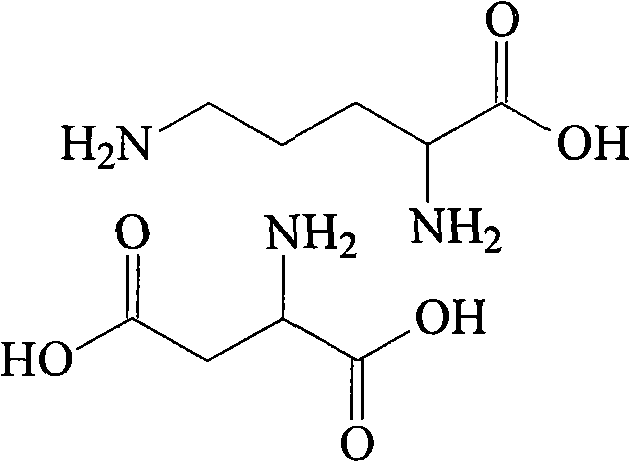
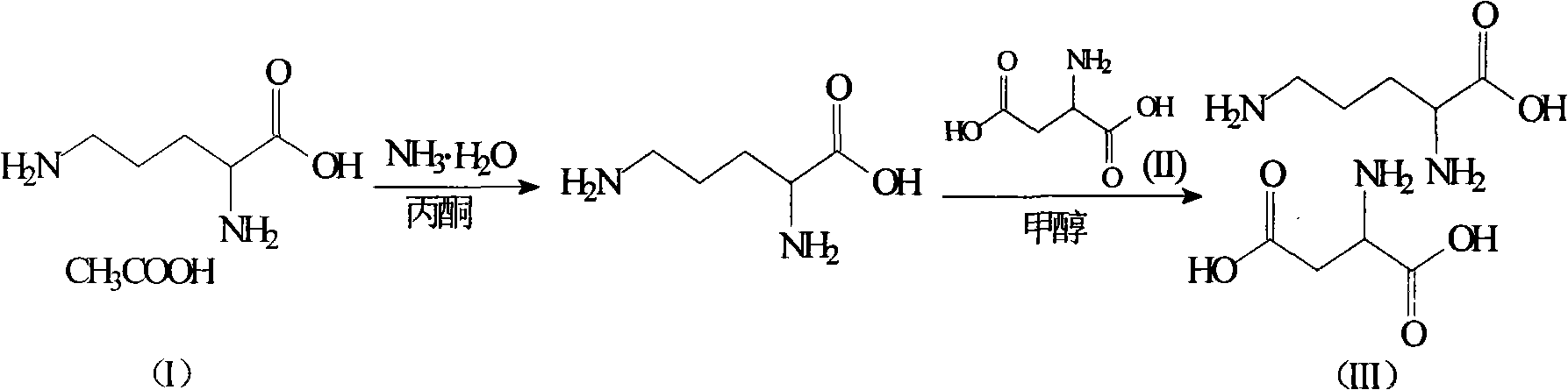
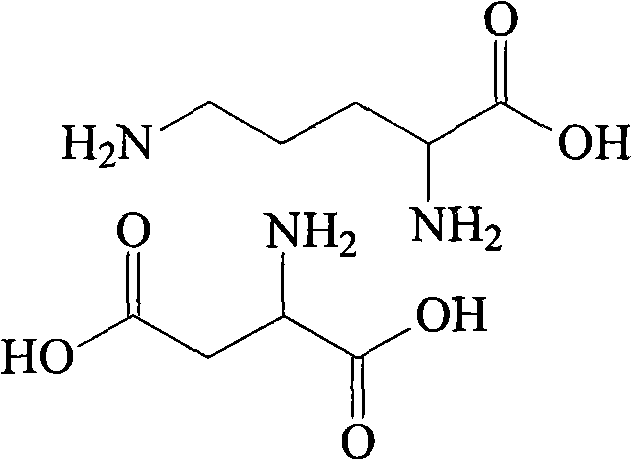
Ornithine and aspartate compound and novel method thereof
A technology of ornithine aspartate and aspartic acid, applied in the field of medicine, can solve the problems of high cost, inconvenient synthesis and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The preparation of embodiment 1 aspartic acid ornithine
[0019] Dissolve 384g of L-ornithine acetate in 500ml of water, then adjust the pH of the system to 7.5 with ammonia water, add 3L of acetone, stir at room temperature for 2 hours to completely precipitate ammonium acetate, filter, and concentrate the filtrate under reduced pressure to 400ml, then add 266g of L-aspartic acid, stir and heat up to 60°C, slowly add 800ml of methanol, the solution starts to become cloudy, then heat and reflux the solution for 40 minutes, then cool to room temperature, filter to obtain a white solid, and then The white solid was added to 900ml of methanol, heated to reflux for beating, cooled to room temperature, filtered, and vacuum-dried at 50°C to obtain 453g of ornithine aspartate with a yield of 85.4%, a purity of 99.5%, and a specific rotation [α] D 20 ° = 26.7°.
Embodiment 2
[0020] The preparation of embodiment 2 aspartic acid ornithine
[0021] Dissolve 384g of L-ornithine acetate in 500ml of water, then adjust the pH of the system to 7.0 with ammonia water, add 3L of acetone, and stir at room temperature for 4 hours to completely precipitate ammonium acetate, filter, and concentrate the filtrate under reduced pressure to 400ml, then add 266g of L-aspartic acid, stir and heat up to 50°C, slowly add 700ml of methanol, the solution starts to become cloudy, then heat the solution to reflux for 20 minutes, then cool to room temperature, filter to obtain a white solid, and then The white solid was added to 800ml of methanol, heated to reflux for beating, cooled to room temperature, filtered, and vacuum-dried at 60°C to obtain 448g of ornithine aspartate with a yield of 84.4%, a purity of 99.6%, and a specific rotation [α] D 20 ° = 26.6°.
Embodiment 3
[0022] The preparation of embodiment 3 aspartic acid ornithine
[0023] Dissolve 384g of L-ornithine acetate in 500ml of water, then adjust the pH of the system to 8.0 with ammonia water, add 3L of acetone, and stir at room temperature for 3 hours to completely precipitate the ammonium acetate, filter, and concentrate the filtrate under reduced pressure to 400ml, then add 266g of L-aspartic acid, stir and heat up to 55°C, slowly add 700ml of methanol, the solution starts to become cloudy, then heat the solution to reflux for 30 minutes, then cool to room temperature, filter to obtain a white solid, and then The white solid was added to 800ml of methanol, heated to reflux for beating, cooled to room temperature, filtered, and vacuum-dried at 55°C to obtain 465g of ornithine aspartate with a yield of 87.6%, a purity of 99.5%, and a specific rotation [α] D 20 ° = 26.7°.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com