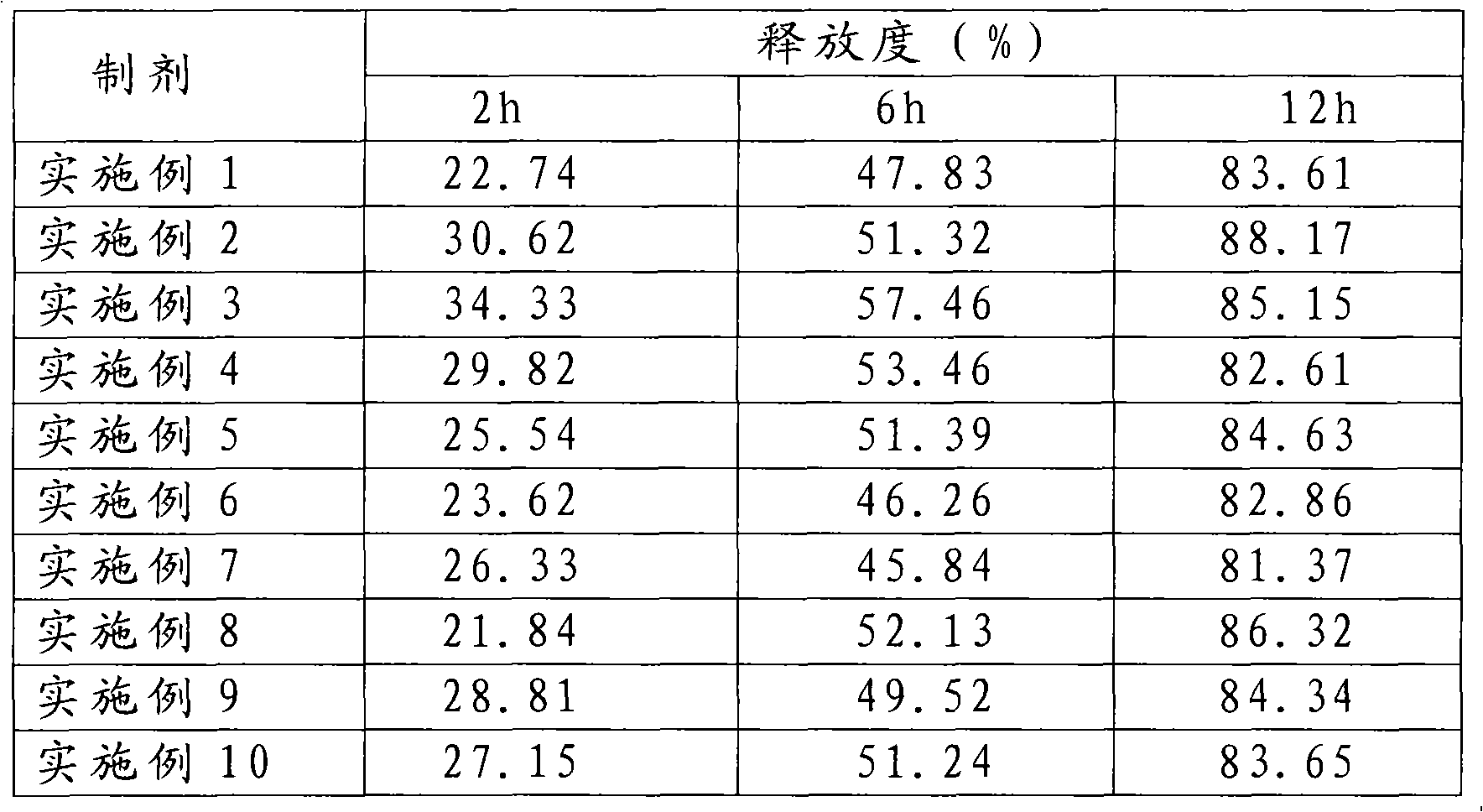
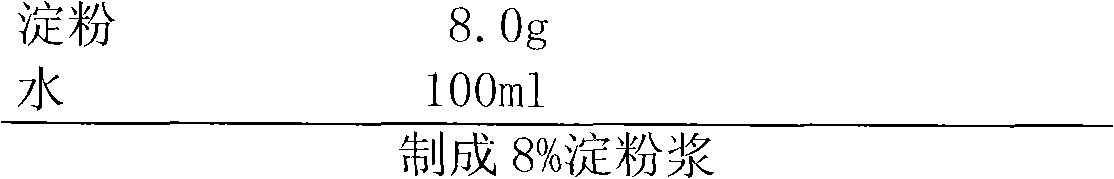
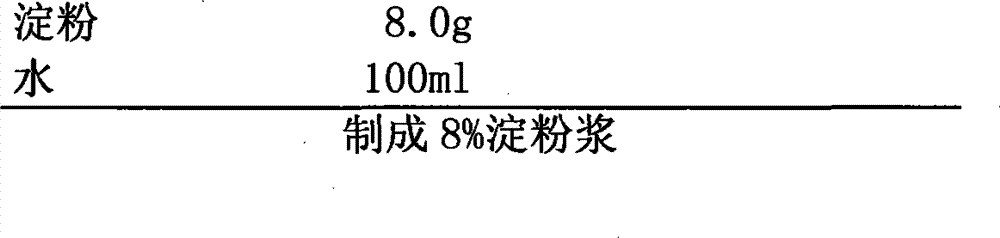
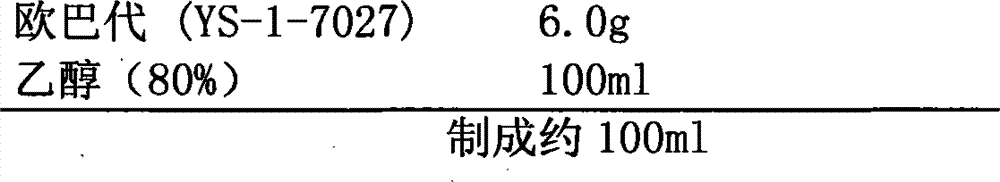
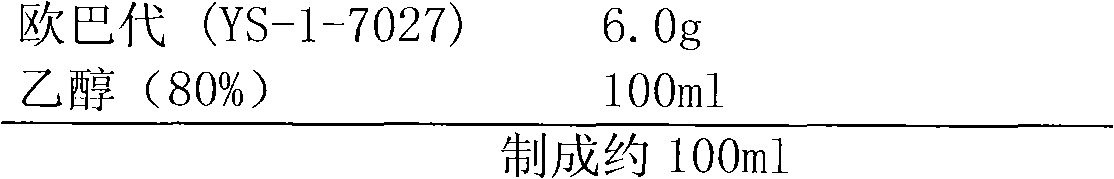Patents
Literature
223 results about "Febuxostat" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
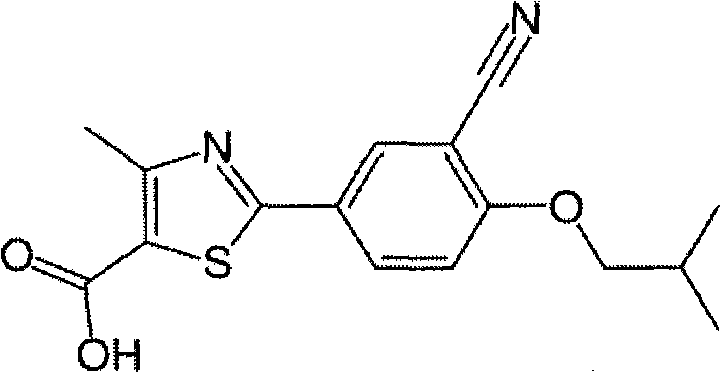
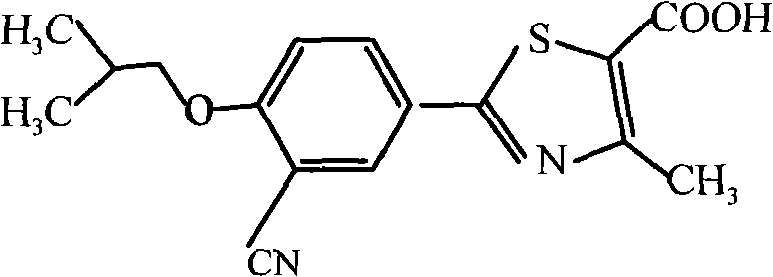
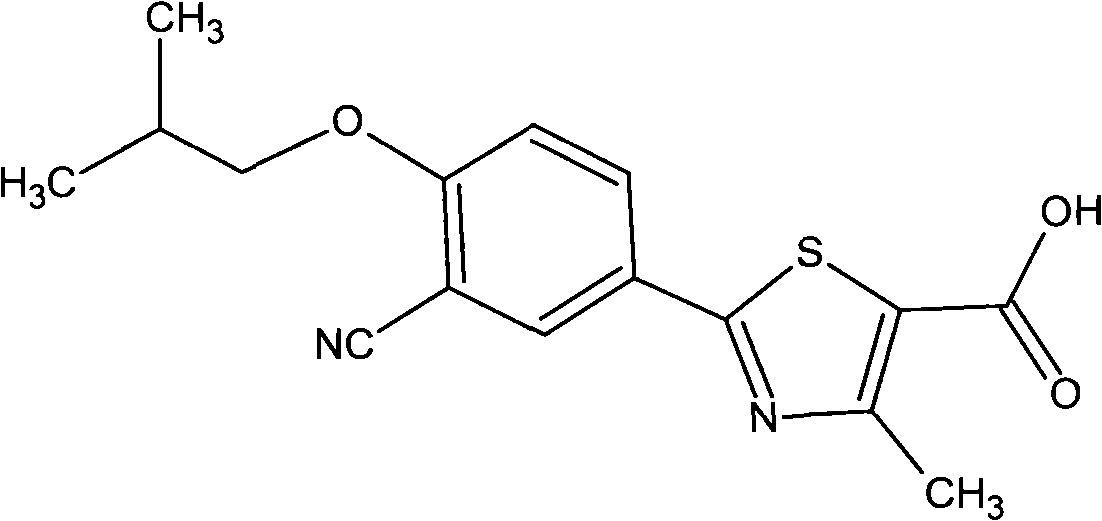
Febuxostat is used to lower uric acid levels in people with gout.
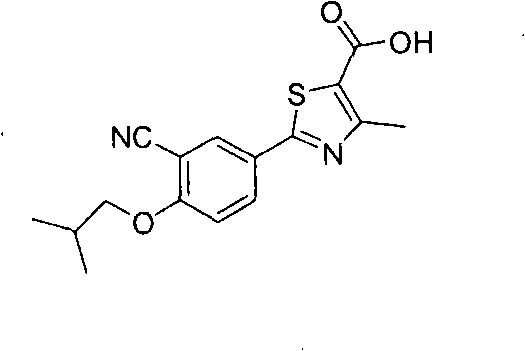
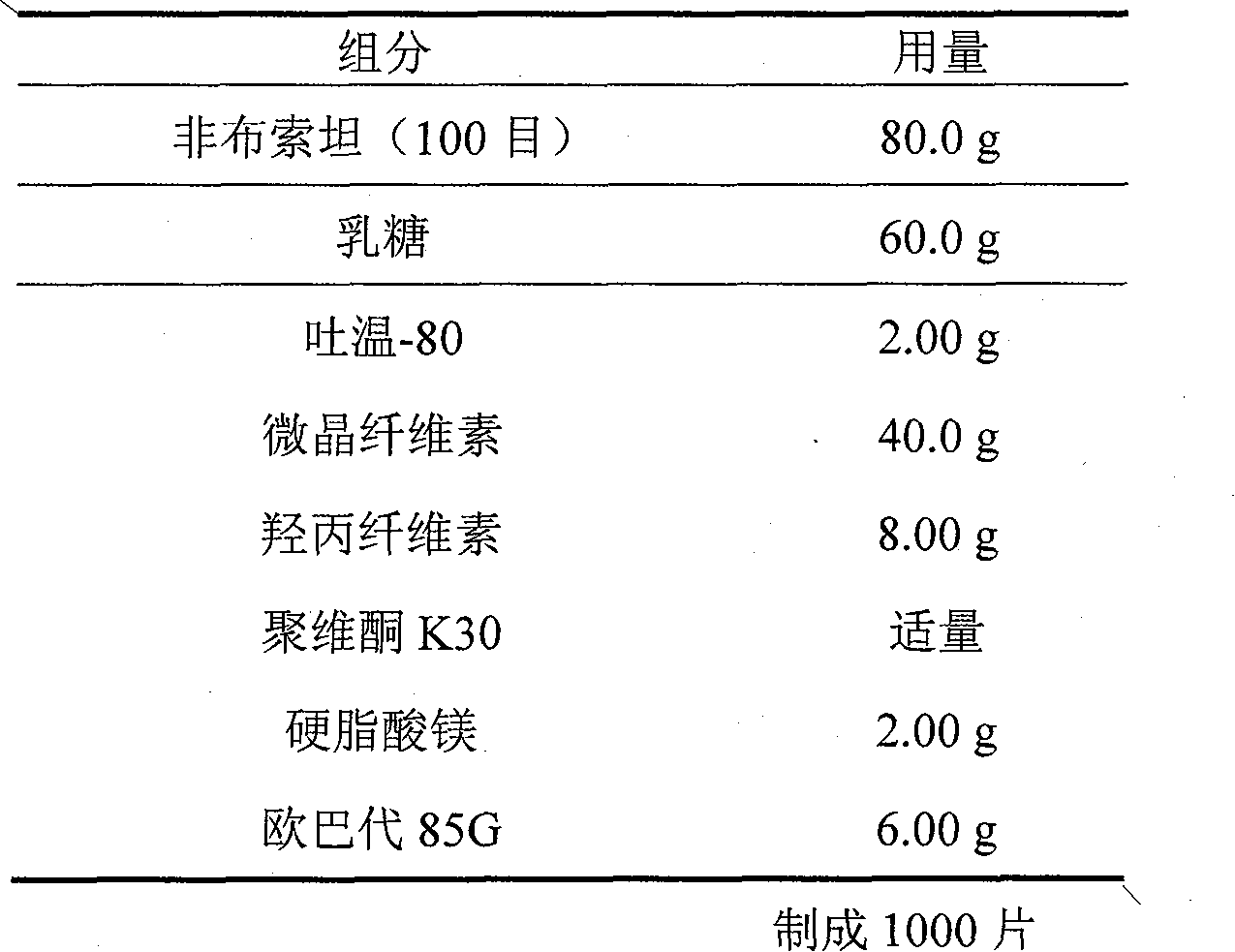
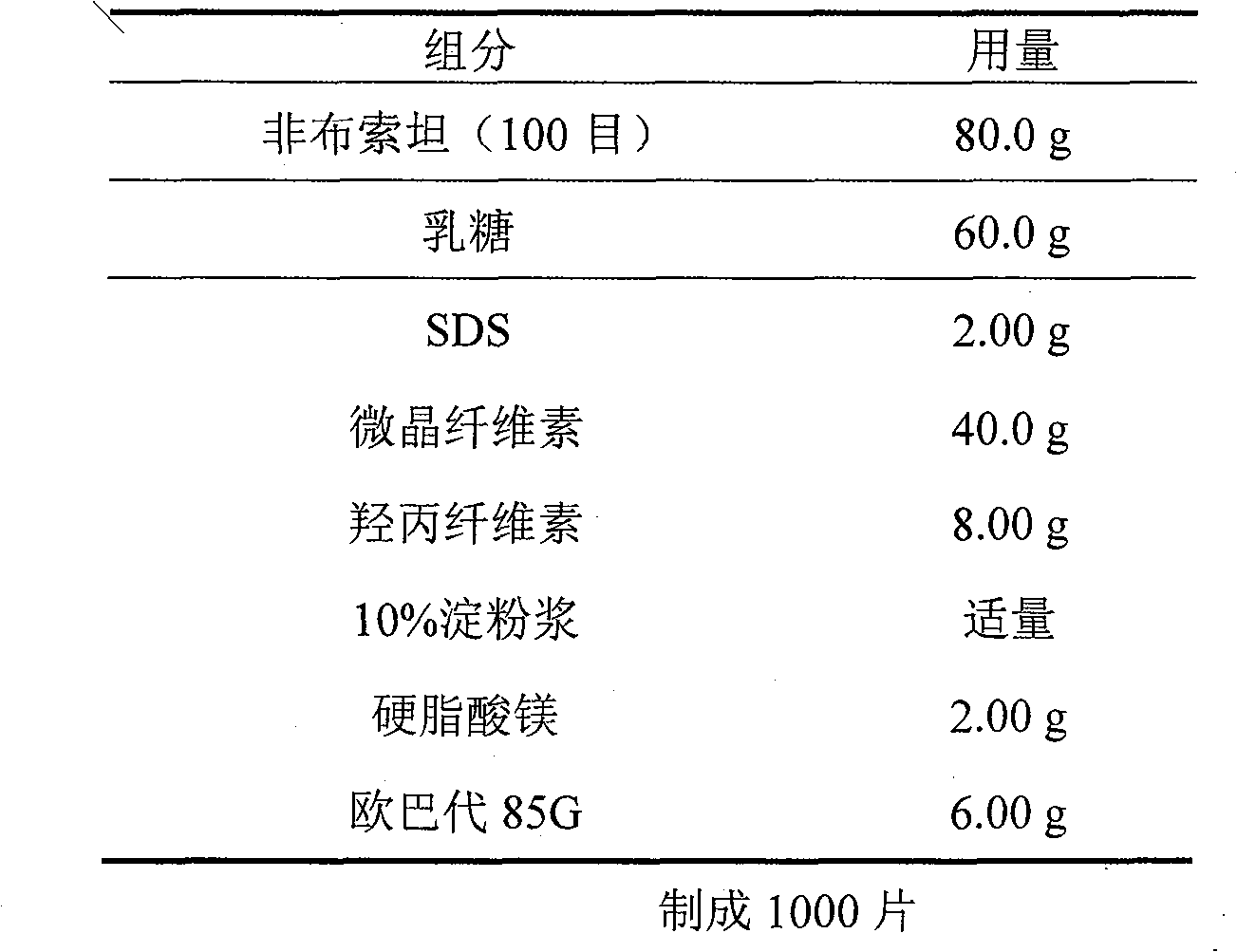
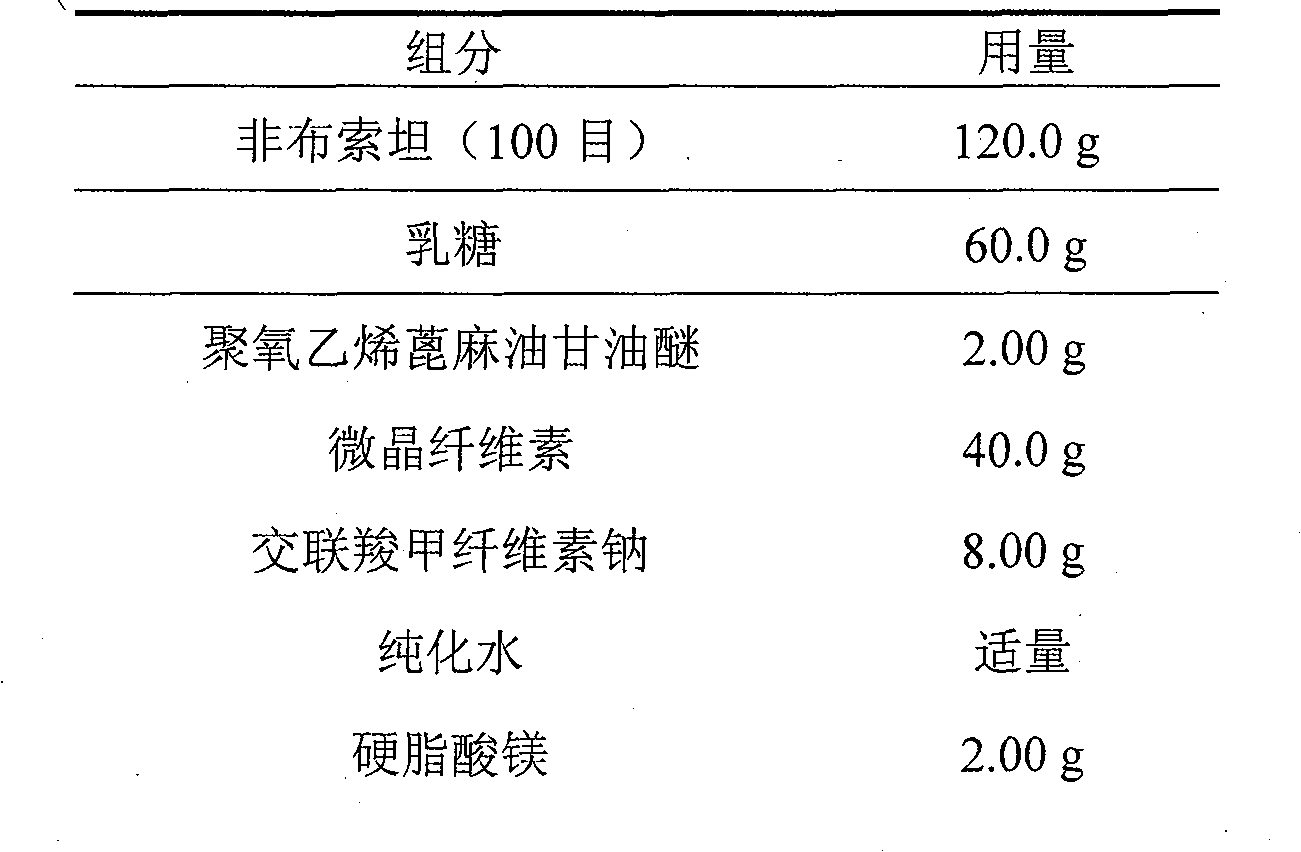
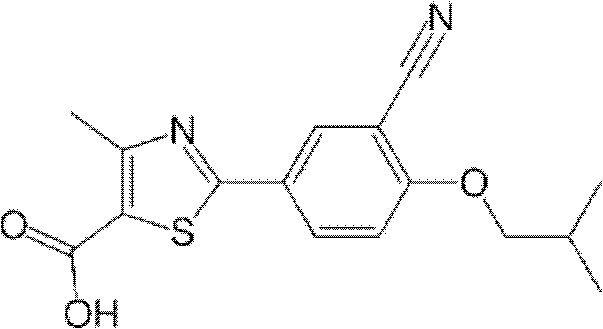
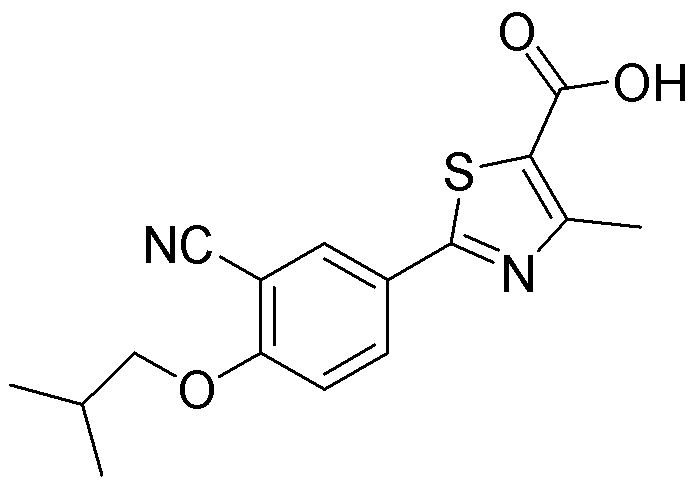
Crystal form and preparation of febuxostat
ActiveCN101412700AImprove stabilityNot prone to crystallizationOrganic active ingredientsOrganic chemistryEthyl esterFebuxostat
The invention provides a crystal form of anti-hyperuricemia drug 2-(3-cyano-4-isooxyphenyl)-4-methyl-5-thiazole formic acid and a method for preparing the same. The method for preparing the crystal form mainly comprises the following steps: heating up and dissolving the 2-(3-cyano-4-isooxyphenyl)-4-methyl-5-thiazole formic acid in ethyl acetate, and cooling down the solution for recrystallizing the solution to obtain the crystal form. The crystal form has better dissolution degree and stability.
Owner:SHANGHAI INST OF PHARMA IND +1
Oral solid preparation of Febuxostat with high-bioavailability and preparation method thereof
ActiveCN101474175AHigh and stable bioavailabilityOrganic active ingredientsSkeletal disorderDiseaseBioavailability
The invention relates to a high bioavailability febuxostat oral solid dosage and a preparation method thereof. The invention is characterized in that the solid dosage comprises: a) febuxostat is C, H, I or J crystal form; b) the average grain diameter of the febuxostat crystal form ranges from 3.5-10mum, preferably 3.5-7mum. The preferable oral solid dosage is tablet, has the advantages of simple preparation technique, low cost and relatively high bioavailability, and can be used for treating diseases relative to extremely-high uric acid, such as gout, and reducing uric acid in blood.
Owner:JIANGSU WANBANG BIOPHARMLS +1
Febuxostat dispersible tablet drug and preparing method thereof
ActiveCN101780073AEasy to prepareLow costOrganic active ingredientsSkeletal disorderAdditive ingredientEther
The invention relates to a febuxostat dispersible tablet drug and a preparing method. The drug is prepared from febuxostat as the active drug ingredient, and acceptable auxiliary ingredients in the dispersible tablet preparation. The febuxostat dispersible tablet drug is characterized in that at least one of polyoxyethylene 40 monostearate ingredient, polyethenoxy ether castor oil ingredient and hydrogenated castor oil polyoxyl ingredient in the auxiliary ingredients is used as a solubilizing agent ingredient and has the usage amount of 0.1-5 times the weight of febuxostat. The drug can obviously enhance the dissolution rate of the insoluble effective drug ingredient of febuxostat, and has the advantages of high drug dispersion degree, high dissolution rate, quick absorption and effect taking, high biological utilization degree, and the like.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD
Febuxostat crystal form and preparation method thereof
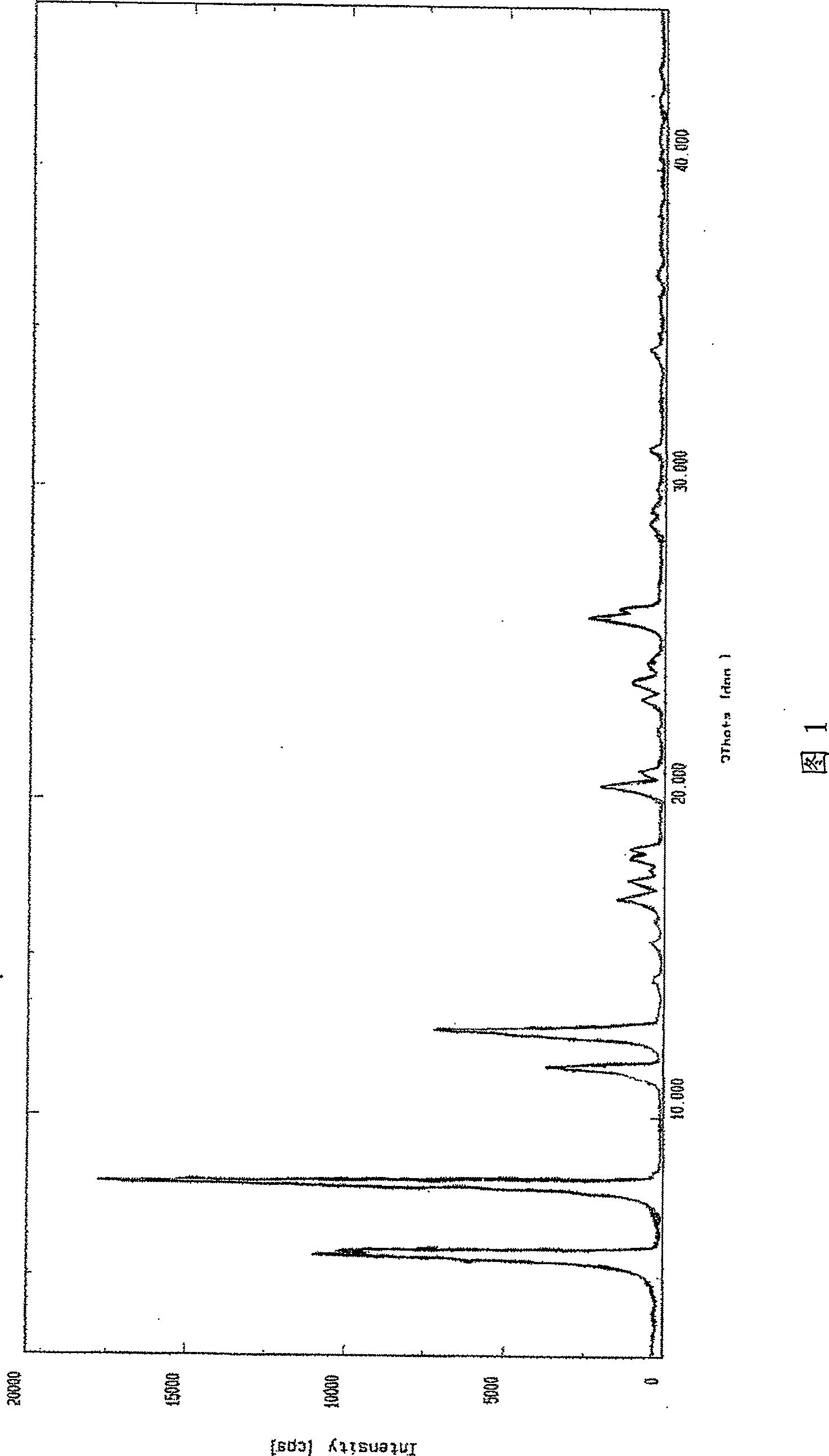
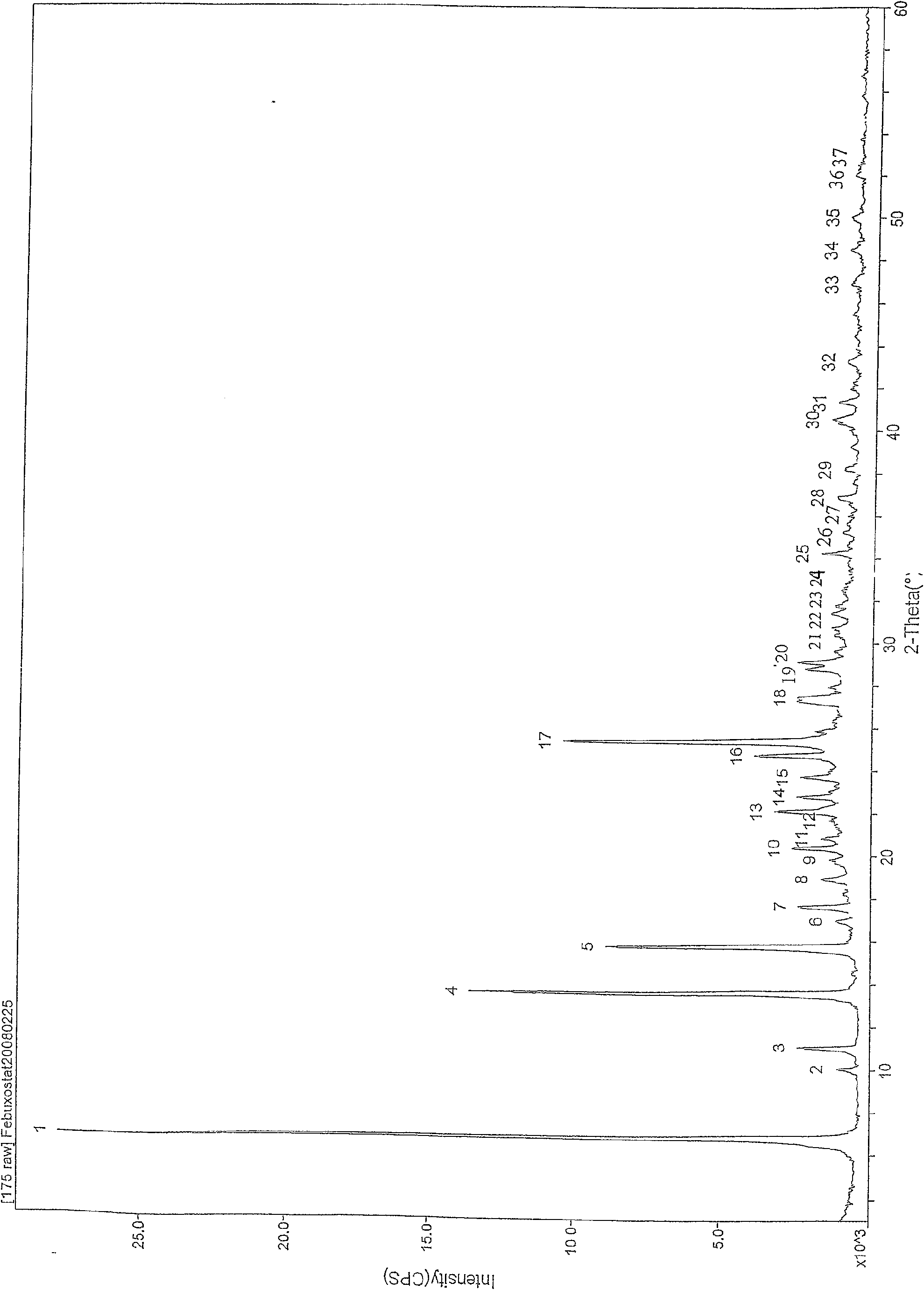
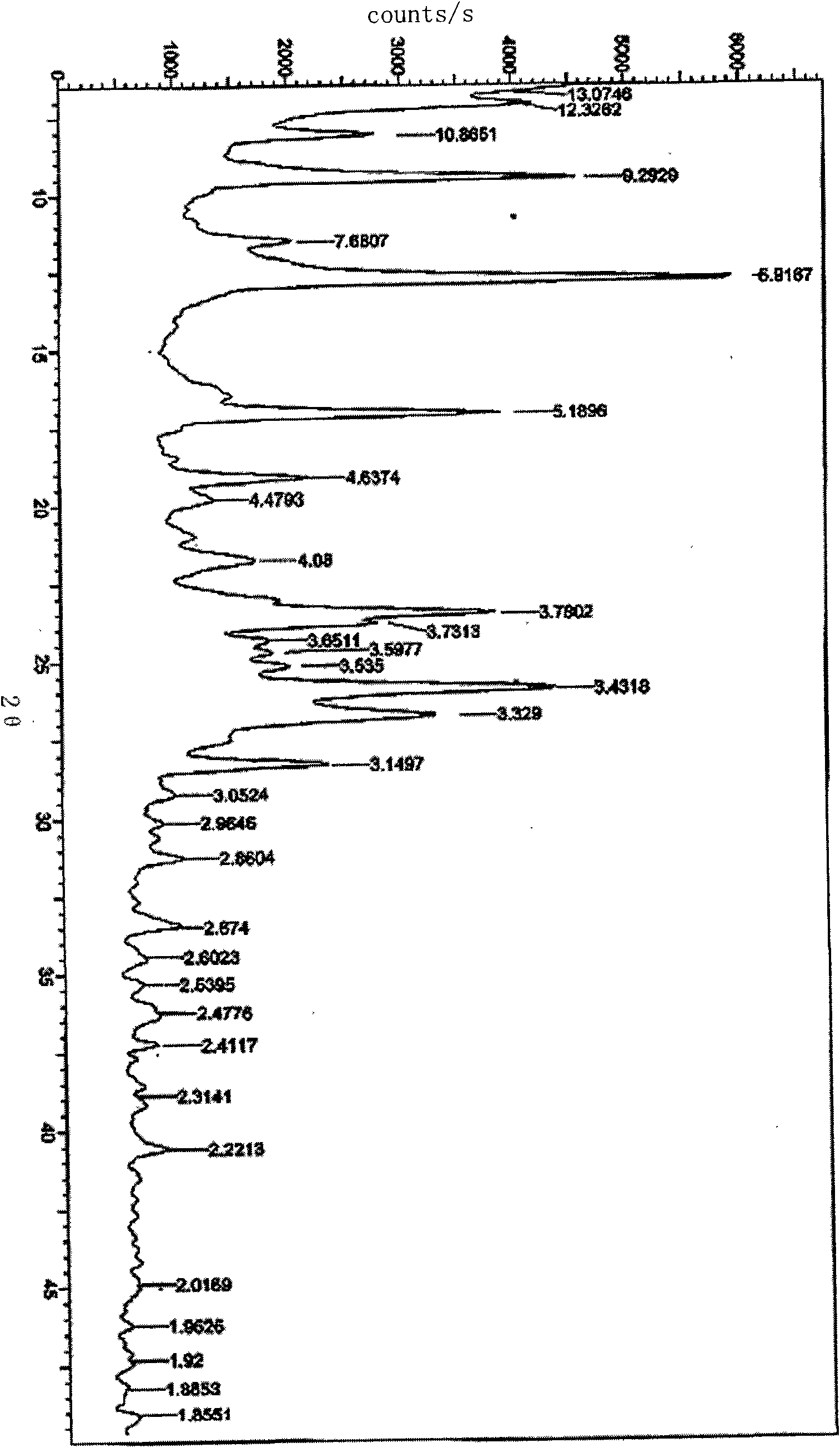
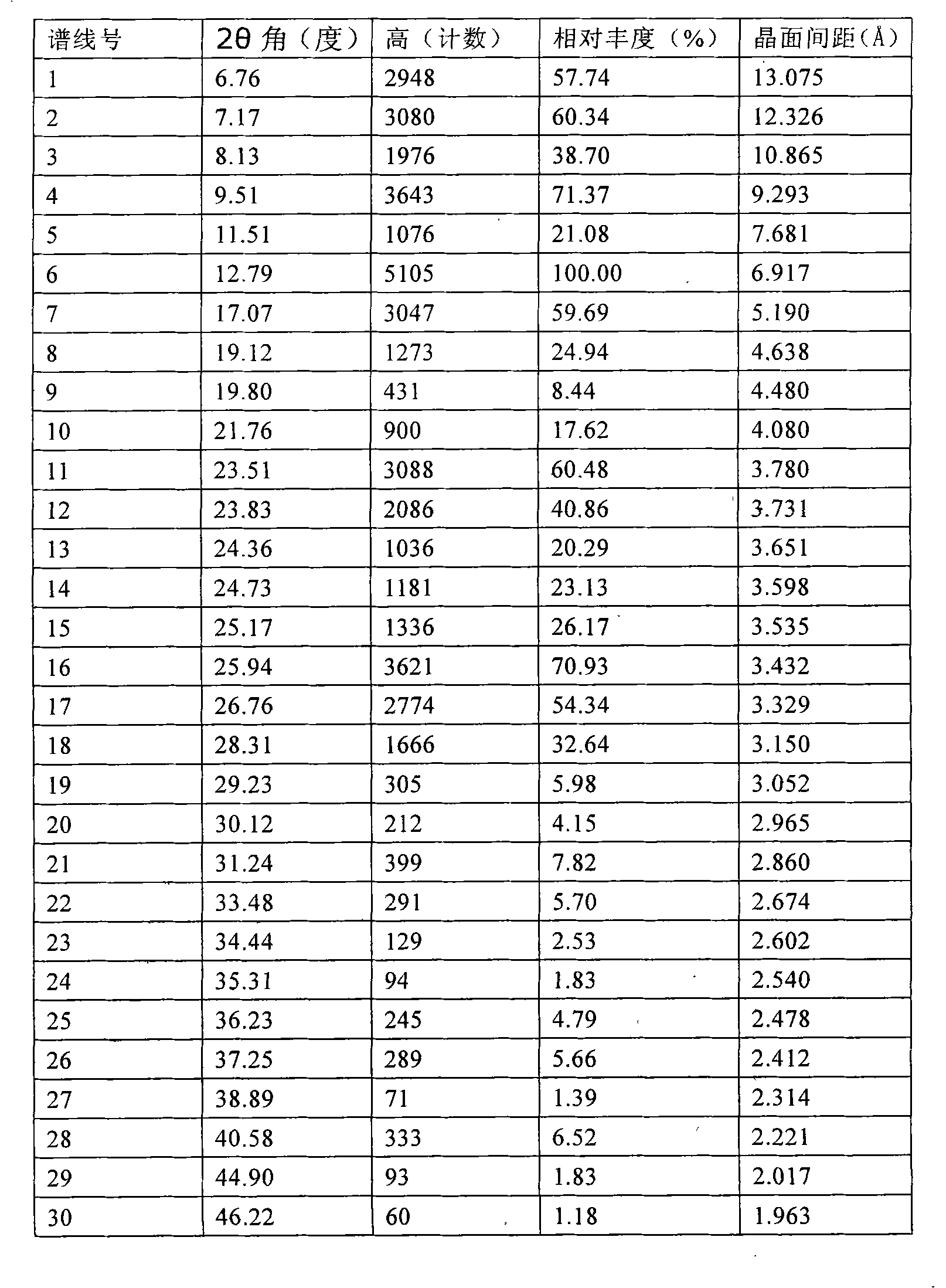
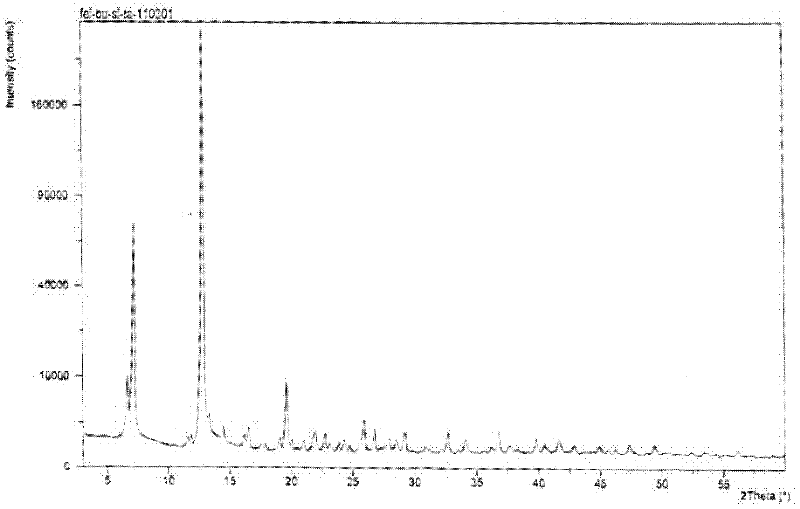
The invention relates to a Febuxostat crystal form. The x-ray powder diffraction has characteristic peaks when a refraction angle 2 theta is at 6.80+ / - 0.2 degree, 11.04+ / - 0.2 degree, 13.56+ / - 0.2 degree, 15.74+ / -0.2 degree, 17.56+ / -0.2 degree, 20.36+ / -0.2 degree, 22.10+ / -0.2 degree, 24.72+ / - 0.2 degree, 25.38+ / - 0.2 degree, 28.80+ / -0.2 degree and 29.10 degrees+ / - 0.2 degree. The invention also provides a preparation method of the Febuxostat crystal form. The crystal form has better stability and is suitable for technically applying preparations and being stored in a long period.
Owner:CSPC OUYI PHARM CO LTD +1
High-purity Febuxostat and preparation method thereof
ActiveCN101781270ALow yieldImprove efficiencyOrganic active ingredientsOrganic chemistryOrganic solventSolvent
The invention belongs to the technical field of pharmaceutical chemistry, in particular to a high-purity compound of 2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methylthiazole-5-carboxylic acid (namely Febuxostat) and a preparation method thereof, wherein the purity thereof is not lower than 99.0%. The method uses a mixed solvent comprising two organic solvents to purify a Febuxostat crude product, and prepares a pharmaceutical composition by mixing a medical carrier and the high-purity compound.
Owner:CHONGQING PHARMA RES INST
Preparation method for febuxostat
InactiveCN103304512ALow costReduce operation processOrganic chemistryHydroxylamine HydrochlorideToxic material
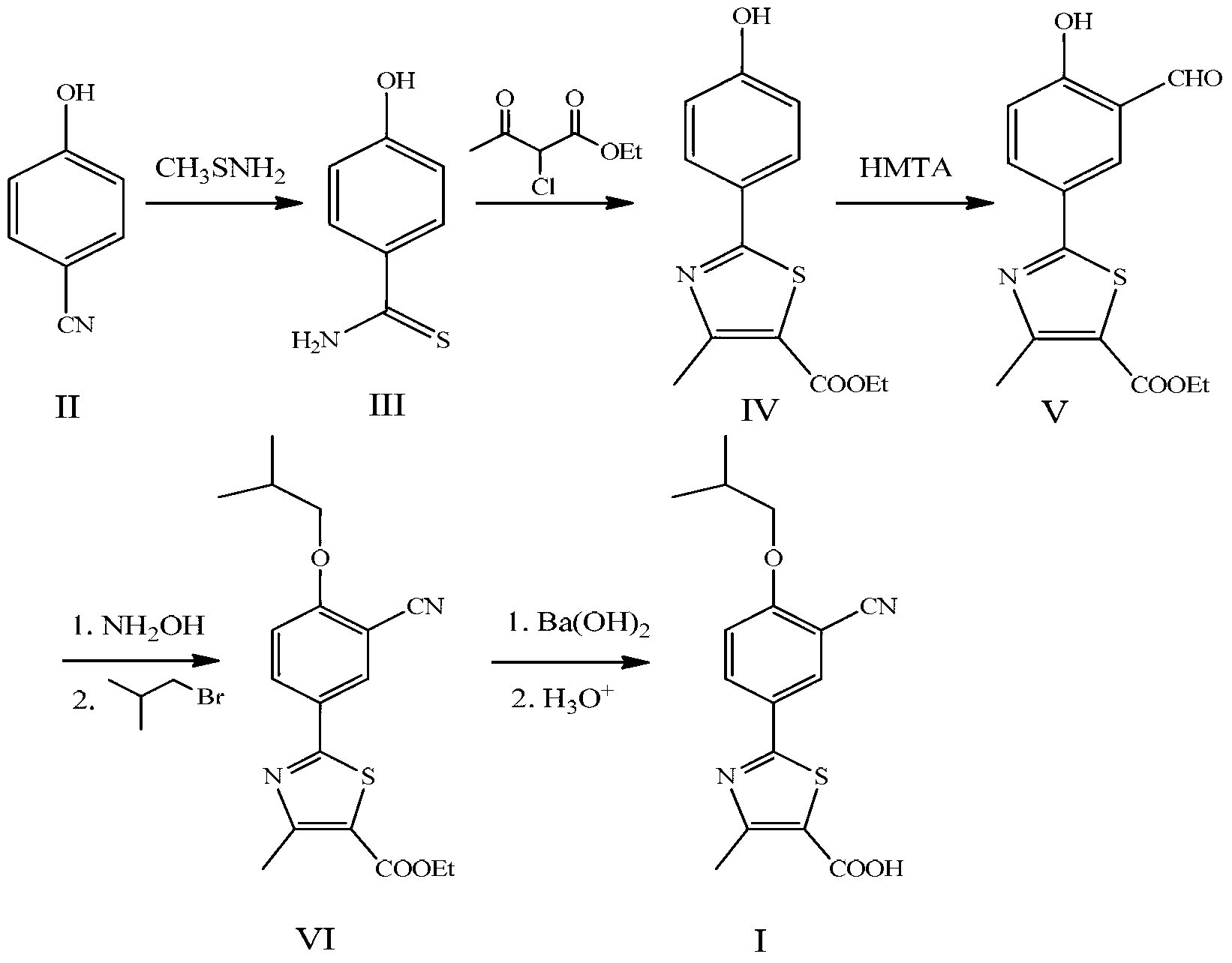
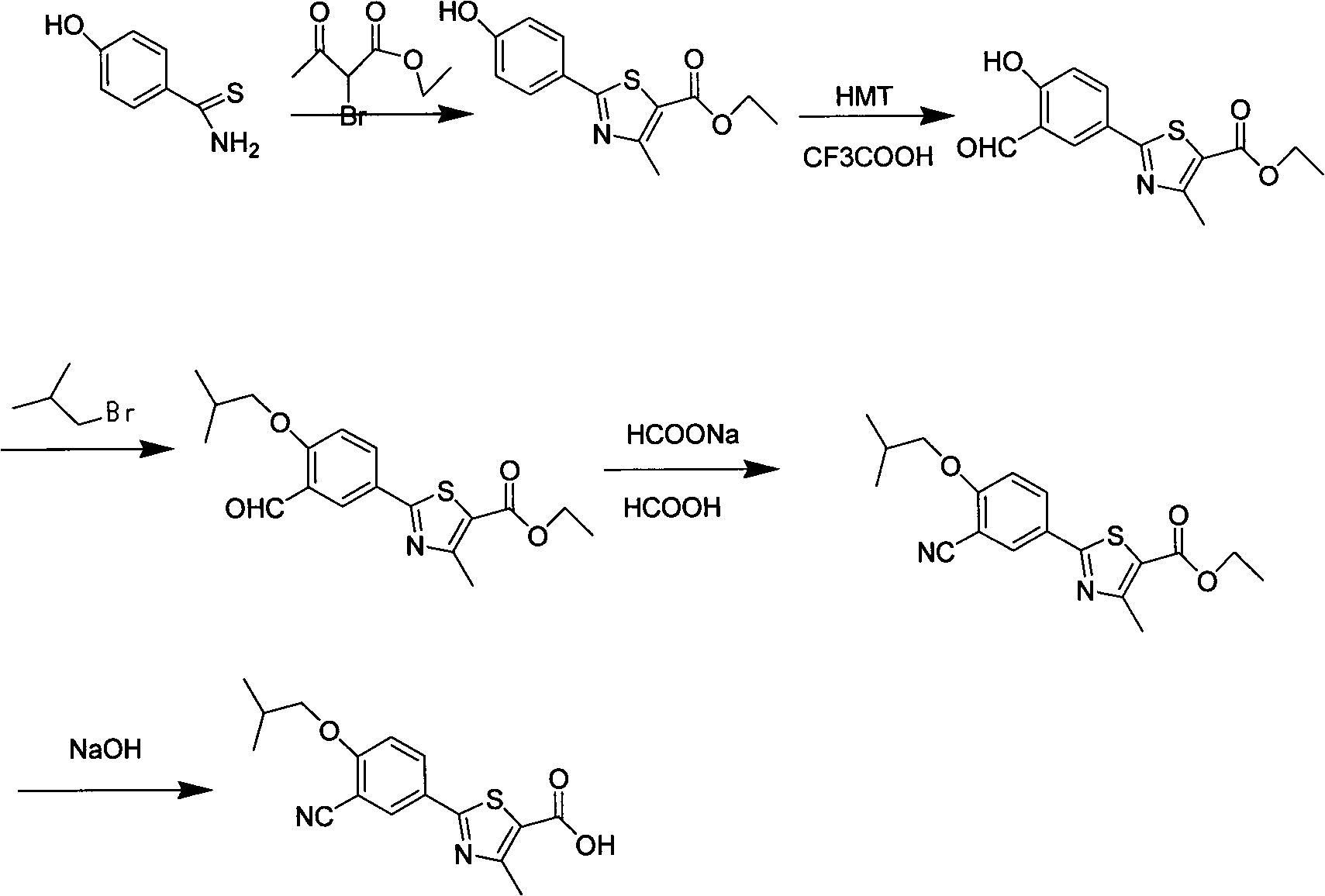
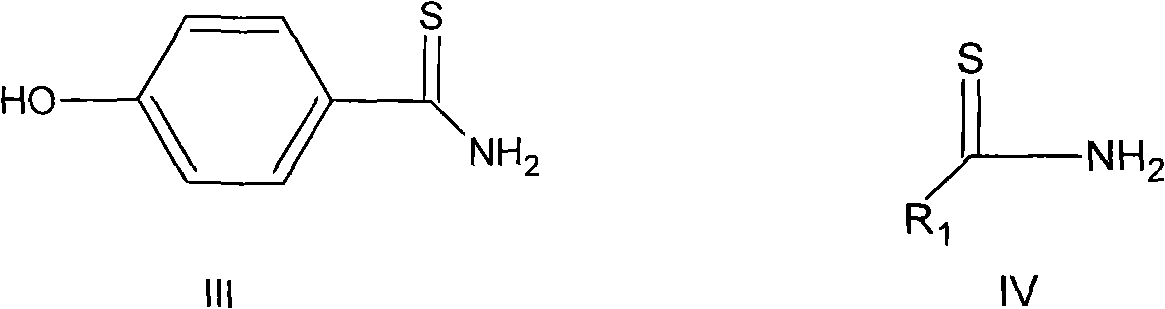
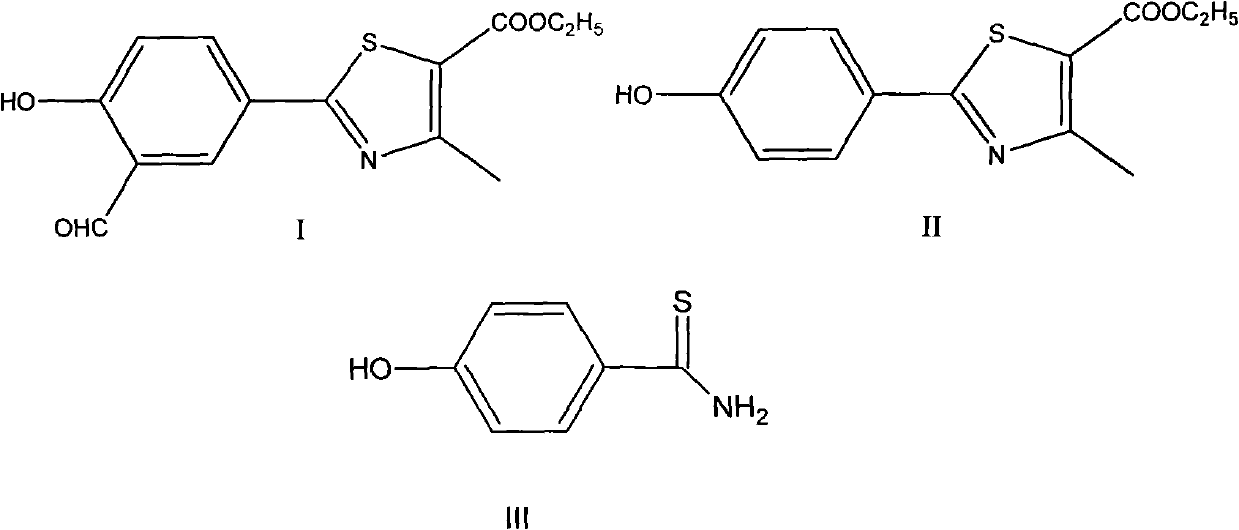
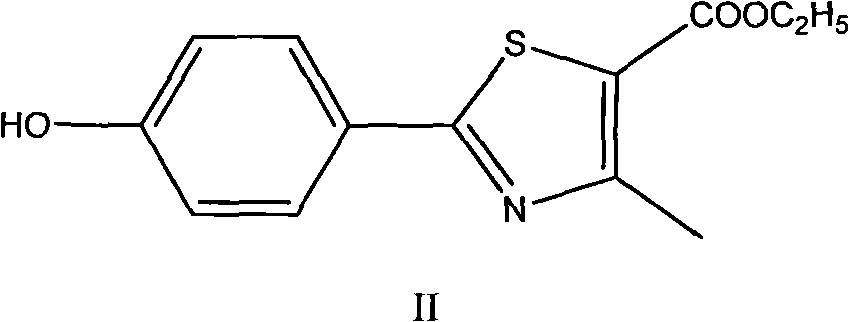
The invention discloses a preparation method for febuxostat. The preparation method for febuxostat comprises the following steps of: by using 4-hydroxybenzonitrile and thioacetamide as raw materials, and reacting in hydrochloric acid solution to prepare 4-hydroxythiobenzamide; carrying out a reaction on 4-hydroxythiobenzamide and 2-chloroacetoacetic acid ethyl ester to prepare 2-(4-hydroxylphenyl)-4-methylthiazol-5-carboxylic acid ethyl ester; carrying out a reaction on the obtained compound and hexamine in the mixed acid system of methanesulfonic acid and trifluoroacetic acid to prepare 2-(3-formyl-4-hydroxylphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester; synthesising 2-(3-nitrile-4-isobutoxylphenyl) -4-methylthiazole-5-carboxylic acid ethyl ester from the compound, hydroxylamine hydrochloride, potassium carbonate, iso-butyl bromide and the like in a polar protonic solvent via a one-pot method; and finally hydrolyzing in an alkaline condition to obtain the target product, namely, febuxostat. The total yield of the preparation method for febuxostat disclosed by the invention is increased to 66%, the separation steps are reduced, any extremely toxic substance is not involved, and the environmental pollution is less.
Owner:SOUTH CHINA UNIV OF TECH
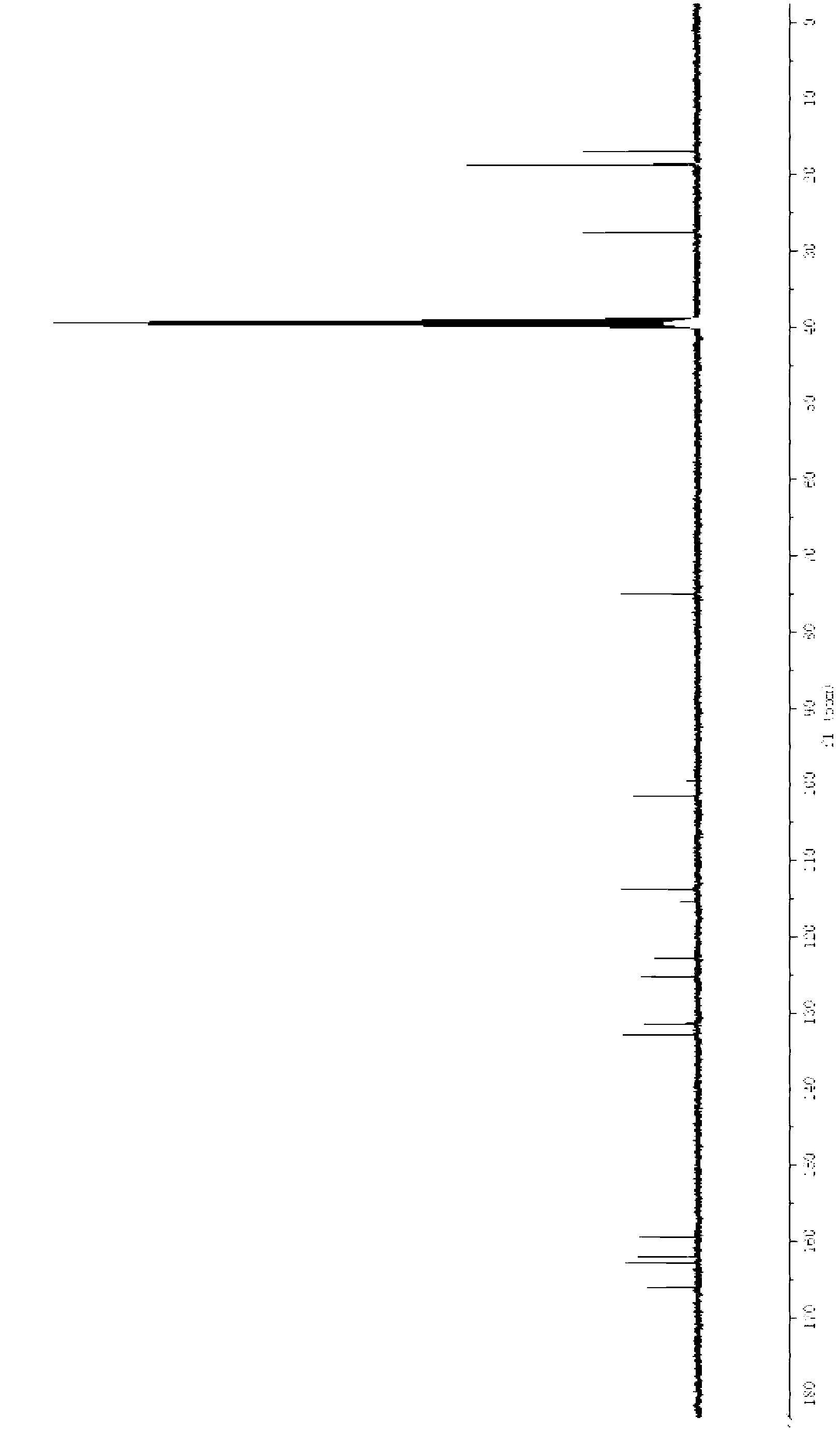
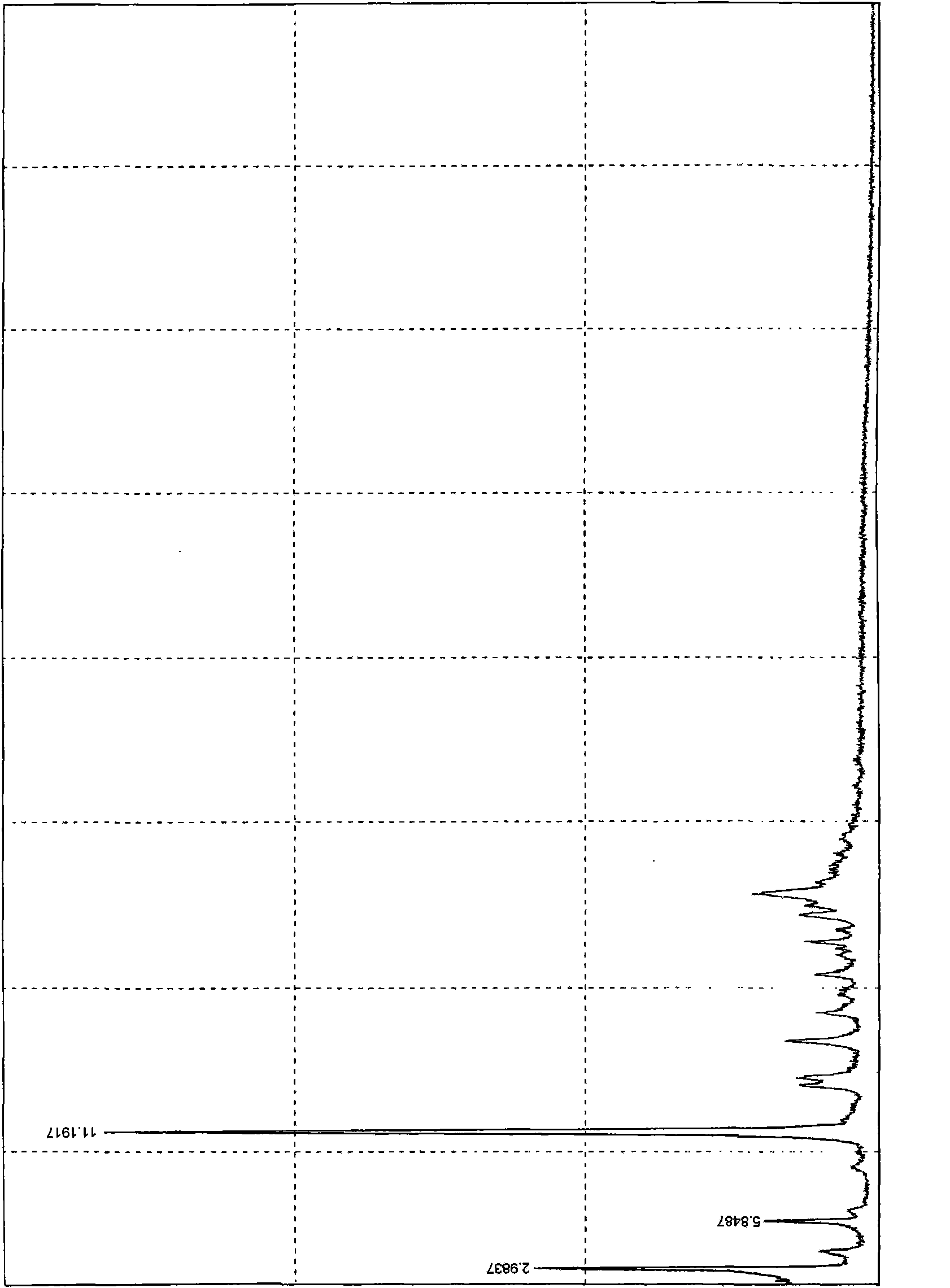
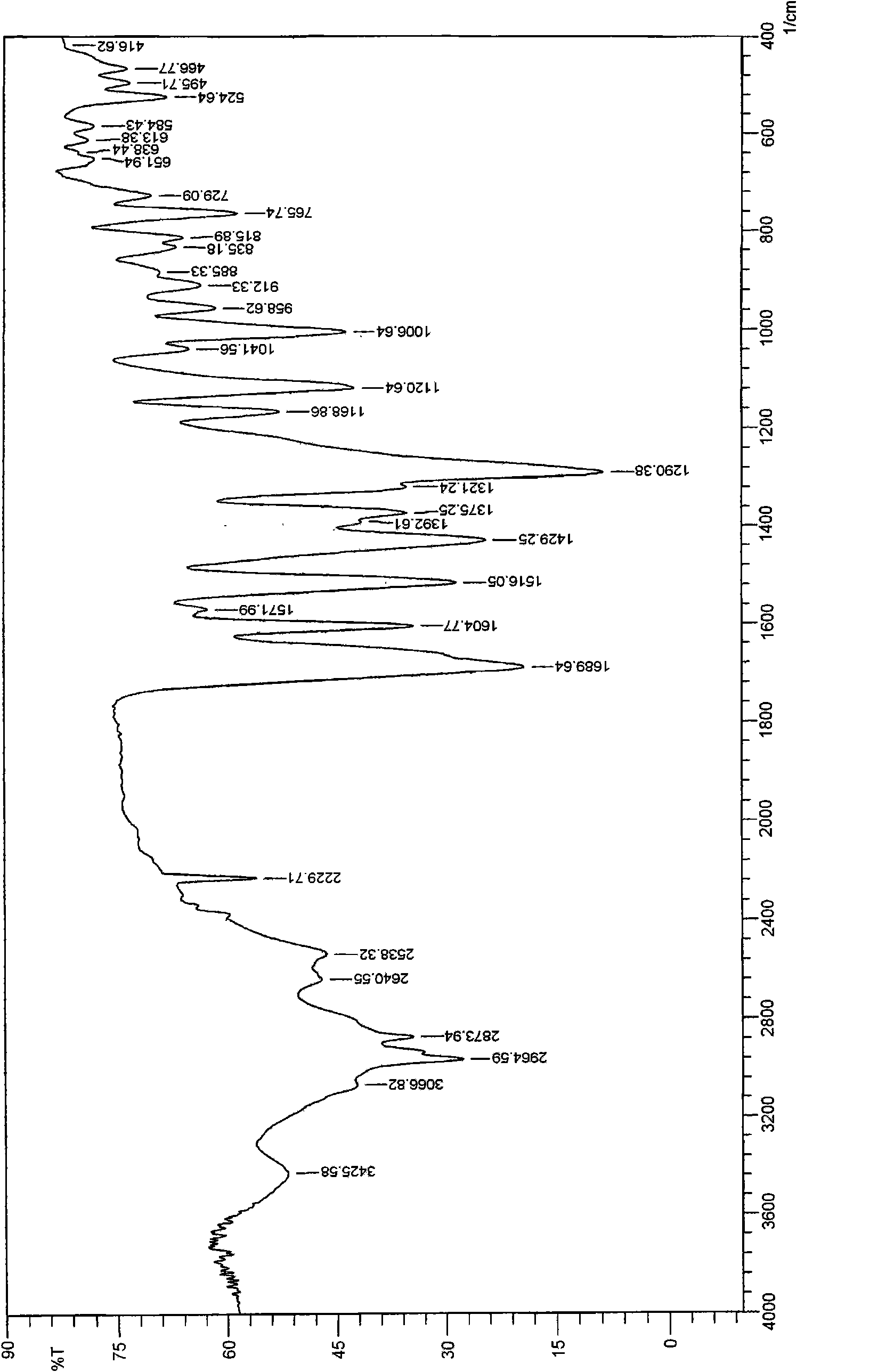
Crystal of febuxostat, preparation method and application in medicaments
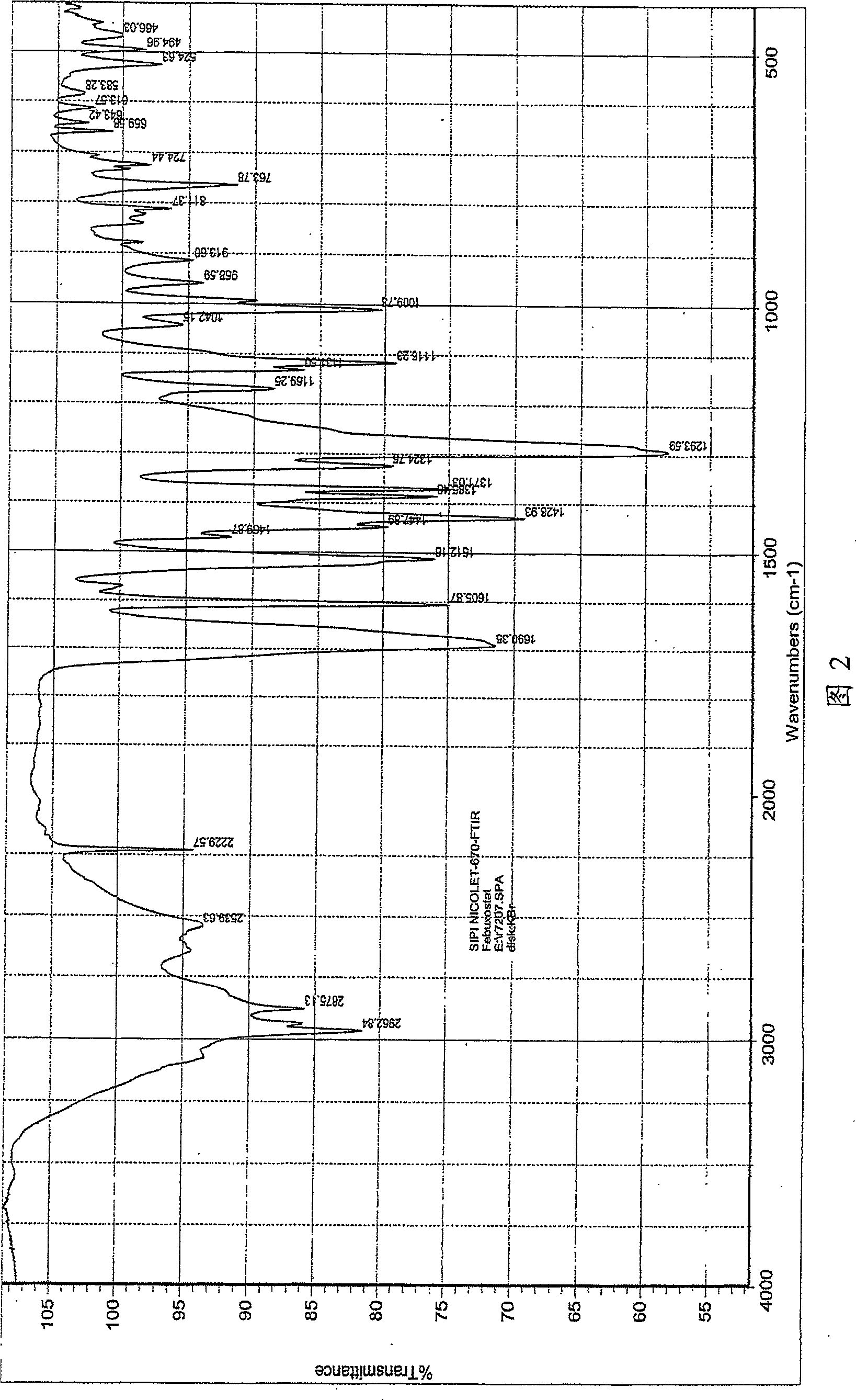
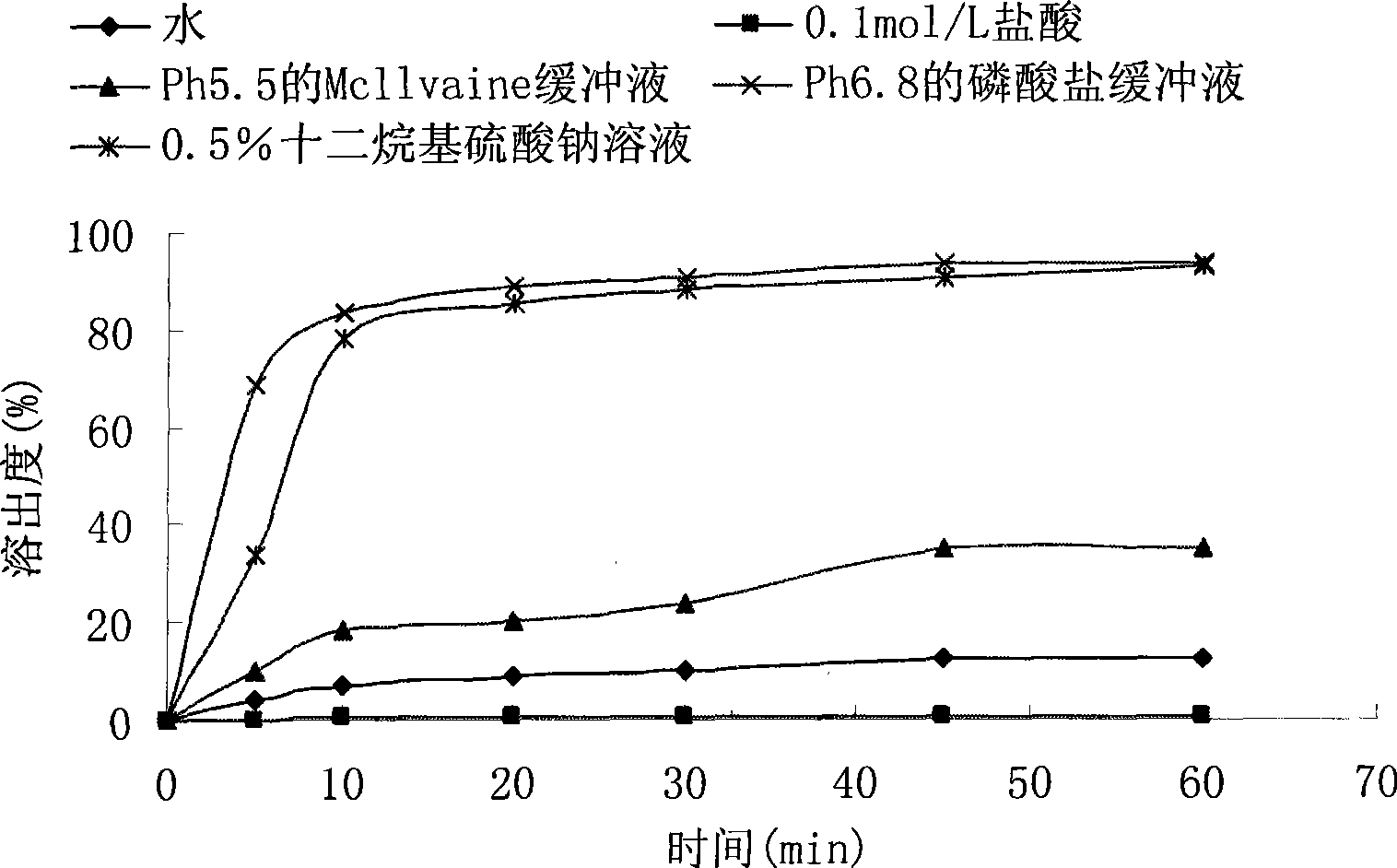
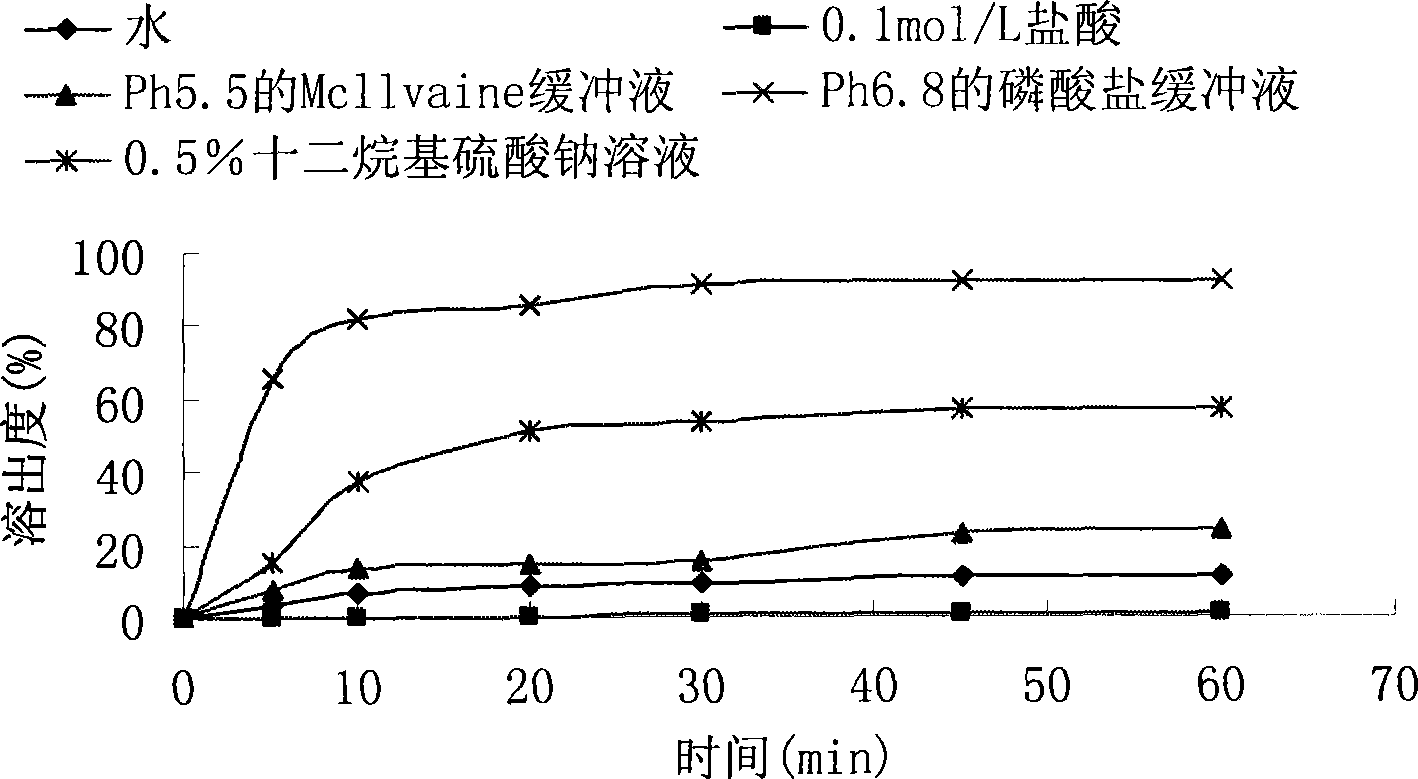
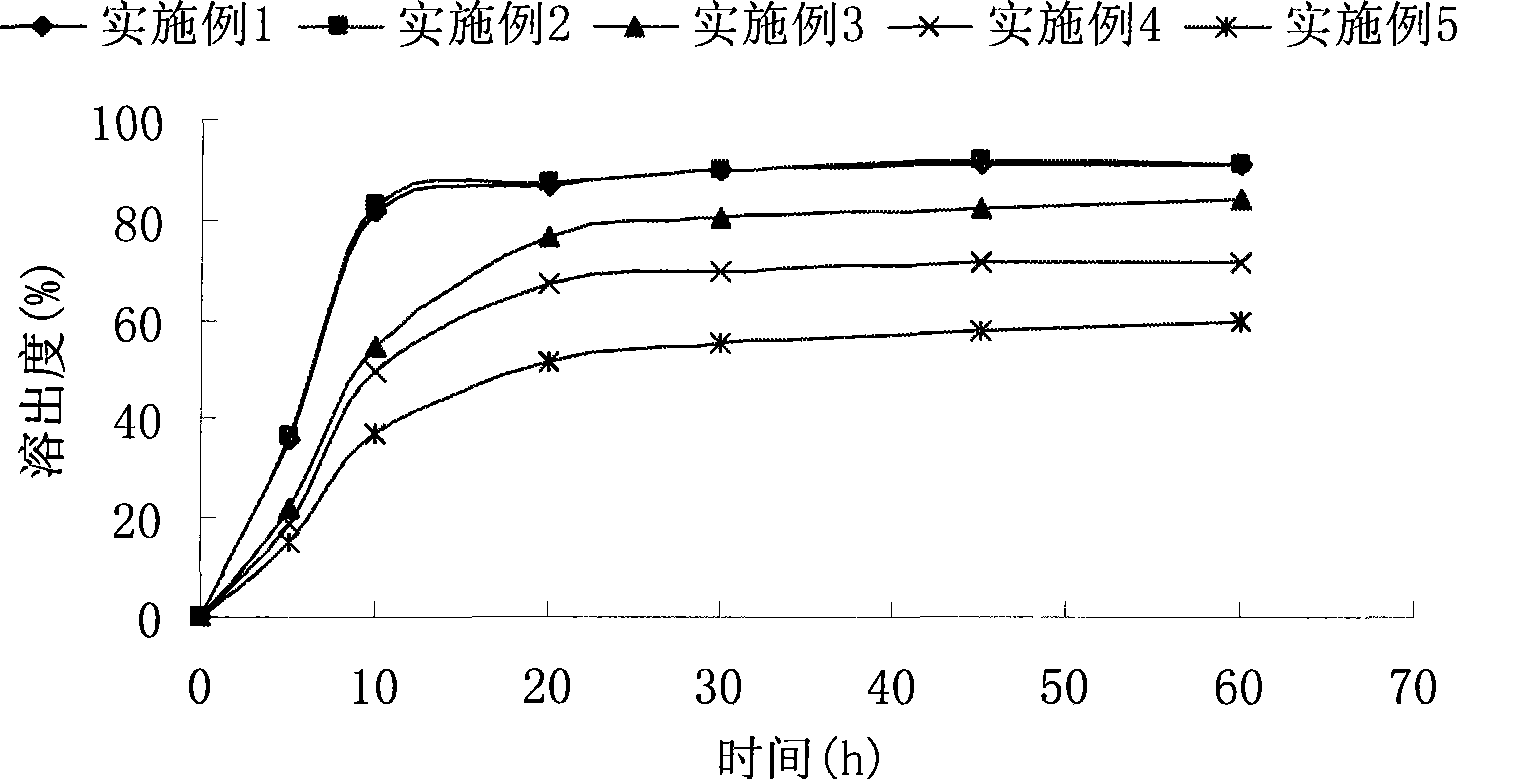
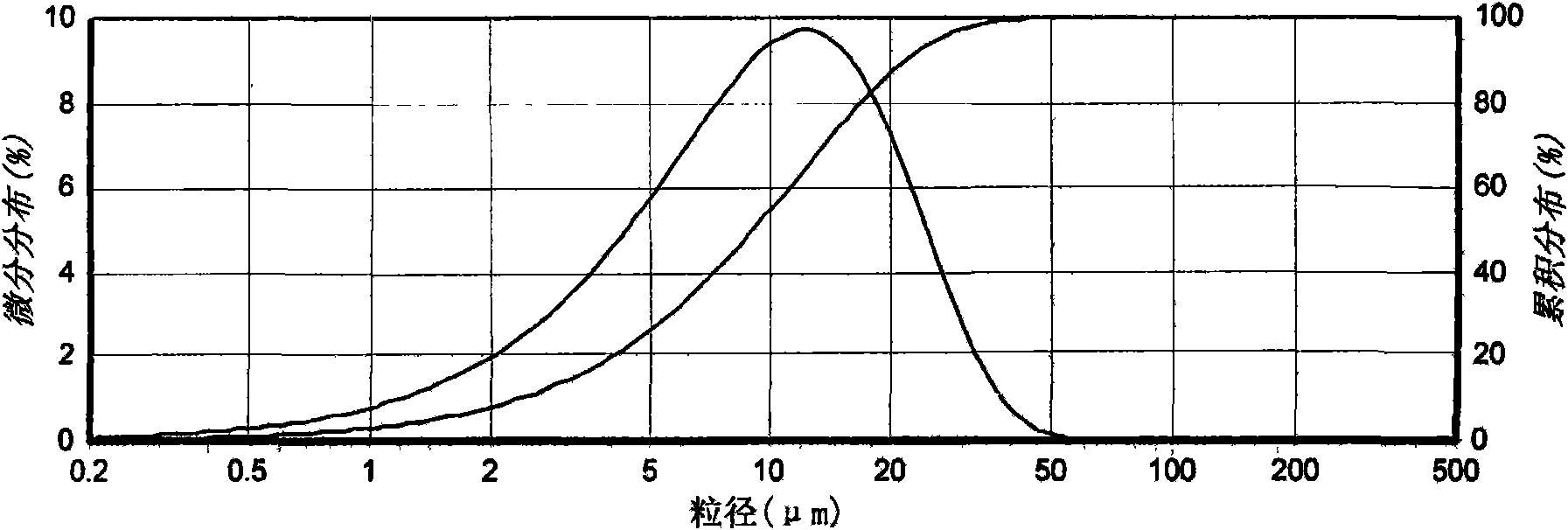
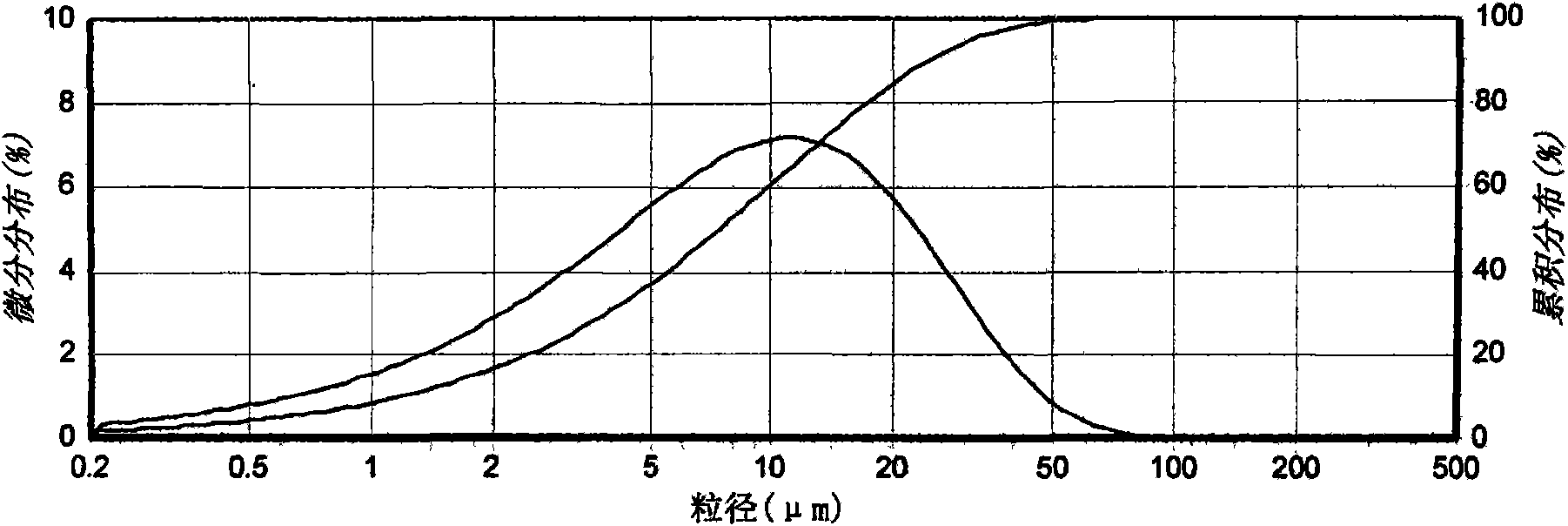
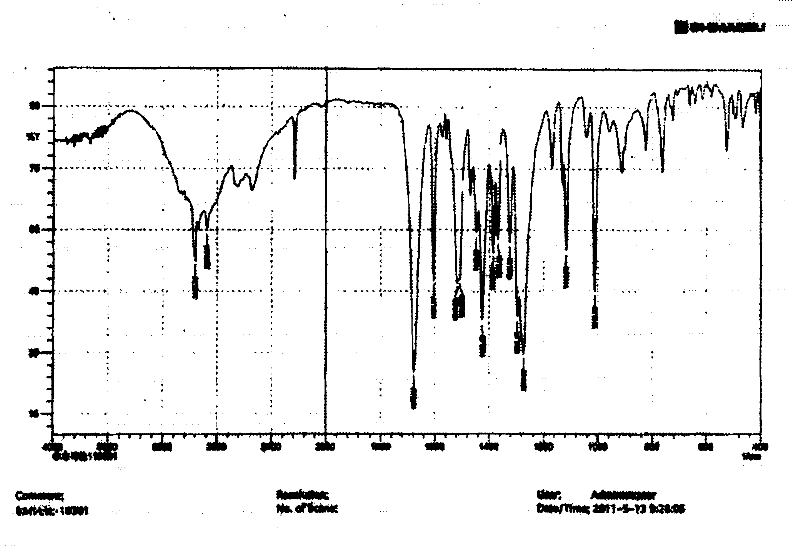
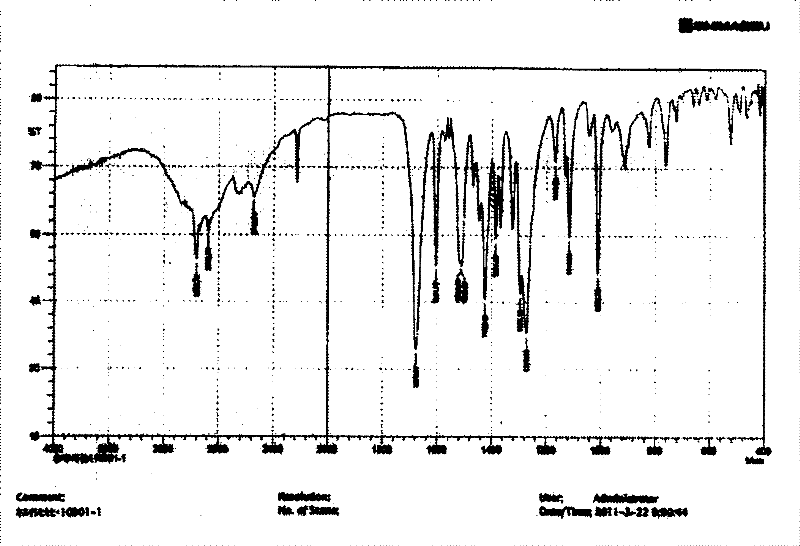
The invention relates to a crystal (type M) of febuxostat, a preparation method and an application in medicaments. The reflected angle 2theta of the crystal X-ray powder diffraction pattern has characteristic absorption peak at about 2.95 degrees, 5.84 degrees, 11.19 degrees, 14.04 degrees, 14.54 degrees, 16.75 degrees, 18.47 degrees, 20.81 degrees, 22.72 degrees, 24.34 degrees and 25.62 degrees, wherein the reflected angle 2theta has three strongest peaks at 11.19 degrees, 2.98 degrees and 5.84 degrees; in an infrared spectrum pattern, the characteristic absorption peak occurs at about 3425cm<1>, 2964cm<1>, 2873cm<1>, 1689cm<1>, 835cm<1>, 815cm<1>, 765cm<1> and 729cm<1>; the particle diameter measured with a laser diffraction method ranges from 0.5 mu m to 50 mu m, the counting particle diameter D90 is less than 40 mu m and the average particle diameter is less than 20 mu m. The crystal can be obtained by recrystalling the febuxostat with toluene; the crystal and pharmaceutically acceptable auxiliary parts can form the useable corresponding medicinal preparation with satisfactory dissolving rate; the dissolution of a tablet in phosphate buffer solution medium with the pH value of 7.2 is greater than or equal to 45% within five minutes and greater than or equal to 95% within 35 minutes.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD
Febuxostat crystal and preparation method and application in medicines thereof
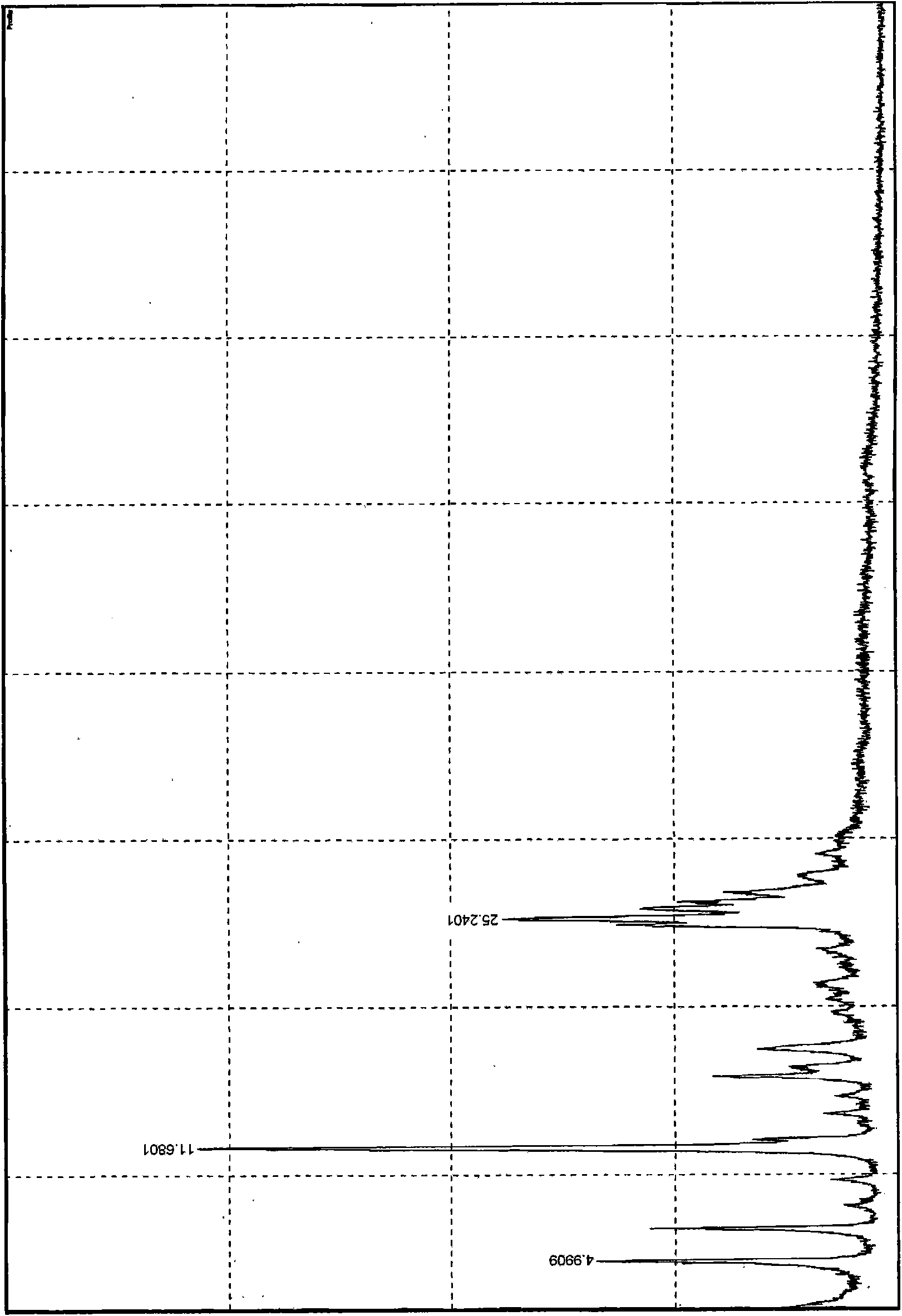
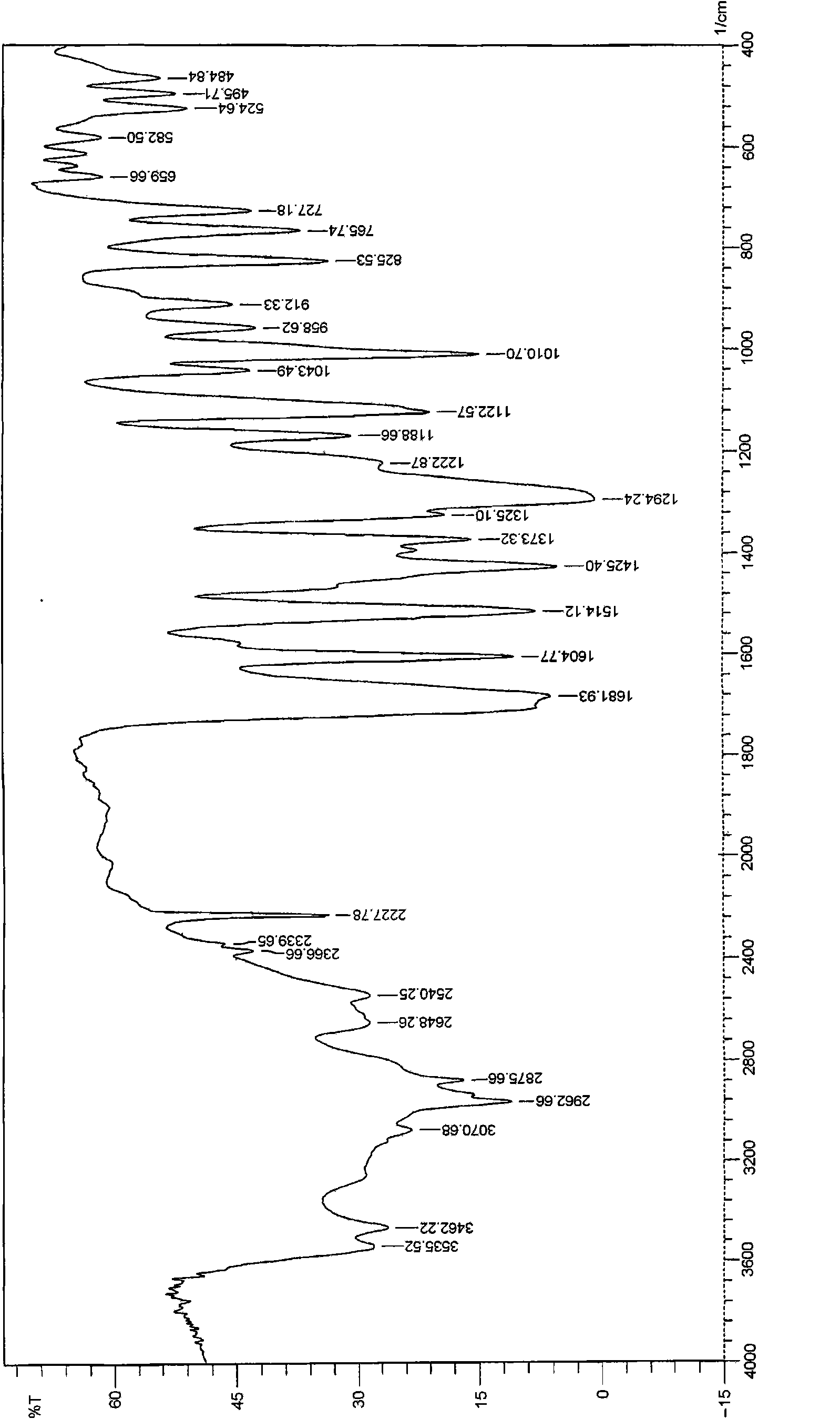
The invention relates to a febuxostat crystal and a preparation method and medical composite thereof. Characteristic absorption peaks appear on the crystal X-ray powder diffraction pattern at the following reflection angle 2 theta: 4.99 degrees, 6.90 degrees, 11.68 degrees, 15.89 degrees, 17.54 degrees, 24.87 degrees, 25.24 degrees, 25.82 degrees, 26.18 degrees and 26.76 degrees; characteristic absorption peaks appear on the infrared spectrogram when the values of wave number are separately about 3535cm<-1>, 3462 cm<-1>, 2962cm<-1>, 2875cm<-1>, 1681cm<-1>, 825cm<-1>, 765cm<-1> and 727cm<-1>; the grain size distribution range measured by the laser diffraction method is 0.5-50mu m, the statistical grain size D90 is less than 40mu m, and the average grain size is less than 20mu m. The febuxostat crystal can be obtained by recrystallizing febuxostat in N,N-dimethylformamide or N,N-dimethylacetamide. The febuxostat crystal and pharmaceutically acceptable auxiliary components can form the corresponding pharmaceutical preparation for use; the pharmaceutical preparation can have satisfactory dissolution rate; and the dissolution rate in 5 minutes of the tablet in phosphate buffer solution medium with a pH value of 7.2 is not less than 45% and in 35 minute is not less than 95%.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD
Febuxostat dispersible tablet and preparation method thereof
InactiveCN101966163ADissolution is rapid and completeImprove bioavailabilityOrganic active ingredientsSkeletal disorderCurative effectMagnesium stearate
The invention relates to a febuxostat dispersible tablet and a preparation method thereof. The febuxostat dispersible tablet comprises febuxostat as a main material and a diluent, a disintegrant, a binder, a flow aid, a lubricant and other assistants as auxiliary materials, wherein the flow aid is silicone dioxide, and the lubricant is magnesium stearate. The febuxostat dispersible tablet is prepared from the following components in percentage by weight: 5-30% of the febuxostat, 20-80% of the diluent, 5-30% of the disintegrant, 0.1-5% of the binder, 1-10% of the silicone dioxide, 0.2-5% of the magnesium stearate and 2-8% of other assistants. The febuxostat dispersible tablet prepared by adding a proper amount of auxiliary materials into crushed febuxostat has the advantages of rapid and complete drug dissolution and stable quality, is suitable for long-term storage and convenient to use, and the bioavailability of human bodies on drugs is increased so as to increase curative effect.
Owner:JIANGSU WANBANG BIOPHARMLS +1
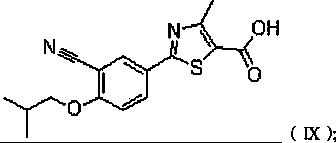
2-(3-cyano-4-isobutoxy phenyl)-4-methyl-5-thiazolyl formic acid of delta-crystal form, as well as preparation method and medicine composite thereof
InactiveCN101805310AImprove humidity stabilityLower uric acidOrganic active ingredientsOrganic chemistryOrganic solventMethyl group
The invention discloses 2-(3-cyano-4-isobutoxy phenyl)-4-methyl-5-thiazolyl formic acid of a new delta-crystal form and a preparation method thereof. The method comprises the steps of: heating a mixture of Febuxostat and absolute ethanol till completely dissolving, and then filtering to obtain an alcoholate; putting the alcoholate in a 80 DEG C baking oven for baking for 2 hours under 2mmHg vacuum decompression to obtain crystals of the delta-crystal form, which has excellent temperature and humidity stability, no any organic solvents or water and is suitable for preparation process and long-term storage. A medicine composite which takes the compound of the crystal form can be used for reducing uric acid in blood and treating all clinical hyperlithic situations, such as uarthritis.
Owner:成都威克药业有限责任公司
Medicinal composition for treating hyperuricemia
InactiveCN101658519AEffective treatmentLow toxicityOrganic active ingredientsSkeletal disorderSide effectBenzbromarone
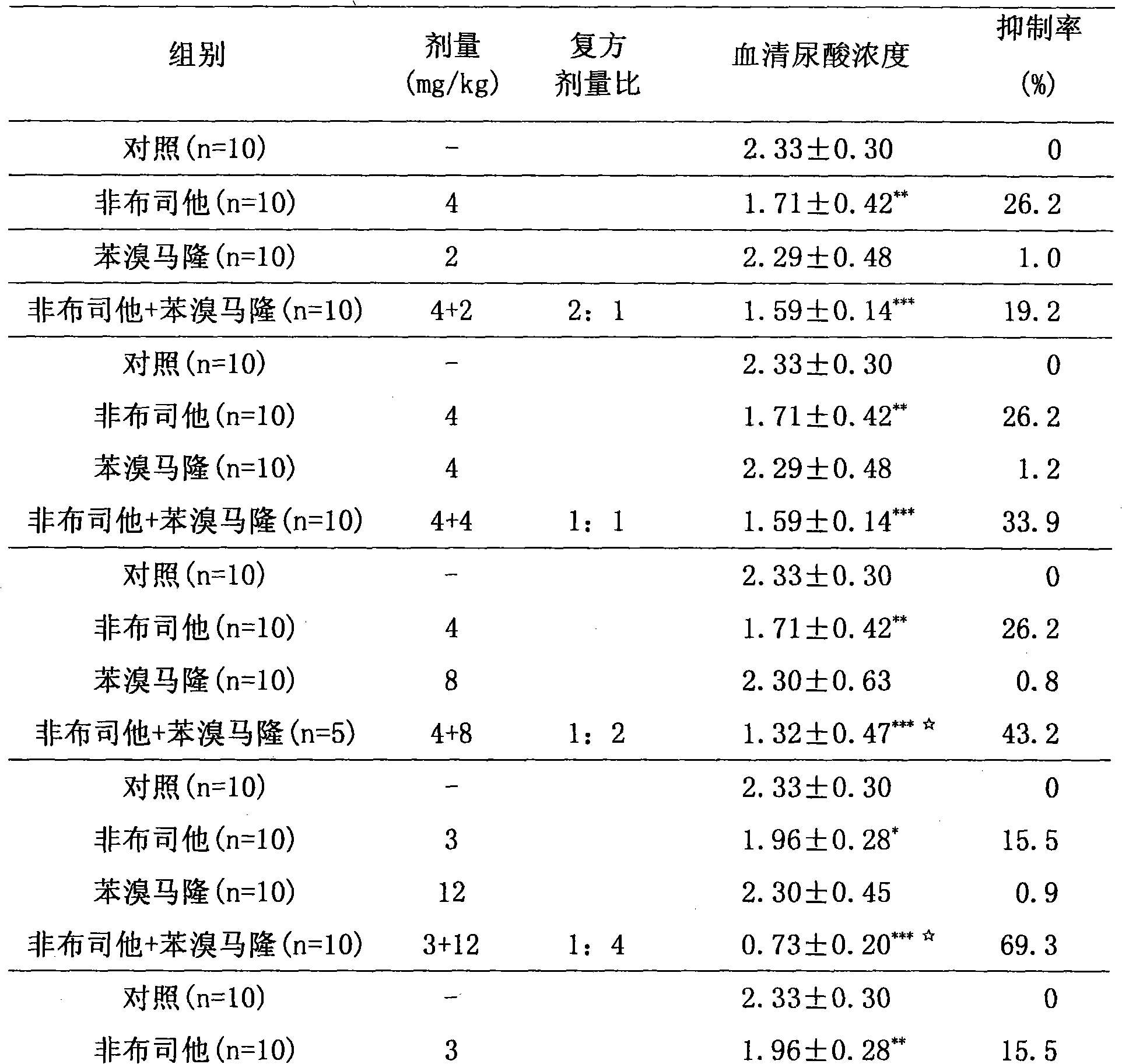
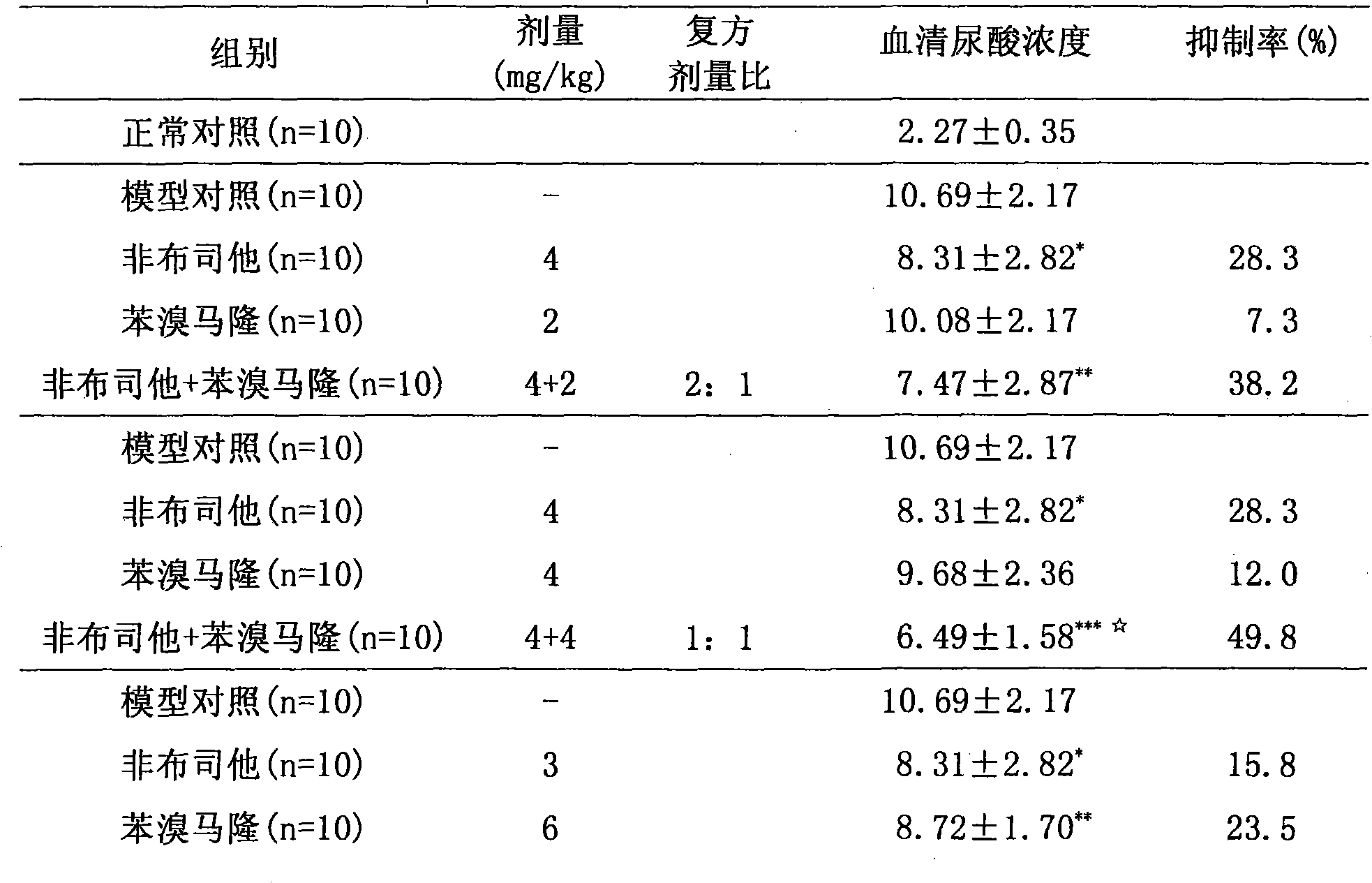
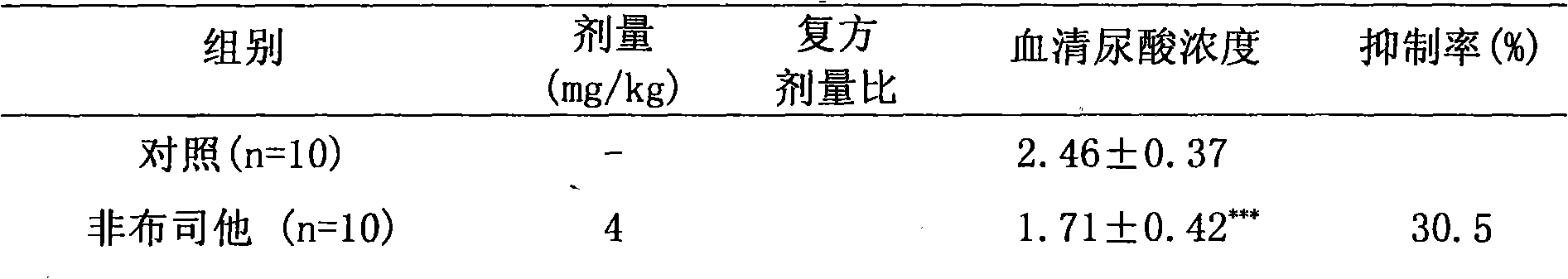
The invention relates to a medicinal composition for treating hyperuricemia, which comprises Febuxostat or derivate thereof, benzbromarone and a pharmaceutically acceptable medicinal carrier, whereinthe ratio in weight portion of the Febuxostat or derivate thereof to the benzbromarone to the pharmaceutically acceptable medicinal carrier is 1:1-4:0.5-100. The medicinal composition can provide extremely beneficial effects of treating the hyperuricemia. Pharmacological tests prove that the medicinal composition has obviously synergistic action, can quickly reduce the concentration of purine trione in blood serum, remarkably improves effect of treating the hyperuricemia, greatly reduces medicament dosage of single component simultaneously, and effectively reduces toxic and side effect of medicament.
Owner:TIANJIN TAIPU PHARMA SCI & TECH DEV +1
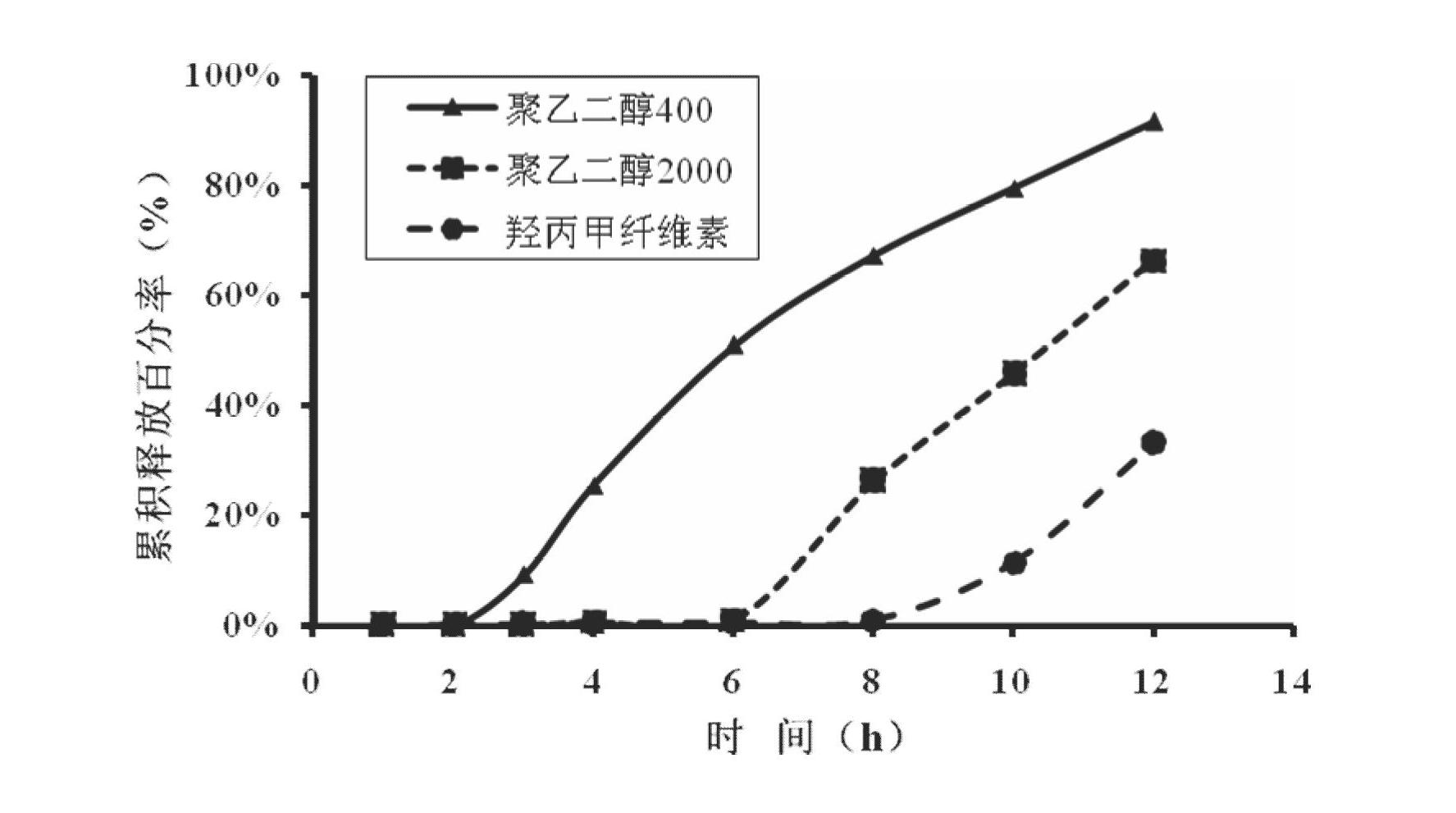
Oral slow/controlled-release preparation containing febuxostat and preparation method thereof
ActiveCN101773498AImprove securityEffective plasma concentrationOrganic active ingredientsSkeletal disorderBlood concentrationBurst effect
The invention provides an oral slow / controlled-release preparation containing febuxostat and a preparation method thereof, which prepare the febuxostat into the long-acting oral slow / controlled-release preparation and can solve the problem that the incidence of an adverse reaction is increased because of the quicker dissolving-out and the burst effect of a common preparation existing in the prior art. The invention has the technical scheme that the oral slow / controlled-release preparation containing the febuxostat comprises the following components by weight percent: 5 to 60 percent of febuxostat, 10 to 50 percent of slow / controlled-release material, 20 to 80 percent of filling auxiliary material, 0.3 to 20 percent of adhesive and 0.1 to 7 percent of lubricant or glidant. Compared with a common quick-release preparation, the slow / controlled-release preparation can keep the effective and stable blood concentration for a longer time, avoids the burst effect of the quick-release preparation, lowers the incidence of the adverse reaction and enhances the application safety.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Febuxostat tablet and preparation method thereof
ActiveCN102488665ALess prescription ingredientsExcipients are easy to getOrganic active ingredientsSkeletal disorderActive agentCombinatorial chemistry
The invention discloses a febuxostat tablet and a preparation method thereof. The febuxostat tablet comprises a tablet core and a coating, the tablet core comprises the following compositions: by weight percentage, from 5% to 30% of febuxostat, from 15% to 60% of filler, from 1% to 20% of disintegrating agent, from 0.1% to 5% of surfactant, from 0.1% to 8% of lubricating agent and a defined amount of adhesive. The febuxostat tablet adopts the high-efficient disintegrating agent within a reasonable proportion range, simultaneously, the febuxostat which is difficultly soluble medicine is dissolved by the aid of the surfactant and the high-efficient disintegrating agent, so that solubility of the febuxostat is improved, and bioavailability of the febuxostat is increased. In addition, the preparation method for the febuxostat tablet is simple, and is controllable in quality and fine in stability.
Owner:KANGYA OF NINGXIA PHARMA
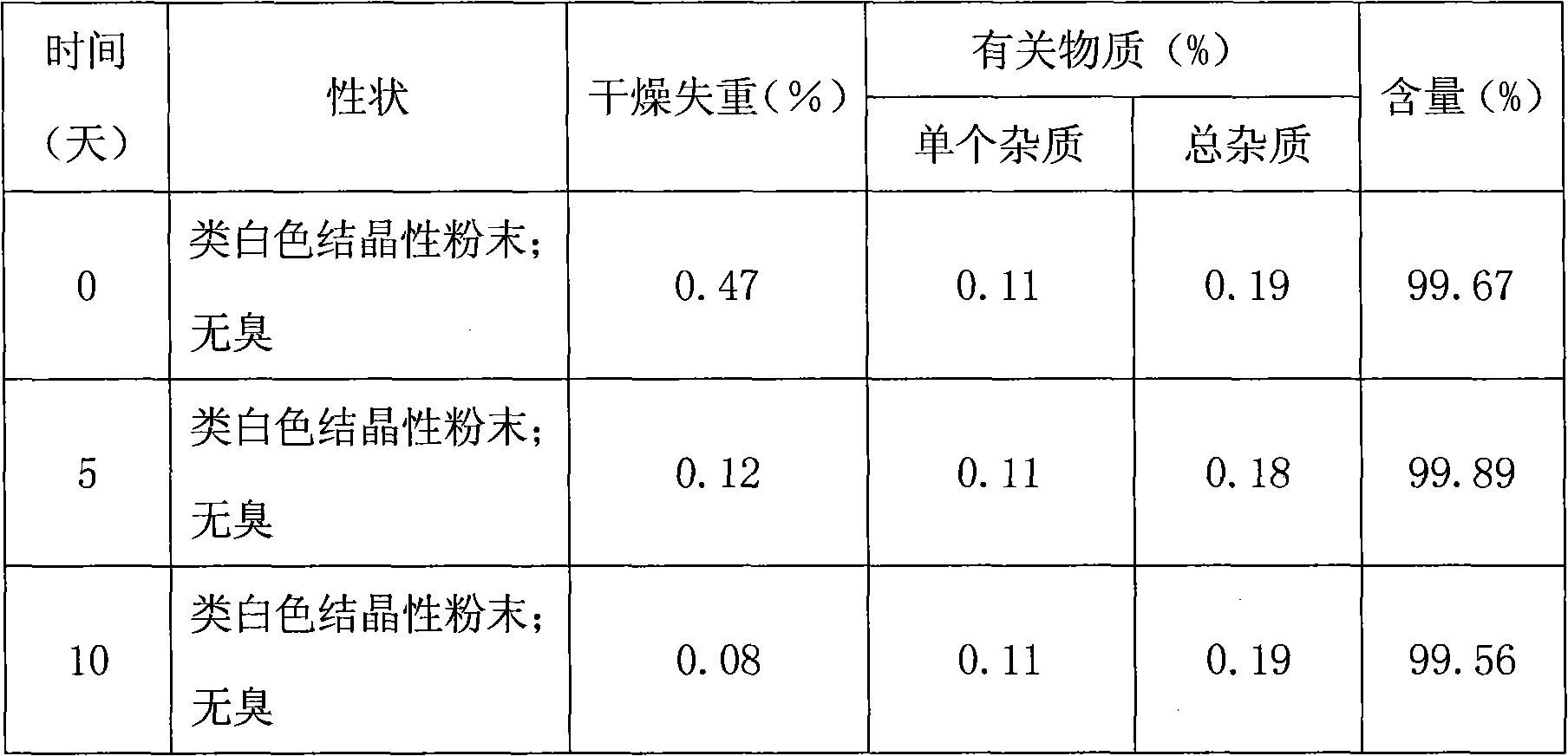
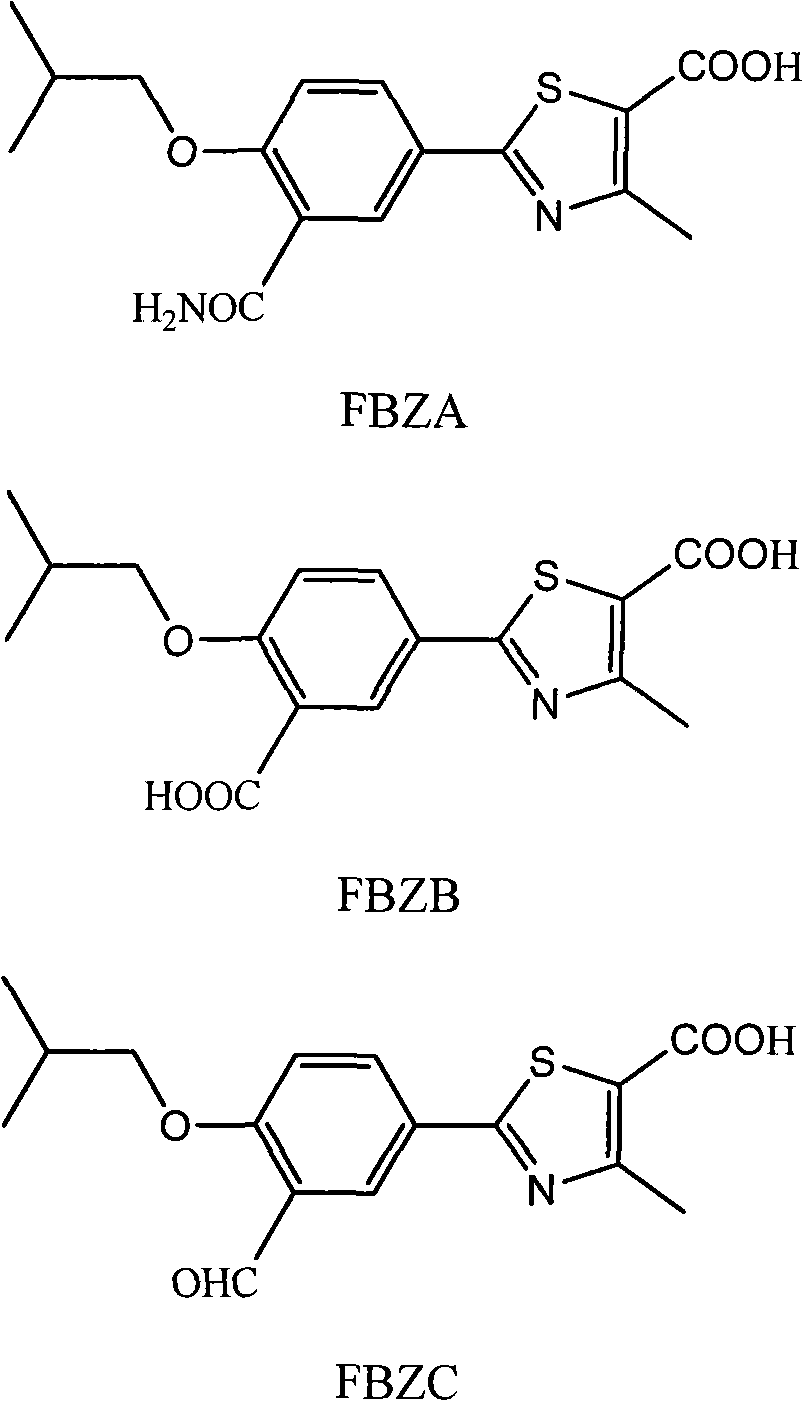
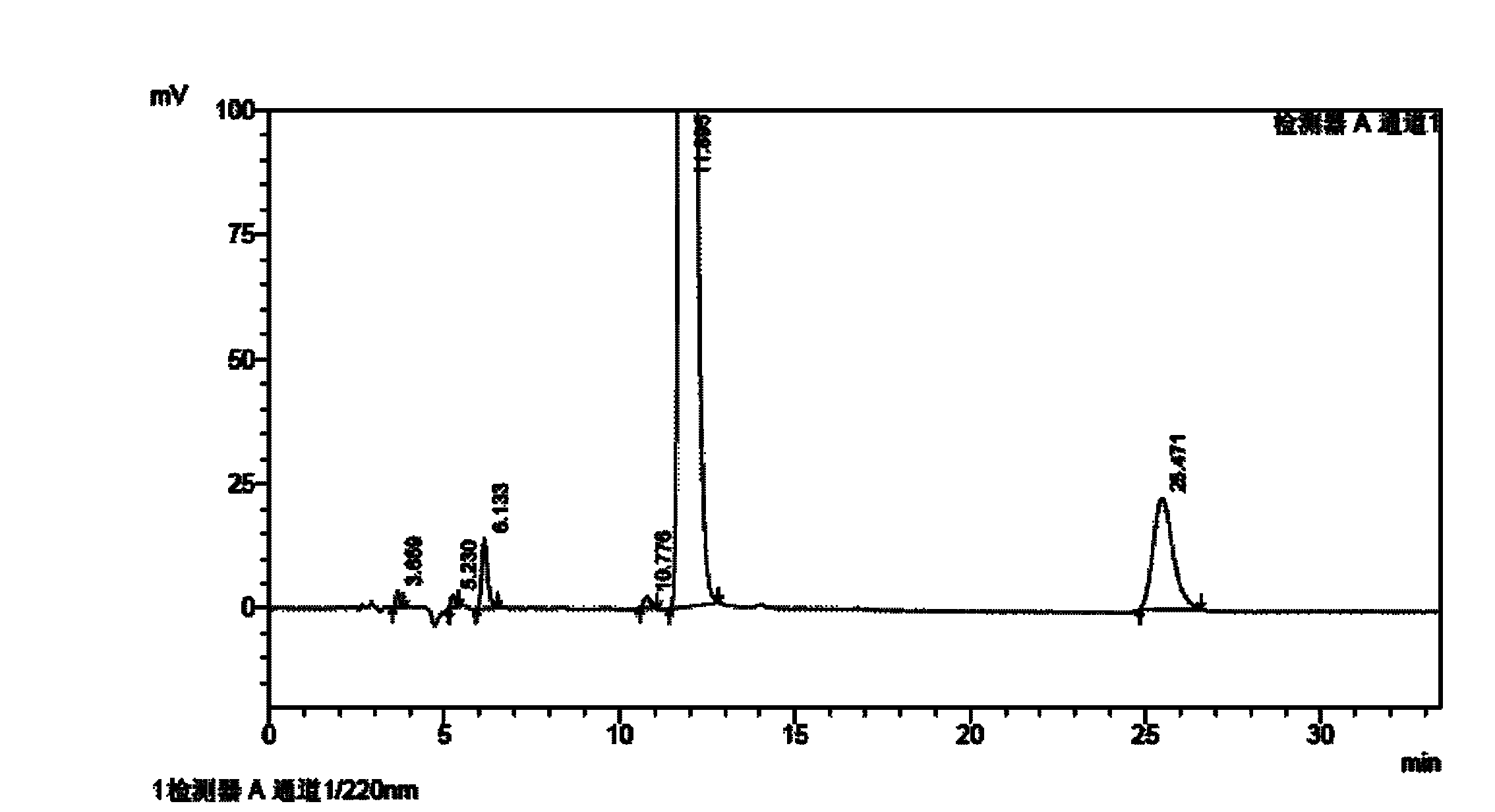
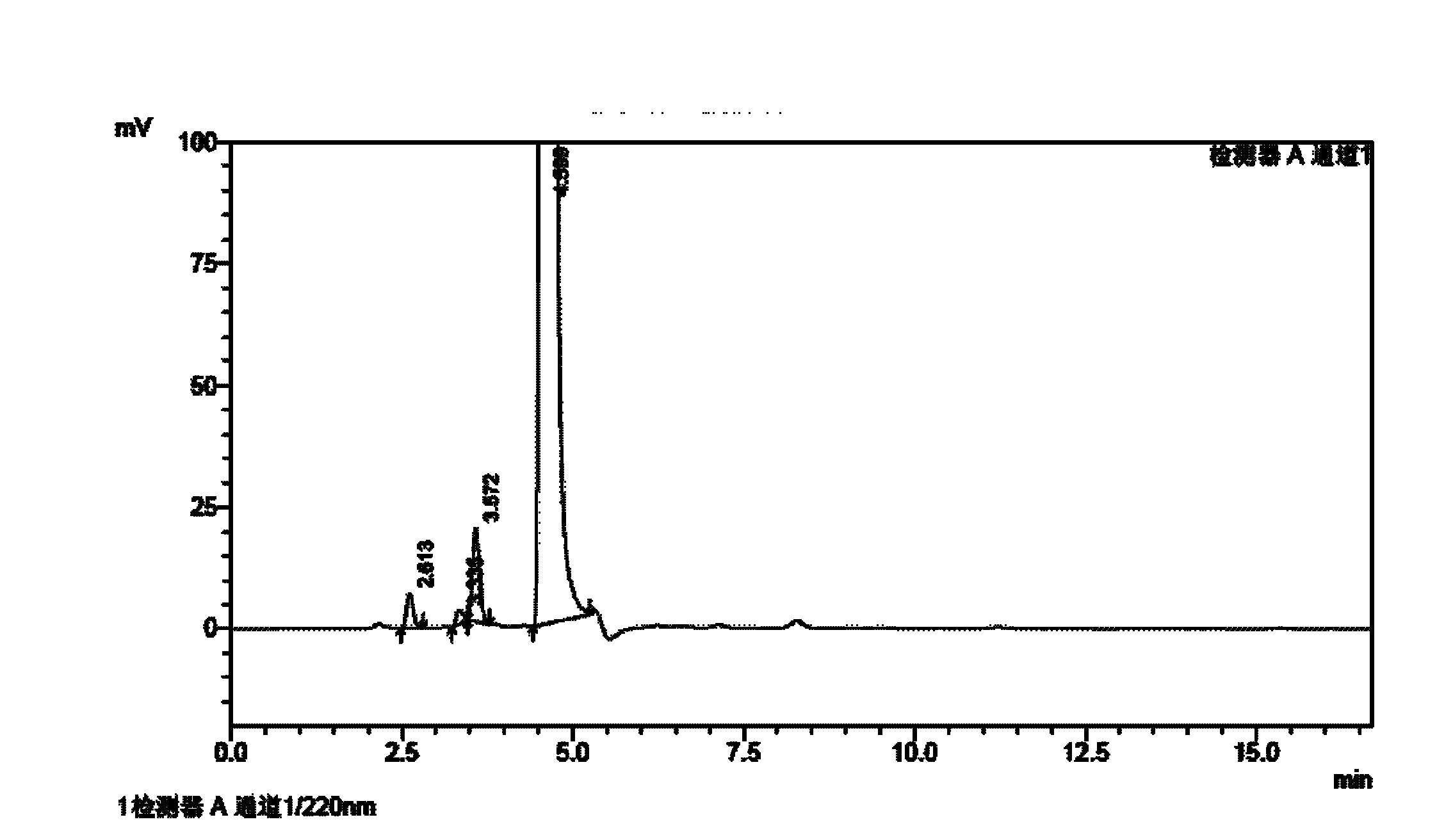
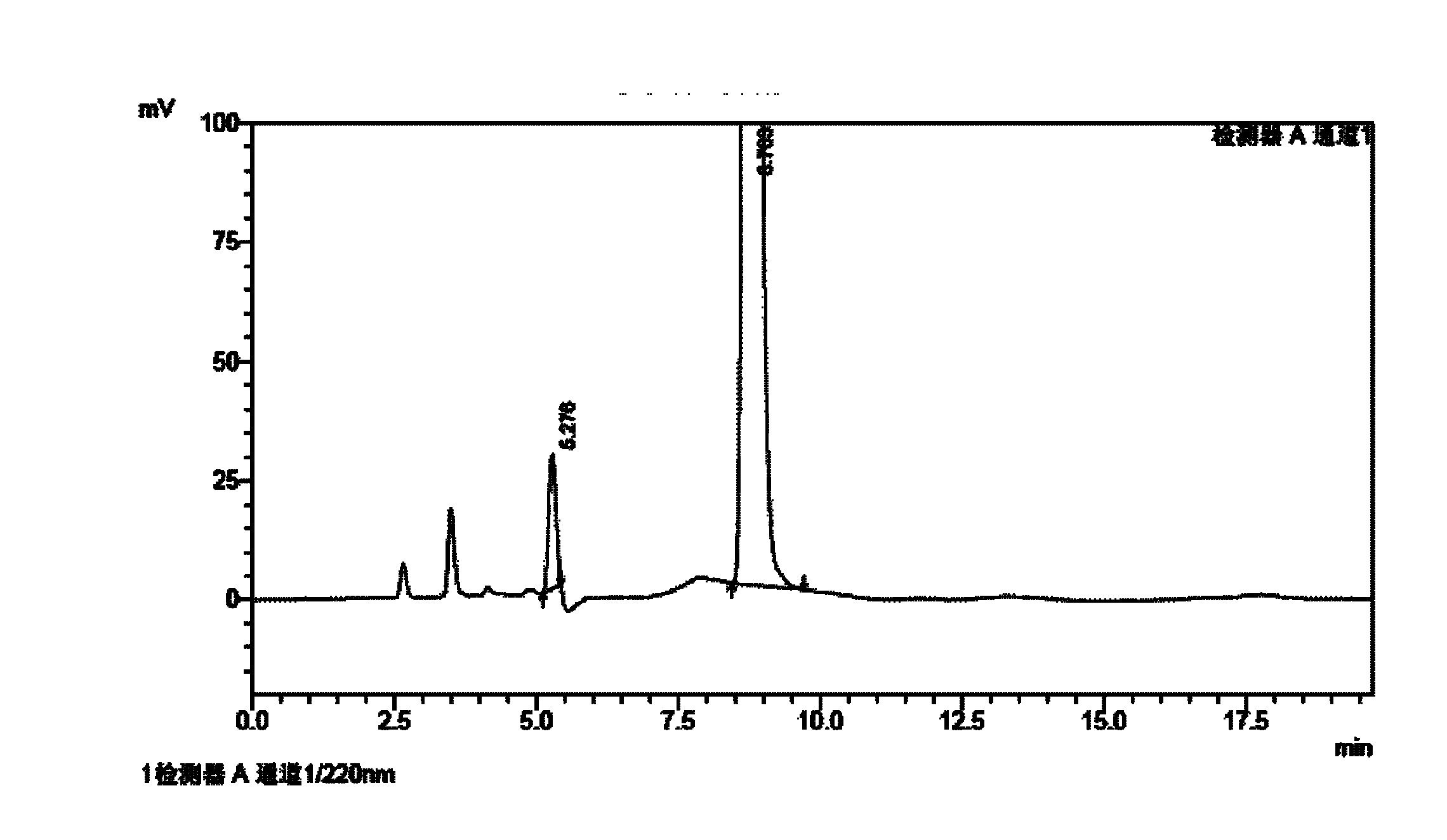
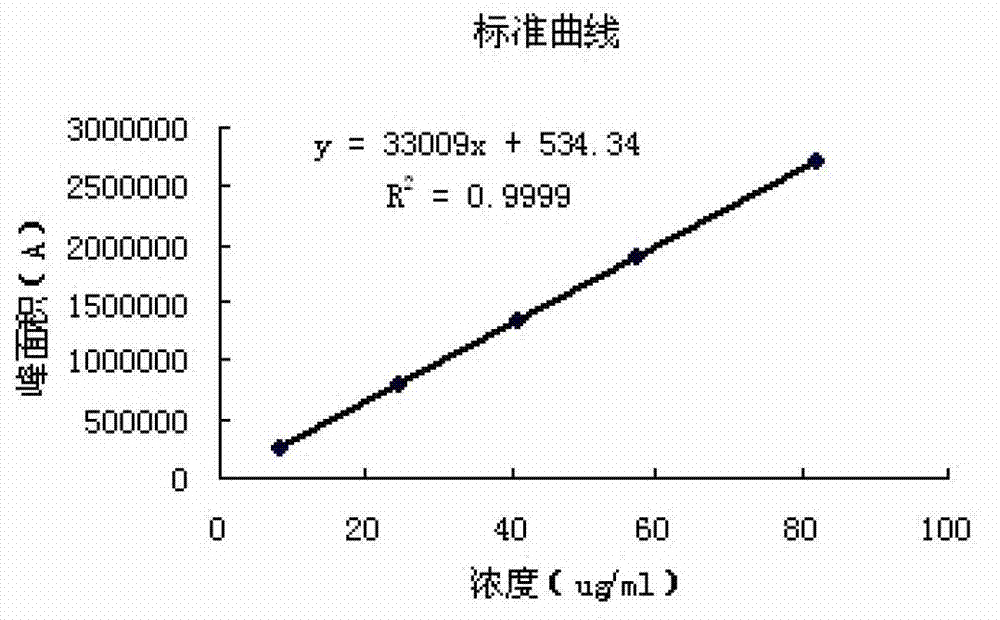
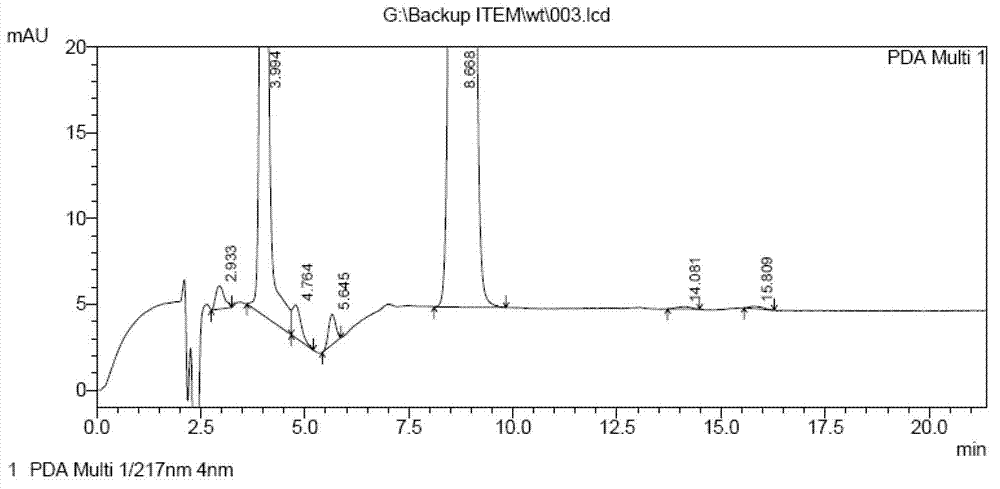
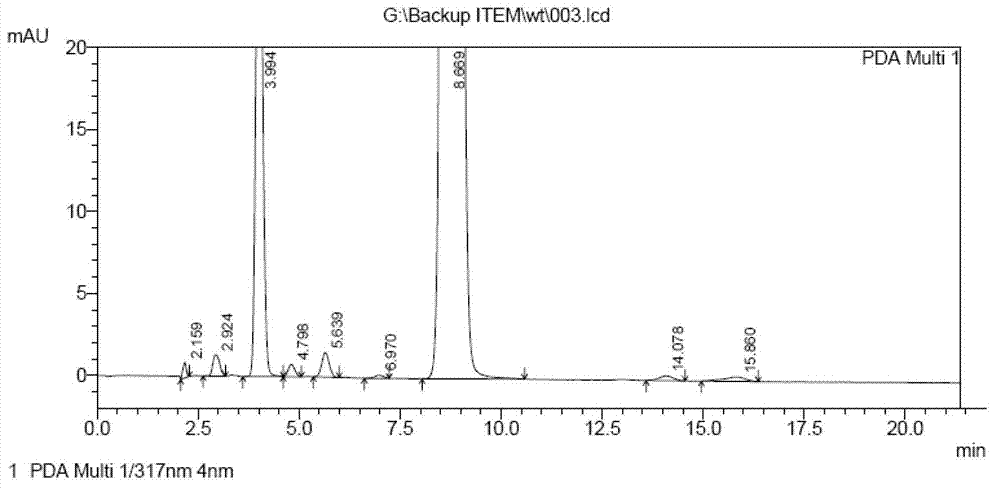
Method for determining impurities in febuxostat and its preparation through high performance liquid chromatography
The invention relates to a medicine analysis method, and especially relates to a method for determining impurities in febuxostat and its preparation through high performance liquid chromatography. The method for detecting and analyzing impurities in febuxostat is established through treating an octadecylsilane bonded silica gel as a filler and a mixed solution comprising a triethylamine buffer solution and methanol as a mobile phase, and is sensitive, specific and comprehensive. The change of the impurities in the production and storage processes of febuxostat can be effectively detected through adopting the method, so the method is of great practical importance for the quality control of febuxostat and its preparation.
Owner:PHARMA RES INST OF BENCAO TIANYUAN OF BEIJING
The preparation method of Febuxostat A crystal form
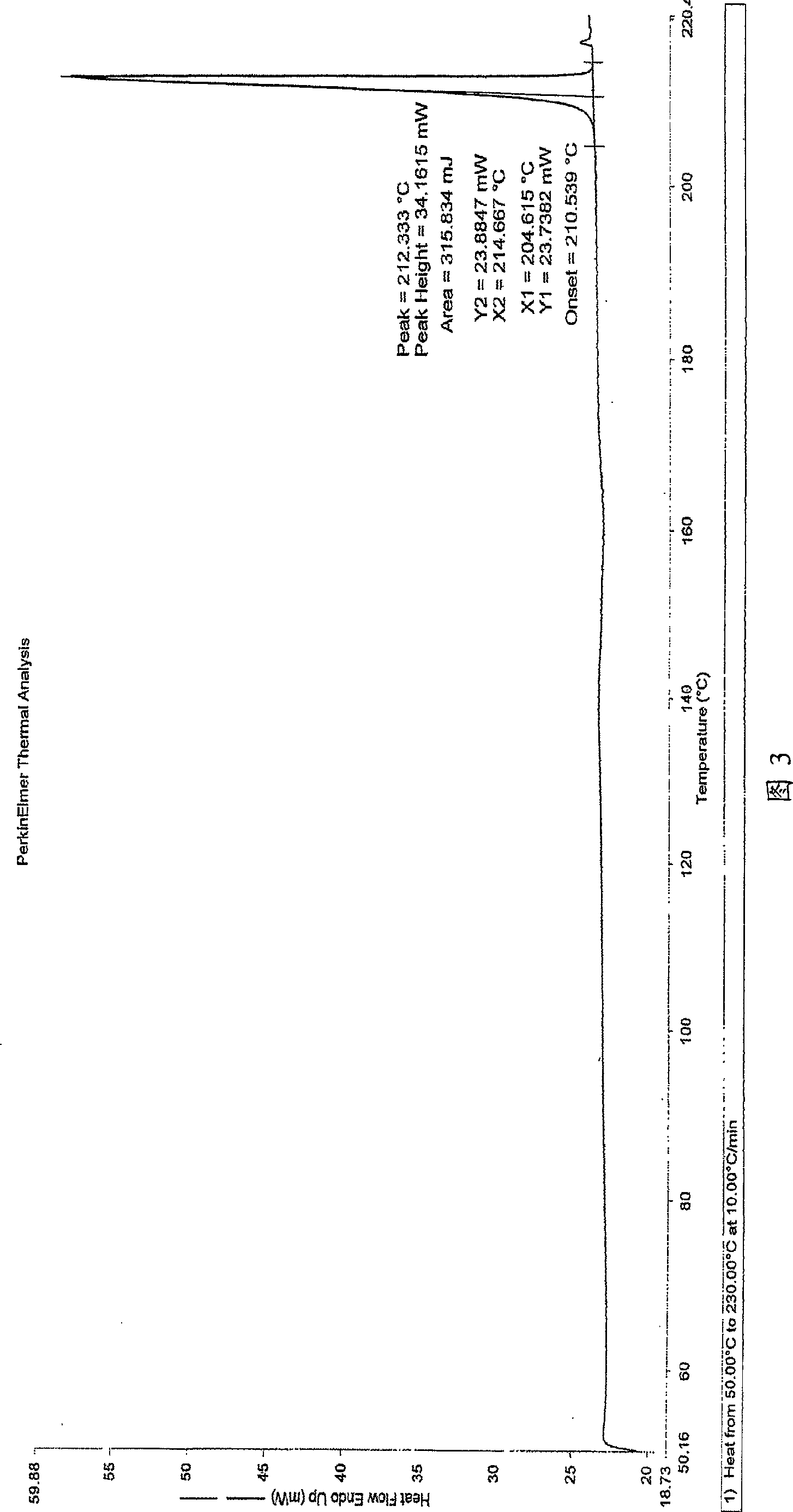
The invention belongs to the field of medicine chemistry and particularly relates to a method for preparing Febuxostat crystal A. The method comprises: dissolving, crystallizing and drying, wherein the dissolving is to dissolve Febuxostat in a solvent with heating in a water bath, the solvent may be RCOCH3 or RCH2OH, R may be methyl or ethyl, the mass / volume ratio of the Febuxostat to the solventranges from 1:5 to 1:20, the Febuxostat is based on gram and the volume of the solvent is based on milliliter; after dissolution, placing the solution in a water bath at 25 to 40 DEG C, standing, stirring for 20 to 40 minutes upon the precipitation of crystals; placing at -15 to 0 DEG C to continue to precipitate crystals for 8 to 10 hours, and filtering; and after filtering, drying at 65 DEG C under vacuum for 6 to 8 hours to obtain the Febuxostat crystal A. The Febuxostat crystal A prepared by the invention is high in purity and yield, the process is simple and easy to implement, the yield is 92.0 to 98.0 percent and the purity is more than 99.90 percent.
Owner:SHANDONG QIDU PHARMA
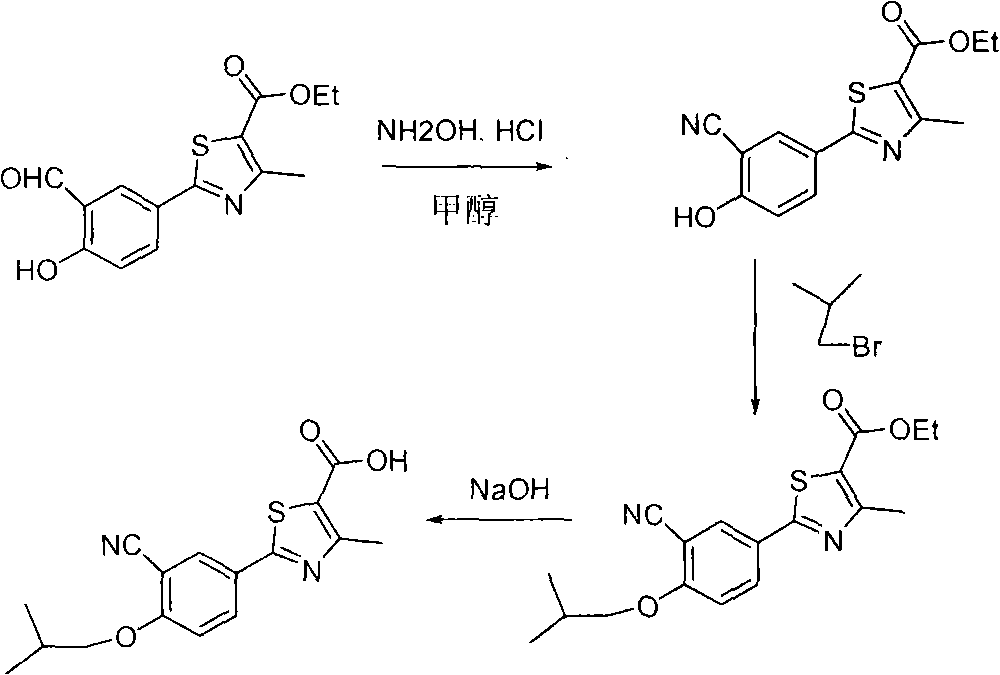
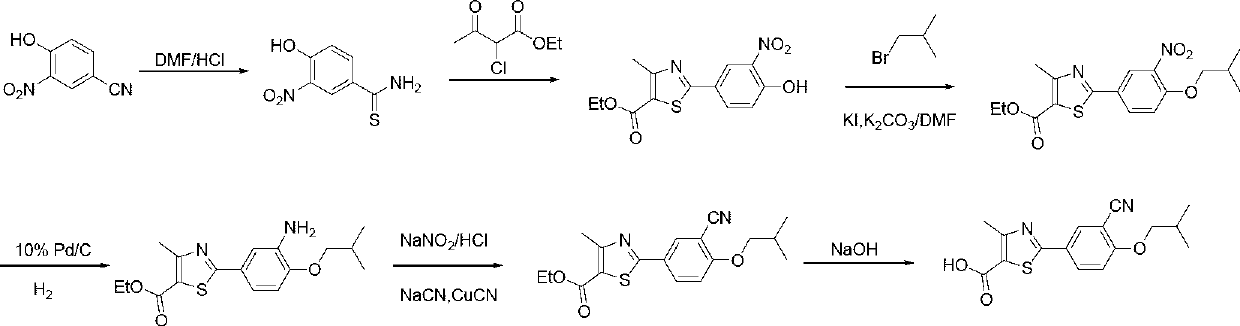
Method for preparing febuxostat intermediate
The invention relates to a method for preparing a febuxostat intermediate, which comprises the following steps of: dissolving 2-[4-hydroxyphenyl]-4-methylthiazol-5-ethyl formate in a mixed acid reaction solvent, adding a certain amount of urotropine, heating to react for 1-36 h at certain temperature, and treating the reaction liquid to obtain corresponding heterocyclic aldehyde.
Owner:CHINA RESOURCES SAIKE PHARMA
Medicine compound containing febuxostat
The invention discloses a medicine compound containing febuxostat; and containing the febuxostat and surfactant, the medicine compound can be stably and quickly dissolved out and is applicable to arthrolithiasis curing.
Owner:AVENTIS PHARMA HAINAN
Method for determining related substances by utilizing high performance liquid chromatography in febuxostat synthesis process
InactiveCN102565225AEfficient determinationGood elution separation effectComponent separationGradient elutionSilica gel
The invention discloses a method for determining related substances by utilizing high performance liquid chromatography in febuxostat synthesis process and belongs to the field of chromatographic analysis. According to the method, octadecylsilane chemically bonded silica gel is taken as the chromatographic column packing, a mixed solvent gradient elution taking organic phase and organic acid solution as aqueous phase serves as mobile phase, wherein the organic phase is acetonitrile or methanol; and the organic acid solution is formic acid solution with the volume percent of 0.1%-0.8% or acetic acid solution with the volume percent of 0.1%-0.8%. Compared with the prior art, the method for determining related substances by utilizing high performance liquid chromatography in febuxostat synthesis process has the characteristics of high analysis sensitiveness, accurate and reliable results and the like, and has good popularization and application values in the quality control field of febuxostat.
Owner:CISEN PHARMA
Preparation method of high-purity febuxostat
The invention relates to a preparation method of high-purity febuxostat. The method comprises the steps: carrying out etherification reaction on ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate and refined bromo-isobutane, then conducting cyaniding and hydrolysis to obtain other crude febuxostat, and recrystalizing to obtain high-purity febuxostat. In febuxostat prepared by using the method, the content of impurity 2-(3-cyano-4-n-propoxy phenyl)-4-methylthiazole-5-formic acid is smaller than 0.10%.
Owner:CHINA RESOURCES SAIKE PHARMA
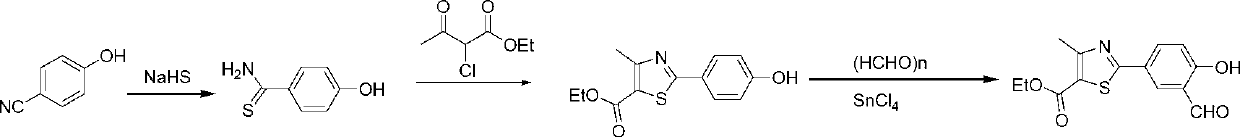
Preparation method of febuxostat intermediate
ActiveCN101665471AReduce usageEasy to industrializeOrganic chemistryHexamethylenetetramineFormylation reaction
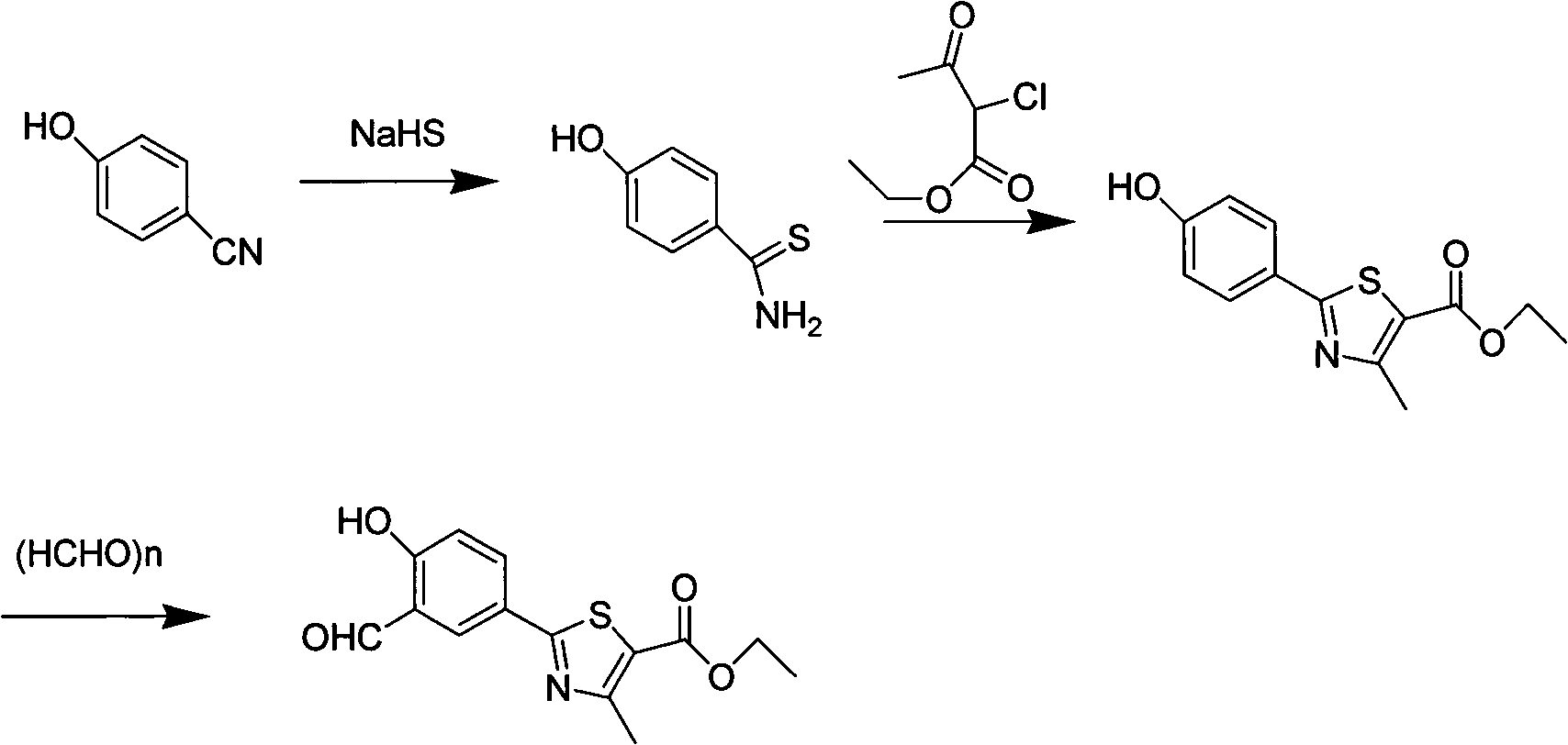
The invention relates to the field of pharmaceutical chemistry, in particular to a preparation method of 2-(3-formoxyl-4-hydroxy phenyl)-4-methyl-thiazole-5-carboxylate as an intermediate of pharmaceutical febuxostat for treating gout; the invention is characterized in that the intermediate is prepared through the reaction of 2-(4-hydroxy phenyl)-4-methyl-thiazole-5-carboxylate and paraformaldehyde under the catalysis of tin tetrachloride. The preparation method avoids the use of trifluoroacetic acid and methenamine as corrosive reagents, the yield of a formylation reaction of the febuxostat and low toxicity paraformaldehyde in acetonitrile can reach more than 95 percent, and the preparation method is easy for industrialization.
Owner:CHINA PHARM UNIV +1
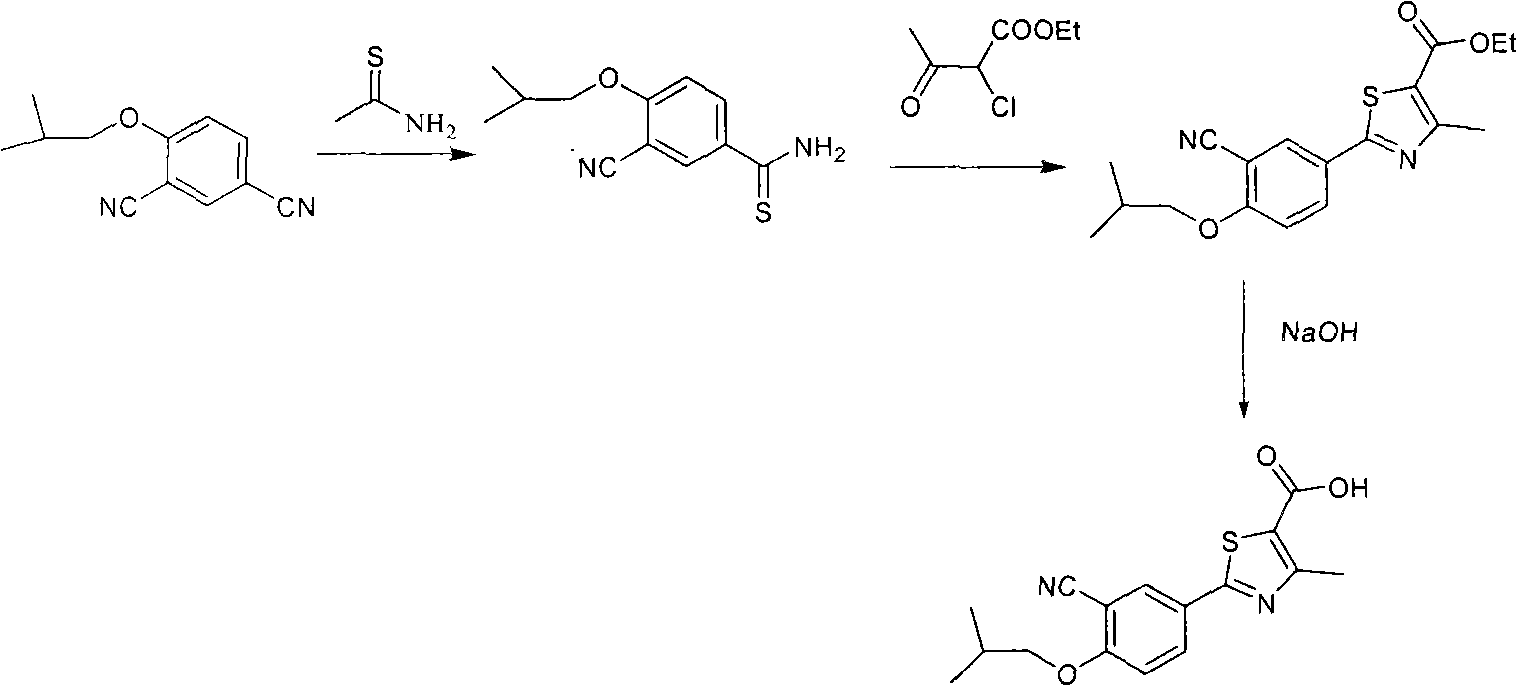
The synthetic method of febuxostat
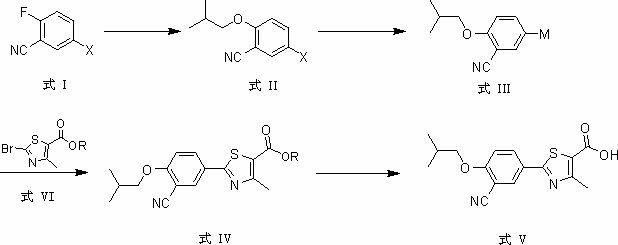
The invention provides a method for synthesizing febuxostat. The method comprises the following steps of: (a) performing aromatic ring substitution reaction on a compound of a formula I to obtain a compound of a formula II; (b) performing halogen-metal exchange reaction on the compound of the formula II to obtain a compound of a formula III; (c) performing coupling reaction on the compound of the formula III and the compound of a formula VI under the action of a metal catalyst to obtain a compound of a formula IV; and (d) performing hydrolysis reaction on the compound of the formula IV to obtain the compound of a formula V, namely febuxostat, wherein X is I or Br; M is selected from boric acid ester or SnBu3; and R is H or an alkyl group. In the method, a convergent synthesis strategy is adopted, and a carbon-carbon bond is formed by applying metal catalyzed aromatic ring coupling reaction in a key step, so that a system in which a benzene ring is coupled with a thiazole ring is established. The method has the advantages of simple and short steps, high yield and low environmental pollution and can be suitable for industrial production.
Owner:ARROMAX PHARMATECH
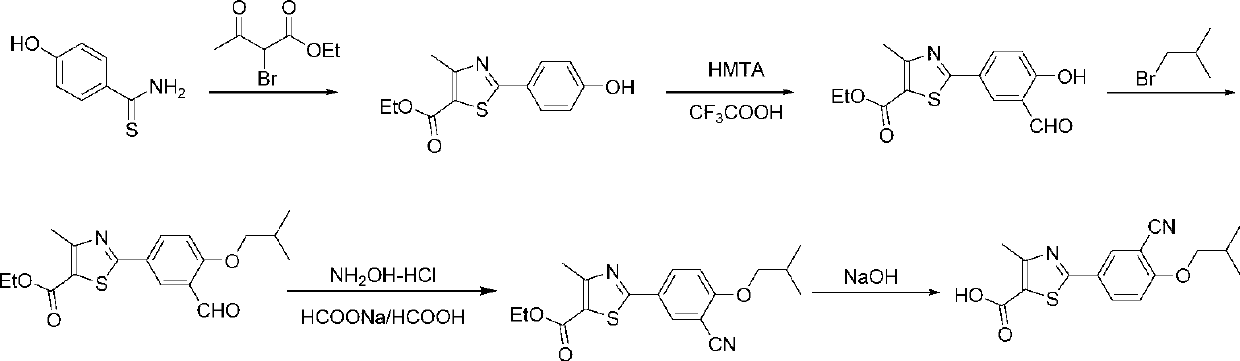
Preparation methods of compound 2-(3-formyl-4-isobutoxy phenyl)-4-methyl thiazole-5-ethyl formate and febuxostat
The invention provides a preparation method of 2-(3-formyl-4-isobutoxy phenyl)-4-methyl thiazole-5-ethyl formate which is obtained by using 4-isobutoxy cyanophenyl as an initial raw material and through a series of reactions. The invention also provides a preparation method of febuxostat, which comprises the following steps: reacting 2-(3-formyl-4-isobutoxy phenyl)-4-methyl thiazole-5-ethyl formate with hydroxylamine hydrochloride under the action of a catalyst to obtain a compound with a structure as shown in formula (VIII); hydrolyzing the compound with the structure as shown in formula (VIII) under an alkaline condition, and performing acidification to obtain febuxostat. The preparation method of the invention prepares febuxostat without using cyanides, and is high in safety. The preparation methods of the invention are simple in operation and high in yield. Experiment results show that the yield of step (A) is up to 90%, the yield of step (B) is up to 85%, the yield of step (C) is up to 90%, the yield of step (D) is up to 90%, and the yield of step (E) is up to 97%.
Owner:ZHEJIANG AUSUN PHARMA
Medicinal composition for treating hyperuricemia
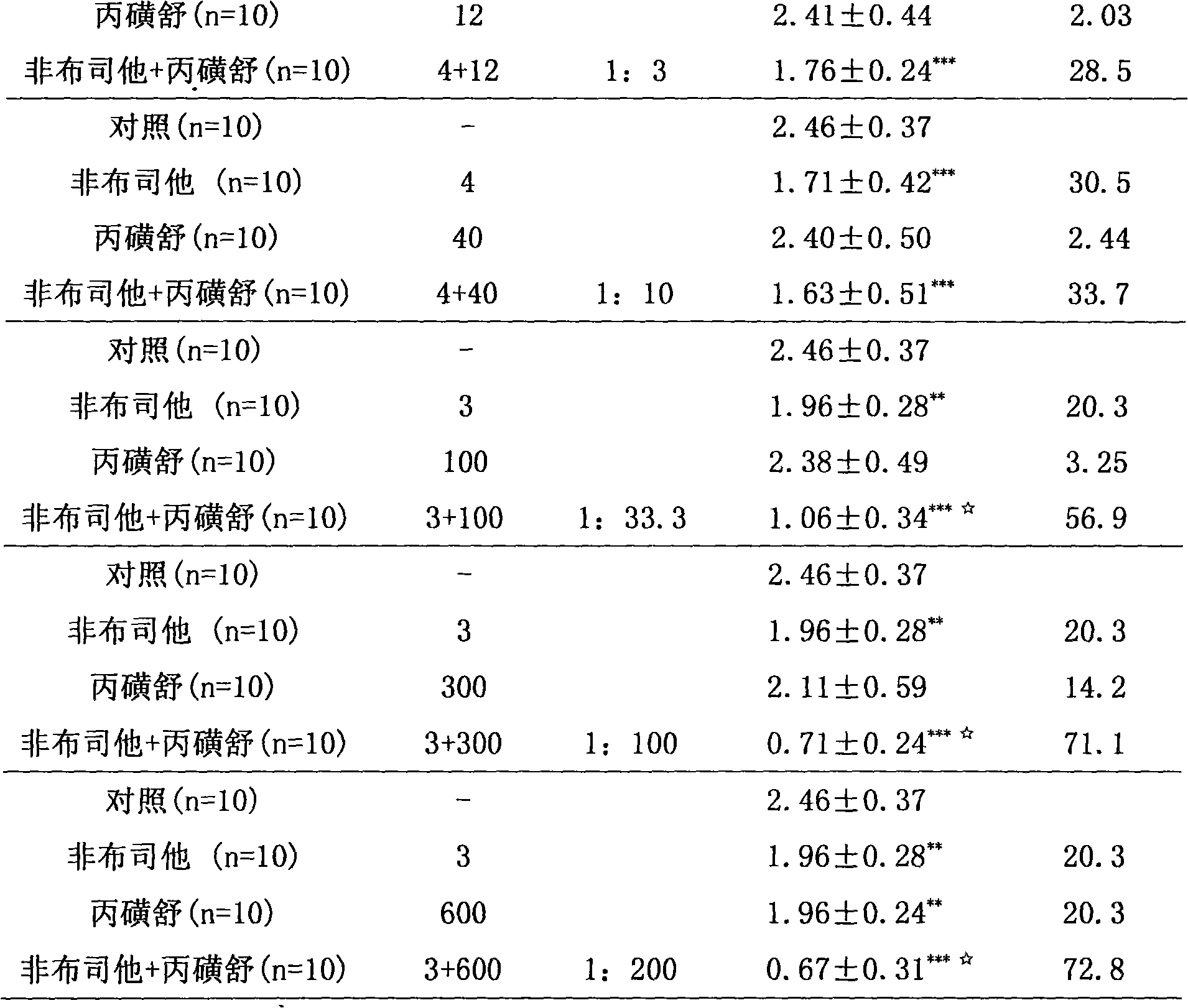
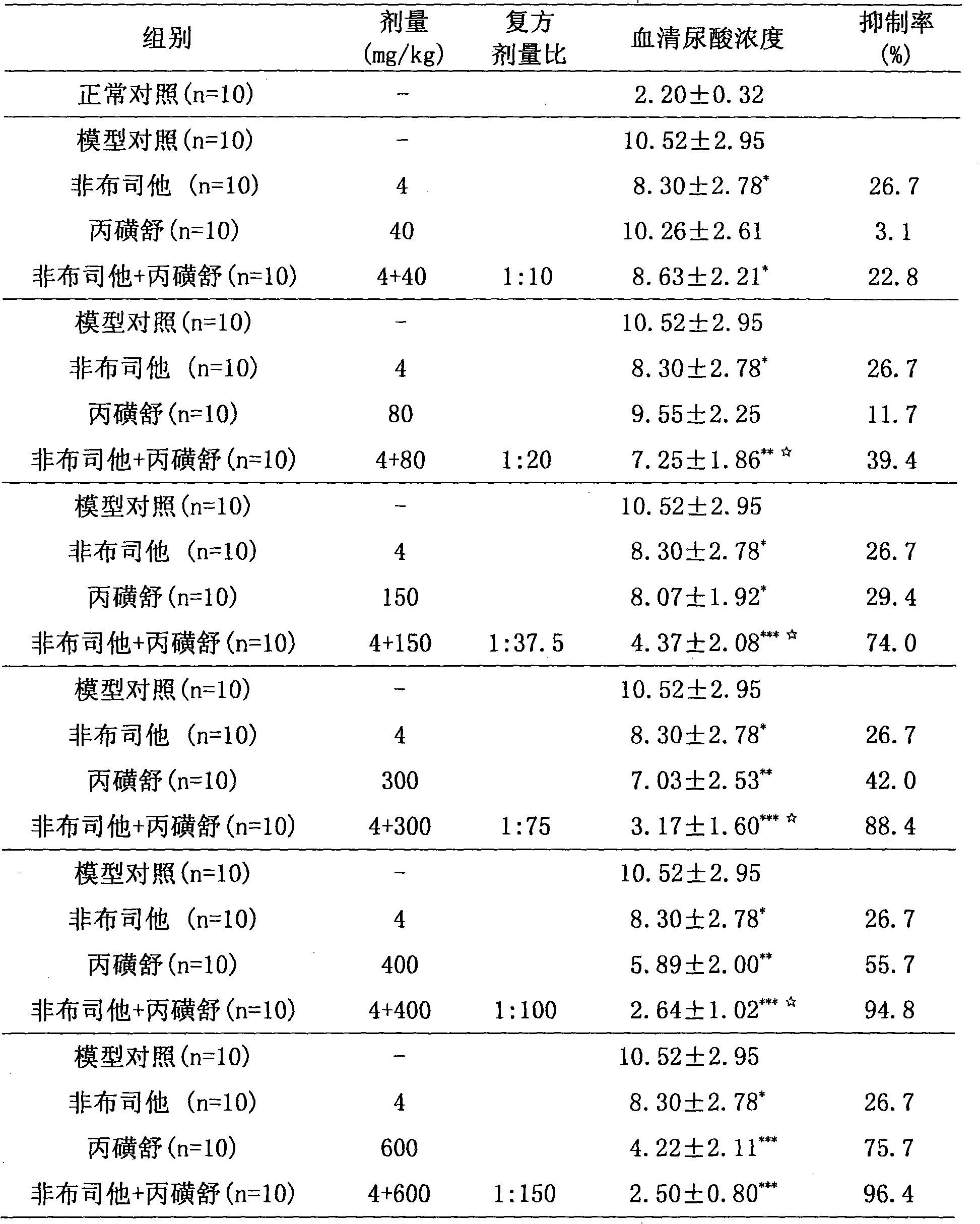
InactiveCN101658520AEffective treatmentLow toxicityOrganic active ingredientsPeptide/protein ingredientsSide effectPurine
The invention relates to a medicinal composition for treating hyperuricemia, which comprises Febuxostat or derivate thereof, probenecid and a pharmaceutically acceptable medicinal carrier, wherein theratio in weight portion of the Febuxostat or derivate thereof to the probenecid to the pharmaceutically acceptable medicinal carrier is 1:10-200:0.5-100. The medicinal composition can provide extremely beneficial effects of treating the hyperuricemia. Pharmacological tests prove that the medicinal composition has obviously synergistic action, can quickly reduce the concentration of purine trionein blood serum, remarkably improves effect of treating the hyperuricemia, greatly reduces medicament dosage of single component simultaneously, and effectively reduces toxic and side effect of medicament.
Owner:TIANJIN TAIPU PHARMA SCI & TECH DEV +1
Preparation method and detection method of febuxostat raw material
InactiveCN103030605AUniform qualityEffective quality controlOrganic chemistryComponent separationHydroxylamineThio-
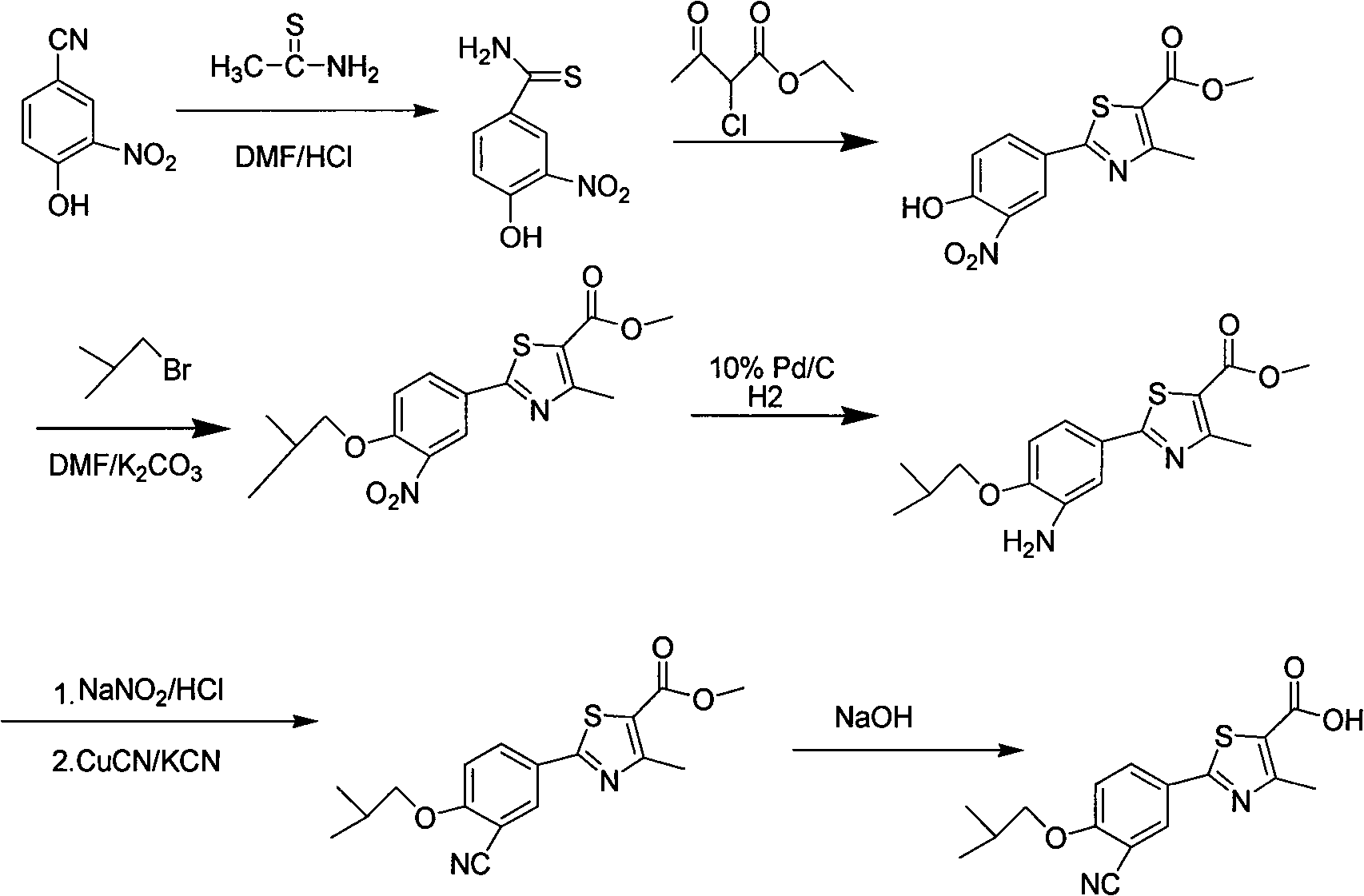
The invention discloses a preparation method and a detection method of febuxostat raw material. The preparation method comprises Cyanophenol, DMF (Dimethyl Formamide) saturated with HCl, and thioacetamide are mixed and react to obtain an intermediateIII; the intermediate III, anhydrous ethyl alcohol, 2-chlorination acetoacetic ester are mixed and react to obtain an intermediate IV; the intermediate IV, hexamethylene tetramine and polyphosphoric acid are mixed and react to obtain an intermediate V; the intermediate V, DMF, anhydrous potassium carbonate and bromo-2-methylpropane are mixed and react to obtain an intermediate VI; the intermediate VI, anhydrous formic acid, oxammonium hydrochloride and sodium formate are mixed and react to obtain an intermediate VII; the intermediate VII, anhydrous ethyl alcohol and sodium hydroxide are mixed and react to obtain a febuxostat crude product; and refining and crystal transformation are performed to the febuxostat crude product to obtain the febuxostat raw material. Aiming at avoiding defects in the prior art, the preparation technology of the febuxostat raw material is optimized, and a systematic, complete and effective component identifying and content measuring method is established.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
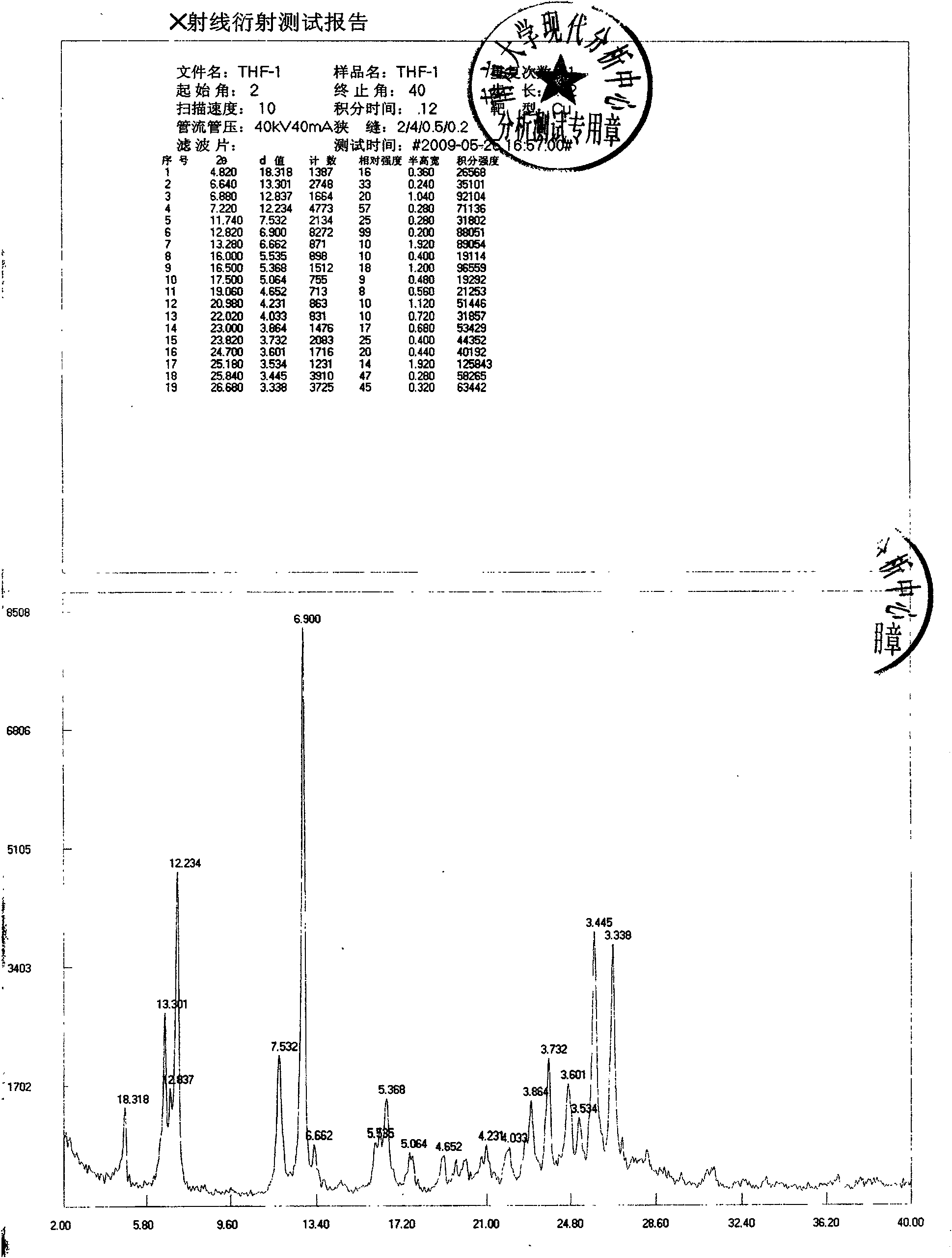
New crystal form of febuxostat and preparation method thereof
ActiveCN101671315ALow hygroscopicityImprove stabilityOrganic active ingredientsOrganic chemistryElectricityDissolution
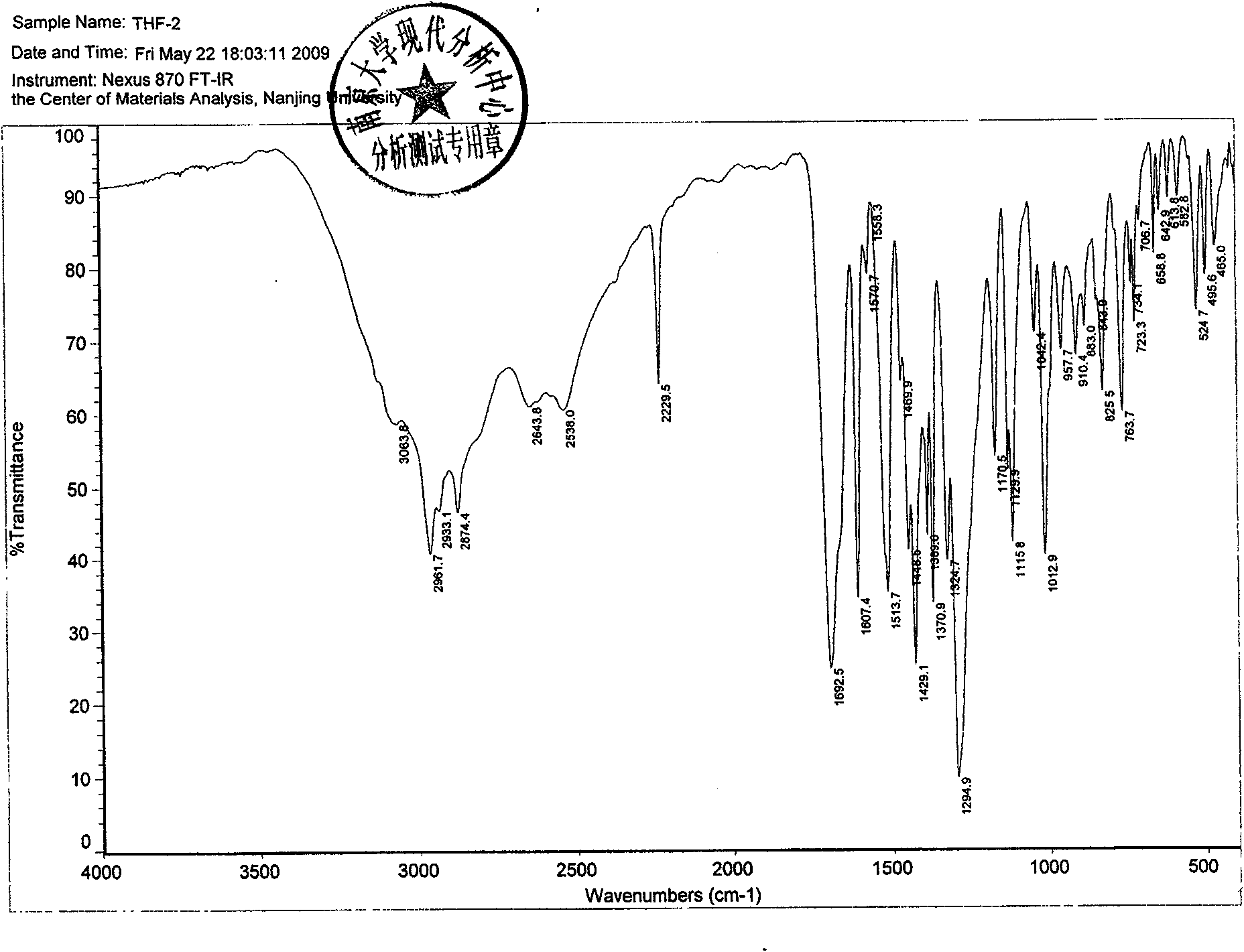
The invention discloses a new crystal form of febuxostat and a preparation method thereof. The preparation method of the crystal form adopts tetrahydrofuran as a solvent, the crystal form is small instatic electricity and has good dissolution rate, other processing manners such as micronization or preparation of solid dispersion and the like are not required during the process of preparing the pharmaceutical preparation, and the preparation with good dissolution rate can be obtained according to the conventional preparation process.
Owner:HEFEI IND PHARMA INST CO LTD
Solid dispersion system of febuxostat and preparation method of solid dispersion system, and pharmaceutical applications
The present invention relates to a solid dispersion system of febuxostat. The solid dispersion system comprises febuxostat and a nanometer skeletal material of colloid silica, and optionally includes a conventional solid dispersion carrier; wherein the conventional solid dispersion carrier is at least one selected from the group consisting of polyethylene glycol, polyvinylpyrrolidone, hydroxypropyl methyl cellulose, sodium alginate, poloxamer and polyacrylic resin. The solid dispersion system can preferably solve the technical problems of febuxostat with poor solubility in water and bioavailability.
Owner:PEKING UNIV
Preparation method of intermediates of Febuxostat
The invention relates to a preparation method of important intermediates, such as 2-(3-formaldehyde radical-4-hydroxyl phenyl)-4-methyl-5-thiazole ethyl formate, of Febuxostat. The method is as follows: reacting thioacetamide and 4-cyanol phenol which are used as raw materials in concentrated hydrochloric acid solution to obtain 4-hydroxythiobenzamide; reacting 4-hydroxythiobenzamide with 2-chloracetyl ethyl acetate to prepare 2-(4-hydroxyl phenyl)-4-methyl-5-thiazole ethyl formate; and reacting 2-(4-hydroxyl phenyl)-4-methyl-5-thiazole ethyl formate with hexamethylenetetramine in the presence of polyphosphoric acid and methanesulfonic acid to obtain 2-(3-formaldehyde radical-4-hydroxyl phenyl)-4-methyl-5-thiazole ethyl formate. In the invention, the operation is simple and convenient, the yield is high, the preparation process is stable, and the production cost is low, thus the preparation method is suitable for industrial production and application.
Owner:XUZHOU WANBANG JINQIAO PHARMA +1
Febuxostat tablet
ActiveCN102895209AImprove solubilityImprove bioavailabilityOrganic active ingredientsPharmaceutical delivery mechanismAdhesiveMedicine
The invention discloses a Febuxostat tablet. The Febuxostat tablet comprises a tablet core and a coating, wherein the tablet core comprises the following components in weight percentage: 5-30 percent of Febuxostat, 15-60 percent of filler, 1-20 percent of disintegrating agent, 0.1-5 percent of surface active agent, 0.1-8 percent of lubricant and a right amount of adhesive. According to the Febuxostat tablet, trough adopting the powerful disintegrating agent within a reasonable proportional region, and meanwhile, through jointly using the surface active agent, the poorly water-soluble drug Febuxostat dissolves out, and further, the dissolvability of the Febuxostat is increased, and the bioavailability of the Febuxostat is improved. Moreover, the Febuxostat tablet is simple in preparation method, controllable in quality and high in stability.
Owner:KANGYA OF NINGXIA PHARMA
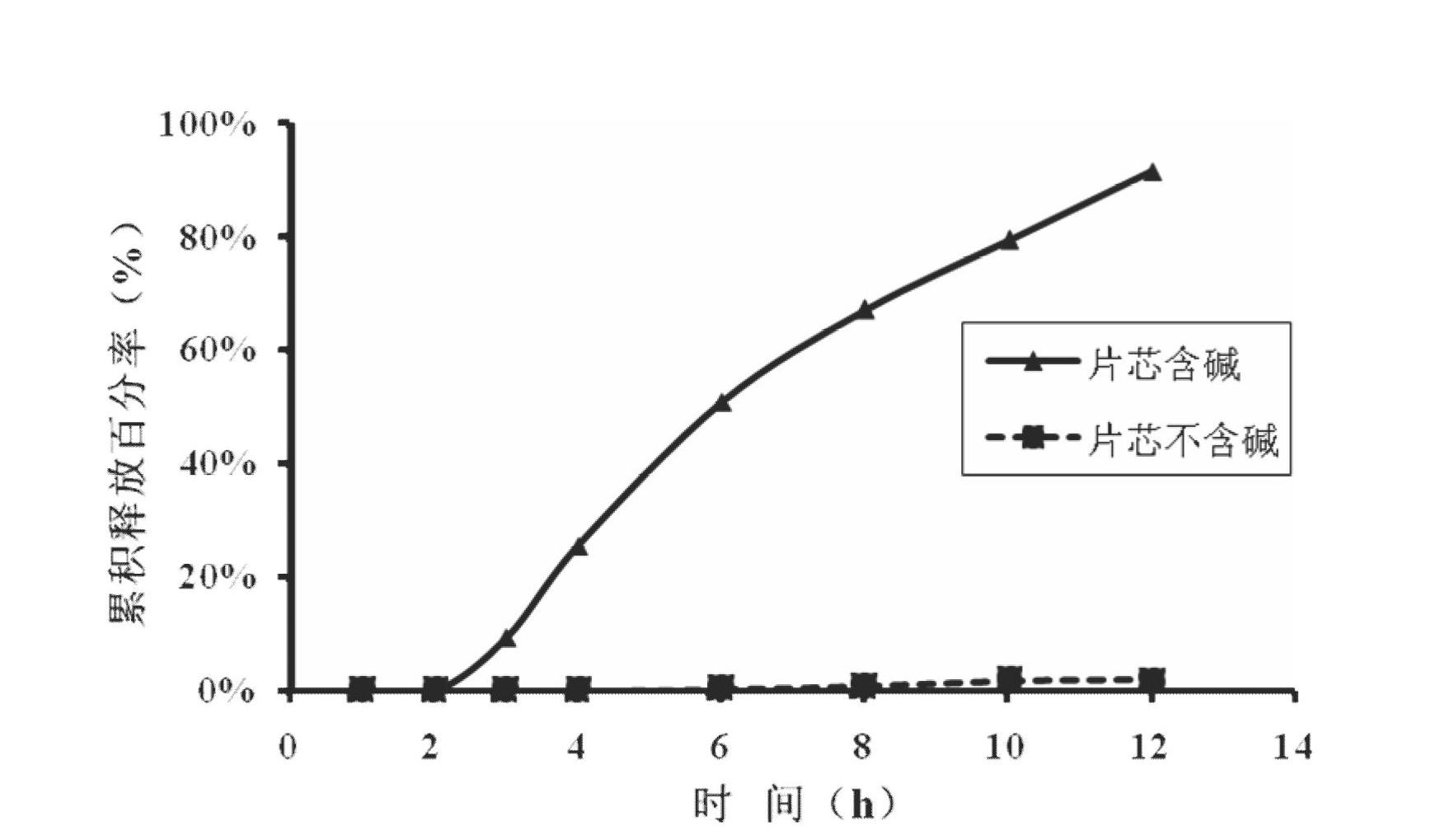
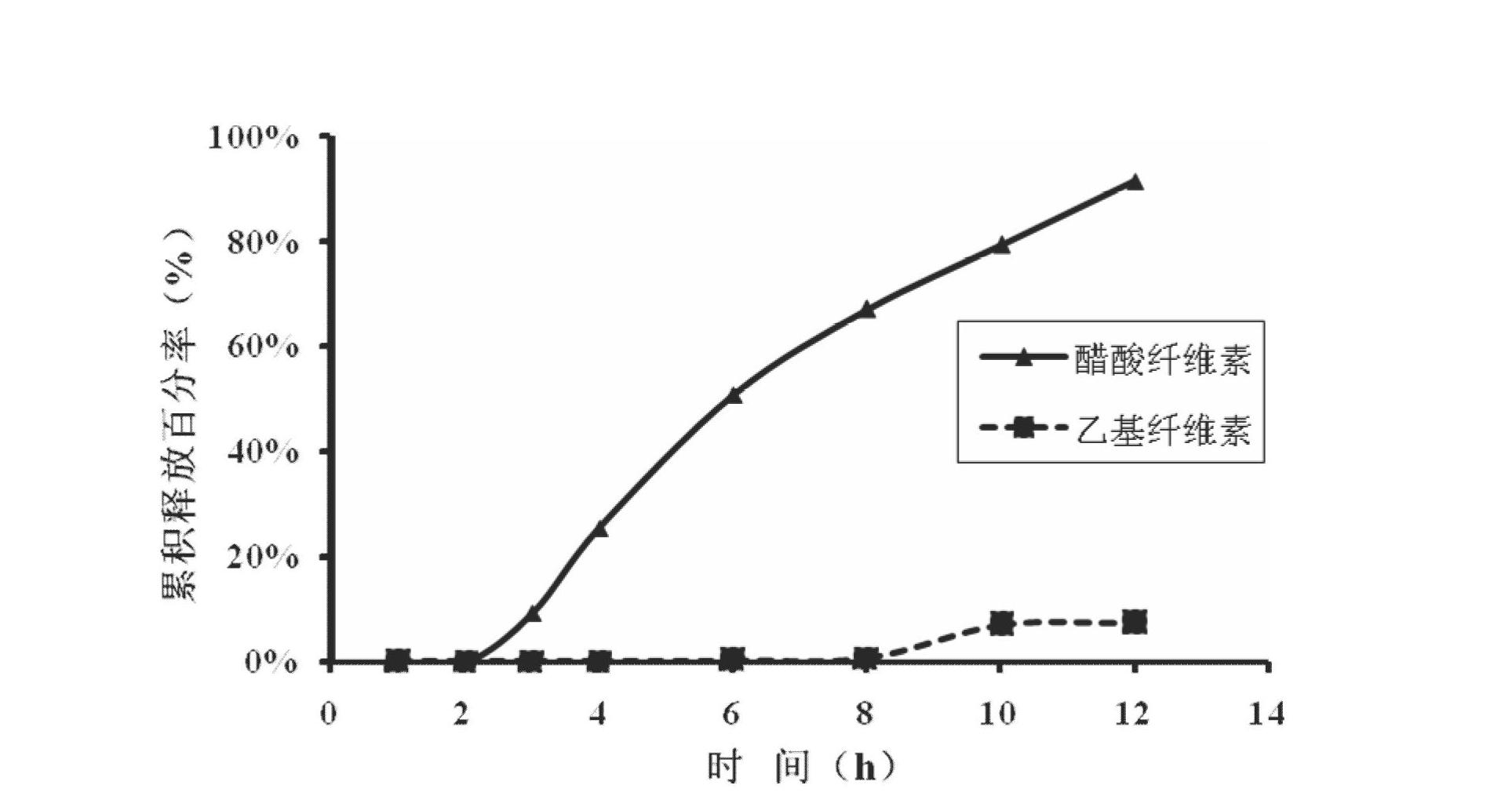
Febuxostat osmotic pump controlled release tablet for treating gout and preparation method
InactiveCN102641255AOrganic active ingredientsInorganic non-active ingredientsCellulose acetateAdhesive
The invention relates to a medical preparation containing a Febuxostat compound and with special physical shape as a characteristic, in particular to a Febuxostat osmotic pump controlled release tablet for treating gout. The osmotic pump controlled release agent comprises a core tablet and a coat and is characterized in that the core tablet is composed of the following components by weight: 10% to 40% of Febuxostat, 30% to 60% of osmotic pressure active substance, 4% to 20% of alkaline substance, 0% to 30% of filler, 1% to 5% of adhesive and 0.2% to 2% of lubricant. The coat is composed of the following components by weight: 40% to 70% of cellulose acetate, 20% to 40% of polyethylene glycol 400 and 10% to 20% of phthalic acid diethyl ester. The tablet resolves the problem that the Febuxostat is slightly soluble medicine and is hard to release by adding the alkaline substance into the core tablet, is capable of releasing over 90% of medicine within 12 hours by screening optimization of film formation materials, porogen and plasticizers in the coat, and satisfies zero level dynamic medicine releasing characteristics. The Febuxostat osmotic pump controlled release tablet for treating gout can be used for treating gout and has the advantages of being durable in effect, safe and easy to produce and prepare.
Owner:SOUTHERN MEDICAL UNIVERSITY
Synthetic method of febuxostat
InactiveCN102964313ARaw materials are cheap and easy to getHigh yieldOrganic chemistrySalicylaldehydePhenyl Ethers
The invention discloses a synthetic method of febuxostat. The synthetic method of febuxostat comprises the following steps: (1) preparing 4-hydroxy-1, 3-phthalic aldehyde; (2) preparing 4-hydroxy-1, 3-diphenyl nitrile; (3) preparing 4-isobutoxy-1, 3-diphenyl nitrile; (4) preparing 2-isobutoxy-5-sulfobenzyl acylamino cyanophenyl; (5) preparing 2-(3-cyan-4-isobutyl phenyl ether)-4-methylthiazole-5-ethyl formate; and (6) preparing febuxostat. According to the synthetic method, the low-price salicylaldehyde is adopted as the starting material and is subjected to aldehydizing, alkylating, thioacid amidating, cyclizing, and hydrolyzing so as to obtain the end product; the toxic agents such as sodium cyanide and the highly-corrosive agent like trifluoroacetic acid are not used; the raw materials in use are low in price and easy to obtain; the reaction condition is mild; the operation is simple and convenient; the total recovery is high; the obtained product is easy to purify; and the industrial production is easy to realize.
Owner:周广连
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com