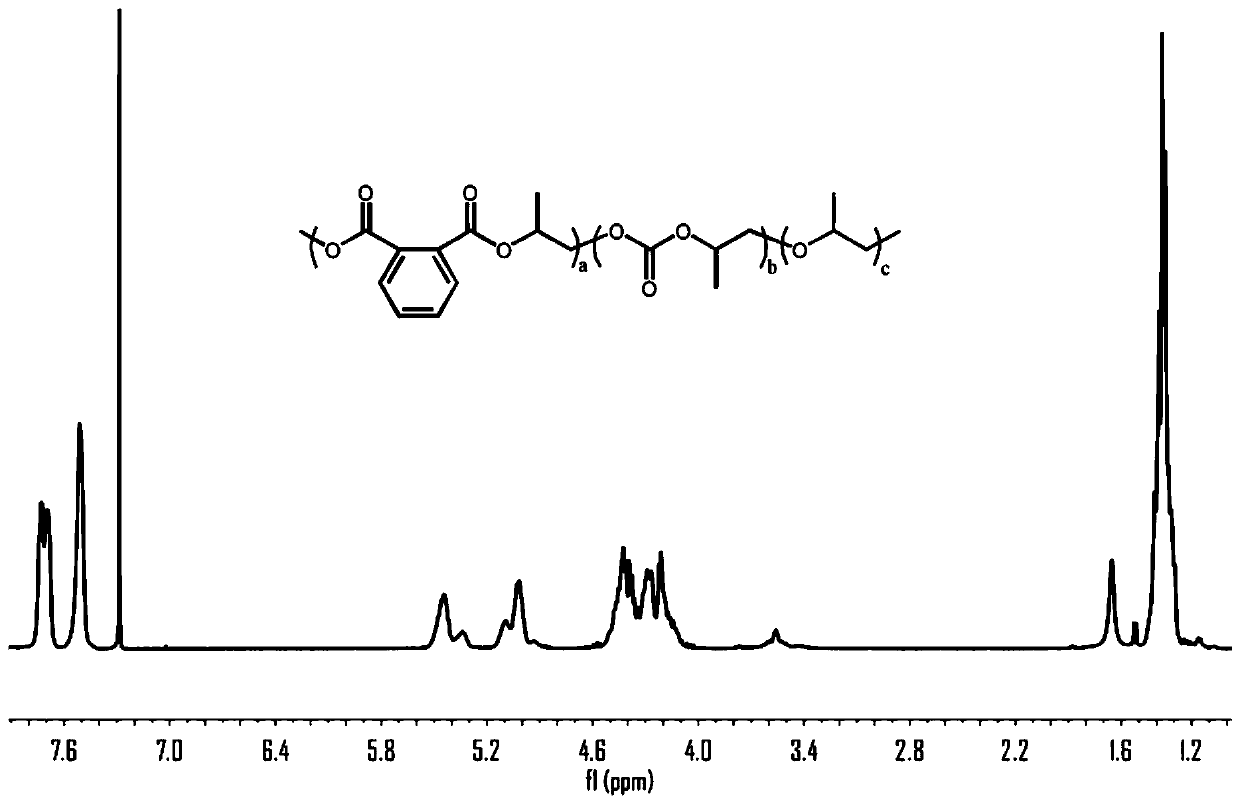
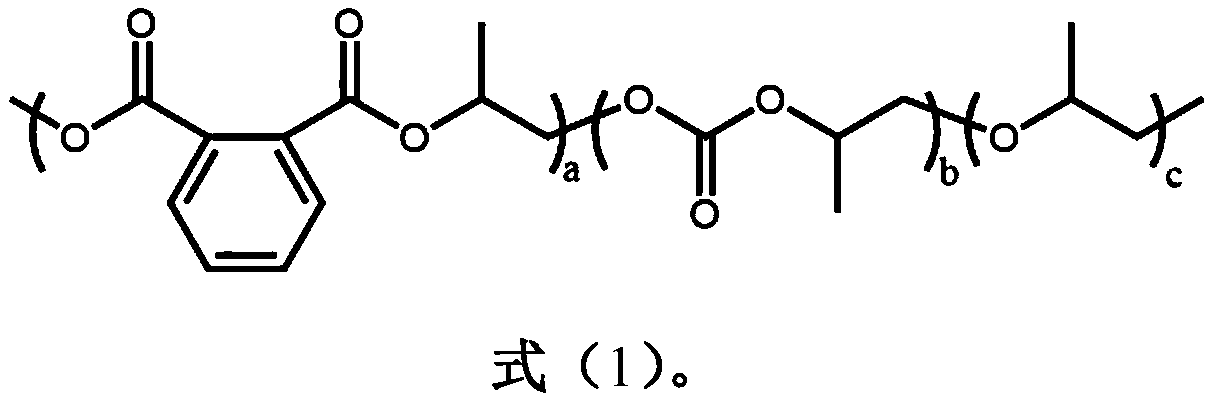
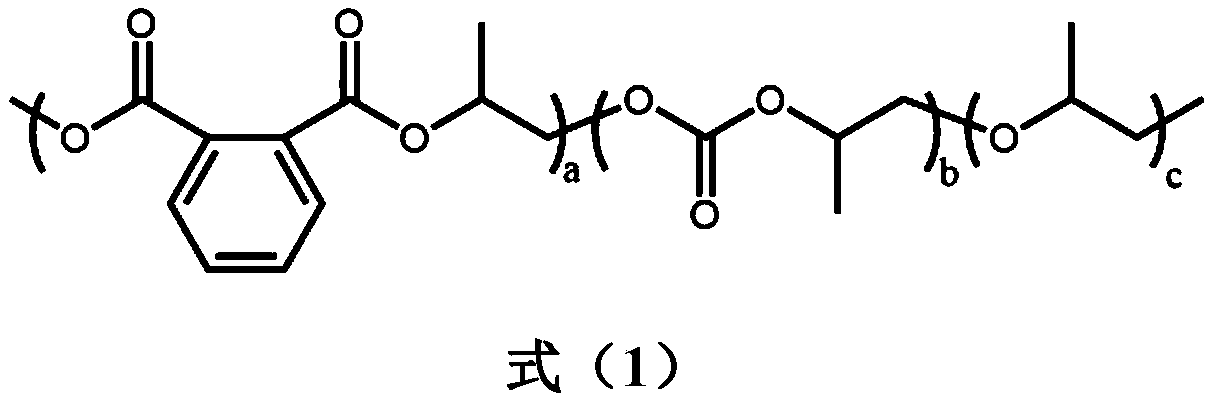
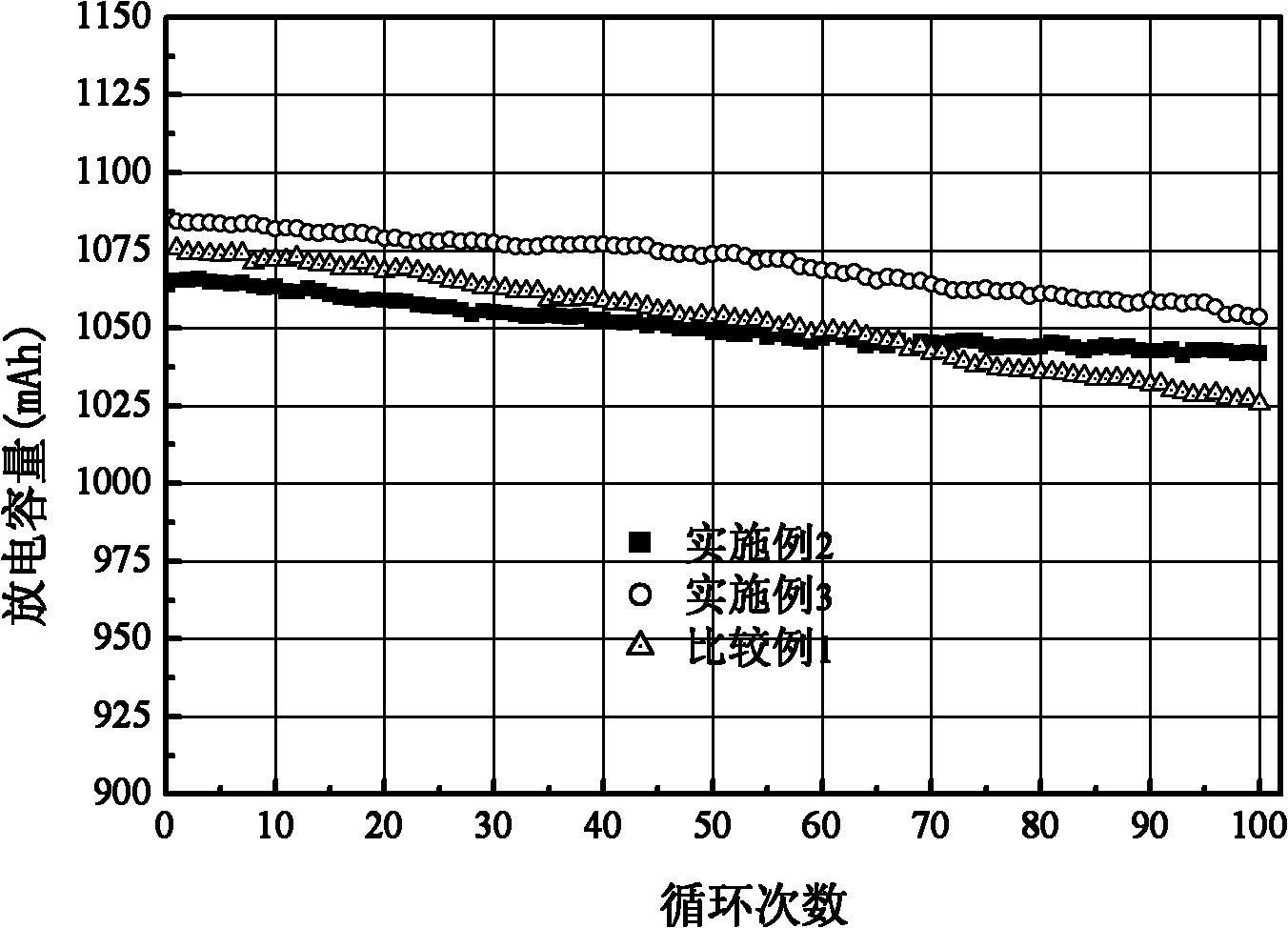
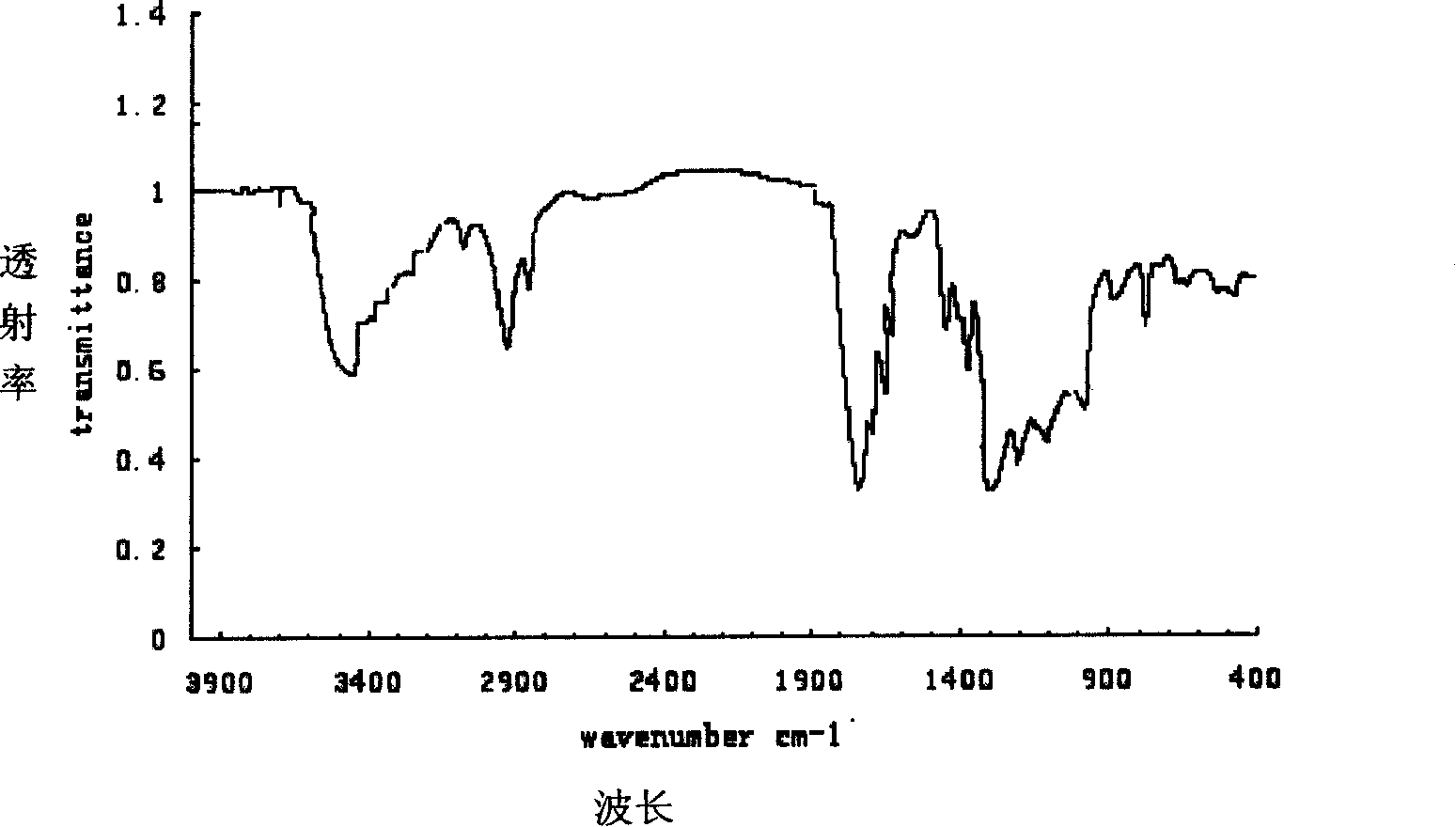
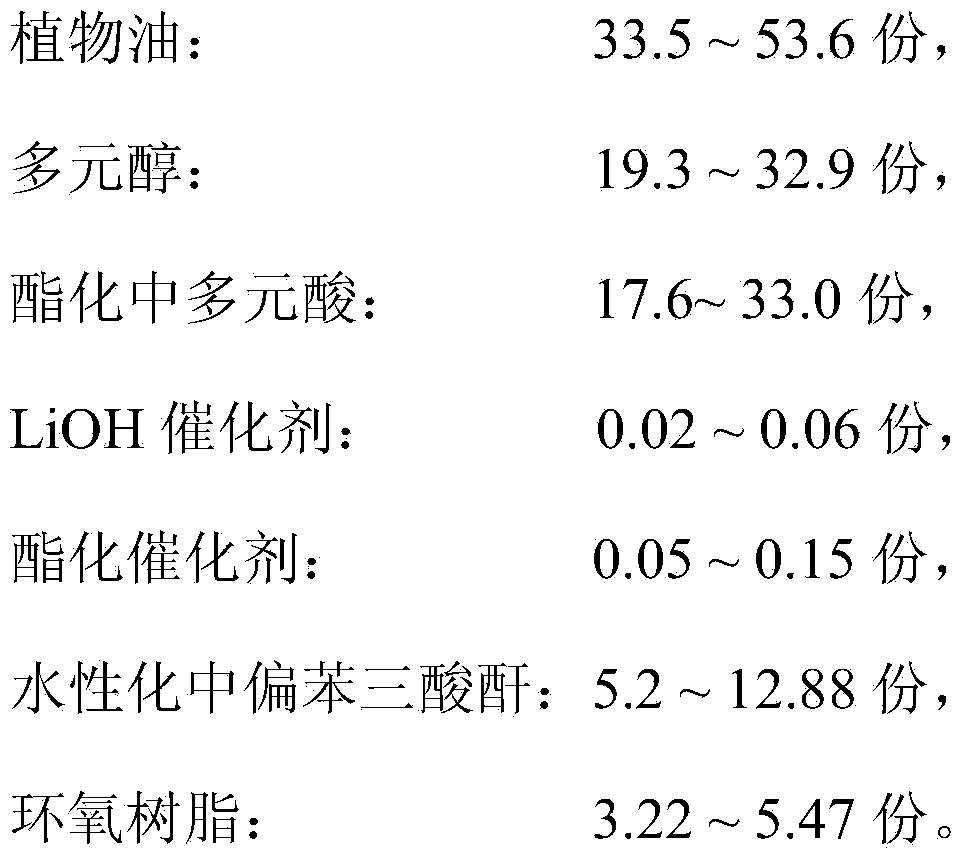
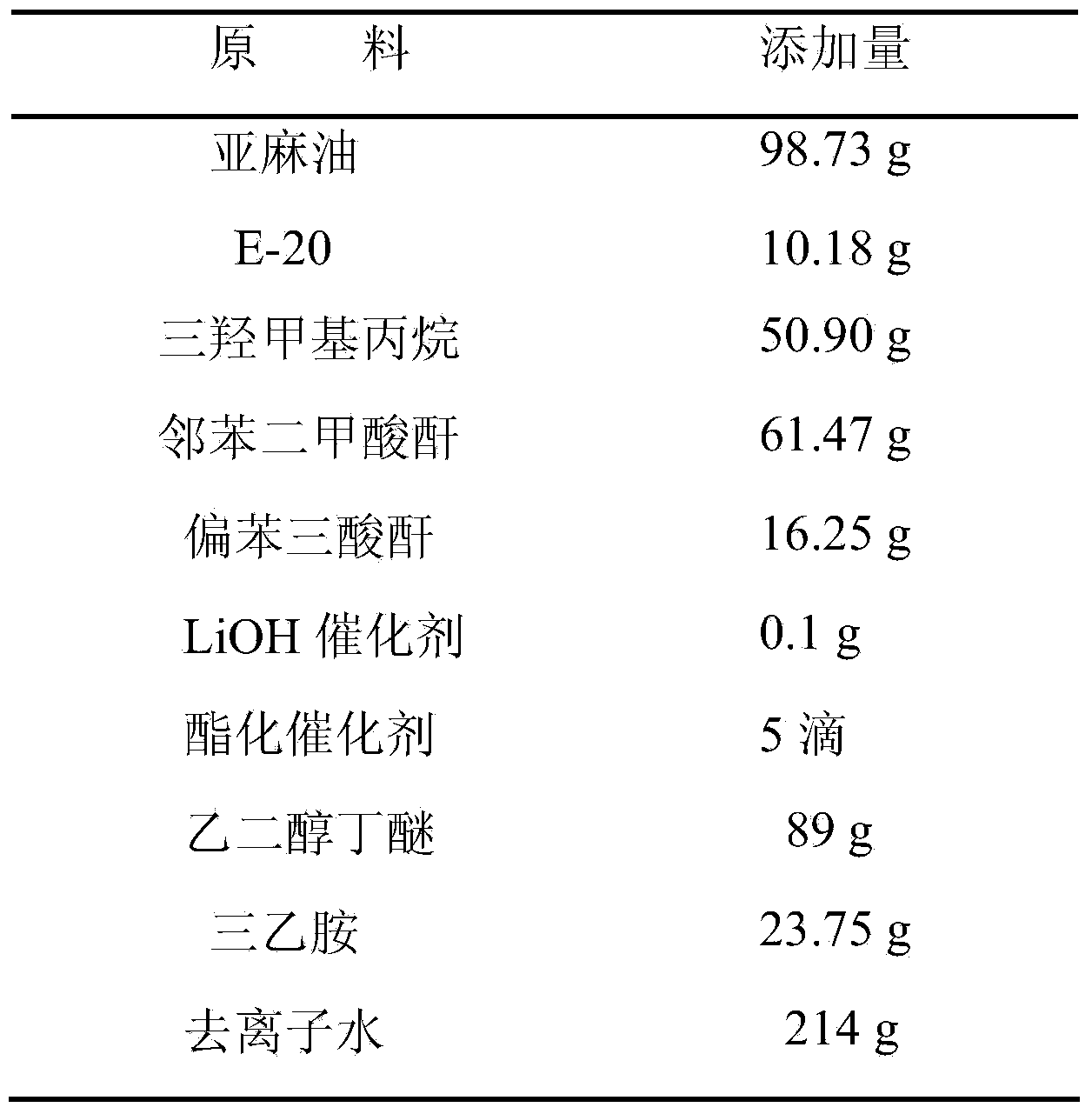
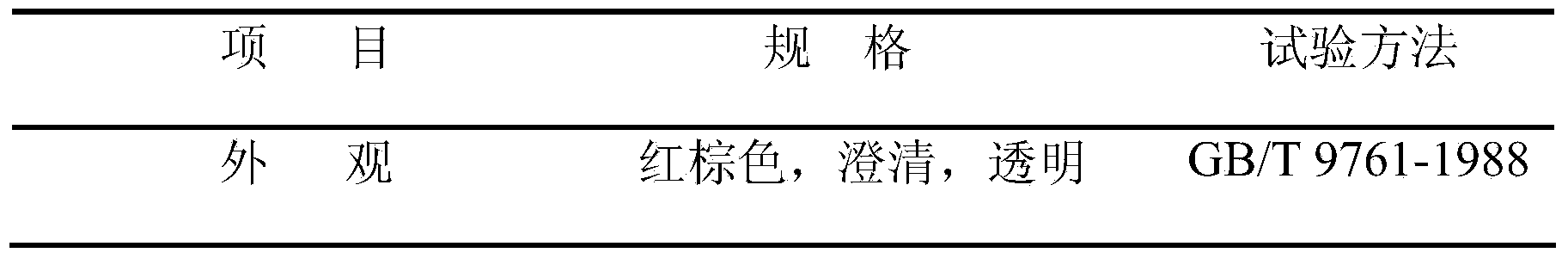
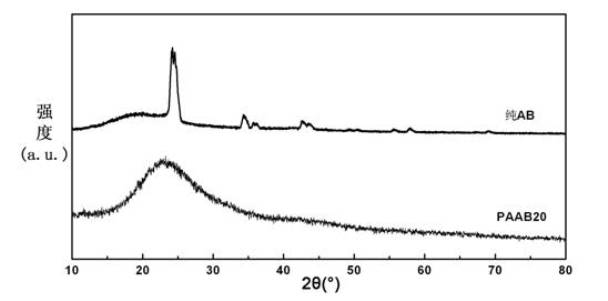
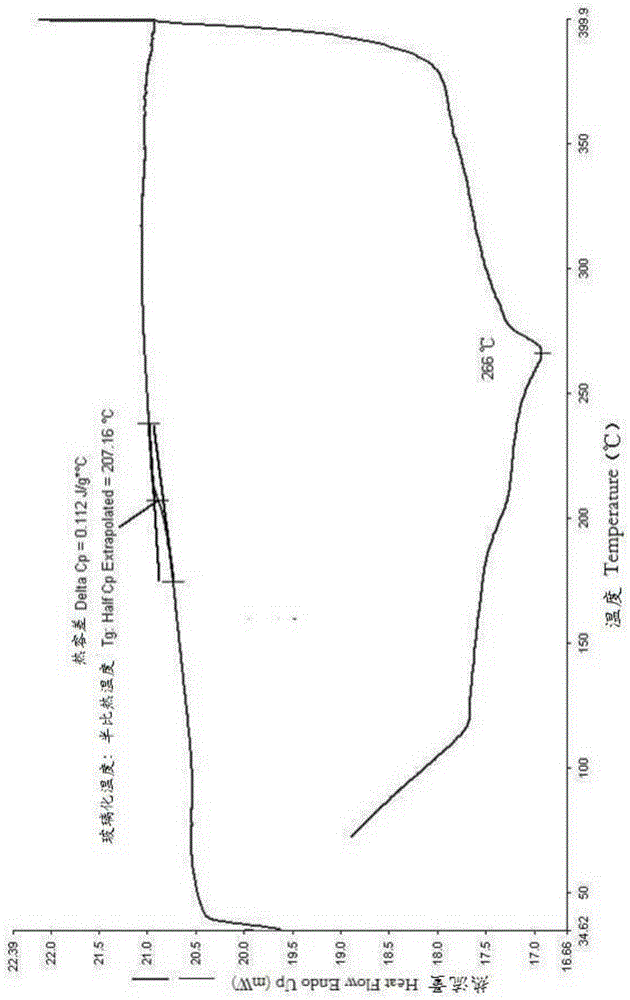
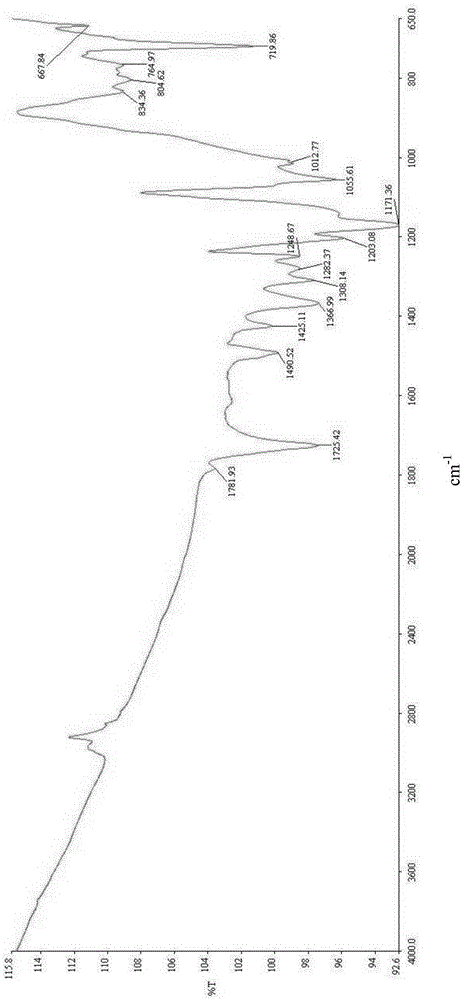
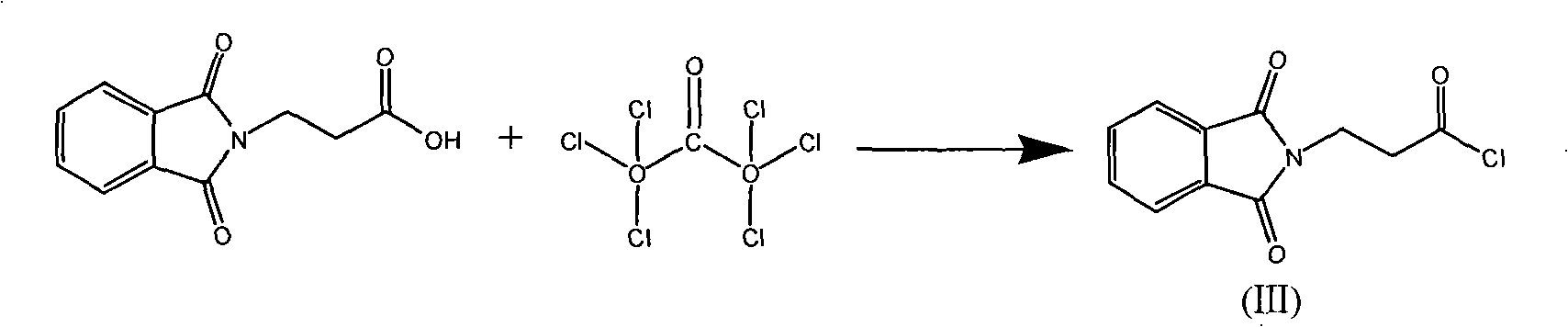
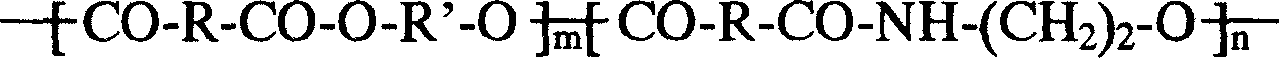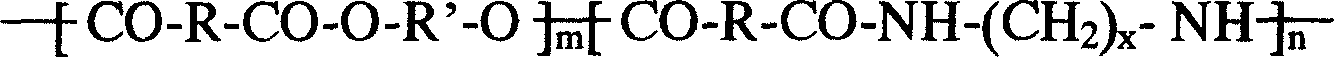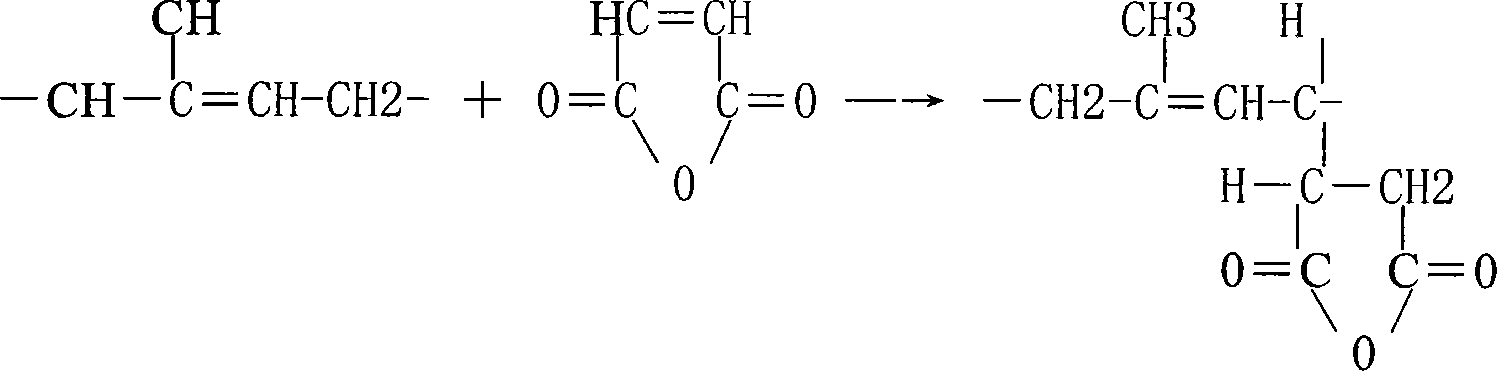Patents
Literature
2462 results about "Phthalic anhydride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phthalic anhydride is the organic compound with the formula C₆H₄(CO)₂O. It is the anhydride of phthalic acid. Phthalic anhydride is a principal commercial form of phthalic acid. It was the first anhydride of a dicarboxylic acid to be used commercially. This white solid is an important industrial chemical, especially for the large-scale production of plasticizers for plastics. In 2000, the worldwide production volume was estimated to be about 3 million tonnes per year.
Composite waterproof plate, ad its manufacturing method and use
InactiveCN1640640AImprove waterproof performanceImprove corrosion resistanceWood working apparatusDomestic articlesPolymer scienceAdhesive
The present invention relates to a composite water-proof material. It is made up by using (by weight portion) 20-70 portions of plant fibre, 10-60 portions of flyash and 5-40 portions of adhesive through the processes of mixing them, die-pressing and polymerization. The described adhesive is formed from maleic anhydride, phthalic anhydride, polyalcohol, styrene or mixture of their homologous compounds, also can include trimeric cyanamide, acrylic acid or rosin and dicyclopentadiene. Its raw material composition also can include 10-40 portions of new and old plastics. Said plate material has strong strength and good water-proof property, so that it has extensive application range.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method to prepare processable polyimides with reactive endogroups using 1,3-bis(3-aminophenoxy)benzene
InactiveUS6288209B1Improved solvent resistance and modulus and elevated use temperatureImproved melt processabilityNon-fibrous pulp additionSynthetic resin layered productsPolymer scienceBackbone chain
Polyimide copolymers were obtained containing 1,3-bis(3-aminophenoxy)benzene (APB) and other diamines and dianhydrides and terminating with the appropriate amount of reactive endcapper. The reactive endcappers studied include but should not be limited to 4-phenylethynyl phthalic anhydride (PEPA), 3-aminophenoxy-4'-phenylethynylbenzophenone (3-APEB), maleic anhydride (MA) and nadic anhydride (5-norbornene-2,3-dicarboxylic anhydride, NA). Homopolymers containing only other diamines and dianhydrides which are not processable under conditions described previously can be made processable by incorporating various amounts of APB, depending on the chemical structures of the diamines and dianhydrides used. By simply changing the ratio of APB to the other diamine in the polyimide backbone, a material with a unique combination of solubility, Tg, Tm, melt viscosity, toughness and elevated temperature mechanical properties can be prepared. The copolymers that result from using APB to enhance processability have a unique combination of properties that include low pressure processing (200 psi and below), long term melt stability (several hours at 300° C. for the phenylethynyl terminated polymers), high toughness, improved solvent resistance, improved adhesive properties, and improved composite mechanical properties. These copolyimides are eminently suitable as adhesives, composite matrices, moldings, films and coatings.
Owner:NASA
Method to prepare processable polyimides with reactive endgroups using 1,3-bis (3-aminophenoxy) benzene
InactiveUS6133401AImprove adhesionImprove composite effectSynthetic resin layered productsThin material handlingSolubilityAdhesive
Polyimide copolymers were obtained containing 1,3-bis(3-aminophenoxy)benzene (APB) and other diamines and dianhydrides and terminating with the appropriate amount of reactive endcapper. The reactive endcappers studied include but should not be limited to 4-phenylethynyl phthalic anhydride (PEPA), 3-aminophenoxy-4'-phenylethynylbenzophenone (3-APEB), maleic anhydride (MA) and nadic anhydride (5-norbornene-2,3-dicarboxylic anhydride, NA). Homopolymers containing only other diamines and dianhydrides which are not processable under conditions described previously can be made processable by incorporating various amounts of APB, depending on the chemical structures of the diamines and dianhydrides used. By simply changing the ratio of APB to the other diamine in the polyimide backbone, a material with a unique combination of solubility, Tg, Tm, melt viscosity, toughness and elevated temperature mechanical properties can be prepared. The copolymers that result from using APB to enhance processability have a unique combination of properties that include low pressure processing (200 psi and below), long term melt stability (several hours at 300 DEG C. for the phenylethynyl terminated polymers), high toughness, improved solvent resistance, improved adhesive properties, and improved composite mechanical properties. These copolyimides are eminently suitable as adhesives, composite matrices, moldings, films and coatings.
Owner:NAT AERONAUTICS & SPACE ADMINSTRATION NASA THE
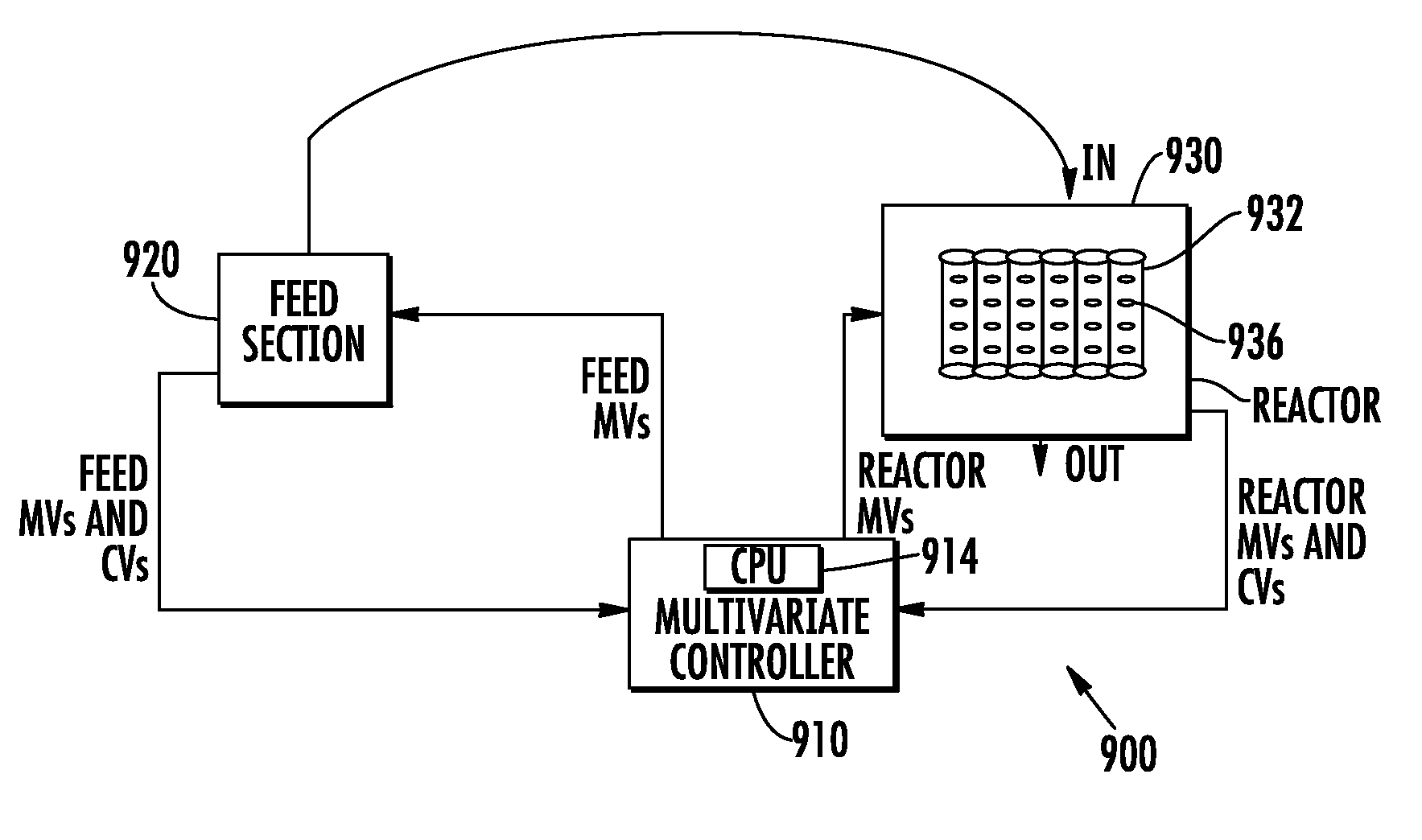
Multivariable process controller and methodology for controlling catalyzed chemical reaction to form phthalic anhydride and other functionalized aromatics
InactiveUS8060258B2Increase productivityMinimizing flow rateSampled-variable control systemsGaseous chemical processesChemical reactionChemical reactor
A multivariable method and process controller is for controlling a catalyzed chemical reaction to form phthalic anhydride (PA), produced by a production unit including a chemical reactor having a plurality of pipes connected in parallel having inner surfaces affixed with at least one solid catalyst. The reactor implements a process for forming PA by receiving flows of reagents including at least one oxidizable substituted aromatic and an oxygen including source gas at one or more inlets of the reactor. A dynamic multivariable model for the process represents the effects of moving a plurality of manipulated variables (MVs) including a flow of the oxygen including source gas and a flow or temperature of the oxidizable substituted aromatic on controlled variables (CVs) including a temperature at a plurality of positions along a length of the pipes. During the process, a first parameter related to performance of the catalyst in producing PA and a second parameter including a temperature at one or more of the plurality of positions in measured. Using the dynamic model, the temperature in the plurality of positions along the length of the reactor are automatically adjusting based on at least the first measured parameter, which permits the temperature profile to be adjusted to compensate for ageing of the catalyst to improve production efficiency.
Owner:HONEYWELL INT INC
Preparation method of biodegradable carbon dioxide-based polyester-polycarbonate terpolymer
The invention discloses a preparation method of a biodegradable carbon dioxide-based polyester-polycarbonate terpolymer. A commercialized, simple and efficient nonmetal triethyl boron / tetra-n-butyl ammonium chloride catalytic initiation system is used for catalyzing and initiating epoxypropane, carbon dioxide and phthalic anhydride to be subjected to a ternary polymerization reaction, and an aromatic polyester structure with the content of 10%-40% is successfully introduced into a main chain of polymethyl ethylene carbonate (PPC). The terpolymer can have different sequence structures; and haverandom and block structures and the like, the glass transition temperature and the thermal stability of PPC are improved, and a catalytic system does not contain metal components, so that the phthalic anhydride modified PPC has extremely high light transmittance, very high melt viscosity and good foaming performance on the premise of keeping the biodegradability of a PPC material. The method canbe used for synthesizing the modified PPC biodegradable material, and widens the application range of the PPC material.
Owner:SUN YAT SEN UNIV
High-temperature lithium ion battery electrolyte and lithium ion battery
ActiveCN102082292AEasy to manufactureEasy to operateCell electrodesSecondary cellsOxalateOrganic solvent
The invention discloses a high-temperature lithium ion battery electrolyte and a lithium ion battery. The electrolyte is prepared by uniformly mixing electrolyte salts, a nonaqueous organic solvent, a high-temperature film-forming additive and high-temperature gas generation inhibiting additives, wherein the high-temperature film-forming additive is lithium bis(oxalate) borate (LiBOB); the LiBOB accounts for 0.2-3% by mass of the mixed lithium salt solution formed by the electrolyte salts and the nonaqueous organic solvent; the high-temperature gas generation inhibiting additive is one or twoof ionic liquid and phthalic anhydride, and accounts for 0.8-9% by mass of the mixed lithium salt solution; and the total content of the added high-temperature film-forming additive and high-temperature gas generation inhibiting additives accounts for 1-11% by mass of the mixed lithium salt solution. The lithium ion battery comprises a cathode, an anode, a diaphragm and the high-temperature lithium ion battery electrolyte. The invention has the advantage of reasonable design, the steps in the preparation processes are simple and convenient to implement, and the prepared electrolyte and the lithium ion battery have excellent comprehensive performance.
Owner:XIAN SAFTY ENERGY TECH
Alkyd resin for wood lacquer and preparation method thereof
The invention discloses alkyd resin for wood lacquer and a preparation method thereof. According to the raw material formula, the alkyd resin for the wood lacquer comprises the following components in percentage by mass: 18.3 to 28.3 percent of soybean oleic acid, 10.2 to 20.2 percent of glycerin, 3.8 to 7.8 percent of pentaerythritol, 17.6 to 21.6 percent of recycled terephthalic acid, 0.05 to 0.2 percent of color substration agent, 0.05 to 0.2 percent of antioxidant, 2.5 to 5.5 percent of phthalic anhydride, 3.2 to 7.2 percent of benzoic acid, 2 to 5 percent of dimethylbenzene, 18 to 30 percent of thinning agent and 0.05 to 0.2 percent of wetting dispersant. Terephthalic acid is adopted, and the unique chemical performance of the terephthalic acid endows the resin with high dryness and weather resistance; waste and inferior terephthalic acid produced by a petrochemical enterprise is utilized, so that the production cost of the resin is lower than that of alkyd resin produced by the conventional method; simultaneously, waste of the petrochemical enterprise is reduced, the environment is prevented from being polluted, and wastes are utilized to save energy and reduce emission.
Owner:HUIZHOU CHANGRUNFA PAINT
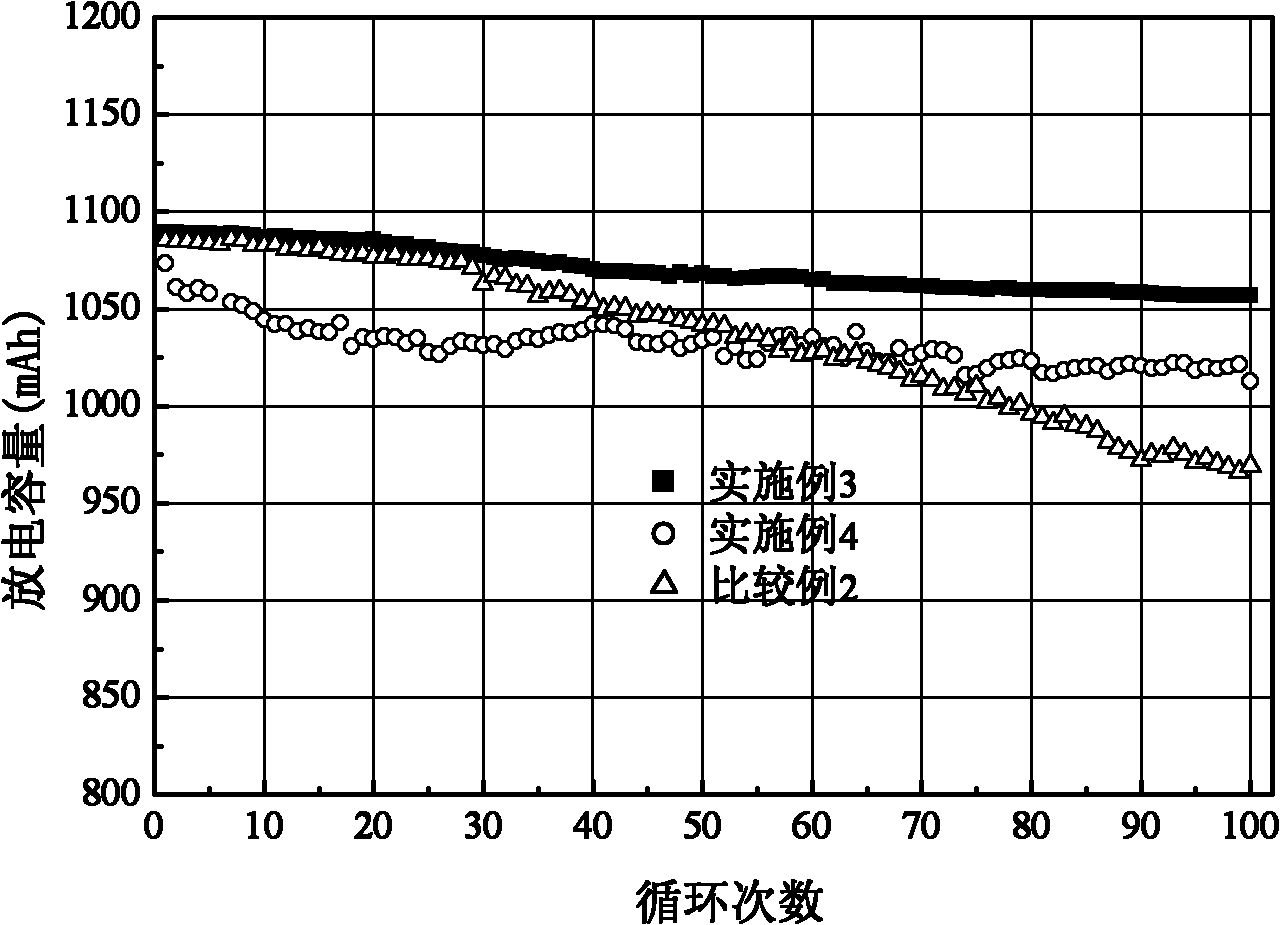
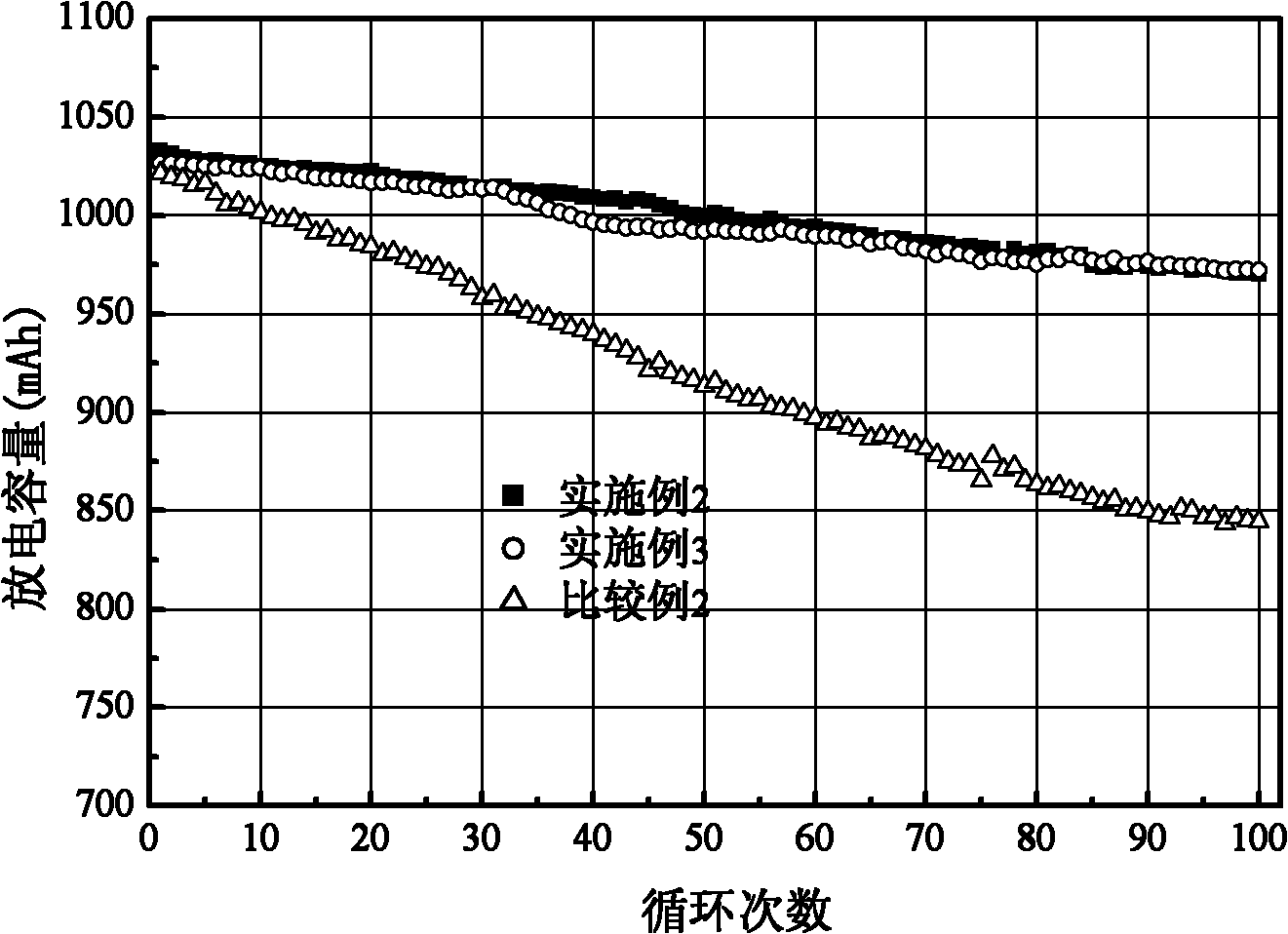
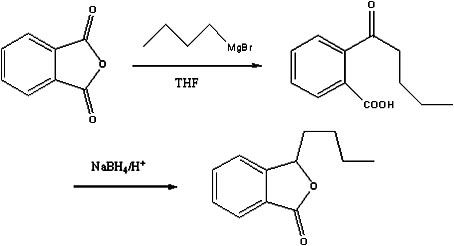
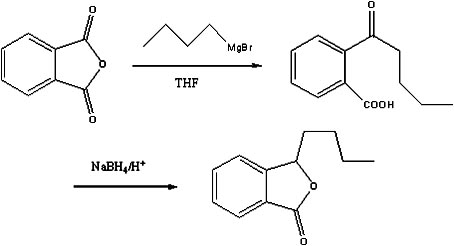
Preparation method of butylphthalide
InactiveCN101962374AReaction raw materials are readily availableFew reaction stepsOrganic chemistryBenzoic acidGrignard reagent
The invention discloses a preparation method of butylphthalide, which comprises the following steps: taking phthalic anhydride as a raw material; enabling the phthalic anhydride to carry out addition reaction with the Grignard reagent of butyl halide to obtain an intermediate of o-valeryl benzoic acid; and then, reducing by sodium borohydride, and carrying out acidic cyclization to obtain the butylphthalide. The phthalic anhydride and the butyl halide which are used as raw materials in the preparation method of the butylphthalide of the invention are commercial products, and the reaction raw materials can be obtained easily. Because the Grignard reaction, the sodium borohydride reduction and the acidic cyclization are classical reactions, the operation is simple, the industrialized production can be realized easily, the yield of the butylphthalide reaches 50-60%, and the purity of the butylphthalide reaches 97-98%.
Owner:SHANGHAI INST OF TECH
H-grade low-temp. beading polyurethane paint wire-covering paint
InactiveCN1394926AEasy to control smoothnessReduce dosagePolyurea/polyurethane coatingsPolyisocyanurateAdipic acid
The present invention relates to a H-grade low-temp. direct-welding enamelled wire enamel, said enamel liquor mainly includes three portions of hydroxyl component, closed type isocyanate component and solvent. The hydroxyl component is formed from raw materials of dihydric alcohol terephthate or amidoimide dihydric alcohol, new pentanediol or 2-methyl-1,3-propanedio, glycerine, 1,1,1-trihydroxymethyl propane, tri(alpha-hydroxyethyl) isocyanate, adipic acid and phthalic anhydride. The acid value of polyester polybasic alcohol is less than 2, hydroxy value is 70-250, number-average molecular weight is 2000-7000 and molecualr weight distribution index is 2-3. The isocyanate component is made up by using general raw material TDI and MDI through the processes of cyclic self-polymerization or addition with small quantity of tribasic alcohol and closing with dimethyl phenol.
Owner:NANJING UNIV
Benzoic anhydride catalyst and preparation method thereof
ActiveCN101422727AImprove performanceImprove working environmentOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsChemical industryGas phase
The invention relates to a catalyst that is used in the catalytic oxidation reaction between raw materials of ortho-xylene and / or naphthalin and gas phase that contains molecular oxygen for producing phthalic anhydride, as well as a preparation method of the catalyst. The catalyst comprises an inert imperforated carrier material and a catalytic active substance layer that is coated on the carrier and contains vanadic oxide, diantimony trioxide, titanium dioxide and a cementing agent, etc. In a preparation process of the catalyst, serum is firstly prepared, viscosity of the catalytic active substance serum is controlled, the carrier is heated, the serum is sprayed onto the carrier in a barrate, and the carrier is dried by hot wind. The catalyst is used for the field of chemical industry.
Owner:CHINA PETROLEUM & CHEM CORP +1
Degradable unsaturated polyesteramide resin and synthesis method thereof
The invention adopts a melt polycondensation method. A few of monomers of C2-5 aliphatic dibasic alcohol, diethylene glycol, polyglycol, fumaric acid, maleic anhydride, lactic acid, glycolic acid, ethanolamine, C2 to C12 aliphatic diamine, glutamic acid, lysine, glycine, etc. are taken as basic materials to be synthesized to obtain non toxic unsaturated polyester-amide resin with adjustable degradation rate and lower cost; wherein, the partial fumaric acid or maleic anhydride can be replaced with phthalic anhydride, isophthalic acid, or adipic acid. The resin yearns for being used as matrix resin of medical bone internal fixation material, tissue engineering scaffold material, bone tissue temporary substitutes, environmental protection type bonding agent, environmental protection type fiberglass reinforced plastics, environmental protection type coating material, disposable tableware, packing material, shopping bags, disposable bags, drug coating or capsule, drug delivery (controlled-release) material, agricultural mulching films, etc., and can be recovered to be utilized.
Owner:HUNAN UNIV
Plaster-shape regenerated rubber and preparation method thereof
InactiveCN101100534ASimple processNo need to consume electricityPlastic recyclingFiberPolymer science
A creamed reclaimed rubber and its production are disclosed. It consists of 20-50 mesh vulcanized rubber powder 100 proportion, paraffin oil as solvent 70-90 proportion, solid coumarone 10-30 proportion as solvent, catalyst and catalyst accessory phthalic acid anhydride 2-5 proportion, methyl aldehyde aqueous solution 4-6 proportion, 1,3-benzenediol 0.2-0.5 proportion. The process is carried out by adding vulcanized rubber powder into vertical de-polymerizer, adding into solvent, catalyst and catalyst accessory successively, closing feeding open, agitating, raising temperature to 160-180 deg. C, controlling pressure to 0.5-0.7 MPa, reacting for 2-2.5 hrs, lowering temperature, releasing gas, discharging and filtering to obtain final product. It's simple, has stable quality, waterproof and antiseptic performances and no environmental pollution. It can be used to synthesize rubber with petrochemical asphalt and to paving road.
Owner:TIANJIN YATENGDA RUBBER & PLASTIC PRODS DEV
Polyurethane resin, preparation method thereof and application thereof to micro cellular elastomer
The invention discloses polyurethane resin, a preparation method thereof and application thereof to a micro cellular elastomer. The polyurethane resin comprises a polyurethane resin component A and a polyurethane resin component B; the polyurethane resin component A and the polyurethane resin component B comprise polyether ester polyol; a ratio of a mole number of active hydrogen in the component A to a mole number of -NCO in the component B is 1:1. The polyether ester polyol is a reaction product of polyether polyol, phthalic anhydride, a double-metal cyanide complex catalyst, epoxypropane, ethylene oxide and a basic catalyst. According to the polyurethane resin and the preparation method and the application thereof, which are disclosed by the invention, under the condition of not adding an open-hole agent and graft polymer polyol, the polyurethane micro cellular elastomer with a low forming density, a high hardness, low shrinkage, high tensile strength and tearing strength, excellent hydrolysis resistance and excellent wear resistance can be prepared, and not only hydrolysis resistance and a forming hardness of the polyurethane micro cellular elastomer are improved, but also the open taphole of the polyurethane micro cellular elastomer is improved, so that cells of the polyurethane micro cellular elastomer are fine and uniform, and shrinkage is low.
Owner:ZHEJIANG HUAFON NEW MATERIALS CO LTD +1
Method for preparing terylene environment protection type dye carrier
The invention relates to a method for preparing a terylene environmental protection type dyeing carrier, which comprises the following steps: (1) alkylamine, dimethylformamide, phthalic anhydride and triethylamine are added into a three-neck flask with the volume of 500mL under the condition of ice bath and are subject to thermal insulation reaction for one hour; then the temperature is increased to 120 DEG C to perform a reflux reaction for four hours; the mixture is dumped into ice water after the reaction is finished; and white crystals are precipitated; (2) according to the mass proportion of 4 to 1, a carrier precursor is added into an emulsifying agent, heated and stirred; and (3) the process formulation is a dispersion dye, 1 to 3 percent owf of carrier, and 0.3g to 0.5g.L<-1> of glacial acetic acid; the bath ratio is 1 to 20; the dyeing temperature is between 85 and 100 DEG C; the thermal insulation time is between 60 and 100 minutes; and the dyeing carrier is washed and aired. The method is suitable for terylene boiling dyeing processes of dyeing and finishing plants, can reduce production cost, and shorten dyeing period. The textile after the dyeing has bright color, pure chromatic light, high brightness, no toxicity, no smell, and low COD value, so the dyeing carrier is an environment-frinedly green auxiliary.
Owner:DONGHUA UNIV
Preparation method of alkyd resin
InactiveCN102408551AImprove adhesionFullnessCosmetic preparationsToilet preparationsPolymer scienceNitrocellulose
The invention relates to a preparation method of alkyd resin. The preparation method comprises the following specific steps: (1) adding neopentyl glycol, trihydroxymethyl propane, phthalic anhydride, hydrogenated phthalic anhydride and antioxidant to a reaction container, and heating to carry out melt esterification on the materials at the temperature of 200-240 DEG C; (2) when esterification is carried out to an acid value being 40-45 mgKOH / g, cooling to 120-160 DEG C, and adding short-chain synthetic fatty acid and an aliphatic hydrocarbon solvent; (3) heating to carry out reflux esterification at the temperature of 180-200 DEG C, and diluting with acetic acid ester organic solvent when the acid value is smaller than or equal to 8 mgKOH / g; and (4) cooling to 100 DEG C and filtering to obtain alkyd resin. The paint prepared from the alkyd resin obtained in the invention and nitrocellulose has the characteristics of rapid drying, high hardness, good fullness, good adhesion force, and good water resistance.
Owner:JIANGSU SANMU GROUP CORPORATION
Acrylic acid ester modified epoxy soybean oil, its preparation method and application thereof
ActiveCN102660387ANovel structureImprove adhesionOrganic chemistryFatty acid chemical modificationPolymer scienceMeth-
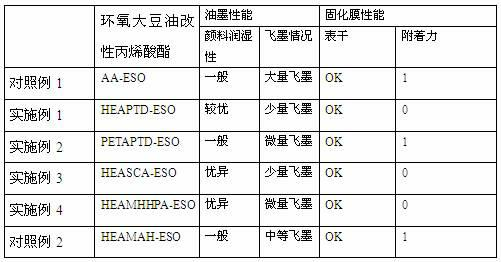
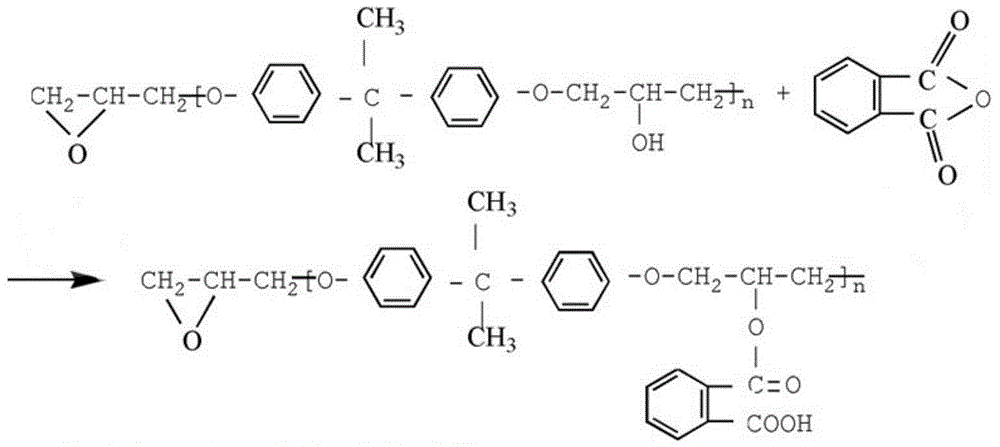
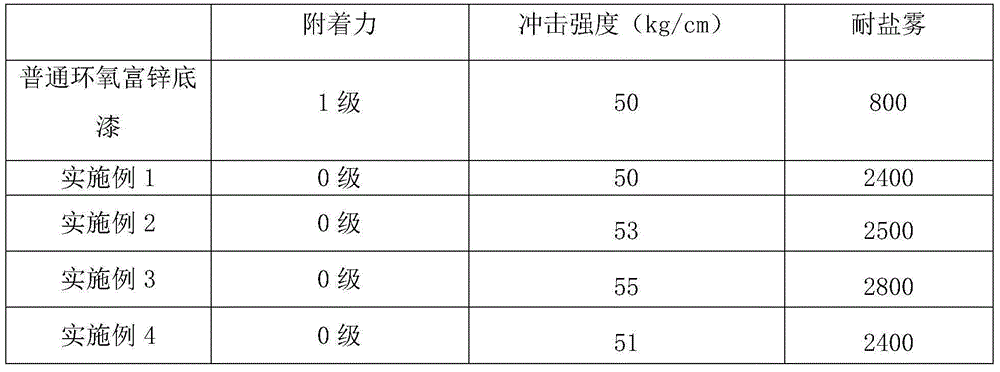
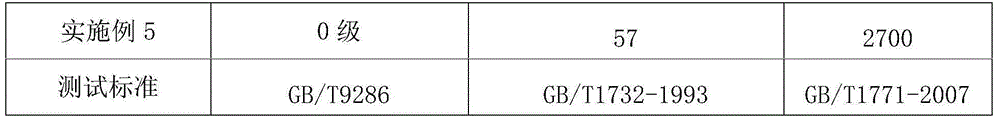
The invention discloses an acrylic acid ester modified epoxy soybean oil, its preparation method and an application thereof. According to the preparation method of the acrylic acid ester modified epoxy soybean oil, acrylate monomer bearing a hydroxyl group reacts with acid anhydride to obtain a half-ester intermediate, and the half-ester intermediate reacts with epoxy soybean oil to obtain the acrylic acid ester modified epoxy soybean oil. The acrylate monomer bearing a hydroxyl group is hydroxyethyl acrylate, hydroxyethyl methacrylate, pentaerythritol triarylate, hydroxypropyl acrylate, hydroxypropyl methacrylate, trimethylol propane diacrylate or trimethylol propane dimethacrylate. Acid anhydride is o phthalic anhydride, succinic anhydride, methyl hexahydrophthalicanhydride, hexahydrophthalic anhydride or tetrahydrophthalic anhydride. The acrylic acid ester modified epoxy soybean oil has a novel structure. When applied in paper printing ink, the acrylic acid ester modified epoxy soybean oil can improve adhesive force of a cured film and also can improve wetability of pigments and remarkably minimize occurrence of misting. The preparation method helps widen the application range of the modified epoxy soybean oil.
Owner:GUANGDONG BOSSIN NOVEL MATERIALS TECH CO LTD
Graphene-modified epoxy zinc-rich primer and preparation method thereof
ActiveCN105713481AGood dispersionEffective dispersionAnti-corrosive paintsEpoxy resin coatingsEpoxySlurry
The invention relates to a graphene-modified epoxy zinc-rich primer and a preparation method thereof, wherein the parts by mass of each component of the primer are: 15-30 parts of modified epoxy resin, 0.3-5 parts of graphene, and butanol 2-8 parts, 4-10 parts of toluene, 0.3-3 parts of anti-settling agent, 70-90 parts of zinc powder, and the modified epoxy resin is phthalic acid modified epoxy resin. The preparation method comprises (1) preparing modified epoxy resin phthalic acid modified epoxy resin; (2) preparing graphene epoxy resin slurry; (3) preparing graphene modified epoxy zinc-rich primer. The present invention reacts the hydroxyl group of the epoxy resin with phthalic anhydride by modifying the epoxy resin, introduces the highly polar carboxyl group, and the epoxy resin itself becomes a dispersant with strong dispersion ability, which can efficiently disperse and chelate graphite Graphene and resin chelate and evenly disperse in the coating system to achieve the best anti-corrosion effect. In addition, the sheet-like structure of graphene makes the paint film denser, and the path for corrosive gas to enter is longer to further enhance the anti-corrosion effect .
Owner:JIANGSU BANGJIE ANTI CORROSION THERMAL INSULATIONTECH
Epoxy-modified water-based alkyd resin and water-based alkyd amino stoving varnish and preparation method of epoxy-modified water-based alkyd resin and water-based alkyd amino stoving varnish.
InactiveCN104072742ASolve the hardnessSolve Adhesion ProblemsPolyester coatingsWater basedVegetable oil
The invention discloses epoxy-modified water-based alkyd resin, water-based alkyd amino stoving varnish and a preparation method of the epoxy-modified water-based alkyd resin and the water-based alkyd amino stoving varnish, belonging to the technical field of high molecular materials. The preparation method comprises the following steps: mixing vegetable oil, bisphenol A glycidyl ether epoxy resin and polyhydric alcohols to synthesize an alcoholysate monoglyceride; then, carrying out esterification reaction with phthalic anhydride, then hydrating by using trimellitic anhydride, and finally neutralizing and dispersing to obtain the epoxy-modified water-based alkyd resin (as a component A); and then, mixing the component A with various types of amino resins (as a component B) and a certain amount of pigments and filler, cosolvent, neutralizer and deionized water to prepare the amino stoving varnish. By using vegetable oil as a main raw material, an extremely small amount of solvent is used, and the method hardly depends on petroleum and is low in cost and the production process is simple and easy to control. The prepared stoving varnish can be widely applied to the fields such as industrial corrosion prevention and belongs to the environment-friendly coating.
Owner:SOUTH CHINA UNIV OF TECH
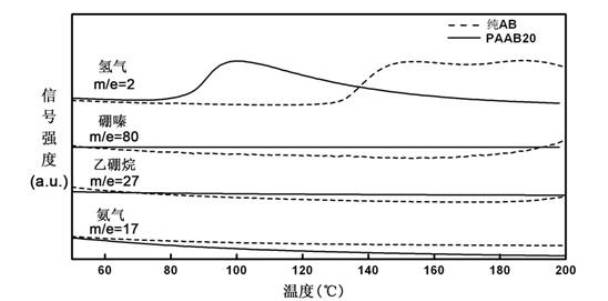
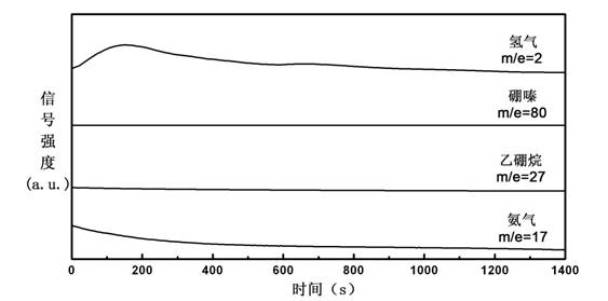
Organic matter and ammonia borane compounded hydrogen storage material and preparation method thereof
ActiveCN102030313ALowering the temperature of thermally liberated hydrogenInhibitionMonoborane/diborane hydridesPolyethylene oxideSolvent
The invention relates to an organic matter and ammonia borane compounded hydrogen storage material. The hydrogen storage material is prepared by compounding the organic matter and the ammonia borane, wherein the organic matter is phthalic anhydride, polyethylene oxide, dextrose, mannitol or mannitol hexaacetic ester. The preparation method comprises the following steps: 1) adding the organic matter to the purified acetonitrile solvent, and stirring for dissolving; 2) dissolving the ammonia borane into the mixing solvent comprising acetonitrile and methanol, and stirring at the temperature of 20 to 70 DEG C to obtain a uniform solution; and 3) carrying out vacuum drying, and removing the solvent, thus obtaining the hydrogen storage material. The invention has the advantages that the ammonia borane and the organic matter are taken as raw materials to prepare the hydrogen storage material at the lower hydrogen discharge temperature; the thermal decomposition and hydrogen discharge temperature of the ammonia borane can be effectively reduced; the generation of harmful gas impurities of borazole, diborane, ammonia and the like is effectively inhibited; the hydrogen storage material has quicker hydrogen discharge kinetics; in addition, the heat discharge amount is less in the hydrogen discharge course; and the enthalpy change of a decomposition reaction approaches to thermal neutrality; and the hydrogen storage material is beneficial to realizing the regeneration of reaction products through a solid-gas reaction or a chemical process under the relatively mild condition.
Owner:NANKAI UNIV
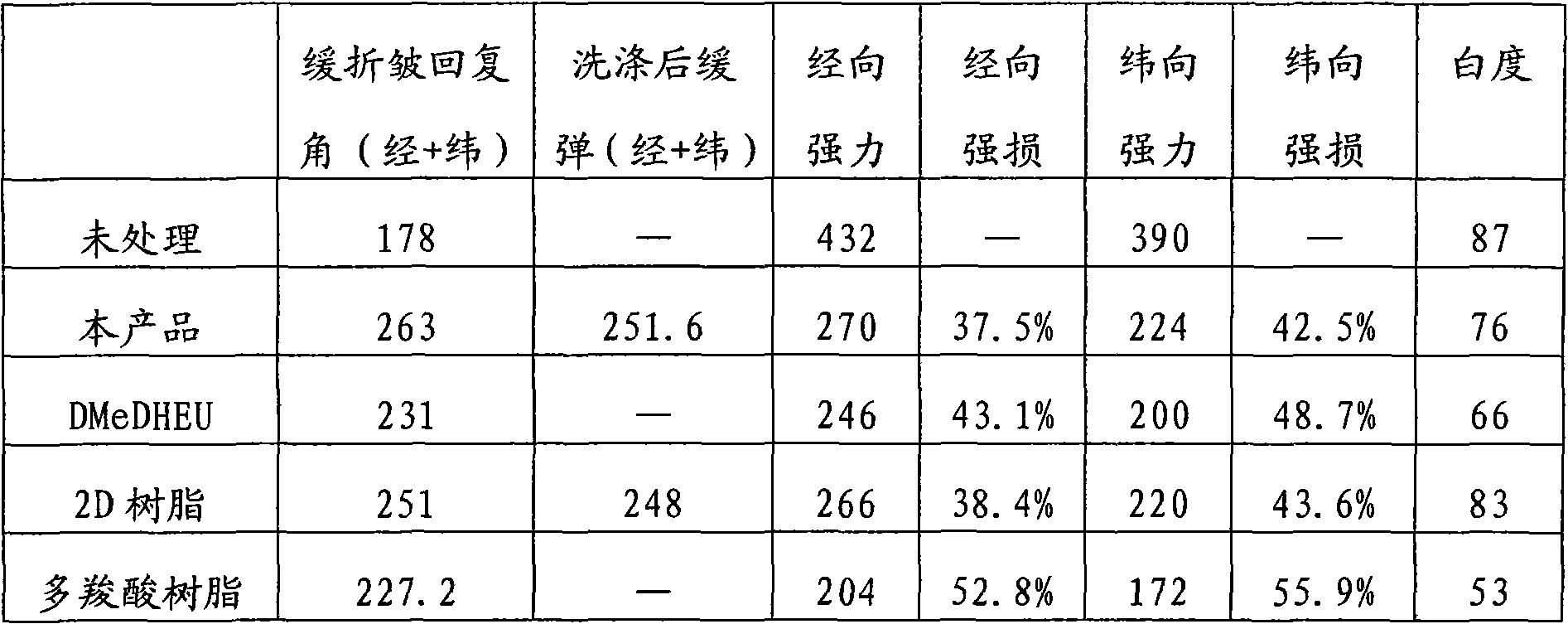
Formaldehydeless permanent press finishing agent for fabric and preparation method and application thereof
ActiveCN101956322AOvercome lossImprove wrinkle resistanceVegetal fibresPotassium persulfatePolymer science
The invention discloses a novel formaldehydeless permanent press finishing agent for a fabric and a preparation method and application thereof. The formaldehydeless permanent press finishing agent for the fabric is prepared by performing a polymerization reaction on acrylamide, glyoxal, phthalic anhydride, sulfuric acid, potassium persulfate and the like. The product of the invention is mainly used for performing noniron finish on the fabric and improving the firmness of a dyed fabric and a printed fabric. Compared with the conventional N-methylol acrylamide resin, the permanent press finishing agent has prominent advantages of excluding of formaldehyde and crease resilience equivalent to or surpassing those of etherfied 2D (etherfied dimethylol dihydroxy ethylene urea) after being used for finishing a cotton fabric; and compared with the conventional formaldehydeless resin, the agent has the advantages of high crease resilience, strength loss less than that of a polycarboxylic formaldehydeless resin, cost lower than those of dimethylol dihydroxy ethylene urea (DMeDHEU) and butanetetracarboxylic acid (BTCA) and cost performance far higher than that of a polycarboxylic resin.
Owner:SICHUAN YIXIN TECH
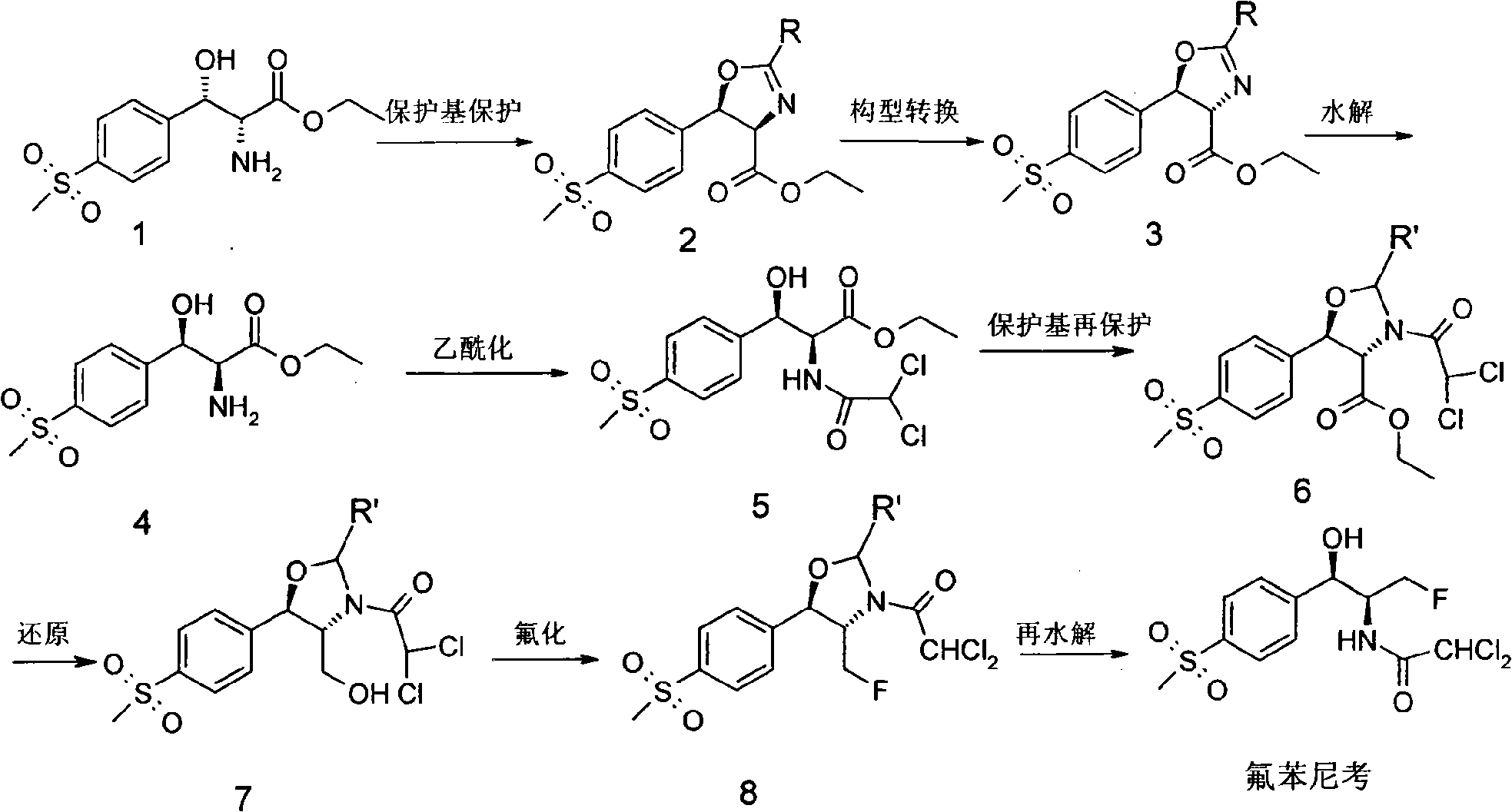
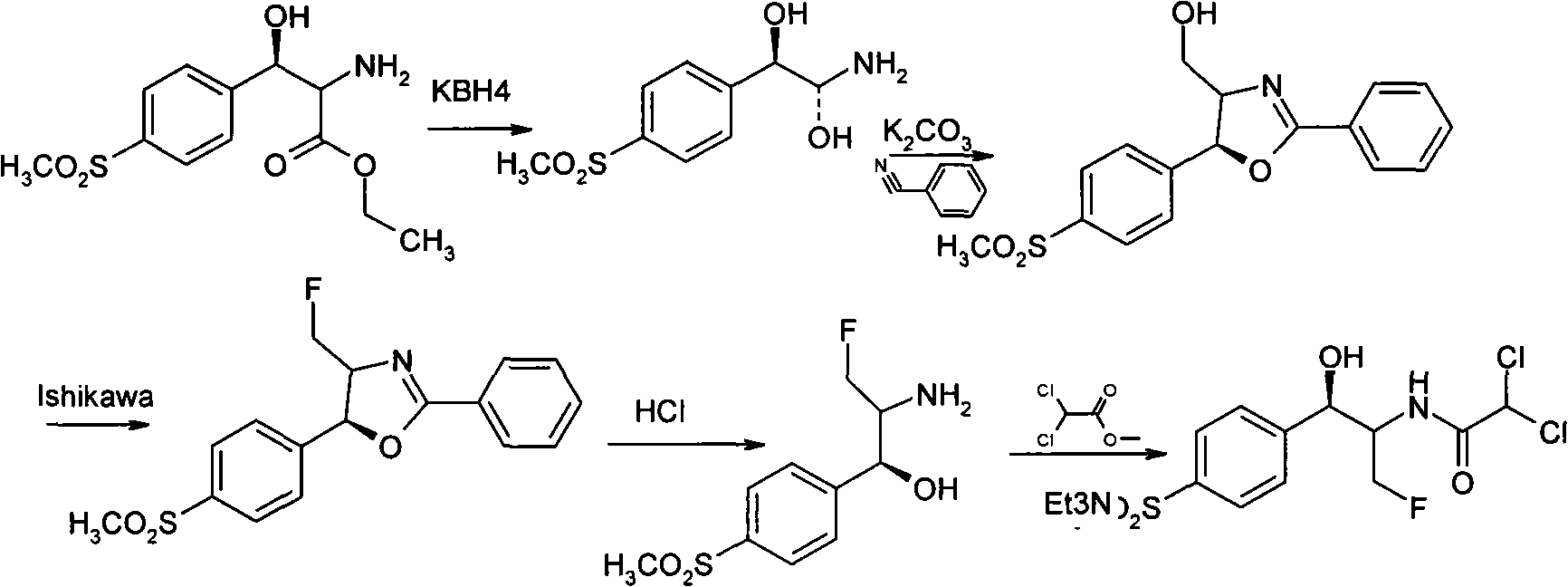
Method for synthesizing florfenicol
InactiveCN101265220AEasy marketEasy to operateAntibacterial agentsOrganic chemistrySodium methoxideSynthesis methods
The invention discloses a synthesis method of synthesizing florfenicol. The synthesis method comprises the following steps: L-threo-(p-(methylsylfonyl) phenyl) serine ethyl ester which is used as raw material is processed by the protection by a protecting group, configurational transition, hydrolyzation, acetylation, re-protection, deoxidation, fluorination, and pre-hydrolyzation, so that the florfenicol is obtained, wherein, the protecting group R is one of benzoyl chloride, phthalic anhydride, cyanophenyl, and allyl compounds; the configurational transition is performed when sodium alcoholate or sodium methoxide exists. The raw material which is used in the method is a byproduct which is generated during the process of preparing thiamphenicol, is easy to get in the market, and is inexpensive; the technological operation is simple, the cost is low, the yield rate is high, and the synthesis method has industrialized value.
Owner:SHANGHAI RECORD PHARM CO LTD
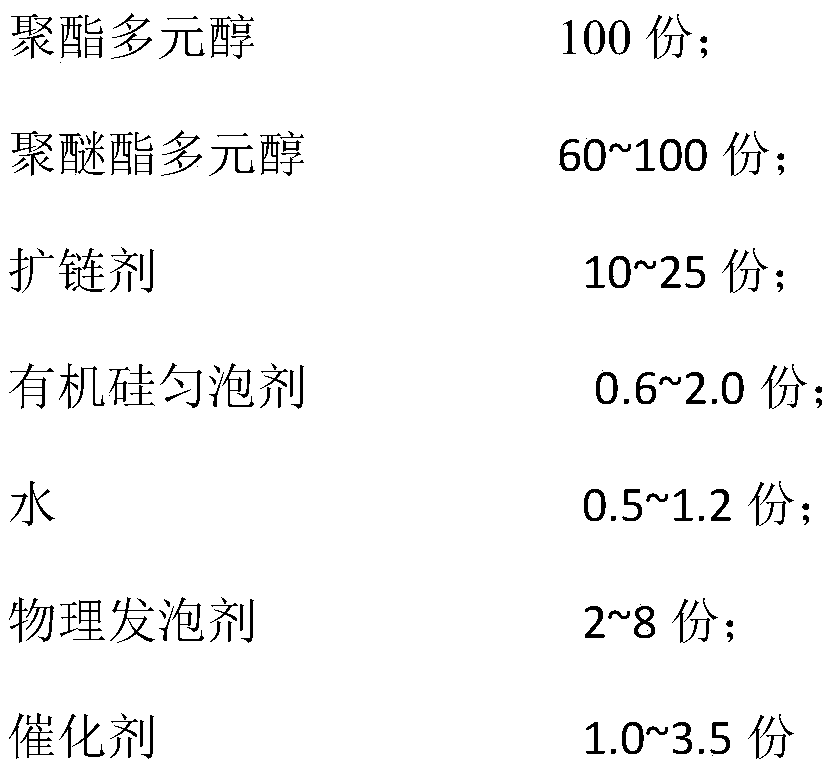
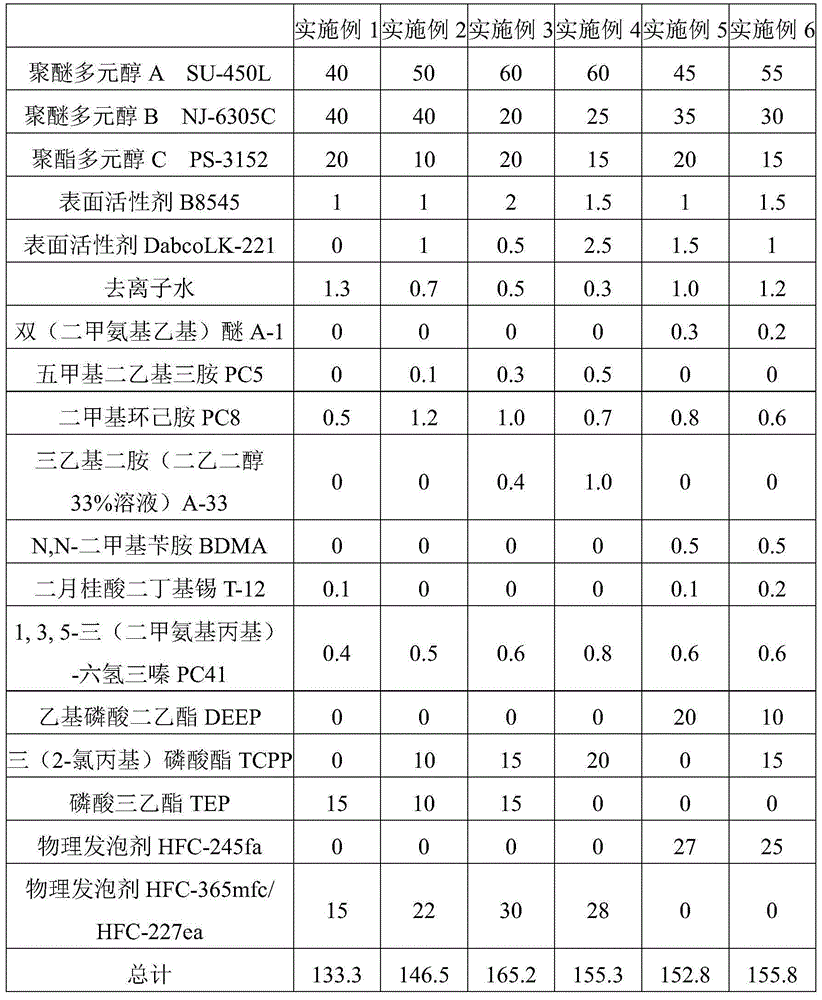
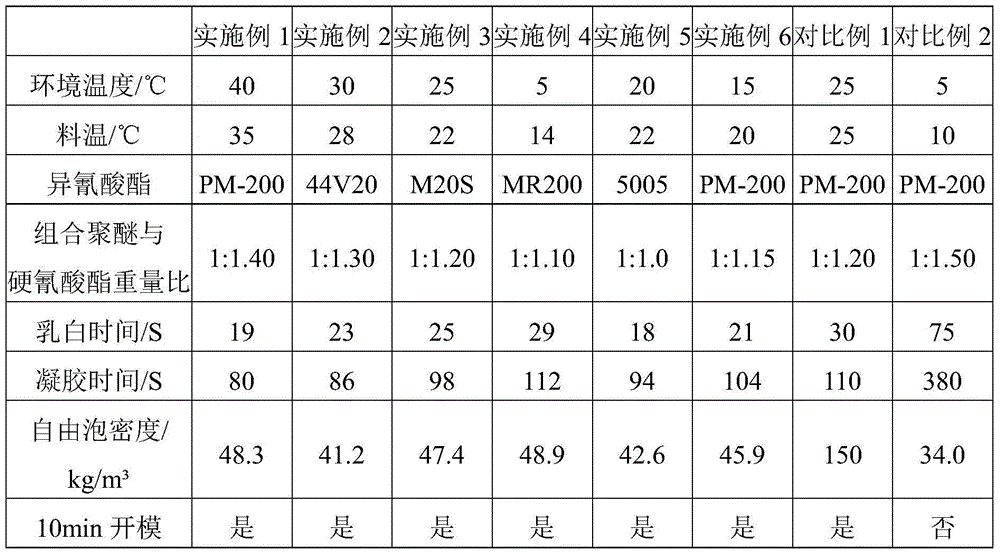
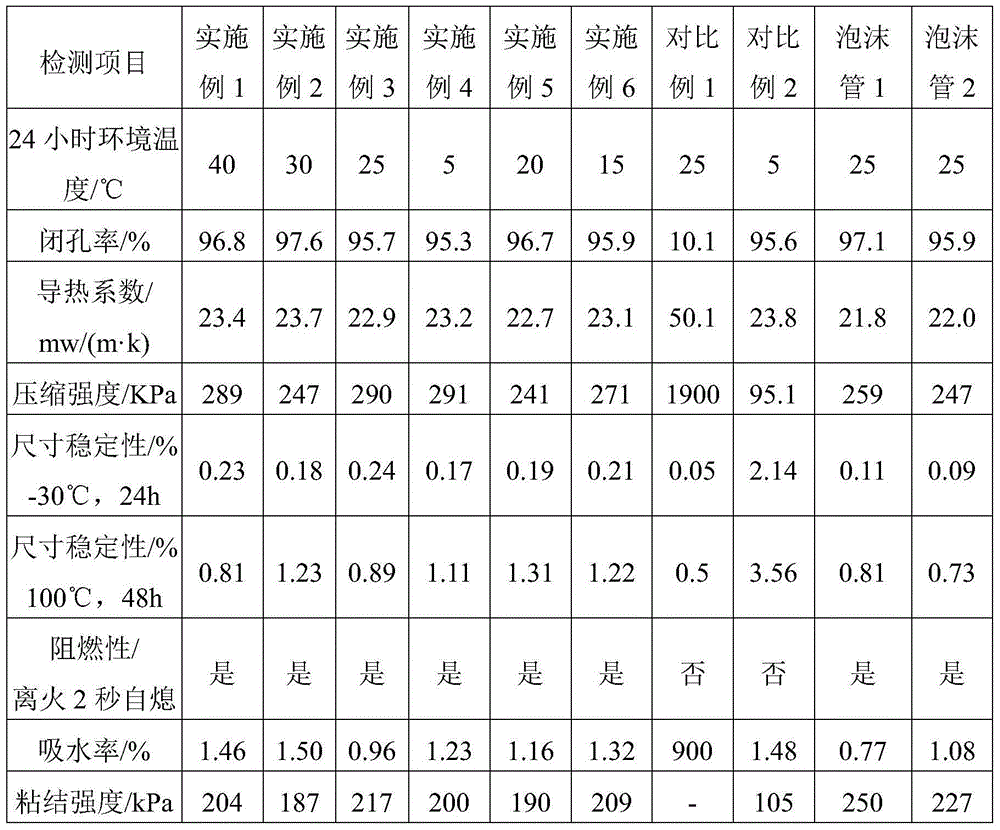
Low-temperature low-conductivity polyurethane foam, raw material composition, combined polyether and preparation method thereof
The invention discloses low-temperature low-conductivity polyurethane foam, a raw material composition, combined polyether and a preparation method thereof. The combined polyether comprises the following components in parts by weight: 30-50 parts of sorbitol-based polyether polyol, 20-40 parts of sucrose-based polyether polyol, 10-20 parts of glyceryl polyether polyol, 5-15 parts of phthalic anhydride polyester polyol, 2.0-3.0 parts of a surfactant, 1.5-2.5 parts of a chemical foamer, 0.3-0.7 part of a catalyst A, 0.2-0.5 part of a catalyst B, 0.8-1.5 part of a catalyst C, and 10-30 parts of 1, 1, 1, 3, 3-perfluoropropane. The total weight of polyether polyol and polyester polyol is 100 parts. The catalysts A, B and C are independently amine catalysts or metal catalysts, respectively, and are different from each other. The polyurethane foam is excellent in performance, strong in low temperature resistance, good in dimensional stability, low in heat conductive coefficient and wide in application range.
Owner:SHANGHAI DONGDA POLYURETHANE
Polymide resin, method for manufacturing the same and thin film thereof
The invention discloses a polymide resin, a method for manufacturing the same and thin film thereof. The polyimide resin is derived from at least two dianhydrides and at least two diamines. The dianhyride is selected from a group consisting of p-phenylenebis (trimellitate anhydride), 4,4'-(hexafluoroisopropylidene)-diphthalic anhydride, and 4,4'-(4,4' -isopropylidenediphenoxy)bis(phthalic anhydride). One of the diamine monomers is 2,2' -bis(trifluoromethyl)benzidine, and the molar ratio of the 2,2'-bis(trifluoromethyl)benzidine to the diamine monomers is between 70% and 90%; and the other diamine monomers are selected from a group consisting of 4,4'-diaminobenzanilide, 4,4'-methylenedianiline, 2,2-bis[4-(4-aminophenoxy)phenyl, 4,4'-diaminodiphenyl-sulfone, 1,3-bis(4-aminophenoxy)benzene, 4,4'-oxydianiline, p-phenylenediamine, 4,4'-diamino-2,2'-dimethyl-1,1'-biphenyl and 2,2-bis[4-(4-aminophenoxy)phenyl]-1,1,1,3,3,3-hexafluoropropane.
Owner:MICROCOSM TECH
Electric-insulation epoxy resin castable for outdoor mutual inductor
ActiveCN103709608AExcellent physical and chemical propertiesHeat resistantPlastic/resin/waxes insulatorsPolymer scienceFirming agent
The invention relates to an electric-insulation epoxy resin castable for an outdoor mutual inductor. The epoxy resin castable comprises the following materials by weight percent: 21-26% of resin, 45-55% of filling, 15-25% of a curing agent and 2-6% of other auxiliary agents, wherein the resin is formed by mixing organic silicon modified epoxy resin, phenolic aldehyde epoxy resin, brominated epoxy resin and bisphenol F-type epoxy resin; the filling is silane-processed silica powder; the curing agent is modified methylhexahydrophthalic anhydride; the other auxiliary agents include a curing catalyst, a plasticizer, a reactive diluent, a silane coupling agent, a coloring agent and an antioxidant. The electric-insulation epoxy resin castable has excellent physical and chemical properties and has the performance of heat resistance, low temperature resistance, ultraviolet irradiation resistance, ageing resistance and the like. The electric-insulation epoxy resin castable is applicable to the electric insulation of the outdoor mutual inductor and other electrical equipment.
Owner:JIANGXI TENGDE IND
Interpenetrating polymer network anticorrosion paint and production method thereof
InactiveCN101492588AShorten drying timeHigh surface hardnessAnti-corrosive paintsEpoxy resin coatingsEpoxyBenzoic anhydride
Owner:衡阳市化工研究所
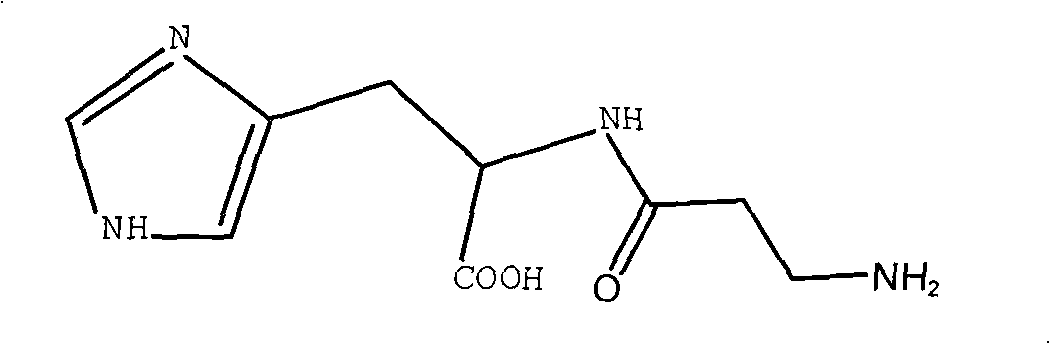
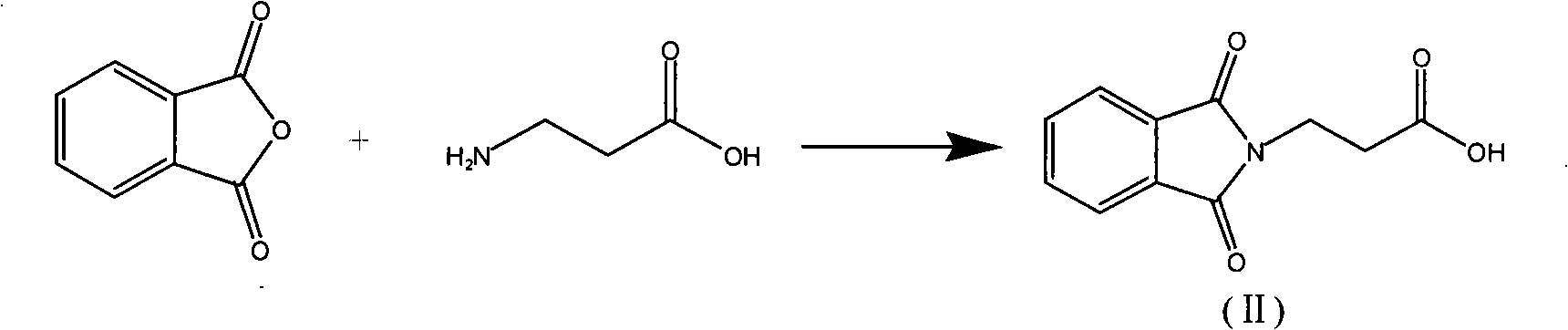
Synthetic method of L-carnosine
ActiveCN101284862AAvoid formingReduce consumptionPeptide preparation methodsBulk chemical productionHydrazine compoundOrganic synthesis
A method for synthesizing L-carnosine belongs to the organic synthesis technical field. The method is as follows: beta-alanine is dissolved in non-polar solvent to be reacted with phthalic anhydride under the catalysis of organic amine, and then phthaloyl-beta-alanine is obtained through water recrystallization; the phthaloyl-beta-alanine is dissolved in solvent to synthesize phthaloyl-beta-alanyl chloride through the chlorinated reagent of acyl chloride phthaloyl-beta-alanine; L-histidine is reacted with hexamethyl disilazane or trimethylchlorosilane to obtain L-histidine trimethylsilane protector; the protector is reacted with the phthaloyl-beta-alanyl chloride to obtain hydrochloride product with the protecting group divested by water, and then neutralization product is obtained by the hydrochloride product obtained through the neutralization condensation reaction of alkaline reagent, thereby obtaining L-carnosine crude product through the hydrazinolysis of the neutralization product by hydrazine hydrate; and the crude product is purified to obtain L-carnosine finished product. The method has low raw material consumption, short reaction procedure and high yield; moreover, the quality of synthesized L-carnosine can meet the requirements of industrial production.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
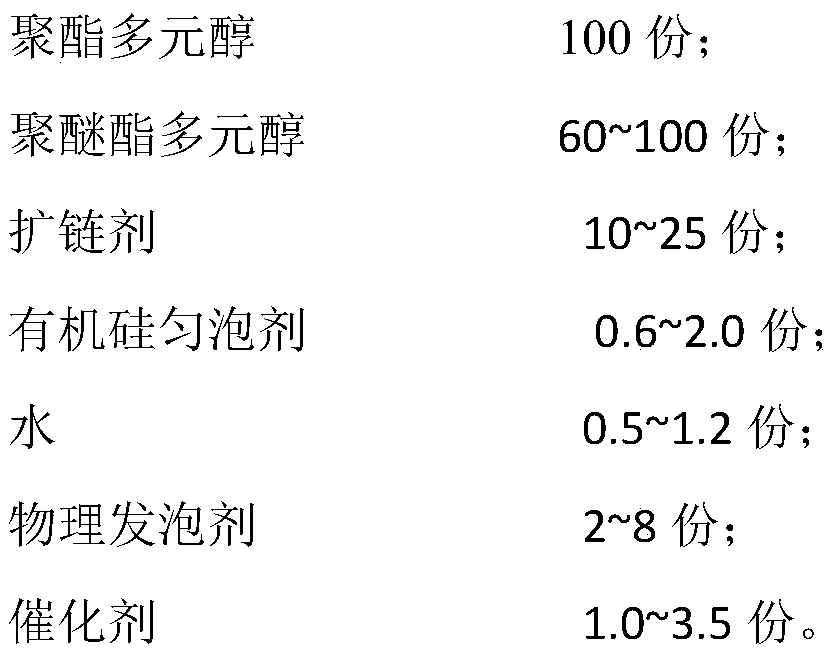
Combined polyether, raw material composition, polyurethane foam as well as preparation method and application thereof
ActiveCN104592471ALow thermal conductivityHigh compressive strengthThermal insulationPipe protection by thermal insulationPolyesterPolyol
The invention discloses combined polyether, a raw material composition, polyurethane foam as well as a preparation method and application thereof. The combined polyether comprises the following components in parts by weight: 100 parts of polyol, 1-4 parts of a surfactant, 0.3-1.3 parts of a chemical foaming agent, 1-3 parts of a catalyst, 15-30 parts of a flame retardant and 15-30 parts of a physical foaming agent, wherein polyol is a mixture of 40-60 parts of polyether polyol A, 20-40 parts of polyester polyol B and 10-20 parts of phthalic anhydride polyol. The foam has the advantages of excellent comprehensive property, low cost, a certain thermal insulation fire-retardant property and is simple in preparation method, safe to operate and wide in application range, high in construction and foam curing speed and short in time from mixing to foam molding curing ( only 10 minutes) and can be used in high-temperature, wind, cold and other 5-40 DEG C terrestrial environments.
Owner:SHANGHAI DONGDA POLYURETHANE
High-temperature-resistant rubber sealing ring for automobiles
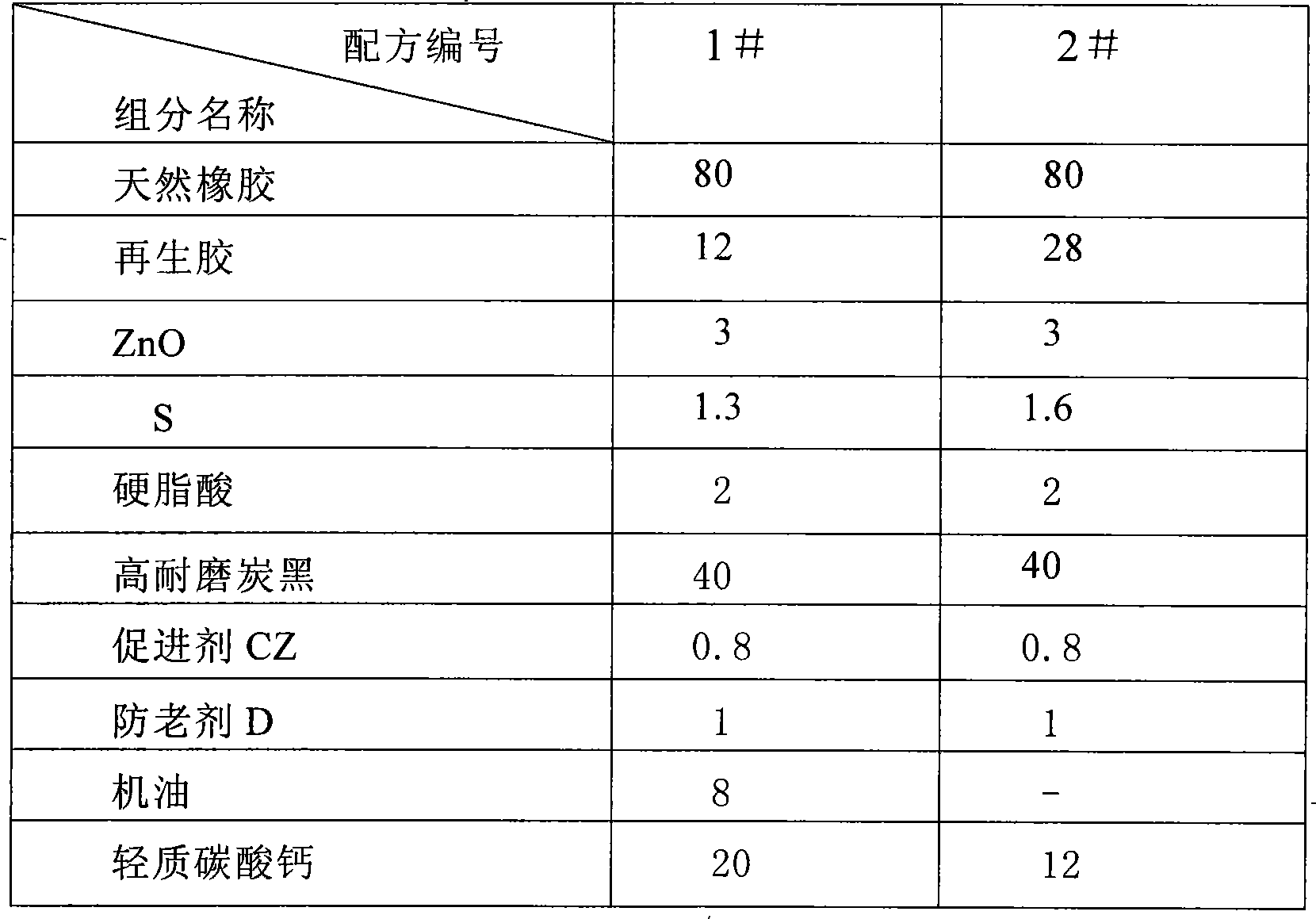
The invention discloses a high-temperature-resistant rubber sealing ring for automobiles. The high-temperature-resistant rubber sealing ring comprises the following raw materials in parts by weight: 70-90 parts of ethylene-propylene-diene-terpolymer rubber, 10-30 parts of chloroprene rubber, 1-4 parts of an anti-aging agent, 0.8-1.5 parts of active zinc oxide, 0.2-0.4 part of magnesium oxide, 0.5-1.0 part of calcium oxide, 0.6-1.5 parts of butyltin mercaptide, 30-40 parts of modified kaolin, 10-15 parts of magnesium methacrylate, 1-3 parts of a promoter, 6-10 parts of soybean oil, 1-2 parts of ethyl methacrylate, 40-80 parts of paraffin oil, 0.2-0.5 part of triallyl phosphate, 1.0-1.5 parts of a crosslinking agent BIBP, 0.5-1.2 parts of an assistant crosslinking agent TAIC, 0.3-0.5 part of microcrystalline wax, 2-3 parts of a vulcanizing agent and 0.5-1 part of phthalic anhydride. The sealing ring disclosed by the invention has strong weather resistance, corrosion resistance and ozone resistance, and can be suitable for being used as the high-temperature-resistant rubber sealing ring for the automobiles.
Owner:TONGLING SANSHENG ELECTRONICS
Unsaturated polyester resin for pultrusion and preparation method for same
The invention discloses unsaturated polyester resin for pultrusion and a preparation method for the same. The resin is mainly made of, by weight, 6400-6800 parts of phthalic anhydride, 4000-4800 parts of maleic anhydride, 1700-2000 parts of 1,2-propylene glycol, 4000-4400 parts of ethylene glycol, 8000-9000 parts of styrene, 1-5 parts of polymerization inhibitor, 1-5 parts of antioxidant and 0.01-1 part of retarding agent. Compared with the prior art, the unsaturated polyester resin has the advantages that the heat distortion temperature of the unsaturated polyester resin is higher than 88 DEG C on the premise of meeting a pultrusion process requirement, and performances such as stretching, compression strength and the like of the unsaturated polyester resin are remarkably enhanced.
Owner:宜兴市兴合树脂有限公司
Synthetic resin and preparation method as well as application thereof
InactiveCN102174217AHigh molecular weightHigh softening pointBuilding insulationsPolyesterPentaerythritol
The invention provides a synthetic resin composition which consists of the following components in percentage by weight: 35 percent of component A and 65 percent of component B, wherein the component A is formed by polymerizing the following monomers in parts by weight: 20 parts of polyethylene, 20 parts of polypropylene, 15 parts of polyester, 8 parts of nylon, 10 parts of phthalic anhydride and 4 parts of pentaerythritol; and the component B is prepared from the following raw materials in parts by weight: 20 parts of pine tar, 8 parts of naphthenic oil, 10 parts of coumarone indene resin, 5 parts of 420 resin and 57 parts of rubber powder. In the resin composition, high molecular material waste can be effectively utilized and changed into things of value, and environmental pollution can be reduced. The invention also provides a preparation method and application of the resin composition.
Owner:方先明
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com