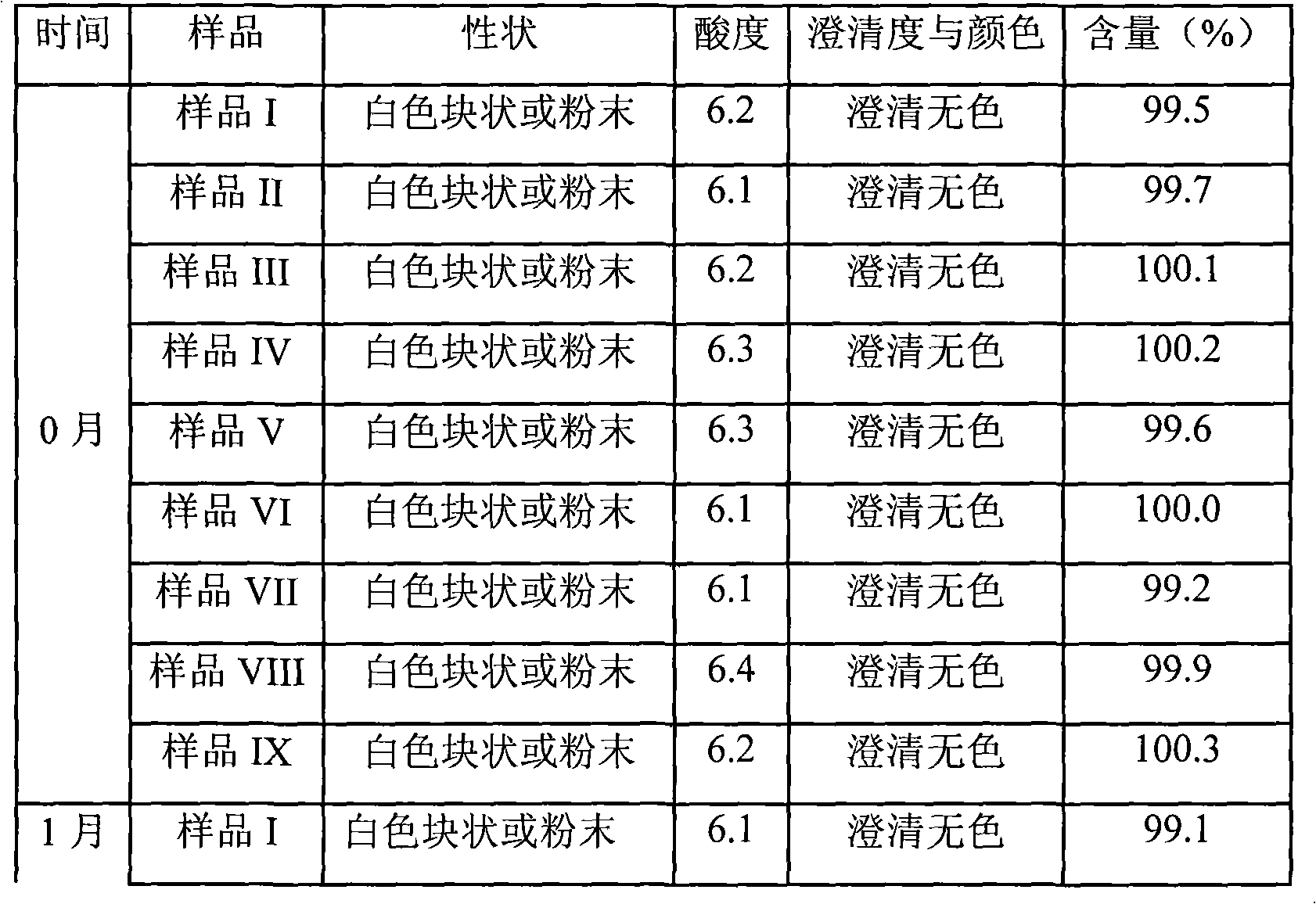
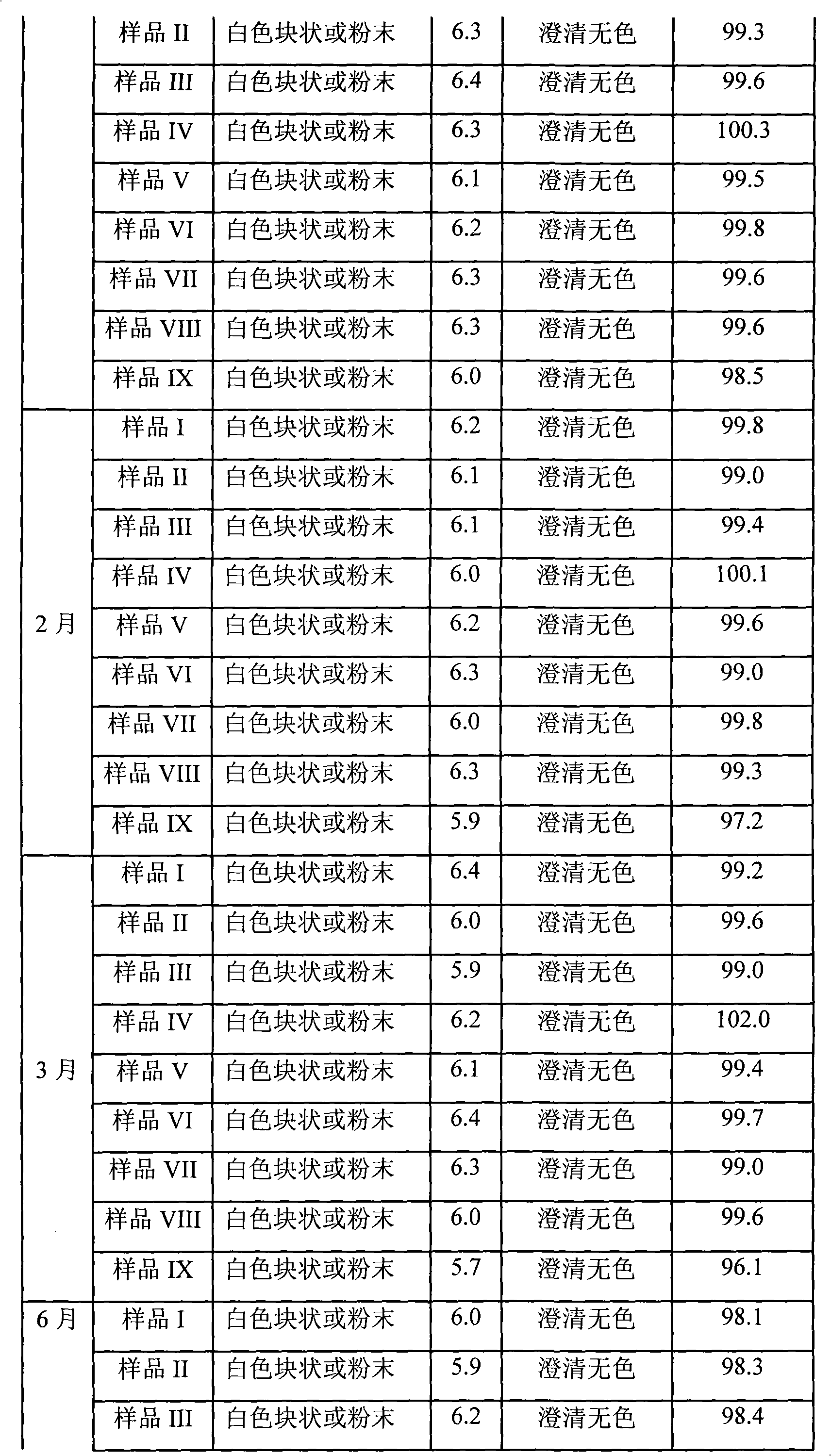
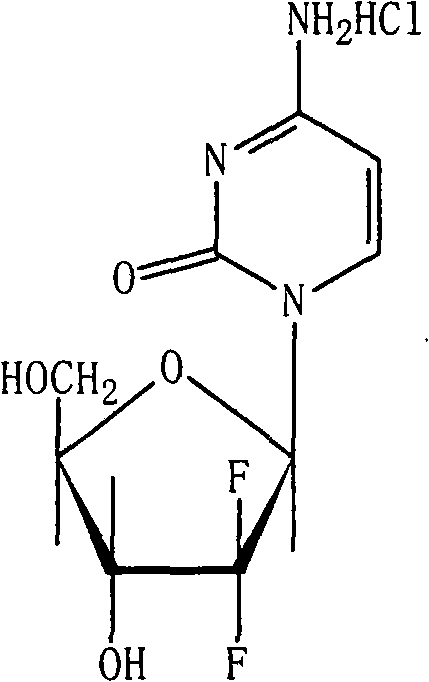
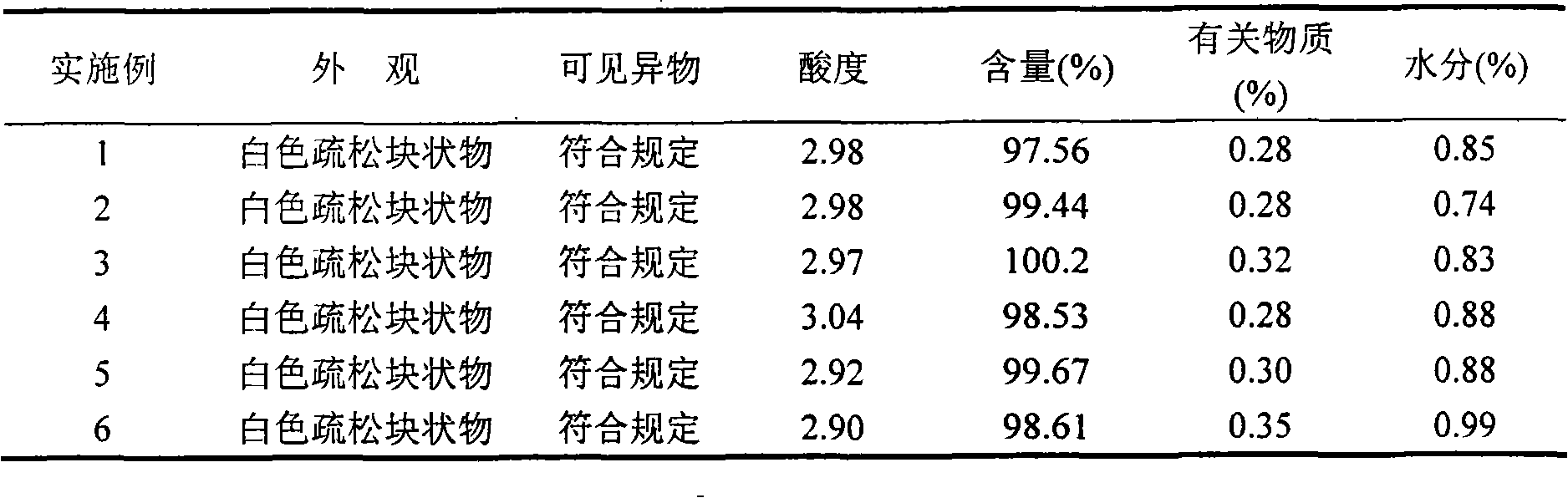
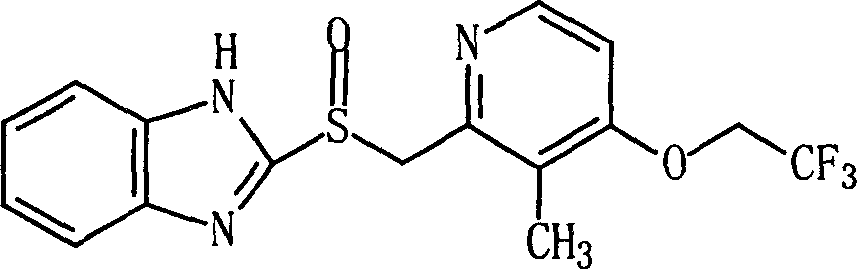
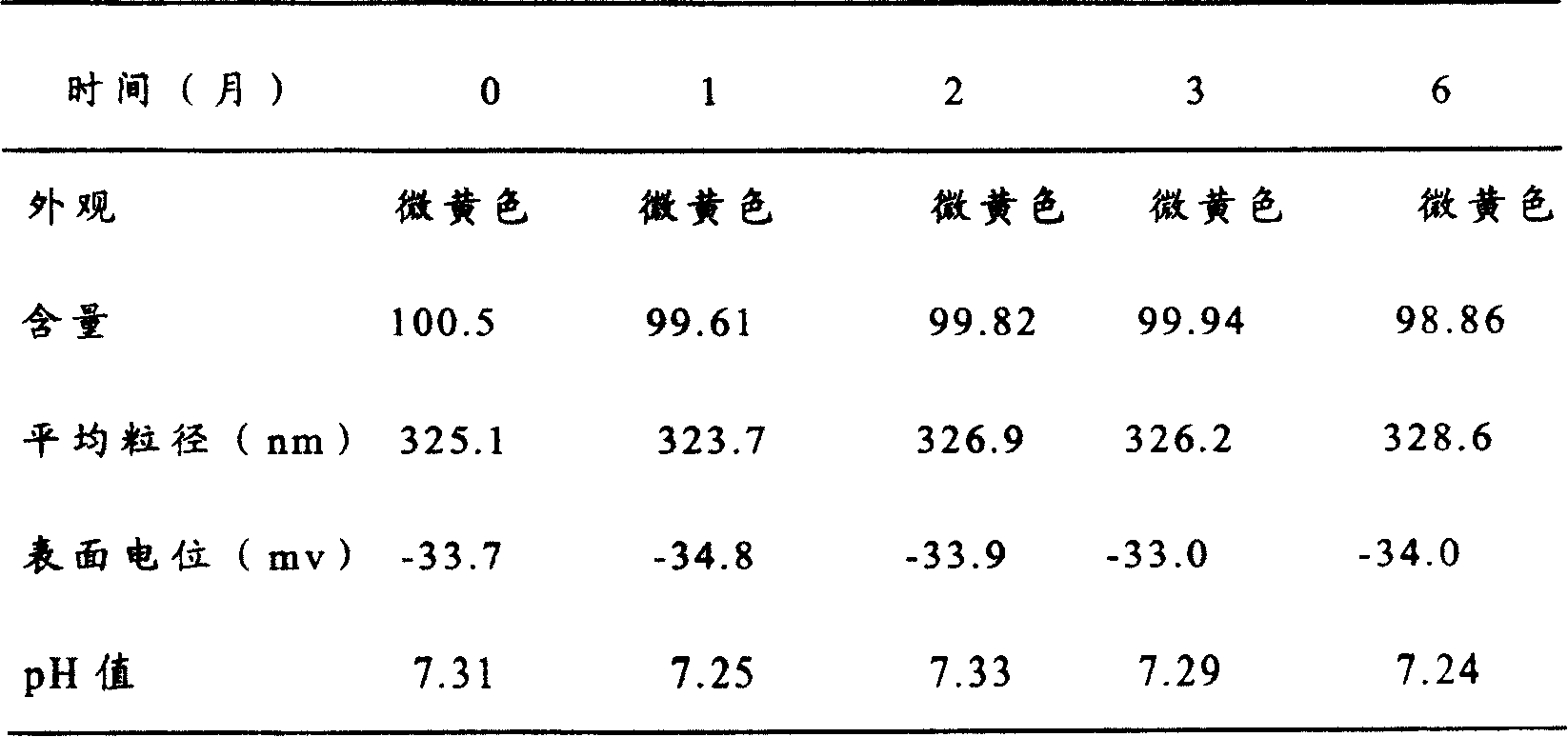
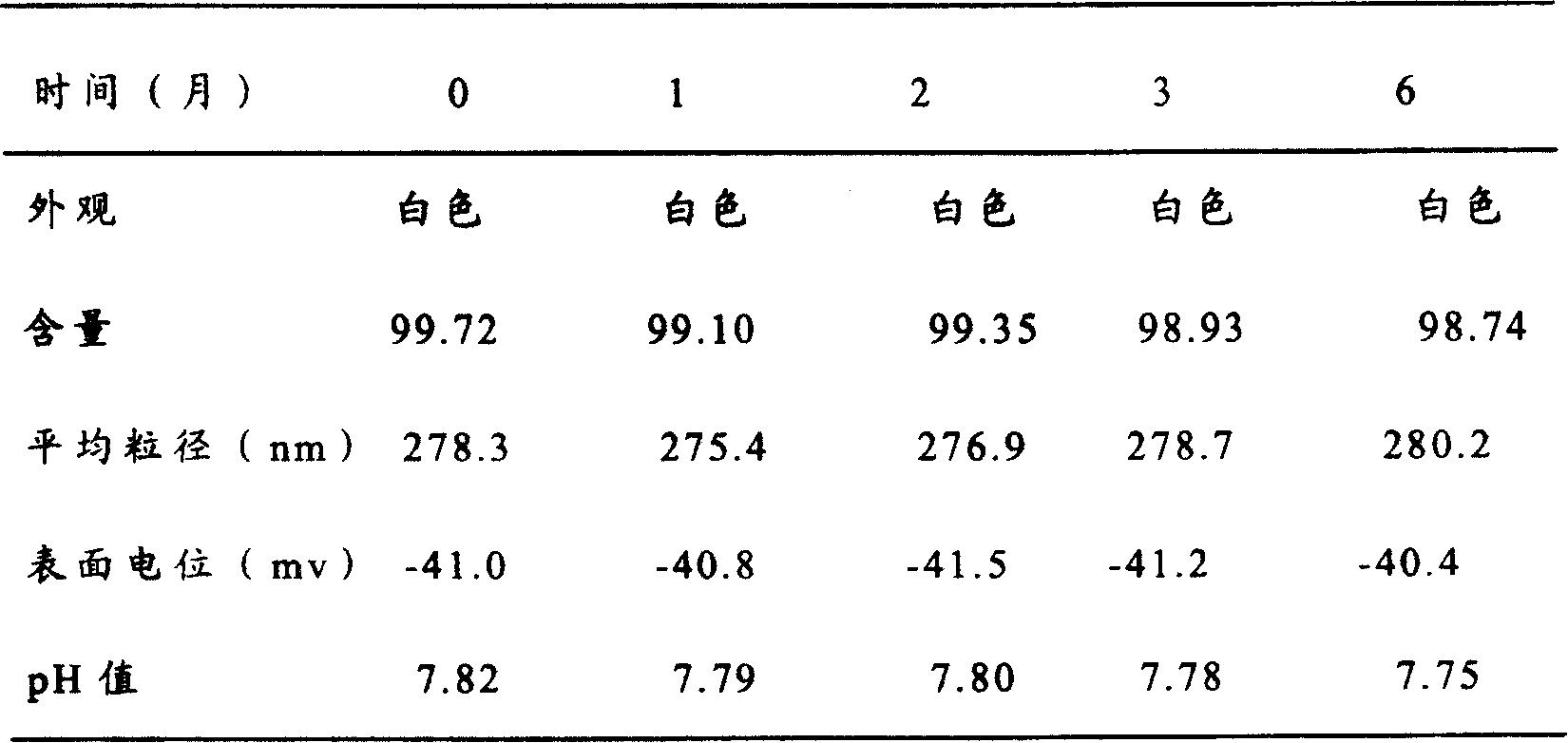
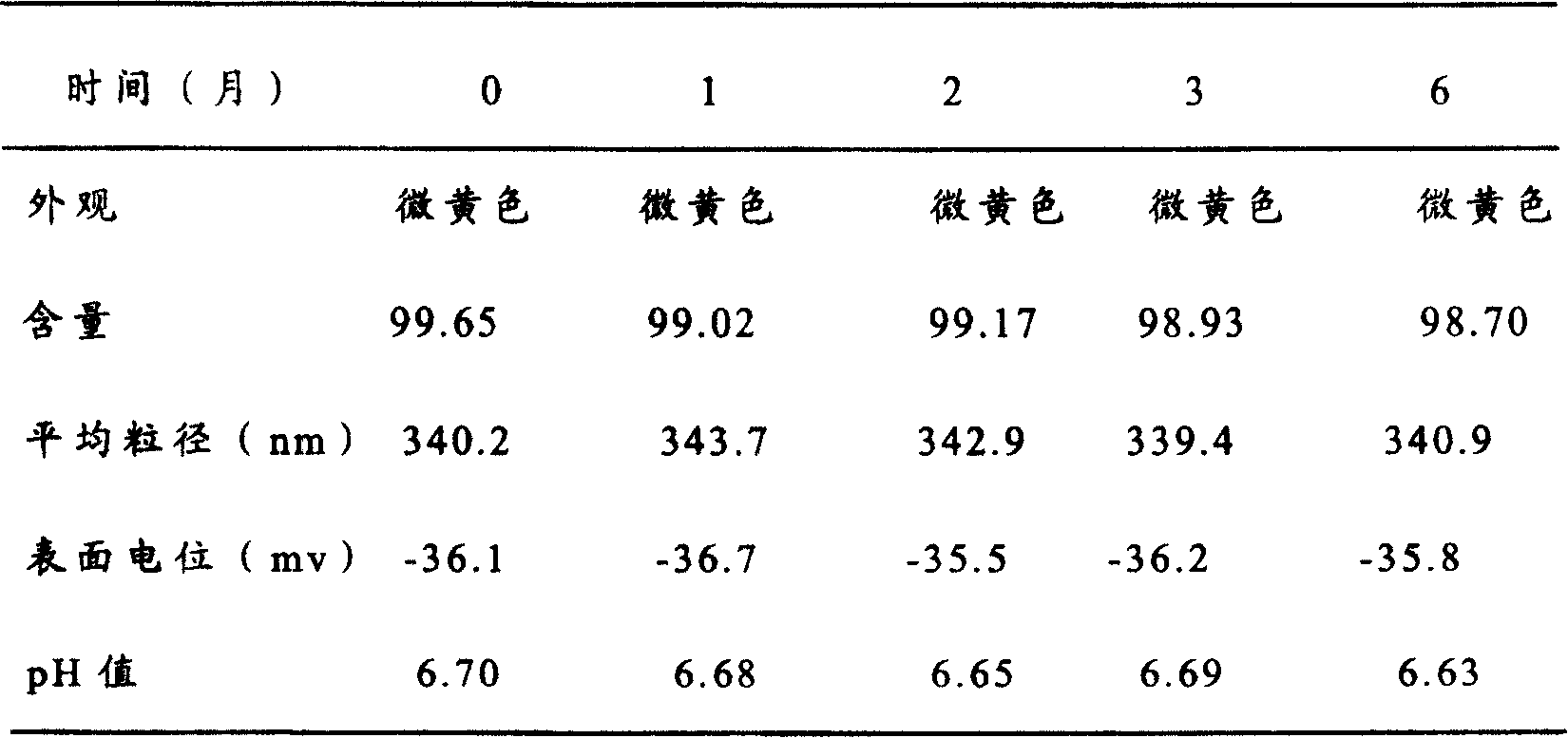
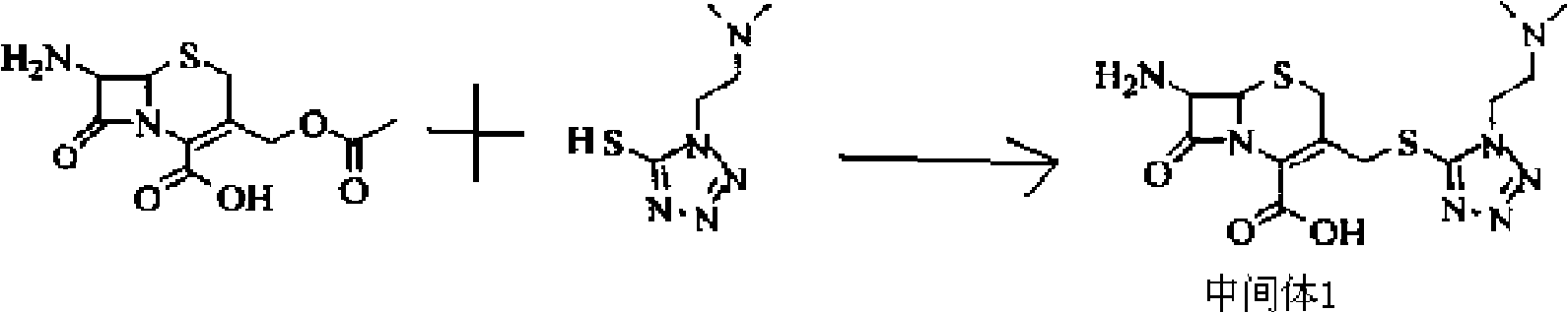Patents
Literature
2194 results about "Powder injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Manufacturing method of multilayer shell-core composite structural part
ActiveCN102009175AShell surface hardness is highHigh surface finishJoint implantsCeramic layered productsComposite constructionHigh surface
The invention discloses a manufacturing method of a multilayer shell-core composite structural part, which comprises the following steps of: (1) respectively preparing feed for injection forming of a core layer, a transition layer and a shell layer, wherein powder in the feed of the core layer and the powder in the feed of the shell layer are selected from one or a mixture of some of metal powder, ceramic powder, or toughened ceramic powder and are different from each other, and the powder in the feed of the transition layer is gradient composite powder; (2) respectively manufacturing blanks of the multilayer shell-core composite structural part layer by layer with a powder injection forming method; (3) degreasing the blanks; and (4) sintering the blanks to obtain the multilayer shell-core composite structural part. The multilayer shell-core composite structural part is manufactured with the powder injection forming method, and has the advantages of high surface hardness, abrasion resistance, uniform thickness of the shell layer, stable and persistent performance, strong binding force between the shell layer and the core layer due to the transition layer, good integral bending strength and good impact toughness and is difficult to crack.
Owner:SUZHOU DINGAN ELECTRONICS TECH
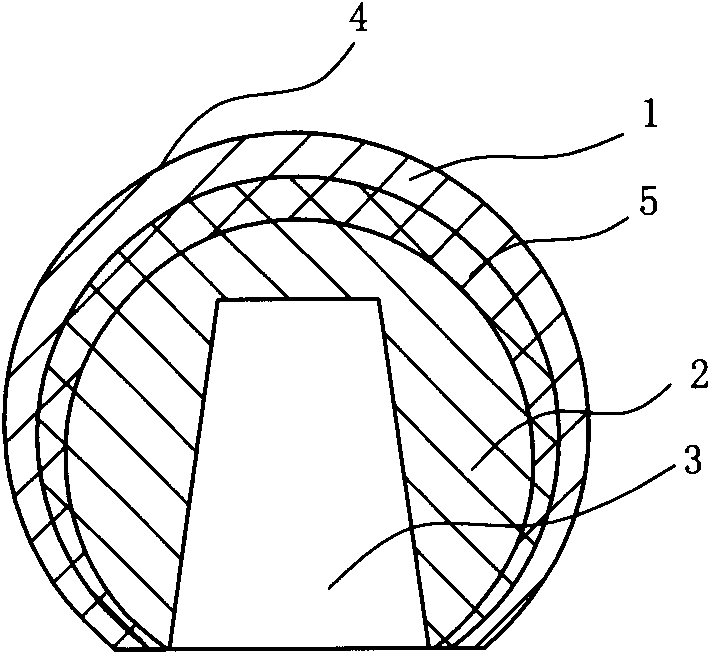
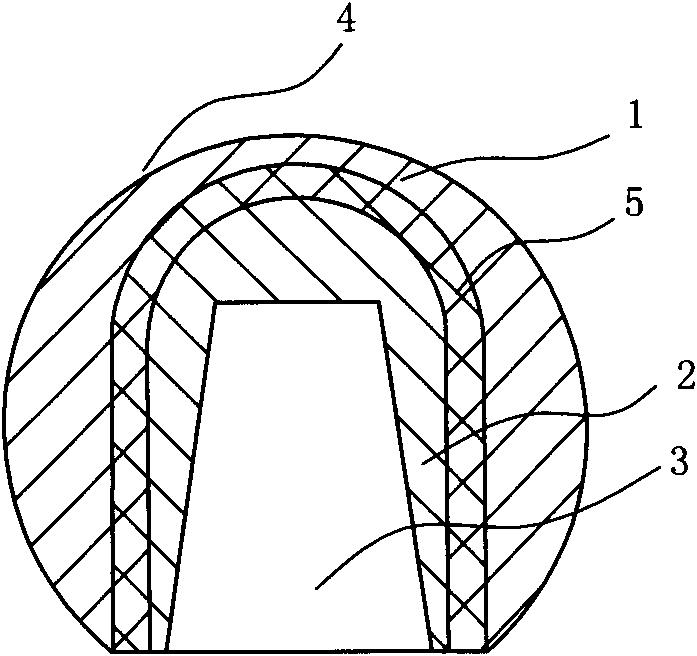
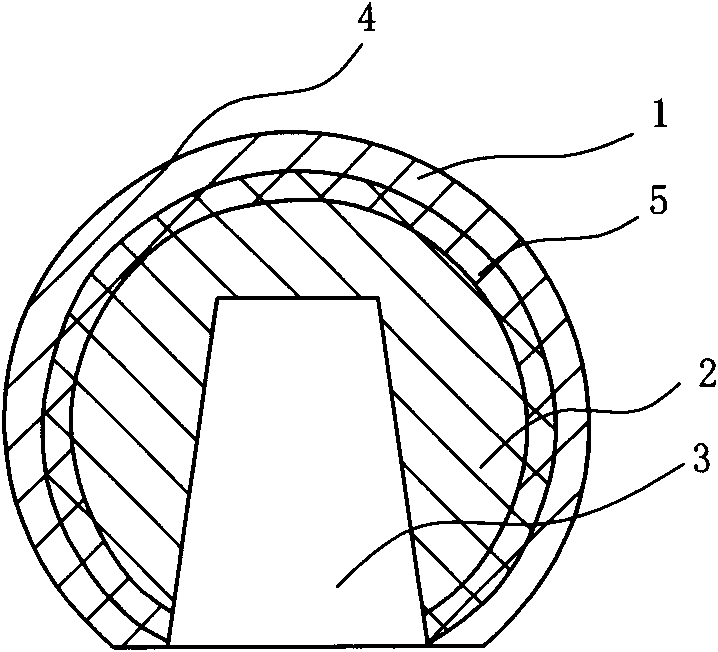
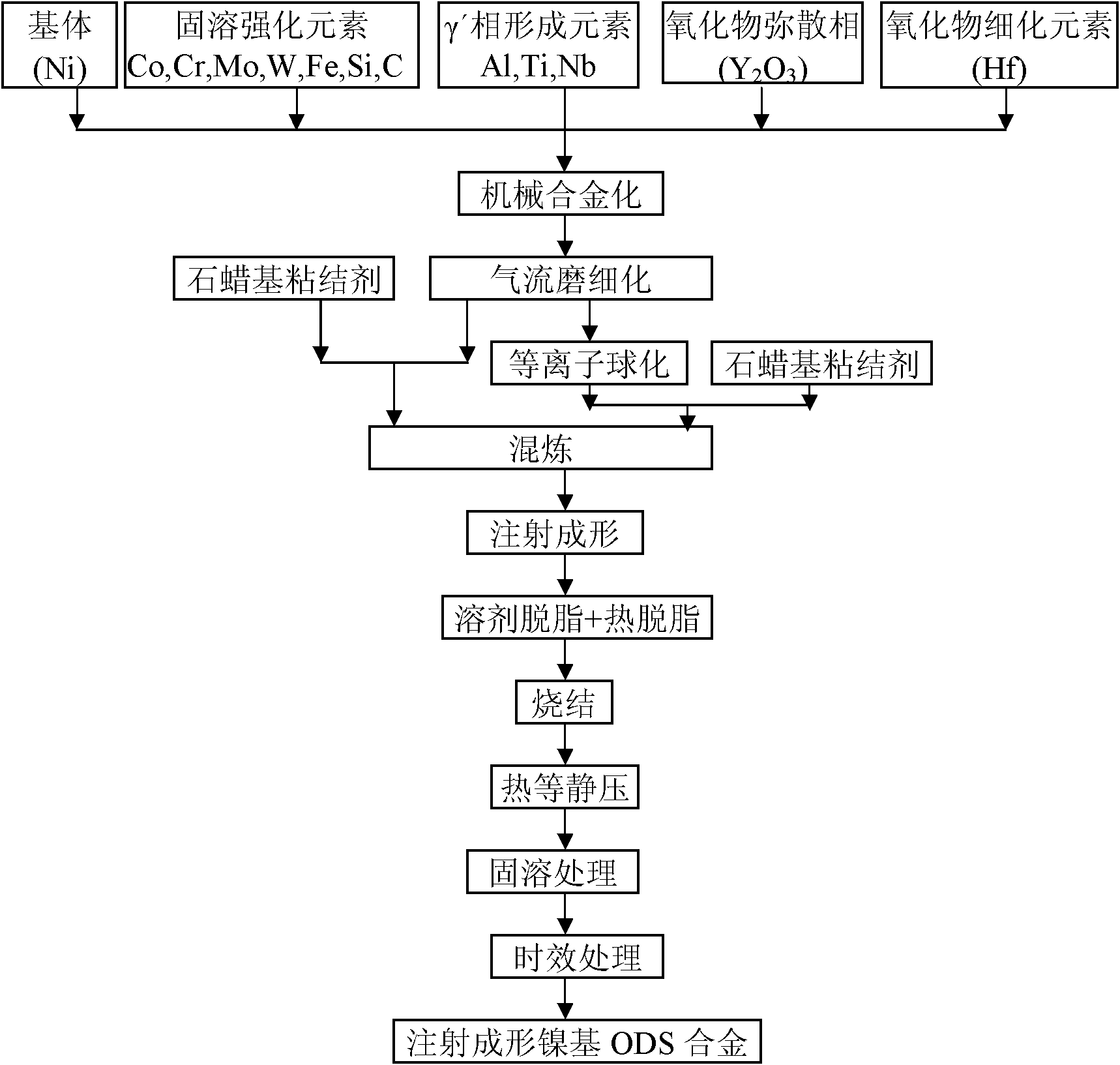
Method for preparing injection-molding nickel-base ODS (oxide dispersion strengthened) alloy
The invention provides a method for preparing a nickel-base ODS (oxide dispersion strengthened) alloy by injection molding, belonging to the technical field of injection molding of powder. The method comprises the following steps: carrying out high-energy ball milling on the raw material powder so that Y2O3 particles are uniformly dispersed in a nickel substrate, refining mechanical alloy powder by jet milling, and carrying out plasma nodularization on the powder which is refined by jet milling; evenly mixing and smelting the powder, which is refined by jet milling and plasma nodularization, and adhesive to obtain a uniform feed material; and carrying out injection molding, two-step degreasing and sintering on the feed material to obtain a sintered blank of which the density is 93-96%, carrying out hot isostatic compaction on the sintered blank so that the sintered blank is completely compact, and finally, carrying solution heat treatment and aging heat treatment to obtain the injection-molding nickel-base ODS alloy. The nickel-base ODS alloy can be prepared into high-precision parts in complex shapes, thereby solving the problem of difficulty in molding of nickel-base ODS alloy. The gamma' phase and the oxide strengthening mechanism are combined to greatly enhance the high-temperature mechanical properties of the nickel-base ODS alloy.
Owner:UNIV OF SCI & TECH BEIJING
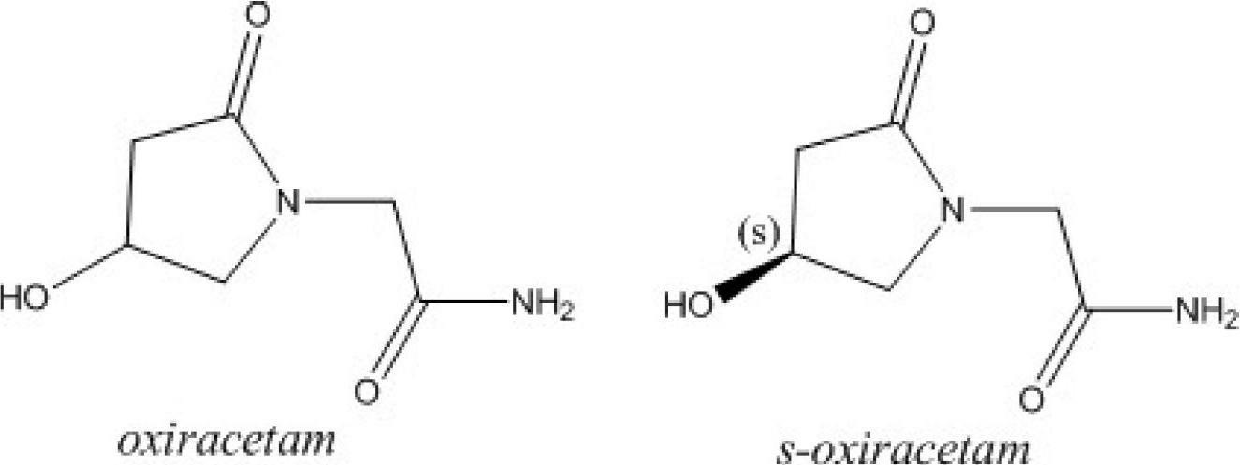
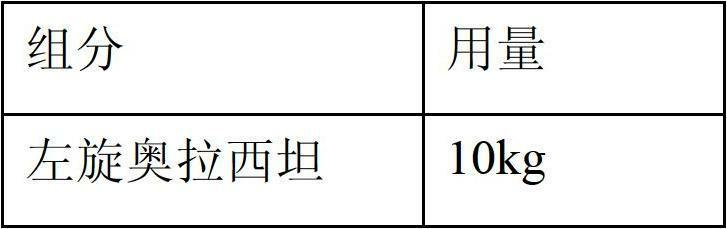
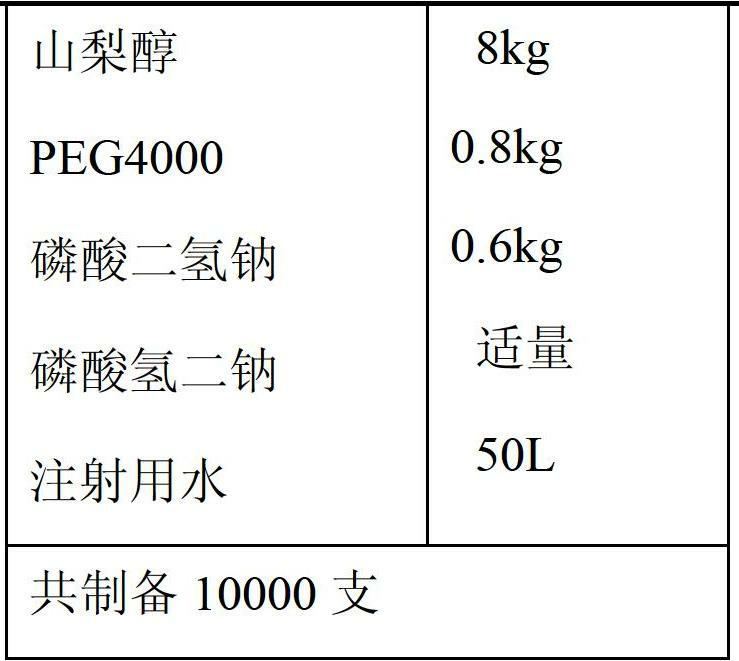
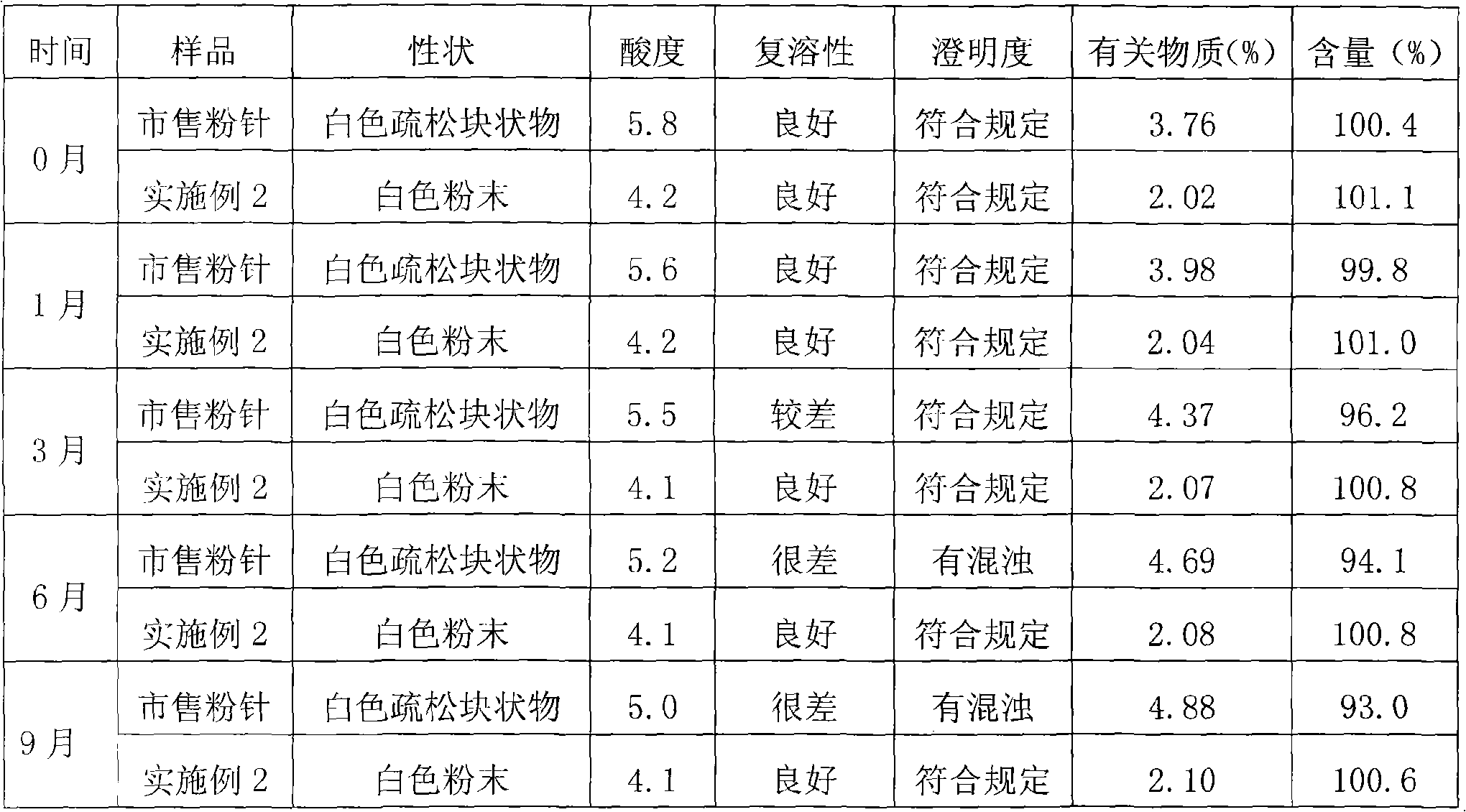
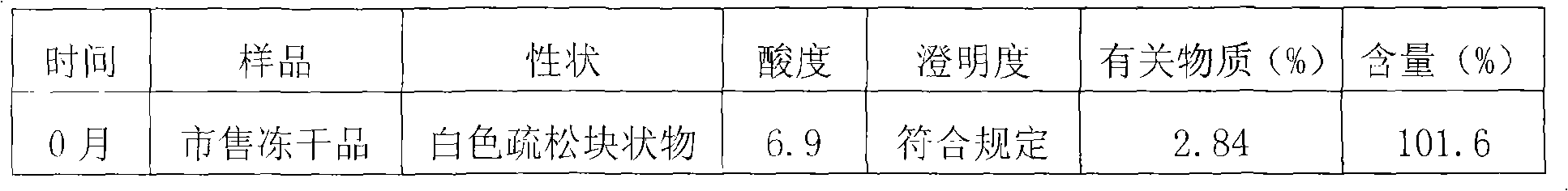
Freeze-dried powder injection of L-oxiracetam and process for preparing freeze-dried powder injection
ActiveCN102670527AFast sublimationShorten freeze-drying timePowder deliveryOrganic active ingredientsPolyethylene glycolEngineering
The invention relates to a freeze-dried powder injection of L-oxiracetam and a process for preparing the freeze-dried powder injection. The freeze-dried powder injection comprises, by weight, 1 part of the L-oxiracetam, 0.1 to 10 parts of an excipient, 0.01 to 1 part of an antisticking agent and 0.01 to 0.5 part of a pH regulator, wherein polyethylene glycol (PEG) series of products serve as the antisticking agent preferably. The freeze-dried powder injection which is prepared on the basis of a prescription according to the process has the advantages of being short in freeze-drying time, attractive in appearance, short in redissolving time and high in stability.
Owner:南京博德生物制药有限公司
Method for preparing blades of adjustable nozzle in use for turbocharger of engine by using powder as raw material
A process for preparing the blades of adjustable nozzle for the turbine booster of engine from raw powder includes proportionally mixing a chosen alloy pwoder with the adhesive prepared from paraffin wax, high-density polyethene, stearic acid and polypropene, pugging, injection molding at 150-175 deg.C under 75-125 MPa, degreasing and sintering.
Owner:UNIV OF SCI & TECH BEIJING
Method for preparing powder injection using superfine communication technique and prepared products
InactiveCN101332188AReduce contentUniform colorAntibacterial agentsOrganic active ingredientsLatamoxefClindamycin Phosphate
The present invention relates to a method of using a superfine pulverizing technology to prepare sterile powder for injection (powder injection) of chemical medicine and the prepared medicine powder injection. Invert sugar, clindamycin phosphate, cefpiramide sodium, cefepime hydrochloride, latamoxef sodium or cefmetazole sodium are preferable as the chemical medicine.
Owner:HAINAN LINGKANG PHARMA CO LTD
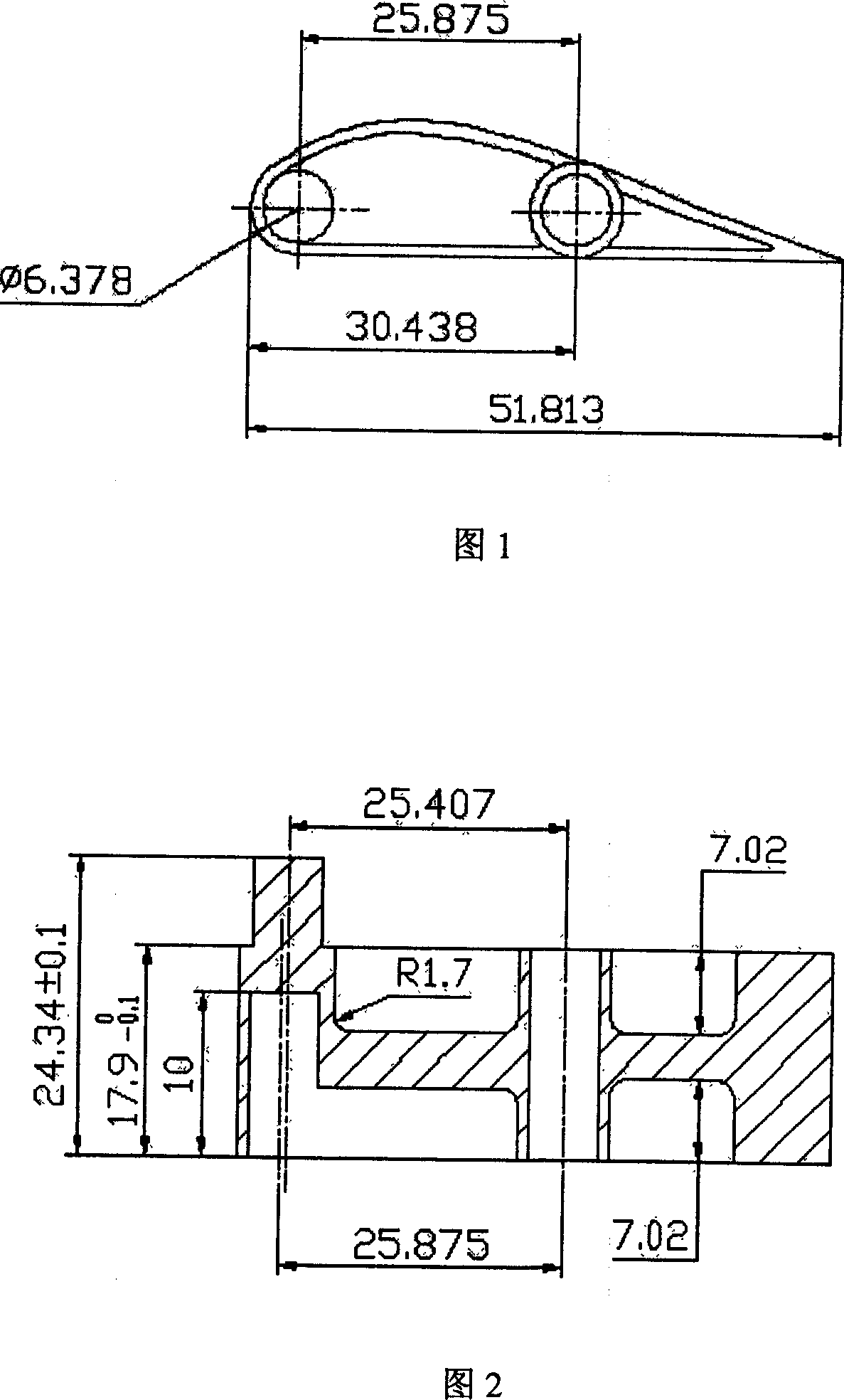
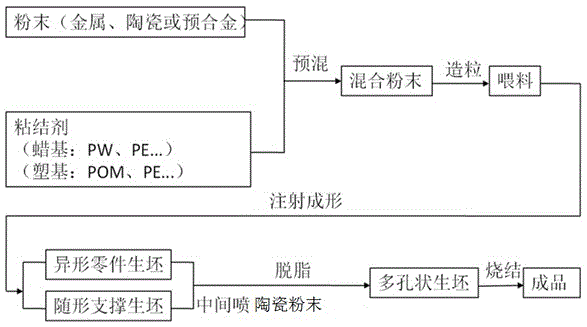
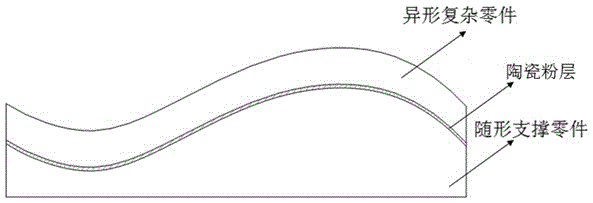
Method for preparing special-shaped complex part through powder injection molding technology
ActiveCN105108154AHas a three-dimensional structureNo fixed radius of curvatureCeramic shaping apparatusPowder injectionPowder injection molding
The invention relates to the technical field of powder injection molding and particularly discloses a method for preparing a special-shaped complex part through a powder injection molding technology. The method includes the steps of mixing and pelleting, injection molding, and degreasing and sintering. In the injection molding step, a green body of the special-shaped complex part and a green body of a profile-followed supporting part corresponding to the special-shaped complex part are prepared; in the degreasing and sintering step, the special-shaped complex part is placed on the profile-followed supporting part corresponding to the special-shaped complex part for degreasing and sintering. According to the method, the problems of deformation, collapse and even breakage in the degreasing and sintering process of the special-shaped complex part can be well solved, the problem that the special-shaped complex part cannot be sintered through a traditional method is effectively solved, and the complexity of the powder injection molding field is lowered.
Owner:SHENZHEN ELEMENT TECH CO LTD
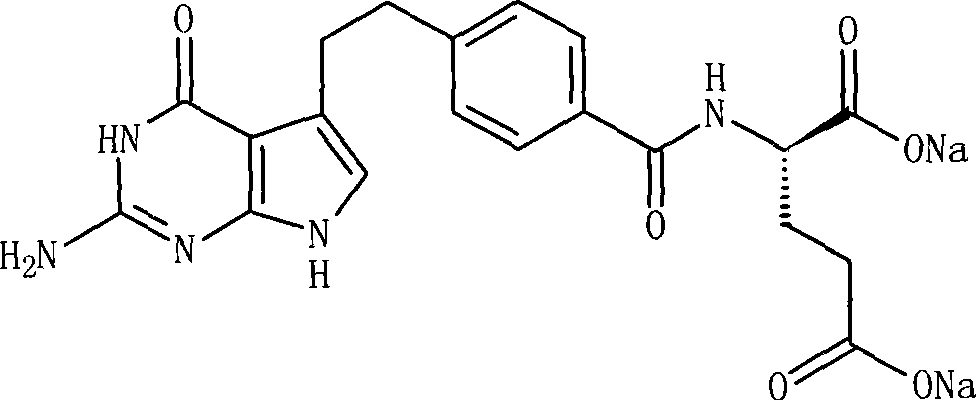
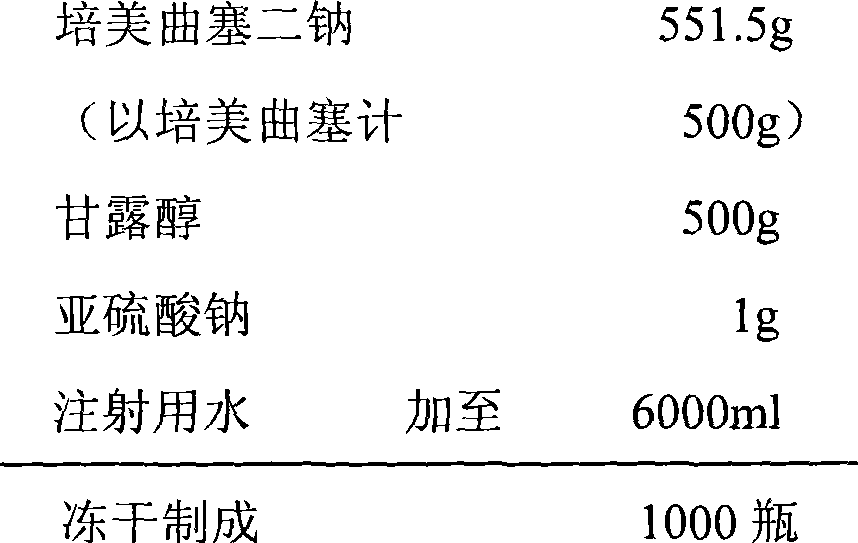
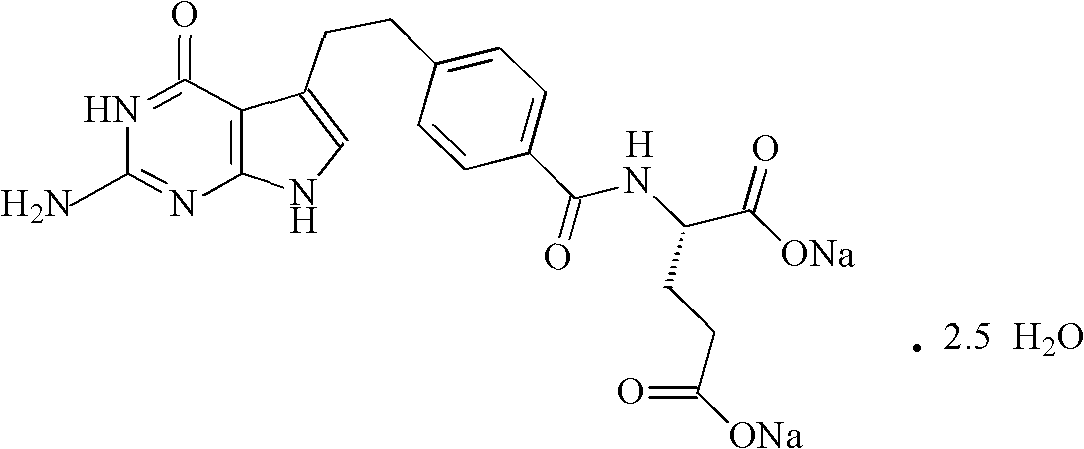
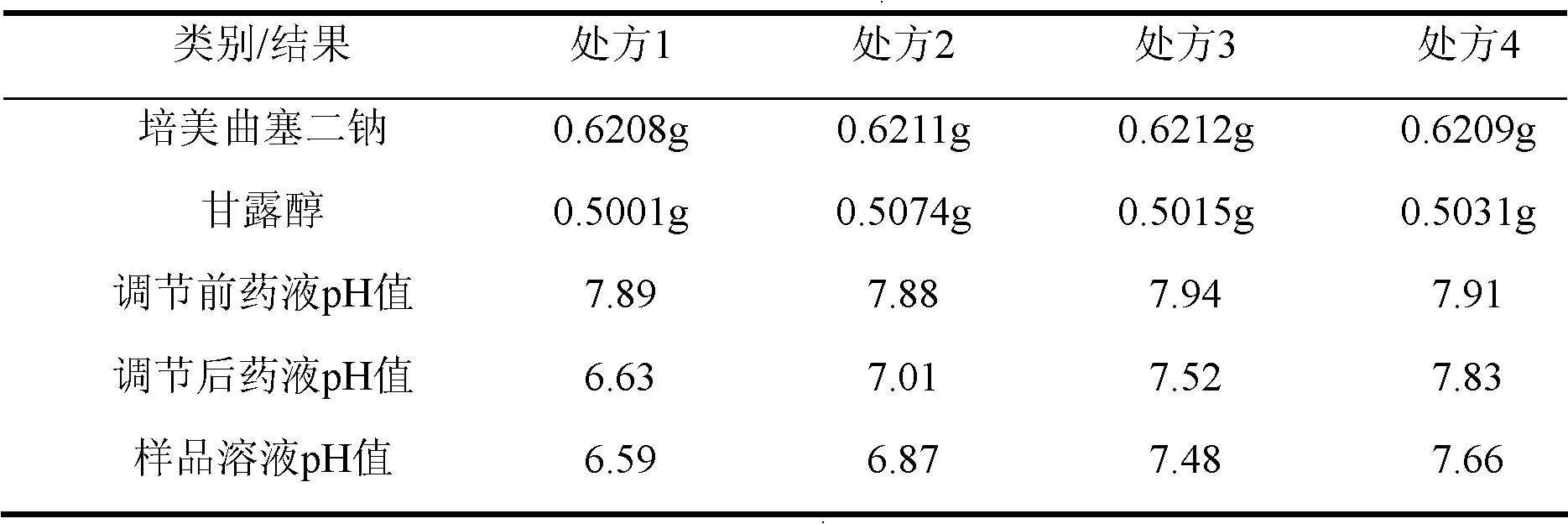
Pemetrexed disodium freeze-dried injection and preparation method thereof
ActiveCN101411710AImprove stabilityLow content of related substancesPowder deliveryOrganic active ingredientsMANNITOL/SORBITOLSulfite salt
The invention relates to a pemetrexed disodium lyophilized powder injection, which consists of pemetrexed disodium, mannitol and sodium sulfite in the following weight portions: 50 portions of the pemetrexed disodium, 10 to 50 portions of mannitol, and 0.1 to 1 portions of sodium sulfite; and the pH value of the pemetrexed disodium lyophilized powder injection is between 7.0 and 8.0. The process for preparing the pemetrexed disodium lyophilized powder injection comprises the following steps: placing the mannitol in a sterile chamber; adding 80 percent of water for injection into the sterile chamber to dissolve the mannitol; adding the sodium sulfite to the mixture after the water for injection is cooled to a temperature of between 15 and 25 DEG C, and evenly stirring the solution for dissolving the sodium sulfite; then, adding the pemetrexed disodium into the solution, and stirring the solution to completely dissolve the pemetrexed disodium and evenly mixing the pemetrexed disodium, and adjusting the pH value of the solution to between 7.0 and 8.0; decarbidizing; after an intermediate compound passes examination, carrying out volume fixing, filtering, filling, partially stopping, traying, lyophilizing, nitrogen aerating, stopping and unboxing, sealing by a plastic-aluminum combined cap, and packaging after passes quality inspection to obtain the pemetrexed disodium lyophilized powder injection. The invention has the advantages of simple preparation process, convenience and practicality, good repeatability and low production cost, and can realize industrial large-scale production easily.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Pantoprazole sodium compound and pharmaceutical composition thereof
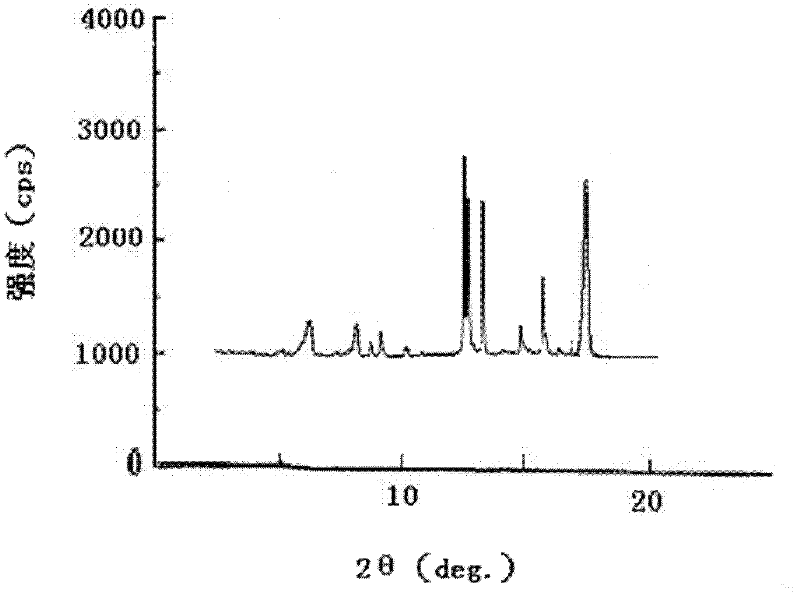
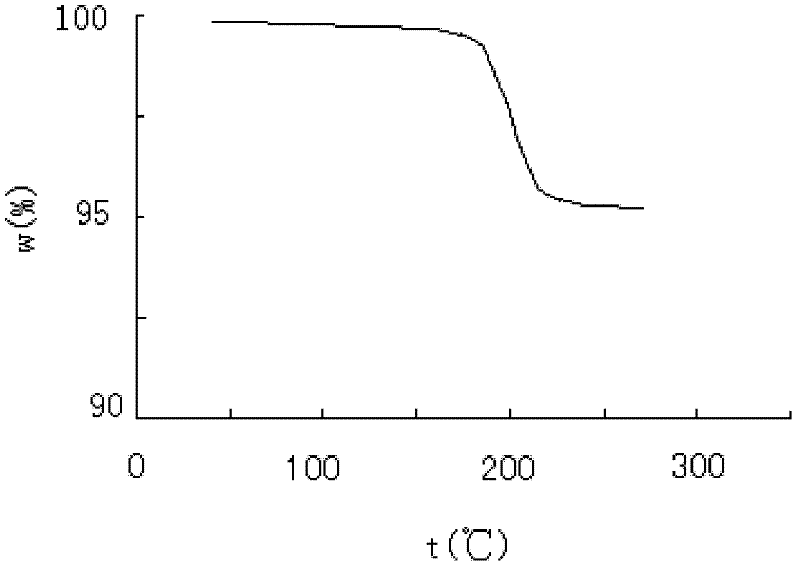
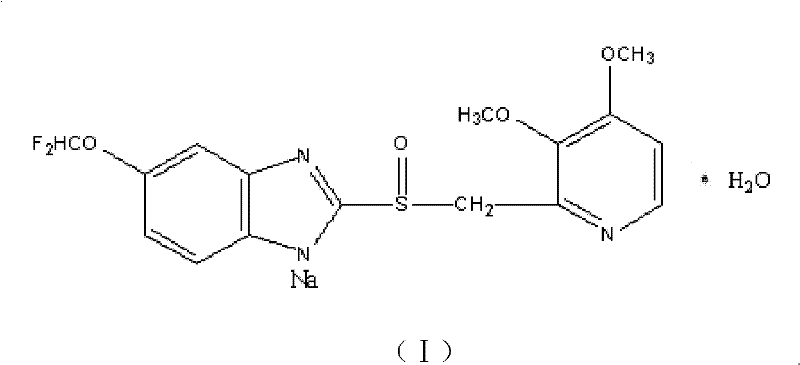
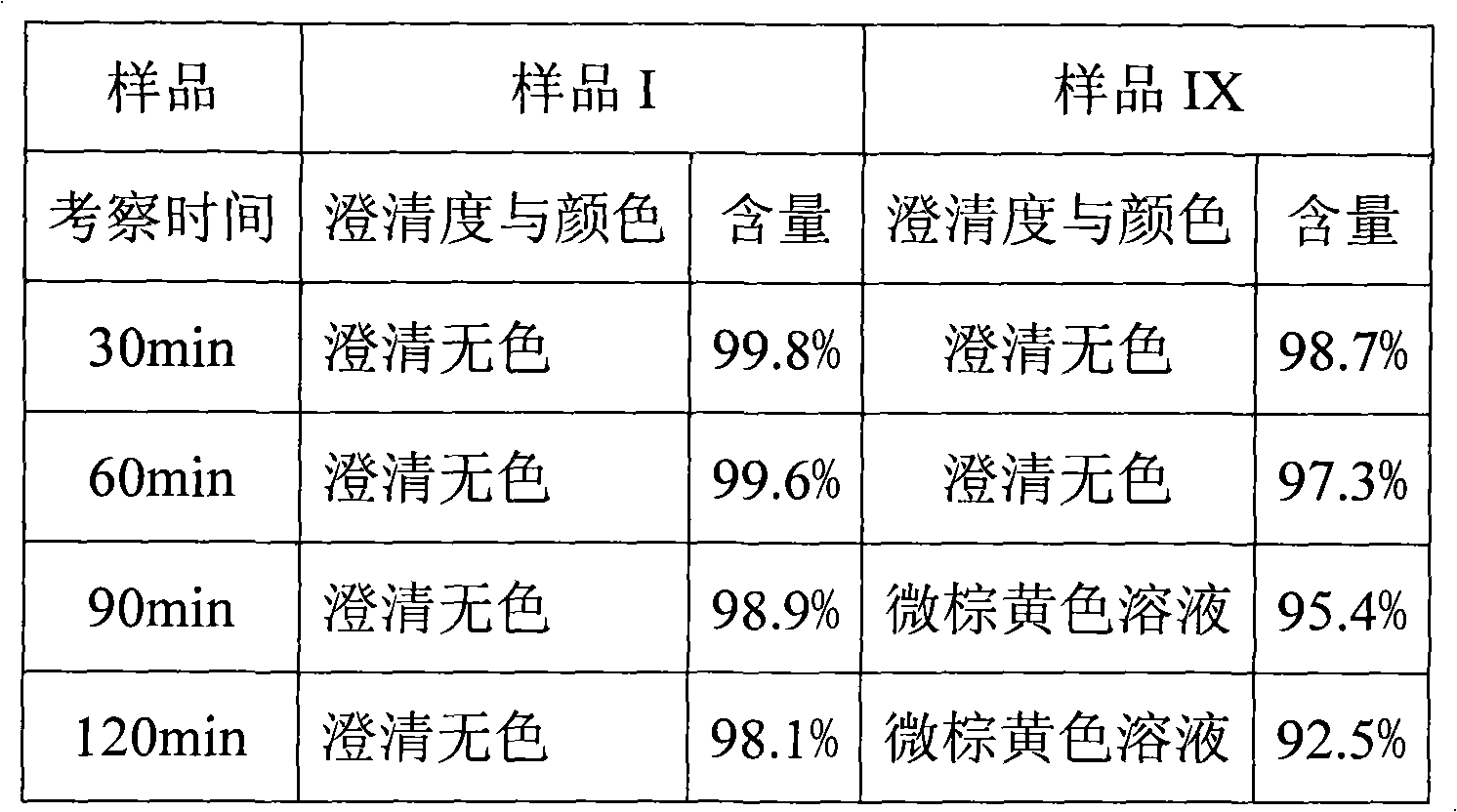
ActiveCN102351844AStable moisture contentNo changeOrganic active ingredientsPowder deliveryFreeze-dryingX-ray
The invention discloses a pantoprazole sodium compound, which is crystal. In X-ray powder diffraction pattern obtained through Cu-Kalpha ray measurement, the characteristic peaks of the pantoprazole sodium compound are shown in positions where 2theta is 12.5, 12.6, 13.2, 16.2 and 17.3. The pantoprazole sodium compound can be used together with multiple freeze-drying supporting agents, the prepared freeze-dried powder injection has the advantages of good redissolution, good transparency after redissolution, low impurity content and the like; and moreover, the use amount of the freeze-drying supporting agent is lower, thus saving the pharmaceutical cost and improving the stability of a drug preparation. The invention also discloses a pharmaceutical composition. The pharmaceutical composition comprises a pharmaceutical active ingredient and pharmaceutical auxiliary materials, wherein the pharmaceutical active ingredient is the pantoprazole sodium compound. The stability of the pharmaceutical composition is obviously superior to that of commercial products, and especially, the stability duration of the pharmaceutical composition after being matched with common infusion fluid is prolonged, thus facilitating the clinical application.
Owner:江西新先锋医药有限公司
Omeprazole freeze-dried powder injection and preparation method thereof
InactiveCN101283986AStable color and lusterLittle side effectsOrganic active ingredientsPowder deliveryOmeprazole SodiumSide effect
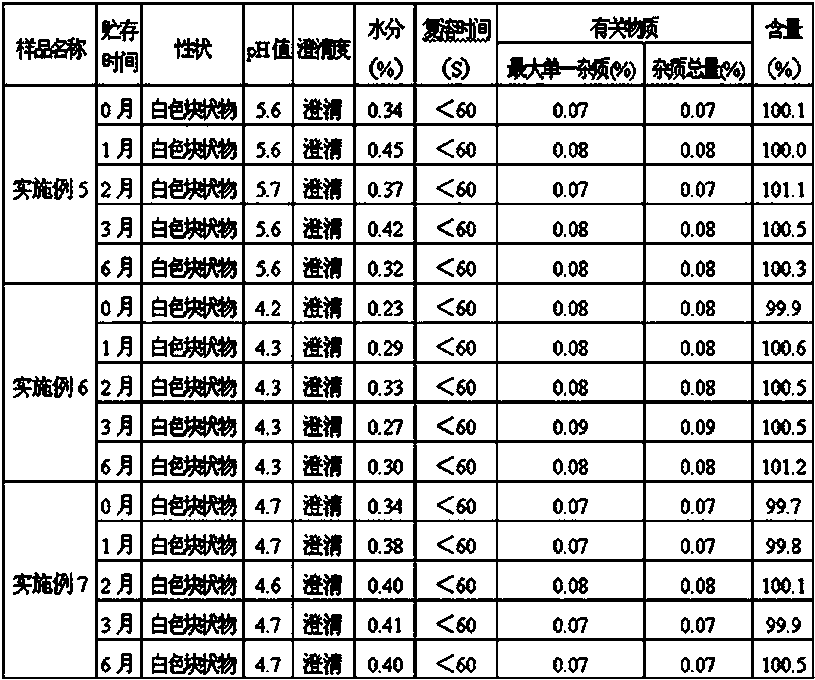
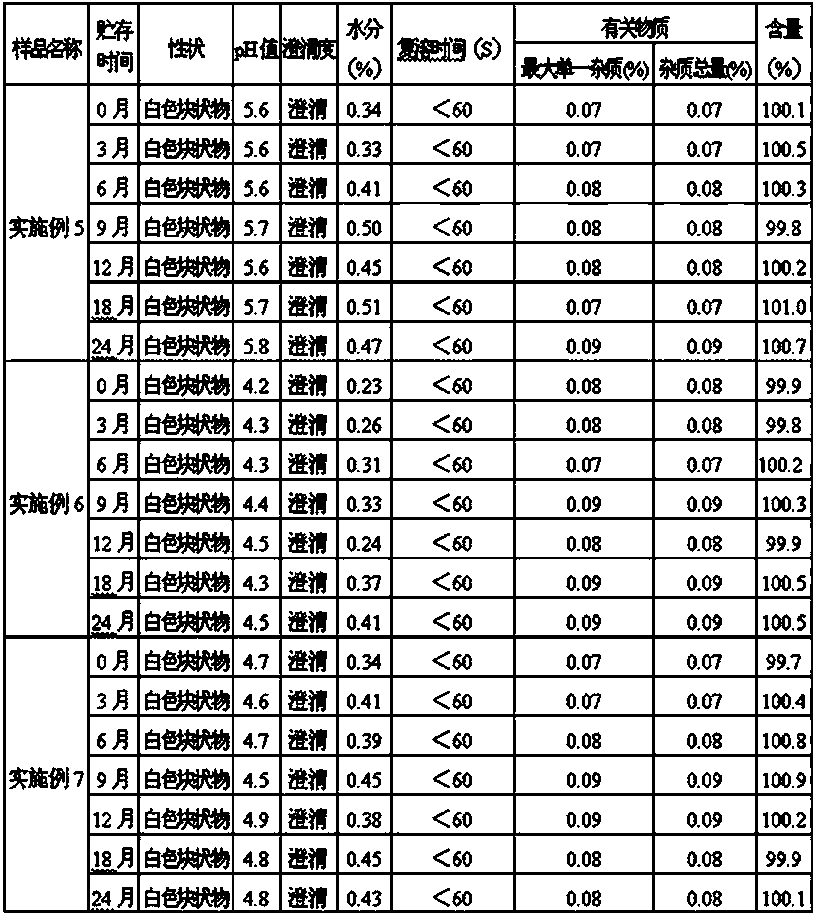
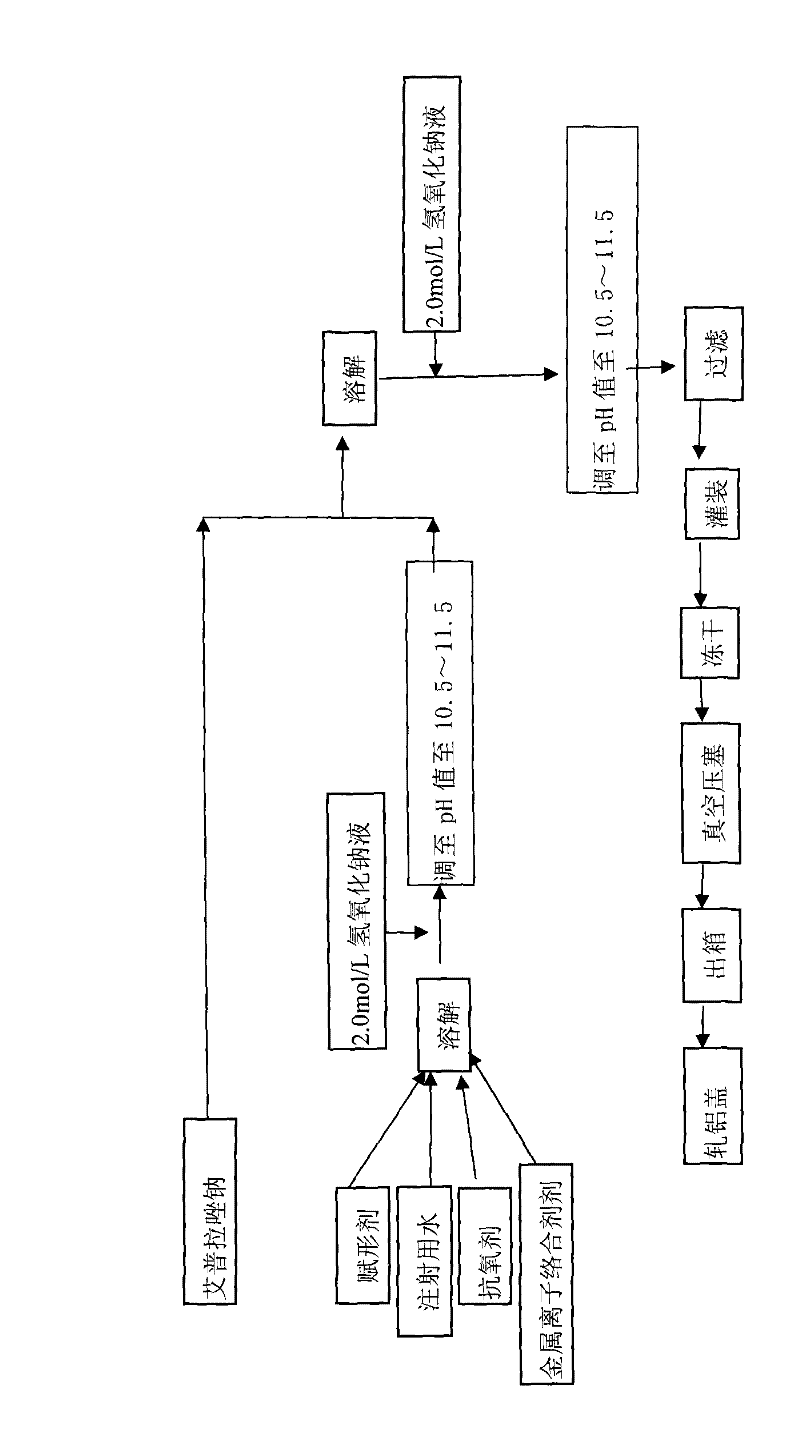
The invention provides an omeprazole sodium lyophilized powder for injection (pH of 10.8-11.2), comprising omeprazole sodium as the main active component, as well as excipient, metal ion complexing agent, stabilizer, antioxidant and pH regulator. The omeprazole sodium lyophilized powder for injection has higher active component content than that of the conventional omeprazole sodium injection, solves solution clarity problem, and effectively prevents loss of bone calcium due to complexation with calcium ion. The omeprazole sodium lyophilized powder for injection also has the advantages of stable color and properties, low side effects, good stability, good redissolution, and convenient application.
Owner:海南瑞基药物研究有限公司
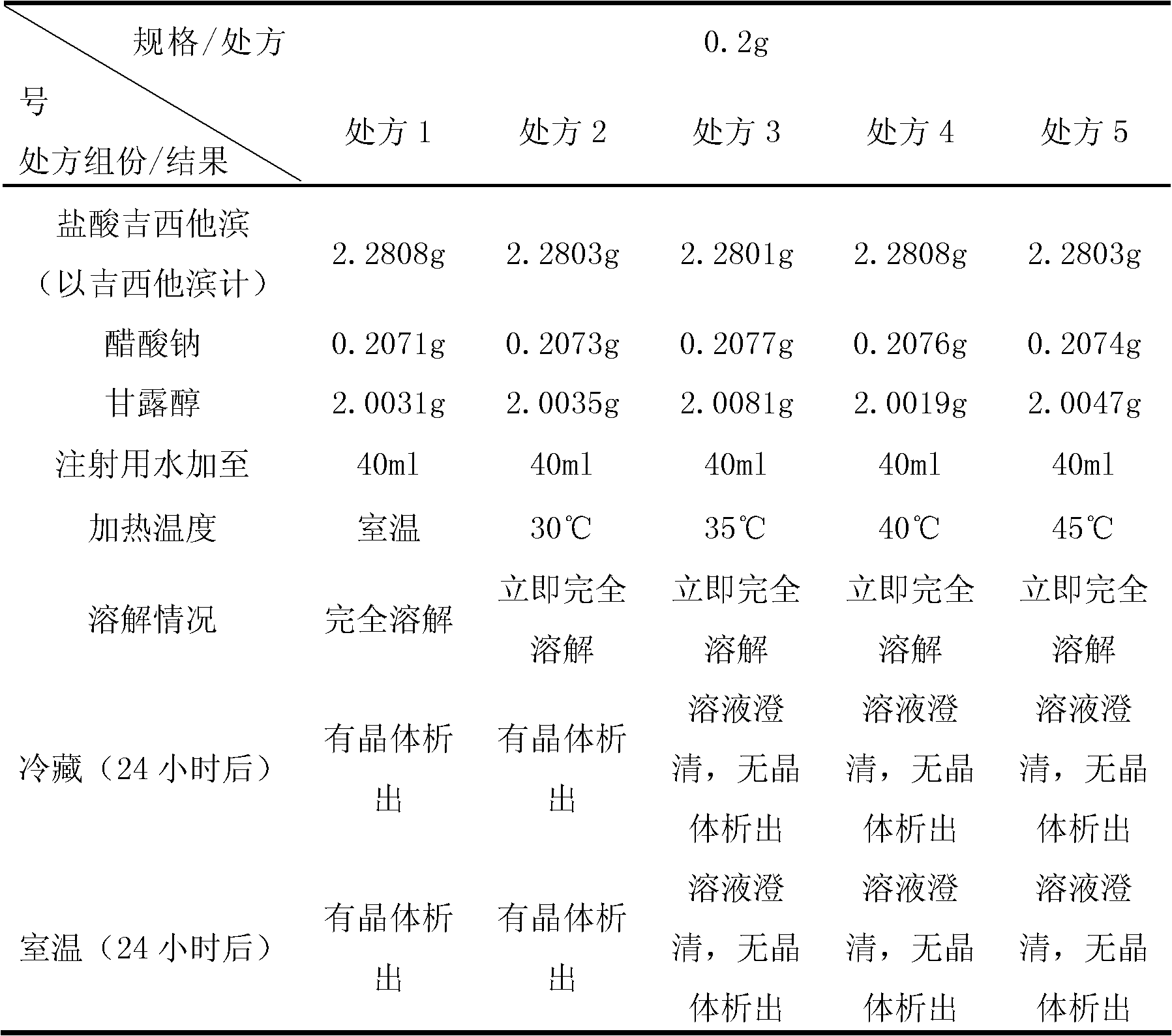
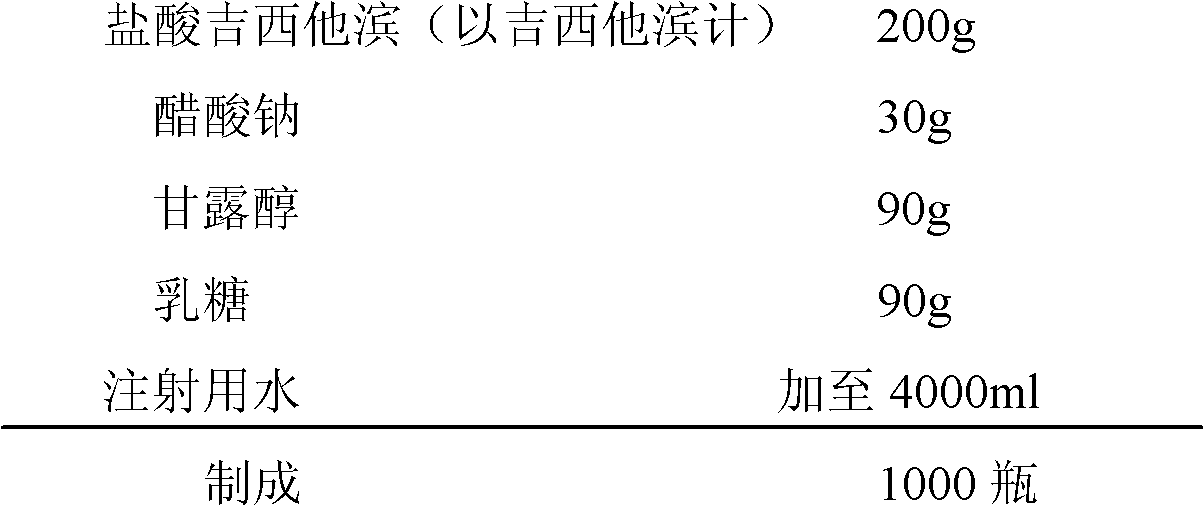
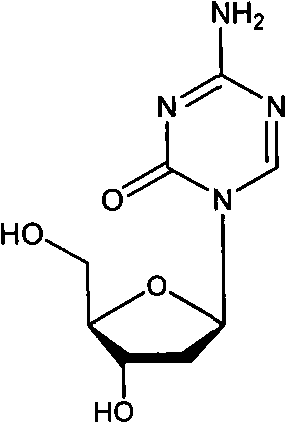
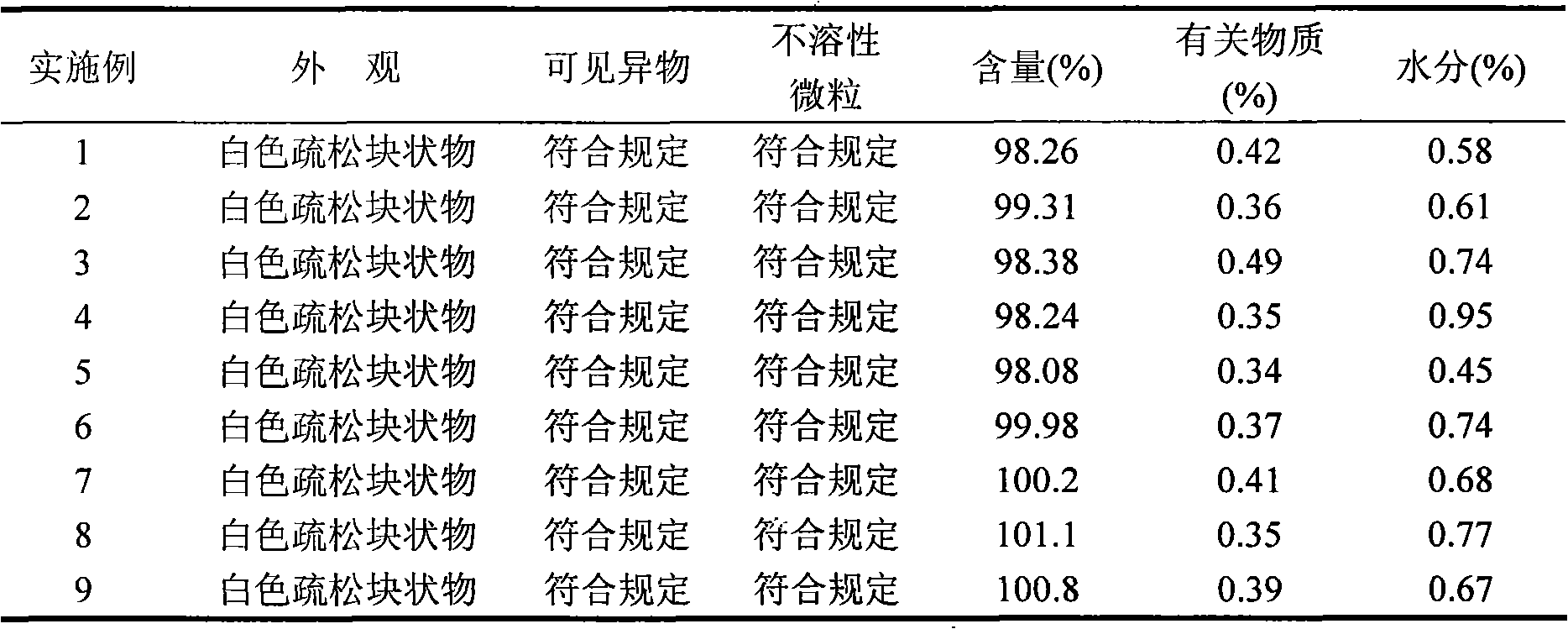
Gemcitabine hydrochloride lyophilized powder injection
ActiveCN101564381AImprove stabilityLow content of related substancesPowder deliveryOrganic active ingredientsPancreas CarcinomaDrug
The invention relates to a gemcitabine hydrochloride lyophilized powder injection and a preparation method thereof. The gemcitabine hydrochloride lyophilized powder injection prepared by the method can be used as a therapeutic medicament for treating middle and late non-small cell lung cancer, pancreatic cancer and the like. The gemcitabine hydrochloride lyophilized powder injection is characterized by consisting of gemcitabine hydrochloride, mannitol and sodium acetate, wherein the weight ratio of the gemcitabine hydrochloride to the mannitol is 1:0.5-5, and the weight ratio of the gemcitabine hydrochloride to the sodium acetate is 1:0.01-0.1. The preparation method comprises the following steps: taking the mannitol and the sodium acetate; dissolving the mannitol and the sodium acetate by adding injection water; adding the gemcitabine hydrochloride to the mixture, stirring and dissolving the mixture, and adjusting the pH to between 2.7 and 3.3; fixing the volume; filtering the product by a 0.22 mu m microporous membrane; filling, dishing up, lyophilizing, and compressing; taking the product out of a box, and tying the product with an aluminum-plastic composite cover; and inspecting the quality, and packaging the product after passing the quality inspection to obtain the gemcitabine hydrochloride lyophilized powder injection.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
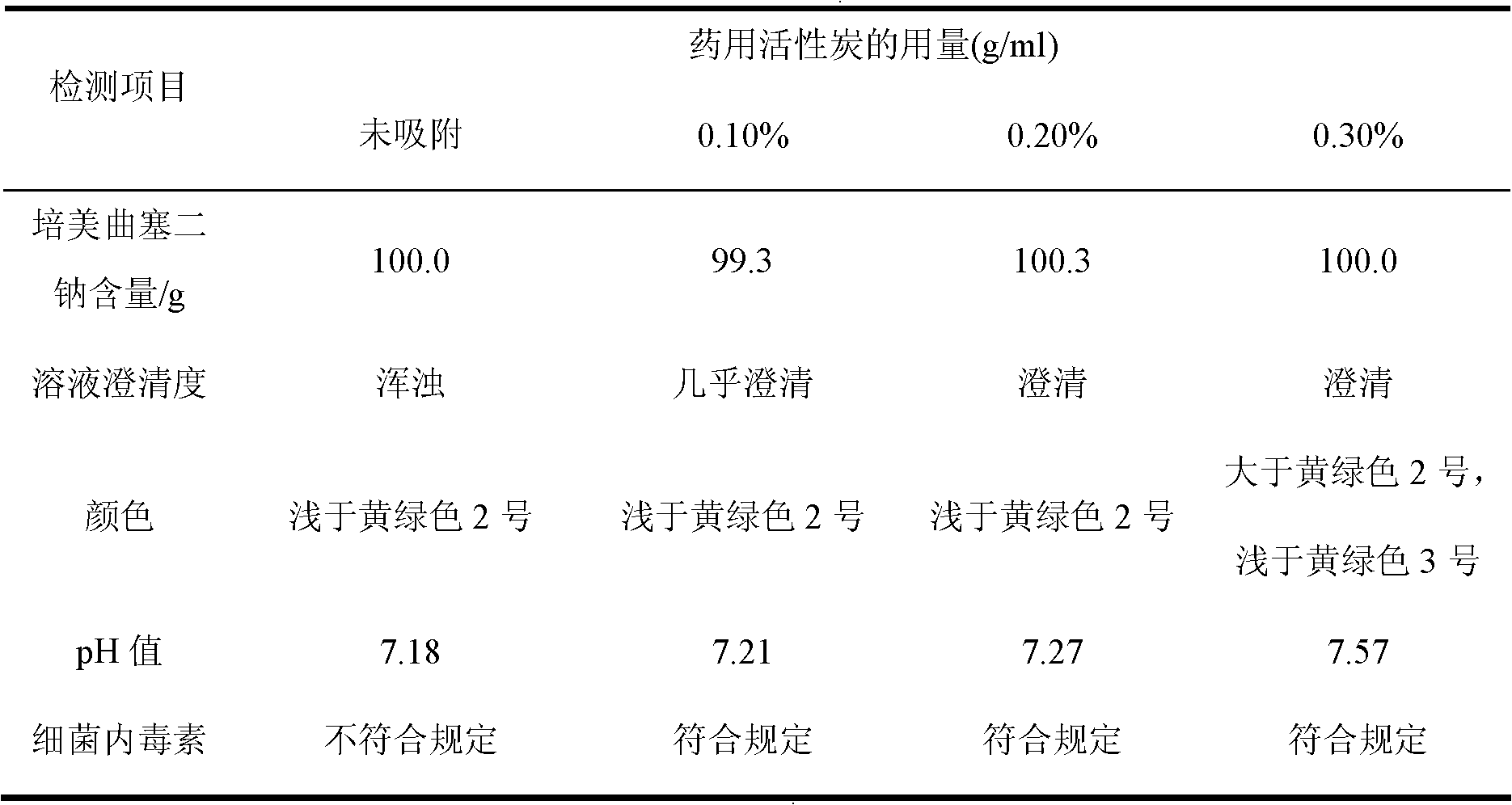
Pemetrexed disodium freeze-dried powder injection and preparation method thereof
ActiveCN102106833AReduce adverse effectsSimple prescriptionOrganic active ingredientsPowder deliveryActivated carbonFreeze-drying
The invention belongs to the technical field of medication, and in particular relates to a pemetrexed disodium freeze-dried powder injection and a preparation method thereof. The pemetrexed disodium freeze-dried powder injection consists of pemetrexed disodium and mannitol, wherein the mass ratio of the mannitol to the pemetrexed disodium is (0.6-2.0):1. The preparation method comprises the following steps: adding injecting water into a liquid preparation tank; adding the pemetrexed disodium weighted according to the formula; stirring until the pemetrexed disodium completely dissolved; adding the mannitol; regulating the pH by utilizing a hydrochloric acid solution or a sodium hydroxide solution; adding activated carbon for decoloration; filtering to remove the carbon; finely filtering with a filter membrane; subpackaging; and freezing and drying. The pemetrexed disodium freeze-dried powder injection has excellent moldability; the appearance of the solution before freezing is clear; the frozen and dry product has good re-dissolubility; and the re-dissolved product has the advantages of good clarity, low impurity content, low moisture content, good stability and controllable quality.
Owner:HAINAN JINRUI PHARMA
Freeze dried combination of Lansoprazole available for linjection and preparation method
InactiveCN1660091AImprove product qualityDoes not reduce efficacyPowder deliveryOrganic active ingredientsLansoprazoleFreeze-drying
A freeze-dried powder injection of lansoprazole is prepared from lansoprazole, cosolvent, alkaline amino acid and water proportionally.
Owner:龙蓓
Freeze-dried minocycline hydrochloride powder injection and its preparing process
A freeze dried minocycline hydrochloride powder as antibacterial injection is prepared from minocycline hydrochloride (0.05-10 wt.portions), freeze dried power supporting agent (10-100) and pH regulator. Its advantages are broad antibacterial sprectrum, high stability to light, heat, oxygen and water, and no pollution.
Owner:于航
Oxiracetam for injection and preparation method thereof
ActiveCN104069074AGood lookingRapid reconstitutionOrganic active ingredientsPowder deliveryFreeze-dryingPowder injection
The invention discloses oxiracetam freeze-dried powder injection and a preparation method thereof; and the oxiracetam freeze-dried powder injection disclosed by the invention is prepared by comprising the following steps: dissolving oxiracetam in water for injection, adding ethanol which is 1.2-2.0% (V / V) of the total volume, preparing into a 200-500 mg / ml oxiracetam solution, and freeze-drying. The oxiracetam freeze-dried powder injection disclosed by the invention is prepared by adopting a proper amount of ethanol with the help of the special freeze-drying process, particularly a special pre-freezing manner; pre-freezing process parameters are established, therefore, the surface of the solution is crystallized at first; the solute on the surface is prevented from being accumulated; and the oxiracetam freeze-dried powder injection prepared by the invention is basically free from accessories, rapid to re-dissolve, good in quality and steady for storage.
Owner:YAOPHARMA CO LTD
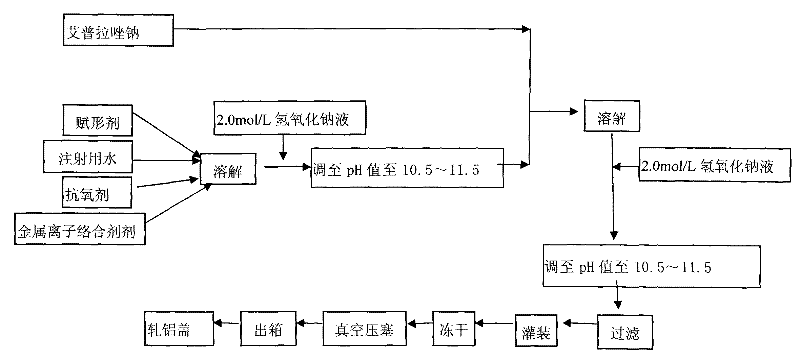
Powder injection for treating peptic ulcers and preparation method thereof
The invention provides an injection for treating a peptic ulcers and a preparation method thereof. The powder injection provided by the invention comprises the active ingredients of Ilaprazole sodium and excipient, wherein the ratio of the both in parts by weight is (1:1)-(1:30), and the preferable ratio is (1:10)-(1:18). The preferable prescription of the powder injection provided by the invention comprises 1 part of Ilaprazole sodium, 1-30 parts of excipient, 0-10 parts of antioxidant and / or 0-0.3 part of metal ion complexing agent; and a right amount of inorganic base is added to regulate the pH value to 9.0-12.0. The Ilaprazole sodium freeze-dried powder injection provided by the invention has stable quality, and is suitable for treating peptic ulcer bleeding and stress ulcers and preventing upper gastrointestinal bleeding caused by serious diseases.
Owner:LIVZON PHARM GRP INC
Aluminum alloy material and production method of cast-rolling stock of decorative strip of same
The invention relates to a 3004 aluminum alloy material and a production method of a cast-rolling stock of a decorative strip of the 3004 aluminum alloy material. The compositions of the aluminum alloy material comprise smaller than or equal to 0.25 percent of Si, 0.20 to 0.60 percent of Fe, 0.10 to 0.15 percent of Cu, 1.0 to 1.20 percent of Mn, 0.85 to 1.25 percent of Mg, smaller than or equal to 0.05 percent of Ti, and the remaining amount of Al. The production method comprises the steps of smelting, refining, on-line degassing, grain refinement, filtration, cast rolling, and the like; during the smelting, 50 percent to 60 percent of electrolytic aluminum liquid and the rest of aluminum alloy waste materials are mixed and smelted; the compositions except Mg are added to the mixture; and after the steps of powder injection refining, slag skimming, magnesium ingot adding, furnace turning-down and refining are conducted, the stock of the strip is cast-rolled. A product of the production method has even and steady chemical compositions and can satisfy the mechanical property requirements of subsequent rolling, so that the yield of subsequent rolling is increased; by using the aluminum alloy waste materials as raw materials, the raw materials are saved; by directly adding the electrolytic aluminum liquid, gas loss in the melting process is reduced, and burning loss in the waste material melting process can also be decreased; and by using a cast-rolling manner to replace a hot-rolling manner, the process is simplified and the production cost is greatly reduced.
Owner:沁阳市鼎建建设发展有限公司
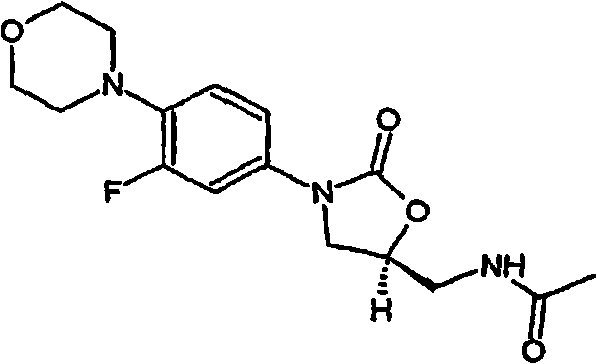
Preparation method of linezolid and preparation thereof
InactiveCN101948442AImprove stabilityExtended shelf lifeAntibacterial agentsPowder deliveryFreeze-dryingNitrobenzene
The invention relates to a preparation method of linezolid and a preparation thereof, in particular to a preparation method of a linezolid raw material and a freeze-dried powder injection thereof. The preparation method mainly comprises the following steps: (1) synthesizing 2(S)-1-amino-3-chloro-2-propanol hydrochloride; (2) synthesizing (S)-N-[2-acetoxyl-3-chloropropyl] acetamide; (3) synthesizing 3-fluoro-4-morpholinyl nitrobenzene; (4) synthesizing N-carbobenzoxy-3-fluoro-4-morpholinyl aniline; (5) synthesizing the linezolid; and (6) preparing the linezolid preparation. The freeze-dried powder injection of the linezolid for injection, which is prepared by the invention, effectively improves the stability of medicines so that the medicines have longer shelf life, can be transported more conveniently and are more favorable for clinical applications. Moreover, the method of the invention for preparing the raw material linezolid and the freeze-dried powder injection thereof is simple and effective and is suitable for large-scale industrial production.
Owner:符健
Fireproof electric meter box
InactiveCN107356793ASimple structureEasy to useTime integral measurementTemperature controlEngineering
The invention discloses a fireproof electric meter box, and the box comprises a housing. The interior of the housing is provided with a temperature control device, an independent power supply, and a plurality of independent fire extinguishing devices. Each fire extinguishing device comprises a powder injection apparatus and a fire extinguishing agent pot, wherein the powder injection apparatus comprises a pressing device and a nozzle, and the nozzle is disposed at one end of the fire extinguishing agent pot. Each pressing device comprises a hydraulic rod and a sealing valve, wherein the sealing valve is disposed in the corresponding nozzle, and one end of the hydraulic rod is fixedly connected with one end of the sealing valve, and the sealing valve comprises a supporting rod. The beneficial effects are that the box is simple in structure, is convenient to use, and can timely find and extinguish fire through the temperature control device and the fire extinguishing devices when fire is mall.
Owner:HEFEI DONGJIU ELECTRICAL CO LTD
Preparation method of polyinosinic acid-polycytidylic acid lyophilized powder injection
InactiveCN102988303ASlow down the rate of oxidative degradationLong validity periodOrganic active ingredientsPowder deliverySide effectOrganic chemistry
The invention discloses a preparation method of a polyinosinic acid-polycytidylic acid lyophilized powder injection. The method comprises the preparation of a polyinosinic acid-polycytidylic acid solution and a preparation of a lyophilized powder injection thereof. According to the invention, proper amounts of polyinosinic acid and polycytidylic acid are respectively dissolved by using normal saline; the mixtures are mixed and stirred, such that the polyinosinic acid-polycytidylic acid solution is prepared; a lyophilization additive is added and well mixed; and filtering, sub-packaging, and lyophilization are carried out. The prepared injection is suitable for frost preservation under a temperature below 0 DEG C. Therefore, expiration date is postponed, oxidative degradation speed is reduced, toxic and side effect are reduced, and stability is improved.
Owner:天津泽世德生物医药有限公司
Clindamycin phosphate freeze-dried powder needle and preparation thereof
InactiveCN101301278ASimple recipeLittle side effectsAntibacterial agentsPowder deliverySide effectFreeze-drying
The invention provides a clindamycin phosphate freeze-dried powder injection, which is prepared by the steps that: clindamycin phosphate solution is added with NaOH and is freeze-dried, wherein, the weight ratio of clindamycin phosphate to the NaOH is between 12 and 18 to 1, and the preferred weight ratio is 16.5 to 1. The clindamycin phosphate freeze-dried powder injection has simple formula and less auxiliary materials, overcomes side effects due to the fact that the auxiliary materials are excessively added, and ensures that patients are safer for use.
Owner:BEIJING JINGWEI SHUNKANG MEDICAL TECH DEV
Lansoprazole lyophilized powder injection and its preparing method
The present invention relates to a lansoprazole freeze-dried powder injection and its preparation method. Said lansoprazole freeze-dried powder injection contains ethylene diamine tetraacetic acid (EDTA) and / or its salt, and is made up by using active component lansoprazole, ethylene diamine tetraacetic acid and / or its salt, stabilizing agent, excipient, pH regulator and injection water through a certain preparation process. Besides, said invention also provides the concrete steps of said preparation process.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
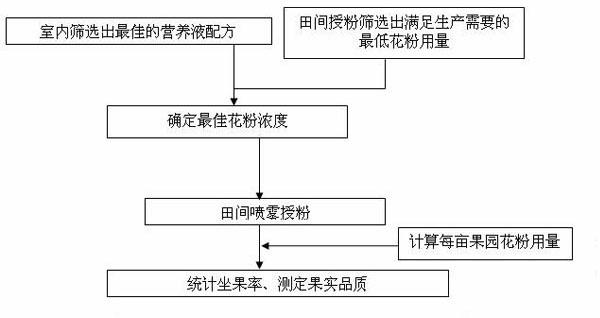
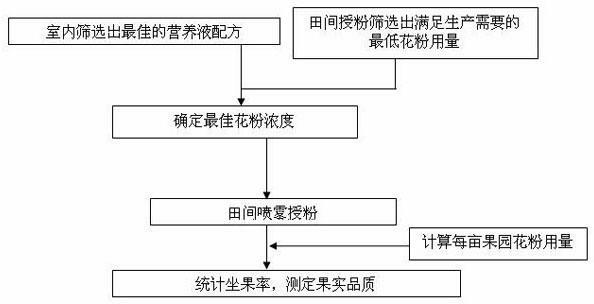
Method with effects of saving cost and improving efficiency for liquid pollination of pear tree
ActiveCN102318549AOvercoming swelling deathOvercome uniformity issuesPlant genotype modificationFruit treeSprayer
The invention provides a method with effects of saving cost and improving efficiency for liquid pollination of pear tree, which belongs to the fruit trees production technical field. The method comprises the following steps: preparing a nutrient solution in full-bloom stage of pear flowers, adding 0.8g pure pollen in every liter of nutrient solution, spraying and pollinating by an electrostatic sprayer to achieve the purpose of artificial pollination. The pollination method has the advantages of simple operation, fast pollination speed, uniform powder injection, concentration, accuracy, pollen and manual work saving, low cost and the like, and solves the practical problems that pollen can not be uniformly dissolved in water in liquid pollination of fruit trees, pollen is easy to adhere with the wall of a container, pollen is obstructed a nozzle, uneven powder injection causes poor pollination effect and the real application of liquid pollination can not be realized for a long time. According to the invention, the new collected pure pollen (germination rate is 60%-80%) in current year required by per mu is 8-11g. In addition, the method of the invention is capable of increasing the moisture and nutrient of pistil stigma and prolonging the fecundation time of stigma pollination, and the fruit quality can not be affected by increasing the fruit setting rate.
Owner:NANJING AGRICULTURAL UNIVERSITY
Gemcitabine hydrochloride lyophilized powder injection and preparation method thereof
ActiveCN102144981AReduce dosageImprove stabilityPowder deliveryOrganic active ingredientsSodium acetateAdjuvant
The invention relates to a gemcitabine hydrochloride lyophilized powder injection and a preparation method thereof. The lyophilized powder injection comprises the following components in parts by weight: 20-30 parts of gemcitabine hydrochloride, 5-9 parts of mannitol, and 3-10 parts of sodium acetate. The freeze-drying step includes the following three stages: a pre-freezing stage, a primary drying stage and a secondary drying stage, and the entire freeze-drying time is lower than 20 hours. The gemcitabine hydrochloride lyophilized powder injection provided by the invention has the advantages of less types and amounts of adjuvants, easily-controlled technological parameters, simple process route, short freeze-drying time, convenience in operation, good repeatability, low contents of related substances, and controllable quality; and the redissolved lyophilized powder injection has good clarity and forming performance. The lyophilized powder injection has stable and controllable quality, is easy to realize industrial production, and can generate considerable economic and social benefits.
Owner:HAINAN JINRUI PHARMA CO LTD
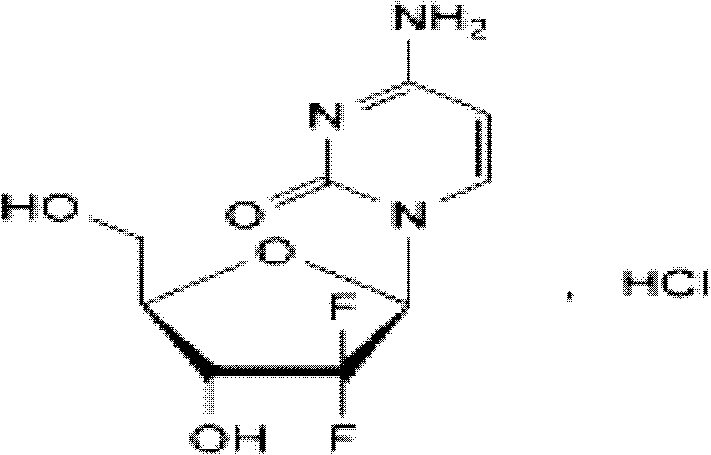
Decitabine freeze-dried powder injection
ActiveCN101584670AImprove stabilityLow content of related substancesOrganic active ingredientsPowder deliveryPhosphateFreeze-drying
The invention relates to a decitabine freeze-dried powder injection and a preparing method thereof. The prepared decitabine freeze-dried powder injection is used for treating myelodysplastic syndrome (MDS). The decitabine freeze-dried powder injection contains decitabine, utilizes the mixed solvent composed of the tert-butyl alcohol and the injection water in the preparation process, wherein the concentration of the decitabine in the mixed solvents is 2.5-5 mg / ml; and the volume ratio of the solvents is: 5-50% of tert-butyl alcohol and the balance of injection water. The potassium dihydrogen phosphate and the sodium hydroxide may be added for the pH regulator. The preparation process comprises the following steps: measuring tert-butyl alcohol, adding injection water, potassium dihydrogen phosphate and sodium hydroxide, stirring and mixing evenly, cooling to 2-15 DEG C, heat preserving, adding decitabine, stirring to dissolve, filtering, filling, plugging, disking, freeze-drying, pressing plug, out box, tying and packing after quality test qualification.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Freeze dried powder injection of red peony root and safflower for treating cardiovascular and cerebrovascular disease and its preparation method
InactiveCN1569201AHigh content of active ingredientsQuality improvementPowder deliveryUnknown materialsDiseaseFreeze-drying
In accordance with the invention, water extraction method is employed to extract the effective components of safflower and radix paeoniae rubrathe, big porous resin adsorption method is employed for purification and refining.
Owner:北京神农坛医药科技有限公司
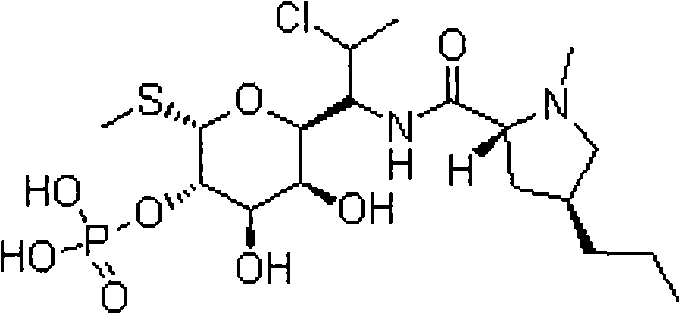
Method for preparing clindamycin phosphate powder injection raw medicine
ActiveCN101439022AChange the dosage ratioChange centrifugationAntibacterial agentsPowder deliverySolubilityClindamycin Phosphate
The invention discloses a preparation method for a clindamycin phosphate powder injection raw medicine which comprises the steps: (a) purification is carried out on a crude product of clindamycin phosphate; (b) crystallization and crystal growth are carried out on filter liquor after the filter liquor is arranged in a crystallization tank; (c) centrifugal separation and washing are carred on crystallization liquor; (d) fast temperature raising and drying are carried out on a clindamycin phosphate wet finished product. The preparation method for the clindamycin phosphate powder injection raw medicine has the advantages of favorable water solubility, uniform crystal form particles, small specific volume, big bulk density, low solvent residue, and the like. The method also simplifies the process operation, and dramatically enhances the product yield.
Owner:华北制药华胜有限公司
Oryzanol composition and its preparation method
ActiveCN100386082CStrong solubilizing abilityQuick effectPowder deliveryOrganic active ingredientsFreeze-dryingSURFACTANT BLEND
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Cefotiam hydrochloride medicament composition sterile powder injection and preparation method thereof
ActiveCN101584665AImprove stabilityHigh purityAntibacterial agentsOrganic active ingredientsCefotiam HydrochlorideSodium carbonate anhydrous
The present invention discloses a cefotiam hydrochloride medicament composition sterile powder injection, including 500 to 600 weight shares of cefotiam hydrochloride and 110 to 150 weight shares of anhydrous sodium carbonate. The cefotiam hydrochloride medicament composition sterile powder injection has advantages of a good stability and a high purity. The invention also discloses a method for preparing the cefotiam hydrochloride medicament composition sterile powder injection, including steps of weighing the cefotiam hydrochloride and the anhydrous sodium carbonate according to the formula amount separately and mixing them in a sterile container uniformly. The method is simple, and the cefotiam hydrochloride prepared by the above method has a good stability.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD
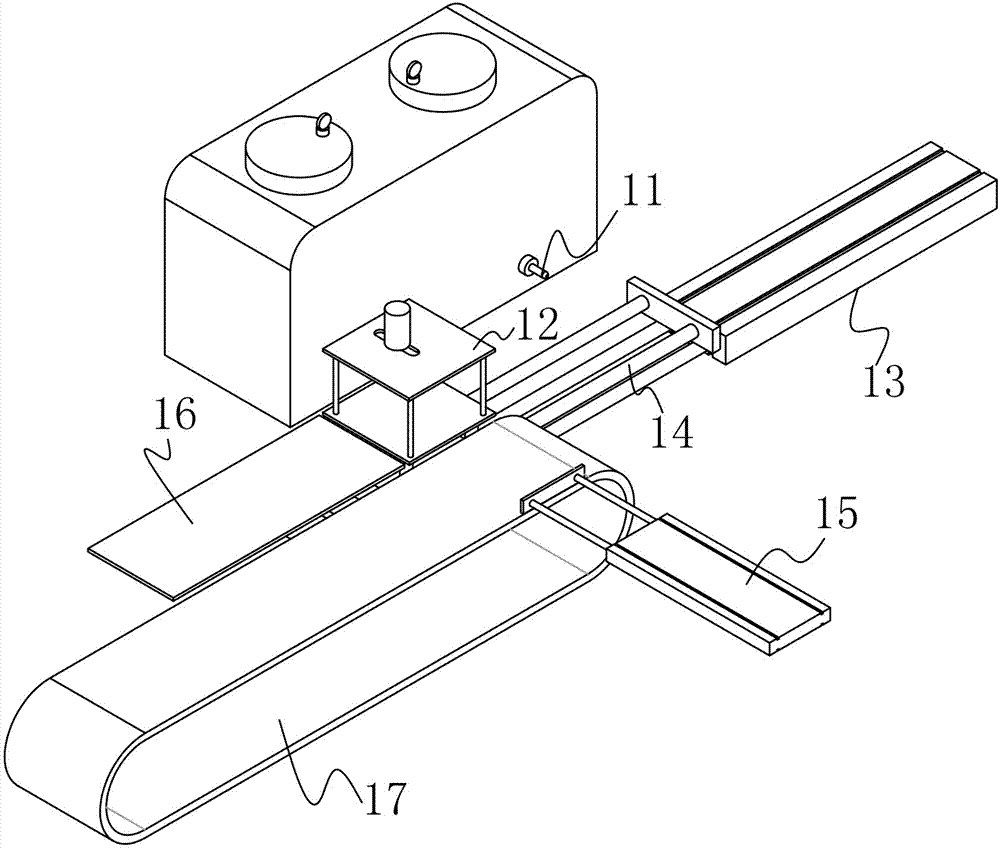
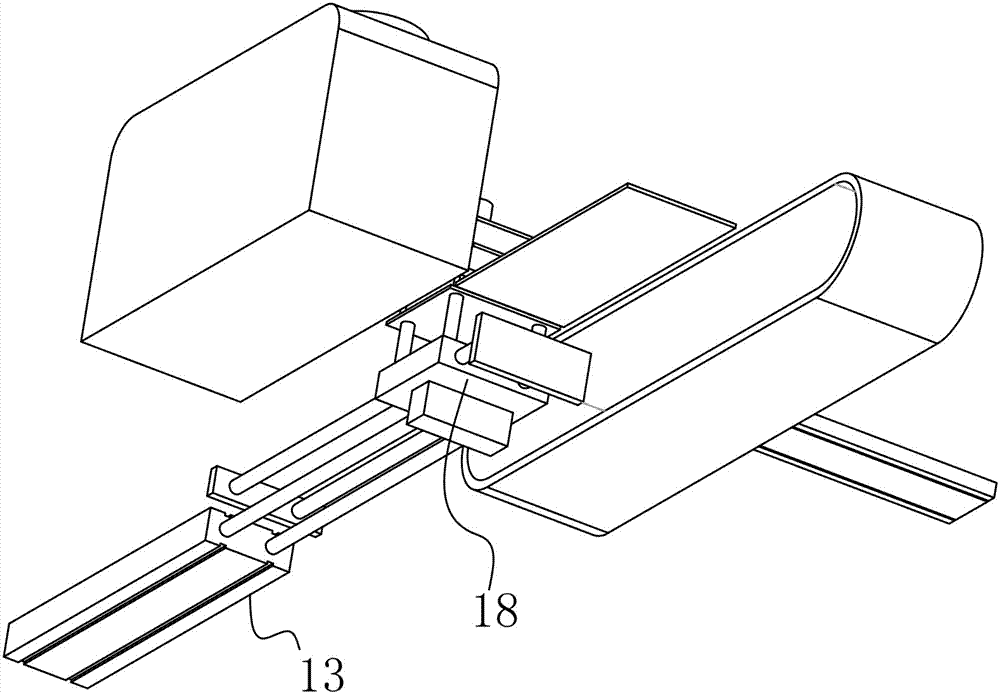
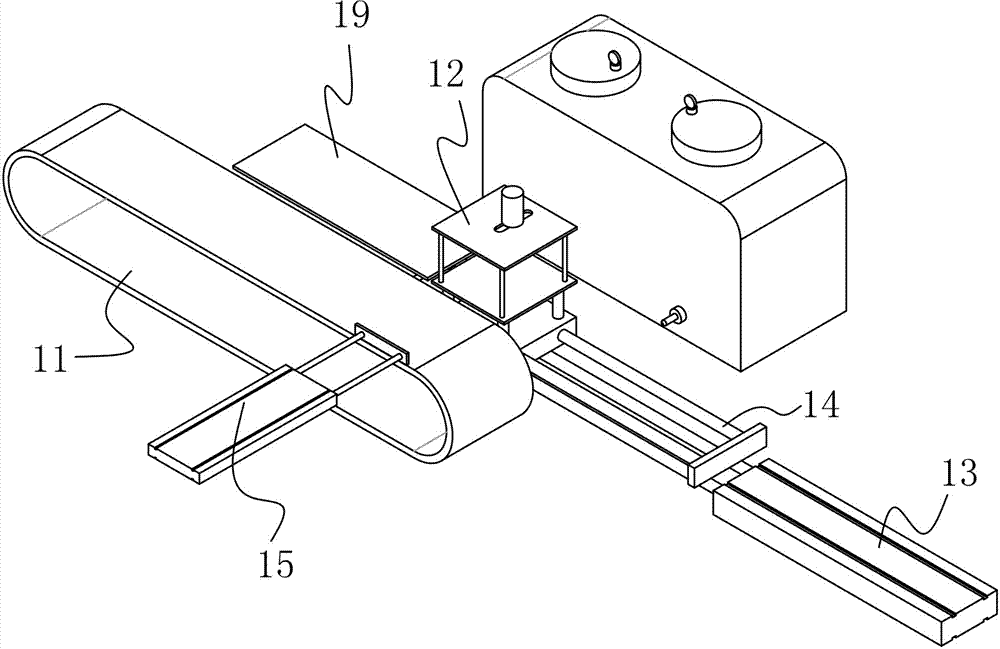
Powder-injection wax-injection integrated structure
The invention discloses a powder-injection wax-injection integrated structure. The integrated structure comprises a wax-injection area and a powder flapping area, wherein the wax-injection area comprises a wax injection nozzle capable of injecting wax; the powder flapping area comprises a powder blowing nozzle capable of blowing powder; the integrated structure also comprises a fixture structure similar to a clamp die and a driving device; the driving device can be used for pushing and pulling the fixture structure to the wax-injection area or the powder flapping area. A manual powder flapping operation procedure is replaced by a mechanical operation mode of firstly flapping the powder and then injecting the wax, the time is saved, the efficiency is improved, and the cost is reduced.
Owner:DONGGUAN SONGYAN ZHIDA IND DESIGN CO LTD
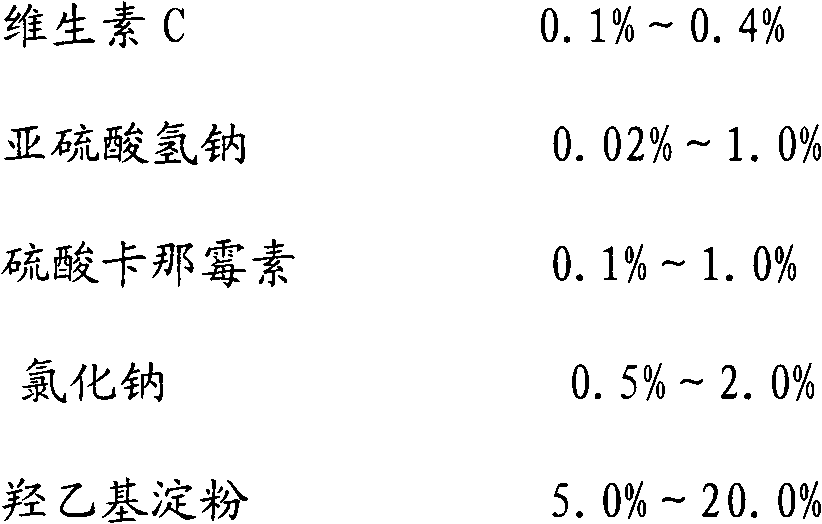
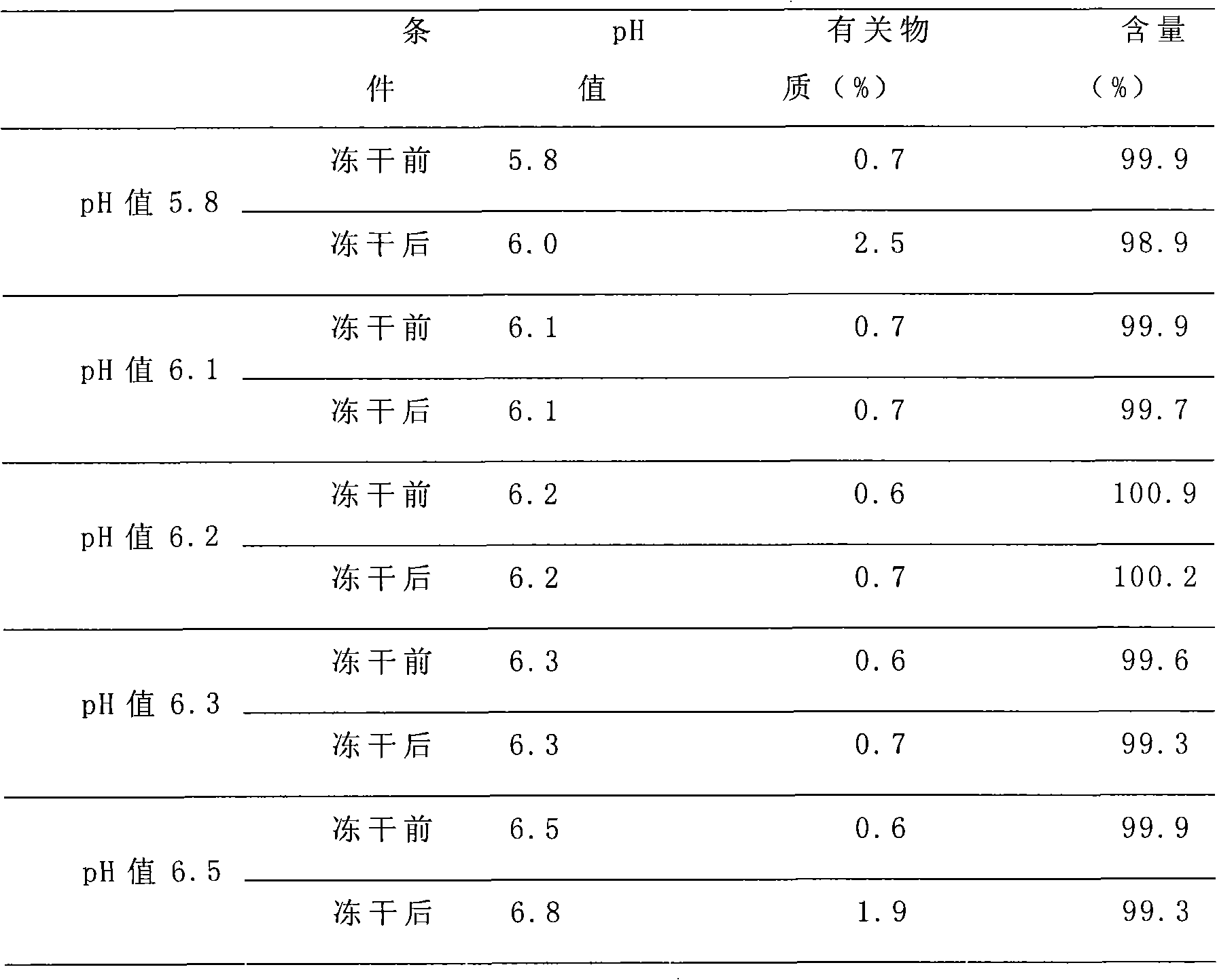
Stable S-oxiracetam preparation for injection and preparation method of same
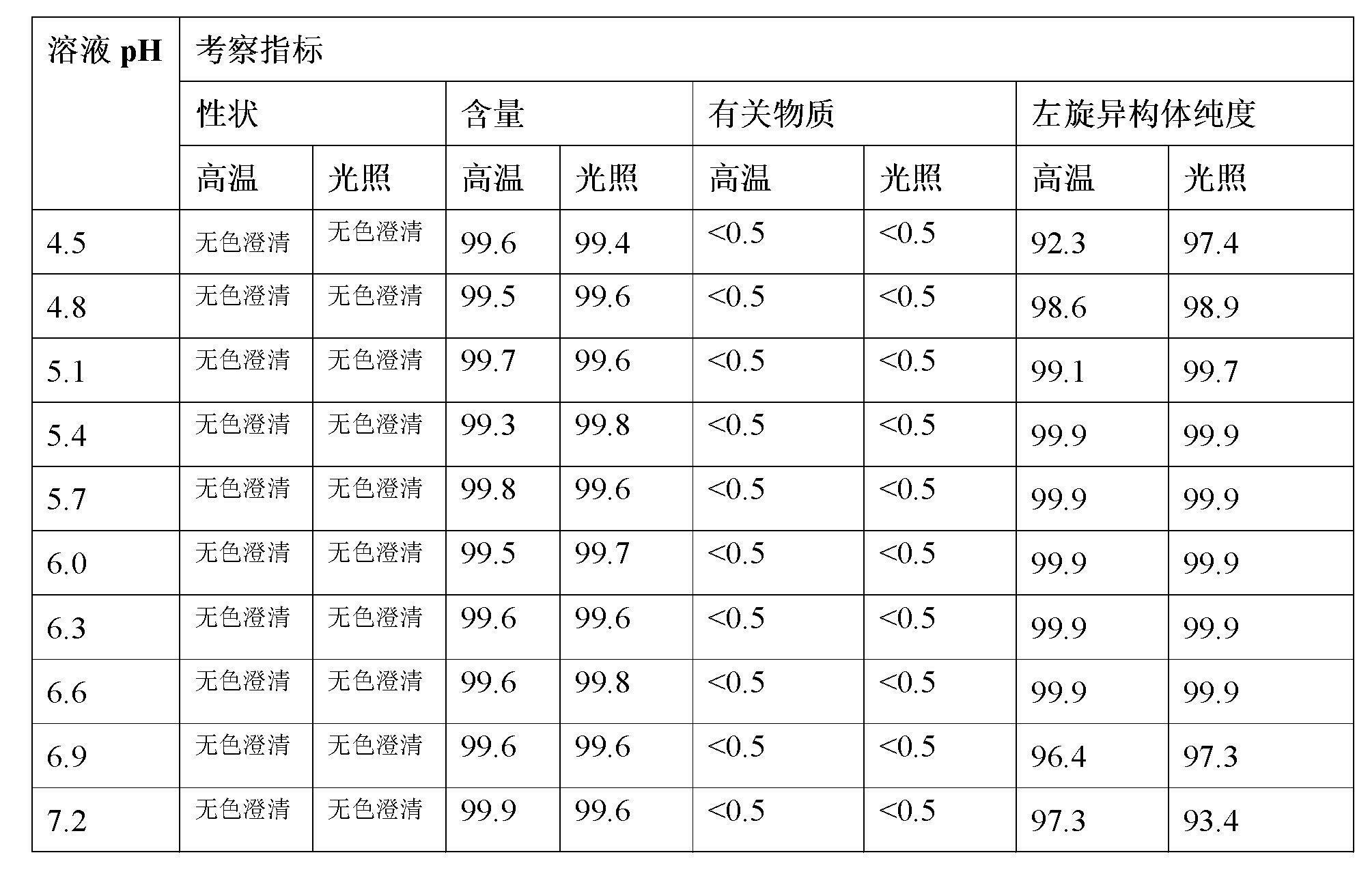
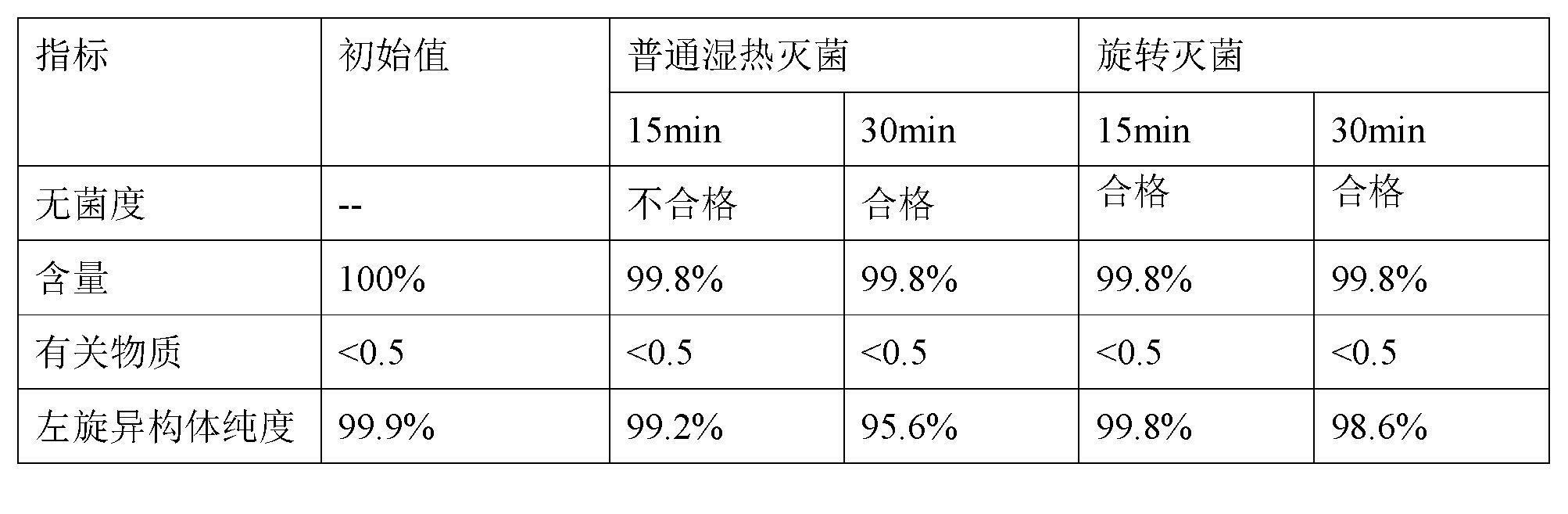
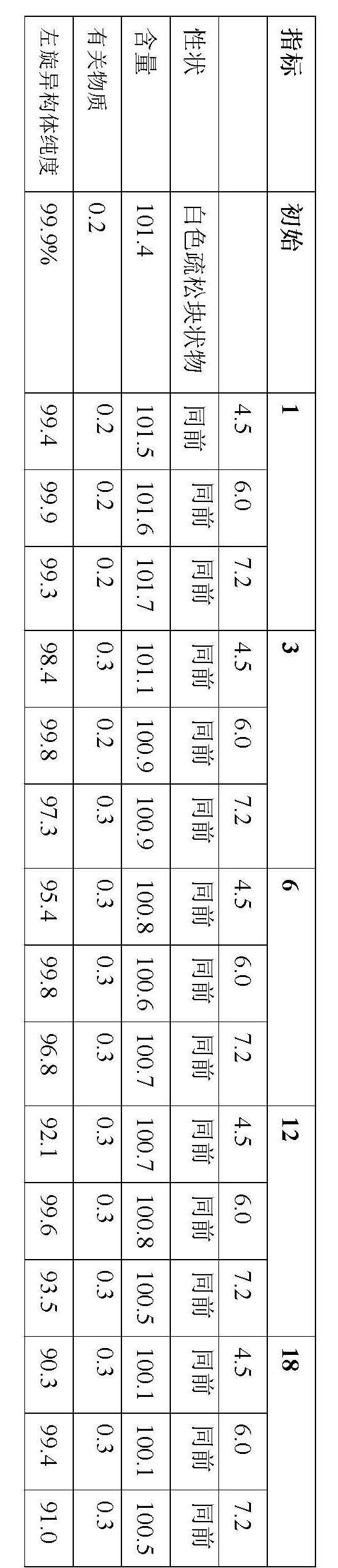
The invention relates to a stable preparation for injection by taking S-oxiracetam as active ingredient. The preparation is a composition for injection, and is formed by the S-oxiracetam or salts thereof serving as active ingredient and pharmaceutically acceptable auxiliary material. To restrain the racemization of the S-oxiracetam, when the powder injection with the active ingredient is prepared, and only the pH value of the S-oxiracetam medicament solution ranges from 4.5 to 7.0, the pH value is between 5.4 to 6.6 preferably, so that the S-oxiracetam medicament solution can be produced, and the final freeze-dried product has acceptable long stability; and moreover, when injection is produced, a rotary steam sterilization method needs to be adopted, terminal rotating sterilization is performed for 15 to 45 minutes under the temperature of 121 DEG C, and the terminal rotating sterilization is performed for 15 to 20 minutes preferably, so that the S-oxiracetam injection with high purity can be obtained and has acceptable long stability.
Owner:FUKANGREN BIO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com