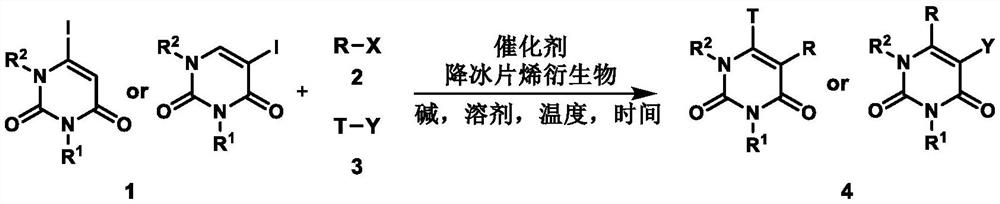
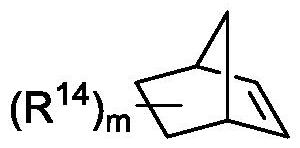
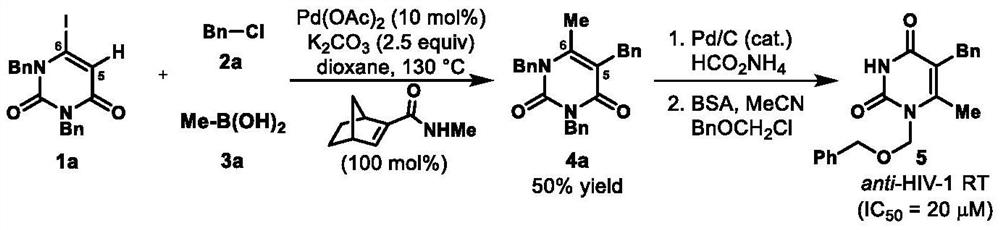
A method for preparing uracil and thymine derivatives
A technology of thymine and uracil, which is applied in the field of efficient preparation of uracil and thymine derivatives, achieving the effects of convenient purification, high step economy, efficient and diversified methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Preparation of 1,3-dibenzyl-5-methylpyrimidine-2,4(1H,3H)-dione-6-d
[0038]
[0039] Under the protection of an inert gas, add Pd(OAc) to a dry 4.0mL reaction bottle equipped with a magnetic stir bar 2(1.1mg, 5mol%), NBE-CONHMe (7.6mg, 50mol%), 1,3-dibenzyl-6-iodopyrimidine-2,4(1H,3H)-dione (0.12 mmol, 1.2equiv) , methyl p-toluenesulfonate (0.15mmol, 1.5equiv), DCO 2 Na (0.1 mmol, 1.0 equiv), potassium carbonate (0.25 mmol, 2.5 equiv) and dried 1,4-dioxane (1.0 mL). The above reaction solution was placed on a heating block preheated to 130° C. and stirred for 48 hours. The reaction was monitored by TLC. After the reaction was complete, it was cooled to room temperature, the mixture was filtered with diatomaceous earth, washed with ethyl acetate, and the solvent was removed under reduced pressure. The crude product was directly separated and purified by column chromatography to obtain the target product (colorless oil, 52%, 80% D). 1 H NMR (400MHz, CDC...
Embodiment 2
[0040] Example 2: Preparation of 1,3-dibenzyl-5-methyl-6-phenylpyrimidine-2,4(1H,3H)-dione
[0041]
[0042] Under the protection of an inert gas, add Pd(OAc) to a dry 4.0mL reaction bottle equipped with a magnetic stir bar 2 (1.1mg, 5mol%), NBE-CONHMe (7.6mg, 50mol%), 1,3-dibenzyl-6-iodopyrimidine-2,4(1H,3H)-dione (0.12 mmol, 1.2 equiv) , methyl p-toluenesulfonate (0.15 mmol, 1.5 equiv), PhBPin (0.1 mmol, 1.0 equiv), potassium carbonate (0.25 mmol, 2.5 equiv) and dried 1,4-dioxane (1.0 mL). The above reaction solution was placed on a heating block preheated to 130° C. and stirred for 48 hours. The reaction was monitored by TLC. After the reaction was complete, it was cooled to room temperature, the mixture was filtered with diatomaceous earth, washed with ethyl acetate, and the solvent was removed under reduced pressure. The crude product was directly separated and purified by column chromatography to obtain the target product (colorless oil, 52%) . 1 H NMR (400MHz, CDC...
Embodiment 3
[0043] Example 3: Preparation of (E)-1,3-dibenzyl-5-methyl-6-styrylpyrimidine-2,4(1H,3H)-dione
[0044]
[0045] Under the protection of an inert gas, add Pd(OAc) to a dry 4.0mL reaction bottle equipped with a magnetic stir bar 2 (1.1mg, 5mol%), NBE-CONHMe (7.6mg, 50mol%), 1,3-dibenzyl-6-iodopyrimidine-2,4(1H,3H)-dione (0.12 mmol, 1.2equiv) , methyl p-toluenesulfonate (0.15 mmol, 1.5 equiv), styrene (0.1 mmol, 1.0 equiv), potassium carbonate (0.25 mmol, 2.5 equiv) and dried 1,4-dioxane (1.0 mL). The above reaction solution was placed on a heating block preheated to 130° C. and stirred for 48 hours. The reaction was monitored by TLC. After the reaction was complete, it was cooled to room temperature. The mixture was filtered with diatomaceous earth, washed with ethyl acetate, and the solvent was removed under reduced pressure. The crude product was directly separated and purified by column chromatography to obtain the target product (colorless oil, 47%) . 1 H NMR (400MHz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com