3, 3-difluoro-3, 4-dihydroquinoline-2 (1H)-one compound and preparation method thereof
A technology of ketone compounds and dihydroquinolines, applied in the direction of organic chemistry, can solve the problems of inability to introduce, lack of step economy and atom economy, lack of substrate universality, etc., to achieve mild conditions and great practical value and socio-economic benefits, environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
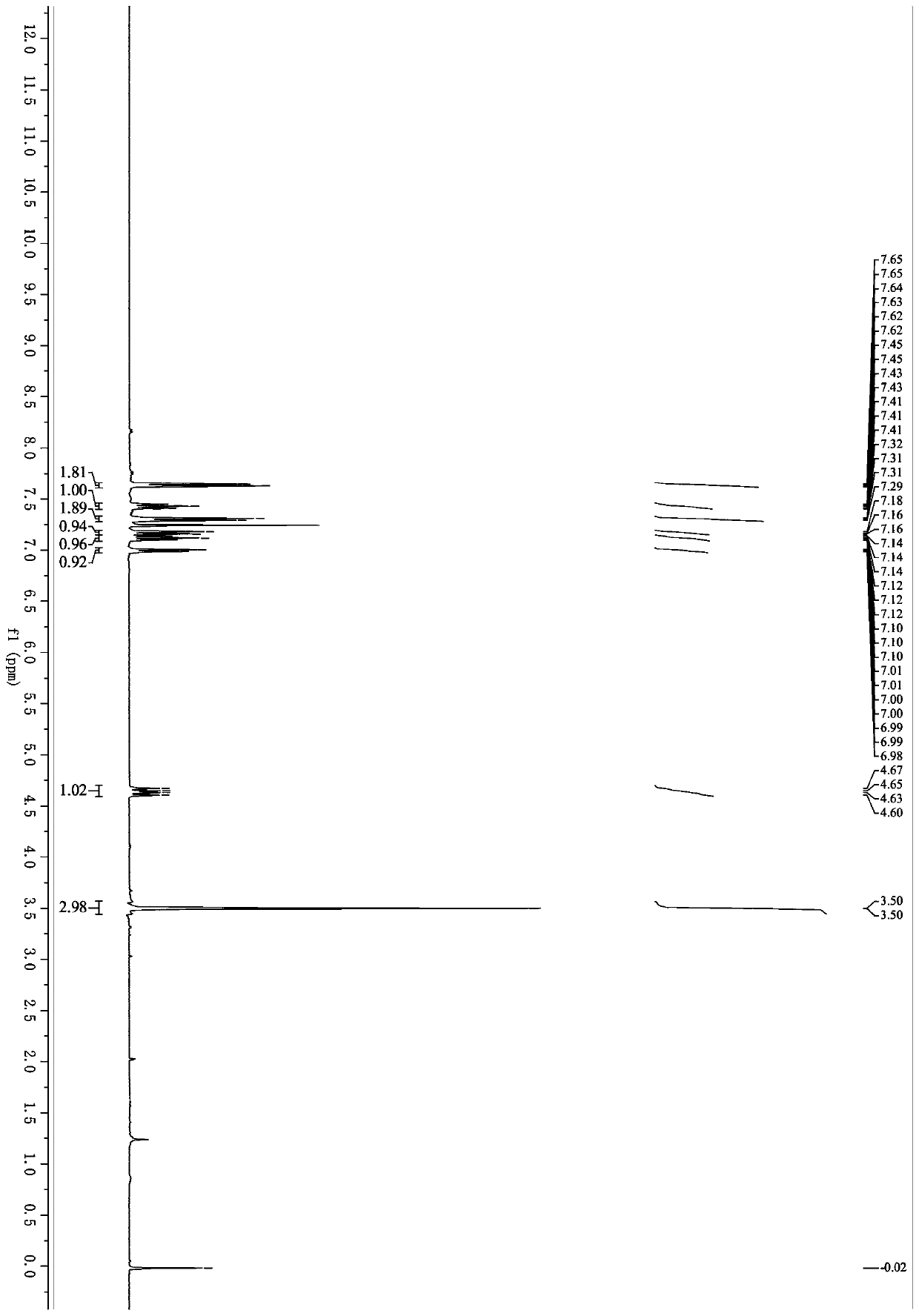
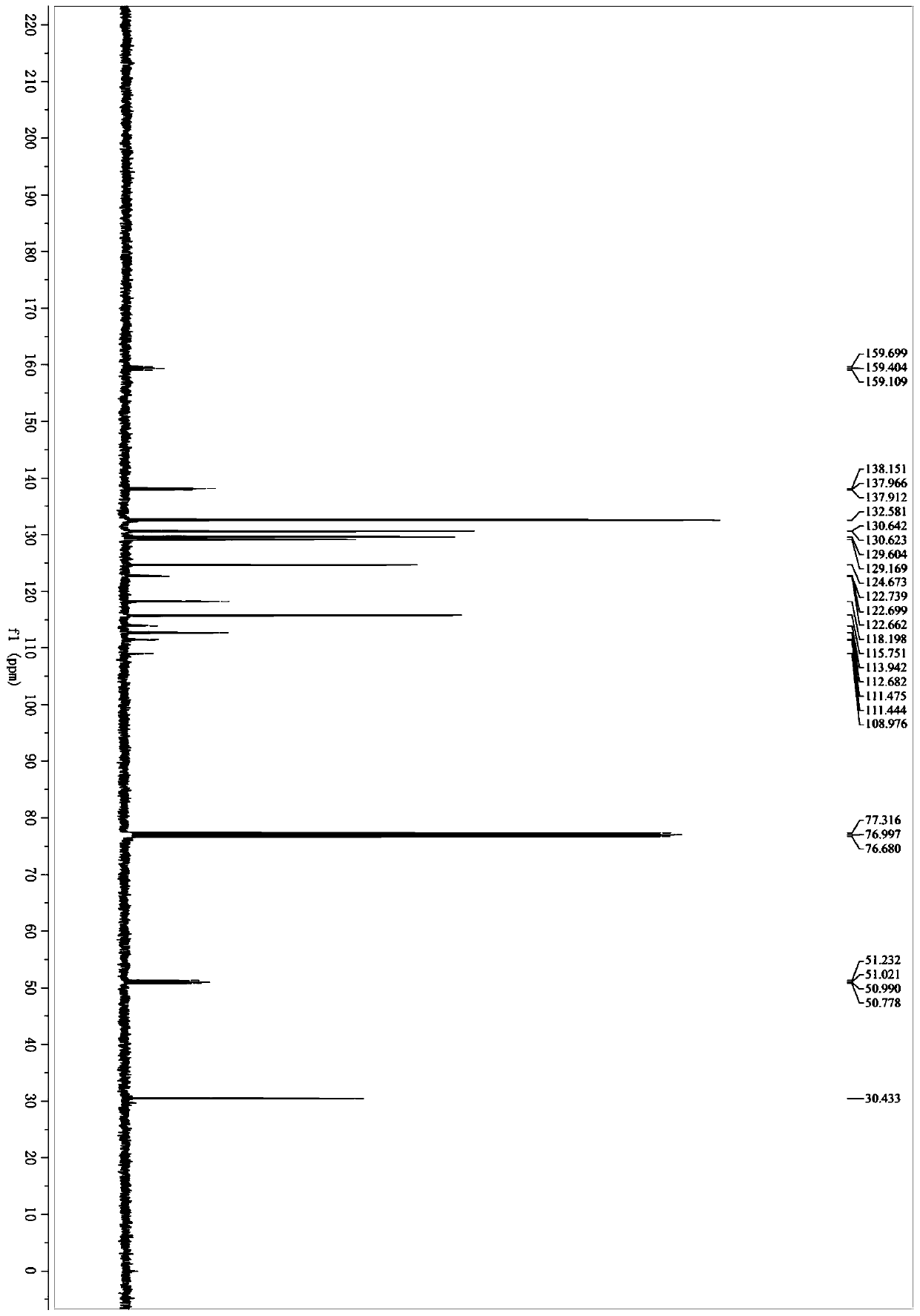
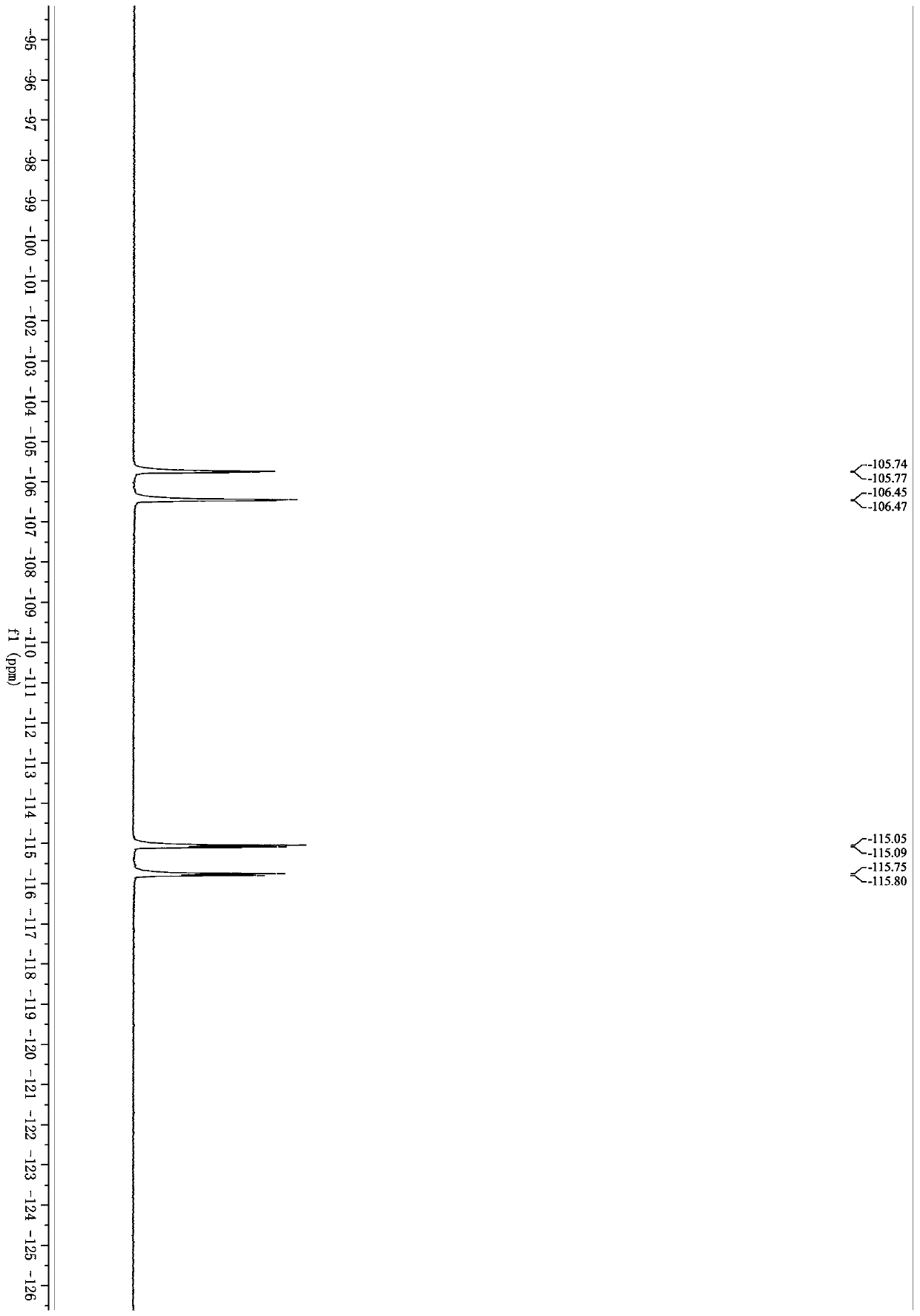
[0055] Example 1: Synthesis of 4-(3,3-difluoro-1-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-4-yl)benzonitrile (1a)
[0056]
[0057] 2-(Methyl(phenyl)amino)-2-glyoxylic acid (134mg, 0.75mmol), 4-(2,2-difluorovinyl)benzonitrile (82mg, 0.50mmol), K 2 S 2 o 8 (270mg, 1.0mmol), and AgNO 3 (8.5mg, 0.050mmol) was placed in a Schlenk tube with a magnetic stir bar. Evacuating the reaction tube and filling it with argon was repeated three times. Acetone (1.5 mL) and distilled water (1.5 mL) were successively added to the reaction. The resulting reaction mixture was stirred at 50°C for 9 hours. The resulting mixture was extracted with ethyl acetate (10 mL x 2), anhydrous Na 2 SO 4 Dry, filter and concentrate in vacuo. The resulting crude product was purified on a silica gel column with ethyl acetate and petroleum ether as eluents to obtain 4-(3,3-difluoro-1-methyl-2-oxo-1,2,3,4-tetrahydro Quinolin-4-yl)benzonitrile, white solid (117mg, yield 79%), mp 203.3-204.4°C; 1 H NMR (40...
Embodiment 2
[0058] Example 2: Synthesis of 3,3-difluoro-1-methyl-4-(p-tolyl)-3,4-dihydroquinolin-2(1H)-one (1b)
[0059]
[0060] 2-(Methyl(phenyl)amino)-2-glyoxylic acid (134mg, 0.75mmol), 1-(2,2-difluorovinyl)-4-toluene (77mg, 0.50mmol), K 2 S 2 o 8 (270mg, 1.0mmol), and AgNO 3 (8.5mg, 0.050mmol) was placed in a Schlenk tube with a magnetic stir bar. Evacuating the reaction tube and filling it with argon was repeated three times. Acetone (1.5 mL) and distilled water (1.5 mL) were successively added to the reaction. The resulting reaction mixture was stirred at 50°C for 9 hours. The resulting mixture was extracted with ethyl acetate (10 mL x 2), anhydrous Na 2 SO 4 Dry, filter and concentrate in vacuo. The resulting crude product was purified on a silica gel column with ethyl acetate and petroleum ether as eluents to give 3,3-difluoro-1-methyl-4-(p-tolyl)-3,4-dihydroquinoline -2(1H)-one, white solid (68mg, yield 47%), mp 157.2-157.3°C; 1 H NMR (400MHz, Chloroform-d) δ7.40 (t...
Embodiment 3
[0061] Example 3: N-(4-(3,3-difluoro-1-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-4-yl)phenyl)acetamide (1c )Synthesis
[0062]
[0063] 2-(Methyl(phenyl)amino)-2-glyoxylic acid (134mg, 0.75mmol), N-(4-(2,2-difluorovinyl)phenyl)acetamide (98mg, 0.50 mmol) ,K 2 S 2 o 8 (270mg, 1.0mmol), and AgNO 3 (8.5mg, 0.050mmol) was placed in a Schlenk tube with a magnetic stir bar. Evacuating the reaction tube and filling it with argon was repeated three times. Acetone (1.5 mL) and distilled water (1.5 mL) were successively added to the reaction. The resulting reaction mixture was stirred at 50°C for 9 hours. The resulting mixture was extracted with ethyl acetate (10 mL x 2), anhydrous Na 2 SO 4 Dry, filter and concentrate in vacuo. The resulting crude product was purified on a silica gel column with ethyl acetate and petroleum ether as eluents to obtain N-(4-(3,3-difluoro-1-methyl-2-oxo-1,2,3,4 -tetrahydroquinolin-4-yl)phenyl)acetamide, white solid (102mg, 62% yield), mp 180.9-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com