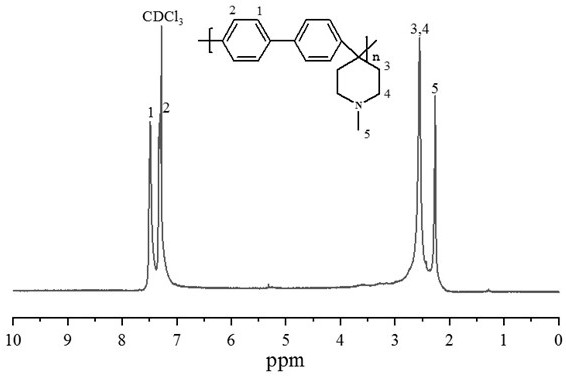
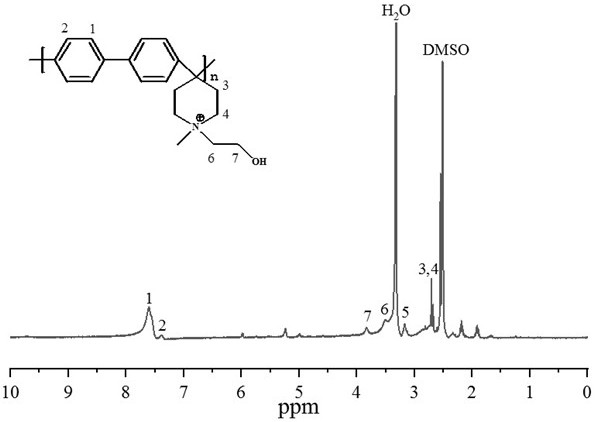
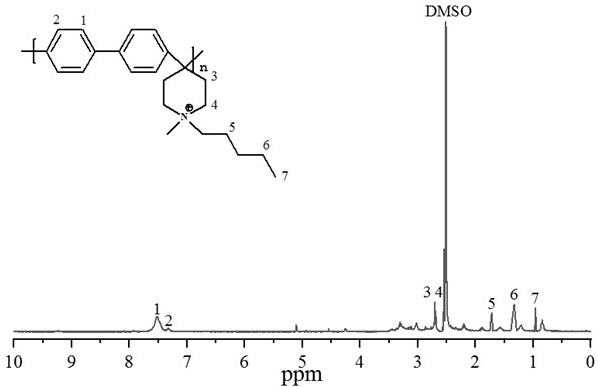
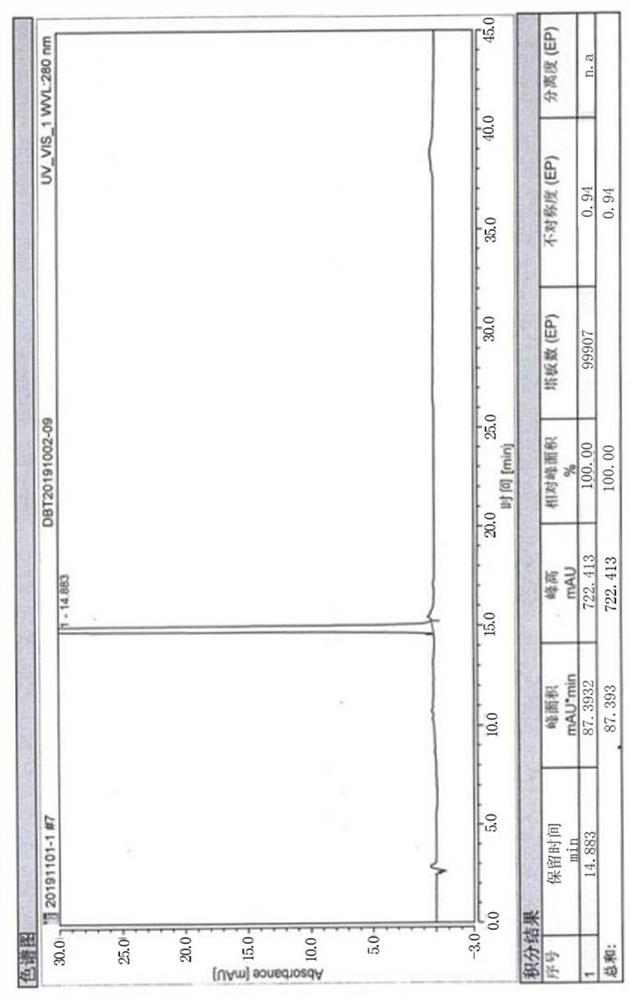
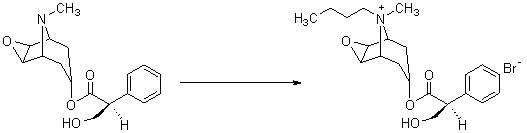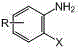Patents
Literature
164 results about "Butyl bromide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Butyl bromide may refer to: 1-Bromobutane 2-Bromobutane 1-Bromo-2-methylpropane 2-Bromo-2-methylpropane
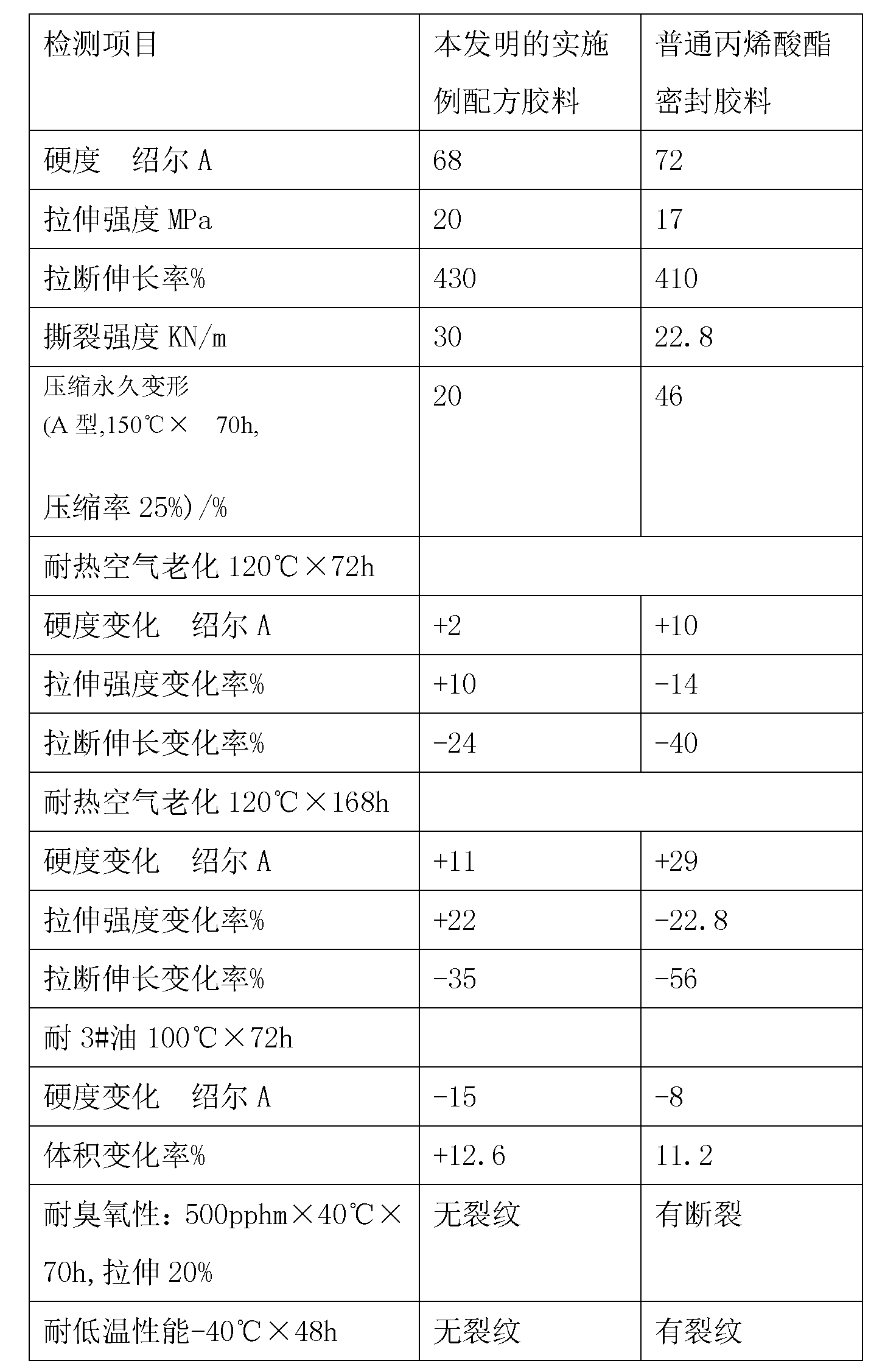
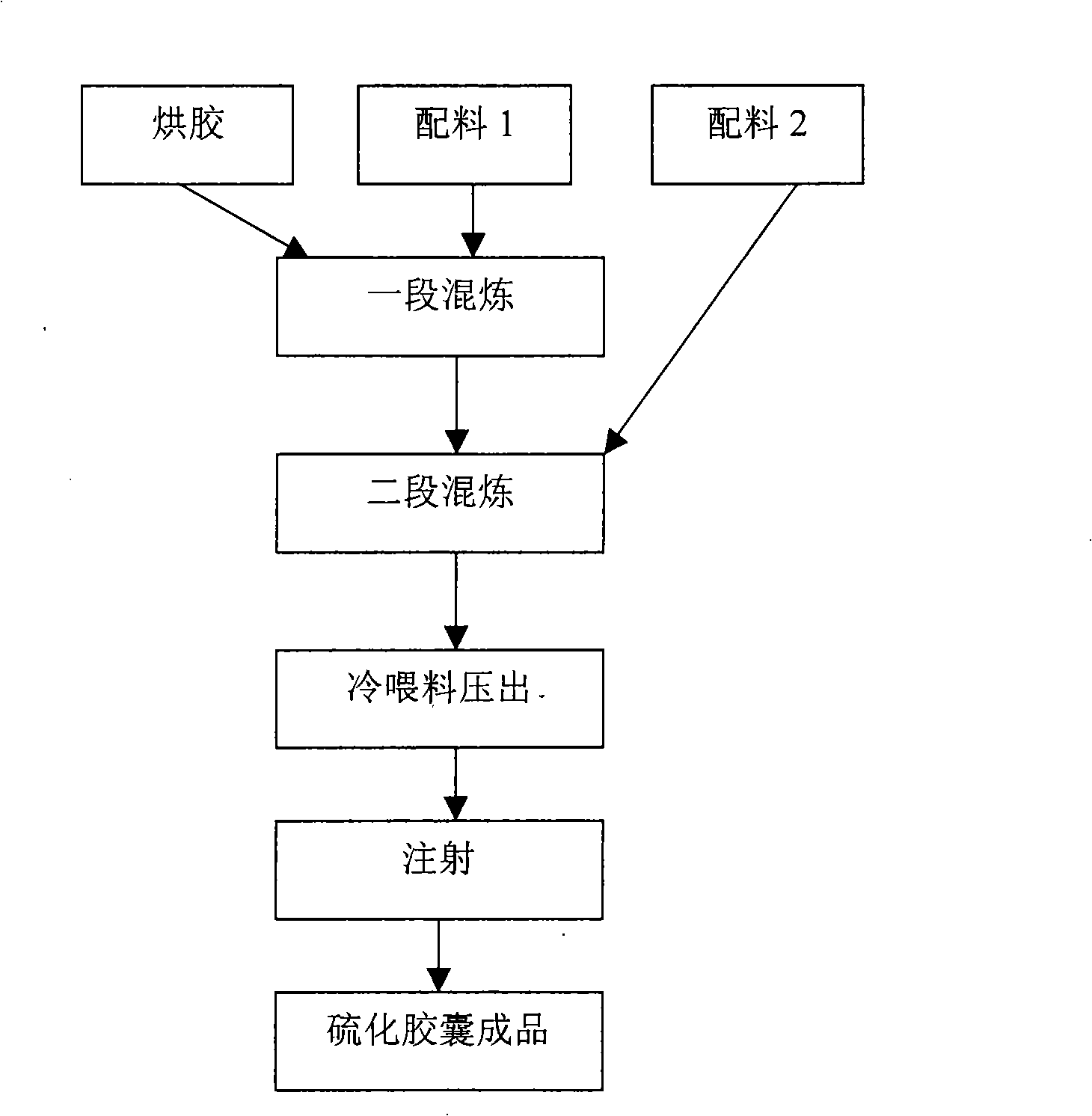
Seal rubber for water pump of automobile and preparation method of seal rubber
The invention discloses a seal rubber for a water pump of an automobile. The seal rubber comprises the following raw materials by weight: 50 to 60 parts of acrylic rubber, 10 to 15 parts of fluorinated silicone rubber, 10 to 15 parts of epichlorohydrin rubber T3100, 10 to 15 parts of butyl bromide rubber 221, 30 to 36 parts of high wear-resisting carbon black N330, 20 to 24 parts of precipitated white carbon black, 4 to 6 parts of light calcium carbonate, 5 to 10 parts of wollastonite in powder, 3 to 5 parts of zirconium tetrafluoride, 5 to 7 parts of barium sulfate, 3 to 5 parts of molybdenum disulfide, 9 to 11 parts of modified iron tailings powder, 1 to 2 parts of vulcanizing agent DCBP, 1 to 2 parts of antioxidant TPPD, 0.5 to 1 parts of accelerant H, 4 to 6 parts of magnesium oxide, 1 to 2 parts of 2-thiol benzimidazole, 1 to 2 parts of paraffin, 1 to 2 parts of isopropyl tri(dioctylpyrophosphate) titanate, and 17 to 19 parts of tri(2-ethylhexyl) acetocitrate. The rubber compound is excellent in ozone resistance, low-temperature resistance and heat resistance, wide in operating temperature wide and environment-friendly in process, and can be used for manufacturing various sealing gaskets.
Owner:马鞍山市中澜橡塑制品有限公司
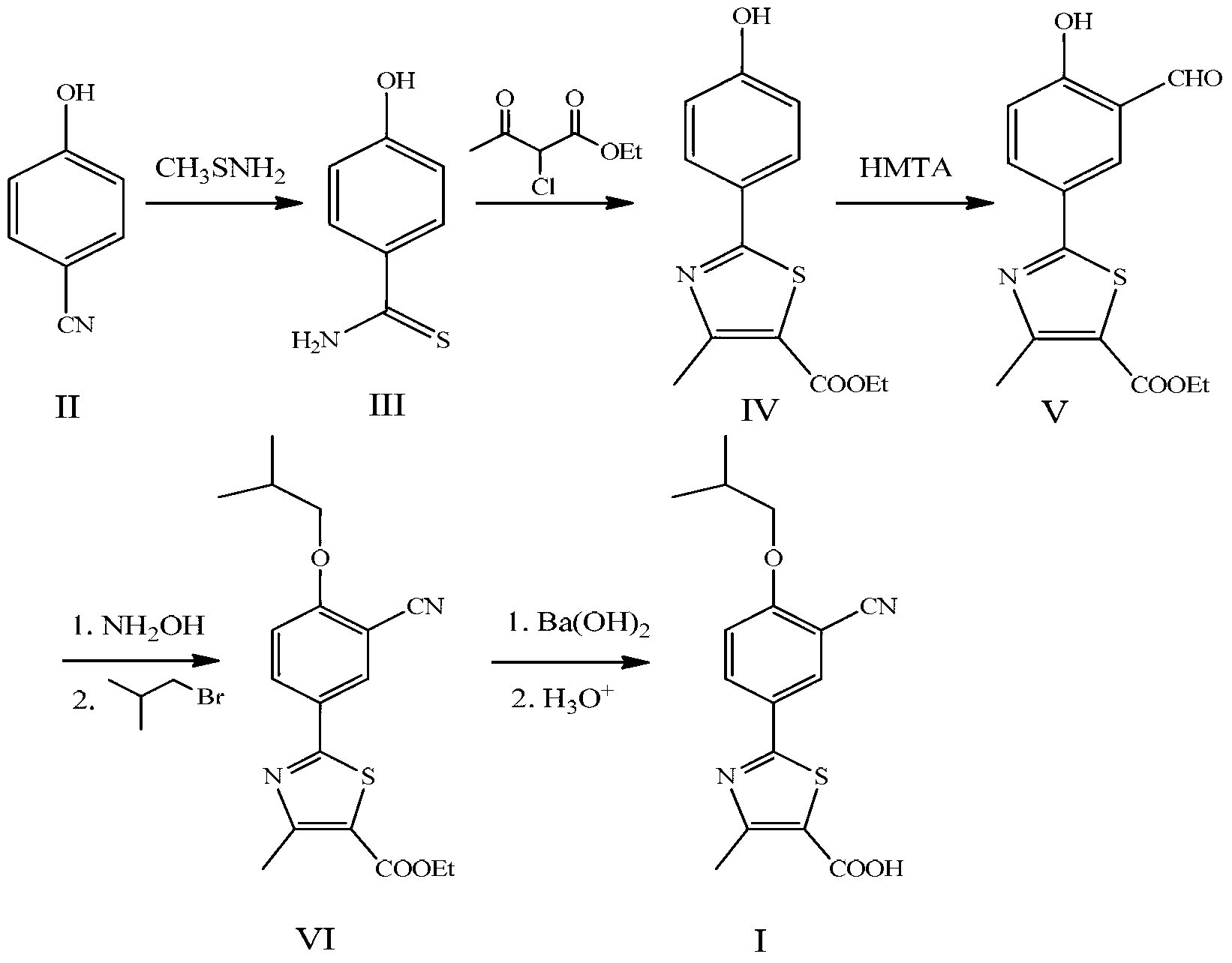
Preparation method for febuxostat
InactiveCN103304512ALow costReduce operation processOrganic chemistryHydroxylamine HydrochlorideToxic material
The invention discloses a preparation method for febuxostat. The preparation method for febuxostat comprises the following steps of: by using 4-hydroxybenzonitrile and thioacetamide as raw materials, and reacting in hydrochloric acid solution to prepare 4-hydroxythiobenzamide; carrying out a reaction on 4-hydroxythiobenzamide and 2-chloroacetoacetic acid ethyl ester to prepare 2-(4-hydroxylphenyl)-4-methylthiazol-5-carboxylic acid ethyl ester; carrying out a reaction on the obtained compound and hexamine in the mixed acid system of methanesulfonic acid and trifluoroacetic acid to prepare 2-(3-formyl-4-hydroxylphenyl)-4-methylthiazole-5-carboxylic acid ethyl ester; synthesising 2-(3-nitrile-4-isobutoxylphenyl) -4-methylthiazole-5-carboxylic acid ethyl ester from the compound, hydroxylamine hydrochloride, potassium carbonate, iso-butyl bromide and the like in a polar protonic solvent via a one-pot method; and finally hydrolyzing in an alkaline condition to obtain the target product, namely, febuxostat. The total yield of the preparation method for febuxostat disclosed by the invention is increased to 66%, the separation steps are reduced, any extremely toxic substance is not involved, and the environmental pollution is less.
Owner:SOUTH CHINA UNIV OF TECH
Brombutyl curing bladder and production method thereof
The invention discloses a butyl bromide sulfuration capsule, which is characterized in that butyl bromide rubber and butyl rubber are taken as a raw rubber body, the capsule also comprises the compositions in weight percentage: 3 to 5 percent of zinc oxide, 0.2 to 2 percent of stearine, 5 to 9 percent of 7530E resin, 0.1 to 0.5 percent of magnesia and 2 to 100 percent of protective wax, wherein, in the raw rubber body, the butyl rubber is between 50 and 90 percent and the butyl bromide rubber is between 10 and 50 percent. In the formulation of the capsule, the butyl bromide rubber and the butyl rubber are taken as the raw rubber body; meanwhile, the butyl bromide rubber is between 10 and 50 percent in the raw rubber. Compared with the butyl rubber, the butyl bromide rubber has the characteristics of good heat-resistant quality, good autohension and high sulfuration speed. The contrast test shows that if the capsule product with the same thickness is made by using the mixture ratio, the sulfuration speed is shortened by half and the physical performance of the product is the same in the condition of the same sulfuration temperature.
Owner:SHANGHAI TIRE & RUBBER TIRE RES INST
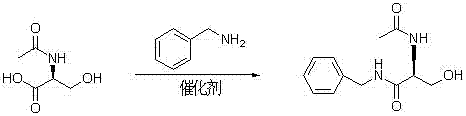
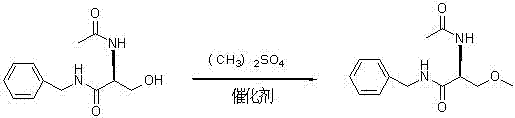
Synthetizing method of lacosamide
InactiveCN103113256AHighlight substantive featuresSignificant progressOrganic compound preparationCarboxylic acid amides preparationPtru catalystAmmonium chloride mixture
The invention provides a synthetizing method of lacosamide. The method comprises the steps of: based on D-serine as a raw material, performing an acylation reaction with acetic anhydride and then performing a condensation reaction with benzylamine; and finally, performing a methylation reaction with dimethyl sulfate, thereby obtaining lacosamide, wherein N,N' dicyclohexylcarbodiimide (DCC) or N,N' carbonyl diimidazole (CDI) is used as a catalyst in the condensation reaction; and a phase transfer catalyst including triethyl benzyl ammonium chloride (TEBA), tetrabutylammonium chloride (TBAC), tetrabutylammonium bromide (TBAB) or tetrabutylammonium hydrogen sulfate (TBAHS) is adopted in the methylation reaction. The method has the advantages of being simple in synthetizing process, moderate in reaction condition, simple in after-treatment, high in yield and high in product purity.
Owner:SUZHOU HONGRUI MEDICAL TECH
Electrochemical synthesis method for tetra-n-butylammonium tribromide
InactiveCN101054680ANothing producedConvenient sourceElectrolysis componentsElectrolytic organic productionElectrolysisSynthesis methods
The present invention relates to an electrochemistry synthetic method of tetributyl tribromide of ammonium and belongs to the field of chemical formulation preparing technology. Sadi synthetic method in accordance with the present invention is as follow: dissolving tetrabutyl ammonium bromide in a aqueous solution of bromium salts such as ammonium bromide, potassium bromide or sodium bromide, magnetic-stirring it until reaching a entire solubilization, and then restirring it after adding organic solvent such as methylene dichloride or acetonitrile therein; putting the solution into an electrolytic tank of non-diaphragm type, selecting platinum meshwork as cathode and anode, inputting a constant electrical current for electroanalysis; electrolyzing for 3 hours under normal temperature and magnetic stirring to obtain tetributyl tribromide of ammonium in oil phase finally; washing the oil phase with distilled water, evaporating the organic solvent off and exposing it to air for a period of time, after the product solidifies, washing it with water, performing a recrystallization with methanol to obtain high-purity tetributyl tribromide of ammonium solid.
Owner:SHANGHAI UNIV
Preparation method of corrosion inhibitor for novel ionic liquid oil field water
ActiveCN104498960ASolve the problem of faster corrosionReduce corrosionHexamethylenetetramineThiourea
The invention relates to a synthetic oilfield flooding compounded corrosion inhibitor which takes imidazole type ionic liquid synthesized by a two-step method as a controlled-release host and the other added compounds as auxiliary controlled-release agents. A preparation method of the corrosion inhibitor for the novel ionic liquid oil field water comprises the following steps: (1) at a certain temperature, mixing N-methylimidazole with n-butyl bromide or benzyl chloride, reacting while stirring, washing and removing unreacted substances after reaction is carried out for hours, thus obtaining halide of N-methylimidazole; (2) mixing the product obtained in the step (1) with a selected compound, adding a solvent, stirring for reacting for a period of time at a certain temperature, extracting a reaction solution, washing, and removing the solvent, thus obtaining the target products; and (3) compounding the products obtained in the step (2) with urotropine, OP-10, potassium iodide and thiourea in a certain proportion, thus obtaining the corrosion inhibitor with extremely high corrosion inhibition efficiency. The preparation method of the corrosion inhibitor for the novel ionic liquid oil field water has the advantages that raw materials are simple and available, the operational process is easy and safe, the reaction time is short, the cost is low, the yield is high, the corrosion inhibition performance is strong, the corrosion inhibitor for the novel ionic liquid oil field water is applicable to industrial large-scale production, and the practicability is strong.
Owner:TANGSHAN NORMAL UNIV
Method for synthesizing quaternary ammonium salt ionic liquid by microwave radiation heating
InactiveCN102344377AGood acid and alkali resistanceReduce volumeOrganic compound preparationAmino compound preparationHalohydrocarbonButyl chloride
The invention provides a method for synthesizing quaternary ammonium salt ionic liquid by microwave radiation heating, which comprises the following steps: mixing tertiary amine compounds (N,N-dimethylethanolamine, trimethylamine or triethanolamine) and halohydrocarbon compounds (allyl chloride, n-butyl chloride, n-butyl bromide, octadecyl chloride) with a molar ratio of 1:1.05-1:2 under a microwave radiation heating condition, putting the mixture into a microwave reactor to perform a microwave reaction with an adjusted microwave power of 100 W-400 W, a heating temperature of 30 DEG C-80 DEG C, and reaction time of 1 min-15 min so as to obtain a crude product, removing residual halohydrocarbon compounds in the crude product to obtain quaternary ammonium salt ionic liquid with halogens as anions; the halohydrocarbon compounds are chlorinated hydrocarbon compounds or brominated hydrocarbon compounds. The preparation method of the invention is simple, does not require the addition of any solvent except reaction raw materials, and can prepare high-purity quaternary ammonium salt ionic liquid simply, efficiently, rapidly, and economically.
Owner:SOUTH CHINA UNIV OF TECH
Method for preparing cyclic carbonate
InactiveCN103641811AClear structureThe synthesis method is simpleOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsQuaternary ammonium cationRare earth metal compounds
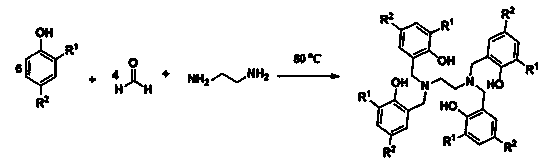
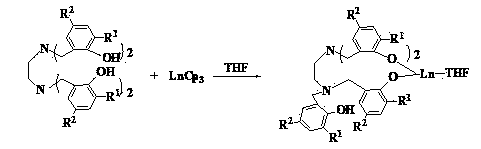
The invention discloses a method for preparing cyclic carbonate. The method specifically comprises the following step: with a quadri-aryloxy bridged rare earth metal compound as a catalyst, catalyzing carbon dioxide and alkylene oxide to react in the present of quaternary ammonium salt, wherein the general formula of the quadriaryloxy bridged rare earth metal compound is LLn(THF), wherein L refers to ethanediamine group bridged quadri-aryloxy, Ln refers to rare earth metal ions, and the quaternary ammonium salt is one of tetrabutylammonium iodide, tetrabutylammonium bromide, tetrabutylammonium chloride, tetraoctyl ammonium bromide, bis(triphenylphosphine) ammonium chloride and benzyl butyl ammonium bromide. The rare earth catalyst in the catalysis system is clear in structure, easy to synthesize, high in catalysis activity, less in using amount, mild in reaction conditions and wide in universality to alkylene oxide. According to the preparation method disclosed by the invention, raw materials are easily available, the reaction conditions are wild, a reaction substrate is wide in universality, the reaction time is short, the yield of the target product, namely the cyclic carbonate is high, and the reaction operation and the posttreatment process are simple.
Owner:SUZHOU UNIV
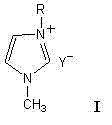
AM/AA/N-beta-CD polymer-ionic liquid [bquin]BF4 composite clay stabilizer and synthesis method thereof
ActiveCN103897121AHas inclusion functionHigh viscosityDrilling compositionSolubilitySodium Bentonite
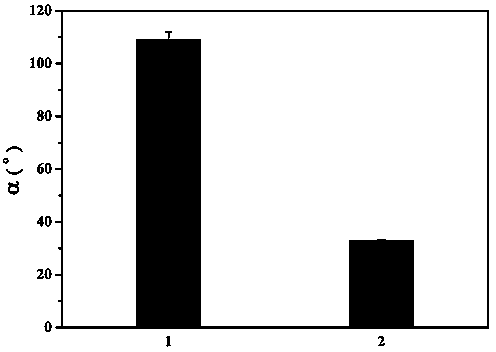
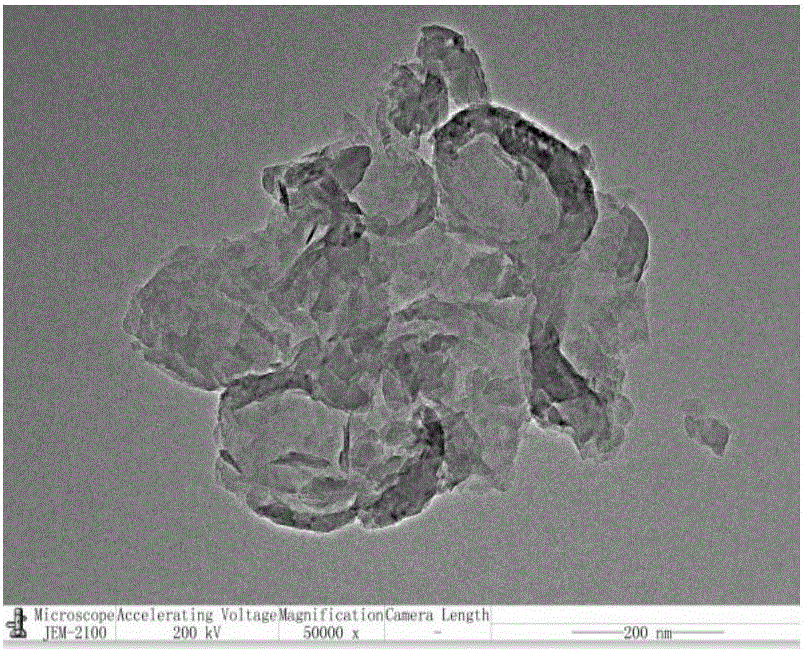
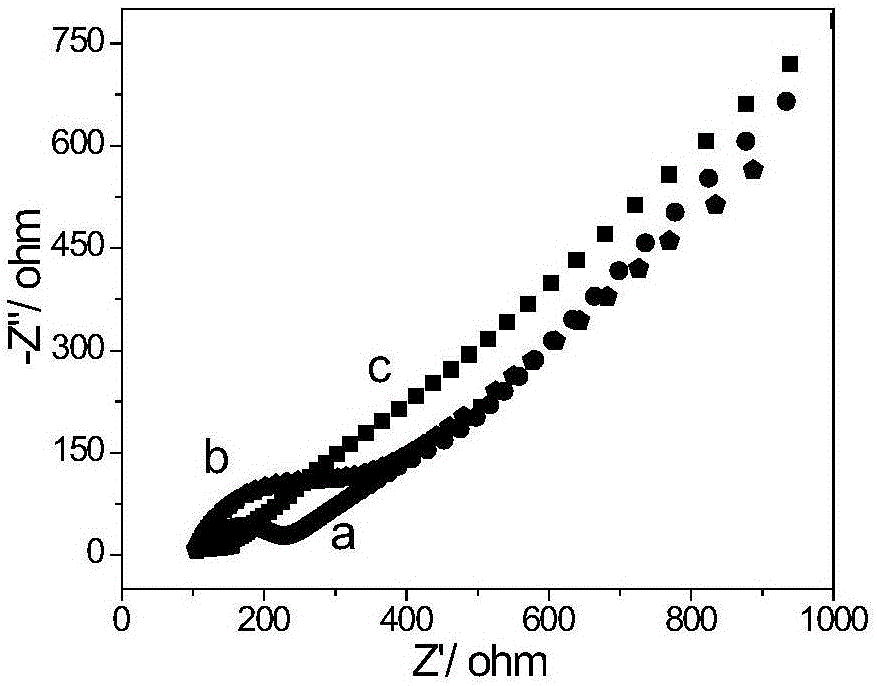
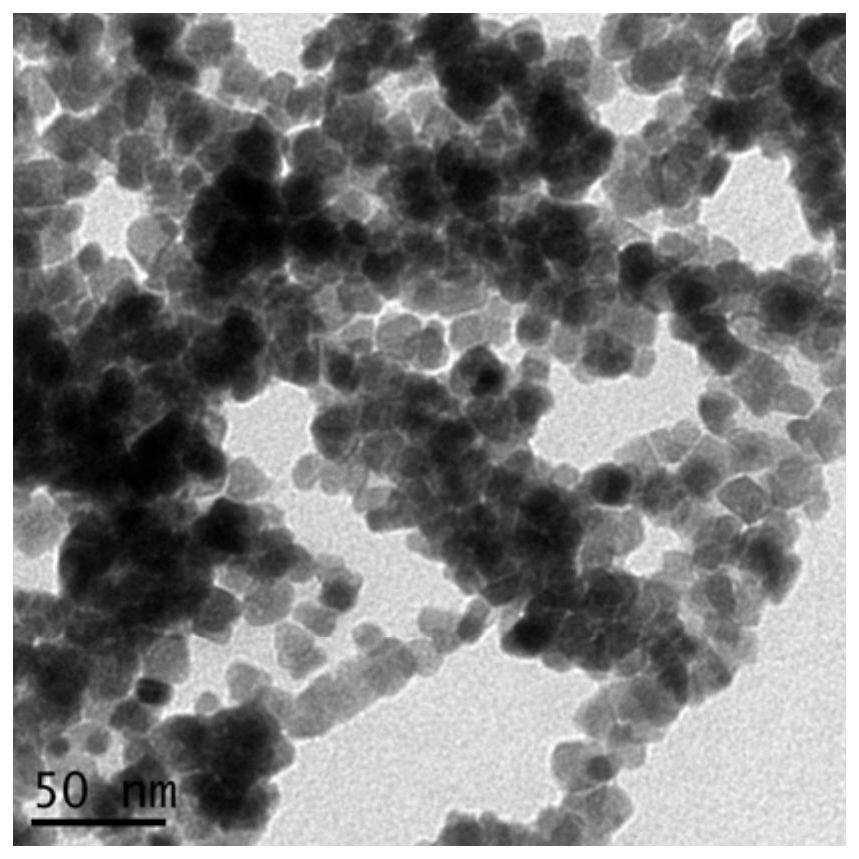
The invention relates to a polymer-ionic liquid composite clay stabilizer used for restraining clay hydration expansion in petroleum industry and a synthesis method of the polymer-ionic liquid composite clay stabilizer. The synthesis method comprises the steps of: reacting single 6-allyl amino beta-cyclodextrin, acrylamide and acrylic acid under initiation of ammonium persulfate and sodium hydrogen sulfite in an aqueous solution under the condition of pH of 7 and the temperature of 35 DEG C, purifying by aqueous solution after 8 hours of reaction and drying to obtain a polymer; refluxing quinoline and n-butyl bromide for 48 hours at 60 DEG C to obtain brominated 1-normal-butyl-quinoline salt, stirring for 3 days with sodium fluoborate in acetonitrile, filtering, carrying out rotary evaporation and drying to obtain tetrafluoroborate 1-normal-butyl-quinoline salt ([bquin]BF4); refluxing the polymer and the [bquin]BF4 for 4 hours at 50 DEG C to obtain a polymer-ionic liquid compound. The polymer-ionic liquid compound has good water solubility, stability and clay expansion restraining power and the antiswelling rate to bentonite can reach 90.3%.
Owner:SOUTHWEST PETROLEUM UNIV
Method for modifying ZSM-5 molecular sieve through organophosphorous compound
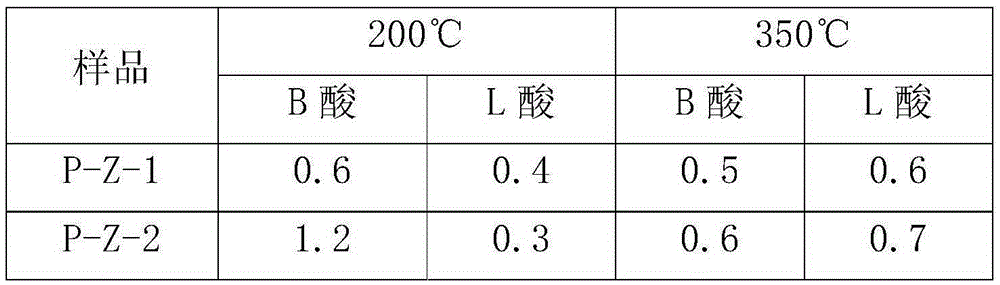
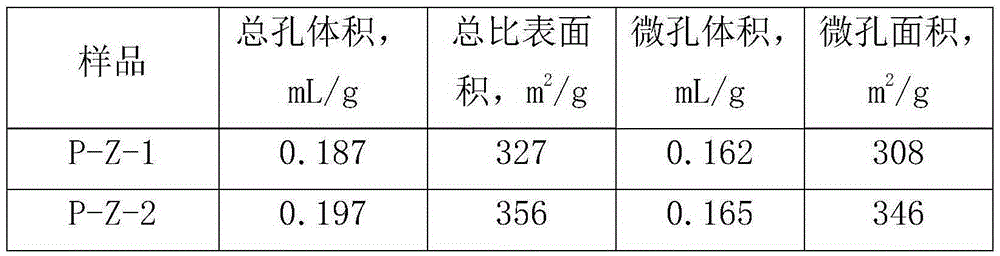
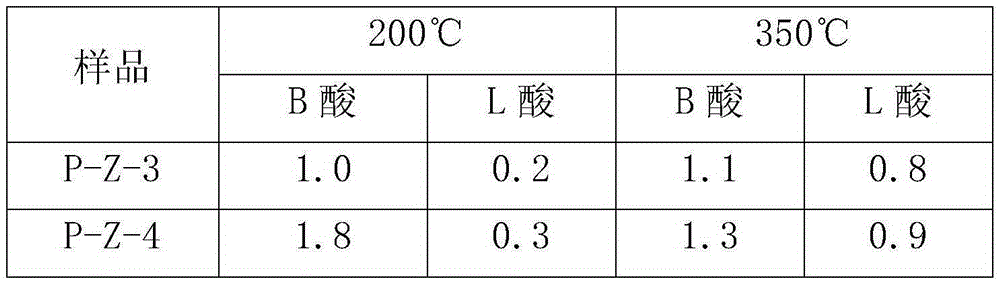
ActiveCN106607081AHigh pore volumeIncrease surface areaCatalytic crackingMolecular sieve catalystsMolecular sieveOrganophosphorous compounds
The invention provides a method for modifying a ZSM-5 molecular sieve through an organophosphorous compound. The method comprises mixing an HZSM-5 molecular sieve and one or more organophosphorous compounds such as tetrabutylphosphonium hydroxide, tetrabutylphosphonium bromide, di-n-butyl phosphate, 1-butylphosphoric acid, tri-n-butylphosphonium oxide, tributylphosphine, fosetyl-aluminum, tetraphenylphosphonium bromide, triphenylethylphosphonium bromide, triphenylbutylphosphonium bromide and triphenylbenzylphosphonium bromide, and drying and calcining the mixture. The organophosphorous compound-modified ZSM-5 molecular sieve has a high pore volume and a high specific surface area and contains more B acid centers.
Owner:CHINA PETROLEUM & CHEM CORP +1
Bituminous paint
InactiveCN103122215AImprove heat resistanceStrong adhesionAnti-corrosive paintsBituminous coatingsNitrobenzeneM-nitrophenol
The invention discloses a bituminous paint which comprises the following raw materials in parts by mass: 1-3 parts of potassium chloride, 2-4 parts of metanitrophenol, 1-2 parts of anthraquinone, 2-5 parts of sodium dodecyl benzene sulfonate, 2-6 parts of tetraethylammonium chloride, 2-5 parts of hexadecyltributylphosphorus tribromide, 1-3 parts of 4-bromo-1-butanol, 3-5 parts of diethylaminoethanol hexanoate and 2-5 parts of praseodymium trifluoroacetylacetone. The bituminous paint disclosed by the invention has the advantages of favorable heat resistance, favorable adhesive force, favorable weather resistance, short drying time and obvious anticorrosion effect, and is convenient, safe and environment-friendly to use in construction.
Owner:桑达化工(南通)有限公司
Visible light triggered material surface graft polymerization based functional modifying method
InactiveCN104194026ARich varietyMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationButyl bromidePolymer science
The invention discloses a visible light triggered material surface graft polymerization based functional modifying method. The functional modifying method comprises the steps of firstly, grafting iso-butyl-bromide on the surface of a material; and carrying out free radical graft copolymerization on the material and other functional vinyl monomers under the irradiation of visible light and in the presence of decacarbonyldimanganese to realize material surface graft modification. The visible light triggered material surface graft polymerization based functional modifying method has the remarkable characteristics: 1, materials which can be modified by using the modifying method are wide in range and can be organic polymer materials, inorganic nonmetallic materials and metal materials; 2, the modifying method is carried out at room temperature, and therefore, the reaction conditions are mild; 3, the modifying method is used for initiating polymerization graft modification under the condition of visible light and is particularly suitable for materials and functional monomers which cannot stably exist under a thermal polymerization condition; and 4, the modifying method is simple and convenient to operate, high in grafting efficiency and speed and strong in universality.
Owner:SUZHOU FENGYA BIOTECH
Synthesizing method of 2- butyl-1,2-benzisothiazoline-3-ketone
InactiveCN102807532AReduce generation costImprove product qualityOrganic chemistryButyl bromideKetone
The invention discloses a synthesizing method of 2- butyl-1,2-benzisothiazoline-3-ketone, which is characterized by comprising the following steps: (1) reacting 1,2-benzisothiazoline-3-ketone (BIT) with alkali so as to obtain 1,2-benzisothiazoline-3-ketone alkali metal salt; and (2) adding the alkali metal salt into an aprotic polar solvent to react with halogenated normal butane, wherein the halogenated normal butane is one or a combination of two or more of 1-n-propylcarbinyl chloride, 1-n-butyl bromide and 1-n-iodobutane, and preferably is the 1-n-butyl bromide. According to the method, the process cost is low, the production quality is good, and three wastes are few.
Owner:LIANYUNGANG SUNLION CHEM
Ionic liquid functional carbon nitride nanosheet modified electrode as well as preparation and application of electrode in chlorphenol detection
ActiveCN106442666ALarge specific surface areaIncrease the active siteMaterial electrochemical variablesProtonationDispersity
The invention discloses an ionic liquid functional C3N4 modified electrode as well as a preparation method and application of the electrode in chlorphenol detection. C3N4 is protonated in acid to prepare a C3N4 nanosheet, and then quaternization is realized with n-butyl bromide in an alkaline condition to prepare an ionic liquid functional C3N4 nanosheet; the catalytic active sites of the obtained ionic liquid functional C3N4 nanosheet are increased while the dispersity, electrical conductivity and electrochemical catalytic performance are obviously improved; and the ionic liquid functional C3N4 nanosheet is dropwise applied to the electrode surface to obtain an ionic liquid functional C3N4 nanosheet modified electrode and is applied to efficient and sensitive detection of 2,4-dichlorophenol in a water medium. According to the invention, the obtained modified electrode imposes low detection limit on 2,4-dichlorophenol and has relatively high selectivity, stability and anti-interference performance and relatively wide linear range; and a quick and sensitive electrochemical method is provided for quantitative detection of 2,4-dichlorophenol.
Owner:江苏乐士源新能源科技有限公司
Process for synthesizing sex pheromone of pine caterpillar
ActiveCN102613177AEasy to operateSynthetic raw materials are readily availableBiocidePreparation by isomerisationGrignard reagentIodide
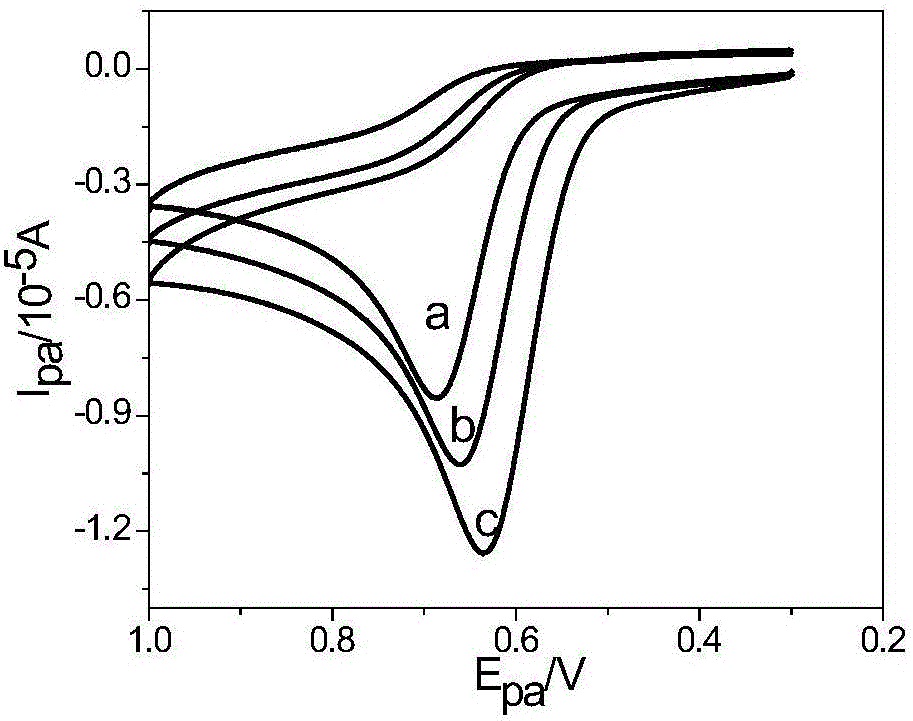
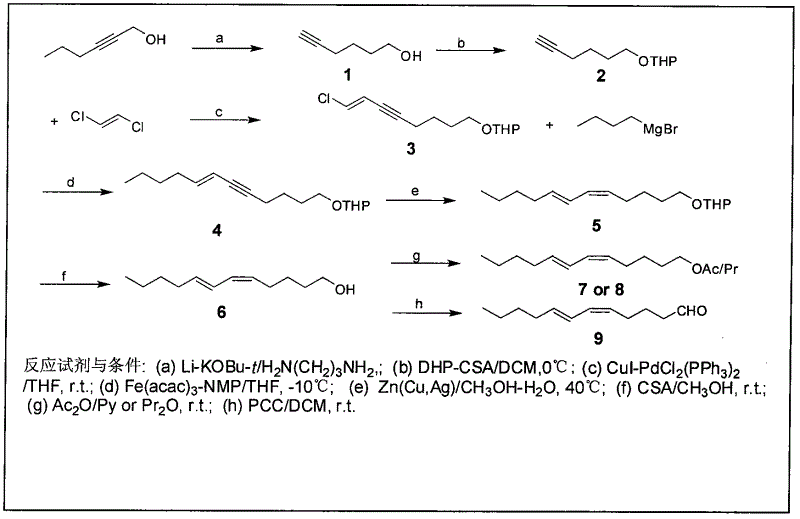
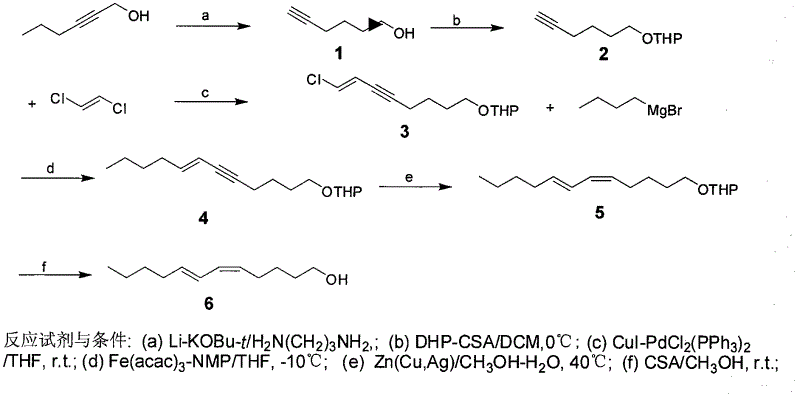
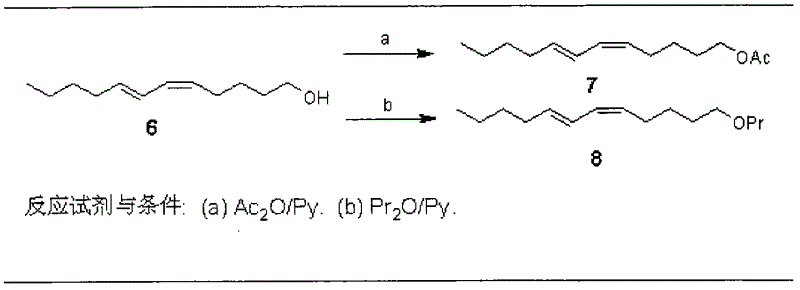
The invention discloses a process for synthesizing sex pheromone of pine caterpillar, which employs 2-hexyne-1-alcohol as an initial raw material, three-bond positional transference is carried out under the effect of lithium and propane diamine to obtain 5-hexyne-1-alcohol; under acidic condition, 5-hexyne-1-alcohol is reacted with dihydropyran to obtain 1-THP-5-hexyne-1-alcohol protected by THP on hydroxyl, Under co-catalysis of metal palladium and cuprous iodide, 1-THP-5-hexyne-1-alcohol and trans-dichloroethylene are subjected to coupling reaction to generate conjugate enyne(7E)-1-THP-8-chlorine-5-alkyne-7-alkene-1-octanol; under the catalysis of metallic iron, (7E)-1-THP-8-chlorine-5-alkyne-7-alkene-1-octanol and a n-Butyl bromide grignard reagent are further subjected to coupling reaction to obtain (7E)-1-THP-5-alkyne-7-alkene-1-dodecanol, under the catalytic reduction of metal zinc, (5Z, 7E)-1-THP-dodecanol dienol; under the camphor sulfonic acid condition, (5Z, 7E)-1-THP-dodecanol dienol removes the THP protective group to obtain the final target product (5Z, 7E)-dodecanol dienol. The method of the invention has the advantages of easily available synthesis raw materials, low cost, mild reaction condition, easy operation, high yield and good stereoselectivity.
Owner:WENZHOU MEDICAL UNIV +1
High-selectivity catalytic synthesis method for 4, 4'-bisphenol F
InactiveCN105016980AImprove stabilityEasy to separateOrganic chemistryOrganic compound preparationPhenolMethyl group
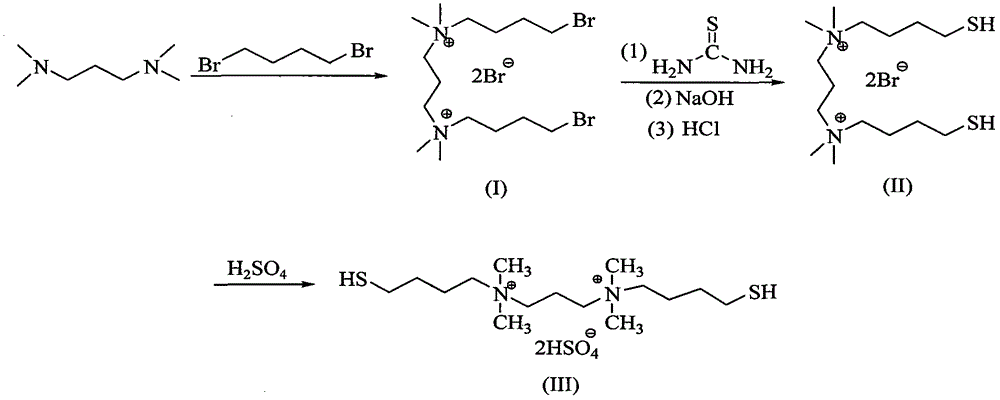
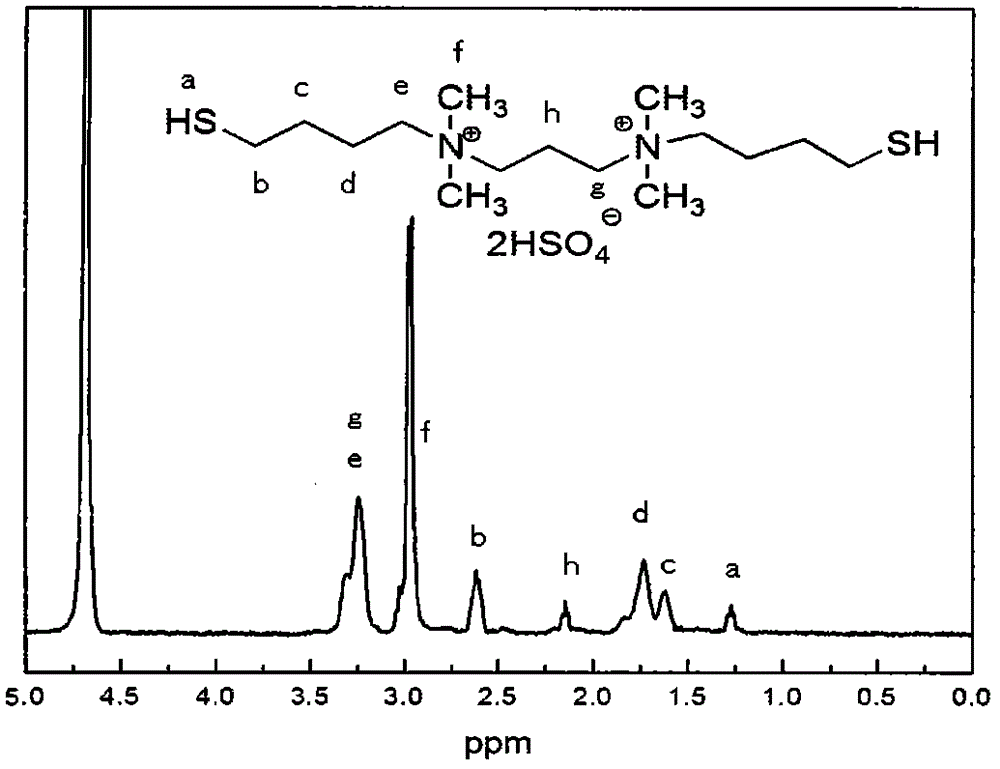
The invention discloses a high-selectivity catalytic synthesis method for 4, 4'-bisphenol F. Ionic liquid N, N-bi(4-sulfydryl) butyl-N, N, N, N-tetramethyl-1, 3-propane diammonium sulfate hydrogen sesquisulfate is used as a catalyst; phenol and formaldehyde are used as raw materials; the 4, 4'-bisphenol F is obtained through high-selectivity catalytic synthesis via condensation reaction. The adopted ion liquid catalyst of N, N-bi(4- sulfydryl) butyl-N, N, N, N- tetramethyl-1, 3-propane diammonium sulfate hydrogen sesquisulfate uses N, N, N, N-tetramethyl-1, 3- propane diamine, 1, 4- dibromobutane and thiourea as raw materials; firstly, butyl bromide is introduced onto diamine through nucleophilic substitution reaction; then, isothiuronium salts are generated through the reaction between thiourea and butyl bromide; sodium hydroxide is used for hydrolyzing the isothiuronium salts to obtain hydrosulfuryl salts; dilute hydrochloric acid acidification is carried out to obtain hydrosulphonyl; finally, concentrated sulfuric acid is used for replacing negative ions into bisulfate ions. When the catalyst is used for bisphenol F catalytic synthesis, the selectivity of the 4, 4'-bisphenol F is greatly improved.
Owner:XIANGTAN UNIV
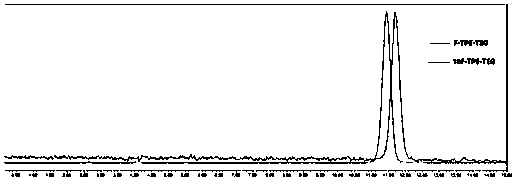
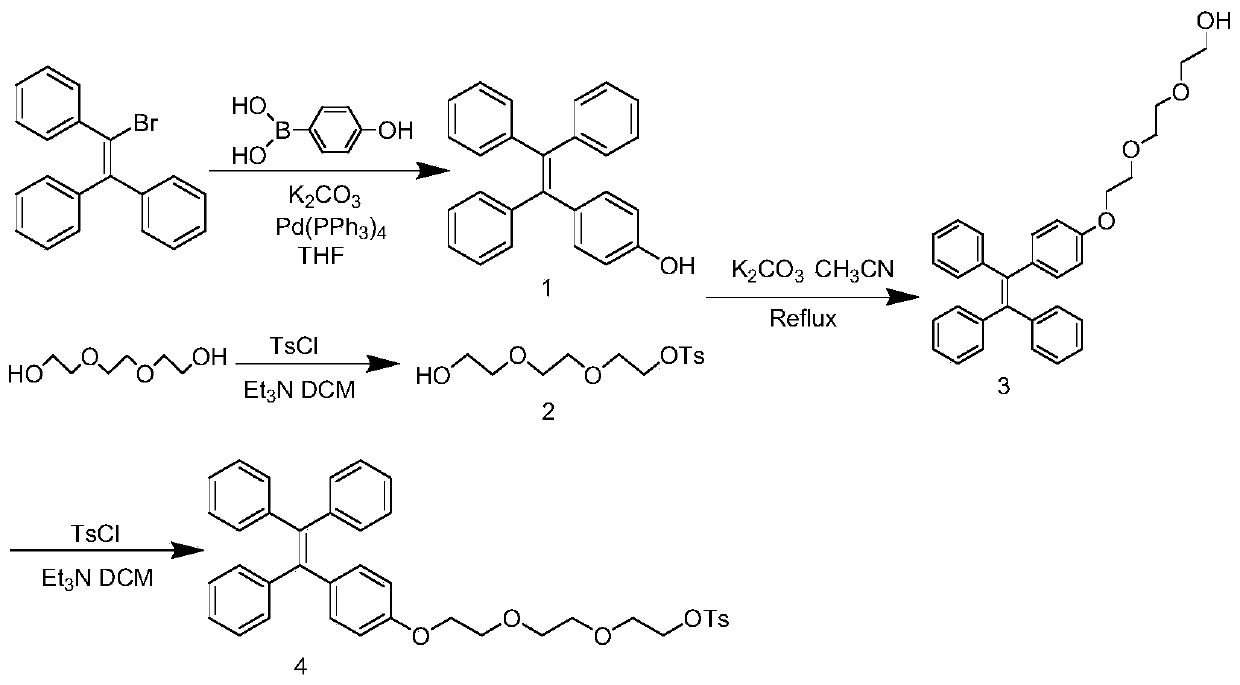
<18>F-labeled aggregation-induced emission (AIE) fluorescent/positron emission tomography (PET) dual-mode probe, and preparation method and application thereof
ActiveCN109867591AStrong resistance to photobleachingStrong penetrating powerOrganic compound preparationSulfonic acid esters preparationSynthesis methodsDual mode
The invention discloses an <18>F-labeled aggregation-induced emission (AIE) fluorescent / positron emission tomography (PET) dual-mode probe, and a preparation method and application thereof. The compound is denoted as <18>F-TPE-TEG, and has a following structure. The synthesis method of a labelled precursor of the compound comprises the following steps: firstly, adding 2-bromo-1,1,2-triphenylethylene, 4-hydroxyphenylboronic acid and tetrabutylammonium bromide into tetrahydrofuran, and taking K2CO3 and tetrakis(triphenylphosphine)palladium as catalysts to carry out a reaction to obtain 4-hydroxytetraphenylethylene; then dissolving triethylene glycol, triethylamine and p-toluenesulfonyl chloride in dichloromethane, and carrying out a reaction to obtain 8-p-toluenesulfonyloxy-3,6-dioxyoctanol;adding the 4-hydroxytetraphenylethylene, K2CO3, the 8-p-toluenesulfonyloxy-3,6-dioxyoctanol into acetonitrile, and carrying out a reaction to obtain 8-tetraphenylethyleneoxy-3,6-dioxyoctanol; and finally, dissolving the 8-tetraphenylethyleneoxy-3,6-dioxyoctanol, p-toluenesulfonyl chloride and triethylamine in dichloromethane, and carrying out a reaction to obtain the labelled precursor. The <18>F-labeled compound can be used as an AIE fluorescent / PET dual-mode probe to be applied to tumor imaging research.
Owner:ZHEJIANG UNIV
Preparation method of scopolamine butylbromide
The invention discloses a preparation method of scopolamine butylbromide, comprising the steps of: (1) subjecting scopolamine and n-butyl bromide to heating under reflux to complete reaction; (2) preparing a crude product of scopolamine butylbromide; and (3) refining the crude product of scopolamine butylbromide. By means of the invention, by utilizing the physicochemical property that n-butyl bromide has excellent dissolubility for scopolamine, but poor dissolubility for reaction product scopolamine butylbromide, the scopolamine and excessive n-butyl bromide are reacted, and thus the yield of scopolamine butylbromide is improved by raising reaction temperature, increasing reaction speed and shortening reaction time. In the preparation method of scopolamine butylbromide, disclosed by the invention, the use of any medium is avoided, the n-butyl bromide is not only used as a raw material for synthesizing scopolamine butylbromide, but also as a reaction solvent with stable properties, excessive n-butyl bromide can be recovered and treated relatively easily after the reaction is finished and can be repeatedly used, and thus, cost reduction is facilitated, and environment is protected.
Owner:LAKERSPHARMA CO LTD
Targeted chitosan gene vector and its preparation and use
InactiveCN1884551AOvercome the disadvantage of low transfection efficiencyHigh transfection efficiencyMicroinjection basedVector-based foreign material introductionSodium acetateFluorescence
This invention provides a kind of preparation method of gene vector which made chitosan as matrix, and made haluronic acid as target molecule. The method is: at 37DEG C, use hydoscine N butyl bromide degrade natural haluronic acid to the desired length, after dislysis and purification then freezing and drying, then according to the specific quality proportion, at 37DEG C coupled with four kinds of chitosans with different molecular weight catalysed by the EDC, and formed different target chitosan gene vector with four kind of different molecular weight. Dissolve the obtained gene vector with 5 mmol / l pH 5.5 buffer solution of acid acetic and sodium acetate to get 0.1 - 10 mg / ml solution, after the 0.22 mm membrane filter, composite with the reporter gene with FP to form a series of composition with quality proportion of vector and plasmid DNA. Using it to transfect Insect cell lines Sf9, can achieve the same or better transfection efficiency with the commercial transfection agent liposome. The effect of using this vector transfect silkworm is excellent. The best transfection effeciency is over 50%. This vector is safe and innocuity, cheap and easy to use.
Owner:HUBEI UNIV
3, 3-difluoro-3, 4-dihydroquinoline-2 (1H)-one compound and preparation method thereof
The invention relates to a 3, 3-difluoro-3, 4-dihydroquinoline-2 (1H)-one compound and a preparation method thereof. AN oxamic acid derivative and gem-difluoroolefin are used as raw materials; in thepresence of a catalyst AgNO3 and an oxidizing agent (NH4)2S2O8, the raw materials are heated to react in acetone / water (v acetone / v water = 1: 1) to synthesize a series of 3, 3-difluoro-3, 4-dihydroquinoline-2(1H)-one compounds. In the reaction formula, the oxamic acid derivative is selected from R1 which is methyl or benzyl and R2 which is methyl, methoxy, fluorine, chlorine, bromine and trifluoromethyl; the gem-difluoroolefin compound is selected from R3 which is a cyano group, a methyl group, a trifluoromethyl group, a methoxy group, a tert-butyl group, bromine, chlorine, an acetamido group, an ester group, a phenyl group and a pyridyl group; and R4 is methyl or hydrogen. According to the method for synthesizing the 3, 3-difluoro-3, 4-dihydroquinoline-2 (1H)-one compound, a CF2 group isintroduced into a 3, 4-dihydroquinoline-2 (1H)-one structure, the synthesis method of the 3, 3-difluoro-3, 4-dihydroquinoline-2 (1H)-one compound is provided, and the method has the advantages of good substrate universality, high atom economy, simple steps, mild condition, easily-available raw materials, environmental protection, wide application and high practical value.
Owner:ZUNYI MEDICAL UNIVERSITY
Synthesis process of tetrabutylammonium bromide
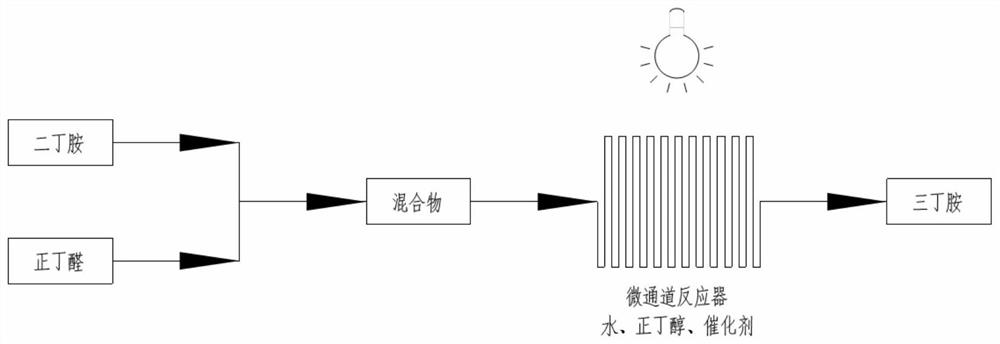
ActiveCN111960948AHigh yieldHigh purityOrganic compound preparationChemical/physical/physico-chemical microreactorsAminationButyraldehyde
The invention discloses a synthesis process of tetrabutylammonium bromide. The process is characterized by comprising the following steps: (1) taking dibutylamine and n-butyraldehyde as initial raw materials, taking water as a hydrogen source and butanol as a sacrificial reagent under the action of a modified titanium dioxide photocatalyst, and preparing tributylamine by a photocatalytic continuous micro-channel reactor through a reductive amination mechanism; and (2) after concentrating the obtained tributylamine, making the tributylamine directly dissolved in the solvent and mixed with a certain proportion of n-bromobutane, and then enter the next step continuous micro-channel reactor, such that the target product TBAB can be obtained at the high yield after the reaction is performed for3-5 h at the temperature of 60-90 DEG C. Compared with the kettle type reaction, the continuous reaction temperature is low, the reaction time is short, and the process is safe and efficient.
Owner:KENTE CATALYSTS INC
Synthesis method of tetrabutylammonium bromide
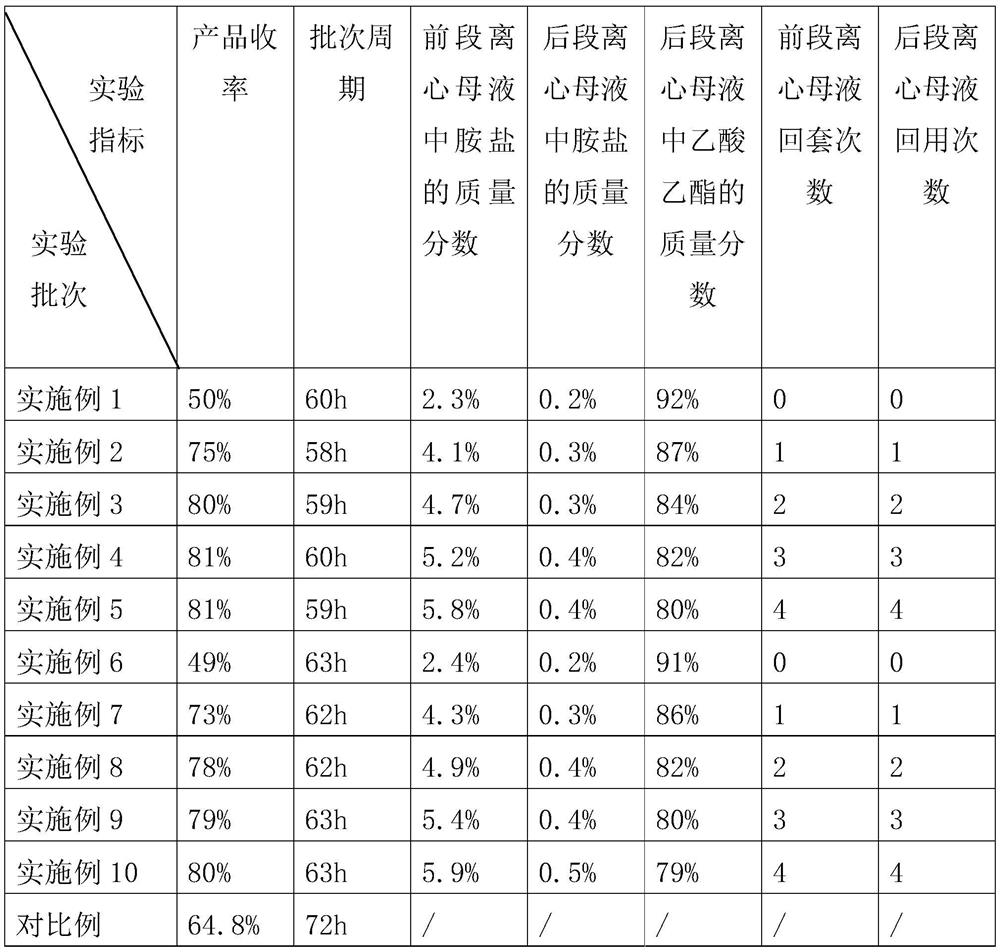
PendingCN113248393AEliminate desolvation operationAvoid evaporation lossAmino compound purification/separationOrganic compound preparationButyl bromidePhysical chemistry
The invention discloses a synthesis method of tetrabutylammonium bromide, which comprises the steps of reflux reaction, crystallization, centrifugation, mother liquor application, centrifugal machine washing and the like, the desolvation operation of dichloroethane is omitted, the evaporation loss of dichloroethane in the desolvation process is avoided, the raw materials are saved, and after the front-section centrifugal mother liquor is supplemented to the formula amount, infinite times of return use can be basically realized, bromobutane and a small amount of tetrabutylammonium bromide contained in the front-section centrifugal mother liquor can be completely put into the production of the next batch, the yield of the product is improved, impurities in the product can be washed away by washing a centrifugal machine with ethyl acetate, the chromaticity of the product is improved, and meanwhile, the post-stage centrifugal mother liquor obtained after centrifugation can be repeatedly used under the condition that the post-stage centrifugal mother liquor is inspected to be qualified, so that raw materials are saved.
Owner:SHANDONG TONGCHENG MEDICINE TECH CO LTD
Method for catalytically synthesizing phenazine compounds in water phase under microwave radiation
The invention discloses a method for catalytically synthesizing phenazine compounds in a water phase under microwave radiation, which comprises the following steps: adding a catalytic amount of catalyst ferric chloride or nickelous chloride, a cocatalyst lithium proline, a substrate substituted 2-haloaniline, a phase-transfer catalyst 4-butyl ammonium chloride, 4-butyl ammonium bisulfate or 4-butyl ammonium bromide, an inorganic alkali or organic alkali and water into a reaction vessel, putting into a microwave reaction instrument, reacting at certain temperature under certain power for some time, concentrating under reduced pressure, and purifying the product by column chromatography. The method for preparing phenazine compounds is friendly to the environment, simple to operate and high in efficiency. Compared with the prior art, the method has the advantages of obviously higher reaction speed than conventional heating, mild reaction conditions, high yield, high safety, low cost and environment friendliness, and is simple to operate.
Owner:FUJIAN MEDICAL UNIV
Ether-bond-free anion exchange membrane of polyaryl piperidine for fuel cell and preparation method of ether-bond-free anion exchange membrane
The invention discloses an ether-bond-free anion exchange membrane of polyaryl piperidine for a fuel cell and a preparation method. The anion exchange membrane is prepared according to different molar ratios of grafted n-butyl bromide to bromoethanol, the molar ratio of grafted n-butyl bromide to bromoethanol is a: b, a and b are both even numbers of 2-8, and a + b = 10. A main chain of the anion exchange membrane is polybiphenyl piperidine without ether bonds, so that the alkaline stability is improved, the alkali resistance is improved, and meanwhile, the microphase separation structure of the anion exchange membrane is changed by grafting bromoethanol and bromo-n-butane with different proportions on the side chain, so that the problem of balance between the alkali resistance and conductivity of the anion exchange membrane is solved; experimental results show that the ion conductivity of the ether-bond-free anion exchange membrane of the polyaryl piperidine is 0.47 S / cm to 0.61 S / cm at the temperature of 80 DEG C, and the thickness of the anion exchange membrane is 22 microns to 26 microns.
Owner:CHANGCHUN UNIV OF TECH
Preparation method of high-purity butylphthalide
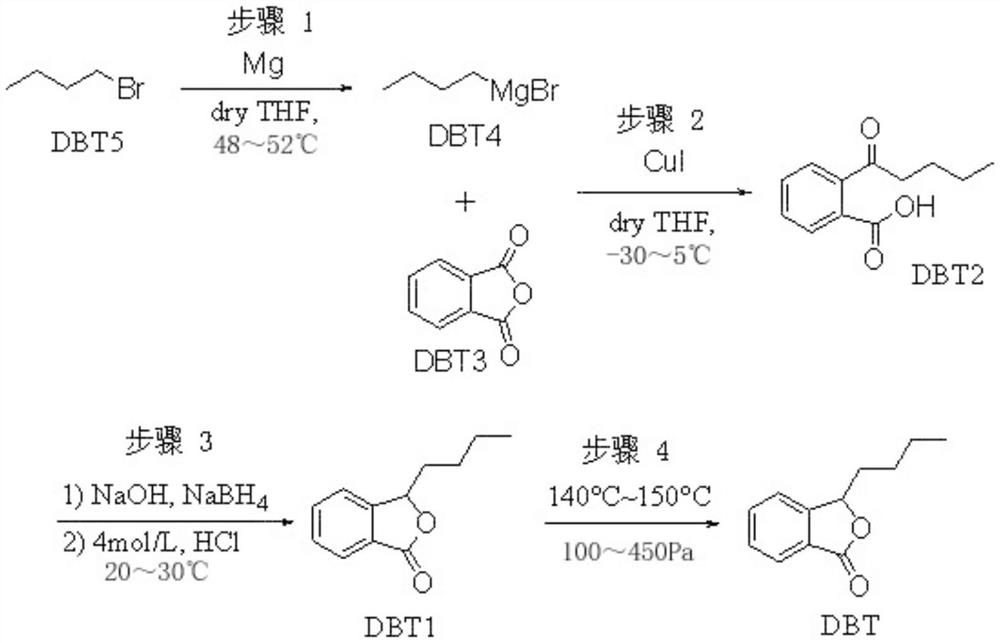
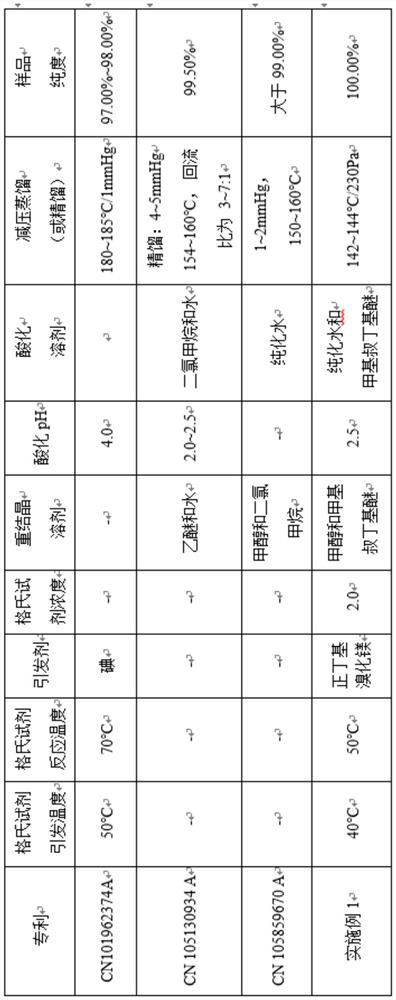
ActiveCN111961018AMild triggering conditionsImprove securityOrganic chemistryBenzoic acidButyl bromide
The invention relates to a preparation method of high-purity butylphthalide. The preparation method comprises the following steps: (1) preparing n-butyl magnesium bromide; (2) synthesizing 2-valerylbenzoic acid; (3) synthesizing butylphthalide; and (4) refining the butylphthalide. Due to the adoption of the technical scheme, the preparation method has the advantages that the n-butyl magnesium bromide is used as an initiator in the step (1), so that the initiation condition is mild, and the safety performance is improved; and the initiation temperature is 38-42 DEG C, and the reaction temperature is only 48-52 DEG C, so that the temperature is easy to implement in the mass production process; the 2-valerylbenzoic acid is used as the raw material, and carbonyl is reduced in a sodium borohydride aqueous solution mode, so that explosion easily caused by a solid feeding mode is avoided, and production safety is guaranteed; and the butylphthalide is refined in a reduced pressure distillationmode, so that the method is simple and easy to implement, and the purity of the obtained high-purity butylphthalide reaches 100%. The synthesis process of butylphthalide is stable, the preparation process is mild and controllable, and the method is suitable for batch production.
Owner:吉林省奇健生物技术有限公司
Preparation method of modified BiOBr visible light catalyst
InactiveCN105833895AEasy to recyclePromote absorptionMolecular sieve catalystsIonic liquidPolyacrylamide
The invention discloses a preparation method of a modified BiBr visible light catalytic film, and belongs to the technical field of light catalysis .Bismuth oxide is adopted as a bismuth source and subjected to nitric acid treatment to obtain bismuth nitrate, butyl bromide pyridine ionic liquid is adopted as a bromine source, the bismuth nitrate and the butyl bromide pyridine ionic liquid are subjected to a reaction to obtain a precursor, then, a reaction is performed inside a stainless steel hydrothermal autoclave with a Teflon liner to obtain BiOBr, zeolite is treated through cationic polyacrylamide, the treated zeolite and BiOBr are subjected to a reaction inside the stainless steel hydrothermal autoclave with the Teflon liner to obtain modified BiOBr, the zeolite can be used as a light catalyst, the BiOBr is modified through addition of zeolite, the absorption band of the visible light catalyst is 570 nm or above, the absorption capacity is enhanced, and the modified BiOBr is easy to recycle .
Owner:陈建峰
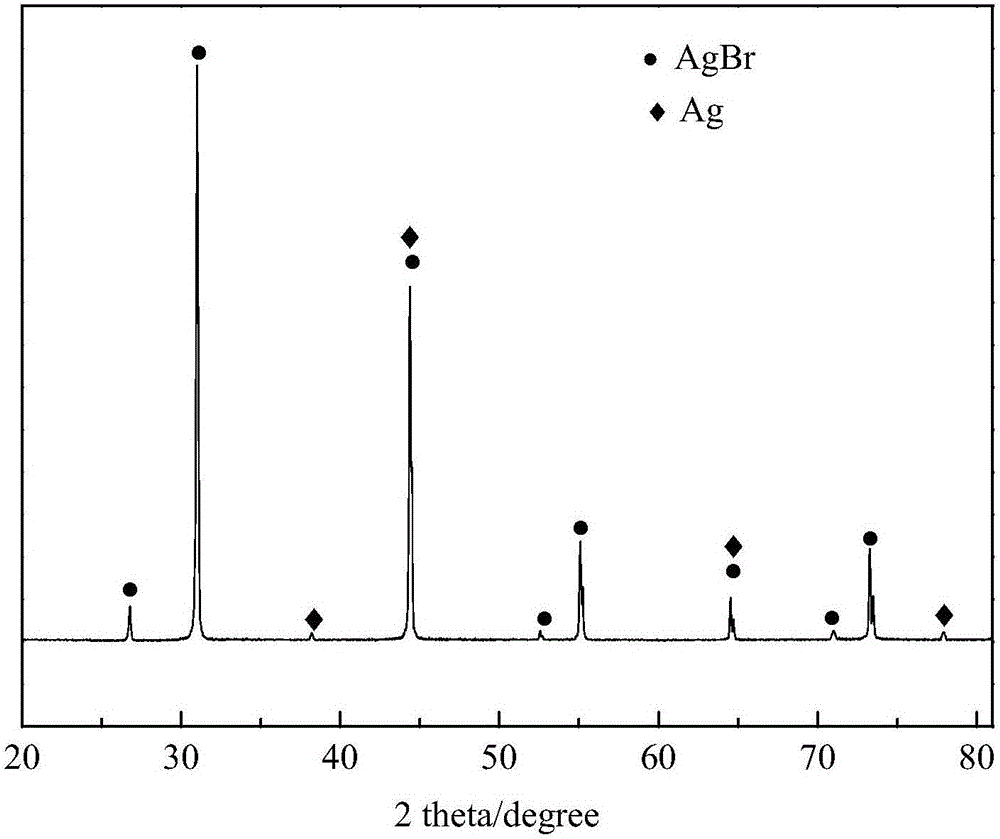
Preparation and application of photocatalyst Ag/ AgBr
InactiveCN106111166ANo change in degradabilityHigh activityWater/sewage treatment by irradiationWater treatment compoundsWater bathsButyl bromide
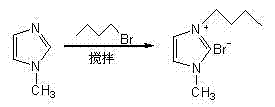
The invention discloses preparation and application of a photocatalyst Ag / AgBr, and belongs to the technical field of nanocatalysts. According to the method, 1-butyl-3-methylimidazole bromine ([BMIM]Br) is prepared from N-methylimidazole and n-butyl bromide under certain conditions, wherein a preparation method comprises the steps that a certain amount of N-methylimidazole and a certain amount of n-butyl bromide are weighed and put into a round-bottom flask to be stirred and refluxed for a period of time in a high-temperature water bath to obtain faint yellow thick liquid, and 1-butyl-3-methylimidazole bromine ([BMIM]Br) which is collected after cooling has the very high catalytic activity in preparation of the photocatalyst Ag-AgBr by serving as a precursor.
Owner:YANCHENG TEACHERS UNIV
Method for synthesizing 7-bromo-6-chloro-4-quinazolinone
ActiveCN102351790ASave raw materialsThe reaction steps are simpleOrganic chemistrySodium bicarbonateNitrogen gas
The invention discloses a method for synthesizing 7-bromo-6-chloro-4-quinazolinone. The method comprises the following steps of: (1) dripping liquid bromine into suspension of m-chlorotoluene, n-butyl bromide and anhydrous ferric trichloride, washing with water and a saturated sodium bicarbonate solution, distilling, and recrystallizing; (2) mixing water, pyridine, tertiary butanol and the like, dissolving a product in the step (1) and potassium permanganate in a mixed solution, heating and refluxing, continuously adding potassium permanganate, filtering, washing with boiling water, regulating the pH value, cooling and filtering; (3) dissolving a product in the step (2) in a mixed solution of stronger ammonia water and ethyl acetate at room temperature under the protection of nitrogen, dripping into suspension of cuprous oxide and stronger ammonia water, adding ethylene diamine tetraacetic acid (EDTA), regulating the pH value, evaporating the ethyl acetate, cooling, and performing suction filtration; and (4) mixing a product in the step (3) and formamide, dissolving in dimethyl formamide (DMF), heating and refluxing, cooling, precipitating, filtering, washing with absolute ethanol, and recrystallizing by using ethylene glycol monomethyl ether. The method has the advantages of cheap raw materials, simplified steps and practicable process, avoids 6-bromoisatin, and is suitable for industrial production.
Owner:JIANGXI WANLI PHARMA CO LTD
Bar code for sulfurizing rubber tyre and its making process
InactiveCN1887981AAchieving wear resistanceMake up for the remaining defectsStampsInksPolyesterScreen printing
The sulfurized rubber tyre bar code is prepared with the materials including butyl bromide, natural rubber, terpene resin, softener, antiager, white carbon black and titanium white powder in certain weight proportion. The preparation process includes the following steps: screen printing bar code and number with the compounded rubber ink on transparent polyester film; coating one layer of white sulfurizing material; and cutting into single bar codes. The bar code is combined with tyre through sulfurizing, and is wear resistant and low in cost. There are bar code and number printed simultaneously for recognition with computer and by human eyes.
Owner:冯秉栋
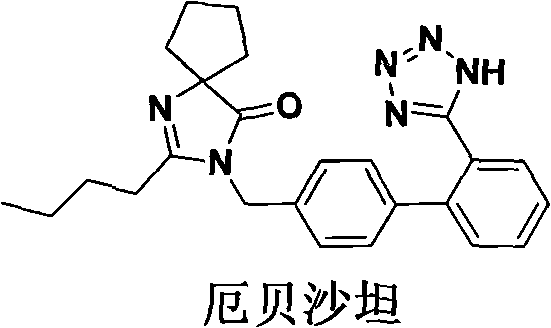
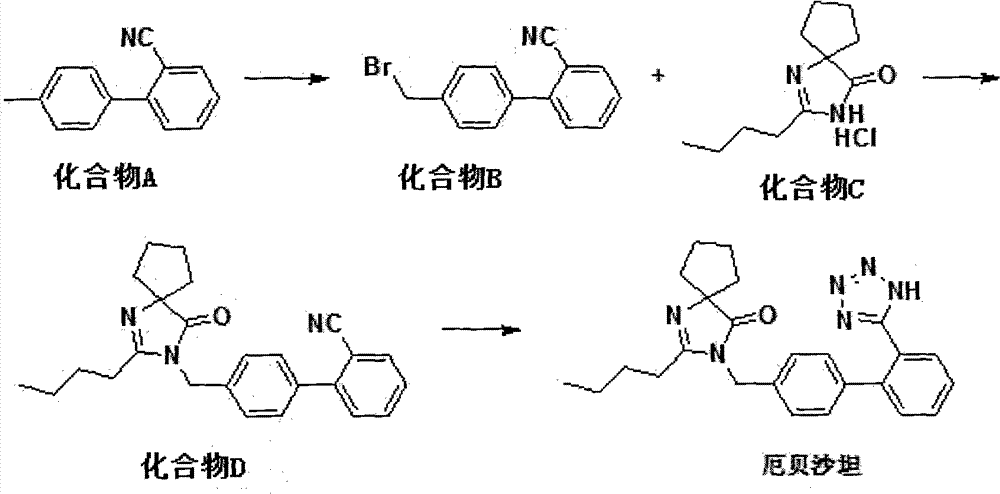
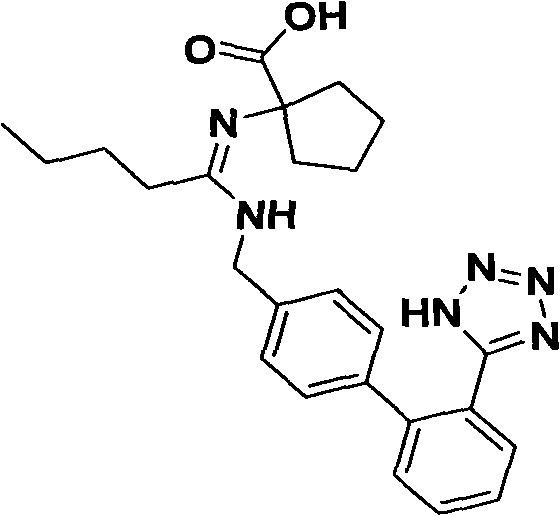
Synthetic route and preparation method of irbesartan
The invention relates to a synthetic route and a preparation method of irbesartan. The method comprises three steps: (1) reacting a compound I (2-cyano-4'-methyl diphenyl), an inorganic salt oxidant, and an inorganic salt reductant in dichloromethane and water to form a compound IRB-02 (2-cyano-4'-bromomethylbiphenyl); (2) reacting a compound IRB-02, a compound IRB-01 (2-butyl-1, 3-diaza spiro [4.4] nonane-1-vinyl-4-ketone hydrochloride), tetrabutylammonium bromide and inorganic alkali in dichloromethane and water to obtain a compound IRB-03 (2-butyl-3-[(2-cyano biphenyl-4-base)methyl]-1,3-diaza spiro [4.4] nonane-1-vinyl-4-ketone); and (3) reacting a compound IRB-03, tetrabutylammonium bromide, zinc chloride and sodium azide in toluene to obtain the irbesartan.
Owner:珠海保税区丽珠合成制药有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

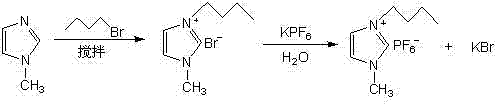

![AM/AA/N-beta-CD polymer-ionic liquid [bquin]BF4 composite clay stabilizer and synthesis method thereof AM/AA/N-beta-CD polymer-ionic liquid [bquin]BF4 composite clay stabilizer and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/e15dbbf8-c72c-4ab6-bf51-8de551180619/140408101615.PNG)
![AM/AA/N-beta-CD polymer-ionic liquid [bquin]BF4 composite clay stabilizer and synthesis method thereof AM/AA/N-beta-CD polymer-ionic liquid [bquin]BF4 composite clay stabilizer and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/e15dbbf8-c72c-4ab6-bf51-8de551180619/140408101620.PNG)
![AM/AA/N-beta-CD polymer-ionic liquid [bquin]BF4 composite clay stabilizer and synthesis method thereof AM/AA/N-beta-CD polymer-ionic liquid [bquin]BF4 composite clay stabilizer and synthesis method thereof](https://images-eureka.patsnap.com/patent_img/e15dbbf8-c72c-4ab6-bf51-8de551180619/140408101624.PNG)