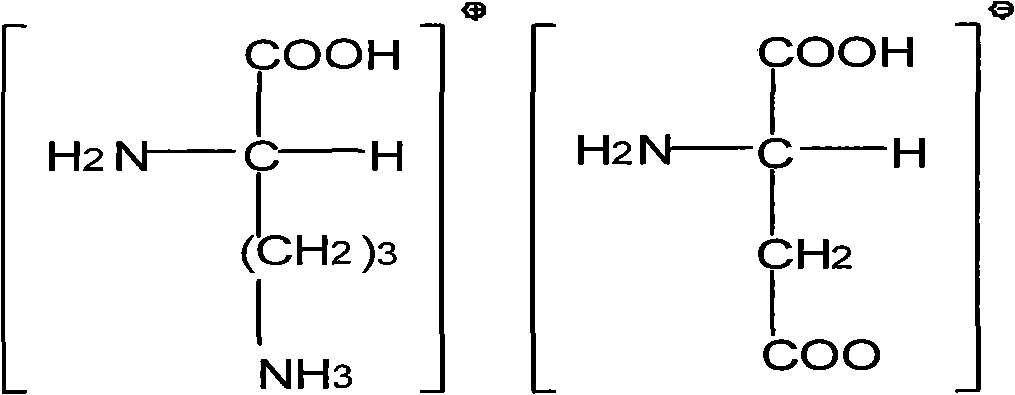Method for preparing ornithine aspartate powder injection for injection
The technology of aspartate ornithine aspartate powder injection and aspartate ornithine aspartate is applied in the field of medicine and can solve the problems of long freeze-drying time, high production cost, easy precipitation of crystals, etc. The effect of short cycle time and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A preparation method of aspartic acid ornithine powder injection for injection, the method comprises the following steps:
[0034] 1. Take 4.5kg of barium hydroxide, add 30L of water to heat and stir to dissolve completely, then add 3.5kg of arginine, heat to 90 or 93 or 95 or 98 or 100°C, continue stirring to hydrolyze arginine, use thin The termination of the reaction was monitored by layer chromatography, once every half an hour, and when no arginine spots were detected, the reaction was carried out for another half an hour to ensure that there was no arginine residue in the reaction solution.
[0035]2, gradually add 50% sulfuric acid solution 2.7kg and aspartic acid 2.7kg in step 1 reaction solution under agitation, and continue to adjust the pH of the solution with 50% sulfuric acid solution to be neutral (about 6.5) and then After no barium sulfate precipitate occurs, continue to stir for 2 hours, filter, and remove the barium sulfate precipitate and unreacted as...
Embodiment 2
[0041] A preparation method of aspartic acid ornithine powder injection for injection, the method comprises the following steps:
[0042] 1. Take 4.5kg Ba(OH) 2 .8H 2 O, add 30L of water, heat and stir to dissolve completely, then add 3.5kg of arginine, heat to 91 or 92 or 94 or 97 or 99°C, continue stirring to hydrolyze the arginine, monitor the termination of the reaction by thin-layer chromatography, Once every half an hour, when no arginine spots can be detected, react for another half an hour to ensure that there is no arginine residue in the reaction solution.
[0043] 2. Gradually add 75% phosphoric acid solution 1.2kg and aspartic acid 2.7kg to step 1 reaction solution under agitation, and continue to adjust the pH of the solution with 75% phosphoric acid solution to be neutral (about 6.5) and then After no barium phosphate precipitate occurs, continue stirring for 2 hours and filter to remove the barium phosphate precipitate and unreacted aspartic acid.
[0044] 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com