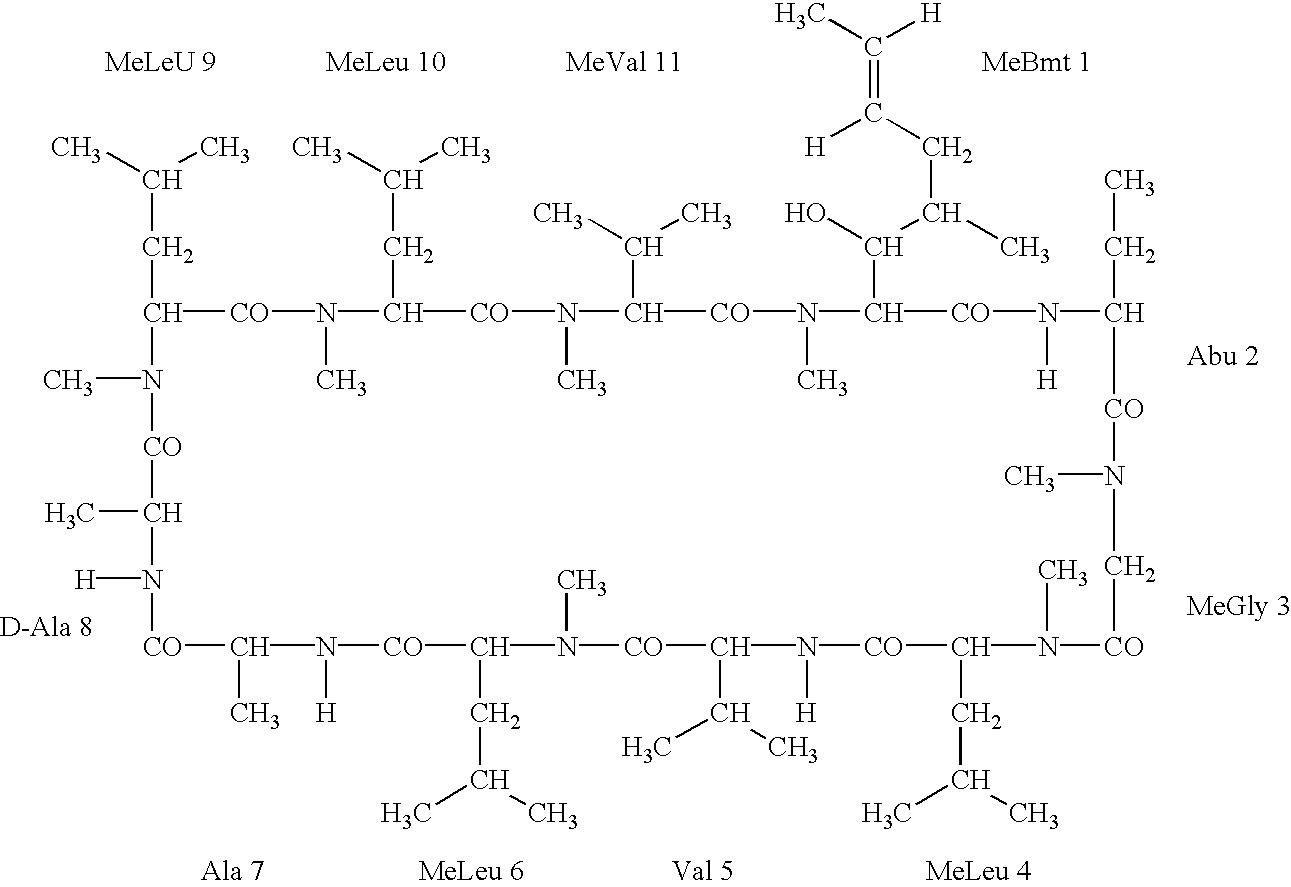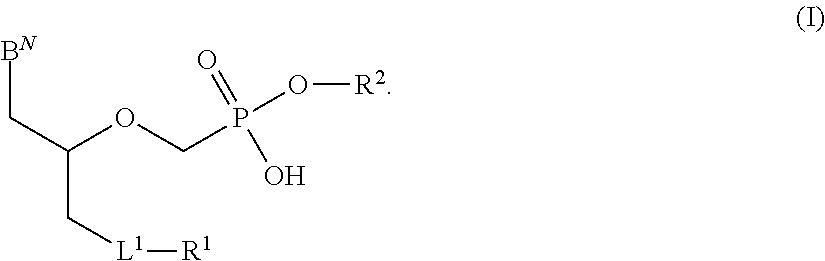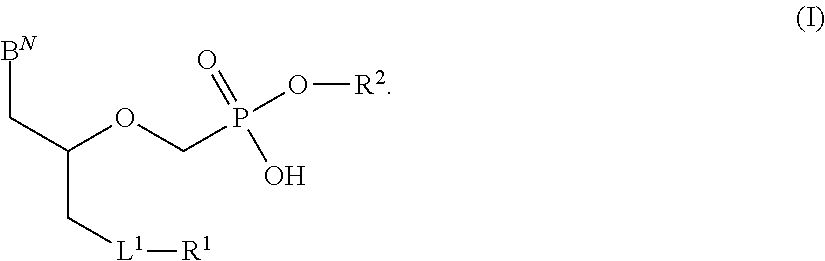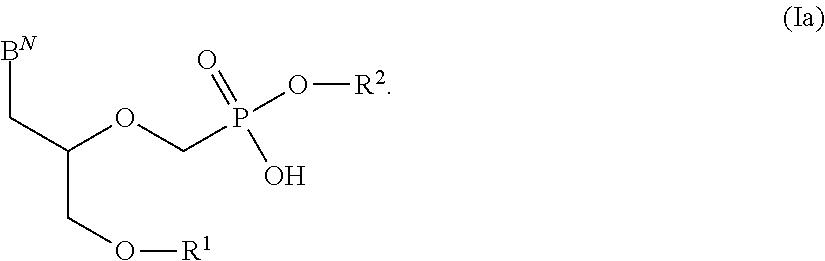Patents
Literature
371 results about "Reduced toxicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Reducing toxicity. Dendrimers can be used to restrict the circulation of the drug in the body. This may avoid side effects, or may allow a higher dose of the drug to be administered than would otherwise be acceptable.
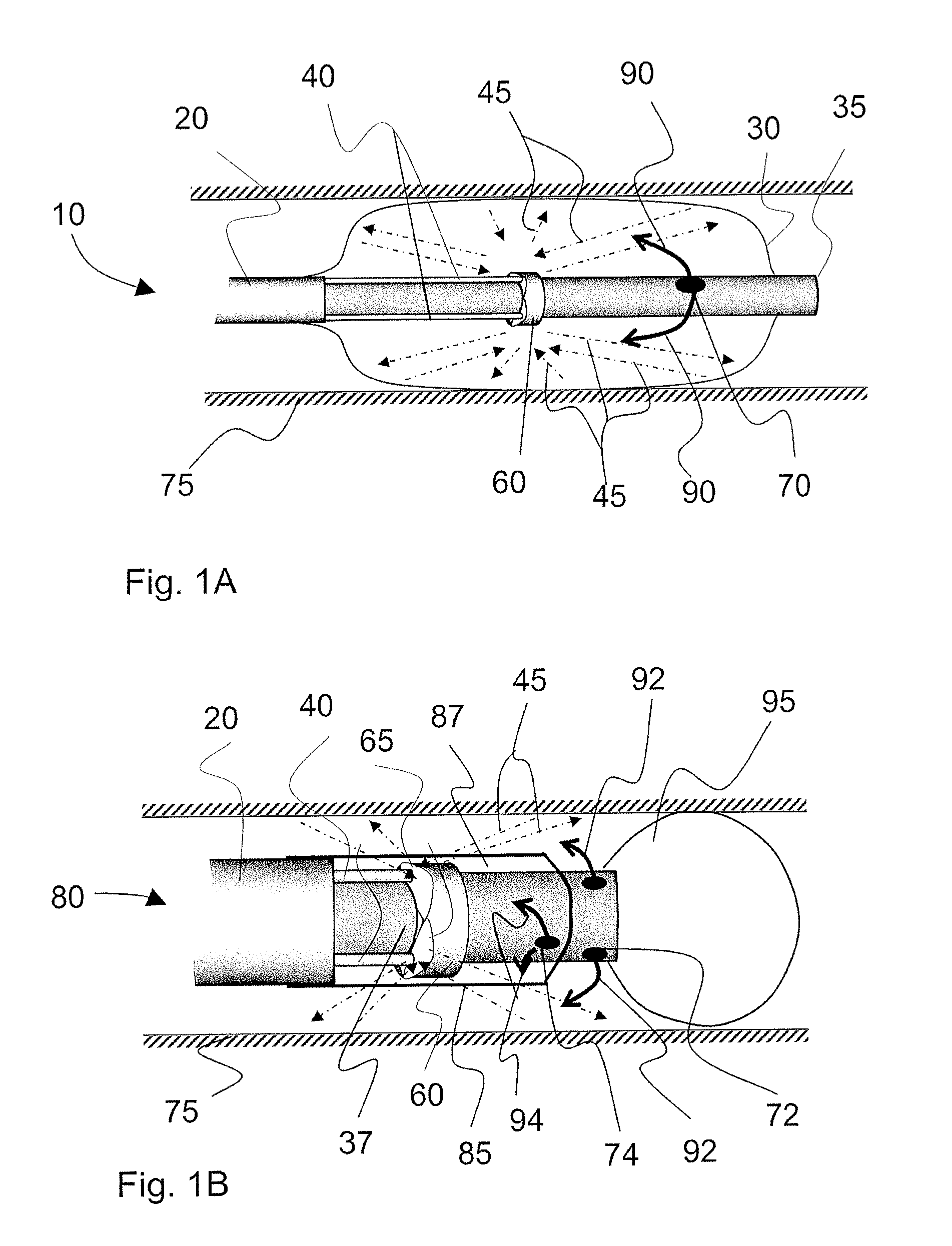
Medical devices having a temporary radiopaque coating
A medical device comprising radiopaque water-dispersible metallic nanoparticles, wherein the nanoparticles are released from the medical device upon implantation of the device. The medical device of the present invention is sufficiently radiopaque for x-ray visualization during implantation, but loses its radiopacity after implantation to allow for subsequent visualization using more sensitive imaging modalities such as CT or MRI.The nanoparticles are formed of a metallic material and have surface modifications that impart water-dispersibility to the nanoparticles. The nanoparticles may be any of the various types of radiopaque water-dispersible metallic nanoparticles that are known in the art. The nanoparticles may be adapted to facilitate clearance through renal filtration or biliary excretion. The nanoparticles may be adapted to reduce tissue accumulation and have reduced toxicity in the human body. The nanoparticles may be applied directly onto the medical device, e.g., as a coating, or be carried on the surface of or within a carrier coating on the medical device, or be dispersed within the pores of a porous layer or porous surface on the medical device. The medical device itself may be biodegradable and may have the nanoparticles embedded within the medical device itself or applied as or within a coating on the biodegradable medical device. The nanoparticles may be released by diffusion through the carrier coating, disruption of hydrogen bonds between the nanoparticles and the carrier coating, degradation of the nanoparticle coating, degradation of the carrier coating, diffusion of the nanoparticles from the medical device, or degradation of the medical device carrying the nanoparticles.
Owner:BOSTON SCI SCIMED INC
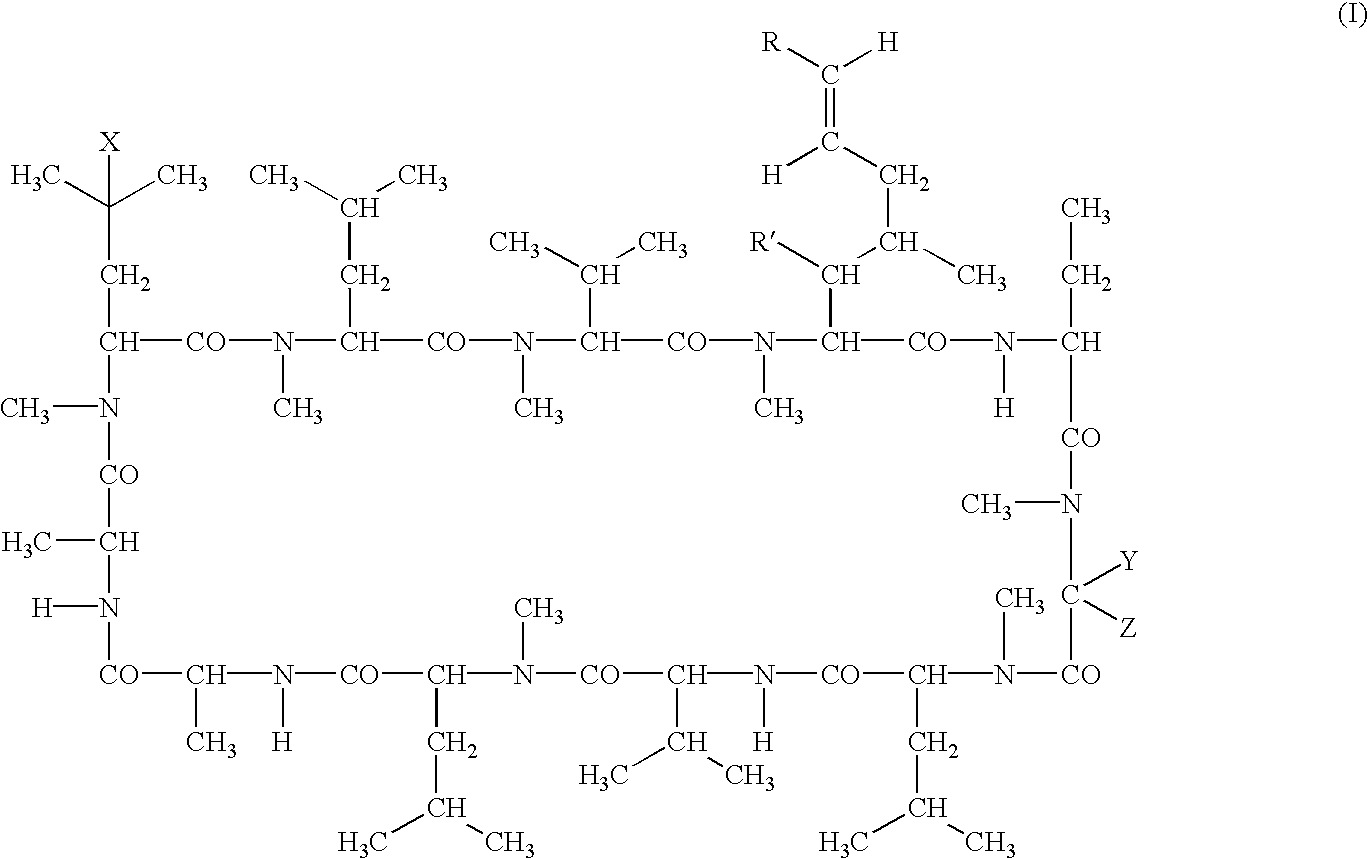
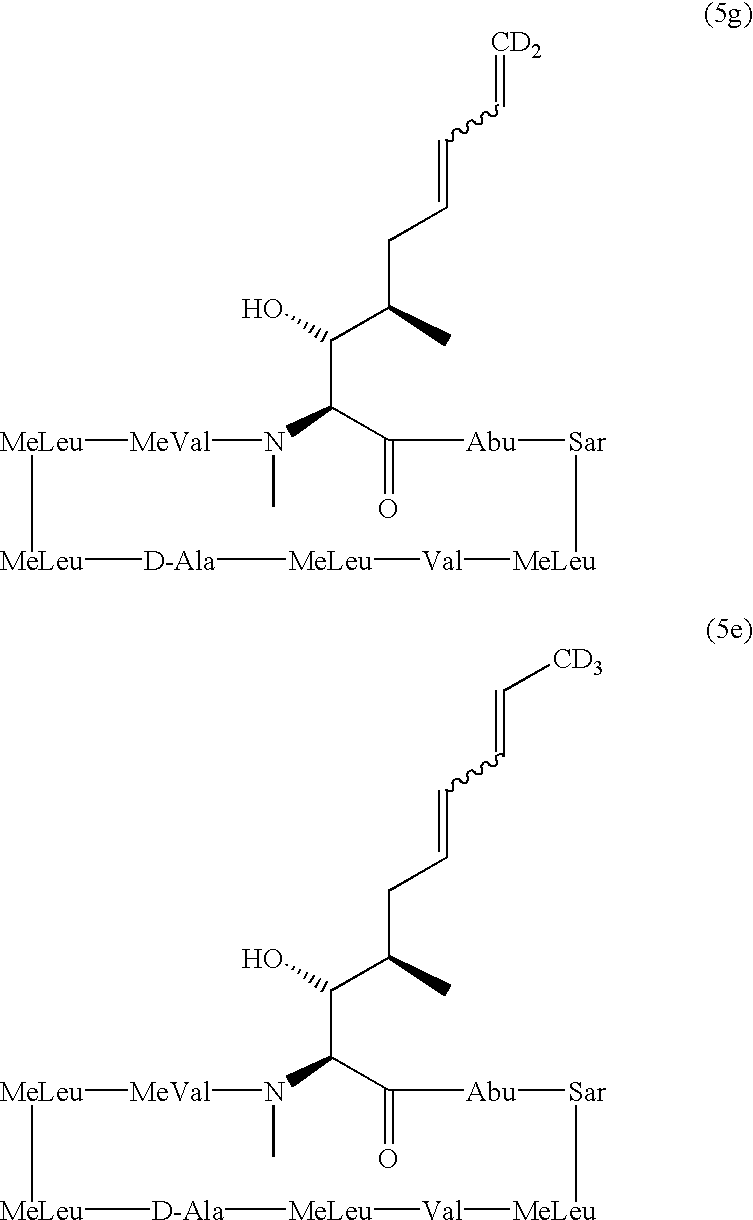
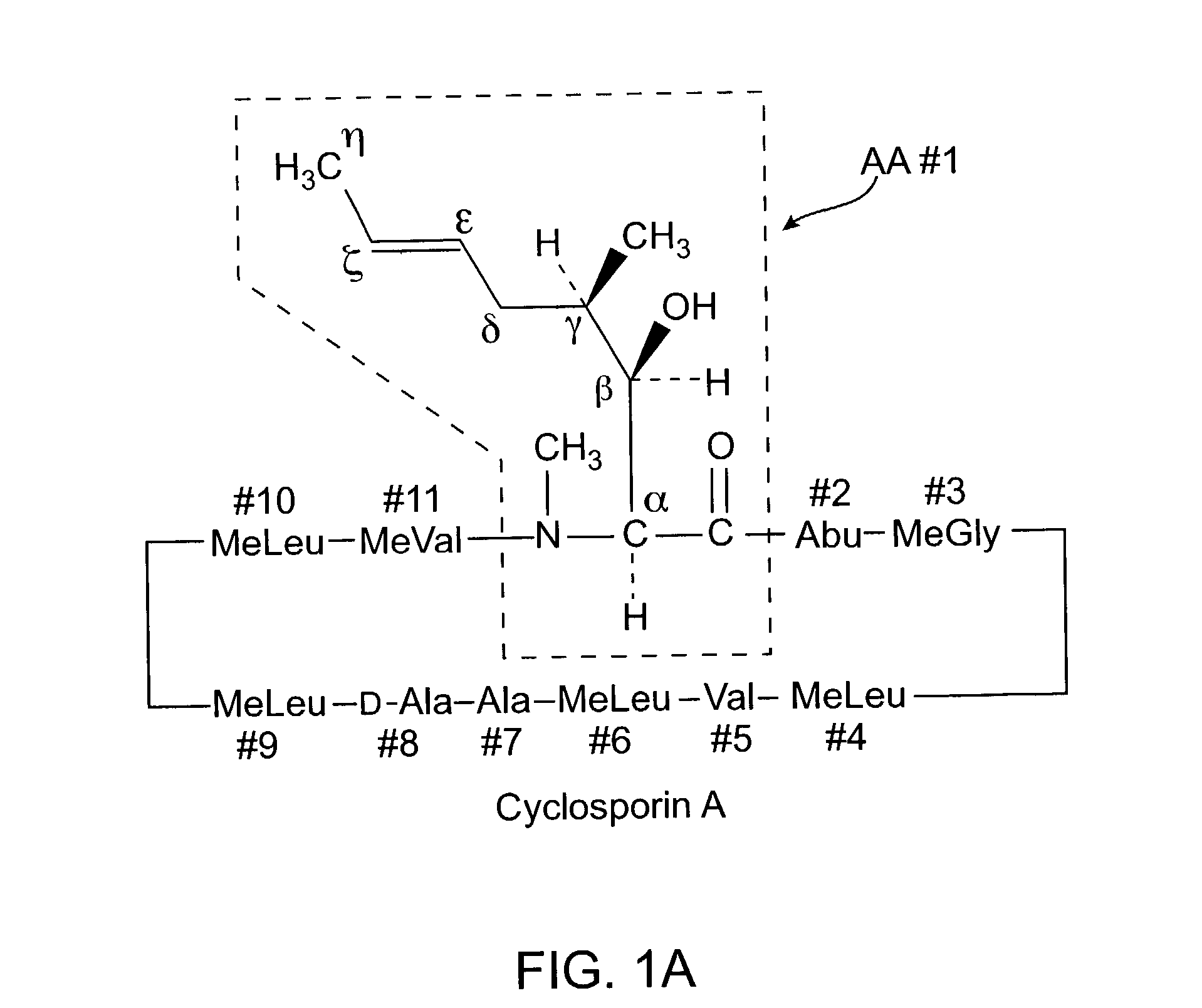
Deuterated cyclosporine analogs and their use as immunomodulating agents
InactiveUS6605593B1Improve practicalityAltered physicochemical and pharmacokinetic propertyNervous disorderAntipyreticCyclosporinsImmunomodulating Agent
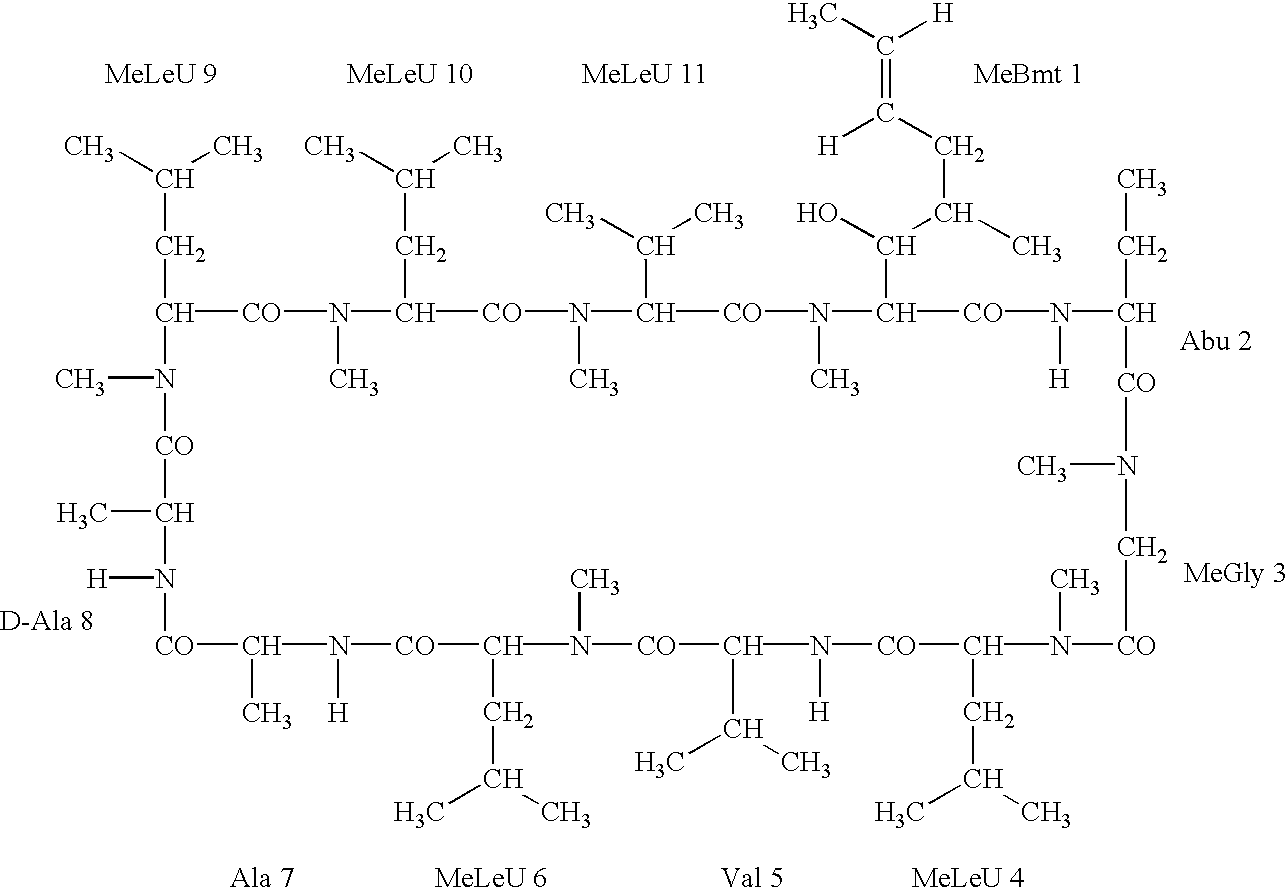
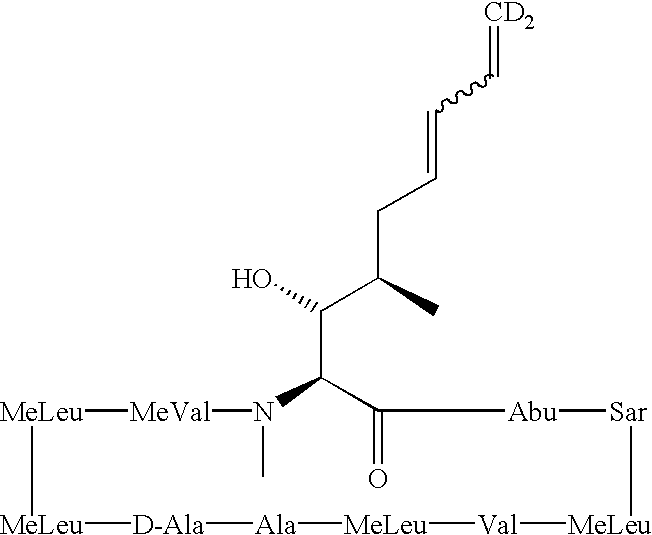
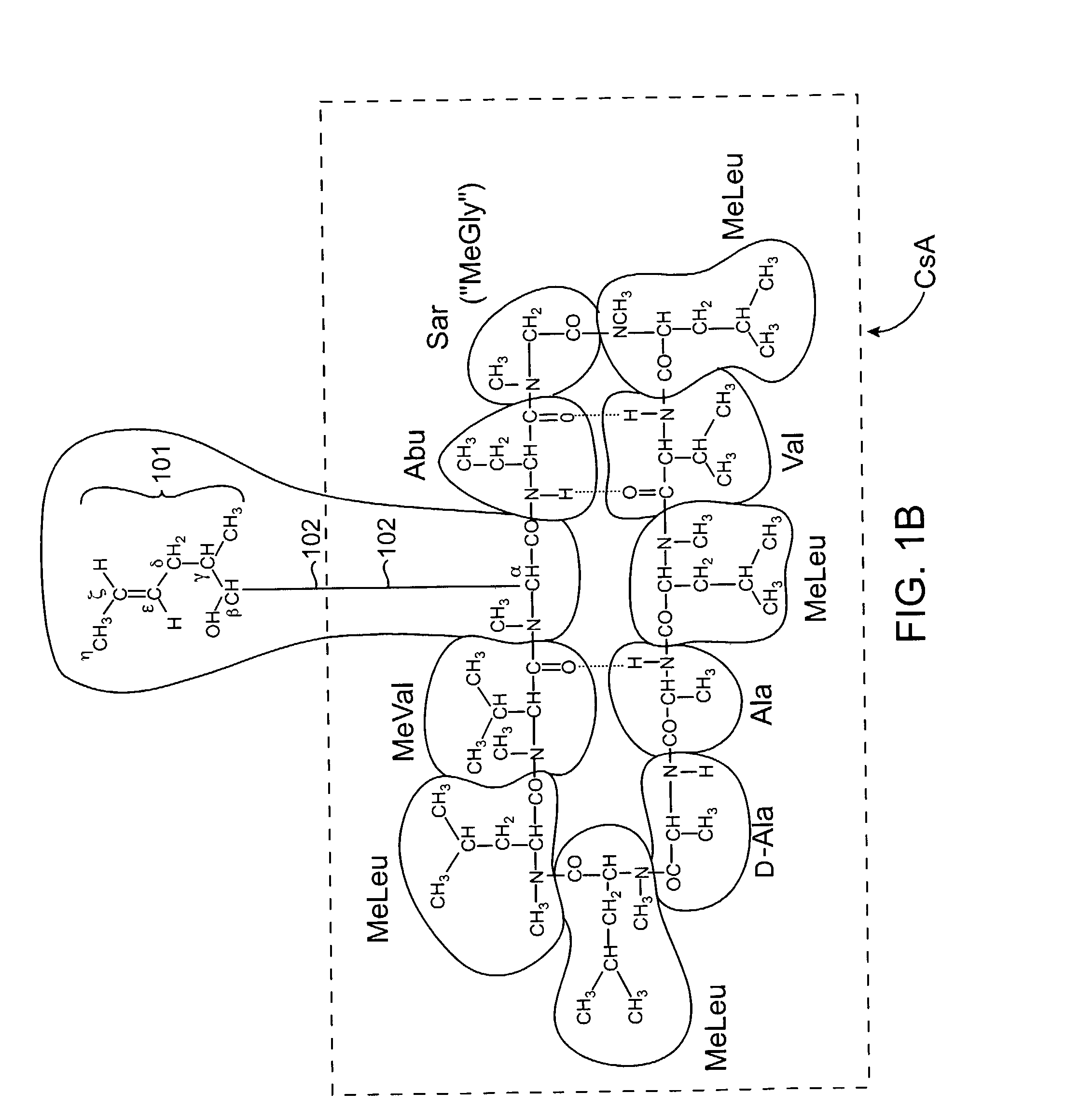
Cyclosporine derivatives are disclosed which possess enhanced efficacy and reduced toxicity over naturally occurring and other presently known cyclosporins and cyclosporine derivatives. The cyclosporine derivatives of the present invention are produced by chemical and isotopic substitution of the cyclosporine A (CsA) molecule by: (1) Chemical substitution and optionally deuterium substitution of amino acid 1; and (2) deuterium substitution at key sites of metabolism of the cyclosporine A molecule such as amino acids 1, 4, 9. Also disclosed are methods of producing the cyclosporine derivatives and method of producing immunosuppression with reduced toxicity with the disclosed cyclosporine derivatives.
Owner:AURINIA PHARMA
Mutant forms of cholera holotoxin as an adjuvant
InactiveUS7332174B2No loss in adjuvanting propertyLow toxicityAntibacterial agentsFungiAntigenAdjuvant
Mutant cholera holotoxins having single or double amino acid substitutions or insertions have reduced toxicity compared to the wild-type cholera holotoxin. The mutant cholera holotoxins are useful as adjuvants in antigenic compositions to enhance the immune response in a vertebrate host to a selected antigen from a pathogenic bacterium, virus, fungus, or parasite, a cancer cell, a tumor cell, an allergen, or a self-molecule.
Owner:WYETH HOLDINGS CORP +1
Mutant forms of cholera holotoxin as an adjuvant
InactiveUS7285281B2No loss in adjuvanting propertyLow toxicityAntibacterial agentsFungiAdjuvantCancer cell
Owner:UNIV OF COLORADO FOUND +1
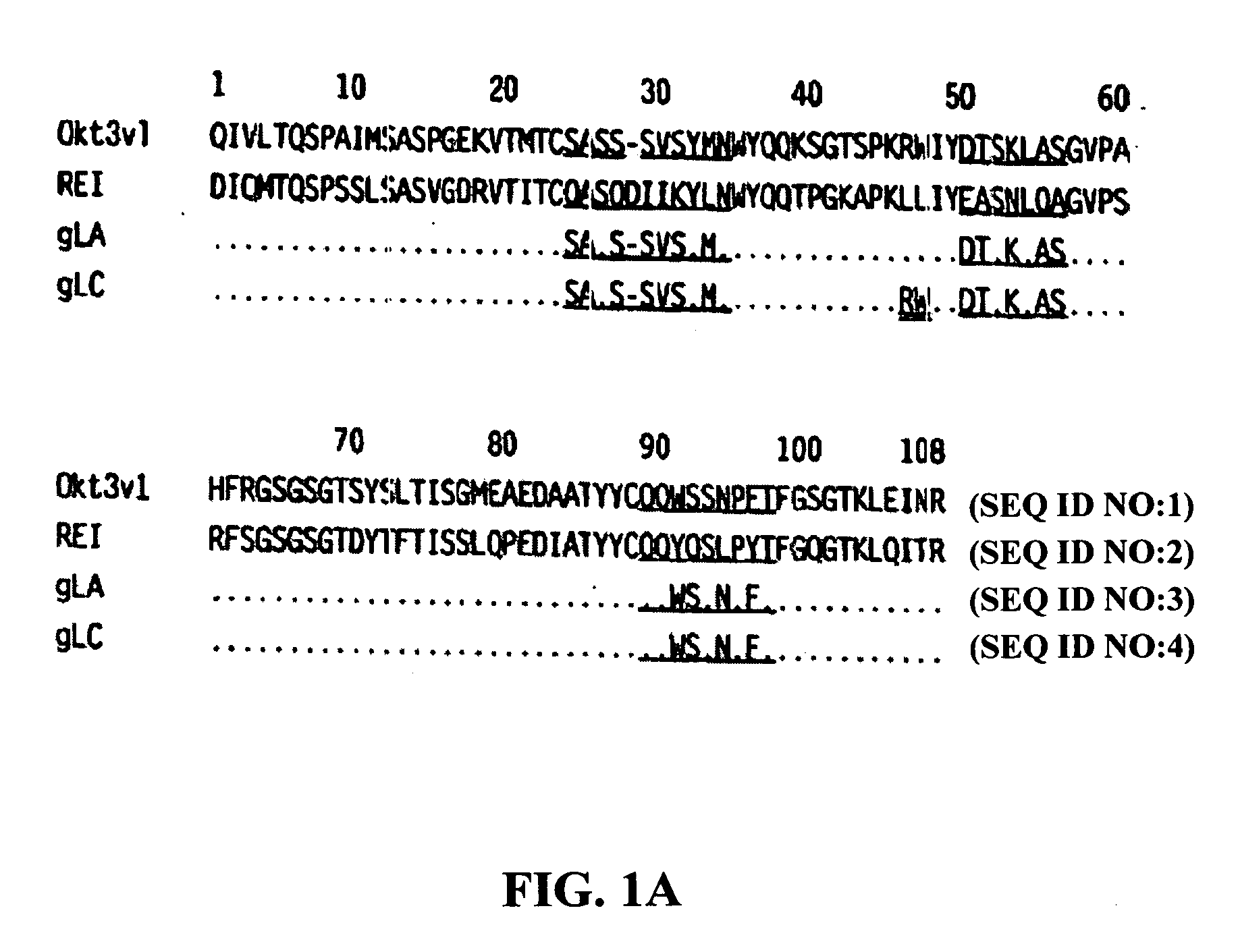
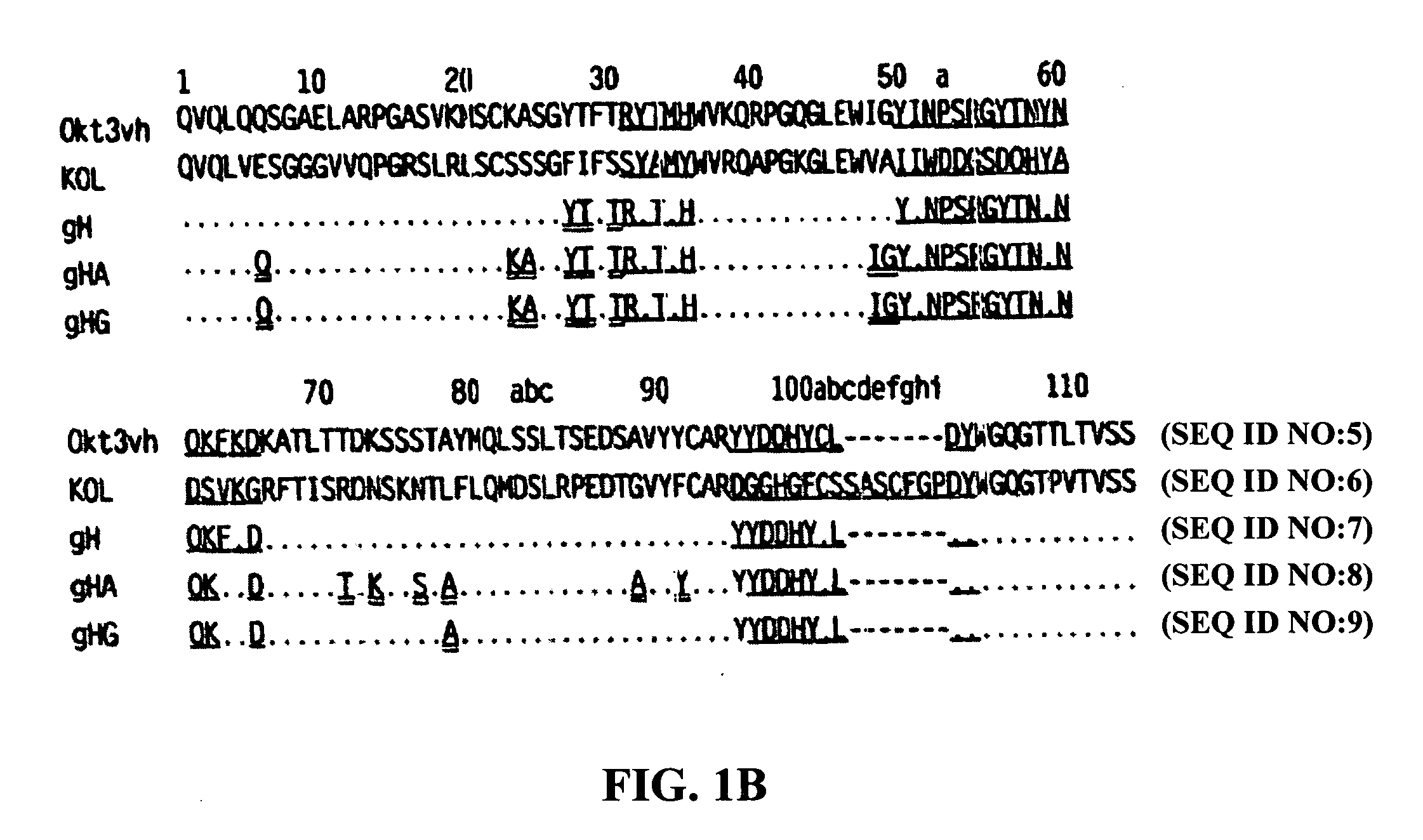
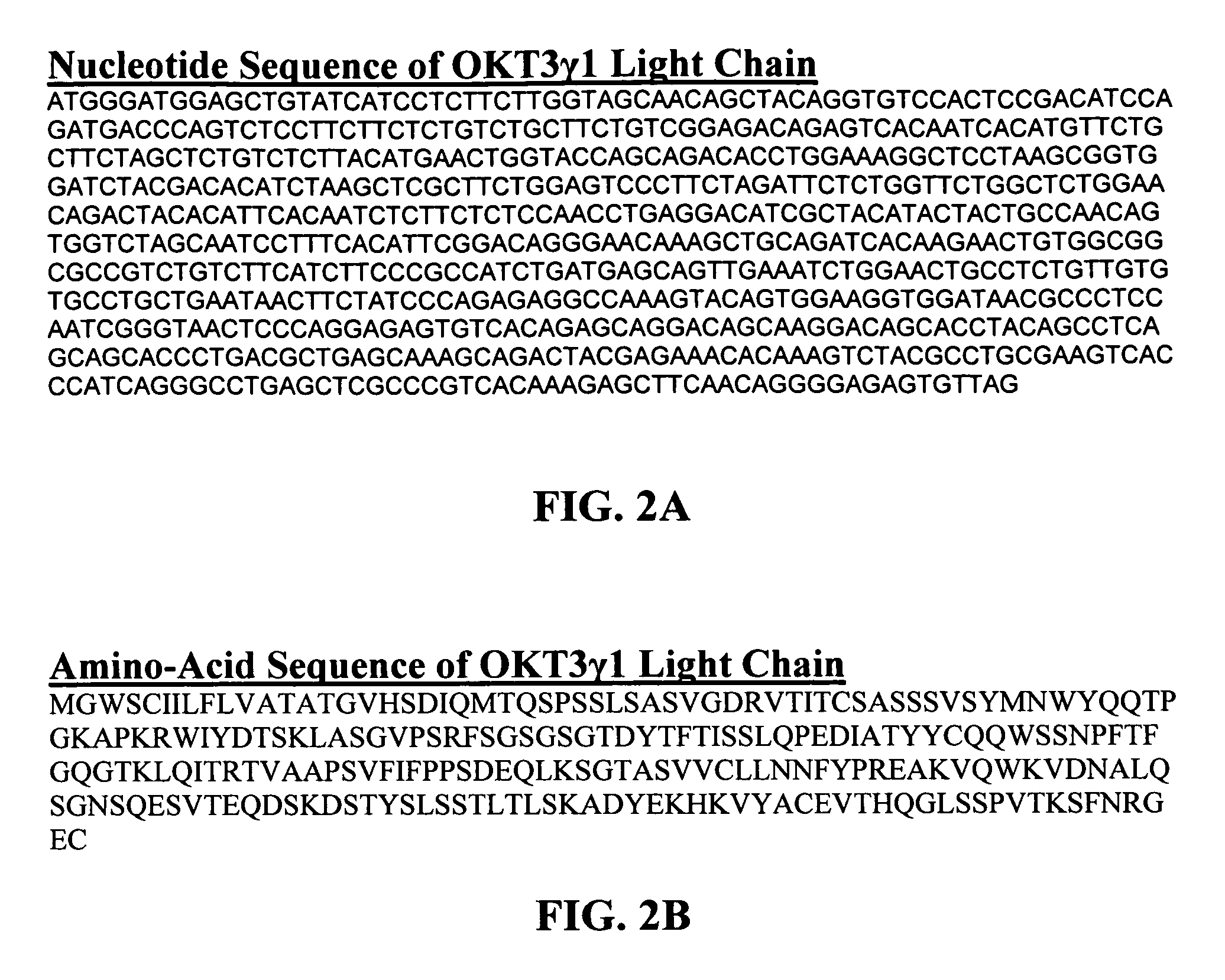
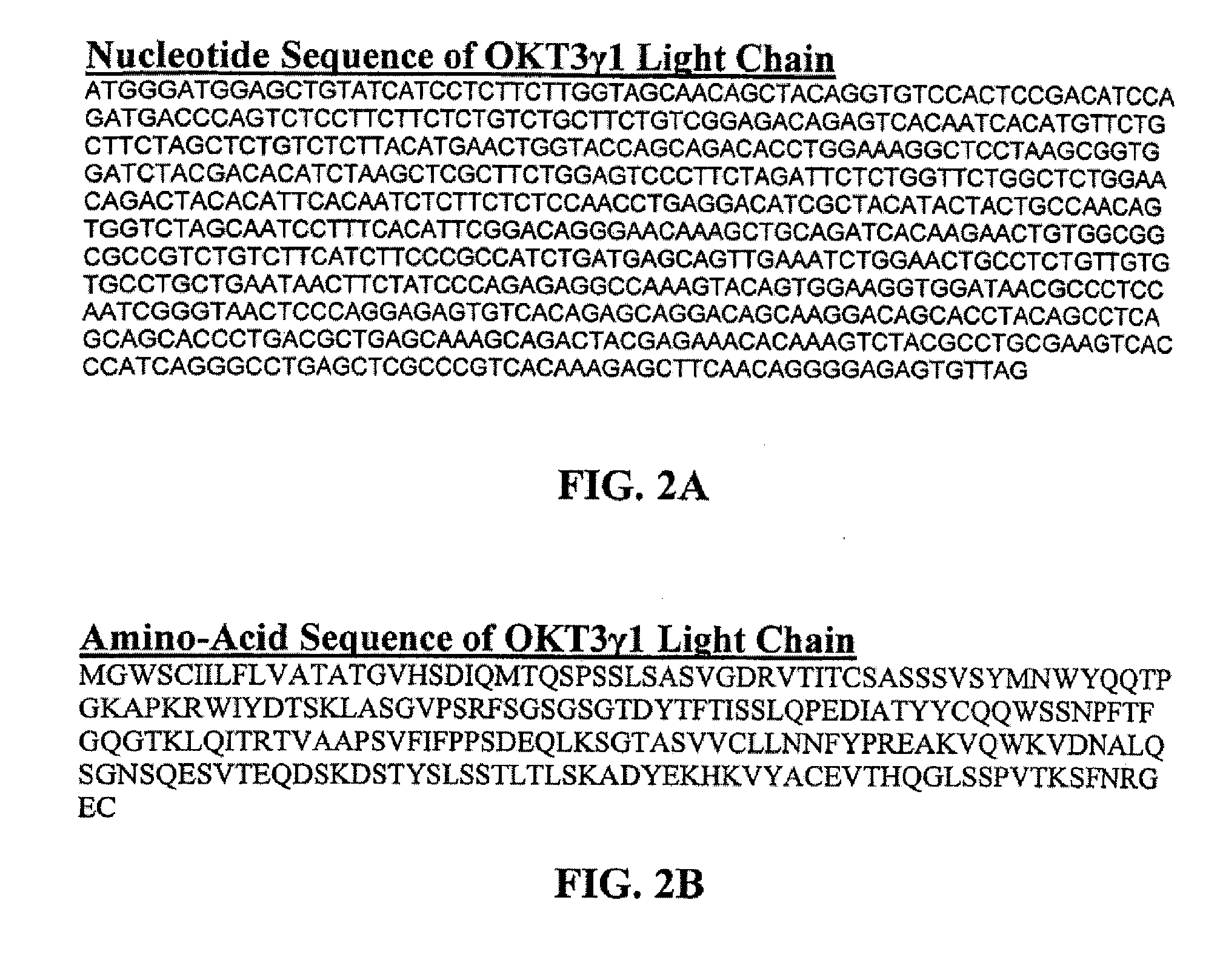
Methods for the treatment of autoimmune disorders using immunosuppressive monoclonal antibodies with reduced toxicity
ActiveUS20070077246A1Treating and preventing and ameliorating symptomSlow and reduce damagePeptide/protein ingredientsAntipyreticAutoimmune conditionAutoimmune disease
The present invention provides methods of treating, preventing or ameliorating the symptoms of T cell-mediated immunological diseases, particularly autoimmune diseases, through the use of anti-CD3 antibodies. In particular, the methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the invention provides for modification of the anti-CD3 antibodies such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-CD3 antibodies.
Owner:PROVENTION BIO INC
Fusogenic lipids and vesicles
InactiveUS20030082154A1Effective protectionReduce deliveryBiocideOrganic active ingredientsLipid formationCell membrane
Novel lipid compounds are provided that may be termed "pro-cationic" in that they are neutral or negatively charged until they are either brought into contact with cellular membranes or are internalized by cells. The lipids have a hydrophobic tail group and a hydrophilic head group, the head group incorporating both a positively and negatively charged region at physiological pH. The hydrophobic tail group is stably connected to the positive region of the head group which in turn is connected to the negative region by a disulfide bond that is susceptible to cleavage by membrane-bound and intracellular factors. Cleavage of the disulfide bond removes the negatively charged region from the head group resulting in a lipid that is cationic and therefor fusogenic with negatively charged cell membranes. Consequently, lipids of the invention are useful as components of liposomes that serve as vehicles for delivering pharmaceutical agents into cells with reduced toxicity.
Owner:IONIS PHARMA INC
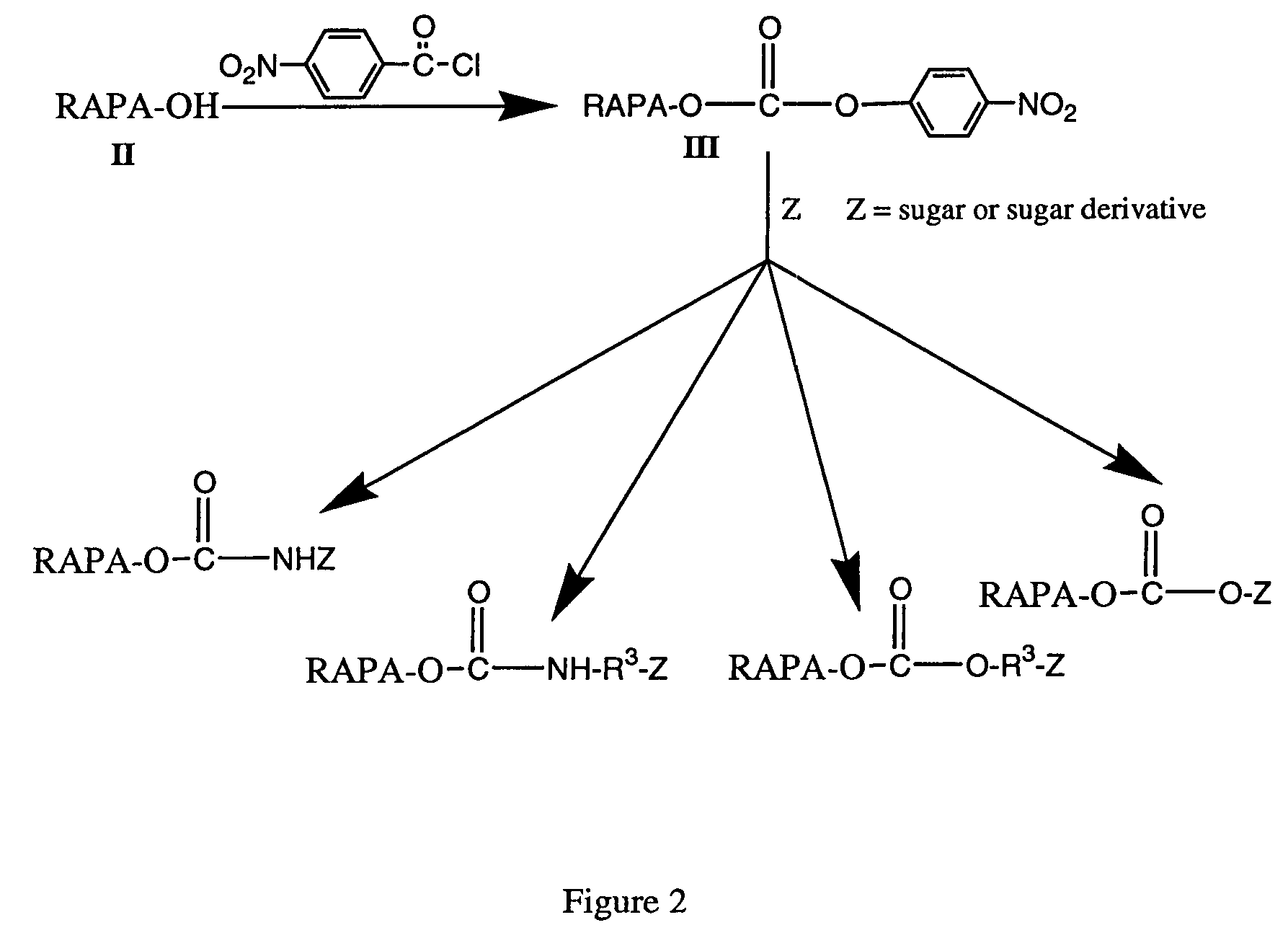
Rapamycin carbohydrate derivatives
InactiveUS7160867B2Low toxicityMore water solubleAntibacterial agentsBiocidePseudosugarsCarbohydrate derivative
This invention provides modified rapamycins that have specific monosaccharide(s), oligosaccharide(s), pseudosugar(s) or derivatives thereof attached through a linker to create rapamycin carbohydrate derivatives having enhanced pharmacokinetic and / or pharmacodynamic profiles. For example, administration of the rapamycin carbohydrate derivative results in altered pharmacokinetic profiles and reduced toxicities. Thus, the present invention provides compounds with characteristics that are distinct from other drugs in its class such as rapamycin.
Owner:ISOTECHNIKA INC
Compositions and methods for tumor-targeted delivery of effector molecules
InactiveUS6962696B1Inhibit tumor growthReduce tumor volumeOrganic active ingredientsHeavy metal active ingredientsTumor targetMelanoma
The present application discloses the preparation and use of attenuated tumor-targeted bacteria vectors for the delivery of one or more primary effector molecule(s) to the site of a solid tumor. The primary effector molecule(s) of the invention is used in the methods of the invention to treat a solid tumor cancer such as a carcinoma, melanoma, lymphoma, or sarcoma. The invention relates to the surprising discovery that effector molecules, which may be toxic when administered systemically to a host, can be delivered locally to tumors by attenuated tumor-targeted bacteria with reduced toxicity to the host. The application also discloses to the delivery of one or more optional effector molecule(s) (termed secondary effector molecules) which may be delivered by the attenuated tumor-targeted bacteria in conjunction with the primary effector molecule(s).
Owner:NANOTHERAPEUTICS INC
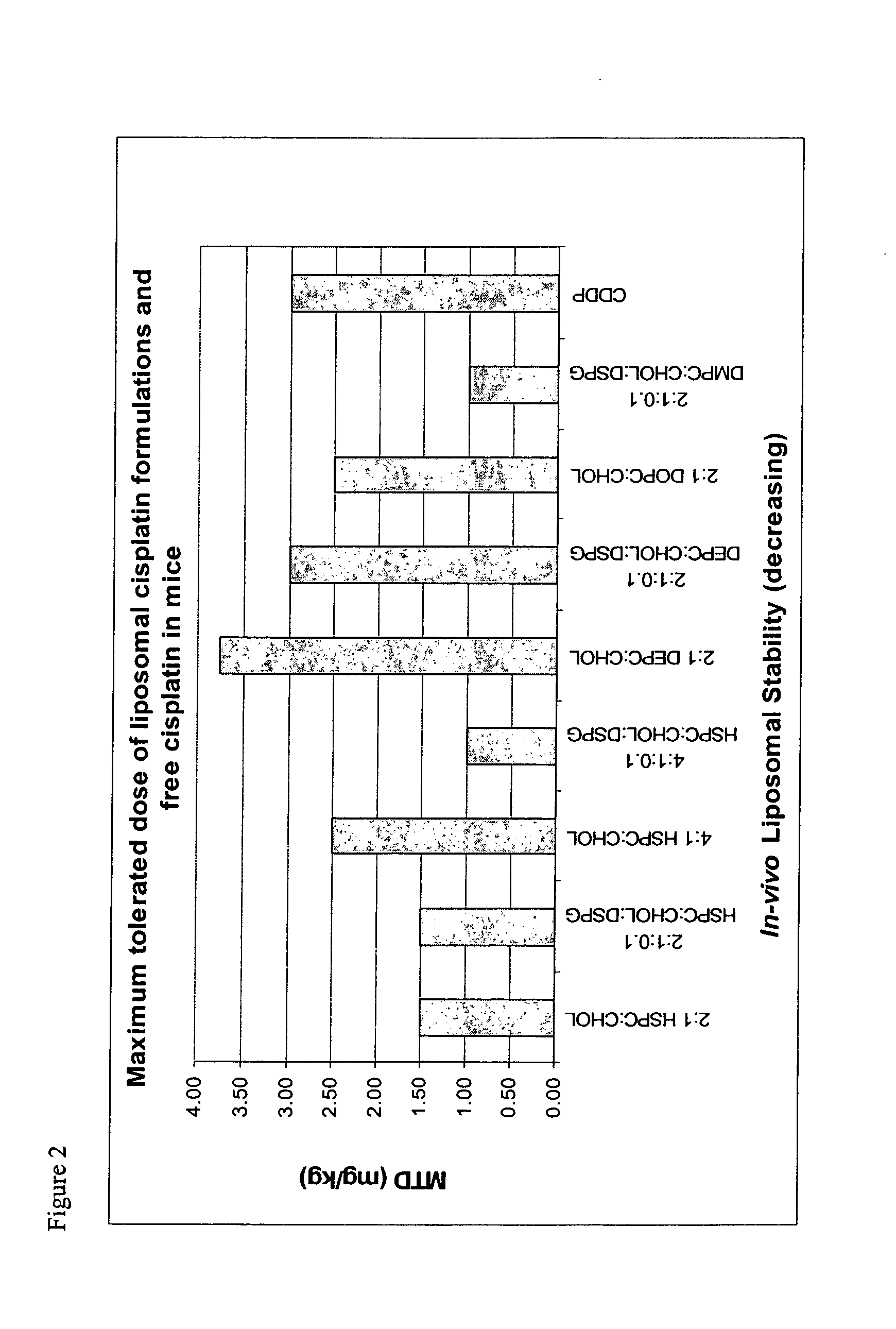
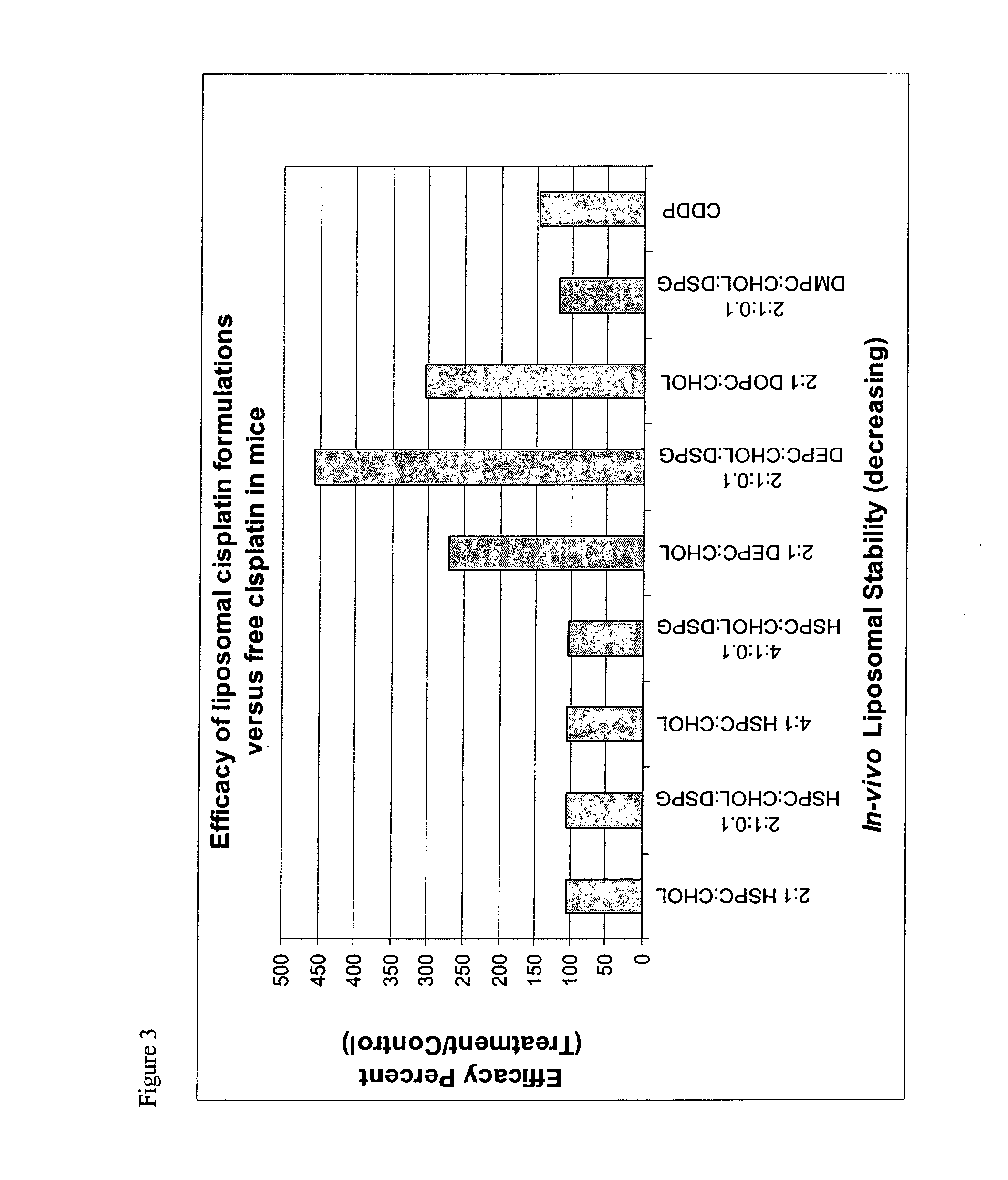
Liposomal formulations
InactiveUS20040156888A1Optimize volumeHeavy metal active ingredientsMicroencapsulation basedMedicineLiposome
The invention provides a formulation comprising a lipophobic therapeutic agent encapsulated in a liposome having improved efficacy and / or reduced toxicity.
Owner:GILEAD SCI INC
Methods for the Treatment of Autoimmune Disorders Using Immunosuppressive Monoclonal Antibodies with Reduced Toxicity
ActiveUS20080095766A1Reduce the possibilityIncreasing concentration of antibodySenses disorderNervous disorderDosing regimenInsulin dependent diabetes
The present invention provides methods of treating, preventing, slowing the progression of, or ameliorating the symptoms of T cell mediated immunological diseases, particularly autoimmune diseases (e.g., autoimmune diabetes (i.e. type 1 diabetes or insulin-dependent diabetes mellitus (IDDM)) and multiple sclerosis) through the use of anti-human CD3 antibodies. The antibodies of the invention of the invention are preferably used in low dose dosing regimens, chronic dosing regimens or regimens that involve redosing after a certain period of time. The methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the methods of the invention provide for use of anti-human CD3 antibodies modified such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-human CD3 antibodies.
Owner:PROVENTION BIO INC
Deuterated cyclosporine analogs and their use as immunomodulating agents
InactiveUS20020132763A1Improve practicalityAltered physicochemical and pharmacokinetic propertyNervous disorderAntipyreticCyclosporinsImmunomodulating Agent
Cyclosporine derivatives are disclosed which possess enhanced efficacy and reduced toxicity over naturally occurring and other presently known cyclosporins and cyclosporine derivatives. The cyclosporine derivatives of the present invention are produced by chemical and isotopic substitution of the cyclosporine A (CsA) molecule by: (1) Chemical substitution and optionally deuterium substitution of amino acid 1; and (2) deuterium substitution at key sites of metabolism of the cyclosporine A molecule such as amino acids 1, 4, 9. Also disclosed are methods of producing the cyclosporine derivatives and method of producing immunosuppression with reduced toxicity with the disclosed cyclosporine derivatives.
Owner:NAICKER SALVARAJ +2
Low toxicity primer compositions for reduced energy ammunition
InactiveUS20100300319A1Increased heat generationHigh sensitivityAmmunition projectilesShotgun ammunitionToxic materialOxidizing agent
A primer composition with reduced toxicity suited for reduced-energy ammunition comprises bismuth (III) oxide as the principal oxidizer and contains a portion of propellant composition mixed therein. This composition may also be used in a cartridge which is otherwise substantially free of any other propellant compound and preferably produces a residue which is substantially free of toxic substances.
Owner:GENERAL DYNAMICS ORDNANCE & TACTICAL SYST CANADA VALLEYFIELD INC
Cyclosporine analogue mixtures and their use as immunomodulating agents
ActiveUS6998385B2Potent immunosuppressantImprove effectivenessSenses disorderNervous disorderCyclosporinsImmunomodulating Agent
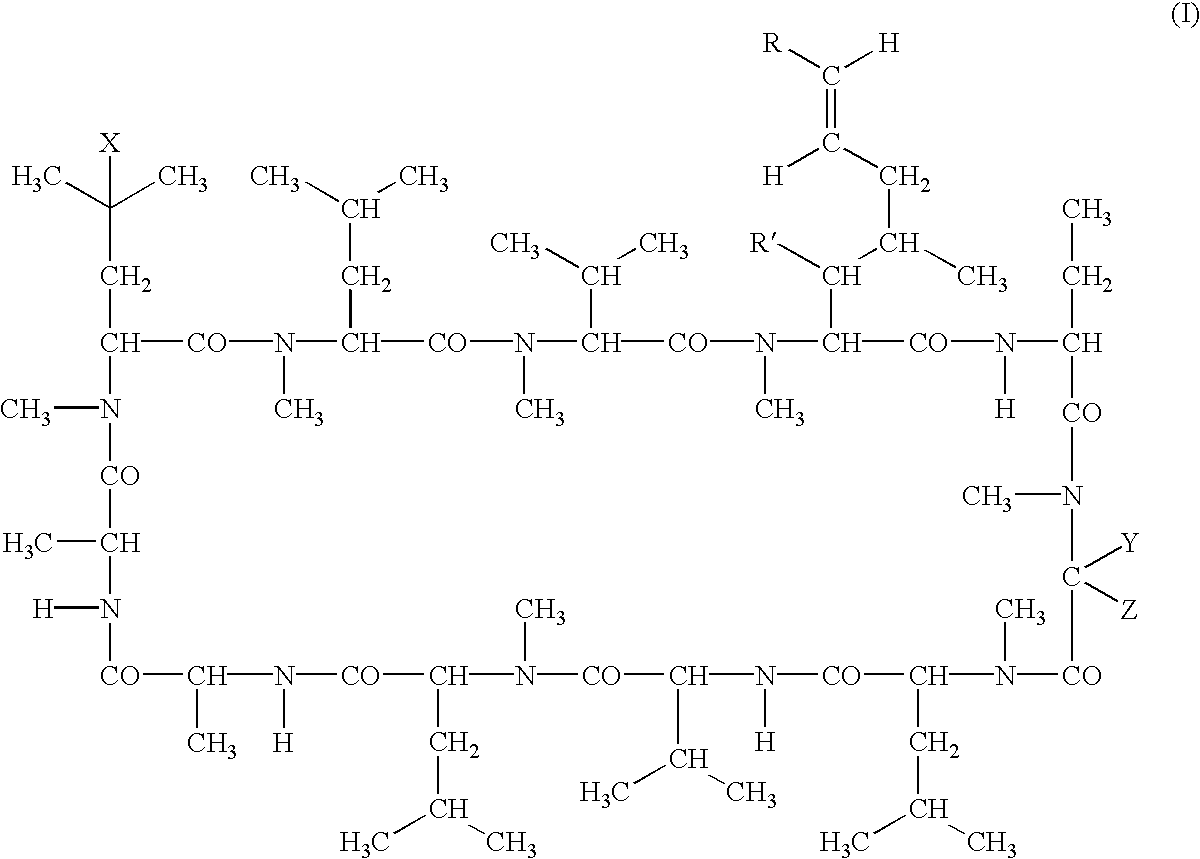
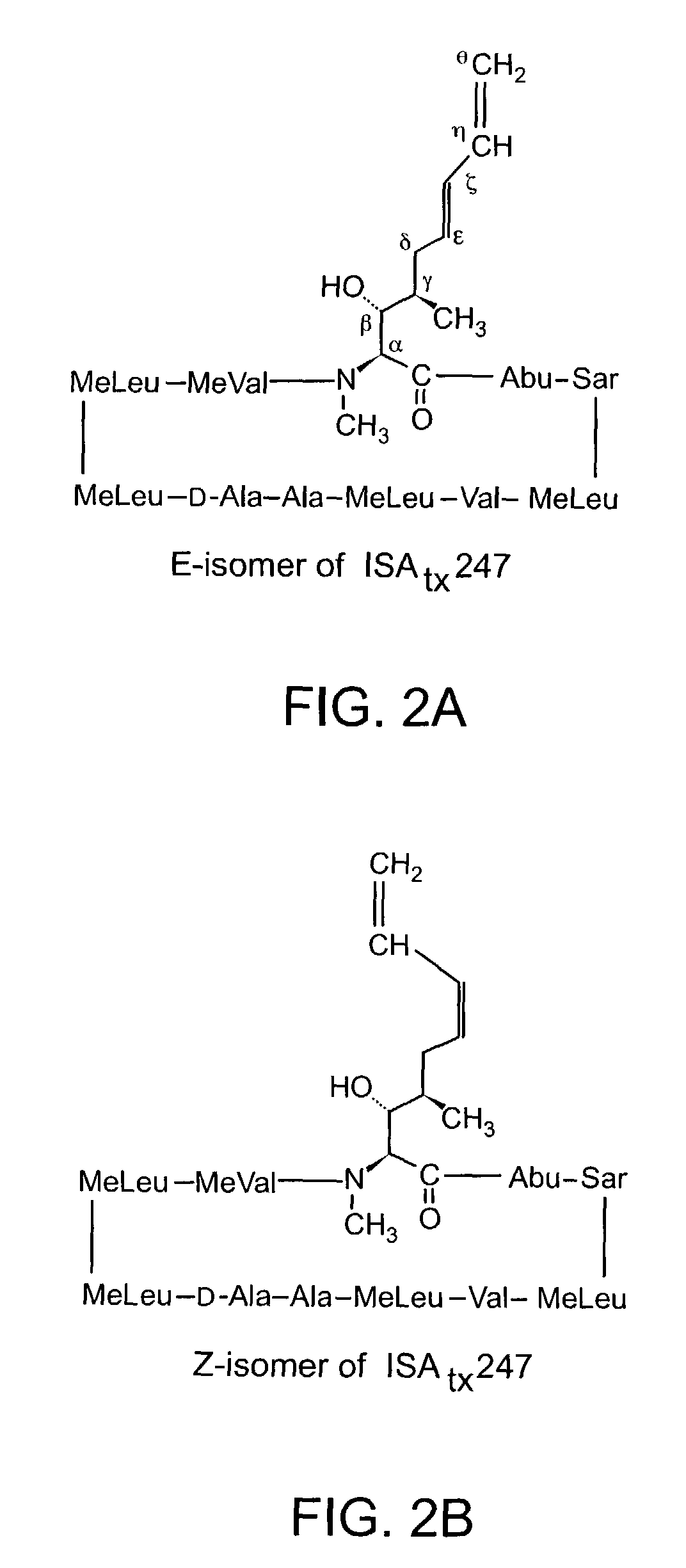
The invention is directed to isomeric mixtures of cyclosporine analogues that are structurally similar to cyclosporine A. The mixtures possess enhanced efficacy and reduced toxicity over the individual isomers and over naturally occurring and other presently known cyclosporines and cyclosporine derivatives. Embodiments of the present invention are directed toward cis and trans-isomers of cyclosporin A analogs referred to as ISATX247, and derivatives thereof. Mixtures of ISATX247 isomers exhibit a combination of enhanced potency and reduced toxicity over the naturally occurring and presently known cyclosporins. ISATX247 isomers and alkylated, arylated, and deuterated derivatives are synthesized by stereoselective pathways where the particular conditions of a reaction determine the degree of stereoselectivity. The ratio of isomers in a mixture may range from about 10 to 90 percent by weight of the (E)-isomer to about 90 to 10 percent by weight of the (Z)-isomer, based on the total weight of the mixture.
Owner:AURINIA PHARMA
Cyclodextrin-based polymers for therapeutics delivery
InactiveUS20060210527A1Improve drug stabilityImprove solubilityNanomedicinePharmaceutical non-active ingredientsPresent methodCyclodextrin
The present invention relates to novel compositions of therapeutic polymeric compounds designed as carriers for small molecule therapeutics delivery and pharmaceutical compositions thereof. In some embodiments, the small molecule therapeutic is attached to the polymer by a photocleavable linker. The polymeric compounds may also employ targeting agents. By selecting from a variety of linker groups and targeting ligands the polymers present methods for controlled delivery of the therapeutic agents. On reaching a targeted site in the body of a patient, the linker can then be cleaved by the shining of ultraviolet, visible, or infrared wavelength light onto the site. The methods provide reduced toxicity and local delivery of therapeutics. The invention also relates to methods of treating subjects with the therapeutic compositions described herein. The invention further relates to methods for conducting a pharmaceutical business comprising manufacturing, licensing, or distributing kits containing or relating to the polymeric compounds described herein.
Owner:CERULEAN PHARMA
Compositions and methods for tumor-targeted delivery of effector molecules
InactiveUS20050249706A1Inhibit tumor growthReduce tumor volumeBiocideOrganic active ingredientsTumor targetAbnormal tissue growth
The present application discloses the preparation and use of attenuated tumor-targeted bacteria vectors for the delivery of one or more primary effector molecule(s) to the site of a solid tumor. The primary effector molecule(s) of the invention is used in the methods of the invention to treat a solid tumor cancer such as a carcinoma, melanoma, lymphoma, or sarcoma. The invention relates to the surprising discovery that effector molecules, which may be toxic when administered systemically to a host, can be delivered locally to tumors by attenuated tumor-targeted bacteria with reduced toxicity to the host. The application also discloses to the delivery of one or more optional effector molecule(s) (termed secondary effector molecules) which may be delivered by the attenuated tumor-targeted bacteria in conjunction with the primary effector molecule(s).
Owner:NANOTHERAPEUTICS INC
Compositions and Methods for Tumor-Targeted Delivery of Effector Molecules
InactiveUS20070298012A1Reduce Toxicity RiskReduce riskHeavy metal active ingredientsBiocideParanasal Sinus CarcinomaMelanoma
The present application discloses the preparation and use of attenuated tumor-targeted bacteria vectors for the delivery of one or more primary effector molecule(s) to the site of a solid tumor. The primary effector molecule(s) of the invention is used in the methods of the invention to treat a solid tumor cancer such as a carcinoma, melanoma, lymphoma, or sarcoma. The invention relates to the surprising discovery that effector molecules, which may be toxic when administered systemically to a host, can be delivered locally to tumors by attenuated tumor-targeted bacteria with reduced toxicity to the host. The application also discloses to the delivery of one or more optional effector molecule(s) (termed secondary effector molecules) which may be delivered by the attenuated tumor-targeted bacteria in conjunction with the primary effector molecule(s).
Owner:NANOTHERAPEUTICS INC
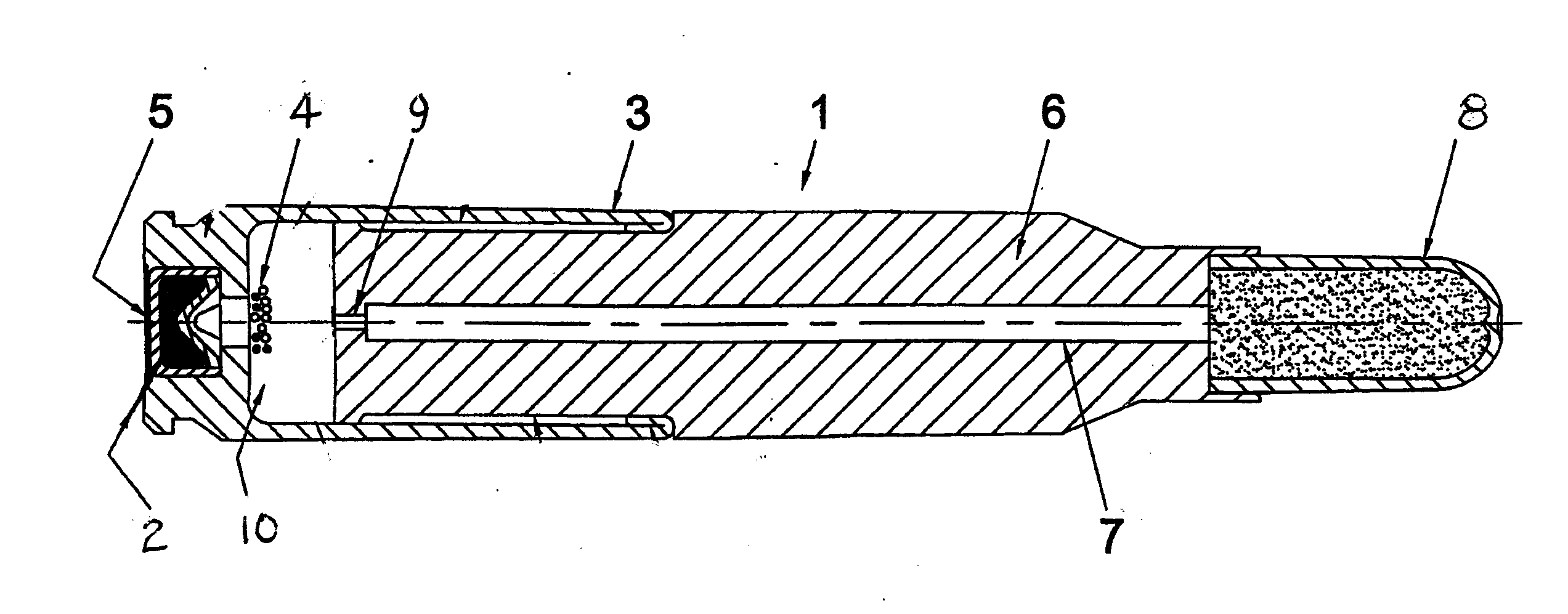
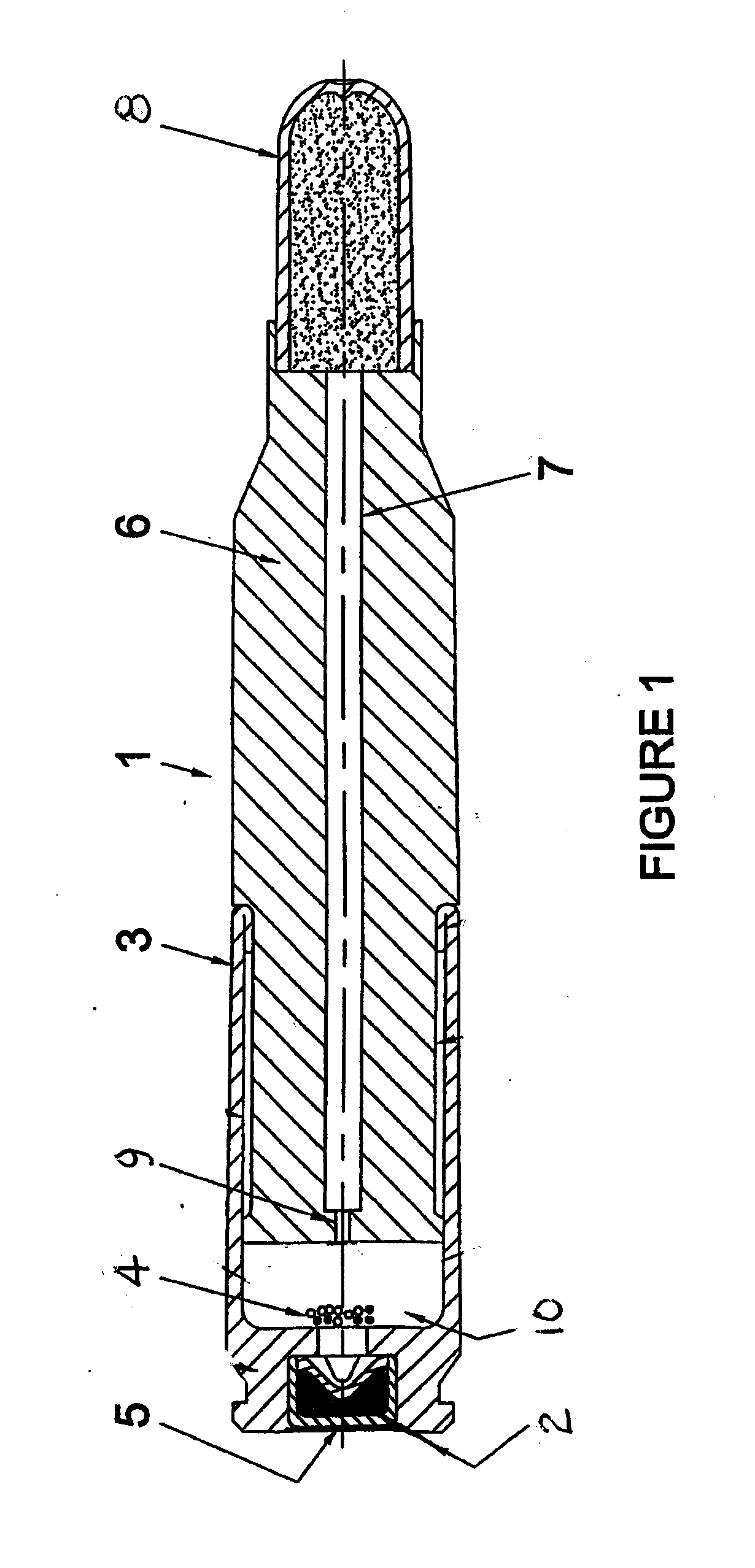
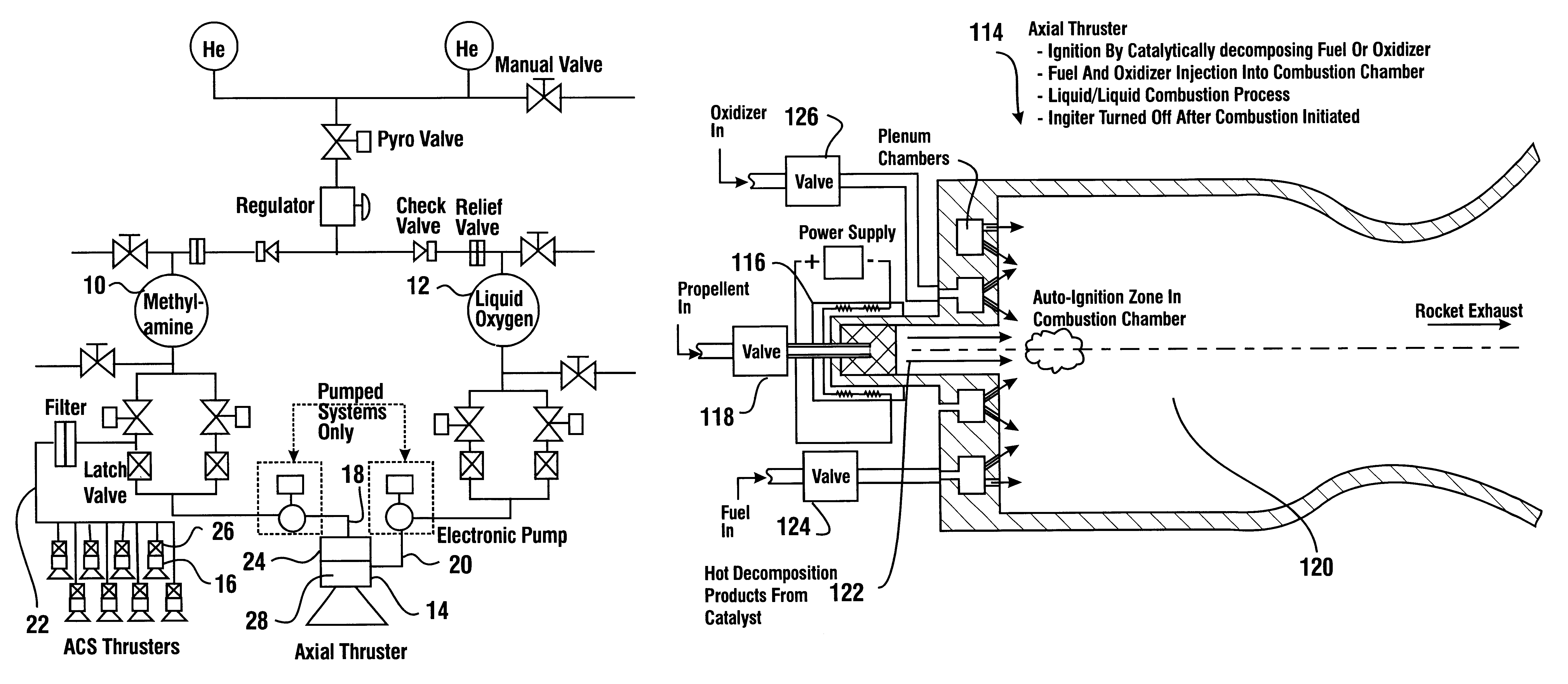
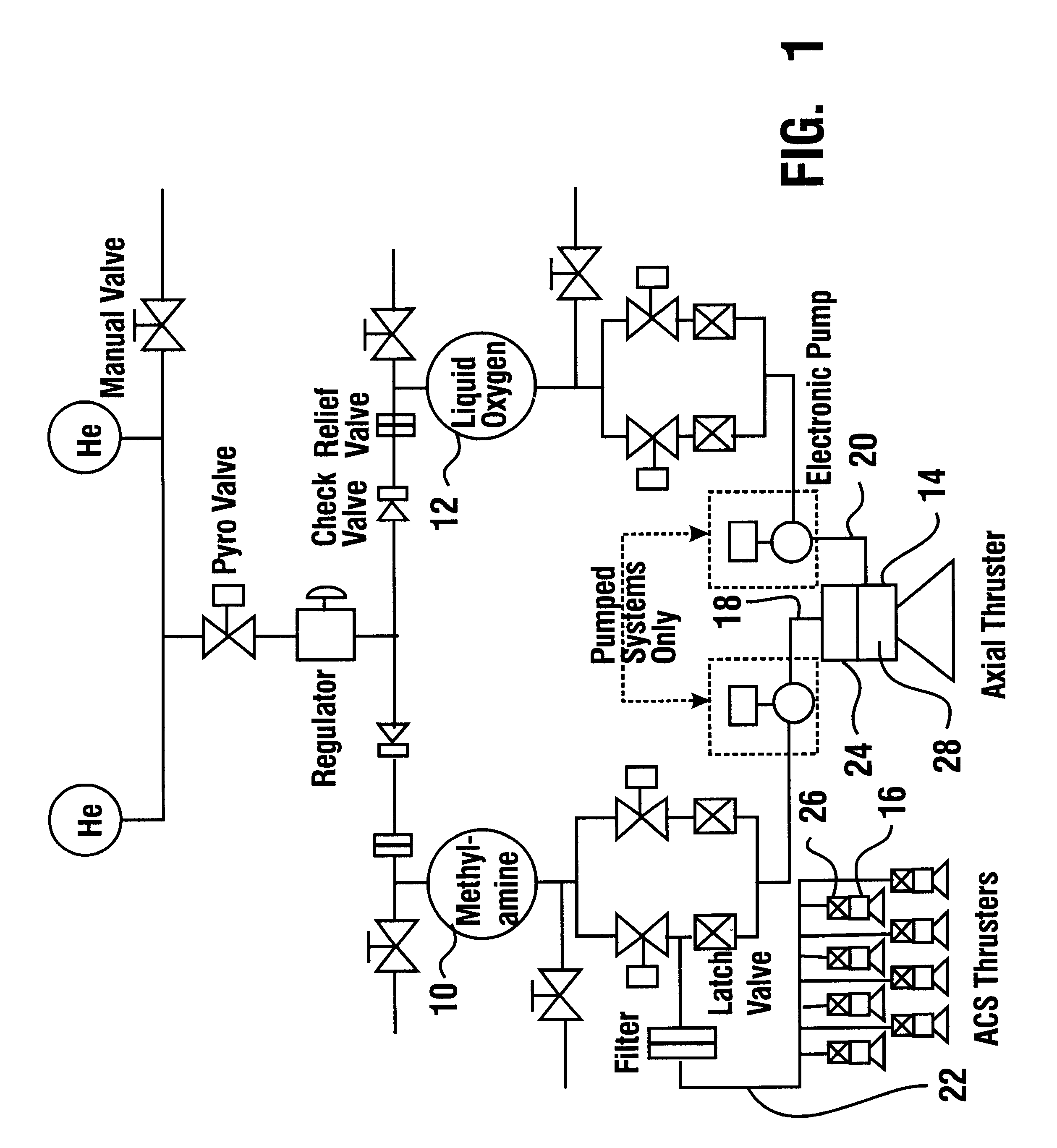
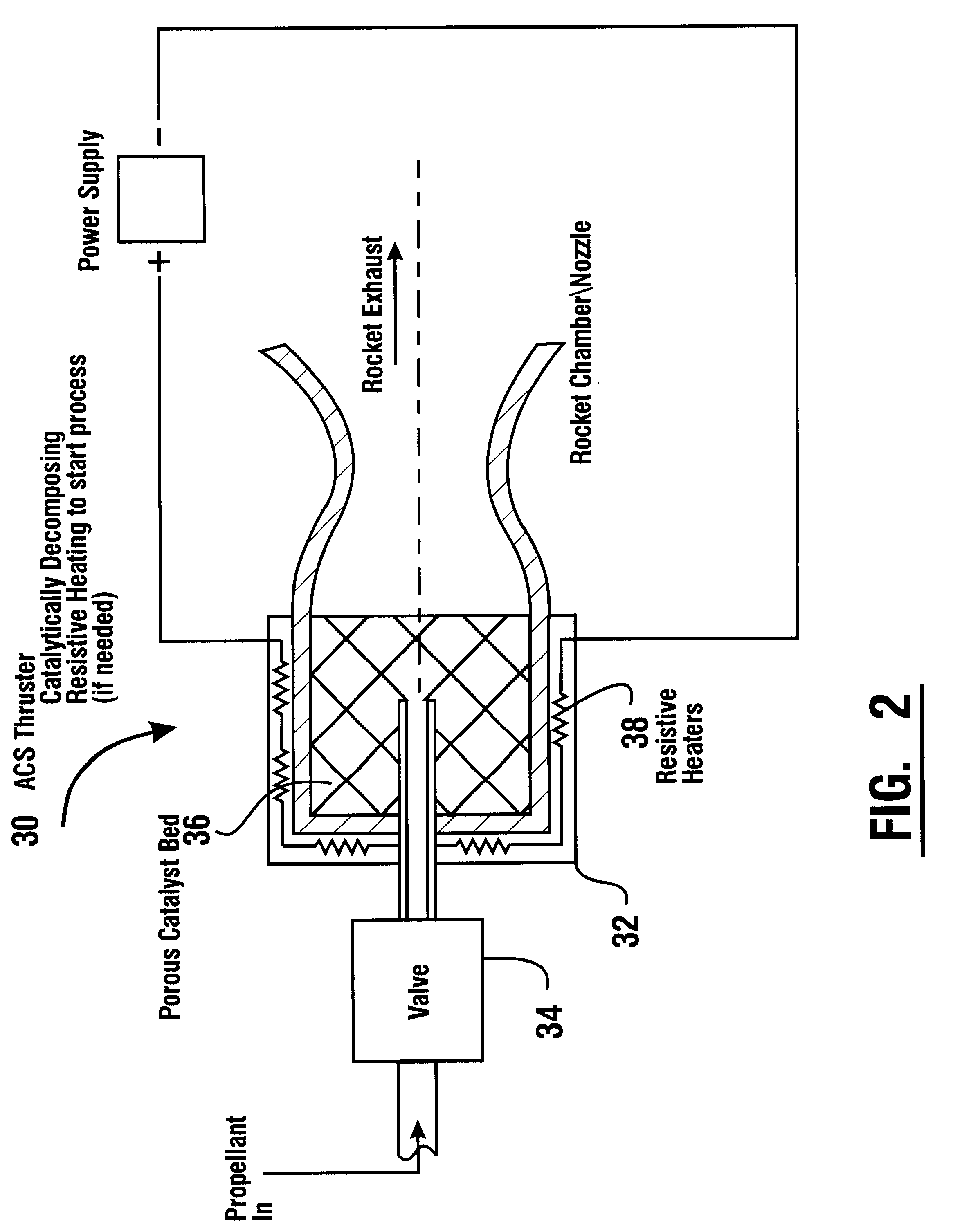
Reduced toxicity fuel satellite propulsion system
InactiveUS6272846B1Low toxicityReduce weightGas turbine plantsExplosivesCombustion chamberVALVE PORT
A reduced toxicity fuel satellite propulsion system including a reduced toxicity propellant supply (10) for consumption in an axial class thruster (14) and an ACS class thruster (16). The system includes suitable valves and conduits (22) for supplying the reduced toxicity propellant to the ACS decomposing element (26) of an ACS thruster. The ACS decomposing element is operative to decompose the reduced toxicity propellant into hot propulsive gases. In addition the system includes suitable valves and conduits (18) for supplying the reduced toxicity propellant to an axial decomposing element (24) of the axial thruster. The axial decomposing element is operative to decompose the reduced toxicity propellant into hot gases. The system further includes suitable valves and conduits (20) for supplying a second propellant (12) to a combustion chamber (28) of the axial thruster, whereby the hot gases and the second propellant auto-ignite and begin the combustion process for producing thrust.
Owner:NASA
Phosphonates with reduced toxicity for treatment of viral infections
There are provided, inter alia, acyclic nucleoside phosphonate compounds having reduced toxicity and enhanced antiviral activity, and pharmaceutically accepted salts and solvates thereof. There are also provided methods of using the disclosed compounds for inhibiting viral RNA-dependent RNA polymerase, inhibiting viral reverse transcriptase, inhibiting replication of virus, including hepatitis C virus or a human retrovirus, and treating a subject infected with a virus, including hepatitis C virus or a human retrovirus.
Owner:RGT UNIV OF CALIFORNIA
Process for reducing the toxicity of hydrocarbons
InactiveUS20050197256A1Low toxicityHydrocarbon by isomerisationHydrocarbon distillationFractional distillationHydrocarbon
This invention relates to a method for reducing the toxicity of a mixture of hydrocarbons by means of fractional distillation, a distillate having a reduced toxicity and a composition including the distillate.
Owner:THE PETROLEUM OIL & GAS CORP OS SOUTH AFRICA PTY LTD
Recombinant human albumin fusion proteins with long-lasting biological effects
ActiveUS7244833B2Good for healthImprove stabilityBacteriaPeptide/protein ingredientsDiseaseHuman albumin
Compositions, kits and methods are provided for promoting general health or for prevention or treatment of diseases by using novel recombinant fusion proteins of human serum albumin (HSA) and bioactive molecules. The bioactive molecules may be a protein or peptide having a biological function in vitro or in vivo, and preferably, having a therapeutic activity when administered to a human. By fusing the bioactive molecule to HSA, stability of the bioactive molecule in vivo can be improved and the therapeutic index increased due to reduced toxicity and longer-lasting therapeutic effects in vivo. In addition, manufacturing processes are provided for efficient, cost-effective production of these recombinant proteins in yeast.
Owner:YU ZAILIN +1
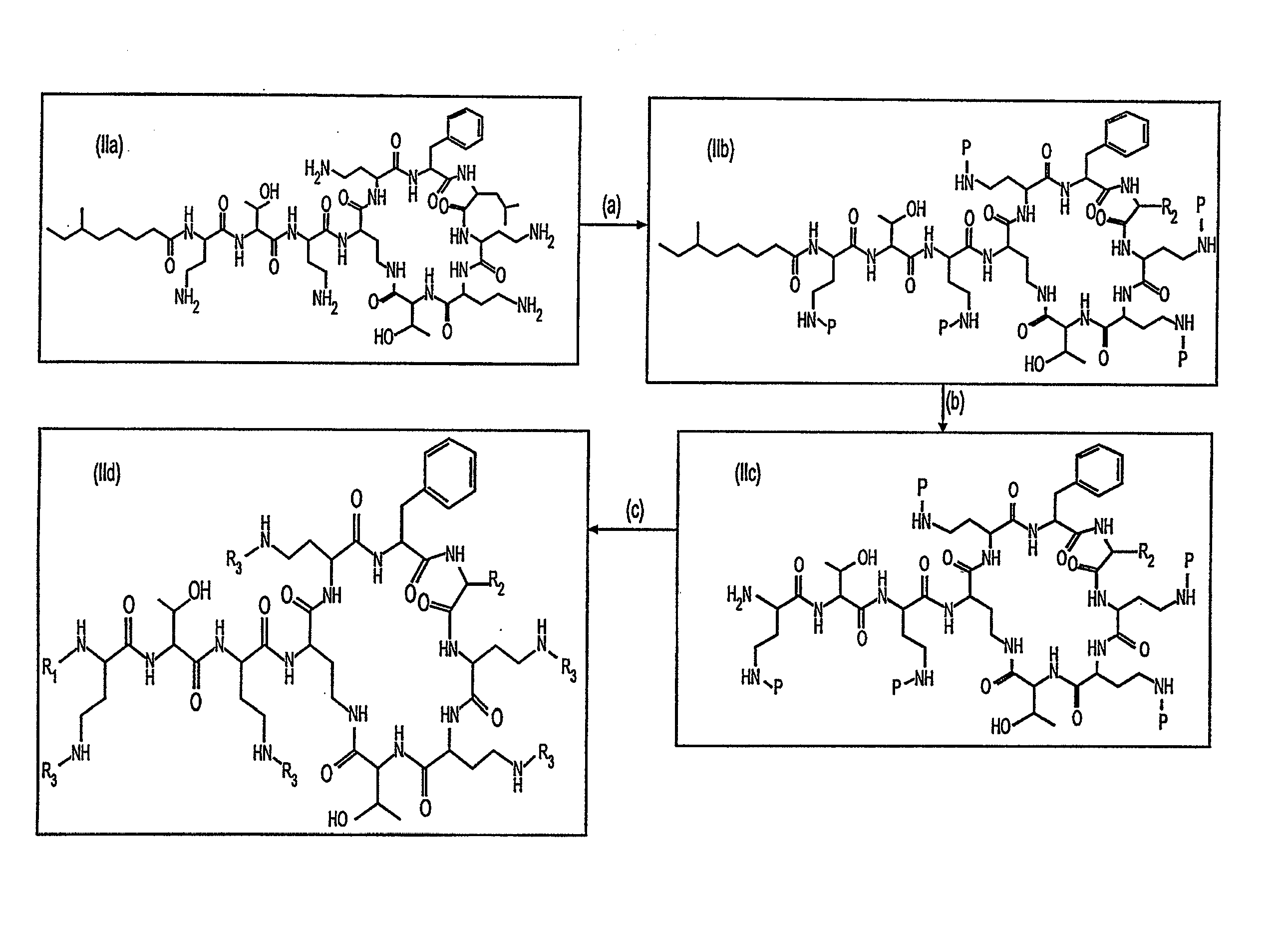
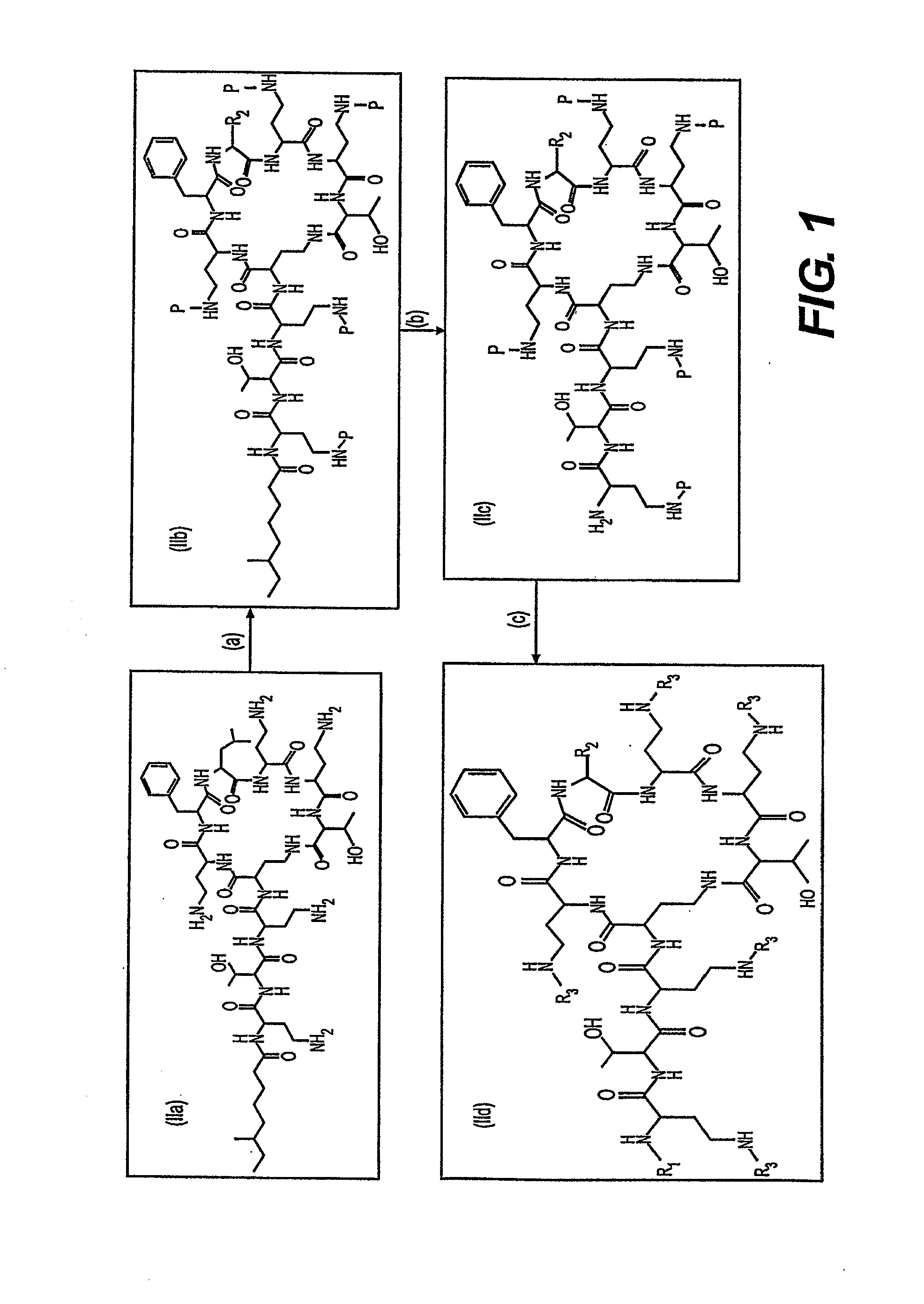
Antibiotic compositions for the treatment of gram negative infections
Provided herein are novel compounds and novel protected compounds that can be derived from polymyxin, including, e.g., polymyxin A. The novel compounds have antibacterial properties against a diverse range of Gram negative bacteria and reduced toxicity compared to polymyxins such as polymyxin A. Also provided are antibacterial pharmaceutical compositions containing the novel compounds and novel protected compounds, as well as methods for preparing the antibacterial compounds and protected compounds.
Owner:BIOSOURCE PHARM INC +1
Fluid media for bio-sensitive applications
InactiveUS20100049182A1Less sensitiveReduce riskDiagnostics using spectroscopySurgical instrument detailsFiberSpectroscopy
Systems, methods, and apparatus for providing a fluid and reduced-toxicity optical media with optical analysis and therapeutic energy delivery. An aspect of the invention provides an aqueous solution of increased-salinity of between about 1% and 35%. An increasing salinity in accordance with the invention provides improved transmissive efficiency at many wavelengths and less toxicity than many existing systems and methods. A catheter having integrated fibers for probing or treating internal lumens or other tissues can include a liquid-inflatable balloon or flushing mechanism using the solution for displacing blood or other obstructions in an optical path between the fiber and targeted tissue. Methods including spectroscopy can be employed with the solution for diagnosing medical conditions associated with diseased vessels or other tissues while reducing the risk of permanent damage resulting from the diagnosis. Additional applications include the deliver of therapeutic radiation externally and internally to tissues through h the solution media.
Owner:CORNOVA
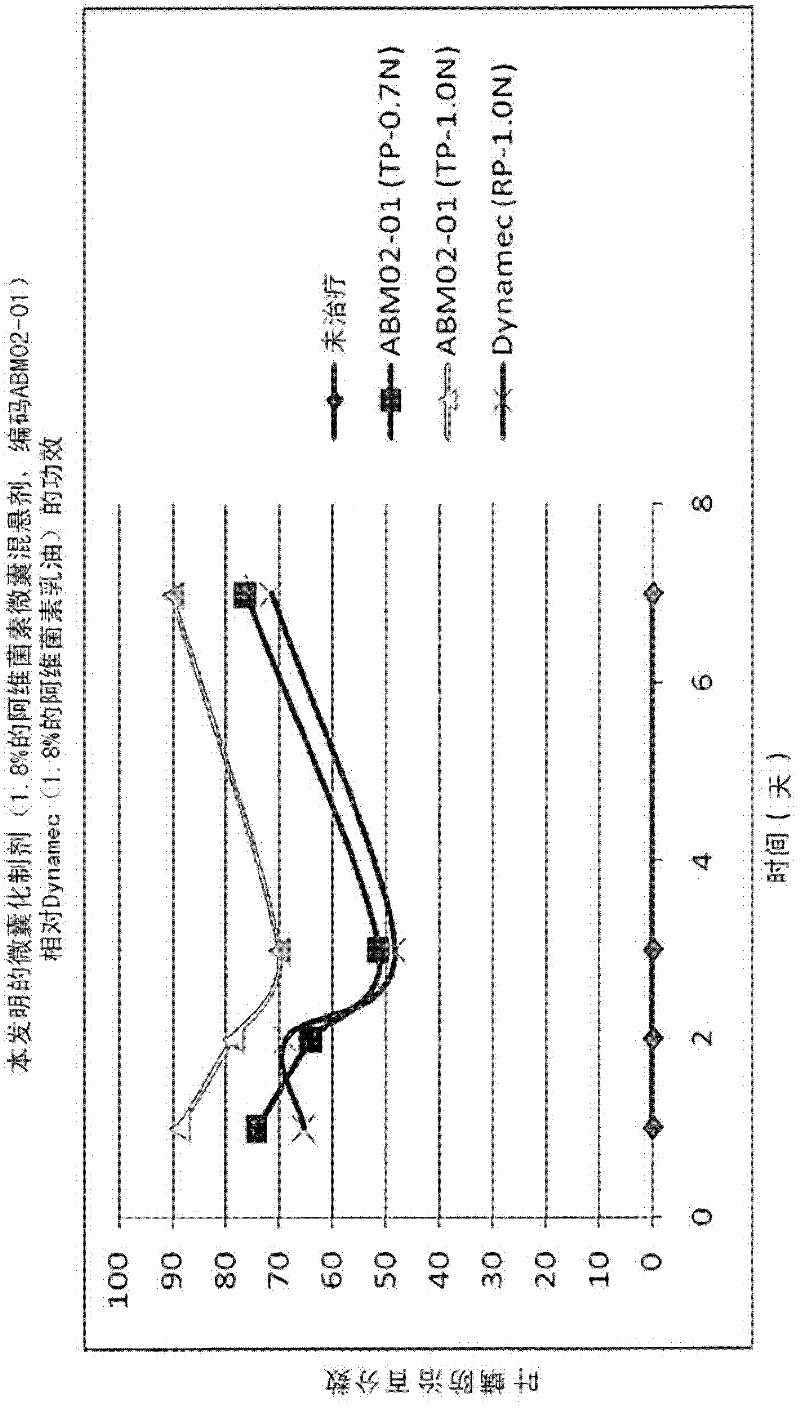
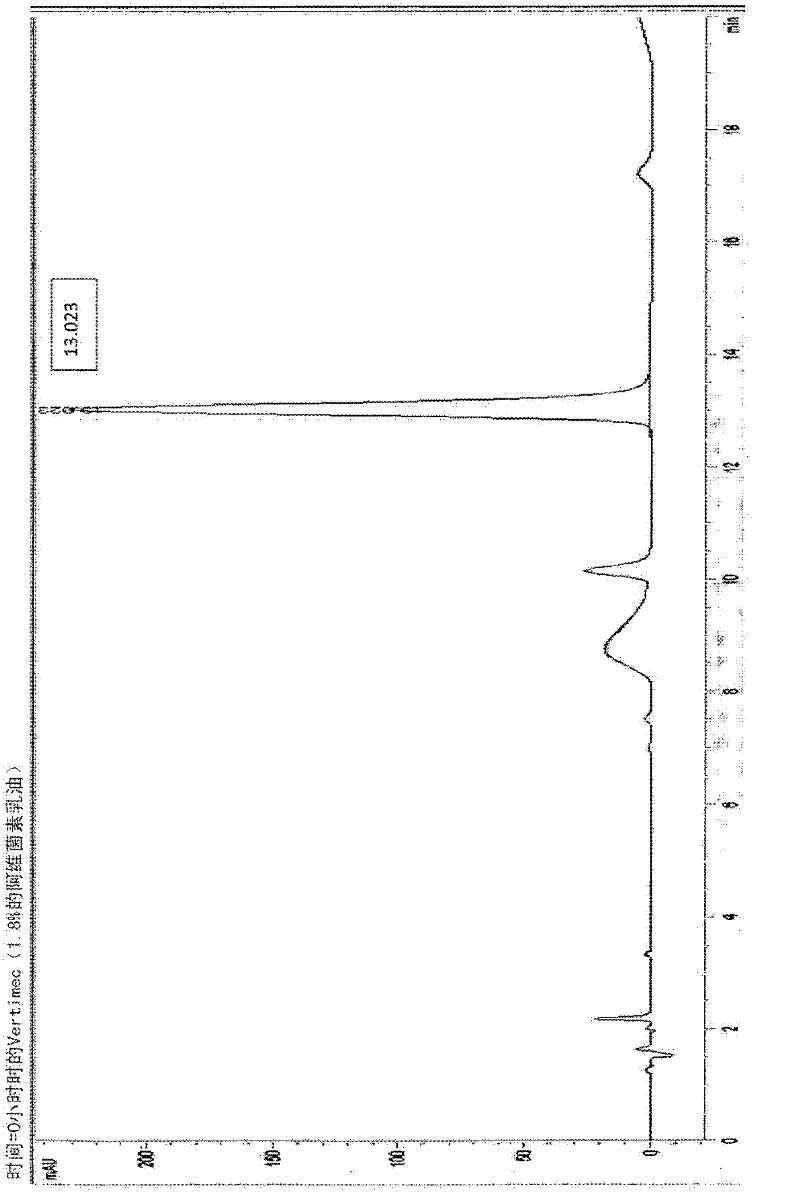
Microcapsules containing macrolide lactones abamectin, milbemectin, avermectins, milbemycins, emamectins, ivermectins and mectins in general
Microencapsulated formulations of macrolide lactones (abamectin, milbemectin, milbemycins emamectin, avermectins, ivermectins) wherein the active ingredient is protected from UV-degradation, with exceptional release characteristics resembling those of an emulsion concentrate or, if desired, of long-lasting effect; further with appropriate rheological properties, and with reduced toxicity. The invention provides a unique microencapsulation process for the chemical stability and biological activity of mectins, e.g. abamectin, and provides microcapsules of mectins to be used in formulations CS, WG / CS, ZC, EC / CS and any formulation type containing microcapsules and combination with other biologically active ingredients.
Owner:GAT MICROENCAPSULATION AG
Antibiotic compositions for the treatment of gram negative infections
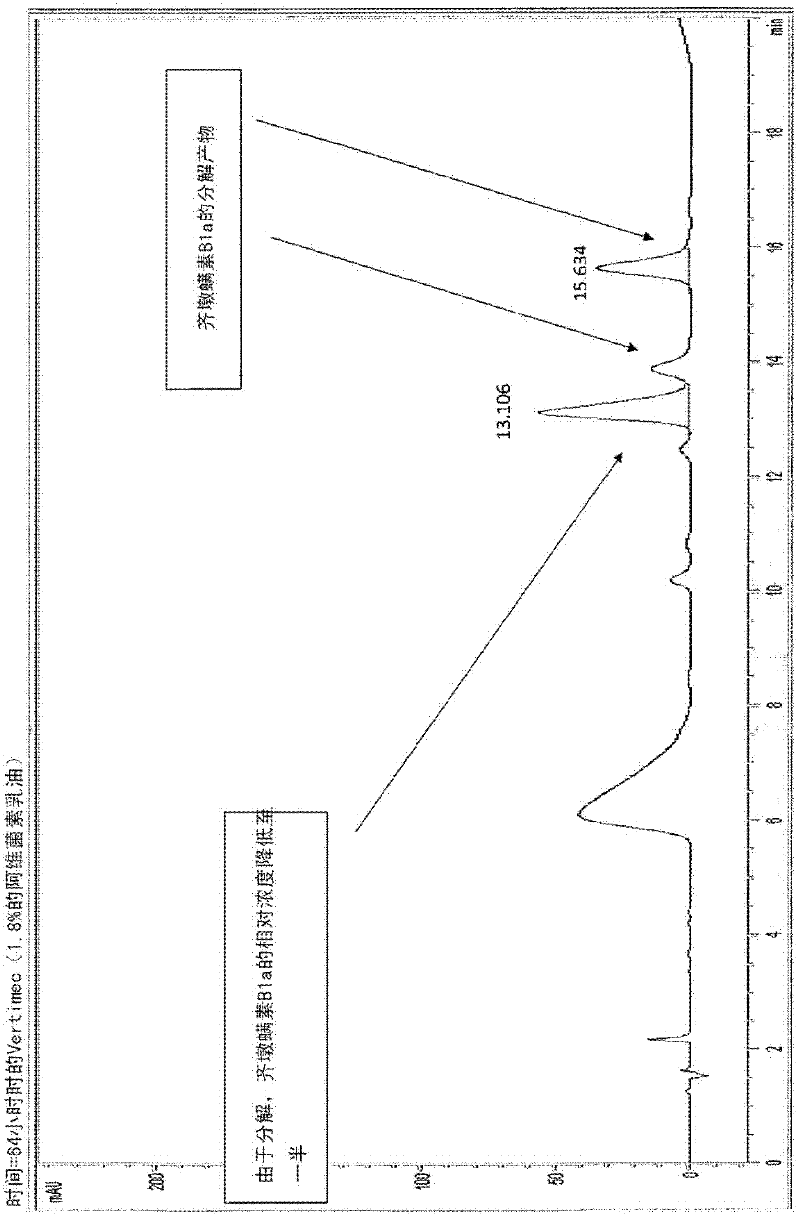
Provided herein are novel compounds and novel protected compounds that can be derived from polymyxin, including, e.g., polymyxin B1 and [Il7] polymyxin B1. The novel compounds have antibacterial properties against a diverse range of gram negative bacteria and reduced toxicity compared to polymyxins such as polymyxin B. Also provided are antibacterial pharmaceutical compositions containing the novel compounds and novel protected compounds, as well as methods for preparing the antibacterial compounds and protected compounds.
Owner:BIOSOURCE PHARM INC
Preparation of amphiphilic ursolic acid-polysaccharide coupled substance and application thereof in treating tumors
ActiveCN103705939AGood water solubilityImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsUrsolic acidTumor therapy
The invention relates to preparation and application of an amphiphilic ursolic acid-polysaccharide coupled substance with antitumor activity. The ursolic acid is chemically modified to substitute C3 site carboxyl group with amino group, the generated aminated ursolic acid is chemically coupled to a polysaccharide framework to form the amphiphilic ursolic acid-polysaccharide coupled substance, and the amphiphilic ursolic acid-polysaccharide coupled substance can be self-assembled into a nano micelle in water. The method is characterized in that the antitumor activity of the chemically modified ursolic acid is obviously enhanced; the hydrophobic inner core formed by the hydrophobic group can physically wrap the antineoplastic drug to obviously improve the solubility of the antineoplastic drug; and the antineoplastic drug which is physically wrapped by the chemically coupled ursolic acid can implement combined chemotherapy, has higher antitumor activity, has the combined synergetic action, and lowers the toxicity. The preparation method is simple and easy to operate, has the advantage of higher yield, and can easily implement industrialization.
Owner:南京泽恒医药技术开发有限公司
Novel formulation of dehydrated lipid vesicles for controlled release of active pharmaceutical ingredient via inhalation
InactiveUS20090047336A1Reduce systemic side effectsLow toxicityOrganic active ingredientsBiocideLipid formationSide effect
A new formulation of dehydrated lipid vesicles employs a vesicle preserver and permits the control of release and delivery of active pharmaceutical ingredients into the respiratory system for treatment in particular of asthma. The typical formulation provides controlled release of the active pharmaceutical ingredient from 0% to 100% from 0 to 72 hours after inhalation, changes the systemic administration to topical administration, allows prolonged therapeutic period for one administration, increased stability, with reduced dose, reduced systemic side effects, reduced toxicity.
Owner:HONG KONG BAPTIST UNIV
Lna oligonucleotide carbohydrate conjugates
The invention provides LNA therapeutics oligonucleotide carbohydrate conjugates with considerably enhanced potency, extended therapeutic index and reduced toxicity.
Owner:F HOFFMANN LA ROCHE & CO AG +1
Treatment regimens using multiple pharmaceutical agents
InactiveUS20140377285A1Lower toxicity levelGood curative effectBiocideMetabolism disorderTreatment regimenReduced toxicity
The present invention provides for methods and pharmaceutical compositions for treating disorders using treatment regimens involving multiple agents. In one aspect, a method of treatment is provided resulting in reduced toxicity and / or synergistic effect by administration according to a described dosing schedule.
Owner:INTELLIKINE
Liquid crystals with reduced toxicity and applications thereof
InactiveUS7303694B2Low toxicityLiquid crystal compositionsMaterial nanotechnologyCell culture mediaLiquid crystal
Liquid crystal compositions that exhibit little or no toxicity with respect to cells include liquid crystals with chemical functional groups such as fluorine atoms, fluorophenyl groups, or difluorophenyl groups. Liquid crystals with little or no toxicity to cell lines may be added to cell culture media or added to components used in cell culture media. Cells may be grown in cell culture media that includes liquid crystals that exhibit little or no toxicity to cells.
Owner:WISCONSIN ALUMNI RES FOUND
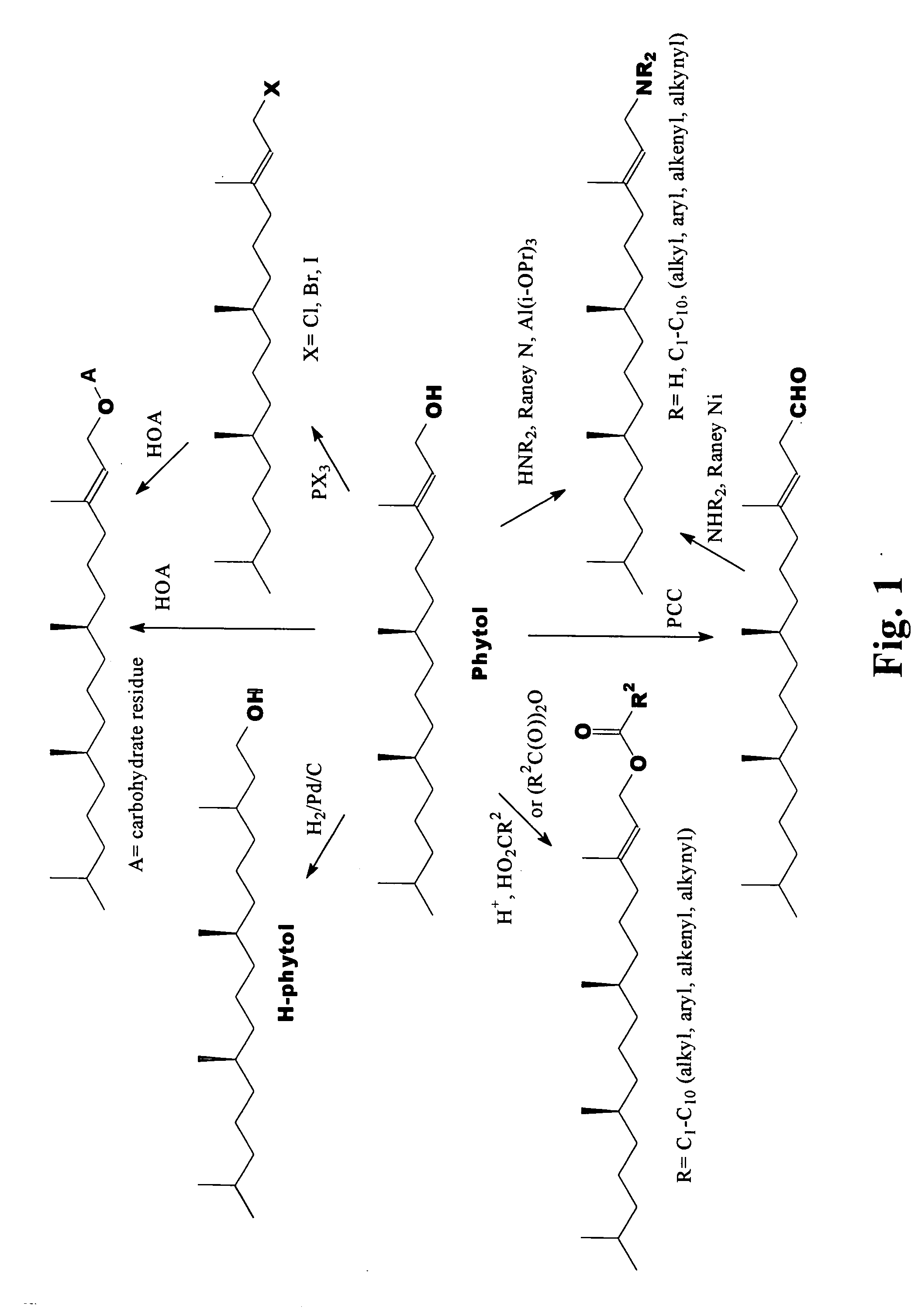
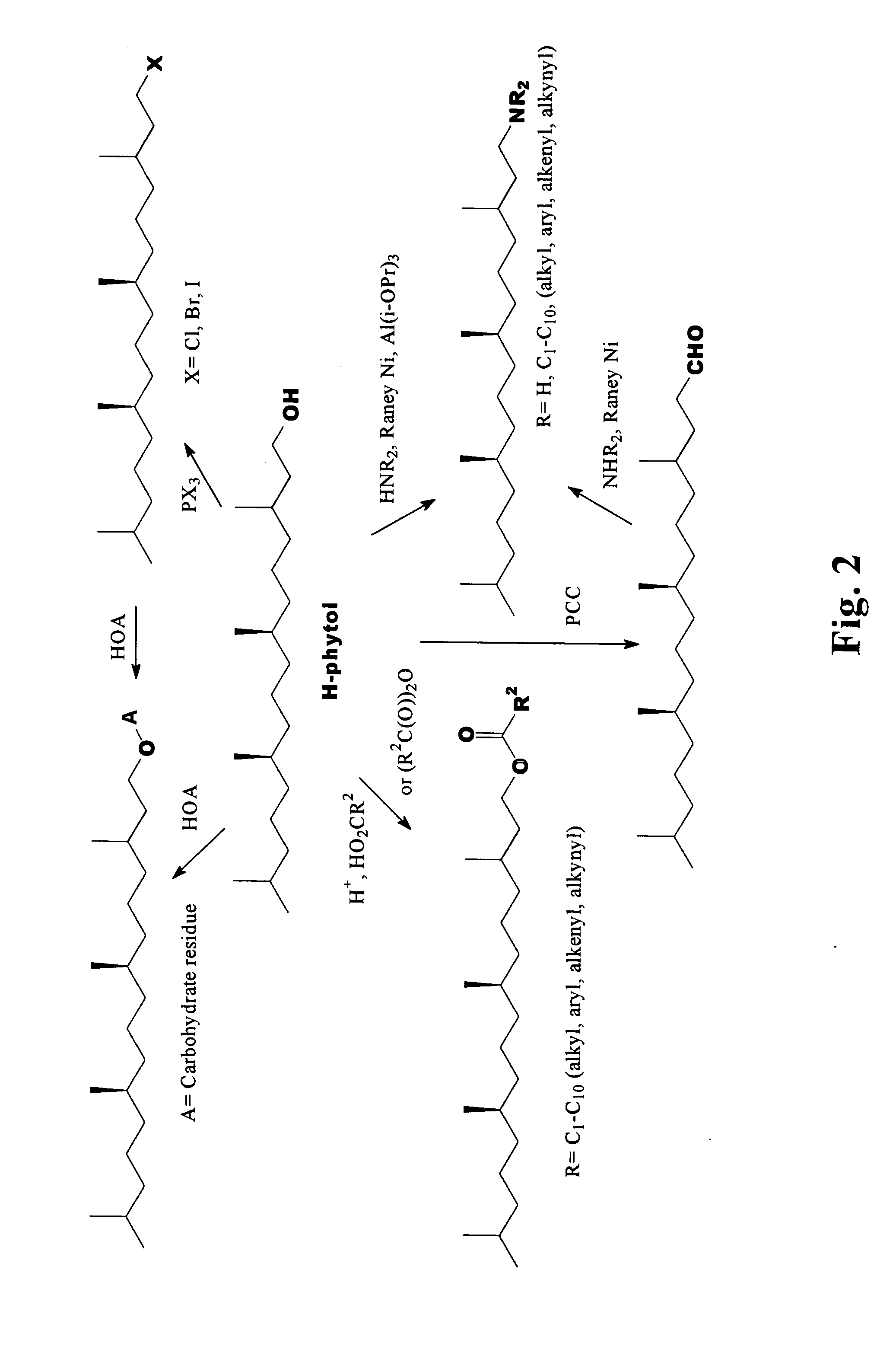
Novel phytol derived immunoadjuvants and their use in vaccine formulations
InactiveUS20050158329A1Improving immunogenicityInduce immunogenic responseBiocideHydroxy compound active ingredientsSide effectParticulate antigen
This invention relates to a novel immunoadjuvant, an adjuvant component, and vaccines containing the adjuvant component. The adjuvant includes phytol or a phytol derivative. The adjuvant component, when combined with a soluble or particulate antigen, provides a vaccine with an enhanced ability to induce both humoral and cytotoxic immune responses while displaying reduced toxicity and / or adverse side effects over vaccines that include the antigen but without the benefit of this adjuvant component.
Owner:GHOSH SWAPAN K
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com