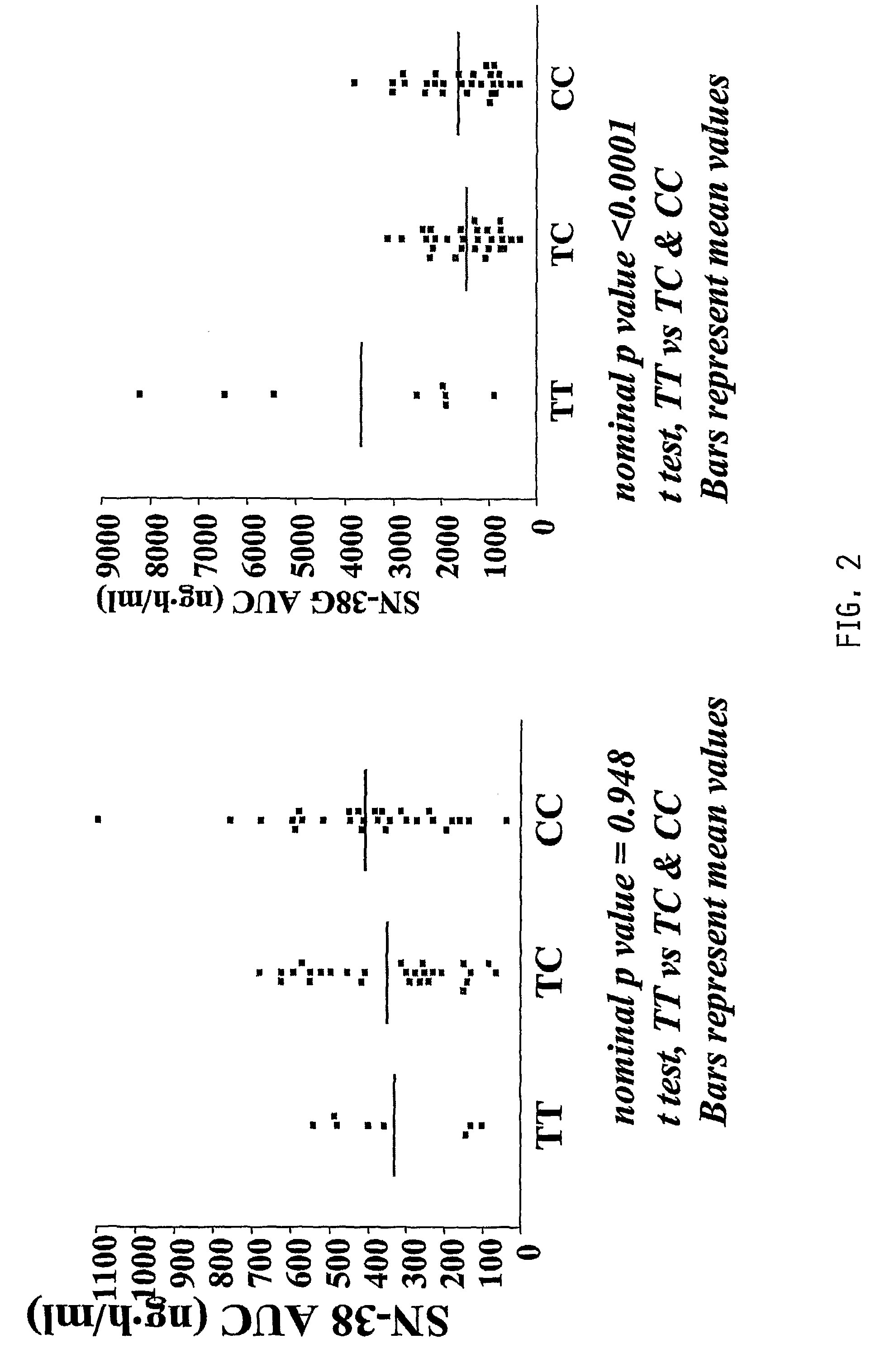
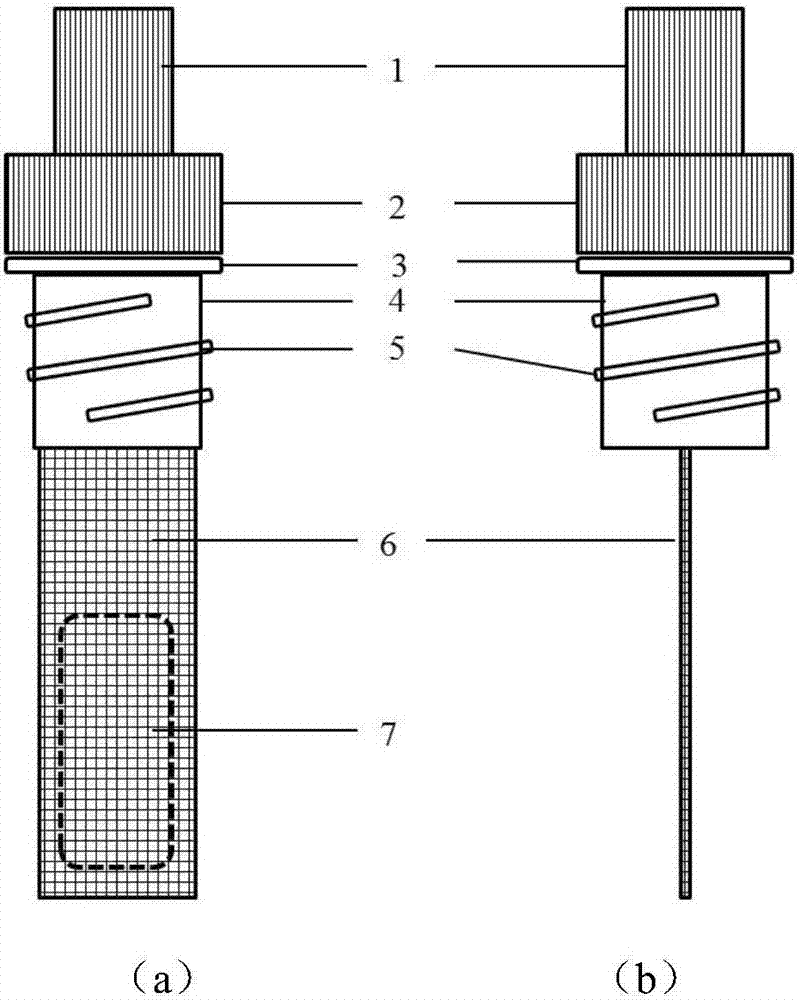
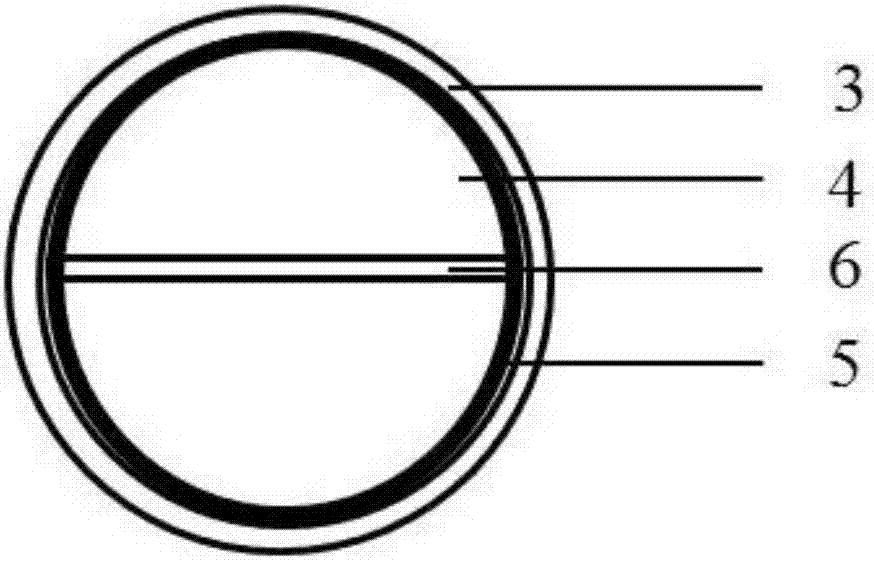
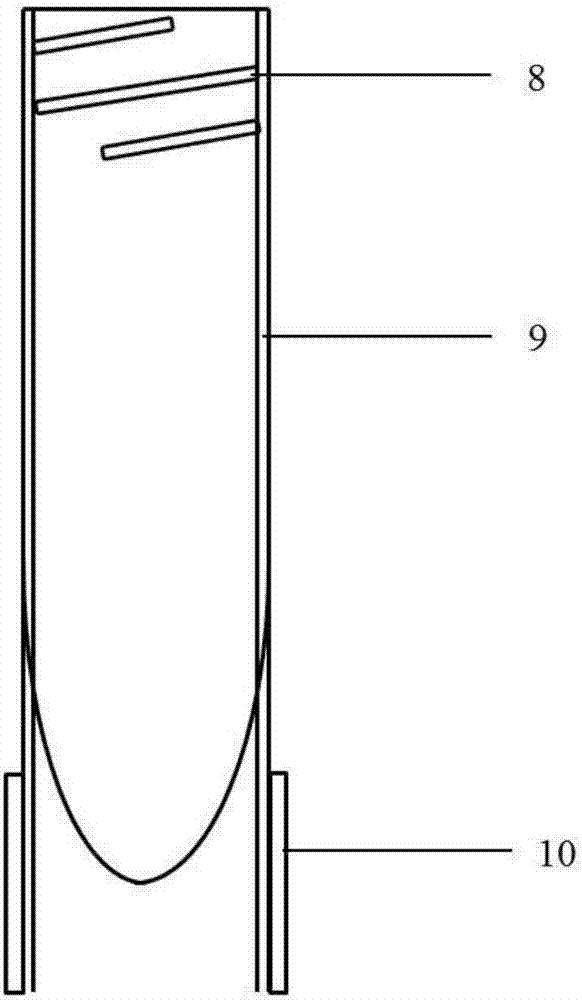
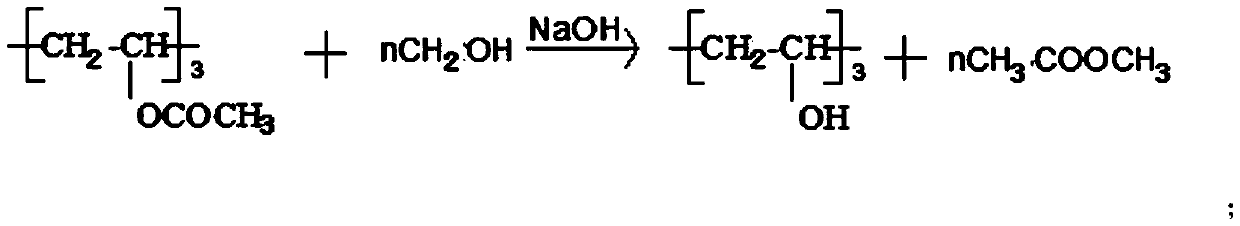Patents
Literature
58results about How to "Reduce Toxicity Risk" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
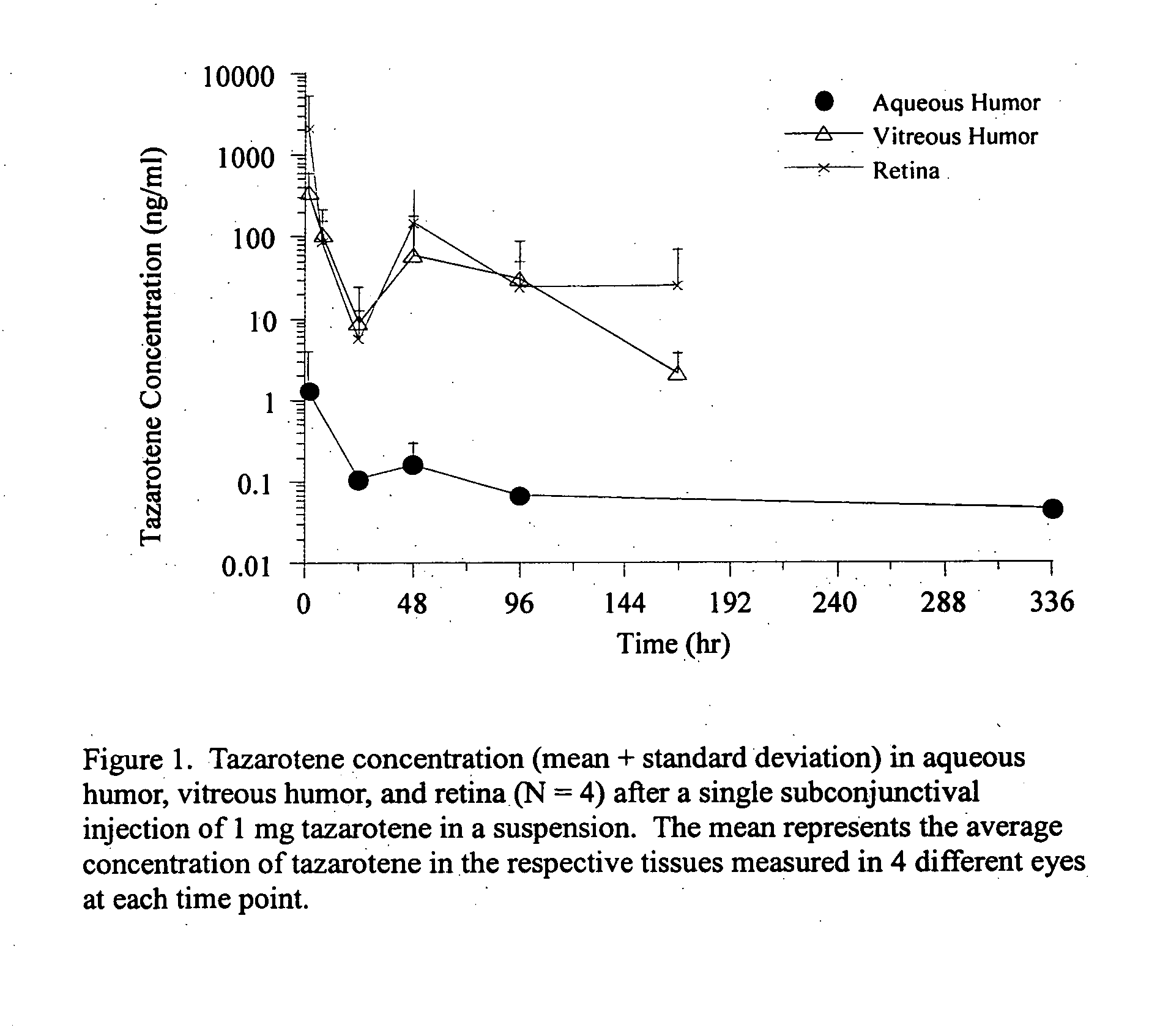
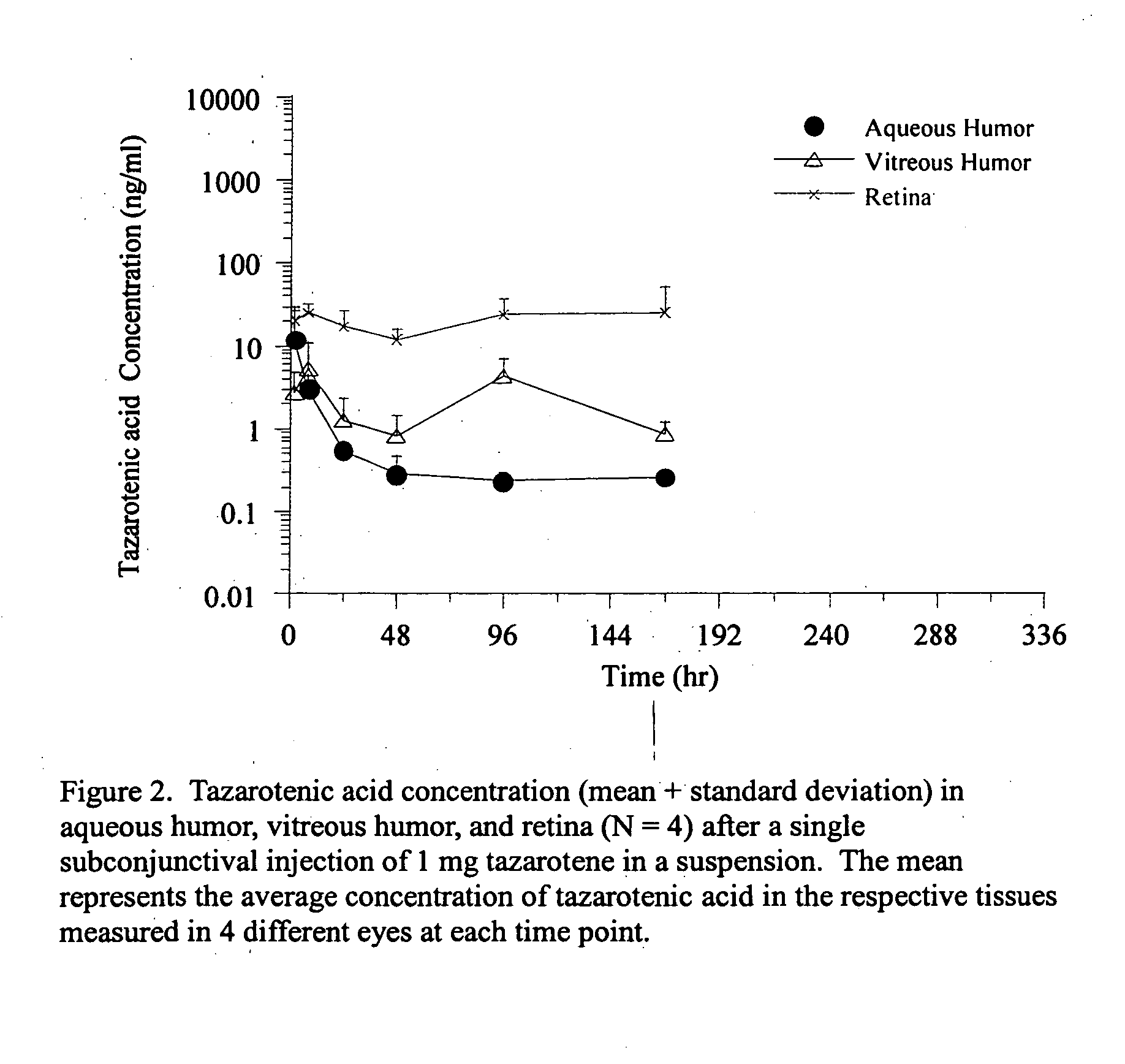
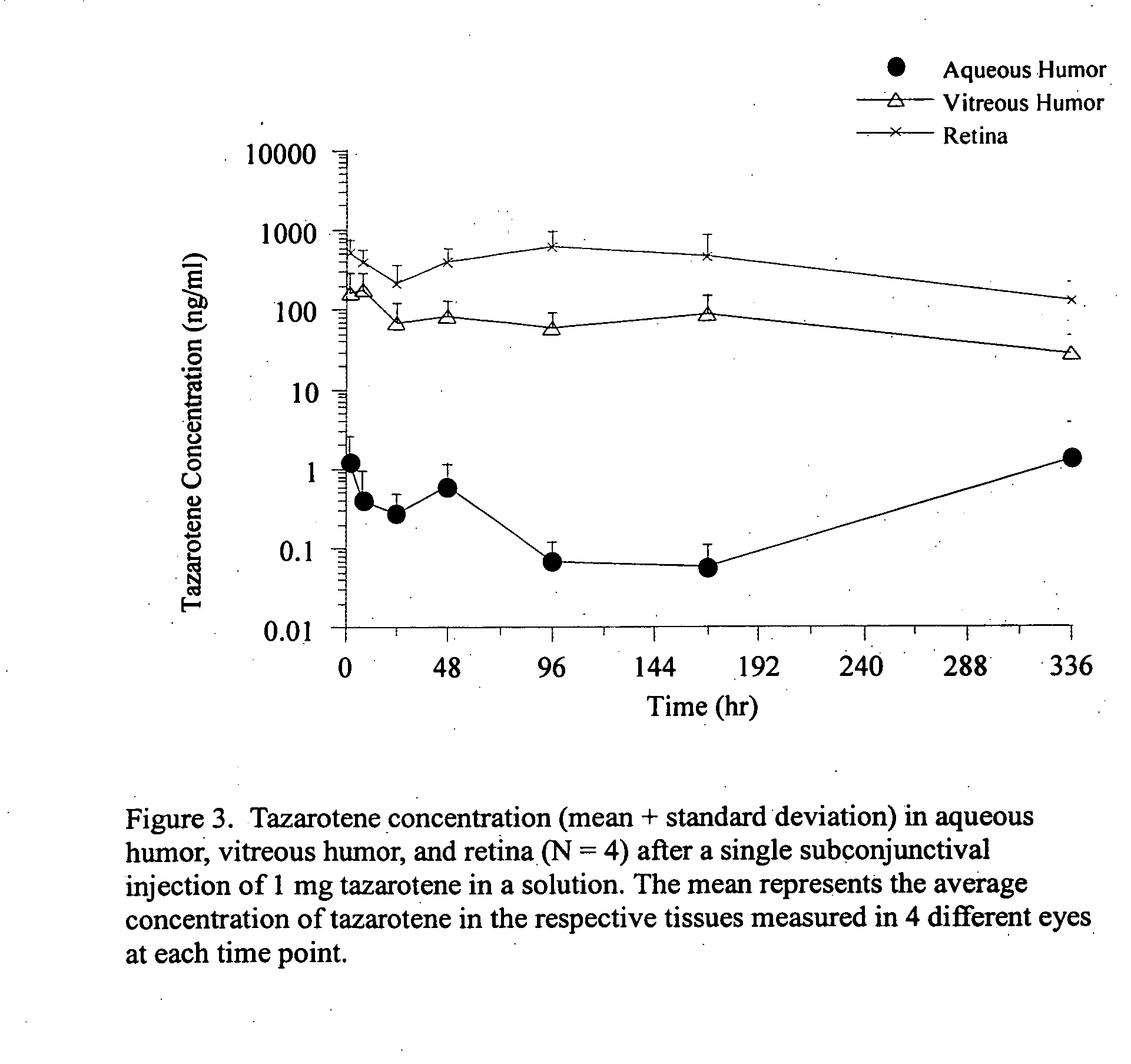
Delivery of an active drug to the posterior part of the eye via subconjunctival or periocular delivery of a prodrug
InactiveUS20050009910A1Prolong the action timeIncrease concentrationBiocideSenses disorderConjunctivaEster prodrug
The present invention relates to method of sustained-delivery of an active drug to a posterior part of an eye of a mammal to treat or prevent a disease or condition affecting said mammal, wherein said disease or condition can be treated or prevented by the action of said active drug upon said posterior part of the eye, comprising administering an effective amount of an ester prodrug of the active drug subconjunctivally or periocularly. Preferably, the active drug is more than about 10 times as active as the prodrug. Other aspects of this invention deal with the treatment of certain diseases by the periocular or subconjunctival delivery of an ester prodrug, and certain pharmaceutical products containing ester prodrugs for periocular or subconjunctival administration.
Owner:ALLERGAN INC
Compositions and methods for tumor-targeted delivery of effector molecules
InactiveUS20040229338A1Reduce Toxicity RiskReduce riskHeavy metal active ingredientsBiocideTumor targetBacilli
The present application discloses the preparation and use of attenuated tumor-targeted bacteria vectors for the delivery of one or more primary effector molecule(s) to the site of a solid tumor. The primary effector molecule(s) of the invention is used in the methods of the invention to treat a solid tumor cancer such as a carcinoma, melanoma, lymphoma, or sarcoma. The invention relates to the surprising discovery that effector molecules, which may be toxic when administered systemically to a host, can be delivered locally to tumors by attenuated tumor-targeted bacteria with reduced toxicity to the host. The application also discloses to the delivery of one or more optional effector molecule(s) (termed secondary effector molecules) which may be delivered by the attenuated tumor-targeted bacteria in conjunction with the primary effector molecule(s).
Owner:NANOTHERAPEUTICS INC
Compositions and Methods for Tumor-Targeted Delivery of Effector Molecules
InactiveUS20070298012A1Reduce Toxicity RiskReduce riskHeavy metal active ingredientsBiocideParanasal Sinus CarcinomaMelanoma
The present application discloses the preparation and use of attenuated tumor-targeted bacteria vectors for the delivery of one or more primary effector molecule(s) to the site of a solid tumor. The primary effector molecule(s) of the invention is used in the methods of the invention to treat a solid tumor cancer such as a carcinoma, melanoma, lymphoma, or sarcoma. The invention relates to the surprising discovery that effector molecules, which may be toxic when administered systemically to a host, can be delivered locally to tumors by attenuated tumor-targeted bacteria with reduced toxicity to the host. The application also discloses to the delivery of one or more optional effector molecule(s) (termed secondary effector molecules) which may be delivered by the attenuated tumor-targeted bacteria in conjunction with the primary effector molecule(s).
Owner:NANOTHERAPEUTICS INC
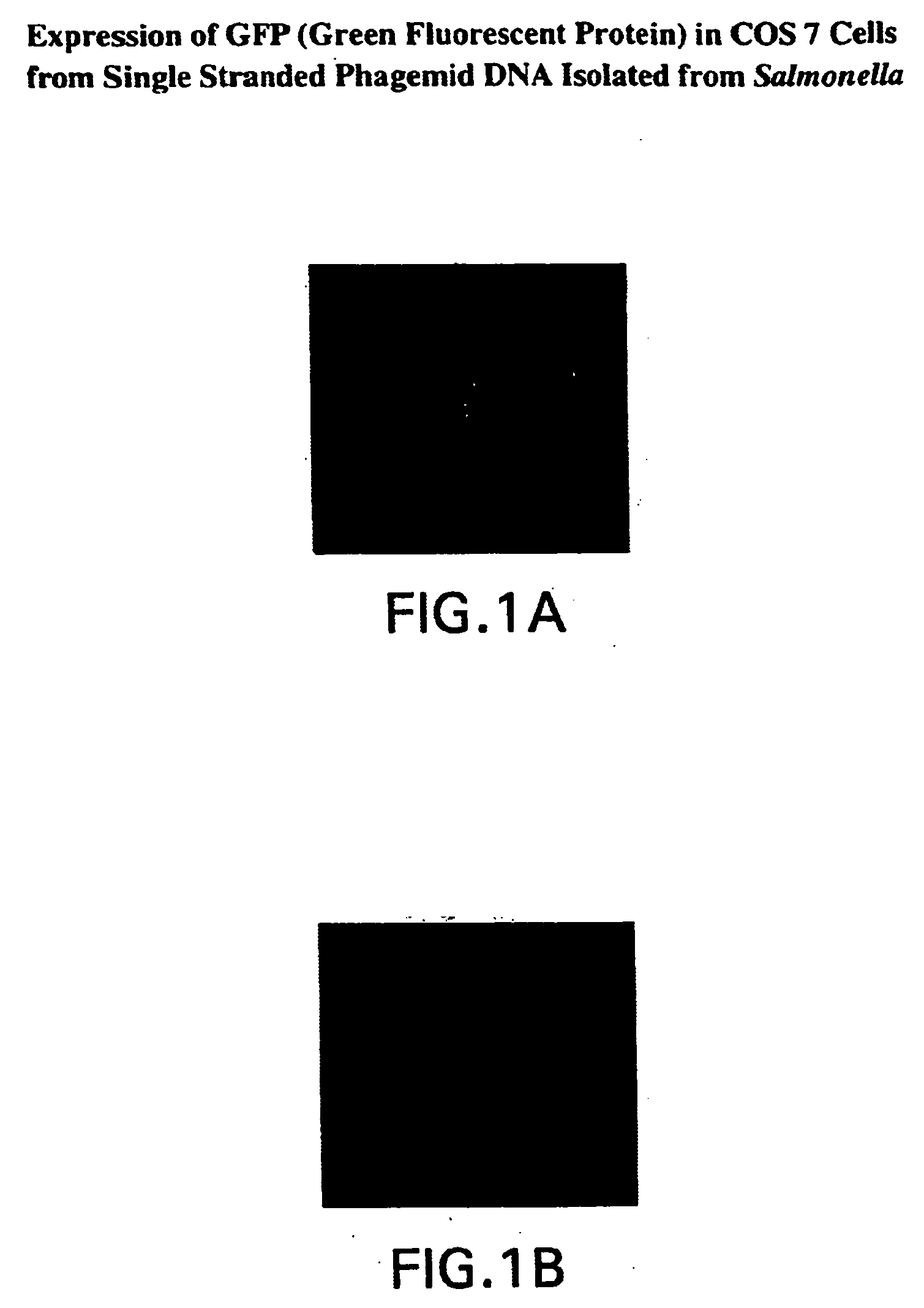
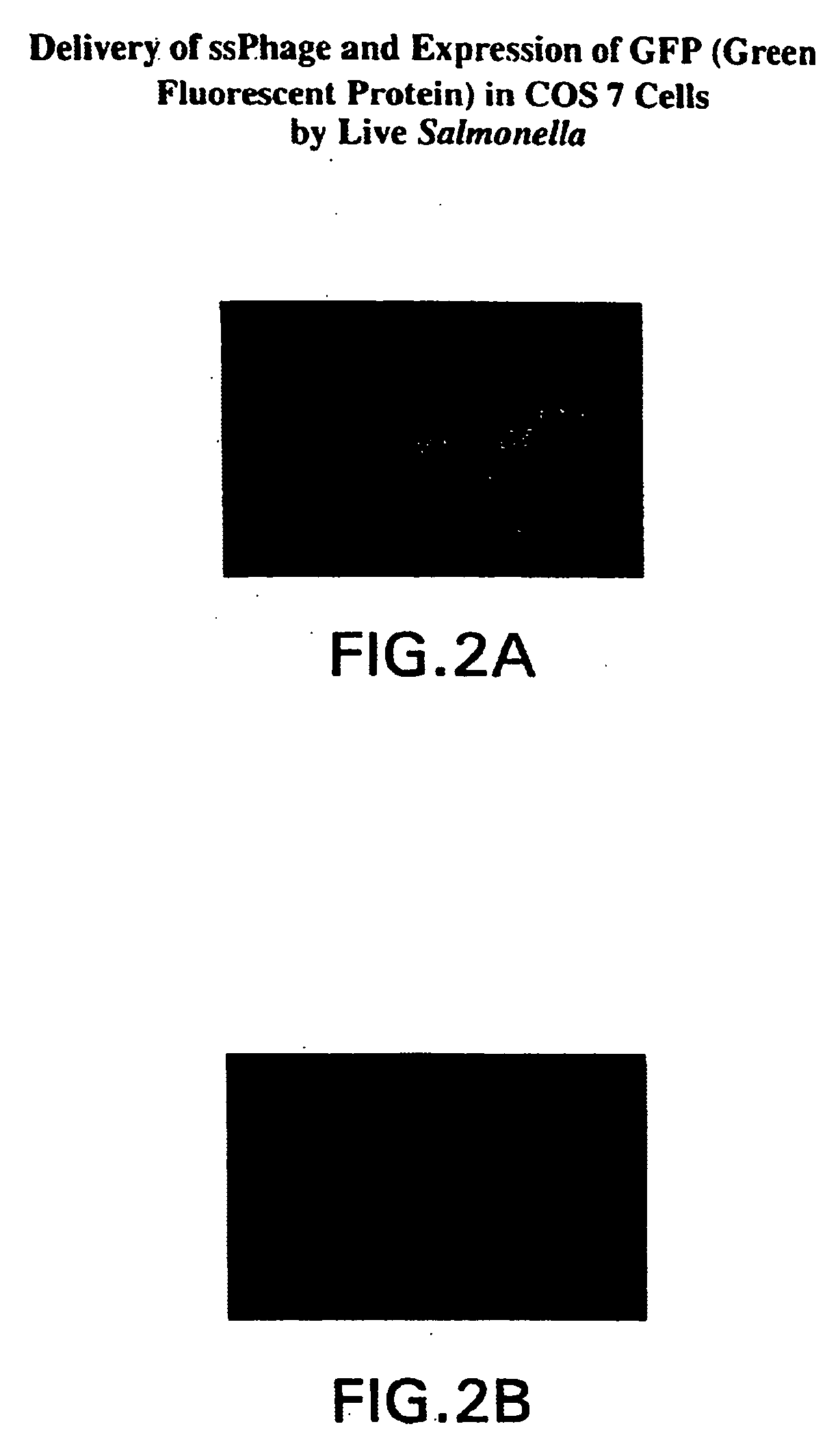
Compositions and methods for delivery of an agent using attenuated Salmonella containing phage
InactiveUS20040219169A1Reduce Toxicity RiskReduce riskBacterial antigen ingredientsBacteriaGene productBacteriophage
Owner:NANOTHERAPEUTICS INC
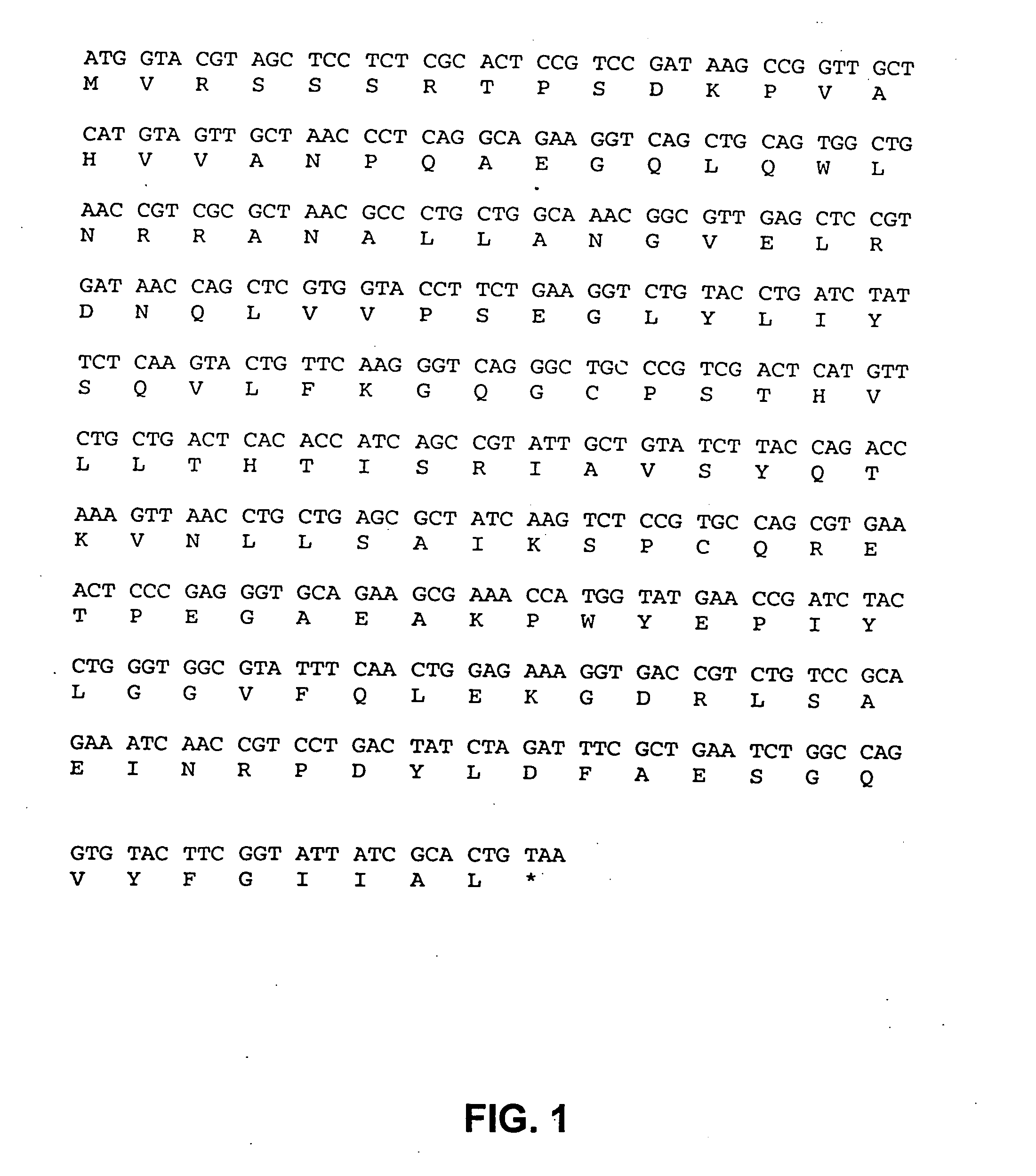
Modified oligonucleotides for telomerase inhibition
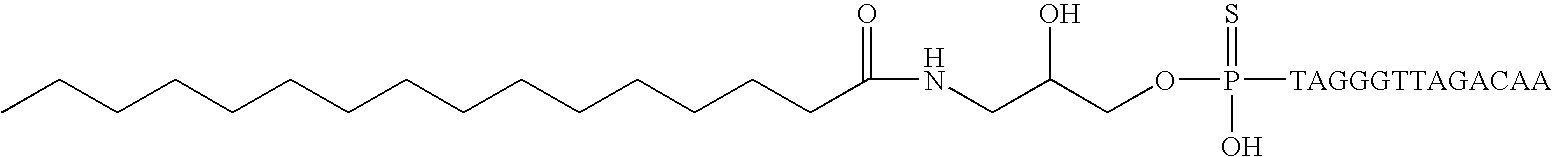
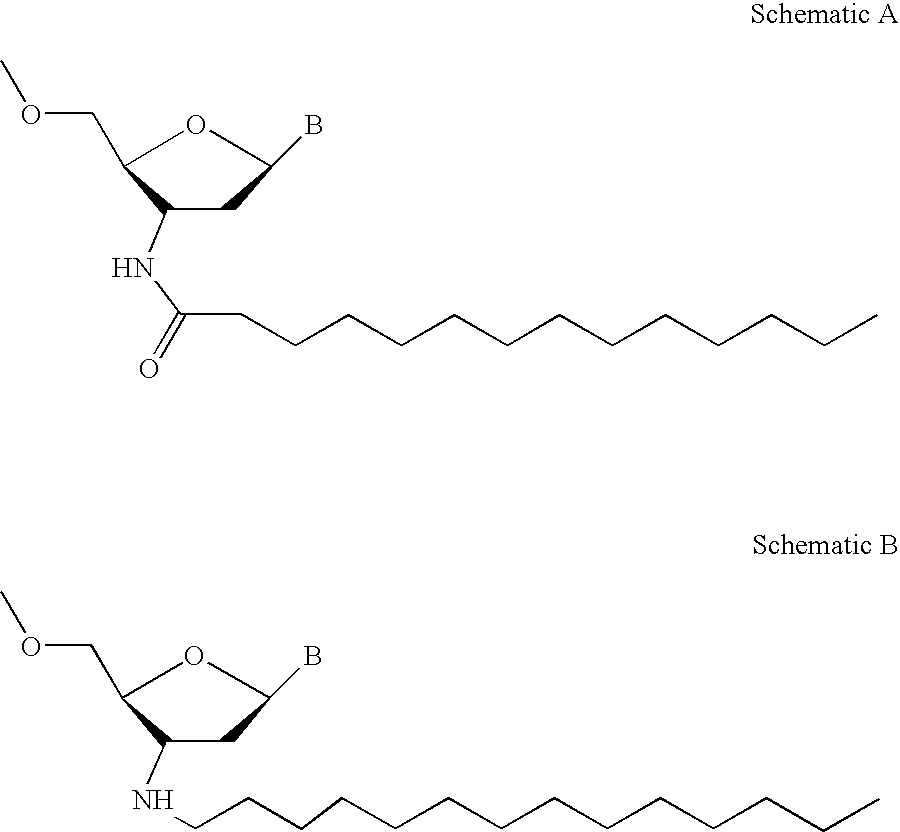
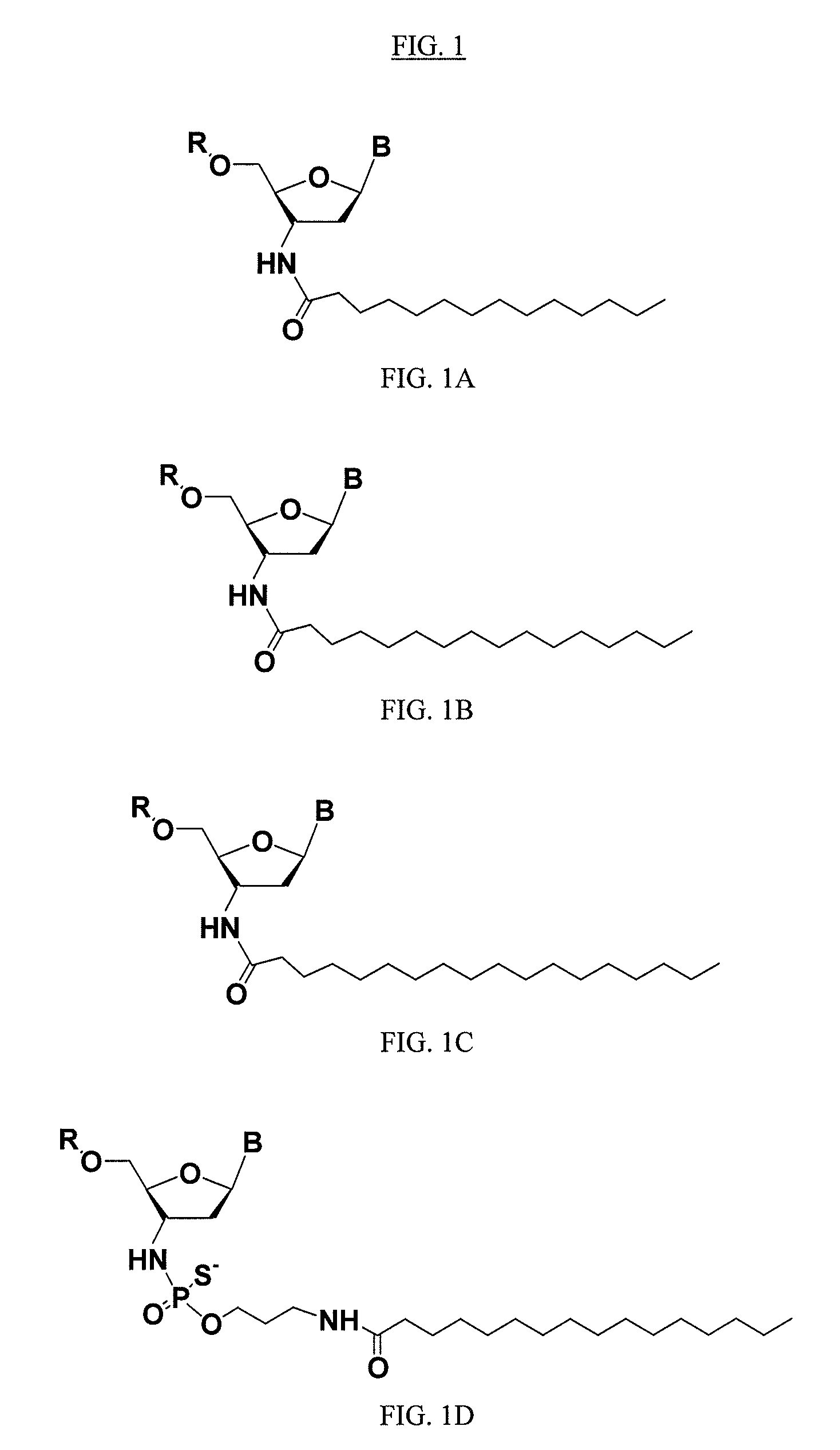
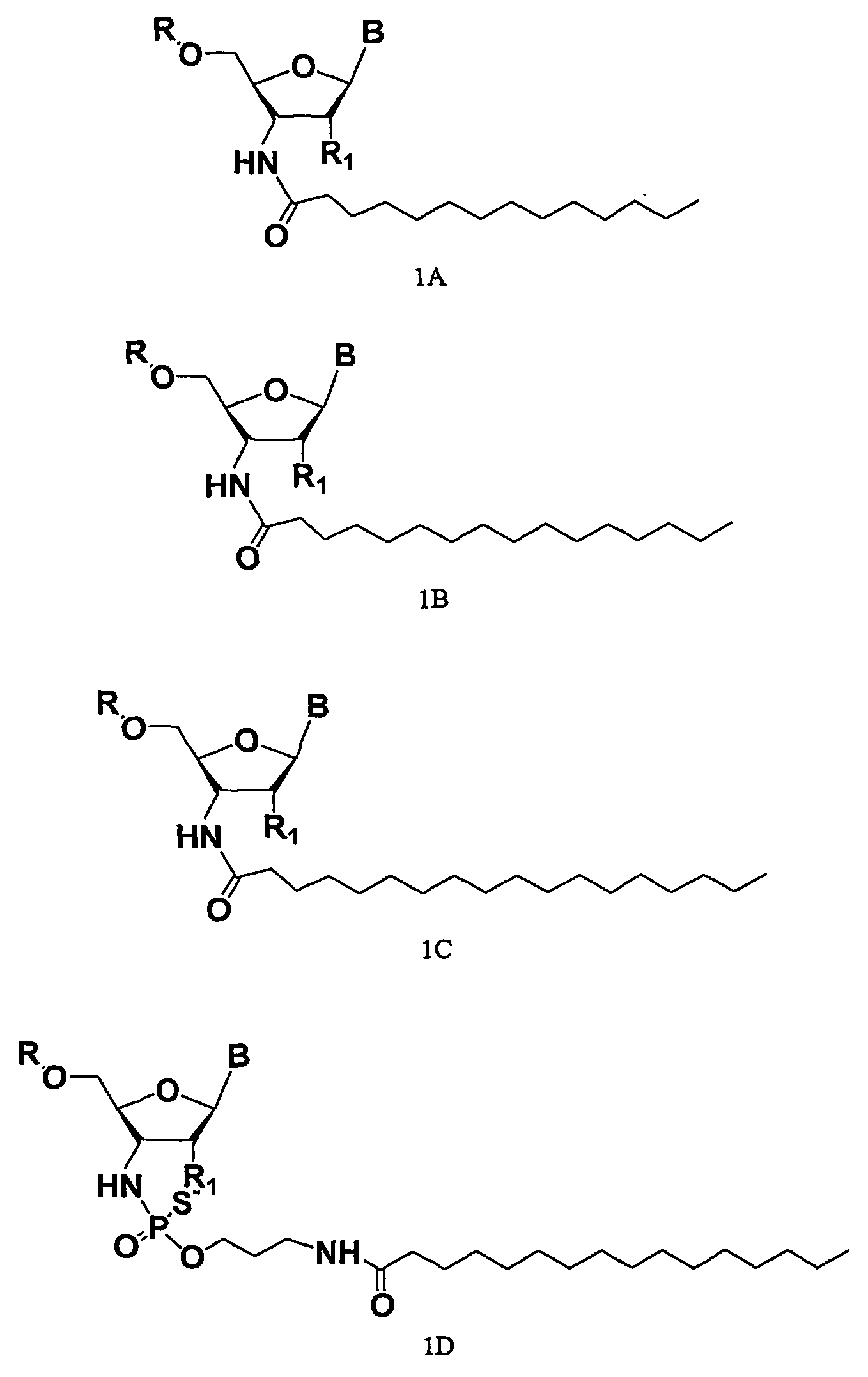
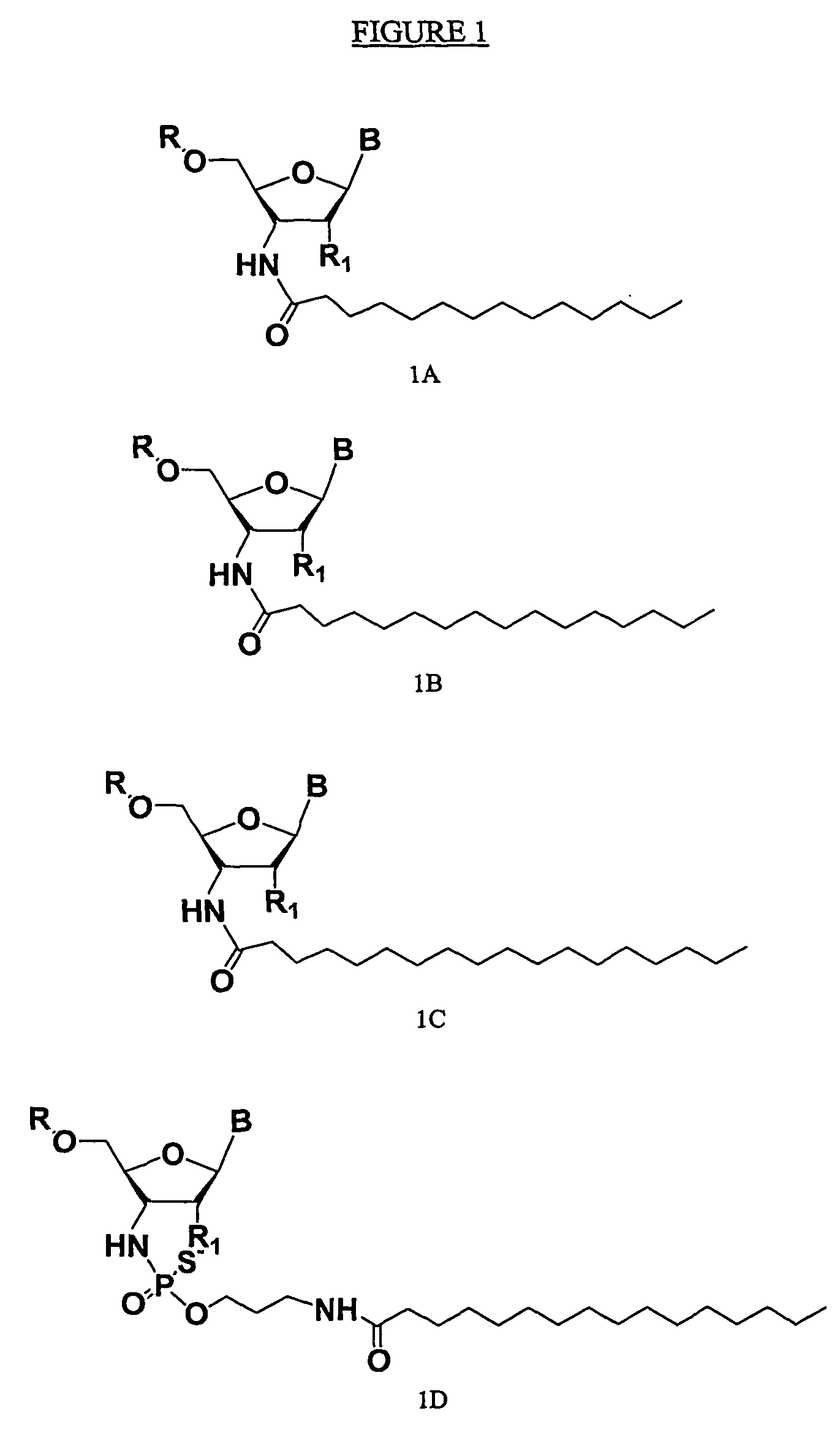
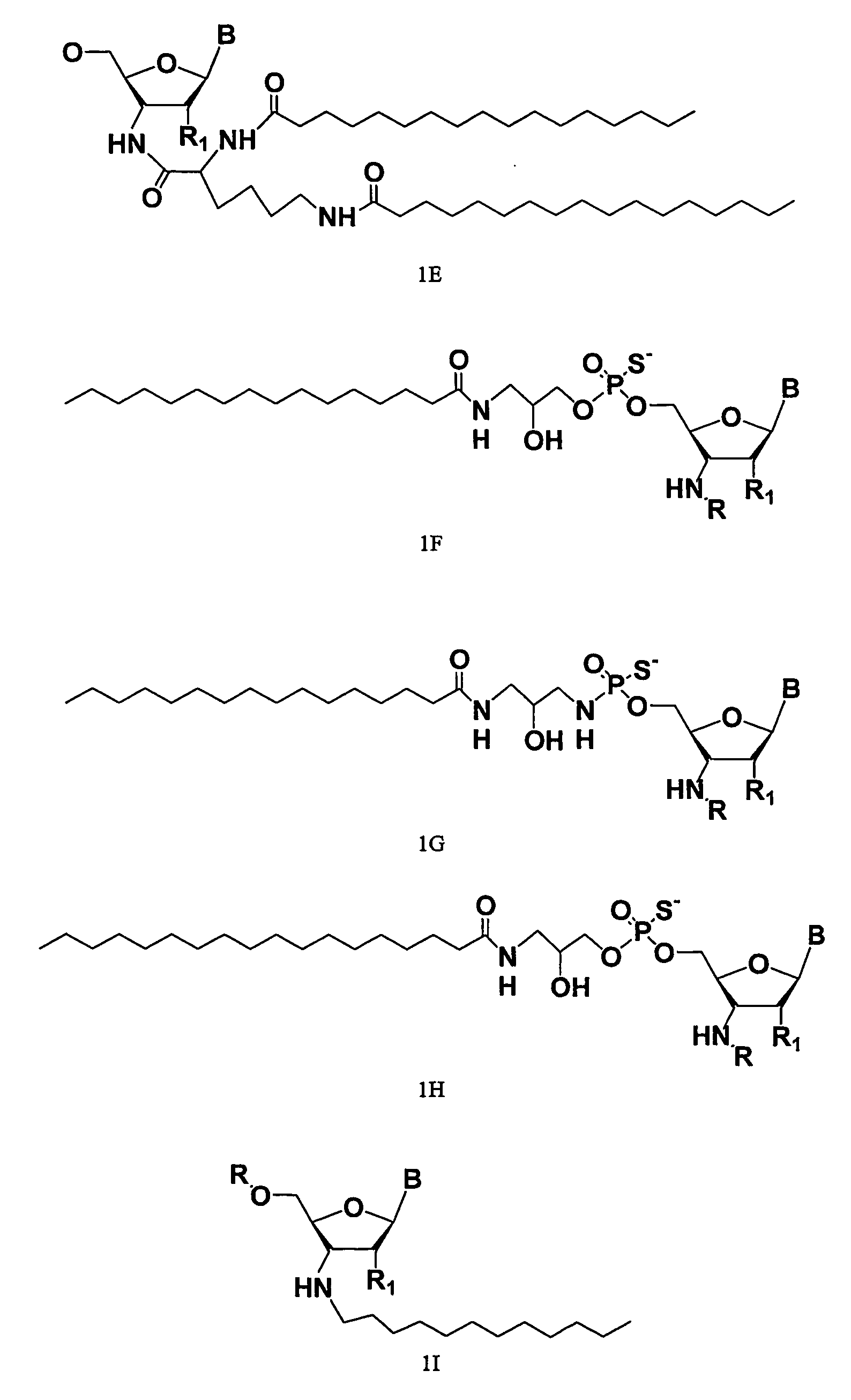
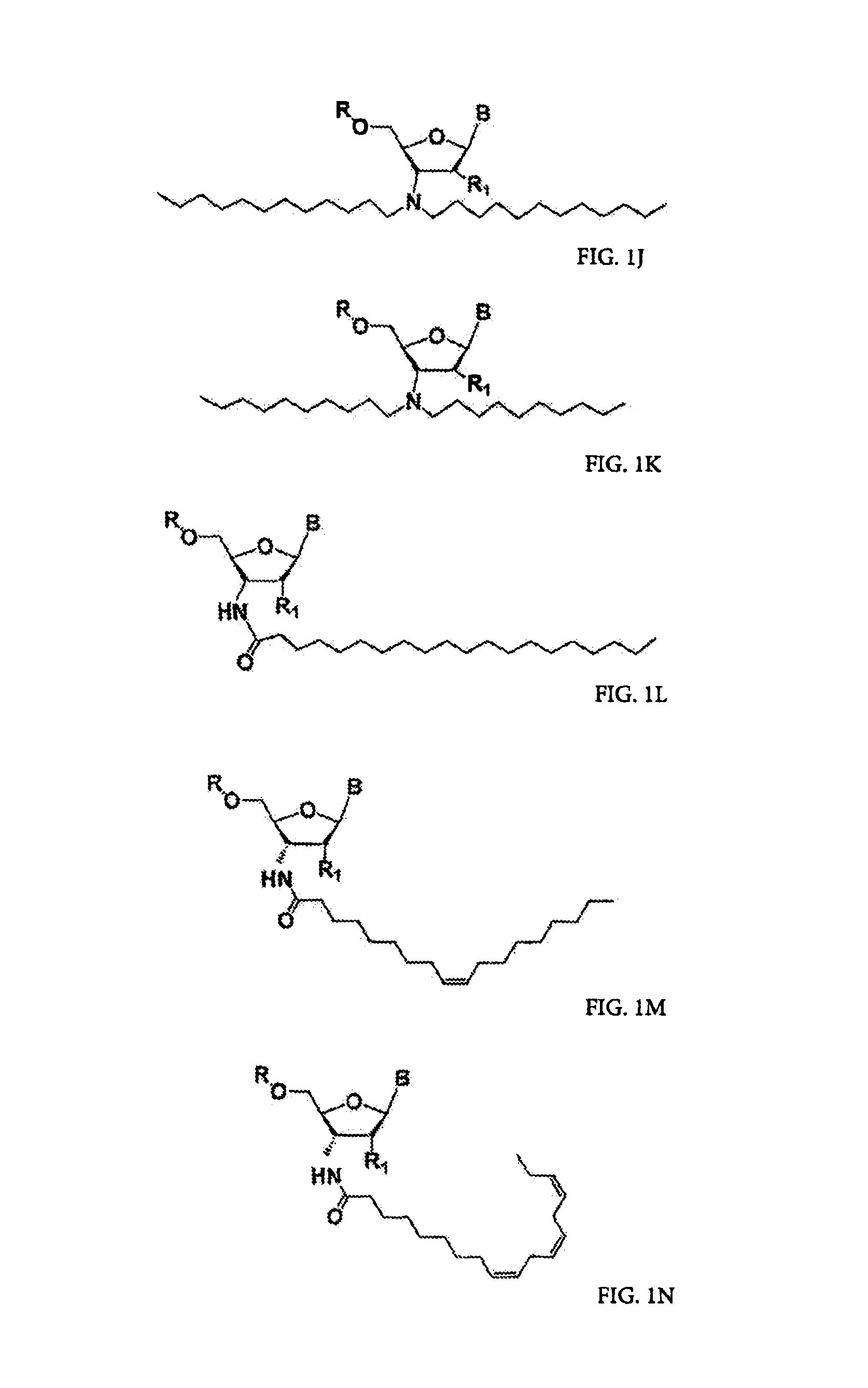
ActiveUS20050113325A1Superior cellular uptake propertyReduce Toxicity RiskBiocideGenetic material ingredientsTelomeraseLipid moiety
Compounds comprising an oligonucleotide moiety covalently linked to a lipid moiety are disclosed. The oligonucleotide moiety comprises a sequence that is complementary to the RNA component of human telomerase. The compounds inhibit telomerase activity in cells with a high potency and have superior cellular uptake characteristics.
Owner:GERON CORPORATION
Modified oligonucleotides for telomerase inhibition
ActiveUS7494982B2Inhibit telomeraseMaintain good propertiesBiocideSugar derivativesTelomeraseLipid moiety
Compounds comprising an oligonucleotide moiety covalently linked to a lipid moiety are disclosed. The oligonucleotide moiety comprises a sequence that is complementary to the RNA component of human telomerase. The compounds inhibit telomerase activity in cells with a high potency and have superior cellular uptake characteristics.
Owner:GERON CORPORATION
Delivery of an active drug to the posterior part of the eye via subconjunctival or periocular delivery of a prodrug
InactiveUS20120157499A1Prolong the action timeIncrease concentrationBiocideSenses disorderConjunctivaDisease
Owner:ALLERGAN INC
Ester combination local anesthetic
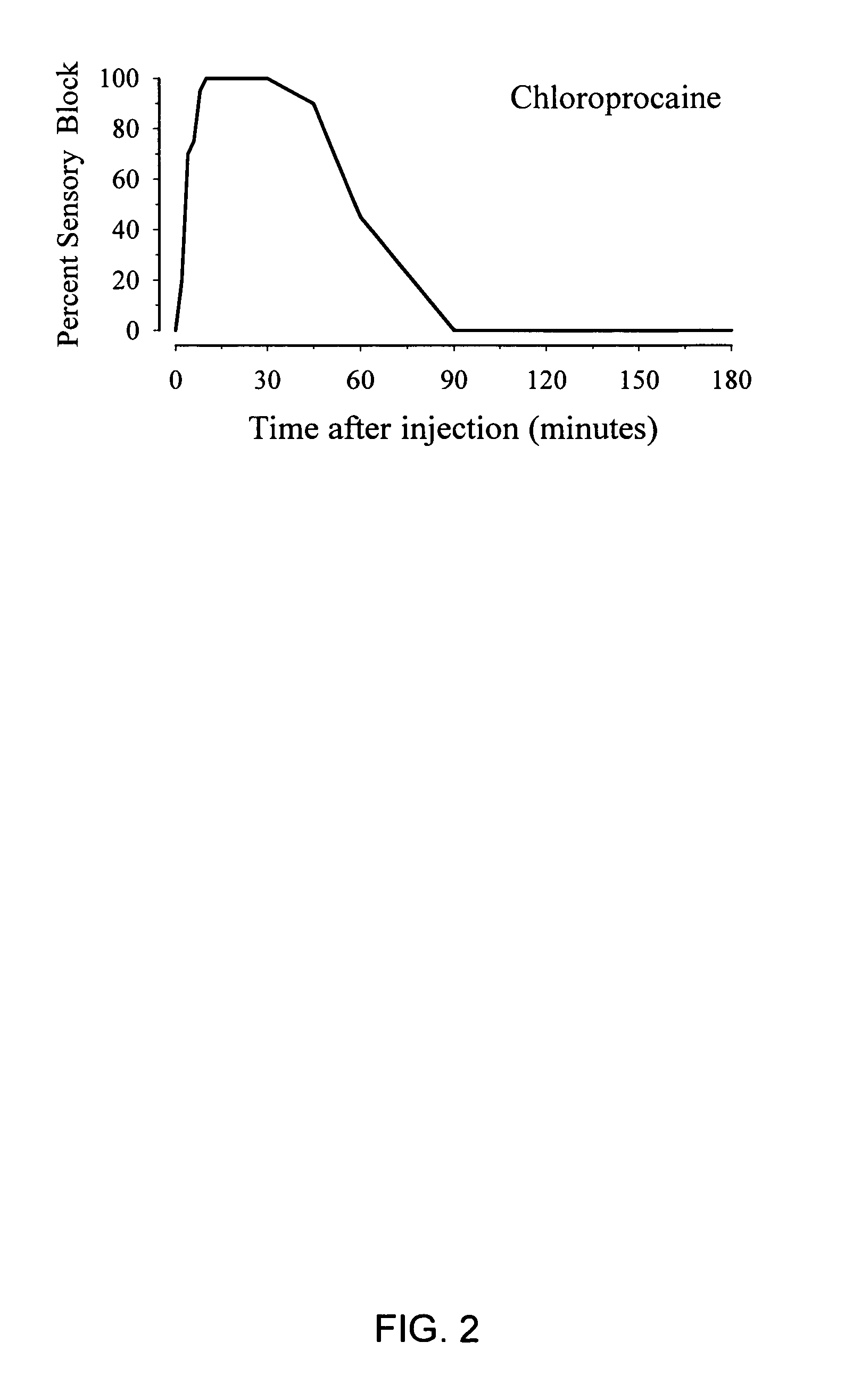
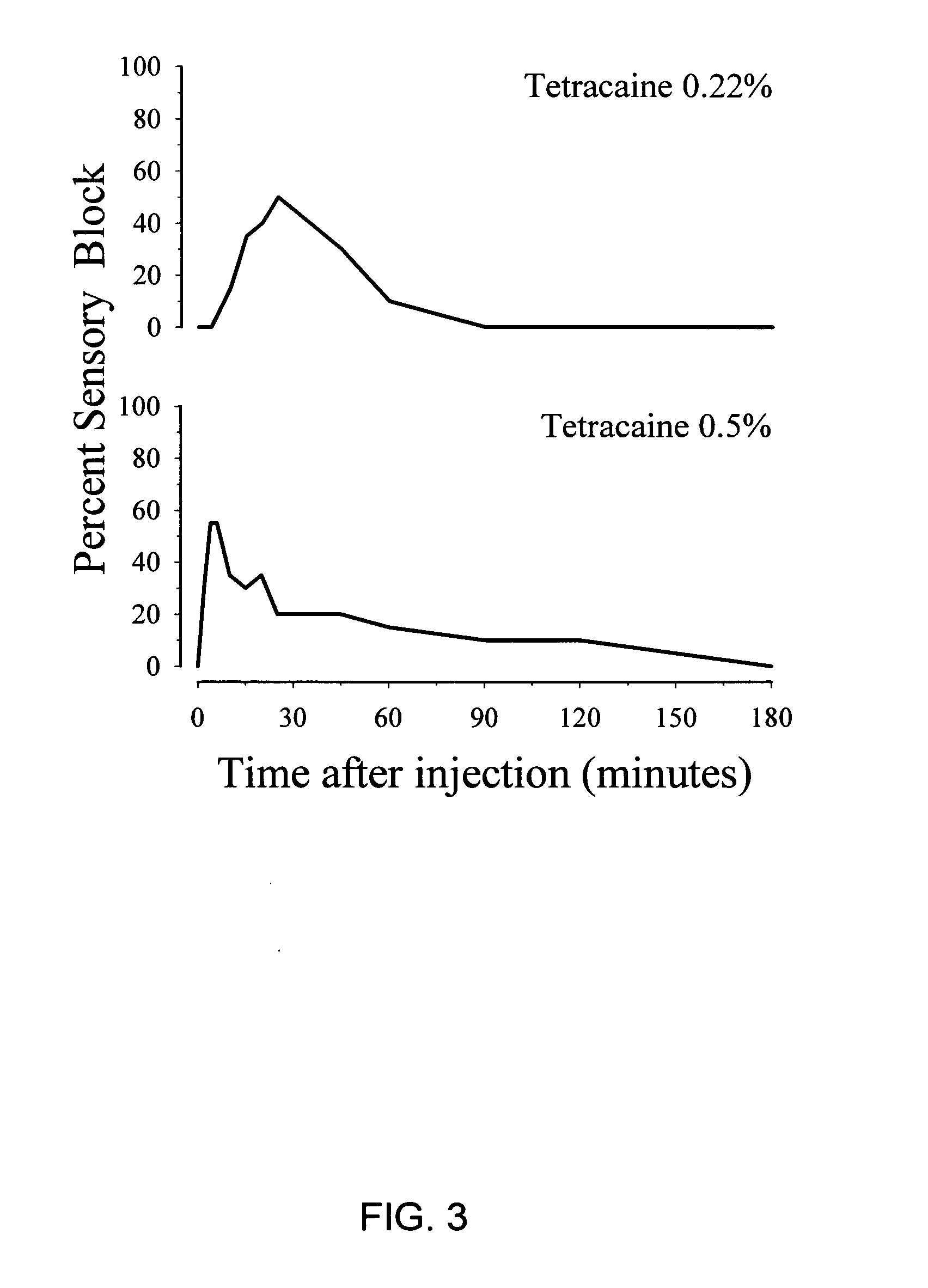
InactiveUS20050137177A1Low risk of toxicityRapid actionBiocideAnimal repellantsPain durationChemical toxicity
The present invention provides compositions and methods for improved local anesthesia and / or analgesia, in which onset of action is rapid, the risk of toxicity is low, and the effect is sustained. More particularly, the present invention provides a combination of at least two ester anesthetics for administration to a subject, where at least one ester anesthetic provides a rapid onset of action and at least one ester anesthetic provides sustained activity. The compositions of the present invention are useful for the production of analgesia and / or anesthesia and are particularly useful for the prophylaxis and / or treatment of pain.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Gelatin porous material prepared by using high inner phase emulsion as template and preparation method of gelatin porous material
The invention discloses a gelatin porous material prepared by using a high inner phase emulsion as a template and a preparation method of the gelatin porous material. A gelatin solution and an organic solvent are stirred and mixed at a high speed to form an oil-in-water type (O / W) high inner phase emulsion, and a continuous phase is crosslinked by using genipin to form a three-dimensional network-state structure, and the three-dimensional network-state structure is frozen and dried to prepare the porous material with a pore diameter of 10-100 mu m and a porosity of more than 70 percent. The gelatin porous material prepared by adopting the preparation method is easily formed, is good in biocompatibility, and can be widely applied to a tissue engineering material.
Owner:SICHUAN UNIV
Methods and compositions relating to the pharmacogenetics of different gene variants
InactiveUS20090017452A1Reduce Toxicity RiskAddress bad outcomesMicrobiological testing/measurementPharmacogeneticsGene product
The present invention is directed to methods and compositions for determining the presence or absence of polymorphisms within an ABCC2, UGT1A1, and / or SLCO1B1 gene and correlating these polymorphisms with activity levels of their gene products and making evaluations regarding the effect on their substrates, particularly those substrates that are drugs. In addition, there are methods and compositions of evaluating the risk of an individual for developing toxicity or adverse event(s) to an ABCC2, UGT1A1, and / or SLCO1B1 substrate. In some embodiments, the invention concerns methods and compositions for determining the presence or absence of ABCC2 3972C>T variant and predicting or anticipating the level of activity of ABCC2 and determining dosages of an ABCC2 drug substrate, such as irinotecan, in a patient. Such methods and compositions can be used to evaluate whether irinotecan-based therapy, or therapy involving other ABCC2 substrates, may pose toxicity problems if given to a particular patient or predicting their efficacy. Alterations in suggested therapy may ensue based on genotyping results.
Owner:RGT UNIV OF CALIFORNIA +1
Large ovarian tissue vitrification freezing carrier and application thereof
PendingCN107535482ALow priceWide selection of materialsDead animal preservationGerm cellsVitrificationEngineering
The invention discloses a large ovarian tissue vitrification freezing carrier. The carrier consists of a carrier inner core and an outer sleeve, wherein the carrier inner core is in threaded connection with the outer sleeve, maintaining high airtightness and preventing cross infection of the ovarian tissue; meanwhile, the user operation is also facilitated; the carrier inner core consists of a handheld part, a cap part, a support part and a bearing part sequentially from top to bottom; the bearing part is clamped and fixed by the support part; when in use, a large ovarian tissue is put on an end part of the bearing part, away from the support part; the end part is a large ovarian tissue loading area; experiments prove that by using the carrier disclosed by the invention, the permeation efficiency of a refrigerant is improved; moreover, the operation is simple, the price is low, batch storage of large ovarian tissues is convenient, and the carrier is suitable for promotion and application.
Owner:SHANDONG UNIV QILU HOSPITAL
Sampling device
ActiveCN105050500AReduce Toxicity RiskPhysiologically relevantSurgical needlesVaccination/ovulation diagnosticsHealth statesNutrient absorption
The present invention relates to a device for sampling internal substance(s) in the gastrointestinal tract of an animal composed of at least one wall which is partially expandable. The invention also relates to a method of orally administering the device to an animal and recovering the device from the stool to carry out analysis on the collected sample for diagnosing the health of the gastrointestinal tract and determining nutrient absorption and digestibility.
Owner:MARS INC
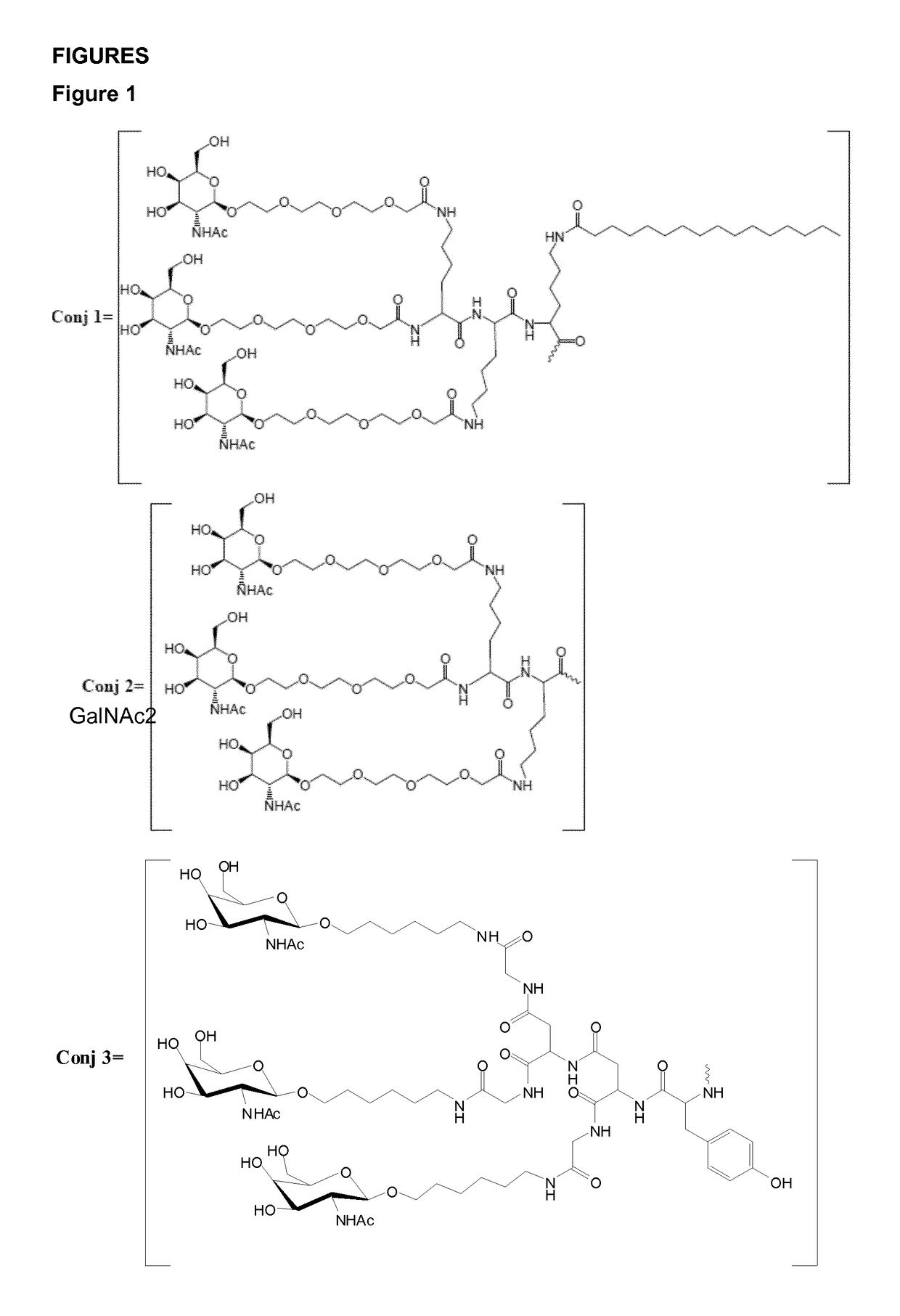
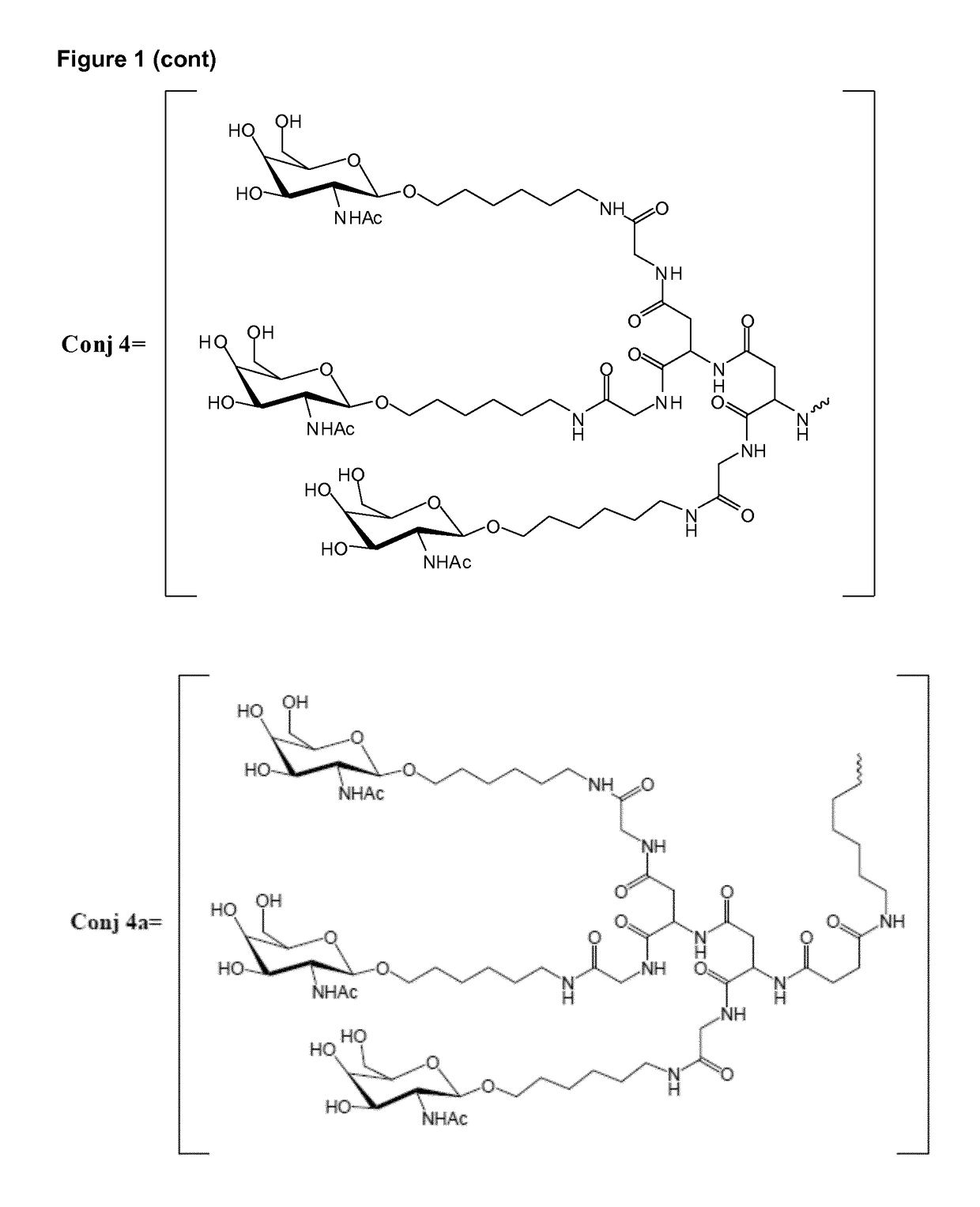
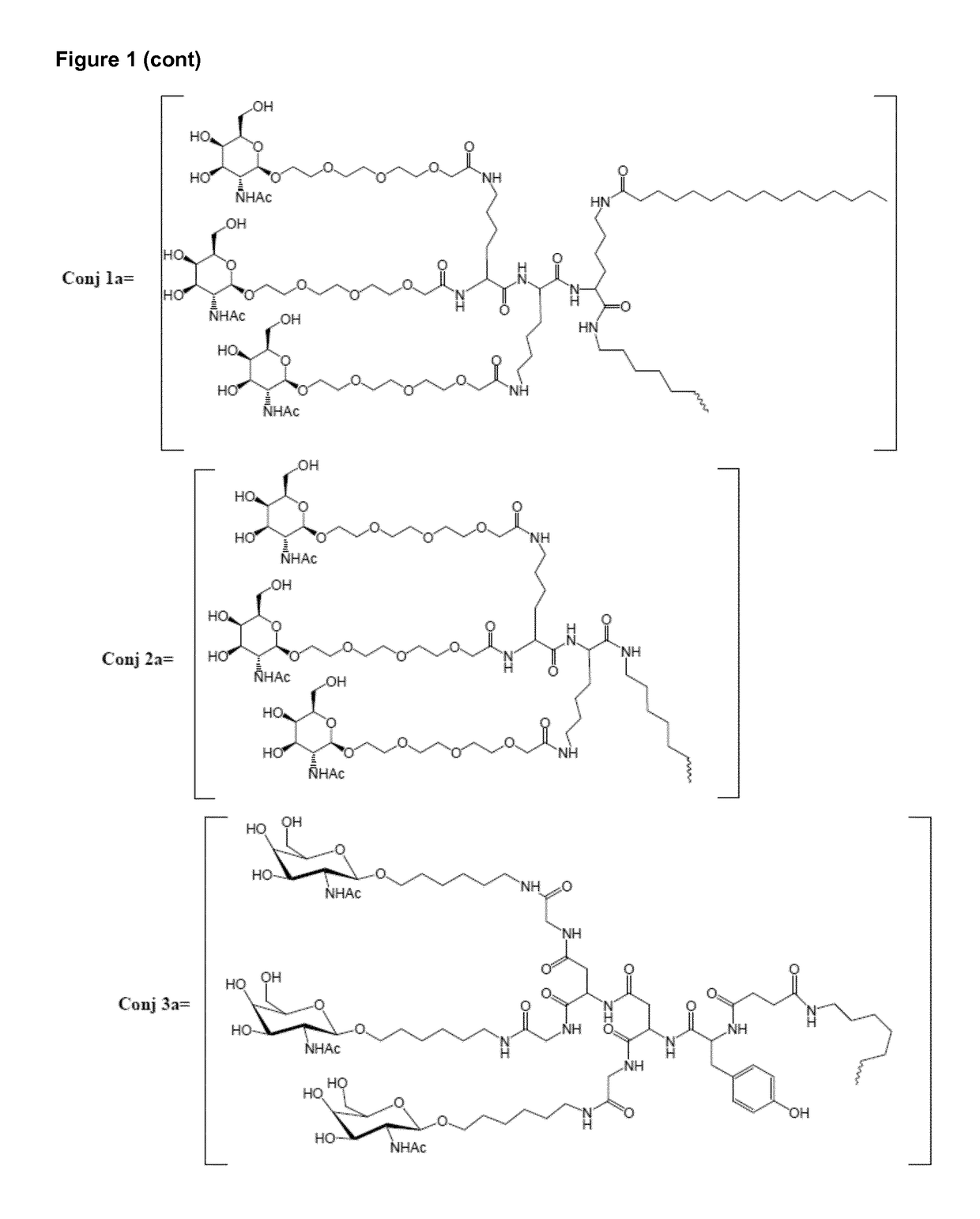
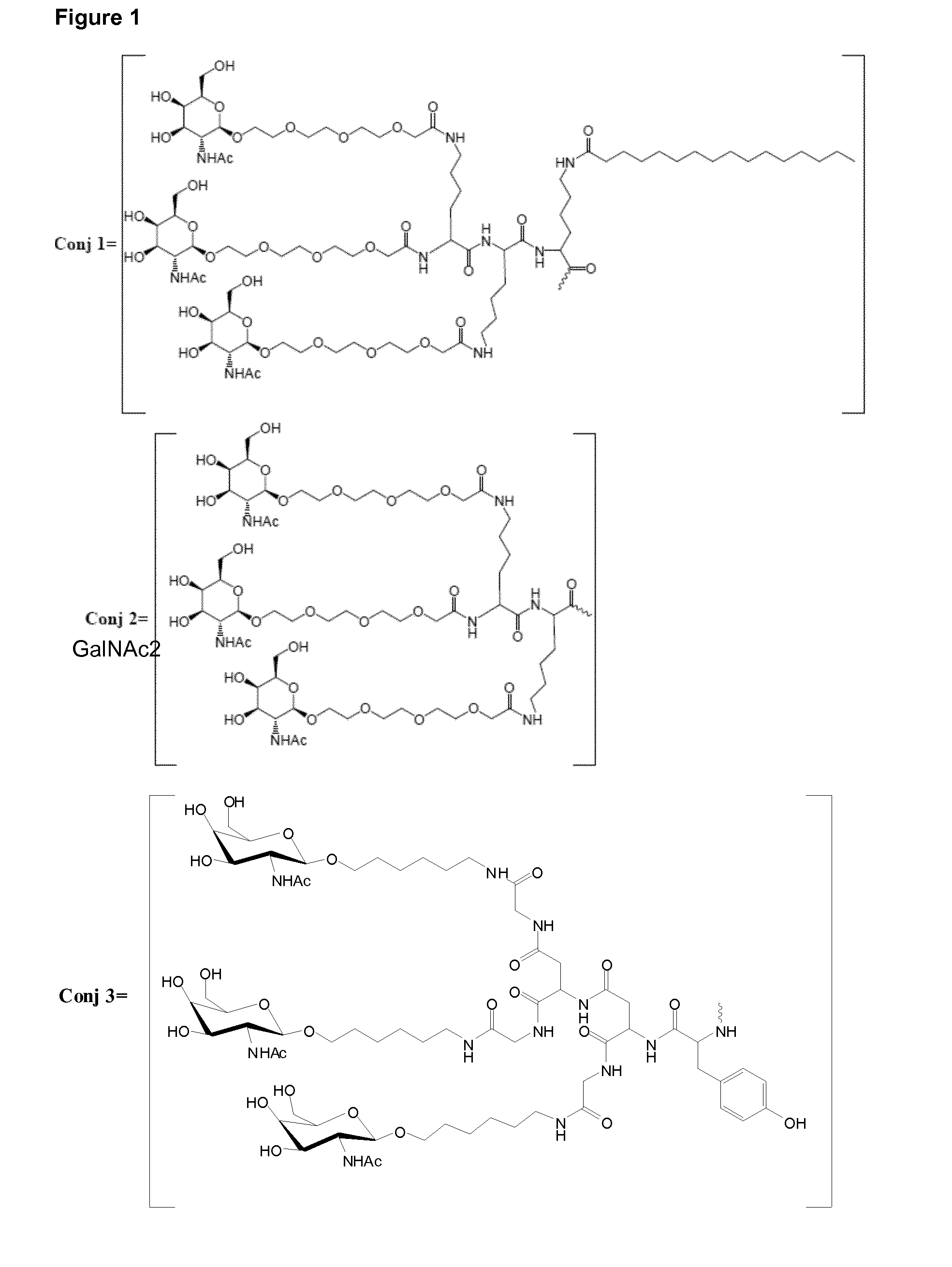
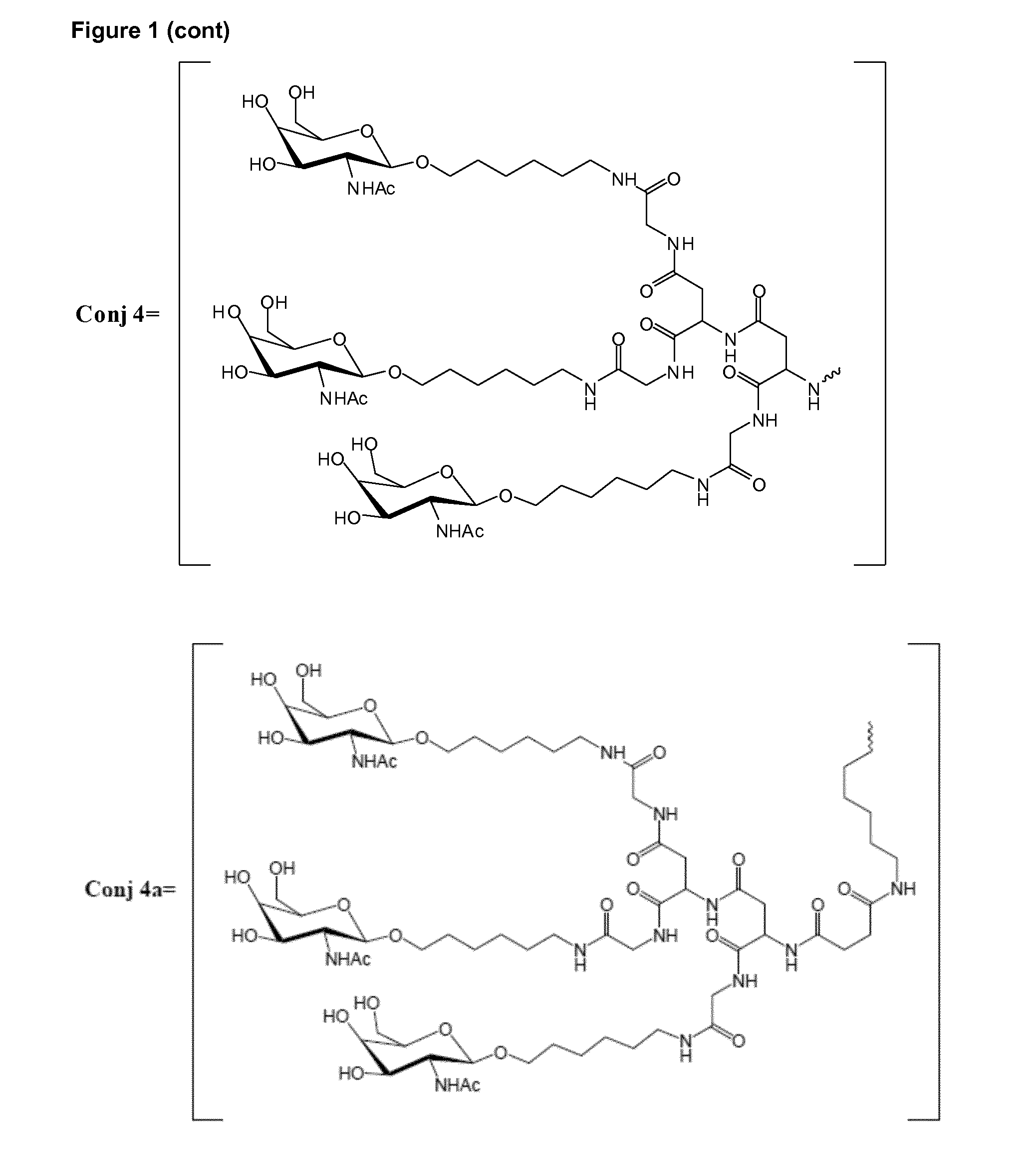
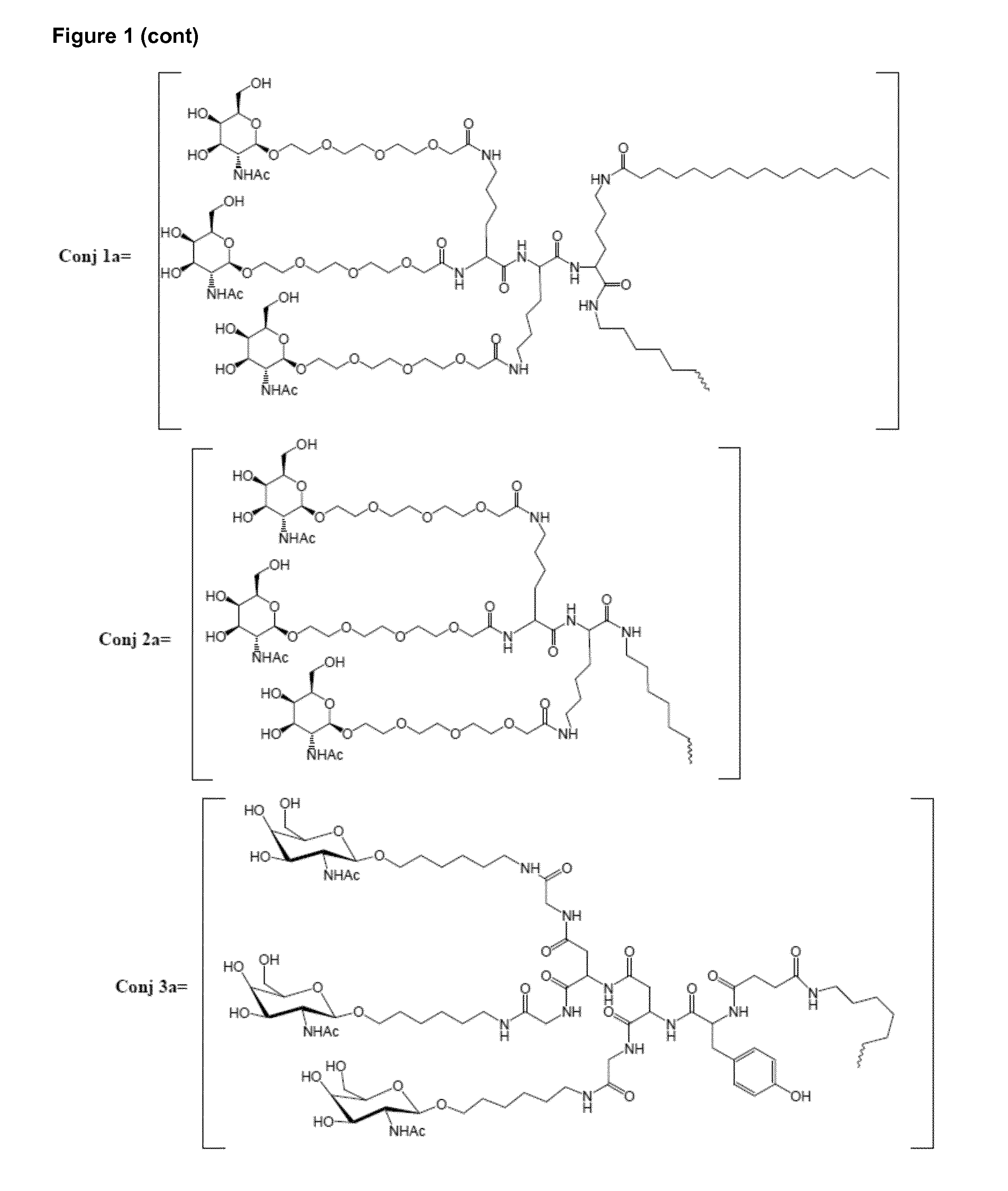
Oligonucleotide conjugates
ActiveUS9879265B2Reduce Toxicity RiskReduce nephrotoxicityOrganic active ingredientsSugar derivativesMedical disorderSubtilisins
The present invention relates to oligomeric compounds and conjugates thereof that target Proprotein Convertase Subtilisin / Kexin type 9 (PCSK9) PCSK9 mRNA in a cell, leading to reduced expression of PCSK9. Reduction of PCSK9 expression is beneficial for a range of medical disorders, such as hypercholesterolemia and related disorders.
Owner:ROCHE INNOVATION CENT COPENHAGEN
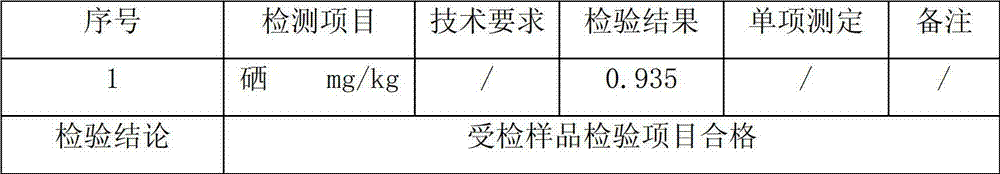
Production method of selenium-rich ecological eggs
InactiveCN103027307ASafe conversionEfficient conversionAnimal feeding stuffFood preparationEpimediumAnimal science
The invention discloses a production method of selenium-rich ecological eggs. The production method comprises the following steps: respectively drying and crushing 25-35 parts by weight of epimedium herb, 50-70 parts by weight of common yam rhizome, 15-25 parts by weight of largehead atractylodes rhizome and 55-65 parts by weight of selenium-rich sainfoin, screening by a 60-80-mesh screen, and uniformly mixing; adding 25-35 parts by weight of selenium-rich medical stone powder, and uniformly mixing to prepare a selenium-rich organic feed additive; uniformly stirring and mixing the selenium-rich organic feed additive and a chicken feed according to the weight ratio of (5-12): 100 to prepare a special chicken feed for later use; and the feeding time by adopting the special chicken feed is 25-28 days, and the chicken feed is adopted for feeding during the rest of time. The selenium-rich organic feed additive used in the production method disclosed by the invention is rich in selenium, further contains a variety of components capable of enhancing immunity and has no toxicity or side effects; and simultaneously, all the components can realize synergy, promote safe and high-efficient transformation of selenium in organisms of laying hens and greatly reduce the risk of producing toxicity in the eggs caused by excessive selenium.
Owner:SHANGHAI YUANKANG SELENIUM BIOTECH
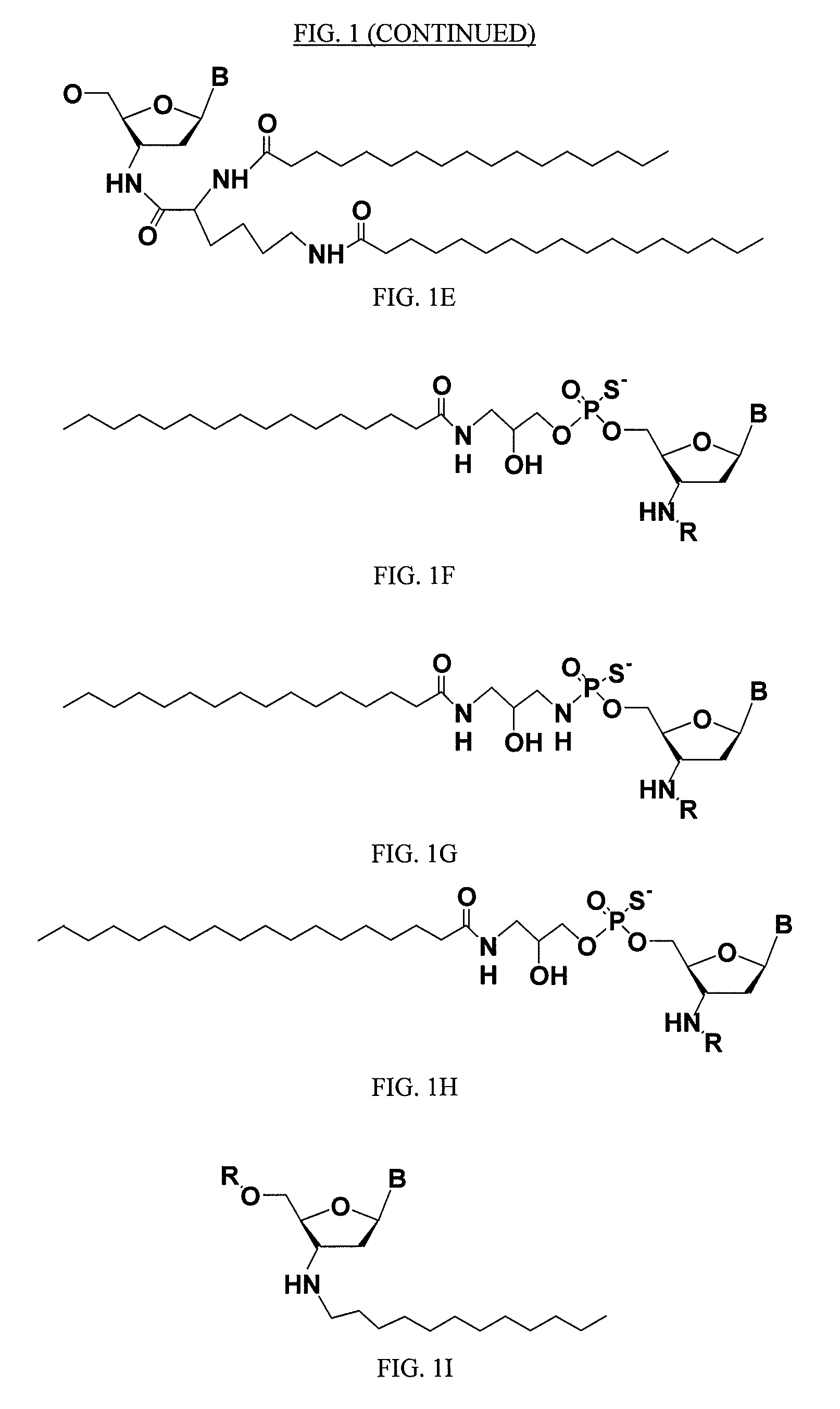
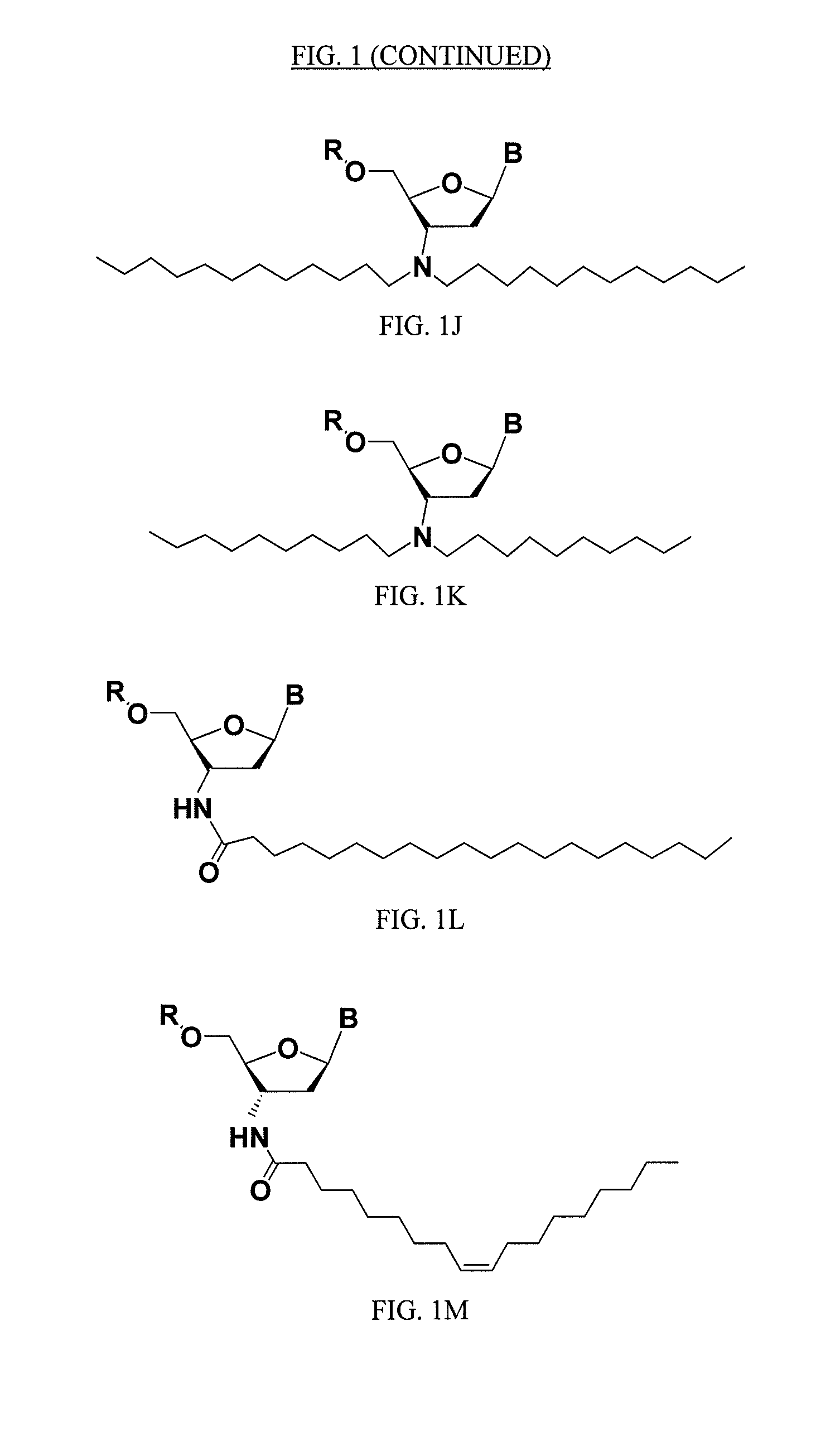
Rna Amidates and Thioamidates for Rnai
ActiveUS20070275919A1Improve the immunityLess criticalOrganic active ingredientsSugar derivativesRegulator geneBiology
The present disclosure relates to RNA amidates and thioamidates useful for RNA interference applications. The RNA amidates and thioamidates contain at least one internucleoside linkage chosen from ribo-N3′→P5′ phosphoramidate (NP) and ribo-N3′→P5′ thiophosphoramidate (NPS) linkages, and optionally further containing at least one covalently conjugated lipid moiety. Compositions comprising the amidates and thioamidates are disclosed, as are methods for their use in modulating gene expression.
Owner:GERON CORPORATION
Plant raw material composition with anti-fatigue effect, preparation method, application and product thereof
ActiveCN101804123ARecovery effectSlow immune response, poor judgment, mental depression" and other fatigue symptomsAntinoxious agentsFood scienceSolubilityRHODIOLA ROSEA ROOT
The invention provides a plant raw material composition with an anti-fatigue effect, a preparation method, an application and a product thereof. The composition is prepared from the following in proportion: 20 to 80 percent of rhodiola rosea plants, 10 to 60 percent of gynostemma pentaphylla plants, and 10 to 60 percent of lycium plants. Auxiliary materials can be added into the composition to prepare health-care products, foods or anti-fatigue medicament. The medicament can obviously improve the fatigue symptom of fatigue people, and improve sleep quality without affecting human normal physiologic indexes. The medicament can improve the oxygen supply capability of a body, and plays a role of resisting fatigue by potentially improving the function of red blood cells and platelets. The medicinal composition uses small effective dosage, has good water solubility, and is suitable for preparing various preparations.
Owner:北京植物世纪营养品有限公司
A kind of gelatin porous material prepared with high internal phase emulsion as a template and its preparation method
The invention discloses a gelatin porous material prepared by using a high internal phase emulsion as a template and a preparation method. Using high-speed stirring to mix gelatin aqueous solution and organic solvent to form an oil-in-water (O / W) high internal phase emulsion, and use genipin to cross-link the continuous phase to form a three-dimensional network structure, and freeze-dry to obtain a pore size of 10-100 μm. Porous materials with a porosity greater than 70%. The material prepared by the invention is easy to form, has good biocompatibility, and can be widely used in tissue engineering materials.
Owner:SICHUAN UNIV
Antibacterial microemulsion gel and preparation method thereof
PendingCN112336685AImprove wettabilityImprove stabilityAntibacterial agentsOrganic active ingredientsAntibacterial activityBiomedicine
The invention relates to antibacterial microemulsion gel and a preparation method thereof. The preparation method of the microemulsion gel takes a Pickering emulsion as a template, and comprises the following steps of (1) preparing a continuous phase which is composed of distilled water, gelatin nanoparticles, aminoglycoside antibiotics and oxidized polysaccharide; and (2) adding a dispersion phase containing antibacterial essential oil into the continuous phase, performing emulsifying to obtain the Pickering emulsion, and performing reaction crosslinking for a certain time to obtain the antibacterial microemulsion gel. The constructed microemulsion gel is intelligent microemulsion gel administrated as required, the release of the antibiotics and antibacterial essential oil can be regulated and controlled according to in-vitro environmental conditions, the antibacterial activity is high, and the microemulsion gel can be applied to the biomedical fields of drug controlled release systems, medical dressings and the like.
Owner:SOUTHWEST JIAOTONG UNIV
Methods and compositions for therapeutic drug monitoring and dosing by point of care pharmacokinetic profiling
InactiveUS20140349862A1Good curative effectReduce dosageOrganic active ingredientsLibrary screeningPoint of careSelf sampling
Disclosed are methods and kits for pharmacokinetic profiling employing point-of-care or point of service self-sampling and allowing for dosage adjustments based on the pharmacokinetic profiles.
Owner:AUTOTELIC
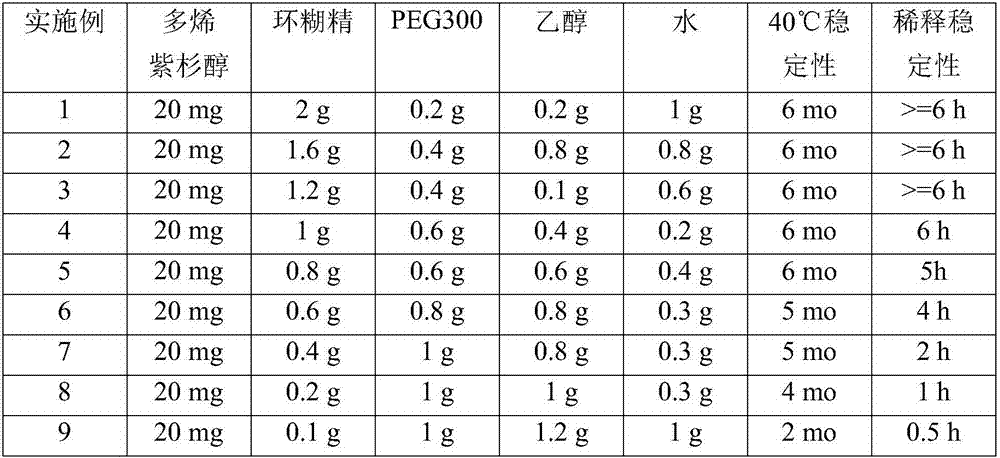
Pharmaceutical compositions containing taxane-cyclodextrin complexes, method of making and methods of use
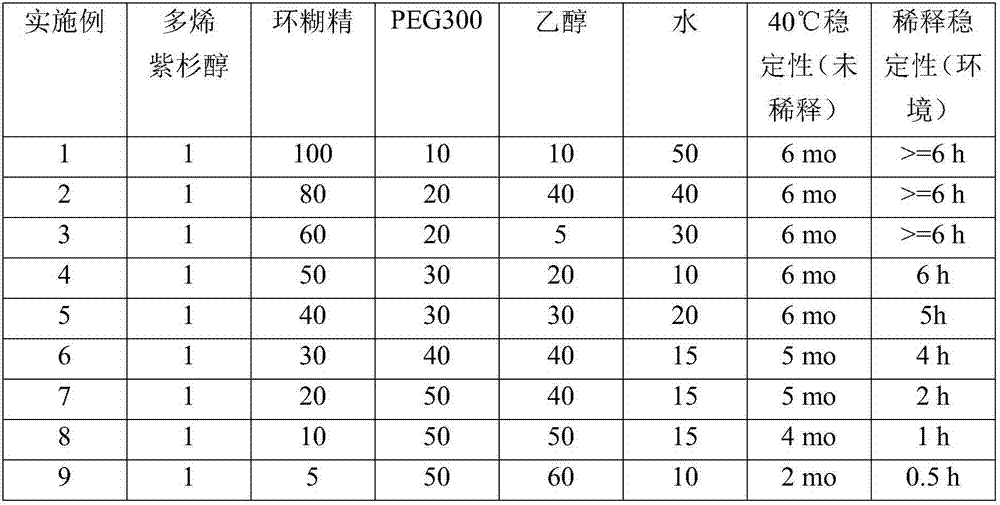
ActiveCN107427486AReduce Toxicity RiskOrganic active ingredientsOrganic non-active ingredientsPolyethylene glycolCyclodextrin
Pharmaceutical formulations for parenteral administration comprising taxane compounds complexed with cyclodextrins and polyethylene glycol, methods of making the pharmaceutical formulations and methods of treating cancer patients using the pharmaceutical formulations.
Owner:HUNAN JINZHUN MEDICAL TECH CO LTD
RNA amidates and thioamidates for RNAi
ActiveUS9133233B2Maintain good propertiesReduce Toxicity RiskSugar derivativesActivity regulationRegulator geneBiology
The present disclosure relates to RNA amidates and thioamidates useful for RNA interference applications. The RNA amidates and thioamidates contain at least one internucleoside linkage chosen from ribo-N3′→P5′ phosphoramidate (NP) and ribo-N3′→P5′ thiophosphoramidate (NPS) linkages, and optionally further containing at least one covalently conjugated lipid moiety. Compositions comprising the amidates and thioamidates are disclosed, as are methods for their use in modulating gene expression.
Owner:GERON CORPORATION
Method for removing element impurities and pigments in sugammadex sodium refined product
The invention discloses a method for removing element impurities and pigments in a sugammadex sodium refined product. The method comprises steps: dissolving the refined sugammadex sodium product intoan aqueous solution; dropwise adding a certain amount of a poor solvent to separate out a small part of sugammadex sodium and a large part of element impurities and pigments in the solution, separating the precipitate, collecting sugammadex sodium contained in an upper mother liquor, and obtaining a product with the large part of element impurities removed through a direct concentration or solventcrystallization method. The obtained product is light in color, the content of element impurities is remarkably reduced, the requirement of ICH for the limit of the element impurities is met, and themethod is easy, convenient and safe to operate, good in economical efficiency and suitable for industrial production.
Owner:HEFEI BOSIKC PHARMTECH CO LTD
Fire extinguisher system and method for extinguishing fires
A system for extinguishing fires is provided which is compliant with ANSI / UL 1254 1999. The system comprises a fire extinguishant liquified gas which is EPA compliant, an ammonium fire extinguishing material, a polymeric gelling agent that interacts with fire extinguishing substances (a) and (b) to promote the formation of a stable, gel-like suspension there between, and a viscosity reducing agent which facilitates the flow of the mixed phase agent from the extinguisher so that a sufficient amount of the fire extinguishing substances (a) and (b) is discharged from the system in response to heat generated by a fire in an area proximate to said fire extinguisher system to extinguish the fire in a manner compliant with ANSI / UL 1254 1999.
Owner:CEASE FIRE
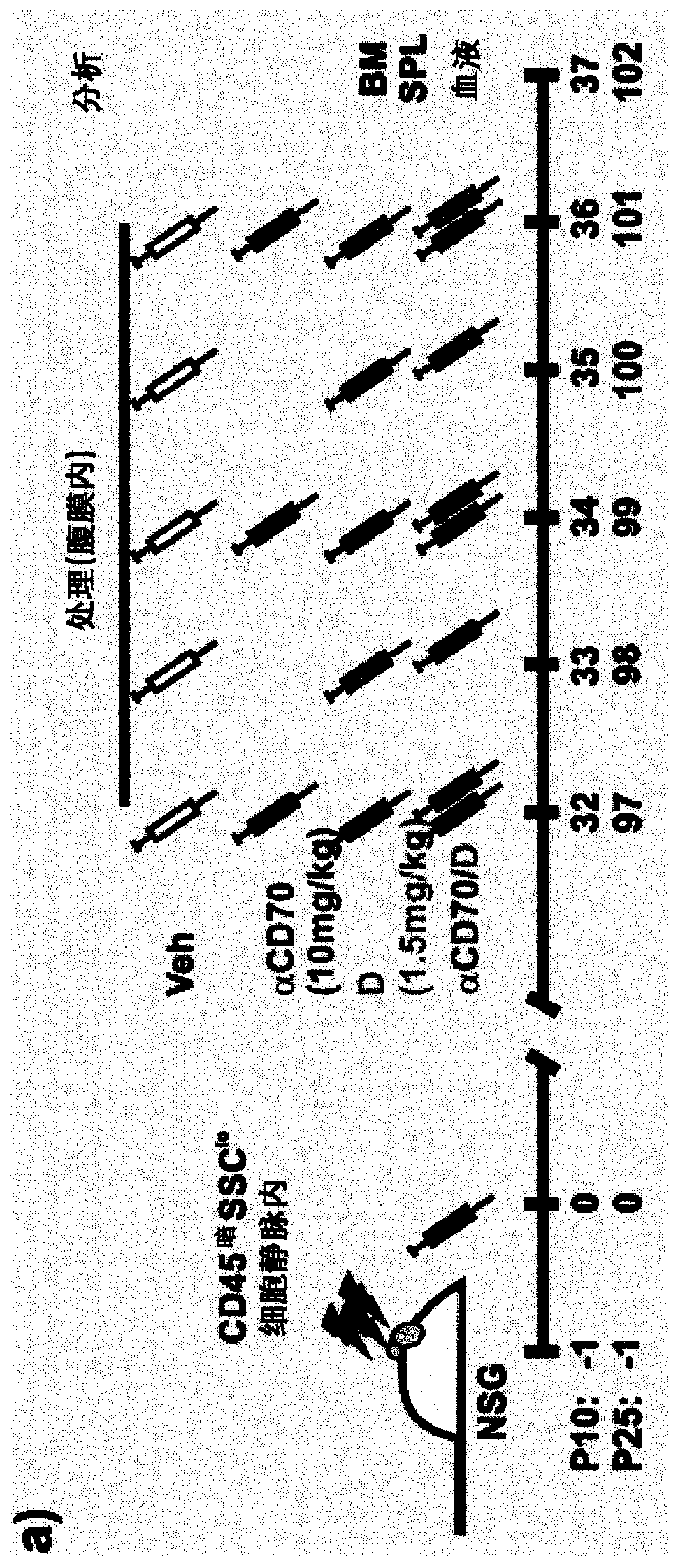
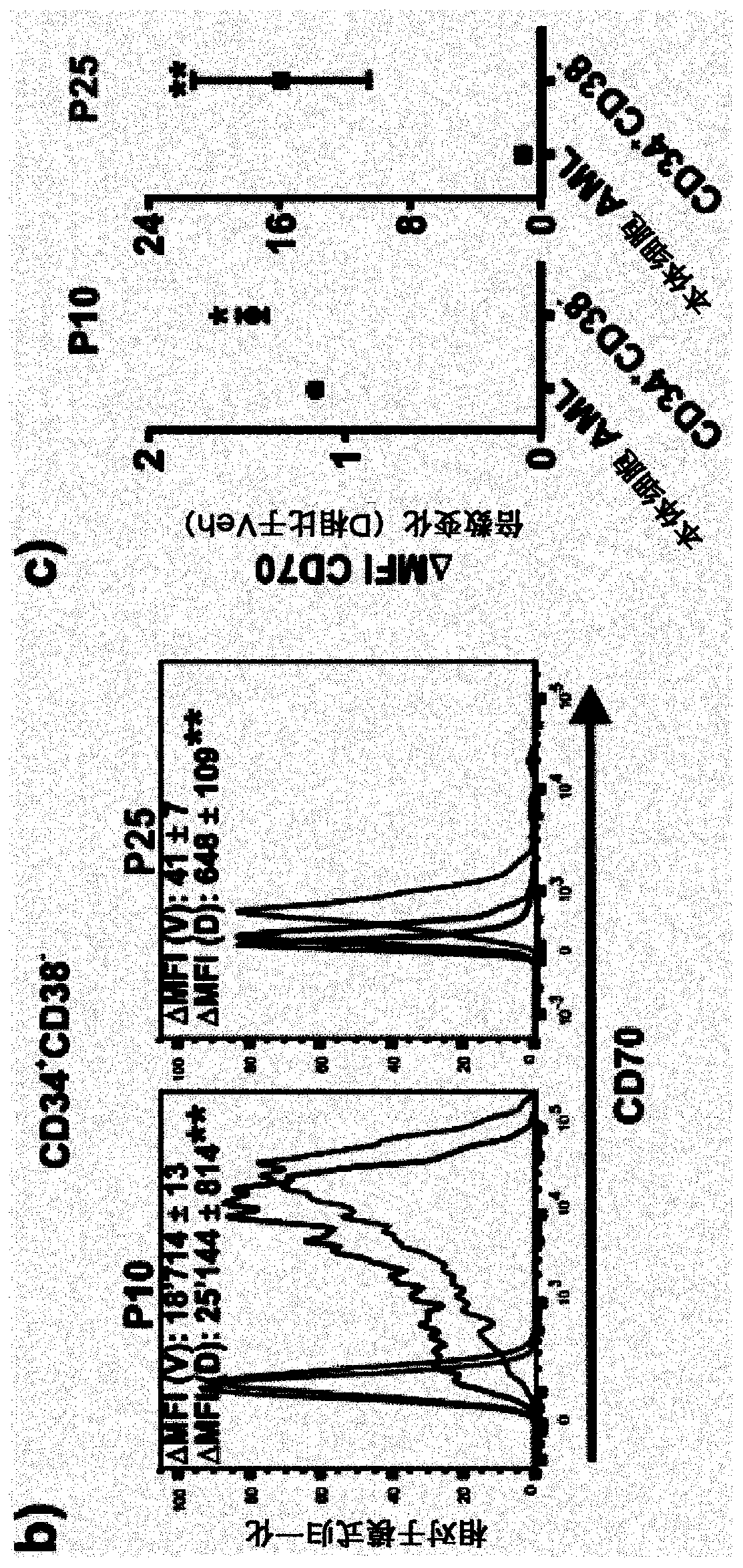
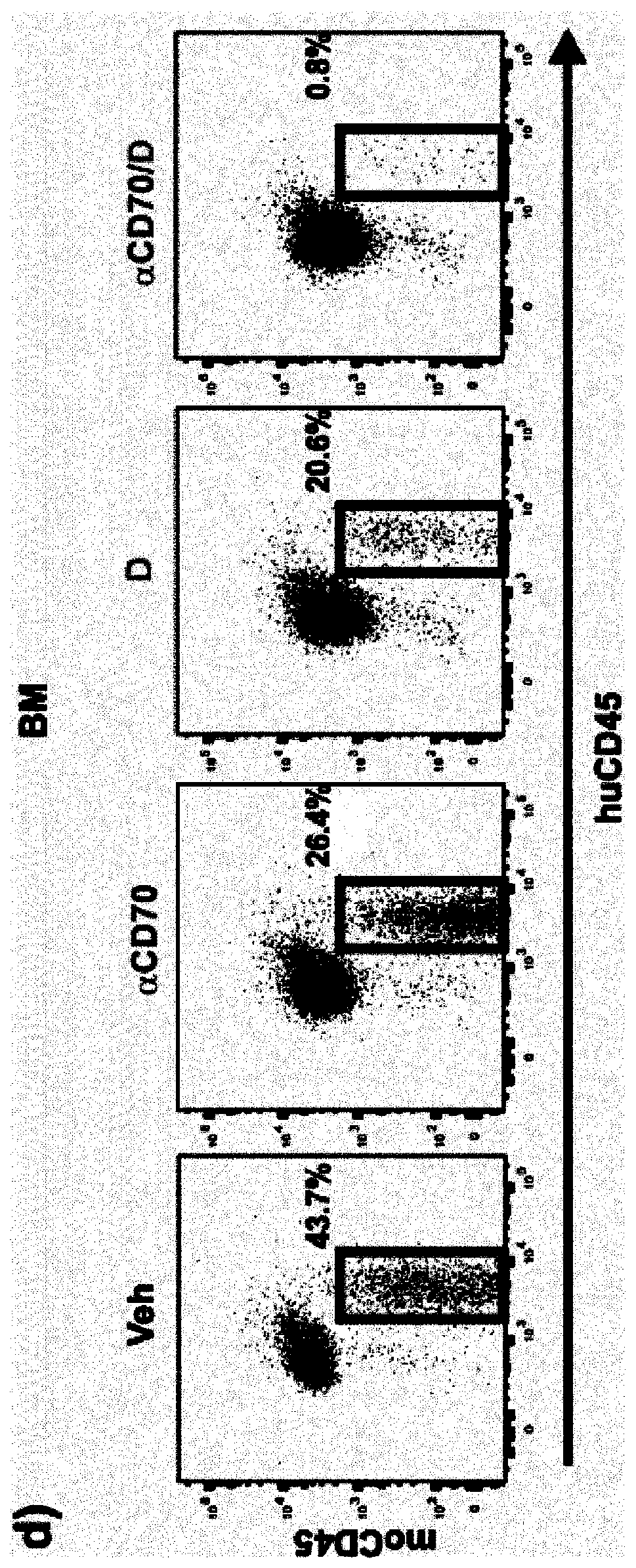
Use of anti cd70 antibody argx-110 to treat acute myeloid leukaemia
PendingCN110730789AReduce the percentage of blastsReduce percentageOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesOncology
Owner:ARGENX BVBA +1
Oligonucleotide conjugates
ActiveUS20160138025A1Reduce Toxicity RiskReduce nephrotoxicityOrganic active ingredientsSpecial deliveryKexinSubtilisin
The present invention relates to oligomeric compounds and conjugates thereof that target Proprotein Convertase Subtilisin / Kexin type 9 (PCSK9) PCSK9 mRNA in a cell, leading to reduced expression of PCSK9. Reduction of PCSK9 expression is beneficial for a range of medical disorders, such as hypercholesterolemia and related disorders.
Owner:ROCHE INNOVATION CENT COPENHAGEN
Power conversion device of gastrointestinal peristalsis pressure sampling capsules
InactiveCN111557693AReduce Toxicity RiskAchieve interactionSurgeryVaccination/ovulation diagnosticsGear drivePeristalsis
The present invention discloses a power conversion device of gastrointestinal peristalsis pressure sampling capsules, which comprises a spring piece, a pressing shaft is fixedly connected to the bottom of the spring piece, a restraining plate is movably connected to the left side of the pressing shaft, and a rotating shaft is movably connected to the bottom of the pressing shaft. Pressure is generated on the spring piece through gastrointestinal peristalsis outside the capsule; the spring piece transmits pressure to the pressing shaft and the pressing shaft transmits the pressure to the rotating shaft; then the rotating shaft transmits pressure to the paddle board; the paddle board transmits pressure to the inclined panel to cause the rotation of the rotor; under natural bounce, by means of movable degree of freedom through rotating shaft, the rotating shaft is free of being stuck by the next slope planel; the rotor rotates to drive the first optical axis to rotate, and the first optical axis drives the first small gear to rotate, then the first small gear drives the first large gear to rotate. The device has the advantages that the toxicity risk is low, pressure generated by gastrointestinal tract peristalsis can be converted into rotation in the capsule, then the capsule is driven to be opened and closed, samples are obtained, and area conversion operation is achieved are achieved.
Owner:WENZHOU FANGZHI BIOTECH
Gleditsia amorphoides seedless pod extract and its use as an agricultural adjuvant
InactiveUS20080113865A1Reduce environmental impactLow costBiocideFungicidesAs elementBean Pod Extract
Extract of the seedless pod of Gleditsia Amorphoides and its use as adjuvant in agriculture, which has a content of soluble solids between 250-270 g / kg; triterpene saponins between 60-75 g / kg; a conductivity (5° Brix) of 6+ / −4 mS / cm; an absorbance of 400 nm (at 1.1% of the product in water)<0.500 UA; a foam (5° Brix) of 160 ml; a (direct) pH equal to 3.9+ / −0.3; while its chemical assessment in g. per 100 g. of the extract expressed as elements amounts to a total Ni=0.51; assimilable P=0.28; K=1.22; S=0.63; Mg=0.08 and Fe=0.0022; while assimilable P (expressed as 05P)=0.64 and K (expressed as KO)=1.47; likewise, the total amount of phenols is approximately 8.5 and 10%, and that of tannins is between 0.9 and 1.5%.
Owner:IDEASUPPLY COM ARGENTINA
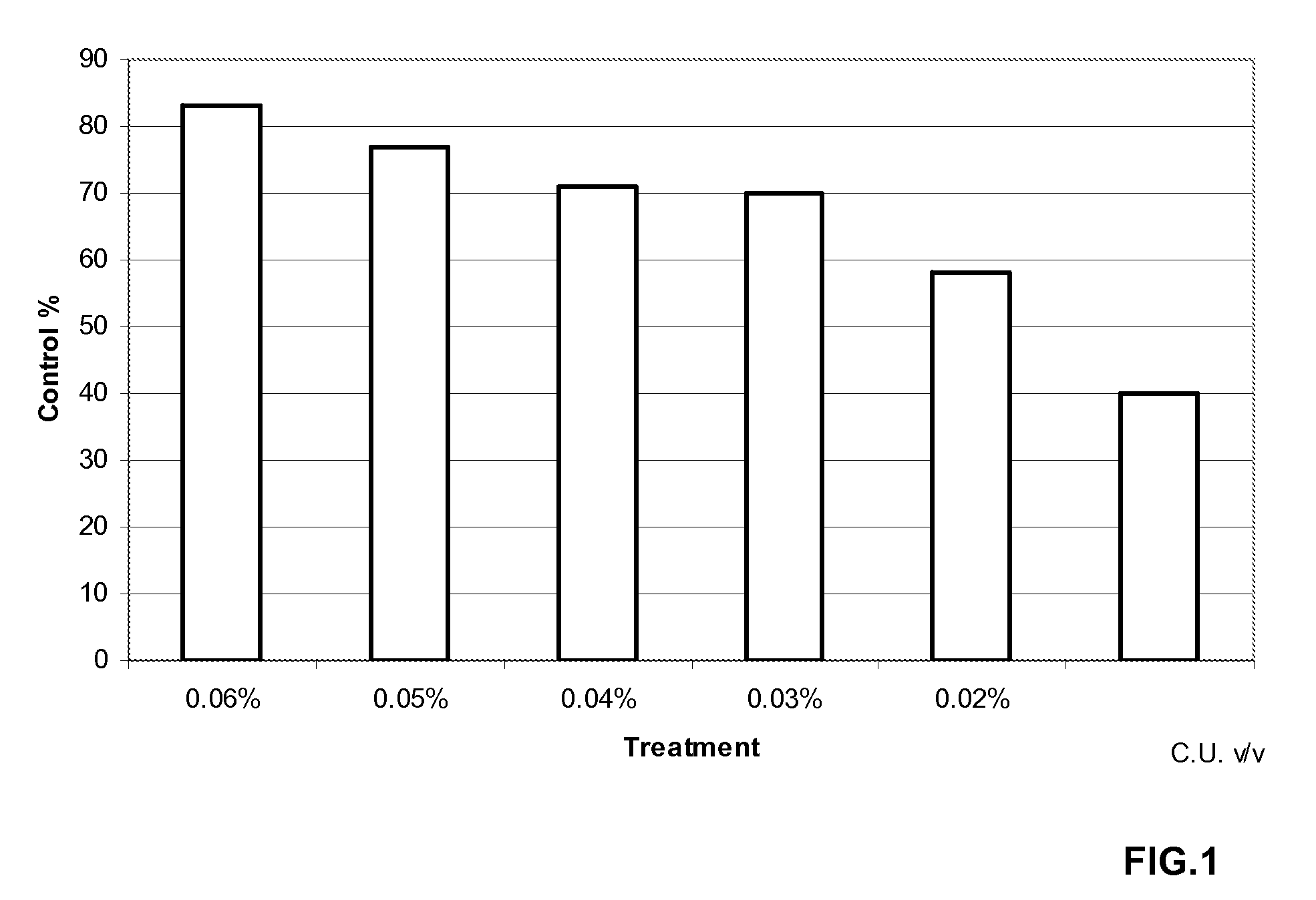
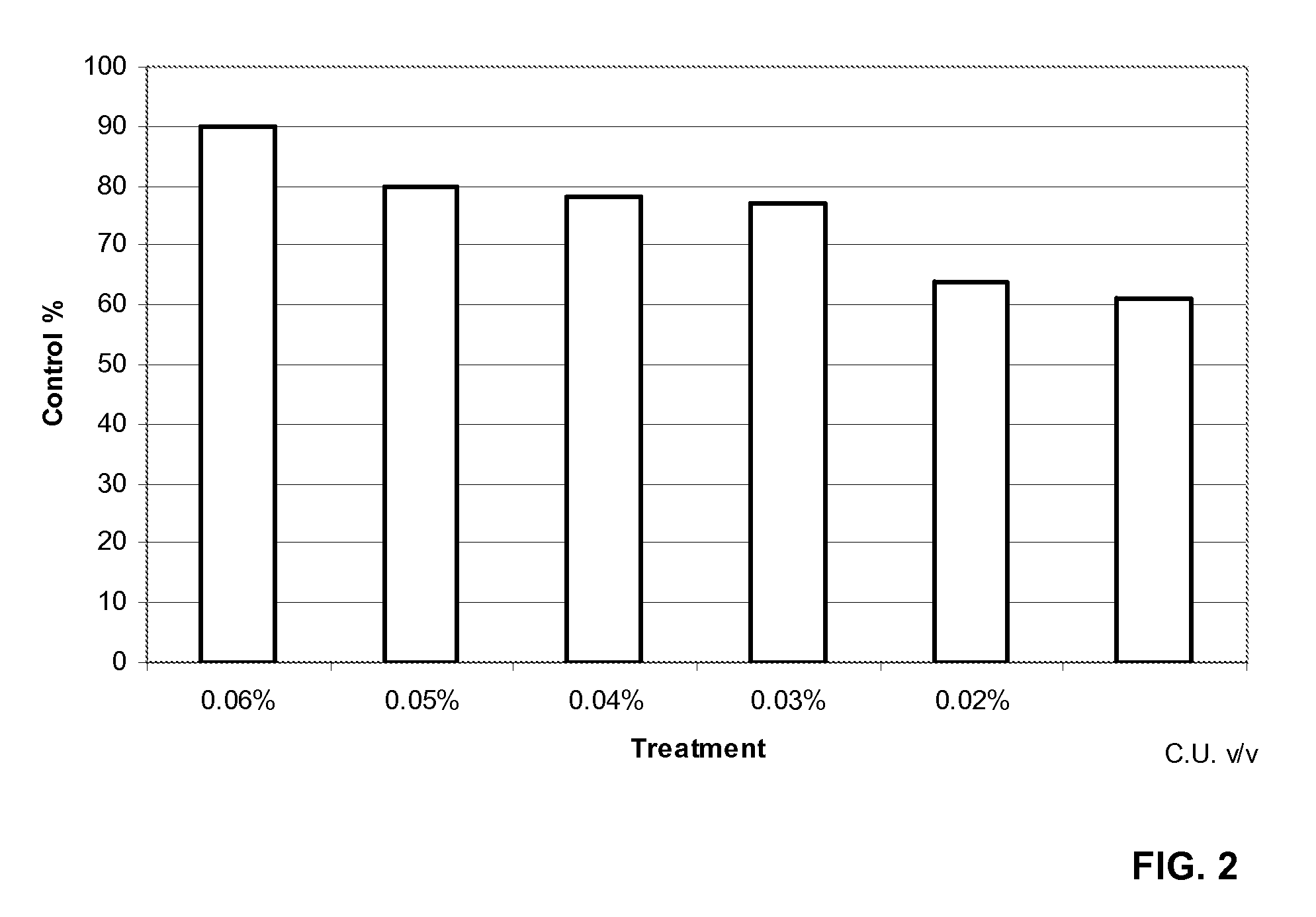
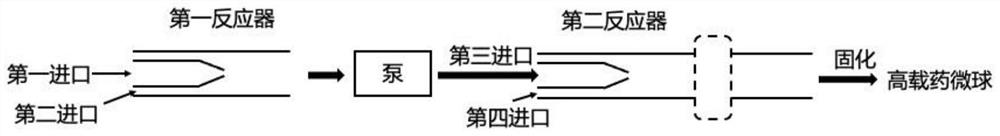
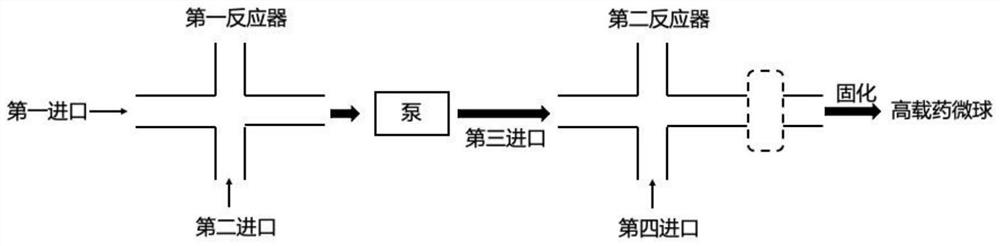
Continuous flow preparation method of high drug-loading microspheres
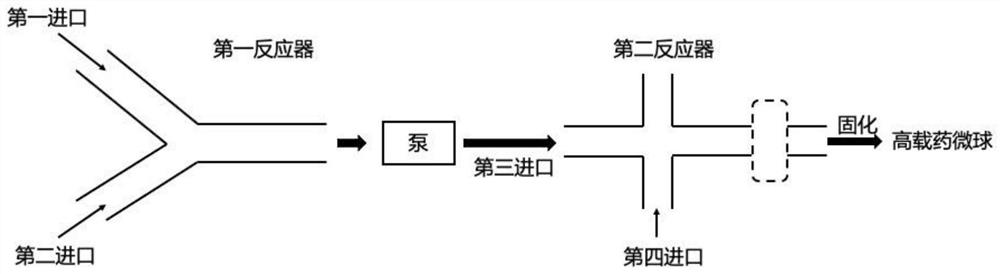
PendingCN114146647APrevent leakageAvoid qualitative differencesPharmaceutical non-active ingredientsMicroballoon preparationDrugs solutionMicrosphere
The invention belongs to the technical field of pharmaceutical preparations, and discloses a continuous flow preparation method of high-drug-loading microspheres. The method comprises the following steps: precipitating an active drug solution by using a poor solvent in which a microsphere matrix material is dissolved to form nanoparticles, directly taking the mixture as an oil phase, mixing the oil phase with a water phase to prepare oil-in-water emulsion droplets, and curing to obtain the high-drug-loading microspheres. The nanoparticles are directly wrapped in one step in continuous flow preparation, the preparation process of the microspheres is simplified, and the application value is high. The prepared high-drug-loading microsphere comprises nanoparticles composed of active drug components and a microsphere matrix material used for controlling drug release, and the particle size is 1-2000 [mu] m; the mass of the active drug component accounts for 5-80% of the mass of the whole microsphere, and the encapsulation efficiency of the active drug is 5-100%. The microspheres prepared through a continuous flow method are high in encapsulation efficiency and drug loading capacity and free of burst release, the treatment efficiency can be effectively improved, and toxic and side effects and adverse reactions can be reduced.
Owner:CHINA PHARM UNIV
Gleditsia amorphoides seedless pod extract and its use as an agricultural adjuvant
InactiveUS20110281726A1Reduce environmental impactLow costBiocideFungicidesBean Pod ExtractAs element
Extract of the seedless pod of Gleditsia Amorphoides and its use as adjuvant in agriculture, which has a content of soluble solids between 250-270 g / kg; triterpene saponins between 60-75 g / kg; a conductivity (5° Brix) of 6+ / −4 mS / cm; an absorbance of 400 nm (at 1.1% of the product in water)<0.500 UA; a foam (5° Brix) of 160 ml; a (direct) pH equal to 3.9+ / −0.3; while its chemical assessment in g. per 100 g. of the extract expressed as elements amounts to a total Ni=0.51; assimilable P=0.28; K=1.22; S=0.63; Mg=0.08 and Fe=0.0022; while assimilable P (expressed as 05P)=0.64 and K (expressed as KO)=1.47; likewise, the total amount of phenols is approximately 8.5 and 10%, and that of tannins is between 0.9 and 1.5%.
Owner:IDEASUPPLY COM ARGENTINA
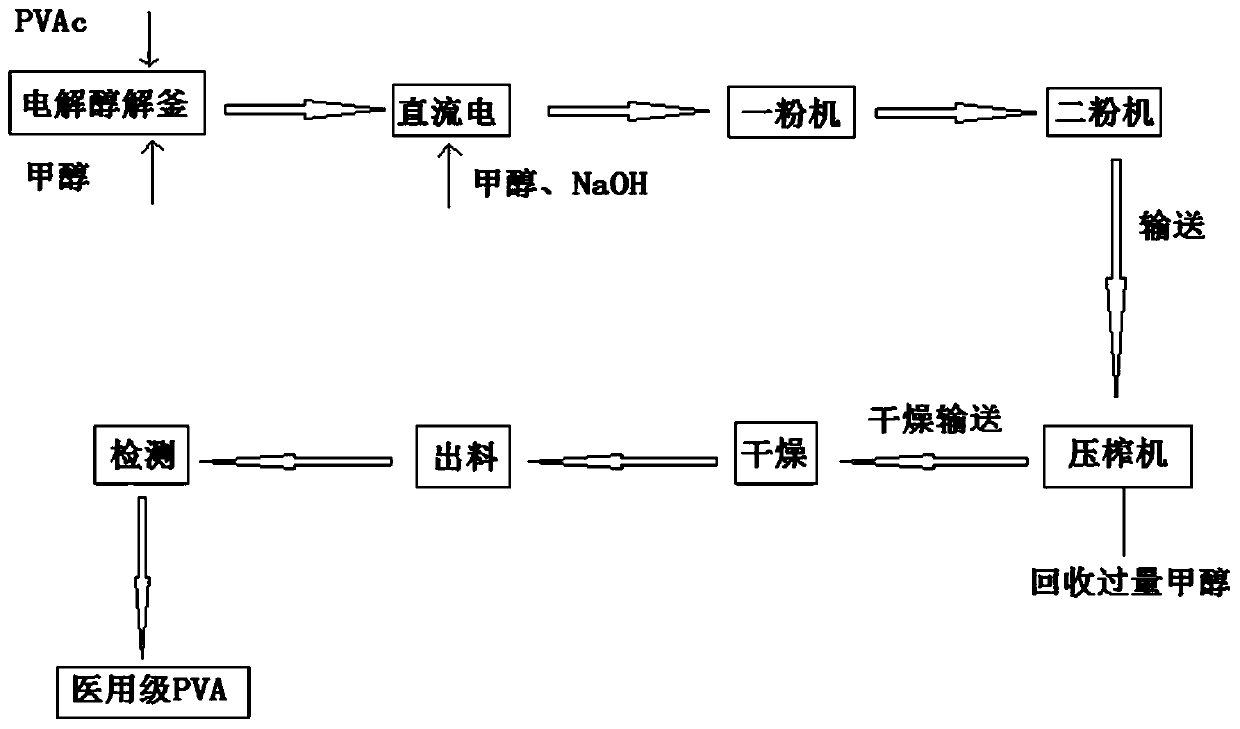
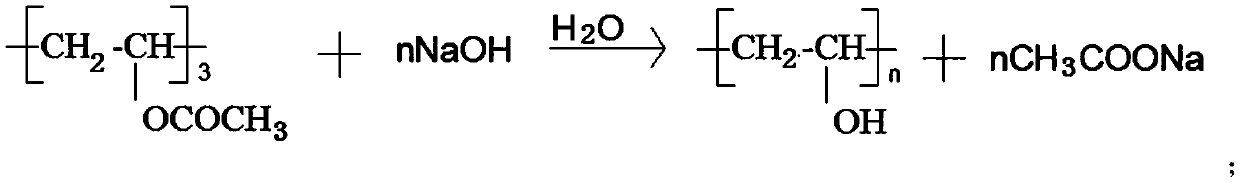
Preparation method of polyvinyl alcohol resin high in alcoholysis degree
The invention discloses a preparation method of high-alcoholysis-degree polyvinyl alcohol resin. The preparation method comprises the following steps: adding methanol into an electrolytic alcoholysiskettle, slowly adding PVAc while stirring, and heating to dissolve to obtain a PVAc / methanol solution; dissolving NaOH in methanol to obtain a NaOH-methanol solution; adding polymethylsiloxane into the electrolytic alcoholysis kettle containing the PVAc / methanol solution, and then applying direct current at a temperature kept to be 45-48 DEG C; reducing the temperature of the PVAc / methanol solution to 28-32 DEG C, and adding the NaOH-methanol solution into the electrolytic alcoholysis kettle for reaction; passing an obtained product through a pulverizer and a squeezer, and drying and discharging to obtain medical-grade polyvinyl alcohol resin. According to the preparation method, monomer residue is reduced, so that energy saving and emission reduction are realized, and the influence of monomers on the chemical and mechanical properties of polyvinyl acetate and polyvinyl alcohol is reduced, so that the product can meet the medical-grade requirements.
Owner:ZHENDE MEDICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com