Preparation method of biodegradable carbon dioxide-based polyester-polycarbonate terpolymer
A technology of polycarbonate terpolymer and carbon dioxide, which is applied in the field of preparation of biodegradable carbon dioxide-based polyester-polycarbonate terpolymer, can solve the problems that have not been reported and limit large-scale application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
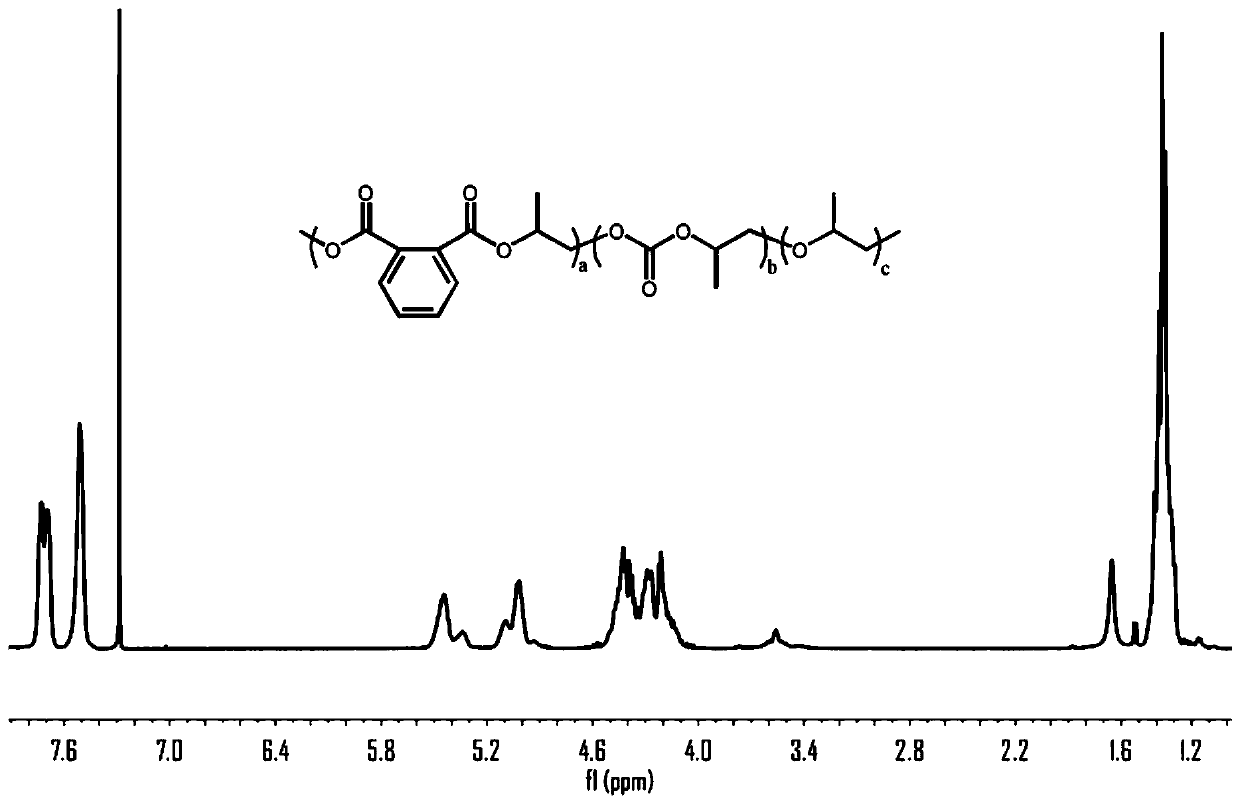
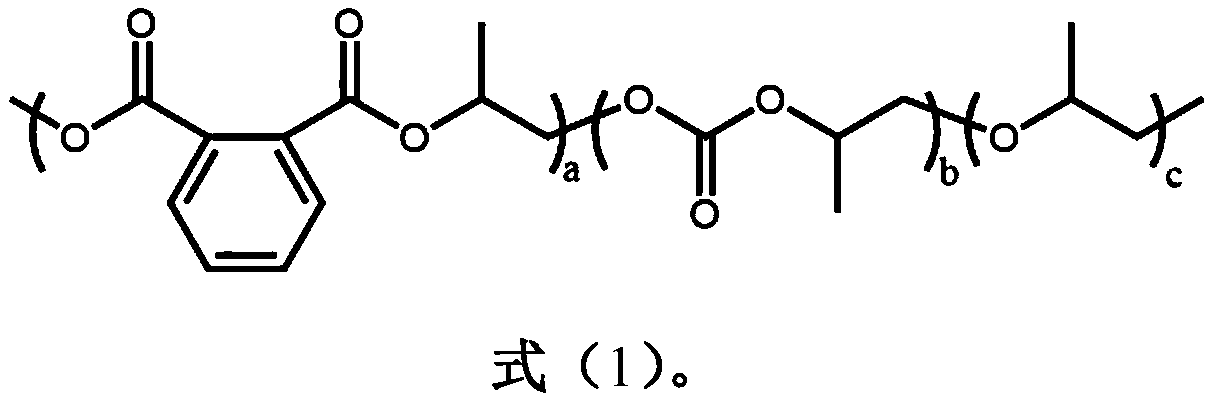
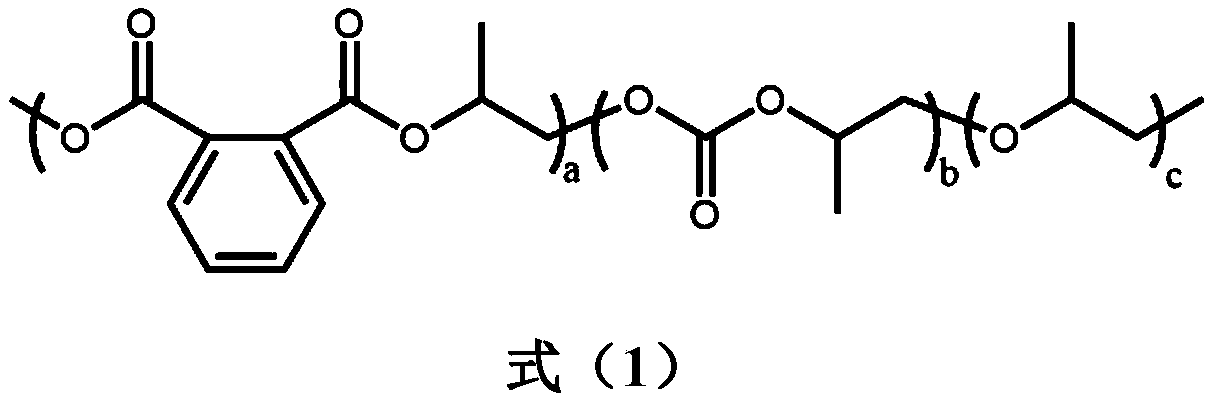
[0020] With a 50mL autoclave as the reaction vessel, in an anhydrous and oxygen-free environment, 1.0g of phthalic anhydride, 3.92g of propylene oxide, 7.5mg of tetra-n-butylammonium chloride, and 54μL of triethylboron solution were sequentially Put it into a high-pressure reactor, fill it with 1.0MPa carbon dioxide, react at 80°C for 12 hours, release the carbon dioxide pressure after the reaction, dissolve the product with dichloromethane, quench the reaction with dilute hydrochloric acid / methanol solution, and precipitate in ethanol to obtain a polymer , After the polymer is vacuum-dried, do molecular weight testing, nuclear magnetic analysis and thermal performance analysis. m n =52.7kDa, PDI=1.16, polyester content 14.8%, polycarbonate content=81.6%, polyether content=3.6%, T g = 40.5°C.
Embodiment 2
[0022] With a 50mL autoclave as the reaction vessel, in an anhydrous and oxygen-free environment, 2.5g of phthalic anhydride, 4.90g of propylene oxide, 9.4mg of tetra-n-butylammonium chloride, and 200μL of triethylboron solution were sequentially Put it into a high-pressure reactor, fill it with 1.0MPa carbon dioxide, react at 80°C for 12 hours, release the carbon dioxide pressure after the reaction, dissolve the product with dichloromethane, quench the reaction with dilute hydrochloric acid / methanol solution, and precipitate in ethanol to obtain a polymer , After the polymer is vacuum-dried, do molecular weight testing, nuclear magnetic analysis and thermal performance analysis. m n =102.8kDa, PDI=1.35, polyester content 39.9%, polycarbonate content=55.2%, polyether content=4.9%, T g =47.4°C
Embodiment 3
[0024] With a 50mL autoclave as the reaction vessel, in an anhydrous and oxygen-free environment, 1.0g of phthalic anhydride, 4.0g of propylene oxide, 7.5mg of tetra-n-butylammonium chloride, and 54μL of triethylboron solution were sequentially Put it into a high-pressure reactor, fill it with 3.0MPa carbon dioxide, react at 80°C for 12 hours, release the carbon dioxide pressure after the reaction, dissolve the product with dichloromethane, quench the reaction with dilute hydrochloric acid / methanol solution, and precipitate in ethanol to obtain a polymer , After the polymer is vacuum-dried, do molecular weight testing, nuclear magnetic analysis and thermal performance analysis. m n =61.2kDa, PDI=1.27, polyester content 33.4%, polycarbonate content=60.8%, polyether content=5.8%, T g = 45.6°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com