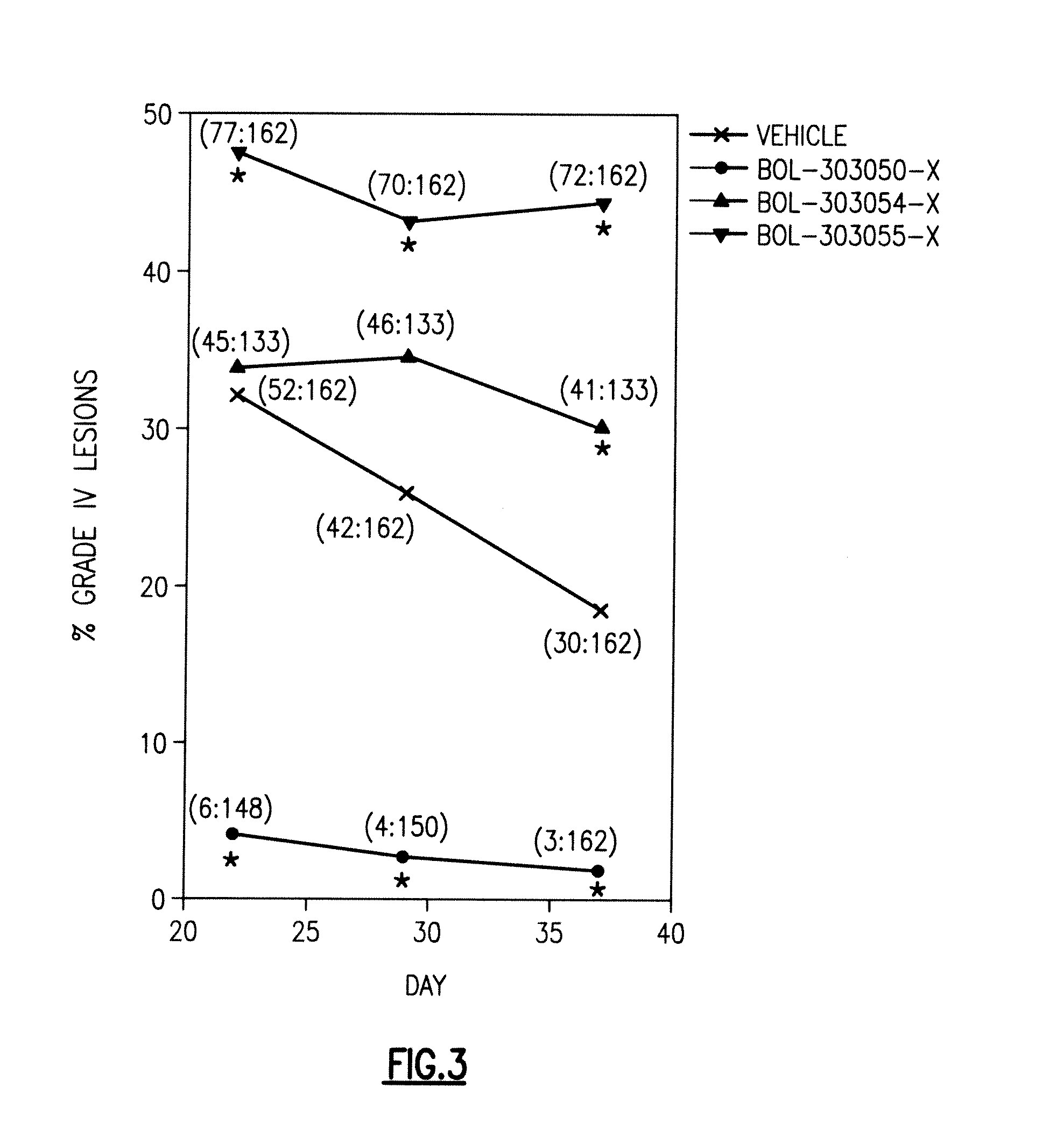
Patents
Literature
122 results about "Vitronectin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
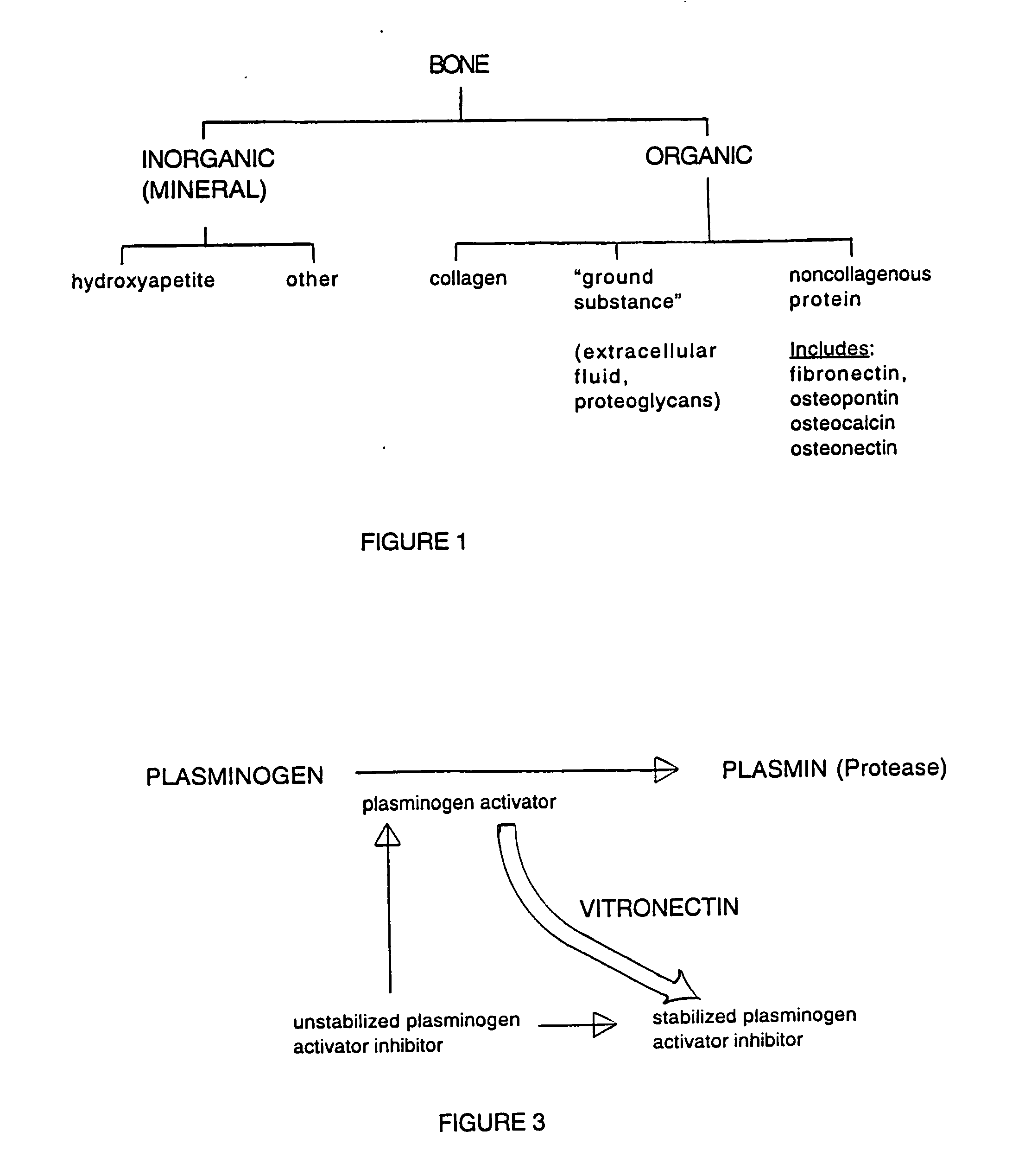
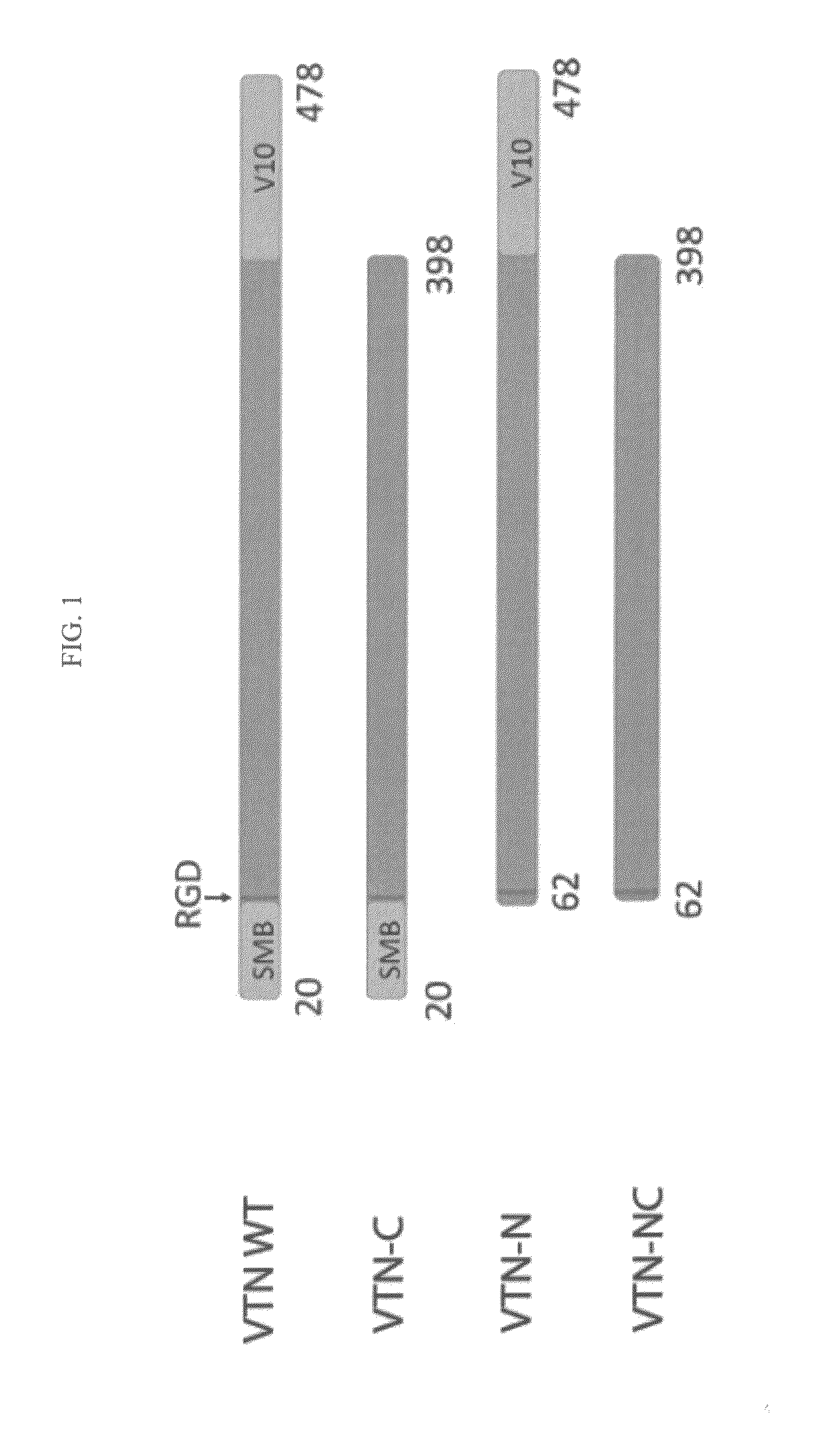
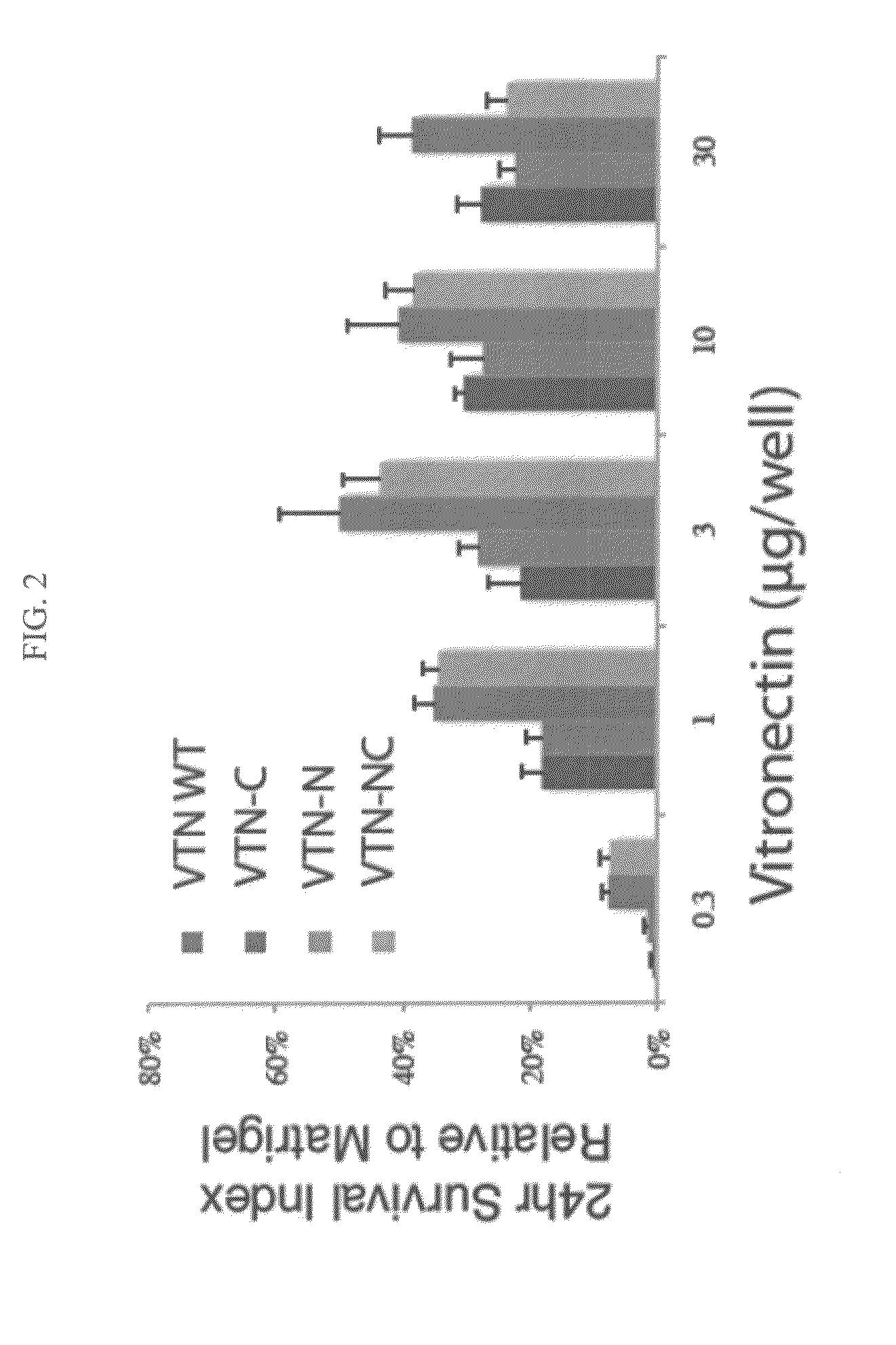
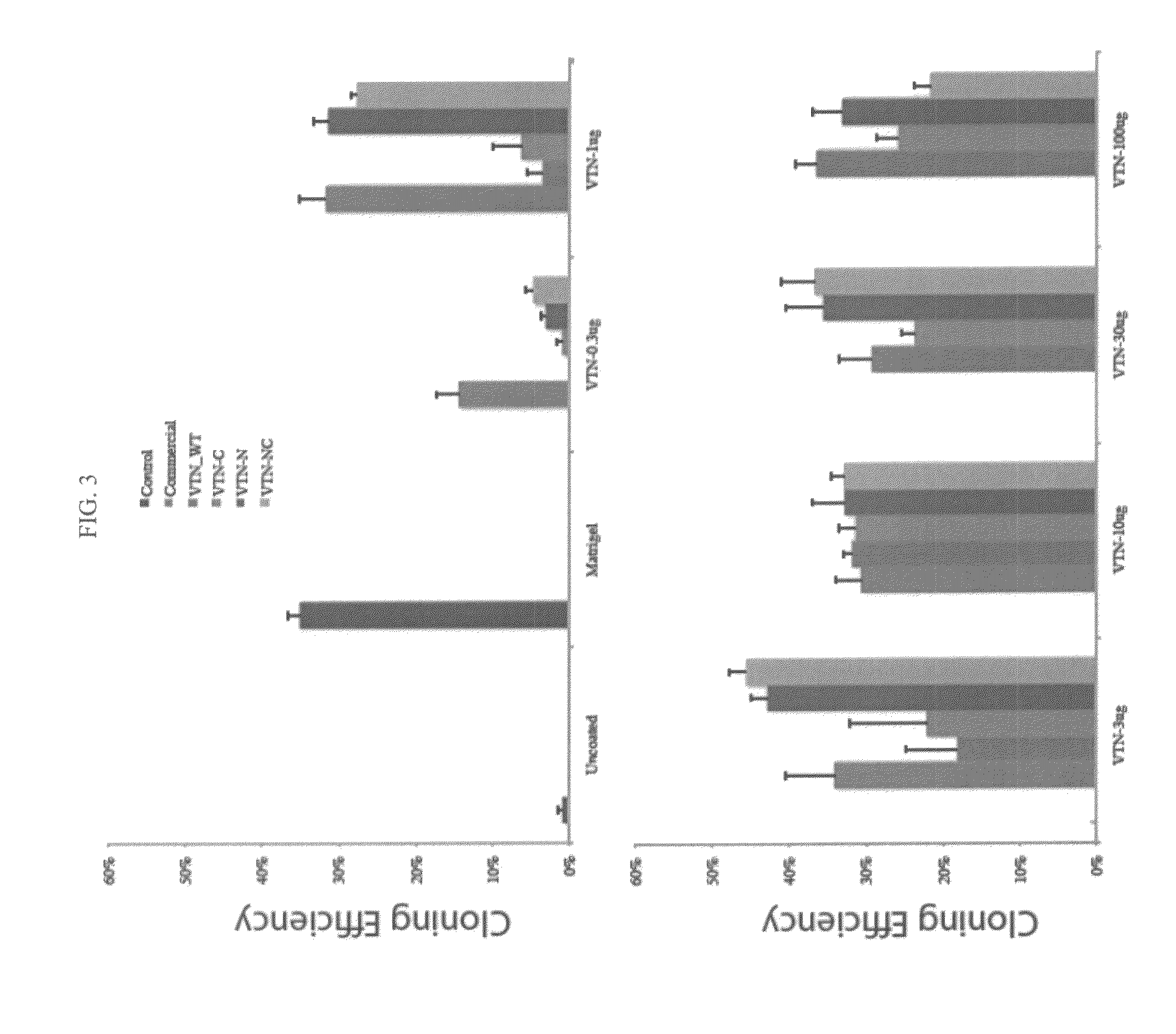
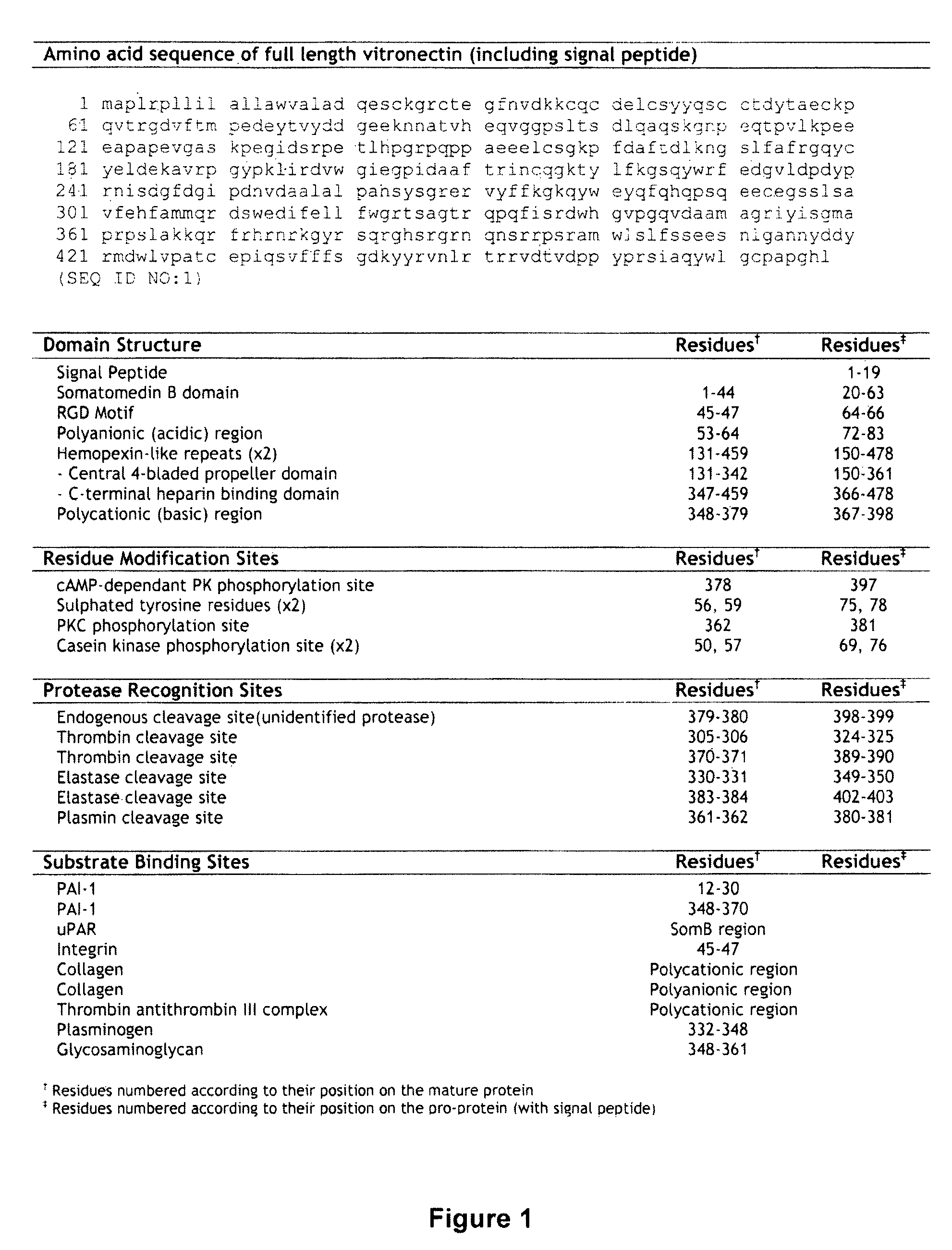
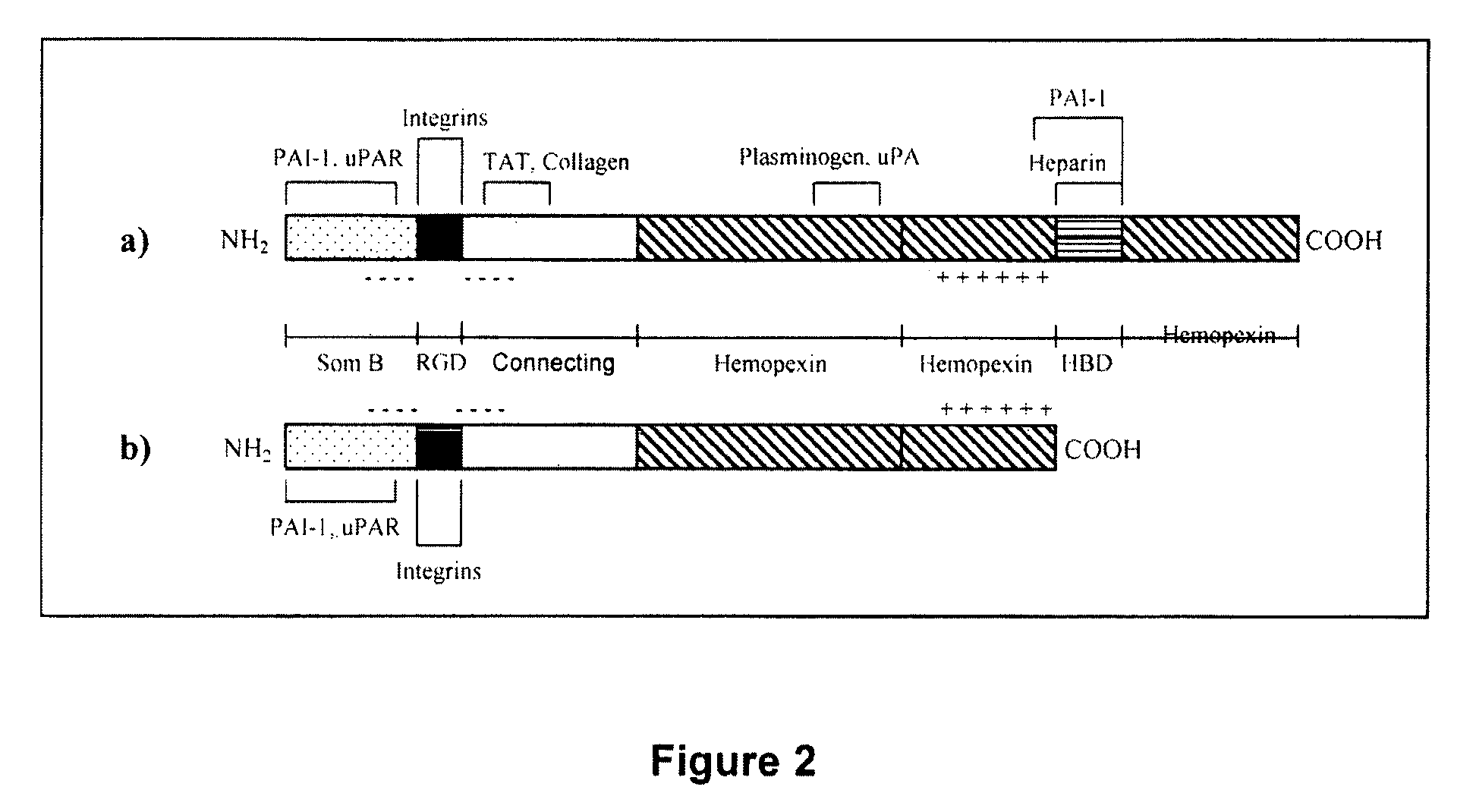
Vitronectin (VTN or VN) is a glycoprotein of the hemopexin family which is abundantly found in serum, the extracellular matrix and bone. In humans it is encoded by the VTN gene. Vitronectin binds to integrin alpha-V beta-3 and thus promotes cell adhesion and spreading. It also inhibits the membrane-damaging effect of the terminal cytolytic complement pathway and binds to several serpins (serine protease inhibitors). It is a secreted protein and exists in either a single chain form or a clipped, two chain form held together by a disulfide bond. Vitronectin has been speculated to be involved in hemostasis and tumor malignancy.
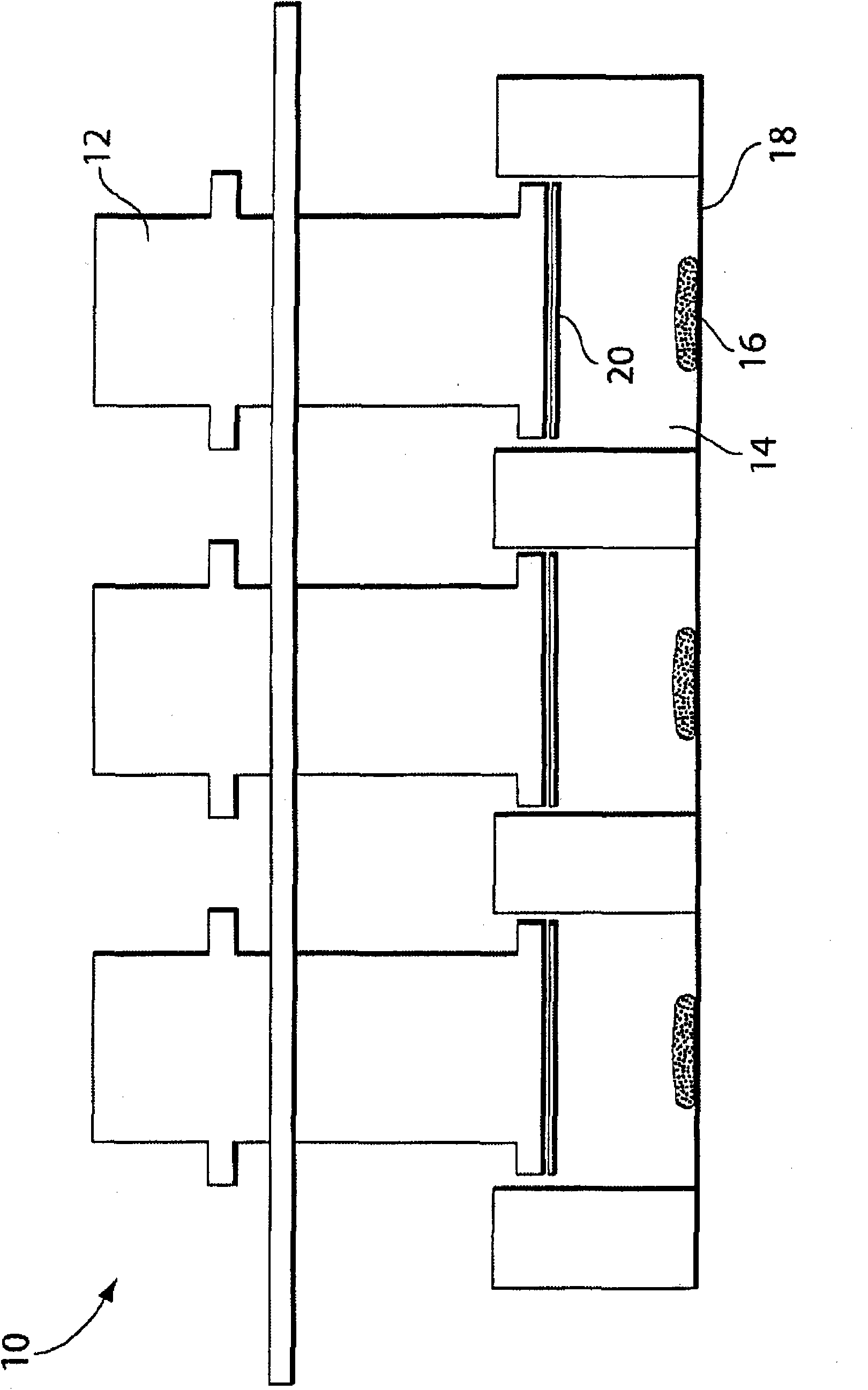
Biodegradable polymer coils for intraluminal implants
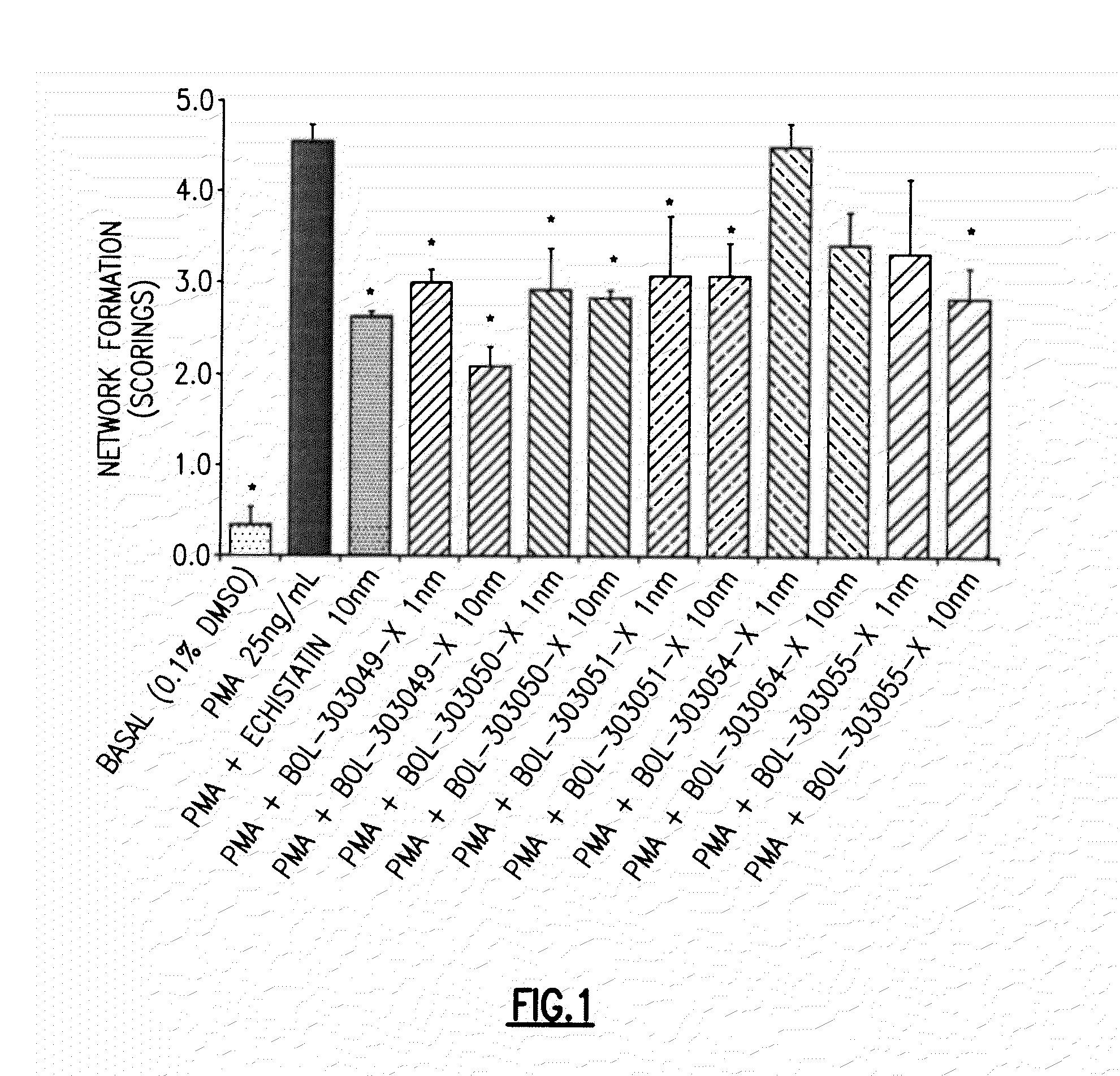
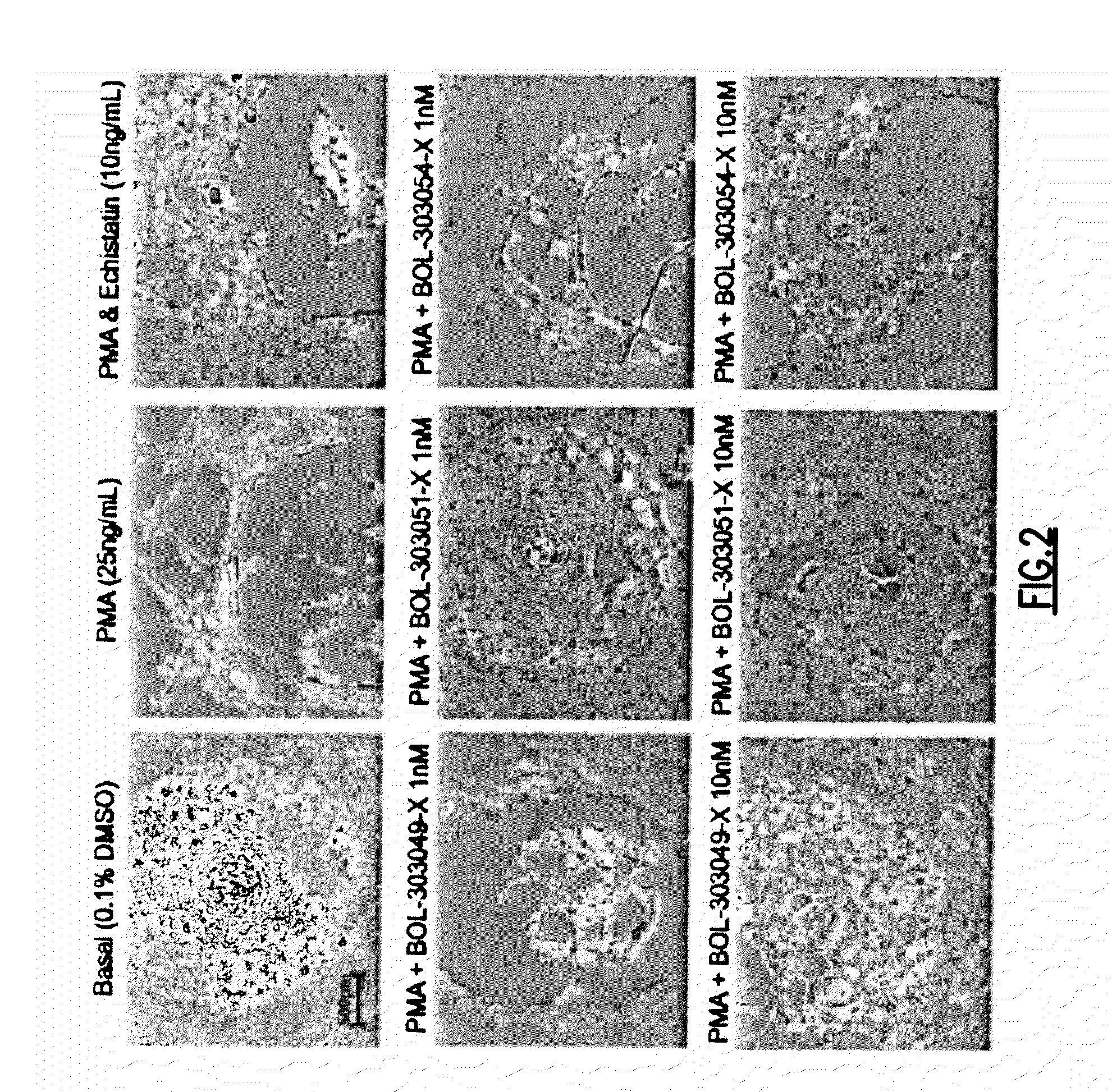
An endovascular cellular manipulation and inflammatory response are elicited from implantation in a vascular compartment or any intraluminal location of a separable coil comprised at least in part of at least one biocompatible and absorbable polymer or protein and growth factors. Typically a catheter associated with the separable coil is used to dispose the coil into a selected body lumen. The biocompatible and absorbable polymer or protein is thrombogenic. The coil further is comprised at least in part of a growth factor or more particularly a vascular endothelial growth factor, a basic fibroblast growth factor or other growth factors. The biocompatible and absorbable polymer is in the illustrated embodiment at least one polymer selected from the group consisting of polyglycolic acid, poly~glycolic acid poly-L-lactic acid copolymers, polycaprolactive, polyhydroxybutyrate / hydroxyvalerate copolymers, poly-L-lactide. Polydioxanone, polycarbonates, and polyanhydrides. The biocompatible and absorbable protein is at least one protein selected from the group consisting of collagen, fibrinogen, fibronectin, vitronectin, laminin, and gelatin. In one embodiment the coil is composed of the biocompatible and absorbable polymer or protein with a radio-opaque material is disposed thereon. Alternatively, the coil is composed of a radio-opaque material, and the biocompatible and absorbable polymer or protein is disposed thereon. This apparatus may be positioned within intracranial aneurysms or any aneurysm in the body as well as within other body cavities.
Owner:RGT UNIV OF CALIFORNIA
Lipoprotein-associated markers for cardiovascular disease
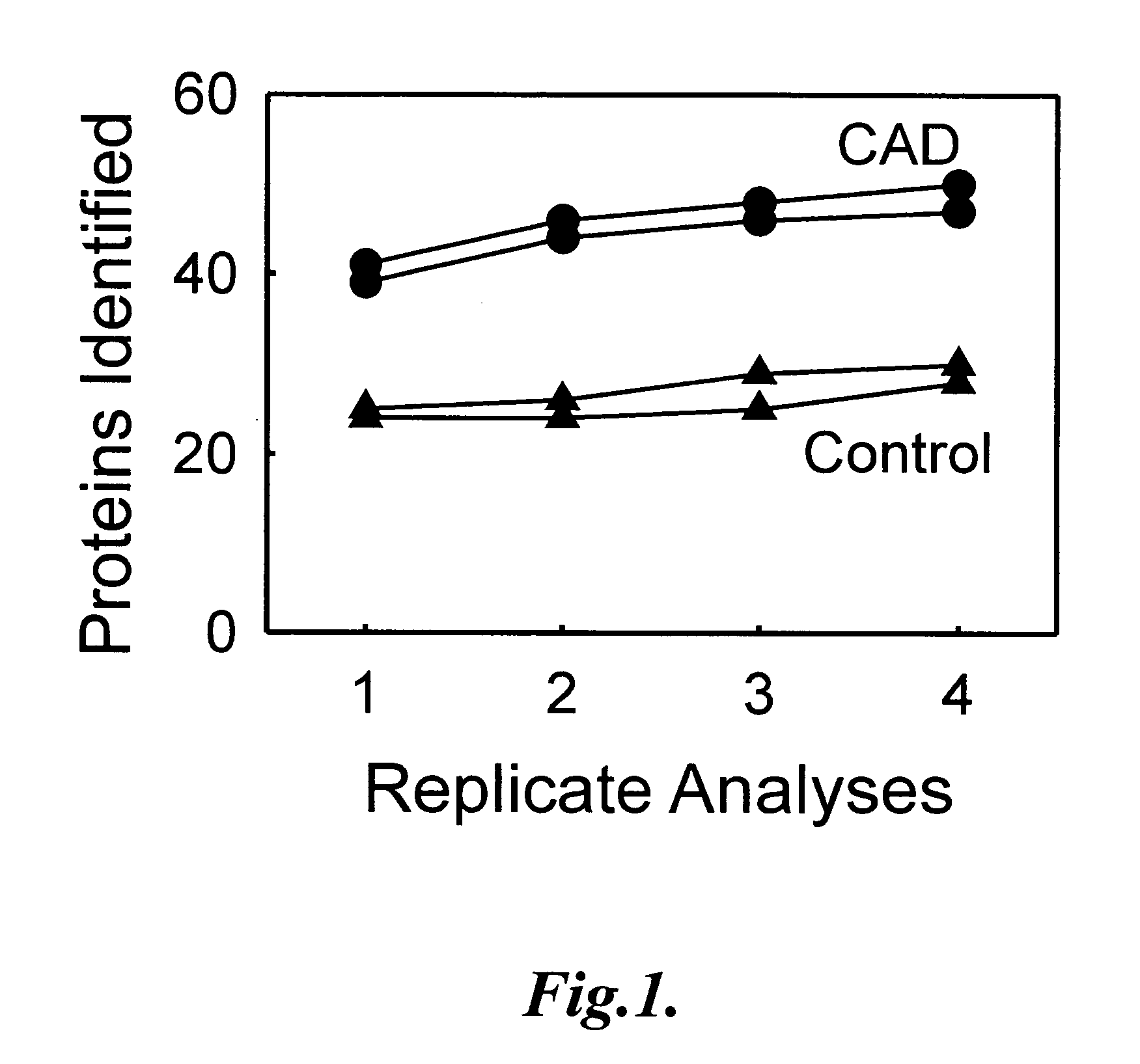
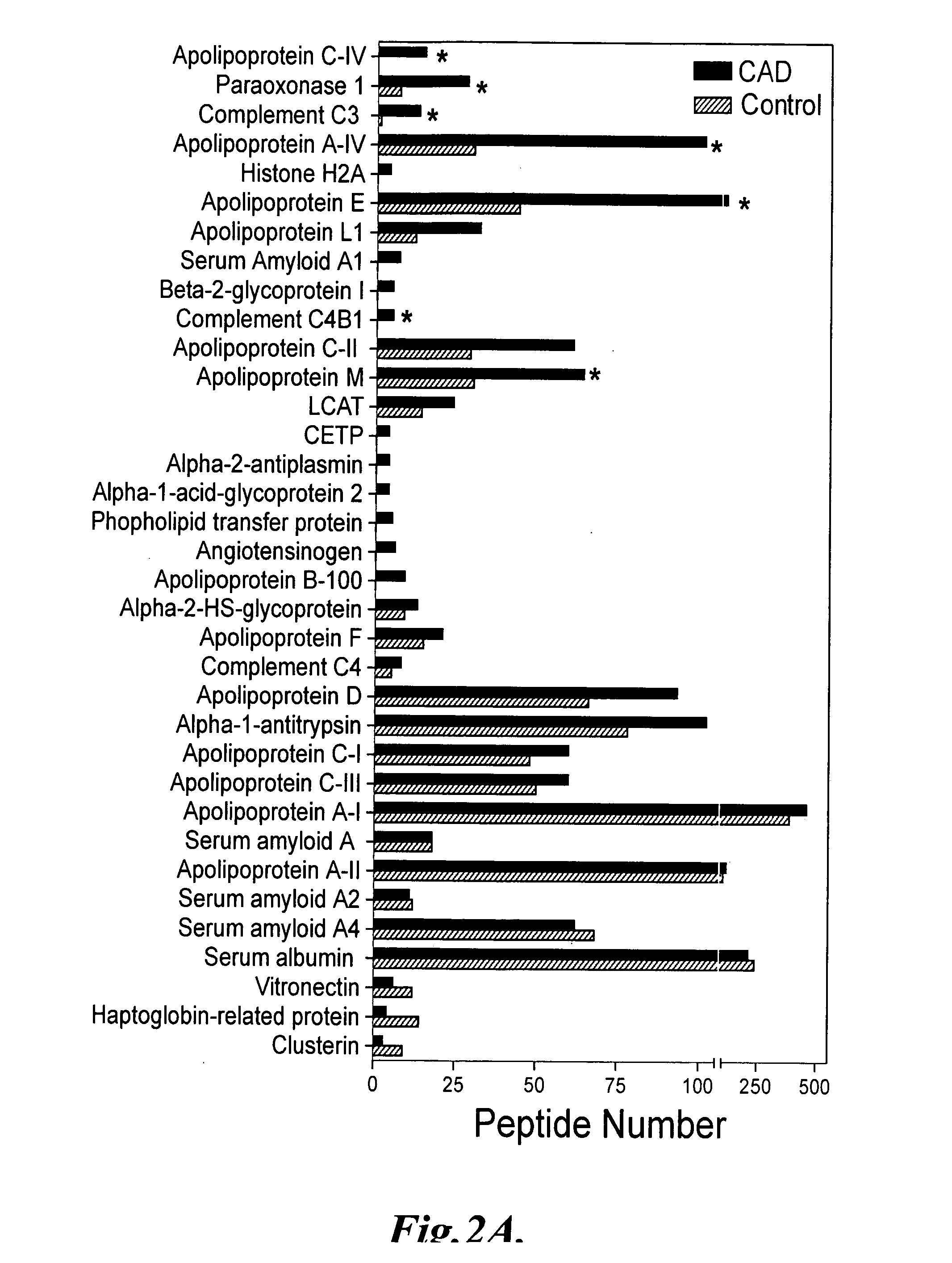
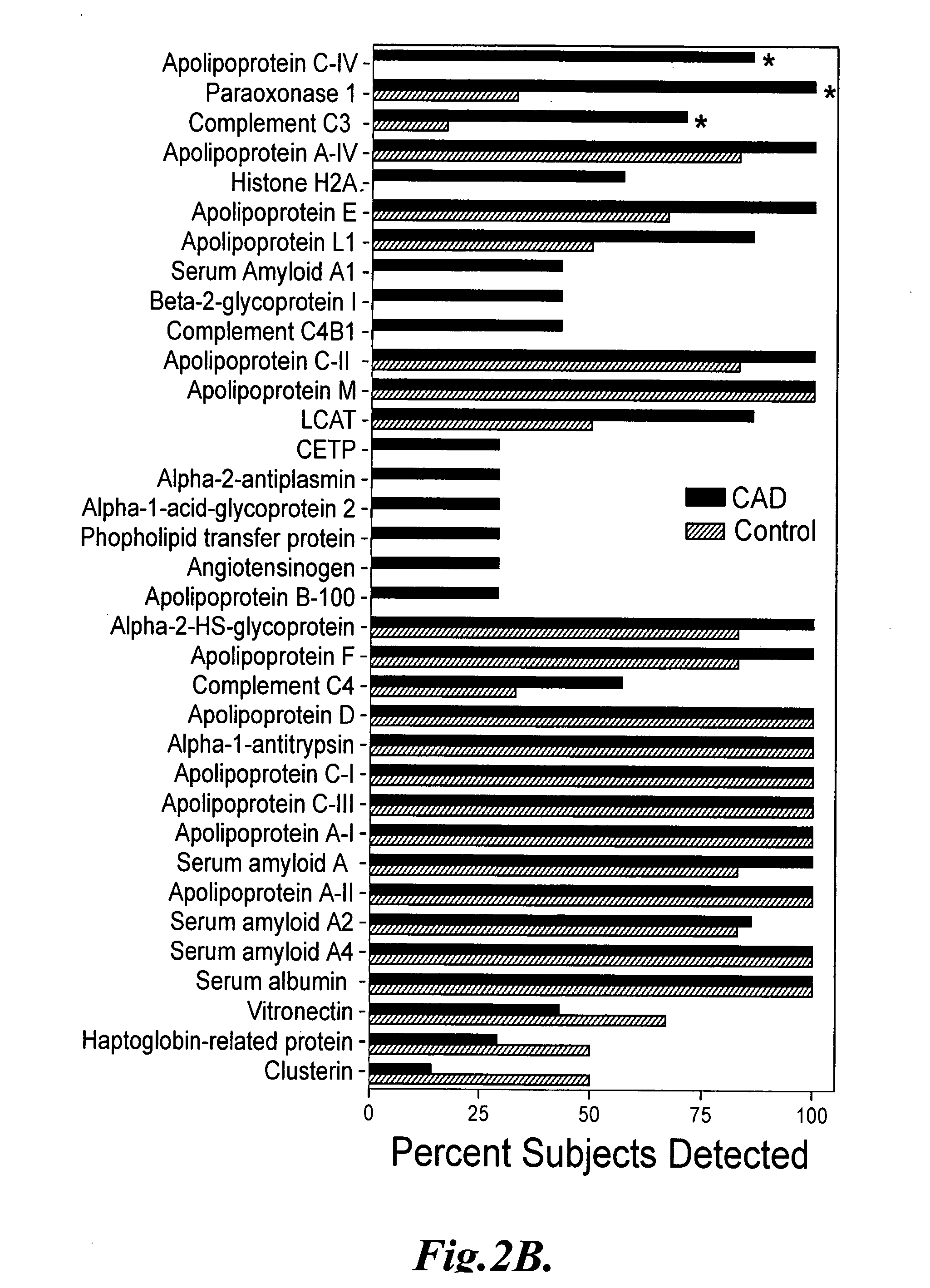
The invention provides methods of screening a mammalian subject to determine if the subject is at risk to develop, or is suffering from, cardiovascular disease. The methods comprise detecting an amount of at least one biomarker in a biological sample, or HDL subfraction thereof, from the subject, and comparing the detected amount of the biomarker to a predetermined value, where a difference between the detected amount and the predetermined value is indicative of the presence or risk of cardiovascular disease in the subject. In some embodiments, the biomarker comprises at least one of ApoC-IV, Paraoxonase 1, C3, C4, ApoA-IV, ApoE, ApoL1, C4B1, Histone H2A, ApoC-II, ApoM, Vitronectin, Haptoglobin-related protein, and Clusterin, or combinations thereof.
Owner:UNIV OF WASHINGTON
Composition and method for bone regeneration
InactiveUS20050147645A1Good effectDipeptide ingredientsTripeptide ingredientsOsteoblastBone formation
A composition for modulating bone regeneration composition comprises a matrix selected from the group consisting of glycolic acid, lactic acid, collagen, demineralized bone, or a combination thereof. A first biologically active molecule comprising a fibronectin is attached to a portion of the matrix, to facilitate osteoblast activity and for promoting an increase in bone formation. A second biologically active molecule comprising a vitronectin, selected for its ability to attract osteoclasts and produce an inhibiting effect on osteoclast activity to thereby promote a decrease in bone resorption, is also attached to a portion of the matrix.
Owner:BUDNY JOHN ARNOLD
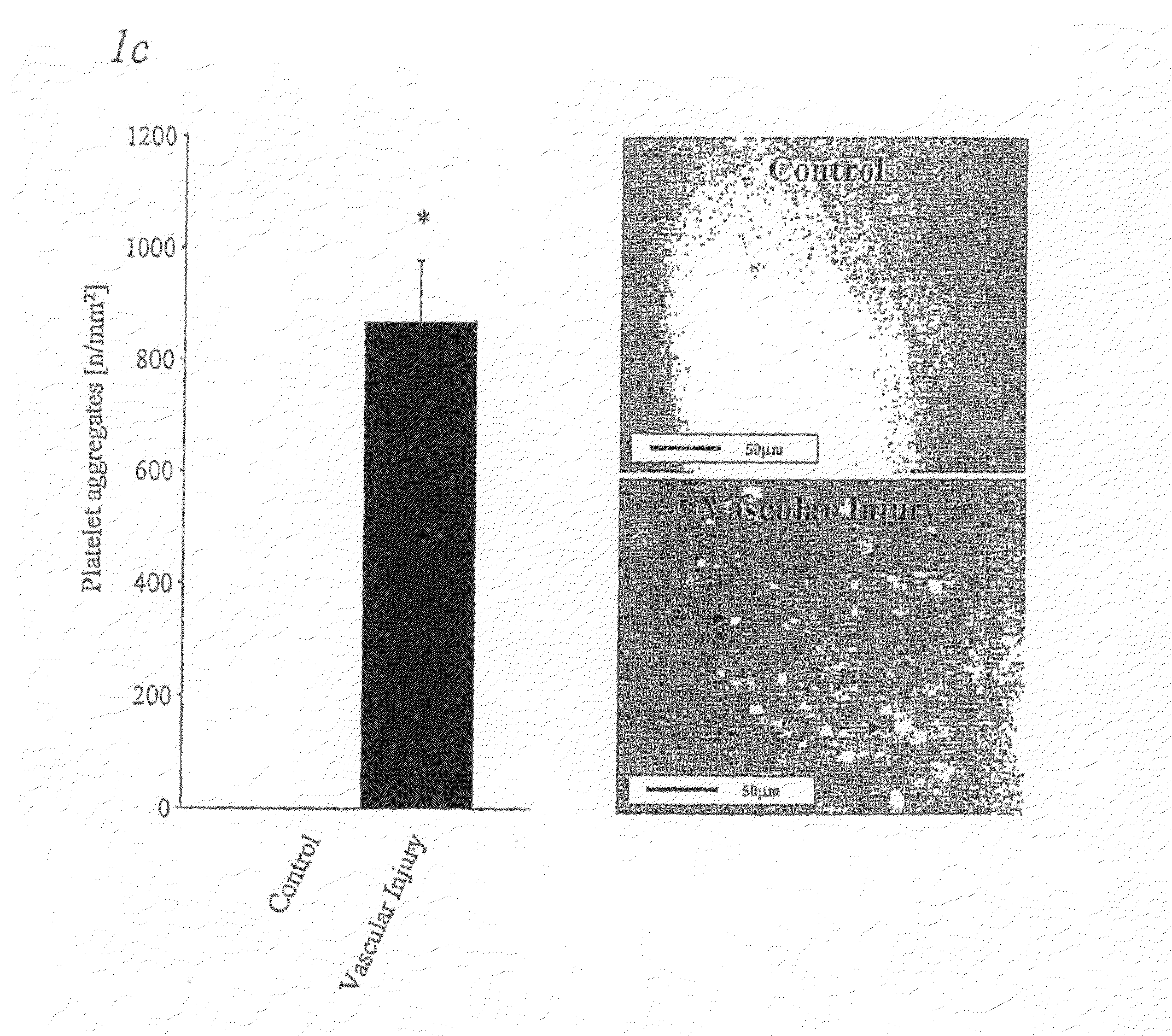
Methods, products and uses involving platelets and/or the vasculature
InactiveUS20090130021A1Inhibits interactionInhibits GPVI interactionVirusesPeptide/protein ingredientsDiseaseVitronectin
The present disclosure relates to agents which interfere with the binding of GPVI to various components. Agents which interfere with GPVI interaction with one or both of fibronectin and vitronectin or sequences thereof are also disclosed. Methods of treating disorders or diseases which involve pathological, dysfunctional or non-pathological interaction of GPVI with fibronectin and / or vitronectin are included in the present disclosure. The invention also relates to uses of agents for the prevention or treatment of disorders arising from blood platelet adhesion and aggregation.
Owner:MUNCH GOTZ +5
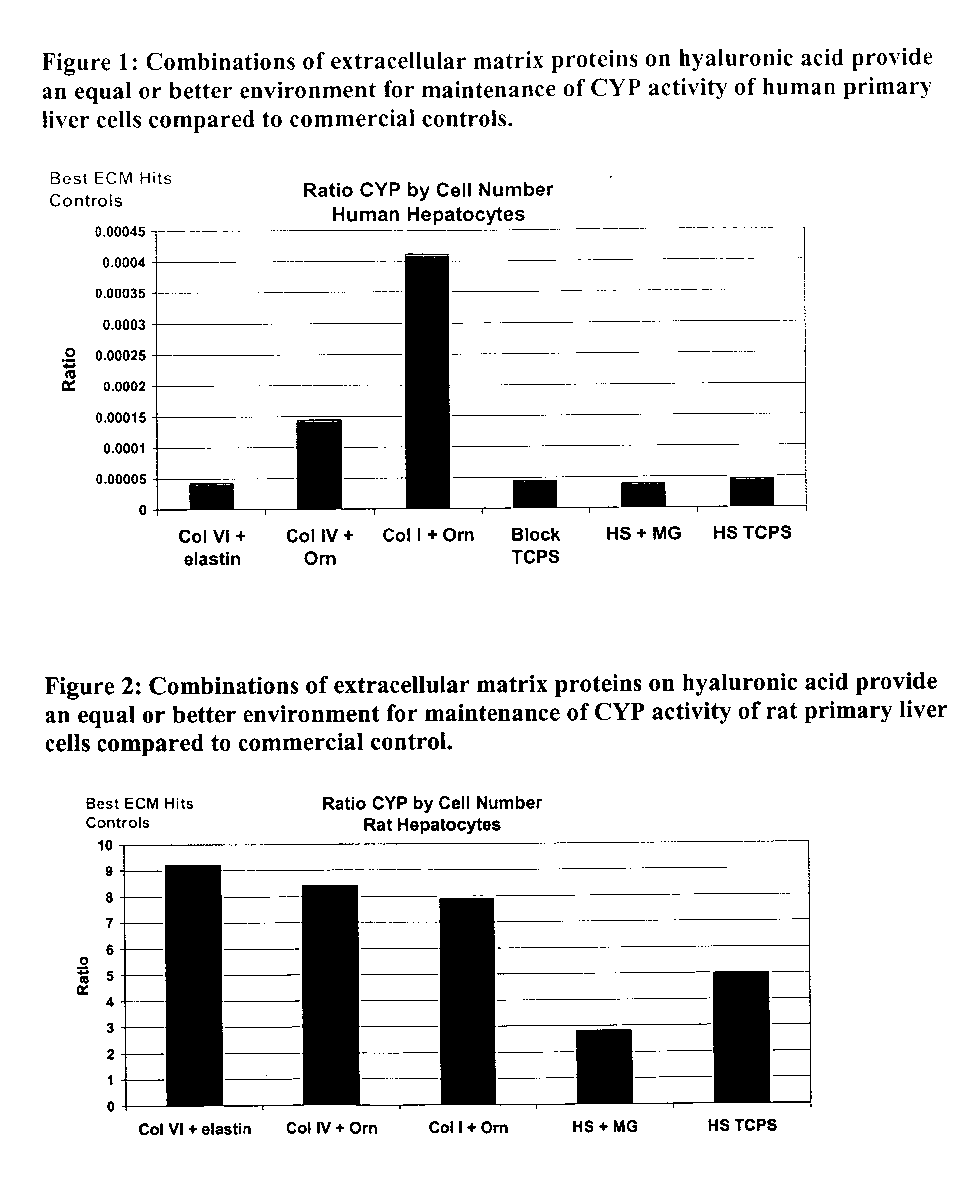
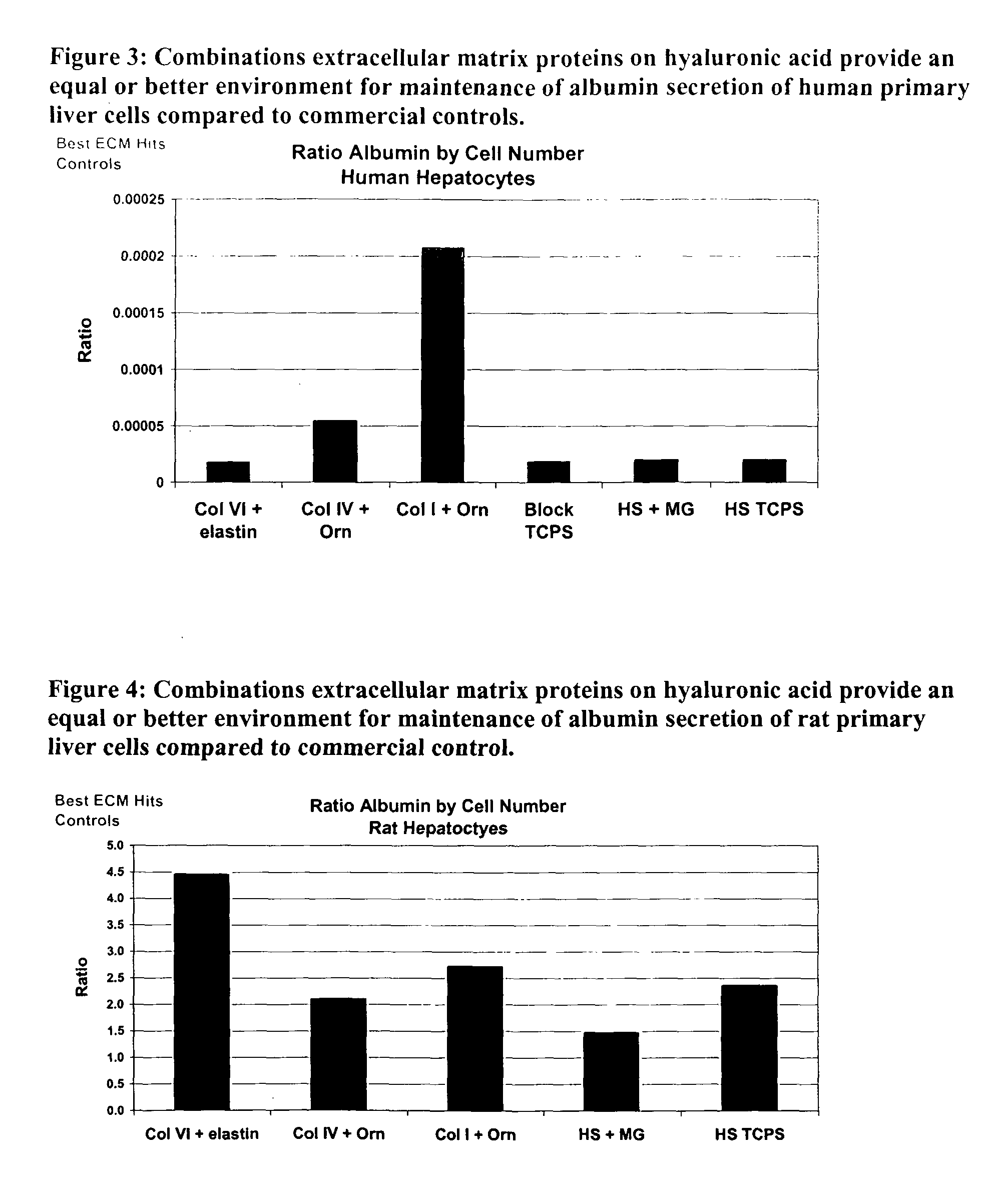
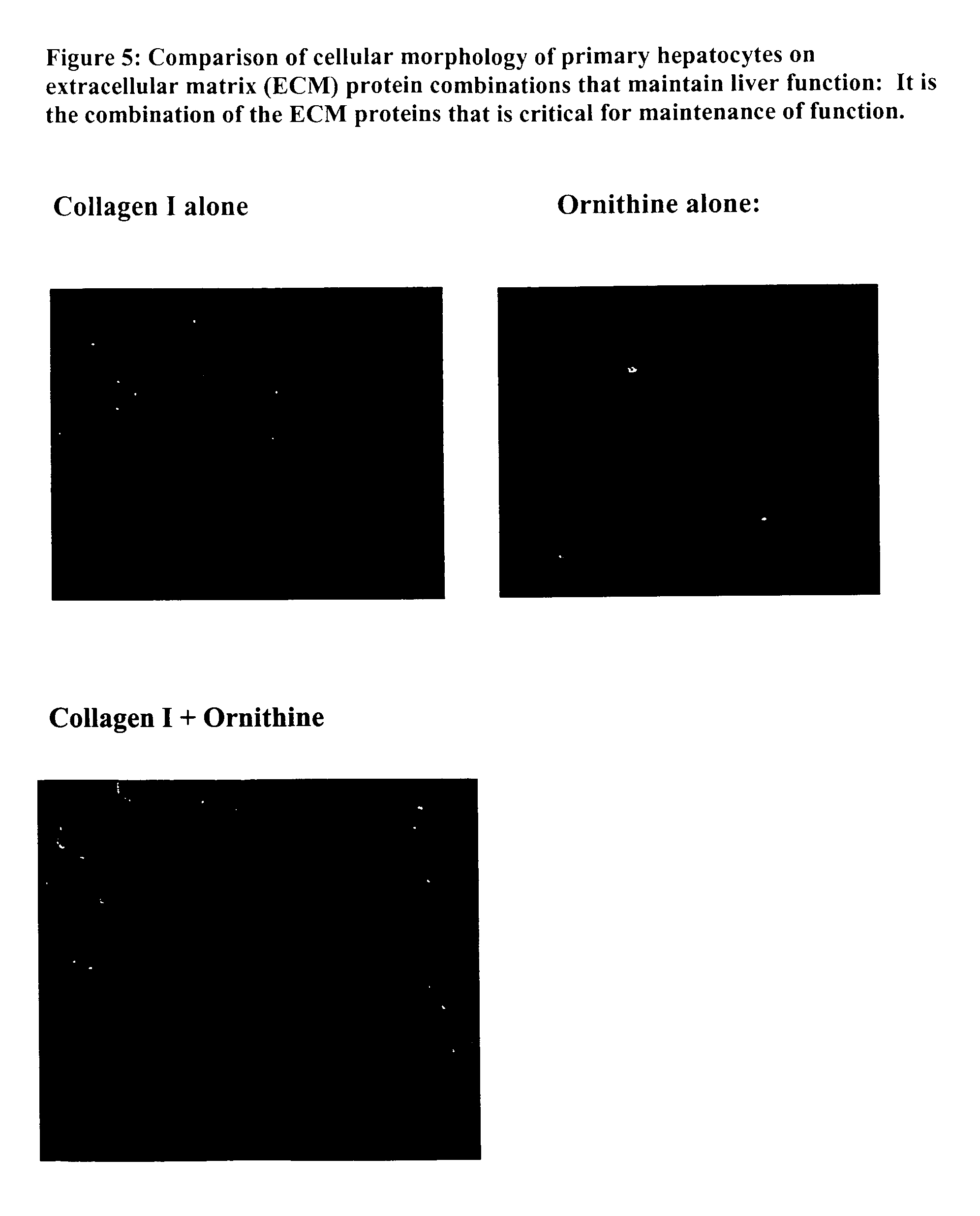
Environments that maintain function of primary liver cells
InactiveUS20050059150A1Good adhesionEasy maintenanceHepatocytesArtificial cell constructsECM ProteinL-Ornithine
Surfaces useful for cell culture comprise a support to which is bound a CAR material, and, bound to the CAR material, an ECM protein, or a biologically active fragment or variant thereof such as elastin, fibronectin, vitronectin, laminin, collagen I, collagen III, collagen IV, and collagen VI. Also, optionally present on the surface is an active factor, preferably a polycationic polymer or a biologically active fragment or variant thereof, such as polyethyleneimine (PEI), poly-D-lysine (PDL), poly-L-lysine (PLL), poly-D-ornithine (PDO) or poly-L-ornithine (PLO). This surface is used in cell culture to promote cell attachment, survival, and / or proliferation of primary liver cells. The invention also relates to methods utilizing this surface, such as methods for attachment, survival, and / or proliferation of cells. Further disclosed is the use of the surface in cell culture with serum-free medium. Methods of screening using the surface of the invention are also disclosed.
Owner:BECTON DICKINSON & CO
Vitronectin-derived cell culture substrate and uses thereof
ActiveUS20120301962A1Improve abilitiesBacteriaNon-embryonic pluripotent stem cellsVitronectinCell culture media
Vitronectin-derived cell culture substrates and methods of using the same for culturing pluripotent stem cells are presented.
Owner:WISCONSIN ALUMNI RES FOUND
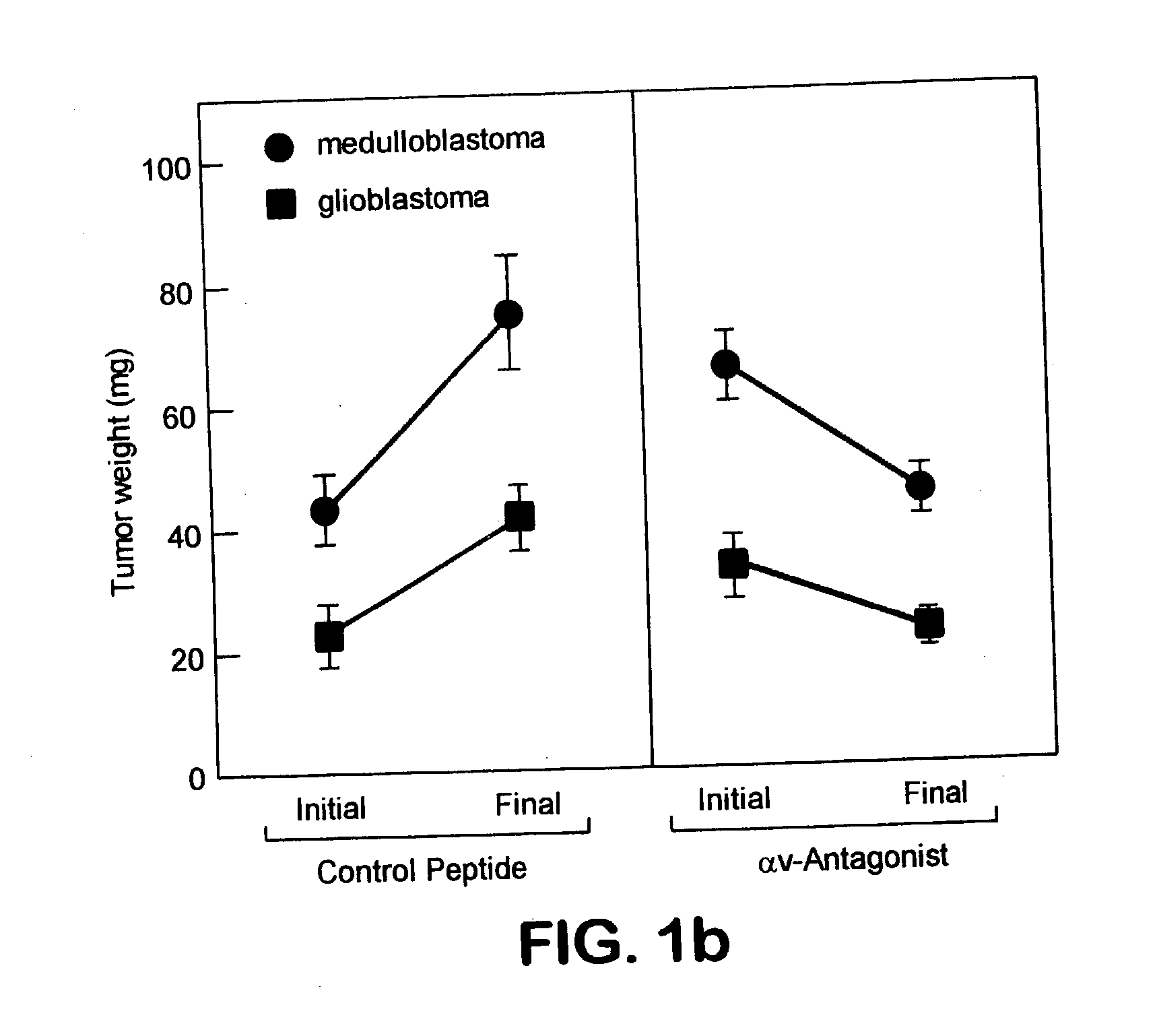
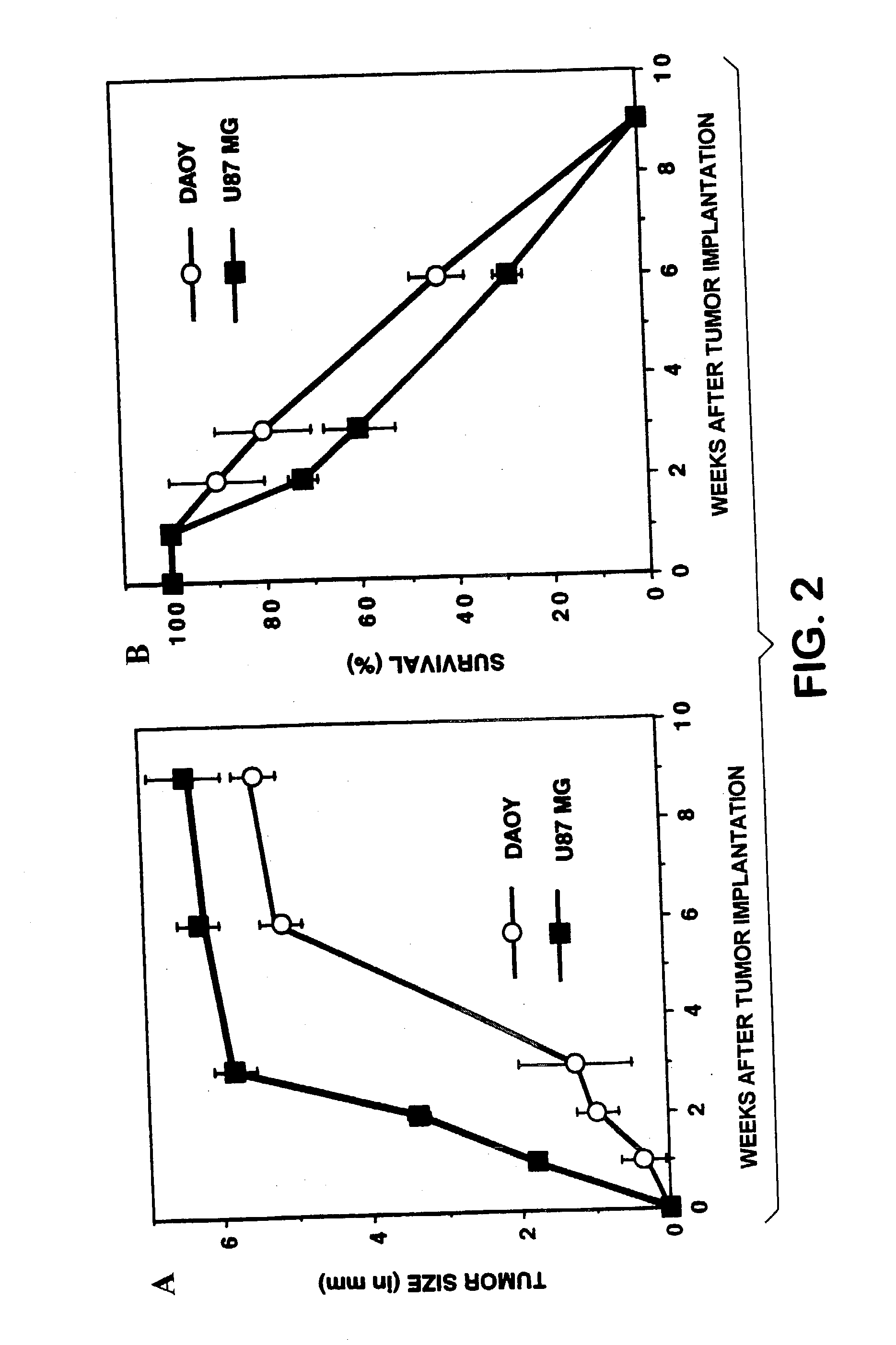
Methods for inhibiting brain tumor growth
InactiveUS20030157098A1Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsTumour tissueCell adhesion
The present invention describes methods for inhibition of tumor growth in the brain, using antagonists of integrins such as alphavbeta3 and alphavbeta5. Antagonists of the present invention can inhibit angiogenesis in brain tumor tissue. They can also inhibit vitronectin and tenascin-mediated cell adhesion and migration in brain tumor cells. They can further induce direct brain tumor cell death.
Owner:CHILDRENS HOSPITAL OF LOS ANGELES
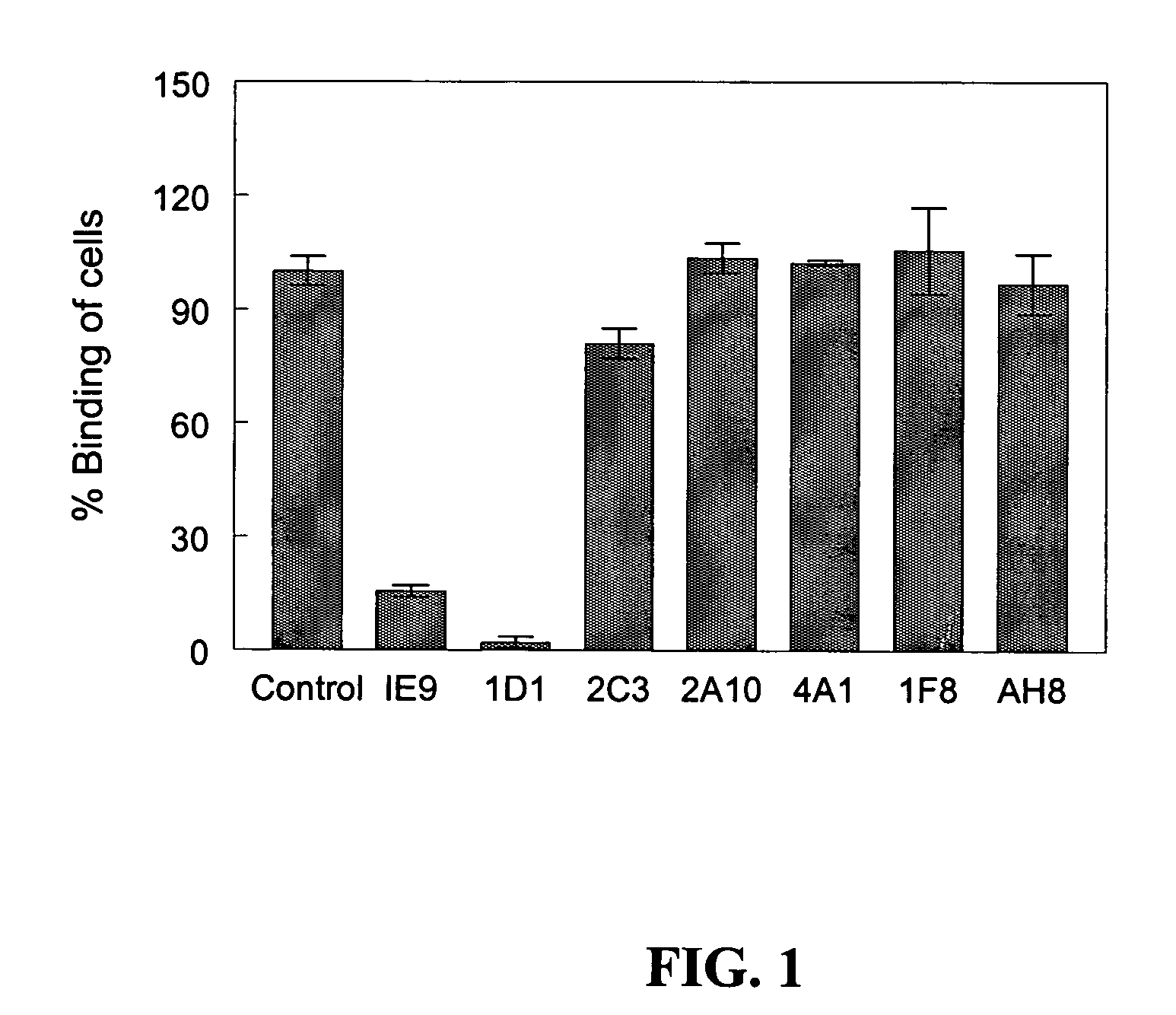
Anti-human vitronectin antibody and methods for making the same
InactiveUS20050048057A1Animal cellsImmunoglobulins against animals/humansVitronectinPercent Diameter Stenosis
The present invention relates to a method for raising a monoclonal antibody against human vitronectin and the purified anti-vitronectin antibody produced by this method. The antibody of the present invention is useful as both a research tool and as an agent for the diagnosis and treatment of cancers, cardiovascular diseases, particularly atherosclerosis and restenosis, osteoporosis, and inflammatory diseases.
Owner:MOLECULAR INNOVATIONS
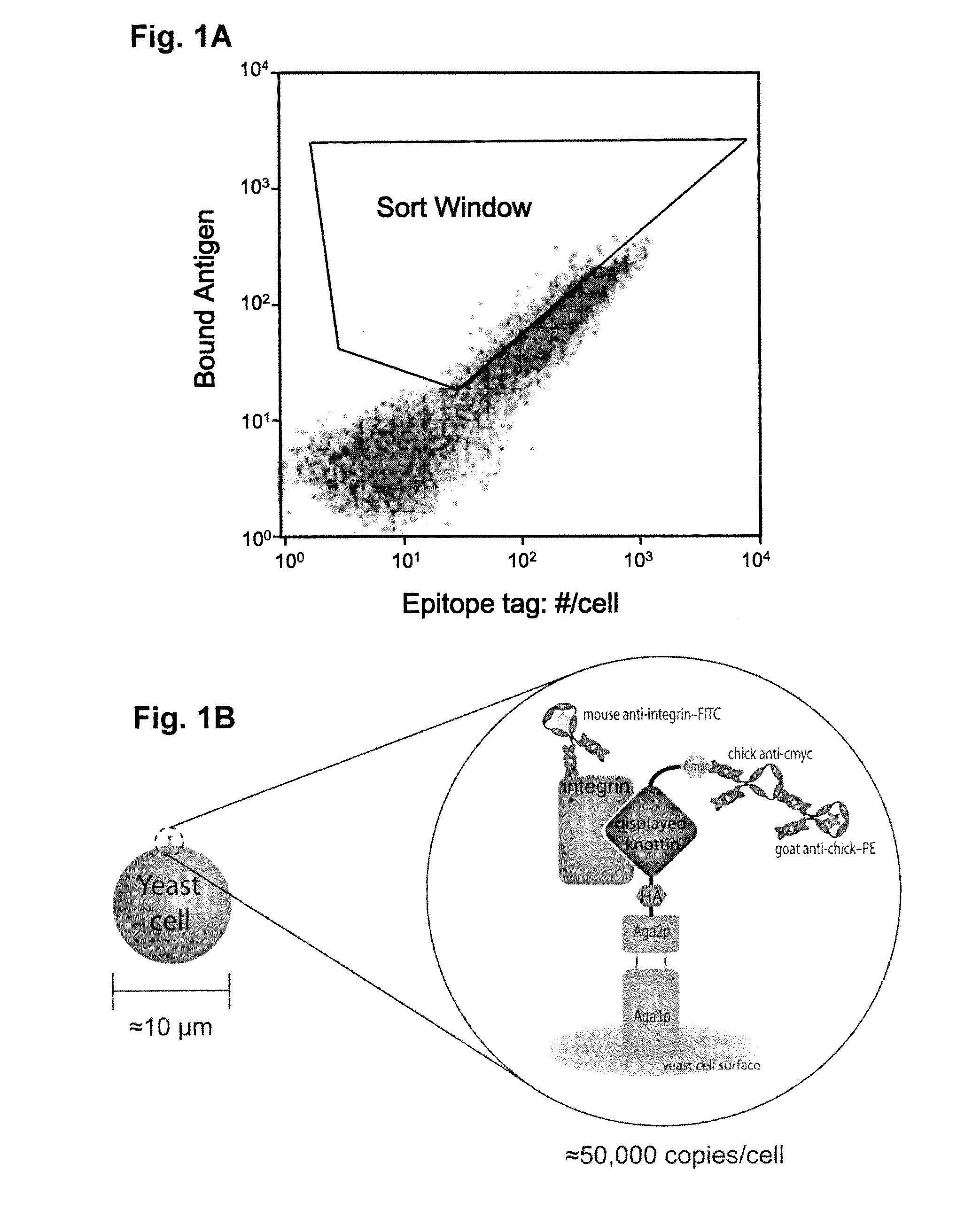
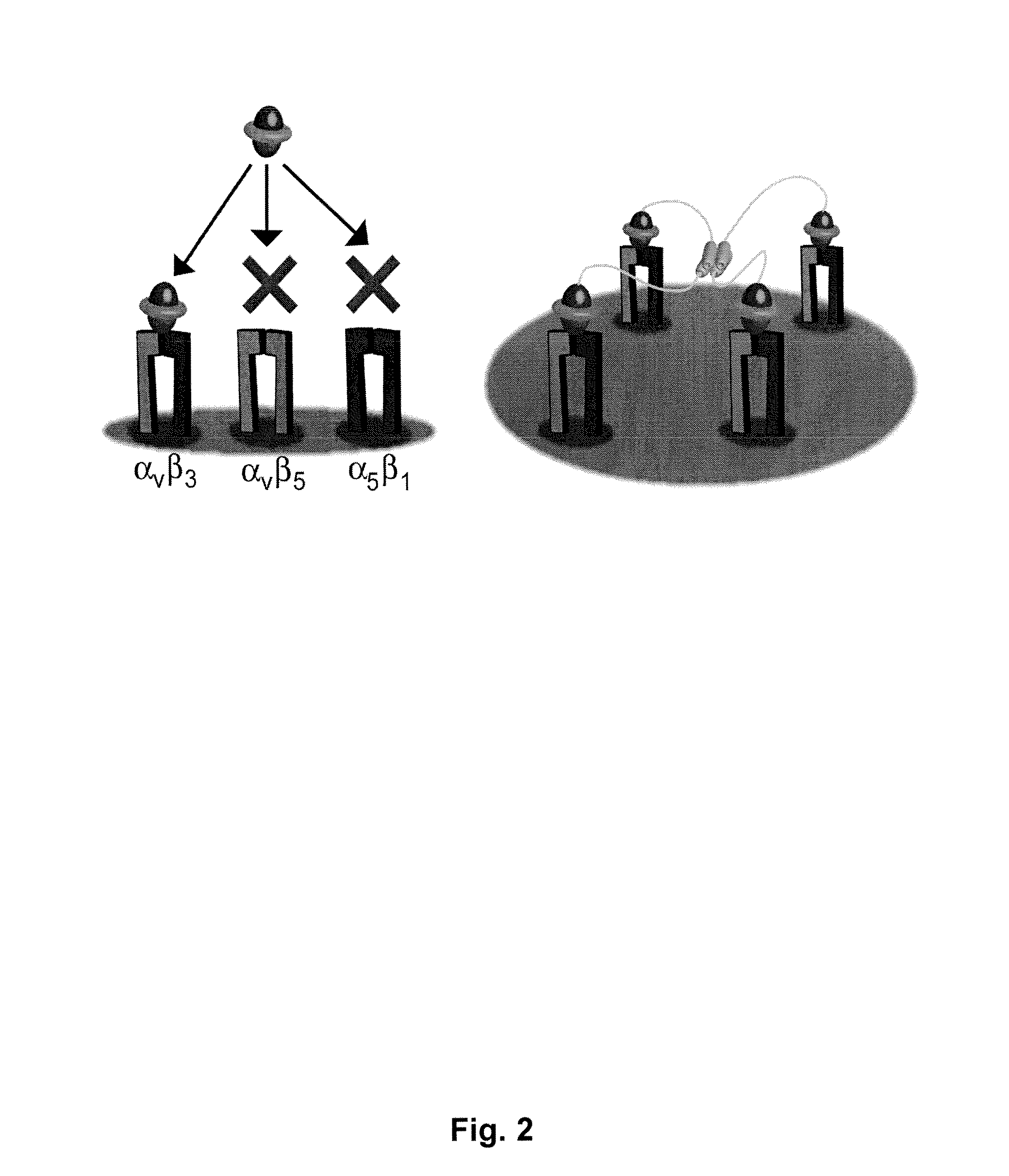
Engineered integrin binding peptides
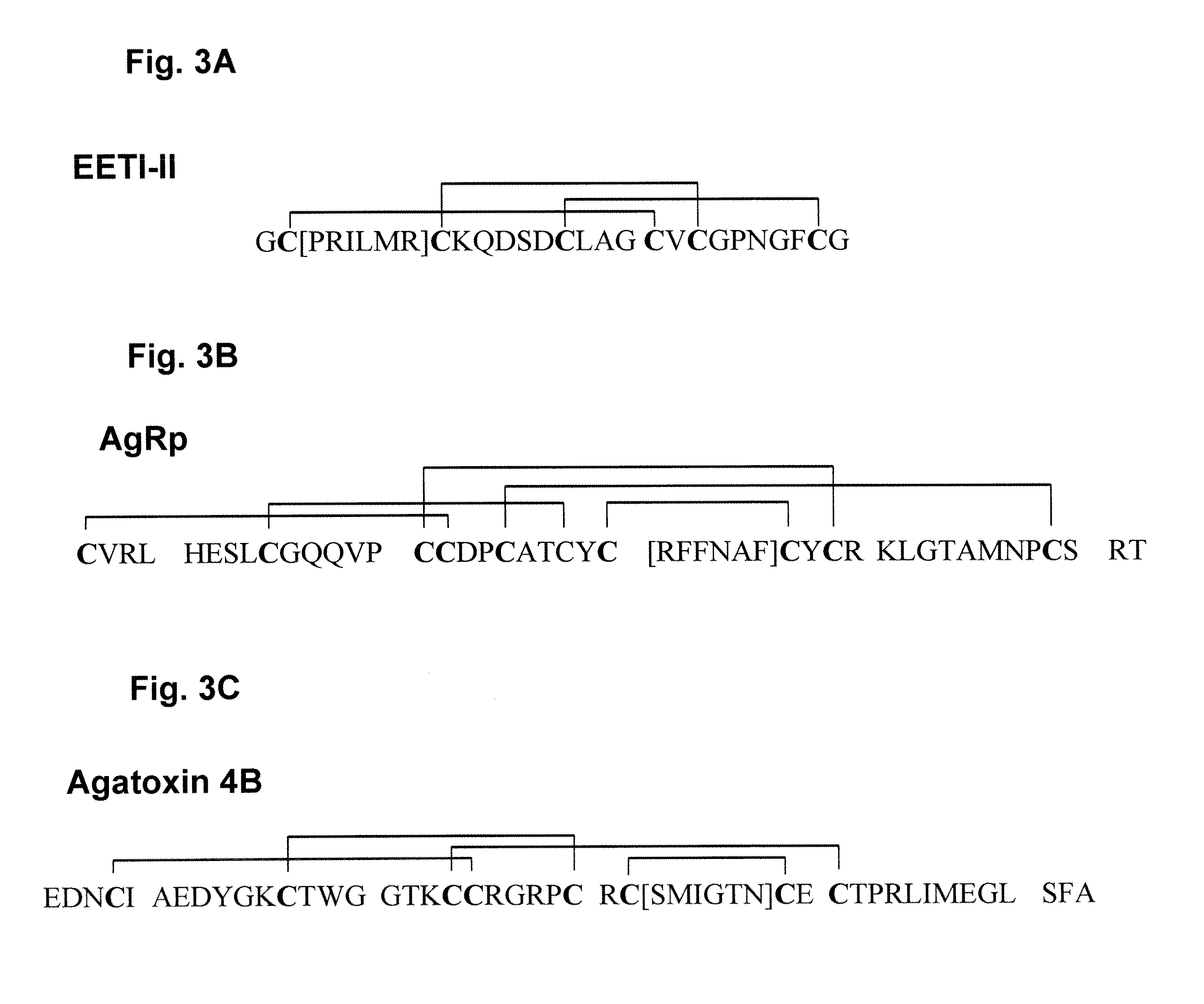
Engineered peptides that bind with high affinity (low equilibrium dissociation constant (Kd)) to the cell surface receptors of fibronectin (α5β1) or vitronectin (αvβ3 and αvβ5 integrins) are disclosed. These peptides are based on a molecular scaffold into which a subsequence containing the RGD integrin-binding motif has been inserted. The subsequence (RGD mimic) comprises about 9-13 amino acids, and the RGD contained within the subsequence can be flanked by a variety of amino acids, the sequence of which was determined by sequential rounds of selection (in vitro evolution). The molecular scaffold is preferably based on a knottin, e.g., EETI (Trypsin inhibitor 2 (Trypsin inhibitor II) (EETI-II) [Ecballium elaterium (Jumping cucumber)], AgRP (Agouti-related protein), and Agatoxin IVB, which peptides have a rigidly defined three-dimensional conformation. It is demonstrated that EETI tolerates mutations in other loops and that the present peptides may be used as imaging agents.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Blocking the migration or metastasis of cancer cells by affecting adhesion proteins and the uses of new compounds thereof
ActiveUS20100004190A1Growth inhibitionReduce adhesionBiocideSugar derivativesDiseaseLymphatic Spread
This invention provides methods, processes, compounds and compositions for modulating the gene expression and modulating the secretion, expression, or synthesis of adhesion proteins or their receptors to cure disease, wherein the modulating comprises positive and negative regulating; wherein comprises inhibiting cancer growth, wherein the adhesion proteins or receptors comprise fibronectin, integrins family, Myosin, vitronectin, collagen, laminin, Glycosylation cell surface proteins, polyglycans, cadherin, heparin, tenascin, CD 54, CAM, elastin and FAK; wherein the methods, processes, compounds and compositions are also for anti-angiogenesis; wherein the cancers comprise breast cancer, leukocyte cancer, liver cancer, ovarian cancer, bladder cancer, prostate cancer, skin cancer, bone cancer, brain cancer, leukemia cancer, lung cancer, colon cancer, CNS cancer, melanoma cancer, renal cancer or cervix cancer.
Owner:PACIFIC ARROW
Human serum-free culture medium and preparation method thereof
ActiveCN102191215AExcellent proliferation rateImprove securityBlood/immune system cellsLipid formationCatalase
The invention discloses a human serum-free culture medium and a preparation method thereof. The human serum-free culture medium uses eleven raw materials, namely human serum albumin solution for treatment, human recombinant insulin solution, human transferrin solution, human cholesterol solution, human catalase solution, 2-mercaptoethanol solution, ascorbic acid solution, linoleic acid solution, ethanolamine solution, human vitronectin solution and L-glutamine solution; the added protein and lipid are both from the blood plasma, serum or tissue of human, the protein is pharmaceutical-grade orhighly purified human protein or human recombinant protein, the protein and lipid do not contains any animal component, the other components all meet the United States Pharmacopoeia or national standards; and the human serum-free culture medium is qualified through the cell culture test and is clinical, safe and reasonable human serum-free culture medium. The proliferation rate of the CIK cell cultured and inducted by the human serum-free culture medium is better than that of the CIK cell cultured and inducted by the culture medium with serum, the cell CD3+CD56+ percentage and the killing rate to the K562 leukemic cell are similar to that of the culture medium with serum. By adopting the human serum-free culture medium, the safety and standardization of cell therapy can be increased.
Owner:湘雅生物医药(湖州)有限公司
Simultaneous imaging of cardiac perfusion and a vitronectin receptor targeted imaging agent
InactiveUS6838074B2In-vivo radioactive preparationsGeneral/multifunctional contrast agentsVitronectinImaging agent
The present invention describes a method of concurrent imaging in a mammal comprising:a) administering to said mammal a vitronectin receptor targeted imaging agent and a perfusion imaging agent; andb) concurrently detecting the vitronectin receptor targeted imaging agent bound at the vitronectin receptor and the perfusion imaging agent; andc) forming an image from the detection of said vitronectin targeted imaging agent and said perfusion imaging agent.
Owner:BRISTOL MYERS SQUIBB CO
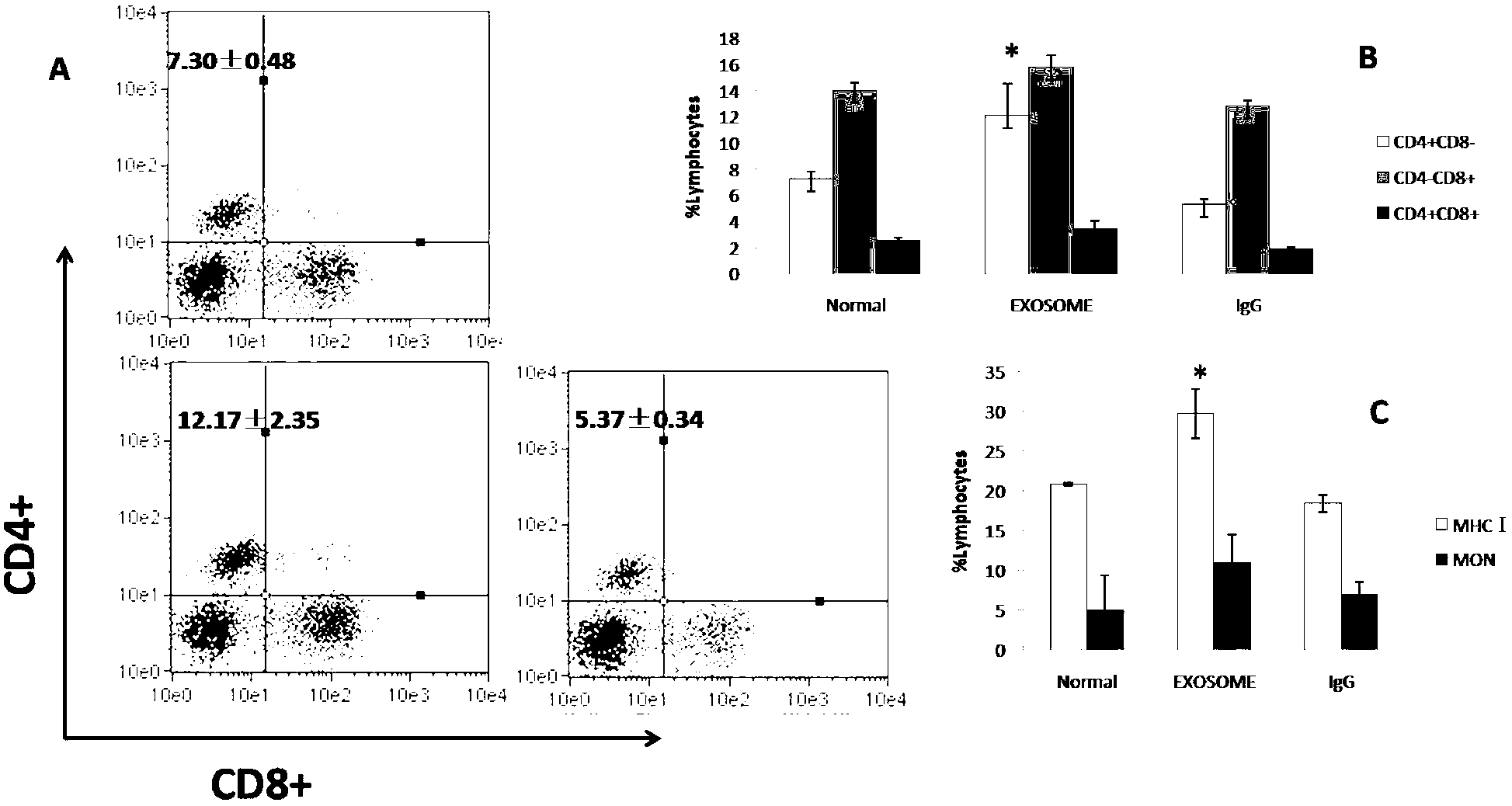
Determination of Exosomel Biomarkers for Predicting Cardiovascular Events
InactiveUS20120309041A1Reduce stepsComplicate to detectMicrobiological testing/measurementImmunoglobulins against animals/humansAmniotic fluidNGAL Protein
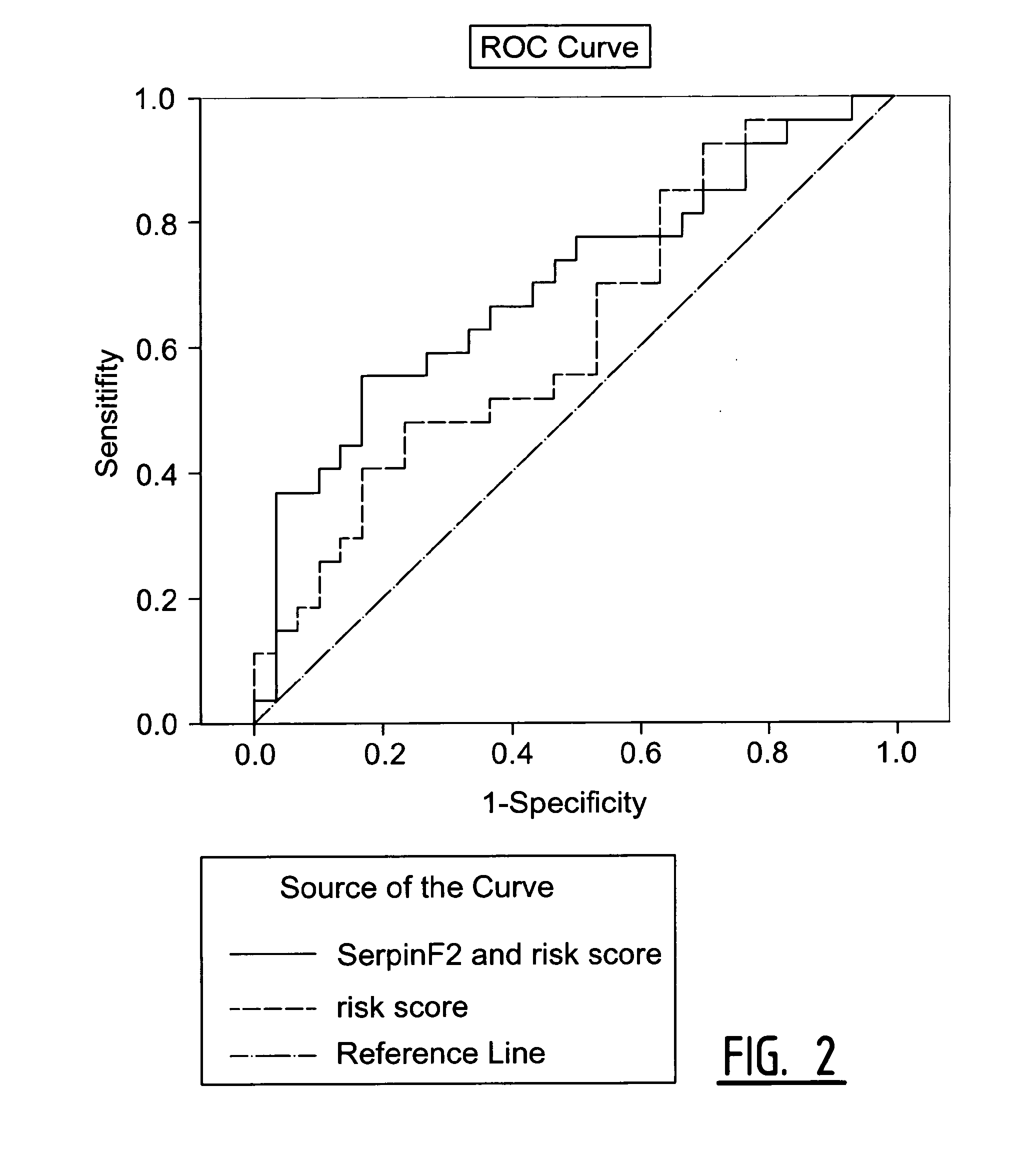
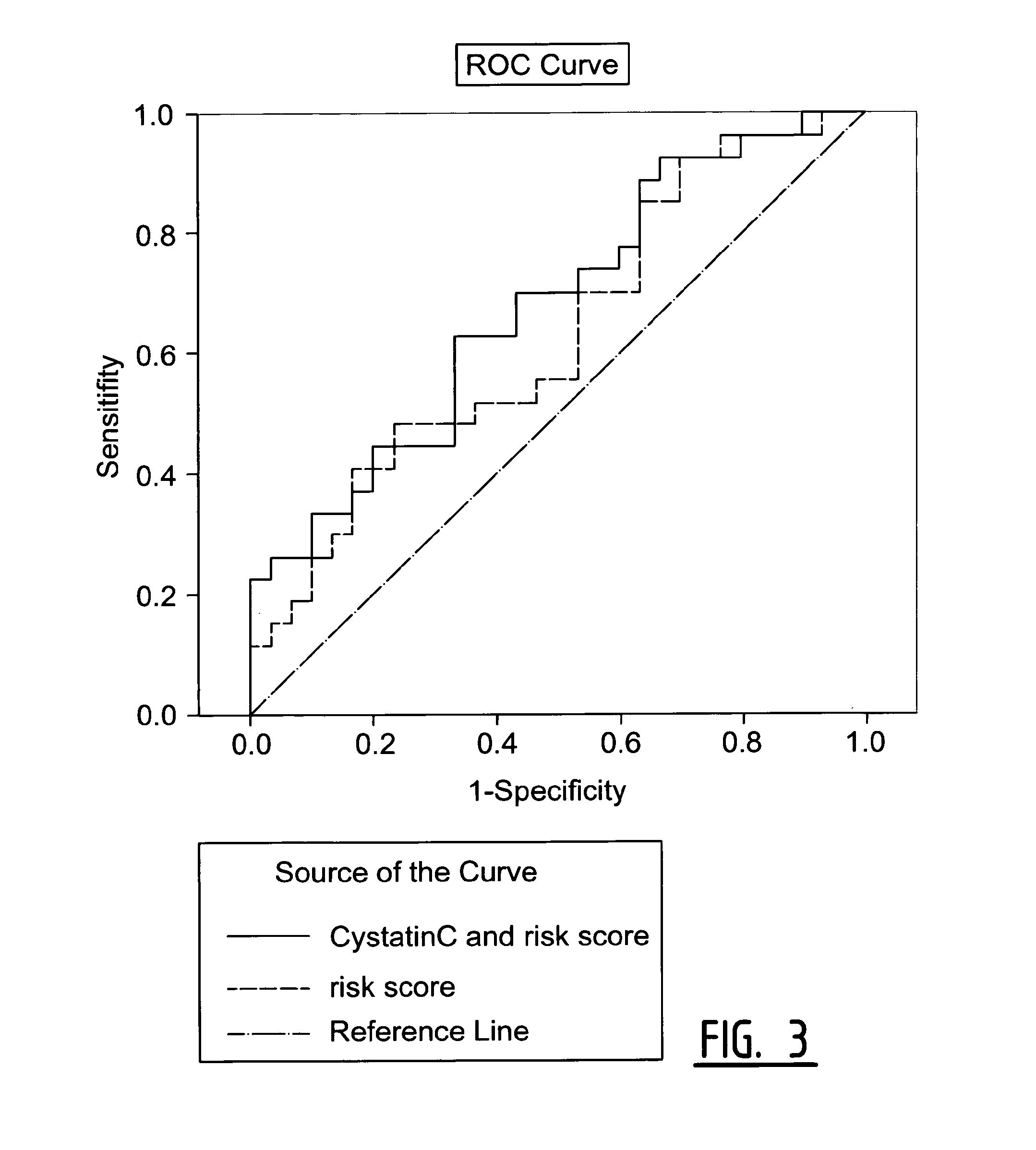
The present invention relates to a method of predicting the risk of a subject developing a cardiovascular event, comprising determining the presence of a biomarker that is indicative of the risk of developing a cardiovascular event in exosomes from the subject. The exosomes are suitably isolated from a body fluid selected from serum, plasma, blood, urine, amniotic fluid, malignant ascites, bronchoalveolar lavage fluid, synovial fluid, breast milk, saliva, in particular serum. The biomarker is selected from the proteins Vitronectin, Serpin F2, CD14, Cystatin C, Plasminogen, Nidogen 2 or any combination of two or more of these proteins.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC
Method for obtaining spinal motoneurons and functional cells thereof based on human iPS (induced pluripotent stem) cells
ActiveCN106701824APromote directed differentiationRapid directed differentiationGenetically modified cellsNervous system cellsDisease phenotypeCells transplantation
The invention belongs to the field of biomedicine, and relates to a safe and reliable reprogrammed method for obtaining human iPS (induced pluripotent stem) cells, a method for simply, rapidly and efficiently inducing and differentiating iPS cells into spinal motoneurons, and a method for economically and efficiently obtaining functional spinal motoneurons and application of the method. Human fibroblasts are infected with Sendai virus carrying a reprogramming factor by using vitronectin as the sole growth medium, and are cultured for 3 weeks to obtain human iPS cells without exogenous serum interference; the iPS cells are efficiently induced and differentiated into spinal motoneurons by a combination of multiple small molecule compounds; and further, an astrocyte conditioned culture medium promotes maturation culture and is applied in the formation of neuromuscular junctions. The functional spinal motoneurons obtained by efficient induction can be applied to simulation of disease phenotype and discussion of pathogenesis, and provides a theoretical basis for the screening of effective therapeutic drugs in the future, and even in the future can be applied to the cell transplantation therapy of diseases.
Owner:THE FIRST AFFILIATED HOSPITAL OF FUJIAN MEDICAL UNIV
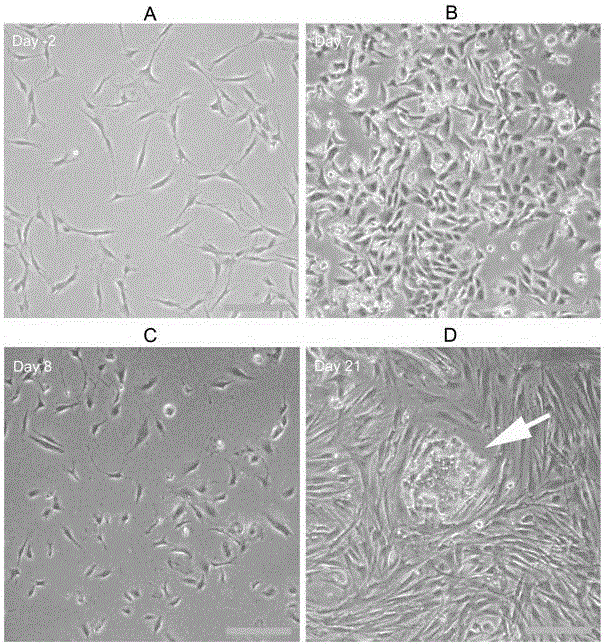
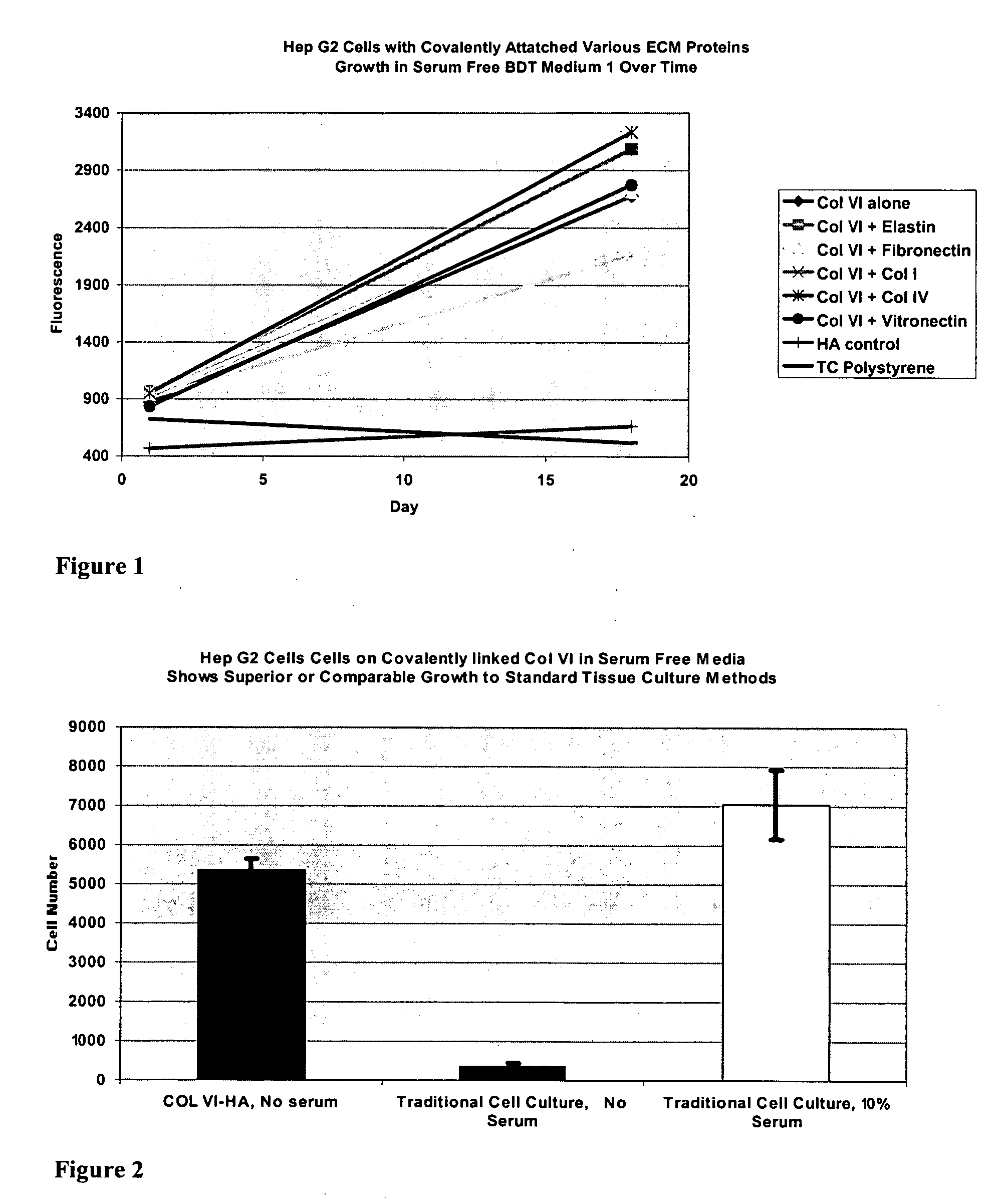
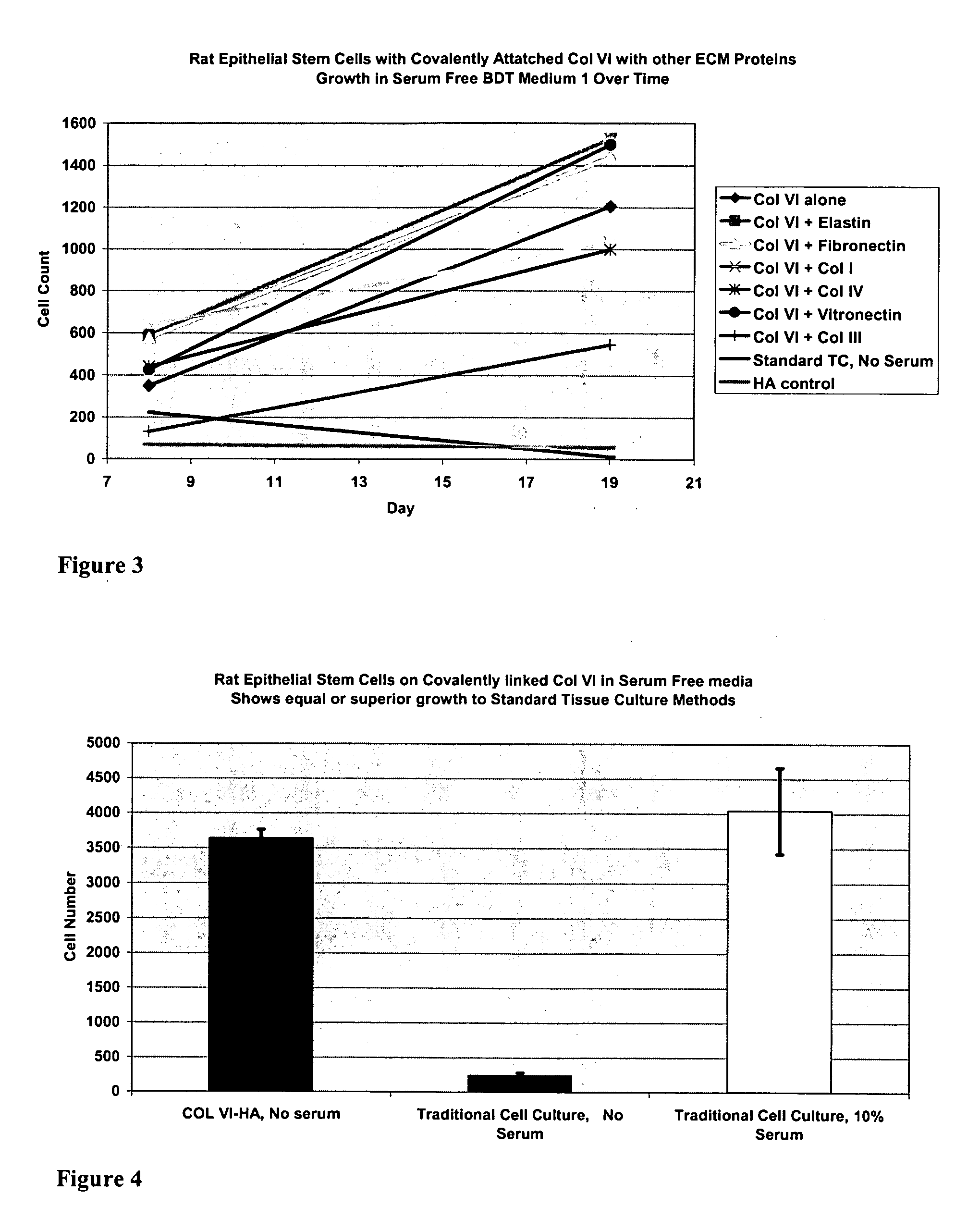
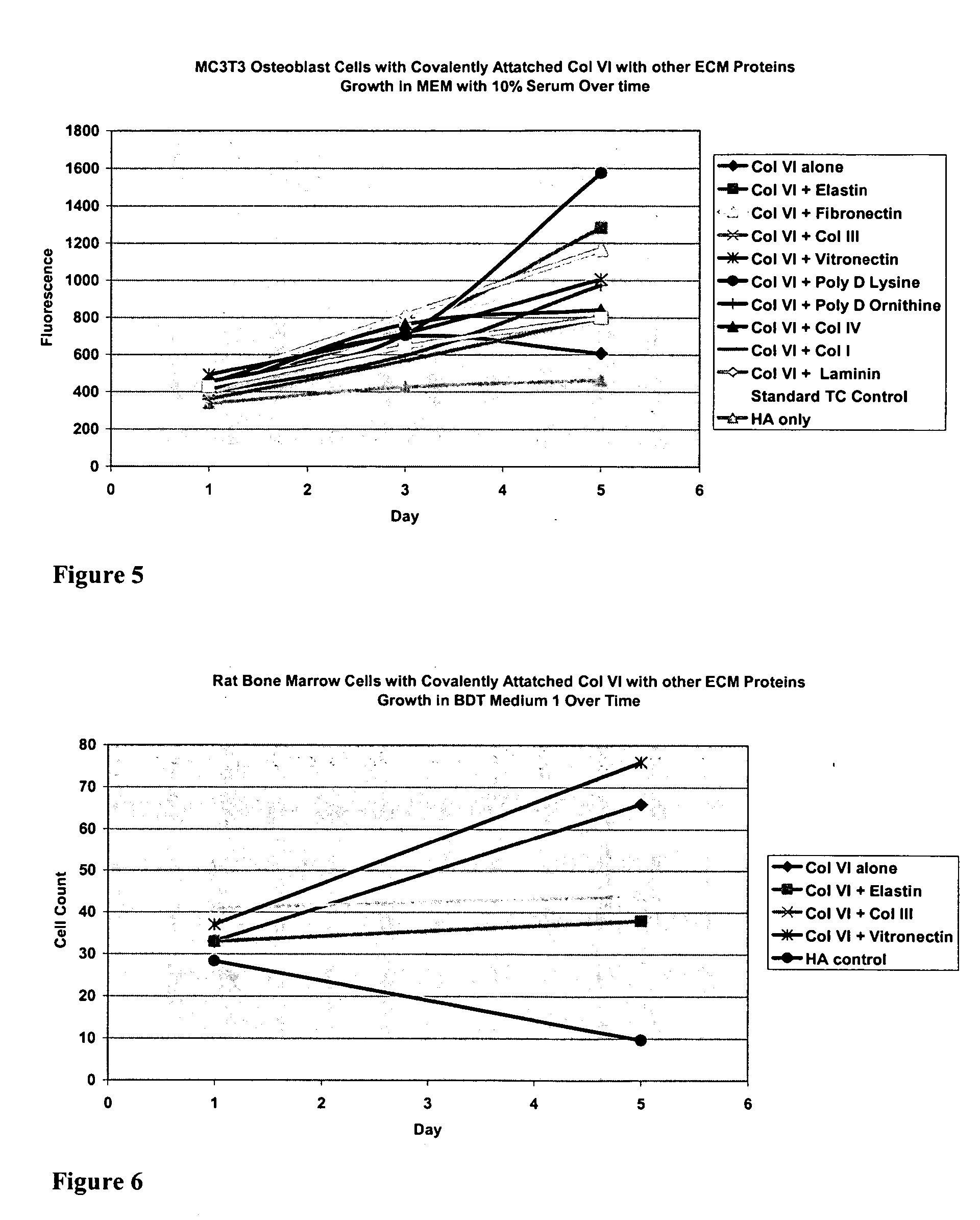
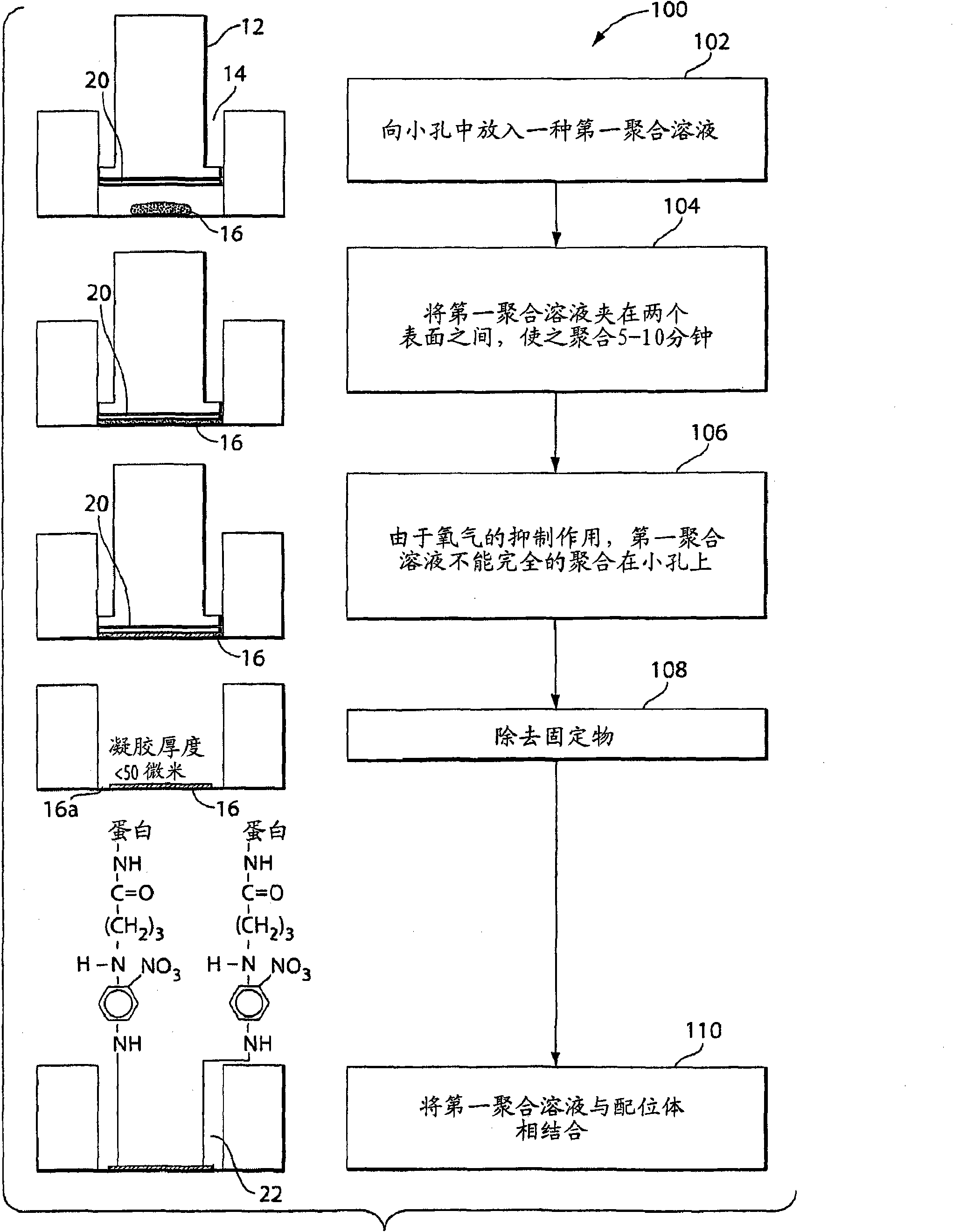
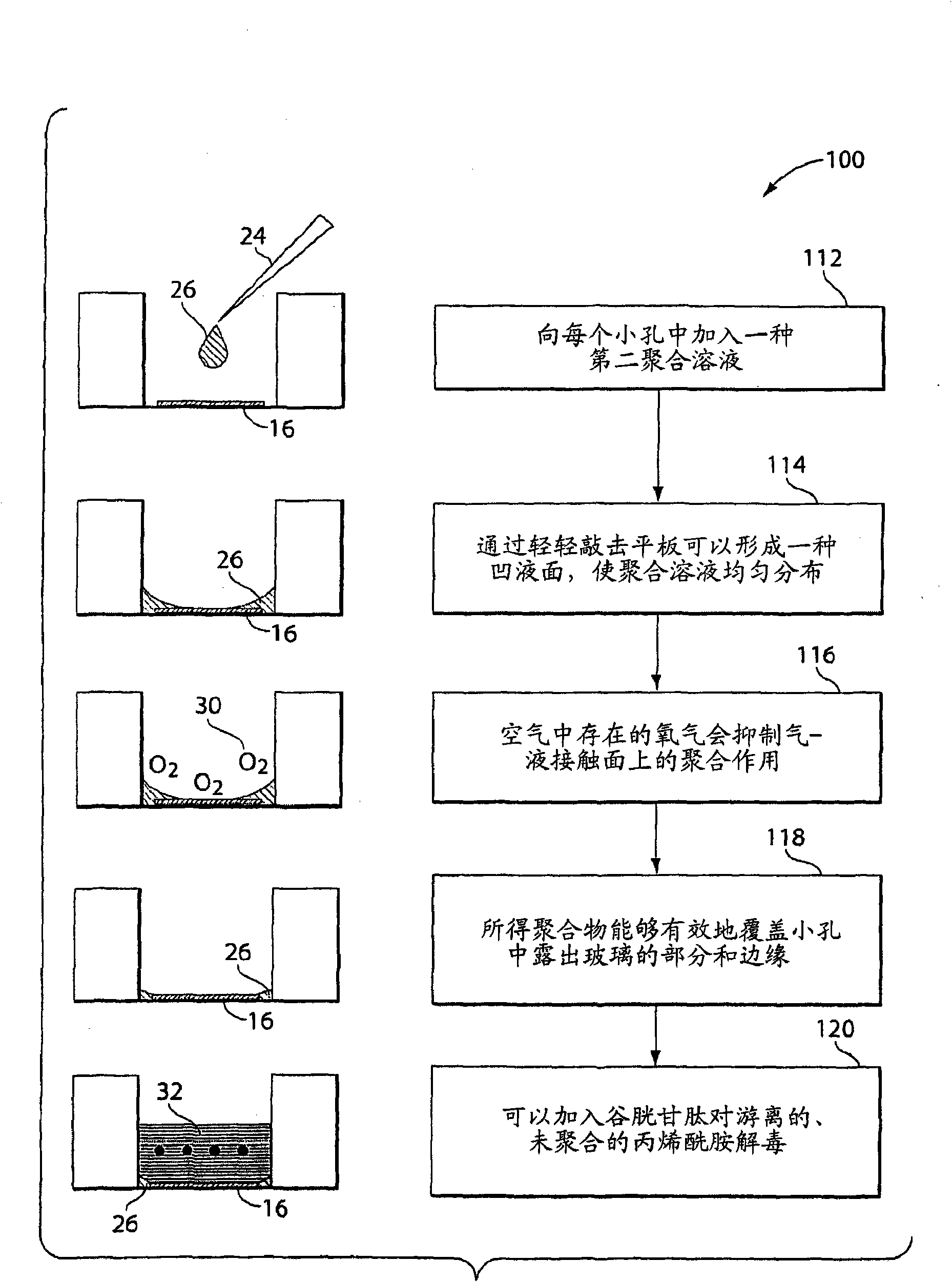
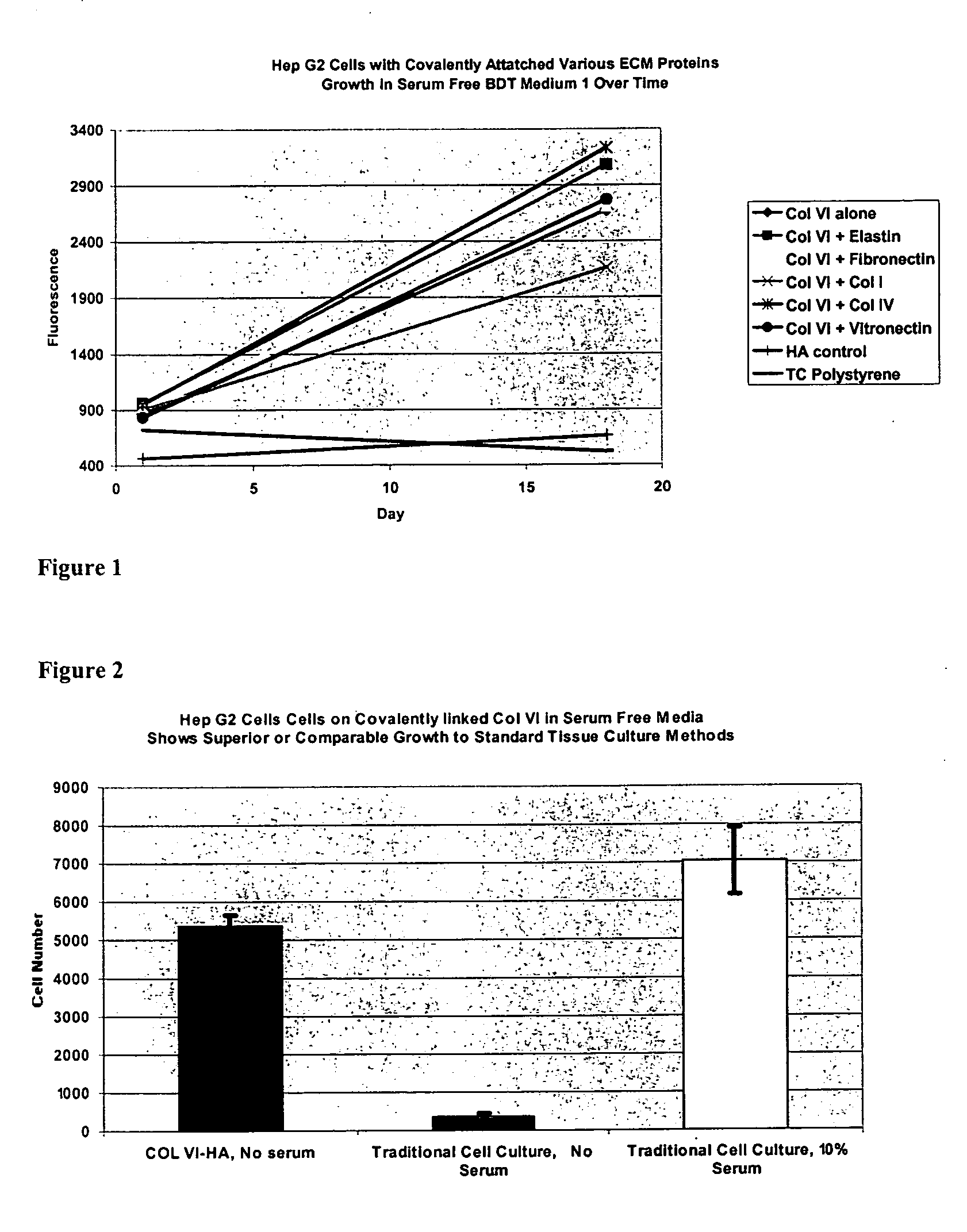
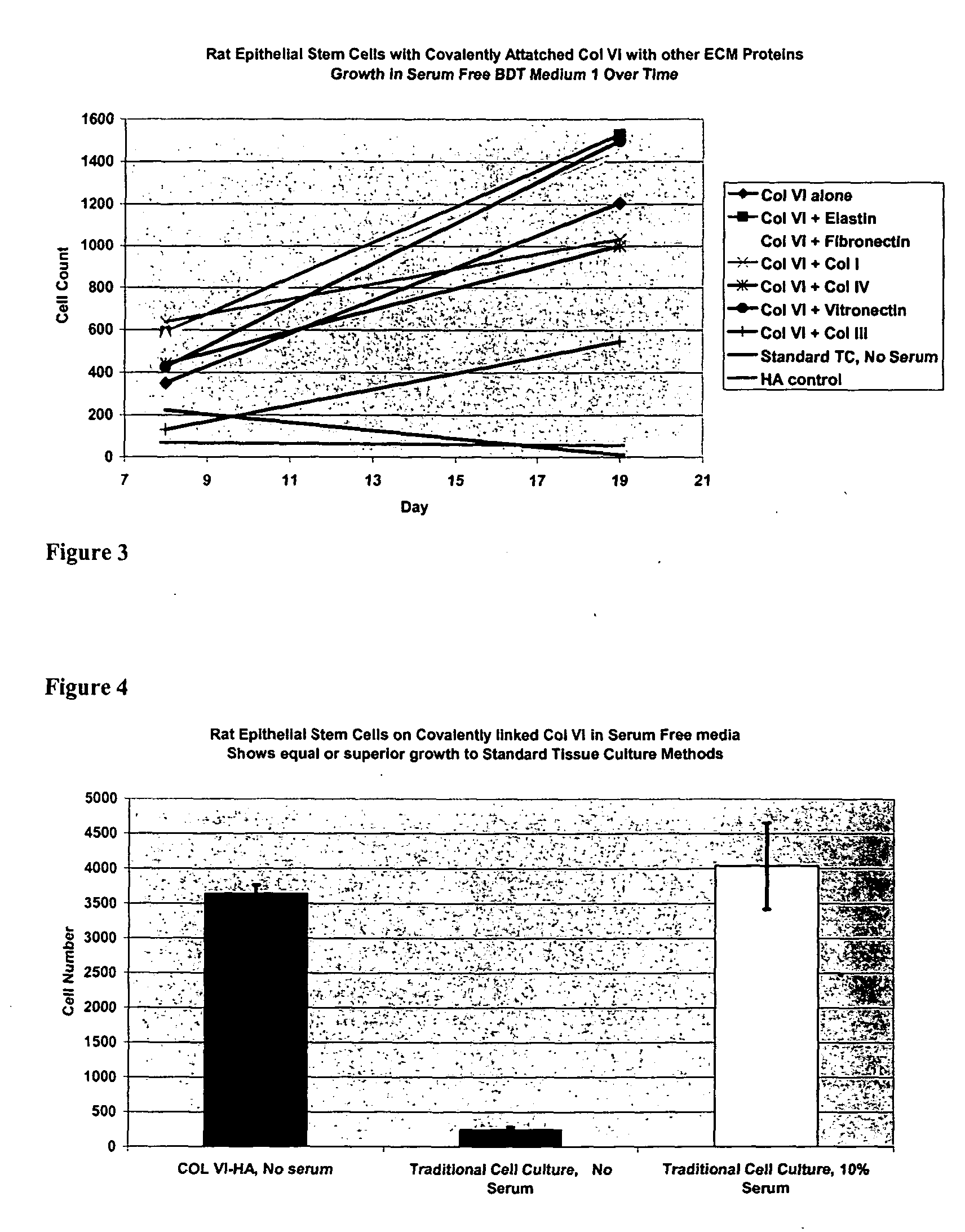
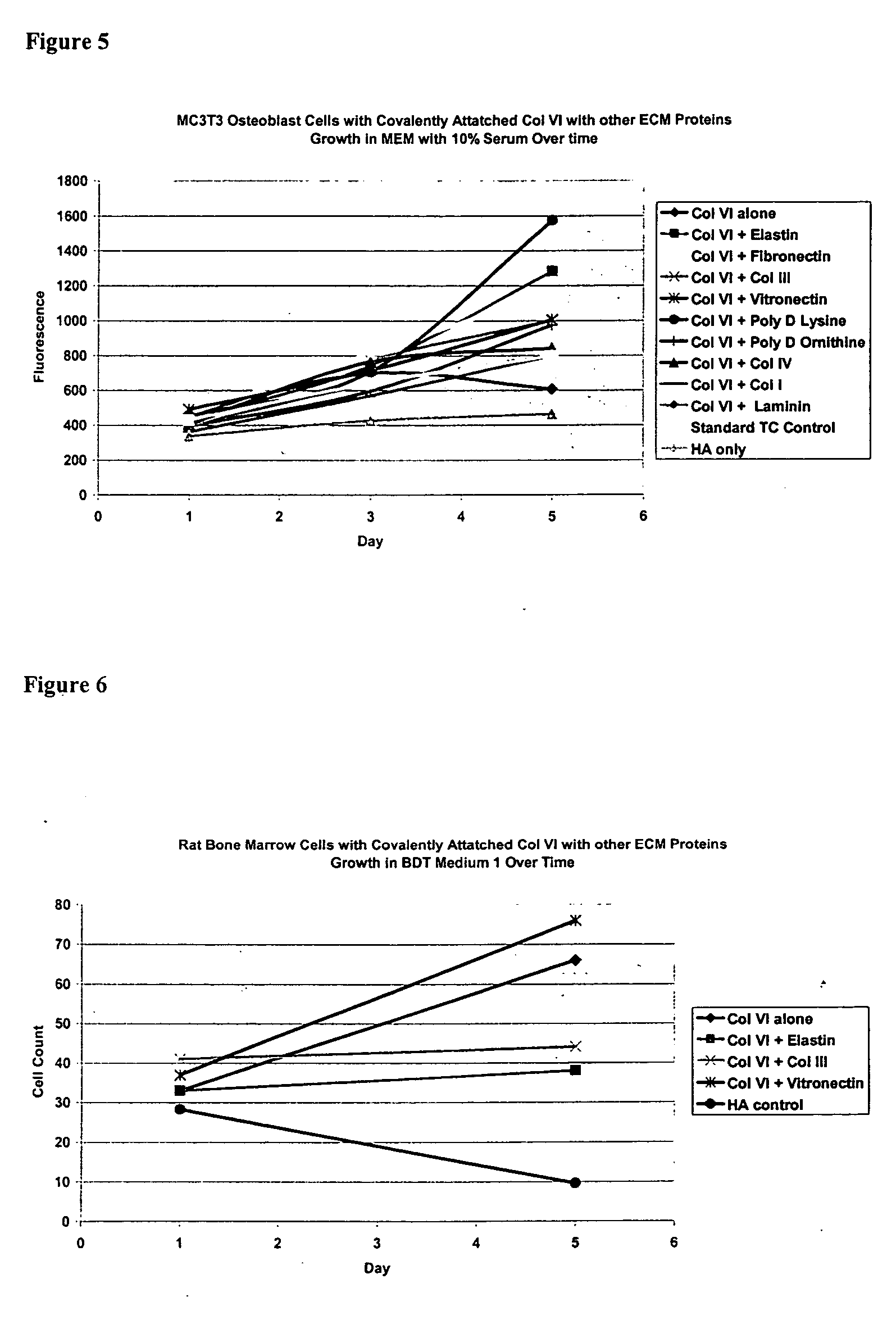
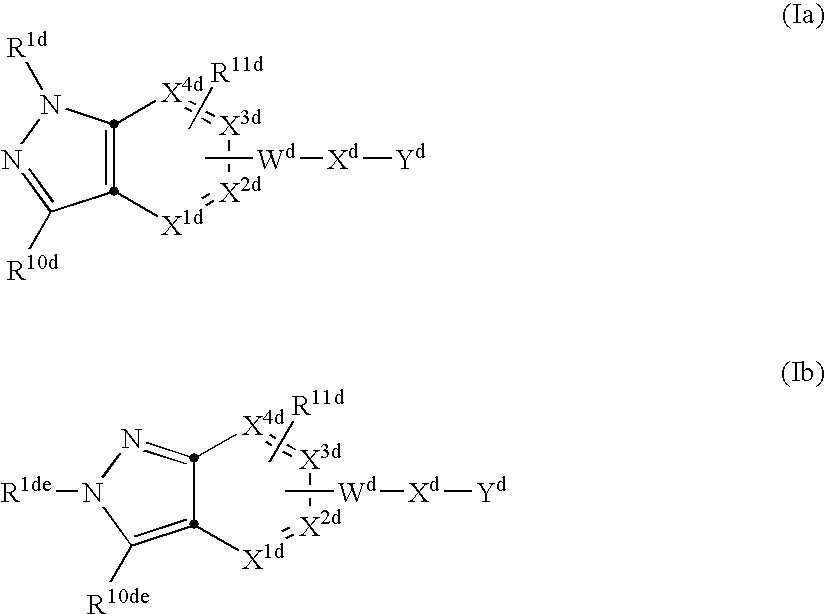
Covalently attached collagen VI for cell attachment and proliferation
Surfaces useful for cell culture comprise a support to which is bound a CAR material, and, bound to the CAR material, collagen VI or a biologically active fragment or variant thereof and, optionally, one or more other ECM proteins (or fragments or variants thereof) such as elastin, fibronectin, vitronectin, tenascin, laminin, entactin, aggrecan, decorin, collagen I, collagen III, and collagen IV. Also, optionally present on the surface is one or more polycationic polymers, such as poly-D-lysine or poly-D-ornithine. This surface is used in cell culture to promote cell attachment, survival, and / or proliferation of a number of different cell types such as (a) liver cells (e.g., HepG2 tumor cells, and a newly discovered line of rat liver epithelial stem cells) (b) osteoblasts, such as the murine cell line MC3T3 cell line and (c) primary bone marrow cells. Kits comprising the surfaces and additional reagents are also disclosed.
Owner:BECTON DICKINSON & CO
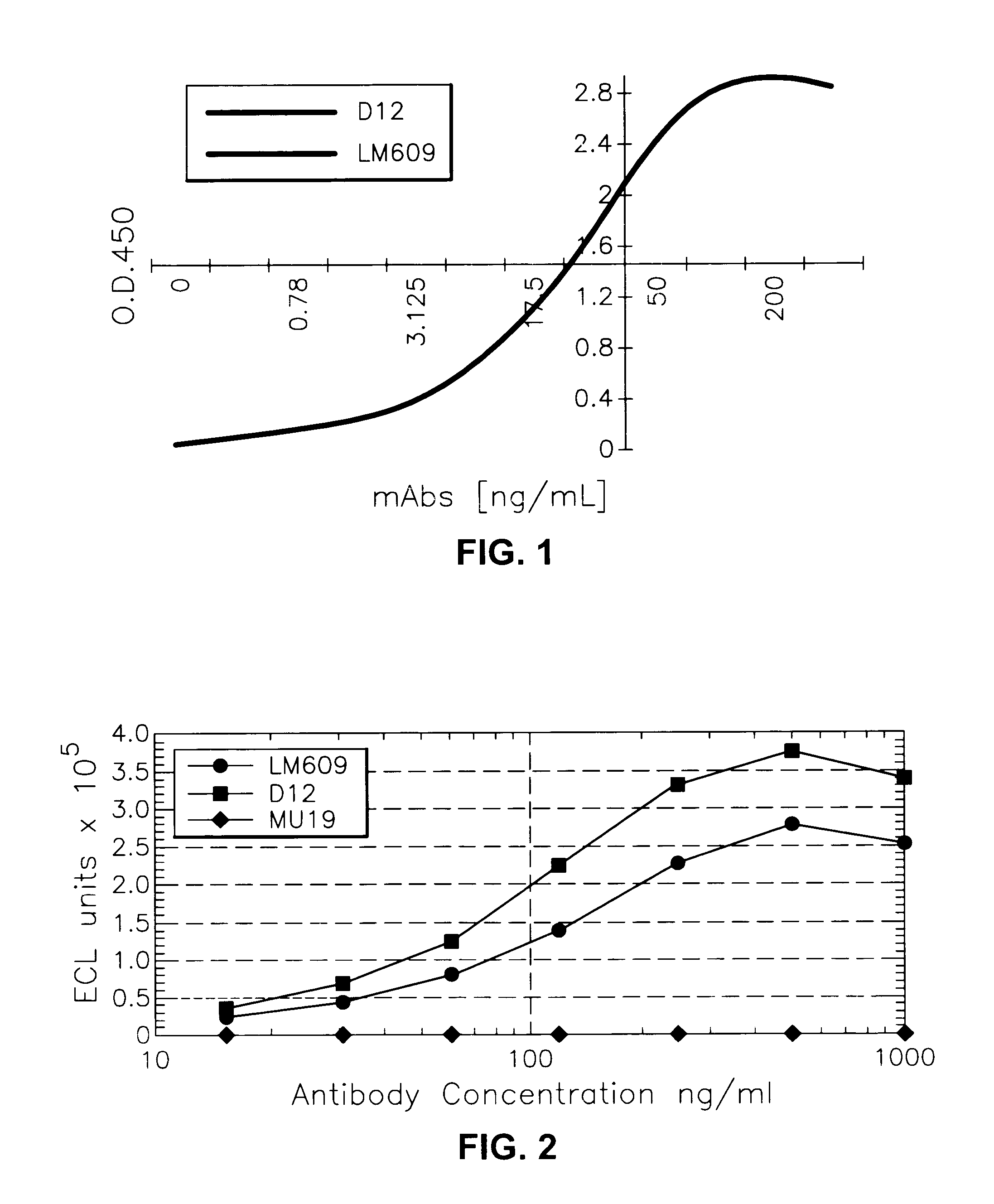
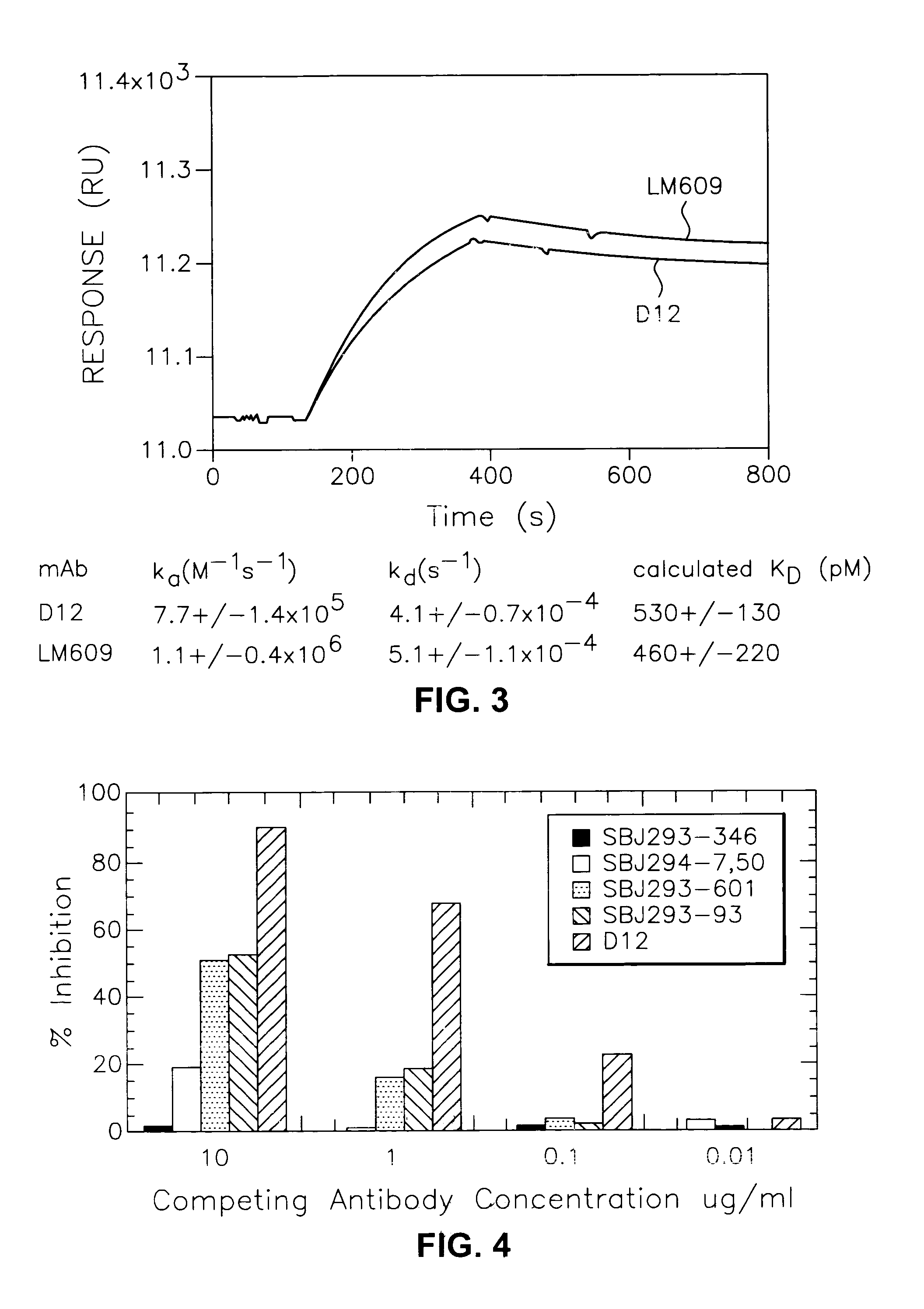
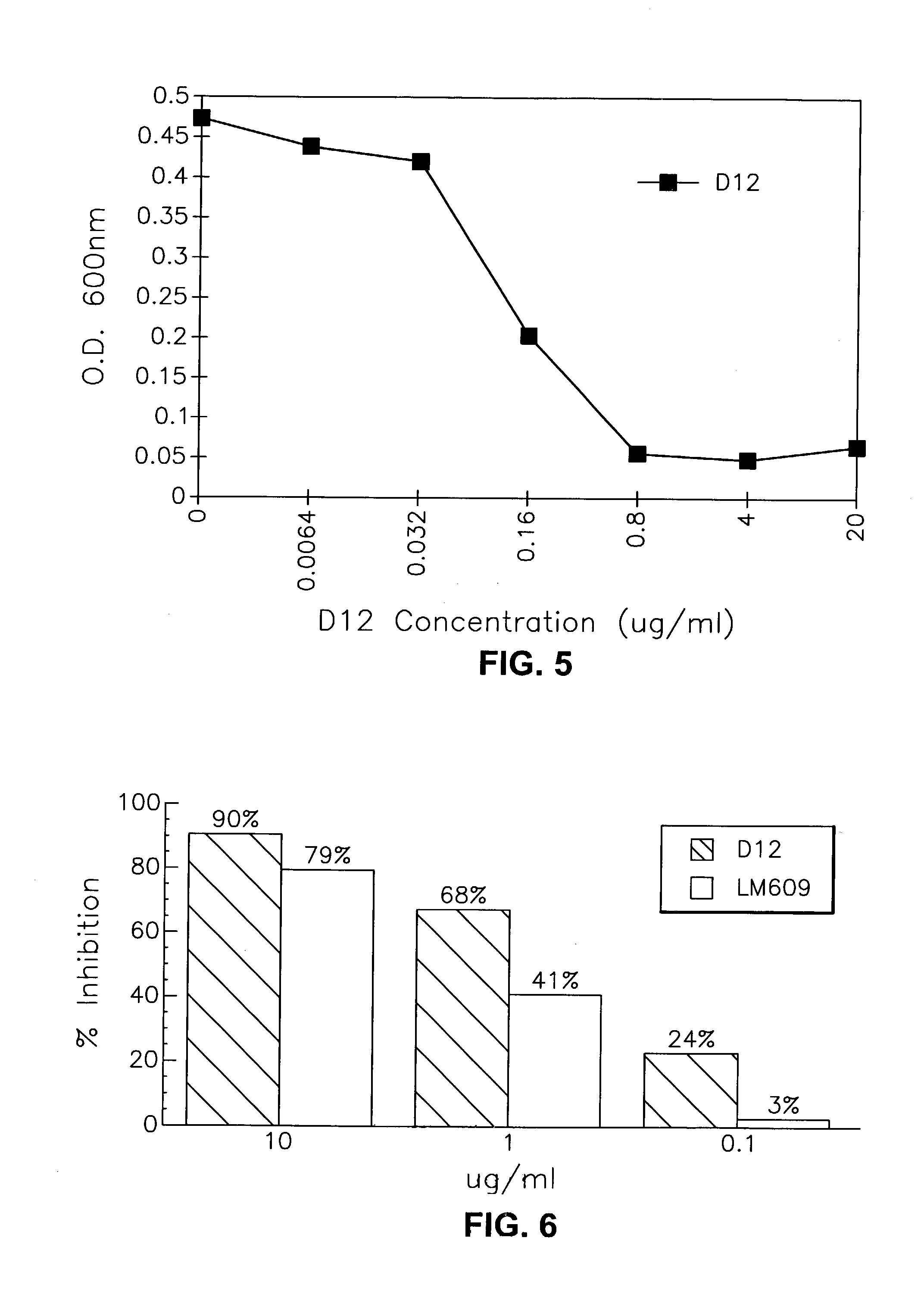
Anti-alphabeta3 humanized monoclonal antibodies
This invention relates to novel humanized and other recombinant or engineered antibodies or monoclonal antibodies to the vitronectin αvβ3 receptor and to the genes encoding same. Such antibodies are useful for the therapeutic and / or prophylactic treatment of αvβ3-mediated disorders, such as restenosis, in human patients.
Owner:SMITHKLINE BECKMAN CORP
Compliant surface multi-well culture plate
InactiveCN101842474ABioreactor/fermenter combinationsBiological substance pretreatmentsCollagen iEngineering
A multi-well plate can be loaded with a range of compliant substrates. Commerically-available assays can be used to test cellular responses across a plate with shear modulus from 50 to 51200 Pascals. Cells can be grown in the plates, and can be manipulated and analyzed. Hydrogels can be attached to the bottom of a well. The plates can support the attachment and growth of different cell types and can be compatible with standard 96-well and 384-well plate assays. The mechanical properties of the hydrogels can be reproducible and stable to increase the shelf life of the substrate. The hydrogel can be compatible with growth of a variety of cell types, various attachment ligands such as collagen I, collagen IV, flbronectin, vitronectin, laminin, or RGD peptides and can be coupled to the gel surface.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Covalently attached collagen VI for cell attachment and proliferation
InactiveUS20050058687A1Promote proliferationEasy SurvivalBioreactor/fermenter combinationsBiological substance pretreatmentsCollagen VIPolymer
Surfaces useful for cell culture comprise a support to which is bound a CAR material, and, bound to the CAR material, collagen VI or a biologically active fragment or variant thereof and, optionally, one or more other ECM proteins (or fragments or variants thereof) such as elastin, fibronectin, vitronectin, tenascin, laminin, entactin, aggrecan, decorin, collagen I, collagen III, and collagen IV. Also, optionally present on the surface is one or more polycationic polymers, such as poly-D-lysine or poly-D-ornithine. This surface is used in cell culture to promote cell attachment, survival, and / or proliferation of a number of different cell types such as (a) liver cells (e.g., HepG2 tumor cells, and a newly discovered line of rat liver epithelial stem cells) (b) osteoblasts, such as the murine cell line MC3T3 cell line and (c) primary bone marrow cells. Kits comprising the surfaces and additional reagents are also disclosed.
Owner:BECTON DICKINSON & CO
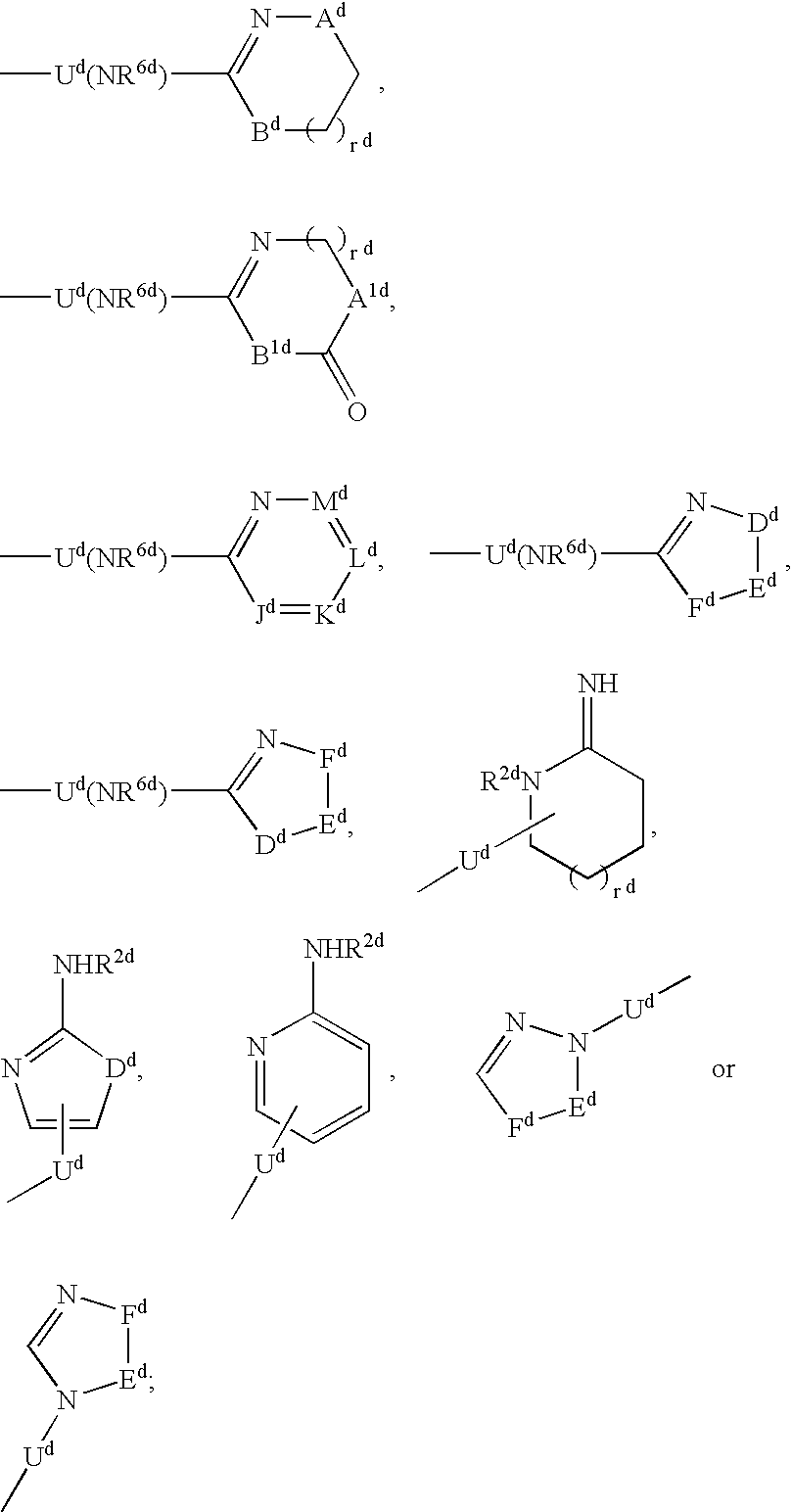
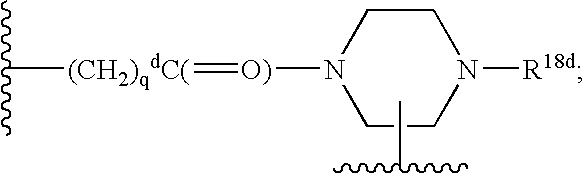
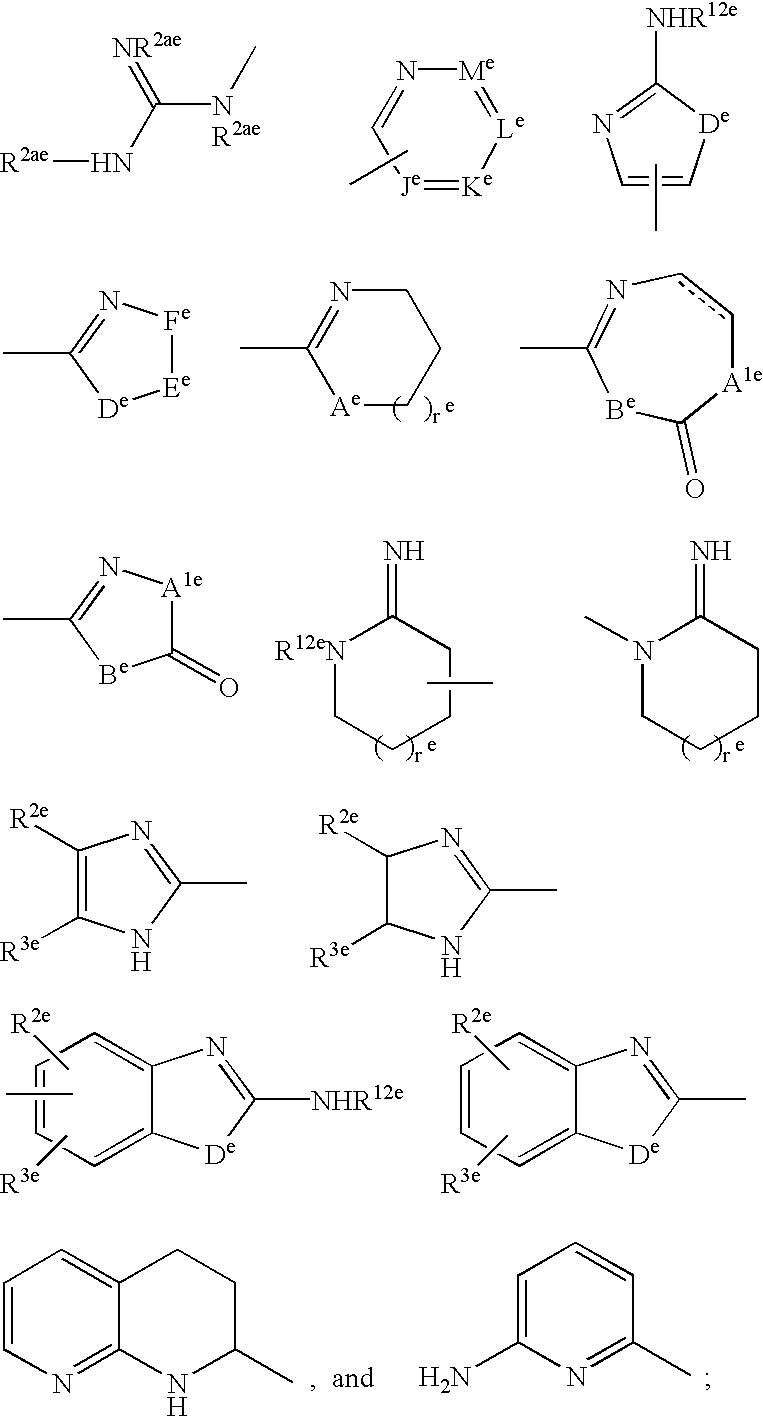
Vitronectin receptor antagonist pharmaceuticals
InactiveUS20050154185A1Improve stabilityGeneral/multifunctional contrast agentsImmunoglobulinsVitronectinUltrasound contrast media
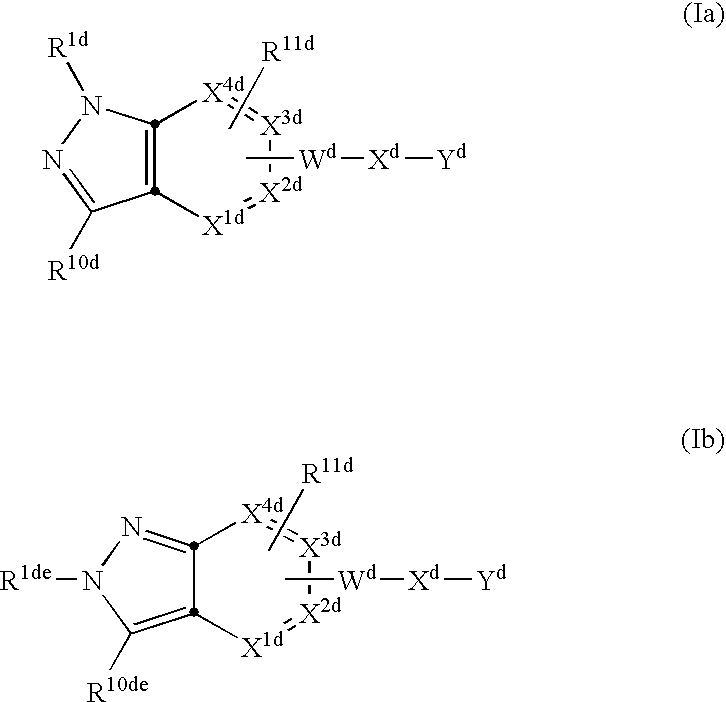
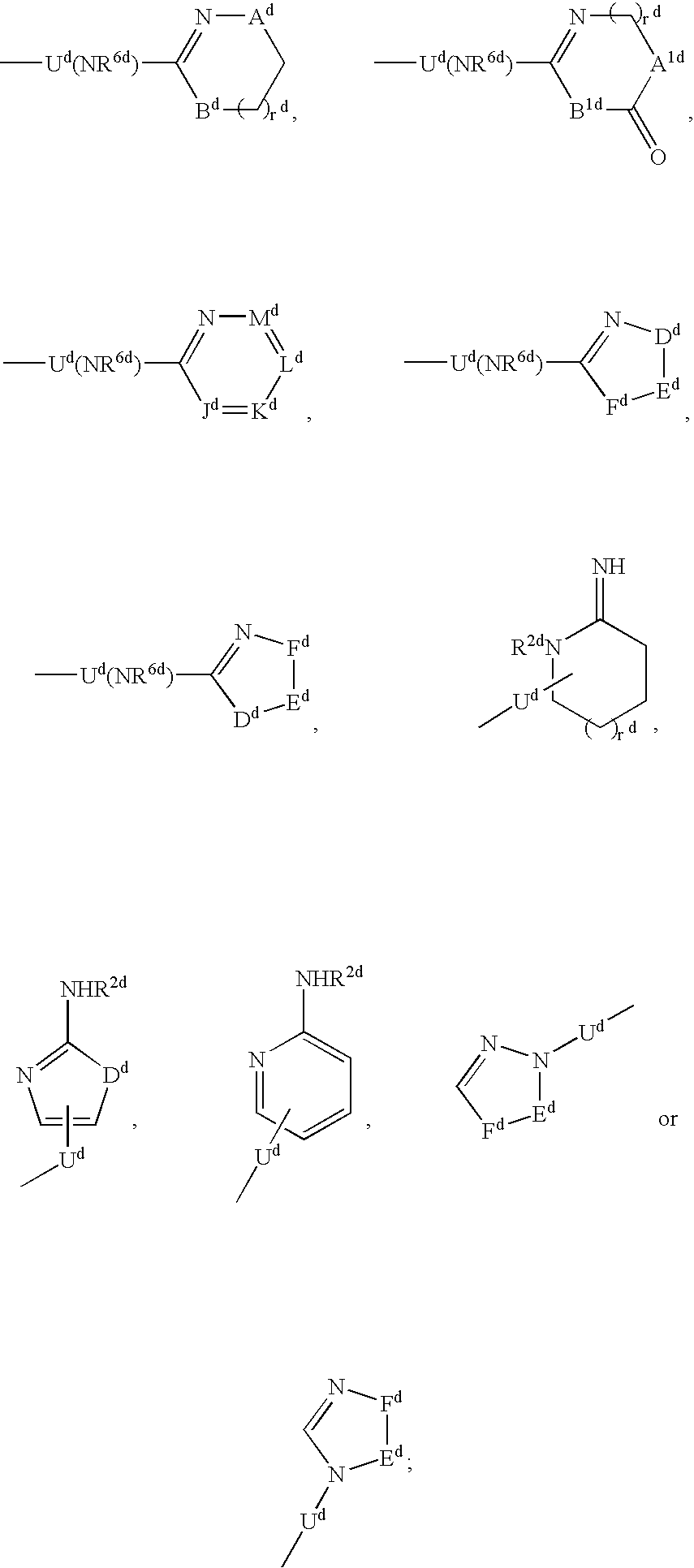
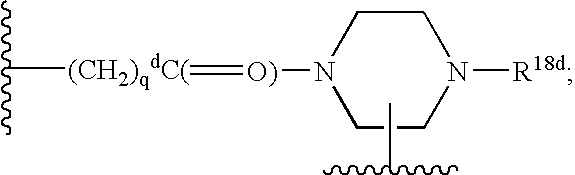
The present invention describes novel compounds of the formula: (Q)d-Ln-Ch, useful for the diagnosis and treatment of cancer, methods of imaging tumors in a patient, and methods of treating cancer in a patient. The present invention also provides novel compounds useful for monitoring therapeutic angiogenesis treatment and destruction of new angiogenic vasculature. The present invention also provides novel compounds useful for imaging atherosclerosis, restenosis, cardiac ischemia, and myocardial reperfusion injury. The present invention also provides novel compounds useful for the treatment of rheumatoid arthritis. The pharmaceuticals are comprised of a targeting moiety that binds to a receptor that is upregulated during angiogenesis, an optional linking group, and a therapeutically effective radioisotope or diagnostically effective imageable moiety. The imageable moiety is a gamma ray or positron emitting radioisotope, a magnetic resonance imaging contrast agent, an X-ray contrast agent, or an ultrasound contrast agent.
Owner:LANTHEUS MEDICAL IMAGING INC
Method for extracting exosome from chicken bile and application of exosome in immunology
InactiveCN102697812AEasy to maintain biological activityExtraction time is shortDigestive systemAntibody ingredientsDiseaseIntestinal structure
The invention belongs to the field of molecular immunology, and in particular relates to an exosome secreted and released from the epidermal cells of a chicken liver, gall bladder and bile duct, and a preparation method of the exosome. The exosome contains hepatogenic proteins, such as C-reactive protein, aspartate aminotransferase, and vitronectin, epithelium-derived mucin and proteins related to an immune reaction, such as immunoglobulin, and can be taken as a biological indicator for studying liver diseases and as an adjuvant for immune reaction for immune-regulation, and can also be takenas a carrier for treating the diseases of livers, gall bladders and intestines.
Owner:SHANDONG AGRICULTURAL UNIVERSITY
Vitronectin:keratinocyte growth factor chimeras
InactiveUS9562086B2Easy to producePeptide/protein ingredientsAntibody mimetics/scaffoldsCancer cellProtein-protein complex
Isolated protein complexes are provided comprising keratinocyte growth factor and vitronectin, or at least domains thereof that enable binding to and activation of both a keratinocyte growth factor receptor and an integrin receptor for vitronectin. These protein complexes include synthetic proteins where the keratinocyte growth factor and vitronectin sequences are joined by a linker sequence. In particular forms, vitronectin sequences do not include a C-terminal heparin binding domain. Also provided are uses of these protein complexes for stimulating or inducing cell migration and / or proliferation in wound healing, tissue engineering, cosmetic and therapeutic treatments such as skin replacement, skin replenishment and treatment of burns where epithelial cell migration is required. In other embodiments, the invention provides inhibition of cancer cell metastasis, particularly in relation to breast cancer.
Owner:FACTOR THERAPEUTICS LTD
Methods and compositions useful for inhibition of αvβ5mediated angiogenesis
InactiveUS7053041B1Inhibit angiogenesisPrevents vascularizationOrganic active ingredientsSenses disorderDiseaseVascular disease
The present invention describes methods for inhibiting angiogenesis in tissues using vitronectin αvβ5 antagonists. The αvβ5-mediated angiogenesis is correlated with exposure to cytokines including vascular endothelial growth factor, transforming growth factor-α and epidermal growth factor. Inhibition of αvβ5-mediated angiogenesis is particularly preferred in vascular endothelial ocular neovascular diseases, in tumor growth and in inflammatory conditions, using therapeutic compositions containing αvβ5 antagonists.
Owner:THE SCRIPPS RES INST
Peptide ligands of the urokinase receptor
InactiveUS7094752B2Peptide/protein ingredientsMicrobiological testing/measurementDiseasePeptide ligand
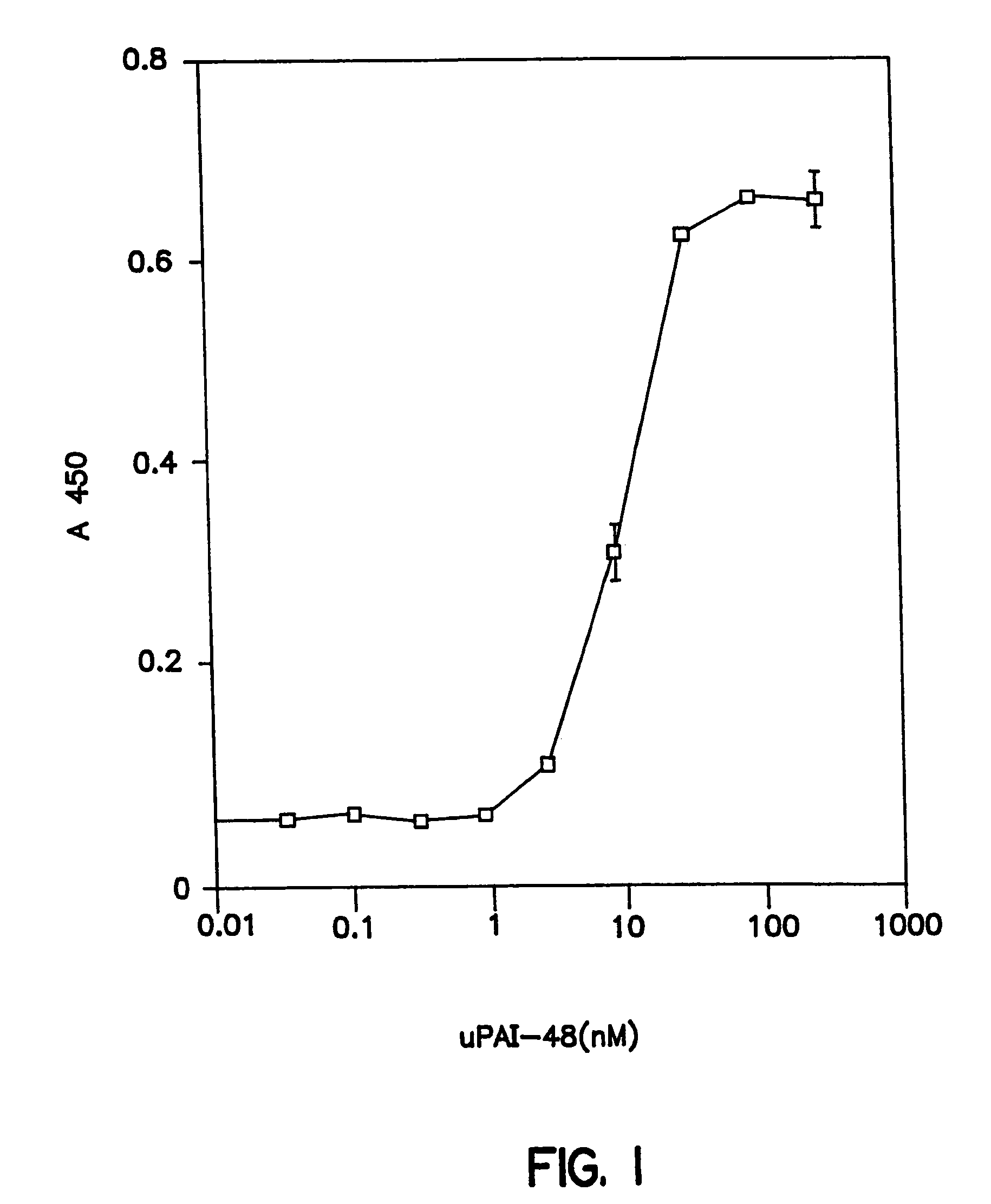
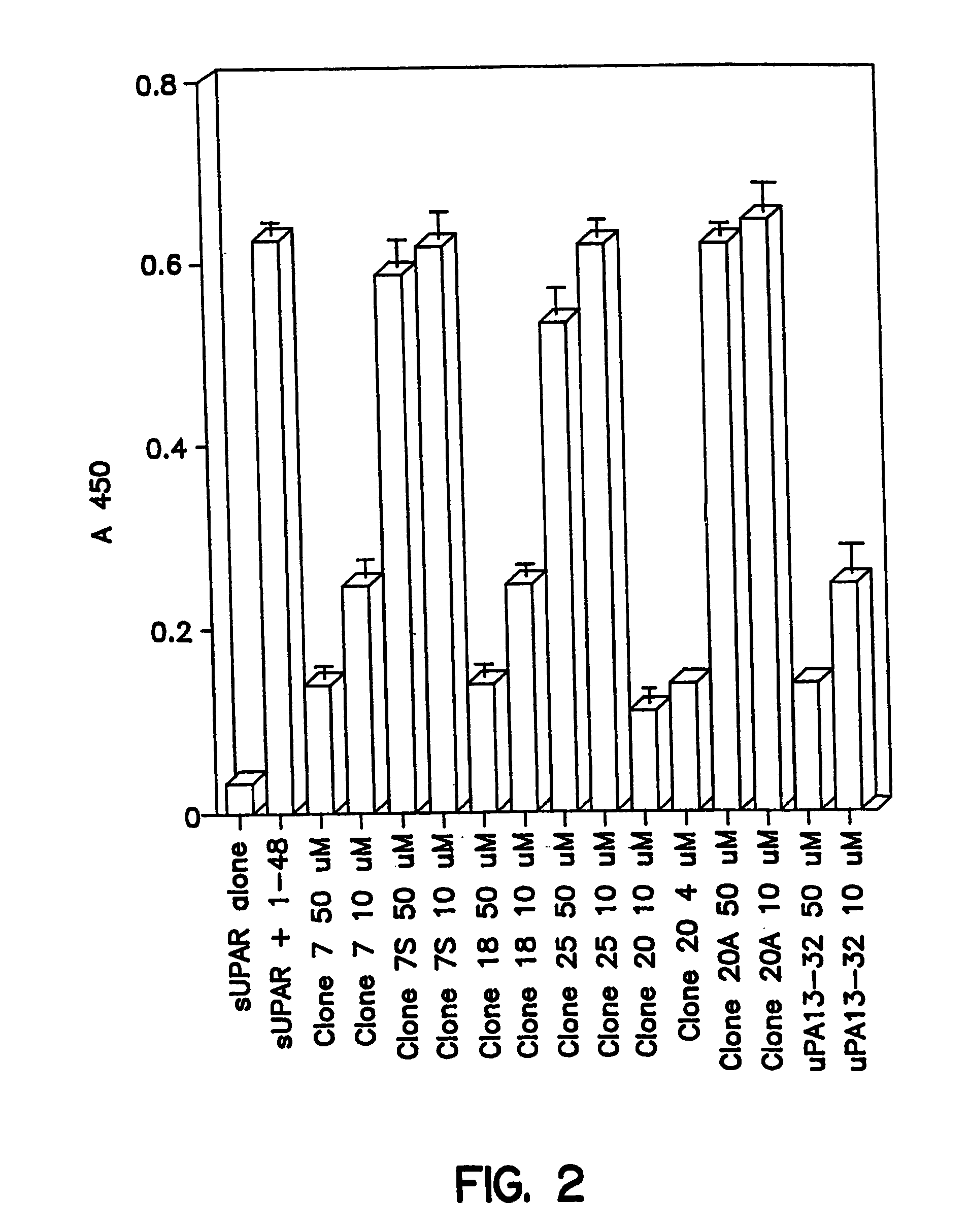
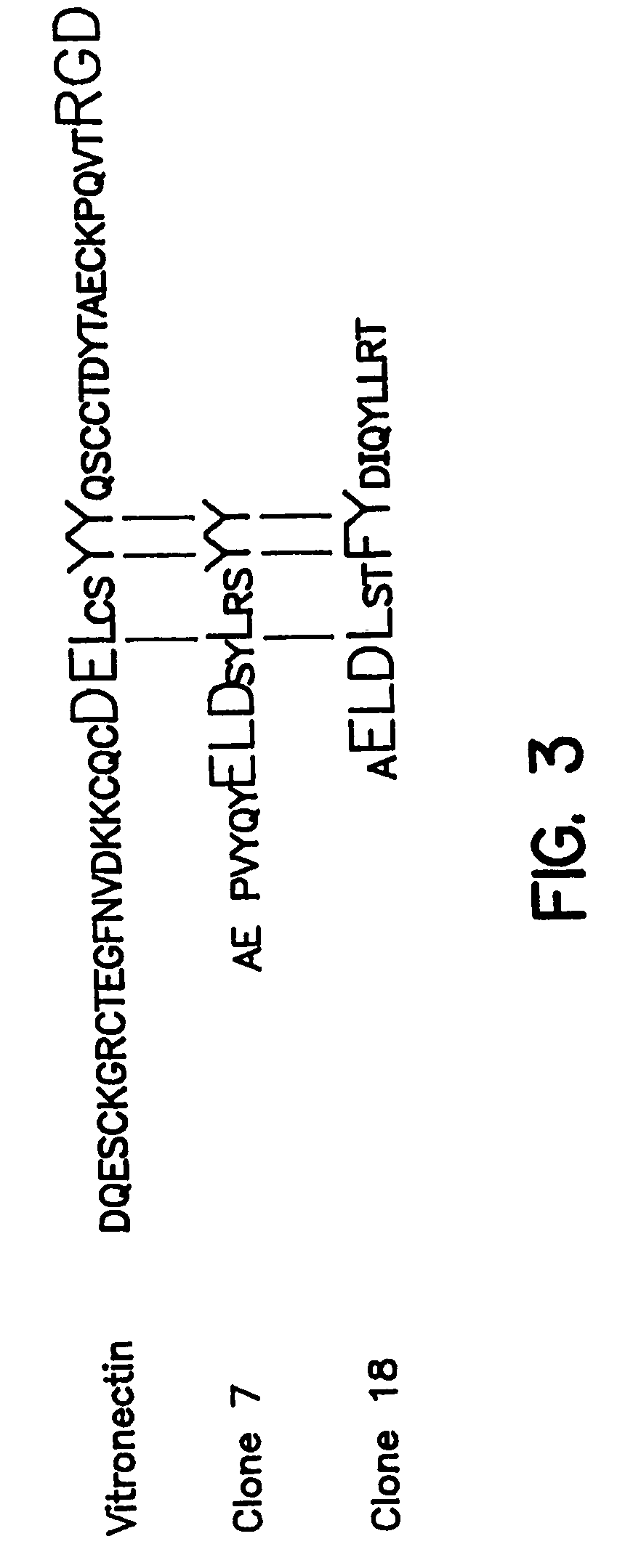
Novel peptides that are capable of binding to uPAR and inhibiting the binding of an integrin and vitronectin are described. Also provided are nucleic acid sequences encoding the novel peptides. Methods for screening for small molecules, other peptides, or peptoids that mimic the antagonistic function of the peptides of the invention are described. The invention has applications in design of therapeutics for treating disorders characterized by upregulation of uPA and uPAR, and cancer and chronic inflammation, cell migration or uPAR: integrin binding interactions, and diagnostical applications to such disorders.
Owner:CHIRON CORP +1
A feeder cell-free culture medium and system
A cell culture medium and system are provided which eliminates or at least reduces the need for feeder cells. The cell culture medium comprises one or more factors that are normally secreted and / or produced by a feeder cell and a synthetic chimeric protein comprising IGF-I and a portion of vitronectin. The cell culture medium is particularly suitable for propagating human embryonic stem cells and keratinocytes. This invention also relates to compositions and methods which utilize the cells cultured in the cell culture medium of the invention.
Owner:QUEENSLAND UNIVERSITY OF TECH
Composition and method for bone regeneration
InactiveUS20030219429A1Good effectPowder deliveryPeptide/protein ingredientsOsteoblastBone formation
A composition for modulating bone regeneration composition comprises a matrix selected from the group consisting of glycolic acid, lactic acid, collagen, demineralized bone, or a combination thereof. A first biologically active molecule comprising a fibronectin is attached to a portion of the matrix, to facilitate osteoblast activity and for promoting an increase in bone formation. A second biologically active molecule comprising a vitronectin, selected for its ability to attract osteoclasts and produce an inhibiting effect on osteoclast activity to thereby promote a decrease in bone resorption, is also attached to a portion of the matrix.
Owner:PHARMACAL BIOTECHNOLOGIES INC
Blocking the migration or metastasis of cancer cells by affecting adhesion proteins and the uses of new compounds thereof
This invention provides methods, processes, compounds and compositions for modulating the gene expression and modulating the secretion, expression, or synthesis of adhesion proteins or their receptors to cure disease, wherein the modulating comprises positive and negative regulating; wherein comprises inhibiting cancer growth, wherein the adhesion proteins or receptors comprise fibronectin, integrins family, Myosin, vitronectin, collagen, laminin, Glycosylation cell surface proteins, polyglycans, cadherin, heparin, tenascin, CD 54, CAM, elastin and FAK; wherein the methods, processes, compounds and compositions are also for anti-angiogenesis; wherein the cancers comprise breast cancer, leukocyte cancer, liver cancer, ovarian cancer, bladder cancer, prostate cancer, skin cancer, bone cancer, brain cancer, leukemia cancer, lung cancer, colon cancer, CNS cancer, melanoma cancer, renal cancer or cervix cancer.
Owner:PACIFIC ARROW
Nucleated pearl lamella conditioning fluid and application thereof
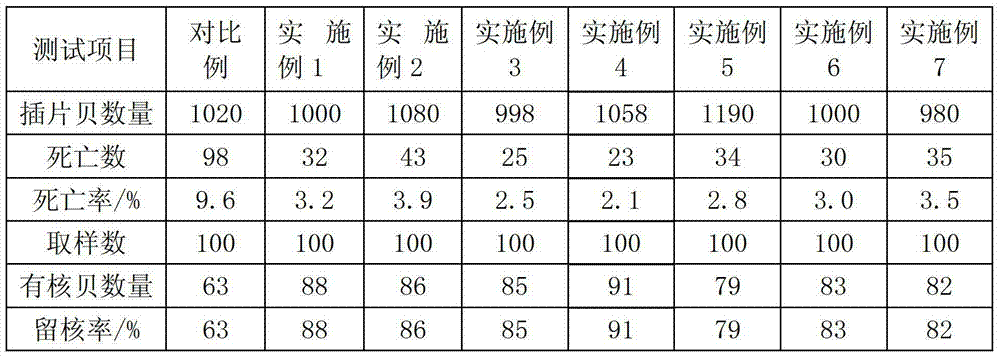
InactiveCN103283661AHigh activityAvoid infectionClimate change adaptationPisciculture and aquariaWound healingCell division
The invention relates to nucleated pearl lamella conditioning fluid in the technical field of pearl culture and application of the nucleated pearl lamella conditioning fluid. The conditioning fluid comprises the following constituents, by mass-to-volume concentration percentage, 1-5% of acid red, 0.01-5% of chondroitin, 0.1-5% of mafenide, 0.1-5% of taurine, 0.75% of sodium chloride, 0.1-5% of hyriopsis cumingii polysaccharide, and 0.05-3% of vitronectin, and allowance being water. The invention further relates to the application of the nucleated pearl lamella conditioning fluid in the pearl culture. The nucleated pearl lamella conditioning fluid can strengthen cell viability, prevent cell infection, and accelerate cell division and wound healing. When the nucleated pearl lamella conditioning fluid is applied to culture pearls, the death rate of the pearls is obviously reduced, and the rate of remaining nucleuses is obviously improved. Meanwhile, lamellas can be rapidly attached to lamella insertion shells, and the lamella insertion shells can rapidly proliferate to grow into pearl sacs. The nucleated pearl lamella conditioning fluid is simple in method, easy to achieve, wide in application range and remarkable in effect, is used for cultivating nucleated pearls by fresh water pearl mussels, and has wide application prospects.
Owner:ZHEJIANG SHANXIAHU PEARL GROUP CO LTD
Simultaneous imaging of cardiac perfusion and a vitronectin receptor targeted imaging agent
InactiveUS7138104B2Minimize potentialIncreased toxicityBiocideGeneral/multifunctional contrast agentsVitronectinImaging agent
The present invention describes a method of concurrent imaging in a mammal comprising:a) administering to said mammal a vitronectin receptor targeted imaging agent and a perfusion imaging agent; andb) concurrently detecting the vitronectin receptor targeted imaging agent bound at the vitronectin receptor and the perfusion imaging agent; andc) forming an image from the detection of said vitronectin targeted imaging agent and said perfusion imaging agent.
Owner:LANTHEUS MEDICAL IMAGING INC
Compositions and methods for treating, reducing, ameliorating, alleviating, or inhibiting progression of, pathogenic ocular neovascularization
InactiveUS20110189174A1Improve treatmentDecrease increaseBiocideOrganic active ingredientsVitronectinOcular neovascularization
A composition for treating, reducing, ameliorating, alleviating, or inhibiting the progression of, pathological ocular neovascularization comprises an integrin or vitronectin receptor antagonist having any one of Formulae I-XI, as defined herein. The composition can further comprise a VEGF inhibitor. Such composition is administered to an ocular environment by a method such as topical application, periocular injection, intravitreal injection, or intravitreal implantation. The composition can be administered alone or in combination with another procedure chosen to enhance the outcome of the treatment.
Owner:BAUSCH & LOMB INC
Injection reagent for cosmetic filling
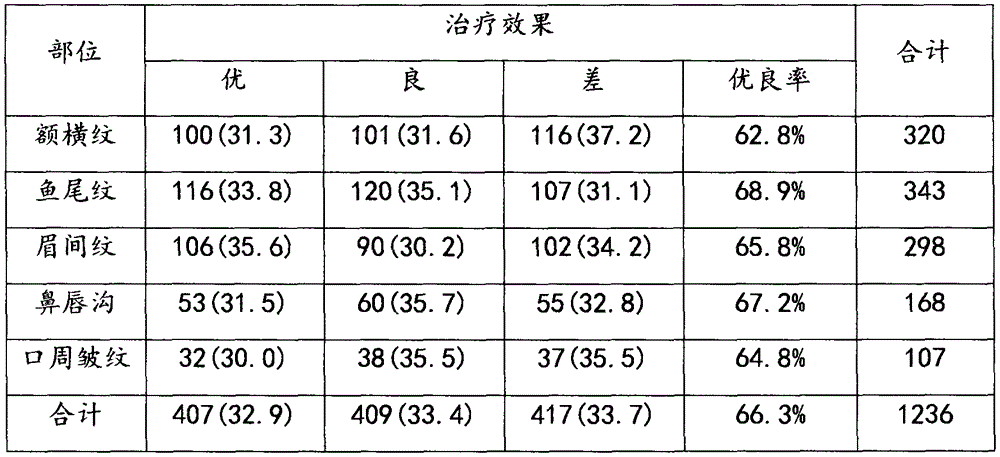
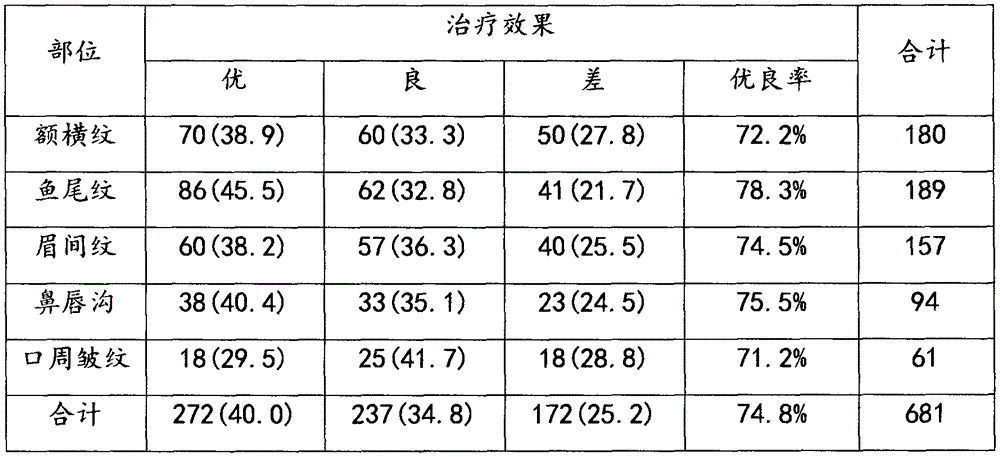
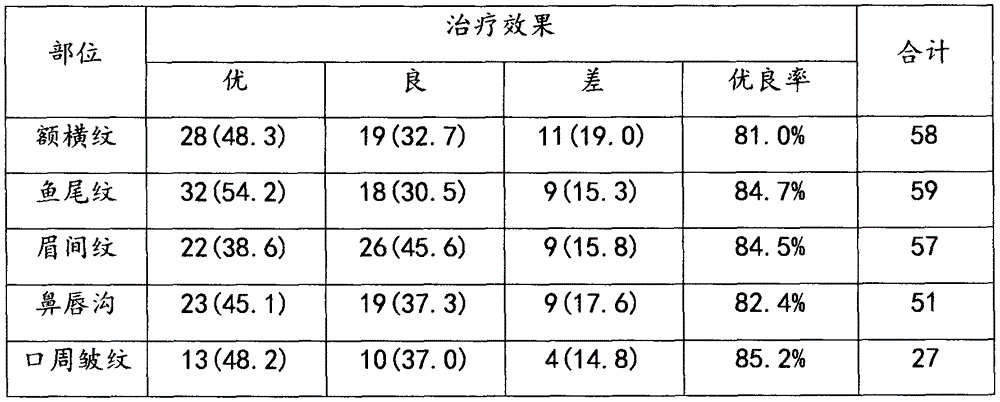
The invention relates to an injection reagent for cosmetic filling, and the injection reagent comprises the following ingredients in percentage by weight: 13-17% of transforming growth factor -beta, 19-23% of platelet-derived growth factor, 9-12% of epidermal growth factor, 2-4% of fibronectin, 2-4% of vitronectin, 32-38% of protease-activated receptor, 1-2% of interleukin 1beta and 2-6% of cross-linking agent. The injection reagent for cosmetic filling is capable of removing frontal cross striation, crow feet, intercilium wrinkle, nasolabial wrinkle, perioral wrinkle and scars, and is free of untoward effects and safe to use.
Owner:白晋
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com