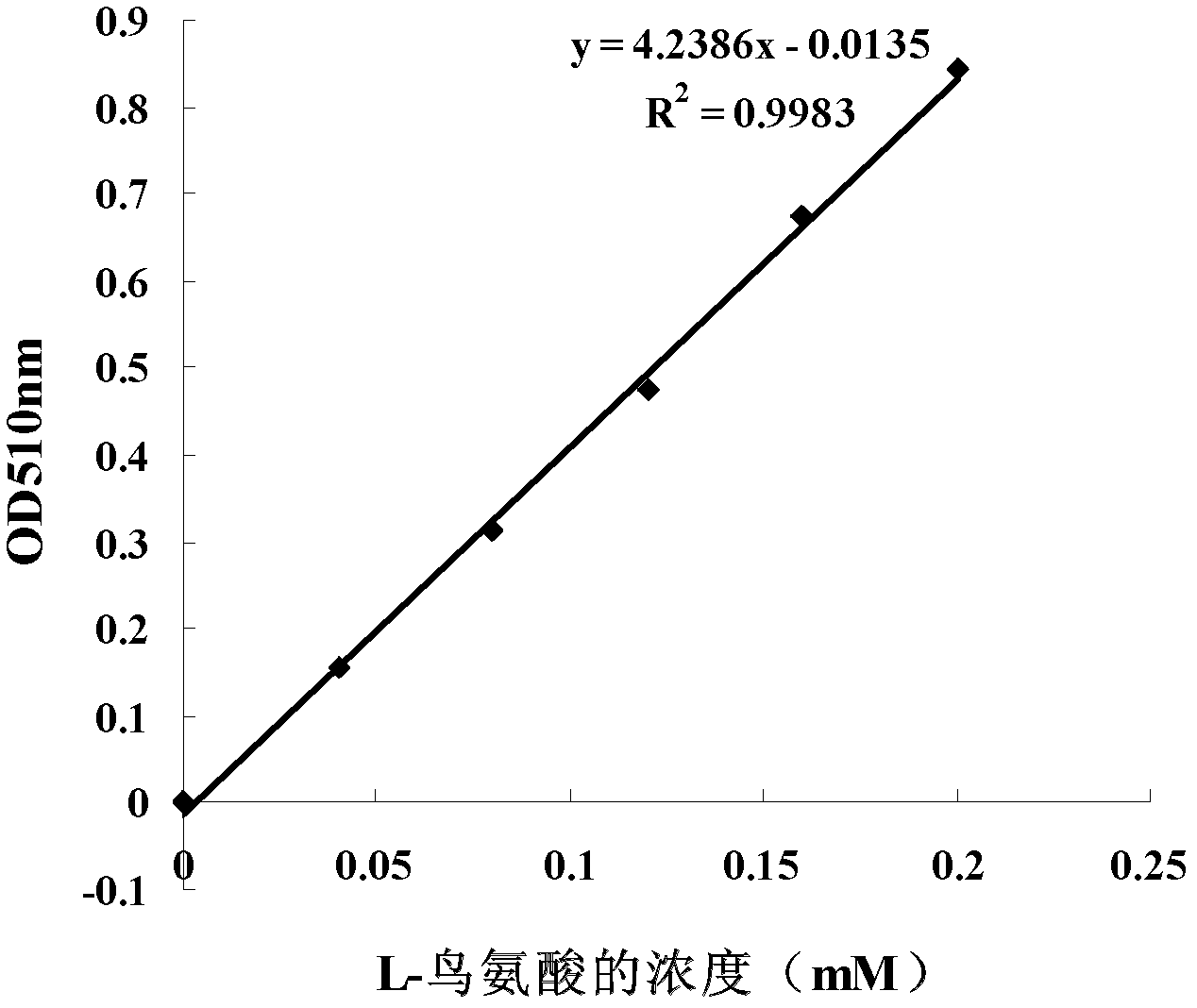
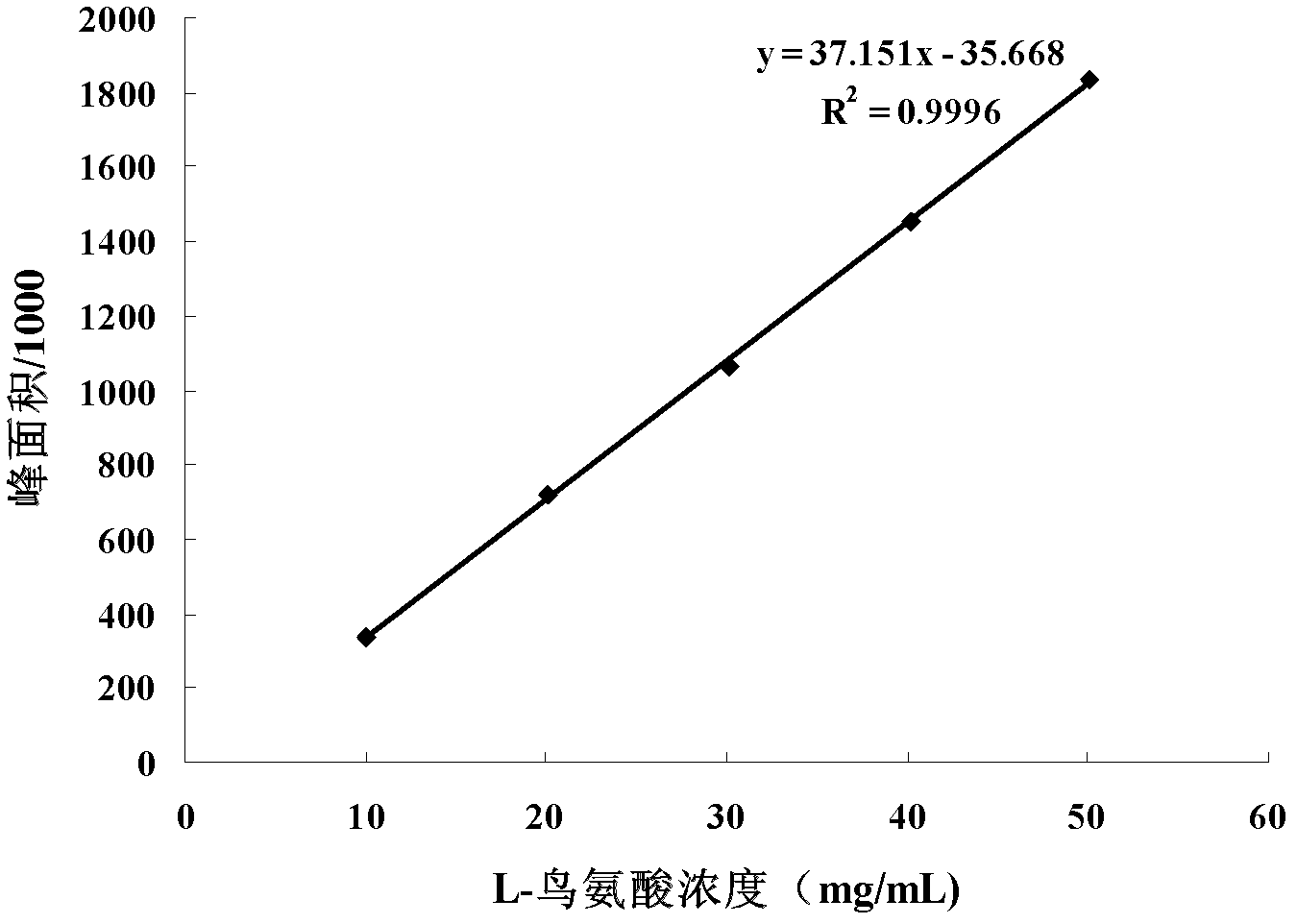
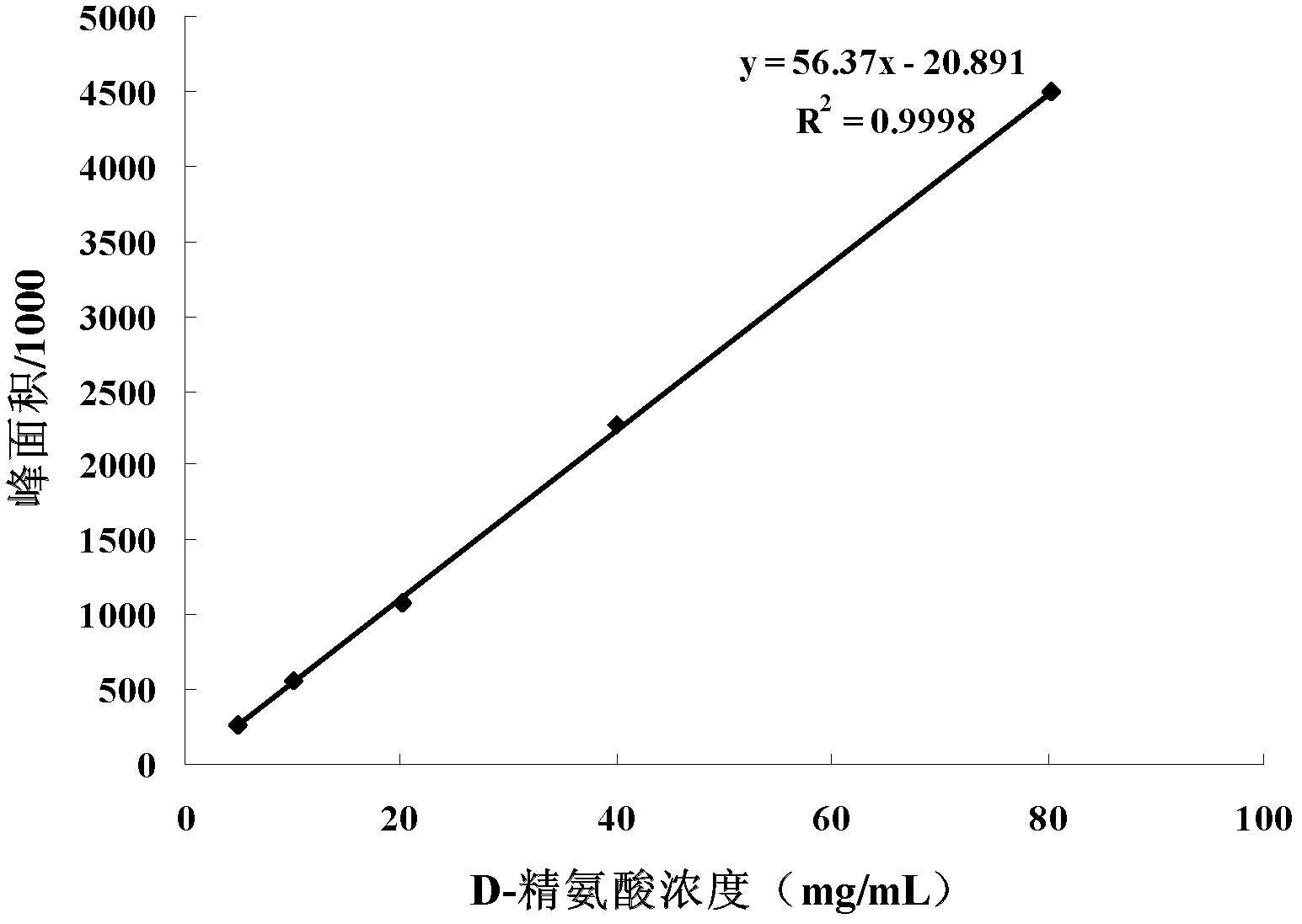
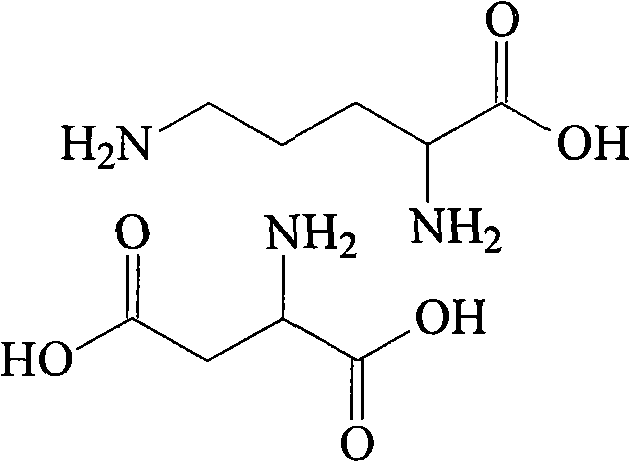
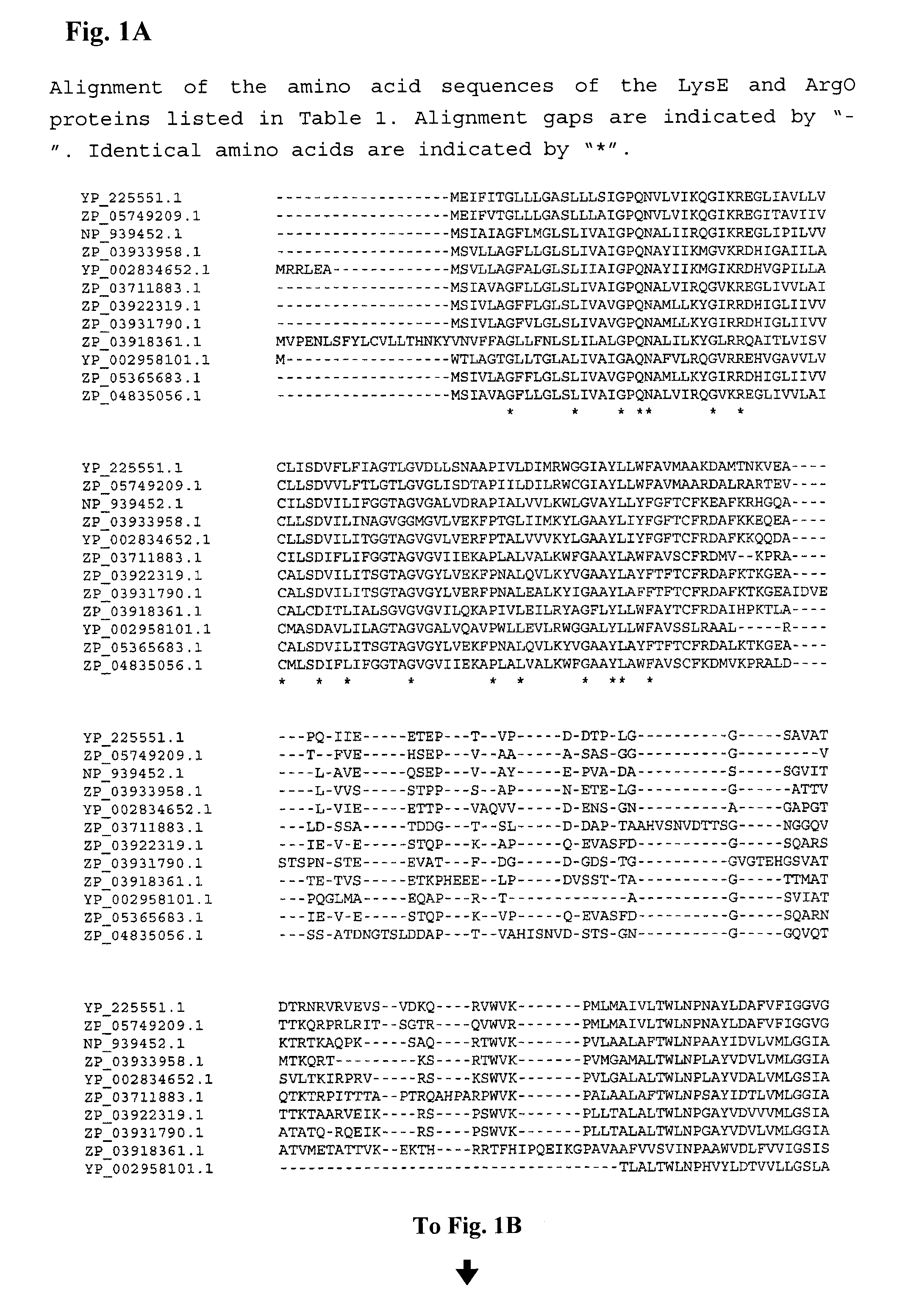
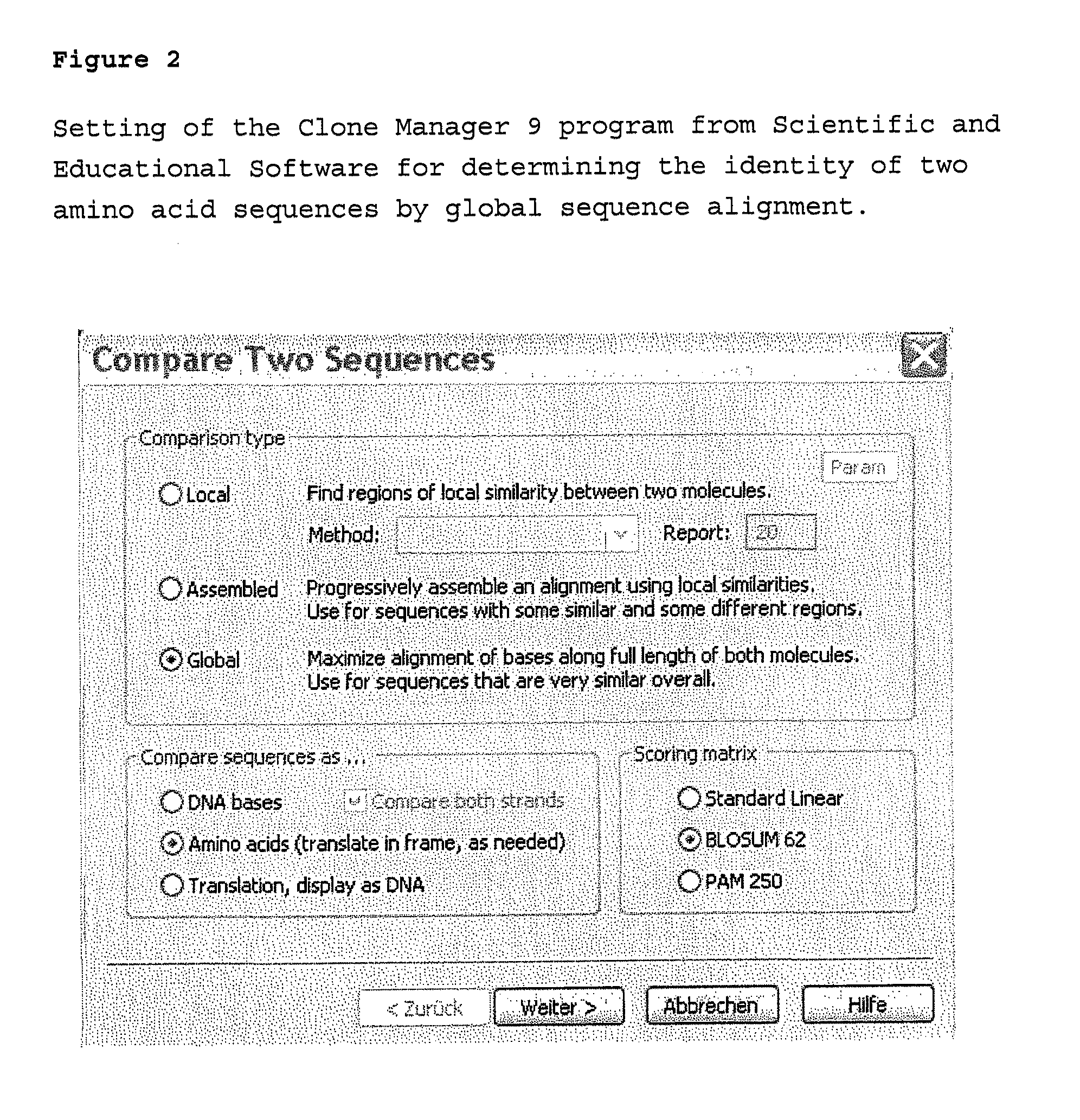
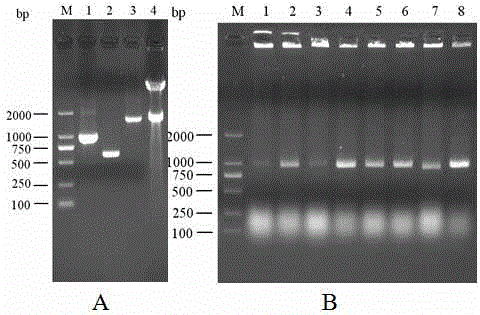
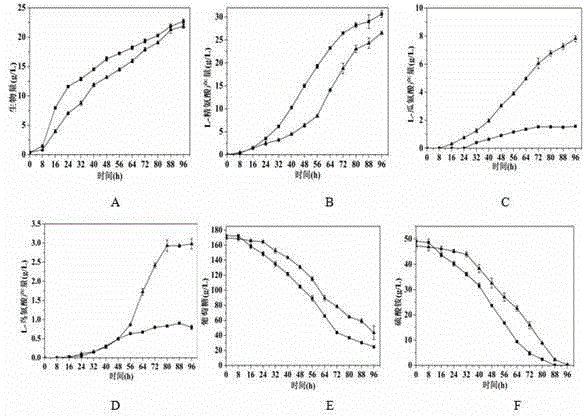
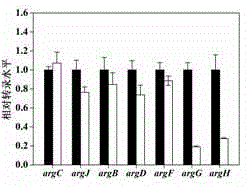
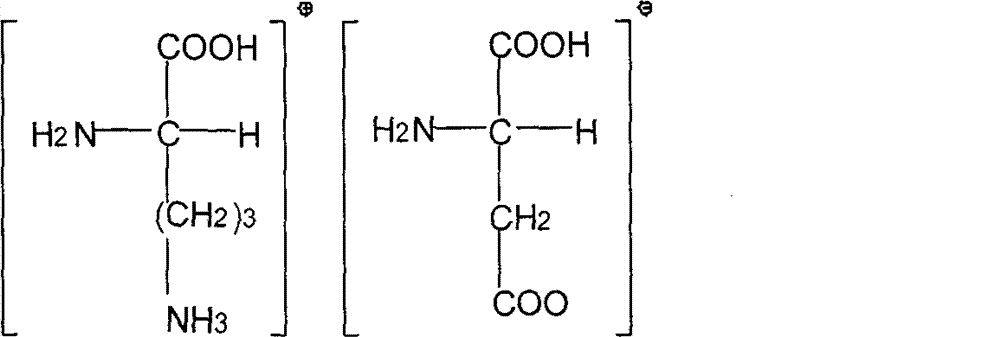
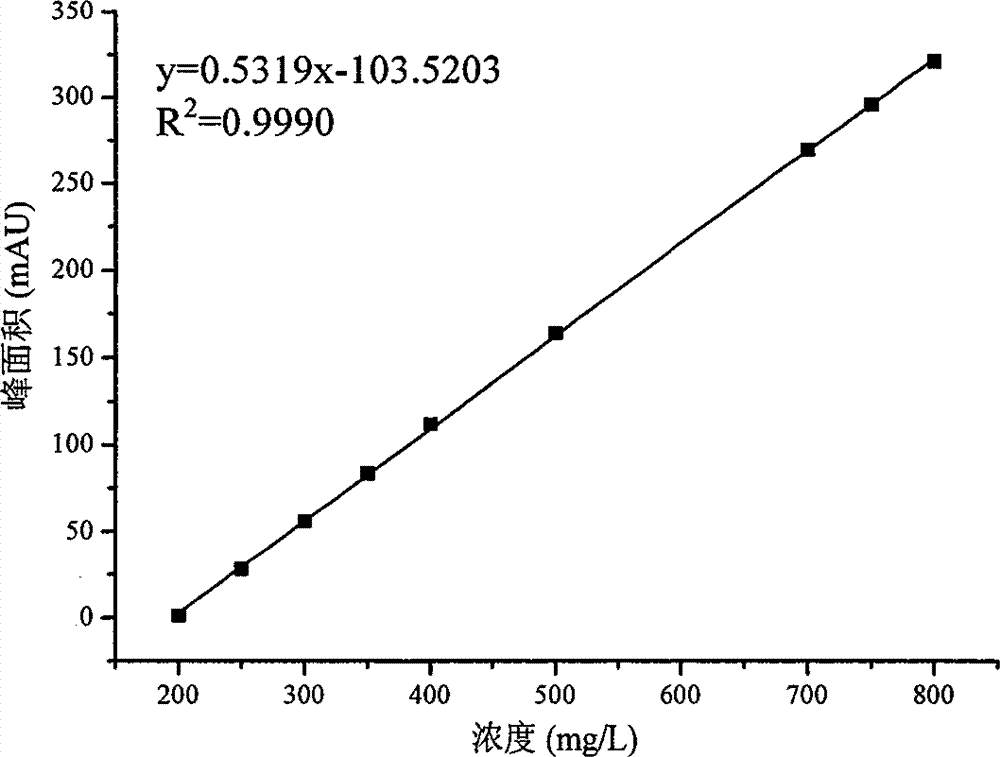
Patents
Literature
161 results about "L-Ornithine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
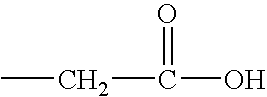
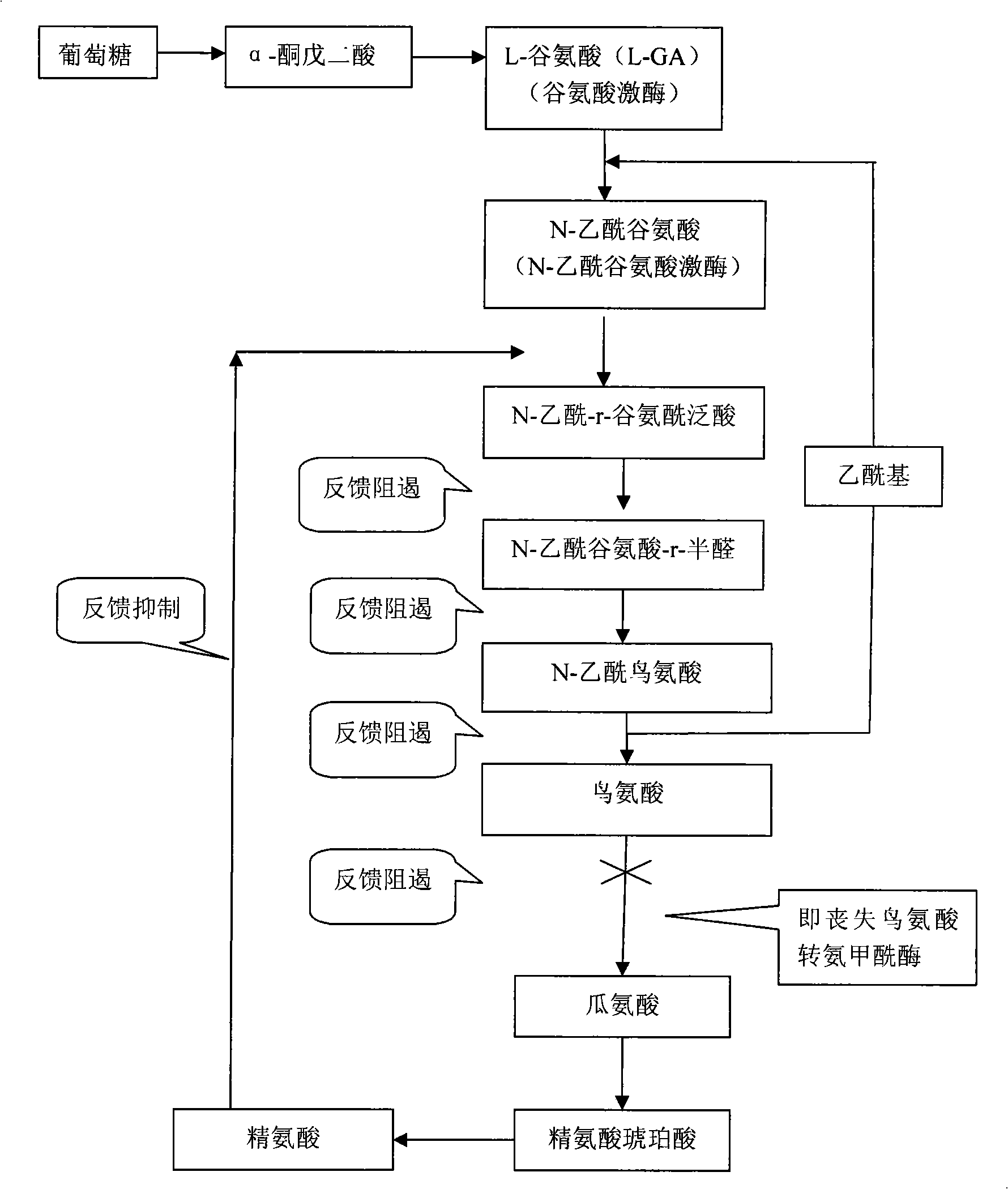
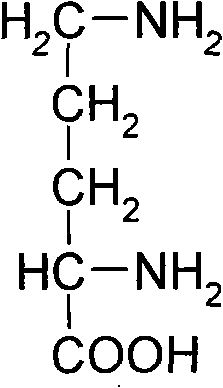
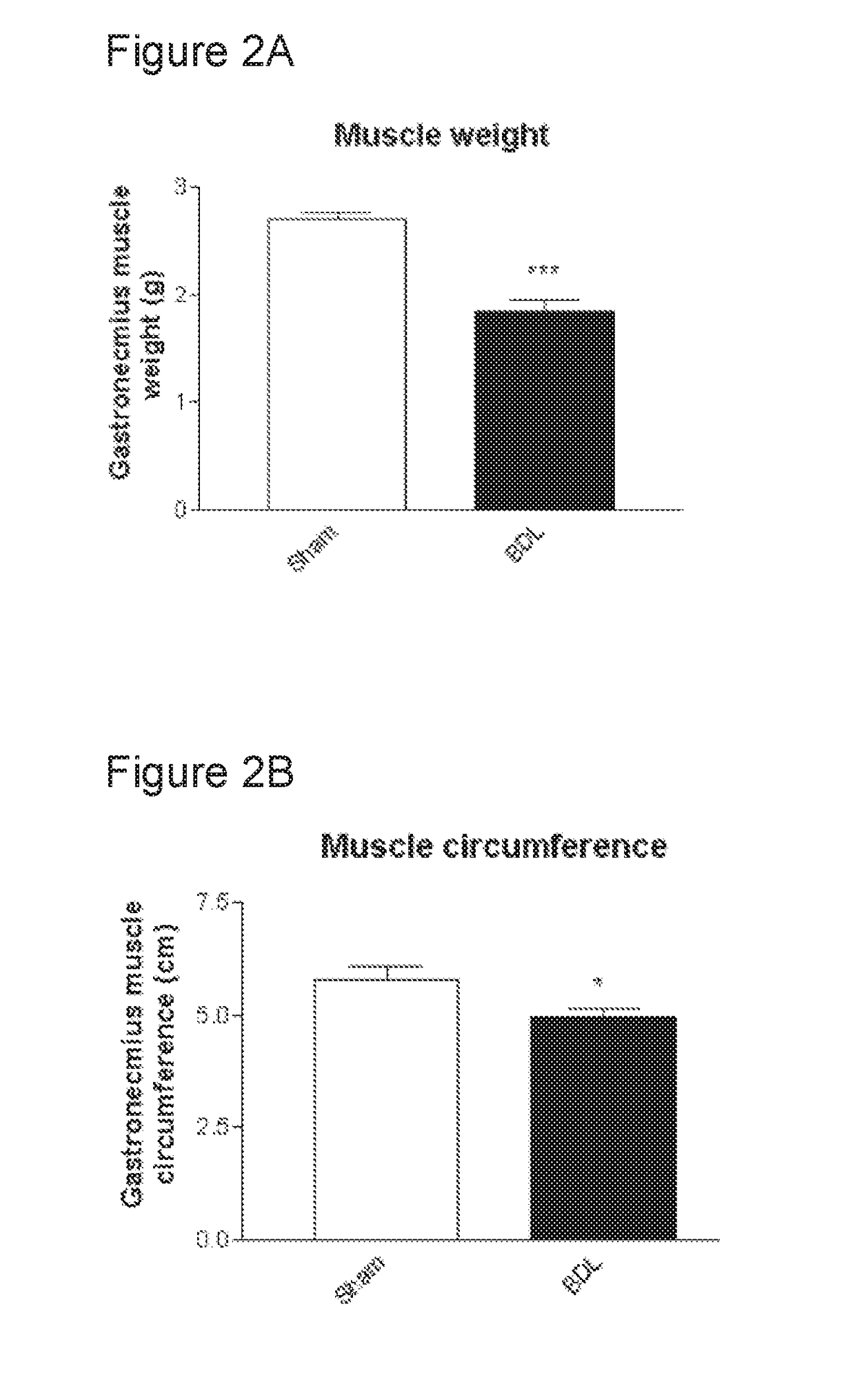
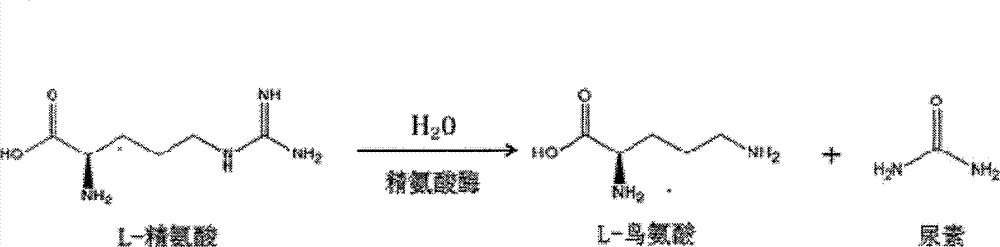
L-Ornithine is a nonprotein amino acid (not used to create proteins) that is an intermediate of the urea cycle, and provision of ornithine to a cell is actually the rate limiting step of the cycle.
Raman spectroscopy for bioprocess operations
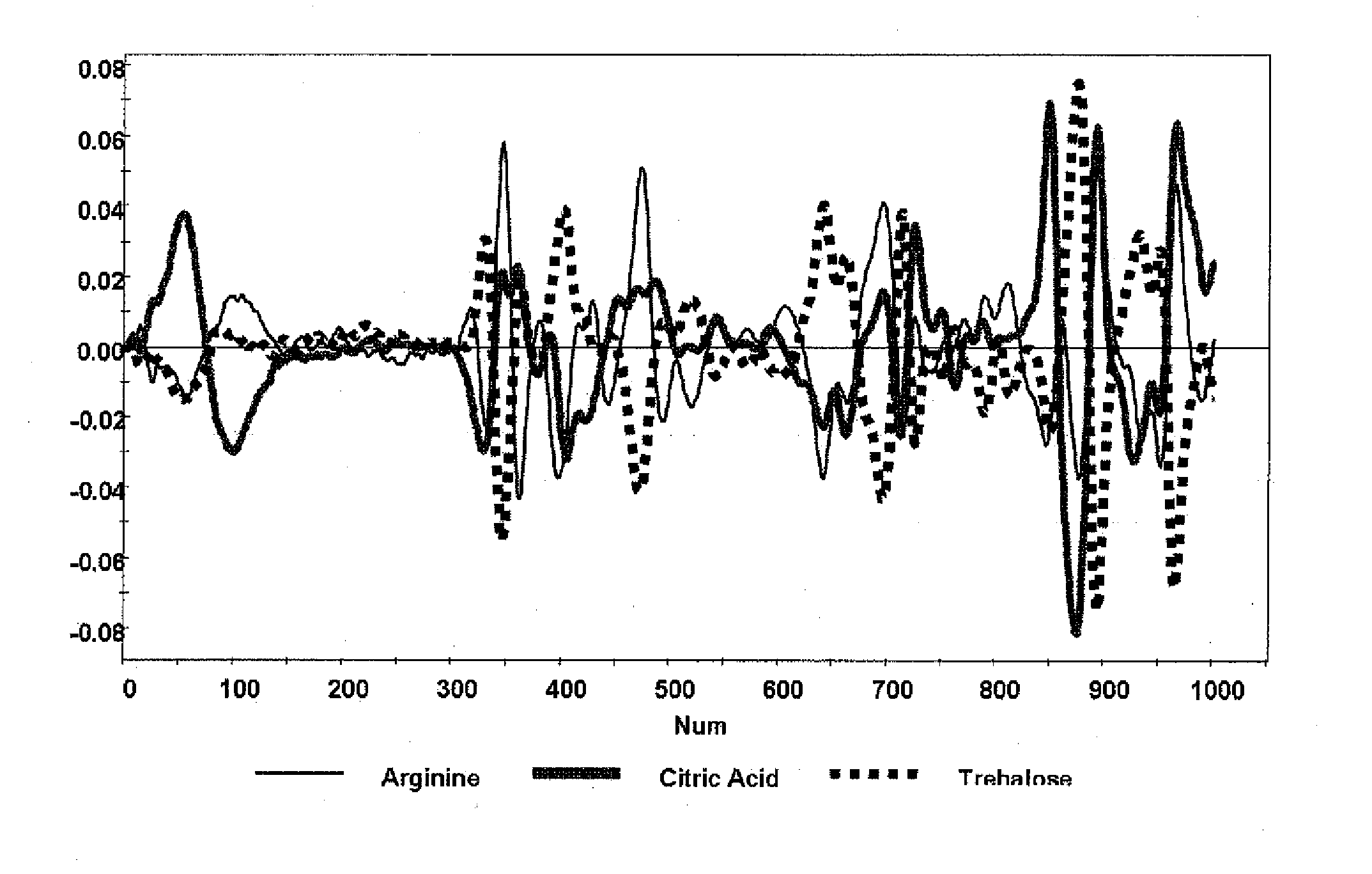
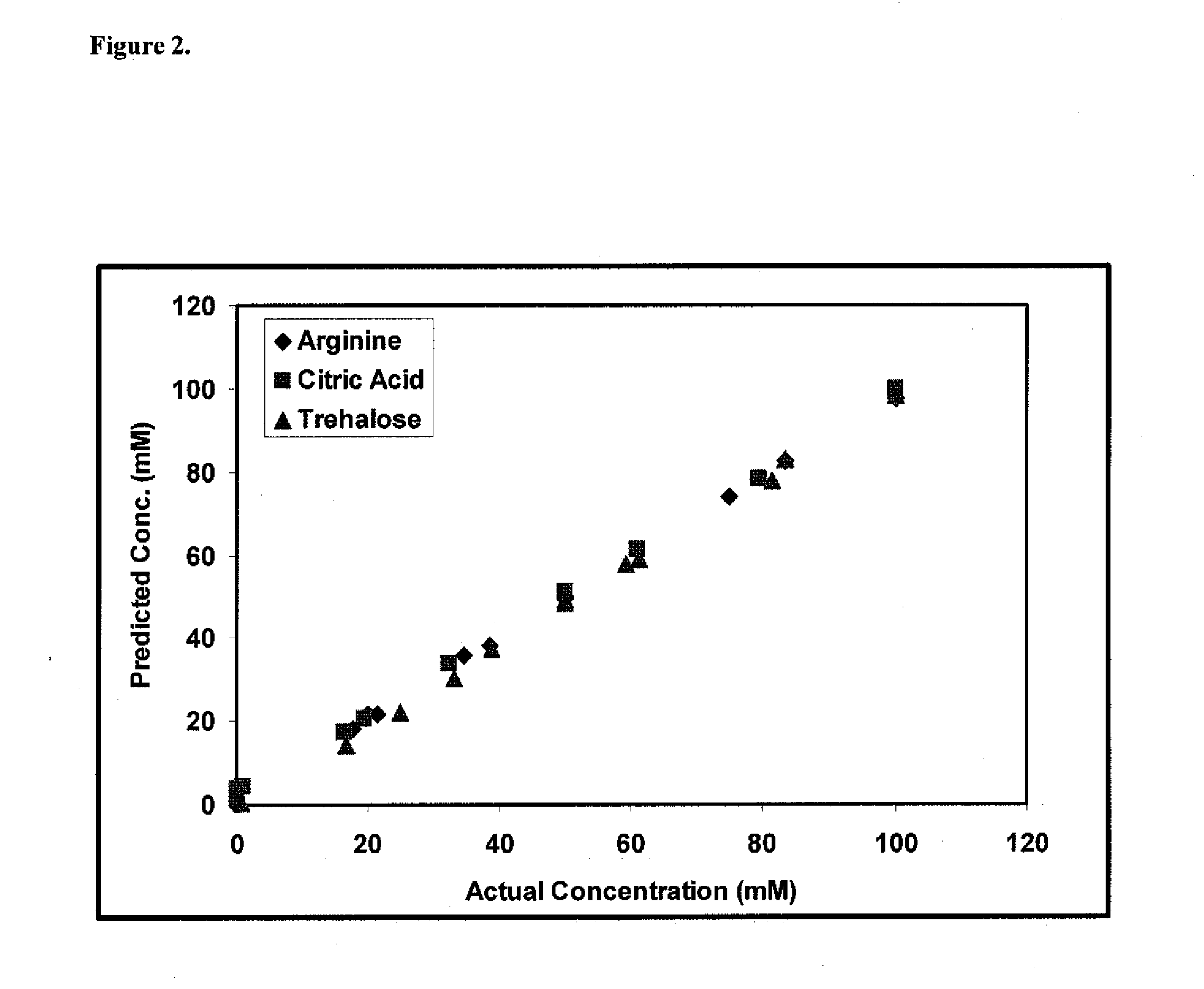
A method of characterizing a multi-component mixture for use in a bioprocess operation that includes providing a multi-component mixture standard with pre-determined amounts of known components; performing a Raman Spectroscopy analysis on the multi-component mixture standard; providing a multi-component test mixture from the bioprocess operation; performing a Raman Spectroscopy analysis on the multi-component test mixture; and comparing the analysis of the multi-component mixture standard and the multi-component test mixture to characterize the multi-component test mixture. In one embodiment, the multi-component mixture standard and the multi-component test mixture both comprise one or more of, at least two, at least three of, or each of, a polysaccharide (e.g. sucrose or mannitol), an amino acid (e.g., L-arginine, L-histidine or L-ornithine), a surfactant (e.g. polysorbate 80) and a pH buffer (e.g., a citrate formulation).
Owner:ABBVIE INC
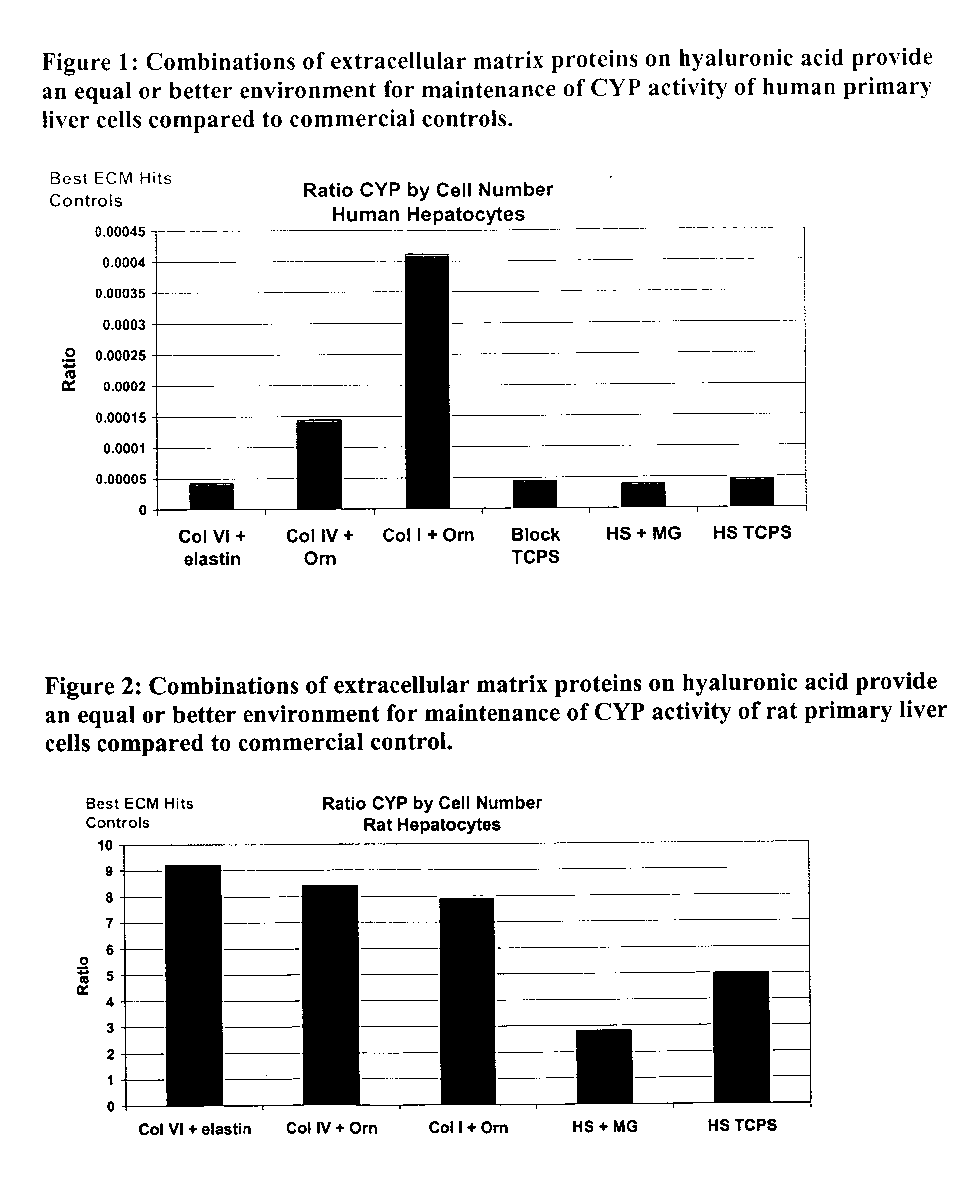
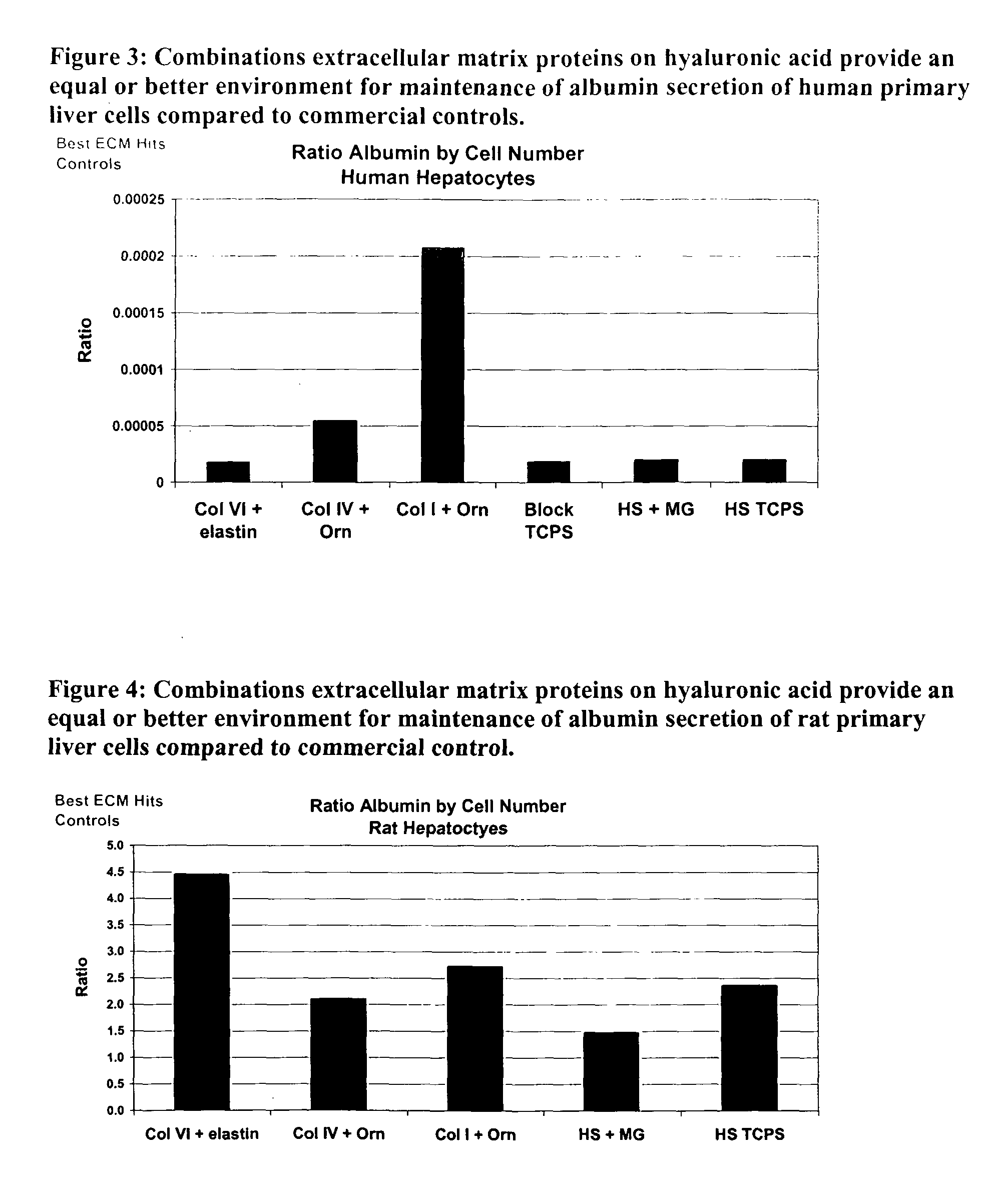
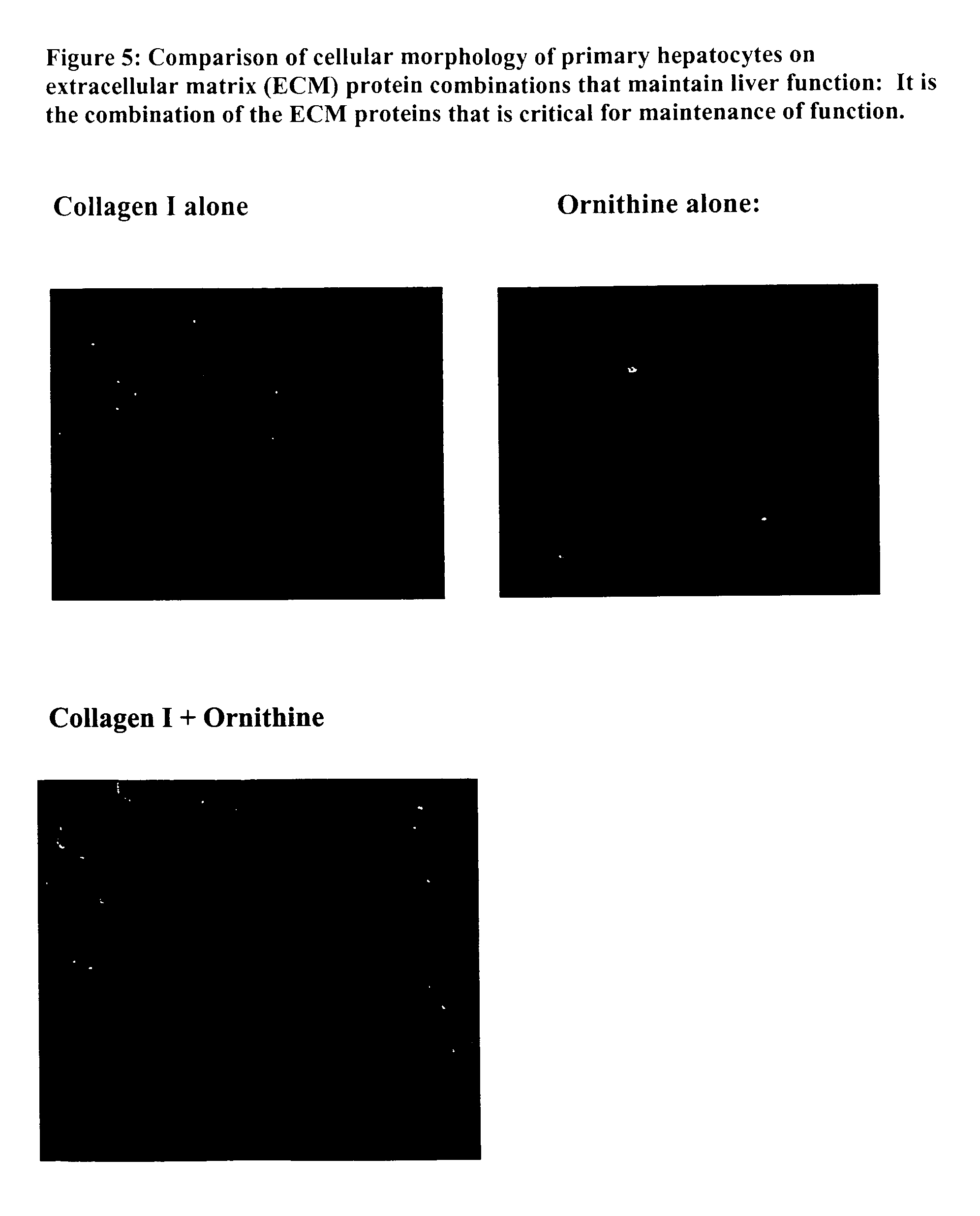
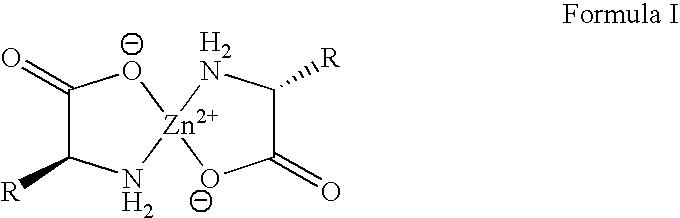
Environments that maintain function of primary liver cells
InactiveUS20050059150A1Good adhesionEasy maintenanceHepatocytesArtificial cell constructsECM ProteinL-Ornithine
Surfaces useful for cell culture comprise a support to which is bound a CAR material, and, bound to the CAR material, an ECM protein, or a biologically active fragment or variant thereof such as elastin, fibronectin, vitronectin, laminin, collagen I, collagen III, collagen IV, and collagen VI. Also, optionally present on the surface is an active factor, preferably a polycationic polymer or a biologically active fragment or variant thereof, such as polyethyleneimine (PEI), poly-D-lysine (PDL), poly-L-lysine (PLL), poly-D-ornithine (PDO) or poly-L-ornithine (PLO). This surface is used in cell culture to promote cell attachment, survival, and / or proliferation of primary liver cells. The invention also relates to methods utilizing this surface, such as methods for attachment, survival, and / or proliferation of cells. Further disclosed is the use of the surface in cell culture with serum-free medium. Methods of screening using the surface of the invention are also disclosed.
Owner:BECTON DICKINSON & CO
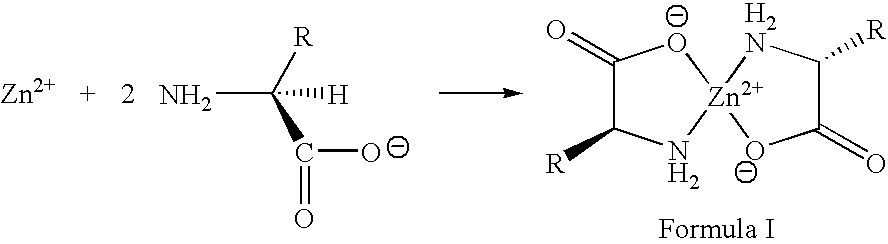
Zinc complexes of natural amino acids for treating elevated copper caused toxicities
The present invention relates to the use of zinc complexes of natural amino acids, especially L-arginine, L-lysine, L-ornithine, and other natural amino acids, in a molar ratio of about 1:2 (metal:amino acid), and formulations thereof. These pharmaceutical compositions offer better tolerated and faster acting regimens than common zinc salts (i.e., acetate, sulfate, etc.) for long term maintenance therapy of diseases caused by abnormal elevated copper levels, such as in Wilson's disease, inflammatory and fibrotic diseases and Alzheimer's disease.
Owner:SSV THERAPEUTICS
Diet food
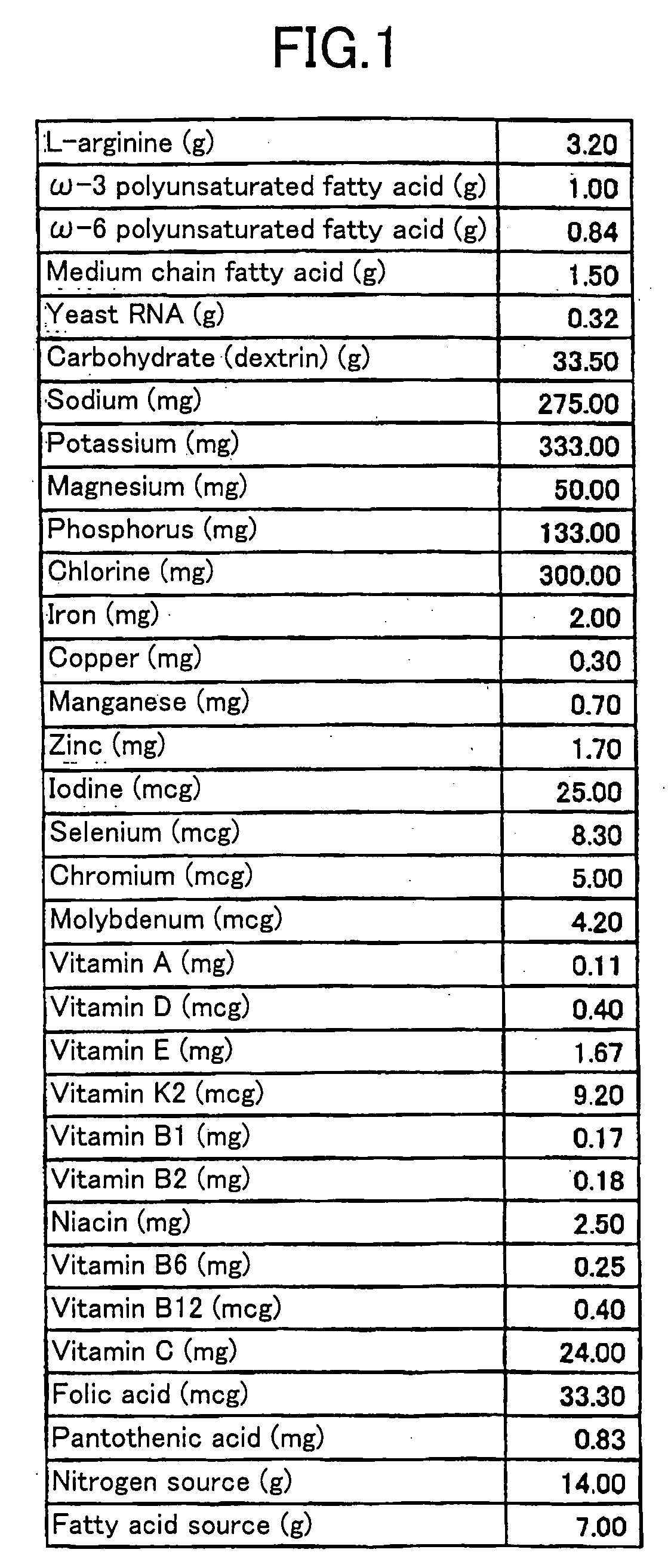
InactiveUS20060280776A1Safe and effectiveEffectively prevent or improve an adult disease dispositionBiocideVitamin food ingredientsΩ 3 pufaArginine
The diet food of this invention having effects to reduce body weight and prevent / improve obesity and atherosclerotic or metabolic disorders includes an ω-3 PUFA or an ω-6 PUFA, and at least one of L-arginine, L-ornithine, an L-arginine precursor and an L-ornithine precursor, and further includes diacylglycerol, a middle or short chain fatty acid, a phytosterol, a nucleo-base, a nucleoside, a nucleic acid, dextrin, various vitamins, various minerals or a probiotics material.
Owner:BBK BIO
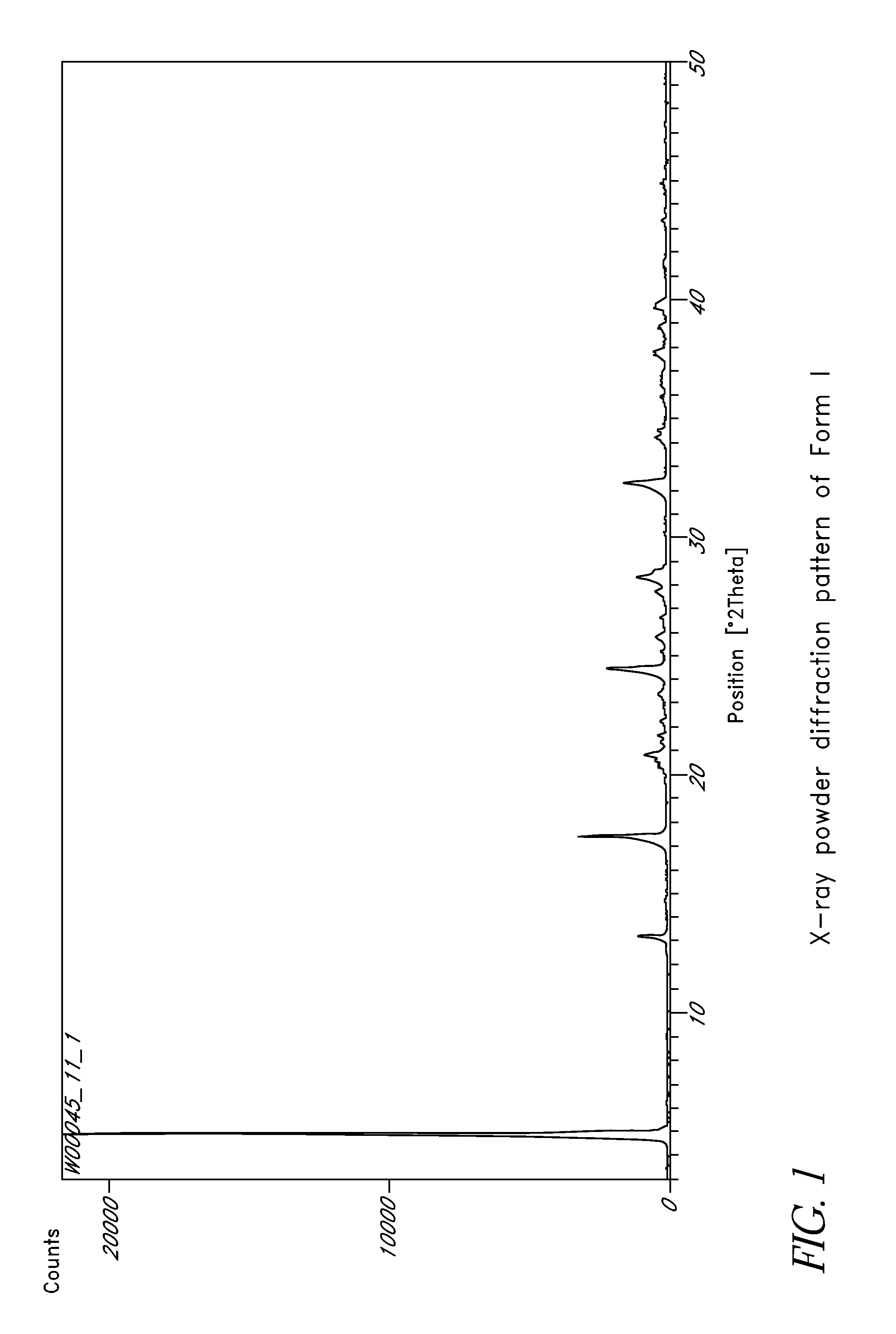
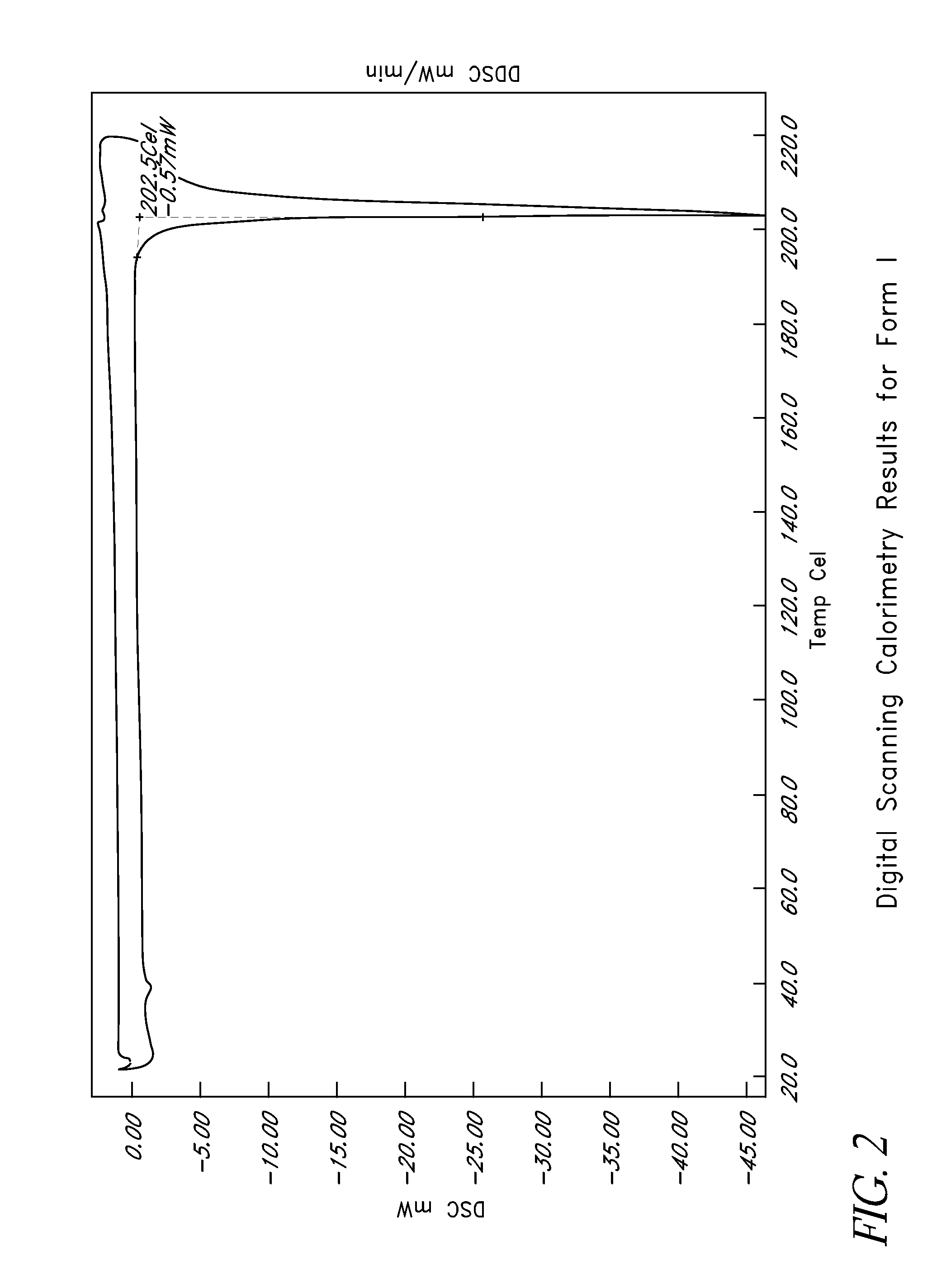
L-ornithine phenyl acetate and methods of making thereof
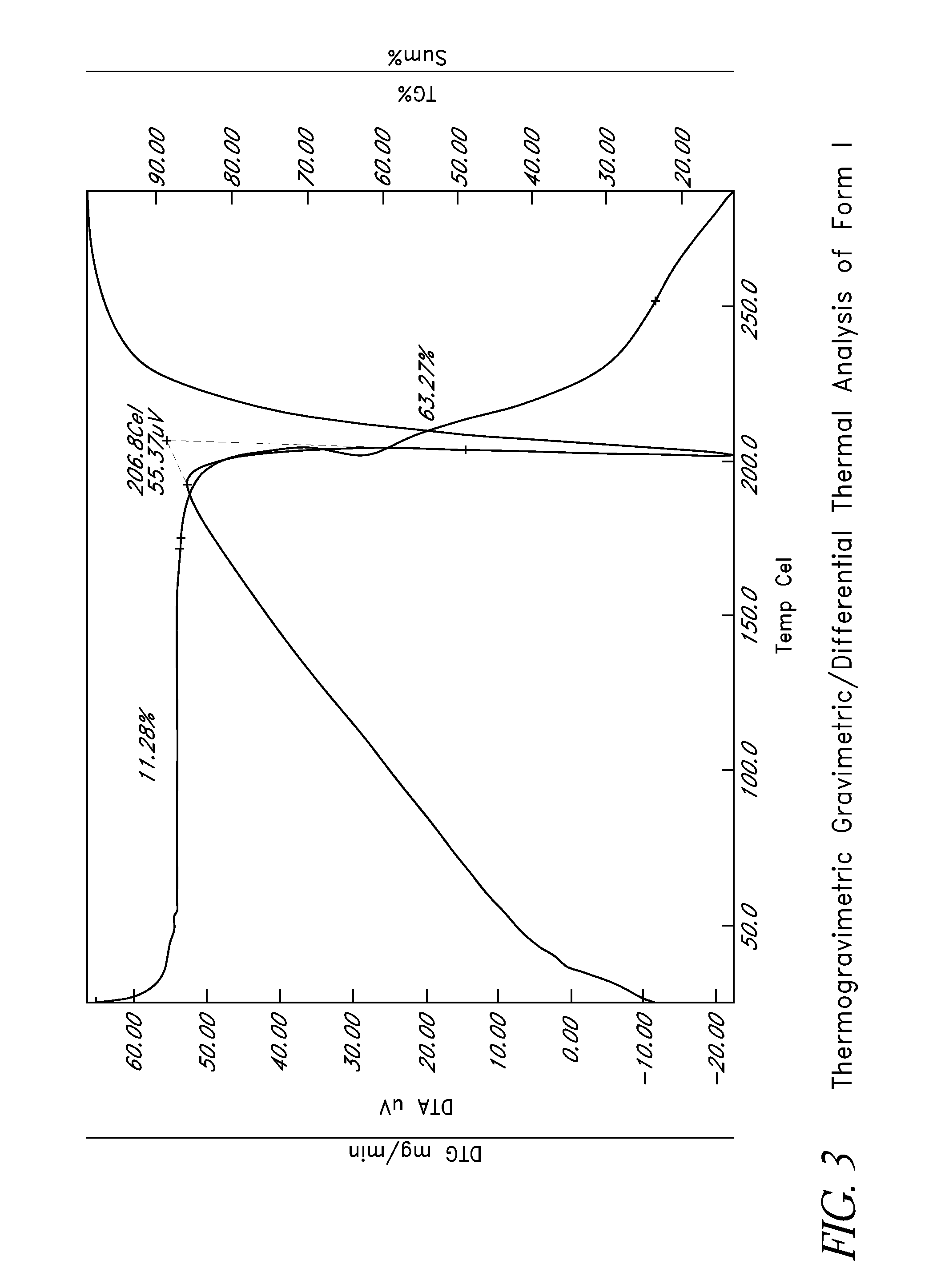
Disclosed herein are crystalline forms of L-ornithine phenyl acetate and methods of making the same. The crystalline form may, in some embodiments, be Forms I, II, III and V, or mixtures thereof. The crystalline forms may be formulated for treating subjects with liver disorders, such as hepatic encephalopathy. Accordingly, some embodiments include formulations and methods of administering L-ornithine phenyl acetate.
Owner:OCERA THERAPEUTICS INC
Pharmaceutical composition for promoting nerve damage restoration and application thereof
The invention belongs to the field of medical health products, and in particular relates to a pharmaceutical composition for promoting nerve injury restoration and an application thereof. The pharmaceutical composition in each unit contains 0.5-8g of L-ornithine, 1-5g of aspartic acid, 3-10g of arginine, 3-10g of vitamin B6, and the balance of accessories and / or other components. The pharmaceutical composition disclosed by the invention can obviously promote the restoration of spinal nerve function, and can be used for preparing medicines of nerve injury restoration; and the pharmaceutical composition has very good curative effect on avute myelitis.
Owner:JIANGSU TONGKAI ZHAOFENG BIOTECHNOLOGY CO LTD
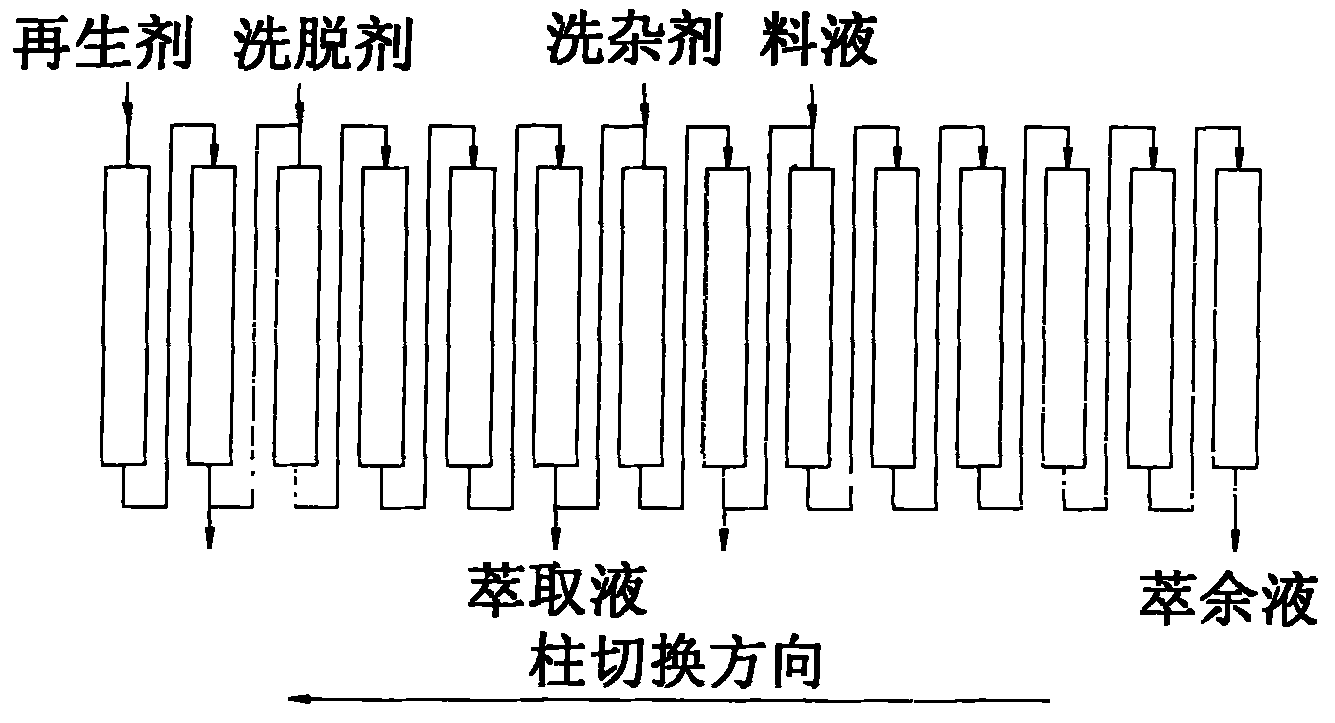
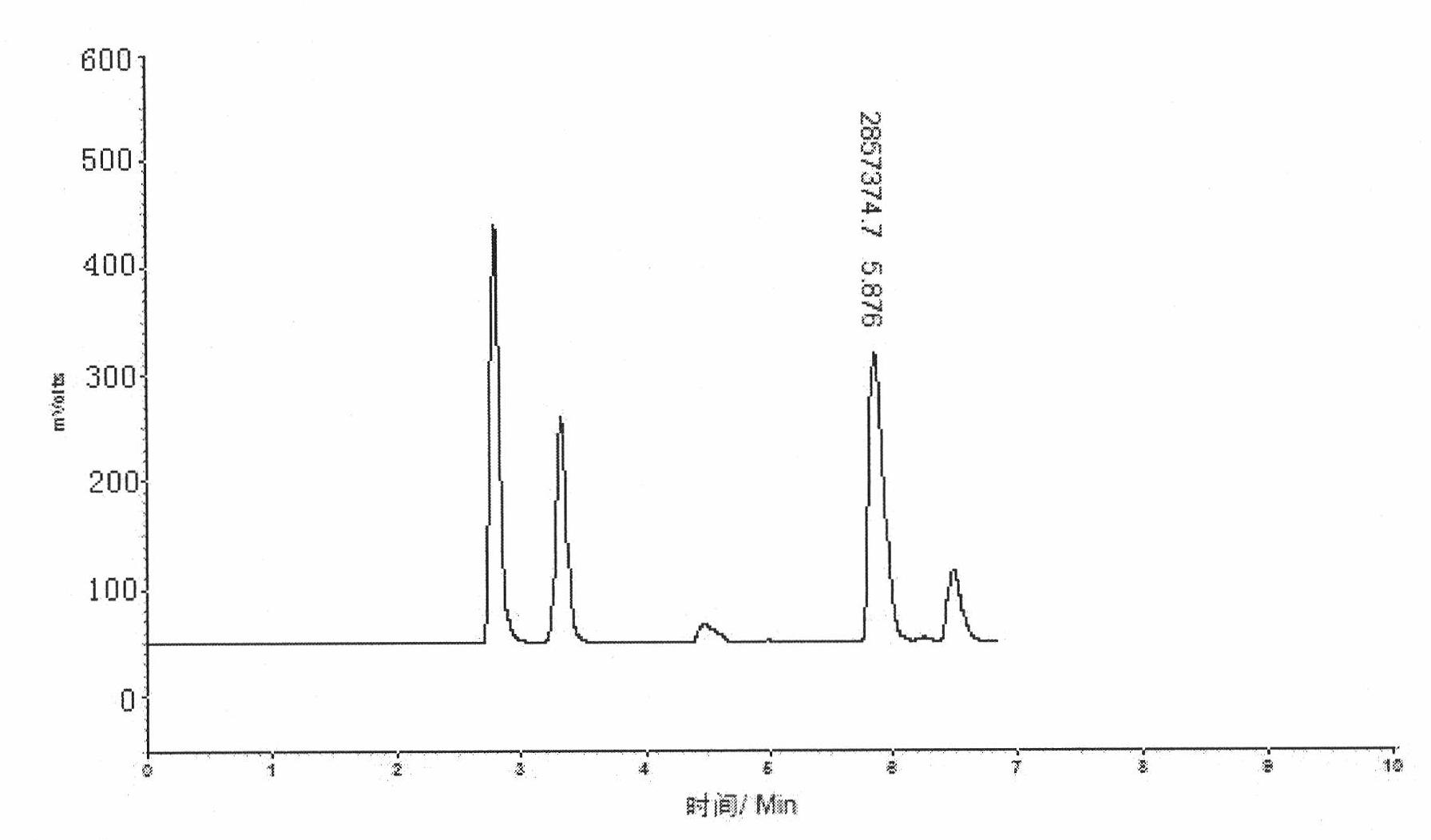
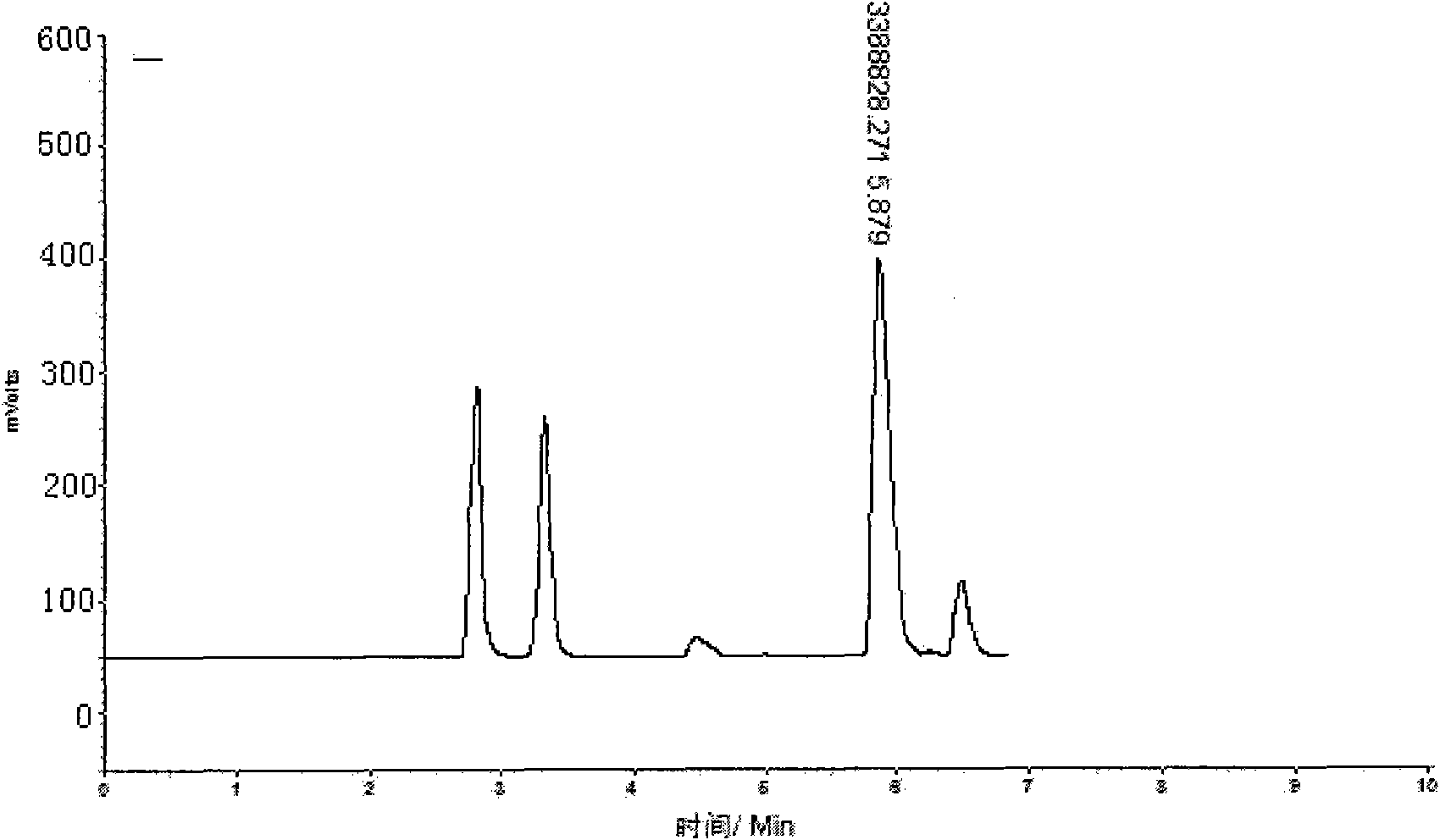
Method for separating and purifying L-ornithine by using simulated moving bed
ActiveCN101774935AImprove adsorption efficiencyFully automatedCation exchanger materialsOrganic compound preparationFiltrationSimulated moving bed
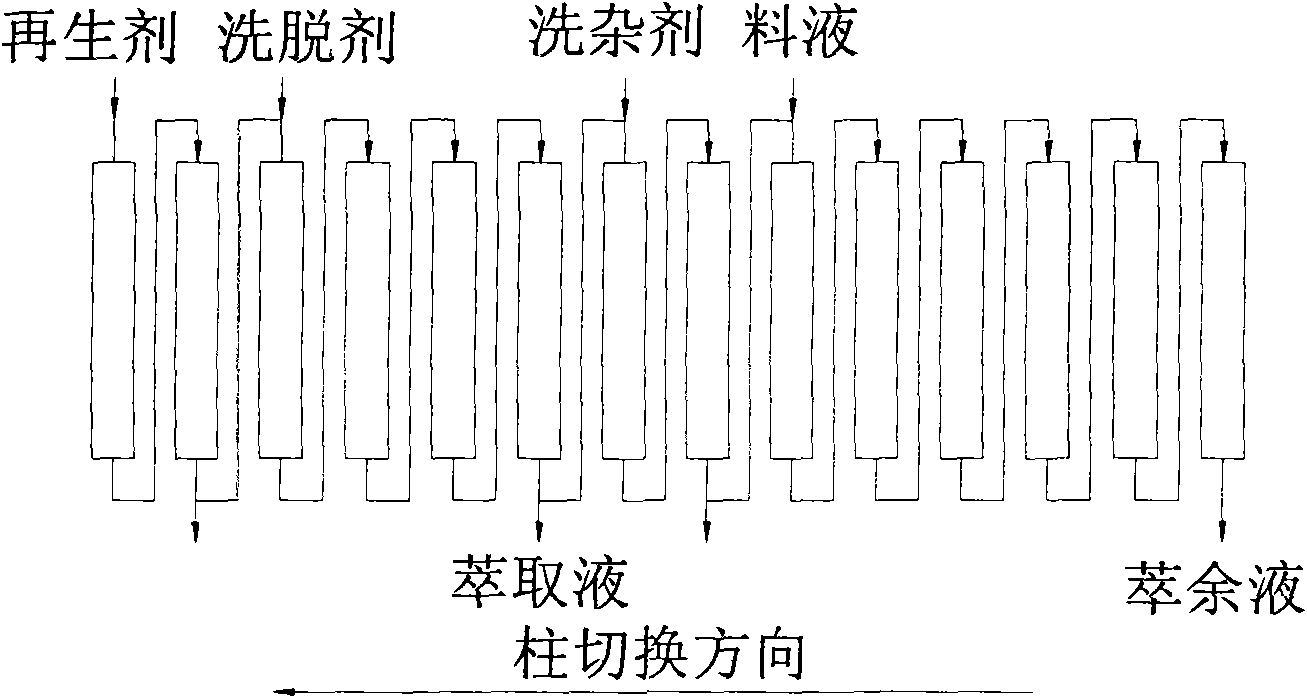
The invention belongs to the field of biological pharmaceutics, and discloses a method for separating and purifying L-ornithine by using a simulated moving bed. The method comprises the following steps: firstly, removing bacteria and solid substances from L-ornithine fermentation solution by using ultra-filtration; secondly, feeding the solution into the simulated moving bed, adsorbing the solution, washing the impurities, eluting the L-ornithine, collecting the elution and regenerating a column; and finally, concentrating and decolorizing the collected elution, adjusting the pH of the elution to between 4.5 and 5.0 with hydrochloric acid, crystallizing the elution, and drying the crystal to obtain L-ornithine hydrochloride crystal. The L-ornithine separated and purified by using the method has the advantages of high yield, high purity, low cost, environmental protection, and suitability for industrialized production.
Owner:NANJING UNIV OF TECH
Corynebacterium glutamicum and method for preparing L-ornithine and salts thereof by using same
ActiveCN101955901AThe fermentation process is simpleEasy to operateBacteriaMicroorganism based processesL-OrnithineCulture mediums
The invention belongs to the field of fermentation engineering and discloses corynebacterium glutamicum and a method for preparing L-ornithine and salts thereof by using the same. The corynebacterium glutamicum is corynebacterium glutamicum 1006 with the collection number of CGMCCNo.3663. The method comprises the following steps of: culturing the corynebacterium glutamicum in a culture medium containing carbon sources, nitrogen sources and inorganic salts at the temperature of between 28 and 30 DEG C to generate L-ornithine fermentation liquor; removing the thalli of the fermentation liquor by using ceramic membranes of 1 to 15 ten thousand Dalton; separating the fermentation liquor by using cation exchange resins to obtain L-ornithine solution; and decoloring and crystallizing the L-ornithine solution by a hydrochloric acid and ethanol method to obtain L-ornithine hydrochloride. The method has the advantages of simple thallus cultivation and contribution to industrialized mass production. The accumulation of the L-ornithine prepared from the corynebacterium glutamicum reaches 35 to 45g / L.
Owner:NANJING UNIV OF TECH
Method for producing L-ornithine by microorganism fermentation
InactiveCN101323866AFast acid productionImprove sugar conversion rateBacteriaMicroorganism based processesSolubilityChemical treatment
The invention provides a method for producing L-ornithine by microbial fermentation, namely, using Corynebacterium glutamate to obtain the strains of CS-189(cit<(-)>+SG<r>) by chemical treatment, and obtain the L-ornithine hydrochloride by fermentation, culture solution micro-filtration by a ceramic membrane, ion exchange resin and extraction by a hydrothermal crystallization method with decompression concentration. The strains used in the method are auxotrophy resistant to sulfaguanidine, which significantly enhances acid yield by fermentation. Meanwhile, aiming at the problem that the solubility of the L-ornithine hydrochloride in water is extremely difficult, the hydrothermal crystallization method is adopted to obtain better extraction rate under the condition that flammable and explosive alcohol is not used. The method simplifies processes and reduces extraction cost.
Owner:上海聚瑞生物技术有限公司
Treatment and prevention of muscle loss using l-ornithine in combination with at least one of phenylacetate and phenylbutyrate
ActiveUS20180221320A1Reducing blood ammoniaImproving muscle metabolismOrganic active ingredientsMicrobiological testing/measurementMuscle lossOrnithine synthesis
Disclosed herein are methods of treating and preventing muscle loss using ornithine in combination with at least one of phenyl acetate and phenylbutyrate.
Owner:OCERA THERAPEUTICS INC
Method for producing L-ornithine hydrochloride
InactiveCN1594282AReduce solubilityNo pollutionOrganic compound preparationAmino-carboxyl compound preparationOrnithine synthesisArginine
The invention discloses a method for producing L-ornithine hydrochloride through weak Ba(OH)2 hydrolysis L-arginine hydrochloric salts, neutralizing with dilute sulfuric acid, removing Ba2+, obtaining L-ornithine hydrochloride crystallized crude product through concentrated crystallization, re-crystallizing in pure water to obtain the pure product.
Owner:WUHAN GRAND HOYO
Method of transforming enzyme to prepare L-ornithine
the invention discloses a preparing method of L-ornithine, which comprises the following steps: preparing inclined-plane seed; selecting culture medium; optimizing converting condition; extracting product. the invention improves converting rate of arginine by 99.5%, which provides basic raw material for medical industry.
Owner:山东泓达生物科技有限公司
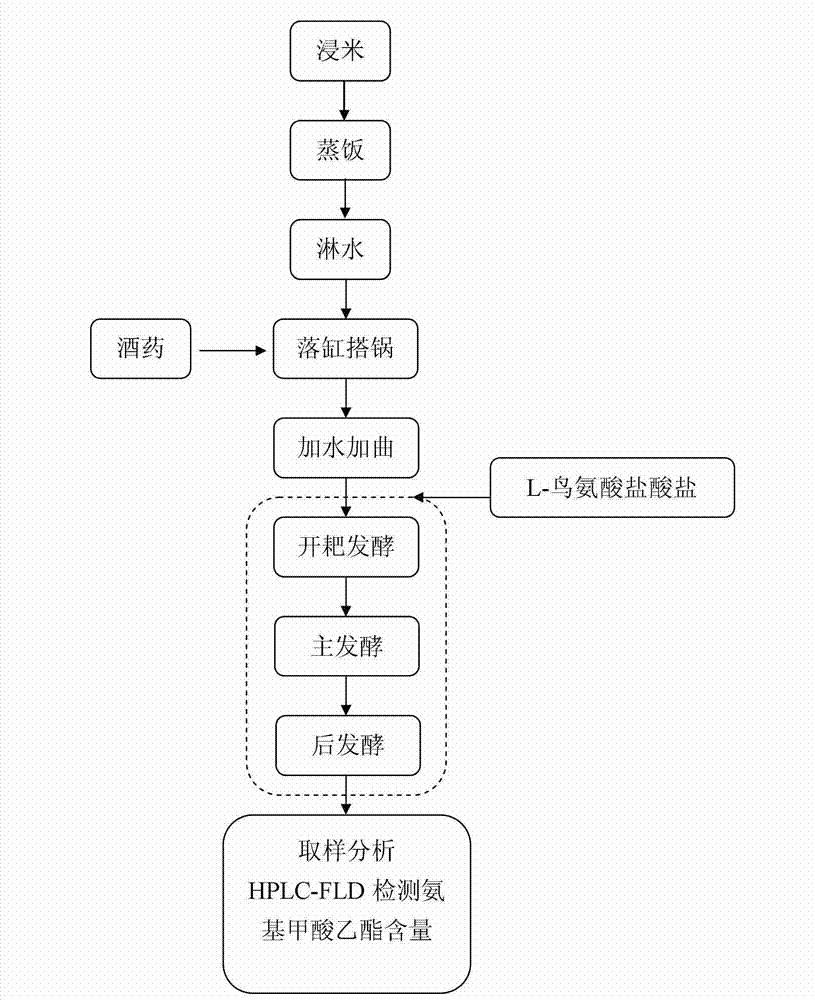
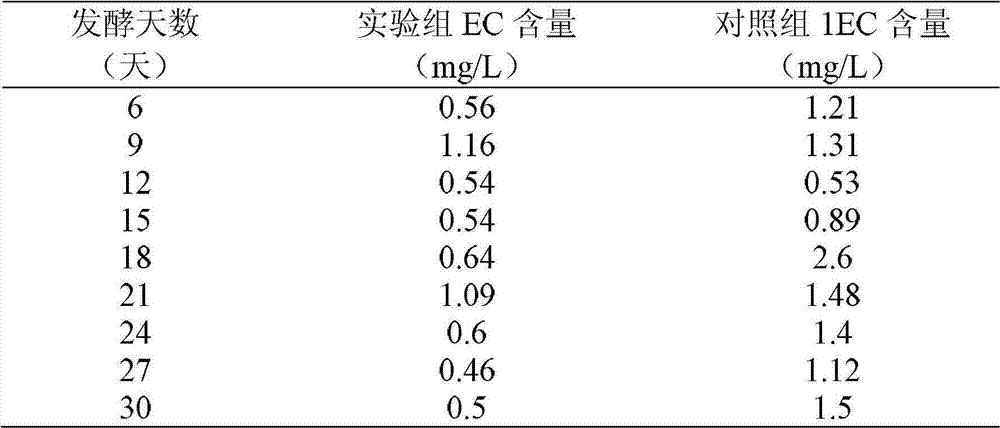
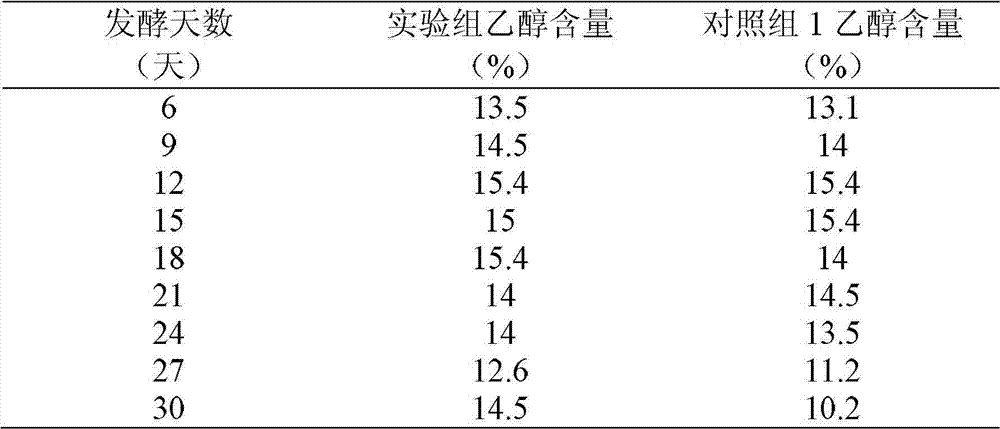
Yellow wine brewing method
The invention belongs to a yellow wine brewing method, which comprises the steps of rice soaking, rice steaming, water spraying, jar falling and pot erecting, yeast and water addition, fermentation and post treatment, wherein L-ornithine monohydrochloride is added into fermentation liquid in the second day to the six day after the jar falling and pot erecting. The yellow wine brewing method has the advantages that the L-ornithine monohydrochloride as an inhibitor is easy to control, the inhibition efficiency is high, and the generation of ethyl carbamate can be effectively inhibited; after the L-ornithine monohydrochloride is dissolved in water, ornithine is generated and participates in uric acid circulation in vivo, no risk and no toxic and harm exist, and no environment pollution is caused; and in addition, the L-ornithine monohydrochloride is easy to obtain, the use is convenient, and the cost is low.
Owner:ZHEJIANG UNIV
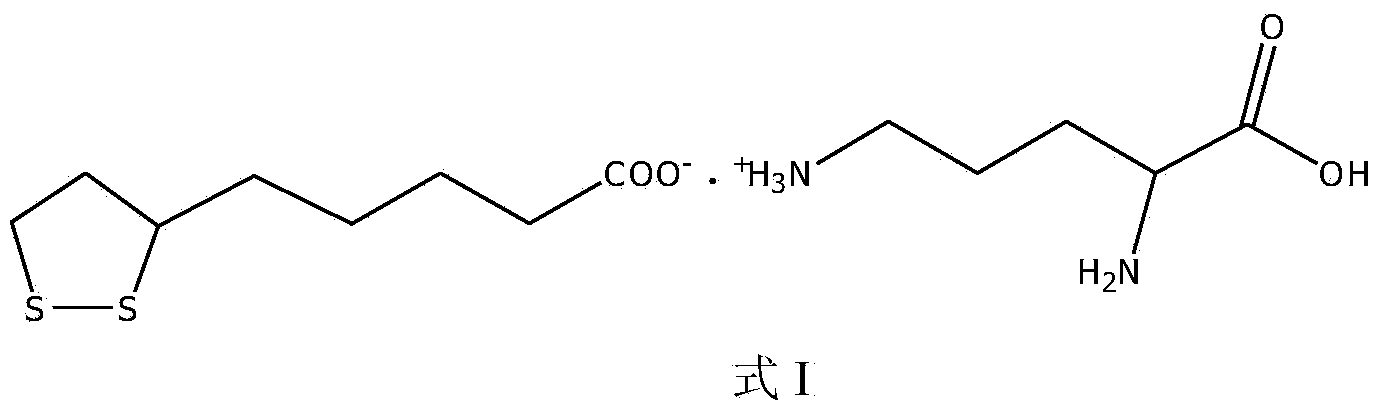
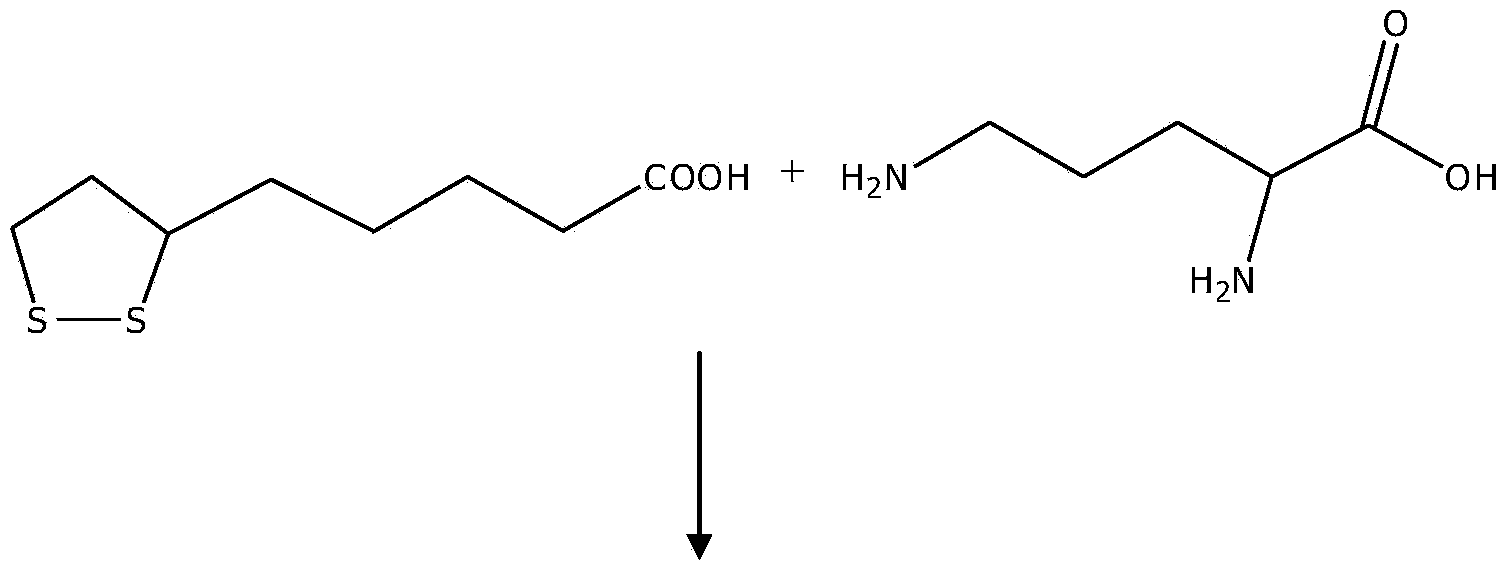
L-ornithine lipoic acid compound salt, and preparation method and application thereof
ActiveCN104387364AAppropriate medicationSimple manufacturing methodOrganic active ingredientsOrganic chemistryHepatic comaFatty liver
The invention belongs to the field of pharmaceutical chemical engineering, and discloses a L-ornithine lipoic acid compound salt, and a preparation method and application thereof. A structural formula of the L-ornithine lipoic acid compound salt is as shown in the formula I. The L-ornithine lipoic acid compound salt has functions of preventing and treating hepatic coma caused by acute and chronic hepatic diseases such as liver cirrhosis, fatty liver, hepatitis and the like and can be used for preparing a medicament for treating hepatic coma. The preparation method for the L-ornithine lipoic acid compound salt is simple and easy to operate and is low in cost; the product is high in purity.
Owner:NANJING UNIV OF TECH
Method for preparing d-arginine hydrochloride and l-ornithine hydrochloride by splitting dl-arginine by microbial enzymatic method
InactiveCN102286602AIncrease enzyme activityIncrease vitalityMicroorganism based processesFermentationEscherichia coliD-Arginine
A method for preparing D-arginine hydrochloride and L-ornithine hydrochloride by splitting DL-arginine by microbial enzymatic method. The present invention utilizes the fermented bacterium of recombinant E. coli or its spray-dried preparation that highly expresses L-arginase as an enzyme source, uses DL-arginine as a substrate, and splits DL-arginine through an enzymatic reaction to produce D-arginine and L-ornithine. The resulting liquid is converted into L-ornithine monohydrochloride by adding hydrochloric acid, then adding a certain volume of ethanol to precipitate it, and collecting the precipitate to obtain L-ornithine hydrochloride; in the supernatant, D- The purification method of arginine is: adsorption and elution of ion exchange resin, concentration and drying under reduced pressure, to obtain D-arginine hydrochloride; this patent provides a new D-arginine hydrochloride and L- Green production process route of ornithine hydrochloride.
Owner:天津启仁医药科技有限公司
Ornithine and aspartate compound and novel method thereof
InactiveCN101880240ALow costSimple processOrganic compound preparationAmino-carboxyl compound preparationSolubilityOrnithine synthesis
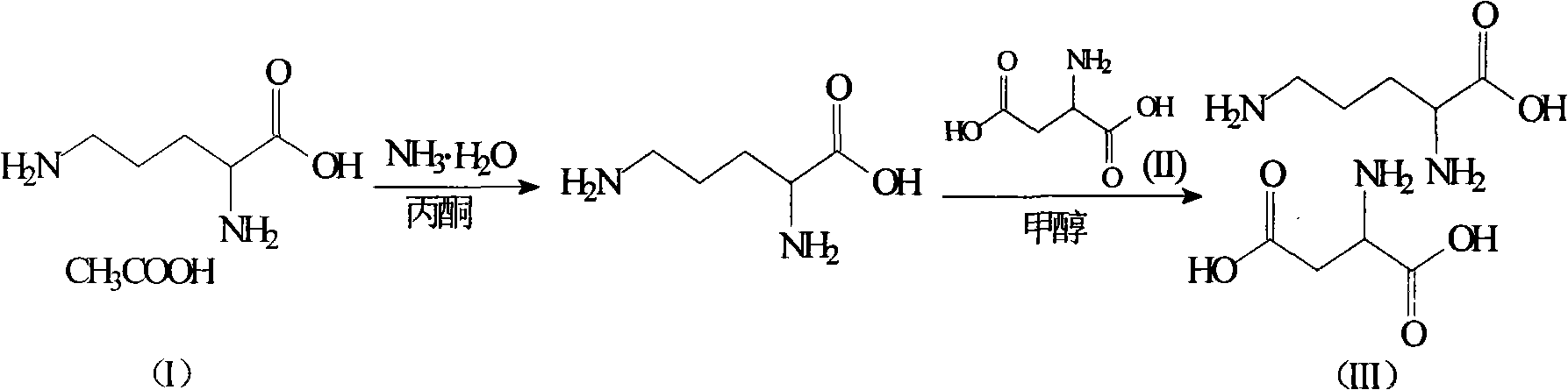
The invention relates to an ornithine aspartate compound and a novel method thereof. L-aspartic acid and L-ornithine acetate are used as starting materials. The method comprises the following steps of: removing ammonium acetate serving as an intermediate product through solubility differences of ornithine and the ammonium acetate in acetone to obtain free alkali of the ornithine; and reacting thefree alkali with the L-aspartic acid to generate the ornithine and aspartate compound. The synthetic method of the ornithine and aspartate compound has the advantages of low cost, simple process, high purity of the obtained product and easy industrial production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Composite for disintoxicating and sobering
The invention discloses a composite (B) for disintoxicating and sobering, which is prepared by glucose and fructose, fruit glucose, honey, L-cysteine, L-alanine, L-ornithine, L-glutamine, L-carnitine according to a certain weight range. In order to enable the effect better, one or more of taurine, L-asparaginic acid or L-aspartate, vitamin B1, vitamin B6, caffeine, L-arginine, L-glutamic acid, L-proline, phaseomannite, vitamin B2, nicotinic acid, folic acid, vitamin B12 and pantothenic acid are also added. The composite can be prepared into liquid preparation and electuary. The composite of the invention can be prepared into food and health food. The invention can quickly disintoxicate, sober, eliminate alcoholism symptom and continued effect of alcohol on a human body, and has no side effect.
Owner:克科
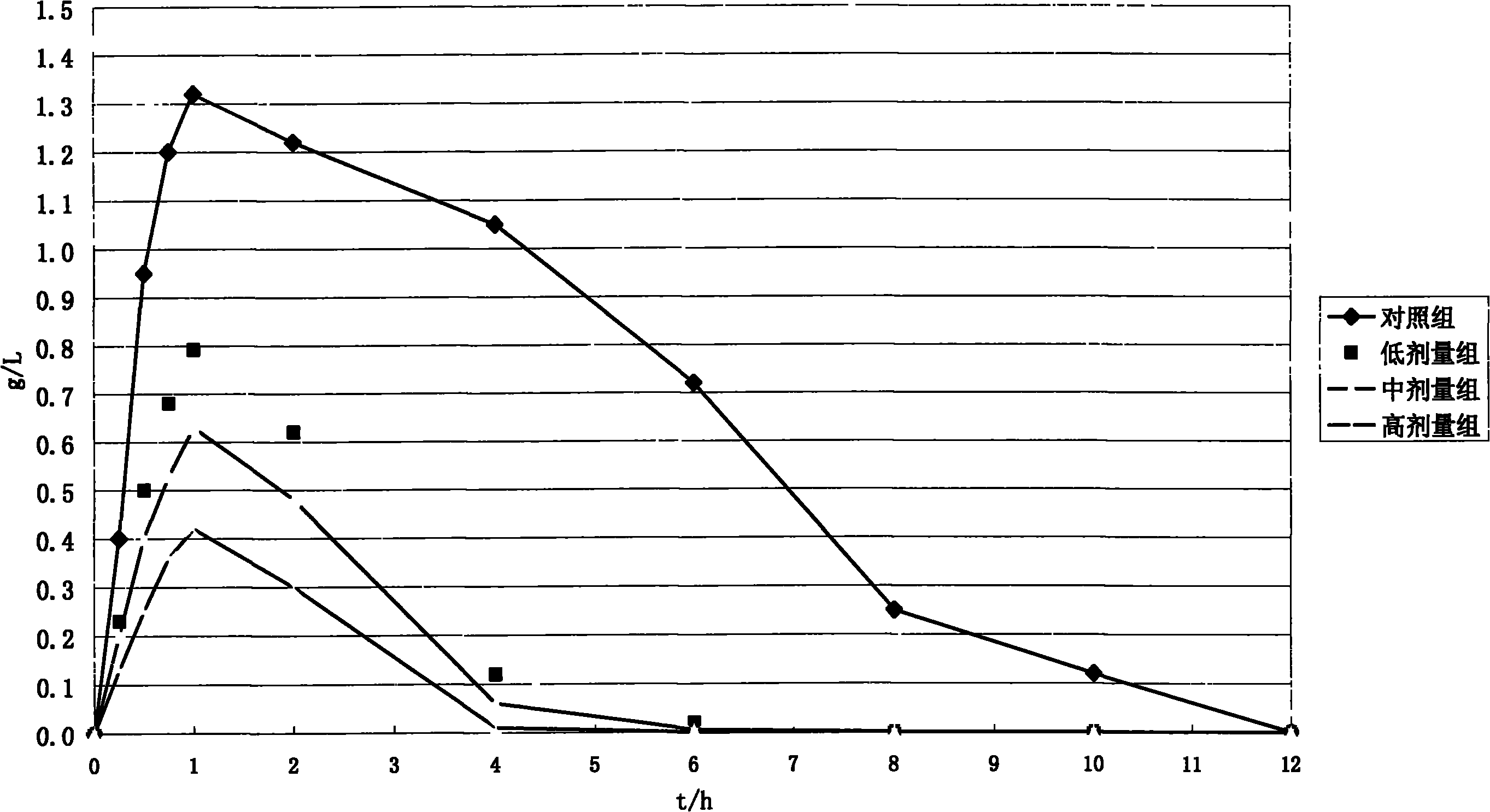
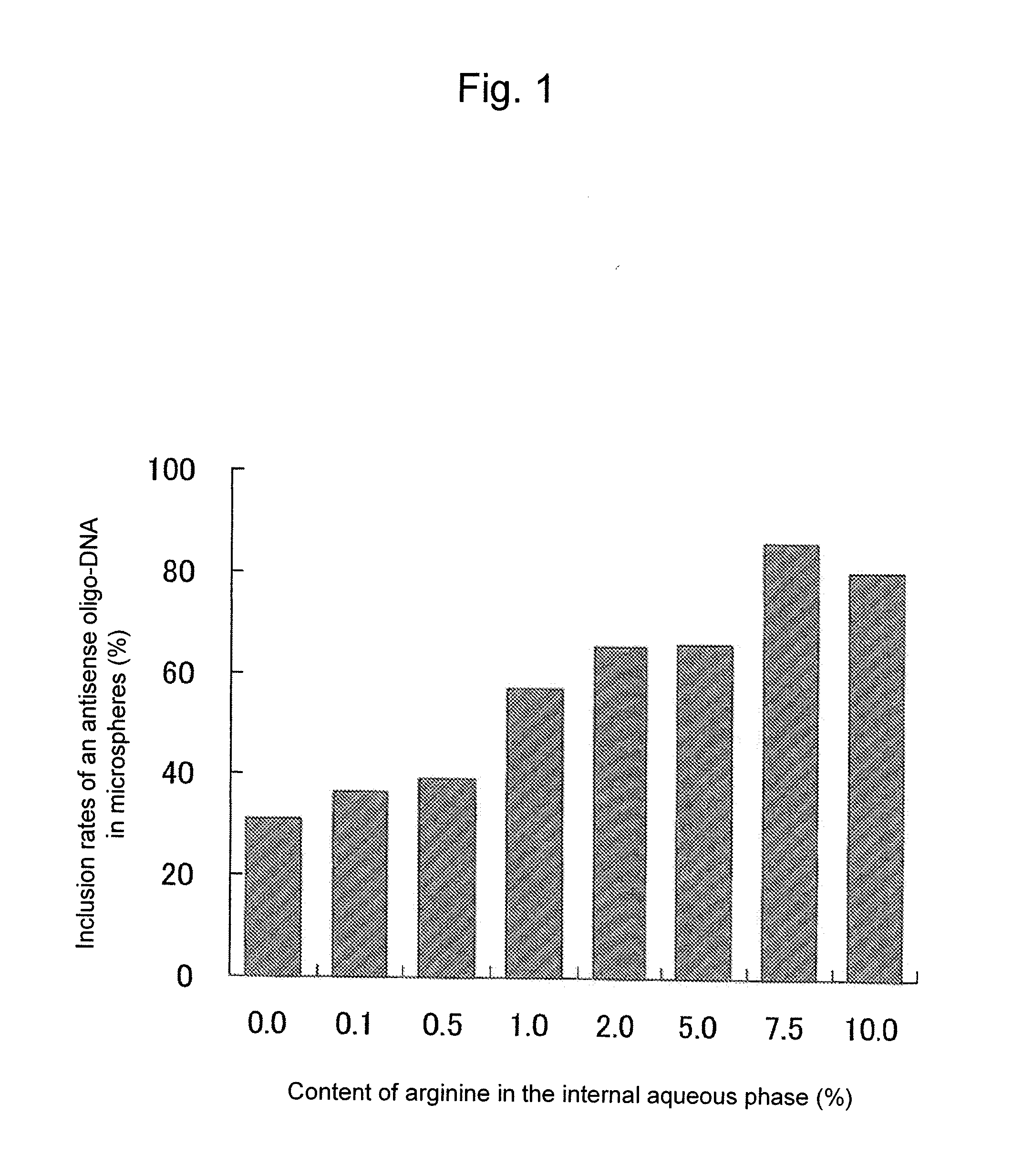
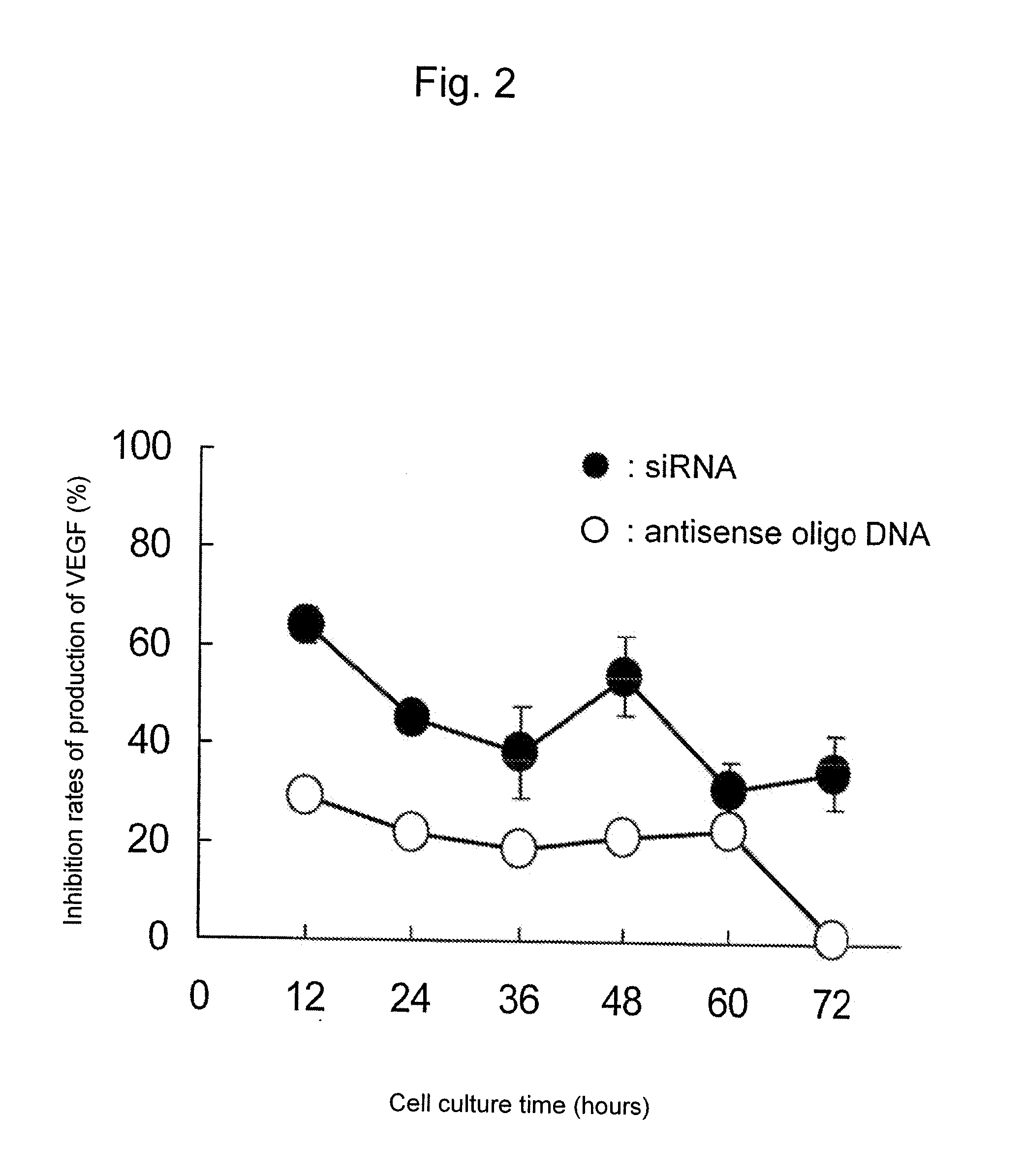
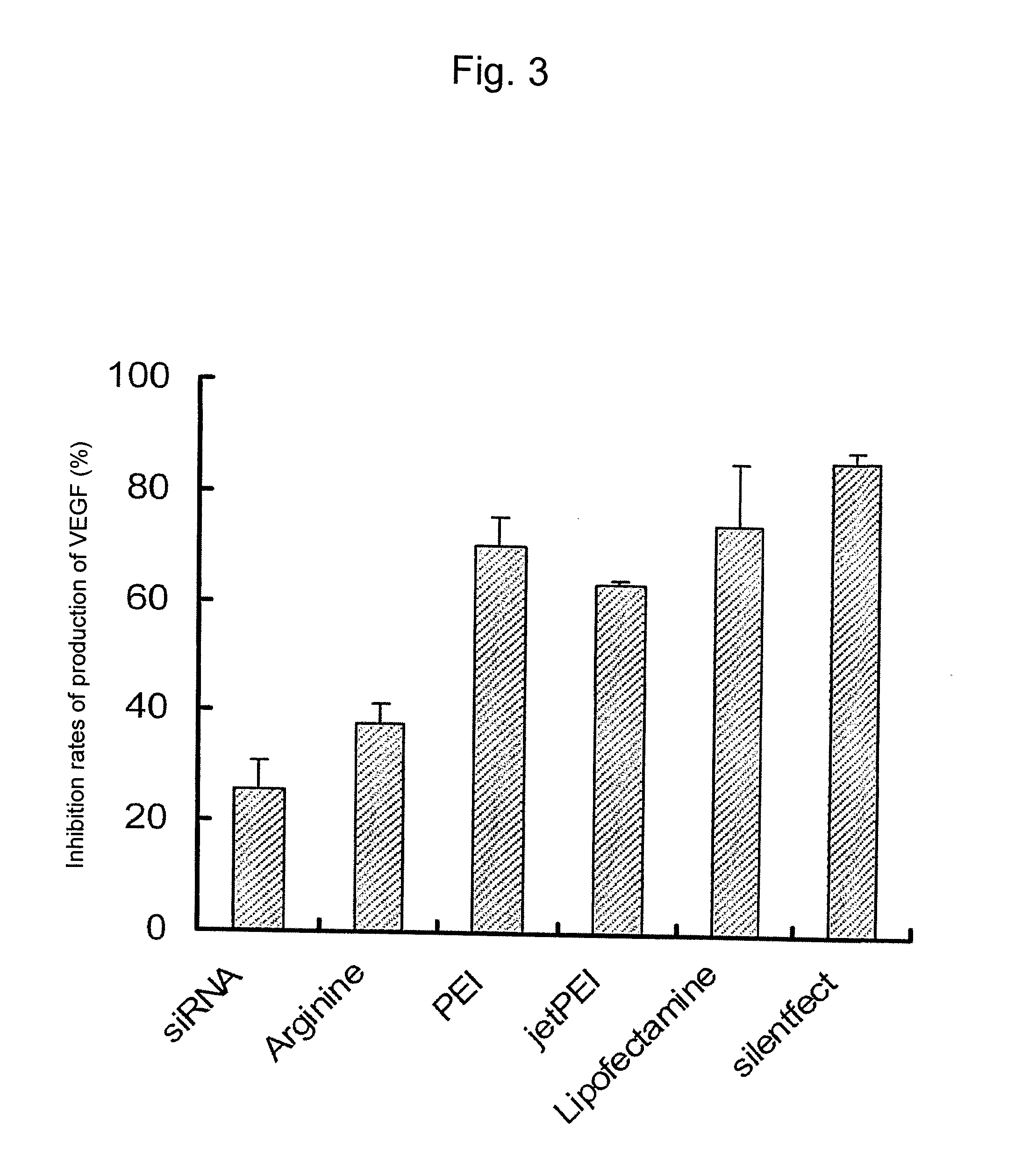
Sustained-release microsphere containing short chain deoxyribonucleic acid or short chain ribonucleic acid and method of producing the same
InactiveUS20100310670A1Inhibit expressionHigh inclusion ratePowder deliveryOrganic active ingredientsDiseaseArginine
A sustained-release microsphere formulation containing a short chain deoxyribonucleic acid or a short chain ribonucleic acid as an active ingredient, which has improved sustained-release properties and long-lasting efficacy, is provided. A fine particle formulation, encapsulating stably a short chain deoxyribonucleic acid or a short chain ribonucleic acid, being capable of inhibiting, for a long period, expression of a specific protein related to a disease, and which can be administered by injection or transmucosally, and a production method of the same are provided. A sustained-release microsphere formulation containing a short chain deoxyribonucleic acid or a short chain ribonucleic acid, particularly siRNA, as an active ingredient, especially a sustained-release microsphere prepared through a w1 / o / w2 type emulsion, is characterized in that a positively charged basic substance, such as arginine, polyethylenimine, a cell permeable peptide, poly-L-lysine or poly-L-ornithine, is included in an in vivo degradable polymer.
Owner:TAKEDA PHARMA CO LTD
Method for producing L-ornithine hydrochloride by genetic engineering bacteria
ActiveCN102191291ASimple procedureFold preciselyBacteriaMicroorganism based processesOrnithine synthesisArginine
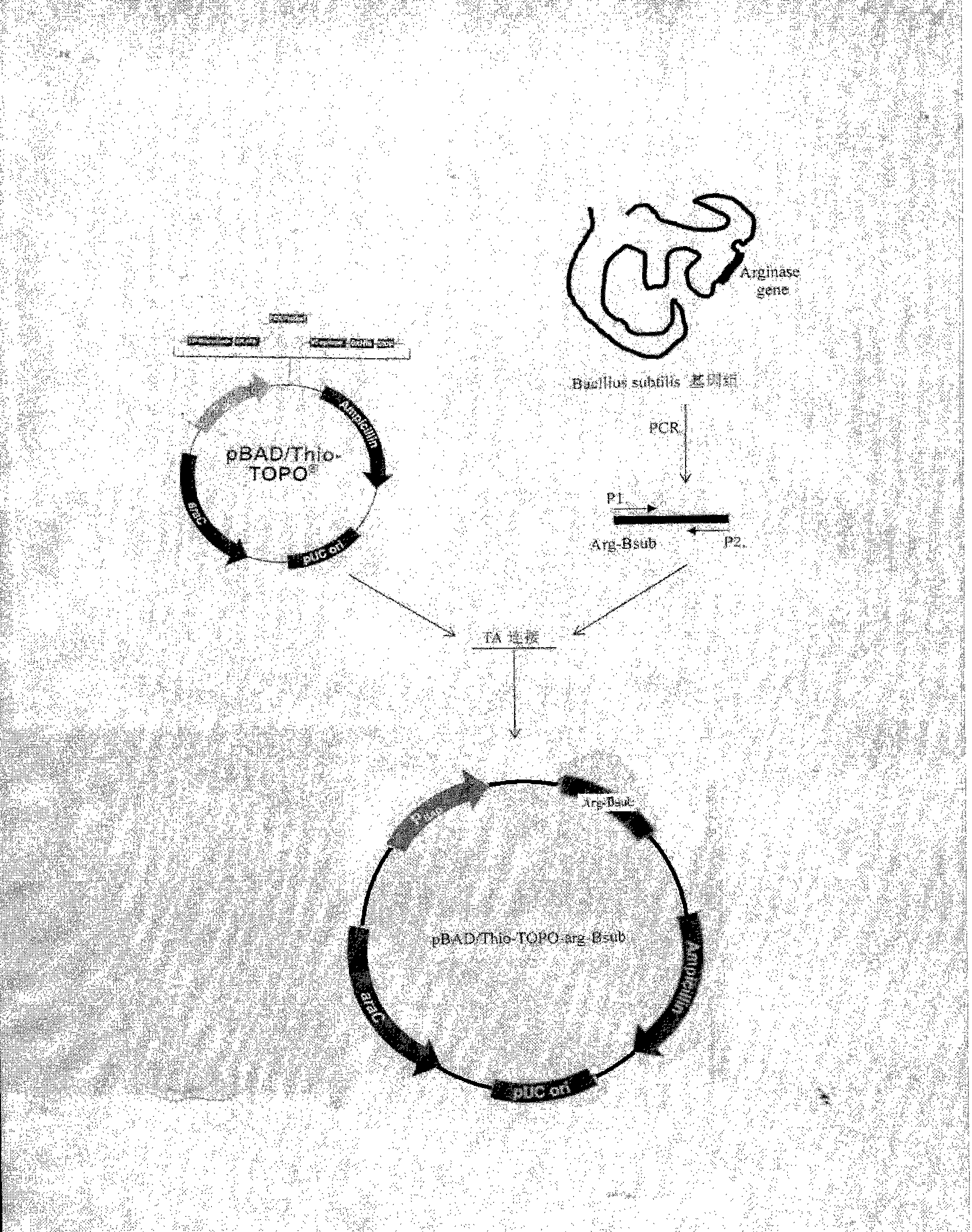
The invention discloses a method for producing L-ornithine hydrochloride by TOP10 / PBAD / Thio-TOPO-Arg-Bsub colibacillus genetic engineering bacteria. The strain can express and generate excess bacillus subtilis arginase, and efficiently converts L-arginine into L-ornithine.
Owner:WUHAN GRAND HOYO
Preparation method of L-ornithine-L-aspartate
InactiveCN101844995ANo pollution in the processLess waste waterOrganic compound preparationAmino-carboxyl compound preparationIon contentL-ornithine L-aspartate
The invention relates to a preparation method of L-ornithine-L-aspartate, which comprises the following steps: mixing L-ornithine hydrochloride and deionized water; adding anion exchange resin and continue stirring to obtain a mixed solution; detecting chloride ion content by utilizing a silver nitrate solution; then adding aspartate; removing the resin and concentrating the filtrate after stirring reaction is ended; adding activated carbon for decoloration and dropwise adding absolute ethyl alcohol; then cooling the decolorized filtrate to the room temperature for separating out crystal; and drying the obtained crystal for 8-30h after the filtering is ended. Compared with the prior art, the preparation method does not generate exhaust gas or waste residues, generates low amount of waste water, basically does not need COD (chemical oxygen demand) and BOD (biochemical oxygen demand), almost does not pollute the environment and has high total product yield which can reach more than 80 percent, does not use poisonous raw materials and has higher product quality.
Owner:SHANGHAI TIANYE CHEM
Method for preparing L-ornithine-L-aspartate salt
InactiveCN102102118AHigh purityIncrease productionOn/in organic carrierFermentationOrnithine aspartateL-ornithine L-aspartate
The invention relates to a method for preparing L-ornithine-L-aspartate salt. The L-ornithine-L-aspartate salt is produced through conversion of arginase. The method comprises the following process steps of: (1) preparing immobilized enzyme; (2) optimizing conversion conditions; and (3) extracting product and refining. Compared with the prior art, the method has the advantages that: production cost is low, the production conditions are mild, impurities in a conversion system are a few, the process steps are simple, the production operation is safe, the purity is high, 300 to 320g of L-ornithine-L-aspartate salt is contained in each liter of reaction solution, the yield is high, the conversion rate of arginase is over 95 percent and the like.
Owner:湖南天成生化科技有限公司
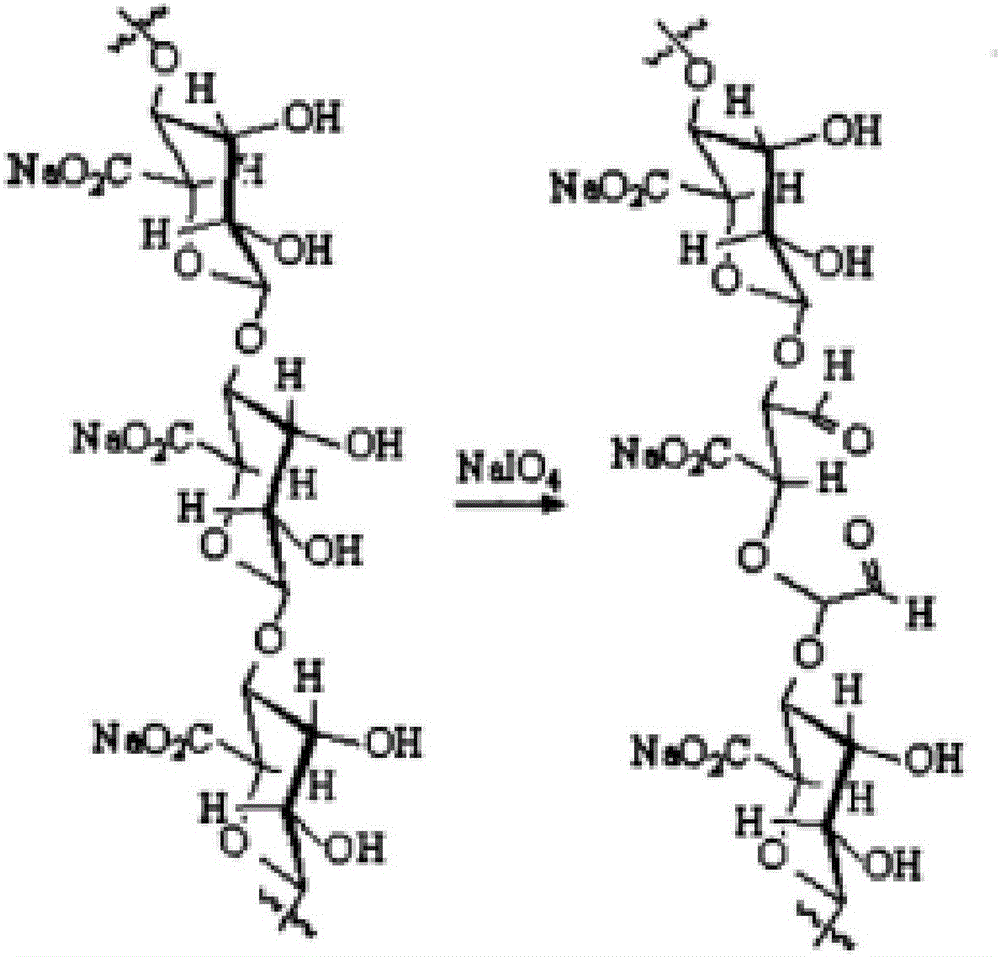
Sodium-alginate-based hydrogel and preparation method thereof
The invention relates to sodium-alginate-based hydrogel and a preparation method thereof. The sodium-alginate-based hydrogel is prepared through steps as follows: 1) 100-300 mg of aminated sodium alginate, 20-100 mg of L-lysine or 25-120 mg of L-arginine or 18-90 mg of L-ornithine and 20-100 mg of nano ferroferric oxide magnetic particles are dissolved in a PBS solution; 2) 50-300 mg of oxidized sodium alginate is dissolved in a borax solution; 3) solutions obtained in steps 1) and 2) are mixed and stirred and react at the constant temperature of 25-40 DEG C for 0.5-24 h, and the sodium-alginate-based hydrogel is obtained. Oxidized sodium alginate is prepared by reacting sodium alginate with an oxidizing agent under the acid condition, and aminated sodium alginate is prepared by modifying sodium alginate with a diamine compounds. The prepared sodium-alginate-based hydrogel has the advantages of being injectable, self-healing, degradable, good in biocompatibility and the like, raw material sources are wide, the preparation process is simple, and industrial production is facilitated.
Owner:WUHAN UNIV OF TECH
Process for the fermentative preparation of L-ornithine
A process for the fermentative preparation of L-ornithine using microorganisms characterized by an increased export of the amino acid.
Owner:EVONIK OPERATIONS GMBH
Method for producing L-arginine, L-ornithine or L-citrulline
It is intended to provide a polypeptide having (i) an amino acid sequence derived from the amino acid sequence represented by SEQ ID NO:1 by substitution of one or more amino acids in the amino acid sequences of from the 20- to the 38-th positions from the N end, or (ii) an amino acid sequence derived from the amino acid sequence represented by SEQ ID NO:1 by substitution of one or more amino acids in the amino acid sequences of from the 20- to the 38-th positions from the N end and deletion, substitution or addition of one or more amino acids in the amino acid sequence of from the first to the 19-th positions or from the 39-th to the 294-th positions, and having an N-acetylglutamate kinase activity.
Owner:KYOWA HAKKO BIO CO LTD
Metabolic transformation method for efficiently improving production capacity of corynebacterium crenatum SYPA5-5 L-arginine
The invention provides a metabolic transformation strategy for efficiently improving production capacity of corynebacterium crenatum SYPA5-5 L-arginine, and belongs to the biological technical field. Histidine at a site 268 in N-acetyl glutamatekinase, NAGK, argB of SYPA-5 is mutated into asparaginate, so that feedback inhibition effect of the L-arginine on NAGK can be effectively relived; a mutation site is precisely introduced to a corresponding position of SYPA5-5 genome, so that yield of the obtained feedback inhibition-preventing bacterial strain (H-7)L-arginine is lowered, and metabolic intermediate (L-ornithine and L-citrulline) is largely accumulated. Transcriptional analysis is carried out on an arg gene cluster of h-7 to find that arg GH transcriptional level is lowered. Arg GH reinforced expression is carried out in H-7, and production capacity of the obtained recombinant bacterial strain H-7-GH, L-arginine is improved by 99.0% in comparison with SYPA5-5. Preliminary optimization is carried out on a fermentation culture medium of H-7-GH, so that yield of the L-arginine is 45.1g / L, which is improved by 49.8% in comparison with that before optimization, and improved by 1.13 times in comparison with SYPA5-5. Feedback inhibition of the L-arginine on NAGK is relieved, and metabolic transformation strategy of the arg GH gene is expressed in a strengthened manner at the same time, so that production capacity of the L-arginine of the SYPA 5-5 is effectively improved.
Owner:JIANGNAN UNIV
Preparation method of ornithine aspartate
InactiveCN102964261AOrganic compound preparationAmino-carboxyl compound preparationInstabilityL-Ornithine
As a drug for treatment of liver diseases, ornithine aspartate has very wide application clinically. However, existing ornithine aspartate preparation methods consist of: taking ornithine hydrochloride as the raw material, using ion exchange resin to remove chloride ions, then employing ammonia water to conduct elution, carrying out heating to remove ammonia, and adding aspartic acid to perform salification, and have the disadvantages of great time consumption, high cost, large acid-base consumption, great pollution, instability of L-ornithine under an alkaline condition during heating for ammonia removing, and easy generation of impurities. The method provided in the invention includes: adopting ornithine hydrochloride as the starting material, employing an electrodialysis method and making use of the principle that charged ions move under a current function to remove chloride ions in the ornithine hydrochloride, adding aspartic acid to perform salification, and conducting refining with methanol or ethanol. The method not only guarantees the stability of L-ornithine, has the characteristics of simplicity, practicability, and low cost, but also substantially alleviates the environmental protection pressure due to less acid-base consumption, and improves the production capability, thus being suitable for scale-up production.
Owner:肖文辉 +1
Preparation method of L-ornithine hydrochloride
InactiveCN1590367AEasy to operateHigh yieldOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisArginine
A process for preparing L-ornithine hydrochloride includes dissolving L-arginine in water, adding crown ether and calcium hydroxide, stirring while heating, adding choline, reaction, cooling, using sulfuric acid to regulate pH value, filtering to remove calcium sulfate, vacuum concentrating, using saturated solution of barium hydroxide to regulate pH value, filtering to remove barium sulfate, using hydrochloric acid to regulate pH value, vacuum concentrating, adding alcohol, cooling and filtering. It has high output rate and purity.
Owner:上海依福瑞实业有限公司
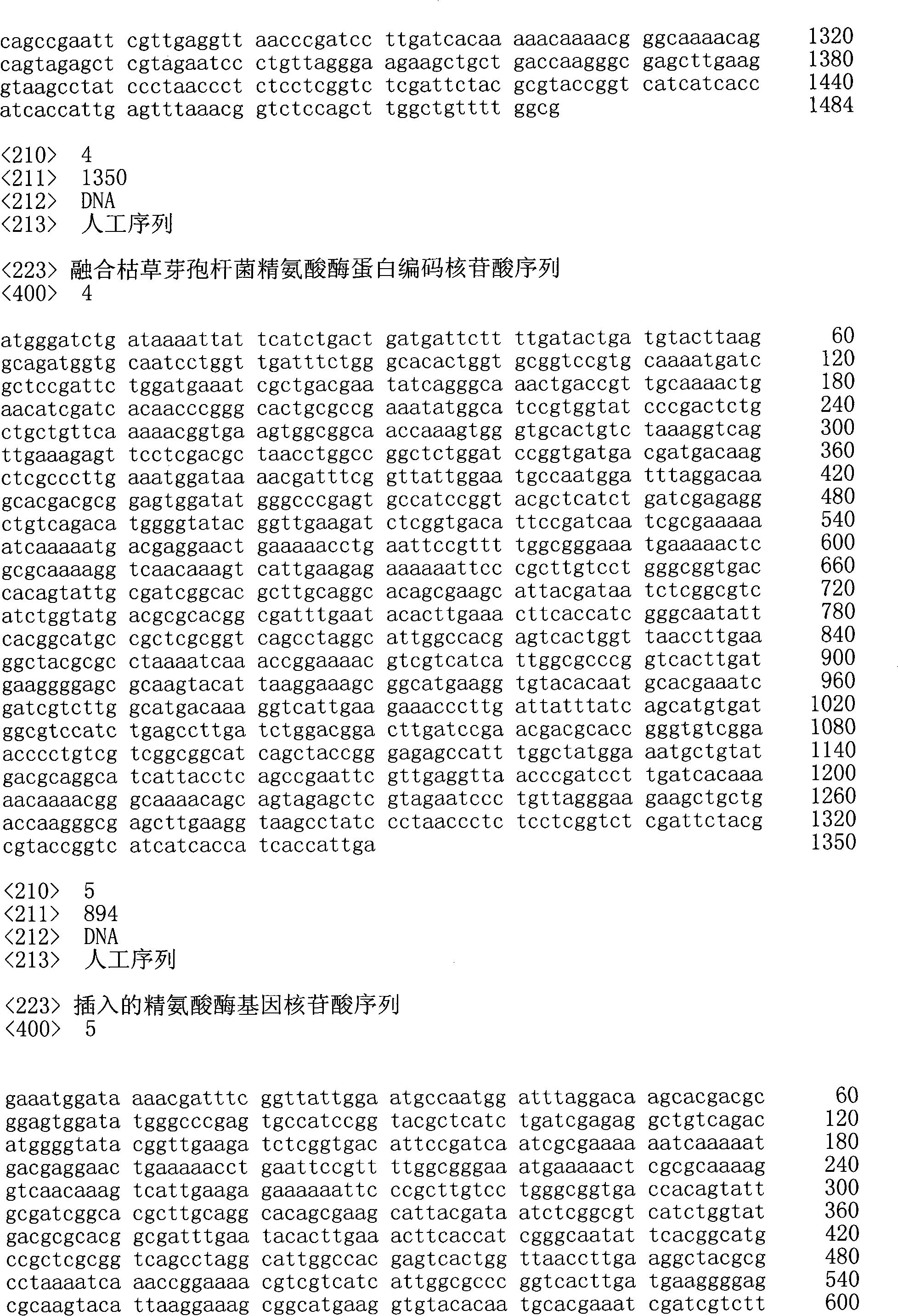
Construction of arginase production engineering bacteria and application of arginase production engineering bacteria in production of L-ornithine
InactiveCN103923936AIncrease vitalityMeet the needs of industrial scale productionBacteriaFermentationEscherichia coliBiotechnology
The invention describes a construction method of arginase production engineering bacteria and application of the arginase production engineering bacteria in production of L-ornithine by transformation of L-arginine, and belongs to the technical field of biological engineering. According to the method, by means of molecular biology, thermophilic bacteria Bacillus caldovelox (DSM411) is cloned to obtain arginase gene, a constructed expression plasmid is transfected into E.coli BL21 (DE3), and expression vector high-copy recombine ant arginase-containing engineering bacteria pET28a-ARG is obtained by kanamycin resistance plate screening. The enzyme can still show high activity after induced expression in E.coli. The bacteria obtained by fermentation can be directly used for transformation without breaking, in the condition of 60 DEG C, the engineering bacteria has higher permeability, and is more conducive to the substrate uptake and product release, the reaction speed is greatly improved, the transformation period is only 2-4h, the ornithine yield can reach 120.1g / L, and the transformation rate is 98.9%.
Owner:JIANGNAN UNIV
Production of mutant strain of aspergillus fumigatus, method of assay for inhibiting siderophore biosynthesis and diagnostic method for detecting likely aspergillus fumigatus infection
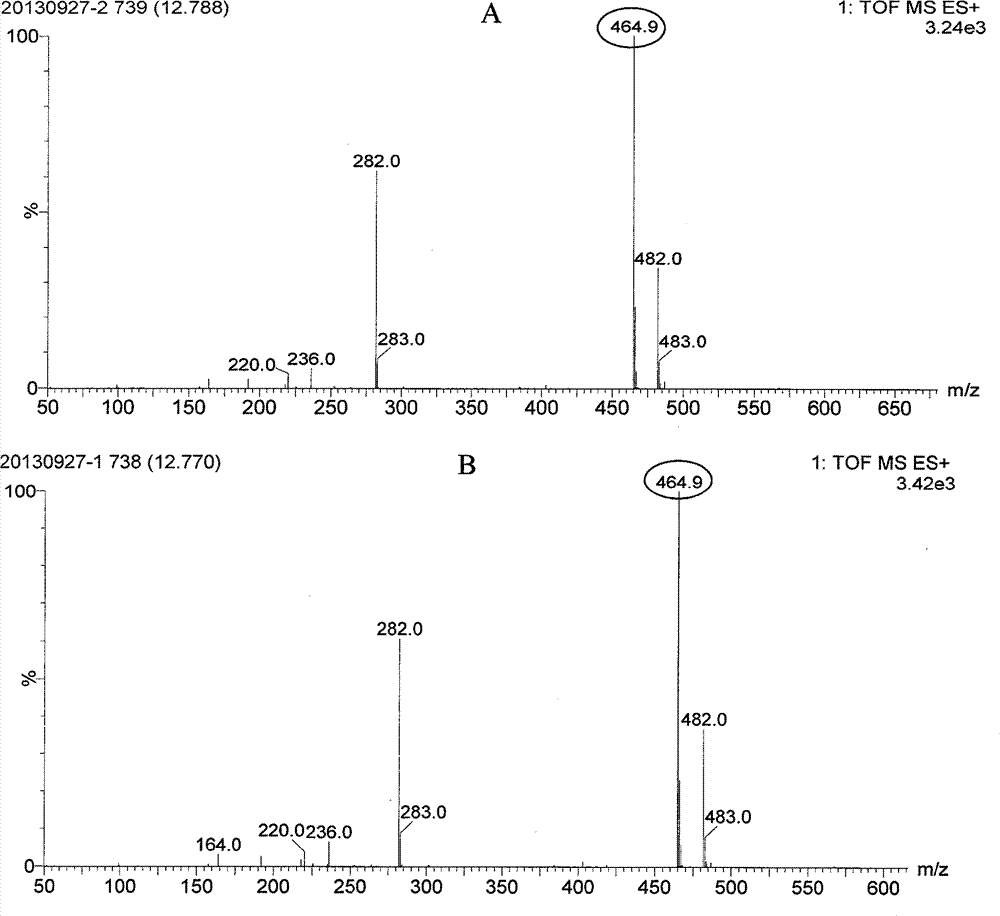
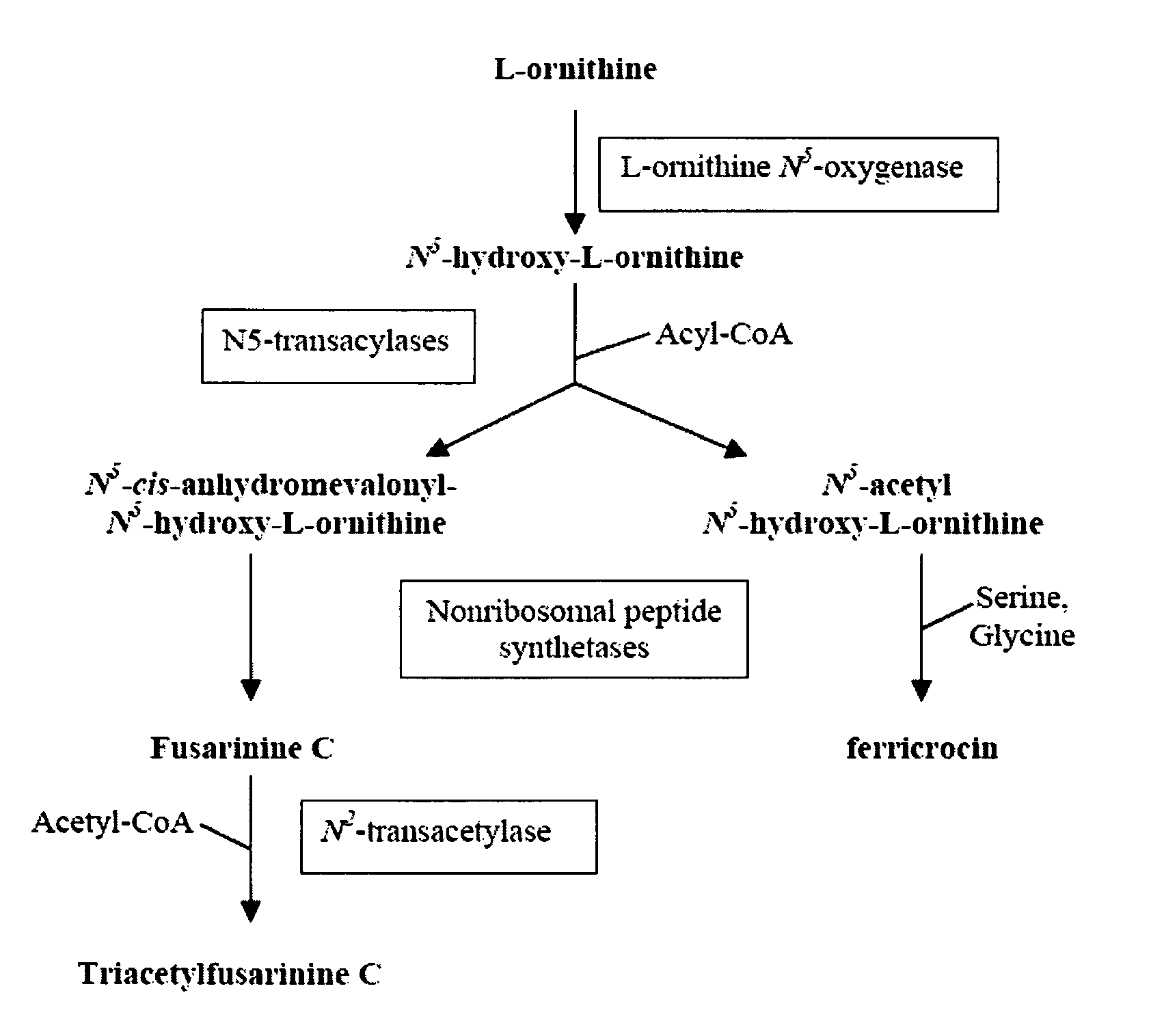
This application relates to the production and characterization of a mutant strain of Aspergillus fumigatus. The application also relates to a method for inhibiting siderophore biosynthesis in Aspergillus fumigatus and an assay for identifying drug candidates or other agents having potential inhibitory activity. The method may comprise, for example, the step of inhibiting an enzyme catalyzing siderophore biosynthesis, such as L-ornithine N5-oxygenase. In one embodiment the siderophore is a hydroxamate siderophore, such as N′N″N′″-triacetylfusarinine C (TAF) or ferricrocin. A method of preventing or treating fungal infections in a patient is also described comprising administering to the patient an agent suitable for inhibiting fungal secretion of siderophores. The method is particularly useful for immunocompromised patients susceptible to fungal infections caused by Aspergillus fumigatus, such as pulmonary aspergillosis. The invention also relates to diagnostic methods for detecting a biomarker indicative of likely A fumigatus infection in vivo, such as serum presence of TAF.
Owner:SIMON FRASER UNIVERSITY
Method for producing L-ornithine hydrochloride through immobilized enzyme process
InactiveCN101851646AProduction operation safetyHigh purityOn/in organic carrierFermentationOrnithine synthesisL-Ornithine
The invention relates to a preparation method for L-ornithine hydrochloride. The L-ornithine hydrochloride is produced through converting arginase. The method comprises the following technological steps: (1) preparing immobilized enzyme; (2) optimizing conversion conditions; and (3) extracting products and refining. Compared with the prior art, the invention has the advantages that the production cost is low, the production conditions are mild, the impurities in the conversion system are less, the technological steps are simple, the production and the operation are safe, the purity is high, one liter of solution contains 100-130g of L-ornithine hydrochloride, the yield is high, the conversion rate of arginine reaches more than 95 percent and the like.
Owner:湖南天成生化科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com