Patents
Literature
36results about How to "Highly economical steps" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
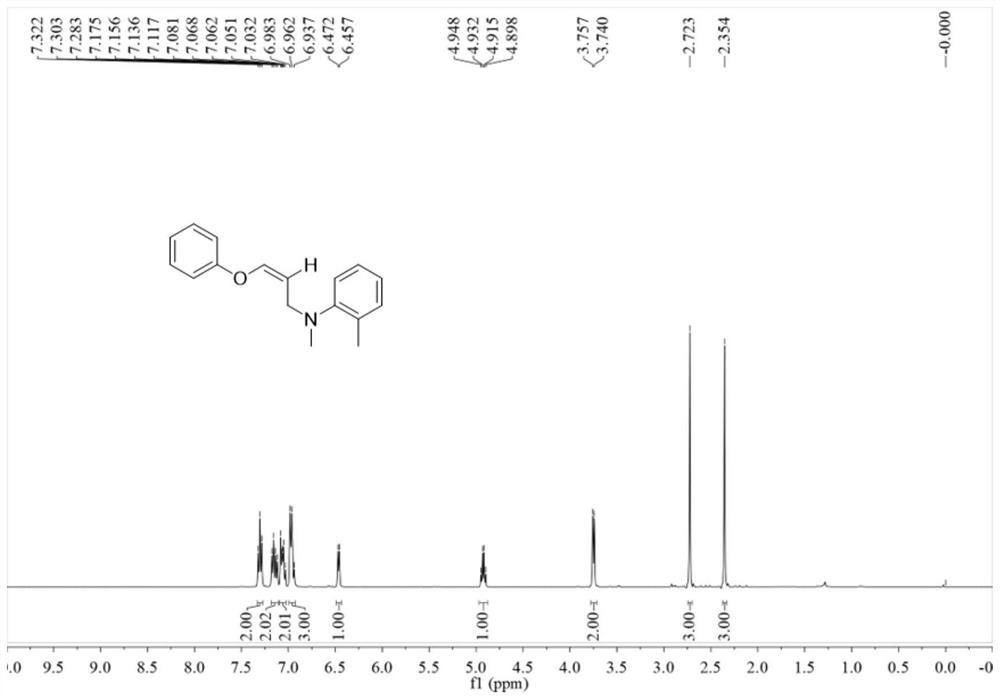
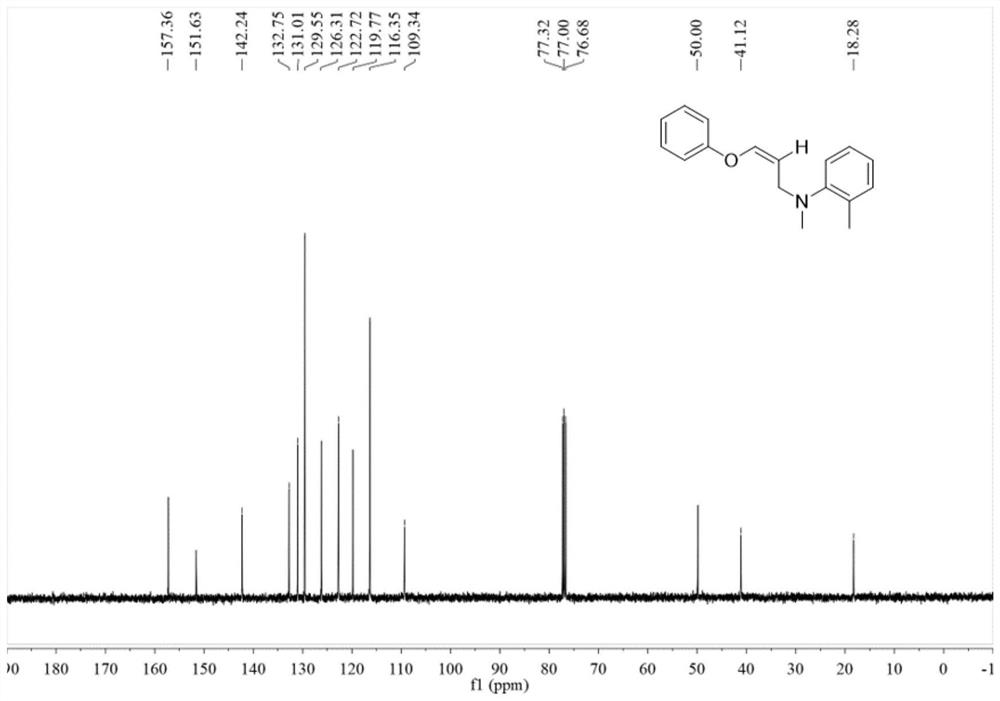
Synthetic method for imidazole[1,2-a]pyridine and 2-butyl-5-chloro-1H-imidazole-4-carboxaldehyde compounds
The invention discloses a novel synthetic method for 3-formyl-imidazole[1,2-a]pyridine and 2-butyl-5-chloro-1H-imidazole-4-carboxaldehyde, and effective application of the method into synthesis of Necopidem and Saripidem. According to the invention, a 3-formyl-imidazole[1,2-a]pyridine system is established by using a cheap and low toxic Cu-catalyed O2 activated intramolecular olefin under dehydrogenation ammoxidation; conditions for the method are mild, reaction substrates are easily available, a catalytic system is simple, operation is simple, tolerance of functional groups is good, and the method is economical and effective and has very important scientific values and realistic significance.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Novel method for synthesizing indolo[1,2-a]quinoxaline derivative
ActiveCN110981877AImprove toleranceThe steps are economicalOrganic chemistryQuinoxalinePalladium catalyst
The invention discloses a novel method for synthesizing an indolo[1,2-a]quinoxaline derivative. The novel synthesis method comprises the following steps: adding a palladium catalyst, an indole compound, acyl chloride, an additive, alkali and a solvent into a glass reaction container, carrying out a reaction under stirring at 110-130 DEG C under the protection of argon, and separating and purifyinga crude reaction product so as to obtain an indolo[1,2-a]quinoxaline compound. According to the invention, a coupling reaction of primary amine-guided acyl chloride and an indole derivative is developed; a series of functionalized indolo[1,2-a]quinoxaline derivatives can be constructed through simple operation; and high step economy and atom economy are achieved. In addition, the reaction in thenovel method provided by the invention has the main advantages of simple and easily-available substrate, single selectivity, good functional group tolerance and high yield.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
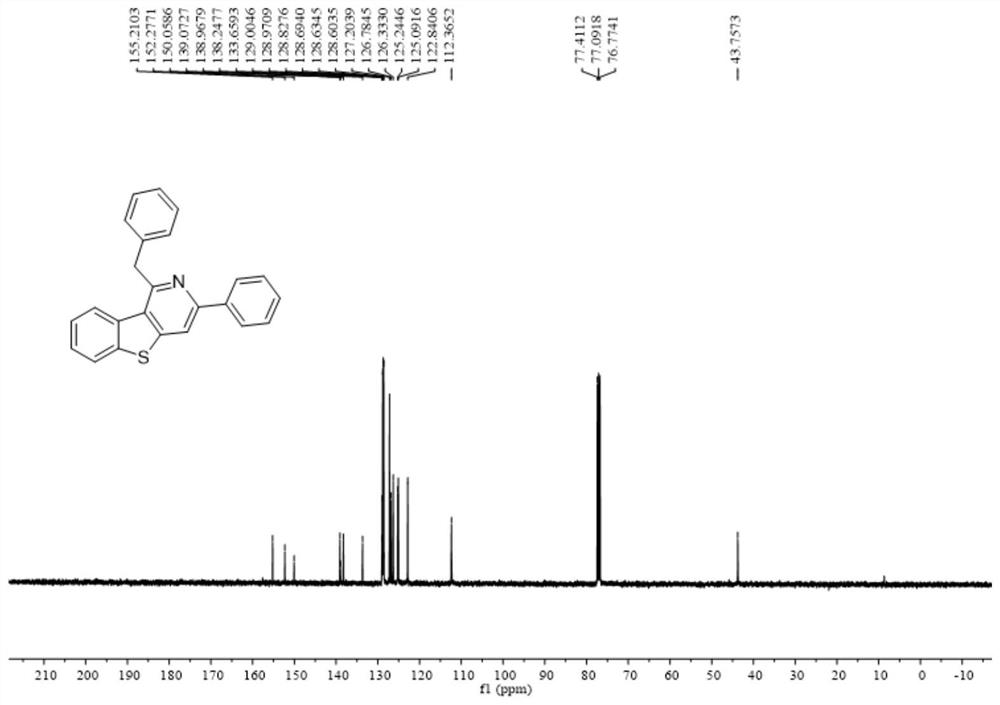
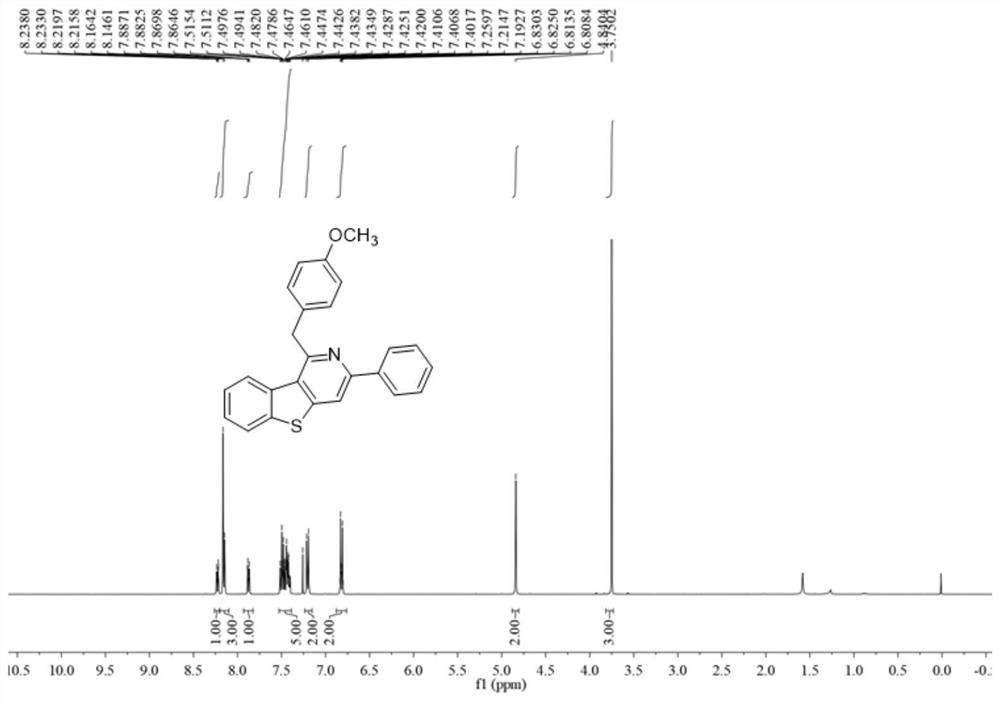
Polysubstituted benzothienopyridine compound and preparation method thereof
InactiveCN112592352AImprove toleranceRaw materials are easy to obtainOrganic chemistryOrganic synthesisCitalopram
The invention belongs to the technical field of organic synthesis, and discloses a polysubstituted benzothienopyridine compound and a preparation method thereof. The preparation method comprises the steps of dissolving nitrile amino metal salt and alkali in an organic solvent in a reactor, stirring to react at the temperature of 90-130 DEG C, and separating and purifying the reaction product to obtain the polysubstituted benzothienopyridine compound, wherein the reaction formula of the preparation method is shown as a formula (I) in the description. According to the method disclosed by the invention, a series of polysubstituted benzothienopyridine compounds are synthesized by taking simple and readily available 2-methyl-3-alkynyl benzothiophene and nitrile as reaction raw materials. The method has the characteristics of simple and readily available raw materials, convenience in operation, no transition metal, high step economy, high atom economy, wide substrate applicability, good tolerance of functional groups and the like; and part of application research is carried out, and a derivative product of a drug molecule citalopram is synthesized.
Owner:SOUTH CHINA UNIV OF TECH
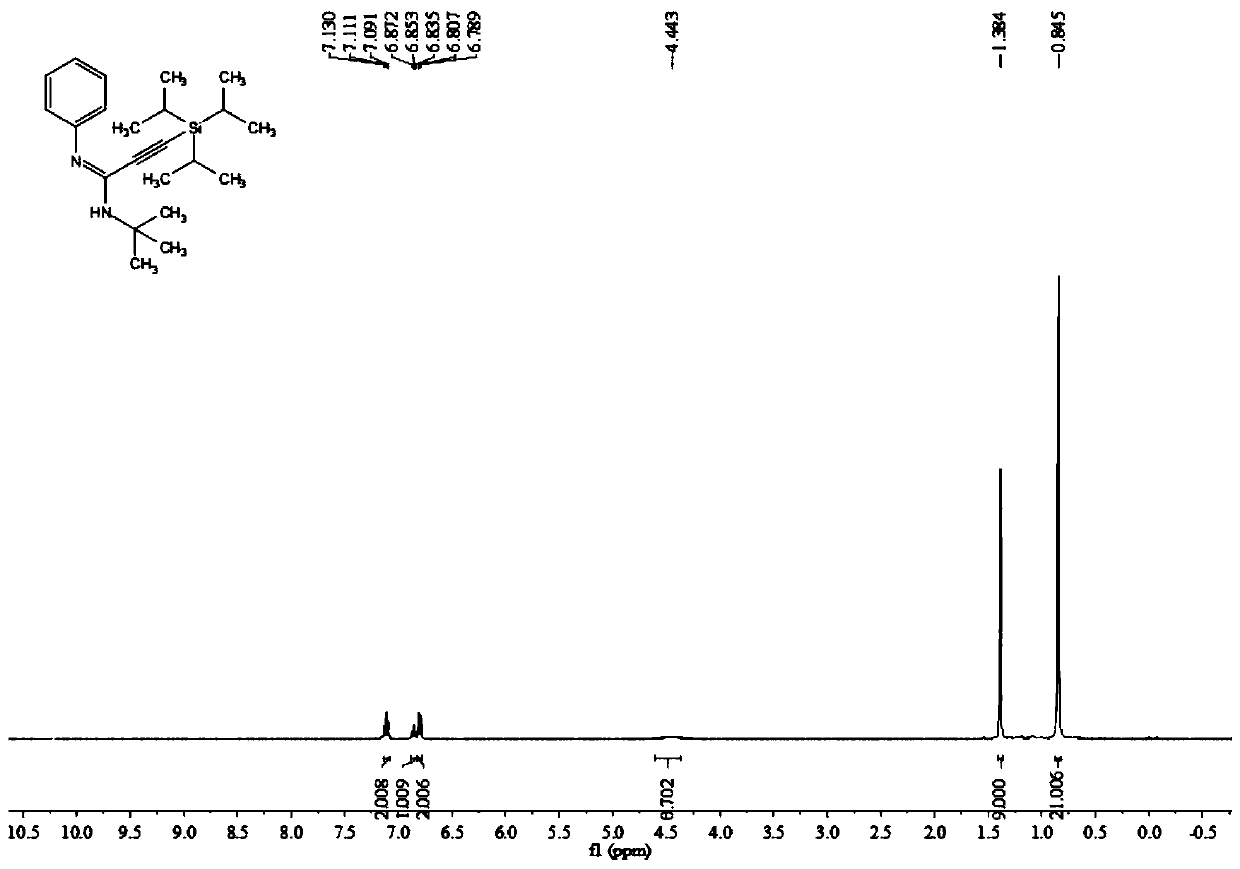
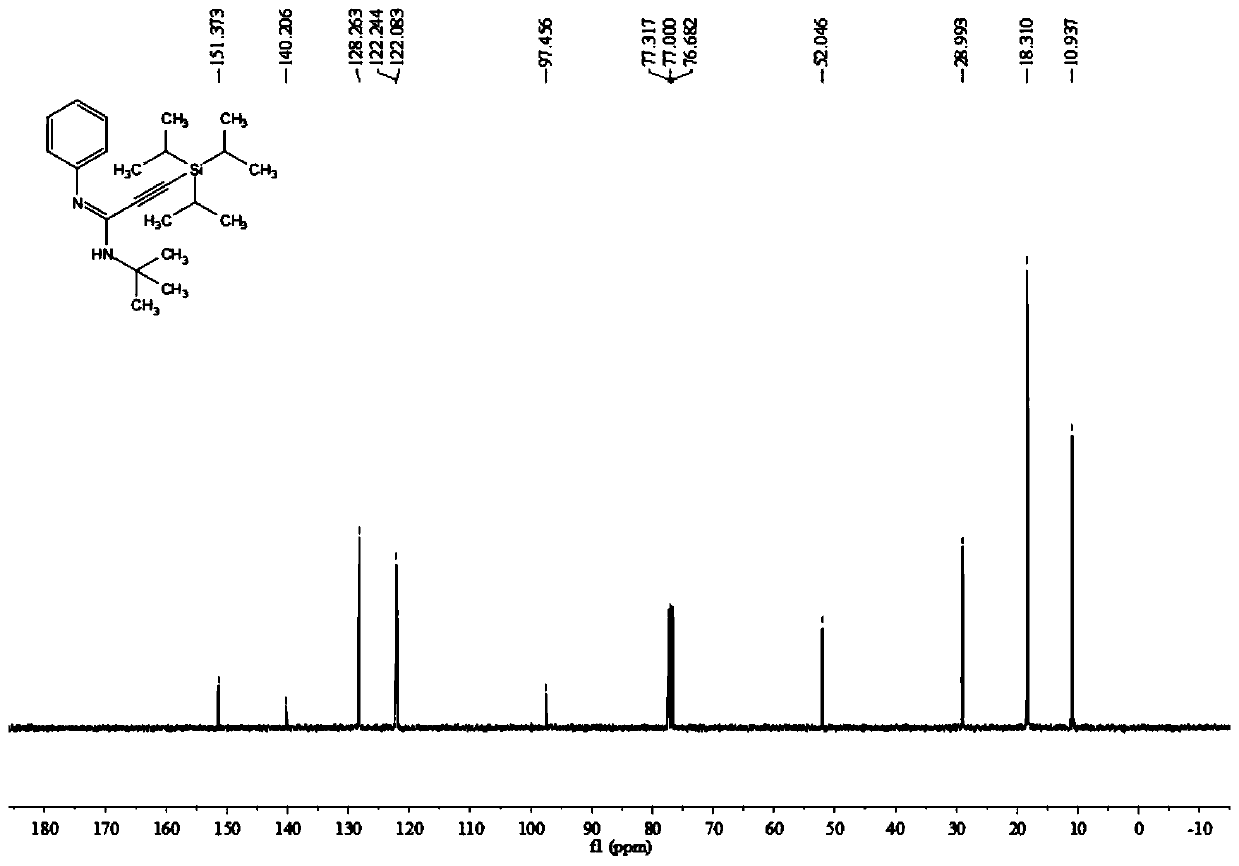
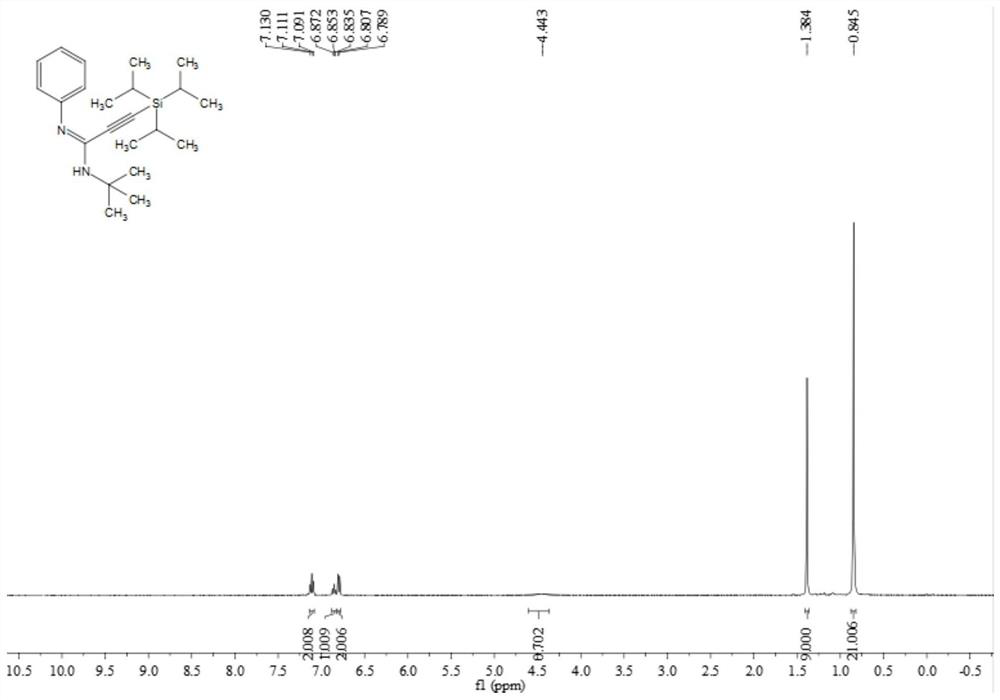
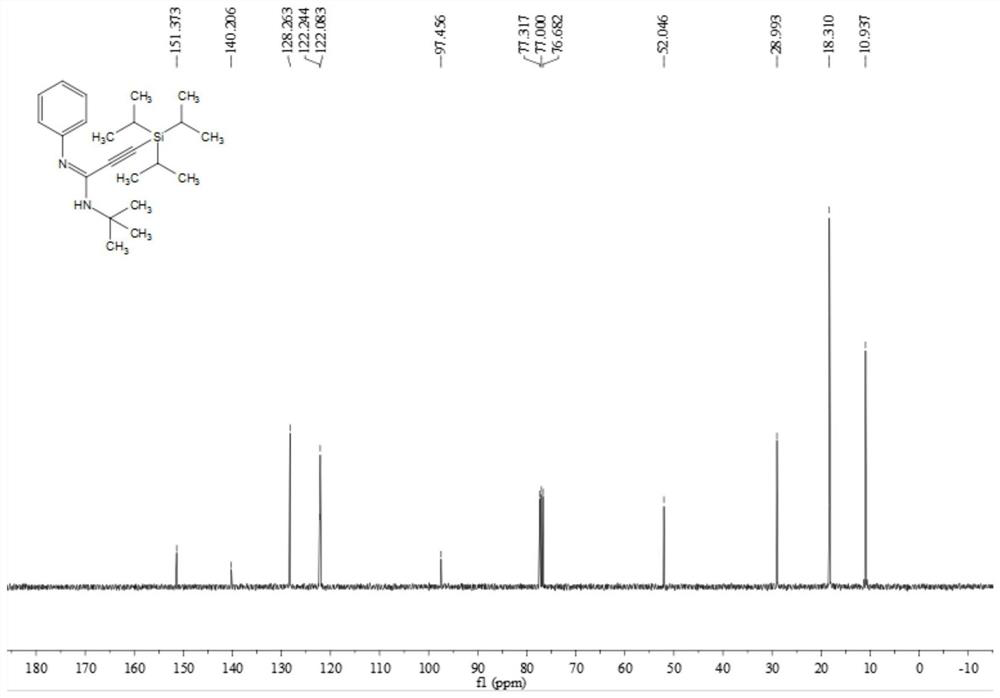
Poly-substituted alkynyl amidine compounds, preparation method and application thereof
ActiveCN110317221AImprove toleranceRaw materials are easy to obtainGroup 4/14 element organic compoundsBulk chemical productionOrganic solventOrganic synthesis
The invention belongs to the technical field of organic synthesis, and discloses poly-substituted alkynyl amidine compounds, a preparation method and an application thereof. The preparation method includes adding and dissolving an alkynyl bromide palladium salt catalyst, an additive and alkali in an organic solvent in a reactor, finally, adding isonitrile and stirring the mixture to react at 90-100 DEG C, and separating and purifying the reaction product to obtain the poly-substituted alkynyl amidine compounds, wherein the reaction formula is shown in a formula (I). The method uses simple andeasily available alkynyl bromide, amine and isonitrile as reaction raw materials to synthesize a series of poly-substituted alkynyl amidine compounds. The method has the characteristics of simple andeasily-available raw materials, convenient operation, mild conditions, high step economy, wide substrate applicability, good tolerance of functional groups and the like; and some of application studies of the method are carried out and a variety of useful nitrogen-containing heterocycles are synthesized.
Owner:SOUTH CHINA UNIV OF TECH
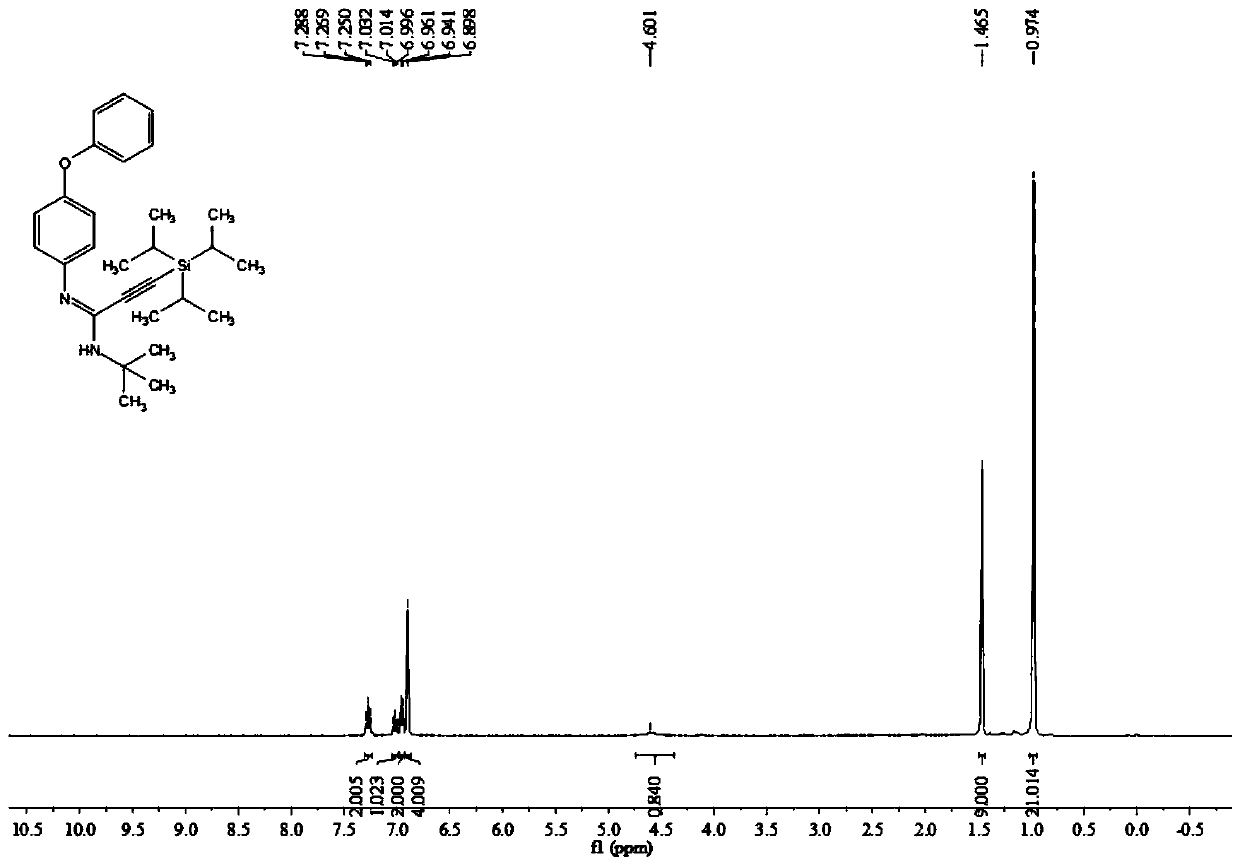
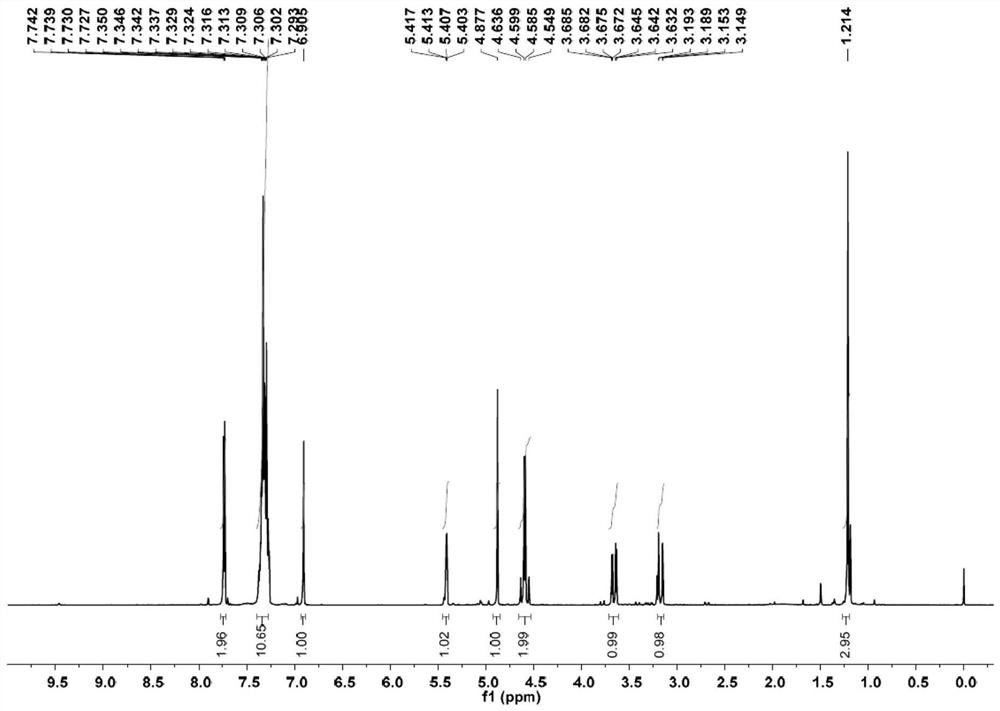
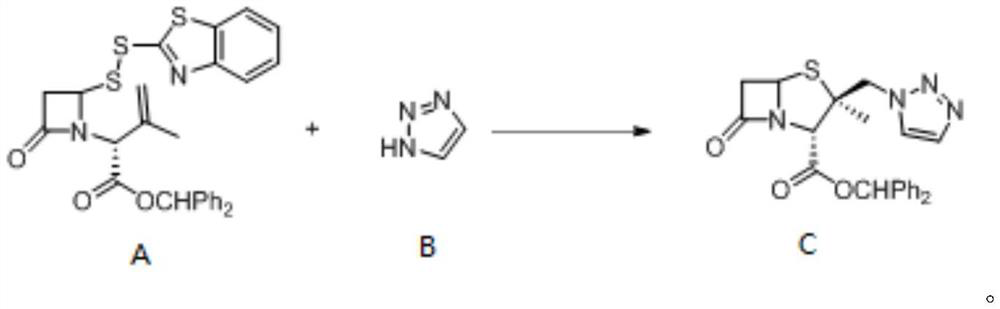
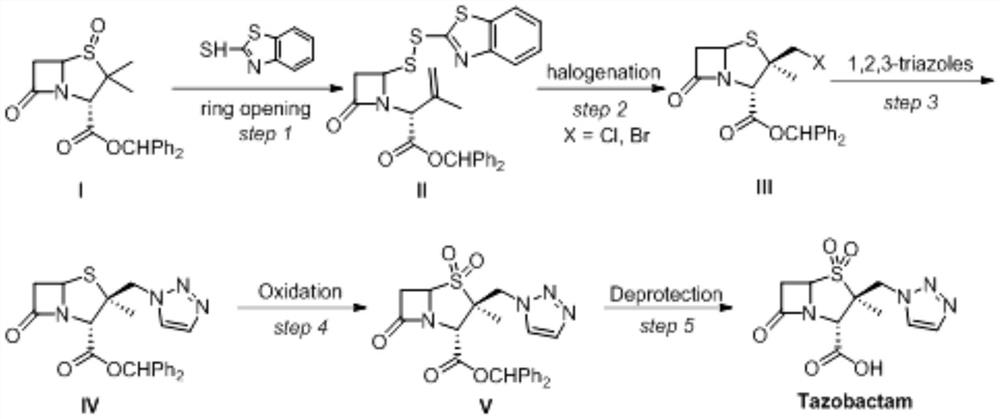
Method for electrochemical synthesis of tazobactam key intermediate
ActiveCN113073348AHigh selectivityFew reaction stepsAnodisationElectrolysis componentsChemical synthesisCompound a
The invention provides a method for electrochemical synthesis of a tazobactam key intermediate. The method comprises the following steps: by taking a disulfide ring-opening compound A and 1, 2, 3-triazole B as reaction substrates, mixing the reaction substrates, electrolyte and a reaction solvent, and then performing electrochemical anodic oxidation to obtain the tazobactam key intermediate C. The method effectively solves the problems of low selectivity, low reaction efficiency or poor economical efficiency and the like during synthesis of 2 beta-triazole methyl penicillic acid diphenylmethyl ester in the prior art.
Owner:JINLIN ASYMCHEM PHARM CO LTD
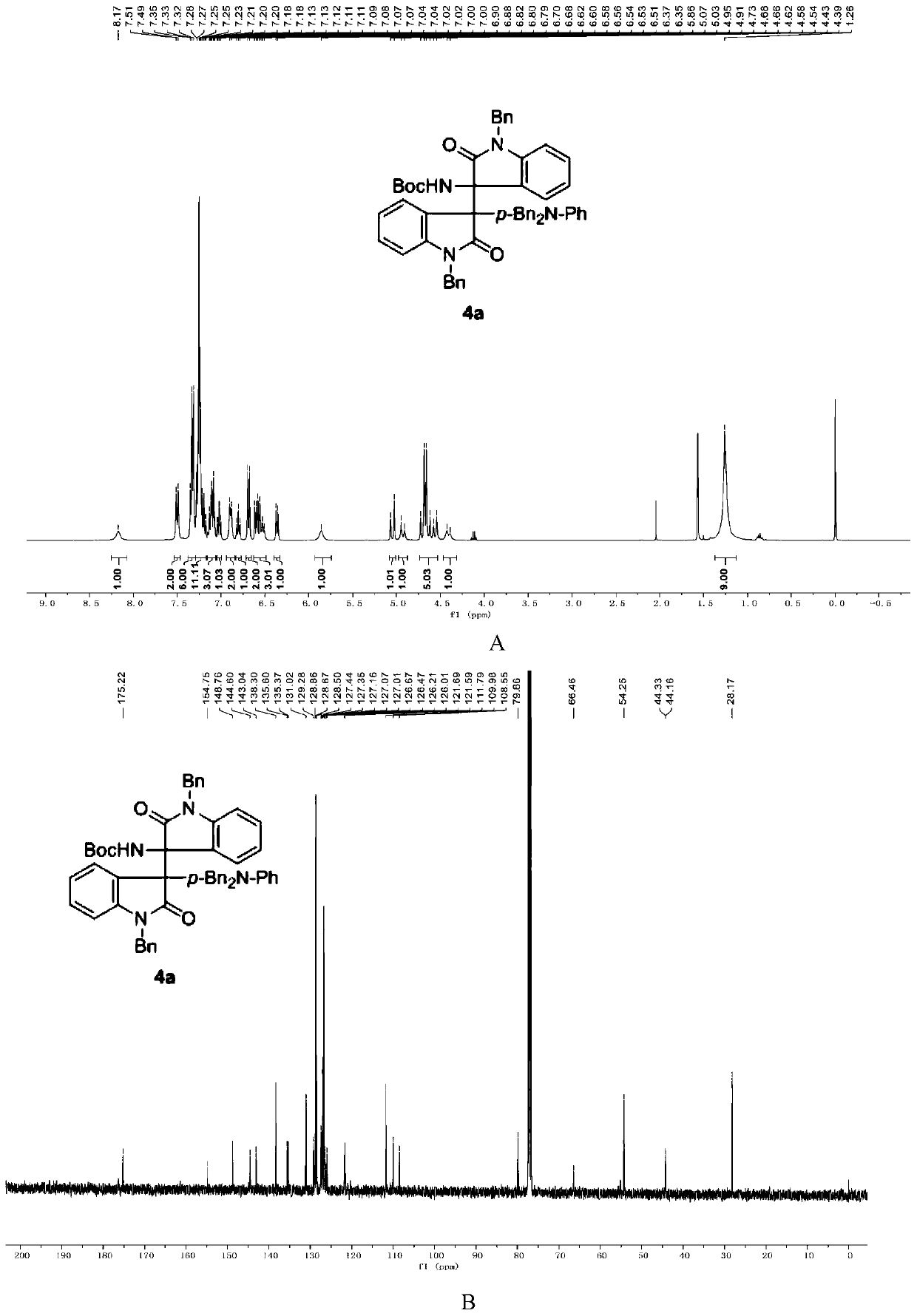
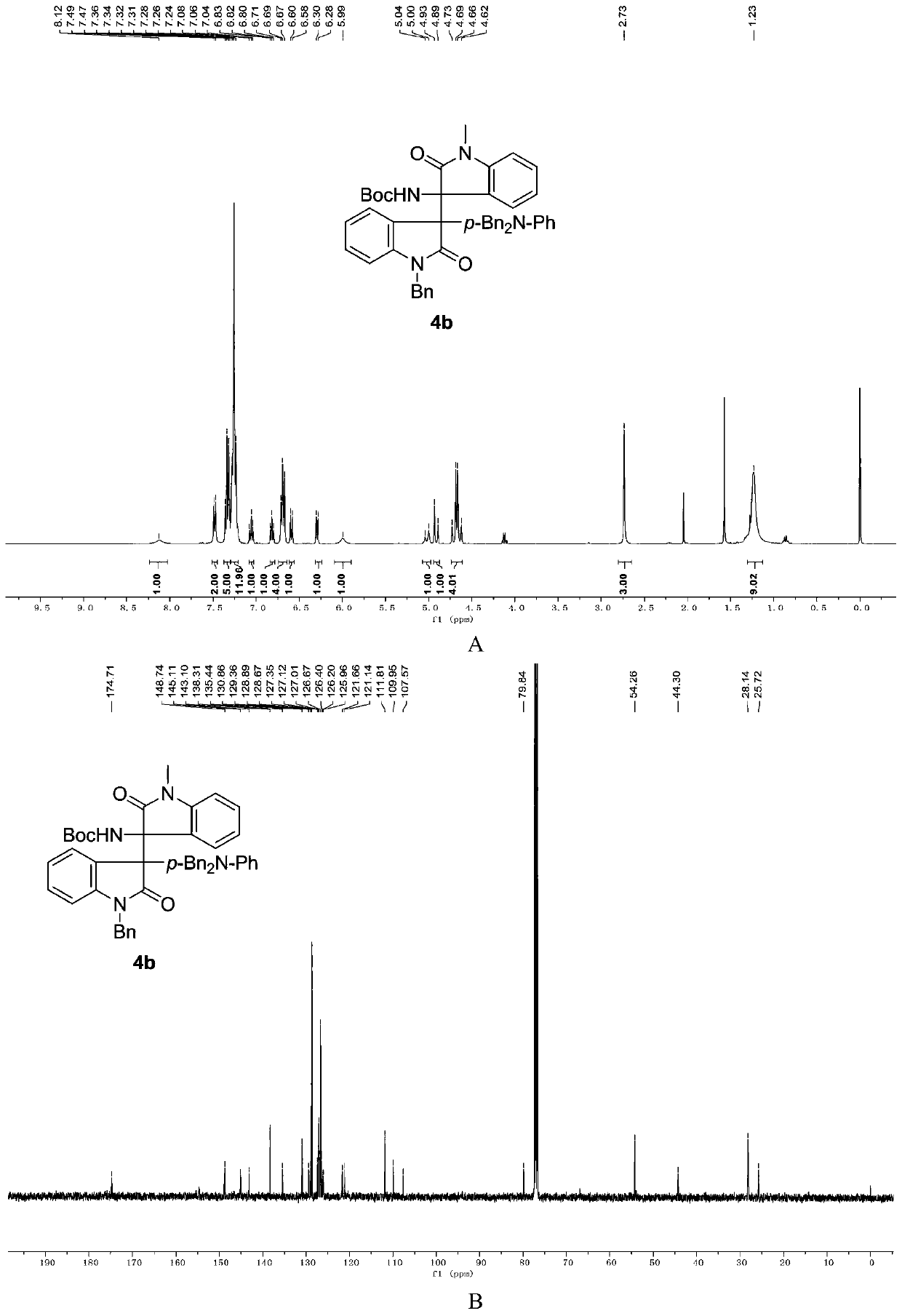
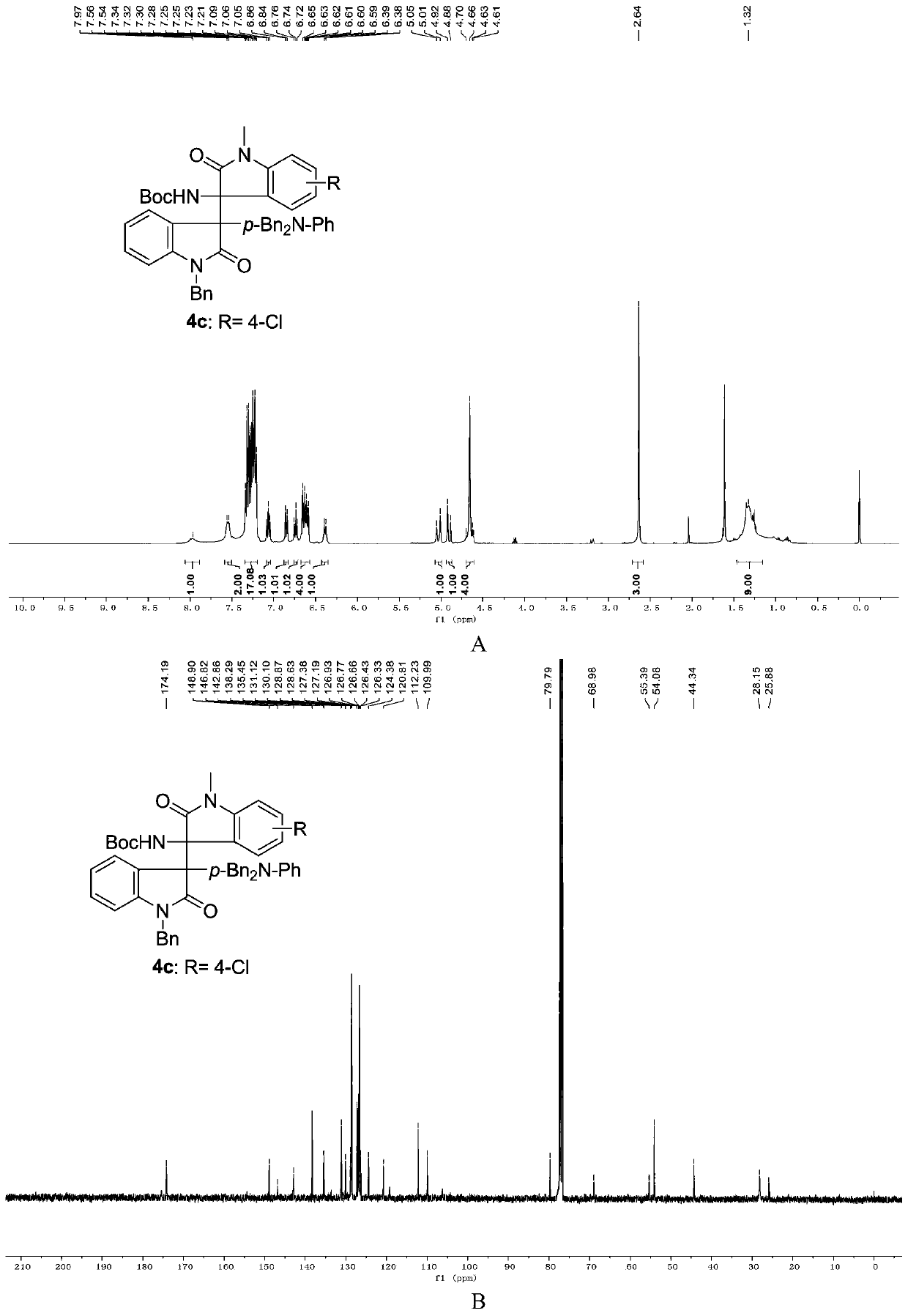
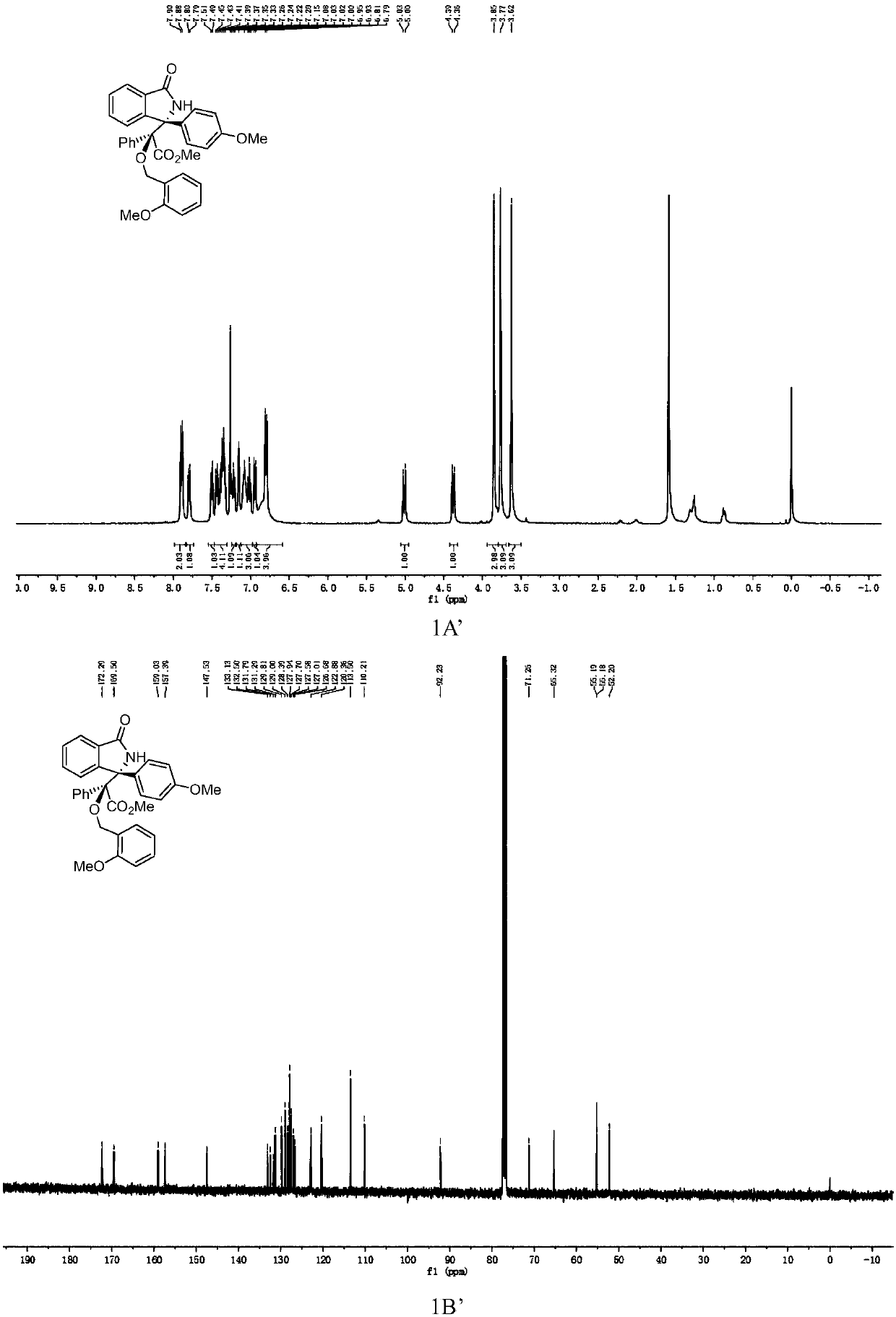
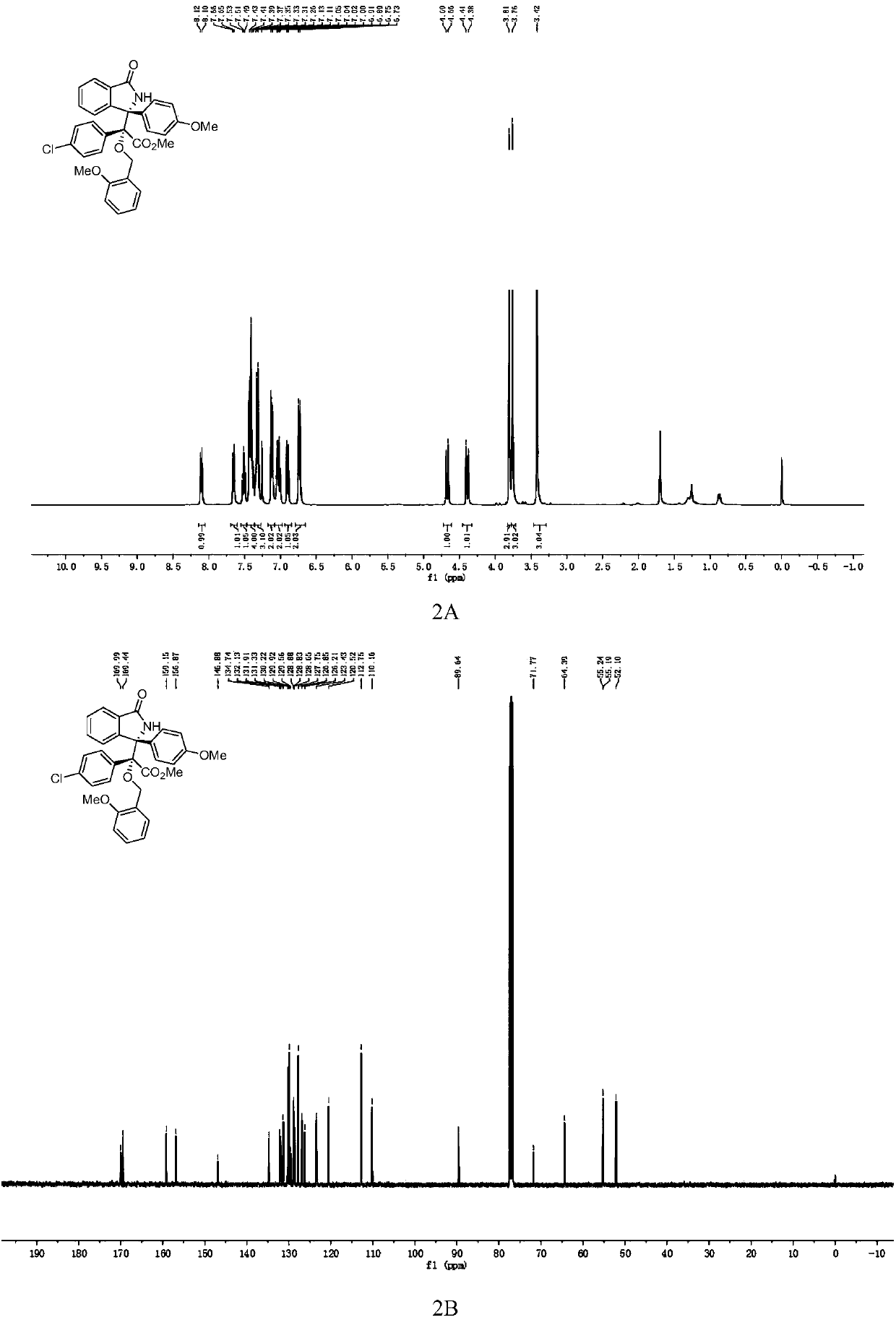
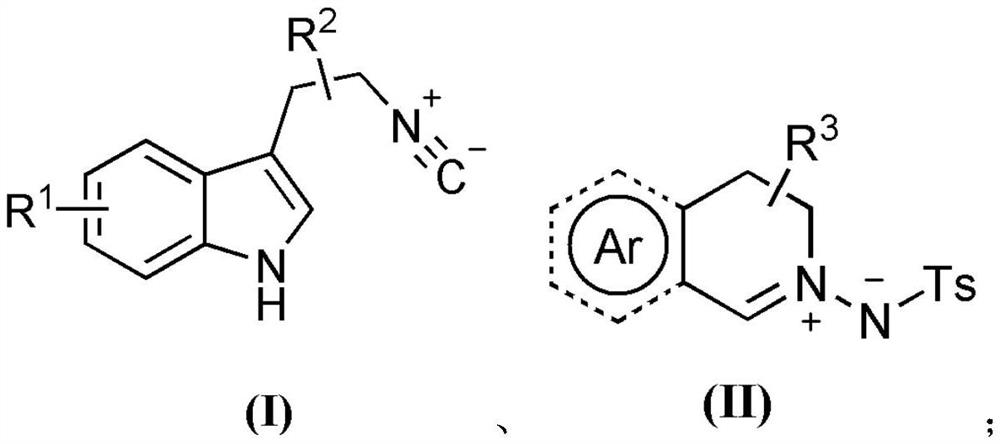
3-aryl-3'-amino bis(quaternary carbon) bis(oxindole) compounds and synthetic method and applications thereof
ActiveCN109776384AMild reaction conditionsFew reaction stepsNervous disorderOrganic chemistrySolventHigh selectivity
3-aryl-3'-amino bis(quaternary carbon) bis(oxindole) compounds and a synthetic method and applications thereof are disclosed. The compounds are prepared from diazo compounds, N,N-disubstituted anilineand isatin imine compound by adopting rhodium acetate as a catalyst, an organic solvent as a solvent and a 4A molecular sieve as an additive through one-step reaction at room temperature. The methodhas advantages of efficient atom economy, high selectivity, a low dosage of the catalyst, simple and safe operation, and the like. The synthesized bis(quaternary carbon) bis(oxindole) compounds can beadopted as important medical and chemical intermediates and have wide application prospects in the medicinal field.
Owner:EAST CHINA NORMAL UNIV
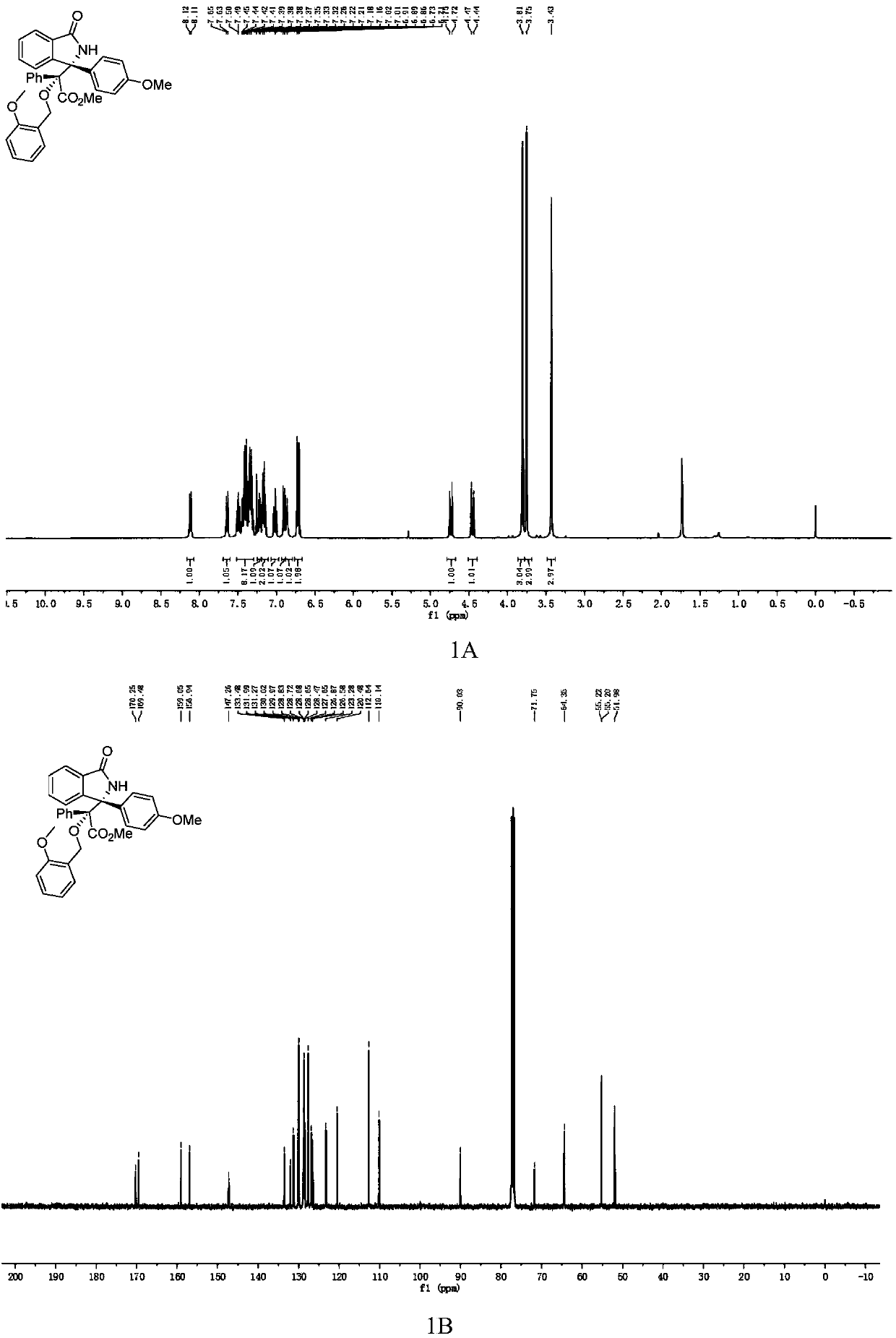
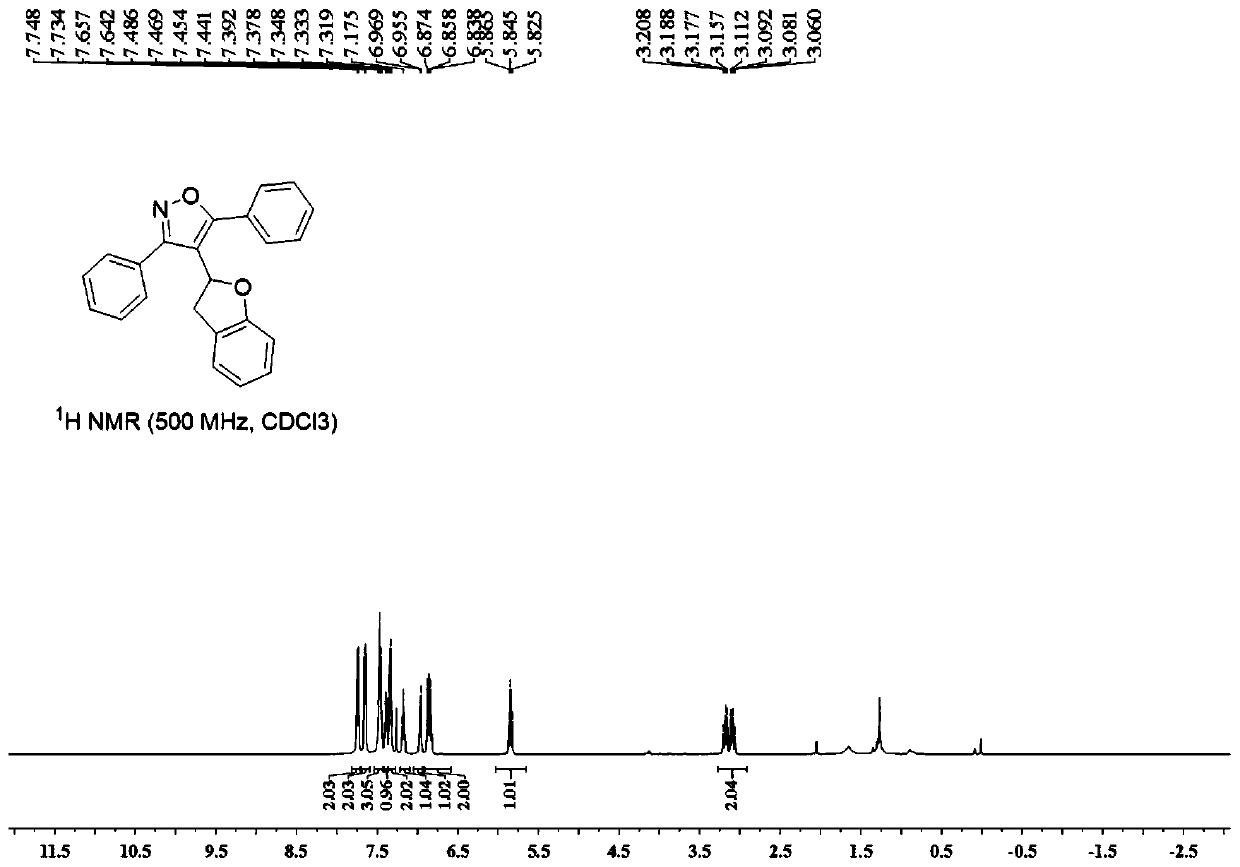
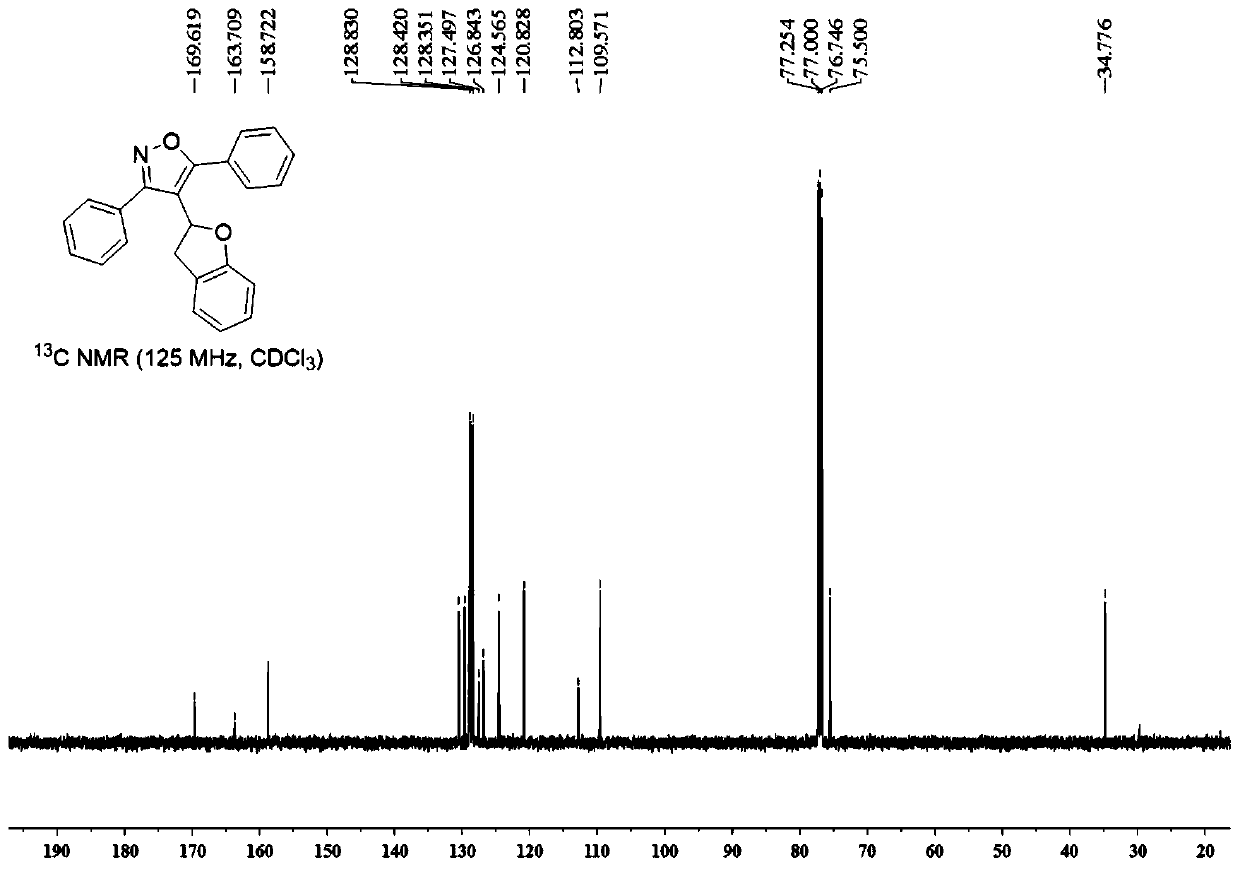
Chiral 3,3-disubstituted isoindoline-1-one derivative, synthesis method and applications thereof
InactiveCN108383771AHigh chiralityAtom economy is highOrganic chemistry methodsAntineoplastic agentsSynthesis methodsPhosphoric acid
The invention discloses a chiral 3,3-disubstituted isoindoline-1-one derivative and a synthesis method thereof. According to the analysis method, a diazo compound, a 3-hydroxyisoindoline-1-one and a benzyl alcohol are used as raw materials, a metal Lewis acid and chiral phosphoric acid are used as catalysts, and a one-step reaction is performed in an organic solvent at a temperature of -20-0 DEG Cby using a molecular sieve defined in the specification as water absorbing agent to obtain the chiral 3,3-disubstituted isoindoline-1-one derivative. According to the present invention, the synthesismethod has advantages of efficient atomic economy, high selectivity, low catalyst consumption, simple and safe operation and the like, and the synthesized chiral 3,3-disubstituted isoindoline-1-one derivative is the important chemical and pharmaceutical intermediate.
Owner:EAST CHINA NORMAL UNIV
Synthetic method for imidazole[1,2-a]pyridine and 2-butyl-5-chloro-1H-imidazole-4-carboxaldehyde compounds
The invention discloses a novel synthetic method for 3-formyl-imidazole[1,2-a]pyridine and 2-butyl-5-chloro-1H-imidazole-4-carboxaldehyde, and effective application of the method into synthesis of Necopidem and Saripidem. According to the invention, a 3-formyl-imidazole[1,2-a]pyridine system is established by using a cheap and low toxic Cu-catalyed O2 activated intramolecular olefin under dehydrogenation ammoxidation; conditions for the method are mild, reaction substrates are easily available, a catalytic system is simple, operation is simple, tolerance of functional groups is good, and the method is economical and effective and has very important scientific values and realistic significance.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
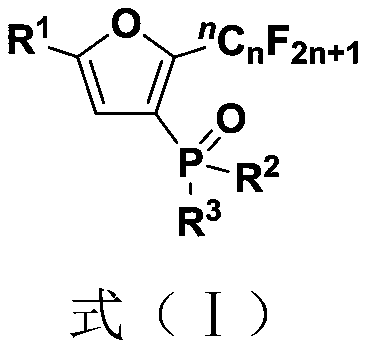
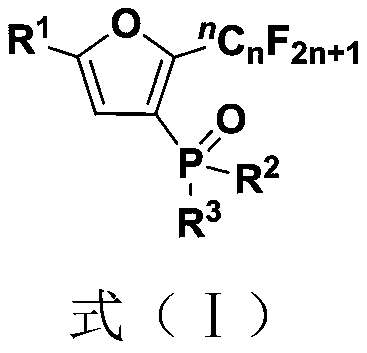
Polysubstituted benzodihydrofuro heterocyclic compound and preparation method and application thereof
ActiveCN111205279AImprove toleranceRaw materials are easy to obtainOrganic chemistryFuranPtru catalyst
The invention belongs to the technical field of organic synthesis, and discloses a polysubstituted benzodihydrofuro heterocyclic compound and a preparation method and application thereof. The preparation method comprises the following steps: adding a palladium salt catalyst, an oxidizing agent, an additive and alkali into a reactor, performing dissolving into an organic solvent, performing stirring to react at 50-70 DEG C, and separating and purifying the reaction product to obtain the polysubstituted benzodihydrofuro heterocyclic compound, the reaction formula of the preparation method is shown as a formula (I). According to the method, the alkyne ketoxime ether and the o-iodophenyl alkenyl ether which are simple and easy to obtain are used as reaction raw materials to synthesize a seriesof polysubstituted benzodihydrofuro heterocyclic compounds, and the method has the characteristics of simple and easy-to-obtain raw materials, convenience in operation, mild conditions, high step economy, wide substrate applicability, good functional group tolerance and the like.
Owner:SOUTH CHINA UNIV OF TECH
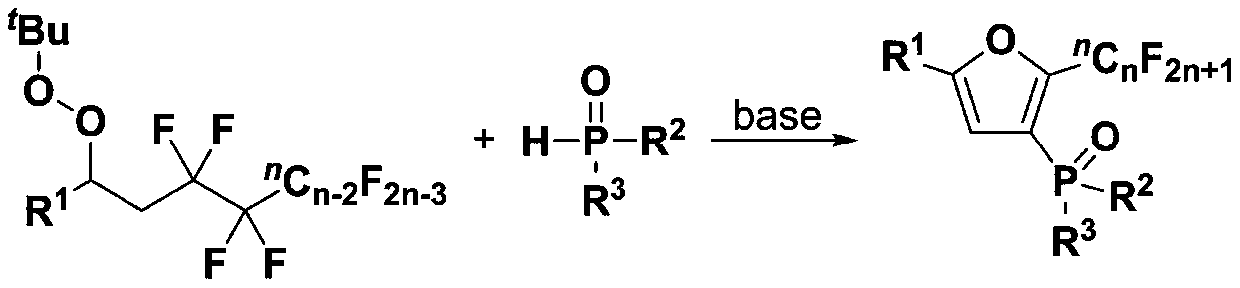
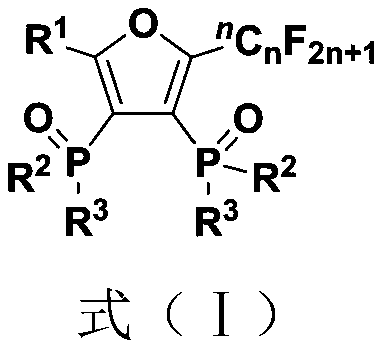
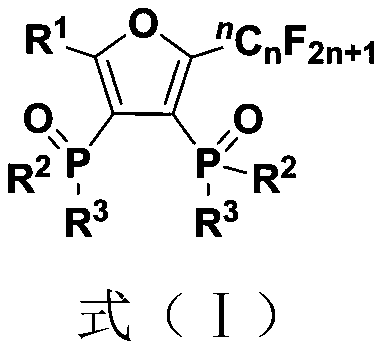
Fluoroalkyl substituted furyl diphosphine oxide compound and preparation method thereof
InactiveCN111574564AWide variety of sourcesEasy to manufactureGroup 5/15 element organic compoundsFuranPhosphine oxide
The invention discloses a fluoroalkyl substituted furyl diphosphine oxide compound and a preparation method thereof. The preparation method comprises the following steps: adding an alkali accelerant and a solvent into a reaction raw material formed by mixing a polyfluoroalkyl substituted peroxide compound and an organic phosphine oxide compound, carrying out stirring and reacting for 1-24 hours inan air atmosphere at 50-90 DEG C, and determining a reaction process by using TLC detection, wherein a reaction product is obtained after the reaction is finished, a molar volume ratio of the polyfluoroalkyl substituted peroxide compound to the organic phosphine oxide compound to the alkali accelerant to the solvent is 1 mmol: (1-3) mmol: (2-6) mmol: (1-3) mL, and the solvent comprises dimethyl sulfoxide and N,N-dimethyl formamide; and washing, extracting and drying the reaction product, and carrying out column chromatography separation to obtain the fluoroalkyl substituted furyl diphosphineoxide compound. According to the method disclosed by the invention, required initial raw materials are simple, wide in source and easy to prepare, and the obtained diphosphine oxide compound has a wide application prospects in the fields of drug synthesis and material research and in related extension fields.
Owner:NANJING UNIV OF TECH
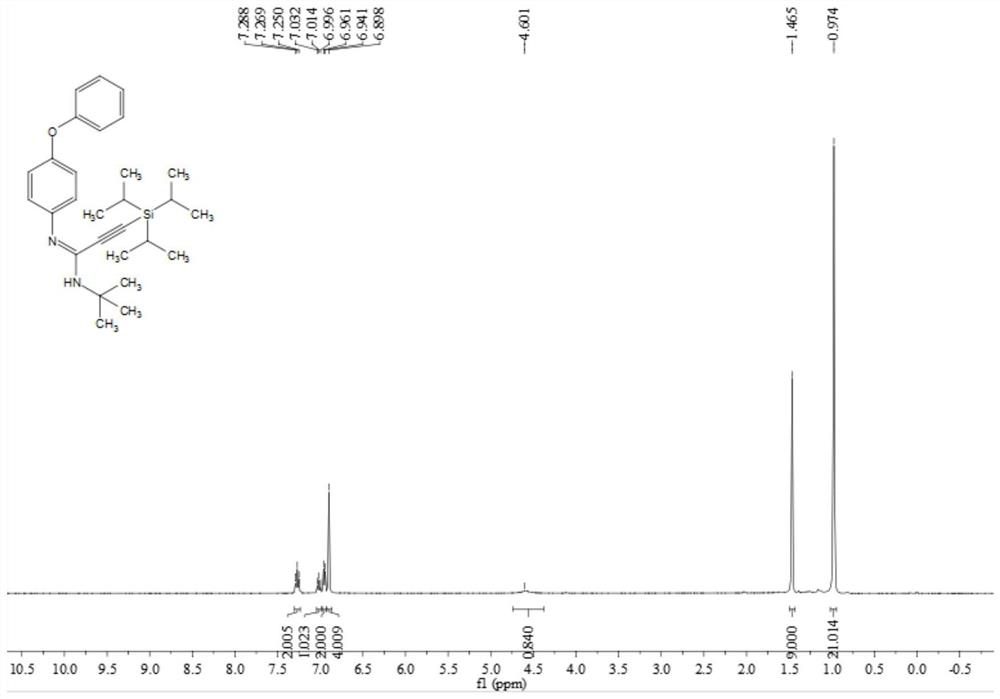
A kind of multi-substituted alkyne amidine compound and its preparation method and application
ActiveCN110317221BImprove toleranceRaw materials are easy to obtainGroup 4/14 element organic compoundsBulk chemical productionOrganic solventPtru catalyst
The invention belongs to the technical field of organic synthesis, and discloses a multi-substituted alkyne amidine compound, a preparation method and application thereof. In the reactor, add alkyne bromide palladium salt catalyst, additives and alkali to dissolve in the organic solvent, and finally add isonitrile and stir the reaction at 90-100°C, the reaction product is separated and purified to obtain polysubstituted alkyne amidine compounds, the above preparation The reaction formula of method is shown in formula (I). The method of the present invention synthesizes a series of multi-substituted alkyne amidine compounds with simple and easy-to-obtain alkyne bromide, amine, and isonitrile as reaction raw materials. The method has the advantages of simple and easy-to-obtain raw materials, convenient operation, mild conditions, high step economy, and It has the characteristics of wide applicability and good tolerance of functional groups; and carried out some application research, and synthesized a variety of useful nitrogen-containing heterocycles.
Owner:SOUTH CHINA UNIV OF TECH
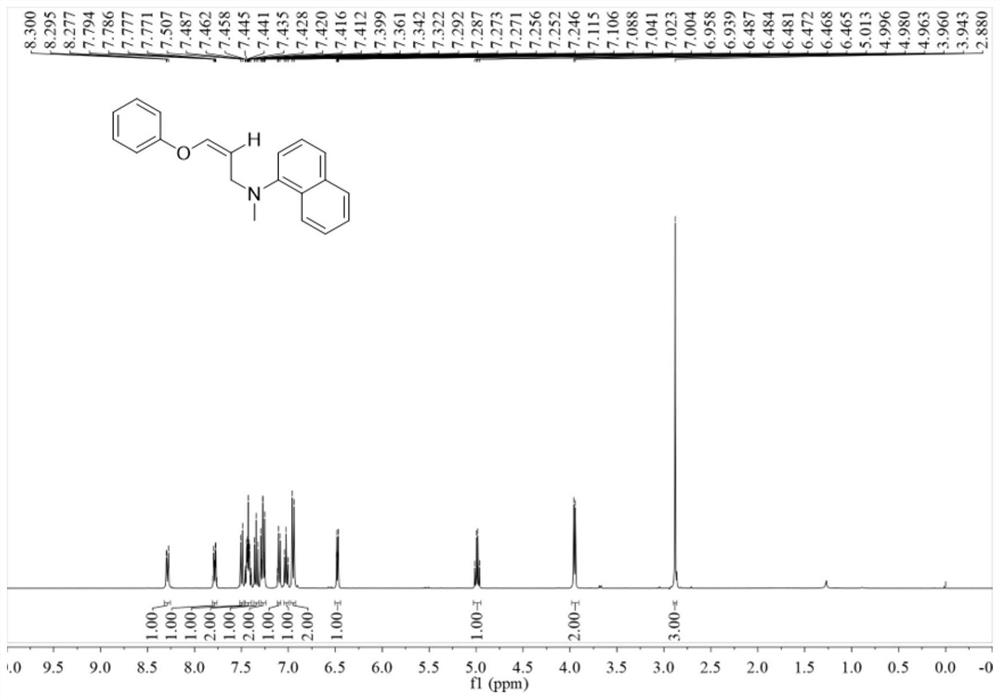
Method for primary amine-directed construction of 6-(4-bromo-2-fluorophenyl)indolo[1,2-a]quinoxaline
The invention relates to a method for primary amine-directed construction of 6-(4-bromo-2-fluorophenyl)indolo[1,2-a]quinoxaline. In a reaction test tube, 2-(1H-indol-1-yl)benzenamine and 4-bromo-2-fluorobenzoyl formic acid are added and taken as raw materials, and an acylation / cyclization reaction is carried out to prepare the 6-(4-bromo-2-fluorophenyl)indolo[1,2-a]quinoxaline in a high yield mode. The method for the primary amine-directed construction of the 6-(4-bromo-2-fluorophenyl)indolo[1,2-a]quinoxaline has the advantages that primary amine is taken as a traceless directional base to increase the step economy of the reaction; the raw materials required for the reaction are cheap and easy to obtain, the conditions are mild, the operation is simple, the selectivity is single, the separation yield is high, the industrial production is facilitated, and the method meets the development needs of green organic chemistry.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
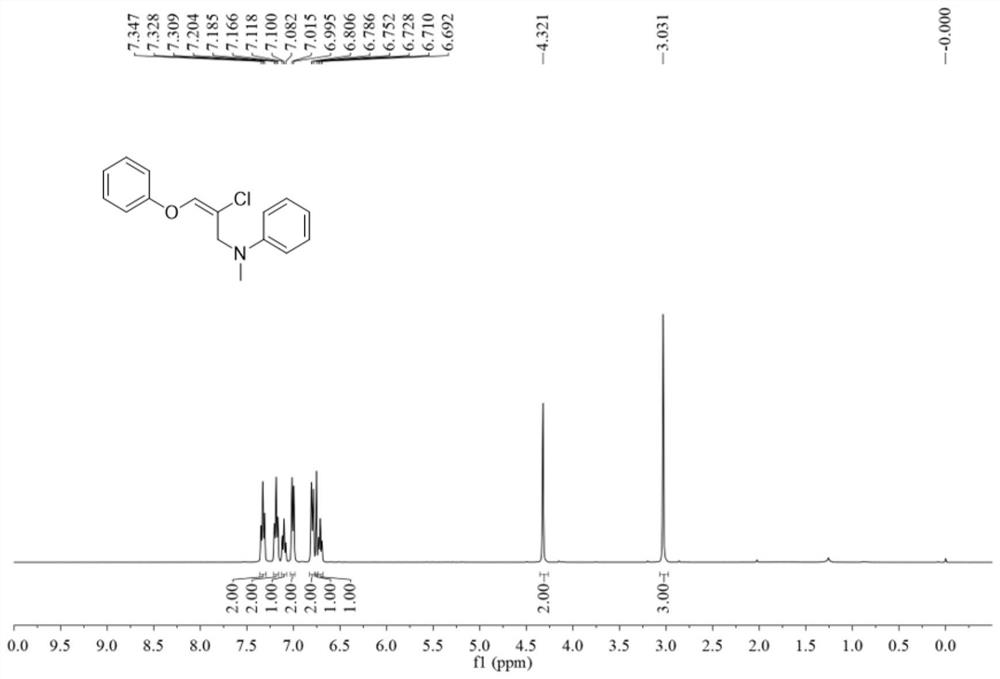
Method for constructing 2-chloro-6-phenylindolo[1,2-a]quinoxaline by primary amine directing
InactiveCN109503596AReduce usageMild reaction conditionsOrganic chemistryQuinoxalineBenzoylformic acid
The invention discloses a method for constructing 2-chloro-6-phenylindolo[1,2-a]quinoxaline by primary amine directing. 4-chloro-2-(1H-indole-1-yl)aniline and benzoyl formic acid are added into a reaction tube and used as raw materials, and high-yield 2-chloro-6-phenylindolo[1,2-a]quinoxaline is obtained by acylation / cyclization reaction. According to the method, primary amine is used as a traceless directing group, and thus the economical efficiency of the reaction steps is increased; and the raw materials required for the reaction are cheap and easy to obtain, conditions are mild, the operation is simple, the selectivity is single and the separation yield is high, therefore, the method facilitates industrial production and is in line with the development needs of green organic chemistry.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
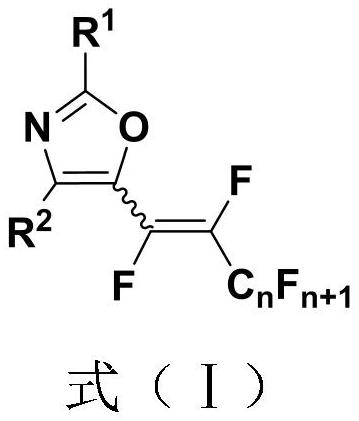
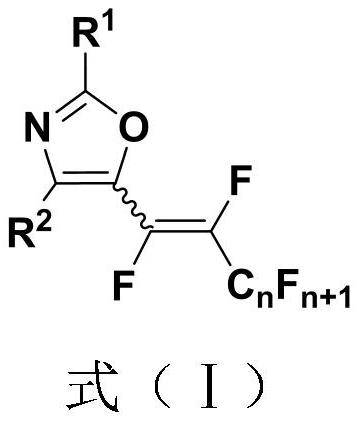
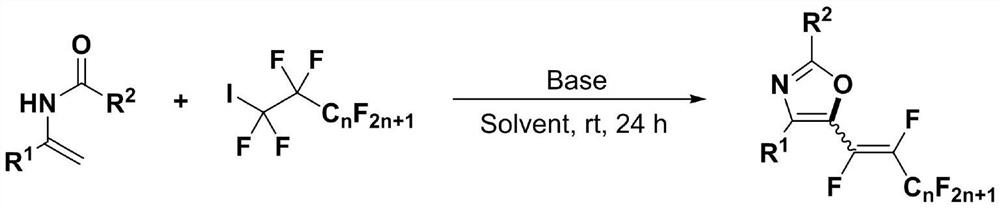
Polyfluoroalkenyl substituted oxazole compound and preparation method thereof
InactiveCN111747904AEfficient formationDiverse functional group toleranceOrganic chemistryPtru catalystNatural product
The invention discloses a polyfluoroalkenyl substituted oxazole compound and a preparation method thereof. The preparation method comprises the following steps: adding a reaction raw material formed by mixing an N-acyl enamine compound and a perfluoroalkyl iodide into an alkali accelerant and a solvent, stirring and reacting for 1-24 hours in an air atmosphere at room temperature, determining a reaction process by TLC detection, obtaining a reaction solution after the reaction is finished, finally, sequentially washing, extracting and drying the reaction solution, and carrying out column chromatography separation to obtain the polyfluoroalkenyl substituted oxazole compound. According to the preparation method, the required raw materials are simple and easy to obtain, various functional group tolerance and substrate ranges are achieved, and natural products and pharmaceutical active molecular skeletons can be introduced, the preparation method disclosed by the invention is mild in reaction condition, does not need an expensive transition metal catalyst, and meets the requirements of green and economic chemistry, and according to the preparation method, a five-membered oxazole heterocyclic ring does not need to be constructed and synthesized in advance, and the highest step economy is achieved.
Owner:NANJING UNIV OF TECH
A kind of multi-substituted benzodihydrofuranoheterocyclic compound and its preparation method and application
ActiveCN111205279BImprove toleranceRaw materials are easy to obtainOrganic chemistryFuranPtru catalyst
The invention belongs to the technical field of organic synthesis, and discloses a multi-substituted benzodihydrofuranoheterocyclic compound, a preparation method and application thereof. Add palladium salt catalyst, oxidizing agent, additive and base into the reactor, then add dissolved in organic solvent, stir and react at 50-70°C, the reaction product is separated and purified to obtain multi-substituted benzodihydrofuranoheterocycles compound, the reaction formula of the above preparation method is shown in formula (I). The method of the present invention synthesizes a series of multi-substituted chromanheterocyclic compounds with simple and easy-to-obtain acetylenone oxime ether and o-iodophenylenyl ether as reaction raw materials. The method has the advantages of simple and easy-to-obtain raw materials, easy operation Convenience, mild conditions, high step economy, wide substrate applicability, good functional group tolerance, etc.
Owner:SOUTH CHINA UNIV OF TECH
A kind of halogenated oxalylamine compound and its preparation method and application
ActiveCN112441934BImprove toleranceRaw materials are easy to obtainOrganic compound preparationAmino-hyroxy compound preparationOrganic synthesisPropylamine
The invention belongs to the technical field of organic synthesis, and discloses a halogenated oxalylamine compound, a preparation method and application thereof. Add copper halide into the reactor, then add it dissolved in an organic solvent, stir and react at 35-45°C, the reaction product is separated and purified to obtain a halogenated oxaallylamine compound, the reaction formula of the above preparation method is as follows: Shown in formula (I). The method of the present invention synthesizes a series of halogenated oxalylamine compounds by using simple and easy-to-obtain allene ethers and aromatic amines as reaction raw materials. The method has the advantages of simple and easy-to-obtain raw materials, convenient operation, mild conditions, and high step economy , wide substrate applicability, and good functional group tolerance.
Owner:SOUTH CHINA UNIV OF TECH
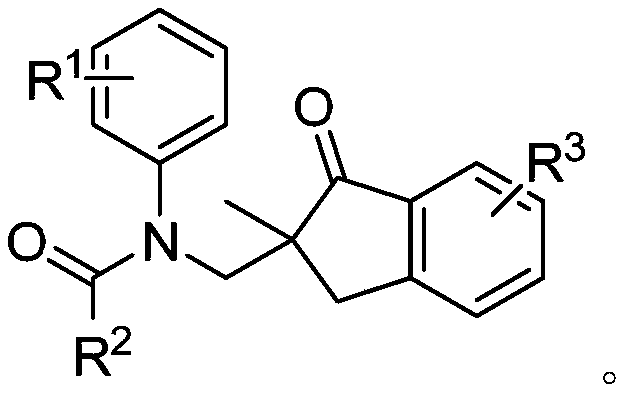
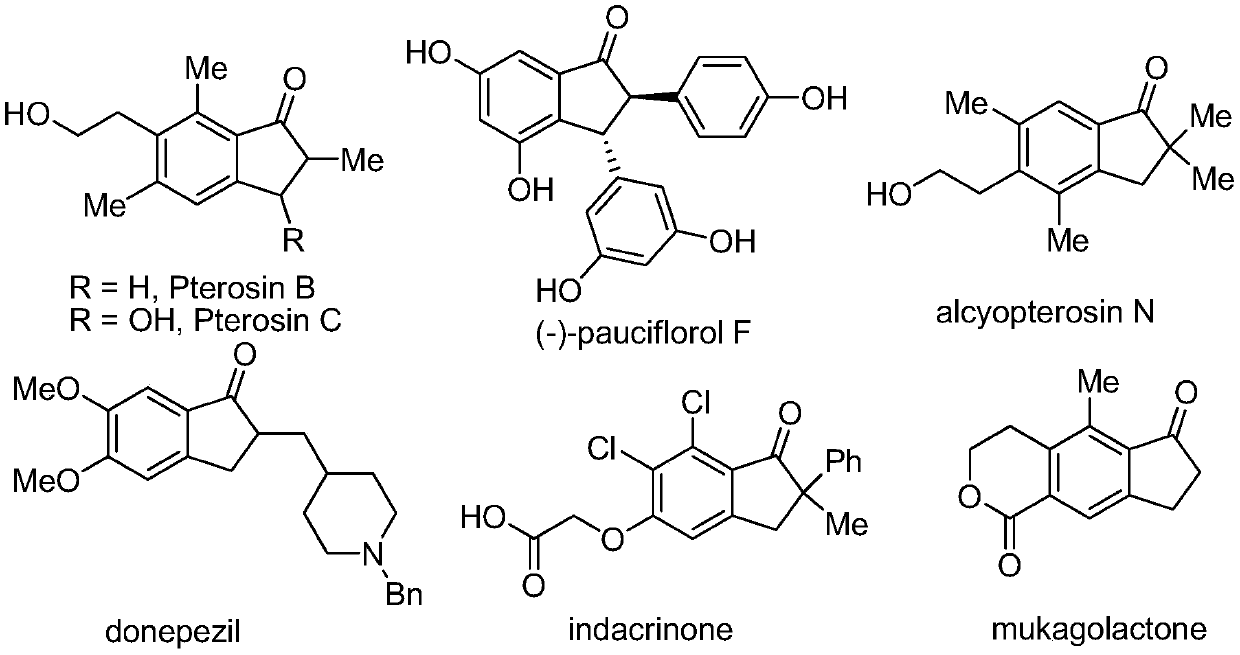
Method for synthesizing 2,2-disubstituted indanone compound
InactiveCN110330442AHighly economical stepsImprove economyOrganic compound preparationCarboxylic acid amides preparationAcetonitrileSolvent
The invention belongs to the field of medicines, organic chemical industry and fine chemical industry and in particular discloses a method for synthesizing a 2,2-disubstituted indanone compound. The method comprises the following specific synthesis steps: taking an N-(2-methyl allyl) amide compound and a 3-aminoindazole compound as raw materials, and heating and reacting the raw materials in an acetonitrile solvent under nitrogen protection at a temperature of 50-100 DEG C for 5-15 hours in presence of a copper salt catalyst, an oxidizing agent and water, thereby obtaining the 2,2-disubstituted indanone compound. The reaction comprises processes such as denitrification, free radical addition and hydrolysis, and involves cleavage of two C-N bonds and formation of two C-C bonds. The method disclosed by the invention opens up a new way to synthesis of the indanone structure.
Owner:CHANGZHOU UNIV
Method for constructing 2-fluorine-6-phenyl indolo[1,2-a] quinoxaline through primary amine guiding
The invention discloses a method for constructing 2-fluorine-6-phenyl indolo[1,2-a] quinoxaline through primary amine guiding. The method comprises the following steps: adding 4-fluorine-2-(1H-indole-1-yl)phenylamine and benzoyl formic acid as the raw material in a reaction test tube, and preparing 2-fluorine-6-phenyl indolo[1,2-a] quinoxaline in high yield through acylation / cyclization reaction.The primary amine is used as a traceless guide base, and the step economy of the reaction is increased; the raw material required by the reaction is cheap and easy to obtain, the condition is mild andthe operation is simple, the selectivity is single, the separation efficiency is high, and the method is in favor of industrial production and conforms to the development demand of the green organicchemistry.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Method for constructing 6-(2-methylphenyl)indolo[1,2-a]quinoxaline guided by primary amine
The invention discloses a method for constructing 6-(2-methylphenyl)indolo[1,2-a]quinoxaline guided by primary amine. The method comprises the following steps: adding 2-(1H-indole-1-base)aniline and 2-methyl phenylglyoxylic acid as raw materials in a reaction test tube to prepare6-(2-methylphenyl)indolo[1,2-a]quinoxaline by virtue of acylation / cyclization reaction. According to the invention, theprimary amine is used as a traceless guiding group, so that the step economical performance of the reaction is improved; moreover, the raw materials required for the reaction are cheap and easily available, the conditions are mild, the operation is simple, the selectivity is single, the separation yield is high, the industrial production is facilitated, and the development requirement of green organic chemistry can be met.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
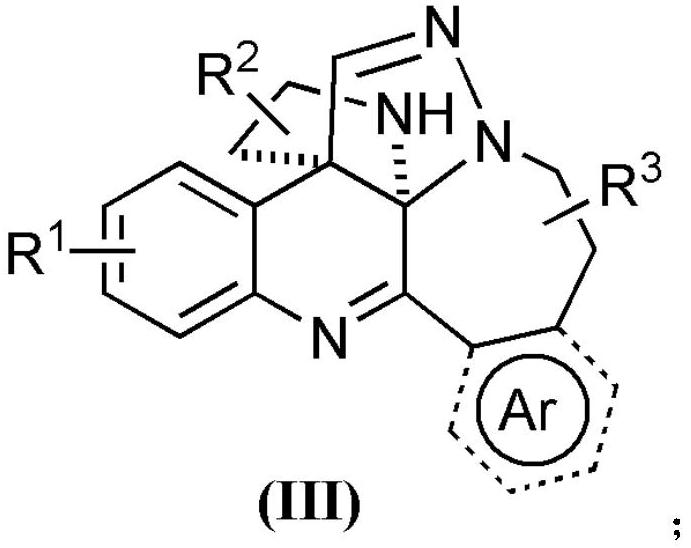
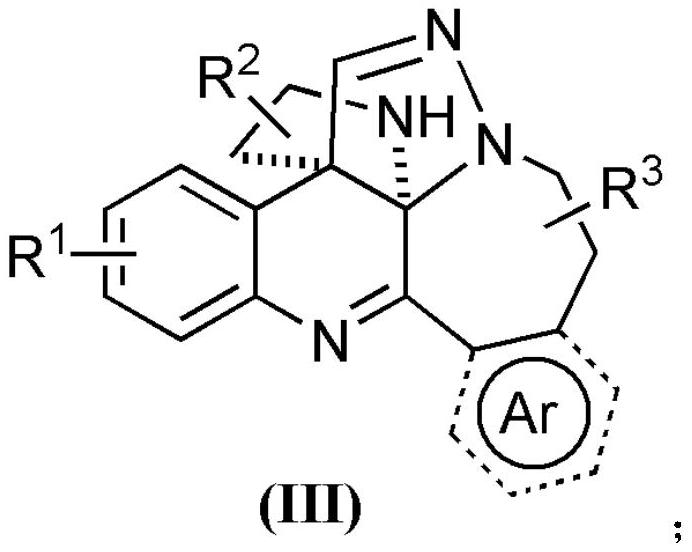
Polycyclic quinoline derivative as well as preparation and application thereof
ActiveCN113845523AHighly economical stepsAtom economy is highOrganic chemistryAntineoplastic agentsQuinolinePhenyl group
The invention relates to a polycyclic quinoline derivative as well as preparation and application thereof. The structure of the polycyclic quinoline derivative is shown as a formula (III), wherein R1 is selected from hydrogen, C1-C5 alkyl, halogen, C6-C10 aryl and C1-C5 alkoxy; R2 is selected from hydrogen, alkyl with 1 to 5 carbon atoms and alkoxycarbonyl with 1 to 5 carbon atoms; R3 is selected from hydrogen and C1-C5 alkyl; and the Ar, shown as the specification, is selected from phenyl, substituted phenyl, naphthyl, pyrrolyl or thienyl, and a substituent group on the substituted phenyl is selected from C1-C5 alkyl, phenyl or halogen. The polycyclic quinoline derivative disclosed by the invention is constructed by utilizing simple raw materials in one step, is mild in reaction condition, environment-friendly and simple to operate, and has tumor inhibition activity.
Owner:SUZHOU UNIV
3-aryl-3'-aminobiquaternary carbon bisoxindole compound and its synthesis method and application
ActiveCN109776384BBiopharmaceutical activityGood diastereoselectivityNervous disorderOrganic chemistrySolvent moleculePtru catalyst
The invention discloses a synthesis method and application of a 3-aryl-3'-aminobiquaternary carbobisoxindole compound, which uses diazo compounds, N,N-disubstituted anilines, and isatinimine compounds as raw materials. Using rhodium acetate as a catalyst, an organic solvent as a solvent, and molecular sieves as an additive, the biquaternary carbon bisoxindole compound is obtained through one-step reaction at room temperature. The synthesis method of the biquaternary carbon bisoxindole compound of the present invention has the advantages of efficient atom economy, high selectivity, low catalyst consumption, simple and safe operation, etc. The synthesized biquaternary carbon bisoxindole compound can be used as an important medicine and Chemical intermediates have broad application prospects in the pharmaceutical field.
Owner:EAST CHINA NORMAL UNIV
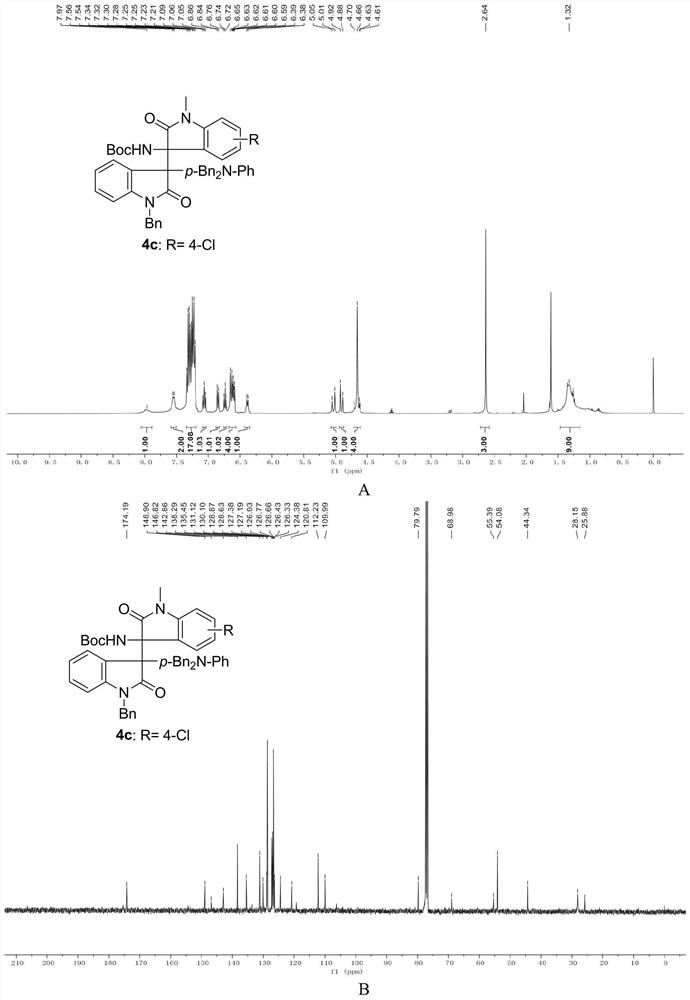
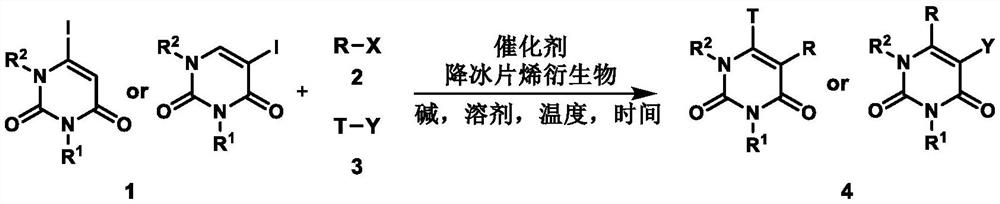
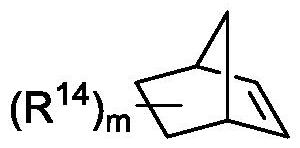
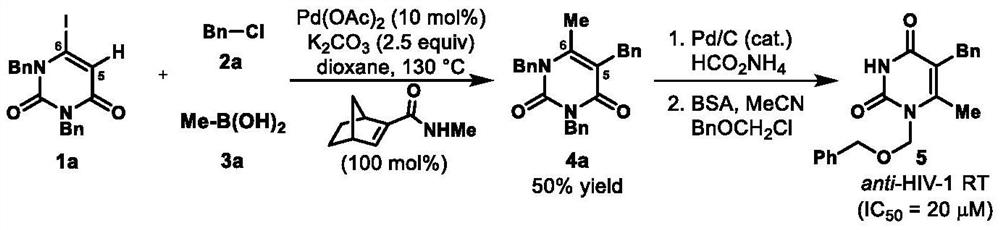
A method for preparing uracil and thymine derivatives
ActiveCN111909100BLow priceEasy to prepareGroup 4/14 element organic compoundsOrganic chemistry methodsAlkanePalladium catalyst
The invention provides a method for efficiently and diversifying the synthesis of uracil and thymine derivatives. In this method, iodouracil and thymine compounds, electrophiles (halogenated alkanes or brominated aromatic compounds), nucleophiles (alkenes, alkynes, borates, etc.), palladium catalysts, norbornene derivatized Dissolve the substance and alkali (potassium carbonate) together in an organic solvent (1,4-dioxane or ethylene glycol dimethyl ether), then stir and react at 50-150°C, and separate and purify after the reaction, which can be efficient and economical , Green synthesis of polysubstituted uracil and thymine compounds. The method has mild conditions, good substrate universality and high yield, and the prepared 2-pyridones and uracils are widely used in the fields of medicinal chemistry and organic chemistry. In addition, a method for synthesizing key intermediates of anti-HIV drugs is also provided, which improves step economy.
Owner:WUHAN UNIV
Method for constructing 8-fluoro-6-phenylindolo[1,2-a]quinoxaline by primary amine directing
InactiveCN109503602ASingle choiceHighly economical stepsOrganic chemistryQuinoxalineBenzoylformic acid
The invention relates to a method for constructing 8-fluoro-6-phenylindolo[1,2-a]quinoxaline by primary amine directing. 2-(4-chloro-1H-indole-1-yl)aniline and benzoyl formic acid are added into a reaction tube and used as raw materials, and high-yield 8-fluoro-6-phenylindolo[1,2-a]quinoxaline is obtained by acylation / cyclization reaction. According to the method, primary amine is used as a traceless directing group, and thus the economical efficiency of the reaction steps is increased; and the raw materials required for the reaction are cheap and easy to obtain, conditions are mild, the operation is simple, the selectivity is single and the separation yield is high, therefore, the method facilitates industrial production and is in line with the development needs of green organic chemistry.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Method for constructing 6-(furan-2-yl)indolo[1,2-a]quinoxaline through primary amine guidance
The invention relates to a method for constructing 6-(furan-2-yl)indolo[1,2-a]quinoxaline through primary amine guidance. The method comprises the following steps: adding 2-(1H-indole-1-yl)aniline and2-furoyl formic acid into a reaction test tube as raw materials, and carrying out acylation / cyclization reaction to prepare the 6-(furan-2-yl)indolo[1,2-a]quinoxaline in a high-yield manner. According to the method provided by the invention, primary amine is used as a traceless guidance group and the economic efficiency of steps of the reaction is increased; the raw materials needed by the reaction are cheap and easy to obtain, conditions are moderate and the operation is simple, the selectivity is single and the separation yield is high; industrial production is facilitated and the development requirements of green organic chemistry are met.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Method for constructing 6-(3-fluorophenyl)indolo[1,2-a]quinoxaline guided by primary amine
The invention relates to a method for constructing 6-(3-fluorophenyl)indolo[1,2-a]quinoxaline guided by primary amine. The method comprises the following steps: adding 2-(1H-indole-1-base)aniline and3-fluorobenzoylformic acid as raw materials in a reaction test tube to prepare 6-(3-fluorophenyl)indolo[1,2-a]quinoxaline by virtue of acylation / cyclization reaction. According to the invention, the primary amine is used as a traceless guiding group, so that the step economical performance of the reaction is improved; and moreover, the raw materials required for the reaction are cheap and easily available, the conditions are mild, the operation is simple, the selectivity is single, the separation yield is high, the industrial production is facilitated, and the development requirement of greenorganic chemistry can be met.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
A kind of oxyallylamine compound and its preparation method and application
ActiveCN112521289BImprove toleranceRaw materials are easy to obtainOrganic compound preparationAmino-hyroxy compound preparationPtru catalystOrganic synthesis
The invention belongs to the technical field of organic synthesis, and discloses an oxalylamine compound, a preparation method and application thereof. Add copper salt catalyst and additives into the reactor, then add dissolved organic solvent, stir and react at 40-60°C, the reaction product is separated and purified to obtain oxalylamine compounds, the reaction formula of the above preparation method As shown in formula (I). The method of the present invention synthesizes a series of oxalylamine compounds with simple and easy-to-obtain allene ethers and amines as reaction raw materials. The method has the advantages of simple and easy-to-obtain raw materials, convenient operation, mild conditions, high atom utilization rate, and economical steps. High stability, wide substrate applicability, good functional group tolerance, etc.
Owner:SOUTH CHINA UNIV OF TECH
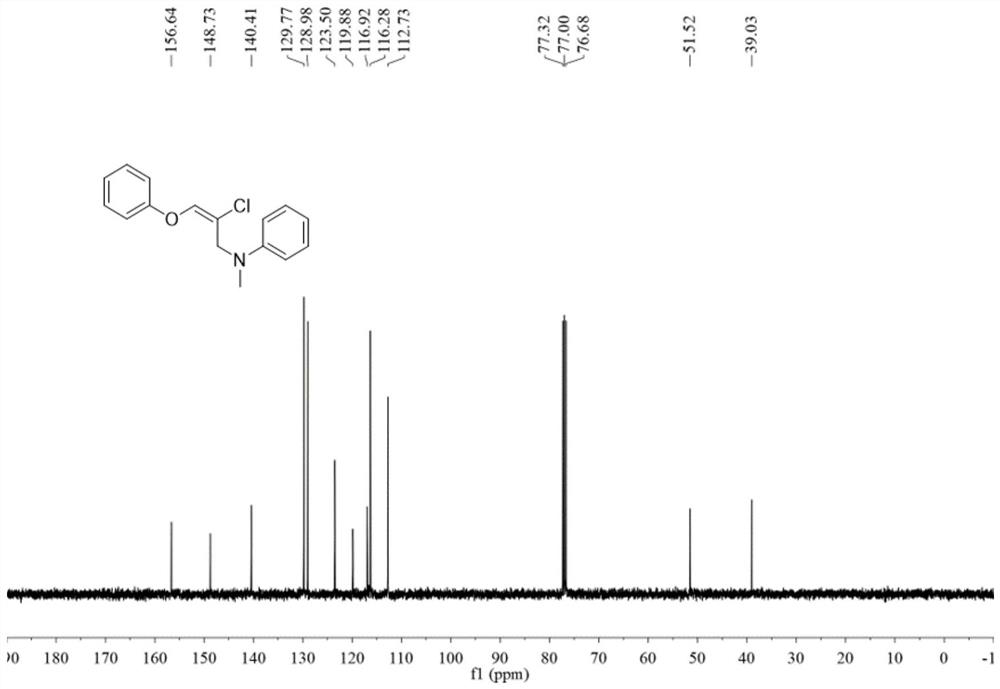
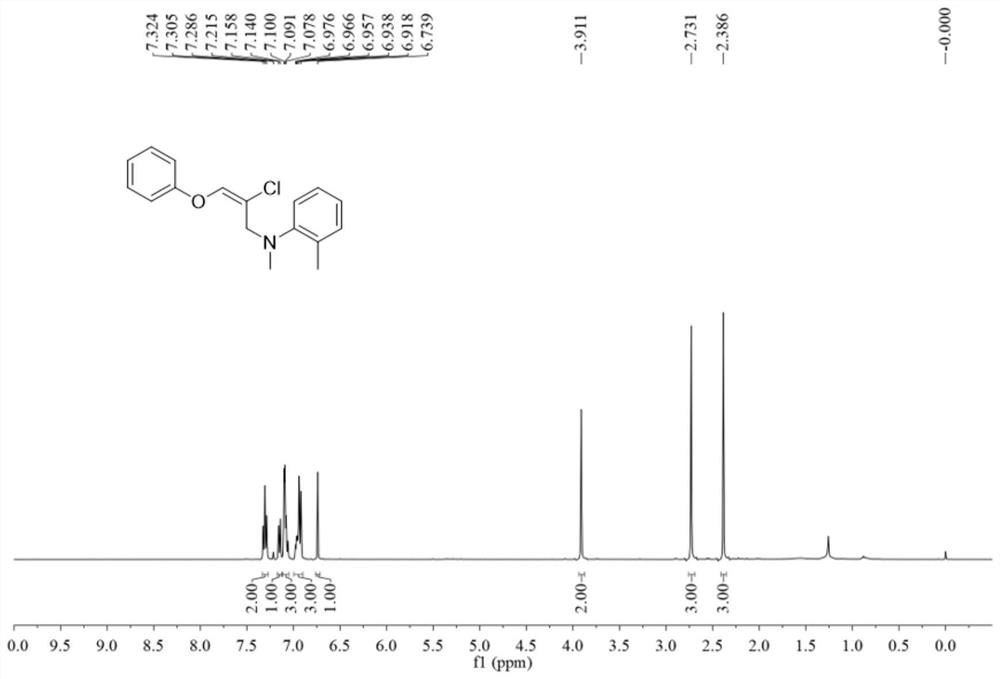
Halogenated oxaallylamine compound as well as preparation method and application thereof
ActiveCN112441934AImprove toleranceRaw materials are easy to obtainOrganic compound preparationAmino-hyroxy compound preparationOrganic solventOrganic synthesis
The invention belongs to the technical field of organic synthesis, and discloses a halogenated oxaallylamine compound as well as a preparation method and application thereof. The preparation method comprises the following steps: adding copper halide into a reactor, then adding an organic solvent, conducting dissolving, conducting stirring and reacting at 35-45 DEG C, and separating and purifying the reaction product to obtain the halogenated oxaallylamine compound. The reaction formula of the preparation method is as shown in a formula (I). According to the method, allene ether and aromatic amine which are simple and easy to obtain are used as reaction raw materials to synthesize a series of halogenated oxaallylamine compounds, and the method has the characteristics of simple and easy-to-obtain raw materials, convenience in operation, mild conditions, high step economy, wide substrate applicability, good functional group tolerance and the like.
Owner:SOUTH CHINA UNIV OF TECH
Fluoroalkyl substituted furyl monophosphine oxide compound and preparation method thereof
InactiveCN111560038AWide variety of sourcesEasy to manufactureGroup 5/15 element organic compoundsFuranPhosphine oxide
The invention discloses a fluoroalkyl substituted furyl monophosphine oxide compound and a preparation method thereof. The preparation method comprises the following steps: adding an alkali promoter and a solvent into a reaction raw material formed by mixing a polyfluoroalkyl substituted peroxide compound and an organic phosphine oxide compound, stirring and reacting for 1-24 h in an air atmosphere at room temperature, determining a reaction process by TLC detection, and obtaining a reaction product after the reaction is finished, wherein the molar volume ratio of the polyfluoroalkyl substituted peroxide compound to the organic phosphine oxide compound to the alkali promoter to the solvent is 1 mmol: (1-3) mmol: (2-6) mmol: (1-3) mL, and the solvent comprises chloroform, tert-butyl alcohol, acetonitrile and dichloromethane; and washing, extracting and drying the reaction product, and carrying out column chromatography separation to obtain the fluoroalkyl substituted furyl monophosphineoxide compound. The method disclosed by the invention is simple in required initial raw materials, wide in source and easy to prepare, and the obtained monophosphine oxide compound has a wide application prospect in the fields of drug synthesis, material research and related extension.
Owner:NANJING UNIV OF TECH
Polycyclic quinoline derivatives and their preparation and application
ActiveCN113845523BHighly economical stepsAtom economy is highOrganic chemistryAntineoplastic agentsArylQuinolizine
The present invention relates to a polycyclic quinoline derivative and its preparation and application. The structure of the polycyclic quinoline derivative is shown in formula (III): wherein, R 1 selected from hydrogen, C 1 ‑C 5 Alkyl, halogen, C 6 ‑C 10 Aryl, C 1 ‑C 5 alkoxy; R 2 selected from hydrogen, C 1 ‑C 5 Alkyl, C 1 ‑C 5 Alkoxycarbonyl; R 3 selected from hydrogen, C 1 ‑C 5 Alkyl; base is selected from phenyl, substituted phenyl, naphthyl, pyrrolyl or thienyl, and the substituent on substituted phenyl is selected from C 1 ‑C 5 Alkyl, phenyl or halogen. The polycyclic quinoline derivative of the present invention is constructed in one step using simple raw materials, has mild reaction conditions, is environmentally friendly, and is simple to operate, and the polycyclic quinoline derivative has tumor suppressing activity.
Owner:SUZHOU UNIV
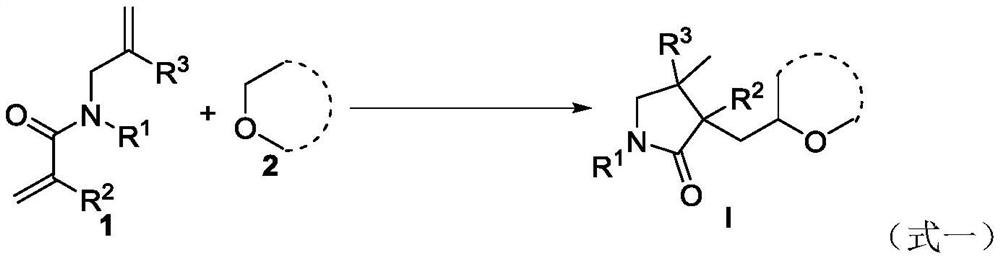
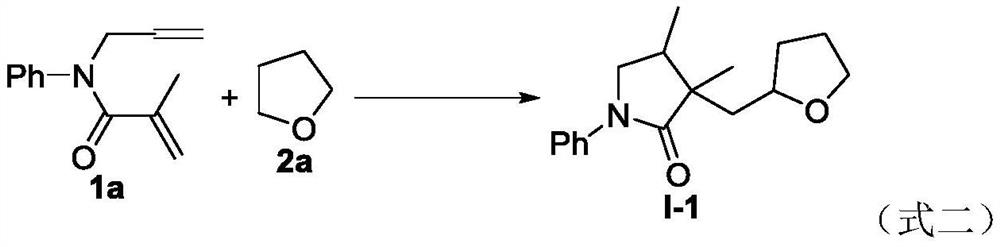
A kind of preparation method of ether substituted 2-pyrrolidone compound
The present invention relates to a kind of preparation method of ether-substituted 2-pyrrolidone compound, the method adds 1,6-diene compound, ether solvent and oxidant into schlenk bottle, then above-mentioned reactor is stirred and reacted under air atmosphere , after the reaction is completed, the target product is obtained through post-processing. The process can be realized only at high temperature without catalyst and alkali, avoids the generation of other free radicals, greatly improves the reaction yield, and shortens the reaction time. The invention has the advantages of simple operation, cheap and easy-to-obtain raw materials, mild reaction conditions, high efficiency and stability, high atom economy and step economy, is especially suitable for industrial production, has good application prospects, and is an ether-substituted 2-pyrrolidone compound It provides a basis for the research and application of the activity of , and also provides a new idea for the free radical cyclization reaction.
Owner:YANGTZE NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Synthetic method for imidazole[1,2-a]pyridine and 2-butyl-5-chloro-1H-imidazole-4-carboxaldehyde compounds Synthetic method for imidazole[1,2-a]pyridine and 2-butyl-5-chloro-1H-imidazole-4-carboxaldehyde compounds](https://images-eureka.patsnap.com/patent_img/fe80eb46-8b88-4348-acbc-81f181076c99/BDA0000087034270000022.PNG)
![Synthetic method for imidazole[1,2-a]pyridine and 2-butyl-5-chloro-1H-imidazole-4-carboxaldehyde compounds Synthetic method for imidazole[1,2-a]pyridine and 2-butyl-5-chloro-1H-imidazole-4-carboxaldehyde compounds](https://images-eureka.patsnap.com/patent_img/fe80eb46-8b88-4348-acbc-81f181076c99/BDA0000087034270000023.PNG)
![Synthetic method for imidazole[1,2-a]pyridine and 2-butyl-5-chloro-1H-imidazole-4-carboxaldehyde compounds Synthetic method for imidazole[1,2-a]pyridine and 2-butyl-5-chloro-1H-imidazole-4-carboxaldehyde compounds](https://images-eureka.patsnap.com/patent_img/fe80eb46-8b88-4348-acbc-81f181076c99/BDA0000087034270000024.PNG)
![Novel method for synthesizing indolo[1,2-a]quinoxaline derivative Novel method for synthesizing indolo[1,2-a]quinoxaline derivative](https://images-eureka.patsnap.com/patent_img/01254e41-7e7f-43cd-9af7-52e2d3858384/HDA0002312117720000011.png)
![Novel method for synthesizing indolo[1,2-a]quinoxaline derivative Novel method for synthesizing indolo[1,2-a]quinoxaline derivative](https://images-eureka.patsnap.com/patent_img/01254e41-7e7f-43cd-9af7-52e2d3858384/HDA0002312117720000012.png)
![Novel method for synthesizing indolo[1,2-a]quinoxaline derivative Novel method for synthesizing indolo[1,2-a]quinoxaline derivative](https://images-eureka.patsnap.com/patent_img/01254e41-7e7f-43cd-9af7-52e2d3858384/HDA0002312117720000021.png)
![Synthetic method for imidazole[1,2-a]pyridine and 2-butyl-5-chloro-1H-imidazole-4-carboxaldehyde compounds Synthetic method for imidazole[1,2-a]pyridine and 2-butyl-5-chloro-1H-imidazole-4-carboxaldehyde compounds](https://images-eureka.patsnap.com/patent_img/51adac45-186e-457b-9728-7740dc619dc5/BDA0000087034270000022.PNG)
![Synthetic method for imidazole[1,2-a]pyridine and 2-butyl-5-chloro-1H-imidazole-4-carboxaldehyde compounds Synthetic method for imidazole[1,2-a]pyridine and 2-butyl-5-chloro-1H-imidazole-4-carboxaldehyde compounds](https://images-eureka.patsnap.com/patent_img/51adac45-186e-457b-9728-7740dc619dc5/BDA0000087034270000023.PNG)
![Synthetic method for imidazole[1,2-a]pyridine and 2-butyl-5-chloro-1H-imidazole-4-carboxaldehyde compounds Synthetic method for imidazole[1,2-a]pyridine and 2-butyl-5-chloro-1H-imidazole-4-carboxaldehyde compounds](https://images-eureka.patsnap.com/patent_img/51adac45-186e-457b-9728-7740dc619dc5/BDA0000087034270000024.PNG)
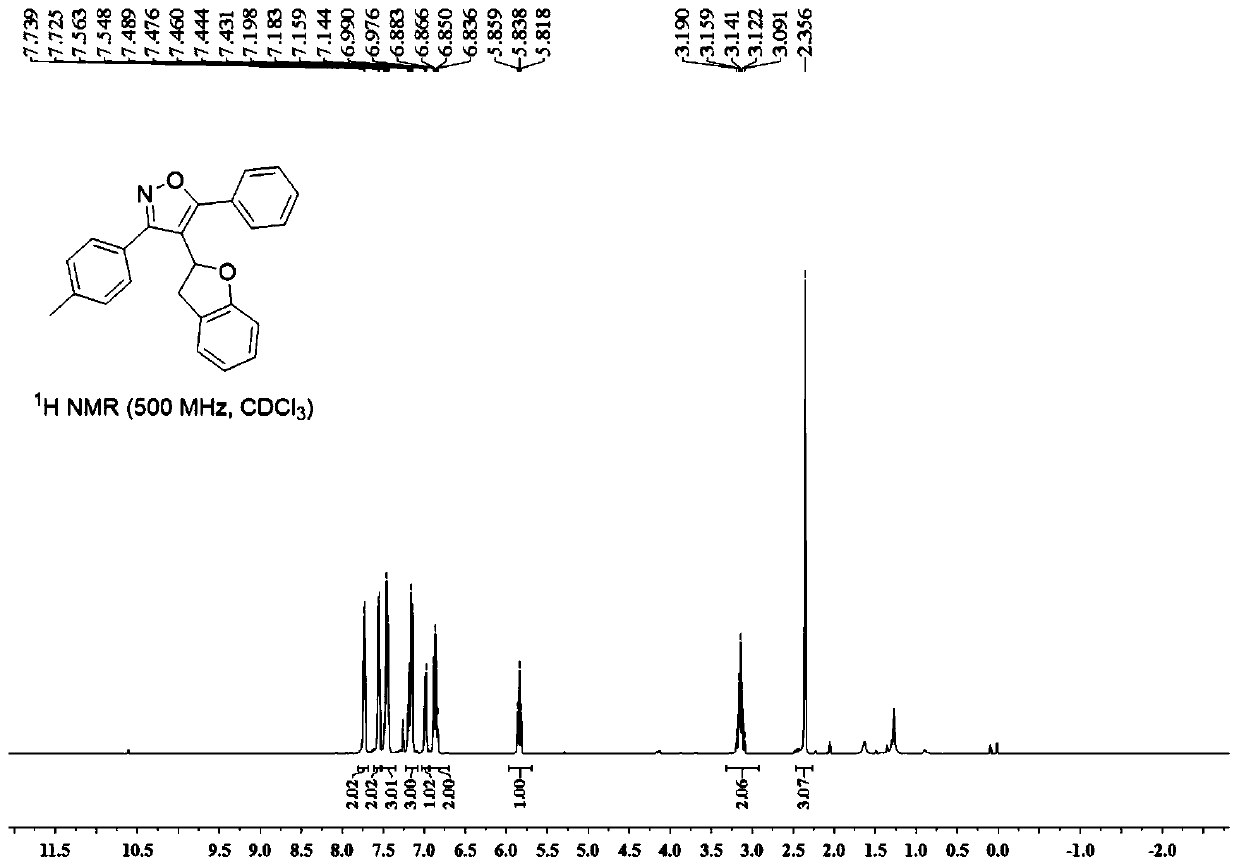
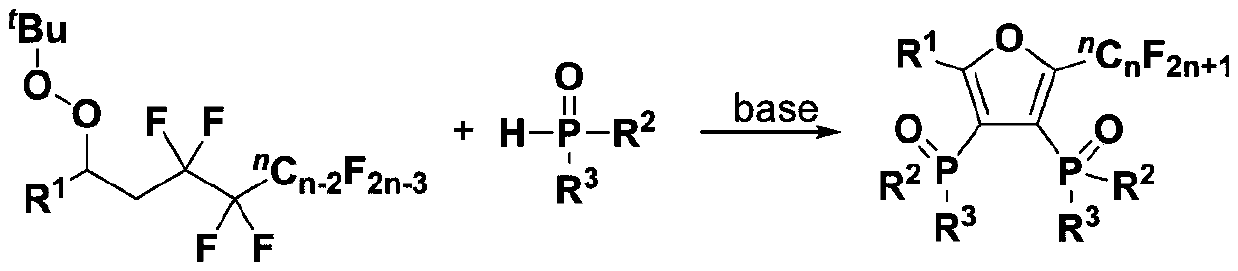
![Method for primary amine-directed construction of 6-(4-bromo-2-fluorophenyl)indolo[1,2-a]quinoxaline Method for primary amine-directed construction of 6-(4-bromo-2-fluorophenyl)indolo[1,2-a]quinoxaline](https://images-eureka.patsnap.com/patent_img/4645c98a-0971-46d2-9478-6549ae124025/HDA0001916915520000011.png)
![Method for primary amine-directed construction of 6-(4-bromo-2-fluorophenyl)indolo[1,2-a]quinoxaline Method for primary amine-directed construction of 6-(4-bromo-2-fluorophenyl)indolo[1,2-a]quinoxaline](https://images-eureka.patsnap.com/patent_img/4645c98a-0971-46d2-9478-6549ae124025/HDA0001916915520000012.png)
![Method for primary amine-directed construction of 6-(4-bromo-2-fluorophenyl)indolo[1,2-a]quinoxaline Method for primary amine-directed construction of 6-(4-bromo-2-fluorophenyl)indolo[1,2-a]quinoxaline](https://images-eureka.patsnap.com/patent_img/4645c98a-0971-46d2-9478-6549ae124025/HDA0001916915520000021.png)
![Method for constructing 2-chloro-6-phenylindolo[1,2-a]quinoxaline by primary amine directing Method for constructing 2-chloro-6-phenylindolo[1,2-a]quinoxaline by primary amine directing](https://images-eureka.patsnap.com/patent_img/4d562754-eea8-4626-8ca2-b14d03548ea4/HDA0001916914750000011.png)
![Method for constructing 2-chloro-6-phenylindolo[1,2-a]quinoxaline by primary amine directing Method for constructing 2-chloro-6-phenylindolo[1,2-a]quinoxaline by primary amine directing](https://images-eureka.patsnap.com/patent_img/4d562754-eea8-4626-8ca2-b14d03548ea4/HDA0001916914750000012.png)
![Method for constructing 2-chloro-6-phenylindolo[1,2-a]quinoxaline by primary amine directing Method for constructing 2-chloro-6-phenylindolo[1,2-a]quinoxaline by primary amine directing](https://images-eureka.patsnap.com/patent_img/4d562754-eea8-4626-8ca2-b14d03548ea4/HDA0001916914750000021.png)
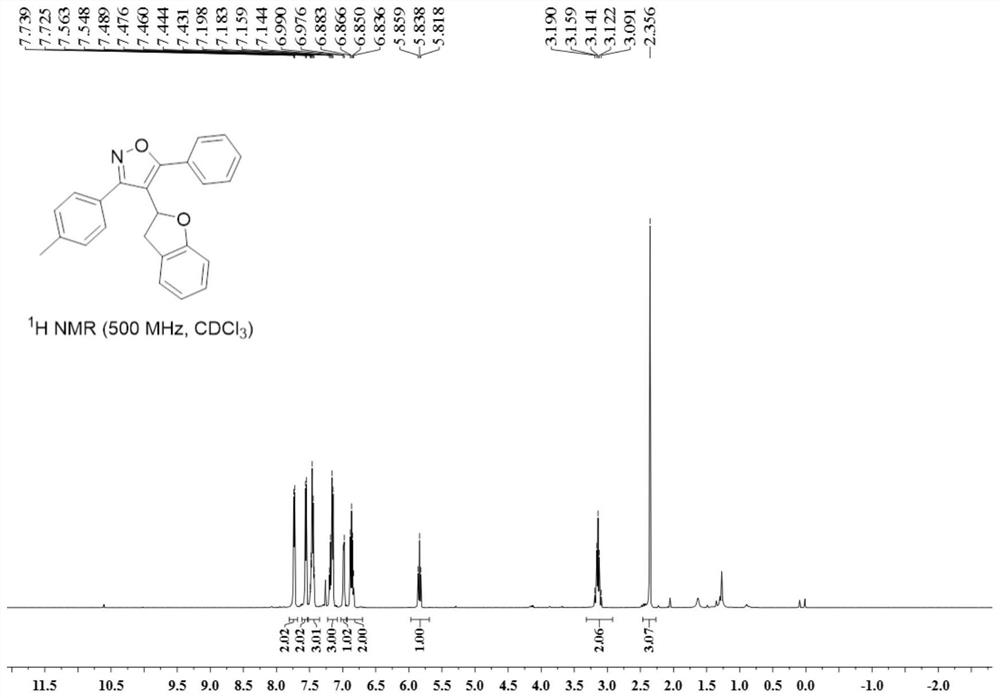
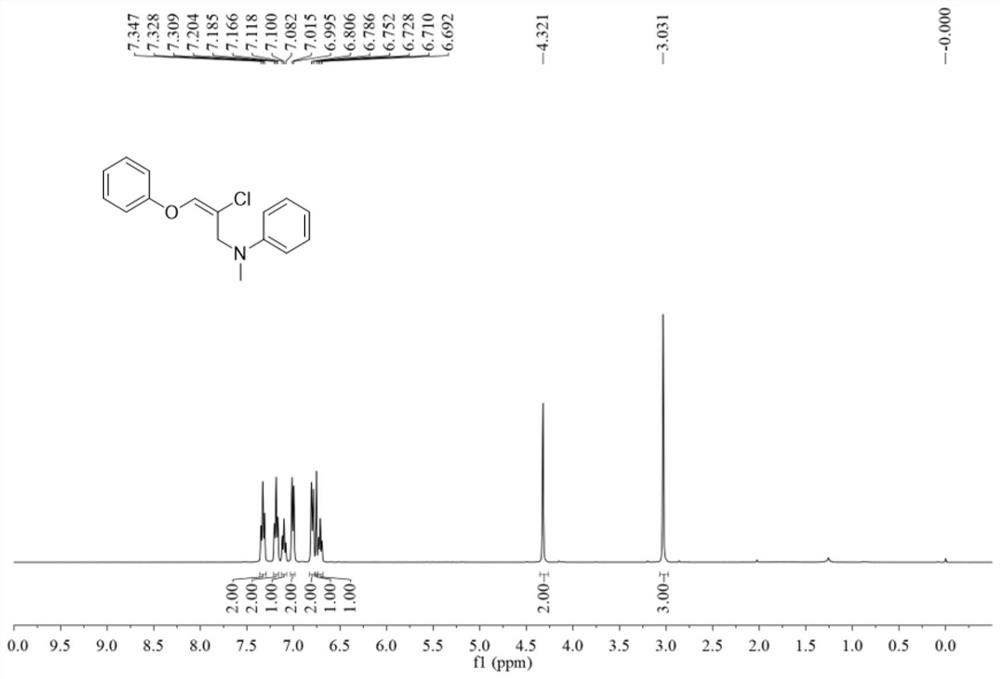
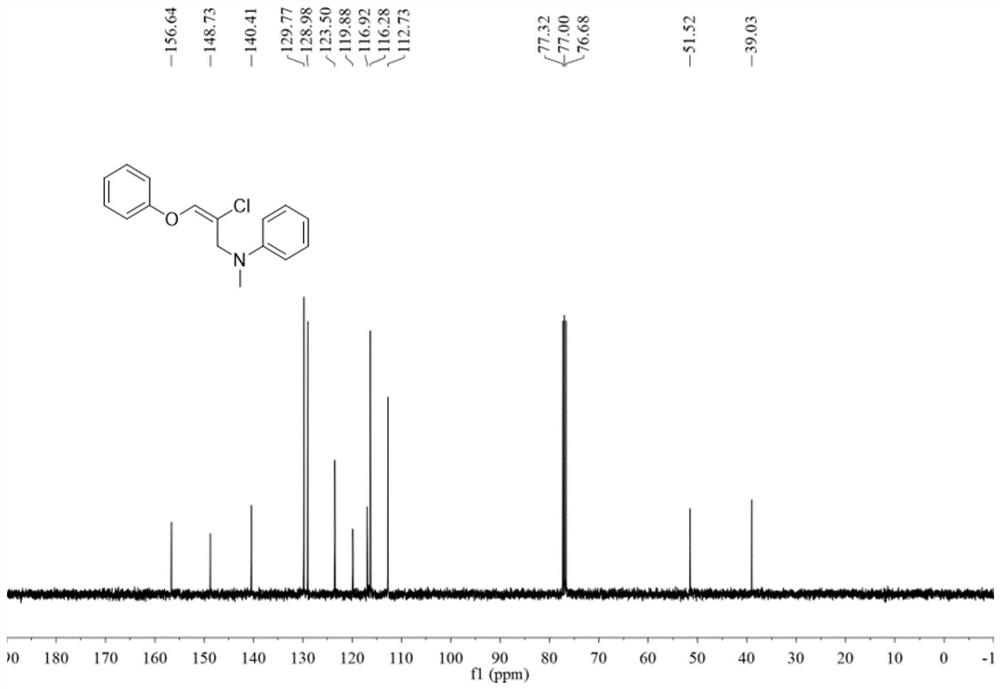
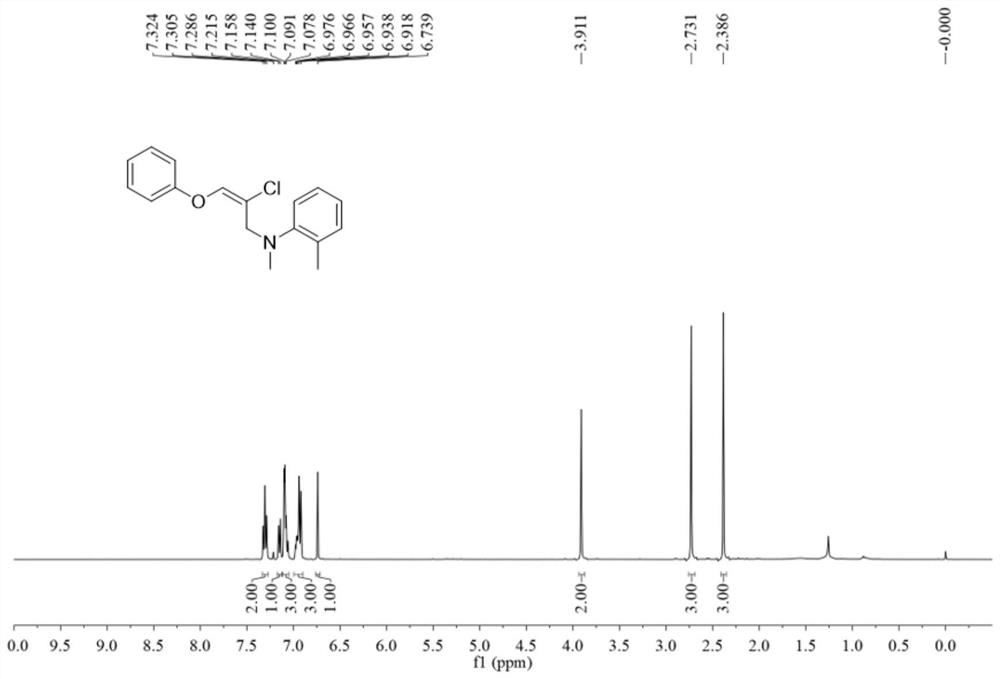
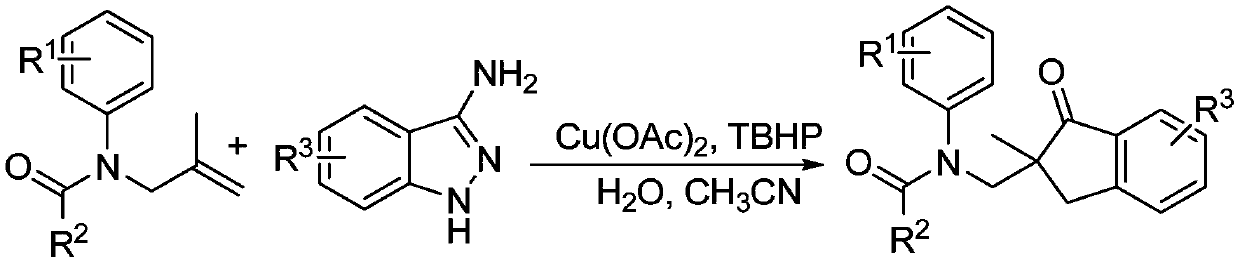
![Method for constructing 2-fluorine-6-phenyl indolo[1,2-a] quinoxaline through primary amine guiding Method for constructing 2-fluorine-6-phenyl indolo[1,2-a] quinoxaline through primary amine guiding](https://images-eureka.patsnap.com/patent_img/6dfd3c41-cac8-4515-8a07-edb3b796a64a/HDA0001916915860000011.png)
![Method for constructing 2-fluorine-6-phenyl indolo[1,2-a] quinoxaline through primary amine guiding Method for constructing 2-fluorine-6-phenyl indolo[1,2-a] quinoxaline through primary amine guiding](https://images-eureka.patsnap.com/patent_img/6dfd3c41-cac8-4515-8a07-edb3b796a64a/HDA0001916915860000012.png)
![Method for constructing 2-fluorine-6-phenyl indolo[1,2-a] quinoxaline through primary amine guiding Method for constructing 2-fluorine-6-phenyl indolo[1,2-a] quinoxaline through primary amine guiding](https://images-eureka.patsnap.com/patent_img/6dfd3c41-cac8-4515-8a07-edb3b796a64a/HDA0001916915860000021.png)
![Method for constructing 6-(2-methylphenyl)indolo[1,2-a]quinoxaline guided by primary amine Method for constructing 6-(2-methylphenyl)indolo[1,2-a]quinoxaline guided by primary amine](https://images-eureka.patsnap.com/patent_img/7b7b4e0f-b87f-44a8-adff-670beaa71695/HDA0001916914630000011.png)
![Method for constructing 6-(2-methylphenyl)indolo[1,2-a]quinoxaline guided by primary amine Method for constructing 6-(2-methylphenyl)indolo[1,2-a]quinoxaline guided by primary amine](https://images-eureka.patsnap.com/patent_img/7b7b4e0f-b87f-44a8-adff-670beaa71695/HDA0001916914630000012.png)
![Method for constructing 6-(2-methylphenyl)indolo[1,2-a]quinoxaline guided by primary amine Method for constructing 6-(2-methylphenyl)indolo[1,2-a]quinoxaline guided by primary amine](https://images-eureka.patsnap.com/patent_img/7b7b4e0f-b87f-44a8-adff-670beaa71695/HDA0001916914630000021.png)
![Method for constructing 8-fluoro-6-phenylindolo[1,2-a]quinoxaline by primary amine directing Method for constructing 8-fluoro-6-phenylindolo[1,2-a]quinoxaline by primary amine directing](https://images-eureka.patsnap.com/patent_img/ac1f5503-ef02-4fef-a8f8-15d3d74f7357/HDA0001916917070000011.png)
![Method for constructing 8-fluoro-6-phenylindolo[1,2-a]quinoxaline by primary amine directing Method for constructing 8-fluoro-6-phenylindolo[1,2-a]quinoxaline by primary amine directing](https://images-eureka.patsnap.com/patent_img/ac1f5503-ef02-4fef-a8f8-15d3d74f7357/HDA0001916917070000012.png)
![Method for constructing 8-fluoro-6-phenylindolo[1,2-a]quinoxaline by primary amine directing Method for constructing 8-fluoro-6-phenylindolo[1,2-a]quinoxaline by primary amine directing](https://images-eureka.patsnap.com/patent_img/ac1f5503-ef02-4fef-a8f8-15d3d74f7357/HDA0001916917070000021.png)
![Method for constructing 6-(furan-2-yl)indolo[1,2-a]quinoxaline through primary amine guidance Method for constructing 6-(furan-2-yl)indolo[1,2-a]quinoxaline through primary amine guidance](https://images-eureka.patsnap.com/patent_img/c05a0109-417f-4820-8a9f-80a843614b4f/HDA0001916914990000011.png)
![Method for constructing 6-(furan-2-yl)indolo[1,2-a]quinoxaline through primary amine guidance Method for constructing 6-(furan-2-yl)indolo[1,2-a]quinoxaline through primary amine guidance](https://images-eureka.patsnap.com/patent_img/c05a0109-417f-4820-8a9f-80a843614b4f/HDA0001916914990000012.png)
![Method for constructing 6-(furan-2-yl)indolo[1,2-a]quinoxaline through primary amine guidance Method for constructing 6-(furan-2-yl)indolo[1,2-a]quinoxaline through primary amine guidance](https://images-eureka.patsnap.com/patent_img/c05a0109-417f-4820-8a9f-80a843614b4f/HDA0001916914990000021.png)
![Method for constructing 6-(3-fluorophenyl)indolo[1,2-a]quinoxaline guided by primary amine Method for constructing 6-(3-fluorophenyl)indolo[1,2-a]quinoxaline guided by primary amine](https://images-eureka.patsnap.com/patent_img/c3dffc72-56a4-451b-84b9-d822eccaccbe/HDA0001916913890000011.png)
![Method for constructing 6-(3-fluorophenyl)indolo[1,2-a]quinoxaline guided by primary amine Method for constructing 6-(3-fluorophenyl)indolo[1,2-a]quinoxaline guided by primary amine](https://images-eureka.patsnap.com/patent_img/c3dffc72-56a4-451b-84b9-d822eccaccbe/HDA0001916913890000012.png)
![Method for constructing 6-(3-fluorophenyl)indolo[1,2-a]quinoxaline guided by primary amine Method for constructing 6-(3-fluorophenyl)indolo[1,2-a]quinoxaline guided by primary amine](https://images-eureka.patsnap.com/patent_img/c3dffc72-56a4-451b-84b9-d822eccaccbe/HDA0001916913890000021.png)