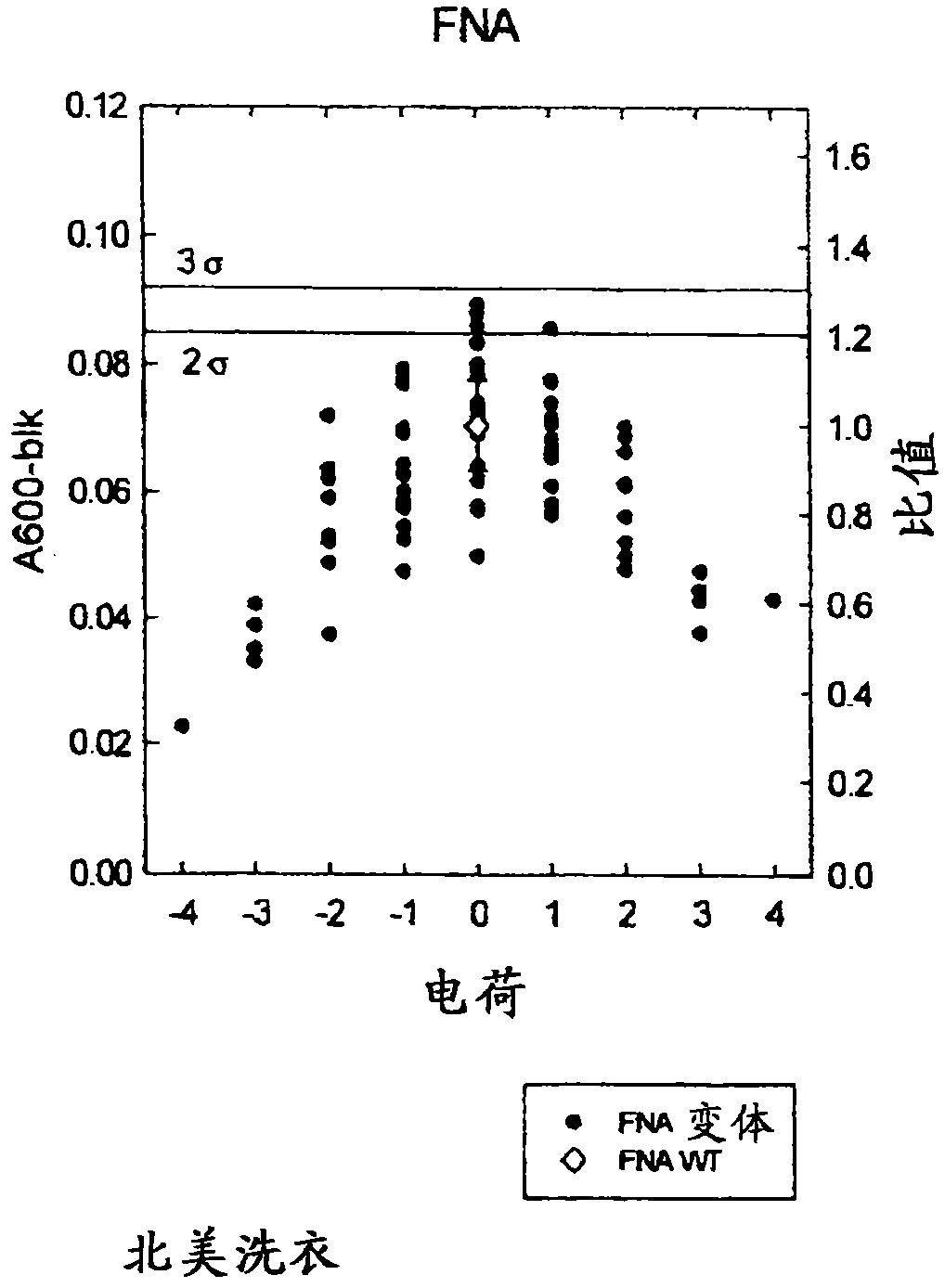
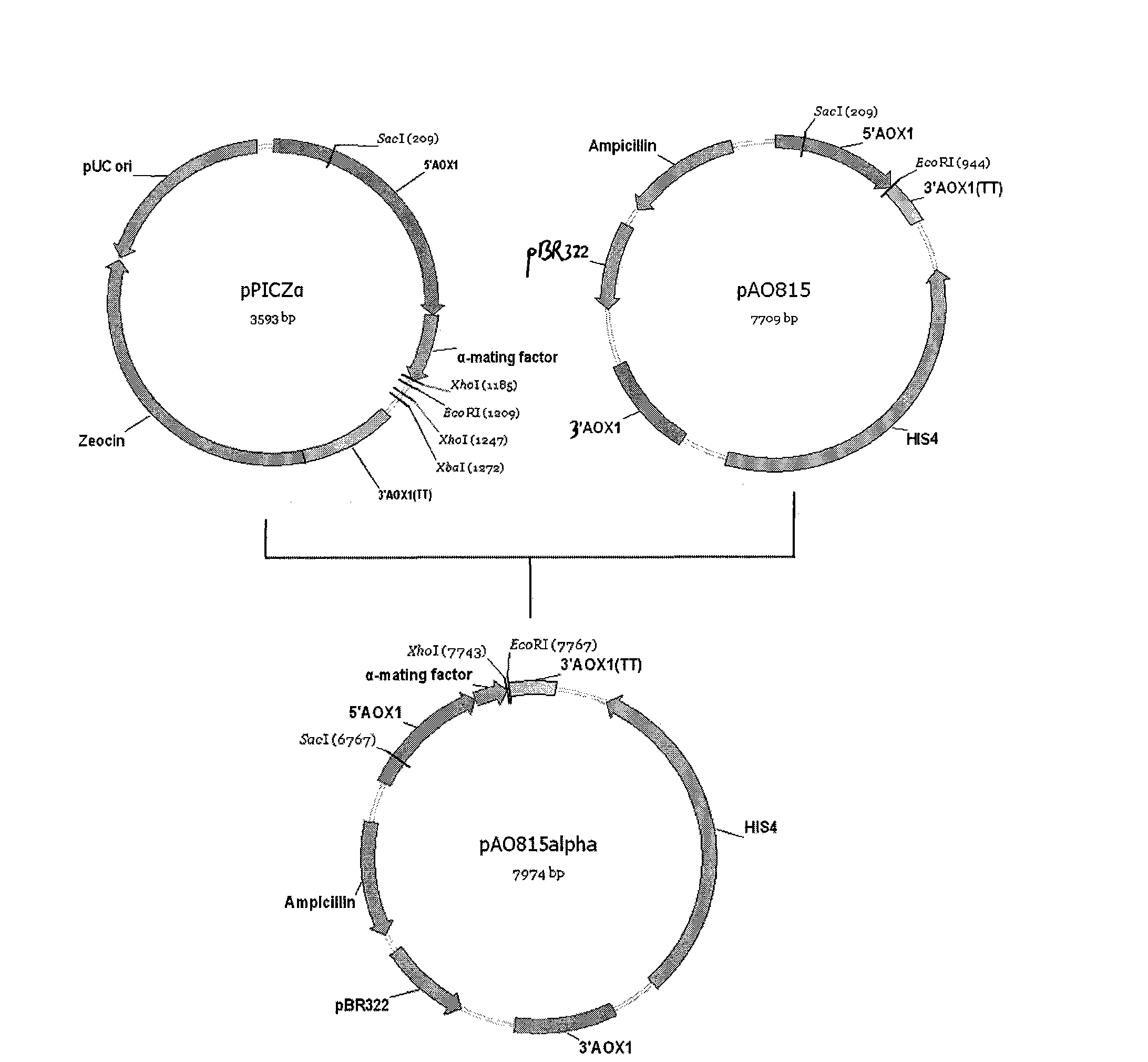
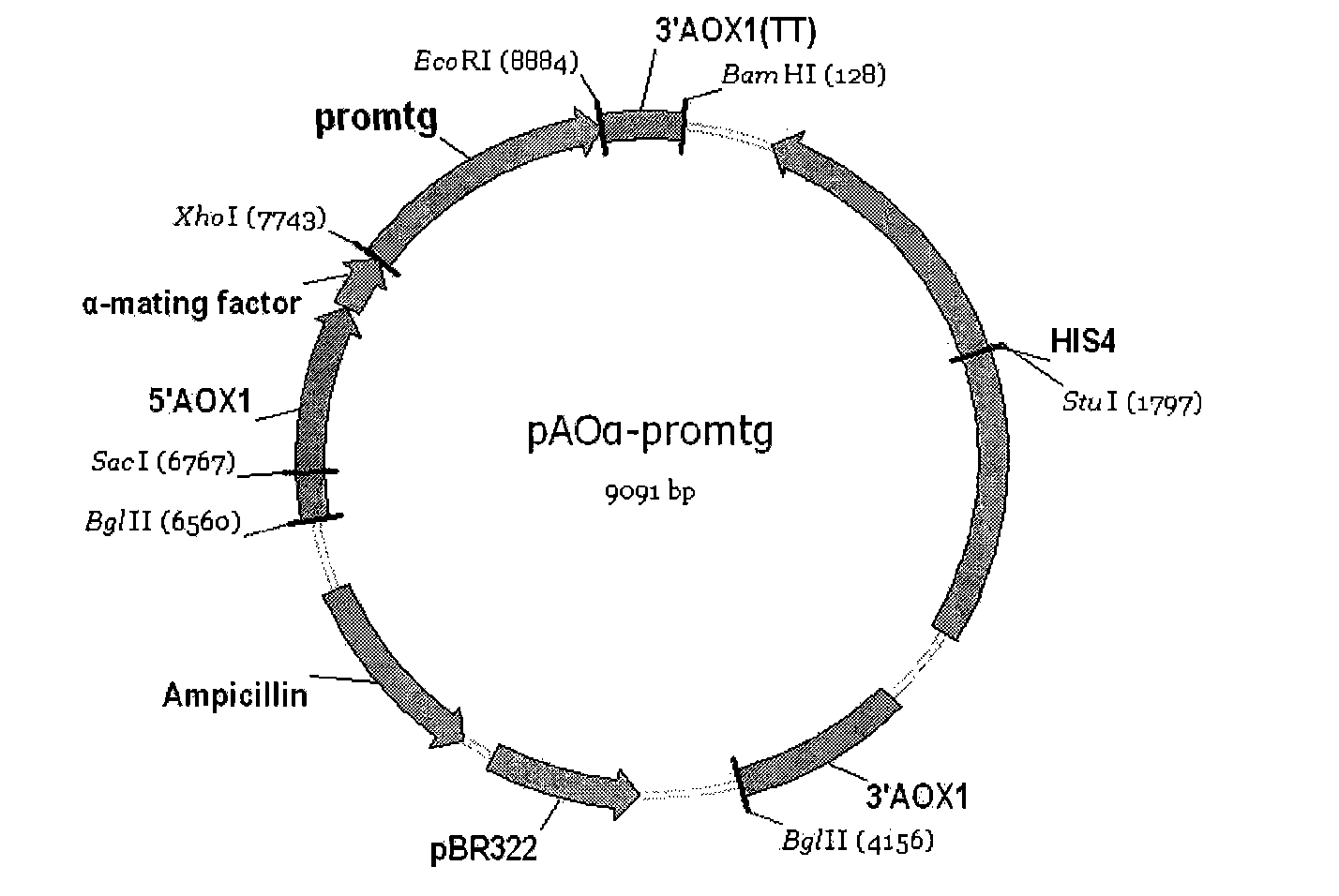
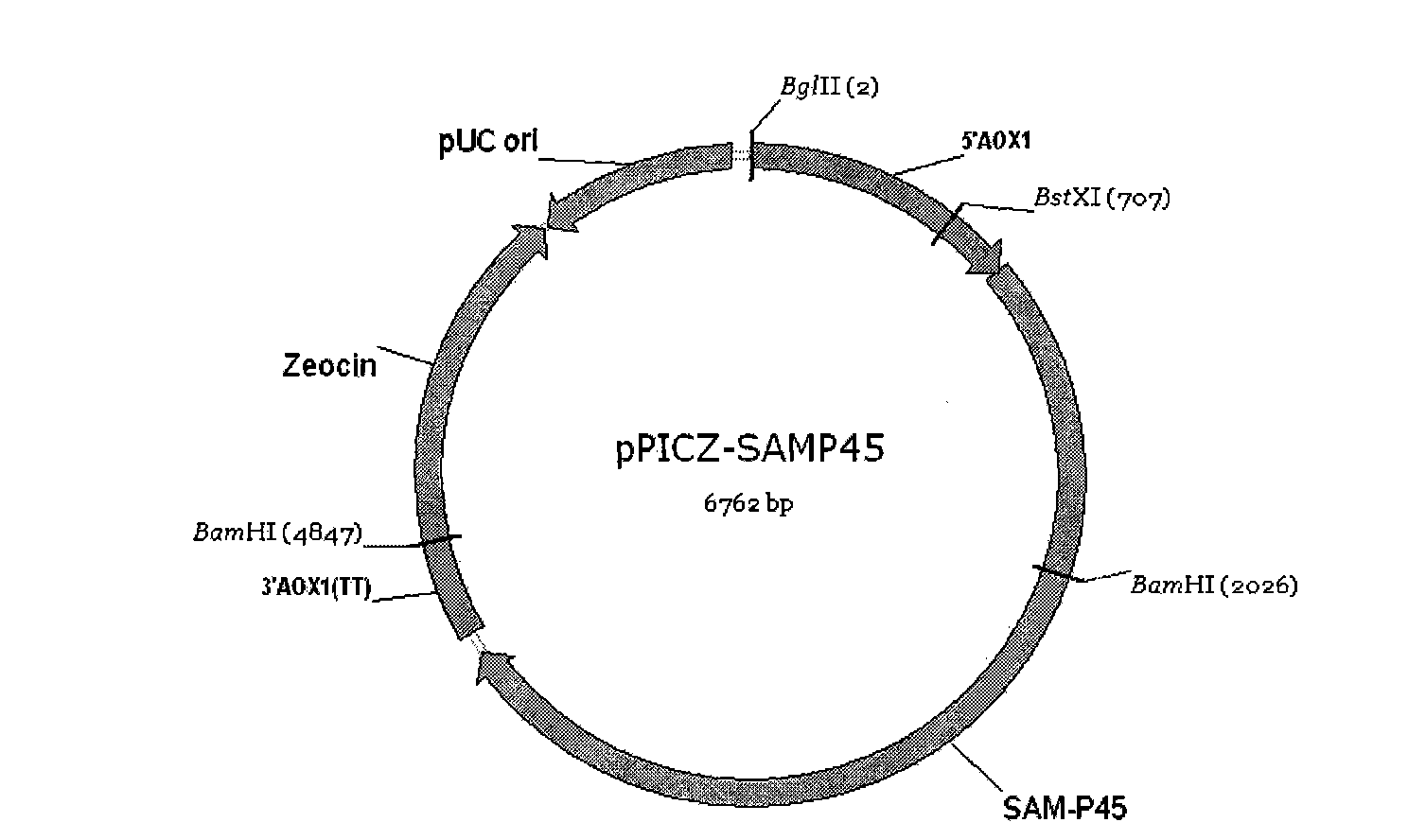
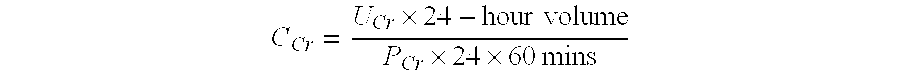Patents
Literature
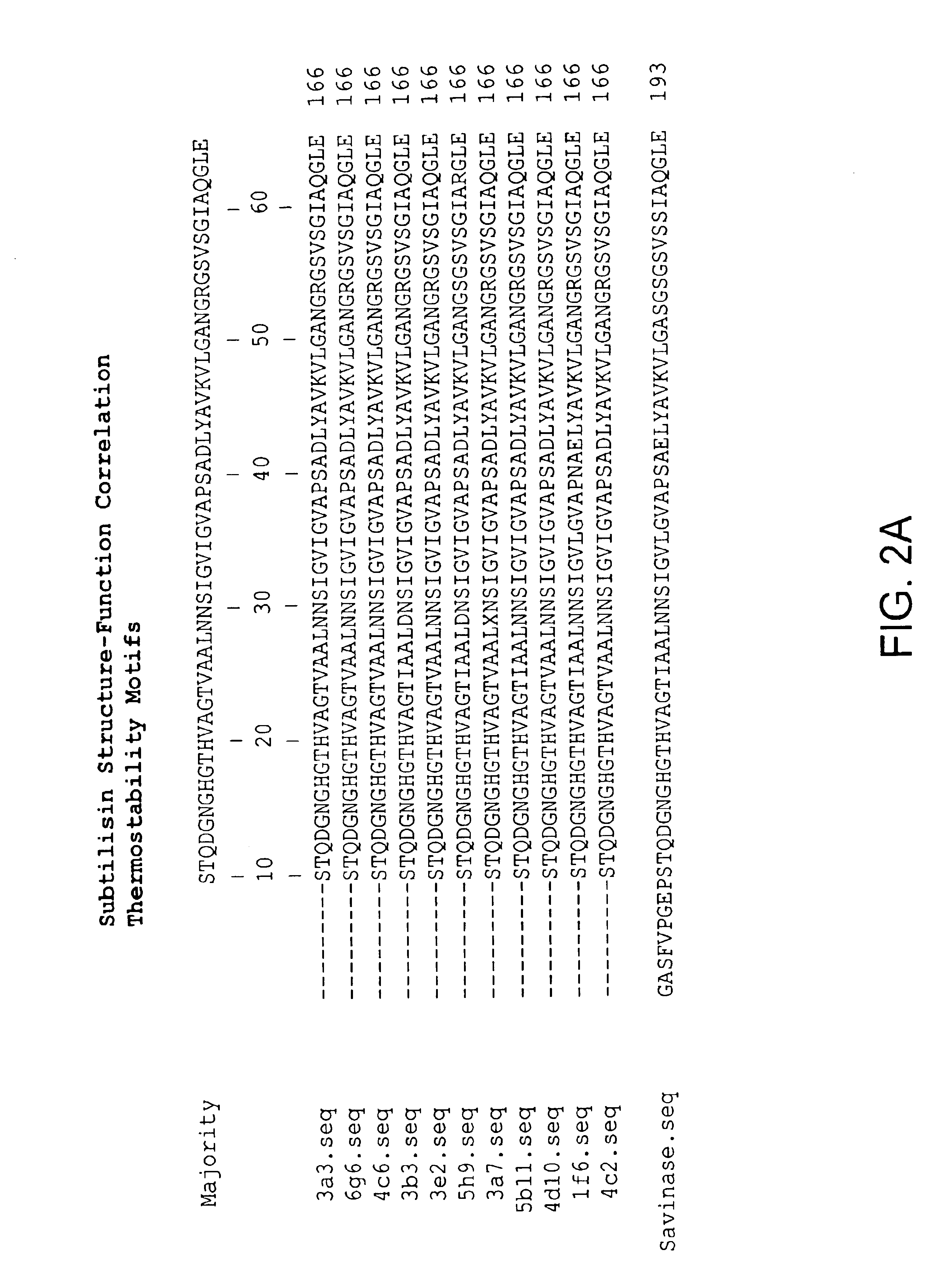
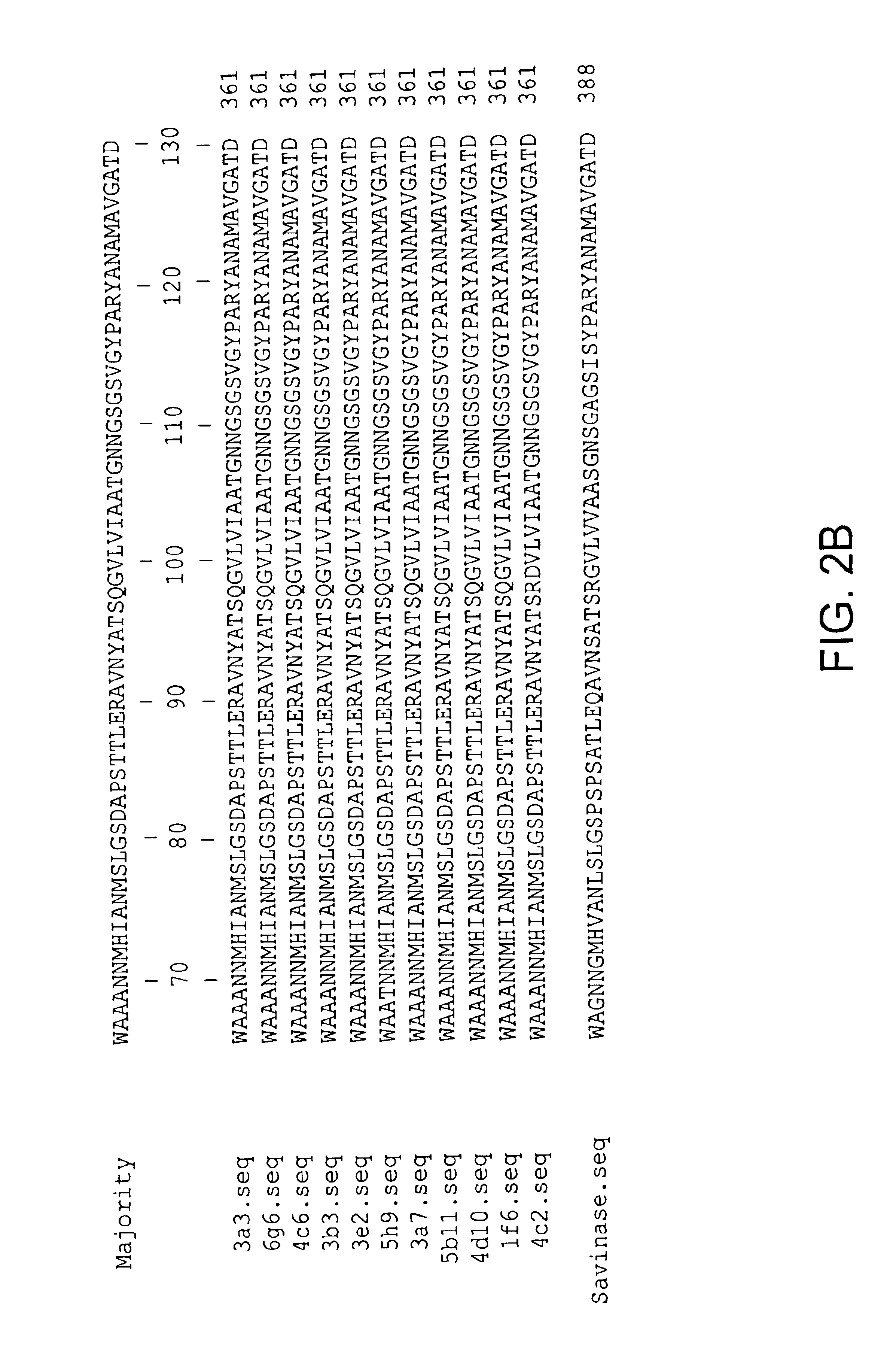
81 results about "Subtilisins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A family of SERINE ENDOPEPTIDASES isolated from Bacillus subtilis. EC 3.4.21.-
Subtilisin variants
New subtilisin homologues (both nucleic acids and proteins) are provided. Compositions which include these new proteins, recombinant cells, shuffling methods involving the new homologues, antibodies to the new homologues, and methods of using the homologues are also provided.
Owner:NOVOZYMES AS +1
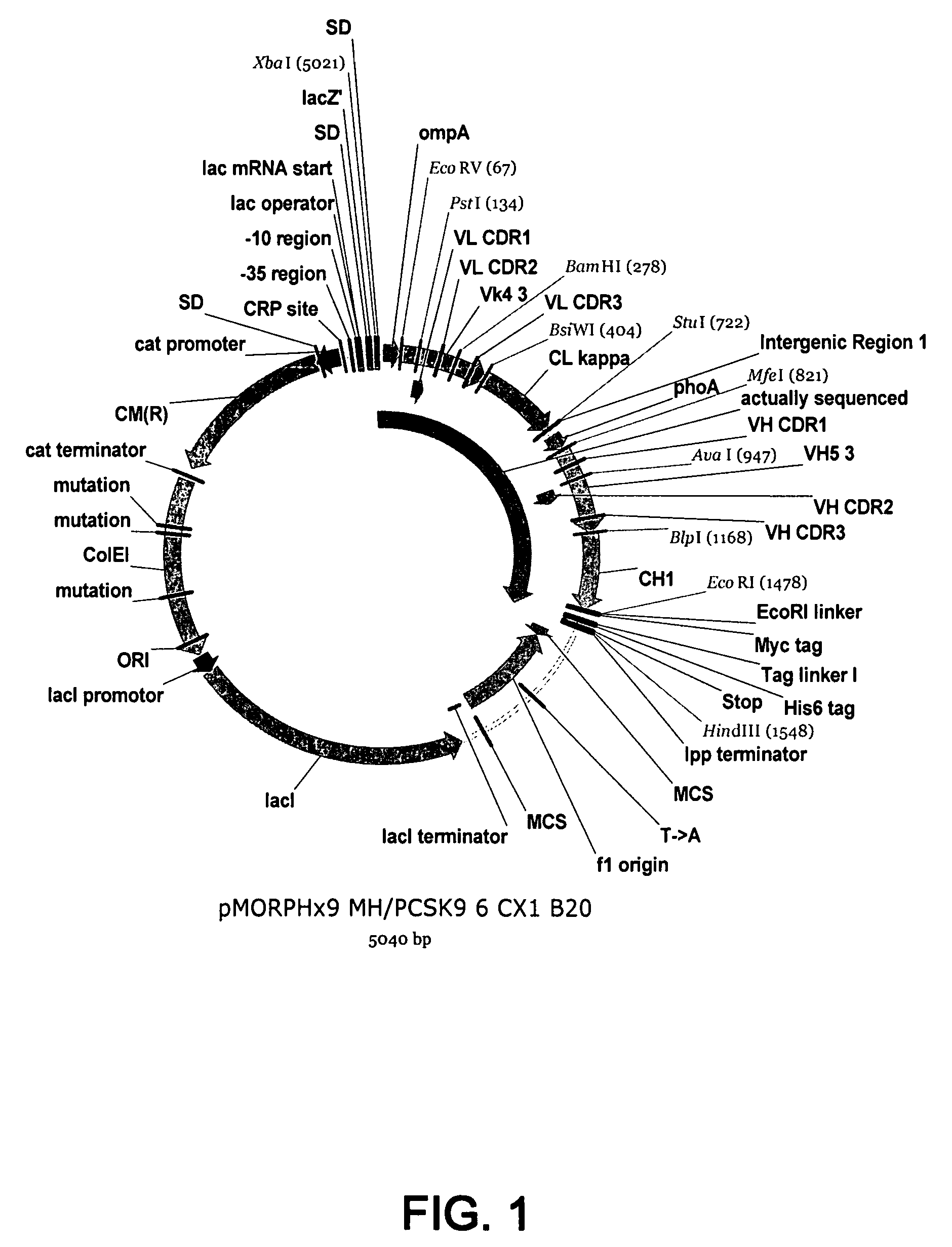
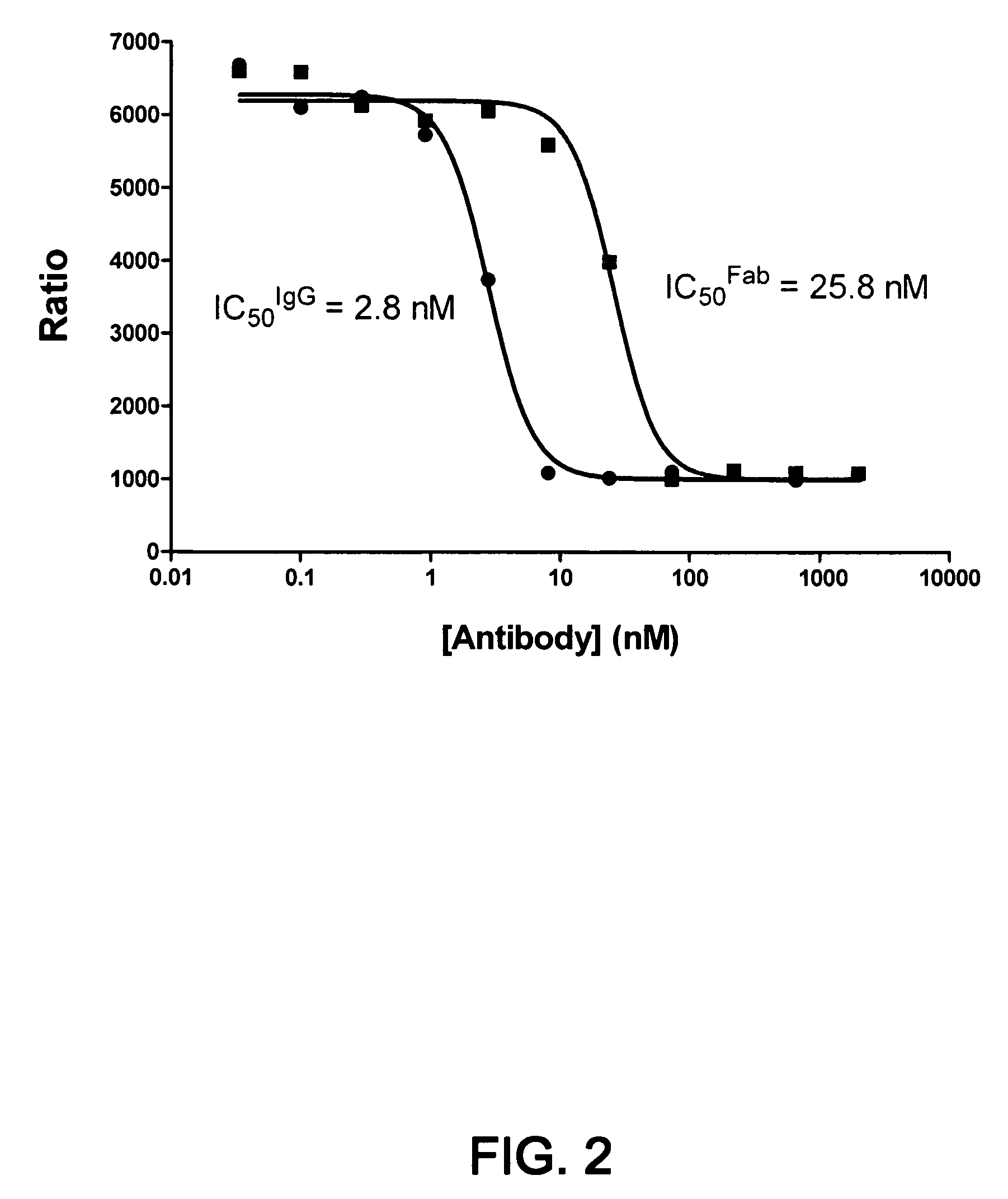
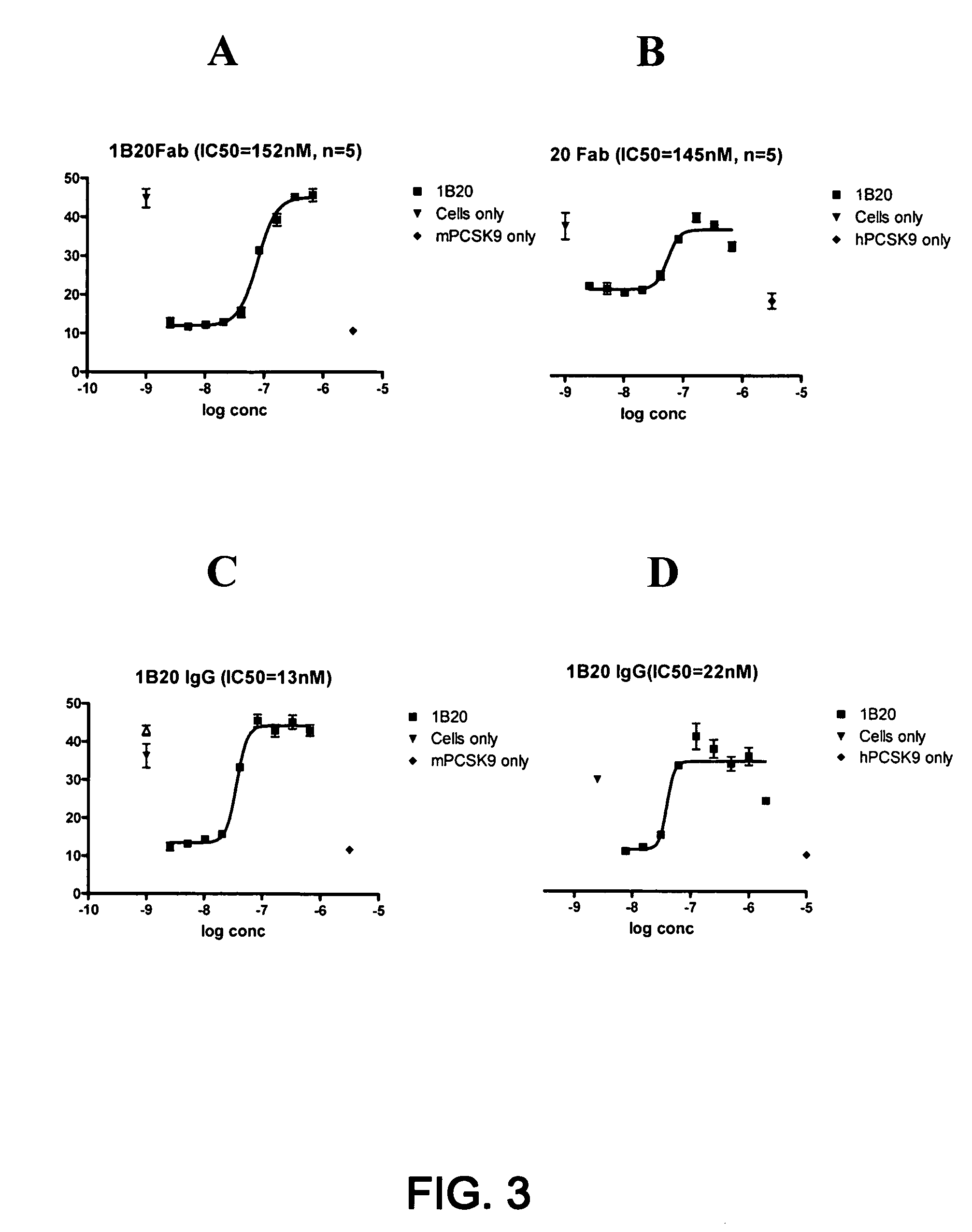
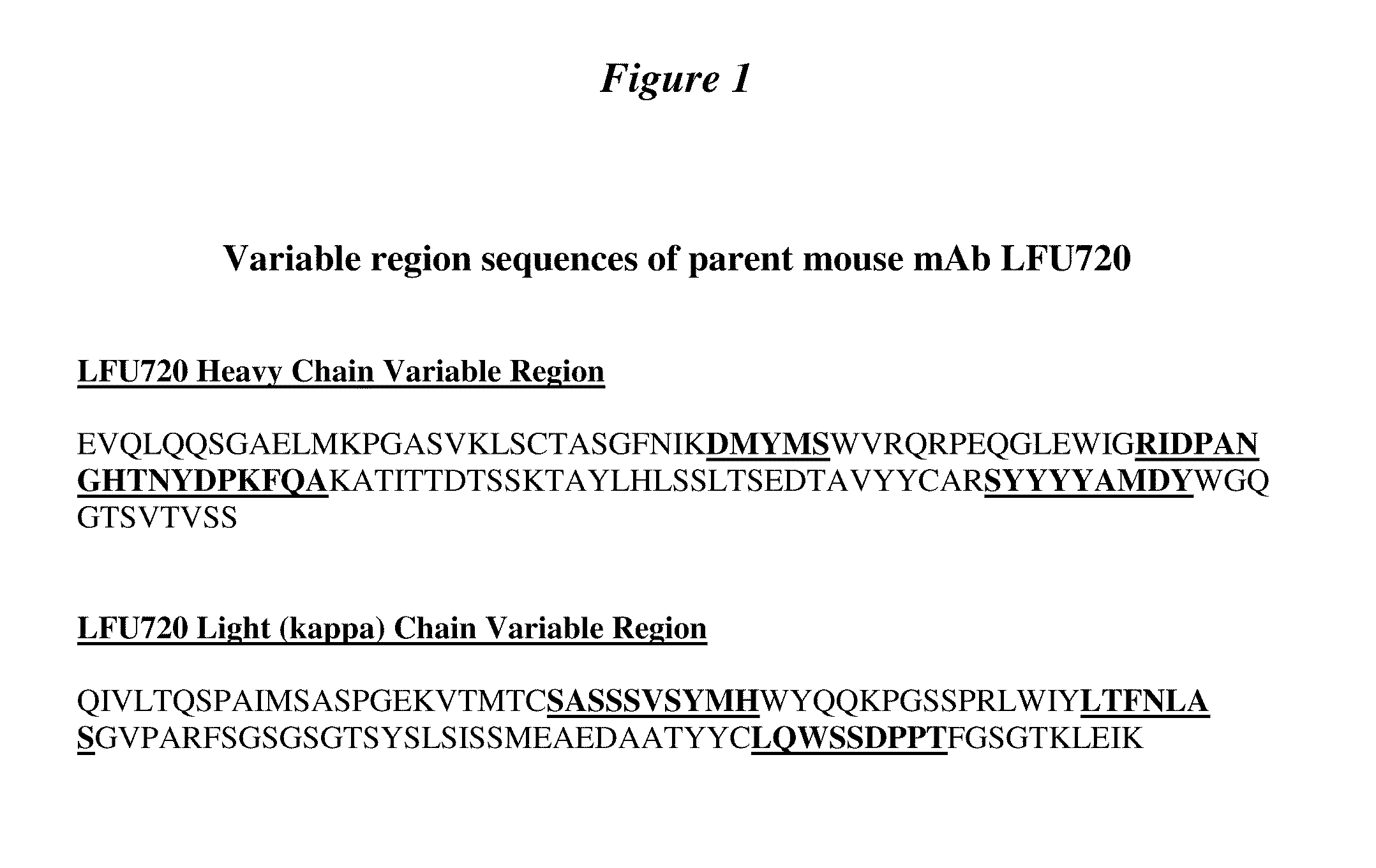
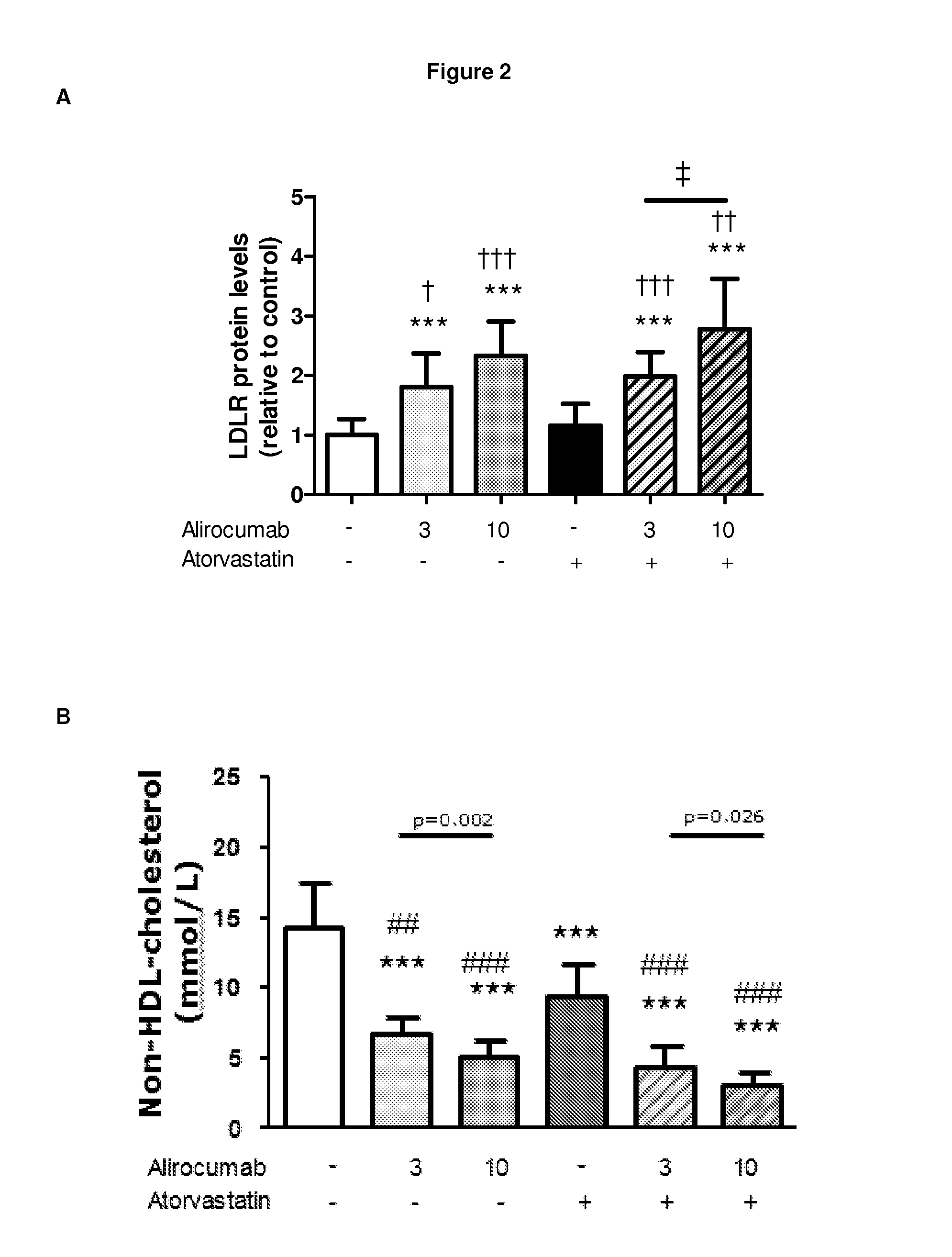
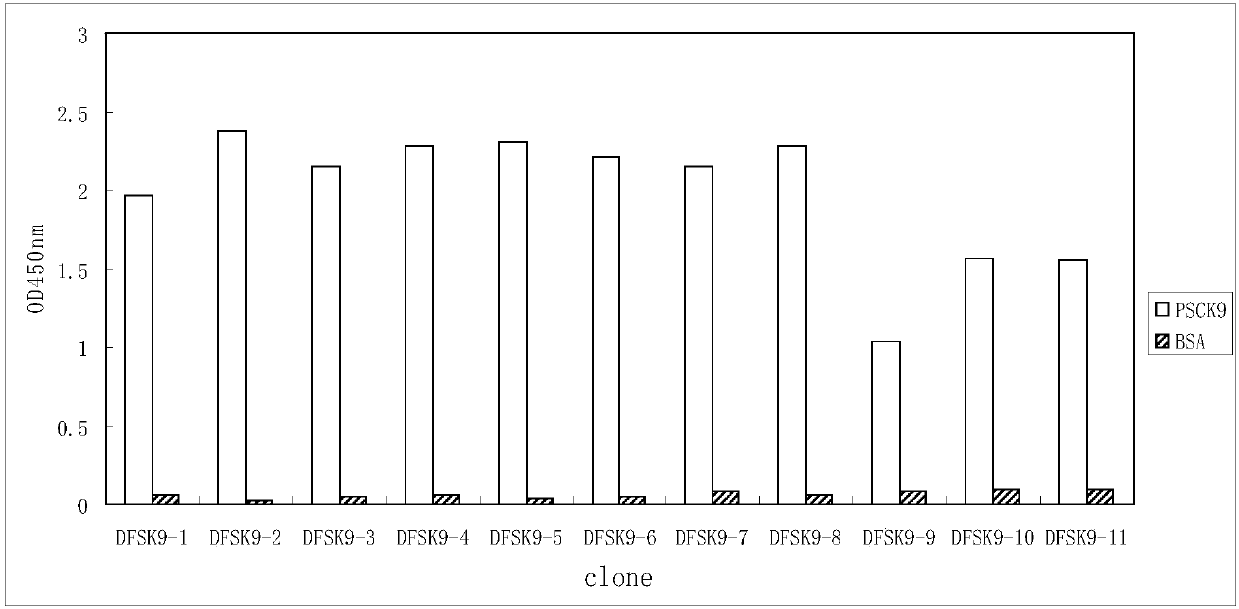
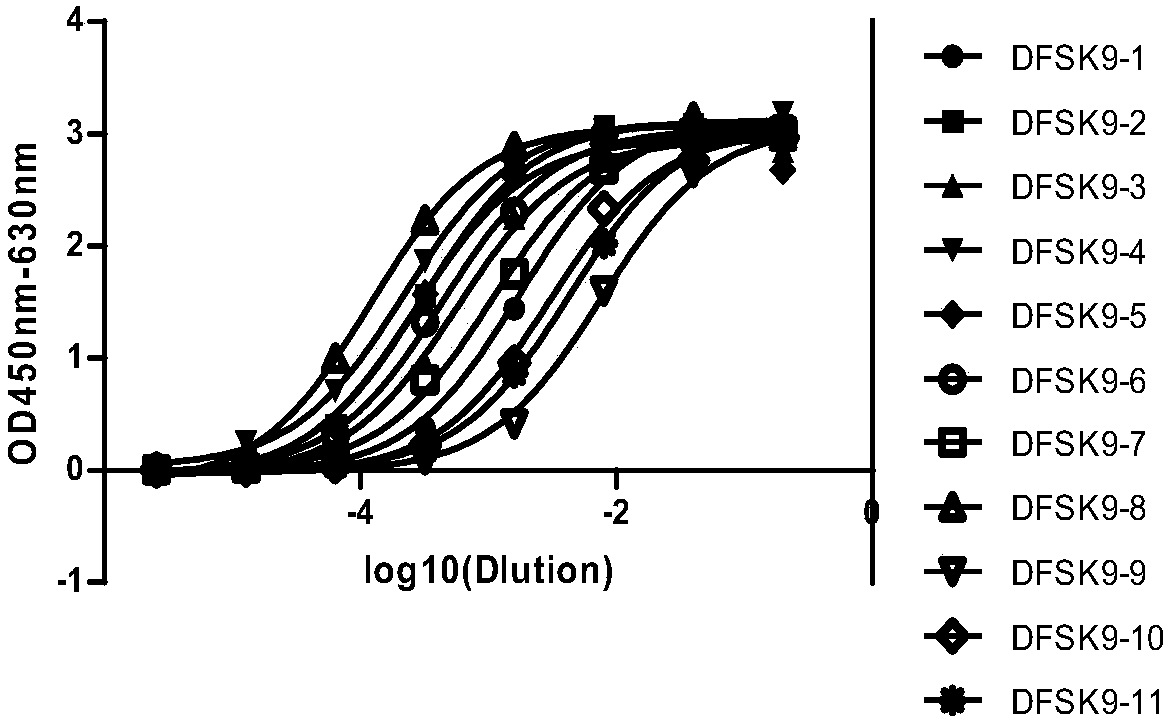
1B20 PCSK9 antagonists
Antagonists of human proprotein convertase subtilisin-kexin type 9 (“PCSK9”) are disclosed. The disclosed antagonists are effective in the inhibition of PCSK9 function and, accordingly, present desirable antagonists for use in the treatment of conditions associated with PCSK9 activity. The present invention also discloses nucleic acid encoding said antagonists, vectors, host cells, and compositions comprising the antagonists. Methods of making PCSK9-specific antagonists as well as methods of using the antagonists for inhibiting or antagonizing PCSK9 function are also disclosed and form important additional aspects of the present disclosure.
Owner:MERCK SHARP & DOHME LLC
Pcsk9 antagonists
ActiveUS20110142849A1Reduce severitySufficient amountPeptide/protein ingredientsGenetic material ingredientsKexinSubtilisins
The present invention provides antibody antagonists against proprotein convertase subtilisin / kexin type 9a (“PCSK9”) and methods of using such antibodies.
Owner:NOVARTIS AG
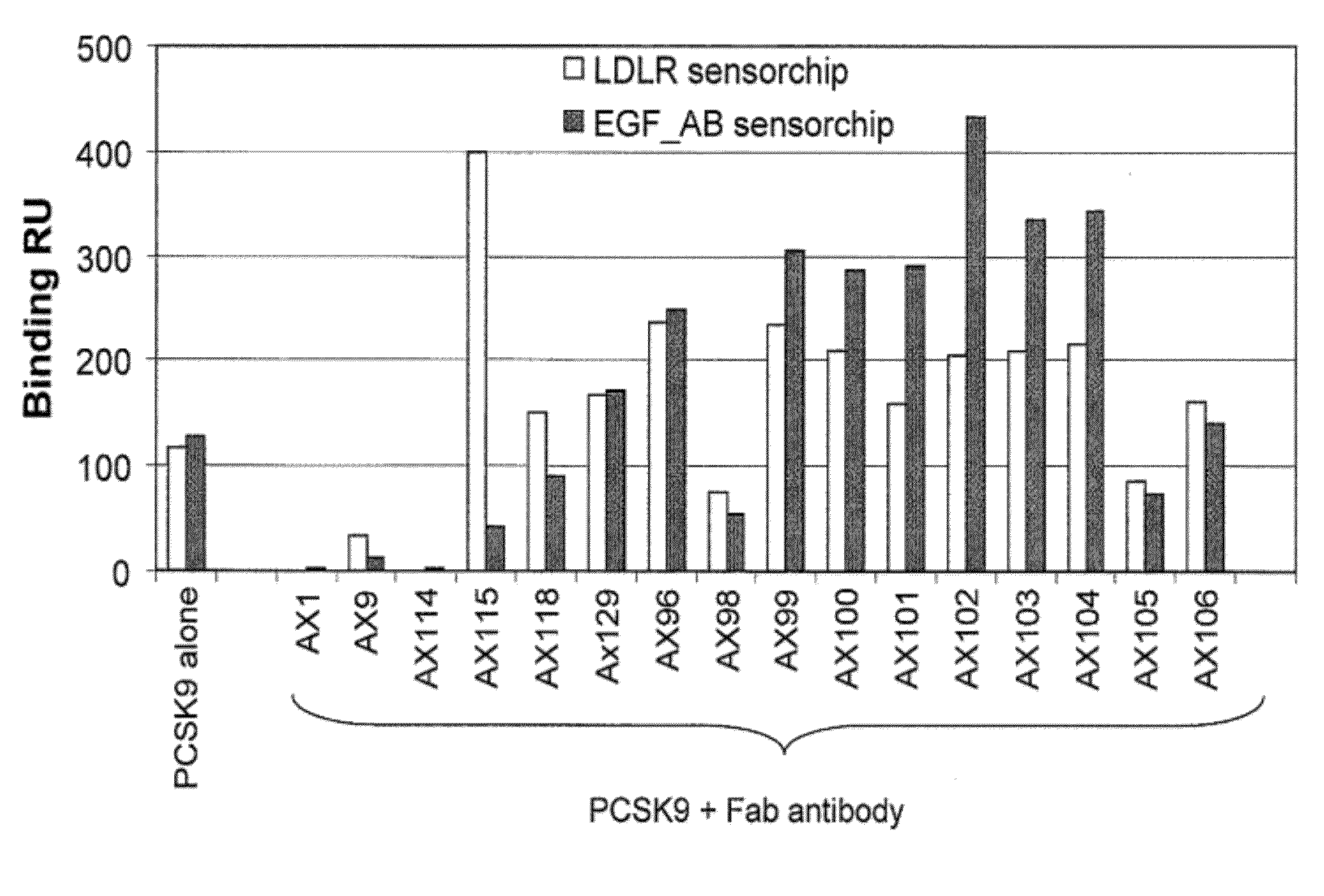
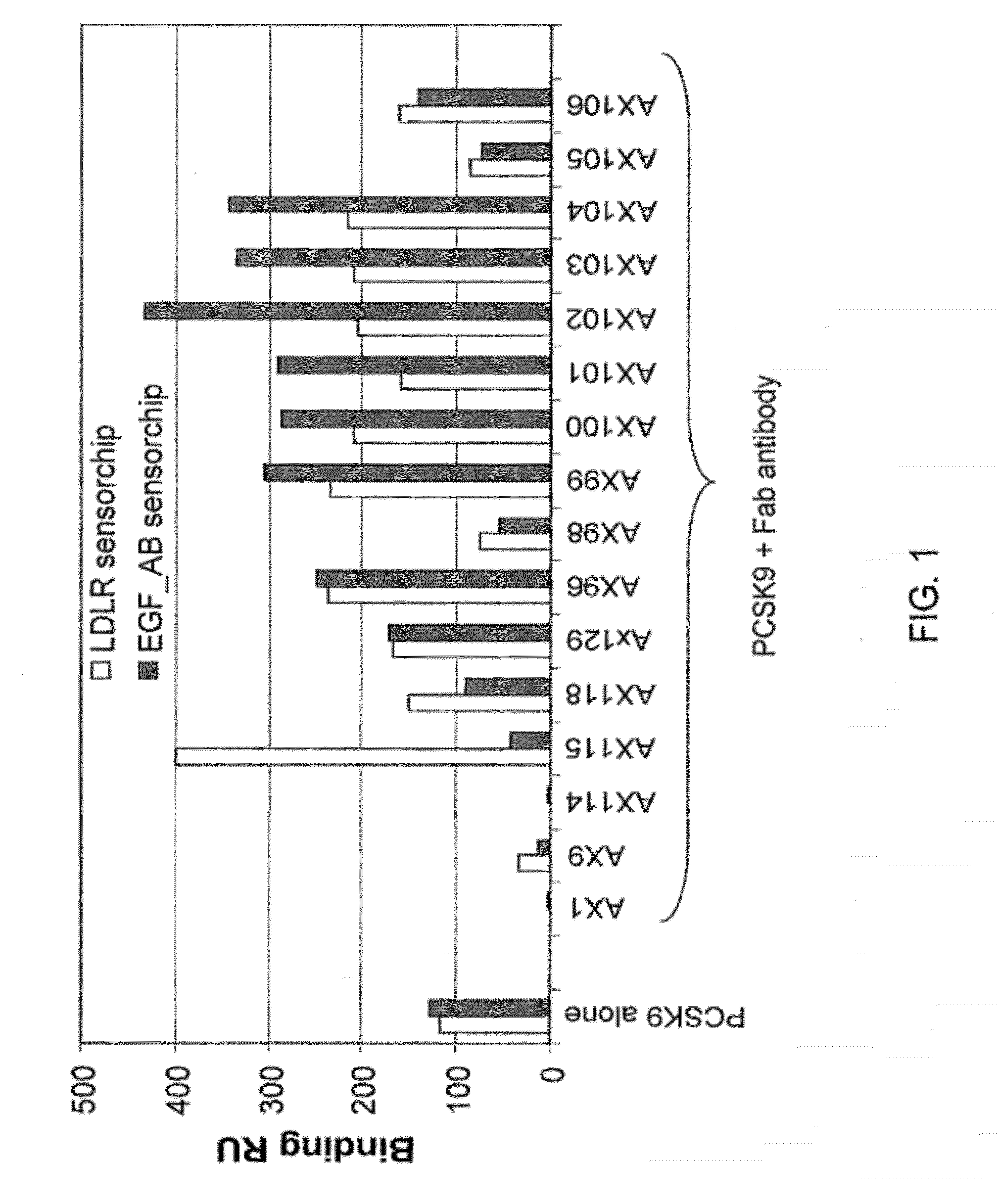
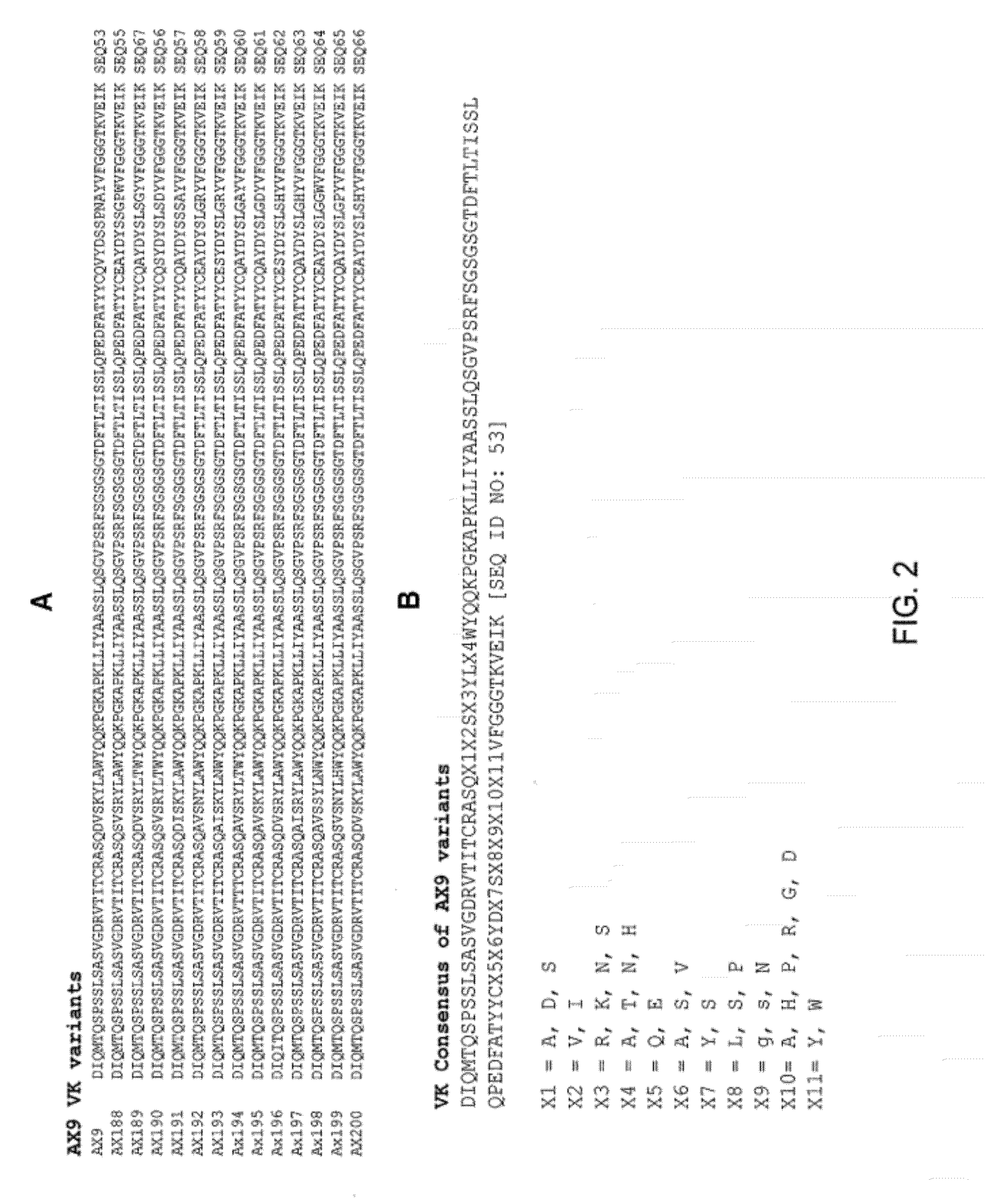
Ax1 and ax189 psck9 antagonists and variants
Antagonists of human proprotein convertase subtilisin-kexin type 9 (“PCSK9”) are disclosed. The disclosed antagonists are effective in the inhibition of PCSK9 function and, accordingly, present desirable antagonists for use in the treatment of conditions associated with PCSK9 activity. The present invention also discloses nucleic acid encoding said antagonists, vectors, host cells, and compositions comprising the antagonists. Methods of making PCSK9-specific antagonists as well as methods of using the antagonists for inhibiting or antagonizing PCSK9 function are also disclosed and form important additional aspects of the present disclosure.
Owner:MERCK SHARP & DOHME LLC
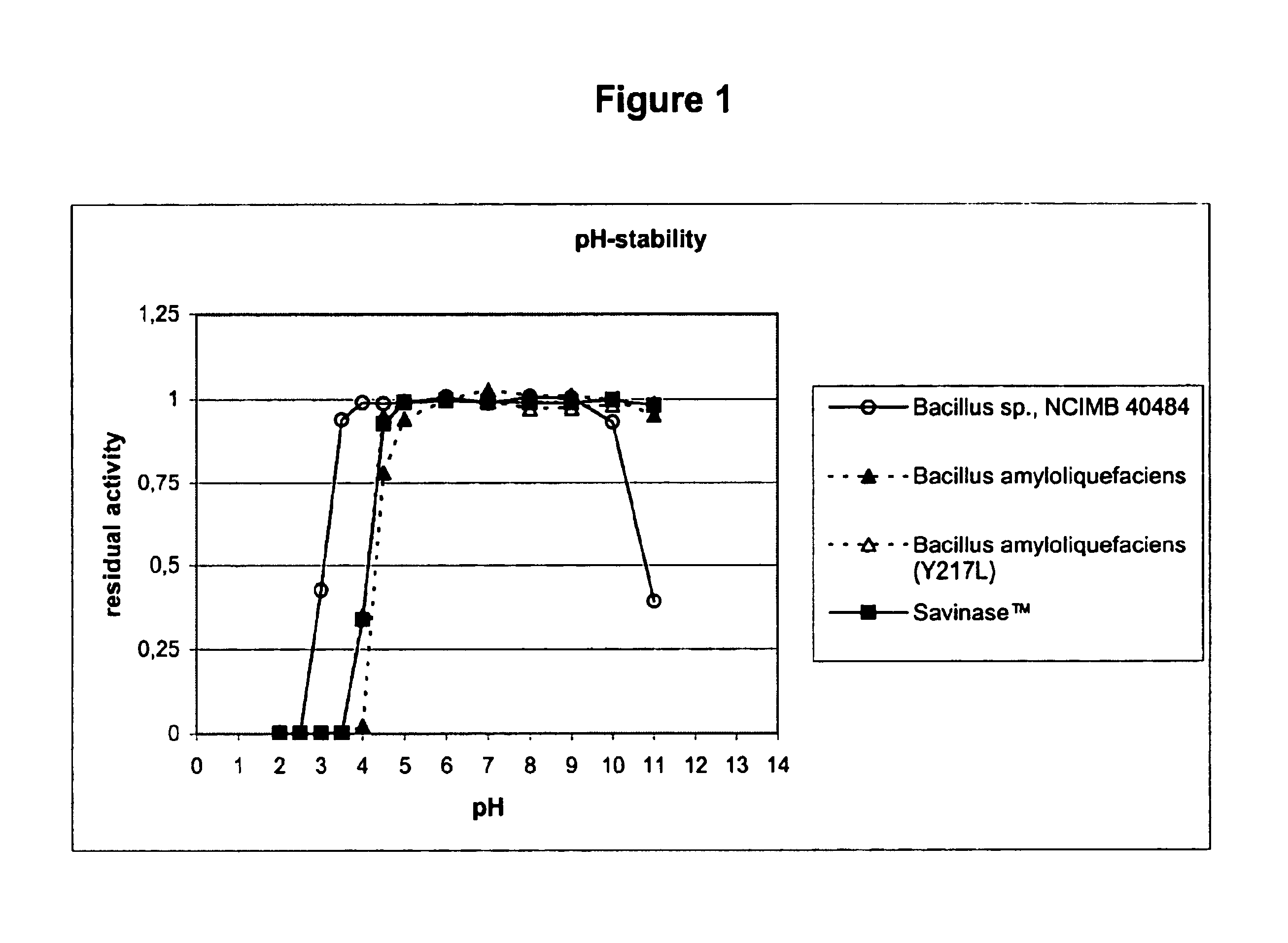
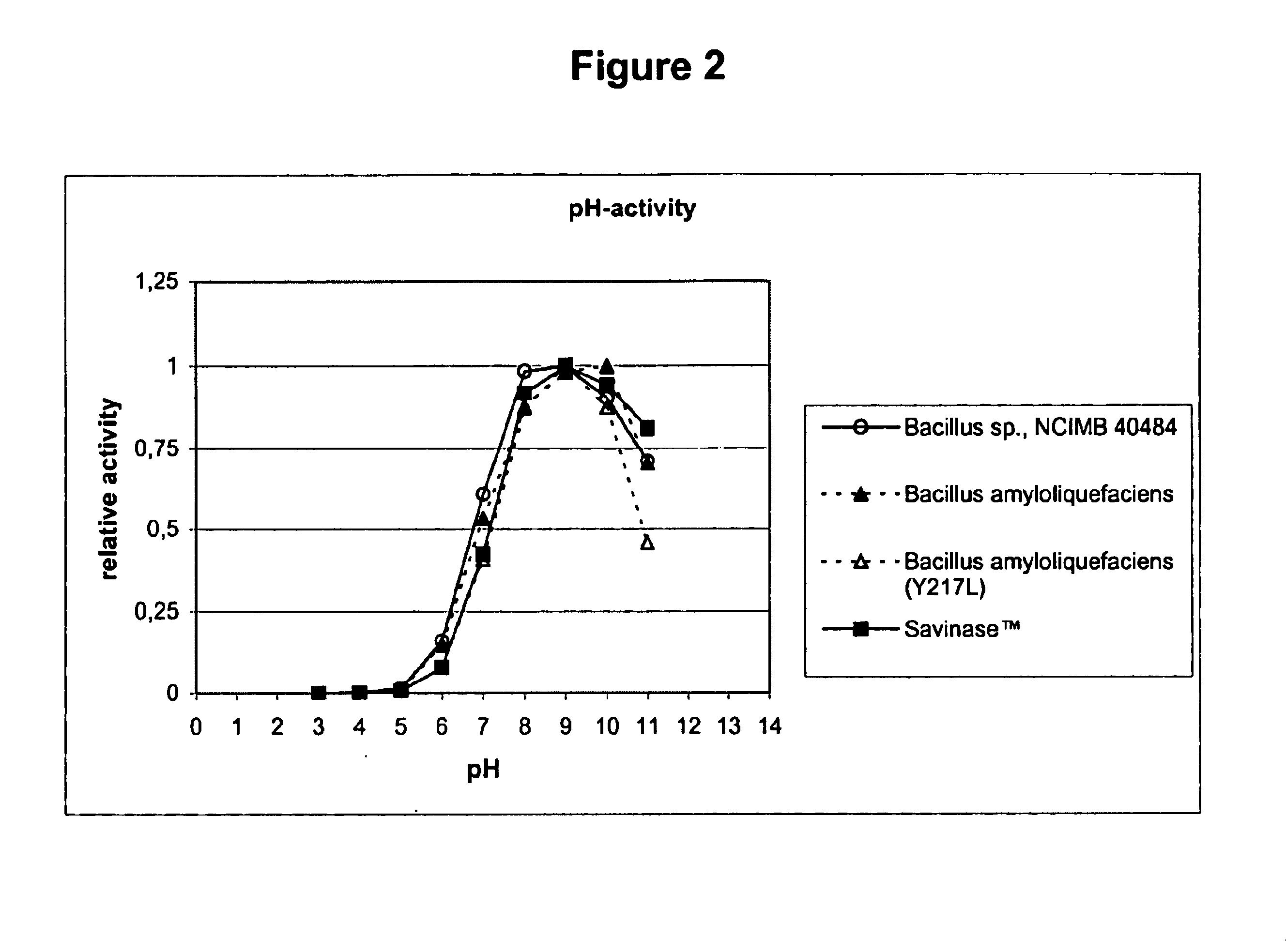
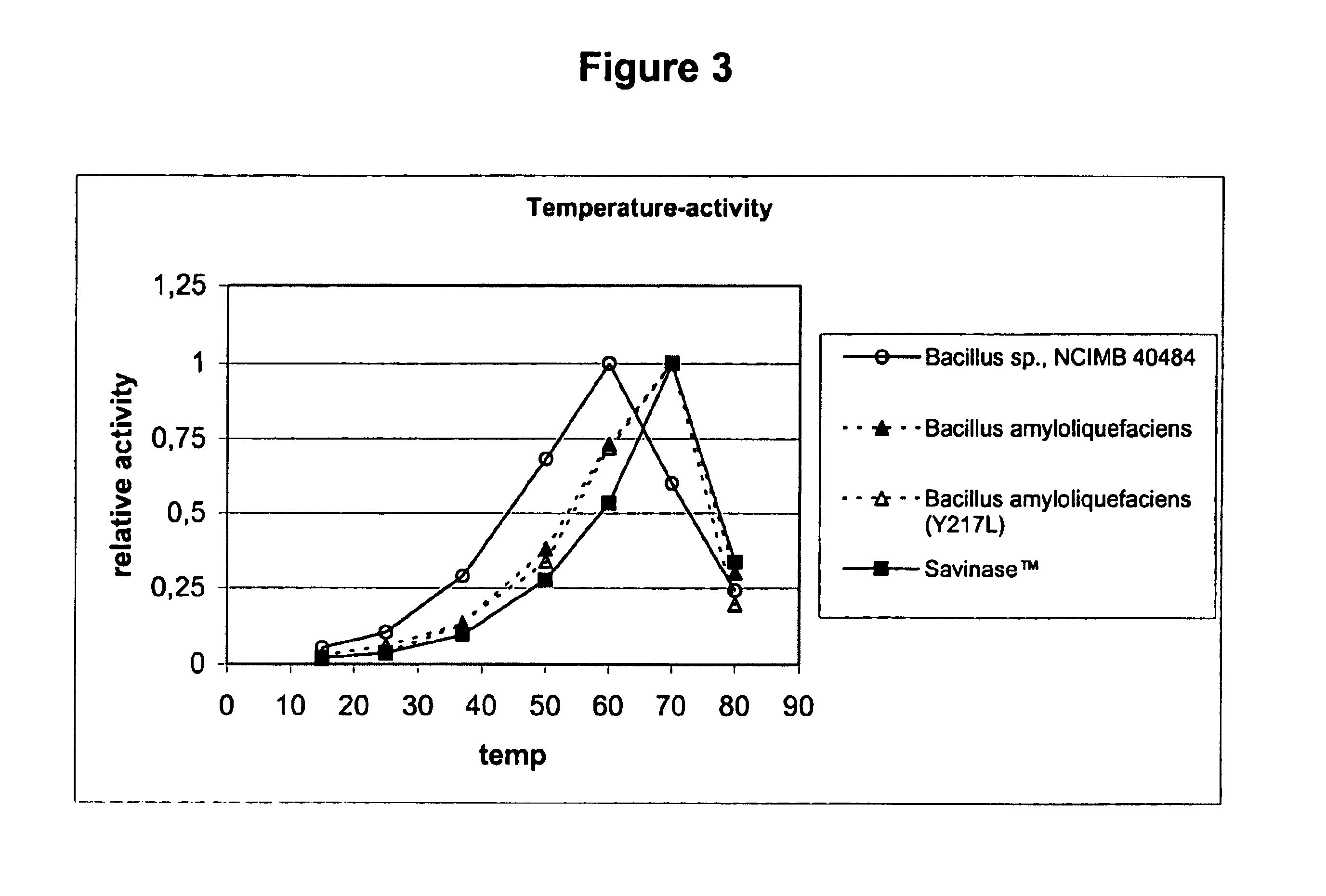
Use of acid-stable subtilisin proteases in animal feed
Acid-stable proteases of the subtilisin family, their use in animal feed, feed-additives and feed compositions containing such proteases, and methods for the treatment of vegetable proteins using such proteases.
Owner:DSM IP ASSETS BV
Calcium free subtilisin mutants
InactiveUS6541234B1Improve thermal stabilityImprove robustnessBacteriaSugar derivativesSubtilisinMutant
Novel calcium free subtilisin mutants are taught, in particular subtilisins which have been mutated to eliminate amino acids 75-83 and which retain enzymatic activity and stability. Recombinant methods for producing same and recombinant DNA encoding for such subtilisin mutants are also provided.
Owner:MARYLAND UNIV OF
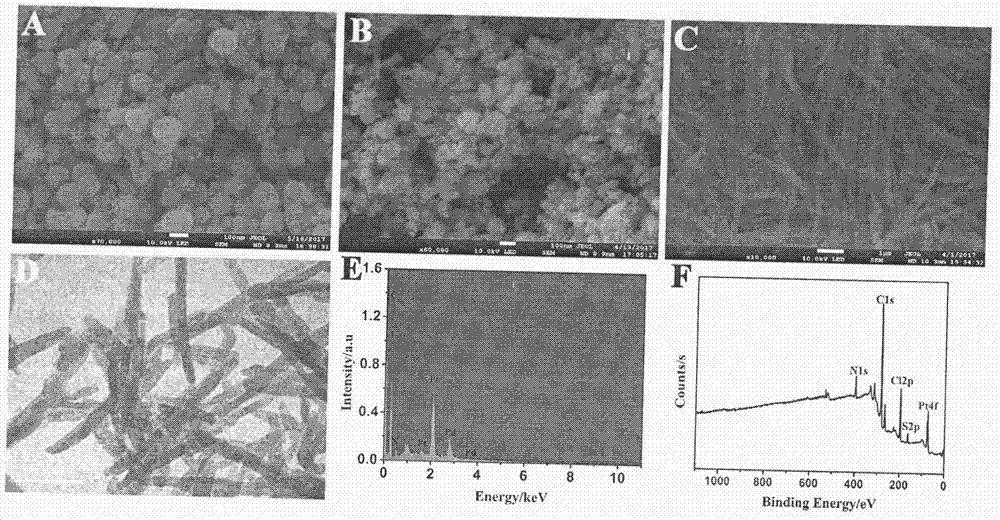
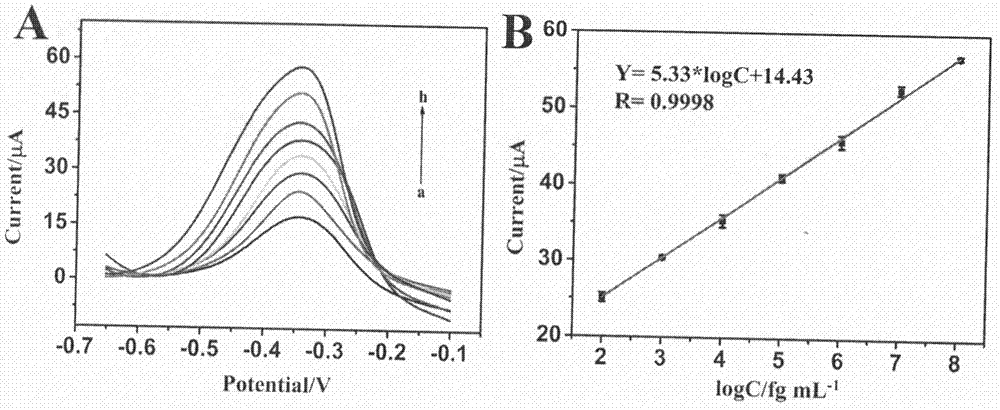
Method for preparing electrochemical immunosensor for pcsk9 protein detection
InactiveCN107389949AImprove conductivityHigh sensitivityBiological testingMaterial electrochemical variablesProtein detectionStaphylococcus aureus
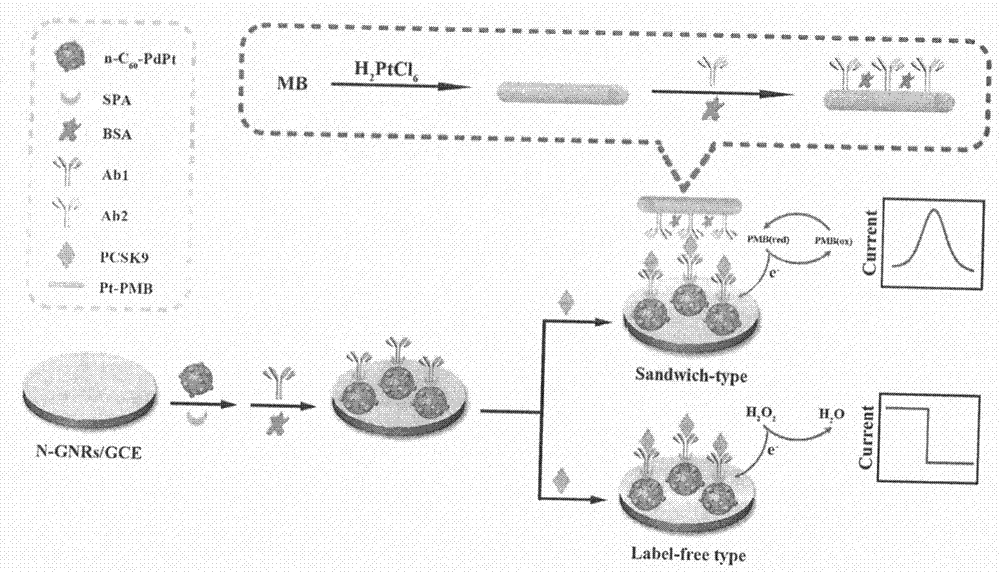
The invention relates to a preparation method and application of an electrochemical immunosensor for a biomarker, namely a recombinant proprotein convertase subtilisin kexin type 9 (pcsk9) protein for predicting and diagnosing cardiovascular diseases, and belongs to the technical field of electrochemical detection. The preparation method is characterized by comprising the following steps: firstly, performing nitrogen doping on a graphene nanobelt (n-gnrs), performing amination on a fullerene-palladium-platinum nanoparticle (n-C60 / pdpt) composite material and a staphylococcus aureus a protein (spa), and performing layer-by-layer self-assembling so as to immobilize a pcsk9 antibody (ab1); mixing the synthesized nanoparticle-poly-methylene blue (pt-pmb) with the pcsk9 antibody (ab2), and preparing a nano beacon, thereby obtaining the electrochemical immunosensor for pcsk9 protein detection. The electrochemical immunosensor has the advantages of being high in sensitivity, good in specificity and rapid and convenient in detection. A novel method for pcsk9 protein detection is provided, and useful information is provided for clinical prediction and diagnosis on cardiovascular diseases.
Owner:CHONGQING MEDICAL UNIVERSITY
Methods of lowering proprotein conversate subtilisin/kexin type 9 (PCSK9)
InactiveUS20140093513A1Convenient treatmentReduce plasma cholesterol levelBiocideSenses disorderSubtilisinsProprotein Convertase Inhibitors
The invention relates to new methods of modulating cholesterol by inhibiting proprotein convertase subtilisin / kexin type 9 (PCSK9) with fatty acid derivatives; and new methods for treating or preventing a metabolic disease comprising the administration of an effective amount of a fatty acid derivative. The present invention is also directed to fatty acid bioative derivatives and their use in the treatment of metabolic diseases.
Owner:CATABASIS PHARMA
Antagonists of pcsk9
Antagonists of human proprotein convertase subtilisin-kexin type 9 ('PCSK9') are disclosed. The disclosed antagonists are effective in the inhibition of PCSK9 function and, accordingly, present desirable antagonists for the use in the treatment of conditions associated with PCSK9 activity. The present invention also discloses nucleic acid encoding said antagonists, vectors, host cells, and compositions comprising the antagonists. Methods of making PCSK9-specific antagonists as well as methods of using the antagonists for inhibiting or antagonizing PCSK9 function are also disclosed and form important additional aspects of the present disclosure.
Owner:SCHERING AG
Methods for producing polypeptidies in protease-deficient mutants of trichoderma
The present invention relates to mutants of a parent Trichoderma strain, comprising a polynucleotide encoding a polypeptide and one or more (several) genes selected from the group consisting of a first subtilisin-like serine protease gene, a first aspartic protease gene, a trypsin-like serine protease gene, a second subtilisin-like serine protease gene, and a second aspartic protease gene, wherein the one or more (several) genes are modified rendering the mutant strain deficient in the production of one or more (several) enzymes selected from the group consisting of a first subtilisin-like serine protease, a first aspartic protease, a trypsin-like serine protease, a second subtilisin-like serine protease, and a second aspartic protease, respectively, compared to the parent Trichoderma strain when cultivated under identical conditions. The present invention also relates to methods of producing a polypeptide in such mutants and methods for producing such mutants.
Owner:NOVOZYMES INC
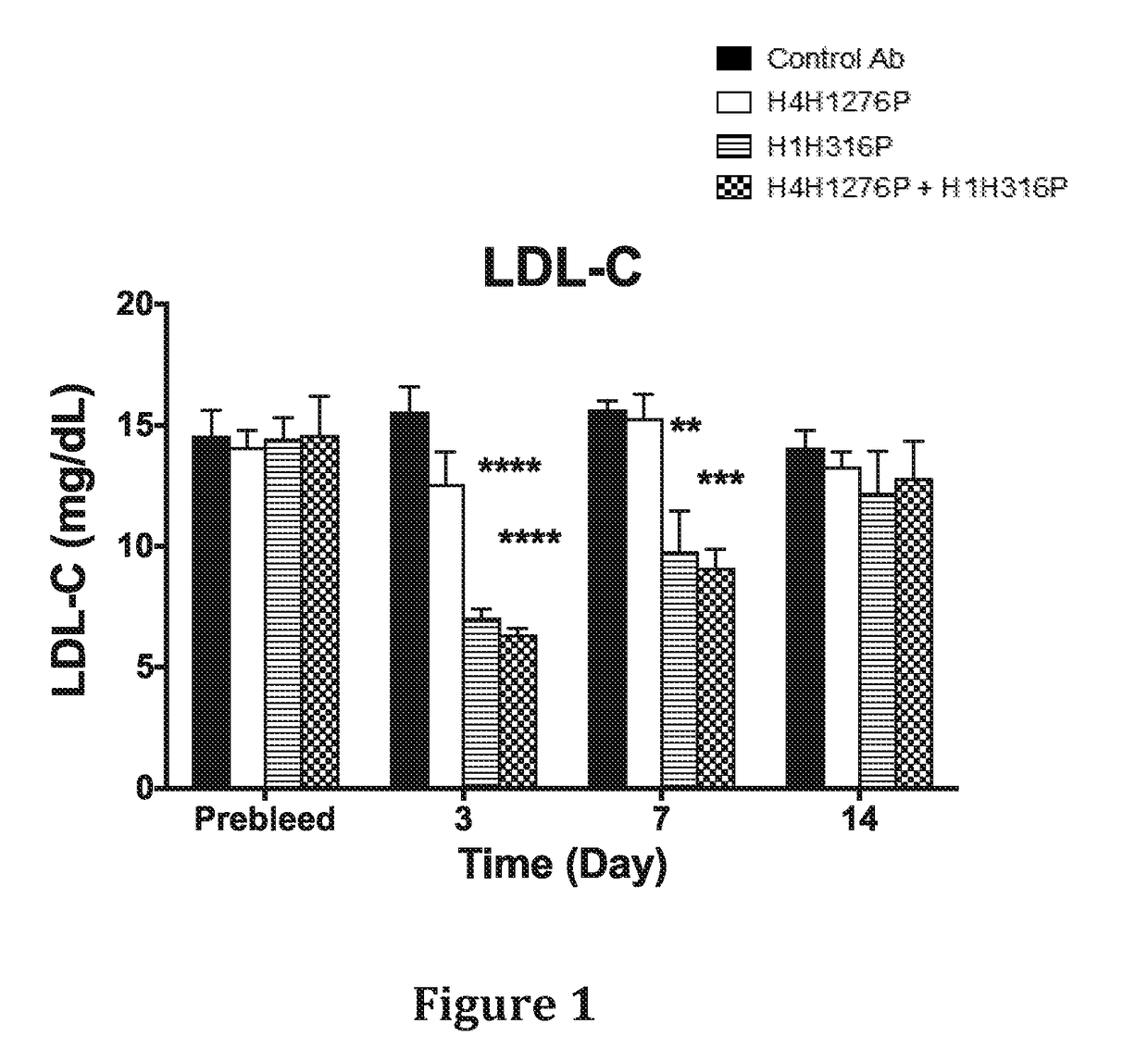
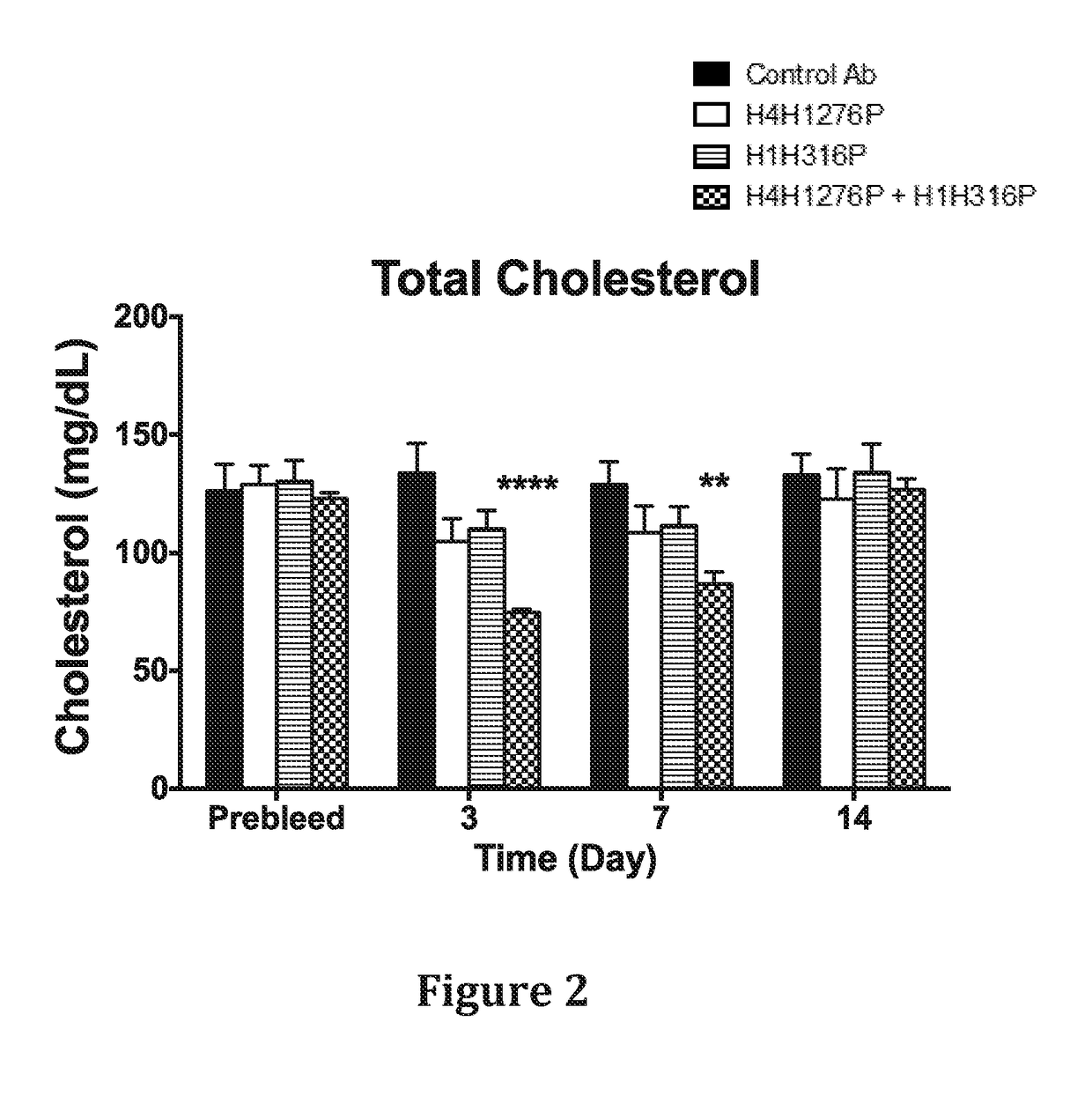
Methods for inhibiting atherosclerosis by administering an inhibitor of pcsk9
ActiveUS20160115246A1Reduces atherosclerotic plaque formationReduce formationOrganic active ingredientsAntibody ingredientsKexinAntigen binding
The present invention provides methods and compositions for inhibiting atherosclerotic plaque formation in a subject. In certain embodiments, the methods of the present invention comprise selecting a subject who has, or is at risk of developing, atherosclerosis, and administering to the subject a pharmaceutical composition comprising a proprotein convertase subtilisin / kexin type 9 (PCSK9) inhibitor. In certain embodiments, the PCSK9 inhibitor is an anti-PCSK9 antibody, or antigen binding protein.
Owner:SANOFI BIOTECH SAS +1
Methods for Producing Polypeptidies in Protease-Deficient Mutants of Trichoderma
The present invention relates to mutants of a parent Trichoderma strain, comprising a polynucleotide encoding a polypeptide and one or more (several) genes selected from the group consisting of a first subtilisin-like serine protease gene, a first aspartic protease gene, a trypsin-like serine protease gene, a second subtilisin-like serine protease gene, and a second aspartic pro-tease gene, wherein the one or more (several) genes are modified rendering the mutant strain deficient in the production of one or more (several) enzymes selected from the group consisting of a first subtilisin-like serine protease, a first aspartic protease, a trypsin-like serine protease, a second subtilisin-like serine protease, and a second aspartic protease, respectively, compared to the parent Trichoderma strain when cultivated under identical conditions. The present invention also relates to methods of producing a polypeptide in such mutants and methods for producing such mutants.
Owner:NOVOZYMES INC
Oligonucleotide conjugates
ActiveUS9879265B2Reduce Toxicity RiskReduce nephrotoxicityOrganic active ingredientsSugar derivativesMedical disorderSubtilisins
The present invention relates to oligomeric compounds and conjugates thereof that target Proprotein Convertase Subtilisin / Kexin type 9 (PCSK9) PCSK9 mRNA in a cell, leading to reduced expression of PCSK9. Reduction of PCSK9 expression is beneficial for a range of medical disorders, such as hypercholesterolemia and related disorders.
Owner:ROCHE INNOVATION CENT COPENHAGEN
Anti-pcsk9 antibodies with ph-dependent binding characteristics
The present invention provides antibodies and antigen-binding fragments thereof that specifically bind proprotein convertase subtilisin / kexin-9 (PCSK9) with greater affinity at neutral pH than at acidic pH. The antibodies of the invention may possess one or more amino acid changes as compared to antibodies that do not exhibit pH-dependent binding properties. For example, the present invention includes anti-PCSK9 antibodies which possess one or more histidine substitutions in one or more complementarity determining regions. The antibodies of the invention, with pH-dependent binding properties, remain in circulation and exhibit cholesterol lowering activity for prolonged periods of time in animal subjects as compared to anti-PCSK9 antibodies that do not exhibit pH-dependent binding properties. The antibodies of the invention are therefore useful for treating diseases and disorders related to elevated HDL cholesterol, wherein the antibodies of the invention can be administered to a patient at a lower dose and / or with less frequent dosing as compared to antibodies that do not exhibit pH-dependent binding properties.
Owner:REGENERON PHARM INC
Seafood seasoning and preparation method thereof
InactiveCN102232536AReasonable ratioIncrease the degree of hydrolysisFood preparationAdditive ingredientSubtilisins
The invention relates to a seafood seasoning and a preparation method thereof. Fishes, shrimps, shellfishes or leftovers thereof are taken as raw materials, ingredients are added, and the seafood seasoning is a granular product prepared by the steps of double-enzyme hydrolysis, vacuum concentration, spray drying and granulation. Compared with the prior art, the preparation method of the seafood seasoning has the advantages that: double enzymes, namely subtilisin and flavourzyme are used for performing protein hydrolysis on low-value fishes, shrimps, shellfishes or leftovers thereof, and the proportion, adding amount, hydrolysis temperature, pH value and hydrolysis time of the bienzyme are strictly controlled, so the higher hydrolysis degree can be obtained, and the bitterness is furthest avoided; and the granular seafood seasoning has a reasonable proportion as well as excellent color, aroma and taste, develops a novel way for high-value utilization of low-value aquatic proteins, and has higher utilization value and broad market prospect.
Owner:FISHERIES RES INST OF FUJIAN
PCSK9 (Proprotein Convertase Subtilisin Kexin Type 9) resistant monoclonal antibody
ActiveCN107698680ABinding blockIncrease intakeMetabolism disorderMammal material medical ingredientsKexinPhage antibodies
The invention relates to the technical field of antibody engineering and in particular discloses a PCSK9 (Proprotein Convertase Subtilisin Kexin Type 9) resistant monoclonal antibody. The monoclonal antibody disclosed by the invention comprises an amino acid sequence coding an antibody variable region and a CDR region. The invention further discloses an acquiring method and application of the monoclonal antibody. The method comprises the following steps: screening a PCSK9 resistant monoclonal antibody from a phage antibody library, performing affinity maturation through a method for constructing the phage antibody library by virtue of strand displacement, performing mutant library-construction screening on light-chain CDR1, 2 and 3 regions of the monoclonal antibody obtained by preliminaryscreening, selecting a monoclonal antibody with high affinity, performing mutant library-construction screening on heavy-chain CDR1, 2 and 3 regions of the monoclonal antibody, and finally screeningthe PCSK9 resistant monoclonal antibody with high affinity. The PCSK9 resistant monoclonal antibody obtained in the invention has excellent affinity to PCSK9, is capable of inhibiting binding betweenthe PCSK9 and ligands thereof, and can be used for treating dyslipidemia, cardiovascular and cerebrovascular diseases and thrombosis-obstructive diseases.
Owner:BEIJING DONGFANG BIOTECH
Compositions and methods comprising variant microbial proteases
InactiveCN102046783ADetergent mixture composition preparationAnimal feeding stuffSubtilisinsProtein engineering
The present invention provides methods for protein engineering. Specifically, the invention provides methods utilizing site evaluation libraries to design libraries that optimize two or more properties of a protein. The present invention also provides variant subtilisins suitable for various uses.
Owner:DANISCO US INC
Serine protease variants having amino acid deletions and substitutions
The present invention relates to variants of serine proteases having decreased immunogenicity relative to their corresponding wild-type proteases. More particularly, the present invention relates to variants having a modified amino acid sequence of a wild-type amino acid sequence, wherein the modified amino acid sequence comprises a deletion and, optionally, a substitution of one or more specifically identified positions corresponding to subtilisin BPN'. The invention further relates to mutant genes encoding such variants and cleaning and personal care compositions comprising such variants.
Owner:THE PROCTER & GAMBLE COMPANY
Recombinant bacterium for expressing glutamine transaminage and application thereof
The invention discloses a recombinant bacterium which is a recombinant methanol nutritional type microzyme containing a glutamine transaminage prosoma gene and a subtilisin type hydrolase gene SAM-P45. The invention also protects the recombinant bacterium as Pichia pastoris GSMTGB, the recombinant bacterium is preserved in CGMCC (address: Datun Road, Chaoyang district, Beijing city, China) on August 25th, 2009, the preserving number is CGMCC No.3249, and the enzyme activity of the glutamine transaminage reaches 0.43u / ml when the recombinant bacterium is induced and fermented for 72 hours.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Mactra veneriformis glycosaminoglycan extraction method
The invention provides a Mactra veneriformis glycosaminoglycan extraction method, which is characterized by comprising the following steps of: weighing the soft part of Mactra veneriformis, adding water to prepare into a homogenate with the ratio of water to material being 3:1, adding neutral subtilisin and trypsin into the homogenate for enzymatic hydrolysis while the ratio of bacillus subtilis neutral proteinase to trypsin is 4:3, the addition of compound enzyme is 1% and the hydrolysis time is 3-5h, boiling and centrifuging the enzymolysis liquid to obtain a clear liquid, adding active carbon for decolouring, followed by vacuum filtration, filtering with the assistance of diatomite, carrying out alcohol precipitation to obtain crude glycosaminoglycan with the yield rate being more than 1.3%, preparing 3% of a sugar solution, centrifuging to remove acidic and neutral proteins, followed by dialysis of a supernatant and alcohol precipitation, preparing 1% of a sugar solution, adding 5% of CTAB, centrifuging, dissolving the precipitate into a KCI solution to obtain a dissociation liquid, carrying out alcohol precipitation, followed by dialysis and alcohol precipitation to obtain high-purity glycosaminoglycan. The method provided by the invention has advantages of mild reaction condition and high yield rate and purity. Lots of proteins can be recovered while extracting glycosaminoglycan. The extracted substance has important effects of removing free radial, resisting oxidation and resisting coagulation. The yield rate of crude glycosaminoglycan reaches over 1.3% and the purity of glycosaminoglycan reaches more than 95%.
Owner:TIANJIN UNIV OF SCI & TECH
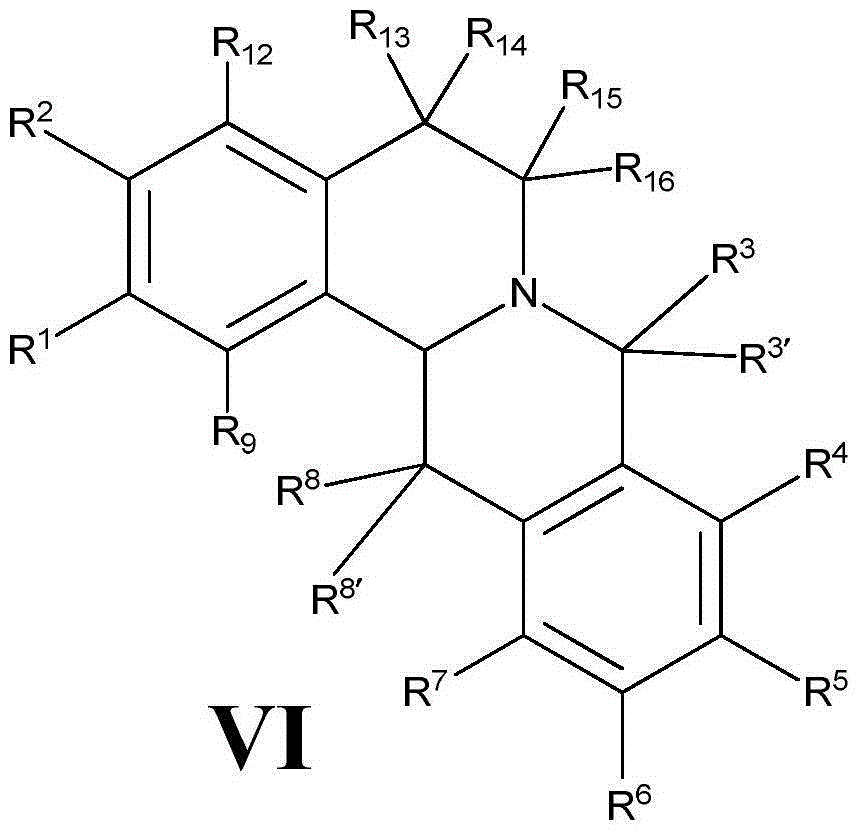
Tetrahydropalmatine derivative and application thereof
InactiveCN105481849AOrganic active ingredientsMetabolism disorderAcute hyperglycaemiaInsulin resistance
The invention relates to a tetrahydropalmatine derivative and application thereof, a compound as shown in a formula (VI) and a preparation method thereof, and an application of the compound in medicine. Specifically, the invention relates to a derivative of the compound as shown in the general formula (VI) and a preparation method thereof, and an application of the derivative as a therapeutic agent in prevention and treatment of hyperlipidemia, hypercholesterolemia, hypertriglyceridemia, hepatic steatosis, diabetes type II, hyperglycemia, adiposis, or insulin resistance and metabolic syndrome. The compound disclosed by the invention also can reduce total cholesterol, low density lipoprotein (LDL)-cholesterol and triglycerides, and increases the expression of a liver LDL receptor and inhibits the expression of proprotein convertase subtilisin / kexin type 9 (PCSK9).
Owner:CHENGDU BESTCHIRALBIO LIMITED LIABILITY
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using a one or more assays configured to detect a kidney injury marker selected from the group consisting of Stanniocalcin-1, Antithrombin-III, Toll-like receptor 2, Triiodothyronine (T3), Thyroxine (T4), Extracellular matrix protein 1, Coagulation factor XIII A chain, Coagulation factor XIII B chain, Interleukin-17F, Interleukin-22, Vitronectin, Progesterone, Estradiol, Growth / differentiation factor 15, and Proprotein convertase subtilisin / kexin type 9 as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
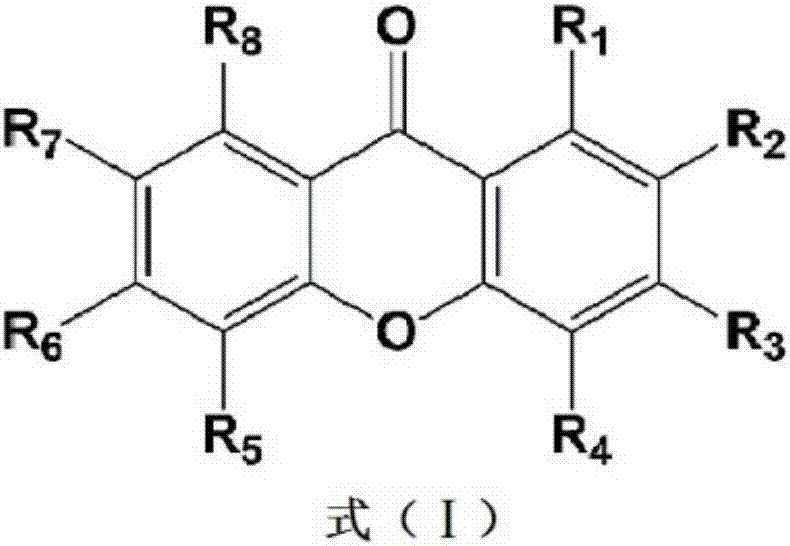
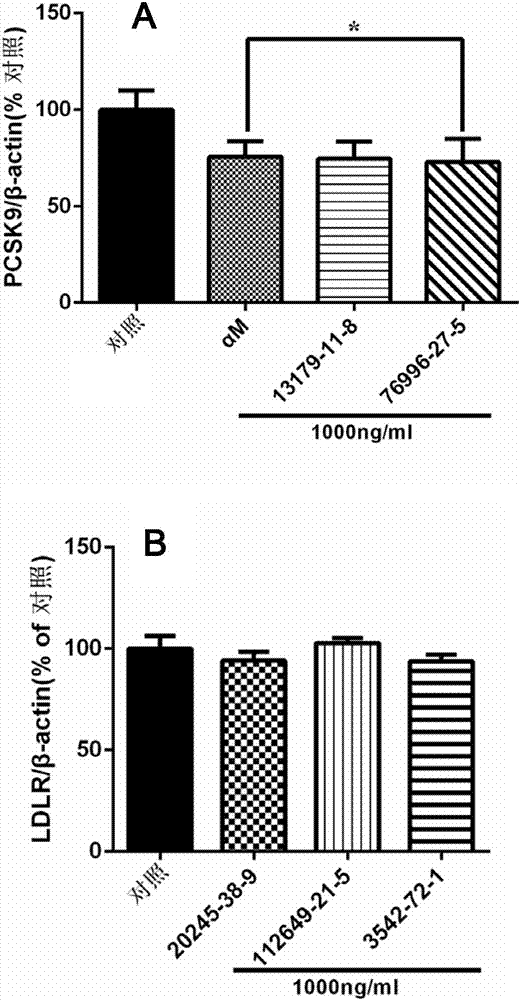
Applications of xanthone compounds and derivatives thereof in preparing medicines for lowering blood lipid
The invention relates to novel applications of xanthone compounds (as shown in the general formula I), derivatives, enantiomers, diastereoisomers and racemes of the xanthone compound, mixtures of the xanthone compounds, the derivatives, the enantiomers, the diastereoisomers and the racemes of the xanthone compounds, pharmaceutically acceptable salts of the xanthone compounds, and compositions of the pharmaceutically acceptable salts in preparing products for preventing and / or treating hyperlipidemia or preparing products for inhibiting pro-protein convertase subtilisin / kexin 9 (PCSK9).
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Antioxidant peony peptide and preparation method thereof
The invention relates to the field of plant extracts, in particular to an antioxidant peony peptide and a preparation method thereof. The peony peptide comprises 6-9 amino acids, and comprises one ormore of tyrosine, cystine and methionine. According to the preparation method, a two-step decomposition method is utilized, ficin proteinase with not high specificity and subtilisin are selected and compounded, firstly, peptide bonds at main glutamic acids in peony seed meal protein are ruptured, and a protein peptide fragment is formed; then a small amount of bromelain without specificity, papainwith specificity and endopeptidase Glu-C with specificity are utilized, and the tyrosine, the cystine and the methionine are retained in peptide chain molecules as much as possible to improve the antioxidant capacity of the protein peptide.
Owner:世堃堂(广东)生物科技有限公司
Methods of reducing a viral infection and kits therefore
A method for treating and / or preventing a proprotein convertase subtilisin / kexin type 9 preproprotein (PCSK9)-susceptible viral infection comprising increasing a PCSK9 activity and / or expression in a biological system infected by the virus, whereby the increased PCSK9 activity and / or expression treats and / or prevents the viral infection in the biological system. Methods of classifying subjects, methods of screening and kits therefore.
Owner:INSTITUT NATIONAL DE LA RECHERCHE SCIENTIFIQUE +1
Use of acid stable protease in animal feed
Acid-stable proteases of the subtilisin family, their use in animal feed, feed-additives and feed compositions
Owner:DSM IP ASSETS BV
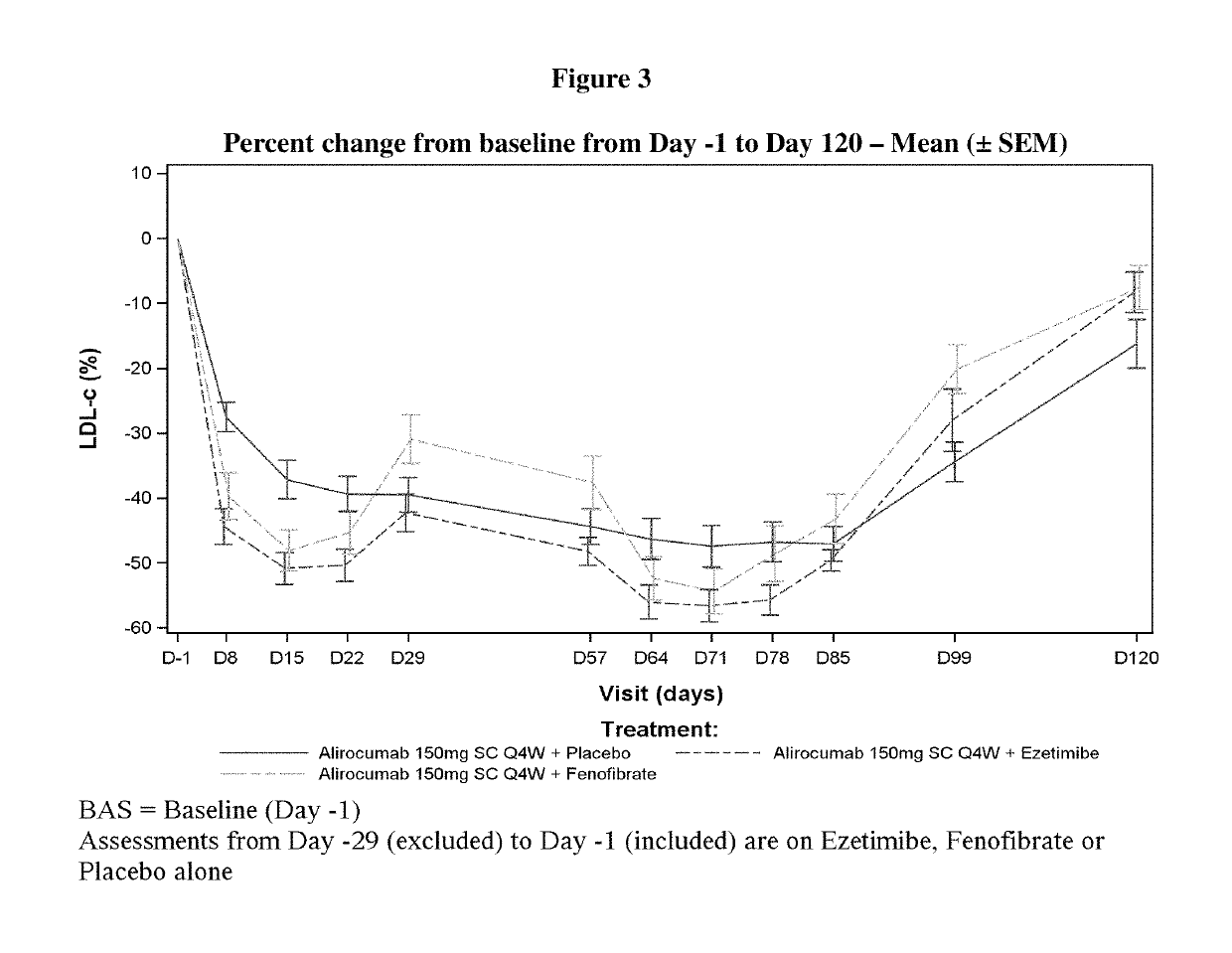
Dosing regimens for use with PCSK9 inhibitors
ActiveUS10428157B2Reducing LDL-CGain is not constantMetabolism disorderAntibody ingredientsKexinDisease
Owner:SANOFI BIOTECH SAS +1
Methods for Treating Patients with Hyperlipidemia by Administering a PCSK9 Inhibitor in Combination with an ANGPTL3 Inhibitor
InactiveUS20170253666A1Treating hyperlipidemiaLower Level RequirementsMetabolism disorderPharmaceutical delivery mechanismAntigenProtein C
The present invention provides methods for treating patients suffering from hypercholesterolemia, wherein the patient is non-responsive to, inadequately controlled by, or intolerant to treatment with a standard lipid modifying therapy. The methods of the invention provide for lowering at least one lipid parameter in the patient by administering a therapeutically effective amount of an antibody or antigen-binding fragment thereof that specifically binds to proprotein convertase subtilisin / kexin type 9 (PCSK9) in combination with a therapeutically effective amount of an antibody that specifically binds to angiopoietin-like protein 3 (ANGPTL3). The combination of an anti-PCSK9 antibody with an anti-ANGPTL3 antibody is useful in treating diseases such as hypercholesterolemia, including familial hypercholesterolemia (FH), both heFH and hoFH, as well as hyperlipidemia, hyperlipoproteinemia and dyslipidemia, including hypertriglyceridemia, chylomicronemia, and to prevent or treat diseases or disorders, for which abnormal lipid metabolism is a risk factor, such as cardiovascular diseases.
Owner:REGENERON PHARM INC
Cervical cell Warburg effect simple detection reagent as well as preparation method and application thereof
ActiveCN107132217AIncreased sensitivityStrong specificityMaterial analysis by observing effect on chemical indicatorQuinolineOxygen
The invention relates to a cervical cell Warburg effect simple detection reagent as well as a preparation method and application thereof. The reagent is composed of a reagent A, a reagent B, a reagent C and a reagent D, wherein the reagent A is 4-6% of a subtilisin isotonic solution; the reagent B is an acetate buffer solution of which the pH value is 3.5-6.0; the reagent C is composed of a 3,3'5,5' tetramethyl benzidine solution with the concentration of 0.1-0.25g / L, a 6-methoxy quinoline solution with the concentration of 1.0-2.5mL / L and a cumene hydroperoxide solution with the concentration of 2-5mL / L; and the reagent D is composed of 0.03-0.07% of a toluidine blue O solution and a triton x-100 solution with the concentration of 2.5-7.5mL / L. The reagent can be used for qualitatively detecting the contents of cellular free ferroprotoporphyrin and active oxygen of cervical exfoliated cells so as to judge the strength of the Warburg effect of cervical cells.
Owner:山东天倪生物技术有限公司
Method for preparing high-uniformity single-order streptavidin tetramer
InactiveCN104962570AImprove uniformityGuaranteed stabilityDepsipeptidesVector-based foreign material introductionBiotin-streptavidin complexWild type
The invention discloses a method for preparing high-uniformity single-order streptavidin tetramer. Recombinant plasmids of wild type streptavidin and activity deletion mutants are prepared separately; engineering bacteria are further prepared; recombined streptavidin is expressed through engineering bacteria fermentation; the obtained wild type and mutant streptavidin inclusion bodies are mixed according to a mass ratio of 1:3; centrifugation is carried out; inclusion body renaturation and separation are carried out to obtain the single-order streptavidin tetramer; and an His8-tag is removed through protease K or bacillus subtilis protease. The preparing method is simple and easy to operate; the single-order streptavidin tetramer does not contain the His8-tag label which has negative effects on the activity; in addition, various factors can be borne, the state of the single-order tetramer is kept, and the method has the uniformity and the stability.
Owner:HUBEI UNIV OF ARTS & SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com