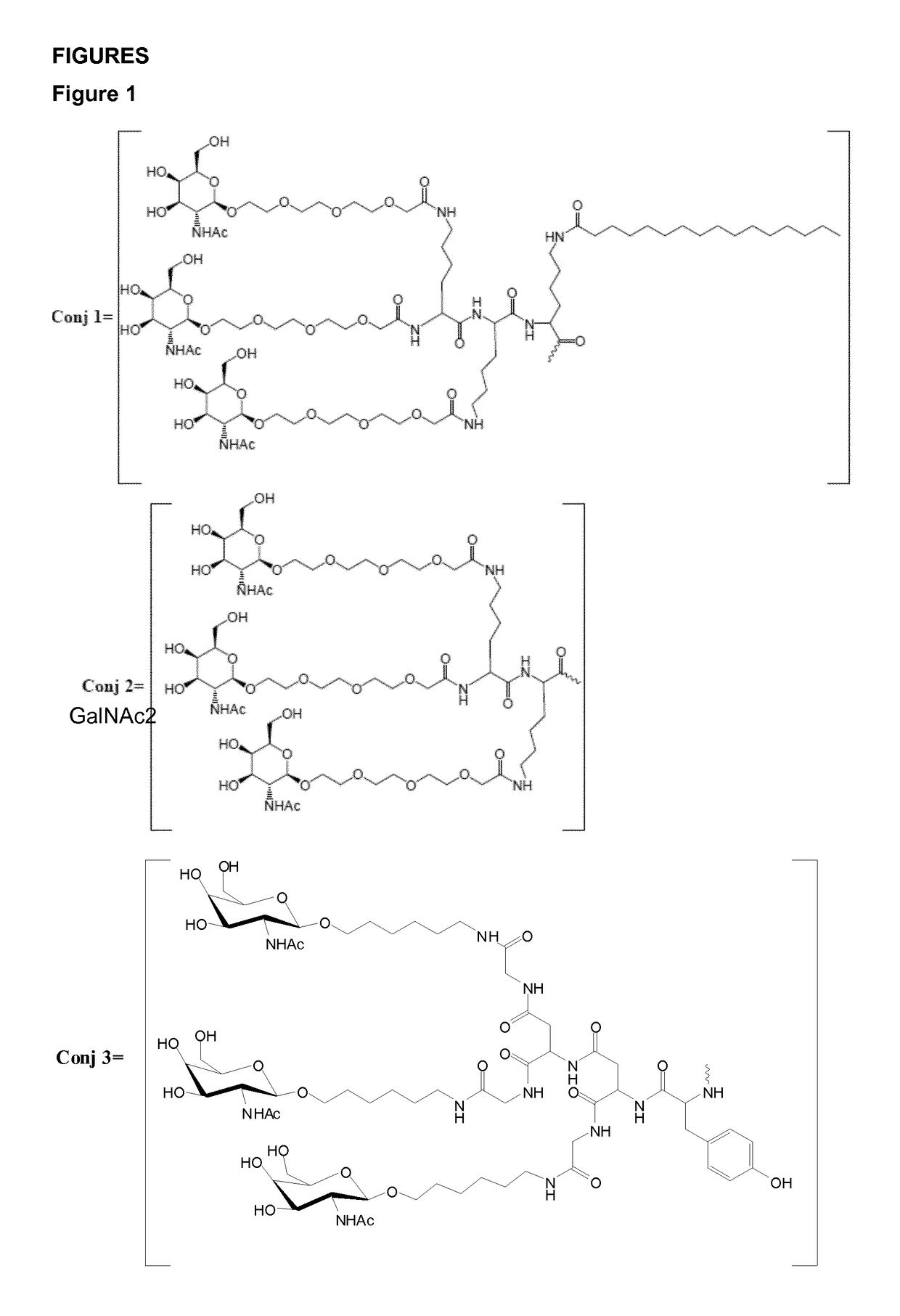
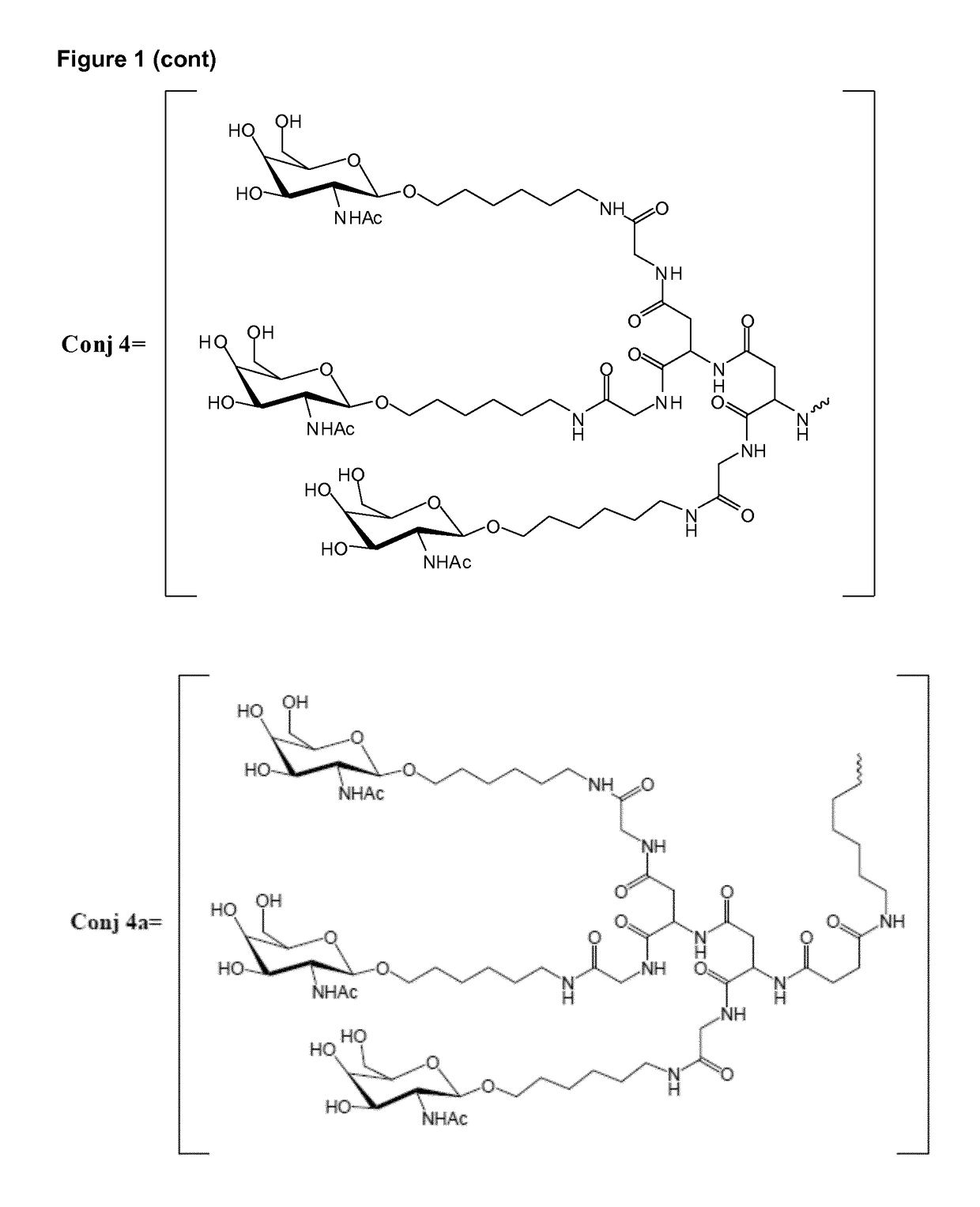
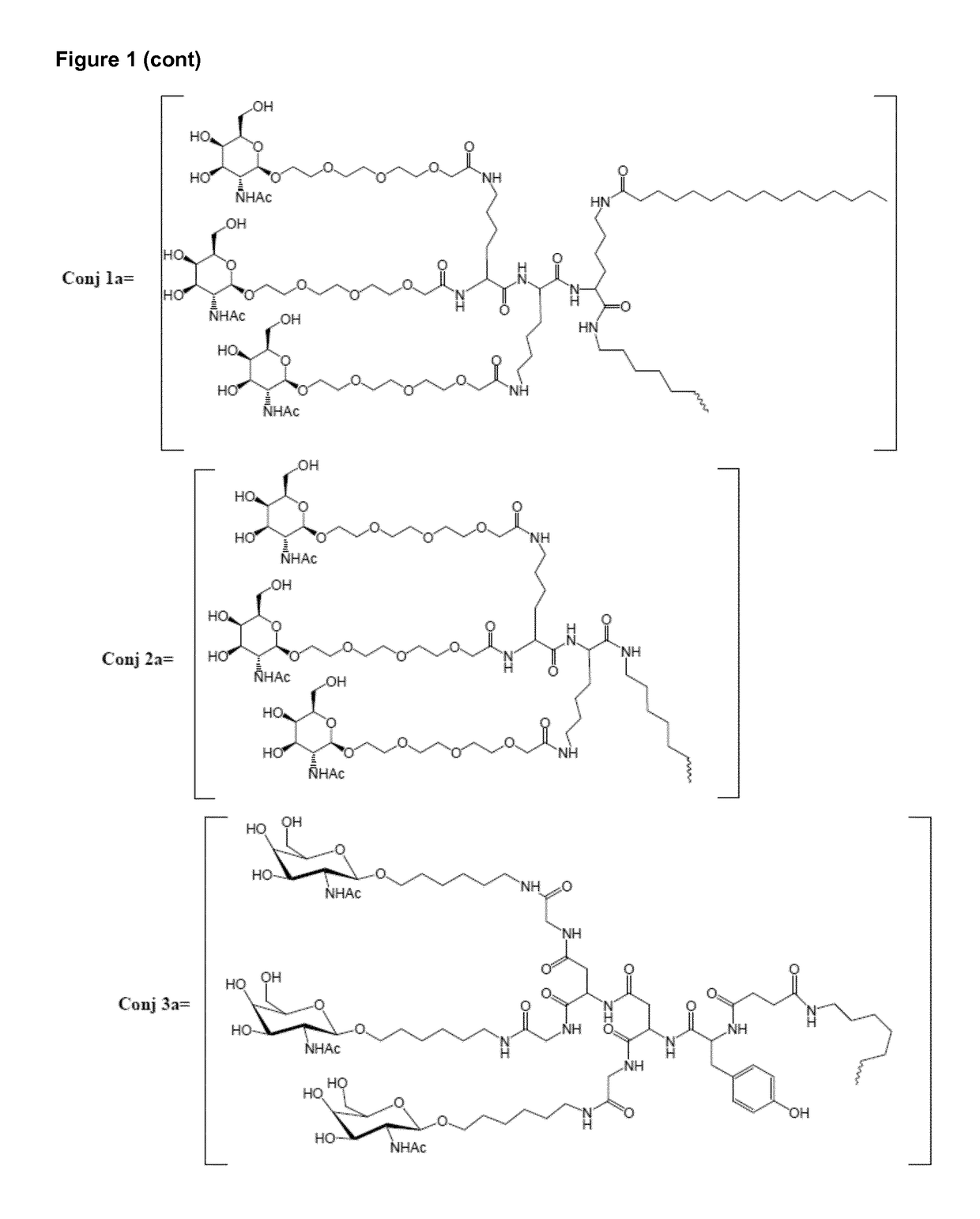
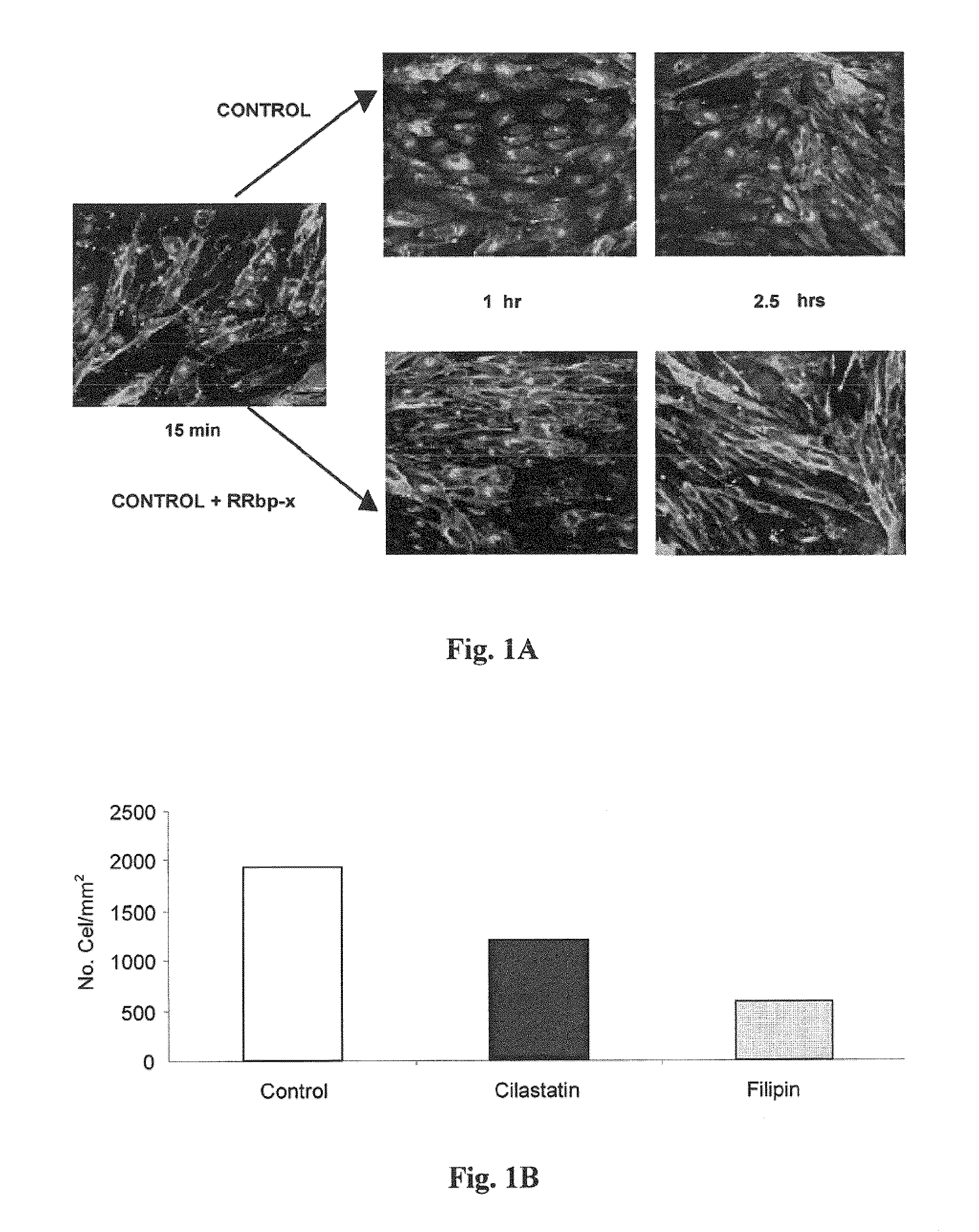
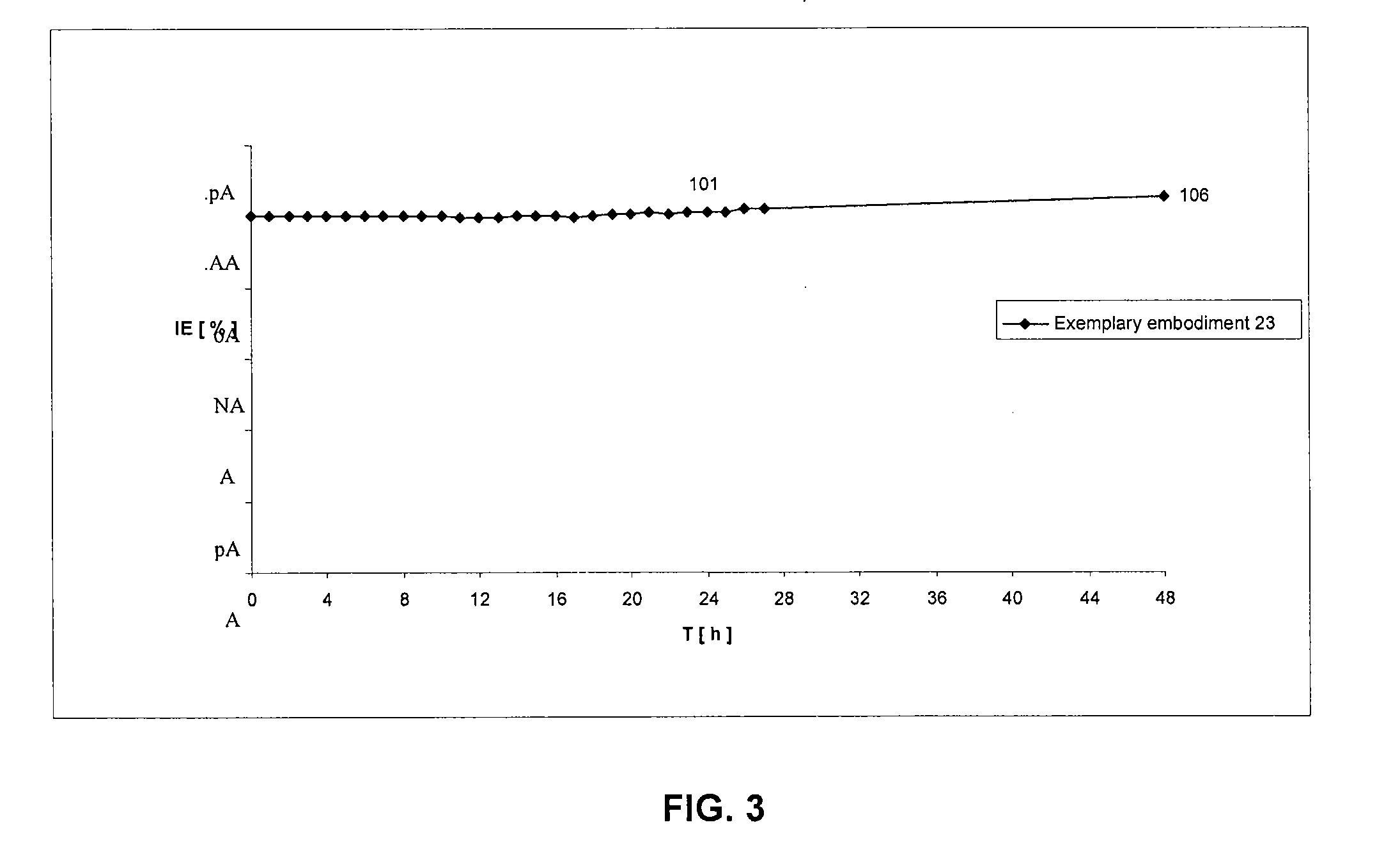
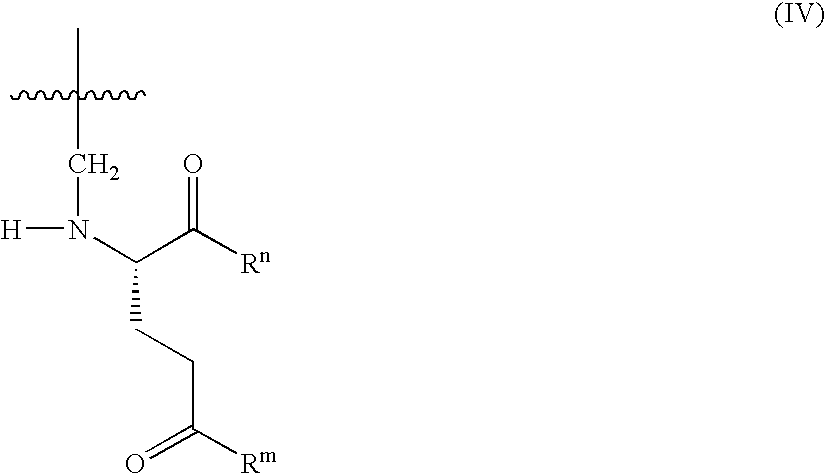
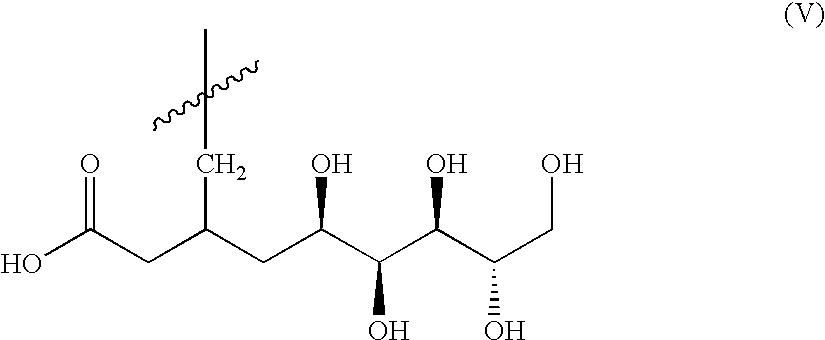
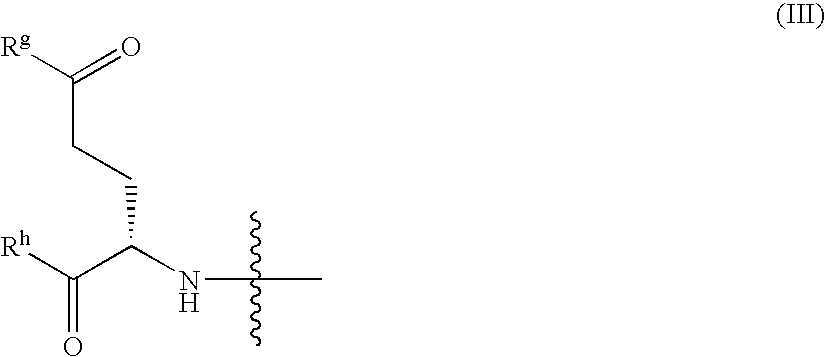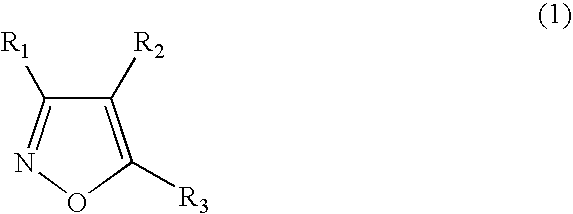Patents
Literature
120results about How to "Reduce nephrotoxicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
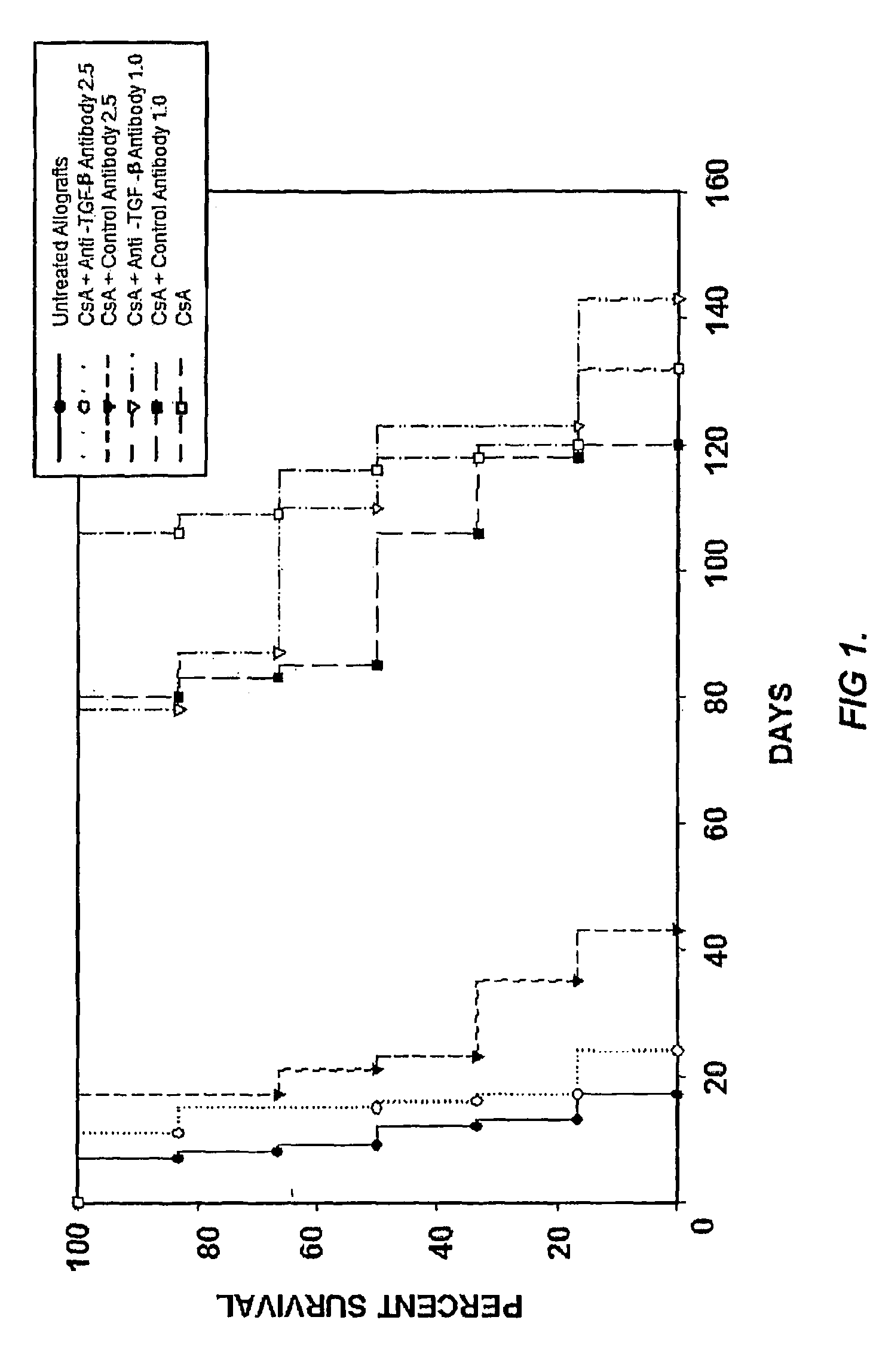
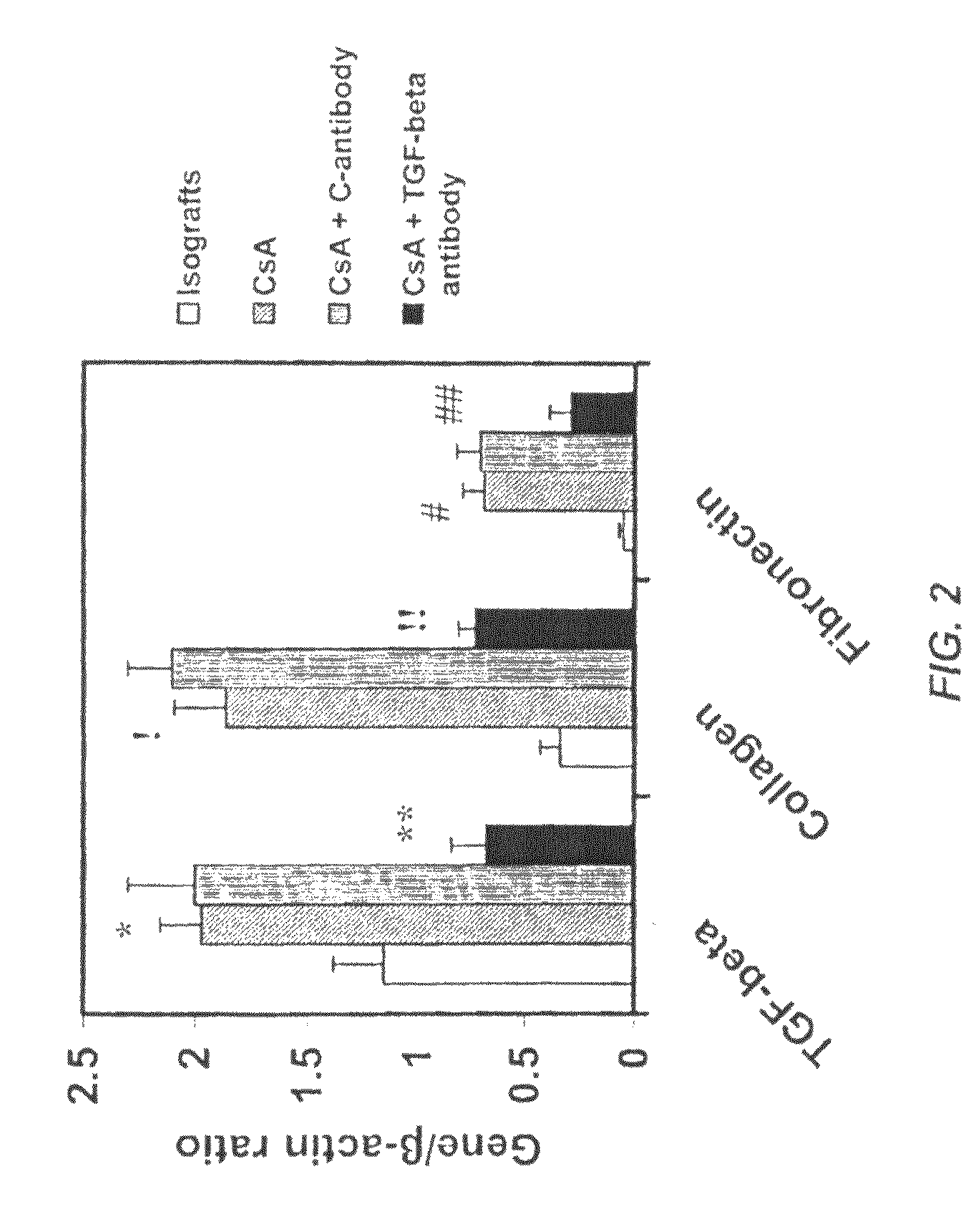
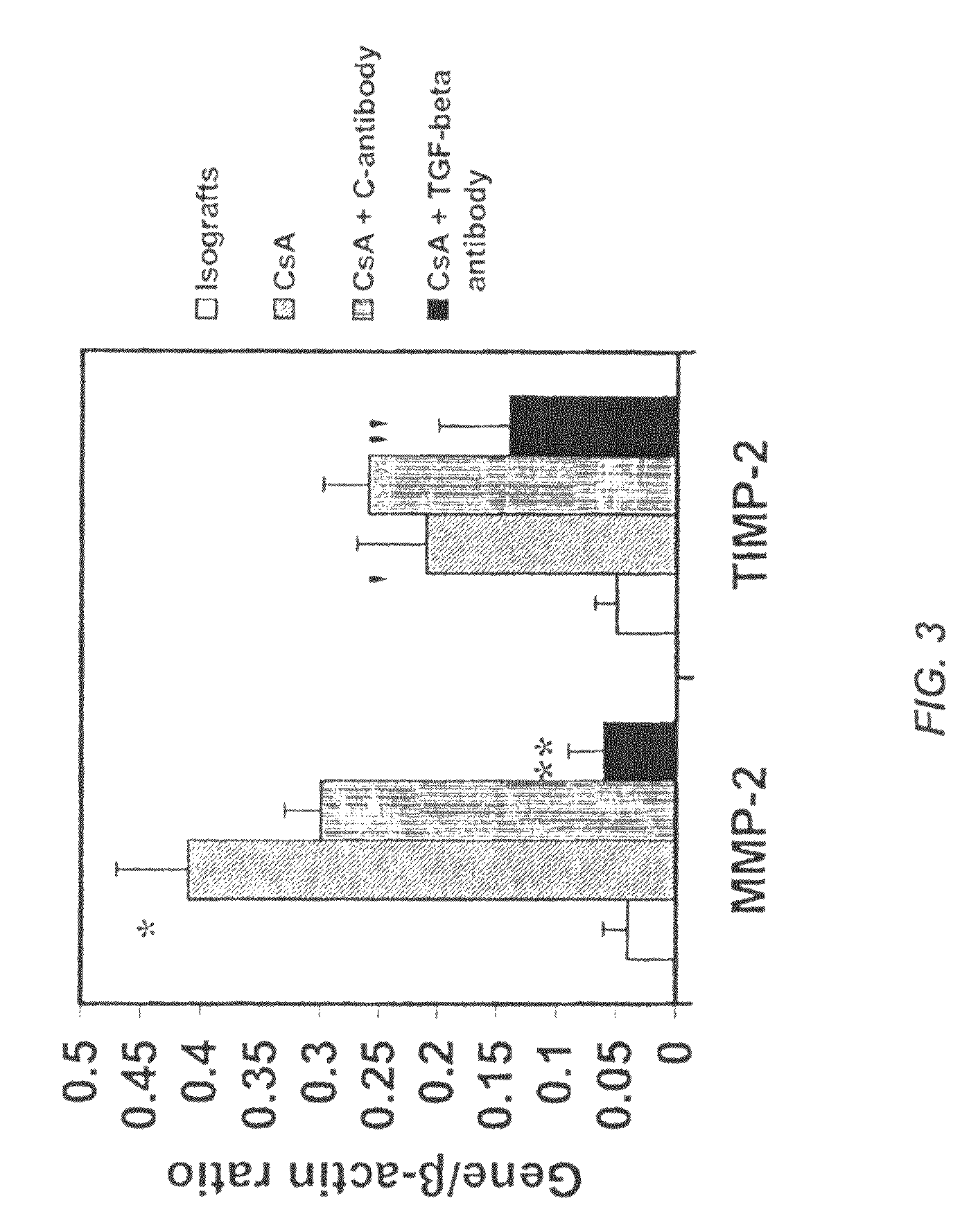
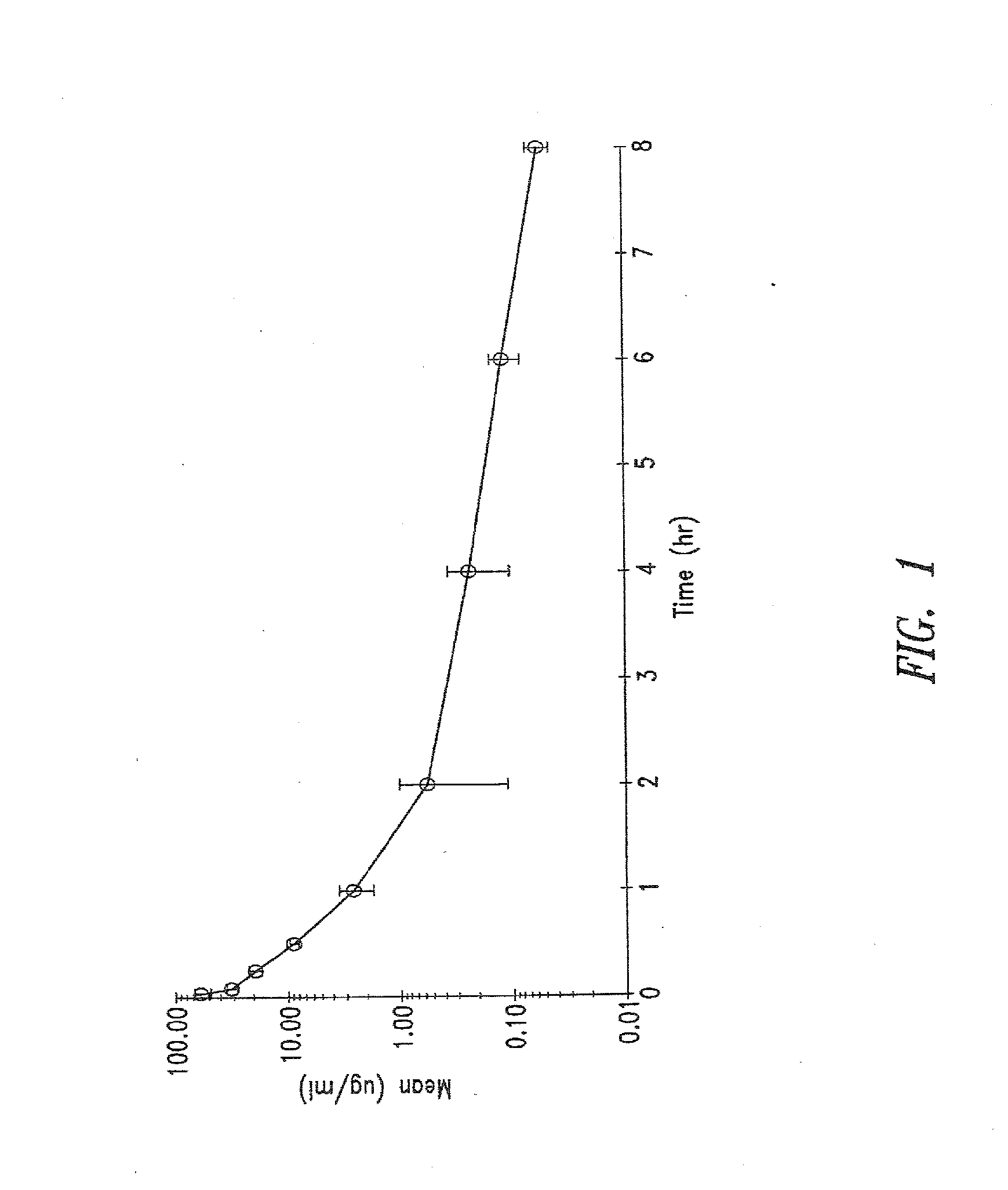
Use of TGF-β antagonists to limit nephrotoxicity of immunosuppressive agents
ActiveUS7867496B2Amelioration of nephrotoxic side effectReduce nephrotoxicityImmunoglobulins against growth factorsUnknown materialsDiseaseSide effect
The disclosure relates to methods of ameliorating nephrotoxic side effects of immunosuppressive agents whose immunosuppressive activity is mediated via upregulation of TGF-β such as, for example, cyclosporine (CsA). The disclosure provides treatment modalities for use in patients that require immunosuppression, e.g., patients at risk of transplant rejection or having an autoimmune disease. In the methods of the invention, a TGF-β antagonist, e.g., an anti-TGF-β antibody, is administered to a patient treated with an immunosuppressive agent. Such a TGF-β antagonist is administered in a therapeutically effective amount sufficient to alleviate the nephrotoxic effects of the immunosuppressive agent without substantially interfering with immunosuppressive activity of the agent.
Owner:GENZYME CORP +1
Aminoglycoside dosing regimens
InactiveUS20120208781A1Reduce nephrotoxicityReduce ototoxicityAntibacterial agentsBiocideAminoglycosideDosing regimen
The present invention provides new aminoglycoside dosing regimens associated with enhanced microbicidal activity and reduced nephrotoxicity, as well as methods of using these dosing regimens to treat various bacterial infections.
Owner:ACHAOGEN
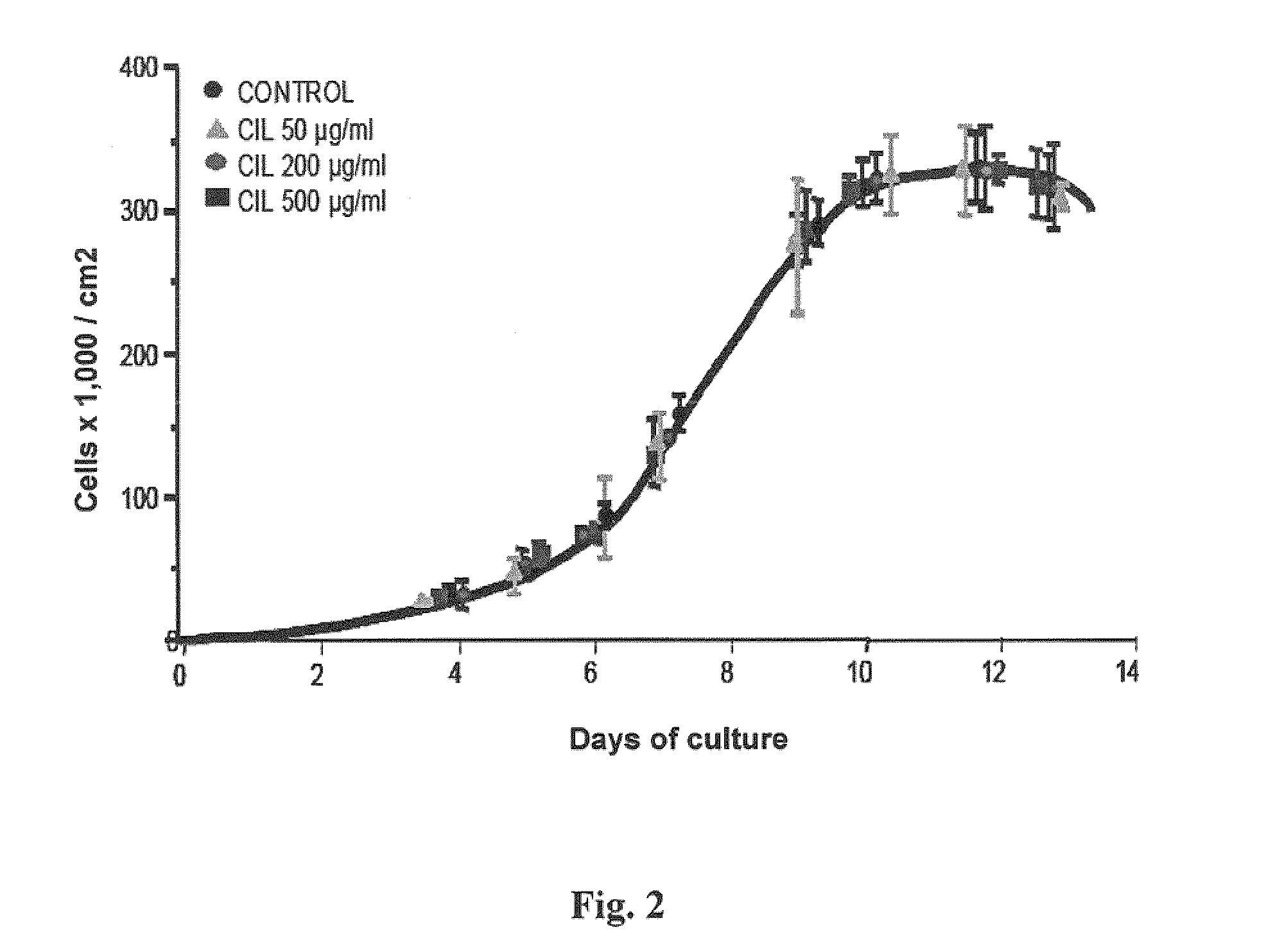
Use of cilastatin to reduce nephrotatoxicity of various compounds
ActiveUS20110165264A1Reduce accumulationReduce harmHalogenated hydrocarbon active ingredientsBiocideSolubilityPharmacy medicine
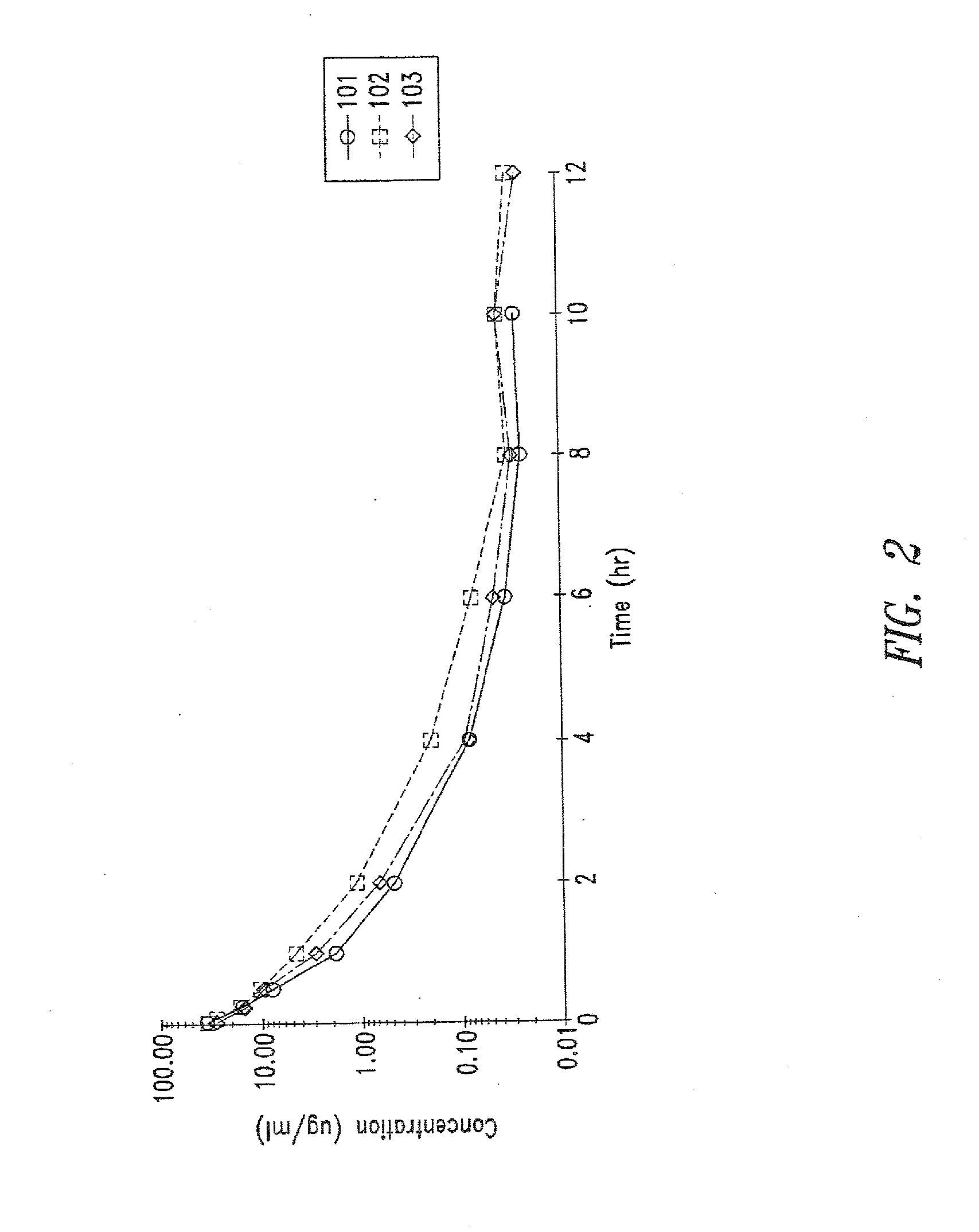
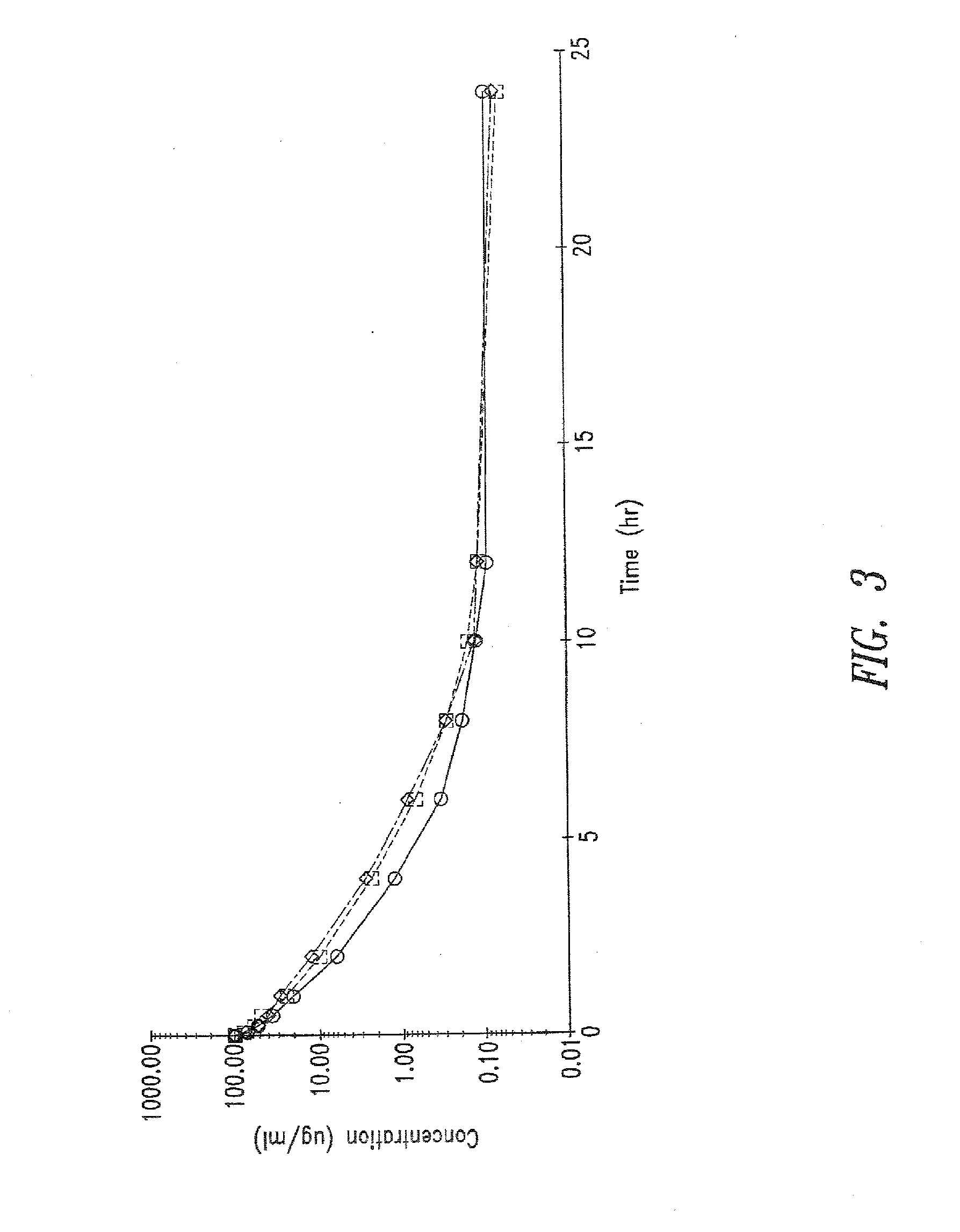
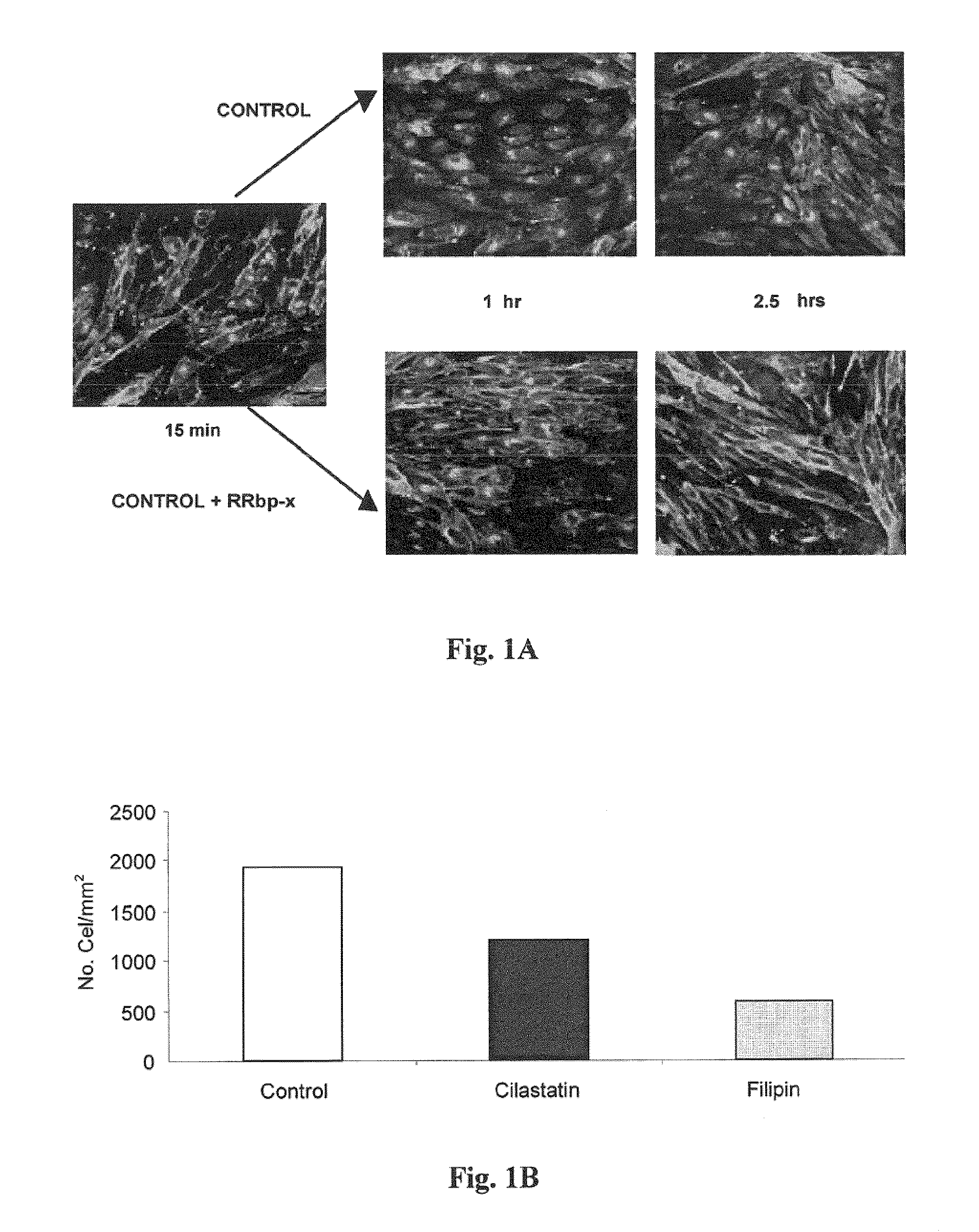
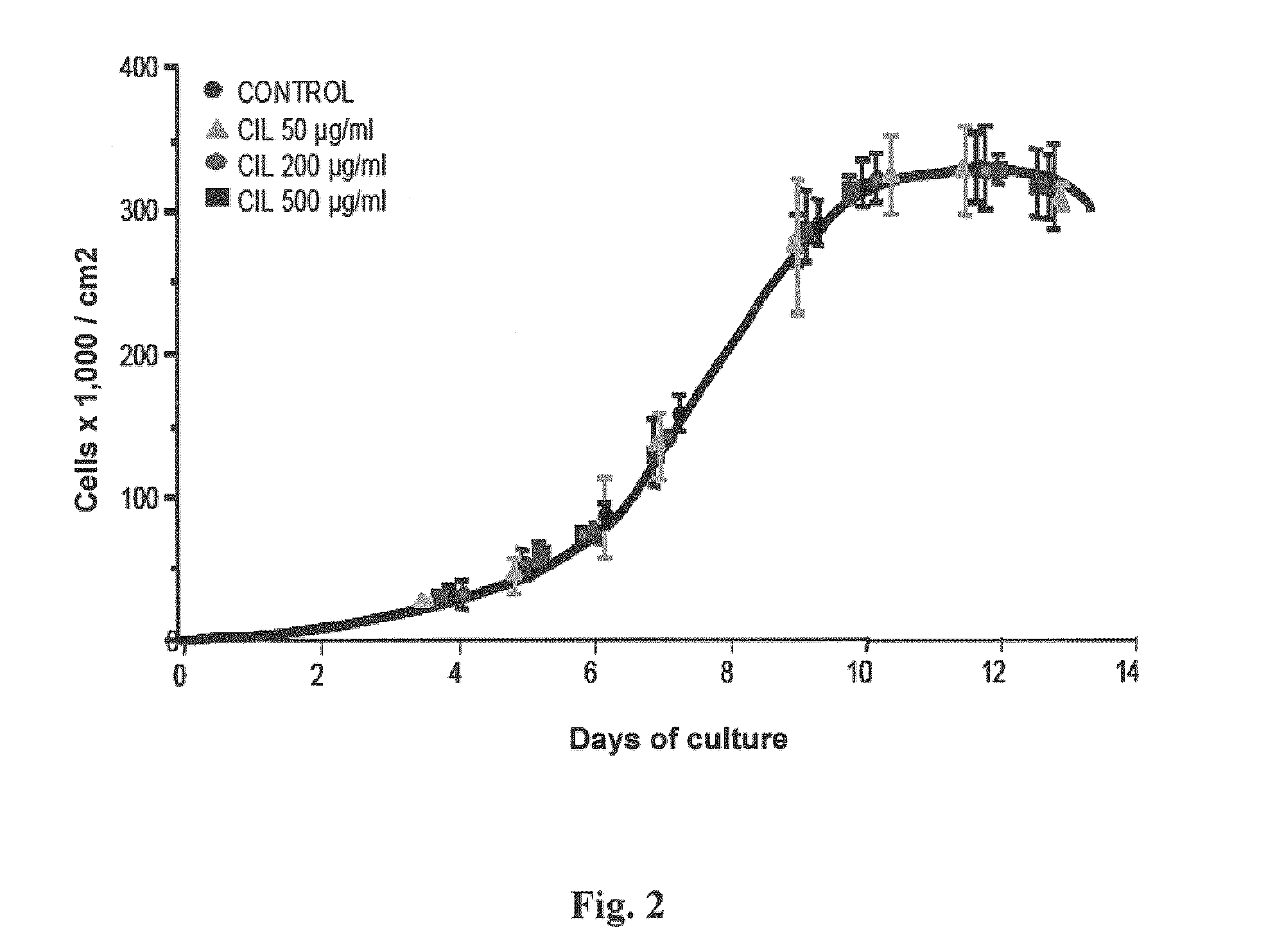
Use of cilastatin to reduce the nephrotoxicity of different compounds. The invention refers to use of cilastatin to prepare a medicinal product to reduce the nephrotoxicity of a nephrotoxic compound that enters the cells of the proximal tubule through cholesterol rafts. The invention is based on the discovery that a great number of nephrotoxic compounds, including drugs, enter the cells of the proximal tubule through the cholesterol rafts, and that cilastatin is able to interfere with this transport mechanism, decreasing the nephrotoxicity of such compounds to a variable extent. The nephroprotective effect is common to compounds of different chemical nature and solubility and is specific for the kidney, causing no interference with the effects of nephrotoxic drugs having their targets in other organs. Therefore, administration of cilastatin allows for decreasing the nephrotoxic effects of different drugs without reducing their therapeutic effects.
Owner:FUNDACION PARA LA INVESTIGACION BIOMEDICA DEL HOSPITAL GREGORIO MARANON
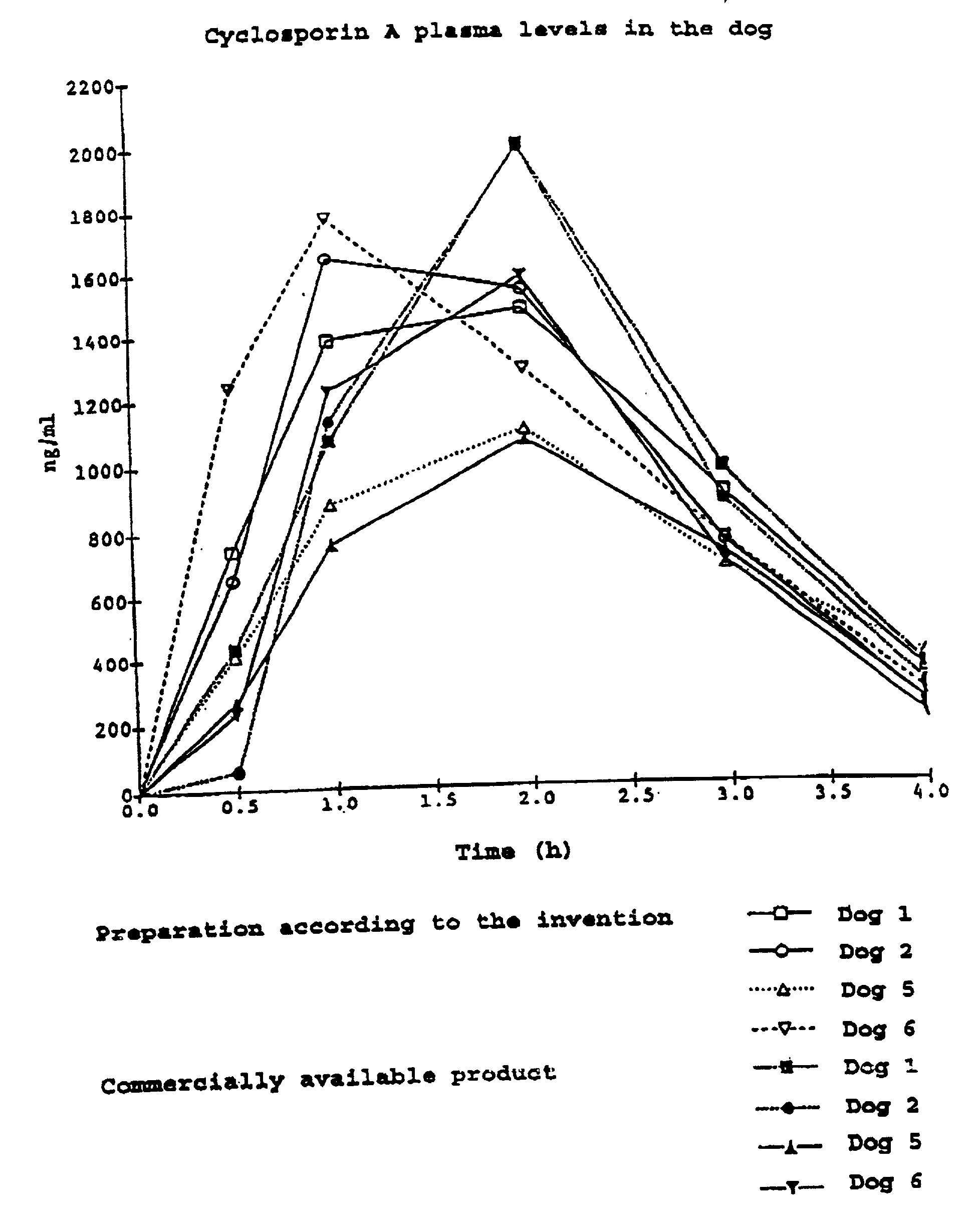
Pharmaceutical preparation with cyclosporin a
InactiveUS20010014665A1Reduce nephrotoxicityReduce impactOrganic active ingredientsBiocideAlcoholVegetable oil
The invention relates to a pharmaceutical preparation which consists of or contains cyclosporin A, an emulsifying .alpha.-tocopherol derivative, an ethoxylation product of vegetable oils, fatty acids or fats as a further emulsifier and a pharmaceutically customary alcohol.
Owner:HEXAL AG
Reconstituted HDL formulation
ActiveUS9125943B2Reduce riskImprove stabilityApolipeptidesPeptide/protein ingredientsApolipoproteins EHigh-density lipoprotein
The present invention relates to reconstituted high density lipoprotein (rHDL) formulations comprising an apolipoprotein, a lipid and a lyophilization stabilizer. Said formulations have reduced renal toxicity and good long-term stability, especially in lyophilized form.
Owner:CSL LTD
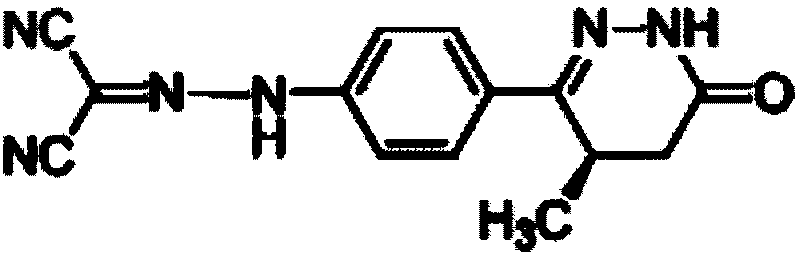
Fluorine-containing optically active composition for anti-infection
ActiveCN101550153AStable in natureLower pHOrganic active ingredientsOrganic chemistrySolubilitySide effect
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE +1
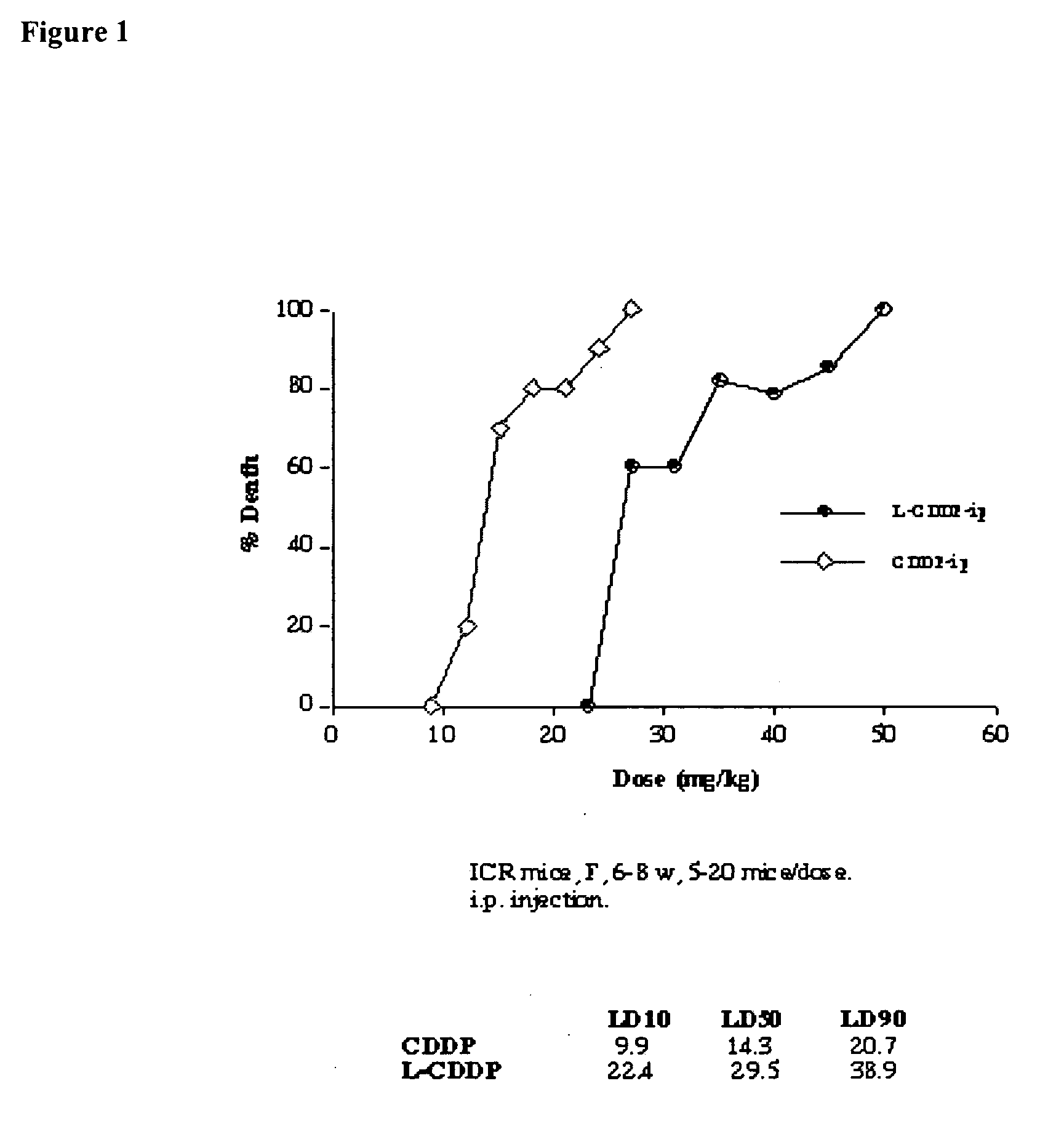
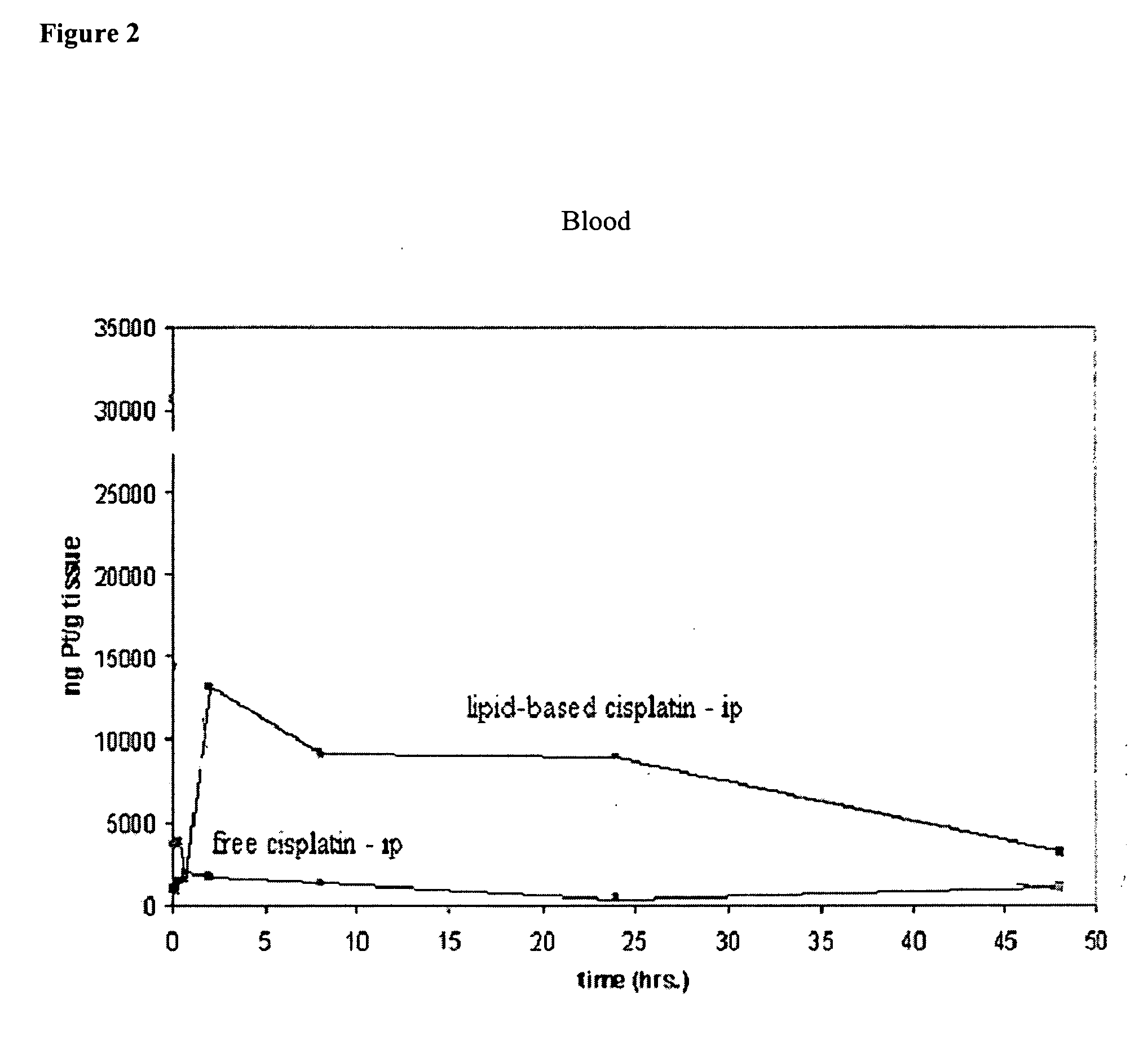
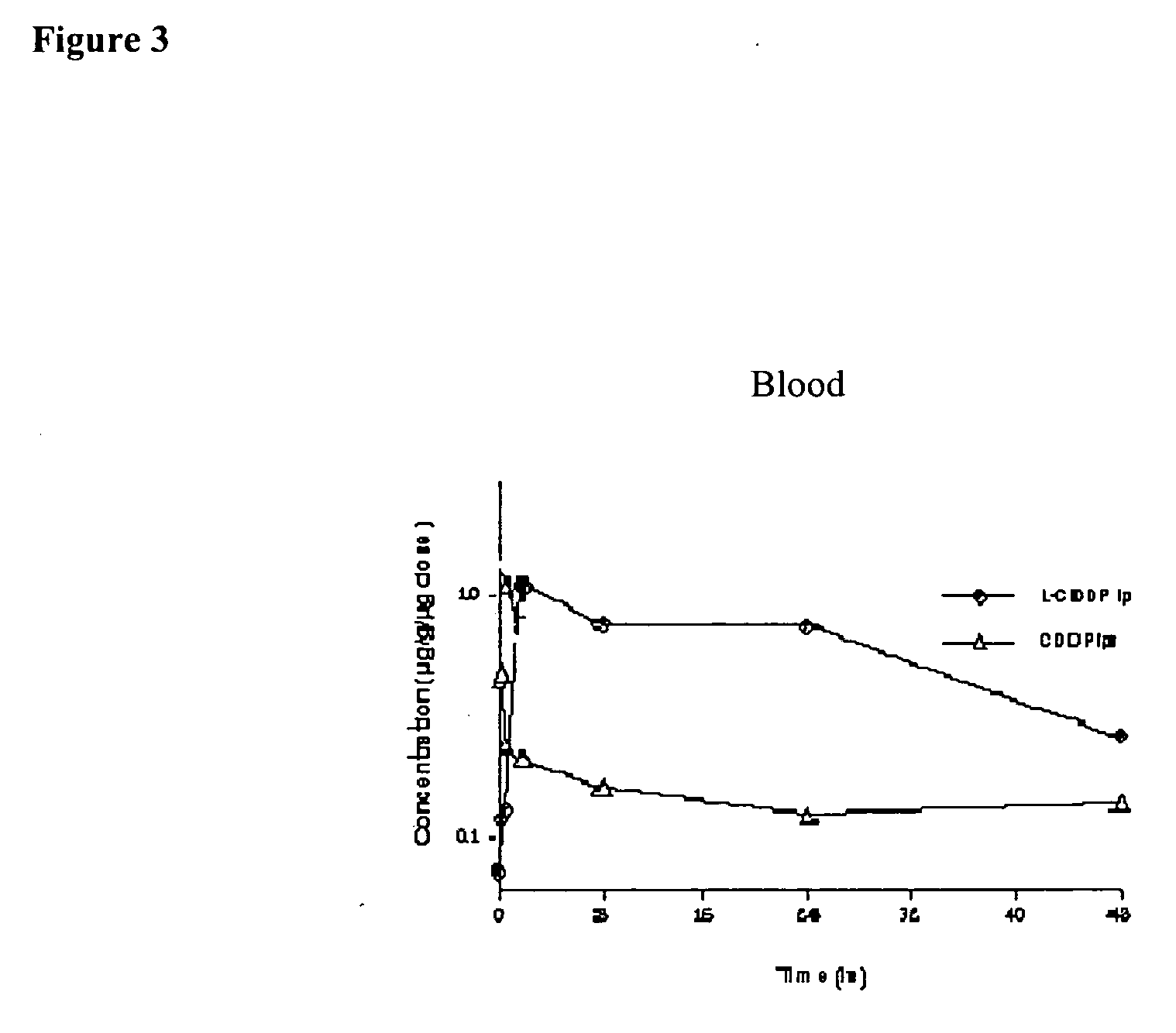
Methods of treating cancer with lipid-based platinum compound formulations administered intraperitoneally
InactiveUS20060246124A1Reduce nephrotoxicityPotent and efficient cancer treatmentBiocideHeavy metal active ingredientsLipid formationAbdominal cavity
Owner:PILKIEWICZ FR G +3
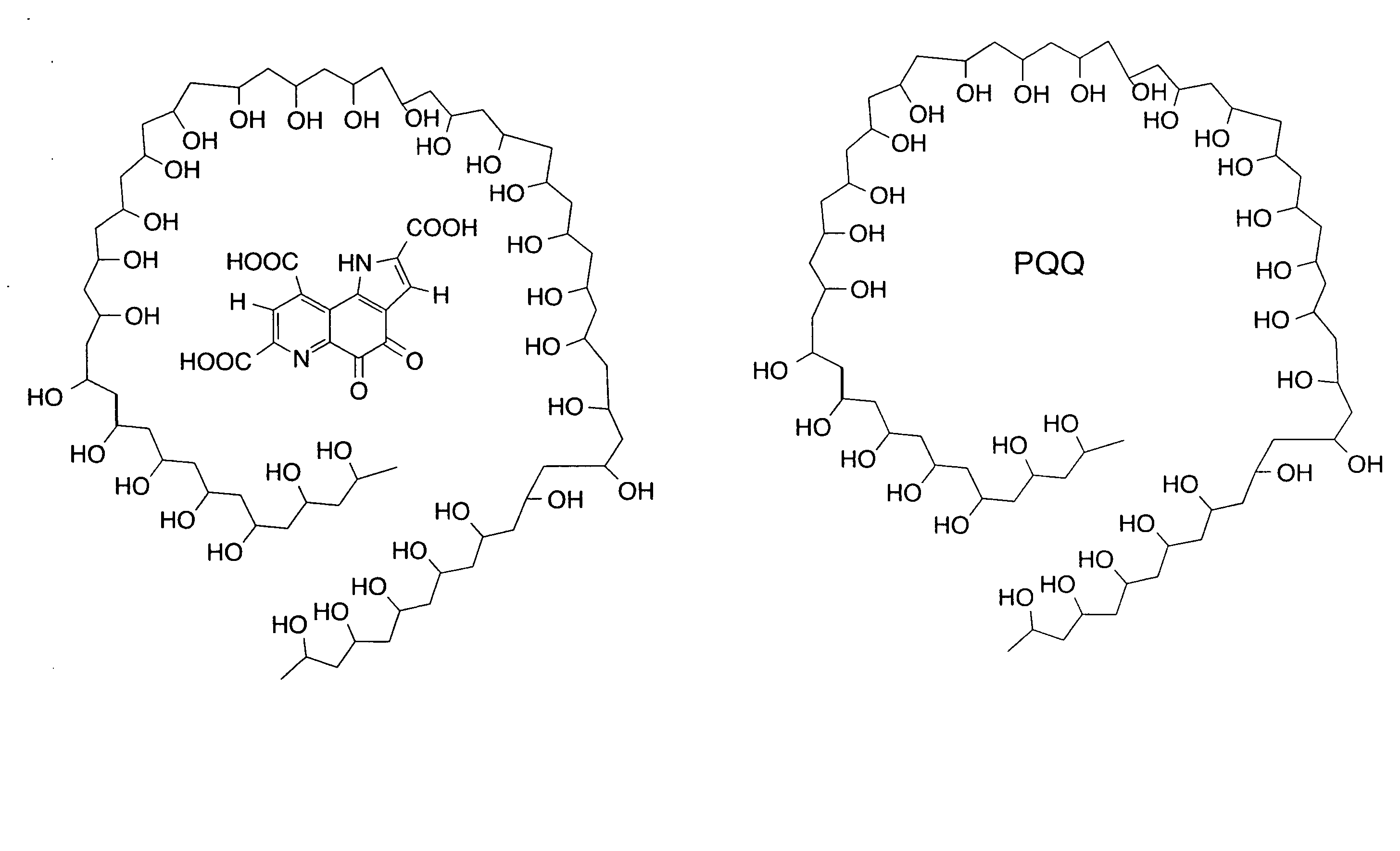
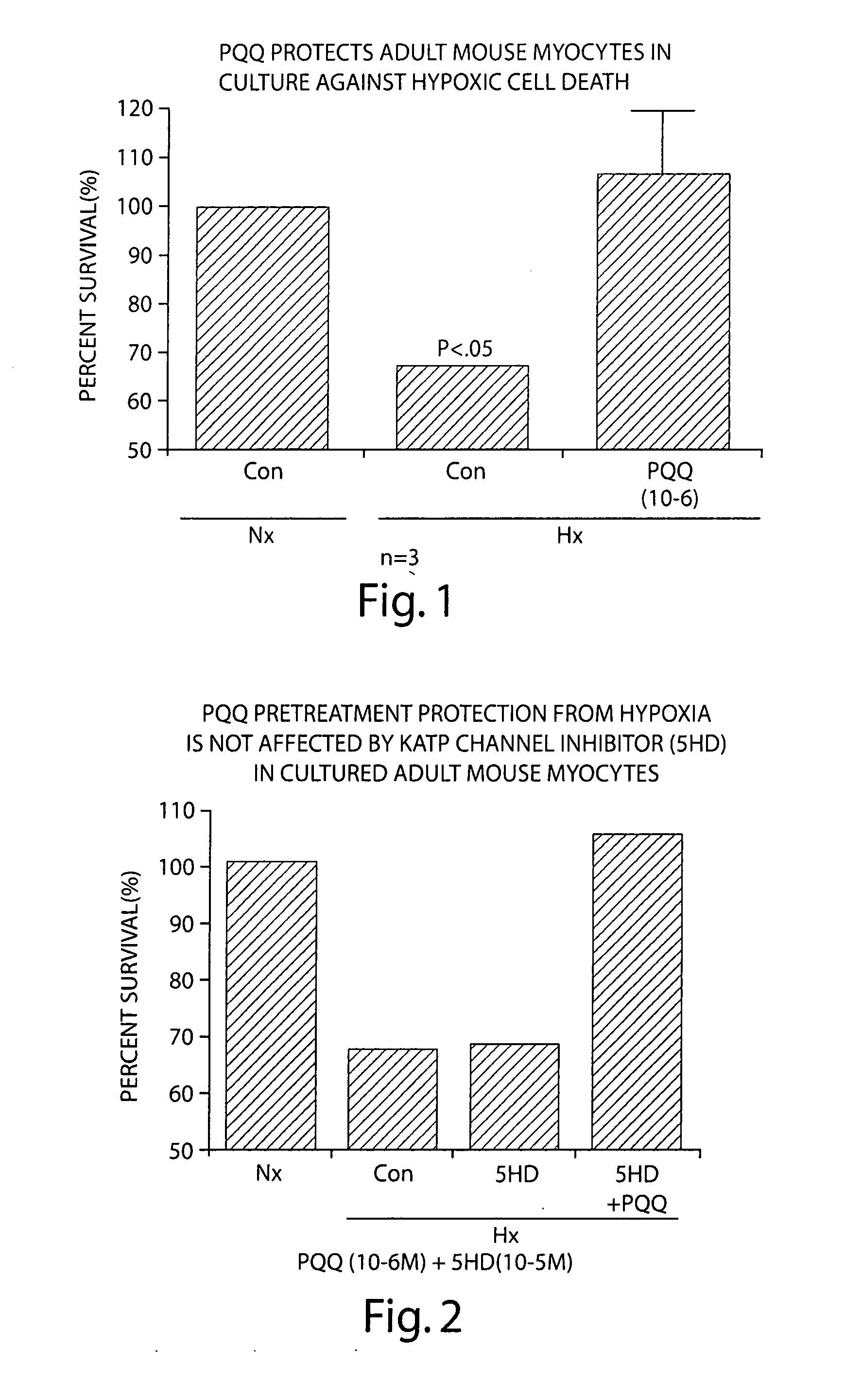
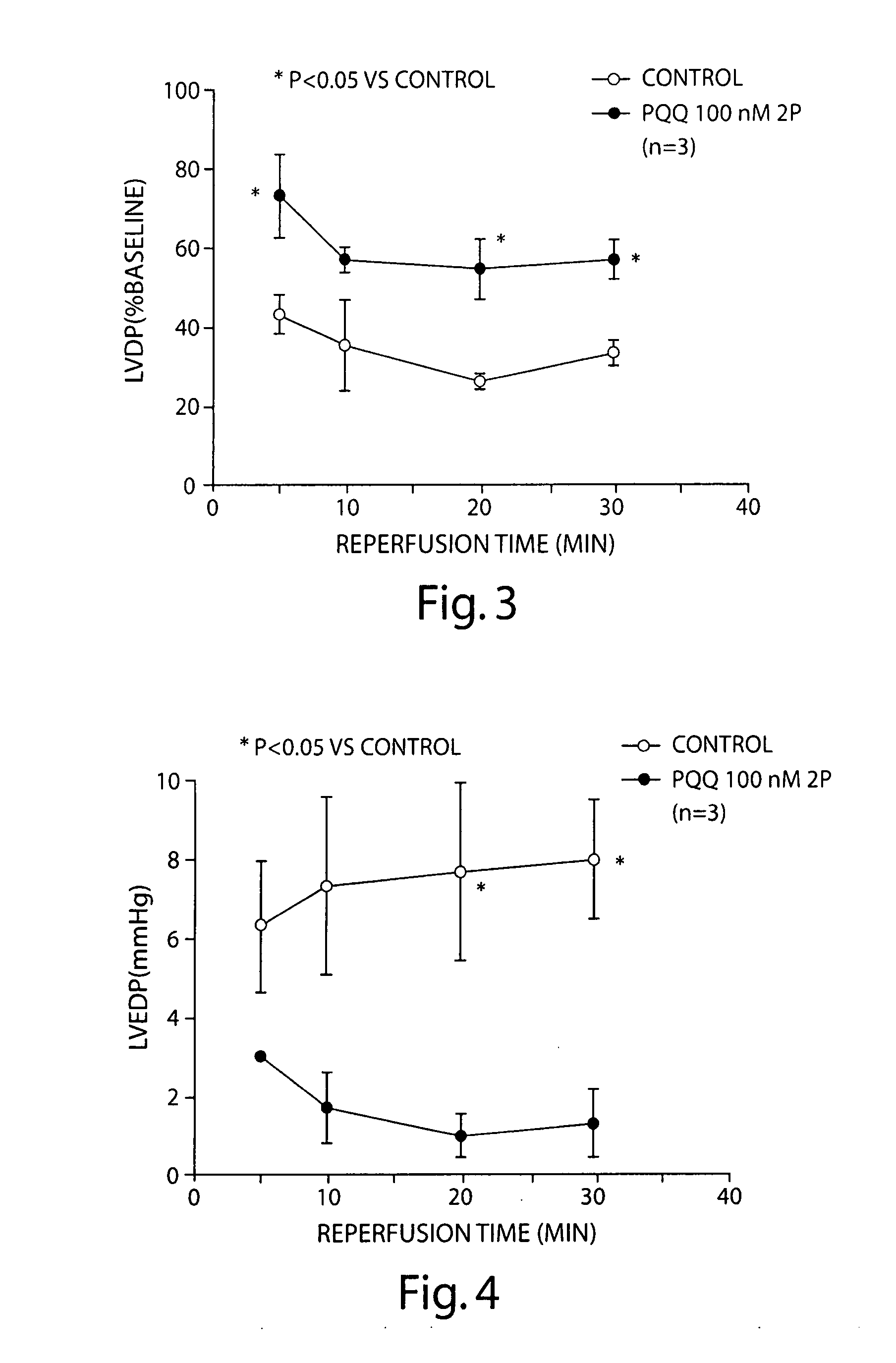
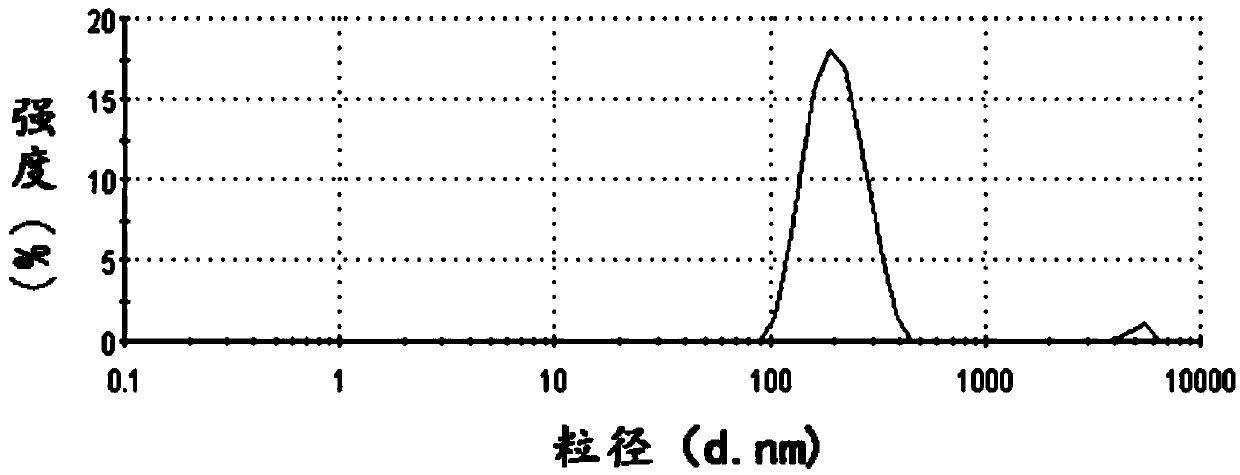
Pyrroloquinoline quinone drugs and methods of use thereof
InactiveUS20080051428A1Reduce nephrotoxicityMyocardial oxidative stress can be prevented or minimizedBiocideUrinary disorderInjury causeHuman patient
The invention includes compositions comprising substantially purified pyrroloquinoline quinone, that are useful in methods for the treatment and prevention of cardiac injury caused by hypoxia or ischemia. The invention also includes methods for the treatment and prevention of cardiac injury comprising contacting a composition of the invention with a human patient.
Owner:CLF MEDICAL TECH ACCELERATION
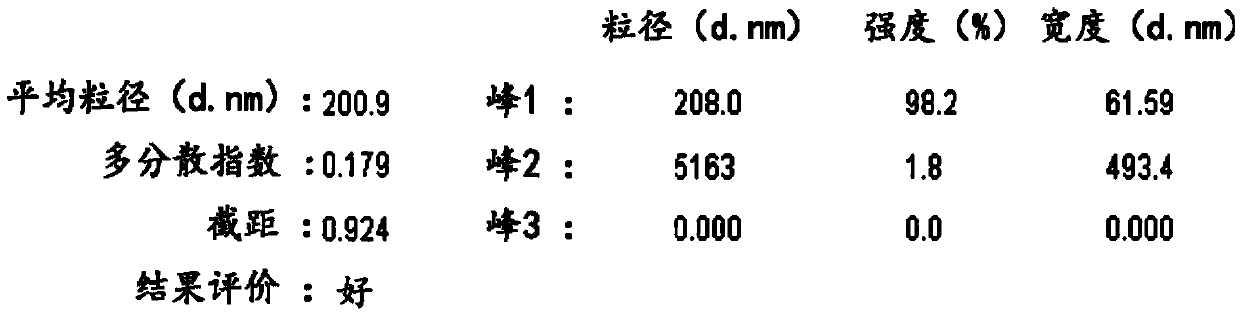
Injection paclitaxel nanocrystal and preparation method thereof
ActiveCN103768046AImprove stabilityImprove solubilityPowder deliveryOrganic active ingredientsMedicineNanocrystal
The invention belongs to the technical field of medicines, relates to a paclitaxel medicinal composition and a preparation method thereof, and in particular relates to an injection paclitaxel nanocrystal preparation and a preparation method thereof as well as application of the preparation for preparing anti-tumor medicines. The paclitaxel nanocrystal belongs to a nano preparation, is small in grain diameter and good in stability, and cannot be easily separated, so that the dissolubility of paclitaxel is remarkably improved, and in-vivo studies prove that the paclitaxel nanocrystal has certain targeting performance, and can be used in anti-tumor treatment.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Cyclic nonapeptide amides
InactiveUS20080070884A1Comparable and improved antibacterial activityImprove stabilityAntibacterial agentsBiocideBACTERIAL INFECTIOUS DISEASESCombinatorial chemistry
The invention relates to cyclic nonapeptide amides and to methods for their preparation and to their use for the production of medicaments for the treatment and / or prophylaxis of diseases, especially bacterial infectious diseases.
Owner:AICURIS GMBH & CO KG
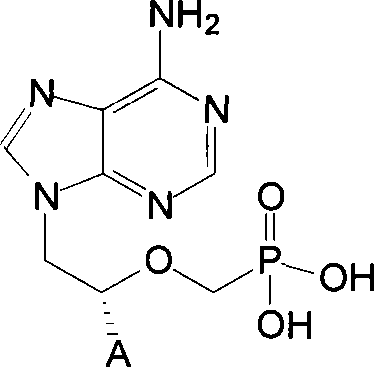
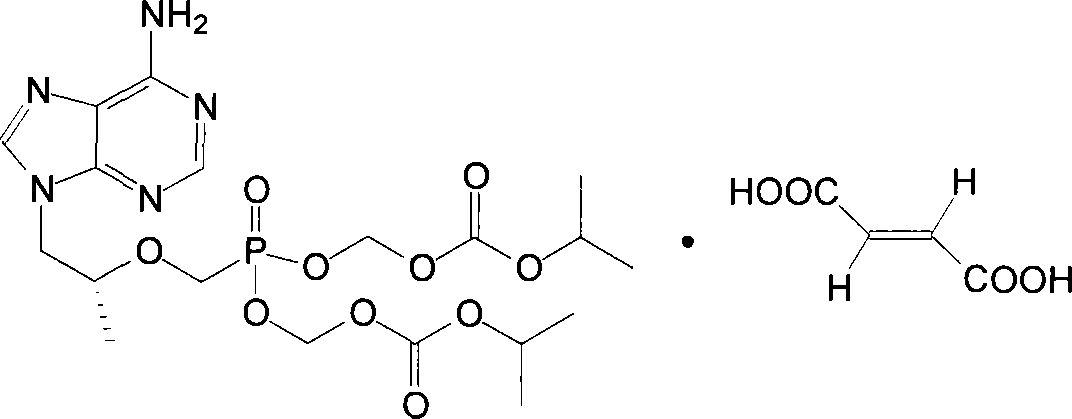
Chemical modified adefovir and tynofovir
ActiveCN1947796AReduced renal clearanceReduce nephrotoxicityPowder deliveryOrganic active ingredientsPolyethylene glycolChemistry
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
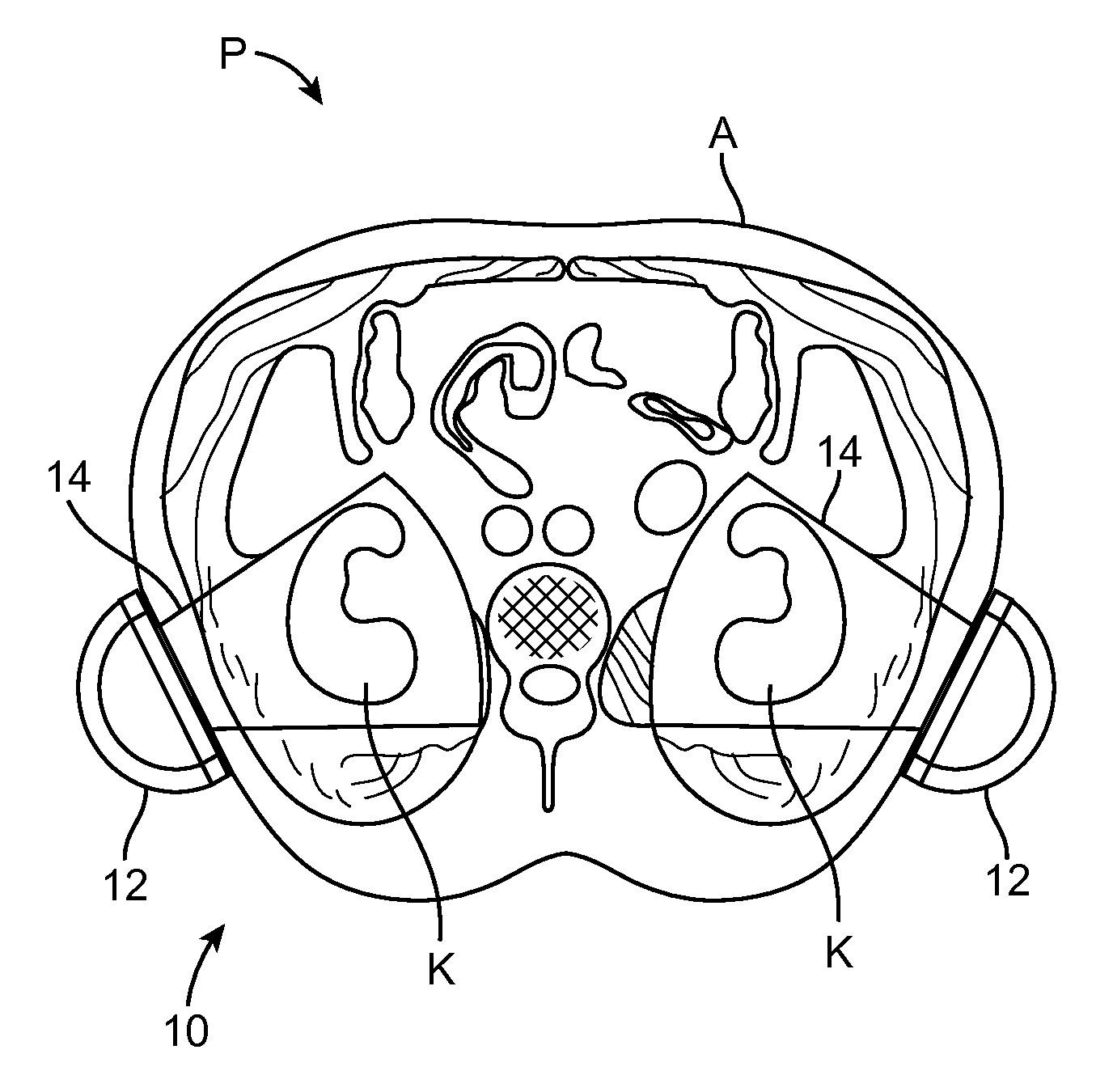
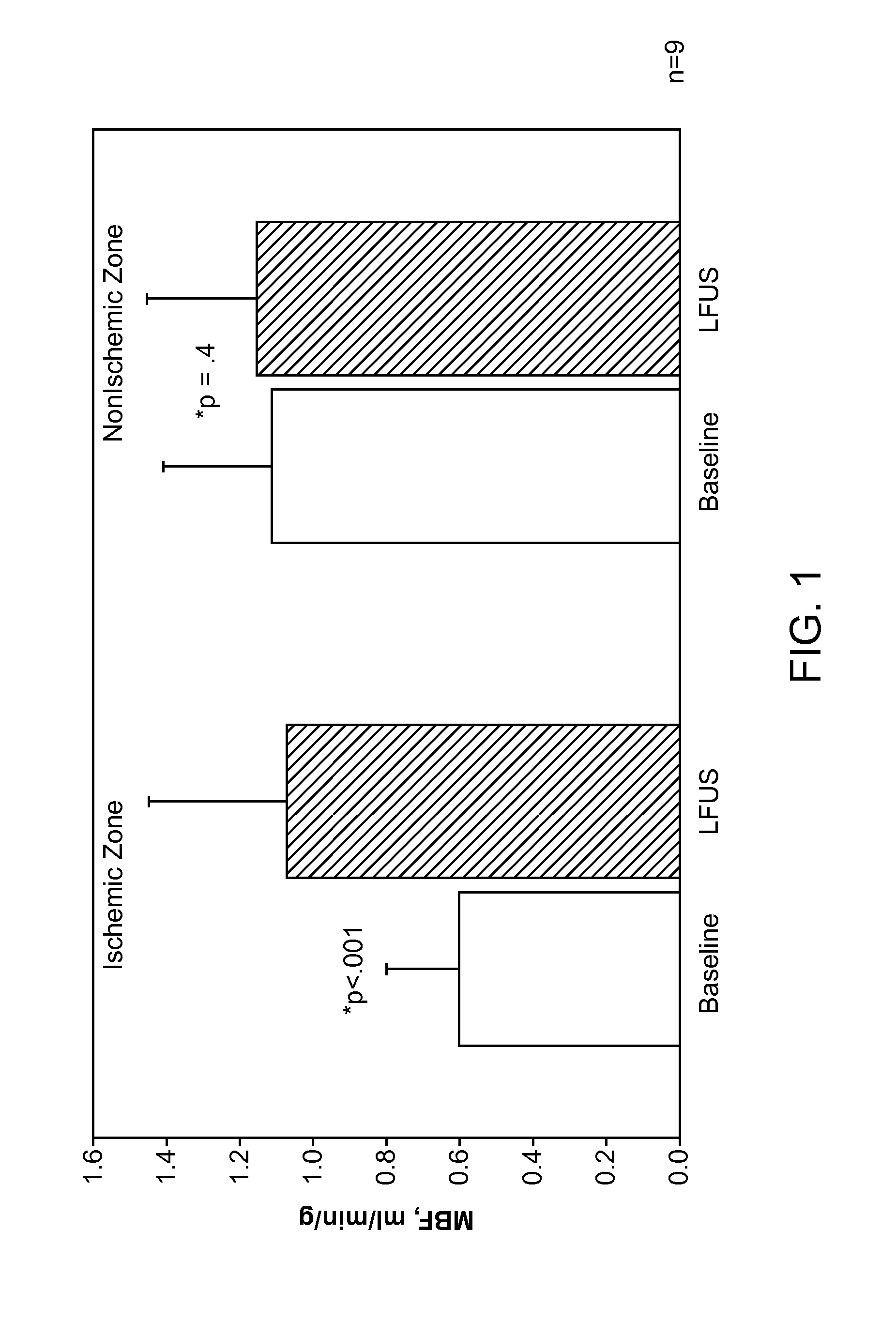
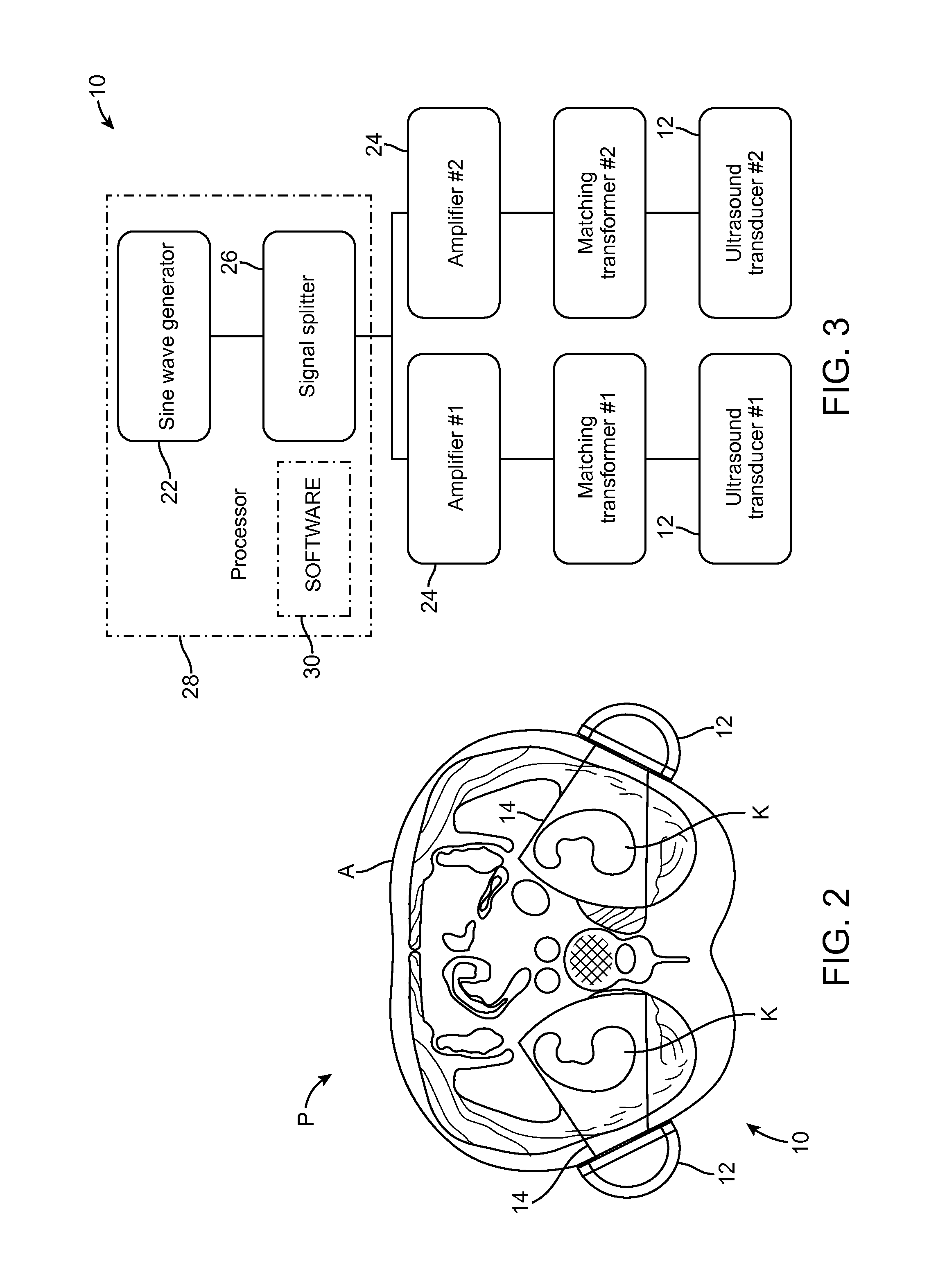
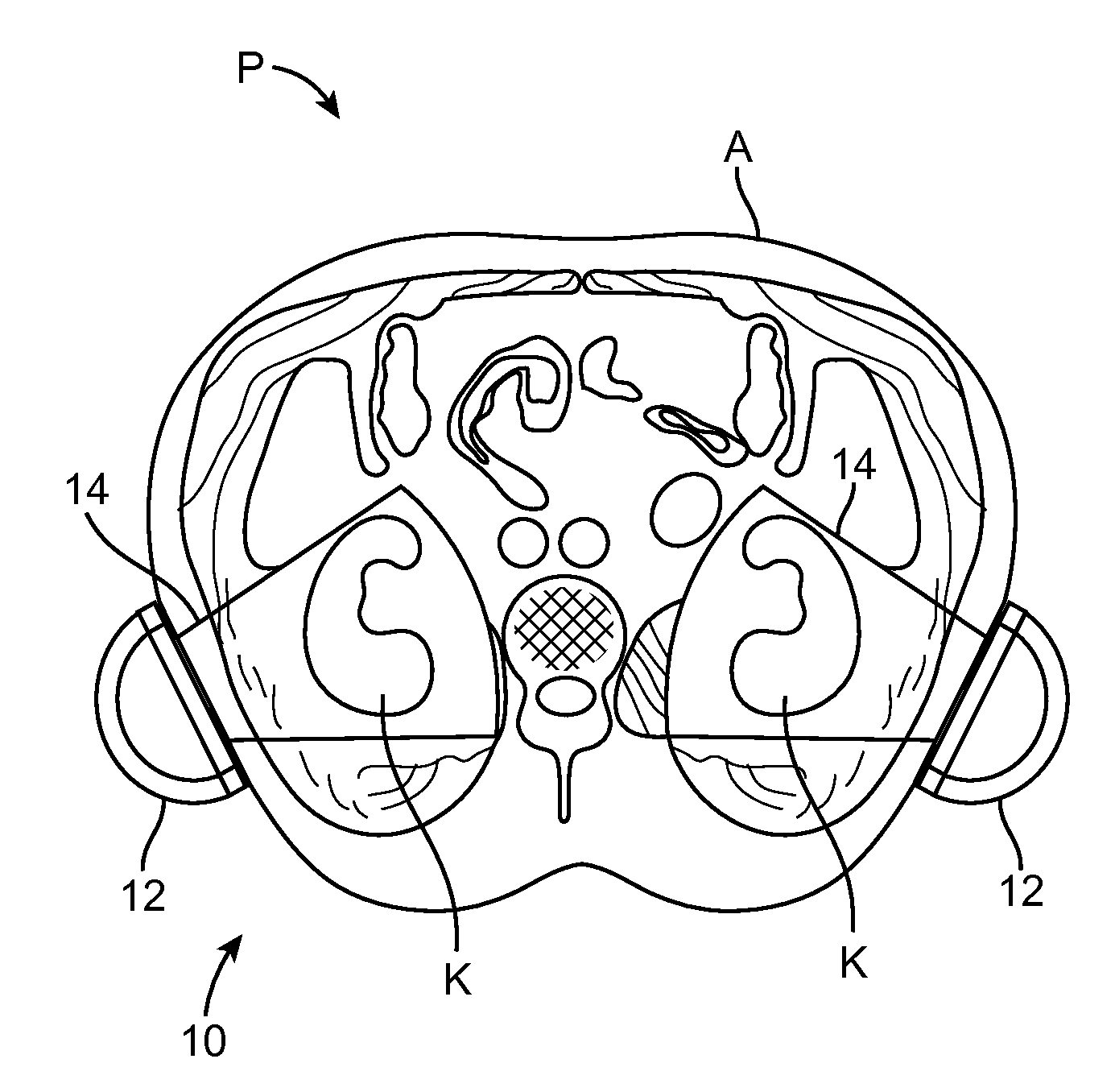
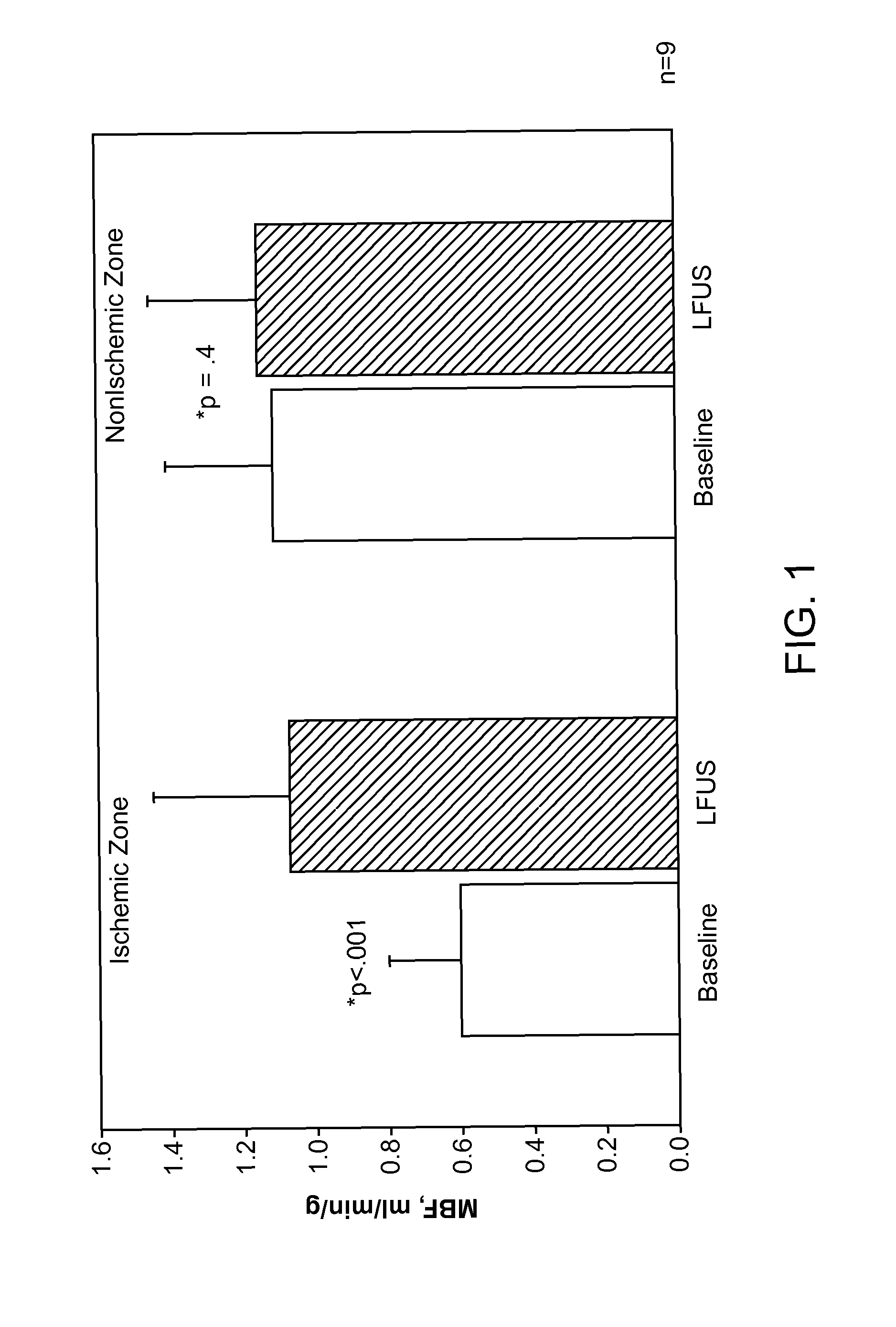
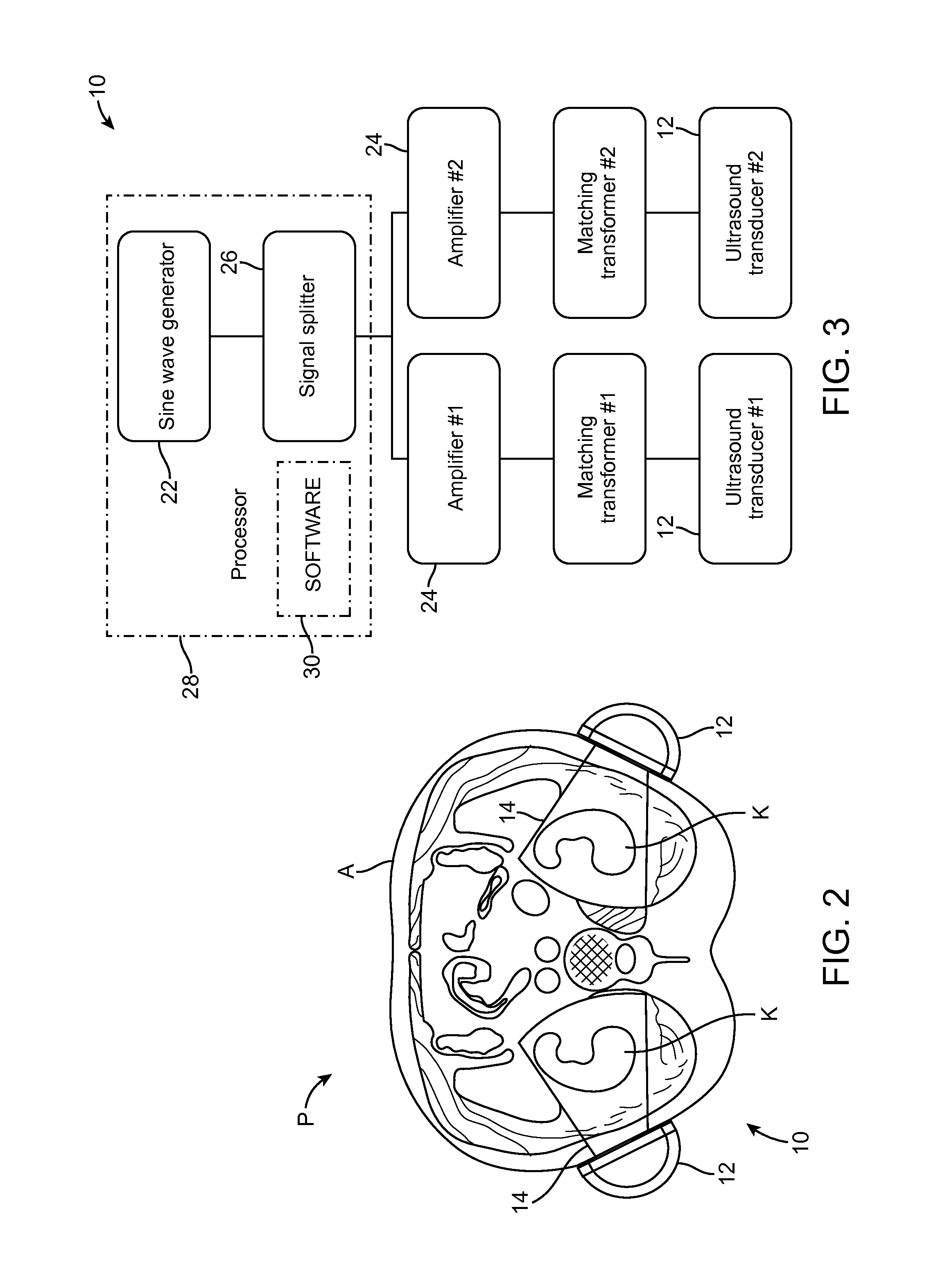
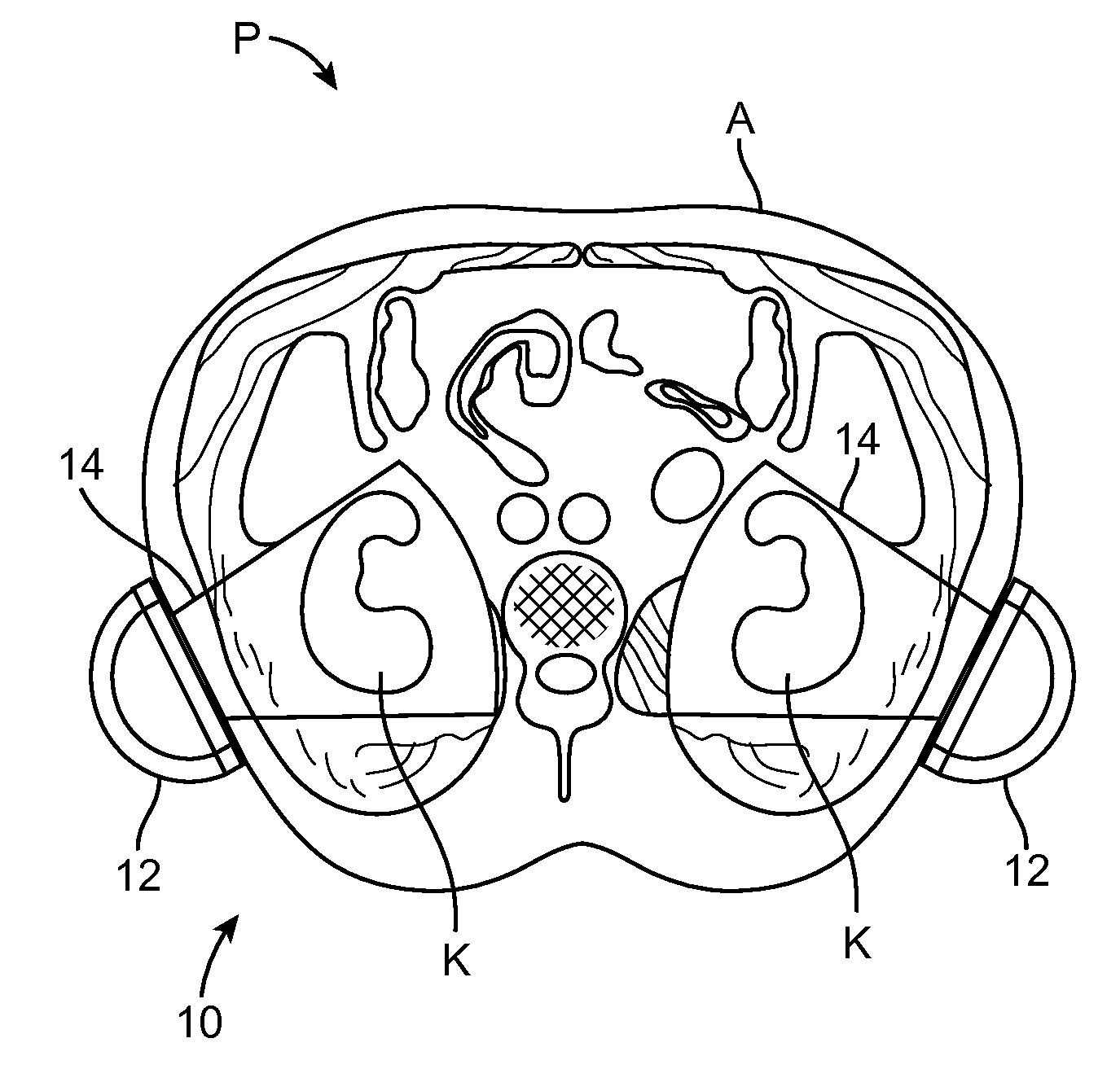
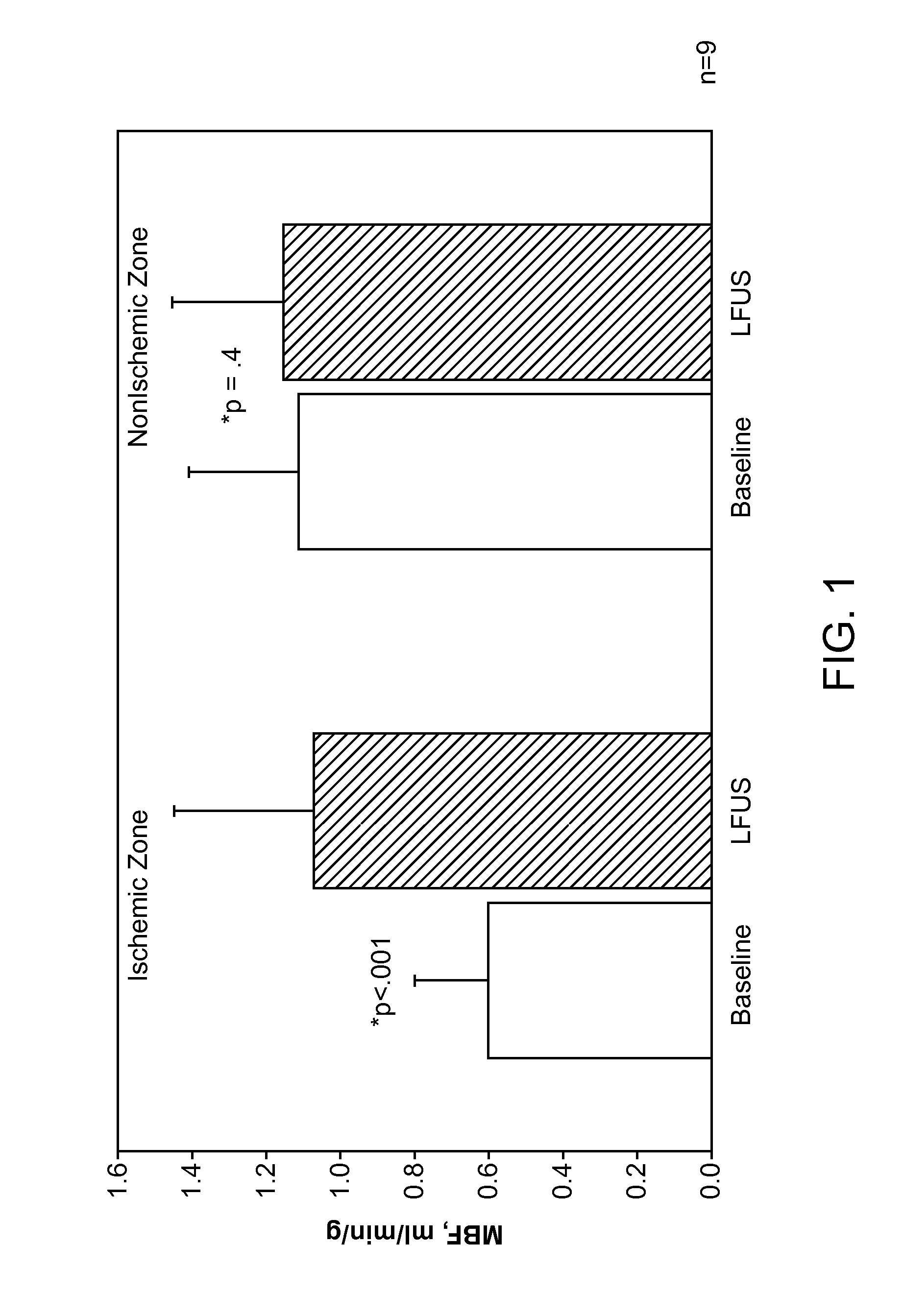
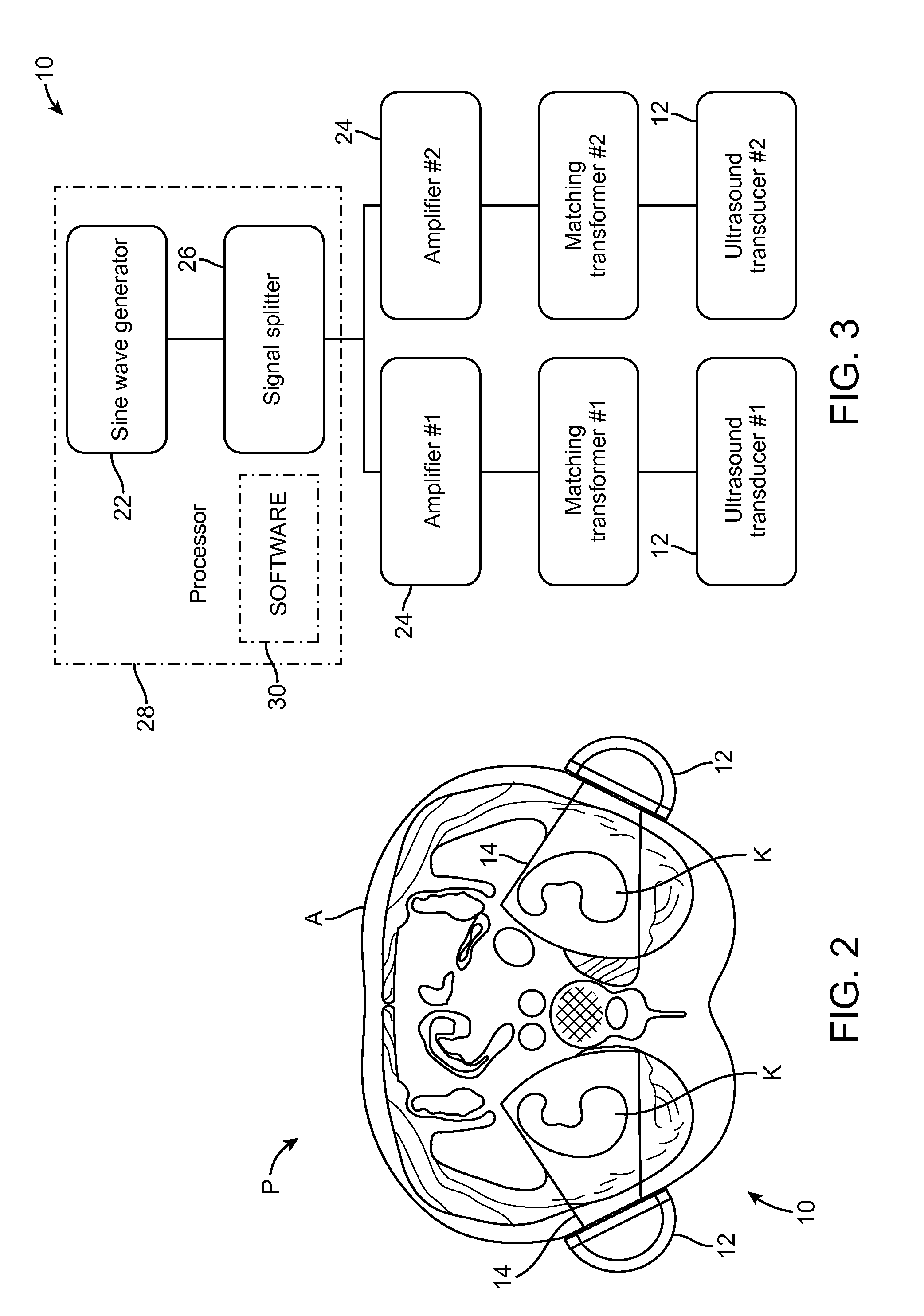
Renal Injury Inhibiting Devices, Systems, and Methods Employing Low-Frequency Ultrasound or Other Cyclical Pressure Energies
ActiveUS20120065501A1Mitigate and avoid injuryIncrease perfusionUltrasound therapyOrgan movement/changes detectionShock waveNitric oxide
Improved devices, systems, and methods treatment of patients can be used to help mitigate injury to the kidneys by applying cyclical mechanical pressure energy at low intensities. The energy often be selectively directed from non-invasive transducers disposed outside the patients. The energy will typically comprise low frequency ultrasound energy, shock wave energy, or the like, and may induce the generation and / or release of nitric oxide, thereby enhancing perfusion and ameliorating tissue damage. Superimposed micro and macro duty cycles may help avoid thermal and other injury to tissues of the patient during treatment. Bilateral treatments are facilitated by a support structure that orients at least one transducer toward each kidney.
Owner:RGT UNIV OF CALIFORNIA +1
Renal injury inhibiting devices, systems, and methods employing low-frequency ultrasound or other cyclical pressure energies
ActiveUS8585597B2Lower levelIncrease perfusionUltrasound therapyOrgan movement/changes detectionEngineeringNitric oxide
Improved devices, systems, and methods treatment of patients can be used to help mitigate injury to the kidneys by applying cyclical mechanical pressure energy at low intensities. The energy often be selectively directed from non-invasive transducers disposed outside the patients. The energy will typically comprise low frequency ultrasound energy, shock wave energy, or the like, and may induce the generation and / or release of nitric oxide, thereby enhancing perfusion and ameliorating tissue damage. Superimposed micro and macro duty cycles may help avoid thermal and other injury to tissues of the patient during treatment. Bilateral treatments are facilitated by a support structure that orients at least one transducer toward each kidney.
Owner:RGT UNIV OF CALIFORNIA +1
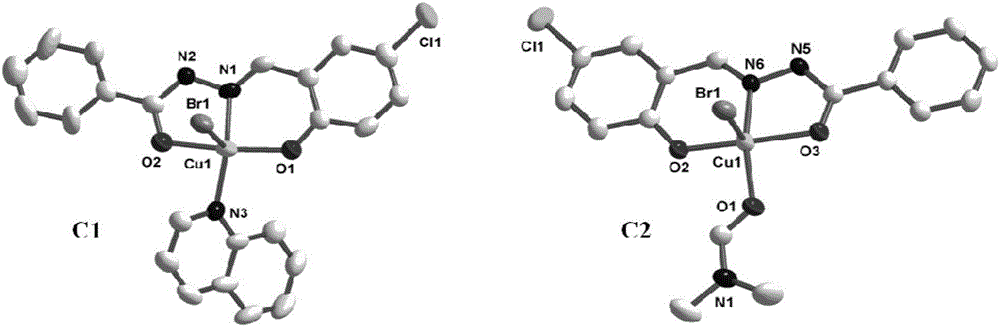
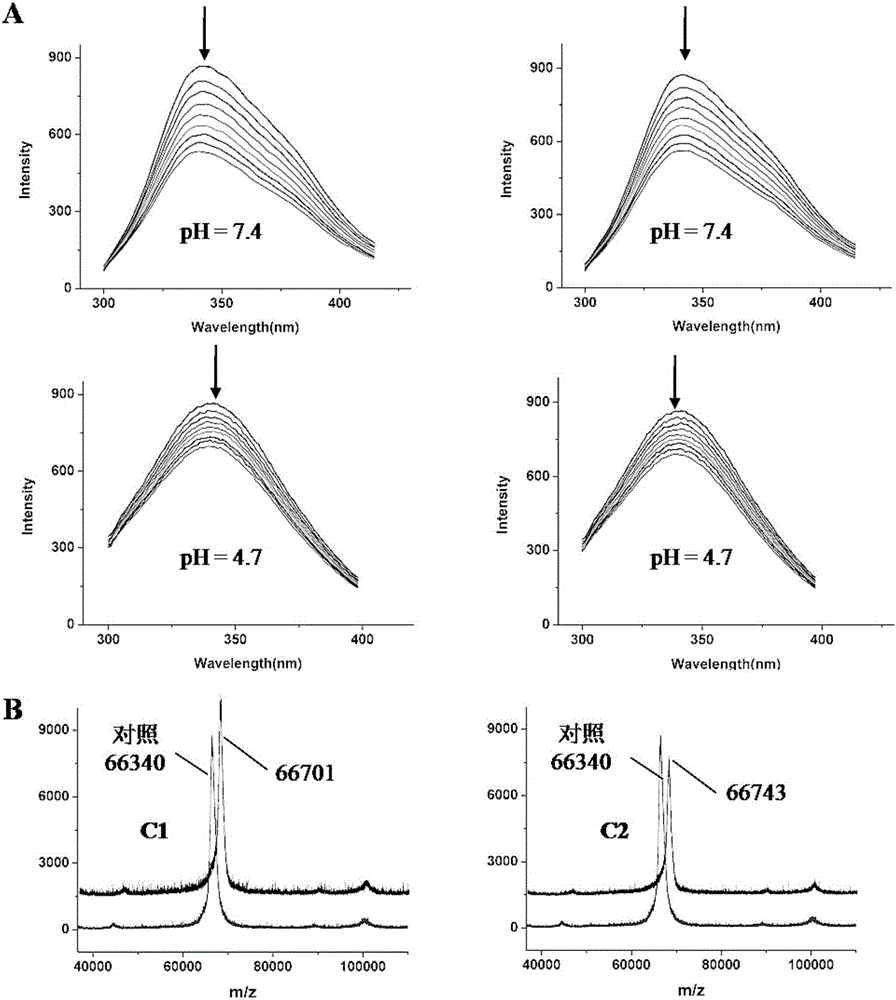
Acylhydrazone schiff base-copper complex-human serum albumin complex and applications thereof
ActiveCN106749343AEvenly dispersedAvoid Prodrug Burst ReleaseGroup 1/11 organic compounds without C-metal linkagesOrganic active ingredientsQuinolineCoordination complex
The invention discloses an acylhydrazone schiff base-copper complex and complex nanoparticles composed of the acylhydrazone schiff base-copper complex and human serum albumin. The chemical formula of the acylhydrazone schiff base-copper complex is [CuBr(BA)(L)] or [CuBr(DMF)(L)], wherein BA is quinoline, L is a compound with a tridentate acylhydrazone schiff base structure, DMF is N,N-dimethyl formamide, and Br-, quinolone or DMF in the complex is coordinated with a copper center as ligands. As prodrugs, the complex nanoparticles composed of the acylhydrazone schiff base-copper complex and human serum albumin not only improve the activity of drugs, but also can reduce the toxicity of drugs, thus having good clinical application values.
Owner:苏州美仑生物科技有限公司
Oligonucleotide conjugates
ActiveUS9879265B2Reduce Toxicity RiskReduce nephrotoxicityOrganic active ingredientsSugar derivativesMedical disorderSubtilisins
The present invention relates to oligomeric compounds and conjugates thereof that target Proprotein Convertase Subtilisin / Kexin type 9 (PCSK9) PCSK9 mRNA in a cell, leading to reduced expression of PCSK9. Reduction of PCSK9 expression is beneficial for a range of medical disorders, such as hypercholesterolemia and related disorders.
Owner:ROCHE INNOVATION CENT COPENHAGEN
Use of cilastatin to reduce nephrotatoxicity of various compounds
ActiveUS9216185B2Reduce accumulationReduce harmHalogenated hydrocarbon active ingredientsHeavy metal active ingredientsSolubilityCholesterol
Use of cilastatin to reduce the nephrotoxicity of different compounds. The invention refers to use of cilastatin to prepare a medicinal product to reduce the nephrotoxicity of a nephrotoxic compound that enters the cells of the proximal tubule through cholesterol rafts. The invention is based on the discovery that a great number of nephrotoxic compounds, including drugs, enter the cells of the proximal tubule through the cholesterol rafts, and that cilastatin is able to interfere with this transport mechanism, decreasing the nephrotoxicity of such compounds to a variable extent. The nephroprotective effect is common to compounds of different chemical nature and solubility and is specific for the kidney, causing no interference with the effects of nephrotoxic drugs having their targets in other organs. Therefore, administration of cilastatin allows for decreasing the nephrotoxic effects of different drugs without reducing their therapeutic effects.
Owner:FUNDACION PARA LA INVESTIGACION BIOMEDICA DEL HOSPITAL GREGORIO MARANON
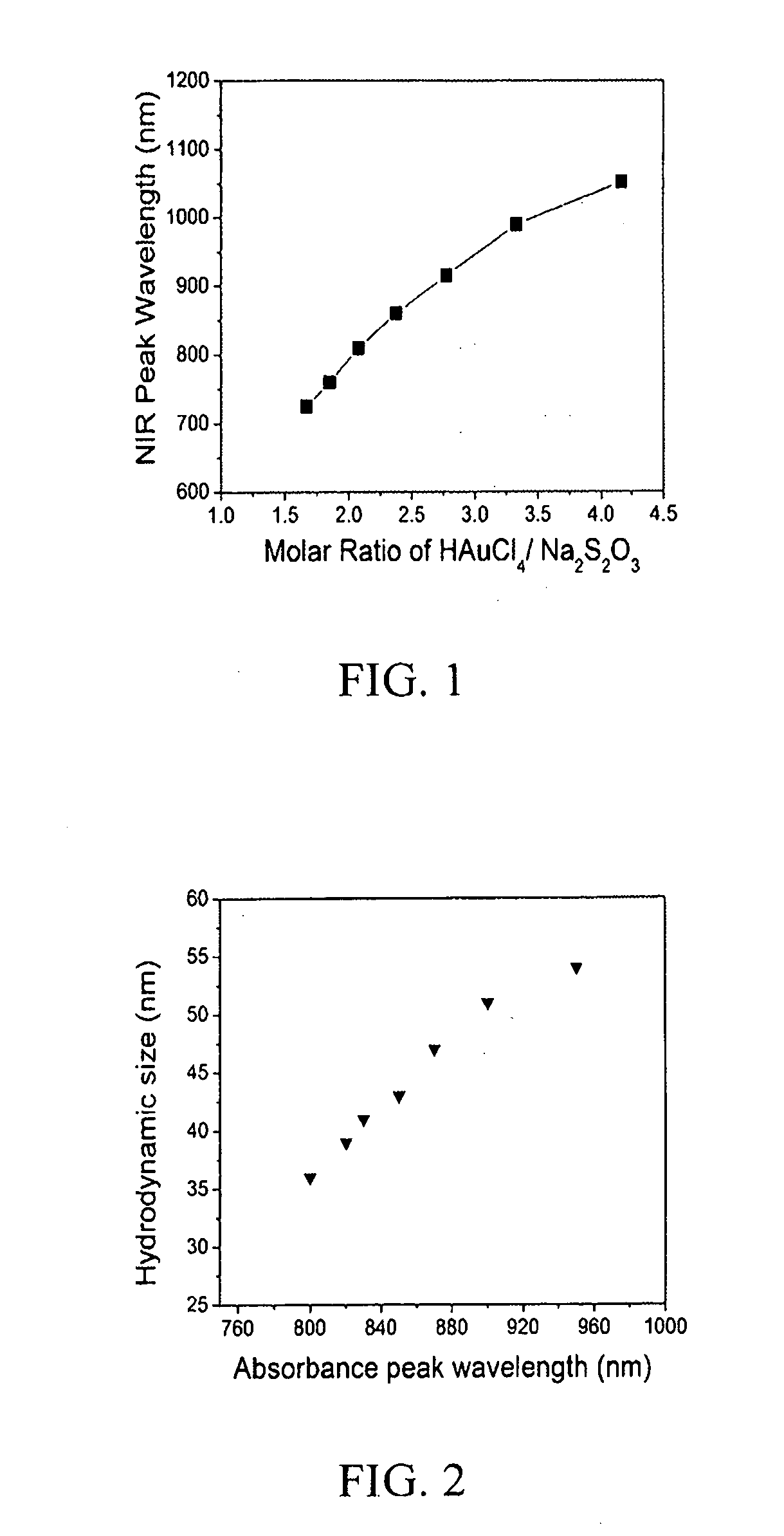
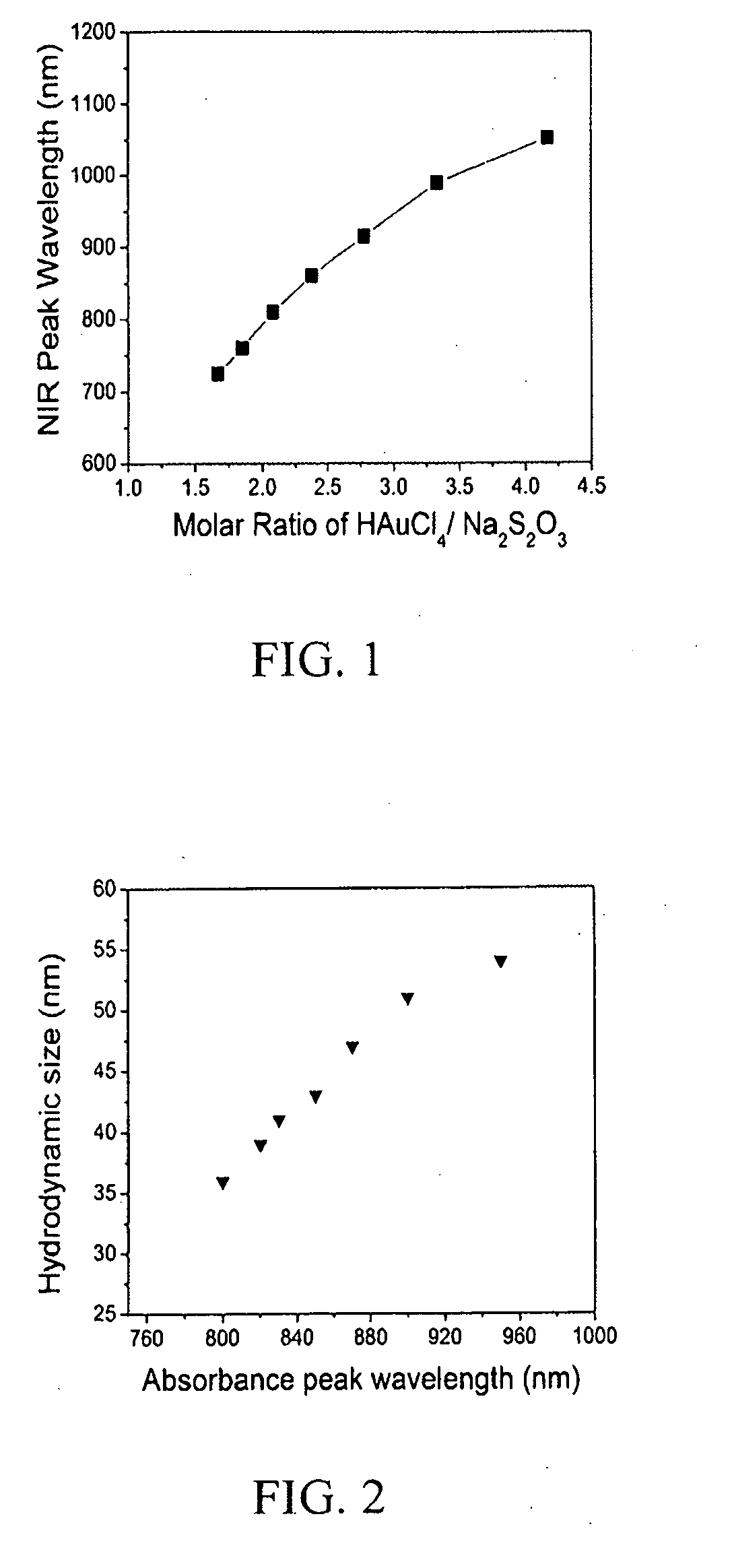
Diagnostic and therapeutic nanoparticles
InactiveUS20110064676A1Easy diagnosisHigh opacityOrganic active ingredientsHeavy metal active ingredientsMRI contrast agentNanoparticle
The present invention relates to diagnostic and therapeutic nanoparticles. More particularly, the present invention relates to creating a hybrid gold / gold sulfide nanoparticle with a chitosan matrix surrounding the metallic nanoparticle and a method for making the same. The chitosan-coated gold / gold sulfide nanoparticles can then be incorporated with additional therapeutic or diagnostic compounds such as iodine, antibodies, or other suitable compounds. The nanoparticles of the present invention have the dual capabilities of absorbing near infrared wavelength light to (1) act as a therapeutic agent by generating heat energy effective for cell ablation or for release of therapeutic compounds embedded in the chitosan matrix and (2) creating diagnostic benefit by incorporation of X-ray or MRI contrast-agents.
Owner:UNIV OF LOUISVILLE RES FOUND INC
Stable butylphthalide sodium chloride injection as well as preparation method and application thereof
PendingCN112386571AImprove solubilityGood water solubilityOrganic active ingredientsInorganic non-active ingredientsSodium Chloride InjectionCyclodextrin
The invention discloses a stable butylphthalide sodium chloride injection as well as a preparation method and application thereof, belongs to the technical field of pharmaceutical preparations, and solves the problems of poor safety and stability of the butylphthalide sodium chloride injection in the prior art. The stable butylphthalide sodium chloride injection comprises a sulfobutyl betacyclodextrin sodium compound, sodium chloride and water. The pH value of the butylphthalide sodium chloride injection is 4.0-5.0. The preparation method comprises the following steps of weighing a prescription amount of sulfobutyl betacyclodextrin sodium, and adding water for dissolving to prepare an auxiliary material solution; weighing a prescription amount of butylphthalide, adding the butylphthalide into the auxiliary material solution, stirring to enable the sulfobutyl betacyclodextrin sodium to include the butylphthalide, and adjusting the pH value of the solution to 4.0-5.0 after the inclusionof the butylphthalide is completed; and filtering, filling and sterilizing to obtain the product. The stable butylphthalide sodium chloride injection as well as the preparation method and applicationthereof are scientific in design and ingenious in thought, and the butylphthalide sodium chloride injection has good stability and safety.
Owner:CHENGDU SHIBEIKANG BIOLOGICAL MEDICINE TECH CO LTD
Glycopeptide disulfide and thioester derivatives
InactiveUS6872804B2High antibacterial activityImprove securityNanomedicinePharmaceutical delivery mechanismThioester synthesisGlycopeptide
Disclosed are disulfide and thioester derivatives of glycopeptides and pharmaceutical compositions containing such glycopeptide derivatives. The disclosed glycopeptide derivatives are useful as antibacterial agents.
Owner:THERAVANCE BIOPHARMA ANTIBIOTICS IP
Entecavir high-density lipoprotein enveloping preparation, and preparation method and use thereof
ActiveCN103655477AImprove securityReduce dosePowder deliveryDigestive systemPhospholipidHigh-density lipoprotein
The invention discloses an entecavir high-density lipoprotein enveloping preparation, and a preparation method and a use thereof. The entecavir high-density lipoprotein enveloping preparation contains entecavir, phospholipid, cholesterol and ApoAl in the weight ratio of 5: 7: 3: (5-20).
Owner:唐为钢
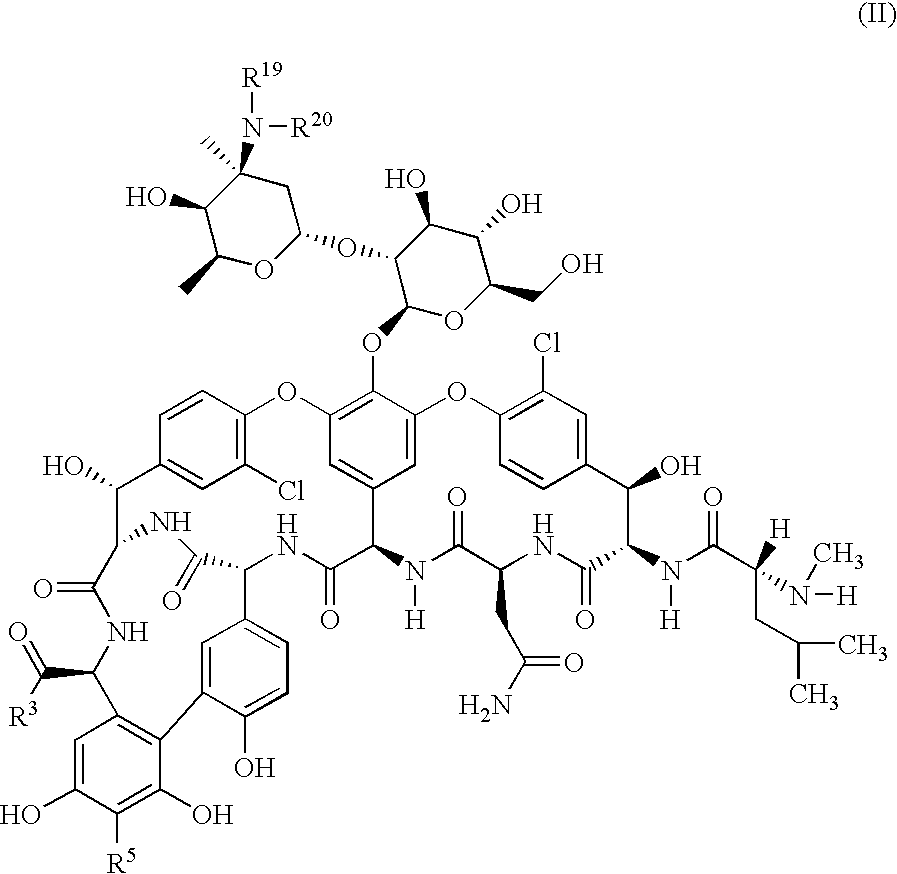
Lysobactin amides
InactiveUS20090203582A1Comparable and improved antibacterial effectImprove toleranceAntibacterial agentsBiocideDiseaseBACTERIAL INFECTIOUS DISEASES
The invention relates to lysobactin amides and methods for their preparation, as well as their use for manufacturing medicaments for the treatment and / or prophylaxis of diseases, in particular bacterial infectious diseases.
Owner:AICURIS GMBH & CO KG
Glycopeptide carboxy-saccharide derivatives
InactiveUS7157554B2Highly effective antibacterial activityReduce accumulationAntibacterial agentsPharmaceutical delivery mechanismGlycopeptideC-terminus
Disclosed are glycopeptide derivatives substituted at the C-terminus and / or the R-terminus with a substituent that comprises one or more saccharide groups and a carboxy group; and pharmaceutical compositions containing such glycopeptide derivatives. The disclosed glycopeptide derivatives are useful as antibacterial agents.
Owner:THERAVANCE BIOPHARMA ANTIBIOTICS IP
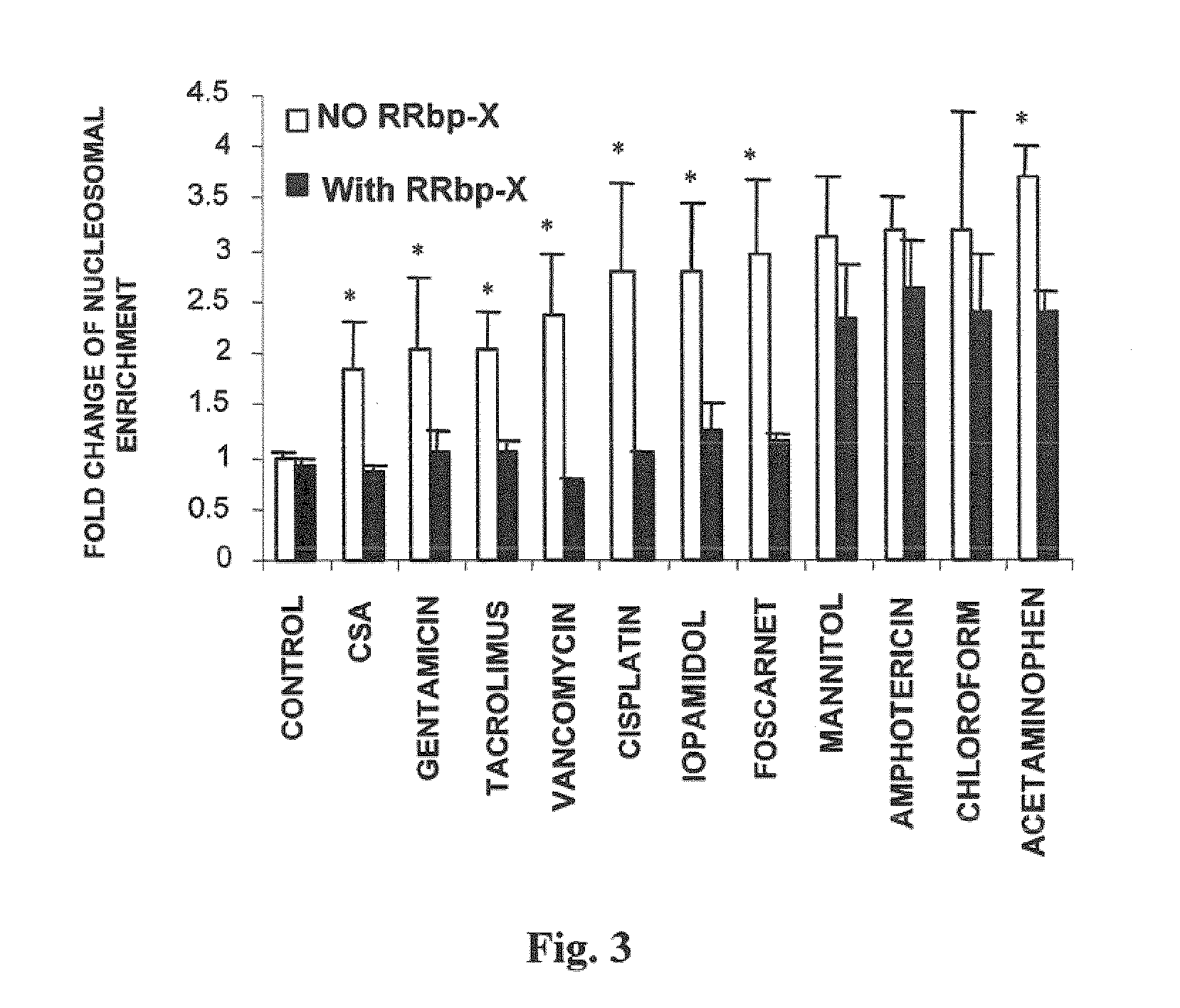
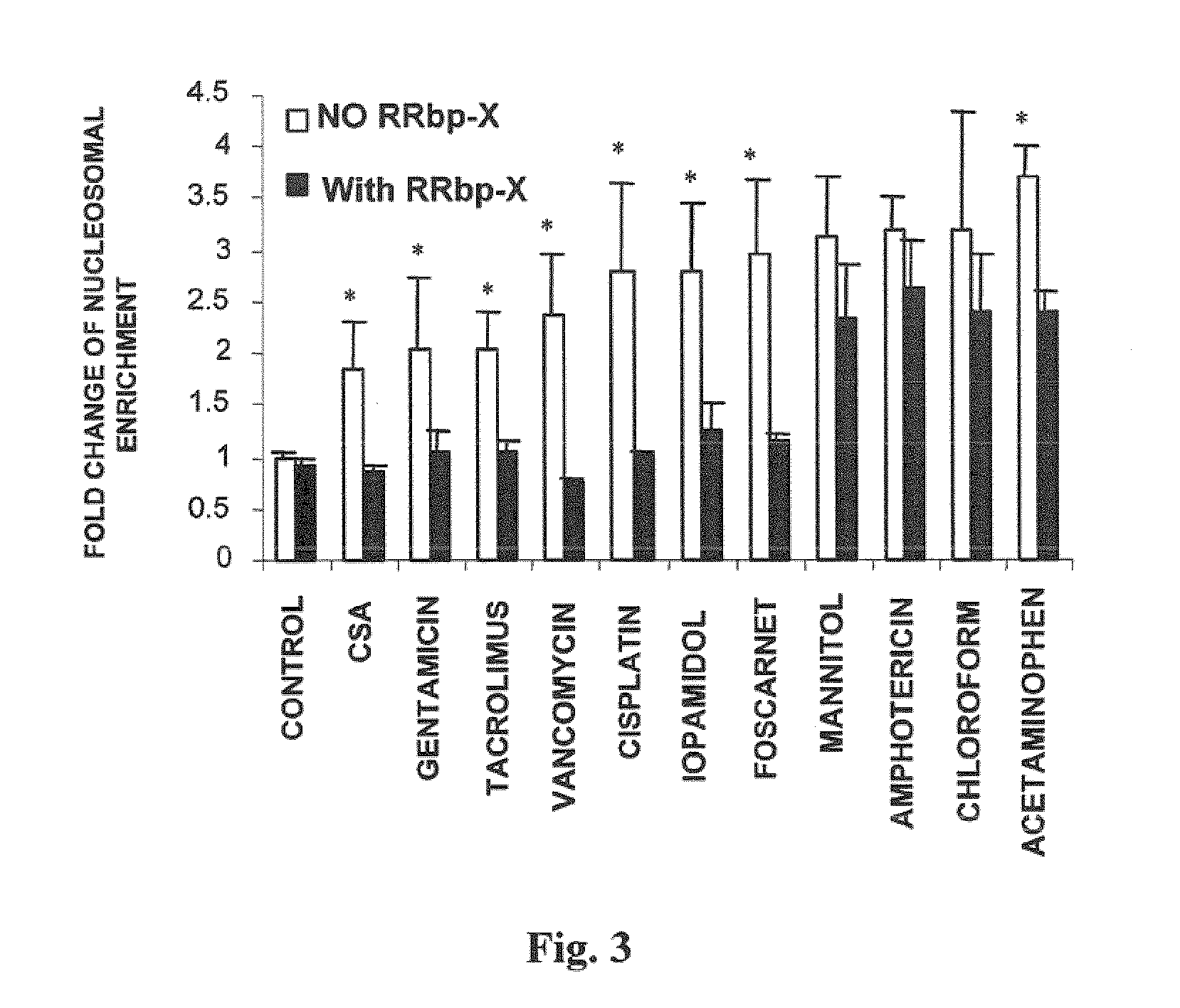
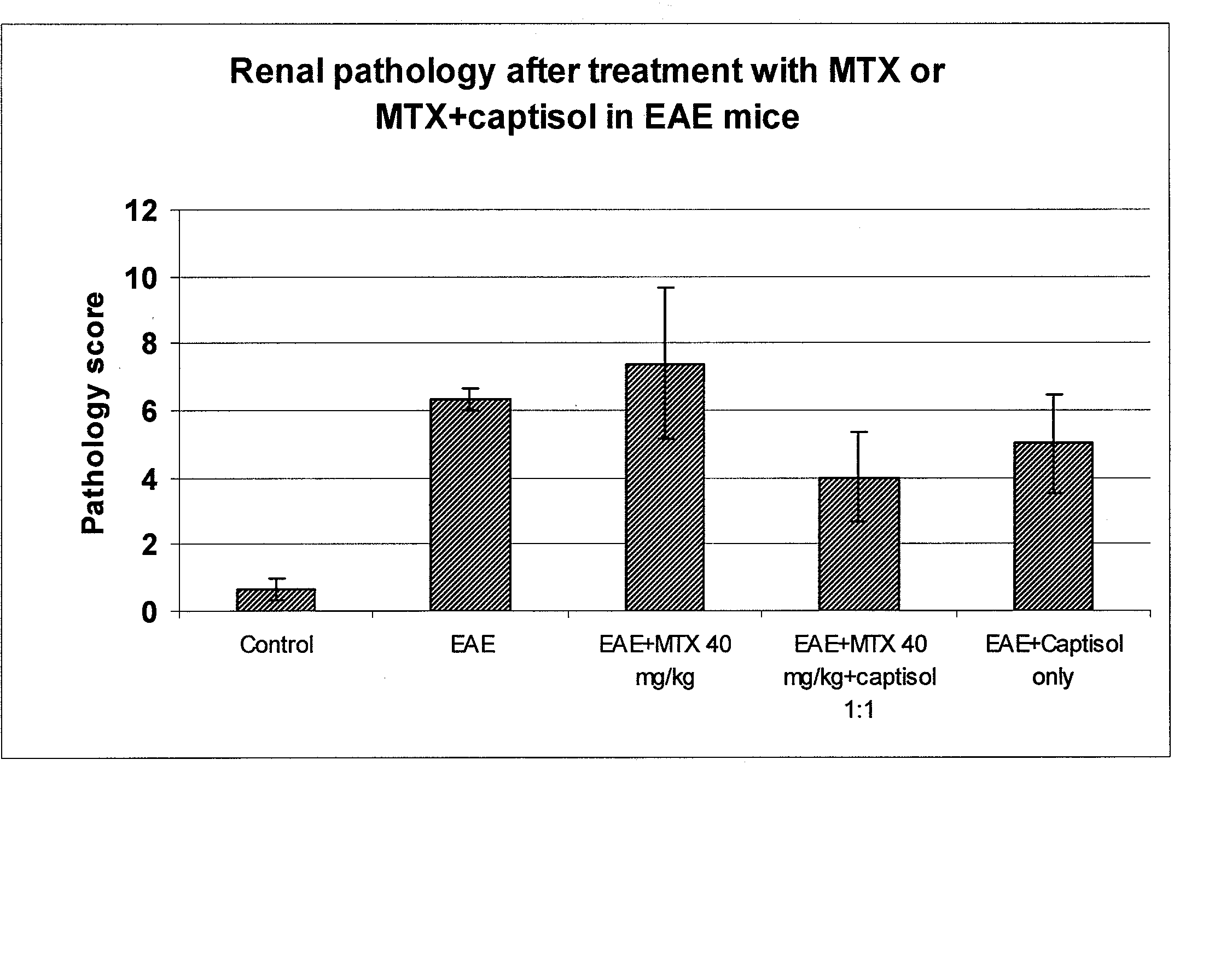
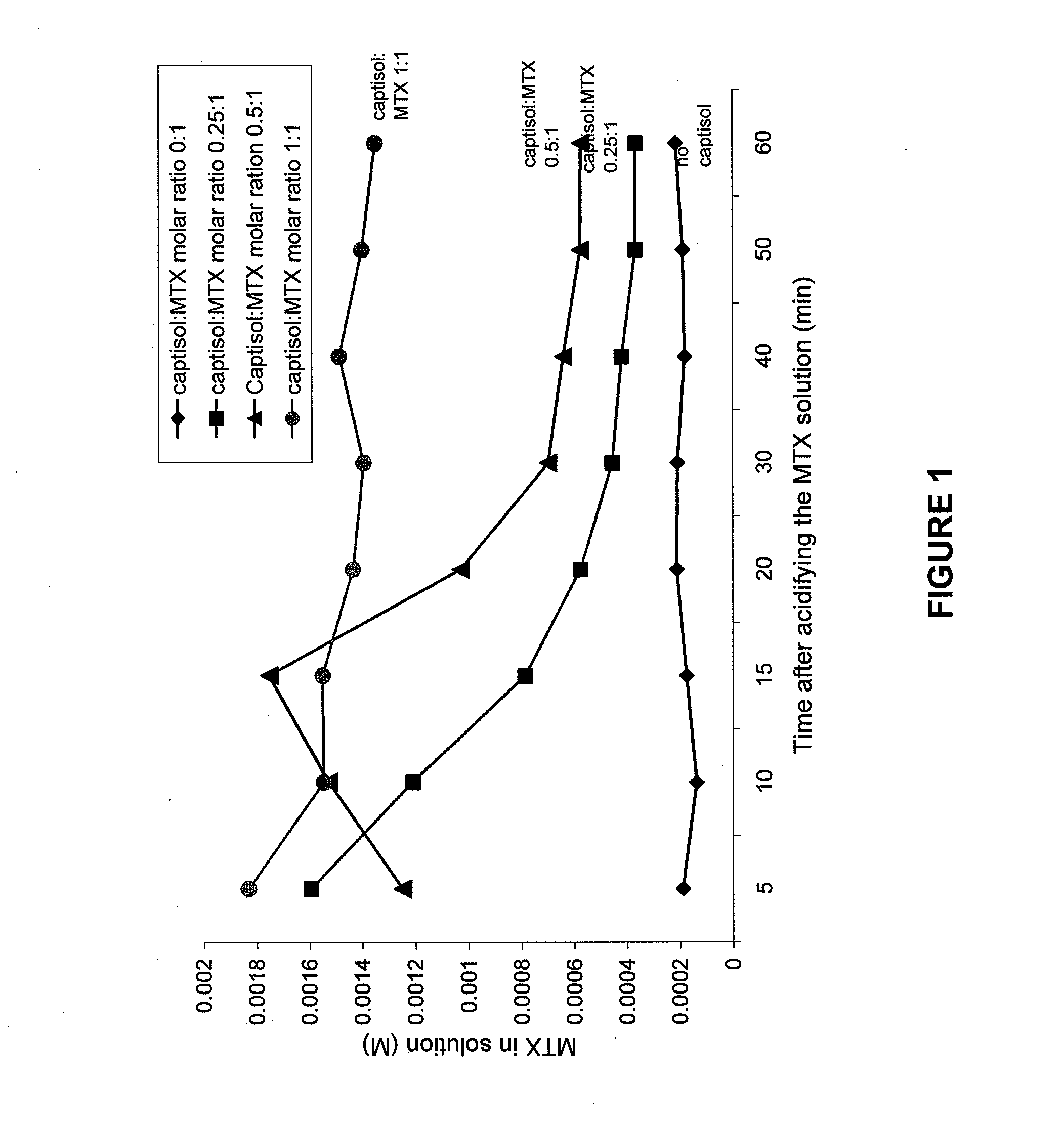
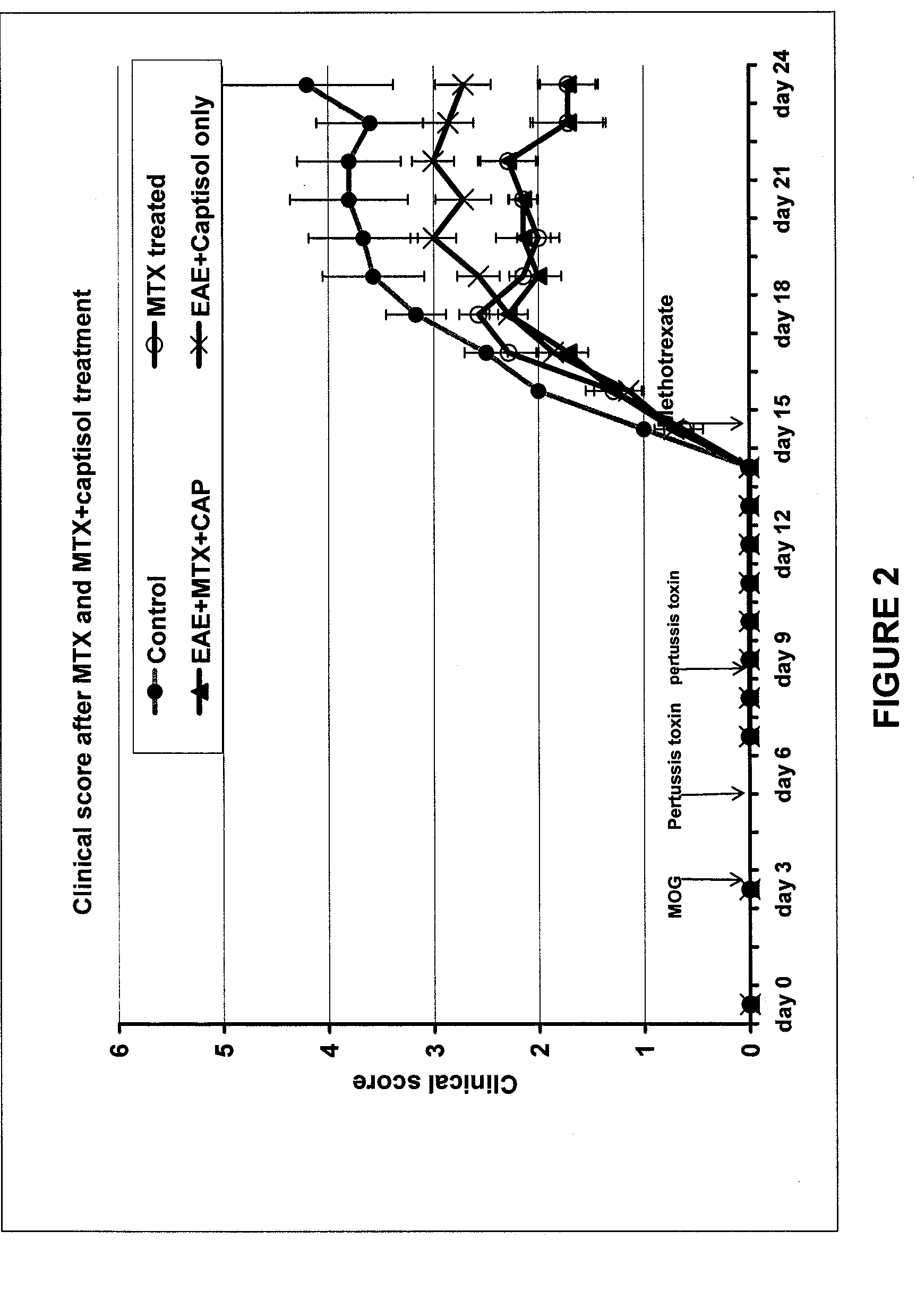
Compositions useful for reducing nephrotoxicity and methods of use thereof
ActiveUS20070270380A1Reduce nephrotoxicityPrevent nephrotoxicityOrganic active ingredientsBiocideNephrotoxicityOligosaccharide
The present invention provides compositions and methods to reduce renal damage caused by nephrotoxic drugs. The invention provides compositions comprising an anionically substituted oligosaccharide, a nephrotoxic drug and a pharmaceutically acceptable carrier, where the oligosaccharide is present in an amount effective for substantially inhibiting the nephrotoxic effect of the drug.
Owner:VERROW PHARMA
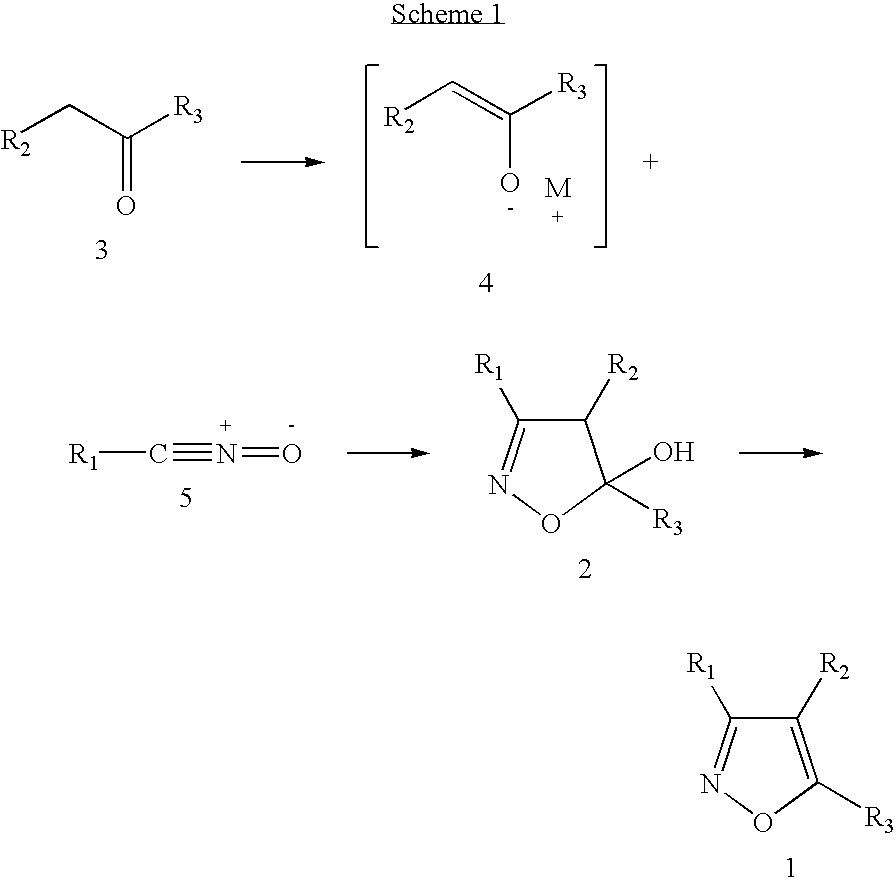
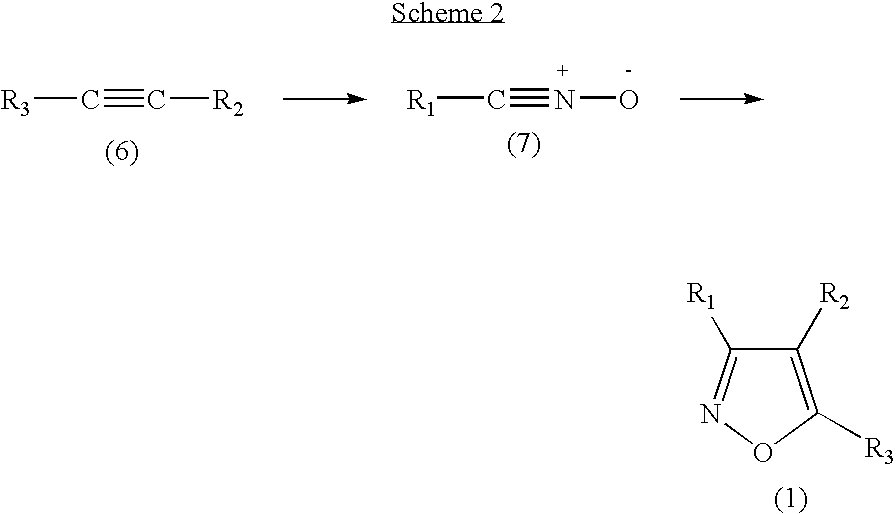
Functionalized diarylisoxazoles inhibitors of ciclooxygenase
The present invention refers to isoxazole derivatives, in particular diarylisoxazole derivatives inhibitors of cyclooxygenase (COX), in particular cyclooxygenase-1 (COX-1), to their pharmaceutical compositions, the process for their preparation and their use for the chemoprevention and treatment of inflammatory syndromes and in the prevention and treatment of carcinomas, in particular intestinal, ovarian and cutaneous carcinomas, in the treatment of pain syndromes, in particular after surgery, and in the cardiovascular field as antithrombotics / vasoprotectives / cardioprotectives.
Owner:UNIV DEGLI STUDI DI BARI +1
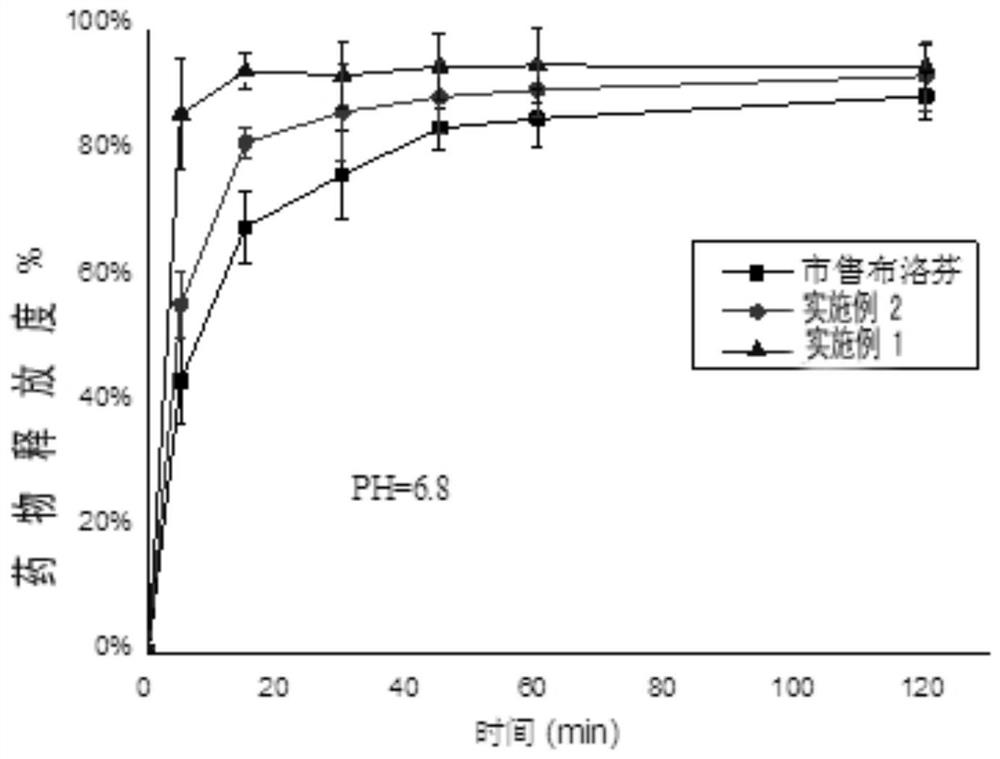
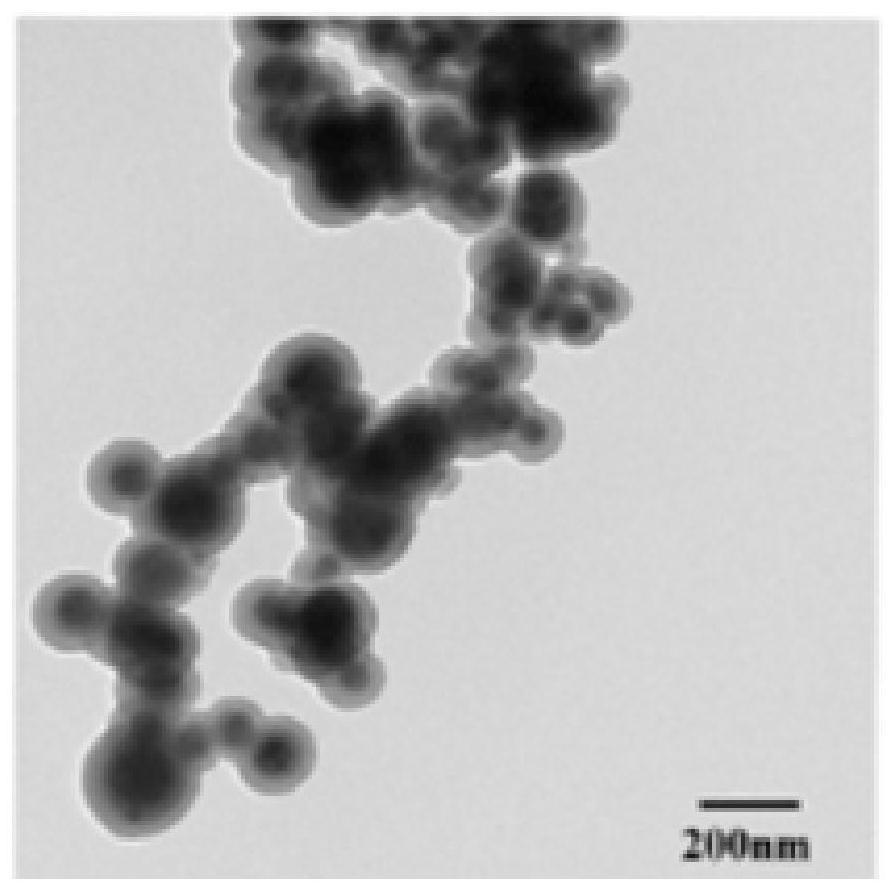
Ibuprofen nanoparticle with low renal toxicity and preparation method thereof
ActiveCN112891311AReduce usageAvoid lostOrganic active ingredientsPowder deliveryNon steroid anti inflammatory drugKidney Toxicity
The invention relates to an ibuprofen nanoparticle with low renal toxicity and a preparation method thereof, and belongs to the technical field of pharmaceutical preparations. The nanoparticle is prepared by placing ibuprofen, a plant-derived functional natural product auxiliary material, a flow aid and ball-milling beads in a ball-milling tank and carrying out a co-grinding reaction at a certain rotating speed, and the ibuprofen is uniformly entrapped in a carrier under the action of mechanical force, so that an ibuprofen nanoparticle product with low renal toxicity is obtained. According to the ibuprofen nanoparticle and the method, the nanoparticle is prepared through a limited preparation method, the operation is simple, the use of an organic solvent is avoided, the problems about raw material loss, thermal decomposition and the like caused by solvent residues and a solvent thermal removal process are prevented, and the advantages of high preparation efficiency, safe and reliable production, low production cost, less pollution and the like are achieved; and meanwhile, a plant-derived macromolecule is adopted as the carrier, so that the renal toxicity of the ibuprofen is reduced while solubilization and synergism are achieved, and the macromolecule is of great importance to safe medication of a non-steroidal anti-inflammatory drug.
Owner:ZHEJIANG UNIV OF TECH
Chemical modified adefovir and tynofovir
ActiveCN1947796BReduced renal clearanceReduce nephrotoxicityPowder deliveryOrganic active ingredientsPolythylene glycolPharmaceutical Substances
Owner:LIANYUNGANG RUNZHONG PHARMA CO LTD
Levosimendan-containing injection medicine preparation and preparation method thereof
InactiveCN108261398AAvoid inconvenienceImprove securityPowder deliveryLyophilised deliveryFreeze-dryingBULK ACTIVE INGREDIENT
The invention provides a levosimendan-containing injection medicine preparation and a preparation method thereof, and belongs to the field of medicine preparations. The preparation can be injection solution or freeze-dried powder and is mainly used for administrating medicines by injection. The injection solution comprises levosimendan serving as an active component or medicinal derivative of thelevosimendan and sulfobutyl beta cyclodextrin serving as a solubilizing stabilizer or medicinal salt of the sulfobutyl beta cyclodextrin, the weight ratio of the active component to the solubilizing stabilizer is 1:10-300, preferably, the weight ratio is 1:40-100, and most preferably, the weight ratio is 1:64-100. According to the preparation, the accessory sulfobutyl beta cyclodextrin has betterwater solubility, less hemolytic action and low renal toxicity as compared with hydroxypropyl beta cyclodextrin and is firstly used as a clathration material of the levosimendan or the medicinal derivative of the levosimendan. Compared with marketed similar injection solution, the injection solution takes water as a solvent, does not contain ethyl alcohol and is better in safety, and a freeze-dried preparation is more stable and easily stored at the room temperature.
Owner:QILU PHARMA CO LTD
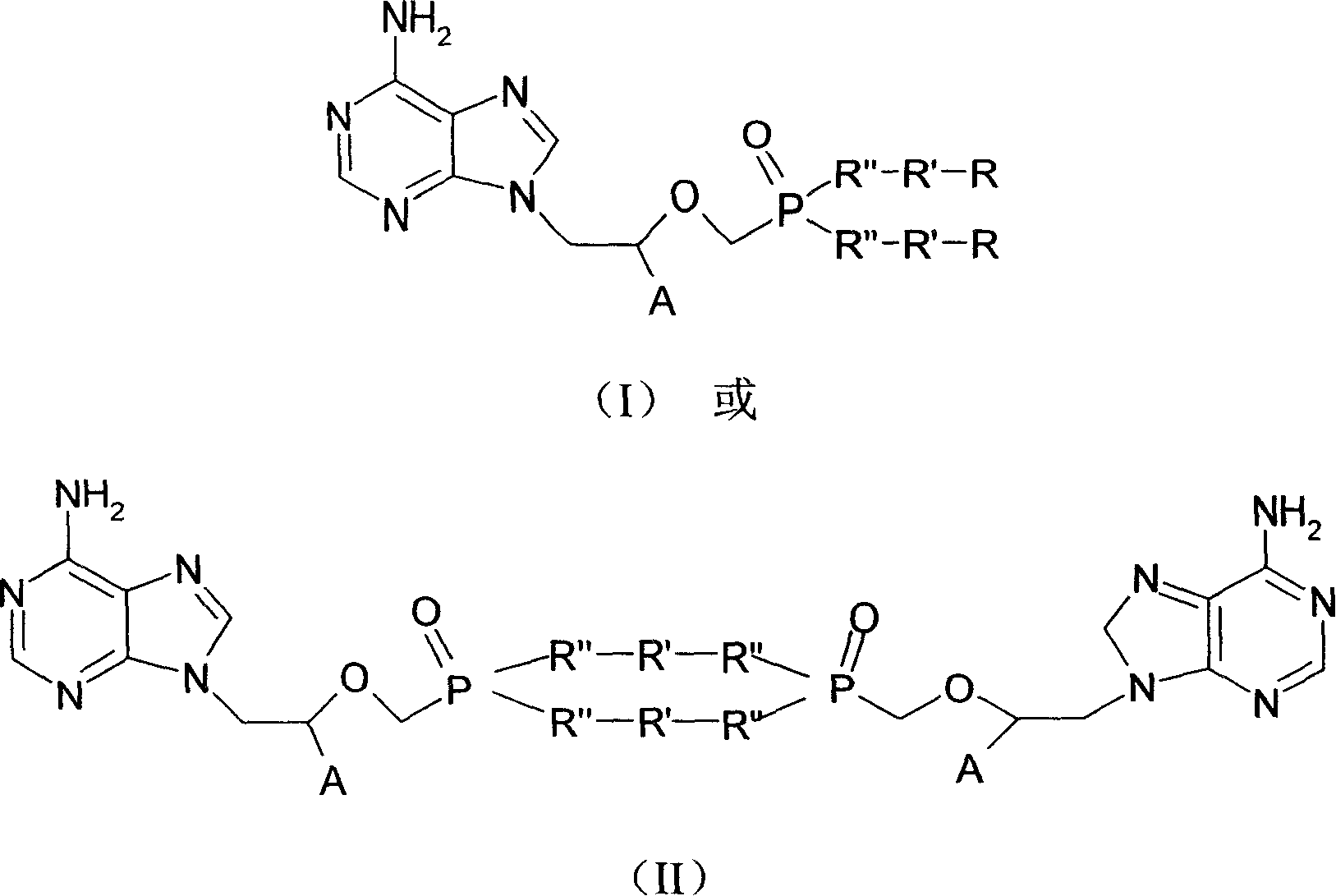
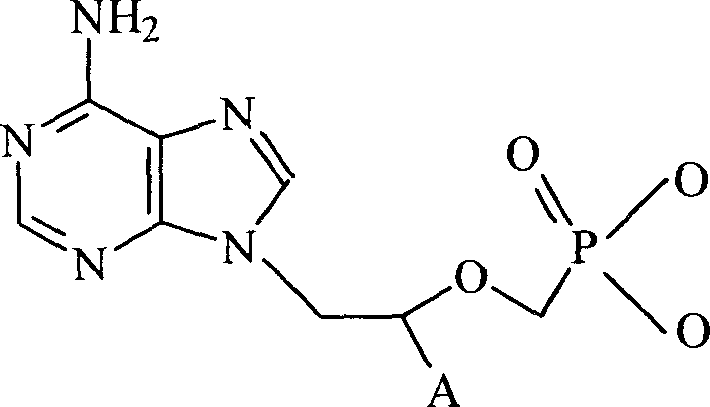
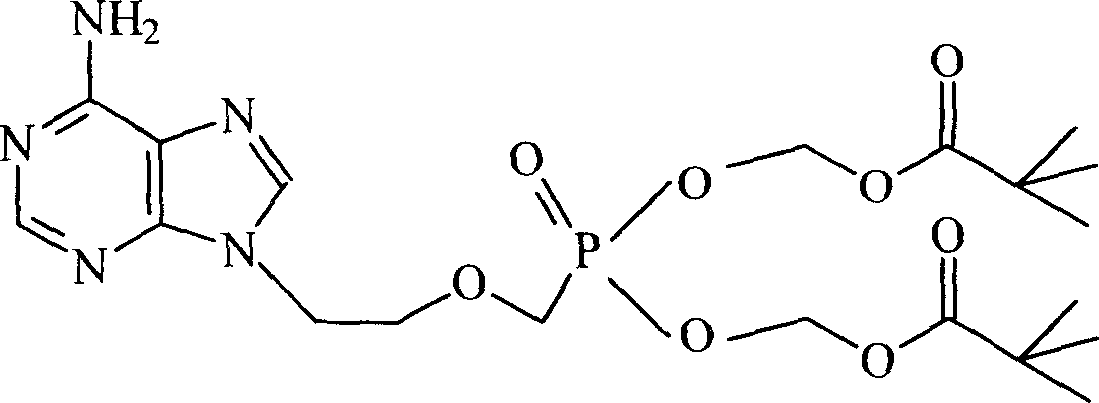
Tenofovir, adefovir and intelligent polymer conjugates and preparation and use thereof
InactiveCN101239189APromote absorptionGood treatment effectOrganic active ingredientsDigestive systemAntiviral drugSmart polymer
The present invention provides a conjugate of tenofovir, addy adefovir and intelligent polymers, a method for preparing the same, and an usage. The invention relates to the technical field that antiviral medicinal molecular tenofovir and addy adefovir are chemical modified, namely, phosphate group of tenofovir or addy adefovir is linked with X group, then X group is linked with intelligent polymers R, or phosphate group of tenofovir or addy adefovir is linked with intelligent polymers R with X function group, and a conjugate of tenofovir, addy adefovir and intelligent polymers with A-X-R-Y formula is formed. The modified tenofovir and addy adefovir forms efficient screen for raw medicine, and improves obviously medicine absorption of intestinal and absorption of cell, improves bioavailability of medicine, at the same time, reduces the kidney cleaning of medicine, and reduces the toxic for kidney, improves security.
Owner:华林 +2
Macro/Micro Duty Cycle Devices, Systems, and Methods Employing Low-Frequency Ultrasound or Other Cyclical Pressure Energies
ActiveUS20120065552A1Mitigate and avoid injuryAvoid thermal and other injuryUltrasound therapyOrgan movement/changes detectionShock waveLoad cycling
Improved devices, systems, and methods treatment of patients can be used to help mitigate injury to the kidneys by applying cyclical mechanical pressure energy at low intensities. The energy often be selectively directed from non-invasive transducers disposed outside the patients. The energy will typically comprise low frequency ultrasound energy, shock wave energy, or the like, and may induce the generation and / or release of nitric oxide, thereby enhancing perfusion and ameliorating tissue damage. Superimposed micro and macro duty cycles may help avoid thermal and other injury to tissues of the patient during treatment. Bilateral treatments are facilitated by a support structure that orients at least one transducer toward each kidney.
Owner:SONOGENIX
Diagnostic and therapeutic nanoparticles
InactiveUS20110064665A1Easy diagnosisHigh opacityHeavy metal active ingredientsOrganic active ingredientsAntibodyBiology
The present invention relates to diagnostic and therapeutic nanoparticles. More particularly, the present invention relates to creating a hybrid gold / gold sulfide nanoparticle with a chitosan matrix surrounding the metallic nanoparticle and a method for making the same. The chitosan-coated gold / gold sulfide nanoparticles can then be incorporated with additional therapeutic or diagnostic compounds such as iodine, antibodies, or other suitable compounds. The nanoparticles of the present invention have the dual capabilities of absorbing near infrared wavelength light to (1) act as a therapeutic agent by generating heat energy effective for cell ablation or for release of therapeutic compounds embedded in the chitosan matrix and (2) creating diagnostic benefit by incorporation of X-ray or MRI contrast agents.
Owner:UNIV OF LOUISVILLE FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com