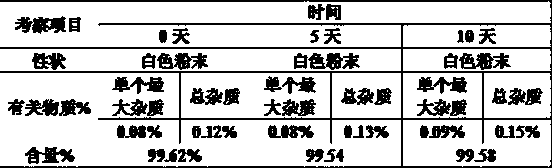
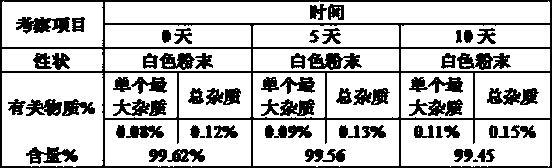
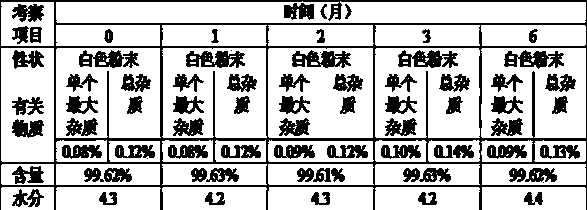
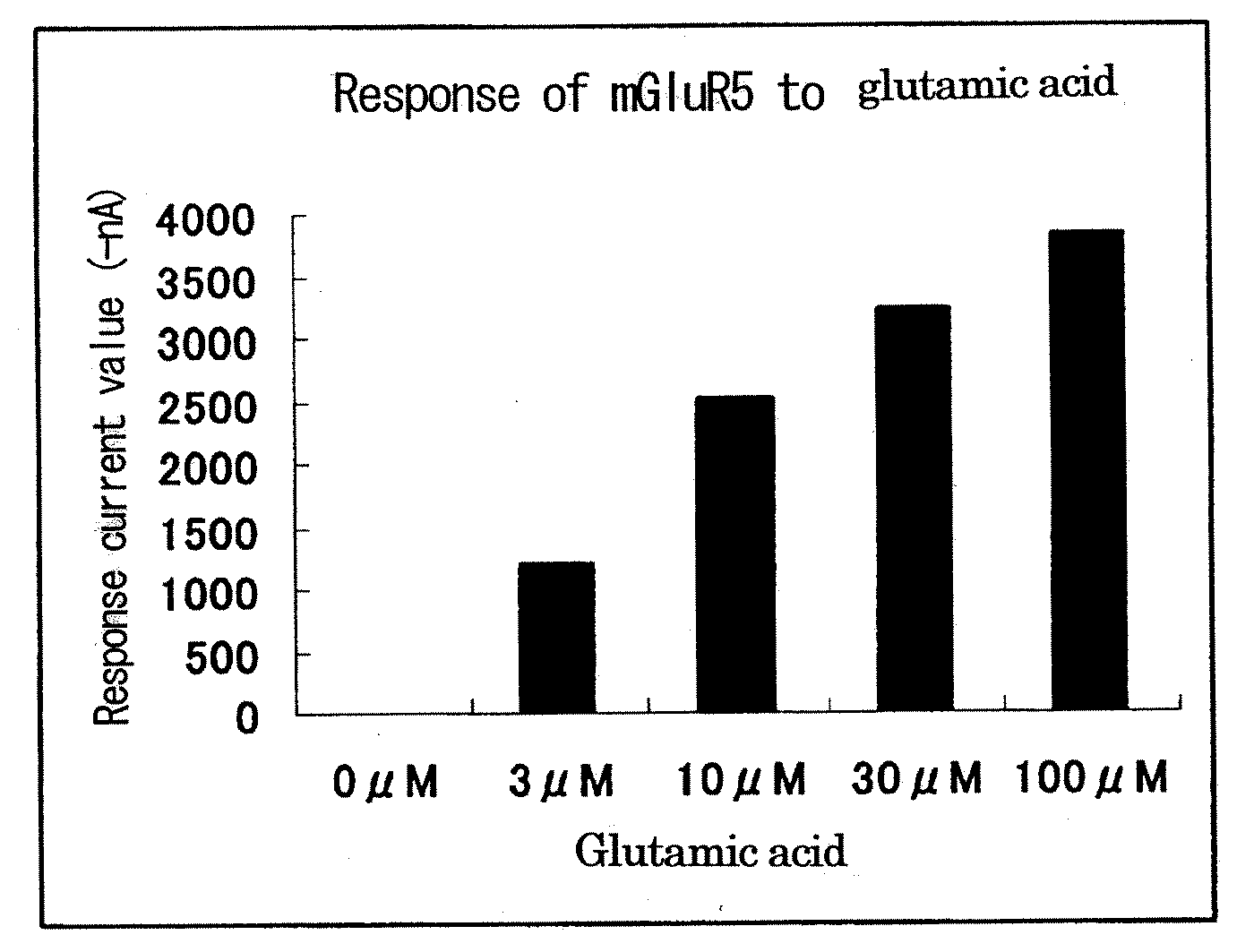
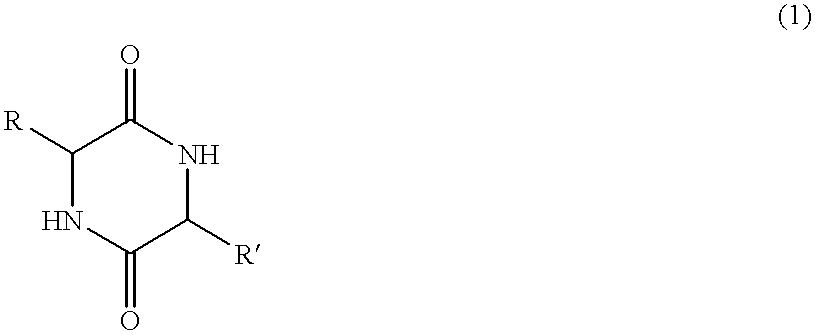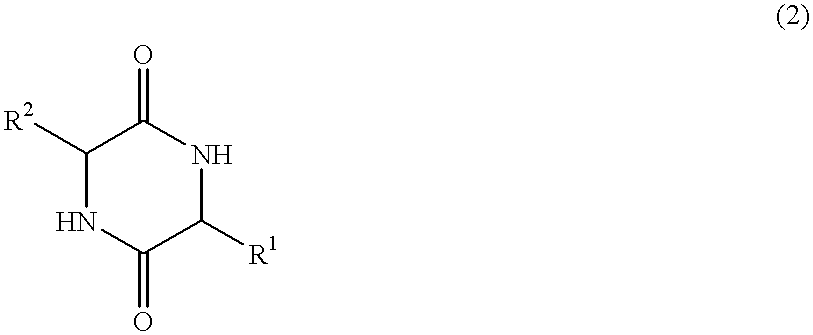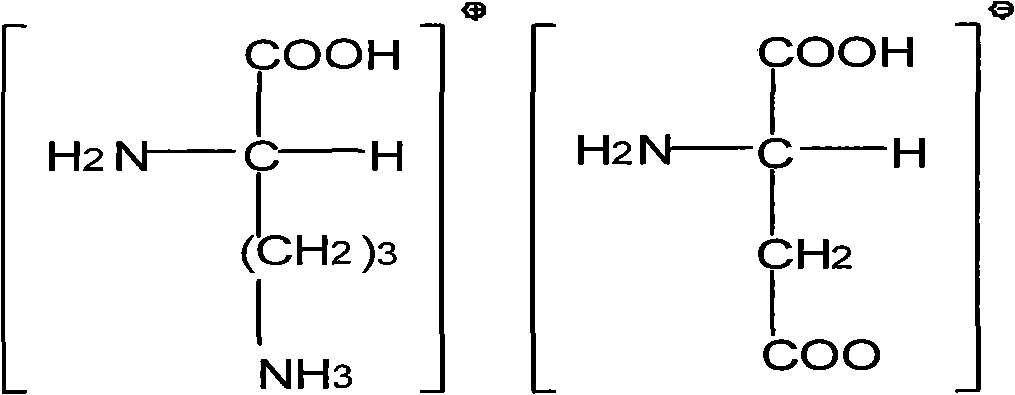Patents
Literature
198 results about "Ornithine aspartate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
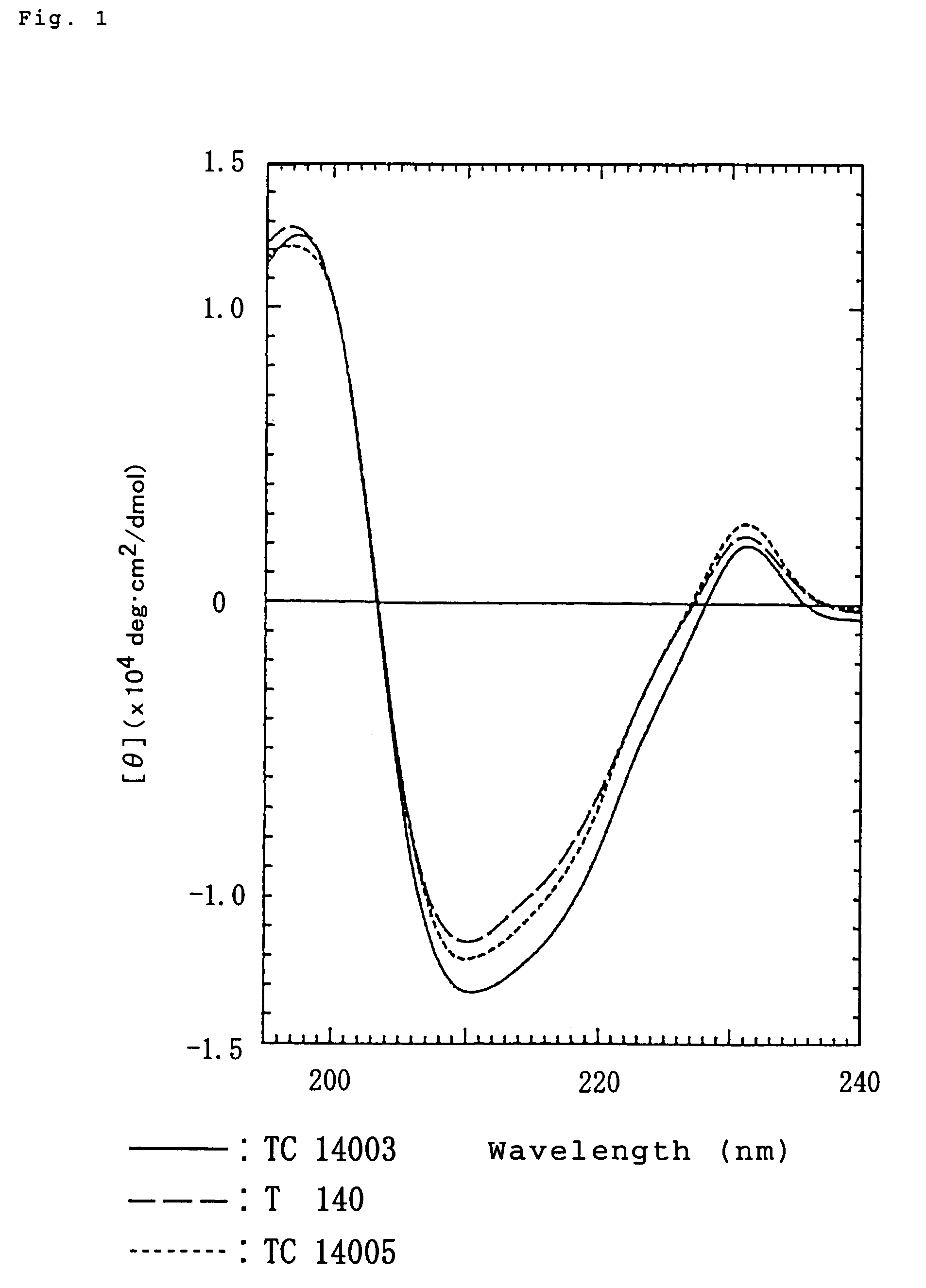
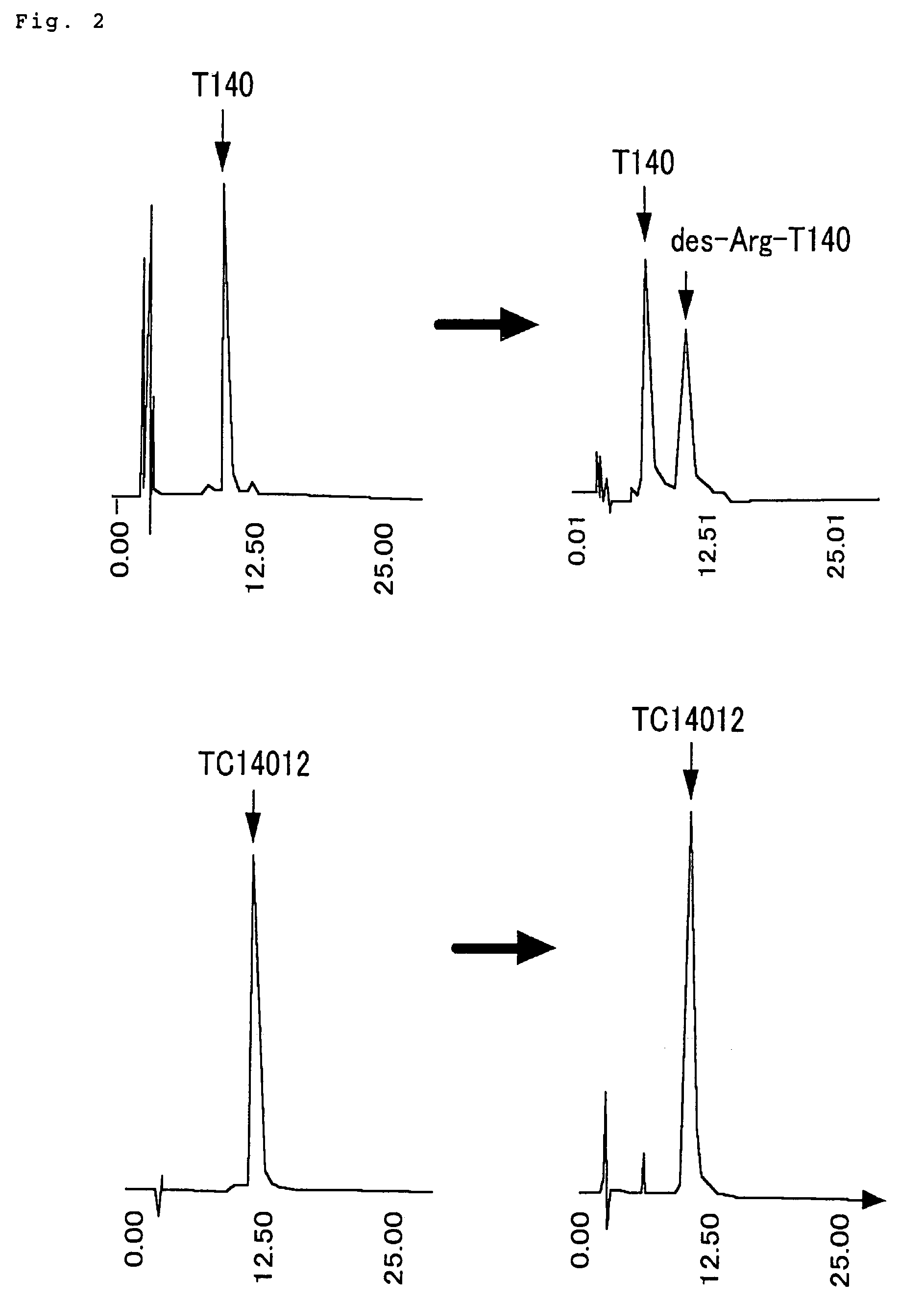
L-Ornithine L-aspartate (LOLA), a stable salt of ornithine and aspartic acid, has been used in the treatment of cirrhosis. Weast, Robert C., ed. (1981). Weber AL, Miller SL (1981). "Reasons for the occurrence of the twenty coded protein amino acids" (PDF). "Ornithine Biosynthesis".
Method of using hydroxycarboxylic acids or related compounds for treating skin changes associated with intrinsic and extrinsic aging
A composition comprising an amphoteric or pseudo-amphoteric agent and a polyhydroxy alpha hydroxyacid existing as a free acid, lactone, or salt, and isomeric or non-isomeric forms thereof is provided. The amphoteric or pseudo-amphoteric agent can be selected from amino acids, dipeptides, aminoaldonic acid, aminouronic acid, lauryl aminoproplyglycine, aminoaldaric acid, neuraminic acid desulfated heparin, deacetylated hyaluronic acid, hyalobiuronic acid, chondrosine, deacetylated chondroitin, creatine, creatinine, hydroxyproline, homocysteine, homocystine, homoserine, ornithine, citrulline, phosphatidylserine, and sphingomyelin. The composition may contain other additives, including cosmetic or pharmaceutical agents for topical treatment of dermatological disorders.
Owner:TRISTRATA TECH
Stabilized Glycosaminoglycan Preparations and Related Methods
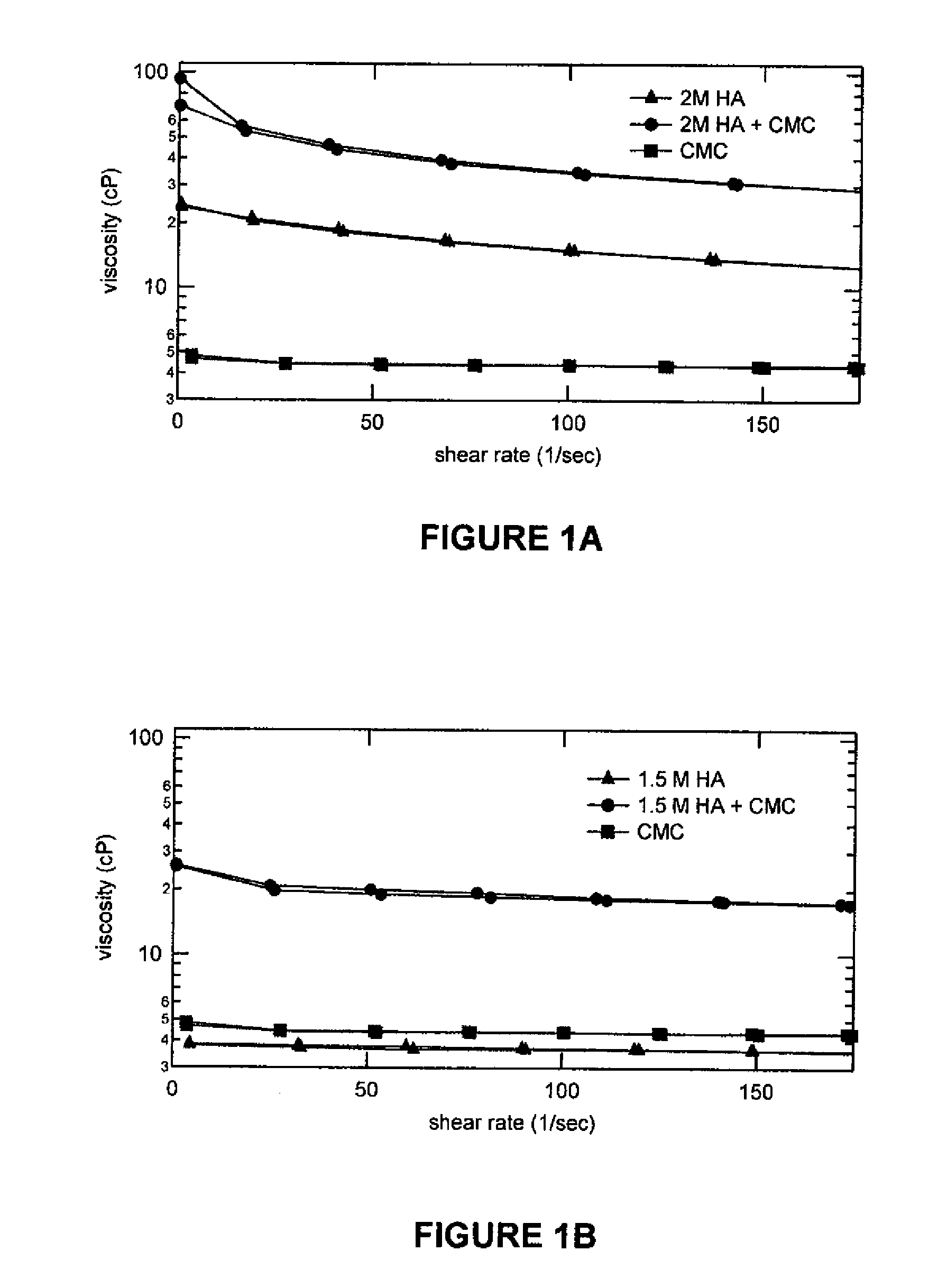
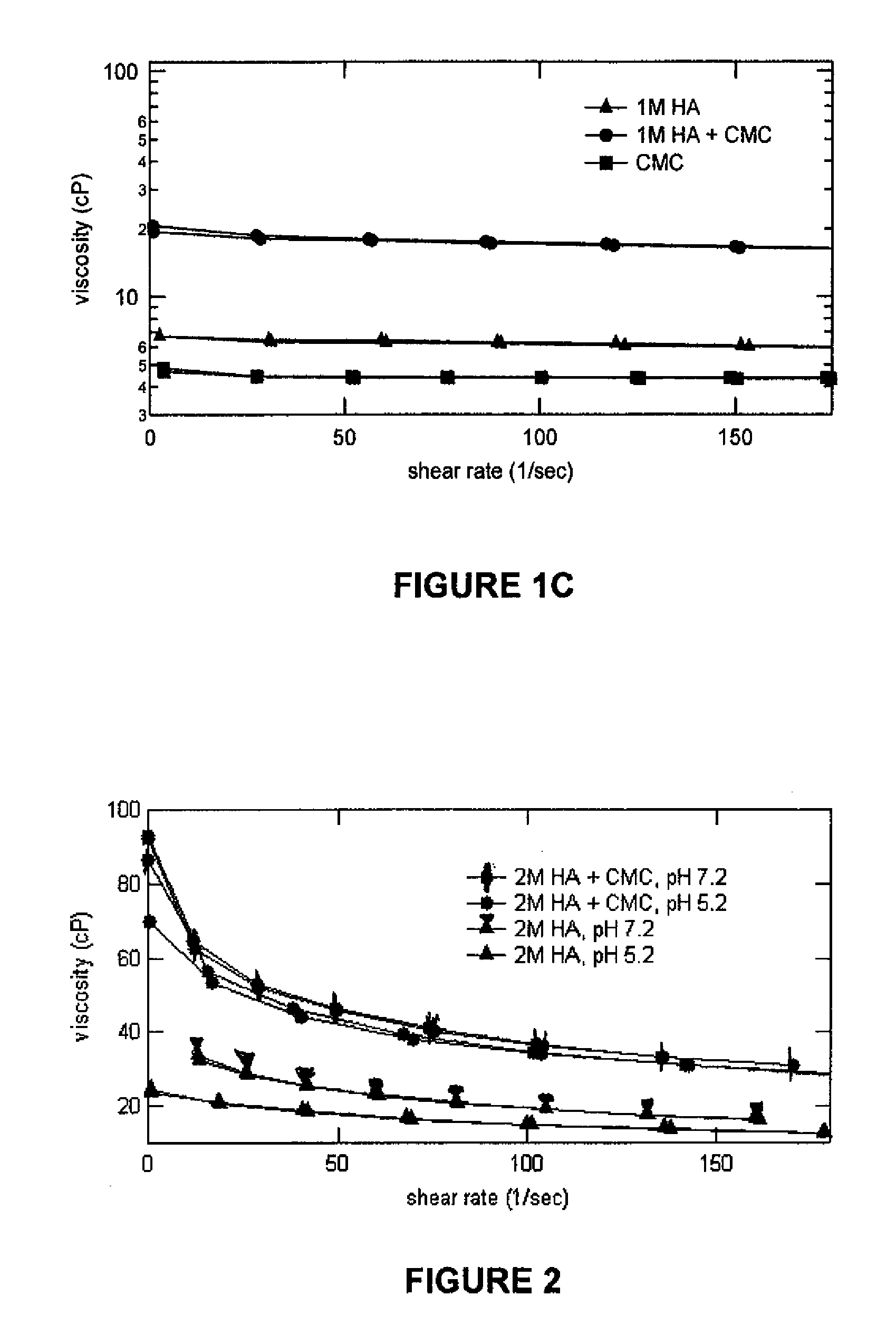
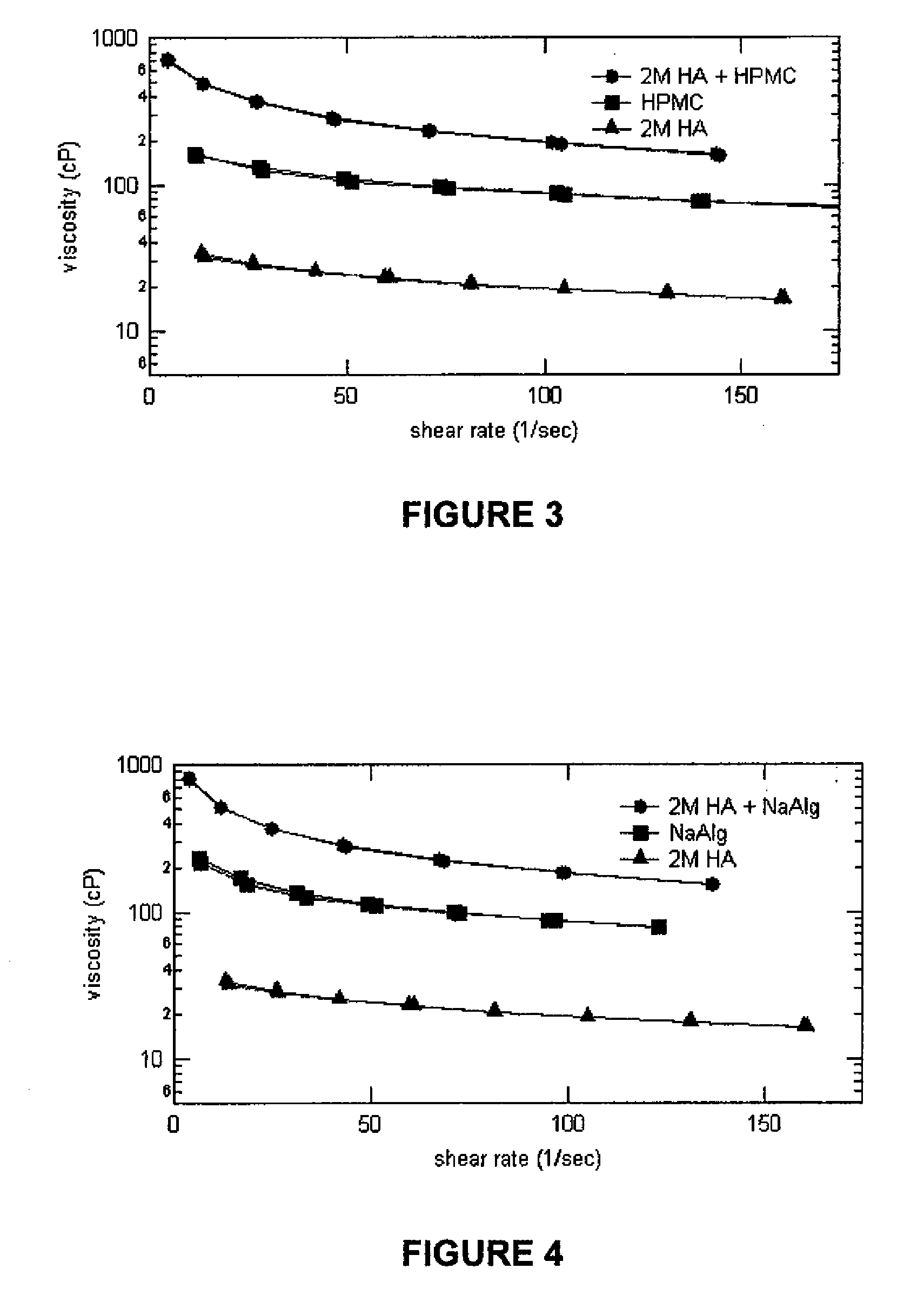
Compositions comprising a glycosaminoglycan (e.g., a hyaluronan, hyaluronic acid, hyaluronate, sodium hyaluronate, dermatan sulfate, karatan sulfate, chondroitin 6-sulfate, heparin, etc.) in combination with at least one component selected from; i) polyglycols (e.g., polyethylene glycol), ii) long chain hydroxy polyanionic polysaccharides (e.g., dextran, sodium alginate, alginic acid, propylene glycol alginate, carboxymethyl cellulose and carboxyethyl cellulose, hydroxyl ethyl starch, hydroxyl propyl methyl cellulose, hydroxy propyl ethyl cellulose, hydroxy propyl cellulose, methyl cellulose, polylysine, polyhistidine, polyhydroxy proline, poly ornithine, polyvinyl pyrolidone, polyvinyl alcohol, chitosan, etc.) and iii) long chain Nitrogen containing polymers (e.g., Polylysine, Polyvinylpyrrolidone, and polyvinyl alcohol). The invention also includes methods for using such compositions (e.g., as substance delivery materials, tissue fillers or bulking agents, as moistening or hydrating agents, etc.)
Owner:S K PHARMA INC
Compositions Comprising Ornithine And Phenylacetate Or Phenylbutyrate For Treating Hepatic Encephalopathy
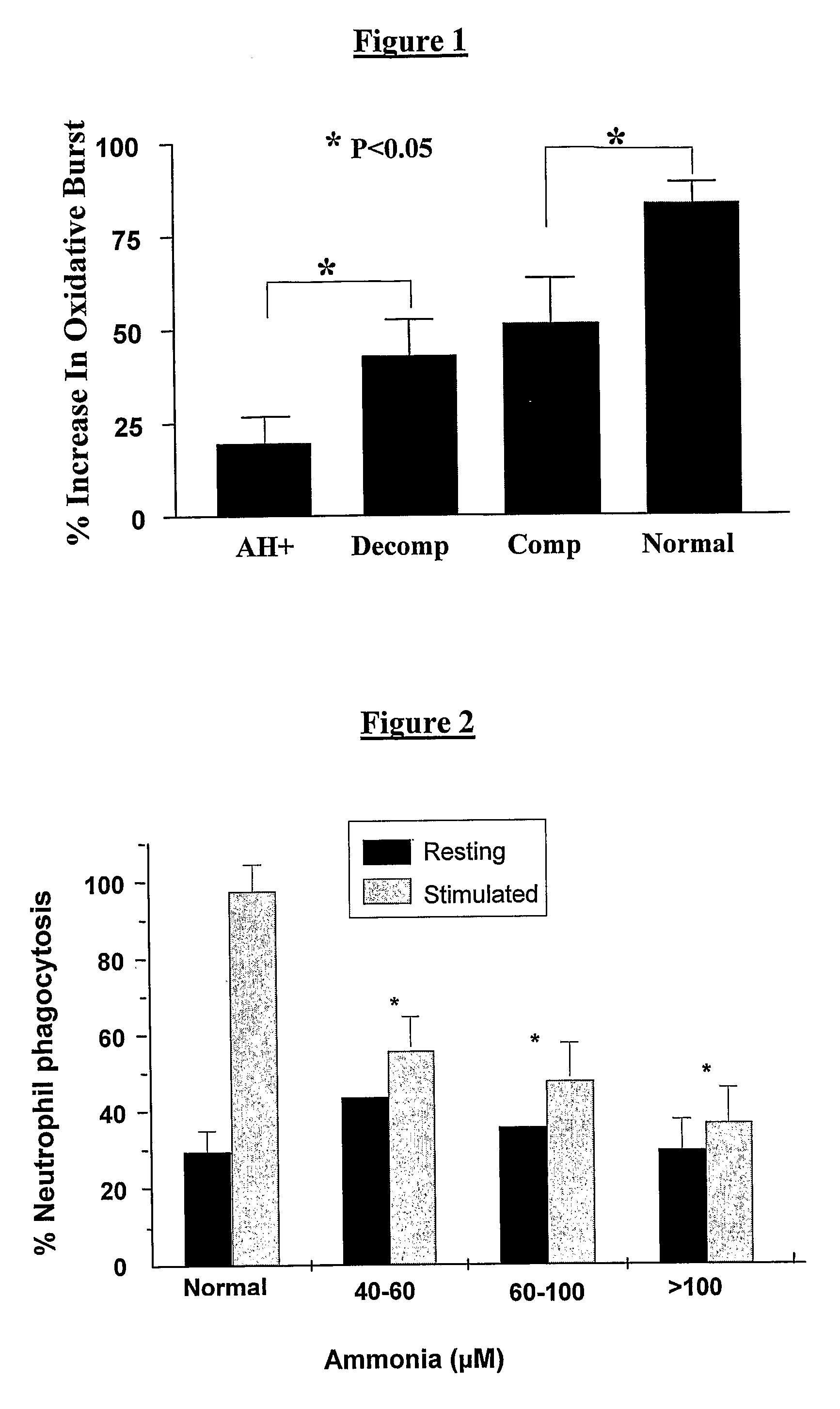
ActiveUS20080119554A1Avoid treatmentAntibacterial agentsBiocideOrnithine aspartateLiver decompensation
The present invention relates to use of ornithine in the manufacture of a medicament for use in combination with at least one of phenylacetate and phenylbutyrate for preventing or treating liver decompensation or hepatic encephalopathy. The invention also relates to use of at least one of phenylacetate and phenylbutyrate in the manufacture of a medicament for use in combination with ornithine for preventing or treating liver decompensation or hepatic encephalopathy.
Owner:UCL BUSINESS PLC
Compositions and methods for glycogen synthesis
InactiveUS20050176827A1Function increaseIncrease insulin sensitivityBiocideOrganic active ingredientsCysteine thiolateTryptophan
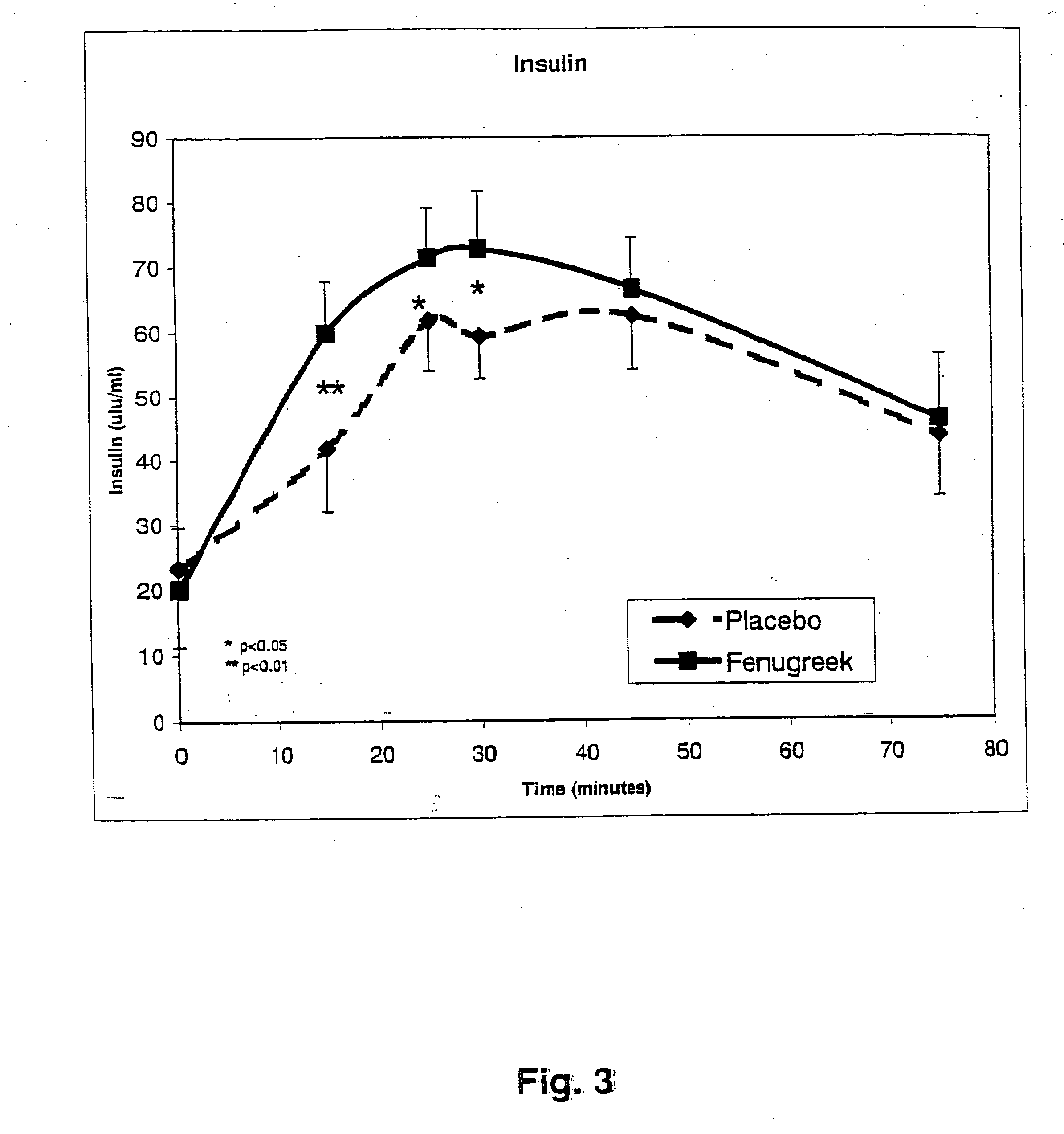
A composition of bio-active compounds and methods for facilitating and supporting the metabolism and transport of glucose and carbohydrates into muscle cells, promoting muscle function and growth, promoting glycogen synthesis, enhancing glucose disposal, stimulating pancreatic beta cells, promoting metabolic recovery, promoting muscle recovery, promoting lean body mass, and promoting fat burning. Preferably, the composition of bio-active compounds includes a combination of 4-hydroxyisoleucine with at least one amino acid selected from the group consisting of arginine, aspartate, threonine, serine, glutamate, proline, glycine, alanine, cysteine, valine, methionine, isoleucine, leucine, tryptophan, phenylalanine, ornithine, lysine, histidine, gamma-amino butyrate and tyrosine. In one presently preferred embodiment of the present invention, the combination is derived, isolated, and / or extracted from fenugreek seeds. Methods for using a novel composition of bio-active compounds from fenugreek seed for facilitating and supporting the metabolism and transport of glucose and carbohydrates into muscle cells, promoting muscle function and growth, promoting glycogen synthesis, enhancing glucose disposal, stimulating pancreatic beta cells, promoting metabolic recovery, promoting muscle recovery, promoting lean body mass, and promoting fat burning are also disclosed, wherein methods comprise the steps of: (1) providing an effective amount of a composition of bio-active compounds derived, isolated, and / or extracted from fenugreek seeds; and (2) administering the composition to a human or animal.
Owner:TSI INC
Method of synthesizing diketopiperazines
InactiveUS6967202B2Prevent unwanted side reactionInhibit side effectsOrganic active ingredientsDipeptide ingredientsSide chainTyrosine
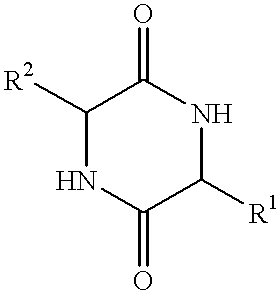
The invention provides a method of synthesizing a diketopiperazine of the formula: wherein:R1 is —CH2COR3, or —CH2CH2COR3;R2 is the side chain of an amino acid selected from the group consisting of glycine, alanine, valine, leucine, isoleucine, serine, threonine, aspartic acid, asparagine, glutamic acid, glutamine, lysine, hydroxylysine, histidine, arginine, phenylalanine, tyrosine, tryptophan, thyroxine, cysteine, methionine, norvaline and ornithine;R3 is —OH, —NH2, —OR4, —NHR4, or —NR4R4; andeach R4 is independently an alkyl, aryl, alkylaryl, or arylalkyl.
Owner:AMPIO PHARMA
Orally administered small peptides synergize statin activity
InactiveUS7148197B2Readily taken up and deliveredMany symptomOrganic active ingredientsPeptide/protein ingredientsThreonineTyrosine
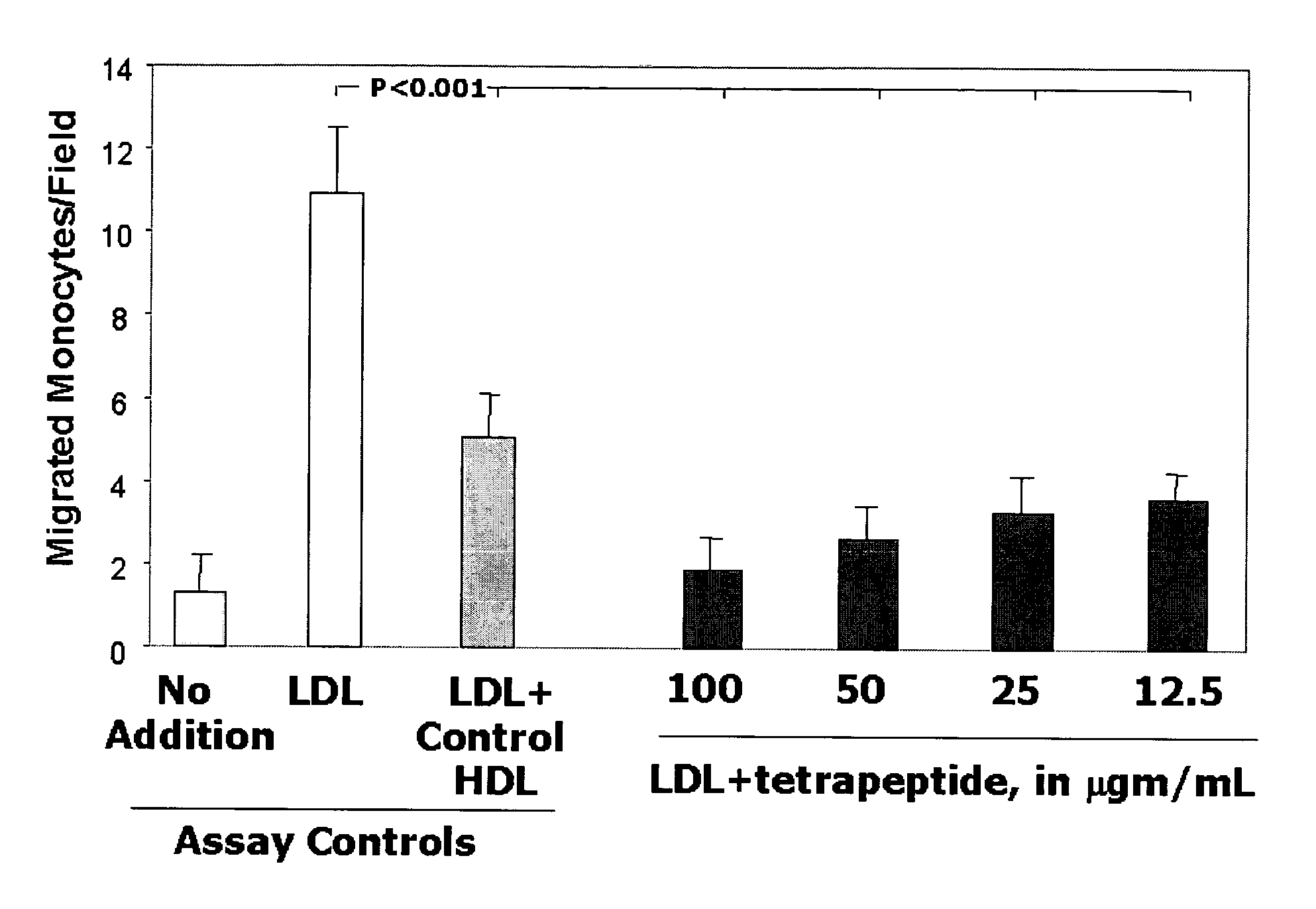
This invention provides novel peptides for the treatment of atherosclerosis. In certain embodiments the peptide is X1-X2-X3-X4 where X1 and X4 are independently selected from the group consisting of alanine (Ala), valine (Val), leucine (Leu), isoleucine (Ile), proline (Pro), phenylalanine (Phe), tryptophan (Trp), methionine (Met), serine (Ser) bearing a hydrophobic protecting group, beta-naphthyl alanine, alpha-naphthyl alanine, norleucine, cyclohexylalanine, threonine (Thr) bearing a hydrophobic protecting group, tyrosine (Tyr) bearing a hydrophobic protecting group, lysine (Lys) bearing a hydrophobic protecting group, arginine (Arg) bearing a hydrophobic protecting group, ornithine (Orn) bearing a hydrophobic protecting group, aspartic acid (Asp) bearing a hydrophobic protecting group, cysteine (Cys) bearing a hydrophobic protecting group, and glutamic acid (Glu) bearing a hydrophobic protecting group; X2 and X3 are independently selected from the group consisting of Asp, Arg, and Glu; and the peptide converts pro-inflammatory HDL to anti-inflammatory HDL or makes anti-inflammatory HDL more anti-inflammatory.
Owner:RGT UNIV OF CALIFORNIA +1
Methods for enhancing the transport of glucose into muscle
InactiveUS20050226948A1Effective quantityLow production costBiocideUnknown materialsCysteine thiolateTryptophan
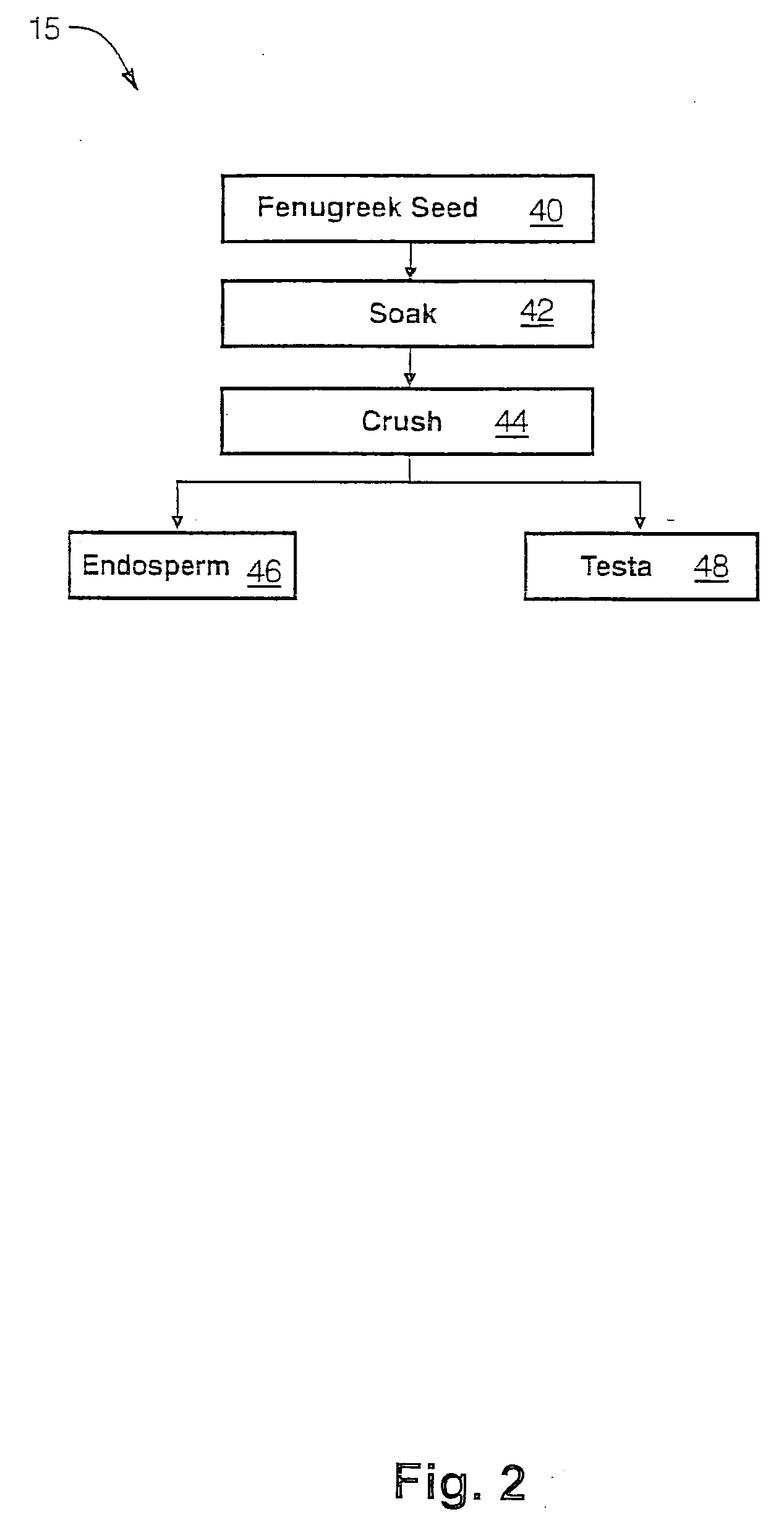
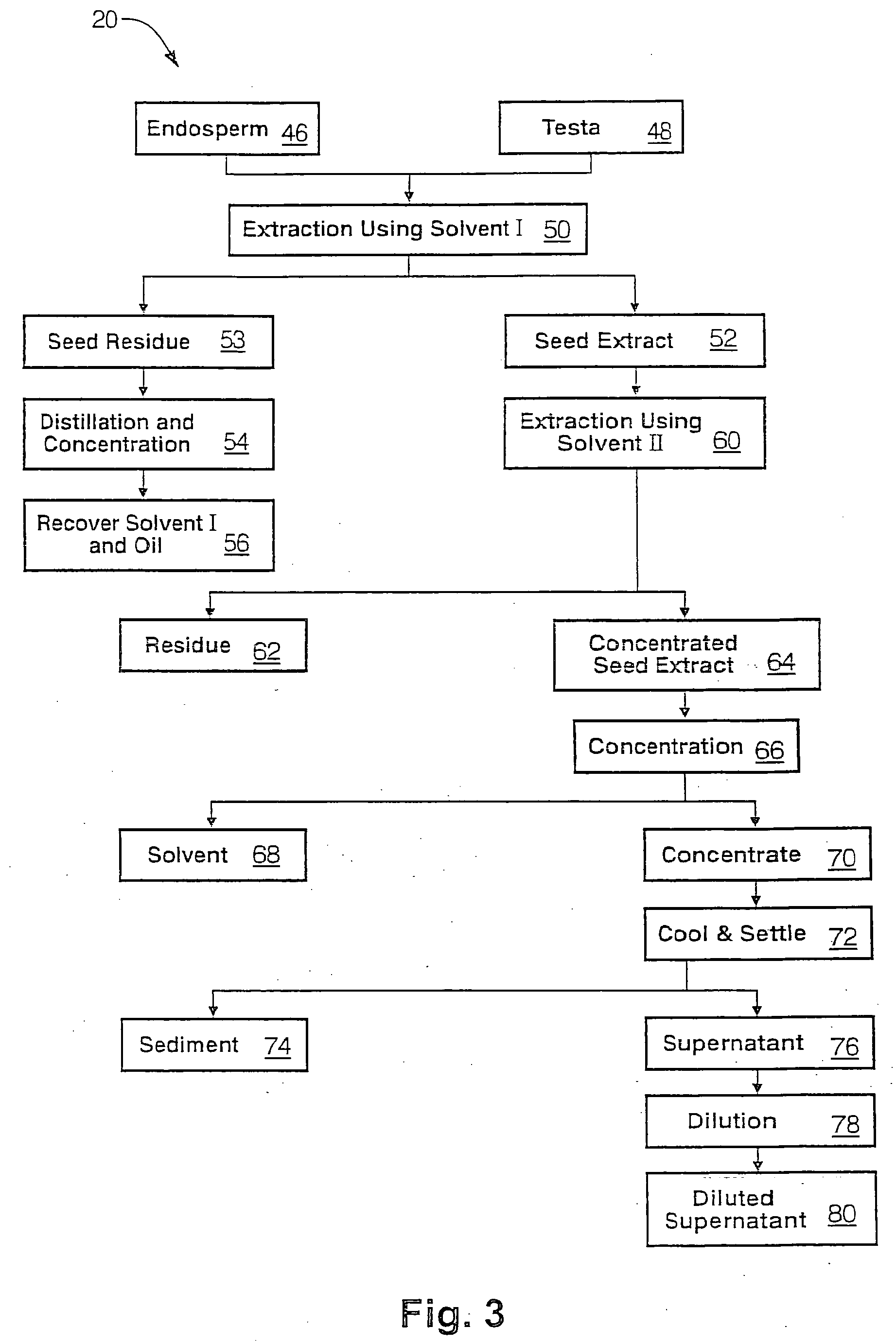
The present invention is directed to novel compositions of bio-active compounds comprising 4-hydroxyisoleucine and one or more compounds selected from the group of amino acids, alkaloids, glycosides, volatile oils, saponins, sapogenins, mannans, flavonoids, fatty acids, vitamins and provitamins, minerals, and carbohydrates. Preferably, the novel compositions of bio-active compounds include 4-hydroxyisoleucine and one or more amino acids selected from the group consisting of arginine, aspartate, threonine, serine, glutamate, proline, glycine, alanine, cysteine, valine, methionine, isoleucine, leucine, tryptophan, phenylalanine, ornithine, proline, lysine, histidine, and gamma-aminobutyrate. The composition of bio-active compounds preferably include between about ten percent and about seventy percent of 4-hydroxyisoleucine and between about twenty percent and about forty percent of other amino acids. The bio-active compounds of the novel composition of the present invention may be derived, isolated, and / or extracted from Fenugreek seeds. A preferred method for extracting the bio-active compounds from Fenugreek seeds includes the steps of: (1) providing a plurality of Fenugreek seeds; (2) preparing the Fenugreek seeds; and (3) extracting a novel composition of bio-active compounds from the Fenugreek seeds, which include a preliminary extraction step and a secondary extraction step. The compositions of bio-active compounds have been found to be helpful in restoring healthy energy balance in humans and animals, aiding in weight management efforts, and for balancing blood sugar levels by way of assisting the body to make more efficient use of existing (i.e., endogenous) insulin.
Owner:TSI INC
Environments that maintain function of primary liver cells
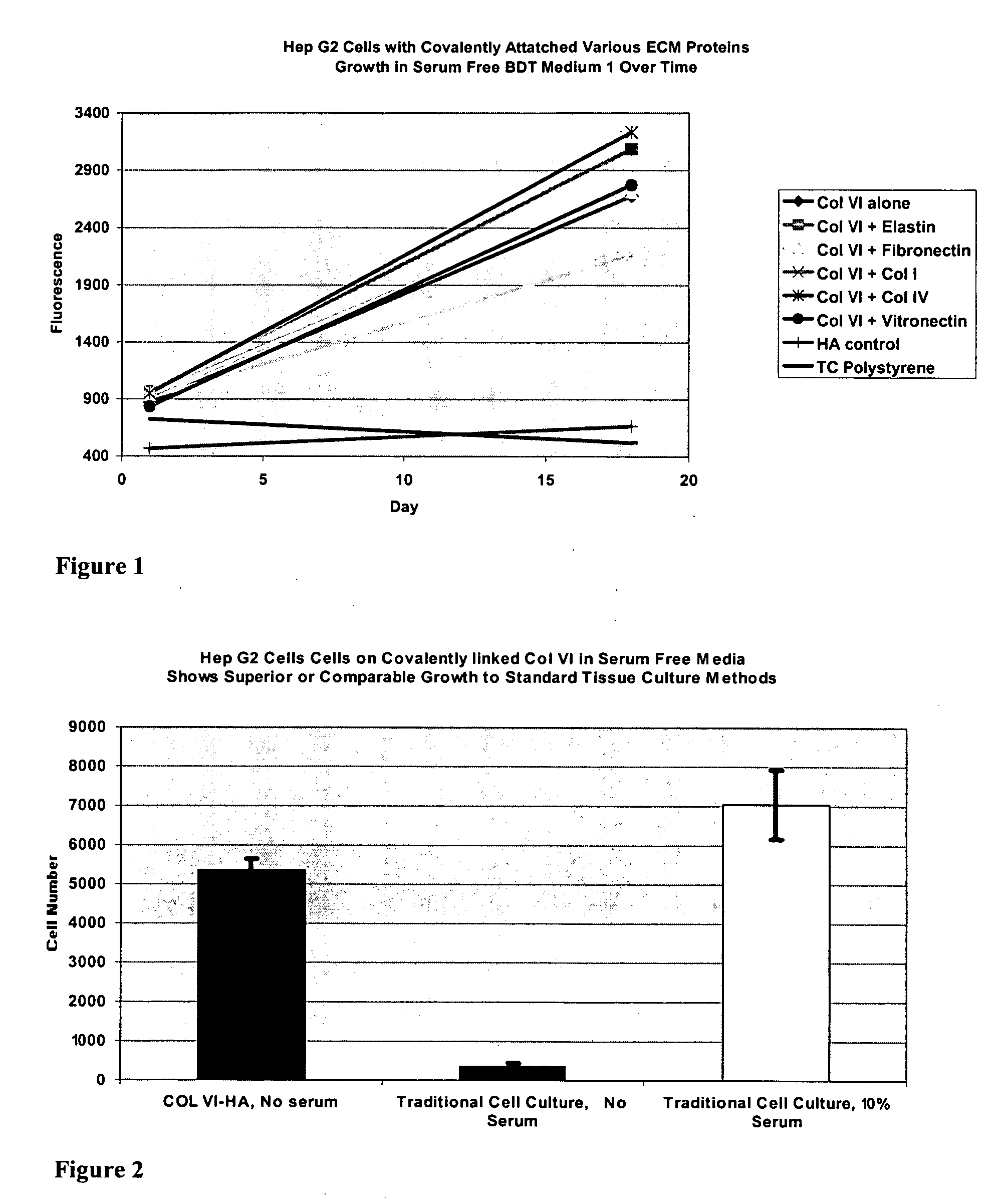
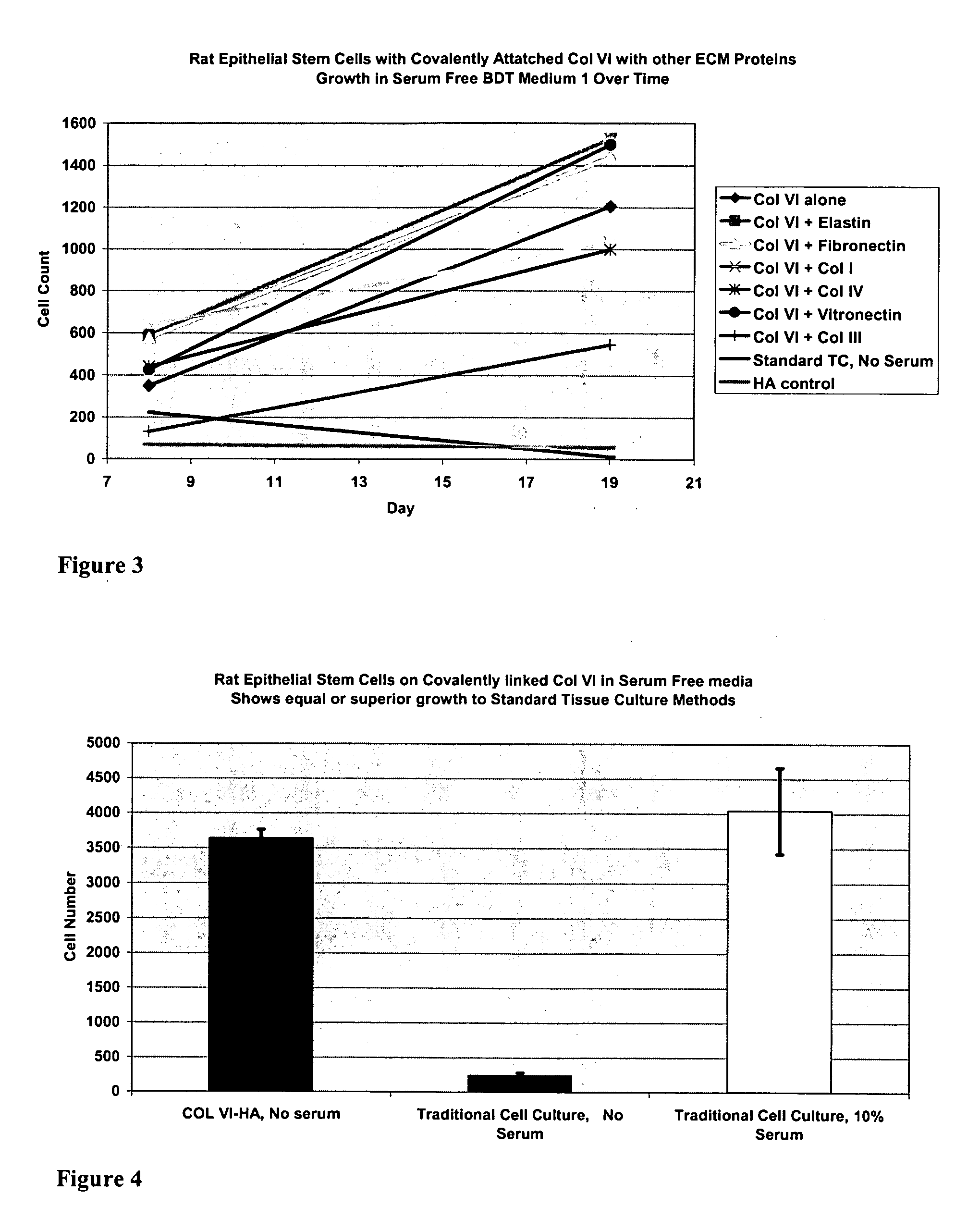
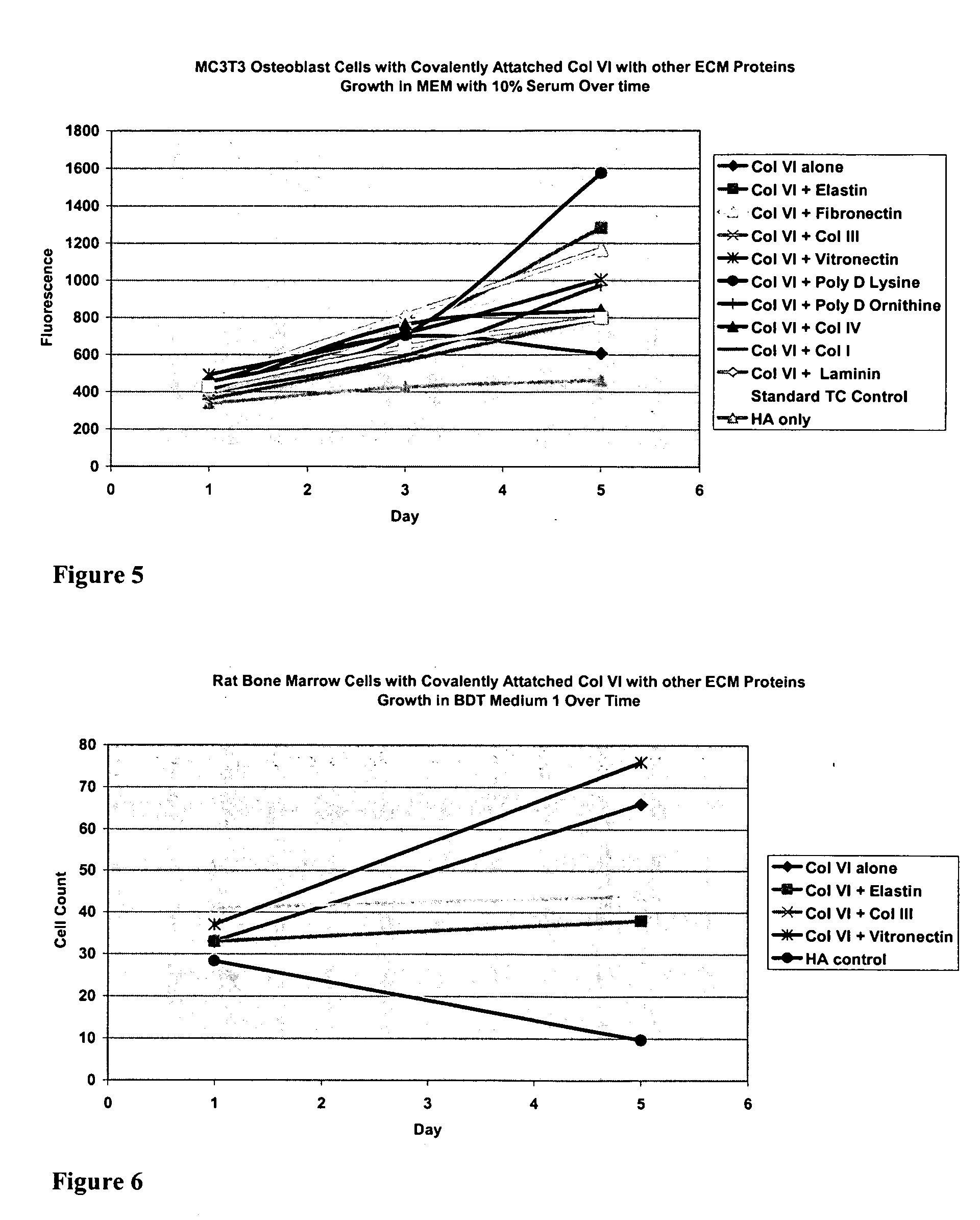
InactiveUS20050059150A1Good adhesionEasy maintenanceHepatocytesArtificial cell constructsECM ProteinL-Ornithine
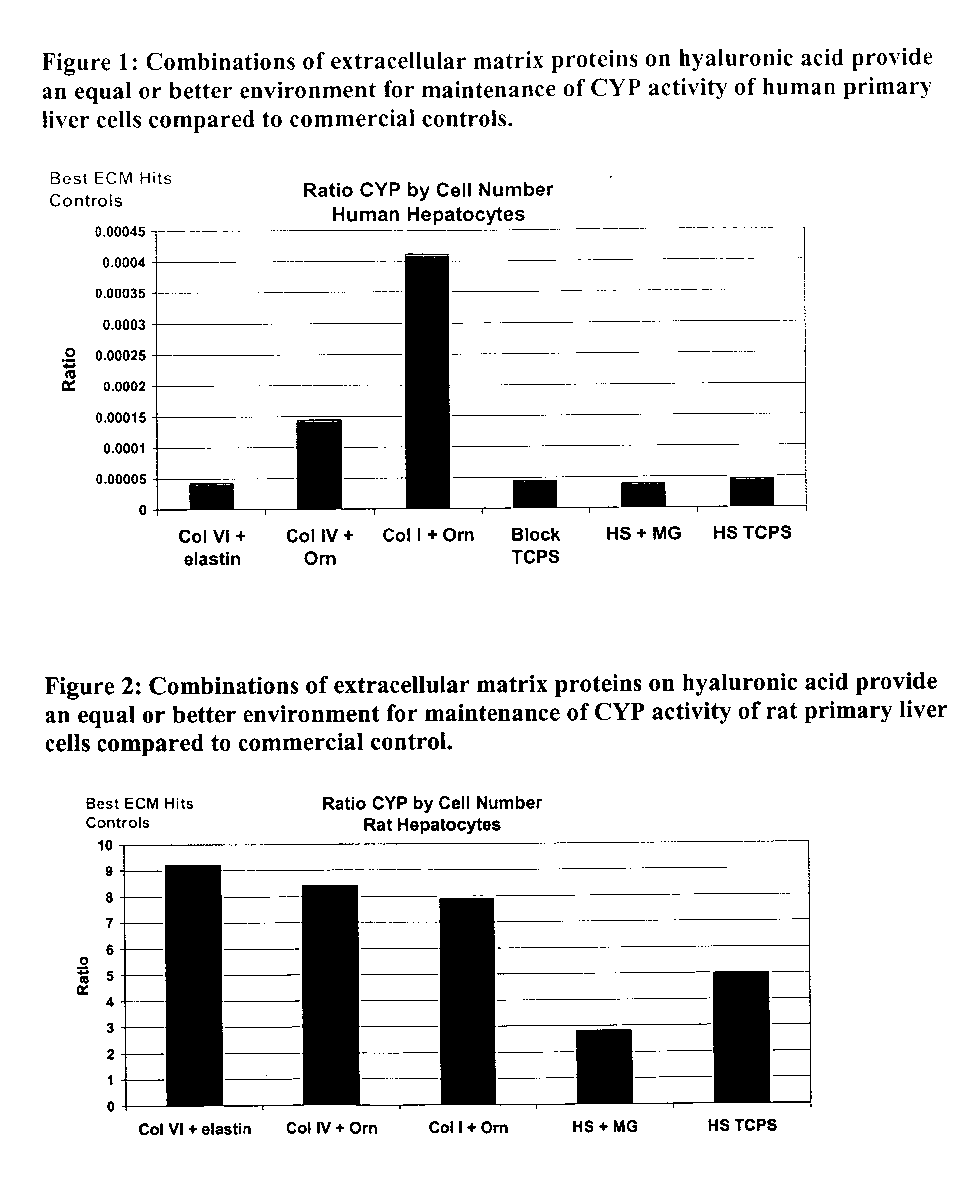
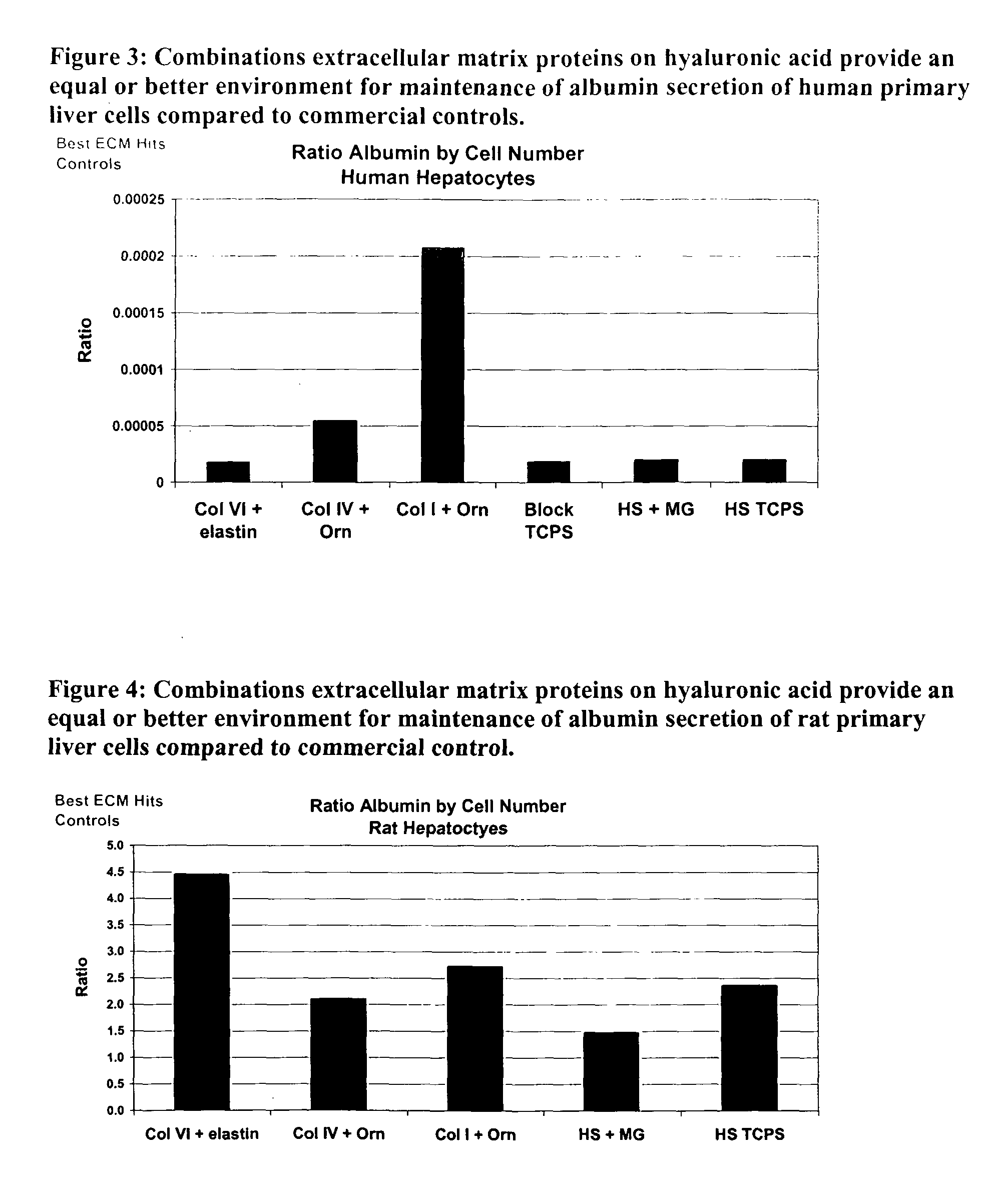
Surfaces useful for cell culture comprise a support to which is bound a CAR material, and, bound to the CAR material, an ECM protein, or a biologically active fragment or variant thereof such as elastin, fibronectin, vitronectin, laminin, collagen I, collagen III, collagen IV, and collagen VI. Also, optionally present on the surface is an active factor, preferably a polycationic polymer or a biologically active fragment or variant thereof, such as polyethyleneimine (PEI), poly-D-lysine (PDL), poly-L-lysine (PLL), poly-D-ornithine (PDO) or poly-L-ornithine (PLO). This surface is used in cell culture to promote cell attachment, survival, and / or proliferation of primary liver cells. The invention also relates to methods utilizing this surface, such as methods for attachment, survival, and / or proliferation of cells. Further disclosed is the use of the surface in cell culture with serum-free medium. Methods of screening using the surface of the invention are also disclosed.
Owner:BECTON DICKINSON & CO
Compound prepn of ornithine and asparagic acid for preventing and treating hepatosis and hepatic encephalopathy and its prepn process
InactiveCN1383815ASimple production methodImprove product qualityPeptide/protein ingredientsDigestive systemOrnithine aspartateChronic liver disease
A compound preparation consists of ornithine or its salt and asparagic acid or its salt as well as proper amount of supplementary material; and is prepared into oral preparation or injection through mechanical mixing and other process. The said compound preparation is used for treating various acute hepatitis, chronic hepatosis and hepatic encephacopathy and has the obvious effects of protecting liver and strengthening physique. The preparation process is simple, short in production period and low in cost.
Owner:刘万忠
Polypeptides having anti-HIV activity and compositions comprising same
InactiveUS7138488B2Improve stabilityAnthropod material medical ingredientsPeptide/protein ingredientsCrystallographyCitrulline
Polypeptides of A1-Arg-A2-Cys-Tyr-A3-A4-X-A5-A6-Cit Cys-A7 (I) or their salts (wherein A1 is hydrogen or a residue of arginine, lysine, ornithine, citrulline, alanine, or the like; A2 is an aromatic amino acid residue; A3, A4 and A6 are each a residue of arginine, lysine, ornithine, citrulline, or alanine; A5 is a residue of tyrosine, phenylalanine, alanine, naphthylalanine, or citrulline; A7 is a lysine or arginine residue whose carboxyl group may be converted into amido; and X is a residue of D-ornithyl-proline, prolyl-D-ornithine, D-lysylproline, or the like, with the proviso that any one of A1, A3, A4, A5, A6 and A7 is a residue of alanine or the like or that X is citrulline or the like).
Owner:NOBUTAKA FUJII PH D +1
Methods for affecting homeostasis and metabolism in a mammalian body
InactiveUS20050233014A1Effective quantityLow production costOrganic active ingredientsBiocideCysteine thiolateTryptophan
The present invention is directed to novel compositions of bio-active compounds comprising 4-hydroxyisoleucine and one or more compounds selected from the group of amino acids, alkaloids, glycosides, volatile oils, saponins, sapogenins, mannans, flavonoids, fatty acids, vitamins and provitamins, minerals, and carbohydrates. Preferably, the novel compositions of bio-active compounds include 4-hydroxyisoleucine and one or more amino acids selected from the group consisting of arginine, aspartate, threonine, serine, glutamate, proline, glycine, alanine, cysteine, valine, methionine, isoleucine, leucine, tryptophan, phenylalanine, ornithine, proline, lysine, histidine, and gamma-aminobutyrate. The composition of bio-active compounds preferably include between about ten percent and about seventy percent of 4-hydroxyisoleucine and between about twenty percent and about forty percent of other amino acids. The bio-active compounds of the novel composition of the present invention may be derived, isolated, and / or extracted from Fenugreek seeds. A preferred method for extracting the bio-active compounds from Fenugreek seeds includes the steps of: (1) providing a plurality of Fenugreek seeds; (2) preparing the Fenugreek seeds; and (3) extracting a novel composition of bio-active compounds from the Fenugreek seeds, which include a preliminary extraction step and a secondary extraction step. The compositions of bio-active compounds have been found to be helpful in restoring healthy energy balance in humans and animals, aiding in weight management efforts, and for balancing blood sugar levels by way of assisting the body to make more efficient use of existing (i.e., endogenous) insulin.
Owner:TSI INC
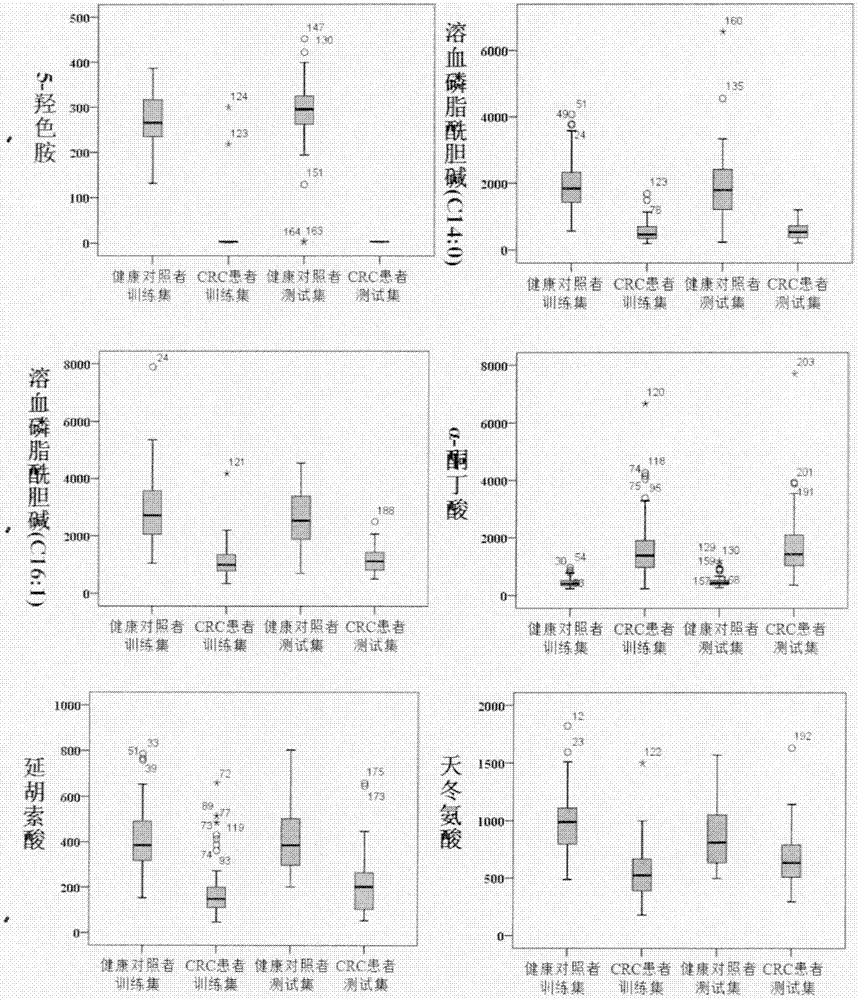
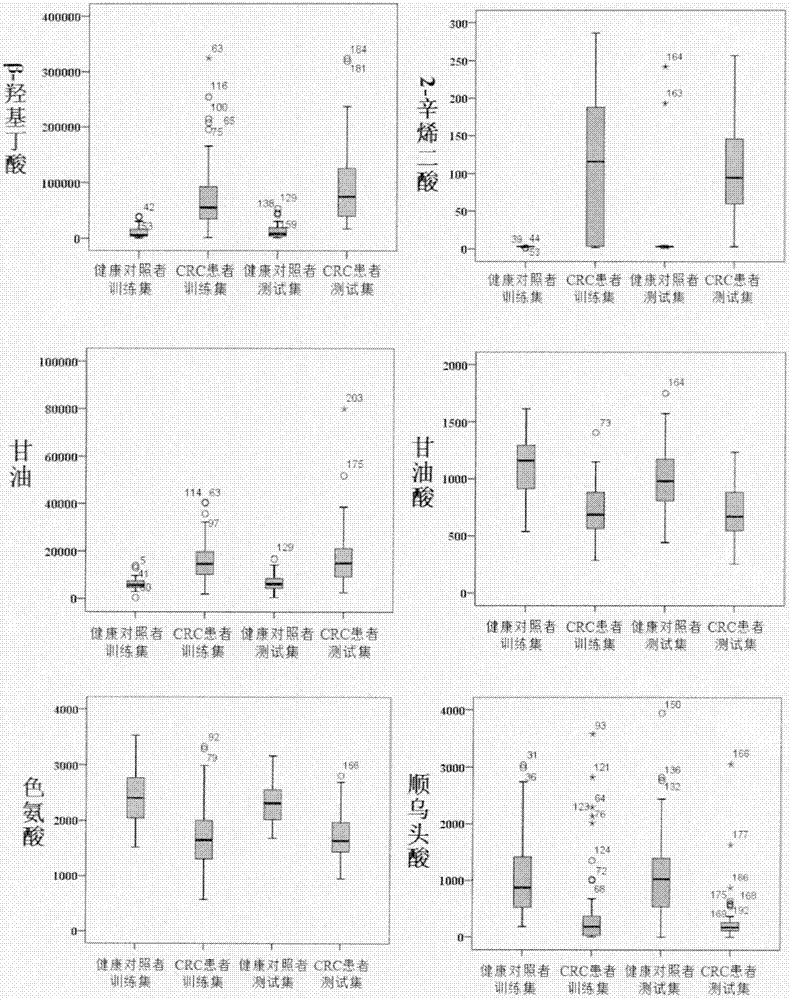
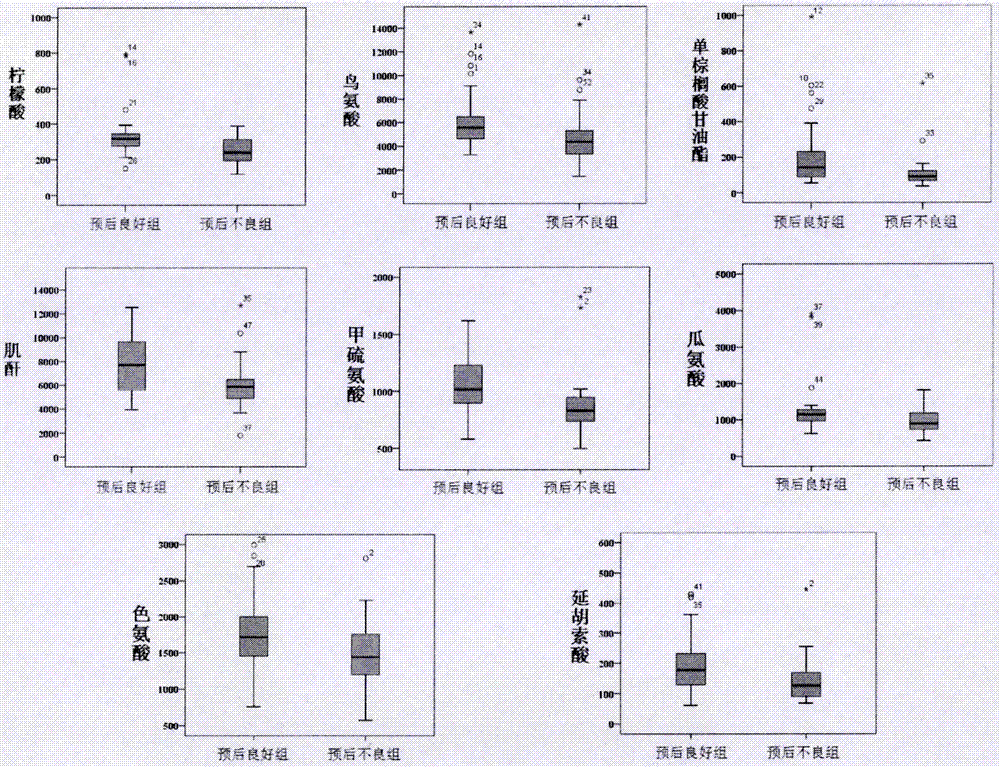
Molecular marker with auxiliary functions of early diagnosis and prognosis monitoring on colorectal cancer, and application thereof
Owner:SHENZHEN UNIV
Novel polypeptide anti-HIV agent containing the same
The present invention provides novel polypeptides of A1-Arg-A2-Cys-Tyr-A3-A4-X-A5-A6-Cit Cys-A7 (I) or their salts (wherein A1 is hydrogen or a residue of arginine, lysine, ornithine, citrulline, alanine, or the like; A2 is an aromatic amino acid residue; A3, A4 and A6 are each a residue of arginine, lysine, ornithine, citrulline, or alanine; A5 is a residue of tyrosine, phenylalanine, alanine, naphthylalanine, or citrulline; A7 is a lysine or arginine residue whose carboxyl group may be converted into amido; and X is a residue of D-ornithyl-proline, prolyl-D-ornithine, D-lysylproline, or the like, with the proviso that any one of A1, A3, A4, A5, A6 and A7 is a residue of alanine or the like or that X is citrulline or the like), and methods of using same in the treatment of HIV.
Owner:BIOKINE THERAPEUTICS LTD
Polypeptides having anti-HIV activity and compositions comprising same
InactiveUS20060264605A1Peptide/protein ingredientsViral antigen ingredientsCrystallographyHydrogen atom
The present invention relates to a polypeptide represented by the formula: A1-Arg-A2-Cys-Tyr-A3-A4-X-A5-A6-Cit-Cys-A7 (I) (wherein A1 represents a hydrogen atom or an arginine, lysine, ornithine, citrulline, alanine residue, etc.; A2 represents an aromatic amino acid residue; A3, A4 and A6 represent an arginine, lysine, ornithine, citrulline or alanine residue, A5 represents a tyrosine, phenylalanine, alanine, naphthylalanine or citrulline residue; A7 represents a lysine or arginine residue in which a carboxyl group may be amidated; X represents a D-ornithyl-proline, prolyl-D-ornithine, D-lysyl-proline residue, etc.; provided that either of A1, A3, A4, A5, A6 and A7 is an alanine residue, etc., or X is citrulline, etc.) or a salt thereof.
Owner:BIOKINE THERAPEUTICS LTD
Use of topical arginine to enhance wound healing
InactiveUS20030091601A1Promote wound healingIncrease in collagen depositionBiocideOrganic active ingredientsEmulsionOrnithine aspartate
The present invention relates to the treatment of wounds by the topical administration of arginine or ornithine to wound sites. There is described herein a wound healing enhancing medicinal composition formulated from the amino acid arginine or a by-product thereof. Topical application of the medicinal composition to the wound site enhances wound healing by increasing collagen biosynthesis. A medical composition of the present invention may be administered alone, on dressings, or in combination with common vehicles for topical application including, for example, oil-based emulsions, gels, creams, ointments, and the like.
Owner:ABAT
Method of using hydroxycarboxylic acids or related compounds for treating skin changes asociated with intrinsic and extrinsic aging
A composition comprising an amphoteric or pseudo-amphoteric agent and a polyhydroxy alpha hydroxyacid existing as a free acid, lactone, or salt, and isomeric or non-isomeric forms thereof is provided. The amphoteric or pseudo-amphoteric agent can be selected from amino acids, dipeptides, aminoaldonic acid, aminouronic acid, lauryl aminoproplyglycine, aminoaldaric acid, neuraminic acid desulfated heparin, deacetylated hyaluronic acid, hyalobiuronic acid, chondrosine, deacetylated chondroitin, creatine, creatinine, hydroxyproline, homocysteine, homocystine, homoserine, ornithine, citrulline, phosphatidylserine, and sphingomyelin. The composition may contain other additives, including cosmetic or pharmaceutical agents for topical treatment of dermatological disorders.
Owner:TRISTRATA TECH
Bifidobacteria viable bacteria preparation and special-purpose protective agent thereof
ActiveCN101016527ASolve the problem of difficult storage at room temperatureSimple methodBacteriaVitamin CActive agent
The invention discloses a preparing method of bifidobacteria bacterial active agent and single-purpose protecting agent, which is characterized by the following: allocating 0.01-5.0 wt glycerin, 0.1-6.0 wt polyvinyl pyrrolidon, 0.1-5.0 carbowax, 1.0-10.0 wt skimmed milk powder, 0.5-15.0 wt mycose, 0.1-5.0 wt glutavene, 0.1-5.0 wt L-aspartic acid, 0.1-5.0 wt vitamin C sodium and 0.1-5.0 wt ornithine hydrochlorate. This invention solves the problem of preservation difficulty at normal temperature with conventional method.
Owner:LIVZON PHARM GRP INC
Soy-bean peptide
ActiveCN1742605AApplicable to the needs of different types of peopleIncrease lean body massVegetable proteins working-upSerum igeLong distance runners
The present invention provides a soybean peptide beverage whose composition includes (by weight portion ratio) cane sugar 36000-14000000, oligo maltose 24000-9600000, soybean oligopeptide 19000-760000, ornithine 400-19000 and creatine 2000-100000. Besides, said invention also provides health-care functions of said soybean peptide beverage.
Owner:BEIJING COMPETITOR SPORTS SCI & TECH
Treatment and prevention of muscle loss using l-ornithine in combination with at least one of phenylacetate and phenylbutyrate
ActiveUS20180221320A1Reducing blood ammoniaImproving muscle metabolismOrganic active ingredientsMicrobiological testing/measurementMuscle lossOrnithine synthesis
Disclosed herein are methods of treating and preventing muscle loss using ornithine in combination with at least one of phenyl acetate and phenylbutyrate.
Owner:OCERA THERAPEUTICS INC
Preparation method of ornithine aspartate
PendingCN104058981ALow costPromote environmental protectionOrganic compound preparationAmino-carboxyl compound preparationOrnithine aspartateIon exchange
The invention provides a preparation method of ornithine aspartate, which comprises the following steps: firstly dissolving ornithine salt in water, adjusting the pH to 8-11 with alkali liquor, obtaining free ornithine by an electrodialysis method, adjusting the pH of the solution to 7-9 with aspartic acid, performing concentration, and adding an alcoholic solution for crystallization. The method of the invention can realize desalting effect, needs no spherical or powdery ion exchange resin materials or column chromatography filling materials, is simple in production process, high in production efficiency, low in cost, more stable in materials during production process, high in product yield, high in purity, less in production waste liquid, and beneficial to environmental protection.
Owner:辽宁科泰生物基因制药股份有限公司
Prophylactic or therapeutic composition for hemoglobinuria or myoglobinuria
A prophylactic or therapeutic composition for hemoglobinuria or myoglobinuria which may occur in humans or animals upon loading of exercise or stress or the like is provided.According to the present invention, the prophylactic or therapeutic composition for hemoglobinuria or myoglobinuria which comprises a branched-chain amino acid such as valine, leucine or isoleucine or a salt thereof, a basic amino acid such as ornithine, arginine, lysine, histidine or citrulline or a salt thereof and glutamine or a salt thereof as active ingredients can be provided.
Owner:KYOWA HAKKO BIO CO LTD
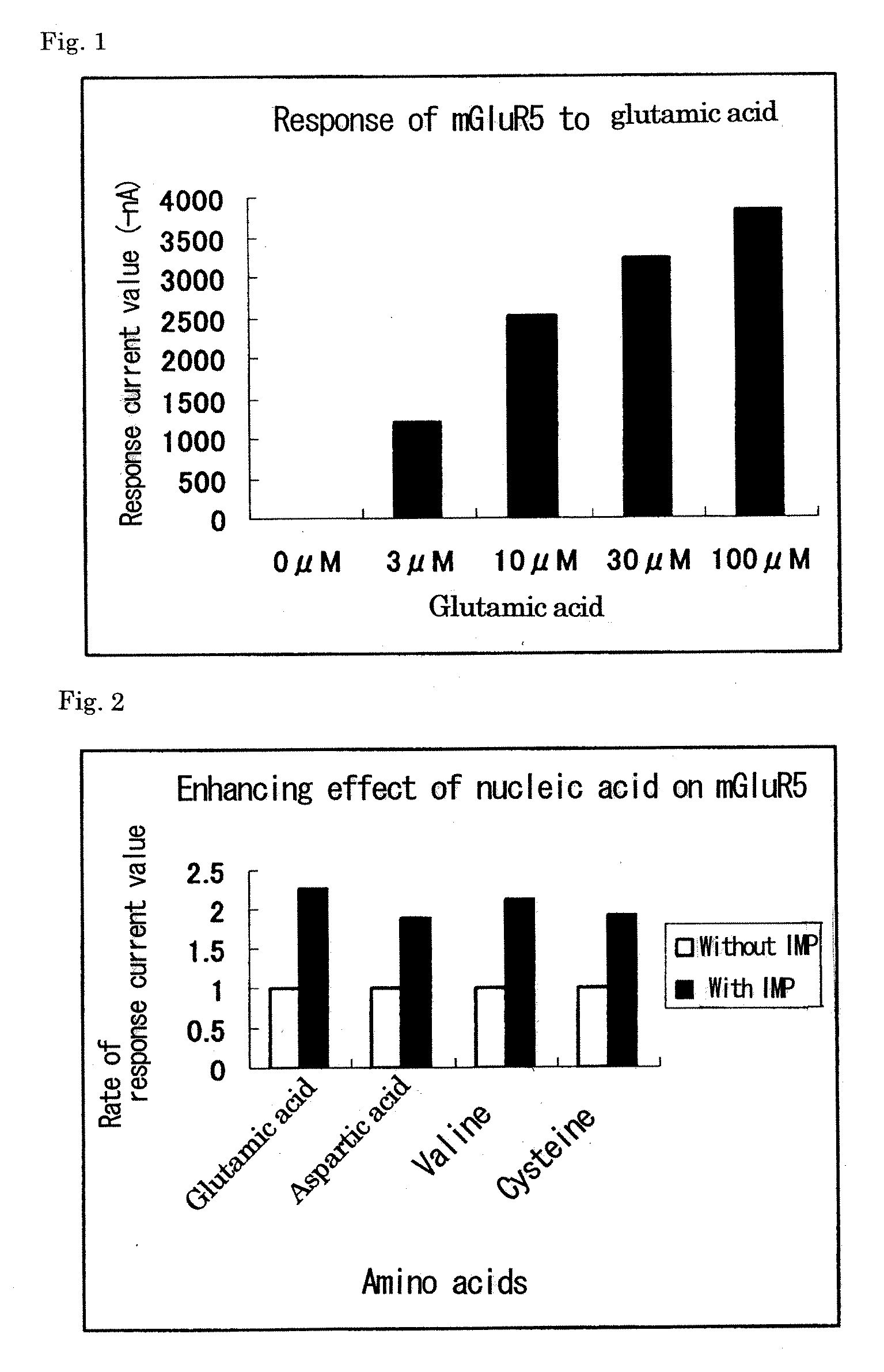
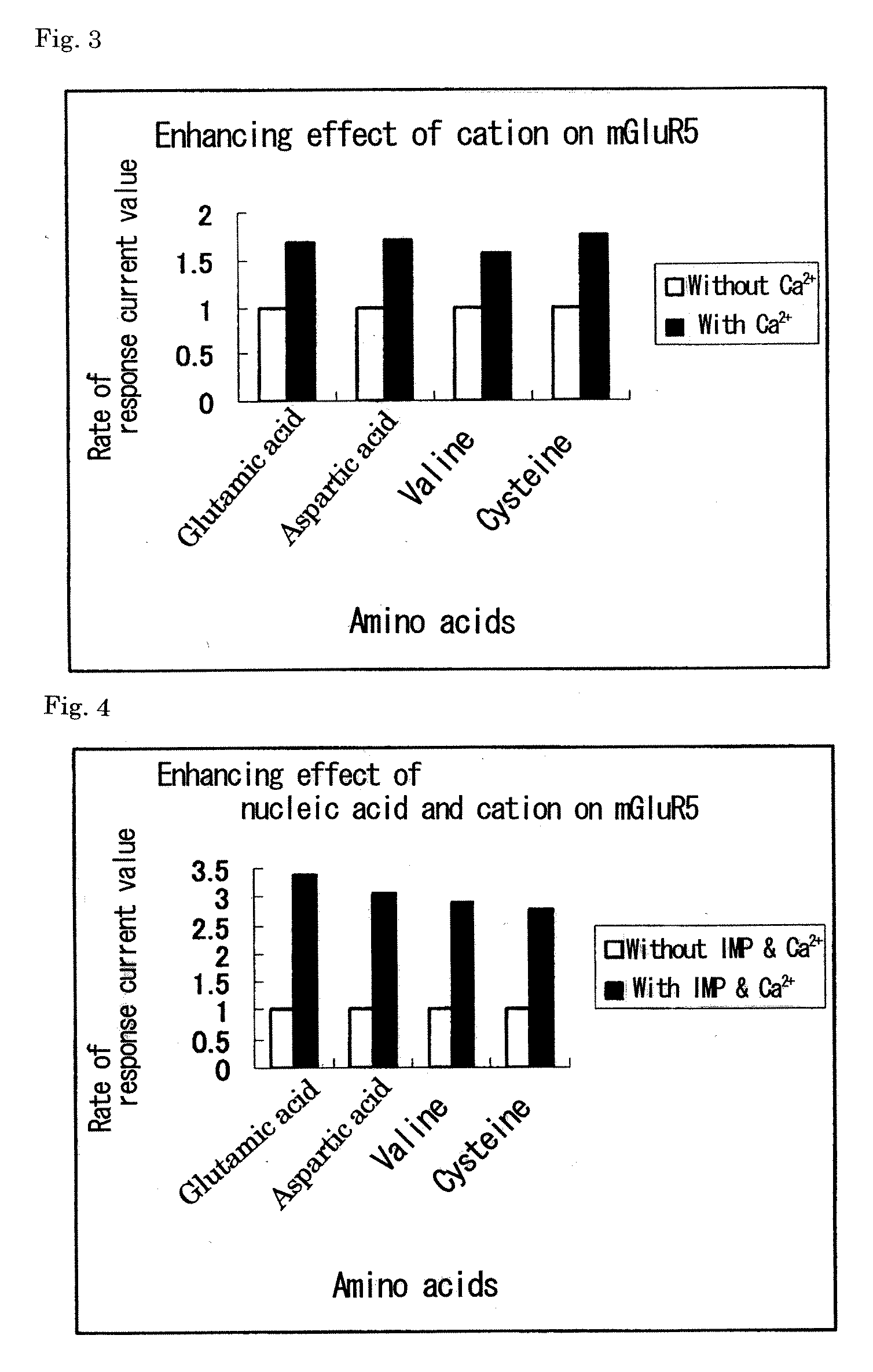
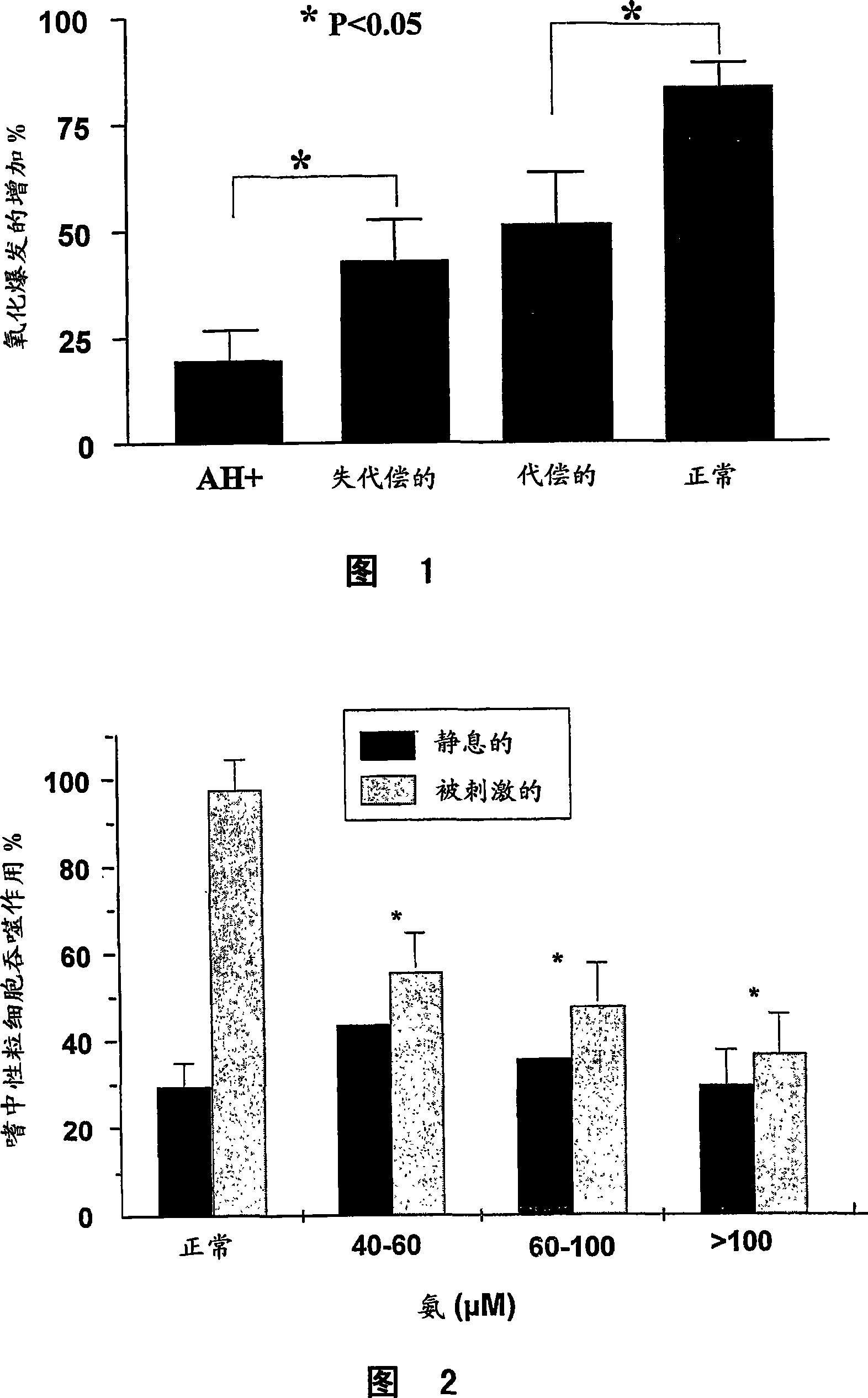
Metabotropic glutamate receptor activator
InactiveUS20080182811A1Raise security concernsHighly safe for a living bodyBiocideSenses disorderTryptophanValine
Amino acids other than glutamic acid are used as a metabotropic glutamate receptor activator. More preferably, aspartic acid, valine and cysteine are used as a group I metabotropic glutamate receptor activator; alanine, arginine, asparagine, aspartic acid, cysteine, glutamine, glycine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, proline, serine, threonine, tryptophan, tyrosine, valine, ornithine, taurine and hydroxyproline are used as a group II metabotropic glutamate receptor activator; and cysteine is used as a group III metabotropic glutamate receptor activator.
Owner:AJINOMOTO CO INC
Compositions comprising ornithine and phenylacetate or phenylbutyrate for treating hepatic encephalopathy
The present invention relates to use of ornithine in the manufacture of a medicament for use in combination with at least one of phenylacetate and phenylbutyrate for preventing or treating liver decompensation or hepatic encephalopathy. The invention also relates to use of at least one of phenylacetate and phenylbutyrate in the manufacture of a medicament for use in combination with ornithine for preventing or treating liver decompensation or hepatic encephalopathy.
Owner:UCL BUSINESS PLC
Specific antigenic mark for rheumatoid arthritis and its use
InactiveCN1712964ASensitive detection meansThe detection method is simpleBiological testingSeronegative rheumatoid arthritisRheumatoid arteritis
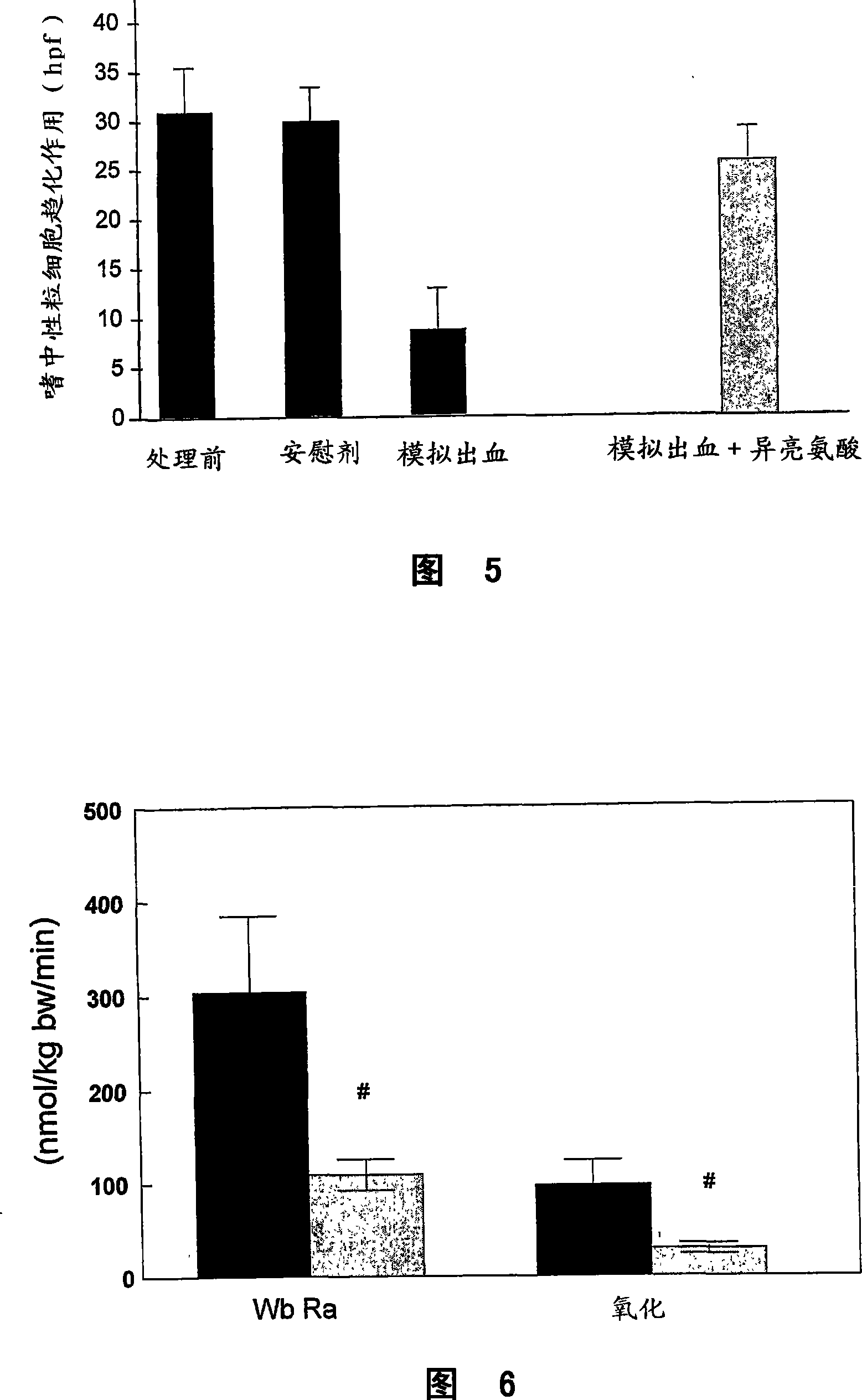
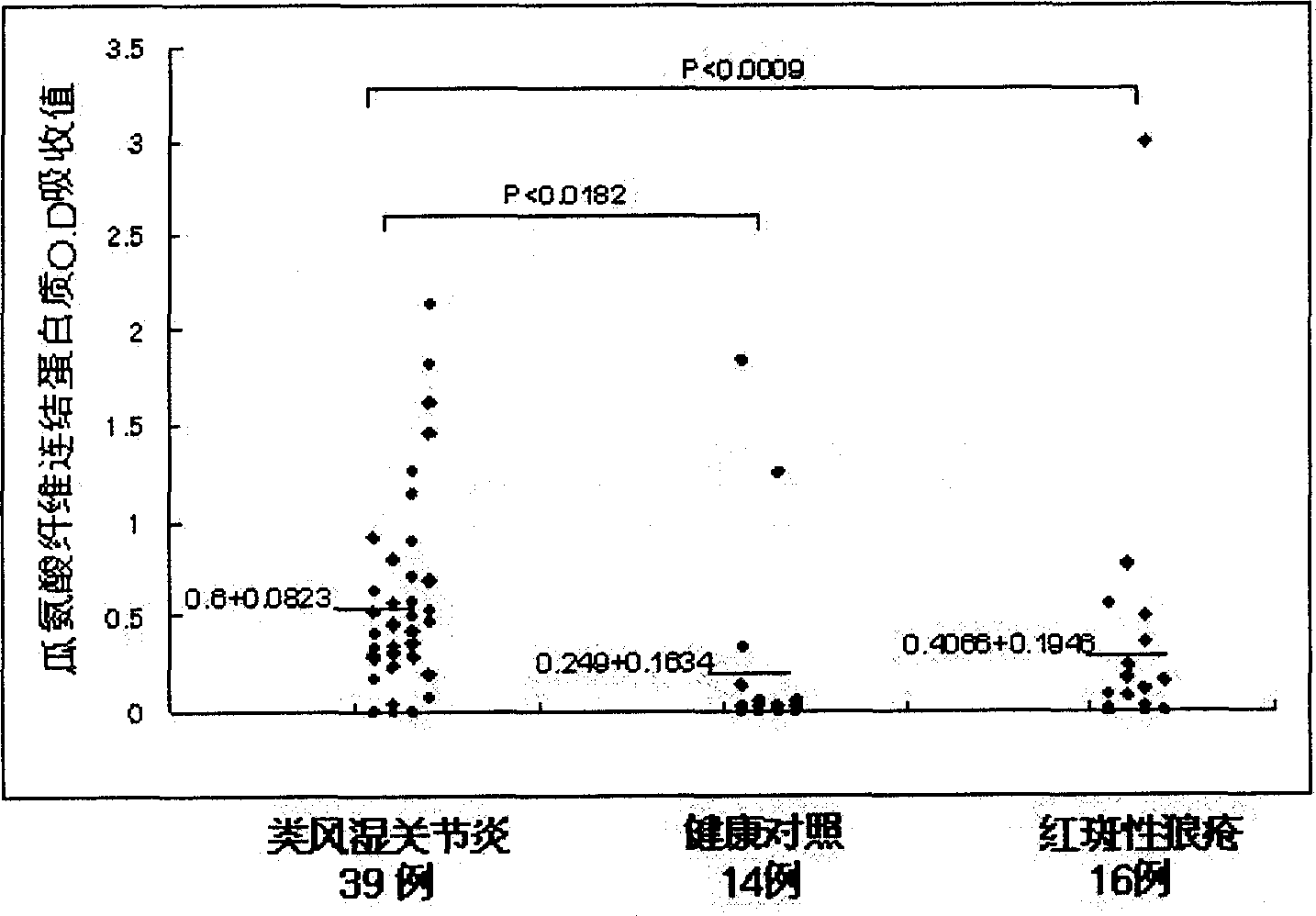
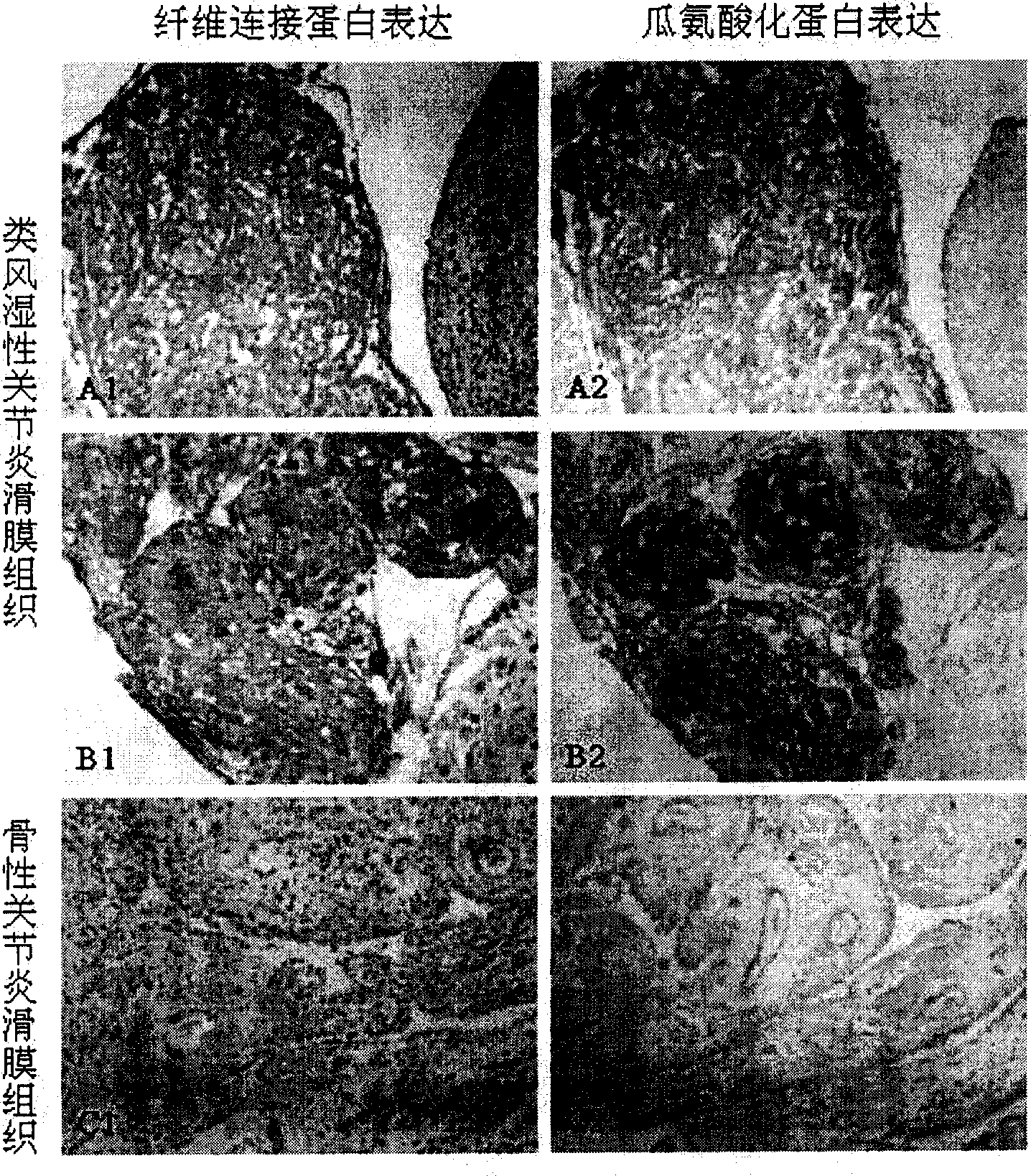
A mark substance of specificity - anti-genicity for atrophic arthritis is carbamyl ornithine fibre connexin existied in blood plasma and joint synovium tissue of atrophic arthritis patient. The invented mark substance has excellent application value in preparing reagent and medicine for diagnosing and curing disease of atrophic arthritis.
Owner:SHANDONG MEDICAL BIO TECH RES CENT
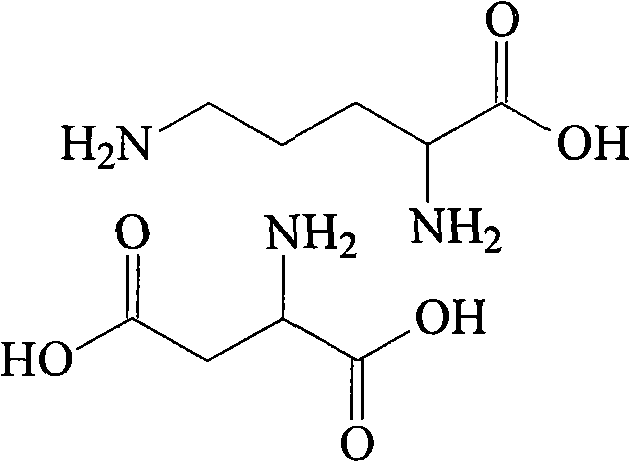
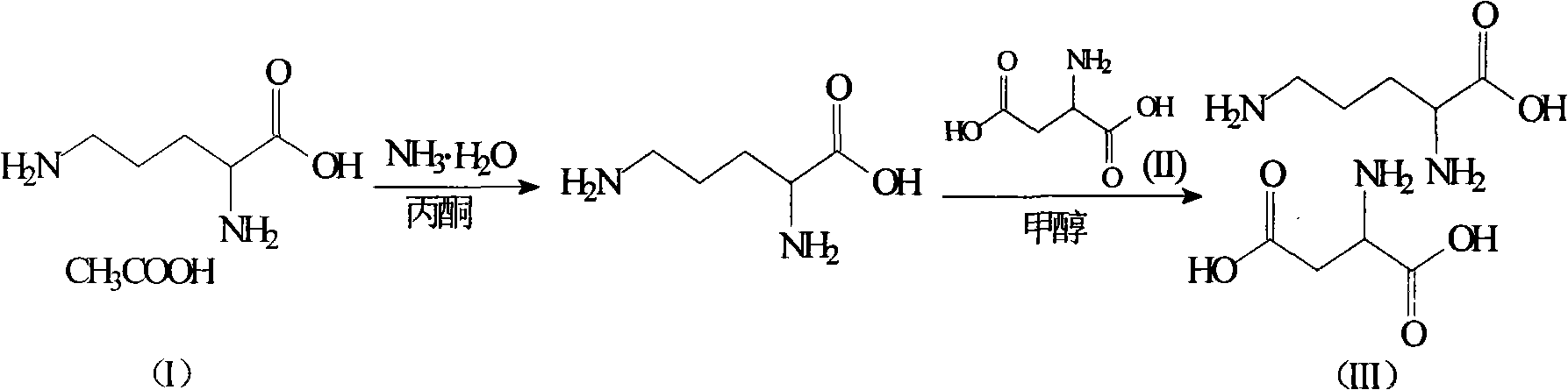
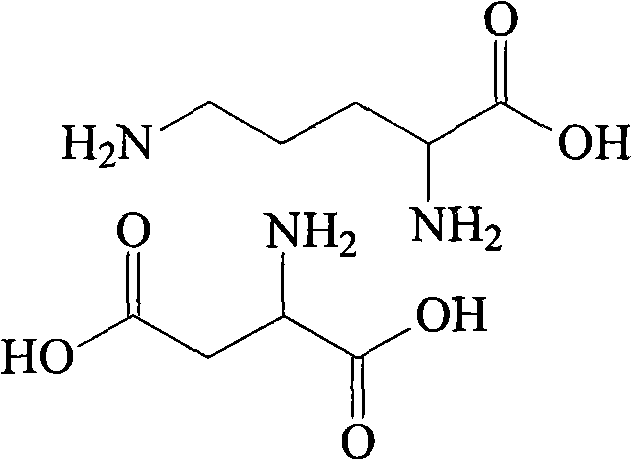
Ornithine and aspartate compound and novel method thereof
InactiveCN101880240ALow costSimple processOrganic compound preparationAmino-carboxyl compound preparationSolubilityOrnithine synthesis
The invention relates to an ornithine aspartate compound and a novel method thereof. L-aspartic acid and L-ornithine acetate are used as starting materials. The method comprises the following steps of: removing ammonium acetate serving as an intermediate product through solubility differences of ornithine and the ammonium acetate in acetone to obtain free alkali of the ornithine; and reacting thefree alkali with the L-aspartic acid to generate the ornithine and aspartate compound. The synthetic method of the ornithine and aspartate compound has the advantages of low cost, simple process, high purity of the obtained product and easy industrial production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Preparation of ornithine aspartate for injection
InactiveCN102475697AAchieve desalinationShorten the timeOrganic active ingredientsPowder deliveryDesalinationOrnithine aspartate
The invention relates to a preparation process of ornithine aspartate for injection. According to the preparation process, a one-step operation of concentration, desalination and purification is carried out on the obtained reacted raw material liquid. The process used in the invention, which has the advantages of simplicity, short production period, and low energy consumption, is very suitable for the industrialized production of ornithine aspartate injections.
Owner:BEIJING KAWIN TECH SHARE HLDG +1
Preparation method of L-ornithine-L-aspartate
InactiveCN101844995ANo pollution in the processLess waste waterOrganic compound preparationAmino-carboxyl compound preparationIon contentL-ornithine L-aspartate
The invention relates to a preparation method of L-ornithine-L-aspartate, which comprises the following steps: mixing L-ornithine hydrochloride and deionized water; adding anion exchange resin and continue stirring to obtain a mixed solution; detecting chloride ion content by utilizing a silver nitrate solution; then adding aspartate; removing the resin and concentrating the filtrate after stirring reaction is ended; adding activated carbon for decoloration and dropwise adding absolute ethyl alcohol; then cooling the decolorized filtrate to the room temperature for separating out crystal; and drying the obtained crystal for 8-30h after the filtering is ended. Compared with the prior art, the preparation method does not generate exhaust gas or waste residues, generates low amount of waste water, basically does not need COD (chemical oxygen demand) and BOD (biochemical oxygen demand), almost does not pollute the environment and has high total product yield which can reach more than 80 percent, does not use poisonous raw materials and has higher product quality.
Owner:SHANGHAI TIANYE CHEM
Covalently attached collagen VI for cell attachment and proliferation
Surfaces useful for cell culture comprise a support to which is bound a CAR material, and, bound to the CAR material, collagen VI or a biologically active fragment or variant thereof and, optionally, one or more other ECM proteins (or fragments or variants thereof) such as elastin, fibronectin, vitronectin, tenascin, laminin, entactin, aggrecan, decorin, collagen I, collagen III, and collagen IV. Also, optionally present on the surface is one or more polycationic polymers, such as poly-D-lysine or poly-D-ornithine. This surface is used in cell culture to promote cell attachment, survival, and / or proliferation of a number of different cell types such as (a) liver cells (e.g., HepG2 tumor cells, and a newly discovered line of rat liver epithelial stem cells) (b) osteoblasts, such as the murine cell line MC3T3 cell line and (c) primary bone marrow cells. Kits comprising the surfaces and additional reagents are also disclosed.
Owner:BECTON DICKINSON & CO
Method for preparing L-ornithine-L-aspartate salt
InactiveCN102102118AHigh purityIncrease productionOn/in organic carrierFermentationOrnithine aspartateL-ornithine L-aspartate
The invention relates to a method for preparing L-ornithine-L-aspartate salt. The L-ornithine-L-aspartate salt is produced through conversion of arginase. The method comprises the following process steps of: (1) preparing immobilized enzyme; (2) optimizing conversion conditions; and (3) extracting product and refining. Compared with the prior art, the method has the advantages that: production cost is low, the production conditions are mild, impurities in a conversion system are a few, the process steps are simple, the production operation is safe, the purity is high, 300 to 320g of L-ornithine-L-aspartate salt is contained in each liter of reaction solution, the yield is high, the conversion rate of arginase is over 95 percent and the like.
Owner:湖南天成生化科技有限公司
Method for preparing ornithine aspartate powder injection for injection
InactiveCN101843587AReduce manufacturing costSimple production equipmentOrganic active ingredientsPowder deliveryDepyrogenationBarium hydroxide
The invention discloses a method for preparing ornithine aspartate powder injection for injection. The method comprises the following steps of: taking arginine, adding the solution of barium hydroxide, stirring to dissolve the arginine, and heating to hydrolyze so as to completely transform the arginine into ornithine; adding aspartate, uniformly stirring, adding a precipitator, filtering to remove barium ion precipitate, and regulating the pH value of the solution to neutrality; adding an inert organic solvent for crystallization, filtering and pumping, adding water for injection to dissolve the crystals, adding active carbon for needle for decoloration and depyrogenation, coarsely filtering to remove carbon, and filtering to make the solution sterile and clarified by using a 0.22 mu m millipore filter; and adding ethanol for crystallization, obtaining ornithine aspartate sterile powder, packaging the powder into a sterilized vial, capping, and rolling an aluminum cover to obtain the ornithine aspartate sterile powder injection for injection. The method has the advantages of increasing the yield, preparing the ornithine aspartate by the one-pot method, along with easy operation, simple and convenient operation, low cost, short production period, and suitability for mass production.
Owner:HUBEI HOPE PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com