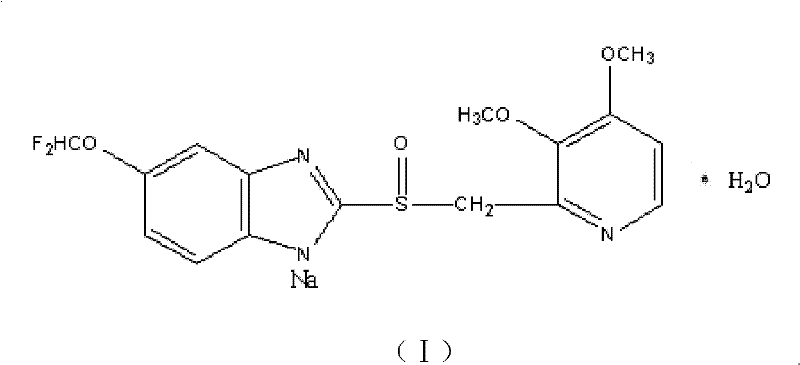
Pantoprazole sodium compound and pharmaceutical composition thereof
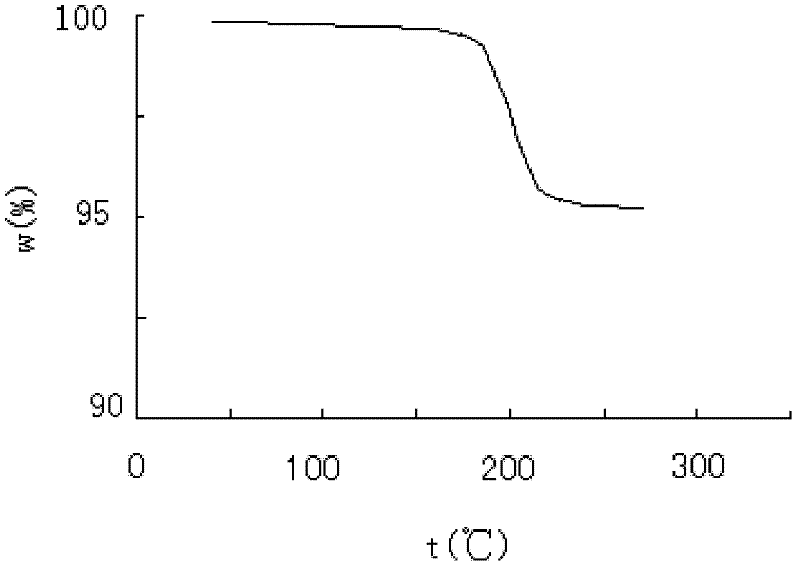
A pantoprazole sodium and compound technology, applied in the field of pantoprazole sodium compounds and pharmaceutical compositions thereof, can solve the problems of limited application, extremely strict requirements on preparation process parameters, limited degree of stability of pharmaceutical preparations, etc. The effect of water stabilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] [embodiment 1] the preparation of pantoprazole sodium compound
[0066] 1) Dissolve pantoprazole sodium powder in a water / methanol mixed solution with a volume ratio of 1:3.5, heat the mixed solution at 45°C, stir at a speed of 900 rpm for 20min, and then filter while hot to obtain the filtrate 1;
[0067] 2) Stir the filtrate 1 at a speed of 1350 rpm, while keeping the temperature of the filtrate 1 at 12.5° C., add pre-cooled diethyl ether to the filtrate 1 until the solution appears white turbidity, and obtain solution 2, wherein the temperature of the pre-cooled diethyl ether 12.5°C;
[0068] 3) Stop stirring, let the solution stand at 12.5°C for 215h to precipitate crystals, filter, wash the filter cake with pre-cooled ether, and vacuum dry the filter cake at 40°C for 18h to obtain the pantoprazole sodium compound.
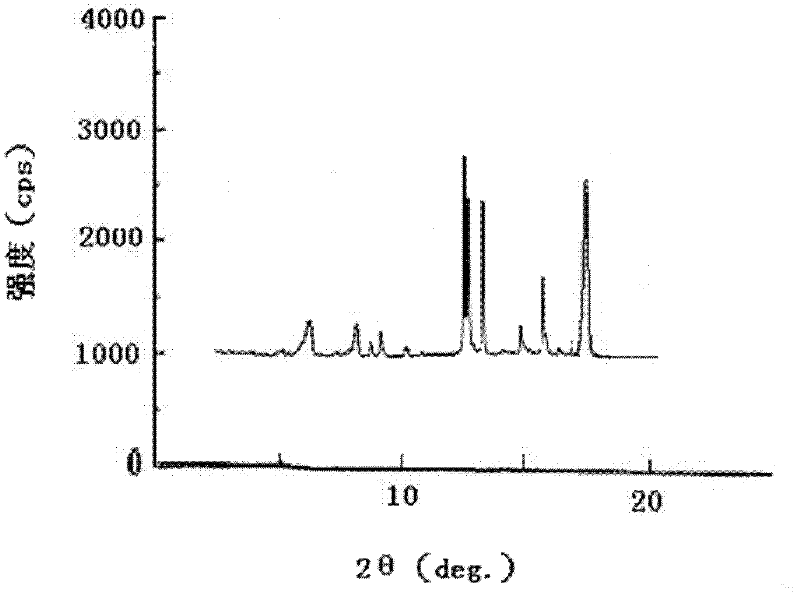
[0069] The obtained pantoprazole sodium compound uses the X-ray powder diffraction figure obtained by Cu-Kα ray measurement (see figure 1 ) in the c...
Embodiment 2
[0070] [embodiment 2] the preparation of pantoprazole sodium compound
[0071] 1) Dissolve pantoprazole sodium powder in a water / methanol mixed solution with a volume ratio of 1:5, heat the mixed solution at 40°C, stir at a speed of 1000 rpm for 20min, and then filter while hot to obtain the filtrate 1;
[0072] 2) Stir the filtrate 1 at a speed of 1500 rpm, while keeping the temperature of the filtrate 1 at 10° C., add pre-cooled diethyl ether to the filtrate 1 until the solution appears white turbidity, and obtain solution 2, wherein the temperature of the pre-cooled diethyl ether 10°C;
[0073] 3) Stop stirring, let the solution stand at 15° C. for 220 h to precipitate crystals, filter, wash the filter cake with pre-cooled ether, and vacuum dry the filter cake at 50° C. for 24 h to obtain the pantoprazole sodium compound.
[0074] The obtained pantoprazole sodium compound, its melting point and the X-ray powder diffraction pattern measured using Cu-K α rays are consistent...
Embodiment 3
[0075] [embodiment 3] the preparation of pantoprazole sodium compound
[0076] 1) Dissolve pantoprazole sodium powder in a water / methanol mixed solution with a volume ratio of 1:2, heat the mixed solution at 50°C, stir at a speed of 800 rpm for 20min, and then filter while it is hot to obtain the filtrate 1;
[0077] 2) Stir the filtrate 1 at a speed of 1200 rpm, while keeping the temperature of the filtrate 1 at 15°C, add pre-cooled ether to the filtrate 1 until the solution appears white turbidity, and obtain solution 2, wherein the temperature of the pre-cooled ether 15°C;
[0078] 3) Stop stirring, let the solution stand at 10° C. for 210 h to precipitate crystals, filter, wash the filter cake with pre-cooled ether, and dry the filter cake 12 under vacuum at 30° C. to obtain the pantoprazole sodium compound.
[0079] The obtained pantoprazole sodium compound, its melting point and the X-ray powder diffraction pattern measured using Cu-K α rays are consistent with those o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com