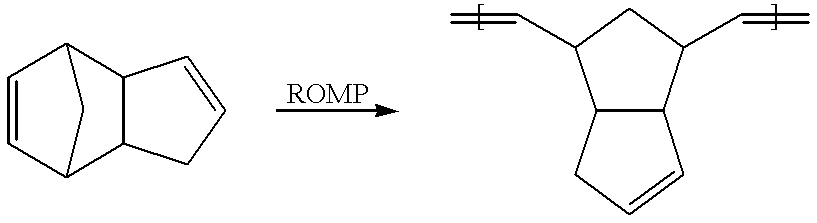
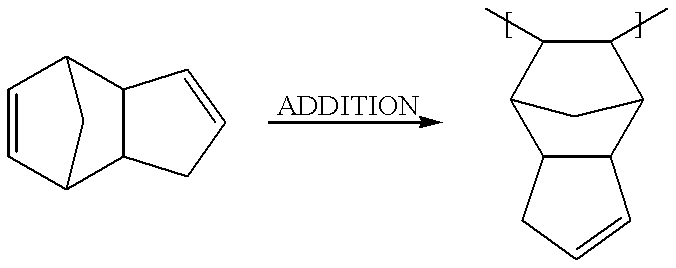
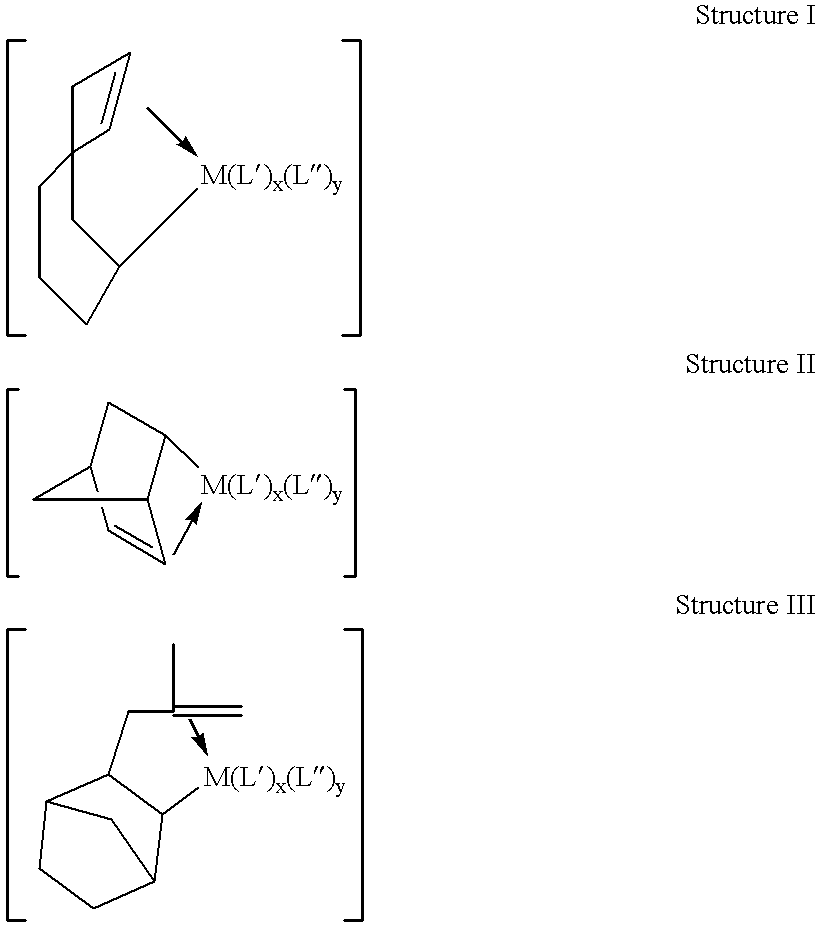
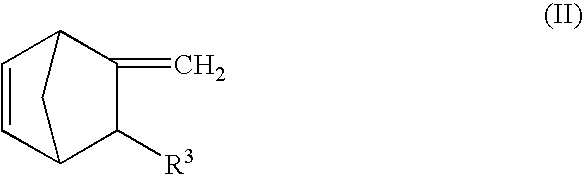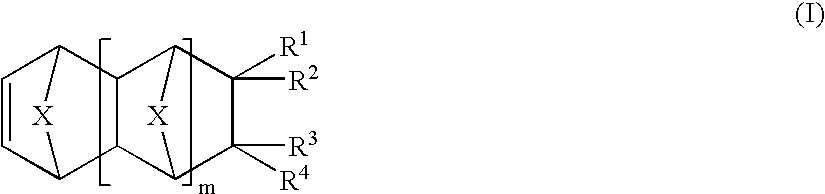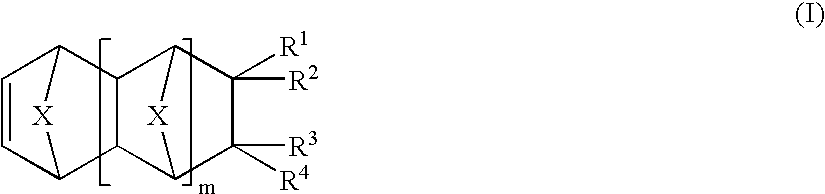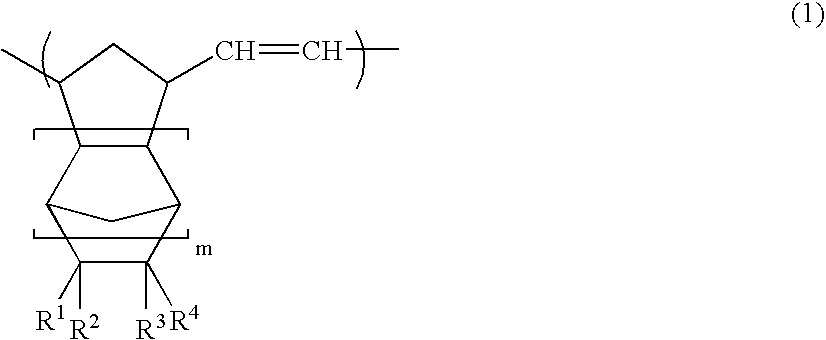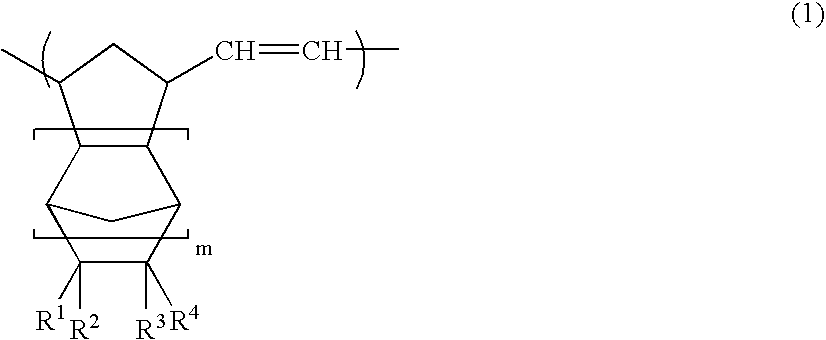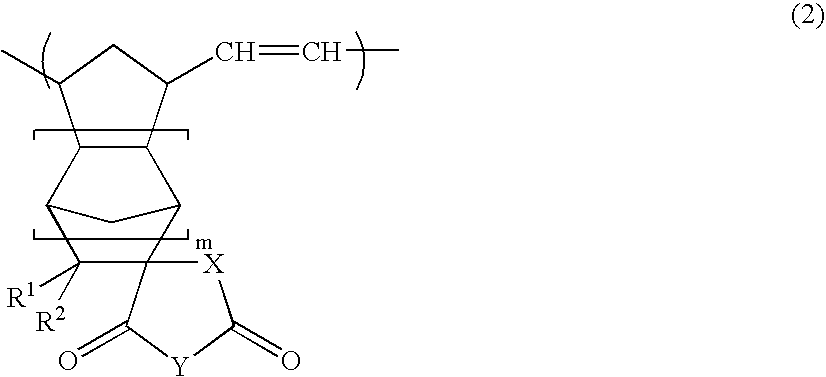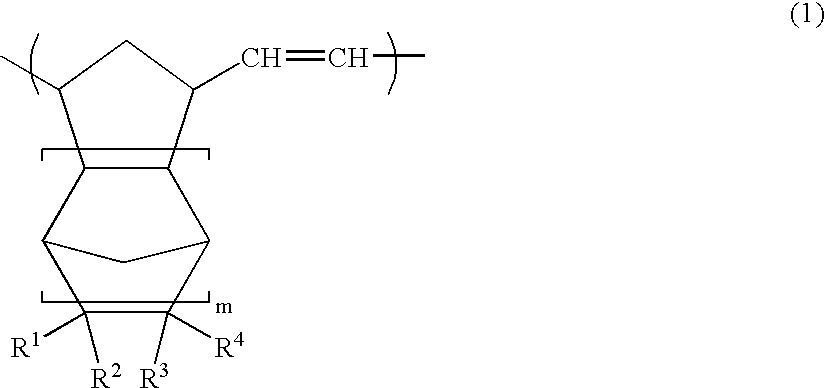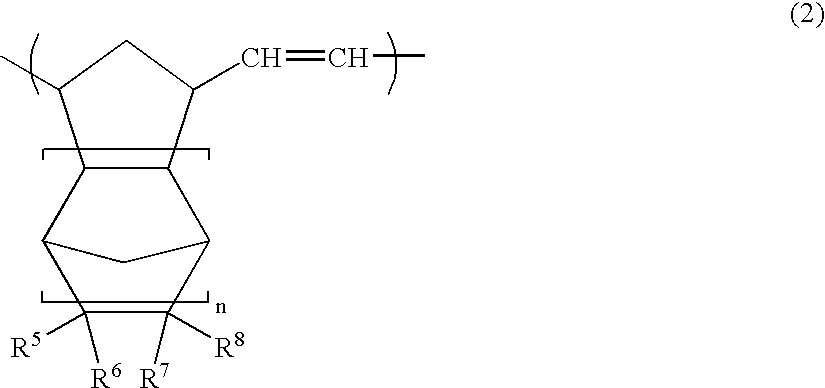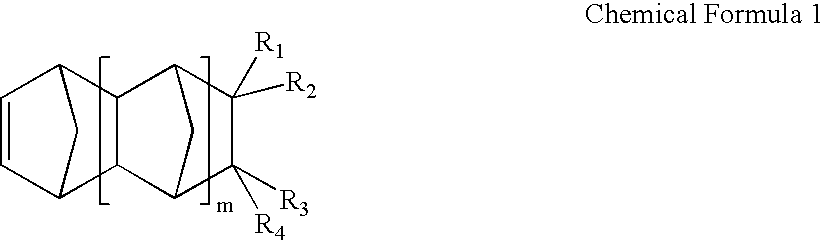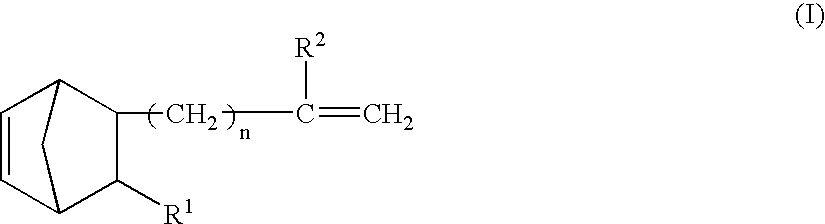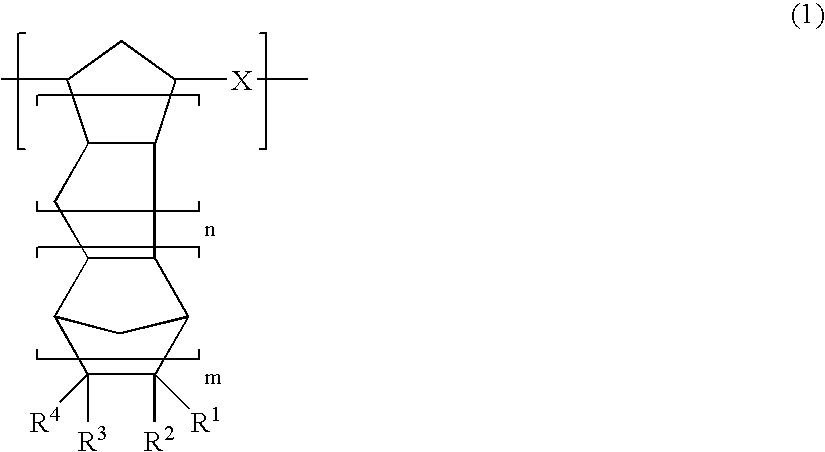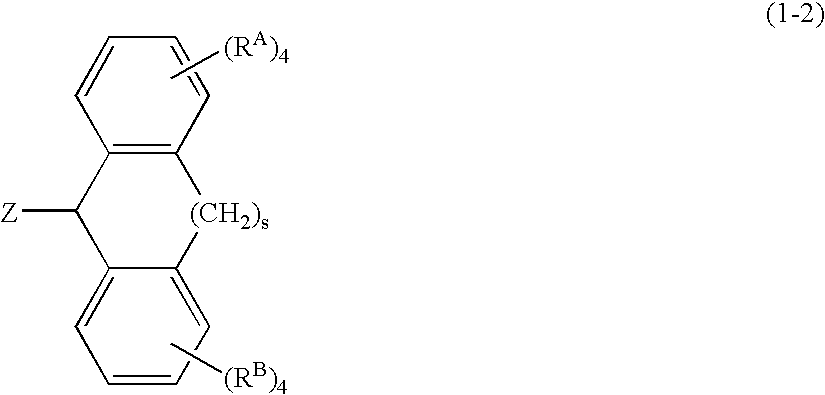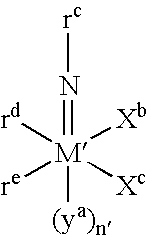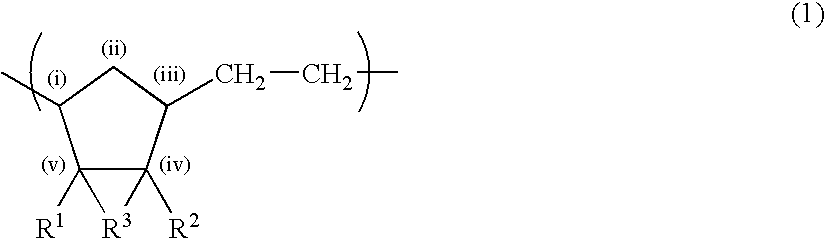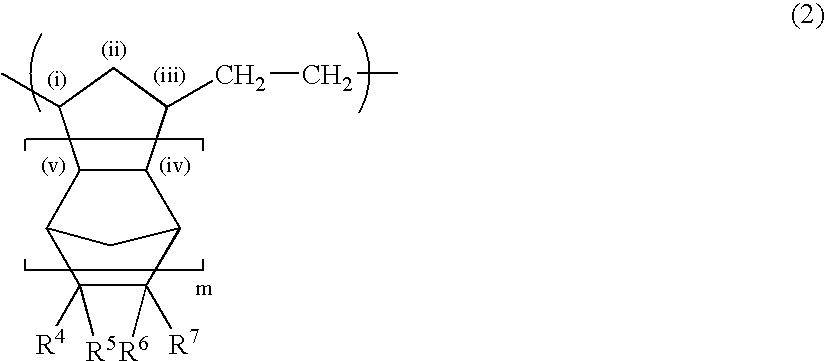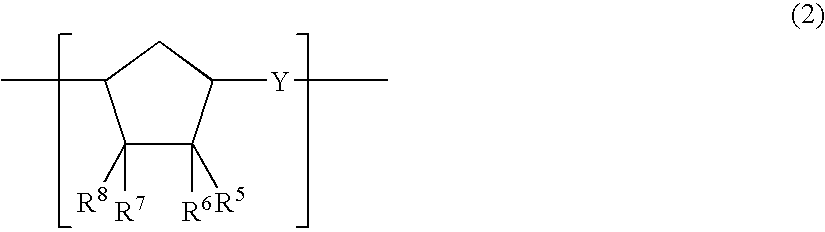Patents
Literature
1243 results about "Norbornene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
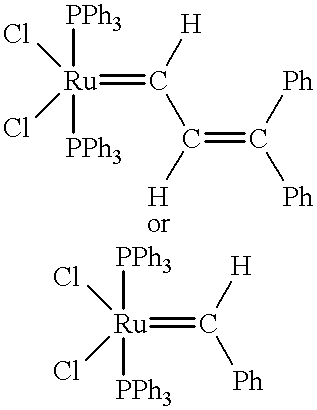
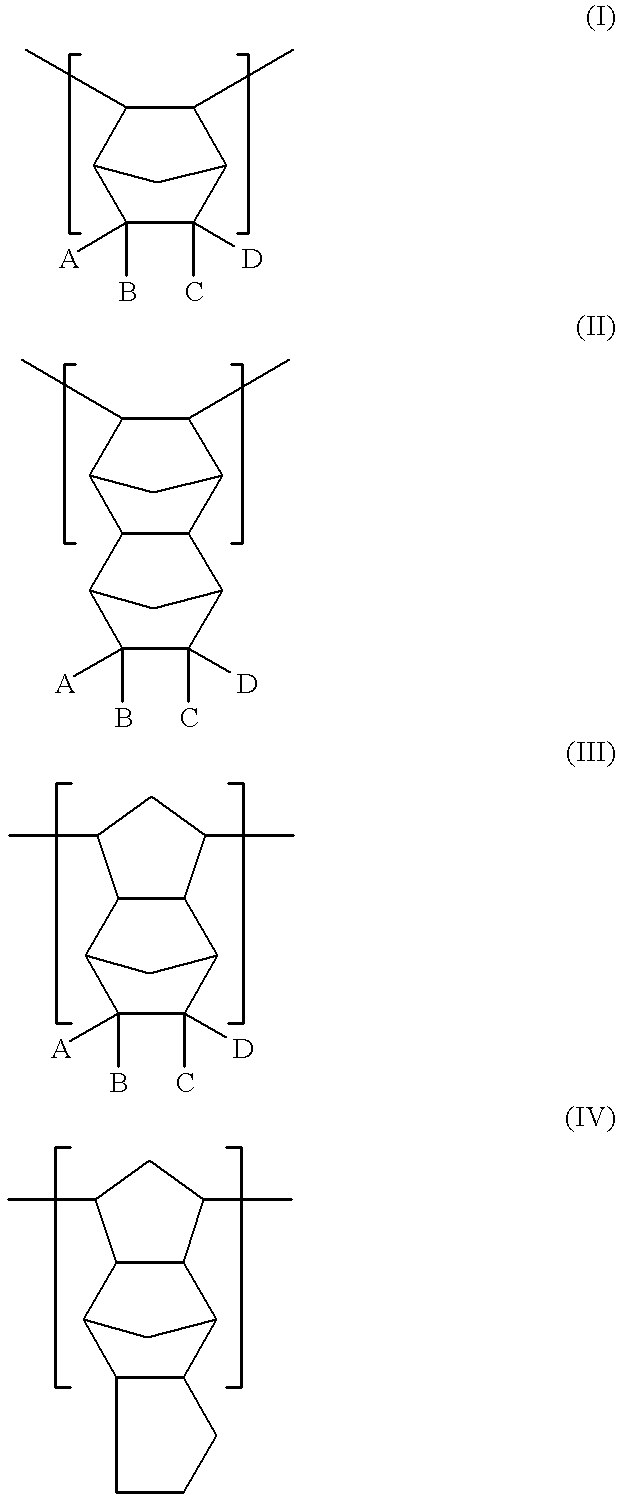
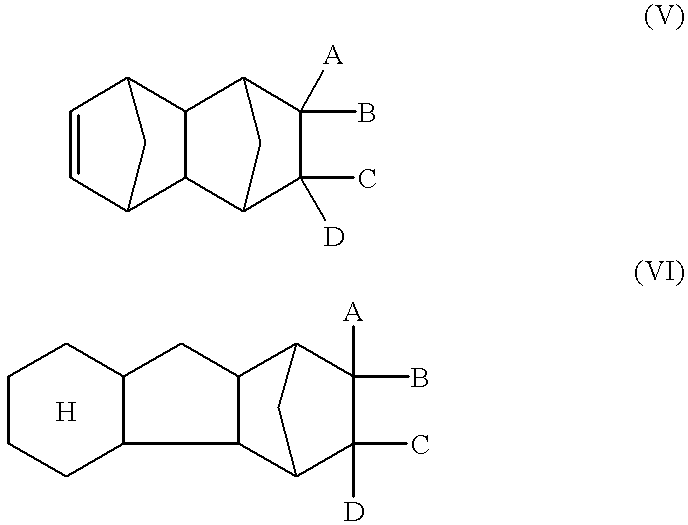
Norbornene or norbornylene or norcamphene is a bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring with a methylene bridge between carbons 1 and 4. The molecule carries a double bond which induces significant ring strain and significant reactivity.
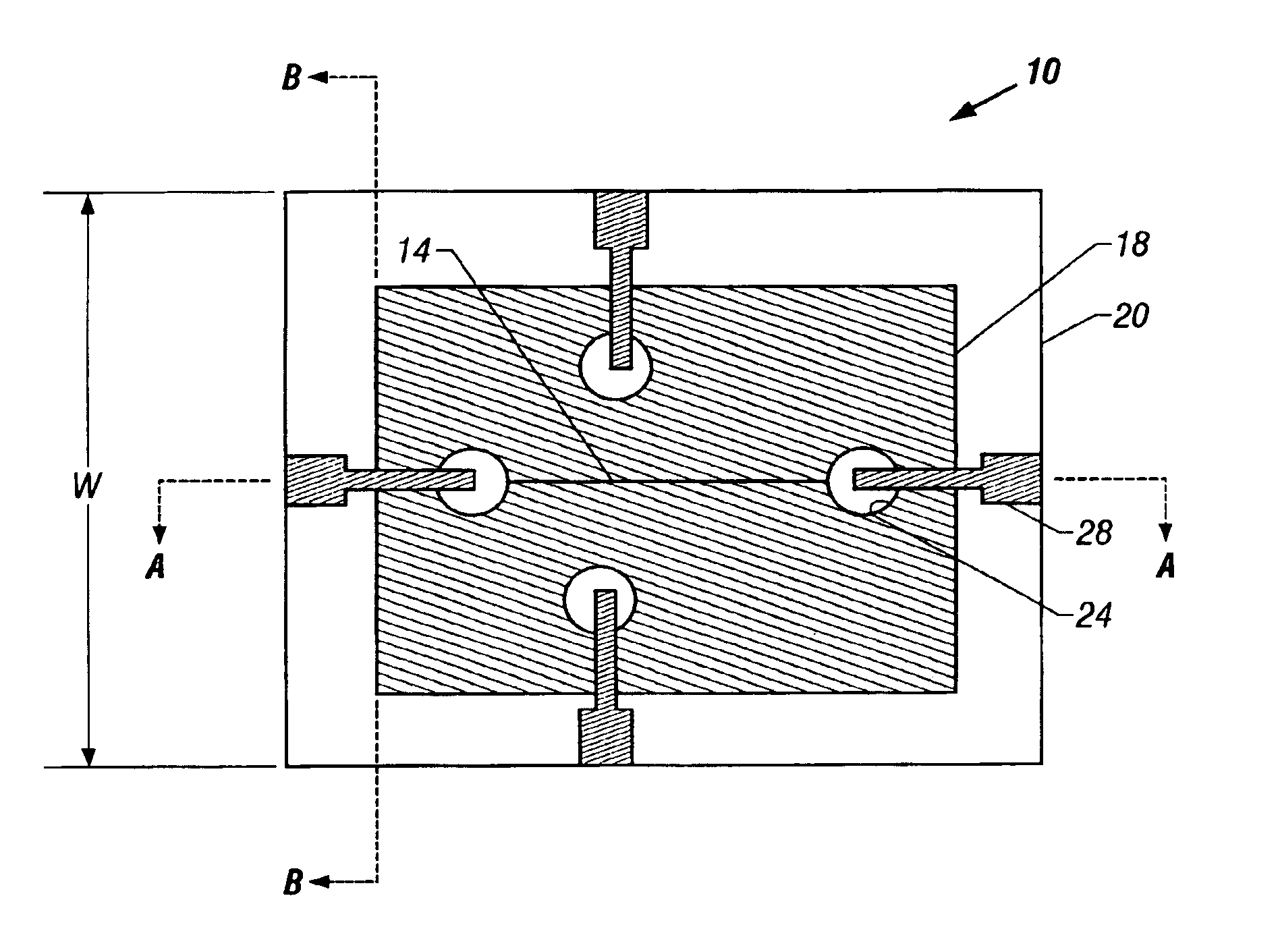
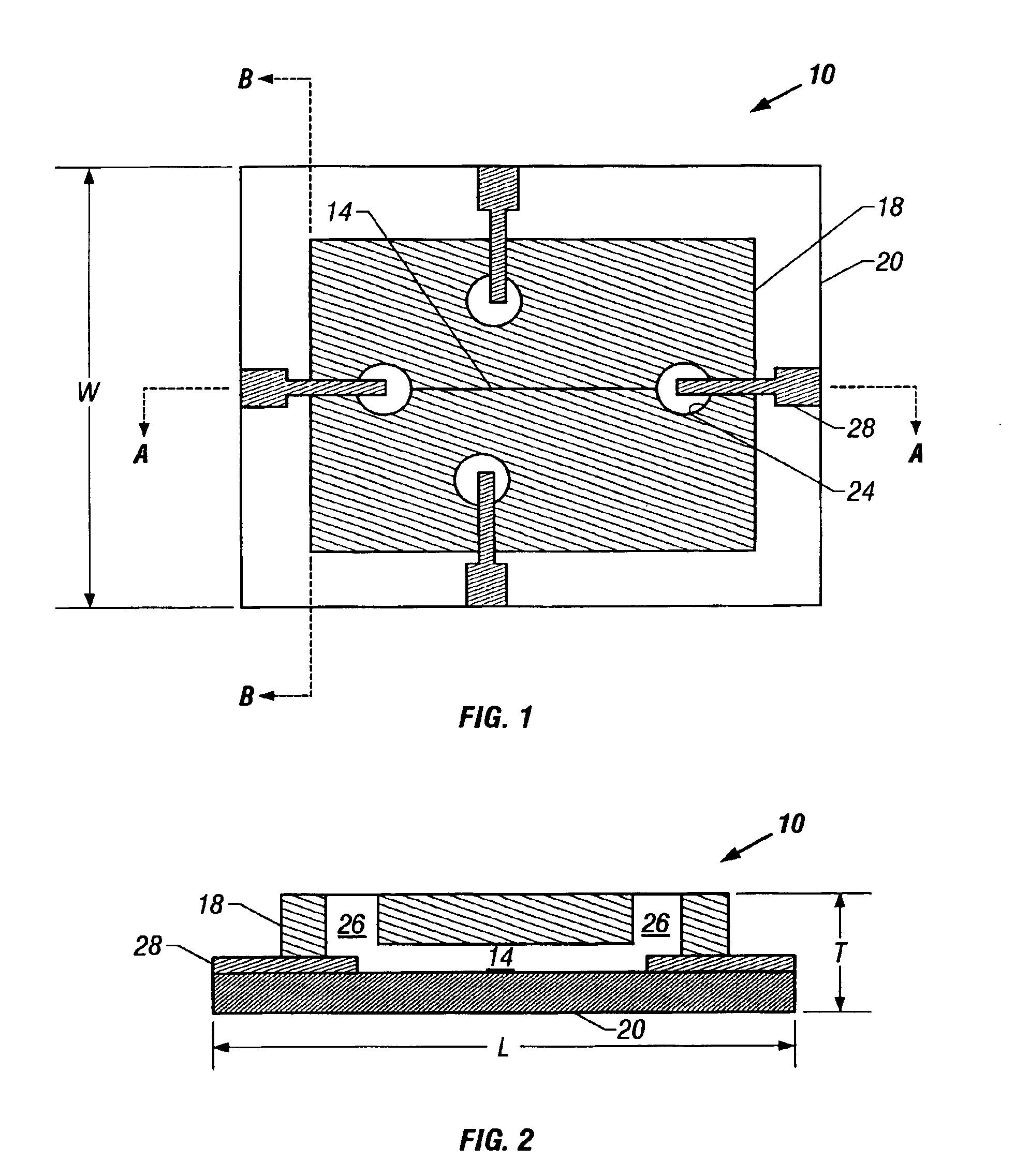
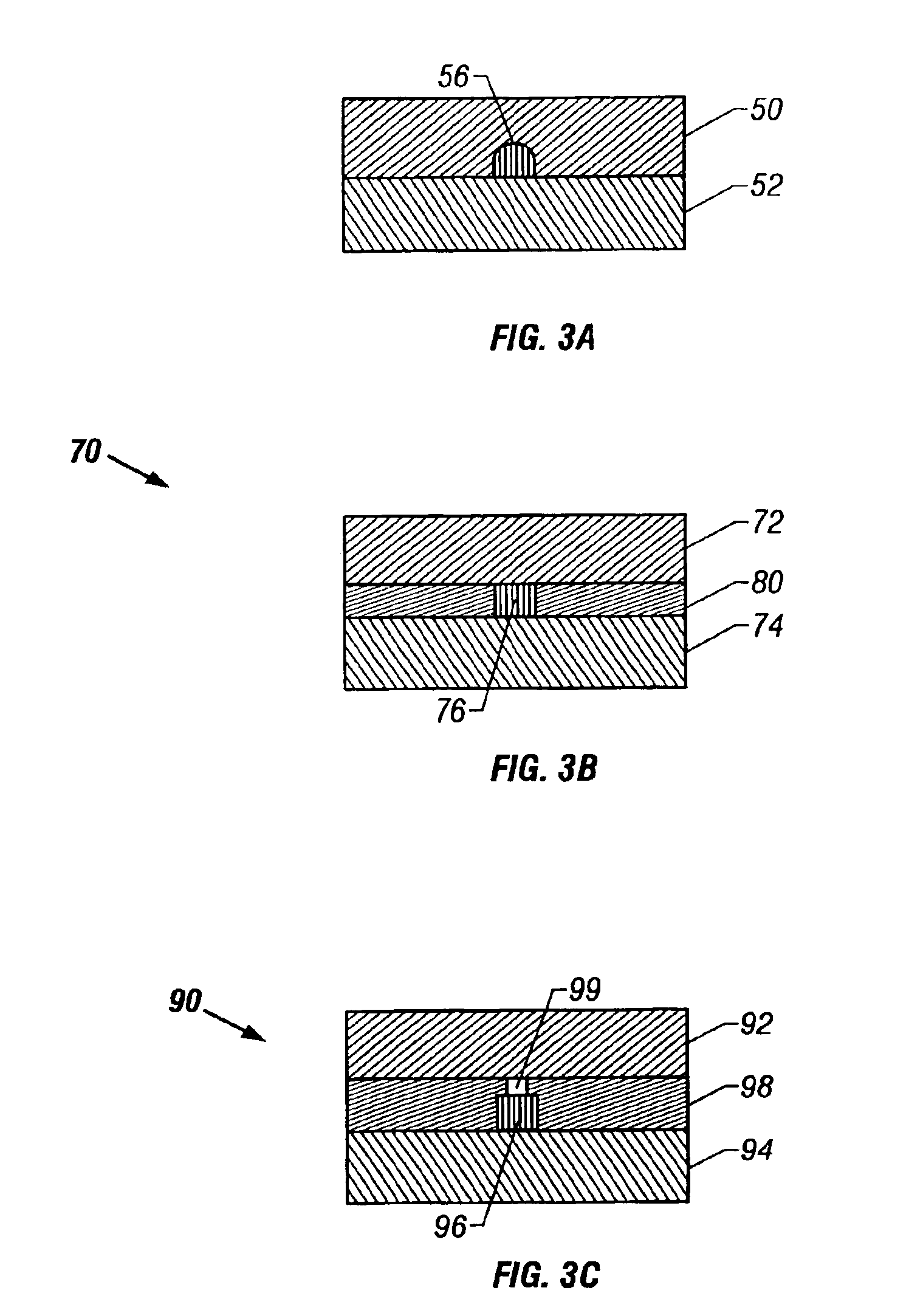
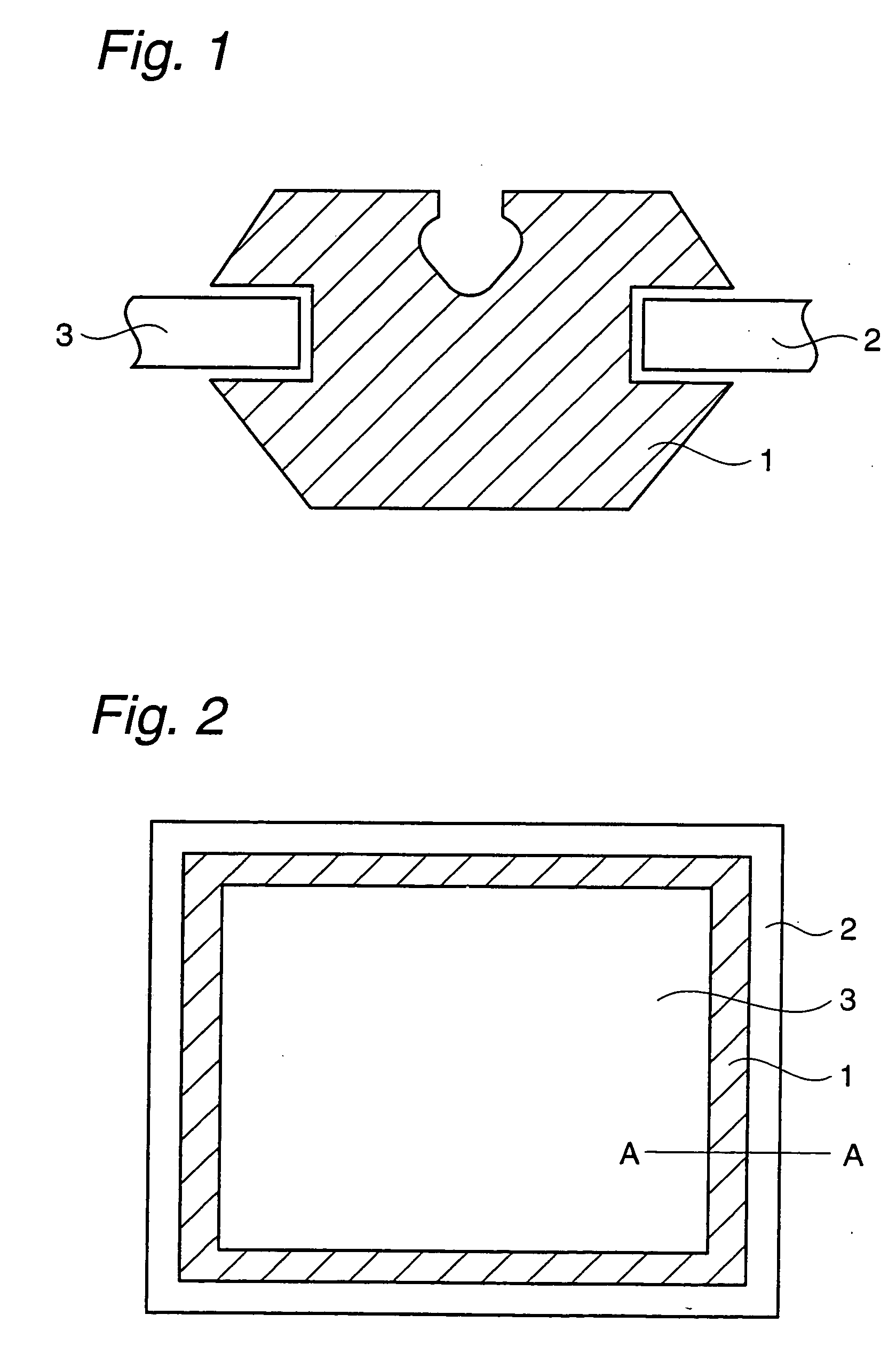
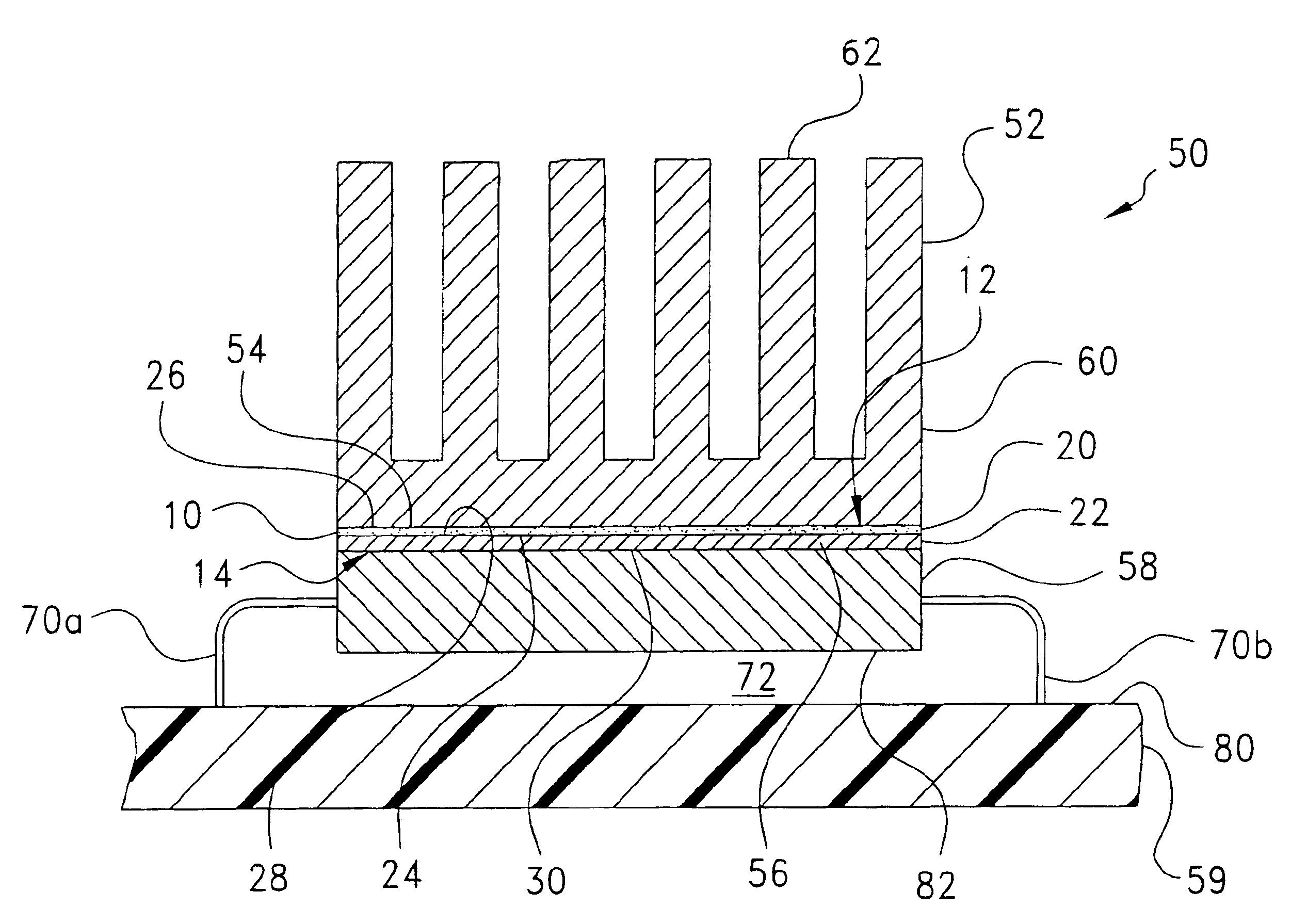
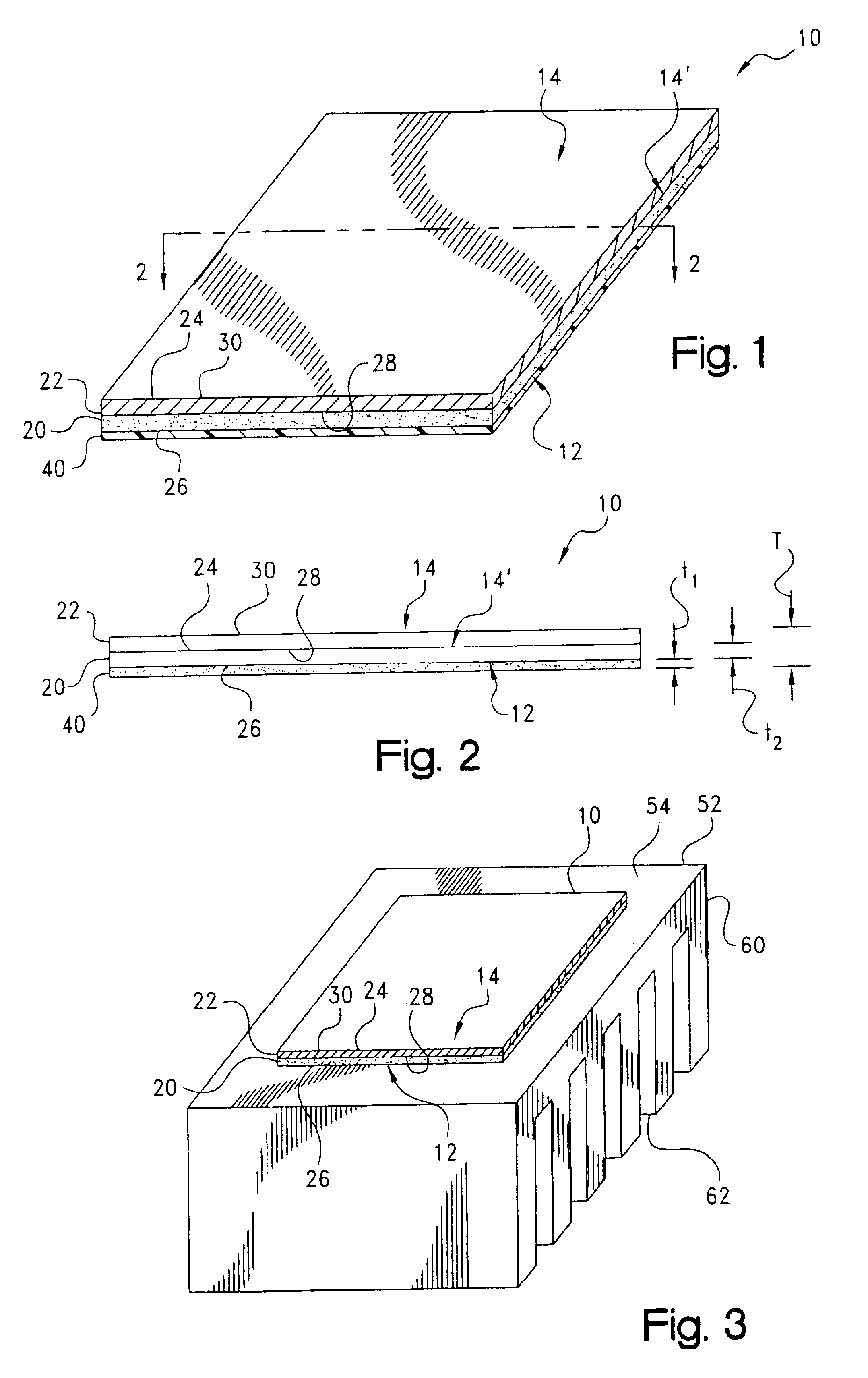
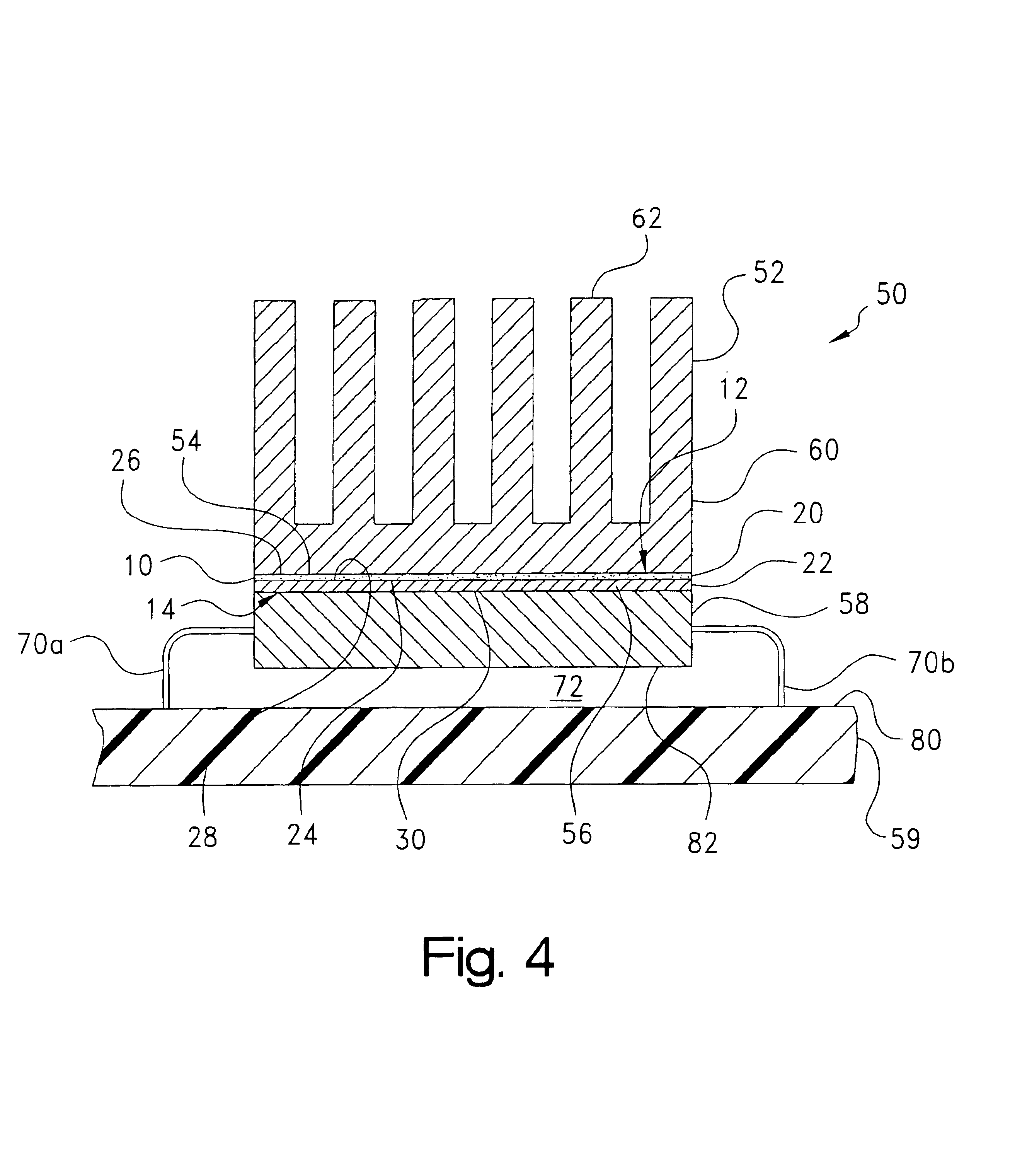
Microfluidic chip having integrated electrodes
InactiveUS6939451B2Reduces the formation of air bubblesSludge treatmentFixed microstructural devicesElectrochemical detectorNorbornene
A microfluidic device having integrated components for conducting chemical operations. Depending upon the desired application, the components include electrodes for manipulating charged entities, heaters, electrochemical detectors, sensors for temperature, pH, fluid flow, and other useful components. The device may be fabricated from a plastic substrate such as, for example, a substantially saturated norbornene based polymer. The components are integrated into the device by adhering an electrically conductive film to the substrate. The film may be made of metal or an electrically conducting ink and is applied to the device through metal deposition, printing, or other methods for applying films. Methods for reducing bubble formation during electrokinetic separation and methods for heating material in a microfluidic device are also disclosed.
Owner:MONOGRAM BIOSCIENCES
Application of supported non-metallocene catalyst in ethene polymerization process by slurry method
The invention discloses an application of load non- metallocene catalyst in the slurry process for vinyl polymerying, the load non- metallocene catalyst and catalyst promoter forming the catalytic system, the alkene polymerization comprising: vinyl homopolymerization, combined polymerization of vinyl with propylene, butylenes, hexane, octane or norbornene; the catalyst carrier being chosen from: inorganic oxide of metallic oxide from IIA, IIIA, IVA, and IVB groups, or oxided mixture and mixing oxide; the catalyst promoter being chosen from: methylaluoxane, ethylaluoxane, isobutylaluoxane, trimethylaluminum,triethylaluminum,triisobutylaluminum,methylaluoxane-trimethylaluminum or methylaluoxane-triethylaluminum; the mole proportion between the catalyst promoter and catalyst being Al / Ti= 1:1-500. The inventioin is characterized by the less methylaluoxane consumption, stable reaction, easy-to-control polymerization temperature and non still-sticking phenomenon. The produced polyolefine possesses perfect granual shape, and the maximum polymer clamp density can reach 0.385 g / ml.
Owner:SINOPEC YANGZI PETROCHEM
Polymeric composites including dicyclopentadiene and related monomers
InactiveUS6310121B1Group 8/9/10/18 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsFiberMonomer composition
Compositions including fibers, fillers, reinforcing agents or other property-enhancing agents added to a polymeric matrix formed from DCPD monomer or related compounds such as norbornene and norbornadiene are disclosed. The composition has a mechanically forgiving matrix due to the polymer with the inherent strength and other physical properties of the enhancement agent according to the invention. Enhancement agents according to the invention generally include inorganic, organic and metallic substances in a variety of forms.
Owner:CYMETECH
Crosslinkable rubber compositions and use thereof
The crosslinkable rubber composition of the invention is crosslinkable by hot air, and a hot-air crosslinked rubber sheet thereof has no scratch on the surface in a hardness test using a pencil of HB and has a compression set of not more than 70% after a heat treatment at 150° C. for 22 hours. The rubber composition comprises an ethylene / α-olefin / non-conjugated polyene random copolymer rubber comprising a specific vinyl end group-containing norbornene compound, a SiH group-containing compound having at least two SiH groups in one molecule, and if necessary, an addition reaction catalyst comprising a platinum group element and a reaction inhibitor. The automobile weatherstrip, hose, rubber vibration insulator, belt, sealing material, expanded product, covered electric wire, electric wire joint, electric insulating part and household rubber product according to the invention comprise the above-mentioned rubber composition. The rubber composition has a high crosslinking rate and excellent productivity to produce crosslinked rubber molded products, is capable of undergoing hot-air crosslinking such as HAV or UHF and is capable of providing crosslinked rubber molded products having excellent compression set resistance, strength properties, heat resistance, weathering resistance and abrasion resistance.
Owner:MITSUI CHEM INC +1
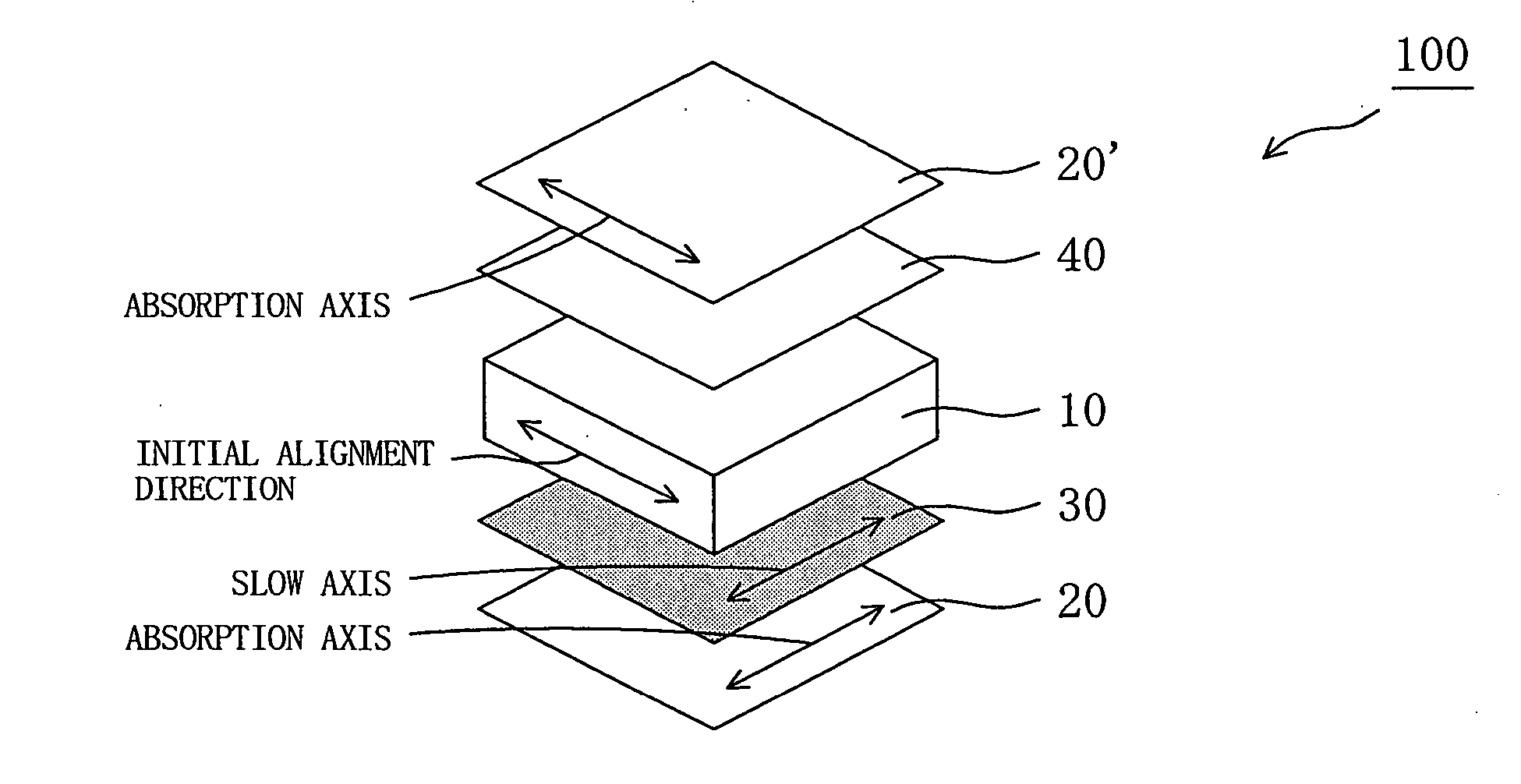
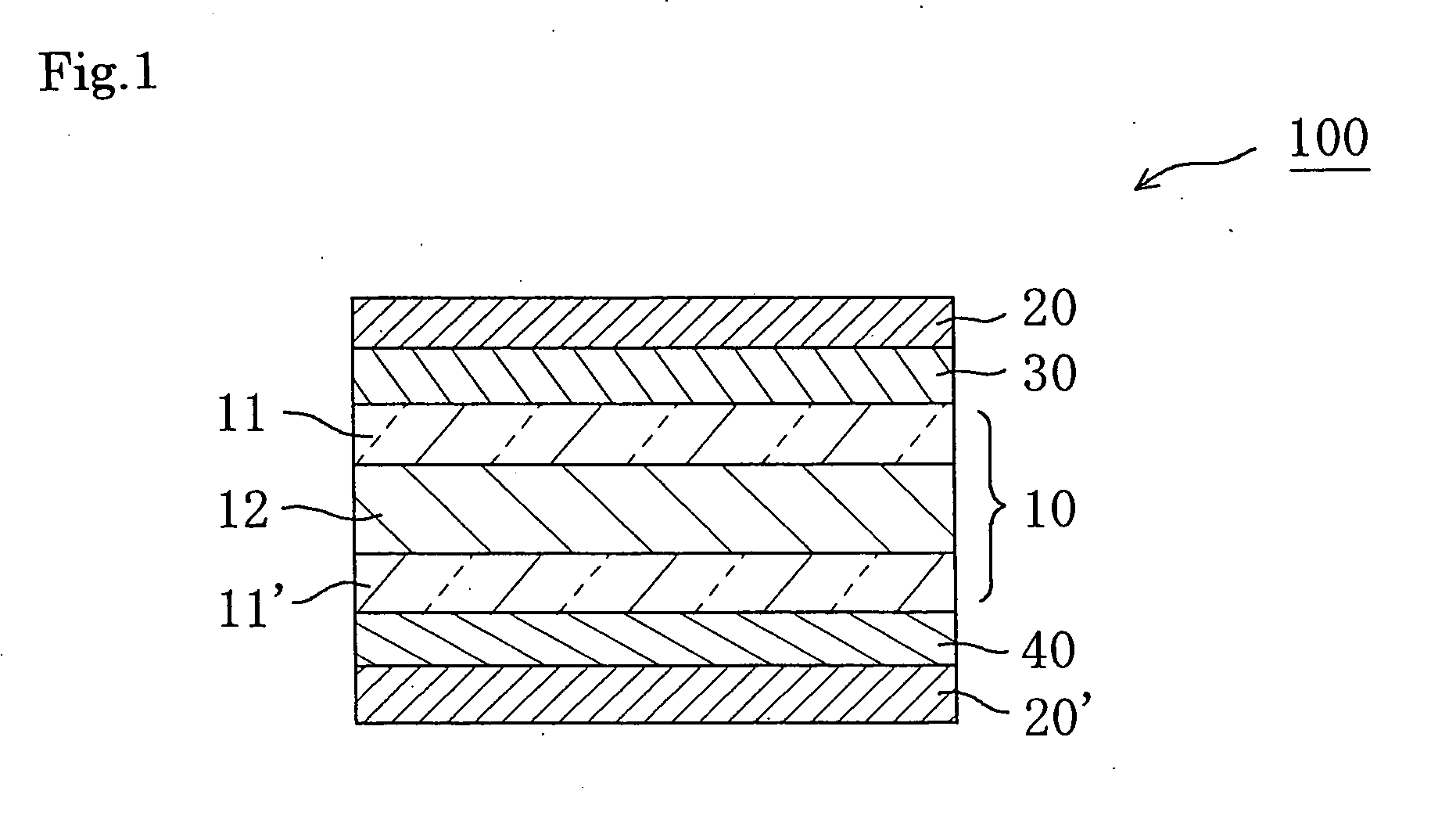
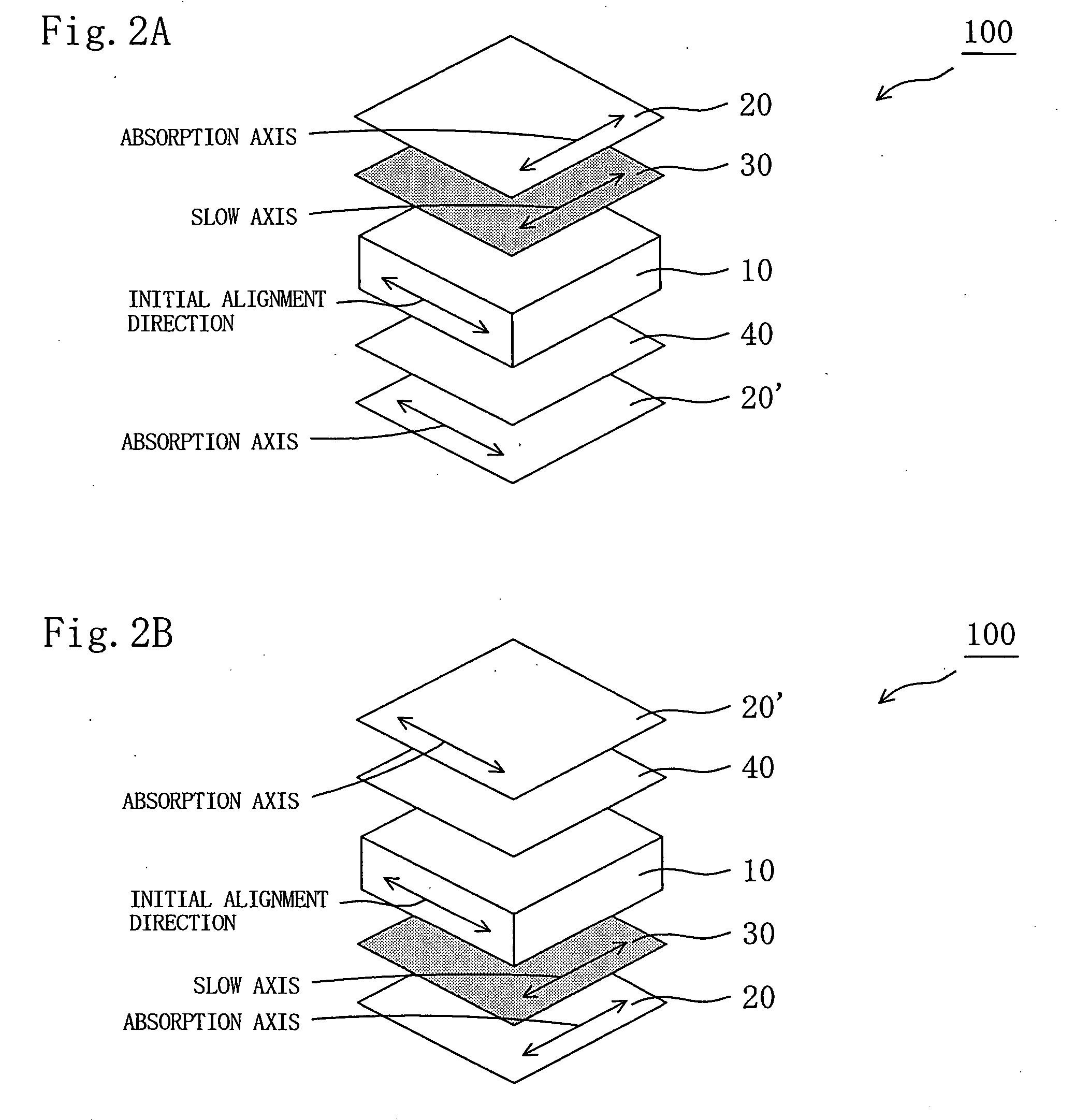
Retardation plate and fabrication method thereof, and plate for circularly polarizing light, 1/2 wave plate and reflection-type liquid crystal display device utilizing the retardation plate
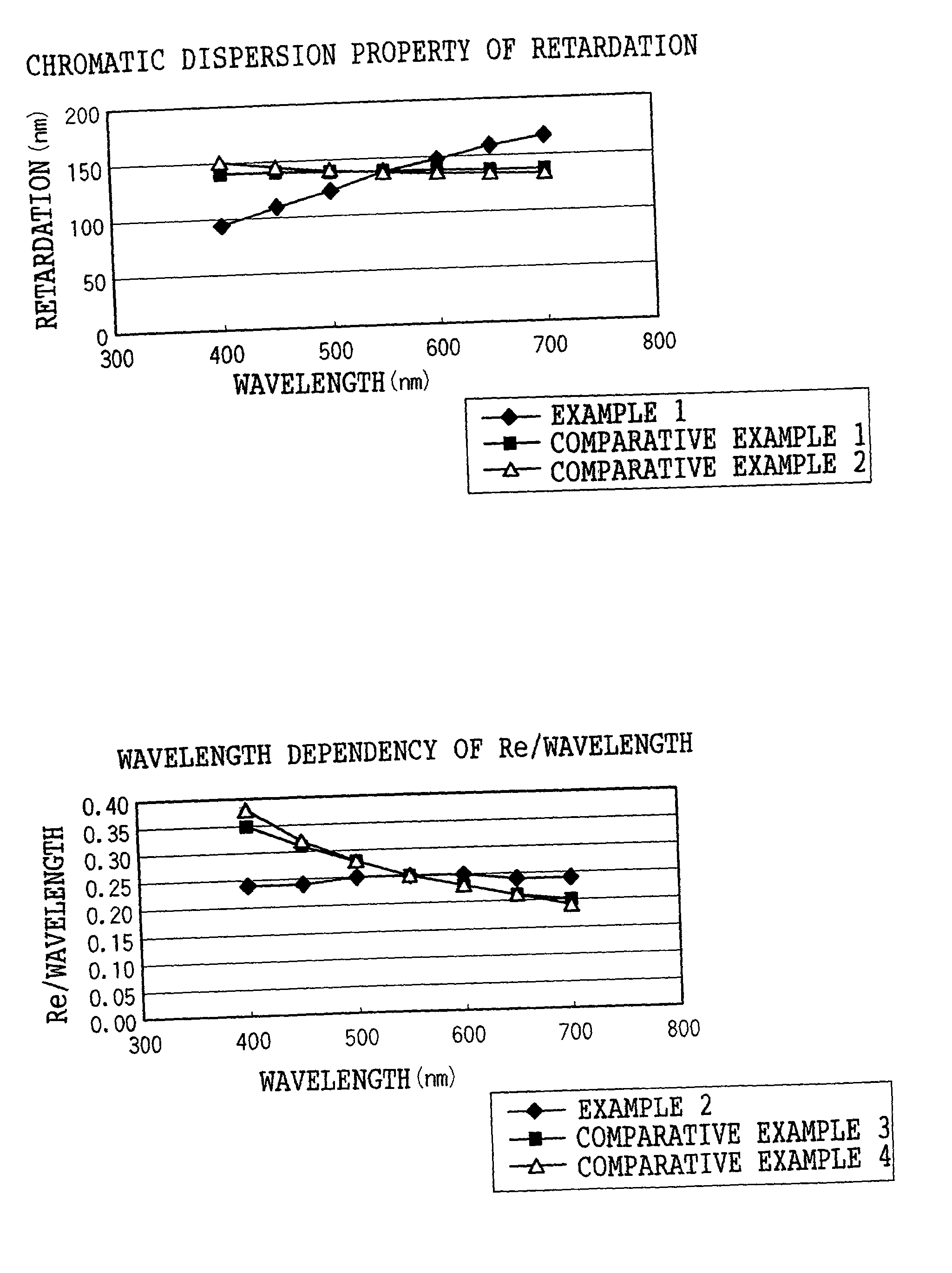
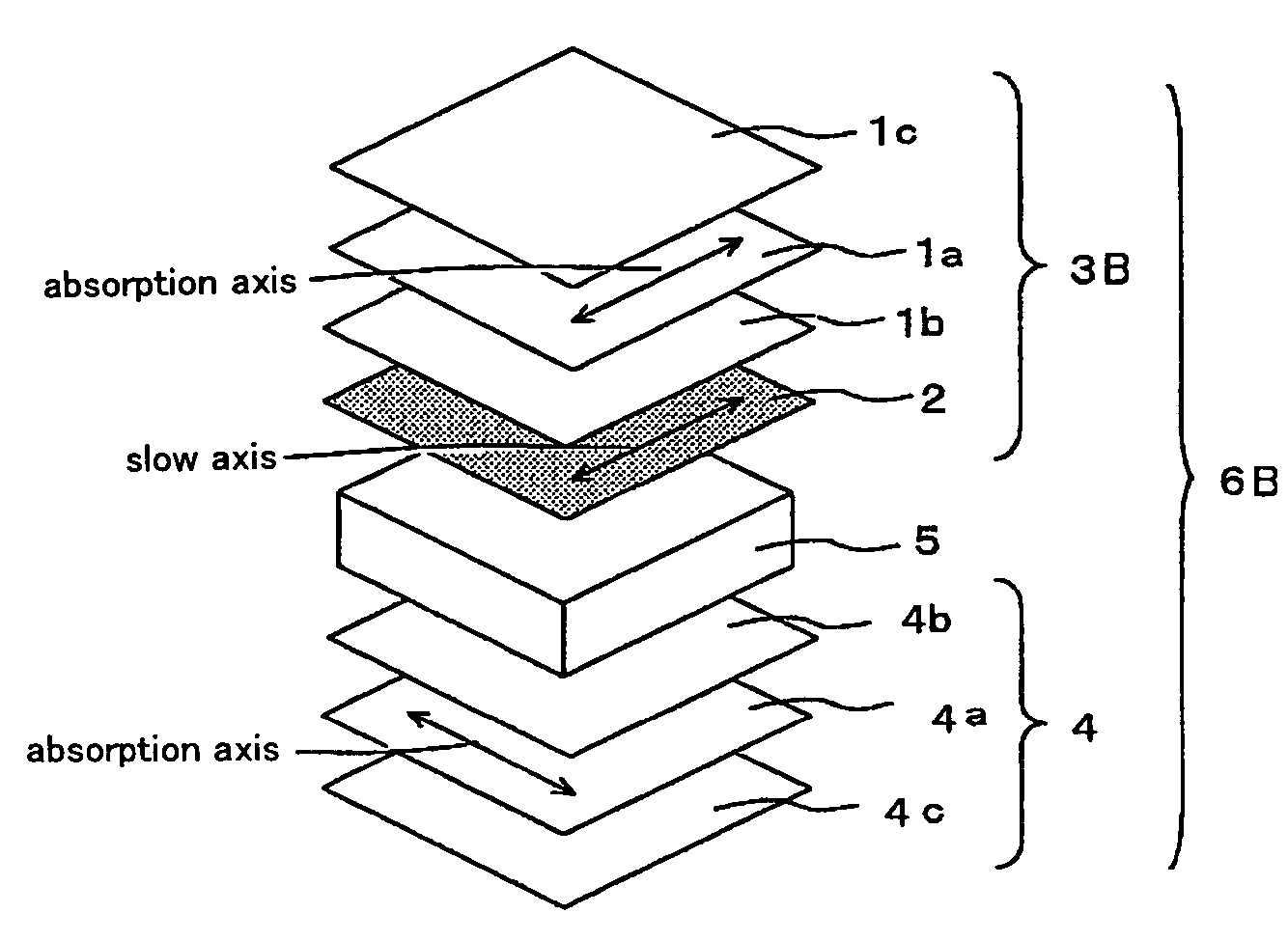
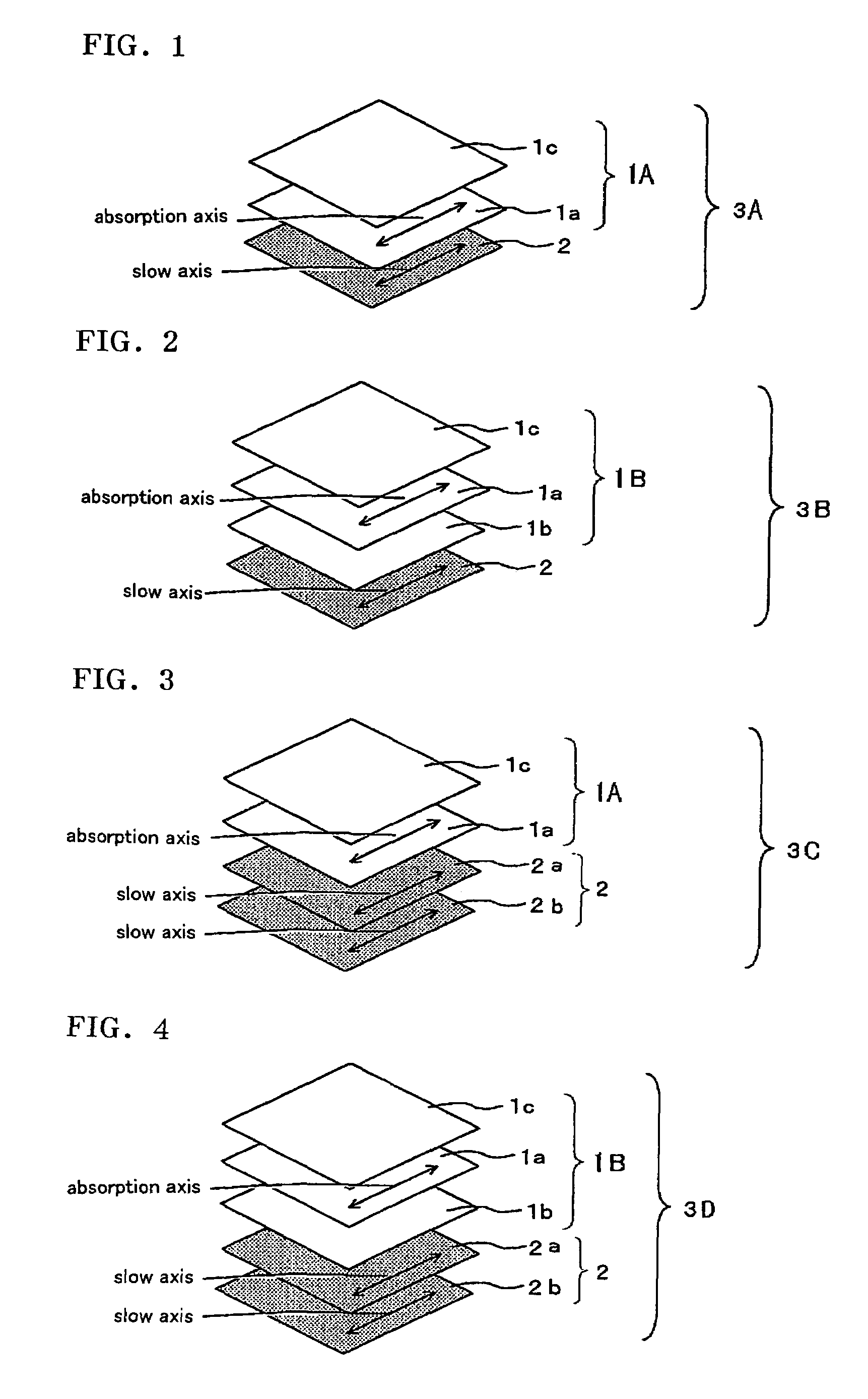
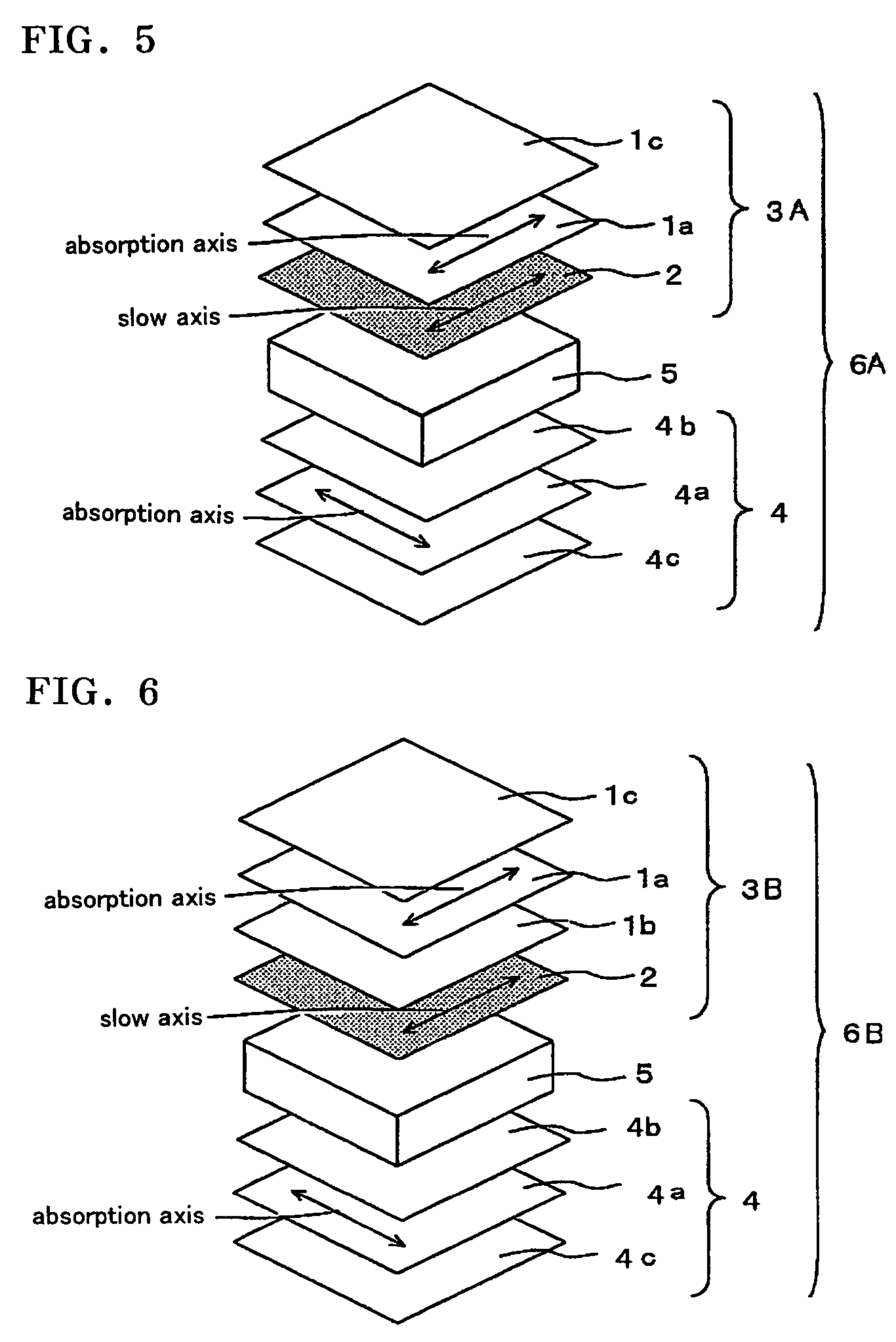
The present invention provides a broad band retardation plate that can be fabricated by a simple process and uniformly retards light incident of the entire visible light region. The retardation plate contains materials including positive or negative intrinsic double refraction values. When retardation values in wavelengths of 450 nm, 550 nm, and 650 nm are defined as Re(450), Re(550) and Re(650), respectively, the retardation plate satisfies the relational expression of Re(450)<Re(550)<Re(650). The retardation plate has a first layer comprising a positive material and a second layer comprising a negative material. The first layer and second layers have double refraction, and are laminated such that lag axes of the both layers are orthogonally crossed. It is preferable that the positive material is a norbornene based polymer and the like.
Owner:FUJIFILM CORP +1
Photosensitive compositions based on polycyclic polymers for low stress, high temperature films
InactiveUS20060020068A1Increase variabilityFilm/foil adhesivesSemiconductor/solid-state device manufacturingAddition polymerNorbornene
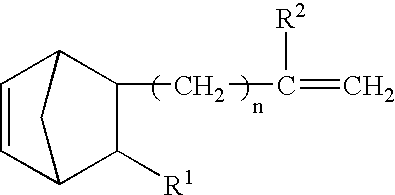
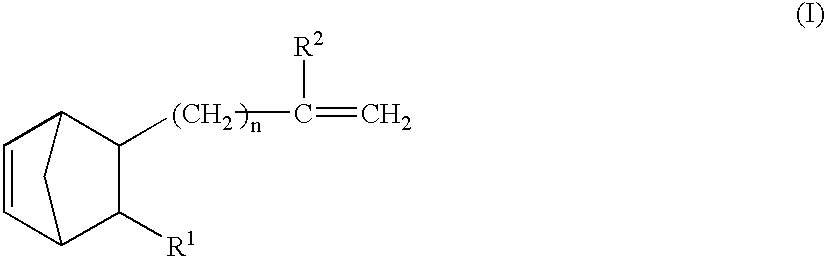
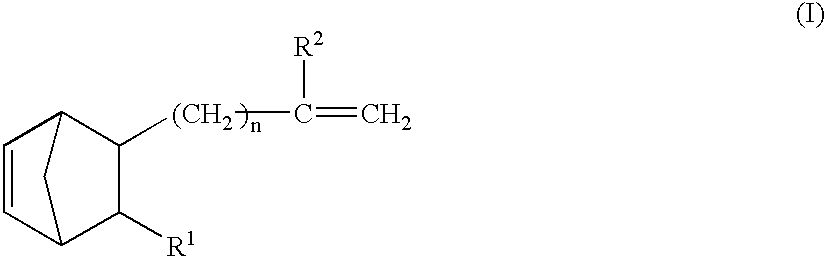
Vinyl addition polymer compositions, methods for forming such compositions, methods for using such compositions to form microelectronic and optoelectronic devices are provided. The vinyl addition polymer encompassed by such compositions has a polymer backbone having two or more distinct types of repeat units derived from norbornene-type monomers independently selected from monomers of Formula I: wherein each of X, m, R1, R2, R3, and R4 is as defined herein and wherein a first type of repeat unit is derived from a glycidyl ether substituted norbornene monomer and a second type of repeat unit is derived from an aralkyl substituted norbornene monomer.
Owner:PROMERUS LLC
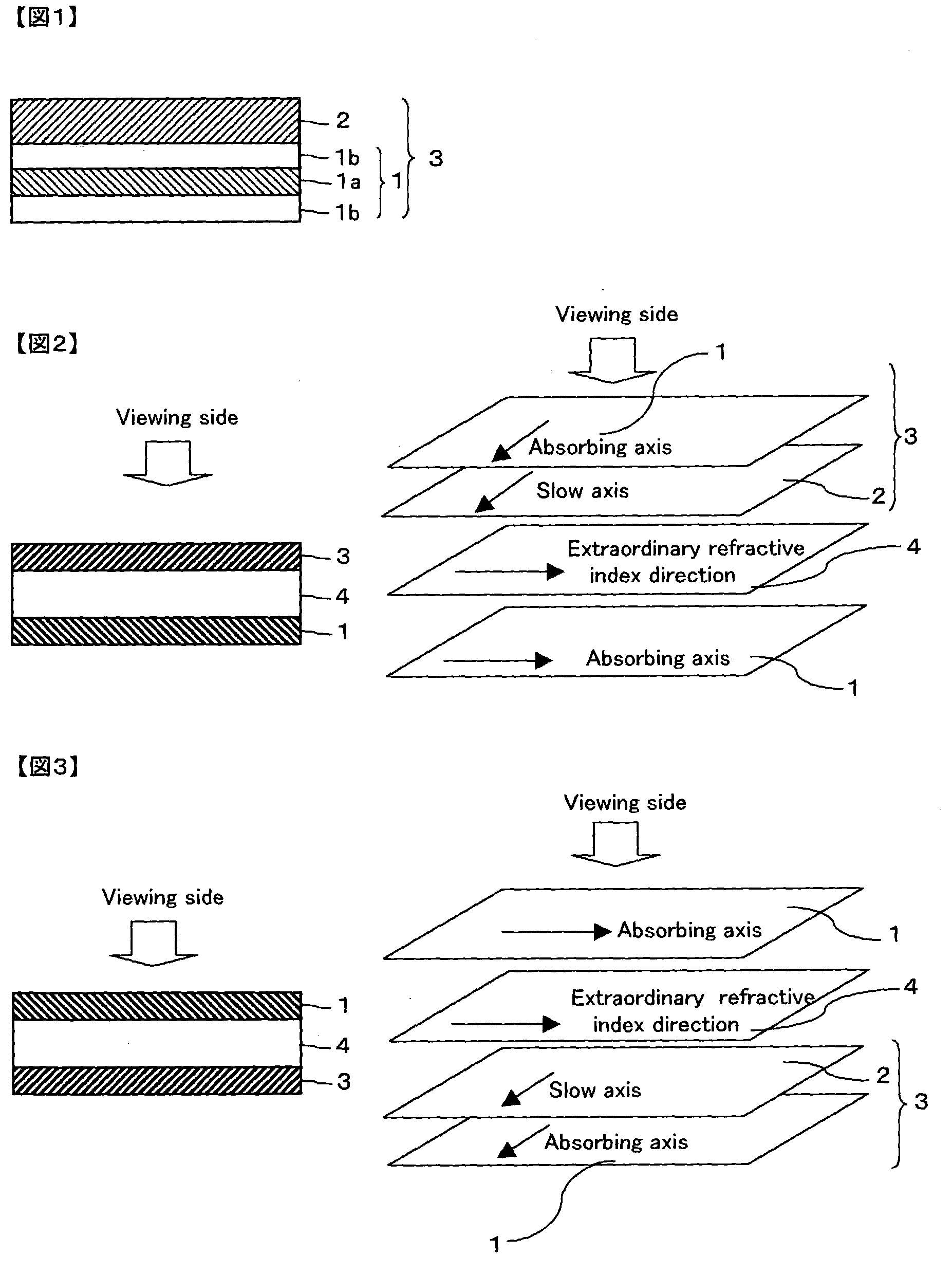
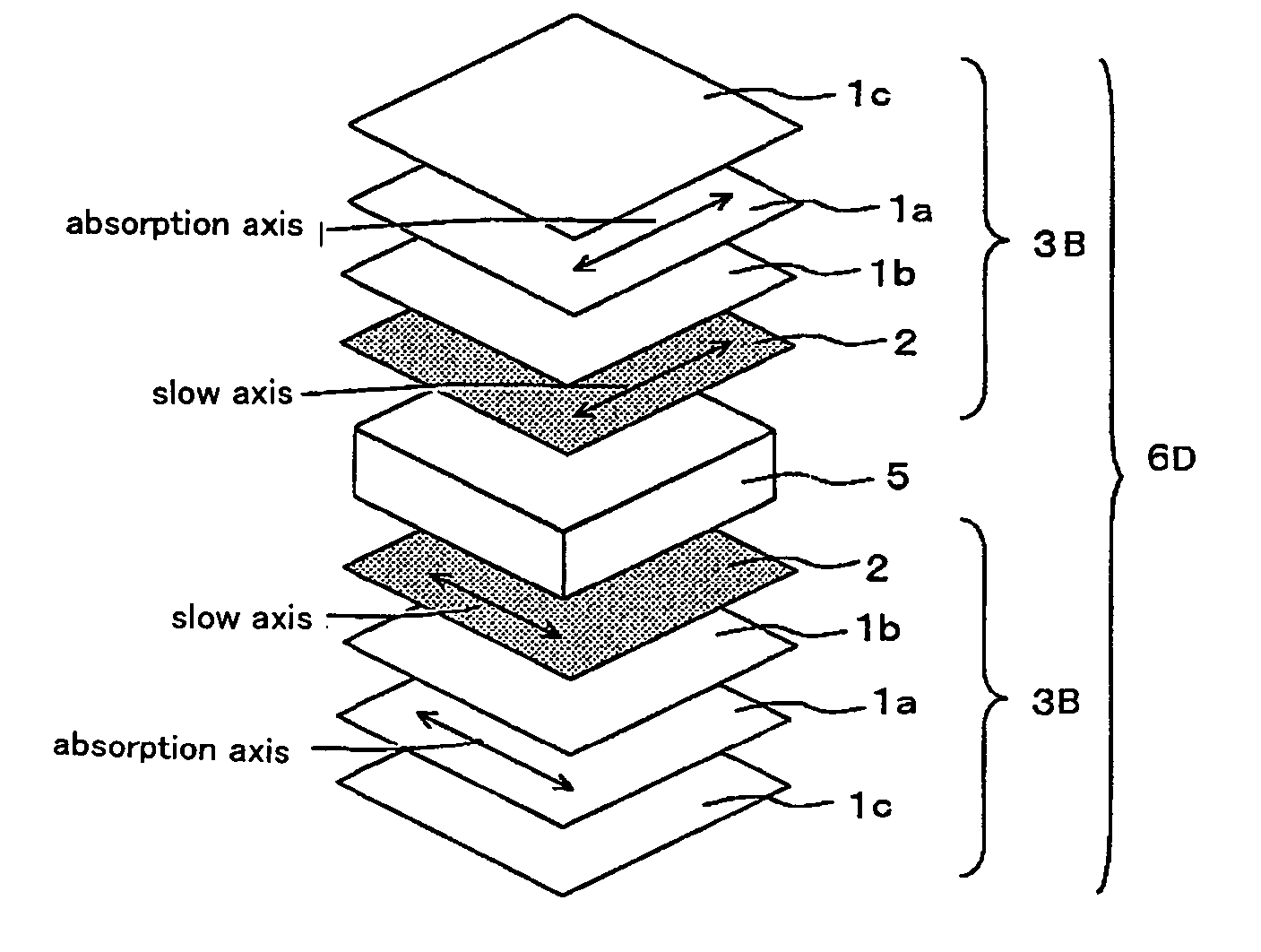
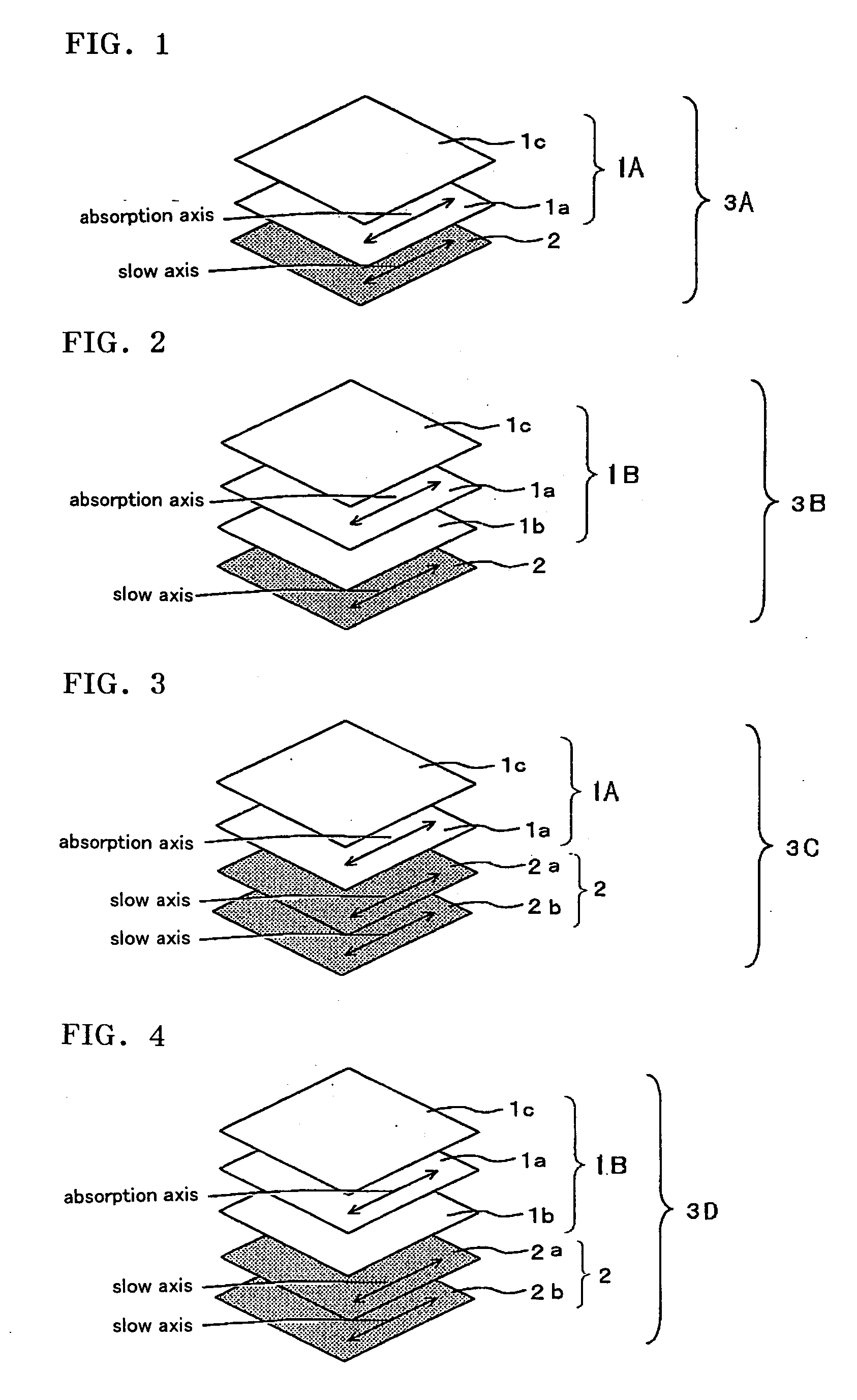
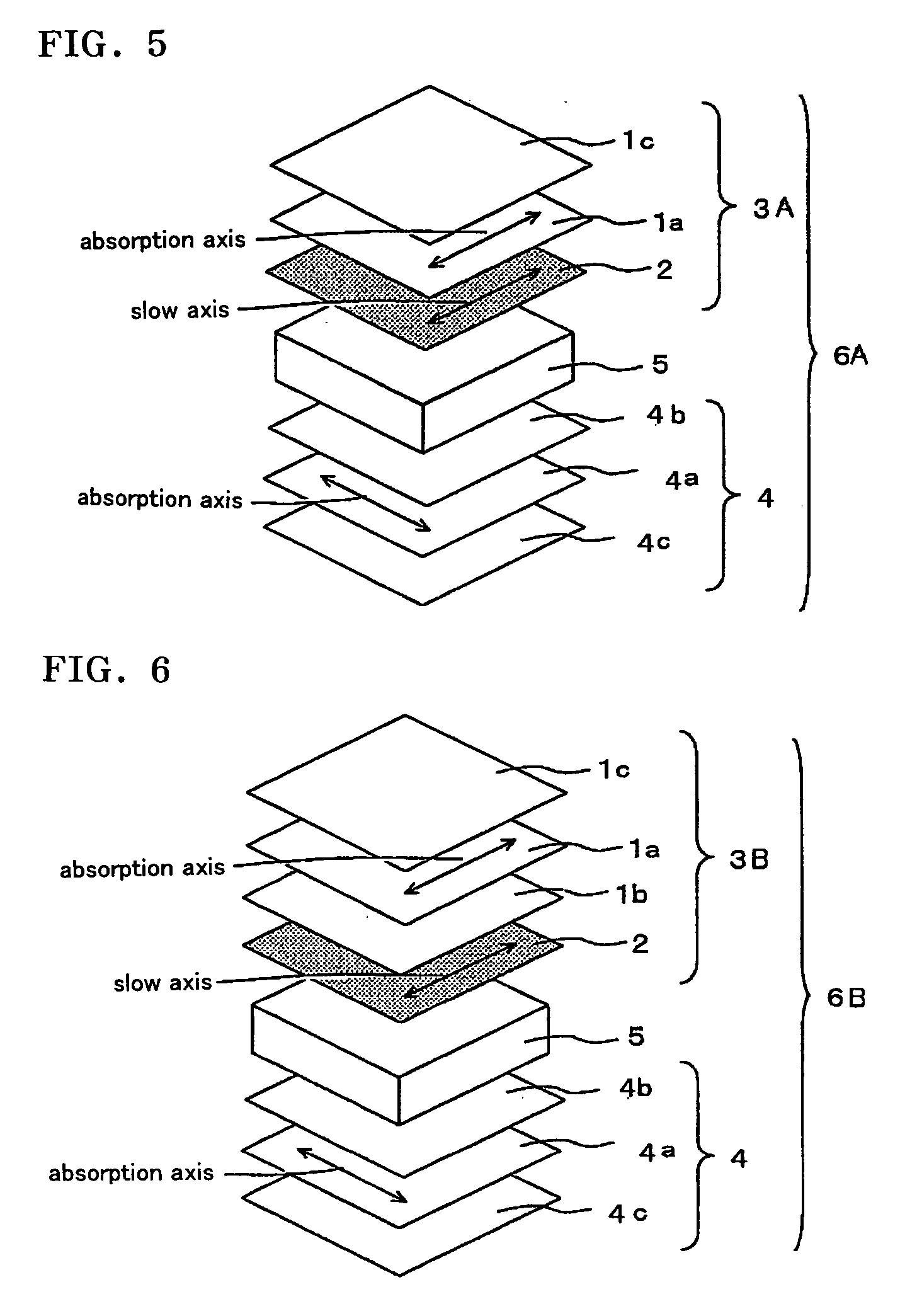
Optical film and display system
InactiveUS20030210370A1Easy to viewIncrease contrastPolarising elementsNon-linear opticsIn planeTectorial membrane
An optical film in which a retardation film is laminated on one side of a polarizing plate, in which a transparent protective film is laminated on both sides of a polarizer, so that an absorbing axis of the polarizing plate and a slow axis of the retardation film may be perpendicular or may be parallel to each other, wherein a value Nz represented by Nz=(nx1-nzi) / (nx1-ny1) satisfies a range of 0.4 through 0.6, and an in-plane retardation Re1=(nx1-ny1)xd1 is 200 through 350 nm, where, a direction of the retardation film in which an in-plane refractive index within the film surface concerned gives a maximum is defined as X-axis, a direction perpendicular to X axis is defined as Y-axis, a thickness direction of the film is defined as Z-axis, refractive indexes in axial direction are defined as nx1, ny1, nz1, respectively, and a thickness of the film is defined as d1 (nm), and the transparent protective films comprise a thermoplastic saturated norbornene resin, may realize an easily viewable display with high contrast ratio in a wide range when applied to a display system and that may provide a retardation value stabilized under conditions of high temperature or high humidity.
Owner:NITTO DENKO CORP
Barrier Packaging Webs Having Metallized Non-Oriented Film
ActiveUS20090110888A1Improve sealingImprove barrier propertiesFlexible coversWrappersThermoplasticFiber
Owner:BEMIS COMPANY INC
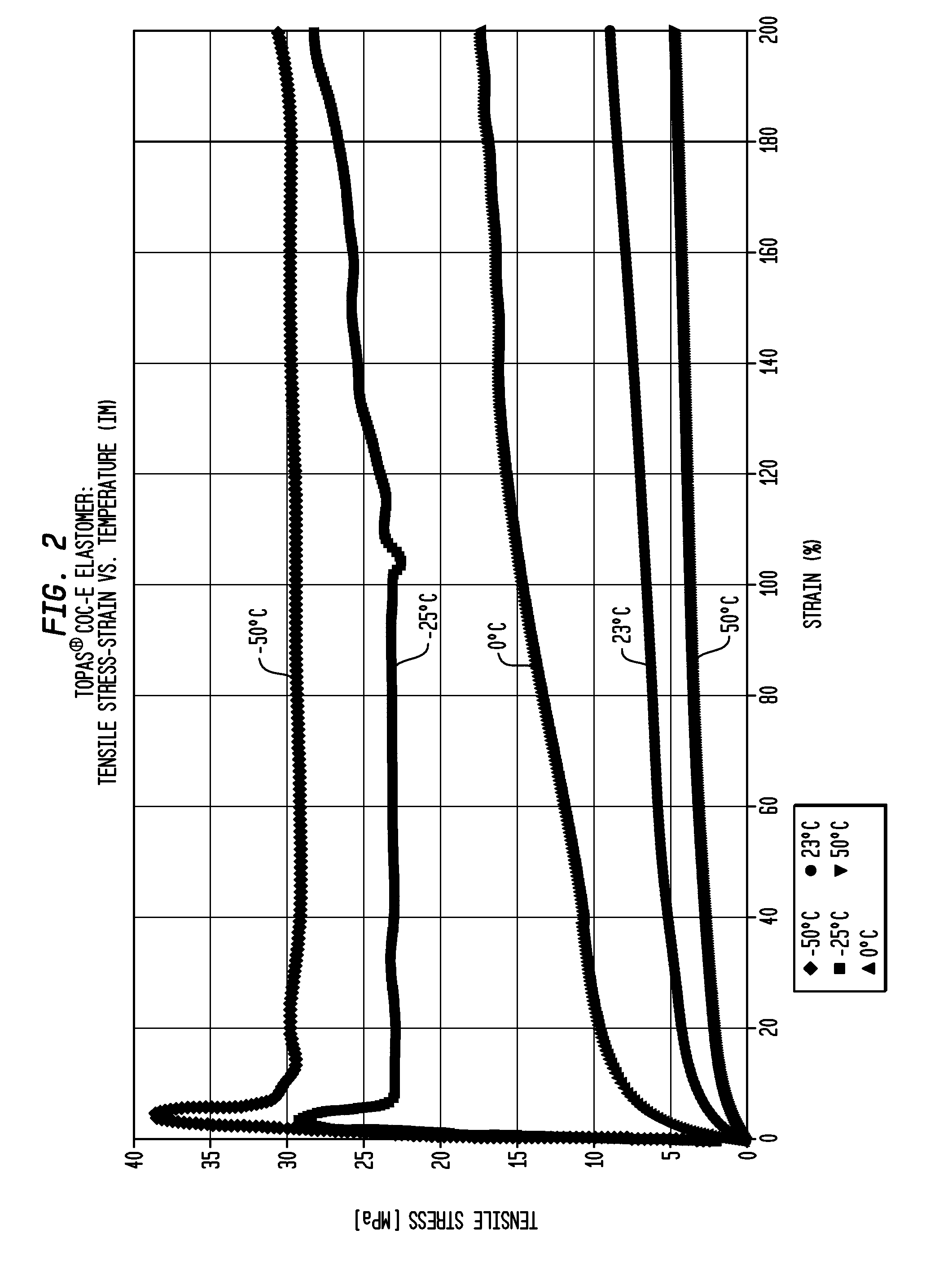
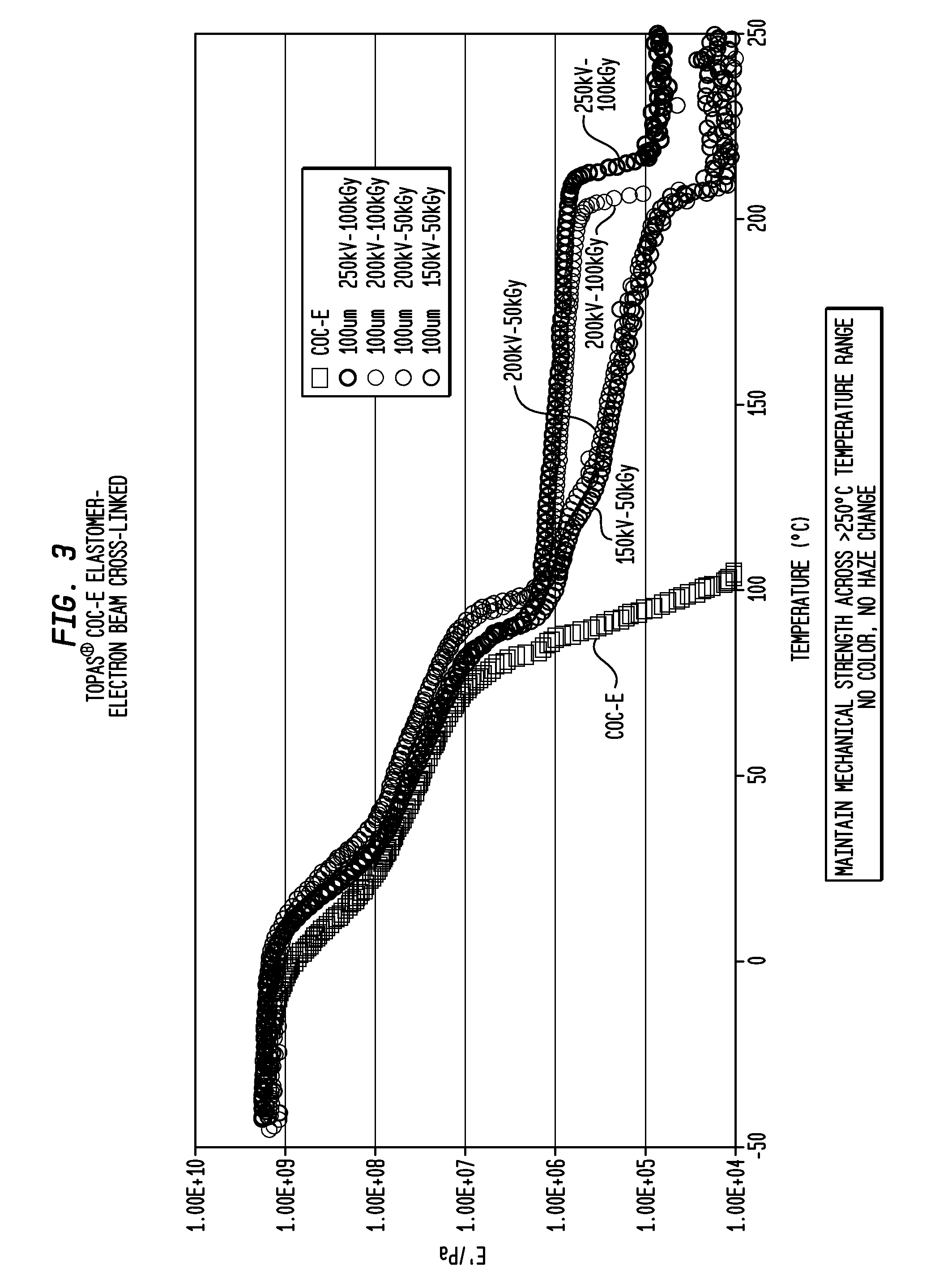
Transparent, Flexible Products Made With Partially Crystalline Cycloolefin Elastomer
InactiveUS20120021151A1Allocation is accurateImprove mechanical propertiesWrappers shrinkageClosuresElastomerNorbornene
Shaped articles made with a partially crystalline, cycloolefin elastomer of norbornene and ethylene typically having at least one glass transition temperature (Tg) in the range of from −10° C. to 15° C. and a crystalline melting temperature in the range of from 60° C. to 125° C. and a % crystallinity by weight in the range of from 5% to 40%. The shaped articles may be in the form of medical tubing; a contact lens mold or component thereof; a container such as a bottle, a squeeze bottle or a squeeze tube; an eyedropper or eyedropper component; an elastomeric closure, optionally a pierceable elastomeric closure or the shaped article is selected from shrink film and / or shrink tubing.
Owner:TOPAS ADVANCED POLYMERS
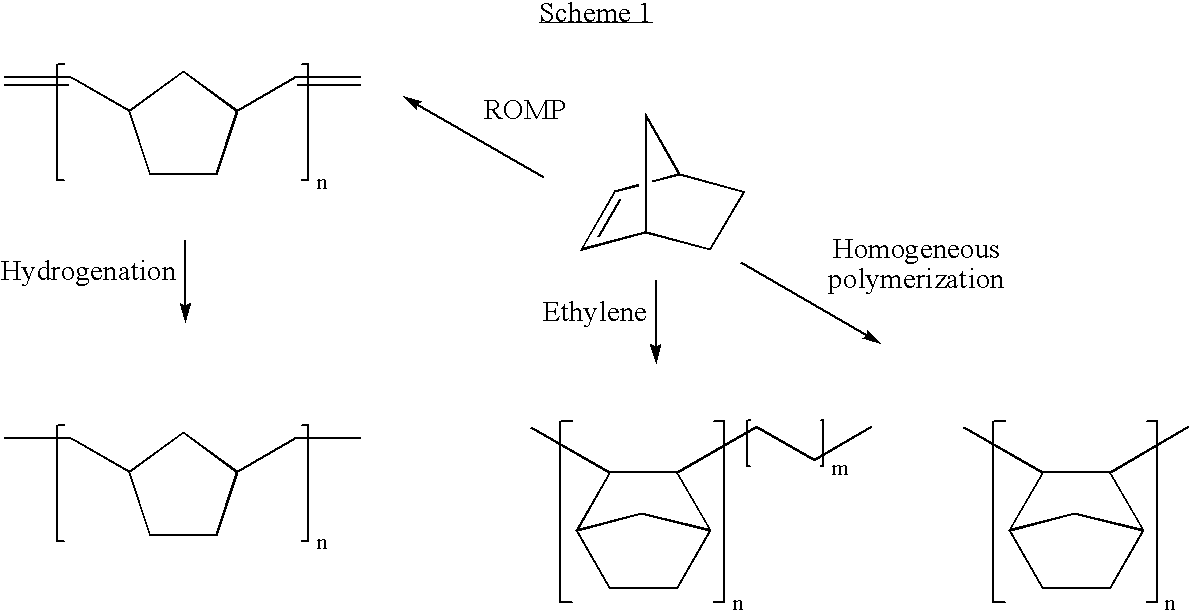
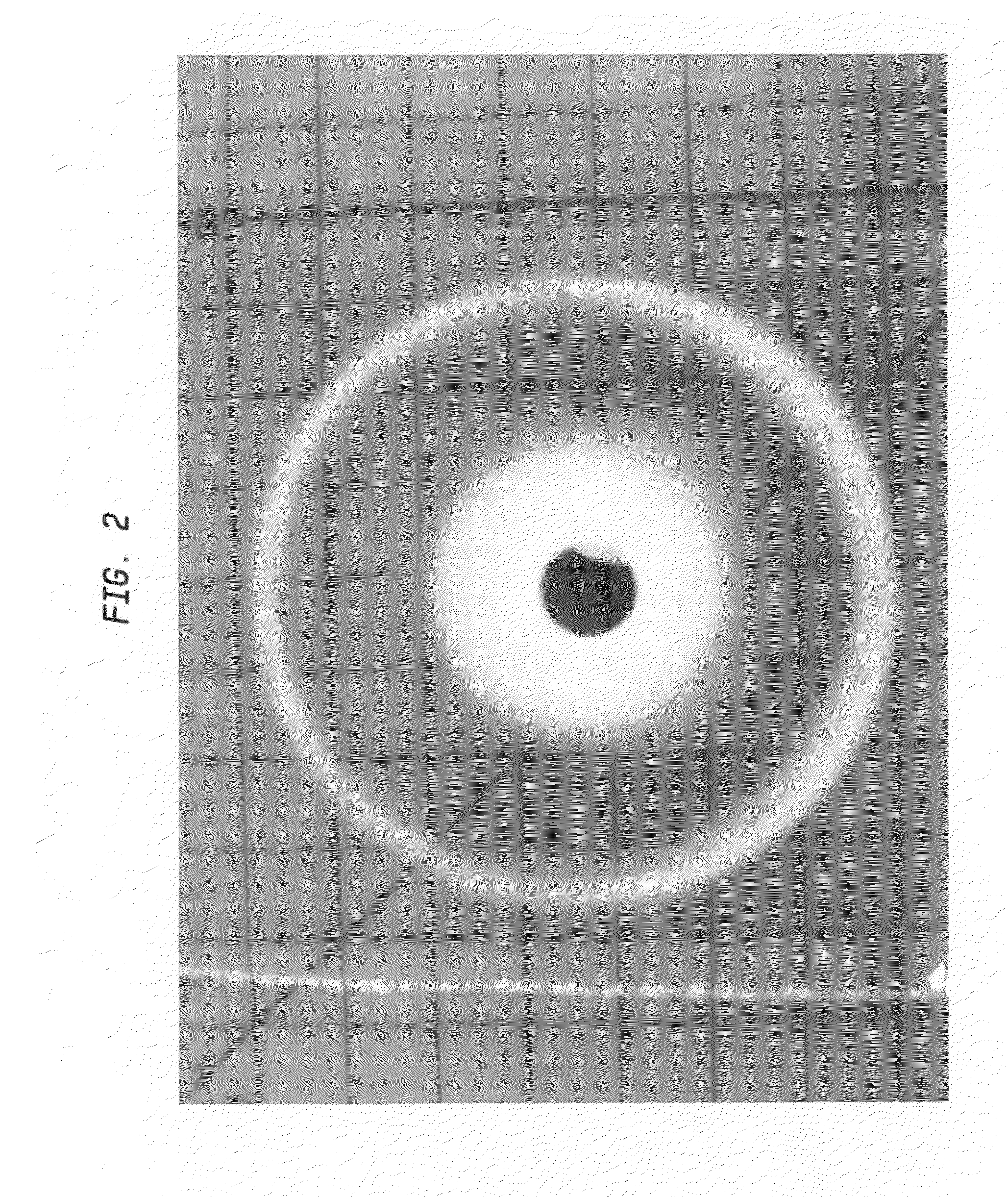
Norbornene-based ring-opening polymerization polymer, product of hydrogenation of norbornene-based ring-opening polymerization polymer, and processes for producing these
A norbornene ring-opened polymer which in the molecule has repeating units represented by the formula (1): wherein R1 represents Q, R2 represents Q or C(═O)R5, R3 represents Q or C(═O)R6, and R4 represents Q or X—C(═O)R7 (wherein Q represents hydrogen, a C1-10 hydrocarbon group, etc.; R5, R6 and R7 each represents hydroxyl, C1-10 alkoxyl, etc., provided that R6 and R7 may be bonded to each other to constitute oxygen, NH, etc.; X represents methylene, etc., provided that when R2 is Q, then R3 is C(═O)R6 and R4 is X—C(═O)R7 and that when R4 is Q, then R2 is C(═O)R5, R3 is C(═O)R6, and the configuration of R2 and R3 is trans, and m is 0 or 1), the polymer having a weight-average molecular weight as determined by gel permeation chromatography of 1,000 to 1,000,000. Also provided is a hydrogenation product of the norbornene ring-opened polymer. The norbornene ring-opened polymer and the hydrogenation product are excellent in heat resistance, electrical properties, etc.
Owner:ZEON CORP
Retardation film, process for producing the same, optical film, image display, liquid crystal panel and liquid crystal display
ActiveUS20060028601A1Retardation valueHard to cause a shift or an unevenness of a retardation valueOptical light guidesNon-linear opticsRefractive indexDisplay device
A retardation film of the present invention comprises a stretched film of a polymer film containing a norbornene-based resin, wherein the stretched film satisfies the following equation (1) and the equation (2); 100 nm≦(nx−ny)·d≦350 nm . . . (1),0.1≦(nx−nz) / (nx−ny)≦0.9 . . . (2), where the refractive indices in the slow axis direction, the fast axis direction and the thickness direction of the film are nx, ny and nz, respectively, d(nm) is thickness of the film, and the slow axis direction is a direction that the refractive index in film plane is maximum. The retardation film is hard to cause a shift or an unevenness of a retardation value due to a stress.
Owner:NITTO DENKO CORP
Mold addition polymerization of norbornene-type monomers using group 10 metal complexes
InactiveUS6350832B1Increase displacementMore efficient in polymerizing polycyclic olefinsCoatingsThermoplasticPolymer science
A catalyst system and a process for the bulk addition polymerization or of polycyclic olefins, such as norbornene, methylnorbornene, ethylnorbornene, butylnorbornene or hexylnorbornene, 1,2,3,4,4a,5,8,8a-octahydro-1,4:5,8-dimethanonapthalene, 5,5'-(1,2-ethanediyl)bisbicyclo[2.2.1]hept-2-ene, and 1,4,4a,4b,5,8,8a,8b-octahydro-1,4:5,8-dimethanobiphenylene are disclosed. The catalyst includes an organonickel or organopalladium transition metal procatalyst and an activator compound. Polymerization can be carried out in a reaction injection molding process to yield thermoplastic and thermoset molded polymeric articles possessing high glass transition temperatures.
Owner:SUMITOMO BAKELITE CO LTD
Thermoplastic vulcanizates from a cyclic olefin rubber, a polyolefin, and a compatiblizer
A blend of a semicrystalline polyolefin, a rubbery polymer containing repeat units from norbornene, and a polymeric compatibilizer is disclosed. This composition has reduced oil swell as compared to thermoplastic vulcanizates from a semicrystalline polyolefin and hydrocarbon rubbers.
Owner:ADVANCED ELASTOMER SYST LP
Open-ring copolymer, hydrogenated open-ring copolymer, process for production of both, and compositions
A ring-opened metathesis copolymer and its hydrogenated product having a desired monomer unit ratio and a high molecular weight and having hydroxyl groups or hydroxycarbonyl groups can be obtained by conducting a ring-opening metathesis copolymerization of a norbornene-type monomer having hydroxyl groups or hydroxycarbonyl groups with an unsubstituted norbornene-type monomer having at least three rings in the presence of a catalyst predominantly comprised of an organic ruthenium compound having coordinated therewith a neutral electron-donating ligand, and, if desired, hydrogenating the resulting copolymer. The thus-obtained ring-opened metathesis copolymer and its hydrogenation product are characterized by having a low water absorption, good adhesion to metal and other materials, good compatibility with a curing agent and other compounds, and high heat resistance, and exhibiting reduced signal retardation and signal noise.
Owner:ZEON CORP
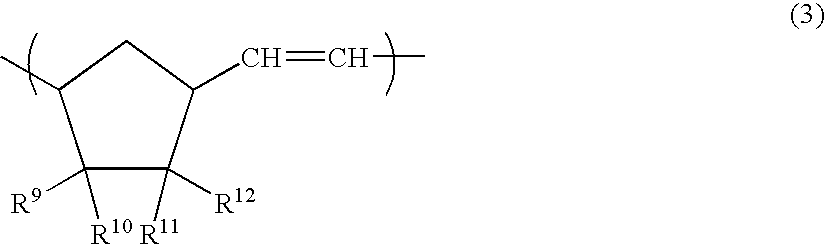
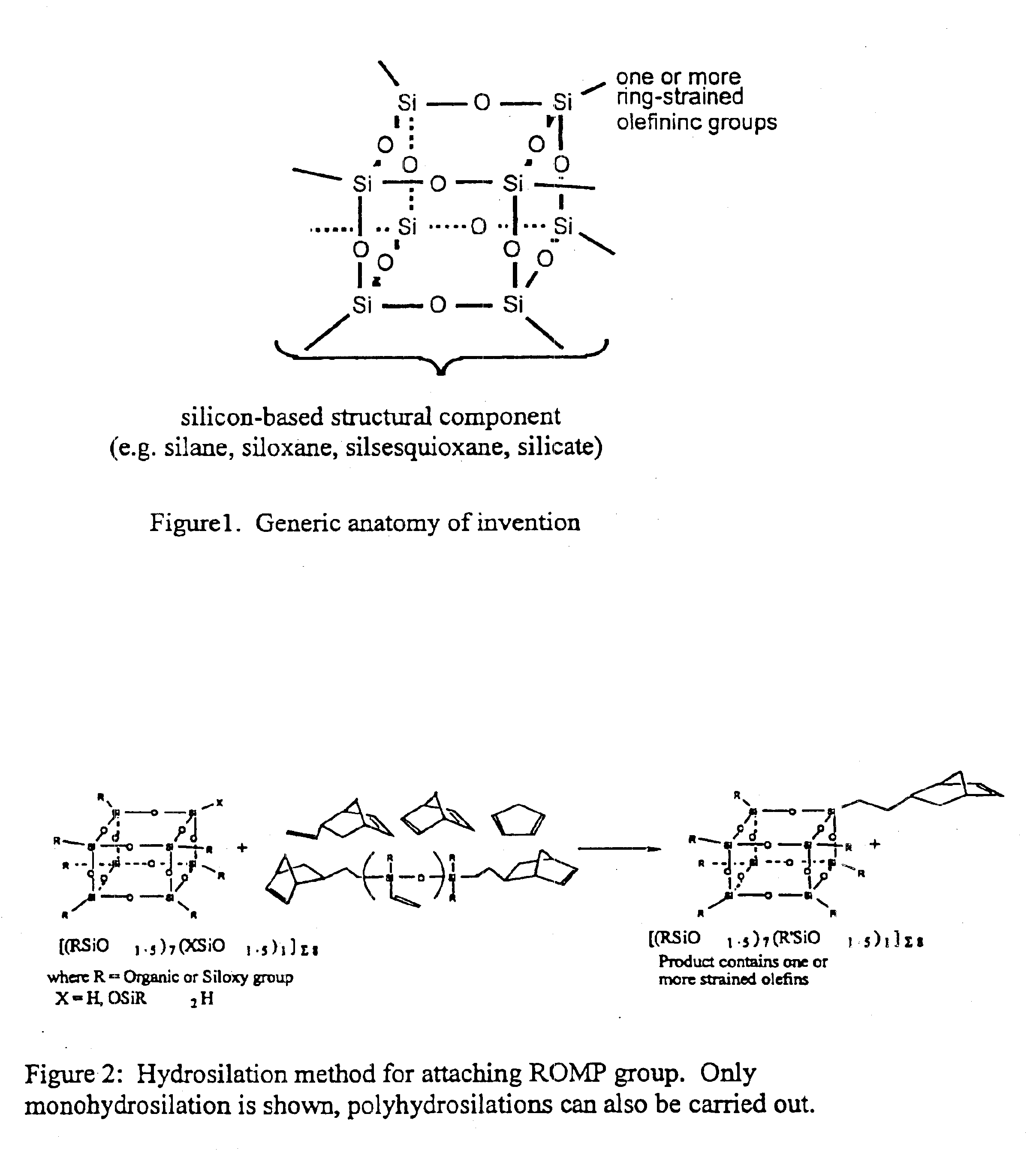
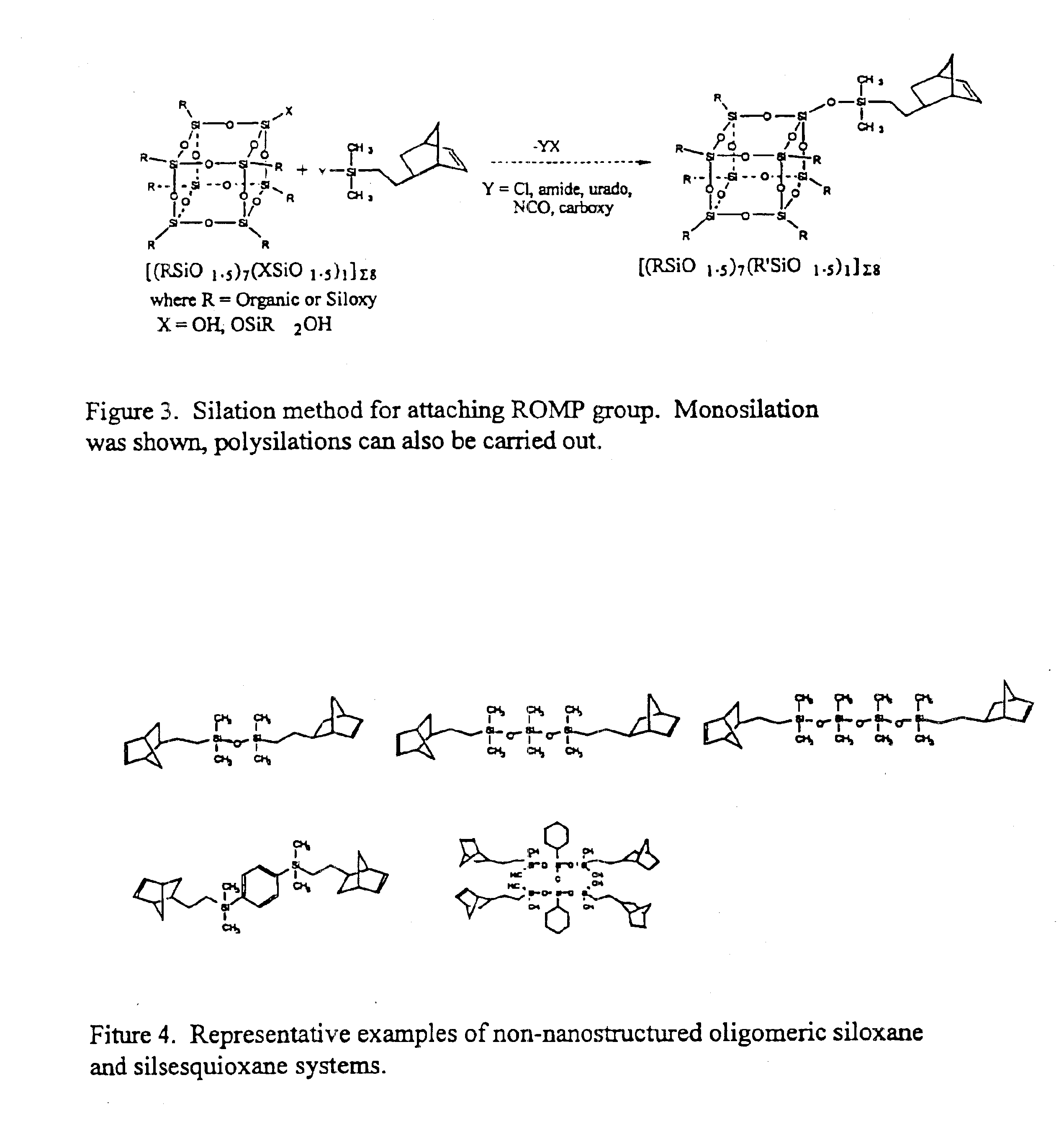
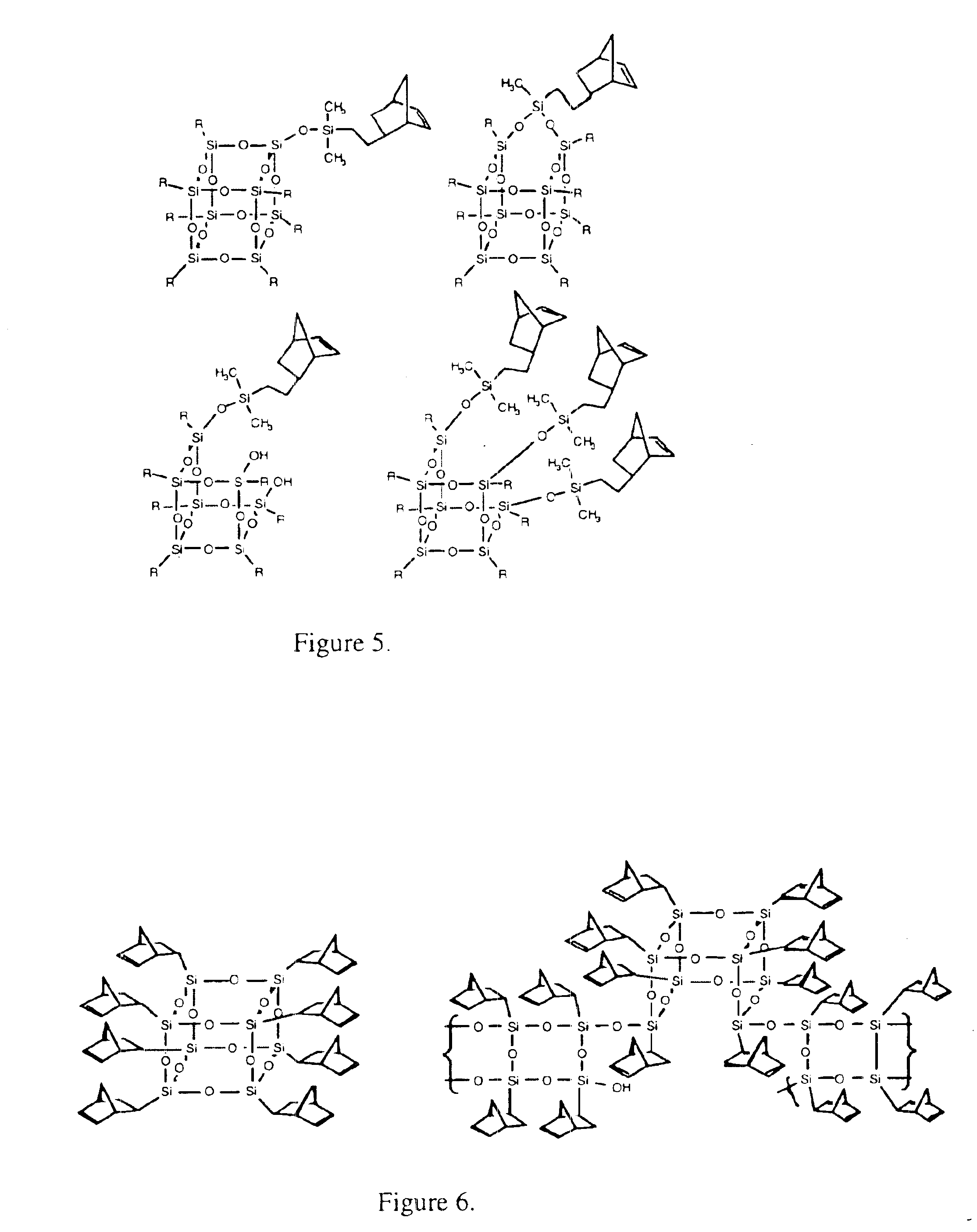
Polyhedral oligomeric -silsesquioxanes, -silicates and -siloxanes bearing ring-strained olefinic functionalities
InactiveUS6911518B2Improving biocompatabilityImprove fire resistanceSilicon organic compoundsGroup 6/16 organic compounds without C-metal linkagesAlkaline earth metalChemical reaction
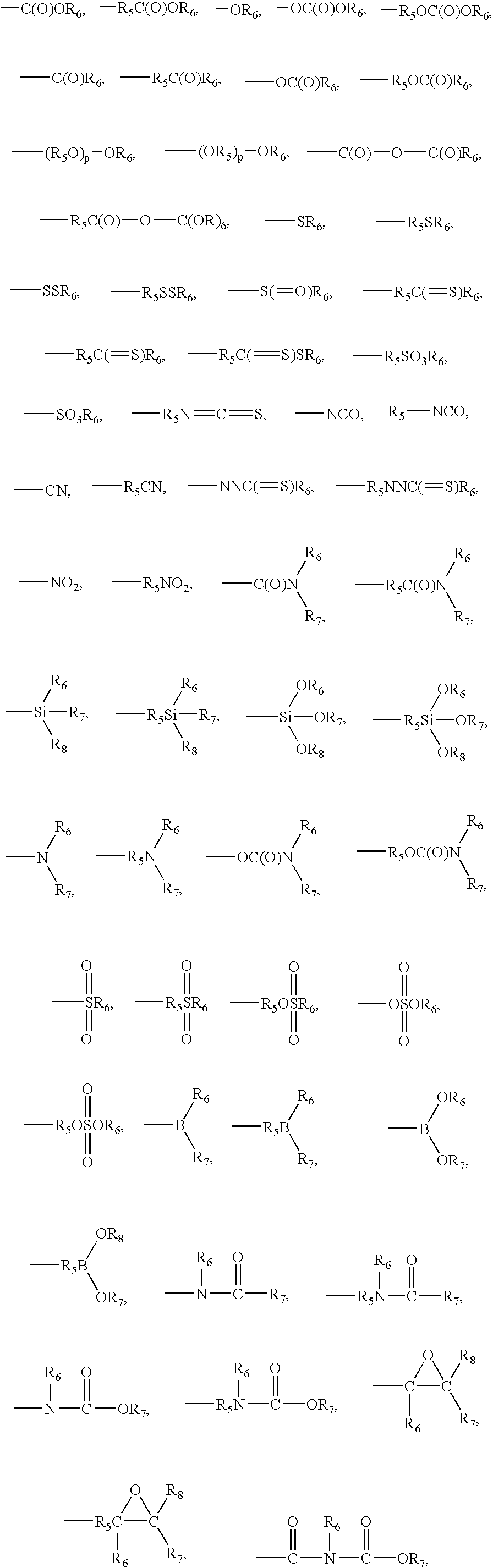
Processes have been developed for the manufacture of polyhedral oligomeric silsesquioxanes (POSS), polysilsesquioxanes, polyhedral oligomeric silicates (POS), and siloxane molecules bearing reactive ring-strained cyclic olefins (e.g. norbornenyl, cyclopentenyl, etc. functionalities). The preferred manufacturing processes employ the silation of siloxides (Si—OA, where A=H, alkaline or alkaline earth metals) with silane reagents that contain at least one reactive ring-strained cyclic olefin functionality [e.g., X3-ySi(CH3)y(CH2)2 where y=1-2 and X=OH, Cl, Br, I, alkoxide OR, acetate OOCR, peroxide OOR, amine NR2, isocyanate NCO, and R]. Alternatively, similar products can be prepared through hydrosilation reactions between silanes containing at least one silicon-hydrogen bond (Si—H) with ring-strained cyclic olefin reagents [e.g., 5-vinyl, 2 norbornene CH2═CH, cyclopentadiene]. The two processes can be effectively practiced using polymeric silsesquioxanes [RSiO1.5]∞ where ∞=1-1,000,000 or higher and which contain unreacted silanol or silane groups at chain terminus or branch points, on POSS nanostructures of formulas [(RSiO1.5)n]Σ#, homoleptic, [(RSiO1.5)m(R′SiO1.5)n]Σ#, heteroleptic, and {(RSiO1.5)m(RXSiO1.0)n}Σ#, functionalized heteroleptic nanostructures, on silanes RSiX3, linear, cyclic, oligomeric and polymeric siloxanes (polymeric formula RX2Si—(OSiRX)m—OSiRX2 where m=0-1000, X=OH, Cl, Br, I, alkoxide OR, acetate OOCR, peroxide OOR, amine NR2, isocyanate NCO, and R). Each of the processes result in new chemical species bearing one or more ring strained olefins that can undergo polymerization, grafting, or other desirable chemical reactions to form polymeric products. These polymeric systems are most desirably utilized in polymerizations for the modification of properties of thermoplastic or thermoset resin systems or for the preparation of polymers with utility in electronics, medical devices, sporting goods, and aerospace as coatings and structural components.
Owner:HYBRID PLASTICS INC
In-Reactor Polymer Blends
This invention relates to in-reactor polymer blends comprising at least 60 mole % of propylene and from 0.01 to 10 mole % of at least one diene selected from the group of C6 to C12 α,ω-diene, norbornadiene, vinyl norbornene and mixtures thereof with the balance being ethylene. The blend comprises first and second polymers having different crystallinities and or different Tg's.
Owner:EXXONMOBIL CHEM PAT INC
Negative C-plate type optical anisotropic film comprising poly cycloolefin and method for preparing the same
ActiveUS7524542B2No optical lossSuperior water absorption resistance and durabilityLiquid crystal compositionsPretreated surfacesAddition polymerLiquid-crystal display
Owner:LG CHEM LTD
Crosslinkable rubber compositions and uses thereof
The crosslinkable rubber composition is crosslinkable at room temperature, has a gelation time at room temperature of 30 days or less, and can prepare a crosslinked rubber sheet by crosslinking the composition at room temperature, wherein the crosslinked rubber sheet has a tensile elongation of 20% or more, and is free of cracks after treatment at 40° C. in a 50 pphm ozone concentration for 96 hr. Specifically, it comprises an ethylene / alpha-olefin / non-conjugated polyene random copolymer rubber comprising a norbornene compound having a specific vinyl end group, an SiH group-containing compound, which has at least two SiH groups in one molecule, and optionally a platinum catalyst, a reaction inhibitor and / or a silane-coupling agent. The sealing, potting and coating materials and adhesives of the present invention comprise the above rubber composition. The rubber composition has a high crosslinking rate at room temperature and excellent productivity, and can prepare crosslinked rubber molded products (including foamed products) having excellent weathering resistance, ozone resistance, heat aging resistance and compression set at low cost. Further, it is suitable for use of electric and electronic parts, transportation machines, civil engineering and construction materials, medical appliances and goods for leisure activities.
Owner:MITSUI CHEM INC
Liquid crystal panel and liquid crystal display apparatus
ActiveUS20060066787A1Increase contrastImprove display uniformityLiquid crystal compositionsThin material handlingPolarizerNorbornene
A liquid crystal panel having improved contrast ratio in an oblique direction and good display evenness without causing shift or unevenness in retardation values due to shrinkage stress of a polarizer or heat of backlight is provided. A liquid crystal panel according to an embodiment of the present invention includes: a liquid crystal cell; a polarizer arranged on both sides of the liquid crystal cell; a first optical element arranged between one polarizer and the liquid crystal cell; and a second optical element arranged between the other polarizer and the liquid crystal cell, wherein: the first optical element comprises a retardation film containing a norbornene-based resin and satisfying the following expressions (1) and (2); and the second optical element has substantially optical isotropy: 240nm≦Re[590]≦350nm (1) 0.20≦Rth[590] / Re[590]≦0.80 (2).
Owner:NITTO DENKO CORP
Crosslinkable rubber compositions and uses thereof
The crosslinkable rubber composition of the invention is crosslinkable by hot air, and a hot-air crosslinked rubber sheet thereof has no scratch on the surface in a hardness test using a pencil of HB and has a compression set of not more than 70% after a heat treatment at 150° C. for 22 hours. The rubber composition comprises an ethylene / α-olefin / non-conjugated polyene random copolymer rubber comprising a specific vinyl end group-containing norbornene compound, a SiH group-containing compound having at least two SiH groups in one molecule, and if necessary, an addition reaction catalyst comprising a platinum group element and a reaction inhibitor. The automobile weatherstrip, hose, rubber vibration insulator, belt, sealing material, expanded product, covered electric wire, electric wire joint, electric insulating part and household rubber product according to the invention comprise the above-mentioned rubber composition. The rubber composition has a high crosslinking rate and excellent productivity to produce crosslinked rubber molded products, is capable of undergoing hot-air crosslinking such as HAV or UHF and is capable of providing crosslinked rubber molded products having excellent compression set resistance, strength properties, heat resistance, weathering resistance and abrasion resistance.
Owner:MITSUI CHEM INC
Melt blends of amorphous cycloolefin polymers and partially crystalline cycloolefin elastomers with improved toughness
ActiveUS20110256373A1Simple compositionImprove toughnessSynthetic resin layered productsThin material handlingElastomerCyclo olefin polymer
A melt-blend resin composition prepared by melt-blending includes from 60 parts to 99 parts per hundred weight resin in the blend of an amorphous cycloolefin polymer composition exhibiting a glass transition temperature in the range of from 30° C. to 200° C.; and from 40 parts to 1 part per hundred weight resin in the blend of a partially crystalline, cycloolefin elastomer of norbornene and ethylene preferably having a glass transition temperature in the range of from −10° C. to 15° C. and a crystalline melting temperature in the range of from 60° C. to 125° C. and a % crystallinity by weight in the range of from 5% to 40%. The partially crystalline, cycloolefin elastomer optionally has a second glass transition temperature at less than −90° C.
Owner:TOPAS ADVANCED POLYMERS
Method to prepare processable polyimides with reactive endogroups using 1,3-bis(3-aminophenoxy)benzene
InactiveUS6288209B1Improved solvent resistance and modulus and elevated use temperatureImproved melt processabilityNon-fibrous pulp additionSynthetic resin layered productsPolymer scienceBackbone chain
Polyimide copolymers were obtained containing 1,3-bis(3-aminophenoxy)benzene (APB) and other diamines and dianhydrides and terminating with the appropriate amount of reactive endcapper. The reactive endcappers studied include but should not be limited to 4-phenylethynyl phthalic anhydride (PEPA), 3-aminophenoxy-4'-phenylethynylbenzophenone (3-APEB), maleic anhydride (MA) and nadic anhydride (5-norbornene-2,3-dicarboxylic anhydride, NA). Homopolymers containing only other diamines and dianhydrides which are not processable under conditions described previously can be made processable by incorporating various amounts of APB, depending on the chemical structures of the diamines and dianhydrides used. By simply changing the ratio of APB to the other diamine in the polyimide backbone, a material with a unique combination of solubility, Tg, Tm, melt viscosity, toughness and elevated temperature mechanical properties can be prepared. The copolymers that result from using APB to enhance processability have a unique combination of properties that include low pressure processing (200 psi and below), long term melt stability (several hours at 300° C. for the phenylethynyl terminated polymers), high toughness, improved solvent resistance, improved adhesive properties, and improved composite mechanical properties. These copolyimides are eminently suitable as adhesives, composite matrices, moldings, films and coatings.
Owner:NASA
Thermoplastic norbornene resin based optical film
InactiveUS20040242823A1Improve surface smoothnessSurface smoothness be controlledLiquid crystal compositionsDiffusing elementsIn planeThermoplastic
An optical film is provided, which displays a positive wavelength dependency across the entire wavelength region from 400 to 800 nm, and is capable of imparting a specified retardation to transmitted light with a single sheet of film. The optical film particularly, includes a thermoplastic norbornene-based resin with a specified structure formed of a structural unit (I) which imparts a positive birefringence and a structural unit (II) which imparts a negative birefringence, and which satisfies particular conditions with respect to DeltaNI(lambda), DeltaNII(lambda), DeltaNI(800) and DeltaNII(800) wherein DeltaNI(lambda) and DeltaNII(lambda) represent the difference between a refractive index Nx(lambda) in an x axis direction at a wavelength lambda, and a refractive index Ny(s) in a y axis direction, and DeltaNI(800) and DeltaNII(800) represent the difference in refractive indexes at a wavelength of 800 nm, and the x axis represents the stretching direction and the y axis represents the in-plane direction perpendicular to the x direction.
Owner:JSR CORPORATIOON
Method to prepare processable polyimides with reactive endgroups using 1,3-bis (3-aminophenoxy) benzene
InactiveUS6133401AImprove adhesionImprove composite effectSynthetic resin layered productsThin material handlingSolubilityAdhesive
Polyimide copolymers were obtained containing 1,3-bis(3-aminophenoxy)benzene (APB) and other diamines and dianhydrides and terminating with the appropriate amount of reactive endcapper. The reactive endcappers studied include but should not be limited to 4-phenylethynyl phthalic anhydride (PEPA), 3-aminophenoxy-4'-phenylethynylbenzophenone (3-APEB), maleic anhydride (MA) and nadic anhydride (5-norbornene-2,3-dicarboxylic anhydride, NA). Homopolymers containing only other diamines and dianhydrides which are not processable under conditions described previously can be made processable by incorporating various amounts of APB, depending on the chemical structures of the diamines and dianhydrides used. By simply changing the ratio of APB to the other diamine in the polyimide backbone, a material with a unique combination of solubility, Tg, Tm, melt viscosity, toughness and elevated temperature mechanical properties can be prepared. The copolymers that result from using APB to enhance processability have a unique combination of properties that include low pressure processing (200 psi and below), long term melt stability (several hours at 300 DEG C. for the phenylethynyl terminated polymers), high toughness, improved solvent resistance, improved adhesive properties, and improved composite mechanical properties. These copolyimides are eminently suitable as adhesives, composite matrices, moldings, films and coatings.
Owner:NAT AERONAUTICS & SPACE ADMINSTRATION NASA THE
High temperature stable thermal interface material
InactiveUS6956739B2Reduce thermal resistanceImprove performanceSemiconductor/solid-state device detailsSynthetic resin layered productsParticulatesLiquid resin
A thermally-conductive interface interposable intermediate a first heat transfer surface and an opposing second heat transfer surface to provide a thermal pathway therebetween. The interface includes a thermally-conductive compound formed into a layer which is conformable between the first and second heat transfer surface. The compound is an admixture of: (a) a liquid resin constituent; and (b) a particulate filler constituent. The liquid resin constituent may be an ethylene-propylene copolymer (EPM) or a terpolymer (EPDM) of ethylene and propylene and a diene which may be ethylidene norbornene (ENB) or dicyclopentadiene (DCPD).
Owner:PARKER INTANGIBLES LLC
Norbornene ring-opened polymer hydrogenated product and process for producing same
A norbornene ring-opened polymer hydrogenated product having a syndiotactic structure and a process for producing the same are disclosed. The ring-opened polymer hydrogenated product contains a repeating unit originating from a polycyclic norbornene monomer with three or more rings in the polymer repeating units, having a weight average molecular weight of 500 to 1,000,000, and having a racemo diad proportion of 51% or more. The process comprises a step of polymerizing a polycyclic norbornene monomer having three or more rings by solution polymerization using a group 6 transition metal compound with a hydroxyl group-containing aryloxy group or the like bonded thereto as a polymerization catalyst to obtain a ring-opened polymer and a step of hydrogenating double bonds in the main chain of the ring-opened polymer.
Owner:ZEON CORP
Thermoplastic norbornene resin based optical film
InactiveUS20040047056A1Improve toughnessEasy to operateMirrorsDiffusing elementsThermoplasticHalogen
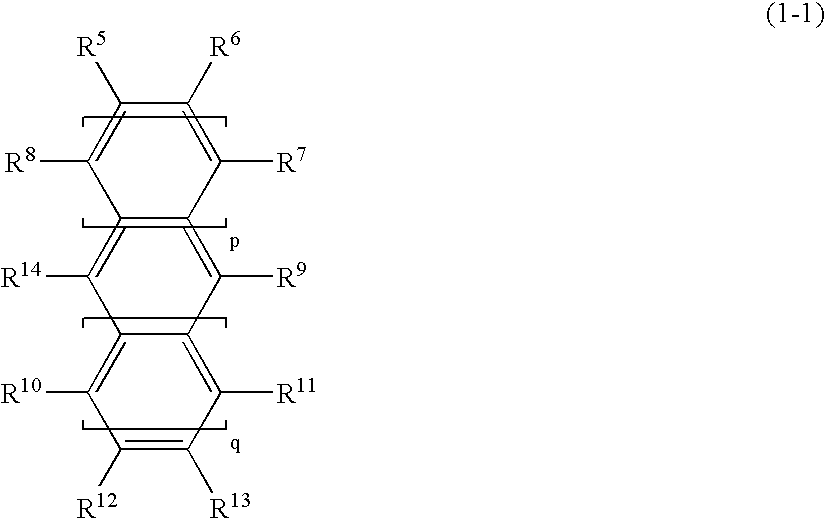
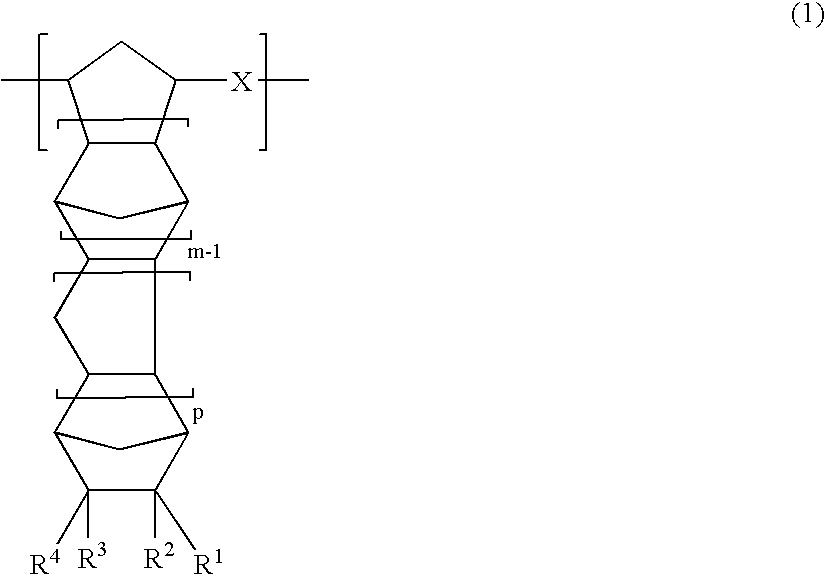
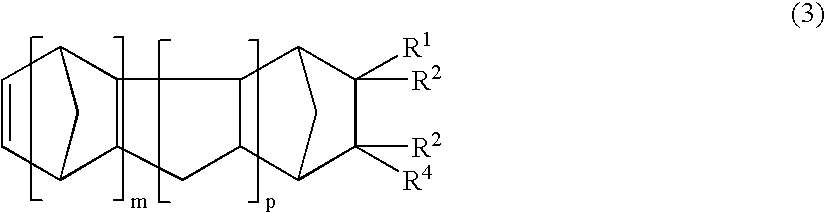
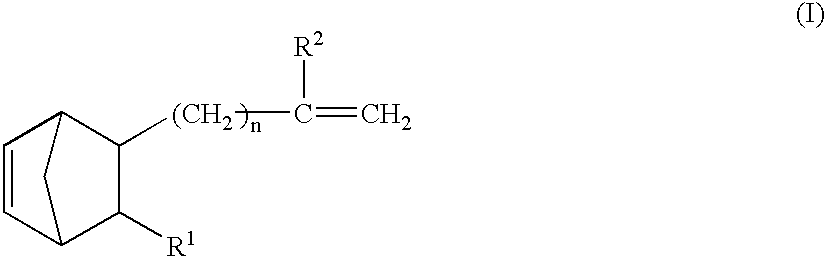
A thermoplastic norbornene resin which displays a high degree of toughness, and for which the exhibited retardation and the wavelength dependency of that retardation can be controlled is provided, which is formed from a copolymer with a structural unit represented by a general formula (1) and a structural unit represented by a general formula (2). [wherein, m is an integer of 1 or greater, p is either 0, or an integer of 1 or greater, each X and each Y represents, independently, either -CH=CH- or -CH2CH2-, and R<1 >to R<8 >each represent, independently, a hydrogen atom, a halogen atom or a hydrocarbon group or the like.]
Owner:JSR CORPORATIOON
Retardation film, process for producing the same, optical film, image display, liquid crystal panel and liquid crystal display
ActiveUS7215839B2Hard to cause a shift or an unevenness of a retardation valueOptical light guidesNon-linear opticsLiquid-crystal displayFilm plane
A retardation film of the present invention comprises a stretched film of a polymer film containing a norbornene-based resin, wherein the stretched film satisfies the following equation (1) and the equation (2); 100 nm≦(nx−ny)·d≦350 nm . . . (1), 0.1≦(nx−nz) / (nx−ny)≦0.9 . . . (2), where the refractive indices in the slow axis direction, the fast axis direction and the thickness direction of the film are nx, ny and nz, respectively, d(nm) is thickness of the film, and the slow axis direction is a direction that the refractive index in film plane is maximum. The retardation film is hard to cause a shift or an unevenness of a retardation value due to a stress.
Owner:NITTO DENKO CORP
Retardation plate and fabrication method thereof, and plate for circularly polarizing light, ½ wave plate and reflection-type liquid crystal display device utilizing the retardation plate
The present invention provides a broad band retardation plate that can be fabricated by a simple process and uniformly retards light incident of the entire visible light region. The retardation plate contains materials including positive or negative intrinsic double refraction values. When retardation values in wavelengths of 450 nm, 550 nm, and 650 nm are defined as Re(450), Re(550) and Re(650), respectively, the retardation plate satisfies the relational expression of Re(450)<Re(550)<Re(650). The retardation plate has a first layer comprising a positive material and a second layer comprising a negative material. The first layer and second layers have double refraction, and are laminated such that lag axes of the both layers are orthogonally crossed. It is preferable that the positive material is a norbornene based polymer and the like.
Owner:FUJIFILM CORP +1
Crosslinkable rubber compositions and uses thereof
The crosslinkable rubber composition is crosslinkable at room temperature, has a gelation time at room temperature of 30 days or less, and can prepare a crosslinked rubber sheet by crosslinking the composition at room temperature, wherein the crosslinked rubber sheet has a tensile elongation of 20% or more, and is free of cracks after treatment at 40° C. in a 50 pphm ozone concentration for 96 hr. Specifically, it comprises an ethylene / alpha-olefin / non-conjugated polyene random copolymer rubber comprising a norbornene compound having a specific vinyl end group, an SiH group-containing compound, which has at least two SiH groups in one molecule, and optionally a platinum catalyst, a reaction inhibitor and / or a silane-coupling agent. The sealing, potting and coating materials and adhesives of the present invention comprise the above rubber composition.
Owner:MITSUI CHEM INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com