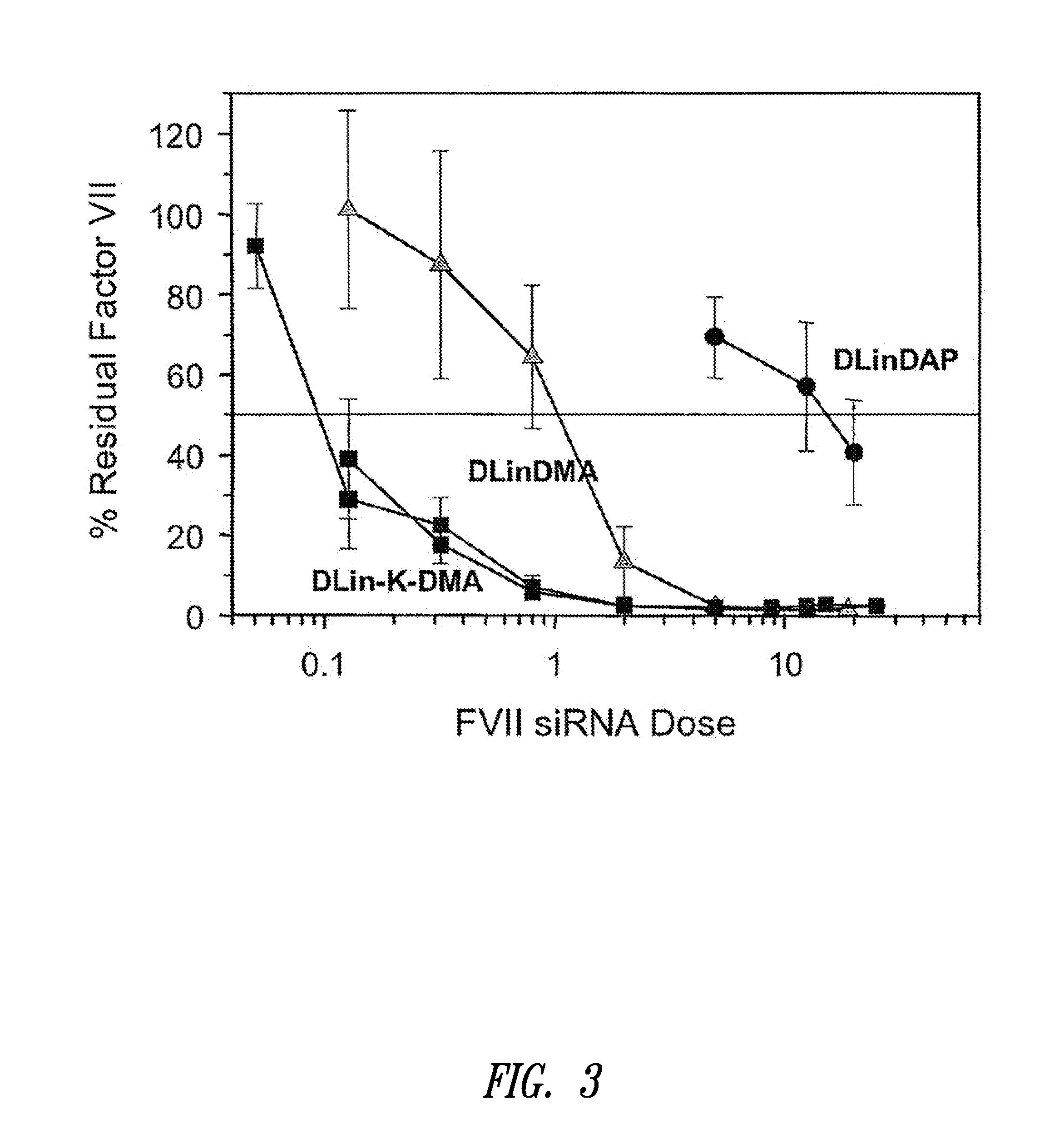
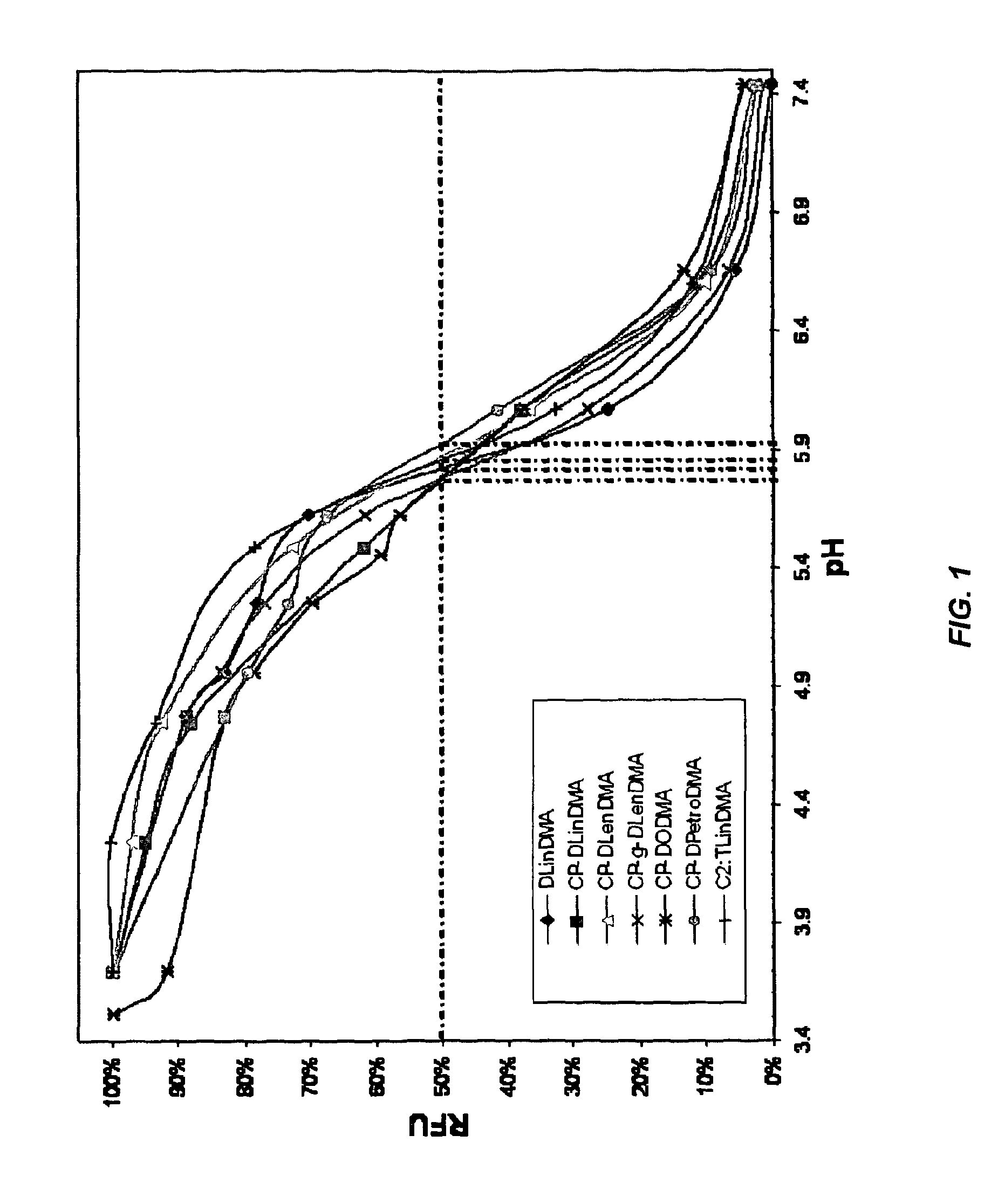
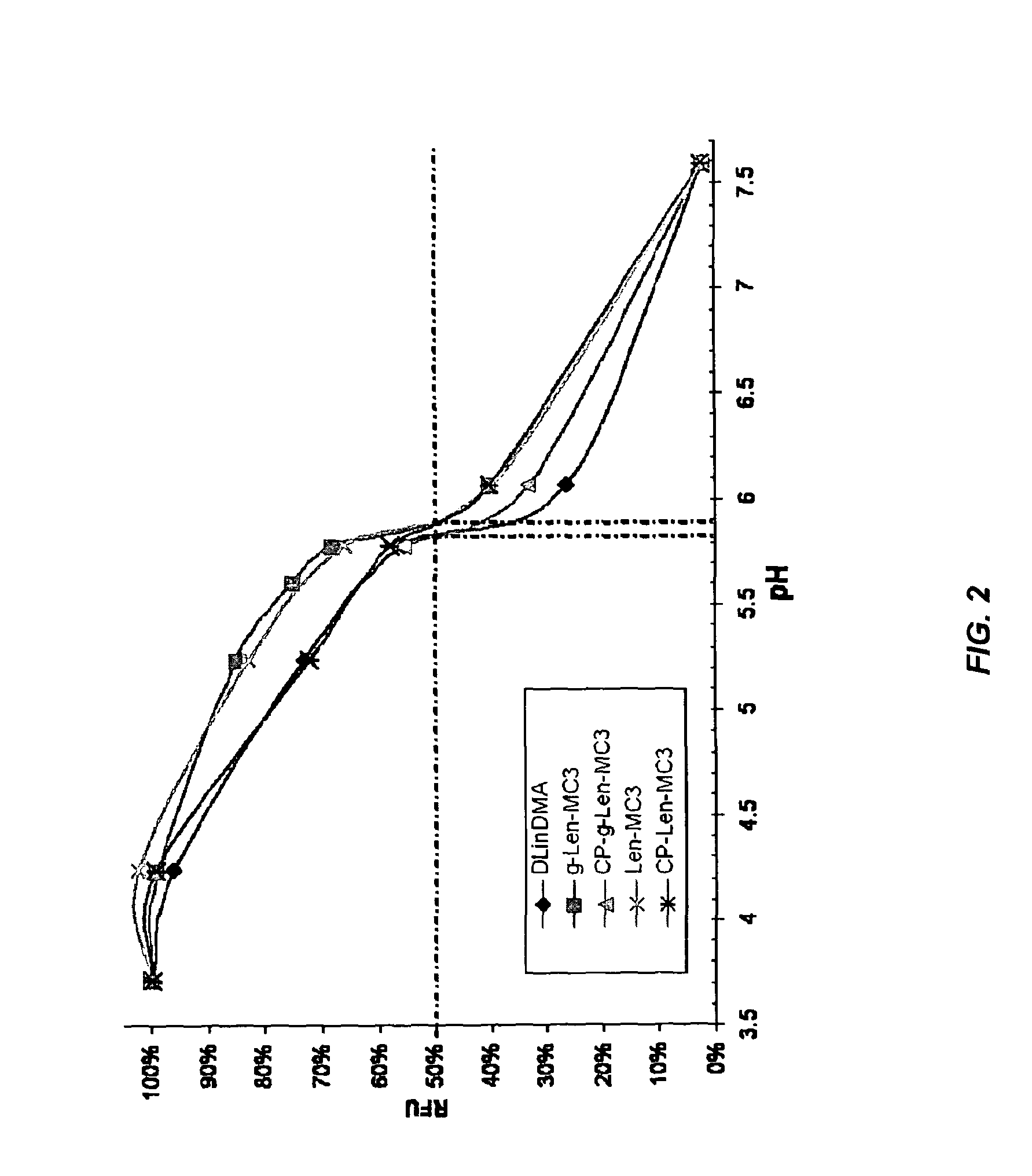
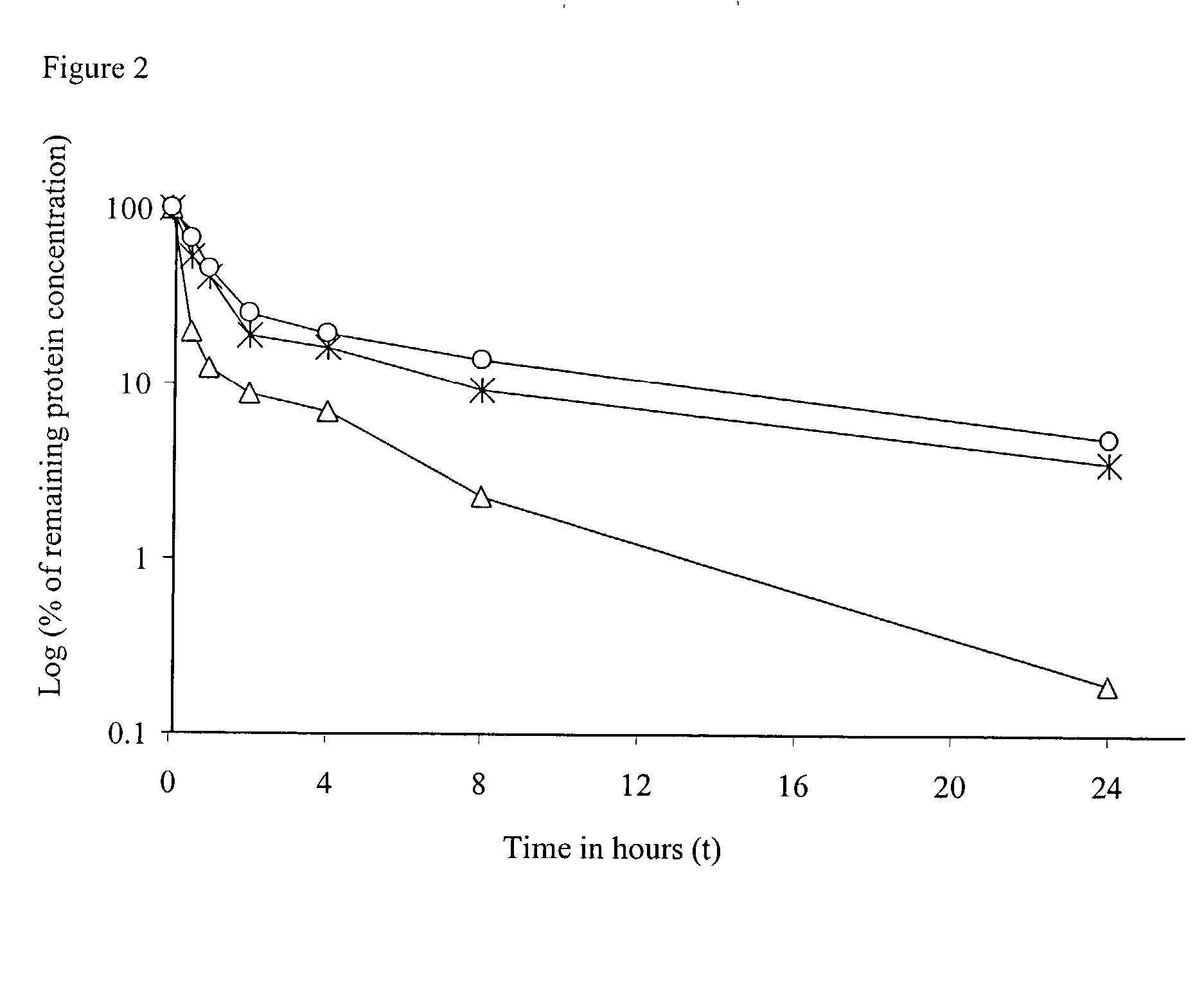
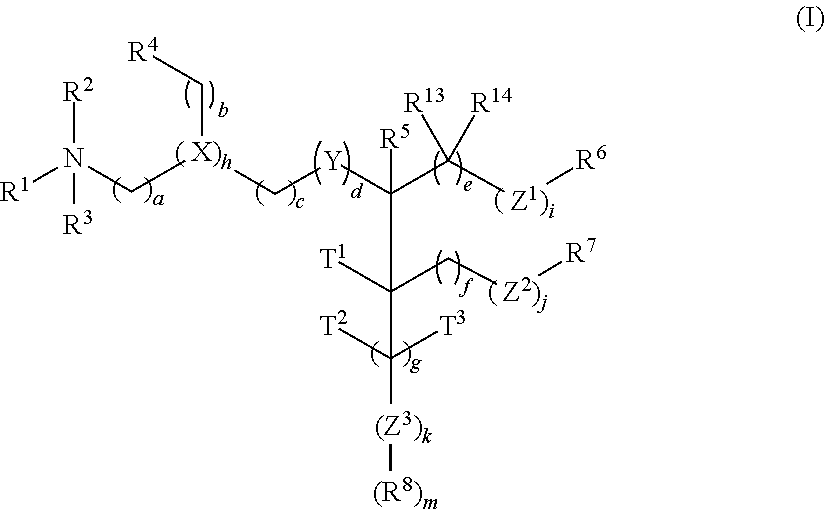
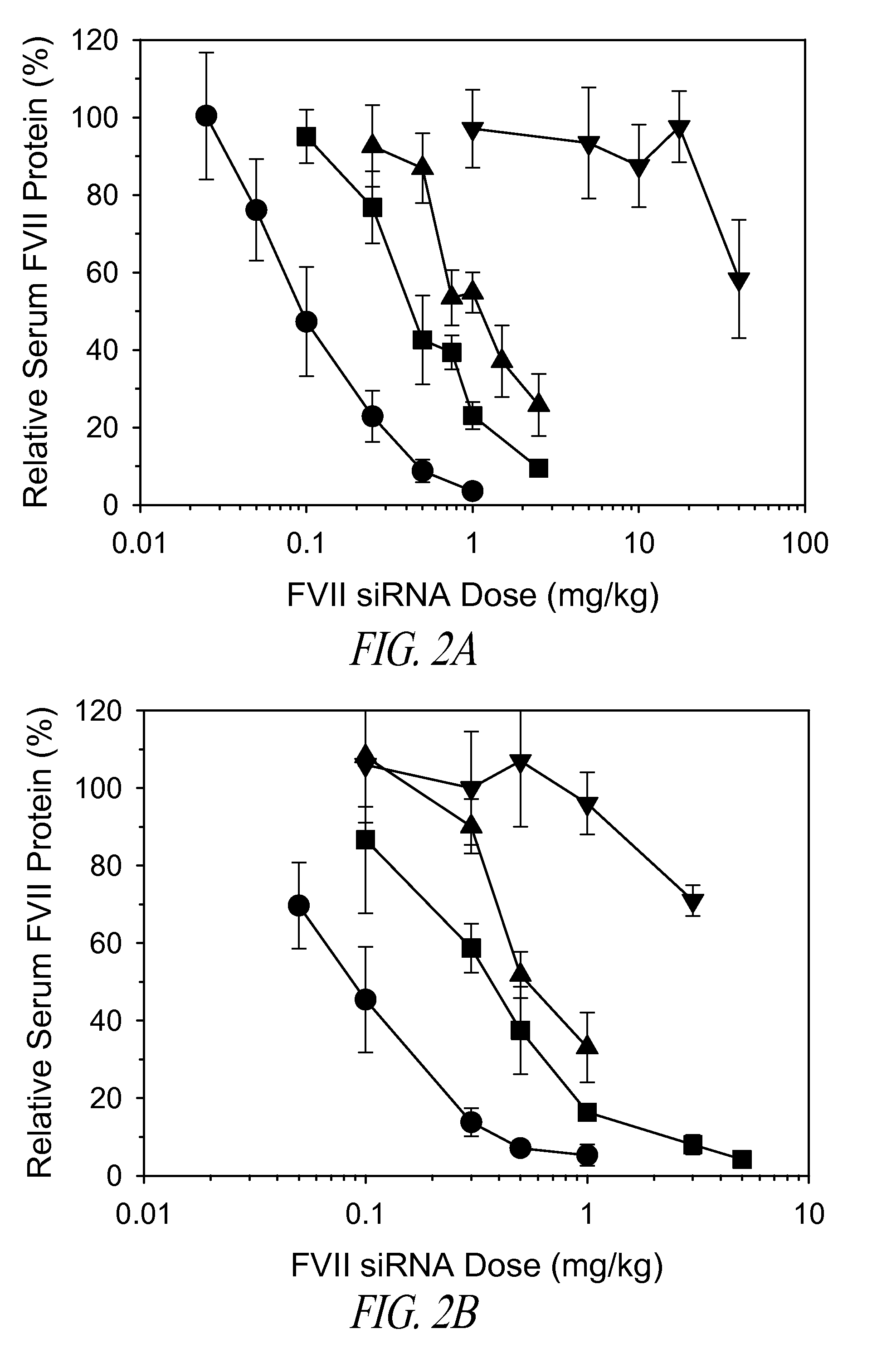
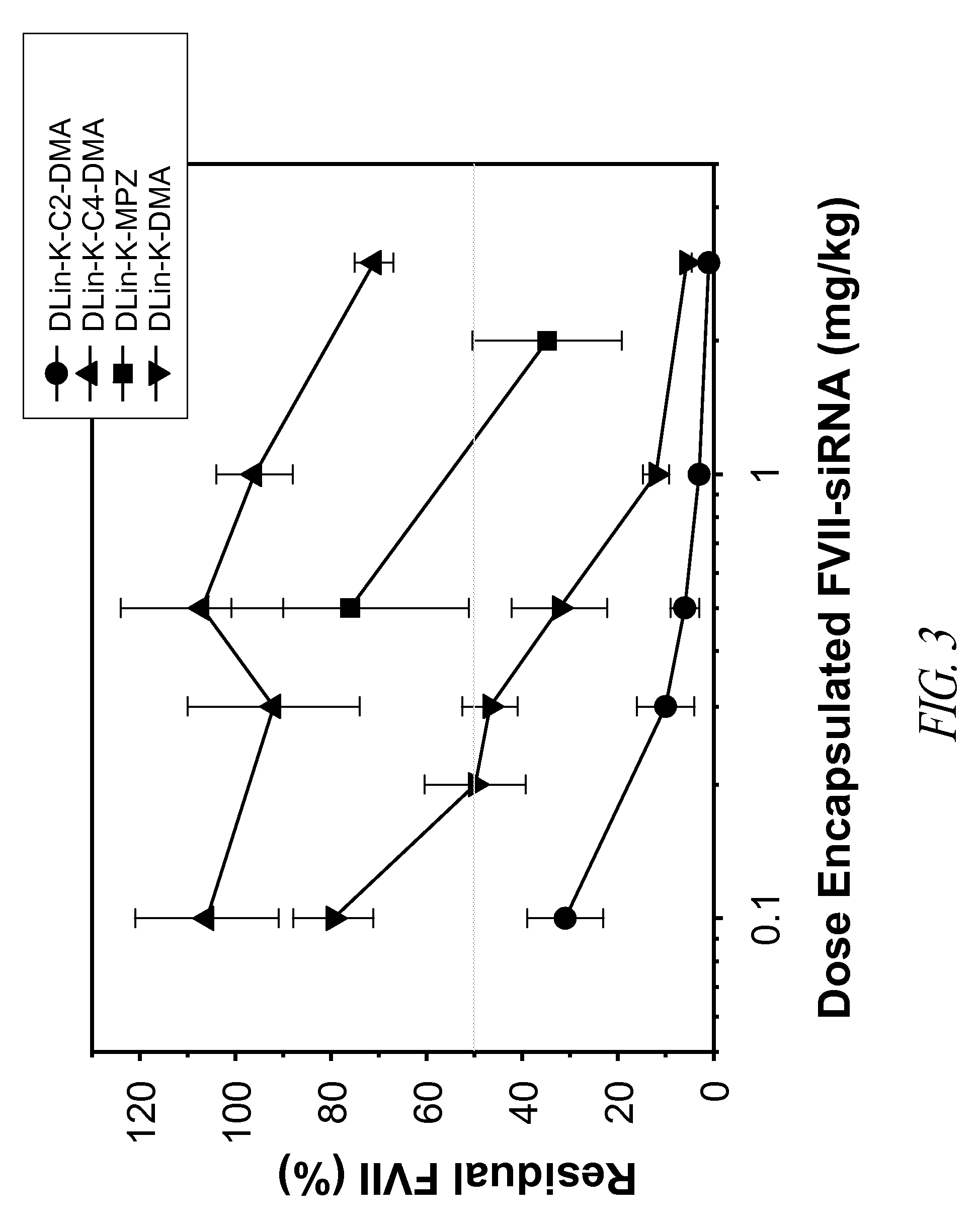
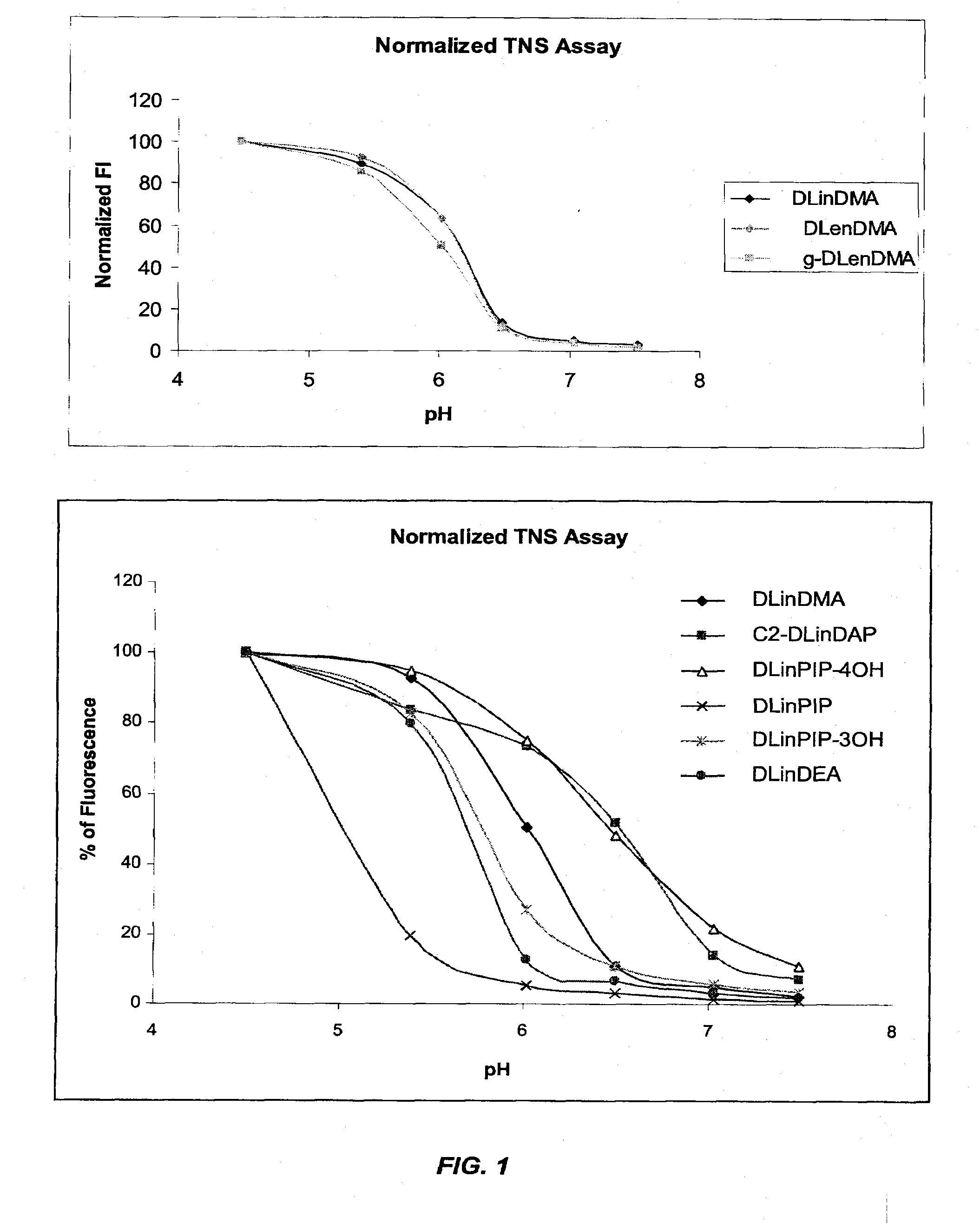
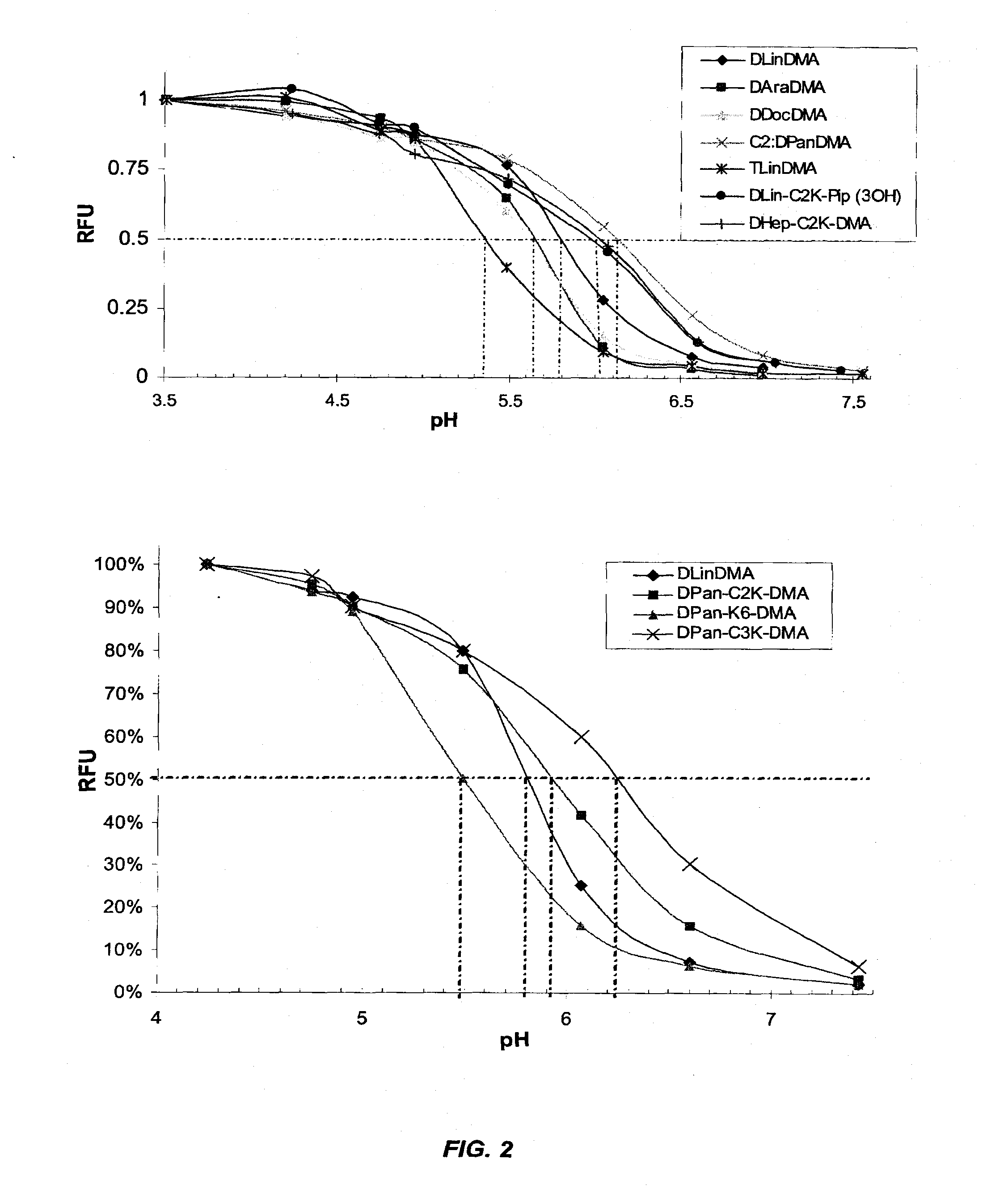
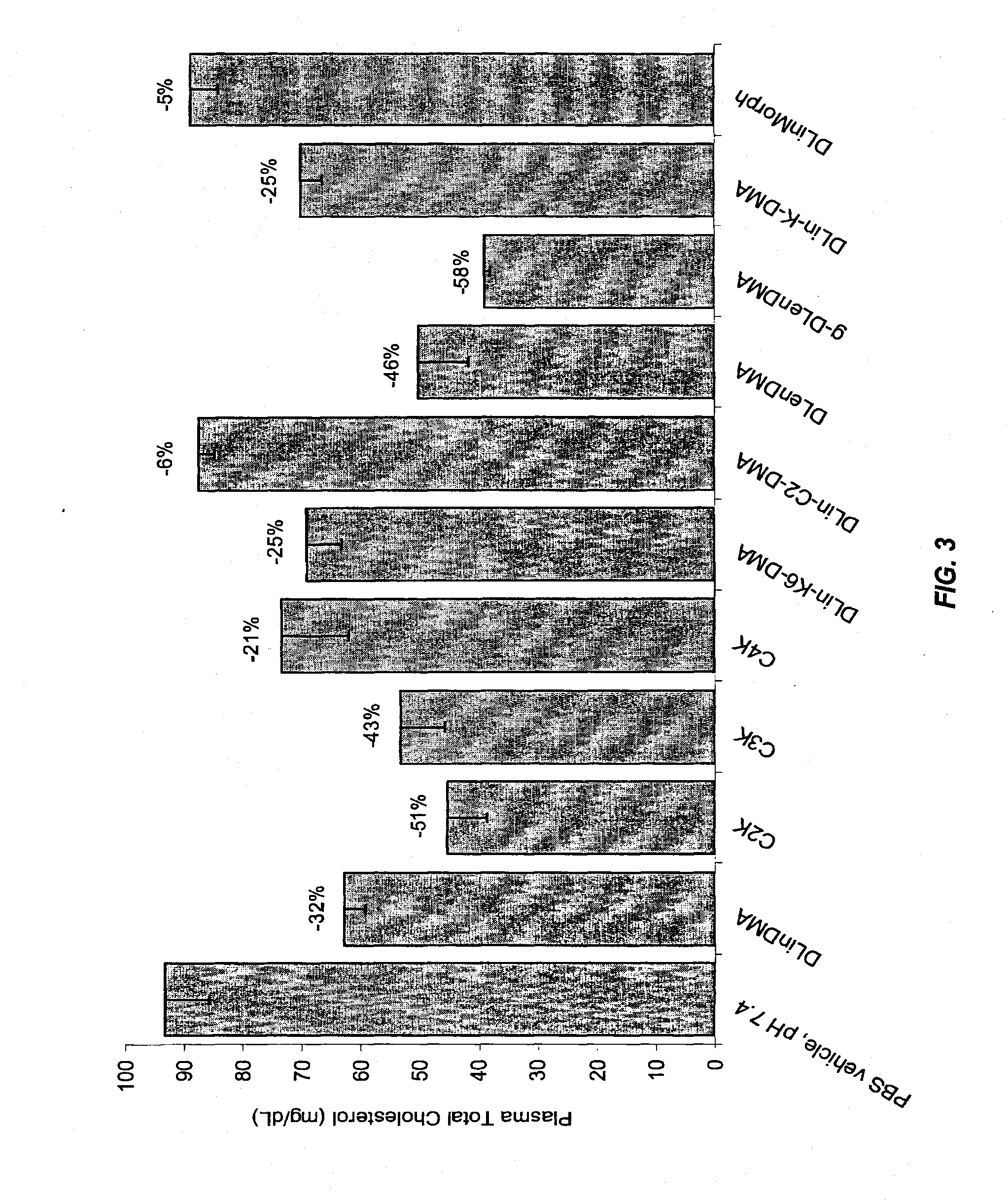
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel cationic lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of a specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:PROTIVA BIOTHERAPEUTICS
Amino lipids and methods for the delivery of nucleic acids
The present invention provides superior compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of specific target proteins at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:ARBUTUS BIOPHARMA CORPORAT ION +1
Cationic lipids and methods for the delivery of therapeutic agents
InactiveUS20120202871A1Cyclic stabilitySize requirementBiocideOrganic active ingredientsLipid formationLipid particle
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel cationic lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of a specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:PROTIVA BIOTHERAPEUTICS
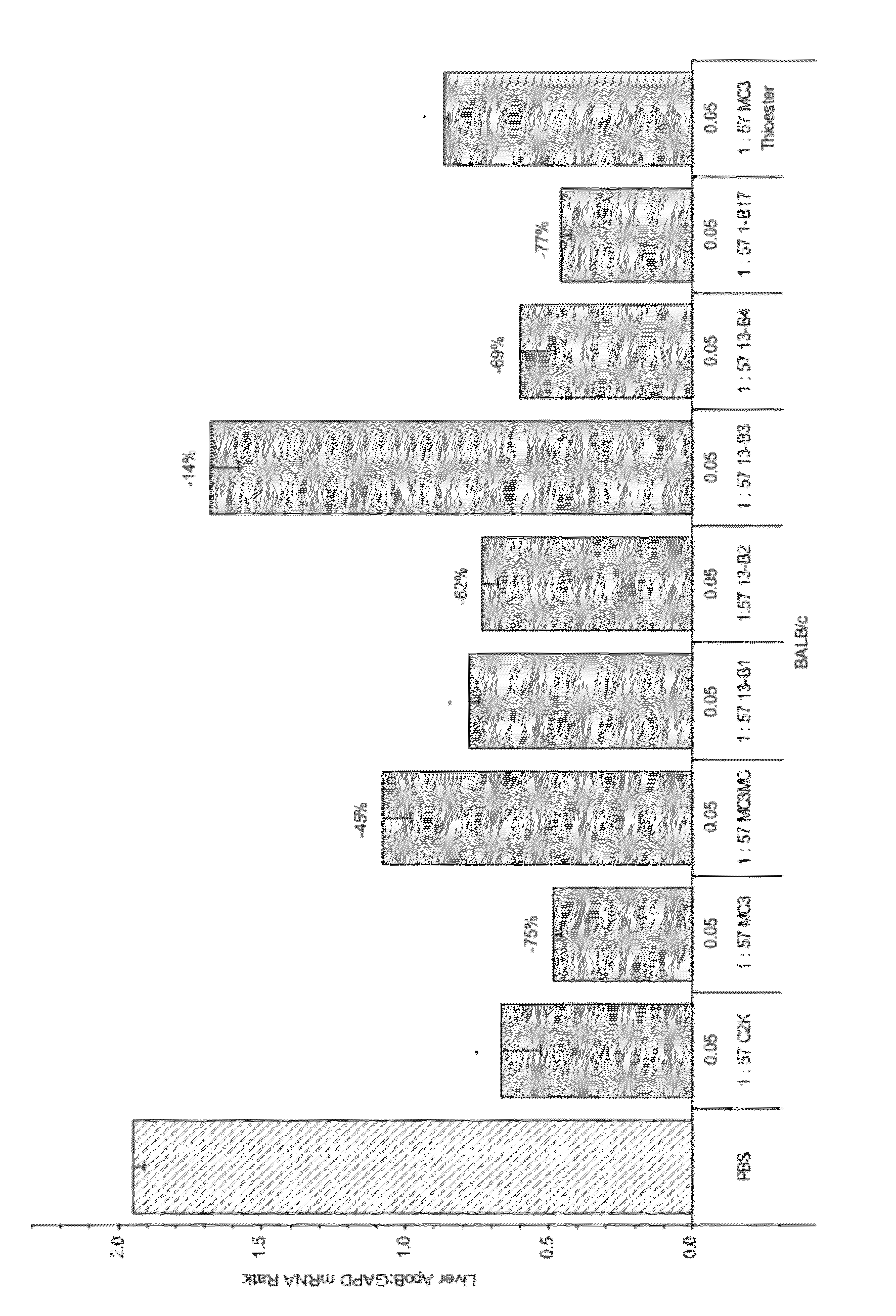
Compositions and methods for silencing apolipoprotein B
InactiveUS8236943B2Improve effectivenessHigh activityOrganic active ingredientsNanotechLipid particleApolipoproteins b
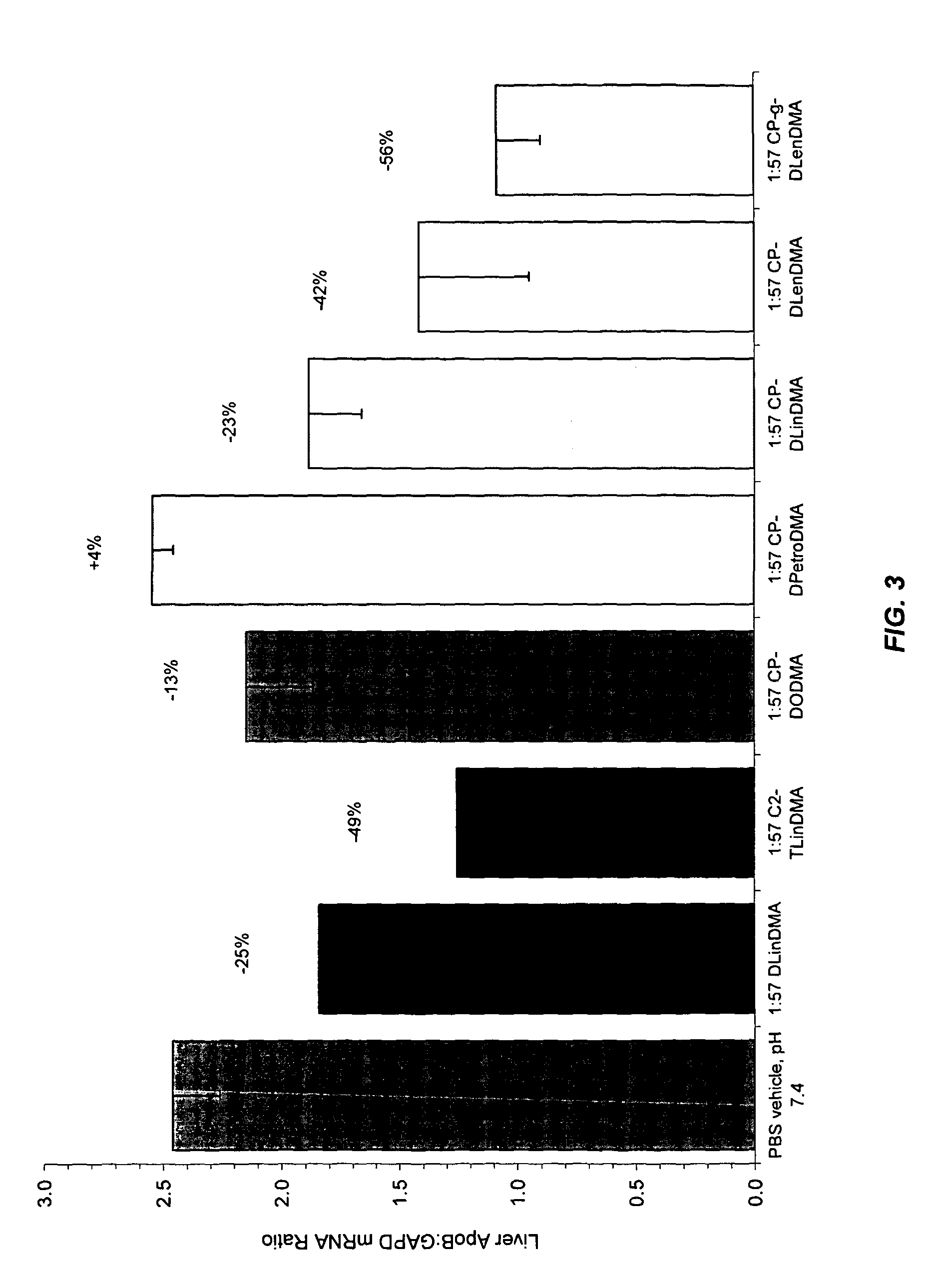
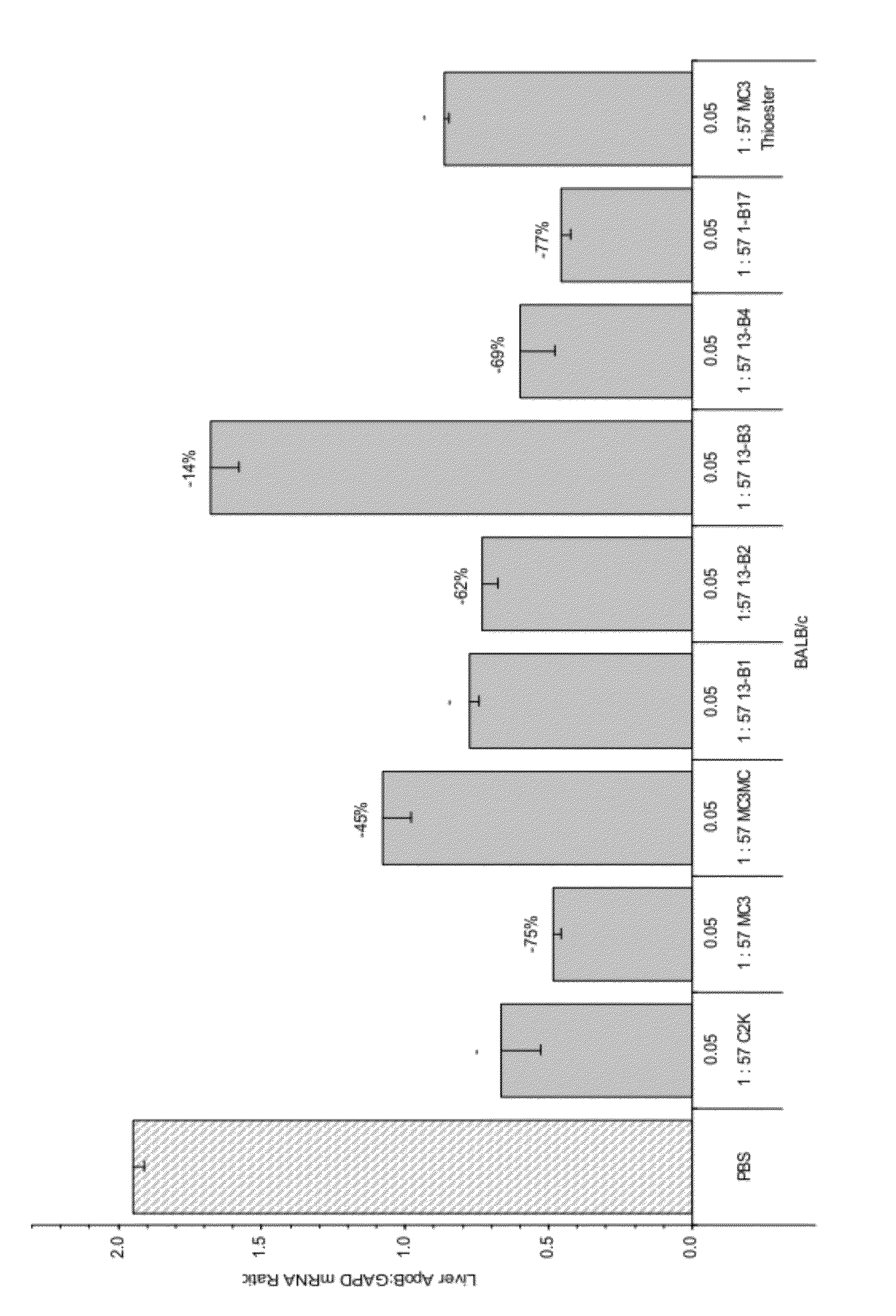
The present invention provides compositions and methods for the delivery of interfering RNAs that silence APOB expression to liver cells. In particular, the nucleic acid-lipid particles provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of APOB at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
Novel cationic lipids and methods of use thereof
InactiveUS20130123338A1Cyclic stabilitySize requirementOrganic active ingredientsBiocideProtein targetLipid particle
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel cationic lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of a specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:PROTIVA BIOTHERAPEUTICS
Advanced drug development and manufacturing
X-ray fluorescence (XRF) spectrometry has been used for detecting binding events and measuring binding selectivities between chemicals and receptors. XRF may also be used for estimating the therapeutic index of a chemical, for estimating the binding selectivity of a chemical versus chemical analogs, for measuring post-translational modifications of proteins, and for drug manufacturing.
Owner:RGT UNIV OF CALIFORNIA
Compositions and methods for the delivery of nucleic acids
InactiveUS20110117125A1Reduce particle aggregationReduce selection requirementsAntibacterial agentsOrganic active ingredientsLipid particleProtein target
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:THE UNIV OF BRITISH COLUMBIA +2
Trialkyl cationic lipids and methods of use thereof
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel cationic lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of a specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:PROTIVA BIOTHERAPEUTICS
Novel cationic lipids and methods of use thereof
ActiveUS20130064894A1Cyclic stabilitySize requirementPowder deliveryBiocideLipid formationLipid particle
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel cationic lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of a specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:PROTIVA BIOTHERAPEUTICS
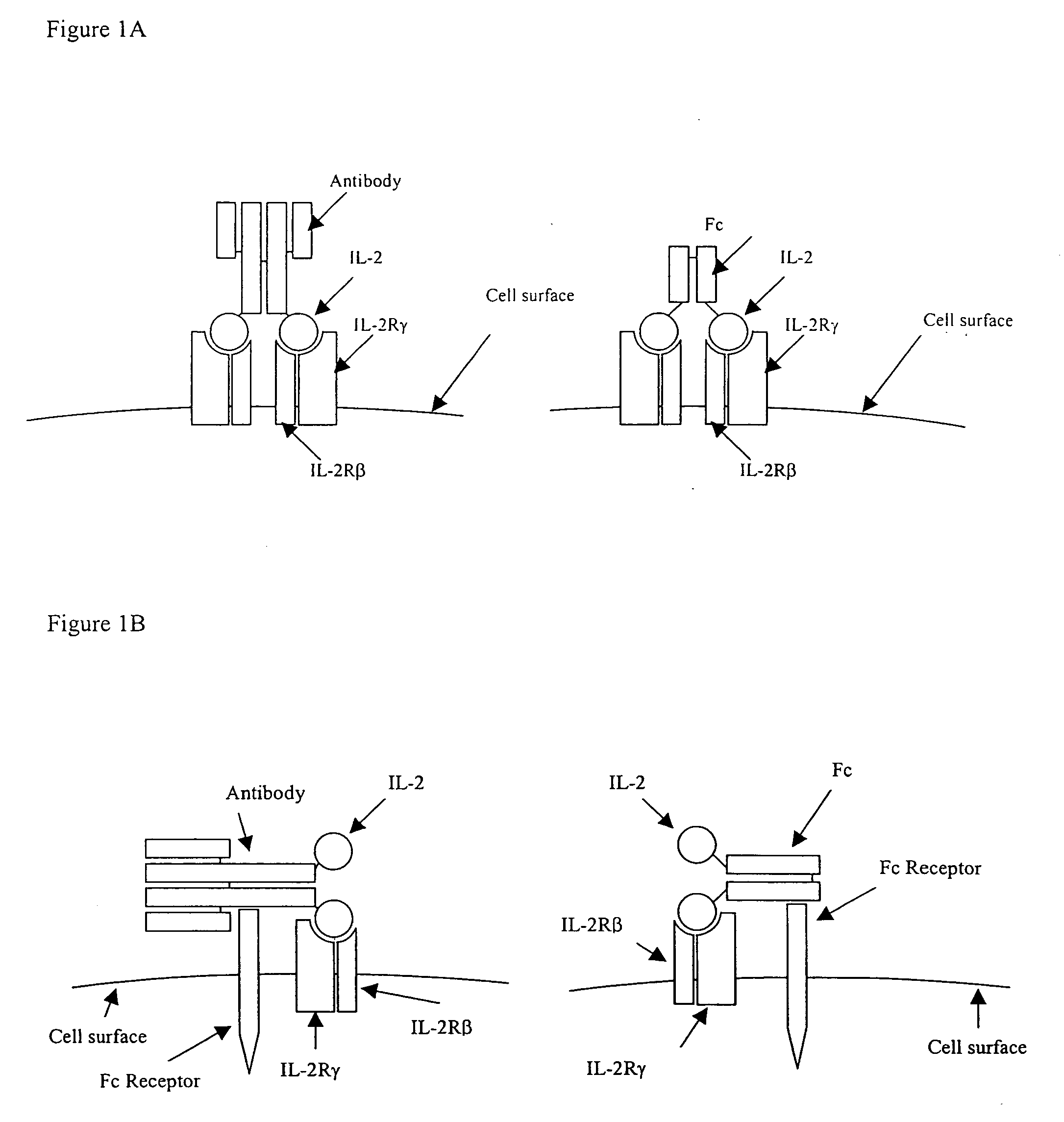
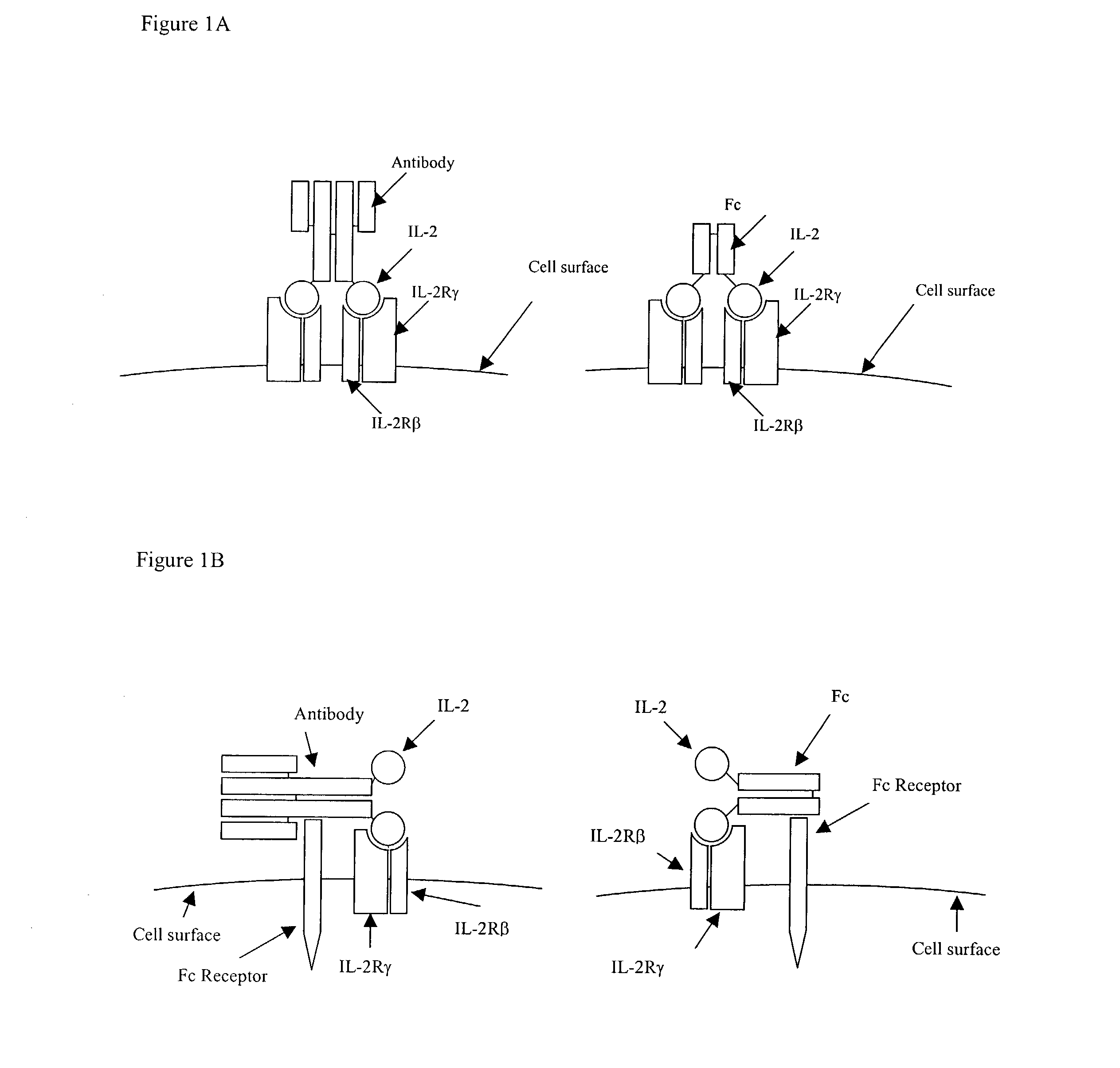
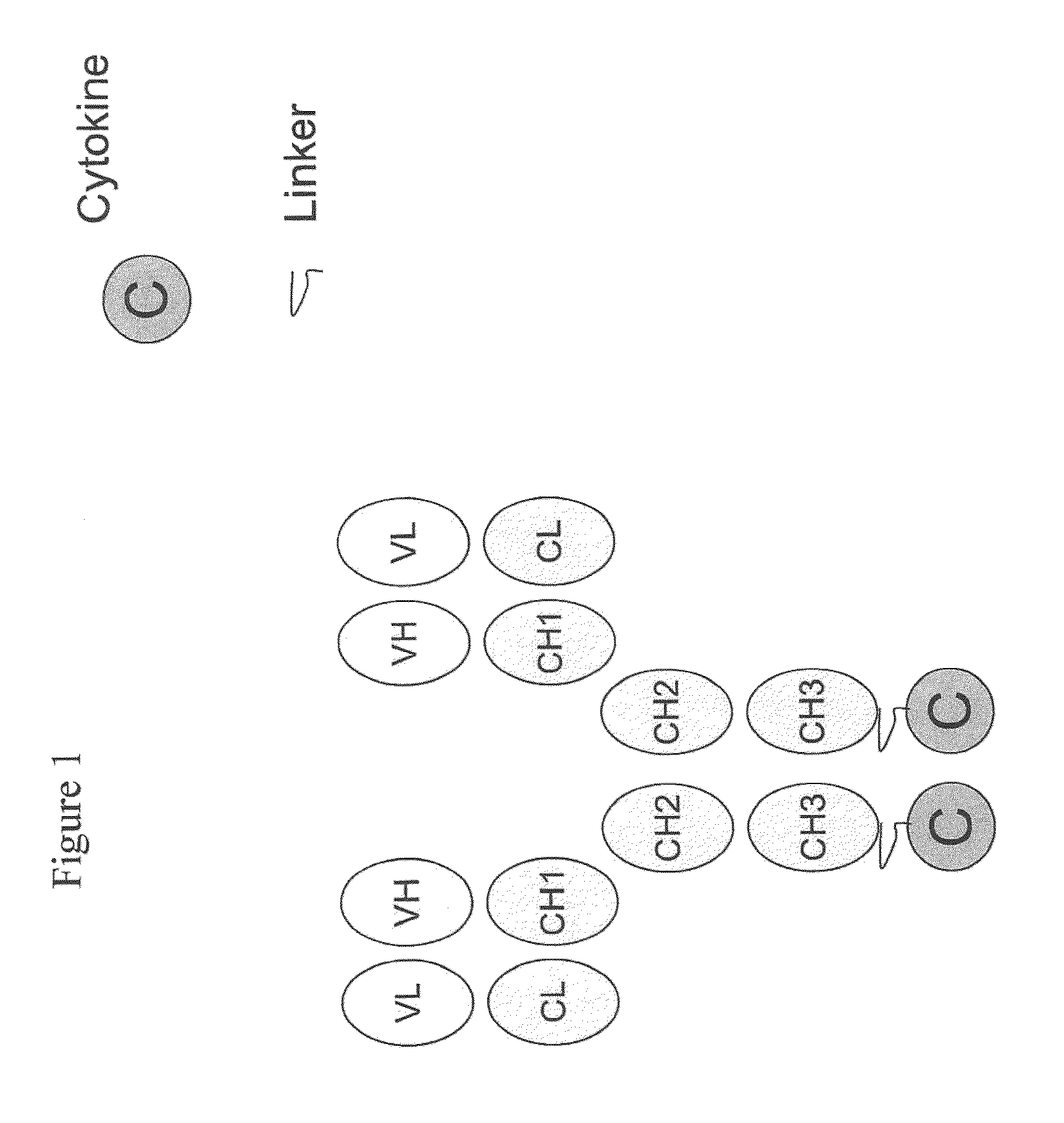
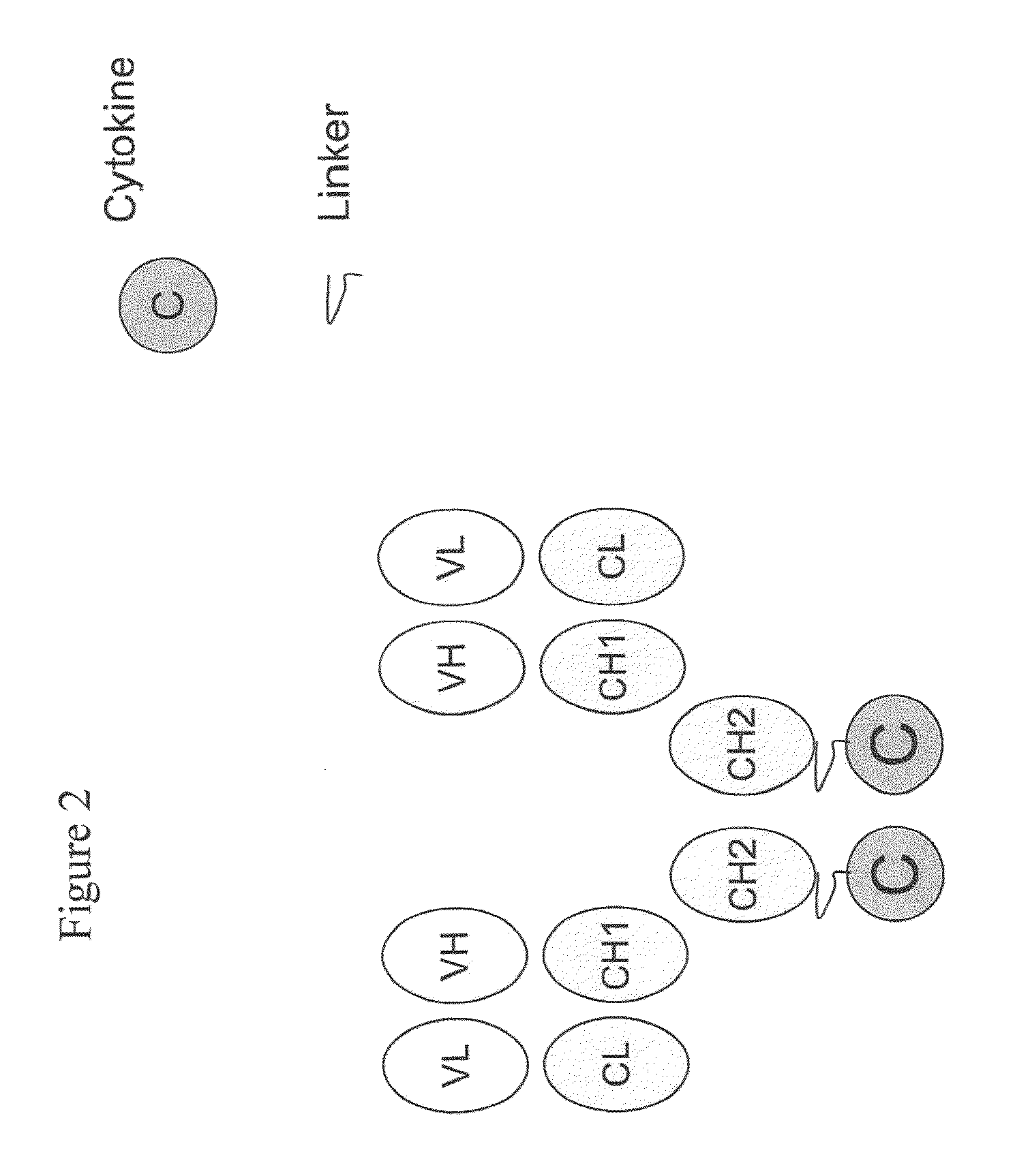
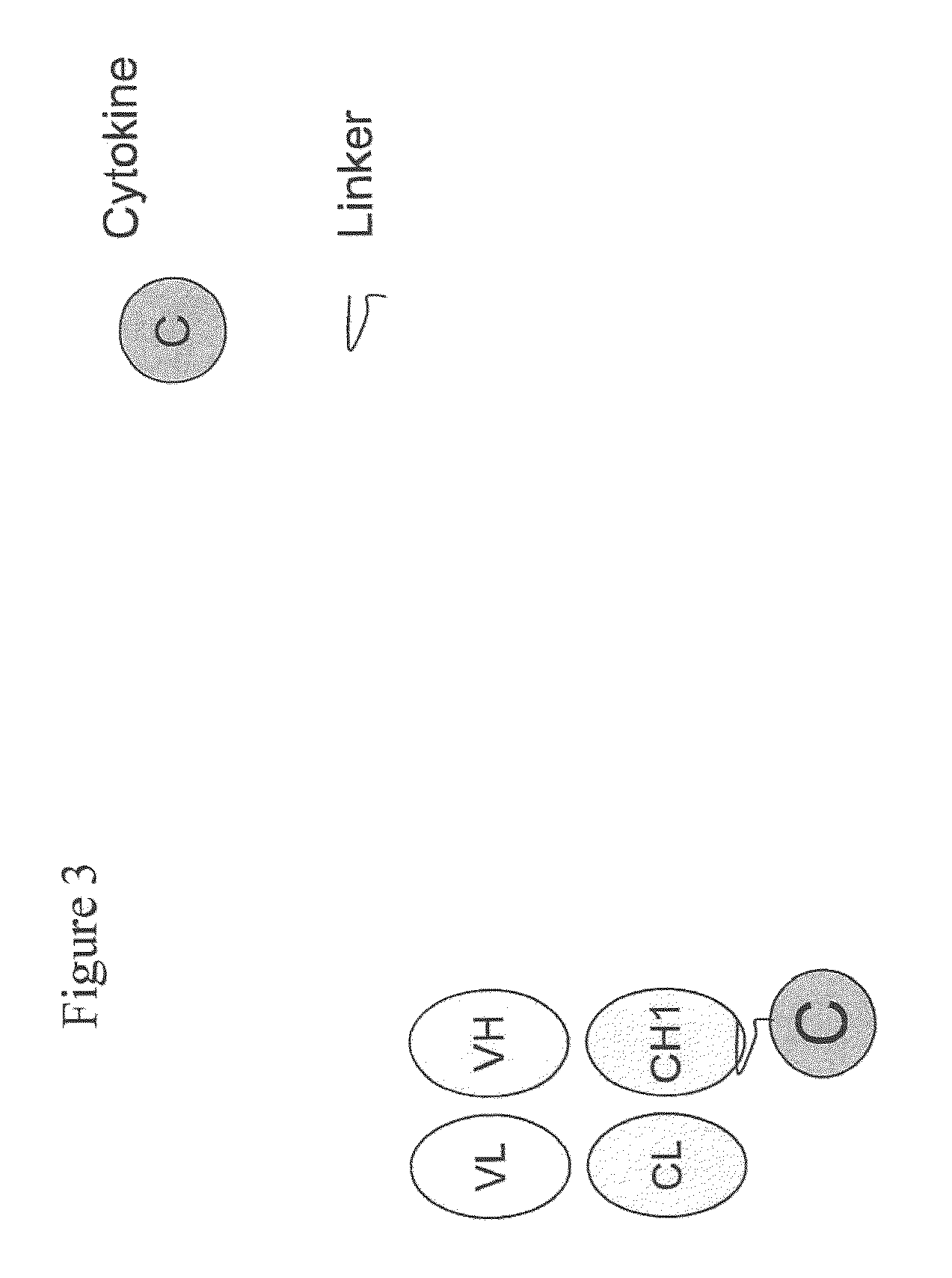
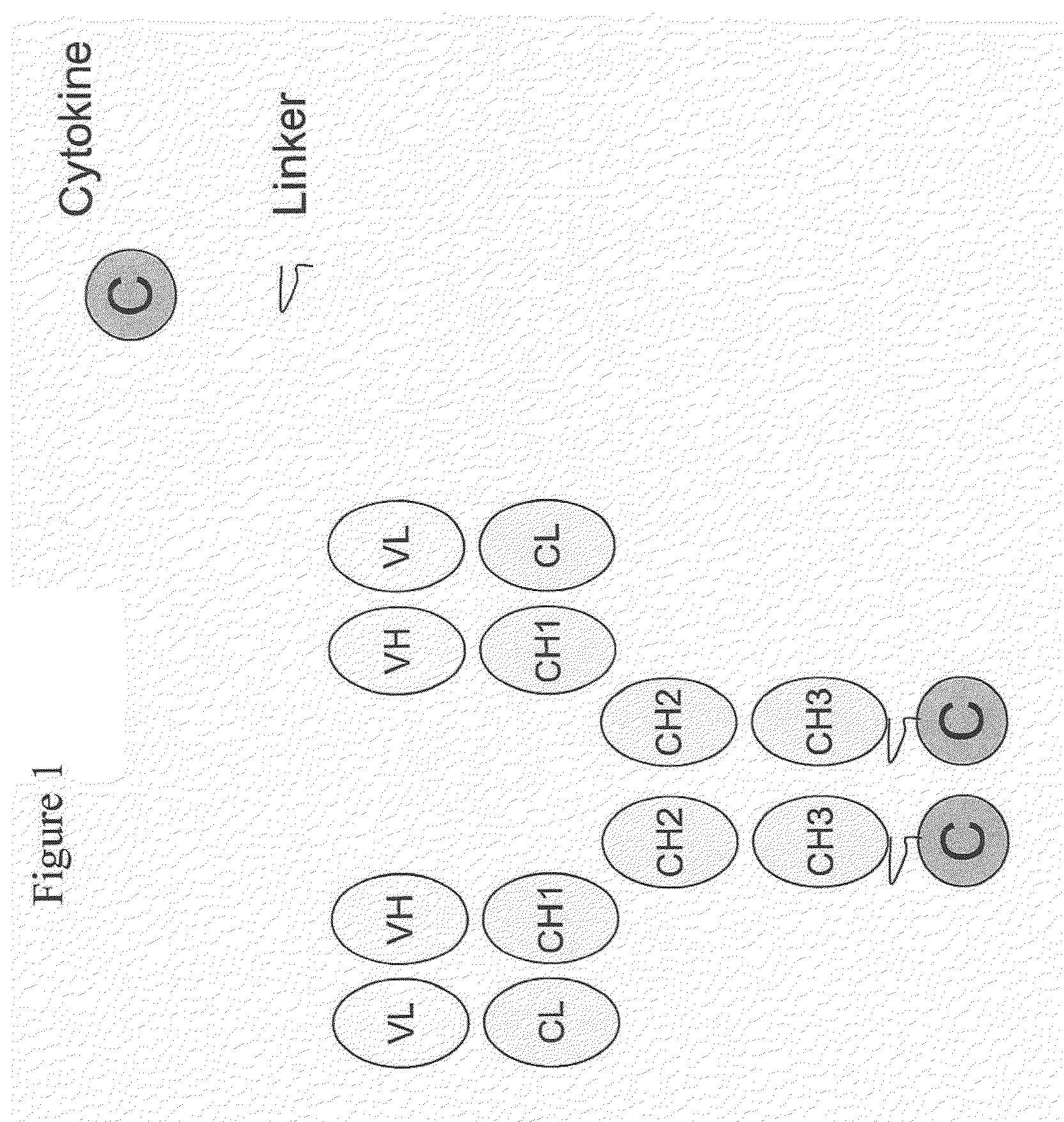
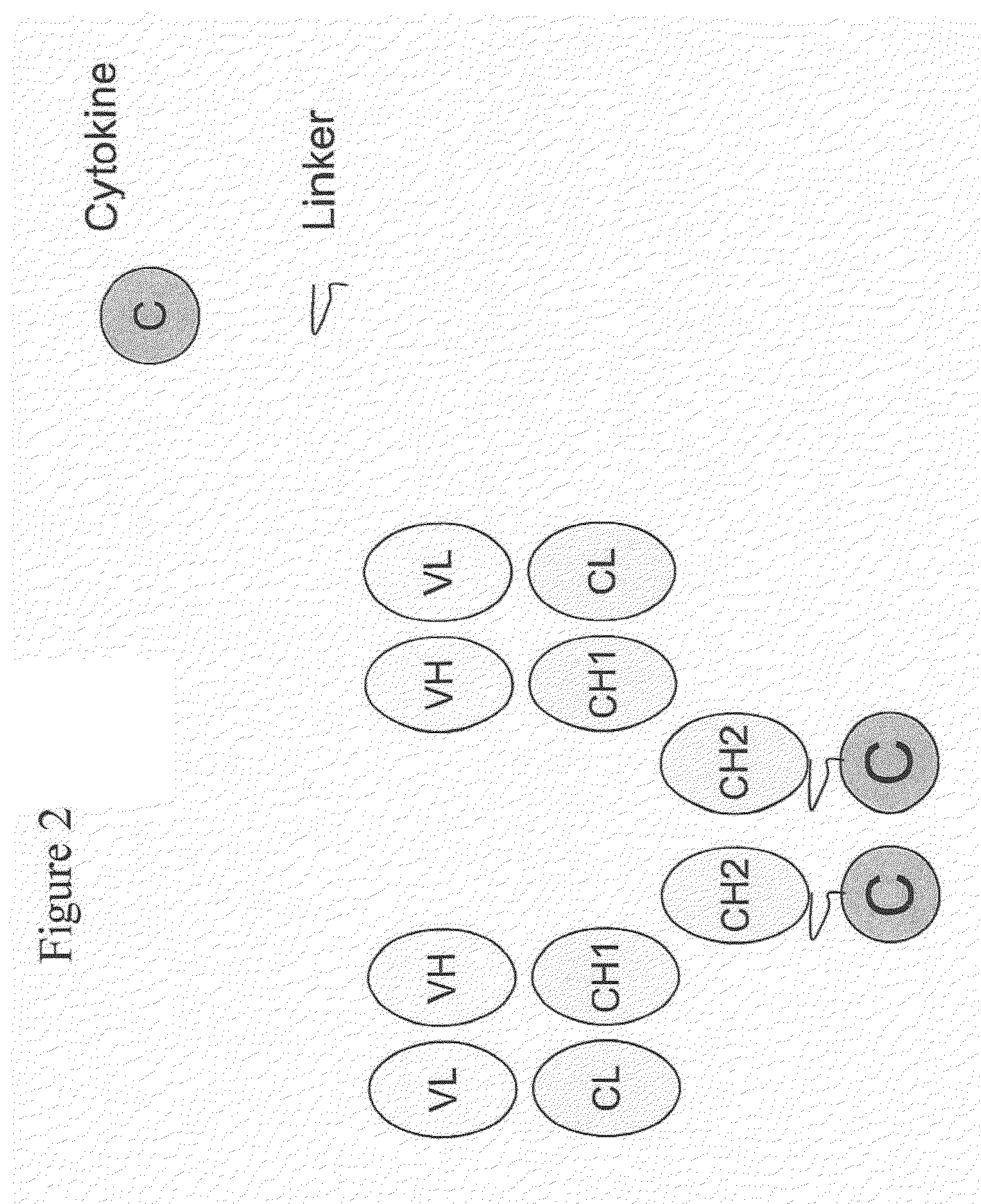
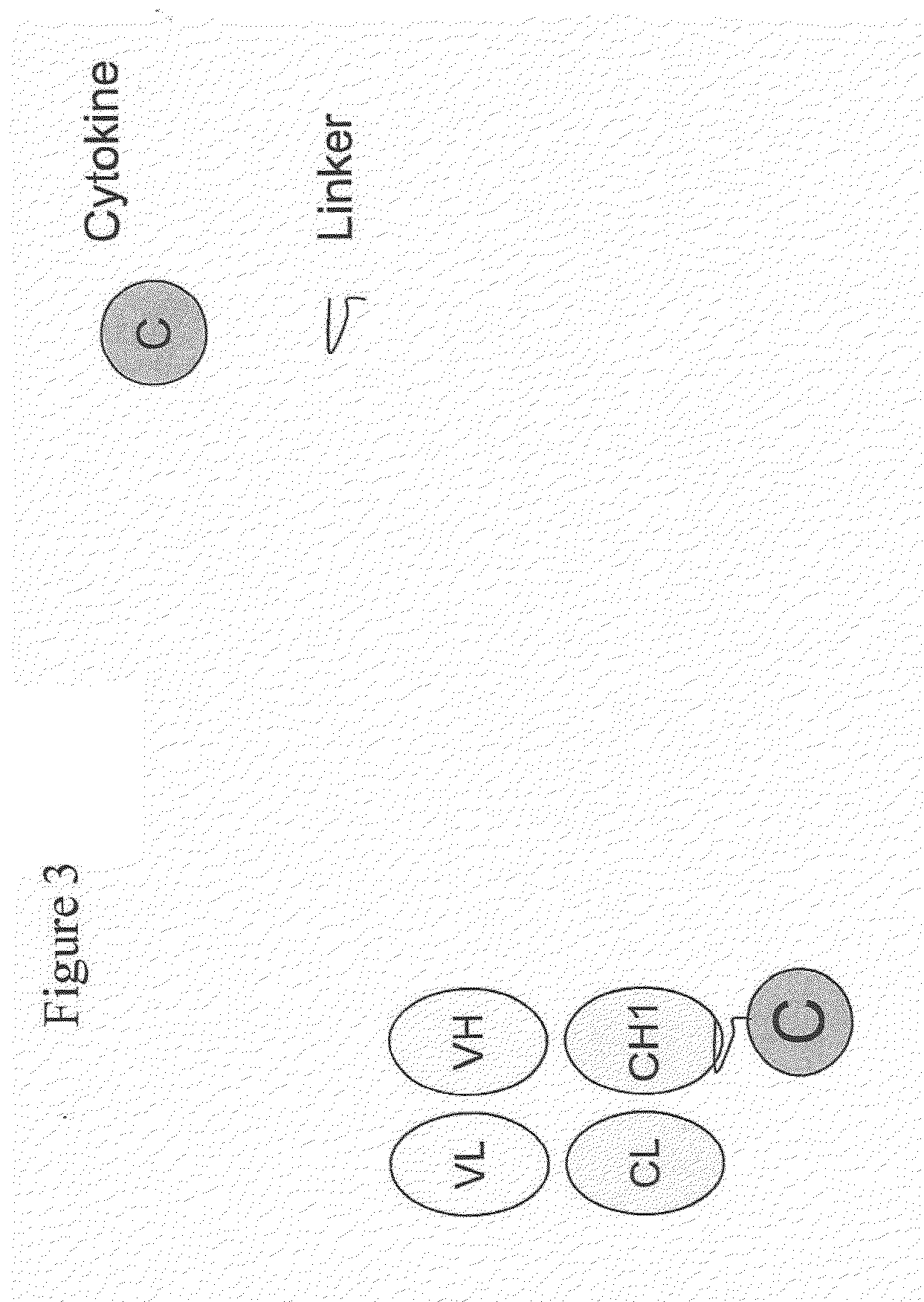
IL-2 fusion proteins with modulated selectivity
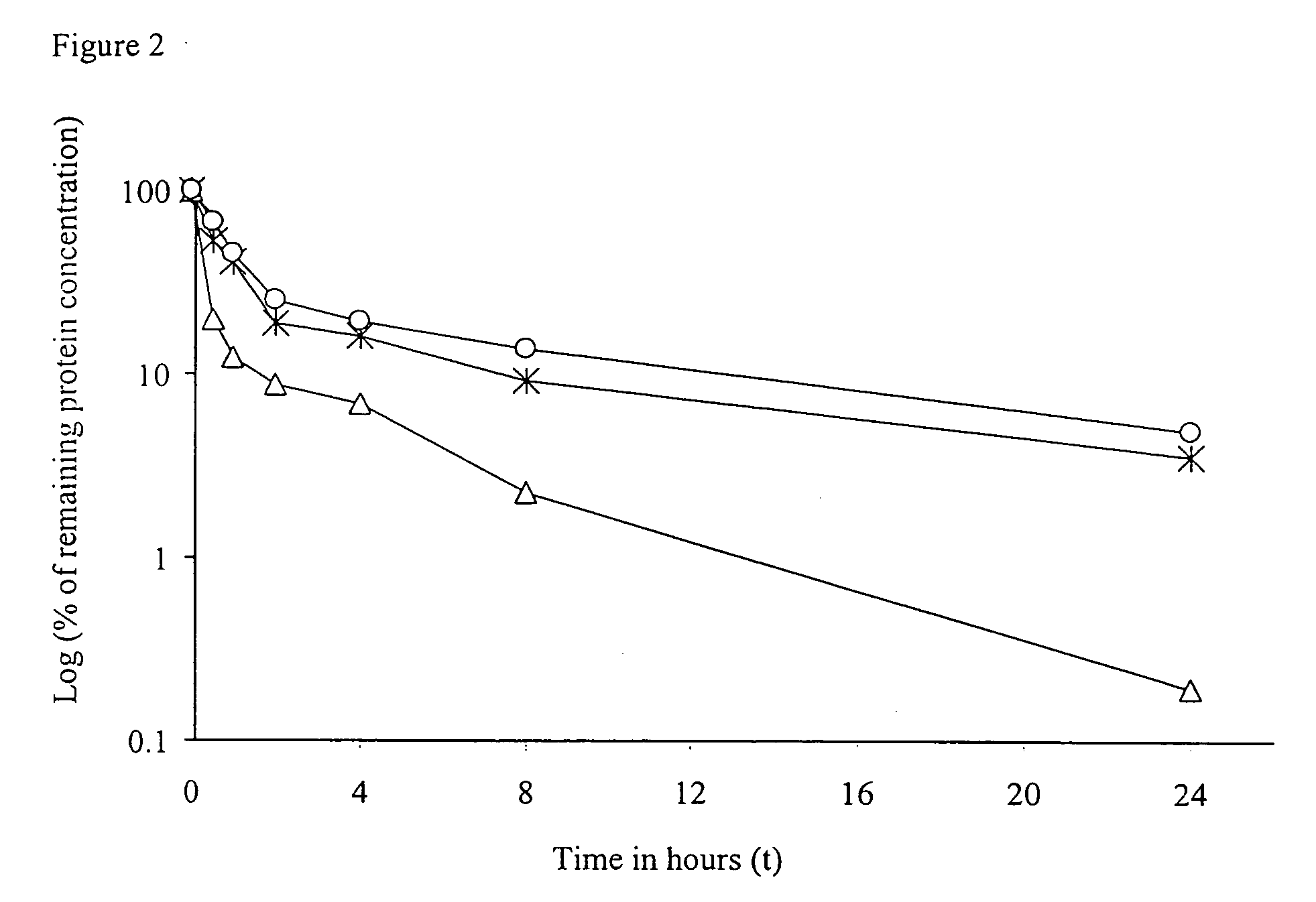
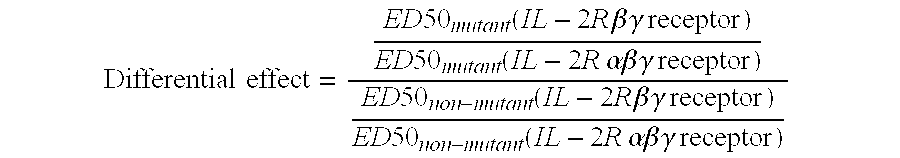
The invention provides cytokine fusion proteins with an increased therapeutic index, and methods to increase the therapeutic index of such fusion proteins. The fusion proteins of the invention are able to bind to more than one type of cytokine receptor expressed on cells and also bind to more than one cell type. In addition, the fusion proteins of the invention exhibit a longer circulating half-life in a patient's body than the corresponding naturally occurring cytokine.
Owner:MERCK PATENT GMBH
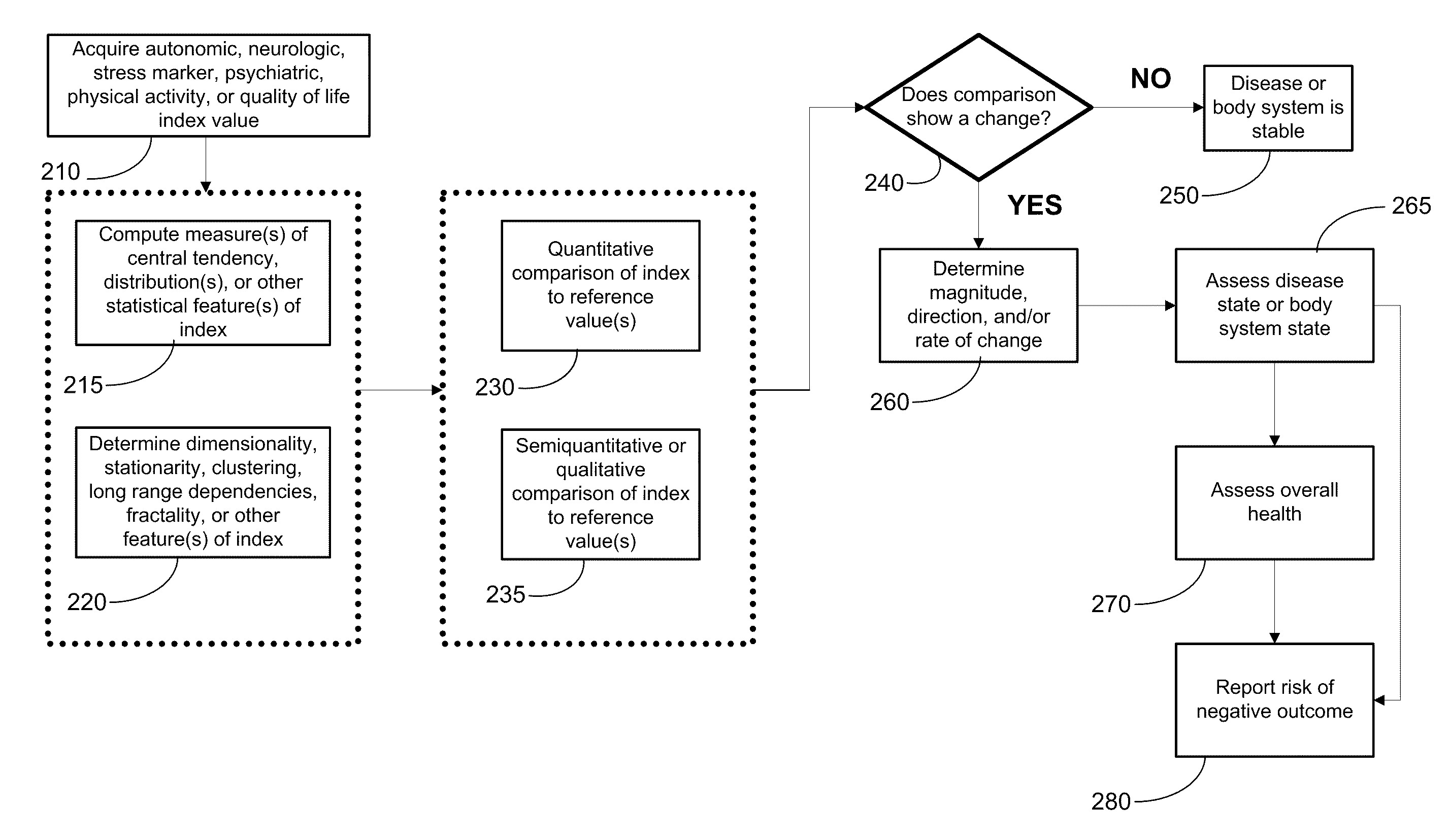
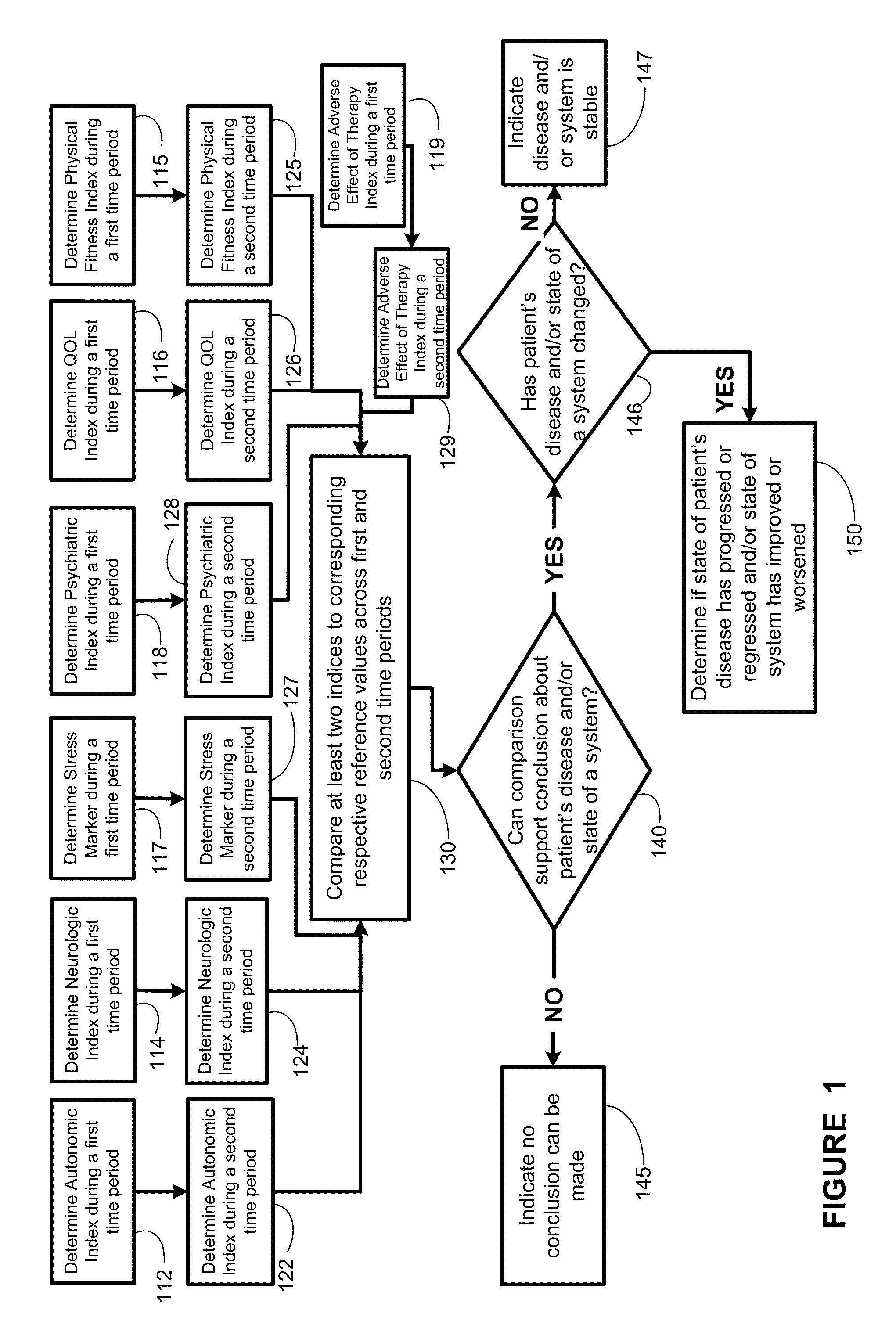
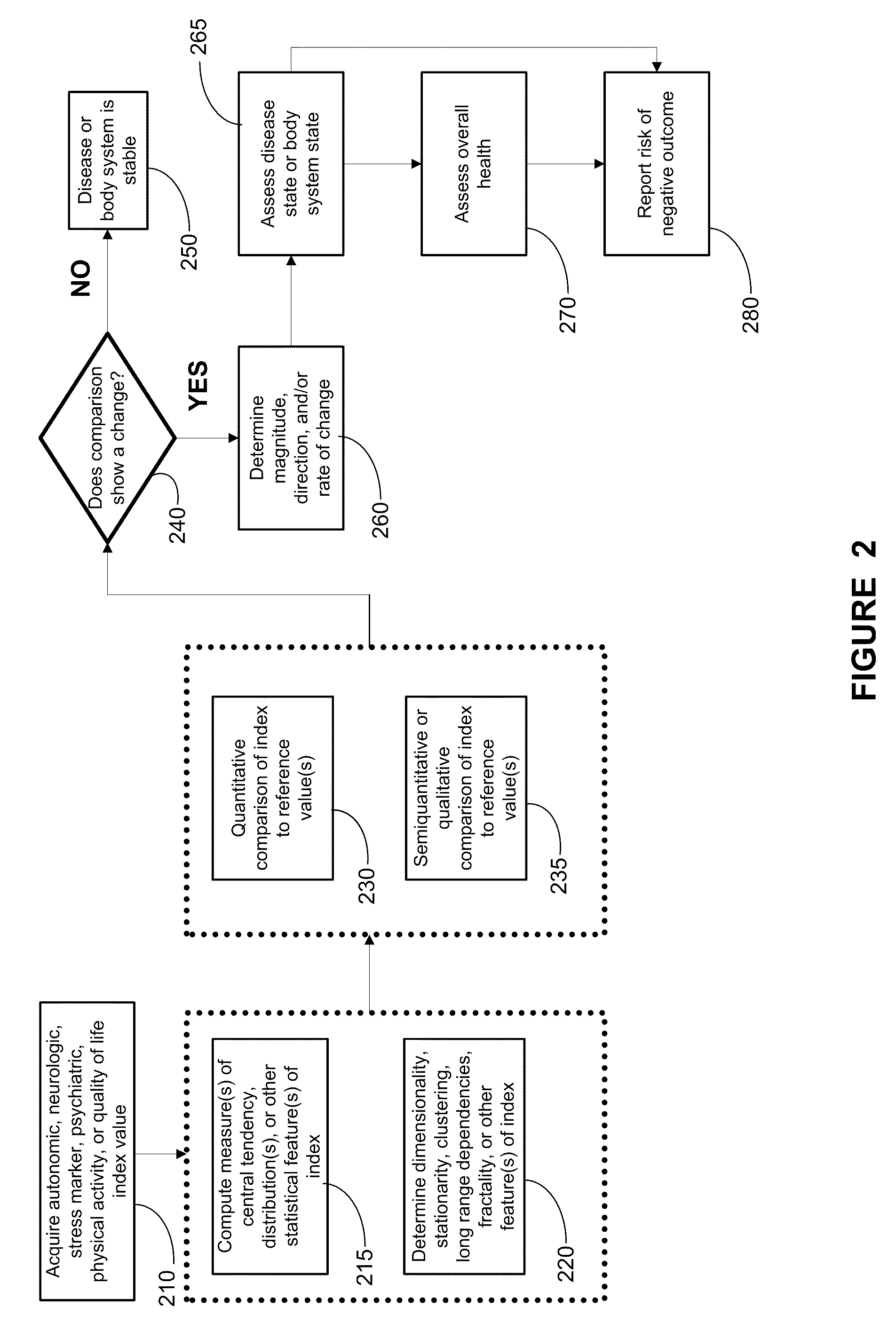
Systems approach to comorbidity assessment
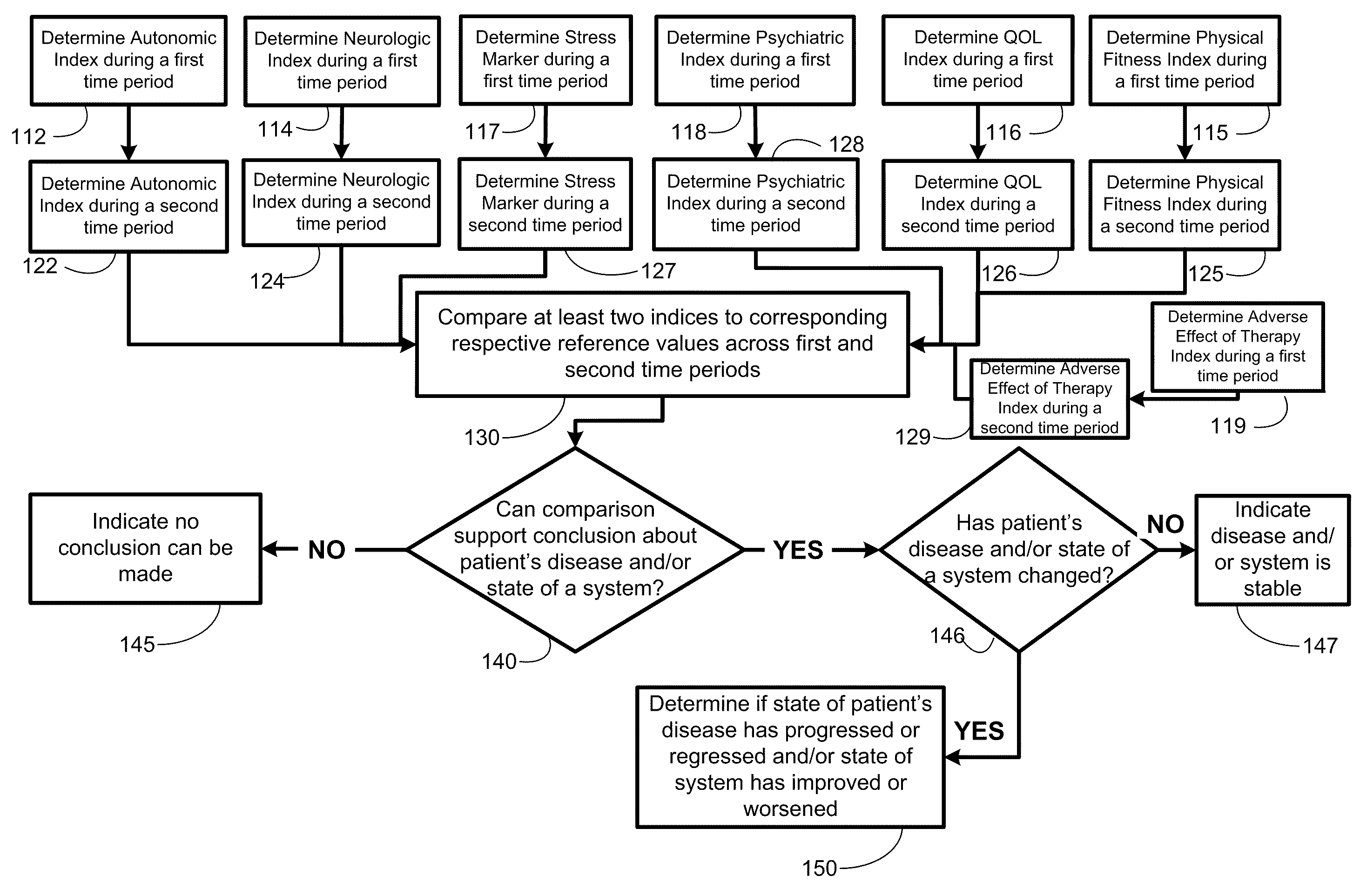
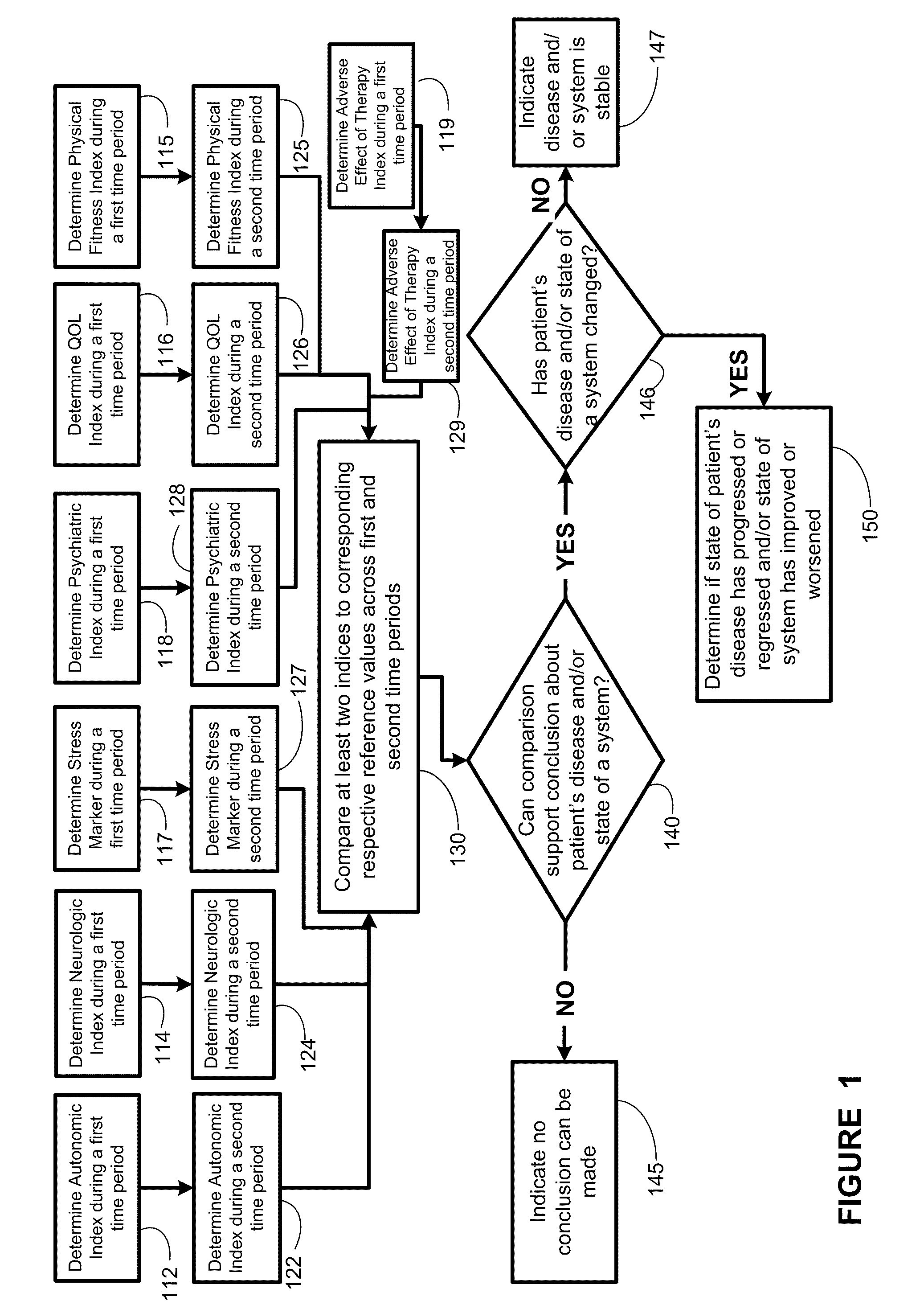
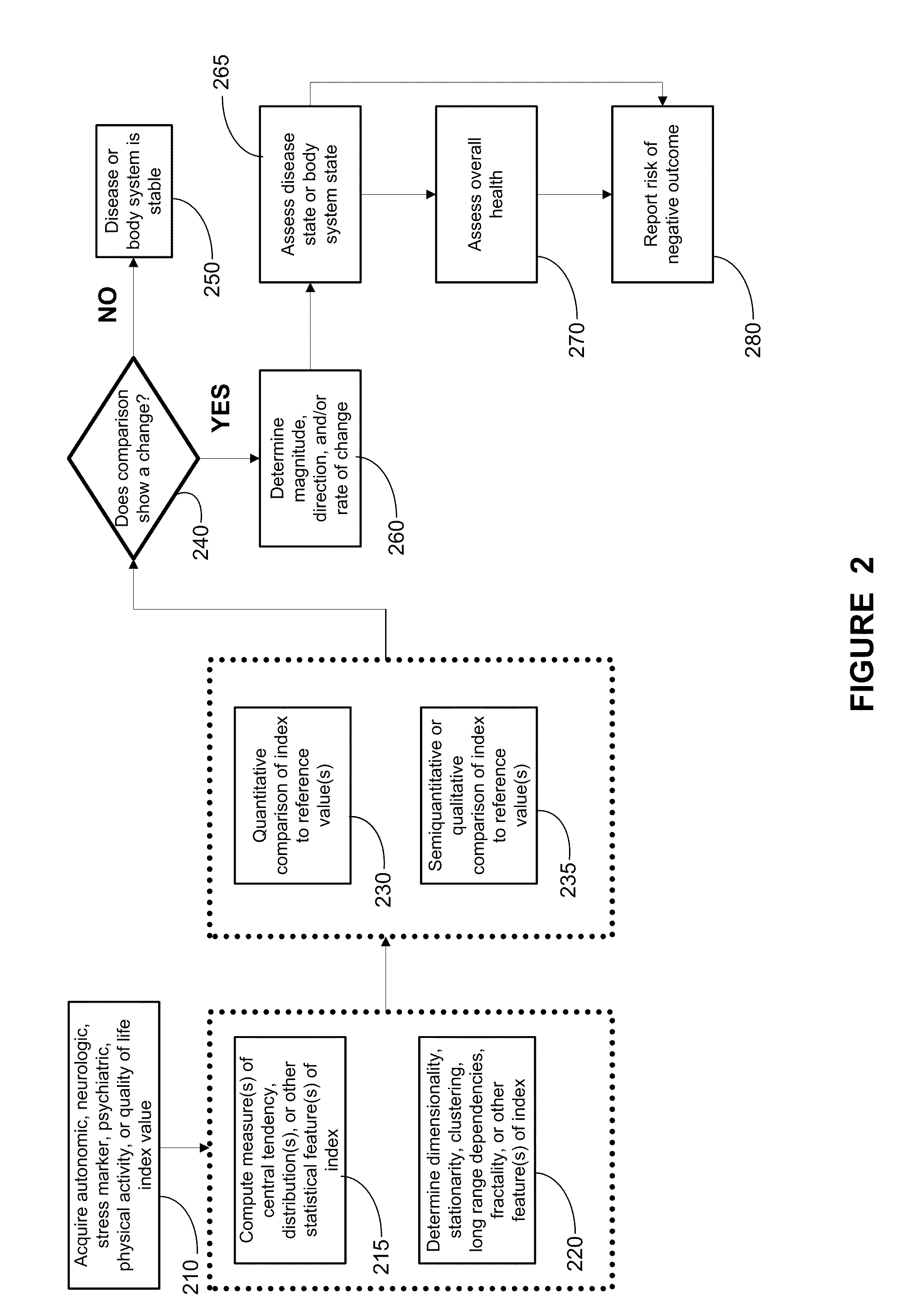
Methods, systems, and apparatus for assessing a state of a comorbidity associated with a primary disease are provided. The methods comprise receiving at least one autonomic index, neurologic index, stress marker index, psychiatric index, endocrine index, adverse effect of therapy index, physical fitness index, or quality of life index of a patient; comparing the at least one index to at least one reference value; and assessing a state of a body system of the patient that is a site of the comorbidity, based on the comparison. A computer readable program storage device encoded with instructions that, when executed by a computer, perform the method described above is also provided. A medical device system capable of implementing the method described above is also provided.
Owner:FLINT HILLS SCI L L C
Methods for determining therapeutic index from gene expression profiles
InactiveUS6222093B1Low therapeutic indexLow indexChemical property predictionSugar derivativesDrug specific IgEMedicine
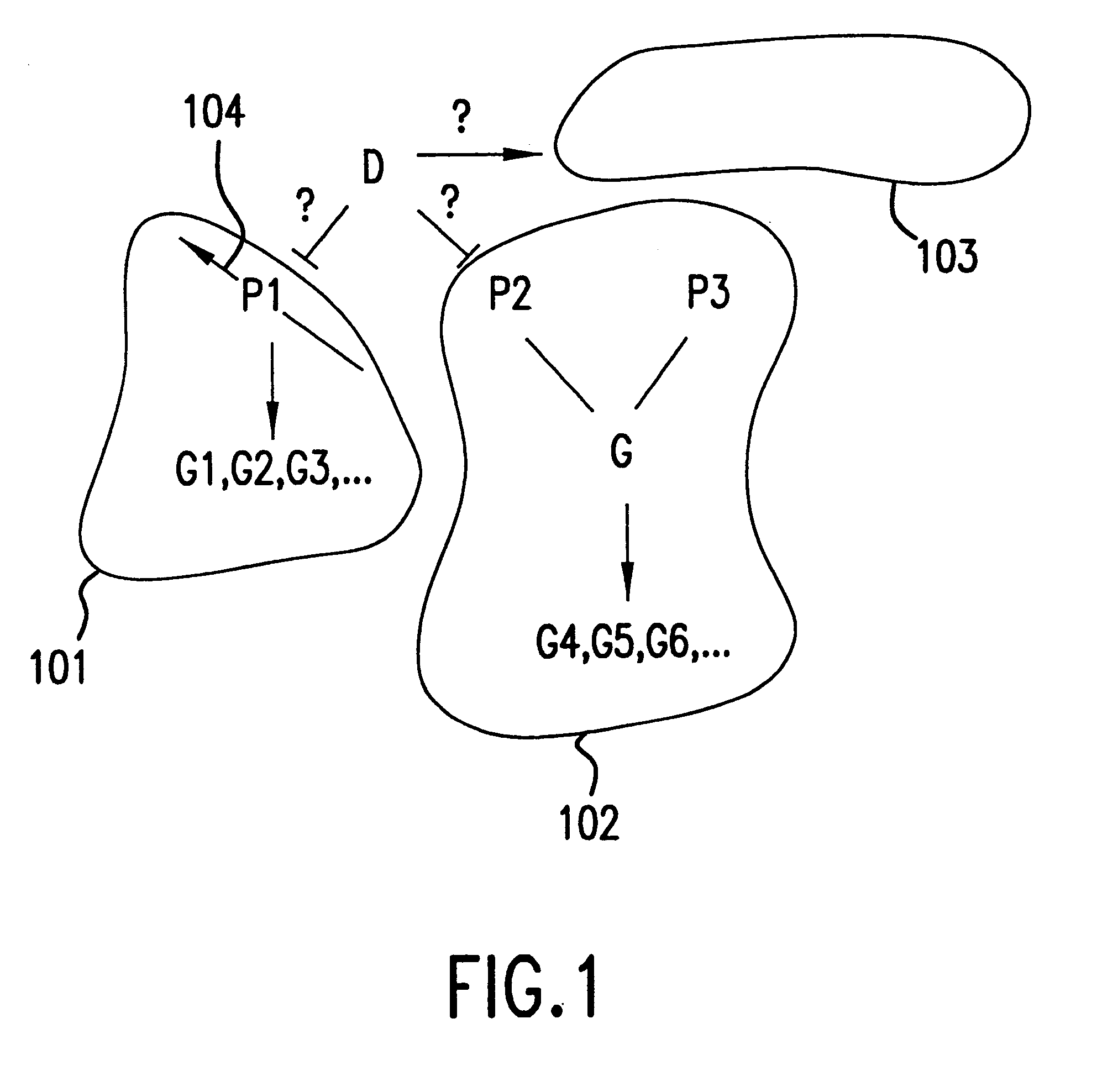
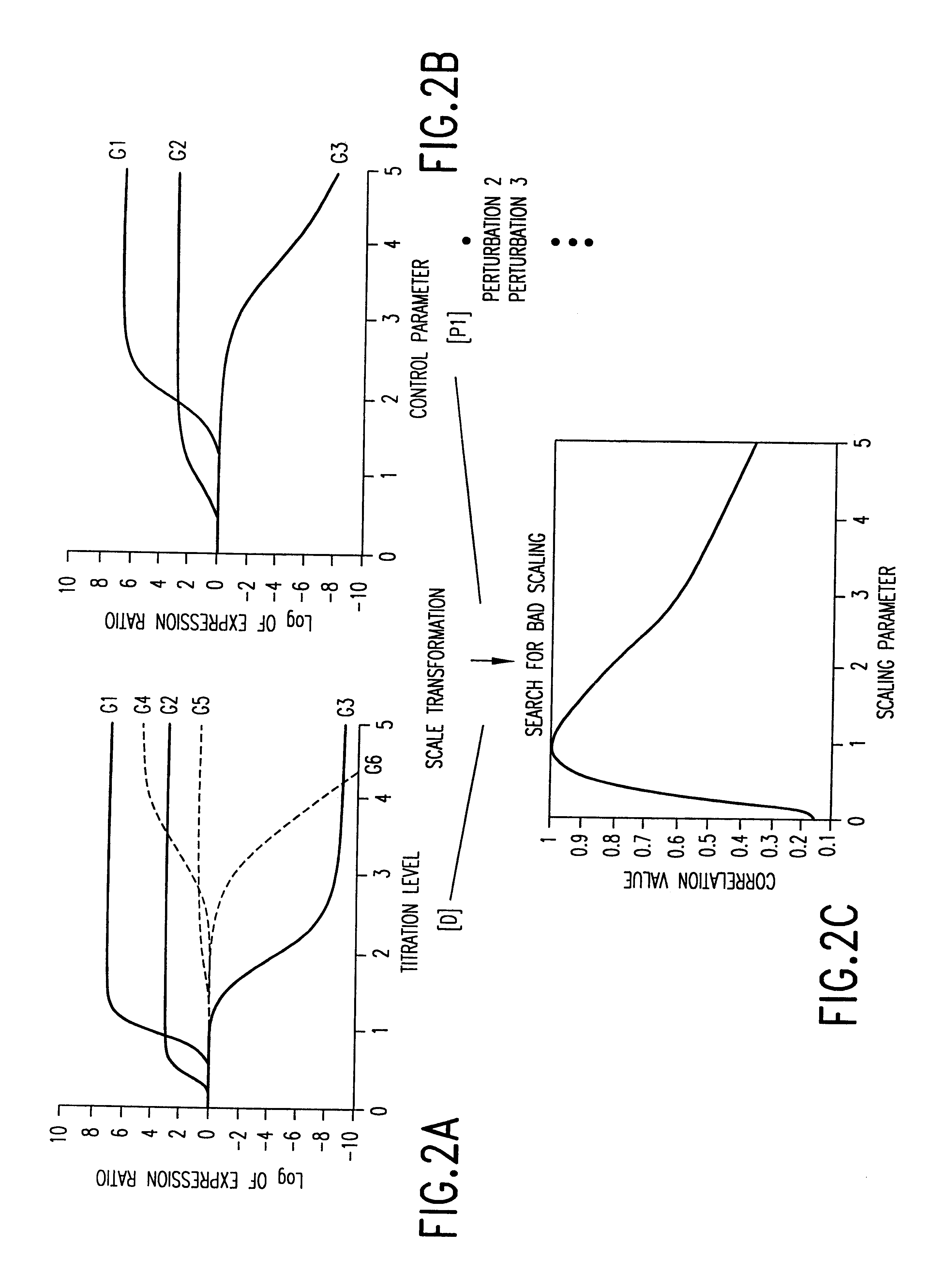
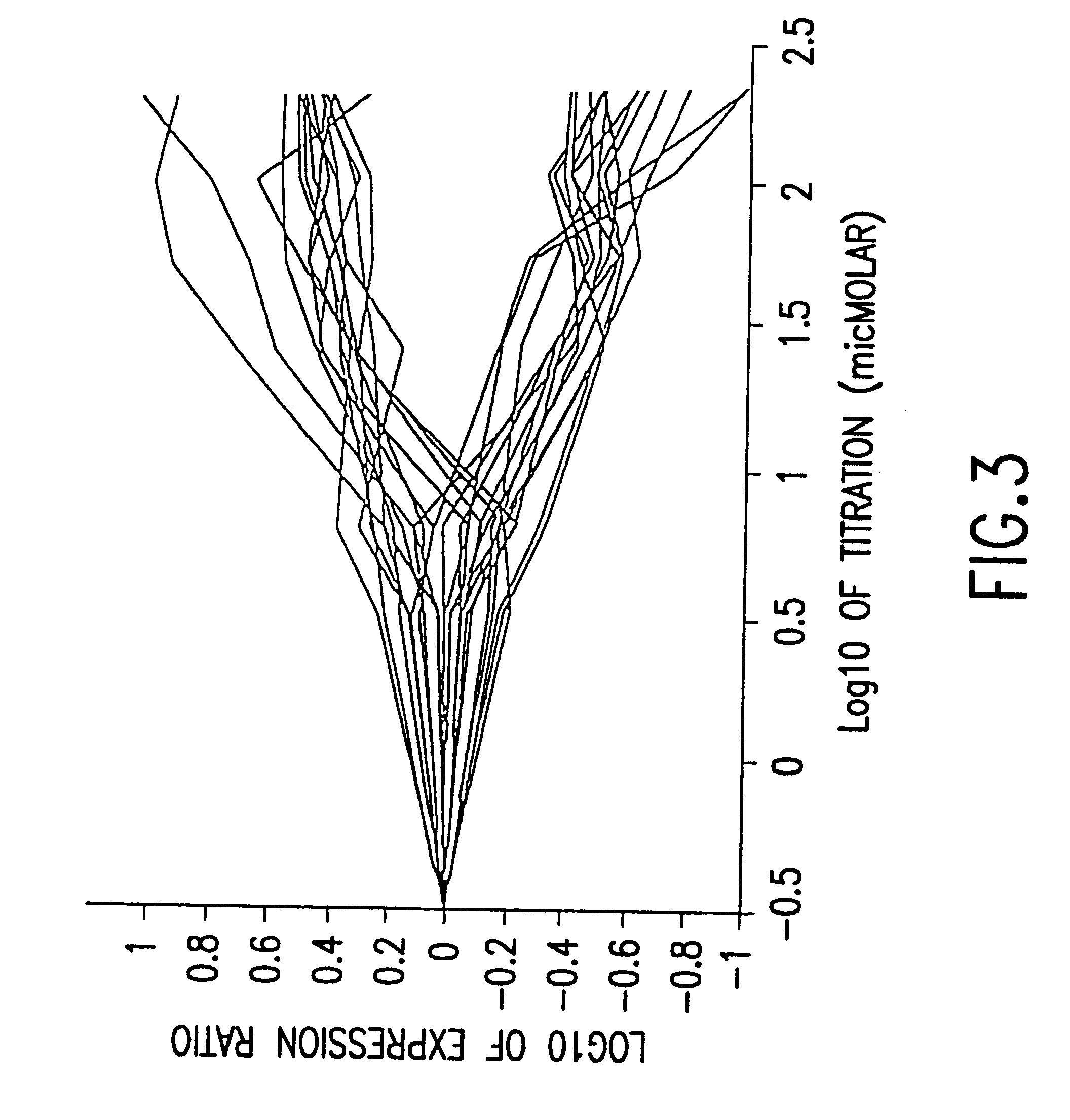
This invention provides methods for determining drug specificity, therapeutic index and effective doses for individual patients. According to the methods of the invention, graded levels of drug are applied to a biological sample or a patient. A plurality of cellular constituents are measured to determine the activity of the drug on a target pathway and at least one off-target pathway. A drug specificity is determined by comparing the target and off target activities of the drug. A therapeutic concentration (or dose) is defined as a concentration (or dose) of the drug that induces certain response in the target pathway. A toxic concentration (or dose) is defined as a concentration (or dose) of the drug that induces certain response in the off target pathway. Therapeutic index is the ratio of the toxic concentration over therapeutic concentration. Methods are also provided to determine an effective dose of a drug for a patient by measuring the activity of the drug on the particular patient.
Owner:MICROSOFT TECH LICENSING LLC
Novel cyclic cationic lipids and methods of use
ActiveUS20130116307A1Cyclic stabilitySize requirementBiocideOrganic active ingredientsLipid particleProtein target
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel cationic lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of a specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:ARBUTUS BIOPHARMA CORPORAT ION
IL-2 fusion proteins with modulated selectivity
The invention provides cytokine fusion proteins with an increased therapeutic index, and methods to increase the therapeutic index of such fusion proteins. The fusion proteins of the invention are able to bind to more than one type of cytokine receptor expressed on cells and also bind to more than one cell type. In addition, the fusion proteins of the invention exhibit a longer circulating half-life in a patient's body than the corresponding naturally occurring cytokine.
Owner:MERCK PATENT GMBH
Novel trialkyl cationic lipids and methods of use thereof
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel cationic lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of a specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:PROTIVA BIOTHERAPEUTICS
Cationic lipids and methods for the delivery of therapeutic agents
Owner:PROTIVA BIOTHERAPEUTICS
Amino lipids and methods for the delivery of nucleic acids
The present invention provides superior compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid to cells in vivo. The compositions of the present invention are highly potent, thereby allowing effective knock-down of specific target proteins at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:ARBUTUS BIOPHARMA CORPORAT ION +1
Nucleic acid carriers for delivery of therapeutic agents
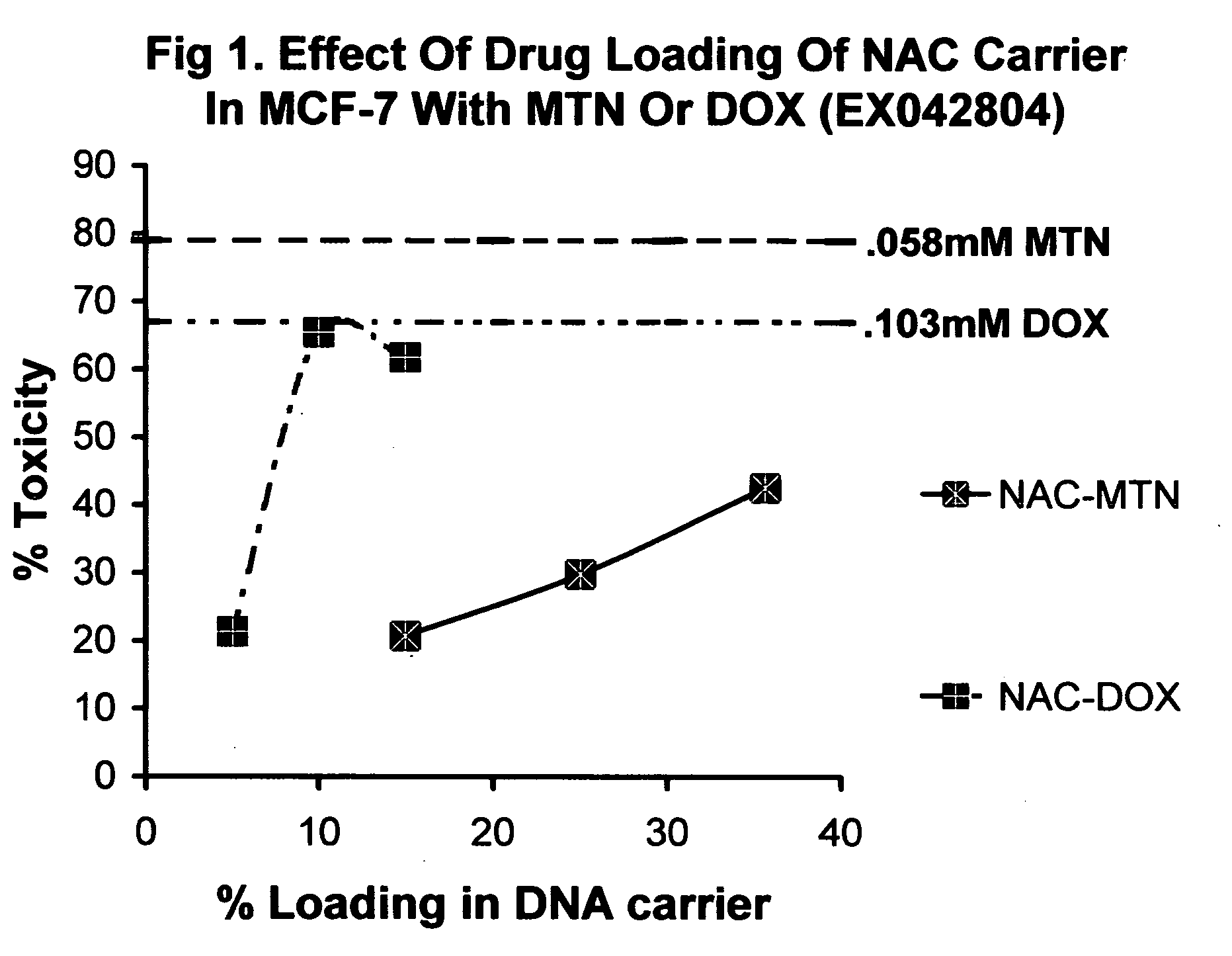
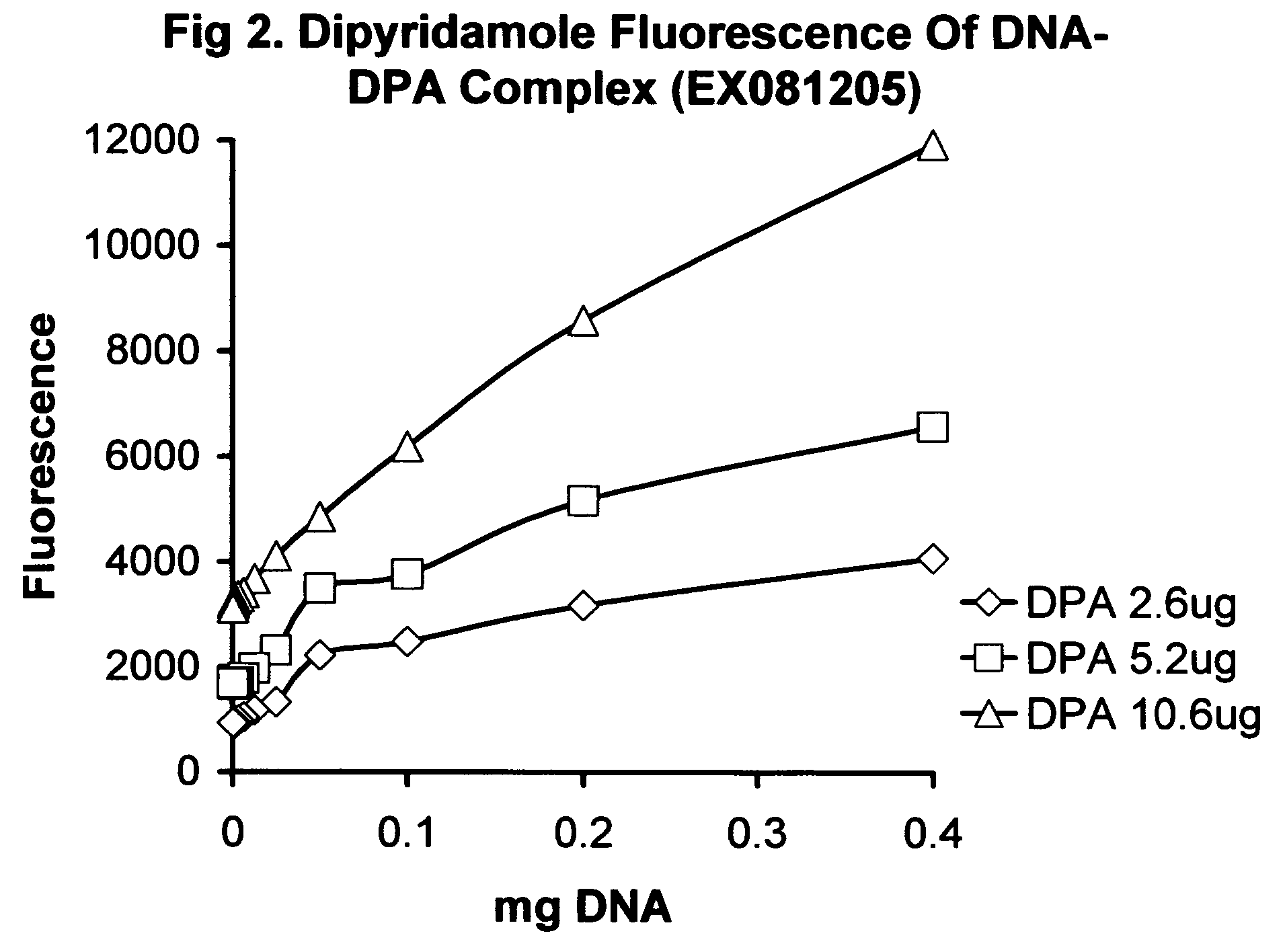
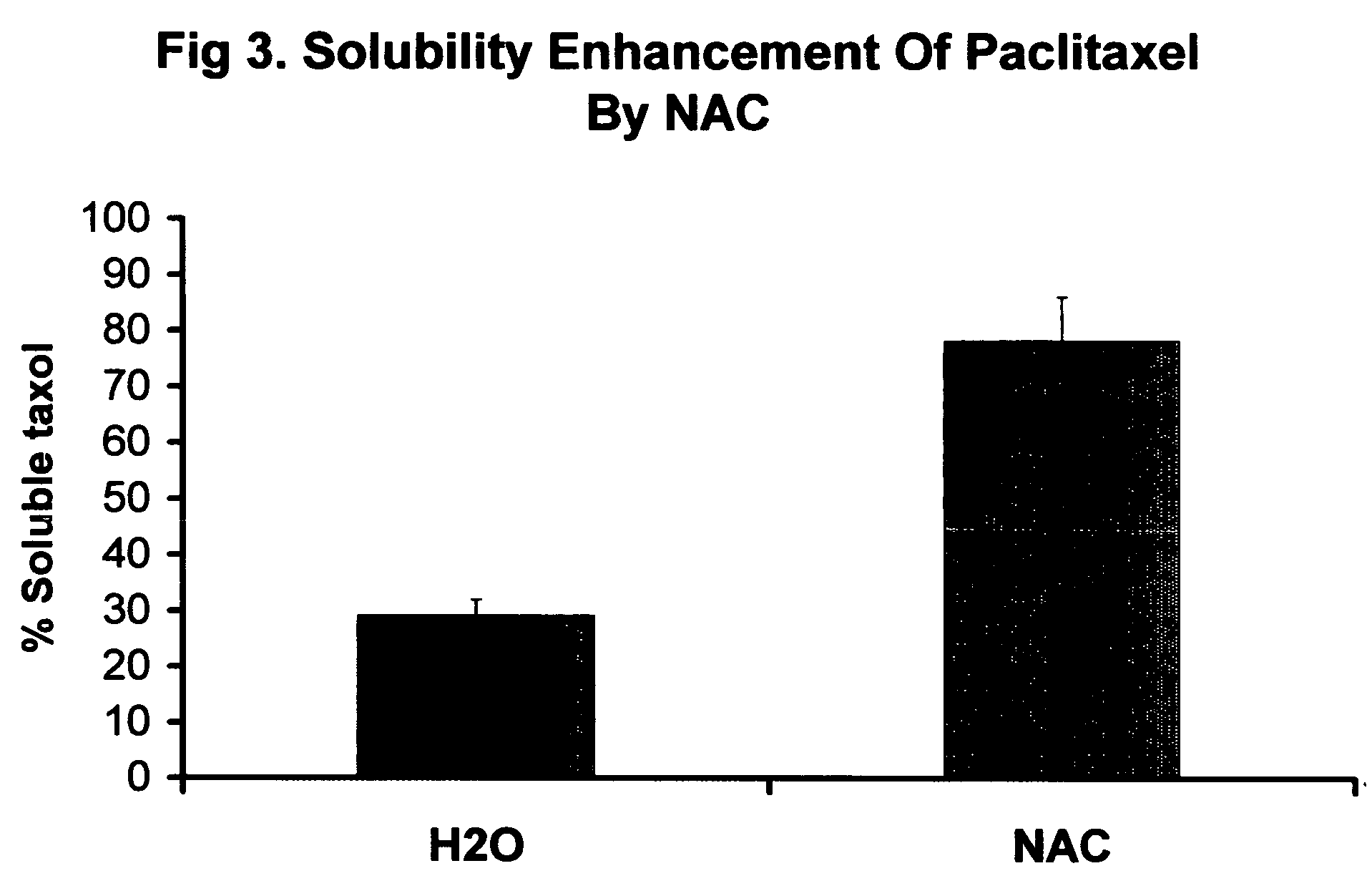
InactiveUS20070225213A1Improve solubilityLow biological effectHeavy metal active ingredientsBiocideIn vivoProliferation rate
Nucleic acid drug carriers comprise a nucleic acid carrier complexed with a drug, wherein the nucleic acid carrier and the drug are associated non-covalently, and optionally other agents such as spacer, transfection agents, and targeting agents. The nucleic acid drug complex are discovered to have permissive or refractory uptake depending on many factors including cell type, proliferation rate, among others. The refractive uptake of the nucleic acid drug complex are shown to be useful in the nucleic acid targeting of drugs, both in vitro and in vivo. Novel drug compositions are disclosed that effectively reduce the toxicity of drugs while maintaining drug activity and enhancing a drug's therapeutic index.
Owner:KOSAK MATTHEW K
Use of WNT inhibitors to augment therapeutic index of chemotherapy
InactiveUS20080075714A1Increase dosePeptide/protein ingredientsAntibody mimetics/scaffoldsIntestinal structureWnt inhibitor
Methods and compositions are provided for the protection of normal cells from cytoreductive therapy that target proliferating cells, by administering an inhibitor of Wnt signaling pathways. Wnt signaling is critically important for homeostasis of the epithelial lining of the adult intestine and other proliferating normal adult tissues.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
L-Threonine derivatives of high therapeutic index
The present invention is directed to a derivative comprised of an L-Threonine bonded to a medicament or drug having a hydroxy, amino, carboxy or acylating derivative thereon. The derivative has the same utility as the drug from which it is made, but it has enhanced therapeutic properties. In fact, the derivatives of the present invention enhance at least one or more therapeutic qualities, as defined herein. The present invention is also directed to pharmaceutical compositions containing same.
Owner:SIGNATURE R & D HLDG LLC
Engineered antibody-interferon mutant fusion molecules
The field of the present invention relates to genetically engineered fusion molecules, methods of making said fusion molecules, and uses thereof in anti-tumor immunotherapies. More specifically, the present invention relates to fusion molecule constructs wherein a tumor associated antigen (TAA) antibody (Ab) serves as a targeting moiety to selectively deliver a cytokine to a tumor cell for purposes of killing or inhibiting the growth or proliferation of said tumor cell. In various embodiments, the engineered fusion molecules comprise a TAA Ab fused to an interferon-alpha (IFN-α) mutant molecule. The engineered Ab-IFN-α mutant fusion molecules of the present invention demonstrate improved therapeutic index and preserved or increased efficacy as compared to Ab-wildtype IFN-α fusion molecules, and / or demonstrate improved PK properties as compared to Ab-wildtype IFN-α fusion molecules.
Owner:IMMUNGENE
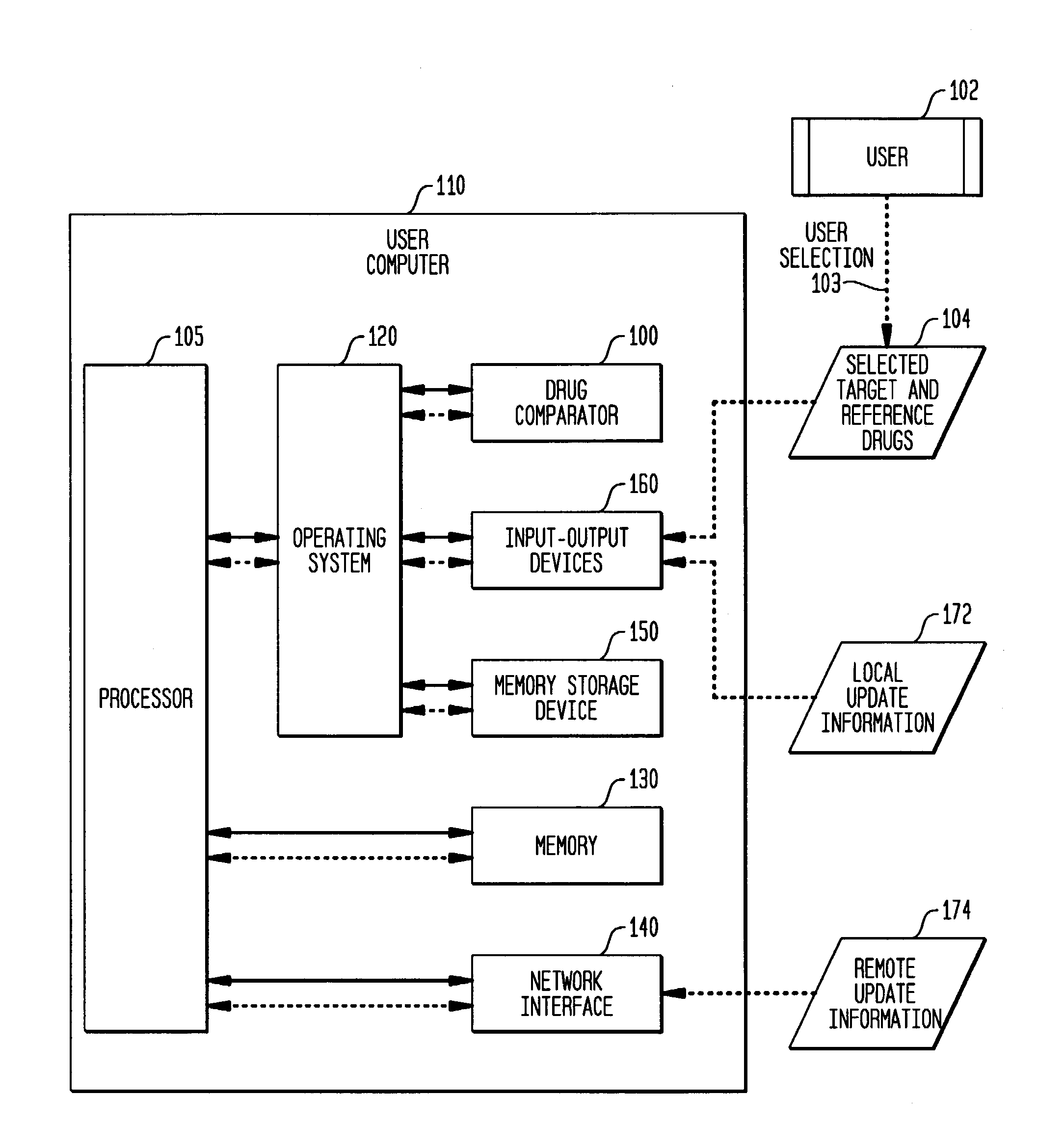
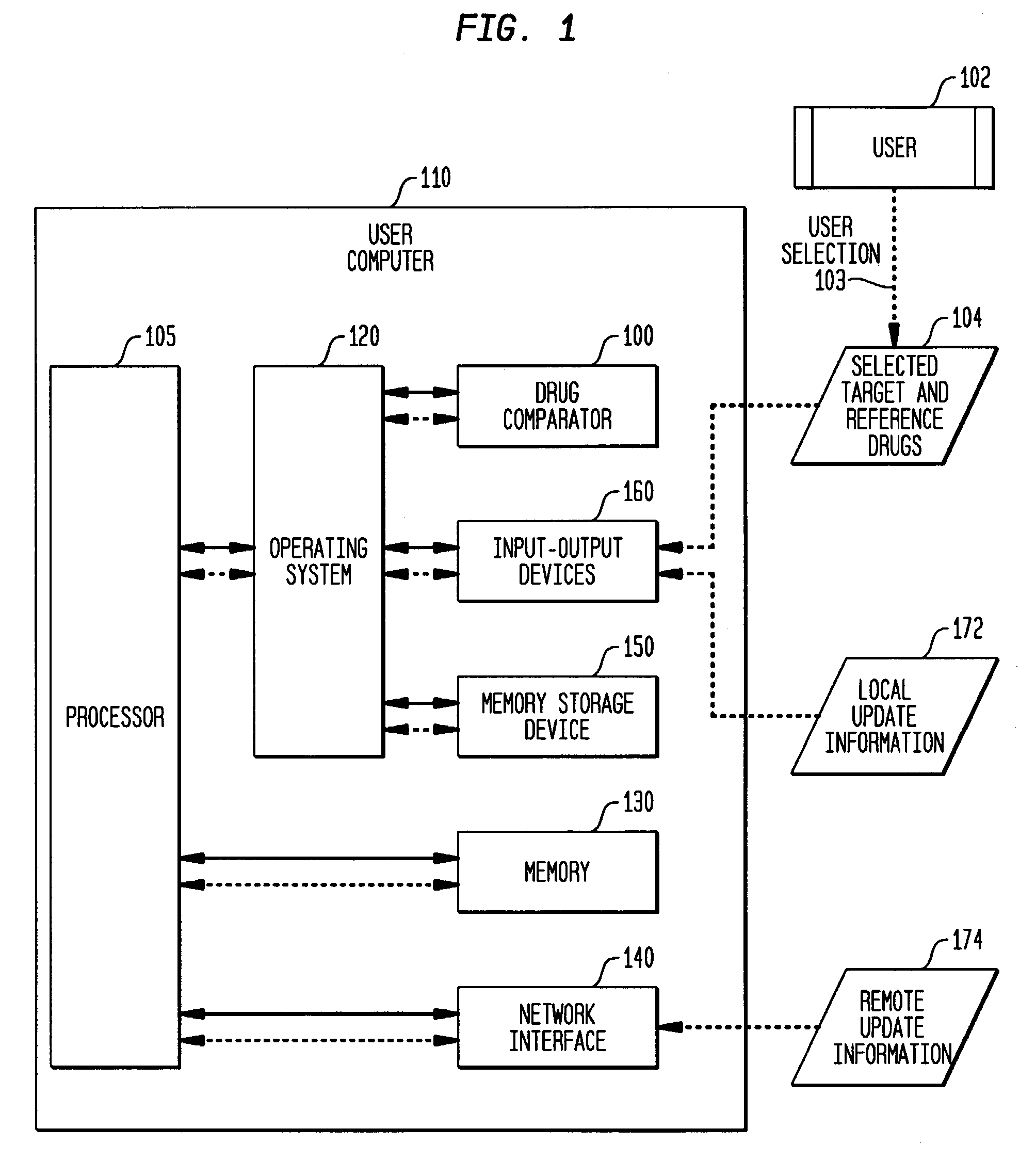
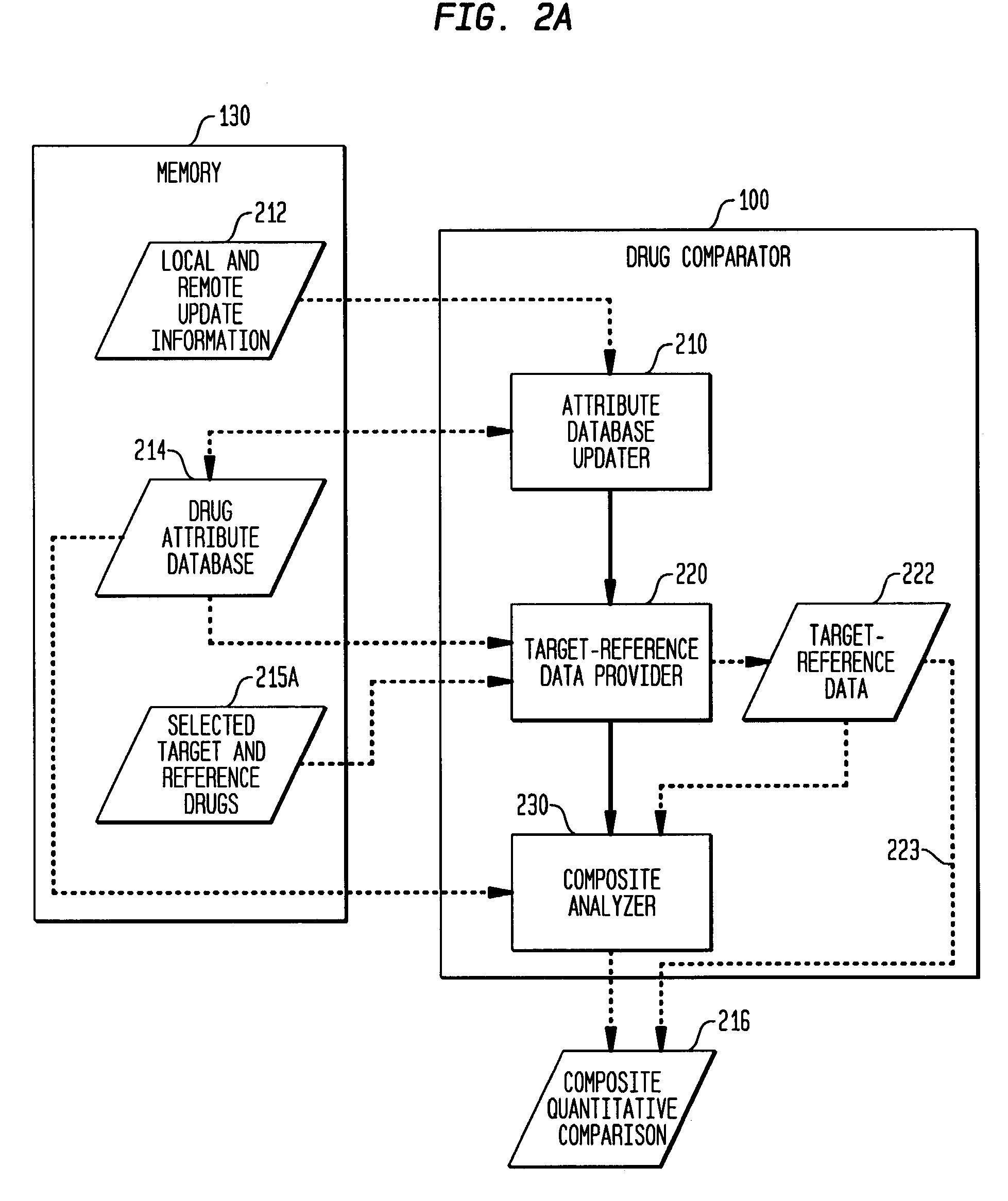
Multi-attribute drug comparison
A computer-implemented apparatus or method, or a software product, for generating a composite quantitative comparison of drug products based on multiple attributes of them. A set of name-attribute similarity scores are generated based on similarities among the names of selected target and reference drugs. A set of product-attribute similarity scores are generated based on similarities among product attributes of the selected target and reference drugs. A target drug confusability score is generated based on the confusability of the target drug as compared to a population of other drugs. The composite quantitative comparison is generated based on a composite of the name-attribute and product-attribute similarity scores, and the target confusability score. A set of one or more severity of confusion scores may also be included in the composite quantitative comparison. These scores are based on one or more indicators of the severity of the consequences to a patient of confusing the target and reference drugs so that, for example, the wrong drug is administered to the patient, or the correct drug is incorrectly administered. The name-attribute similarity scores may be generated based on orthographic, phonetic, and / or phonological analysis. The product-attribute similarity scores may be generated based on the drugs'strengths, indications, dosages, administration routes, manufacturers, pharmacological categories, storage requirements, colors, shapes, legal standing, trademark description, and / or other attributes. The composite quantitative comparison may include severity-weighted similarity scores or both similarity scores and severity of confusion scores. The severity of confusion indicators may include a therapeutic index and / or a contraindication index.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
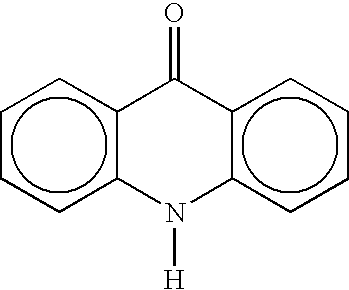
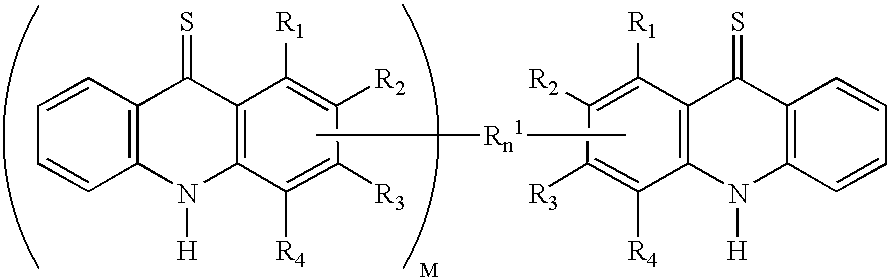
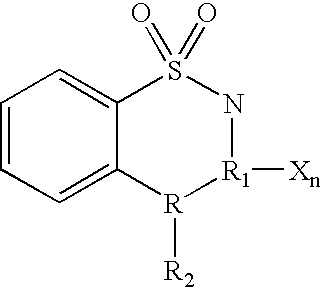
Cyclin dependent kinase (CDK)4 inhibitors and their use for treating cancer
Certain derivatives of acridones and benzothiadiazines have been found to have anti-cancer properties by virtue of their specific inhibition of the cyclin D dependent kinase CDK4. These molecules inhibit CDK4 activity more than they inhibit the activity of other such kinases (e.g. CDC2 and CDK2). This specificity results in an improved therapeutic index when used as drugs to treat susceptible cancers.
Owner:UNITED STATES OF AMERICA +2
Cationic lipids and methods for the delivery of therapeutic agents
InactiveUS20140134260A1Cyclic stabilitySize requirementOrganic active ingredientsBiocideLipid formationProtein target
The present invention provides compositions and methods for the delivery of therapeutic agents to cells. In particular, these include novel cationic lipids and nucleic acid-lipid particles that provide efficient encapsulation of nucleic acids and efficient delivery of the encapsulated nucleic acid in vivo. The compositions of the present invention are highly potent, thereby allowing effective know-down of a specific target protein at relatively low doses. In addition, the compositions and methods of the present invention are less toxic and provide a greater therapeutic index compared to compositions and methods previously known in the art.
Owner:PROTIVA BIOTHERAPEUTICS
Engineered antibody-interferon mutant fusion molecules
ActiveUS20130230517A1Peptide/protein ingredientsAntibody mimetics/scaffoldsWild typeInterferon alpha
The field of the present invention relates to genetically engineered fusion molecules, methods of making said fusion molecules, and uses thereof in anti-tumor immunotherapies. More specifically, the present invention relates to fusion molecule constructs wherein a tumor associated antigen (TAA) antibody (Ab) serves as a targeting moiety to selectively deliver a cytokine to a tumor cell for purposes of killing or inhibiting the growth or proliferation of said tumor cell. In various embodiments, the engineered fusion molecules comprise a TAA Ab fused to an interferon-alpha (IFN-α) mutant molecule. The engineered Ab-IFN-α mutant fusion molecules of the present invention demonstrate improved therapeutic index and preserved or increased efficacy as compared to Ab-wildtype IFN-α fusion molecules, and / or demonstrate improved PK properties as compared to Ab-wildtype IFN-α fusion molecules.
Owner:IMMUNGENE
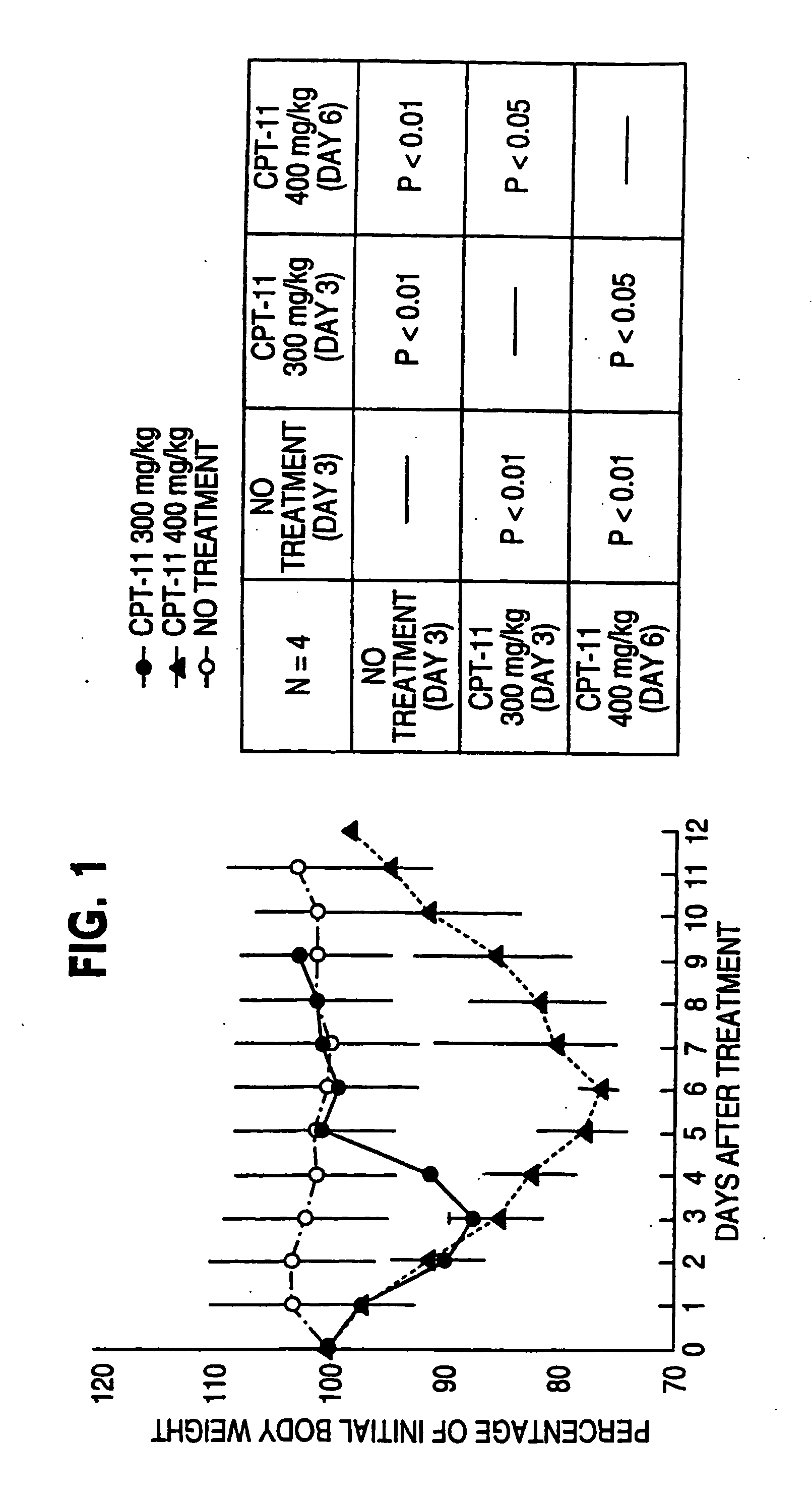
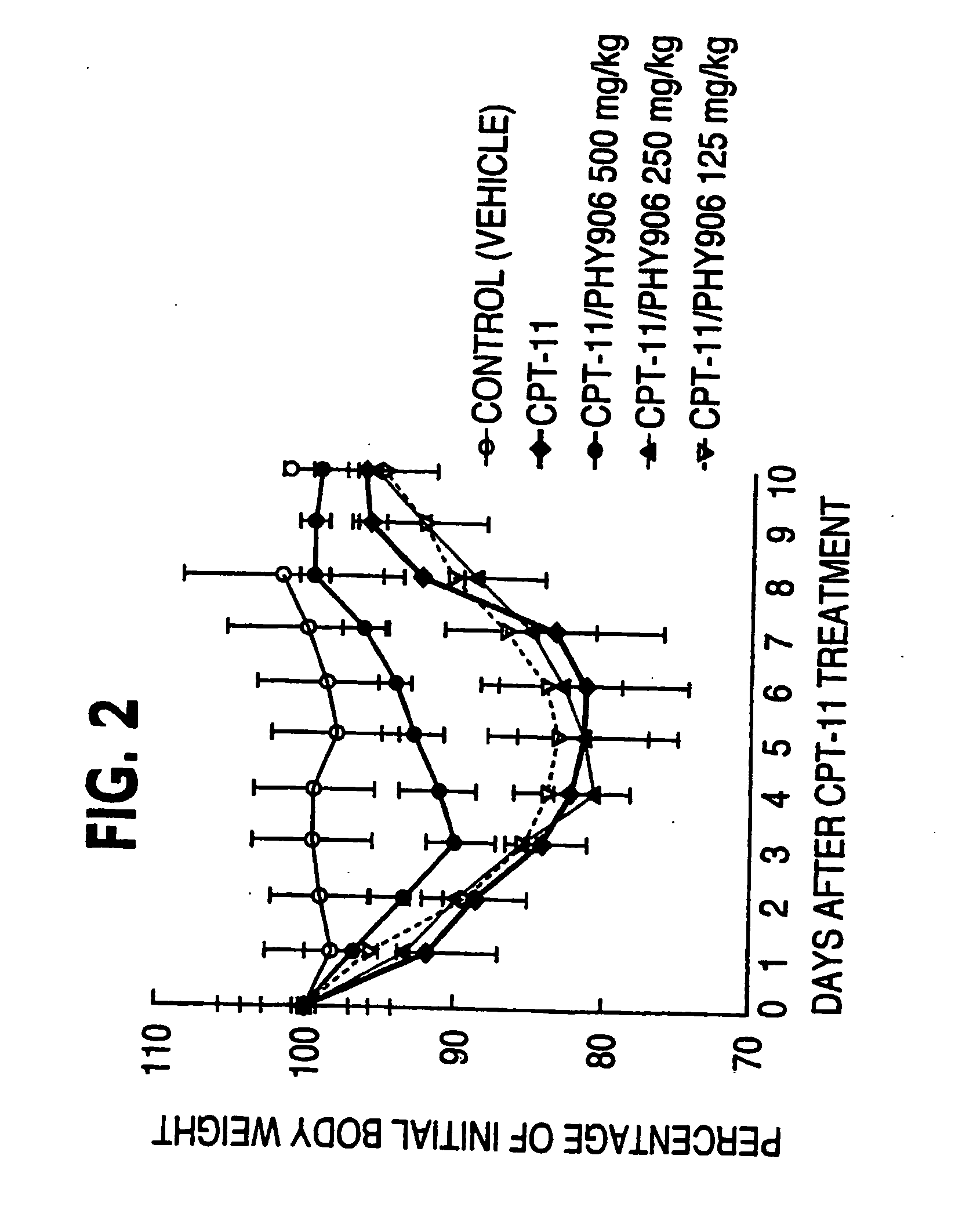
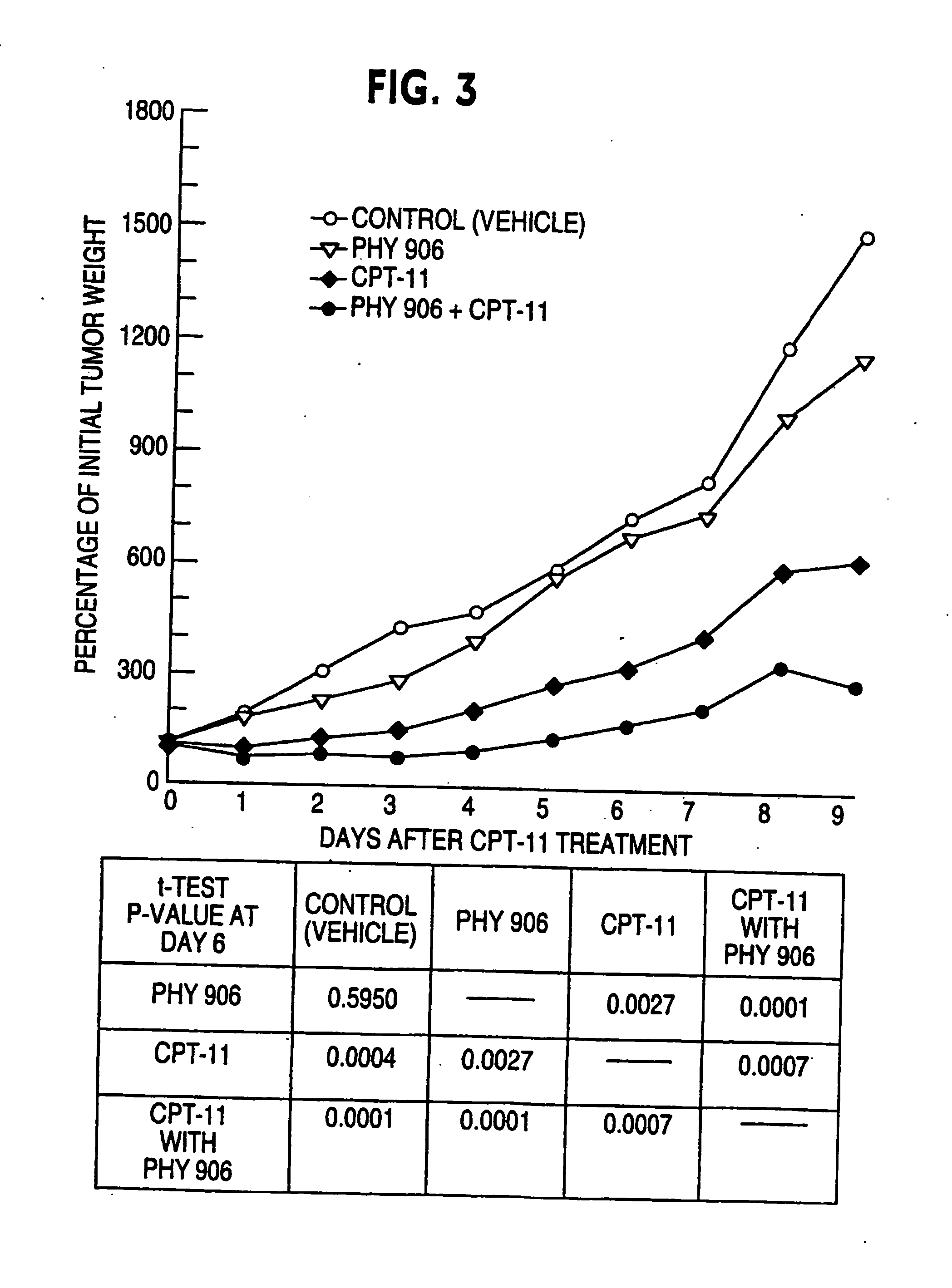
Herbal composition PHY906 and its use in chemotherapy
InactiveUS20050196473A1High indexModulating hematopoietic activityBiocideAntiviralsQuality of lifeNeoplasm
This invention provides herbal compositions useful for increasing the therapeutic index of drugs, including those used in the treatment of disease, especially viral infections and neoplasms of cancer. This invention provides methods useful for improving the quality of life of an individual undergoing chemotherapy. Furthermore, this invention improves the treatment of disease by increasing the therapeutic index of chemotherapy drugs by administering the herbal composition PHY906 to a person undergoing such chemotherapy.
Owner:YALE UNIV
Systems approach to disease state and health assessment
Methods, systems, and apparatus for assessing a state of an epilepsy disease or a comorbidity thereof are provided. The methods comprise receiving at least one autonomic index, neurologic index, stress marker index, psychiatric index, endocrine index, adverse effect of therapy index, physical fitness index, or quality of life index of a patient; comparing the at least one index to at least one reference value; and assessing a state of an epilepsy disease or a body system of the patient based on the comparison. A computer readable program storage device encoded with instructions that, when executed by a computer, perform the method described above is also provided. A medical device system capable of implementing the method described above is also provided.
Owner:FLINT HILLS SCI L L C
Recombinant human albumin fusion proteins with long-lasting biological effects
ActiveUS7244833B2Good for healthImprove stabilityBacteriaPeptide/protein ingredientsDiseaseHuman albumin
Compositions, kits and methods are provided for promoting general health or for prevention or treatment of diseases by using novel recombinant fusion proteins of human serum albumin (HSA) and bioactive molecules. The bioactive molecules may be a protein or peptide having a biological function in vitro or in vivo, and preferably, having a therapeutic activity when administered to a human. By fusing the bioactive molecule to HSA, stability of the bioactive molecule in vivo can be improved and the therapeutic index increased due to reduced toxicity and longer-lasting therapeutic effects in vivo. In addition, manufacturing processes are provided for efficient, cost-effective production of these recombinant proteins in yeast.
Owner:YU ZAILIN +1
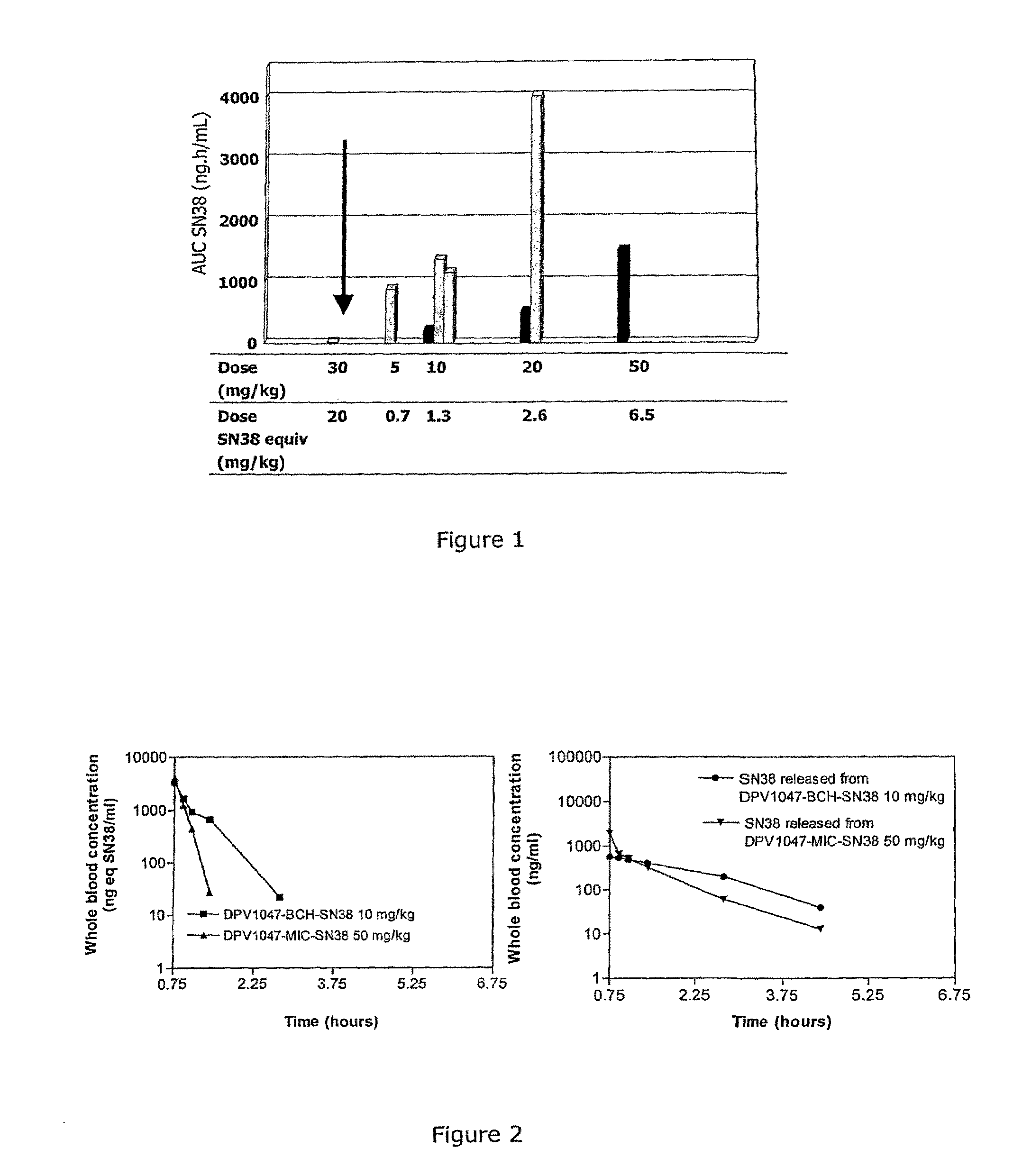
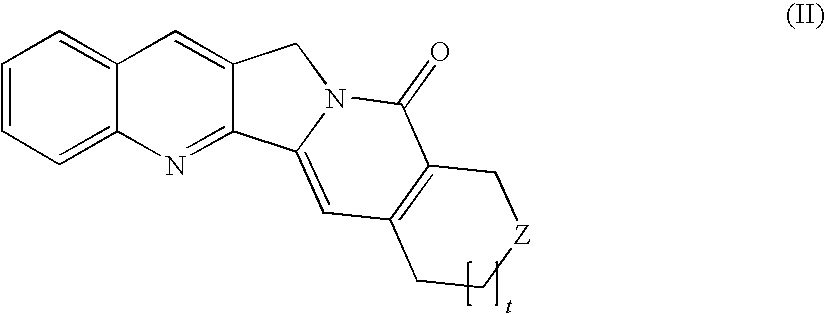
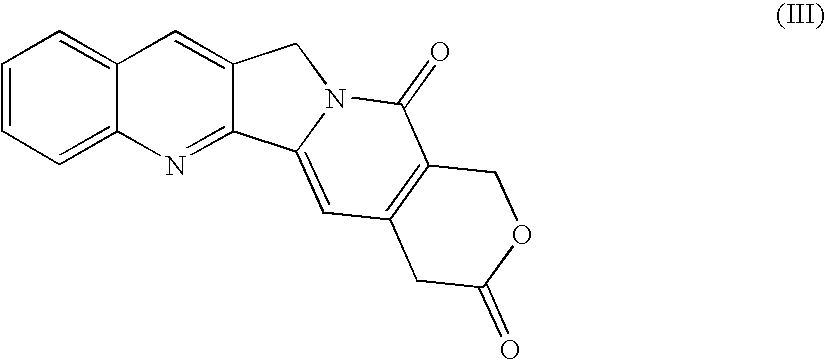
Camptothecin-peptide conjugates and pharmaceutical compositions containing the same
InactiveUS20100015136A1Improve solubilityModify pharmacokineticsPeptide/protein ingredientsAntibody ingredientsBULK ACTIVE INGREDIENTAqueous solubility
The present invention relates to a novel compound of use in the improved delivery of therapeutic drug agents into target cells or tissues, composition comprising the same and uses thereof. The compound is more specifically a conjugate of a peptide moiety and a camptothecin, a derivative or analog thereof which provides numerous benefits, including enhancement in terms of aqueous solubility, pharmacokinetics and tissue distribution, enlargement of the therapeutic index, and limitation of the inter-patient metabolic variability, as well as improvement of delivery of the biologically active ingredient to the target cells or tissues.
Owner:DRAIS PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com