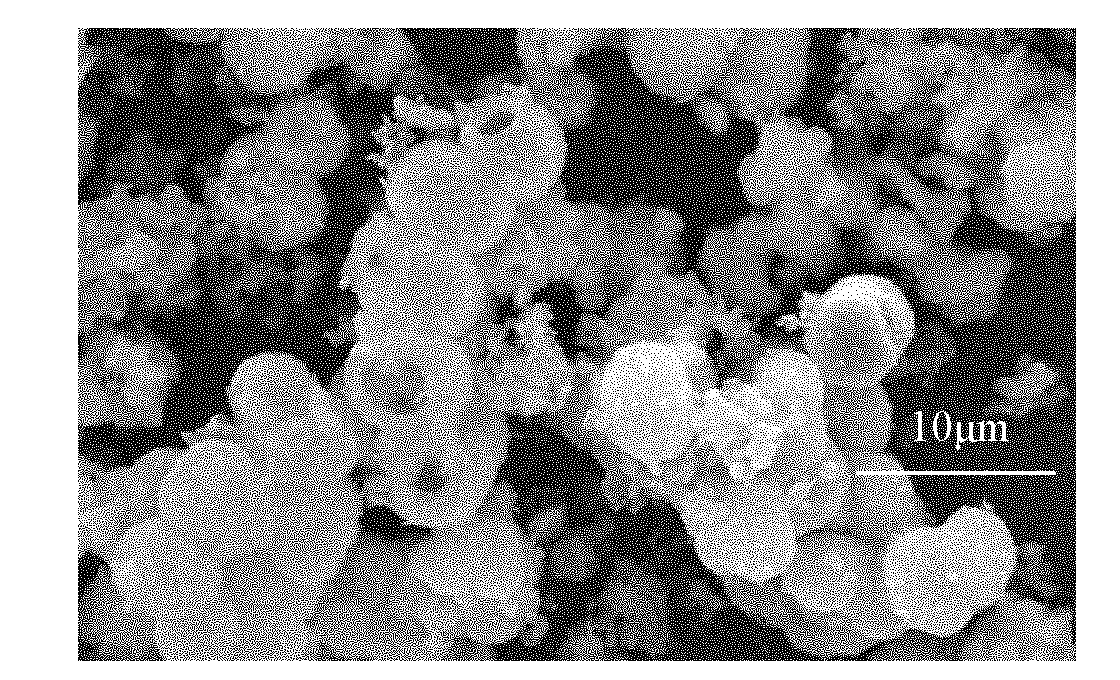Patents
Literature
2194 results about "Powder diffraction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Powder diffraction is a scientific technique using X-ray, neutron, or electron diffraction on powder or microcrystalline samples for structural characterization of materials. An instrument dedicated to performing such powder measurements is called a powder diffractometer.
Aggregate of carbon nanotubes, dispersion thereof and conductive film using the same
InactiveUS20090001326A1Good dispersionQuality improvementMaterial nanotechnologyNon-metal conductorsCombustionX-ray
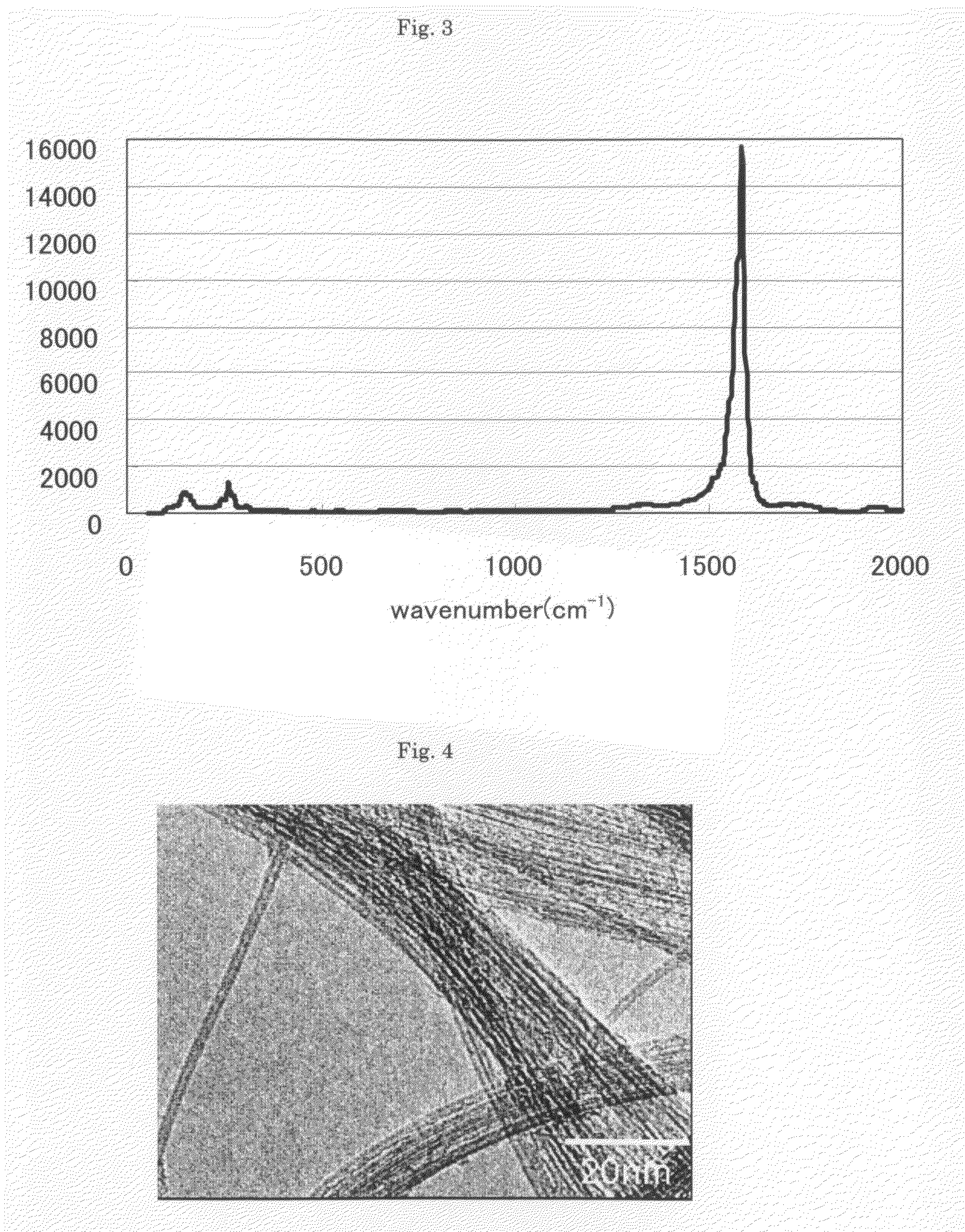
Provided is an aggregate of carbon nanotubes satisfying (1) there is a 2θ peak at 24°±2° by X-ray powder diffraction analysis; (2) a height ratio (G / D ratio) of G band to D band by Raman spectroscopic analysis of wavelength 532 nm is 30 or more; and (3) a combustion peak temperature is 550° C. or more, and 700° C. or less. The present invention provides an aggregate of carbon nanotubes excellent in dispersibility while high quality, giving a film, molded article, membrane or the like having excellent characteristics
Owner:TORAY IND INC
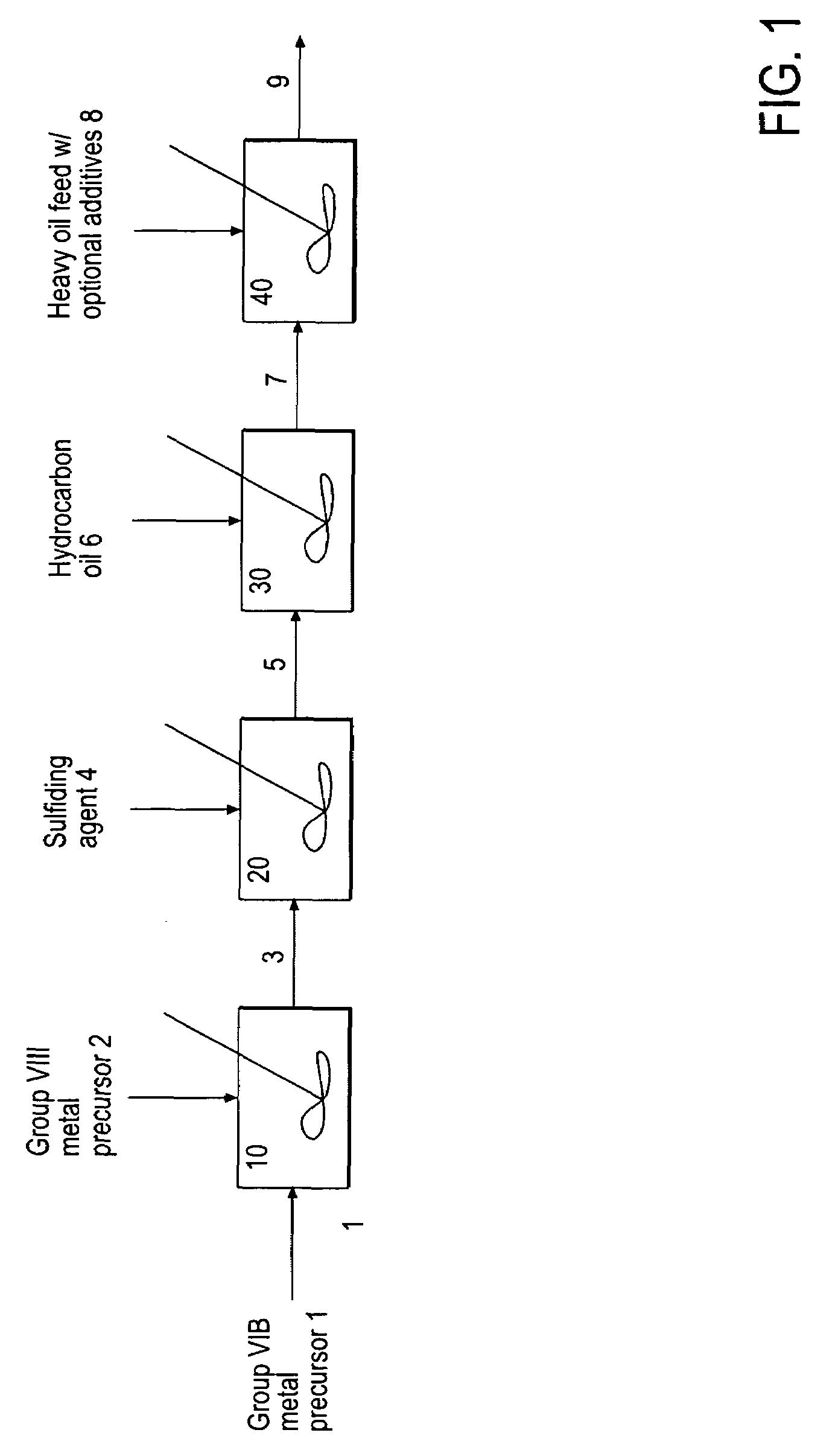
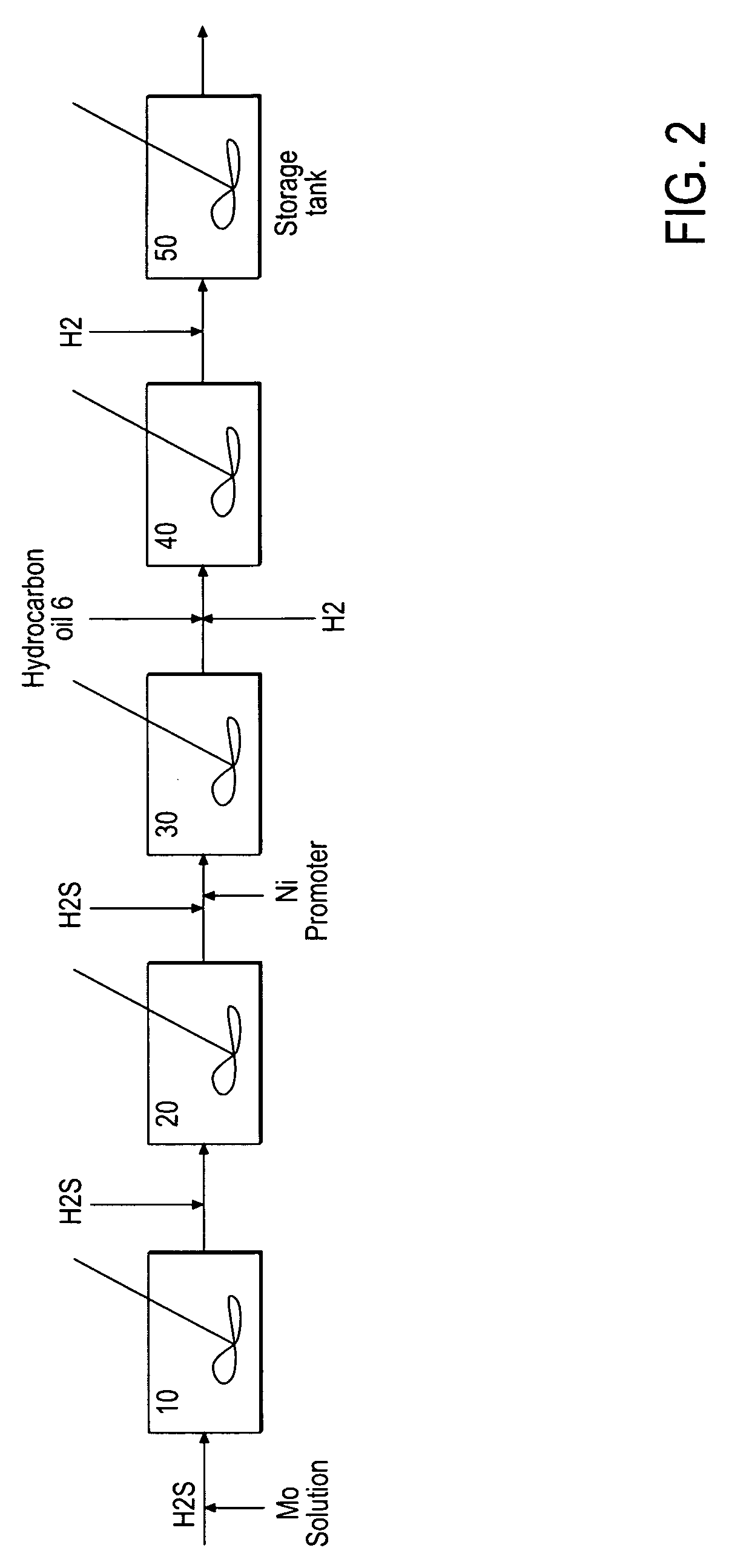
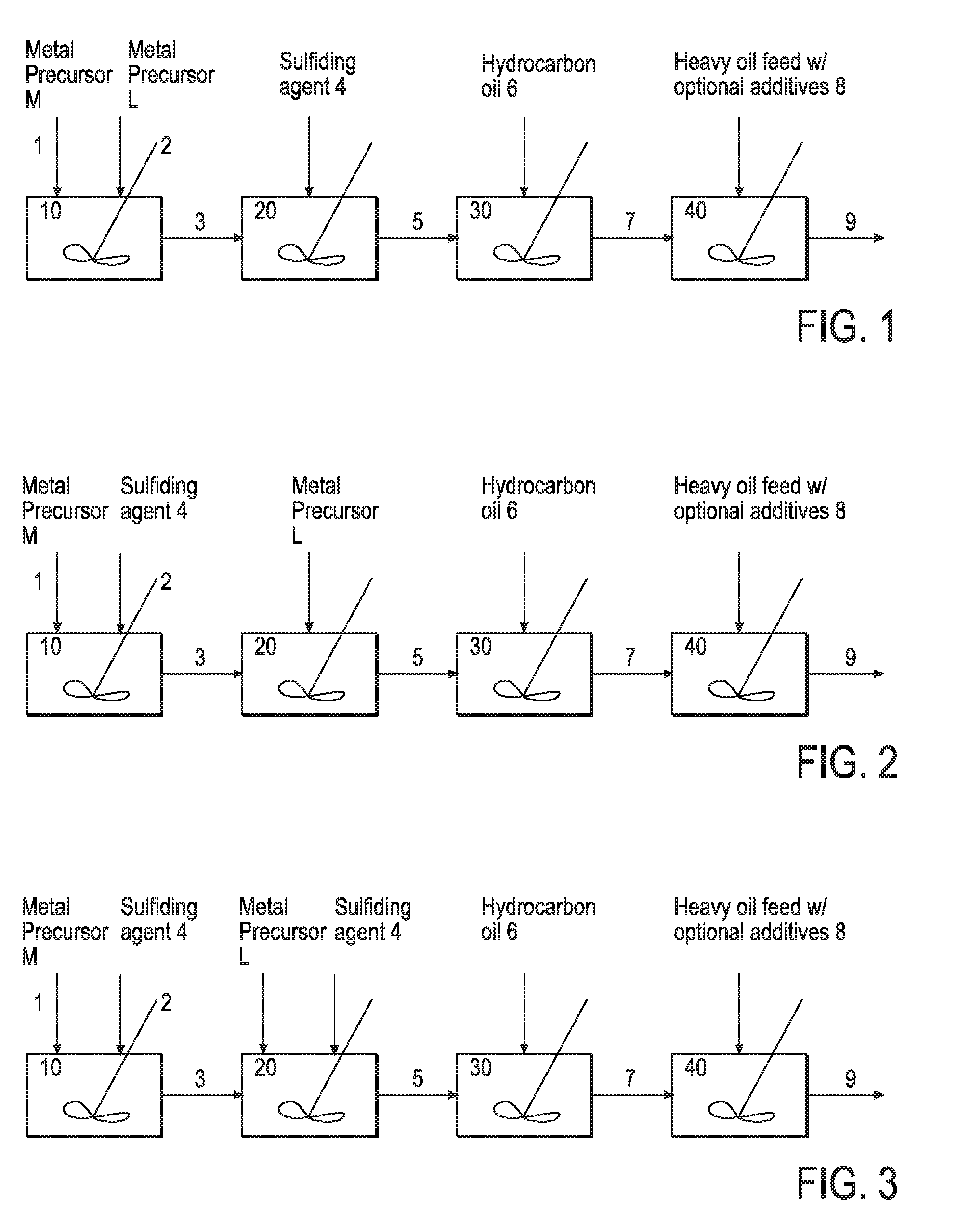
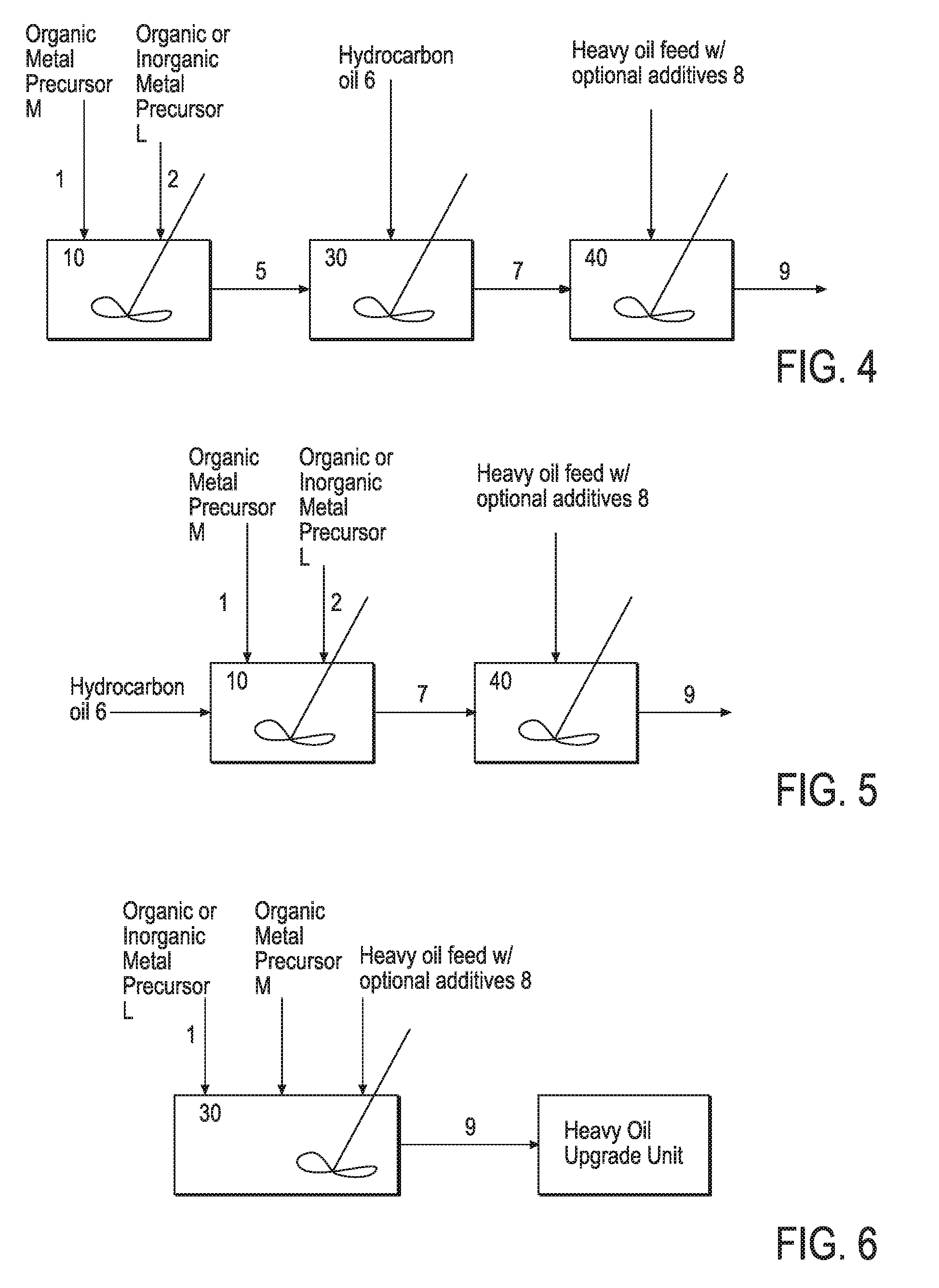
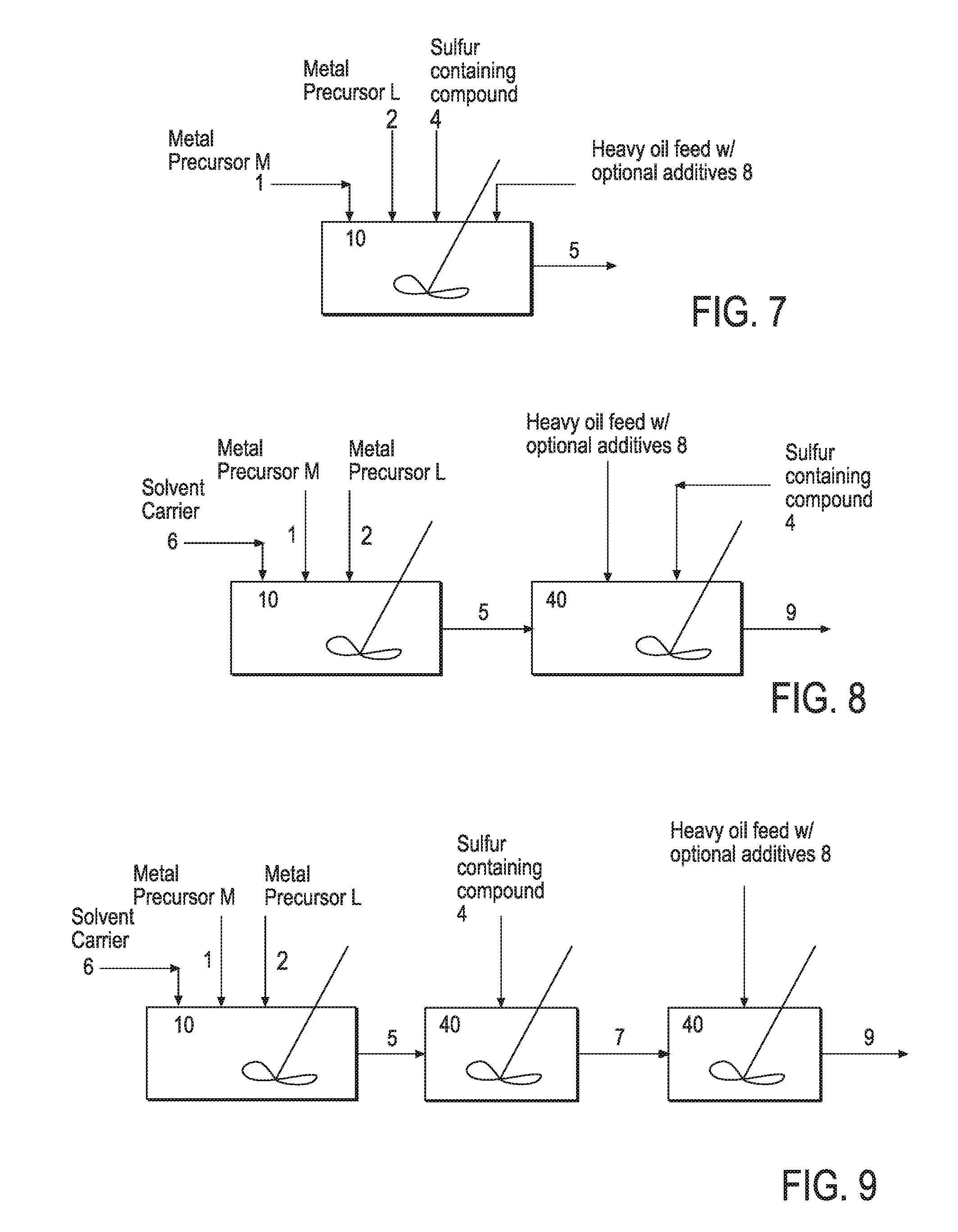
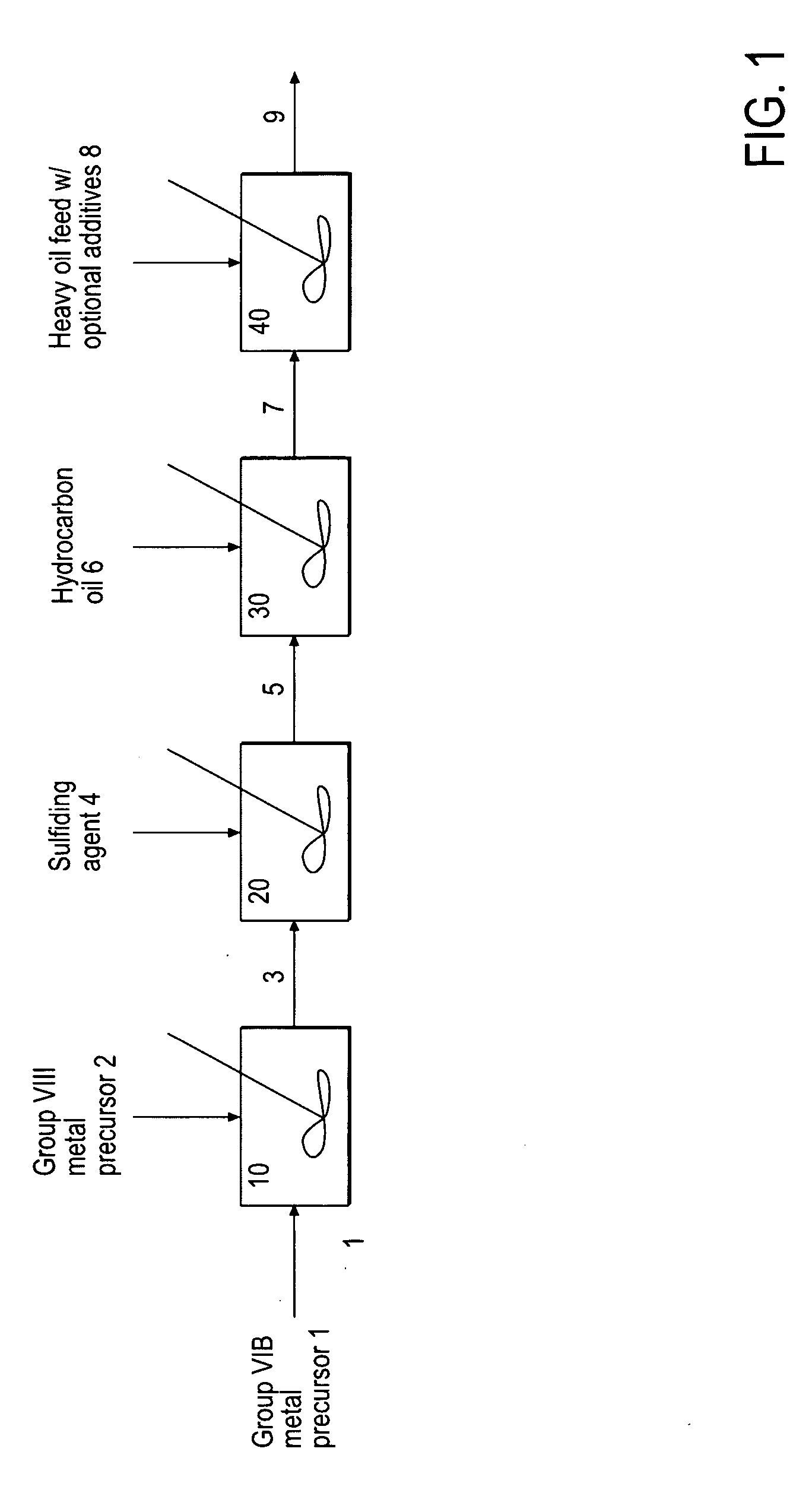
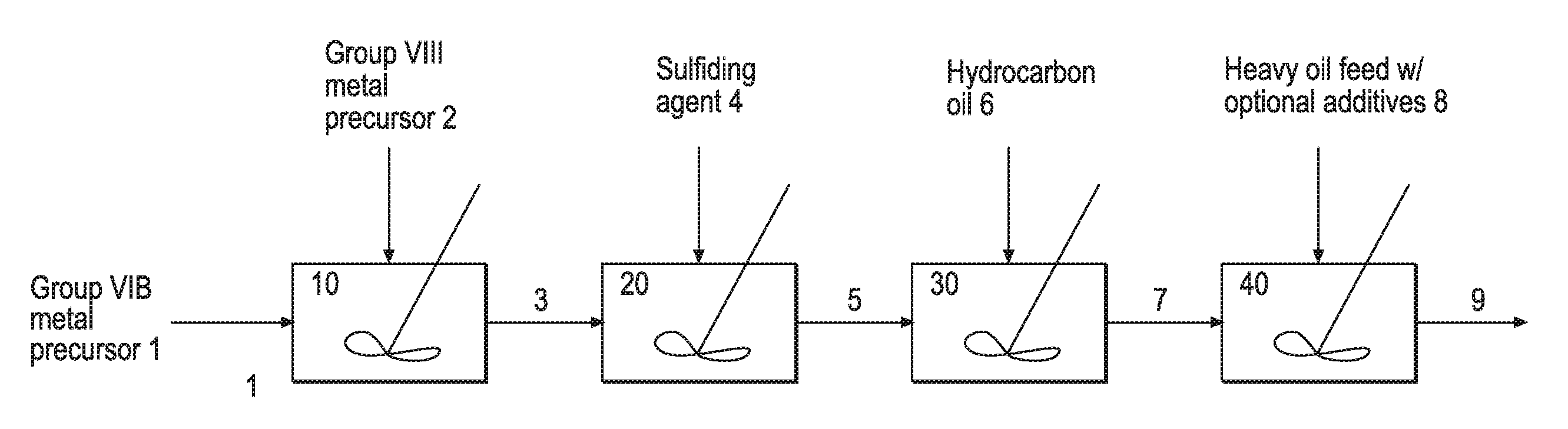
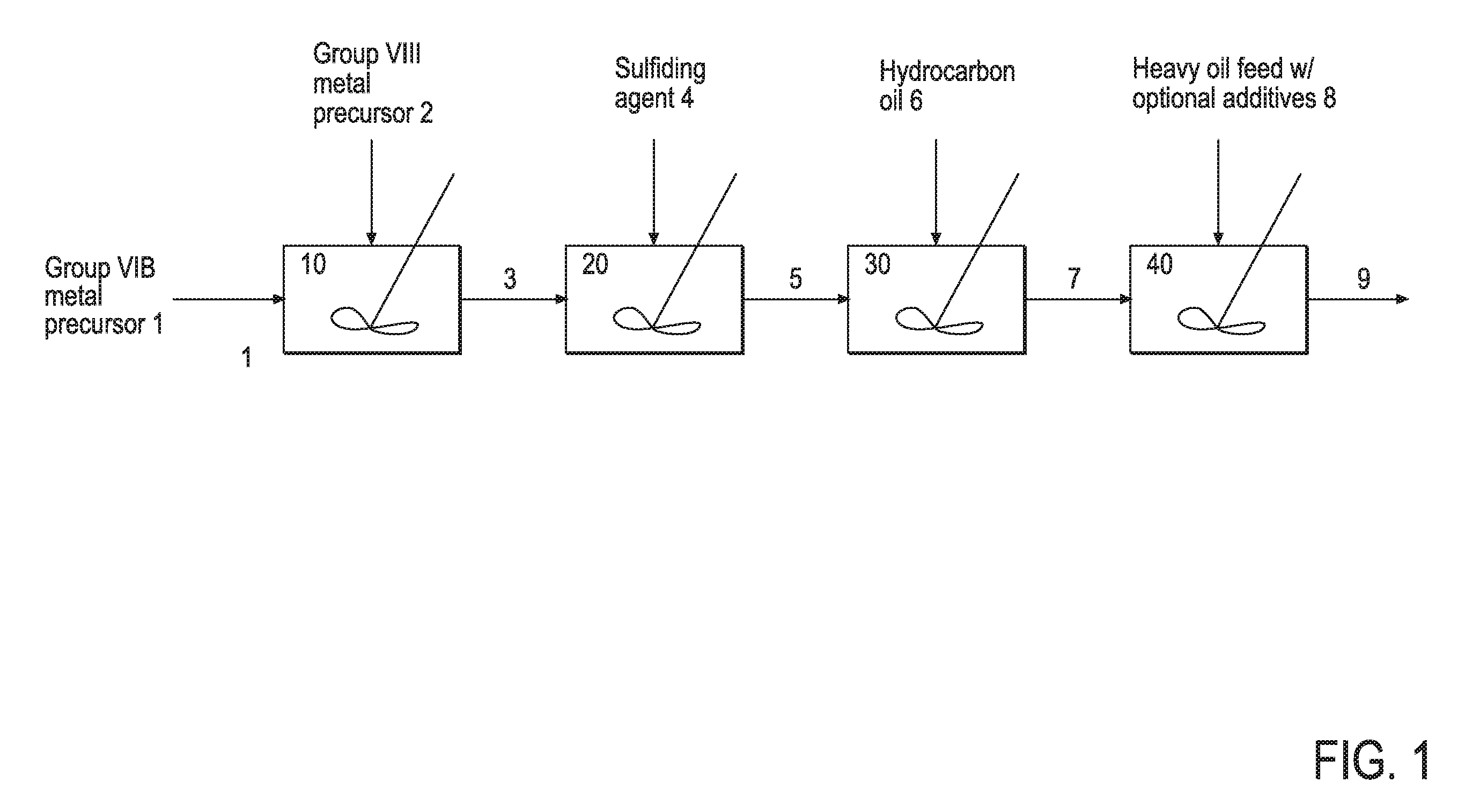
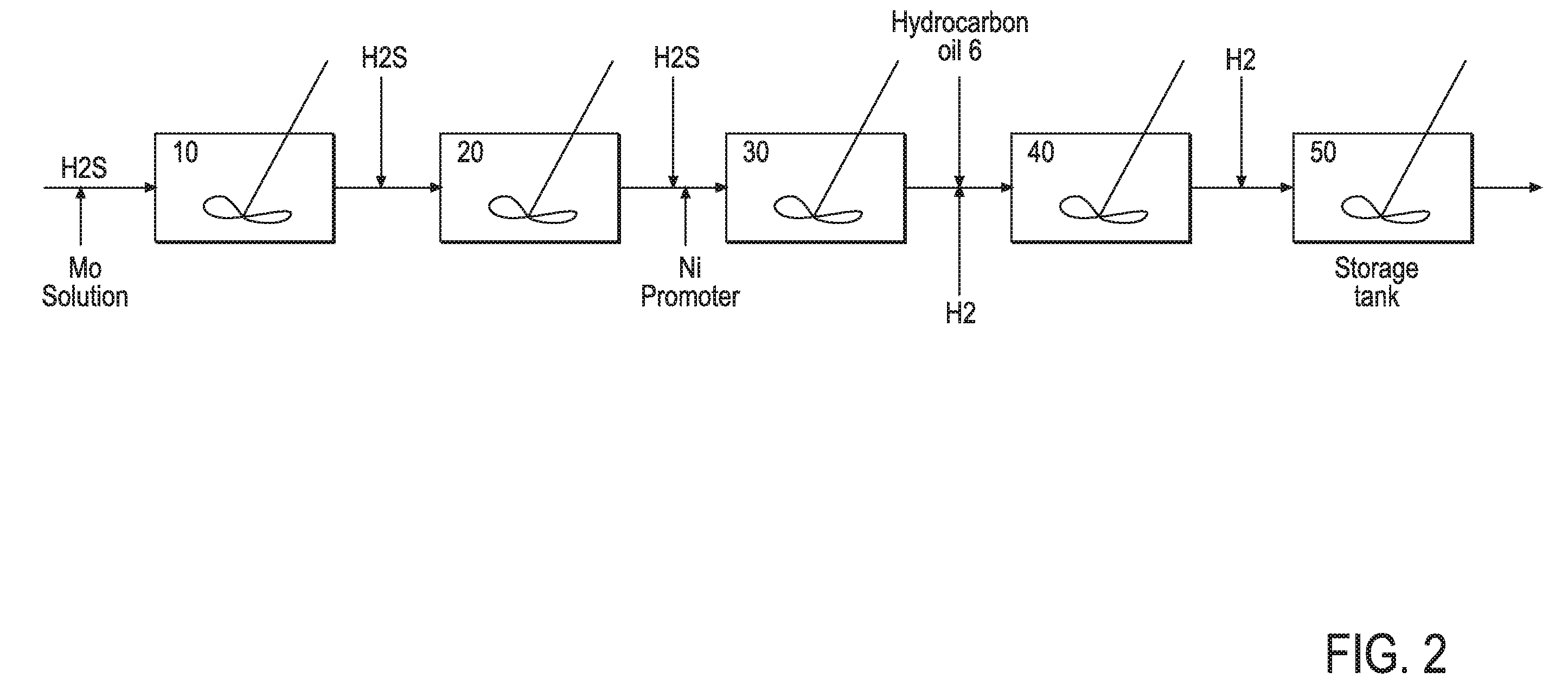
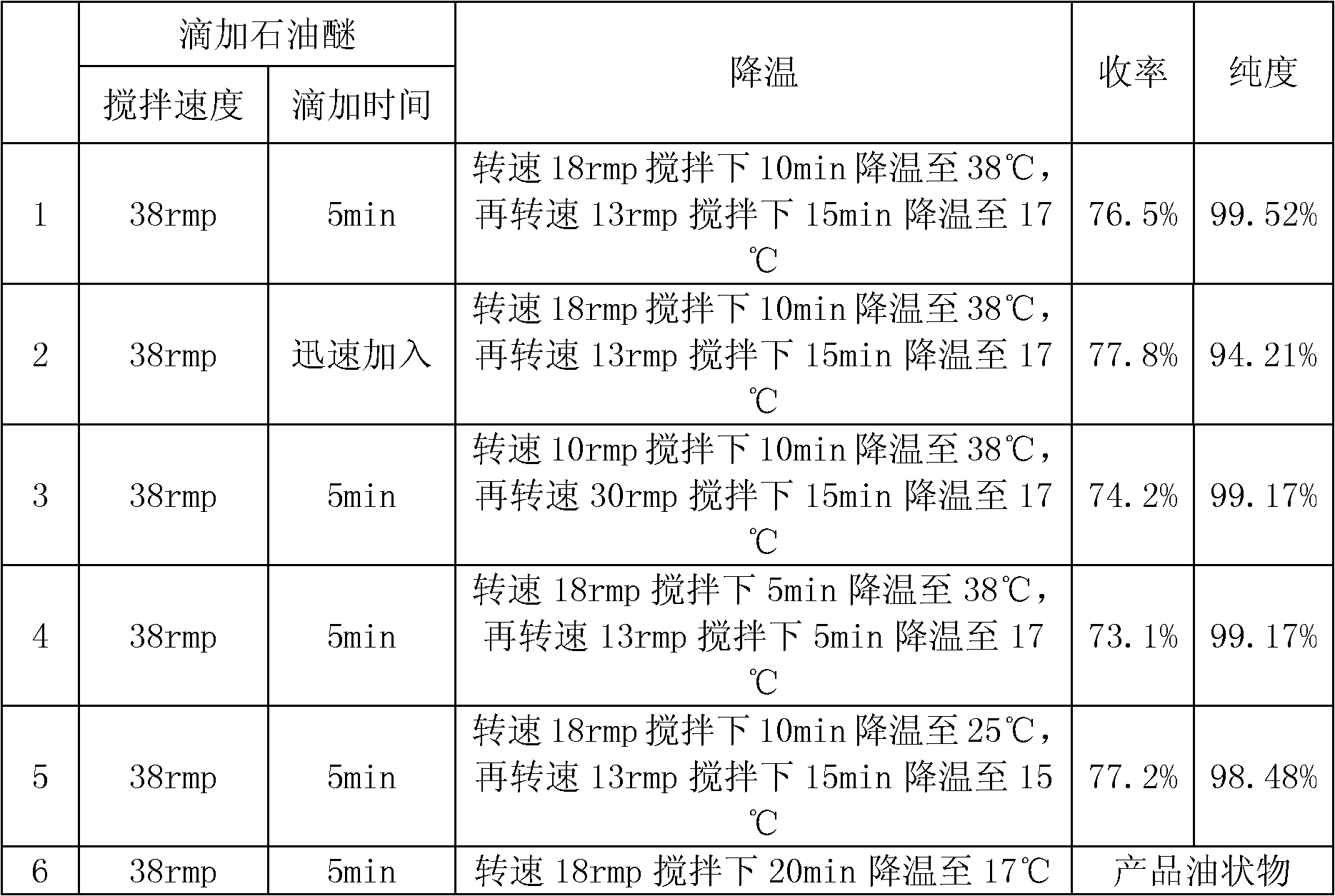
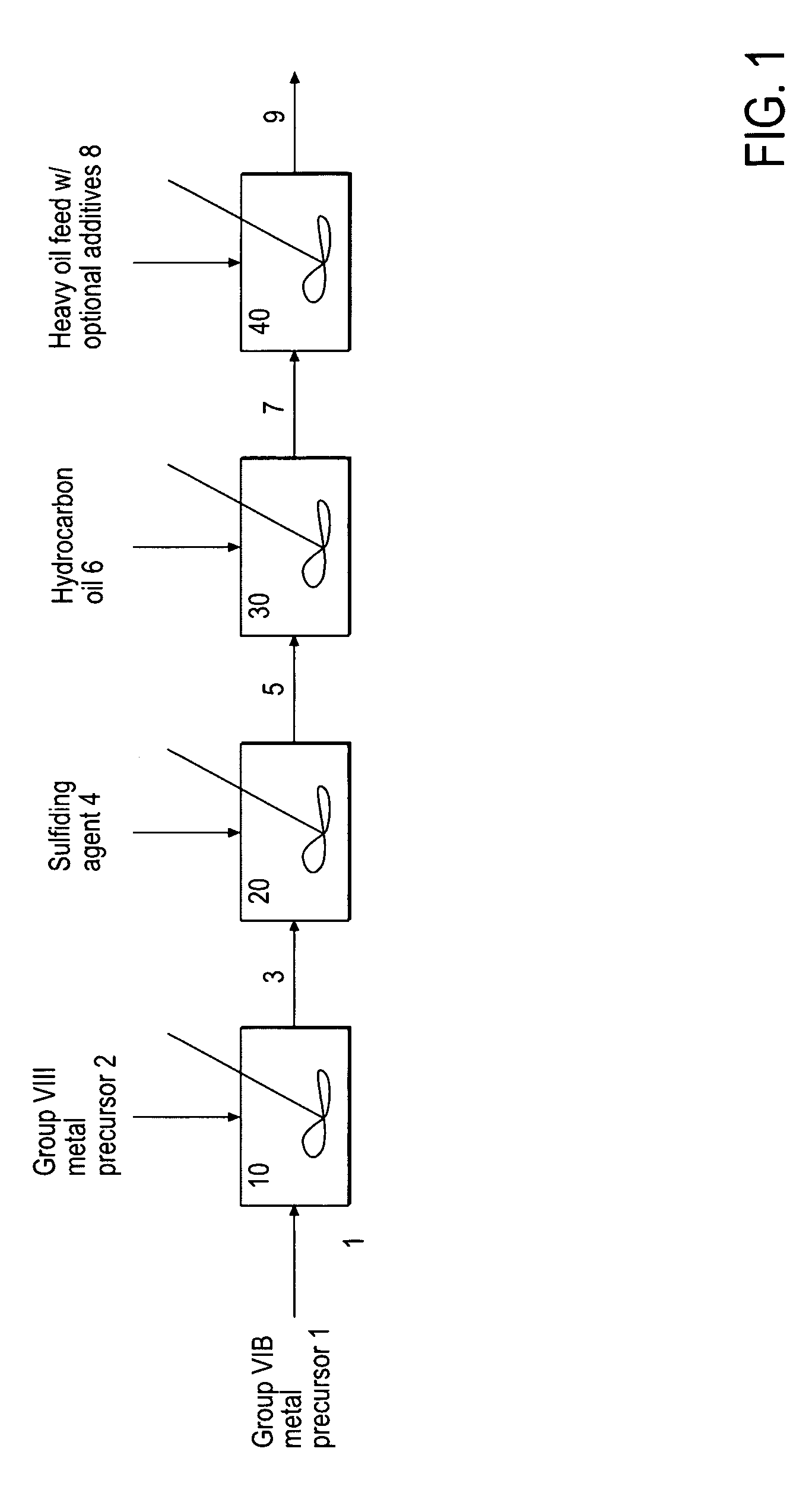
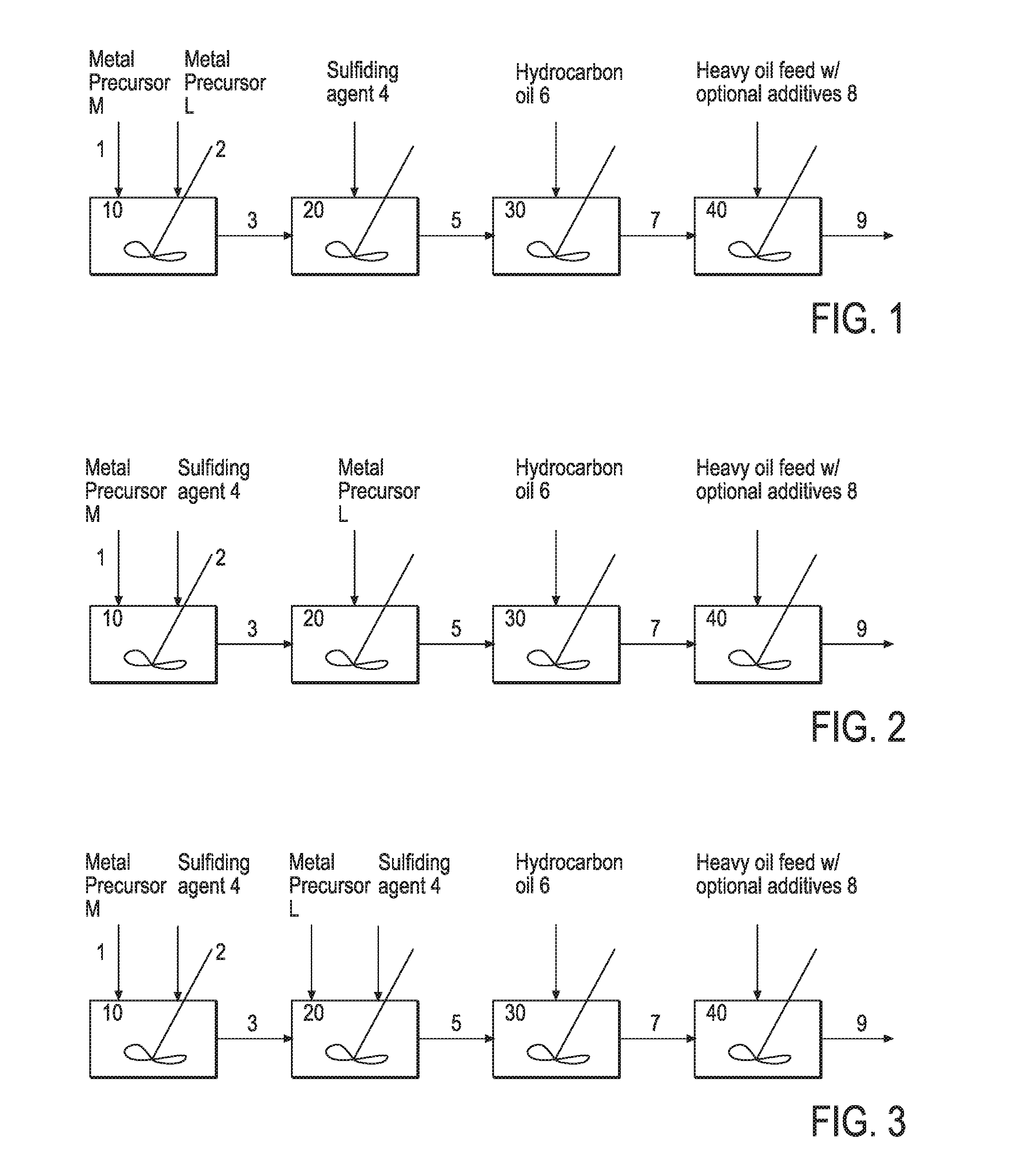
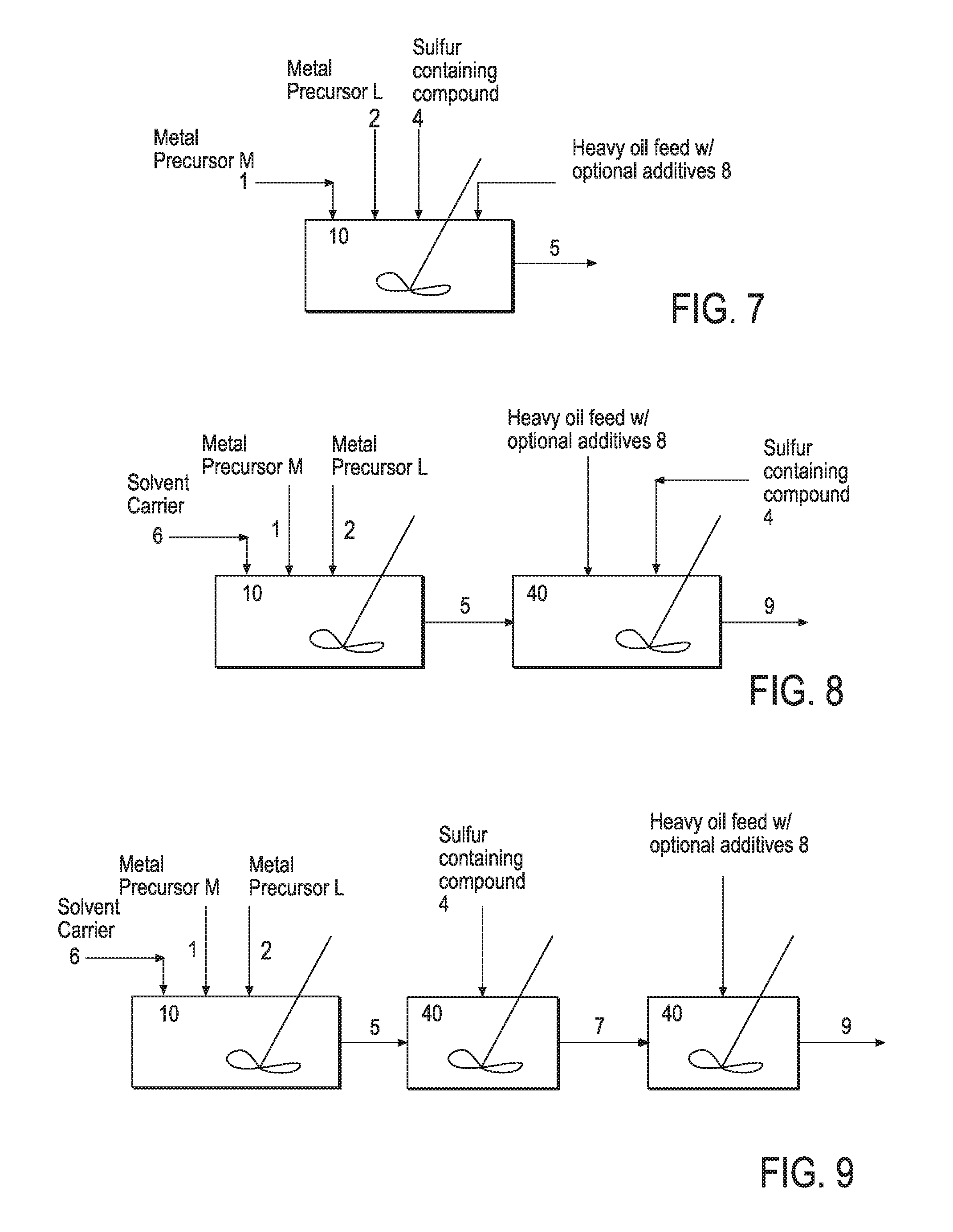
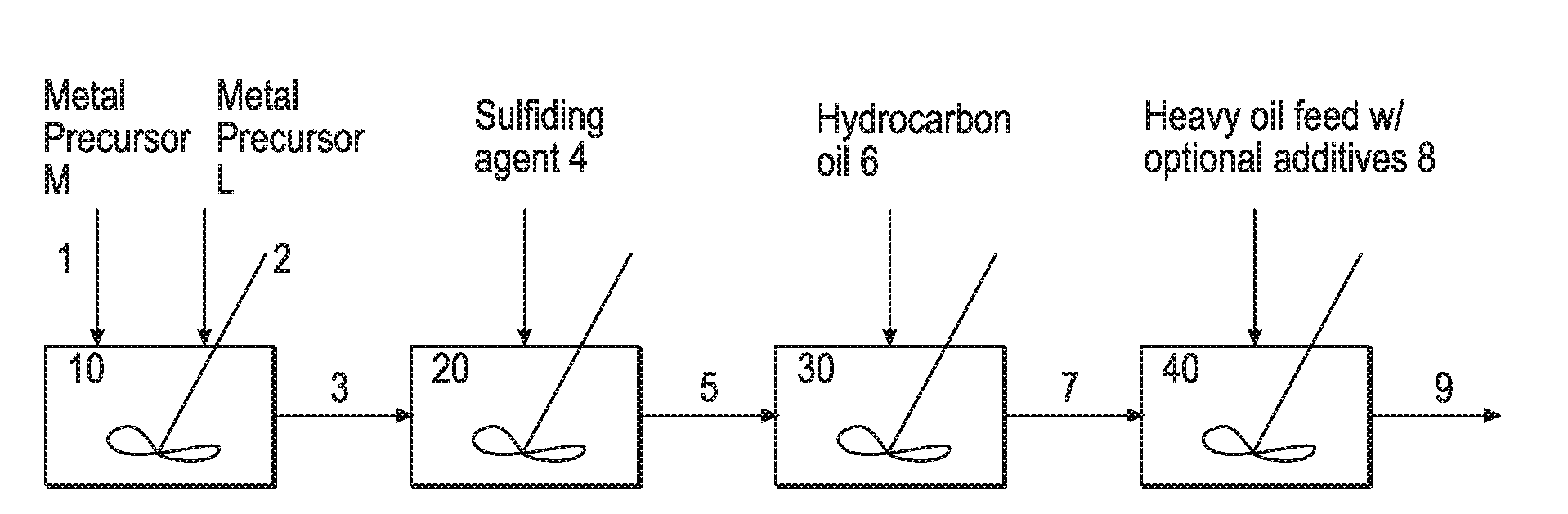
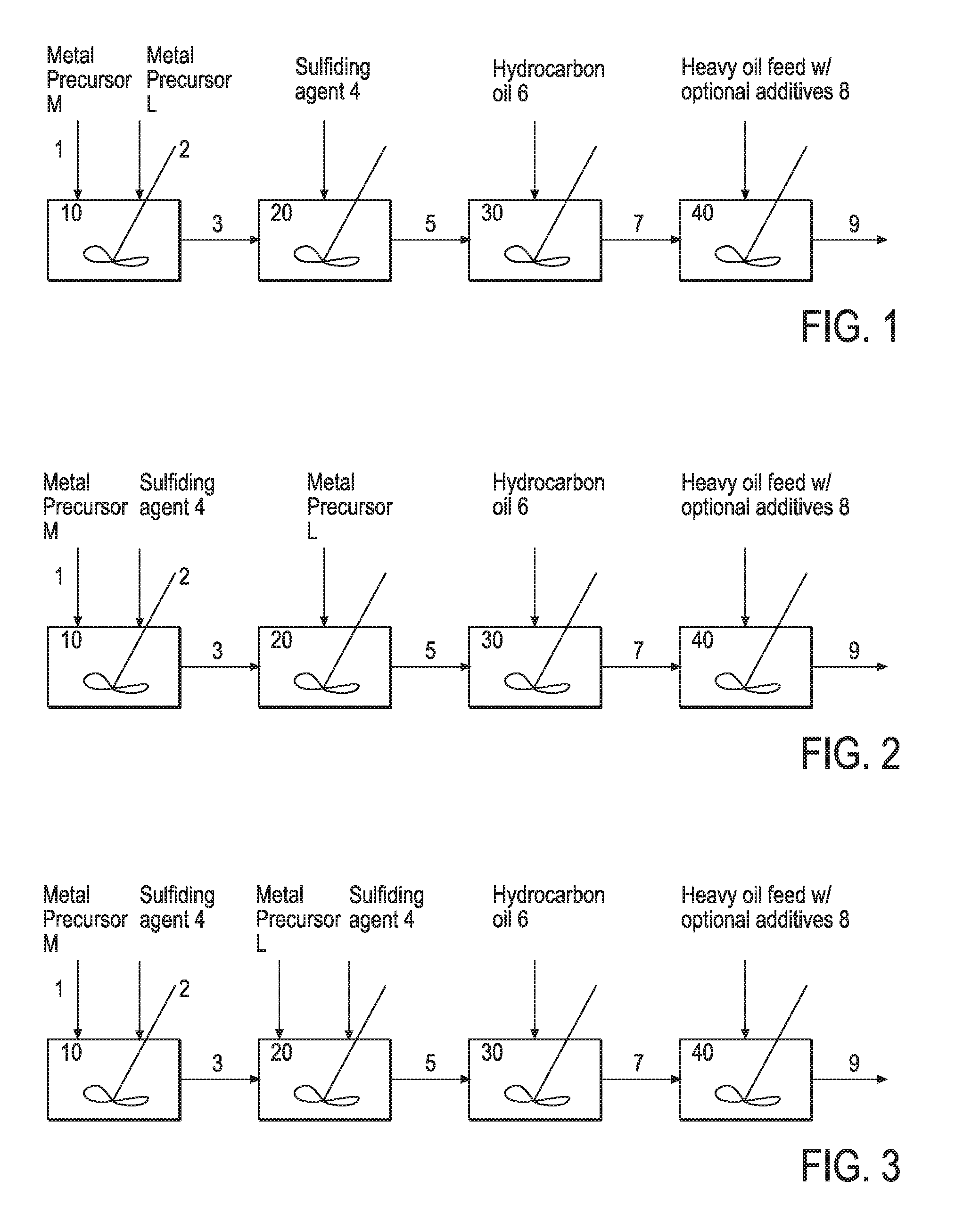
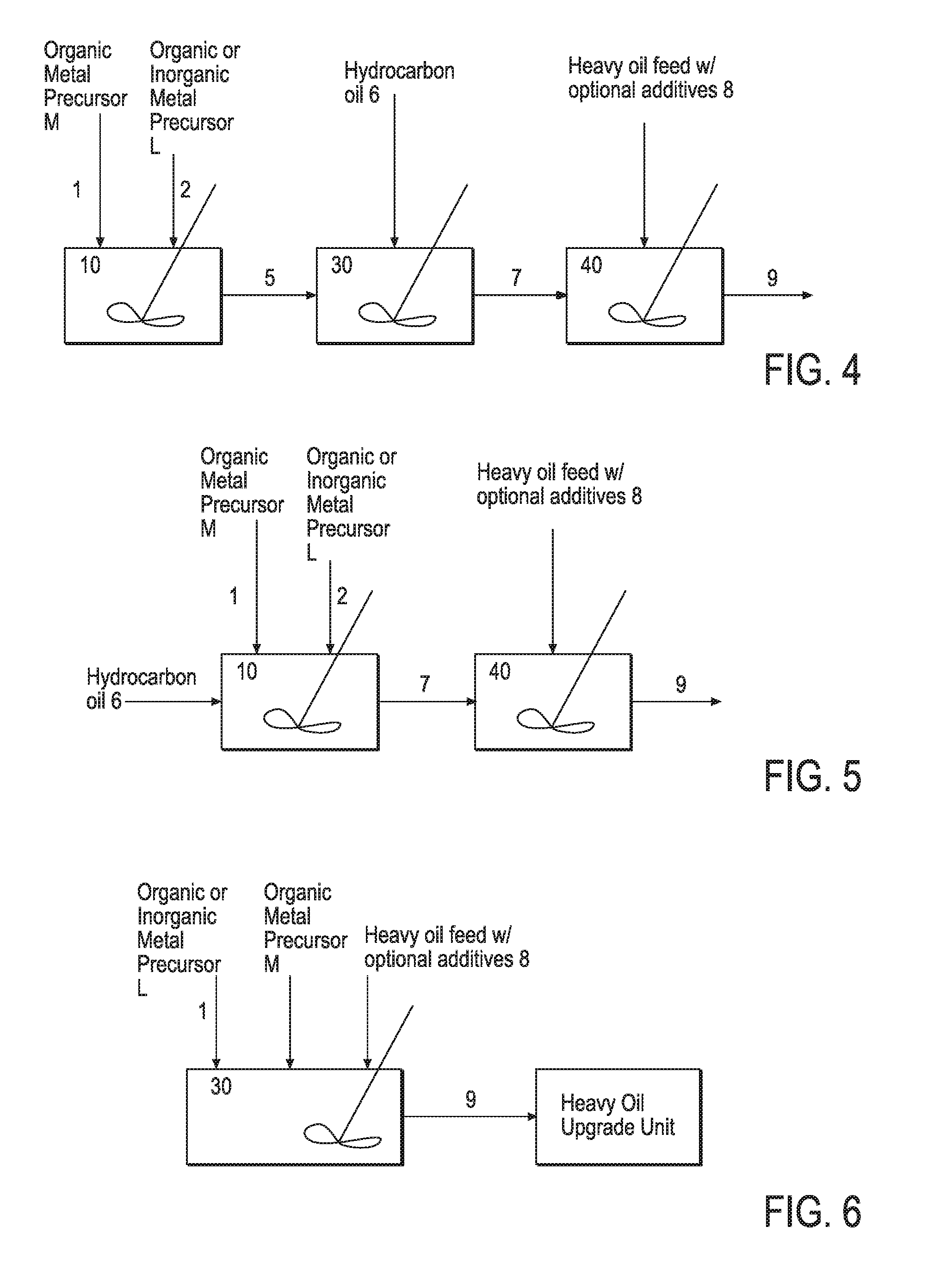
Hydroprocessing bulk catalyst and uses thereof
ActiveUS20090057201A1Inorganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsNon noble metalPowder diffraction
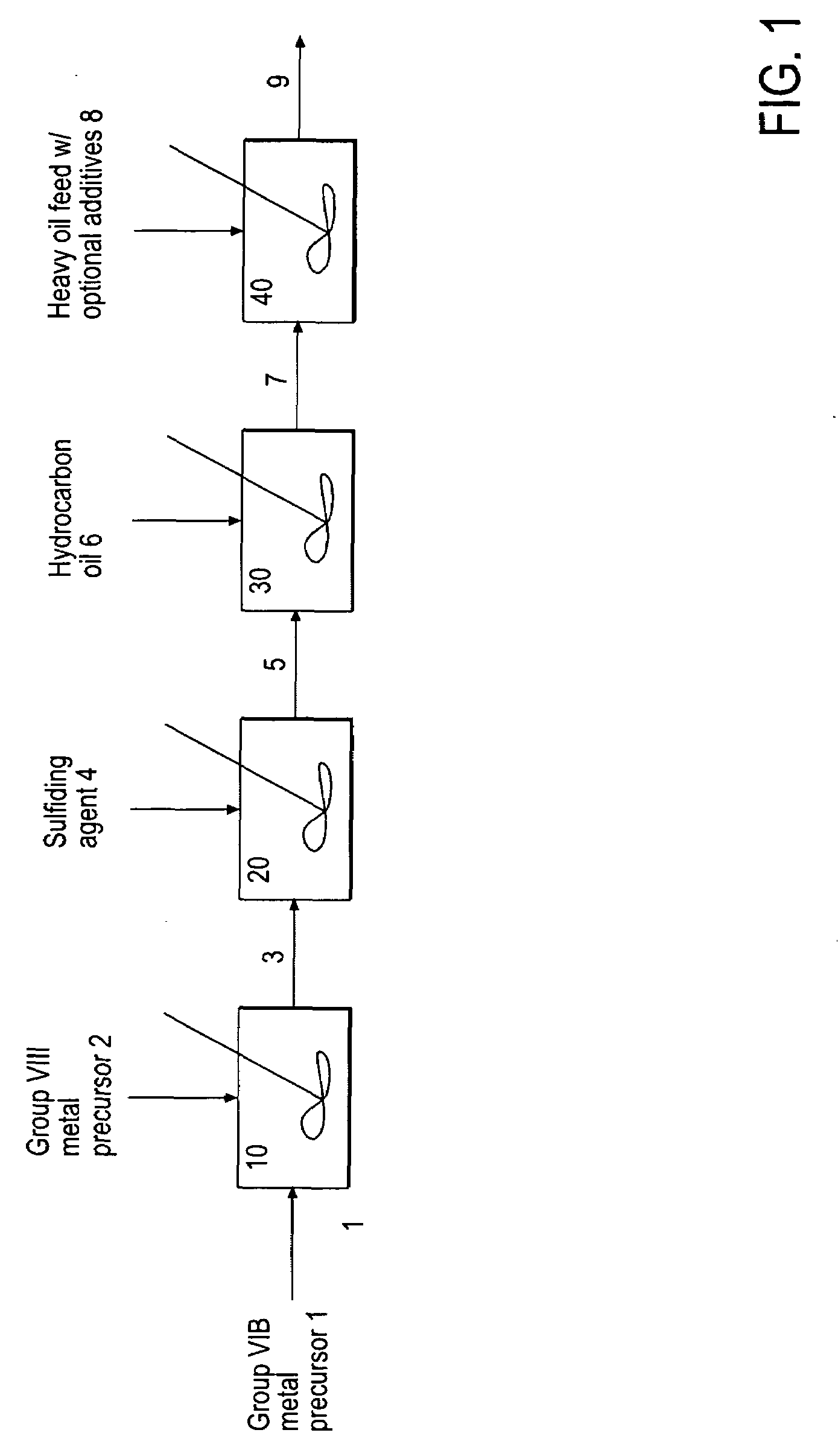
A hydroprocessing bulk catalyst is provided. A process to prepare hydroprocessing bulk catalysts is also provided. The hydroprocessing catalyst has the formula (Mt)d(Lu)b(Sv)d(Cw)e(Hx)f(Oy)g(Nz)h, wherein M is at least one group VIB metal; promoter metal L is optional and if present, L is at least one Group VIII non-noble metal; t, u, v, w, x, y, z representing the total charge for each of the components (M, L, S, C, H, O and N, respectively); ta+ub+vd+we+xf+yg+zh=0; 0 =<b; and 0=<b / a=<5, (a+0.5b)<=d<=(5a+2b), 0<=e<=11(a+b), 0<=f<=7(a+b), 0<=g<=5(a+b), 0<=h<=0.5(a+b). The catalyst has an X-ray powder diffraction pattern with at least one broad diffraction peak at any of Bragg angles: 8 to 18°, 32 to 40°, and 55 to 65° (from 0 to 70° 2-θ scale). In one embodiment, the catalyst is prepared by sulfiding at least one Group VIB metal compound and optionally at least one group VIII metal compound with a sulfiding agent forming a catalyst precursor; and mixing the catalyst precursor with a hydrocarbon compound to form the hydroprocessing catalyst composition.
Owner:CHEVROU USA INC
Transmission-geometry electrochemical cell for in-situ scattering and spectroscopy investigations
ActiveUS20140093052A1Continuous measurementContinuous monitoringMaterial analysis using wave/particle radiationSecondary cellsSpectroscopyX-ray
The present invention relates to a test chamber that can be used to perform a variety of X-ray and neutron spectroscopy experiments including powder diffraction, small-angle scattering, X-ray absorption spectroscopy, and pair distribution functions, such chamber comprising a first electrode with an X-ray transparent window; a second electrode with an X-ray transparent window; a plurality of insulating gaskets providing a hermetic seal around the sample and preventing contact between said first and second electrodes; and an insulating housing into which the first electrode is secured.
Owner:UCHICAGO ARGONNE LLC
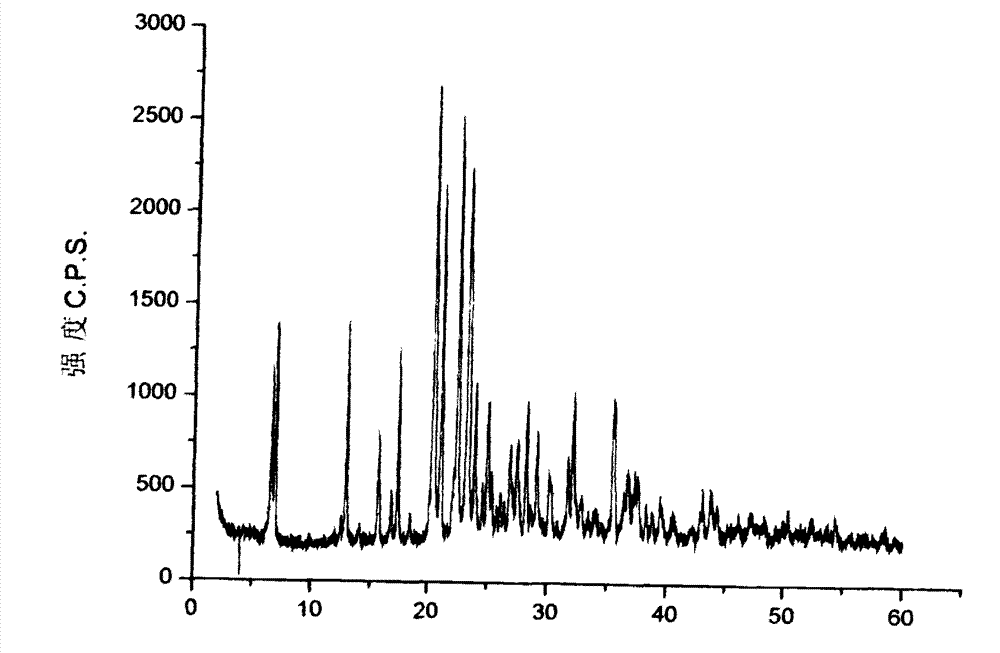
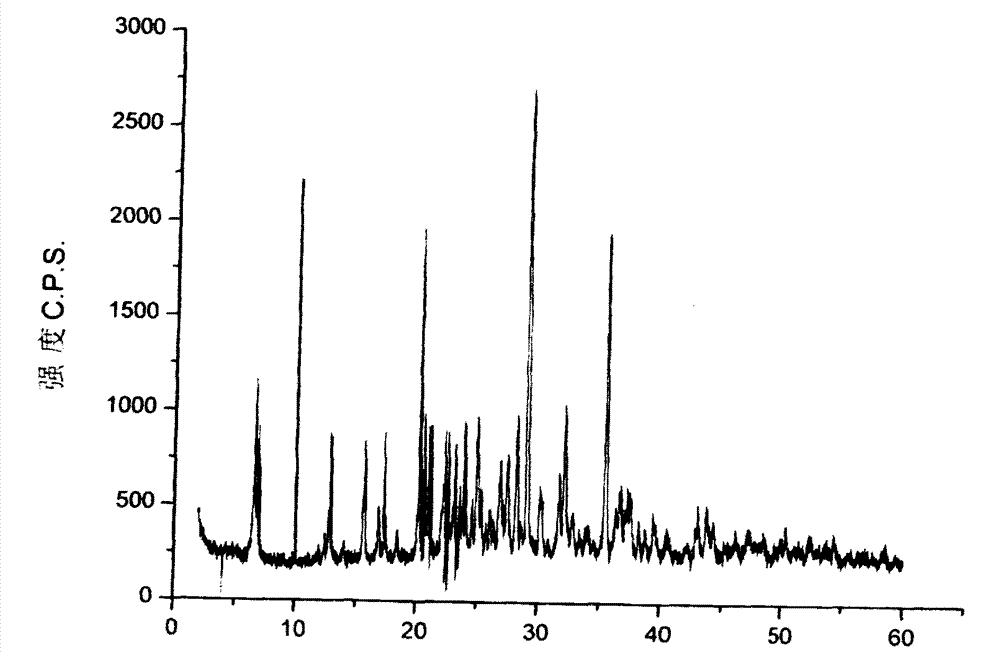
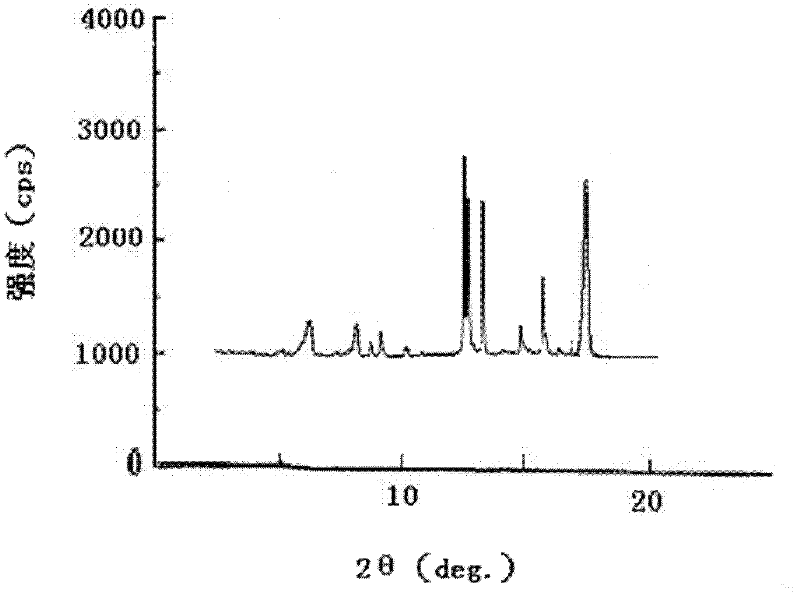
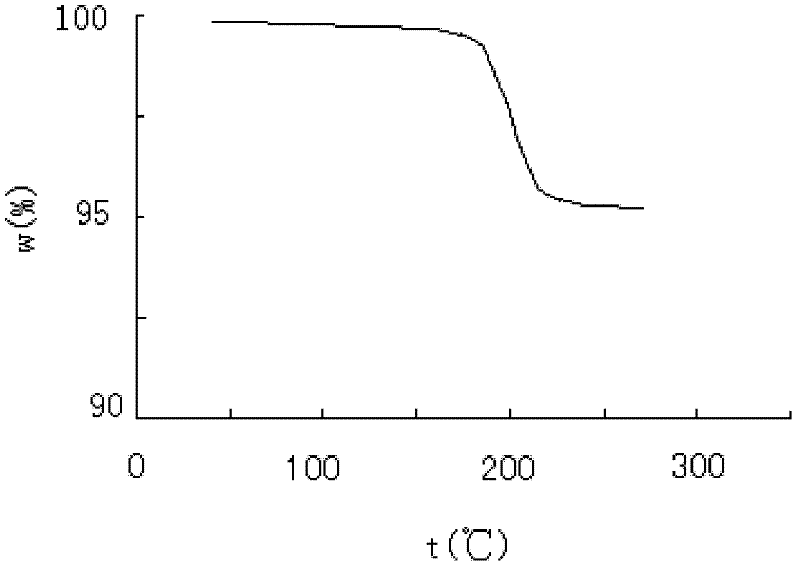
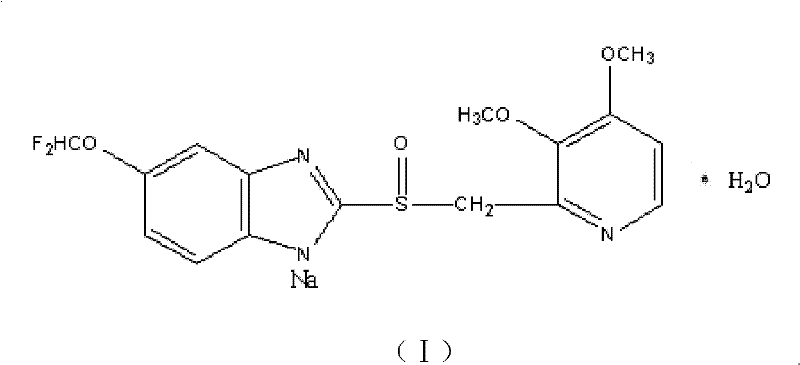
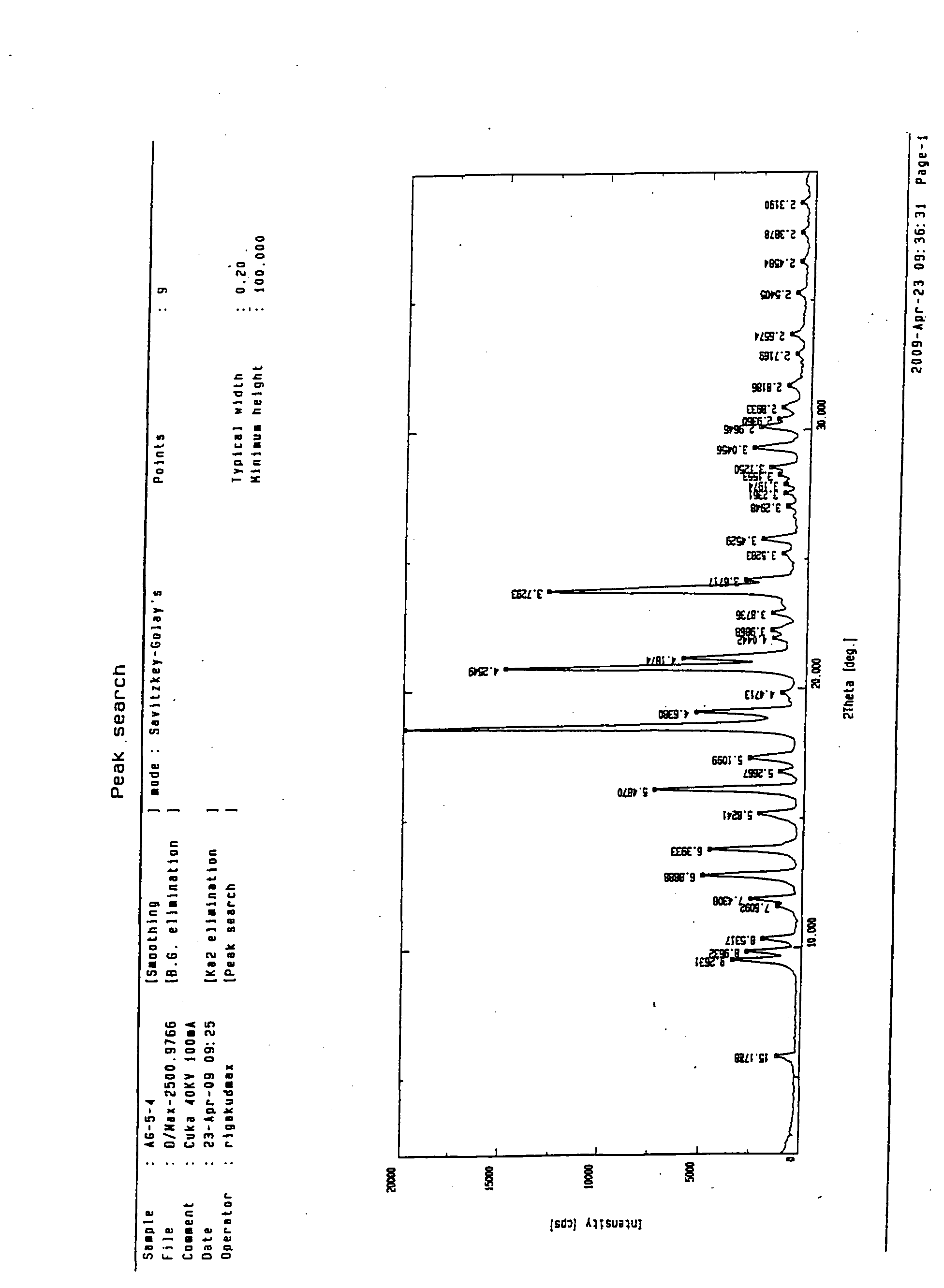
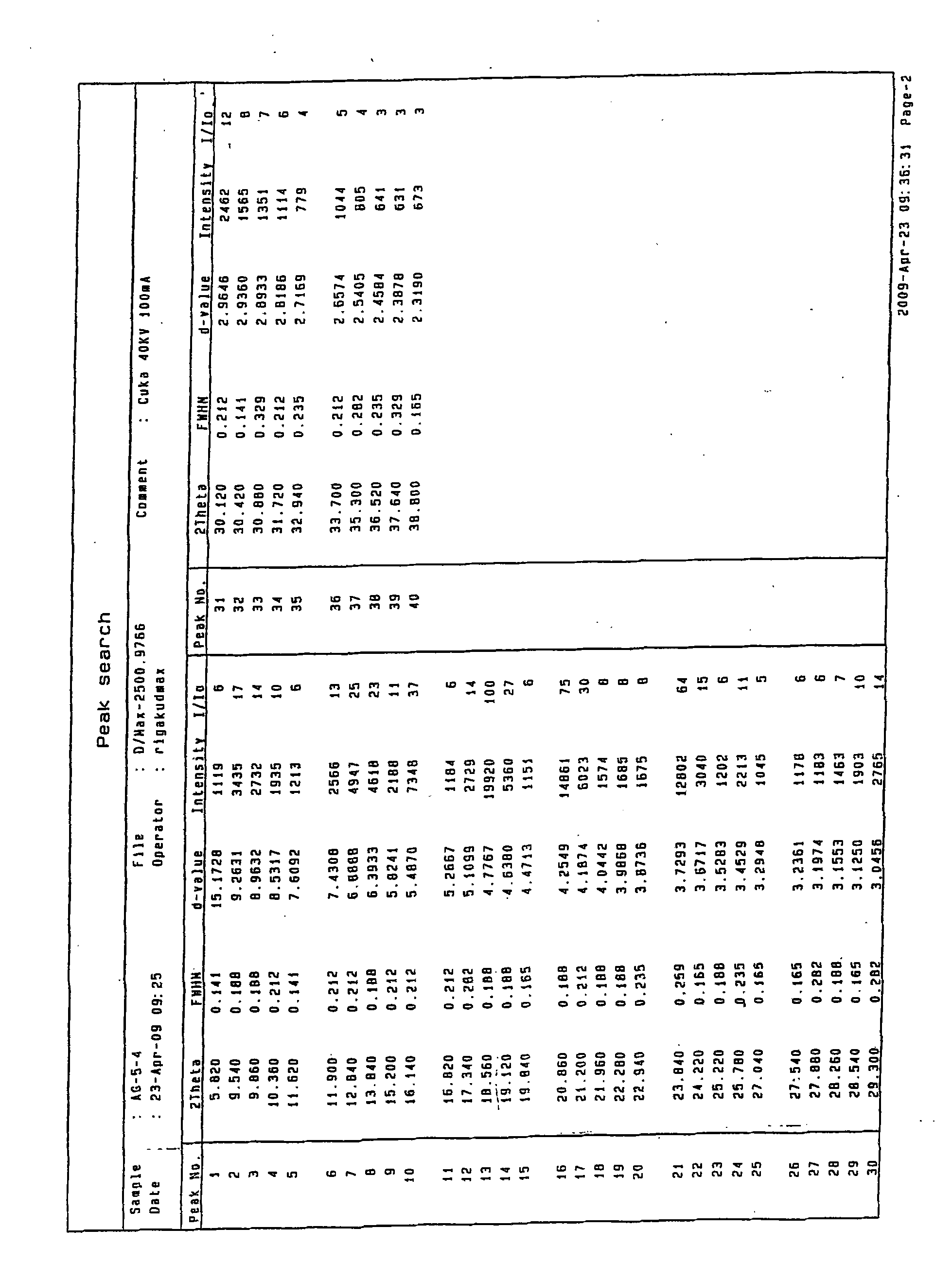
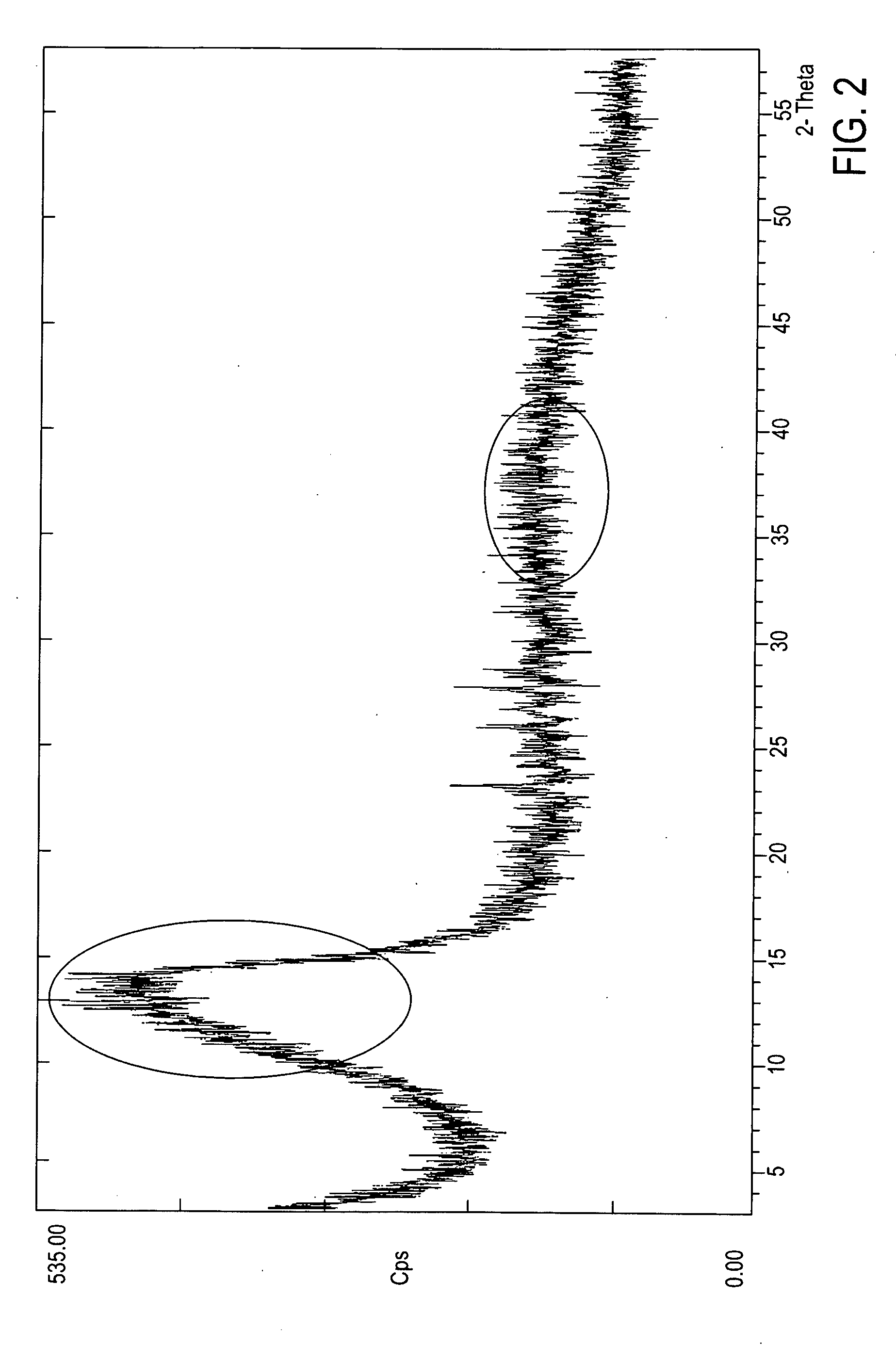
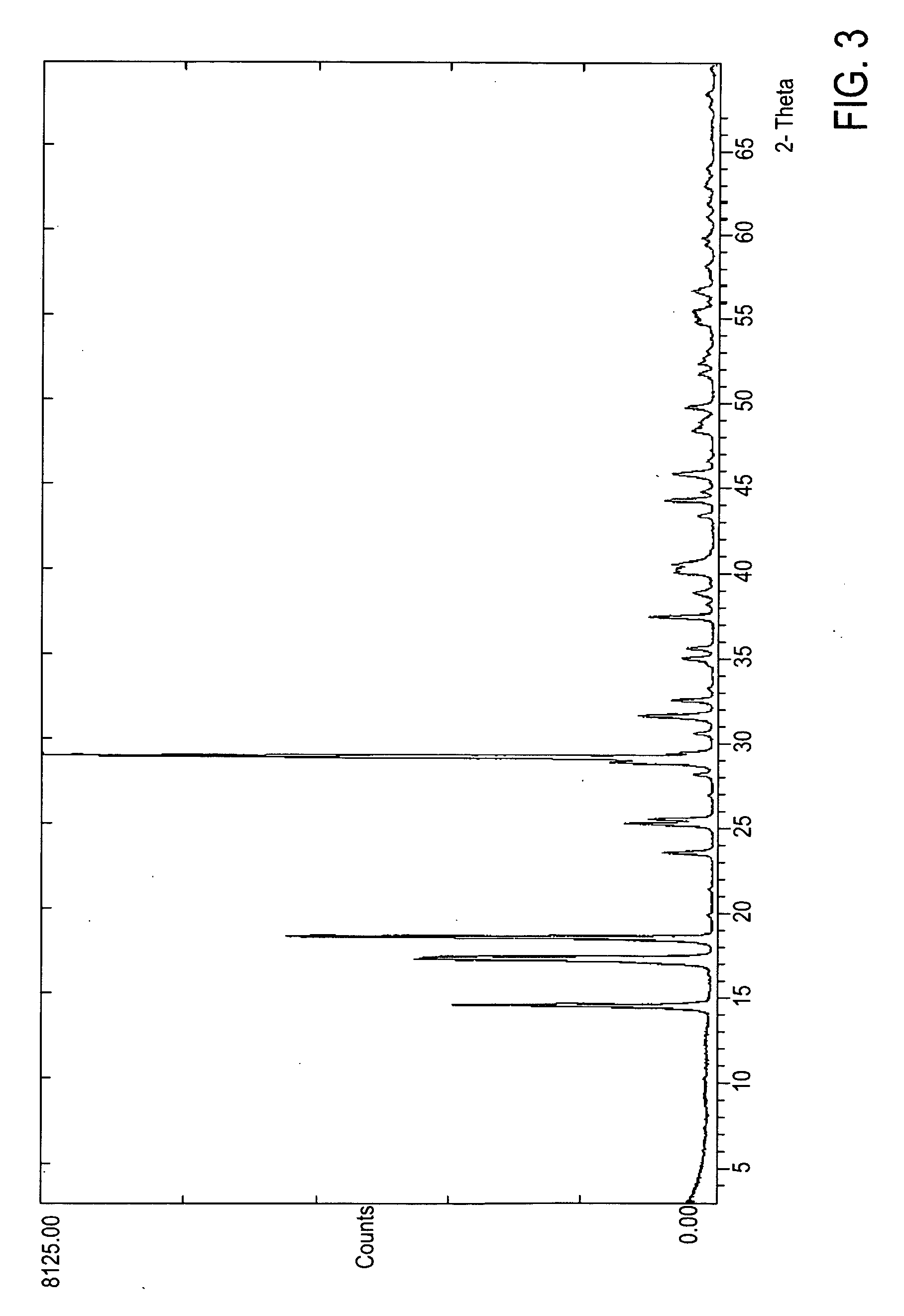
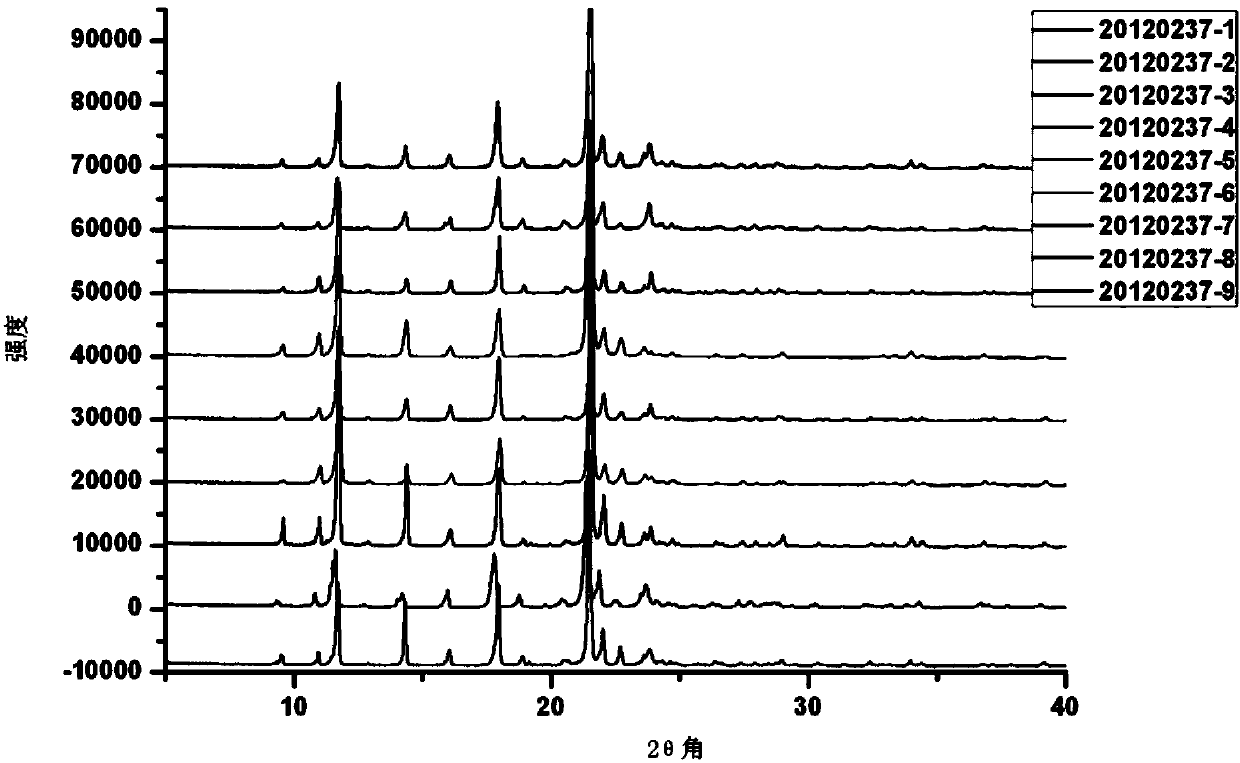
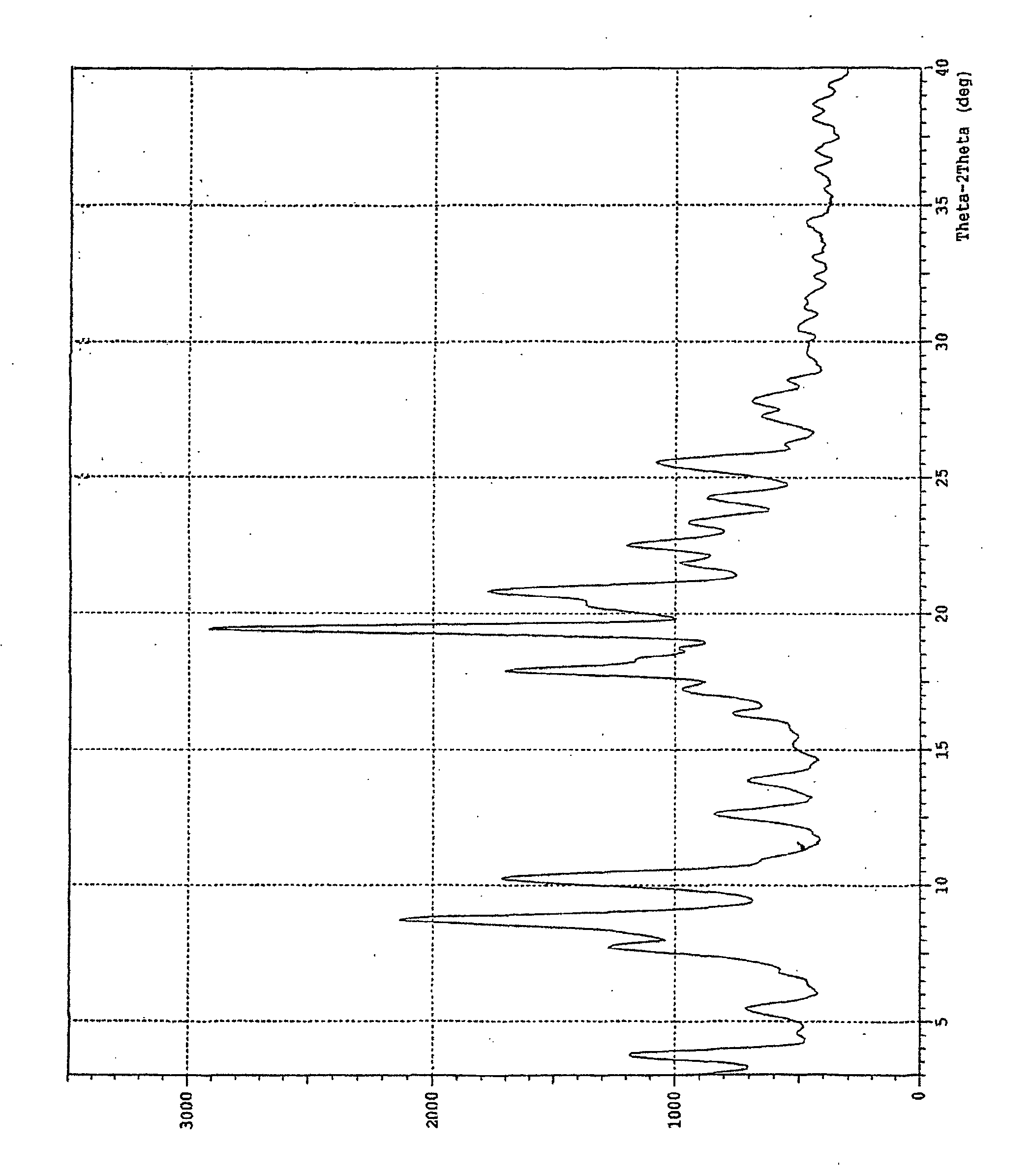
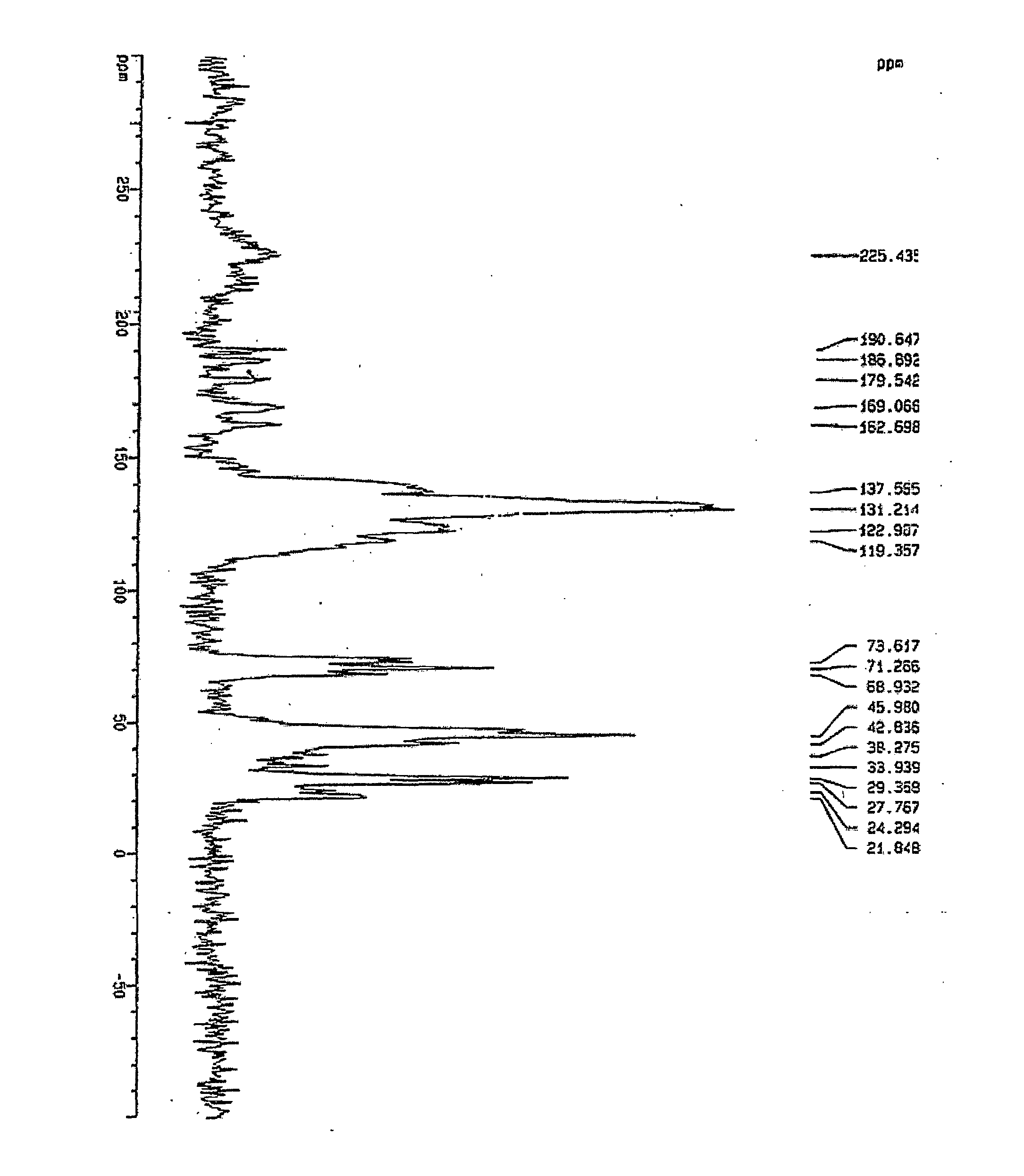
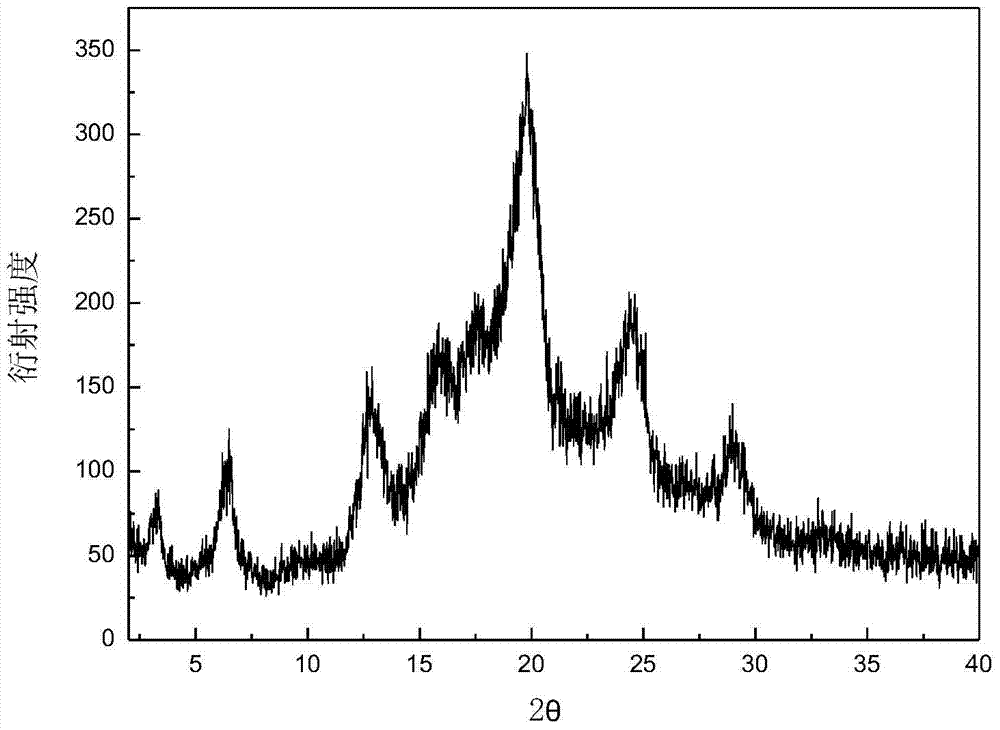
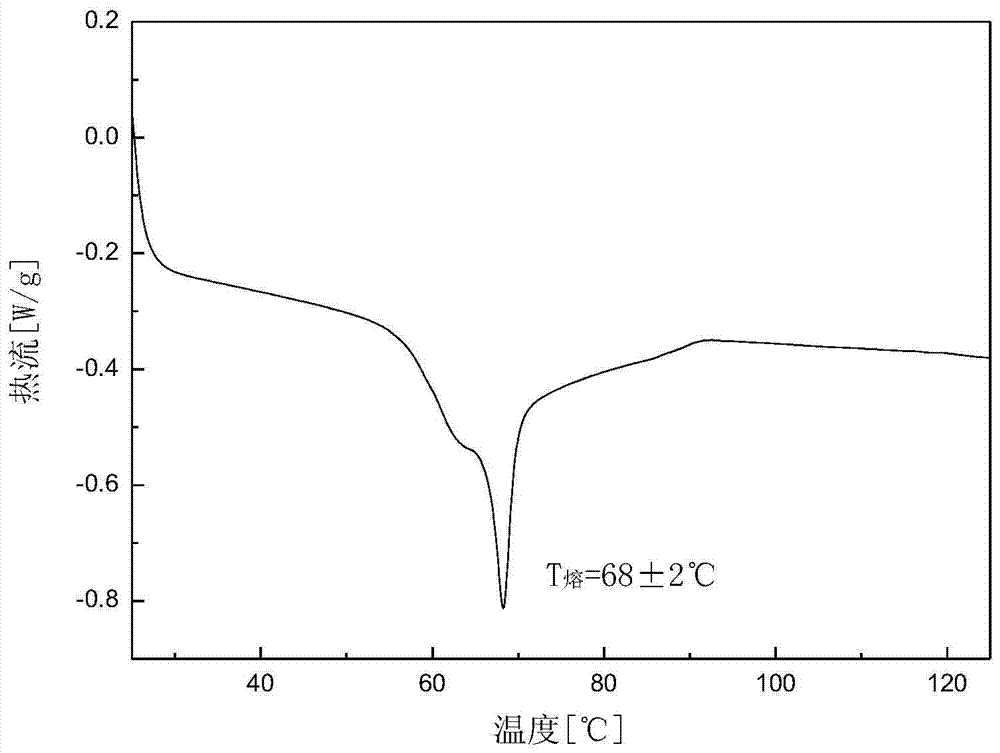
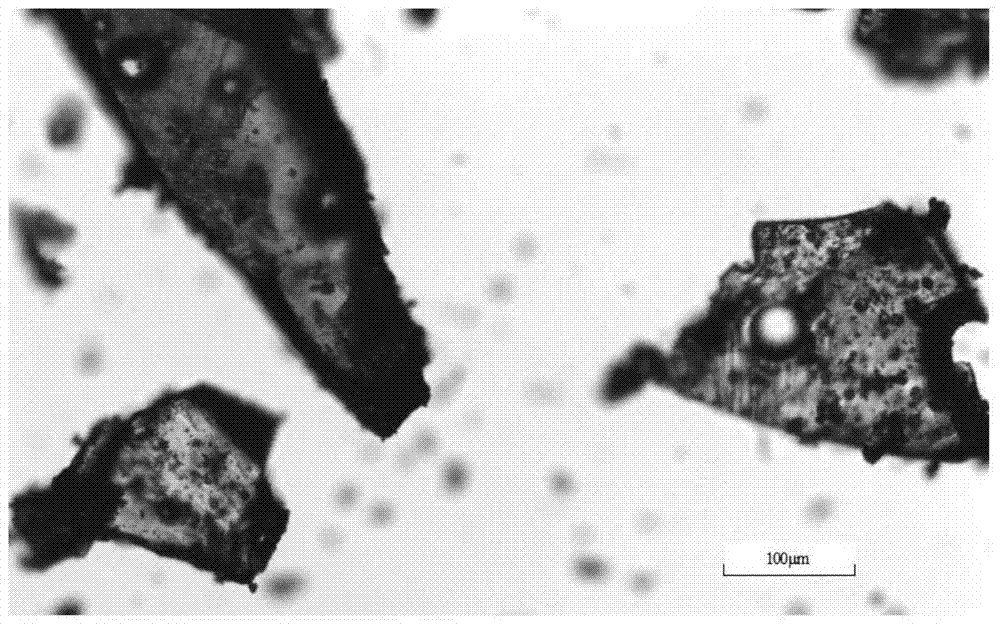
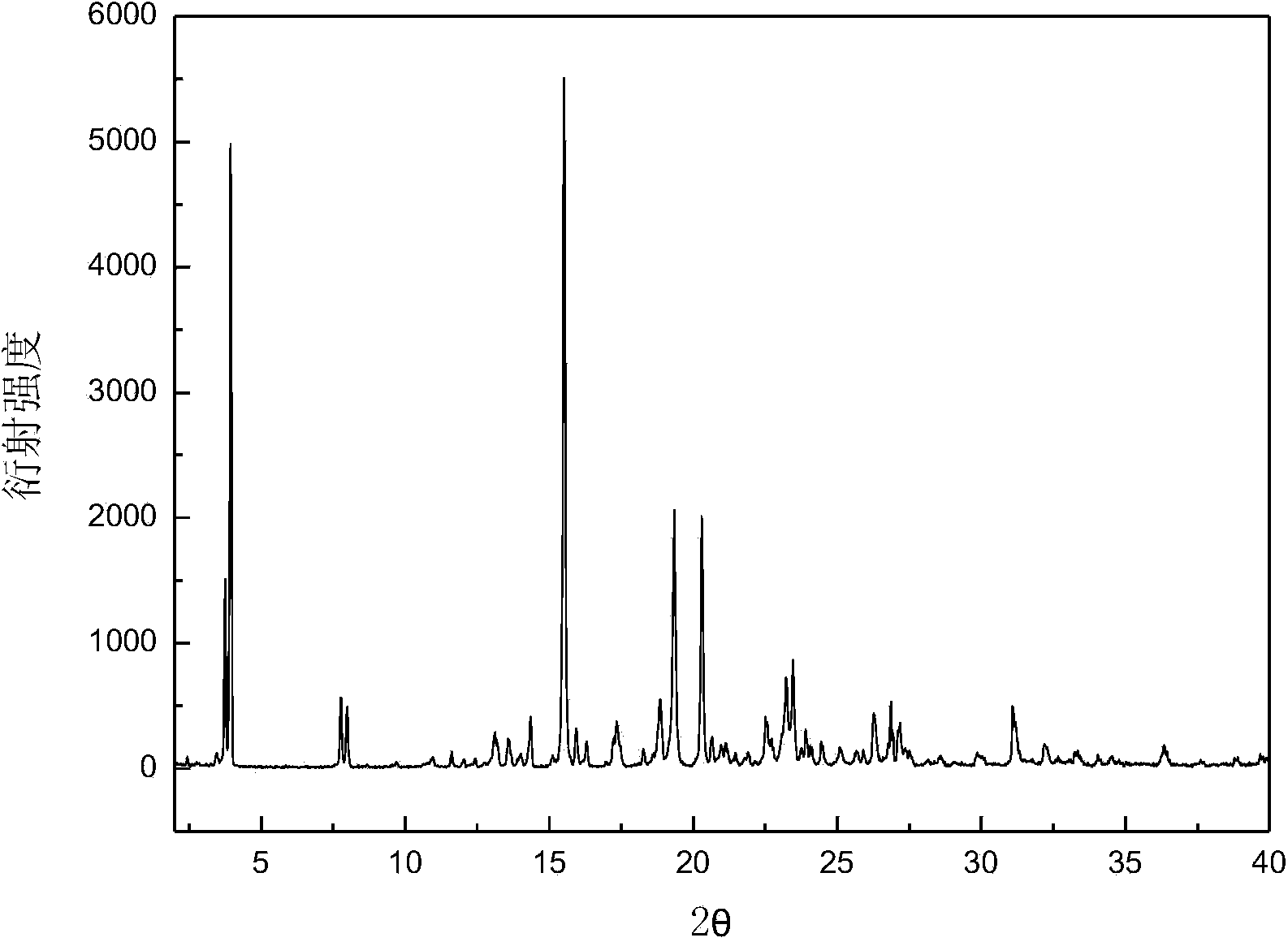
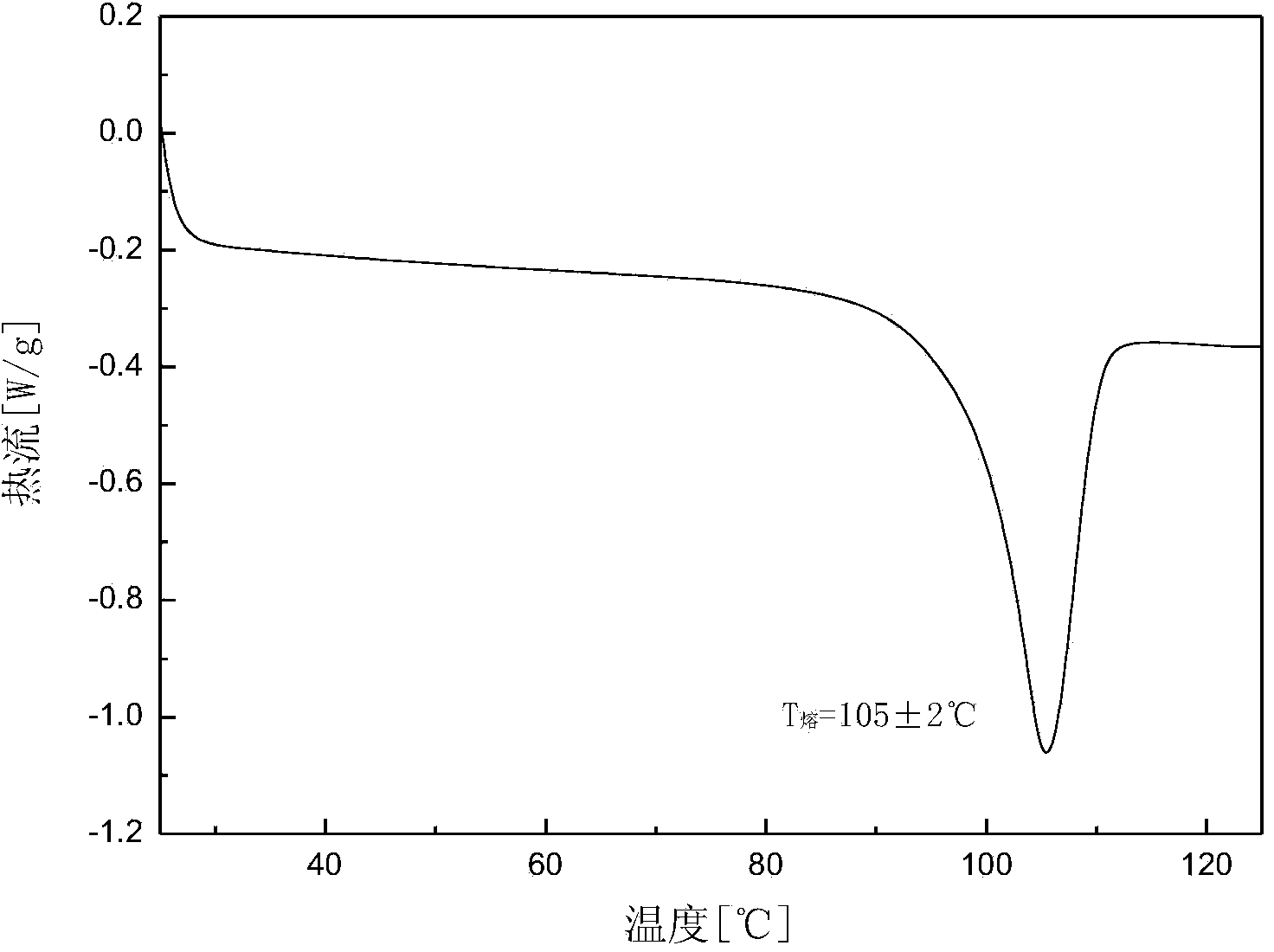
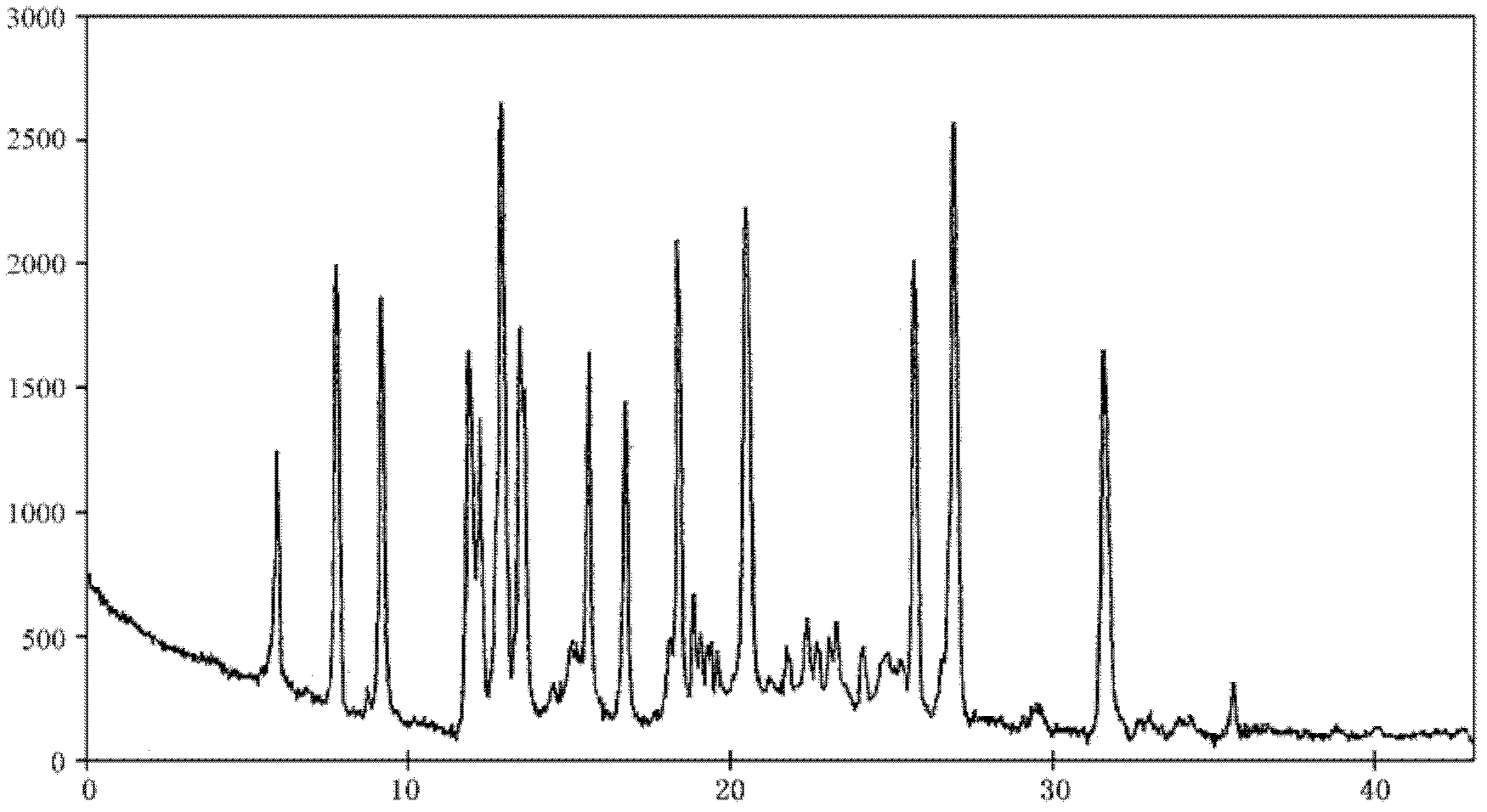
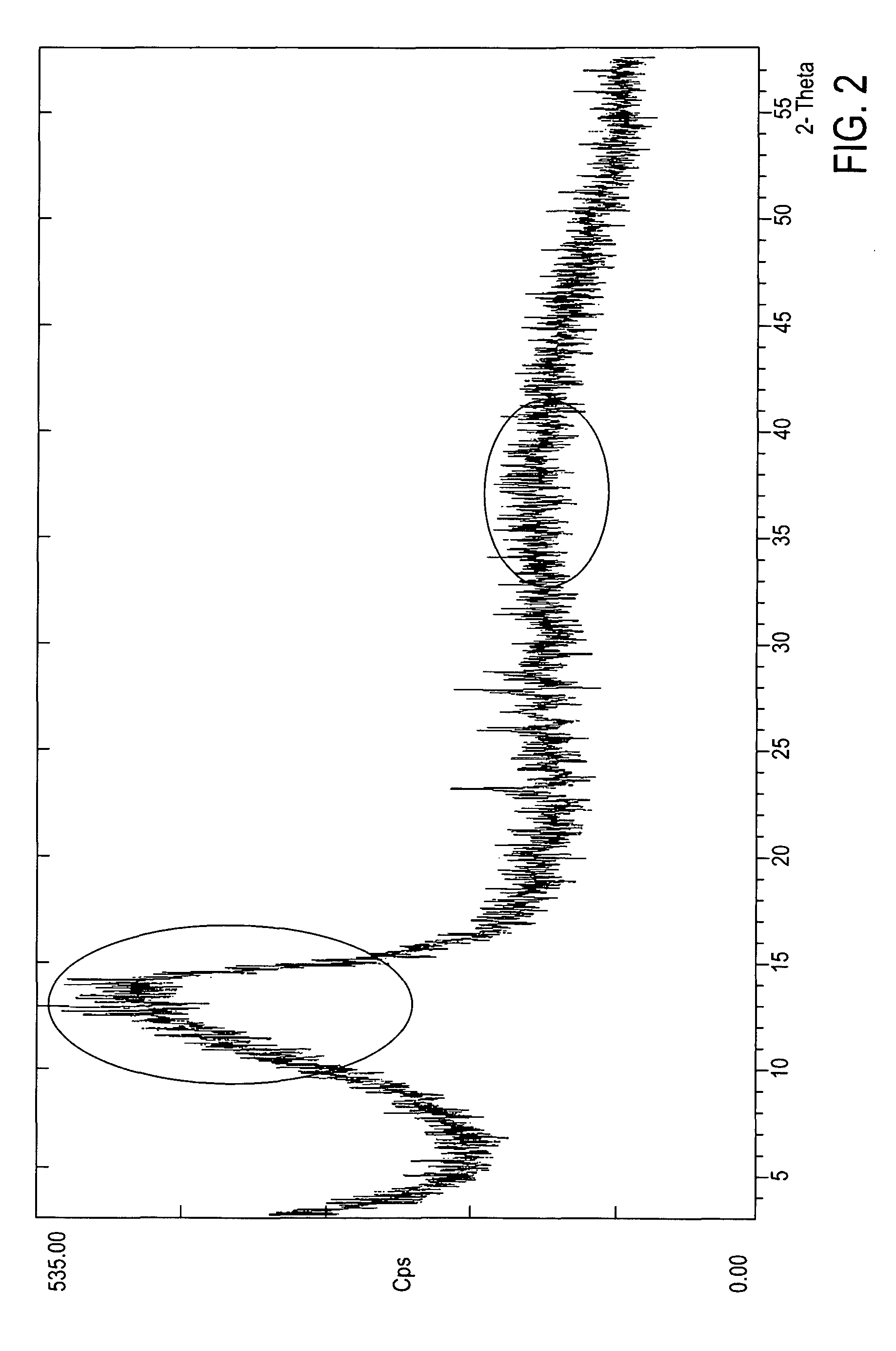
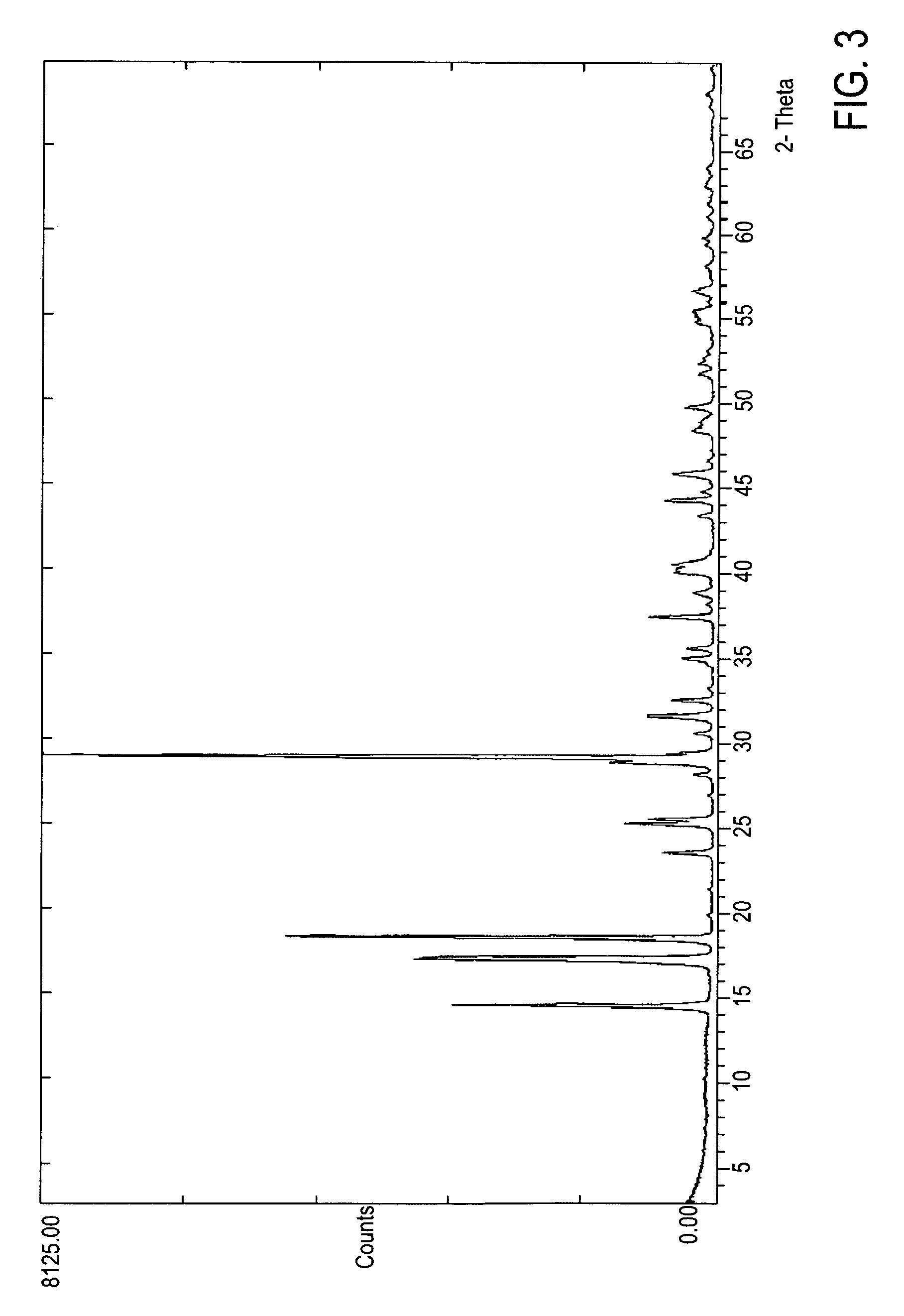
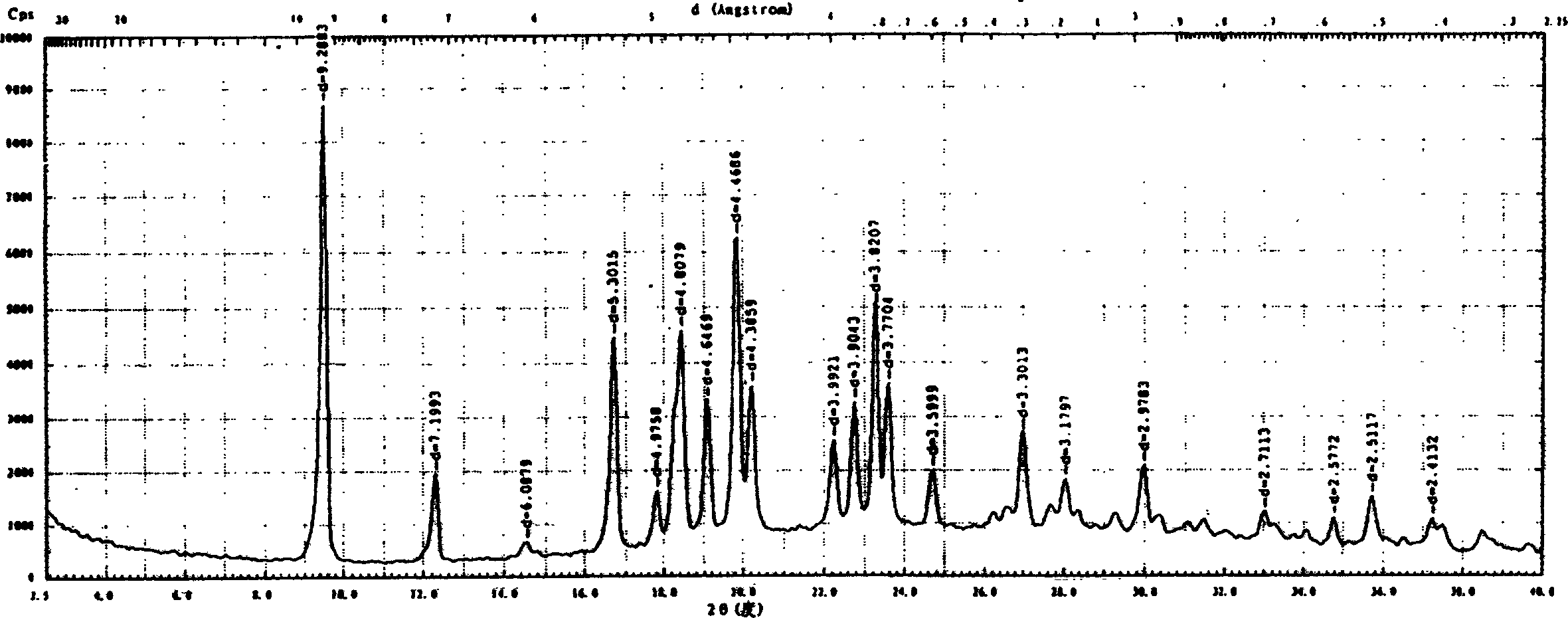
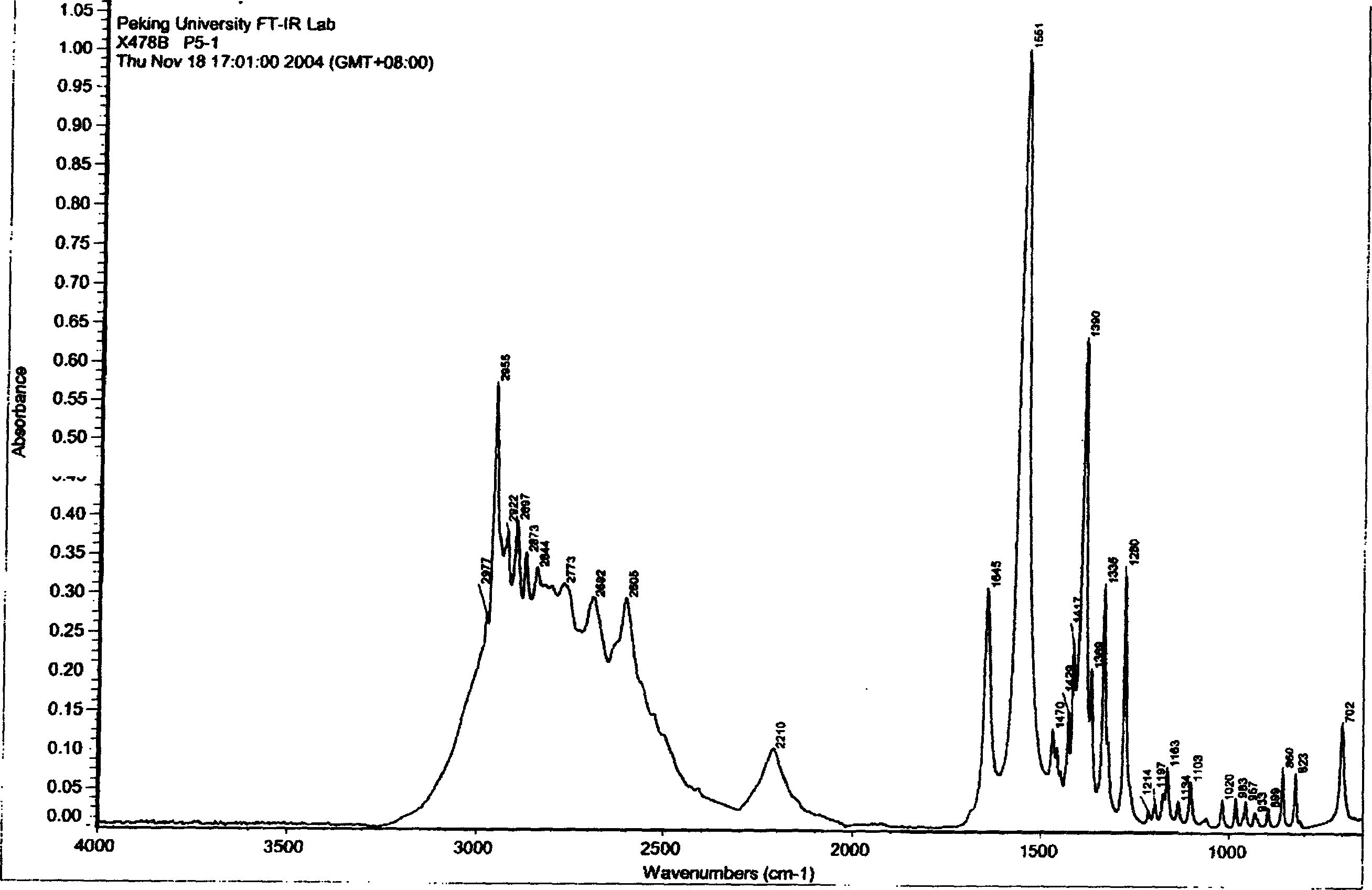
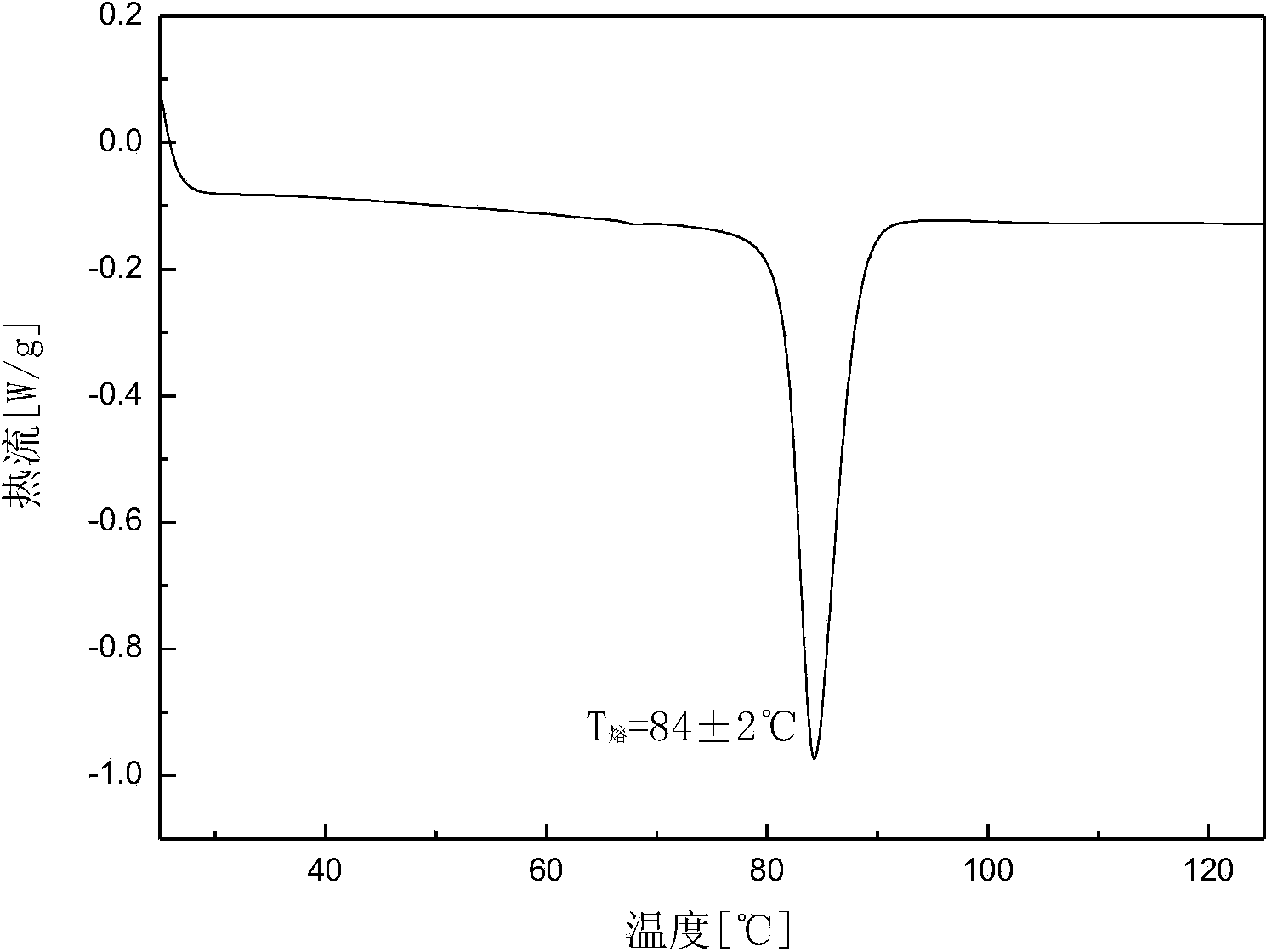
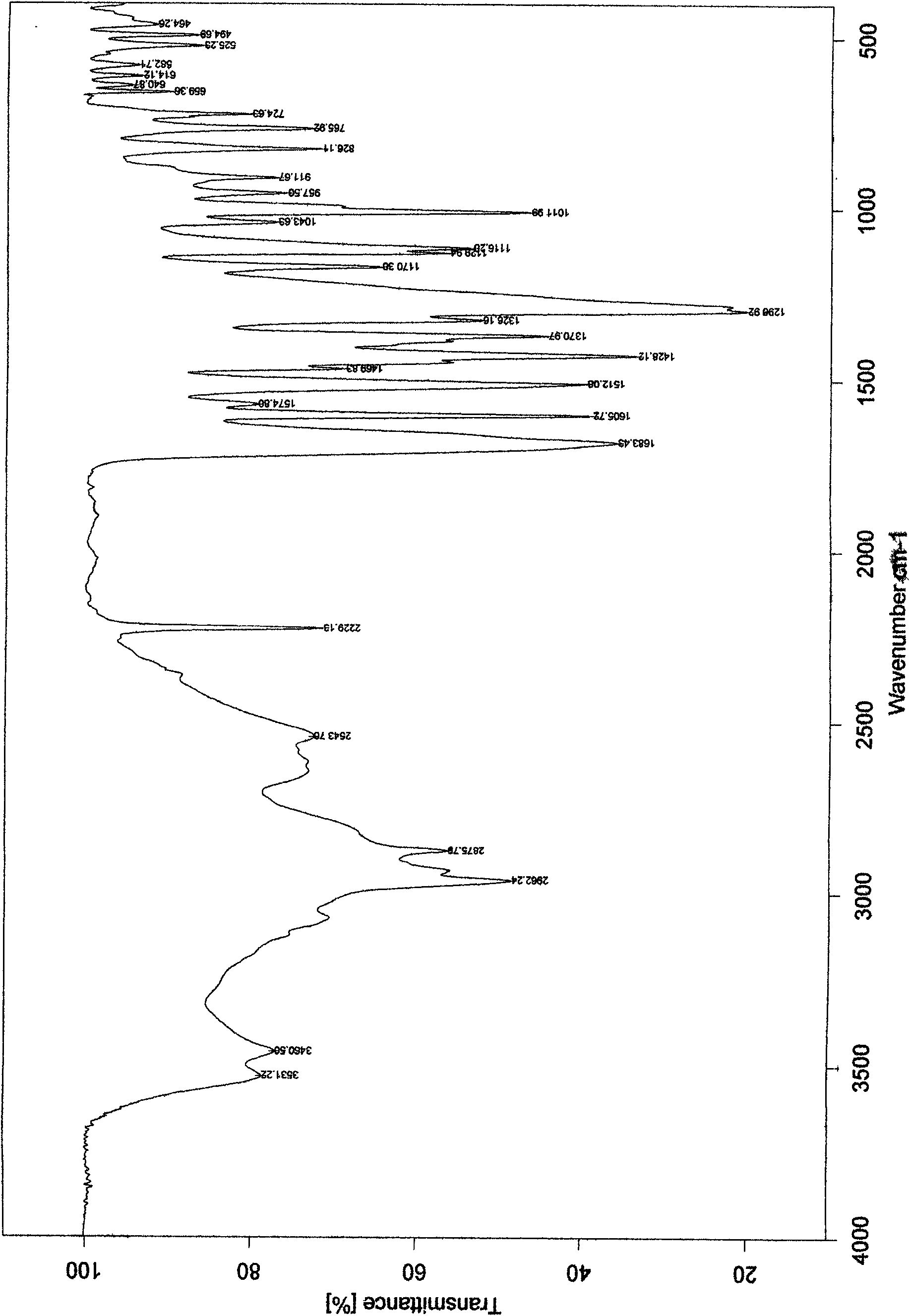
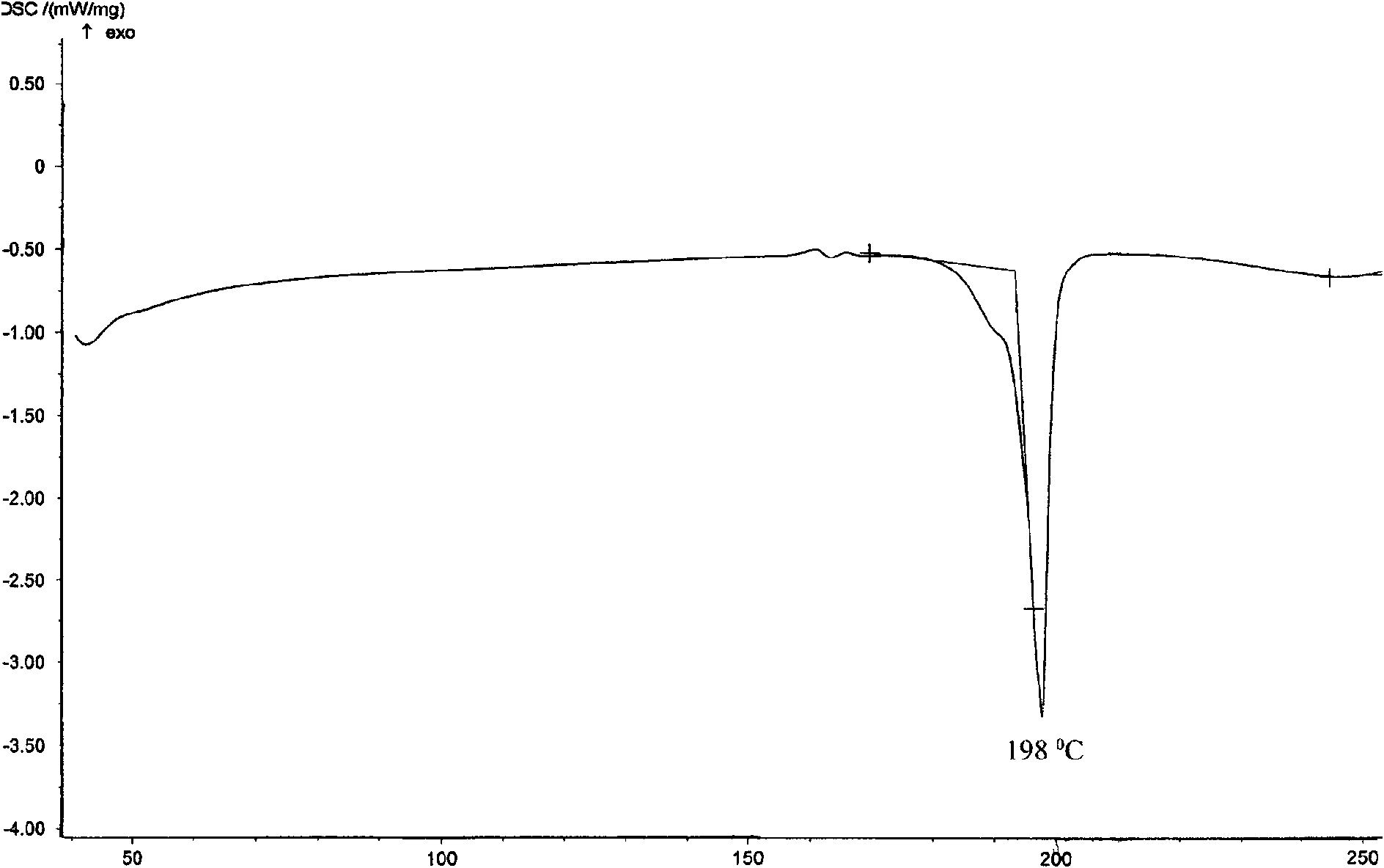
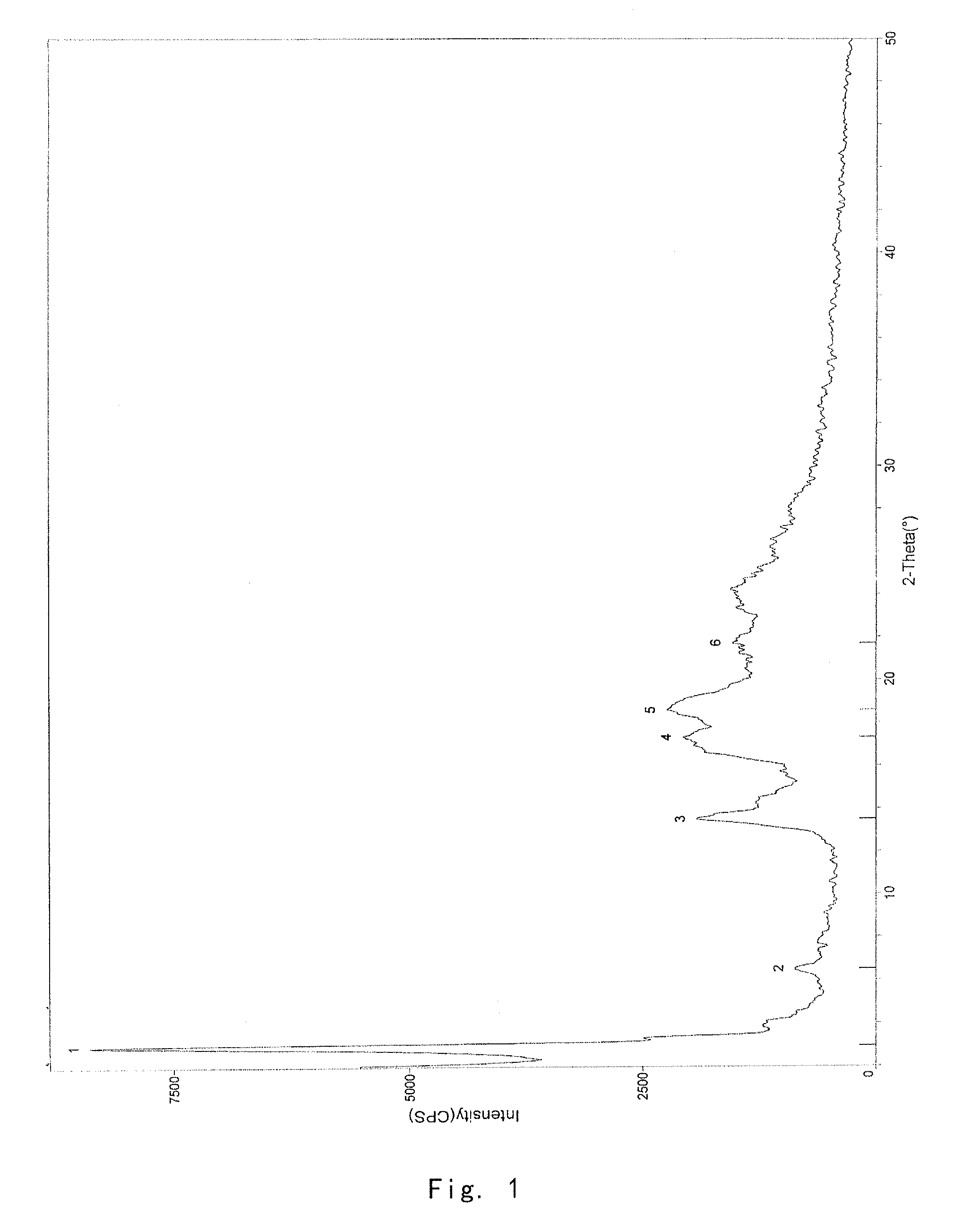
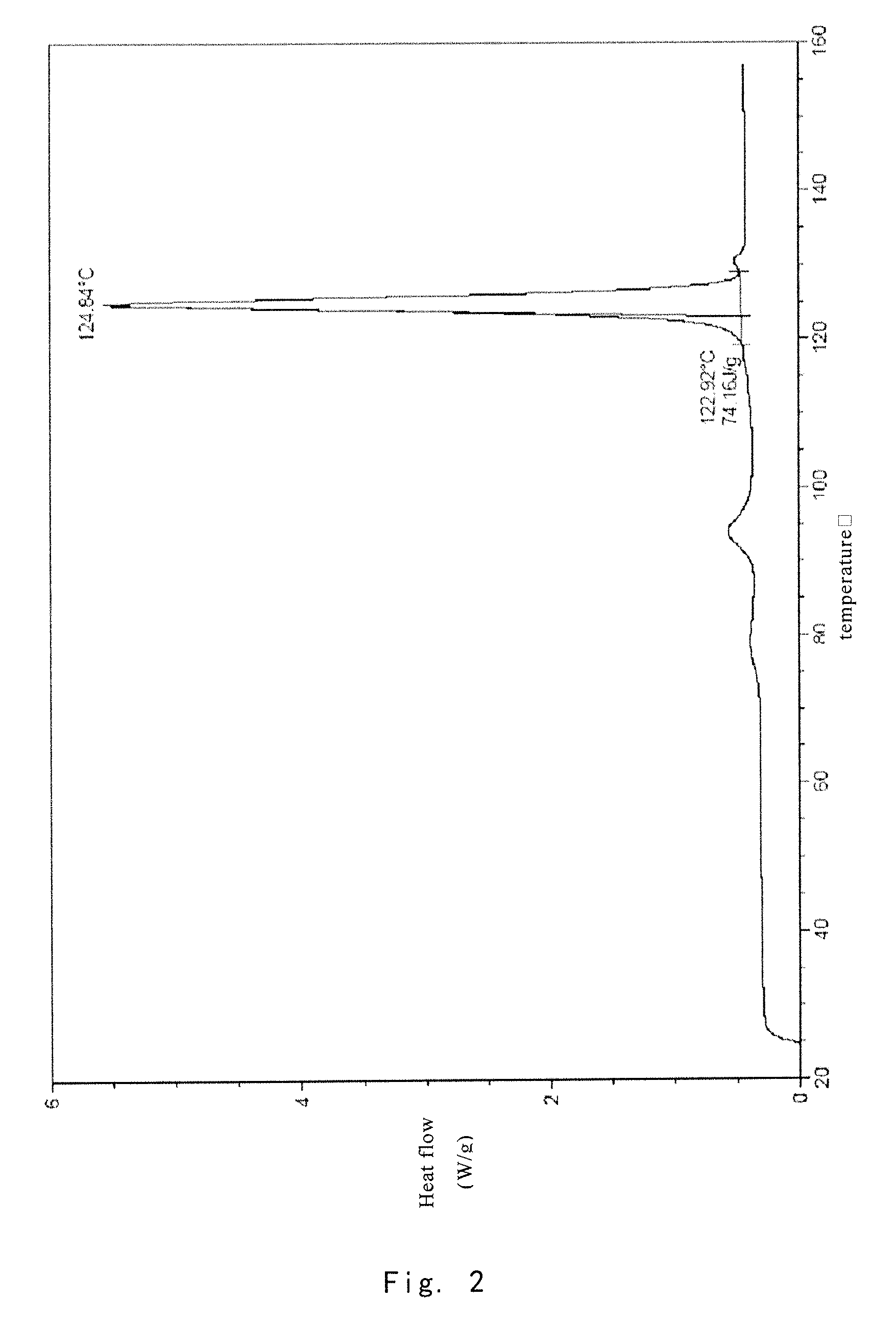
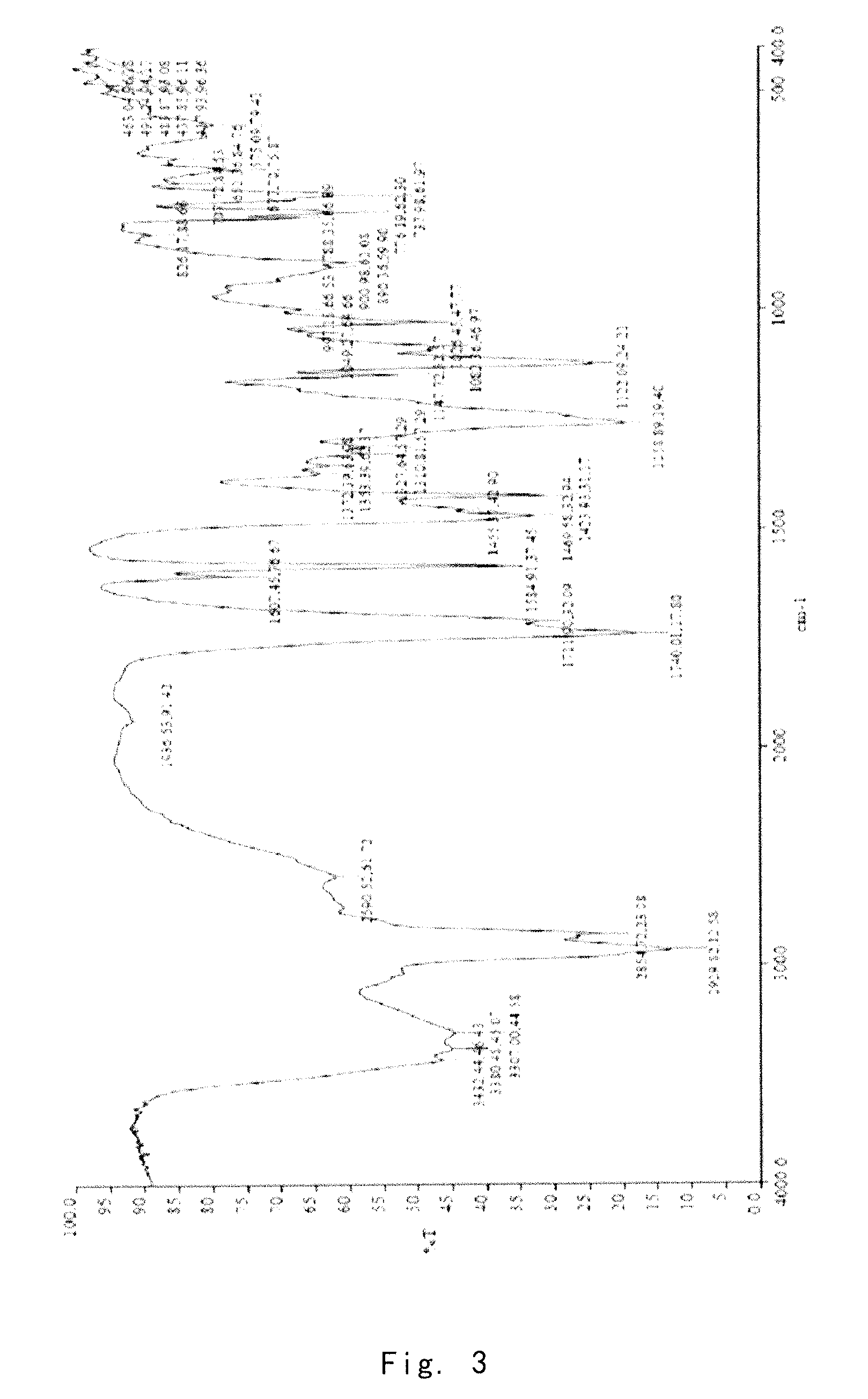
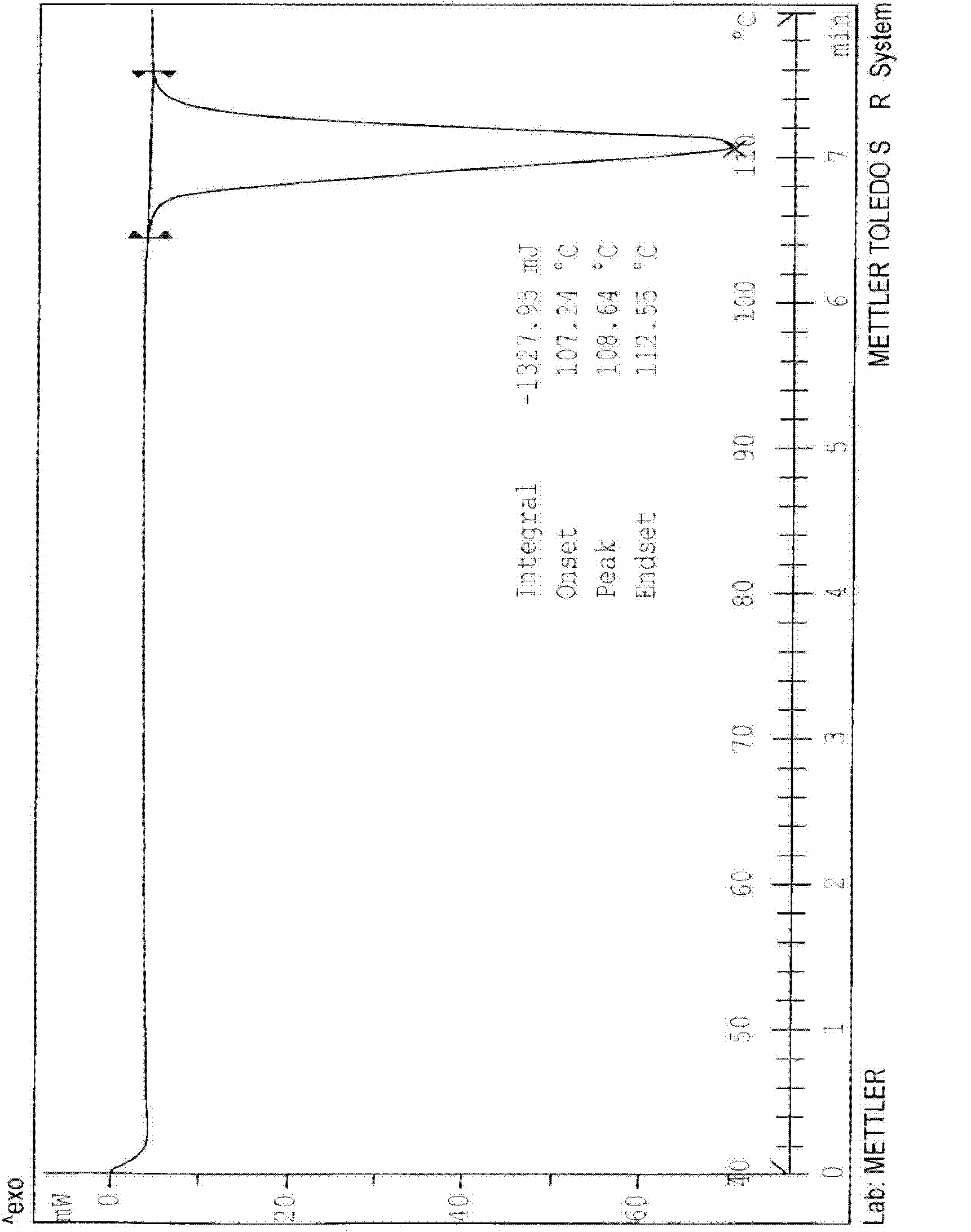
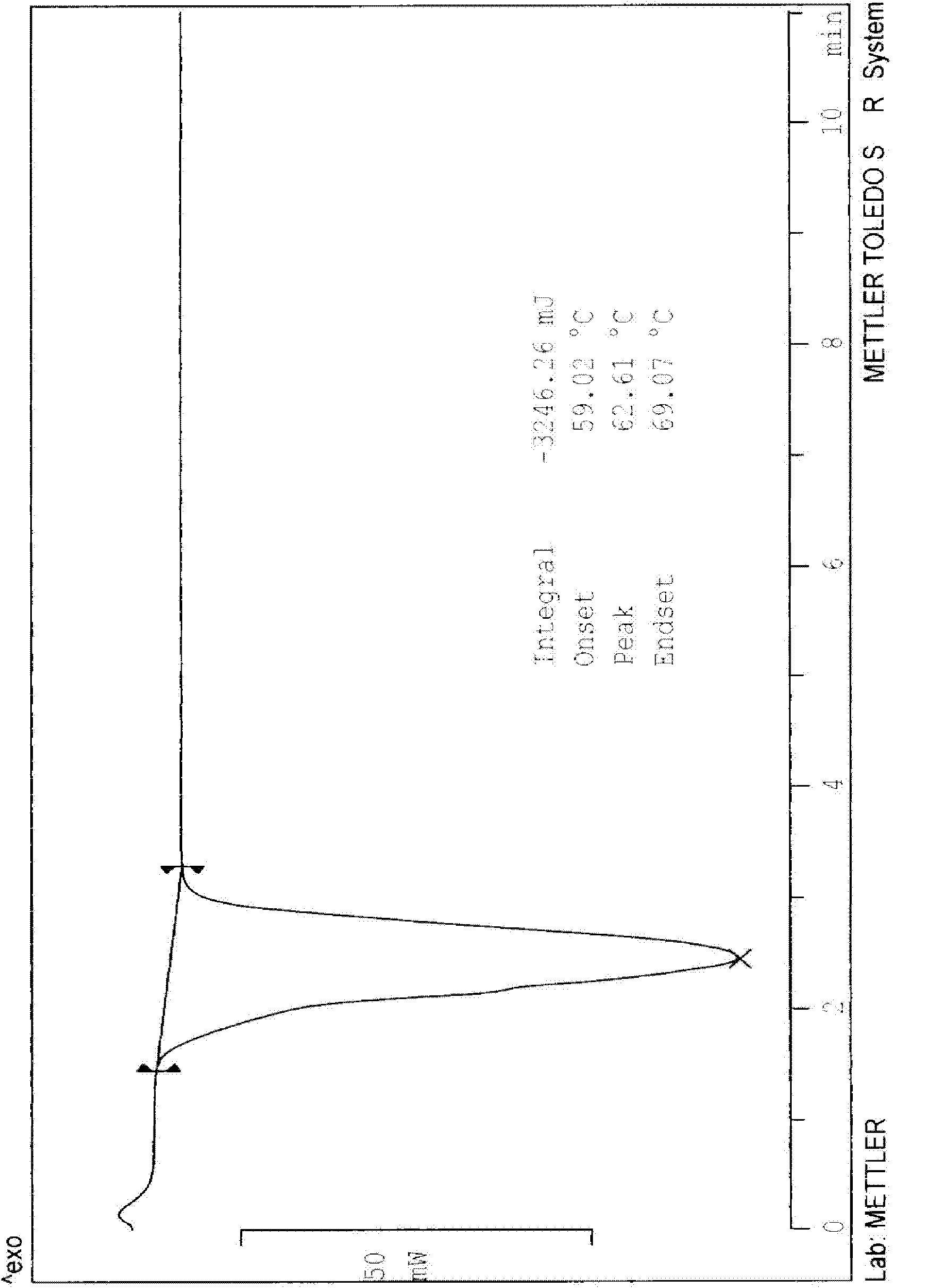
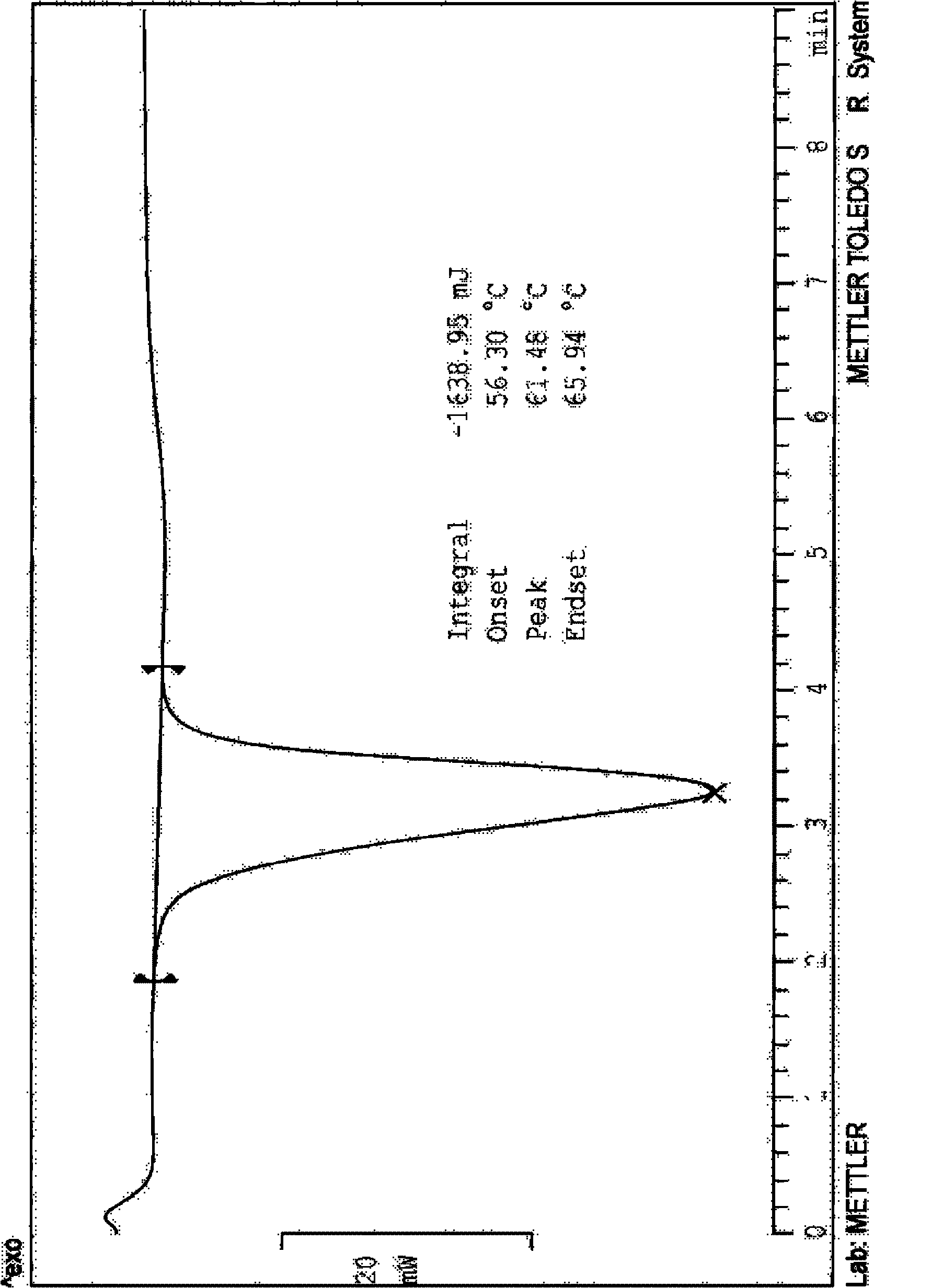
Crystalline form of 1-(beta-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate
A novel crystal form of 1-(β-D-glucopyranosyl)-4-methyl-3-[5-(4-fluorophenyl)-2-thienylmethyl]benzene hemihydrate, and having favorable characteristics, is characterized by its x-ray powder diffraction pattern and / or by its infra-red spectrum.
Owner:MITSUBISHI TANABE PHARMA CORP
Hydroprocessing bulk catalyst and uses thereof
ActiveUS7737073B2Inorganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsTO-18X-ray
A hydroprocessing bulk catalyst is provided. A process to prepare hydroprocessing bulk catalysts is also provided. The hydroprocessing catalyst has the formula (Mt)a(Lu)b(Sv)d(Cw)e(Hx)f(Oy)g(Nz)h, wherein M is at least one group VIB metal; promoter metal L is optional and if present, L is at least one Group VIII non-noble metal; t, u, v, w, x, y, z representing the total charge for each of the components (M, L, S, C, H, O and N, respectively); ta+ub+vd+we+xf+yg+zh=0; 0=<b; and 0=<b / a=<5, (a+0.5b)<=d<=(5a+2b), 0<=e<=11(a+b), 0<=f<=7(a+b), 0<=g<=5(a+b), 0<=h<=0.5(a+b). The catalyst has an X-ray powder diffraction pattern with at least one broad diffraction peak at any of Bragg angles: 8 to 18°, 32 to 40°, and 55 to 65° (from 0 to 70° 2-θ scale). In one embodiment, the catalyst is prepared by sulfiding at least one Group VIB metal compound and optionally at least one group VIII metal compound with a sulfiding agent forming a catalyst precursor; and mixing the catalyst precursor with a hydrocarbon compound to form the hydroprocessing catalyst composition.
Owner:CHEVROU USA INC
Ambroxol hydrochloride compound and medicine composition thereof
The invention discloses an ambroxol hydrochloride crystal and a medicine composition prepared by the ambroxol hydrochloride crystal. The ambroxol hydrochloride crystal shows specific diffractive peaks in parts of 6.9 degrees, 7.2 degrees, 12.8 degrees, 15.6 degrees, 17.5 degrees, 20 degrees, 21 degrees, 22 degrees and 24 degrees in an X-ray powder diffraction pattern which is shown in form of 2 Theta + / -0.2 degrees. The prepared ambroxol hydrochloride crystal is high in yield and liquidity; and the amount of lubricant in original prescription is halved, so that the weight and uniformity of tablets can meet the specification.
Owner:SHENYANG XINMA PHARMA +2
Pantoprazole sodium compound and pharmaceutical composition thereof
ActiveCN102351844AStable moisture contentNo changeOrganic active ingredientsPowder deliveryFreeze-dryingX-ray
The invention discloses a pantoprazole sodium compound, which is crystal. In X-ray powder diffraction pattern obtained through Cu-Kalpha ray measurement, the characteristic peaks of the pantoprazole sodium compound are shown in positions where 2theta is 12.5, 12.6, 13.2, 16.2 and 17.3. The pantoprazole sodium compound can be used together with multiple freeze-drying supporting agents, the prepared freeze-dried powder injection has the advantages of good redissolution, good transparency after redissolution, low impurity content and the like; and moreover, the use amount of the freeze-drying supporting agent is lower, thus saving the pharmaceutical cost and improving the stability of a drug preparation. The invention also discloses a pharmaceutical composition. The pharmaceutical composition comprises a pharmaceutical active ingredient and pharmaceutical auxiliary materials, wherein the pharmaceutical active ingredient is the pantoprazole sodium compound. The stability of the pharmaceutical composition is obviously superior to that of commercial products, and especially, the stability duration of the pharmaceutical composition after being matched with common infusion fluid is prolonged, thus facilitating the clinical application.
Owner:江西新先锋医药有限公司
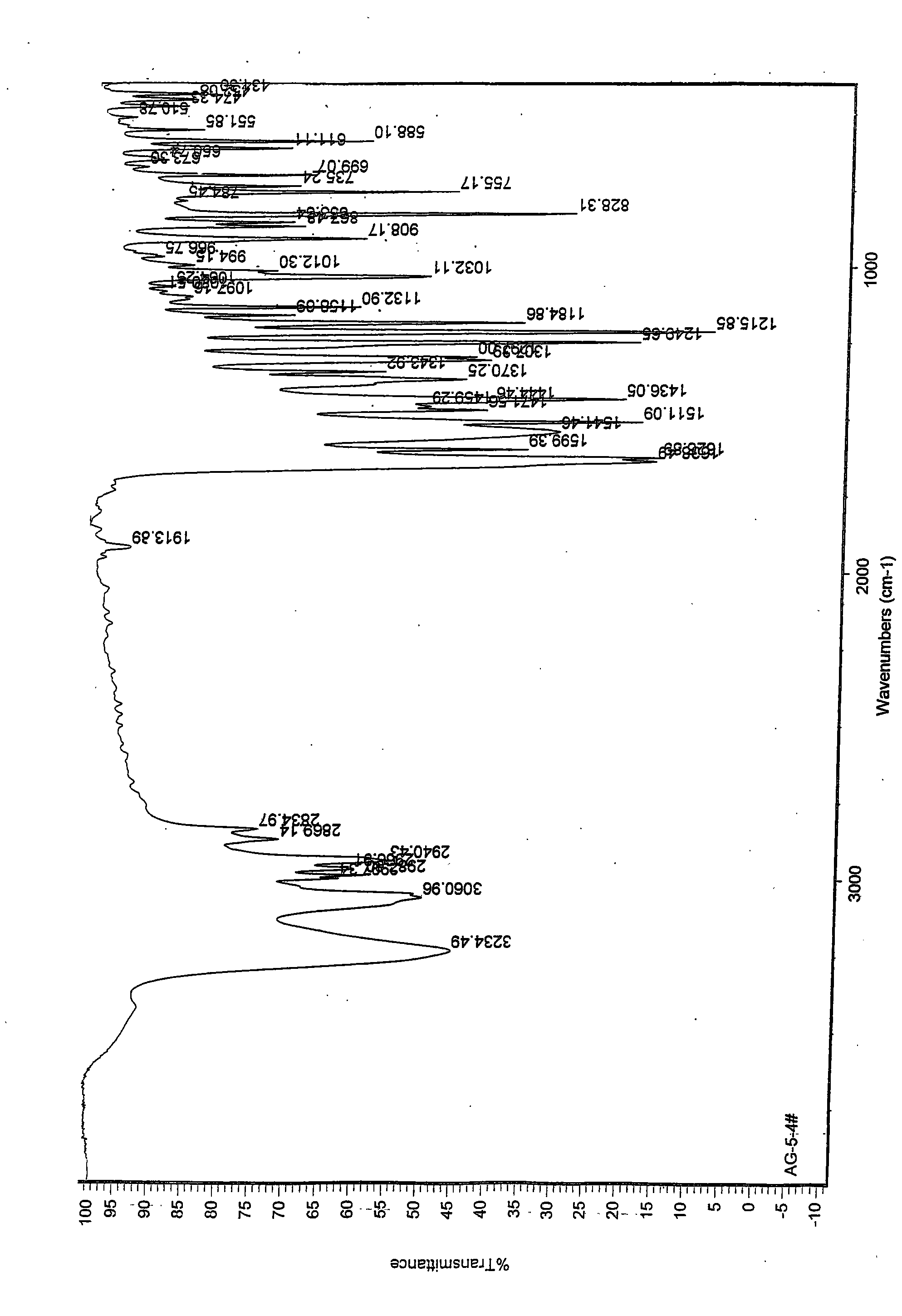
Agomelatine and medicine composition thereof
ActiveCN101781226AQuality improvementImprove solubilityOrganic active ingredientsNervous disorderK-alphaPowder diffraction
The invention discloses a medicine of Agomelatine for treating depression, in particular an Agomelatine crystal and a medicine composition thereof. The Agomelatine crystal is irradiated by using Cu-K alpha; and an X-ray powder diffraction spectrum expressed by the degree of 2theta of the Agomelatine crystal has characteristic diffraction peaks at positions of 12.84, 13.84, 16.14, 14.18, 18.56, 19.12, 20.86, 21.20 and 23.84, and an infrared absorption spectrum of the Agomelatine crystal has characteristic diffraction peaks approximately at position of 3234, 3060, 2940, 1638, 1511, 1436, 1249, 1215, 1184, 1032, 908, 828, 755 and 588cm-1. The DSP heat absorption transition is 97.6 DEG C. The invention further discloses application of the medicine composition containing the Agomelatine crystal as an active effective component to the preparation of medicines for treating the depression.
Owner:TIPR PHARM CO LTD
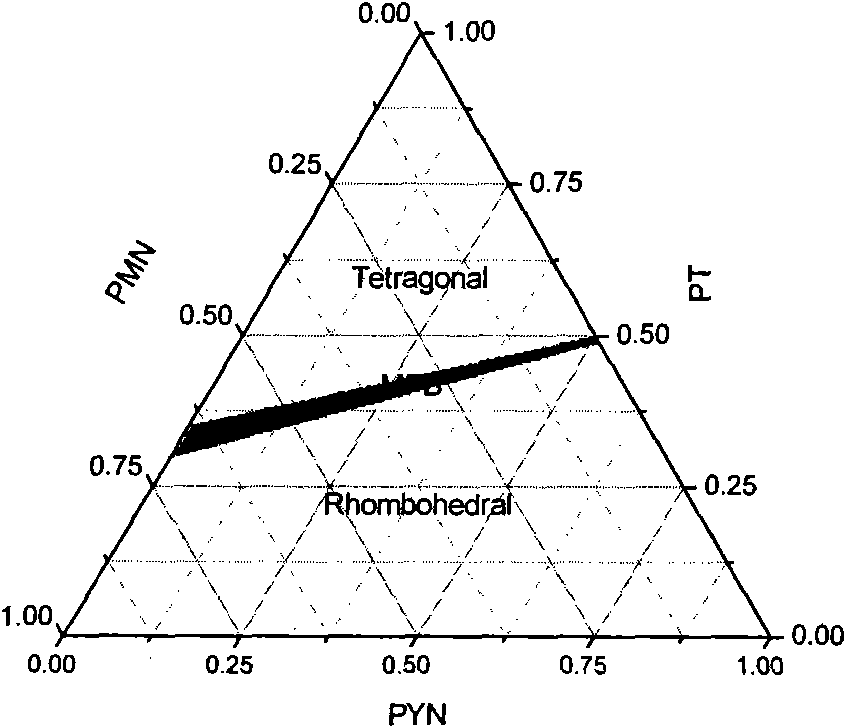
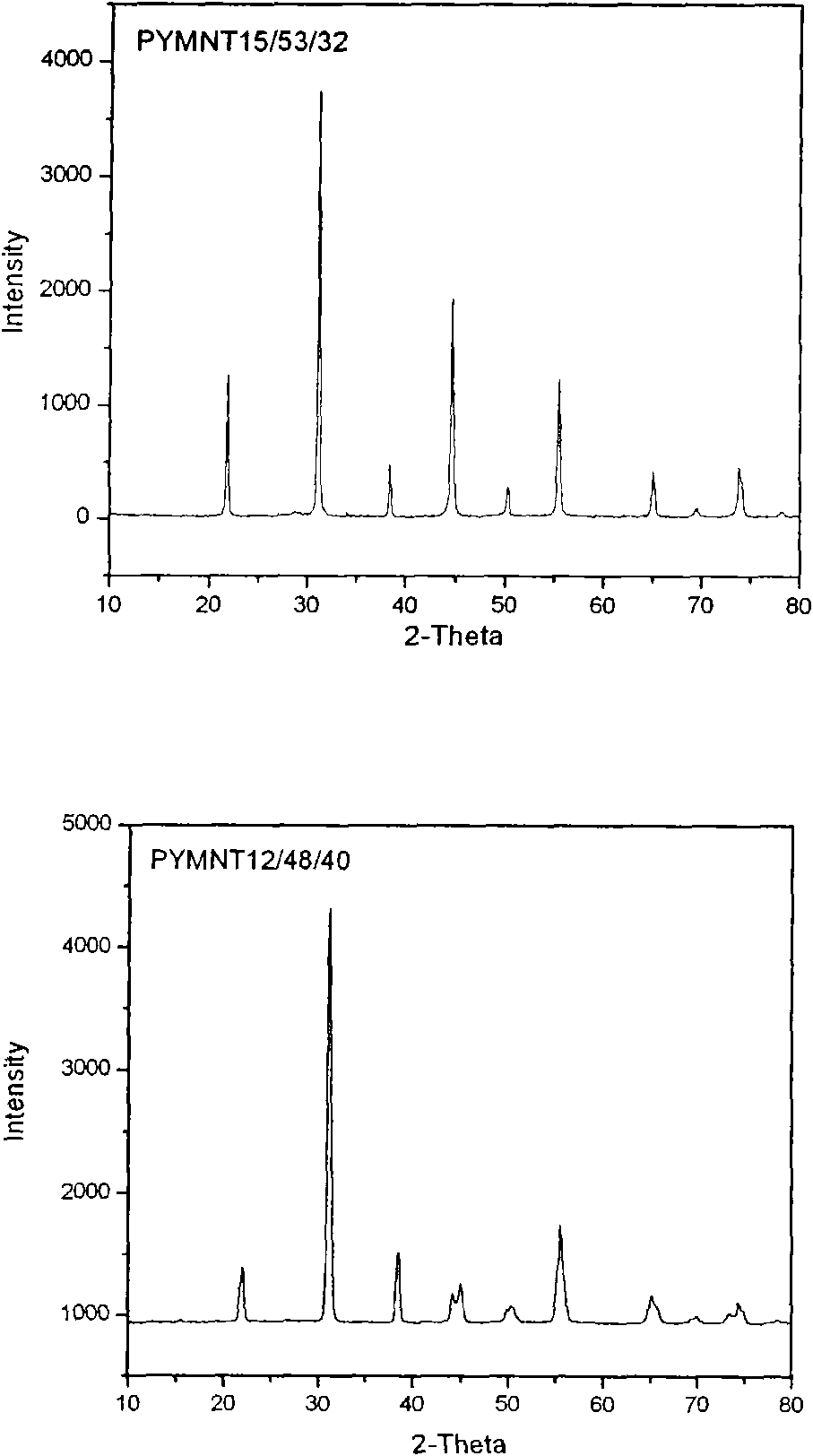
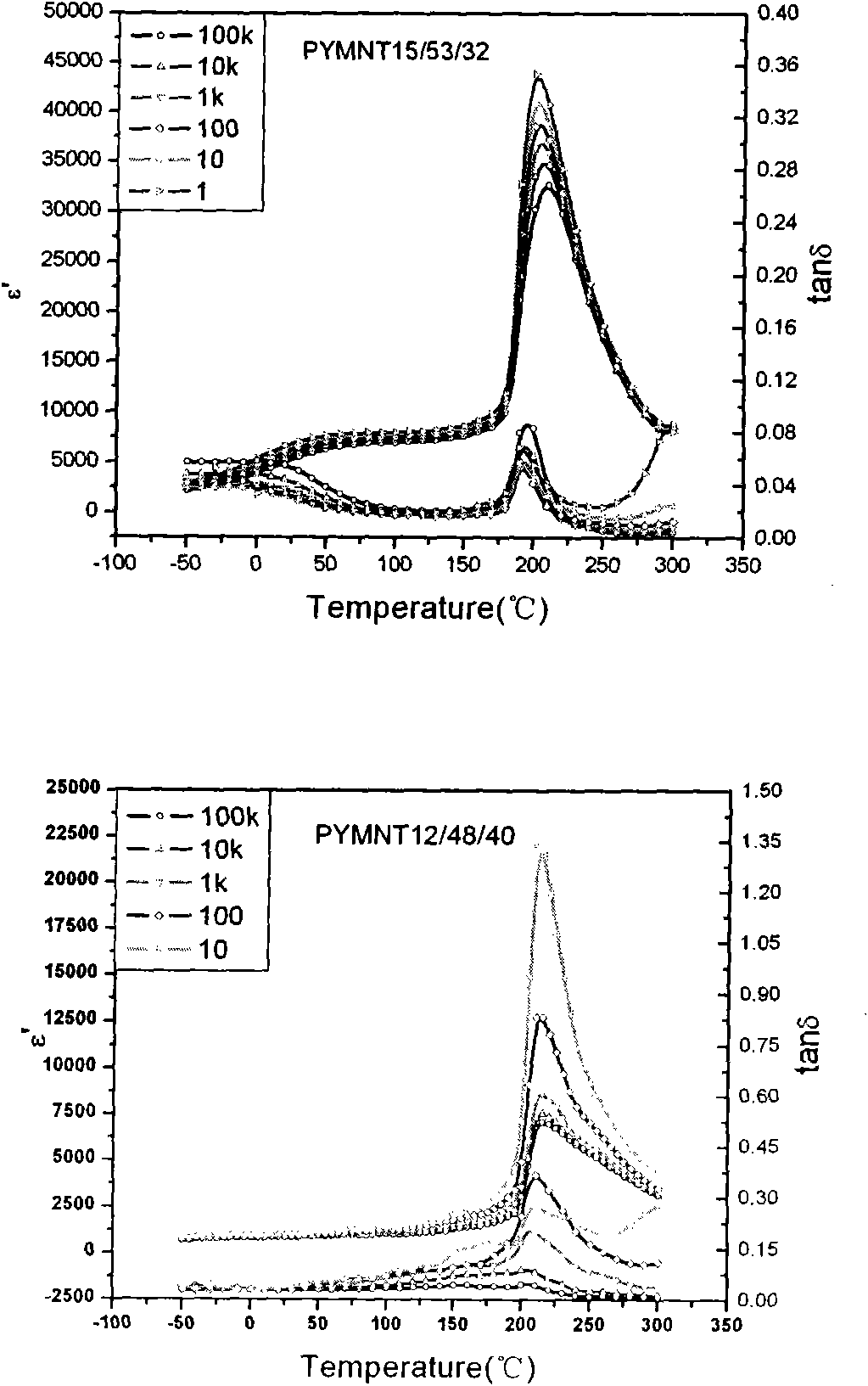
Novel ferroelectric single-crystal lead ytterbium niobate-lead magnesium niobate-lead titanate
InactiveCN102051685APolycrystalline material growthFrom melt solutionsCrystal rotationSingle crystal
The invention relates to the growth, the structures and the properties of novel ferroelectric single-crystal lead ytterbium niobate-lead magnesium niobate-lead titanate. The crystal belongs to a perovskite structure, has an MPB region and has a chemical formula of (1-x-y)Pb(Yb1 / 2Nb1 / 2)O3-xPb(Mg1 / 3Nb2 / 3)O3-yPbTiO3 which is short for PYMNT or PYN-PMN-PT. By adopting a top crystal-seeded method, the crystal with large size and high quality can grow under the conditions that the growth temperature of the crystal is 950-1100 DEG C, the crystal rotation speed is 5-30rpm, and the cooling speed is 0.2-5 DEG C / day, and the grown crystal exposes a 001 natural growth surface. Through X-ray powder diffraction, the system is confirmed as the perovskite structure; and through ferroelectric, dielectric and piezoelectric measurement, the ferroelectricity, the dielectric property and the piezoelectricity of the crystal are analyzed. The crystal has high Curie temperature and trigonal-tetragonal phase transition temperature, large piezoelectric constant and electromechanical coupling factor, high dielectric constant and low dielectric loss and better heat stability. The crystal can be widely applied to devices in the piezoelectric fields of ultrasonically medical imaging, sonar probes, actuators, ultrasonic motors, and the like.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
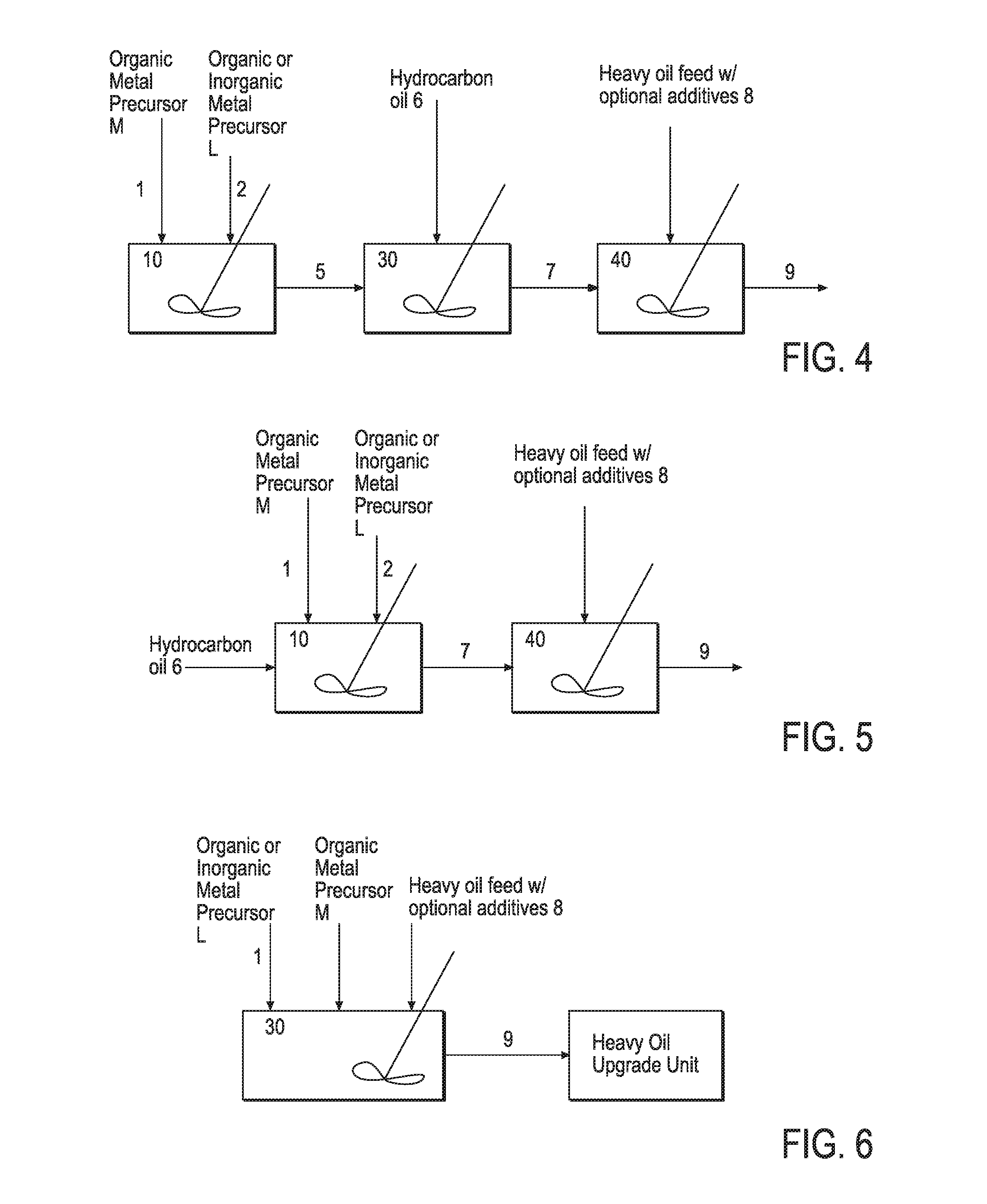
Hydroprocessing bulk catalyst and methods of making thereof
ActiveUS8372776B2Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationLanthanideX-ray
A hydroprocessing bulk catalyst is provided. A process to prepare hydroprocessing bulk catalysts is also provided. The hydroprocessing catalyst has the formula (Rp)i(Mt)a(Lu)b(Sv)d(Cw)e(Hx)f(Oy)g(Nz)h, wherein M is at least at least a “d” block element metal; L is also at least a “d” block element metal, but different from M; t, u, v, w, x, y, z representing the total charge for each of the components (M, L, S, C, H, O and N, respectively); R is optional and in one embodiment, R is a lanthanoid element metal; 0<=i<=1; pi+ta+ub+vd+we+xf+yg+zh=0; 0<b; 0<b / a=<5; 0.5(a+b)<=d<=5(a+b); 0 <e<=11(a+b); 0<f<=7(a+b); 0<g<=5(a+b); 0<h<=2(a+b). The catalyst has an X-ray powder diffraction pattern with at least three diffractions peak located at 2-θ angles of greater than 25°. In one embodiment, the catalyst is prepared by forming at least a sulfided catalyst precursors from at least two “d” block element metals; and mixing the catalyst precursor with a hydrocarbon compound to form the hydroprocessing catalyst composition. In another embodiment, the catalyst is prepared by the thermal decomposition of an oil dispersible sulfur containing organic metal precursor upon contact with a hydrocarbon oil, generating a slurry catalyst. In yet another embodiment, the catalyst is prepared from an in-situ or ex-situ sulfidation of “d block element metal precursors in a solvent carrier.
Owner:CHEVROU USA INC
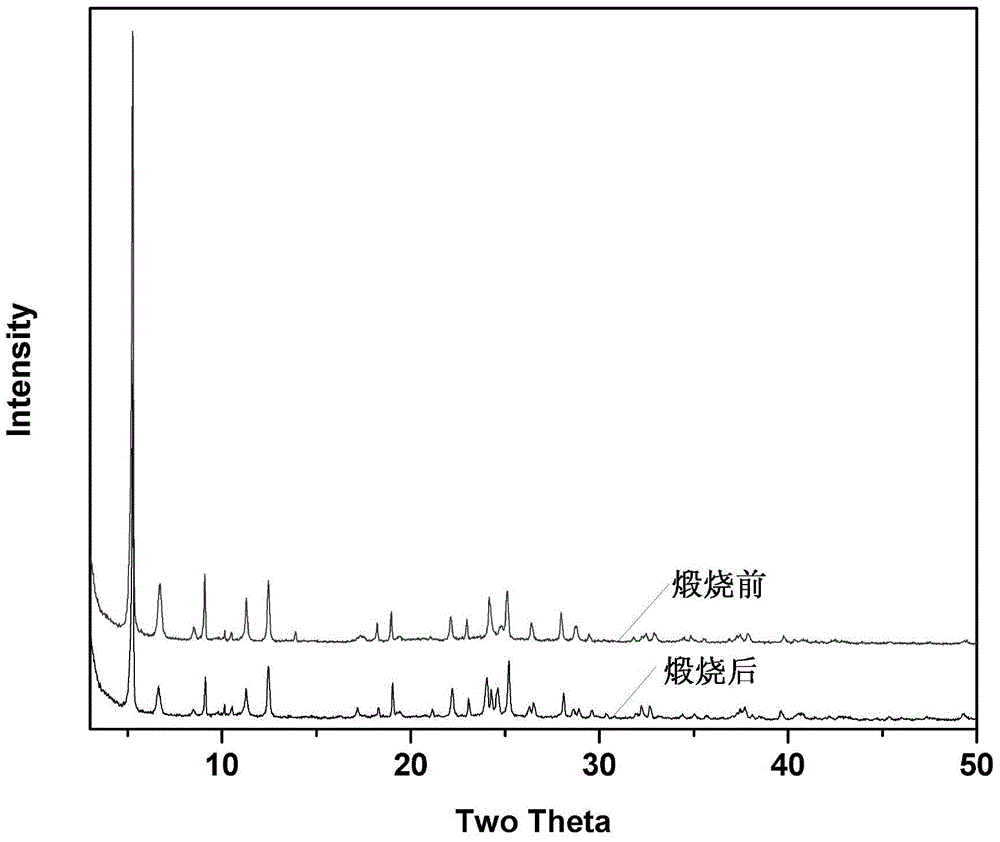
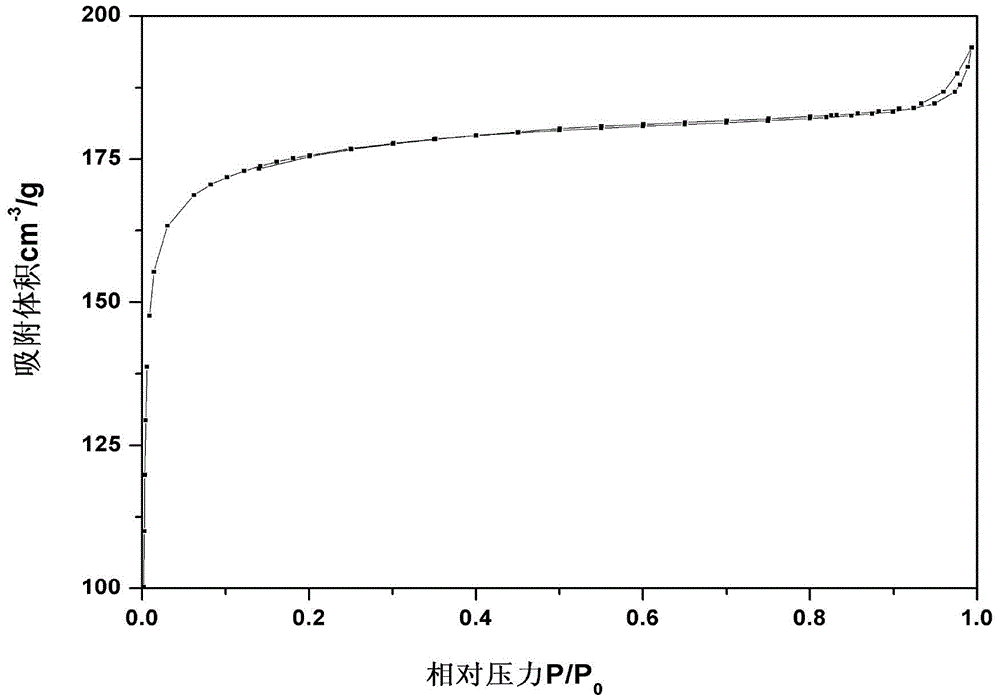
Super-macroporous silicate molecular sieve NUD-1 and preparation method thereof
InactiveCN104370296AMolecular sieve catalystsMolecular-sieve and base-exchange compoundsMolecular sieveHeteroatom
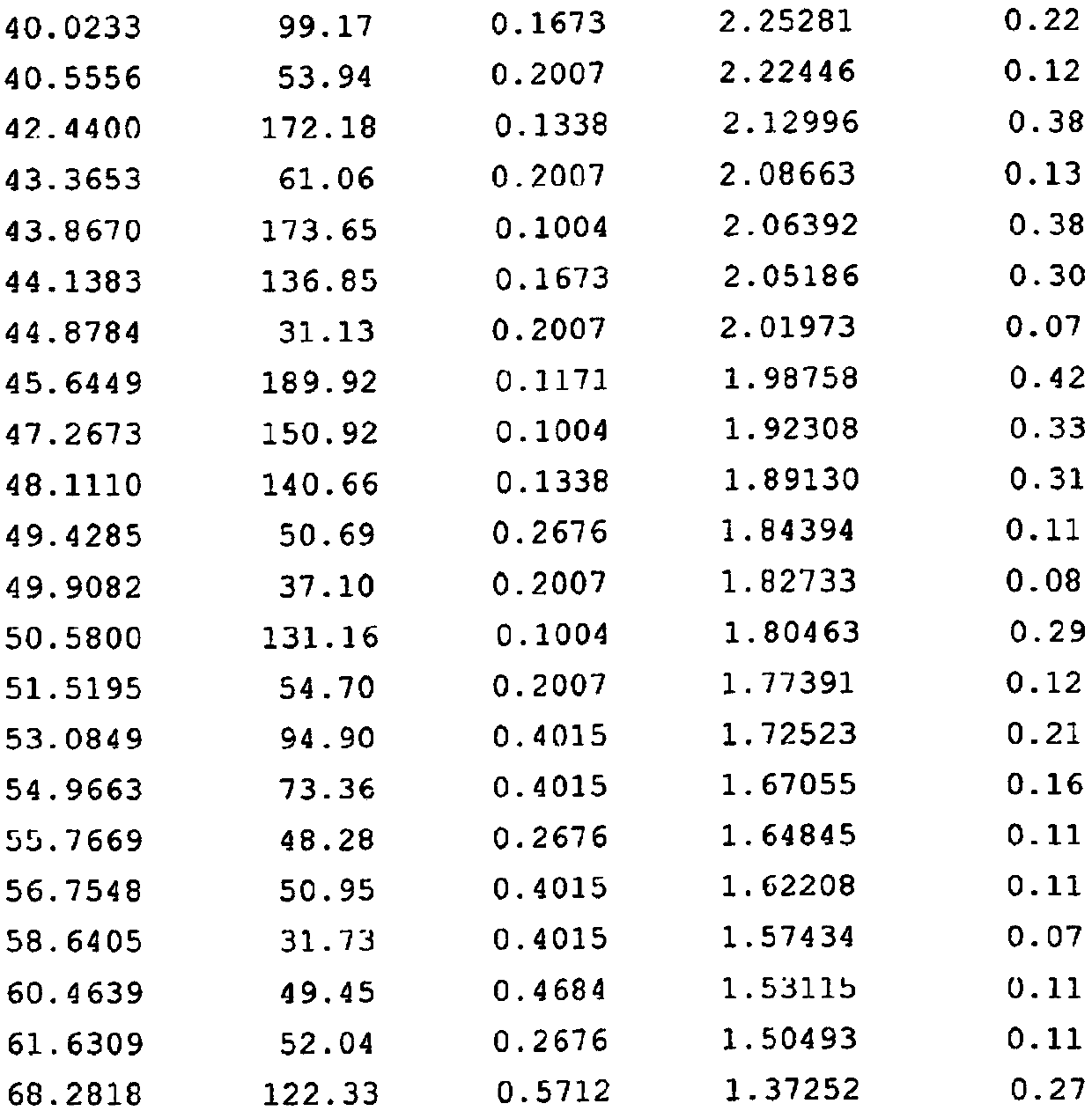
The invention discloses a super-macroporous silicate molecular sieve material and a preparation method thereof, the molecular sieve has the X ray powder diffraction characteristic shown in Table 1, is obtained by hydrothermal synthesis method, has a three-dimensional pore structure of 18 * 12 * 10, good thermal stability, and larger specific surface area, can be incorporated into heteroatom, and has potential application values in petrochemistry, fine chemical engineering and life science and other fields.
Owner:NANJING UNIV
Hydroprocessing Bulk Catalyst and Uses Thereof
ActiveUS20090200204A1Inorganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsNon noble metalPowder diffraction
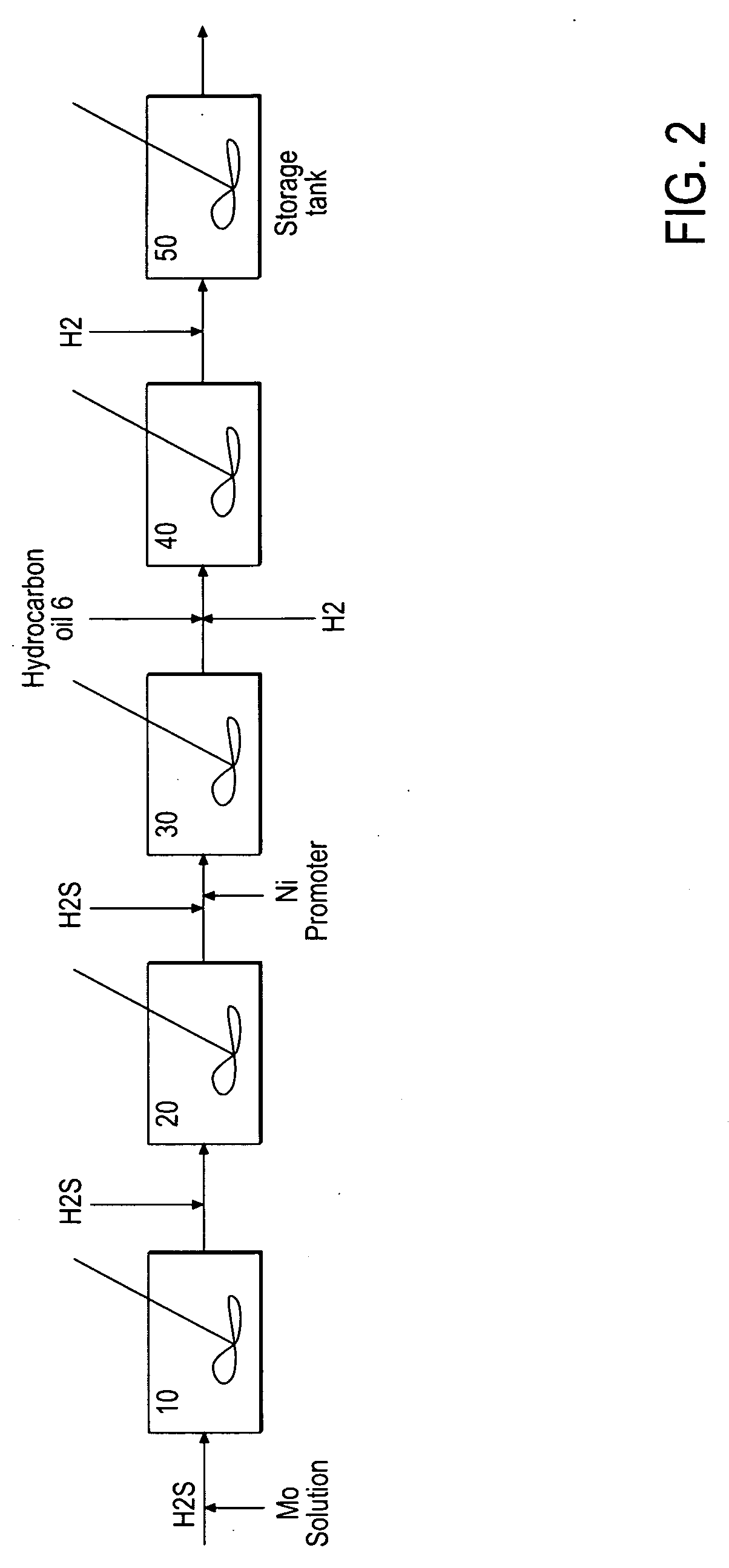
A hydroprocessing catalyst is provided. The hydroprocessing catalyst has the formula (Mt)a(Xu)b(Sv)d(Cw)e(Hx)f(Oy)g(Nz)h, wherein M is at least one group VIB metal; X is at least one Group VIII non-noble metal; t, u, v, w, x, y, z representing the total charge for each of the components (M, X, S, C, H, O and N, respectively); ta+ub+vd+we+xf+yg+zh=0; and 0=<b / a=<5, (a+0.5b)<=d<=(5a+2b), 0<=e<=11(a+b), 0<=f<=7(a+b), 0<=g<=5(a+b), 0<=h<=0.5(a+b). The catalyst has an X-ray powder diffraction pattern with at least one broad diffraction peak at any of Bragg angles: 8 to 18°, 32 to 40°, and 55 to 65° (from 0 to 70° 2-θ scale). In one embodiment, the at least one diffraction peak is greater than 2 degrees wide at ½ height.
Owner:CHEVROU USA INC
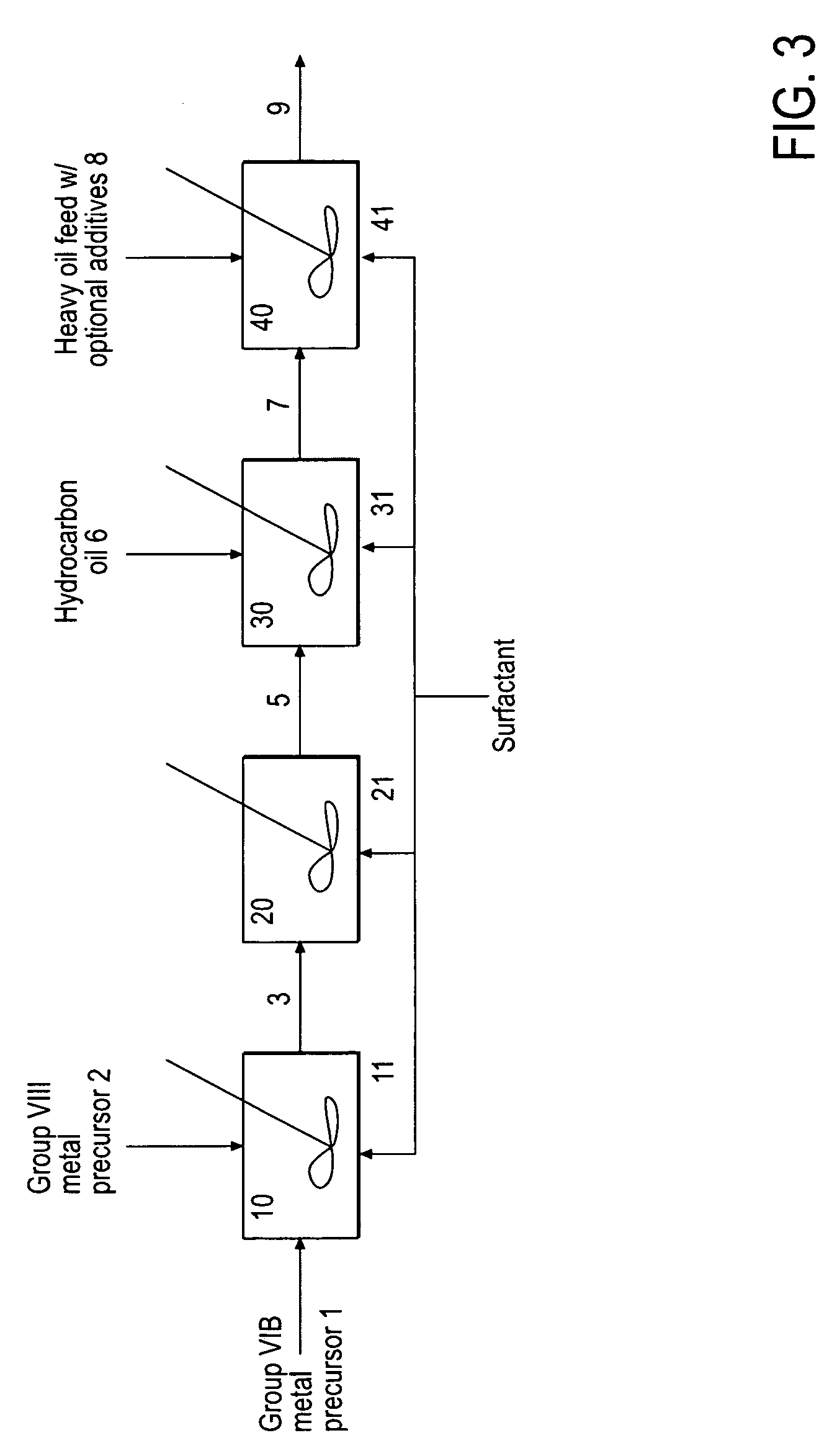
Hydroprocessing Bulk Catalyst and Uses Thereof
ActiveUS20090054225A1Inorganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsTO-18X-ray
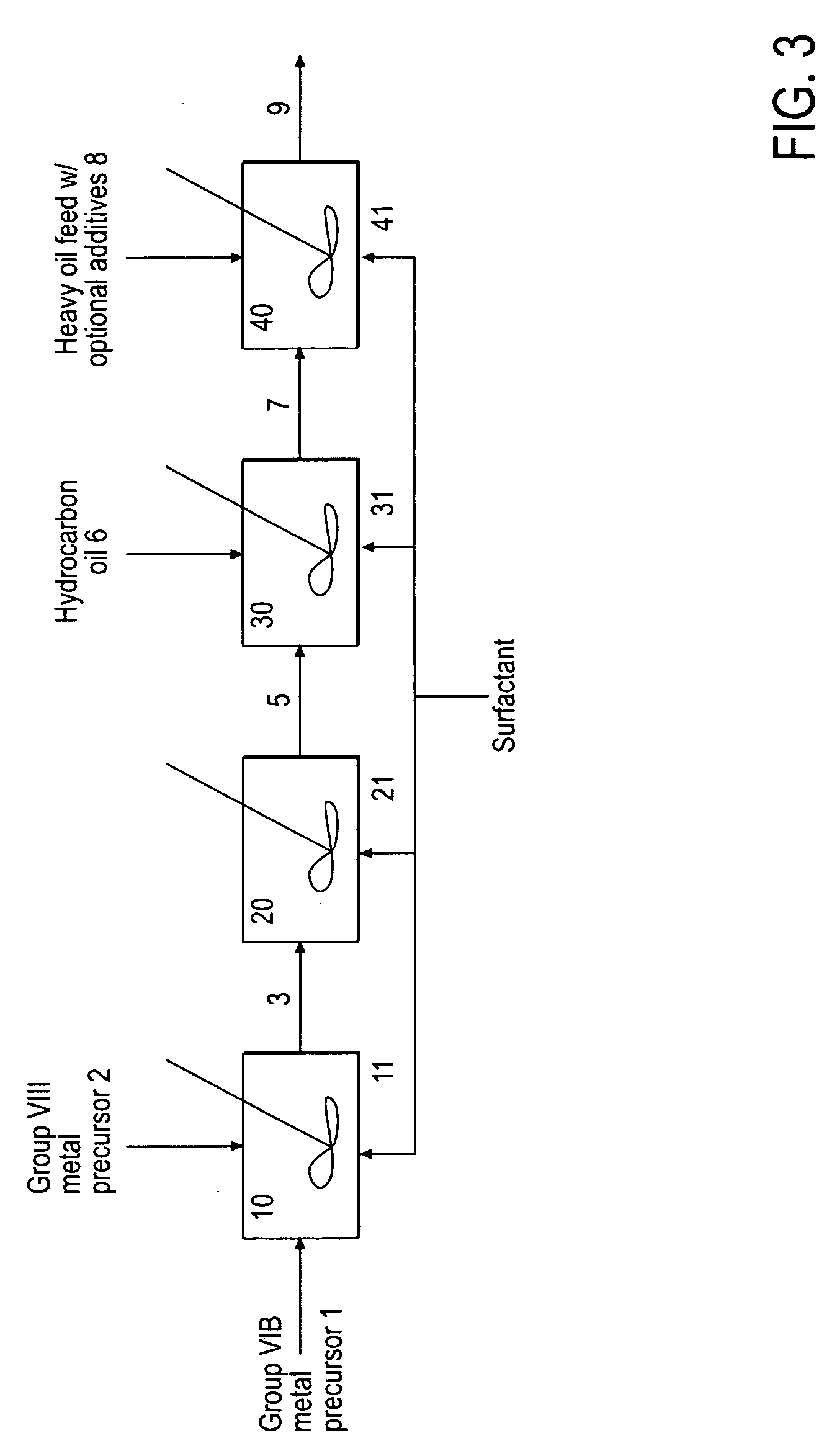
A hydroprocessing bulk catalyst is provided. A process to prepare hydroprocessing bulk catalysts is also provided. The hydroprocessing catalyst has the formula (Mt)a(Lu)b(Sv)d(Cw)e(Hx)f(Oy)gNz)h, wherein M is at least one group VIB metal; L is optional and if present, L is at least one Group VIII non-noble metal; t, u, v, w, x, y, z representing the total charge for each of the components (M, L, S, C, H, O and N, respectively); ta+ub+vd+we+xf+yg+zh=0; 0=<b; and 0=<b / a=<5, (a+0.5b)<=d<=(5a+2b), 0<=e<=11(a+b), 0<=f<=7(a+b), 0<=g<=5(a+b), 0<=h<=0.5(a+b). The catalyst has an X-ray powder diffraction pattern with at least one broad diffraction peak at any of Bragg angles: 8 to 18°, 32 to 40°, and 55 to 65° (from 0 to 70° 2-θ scale). In one embodiment, the catalyst is prepared by sulfiding at least one Group VIB metal compound and optionally at least one group VIII metal compound with a sulfiding agent forming a catalyst precursor; and mixing the catalyst precursor with a hydrocarbon compound to form the hydroprocessing catalyst composition, and wherein at least a surfactant is employed in making the preparation.
Owner:CHEVROU USA INC
2-(4'-methoxy phenoxy)-4-nitro methanesulfonanilide crystal form and preparation method thereof
ActiveCN103553984AEfficient manufacturingOrganic chemistryOrganic compound preparationPowder diffractionCrystal
The invention provides a 2-(4'-methoxy phenoxy)-4-nitro methanesulfonanilide crystal form and a preparation method thereof. In an X-ray powder diffraction pattern of the 2-(4'-methoxy phenoxy)-4-nitro methanesulfonanilide crystal form, X-ray diffraction peaks appear at 2 theta of 11.6+ / -0.2 DEG, 17.9+ / -0.2 DEG and 21.4+ / -0.2 DEG. The crystal form has high purity.
Owner:WUHAN OPTICS VALLEY HUMANWELL BIO PHARMA +2
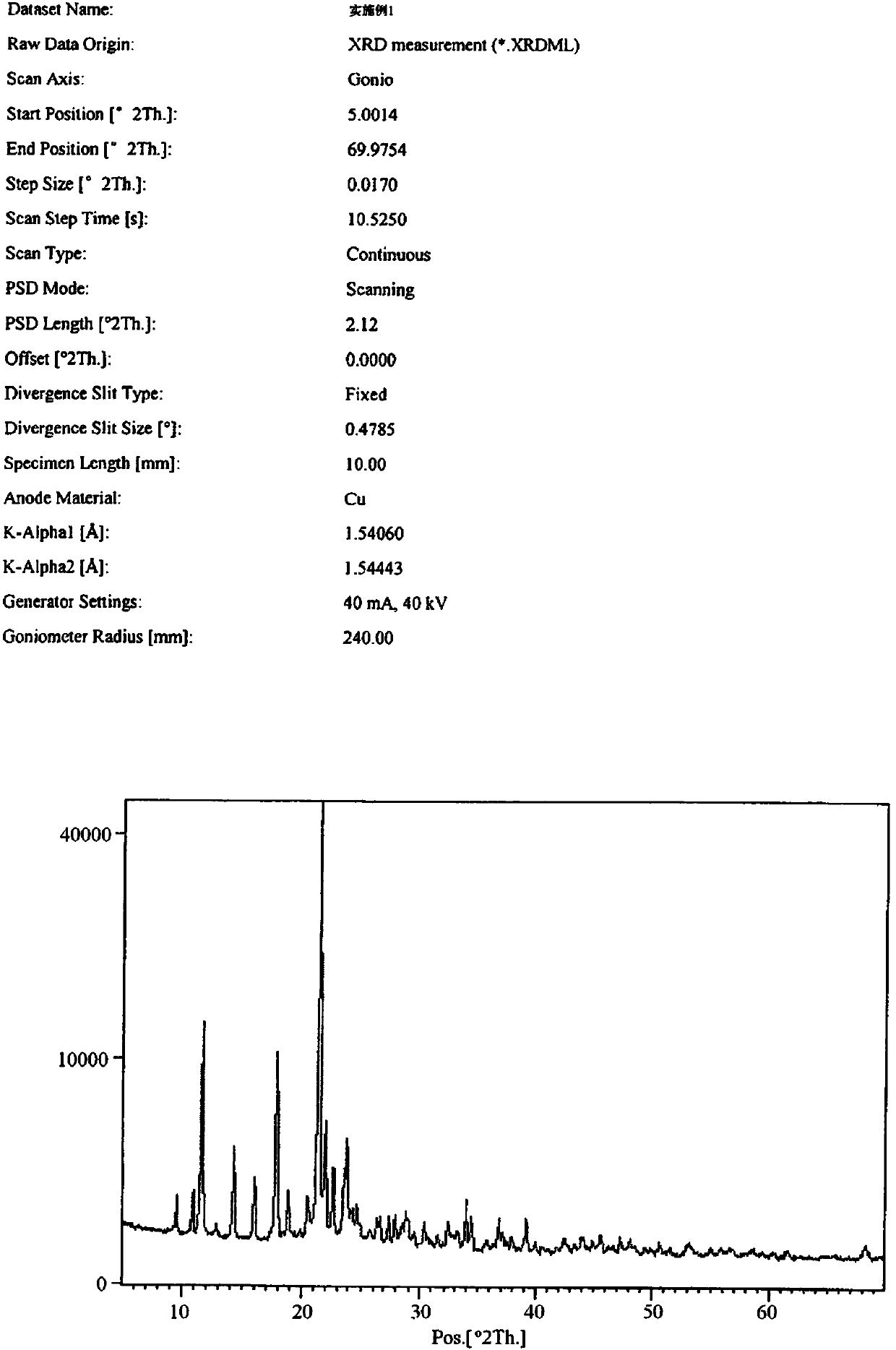
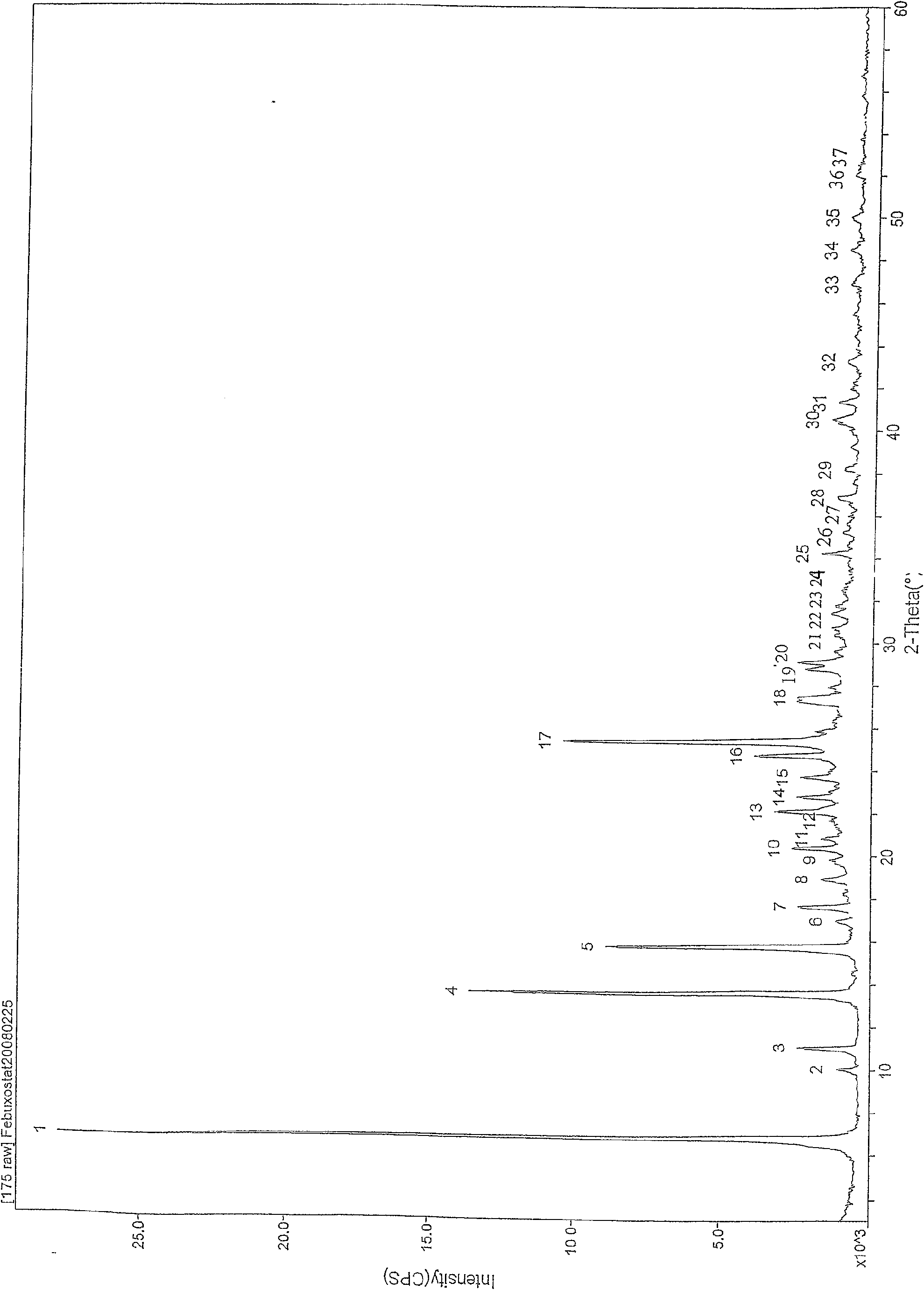
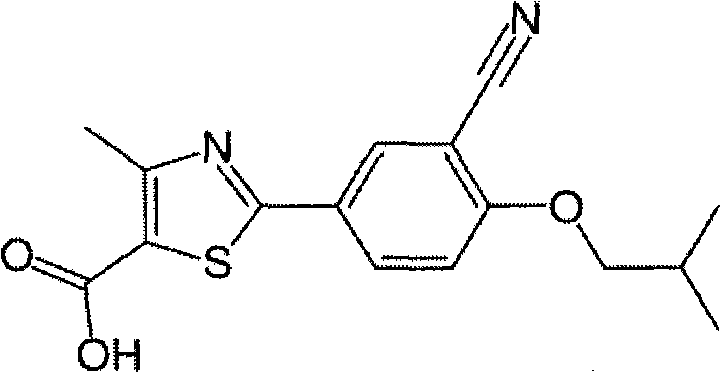
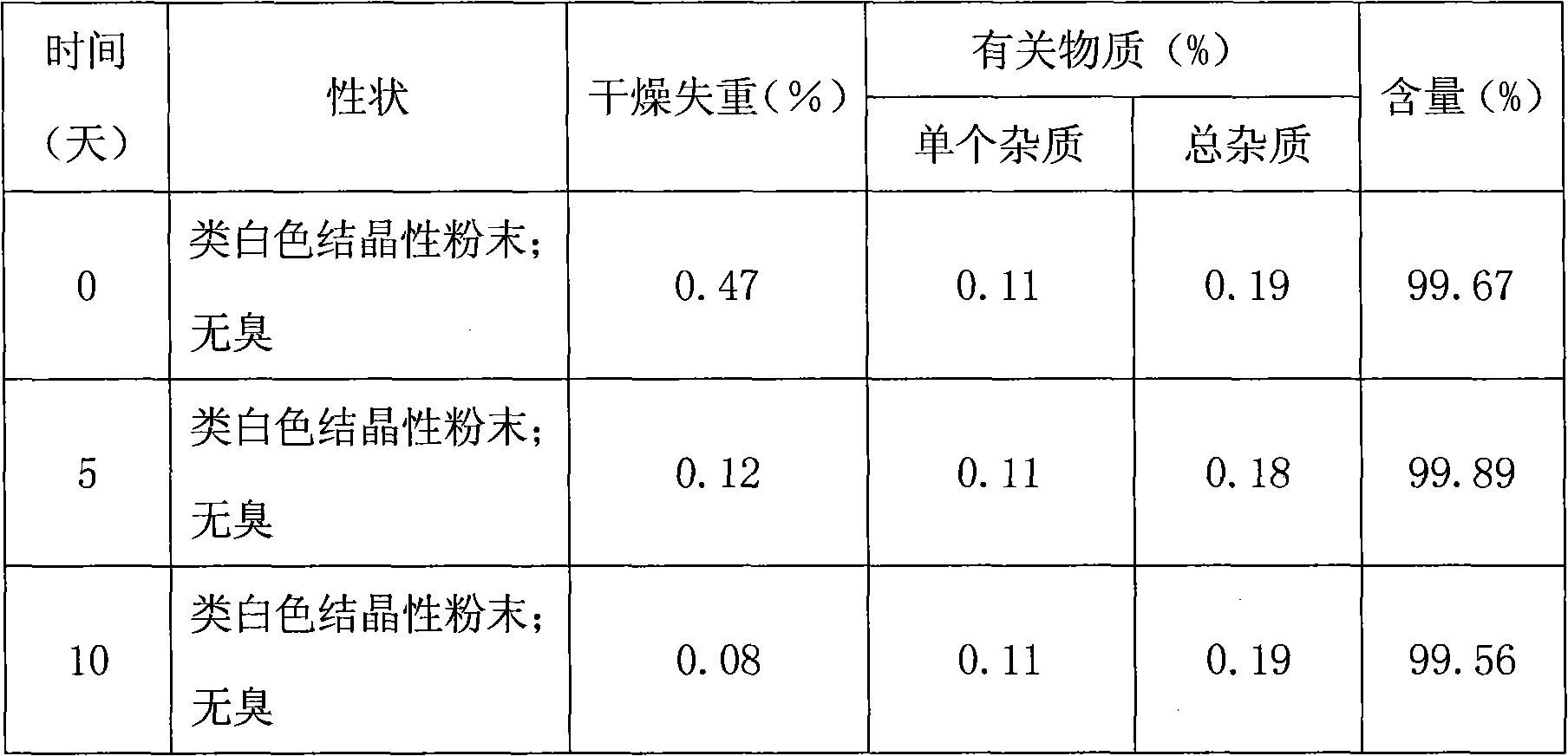
Febuxostat crystal form and preparation method thereof
The invention relates to a Febuxostat crystal form. The x-ray powder diffraction has characteristic peaks when a refraction angle 2 theta is at 6.80+ / - 0.2 degree, 11.04+ / - 0.2 degree, 13.56+ / - 0.2 degree, 15.74+ / -0.2 degree, 17.56+ / -0.2 degree, 20.36+ / -0.2 degree, 22.10+ / -0.2 degree, 24.72+ / - 0.2 degree, 25.38+ / - 0.2 degree, 28.80+ / -0.2 degree and 29.10 degrees+ / - 0.2 degree. The invention also provides a preparation method of the Febuxostat crystal form. The crystal form has better stability and is suitable for technically applying preparations and being stored in a long period.
Owner:CSPC OUYI PHARM CO LTD +1
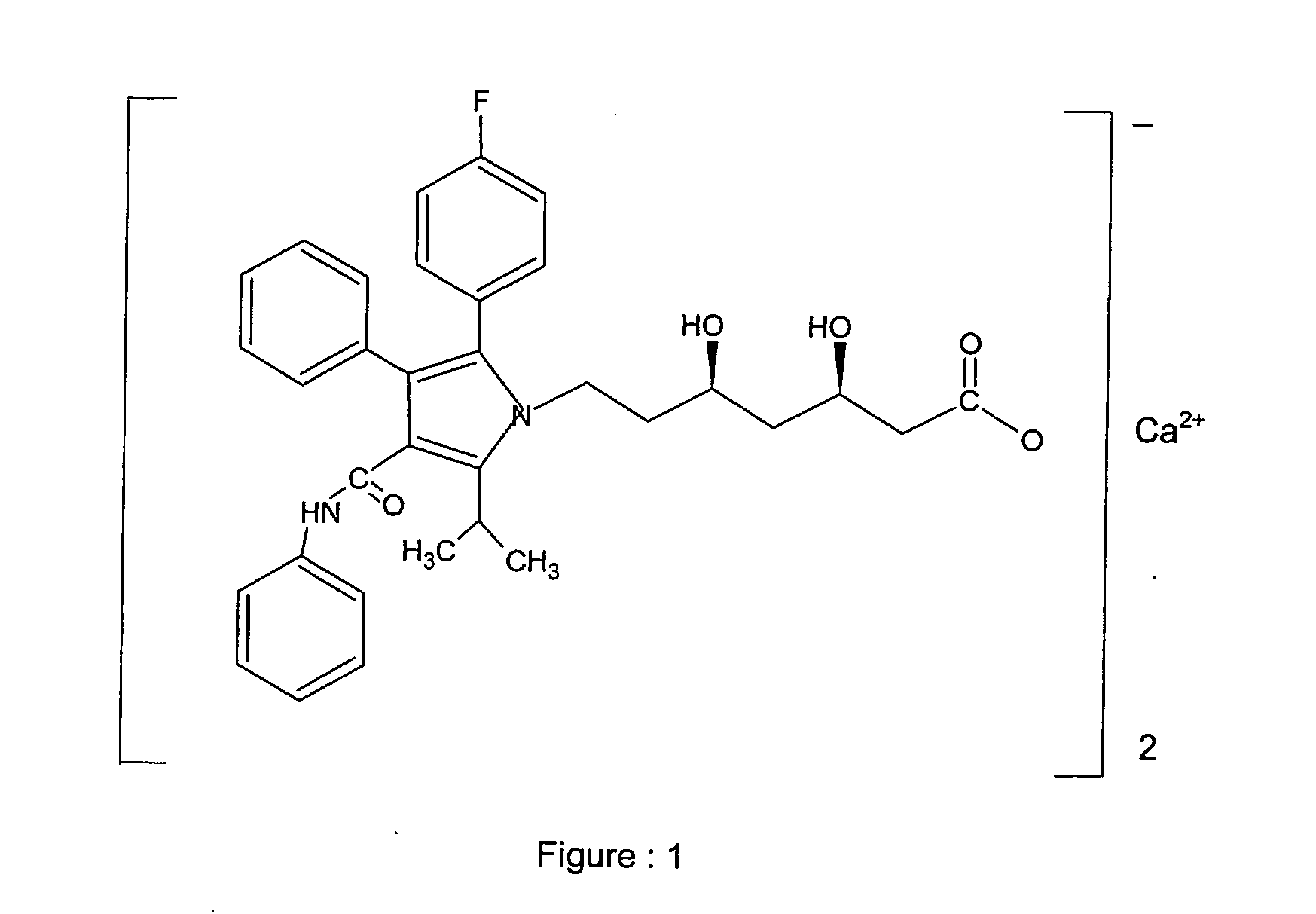
Atorvastatin calcium form vi or hydrates thereof
InactiveUS20060122403A1High purityImprove solubilityOrganic chemistryMetabolism disorderX-rayPowder diffraction
Atorvastatin calcium Form VI Or hydrates thereof, characterized by its X-ray powder diffraction and / or solid state NMR is described, as well as methods for the preparation of the same.
Owner:SURI SANJAY +3
C crystal form of canagliflozin and crystal preparation method of canagliflozin
InactiveCN103936725AImprove stabilityImprove solubilityNervous disorderOrganic chemistrySolubilityX-ray
The invention discloses a C crystal form of canagliflozin and a crystal preparation method of the canagliflozin. The X-ray powder of the canagliflozin, which is diffracted at diffraction angles 2 theta which are equal to 3.4 + / - 0.1, 6.5 + / - 0.1, 12.7 + / - 0.1, 15.8 + / - 0.1, 19.8 + / - 0.1, 24.3 + / - 0.1, 24.8 + / - 0.1 and 29.1 + / - 0.1, has characteristic peaks and is called as C crystal form canagliflozin. The method has the beneficial effects of being simple to operate and less in energy consumption. The prepared novel crystal form product is good in stability, large in solubility and rapid in dissolution rate; the purity of the novel crystal product is higher than 99% and the yield of the novel crystal product is 91% or so; the purity, the color and the shape of the novel crystal product are not changed after the novel crystal product is stored for 100 days at a normal temperature under a drying condition. The novel crystal product can be easily smashed and is easily compatible with the dosage forms of medicine compositions as well as is low in cost. Thus, the novel crystal product is easily suitable for large-scale commercial and industrial applications.
Owner:TIANJIN UNIV +1
Canagliflozin of crystal form A, and crystallization preparation method thereof
InactiveCN103980261AEasy to joinEasy to operateSenses disorderNervous disorderCanagliflozinEnergy consumption
The invention discloses a canagliflozin of crystal form A, and a crystallization preparation method thereof. The X-ray powder diffraction pattern of the canagliflozin of crystal form A has characteristic peaks at diffraction angles 2theta with the values of 3.7+ / -0.1, 3.9+ / -0.1, 7.7+ / -0.1, 7.9+ / -0.1, 11.5+ / -0.1, 13.1+ / -0.1, 13.5+ / -0.1, 14.3+ / -0.1, 15.5+ / -0.1, 17.3+ / -0.1, 18.8+ / -0.1, 19.3+ / -0.1, 20.3+ / -0.1, 22.5+ / -0.1, 22.7+ / -0.1, 23.2+ / -0.1 and 23.4+ / -0.1. The preparation method has the advantages of simplicity and easy operation of operation steps, short time and less energy consumption. The above product prepared in the invention has a good stability, high a purity of above 99%, and has a yield of about 90%, and the purity, the color and the form of the product are unchanged after the product is stored under normal temperature dry conditions for 100d.
Owner:TIANJIN UNIV +1
Lansoprazole crystalline compound, enteric capsule thereof and preparation method of Lansoprazole crystalline compound
ActiveCN102558154AImprove medication safetyImprove stabilityOrganic active ingredientsOrganic chemistryLansoprazoleCrospovidones
The invention relates to a lansoprazole crystalline compound. An X-ray powder diffraction pattern represented by a diffraction angle of 2 theta + / - 0.2 DEG displays feature diffraction peaks at the positions of 5.8 DEG, 7.5 DEG, 9.1 DEG, 11.8 DEG, 12.1 DEG, 12.8 DEG, 13.3 DEG, 15.6 DEG, 16.7 DEG, 18.3 DEG, 20.4 DEG, 25.7 DEG, 26.8 DEG and 31.5 DEG. The invention also relates to a lansoprazole enteric capsule containing the lansoprazole crystalline compound. The lansoprazole enteric capsule comprises 20 to 60 parts of l crystalline compound, 90 to 140 parts of microcrystalline cellulose, 1.5 to 3.5 parts of disodium hydrogen phosphate, 2 to 5 parts of anhydrous sodium sulphite, 1 to 10 parts of crospovidone, 0.8 to 4.2 parts of lauryl sodium sulfate, 2 to 8 parts of povidone K30 and 1 to 3 parts of magnesium stearate.
Owner:HAINAN JINRUI PHARMA CO LTD
Hydroprocessing bulk catalyst and uses thereof
ActiveUS7737072B2Inorganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsTO-18X-ray
A hydroprocessing catalyst is provided. The hydroprocessing catalyst has the formula (Mt)a(Xu)b(Sv)d(Cw)e(Hx)f(Oy)g(Nz)h, wherein M is at least one group VIB metal; X is at least one Group VIII non-noble metal; t, u, v, w, x, y, z representing the total charge for each of the components (M, X, S, C, H, O and N, respectively); ta+ub+vd+we+xf+yg+zh=0; and 0=<b / a=<5, (a+0.5b)<=d<=(5a+2b), 0<=e<=11(a+b), 0<=f<=7(a+b), 0<=g<=5(a+b), 0<=h<=0.5(a+b). The catalyst has an X-ray powder diffraction pattern with at least one broad diffraction peak at any of Bragg angles: 8 to 18°, 32 to 40°, and 55 to 65° (from 0 to 70° 2-θ scale). In one embodiment, the at least one diffraction peak is greater than 2 degrees wide at ½ height.
Owner:CHEVROU USA INC
Novel pregabalin crystalline form and its preparing process
InactiveCN1634869AImprove stabilitySuitable for mass productionOrganic active ingredientsNervous disorderPregabalinMedicine
The invention relates to a novel crystalline form of pregabalin drug for treating epilepsy and neuropathic pain and its preparation method. Compared to others pregabalin with agraphitic form and other forms, pregabalin with the novel crystalline form has good stability and is suitable for mass production. Pregabalin with the novel crystalline form uses Cu-Ka radiation and its X-ray powder diffraction has characteristic peak at 9.5(9.3), 12.3(7.2), 16.7(5.3), 18.4(4.8), 19.9(4.5) and 23.3(3.8) with the coordinate of 2-theta and interplanar spacing (d).
Owner:FUKANGREN BIO PHARMA
Crystal form F of ibrutinib and preparation method
InactiveCN105646498AImprove stabilityHigh purityOrganic active ingredientsOrganic chemistry methodsSolubilitySolvent
The invention discloses a crystal form F of ibrutinib. The crystal form F is characterized in that X-ray powder diffraction (X-RPD) which adopts Cu-Kalpha radiation and is represented with a 2theta angle has diffraction peaks in positions at angles of 3.7 degrees plus or minus 0.2 degrees, 6.7 degrees plus or minus 0.2 degrees, 13.2 degrees plus or minus 0.2 degrees, 16.1 degrees plus or minus 0.2 degrees, 19.1 degrees plus or minus 0.2 degrees, 20.0 degrees plus or minus 0.2 degrees, 23.8 degrees plus or minus 0.2 degrees and 24.6 degrees plus or minus 0.2 degrees. Related solvents in a preparation process of the crystal form F are cheap, the conditions are mild, the operation is simple, good controllability and reproducibility are realized, further, the prepared crystal form has great stability, the HPLC (high performance liquid chromatography) purity is higher than 99%, and the phenomenon of crystal transformation can be avoided; besides, the solubility is high, the dissolubility is good, and the bioavailability is high.
Owner:孙霖
Canagliflozin of crystal form B, and crystallization preparation method thereof
InactiveCN103980262AImprove solubilityFast dissolution rateSenses disorderNervous disorderSolubilityX-ray
The invention discloses a canagliflozin of crystal form B, and a crystallization preparation method thereof. The X-ray powder diffraction pattern of the canagliflozin of crystal form B has characteristic peaks at diffraction angles 2theta with the values of 3.4+ / -0.1, 6.6+ / -0.1, 12.6+ / -0.1, 13.2+ / -0.1, 15.3+ / -0.1, 15.6+ / -0.1, 16.5+ / -0.1, 19.4+ / -0.1, 19.8+ / -0.1 and 23.7+ / -0.1. The method has the advantages of high efficiency, easy operation and short time. Canagliflozin crystals of crystal form B prepared in the invention have a large solubility and a fast dissolving rate, the above product has a purity of above 99%, and has a yield of about 95%, and the purity, the color and the form of the product are unchanged after the product is stored under normal temperature dry conditions for 100d. The product has the advantages of easy crushing, easy addition of medicinal compositions, low cost, and easy commercial industrial large-scale enforcement.
Owner:TIANJIN UNIV +1
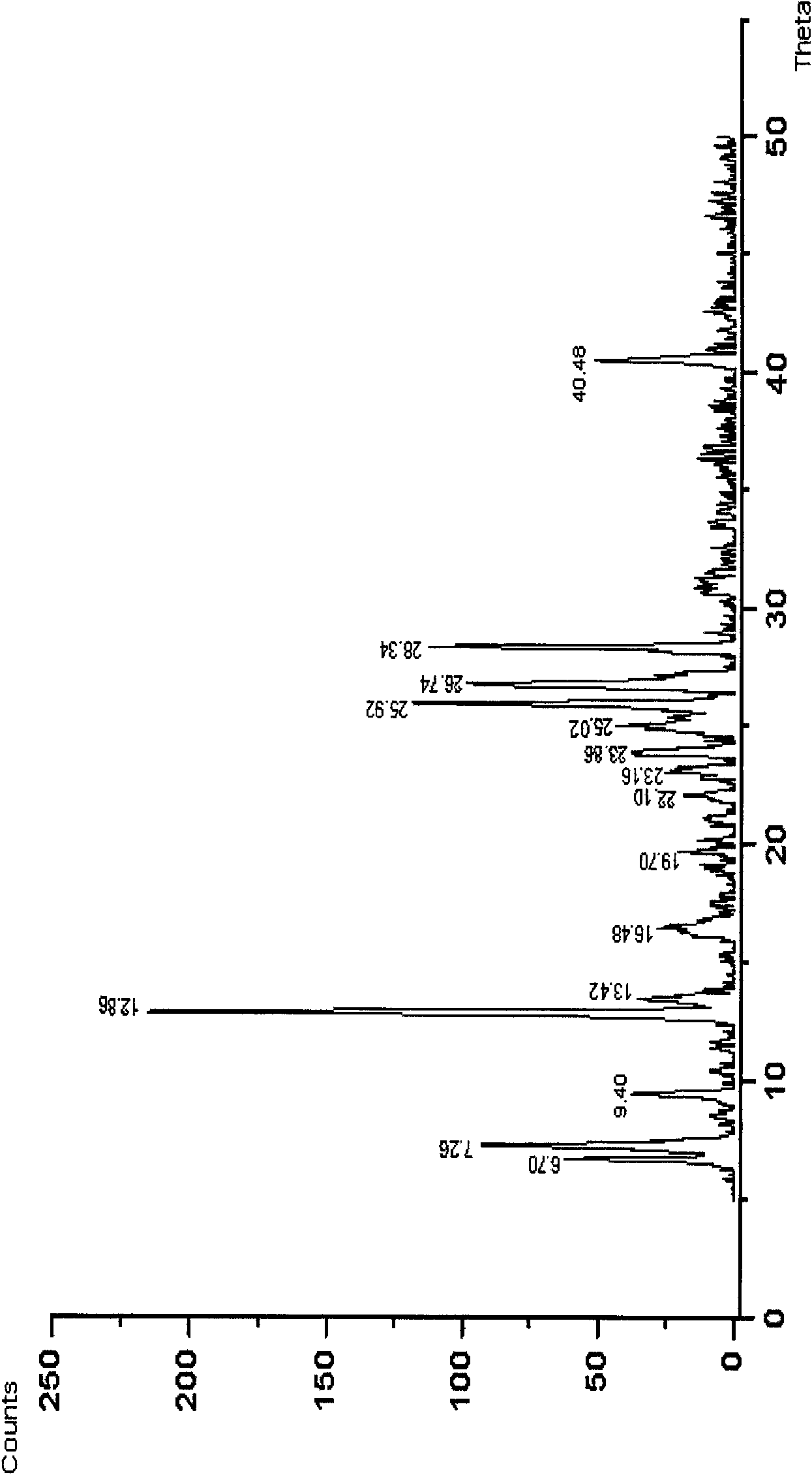
Uloric crystal and preparation method thereof
The invention relates to the field of medicinal chemistry, in particular to an Uloric crystal and a preparation method thereof. The Uloric crystal is characterized in that x-ray powder diffraction feature absorption peak (2theta) values have feature peaks at the parts of 6.70 degrees, 7.26 degrees, 9.40 degrees, 12.86 degrees, 13.42 degrees, 16.48 degrees, 19.70 degrees, 22.10 degrees, 23.16 degrees, 23.86 degrees, 25.02 degrees, 25.92 degrees, 26.74 degrees, 28.34 degrees and 40.48 degrees. An Uloric alpha crystal form of the invention has simple process operation and low cost. Only Uloric needs to be dissolved in a single solvent of ethylene glycol monomethyl ether so as to avoid solvation crystal habit, and the crystal form is stable and has good reproducibility. Moreover, the Uloric alpha crystal form has good solubility and better dissolution rate than A crystals in a solid preparation.
Owner:CHINA PHARM UNIV +1
Hydroprocessing Bulk Catalyst and Methods of Making Thereof
ActiveUS20110124498A1Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationLanthanideX-ray
A hydroprocessing bulk catalyst is provided. A process to prepare hydroprocessing bulk catalysts is also provided. The hydroprocessing catalyst has the formula (Rp)i(Mt)a(Lu)b(Sv)d(Cw)e(Hx)f(Oy)g(Nz)h, wherein M is at least at least a “d” block element metal; L is also at least a “d” block element metal, but different from M; t, u, v, w, x, y, z representing the total charge for each of the components (M, L, S, C, H, O and N, respectively); R is optional and in one embodiment, R is a lanthanoid element metal; 0<=i<=1; pi+ta+ub+vd+we+xf+yg+zh=0; 0<b; 0<b / a=<5; 0.5(a+b)<=d<=5(a+b); 0<e<=11(a+b); 0<f<=7(a+b); 0<g<=5(a+b); 0<h<=2(a+b). The catalyst has an X-ray powder diffraction pattern with at least three diffractions peak located at 2-θ angles of greater than 25°. In one embodiment, the catalyst is prepared by forming at least a sulfided catalyst precursors from at least two “d” block element metals; and mixing the catalyst precursor with a hydrocarbon compound to form the hydroprocessing catalyst composition. In another embodiment, the catalyst is prepared by the thermal decomposition of an oil dispersible sulfur containing organic metal precursor upon contact with a hydrocarbon oil, generating a slurry catalyst. In yet another embodiment, the catalyst is prepared from an in-situ or ex-situ sulfidation of “d block element metal precursors in a solvent carrier.
Owner:CHEVROU USA INC
Crystal form of prostaglandin analogue, and preparation method and use thereof
ActiveUS9278903B2Unique stabilityOrganic compound preparationCardiovascular disorderX-rayPowder diffraction
Provided are a crystal form A of a compound having the structure as represented by formula I, and preparation method and use thereof. The X-ray powder diffraction (XRPD) chart of the crystal form A has characteristic peaks at the following diffraction angles: 2.9±0.2°, 13.6±0.2°, 17.3±0.2° and 18.6±0.2°.
Owner:SHANGHAI TECHWELL BIOPHARMACEUTICALS CO LTD
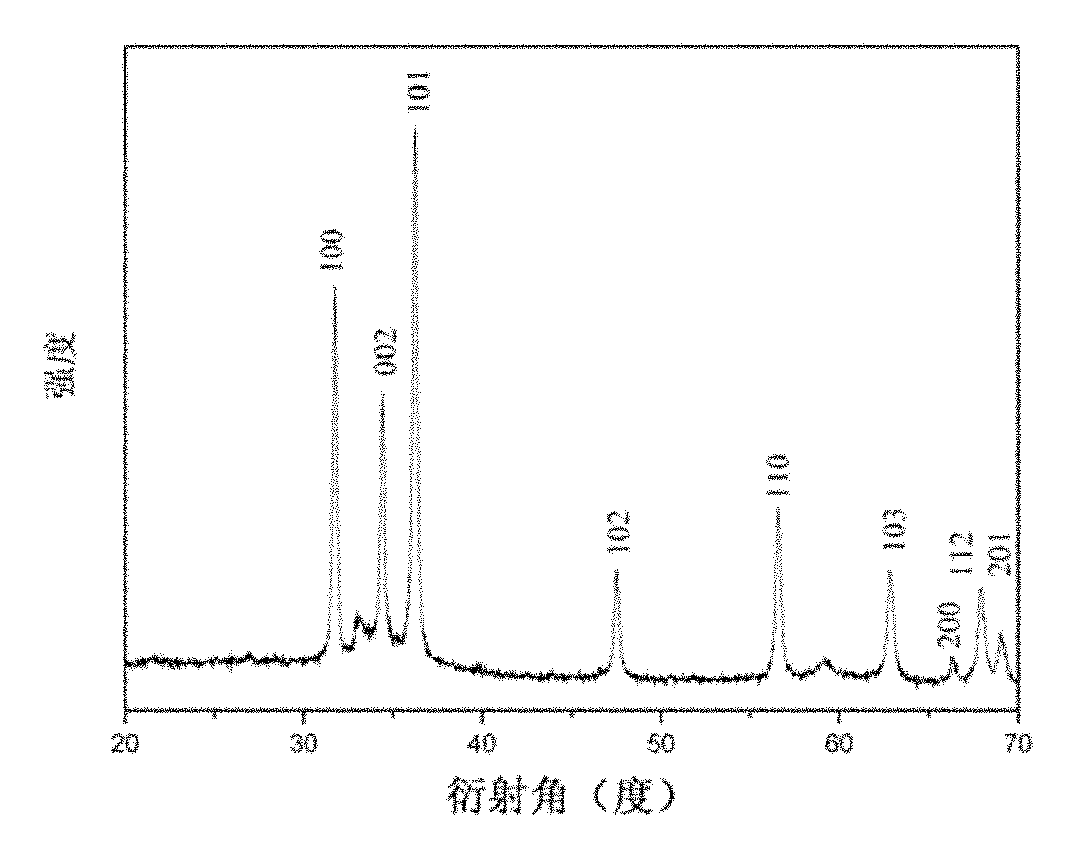
Zinc oxide hollow microspheres and preparation method thereof
InactiveCN101948130AEasy to preparePreparation method low temperatureZinc oxides/hydroxidesMicrosphereHigh pressure
The invention discloses zinc oxide hollow microspheres and a preparation method thereof. The invention relates to an inorganic nano material, and provides the zinc oxide hollow microspheres and the preparation method thereof. The zinc oxide hollow microspheres are made of wurtzite structural zinc oxide with powder diffraction standard joint committee number 36-1451, the diameters of the microspheres are 5 to 8 microns, and the thicknesses of the sphere walls are 0.5 to 1 micron. The preparation method comprises the following steps of: dissolving zinc nitrate hexahydrate and hexamethylene tetramine into water to obtain solution A, and adding sodium citrate into the solution A to obtain solution B; putting the solution B into a closed high pressure reactor, and putting the high pressure reactor into a drying oven to perform hydrothermal reaction; and after the hydrothermal reaction, cooling the reaction product to room temperature, opening the high pressure reactor, and filtering, washing and drying the sediment to obtain the zinc oxide hollow microspheres. The preparation method has the advantages of simplicity, convenience, low temperature, high yield and low sample dislocation density; and the zinc oxide hollow microspheres have broad application value in the fields of medicament release, photocatalysis, dye-sensitized solar cells and the like.
Owner:XIAMEN UNIV
Copolymer containing amorphous agomelatine, and preparation method, pharmaceutical composition and application thereof
ActiveCN102716493AHigh dissolution rateFast absorptionOrganic active ingredientsNervous disorderX-raySolvent
The invention provides a copolymer containing amorphous agomelatine. The detection of a sample of the copolymer by Differential Scanning Calorimetry (DSC) and X-ray powder diffraction shows that the sample has stable properties. The copolymer mainly comprises agomelatine raw material, carrier and solvent. The invention also provides a preparation method of the copolymer containing amorphous agomelatine, and the method has good reproducibility. The invention further provides a pharmaceutical composition containing the copolymer containing amorphous agomelatine and an application of the copolymer containing amorphous agomelatine in preparing medicines for treating depression.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Organic-inorganic hybrid silicates and metal-silicates having an ordered structure
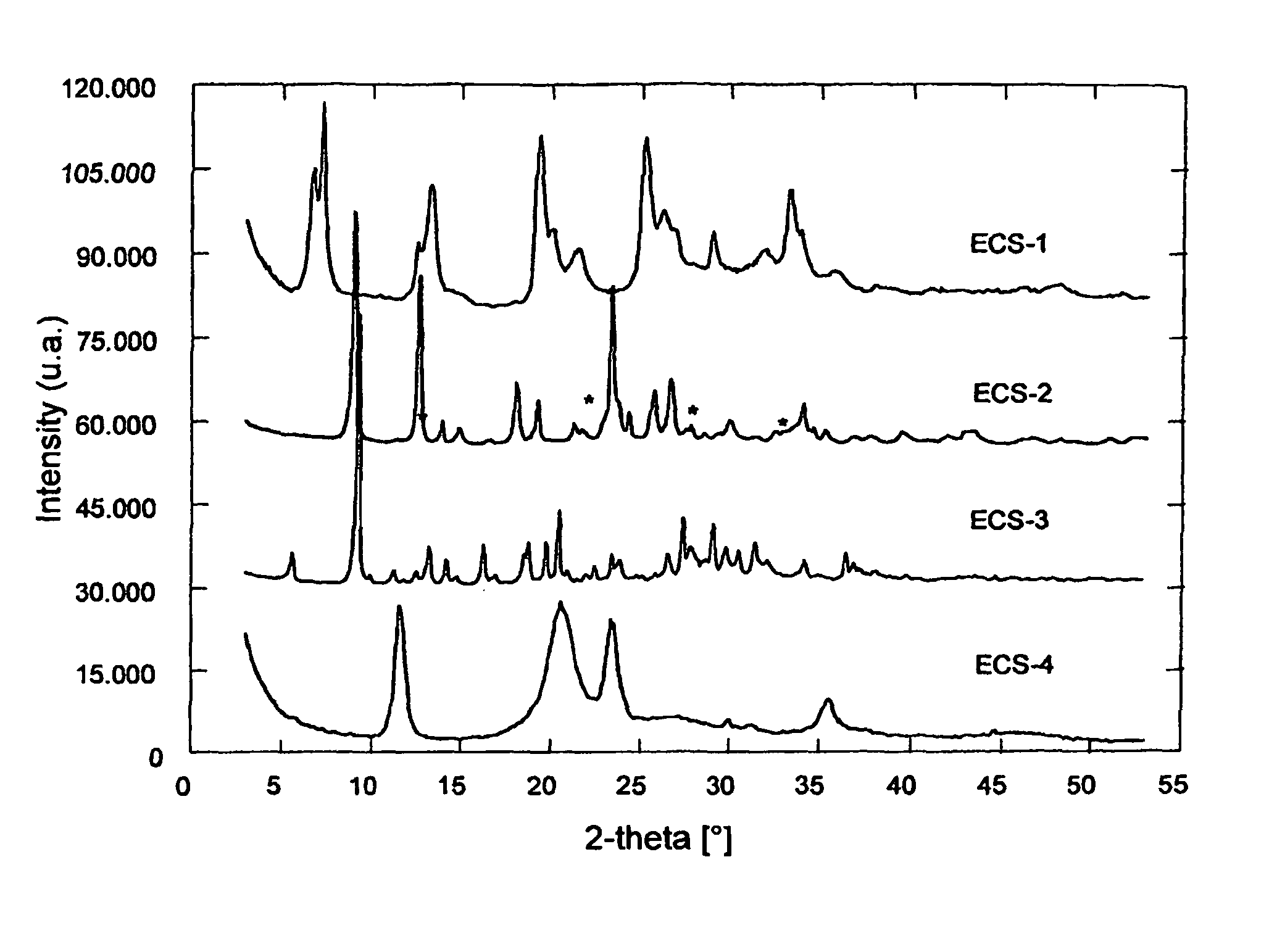
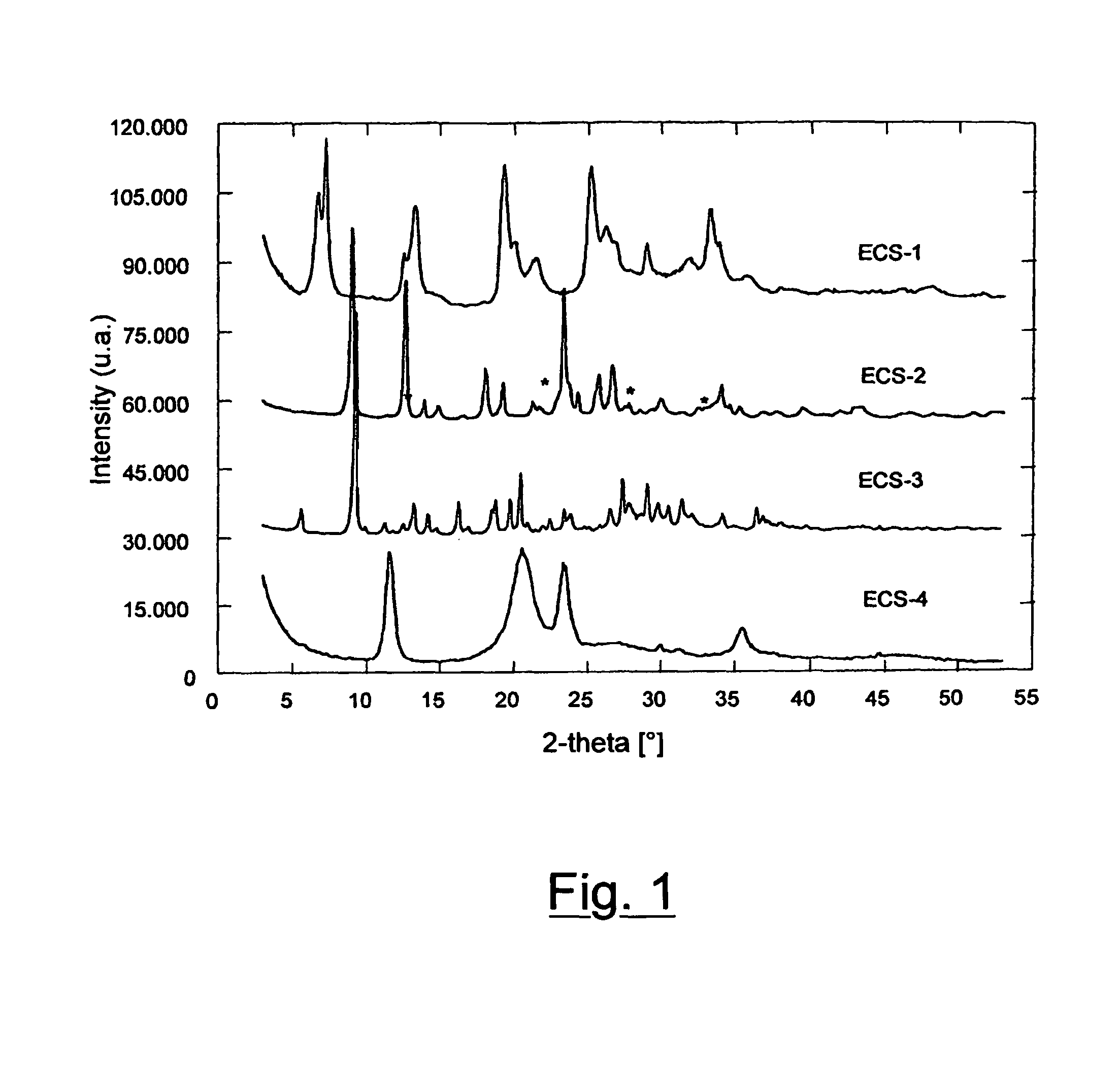
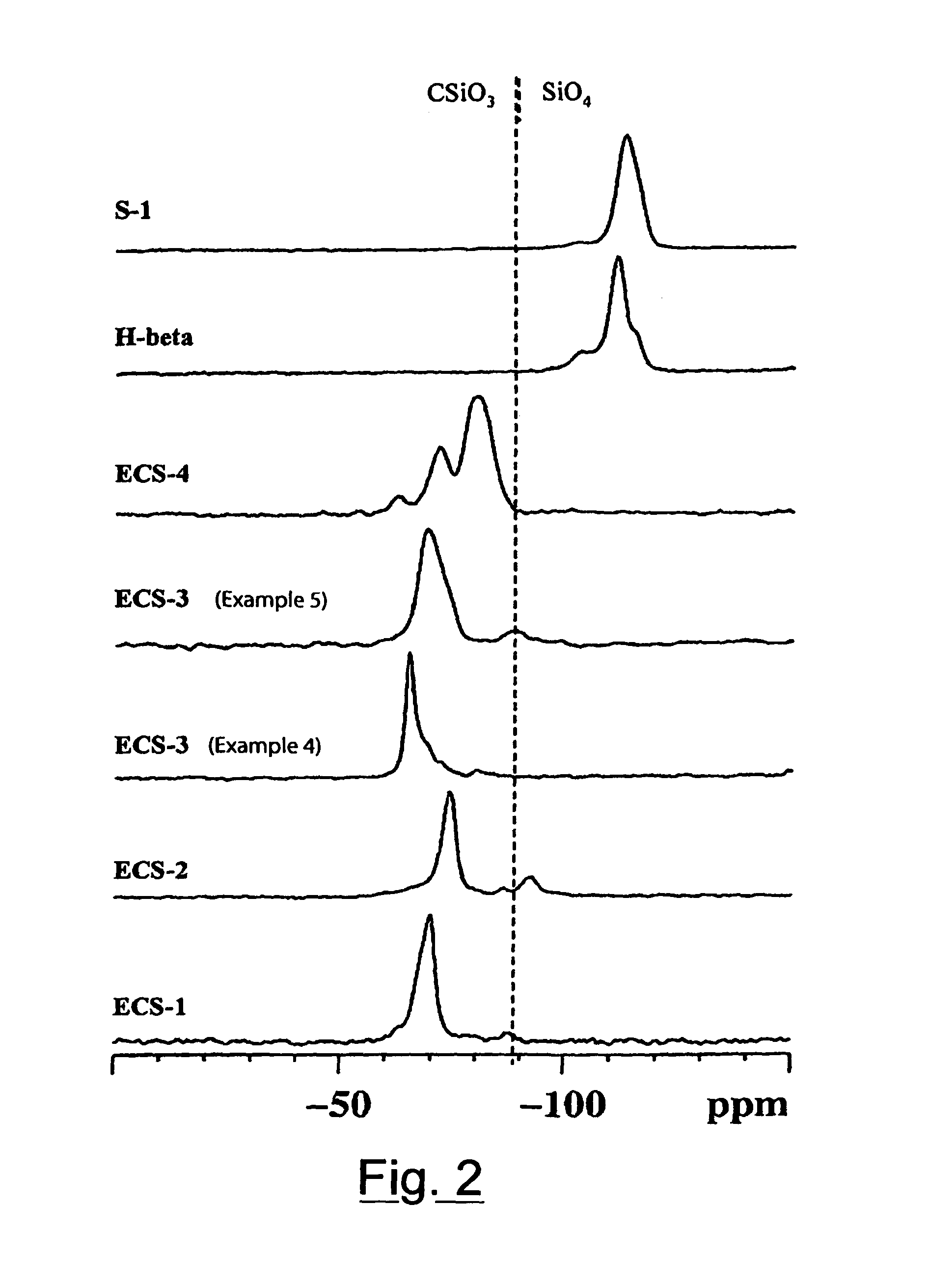
The present invention relates to new organic-inorganic hybrid silicates and metal-silicates called ECS, characterized by an X-ray powder diffraction pattern with reflections exclusively at angular values higher than 4.0° of 2θ, preferably at angular values higher than 4.7°, and an ordered structure containing structural units having formula (a) wherein R is an organic group: Formula (a) and possibly containing one or more elements T selected from groups III B, IV B, V B and transition metals, with a Si / (Si +T) molar ratio in said structure higher than 0.3 and lower than or equal to 1, wherein Si is the silicon contained in the structural unit (a). A process is also described, starting from disilanes, for the preparation of these materials, which does not include the use of templates or surfactants. These materials can be used as molecular sieves, adsorbents, in the field of catalysis, in the field of electronics, in the field of sensors, in the field of nanotechnologies.
Owner:ENI SPA
Hydroprocessing Bulk Catalyst and Methods of Making Thereof
ActiveUS20110124493A1Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationX-rayLanthanide
A hydroprocessing bulk catalyst is provided. A process to prepare hydroprocessing bulk catalysts is also provided. The hydroprocessing catalyst has the formula (RP)i(Mt)a(Lu)b(Sv)d(Cw)e(Hx)f(Oy)g(Nz)h, wherein M is at least at least a “d” block element metal; L is also at least a “d” block element metal, but different from M; t, u, v, w, x, y, z representing the total charge for each of the components (M, L, S, C, H, O and N, respectively); R is optional and in one embodiment, R is a lanthanoid element metal; 0<=i<=1; pi+ta+ub+vd+we+xf+yg+zh=0; 0<b; 0<b / a=<5; 0.5 (a+b)<=d<=5(a+b); 0<e<=11(a+b); 0<f<=7(a+b); 0<g<=5(a+b); 0<h<=2(a+b). The catalyst has an X-ray powder diffraction pattern with at least three diffractions peak located at 2-θ angles of greater than 25°. In one embodiment, the catalyst is prepared by forming at least a sulfided catalyst precursors from at least two “d” block element metals; and mixing the catalyst precursor with a hydrocarbon compound to form the hydroprocessing catalyst composition. In another embodiment, the catalyst is prepared by the thermal decomposition of an oil dispersible sulfur containing organic metal precursor upon contact with a hydrocarbon oil, generating a slurry catalyst. In yet another embodiment, the catalyst is prepared from an in-situ or ex-situ sulfidation of “d block element metal precursors in solvent carrier.
Owner:CHEVROU USA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Crystalline form of 1-(beta-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate Crystalline form of 1-(beta-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate](https://images-eureka.patsnap.com/patent_img/6684e991-76ee-477e-8225-4b4dd6d2c62b/US20080146515A1-20080619-D00000.png)
![Crystalline form of 1-(beta-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate Crystalline form of 1-(beta-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate](https://images-eureka.patsnap.com/patent_img/6684e991-76ee-477e-8225-4b4dd6d2c62b/US20080146515A1-20080619-D00001.png)
![Crystalline form of 1-(beta-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate Crystalline form of 1-(beta-D-glucopyransoyl)-4-methyl-3-[5-(4-fluorophenyl)-2- thienylmethyl]benzene hemihydrate](https://images-eureka.patsnap.com/patent_img/6684e991-76ee-477e-8225-4b4dd6d2c62b/US20080146515A1-20080619-D00002.png)